- 1Department of Infectious Diseases, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2Department of Gastroenterology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Background: Ulcerative colitis (UC) patients with relapsed disease are most likely to suffer from anxiety and depression. Increasing data indicates that psychological issues can change the composition of intestinal flora. Thus, we aim to seek the variation of intestinal microbiota composition in remission UC patients with anxiety and depression in Northwest China.
Results: In this study, 45 UC patients in remission were enrolled. The incidence of anxiety was 33.3%, and the prevalence of depression was 22.2%. There was no statistical difference in the alpha diversity of fecal microbiota, while beta diversity had a significant difference between the anxiety group and the non-anxiety group and the depression group and the non-depression group. Species composition analysis results showed that the ratio of Bifidobacterium and Lactobacilales significantly decreased. At the same time, the proportion of Escherichia-Shigella and Proteus_mirabilis increased in the anxiety group, and the ratio of Faecalibacterium and Bifidobacterium significantly decreased. In contrast, Escherichia-Shigella increased in the depression group at the gene levels.
Conclusion: Anxiety and depression still exist in UC patients even in the remission period. We first identify that the proportion of probiotics decreases while the proportion of pathogens increases in UC patients with anxiety and depression. These findings may provide a new pathophysiological mechanism for the recurrence of disease caused by impaired psychological function and a new method for the treatment strategy of UC patients with psychological issues.
Introduction
Inflammatory bowel diseases, Ulcerative Colitis (UC), and Crohn's disease are chronic idiopathic disorders causing inflammation of the gastrointestinal tract (Ng et al., 2017). The proportion of ulcerative colitis (UC) in IBD in China is significantly higher than in Western countries, which is a chronic inflammatory disease affecting the mucosa of the colon and rectum (Yang et al., 2022).
The most common psychological issues related to UC are anxiety and depression. The chronic inflammation associated with UC makes patients prone to psychological issues owing to their early diagnosis and lifelong symptoms (Neuendorf et al., 2016; Prendergast et al., 2022). There is a high prevalence of anxiety and depression in patients with UC, with up to one-third of patients experiencing anxiety symptoms and a quarter experiencing depression (Yu et al., 2023); female gender, lack of social support, and disease activity were associated with depression and anxiety in UC (Mikocka-Walus et al., 2016; Neuendorf et al., 2016; Williet and Sarter, 2017).
The relationship between UC and unhealthy psychological conditions seems bidirectional. The presence of abnormal anxiety scores at the baseline was associated with the later need for glucocorticosteroids or flare of UC activity and escalation of therapy in remission UC patients (Fairbrass et al., 2021), and depression at the baseline was related to an increased risk of aggressive UC at clinical follow-up (Kim et al., 2023). Despite this, the underlying mechanisms and association between psychological stress and IBD remain poorly understood.
The interaction between the brain and intestine mainly depends on the brain–gut axis. With the gradual understanding of the intestinal flora and its functions, the original “gut–brain axis” has been asserted to be the “gut–brain–bacteria axis” (Gracie et al., 2018; Basiji et al., 2023). Recent research studies revealed that intestinal flora can be affected by human psychological health (Wilkinson et al., 2019). Catecholamines can be released by sympathetic excitation by changing the colonization pattern of intestinal flora on the mucosal surface and leading to intestinal flora across the intestinal mucosa, which could mediate immune responses and cause intestinal inflammation (Mohajeri et al., 2018).
Taken together, anxiety and depression are common comorbidities in UC patients that can cause a flare of UC activity and escalation of therapy. The latest research finds that intestinal flora can be influenced by impaired psychological function. Thus, we aim to seek the variation of intestinal microbiota among UC patients with depression and anxiety, finding the potential pathophysiological mechanism for the recurrence of disease caused by impaired psychological conditions.
Materials and methods
Study population
From July 2021 to May 2022, this cross-sectional study was conducted at the First Affiliated Hospital of Xi'an Jiaotong University. All the UC subjects were in line with the Chinese consensus on diagnosis and treatment in IBD 2018 (Wu et al., 2018), and all the participants were in clinical remission (Mayo score ≤ 2 and no single subscore >1). Patients with other psychiatric disorders or who used antibiotics and probiotics in 1 month were excluded.
Data collection
The information on the demographic and disease characteristics of UC patients was collected via an electronic medical record system. The patient's psychological condition was evaluated by accomplishing Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983). Based on 16S rDNA sequencing technology, we analyzed alpha and beta diversities in fecal microbiota and the species composition differences of UC patients between positive and negative groups using bioinformatics.
Microbial profiling
Fecal samples of all UC patients were collected and prepared. DNA was extracted from the fecal samples (HiPure Stool DNA Kits, D3141, Guangzhou Meiji Biotechnology Company, China), and the V3–V4 hypervariable region of bacterial 16S rRNA gene was sequenced (341F:CCTACGGGNGGCWGCAG 806R GGACTACHVGGGTATCTAAT). At a 98% similarity level, sequences were grouped into OTUs. In at least one sample, OTUs with relative abundances above 0.3% were retained.
Questionnaires
The Hospital Anxiety and Depression Scale was used to assess patients' psychological conditions. The scale has two subscales, the Hospital Depression Scale (HDS) and the Hospital Anxiety Scale (HAS), both with a total score of 0–21. In previous studies, 8 is usually divided into the cutoff score of anxiety and depression.
Statistical analysis
Alpha diversity refers to the Microbiota species diversity and evenness. A paired t-test of the Shannon Index was used to compare alpha diversity between different groups. A P-value of < 0.05 represented that the alpha diversity of different groups has statistical differences. Beta diversity analysis refers to the species differences among different bacterial communities. This study used principal coordinate analysis (PCA) that could find the differences in complex samples by reducing dimensions. We used Welch's test to analyze species composition differences, and a P-value of < 0.05 is the significant statistical threshold.
Results
Study population
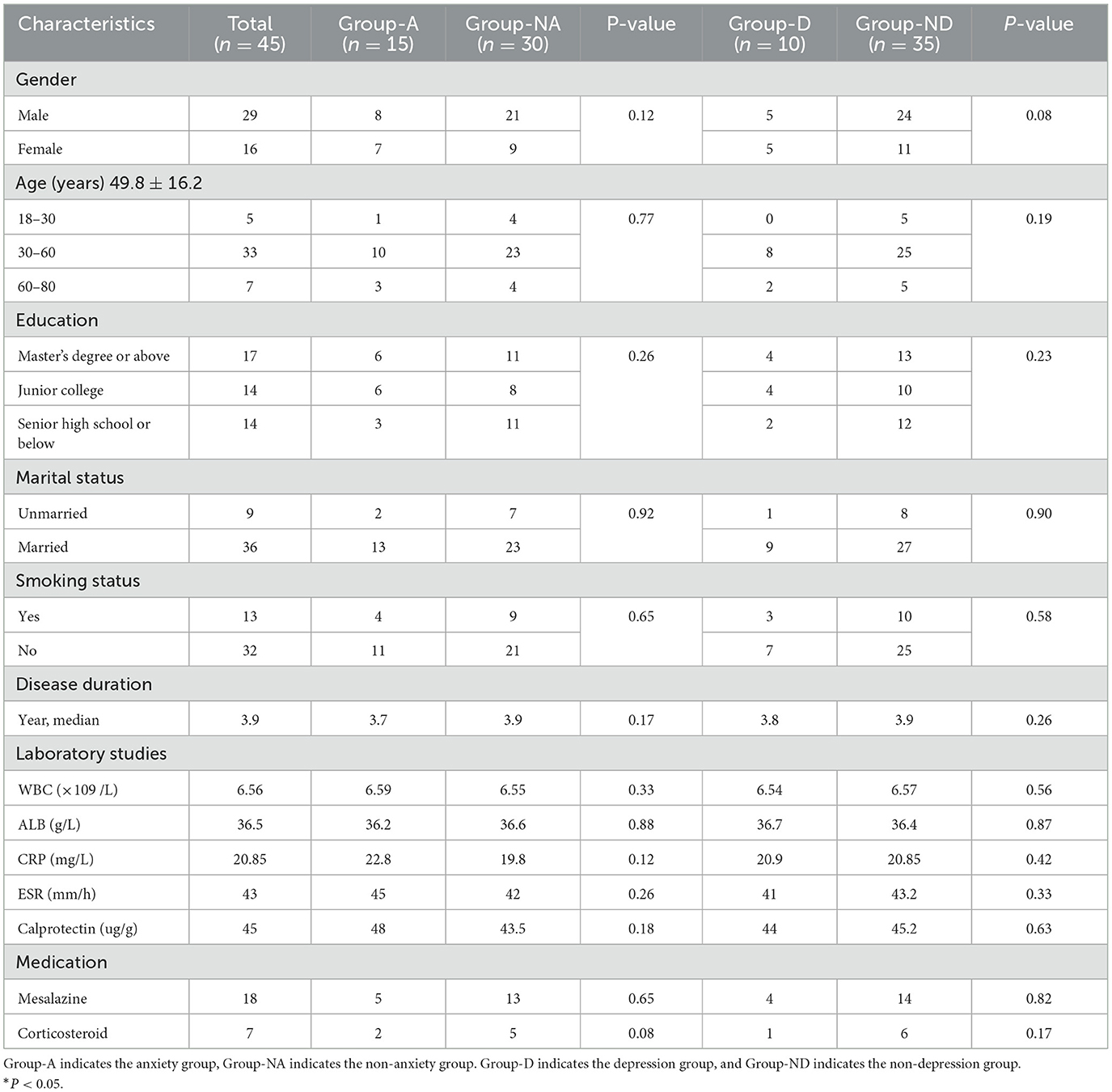
A total of 45 UC patients were included in this study, and all of them had a median age of 49.8 ± 16.2 years. In terms of the duration of the disease, the participants had a median of 3.9 (IQR: 1.3–7.9) years; all of the participants were in remission period. In total, 31% of the patients had education of high school or below, and other demographic and disease characteristics of UC patients were summarized in Table 1.
Prevalence of anxiety and depression in remission UC patients
According to the findings of the HADS questionnaire, anxiety symptoms were found in 15 out of 45 UC patients, while depression symptoms were found in 10 out of 45 UC patients (Table 1). The prevalence of anxiety and depression is 33.3% and 22.2%, respectively. Further statistical analysis showed that gender, age, education level, marital status, disease duration, and medication did not differ between the groups (P > 0.05) (Table 1).
Comparison of Alpha diversity between the groups
The dilution curve of all fecal samples shows that the 30,000 tag sequence amounts that we provided were sufficient to cover most taxa and flora (Figure 1A). Alpha diversity represents the flora species richness and evenness, of which the Shannon index is the most representative index. Using the Shannon index, there were no significant differences in the species richness and diversity between the anxiety, non-anxiety, depression, and non-depression groups in remission UC (P > 0.05) (Figures 1B, C). The findings revealed that anxiety and depression did not affect the alpha diversity.
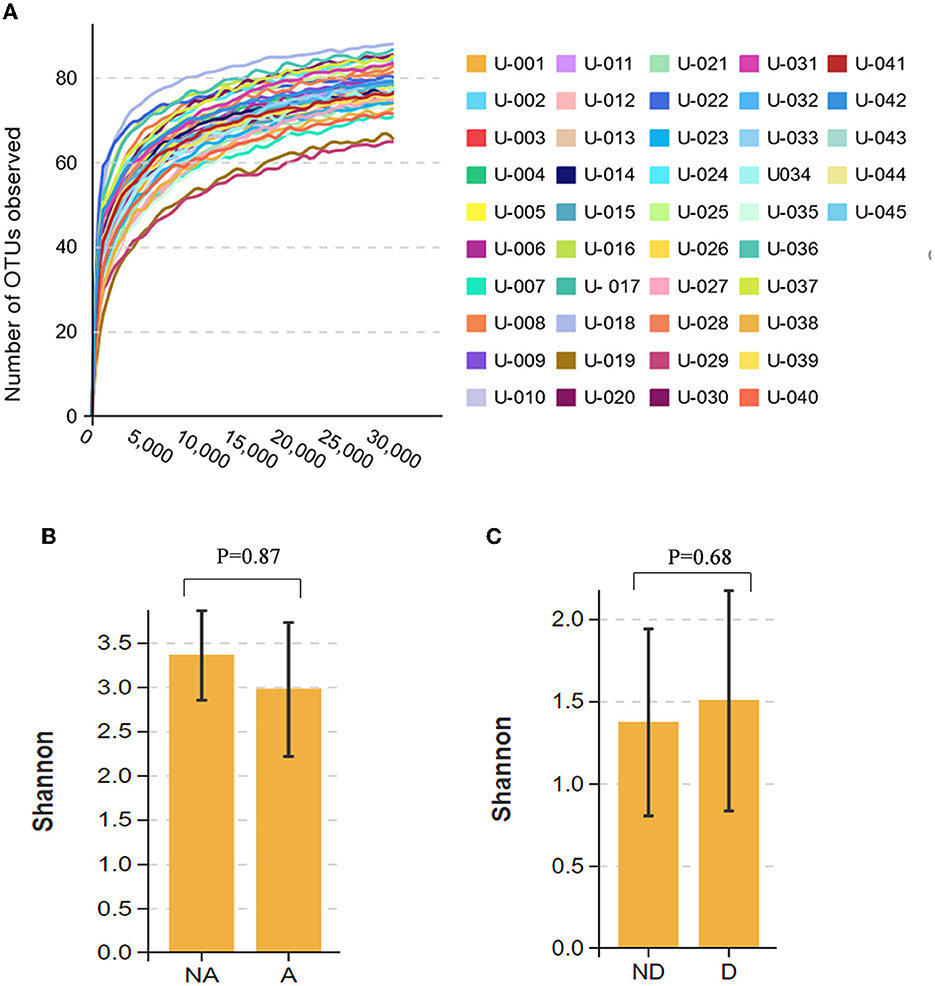
Figure 1. Comparison of alpha diversity between the groups. (A) The dilution curve of the fecal flora of all participants. (B) Shannon index comparison between the anxiety and the non-anxiety groups. (C) Shannon index comparison between the depression and non-depression groups. A indicates the anxiety group, and NA indicates the non-anxiety group. D indicates the depression group, and ND indicates the non-depression group.
Comparison of Beta diversity between the groups
Beta diversity refers to the comparison of species diversity among communities. The principal coordinate analysis (PCA) result showed that the yellow dots represented the anxiety group samples, and the blue dots represented the samples of the non-anxiety group. PCo1 contributes to the species variance of 30.04%, and PCo2 contributes to the species variance of 14.89% (Figure 2A). We found that all samples were divided into two clusters, indicating that the beta diversity was different between the anxiety and non-anxiety groups. The further analysis of similarity (ANOSIM) statistically indicated that anxiety changed the composition of the intestinal flora community in remission UC (R = 0.098, P = 0.009) (Figure 2C).
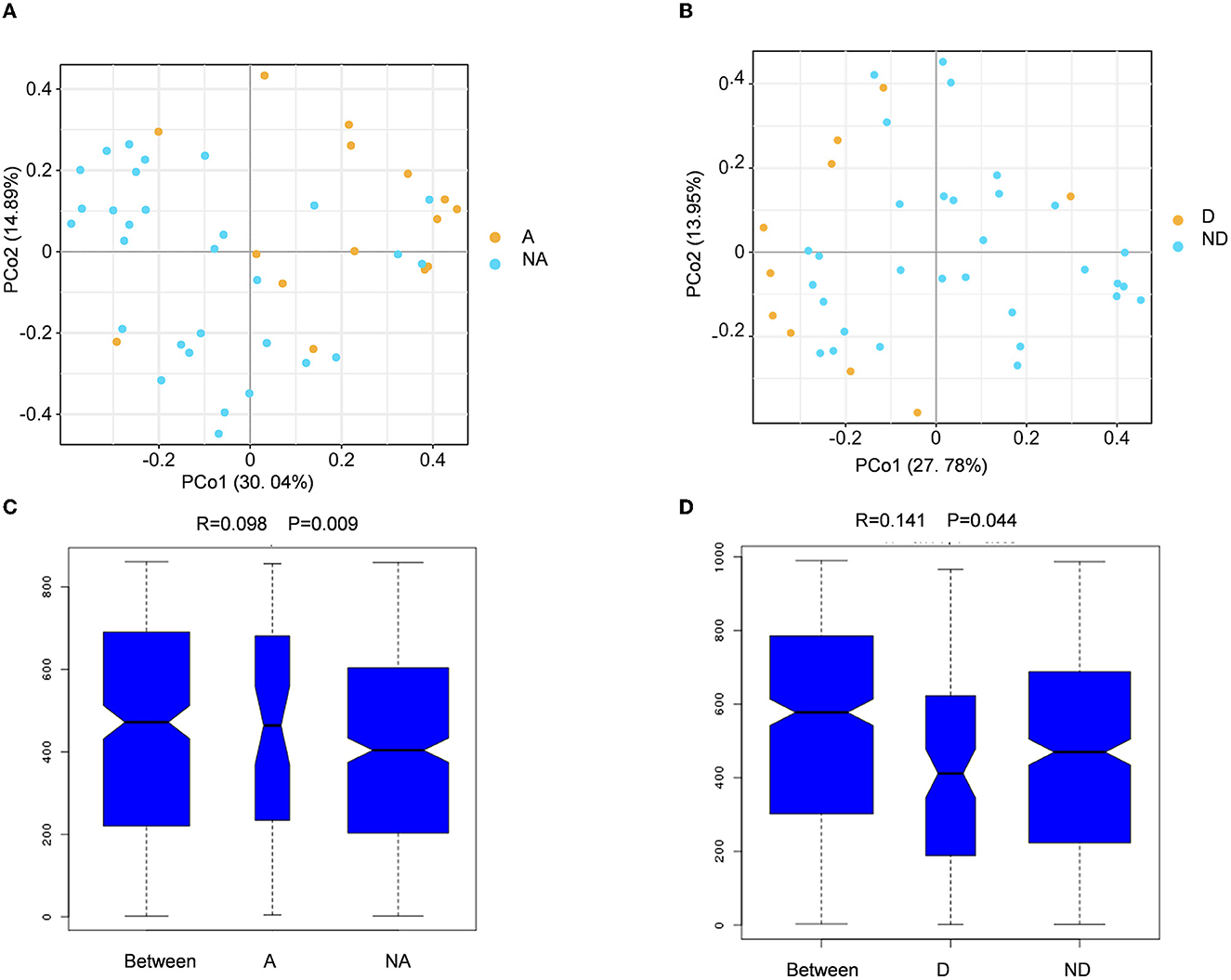
Figure 2. Fecal flora compositional differences associated with anxiety or depression. (A, B) The principal coordinate analysis (PCA) results between the anxiety group, non-anxiety group, depression group, and non-depression group. (C, D) Analysis of similarity between the groups. A indicates the anxiety group, and NA indicates the non-anxiety group. D shows the depression group. ND indicates the non-depression group.
The principal coordinate analysis revealed that PC1 contributed 27.78% and the PC2 provided 13.95% to species variance between the depression group and the non-depression group when compared with the depression group (Figure 2B). The ANOSIM analysis revealed that the two groups have different bacterial community structures (R = 0.141, P = 0.044) (Figure 2D). There was a significant association between anxiety and depression and beta diversity of fecal microbiota in UC patients in remission.
Species composition analysis with anxiety and depression
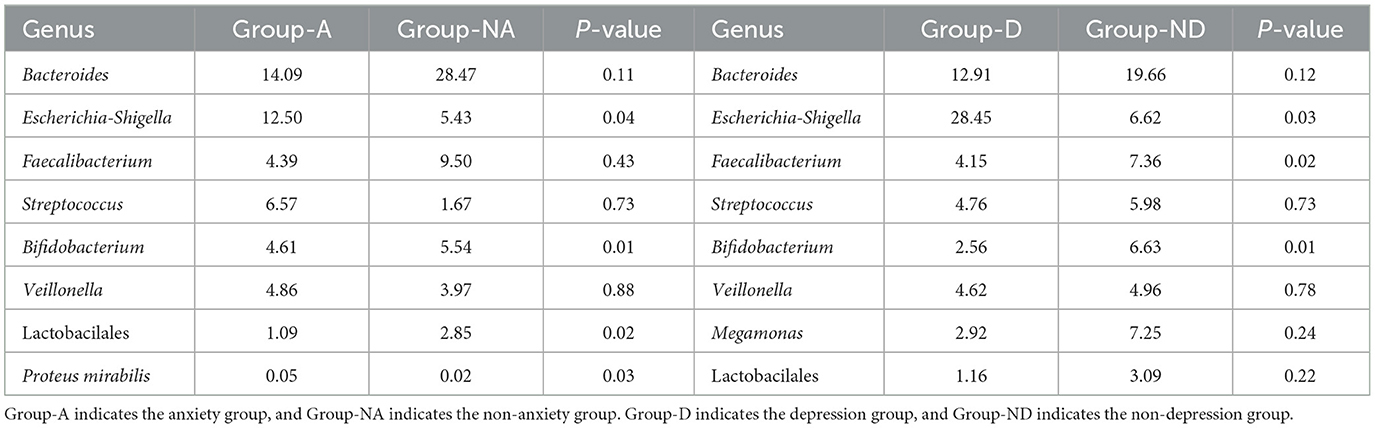
As shown in the species stacking chart (Figure 3A) and Table 2, the fecal flora with the largest composition ratio of the non-anxiety group was Bacteroides, Faecalibacterium, and Megamonas, while the main composition flora of the anxiety group was Bacteroides, Escherichia-Shigella, and Megamonas. Compared with the non-anxiety group, Bifidobacterium and Lactobacillus statistically decreased (P < 0.05), and Escherichia-Shigella and Proteus_mirabilis increased in the anxiety group (P < 0.05).
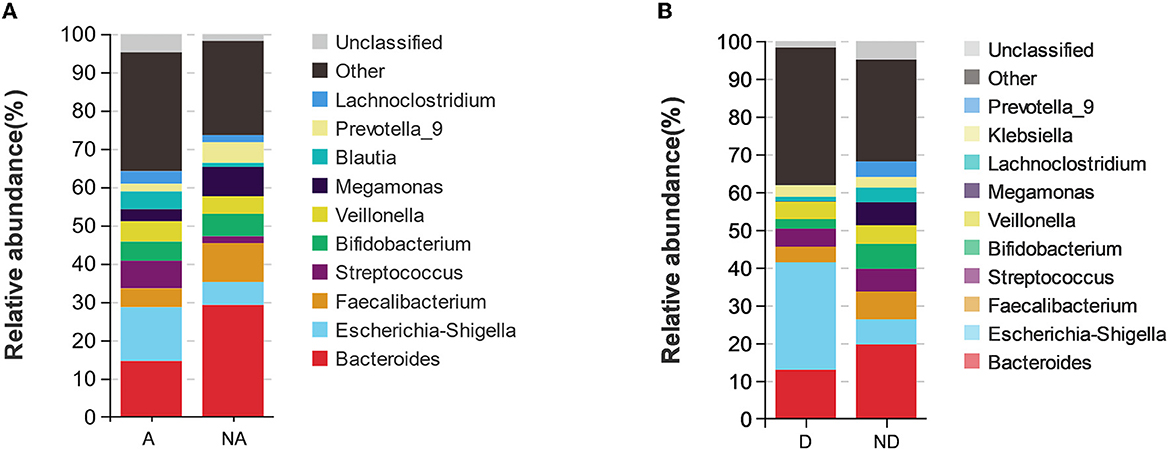
Figure 3. Species stacking chart between different groups. Species stacking chart shows the species proportions at the genus level in different groups in remission UC. (A) Species stacking chart between anxiety group and non-anxiety group. (B) Species stacking chart between depression group and non-depression group. A indicates the anxiety group, and NA indicates the non-anxiety group. D indicates the depression group, and ND indicates the non-depression group.
According to the depression group, Bacteroides, Escherichia, Shigella, and Streptococcus had the largest fecal flora composition ratio. However, the proportion of fecal flora in the non-depression group was Bacteroides, Faecalibacterium, and Escherichia-Shigella. Compared with the non-depression group (Figure 3B), Faecalibacterium and Bifidobacterium were statistically decreased (P < 0.05), and Escherichia-Shigella was statistically increased (P < 0.05) in remission UC with depression.
The variation characteristics of microbiota between patients only had anxiety/depression and suffered from both
Among 45 UC patients, six patients had both anxiety and depression symptoms (Figure 4A), nine patients had only anxiety symptoms, and four patients had only depression symptoms. The Wayne diagram is shown in Figure 4A. We analyzed the microbial communities of these three groups, but there were no statistically significant differences in alpha and beta diversity between the groups (Figures 4B–D).
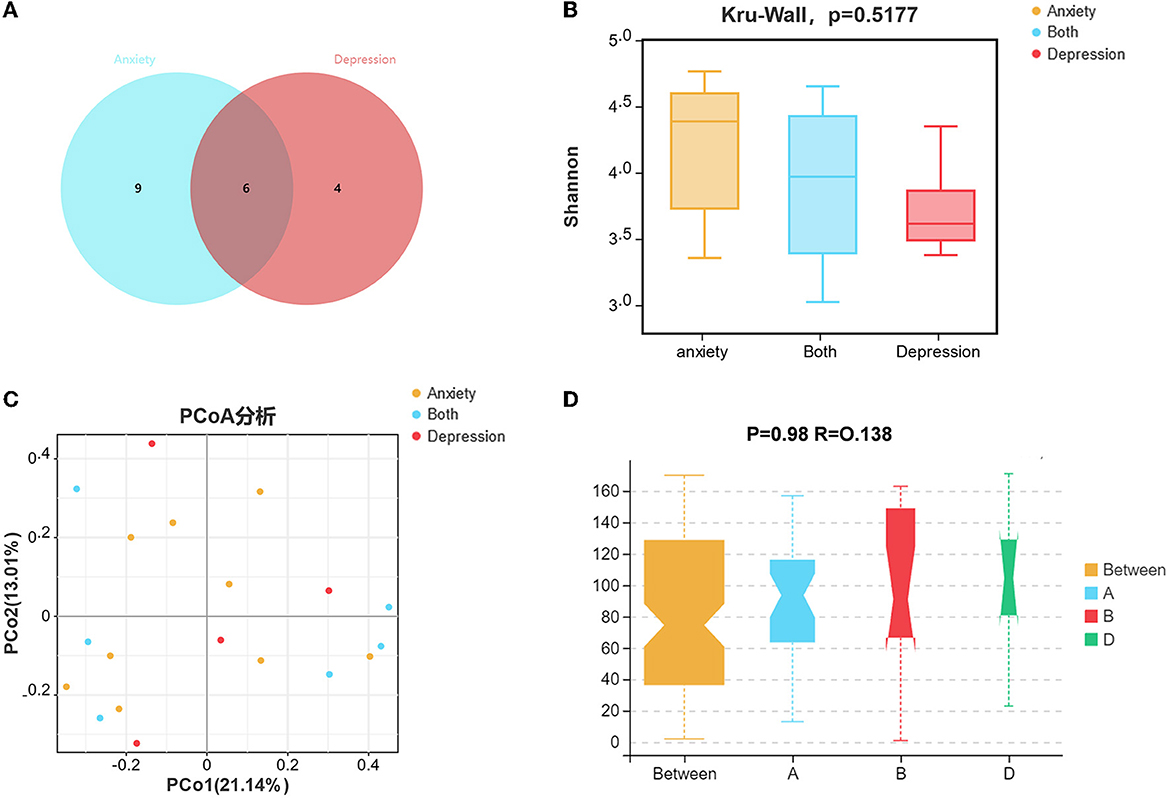
Figure 4. The variation characteristics of microbiota between patients who only had anxiety/depression symptoms and both suffered. (A) Venn diagram of patients only had anxiety/depression symptoms and both suffered. (B) Shannon index comparison between patients only had anxiety/depression symptoms and both suffered. (C) The principal coordinate analysis (PCA) results between the groups. (D) Analysis of Similarity between the groups.
High abundance of Escherichia-Shigella may be associated with disease recurrence in remission UC patients
Our research results found that Escherichia-Shigella significantly increased in both the anxiety and depression groups. Due to the lack of a gold standard, we used the quartile method to classify the relative abundance of Escherichia-Shigella among all participants, mainly including the high content group (n = 11), middle content group (n = 23), and low content group (n = 11). The relative abundance of Escherichia-Shigella in each group of patients is shown in Figure 5A. We followed up the disease recurrence rates of high and low content groups for 6 months which were 36.3% and 9% (Figure 5B) (chi-square test, P < 0.05).
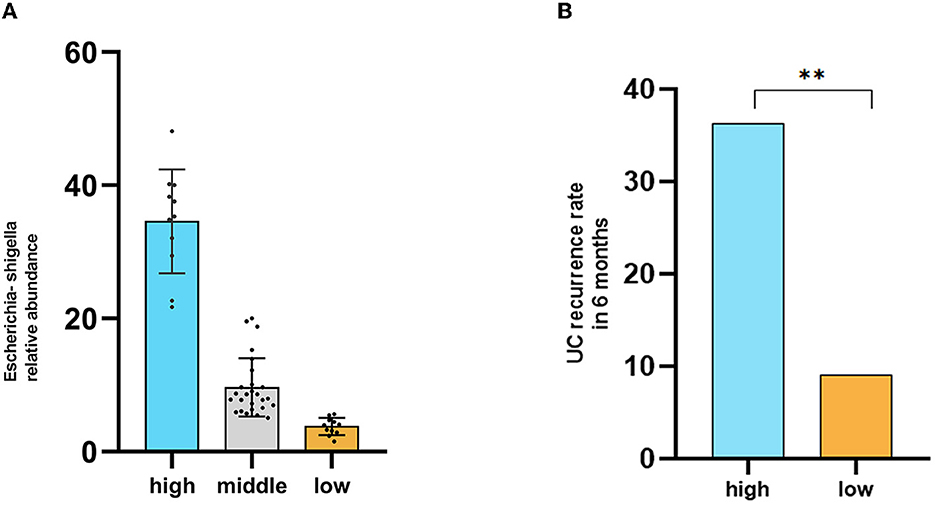
Figure 5. High abundance of Escherichia-Shigella may be associated with disease recurrence in remission UC patients. (A) Groups based on the abundance of Escherichia-Shigella using the quartile method. (B) Comparison of disease recurrence rates within 6 months. High indicates the high Escherichia-Shigella abundance group, middle indicates the middle Escherichia-Shigella abundance group, and low indicates the low Escherichia-Shigella abundance group.
Discussion
We carried out a cross-sectional study in UC that aims to examine the incidence of anxiety and depression in disease remission and the variation of fecal microbiota in anxious and depressed UC patients in remission.
Our study results showed that even in the remission period, the prevalence of anxiety was 33.3%, and depression was 22.2%, which was more than twice the incidence of anxiety and depression in healthy individuals. Anxiety and depression are more prevalent in UC patients with remission due to repeated illnesses, hormones, economic hardship, and other factors (Sidebottom et al., 2021). In some clinical studies, anxiety and depression trigger disease recurrence, surgery, or hospitalization (Regueiro et al., 2016). Therefore, doctors should be careful regarding anxiety and depression in UC patients, even in the remission period (Gao, 2021; Jordi et al., 2022).
Alpha diversity was not significantly different between remission UC patients with anxiety/depression (UCA/UCD) and UC without anxiety/depression (UCNA/UCND), but beta diversity was significantly different (P < 0.05). Consistent with our research results, the latest research suggests that psychological stress could change the composition of intestinal microbiota and promote intestinal inflammation (Bailey et al., 2011; Li, 2019). Increasing attention has been placed on the brain's and microbiota's interactive regulatory mechanism during disease progression (Morais et al., 2021).
We further found that the abundance of harmful or pathogenic bacteria increased, and the proportion of probiotics decreased in UCA/UCD compared with UCNA/UCND in the remission period. In previous animal experiments, acute and chronic stress could both promote the expansion of pathogens and activate the mucosal immune response, and antibiotic treatment eliminated the intestinal microbiota factor and the difference between the stressed and non-stressed groups (Ge et al., 2022). Microbial metabolites also participate in the pathogenesis of colitis. Short-chain fatty acids (SCFAs), one of the important metabolites of intestinal flora, play a major role in several physiological processes, including maintaining the intestinal barrier, inhibiting opportunistic intestinal pathogen colonization, and regulating the Treg cell balance (Bravo et al., 2011). In addition, the components of some harmful bacteria, such as LPS and some types of proteases, can destroy the production of tight junction proteins, thus damaging the intestinal barrier and causing intestinal inflammation (Zou et al., 2022).
We found that the abundance of Bifidobacterium and Lactobacillus decreased, while the abundance of Escherichia-Shigella and Proteus_mirabilis increased in the anxiety group. The abundance of Bifidobacterium and Faecalibacterium decreased in the depression group, while the abundance of Shigella increased at the genus level. In the study by Bravo, Lactobacillus was shown to be able to improve anxiety and depression-related behavior in BALB/c mice; both Bifidobacterium and Lactobacillus have been proven to improve anxiety and depression (Sudo et al., 2004). Anxiety symptom of animals supplemented with rhamnose-Lactobacillus was improved and accompanied by changing the expression of the γ-aminobutyric acid receptor (Štofilová and Kvaková, 2022). Human studies also showed that intestinal microbes played a role in regulating depression and anxiety. The mental health of petrochemical workers improved for 6 weeks after eating probiotic yogurt or multi-species probiotic capsules, improving their mental health for 6 weeks (Kim et al., 2019). In a recent randomized double-blind controlled trial in central Iran, the clinical symptoms of patients with severe depression were improved compared with those of the placebo group, after 8 weeks of treatment by probiotics (including Lactobacillus acidophilus, Lactobacillus lactis, and Bifidobacterium) (Mohammadi et al., 2015; Akkasheh et al., 2016). Probiotics could, therefore, be used to improve the symptoms of UC patients in remission with anxiety and depression.
Probiotic treatment currently includes probiotics and fecal microbiota transplantation (FMT) (Kilinçarslan and Evrensel, 2020). FMT has become an attractive therapeutic strategy. Some studies have shown that the severity of anxiety and depression in IBD patients is improved after FMT (Jang et al., 2019; Qiu et al., 2023). Based on those studies, we believe that for UCA/UCD patients in the remission stage, oral probiotics can be added in order to improve symptoms and disease recurrence. In addition to traditional psychotherapy and drug therapy, FMT may become an important method to improve the mental state of UC patients with severe anxiety and depression.
Interestingly, we followed up the disease recurrence rates of high and low Escherichia-Shigella content groups for 6 months were 36.3% and 9%. Consistent with our results, Escherichia coli is closely related to localization and recurrence of UC. As an antibacterial therapy, phage therapy has the advantage of precise targeting compared with antibiotics and can be used to regulate intestinal flora and kill multiple types of drug-resistant bacteria (Zheng et al., 2022).
However, our study is a single-center study with insufficient subjects to represent all UC patients. The sample size should be further expanded, and the conclusion should be verified in multi-centers.
Conclusion
This study enrolled 45 UC patients in remission. The majority of UC patients remain anxious and depressed even in the remission period. In this study, we found that probiotics decreased, and conditional pathogenic bacteria were more prevalent in UC patients in remission with anxiety/depression compared with UC in the remission stage without anxiety/depression. Therefore, supplementing probiotics may be an important method to prevent the recurrence of disease caused by impaired psychological function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the First Affiliated Hospital of Xi'an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
YL designed the study. LX drafted the manuscript and performed the experiments. YH revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the key projects of research and development in Shaanxi province, China (2022SF-135).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
UC, Ulcerative colitis; HADS, Hospital Anxiety and Depression Scale; PCA, Principal coordinate analysis; ANOSIM, Analysis of similarity; FMT, Fecal bacteria transplantation.
References
Akkasheh, G., Kashani-Poor, Z., Tajabadi-Ebrahimi, M., Jafari, P., Akbari, H., Taghizadeh, M., et al. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 32, 315–320. doi: 10.1016/j.nut.2015.09.003
Bailey, M. T., Dowd, S. E., Galley, J. D., Hufnagle, A. R., Allen, R. G., and Lyte, M. (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 25, 397–407. doi: 10.1016/j.bbi.2010.10.023
Basiji, K., Sendani, A. A., Ghavami, S. B., Farmani, M., Kazemifard, N., Sadeghi, A., et al. (2023). The critical role of gut-brain axis microbiome in mental disorders. Metab. Brain Dis. 3, 1248. doi: 10.1007/s11011-023-01248-w
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. P Natl. Acad. Sci. USA. 108, 16050–16055 doi: 10.1073/pnas.1102999108
Fairbrass, K. M., Gracie, D. J., and Ford, A. C. (2021). Longitudinal follow-up study: effect of psychological co-morbidity on the prognosis of inflammatory bowel disease. Aliment Pharmacol. Ther. 54, 441–450. doi: 10.1111/apt.16454
Gao, X, Tang, Y, Lei, N, Luo, Y, Chen, P, and Liang, C. (2021). Symptoms of anxiety/depression is associated with more aggressive inflammatory bowel disease. Sci. Rep. 11, 1440. doi: 10.1038/s41598-021-81213-8
Ge, L., Liu, S., Li, S., Yang, J., Hu, G., Xu, C., et al. (2022). Psychological stress in inflammatory bowel disease: psychoneuro-immunological insights into bidirectional gut-brain communications. Front. Immunol. 6, 6578. doi: 10.3389/fimmu.2022.1016578
Gracie, D. J., Guthrie, E. A., Hamlin, P. J., and Ford, A. C. (2018). Bi-directionality of brain-gut interactions in patients with inflammatory bowel disease. Gastroenterology 154, 1635–1646. doi: 10.1053/j.gastro.2018.01.027
Jang, H. M., Lee, K. E., and Kim, D. H. (2019). The preventive and curative effects of lactobacillus reuteri NK33 and bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 11:819. doi: 10.3390/nu11040819
Jordi, S. B. U., Lang, B. M., Auschra, B., von Känel, R., Biedermann, L., Greuter, T., et al. (2022). Depressive symptoms predict clinical recurrence of inflammatory bowel disease. Inflamm. Bowel Dis. 28, 560–571. doi: 10.1093/ibd/izab136
Kilinçarslan, S., and Evrensel, A. (2020). The effect of fecal microbiota transplantation on psychiatric symptoms among patients with inflammatory bowel disease: an experimental study. Actas Esp Psiquiatr. 48, 1–7. doi: 10.7717/peerj.4663/table-3
Kim, S., Lee, S., Han, K., Koh, S.-J., Im, J. P., Kim, J. S., et al. (2023). Depression and anxiety are associated with poor outcomes in patients with inflammatory bowel disease: a nationwide population-based cohort study in South Korea. Gen. Hosp. Psychiatry 81, 68–75. doi: 10.1016/j.genhosppsych.2023.01.015
Kim, S. K., Guevarra, R. B., Kim, Y. T., Kwon, J., Kim, H., Cho, J. H., et al. (2019). Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 29, 1335–1340. doi: 10.4014/jmb.1906.06064
Li, N, Wang, Q, Wang, Y, Sun, A, Lin, Y, Jin, Y. (2019). Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 22, 592–602. doi: 10.1080/10253890.2019.1617267
Mikocka-Walus, A., Knowles, S. R., Keefer, L., and Graff, L. (2016). Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm. Bowel Dis. 22, 752–762. doi: 10.1097/MIB.0000000000000620
Mohajeri, M. H., La Fata, G., Steinert, R. E., and Weber, P. (2018). Relationship between the gut microbiota and brain function. Nutr. Rev. 76, 481–486. doi: 10.1093/nutrit/nuy009
Mohammadi, A. A., Jazayeri, S., Khosravi-Darani, K., Solati, Z., Mohammadpour, N., Asemi, Z., et al. (2015). The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosc. 19, 387–395. doi: 10.1179/1476830515Y.0000000023
Morais, L. H., Schreiber, H. L., and Mazmanian, S. K. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Neuendorf, R., Harding, A., Stello, N., Hanes, D., and Wahbeh, H. (2016). Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J. Psychosom. Res. 87, 70–80. doi: 10.1016/j.jpsychores.2016.06.001
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778. doi: 10.1016/S0140-6736(17)32448-0
Prendergast, K. L., Gowey, M. A., Barnes, M. J., Keller, C. V., Horne, C., and Young, J. (2022). Treating anxiety and depression in inflammatory bowel disease: a systematic review. Psychol. Health 37, 105–130. doi: 10.1080/08870446.2020.1867135
Qiu, Z., Luo, D., Yin, H., Chen, Y., Zhou, Z., Zhang, J., et al. (2023). Lactiplantibacillus plantarum N-1 improves autism-like behavior and gut microbiota in mouse. Front. Microbiol. 14, 1134517. doi: 10.3389/fmicb.2023.1134517
Regueiro, M., Greer, J. B., and Eva, S. (2016). Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology 152, 430–439. doi: 10.1053/j.gastro.2016.10.036
Sidebottom, A. M., Rodriguez, T. G., Karpin, J. E., and Rubin, D. T. (2021). Clinical and translational considerations for understanding depression and anxiety in patients with inflammatory bowel disease. Gastroenterol Res. Pract. 2021:6689443. doi: 10.1155/2021/6689443
Štofilová, J., and Kvaková, M. (2022). Probiotic-based intervention in the treatment of ulcerative colitis: conventional and new approaches. Biomedicines 10, 2236. doi: 10.3390/biomedicines10092236
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X. N., et al. (2004). Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 558, 263–275. doi: 10.1113/jphysiol.2004.063388
Wilkinson, B., Trick, L., Knight, A., Valton, V., Goodhand, J., Kennedy, N. A., et al. (2019). Factors associated with depression in people with inflammatory bowel disease: the relationship between active disease and biases in neurocognitive processing. Neurogastroenterol. Motil. 31, 13647. doi: 10.1111/nmo.13647
Williet, N., and Sarter, H. (2017). Patient-reported outcomes in a French nationwide survey of inflammatory bowel disease patients. J. Crohns. Colitis. 11, 165–174. doi: 10.1093/ecco-jcc/jjw145
Wu, K. C., Liang, J., Ran, Z. H., Qian, J. M., Yang, H., and Chen, M. H. (2018). Consensus on diagnosis and treatment of Inflammatory bowel disease (2018 Beijing). Chin. J. Pract. Int. Med. 38, 796–813. doi: 10.19538/j.nk2018090106
Yang, H., Zhou, R., Bai, X., Guo, M., Ruan, G., Wang, L., et al. (2022). Trend and geographic variation in incidence and prevalence of inflammatory bowel disease in regions Across China: a nationwide employee study between 2013 and 2016. Front. Med. 9, 900251. doi: 10.3389/fmed.2022.900251
Yu, R., Liu, C., Zhang, J., Li, J., Tian, S., Ding, F., and Dong, W. (2023). Correlation analysis between disease activity and anxiety, depression, sleep disturbance, and quality of life in patients with inflammatory bowel disease. Nat. Sci. Sleep 3, 407–421. doi: 10.2147/NSS.S407388
Zheng, L., Duan, S. L., Dai, Y. C., and Wu, S. C. (2022). Role of adherent invasive Escherichia coli in pathogenesis of inflammatory bowel disease. World J. Clin. Cases. 10, 11671–11689. doi: 10.12998/wjcc.v10.i32.11671
Zigmond, A. S., and Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x
Keywords: Ulcerative Colitis, psychological issues, fecal microbiota, probiotics, Escherichia Shigella
Citation: Xu L, Li Y and He Y (2023) The variation characteristics of fecal microbiota in remission UC patients with anxiety and depression. Front. Microbiol. 14:1237256. doi: 10.3389/fmicb.2023.1237256
Received: 12 June 2023; Accepted: 16 August 2023;
Published: 07 September 2023.
Edited by:
Giovanni Tarantino, University of Naples Federico II, ItalyReviewed by:
Shaghayegh Baradaran Ghavami, Shahid Beheshti University of Medical Sciences, IranNing Dai, China Academy of Chinese Medical Sciences, China
Copyright © 2023 Xu, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingli He, aGV5aW5nbGkyMDAwQHhqdHVmaC5lZHUuY24=
 Lingyun Xu1
Lingyun Xu1 Yingli He
Yingli He