- 1Department of Clinical Laboratory, Fujian Medical University Union Hospital, Fujian, China
- 2Department of Clinical Laboratory, The 910 Hospital of Joint Logistics Support Force of Chinese People's Liberation Army, Fujian, China
Background: Staphylococcus aureus (S. aureus) is the most common causative agent of burn wound infection, that often leads to high morbidity and mortality. However, there is not enough knowledge about the molecular epidemiology and antimicrobial susceptibility of S. aureus isolates from burn wound infections in Fujian, China.
Methods: Between 2016 and 2021, 90 S. aureus isolates were collected from burn wound infections in Fujian, China, including 59 methicillin-resistant (MRSA) strains and 31 methicillin-susceptible (MSSA) strains. These were investigated for molecular characteristics, virulence genes, biofilms, and antimicrobial susceptibility. All the isolates were genotyped by multilocus sequence typing (MLST), spa typing, agr typing, and SCCmec typing. Conventional PCR was performed for the detection of virulence genes. Biofilm formation capacity was assessed by tissue culture plate assay (TCP). The antimicrobial susceptibility of the isolates was evaluated using the dilution method.
Results: In total, 37 sequence types (ST) and 34 Staphylococcal protein A (spa) types (including a new type named spa-t20720) were identified based on multilocus sequence typing (MLST) and spa typing, respectively. CC8-ST239-t030-agrI-SCCmecIII (57.6%,34/59) and CC7-ST7-t091-agrI (16.1%, 5/31) represented the main clone of MRSA and MSSA isolates, respectively. Antibiotic susceptibility testing identified a significant difference in resistance rates between ST239 and non-ST239 isolates (p < 0.05). Twelve virulence genes were detected, of which the most common were icaA and icaD (both 100%), followed by icaB and icaC (both 96.7%), icaR (95.6%), lukED (81.1%), lukAB (62.2%), pvl (50%), hlgBC (26.7%), and eta (4.4%). Moreover, lukAB, hlgBC, agrI, and agrIII were significantly correlated with burn severity (p < 0.05). MRSA isolates were less likely, compared with MSSA isolates, to carry pvl, lukAB, and hlgBC (p < 0.05). A new spa type, t20720, was identified that contains pvl, lukED, lukAB, hlgBC, icaA, icaB, icaC, icaD, and icaR genes and has strong biofilm formation ability.
Conclusion: CC8-ST239-t030-agrI-SCCmecIII and CC7-ST-7-t091-agrI were the prevalent molecular signatures of MRSA and MSSA isolates from burn wound infections in Fujian, China, respectively. The newly identified spa-t20720 isolate, which carries a wide range of virulence genes and has strong biofilm formation ability, requires special clinical attention.
Introduction
In China, millions of burn injuries occur each year, and over 70% of mortality in burn wards occurs due to infection (Kalligeros et al., 2019). A study on the distribution of pathogens in burn patients in China from 2006 to 2019 showed that 87.98% (7,003/7960) of burn wounds contained pathogenic strains, and S. aureus was the most commonly isolated pathogen (Chen et al., 2021). Notably, burn wound infection is an important source of systemic infection. S. aureus, through burn wounds, can reach blood, organs, and tissues producing toxins causing systemic clinical symptoms and even septic shock, which has a poor prognosis and high mortality rate (Tong et al., 2015). S. aureus can also form biofilms causing persistent infections that delay wound healing (Sabirova et al., 2015).
Staphylococcus aureus isolates, with genome sequence differences of up to 22%, have high genetic diversity and rich phenotypes (Cuteri et al., 2004). Typically, the molecular characteristics of S. aureus isolates differ depending on the region. ST59 is the predominant strain in China (Wang et al., 2022), while ST8 and ST121 are common in the USA (O'Hara et al., 2012; Rao et al., 2015). Meanwhile, the molecular epidemiology of S. aureus also varies with different types of infections. Wang et al. reported that in pediatric patients with bloodstream infections, major methicillin-susceptible S. aureus (MSSA) clones belonged to ST188 and ST7 types, while the predominant methicillin-resistant S. aureus (MRSA) clone was ST59 (Wang et al., 2018).
There is limited information on the epidemiological characteristics of S. aureus isolates from burn wound infections worldwide. ST250-t928 was the major MRSA clone in a Ghanaian burn unit (Amissah et al., 2017), whereas ST239-SCCmec III/t037 was the major MRSA clone from burn wound patients in Iran (Goudarzi et al., 2017). Other studies on burn wounds have focused on the distribution and drug resistance analysis of pathogenic bacteria (Chen et al., 2021; Ozlu and Basaran, 2022). Therefore, we isolated S. aureus isolates from burn patients in the Fujian province (China) to better understand their epidemiological trends and molecular characteristics. Our data may help manage clinical S. aureus burn infections in this geographical area.
Methods
Staphylococcus aureus isolates
In total, 90 non-duplicated S. aureus clinical isolates were obtained from burn wound infections from five hospitals between 2016 and 2021, which were located in five different areas of Fujian province, including Quanzhou (the 910 Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army), Zhangzhou (the 909 Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army), Xiamen (the 73rd Army Hospital of the Army), Fuzhou (Fujian Medical University Union Hospital), and Ningde (Mindong Hospital of Ningde). This retrospective study examined data and bacterial strains from the admitted patients who met the following conditions. First, they had no apparent infection symptoms during the incubation period. Second, the infection occurred 48 h after admission. Third, the clinical specimens collected from burn wounds were identified as MRSA or MSSA in the laboratory. The strains were identified by VITEK 2 Compact system and GP Test Kit (bioMerieux, France).
Burn severity grading
Burn severity grading (BSG) was performed based on the judgment criteria of the 1970 China Burn Conference-Mild burns: a second-degree burn with a total area of <10%; Moderate burns: a second-degree burn with a total area of 11–30% or a third-degree burn with an area of ≤9%; Severe burn: a second-degree burn with a total area of 31–50% or a third-degree burn with an area of 10–20%; Extra-severe burn: a patient with a total burn area of ≥50% or a third-degree burn with an area of ≥20%.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed using the VITEK 2 Compact system and GP-67 Test Kit (bioMerieux, France). Fourteen antibiotics, including penicillin (PEN), oxacillin (OXA), erythromycin (ERY), clindamycin (CLI), tetracycline (TET), gentamicin (GEN), ciprofloxacin (CIP), levofloxacin (LEV), rifampicin (RIF), trimethoprim/sulfamethoxazole (SXT), quinupristin/dalfopristin (QD), linezolid (LZD), tigecycline (TCG), and vancomycin (VAN), were used. S. aureus ATCC 25923 and 29213 strains were used as quality control strains, and the results were interpreted following the Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSIM100, 2022, 32nd edition).
Staphylococcus aureus protein A(spa) typing
Genomic DNA from S. aureus isolates was extracted using the Ezup Column Bacteria Genomic DNA Purification Kit (Sangon Biotech, Shanghai) and stored at −20°C. The amplification of the spa repeat region was carried out as described in previous studies (Uhlén et al., 1984). The resulting amplicons were sent to Sangon Biotech (Shanghai, China) for DNA sequencing, followed by analysis using the Ridom web server.1 Sequences were submitted to the Ridom web server (see footnote 1) to distinguish whether those strains were new spa types. When sequences could not be categorized, isolates will be defined as non-typable (NT). Primers were listed in Supplementary File S1.
Multilocus sequence typing
Multilocus sequence typing (MLST) was carried out following the previously described protocol. Seven housekeeping genes (arc, aro, glp, gmk, pta, tpi, and yqil) were PCR amplified (He et al., 2018). The resulting amplicons were sent to Sangon Biotech (Shanghai, China) for DNA sequencing, and the results were compared with known alleles in the MLST database2 for MLST of the isolates. Related STs were clustered using eBURST, and evolutionary trees were constructed in the MEGA6 software. Primers were listed in Supplementary File S1.
agr typing
The agr typing of isolates was performed by multiplex PCR following the previously described protocol (He et al., 2018), and in total, four types (I-IV) were examined. S. aureus isolates that could not be categorized into any known type were defined as NT. Primers were listed in Supplementary File S1.
Staphylococcus chromosomal cassette mec (SCCmec) typing
MRSA isolates were subjected to SCCmec typing (I, II, III, IVa, IVb, IVc, IVd, and V) by multiplex PCR, as described previously (He et al., 2018). All isolates were also examined for the mecA gene. MRSA isolates that could not be classified were defined as NT. Primers were listed in Supplementary File S1.
Detection of virulence genes
We detected 12 virulence genes (including 5 leukotoxin genes, pvl, lukED, lukAB, hlgBC) (He et al., 2018), and lukMF′ (Jarraud et al., 2002); 2 exfoliative toxin genes, eta and etb (Li et al., 2018); 5 ica operon genes, icaA, icaB, icaC, icaD, and icaR (Mahmoudi et al., 2019) were detected by PCR as described previously. Primers were listed in Supplementary File S1.
Detection of biofilm formation ability
Biofilm formation ability was examined using the tissue culture plate assay (TCP) as described previously with some modifications (Gad et al., 2009). Strains, stored at −80°C, were inoculated on a Columbia Blood Agar plate for overnight culture at 35°C. Then, bacteria were selected and inoculated in fresh Trypticase Soy Broth (TSB) and cultured at 35°C for 24 h. The bacterial solution was diluted to 1.5 × 108 CFU/mL with TSB. 200 μl/well of the respective diluted bacterial solution was added to a sterile 96-well cell culture plate; each strain was plated in triplicate. Equal amounts of TSB broth were added to the negative control wells. S. aureus ATCC29213 was used as the positive control strain. The culture solution was discarded from the well, and the wells were gently washed with sterile phosphate buffer saline (PBS; pH 7.2) three times to remove any floating bacteria. Next, 200 μL methanol was added to each well for fixation of bacterial cells for 15 min. After discarding the methanol and air drying, 200 μl of 1% crystal violet was added to the respective well and incubated for 30 min, followed by the wells washing (thrice) with sterile PBS. Finally, 200 μl 95% ethanol was added to respective wells to dissolve the dye crystals, and the plate optical density was recorded at 595 nm. The degree of biofilm production was scored as follows: absent (OD < ODc), weak (ODc < OD < 1.5 × ODc), moderate (1.5 × ODc < OD < 2 × ODc), and strong (OD > 2 × ODc) (Wu et al., 2019; Velázquez-Suárez et al., 2021).
Statistical analysis
Statistical analysis of data was performed using the SPSS 22.0 software (IBM Corporation, Armonk, NY, USA). The data were compared and analyzed using the chi-square or Fisher’s exact test. All statistical tests were two-tailed. Data with p < 0.05 were considered statistically significant, and those with p < 0.01 were considered of high statistical significance.
Results
Basic information and burn severity grading
In total, ninety burn patients (60 males and 30 females, aged 1–94 years, mean age: 29.85 ± 17.84 years) were included in this study; 34, 27, 5, and 24 burn patients were categorized as mild, moderate, severe, and extra severe burn cases, respectively.
MLST, spa, agr, and SCCmec typing
We obtained 90 S. aureus isolates, including 59 MRSA and 31 MSSA, from 5 hospitals in Fujian province, China. Concerning MRSA, 16 distinct STs were identified, and ST239 (66.1%, 39/59) was the most common MRSA. MSSA showed higher diversity than MRSA; ST 7 (16.1%, 5/31) was the most prevalent MSSA. ST7395, ST965, and ST6829 were found in both MRSA and MSSA. eBURST investigation identified 37 STs belonging to 7 groups and 5 singletons (Table 1 and Figure 1). Furthermore, we generated phylogenetic trees of STs to investigate their heterogeneity. STs were clustered into several clades, indicating their high diversity. Different independent branches belonging to ST239 indicated that this subtype originated from different evolutionary clades (Figure 2).
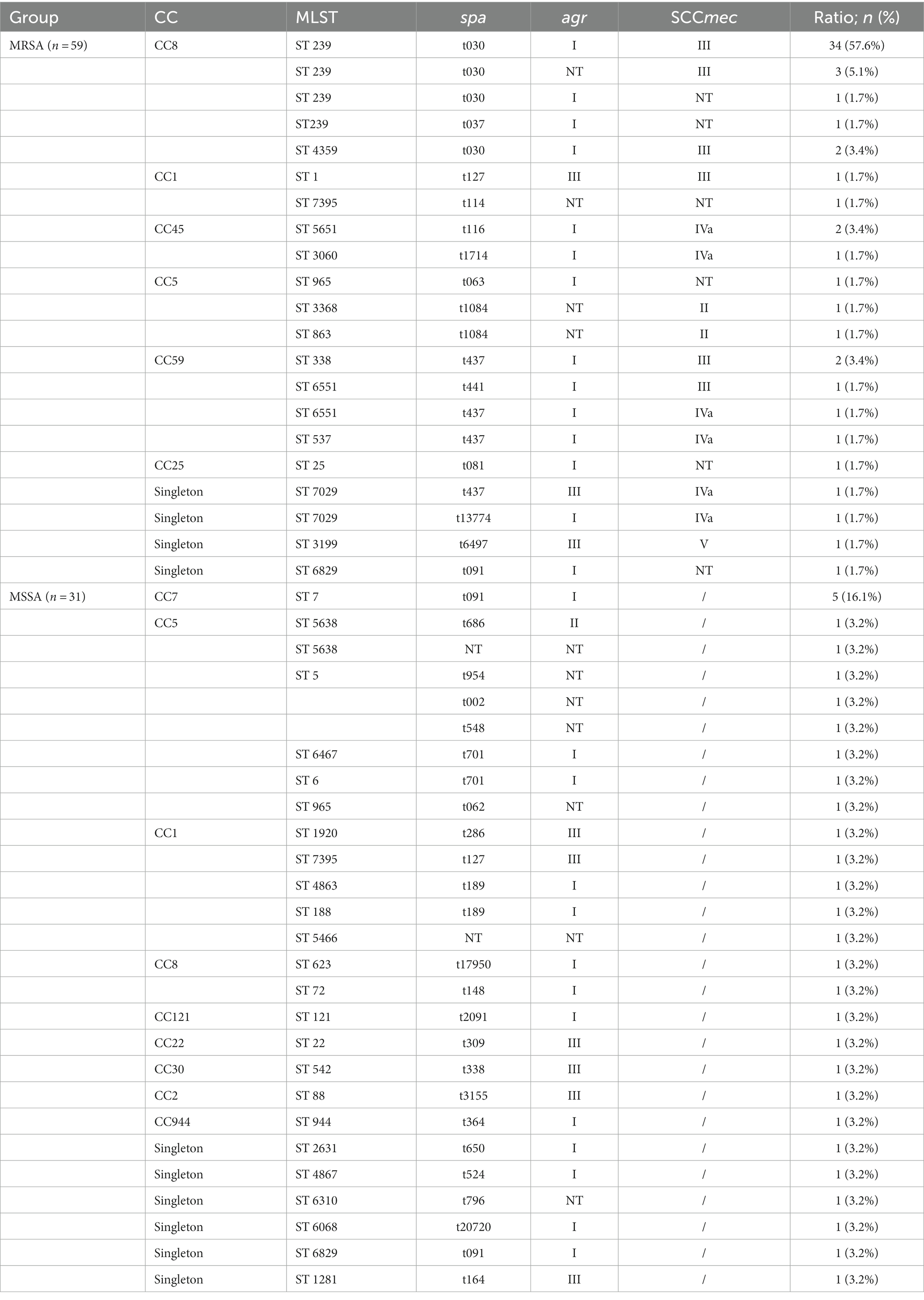
Table 1. Molecular characteristics of the 90 Staphylococcus aureus isolates collected in this study.
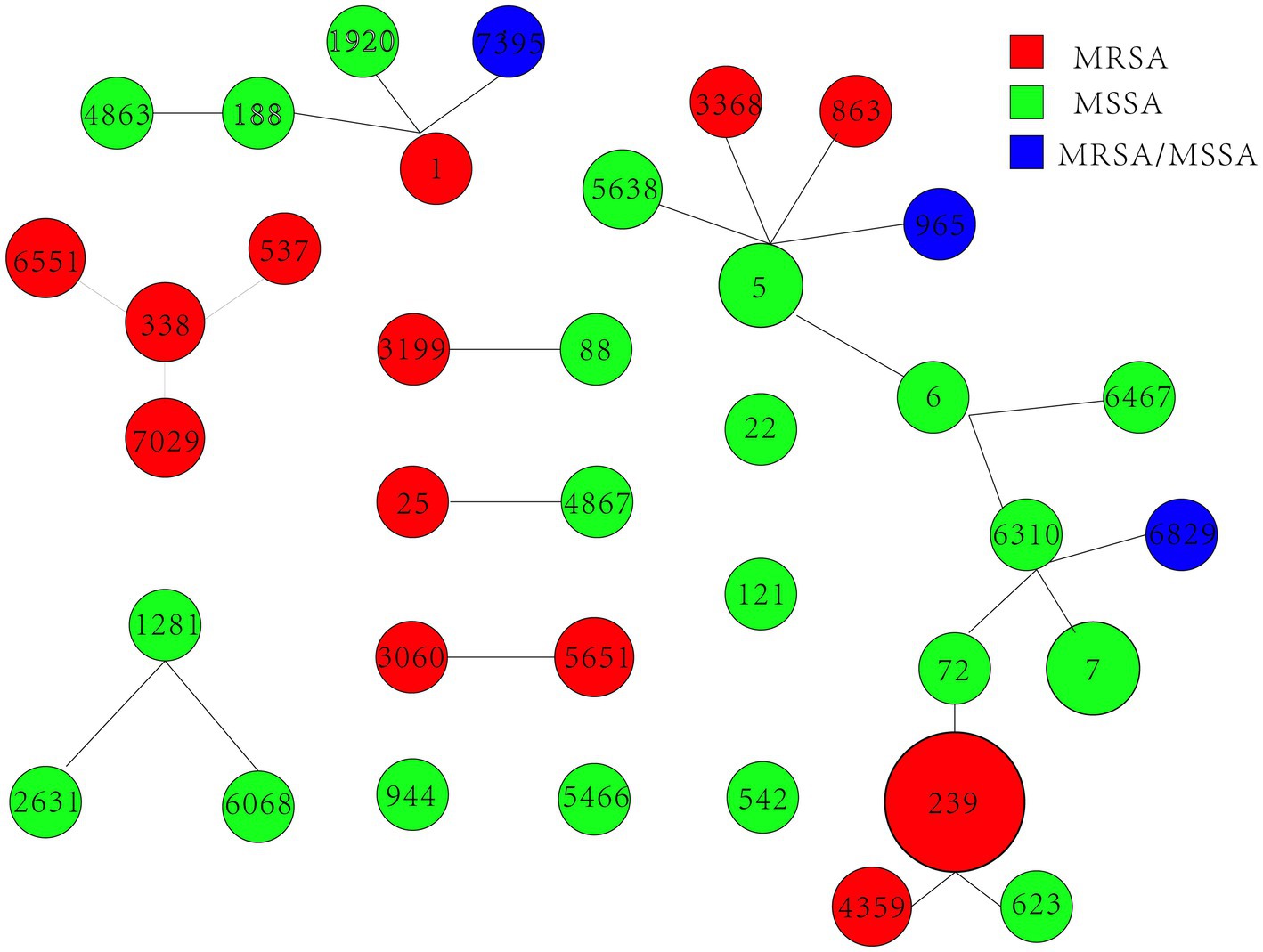
Figure 1. Distribution of STs in the clonal complexes. eBURST graph generated from the MLST data shows the relationships among the 90 S. aureus isolates. Each number denotes an MLST. STs linked by a line belong to the same cluster, and the circular area indicates the prevalence of those STs in the MLST data.
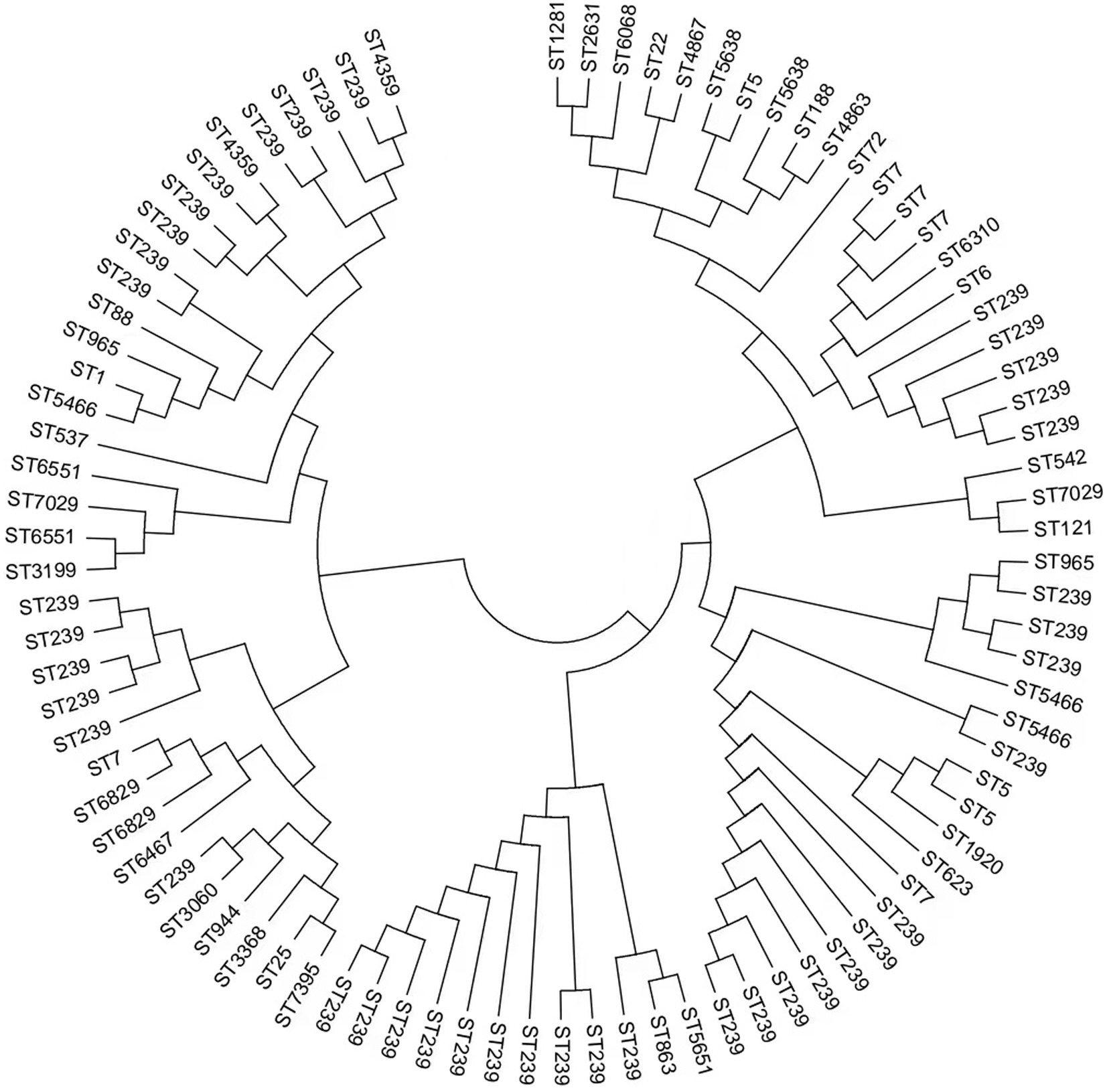
Figure 2. Neighbor-joining phylogeny of the 90 S. aureus isolates. The neighbor-joining (NJ) tree was constructed using the concatenated sequences of the multilocus sequence typing (MLST) scheme.
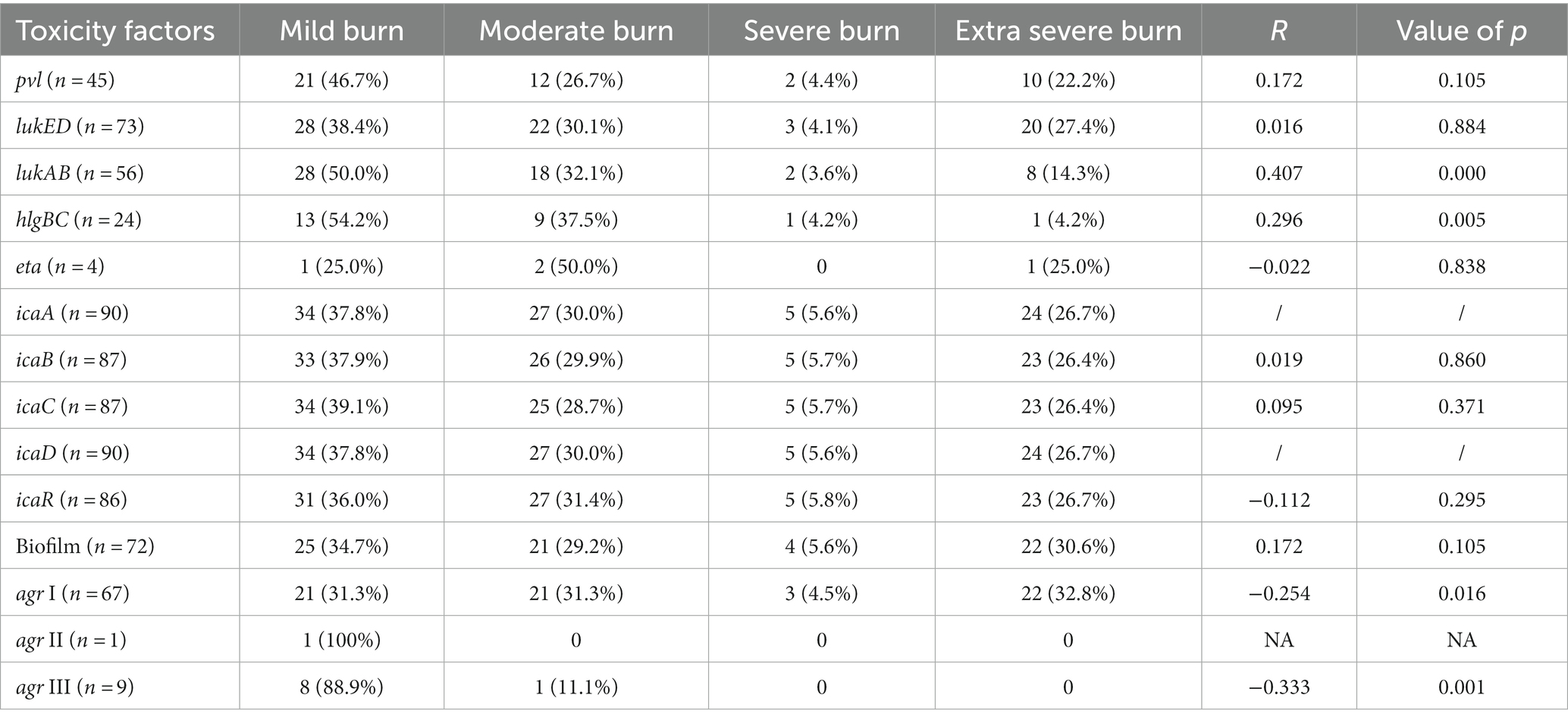
The spa typing classified 59 MRSA isolates into 14 types; t030 was the most frequent type (67.8%, 40/59), followed by t437 (8.47%, 5/59). Among the 31 MSSA isolates, 22 genotypes were found, and t091 (19.4%,6/31) was the most present. Notably, we identified a new type, t20720, which has strong biofilm-forming ability and contains genes pvl, lukED, lukAB, hlgCB, lukED, lukAB, hlgCB, icaA, icaB, icaC, icaD, and icaR. Concerning agr typing, we identified three types: agrI (74.4%,67/90) was the most common, followed by agrIII (10%,9/90). Interestingly, agrI and agrIII exhibited a strong correlation with burn severity (p < 0.05) (Table 2).
Among the 59 MRSA isolates, four SCCmec subtypes (II, III, Iva, and V) were found. The most common was subtype III (72.9%,43/59), followed by subtype IVa (11.9%,7/59), and then subtype II (3.4%, 2/59). Nine isolates were classified as NT for SCCmec typing. All 59 MRSA isolates were mecA-positive. Based on combined (MLST, spa, agr, and SCCmec)typing, the major molecular clone of MRSA was CC8-ST239-t030-agrI-SCCmecIII (55.9%,33/59), while the major molecular clone of MSSA was CC7-ST7-t091-agrI (16.13%,5/31) (Table 1).
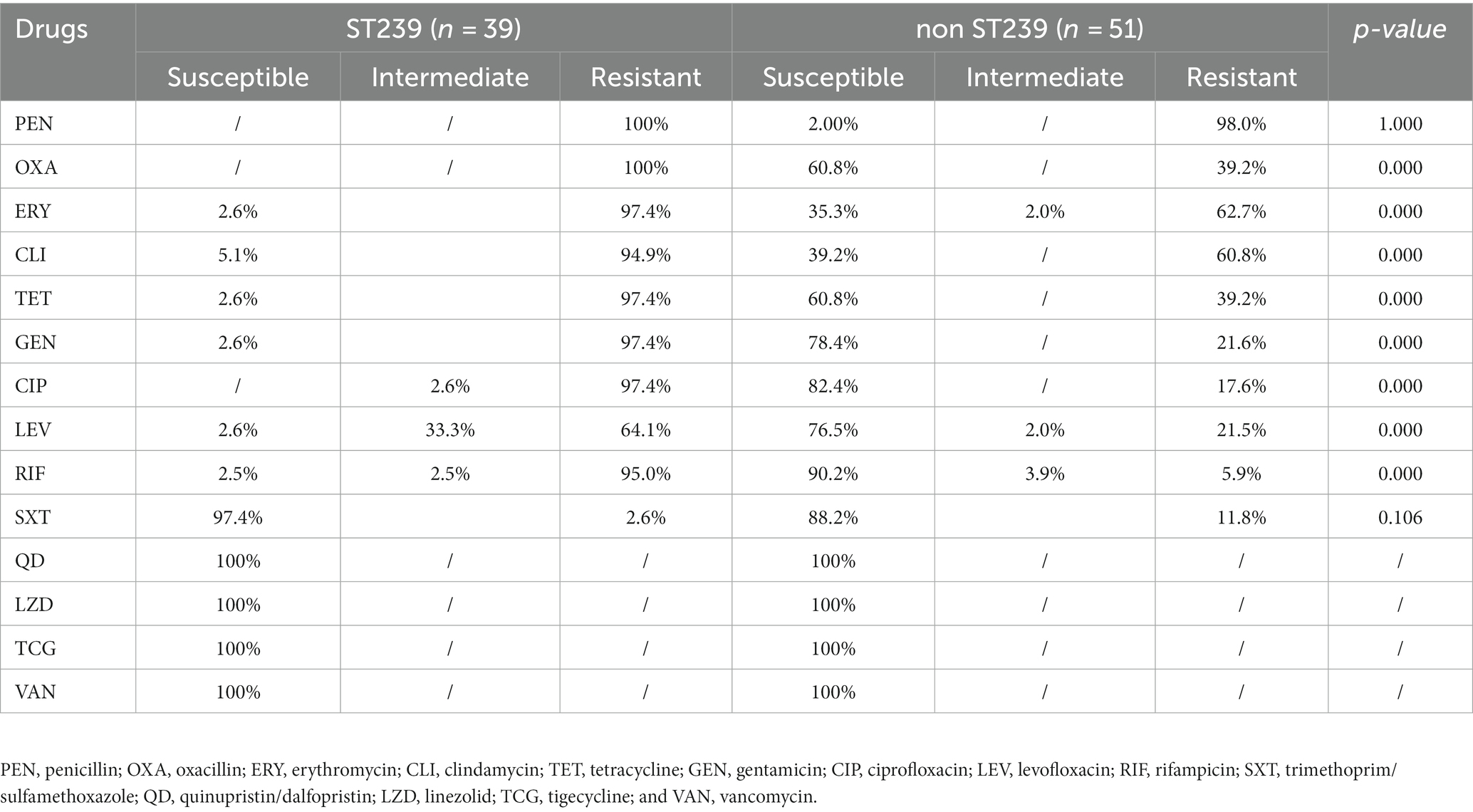
Antimicrobial susceptibility of ST239 and non-ST239
ST239 is a widespread burn wound infection in Fujian, China. Therefore, we compared its antimicrobial susceptibility with non-ST239. Statistical analysis revealed that ST239 isolates had significantly higher resistance than non-ST239 isolates for OXA, ERY, CLI, TET, GEN, CIP, LEV, and RIF antibiotics (p < 0.05). No S. aureus isolate was resistant to QD, VAN, LZD, and TCG antibiotics (Table 3).
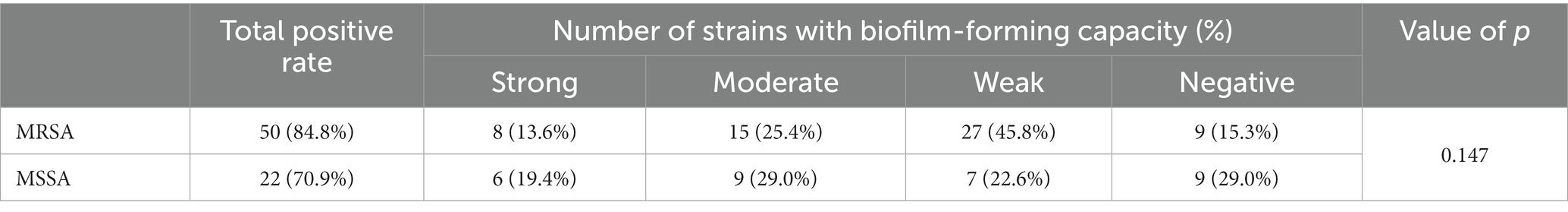
Biofilm formation ability
As quantified by TCD, of the 90 strains of S. aureus studied, 80% (72/90) of isolates were biofilm producers in varying degrees. A total of 37.8% (34/90) of the strains had a weak ability to form biofilms, 26.7%(24/90) had moderate ability, and 15.6%(14/90) strong ability. No significant difference between MRSA and MSSA (p > 0.05). The result of biofilm formation is shown in Table 4.
Virulence gene profiles
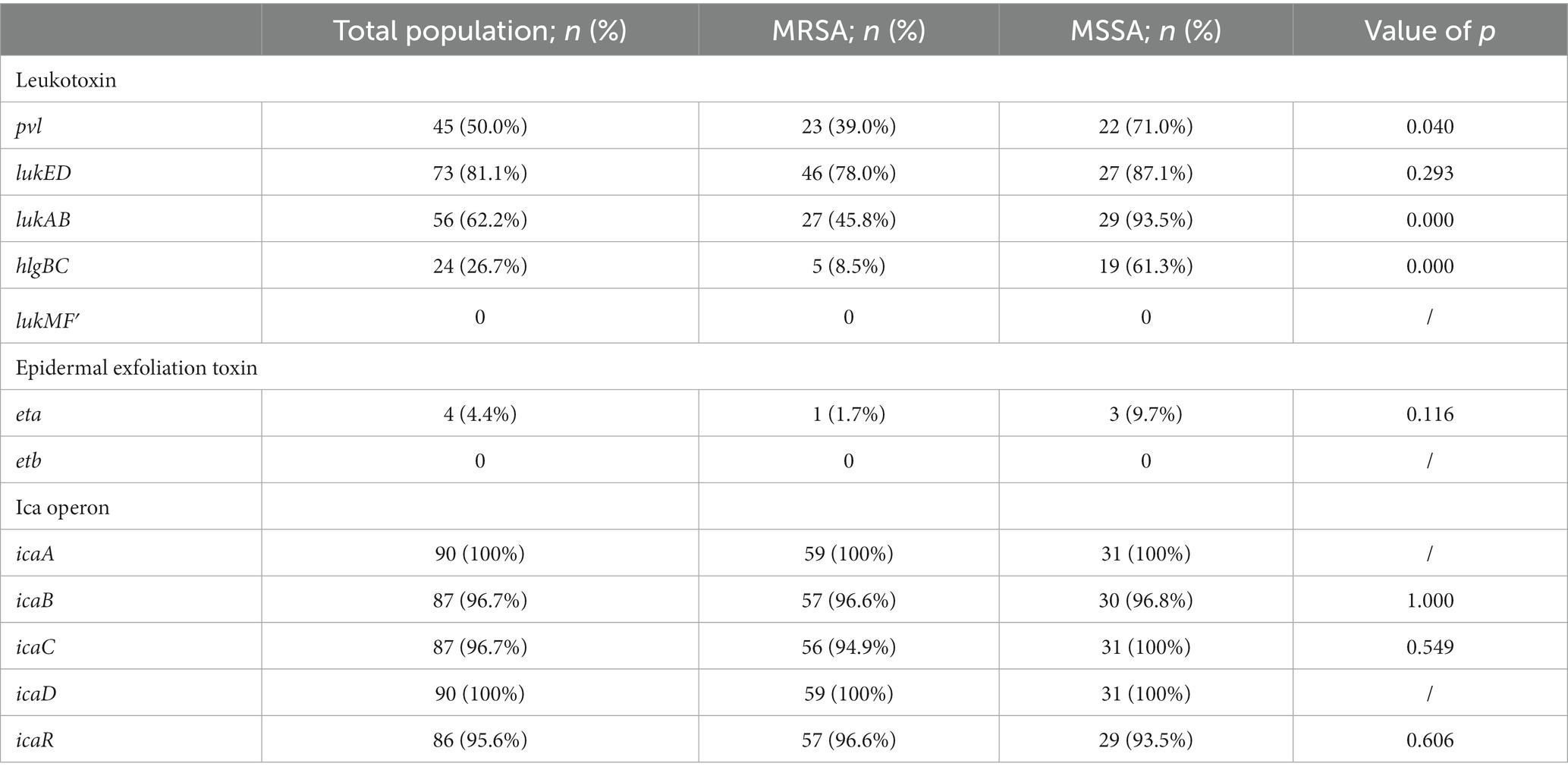
Over 90% of burn wound S. aureus isolates had icaA (100%), icaD (100%), icaB (96.7%), icaC (96.7%), and icaR (95.6%) virulence genes. Over 50% of burn wound S. aureus isolates had lukED (81.1%), lukAB (62.2%), and pvl (50.0%) virulence genes. Meanwhile, hlgBC (26.7%) and eta (4.4%) were the least prevalent virulence genes among burn wound S. aureus isolates. None of the isolates carried lukMF′and etb genes. MRSA isolates were less likely, compared with MSSA isolates, to carry pvl, lukAB, and hlgBC (p < 0.05) (Table 5).
lukAB and hlgBC genes showed a good correlation with BSG (p < 0.05). However, pvl, lukED, eta, icaA, icaB, icaC, icaD, icaR, and biofilm formation ability showed no correlation with BSG (Table 2).The distribution of molecular types in S. aureus isolates from burn wound infections is shown in Table 6.
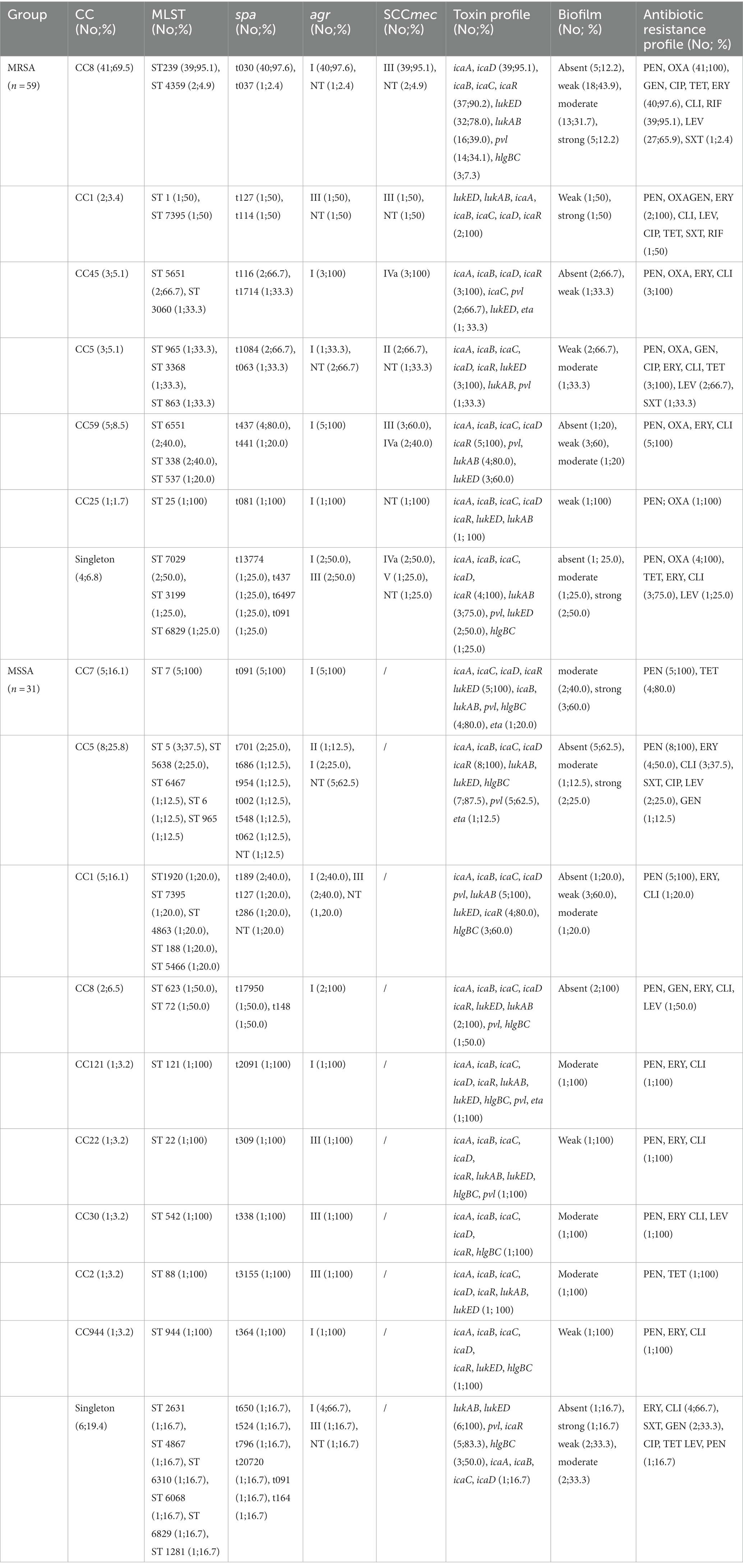
Table 6. Distribution of molecular types in Staphylococcus aureus isolates from burn wound infections.
Discussion
Here, we conducted a multicenter prospective study to determine the molecular epidemiology and antimicrobial susceptibility of S. aureus isolates from burn wound infections. This study presents an extensive collection of 90 S. aureus isolates collected from five different areas in Fujian, China, covering a substantial geographical zone of the city. This is so far a major multicenter study of burn wound infections from Fujian, China.
Staphylococcus aureus burn wound infection has a high incidence rate and mortality (Tong et al., 2015). Skin, the first line of defense against microbial invasion, becomes more prone to infection after burns. S. aureus secretes thick exudates during wound infections, causing inflammation. Tissue necrosis and graft dissolution occur in the late residual of wound burns. Without proper treatment, the wound continues to deteriorate causing severe complications, such as systemic infection or sepsis, leading to high mortality. Previous studies mainly focused on MRSA isolates (Rodrigues et al., 2013; Amissah et al., 2017). However, MSSA is an important opportunistic pathogen in burn wound infections (Dad et al., 2022). Our study focused on S. aureus infections in burn wounds, including MRSA and MSSA. Our result showed that the biofilm formation ability of MSSA was the same as that of MRSA (p > 0.05). Moreover, MSSA has a higher toxicity than MRSA. MRSA was less likely, compared with MSSA, to carry pvl, lukAB, and hlgBC (p < 0.05). These results suggest that MSSA is equally important in burn centers as MRSA.
The molecular epidemiology of S. aureus varies with the types of wound infections. For instance, ST88 and ST152 were the most abundant STs from chronically infected wounds (Wolters et al., 2020). ST59, ST8, and ST45 were the three leading MRSA isolates from skin and soft tissue infections (SSTIs) (Lee et al., 2022). ST30 was the most frequent clonal isolate from wound-related infections in Tehran, Iran (Goudarzi et al., 2019). In this study, CC8-ST239-t030-agrI-SCCmec III was the main clone of MRSA isolates. The possible reason for the spread of ST239 in burn wound infections could be its multidrug resistance, enabling its survival and spread in hospitals (Tong et al., 2015). Antimicrobial susceptibility tests showed high resistance of ST239 against OXA, ERY, CLI, TET, GEN, CIP, LEV, and RIF antibiotics (p < 0.05). The burn wound provides an ideal environment for the growth and reproduction of ST239 (Keen et al., 2010). A previous study showed that CC8-ST239-t030-agrI-SCCmecIII was the most common clone in burn bloodstream infection (Liu et al., 2018). This highlights the importance of molecular surveillance in burn wound patients for timely and effective control of bloodstream infection.
SCCmec types can be identified between hospital-acquired methicillin-resistant S. aureus (HA-MRSA) and community-acquired methicillin-resistant S. aureus (CA-MRSA). HA-MRSA usually carries SCCmec I, II, or III, whereas CA-MRSA usually carries SCCmec IV or V (Cocchi et al., 2011). SCCmec IV and SCCmecV types were generally observed in community-acquired infections, but those strains were detected in this study and were acquired in hospitals. The result suggested that CA-MRSA-based clones may have successfully invaded hospital facilities. The molecular characteristics between CA-MRSA and HA-MRSA are blurring, consistent with previous study (Yu et al., 2022). The increased spread of CA-MRSA in the hospital environment may be related to the smaller size of SCCmecIVa and SCCmecV genes compared to other S. aureus strains (Zhang et al., 2005).
Our analysis also highlights the growing public health threat from livestock-associated methicillin-resistant S. aureus (LA-MRSA) clones. Here, we identified CC1-ST1-t127 carrying lukED, lukAB, and icaABCDR genes in MRSA isolates. Notably, CC1-ST1-t127 is one of the major LA-MRSA lineages and has been associated with pigs across European countries (Merialdi et al., 2019).. There have been reports of human CC1-ST1-t127 infections. CC1-ST1-t127 was the sixth most prevalent clone isolated from human invasive infections in Europe (Grundmann et al., 2010) and the most common clone isolated from nursing homes in Shanghai, China (He et al., 2021). It was the fourth most prevalent MSSA isolate from a children’s hospital in Beijing, China (Wu et al., 2010). A previous study showed that about 77–86% of people with occupational contact with pigs carried LA-MRSA in their nasal cavities (Cuny et al., 2015). Fujian, situated on the southeast coast of China, is a leading modern characteristic agricultural production base, with livestock and poultry industries being its famous sectors. This may promote the spread of LA-MRSA in the Fujian region. Farmers and veterinarians who have contact with livestock are advised to undergo MRSA colonization screening upon hospitalization to prevent the clinical transmission of LA-MRSA.
MSSA has been shown to have higher genetic diversity and broader geographic distribution than MRSA (Wang et al., 2018). In this study, 24 different STs were identified from 31 MSSA isolates from burn wound infections, indicating their higher genetic diversity. The molecular signature of MSSA strains was CC7-ST7-t091-agrI. ST7 is a frequently seen MSSA type associated with foodborne illness (Guo et al., 2023). After the COVID-19 pandemic, ST7 isolates of S. aureus have become very prevalent in Wuhan, China. Notably, ST7 has strong abilities to acquire the SCCmec element and virulence genes under the pressure of disinfectant agents (Gu et al., 2023). All of this suggests that it is crucial to enhance the monitoring of ST7 variants of S. aureus. This study also identified ST22 strains of S. aureus that harbor pvl genes in MSSA isolates. Notably, ST22 was found to be the main MRSA clone in European countries, Australia, New Zealand, and Singapore[38]. A unique ST22 MRSA clone carrying the pvl and toxic shock syndrome toxin-1-encoding genes, named ST22-PT, was reported recently (Yamaguchi et al., 2022) and has sporadically been isolated in several countries (Boswihi et al., 2022). ST22-PT is highly virulent and causes fatal necrotizing pneumonia and sepsis (Uda et al., 2020). MSSA can become MRSA by acquiring SCCmec (Hayakawa et al., 2020); therefore, it is important to prevent the spread of ST22-pvl MSSA to MRSA.
S. aureus secretes a variety of virulence factors, which provide protection from the host’s innate immune response through various mechanisms and promote infection (Kim et al., 2012). pvl causes cell death and inflammation by acting on the cell membranes of macrophages, monocytes, and polymorphonuclear cells (Shallcross et al., 2013). pvl has high toxicity and a high mortality rate (Leistner et al., 2022). pvl has been associated with community-acquired staphylococcal infections, particularly those caused by CA-MRSA (Vandenesch et al., 2003). However, pvl was detected in hospital-acquired infections in previous studies (Benoit et al., 2008; Zhao et al., 2022), similar to ours. About 50% of the strains in our study carried the pvl gene. However, there have been differences in the detection rates of pvl reported from different provinces in China: Guangdong (27.2%) (Liang et al., 2019), Hainan (47.6%) (Li et al., 2019), Yunnan (92.4%) (Ma et al., 2022), Heilongjiang (13.3%) (Yang et al., 2021). Possibly, S. aureus strains gain the pvl virulence gene through the bacteriophage lysogenic transformation indicating the horizontal transmission of this gene (Monecke et al., 2012). In case of potential pvl infection, personal protective equipment should be used, and isolation should be enforced to prevent the spreading of that bacterial strain.
The ica locus (icaADBC) encodes the enzyme N-acetyl-glucosaminyltranferase, which participates in the production of polysaccharide intercellular adhesion (PIA), facilitating the biofilm formation of S. aureus (Cue et al., 2012). A previous study has reported differences in the detection rate of icaADBC in the biofilm formation process of S. aureus, and the absence of a certain icaADBC may not necessarily affect the biofilm formation (Ghaioumy et al., 2021). This also explains the slightly higher detection rates of icaA and icaD (both 100%) than those of icaB and icaC (both 96.7%) in this study. Possibly, S. aureus can form biofilms through cell wall anchoring protein without PIA (Schneewind and Sortases, 2019).
Besides, there are some other notable findings from this study. Firstly, ST7395, ST965, and ST6829 were found in MRSA and MSSA isolates. The result indicated that some MSSA strains provided a stable genomic environment for integrating SCCmec elements, which helped transfer between isolates (Zhu et al., 2022). Secondly, consistent with previous studies (Javdan et al., 2019), agrI and agrIII were related to the severity of burns. However, the correlation reasons were not clear and demanded further investigation. Thirdly, we found a new spa type t20720 of S. aureus in MSSA, which has strong biofilm formation ability and carries the pvl, lukED, lukAB, hlgCB, and icaABCDR virulence genes. This strain should be closely monitored.
Conclusion
In conclusion, CC8-ST239-t030-agrI-SCCmecIII and CC7-ST-7-t091-agrI were the prevalent molecular signature of MRSA and MSSA isolates from burn wound infections in Fujian, China, respectively. Virulence genes were widely distributed among S. aureus, and these strains could easily form biofilms. ST239 exhibited high drug resistance, and the newly identified spa-t20720 isolate requires special clinical attention. Prevention and control measures must be implemented to reduce the spread of these highly virulent and resistant strains.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
BL and XH designed the study and obtained the funding. XH and SZ performed the experiments. DX and GZ performed the statistical analysis. XH wrote the manuscript. BL and XD contributed to manuscript revision. YC contributed the materials. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Plan Project of Quanzhou, Fujian (grant number: 2020N54s) and Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare.
Acknowledgments
We thank Huina Liu (the 909 Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army), Suxin Wang (the 73rd Army Hospital of the Army), and Fanhui Kong (Mindong Hospital of Ningde) for their assistance with strains collection and cases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1236497/full#supplementary-material
Abbreviations
CA-MRSA, Community-acquired methicillin-resistant Staphylococcus aureus; HA-MRSA, Hospital-acquired methicillin-resistant Staphylococcus aureus; LA-MRSA, Livestock-associated methicillin-resistant Staphylococcus aureus; CC, clonal complex; MLST, multilocus sequence typing; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; pvl, Panton-Valentine Leukotoxin; S. aureus, Staphylococcus aureus; SCCmec, Staphylococcus chromosomal cassette mec; NT, non-typeable.
Footnotes
References
Aires-de-Sousa, M., Conceição, T., and de Lencastre, H. (2006). Unusually high prevalence of nosocomial Panton-valentine leukocidin-positive Staphylococcus aureus isolates in Cape Verde Islands. J. Clin. Microbiol. 44, 3790–3793. doi: 10.1128/JCM.01192-06
Amissah, N. A., van Dam, L., Ablordey, A., Ampomah, O. W., Prah, I., Tetteh, C. S., et al. (2017). Epidemiology of Staphylococcus aureus in a burn unit of a tertiary care center in Ghana. PLoS One 12:e0181072. doi: 10.1371/journal.pone.0181072
Benoit, S. R., Estivariz, C., Mogdasy, C., Pedreira, W., Galiana, A., Galiana, A., et al. (2008). Community strains of methicillin-resistant Staphylococcus aureus as potential cause of healthcare-associated infections, Uruguay, 2002-2004. Emerg. Infect. Dis. 14, 1216–1223. doi: 10.3201/eid1408.071183
Boswihi, S. S., Verghese, T., and Udo, E. E. (2022). Diversity of clonal complex 22 methicillin-resistant Staphylococcus aureus isolates in Kuwait hospitals. Front. Microbiol. 13:970924. doi: 10.3389/fmicb.2022.970924
Chen, H., Yang, L., Cheng, L., Hu, X. H., and Shen, Y. M. (2021). Distribution and drug resistance of pathogens in burn patients in China from 2006 to 2019. World J. Clin. Cases 9, 2228–2237. doi: 10.12998/wjcc.v9.i10.2228
Cocchi, P., Cariani, L., Favari, F., Lambiase, A., Fiscarelli, E., Gioffré, F. V., et al. (2011). Molecular epidemiology of meticillin-resistant Staphylococcus aureus in Italian cystic fibrosis patients: a national overview. J. Cyst. Fibros. 10, 407–411. doi: 10.1016/j.jcf.2011.06.005
Cue, D., Lei, M. G., and Lee, C. Y. (2012). Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell. Infect. Microbiol. 2:38. doi: 10.3389/fcimb.2012.00038
Cuny, C., Wieler, L. H., and Witte, W. (2015). Livestock-associated MRSA: the impact on humans. Antibiotics (Basel) 4, 521–543. doi: 10.3390/antibiotics4040521
Cuteri, V., Marenzoni, M. L., Mazzolla, R., Tosti, N., Merletti, L., Arcioni, S., et al. (2004). Staphylococcus aureus: study of genomic similarity of strains isolated in veterinary pathology using amplified fragment length polymorphism (AFLP). Comp. Immunol. Microbiol. Infect. Dis. 27, 247–253. doi: 10.1016/j.cimid.2003.11.003
Dad, V., Ahmadrajabi, R., Esfahani, S., and Saffari, F. (2022). Comparative study of Staphylococcus aureus from burn patients and healthcare workers in a burn center, Yazd, Iran. Wien Med Wochenschr 172, 256–260. doi: 10.1007/s10354-021-00863-5
Gad, G. F., El-Feky, M. A., El-Rehewy, M. S., Hassan, M. A., Abolella, H., and El-Baky, R. M. (2009). Detection of icaA, icaD genes and biofilm production by Staphylococcus aureus and Staphylococcus epidermidis isolated from urinary tract catheterized patients. J. Infect. Dev. Ctries. 3, 342–351. doi: 10.3855/jidc.241
Ghaioumy, R., Tabatabaeifar, F., Mozafarinia, K., Mianroodi, A. A., Isaei, E., Morones-Ramírez, J. R., et al. (2021). Biofilm formation and molecular analysis of intercellular adhesion gene cluster (icaABCD) among Staphylococcus aureus strains isolated from children with adenoiditis. Iran J. Microbiol. 13, 458–463. doi: 10.18502/ijm.v13i4.6969
Goudarzi, M., Bahramian, M., Satarzadeh Tabrizi, M., Udo, E. E., Figueiredo, A. M., Fazeli, M., et al. (2017). Genetic diversity of methicillin resistant Staphylococcus aureus strains isolated from burn patients in Iran: ST239-SCCmec III/t037 emerges as the major clone. Microb. Pathog. 105, 1–7. doi: 10.1016/j.micpath.2017.02.004
Goudarzi, M., Fazeli, M., Pouriran, R., and Eslami, G. (2019). Genotype distribution of Panton-valentine Leukocidin (PVL)-positive Staphylococcus aureus strains isolated from wound-related infections: a three-year multi-center study in Tehran, Iran. Jpn. J. Infect. Dis. 72, 306–311. doi: 10.7883/yoken.JJID.2018.406
Grundmann, H., Aanensen, D. M., van den Wijngaard, C. C., Spratt, B. G., Harmsen, D., and Friedrich, A. W. (2010). Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215. doi: 10.1371/journal.pmed.1000215
Gu, J., Shen, S., Xiong, M., Zhao, J., Tian, H., Xiao, X., et al. (2023). ST7 becomes one of the Most common Staphylococcus aureus clones after the COVID-19 epidemic in the City of Wuhan, China. Infect. Drug. Resist. 16, 843–852. doi: 10.2147/IDR.S401069.eCollection2023
Guo, Y., Yu, X., Wang, J., Hua, D., You, Y., Wu, Q., et al. (2023). A food poisoning caused by ST7 staphylococcal aureus harboring sea gene in Hainan province, China. Front. Microbiol. 14:1110720. doi: 10.3389/fmicb.2023.1110720.eCollection2023
Hayakawa, K., Yamaguchi, T., Ono, D., Suzuki, H., Kamiyama, J., Taguchi, S., et al. (2020). Two cases of Intrafamilial transmission of community-acquired methicillin-resistant Staphylococcus aureus producing both PVL and TSST-1 causing fatal necrotizing pneumonia and sepsis. Infect. Drug Resist. 13, 2921–2927. doi: 10.2147/IDR.S262123.eCollection2020
He, W. P., Gu, F. F., Zhang, J., Li, X. X., Xiao, S. Z., Zeng, Q., et al. (2021). Molecular characteristics and risk factor analysis of Staphylococcus aureus colonization put insight into CC1 colonization in three nursing homes in Shanghai. PLoS One 16:e0253858. doi: 10.1371/journal.pone.0253858
He, C., Xu, S., Zhao, H., Hu, F., Xu, X., Jin, S., et al. (2018). Leukotoxin and pyrogenic toxin Superantigen gene backgrounds in bloodstream and wound Staphylococcus aureus isolates from eastern region of China. BMC Infect. Dis. 18:395. doi: 10.1186/s12879-018-3297-0
Jarraud, S., Mougel, C., Thioulouse, J., Lina, G., Meugnier, H., Forey, F., et al. (2002). Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70, 631–641. doi: 10.1128/IAI.70.2.631-641.2002
Javdan, S., Narimani, T., Shahini Shams Abadi, M., and Gholipour, A. (2019). Agr typing of Staphylococcus aureus species isolated from clinical samples in training hospitals of Isfahan and Shahrekord. BMC. Res. Notes 12:363. doi: 10.1186/s13104-019-4396-8
Kalligeros, M., Shehadeh, F., Karageorgos, S. A., Zacharioudakis, I. M., and Mylonakis, E. (2019). MRSA colonization and acquisition in the burn unit: a systematic review and meta-analysis. Burns 45, 1528–1536. doi: 10.1016/j.burns.2019.05.014
Keen, E. F. 3rd, Robinson, B. J., Hospenthal, D. R., Aldous, W. K., Wolf, S. E., Chung, K. K., et al. (2010). Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 36, 819–825. doi: 10.1016/j.burns.2009.10.013
Kim, H. K., Thammavongsa, V., Schneewind, O., and Missiakas, D. (2012). Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr. Opin. Microbiol. 15, 92–99. doi: 10.1016/j.mib.2011.10.012
Lee, C. Y., Fang, Y. P., Wu, T. H., Chang, Y. F., and Sung, C. H. (2022). Sequence types 8, 59, and 45 methicillin resistant Staphylococcus aureus as the predominant strains causing skin and soft tissue infections in Taiwan's prisons and jails. J. Microbiol. Immunol. Infect. 55, 1239–1245. doi: 10.1016/j.jmii.2021.08.013
Leistner, R., Hanitsch, L. G., Krüger, R., Lindner, A. K., Stegemann, M. S., and Nurjadi, D. (2022). Skin infections due to Panton-valentine Leukocidin-producing S. aureus. Dtsch. Arztebl. Int. 119, 775–784. doi: 10.3238/arztebl.m2022.0308
Li, X., Fang, F., Zhao, J., Lou, N., Li, C., Huang, T., et al. (2018). Molecular characteristics and virulence gene profiles of Staphylococcus aureus causing bloodstream infection. Braz. J. Infect. Dis. 22, 487–494. doi: 10.1016/j.bjid.2018.12.001
Li, X., Huang, T., Xu, K., Li, C., and Li, Y. (2019). Molecular characteristics and virulence gene profiles of Staphylococcus aureus isolates in Hainan, China. BMC Infect. Dis. 19:873. doi: 10.1186/s12879-019-4547-5
Liang, Y., Tu, C., Tan, C., El-Sayed Ahmed, M. A. E., Dai, M., Xia, Y., et al. (2019). Antimicrobial resistance, virulence genes profiling and molecular relatedness of methicillin-resistant Staphylococcus aureus strains isolated from hospitalized patients in Guangdong Province, China. Infect. Drug Resist. 12, 447–459. doi: 10.2147/IDR.S192611.eCollection2019
Liu, Y., Du, F. L., Liu, P. P., Mei, Y. F., Wan, L. G., Wei, D. D., et al. (2018). Molecular epidemiology and virulence features of Staphylococcus aureus bloodstream isolates in a regional burn Center in China, 2012-2016. Microb. Drug Resist. 24, 1354–1360. doi: 10.1089/mdr.2017.0209
Ma, M., Tao, L., Li, X., Liang, Y., Li, J., Wang, H., et al. (2022). Changes in molecular characteristics and antimicrobial resistance of invasive Staphylococcus aureus infection strains isolated from children in Kunming, China during the COVID-19 epidemic. Front. Microbiol. 13:944078. doi: 10.3389/fmicb.2022.1075041
Mahmoudi, H., Pourhajibagher, M., Chiniforush, N., Soltanian, A. R., Alikhani, M. Y., and Bahador, A. (2019). Biofilm formation and antibiotic resistance in meticillin-resistant and meticillin-sensitive Staphylococcus aureus isolated from burns. J. Wound Care 28, 66–73. doi: 10.12968/jowc.2019.28.2.66
Merialdi, G., Feltrin, F., Gaetarelli, B., Lombardi, G., Iurescia, M., Alba, P., et al. (2019). Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) spa type t127, sequence type (ST)1, quickly spreads and persists among young pigs. Pathog. Dis. 77:ftz033. doi: 10.1093/femspd/ftz033
Monecke, S., Engelmann, I., Archambault, M., Coleman, D. C., Coombs, G. W., Cortez de Jäckel, S., et al. (2012). Distribution of SCCmec-associated phenol-soluble modulin in staphylococci. Mol. Cell. Probes 26, 99–103. doi: 10.1016/j.mcp.2012.01.001
O'Hara, F. P., Amrine-Madsen, H., Mera, R. M., Brown, M. L., Close, N. M., Suaya, J. A., et al. (2012). Molecular characterization of Staphylococcus aureus in the United States 2004-2008 reveals the rapid expansion of USA300 among inpatients and outpatients. Microb. Drug Resist. 18, 555–561. doi: 10.1089/mdr.2012.0056
Ozlu, O., and Basaran, A. (2022). Infections in patients with major burns: a retrospective study of a burn intensive care unit. J. Burn Care Res. 43, 926–930. doi: 10.1093/jbcr/irab222
Rao, Q., Shang, W., Hu, X., and Rao, X. (2015). Staphylococcus aureus ST121: a globally disseminated hypervirulent clone. J. Med. Microbiol. 64, 1462–1473. doi: 10.1099/jmm.0.000185
Rodrigues, M. V., Fortaleza, C. M., Riboli, D. F., Rocha, R. S., Rocha, C., and da Cunha, M. L. (2013). Molecular epidemiology of methicillin-resistant Staphylococcus aureus in a burn unit from Brazil. Burns 39, 1242–1249. doi: 10.1016/j.burns.2013.02.006
Sabirova, J. S., Hernalsteens, J. P., De Backer, S., Xavier, B. B., Moons, P., Turlej-Rogacka, A., et al. (2015). Fatty acid kinase a is an important determinant of biofilm formation in Staphylococcus aureus USA300. BMC Genomics 16:861. doi: 10.1186/s12864-015-1956-8
Schneewind, O., and Sortases, M. D. (2019). Surface proteins, and their roles in Staphylococcus aureus disease and vaccine development. Microbiol Spectr 7. doi: 10.1128/microbiolspec.PSIB-0004-2018
Shallcross, L. J., Fragaszy, E., Johnson, A. M., and Hayward, A. C. (2013). The role of the Panton-valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 13, 43–54. doi: 10.1016/S1473-3099(12)70238-4
Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L., and Fowler, V. G. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi: 10.1128/CMR.00134-14
Uda, K., Uehara, Y., Morimoto, Y., Hiramatsu, K., and Miyairi, I. (2020). A pediatric case of septic arthritis caused by methicillin-resistant Staphylococcus aureus with Panton-valentine Leukocidin and toxic shock syndrome Toxin-1. Jpn. J. Infect. Dis. 73, 259–262. doi: 10.7883/yoken.JJID.2019.436
Uhlén, M., Guss, B., Nilsson, B., Gatenbeck, S., Philipson, L., and Lindberg, M. (1984). Complete sequence of the staphylococcal gene encoding protein a. a gene evolved through multiple duplications. J. Biol. Chem. 259, 1695–1702. doi: 10.1016/S0021-9258(17)43463-6
Vandenesch, F., Naimi, T., Enright, M. C., Lina, G., Nimmo, G. R., Heffernan, H., et al. (2003). Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9, 978–984. doi: 10.3201/eid0908.030089
Velázquez-Suárez, C., Cebrián, R., Gasca-Capote, C., Sorlózano-Puerto, A., Gutiérrez-Fernández, J., Martínez-Bueno, M., et al. (2021). Antimicrobial activity of the circular Bacteriocin AS-48 against clinical multidrug-resistant Staphylococcus aureus. Antibiotics (Basel). 30:925. doi: 10.3390/antibiotics10080925
Wang, X., Liu, Q., Zhang, H., Li, X., Huang, W., Fu, Q., et al. (2018). Molecular characteristics of community-associated Staphylococcus aureus isolates from pediatric patients with bloodstream infections between 2012 and 2017 in Shanghai, China. Front. Microbiol. 9:1211. doi: 10.3389/fmicb.2018.01211.eCollection2018
Wang, B., Xu, Y., Zhao, H., Wang, X., Rao, L., Guo, Y., et al. (2022). Methicillin-resistant Staphylococcus aureus in China: a multicentre longitudinal study and whole-genome sequencing. Emerg Microbes Infect. 11, 532–542. doi: 10.1080/22221751.2022.2032373
Wolters, M., Frickmann, H., Christner, M., Both, A., Rohde, H., Oppong, K., et al. (2020). Molecular characterization of Staphylococcus aureus isolated from chronic infected wounds in rural Ghana. Microorganisms 8:2052. doi: 10.3390/microorganisms8122052
Wu, J., Lu, A. D., Zhang, L. P., Zuo, Y. X., and Jia, Y. P. (2019). Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi 40, 52–57. doi: 10.3760/cma.j.issn.0253-2727.2019.01.010
Wu, D., Wang, Q., Yang, Y., Geng, W., Wang, Q., Yu, S., et al. (2010). Epidemiology and molecular characteristics of community-associated methicillin-resistant and methicillin-susceptible Staphylococcus aureus from skin/soft tissue infections in a children's hospital in Beijing, China. Diagn. Microbiol. Infect. Dis. 67, 1–8. doi: 10.1016/j.diagmicrobio.2009.12.006
Yamaguchi, T., Nakamura, I., Sato, T., Ono, D., Sato, A., Sonoda, S., et al. (2022). Changes in the genotypic characteristics of community-acquired methicillin-resistant Staphylococcus aureus collected in 244 medical facilities in Japan between 2010 and 2018: a Nationwide surveillance. Microbiol. Spectr. 10:e0227221. doi: 10.1128/spectrum.02272-21
Yang, X., Zhao, J., Wang, Y., Wu, J., Wang, X., Wang, Y., et al. (2021). Molecular epidemiology of methicillin-resistant Staphylococcus aureus in hospitalized patients in eastern Heilongjiang Province, China. Infect. Drug Resist. 14, 1635–1643. doi: 10.2147/IDR.S307856.eCollection2021
Yu, C. H., Shen, S., Huang, K. A., and Huang, Y. C. (2022). The trend of environmental and clinical methicillin-resistant Staphylococcus aureus in a hospital in Taiwan: impact of USA300. J. Microbiol. Immunol. Infect. 55, 241–248. doi: 10.1016/j.jmii.2021.03.020
Zhang, K., McClure, J. A., Elsayed, S., Louie, T., and Conly, J. M. (2005). Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43, 5026–5033. doi: 10.1128/JCM.43.10.5026-5033.2005
Zhao, R., Wang, X., Wang, X., Du, B., Xu, K., Zhang, F., et al. (2022). Molecular characterization and virulence gene profiling of methicillin-resistant Staphylococcus aureus associated with bloodstream infections in southern China. Front. Microbiol. 13:1008052. doi: 10.3389/fmicb.2022.1008052.eCollection2022
Zhu, H., Luo, H., Zhong, Q., Cao, X., Gu, S., Peng, S., et al. (2022). Comparison of molecular characteristics between methicillin-resistant and -susceptible Staphylococcus aureus clinical isolates by whole-genome sequencing. Infect. Drug Resist. 15, 2949–2958. doi: 10.2147/IDR.S359654.eCollection2022
Keywords: S. aureus virulence genes, molecular typing, antimicrobial susceptibility, burn wound infection, biofilm-forming isolates
Citation: Hong X, Zhou S, Dai X, Xie D, Cai Y, Zhao G and Li B (2023) Molecular typing and characterization of Staphylococcus aureus isolates from burn wound infections in Fujian, China. Front. Microbiol. 14:1236497. doi: 10.3389/fmicb.2023.1236497
Edited by:
Asad U. Khan, Aligarh Muslim University, IndiaReviewed by:
Hussein O. M. Al-Dahmoshi, University of Babylon, IraqAlaa Abouelfetouh, Alexandria University, Egypt
Copyright © 2023 Hong, Zhou, Dai, Xie, Cai, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Li, bGVvbmxlZTMwN0Bob3RtYWlsLmNvbQ==
 Xiaolan Hong1,2
Xiaolan Hong1,2 Bin Li
Bin Li