- 1Ist Department of Oncological Gynaecology and Gynaecology, Medical University in Lublin, Lublin, Poland
- 2Department of Pharmaceutical Microbiology, Faculty of Pharmacy, Medical University in Lublin, Lublin, Poland
- 3Department of Obstetrics and Pathology of Pregnancy, Medical University in Lublin, Lublin, Poland
- 4Independent Laboratory of Cancer Diagnostics and Immunology, Medical University in Lublin, Lublin, Poland
- 5Frauenklinik, Marienhospital Witten, Witten, Germany
Introduction: A healthy vaginal microbiota is represented mainly by Lactobacillus spp. and plays a vital role in maintaining the functional balance in the vaginal environment. Scientists have drawn attention to possible correlations between the vaginal microbiome and gynecological neoplasms. Several recent studies have shown a potential link between the vaginal microbiome and the risk of developing cervical cancer from human papillomavirus (HPV) infection. This study aimed to compare the prevalence and abundance of various lactic acid bacteria species (LABs) in vaginal swabs from healthy controls and patients with abnormal Pap smear results.
Methods: The study included 100 women (79 patients with abnormal cervical Pap smear results and 21 controls) from whom vaginal swabs were collected. Real-time quantitative PCR was used to determine seven lactic acid bacteria (LAB) species and their quantities.
Results: Most patients were colonized by two Lactobacillus species, primarily Lactobacillus gasseri (93%) and L. crispatus (83%). Patient age and place of residence were associated with the diversity of LAB in the vaginal microbiota. The abundance of L. delbrueckii in the vaginal microbiota increased, whereas the abundance of L. gasseri abundance decreased, with patient age. Lactobacillus acidophilus and Limosilactobacillus fermentum were significantly more often detected in patients living in rural versus urban areas. Statistical analysis did not show any significant differences in LAB between groups of patients with various changes on smear tests.
Discussion: The degree of dysplastic changes in the endothelium or the presence of a group of atypical cervical stratified epithelial cells was not associated with significant changes in the studied vaginal bacteria.
1. Introduction
Bacteria, viruses, archaea, yeast, and fungi form the human microbiome (Marchesi and Ravel, 2015; Sender et al., 2016; Requena and Velasco, 2021), which colonizes the skin, genitourinary system, oral cavity, and intestinal tract. The human microbiota establishes a symbiotic relationship with the host and plays a vital role in maintaining the host’s physiological homeostasis (Dominguez-Bello et al., 2019). The composition of the microbiota differs depending on the site of microbial colonization. A healthy vaginal microbiota is represented mainly by Lactobacillus spp. including L. crispatus, L. jensenii, L. gasseri, and L. iners (Requena and Velasco, 2021), whereas Prevotella, Gardnerella, Atopobium, Sneathia, Bifidobacterium, Megasphaera, and Anaerococcus are associated with vaginosis (Moreno and Simon, 2018). The microbiota plays a vital role in maintaining functional balance in the vaginal environment and in preventing colonization by pathogens and the overgrowth of commensal microorganisms. Lactobacillus spp. stabilize the vaginal microbiota by producing antimicrobial components such as hydrogen peroxide, lactic acid, and bacteriocin-like compounds. The ability to adhere and compete with other harmful microorganisms is also crucial (Borges et al., 2014; Tachedjian et al., 2017). In most areas of the human body, a highly species-diverse microbiome is a sign of health (Turnbaugh et al., 2007; Flores et al., 2014). However, for the female reproductive system, a healthy state is more often associated with low microbial diversity and the dominance of one or more Lactobacillus species (Ravel et al., 2011; Liu et al., 2013; MacIntyre et al., 2015). An increasing number of publications indicate a correlation between disturbances of the vaginal microbiota, bacterial vaginosis, and the development of fungal, bacterial, and viral infections (Marchesi and Ravel, 2015; Mitra et al., 2016; Sender et al., 2016; Requena and Velasco, 2021). Some studies have demonstrated a correlation between bacterial vaginosis and increased persistence of human papillomavirus (HPV) infections as well as the development of cervical intraepithelial neoplasia (Gillet et al., 2011; King et al., 2011; Gillet et al., 2012; Guo et al., 2012; Champer et al., 2018). Women with persistent HPV infections have a more species-diverse microbiome, rich in L. gasseri and Gardnerella vaginalis and at the same time with a low abundance of Lactobacilli (Gao et al., 2013; Lee et al., 2013). The rate of resolution of HPV infection and development cervical cancer might also depend on the composition of the vaginal microbiome (Brotman et al., 2014). Apart from vaginal microbiota, also cervical microbiome is important for female’s health. Higher cervical species diversity has been demonstrated in severer cervical intraepithelial neoplasia of reproductive-age women (Wu et al., 2021). More complex composition of cervical microbiota which tends to progress with the aggravation of lesions as well as the prevalence of anaerobic bacteria were found in women with cervical pathology. Disturbed cervical microbial community is associated with cervical intraepithelial neoplasia and cervical cancer (Wang et al., 2022). Lower relative abundance of Lactobacillus as well as presence of Porphyromonas, Prevotella and Campylobacter and Sneathia have been suggested to promote the development of cervical pathology.
Cervical cancer is the fourth most common cancer in the world in terms of both incidence and mortality. In 2018, nearly 570,000 new patients were diagnosed globally, and 311,000 women died from cervical cancer (Arbyn et al., 2020). This cancer can be prevented through early diagnosis and treatment of precancerous states, but it remains the most common cancer in most low and middle-income countries (Arbyn et al., 2020). This study aimed to compare the distribution of 7 various vaginal Lactobacillus spp. between Polish women with abnormal Pap smear results (study group) and healthy individuals (control group) based on quantitative analysis of bacteria in vaginal swabs. We also assessed correlations between the vaginal microbiota and patient age, place of residence (urban vs. rural), HPV status, menopausal status, and history of vaginal infection within the past 3 months.
2. Materials and methods
2.1. Participants
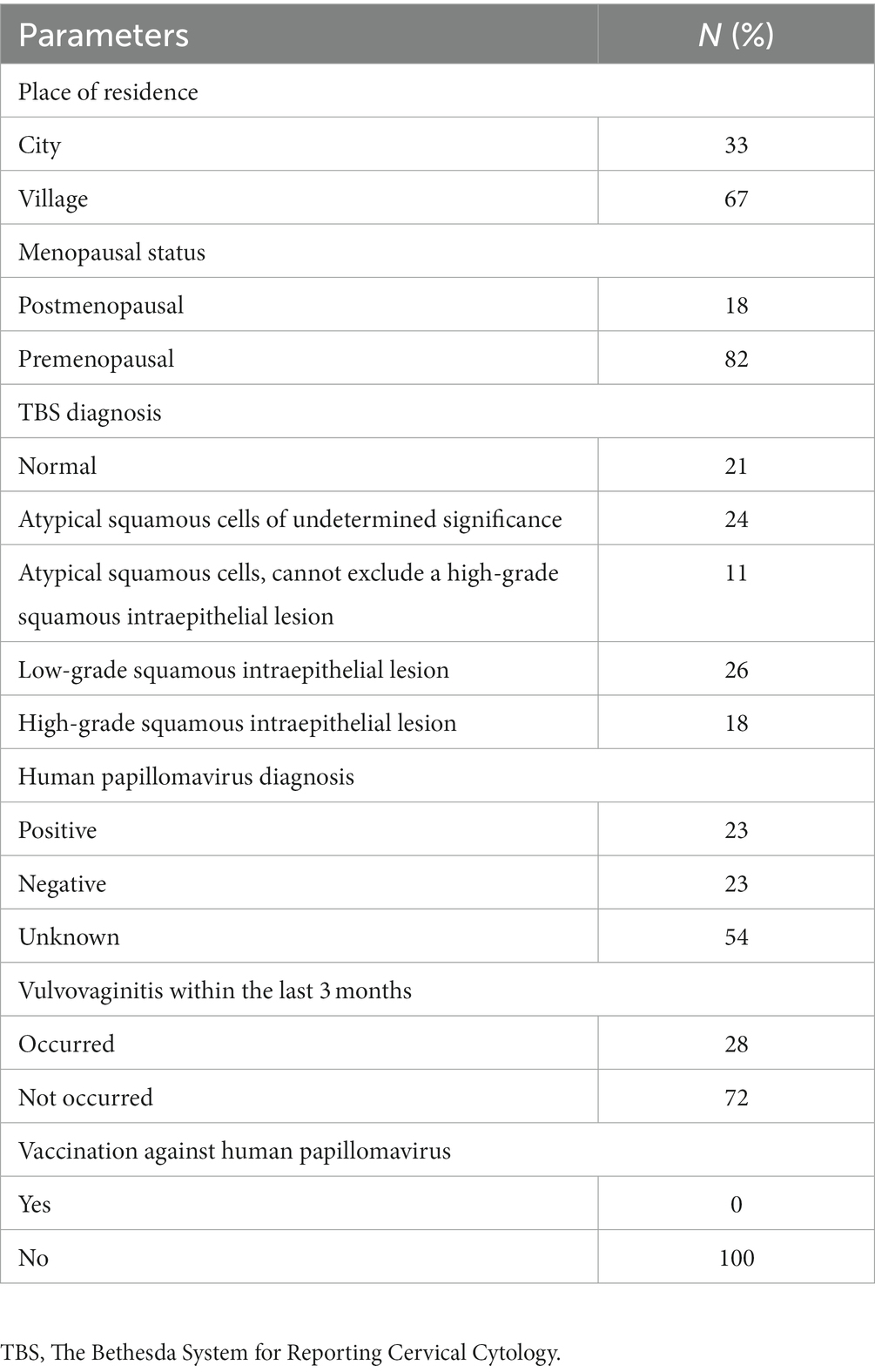
The study included 100 patients (aged 18–72 years) from Ist Department of Gynecological Oncology and Gynecology, Medical University in Lublin, Poland. Inclusion criteria were admission to Gynecology Department and expressed willingness to participate in the project. Exclusion criteria were lack of consent, undergoing surgical procedures in the lower genital area, age below 18, inability to collect accurate data for the project. Patients with abnormal Pap smear results were included in the study group, and healthy individuals were used as a control group. TBS system, which provides a uniform format and standardized lexicon for cervical/vaginal cytology reports was used to classify abnormal Pap smears (Chatterjee et al., 2000). Vaginal swabs were collected from all study participants from October 2014 to November 2017. Considering the size of women population aged 18–72 years in Poland is approximately 9.5 mLn, the margin of error of the measured value at the 95% confidence interval is ±6% when tested 100 women.
This study was approved by the Bioethics Committee of Medical University in Lublin KE – 0254/174/2014 and was conducted in accordance with the fundamental ethical principles described in the Declaration of Helsinki (World Medical Association, 2013). All participants signed informed consent to participate in this study.
2.2. Vaginal swabs for genetic testing
Vaginal swabs were collected from patients using sterile silicone brushes (Genomic Micro AX Swab Gravity Plus kit, A&A Biotechnology). Swabs were placed in 1.5 ml tubes with 700 μL Spheroid Lysis Buffer (SLB) (A&A Biotechnology). All specimens were stored in ultralow freezer at -80°C until further analyses. DNA isolation was performed within 6 months from the date of swab collection. The extraction resulted in high-quality DNA that is free of inhibitors for reproducible results.
2.3. Real-time quantitative PCR
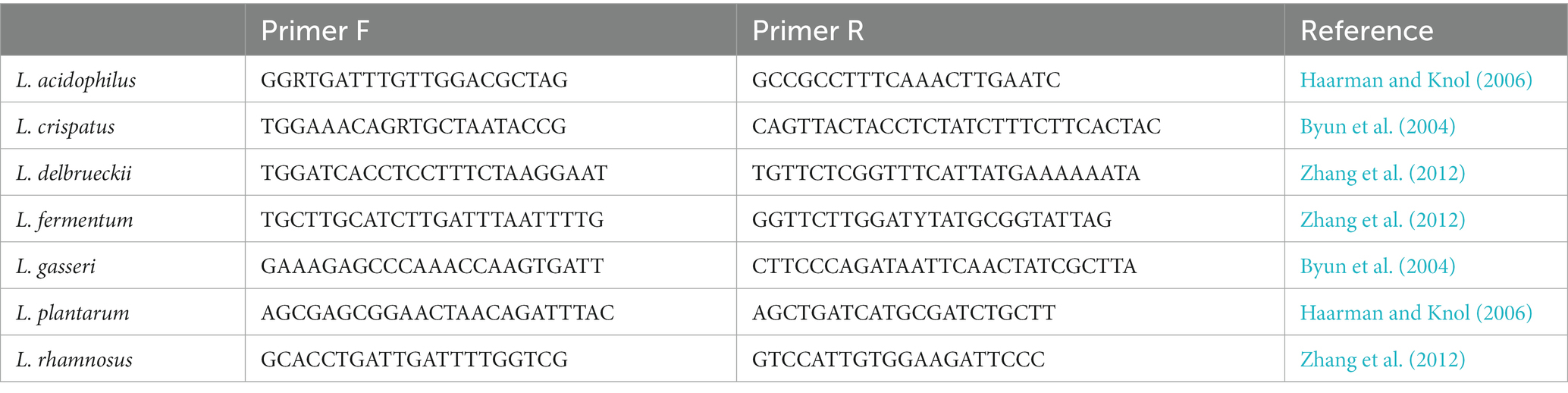
Real-time quantitative PCR was performed on 100 samples of DNA isolated from vaginal swabs from 79 patients with abnormalities in the smear test (50 patients with low-grade and 29 patients with high-grade epithelial lesions) and 21 healthy patients (control group) using LightCycler 96 (Roche). The quantitative analysis included seven Lactobacillus species (Lactobacillus acidophilus, Lactobacillus crispatus, Lactobacillus delbrueckii, Limosilactobacillus fermentum, Lactobacillus gasseri, Lactiplantibacillus plantarum, and Lacticaseibacillus rhamnosus). The quality control (QC) of the implementation procedure included IC (internal control) with amplification for the IC within normal limits, HC (high copy) control, LC (low copy) control, a normal control (NC) with amplification for IPC, and NTC (no template control) with no amplification. Standards, positive controls, and negative controls were performed in triplicate for each analysis. The primers (Genomed, Warsaw, Poland) presented in Table 1 were used for the detection and quantification of the seven LAB species.
2.4. Statistical analysis
The Shapiro–Wilk test was used to check the normality of the data distribution. The verification of statistical hypotheses was based on non-parametric tests. The non-parametric Mann–Whitney test was used to compare the two groups (study and control). The non-parametric Kruskal-Wallis test was used to compare more than two groups (if this test showed a statistically significant difference between the groups, the post-hoc test was used for further comparisons). Spearman’s rank correlation coefficients were calculated to analyze the coexistence of various bacterial species. The Pearson Chi-square test and Fisher’s exact test were used to evaluate the relationship between two qualitative features. STATISTICA 13.1 was used to perform statistical analyses. A p value of <0.05 was considered statistically significant.
3. Results
3.1. Clinical background of the study subjects
In our study, most females were premenopausal. Over 50% of study participants have not been tested for HPV infection and none of them have been vaccinated against it. Vulvovaginitis within lat 3 months was reported in 28% of enrolled females. The results of smear test were normal in 21% of participants, ASC-US was found in 24%, ASC-H in 11%, LSIL in 26% and HSIL in 18% of females. The demographic and clinical data of participants are summarized in Table 2. The vaginal microbiota of most participants (55%) was dominated by two of the seven LABs investigated. Three species were observed in 17% of the examined participants, four in 11%, and five in 3% of participants. In 2% of patients, none of the studied LABs was found. Two LABs tested were detected in 53.16% of the vaginal swabs collected from patients with abnormal Pap smear results (study group) and in 61.91% of individuals from the control group (Figure 1).
3.2. Associations between clinical findings and Lactobacilli
There were no statistically significant differences in identified LAB species between groups of patients with normal Pap smear results and those with ASC-US, ASC-H, LSIL, and HSIL (p > 0.05). In general, L. gasseri and L. crispatus were the most common LAB species detected in all enrolled patients (Table 3). L. gasseri and L. crispatus were also most detected in patients with ASC-US, ASC-H, LSIL, and HSIL. The occurrence of LAB species in participants with various Pap smear results is summarized in Table 3.

Table 3. Prevalence of LAB species detected in enrolled participants in relation to cervical Pap smear result.
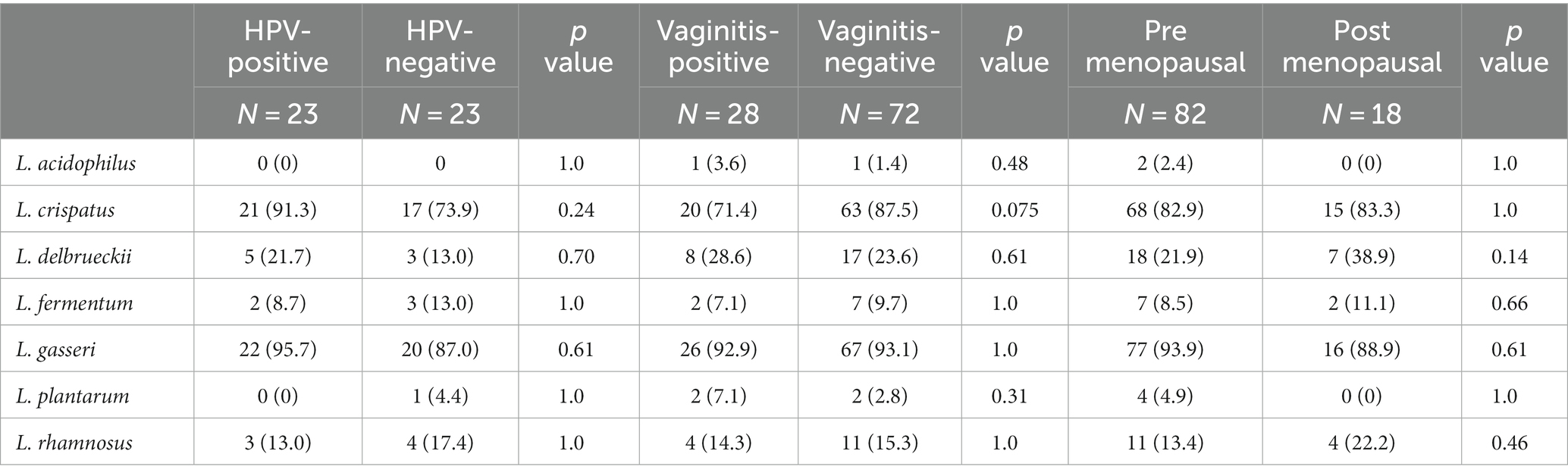
The quantitative real-time PCR analysis also demonstrated the numbers of L. crispatus and L. gasseri cells were the highest. Statistical analysis did not show any significant differences in the number of cells of each species between the studied subgroups of patients (Figure 2). What’s interesting, L. acidophilus was observed only in patients with HSIL and normal smear, while L. plantarum in women with ASC-US, LSIL and normal smear.
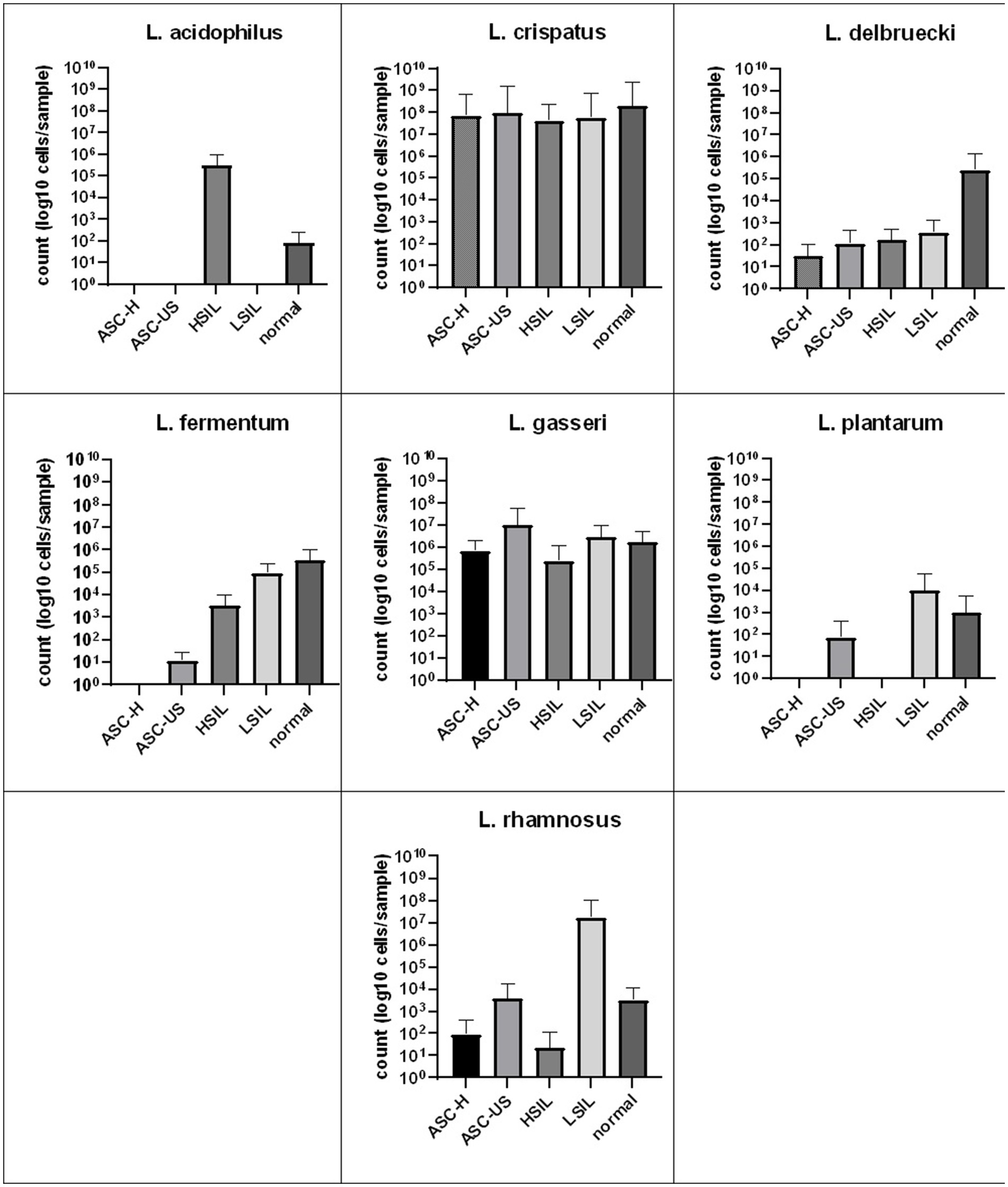
Figure 2. Comparison of the average number of LAB bacterial cells detected by real-time PCR from vaginal swabs in relation to cervical Pap smear result. SD, standard deviation; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion.
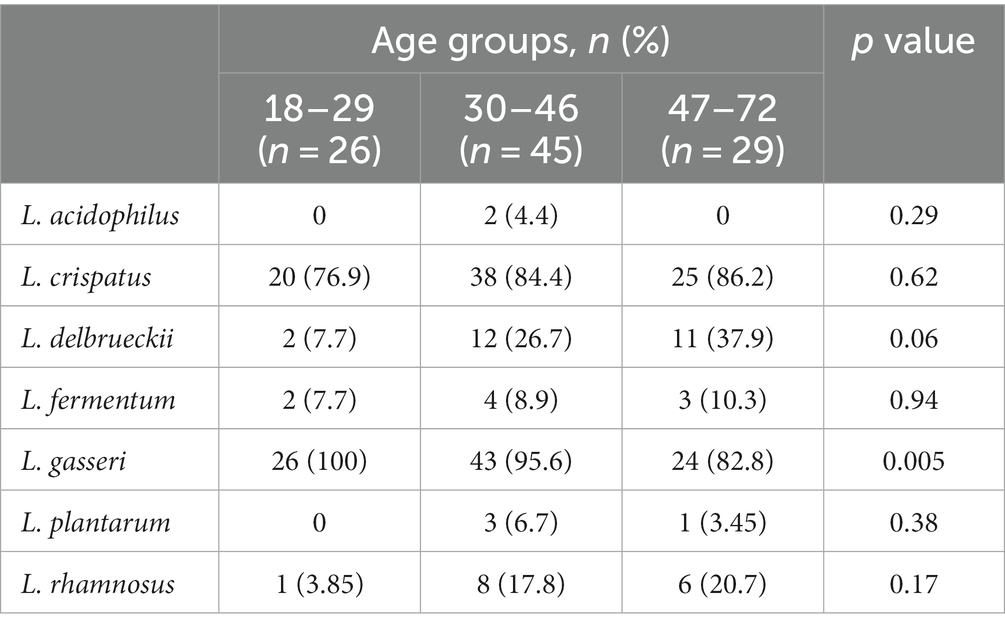
Moreover, analysis of the species distribution in different age groups showed that the abundance of L. delbrueckii significantly increased, whereas that of L. gasseri decreased, with age (Table 4). Statistical analysis of L. gasseri in three age groups also showed a statistically significantly higher abundance of this bacterium in swabs taken from patients aged 18–29 years (p = 0.0046). L. delbrueckii was significantly more abundant in swabs obtained from the oldest group of patients (47–72 years) (p = 0.057).
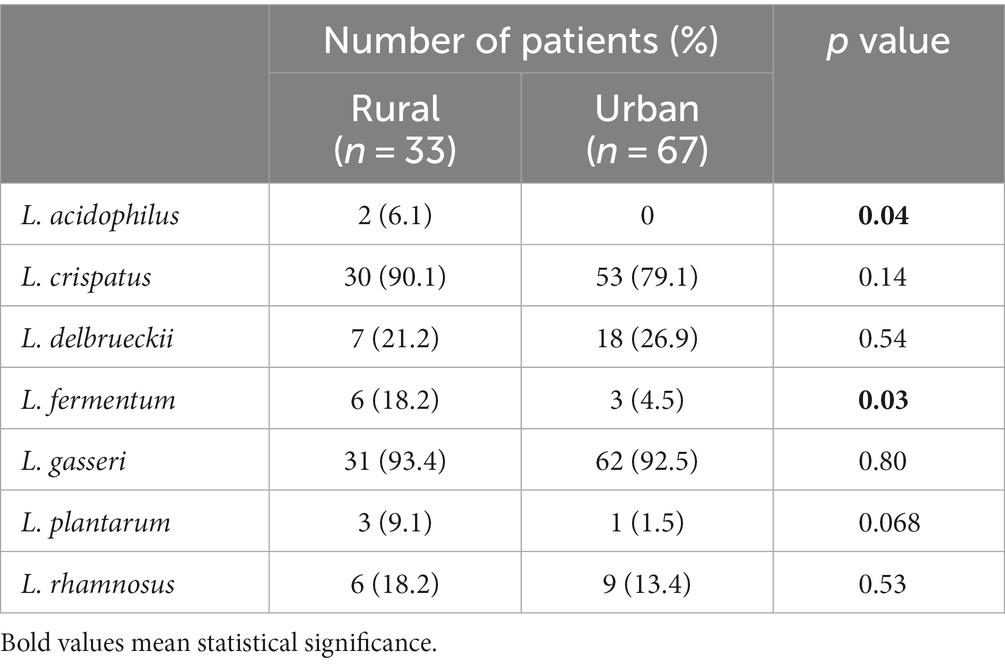
Lactobacillus acidophilus (6.1%) and L. fermentum (18.2%) were found significantly more frequent among women living in rural areas than among those living in urban areas (L. acidophilus 0%, L. fermentum 4.5%) (Table 5). Also, the abundance of L. acidophilus cells (range 0–5.7×106 vs. 0, p = 0.044) and L. fermentum cells (range 0–7.2×107 vs. 0) was higher in women living in rural areas than in those living in urban areas (0–1.6×106, p = 0.029).
There were no statistically significant differences in the vaginal microbiota in patients HPV positive (23 patients) and without HPV infection (23 patients), in those with and without vaginitis within the last 3 months, and between premenopausal and post-menopausal participants (Table 6).
3.3. Correlations between different Lactobacilli species
In patients with abnormal cervical Pap smear result significant positive correlations were identified for L. delbrueckii and L. gasseri (r = 0.79, p = 0.048), and negative correlation between L. fermentum and L. plantarum (r = −0.79, p = 0.048). In healthy control group, significant correlation between L. delbrueckii and L. rhamnosus (r = 0.59, p = 0.005) and L. pantarum and L. fermentum (r = 0.72, p = 0.0002) was detected indicating the coexistence of these species (Figure 3).
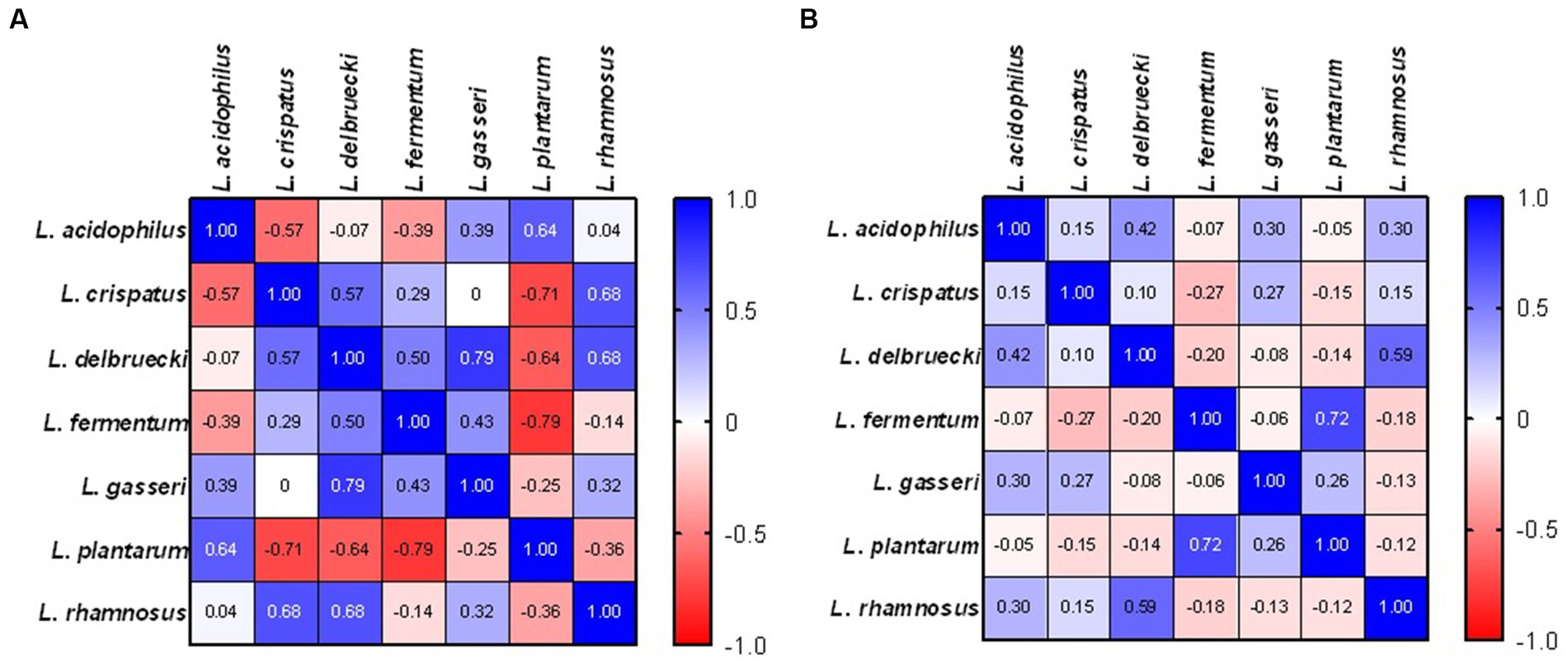
Figure 3. Correlations indicating the co-occurrence of individual LAB species in patients with abnormal cervical Pap smear result (A) and healthy control (B).
4. Discussion
The vaginal microbiome plays a vital role in keeping the female reproductive system healthy. Bacterial vaginosis is a state in which the natural balance of bacteria in the vagina is disturbed, which has been linked to a wide variety of health problems, including a higher risk of sexually transmitted diseases, cystitis, postoperative complications, infertility, miscarriages, premature births, intrauterine infections, cervical and uterus infections, dysplasia, and cervical cancer. Also, cervical microbiota is crucial since the cervix is the place of infections caused by pathogens such as human immunodeficiency virus (HIV) and HPV, Chlamydia trachomatis, or Neisseria gonorrhoeae.
In our present study, vaginal microbiota of most participants in both groups was dominated by two of the seven LABs investigated. Three species were observed in 17% of the examined participants, four in 11%, and five in 3% of participants. This study revealed the presence of LAB belonging to seven species (L. acidophilus, L. crispatus, L. delbrueckii, L. fermentum., L. gasseri, L. plantarum, and L. rhamnosus) in the material from vaginal swabs, with L. gasseri being most frequently detected, and L. crispatus being most abundant one. The dominance of Lactobacillus crispatus and L. gasseri is observed in community state types (CST) I and II group, respectively (Ravel et al., 2011). The presence of L. gasseri was suggested to be associated with rapid clearance of acute HPV infection (Brotman et al., 2014). It has to be noted that HPV infection itself was demonstrated to modify the mucosal metabolism and host immunity, thus promoting changes in the vaginal microbiota (Scott et al., 1999). The results of studies indicate that Lactobacillus species are capable of adhering tightly to the vaginal epithelium, covering its surface, thus protecting the vagina from colonization by pathogenic microorganisms (Petricevic et al., 2014; Mitra et al., 2016). The presence of microbiome dominated by Lactobacilli is related with well-balanced immune-tolerant vaginal microenvironment (Donnarumma et al., 2014). However, it was observed that not all the species equally contributed.
Lactobacillus gasseri was the most frequently detected species in our study, whereas L. crispatus was the most abundant one. Similar results were obtained by De Backer et al. (2007), who examined 71 vaginal swabs collected from Belgian women. They demonstrated that, among four Lactobacillus species (L. crispatus, L. iners, L. jensenii, and L. gasseri), L. crispatus was the most common (detected in 93% of the samples); L. iners was detected in 75% of swabs, L. gasseri in 73%, and L. jensenii in 46%. In another study, L. crispatus was the most abundant Lactobacillus species in the samples collected from healthy women and from women with bacterial vaginosis (Zozaya-Hinchliffe et al., 2010). In the vaginal and cervical swabs of 96 women from Florida, L. iners (55%), L. crispatus (29%), L. gasseri (13%), and L. jensenii (13%) were the most common species. In another American study, L. iners was the most abundant species in healthy women and in those with bacterial vaginosis (Pavlova et al., 2002). A meta-analysis by Norenhag et al. (2020) showed that Lactobacillus in the vaginal microbiome of 55% of HPV-positive and 38% of HPV-negative women was sparse. However, in 44% of HPV-positive and 58% of HPV-negative women, the vaginal microbiome was rich in L. crispatus and L. iners.
In this study, we failed to observe statistically significant differences between groups of patients with normal Pap smear results and those with ASC-US, ASC-H, LSIL, and HSIL. The number of cells of a given bacteria appears to be of higher importance than the presence itself. In our study, quantitative analysis demonstrated the highest abundance of L. crispatus and L. gasseri cells. However, again we failed to show any significant differences in cells number of each species between the studied subgroups of patients. No statistically significant differences in the vaginal microbiota in HPV positive and HPV negative patients, in those with and without vaginitis within the last 3 months, and between premenopausal and post-menopausal participants were observed. In contrast, a study among African women showed that a cervical microbiota dominated by Lactobacillus spp. (L. crispatus, L. inners) was associated with a lower incidence of HIV, herpes simplex virus 2 (HSV-2), and high-risk HPV strains (Borgdorff et al., 2014). The abundance of L. gasseri and L. iners in the vagina has been associated with the rapid resolution of HPV infection. By contrast, low levels of Lactobacillus spp. and high levels of Atopobium spp. have been associated with slower resolution of HPV infection (Gao et al., 2013; Lee et al., 2013; Brotman et al., 2014). Vaginal infections with C. trachomatis have also been suggested to enhance women’s susceptibility to HPV infections via altering the composition of the vaginal microbiota (Silva et al., 2014). Several studies have focused on the correlation between the vaginal microbiome and cervical dysplasia. Overall, a vaginal microbiome rich in L. crispatus occurs in a healthy state (Oh et al., 2015; Audirac-Chalifour et al., 2016; Seo et al., 2016), whereas L. iners seems to be related to cervical cancer either alone or associated with HPV infection (Oh et al., 2015; Piyathilake et al., 2016; Seo et al., 2016). However, another study showed the opposite relationship, with L. iners associated with a reduced risk of cervical intraepithelial neoplasia and cervical cancer (Audirac-Chalifour et al., 2016). Other Lactobacillus species have also shown differing associations with cervical dysplasia (Mitra et al., 2015; Piyathilake et al., 2016).
In this study, we observed a strong positive correlation between L. delbrueckii and L. gasseri as well as negative correlation between L. fermentum and L. plantarum in patients with abnormal cervical Pap smear. In turn, in healthy control group, the presence of L. delbrueckii correlated with L. rhamnosus as well as between L. pantarum and L. fermentum. We did not observe correlation between L. crispatus and any other species. Similary, Gajer et al. (2012) reported that this bacteria is rarely coexisting with other bacterial species since it is either strongly dominant, or absent. Moreover, it was suggested that L. crispatus is associated with protection from pathogens and is the least likely to transition into CST IV. Women with such microbiota are less likely to become infected with Herpes simplex virus (HSV)-2 and HIV, as well HPV (Borgdorff et al., 2014). Vaginal microbial communities exist in a state of dynamic equilibrium which provides resilience (Ravel et al., 2011). The coexistence of various Lactobacillus species in the vaginal milieu enables the maintenance of homeostatic stage. Bacteria show different pH preference, growth patterns and lactic acid production capability (Mehta et al., 2020). The coexistence preferences have been shown to play a key role in the modulation of bacterial taxa composition in the vaginal ecosystem (Verstraelen et al., 2009).
In our study, L. acidophilus was observed only in patients with HSIL and normal smear, while L. plantarum in women with ASC-US, LSIL and normal smear. Similarly, Kwasniewski et al. (2018) observed the prevalence of Lactobacillus acidophilus, but also Lactobacillus iners and Lactobacillus crispatus in women diagnosed with HSIL. Moreover, in their study Lactobacillus acidophilus and Lactobacillus iners were predominant in CST diagnosed with LSIL HPV(+), while Lactobacillus crispatus was absent in smears.
Moreover, in our study, the analysis of the species distribution in different age groups showed that the abundance of L. delbrueckii significantly increased, whereas that of L. gasseri decreased, with age. L. acidophilus and L. fermentum were significantly more prevalent and the number of their cells were much higher among women living in rural areas compared with those living in urban areas.
Apart from the area of residence, the composition of the vaginal microbiota differs between various races. The composition of vaginal microbiome differs between women in different geographic regions therefore, the comparison of results obtained in studies of various ethnic groups show discrepancies. Studies have shown that the variety of Lactobacillus species in the vagina is limited to three to seven species and strictly depends on the population of the studied women (Pavlova et al., 2002; Jin et al., 2007). Studies of Polish women indicated that their vaginal regions are most frequently colonized by three species of Lactobacillus (Pytka et al., 2019), with L. crispatus, L. gasseri, and L. jensenii being the most frequent. However, vaginal microbiota of Swedish healthy women is dominated in 78% by single LAB species. The most prevalent are: L. crispatus, L. gasseri, L. iners and L. jensenii (Vasquez et al., 2002). All isolated vaginal Lactobacillus strains identified in German women belonged to three species: Lactobacillus crispatus (56%), Lactobacillus jensenii (26%), and Lactobacillus gasseri (18%) (Hütt et al., 2016). Researchers from Turkey obtained similar results. They reported that, among 56 identified species 32% belonged to L. crispatus, 30% to L. gasseri, and 13.8% to L. jensenii (Eryilmaz et al., 2018). In an Italian study, 261 vaginal Lactobacillus species were detected, with L. gasseri (28%), L. salivarius (20%), L. crispatus (18%), L. helveticus (13%), L. fermentum (10%), and L. rhamnosus (10%) being the most common (Pino et al., 2019). The prevalence of L. crispatus and L. iners in vaginal was found to be lower in German females (42.4%), Indonesian population (45.0%), Kenyan women (34.4%) and Afro-Caribbean women (26.1%) compared to African American women (91.8%) (Roachford et al., 2022). Similarly, Ravel et al. (2011) observed that vaginal bacterial communities in North American women representing four ethnic groups (white, black, Hispanic, and Asian) were dominated by species of Lactobacillus. Lactobacillus groups I, II, III, and V were identified in 80.2 and 89.7% of Asian and white women, respectively, however, in only 59.6 and 61.9% of Hispanic and black women, respectively. Moreover, it was revealed that community group IV (diverse group) was overrepresented in Hispanic (34.3%) and black (38.9%) women in comparison to Asian (17.6%) and white (9.3%) women which suggest that such vaginal bacterial communities appear normal in black and Hispanic women (Ravel et al., 2011). The higher prevalence of communities not dominated by Lactobacillus sp. (cluster IV) in Hispanic and black females could be associated with differences in vaginal pH (5.0 ± 0.59 in Hispanic) and (pH 4.7 ± 1.04 in black females) in comparison to Asian (pH 4.4 ± 0.59) and white (pH 4.2 ± 0.3) women. Therefore, it appears that vaginal microbiome can change under factors such as glycogen content in epithelial cells, pH, hormone levels, damage caused by sexual intercourse, type of contraception, age, and antibiotic therapy (Valenta, 2005). As commonly known, the v environment is acidic at a pH of 4–4.5. 93 species of the genus Lactobacillus producing lactic acid are responsible for creating such conditions, which hinder colonization by pathogens. Nevertheless, after menopause, when estrogen levels decrease, the vaginal microbiome changes, species diversity increases, and Lactobacillus spp. co-exist with other species. As a result, vaginal pH and susceptibility to vaginal infections increase (Miller et al., 2016).
This study also shows that HPV infection is not recognized by many females as important risk factor for cervical cancer. Only less than 50% of women in this study have ever been tested for HPV, and none of them have been vaccinated against it. Persistent oncogenic HPV infections are essential for cervical oncogenesis. Studies indicate the involvement of the vaginal microbiota in the persistence or resolution of HPV infection. In a study on twins, Lee et al. (2013) showed that, when one of the twins is HPV-positive, there is a significant change in the vaginal microbiota, an increase in species diversity, and a decrease in the number of Lactobacillus spp. bacterial cells, compared with the healthy twin. Mitra et al. (2016) showed that the increasing diversity of the vaginal microbiome and the decreasing number of Lactobacillus cells are associated with the progression of cervical pathological changes.
The study was limited by the random selection of the study population and the limited possibility of extrapolating the results to the general population. We also did not include patients after HPV vaccination, which is not a very popular vaccination in women over 18 years of age in Poland. Further research is needed, particularly to determine the composition of the vaginal microbiota in women who have been vaccinated against HPV. Next limitation could be the use the assay based on qPCR amplification of a species-specific genetic region of the relevant microbe. Then, some species reported in other studies carried out with the use of next generation sequencing technique were not detected, i.e., L. iners or L. jensenii. Although the study has limitations, the results have the potential to be implemented in practice. There are increasing numbers of reports that vaginal application of certain dietary supplements can affect the vaginal microbiome in patients with abnormal cytology, resulting in regression of abnormal cytology and a return to normal cytology. This could be related to the influence of the vaginal microbiome bacteria on HPV or due to another mechanism. Determining the exact composition of the vaginal microbiome of healthy women in Poland might influence the use of this knowledge in the production of these preparations.
5. Conclusion
The data obtained in this study suggest that two Lactobacillus species (L. gasseri and L. crispatus) were predominant in the vaginal microbiota of the studied population of Polish women, regardless of smear test results. In this study, patient age and place of residence were associated with the diversity of Lactobacillus spp. in the vaginal microbiota. The abundance of L. delbrueckii in the vaginal microbiota increased, whereas that of L. gasseri decreased, with age. L. acidophilus and L. fermentum were significantly more frequently detected in women living in rural versus urban areas. The degree of dysplastic changes in the endothelium or the presence of a group of atypical cervical stratified epithelial cells was not associated with significant changes in the studied vaginal bacteria. A similar lack of significant differences in the qualitative and quantitative composition of the vaginal microbiota was also observed when analyzing associations with menopausal status, HPV infection, or vaginitis within 3 months before the study.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Available upon request. Requests to access these datasets should be directed to YmJhcmN6eW5za2lAcG9jenRhLm9uZXQucGw=.
Ethics statement
The studies involving humans were approved by Bioethics Committee of Medical University in Lublin KE – 0254/174/2014. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KF: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. BB: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Project administration. RS: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. AK: Writing – review & editing. AM: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. JK: Conceptualization, Formal analysis, Funding acquisition, Investigation, Resources, Supervision, Validation, Visualization, Writing – review & editing. EW: Writing – review & editing. IK-G: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Investigation, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The equipment used in this research was funded by European Commission grant number POPW.01.03.00-06-010/09-00 in the Operational Program Development of Eastern Poland 2007–2013, Priority Axis I, Modern Economy, Operation 1.3. Supporting Innovation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arbyn, M., Weiderpass, E., Bruni, L., de Sanjosé, S., Saraiya, M., Ferlay, J., et al. (2020). Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob. Health 8, e191–e203. doi: 10.1016/S2214-109X(19)30482-6
Audirac-Chalifour, A., Torres-Poveda, K., Bahena-Román, M., Téllez-Sosa, J., Martínez-Barnetche, J., Cortina-Ceballos, B., et al. (2016). Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS One 11:e0153274. doi: 10.1371/journal.pone.0153274
Borgdorff, H., Tsivtsivadze, E., Verhelst, R., Marzorati, M., Jurriaans, S., Ndayisaba, G. F., et al. (2014). Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 8, 1781–1793. doi: 10.1038/ismej.2014.26
Borges, S., Silva, J., and Teixeira, P. (2014). The role of Lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 289, 479–489. doi: 10.1007/s00404-013-3064-9
Brotman, R. M., Shardell, M. D., Gajer, P., Tracy, J. K., Zenilman, J. M., Ravel, J., et al. (2014). The interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 210, 1723–1733. doi: 10.1093/infdis/jiu330
Byun, R., Nadkarni, M. A., Chhour, K. L., Martin, F. E., Jacques, N. A., and Hunter, N. (2004). Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J. Clin. Microbiol. 42, 3128–3136. doi: 10.1128/JCM.42.7.3128-3136.2004
Champer, M., Wong, A. M., Champer, J., Brito, I. L., Messer, P. W., Hou, J. Y., et al. (2018). The role of the vaginal microbiome in gynaecological cancer. BJOG 125, 309–315. doi: 10.1111/1471-0528.14631
Chatterjee, T., Gill, S. S., and Rac, R. (2000). Standardization of cervical/vaginal cytopathology reporting: the Bethesda system (TBS) for reporting cervical/vaginal cytologic diagnoses. Med. J. Armed Forces India 56, 45–49. doi: 10.1016/S0377-1237(17)30090-4
De Backer, E., Verhelst, R., Verstraelen, H., Alqumber, M. A., Burton, J. P., Tagg, J. R., et al. (2007). Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC Microbiol. 7, 1–13. doi: 10.1186/1471-2180-7-115
Dominguez-Bello, M. G., Godoy-Vitorino, F., Knight, R., and Blaser, M. J. (2019). Role of the microbiome in human development. Gut 68, 1108–1114. doi: 10.1136/gutjnl-2018-317503
Donnarumma, G., Molinaro, A., Cimini, D., De Castro, C., Valli, V., De Gregorio, V., et al. (2014). Lactobacillus crispatus L1: high cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol. 14:137. doi: 10.1186/1471-2180-14-137
Eryilmaz, M., Gurpinar, S. S., Palabiyik, I. M., Guriz, H., and Gerceker, D. (2018). Molecular identification and antimicrobial activity of vaginal Lactobacillus sp. Curr. Pharm. Biotechnol. 19, 1241–1247. doi: 10.2174/1389201020666190110164123
Flores, G. E., Caporaso, J. G., Henley, J. B., Rideout, J. R., Domogala, D., Chase, J., et al. (2014). Temporal variability is a personalized feature of the human microbiome. Genome Biol. 15, 1–13. doi: 10.1186/s13059-014-0531-y
Gajer, P., Brotman, R. M., Bai, G., Sakamoto, J., Schütte, U. M. E., Zhong, X., et al. (2012). Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132ra52–32ra52. doi: 10.1126/scitranslmed.3003605
Gao, W., Weng, J., Gao, Y., and Chen, X. (2013). Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect. Dis. 13, 1–10. doi: 10.1186/1471-2334-13-271
Gillet, E., Meys, J. F., Verstraelen, H., Bosire, C., De Sutter, P., Temmerman, M., et al. (2011). Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect. Dis. 11, 1–9. doi: 10.1186/1471-2334-11-10
Gillet, E., Meys, J. F., Verstraelen, H., Verhelst, R., De Sutter, P., Temmerman, M., et al. (2012). Association between bacterial vaginosis and cervical intraepithelial neoplasia: systematic review and meta-analysis. PLoS One 7:e45201. doi: 10.1371/journal.pone.0045201
Guo, Y. L., You, K., Qiao, J., Zhao, Y. M., and Geng, L. (2012). Bacterial vaginosis is conducive to the persistence of HPV infection. Int. J. STD AIDS 23, 581–584. doi: 10.1258/ijsa.2012.011342
Haarman, M., and Knol, J. (2006). Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 72, 2359–2365. doi: 10.1128/AEM.72.4.2359-2365.2006
Hütt, P., Lapp, E., Štšepetova, J., Smidt, I., Taelma, H., Borovkova, N., et al. (2016). Characterisation of probiotic properties in human vaginal Lactobacilli strains. Microb. Ecol. Health Dis. 27:30484. doi: 10.3402/mehd.v27.30484
Jin, L., Tao, L., Pavlova, S. I., So, J. S., Kiwanuka, N., Namukwaya, Z., et al. (2007). Species diversity and relative abundance of vaginal lactic acid bacteria from women in Uganda and Korea. J. Appl. Microbiol. 0:3147. doi: 10.1111/j.1365-2672.2006.03147.x
King, C. C., Jamieson, D. J., Wiener, J., Cu-Uvin, S., Klein, R. S., Rompalo, A. M., et al. (2011). Bacterial vaginosis and the natural history of human papillomavirus. Infect. Dis. Obstet. Gynecol. 2011, 1–8. doi: 10.1155/2011/319460
Kwasniewski, W., Wolun-Cholewa, M., Kotarski, J., Warchol, W., Kuzma, D., Kwasniewska, A., et al. (2018). Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol. Lett. 16, 7035–7047. doi: 10.3892/ol.2018.9509
Lee, J. E., Lee, S., Lee, H., Song, Y. M., Lee, K., Han, M. J., et al. (2013). Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8:e63514. doi: 10.1371/journal.pone.0063514
Liu, M. B., Xu, S. R., He, Y., Deng, G. H., Sheng, H. F., Huang, X. M., et al. (2013). Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS One 8:e79812. doi: 10.1371/journal.pone.0079812
MacIntyre, D. A., Chandiramani, M., Lee, Y. S., Kindinger, L., Smith, A., Angelopoulos, N., et al. (2015). The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 5, 1–9. doi: 10.1038/srep08988
Marchesi, J. R., and Ravel, J. (2015). The vocabulary of microbiome research: a proposal. Microbiome 3, 1–3. doi: 10.1186/s40168-015-0094-5
Mehta, O., Ghosh, T. S., Akansha Kothidar, M., Gowtham, R., Mitra, R., Kshetrapal, P., et al. (2020). Vaginal microbiome of pregnant Indian women: insights into the genome of dominant Lactobacillus species. Microb. Ecol. 80, 487–499. doi: 10.1007/s00248-020-01501-0
Miller, E. A., Beasley, D. E., Dunn, R. R., and Archie, E. A. (2016). Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front. Microbiol. 7, 1–13. doi: 10.3389/fmicb.2016.01936
Mitra, A., MacIntyre, D. A., Lee, Y. S., Smith, A., Marchesi, J. R., Lehne, B., et al. (2015). Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 5, 1–11. doi: 10.1038/srep16865
Mitra, A., MacIntyre, D. A., Marchesi, J. R., Lee, Y. S., Bennett, P. R., and Kyrgiou, M. (2016). The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 4, 58–15. doi: 10.1186/s40168-016-0203-0
Moreno, I., and Simon, C. (2018). Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod. Med. Biol. 18, 40–50. doi: 10.1002/rmb2.12249
Norenhag, J., Du, J., Olovsson, M., Verstraelen, H., Engstrand, L., and Brusselaers, N. (2020). The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG 127, 171–180. doi: 10.1111/1471-0528.15854
Oh, H. Y., Kim, B. S., Seo, S. S., Kong, J. S., Lee, J. K., Park, S. Y., et al. (2015). The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect. 21, 674.e1–674.e9. doi: 10.1016/j.cmi.2015.02.026
Pavlova, S. I., Kilic, A. O., Kilic, S. S., So, J. S., Nader-Macias, M. E., Simoes, J. A., et al. (2002). Genetic diversity of vaginal Lactobacilli from women in different countries based on 16S rRNA gene sequences. J. Appl. Microbiol. 92, 451–459. doi: 10.1046/j.1365-2672.2002.01547.x
Petricevic, L., Domig, K. J., Nierscher, F. J., Sandhofer, M. J., Fidesser, M., Krondorfer, I., et al. (2014). Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 4:5136. doi: 10.1038/srep05136
Pino, A., Bartolo, E., Caggia, C., Cianci, A., and Randazzo, C. L. (2019). Detection of vaginal Lactobacilli as probiotic candidates. Sci. Rep. 9, 3355–3310. doi: 10.1038/s41598-019-40304-3
Piyathilake, C. J., Ollberding, N. J., Kumar, R., Macaluso, M., Alvarez, R. D., and Morrow, C. D. (2016). Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev. Res. 9, 357–366. doi: 10.1158/1940-6207.CAPR-15-0350
Pytka, M., Kordowska-Wiater, M., and Jarocki, P. (2019). Microbiome of the women’s genital system. Adv. Microbiol. 58, 227–236. doi: 10.21307/PM-2019.58.3.227
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 108, 4680–4687. doi: 10.1073/pnas.1002611107
Requena, T., and Velasco, M. (2021). The human microbiome in sickness and in health. Rev. Clin. Esp. 221, 233–240. doi: 10.1016/j.rceng.2019.07.018
Roachford, O. S. E., Alleyne, A. T., and Nelson, K. E. (2022). Insights into the vaginal microbiome in a diverse group of women of African, Asian and European ancestries. PeerJ 10:e14449. doi: 10.7717/peerj.14449
Scott, M., Stites, D. P., and Moscicki, A. B. (1999). Th1 cytokine patterns in cervical human papillomavirus infection. Clin. Diagn. Lab. Immunol. 6, 751–755. doi: 10.1128/CDLI.6.5.751-755.1999
Sender, R., Fuchs, S., and Milo, R. (2016). Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cells 164, 337–340. doi: 10.1016/j.cell.2016.01.013
Seo, S. S., Oh, H. Y., Lee, J. K., Kong, J. S., Lee, D. O., and Kim, M. K. (2016). The combined effect of diet and cervical microbiome on the risk of cervical intraepithelial neoplasia. Clin. Nutr. 35, 1434–1441. doi: 10.1016/j.clnu.2016.03.019
Silva, J., Cerqueira, F., and Medeiros, R. (2014). Chlamydia trachomatis infection: implications for HPV status and cervical cancer. Arch. Gynecol. Obstet. 289, 715–723. doi: 10.1007/s00404-013-3122-3
Tachedjian, G., Aldunate, M., Bradshaw, C. S., and Cone, R. A. (2017). The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 168, 782–792. doi: 10.1016/j.resmic.2017.04.001
Turnbaugh, P. J., Ley, R. E., Hamady, M., Fraser-Liggett, C. M., Knight, R., and Gordon, J. I. (2007). The human microbiome project. Nature 449, 804–810. doi: 10.1038/nature06244
Valenta, C. (2005). The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 57, 1692–1712. doi: 10.1016/j.addr.2005.07.004
Vasquez, A., Jakobsson, T., Ahrne, S., Forsum, U., and Molin, G. (2002). Vaginal Lactobacillus flora of healthy Swedish women. J. Clin. Microbiol. 40, 2746–2749. doi: 10.1128/JCM.40.8.2746-2749.2002
Verstraelen, H., Verhelst, R., Claeys, G., De Backer, E., Temmerman, M., and Vaneechoutte, M. (2009). Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol. 9:116. doi: 10.1186/1471-2180-9-116
Wang, H., Jiang, Y., Liang, Y., Wei, L., Zhang, W., and Li, L. (2022). Observation of the cervical microbiome in the progression of cervical intraepithelial neoplasia. BMC Cancer 22:362. doi: 10.1186/s12885-022-09452-0
World Medical Association . (2013). Ethical principles for medical research involving human subjects. JAMA. 310, 2191–2194. doi: 10.1001/jama.2013.281053
Wu, S., Ding, X., Kong, Y., Acharya, S., Huaqian, W., Huang, C., et al. (2021). The feature of cervical microbiota associated with the progression of cervical cancer among reproductive females. Gynecol. Oncol. 163, 348–357. doi: 10.1016/j.ygyno.2021.08.016
Zhang, R., Daroczy, K., Xiao, B., Yu, L., Chen, R., and Liao, Q. (2012). Qualitative and semiquantitative analysis of Lactobacillus species in the vaginas of healthy fertile and postmenopausal Chinese women. J. Med. Microbiol. 61, 729–739. doi: 10.1099/jmm.0.038687-0
Keywords: vaginal microbiota, Lactobacillus, L. gasseri, L. crispatus, HPV, cervical cancer
Citation: Frąszczak K, Barczyński B, Siwiec R, Kondracka A, Malm A, Kotarski J, Witt E and Korona-Głowniak I (2023) The analysis of Lactobacillus spp. distribution in the vaginal microbiota of Polish women with abnormal Pap smear result. Front. Microbiol. 14:1257587. doi: 10.3389/fmicb.2023.1257587
Edited by:
Alina Maria Holban, University of Bucharest, RomaniaReviewed by:
Angela T. Alleyne, The University of the West Indies, Cave Hill, BarbadosReet Mändar, University of Tartu, Estonia
Copyright © 2023 Frąszczak, Barczyński, Siwiec, Kondracka, Malm, Kotarski, Witt and Korona-Głowniak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bartłomiej Barczyński, YmJhcmN6eW5za2lAcG9jenRhLm9uZXQucGw=
 Karolina Frąszczak1
Karolina Frąszczak1 Bartłomiej Barczyński
Bartłomiej Barczyński Anna Malm
Anna Malm Jan Kotarski
Jan Kotarski Izabela Korona-Głowniak
Izabela Korona-Głowniak