- 1The Key Laboratory for Quality Improvement of Agricultural Products of Zhejiang Province, College of Advanced Agricultural Sciences, Zhejiang A&F University, Hangzhou, China
- 2National-Regional Joint Engineering Research Center for Soil Pollution Control and Remediation in South China, Guangdong Key Laboratory of Integrated Agro-environmental Pollution Control and Management, Institute of Eco-environmental and Soil Sciences, Guangdong Academy of Sciences, Guangzhou, China
Reactive oxygen species (ROS) are important for plant defense against fungal attack. As a necrotrophic fungus, Botrytis cinerea can exploit ROS that originated from both sides of the host and pathogen during interaction to facilitate its infestation. Meanwhile, B. cinerea needs to exert an efficient oxidative stress responsive system to balance the intracellular redox state when encountering deleterious ROS levels. However, the machinery applied by B. cinerea to cope with ROS remains obscure. Herein, we investigated the role of the transcription factor BcMsn2 in regulating B. cinerea redox homeostasis. Disruption of the BcMsn2 gene severely impaired vegetative growth, sclerotium formation, conidial yield, and fungal virulence. The intracellular oxidative homeostasis of the ∆bcmsn2 mutant was disrupted, leading to significantly elevated levels of ROS and reduced activities of enzymes closely associated with oxygen stress, such as catalase (CAT) and superoxide dismutase (SOD). RNA-Seq and qRT-PCR analyses showed remarkable downregulation of the expression of several genes encoding ROS scavenging factors involved in maintaining the redox homeostasis in ∆bcmsn2, suggesting that BcMsn2 functions as a transcriptional regulator of these genes. Our findings indicated that BcMsn2 plays an indispensable role in maintaining the equilibrium of the redox state in B. cinerea, and intracellular ROS serve as signaling molecules that regulate the growth, asexual reproduction, and virulence of this pathogen.
1. Introduction
Botrytis cinerea is a necrotrophic ascomycete fungus that can infect over 1,400 species, causing massive economic losses worldwide annually (Williamson et al., 2007). Botrytis cinerea has a broad host range, is aggressive, and causes substantial damage; therefore, it is considered as one of the most important plant pathogens by fungal plant pathologists (Dean et al., 2012). The life cycle of B. cinerea has been extensively studied, revealing a predominantly asexual mode of reproduction. The production of conidia is regulated by the external environment and precisely regulated by the circadian system in cells (Martinez et al., 2003; Yoshida et al., 2011; Cohrs et al., 2016; Rascle et al., 2018). Previous studies have identified several genes encoding proteins involved in conidiation, including light-responsive transcriptional regulator genes BcREG1 and BcLTF3 (Michielse et al., 2011; Brandhoff et al., 2017), genes encoding components of the cAMP-dependent pathway (BCG1, BCG3, and BcPKA1) (Doehlemann et al., 2006; Williamson et al., 2007; Schumacher et al., 2008), the gene encoding the Gβ-subunit of the heterotrimeric G protein, BcGB1 (Williamson et al., 2007; Tang et al., 2021), and the MAP kinase-encoding genes BcBMP1 and BcSAK1 (Doehlemann et al., 2006; Segmüller et al., 2007). Therefore, understanding the growth, conidiation, and pathogenic mechanisms of B. cinerea is key to the prevention and control of gray mold.
Reactive oxygen species (ROS) are small molecules, including hydrogen peroxide (H2O2), the superoxide anion radical (O2−), and the hydroxyl radical (OH), which exhibit high oxidative activity. They are generated by plants and pathogens during the infection stage, and are also produced continuously as byproducts of various metabolic pathways in cellular components, such as mitochondria, peroxisomes, and chloroplasts, with mitochondria being the main source of intracellular ROS (Viefhues et al., 2014). Excess intracellular ROS can damage macromolecules and even lead to cell death. Phytopathogenic fungi have developed sophisticated defense systems to maintain redox homeostasis. Enzymatic ROS scavenging mechanisms involve various peroxidases, such as peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT). However, ROS can also act as signaling molecules to regulate growth, sporulation, and virulence. The generation and scavenging of ROS exhibit circadian rhythms, a fundamental biological phenomenon observed in various species that plays a direct role in many physiological processes (Mailloux, 2015; Fanjul-Moles and López-Riquelme, 2016; Milkovic et al., 2019). A certain range of ROS can promote sclerotial differentiation and conidia germination in Sclerotinia sclerotiorum (Georgiou et al., 2000; Papapostolou et al., 2014). In Neurospora crassa, the ROS oscillation observed under free-running conditions might be produced by Cat1 [6]. In B. cinerea, the circadian oscillator BcFRQ1 plays a crucial role in conidiation, sclerotia formation, and virulence (Hevia et al., 2015).
In Saccharomyces cerevisiae, regulation of ROS is associated with the rapid induction of at least 115 proteins, such as Yap1, Skn7, and Msn2/4 (Kuge and Jones, 1994; Morgan, 1997; Godon et al., 1998; Charizanis et al., 1999; Lee et al., 1999). Msn2/4 directly induces the expression of genes encoding antioxidant enzymes, such as catalase, superoxide dismutases, and peroxidases (Hasan et al., 2002; Drakulic et al., 2005). Orthologs of Msn2 have been characterized in several fungi, including Aspergillus parasiticus, Magnaporthe oryzae, Candida albicans, and Sclerotinia sclerotiorum. The msnA deletion strains of A. parasiticus and A. flavus exhibited retarded colony growth with increased conidiation and elevated levels of ROS (Chang et al., 2011). MoMsn2 in M. oryzae is required for aerial hyphal growth and conidiation (Zhang et al., 2014).
In this study, a mutation in BcMsn2, a novel zinc finger transcription factor, resulted in severely retarded vegetative growth, significantly delayed sclerotium formation, a remarkable decrease in conidial yield, and defects in host plant infection. However, the mechanisms underlying the functions of BcMsn2 remain unknown. Herein, we found that BcMsn2 deletion mutants exhibited increased intracellular levels of ROS, leading to irreversible disruption of the cellular redox state. The activities of enzymes closely related to oxygen stress, such as CAT and SOD, were reduced. Additionally, using RNA sequencing (RNA-seq) and quantitative real-time reverse transcription PCR (qRT-PCR) analyses, we observed significant downregulation of several ROS scavenging genes involved in maintaining the redox homeostasis in the ∆bcmsn2 mutant. These findings indicated that BcMsn2 might function by maintaining the redox equilibrium, and intracellular ROS might act as second messengers to regulate growth, asexual reproduction, and virulence in B. cinerea.
2. Materials and methods
2.1. Strains, media, and growth conditions
The wild-type B. cinerea strain B05.10 was used for the transformation experiments in this study (van Kan et al., 1997). Strain B05.10 was cultured on potato dextrose agar (PDA) plates. Potato dextrose broth (PDB) served as the liquid medium for mycelium collection. The BcMsn2 gene deletion mutants and complemented strains were maintained on PDA supplemented with 100 μg/mL hygromycin B (Sigma, St. Louis, MO, United States) or 100 μg/mL nourseothricin (Jena Bioscience, Jena, Germany), respectively. SH agar medium containing 0.6 M sucrose, 4 mM Tris–HCl, 1 mM (NH4)2HPO4, and 1.2% agar was used to regenerate B. cinerea protoplasts.
2.2. Generation of the BcMsn2 deletion and complementation mutants
The sequence of BcMsn2 (Gene ID: Bcin01g05160) was obtained from the Ensembl fungi.1 To investigate the biological function of BcMsn2 in B. cinerea, we generated BcMsn2 deletion mutants through protoplast formation and transformation (Gronover et al., 2001).
The flanking sequences of the BcMsn2 gene were amplified from B05.10 genomic DNA using the primer pair P1/P2 and P5/P6 (all primers are shown in Table S1). The sequence of the gene encoding hygromycin B phosphotransferase (HPH) was amplified from the pBS-HPH1 vector using the primer pair P3/P4. The gene replacement construct was created by double-joint PCR (DJ-PCR) (Yu et al., 2004) (Supplementary Figure S1A). Three deletion mutants, ∆bcmsn2-1a1, ∆bcmsn2-3b1, and ∆bcmsn2-4a1 were identified among 50 hygromycin-resistant transformants using PCR analysis with the primers Gene-F and Gene-R (Supplementary Figure S1B and Supplementary Table S1). All deletion mutants showed identical phenotypic characteristics.
The BcMsn2 deletion mutants ∆bcmsn2-3b1 and ∆bcmsn2-4a1 were complemented with the complete BcMsn2 gene. To construct the p1300-NAT1 vector, the NAT1 gene was amplified using primers P13/P14 from the pD-NAT1 vector (Kuck and Hoff, 2006). The PCR product was cloned into the XhoI site of p1300BAR. The complemented BcMsn2 gene was amplified from the genome of the parental strain B05.10 using the P9/P10 primer pair. The resulting PCR product was cloned into the EcoRI-BamHI sites of p1300-NAT1 to generate the complementation vector, p1300-BcMsn2-C. BcMsn2 in this vector was sequenced to verify that no errors were present in this sequence before transformation into the BcMsn2 deletion mutant using Agrobacterium tumefaciens-mediated transformation (AtMT) (Rolland et al., 2003). The hygromycin-resistant and nourseothricin-resistant BcMsn2 complemented mutants were identified using PCR analysis with the primers Gene-F and Gene-R. The transformant ∆bcmsn2-C that had approximately similar expression levels of the BcMsn2 gene to the wild-type B05.10 strain was selected by qPCR.
2.3. Yeast complementation assay
The BcMsn2 gene was amplified with primers pYES2-F/pYES2-R and cloned into the HindIII-BamHI sites of the yeast expression vector pYES2, generating the vector pYES2-BcMsn2. Transformation of pYES2-BcMsn2 into a ∆msn2 mutant of S. cerevisiae was carried out using the lithium acetate method (Schiestl and Gietz, 1989). The yeast transformants were grown on YPRG medium (1% yeast extract, 2% peptone, 1% galactose, 1% raffinose, 1.5% agar). The wild-type strain BY4741, the ∆scmsn2 mutant, and the ∆scmsn2 mutant transformed with empty pYES2 vector were used as controls.
2.4. Study of the conidiation, hyphal growth and sclerotia, and assays of pathogenicity
Hyphae of the wild-type strain, BcMsn2 deletion mutants, and complemented strains were incubated on PDA plates at 22°C for 2 days. A mycelial agar plug (5 mm in diameter) was cut and transferred onto the center of a fresh PDA plate. The plates were incubated at 22°C. Radial growth was determined by measuring the colony diameter daily for 5 consecutive days. Sclerotia production was observed for 1 month under dark conditions at 22°C.
For the conidiation assay, conidia numbers were counted after 3, 5, and 7 days of incubation on PDA plates. Seven-day-old conidia were also used for size statistics and germination experiments in GB5 medium (Guan et al., 2020), At least 100 conidia were counted per replicate in each experiment. Three replicates were performed for each conidia age.
For the pathogenicity assays, the leaves of beans, and the fruits of tomato, pear, and apple were inoculated with mycelial plugs (2-day-old, 5 mm in diameter) of each strain. Inoculated materials were kept in Petri dishes with high humidity at 22°C for 3 days, and the lesion diameters were measured daily.
2.5. Assays of ROS and antioxidant enzyme activity
For ROS detection using nitroblue tetrazolium (NBT) staining, plates with three-day-old cultures of each strain were stained with 0.05% NBT, and the reaction was terminated using anhydrous ethanol after 1 h (Jing et al., 2020). The staining results were observed under a stereomicroscope. ROS detection using 2′, 7’-Dichlorodihydrofluorescein diacetate (H2DCF-DA) was performed using Reactive Oxygen Species Assay Kit (Code No. S0033S, Beyotime Biotechnology, Jiangsu, China).
The conidia suspension at a final concentration of 1 × 104 was added to 50 mL of PDB medium and incubated in a shaker for 3 days. The mycelia of each strain were collected, weighed, and 2.0 g of mycelium was added to 10 mL PBS [0.1 M, pH 7.4, containing 3% polyvinylpyrrolidone (PVP)]. It was then ground into a 10% tissue homogenate, and the crude extract was obtained by centrifugation at 4000 rpm, 4°C. H2O2, CAT, POD, SOD, and malondialdehyde (MDA), used to indicate enzyme activity, were measured using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6. Detection membrane potential of mitochondria
Mitochondrial membrane potential was detected with Enhanced mitochondrial membrane potential assay kit with JC-1 (Beyotime Biotechnology, C2003S). The final concentration of 1 × 106 conidia suspensions of B. cinerea were placed in 50 mL PDB medium and incubated in a shaker for 12 h. 1 mL suspensions were centrifuged at 8000 rpm for 5 min, then the precipitates were resuspended in 500 μL JC-1 (1×) and incubated in dark place for 20 min at 37°C. Centrifuge and resuspend the precipitate in 500 μL PBS to remove the excessive JC-1. Green fluorescence and red fluorescence were observed using the laser scanning confocal microscope. Fluorescence intensities were counted using ImageJ software.
2.7. RNA-Seq analysis
Mycelia of the wild-type strain B05.10 and ∆bcmsn2 were collected from 10-h PDA cultures. Total RNA samples were extracted using the High-Salt Solution for Precipitation (Plant) kit (Code No.9193, Takara, Dalian, China). Two biological replicates were prepared for each strain. Library construction and sequencing were performed to generate 50 bp reads. The RNA-Seq reads were mapped to the reference genome (ASM83294v1, Ensembl fungi v53; ENA accession number GCA_000143535) using HISAT2 (Pertea et al., 2016). The mapping statistics are shown in Supplementary Table S2. Differentially expressed transcripts were identified using DESeq2 (Love et al., 2014) based on transcript abundance calculated by Salmon (Patro et al., 2017). Transcripts with |log2 fold change| ≥ 1 and adjusted value of p ≤0.05 were considered differentially expressed. The data generated in this project have been deposited in the NCBI database with accession numbers SRR23730092–SRR23730095.
2.8. qRT-PCR analysis
The same amounts of conidia of different strains were inoculated into PDB medium and cultured in a shaker at 22°C and 180 rpm for 2 days. Total RNA samples of the wild-type B05.10 and deletion mutants were utilized for genomic DNA (gDNA)-free cDNA synthesis using the PrimeScript™ RT reagent Kit with gDNA Eraser (Code No. RR047A, Takara). Quantitative real-time PCR amplification of the cDNA was carried out using the BIO-RAD Real-Time PCR Detection System (Bio-Rad, Hercules, CA, United States), with the TB Green® Premix Ex Taq™ (Code No. RR420A, Takara). The GAPDH gene (encoding glyceraldehyde-3-phosphate dehydrogenase) was used as a reference gene to normalize the target gene expression and correct for sample-to-sample variation (Ren et al., 2017). The relative transcript abundances of selected transcripts were calculated using the 2−∆∆Ct method from the mean of three independent determinations of the threshold cycle (Livak and Schmittgen, 2001). The primers used in this study are shown in Supplementary Table S1.
3. Results
3.1. Identification and deletion of BcMsn2 in Botrytis cinerea
In this study, we identified a novel protein named as BcMsn2 (Gene ID: Bcin01p05160) in B. cinerea. Sequence analysis confirmed that the coding region of BcMsn2 gene has one intron and two exons, and the coding sequence (CDS) comprises 1806 bp, encoding a polypeptide of 601 amino acid, consistent with the annotation in the genome database. The predicted BcMsn2 protein shared 66.6% identity with its S. cerevisiae counterpart MSN2. Analysis of BcMsn2 in the Conserved Domain Database revealed the presence of two conserved C2H2 zinc finger DNA-binding domains located at amino acid positions 484–535, characterized by the following pattern [#-X-C-X(1–5)-C-X3-#-X5-#-X2-H-X(3–6)-(H/C)] (Figure 1A). Phylogenetic analysis showed that BcMsn2 was most closely related to the MSN2 protein from S. sclerotiorum (Figure 1B). To investigate the function of BcMsn2, gene deletion mutants were generated via homologous recombination (Supplementary Figure S1A), and the hygromycin-resistant transformants were initially screened using PCR analysis (Supplementary Figure S1B). The BcMsn2 deletion mutant was complemented by introducing the complete BcMsn2 gene using AtMT, and the nourseothricin-resistant transformants were confirmed by PCR (Figure S1B). Additionally, to assess the functionality of BcMsn2 in S. cerevisiae, the open reading frame of BcMsn2 was cloned into the pYES2 vector and transformed into a ∆msn2 mutant of S. cerevisiae, which is known to be hypersensitive to oxidative stress. This transformation excluded any potential interference from the empty pYES2 vector (Figure 1C). The yeast transformants containing the pYES2-BcMsn2 construct exhibited restored growth of the ∆scmsn2 mutant on medium supplemented with 3 mM H2O2 (Figure 1C). These findings indicated that BcMsn2 possesses a conserved function in the oxidative stress response pathway.
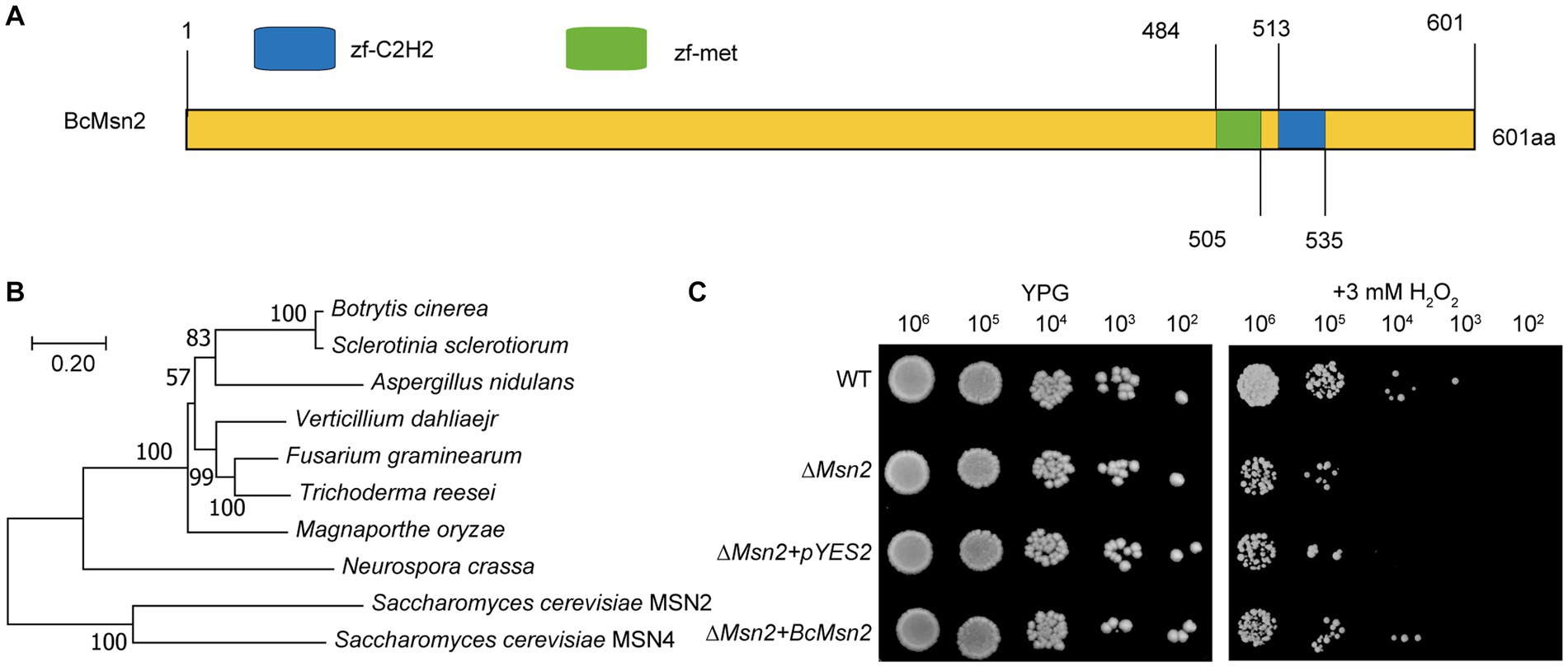
Figure 1. Sequence and phylogenetic analysis of Msn2. (A) Sequence analysis of BcMsn2 in B. cinerea. Zf-C2H2: A zinc finger is part of a protein that can bind to DNA. zf-met: A zinc-finger domain with the CxxCx(12)Hx(6)H motif. (B) The phylogenetic tree of Msn2 with homologs gene from other fungal species was constructed using MEGA version 11. Numbers at the nodes in the rooted tree represent the bootstrap value after 1000 replications. The bar indicates 0.20 distance units. (C) Yeast cells (102 to 106 cells per milliliter) of WT, ∆scmsn2 mutant, and ∆scmsn2/BcMsn2 or ∆scmsn2/pYES2 transformants were assayed for growth on 1% yeast extract, 2% peptone, 3% glycerol (galactose) plates, with or without 3 mM H2O2.
3.2. BcMsn2 plays a critical role in cellular ROS balance
To investigate the involvement of BcMsn2 in maintaining the ROS balance, we measured the H2O2 content in the ∆bcmsn2 mutants and observed a 35–50% increase compared with that in the wild type (WT) and ∆bcmsn2-C strains. We assessed O2·- levels by using NBT staining, and similar to H2O2, we observed a significant accumulation of O2·- in the ∆bcmsn2 mycelium (Figure 2A). Furthermore, to detect ROS, we stained the conidia using H2DCF-DA. The conidial ROS content in the ∆bcmsn2 mutant was 200% higher than that of the WT, which is consistent with the previously measured elevated H2O2 and O2- levels (Figure 2B). The enzyme activities of CAT, POD, and SOD were determined in each strain. The results demonstrated a significant decrease in CAT and SOD activities, while POD activity was increased in the ∆bcmsn2 mutant (Figure 2B). Further research found the mitochondrial membrane potential of the two mutants significantly decreased and were subjected to more mitochondrial damage. Changes in membrane is a central feature of mitochondrial health. Mitochondrial membrane potential depolarization is a good indicator to evaluate mitochondrial dysfunction. When the mitochondrial membrane potential is high, JC-1 forms J-aggregates that produce red fluorescence; when the potential is low, JC-1 is a monomer that produces green fluorescence. Changes in mitochondrial membrane potential were detected by red and green fluorescence transformation. The proportion of mitochondrial depolarization is measured by the relative proportion of red and green fluorescence. We detected membrane potential of mitochondria by JC-1 staining and found more green fluorescence and a lower relative ratio of red/green fluorescence in the two mutants (∆bcmsn2-3b1 and ∆bcmsn2-4a1) (Figures 2C,D). These findings indicated the ∆bcmsn2 mutants have higher intracellular ROS level that damage mitochondrial membranes.
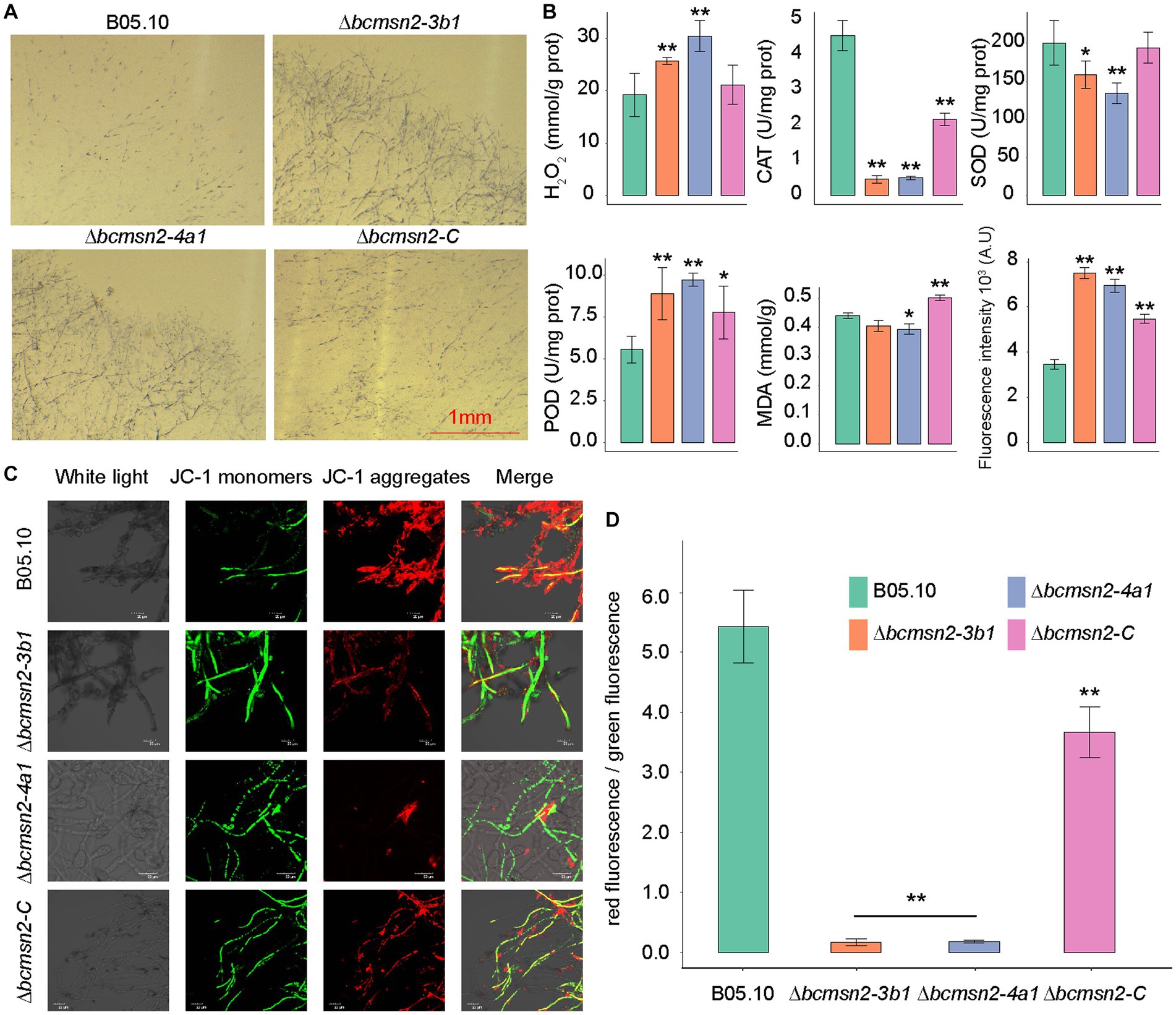
Figure 2. ∆bcmsn2 displayed a higher H2O2 content, increased POD activity, decreased CAT and POD activities, higher ROS levels, and lower mitochondrial membrane potential than the WT and ∆bcmsn2-C. (A) Each strain at 3 days old was stained with 0.05% NBT. Bar = 1 mm. (B) Comparisons of H2O2, CAT, SOD, POD, MDA, and ROS among the WT, ∆bcmsn2, and ∆bcmsn2-C after incubation in PDB for 4 days. (*p< 0.05, **p < 0.01). (C) Detection of mitochondrial membrane potential using the laser scanning confocal microscope. (D) Relative ratio of red/green fluorescence after JC-1 dye.
3.3. Effect of BcMsn2 deletion on vegetative growth and reproductive differentiation
To test whether BcMsn2 is involved in conidial morphogenesis, we determined the ability of each strain to produce conidia at 3, 5, and 7 dpi on PDA plates. Analysis of conidial morphology revealed that the conidia produced by ∆bcmsn2 were significantly different from those of WT in length and width, with a 39% increase in length (10.60 ± 1.46 μm, 14.73 ± 2.08 μm, and 12.83 ± 1.17 μm for conidial length produced by the WT, ∆bcmsn2, and ∆bcmsn2-C strains, respectively) and an 18% increase in width (7.66 ± 0.75 μm, 9.03 ± 1.22 μm, and 7.48 ± 1.17 μm for conidial width produced by the WT, ∆bcmsn2, and ∆bcmsn2-C strains, respectively) (Figures 3A,B). These results suggested that the loss of BcMsn2 in B. cinerea increased the conidial size of the pathogen. In addition, our data showed that the conidia produced by ∆bcmsn2 could be collected at 3 dpi, whereas the WT conidia could be collected at 5 dpi. However, the total number of conidia per unit area of ∆bcmsn2 at 7 dpi was only 24% of that of the wild type strain (4.43 × 105 ± 3.36 × 104 cm−1, 1.06 × 105 ± 3.94 × 104 cm−1, 3.94 × 105 ± 3.28 × 104 cm−1 for conidia production per unit area by the WT, ∆bcmsn2, and ∆bcmsn2-C strains, respectively) (Figure 3C). When incubated on GB5 medium, approximately 75% of the B05.10 conidia germinated at 4 h. Under the same conditions, the germination rate of ∆bcmsn2 conidia was 98% (Figure 3D). Overall, our results demonstrate that BcMsn2 controls conidial morphogenesis and is dispensable for the radial and lateral growth of conidiation in B. cinerea.
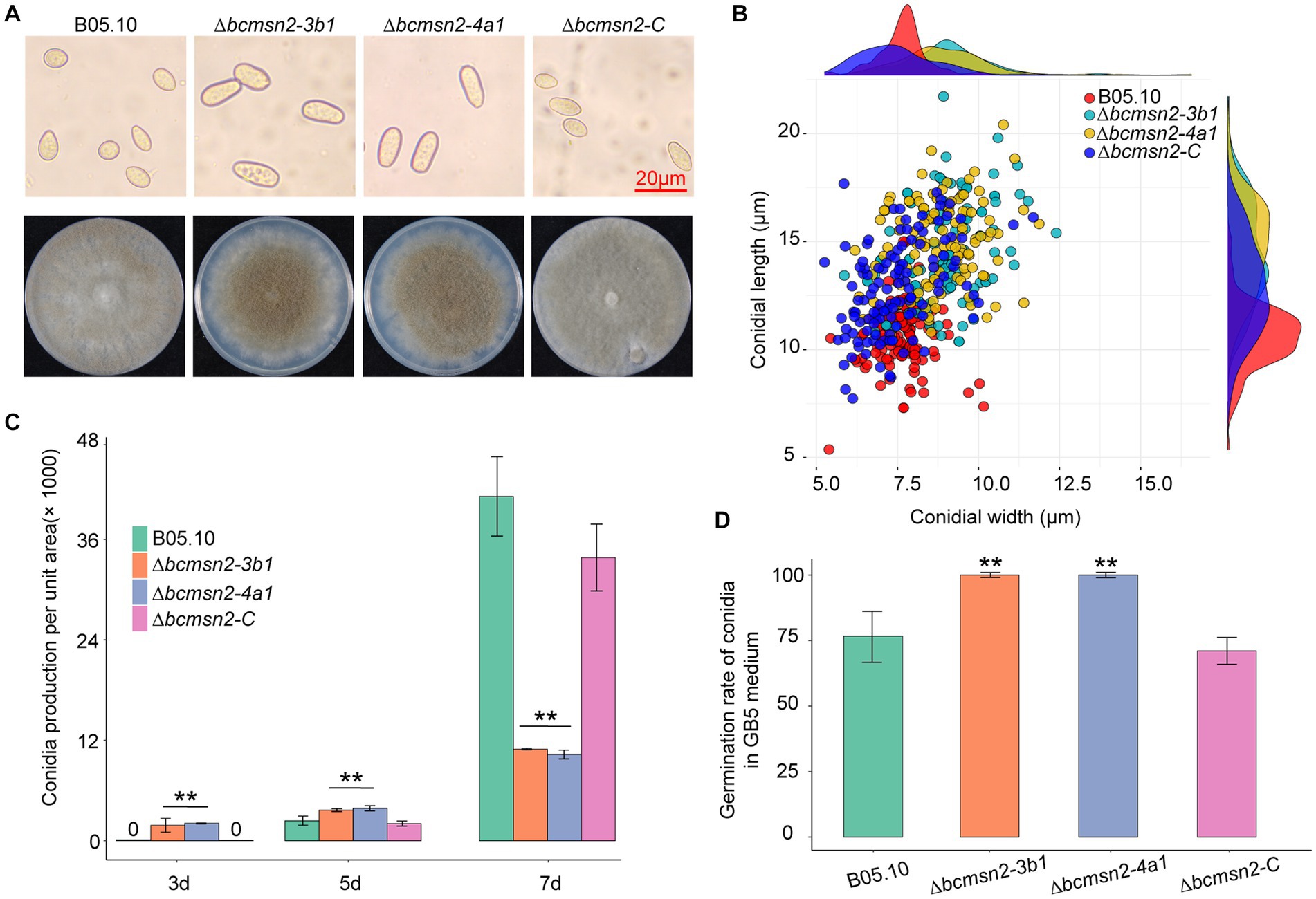
Figure 3. The effect of BcMsn2 knockout on spore morphology, germination, and conidia production. (A) Conidia of the ∆bcmsn2 mutants displayed abnormal morphology with elongation observed under the microscope Bar = 20 mm. The conidial layer was also more concentrated in the 7-day-old colonies. (B) Conidia length and width statistics. (C) The conidia production of the strains cultured on PDA plates was investigated at 3, 5, and 7 days. (D) The conidial germination rate of the strains cultured on GB5 was investigated at 4 hours. (*p < 0.05, **p < 0.01).
To determine the function of BcMsn2 in hyphal growth, ∆bcmsn2 and the complementary strain ∆bcmsn2-C were cultured on the PDA media for 4 days, after which the colony morphology was observed. The results demonstrated that the ∆bcmsn2 mutants showed a decreased colony diameter compared with that of the WT (Figure 4A). The colony diameter (at 4 days post inoculation (dpi)) and the mycelium growth rate (at 3 dpi) were reduced by approximately 53% (Figure 4B). Compared with the WT, ∆bcmsn2 showed increased conidiophore formation (Figure 4C). To investigate the role of BcMsn2 in sclerotia formation, ∆bcmsn2 and the complementary strain ∆bcmsn2-C were cultured on the PDA media for 35 days in the dark. The results demonstrated that the formation of sclerotia was dramatically delayed by 14 days in ∆bcmsn2 compared with that in B05.10. The relative area and number of sclerotia increased after culturing on PDA media for one month; however, the difference among the strains was not significant (Figures 4A,D). Overall, these results demonstrated that loss of BcMsn2 in B. cinerea effected hyphal growth and sclerotia formation.
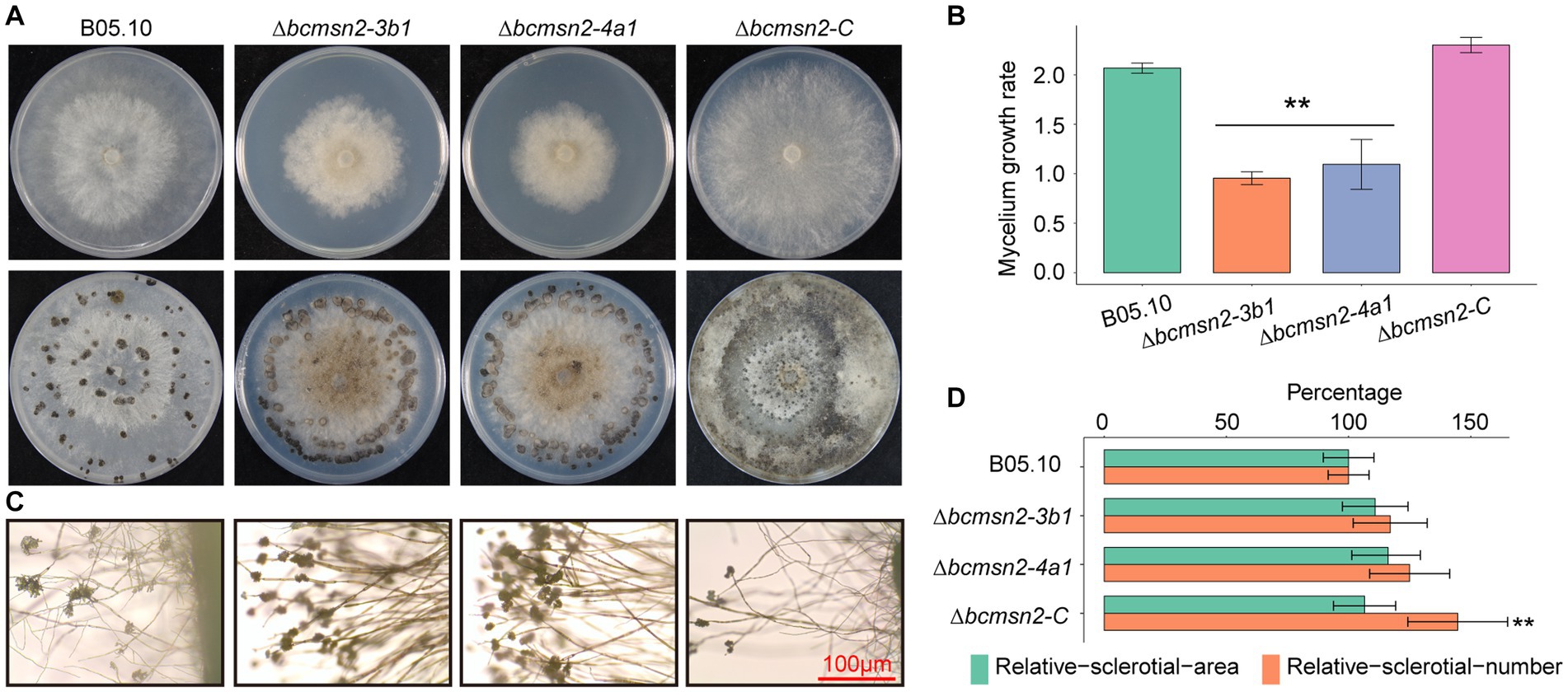
Figure 4. BcMsn2 is required for hyphal growth and conidiation in B. cinerea. (A) BcMsn2 mediates B. cinerea aerial mycelium (upper panel) and sclerotia (lower panel) production. Aerial mycelium and sclerotia of the wild-type (WT), ∆bcmsn2, and complemented strains were inoculated on PDA plates at 20°C for 4 days and 35 days in the dark, respectively. (B) The mycelium growth rate on PDA plates at 20°C for 3 days. Error bars represent the standard deviation and asterisks represent the significant difference (*p < 0.05, **p < 0.01). (C) Conidial and conidiophore formation were observed under a light microscope. Bar = 100 μm. (D) Quantification of the number and area of sclerotia.
3.4. BcMsn2 plays a critical role in virulence
To determine the role of BcMsn2 in B. cinerea pathogenicity, infection assays were conducted using different plant tissues (the leaves of beans, the fruits of tomato, pear, and apple), which serve as entry points for mycelia (Figure 5A). At 60 h post-inoculation (hpi), the lesion diameters of ∆bcmsn2 mutants on apple pear, and tomato fruits, and bean leaves were reduced by 53, 30, 24, and 50%, respectively, compared with those of WT (Figure 5B). These data suggested that BcMsn2 plays an important role in the invasive growth of B. cinerea in planta.
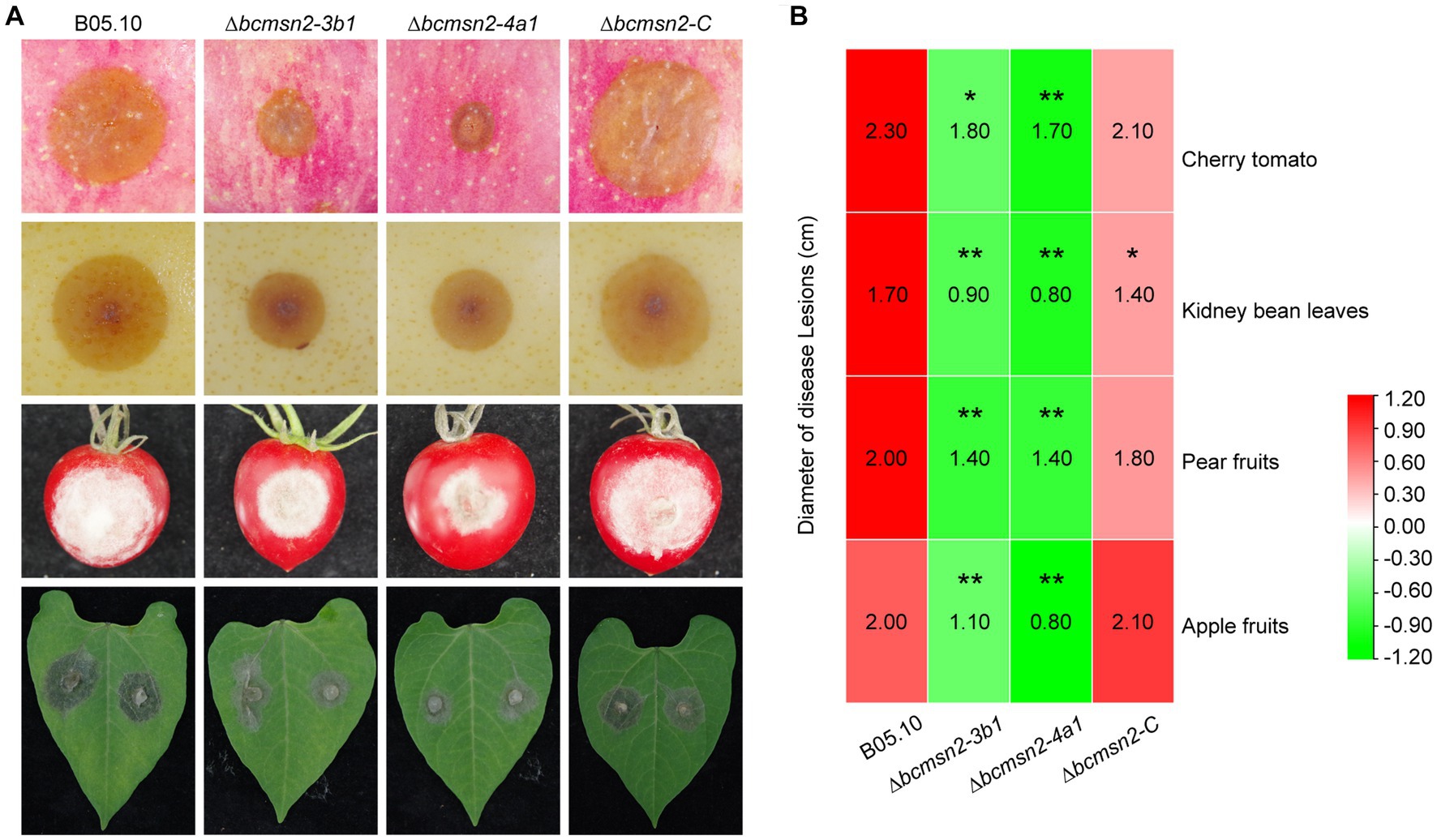
Figure 5. ∆bcmsn2 displayed lower virulence. (A) Pathogenicity assays of each strain on the leaves of bean, and the fruits of tomato, pear, and apple. Disease symptoms were photographed at 60 h post-inoculation (hpi). (B) The diameters of disease lesions of each strain shown in (A). (*p < 0.05, **p < 0.01).
3.5. BcMsn2 regulates cellular ROS scavenging genes
To investigate the functions of BcMsn2 in B. cinerea, RNA-seq analysis was performed using RNA samples isolated from the vegetative hyphae of the WT B05.10 and ∆bcmsn2 from 3-day PDA cultures. Compared with the WT, 2847 differentially expressed genes (1,346 upregulated and 1,501 downregulated) were detected in the ∆bcmsn2 mutant (Figure 6A). The upregulated genes in ∆bcmsn2 were enriched in the cellular nitrogen compound metabolic processes, while the downregulated genes were enriched in methionine biosynthetic/metabolic processes (Figure 6B). To validate the expression pattern of ROS-related genes, eight ROS-related genes were selected, and their expression patterns were confirmed using qRT-PCR. The expression of ROS-related genes was consistent with the expected results according the RNA-Seq analysis (Figure 6C). These results indicate that BcMsn2 is involved in a number of cellular amine metabolic processes, including ROS regulation.
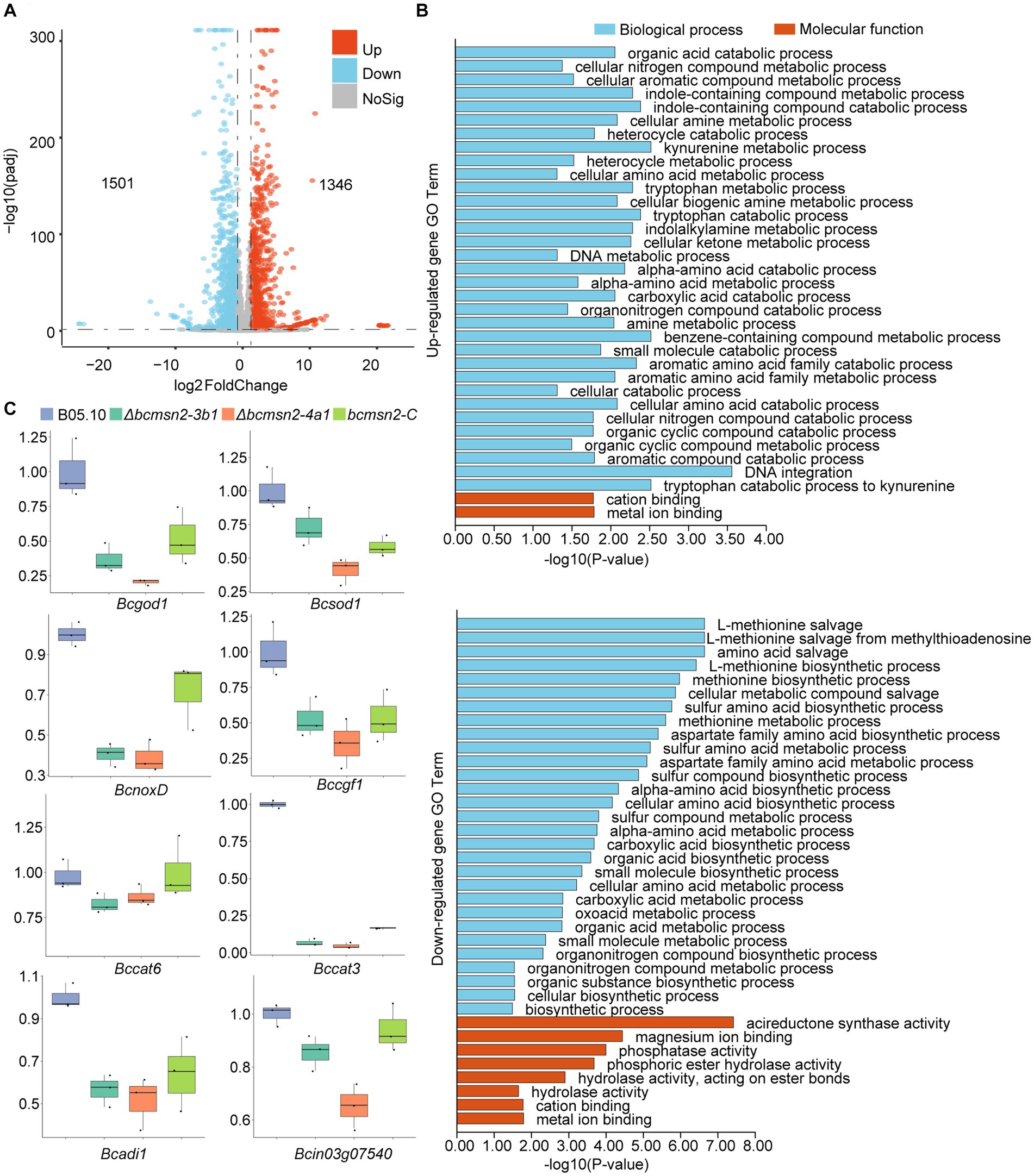
Figure 6. RNA-Seq analysis of the ∆bcmsn2 strain. (A) Volcano plot of the significantly upregulated and downregulated genes in the ∆bcmsn2 mutant (|log2 Fold Change| ≥ 1, p-value <0.05) compared with those in the wild-type. The numbers of differentially expressed genes were calculated with data from two biological replicates. (B) Enriched gene ontology (GO) terms associated with the upregulated and downregulated genes in the ∆bcmsn2 mutants. (C) qRT-PCR detection of the expression of ROS-related genes.
4. Discussion
The imbalance between ROS production and clearance can lead to oxidative stress, which is pervasive in the lifestyle of organisms and their interactions with their host (Saxena et al., 2016). Consequently, many organisms have developed oxidative stress response (OSR) mechanisms to scavenge elevated intracellular ROS levels (Yaakoub et al., 2022). In the yeast S. cerevisiae, the transcription factors Msn2 and Msn4 regulate 200 genes in response to various stresses, including heat shock, osmotic shock, low pH, sorbic acid, glucose starvation, oxidative stress, and high ethanol concentrations (Martínez-Pastor et al., 1996; Schmitt and McEntee, 1996; Gasch et al., 2000; Causton et al., 2001). Msn2 and Msn4 of S. cerevisiae are two C2H2 transcription factors involved in the Ras-cAMP pathway that is regulated by Ras-cAMP protein kinase A (Ras-cAMP-PKA), which activates genes containing the stress response element (STRE: CCCCT) to protect against oxidative stress and to maintain a reduced cellular redox balance (Martínez-Pastor et al., 1996; Schmitt and McEntee, 1996; Beck and Hall, 1999). Overexpression of Msn2 increases oxidation tolerance by enhancing Msn2-mediated proline binding (Mat Nanyan and Takagi, 2020). However, we only identified one homologous gene of Msn2/4 from S. cerevisiae in B. cinerea, which exhibits 66.6% and 64.9% identity with Msn2 and Msn4, respectively. This might be attributed to functional divergence, because Msn2 and Msn4 are functionally redundant in S. cerevisiae (Gasch et al., 2000). Different with S. cerevisiae that is a single celled eukaryotic organism and has no pathogenicity to hosts, B. cinerea are more related to filamentous pathogenic fungi. It was reported that ∆Bbmsn2 and ∆Mrmsn2 showed remarkable defects in conidial yield (40% decrease) and virulence (25% decrease) in two entomopathogenic fungi Beauveria bassiana (Bb) and Metarhizium robertsii (Mr) (Liu et al., 2013). Disruption of the MoMsn2 gene of M. oryzae resulted in defects in aerial hyphal growth, conidial production, and infection of host plants (Zhang et al., 2014). Similar phenotypes indicated that the function of the Msn2 gene in filamentous fungi is conserved.
Disruption of the BcMsn2 gene led to a significant increase in intracellular ROS levels. The involvement of BcMsn2 in the regulation of Sod, Cat, and NoxD genes in B. cinerea was confirmed through RNA-seq and qRT-PCR analyses. The downregulated genes were enriched in the methionine biosynthetic/metabolic processes. Methionine sulfoxide reductases (MsrA/Mxr1 and MsrB/Mxr2) reduce free oxidized methionine by acquiring electrons from the thioredoxin-reducing or glutathione system (García-Santamarina et al., 2013). The findings indicated that BcMsn2 might participate in oxidative stress and intracellular redox homeostasis by regulating Msr activity. Although SOD and CAT exhibit functional redundancy in B. cinerea, the loss of Cat3 is not compensated for by other catalases, resulting in increased development of aerial hyphae and conidia formation in N. crassa (Michán et al., 2003). The NADPH oxidase (NOX) complex in the plasma membrane is a primary ROS-producing protein complex (Li et al., 2019); however, NOX enzymes are not major contributors to ROS production in B. cinerea (Segmüller et al., 2008). Enzymatic ROS scavenging mechanisms are vital for suppressing toxic ROS levels. These results indicated that the BcMsn2 plays an important role in maintaining the balance between oxidizing and reducing equivalents, thereby regulating the intracellular redox state. Cellular ROS concentrations exhibit circadian oscillations (Yoshida et al., 2011). BcFRQ1 functions as a core negative clock element in the circadian system, regulating the conidiation and pathogenicity of B. cinerea (Hevia et al., 2015). It has been demonstrated that the ROS concentration regulates the transcriptional function of WCC, a transcription factor that promotes frq expression in N. crassa (Yoshida et al., 2011). The RNA-seq results identified a related gene (Gene ID: Bcin07g04440) with downregulated expression, which might be regulated by BcMsn2 and is involved in the cross-talk between the cellular redox state and the circadian system, although further investigation is needed to enhance our understanding of this process.
In terms of growth, the BcMsn2 knockout mutant exhibited a highly significant reduction in the hyphal growth rate, earlier conidial production and germination, but a decrease in the total amount of conidia produced, and a severe delay in sclerotium formation. Excessive ROS within ∆bcmsn2 conidia might account for the unusually active conidia but slower growth rate. Hyperoxidant states are a primary driving force for fungal differentiation (Aguirre et al., 2006; Yoshida et al., 2011). Studies have indicated that the differentiation of lateral-chained and terminal sclerotia in S. rolfsii and S. sclerotiorum is reduced by approximately 30% when the H2O2 concentration is low (1–3 mM). By contrast, higher concentrations of H2O2 (5–8 mM) promote sclerotial differentiation (Papapostolou et al., 2014). BcNOXA and BcNOXD interact with each other, and BcNOXA is essential for the formation of sclerotia (Segmüller et al., 2008; Siegmund et al., 2015). Thus, decreased expression of BcNoxD might be the reason for delayed sclerotial formation in the BcMsn2 knockout mutant.
In this article, we found knockout of BcMsn2 resulted in a significant decrease in pathogenicity of B. cinerea on apple pear, and tomato fruits, and bean leave. These results indicated BcMsn2 was also identified as an important virulence determinant, similar to its reported involvement in virulence in M. oryzae. MoMsn2 is essential for sporulation and conidiophore development, and the ∆Momsn2 mutant lost its pathogenicity (Zhang et al., 2014). When B. cinerea infects plants, the plants undergo a so-called ‘oxidative burst’, producing ROS as a crucial component of their defense responses (Govrin and Levine, 2000). Sod knockout had a suppressive effect on pathogenicity (Rolke et al., 2004). The balanced redox status maintained by the thioredoxin system is essential for the development and pathogenesis of B. cinerea (Viefhues et al., 2014; Liang et al., 2022). However, there is a contrary view, suggesting that ROS-degrading enzymes and other central components of cellular redox status are not essential for pathogenesis (Temme and Tudzynski, 2009).
In summary, unraveling the regulation mechanisms of BcMsn2 is of significant importance in understanding its function in B. cinerea. This study demonstrated that BcMsn2 is a key gene in the regulation of intracellular redox homeostasis in B. cinerea. ROS derived from fungi play critical roles in various development processes, including conidia and sclerotia formation, as well as pathogenesis. Many studies have reported the close association between ROS and the circadian rhythm (Yoshida et al., 2011; Ayer et al., 2014; Hevia et al., 2015); however, limited studies have been conducted in B. cinerea. Further investigation will enhance our understanding of how the circadian clock modulates the pathogenic potential of this pathogen at different times of the day.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
PL: formal analysis, resources, validation, visualization and writing–review and editing. KW and JW: formal analysis, resources, validation, investigation, methodology, visualization, and writing–original draft. CX: formal analysis and investigation. SY: writing–review and editing. LM: conceptualization. HS: conceptualization, funding acquisition, methodology, project administration and writing–review and editing. All authors have read and approved the final version of the manuscript.
Funding
This research was supported by the National Science Foundation of China [grant number 31301619] and Zhejiang Provincial Natural Science Foundation of China [grant number LY19C140004].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1247072/full#supplementary-material
Footnotes
References
Aguirre, J., Hansberg, W., and Navarro, R. (2006). Fungal responses to reactive oxygen species. Med. Mycol. 44, 101–107. doi: 10.1080/13693780600900080
Ayer, A., Gourlay, C. W., and Dawes, I. W. (2014). Cellular redox homeostasis, reactive oxygen species and replicative ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 14, 60–72. doi: 10.1111/1567-1364.12114
Beck, T., and Hall, M. N. (1999). The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402, 689–692. doi: 10.1038/45287
Brandhoff, B., Simon, A., Dornieden, A., and Schumacher, J. (2017). Regulation of conidiation in Botrytis cinerea involves the light-responsive transcriptional regulators BcLTF3 and BcREG1. Curr. Genet. 63, 931–949. doi: 10.1007/s00294-017-0692-9
Causton, H. C., Ren, B., Koh, S. S., Harbison, C. T., Kanin, E., Jennings, E. G., et al. (2001). Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12, 323–337. doi: 10.1091/mbc.12.2.323
Chang, P.-K., Scharfenstein, L. L., Luo, M., Mahoney, N., Molyneux, R. J., Yu, J., et al. (2011). Loss of msnA, a putative stress regulatory gene, in aspergillus parasiticus and aspergillus flavus increased production of conidia, aflatoxins and kojic acid. Toxins 3, 82–104. doi: 10.3390/toxins3010082
Charizanis, C., Juhnke, H., Krems, B., and Entian, K.-D. (1999). The oxidative stress response mediated via Pos9/Skn7 is negatively regulated by the Ras/PKA pathway in Saccharomyces cerevisiae. Mol. Gen. Genet. 261, 740–752. doi: 10.1007/s004380050017
Cohrs, K. C., Simon, A., Viaud, M., and Schumacher, J. (2016). Light governs asexual differentiation in the grey mould fungus Botrytis cinerea via the putative transcription factor BcLTF2. Environ. Microbiol. 18, 4068–4086. doi: 10.1111/1462-2920.13431
Dean, R., van Kan, J. A. L., Pretorius, Z. A., Hammond-Kosack, K. E., di Pietro, A. N., Spanu, P. D., et al. (2012). The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
Doehlemann, G., Berndt, P., and Hahn, M. (2006). Different signalling pathways involving a Gα protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol. Microbiol. 59, 821–835. doi: 10.1111/j.1365-2958.2005.04991.x
Drakulic, T., Temple, M., Guido, R., Jarolim, S., Breitenbach, M., Attfield, P., et al. (2005). Involvement of oxidative stress response genes in redox homeostasis, the level of reactive oxygen species, and ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 5, 1215–1228. doi: 10.1016/j.femsyr.2005.06.001
Fanjul-Moles, M. L., and López-Riquelme, G. O. (2016). Relationship between oxidative stress, circadian rhythms, and AMD. Oxidative Med. Cell. Longev. 2016:7420637. doi: 10.1155/2016/7420637
García-Santamarina, S., Boronat, S., Ayté, J., and Hidalgo, E. (2013). Methionine sulphoxide reductases revisited: free methionine as a primary target of H2O2 stress in auxotrophic fission yeast. Mol. Microbiol. 90, 1113–1124. doi: 10.1111/mmi.12420
Gasch, A. P., Spellman, P. T., Kao, C. M., Carmel-Harel, O., Eisen, M. B., Storz, G., et al. (2000). Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257. doi: 10.1091/mbc.11.12.4241
Georgiou, C. D., Tairis, N., and Sotiropoulou, A. (2000). Hydroxyl radical scavengers inhibit sclerotial differentiation and growth in Sclerotinia sclerotiorum and Rhizoctonia solani. Mycol. Res. 104, 1191–1196. doi: 10.1017/S0953756200002707
Godon, C., Lagniel, G., Lee, J., Buhler, J.-M., Kieffer, S., Perrot, M., et al. (1998). The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 273, 22480–22489. doi: 10.1074/jbc.273.35.22480
Govrin, E. M., and Levine, A. (2000). The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 10, 751–757. doi: 10.1016/S0960-9822(00)00560-1
Gronover, C. S., Kasulke, D., Tudzynski, P., and Tudzynski, B. (2001). The role of G protein alpha subunits in the infection process of the gray mold fungus Botrytis cinerea. Mol. Plant-Microbe Interact. 14, 1293–1302. doi: 10.1094/mpmi.2001.14.11.1293
Guan, W., Feng, J., Wang, R., Ma, Z., Wang, W., Wang, K., et al. (2020). Functional analysis of the exocyst subunit BcExo70 in Botrytis cinerea. Curr. Genet. 66, 85–95. doi: 10.1007/s00294-019-01002-9
Hasan, R., Leroy, C., Isnard, A.-D., Labarre, J., Boy-Marcotte, E., and Toledano, M. B. (2002). The control of the yeast H2O2 response by the Msn2/4 transcription factors. Mol. Microbiol. 45, 233–241. doi: 10.1046/j.1365-2958.2002.03011.x
Hevia, M. A., Canessa, P., Müller-Esparza, H., and Larrondo, L. F. (2015). A circadian oscillator in the fungus Botrytis cinerea regulates virulence when infecting Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 112, 8744–8749. doi: 10.1073/pnas.1508432112
Jing, Y., Shen, N., Zheng, X., Fu, A., Zhao, F., Lan, W., et al. (2020). Danger-associated peptide regulates root immune responses and root growth by affecting ROS formation in Arabidopsis. Int. J. Mol. Sci. 21:4590. doi: 10.3390/ijms21134590
Kuck, U., and Hoff, B. (2006). Application of the nourseothricin acetyltransferase gene (nat1) as dominant marker for the transformation of filamentous fungi. Fungal Genet. Biol. 53, 9–11. doi: 10.4148/1941-4765.1106
Kuge, S., and Jones, N. (1994). YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 13, 655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x
Lee, J., Godon, C., Lagniel, G., Spector, D., Garin, J., Labarre, J., et al. (1999). Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 274, 16040–16046. doi: 10.1074/jbc.274.23.16040
Li, H., Tian, S., and Qin, G. (2019). NADPH oxidase is crucial for the cellular redox homeostasis in fungal pathogen Botrytis cinerea. Mol. Plant-Microbe Interact. 32, 1508–1516. doi: 10.1094/MPMI-05-19-0124-R
Liang, M., Dong, L., and Deng, Y. Z. (2022). Circadian redox rhythm in plant–fungal pathogen interactions. Antioxid. Redox Signal. 37, 726–738. doi: 10.1089/ars.2021.0281
Liu, Q., Ying, S.-H., Li, J.-G., Tian, C.-G., and Feng, M.-G. (2013). Insight into the transcriptional regulation of Msn2 required for conidiation, multi-stress responses and virulence of two entomopathogenic fungi. Fungal Genet. Biol. 54, 42–51. doi: 10.1016/j.fgb.2013.02.008
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Love, M. I., Huber, W., and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550–521. doi: 10.1186/s13059-014-0550-8
Mailloux, R. J. (2015). Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 4, 381–398. doi: 10.1016/j.redox.2015.02.001
Martinez, F., Blancard, D., Lecomte, P., Levis, C., Dubos, B., and Fermaud, M. (2003). Phenotypic differences between vacuma and transposa subpopulations of Botrytis cinerea. Eur. J. Plant Pathol. 109, 479–488. doi: 10.1023/A:1024222206991
Martínez-Pastor, M. T., Marchler, G., Schüller, C., Marchler-Bauer, A., Ruis, H., and Estruch, F. (1996). The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15, 2227–2235. doi: 10.1002/j.1460-2075.1996.tb00576.x
Mat Nanyan, N. S. B., and Takagi, H. (2020). Proline homeostasis in Saccharomyces cerevisiae: how does the stress-responsive transcription factor Msn2 play a role? Front. Genet. 11:438. doi: 10.3389/fgene.2020.00438
Michán, S., Lledías, F., and Hansberg, W. (2003). Asexual development is increased in Neurospora Crassa cat-3-null mutant strains. Eukaryot. Cell 2, 798–808. doi: 10.1128/EC.2.4.798-808.2003
Michielse, C. B., Becker, M., Heller, J., Moraga, J., Collado, I. G., and Tudzynski, P. (2011). The Botrytis cinerea Reg1 protein, a putative transcriptional regulator, is required for pathogenicity, conidiogenesis, and the production of secondary metabolites. Mol. Plant-Microbe Interact. 24, 1074–1085. doi: 10.1094/MPMI-01-11-0007
Milkovic, L., Cipak Gasparovic, A., Cindric, M., Mouthuy, P.-A., and Zarkovic, N. (2019). Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells 8:793. doi: 10.3390/cells8080793
Morgan, B. A. (1997). The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 16, 1035–1044. doi: 10.1093/emboj/16.5.1035
Papapostolou, I., Sideri, M., and Georgiou, C. D. (2014). Cell proliferating and differentiating role of H2O2 in Sclerotium rolfsii and Sclerotinia sclerotiorum. Microbiol. Res. 169, 527–532. doi: 10.1016/j.micres.2013.12.002
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A., and Kingsford, C. (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419. doi: 10.1038/nmeth.4197
Pertea, M., Kim, D., Pertea, G. M., Leek, J. T., and Salzberg, S. L. (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11, 1650–1667. doi: 10.1038/nprot.2016.095
Rascle, C., Dieryckx, C., Dupuy, J. W., Muszkieta, L., Souibgui, E., Droux, M., et al. (2018). The pH regulator PacC: a host-dependent virulence factor in Botrytis cinerea. Environ. Microbiol. Rep. 10, 555–568. doi: 10.1111/1758-2229.12663
Ren, H., Wu, X., Lyu, Y., Zhou, H., Xie, X., Zhang, X., et al. (2017). Selection of reliable reference genes for gene expression studies in Botrytis cinerea. J. Microbiol. Methods 142, 71–75. doi: 10.1016/j.mimet.2017.09.006
Rolke, Y., Liu, S., Quidde, T., Williamson, B., Schouten, A., Weltring, K.-M., et al. (2004). Functional analysis of H2O2-generating systems in Botrytis cinerea: the major cu-Zn-superoxide dismutase (BCSOD1) contributes to virulence on french bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol. Plant Pathol. 5, 17–27. doi: 10.1111/j.1364-3703.2004.00201.x
Rolland, S., Jobic, C., Fèvre, M., and Bruel, C. (2003). Agrobacterium-mediated transformation of Botrytis cinerea, simple purification of monokaryotic transformants and rapid conidia-based identification of the transfer-DNA host genomic DNA flanking sequences. Curr. Genet. 44, 164–171. doi: 10.1007/s00294-003-0438-8
Saxena, I., Srikanth, S., and Chen, Z. (2016). Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 7:570. doi: 10.3389/fpls.2016.00570
Schiestl, R. H., and Gietz, R. D. (1989). High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16, 339–346. doi: 10.1007/BF00340712
Schmitt, A. P., and McEntee, K. (1996). Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 93, 5777–5782. doi: 10.1073/pnas.93.12.5777
Schumacher, J., Kokkelink, L., Huesmann, C., Jimenez-Teja, D., Collado, I. G., Barakat, R., et al. (2008). The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant-Microbe Interact. 21, 1443–1459. doi: 10.1094/MPMI-21-11-1443
Segmüller, N., Ellendorf, U., Tudzynski, B., and Tudzynski, P. (2007). BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot. Cell 6, 211–221. doi: 10.1128/EC.00153-06
Segmüller, N., Kokkelink, L., Giesbert, S., Odinius, D., van Kan, J., and Tudzynski, P. (2008). NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant-Microbe Interact. 21, 808–819. doi: 10.1094/MPMI-21-6-0808
Siegmund, U., Marschall, R., and Tudzynski, P. (2015). BcNoxD, a putative ER protein, is a new component of the NADPH oxidase complex in Botrytis cinerea. Mol. Microbiol. 95, 988–1005. doi: 10.1111/mmi.12869
Tang, J., Wu, M., Zhang, J., Li, G., and Yang, L. (2021). Botrytis cinerea G protein β subunit Bcgb1 controls growth, development and virulence by regulating cAMP signaling and MAPK signaling. J. Fungi 7:431. doi: 10.3390/jof7060431
Temme, N., and Tudzynski, P. (2009). Does Botrytis cinerea ignore H2O2-induced oxidative stress during infection? Characterization of Botrytis activator protein 1. Mol. Plant-Microbe Interact. 22, 987–998. doi: 10.1094/MPMI-22-8-0987
van Kan, J. A. L., van’t Klooster, J. W., Wagemakers, C. A. M., Dees, D. C. T., and van der Vlugt-Bergmans, C. J. B. (1997). Cutinase a of Botrytis cinerea is expressed, but not essential, during penetration of gerbera and tomato. Mol. Plant-Microbe Interact. 10, 30–38. doi: 10.1094/MPMI.1997.10.1.30
Viefhues, A., Heller, J., Temme, N., and Tudzynski, P. (2014). Redox systems in Botrytis cinerea: impact on development and virulence. Mol. Plant-Microbe Interact. 27, 858–874. doi: 10.1094/MPMI-01-14-0012-R
Williamson, B., Tudzynski, B., Tudzynski, P., and Van Kan, J. A. L. (2007). Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8, 561–580. doi: 10.1111/j.1364-3703.2007.00417.x
Yaakoub, H., Mina, S., Calenda, A., Bouchara, J.-P., and Papon, N. (2022). Oxidative stress response pathways in fungi. Cell. Mol. Life Sci. 79:333. doi: 10.1007/s00018-022-04353-8
Yoshida, Y., Iigusa, H., Wang, N., and Hasunuma, K. (2011). Cross-talk between the cellular redox state and the circadian system in Neurospora. PLoS One 6:e28227. doi: 10.1371/journal.pone.0028227
Yu, J.-H., Hamari, Z., Han, K.-H., Seo, J.-A., Reyes-Domínguez, Y., and Scazzocchio, C. (2004). Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981. doi: 10.1016/j.fgb.2004.08.001
Keywords: Botrytis cinerea, zinc finger protein, ROS, transcriptional regulator, RNA-Seq
Citation: Lu P, Wang K, Wang J, Xia C, Yang S, Ma L and Shi H (2023) A novel zinc finger transcription factor, BcMsn2, is involved in growth, development, and virulence in Botrytis cinerea. Front. Microbiol. 14:1247072. doi: 10.3389/fmicb.2023.1247072
Edited by:
Huangen Ding, Louisiana State University, United StatesReviewed by:
Arunkumar Venkatesan, Upstate Medical University, United StatesJiaoyu Wang, Zhejiang Academy of Agricultural Sciences, China
Copyright © 2023 Lu, Wang, Wang, Xia, Yang, Ma and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Ma, bGlhbmdtMjAwOEBvdXRsb29rLmNvbQ==; Haojie Shi, c2hqQHphZnUuZWR1LmNu
†These authors have contributed equally to this work
 Ping Lu1†
Ping Lu1† Liang Ma
Liang Ma Haojie Shi
Haojie Shi