- 1Department of Infectious Disease, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department and Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Clinical Laboratory, Center for Gene Diagnosis, and Program of Clinical Laboratory Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
Background: Severe fever with thrombocytopenia syndrome (SFTS) is an emerging zoonosis caused by a novel bunyavirus. Until recently, the SFTS related acute kidney injury (AKI) was largely unexplored. This study aimed to investigate the clinical characteristics and outcomes of AKI in patients with SFTS.
Methods: The non-AKI and AKI groups were compared in terms of general characteristics, clinical features, laboratory parameters and cumulative survival rate. The independent risk factors for in-hospital mortality in patients with SFTS were analyzed by multivariate logistic regression to identify the population with poor prognosis.
Results: A total of 208 consecutive patients diagnosed with SFTS were enrolled, including 153 (73.6%) patients in the non-AKI group and 55 (26.4%) patients in the AKI group. Compared with patients without AKI, patients with AKI were older and had a higher frequency of diabetes. Among these laboratory parameters, platelet count, albumin and fibrinogen levels of patients with AKI were identified to be significantly lower than those of patients without AKI, while ALT, AST, ALP, triglyceride, LDH, BUN, uric acid, creatine, Cys-C, β2-MG, potassium, AMY, lipase, CK-MB, TnI, BNP, APTT, thrombin time, D-dimer, CRP, IL-6, PCT and ESR levels were significantly higher in patients with AKI. A higher SFTS viral load was also detected in the AKI patients than in the non-AKI patients. The cumulative survival rates of patients at AKI stage 2 or 3 were significantly lower than those of patients without AKI or at AKI stage 1. However, there was no significant difference in the cumulative survival rates between patients without AKI and those with stage 1 AKI. Univariate and multivariate binary logistic regression analyses demonstrated that stage 2 or 3 AKI was an independent risk factor for in-hospital mortality in patients with SFTS.
Conclusion: AKI is associated with poor outcomes in patients with SFTS, especially patients at AKI stage 2 or 3, who generally have high mortality. Our findings support the importance of early identification and timely treatment of AKI in patients with SFTS.
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease caused by a novel bunyavirus that was reported in rural areas of China in 2009 (Yu et al., 2011). SFTS is mainly transmitted by contact with ticks, and can be transmitted among humans (Bao et al., 2011), even direct infection of SFTS virus from cats and dogs to humans has been reported, which is recognized as a social problem (Park et al., 2019; Yamanaka et al., 2020). Patients with mild SFTS can present with fever, thrombocytopenia, anorexia, dizziness, headache, nausea, vomiting and diarrhea, while patients with serious SFTS might develop multiple organ dysfunction syndrome including encephalopathy, cardiac failure, acute respiratory distress syndrome, severe pancreatitis, acute kidney injury (AKI), and disseminated intravascular coagulation, with a reported mortality rate ranging from 2.5% to 30% in various epidemic areas (Zhang et al., 2012; Liu S. et al., 2014; Li et al., 2018). The extensive distribution, and high morbidity and mortality of SFTS make it a significant newly emerging infectious disease in China.
Critically ill patients with SFTS always have a high mortality rate, and the clinical manifestations and laboratory parameters could be used to predict the outcomes of patients with SFTS, which have been explored with high interest (Wen et al., 2014; Xu et al., 2018). Among these definite predictors, elevated serum creatinine (sCr) levels have been frequently demonstrated to be closely related to poor prognosis (Cui et al., 2015; Nie et al., 2020; He et al., 2021). The elevated sCr levels in a short time typically represent AKI. It suggests that AKI may be closely related to the adverse outcomes of patients with SFTS. Hence, a comprehensive understanding of the clinical characterization of AKI and its associated outcomes is crucial for formulating personalized treatment strategies to improve the prognosis of patients with SFTS. However, until recently, the data for AKI in SFTS were limited. We performed a study to investigate the incidence, clinical manifestations and outcomes of AKI in a retrospective cohort of hospitalized patients with SFTS.
Methods
Patients
A total of 208 consecutive patients with SFTS admitted to the Department of Infectious Disease, Zhongnan Hospital of Wuhan University between August 2016 and June 2023 were included in a retrospective cohort. The patients were then divided into the AKI (n = 153) and non-AKI groups (n = 55) according to the presence of AKI. According to the current Kidney Disease: Improving Global Outcomes (KDIGO) staging criteria for AKI, patients in the AKI group were further divided into three subgroups: stage 1 AKI group (n = 23), stage 2 AKI group (n = 17) and stage 3 AKI group (n = 15).
The criteria of diagnosing SFTS were as follows (Liu Q. et al., 2014): (1) acute fever (temperature >37.3°C for over 24 h) with thrombocytopenia; (2) laboratory-confirmed SFTS virus infection by detection of viral RNA through polymerase chain reaction assay. Patients were excluded if they fulfilled one or more of the following criteria: (1) age ≤18 years or ≥80 years, (2) reduced glomerular filtration rate or chronic kidney disease had been confirmed before admission, (3) previous kidney transplantation; (4) the presence of preterminal comorbidities (heart disease New York Heart Association III–IV, severe chronic obstructive pulmonary disease), (5) any other types of immunodeficiency. (6) history of malignant tumor.
Diagnosis of AKI was based on the KDIGO guidelines (Palevsky et al., 2013): (1) increase in sCr by ≥26.5 μmol/L (or ≥50%) within 48 h; or (2) increase in sCr to ≥1.5 times the baseline value, which is known or presumed to have within the previous 7 days; or (3) urine volume <0.5 mL/kg/h for 6 h. The KDIGO criteria for AKI staging were as follows: (1) AKI stage 1, increase in sCr by 1.5–1.9 times the baseline value, or increase in sCr by 26.5 μmol/L within 48 h, or urine volume <0.5 mL/kg/h and >0.3 mL/kg/h for 6–12 h; (2) AKI stage 2, increase in sCr by 2.0–2.9 times the baseline value, or urine volume <0.5 mL/kg/h and >0.3 mL/kg/h for ≥12 h; (3) AKI stage 3, increase in sCr by 3.0 times the baseline value, or increase in sCr to 353.6 μmol/L, or start of renal replacement therapy, or urine volume <0.3 mL/kg/h for ≥24 h.
During hospitalization, all patients received routine supportive treatment, including bed rest, adequate nutritional support, intensive care and monitoring. Complications including coagulation disorders, thrombocytopenia crisis, neutropenia, acute respiratory distress syndrome, septic shock and AKI were closely monitored and managed immediately.
Data collection
The medical records of patients with SFTS were reviewed, demographic details, comorbid conditions, symptoms, signs and laboratory tests data including white blood cell (WBC) count and percentage, neutrophil count and percentage, lymphocyte count and percentage, hemoglobin, platelet (PLT) count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin, alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), lactate dehydrogenase (LDH), total cholesterol (TC), triglyceride (TG), blood urea nitrogen (BUN), uric acid, sCr, cystatin C (Cys-C), β2-microglobulin (β2-MG), sodium, potassium, calcium, magnesium, chlorine, phosphorus, amylase (AMY), lipase, creatinine kinase (CK), creatinine kinase myocardial B fraction (CK-MB), troponin I (TnI), brain natriuretic peptide (BNP), prothrombin time (PT), international normalized ratio (INR), prothrombin activity (PTA), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen, D-dimer, C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), erythrocyte sedimentation rate (ESR), SFTS viral load, fecal occult blood test (OBT), urine protein test (UPT), urine occult blood test (UOBT) and survival time were collected at the time of AKI diagnosis.
Statistical analysis
All data were analyzed with IBM SPSS statistical analysis software (version 23.0, Chicago, United States), and p < 0.05 (two-sided) was considered statistically significant. Categorical variables were shown as numbers (percentages) and were compared by the Chi-square test or Fisher’s exact test. Continuous variables were shown as the means ± standard deviations for data with a normal distribution or medians with interquartile ranges (P25–P75) for data with a non-normal distribution, which were compared by the student’s t-test or Mann–Whitney U test, respectively. Correlations between numerical laboratory parameters were analyzed using Spearman correlation analysis. The cumulative survival rates of patients were evaluated using the Kaplan–Meier method and were compared by the log-rank test. Logistic regression analysis was conducted to identify the risk factors for fatal outcome of patients. All clinical data including demographic, comorbid conditions, clinical symptoms and laboratory indicators were included for univariate analysis, from which biologically plausible variables with p-value <0.05 were entered into the multivariate model by a stepwise method.
Results
Demographic and clinical manifestations
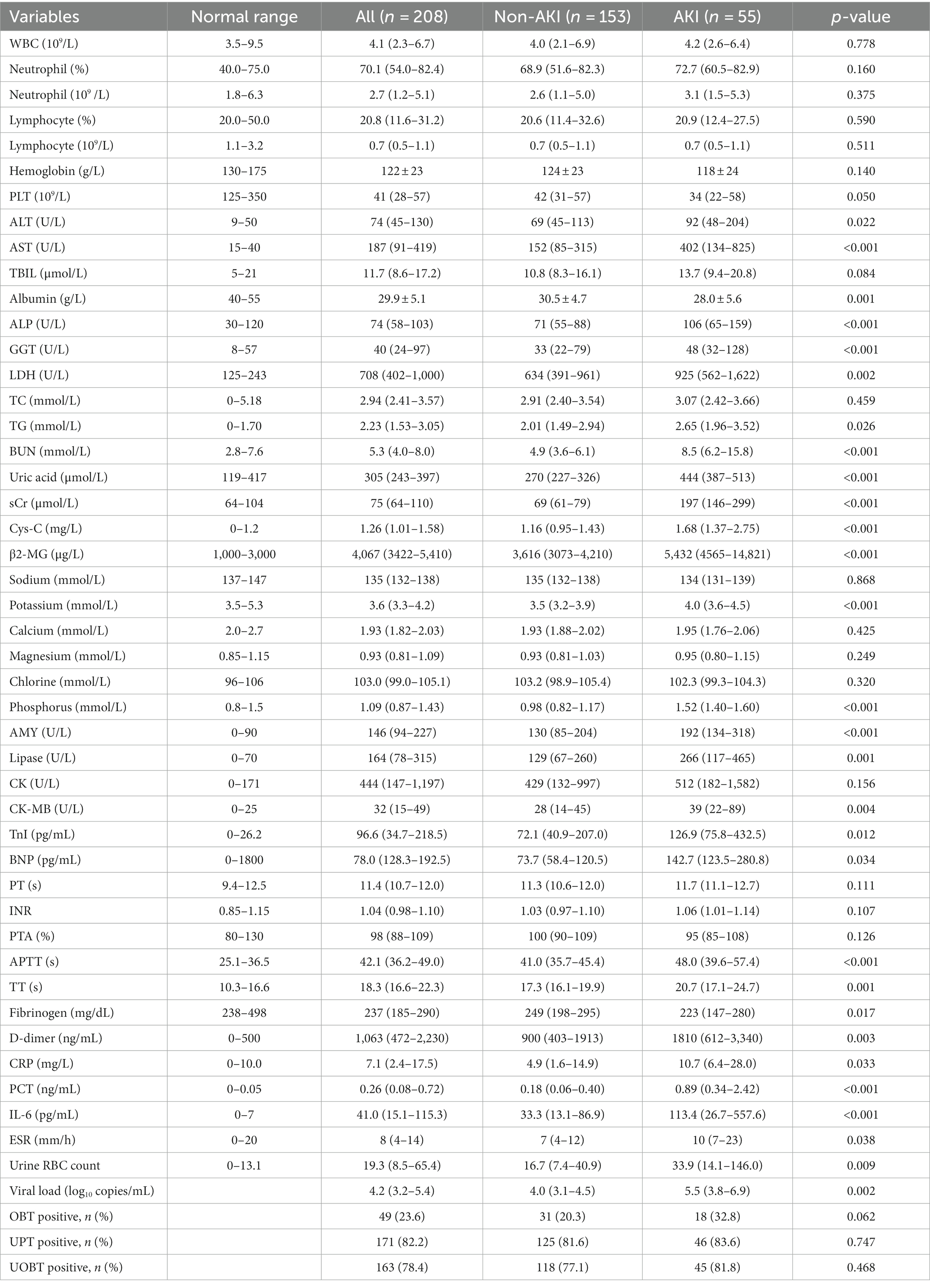
A total of 208 consecutive patients diagnosed with SFTS were enrolled, including 153 patients in the non-AKI group and 55 patients in the AKI group. Among these patients, 48 patients were diagnosed with AKI at admission, and seven patients were diagnosed with AKI during hospitalization. The time between symptom onset and diagnosis of AKI was 8 (7–12) days. Compared with patients without AKI, patients with AKI were older, and had a higher frequency of diabetes and more days from onset to admission. No difference was observed in the frequency of sex or hypertension between the two groups. Among all these clinical manifestations, headache, anorexia, abdominal pain, diarrhea, petechia, encephalopathy and hepatosplenomegaly were significantly overrepresented in patients in the AKI group compared with patients in the non-AKI group. However, there was no significant difference in other clinical features including dizziness, cough, sputum, chest distress, nausea, vomiting and the frequency of fever >38°C (Table 1).
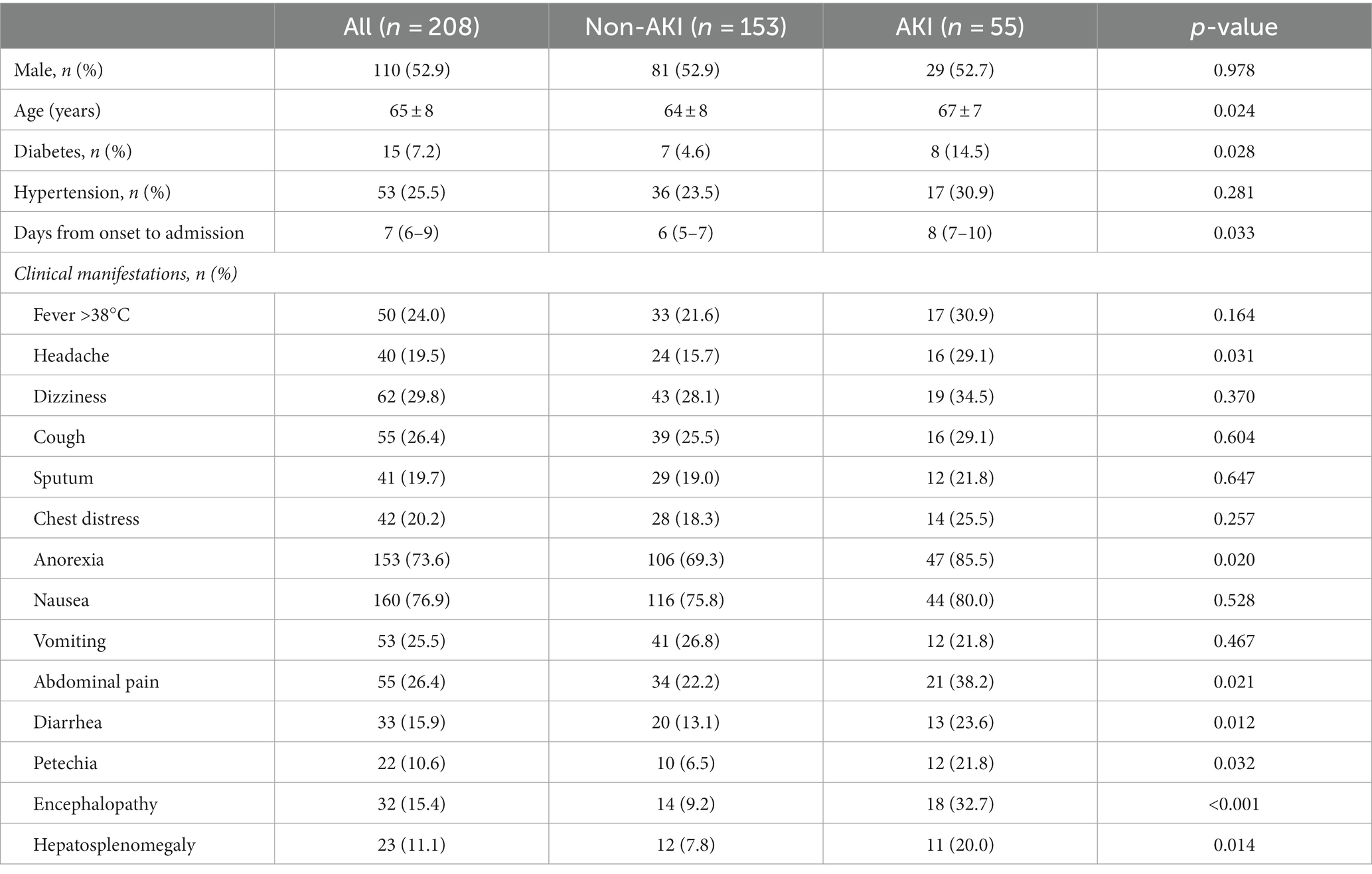
Table 1. Comparison of demographics, comorbid conditions and clinical symptoms between the non-AKI and AKI groups.
Laboratory test results
Among these laboratory parameters, PLT count, albumin and fibrinogen levels of patients with AKI were identified to be significantly lower than those of patients without AKI, while ALT, AST, ALP, LDH, TG, BUN, uric acid, sCr, Cys-C, β2-MG, potassium, phosphorus, AMY, lipase, CK-MB, TnI, BNP, APTT, TT, D-dimer, CRP, IL-6, PCT and ESR levels were significantly higher in patients with AKI. A higher SFTS viral load was also detected in the AKI patients than in the non-AKI patients. In terms of routine urine test examinations, the urine RBC count in the AKI patients was significantly higher than that in the non-AKI patients. No significant difference was observed for WBC, neutrophil and lymphocyte counts, TBIL, TC, sodium, PT, INR, APTT or frequency of OBT positivity between the two groups (Table 2).
Spearman correlation analysis was conducted to analyze the correlation between sCr and other laboratory parameters. The results indicated that sCr was positively correlated with ALT, AST, TBIL, GGT, ALP, BUN, uric acid, Cys-C, AMY, lipase, CK, CK-MB, TnI, PT, INR, APTT and TT, but was negatively correlated with PLT count, albumin, urine volume and fibrinogen (Figure 1).

Figure 1. Spearman correlation analysis was carried out to analyze the relationship between the levels of sCr and other laboratory parameters in patients with SFTS. (A) Correlation analysis between sCr and urine volume. (B) Correlation analysis between sCr and uric acid. (C) Correlation analysis between sCr and BUN. (D) Correlation analysis between sCr and Cys-C. (E) Correlation analysis between sCr and WBC count. (F) Correlation analysis between sCr and neutrophil count. (G) Correlation analysis between sCr and lymphocyte count. (H) Correlation analysis between sCr and hemoglobin. (I) Correlation analysis between sCr and PLT count. (J) Correlation analysis between sCr and ALT. (K) Correlation analysis between sCr and AST. (L) Correlation analysis between sCr and TBIL. (M) Correlation analysis between sCr and ALP. (N) Correlation analysis between sCr and GGT. (O) Correlation analysis between sCr and albumin. (P) Correlation analysis between sCr and AMY. (Q) Correlation analysis between sCr and lipase. (R) Correlation analysis between sCr and CK. (S) Correlation analysis between sCr and CK-MB. (T) Correlation analysis between sCr and TnI. (U) Correlation analysis between sCr and PT. (V) Correlation analysis between sCr and INR. (W) Correlation analysis between sCr and TT. (X) Correlation analysis between sCr and Fibrinogen.
Prognosis classification of patients with SFTS
As shown in Figure 2A, the cumulative survival rate of patients in the AKI group was significantly lower than that in the non-AKI group (93.4% vs. 50.9%, p < 0.001). As shown in Figure 2B, patients in the AKI group were divided into three subgroups, and the cumulative survival rates of patients at AKI stages 1, 2 and 3 were 82.6%, 35.3% and 20.0%, respectively. Both the cumulative survival rates of patients at AKI stage 2 or 3 were significantly lower than those of patients without AKI (all p < 0.001). However, there was no significant difference in the cumulative survival rates between the stage 1 AKI and non-AKI groups (p = 0.066). Moreover, compared with patients at AKI stage 1, patients at AKI stage 2 or 3 also had significantly lower cumulative survival rates (p = 0.003, p < 0.001, respectively). Moreover, the clinical features and laboratory parameters of patients at AKI stages 1, 2 and 3 were compared. We revealed that patients with stage 2 or 3 AKI had more days from onset to admission, and higher serum levels of ALT, AST, LDH, uric acid, Cys-C, β2-MG, AMY, lipase, CK-MB, TnI, BNP, D-dimer, CRP, PCT, IL-6, ESR, viral load and urine RBC count than patients with stage 1 AKI (Supplementary Table S1).
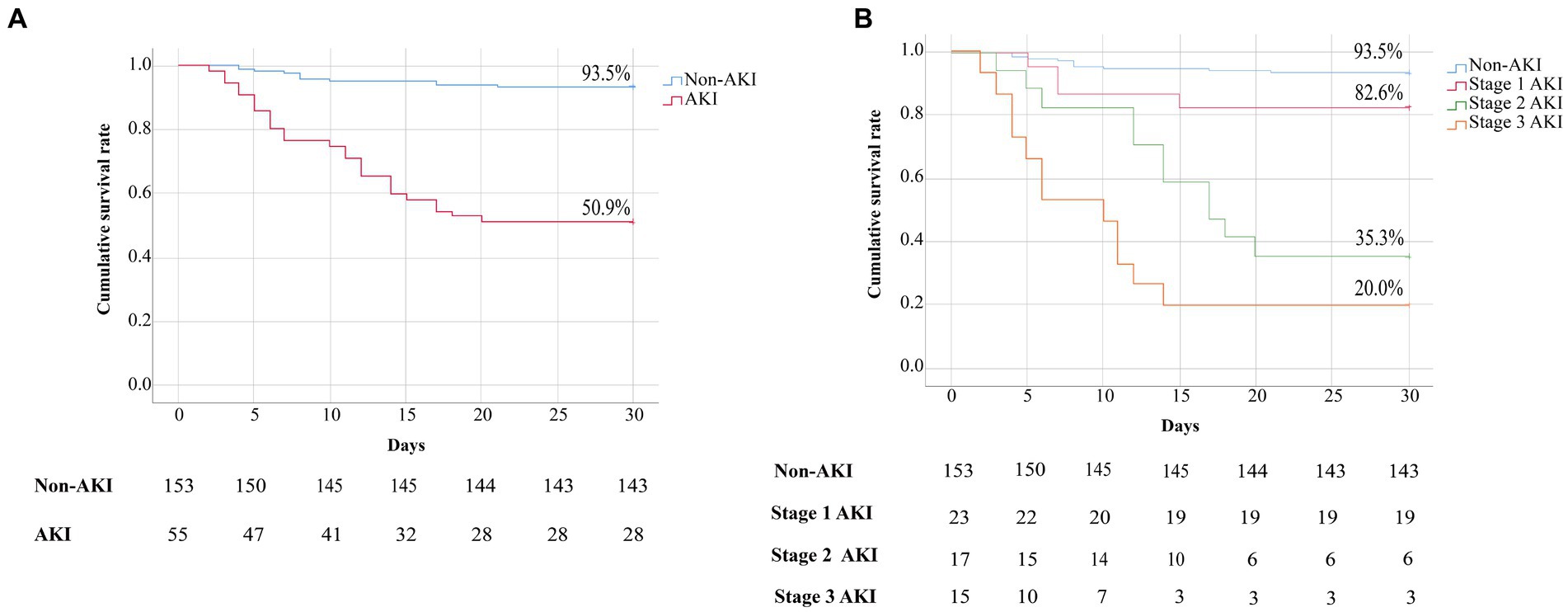
Figure 2. (A) Kaplan–Meier curves showing the cumulative survival rates of patients in the non-AKI and AKI groups. Comparison of the survival estimates was performed using the log rank test, non-AKI group vs. AKI group, p < 0.001. (B) Kaplan–Meier curves showing the cumulative survival rates of patients with SFTS based on AKI stage. Comparison of the survival estimates was performed using the log rank test, non-AKI group vs. stage 1 AKI group, p = 0.481; non-AKI group vs. stage 2 AKI or stage 3 AKI group, all p < 0.001; stage 1 AKI group vs. stage 2 AKI or stage 3 AKI group, p = 0.003, p < 0.001, respectively.
Risk factors for fatal outcomes
Univariable logistic regression analyses revealed that the presence of petechia, encephalopathy, hepatosplenomegaly and stage 2 or 3 AKI, old age, and decreased albumin, low fibrinogen, and elevated levels of AST, ALP, GGT, LDH, TG, uric acid, AMY, lipase, CK-MB, PT, APTT, TT, IL-6 and viral load were risk factors for fatal outcomes in patients with SFTS. The above variables were used for multivariable logistic regression analyses. It demonstrated that stage 2 or 3 AKI (OR, 10.329; 95% CI, 4.815–21.244), encephalopathy (OR, 5.935; 95% CI, 3.056–13.903), elevated levels of LDH (OR, 1.002; 95% CI, 1.001–1.003) and viral load (OR, 2.415; 95% CI, 1.702–4.026) were independent risk factors for mortality in patients with SFTS (Table 3).
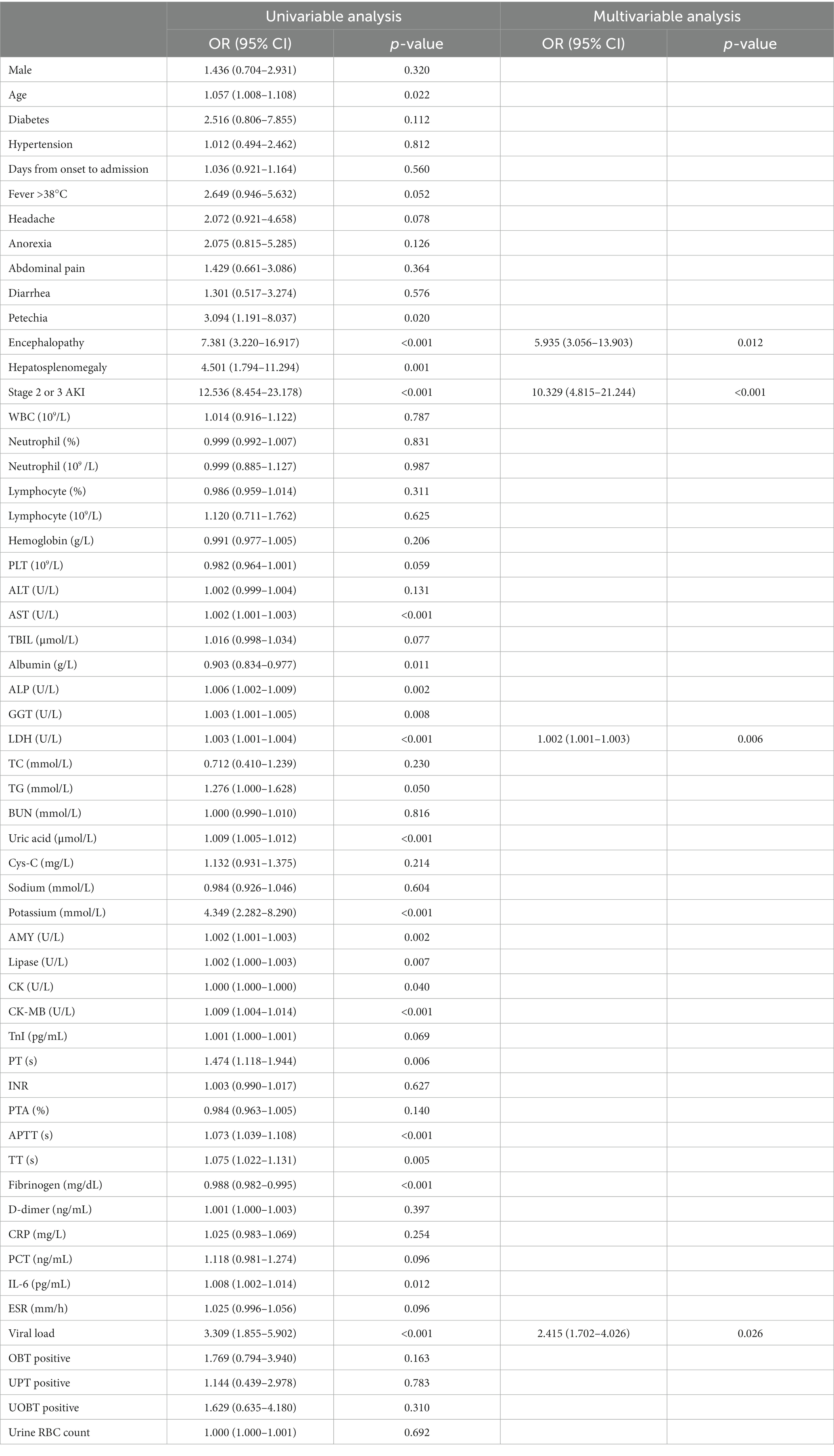
Table 3. Univariable and multivariable logistic regression analyses of in-hospital mortality in patients with SFTS.
Longitudinal changes in laboratory parameters in patients without AKI or at AKI stages 1, 2 and 3
Subgroup patients with longitudinal laboratory test results were analyzed to investigate their serial changes with respect to disease severity. Levels of 22 laboratory parameters at four time points were collected and presented. Compared with patients without AKI or with stage 1 AKI, patients with stage 2 or 3 AKI showed a significant increase in serum concentrations of sCr, BUN, Cys-C, β2-MG, AST, TBIL, ALP, GGT, LDH, BUN, AMY, lipase, CK-MB, TnI, TT, D-dimer, CRP, IL-6, and PCT and significantly reduced serum levels of albumin and PLT count during the course of hospitalization (see Figure 3).
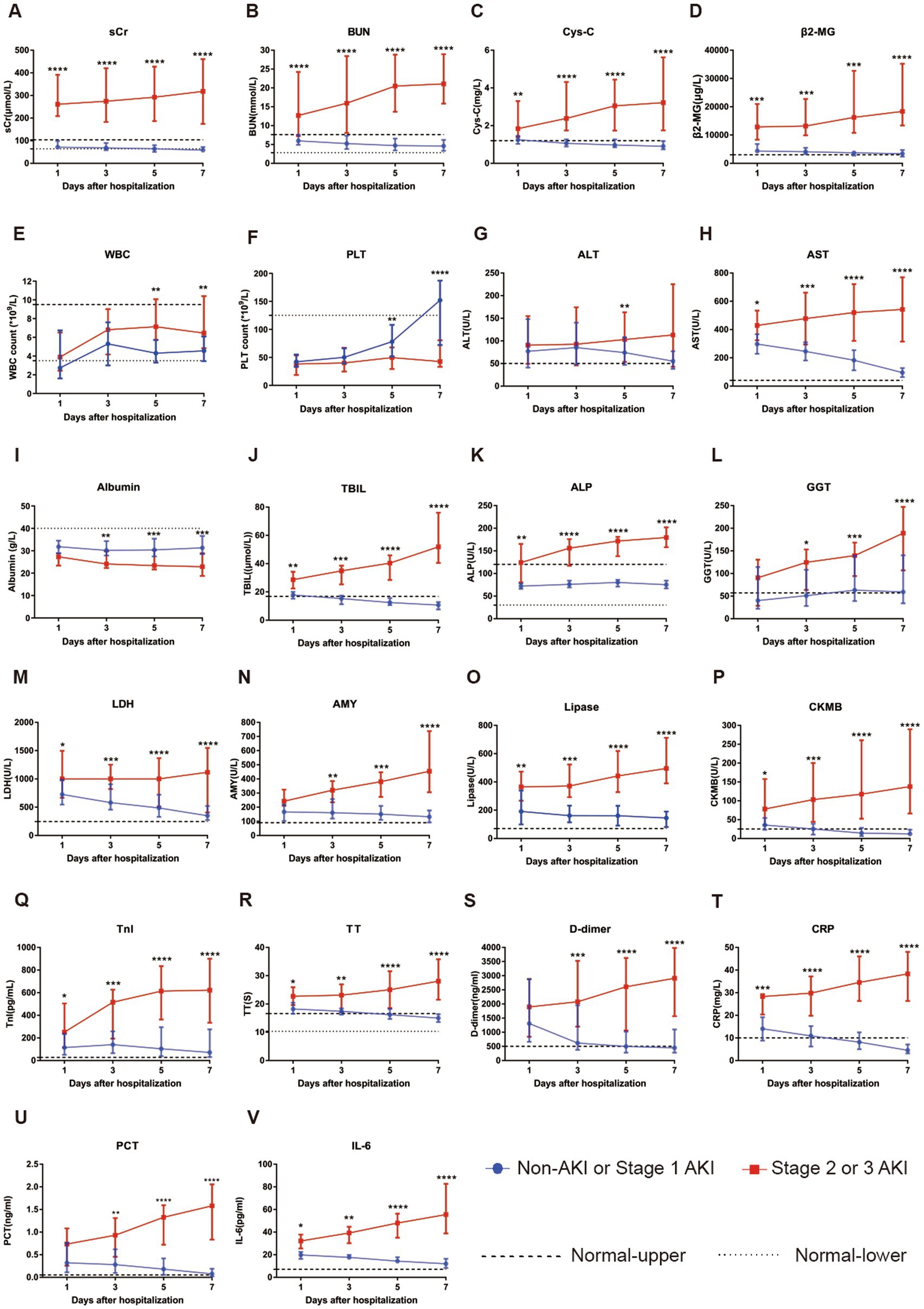
Figure 3. Evaluation of serial laboratory parameters for the non-AKI or stage 1 AKI group (n = 51) vs. stage 2 or 3 group (n = 16) of SFTS patients during hospitalization. (A) The longitudinal changes in sCr between the two groups. (B) The longitudinal changes in BUN between the two groups. (C) The longitudinal changes in Cys-C between the two groups. (D) The longitudinal changes in β2-MG between the two groups. (E) The longitudinal changes in WBC count between the two groups. (F) The longitudinal changes in PLT count between the two groups. (G) The longitudinal changes in ALT between the two groups. (H) The longitudinal changes in AST between the two groups. (I) The longitudinal changes in albumin between the two groups. (J) The longitudinal changes in TBIL between the two groups. (K) The longitudinal changes in ALP between the two groups. (L) The longitudinal changes in GGT between the two groups. (M) The longitudinal changes in LDH between the two groups. (N) The longitudinal changes in AMY between the two groups. (O) The longitudinal changes in lipase between the two groups. (P) The longitudinal changes in CKMB between the two groups. (Q) The longitudinal changes in TnI between the two groups. (R) The longitudinal changes in TT between the two groups. (S) The longitudinal changes in D-dimer between the two groups. (T) The longitudinal changes in CRP between the two groups. (U) The longitudinal changes in PCT between the two groups. (V) The longitudinal changes in IL-6 between the two groups. All data are presented as medians with interquartile ranges (P25–P75). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Normal-upper: the upper limit of the normal value range. Normal-lower: the lower limit of the normal value range.
Discussion
AKI is a clinical syndrome caused by a sudden decrease in renal excretory function, with the accumulation of nitrogen metabolism waste such as creatinine and urea. Other common clinical and laboratory presentations include reduced urine output, resulting in the accumulation of metabolic acids, potassium and phosphate. It is a frequent complication in hospital patients and very common in critically ill patients (Bellomo et al., 2012; Gonsalez et al., 2019). AKI in patients with SFTS was also observed in previous studies (Cui et al., 2015; Jia et al., 2017; Wang et al., 2019; He et al., 2021), yet with very little information on its incidence, clinical characteristics and prognosis.
To our knowledge, this is the first report of the clinical features and outcomes of AKI in SFTS. In the present study, we demonstrated the incidence of AKI in 26.4% of hospitalized SFTS patients. We also revealed that the patients with AKI showed higher decreased levels of albumin and fibrinogen, and elevated levels of AST, TBIL, ALP, LDH, BUN, uric acid, sCr, AMY, lipase, CKMB, TnI, APTT, TT and D-dimer than patients without AKI during hospitalization. Spearman correlation analysis also showed that sCr was closely correlated with these laboratory parameters. They are generally associated with fatal outcomes, and are commonly used to reflect the heart, liver, pancreas, kidney and coagulation functions, and their extremely abnormal levels reveal severe organ injury and dysfunction, which usually occur in critically ill patients, who should receive more attention, specifically by developing new therapeutic and prevention strategies to be used in clinical practice. Furthermore, we revealed that nonspecific symptoms including abdominal pain, diarrhea, encephalopathy and hepatosplenomegaly were more frequent in patients with AKI. Some studies have reported that encephalopathy and hepatosplenomegaly are independent predictors of mortality in patients with SFTS (Liu et al., 2013; Gong et al., 2021). It revealed that AKI was often linked with other severe complications, leading to poor prognosis.
A number of studies have revealed that a high viral load is generally associated with poor outcome. Which can be used to predict the mortality of patients with SFTS (Liu et al., 2016; Yang et al., 2016; Jo et al., 2022; Wang et al., 2022). We also found that the viral load was an independent risk factor for the prognosis of patients with SFTS. In the present study, the viral load was significantly higher in the AKI group than in the non-AKI group. At least in part, it could explain why patients in the AKI group have a higher mortality rate. Although the mechanisms of AKI in patients with SFTS are unclear, some studies reported that the SFTS virus was detected in the kidneys (Chen et al., 2012; Jin et al., 2012). Recently, a report showed that SFTS viral load in urine is an independent predictor of the incidence of AKI (Zhang et al., 2023). It implies that the SFTS virus may directly induce renal damage. Moreover, a large number of studies have shown that the SFTS virus can substantially produce inflammatory cytokines and chemokines, leading to cytokine storms (Khalil et al., 2021; Wang et al., 2021; Gao et al., 2022). It can promote kidney vascular permeability, and even microcirculatory dysfunction, and it can induce thrombosis and capillary leak syndrome, resulting in disseminated intravascular coagulation. In addition, these high serum levels of cytokines can directly cause the death of renal endothelial and tubular epithelial cells (Ahmadian et al., 2021; Legrand et al., 2021).
In the present study, we used the less sensitive sCr-based criteria to identify AKI. Although inulin clearance and radionuclide renal imaging are more sensitive, they are inconvenient and costly. Nonetheless, sCr measurement is still the most practical and widely accepted method for evaluating renal function in patients with AKI at present (Moledina et al., 2017; Moledina and Parikh, 2018). Some biomarkers of AKI including Cys-C and β2-MG were also evaluated in patients with SFTS. Cys-C is an elegant indicator of glomerular filtration rate because it is not affected by factors such as muscle mass, diet, drugs, sex and tubular secretion, and it is a reliable parameter for evaluating and predicting AKI in critically ill patients (Mårtensson et al., 2012; Luft, 2021). β2-MG has also traditionally been used to evaluate renal tubular function, and elevated β2-MG is typically accompanied by kidney injury (Bagshaw et al., 2007). Our results also showed that the serum levels of Cys-C and β2-MG in the AKI group were significantly higher than those in the non-AKI group.
Importantly, our findings confirmed that stage 2 or 3 AKI was a strong independent risk factor for the prognosis in patients with SFTS, and patients at AKI stage 2 or 3 showed a significantly higher in-hospital mortality than patients without AKI or at AKI stage 1. Some previous studies of other diseases also had yielded similar results. A large observational study demonstrated that the mortality of hospitalized patients at AKI stage 2 or 3 was approximately 2 or 4 times higher than that of patients at AKI stage 1, respectively (Khadzhynov et al., 2019). A retrospective analysis of the cohort of patients from the North American Consortium for the Study of End-Stage Liver Disease study on the complications of patients with decompensated cirrhosis revealed that patients at AKI stage 2 or 3 had higher model for end-stage liver disease scores, presence of systemic inflammatory response syndrome and second infections, resulting in a lower survival rate (Wong et al., 2021). Moreover, we compared the longitudinal changes in laboratory parameters of patients in the non-AKI, stage 1, 2 and 3 AKI groups. It revealed that laboratory indicators reflecting organ injury and inflammation returned to the normal range in patients without AKI or at AKI stage 1, but these parameters were aggravated continuously in patients at AKI stage 2 or 3 during hospitalization. These results could explain the higher mortality of patients at AKI stage 2 or 3.
Limitations of our study include the observational and retrospective study design. Additionally, the sample size of this study was relatively small. Therefore, the subsequent analysis of risk factors for AKI development in SFTS was not conducted.
At the same time, due to the limited laboratory resources in our hospital, some other AKI biomarkers including neutrophil gelatinase-associated lipocalin, kidney injury molecule-1 and interleukin-18 could not be measured. Finally, because this was a single-center study, the observations made here could not be extrapolated to other centers.
Conclusion
In conclusion, we found a high incidence of AKI in patients with SFTS, and AKI was associated with poor prognosis. Most importantly, we revealed that patients at AKI stage 2 or 3 had higher mortality. These results provide strong evidence to emphasize that patients with SFTS should be carefully monitored for the development of AKI and corresponding measures should be taken to prevent it, and further explorations are required to identify the risk factors for the development of AKI.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Zhongnan Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the written informed consent was waived due to the nature of the retrospective study and pandemic nature of the disease.
Author contributions
ZZ and XH were involved in patient data analysis and wrote the manuscript drafting. QJ and WH were mainly responsible for the data collection. AL, LD, and YX were responsible for the study design and critical revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Key Research and Development Program of Hubei Province, China (2020BCB025).
Acknowledgments
The authors wish to thank the patient for participating in this study and all the staff members at our institution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1236091/full#supplementary-material
References
Ahmadian, E., Hosseiniyan Khatibi, S. M., Razi Soofiyani, S., Abediazar, S., Shoja, M. M., Ardalan, M., et al. (2021). Covid-19 and kidney injury: pathophysiology and molecular mechanisms. Rev. Med. Virol. 31:e2176. doi: 10.1002/rmv.2176
Bagshaw, S. M., Langenberg, C., Haase, M., Wan, L., May, C. N., and Bellomo, R. (2007). Urinary biomarkers in septic acute kidney injury. Intensive Care Med. 33, 1285–1296. doi: 10.1007/s00134-007-0656-5
Bao, C. J., Guo, X. L., Qi, X., Hu, J. L., Zhou, M. H., Varma, J. K., et al. (2011). A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin. Infect. Dis. 53, 1208–1214. doi: 10.1093/cid/cir732
Bellomo, R., Kellum, J. A., and Ronco, C. (2012). Acute kidney injury. Lancet 380, 756–766. doi: 10.1016/S0140-6736(11)61454-2
Chen, X., Cong, M., Li, M., Kang, Y., Feng, Y., Plyusnin, A., et al. (2012). Infection and pathogenesis of Huaiyangshan virus (a novel tick-borne bunyavirus) in laboratory rodents. J. Gen. Virol. 93, 1288–1293. doi: 10.1099/vir.0.041053-0
Cui, N., Liu, R., Lu, Q., Wang, L., Qin, S., Yang, Z., et al. (2015). Severe fever with thrombocytopenia syndrome bunyavirus-related human encephalitis. J. Infect. 70, 52–59. doi: 10.1016/j.jinf.2014.08.001
Gao, C., Yu, Y., Wen, C., Li, Z., Ding, H., Qi, X., et al. (2022). Nonstructural protein NSs activates inflammasome and pyroptosis through interaction with NLRP3 in human microglial cells infected with severe fever with thrombocytopenia syndrome bandavirus. J. Virol. 96:e0016722. doi: 10.1128/jvi.00167-22
Gong, L., Zhang, L., Wu, J., Lu, S., Lyu, Y., Zhu, M., et al. (2021). Clinical progress and risk factors for death from severe fever with thrombocytopenia syndrome: a multihospital retrospective investigation in Anhui, China. Am. J. Trop. Med. Hyg. 104, 1425–1431. doi: 10.4269/ajtmh.20-0270
Gonsalez, S. R., Cortês, A. L., Silva, R. C. D., Lowe, J., Prieto, M. C., and Silva Lara, L. D. (2019). Acute kidney injury overview: from basic findings to new prevention and therapy strategies. Pharmacol. Ther. 200, 1–12. doi: 10.1016/j.pharmthera.2019.04.001
He, F., Zheng, X., and Zhang, Z. (2021). Clinical features of severe fever with thrombocytopenia syndrome and analysis of risk factors for mortality. BMC Infect. Dis. 21:1253. doi: 10.1186/s12879-021-06946-3
Jia, B., Yan, X., Chen, Y., Wang, G., Liu, Y., Xu, B., et al. (2017). A scoring model for predicting prognosis of patients with severe fever with thrombocytopenia syndrome. PLoS Neglect. Trop. Dis. 11:e5909. doi: 10.1371/journal.pntd.0005909
Jin, C., Liang, M., Ning, J., Gu, W., Jiang, H., Wu, W., et al. (2012). Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc. Natl. Acad. Sci. U.S.A. 109, 10053–10058. doi: 10.1073/pnas.1120246109
Jo, H., Kim, J., Hwang, S., Seo, J., Kim, D. Y., Yun, N., et al. (2022). Viral load as a factor affecting the fatality of patients suffering from severe fever with thrombocytopenia syndrome. Viruses 14:881. doi: 10.3390/v14050881
Khadzhynov, D., Schmidt, D., Hardt, J., Rauch, G., Gocke, P., Eckardt, K. U., et al. (2019). The incidence of acute kidney injury and associated hospital mortality. Dtsch. Arztebl. Int. 116, 397–404. doi: 10.3238/arztebl.2019.0397
Khalil, J., Yamada, S., Tsukamoto, Y., Abe, H., Shimojima, M., Kato, H., et al. (2021). The nonstructural protein NSs of severe fever with thrombocytopenia syndrome virus causes a cytokine storm through the hyperactivation of NF-κb. Mol. Cell. Biol. 41:e0054220. doi: 10.1128/MCB.00542-20
Legrand, M., Bell, S., Forni, L., Joannidis, M., Koyner, J. L., Liu, K., et al. (2021). Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 17, 751–764. doi: 10.1038/s41581-021-00452-0
Li, S., Li, Y., Wang, Q., Yu, X., Liu, M., Xie, H., et al. (2018). Multiple organ involvement in severe fever with thrombocytopenia syndrome: an immunohistochemical finding in a fatal case. Virol. J. 15:97. doi: 10.1186/s12985-018-1006-7
Liu, S., Chai, C., Wang, C., Amer, S., Lv, H., He, H., et al. (2014). Systematic review of severe fever with thrombocytopenia syndrome: virology, epidemiology, and clinical characteristics. Rev. Med. Virol. 24, 90–102. doi: 10.1002/rmv.1776
Liu, Q., He, B., Huang, S. Y., Wei, F., and Zhu, X. Q. (2014). Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 14, 763–772. doi: 10.1016/S1473-3099(14)70718-2
Liu, M., Lei, X., and Yu, X. (2016). Meta-analysis of the clinical and laboratory parameters of SFTS patients in China. Virol. J. 13:198. doi: 10.1186/s12985-016-0661-9
Liu, W., Lu, Q., Zhuang, L. U., Hu, C., Yuan, C., And Fan, X., et al. (2013). Case-fatality ratio and effectiveness of ribavirin therapy among hospitalized patients in China who had severe fever with thrombocytopenia syndrome. Clin. Infect. Dis. 57, 1292–1299. doi: 10.1093/cid/cit530
Luft, F. C. (2021). Biomarkers and predicting acute kidney injury. Acta Physiol. 231:e13479. doi: 10.1111/apha.13479
Mårtensson, J., Martling, C. R., and Bell, M. (2012). Novel biomarkers of acute kidney injury and failure: clinical applicability. Brit. J. Anaesth. 109, 843–850. doi: 10.1093/bja/aes357
Moledina, D. G., Hall, I. E., Thiessen-Philbrook, H., Reese, P. P., Weng, F. L., Schröppel, B., et al. (2017). Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am. J. Kidney Dis. 70, 807–816. doi: 10.1053/j.ajkd.2017.06.031
Moledina, D. G., and Parikh, C. R. (2018). Phenotyping of acute kidney injury: beyond serum creatinine. Semin. Nephrol. 38, 3–11. doi: 10.1016/j.semnephrol.2017.09.002
Nie, Q., Wang, D., Ning, Z., Li, T., Tian, X., Bian, P., et al. (2020). Analysis of severe fever with thrombocytopenia syndrome in critical ill patients in central China. Shock 54, 451–457. doi: 10.1097/SHK.0000000000001527
Palevsky, P. M., Liu, K. D., Brophy, P. D., Chawla, L. S., Parikh, C. R., Thakar, C. V., et al. (2013). KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 61, 649–672. doi: 10.1053/j.ajkd.2013.02.349
Park, E., Shimojima, M., Nagata, N., Ami, Y., Yoshikawa, T., Iwata-Yoshikawa, N., et al. (2019). Severe fever with thrombocytopenia syndrome phlebovirus causes lethal viral hemorrhagic fever in cats. Sci. Rep. 9:11990. doi: 10.1038/s41598-019-48317-8
Wang, Y., Han, S., Ran, R., Li, A., Liu, H., Liu, M., et al. (2021). A longitudinal sampling study of transcriptomic and epigenetic profiles in patients with thrombocytopenia syndrome. Nat. Commun. 12:5629. doi: 10.1038/s41467-021-25804-z
Wang, Y., Song, Z., Wei, X., Yuan, H., Xu, X., Liang, H., et al. (2022). Clinical laboratory parameters and fatality of severe fever with thrombocytopenia syndrome patients: a systematic review and meta-analysis. PLoS Neglect. Trop. Dis. 16:e10489. doi: 10.1371/journal.pntd.0010489
Wang, L., Wan, G., Shen, Y., Zhao, Z., Lin, L., Zhang, W., et al. (2019). A nomogram to predict mortality in patients with severe fever with thrombocytopenia syndrome at the early stage—a multicenter study in China. PLoS Neglect. Trop. Dis. 13:e7829. doi: 10.1371/journal.pntd.0007829
Wen, H., Zhao, L., Zhai, S., Chi, Y., Cui, F., Wang, D., et al. (2014). Severe fever with thrombocytopenia syndrome, Shandong province, China, 2011. Emerg. Infect. Dis. 20, 1–5. doi: 10.3201/eid2001.120532
Wong, F., Reddy, K. R., Tandon, P., O'Leary, J. G., Garcia-Tsao, G., Vargas, H. E., et al. (2021). Progression of stage 2 and 3 acute kidney injury in patients with decompensated cirrhosis and ascites. Clin. Gastroenterol. Hepatol. 19, 1661–1669. doi: 10.1016/j.cgh.2020.08.025
Xu, X., Sun, Z., Liu, J., Zhang, J., Liu, T., Mu, X., et al. (2018). Analysis of clinical features and early warning indicators of death from severe fever with thrombocytopenia syndrome. Int. J. Infect. Dis. 73, 43–48. doi: 10.1016/j.ijid.2018.05.013
Yamanaka, A., Kirino, Y., Fujimoto, S., Ueda, N., Himeji, D., Miura, M., et al. (2020). Direct transmission of severe fever with thrombocytopenia syndrome virus from domestic cat to veterinary personnel. Emerg. Infect. Dis. 26, 2994–2998. doi: 10.3201/eid2612.191513
Yang, Z., Hu, J., Lu, Q., Guo, C., Cui, N., Peng, W., et al. (2016). The prospective evaluation of viral loads in patients with severe fever with thrombocytopenia syndrome. J. Clin. Virol. 78, 123–128. doi: 10.1016/j.jcv.2016.03.017
Yu, X. J., Liang, M. F., Zhang, S. Y., Liu, Y., Li, J. D., Sun, Y. L., et al. (2011). Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364, 1523–1532. doi: 10.1056/NEJMoa1010095
Zhang, Y., He, Y., Dai, Y., Xiong, Y., Zheng, H., Zhou, D., et al. (2012). Hemorrhagic fever caused by a novel bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin. Infect. Dis. 54, 527–533. doi: 10.1093/cid/cir804
Zhang, Q., Zhao, J., Dai, Y., Jiang, Z., Chen, T., Hu, N., et al. (2023). A high viral load in urine correlates with acute kidney injury and poor outcomes in hospitalized patients with severe fever with thrombocytopenia syndrome: a noninvasive and convenient prognostic marker. Open Forum Infect. Dis. 10:ofad085. doi: 10.1093/ofid/ofad085
Glossary
Keywords: severe fever with thrombocytopenia syndrome, acute kidney injury, clinical characteristics, risk factor, mortality
Citation: Zhang Z, Hu X, Jiang Q, Hu W, Li A, Deng L and Xiong Y (2023) Clinical characteristics and outcomes of acute kidney injury in patients with severe fever with thrombocytopenia syndrome. Front. Microbiol. 14:1236091. doi: 10.3389/fmicb.2023.1236091
Edited by:
Lei Deng, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Masaki Yasukawa, Ehime Prefectural University of Health Sciences, JapanHao Li, Beijing Institute of Microbiology and Epidemiology, China
Copyright © 2023 Zhang, Hu, Jiang, Hu, Li, Deng and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anling Li, YW5saW5nODg4QDEyNi5jb20=; Liping Deng, ZGVuZ2xpcGluZ0B3aHUuZWR1LmNu; Yong Xiong, eW9uZ3hpb25nNjRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhongwei Zhang
Zhongwei Zhang Xue Hu2†
Xue Hu2† Anling Li
Anling Li Yong Xiong
Yong Xiong