
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 29 June 2023
Sec. Systems Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1216722
This article is part of the Research Topic One-Health Meets Microbiota: Interactions between Digestive Tract Microbiota, Host, and Environment View all 15 articles
 Qixian Feng1†
Qixian Feng1† Juan Zhang1†
Juan Zhang1† Wenqing Ling1†
Wenqing Ling1† Abraham Allan Degen2
Abraham Allan Degen2 Yi Zhou1
Yi Zhou1 Chenyan Ge1
Chenyan Ge1 Fulin Yang1*
Fulin Yang1* Jing Zhou3*
Jing Zhou3*The aim of this study was to compare the effect of different additives on nutritional quality, fermentation variables and microbial diversity of hybrid Pennisetum silages. A control (CK – no additives) and seven treatments were tested, namely, Lactiplantibacillus plantarum (LP), Lentilactobacillus buchneri (LB), propionic acid (PA), calcium propionate (CAP), LP + LB; LP + PA and LP + CAP. In comparison with CK, all treatments increased the contents of crude protein and lactic acid, decreased the content of butyric acid, and altered the bacterial communities of the silage. Except for the CAP and LP + CAP treatments, the additives decreased pH and the ammonia nitrogen:total nitrogen (NH3-N:TN) ratio. The results of principal component analysis revealed that the PA, LP + PA and LP + LB treatments ranked as the top three silages. The PA and LP + PA treatments exhibited higher water-soluble carbohydrate content, but lower pH, and NH3-N:TN ratio than the other treatments. With the PA and LP + PA treatments, the relative abundances of Lactobacillus and Enterobacter decreased, and of Proteobacteria and Delftia increased, while the carbohydrate metabolism of the microorganisms improved. The LP and LB treatments reduced the Shannon and Simpson diversities. In the beta diversity, PA and LP + PA separated from the other treatments, indicating that there were differences in the composition of bacterial species. The relative abundance of Lactobacillus increased in the LP and LB treatments and of Leucanostoc and Weissella increased in the CAP and LP + CAP treatments. In summary, the addition of L. plantarum, L. buchneri, propionic acid, calcium propionate, and their combinations improved fermentation quality, inhibited harmful bacteria and conserved the nutrients of hybrid Pennisetum.
With the rapid development of the livestock industry and the increased demand for animal products, more animal feed is needed. This has increased the cost of animal feed and the competition for land between food for humans and feed for livestock (Sandström et al., 2022; Zhao et al., 2022), which has led to utilization of different forage resources for ruminants (Li D. et al., 2019; Wang N. et al., 2022). Hybrid Pennisetum (Pennisetum americanum × Pennisetum purpureum) has been incorporated into animal feed and has great potential to be used for ruminants. It is distributed widely in southern China, and is characterized by low cultivation input, high biomass, and strong stress tolerance (Song et al., 2019; Cai et al., 2020; Wu et al., 2020).
Ensiling is a traditional method of preserving forage (Ren et al., 2018; Drouin et al., 2021). However, the hybrid Pennisetum is difficult to ensile because of its low lactic acid bacteria (LAB) count (Shah et al., 2020). Bacterial inoculants and chemicals are often added to improve the fermentation and nutrient qualities of the silage, and to inhibit the activity of harmful microbiota (Queiroz et al., 2018; He Q. et al., 2021). The LAB inoculants are known for their ability to alter fermentation patterns, and are added widely to improve fermentation in the production of silage (Ogunade et al., 2017). Lactiplantibacillus plantarum, the most common additive, is a homofermentative LAB that produces lactic acid efficiently and reduces the pH rapidly (Xu et al., 2021; Xian et al., 2022). Lentilactobacillus buchneri, also a heterofermentative LAB, produces acetic acid during fermentation, inhibits yeast and mold, improves aerobic stability and reduces feed loss (Romero et al., 2017; Zhang et al., 2020). Propionic acid is an aerobic microbial inhibitor that affects nitrogen conversion and reduces the degradation of protein by acidizing the silage or limiting the activity of undesirable bacteria at the early stage of fermentation (Carvalho et al., 2012; He et al., 2020; Ren et al., 2021). Due to the volatility of propionic acid and its relatively short residual time, calcium propionate (CAP), which has the same antibacterial effect as propionic acid after ionization in water, was developed (Xiong et al., 2017). The above-mentioned additives have advantages, but little information is available on their effect on silage quality and the bacterial community of hybrid Pennisetum. To fill this knowledge gap, we compared the effects of L. plantarum, L. buchneri, propionic acid, calcium propionate, and their combinations on the chemical composition, fermentation quality, and microbial community of hybrid Pennisetum.
Hybrid Pennisetum was harvested in Fujian Province (117.93 °E, 26.79 °N, subtropical monsoon climate) in May 2021 by manually mowing at 8–10 cm above ground level, and was transported to the laboratory immediately. The Pennisetum was spread out evenly, and air-dried for 6 h, resulting in a dry matter (DM) content of 181.3 g/kg fresh weight (FW), and was chopped into 1–2 cm lengths using a paper cutter. The following were added to the Pennisetum: (1) distilled water (CK); (2) L. plantarum (LP, provided by Fujian Academy of Agricultural Sciences, viable counts ≥1 × 106 cfu/g FW); (3) L. buchneri (LB, BNCC187961, Beijing Beina Chuanglian Biotechnology Institute, Beijing, China, viable counts ≥1 × 106 cfu/g FW); (4) propionic acid (PA, 0.5% FW, analytical pure, Fuzhou Mili Biotechnology Co., Ltd., Fuzhou, China); (5) calcium propionate (CAP, 0.5% FW, Fuzhou Mili Biotechnology Co., Ltd., Fuzhou, China); (6) LP + LB; (7) LP + PA; and (8) LP + CAP. Each additive was dissolved in 10 mL sterile water and sprayed evenly onto the surface of the Pennisetum (CK was sprayed with an equal volume of distilled water). Subsequently, 400 g of the sprayed hybrid Pennisetum samples were placed in a polyethylene bag (248 mm × 344 mm), with 3 replicates for each treatment. The bags were vacuum sealed, and ensiled at room temperature of 26°C for 30 or 60 days.
After ensiling, DM content of the silage was determined by oven drying at 65°C for 48 h, and the oven-dried samples were sieved through a 0.425 mm screen. The content of water-soluble carbohydrates (WSC) was determined by anthrone sulfuric acid colorimetry (Fu and Diao, 2007); total nitrogen (TN) was determined using an automatic nitrogen analyzer (K9840 Kjeldahl, Hanon, Jinan, China), the crude protein (CP) content was calculated as TN × 6.25; and the neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were measured following Van Soest et al. (1991).
Ten g of sample were added to 90 mL of distilled water for 24 h at 4°C, and filtered through 4 layers of gauze for pH determination (pHS-3D, Shandong, China). Ammonia nitrogen (NH3-N) was determined by phenol sodium hypochlorite colorimetry (Arthur Thomas, 1977), and lactic, acetic, propionic, and butyric acids were determined using high-performance liquid chromatography (Wang et al., 2020).
The pH and microbial counts of the treatments after 60 days of ensiling were determined at 0, 3, 6, and 9 days after aerobic exposure. The pH was determined as described previously. The methods of Dong et al. (2017) were used to measure the counts of LAB, yeast, and aerobic bacteria during aerobic exposure with the de Man Rogosa Sharpe medium, Potato Dextrose Agar medium, and Plate Count Agar medium, respectively (Fuzhou Mili Biotechnology Co., Ltd., Fuzhou, China). No antibiotics were added to the culture media.
After 60 days of ensiling, a sample of each treatment was stored at −80°C for microbial diversity determination. High throughput sequencing was performed in triplicate, and total DNA was extracted using the CTAB/SDS method to check DNA concentration and purity with a 1% agarose gel. The 16S rDNA gene of the bacterial V3 ~ V4 hypervariable region was obtained by primer sequences 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTAC HVGGGTWTCTAAT) (Ling et al., 2022). PCR amplicons were identified by agarose gel electrophoresis using NEXTFLEX® Rapid DNA-Seq Kit for Miseq library construction and sequencing. After the library qualified, it was sent to Majorbio BioPharm Technology Co. Ltd. (Shanghai, China) for sequencing. The library was paired-end sequenced based on the Illumina Novaseq sequencing platform resulting in a complete microbial community.
Raw fastq files were demultiplexed and quality filtered using Trimomatic, and further merged by FLASH software. Uparse software (Uparse v7.0.1001)1 was used to cluster the entire high-quality sequences of all samples, and, by default, the sequences were clustered to operational taxonomic units (OTUs) with 97% similarity (He L. et al., 2021). Alpha diversity was determined using species richness indices (Ace and Chao 1) and species diversity indices (Shannon and Simpson) (Zheng et al., 2020). Beta diversity was determined using principal coordinates analysis (PCoA), and was further analyzed using the ANOSIM test (Dong et al., 2017). The metabolic function of bacteria was predicted by comparing the Kyoto Encyclopedia of Genes and Genomes (KEGG) database with the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2) (Langille et al., 2013). Spearman’s correlation tested the relationships between silage quality and the relative abundances of bacteria at the genus levels in each treatment. Raw sequencing files and associated metadata have been deposited in NCBI’s Sequence Read Archive (PRJNA946341).
The data of silage quality were analyzed by the GLM program in SPSS software (version 26.0, Chicago, IL, United States) with ensiling time, additive treatment and their interaction. The principal component analysis used SPSS software. The eigenvalues of the variance matrix, the variance contribution rate and the weight coefficient of each factor were calculated to generate the principal component equation. The principal component comprehensive score was calculated by standardizing the original data into the principal component equation (Tharangani et al., 2021).
The DM and WSC contents of hybrid Pennisetum silage were affected by ensiling time, additives and their interactions (Table 1). When compared to CK, the LP treatment had a lesser (p < 0.05) DM content, while the LP + LB, PA, LP + PA, CAP and LP + CAP treatments had greater (p < 0.05) DM contents. The loss of DM from silage was due to the breakdown of nutrients, as aerobic microbes converted carbohydrates to water, carbon dioxide, and heat (Haas et al., 2011). In the present study, the DM content in the PA treatment was greater than in the other treatments after 60 days of ensiling. It is likely that the addition of propionic acid inhibited the growth of undesirable microorganisms and reduced their consumption of nutrients (Li et al., 2022). Propionic acid has antifungal properties by maintaining its activity on the surface of microorganisms and competing with amino acids for enzyme activity sites or by altering the cell permeability of the organisms (Gheller et al., 2021).
With the prolongation of ensiling, the CP content in each treatment decreased. The degradation of protein during ensiling involves a series of plant and microbial enzymes (Xiong et al., 2017). Proteins are converted into free amino acids and peptides through the catalytic hydrolysis of plant enzymes, and then are degraded further (Zheng et al., 2022). In the present study, the CP content was greater (p < 0.05) with each additive than in CK. Previous studies reported that propionic acid inhibited Clostridia and Enterobacteria effectively, as these bacteria were poor acid resistant bacteria, and reduced protein breakdown (Ali and Tahir, 2021). Adding calcium propionate also reduced CP loss, but less so than propionate, which might be due to the lesser concentration of dissociated propionate ions with CAP. The increase (p < 0.05) of CP content with the LB treatment most likely involved propionic acid. L. buchneri produces 1,2-propanediol from sugars and then propionic acid in the metabolic process, resulting in a bacteriostatic effect (Ling et al., 2022).
The WSC content of all silages was lower (p < 0.05) at 60 days than at 30 days of ensiling. The WSC serves as an energy source for microorganisms and its consumption implies microbial activity (Gheller et al., 2021). The WSC is converted into organic acid to reduce the pH of the silage (Zhang et al., 2019; Zhu et al., 2022). The NDF content of silage was reduced (p < 0.05) with the LP + LB treatments. Similarly, Du et al. (2022) reported that the content of NDF in ryegrass silage inoculated with L. plantarum, L. buchneri and L. casei was reduced after 60 days of ensiling. When ensiled for 30 days, the PA and LP + PA treatments had a lesser (p < 0.05) NDF content than CK. This could be due to the increase in total organic acids after the addition of PA, which could hydrolyze digestible cell walls (Jiang et al., 2020; Ren et al., 2020).
Table 2 presents the effect of different additives on the silage fermentation parameters of hybrid Pennisetum. A pH in the range of 3.6–4.2 for silage is considered optimal, as it effectively reduces undesirable microorganisms (Lv et al., 2020; Bao et al., 2023). In the current study, the pH was below 4.2 at 30 days of ensiling in all treatments except for CAP, LP + CAP and LB, in which the pHs were greater (p < 0.05) than for CK. Li M. et al. (2019) concluded that CAP led to an increase in buffering energy and, thus, a rise in pH in the silage, which could explain the results in the present study. L. plantarum, which is regarded as the most commonly used homofermentative LAB, has the ability to reduce pH rapidly (Muck et al., 2018; Bai et al., 2022). L. buchneri could improve the aerobic stability of silage (Magnusson and Schnürer, 2001), and when combined with L. plantarum, reduced the pH at the initial stage of fermentation. Consequently, the pH of the LP + LB treatment was lesser than the LP and LB treatments.
After 30 days of ensiling, the LB and LP + LB treatments increased acetic acid content, and after 60 days of ensiling all treatments had greater (p < 0.05) lactic acid content than CK. The LP treatment had the lowest pH and highest lactic acid content of all treatments. It is likely that lactic acid has a lower pH than other organic acids and plays a vital role during fermentation (Jaipolsaen et al., 2021). In this study, the concentration of lactic acid at 30 days was greater (p < 0.05) than at 60 days of ensiling in all treatments, except for the PA treatment. Concomitantly, there was a decrease in acetic acid content, which implied that homolatic fermentation dominated. Li et al. (2018) concluded that the addition of L. plantarum enhanced the quality of alfalfa silage. At 30 days of ensiling, the LP + LB, PA, and LP + CAP treatments had greater (p < 0.05), but the LP, LB and CAP treatments (p < 0.05) had lesser concentrations of propionic acid than CK. The increase in propionic acid content was likely a result of lactic acid being consumed by L. buchneri (Li et al., 2018). With the prolongation of the ensiling period, the content of propionic acid in the CAP treatment increased (p < 0.05), which could be attributed to the dissociation of corresponding organic acid salts (Dai et al., 2022), as was reported by Wen et al. (2017). Kung et al. (2018) reported that butyric acid below 5 g/kg DM was optimal for high quality fermentation, and the butyric acid content of all treatments in the present study met this criterium. After an increase in the ensiling period, the content of butyric acid was lesser (p < 0.05) in the LP, PA, LP + PA and CAP treatments than in CK. The production of silage undergoes dynamic enzymatic and microbial processes of which the degradation of proteins is one of the most crucial stages (Wang S. et al., 2022). In the present study, the NH3-N:TN ratios in the PA and LP + PA treatments were lesser (p < 0.05) than in the other treatments, probably because the lower pH inhibited the activity of the protease (Tian H. et al., 2022). In addition, the NH3-N:TN ratios in the LP, LB and LP + LB treatments were lesser (p < 0.05) than in CK. L. plantarum inhibits protein degradation through its effect on enzymes and microorganisms (Xian et al., 2022), whereas, L. buchneri, through its bacteriostatic effect, reduces the degradation of protein by undesirable microorganisms. The combination of L. buchneri and L. plantarum had a synergistic effect in reducing the degradation of protein.
After 30 days of ensiling, CP was correlated positively (p < 0.05) with DM content, but negatively (p < 0.05) with NDF and ADF contents (Figure 1A). After 60 days of ensiling, CP content was correlated positively (p < 0.05) with lactic acid content and negatively (p < 0.001) with butyric acid content (Figure 1B). The WSC content was correlated negatively with acetic acid content (p < 0.05), pH (p < 0.01) and the NH3-N:TN ratio (p < 0.001). Moreover, the WSC content was correlated negatively with pH as a result of the lactic acid produced by LAB, with WSC as a substrate (Filya, 2003). Lactic acid plays the major role in reducing the pH of silage (Fu et al., 2022). After 30 days of ensiling, there was a positive correlation (p < 0.05) between pH and the NH3-N:TN ratio, and this correlation was stronger after 60 days of ensiling. Generally, NH3-N accumulates continuously during fermentation (Dong et al., 2022a). When LAB dominated in the late stages of ensiling, lactic acid was produced by the fermentation of plant biomass and the pH was reduced to a level that inhibited the activity of ammonia nitrogen producing bacteria (Fan et al., 2022). This could explain the correlation between pH and the NH3-N:TN ratio.
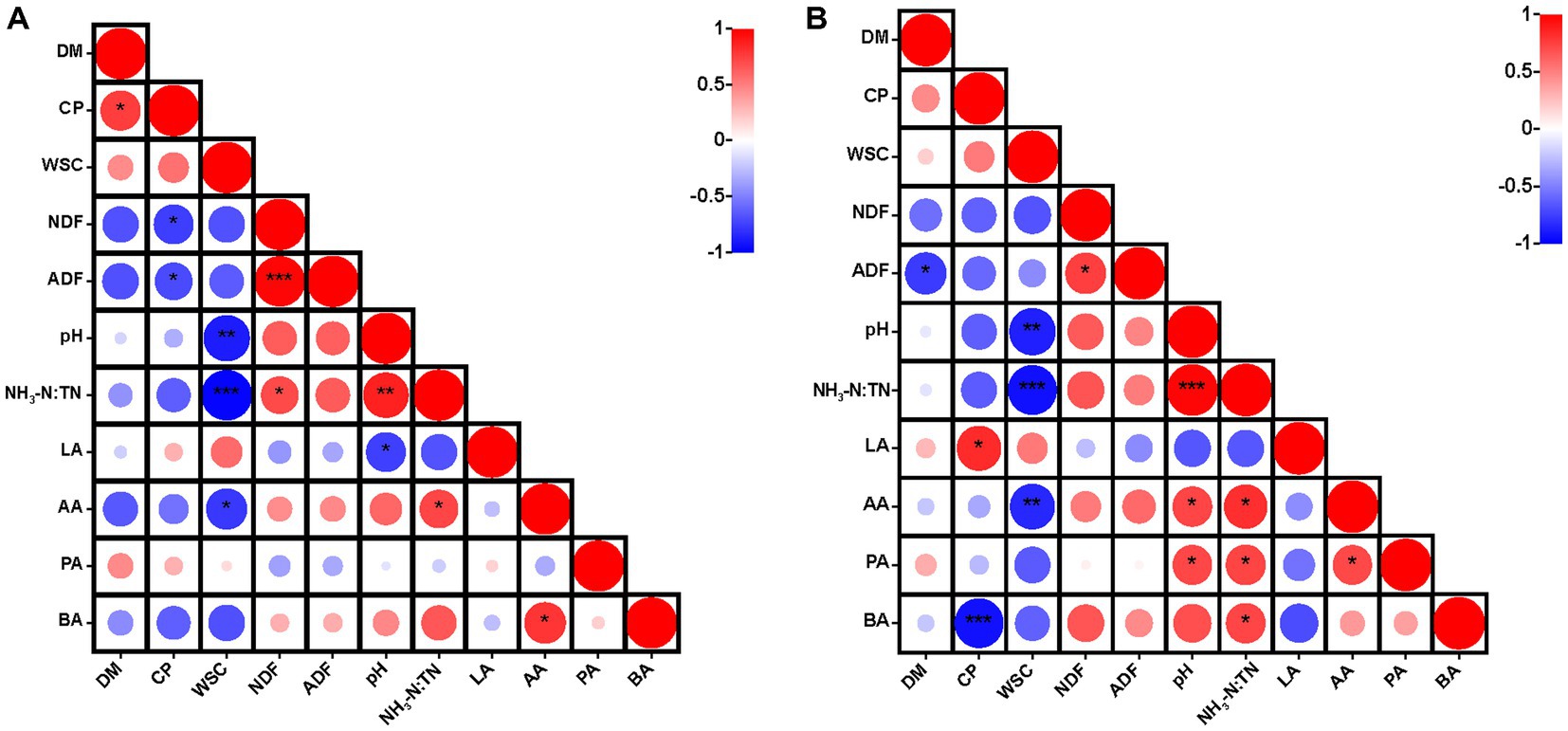
Figure 1. Heatmap of Pearson correlations between nutritional quality and fermentation parameters after 30 days (A) and 60 days (B) of ensiling hybrid Pennisetum. *p < 0.05, **p < 0.01, ***p < 0.001. DM, dry matter; CP, crude protein; WSC, water-soluble carbohydrates; NDF, neutral detergent fiber; ADF, acid detergent fiber; NH3-N:TN, ammonia nitrogen:total nitrogen ratio; LA, lactic acid; AA, acetic acid; PA, propionic acid; BA, butyric acid.
Principal component analysis (PCA) not only reduces the loss of original information, but also simplifies multiple related indicators into independent components, and, subsequently, assesses the indicators based on the difference in principal component scores (Gallo et al., 2013). In the present study, the PCA of 11 indicators of hybrid Pennisetum silage ensiled for different time lengths was carried out. The results of the PCA after 30 days of ensiling are presented in Tables 3, 4. The cumulative contribution of three extracted principal components, based on the characteristic value >1, reached 86.7%, that is, 86.7% of the original index was retained. The positive load value of the NH3-N:TN ratio and the negative load value of WSC were the greatest in the first principal component in their corresponding eigenvector, indicating that the WSC content could limit the silage quality of hybrid Pennisetum. In the second principal component, the positive load value of DM content and the negative load value of lactic acid were the greatest, indicating that the content of lactic acid was the chief factor limiting the quality of hybrid Pennisetum silage. The butyric acid content positive load value and the NDF negative load value were the greatest in the third principal component, which meant that the NDF content held a dominant position in limiting the quality of hybrid Pennisetum silage. The first principal component was correlated positively with NH3-N:TN, NDF, ADF and pH, and negatively with CP and WSC. The second principal component was correlated positively with DM, pH and propionic acid, and negatively with lactic acid and WSC, and the third principal component was correlated positively with organic acids such as butyric acid. After 60 days of ensiling, the cumulative contribution rate of the three extracted principal components, based on the characteristic value >1, reached 89.7% (Tables 3, 4). Similar to ensiling for 30 days, WSC, ADF and butyric acid were the main limiting factors of the principal components after ensiling for 60 days.
We concluded that the lower composite scores indicated better silage quality according to the composite of each original index and the proportion of principal components. Therefore, the top three treatments were LP + PA, PA, and LP + LB.
The changes in pH and relative abundances of microorganisms during aerobic exposure after 60 days of ensiling are presented in Table 5. The resistance against spoilage varies greatly among silages, and different additives are used to prevent aerobic spoilage (Puntillo et al., 2020; Ferrero et al., 2021). With an increase in aerobic exposure, the pH increased (p < 0.05) in all treatments, except for the PA and LP + PA treatments. When the pH of the silage increases by 0.5 after aerobic exposure, it could be regarded as aerobic deterioration (Mu et al., 2021). In the current study, the pH of only the PA and LP + PA treatments did not increase by 0.5 after 6 days of aerobic exposure. The pH of the CAP and LP + CAP treatments were always greater (p < 0.05) than that of CK during aerobic exposure. During aerobic exposure, the abundance of LAB in the CK, LP, LB and LP + LB treatments displayed an increasing trend between days 0 and 6 and then a decreasing trend between days 6 and 9 (p < 0.05), while the abundance of LAB in the PA, LP + PA, CAP and LP + CAP treatments displayed an increasing trend (p < 0.05). When the number of yeasts exceeded 5 log10 cfu/g FW, the silage was prone to aerobic spoilage (Chen et al., 2016). In this study, the number of yeasts in each treatment, except for the organic acid treatment, exceeded this number at 6 days of aerobic exposure, indicating a trend of aerobic spoilage (Wang et al., 2014). The activity of aerobic microorganisms increased after the silage was exposed to air, and they used lactic acid, sugars and amino acids to produce heat continuously, resulting in aerobic putrefaction (Hu et al., 2009; Zhou et al., 2019). In this study, PA and LP + PA inhibited the proliferation of aerobic microorganisms, while the abundance of aerobic bacteria in the other treatments exhibited an increasing trend. The counts of yeast and aerobic bacteria in the PA treatment during aerobic exposure were lower (p < 0.05) than in other treatments, while the pH remained stable, and was lower (p < 0.05) than in other treatments on day 9, which improved the aerobic stability of the silage. The LB treatment failed to inhibit the proliferation of yeast, resulting in an increase (p < 0.05) in pH during aerobic exposure. It is likely that the undesirable microorganisms used the high content of residual lactic acid as a substrate after aerobic exposure (Rabelo et al., 2019).
After sequencing and quality control, a total of 1,044,312 optimized sequences were obtained. According to the 3% difference, a total of 1985 OTUs were obtained by OTU clustering. In total, 88 OTUs were shared by 8 processing units, accounting for 4.43% of all OTUs (Figure 2). CK, LP, LB, LP + LB, PA, LP + PA, CAP and LP + CAP had 11, 1, 2, 3, 63, 102, 5 and 16 OTUs, respectively. Alpha-diversity reflects the microbial abundance and species diversity of a single sample (Xian et al., 2022). Chao1 and ACE diversities are commonly used to measure species richness, while Shannon and Simpson indices are used to measure species diversity (Dong et al., 2020). Compared to CK, except for the LB treatment, the ACE index of all treatments increased, with the LP + CAP treatment being higher than the other treatments (Figure 3). The microbial community within the crop formed in the field, and when the stable environment was disrupted, the microorganisms that were not adapted to the fermentation system were eliminated, and the more adaptable microorganisms dominated in the new environment (Dong et al., 2022b). In this study, the Simpson index of LP was greater (p < 0.05) than the other treatments, while the Chao 1 index was greater (p < 0.05) and the ACE were lesser (p < 0.05) in the LP treatment than CK. Compared to CK, Shannon indices of LP and LB and the Simpson index of LP + CAP were lesser (p < 0.05), while the Shannon index of LP + CAP and the Simpson indices of LP, LB and LP + LB were greater (p < 0.05) than CK. The Shannon indices of LP + LB, LP + PA and LP + CAP were greater but the indices were lesser (p < 0.05) than the LP treatment (p < 0.05). The change in alpha diversity among silages was caused by the dynamic response of microorganisms (Feng et al., 2022). The composition and function of bacteria could differ during the period of ensiling (Sepehri and Sarrafzadeh, 2019).
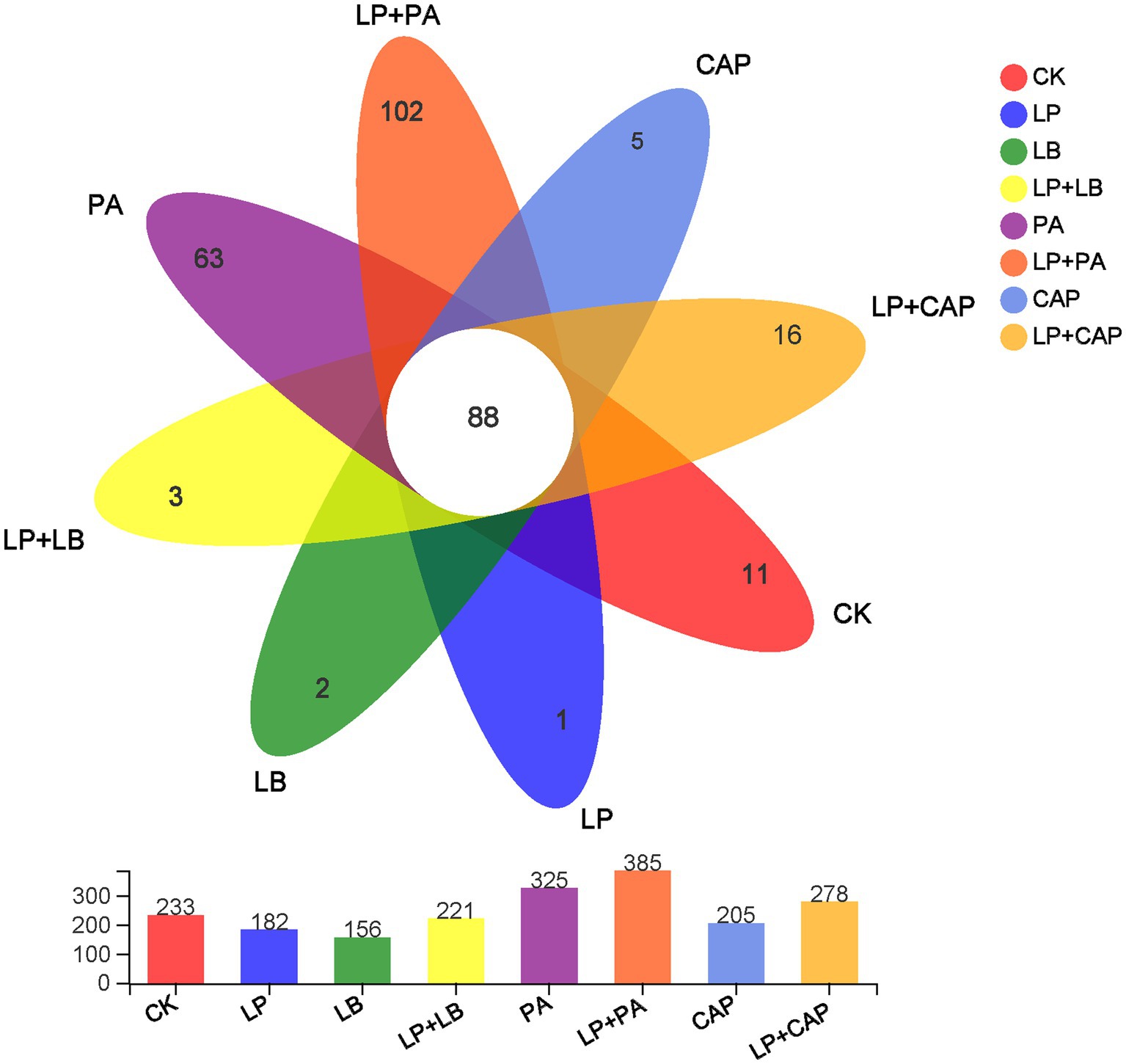
Figure 2. Petal diagram illustrating the degree of overlap of bacterial operational taxonomic units (OTUs) in the 8 silages. CK, distilled water; LP, L. plantarum; LB, L. buchneri; LP + LB, L. plantarum and L. buchneri; PA, propionic acid; LP + PA, L. plantarum and propionic acid; CAP, calcium propionate; LP + CAP, L. plantarum and calcium propionate.
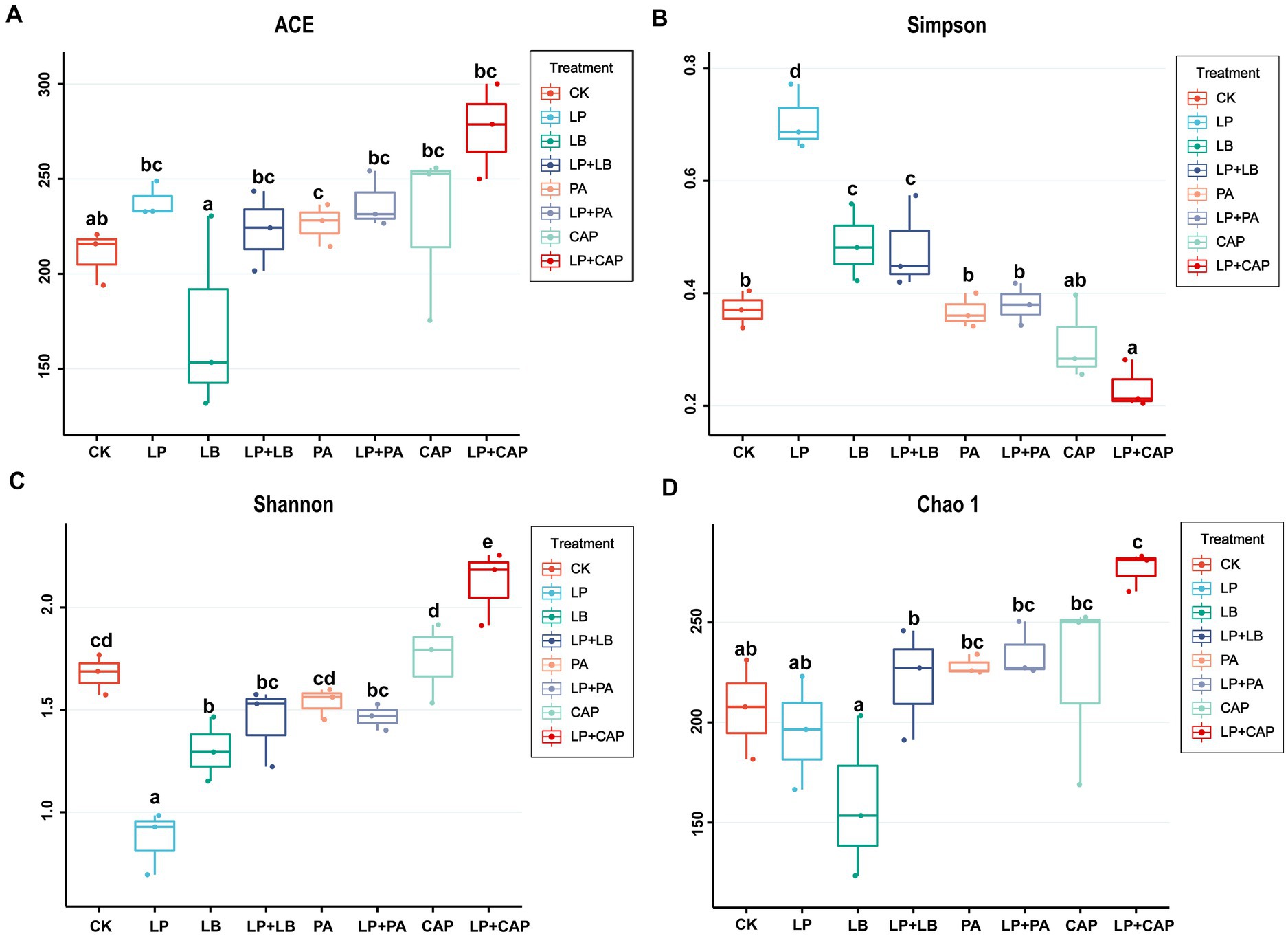
Figure 3. Effects of different additives on alpha diversity of hybrid Pennisetum silage. ACE (A), Simpson (B), Shannon (C) and Chao1 (D) indexes are used to reflected alpha diversity. CK, distilled water; LP, L. plantarum; LB, L. buchneri; LP + LB, L. plantarum and L. buchneri; PA, propionic acid; LP + PA, L. plantarum and propionic acid; CAP, calcium propionate; LP + CAP, L. plantarum and calcium propionate. Means with different lowercase letters differ from each other (p < 0.05).
The PCoA based on the Bray–Curtis dissimilarity displayed distinct clusters among the eight silages (Figure 4). Further analysis through ANOSIM revealed that the results were reliable (R = 0.73, p = 0.001). According to PCoA, CK and LP + LB were clustered in the second and third quadrants, LP and LB in the third quadrant, PA and LP + PA mainly in the fourth quadrant, and CAP and LP + CAP mainly in the second quadrant. In addition, PA and LP + PA were separated from the other treatments, indicating that there were differences in the composition of species; whereas, CK was relatively close to LP, LB, and LP + LB, indicating that the composition of species was similar among these treatments. These results demonstrated that different additive treatments had significant effects on the bacterial community of hybrid Pennisetum silage (Tian J. et al., 2022).
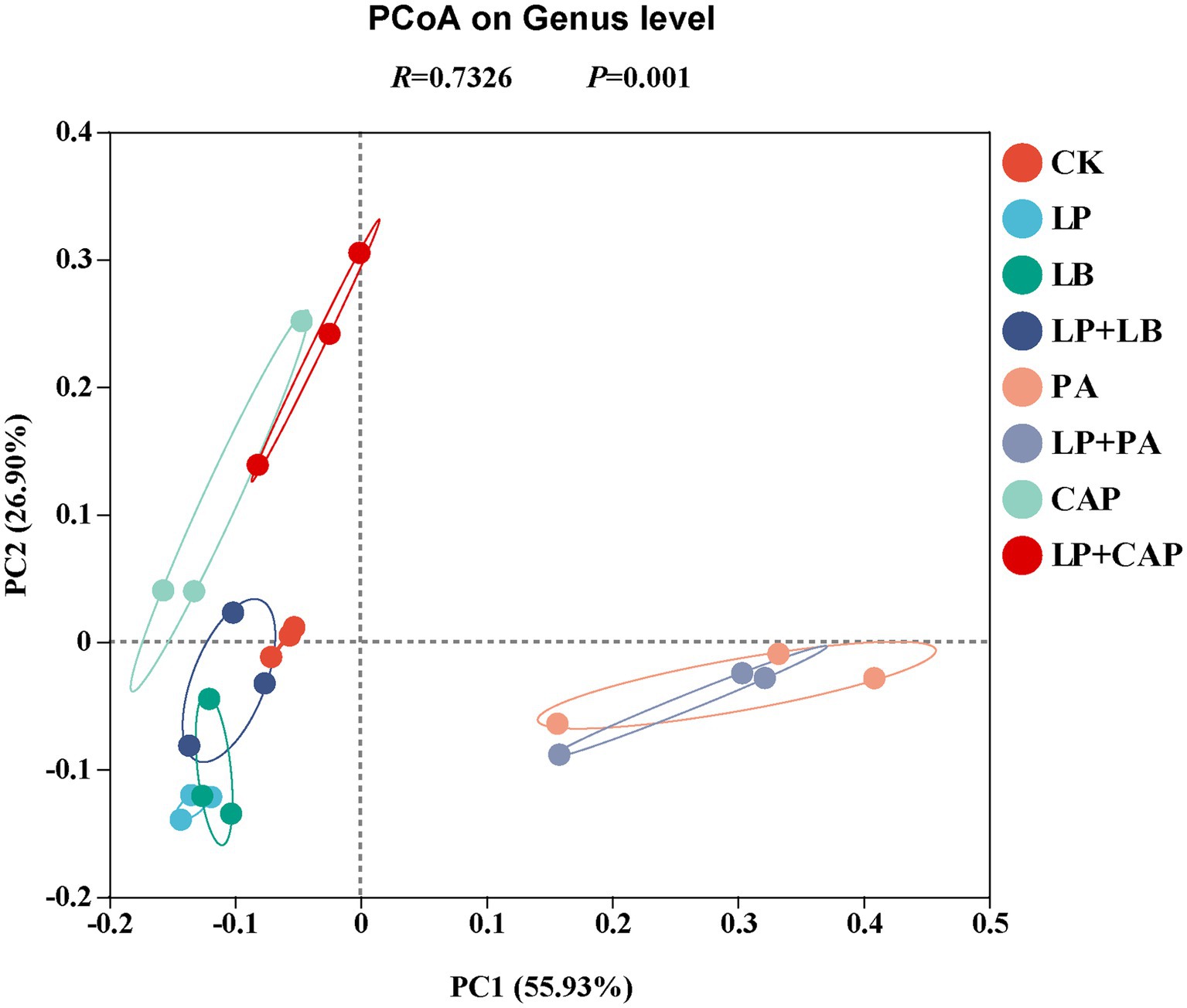
Figure 4. Principal coordinate analysis (PCoA) of microbial diversity of hybrid Pennisetum silage treated with different additives. CK, distilled water; LP, L. plantarum; LB, L. buchneri; LP + LB, L. plantarum and L. buchneri; PA, propionic acid; LP + PA, L. plantarum and propionic acid; CAP, calcium propionate; LP + CAP, L. plantarum and calcium propionate.
After 60 days of ensiling hybrid Pennisetum, the main bacterial phylum was Firmicutes, followed by Proteobacteria and Cyanobacteria (Figure 5A). Liu et al. (2019) reported that Firmicutes and Proteobacteria were the most abundant phyla of barley silage at any point during the ensiling process with or without LAB inoculants, and continued to rise to 99% of the total bacteria at 60 days of ensiling. Most bacteria involved in lactic acid fermentation belong to Firmicutes and Proteobacteria and they play important roles in an anaerobic environment (Yuan et al., 2020). The relative abundance of Firmicutes was lesser (p < 0.05) and of Proteobacteria was greater (p < 0.05) in PA and LP + PA than in CK, while there was no difference (p > 0.05) in the other treatments. The relative abundance of Cyanobacteria in the LP and CAP treatments was lesser (p < 0.05), and in the LP + PA treatment was greater (p < 0.05) than in CK. Cyanobacteria is often found in tropical herbage and could be replaced by Lactobacillus and Enterobacteria after fermentation, but further studies are needed to determine their roles in silage production (Li et al., 2019). The relative abundance of Actinobacteriota in LB was lesser (p < 0.05), while of Actinobacteriota in CAP and LP + CAP was greater (p < 0.05) than in CK. When mixed with L. plantarum, the relative abundances of Firmicutes and Cyanobacteria in LP + PA were greater (p < 0.05), while the relative abundance of Proteobacteria was lesser (p < 0.05) than in LP. The relative abundance of Actinobacteriota in the LP + PA and LP + CAP treatments was greater (p < 0.05) than in the LP treatment. It was reported that Actinobacteriota had the potential of bioremediation to degrade pesticides and heavy metals (Alvarez et al., 2017).
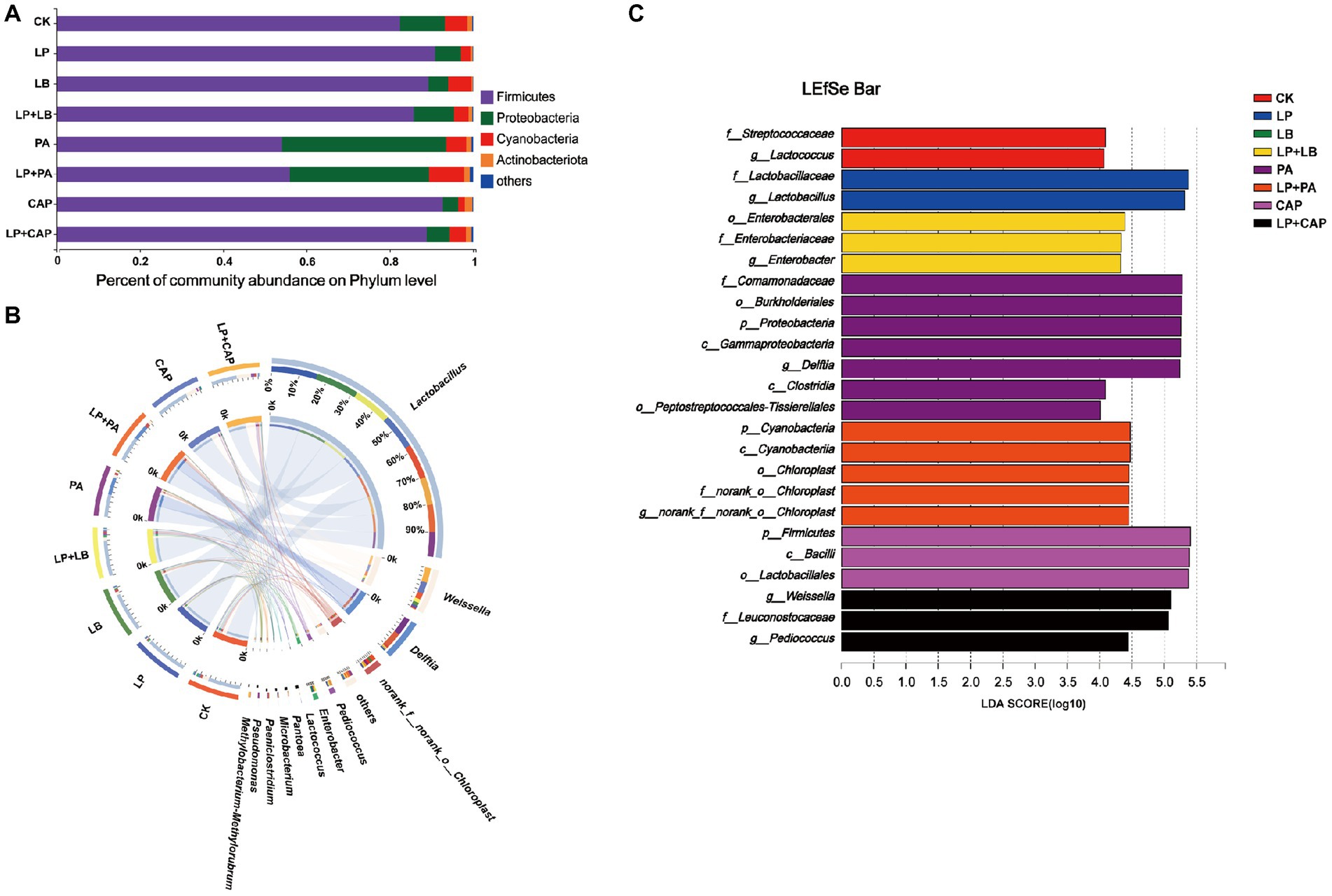
Figure 5. Bacterial abundance at phylum (A) and genus (B) levels of hybrid Pennisetum silage. Linear discriminant analysis effect size (LEfSe) of hybrid Pennisetum silage treated with different additives (C). CK, distilled water; LP, L. plantarum; LB, L. buchneri; LP + LB, L. plantarum and L. buchneri; PA, propionic acid; LP + PA, L. plantarum and propionic acid; CAP, calcium propionate; LP + CAP, L. plantarum and calcium propionate.
The bacterial genera after 60 days of ensiling hybrid Pennisetum is presented in Figure 5B. Lactobacillus was the dominant genus in each treatment. Xu et al. (2020) reported that Lactobacillus was often the most important microbe in the late stages of ensiling, which, together with Weissella and Pediococcus, were the main producers of lactic acid. The relative abundance of Lactobacillus in LP (85.4%) was greater (p < 0.05), but in PA (46.8%) was lesser (p < 0.05) than in CK. The relative abundance of Weissella in the LP, LB, PA and LP + PA treatments was lesser (p < 0.05) than in CK (12.7%). Weissella belongs to the heterofermentative bacteria and consumes WSC to produce a mixture of lactic acid and acetic acid (Huang et al., 2021; Sun et al., 2021). The relative abundance of Delftia in the PA and LP + PA treatments was greater (p < 0.05) than in CK. Delftia is often present in soil and plants and promotes the growth and bioremediation of plants (Liu et al., 2018; Bhat et al., 2022). Dong et al. (2022b) concluded that the low pH of the silage might be due to an accumulation of nitrite in the silage. This occurred because Delftia, as a reductant of nitrate, could not reduce nitrite further. In the acidic environment of silage, nitrite could be converted into nitrogen oxides to reduce the pH of silage. The addition of organic acids reduced the relative abundances of Klebsiella, Paenibacillus, and Enterobacter in corn silage (Jiang et al., 2020). In the present study, the relative abundances of Enterobacter in the PA and LP + PA treatments and of Enterobacterin in the LP + LB treatment were greater (p < 0.05) than in CK. Guo et al. (2020) reported that Enterobacter was one of the dominant bacteria during ensiling, especially in silage treated with LAB. However, Enterobacter was unwanted due to nutrient loss caused by acetic acid fermentation (Ni et al., 2017b), although most Enterobacter bacteria in silage were considered non-pathogenic (Santos et al., 2016). The relative abundances of Weissella and Pediococcus in LP + CAP were greater (p < 0.05) than in LP, while the relative abundances of Delftia and norank_o__Chloroplast in LP + PA were greater (p < 0.05), but the relative abundance of Enterobacter in LP + PA and LP + CAP was lesser (p < 0.05) than in CK. The relative abundance of Methylobactere-methylorubrum in LP + CAP (1.42%) was greater (p < 0.05) than in the other treatments. Methylobactery-methylorubrum is a gram negative, rod-shaped, strictly aerobic bacteria that can utilize methanol and other reduced one-carbon compounds via the serine pathway (Dong et al., 2022b). The high pH of the LP + CAP treatment after 60 days of ensiling was consistent with the neutrophilic property of Methylobacter-methylorubrum (Knief et al., 2012). Methylobacterium was the dominant genus in alfalfa silage (Ni et al., 2017a; Ogunade et al., 2018).
LEfSe was used to analyze the different bacteria species in each treatment. A total of 25 species with relative abundance differences was identified in the 8 treatments (LDA score > 4) (Figure 5C). Proteobacteria and Delftia were biomarkers of the PA treatment at the phylum and genus levels, respectively, while Cyanobacteria and Enterobacter were biomarkers of the LP + PA treatment. Firmicutes was the most abundant phylum in the CAP treatment. In addition, the highest abundances of each treatment at the genus level were as follows: Lactococcus in CK, Lactobacillus in LP, and Weissella and Pediococcus in LP + CAP. Previous studies reported that Pediococcus was generally highly abundant in silage with high pH (Zhao et al., 2022; Zong et al., 2022), and the treatment with LP + CAP in this study had the highest pH at 60 days of ensiling (Table 2).
Figures 6A,B present the second-level classification of microbial community metabolic functions. The metabolic pathway with the greatest abundance in all treatments was carbohydrate metabolism. This metabolism was greater in the PA and LP + PA treatments than in the other treatments, perhaps due to the their high WSC content (Table 1). In addition, the lactic acid content in the PA and LP + PA treatments was greater (p < 0.05) than in CK, which most likely was due to carbohydrate metabolism. The metabolism abundances of the CAP, LP + CAP, PA and LP + PA treatments were greater than CK (p < 0.05), which might be due to the response of microorganisms to long-term acid stress in silage (Bai et al., 2022). The metabolic abundances of terpenoids and polyketides were predicted to be relatively high in the PA and LP + PA treatments. Terpenoids are natural compounds, mainly in Chinese herbal medicine, and are reputed to possess antibacterial and antioxidant properties (Elshamy et al., 2016; Tian et al., 2017). Further predictions of the third-level metabolic pathways of carbohydrate metabolism are presented in Figure 6C. The PA and LP + PA treatments had similar metabolic functions in all carbohydrates, and both had greater metabolism of pyruvate, propanoate, butanoate, ascorbate and aldarate than CK. It was reported that pyruvate metabolism was related to the formation of organic acids such as lactic acid, α-acetolactic acid, acetic acid, and formic acid (Dong et al., 2022a). Pyruvate, an intermediate in the glycolytic pathway, is crucial in lactic acid generation by LAB utilizing WSC, and can interconvert sugars, fats, and amino acids through the acetyl CoA and tricarboxylic acid cycles. As mentioned above, the lactic acid contents of the PA and LP + PA treatments were greater than in CK, and the pH was the lowest among all treatments, which might be related to the up-regulation of this pathway. In addition, the increase in the metabolism of ascorbate and aldarate, C5-branched dibasic acid and inositol phosphate, and glyoxylate and dicarboxylate suggests the consumption of sugar (Yin et al., 2022).
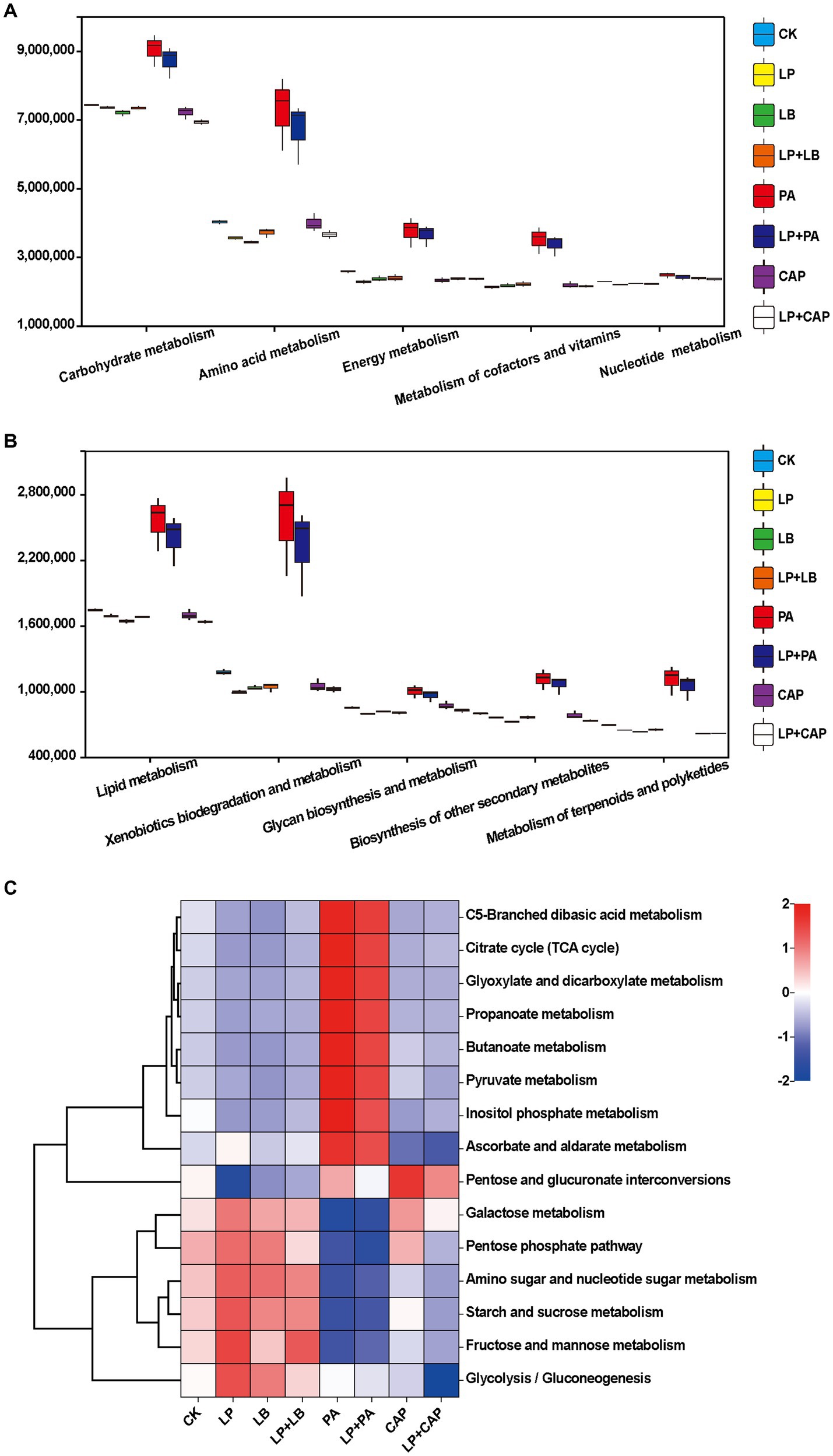
Figure 6. Prediction of microbial metabolic functions of hybrid Pennisetum silage. The second-level (A,B) and third-level (C) classification of microbial community metabolic functions. CK, distilled water; LP, L. plantarum; LB, L. buchneri; LP + LB, L. plantarum and L. buchneri; PA, propionic acid; LP + PA, L. plantarum and propionic acid; CAP, calcium propionate; LP + CAP, L. plantarum and calcium propionate.
Spearman’s correlation tested the relationships between microbial communities at the genus level and nutrients and fermentation characteristics of hybrid Pennisetum silage. Lactococcus correlated positively with ADF (r = 0.44, p < 0.05), and negatively with CP (r = −0.53, p < 0.01) (Figure 7). Moreover, WSC correlated positively with Pseudomonas (r = 0.68, p < 0.001) and Delftia (r = 0.66, p < 0.001), and negatively with Pediococcus (r = −0.85, p < 0.001) and Weissella (r = −0.76, p < 0.001). WSC could be used by microorganisms as a substrate, so it was not surprising that WSC correlated negatively with microorganisms (Zheng et al., 2022). In addition, Enterobacter (r = −0.52, p < 0.01), Pantoea (r = −0.67, p < 0.001), and Microbacterium (r = −0.65, p < 0.001) were correlated negatively with CP. Previous studies had shown that certain Enterobacter were proteolytic, which could cause the loss of protein from silage (Yang et al., 2020). In this study, Weissella was correlated negatively with lactic acid content (r = −0.65, p < 0.001) and positively with acetic acid (r = 0.62, p < 0.01), propionic acid (r = 0.68, p < 0.001), butyric acid (r = 0.87, p < 0.001), pH (r = 0.87, p < 0.001) and NH3-N:TN (r = 0.76, p < 0.001). Results were consistent with those of Zheng et al. (2022) who reported that pH and the concentration of acetic acid were correlated positively with the abundance of Weissella. Furthermore, Pediococcus was correlated positively with NH3-N:TN (r = 0.83, p < 0.001) and acetic acid (r = 0.86, p < 0.001). Several studies indicated that Pediococcus possessed probiotics properties (Fugaban et al., 2021; Jiang et al., 2021). According to Yang et al. (2019), Pediococcus plays a major role in the initial stage of ensiling by helping to create an anaerobic environment that is suitable for LAB growth. Microbacterium might reduce silage quality as this bacterium correlated negatively with lactic acid and CP and positively with pH, butyric acid and NH3-N:TN. Microbacterium is a gram-positive bacterium belonging to Actinobacteria, and is generally isolated from terrestrial and aquatic ecosystems (Marchant et al., 2006). It was reported Microbacterium had the ability to degrade hydrocarbons and complex polysaccharides (Cordovez et al., 2018). However, its specific role in silage production warrants further research.
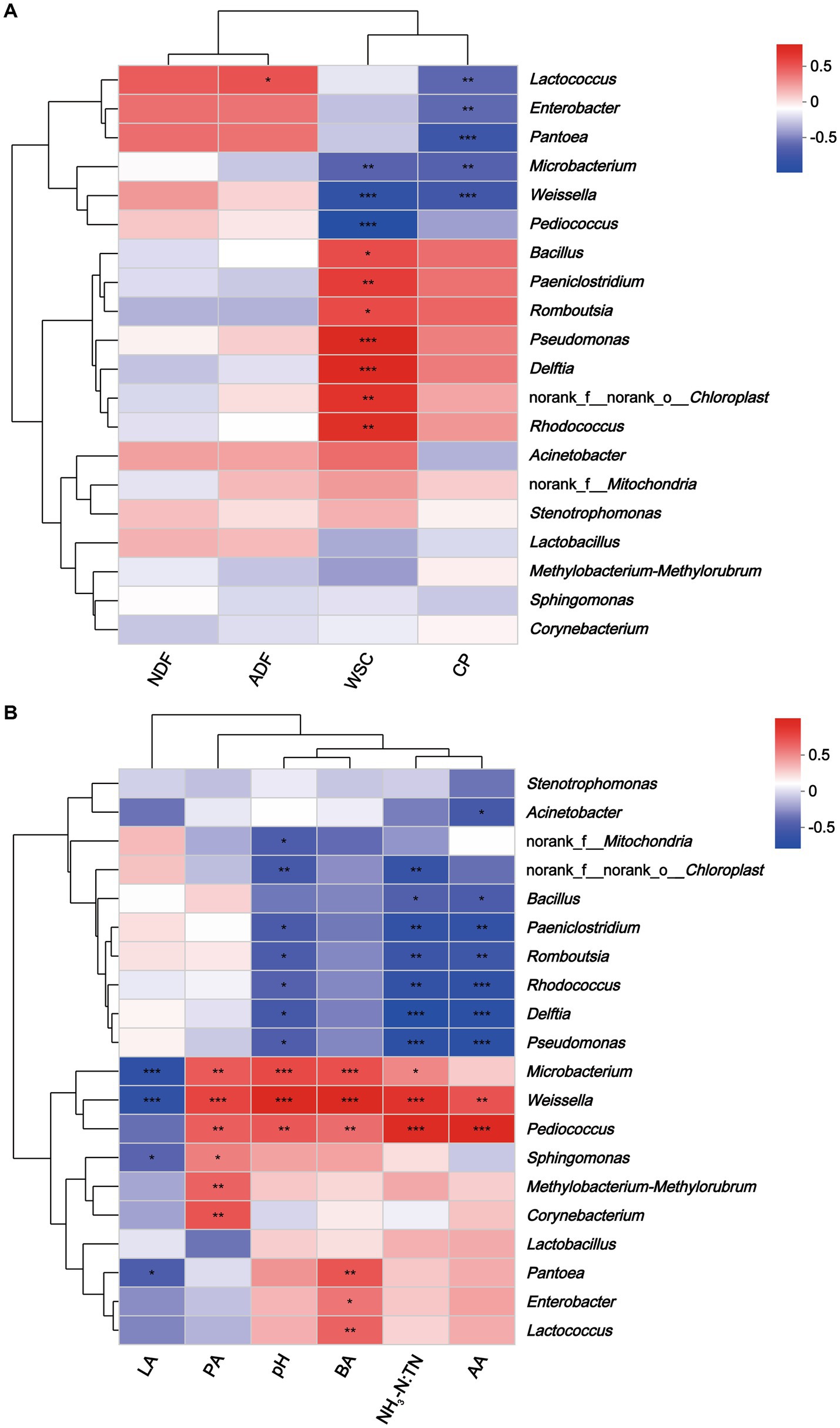
Figure 7. Heatmap of Spearman correlations between nutritional composition (A), fermentation parameters (B) and bacterial abundance of hybrid Pennisetum silage. *p < 0.05; **p < 0.01, ***p < 0.001. NDF, neutral detergent fiber; ADF, acid detergent fiber; WSC, water-soluble carbohydrate; CP, crude protein; LA, lactic acid; AA, acidic acid; PA, propionic acid; BA, butyric acid; NH3-N:TN, ammonia nitrogen:total nitrogen ratio.
Additives affected the quality of hybrid Pennisetum silage by increasing crude protein and lactic acid contents and inhibiting the growth of undesirable bacteria. Principal component analysis revealed that the silage quality of the PA, LP + PA and LB + LP treatments ranked as the top three of the seven treatments. The synergistic effect of L. plantarum combined with L. buchneri improved the quality of silage more so than any one of them alone. The addition of propionic acid was very beneficial, as it increased the relative abundance of Delftia, inhibited the activity of Enterobacter, maintained pH, butyric acid and the NH3-N:TN ratio at low levels and reduced the contents of NDF and ADF. In summary, L. plantarum, L. buchneri, propionic acid, calcium propionate and their combinations could improve the silage of hybrid Pennisetum, which would mitigate the shortage of feed for livestock.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA946341.
QF, JuZ, and WL: writing—original draft, formal analysis, and data curation. AD and CG: writing—review and editing. YZ: formal analysis and data curation. FY and JiZ: funding acquisition, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (42075116 and 32101418), the Fujian Provincial Outstanding Youth Fund Projects (2023J01313545), and the Fujian Provincial Subsidy Project for Science and Technology Special Commissioner (2022S2071).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, M. F., and Tahir, M. (2021). An overview on the factors affecting water-soluble carbohydrates concentration during ensiling of silage. J. Plant Environ. 3, 63–80. doi: 10.33687/jpe.003.01.3702
Alvarez, A., Saez, J. M., Davila Costa, J. S., Colin, V. L., Fuentes, M. S., Cuozzo, S. A., et al. (2017). Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 166, 41–62. doi: 10.1016/j.chemosphere.2016.09.070
Arthur Thomas, T. (1977). An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agr. 28, 639–642. doi: 10.1002/jsfa.2740280711
Bai, J., Ding, Z., Su, R., Wang, M., Cheng, M., Xie, D., et al. (2022). Storage temperature is more effective than lactic acid bacteria inoculations in manipulating fermentation and bacterial community diversity, co-occurrence and functionality of the whole-plant corn silage. Microbiol. Spectr. 10:e0010122. doi: 10.1128/spectrum.00101-22
Bao, X., Guo, G., Huo, W., Li, Q., Xu, Q., and Chen, L. (2023). Ensiling pretreatment fortified with laccase and microbial inoculants enhances biomass preservation and bioethanol production of alfalfa stems. Sci. Total Environ. 857:159442. doi: 10.1016/j.scitotenv.2022.159442
Bhat, S. V., Maughan, H., Cameron, A. D. S., and Yost, C. K. (2022). Phylogenomic analysis of the genus Delftia reveals distinct major lineages with ecological specializations. Microb. Genom. 8:mgen000864. doi: 10.1099/mgen.0.000864
Cai, C., Wang, L., Wang, G., Hao, J., Bai, X., Wang, Z., et al. (2020). Effects of dry explosion pretreatment on physicochemical and fuel properties of hybrid Pennisetum (Pennisetum americanum × P. purpureum). Bioresour. Technol. 297:122508. doi: 10.1016/j.biortech.2019.122508
Carvalho, B. F., Ávila, C. L. S., Pinto, J. C., Pereira, M. N., and Schwan, R. F. (2012). Effects of propionic acid and Lactobacillus buchneri (UFLA SIL 72) addition on fermentative and microbiological characteristics of sugar cane silage treated with and without calcium oxide. Grass Forage Sci. 67, 462–471. doi: 10.1111/j.1365-2494.2012.00863.x
Chen, L., Guo, G., Yuan, X., Zhang, J., Li, J., and Shao, T. (2016). Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat–common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 96, 1678–1685. doi: 10.1002/jsfa.7271
Cordovez, V., Schop, S., Hordijk, K., Dupré de Boulois, H., Coppens, F., Hanssen, I., et al. (2018). Priming of plant growth promotion by volatiles of root-associated Microbacterium spp. Appl. Environ. Microbiol. 84, e01865–e01818. doi: 10.1128/AEM.01865-18
Dai, T., Dong, D., Wang, S., Zong, C., Yin, X., Xu, G., et al. (2022). Assessment of organic acid salts on fermentation quality, aerobic stability, and in vitro rumen digestibility of total mixed ration silage. Trop. Anim. Health Prod. 54:261. doi: 10.1007/s11250-022-03249-w
Dong, Z., Li, J., Wang, S., Dong, D., and Shao, T. (2022a). Diurnal variation of epiphytic microbiota: an unignorable factor affecting the anaerobic fermentation characteristics of sorghum-sudangrass hybrid silage. Microbiol. Spectr. 11:e0340422. doi: 10.1128/spectrum.03404-22
Dong, Z., Li, J., Wang, S., Dong, D., and Shao, T. (2022b). Time of day for harvest affects the fermentation parameters, bacterial community, and metabolic characteristics of sorghum-sudangrass hybrid silage. mSphere 7:e0016822. doi: 10.1128/msphere.00168-22
Dong, Z., Shao, T., Li, J., Yang, L., and Yuan, X. (2020). Effect of alfalfa microbiota on fermentation quality and bacterial community succession in fresh or sterile Napier grass silages. J. Dairy Sci. 103, 4288–4301. doi: 10.3168/jds.2019-16961
Dong, Z., Yuan, X., Wen, A., Desta, S. T., and Shao, T. (2017). Effects of calcium propionate on the fermentation quality and aerobic stability of alfalfa silage. Asian Australas J. Anim. Sci. 30, 1278–1284. doi: 10.5713/ajas.16.0956
Drouin, P., Tremblay, J., Renaud, J., and Apper, E. (2021). Microbiota succession during aerobic stability of maize silage inoculated with Lentilactobacillus buchneri NCIMB 40788 and Lentilactobacillus hilgardii CNCM-I-4785. Microbiol. Open 10:e1153. doi: 10.1002/mbo3.1153
Du, E., Zhao, N., Guo, W., Fan, Q., Wei, J., and Xu, Z. (2022). Effects of konjac flour and Lactiplantibacillus plantarum on fermentation quality, aerobic stability, and microbial community of high-moisture forage rape silages. Fermentation 8:348. doi: 10.3390/fermentation8080348
Elshamy, A. I., Nassar, M. I., Mohamed, T. A., and Hegazy, M. E. (2016). Chemical and biological profile of Cespitularia species: a mini review. J. Adv. Res. 7, 209–224. doi: 10.1016/j.jare.2015.07.003
Fan, X., Xie, Z., Cheng, Q., Li, M., Long, J., Lei, Y., et al. (2022). Fermentation quality, bacterial community, and predicted functional profiles in silage prepared with alfalfa, perennial ryegrass and their mixture in the karst region. Front. Microbiol. 13:1062515. doi: 10.3389/fmicb.2022.1062515
Feng, Q., Shi, W., Chen, S., Degen, A. A., Qi, Y., Yang, F., et al. (2022). Addition of organic acids and Lactobacillus acidophilus to the leguminous forage Chamaecrista rotundifolia improved the quality and decreased harmful bacteria of the silage. Animals 12:2260. doi: 10.3390/ani12172260
Ferrero, F., Tabacco, E., and Borreani, G. (2021). Lentilactobacillus hilgardii inoculum, dry matter contents at harvest and length of conservation affect fermentation characteristics and aerobic stability of corn silage. Front. Microbiol. 12:675563. doi: 10.3389/fmicb.2021.675563
Filya, I. (2003). Nutritive value of whole crop wheat silage harvested at three stages of maturity. Anim. Feed Sci. Tech. 103, 85–95. doi: 10.1016/S0377-8401(02)00284-5
Fu, T., and Diao, Q. (2007). The effect of propionic acid on the fermentation and aerobic stability of maize silage. J. Anim. Feed Sci. 16, 48–53. doi: 10.22358/jafs/74417/2007
Fu, Z., Sun, L., Hou, M., Hao, J., Lu, Q., Liu, T., et al. (2022). Effects of different harvest frequencies on microbial community and metabolomic properties of annual ryegrass silage. Front. Microbiol. 13:971449. doi: 10.3389/fmicb.2022.971449
Fugaban, J. I. I., Vazquez Bucheli, J. E., Kim, B., Holzapfel, W. H., and Todorov, S. D. (2021). Safety and beneficial properties of bacteriocinogenic Pediococcus acidilactici and Pediococcus pentosaceus isolated from silage. Lett. Appl. Microbiol. 73, 725–734. doi: 10.1111/lam.13562
Gallo, A., Moschini, M., Cerioli, C., and Masoero, F. (2013). Use of principal component analysis to classify forages and predict their calculated energy content. Animal 7, 930–939. doi: 10.1017/S1751731112002467
Gheller, L. S., Ghizzi, L. G., Takiya, C. S., Grigoletto, N. T., Silva, T. B., Marques, J. A., et al. (2021). Different organic acid preparations on fermentation and microbiological profile, chemical composition, and aerobic stability of whole-plant corn silage. Anim. Feed Sci. Tech. 281:115083. doi: 10.1016/j.anifeedsci.2021.115083
Guo, L., Yao, D., Li, D., Lin, Y., Bureenok, S., Ni, K., et al. (2020). Effects of lactic acid bacteria isolated from rumen fluid and feces of dairy cows on fermentation quality, microbial community, and in vitro digestibility of alfalfa silage. Front. Microbiol. 10:2998. doi: 10.3389/fmicb.2019.02998
Haas, B. J., Gevers, D., Earl, A. M., Feldgarden, M., Ward, D. V., Giannoukos, G., et al. (2011). Chimeric 16S rRNA sequence formation and detection in sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504. doi: 10.1101/gr.112730.110
He, L., Li, S., Wang, C., Chen, X., and Zhang, Q. (2021). Effects of vanillic acid on dynamic fermentation parameter, nitrogen distribution, bacterial community, and enzymatic hydrolysis of stylo silage. Front. Microbiol. 12:690801. doi: 10.3389/fmicb.2021.690801
He, Q., Zhou, W., Chen, X., and Zhang, Q. (2021). Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus plantarum. J. Clean. Prod. 329:129792. doi: 10.1016/j.jclepro.2021.129792
He, L., Zhou, W., Xing, Y., Pian, R., Chen, X., and Zhang, Q. (2020). Improving the quality of rice straw silage with Moringa oleifera leaves and propionic acid: fermentation, nutrition, aerobic stability and microbial communities. Bioresour. Technol. 299:122579. doi: 10.1016/j.biortech.2019.122579
Hu, W., Schmidt, R. J., McDonell, E. E., Klingerman, C. M., and Kung, L. (2009). The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 92, 3907–3914. doi: 10.3168/jds.2008-1788
Huang, Y., Liang, L., Dai, S., Wu, C., Chen, C., and Hao, J. (2021). Effect of different regions and ensiling periods on fermentation quality and the bacterial community of whole-plant maize silage. Front. Microbiol. 12:743695. doi: 10.3389/fmicb.2021.743695
Jaipolsaen, N., Sangsritavong, S., Uengwetwanit, T., Angthong, P., Plengvidhya, V., Rungrassamee, W., et al. (2021). Comparison of the effects of microbial inoculants on fermentation quality and microbiota in Napier grass (Pennisetum purpureum) and corn (Zea mays L.) silage. Front. Microbiol. 12:784535. doi: 10.3389/fmicb.2021.784535
Jiang, S., Cai, L., Lv, L., and Li, L. (2021). Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Factories 20:45. doi: 10.1186/s12934-021-01537-y
Jiang, F., Cheng, H., Liu, D., Wei, C., An, W., Wang, Y., et al. (2020). Treatment of whole-plant corn silage with lactic acid bacteria and organic acid enhances quality by elevating acid content, reducing pH, and inhibiting undesirable microorganisms. Front. Microbiol. 11:593088. doi: 10.3389/fmicb.2020.593088
Knief, C., Delmotte, N., Chaffron, S., Stark, M., Innerebner, G., Von Wassmann, R., et al. (2012). Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 6, 1378–1390. doi: 10.1038/ismej.2011.192
Kung, L. Jr., Shaver, R., Grant, R., and Schmidt, R. (2018). Silage review: interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 101, 4020–4033. doi: 10.3168/jds.2017-13909
Langille, M. G., Zaneveld, J., Caporaso, J. G., McDonald, D., Knights, D., Reyes, J. A., et al. (2013). Predictive functional profiling of microbial communities using 16SrRNA marker gene sequences. Nat. Biotechnol. 31, 814–821. doi: 10.1038/nbt.2676
Li, D., Ni, K., Zhang, Y., Lin, Y., and Yang, F. (2018). Influence of lactic acid bacteria, cellulase, cellulase-producing Bacillus pumilus and their combinations on alfalfa silage quality. J. Integr. Agr. 17, 2768–2782. doi: 10.1016/S2095-3119(18)62060-X
Li, D., Ni, K., Zhang, Y., Lin, Y., and Yang, F. (2019). Fermentation on characteristics, chemical composition and microbial community of tropical forage silage under different temperatures. Asian Australas J. Anim. Sci. 32, 665–674. doi: 10.5713/ajas.18.0085
Li, M., Yu, Q., Xu, J., Sun, H., Cheng, Q., Xie, Y., et al. (2022). Effect of different organic acid additives on the fermentation quality and bacterial community of paper mulberry (Broussonetia papyrifera) silage. Front. Microbiol. 13:1038549. doi: 10.3389/fmicb.2022.1038549
Li, M., Zi, X., Tang, J., Zhou, H., and Cai, Y. (2019). Silage fermentation, chemical composition and ruminal degradation of king grass, cassava foliage and their mixture. Grassl. Sci. 65, 210–215. doi: 10.1111/grs.12235
Ling, W., Zhang, L., Feng, Q., Degen, A. A., Li, J., Qi, Y., et al. (2022). Effects of different additives on fermentation quality, microbial communities, and rumen degradation of alfalfa silage. Fermentation 8:660. doi: 10.3390/fermentation8110660
Liu, B., Huan, H., Gu, H., Xu, N., Shen, Q., and Ding, C. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Liu, Y., Tie, B., Li, Y., Lei, M., Wei, X., Liu, X., et al. (2018). Inoculation of soil with cadmium-resistant bacterium Delftia sp. B9 reduces cadmium accumulation in rice (Oryza sativa L.) grains. Ecotoxicol. Environ. Saf. 163, 223–229. doi: 10.1016/j.ecoenv.2018.07.081
Lv, J., Fang, X., Feng, G., Zhang, G., Zhao, C., Zhang, Y., et al. (2020). Effects of sodium formate and calcium propionate additives on the fermentation quality and microbial community of wet brewers grains after short-term storage. Animals 10:1608. doi: 10.3390/ani10091608
Magnusson, J., and Schnürer, J. (2001). Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67, 1–5. doi: 10.1128/AEM.67.1.1-5.2001
Marchant, R., Sharkey, F. H., Banat, I. M., Rahman, T. J., and Perfumo, A. (2006). The degradation of n-hexadecane in soil by thermophilic geobacilli. FEMS Microbiol. Ecol. 56, 44–54. doi: 10.1111/j.1574-6941.2006.00061.x
Mu, L., Xie, Z., Hu, L., Chen, G., and Zhang, Z. (2021). Lactobacillus plantarum and molasses alter dynamic chemical composition, microbial community, and aerobic stability of mixed (amaranth and rice straw) silage. J. Sci. Food Agric. 101, 5225–5235. doi: 10.1002/jsfa.11171
Muck, R. E., Nadeau, E. M. G., McAllister, T. A., Contreras-Govea, F. E., Santos, M. C., and Kung, L. Jr. (2018). Silage review: recent advances and future uses of silage additives. J. Dairy Sci. 101, 3980–4000. doi: 10.3168/jds.2017-13839
Ni, K., Minh, T. T., Tu, T. T., Tsuruta, T., Pang, H., and Nishino, N. (2017a). Comparative microbiota assessment of wilted Italian ryegrass, whole crop corn, and wilted alfalfa silage using denaturing gradient gel electrophoresis and next-generation sequencing. Appl. Microbiol. Biotechnol. 101, 1385–1394. doi: 10.1007/s00253-016-7900-2
Ni, K., Wang, F., Zhu, B., Yang, J., Zhou, G., Pan, Y., et al. (2017b). Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 238, 706–715. doi: 10.1016/j.biortech.2017.04.055
Ogunade, I. M., Jiang, Y., Kim, D. H., Cervantes, A. A. P., Arriola, K. G., Vyas, D., et al. (2017). Fate of Escherichia coli O157:H7 and bacterial diversity in corn silage contaminated with the pathogen and treated with chemical or microbial additives. J. Dairy Sci. 100, 1780–1794. doi: 10.3168/jds.2016-11745
Ogunade, I. M., Jiang, Y., Pech Cervantes, A. A., Kim, D. H., Oliveira, A. S., Vyas, D., et al. (2018). Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 101, 2048–2059. doi: 10.3168/jds.2017-12876
Puntillo, M., Gaggiotti, M., Oteiza, J. M., Binetti, A., Massera, A., and Vinderola, G. (2020). Potential of lactic acid bacteria isolated from different forages as silage inoculants for improving fermentation quality and aerobic stability. Front. Microbiol. 11:586716. doi: 10.3389/fmicb.2020.586716
Queiroz, O. C. M., Ogunade, I. M., Weinberg, Z., and Adesogan, A. T. (2018). Silage review: foodborne pathogens in silage and their mitigation by silage additives. J. Dairy Sci. 101, 4132–4142. doi: 10.3168/jds.2017-13901
Rabelo, C. H. S., Härter, C. J., Ávila, C. L. D. S., and Reis, R. A. (2019). Meta-analysis of the effects of Lactobacillus plantarum and Lactobacillus buchneri on fermentation, chemical composition and aerobic stability of sugarcane silage. Grassl. Sci. 65, 3–12. doi: 10.1111/grs.12215
Ren, H., Feng, Y., Pei, J., Li, J., Wang, Z., Fu, S., et al. (2020). Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 307:123238. doi: 10.1016/j.biortech.2020.123238
Ren, H., Sun, W., Yan, Z., Zhang, Y., Wang, Z., Song, B., et al. (2021). Bioaugmentation of sweet sorghum ensiling with rumen fluid: fermentation characteristics, chemical composition, microbial community, and enzymatic digestibility of silages. J. Clean. Prod. 294:126308. doi: 10.1016/j.jclepro.2021.126308
Ren, H., Wang, C., Fan, W., Zhang, B., Li, Z., and Li, D. (2018). Effects of formic or acetic acid on the storage quality of mixed air-dried corn stover and cabbage waste, and microbial community analysis. Food Technol. Biotechnol. 56, 71–82. doi: 10.17113/ftb.56.01.18.5455
Romero, J. J., Zhao, Y., Balseca-Paredes, M. A., Tiezzi, F., Gutierrez-Rodriguez, E., and Castillo, M. S. (2017). Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silage. J. Dairy Sci. 100, 1812–1828. doi: 10.3168/jds.2016-11642
Sandström, V., Chrysafi, A., Lamminen, M., Troell, M., Jalava, M., Piipponen, J., et al. (2022). Food system by-products upcycled in livestock and aquaculture feeds can increase global food supply. Nat. Food 3, 729–740. doi: 10.1038/s43016-022-00589-6
Santos, A. O., Ávila, C. L., Pinto, J. C., Carvalho, B. F., Dias, D. R., and Schwan, R. F. (2016). Fermentative profile and bacterial diversity of corn silages inoculated with new tropical lactic acid bacteria. J. Appl. Microbiol. 120, 266–279. doi: 10.1111/jam.12980
Sepehri, A., and Sarrafzadeh, M.-H. (2019). Activity enhancement of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria in activated sludge process: metabolite reduction and CO2 mitigation intensification process. Appl. Water Sci. 9:131. doi: 10.1007/s13201-019-1017-6
Shah, A. A., Wu, J., Qian, C., Liu, Z., Mobashar, M., Tao, Z., et al. (2020). Ensiling of whole-plant hybrid pennisetum with natamycin and Lactobacillus plantarum impacts on fermentation characteristics and meta-genomic microbial community at low temperature. J. Sci. Food Agric. 100, 3378–3385. doi: 10.1002/jsfa.10371
Song, X., Yue, X., Chen, W., Jiang, H., Han, Y., and Li, X. (2019). Detection of cadmium risk to the photosynthetic performance of hybrid Pennisetum. Front. Plant Sci. 10:798. doi: 10.3389/fpls.2019.00798
Sun, L., Bai, C., Xu, H., Na, N., Jiang, Y., Yin, G., et al. (2021). Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front. Microbiol. 12:655095. doi: 10.3389/fmicb.2021.655095
Tharangani, R. M. H., Yakun, C., Zhao, L. S., Ma, L., Liu, H. L., Su, S. L., et al. (2021). Corn silage quality index: an index combining milk yield, silage nutritional and fermentation parameters. Anim. Feed Sci. Tech. 273:114817. doi: 10.1016/j.anifeedsci.2021.114817
Tian, J., Lai, D., and Zhou, L. (2017). Secondary metabolites from acremonium fungi: diverse structures and bioactivities. Mini Rev. Med. Chem. 17, 603–632. doi: 10.2174/1389557516666160914194134
Tian, J., Yin, X., and Zhang, J. (2022). Effects of wilting during a cloudy day and storage temperature on the fermentation quality and microbial community of Napier grass silage. J. Sci. Food Agric. 102, 4384–4391. doi: 10.1002/jsfa.11792
Tian, H., Zhu, Y., Dai, M., Li, T., Guo, Y., Deng, M., et al. (2022). Additives altered bacterial communities and metabolic profiles in silage hybrid Pennisetum. Front. Microbiol. 12:770728. doi: 10.3389/fmicb.2021.770728
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang, S., Li, J., Zhao, J., Dong, Z., Dong, D., and Shao, T. (2022). Dynamics of the bacterial communities and predicted functional profiles in wilted alfalfa silage. J. Appl. Microbiol. 132, 2613–2624. doi: 10.1111/jam.15417
Wang, S., Sun, Y., Zhao, J., Dong, Z., Li, J., Nazar, M., et al. (2020). Assessment of inoculating various epiphytic microbiota on fermentative profile and microbial community dynamics in sterile Italian ryegrass. J. Appl. Microbiol. 129, 509–520. doi: 10.1111/jam.14636
Wang, N., Xiong, Y., Wang, X., Guo, L., Lin, Y., Ni, K., et al. (2022). Effects of Lactobacillus plantarum on fermentation quality and anti-nutritional factors of paper mulberry silage. Fermentation 8:144. doi: 10.3390/fermentation8040144
Wang, M., Yang, C., Jia, L., and Yu, K. (2014). Effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation characteristics and aerobic stability of whipgrass silage in laboratory silos. Grassl. Sci. 60, 233–239. doi: 10.1111/grs.12065
Wen, A., Yuan, X., Wang, J., Desta, S. T., and Shao, T. (2017). Effects of four short-chain fatty acids or salts on dynamics of fermentation and microbial characteristics of alfalfa silage. Anim. Feed Sci. Tech. 223, 141–148. doi: 10.1016/j.anifeedsci.2016.11.017
Wu, P., Li, L., Jiang, J., Sun, Y., Yuan, Z., Feng, X., et al. (2020). Effects of fermentative and non-fermentative additives on silage quality and anaerobic digestion performance of Pennisetum purpureum. Bioresour. Technol. 297:122425. doi: 10.1016/j.biortech.2019.122425
Xian, Z., Wu, J., Deng, M., Wang, M., Tian, H., Liu, D., et al. (2022). Effects of cellulase and Lactiplantibacillus plantarum on the fermentation parameters, nutrients, and bacterial community in Cassia alata silage. Front. Microbiol. 13:926065. doi: 10.3389/fmicb.2022.926065
Xiong, Y., Meng, Q., Jie, G., Tang, X., and Zhang, H. (2017). Effects of relative humidity on animal health and welfare. J. Integr. Agric. 16, 1653–1658. doi: 10.1016/S2095-3119(16)61532-0
Xu, D., Ding, Z., Wang, M., Bai, J., Ke, W., Zhang, Y., et al. (2020). Characterization of the microbial community, metabolome and biotransformation of phenolic compounds of sainfoin (Onobrychis viciifolia) silage ensiled with or without inoculation of Lactobacillus plantarum. Bioresour. Technol. 316:123910. doi: 10.1016/j.biortech.2020.123910
Xu, D., Wang, N., Rinne, M., Ke, W., Weinberg, Z. G., Da, M., et al. (2021). The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 14, 561–576. doi: 10.1111/1751-7915.13623
Yang, F., Wang, Y., Zhao, S., and Wang, Y. (2020). Lactobacillus plantarum inoculants delay spoilage of high moisture alfalfa silages by regulating bacterial community composition. Front. Microbiol. 11:1989. doi: 10.3389/fmicb.2020.01989
Yang, L., Yuan, X., Li, J., Dong, Z., and Shao, T. (2019). Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 275, 280–287. doi: 10.1016/j.biortech.2018.12.067
Yin, Q., Ji, J., Zhang, R., Duan, Z., Xie, H., Chen, Z., et al. (2022). Identification and verification of key taste components in wampee using widely targeted metabolomics. Food Chem. X 13:100261. doi: 10.1016/j.fochx.2022.100261
Yuan, X., Dong, Z., Li, J., and Shao, T. (2020). Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci. 75, 37–44. doi: 10.1111/gfs.12455
Zhang, Y., Li, L., Kang, X., Sun, Y., Yuan, Z., Xing, T., et al. (2019). Improving methane production from Pennisetum hybrid by monitoring plant height and ensiling pretreatment. Renew. Energ. 141, 57–63. doi: 10.1016/j.renene.2019.03.084
Zhang, G., Wang, J., and Zeng, Y. (2020). A modified rabbit model of tracheal stenosis and a household endoscope. More simplicity and accessibility. Acta Cir. Bras. 35:e351104. doi: 10.1590/ACB351104
Zhao, M., Zhang, H., Pan, G., Yin, H., Sun, J., Yu, Z., et al. (2022). Effect of exogenous microorganisms on the fermentation quality, nitrate degradation and bacterial community of sorghum-sudangrass silage. Front. Microbiol. 13:1052837. doi: 10.3389/fmicb.2022.1052837
Zheng, Y., Li, M., Xu, J., Sun, H., Cheng, Q., Xie, Y., et al. (2022). Effects of different cutting methods and additives on the fermentation quality and microbial community of Saccharum arundinaceum silage. Front. Microbiol. 13:999881. doi: 10.3389/fmicb.2022.999881
Zheng, P., Yang, J., Li, Y., Wu, J., Liang, W., Yin, B., et al. (2020). Gut microbial signatures can discriminate unipolar from bipolar depression. Adv. Sci. 7:1902862. doi: 10.1002/advs.201902862
Zhou, Y., Chen, Y., Guo, J., Shen, Y., and Yang, J. (2019). The correlations and spatial characteristics of microbiome and silage quality by reusing of citrus waste in a family-scale bunker silo. J. Clean. Prod. 226, 407–418. doi: 10.1016/j.jclepro.2019.04.075
Zhu, Y., Xiong, H., Wen, Z., Tian, H., Chen, Y., Wu, L., et al. (2022). Effects of different concentrations of Lactobacillus plantarum and Bacillus licheniformis on silage quality, in vitro fermentation and microbial community of hybrid Pennisetum. Animals 12:1752. doi: 10.3390/ani12141752
Keywords: calcium propionate, hybrid Pennisetum, silage, Lactiplantibacillus plantarum, microbial diversity, propionic acid
Citation: Feng Q, Zhang J, Ling W, Degen AA, Zhou Y, Ge C, Yang F and Zhou J (2023) Ensiling hybrid Pennisetum with lactic acid bacteria or organic acids improved the fermentation quality and bacterial community. Front. Microbiol. 14:1216722. doi: 10.3389/fmicb.2023.1216722
Received: 04 May 2023; Accepted: 12 June 2023;
Published: 29 June 2023.
Edited by:
Xiaodan Huang, Lanzhou University, ChinaReviewed by:
Roxana Beatriz Medina, CONICET Centro de Referencia para Lactobacilos (CERELA), ArgentinaCopyright © 2023 Feng, Zhang, Ling, Degen, Zhou, Ge, Yang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fulin Yang, ZnVsaW4ueWFuZ0BmYWZ1LmVkdS5jbg==; Jing Zhou, emhvdWppbmdfbHpAZmFmdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.