
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 13 September 2023
Sec. Food Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1224085
This article is part of the Research TopicNatural Compounds and Novel Sources of Antimicrobial Agents for Food Preservation and Biofilm Control, Volume IIView all 7 articles
Multidrug-resistant bacteria such as Staphylococcus aureus constitute a global health problem. Gram-positive S. aureus secretes various toxins associated with its pathogenesis, and its biofilm formation plays an important role in antibiotic tolerance and virulence. Hence, we investigated if the metabolites of vitamin A1 might diminish S. aureus biofilm formation and toxin production. Of the three retinoic acids examined, 13-cis-retinoic acid at 10 μg/mL significantly decreased S. aureus biofilm formation without affecting its planktonic cell growth (MIC >400 μg/mL) and also inhibited biofilm formation by Staphylococcus epidermidis (MIC >400 μg/mL), but less affected biofilm formation by a uropathogenic Escherichia coli strain, a Vibrio strain, or a fungal Candida strain. Notably, 13-cis-retinoic acid and all-trans-retinoic acid significantly inhibited the hemolytic activity and staphyloxanthin production by S. aureus. Furthermore, transcriptional analysis disclosed that 13-cis-retinoic acid repressed the expressions of virulence- and biofilm-related genes, such as the two-component arlRS system, α-hemolysin hla, nuclease (nuc1 and nuc2), and psmα (phenol soluble modulins α) in S. aureus. In addition, plant and nematode toxicity assays showed that 13-cis-retinoic acid was only mildly toxic at concentrations many folds higher than its effective antibiofilm concentrations. These findings suggest that metabolites of vitamin A1, particularly 13-cis-retinoic acid, might be useful for suppressing biofilm formation and the virulence characteristics of S. aureus.
Infections caused by drug-resistant bacteria are increasing globally, but the rate of novel antibiotic discovery has declined continuously over past decades. Accordingly, other treatment strategies, such as antitoxin and antibiofilm-based approaches, are being investigated to cope with drug-resistant microbes. Unlike antimicrobial agents, antivirulence compounds are required to reduce the virulence characteristics of microbes without negatively affecting cell growth because microbial killing is associated with a higher risk of developing drug resistance (Dickey et al., 2017).
Staphylococcus aureus is a main cause of community-acquired and nosocomial infections due to its multidrug resistance. This bacterium produces various virulence factors, such as hemolysin, enterotoxins, and immune evasive staphyloxanthin, and causes diverse life-threatening infections, including bacteremia, pulmonary infections, gastroenteritis, toxic shock syndrome, and skin infections (Tong et al., 2015). S. aureus readily forms chronic biofilms on host cells and medical devices and implants. Moreover, this ability to form biofilms plays critical roles in antibiotic tolerance and virulence and significantly increases morbidity and mortality rates, particularly when associated with indwelling medical devices (Moormeier and Bayles, 2017). Hence, inhibiting virulence factor production and biofilm formation (Winkelströter et al., 2014; Park et al., 2022) offer alternative means of fighting recalcitrant S. aureus infections.
Although the antibiofilm potential of vitamins has long been suggested, antibiofilm activities have been attributed to relatively few, such as vitamin B12 against Pseudomonas aeruginosa (Lee et al., 2012), vitamin C against Escherichia coli (Shivaprasad et al., 2021), and Klebsiella pneumoniae (Xu et al., 2022), and vitamin D against S. aureus (Vergalito et al., 2018). However, no study has yet investigated the antibiofilm characteristics of vitamin metabolites.
In this study, we sought to identify a compound that inhibits biofilm formation and toxin production of S. aureus without killing the bacterium. Three metabolites of vitamin A1 (all-trans-retinoic acid, 9-cis-retinoic acid, and 13-cis-retinoic acid) were initially investigated for their antibiofilm activity against S. aureus. The most active, 13-cis-retinoic acid, was further investigated for the activity against three other Staphylococcus strains, a Staphylococcus epidermidis strain, an uropathogenic Escherichia coli strain, a Vibrio parahaemolyticus strain, and a Candida strain. Live imaging microscopy, scanning electron microscopy, qRT-PCR, and hemolysis and lipase activities were used to investigate how 13-cis-retinoic acid affects biofilm formation and toxin production of S. aureus. In addition, the toxicity of 13-cis-retinoic acid was investigated using nematode Caenorhabditis elegans and plant Brassica rapa models, and ADME simulation was performed.
Two methicillin-sensitive S. aureus strains (MSSA; ATCC 6538 and ATCC 25923) and two methicillin-resistant S. aureus strains (MRSA 33591 and MW2), an S. epidermidis strain (ATCC 14990), a fungal Candida albicans DAY185 strain, a uropathogenic E. coli O6:H1 strain CFT073 (ATCC 700928), and a Vibrio parahaemolyticus strain ATCC 17802 were used. Cultures of MSSA ATCC 6538, ATCC 25923, and S. epidermidis strains were performed in Luria-Bertani (LB) broth, and MRSA ATCC 33591 and MW2 strains were cultivated in LB medium containing 0.2% glucose at 37°C and 30°C. Candida albicans DAY185 was cultured in potato dextrose broth (PDB). UPEC and V. parahaemolyticus were cultured in nutrient broth (NB) and LB supplemented with 3% (w/v) NaCl (mLB) at 37°C, respectively. All-trans-retinoic acid, 9-cis-retinoic acid, 13-cis-retinoic acid, and crystal violet were obtained from Sigma-Aldrich (St. Louis, MO, United States). Dimethyl sulfoxide (DMSO) was used to dissolve retinoic acids, and DMSO (0.1% v/v) was used as a control and it did not affect cell growth or biofilm formation. For planktonic cell growth assay, colony-forming units (CFU) were determined after incubating S. aureus cells in 96-well plates in LB medium with or without 13-cis-retinoic acid for 24 h.
A crystal violet staining assay was conducted using 96-well plates, as previously reported (Lee et al., 2021). S. aureus cells (~107 CFU/mL) were inoculated into LB medium and retinoic acids were added at 0, 2, 5, 10, 20, 50, or 100 μg/mL to the wells of 96-well plates and cultivated for 24 h at 37°C without agitation. Biofilm formation was measured by discarding planktonic cells and washing the plates three times with distilled water. Biofilm cells were then stained with 0.1% crystal violet (300 μL) for 20 min and washed three times with water to remove crystal violet. Crystal violet stained cells were then extracted with 95% ethanol (300 μL) by shaking vigorously. Absorbances were measured at 570 nm (OD570) using a Multiskan plate reader (Thermo Fisher Scientific, Waltham, MA, United States). Biofilm formation results are obtained from three independent cultures of six replicate wells.
After forming S. aureus biofilms in 96-well plates in the presence or absence of 13-cis-retinoic acid (0, 2, 5, or 10 μg/mL) for 24 h at 37°C, planktonic cells were removed by washing three times with distilled water, and live biofilm cells were observed by the iRiS™ Digital Cell Imaging System (Logos Biosystems, Anyang, Korea). Color-coded 3D biofilm images were generated using ImageJ.1
Also, SEM was used to observe biofilm reduction by 13-cis-retinoic acid, as previously reported (Park et al., 2022). S. aureus ATCC 6538 cells (~107 CFU/mL) were inoculated into 1 mL of fresh LB medium with or without 13-cis-retinoic acid (0, 2, 5, or 10 μg/mL) in a 96-well plate. A piece of nylon membrane (~ 0.16 cm2) was placed in each well, and S. aureus cells were cultured for 24 h at 37°C without agitation. Biofilms developed on the membrane were then fixed with a glutaraldehyde (2.5%) and formaldehyde (2%) for 24 h, post-fixed with OsO4 (1%), and dehydrated with ethanol and isoamyl acetate (99%). After drying biofilms using critical-point dryer (HCP-2, Hitachi, Tokyo, Japan), biofilm cells were coated with Precision Etching Coating System (Gatan, Inc., Pleasanton, United States) and observed under a field emission scanning electron microscope S-4800 (Hitachi, Tokyo, Japan).
The hemolysis of sheep blood cells (MBcell, Seoul, Korea) was investigated as described previously (Kim et al., 2022a). S. aureus ATCC 6538 cells (~2 × 107 CFU/mL) were diluted in 2 mL of fresh LB medium, cultivated with retinoic acids (0, 0.5, 1, 2, 5, or 10 μg/mL) for 24 h with 250 rpm shaking. Fresh sheep blood cells were collected by centrifugation at 3,000 × g for 2 min, the red blood cells were then cleaned three times with PBS and resuspended gently in PBS buffer (3.3%). S. aureus cell culture (100 μL) was then added to 1 mL of red blood cells and incubated for 4 h at 37°C with shaking at 250 rpm. The mixtures were centrifugated at 16,600 × g for 10 min, and the absorbances of supernatants were measured at 543 nm.
Staphylococcus aureus ATCC 6538 cells (~2 × 107 CFU/mL) were inoculated into LB medium (2 mL) in 14-mL tubes and incubated for 24 h at 37°C with 13-cis-retinoic acid (0, 10, 20, 50, or 100 μg/mL) with shaking at 250 rpm. Staphyloxanthin levels were assessed optically, as previously described (De Souza Feitosa Lima et al., 2019; Kim et al., 2022a).
To quantify the effect of 13-cis-retinoic acid on extracellular lipase production, S. aureus ATCC 6538 cells (~2×107 CFU/mL) were inoculated into LB medium (2 mL) in 14-mL tubes and incubated for 20 h at 37°C with 250 rpm shaking with or without 13-cis-retinoic acid (0, 2, 5, 10, 20, or 50 μg/mL), as previously reported (Lee et al., 2022). Briefly, culture supernatants (0.1 mL) were mixed with 0.9 mL of substrate solution (10% of buffer A with 3 mg/mL of p-nitrophenyl palmitate in isopropyl alcohol and 90% of buffer B with 1 mg/mL of gum arabic and 2 mg/mL sodium deoxycholate in 50 mM Na2PO4 buffer and then heated at 40°C for 30 min). The reactions were stopped by adding 1 M Na2CO3. Absorbances of the reaction supernatant were measured at 405 nm.
S. aureus ATCC 6538 cells at OD600 of 0.05 were inoculated to 15 mL of LB medium in a 250 mL flat-bottomed flask and incubated for 6 h at 37°C with shaking at 250 rpm with or without 13-cis-retinoic acid (100 μg/mL). Cells were then treated with RNase inhibitor (RNAlater, Ambion, TX, USA) for preventing RNA degradation and harvested by centrifugation at 16,600 × g for 1 min. Total RNA was purified using an RNA isolation/purification kit (Qiagen RNeasy Mini Kit, Valencia, CA, USA), and additional step for cell lysis was performed using glass beads to enhance cell disruption. Briefly, acid-washed glass beads (Sigma-Aldrich, 150–212 μm, ~10 x vol. of cell pellet) were added in lysis buffer. The mixture was vortexed vigorously for 50 s and chilled down on ice between each vortex for 50 s, which was repeated twelve times. After breaking cells, supernatant was collected by centrifugation at 16,000 x g for 10 min and the rest of the procedure was followed by the manufacturer’s guidelines. qRT-PCR was applied to analyze the transcript levels of 32 biofilm- and toxin-related genes (agrA, agrB, agrC, agrD, alsS, arlR, arlS, aur, clp9, coa, fibA, fibB, hla, icaA, icaR, isaA, lrgB, nuc1, nuc2, psmα, rbf, RNAIII, saeR, saeS, sarA, sarZ, seb, sigB, srrA, srrB, spa, and yycF) in S. aureus ATCC 6538 cells. Primers used are listed in Supplementary Table S1, and 16s rRNA was used as the housekeeping control. qRT-PCR was performed as previously described (Lee et al., 2022) using an SYBR™ Green qPCR Master Mix (Applied Biosystems, Foster City, United States) and an ABI StepOne Real-Time PCR System (Applied Biosystems). The changes of each gene expression were determined using two independent cultures and four reactions per gene.
Brassica rapa (Chinese cabbage) seeds were soaked in sterile H2O for 16 h, rinsed with water three times, sterilized by soaking in 95% ethanol first and then 3% sodium hypochlorite (both for 15 min) at 25°C, and rinsed with sterile H2O three times. Ten seeds per plate were carefully placed on Murashige and Skoog soft agar plates containing 0.7% agar and 0.86 g/L Murashige and Skoog (MS) and 13-cis-retinoic acid at 0, 20, 50, and 100 μg/mL, and then incubated at 25°C for 5 days. Seed germination rates and seedling lengths were then measured. Four independent cultures were used.
Caenorhabditis elegans fer-15(b26); fem-1(hc17) strain was used to investigate the chemical toxicity of 13-cis-retinoic acid, as previously described (Kim et al., 2022b). Synchronized nematodes were carefully washed two times with M9 buffer (3 g/L KH2PO4, 6 g/L Na2HPO4, 5 g/L NaCl, 1 mM MgSO4). Approximately 40 worms were placed into the each well of 96-well plates containing M9 buffer (200 mL) and 13-cis-retinoic acid (50, 100, 200, or 500 μg/mL). Then, the plates were incubated for 10 days at 25°C. Four independent cultures were used. Survived nematode percentages was determined by responses to LED lights for 30 s and an iRiS™ Digital Cell Imaging System (Logos BioSystems).
The drug-like properties of 13-cis-retinoic acid were evaluated using ADME software. The online web servers, viz, PreADMET2 Molinspiration3 and Gusar4 were accessed on March 15, 2023.
All results were analyzed by one-way ANOVA followed by Dunnett’s test in SPSS version 23 (SPSS Inc., Chicago, IL, United States). Results are presented as averages and standard deviations, and p values <0.05 are considered as a significant change.
The antibiofilm efficacies of three vitamin A1 metabolites, namely, all-trans-retinoic acid, 9-cis-retinoic acid, and 13-cis-retinoic acid, were initially tested at concentrations up to 100 μg/mL to investigate their effects on methicillin-sensitive S. aureus (MSSA 6538). Of these compounds, 13-cis-retinoic acid significantly inhibited biofilm formation, all-trans-retinoic acid had a weak inhibitory effect, but 9-cis-retinoic acid had no effect (Figures 1A–C). More specifically, 13-cis-retinoic acid at 10 μg/mL reduced S. aureus biofilm formation by 91%, while all-trans-retinoic acid at 100 μg/mL inhibited the biofilm formation by 34%. Also, the antimicrobial activity of 13-cis-retinoic acid was investigated by measuring colony-forming units, and at 50 μg/mL, it slightly delayed planktonic cell growth with a MIC of >400 μg/mL (Figure 1D). These results showed that the observed antibiofilm activity of 13-cis-retinoic acid was mainly due to its ability to inhibit biofilm formation rather than cell growth inhibition.

Figure 1. Effects of retinoic acids on biofilm formation and cell growth. Biofilm formation by S. aureus ATCC 6538 in the presence of 9-cis-retinoic acid (A), all-trans-retinoic acid (B), and 13-cis-retinoic acid (C). Cell growth of S. aureus ATCC 6538 in the presence of 13-cis-retinoic acid (D) in 96-well polystyrene plates after culture for 24 h. *p < 0.05 vs. non-treated controls (None).
Further biofilm assays were performed on another MSSA 25923 strain and two methicillin-resistant S. aureus strains (MRSA 33591 and MW2). 13-cis-Retinoic acid potently reduced biofilm formation by MSSA 25923, MRSA 33591, and MRSA MW2 strains with MICs of >400 μg/mL (Figures 2A–C). Specifically, 13-cis-retinoic acid at 10 and 20 μg/mL decreased biofilm formation by MSSA 25923, MRSA 33591, and MRSA MW2 strains by ≥84%.
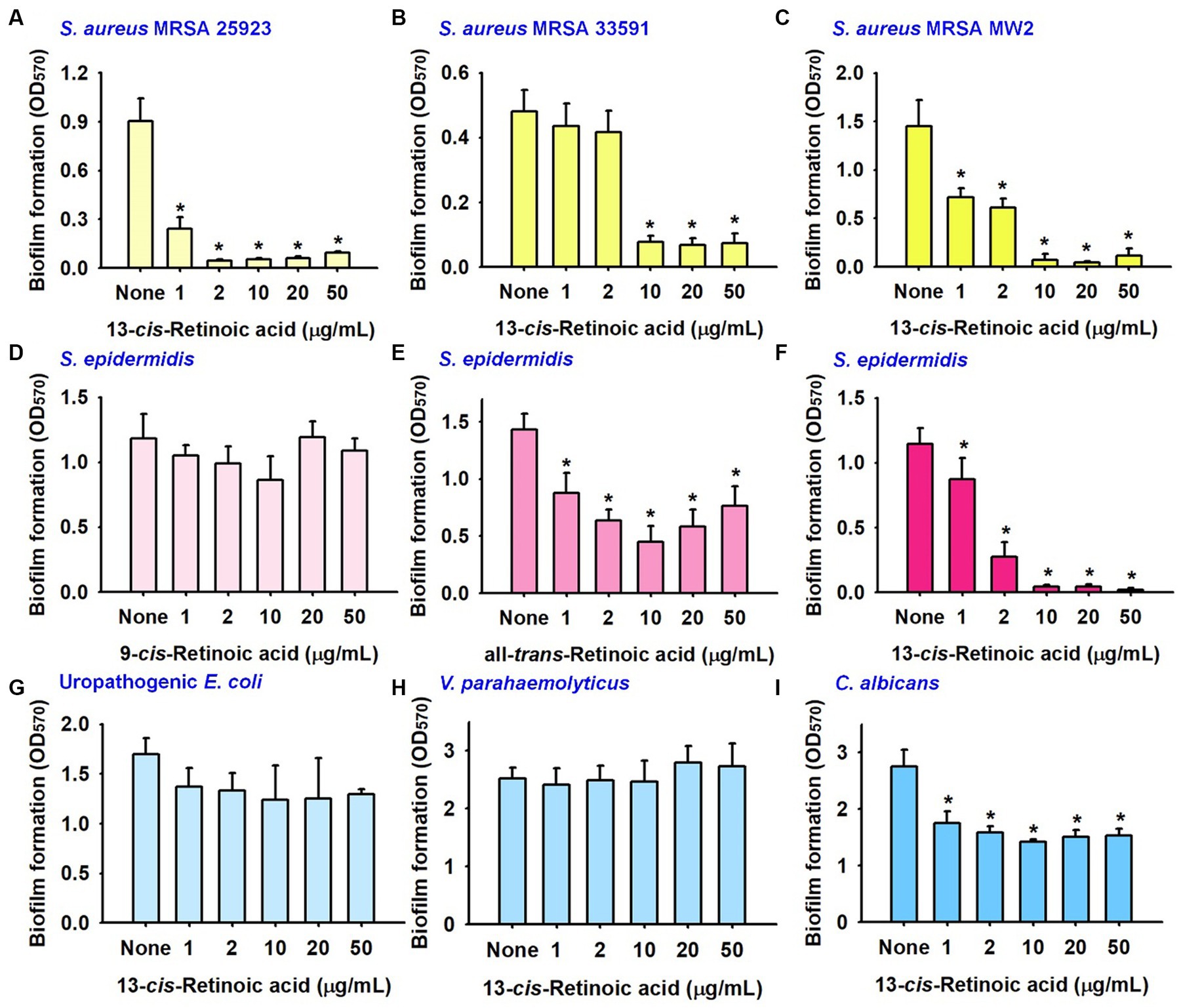
Figure 2. Inhibitory effects of retinoic acids on other biofilms. Biofilm formation by S. aureus ATCC 25923 (A), MRSA 33951 (B), MRSA MW2 (C), S. epidermidis (D–F), uropathogenic E. coli strain (G), V. parahaemolyticus (H), and C. albicans (I) in 96-well polystyrene plates after culture for 24 h. *p < 0.05 vs. non-treated controls (None).
Also, the antibiofilm activities of three retinoic acids were investigated with a S. epidermidis strain. As was observed for S. aureus, S. epidermidis biofilms were strongly inhibited by 13-cis-retinoic acid, weakly inhibited by all-trans-retinoic acid, but unaffected by 9-cis-retinoic acid (Figures 2D–F).
The impact of 13-cis-retinoic acid on other biofilms was also investigated with a uropathogenic Escherichia coli strain, an aquatic pathogenic Vibrio parahaemolyticus strain, and a fungal Candida albicans strain. Unlike that observed for the five Staphylococcal biofilms, 13-cis-retinoic acid up to 50 μg/mL did not reduce biofilm formation by two Gram-negative pathogens (E. coli and V. parahaemolyticus) (Figures 2G,H) and only exhibited weak antibiofilm activity against C. albicans (Figure 2I). These results indicate that 13-cis-retinoic acid is active against Gram-positive Staphylococcal biofilms but not against Gram-negative bacteria.
Live imaging microscopy and SEM were utilized to observe biofilm reduction. For untreated biofilms, 3D color images obtained by bright-field microscopy were green, indicating dense biofilms, whereas 13-cis-retinoic acid at 2–10 μg/mL produced yellow to red colors, indicating weak to no biofilm formation (Figure 3A). SEM analysis also showed that 13-cis-retinoic acid markedly diminished the numbers of S. aureus cells in biofilms but did not affect S. aureus cell morphology (Figure 3B). These observations confirmed that 13-cis-retinoic acid at 2–10 μg/mL significantly inhibited S. aureus biofilm formation without affecting cell morphology.
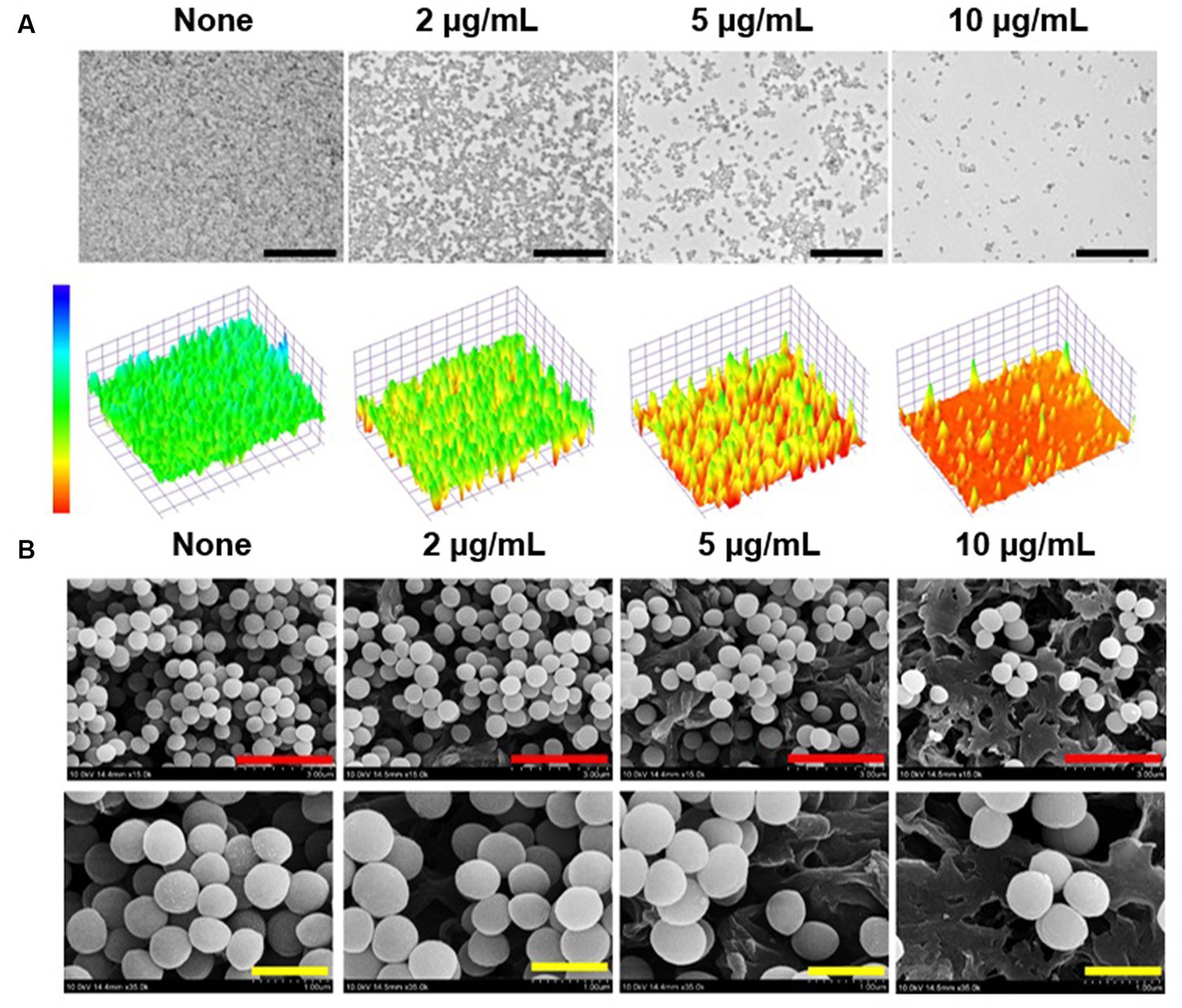
Figure 3. Antibiofilm effects of 13-cis-retinoic acid on S. aureus. Constructed color-coded 3D images of MSSA 6538 biofilms after culture for 24 h in the presence of 13-cis-retinoic acid (A), and corresponding SEM images (B). Black, red, and yellow scale bars represent 50, 3, and 1 μm, respectively.
S. aureus is known to produce various virulence factors, including hemolysins, staphyloxanthin, and extracellular lipase, and thus, we investigated the effects of the three retinoic acids on their levels. Notably, all-trans-retinoic acid and 13-cis-retinoic acid dose-dependently reduced the red blood cell hemolytic activity of S. aureus, while 9-cis-retinoic acid showed only weak anti-hemolytic activity (Figure 4A). For example, all-trans-retinoic acid and 13-cis-retinoic acid at 50 μg/mL inhibited hemolytic activity by 84 and 83%, respectively, which partially reflected their antibiofilm activities (Figure 1).
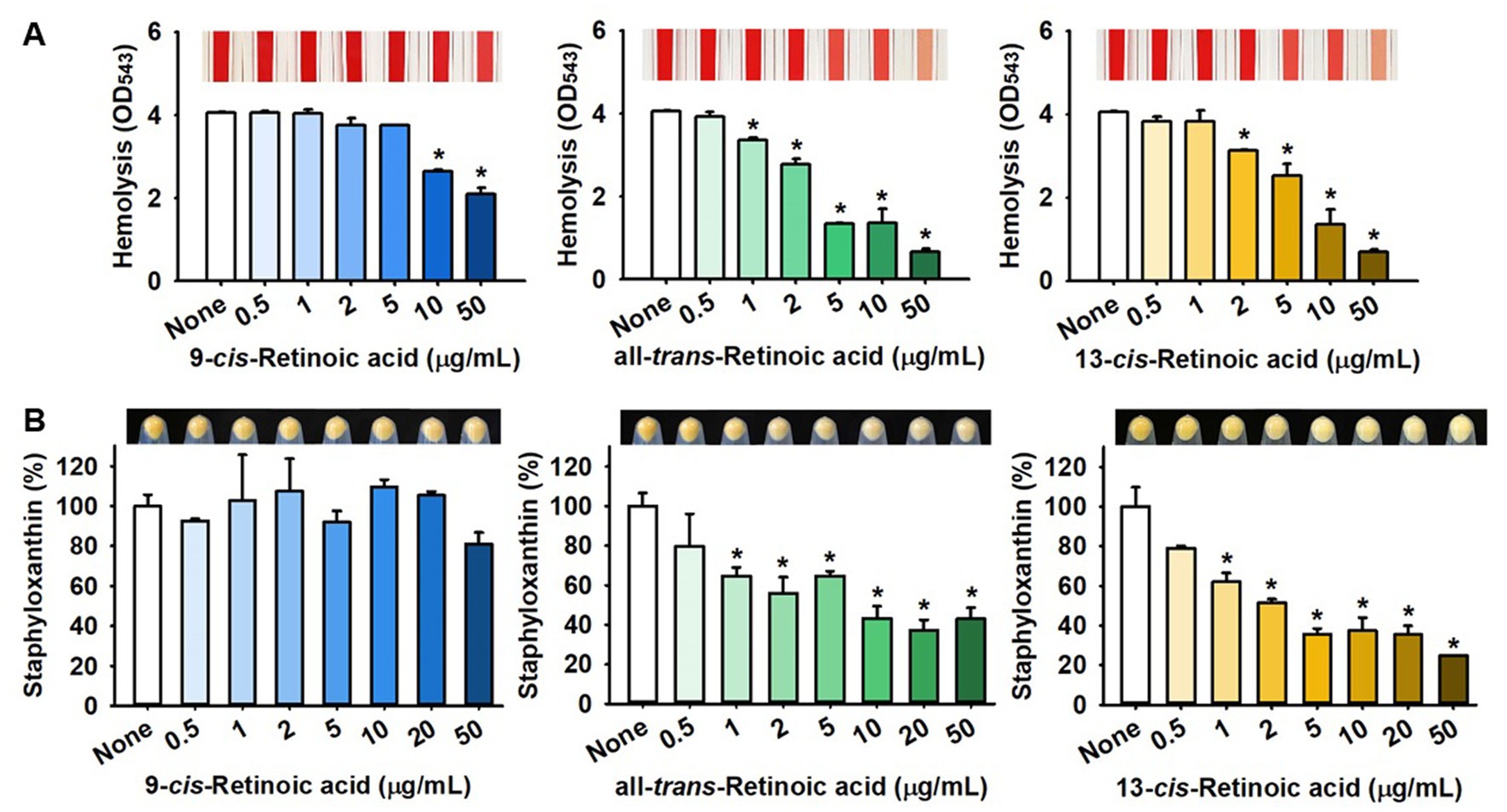
Figure 4. Effects of 13-cis-retinoic acid on hemolytic activity and staphyloxanthin production in S. aureus. Hemolysis (A) and staphyloxanthin production (B). *p < 0.05 vs. non-treated controls (None).
Also, the effects of the three retinoic acids on yellow staphyloxanthin production in S. aureus were investigated. Interestingly, all-trans-retinoic acid and 13-cis-retinoic acid dose-dependently inhibited staphyloxanthin production, whereas 9-cis-retinoic acid did not, which paralleled our biofilm inhibition and hemolytic activity results (Figure 4B). However, 13-cis-retinoic acid did not affect extracellular lipase activity at concentrations of <50 μg/mL (Supplementary Figure S1).
To investigate the molecular mechanisms responsible for the antibiofilm and antitoxin activities of 13-cis-retinoic acid on S. aureus, we used qRT-PCR to assess the expressions of 32 selected biofilm-, toxin-related genes and regulatory genes in S. aureus MSSA 6538 cells. 13-cis-Retinoic acid for 6 h incubation significantly downregulated the gene expression of the arlRS two-component system, α-hemolysin (hla), nuclease (nuc1 and nuc2), coagulase coaA, staphylococcal antigen A isaA, antiholin-like protein lrgB, and psmα (phenol soluble modulins α) but slightly upregulated the expression of the transcriptional regulator sarA and sarZ. However, the expression of other genes tested was unchanged (Figure 5). Notably, 13-cis-retinoic acid suppressed hla expression 13-fold, which matches with its inhibitory effect on S. aureus hemolytic activity.
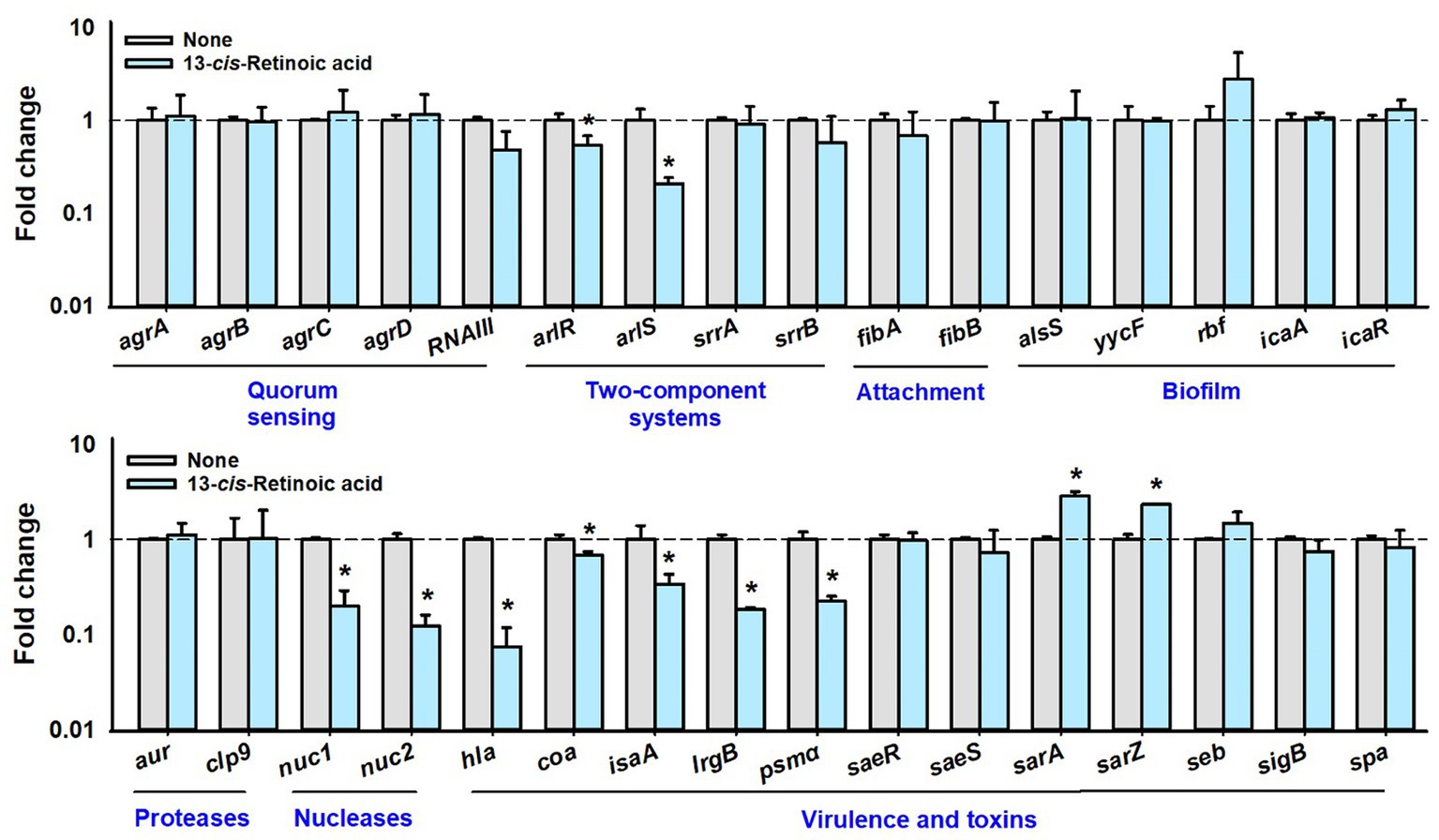
Figure 5. Effects of 13-cis-retinoic acid on gene expressions. Relative transcriptional profiles of biofilm- and virulence-related genes in S. aureus cells treated with 13-cis-retinoic acid at 100 μg/mL for 6 h with shaking at 250 rpm. Fold changes indicate transcriptional differences observed in treated vs. untreated (None) cells as determined by qRT-PCR. 16s rRNA was used as the housekeeping gene. *p < 0.05 vs. non-treated controls.
Further qRT-PCR has been conducted with a different growth condition of 10 h contact with 13-cis-retinoic acid (stationary growth phase) instead of 6 h contact (exponential growth phase). The changes of gene expression were attenuated since only the two-component kinase arlS was downregulated and other genes including QS-related genes were less affected after 10 h incubation (Supplementary Figure S2). The result indicates that gene expression is a dynamic process depending on incubation and growth stages.
Chemical toxicity assessments of 13-cis-retinoic acid were performed using a B. rapa germination assay and a C. elegans model. Interestingly, 13-cis-retinoic acid dose-dependently increased (not decreased) plant root growth for 4 days (Figures 6A,C) and also slightly increased the seed germination rate (Figure 6B). In the nematode model, incubation with 13-cis-retinoic acid up to 50 μg/mL for 10 days was non-toxic (Figure 6D). While incubation for 8 days at concentrations of ≤500 μg/mL had no acute toxic effect, mild toxicity was observed for old nematodes after 8 days. These results suggest that 13-cis-retinoic acid may not be toxic to plants or nematodes in its antibiofilm concentration range (2–10 μg/mL).
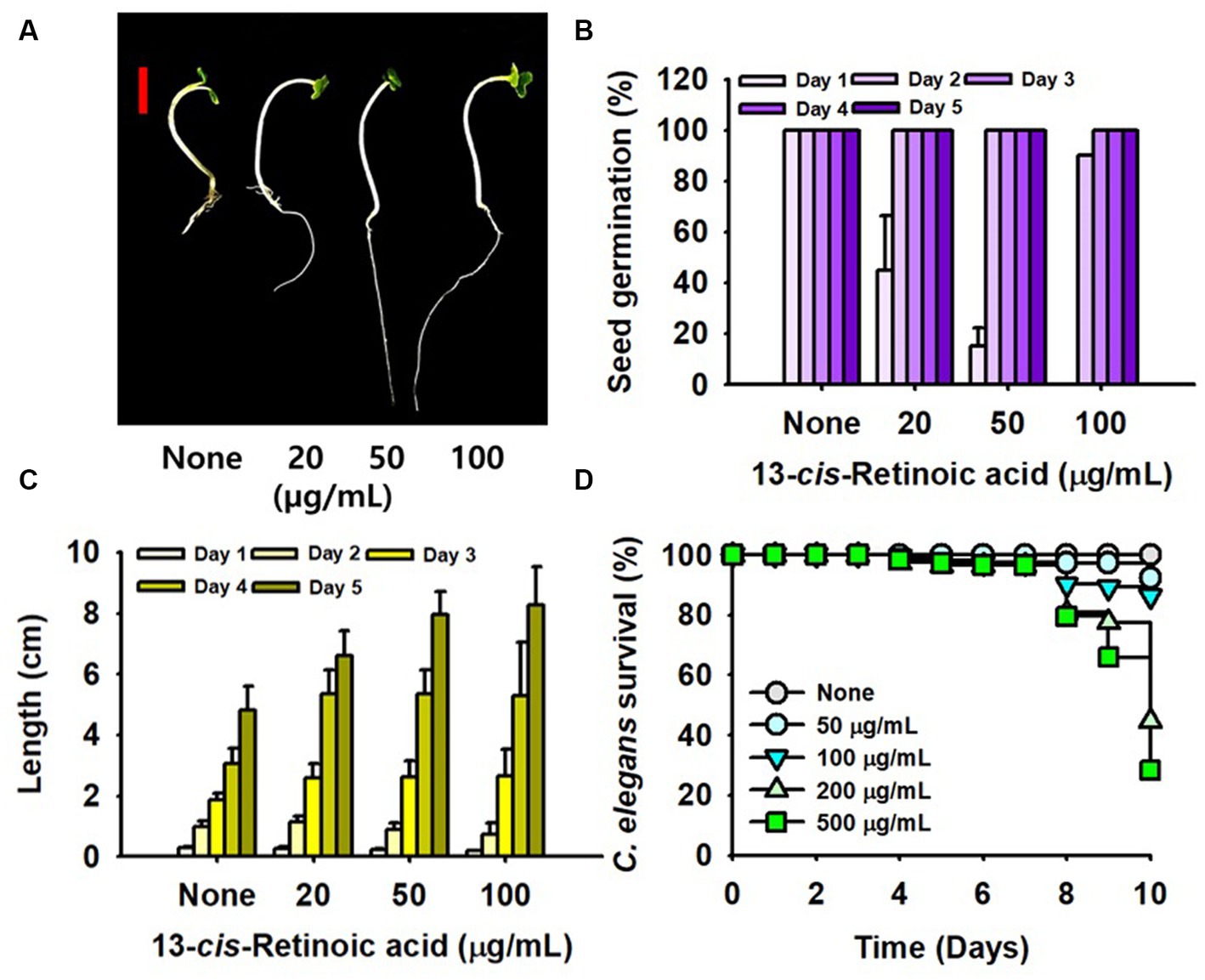
Figure 6. Toxicity of 13-cis-retinoic acid in the plant and nematode models. B. rapa seed growth (A), germination rate (B), and total length (C) cultured with or without different concentrations of 13-cis-retinoic acid at 25°C. (D) C. elegans survival was assessed in the presence or absence of 13-cis-retinoic acid for 10 days. The red scale bar in (A) represents 1 cm.
In silico ADME profiling showed 13-cis-retinoic acid violated one (miLogP <5) of Lipinski’s Rule of Five but had acceptable human intestinal adsorption and skin barrier permeability and no fish toxicity. The ADME parameters investigated are detailed in Supplementary Table S2.
This study demonstrates that retinoic acids, especially 13-cis-retinoic acid, inhibit biofilm formation of S. aureus and reduce its hemolytic activity and ability to produce staphyloxanthin without affecting its planktonic growth. Plant and nematode models and ADME analysis showed that 13-cis-retinoic acid is non-toxic at the active concentrations.
Retinoic acids are metabolites of all-trans retinol (vitamin A1), which is essential for the development of animals. All-trans-retinoic acid is the most abundant retinoic acid in nature, and its isomers, which include 9-cis-retinoic acid and 13-cis-retinoic acid, are present at markedly lower levels (Rühl et al., 2018). For example, the serum level of 13-cis-retinoic acid was the rage of 1.2–5.39 ng/mL in human (Li et al., 2019; Yang et al., 2020). The usage of 13-cis-retinoic acid was approved by the FDA in 1982 for the treatment of severe acne and has been shown to influence cellular differentiation, cell-cycle progression, cell survival, and apoptosis (Layton, 2009). Interestingly, 13-cis-retinoic acid was superior to all-trans-retinoic acid and 9-cis-retinoic acid for sebum suppression (Geiger et al., 1996).
It has been well established that acne vulgaris-associated inflammation may be exacerbated by Cutibacterium acnes and S. aureus (acne-associated bacteria) biofilm formation (Jahns et al., 2012; Tyner and Patel, 2016), which suggests the inhibitory effect of 13-cis-retinoic acid on S. aureus biofilm formation might be useful for treating acne. Therefore, we suggest studies be conducted to determine the impact of 13-cis-retinoic acid on anaerobic C. acnes.
Our transcriptomic study showed that 13-cis-retinoic acid repressed the expressions of regulatory arlRS genes and virulence factor genes (nuclease nuc1 and nuc2, psmα, and α-hemolysin hla) in S. aureus (Figure 5). The ArlRS two-component system affects several cellular processes in S. aureus, including biofilm formation, autolysis, capsule synthesis and virulence (Crosby et al., 2020). Mutations in arlRS were reported to promote S. aureus biofilm formation (Toledo-Arana et al., 2005) and to inhibit adhesion to human endothelial cells and vascular structures (Kwiecinski et al., 2019). ArlRS has also been reported to be important for virulence in several animal infection models (Toledo-Arana et al., 2005). The nucleases Nuc1 and Nuc2 proteins are involved in biofilm structure and bacterial aggregation (Beenken et al., 2012; Yu et al., 2021), and phenol-soluble modulins (PSMs) are a family of toxins that act as key biofilm structuring factors in S. aureus (Periasamy et al., 2012). These previous studies support our findings that 13-cis-retinoic acid down-regulates these important biofilm regulators and thus inhibits biofilm formation.
Our observations indicate that 13-cis-retinoic acid inhibits hemolytic activities (Figure 4) by suppressing the gene expression of α-hemolysin hla (Figure 5), a toxin that plays an important role in the pathogenesis of S. aureus infections by causing hemolysis (Divyakolu et al., 2019) and positively regulating S. aureus biofilm formation (Caiazza and O'Toole, 2003). Previous studies have shown that stilbenoid (Lee et al., 2014b), several flavonoids (Cho et al., 2015), alizarin (Lee et al., 2016), clemastine (Shang et al., 2022), diclazuril (Zheng et al., 2021), tetramethylbutylhydroquinone (Kim et al., 2022), nerolidol (Lee et al., 2014a), 10-hydroxy-2-decenoic acid (Gao et al., 2022), petroselinic acid (Lee et al., 2022), cis-11-eicosenoic acid (Lee et al., 2017), and lapatinib (Liu et al., 2022) have antibiofilm and anti-hemolytic effects on S. aureus. These findings suggest a positive relationship exists between antibiofilm and anti-hemolysis activities. Interestingly, structural comparisons of these compounds indicate that a hydroxyl or acid group and an alkyl chain with that of retinoic acid positively influence antibiofilm and anti-hemolysis activities (Figure 7). We suggest molecular docking studies be conducted on Hla protein and these compounds to identify some possible targets in Hla.
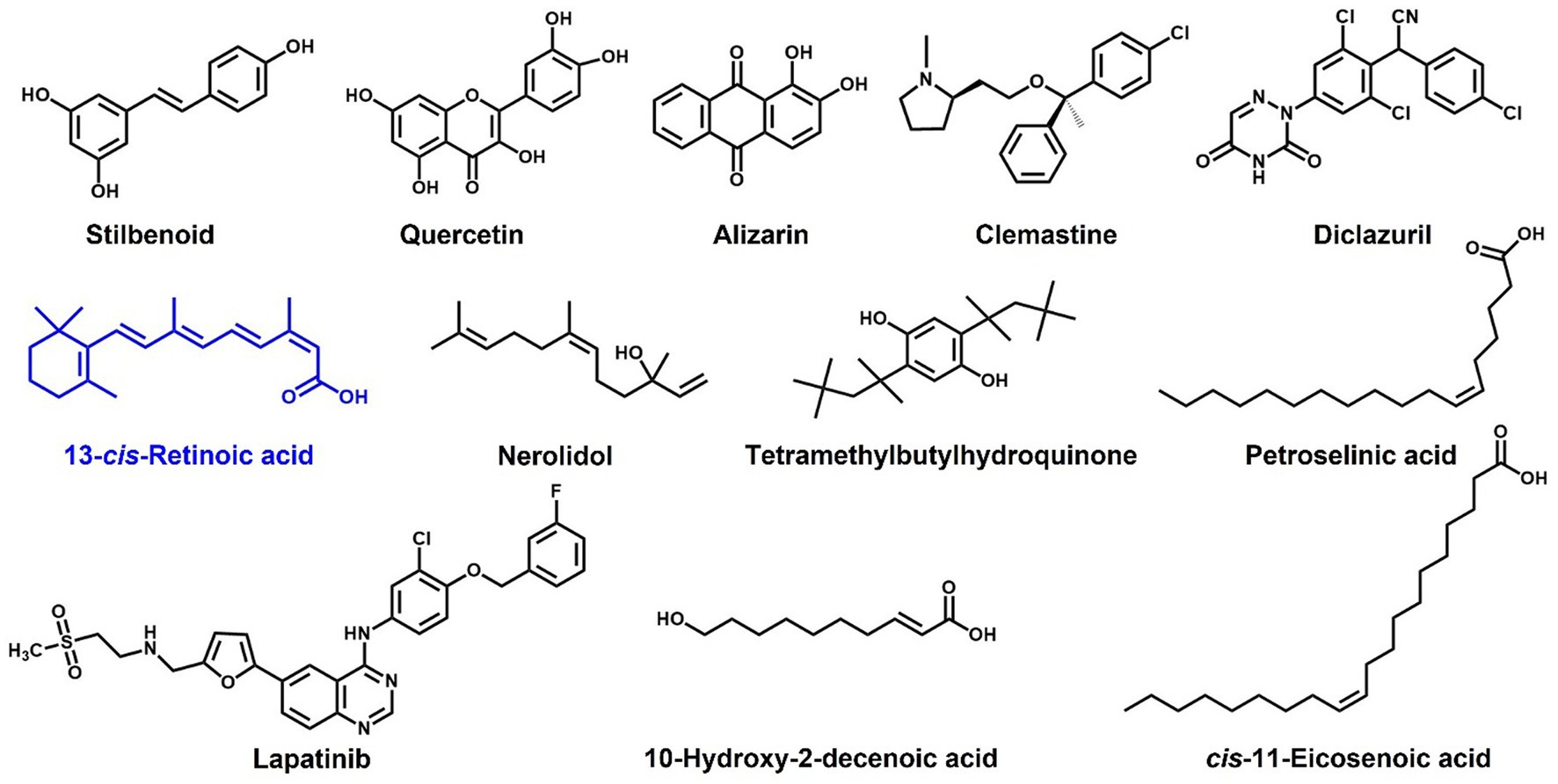
Figure 7. Structures of compounds reported to have antibiofilm and anti-hemolytic effects on S. aureus.
Notably, 13-cis-retinoic acid did not affect the expressions of agr or RNAIII quorum sensing system (Figure 5), which is somewhat intriguing as the Agr system contributes to S. aureus biofilm formation and biofilm dispersal (Boles and Horswill, 2011), and RNAIII is a key effector of Agr system that binds to AgrA and positively regulates hemolysins (Koenig et al., 2004). Thus, our transcriptomic results (Figure 5) indicate that the inhibitions of biofilm formation and hemolysis by 13-cis-retinoic acid are less associated with the Agr and RNAIII systems.
It has been previously shown that oral administration of 13-cis-retinoic acid has no direct antimicrobial effect (Layton, 2009), which concurs with our results (Figure 1D). Oral 13-cis-retinoic acid has been reported to be an effective acne treatment but may induce mood changes and mucocutaneous problems (Layton, 2009). However, our toxicity (Figure 6) and ADME results (Supplementary Table S2) suggest that 13-cis-retinoic acid is environmentally non-toxic and displays acceptable skin permeability, which suggests oral or dermal administration might be a feasible way of treating biofilm-associated S. aureus infections.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The manuscript presents research on animals that do not require ethical approval for their study.
JM, YT, and JL: conceptualization. IP and J-HL: methodology, software, validation, formal analysis, investigation, data curation, and visualization. JM and JL: resources. JL: writing of the manuscript and project administration. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A3A04037486), the NRF funded by the Korean government (MSIT) (2021R1A2C1008368), and by the Priority Research Center Program of the NRF funded by the Ministry of Education (2014R1A6A1031189).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1224085/full#supplementary-material
1. ^https://imagej.nih.gov/ij/index.html
2. ^https://preadmet.qsarhub.com/
Beenken, K. E., Spencer, H., Griffin, L. M., and Smeltzer, M. S. (2012). Impact of extracellular nuclease production on the biofilm phenotype of Staphylococcus aureus under in vitro and in vivo conditions. Infect. Immun. 80, 1634–1638. doi: 10.1128/iai.06134-11
Boles, B. R., and Horswill, A. R. (2011). Staphylococcal biofilm disassembly. Trends Microbiol. 19, 449–455. doi: 10.1016/j.tim.2011.06.004
Caiazza, N. C., and O'Toole, G. A. (2003). Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 185, 3214–3217. doi: 10.1128/jb.185.10.3214-3217.2003
Cho, H. S., Lee, J.-H., Cho, M. H., and Lee, J. (2015). Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 31, 1–11. doi: 10.1080/08927014.2014.991319
Crosby, H. A., Tiwari, N., Kwiecinski, J. M., Xu, Z., Dykstra, A., Jenul, C., et al. (2020). The Staphylococcus aureus ArlRS two-component system regulates virulence factor expression through MgrA. Mol. Microbiol. 113, 103–122. doi: 10.1111/mmi.14404
De Souza Feitosa Lima, I. M., Zagmignan, A., Santos, D. M., Maia, H. S., Dos Santos Silva, L., Da Silva Cutrim, B., et al. (2019). Schinus terebinthifolia leaf lectin (SteLL) has anti-infective action and modulates the response of Staphylococcus aureus-infected macrophages. Sci. Rep. 9:18159. doi: 10.1038/s41598-019-54616-x
Dickey, S. W., Cheung, G. Y. C., and Otto, M. (2017). Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 16, 457–471. doi: 10.1038/nrd.2017.23
Divyakolu, S., Chikkala, R., Ratnakar, K. S., and Sritharan, V. (2019). Hemolysins of Staphylococcus aureus—an update on their biology, role in pathogenesis and as targets for anti-virulence therapy. Adv. Infect. Dis. 9, 80–104. doi: 10.4236/aid.2019.92007
Gao, K., Su, B., Dai, J., Li, P., Wang, R., and Yang, X. (2022). Anti-biofilm and anti-hemolysis activities of 10-hydroxy-2-decenoic acid against Staphylococcus aureus. Molecules 27:1485. doi: 10.3390/molecules27051485
Geiger, J. M., Hommel, L., Harms, M., and Saurat, J. H. (1996). Oral 13-cis retinoic acid is superior to 9-cis retinoic acid in sebosuppression in human beings. J. Am. Acad. Dermatol. 34, 513–515. doi: 10.1016/s0190-9622(96)90462-4
Jahns, A. C., Lundskog, B., Ganceviciene, R., Palmer, R. H., Golovleva, I., Zouboulis, C. C., et al. (2012). An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case–control study. Br. J. Dermatol. 167, 50–58. doi: 10.1111/j.1365-2133.2012.10897.x
Kim, Y.-G., Kim, S., Cho, K. H., Lee, J.-H., and Lee, J. (2022a). Antibiofilm activities of cinnamaldehyde analogs against Uropathogenic Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 23:7225. doi: 10.3390/ijms23137225
Kim, S., Lee, J.-H., Kim, Y. -G., Tan, Y., and Lee, J. (2022). Hydroquinones inhibit biofilm formation and virulence factor production in Staphylococcus aureus. Int. J. Mol. Sci. 23:10683. doi: 10.3390/ijms231810683
Kim, Y.-G., Lee, J.-H., Park, S., Khadke, S. K., Shim, J. J., and Lee, J. (2022b). Hydroquinones including tetrachlorohydroquinone inhibit Candida albicans biofilm formation by repressing hyphae-related genes. Microbiol. Spectr. 10, e02536–e02522. doi: 10.1128/spectrum.02536-22
Koenig, R. L., Ray, J. L., Maleki, S. J., Smeltzer, M. S., and Hurlburt, B. K. (2004). Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J. Bacteriol. 186, 7549–7555. doi: 10.1128/jb.186.22.7549-7555.2004
Kwiecinski, J. M., Crosby, H. A., Valotteau, C., Hippensteel, J. A., Nayak, M. K., Chauhan, A. K., et al. (2019). Staphylococcus aureus adhesion in endovascular infections is controlled by the ArlRS-MgrA signaling cascade. PLoS Pathog. 15:e1007800. doi: 10.1371/journal.ppat.1007800
Layton, A. (2009). The use of isotretinoin in acne. Dermatoendocrinology 1, 162–169. doi: 10.4161/derm.1.3.9364
Lee, K. M., Go, J., Yoon, M. Y., Park, Y., Kim, S. C., Yong, D. E., et al. (2012). Vitamin B12-mediated restoration of defective anaerobic growth leads to reduced biofilm formation in Pseudomonas aeruginosa. Infect. Immun. 80, 1639–1649. doi: 10.1128/iai.06161-11
Lee, J.-H., Kim, Y.-G., Khadke, S. K., and Lee, J. (2021). Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol. Microb. Biotechnol. 14, 1353–1366. doi: 10.1111/1751-7915.13710
Lee, J.-H., Kim, Y.-G., and Lee, J. (2022). Inhibition of Staphylococcus aureus biofilm formation and virulence factor production by petroselinic acid and other unsaturated C18 fatty acids. Microbiol. Spectr. 10:e0133022. doi: 10.1128/spectrum.01330-22
Lee, J.-H., Kim, Y.-G., Park, J. G., and Lee, J. (2017). Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 80, 74–82. doi: 10.1016/j.foodcont.2017.04.035
Lee, J.-H., Kim, Y.-G., Ryu, S. Y., and Lee, J. (2016). Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 6:19267. doi: 10.1038/srep19267
Lee, K., Lee, J.-H., Kim, S. I., Cho, M. H., and Lee, J. (2014a). Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 98, 9447–9457. doi: 10.1007/s00253-014-5903-4
Lee, K., Lee, J.-H., Ryu, S. Y., Cho, M. H., and Lee, J. (2014b). Stilbenes reduce Staphylococcus aureus hemolysis, biofilm formation, and virulence. Foodborne Pathog. Dis. 11, 710–717. doi: 10.1089/fpd.2014.1758
Li, X. B., Liu, T., Fan, L., Gao, Q., Peng, Q., Cai, T., et al. (2019). Circulating serum level of retinoic acid and hip fractures among postmenopausal women. J. Am. Geriatr. Soc. 67, 336–341. doi: 10.1111/jgs.15667
Liu, Y., Shi, Y., Cheng, H., Chen, J., Wang, Z., Meng, Q., et al. (2022). Lapatinib acts against biofilm formation and the hemolytic activity of staphylococcus aureus. ACS Omega 7, 9004–9014. doi: 10.1021/acsomega.2c00174
Moormeier, D. E., and Bayles, K. W. (2017). Staphylococcus aureus biofilm: a complex developmental organism. Mol. Microbiol. 104, 365–376. doi: 10.1111/mmi.13634
Park, S., Lee, J.-H., Kim, Y.-G., Hu, L., and Lee, J. (2022). Fatty acids as aminoglycoside antibiotic adjuvants against Staphylococcus aureus. Front. Microbiol. 13:876932. doi: 10.3389/fmicb.2022.876932
Periasamy, S., Joo, H.-S., Duong, A. C., Bach, T.-H. L., Tan, V. Y., Chatterjee, S. S., et al. (2012). How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. U. S. A. 109, 1281–1286. doi: 10.1073/pnas.1115006109
Rühl, R., Krężel, W., and de Lera, A. R. (2018). 9-Cis-13,14-dihydroretinoic acid, a new endogenous mammalian ligand of retinoid X receptor and the active ligand of a potential new vitamin a category: vitamin A5. Nutr. Rev. 76, 929–941. doi: 10.1093/nutrit/nuy057
Shang, Y., Guo, J., Zhao, Y., Chen, J., Meng, Q., Qu, D., et al. (2022). Clemastine inhibits the biofilm and hemolytic of Staphylococcus aureus through the GdpP protein. Microbiol. Spectr. 10:e0054121. doi: 10.1128/spectrum.00541-21
Shivaprasad, D. P., Taneja, N. K., Lakra, A., and Sachdev, D. (2021). In vitro and in situ abrogation of biofilm formation in E. coli by vitamin C through ROS generation, disruption of quorum sensing and exopolysaccharide production. Food Chem. 341:128171. doi: 10.1016/j.foodchem.2020.128171
Toledo-Arana, A., Merino, N., Vergara-Irigaray, M., Débarbouillé, M., Penadés, J. R., and Lasa, I. (2005). Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J. Bacteriol. 187, 5318–5329. doi: 10.1128/jb.187.15.5318-5329.2005
Tong, S. Y. C., Davis, J. S., Eichenberger, E., Holland, T. L., and Fowler, V. G. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi: 10.1128/CMR.00134-14
Tyner, H., and Patel, R. (2016). Propionibacterium acnes biofilm–a sanctuary for Staphylococcus aureus? Anaerobe 40, 63–67. doi: 10.1016/j.anaerobe.2016.05.014
Vergalito, F., Pietrangelo, L., Petronio Petronio, G., Colitto, F., Alfio Cutuli, M., Magnifico, I., et al. (2018). Vitamin E for prevention of biofilm-caused healthcare-associated infections. Open Med. 15, 14–21. doi: 10.1515/med-2020-0004
Winkelströter, L. K., Teixeira, F. B. D. R., Silva, E. P., Alves, V. F., and De Martinis, E. C. P. (2014). Unraveling microbial biofilms of importance for food microbiology. Microb. Ecol. 68, 35–46. doi: 10.1007/s00248-013-0347-4
Xu, C., Dong, N., Chen, K., Yang, X., Zeng, P., Hou, C., et al. (2022). Bactericidal, anti-biofilm, and anti-virulence activity of vitamin C against carbapenem-resistant hypervirulent Klebsiella pneumoniae. iScience 25:103894. doi: 10.1016/j.isci.2022.103894
Yang, C.-D., Cheng, M.-L., Liu, W., and Zeng, D.-H. (2020). Association of serum retinoic acid with depression in patients with acute ischemic stroke. Aging (Albany NY) 12:2647. doi: 10.18632/aging.102767
Yu, J., Jiang, F., Zhang, F., Hamushan, M., Du, J., Mao, Y., et al. (2021). Thermonucleases contribute to Staphylococcus aureus biofilm formation in implant-associated infections–a redundant and complementary story. Front. Cell. Infect. Microbiol. 12:687888. doi: 10.3389/fmicb.2021.687888
Keywords: antivirulence, biofilm, hemolysis, retinoic acid, Staphylococcus aureus , vitamin A1
Citation: Park I, Lee J-H, Ma JY, Tan Y and Lee J (2023) Antivirulence activities of retinoic acids against Staphylococcus aureus. Front. Microbiol. 14:1224085. doi: 10.3389/fmicb.2023.1224085
Received: 17 May 2023; Accepted: 23 August 2023;
Published: 13 September 2023.
Edited by:
Fabricio Luiz Tulini, Federal University of Western Bahia, BrazilReviewed by:
Zhen Luo, Central South University, ChinaCopyright © 2023 Park, Lee, Ma, Tan and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jintae Lee, anRsZWVAeW51LmFjLmty
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.