
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 22 August 2023
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1226981
This article is part of the Research Topic Synergistic Effects of Antimicrobial Peptides in Overcoming Resistance View all 5 articles
Polymyxin B has been used as a last-line therapy for the treatment of carbapenem-resistant gram-negative bacterial infection. The pharmacokinetic/pharmacodynamic index (AUC/MIC) of polymyxin B has not been clinically evaluated, given that the broth microdilution method for polymyxin susceptibility testing is rarely used in hospitals. This study analyzed data from 77 patients with carbapenem-resistant Klebsiella pneumoniae infections. Among the samples, 63 K. pneumoniae isolates had MIC values of 1.0 mg/L as measured by broth microdilution but 0.5 mg/L as measured using the Vitek 2 system. Polymyxin B AUC/MIC was significantly associated with clinical response (p = 0.002) but not with 30-day all-cause mortality (p = 0.054). With a target AUC/MIC value of 50, Monte Carlo simulations showed that a fixed dose of 100 mg/12 h and three weight-based regimens (1.25 mg/kg/12 h for 80 kg and 1.5 mg/kg/12 h for 70 kg/80 kg) achieved a cumulative fraction of response >90% regardless of renal function, but the risk of nephrotoxicity was high. For patients with carbapenem-resistant K. pneumoniae infections, the underestimation of polymyxin resistance in automated systems need to be taken into account when optimizing polymyxin B dosing based on pharmacokinetic/pharmacodynamic principles.
Infections caused by antimicrobial resistance isolates are a major threat to public health (Algammal et al., 2023). Carbapenem-resistant gram-negative bacteria (CR-GNB) are the main contributors to infectious diseases caused by multidrug-resistant bacteria (Karampatakis et al., 2023). Polymyxins (i.e., colistin and polymyxin B) have been used as alternatives for the treatment of infections caused by CR-GNB. Unfortunately, these drugs have wide inter-individual variability in their pharmacokinetics (PK) and a narrow therapeutic index (Nang et al., 2021).
Recent studies have revealed that the ratio of the free-drug area under the concentration–time curve to the minimum inhibitory concentration (fAUC/MIC) is the most predictive PK/pharmacodynamics (PK/PD) index for polymyxin B (Tsuji et al., 2019). In our previous study, we demonstrated that an AUCss,24h threshold of 50–100 mg·h/L was a good predictor of clinical response and acute kidney injury in a real-world cohort of patients treated with polymyxin B for CR-GNB infections. However, MIC values were not available because an automated system was used (Yang et al., 2022).
Broth microdilution (BMD) assay is a reference method recommended by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for antimicrobial susceptibility testing (AST) of polymyxins (Satlin et al., 2020), but automated systems are more commonly used in clinical laboratories. At our hospital, the MICs of all included isolates were ≤0.5 mg/L (Yang et al., 2022). Considering that automated systems may underestimate MIC for polymyxins (Pfennigwerth et al., 2019; Zhu et al., 2021), this study aimed to measure the MIC of polymyxins via BMD, in order to evaluate the correlation between AUC/MIC ratio in polymyxin B and clinical outcome in patients with Carbapenem-resistant Klebsiella pneumoniae (CRKP) infections, and to explore optimal dosing regimens using Monte Carlo simulations based on PK/PD target.
Data were derived from a previous retrospective study conducted at the First Affiliated Hospital of Zhengzhou University from April 2018 to March 2022 (Yang et al., 2022). This study was approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University (No. 2020-KY-0318) and was registered with the Chinese Clinical Trial Register (No. ChiCTR2100043208).
Patients with CRPK infection whose strains were collected before polymyxin B treatment were included in this study. The primary endpoints were clinical response and 30-day all-cause mortality (Yang et al., 2022). Clinical response was considered at the end of treatment by two physicians: disappearance or improvement of clinical symptoms (body temperature < 38.0°C), radiological resolution of signs of infection, and improved biochemical indicators of infection (≥30% decrease in total peripheral white blood cell count or C-reactive protein level). Patients who did not meet all the above criteria were classified as cases of clinical failure.
A total of 63 bronchoalveolar lavage fluid samples, 6 blood samples, 4 hydrothorax and as cite samples, 2 cerebrospinal fluid samples, and 2 skin tissue pus samples were collected. Species identification and AST were performed using a Vitek® MS MALDI-TOF system (bioMérieux, Marcy-l’Etoile, France) and a Vitek® 2 COMPACT automated system with Vitek® 2 AST cards (0.5–16 mg/L of colistin), respectively. Polymyxin B reference MICs were performed retrospectively from frozen isolates on BMD panels (Wenzhou Kangtai Biotechnology Co., LTD, China). Briefly, 2-fold dilutions ranging from 0.25 to 32 mg/L of polymyxin B were prepared in 96-well plates, using a final inoculum of 5 × 105 cfu/mL of each isolate in sterile water. Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as susceptible controls. CRKP was defined as cases in which K. pneumoniae was non-susceptible to at least one carbapenem antibiotic (CLSI, 2018). Carbapenem resistance was defined as an MIC breakpoint of ≥4 mg/L for meropenem/imipenem/doripenem or ≥2 mg/L for ertapenem (CLSI, 2020). Polymyxin breakpoints of susceptibility ≤2 mg/L/resistance >2 mg/L were applied based on United States Committee on Antimicrobial Susceptibility Testing (USCAST) criteria (CLSI, 2015).
Rates of essential agreement (EA), category agreement (CA), very major error (VME), and major error (ME) were estimated using BMD as the reference method. According to CLSI recommendations, a method must exhibit CA ≥ 90%, EA ≥ 90%, VME < 3%, and ME <3% in order to be considered acceptable (Pogue et al., 2020).
As for the PK study, two blood samples were collected before infusion (C0h) and 2 h after the start of infusion (C2h), at least 3 days after polymyxin B treatment. Plasma concentrations were determined using a validated ultra-performance liquid chromatography–tandem mass spectrometry method previously published by our laboratory (Wang et al., 2020a).
AUCss,24h was estimated using the Bayesian priors of our previously published population PK model using the Phoenix® NLME software package (v8.3, Pharsight, Mountain View, CA, United States) (Wang et al., 2020b). Monte Carlo simulations with 1,000 subjects were performed on fixed and weight-based regimens based on the population PK model. Loading dose was twice the maintenance dose, and infusion time was 1 h. The trapezoidal rule was used to calculate the AUC across 24 h.
Using an AUC/MIC of 50 as the PK/PD target, a probability of target attainment (PTA) value >90% was considered to represent effectiveness. The cumulative fraction of response (CFR) was the sum of the isolates’ frequency for each MIC multiplied by the PTA, and a value of 90% was considered to represent effectiveness. In addition, the probability (%) of achieving the target AUC (50–100 mg·h/L) was calculated, and an AUC of >100 mg·h/L was taken as a predictor of nephrotoxicity, according to international consensus guidelines (Tsuji et al., 2019).
Statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 (SPSS Inc., Chicago, IL, United States). Continuous variables are presented in the form of median (interquartile range, IQR) and were analyzed using the Mann–Whitney U test. Categorical variables are presented in the form of percentage/frequency (%) and were analyzed using the Chi-square test or Fisher’s exact test. Receiver operating characteristic (ROC) curves was used to explore the relationship between PK/PD index and outcome. A p value < 0.05 was considered statistically significant.
A total of 77 patients were included for analysis (Table 1). All isolates were susceptible to polymyxin B. The MIC by BMD was 1.0 mg/L for 63 isolates, 0.5 mg/L for 10, 2.0 mg/L for three, and 0.25 mg/L for one. The automated AST system showed acceptable levels of CA (100%), EA (96.1%), ME (0%), and VME (0%). In addition, results on the susceptibility of CRKP isolates to antimicrobials are shown in Supplementary Table S1.
At the end of polymyxin B treatment, a clinical response was observed in 49 cases (63.6%). Patients with clinical failure had a lower median AUC/MIC than those who exhibited a clinical response (p = 0.002; Figure 1A). Among all patients, 30-day all-cause mortality was 37.7% (29/77), with and the median survival time among patients who did not survive was 9 days (IQR 5-17.5). No significant difference in AUC/MIC between survivors and non-survivors was observed (p = 0.054; Figure 1A).
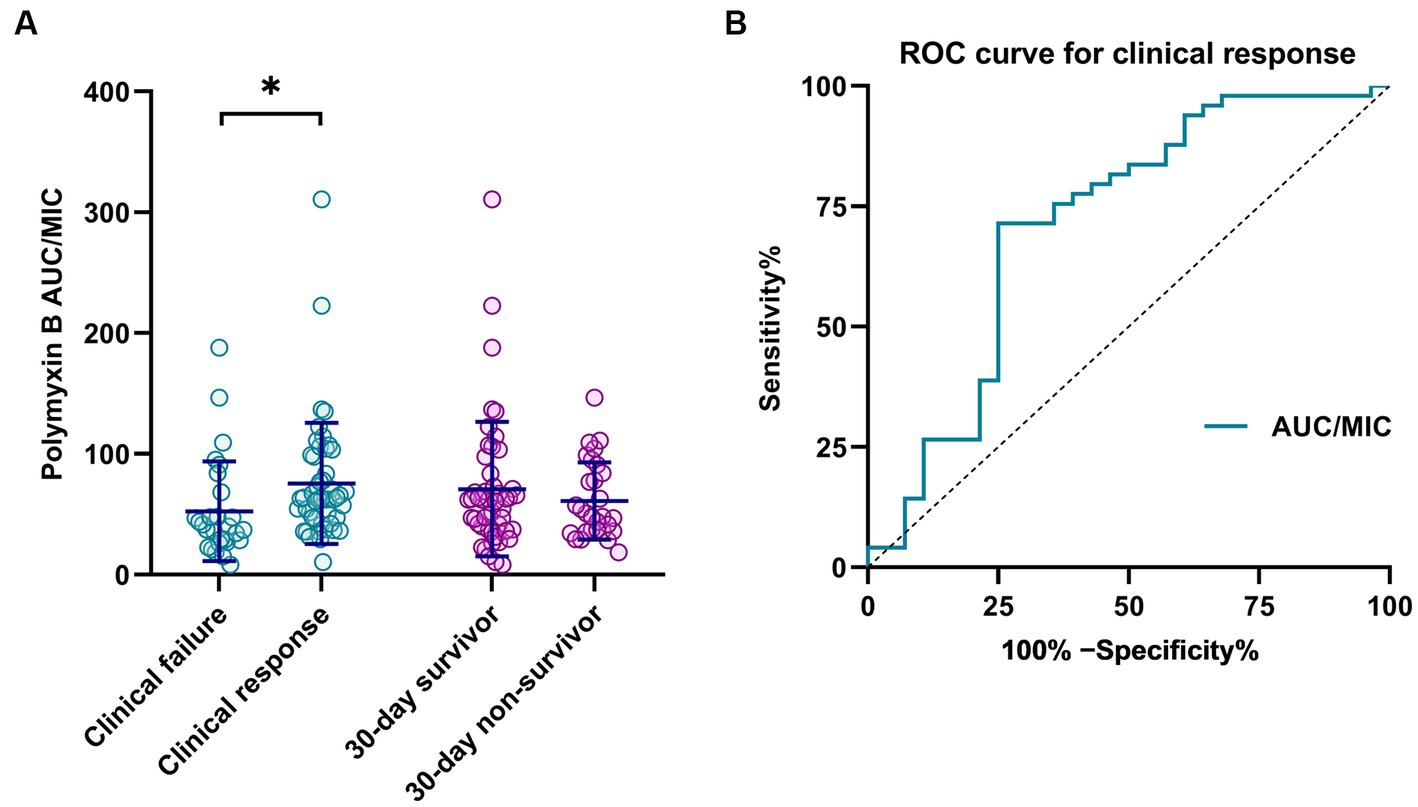
Figure 1. (A) Correlation of polymyxin B clinical outcome with PK/PD parameters (AUC/MIC). *p < 0.05. (B) ROC curve for polymyxin B PK/PD as a predictor of clinical response.
The area under the ROC curve for AUC/MIC as a predictor of clinical response was 0.714 (95% CI 0.583–0.844; p = 0.002; Figure 1B). The optimal cut-off, at the maximum Youden index (0.464), corresponded to an AUC/MIC value of 49.3, with predictive sensitivity and specificity of 71.4 and 73.5%, respectively.
In addition, several potential risk factors that may have affected clinical outcomes were evaluated (Table 2). Platelets, occurrence of septic shock, mechanical ventilation, and AUC were independently associated with clinical response and mortality (p < 0.05). AUC/MIC and AUC/MIC ≥50 only had significant association with clinical response (p < 0.05). Furthermore, sample type had no significant association with clinical outcome (Supplementary Table S2).
Taking AUC/MIC ≥ 50 as the PK/PD target, PTAs for various regimens against MIC distribution are shown in Figure 2 and Supplementary Figure S1. Additionally, CFR, the probability of target AUC (50–100 mg·h/L), and nephrotoxicity (AUC > 100 mg·h/L) are shown in Table 3 and Supplementary Table S3.
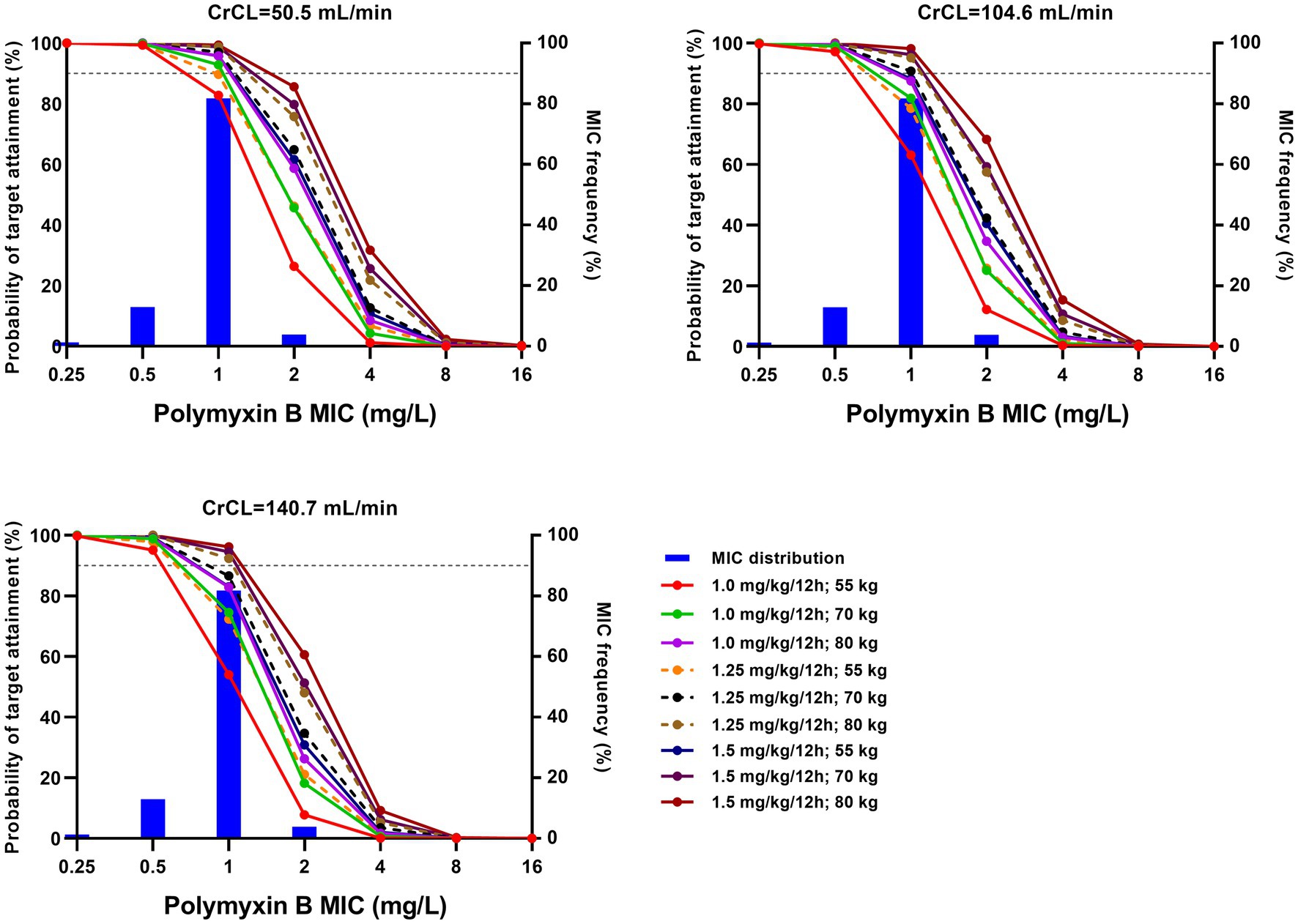
Figure 2. Probability of target attainment (PTA) for various regimens with different creatinine clearance (CrCL). The target was an area under the curve/minimum inhibitory concentration (AUC/MIC) ≥ 50. Histograms represent the frequency distribution as established by broth microdilution. Horizontal dotted lines represent 90% PTA.
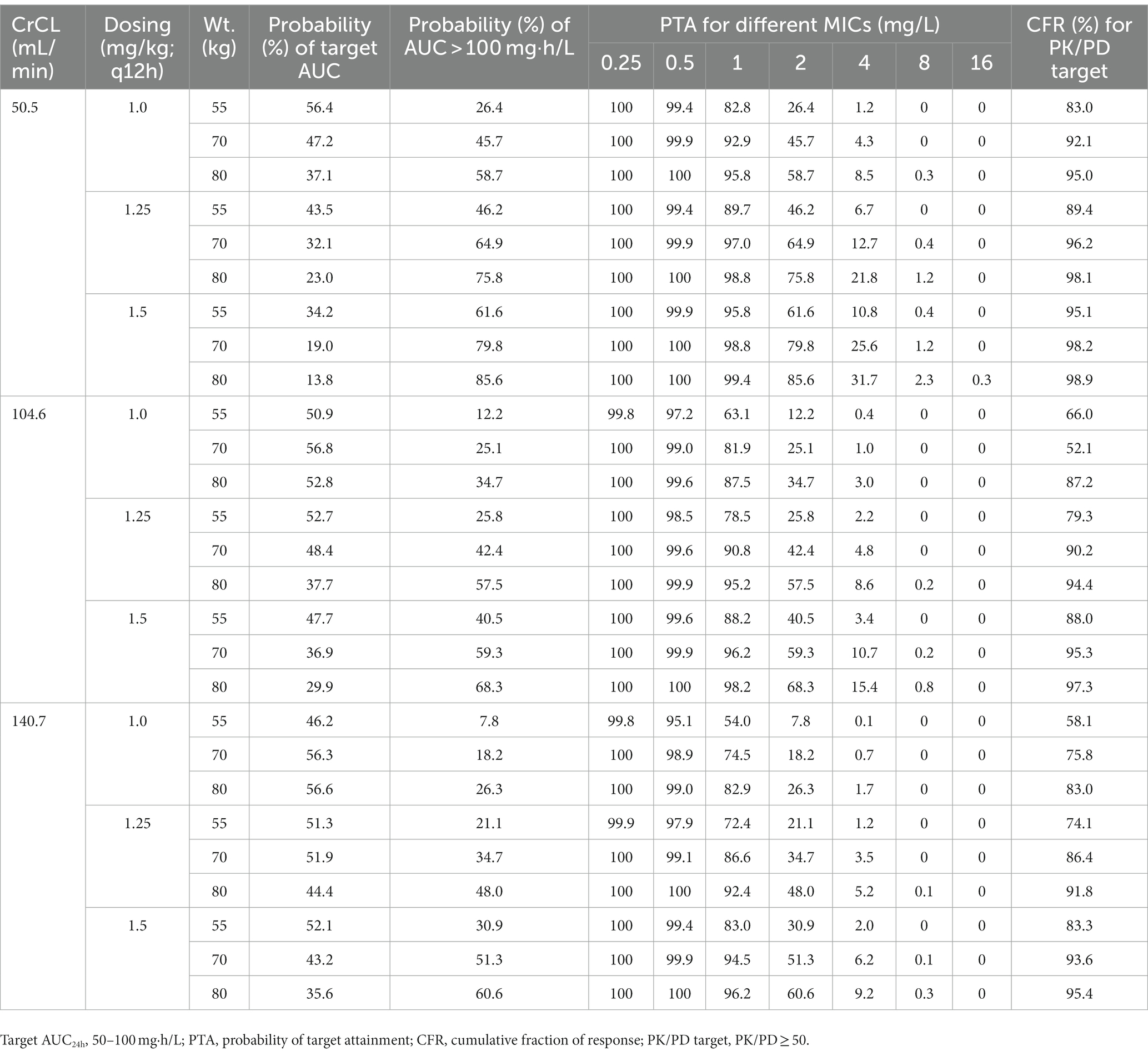
Table 3. Probability of achievement of target AUC, PTA, and CFR for different polymyxin B regimens according to the 10th, 50th, and 90th percentiles of creatinine clearance (CrCL) and weight (Wt.).
As manual preparation of BMD plates is extremely labor intensive, these have been almost entirely replaced by gradient strips or automated systems in clinical laboratories (Pfennigwerth et al., 2019). In this study, most (81.8%) polymyxin MICs of CRKP isolates tested using the Vitek 2 system were one-fold lower dilutions than BMD, which is consistent with other reports (Pfennigwerth et al., 2019; Zhu et al., 2021). Polymyxin B (CID: 9833652) and colistin (CID: 5311054) are lipopeptide components with large-molecule and amphiphilic properties [National Center for Biotechnology Information (NCBI), 2023]. These physicochemical characteristics can affect the accuracy of polymyxin MIC values measured via disk diffusion or using an automated system (Nang et al., 2021). Therefore, it is necessary to note that automated systems may underestimate MIC values when optimizing polymyxin B dose based on the PK/PD principle.
The current study showed that an AUC/MIC rate of >49.3 was significantly associated with clinical response, which was in line with the PK/PD index derived from murine infection models (Lakota et al., 2018) and the target AUC reported in our previous study (Yang et al., 2022). This may be because polymyxin MICs were mostly (81.8%) 1.0 mg/L. Nevertheless, the univariate analysis showed that AUC/MIC ≥50 had no correlation with mortality (p > 0.05). This can be attributed to the fact that patients with CRKP infections suffered from serious underlying diseases and were in poor physical condition, both of which are likely to have affected their clinical outcomes (Liu et al., 2023).
With a target AUC/MIC value of 50, Monte Carlo simulations showed that high dosage with low creatinine clearance (CrCL) resulted in a high PTA (Figure 2; Supplementary Figure S1). All regimens achieved >90% PTA at MICs ≤0.5 mg/L, and no regimen achieved >90% PTA at MICs ≥2.0 mg/L, which was in agreement with previous reports (Miglis et al., 2018; Xie et al., 2020; Wang et al., 2022; Yu et al., 2022). For MICs of 1.0 mg/L, a fixed regimen (100 mg/12 h) and three weight-based regimens (1.25 mg/kg for 80 kg/12 h, 1.5 mg/kg for 70 kg/12 h and 80 kg/12 h) achieved >90% PTA.
Given that accurate MIC values for polymyxins are not always available in clinical practice, CFR may be more useful than PTA for empirical dosing. As shown in Table 3 and Supplementary Table S3, only the above four regimens achieved a CFR > 90% against CRKP, regardless of renal function. However, these regimens also led to a high probability of AUC > 100 mg·h/L (at least 44.7%). Based on a population PK model of healthy Chinese subjects, Bian et al. suggested that polymyxin B dosing regimens of 1.0–1.5 mg/kg/12 h are appropriate for K. pneumoniae (Bian et al., 2021); however, this study did not consider toxic exposure and the differences between healthy subjects and patients in terms of PK characteristics.
The present study has several limitations. First, given that this was a single-center retrospective study with a small sample size, the power of the estimated PK/PD index in predicting clinical outcomes was limited, and risk factors for poor clinical outcome (such as underlying diseases, severity of illness, use of combination therapy, and variability in PK parameters and MICs) were not investigated. Second, only total-drug AUC was estimated in this study, and therefore the impact of free drug concentrations on PK/PD metrics needs to be further investigated. Third, K. pneumoniae isolates present resistance to antimicrobial agents via one or more mechanisms, including production of specified enzymes, decreased cell permeability through loss of OMPs, overexpression of efflux pumps, and modification of the target of the antimicrobial agent (Ahmadi et al., 2021, 2022; Karampatakis et al., 2023). Resistance gene testing can reveal the resistance characteristics and transmission trends of CRKP strains in the relevant region and assist in antibiotic treatment; this approach requires further in-depth research.
In conclusion, comparison of BMD and the Vitek 2 system indicated that the polymyxin MICs of CRKP might be underestimated by a one-fold level of dilution by the Vitek 2 system in our hospital. With a target AUC/MIC value of 50, empirical dosages of 100 mg/12 h, 1.25 mg/kg/12 h at 80 kg, or 1.5 mg/kg/12 h at both 70 kg and 80 kg could achieve effective therapeutic outcomes, but efficacy needs to be balanced against the potential for nephrotoxicity.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University. The ethics committee waived the requirement of written informed consent for participation.
PW and SL contributed to the data acquisition, analysis, and interpretation. PW contributed to manuscript preparation. GQ performed the experiments. JY supervised the research and revised the manuscript. MX and TS designed the study. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key R&D Program of China (Grant No. 2020YFC2008304) and the Educational Committee of Henan Province (23A350006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1226981/full#supplementary-material
Ahmadi, Z., Noormohammadi, Z., Behzadi, P., and Ranjbar, R. (2022). Molecular detection of gyrA mutation in clinical strains of Klebsiella pneumoniae. Iran. J. Public Health 51, 2334–2339. doi: 10.18502/ijph.v51i10.10992
Ahmadi, M., Ranjbar, R., Behzadi, P., and Mohammadian, T. (2021). Virulence factors, antibiotic resistance patterns, and molecular types of clinical isolates of Klebsiella Pneumoniae. Expert Rev. Anti-Infect. Ther. 20, 463–472. doi: 10.1080/14787210.2022.1990040
Algammal, A., Hetta, H. F., Mabrok, M., and Behzadi, P. (2023). Editorial: Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front. Microbiol. 14:1135614. doi: 10.3389/fmicb.2023.1135614
Bian, X., Liu, X., Hu, F., Feng, M., Chen, Y., Bergen, P. J., et al. (2021). Pharmacokinetic/pharmacodynamic based breakpoints of polymyxin B for bloodstream infections caused by multidrug-resistant Gram-negative pathogens. Front. Pharmacol. 12:785893. doi: 10.3389/fphar.2021.785893
CLSI (2015). Verification of commercial microbial identification and antimicrobial susceptibility testing systems. 1st Edn Available at: https://clsi.org/standards/products/microbiology/documents/m52/.
CLSI (2018). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th Edn Available at: https://clsi.org/standards/products/microbiology/documents/m07/.
CLSI (2020). Performance Standards for Antimicrobial Susceptibility Testing. 31st Edn CLSI supplement M100 Available at: https://clsi.org/standards/products/microbiology/documents/m100/.
Karampatakis, T., Tsergouli, K., and Behzadi, P. (2023). Carbapenem-resistant Klebsiella pneumoniae: virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics (Basel) 12:234. doi: 10.3390/antibiotics12020234
Lakota, E. A., Landersdorfer, C. B., Nation, R. L., Li, J., Kaye, K. S., Rao, G. G., et al. (2018). Personalizing polymyxin B dosing using an adaptive feedback control algorithm. Antimicrob. Agents Ch. 62, e00483–e00418. doi: 10.1128/AAC.00483-18
Liu, S. H., Wu, Y., Qi, S., Shao, H. Z., Feng, M., Xing, L. H., et al. (2023). Polymyxin B therapy based on therapeutic drug monitoring in carbapenem-resistant organisms sepsis: the PMB-CROS randomized clinical trial. Crit. Care 27:232. doi: 10.1186/s13054-023-04522-6
Miglis, C., Rhodes, N. J., Avedissian, S. N., Kubin, C. J., Yin, M. T., Nelson, B. C., et al. (2018). Population pharmacokinetics of polymyxin B in acutely ill adult patients. Antimicrob. Agents Ch. 62, e01475–e01417. doi: 10.1128/AAC.01475-17
Nang, S. C., Azad, M. A. K., Velkov, T., Zhou, Q. T., and Li, J. (2021). Rescuing the last-line polymyxins: achievements and challenges. Pharmacol. Rev. 73, 679–728. doi: 10.1124/pharmrev.120.000020
National Center for Biotechnology Information. (2023) Available at: https://www.ncbi.nlm.nih.gov/pccompound/.
Pfennigwerth, N., Kaminski, A., Korte-Berwanger, M., Pfeifer, Y., Simon, M., Werner, G., et al. (2019). Evaluation of six commercial products for colistin susceptibility testing in Enterobacterales. Clin. Microbiol. Infect. 25, 1385–1389. doi: 10.1016/j.cmi.2019.03.017
Pogue, J. M., Jones, R. N., Bradley, J. S., Andes, D. R., Bhavnani, S. M., Drusano, G. L., et al. (2020). Polymyxin Susceptibility Testing and Interpretive Breakpoints: Recommendations from the United States Committee on Antimicrobial Susceptibility Testing (USCAST). Antimicrob. Agents Ch. 64, e01495–e01419. doi: 10.1128/aac.01495-19
Satlin, M. J., Lewis, J. S., Weinstein, M. P., Jean, P., Humphries, R. M., Gunnar, K., et al. (2020). Clinical and laboratory standards institute and european committee on antimicrobial susceptibility testing position statements on polymyxin B and colistin clinical breakpoints. Clin. Infect. Dis. 71, e523–e529. doi: 10.1093/cid/ciaa121
Tsuji, B. T., Pogue, J. M., Zavascki, A. P., Paul, M., Daikos, G. L., Forrest, A., et al. (2019). International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39, 10–39. doi: 10.1002/phar.2209
Wang, P. L., Zhang, Q. W., Qin, Z. F., Xing, H., Xu, M., Pei, H., et al. (2020a). A simple and robust LC-MS/MS analytical method for therapeutic drug monitoring of plasma and cerebrospinal fluid polymyxin B1 and B2. Ther. Drug Monit. 42, 716–723. doi: 10.1097/FTD.0000000000000754
Wang, P. L., Zhang, Q. W., Zhu, Z. F., Feng, M., Sun, T. W., Yang, J., et al. (2020b). Population pharmacokinetics and limited sampling strategy for therapeutic drug monitoring of polymyxin B in Chinese patients with multidrug-resistant Gram-negative bacterial infections. Front. Pharmacol. 11:829. doi: 10.3389/fphar.2020.00829
Wang, P. L., Xing, H., Zhang, F., Liu, S. H., Lu, Y. Q., Zhang, X. J., et al. (2022). Population pharmacokinetics of polymyxin B in critically ill patients receiving continuous venovenous haemofiltration. Int. J. Antimicrob. Agents 60, 106599. doi: 10.1016/j.ijantimicag.2022.106599
Xie, J., Roberts, J. A., Lipman, J., Cai, Y., Wang, H., Zhao, N., Xu, X., Yang, S., Li, Y., and Zhang, K. (2020). Pharmacokinetic/pharmacodynamic adequacy of polymyxin B against extensively drug-resistant Gram-negative bacteria in critically ill, general ward and cystic fibrosis patient populations. Int. J. Antimicrob. Ag. 55:05943. doi: 10.1016/j.ijantimicag.2020.105943
Yang, J., Liu, S. H., Lu, J. L., Sun, T. W., Wang, P. L., and Zhang, X. J. (2022). An area under the concentration-time curve threshold as a predictor of efficacy and nephrotoxicity for individualizing polymyxin B dosing in patients with carbapenem-resistant gram-negative bacteria. Crit. Care 26:320. doi: 10.1186/s13054-022-04195-7
Yu, Z., Liu, X., Du, X., Chen, H., Zhao, F., Zhou, Z., et al. (2022). Pharmacokinetics/pharmacodynamics of polymyxin B in patients with bloodstream infection caused by carbapenem-resistant Klebsiella pneumoniae. Front. Pharmacol. 13:975066. doi: 10.3389/fphar.2022.975066
Keywords: Carbapenem-resistant Klebsiella pneumonia, polymyxin B, antimicrobial susceptibility testing, AUC/MIC, Monte Carlo simulation
Citation: Wang P, Liu S, Qi G, Xu M, Sun T and Yang J (2023) Evaluation of polymyxin B AUC/MIC ratio for dose optimization in patients with carbapenem-resistant Klebsiella pneumoniae infection. Front. Microbiol. 14:1226981. doi: 10.3389/fmicb.2023.1226981
Received: 22 May 2023; Accepted: 31 July 2023;
Published: 22 August 2023.
Edited by:
Matthew Gavino Donadu, University of Sassari, ItalyReviewed by:
Payam Behzadi, Islamic Azad University, ShahreQods, IranCopyright © 2023 Wang, Liu, Qi, Xu, Sun and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Xu, eHVtaW4zNTY4QHNpbmEuY29t; Tongwen Sun, c3VudG9uZ3dlbkAxNjMuY29t; Jing Yang, amluZ3lhbmdfMDEwMUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.