- 1Plant Genetics Lab, Gembloux Agro-Bio Tech, University of Liѐge, Gembloux, Belgium
- 2Virology Lab, Center for Agricultural Biochemistry and Biotechnology, University of Agriculture, Faisalabad, Faisalabad, Pakistan
- 3Department of Bioinformatics and Biotechnology, Government College University, Faisalabad, Pakistan
- 4Department of Integrative Biotechnology, Sungkyunkwan University, Suwon, Republic of Korea
- 5Risk Assessment Research Center, Korea Institute of Ocean Science and Technology, Geoje, Republic of Korea
- 6Microbiology Lab, Department of Zoology, Government College University, Lahore, Pakistan
Circular single-stranded DNA viruses of the family Geminiviridae encode replication-associated protein (Rep), which is a multifunctional protein involved in virus DNA replication, transcription of virus genes, and suppression of host defense responses. Geminivirus genomes are replicated through the interaction between virus Rep and several host proteins. The Rep also interacts with itself and the virus replication enhancer protein (REn), which is another essential component of the geminivirus replicase complex that interacts with host DNA polymerases α and δ. Recent studies revealed the structural and functional complexities of geminivirus Rep, which is believed to have evolved from plasmids containing a signature domain (HUH) for single-stranded DNA binding with nuclease activity. The Rep coding sequence encompasses the entire coding sequence for AC4, which is intricately embedded within it, and performs several overlapping functions like Rep, supporting virus infection. This review investigated the structural and functional diversity of the geminivirus Rep.
Introduction
Single-stranded DNA (ssDNA) viruses infect a wide variety of living organisms. According to a recent metagenomic sequencing analysis, these intracellular obligate parasites are widespread and have a diverse host range (Krupovic et al., 2020). Following the molecular structure and phylogeny of replication-associated protein (Rep), the ssDNA viruses have been categorized as circular Rep-encoding single-stranded (CRESS) DNA viruses (phylum: Cressdnaviricota). Certain CRESS DNA viruses of eukaryotic origin encode only Rep and coat protein genes. Due to the HUH (two histidine residues separated by hydrophobic residues) endonuclease domain in the Rep, most DNA viruses replicate using a rolling circle mechanism (Chandler et al., 2013). Like other CRESS DNA viruses, members of the family Geminiviridae replicate via rolling circle replication (RCR). In addition, the geminivirus Rep (also called AC1, C1, or AL1 in the literature) interacts with the host DNA replication factors for virus DNA replication. The origin of the geminivirus Rep coding sequence is predicted from phytoplasma plasmids using the metagenomic sequence analysis (Krupovic et al., 2009; Kazlauskas et al., 2019). However, the possibility that this shared domain organization in both Rep coding sequences is either the result of convergent evolution or reflects a more recent common ancestry cannot be ruled out at this point. Besides Rep, the geminivirus replication-enhancer protein (REn or AC3) also plays an ancillary role in virus replication.
Genome duplication during the cell cycle is highly regulated in all eukaryotes and is fundamental to preserving genome integrity (Alabert and Groth, 2012; MacAlpine and Almouzni, 2013). This duplication process is known as DNA replication, which aids an organism in transmitting its genetic information to the daughter cells produced during cell division to maintain continuity between generations. DNA replication occurs in three steps: initiation, wherein pairs of replication forks assemble at the replication origin; elongation, wherein forks copy the chromosomes by semiconservative DNA synthesis; and termination, wherein converging replication forks meet (Balakrishnan and Bambara, 2013; Costa et al., 2013). From bacterial to eukaryotic cells, the DNA replication process is highly organized and follows a strict rule of only once per cell cycle (Siddiqui et al., 2013; Skarstad and Katayama, 2013). Geminiviruses largely depend on the host cell machinery and metabolism for replication, transcription, translation, and assembly processes. They manipulate the cells to progress from the resting phase (G0 or G1) to the S phase to create a favorable environment for their replication. This ability allows tropism for cells that otherwise might not be able to support virus DNA replication (De Beeck and Caillet-Fauquet, 1997; Reed et al., 2004). Several host factors have been identified that physically interact with geminivirus Rep. This manuscript reviews the progress in determining the molecular structures and host factors that interact with geminivirus Rep.
Diverse genomes of geminiviruses
Geminiviruses, with more than 520 species, are recognized by the International Committee on Taxonomy of Viruses (ICTV) as one of the most numerous and diverse groups of plant viruses, causing a wide range of diseases in economically important plants (Zerbini et al., 2017). Naturally, geminiviruses are transmitted by different hemipterous insects. They have been classified into fourteen genera: Becurto-, Begomo-, Capula-, Citloda-, Curto-, Eragro-, Grablo-, Maldo-, Mastre-, Mulcrile-, Ogun-, Topile-, Topocu-, and Turncurto-virus, based on the genome organization, host range, insect vector type, and phylogeny (King et al., 2018; Roumagnac et al., 2022). Members of the genus Begomovirus have genomes that are either monopartite, with a single genomic component, DNA-A, of ∼2.6–3.0 kilobases (kb), or bipartite, with two ∼2.6 kb genomic components, DNA-A and DNA-B, together making up the genomic size of around ∼5.2 kb. DNA-A and DNA-B components are packed into twinned quasi-icosahedral virions (Zerbini et al., 2017). Members of other genera comprise the monopartite genome (Zerbini et al., 2017). Geminiviruses have variable genomic structures depending on the genus type. Generally, the DNA-A component encodes four proteins, Rep, REn, transcription activator protein (TrAP/AC2), and pathogenesis/symptom determinant protein (C4/AC4) in the complementary-sense orientation, and two proteins, coat protein (CP/AV1) and pre-coat protein (AV2) in the virion-sense orientation (Devendran et al., 2022). In addition, the geminivirus genomes have been recently identified to encode several small proteins, which have predicted roles in the sub-cellular location and pathogenicity of associated viruses (Gong et al., 2021; Zhao et al., 2022). The DNA-B component encodes movement protein (MP/BC1) in the complementary-sense orientation and nuclear shuttle protein (NSP/BV1) in the virion-sense orientation (Zerbini et al., 2017). Both DNA-A and DNA-B components share a common region (CR) of 180–200 nucleotides that usually spans most of the intergenic region (IR). The IR is a non-coding region encoded by all geminiviruses, which contains cis-acting regulatory elements for gene expression, a predicted hairpin structure containing the conserved (among most geminiviruses) nonanucleotide sequence (TAATATTAC) as part of the loop, and small repeated sequences, known as “iterons,” which are sequence-specific binding sites for Rep. Together, the iterons and hairpin form the origin of replication (ori).
Begomoviruses in the Old World (OW) and a few mastreviruses have been associated with ∼1.4 kb-sized ssDNA molecules referred to as alphasatellites and betasatellites (Zhou, 2013; Kumar et al., 2014; Hamza et al., 2018) and recently assigned to families, Alphasatellitidae and Tolecusatellitidae, respectively (Adams et al., 2017). Alphasatellites contain an ori similar to other members of the family Nanoviridae and encode a nanovirus-like Rep (280–315 amino acids), which enables them to self-replicate. However, they require helper virus components for inter- and intracellular movement and, in exchange, provide helper viruses with selective advantages by acting as suppressors of gene silencing (Nawaz-ul-Rehman et al., 2010). Alternatively, betasatellites do not code for any replication gene and completely depend on the helper component, DNA-A, for their replication, encapsidation, and vector transmission. However, they encode a betaC1 (βC1) protein, which is a suppressor of gene silencing and serves as a major pathogenicity determinant (Zhou, 2013).
Geminivirus infection occurs in host-differentiated cells, where the virus reprograms the host cell cycle to create a permissive environment for its genome replication (Gutierrez, 2000). Geminiviruses use only two proteins for virus DNA replication, Rep and REn. These virus proteins interact with each other and various host factors, directing the host cell to form replication complexes required for virus DNA replication (Figure 1; Fontes et al., 1994; Gutierrez, 2000). Geminivirus Rep directs virus-specific recognition of its cognate ori at the virus genome and starts virus DNA replication (Fontes et al., 1994). Additionally, Rep regulates its own transcription and the expression of virion-sense genes in some geminiviruses (Hofer et al., 1992). Initial experiments demonstrated that Rep is essential for virus DNA replication, where Tomato golden mosaic virus (TGMV) Rep overexpression in Nicotiana benthamiana plants supported efficient virus replication mutated for its Rep (Hanley-Bowdoin et al., 1990). Further experiments demonstrated interesting applications of Rep, where overexpression of the mutated version of Bean golden mosaic virus (BGMV) Rep resulted in partial resistance in bean plants to the respective viruses (Faria et al., 2006).
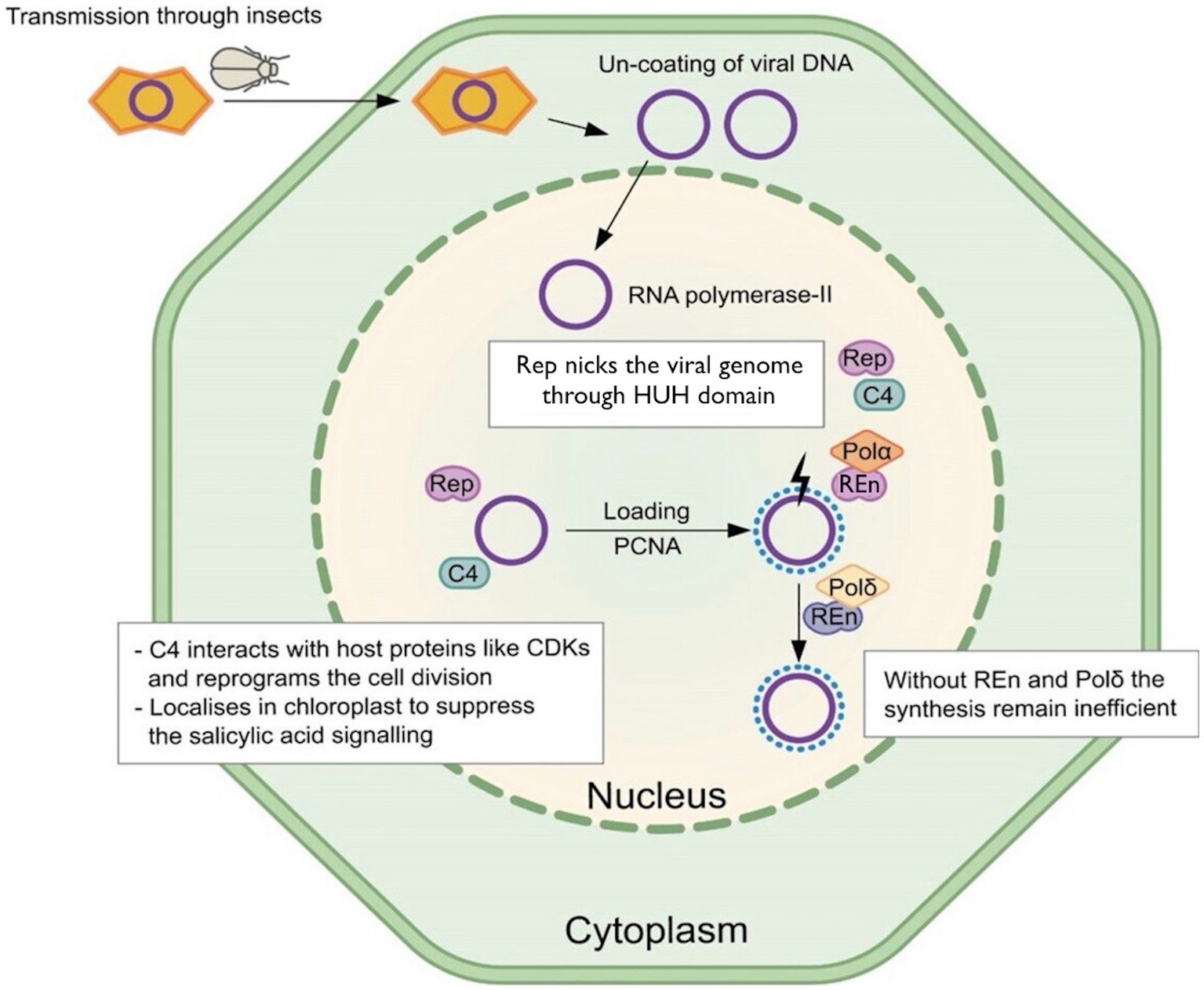
Figure 1. Model for Rep functions. The Rep recruits PCNA to interact with DNA. It binds with nonanucleotide through the HUH domain and starts the rolling circle replication. The C4 protein coded by polycistronic Rep is multifunctional and targets the salicylic acid-based defense pathway. Without REn and Polδ interaction, the replication remains inefficient.
Rep proteins vary at the species level but share similar structures
Geminivirus Rep is the most conserved protein in sequence, position, and function (Figure 2), while its structure and folding patterns present slight variability among different genera (Figure 3 and Supplementary Figure S1; explained below). It shares domain resemblance with the replication protein of a eubacterial plasmid, implying a strong evolutionary link between these proteins (Ilyina and Koonin, 1992; Koonin and Ilyina, 1992). This is consistent with the idea of geminivirus evolution from prokaryotic ssDNA plasmids (Oshima et al., 2001; Krupovic et al., 2009). Rep, with a size of approximately 360 amino acids, transcribes from a bidirectional core promoter located in the IR except for Mastre-, Capula, Becurto, and Grablo-virus Rep (C1:C2), which is a spliced version of C1 and C2 open reading frames (ORFs) (Hanley-Bowdoin et al., 1999, 2013; Varsani et al., 2017). In addition to Rep, Mastre-, Capula-, Becurto-, and Grablo-virus also code for replication-associated protein A (RepA/C1) with sizes varying from 260 to 300 amino acids, transcribed from ORF C1 (Hofer et al., 1992; Collin et al., 1996; Gutierrez et al., 2004; Varsani et al., 2017).
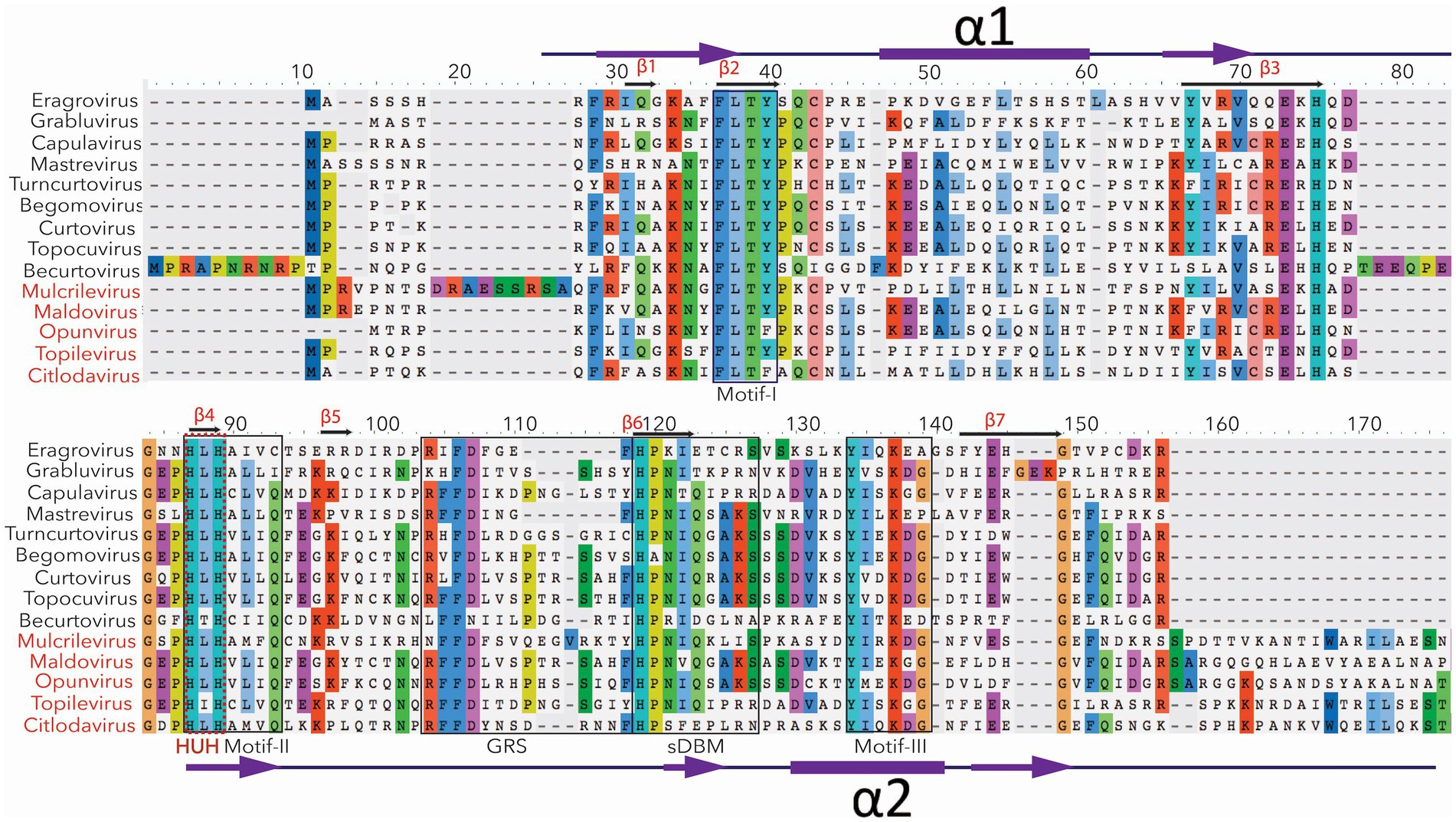
Figure 2. The conserved sequence motifs for Rep of fourteen recognized genera of geminiviruses. The most conserved amino acids are mentioned in different colors. For example, motifs 1, 2, and 3 are conserved among all the geminiviruses. The critical HUH domain of CRESS DNA viruses is mentioned in the red text. HUH domain is involved in rolling circle amplification. The newly recognized five genera (mentioned with red color) have relatively (20%) larger Rep. The conserved part of their Rep is presented here.
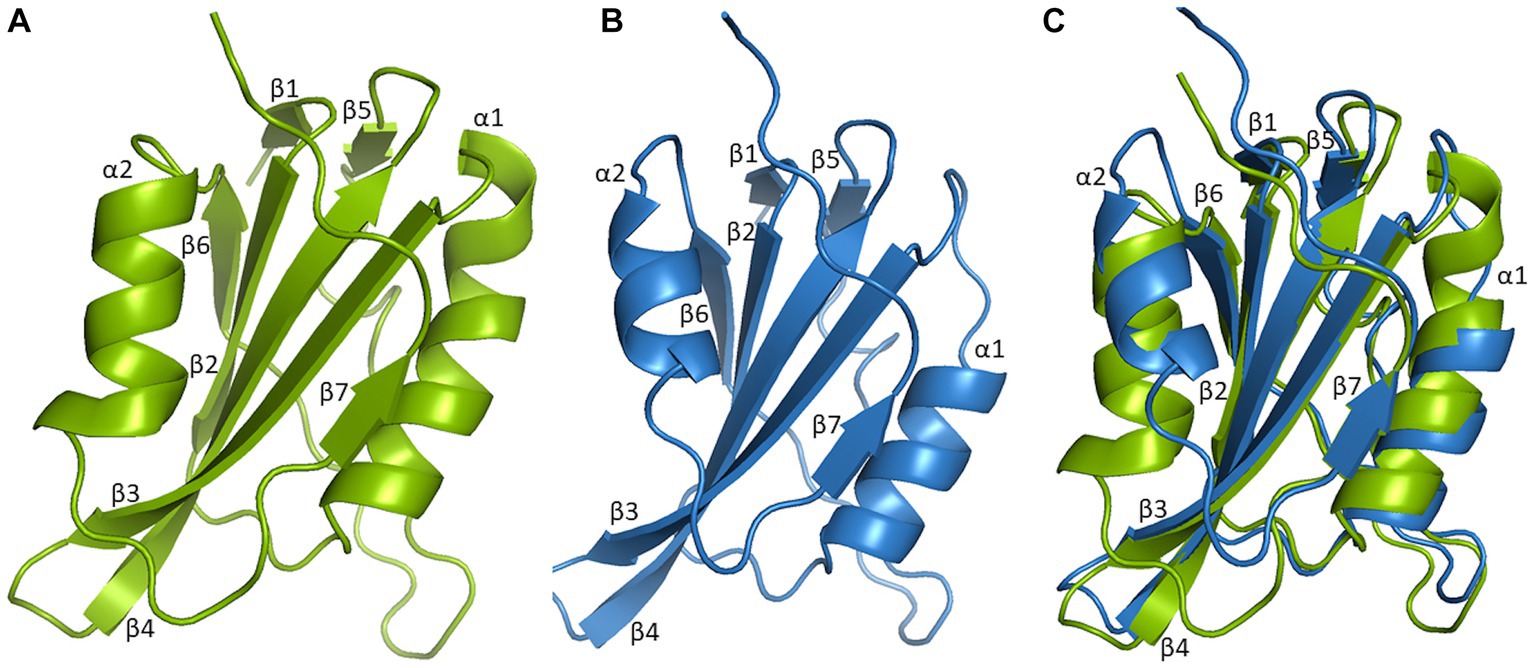
Figure 3. Three-dimensional structural comparison of N-terminal of Mastrevirus and Begomovirus Rep. Protein models were build using Swiss-model software and visualized in Pymol for Rep of Mastrevirus, Wheat dwarf virus (WDV) (A), Begomovirus, Tomato yellow leaf curl virus (TYLCV) (B). Superimposition of WDV Rep and TYLCV Rep structure (C) indicates highly conserved structures between different two genera, comprised of five antiparallel β-sheets, β2, β3, β4, β6, β7 in the center, flanked by two β-sheets, β1, β5 and two α-helices, α1 and α2 are shown. However, two α-helices are smaller in TYLCV Rep compared to WDV Rep.
The N-terminal of geminivirus Rep comprises three domains: DNA binding (amino acids, 1–130), DNA cleavage and ligation (amino acids, 1–120), and oligomerization (amino acids, 120–180) domains. It also contains the binding site for various host proteins, such as proliferating cell nuclear antigen (PCNA), retinoblastoma-related (RBR) protein, SUMO (Small Ubiquitin-like Modifiers)-conjugating enzymes (SCE), Geminivirus Rep-interacting kinase (GRIK), and adenosine triphosphatase (ATPase), as well as geminivirus protein REn. The DNA-binding and cleavage/ligation domains contain three motifs (I, II, and III), which are involved in DNA binding, metal binding, and catalyzing endonuclease activity, respectively (Koonin and Ilyina, 1992). Furthermore, it comprises two alpha-helices (helix αI and αII) and a geminivirus conserved motif designated as the geminivirus Rep sequence (GRS), required for replication initiation (Figure 2; Orozco and Hanley-Bowdoin, 1998; Nash et al., 2011).
The C-terminal contains the ATPase domain, including Walker A and Walker B, which are required for the activities of ATPase, helicase, and DNA unwinding process during replication (Desbiez et al., 1995; Orozco et al., 1997; Choudhury et al., 2006; Clérot and Bernardi, 2006; George et al., 2014). Additionally, another relatively conserved cyclin interaction motif (RXL) has been discovered at the C-terminal close to the Walker A motif among all geminivirus genera (except Becurtovirus) and has been demonstrated to be indispensable for geminivirus genome replication as a mutation of the RXL motif failed to replicate the virus genome in N. benthamiana and fission yeast (Nash et al., 2011; Hipp et al., 2014).
So far, the crystal structure of the Wheat dwarf virus (WDV) Rep HUH endonuclease domain and the NMR structure of the catalytic domain of Tomato yellow leaf curl virus (TYLCV) Rep have been solved (Campos-Olivas et al., 2002; Everett et al., 2019). A three-dimensional structural comparison of the catalytic domain of TYLCV and WDV Rep indicated that it consists of five-stranded antiparallel β-sheets (β2, β3, β4, β6, and β7) in the center, flanked by two-stranded β-sheets (β1 and β5), a β-hairpin, and two α-helices (Figure 3). However, two α-helices appeared more compact in begomoviruses compared to mastreviruses. Intriguingly, Rep of most of the geminivirus genera shared a relatively conserved structure and folding patterns; however, three genera, including Eragro-, Topile-, and Capula-virus, lack two-stranded β-sheets (β1 and β5) in the N-terminal (Supplementary Figure S1). The HUH endonuclease domain directs a metal ion to enable the Rep nicking activity of a specific ssDNA sequence. Therefore, solving its structure provides vital insights into the Rep recognition of ssDNA and sequence specificity (Everett et al., 2019).
Sequence analysis of Chili leaf curl virus (ChiLCV) Rep predicted the presence of a nuclear localization signal between the amino acid residues 244 and 273 (Kushwaha et al., 2017). However, a recent study has reported an auxiliary role of lysine residues at the N-terminal (between amino acid residues, 40–108) of Rep for its nuclear localization, and these lysine residues are highly conserved among various geminiviruses. Mutational analysis of the TYLCV Rep single lysine residue (K67A) significantly increased Rep nuclear exclusion, which was further increased when mutations K71A or K101A alone or in combination were added to K67A (Maio et al., 2019).
The Mastrevirus RepA is structurally similar in its N-terminal region to Rep, containing DNA binding, cleavage/ligation, and oligomerization domains. However, the functions of these RepA domains have not been experimentally described. The C-terminal of RepA includes the promoter transactivation domain (TRA) and binding sites for RBR and GRAB (geminivirus RepA binding) transcription factors (Horváth et al., 1998; Liu et al., 1999; Xie et al., 1999; Gutierrez et al., 2004; Hefferon et al., 2006). Mutational studies in Maize streak virus (MSV) RepA have shown that it is necessary for efficient virus DNA replication and is involved in ssDNA production (Ruschhaupt et al., 2013).
AC4 coding sequence lies within Rep coding sequence
The coding region for AC4 in both monopartite and bipartite geminiviruses is generally embedded within the Rep (Fondong, 2019) and is absolutely required for virus infection (Hipp et al., 2014; Carluccio et al., 2018). However, depending on the geminivirus species, transgenic plants expressing AC4 display distinct phenotypes (Mills-Lujan and Deom, 2010; Luna et al., 2012). According to the unique target signals, such as a chloroplast transit peptide (cTP) and acylation sites, AC4 has been discovered to interact with membranes in the chloroplast, plasma membrane, nucleus, and cytoplasm, which could account for these functional distinctions among geminivirus AC4 proteins (Luna and Lozano-Durán, 2020). For instance, the AC4 of apple geminivirus is a symptom determinant and is targeted to the nucleus, plasma membrane, and chloroplast (Zhan et al., 2018).
The geminivirus AC4 protein has multiple overlapping functions, just like Rep. One of the functional characteristics of AC4 is its prominent role in reprogramming the cellular environment for virus infection. The cell cycle is tightly regulated by the interplay between cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors (CKIs). Recently, Tomato leaf curl Yunnan virus (TLCYnV) C4 was found to interact with and sequester the phosphorylation ability of glycogen synthase kinase 3 (GSK3) in N. benthamiana, a SHAGGY-like kinase and an inhibitor of cyclin CycD1;1, designated as SKη. This interaction leads to the accumulation of CycD1;1, which then forms a complex with CDK and induces cell division (S phase) that enables virus DNA replication (Mei et al., 2018). Beet severe curly top virus (BSCTV)-encoded C4-mediated upregulation of RING finger E3 ligase (RKP) in Arabidopsis plants is yet another strategy to induce cell cycle progression by Curtovirus (Lai et al., 2009). Furthermore, RKP interacts with and downregulates the activity of the cell cycle inhibitors, and upregulates E2F activity, thereby inducing cell proliferation by stimulating cell entry from G1 to S (Cheng et al., 2013).
The AC4 protein of many geminiviruses suppresses the RNA silencing pathway in plants. For example, the AC4 of Mungbean yellow mosaic virus (MYMV) targets BARLEY ANY MERISTEM 1 (BAM1), a receptor-like kinase that is a positive regulator of cell-to-cell movement of RNAi signals (Carluccio et al., 2018). Likewise, TYLCV C4 interacts with both BAM1 and BAM2, thus regulating the intercellular spread of RNA silencing signals (Rosas-Diaz et al., 2018). In addition, the AC4 protein of the Cotton leaf curl Multan virus (CLCuMuV) targets S-adenosyl methionine synthetase activity, a critical enzyme of the host methyl cycle, thus suppressing both transcriptional and post-transcriptional gene silencing (Ismayil et al., 2018). Similarly, Tomato leaf curl New Delhi virus (ToLCNDV) C4 protein interacts with the host AGO2 protein and influences RNA silencing and cytosine methylation of the virus genome (Vinutha et al., 2018). Furthermore, Tomato leaf curl Palampur virus (ToLCPalV) AC4 has also been described as a suppressor of gene silencing (Kulshreshtha et al., 2019).
Owing to the co-existence of two subcellular targeting signals, an N-myristoylation site and cTP, the geminivirus AC4 has also been implicated in suppressing chloroplast-mediated plant defense mechanisms. For instance, TYLCV-C4 is transported from the plasma membrane to the chloroplast upon activation of plant defense and suppresses salicylic acid-mediated defense communication between the nucleus and the chloroplast (Medina-Puche et al., 2020). Other functions have also been reported for geminivirus AC4; for instance, TYLCV C4 has been implicated in virus transport inside phloem tissues (Rojas et al., 2001).
Rep interacts with virus/satellite DNA and initiates replication
The geminivirus Rep starts RCR by locating and attaching to the CR, which contains the iteron sequences between the TATA box and the transcription start site (Fontes et al., 1992). Rep recognizes the virus-specific ori, and this recognition mechanism depends on the architecture of the ori, which appeared to be different between Begomovirus and Mastrevirus (Gutierrez et al., 2004). The ori in Mastrevirus consists of a large cis-acting region with several Rep-binding sites, whereas the ori in Begomovirus has a single Rep-binding site (Fontes et al., 1992; Sanz-Burgos and Gutiérrez, 1998; Castellano et al., 1999). However, various begomovirus species have different iteron sequences and ways of attaching Rep to these repetitive regions. For instance, the iteron sequence in the TGMV consists of 13 bp sections with two 5 bp repeats. In vitro binding analysis showed that Rep binding to these iteron sequences is necessary to initiate virus DNA replication (Fontes et al., 1994). However, Tomato leaf curl virus (TLCV) Rep-binding regions consist of direct repeat “GGTGTCT” elements in CR, as demonstrated by footprinting analysis (Akbar Behjatnia et al., 1998). In addition, Mungbean yellow mosaic Indian virus (MYMIV) contains an additional iteron sequence downstream of CR (Singh et al., 2008). In the case of mastreviruses, Rep binds to three sites, located upstream and downstream of the ori and at the base of the stem-loop structure (Laufs et al., 1995; Castellano et al., 1999).
Once Rep binds to the ori using its binding motif I, it cleaves the phosphodiester bond between the seventh and eighth residues of the invariant loop structure 5’TAATATTAC3′ that involves its motif II (Heyraud-Nitschke et al., 1995; Laufs et al., 1995; Orozco and Hanley-Bowdoin, 1998; Arguello-Astorga et al., 2004). Another sequence in motif III is also involved in cleavage and binding activity (Orozco et al., 1997), thus providing a free 3’OH end to start the synthesis of nascent virus DNA by host polymerases. The elongation involves extending this 3′ end using a complementary strand with the help of host polymerases and associated factors. This is evident from Rep interaction with various host factors, such as replication protein A 32 (RPA32), PCNA, replication factor C (RFC), and minichromosome maintenance 2 (MCM2) (Luque et al., 2002; Bagewadi et al., 2004; Singh et al., 2007). Rep recognizes and cleaves the nonanucleotide in newly formed viral circular DNA and lures its 3’ end to a previously synthesized 5′ end using its DNA-binding domain located in the N-terminus, resulting in the formation of a new viral genome-sized unit (Laufs et al., 1995). In addition to motifs I–III, binding and cleavage activities by two α-helices located between motifs I and II are necessary for virus DNA replication (Orozco and Hanley-Bowdoin, 1998). Moreover, GRS is required for Rep binding. The artificial zinc-finger protein-based mutation of GRS in TYLCV Rep lost its ability to cleave and initiate virus DNA replication (Nash et al., 2011). Many other cis-elements, such as TATA and G boxes, CA and AG motifs, and spacing between the Rep-binding site and hairpin, have also been demonstrated to control virus DNA replication (Orozco et al., 1997). The oligomerization domain located in the center of the Rep is crucial for Rep oligomerization and ultimately for virus DNA replication, as TGMV mutants for its oligomerization domain are defective in replication (Orozco et al., 1997). Rep oligomerization has also been illustrated for mastreviruses, MSV and WDV (Horváth et al., 1998; Sanz-Burgos and Gutiérrez, 1998; Castellano et al., 1999; Missich et al., 2000). In the case of WDV, both Rep and RepA form large oligomeric nucleoprotein C and V complexes by binding near the TATA box of complementary and virion sense promoters, respectively. However, DNase1 footprinting analysis demonstrated that their binding regions were not identical. In plants, bimolecular fluorescence complementation (BiFC) assay in N. benthamiana demonstrated Abutilon mosaic virus (AbMV) Rep self-interaction to form oligomers and their accumulation in the nucleoplasm (Krenz et al., 2011).
Besides Rep, REn actively contributes to virus DNA replication. It recruits host DNA polymerase alpha (α) and delta (δ) to the replicase complex and also interacts with virus Rep (Settlage et al., 1996, 2005; Wu et al., 2021). In vitro studies with the Tomato leaf curl Kerala virus (ToLCKeV) have shown that REn enhances the ATPase activity of Rep (Pasumarthy et al., 2010). REn also modulates the cellular environment by interacting with host RBR and NAC domain-containing transcription factors (Selth et al., 2005; Settlage et al., 2005). The ability of REn to oligomerize may also play a role in virus DNA replication, as evidenced by interacting TGMV and BGMV Rep and REn, which allowed the formation of heteromultimeric units and virus DNA replication in tobacco protoplasts (Settlage et al., 1996). Geminivirus CP might also regulate the virus DNA replication as its strong interaction with Rep has been illustrated for MYMIV, which results in the downregulation of Rep nicking and ligation ability (Malik et al., 2005).
The Rep protein allows replication of its cognate DNA-B component by binding to specific cis-elements in the CR (Lazarowitz et al., 1992). However, the synthesis of viable pseudo-recombinants between TGMV DNA-A and Tomato yellow spot virus (ToYSV) DNA-B, which do not have identical Rep-binding regions, demonstrates that trans-replication of only its cognate DNA-B molecule is not a strict rule (Andrade et al., 2006).
Monopartite begomoviruses are often associated with betasatellites, which are necessary to induce distinctive disease symptoms. However, betasatellites depend on the helper virus for replication, movement, and encapsidation of their genomes. Betasatellite DNA can be replicated by distinct geminiviruses, but they prefer cognate over non-cognate helper viruses (Qing and Zhou, 2009). For example, Cotton leaf curl Multan betasatellite (CLCuMuB) and Ageratum yellow vein betasatellite (AYVB) can be trans-replicated using CLCuMuV, Ageratum yellow vein virus (AYVV), and Eupatorium yellow vein virus (EpYVV), but not by Honeysuckle yellow vein virus (HYVV) (Saunders et al., 2008). It has been reported that Tobacco curly shoot betasatellite (TbCSB) and Tomato yellow leaf curl China betasatellite (TYLCNB) can be trans-replicated by their non-cognate helper viruses, but when co-inoculated, cognate betasatellites predominated over non-cognate partners in the later stages of infection (Qing and Zhou, 2009). These studies found that, while trans-replication of betasatellites occurs from non-cognate helper viruses, there is some specificity for trans-replication of heterologous satellite DNA.
Similar to Rep binding sequences in the virus ori, different sequence motifs have been identified in betasatellites for the Rep binding of the helper virus. For example, a G motif at 143 bp upstream of βC1 has been identified in CLCuMuB, which is important for betasatellite DNA replication (Eini et al., 2009). The sequences within the 26–295 nt region were essential for Rep binding and TLCV satellite DNA replication (Li et al., 2007). The involvement of the satellite conserved region (SCR) region in the DNA replication of AYVB, TYLCCNB, and TbCSB has been experimentally indicated (Saunders et al., 2008; Zhang et al., 2016). DNase I footprinting analysis revealed that betasatellites contain homologous iteron-like sequences of approximately 260 nt known as Rep-binding motifs (RBM) for specific recognition by Rep of their cognate helper viruses. This RBM is located immediately upstream of the satellite-conserved rolling circle cruciform structure and has a higher Rep-binding affinity for cognate helper viruses than non-cognate helper viruses (Zhang et al., 2016). The deletion of the RBM motif demonstrated its important role in efficient betasatellite DNA replication. However, more recently, cis-elements have been characterized for TbCSB for specific Rep binding by its cognate helper virus, Tobacco curly shoot virus (TbCSV) (Xu et al., 2019). Competitive DNA-binding assays revealed that TbCSB contains two iteron-like repeat elements (5′-GGACC-3′), which are identical to repeat sequences in TbCSV that are responsible for specific Rep binding; when these sequences were mutated, TbCSB lost its ability to prefer cognate helper virus-mediated replication (Xu et al., 2019). Sequence analysis has revealed that, interestingly, species-specific repeats in betasatellites are homologous to the repeats in cognate helper viruses. In addition to specific repeats, general repeats (5′-GGTAAAT-3′) have been identified upstream of SCR that allow Rep binding by non-cognate helper viruses.
Rep as RNAi suppressor and pathogenicity protein
Plants use cytosine and histone methylation as a defense against invading geminiviruses, thus regulating virus DNA replication and transcription of virus genes (Raja et al., 2010). Arabidopsis plants mutated for methylation such as cytosine methyltransferases (CMT) and histone methyltransferases (MET) supported high levels of geminivirus DNA replication, and virus DNA isolated from such plants was less methylated (Raja et al., 2008). In vitro methylation of geminivirus DNA results in reduced virus DNA replication in tobacco protoplasts, primarily due to the downregulation of virus gene transcription (Brough et al., 1992; Ermark et al., 1993). Another study found that geminiviruses’ heterogeneous linear double-stranded (ds) DNA form was preferentially methylated among different replicative intermediates, namely, open circular (oc), covalently closed circular (ccc), and heterogeneous linear DNA (Paprotka et al., 2011). However, to counter-defense the host response, geminiviruses encode several suppressors of host-mediated transcriptional gene silencing (TGS) and post-transcriptional gene silencing (PTGS) (Rishishwar and Dasgupta, 2019). Geminivirus Rep-mediated modification of the plant epigenome and downregulation of host methyl cycle enzymes, MET, and CMT, have been demonstrated in N. benthamiana (Rodríguez‐Negrete et al., 2013). In the case of mastreviruses, both Rep and RepA of WDV suppress the spread of RNA silencing signals, thus inducing both local and systemic silencing suppression of the GFP gene (Liu et al., 2014; Wang et al., 2014). Gel mobility shift assays showed that the Rep of WDV binds to 21 and 24 nt small interfering RNA (siRNA) duplexes and single-stranded (ss)-siRNA interfering host mechanism of PTGS. Moreover, deletion mutagenesis of the Rep showed that both N- and C-terminals are not required for silencing suppression, while the N-terminal is required for pathogenicity (Wang et al., 2014).
In addition to geminivirus Rep, alphasatellite-encoded Rep also induced PTGS suppression. The Rep from various alphasatellites, such as Gossypium darwinii symptomless alphasatellites (GDarSLA), Gossypium mustelinium symptomless alphasatellite (GMusSLA), and Cotton leaf curl Multan alphasatellites (CLCuMuA), are known PTGS suppressors (Nawaz-ul-Rehman et al., 2010; Abbas et al., 2019). However, the exact mechanisms of RNAi suppression remain largely unknown.
Rep as a transcription regulator
Rep has been shown to regulate the transcription of early and late-expressing virus genes (Eagle et al., 1994; Shung and Sunter, 2007). Rep binds to the iteron sequences at CR and represses the activity of its own promoter, which leads to the expression of TrAP and REn as the transcription start site of these genes lies within the coding region of Rep. However, replication initiation and transcriptional autoregulation are unrelated, as Rep mutants that are unable to initiate replication could still regulate the transcription of virus genes (Eagle et al., 1994). Mutational analysis identified an RGG motif between 124 and 126 amino acids in the Rep of Tomato yellow leaf curl Sardinia virus (TYLCSV) responsible for its transcription autoregulation activity (Sardo et al., 2011). Furthermore, geminivirus Rep-mediated recruitment of host machinery involved in post-translational modification at virus chromatin has been associated with the activation of virus gene transcription (explained below) (Lee et al., 2007; Kushwaha et al., 2017).
Global geminivirus Rep–host proteins interaction studies
Upon geminivirus infection, Rep is the first virus protein synthesized and is responsible for reprogramming the host cell cycle for virus DNA replication and transcription of other virus genes. Therefore, it interacts with many host proteins implicated in replication, post-translational modification, and defense machinery (Table 1). RBR proteins are the key factors controlling cell cycle progression in both plants and animals. A similar role in controlling virus DNA replication has been described in wheat cells, where virus DNA accumulation is significantly reduced upon expression of the RBR protein (Xie et al., 1996). Both Rep and RepA of WDV interact with plant RBR proteins through their LXCXE motif. This interaction is important for geminivirus DNA replication as its disruption abolishes virus DNA replication in wheat cells (Xie et al., 1995). However, contrary to WDV RepA, the other geminivirus Rep does not contain the LXCXE motif; instead, it binds to the RBR protein through other motifs. For example, TGMV Rep interacts with RBR protein via its novel helix 4 motif, which is located between Rep’s 101 and 180 amino acid regions. Rep’s helix 4 motif is made up of a hydrophobic core flanked by charged amino acid residues, and these residues play an important role in Rep-RBR binding. In addition to Rep, REn interacts with the RBR protein through polar residues at the N- and C-terminals (Settlage et al., 2001, 2005).
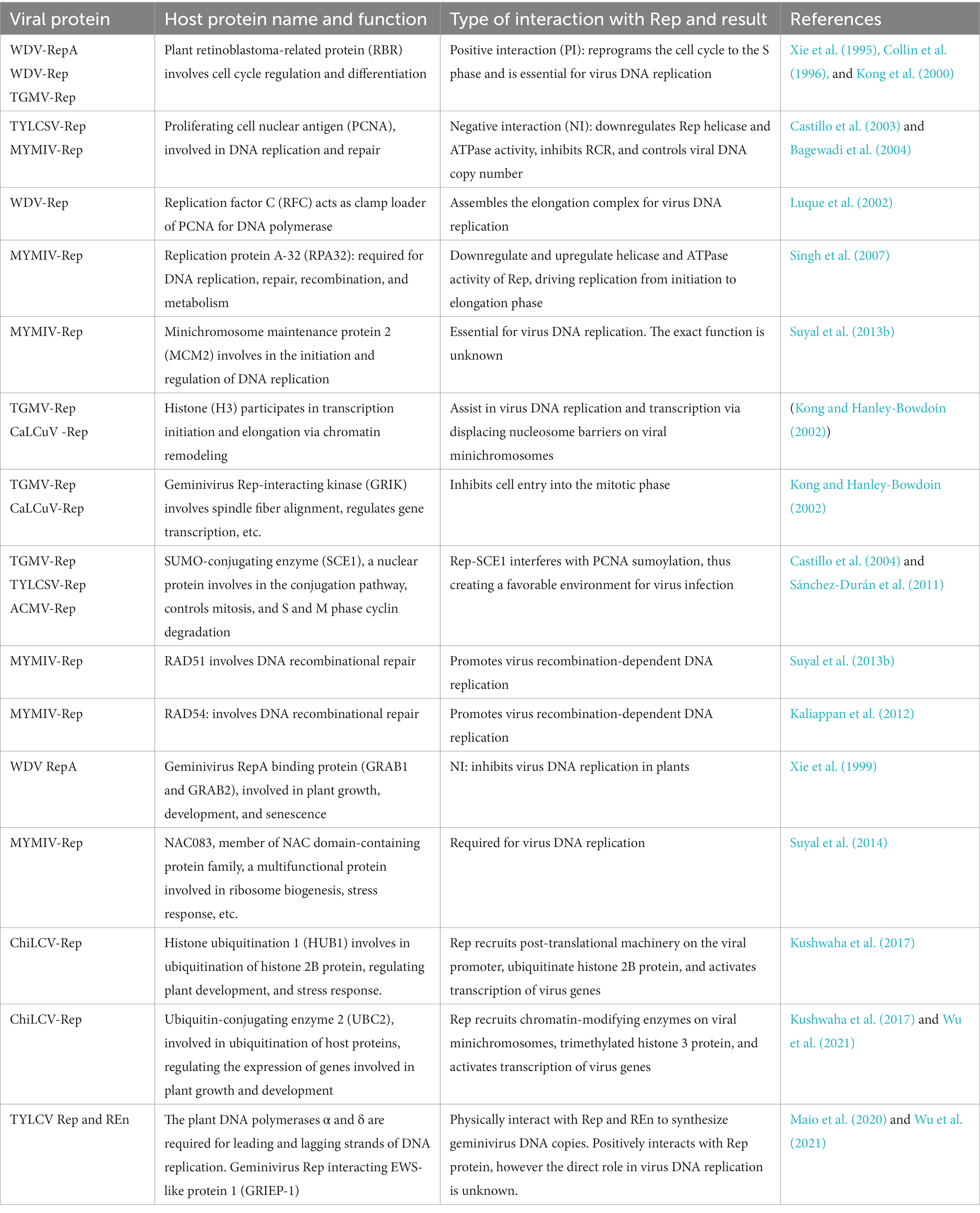
Table 1. Host factors interacting with geminivirus Rep, their function in the plant cell, and virus infection.
Geminivirus infection induces the expression of PCNA, as evident from its high accumulation in the infected mature cells (Nagar et al., 2002). Both Rep and REn of TYLCSV and Indian mung bean yellow mosaic virus (IMYMV) interact with tomato, tobacco, and pea PCNA, respectively (Schalk et al., 1989; Castillo et al., 2003). Additionally, Rep of OW geminiviruses, including Pedilanthus leaf curl virus (PeLCV) and CLCuMuV, has been shown to interact with not only plant PCNA but also yeast PCNA, suggesting a high conservation of Rep binding domains in PCNA among eukaryotic species (Shakir et al., 2021). The middle domain of TYLCV-REn is comprised of hydrophobic residues, and the 132–180 amino acid stretch of TYLCV Rep has been described as crucial for interactions with PCNA (Bagewadi et al., 2004; Settlage et al., 2005). PCNA inhibits the site-specific nicking and ATPase activities of Rep. Since RCR of viruses largely depends on Rep helicase and ATPase activities, it has been proposed that PCNA might act as an inhibitor of RCR, controlling the virus DNA copy number in the infected plant cells (Bagewadi et al., 2004).
The WDV Rep binds to and recruits the larger subunit of replication factor C (RFC) to the 3′ end of nicked geminivirus DNA, assisting in the assembly of the virus DNA replication elongation complex in wheat cells (Luque et al., 2002). In vitro studies have demonstrated that the geminivirus Rep interacts with replication protein A (RPA), a single-stranded (ss) DNA-binding protein primarily involved in replication, repair, and recombination (Singh et al., 2007). The 32 kDa subunit of RPA (RPA32) interacts with the C-terminal of MYMIV-Rep and regulates its helicase and ATPase activities, respectively. Therefore, it has been speculated that this interaction might inhibit RCR initiation and play an important role in DNA strand elongation. In planta, experiments confirmed that RPA32 positively influences MYMIV DNA replication (Singh et al., 2007). Furthermore, MYMIV-Rep interacts with the minichromosome maintenance 2 (MCM2) subunit, which is the licensing factor of the DNA replication machinery. Yeast and Arabidopsis plant mutants for MCM2 displayed reduced virus DNA replication. However, the exact role of MCM2 in viral DNA replication remains unclear (Suyal et al., 2013a).
The assembly of virus genomic DNA as minichromosomes has been previously described for AbMV (Pilartz and Jeske, 2003). Cabbage leaf curl virus (CaLCuV) and TGMV Rep interact with the histone-3 (H3) protein of Arabidopsis. This interaction might help displace histones from the virus nucleosomes, thus helping in virus DNA replication and transcription processes (Kong and Hanley-Bowdoin, 2002). Moreover, ChiLCV Rep interacts with host proteins involved in post-translational modification, N. benthamiana-encoded histone ubiquitination-1 (NbHUB1) and ubiquitin-conjugating enzyme 2 (NbUBC2), and recruits these proteins on the promoter sites at virus minichromosomes (Kushwaha et al., 2017). This recruitment aids in the ubiquitination of H2B and methylation of H3 proteins at virus chromatin, which is associated with RNA polymerase II activation and promoter modification, both of which result in transcription activation (Lee et al., 2007; Kushwaha et al., 2017).
Rep also engages in the geminivirus-related Ser/Thr kinase known as GRIK and the kinesin known as the geminivirus Rep interacting motor protein (GRIMP) (Kong and Hanley-Bowdoin, 2002) that attaches to the spindle fibers. Thus, Rep interaction with kinesin might inhibit cell entry into the mitotic phase. Rep-interacting kinases GRIK1 and GRIK2 accumulate to high levels in CaLCuV-infected tobacco leaves. While these kinases share homology with yeast Sucrose Nonfermenting 1 (SNF1) and mammalian AMPK and with SNF1-related kinases (SnRK1) in plants, it has been hypothesized that these kinases activate the SnRK1 signaling cascade, ensuring the availability of prerequisite precursors and energy sources for virus propagation. In exchange, TGMV-encoded Rep binding to the virus genome is impaired by SnRK1-mediated phosphorylation of the binding domain’s Ser-97 residue, which eventually prevents virus DNA replication (Shen et al., 2018).
Geminiviruses are also known to replicate by recombination-dependent replication (RDR) in addition to RCR (Jeske et al., 2001). Rep interacts with the repair/recombination proteins RAD51 and RAD54 to aid RDR of the virus genome (Kaliappan et al., 2012; Suyal et al., 2013b). Higher expression of RAD51 has been reported in plant cells infected with MYMIV (Suyal et al., 2013b). Complementation assays described the role of these proteins in virus DNA replication, where yeast mutants for rad51 and rad54 displayed reduced virus DNA replication while it was restored upon expression of RAD51 and RAD54 proteins. The binding domains have been mapped to the N-terminal of RAD54 and the oligomerization domain of MYMIV Rep (Kaliappan et al., 2012). In vitro studies have shown that this interaction enhances both the helicase and ATPase activities of Rep. However, the studies on Arabidopsis revealed that the recombination mediator protein RAD51D (a paralog of RAD51) increases Euphorbia yellow mosaic virus (EYMV) DNA replication (Richter et al., 2015, 2016). This implies the possible role of other recombination proteins in virus DNA replication and functional redundancy among them (Castillo et al., 2004).
The Rep of TYLCSV, TGMV, and African cassava mosaic virus (ACMV) interact with sumo-conjugating enzyme 1 (SCE1) to enhance virus DNA replication (Castillo et al., 2004; Sánchez-Durán et al., 2011). One such host protein, PCNA, is involved in virus DNA replication, which controls the cell cycle, DNA replication, and DNA repair. PCNA shifts among these functions through post-translational modifications. Using a reconstituted sumoylation system in E. coli, it has been demonstrated that tomato PCNA sumoylation occurs at two residues, K164 and K254, which play a vital role in DNA metabolism. However, geminiviruses’ Rep expression suppresses this PCNA sumoylation, thus creating a favorable environment for virus DNA replication (Arroyo-Mateos et al., 2018). The lysine residues (K68 and K96) at the N-terminus of TGMV Rep were used in mapping studies to pinpoint the location of this interaction. In vivo experiments with the mutation in lysine residues of the Rep did not result in successful virus DNA replication (Castillo et al., 2004; Sánchez-Durán et al., 2011). Furthermore, transgenic tobacco plants mutated for SCE1 did not support virus DNA replication, suggesting the importance of these enzymes in virus DNA replication.
Moreover, the C-terminal of WDV RepA interacts with the N-terminal domains of GRAB1 and GRAB2, belonging to the NAC domain-containing protein family, which is involved in plant development, senescence, and stress response (Xie et al., 1995). When GRAB was expressed in cultivated wheat cells, WDV DNA replication was reduced, indicating a negative interaction between RepA and GRAB proteins. Furthermore, MYMIV Rep has been shown to interact with Arabidopsis NAC083, another NAC domain-containing protein and host transcription factor (Suyal et al., 2014). However, the precise function of the Rep-NAC083 interaction in the virus infection cycle has not yet been thoroughly examined (Suyal et al., 2014).
Recently, a global interaction network between the TYLCV and N. benthamiana proteins has been presented, which provides a comprehensive overview of potential host proteins targeted by TYLCV during infection (Wang et al., 2017). Additionally, the TYLCV Rep also interacts with EWS-like protein-1 (GRIEP1) and the subunit 4A of the THO complex known as ALY1 (Maio et al., 2020). The direct involvement of GRIEP1 and ALY1 in the virus DNA replication remains unclear. However, their cellular function is to control RNA splicing and the transport of mRNA from the nucleus to the cytoplasm. These observations indicate the indirect involvement of certain host factors in the geminivirus infection cycle.
Conclusion
Geminiviruses reshape the host’s intercellular environment for their infection and counter-defense against host resistance. Due to their small genome size and limited coding capacity, geminivirus proteins have evolved to perform multiple functions and interact with several host factors involved in cellular pathways. One such virus protein is Rep, which is expressed early in the virus infection and has evolved to possess multiple domains to perform diverse functions in the host cell. Rep is one of the highly conserved proteins, structurally and functionally, across geminivirus species and genera, which facilitates virus DNA replication through reprogramming the host cell cycle and mediating the initiation, elongation, and termination of virus and associated DNA-B and betasatellite genome replication. It also controls the transcription of other virus genes, including TrAP, REn, and its own. Moreover, it acts as a pathogenicity determinant by functioning as a suppressor of gene silencing, which is one of the basic host anti-viral defense responses. Furthermore, the AC4 coding sequence embedded with the Rep coding sequence plays multiple overlapping functions as Rep, including cell cycle progression, RNA silencing suppression, and the intercellular movement of virus DNA molecules.
Future directions
From the work of the past many years, it is clear that geminivirus–host interaction is complex and involves diverse cellular pathways, ranging from cell cycle to gene silencing. Rep interaction with several host cellular factors has been described in several geminivirus species, but the exact underlying mechanisms remain to be examined in detail for several of these interactions. The study of geminivirus DNA replication events, a better understanding of the crosstalk between Rep and cellular factors, and the effect of these interactions on cellular pathways will increase our understanding of how viruses manipulate host cellular machinery and how certain pathways contribute to disease. Moreover, a global virus–host interaction network will also be instrumental in increasing our understanding of which interactions are conserved and critical for virus infection and how to disrupt them without disturbing plant growth and development. Finally, developing broad-spectrum resistance to geminivirus infections will enable us to target the most conserved pathways among all geminivirus species.
Author contributions
SSh and MN-u-R: conceptualization. SSh, MM, IL, and SSe: software and drawing. SSh and NN: review of literature. SSh, SL, and MQ: writing and editing. SSh and MN-u-R: supervision and final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by a 2022 fund from the Research of Animal and Plant Quarantine Agency, Korea, and from the Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries, Korea (20210466).
Acknowledgments
The authors are thankful to the virology lab members and the Higher Education Commission of Pakistan for their support in preparing the manuscript in collaboration with Celtech Lab, South Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1224221/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Three-dimensional structural comparison of N-terminal of geminivirus Rep. Protein models were build using Swiss-model software and visualized in Pymol for the representative species of Mastrevirus (a), Begomovirus (b), Curtovirus (c), Grabulavirus (d), Becurtovirus (e), Citlodavirus (f), Turncurtovirus (g), Multrilevirus (h), Opunvirus (i), Topocovirus (j), Maldovirus (k), overlay of a-k (l), Eragrovirus (m), Topilevirus (n), Capulavirus (o), overlay of m-o (p). Superimposition of 11 different genera (a-k) of family geminiviridae indicates highly conserved structures comprised of five anitparallel β-sheets, β2, β3, β4, β6, β7 in the center, flanked by two β-sheets, β1 and β2 and two α-helices, α1 and α2 (l). However, two β-sheets, β1 and β2 are missing in three genera, Eragrovirus, Topilevirus, and Capulavirus Rep (p).
References
Abbas, Q., Amin, I., Mansoor, S., Shafiq, M., Wassenegger, M., and Briddon, R. W. (2019). The rep proteins encoded by alphasatellites restore expression of a transcriptionally silenced green fluorescent protein transgene in Nicotiana benthamiana. Virus 30, 101–105. doi: 10.1007/s13337-017-0413-5
Adams, M. J., Lefkowitz, E. J., King, A. M., Harrach, B., Harrison, R. L., Knowles, N. J., et al. (2017). Changes to taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses (2017). Arch. Virol. 162, 2505–2538. doi: 10.1007/s00705-017-3358-5
Akbar Behjatnia, S. A., Dry, I. B., and Rezaian, M. A. (1998). Identification of the replication-associated protein binding domain within the intergenic region of tomato leaf curl geminivirus. Nucleic Acids Res. 26, 925–931. doi: 10.1093/nar/26.4.925
Alabert, C., and Groth, A. (2012). Chromatin replication and epigenome maintenance. Nat. Rev. Mol. Cell. Biol. 13, 153–167. doi: 10.1038/nrm3288
Andrade, E., Manhani, G., Alfenas, P., Calegario, R., Fontes, E., and Zerbini, F. (2006). Tomato yellow spot virus, a tomato-infecting begomovirus from Brazil with a closer relationship to viruses from Sida sp., forms pseudorecombinants with begomoviruses from tomato but not from Sida. J. Gen. Virol. 87, 3687–3696. doi: 10.1099/vir.0.82279-0
Arguello-Astorga, G., Lopez-Ochoa, L., Kong, L.-J., Orozco, B. M., Settlage, S. B., and Hanley-Bowdoin, L. (2004). A novel motif in geminivirus replication proteins interacts with the plant retinoblastoma-related protein. J. Virol. 78, 4817–4826. doi: 10.1128/JVI.78.9.4817-4826.2004
Arroyo-Mateos, M., Sabarit, B., Maio, F., Sánchez-Durán, M. A., Rosas-Díaz, T., Prins, M., et al. (2018). Geminivirus replication protein impairs SUMO conjugation of proliferating cellular nuclear antigen at two acceptor sites. J. Virol. 92:e00611-18. doi: 10.1128/JVI.00611-18
Bagewadi, B., Chen, S., Lal, S. K., Choudhury, N. R., and Mukherjee, S. K. (2004). PCNA interacts with Indian mung bean yellow mosaic virus rep and downregulates rep activity. J. Virol. 78, 11890–11903. doi: 10.1128/JVI.78.21.11890-11903.2004
Balakrishnan, L., and Bambara, R. A. (2013). Okazaki fragment metabolism. Cold Spring Harb. Perspect. Biol. 5:a010173. doi: 10.1101/cshperspect.a010173
Brough, C. L., Gardiner, W. E., Inamdar, N. M., Zhang, X.-Y., Ehrlich, M., and Bisaro, D. M. (1992). DNA methylation inhibits propagation of tomato golden mosaic virus DNA in transfected protoplasts. Plant Mol. Biol. 18, 703–712. doi: 10.1007/BF00020012
Campos-Olivas, R., Louis, J. M., Clérot, D., Gronenborn, B., and Gronenborn, A. M. (2002). The structure of a replication initiator unites diverse aspects of nucleic acid metabolism. Proc. Natl. Acad. Sci. 99, 10310–10315. doi: 10.1073/pnas.152342699
Carluccio, A. V., Prigigallo, M. I., Rosas-Diaz, T., Lozano-Duran, R., and Stavolone, L. (2018). S‐acylation mediates Mungbean yellow mosaic virus AC4 localization to the plasma membrane and in turns gene silencing suppression. PLoS Pathog. 14:e1007207. doi: 10.1371/journal.ppat.1007207
Castellano, M. M., Sanz-Burgos, A. P., and Gutiérrez, C. (1999). Initiation of DNA replication in a eukaryotic rolling-circle replicon: identification of multiple DNA-protein complexes at the geminivirus origin. J. Mol. Biol. 290, 639–652. doi: 10.1006/jmbi.1999.2916
Castillo, A. G., Collinet, D., Deret, S., Kashoggi, A., and Bejarano, E. R. (2003). Dual interaction of plant PCNA with geminivirus replication accessory protein (Ren) and viral replication protein (rep). Virology 312, 381–394. doi: 10.1016/S0042-6822(03)00234-4
Castillo, A., Kong, L., Hanley-Bowdoin, L., and Bejarano, E. (2004). Interaction between a geminivirus replication protein and the plant sumoylation system. J. Virol. 78, 2758–2769. doi: 10.1128/JVI.78.6.2758-2769.2004
Chandler, M., De La Cruz, F., Dyda, F., Hickman, A. B., Moncalian, G., and Ton-Hoang, B. (2013). Breaking and joining single-stranded DNA: the HUH endonuclease superfamily. Nat. Rev. Microbiol. 11, 525–538. doi: 10.1038/nrmicro3067
Cheng, Y., Cao, L., Wang, S., Li, Y., Shi, X., Liu, H., et al. (2013). Downregulation of multiple CDK inhibitor ICK/KRP genes upregulates the E2F pathway and increases cell proliferation, and organ and seed sizes in Arabidopsis. Plant J. 75, 642–655. doi: 10.1111/tpj.12228
Choudhury, N. R., Malik, P. S., Singh, D. K., Islam, M. N., Kaliappan, K., and Mukherjee, S. K. (2006). The oligomeric rep protein of Mungbean yellow mosaic India virus (MYMIV) is a likely replicative helicase. Nucleic Acids Res. 34, 6362–6377. doi: 10.1093/nar/gkl903
Clérot, D., and Bernardi, F. (2006). DNA helicase activity is associated with the replication initiator protein rep of tomato yellow leaf curl geminivirus. J. Virol. 80, 11322–11330. doi: 10.1128/JVI.00924-06
Collin, S., Fernández-lobato, M., Gooding, P. S., Mullineaux, P. M., and Fenoll, C. (1996). The two nonstructural proteins from wheat dwarf virus involved in viral gene expression and replication are retinoblastoma-binding proteins. Virology 219, 324–329. doi: 10.1006/viro.1996.0256
Costa, A., Hood, I. V., and Berger, J. M. (2013). Mechanisms for initiating cellular DNA replication. Ann. Rev. Biochem. 82, 25–54. doi: 10.1146/annurev-biochem-052610-094414
De Beeck, A. O., and Caillet-Fauquet, P. (1997). “Viruses and the cell cycle” in Progress in cell cycle research. eds. L. Meijer, S. Guidet, and M. Philippe (Boston, MA: Springer).
Desbiez, C., David, C., Mettouchi, A., Laufs, J., and Gronenborn, B. (1995). Rep protein of tomato yellow leaf curl geminivirus has an ATPase activity required for viral DNA replication. Proc. Natl. Acad. Sci. 92, 5640–5644. doi: 10.1073/pnas.92.12.5640
Devendran, R., Namgial, T., Reddy, K. K., Kumar, M., Zarreen, F., and Chakraborty, S. (2022). Insights into the multifunctional roles of geminivirus-encoded proteins in pathogenesis. Archiv. Virol. 167, 307–326. doi: 10.1007/s00705-021-05338-x
Eagle, P. A., Orozco, B. M., and Hanley-Bowdoin, L. (1994). A DNA sequence required for geminivirus replication also mediates transcriptional regulation. Plant Cell 6, 1157–1170. doi: 10.1105/tpc.6.8.1157
Eini, O., Behjatnia, S. A., Dogra, S., Dry, I. B., Randles, J. W., and Rezaian, M. A. (2009). Identification of sequence elements regulating promoter activity and replication of a monopartite begomovirus-associated DNA β satellite. J. Gen. Virol. 90, 253–260. doi: 10.1099/vir.0.002980-0
Ermark, G., Paszkowski, U., Wohlmuth, M., Scheid, O. M., and Paszkowski, J. (1993). Cytosine methylation inhibits replication of African cassava mosaic virus by two distinct mechanisms. Nucleic Acids Res. 21, 3445–3450. doi: 10.1093/nar/21.15.3445
Everett, B. A., Litzau, L. A., Tompkins, K., Shi, K., Nelson, A., Aihara, H., et al. (2019). Crystal structure of the wheat dwarf virus rep domain. Acta Crystallogr. Sect. F 75, 744–749. doi: 10.1107/S2053230X19015796
Faria, J. C., Albino, M. M., Dias, B. B., Cançado, L. J., da Cunha, N. B., Silva, L. M., et al. (2006). Partial resistance to bean golden mosaic virus in a transgenic common bean (Phaseolus vulgaris L.) line expressing a mutated rep gene. Plant Sci. 171, 565–571. doi: 10.1016/j.plantsci.2006.06.010
Fondong, V. N. (2019). The ever-expanding role of C4/AC4 in geminivirus infection: punching above its weight? Mol. Plant 12, 145–147. doi: 10.1016/j.molp.2018.12.006
Fontes, E., Eagle, P. A., Sipe, P. S., Luckow, V. A., and Hanley-Bowdoin, L. (1994). Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J. Biol. Chem. 269, 8459–8465. doi: 10.1016/S0021-9258(17)37216-2
Fontes, E., Luckow, V. A., and Hanley-Bowdoin, L. (1992). A geminivirus replication protein is a sequence-specific DNA binding protein. Plant Cell 4, 597–608. doi: 10.1105/tpc.4.5.597
George, B., Ruhel, R., Mazumder, M., Sharma, V. K., Jain, S. K., Gourinath, S., et al. (2014). Mutational analysis of the helicase domain of a replication initiator protein reveals critical roles of Lys 272 of the B′ motif and Lys 289 of the β-hairpin loop in geminivirus replication. J. Gen. Virol. 95, 1591–1602. doi: 10.1099/vir.0.064923-0
Gong, P., Tan, H., Zhao, S., Li, H., Liu, H., Ma, Y., et al. (2021). Geminiviruses encode additional small proteins with specific subcellular localizations and virulence function. Nat. Commun. 12, 1–11. doi: 10.1038/s41467-021-24617-4
Gutierrez, C. (2000). DNA replication and cell cycle in plants: learning from geminiviruses. EMBO J. 19, 792–799. doi: 10.1093/emboj/19.5.792
Gutierrez, C., Ramirez-Parra, E., Castellano, M. M., Sanz-Burgos, A. P., Luque, A., and Missich, R. (2004). Geminivirus DNA replication and cell cycle interactions. Vet. Microbiol. 98, 111–119. doi: 10.1016/j.vetmic.2003.10.012
Hamza, M., Tahir, M. N., Mustafa, R., Kamal, H., Khan, M. Z., Mansoor, S., et al. (2018). Identification of a dicot infecting mastrevirus along with alpha-and betasatellite associated with leaf curl disease of spinach (Spinacia oleracea) in Pakistan. Virus Res. 256, 174–182. doi: 10.1016/j.virusres.2018.08.017
Hanley-Bowdoin, L., Bejarano, E. R., Robertson, D., and Mansoor, S. (2013). Geminiviruses: masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 11, 777–788. doi: 10.1038/nrmicro3117
Hanley-Bowdoin, L., Elmer, J. S., and Rogers, S. G. (1990). Expression of functional replication protein from tomato golden mosaic virus in transgenic tobacco plants. Proc. Natl. Acad. Sci. 87, 1446–1450. doi: 10.1073/pnas.87.4.1446
Hanley-Bowdoin, L., Settlage, S. B., Orozco, B. M., Nagar, S., and Robertson, D. (1999). Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Plant Sci. 18, 71–106.
Hefferon, K. L., Moon, Y. S., and Fan, Y. (2006). Multi‐tasking of nonstructural gene products is required for bean yellow dwarf geminivirus transcriptional regulation. FEBS J. 273, 4482–4494. doi: 10.1111/j.1742-4658.2006.05454.x
Heyraud-Nitschke, F., Schumacher, S., Laufs, J., Schaefer, S., Schell, J., and Gronenborn, B. (1995). Determination of the origin cleavage and joining domain of geminivirus rep proteins. Nucleic Acid Res. 23, 910–916. doi: 10.1093/nar/23.6.910
Hipp, K., Rau, P., Schäfer, B., Gronenborn, B., and Jeske, H. (2014). The RXL motif of the African cassava mosaic virus rep protein is necessary for rereplication of yeast DNA and viral infection in plants. Virology 462-463, 189–198. doi: 10.1016/j.virol.2014.06.002
Hofer, J., Dekker, E. L., Reynolds, H. V., Woolston, C. J., Cox, B. S., and Mullineaux, P. M. (1992). Coordinate regulation of replication and virion sense gene expression in wheat dwarf virus. Plant Cell 4, 213–223. doi: 10.1105/tpc.4.2.213
Horváth, G. V., Pettkó-Szandtner, A., Nikovics, K., Bilgin, M., Boulton, M., Davies, J. W., et al. (1998). Prediction of functional regions of the maize streak virus replication-associated proteins by protein-protein interaction analysis. Plant Mol. Biol. 38, 699–712. doi: 10.1023/A:1006076316887
Ilyina, T. V., and Koonin, E. V. (1992). Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acid Res. 20, 3279–3285. doi: 10.1093/nar/20.13.3279
Ismayil, A., Haxim, Y., Wang, Y., Li, H., Qian, L., Han, T., et al. (2018). Cotton leaf curl Multan virus C4 protein suppresses both transcriptional and post-transcriptional gene silencing by interacting with SAM synthetase. PLoS Pathog. 14:e1007282. doi: 10.1371/journal.ppat.1007282
Jeske, H., Lütgemeier, M., and Preiß, W. (2001). DNA forms indicate rolling circle and recombination-dependent replication of Abutilon mosaic virus. EMBO J. 20, 6158–6167. doi: 10.1093/emboj/20.21.6158
Kaliappan, K., Choudhury, N. R., Suyal, G., and Mukherjee, S. K. (2012). A novel role for RAD54: this host protein modulates geminiviral DNA replication. FASEB J. 26, 1142–1160. doi: 10.1096/fj.11-188508
Kazlauskas, D., Varsani, A., Koonin, E. V., and Krupovic, M. (2019). Multiple origins of prokaryotic and eukaryotic single-stranded DNA viruses from bacterial and archaeal plasmids. Nat. Commun. 10, 1–12. doi: 10.1038/s41467-019-11433-0
King, A. M., Lefkowitz, E. J., Mushegian, A. R., Adams, M. J., Dutilh, B. E., Gorbalenya, A. E., et al. (2018). Changes to taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses (2018). Arch. Virol. 163, 2601–2631. doi: 10.1007/s00705-018-3847-1
Kong, L.-J., and Hanley-Bowdoin, L. (2002). A geminivirus replication protein interacts with a protein kinase and a motor protein that display different expression patterns during plant development and infection. Plant Cell 14, 1817–1832. doi: 10.1105/tpc.003681
Kong, L.-J., Orozco, B. M., Roe, J. L., Nagar, S., Ou, S., Feiler, H. S., et al. (2000). A geminivirus replication protein interacts with the retinoblastoma protein through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J. 19, 3485–3495. doi: 10.1093/emboj/19.13.3485
Koonin, E. V., and Ilyina, T. V. (1992). Geminivirus replication proteins are related to prokaryotic plasmid rolling circle DNA replication initiator proteins. J. Gen. Virol. 73, 2763–2766. doi: 10.1099/0022-1317-73-10-2763
Krenz, B., Neugart, F., Kleinow, T., and Jeske, H. (2011). Self-interaction of Abutilon mosaic virus replication initiator protein (rep) in plant cell nuclei. Virus Res. 161, 194–197. doi: 10.1016/j.virusres.2011.07.020
Krupovic, M., Ravantti, J. J., and Bamford, D. H. (2009). Geminiviruses: a tale of a plasmid becoming a virus. BMC Evol. Biol. 9, 112–111. doi: 10.1186/1471-2148-9-112
Krupovic, M., Varsani, A., Kazlauskas, D., Breitbart, M., Delwart, E., Rosario, K., et al. (2020). Cressdnaviricota: a virus phylum unifying seven families of rep-encoding viruses with single-stranded, circular DNA genomes. J. Virol. 94:e00582-20. doi: 10.1128/JVI.00582-20
Kulshreshtha, A., Kumar, Y., Roshan, P., Bhattacharjee, B., Mukherjee, S. K., and Hallan, V. (2019). AC4 protein of tomato leaf curl Palampur virus is an RNA silencing suppressor and a pathogenicity determinant. Microb. Pathog. 135:103636. doi: 10.1016/j.micpath.2019.103636
Kumar, J., Kumar, J., Singh, S. P., and Tuli, R. (2014). Association of satellites with a mastrevirus in natural infection: complexity of wheat dwarf India virus disease. J. Virol. 88, 7093–7104. doi: 10.1128/JVI.02911-13
Kushwaha, N. K., Bhardwaj, M., and Chakraborty, S. (2017). The replication initiator protein of a geminivirus interacts with host monoubiquitination machinery and stimulates transcription of the viral genome. PLoS Pathog. 13:e1006587. doi: 10.1371/journal.ppat.1006587
Lai, J., Chen, H., Teng, K., Zhao, Q., Zhang, Z., Li, Y., et al. (2009). RKP, a RING finger E3 ligase induced by BSCTV C4 protein, affects geminivirus infection by regulation of the plant cell cycle. Plant J. 57, 905–917. doi: 10.1111/j.1365-313X.2008.03737.x
Laufs, J., Jupin, I., David, C., Schumacher, S., Heyraud-Nitschke, F., and Gronenborn, B. (1995). Geminivirus replication: genetic and biochemical characterization of rep protein function, a review. Biochimie 77, 765–773. doi: 10.1016/0300-9084(96)88194-6
Lazarowitz, S. G., Wu, L. C., Rogers, S. G., and Elmer, J. S. (1992). Sequence-specific interaction with the viral AL1 protein identifies a geminivirus DNA replication origin. Plant Cell 4, 799–809. doi: 10.1105/tpc.4.7.799
Lee, J.-S., Shukla, A., Schneider, J., Swanson, S. K., Washburn, M. P., Florens, L., et al. (2007). Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cells 131, 1084–1096. doi: 10.1016/j.cell.2007.09.046
Li, D., Behjatnia, S. A., Dry, I. B., Randles, J. W., Eini, O., and Rezaian, M. A. (2007). Genomic regions of tomato leaf curl virus DNA satellite required for replication and for satellite-mediated delivery of heterologous DNAs. J. Gen. Virol. 88, 2073–2077. doi: 10.1099/vir.0.82853-0
Liu, Y., Jin, W., Wang, L., and Wang, X. (2014). Replication-associated proteins encoded by wheat dwarf virus act as RNA silencing suppressors. Virus Res. 190, 34–39. doi: 10.1016/j.virusres.2014.06.014
Liu, L., Saunders, K., Thomas, C. L., Davies, J. W., and Stanley, J. (1999). Bean yellow dwarf virus RepA, but not rep, binds to maize retinoblastoma protein, and the virus tolerates mutations in the consensus binding motif. Virology 256, 270–279. doi: 10.1006/viro.1999.9616
Luna, A. P., and Lozano-Durán, R. (2020). Geminivirus-encoded proteins: not all positional homologs are made equal. Front. Microbiol. 11:878. doi: 10.3389/fmicb.2020.00878
Luna, A. P., Morilla, G., Voinnet, O., and Bejarano, E. R. (2012). Functional analysis of gene-silencing suppressors from tomato yellow leaf curl disease viruses. Mol. Plant Microbe Interact. 25, 1294–1306. doi: 10.1094/MPMI-04-12-0094-R
Luque, A., Sanz-Burgos, A. P., Ramirez-Parra, E., Castellano, M. M., and Gutierrez, C. (2002). Interaction of geminivirus rep protein with replication factor C and its potential role during geminivirus DNA replication. Virology 302, 83–94. doi: 10.1006/viro.2002.1599
MacAlpine, D. M., and Almouzni, G. (2013). Chromatin and DNA replication. Cold Spring Harb. Perspect. Biol. 5:a010207. doi: 10.1101/cshperspect.a010207
Maio, F., Arroyo-Mateos, M., Bobay, B. G., Bejarano, E. R., Prins, M., and van den Burg, H. A. (2019). A lysine residue essential for geminivirus replication also controls nuclear localization of the tomato yellow leaf curl virus rep protein. J. Virol. 93, e01910–e01918. doi: 10.1128/JVI.01910-18
Maio, F., Helderman, T. A., Arroyo-Mateos, M., van der Wolf, M., Boeren, S., Prins, M., et al. (2020). Identification of tomato proteins that interact with replication initiator protein (rep) of the geminivirus TYLCV. Front. Plant Sci. 11:1069. doi: 10.3389/fpls.2020.01069
Malik, P. S., Kumar, V., Bagewadi, B., and Mukherjee, S. K. (2005). Interaction between coat protein and replication initiation protein of mung bean yellow mosaic India virus might lead to control of viral DNA replication. Virology 337, 273–283. doi: 10.1016/j.virol.2005.04.030
Medina-Puche, L., Tan, H., Dogra, V., Wu, M., Rosas-Diaz, T., Wang, L., et al. (2020). A defense pathway linking plasma membrane and chloroplasts and co-opted by pathogens. Cells 182, 1109–1124. doi: 10.1016/j.cell.2020.07.020
Mei, Y., Yang, X., Huang, C., Zhang, X., and Zhou, X. (2018). Tomato leaf curl Yunnan virus-encoded C4 induces cell division through enhancing stability of cyclin D 1.1 via impairing NbSKη-mediated phosphorylation in Nicotiana benthamiana. PLoS Pathog. 14:e1006789. doi: 10.1371/journal.ppat.1006789
Mills-Lujan, K., and Deom, C. M. (2010). Geminivirus C4 protein alters Arabidopsis development. Protoplasma 239, 95–110. doi: 10.1007/s00709-009-0086-z
Missich, R., Ramirez-Parra, E., and Gutierrez, C. (2000). Relationship of oligomerization to DNA binding of wheat dwarf virus RepA and rep proteins. Virology 273, 178–188. doi: 10.1006/viro.2000.0412
Nagar, S., Hanley-Bowdoin, L., and Robertson, D. (2002). Host DNA replication is induced by geminivirus infection of differentiated plant cells. Plant Cell 14, 2995–3007. doi: 10.1105/tpc.005777
Nash, T. E., Dallas, M. B., Reyes, M. I., Buhrman, G. K., Ascencio-Ibanez, J. T., and Hanley-Bowdoin, L. (2011). Functional analysis of a novel motif conserved across geminivirus rep proteins. J. Virol. 85, 1182–1192. doi: 10.1128/JVI.02143-10
Nawaz-ul-Rehman, M. S., Nahid, N., Mansoor, S., Briddon, R. W., and Fauquet, C. M. (2010). Post-transcriptional gene silencing suppressor activity of two non-pathogenic alphasatellites associated with a begomovirus. Virology 405, 300–308. doi: 10.1016/j.virol.2010.06.024
Orozco, B. M., and Hanley-Bowdoin, L. (1998). Conserved sequence and structural motifs contribute to the DNA binding and cleavage activities of a geminivirus replication protein. J. Biol. Chem. 273, 24448–24456. doi: 10.1074/jbc.273.38.24448
Orozco, B. M., Miller, A. B., Settlage, S. B., and Hanley-Bowdoin, L. (1997). Functional domains of a geminivirus replication protein. J. Biol. Chem. 272, 9840–9846. doi: 10.1074/jbc.272.15.9840
Oshima, K., Kakizawa, S., Nishigawa, H., Kuboyama, T., Miyata, S.-I., Ugaki, M., et al. (2001). A plasmid of phytoplasma encodes a unique replication protein having both plasmid-and virus-like domains: clue to viral ancestry or result of virus/plasmid recombination? Virology 285, 270–277. doi: 10.1006/viro.2001.0938
Paprotka, T., Deuschle, K., Metzler, V., and Jeske, H. (2011). Conformation-selective methylation of geminivirus DNA. J. Virol. 85, 12001–12012. doi: 10.1128/JVI.05567-11
Pasumarthy, K. K., Choudhury, N. R., and Mukherjee, S. K. (2010). Tomato leaf curl Kerala virus (ToLCKeV) AC3 protein forms a higher order oligomer and enhances ATPase activity of replication initiator protein (rep/AC1). Virol. J. 7, 1–8. doi: 10.1186/1743-422X-7-128
Pilartz, M., and Jeske, H. (2003). Mapping of abutilon mosaic geminivirus minichromosomes. J. Virol. 77, 10808–10818. doi: 10.1128/JVI.77.20.10808-10818.2003
Qing, L., and Zhou, X. (2009). Trans-replication of, and competition between, DNA β satellites in plants inoculated with tomato yellow leaf curl China virus and tobacco curly shoot virus. Phytopathology 99, 716–720. doi: 10.1094/PHYTO-99-6-0716
Raja, P., Sanville, B. C., Buchmann, R. C., and Bisaro, D. M. (2008). Viral genome methylation as an epigenetic defense against geminiviruses. J. Virol. 82, 8997–9007. doi: 10.1128/JVI.00719-08
Raja, P., Wolf, J. N., and Bisaro, D. M. (2010). RNA silencing directed against geminiviruses: post-transcriptional and epigenetic components. Biochim. Biophys. Acta Gene Regul. Mech. 1799, 337–351. doi: 10.1016/j.bbagrm.2010.01.004
Reed, S. M., Bayly, W. M., and Sellon, D. C. (2004). Mechanisms of infectious disease. Equine Int. Med. 2004, 59–109. doi: 10.1016/B0-72-169777-1/50004-4
Richter, K. S., Ende, L., and Jeske, H. (2015). Rad54 is not essential for any geminiviral replication mode in planta. Plant Mol. Biol. 87, 193–202. doi: 10.1007/s11103-014-0270-1
Richter, K. S., Serra, H., White, C. I., and Jeske, H. (2016). The recombination mediator RAD51D promotes geminiviral infection. Virology 493, 113–127. doi: 10.1016/j.virol.2016.03.014
Rishishwar, R., and Dasgupta, I. (2019). Suppressors of RNA silencing encoded by geminiviruses and associated DNA satellites. Virus 30, 58–65. doi: 10.1007/s13337-018-0418-8
Rodríguez‐Negrete, E., Lozano‐Durán, R., Piedra‐Aguilera, A., Cruzado, L., Bejarano, E. R., and Castillo, A. G. (2013). GeminivirusRep protein interferes with the plantDNAmethylation machinery and suppresses transcriptional gene silencing. New Phytol. 199, 464–475. doi: 10.1111/nph.12286
Rojas, M. R., Jiang, H., Salati, R., Xoconostle-Cázares, B., Sudarshana, M., Lucas, W. J., et al. (2001). Functional analysis of proteins involved in movement of the monopartite begomovirus, tomato yellow leaf curl virus. Virology 291, 110–125. doi: 10.1006/viro.2001.1194
Rosas-Diaz, T., Zhang, D., Fan, P., Wang, L., Ding, X., Jiang, Y., et al. (2018). A virus-targeted plant receptor-like kinase promotes cell-to-cell spread of RNAi. Proc. Natl. Acad. Sci. U. S. A. 115, 1388–1393. doi: 10.1073/pnas.1715556115
Roumagnac, P., Lett, J.-M., Fiallo-Olivé, E., Navas-Castillo, J., Zerbini, F. M., Martin, D. P., et al. (2022). Establishment of five new genera in the family Geminiviridae: Citlodavirus, Maldovirus, Mulcrilevirus, Opunvirus, and Topilevirus. Arch. Virol. 167, 695–710. doi: 10.1007/s00705-021-05309-2
Ruschhaupt, M., Martin, D. P., Lakay, F., Bezuidenhout, M., Rybicki, E. P., Jeske, H., et al. (2013). Replication modes of maize streak virus mutants lacking RepA or the RepA–pRBR interaction motif. Virology 442, 173–179. doi: 10.1016/j.virol.2013.04.012
Sánchez-Durán, M. A., Dallas, M. B., Ascencio-Ibanez, J. T., Reyes, M. I., Arroyo-Mateos, M., Ruiz-Albert, J., et al. (2011). Interaction between geminivirus replication protein and the SUMO-conjugating enzyme is required for viral infection. J. Virol. 85, 9789–9800. doi: 10.1128/JVI.02566-10
Sanz-Burgos, A. P., and Gutiérrez, C. (1998). Organization of thecis-acting element required for wheat dwarf Geminivirus DNA replication and visualization of a rep protein–DNA complex. Virology 243, 119–129. doi: 10.1006/viro.1998.9037
Sardo, L., Lucioli, A., Tavazza, M., Masenga, V., Tavazza, R., Accotto, G. P., et al. (2011). An RGG sequence in the replication-associated protein (rep) of tomato yellow leaf curl Sardinia virus is involved in transcriptional repression and severely impacts resistance in rep-expressing plants. J. Gen. Virol. 92, 204–209. doi: 10.1099/vir.0.025817-0
Saunders, K., Briddon, R. W., and Stanley, J. (2008). Replication promiscuity of DNA-β satellites associated with monopartite begomoviruses; deletion mutagenesis of the Ageratum yellow vein virus DNA-β satellite localizes sequences involved in replication. J. Gen. Virol. 89, 3165–3172. doi: 10.1099/vir.0.2008/003848-0
Schalk, H.-J., Matzeit, V., Schiller, B., Schell, J., and Gronenborn, B. (1989). Wheat dwarf virus, a geminivirus of graminaceous plants needs splicing for replication. EMBO J. 8, 359–364. doi: 10.1002/j.1460-2075.1989.tb03385.x
Selth, L. A., Dogra, S. C., Rasheed, M. S., Healy, H., Randles, J. W., and Rezaian, M. A. (2005). A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication. Plant Cell 17, 311–325. doi: 10.1105/tpc.104.027235
Settlage, S. B., Miller, A. B., Gruissem, W., and Hanley-Bowdoin, L. (2001). Dual interaction of a geminivirus replication accessory factor with a viral replication protein and a plant cell cycle regulator. Virology 279, 570–576. doi: 10.1006/viro.2000.0719
Settlage, S. B., Miller, A. B., and Hanley-Bowdoin, L. (1996). Interactions between geminivirus replication proteins. J. Virol. 70, 6790–6795. doi: 10.1128/jvi.70.10.6790-6795.1996
Settlage, S. B., See, R. G., and Hanley-Bowdoin, L. (2005). Geminivirus C3 protein: replication replication enhancement and protein interactions. J. Virol. 79, 9885–9895. doi: 10.1128/JVI.79.15.9885-9895.2005
Shakir, S., Jander, G., Nahid, N., Mubin, M., Younus, A., and Nawaz-Ul-Rehman, M. S. (2021). Interaction of eukaryotic proliferating cell nuclear antigen (PCNA) with the replication-associated protein (rep) of cotton leaf curl Multan virus and pedilanthus leaf curl virus. 3 Biotech 11:14. doi: 10.1007/s13205-020-02499-5
Shen, W., Bobay, B. G., Greeley, L. A., Reyes, M. I., Rajabu, C. A., Blackburn, R. K., et al. (2018). Sucrose nonfermenting 1-related protein kinase 1 phosphorylates a geminivirus rep protein to impair viral replication and infection. Plant Physiol. 178, 372–389. doi: 10.1104/pp.18.00268
Shung, C.-Y., and Sunter, G. (2007). AL1-dependent repression of transcription enhances expression of tomato golden mosaic virus AL2 and AL3. Virology 364, 112–122. doi: 10.1016/j.virol.2007.03.006
Siddiqui, K., On, K. F., and Diffley, J. F. (2013). Regulating DNA replication in eukarya. Cold Spring Harb. Perspect. Biol. 5:a012930. doi: 10.1101/cshperspect.a012930
Singh, D. K., Islam, M. N., Choudhury, N. R., Karjee, S., and Mukherjee, S. K. (2007). The 32 kDa subunit of replication protein a (RPA) participates in the DNA replication of mung bean yellow mosaic India virus (MYMIV) by interacting with the viral rep protein. Nucleic Acids Res. 35, 755–770. doi: 10.1093/nar/gkl1088
Singh, D. K., Malik, P. S., Choudhury, N. R., and Mukherjee, S. K. (2008). MYMIV replication initiator protein (rep): roles at the initiation and elongation steps of MYMIV DNA replication. Virology 380, 75–83. doi: 10.1016/j.virol.2008.07.010
Skarstad, K., and Katayama, T. (2013). Regulating DNA replication in bacteria. Cold Spring Harb. Perspect. Biol. 5:a012922. doi: 10.1101/cshperspect.a012922
Suyal, G., Mukherjee, S. K., and Choudhury, N. R. (2013a). The host factor RAD51 is involved in mungbean yellow mosaic India virus (MYMIV) DNA replication. Arch. Gesamte Virusforsch. 158, 1931–1941. doi: 10.1007/s00705-013-1675-x
Suyal, G., Mukherjee, S. K., Srivastava, P. S., and Choudhury, N. R. (2013b). Arabidopsis thaliana MCM2 plays role (s) in mungbean yellow mosaic India virus (MYMIV) DNA replication. Arch. Virol. 158, 981–992. doi: 10.1007/s00705-012-1563-9
Suyal, G., Rana, V. S., Mukherjee, S. K., Wajid, S., and Choudhury, N. R. (2014). Arabidopsis thaliana NAC083 protein interacts with Mungbean yellow mosaic India virus (MYMIV) rep protein. Virus Genes 48, 486–493. doi: 10.1007/s11262-013-1028-6
Varsani, A., Roumagnac, P., Fuchs, M., Navas-Castillo, J., Moriones, E., Idris, A., et al. (2017). Capulavirus and Grablovirus: two new genera in the family Geminiviridae. Arch. Virol. 162, 1819–1831. doi: 10.1007/s00705-017-3268-6
Vinutha, T., Kumar, G., Garg, V., Canto, T., Palukaitis, P., Ramesh, S., et al. (2018). Tomato geminivirus encoded RNAi suppressor protein, AC4 interacts with host AGO4 and precludes viral DNA methylation. Gene 678, 184–195. doi: 10.1016/j.gene.2018.08.009
Wang, Y., Dang, M., Hou, H., Mei, Y., Qian, Y., and Zhou, X. (2014). Identification of an RNA silencing suppressor encoded by a mastrevirus. J. Gen. Virol. 95, 2082–2088. doi: 10.1099/vir.0.064246-0
Wang, L., Ding, X., Xiao, J., Jiménez-Gόngora, T., Liu, R., and Lozano-Durán, R. (2017). Inference of a geminivirus− host protein− protein interaction network through affinity purification and mass spectrometry analysis. Viruses 9:275. doi: 10.3390/v9100275
Wu, M., Wei, H., Tan, H., Pan, S., Liu, Q., Bejarano, E. R., et al. (2021). Plant DNA polymerases α and δ mediate replication of geminiviruses. Nat. Commun. 12, 1–10. doi: 10.1038/s41467-021-23013-2
Xie, Q., Sanz-Burgos, A. P., Guo, H., García, J. A., and Gutiérrez, C. (1999). GRAB proteins, novel members of the NAC domain family, isolated by their interaction with a geminivirus protein. Plant Mol. Biol. 39, 647–656. doi: 10.1023/A:1006138221874
Xie, Q., Sanz‐Burgos, A., Hannon, G., and Gutiérrez, C. (1996). Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins. EMBO J. 15, 4900–4908. doi: 10.1002/j.1460-2075.1996.tb00870.x
Xie, Q., Suárez‐López, P., and Gutierrez, C. (1995). Identification and analysis of a retinoblastoma binding motif in the replication protein of a plant DNA virus: requirement for efficient viral DNA replication. EMBO J. 14, 4073–4082. doi: 10.1002/j.1460-2075.1995.tb00079.x
Xu, X., Qian, Y., Wang, Y., Li, Z., and Zhou, X. (2019). Iterons homologous to helper geminiviruses are essential for efficient replication of betasatellites. J. Virol. 93, e01532–e01518. doi: 10.1128/JVI.01532-18
Zerbini, F. M., Briddon, R. W., Idris, A., Martin, D. P., Moriones, E., Navas-Castillo, J., et al. (2017). ICTV virus taxonomy profile: Geminiviridae. J. Gen. Virol. 98:131. doi: 10.1099/jgv.0.000738
Zhan, B., Zhao, W., Li, S., Yang, X., and Zhou, X. (2018). Functional scanning of apple geminivirus proteins as symptom determinants and suppressors of posttranscriptional gene silencing. Viruses 10:488. doi: 10.3390/v10090488
Zhang, T., Xu, X., Huang, C., Qian, Y., Li, Z., and Zhou, X. (2016). A novel DNA motif contributes to selective replication of a geminivirus-associated betasatellite by a helper virus-encoded replication-related protein. J. Virol. 90, 2077–2089. doi: 10.1128/JVI.02290-15
Zhao, S., Gong, P., Ren, Y., Liu, H., Li, H., Li, F., et al. (2022). The novel C5 protein from tomato yellow leaf curl virus is a virulence factor and suppressor of gene silencing. Stress Biol. 2:19. doi: 10.1007/s44154-022-00044-3
Keywords: geminiviruses, replication, pathogenicity, virus interaction, host factors
Citation: Shakir S, Mubin M, Nahid N, Serfraz S, Qureshi MA, Lee T-K, Liaqat I, Lee S and Nawaz-ul-Rehman MS (2023) REPercussions: how geminiviruses recruit host factors for replication. Front. Microbiol. 14:1224221. doi: 10.3389/fmicb.2023.1224221
Edited by:
Ashish Srivastava, Amity University, IndiaReviewed by:
Marcos de la Peña, Polytechnic University of Valencia, SpainCharith Raj Adkar-Purushothama, Université de Sherbrooke, Canada
Copyright © 2023 Shakir, Mubin, Nahid, Serfraz, Qureshi, Lee, Liaqat, Lee and Nawaz-ul-Rehman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Shah Nawaz-ul-Rehman, bXNuYXdhenVscmVobWFuQHVhZi5lZHUucGs=; Sukchan Lee, Y2VsbDR1QHNra3UuZWR1
 Sara Shakir1
Sara Shakir1 Muhammad Mubin
Muhammad Mubin Saad Serfraz
Saad Serfraz Muhammad Amir Qureshi
Muhammad Amir Qureshi Taek-Kyun Lee
Taek-Kyun Lee Iram Liaqat
Iram Liaqat Sukchan Lee
Sukchan Lee Muhammad Shah Nawaz-ul-Rehman
Muhammad Shah Nawaz-ul-Rehman