- 1Department of Pharmacology and Toxicology, Medical University of Lodz, Łódź, Poland
- 2Department of Toxicology, Medical University of Gdansk, Gdańsk, Poland
- 3Department of Pharmaceutical Microbiology, Medical University of Gdansk, Gdańsk, Poland
The intake of psychobiotic bacteria appears to be a promising adjunct to neuropsychiatric treatment, and their consumption may even be beneficial for healthy people in terms of mental functioning. The psychobiotics’ mechanism of action is largely outlined by the gut-brain axis; however, it is not fully understood. Based on very recent studies, we provide compelling evidence to suggest a novel understanding of this mechanism: bacterial extracellular vesicles appear to mediate many known effects that psychobiotic bacteria exert on the brain. In this mini-review paper, we characterize the extracellular vesicles derived from psychobiotic bacteria to demonstrate that they can be absorbed from the gastrointestinal tract, penetrate to the brain, and carry the intracellular content to exert beneficial multidirectional action. Specifically, by regulating epigenetic factors, extracellular vesicles from psychobiotics appear to enhance expression of neurotrophic molecules, improve serotonergic neurotransmission, and likely supply astrocytes with glycolytic enzymes to favor neuroprotective mechanisms. As a result, some data suggest an antidepressant action of extracellular vesicles that originate even from taxonomically remote psychobiotic bacteria. As such, these extracellular vesicles may be regarded as postbiotics of potentially therapeutic application. The mini-review is enriched with illustrations to better introduce the complex nature of brain signaling mediated by bacterial extracellular vesicles and indicates knowledge gaps that require scientific exploration before further progress is made. In conclusion, bacterial extracellular vesicles appear to represent the missing piece of the puzzle in the mechanism of action of psychobiotics.
1. Introduction
During the last few decades, studies have revealed the fascinating connection between human gut microorganisms and neuropsychological functioning. The gut is home to a unique and diverse community of microbes, collectively known as the gut microbiota. This composed microecosystem plays a crucial role in our overall condition, including mental health (Cuesta et al., 2021). Probiotics—being defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (Hill et al., 2014)—appear to be effective in advantageous modulation of the gut microbiota composition or function (Hemarajata and Versalovic, 2013; Morelli and Patrone, 2015; Oliphant and Claud, 2022). More specifically, the term psychobiotics (Dinan et al., 2013) has been coined to differentiate the gut microbiota modulating preparations that influence the brain function and mental health. Although currently psychobiotics encompass both probiotics and prebiotics (nutritional factors for the intestinal microbiota; Sarkar et al., 2016), in this review article, we refer to the original definition of psychobiotics as mental health-benefiting live microorganisms (Sarkar et al., 2016), such as Lactobacillus helveticus and Bifidobacterium longum (Messaoudi et al., 2011), or Akkermansia muciniphila (Ding et al., 2021), among others.
Recent research shed light on the role of psychobiotics on the gut microbiome and the diverse range of neuropsychiatric disorders. Individuals suffering from depression and anxiety tend to have lower abundance of beneficial gut bacteria with their functional impairment (Jiang et al., 2015; Aizawa et al., 2016; Kelly et al., 2016; Peter et al., 2018; Kazemi et al., 2019), while taking probiotics has been found to reduce depressive and anxiety symptoms (Huang et al., 2017; Ng et al., 2018; Reis et al., 2018; Goh et al., 2019; Liu et al., 2019; Chao et al., 2020; Cohen Kadosh et al., 2021; El Dib et al., 2021). Also, healthy people may benefit from the use of psychobiotics in terms of mental functioning (Messaoudi et al., 2011; Huang et al., 2017; Chao et al., 2020). Moreover, psychobiotics appear to reverse the neurocognitive deterioration in Alzheimer’s disease and mild cognitive impairment (Den et al., 2020; Liu et al., 2023). These findings provide compelling evidence that bacterial psychobiotics may play a vital role in neuropsychiatric treatment and general well-being.
The gut-brain axis (GBA) has been outlined as a framework for mechanism of action of psychobiotics. GBA is a bidirectional communication network between the intestine (together with the residing microbiota) and the brain. This communication is mediated by multiple pathways and messengers, such as the vagus nerve (Breit et al., 2018), the hypothalamic–pituitary–adrenal axis (Frankiensztajn et al., 2020), microbiota-derived neurotransmitters and their precursors (Tillmann et al., 2018; Chen et al., 2021), neurotrophic factors (Ait-Belgnaoui et al., 2014; Mohammadi et al., 2019; Heidarzadeh-Rad et al., 2020), specific bacterial metabolites such as short chain fatty acids (O’Riordan et al., 2022; Karbownik et al., 2023), and immune system, including the modulation of cytokine release (Mohammadi et al., 2019; Rutsch et al., 2020; Partrick et al., 2021). However, the psychobiotics’ mechanism of action is still a mystery to the scientists. Recently, another factor has emerged as potentially shaping the GBA function following the use of psychiobiotics. This factor includes bacterial extracellular vesicles (EVs). It appears that EVs represent the missing piece of the puzzle in GBA and the mechanism of action of psychobiotics.
Psychobiotic bacteria within the gut—as all the living cells—produce and release nanosized EVs carrying bacterial cellular components (Cuesta et al., 2021). Bacterial EVs contribute to communication not only between the microbes, but also at the inter-kingdom level to affect the host cells (Haas-Neill and Forsythe, 2020; Cuesta et al., 2021; Pirolli et al., 2021). Bacterial EVs are small enough to be absorbed from the gastrointestinal tract and penetrate to the brain. In this way, EVs cargo a range of foreign bioactive compounds to affect the central nervous system (CNS) function. In this review, we decipher the role of bacterial EVs in the mechanism of action of psychobiotics by characterizing the nature of probiotic EVs, suggesting the way of their distribution to the CNS, and providing evidence for their action therein. We argue that bacterial EVs represent the novel mechanism of action of psychobiotics and highlight the future directions in relevant research.
2. Biogenesis and characteristics of bacteria-derived EVs
Bacteria have been reported to secrete several types of vesicles. While their biogenesis has not been completely explained, the structure and cargo of the bacterial EVs is dependent on the parental cell and is likely reflected in pharmacological effect under exposition (Toyofuku et al., 2019). In this section, we present only the outline of the current state of knowledge on this topic (Figure 1).

Figure 1. Biogenesis and characteristics of bacteria-derived extracellular vesicles (EVs). Outer-inner membrane vesicles (OIMVs) are formed from Gram-negative bacteria through blebbing or in “explosive cell lysis” process. OIMVs are considered to contain all functional components of parental cells including lipopolysaccharide (LPS) and the fragments of chromosomal deoxyribonucleic acid. Outer membrane vesicles (OMVs) are the most abundant vesicle type secreted by Gram-negative bacteria. OMVs are formed through blebbing and predominantly contain periplasmic content of the parental cell. In the case of Gram-positive bacteria, the mechanism of cytoplasmic membrane vesicles (CMVs) generation is based on selective degradation of peptidoglycan cell wall layer. It might lead to the cell death which is termed “bubbling cell lysis” Created with BioRender.com.
Outer membrane vesicles (OMVs) are the main type of EVs released by Gram-negative bacteria. OMVs are formed from the outer membrane of the cell and are packed with periplasmic material. Some works have also reported the presence of cytosolic proteins as well as genetic material [deoxyribonucleic (DNA) and ribonucleic acids (RNA)] in OMVs isolates (Schwechheimer and Kuehn, 2015; Lee et al., 2016). The secretion of OMVs might be initiated by the local polarization of the membrane (Mashburn-Warren et al., 2008) or intercalation of chemicals into outer membrane (Kobayashi et al., 2000; Florez et al., 2017). Both triggers are assumed to disturb the curvature of membrane which leads to OMVs formation. Increased turgor pressure in periplasmic space, due to the accumulation of biochemicals like misfolded proteins or phospholipids, have also been associated with outer membrane vesiculation (McBroom and Kuehn, 2007; Roier et al., 2016). Small quorum-sensing molecules and proteins additionally stimulate vesicle production (McMillan and Kuehn, 2021).
The OIMVs are double bilayered vesicles, enveloping a fragment of cytosol with a coat composed of inner (cellular) and outer membrane. Thus, OIMVs are considered to contain all functional components of parental cell including lipopolysaccharide (LPS) and the fragments of chromosomal DNA (Baeza et al., 2021). OIMVs might be formed similarly to OMVs through blebbing as a result of autolysin activity that transiently breaks down peptidoglycan to allow OIMV release (Pérez-Cruz et al., 2013; McMillan and Kuehn, 2021). Alternatively, OIMVs might be secreted in “explosive cell lysis” process (Turnbull et al., 2016; Baeza et al., 2021). OIMVs are less abundant fraction than OMVs (their content does not typically exceed few percent of total EVs population during the logarithmic growth phase; Pérez-Cruz et al., 2013; Baeza et al., 2021). Although the size of both types of vesicles is dependent on bacterial species and strain, the diameter of OIMVs typically exceeds 100 nm, and OMVs are usually smaller than OIMVs (Pérez-Cruz et al., 2013).
In the case of Gram-positive bacteria, the mechanism of cytoplasmic membrane vesicles (CMVs) generation is based on selective degradation of thick peptidoglycan cell wall layer. It might lead to the death of the cell which is termed “bubbling cell lysis” (Liu et al., 2018; McMillan and Kuehn, 2021). It is assumed to be analogous to explosive cell lysis occurring in Gram-negative bacteria. Both are initiated by phages or stress conditions like DNA damage (SOS response) or perforation of peptidoglycan by exogenous factors (e.g., enzymes or antibiotics). In response, the expression of endolysins and fragmentation of peptidoglycan occurs. Perforation of thick peptidoglycan in Gram-positive bacteria leads to the “leakage” of cell interior (through the pores) in a form of vesicles (“bubbles”) while in Gram-negative the process is more rapid (cell “explodes”). Nevertheless, CMVs released in this process typically feature 20–200 nm in size and contain all cellular components of parental cell, and reflects the cell condition at the time of death (Toyofuku et al., 2019).
3. Signaling and distribution of psychobiotic EVs to the brain
Extracellular vesicles may represent the efficient nanostructure for transport of bacterial bioactive compounds to the human brain. After being absorbed from the gastrointestinal tract (Stentz et al., 2018), the most likely mechanism of EVs distribution to the CNS is by (1) crossing the blood–brain barrier (BBB; Matsumoto et al., 2017). Other potential ways include (2) vagal nerve transport and (3) activated leukocyte trafficking to the brain (Figure 2).
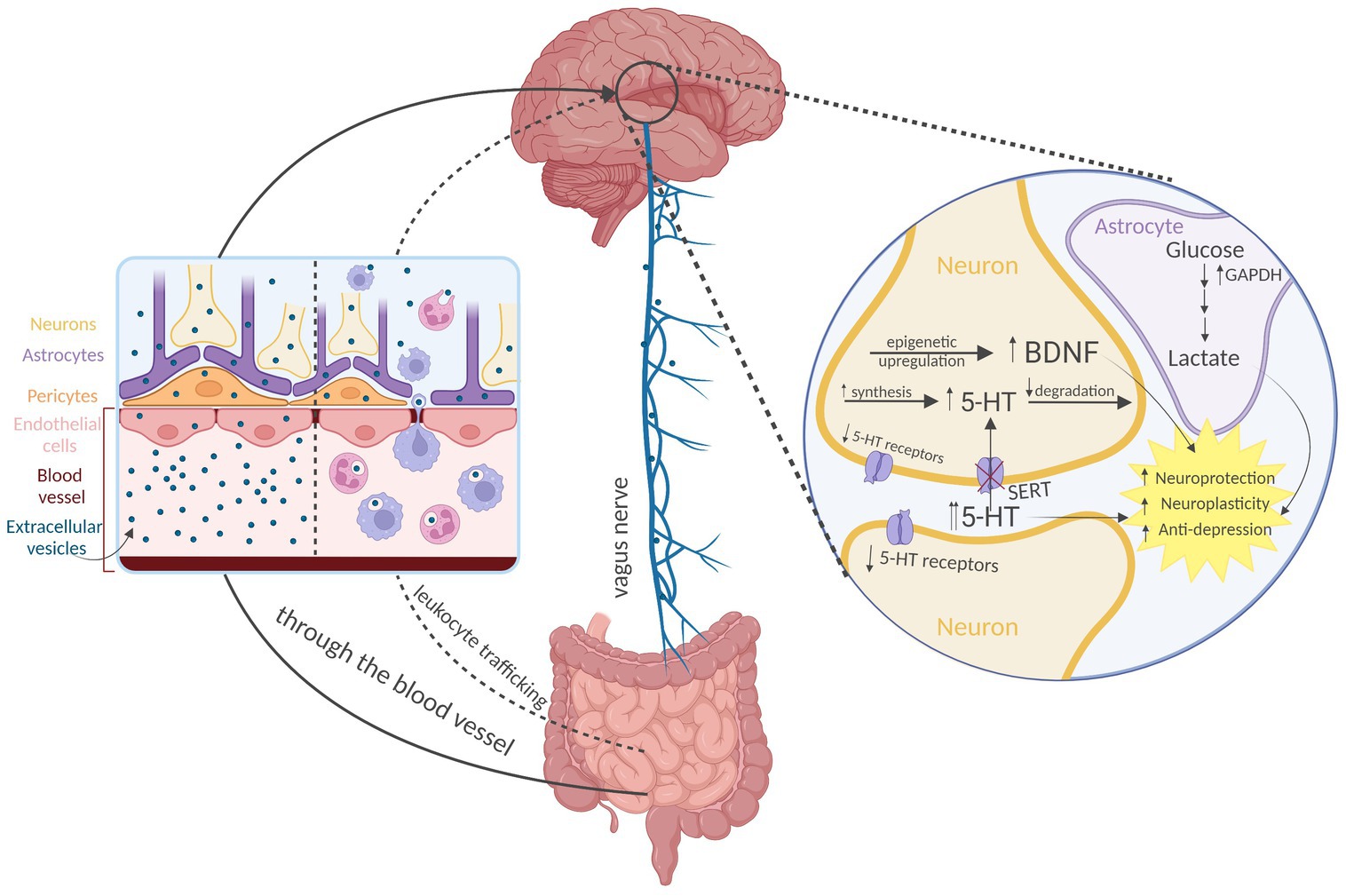
Figure 2. Distribution and action of the psychobiotic bacteria-derived extracellular vesicles (EVs) on the brain. Direct crossing of the blood–brain barrier appears to be the predominant way of EVs transport to the brain. Other routes include vagal nerve transport and activated leukocyte trafficking. According to the current evidence, EVs from psychobiotic bacteria produce antidepressant-like effect mediated by epigenetic upregulation of neurotrophic factors (e.g., brain-derived neurotrophic factor, BDNF), modulation of serotonergic system expression, and possibly by supplementation the astrocytes with glycolytic enzymes (glyceraldehyde 3-phosphate dehydrogenase, GAPDH). 5-HT—serotonin, and SERT—5HT transporter Created with BioRender.com.
A set of in vitro experiments have been performed to document the EVs transport through the intact BBB. In the study by Morad et al., an in vitro static BBB model has been constructed. This was a two-channel microfluidic culture device that contained a vascular channel lined by induced pluripotent stem-derived human microvascular endothelial cells, which were separated by a porous extracellular matrix-coated membrane from an abluminal channel containing primary human astrocytes and pericytes (Morad et al., 2019). In this way, the model could replicate the barrier function of in vivo intact human BBB (Park et al., 2019). By labeling tumor-derived 150 nm size EVs and flowing them through the vascular channel, a fluorescent signal was then detected in the abluminal chamber and increased significantly over time. Fluorescence microscopy analysis confirmed the presence of EVs that were taken up by astrocytes in the abluminal chamber. These findings demonstrate that EVs can interact with endothelial cells under flow conditions and continuously cross the endothelial layer through transcytosis (Morad et al., 2019).
Furthermore, a zebrafish model was used for in vivo studies to explore transcytosis of EVs through the BBB. Zebrafish develop a mature BBB at 3 days postfertilization and are a suitable model for BBB studies. Intracardiac injection of the brain-seeking EVs into zebrafish was performed and their distribution in the brain was monitored through live imaging. The results of this study showed that EVs were taken up by multiple cells within the brain parenchyma, proving their ability to cross the BBB in vivo. Furthermore, transport of EV-containing endocytic vesicles within endothelial cells could be observed using time-lapse imaging. As in transcytosis, these vesicles moved toward the plasma membrane and fused with the membrane. Most importantly, the BBB remained intact throughout the duration of these experiments, highlighting the absence of an inflammatory process (Morad et al., 2019).
Although several other papers similarly documented the passage of EVs of various origin through the intact BBB (Banks et al., 2020; Jakubec et al., 2020; Morales-Prieto et al., 2022; Zhou et al., 2022), the other mechanisms appear to exist as well. A second pathway that bacterial EVs may penetrate to the brain is through the vagal nerve. Paenalcaligenes hominis isolated from the feces of elderly people and aged mice were transplanted into young mice, and bacterial EVs-mediated cognitive impairment and colonic inflammation was noted (Lee et al., 2020). Specifically, Lee et al. (2020) orally gavaged conjugated P. hominis EVs or LPS in mice. The results showed that conjugated EVs and LPS were detectable in microglial cells and in the pyramidal region of the hippocampus. Furthermore, conjugated EVs were more abundant than LPS. However, following vagotomy, conjugated EVs abundance in this region was significantly reduced, unlike the conjugated LPS, which was not affected. Additionally, bacterial 16S ribosomal DNA levels in the hippocampus were increased after oral gavage of P. hominis or its EVs, whereas the process was inhibited by vagotomy. These findings suggest that EVs of P. hominis are orally absorbed and may be able to exert their actions on the brain at least partially through transport via the vagus nerve (Lee et al., 2020).
Other putative mechanisms that have not been extensively studied include EVs crossing the BBB via activated leukocytes trafficking to the brain. Bacterial peptidoglycans, which are a component of EVs, have been identified in the brains of patients with multiple sclerosis, which are heavily infiltrated with blood derived leukocytes. This suggests that these immune cells could be a source of EVs entry into the CNS, although it was not directly investigated (Schrijver et al., 2001). This mechanism of action is also common for viruses’ (size 20–200 nm) ability to reach and infect the brain (Wurdinger et al., 2012; Branton et al., 2013).
4. Effects of EVs derived from psychobiotics on the CNS
Mounting evidence confirms that pathogenic bacteria EVs produce deleterious effects on the CNS function, whereas probiotic bacteria EVs exert beneficial effects on peripheral tissues (Cuesta et al., 2021; Pirolli et al., 2021). This provides indirect evidence for advantageous CNS action of EVs from psychobiotics. Depending on the species of origin, bacterial EVs may have both beneficial and detrimental effects on the CNS, with the latter being more extensively studied (Cuesta et al., 2021). Post-mortem brain samples from patients with Alzheimer’s disease have shown the presence of bacterial nucleic acids that could have been transported in EVs (Emery et al., 2017; Cuesta et al., 2021; Vandendriessche et al., 2021). EVs from the periodontal pathogen Aggregatibacter actinomycetemcomitans were shown to cross the BBB after intracardiac injection to mice, carrying bacterial extracellular nucleic acids, which increased expression of tumor necrosis factor alpha, a known pro-inflammatory cytokine, in the brain cortex (Han et al., 2019). On the other hand, probiotic bacteria EVs from lactobacilli (Al-Nedawi et al., 2015; Behzadi et al., 2017; Li et al., 2017; Seo et al., 2018; Choi et al., 2020; Kim et al., 2020), bifidobacteria (Kim et al., 2016), as well as other probiotic genera (de Rodovalho et al., 2020), were found to exert beneficial anti-inflammatory action through various mechanisms as tested in human peripheral cells or animal models relevant to non-CNS disorders.
However, very recent literature provides direct evidence for CNS-relevant advantageous action of EVs from psychobiotics (Figure 2). Choi et al. (2019) investigated the therapeutic potential of EVs derived from Lactiplantibacillus plantarum on depression model. The authors induced depressive-like behavior in stressed mice and administered intraperitoneal injections of L. plantarum EVs. The results showed a reduction in depressive-like behavior, an increase in brain-derived neurotrophic factor (BDNF) gene expression in the hippocampi, and an additional increase in BDNF expression in a glucocorticoid-suppressed mouse neuronal cell line culture following L. plantarum-derived EVs treatment. This evidence was the first to suggest that EVs from psychobiotics may be beneficial at both molecular and functional levels. This indicated the potential for psychobiotic EVs as a therapeutic option for depression, and supported the notion that EVs play a significant role in the psychobiotics’ mechanism of action (Choi et al., 2019).
A follow-up study conducted by the same research team has suggested that the effect is not limited to EVs from just one bacterial psychobiotic strain. Choi et al. (2022) induced chronic stress-like conditions in mice and administered EVs isolated from taxonomically different potential psychobiotic bacteria L. plantarum, Bacillus subtilis, or Akkermansia muciniphila. The authors noted that all the EVs produced an anti-depressive-like effect and restored diminished hippocampal BDNF as well as neurotrophin-3 and -4/5 expression; however, the behavioral effect was the most pronounced in case of EVs from L. plantarum, and EVs from A. muciniphila affected partially different epigenetic regulators of the expression of tested neurotrophic factors than that of L. plantarum and B. subtilis. The superior effect of L. plantarum EVs was not surprising as the authors found particularly remarkable stress-induced decrease in lactobacilli abundance in the gut microbiota in the tested animal model, which produced a gap for therapeutic response from the relevant EVs (Choi et al., 2022).
It becomes clearer that EVs from psychobiotic bacteria produce more versatile CNS-relevant effects than that mediated by neurotrophic factors. A study by Yaghoubfar et al. (2020) has shed light on the effects of EVs from potential psychobiotic A. muciniphila (Ding et al., 2021) on expression of serotonergic system in mice hippocampi. Oral treatment with the EVs led to an increase in the messenger RNA expression of the gene encoding rate limiting serotonin (5-HT) synthesizing enzyme tryptophan hydroxylase, whereas a decrease in degrading enzyme monoamine oxidase was noted. Also, 5-HT level was found to be increased, possibly extracellularly, as the expression of 5-HT transporter reduced. At the same time, the expression of some 5-HT receptors was decreased. As the treatment with Akkermansia EVs in the experiment lasted for 4 weeks (Yaghoubfar et al., 2020), the resulted serotonergic regulation may be comparable to that following chronic and therapeutically relevant treatment with antidepressants (Artigas, 2013; Gray et al., 2013; Bowman and Daws, 2019).
Another proposed mechanism for the beneficial effects of EVs from psychobiotics involves glycolytic enzymes. As a byproduct of glycolysis, astrocytes produce lactate, and it happens regardless of sufficient oxygen availability (Takahashi, 2021). Astrocyte-derived lactate is believed to fuel neurons, support synaptic plasticity processes (Suzuki et al., 2011) and its transport may prevent depression (Yao et al., 2023). Thus, high glycolytic activity in astrocytes may be beneficial (Takahashi, 2021). In a study by Bajic et al. (2020), EVs released by the dairy isolate L. plantarum were found to be enriched in enzymes involved in central metabolic pathways, including glycolysis, in particular, glyceraldehyde 3-phosphate dehydrogenase. This suggests that glycolytic enzymes may be supplemented by psychobiotic EVs to astrocytes, thus contributing to neuroprotection, however, a direct causation needs to be examined.
A study by Yang et al. (2022) explored the potential of EVs derived from L. plantarum against ischemic brain injury. Although this condition represents an acute clinical event, post-stroke complications typically include depression and cognitive deterioration (Wijeratne and Sales, 2021). The results showed that EVs from Lactiplantibacillus significantly reduced brain damage and improved neurological function in mice following a stroke. Moreover, they reduced infarct size and decreased neurological deficits. The mechanism of this protective effect involved the regulation of a specific microRNA, miR-101a-3p, and its downstream targets, c-Fos and transforming growth factor-β, leading to the inhibition of neuron apoptosis. Importantly, miR-101a-3p could also serve as a marker for neurological recovery in ischemic stroke patients. This finding may further shed light on the EVs-mediated mechanism of CNS-relevant action of psychobiotic bacteria (Yang et al., 2022).
5. Discussion
Gut-brain axis outlines complex mechanisms in which psychobiotics exert action on the brain. Until recently, it has not been appreciated that the GBA-mediated action may be additionally evoked through bacterial secreted EVs; this topic has been neglected in majority of recent review papers in the area of psychobiotics’ mechanism of action (Del Toro-Barbosa et al., 2020; Dey and Mookherjee, 2021; Johnson et al., 2021; Tremblay et al., 2021; Yang et al., 2021; Magalhães-Guedes, 2022; Suda and Matsuda, 2022). Nevertheless, here we provide substantial evidence supporting such supposition. EVs from psychobiotic bacteria are small enough to be absorbed from the gastrointestinal tract and transported to the brain, where they can interact with the CNS components, affecting various range of brain processes. EVs from psychobiotics—even originated from taxonomically remote bacteria—may produce antidepressant effects. This may be through modulation of the expression of neurotrophic factors (Choi et al., 2019, 2022), neurotransmitter regulation (Yaghoubfar et al., 2020), or possible supplementation of the astrocytes with glycolytic enzymes (Bajic et al., 2020), among the investigated mechanisms. There is also a large body of evidence supporting anti-inflammatory action of probiotic EVs (Al-Nedawi et al., 2015; Kim et al., 2016, 2020; Behzadi et al., 2017; Li et al., 2017; Seo et al., 2018; Choi et al., 2020; de Rodovalho et al., 2020), however, none was performed in the CNS-relevant model. Collectively, EVs appear to intermediate a great deal of known mechanisms within the GBA. Moreover, psychobiotic bacteria-derived EVs may represent the “concentrated” messenger to the brain, as some of their effects were multiplied in comparison to administration of parental psychobiotic bacteria (Yaghoubfar et al., 2020). In this light, EVs may represent the missing piece of the puzzle in mechanism of action of psychobiotics.
Psychobiotics encompass Gram-positive and Gram-negative bacteria. Despite their differences, numerous bacteria from both these groups were shown to secrete EVs and to feature similar pharmacological effect in the GBA (Choi et al., 2022). On the other hand, EVs even from the same bacteria species (e.g., Escherichia coli) may have the opposite strain-dependent pro- (Imamiya et al., 2023) or anti-inflammatory properties (Güttsches et al., 2012), and this phenomenon results from the altered form of LPS expressed by the parental bacteria (Güttsches et al., 2012). Little is known about specific ingredients and markers of bacterial EVs [e.g., fatty (Schroeder and Bäckhed, 2016; Champagne-Jorgensen et al., 2021) or ribonucleic acids (Lee et al., 2020), kinases (Gradowski et al., 2020), and other enzymes (Bajic et al., 2020)] that translate to the properties of psychobiotics. Scientific efforts toward their identification are urgently needed to understand EVs-mediated mechanism of action.
Limited knowledge on the biogenesis of bacterial EVs and their still evolving classification make it difficult to unequivocally identify the type of vesicles responsible for certain CNS-relevant effects. Since 2018, the updated recommendation of International Society of Extracellular Vesicles considering, inter alia, isolates characterization has increased the reporting standards (Théry et al., 2018), with the hope for deciphering relationship between probiotic EVs quality and their pharmacological effects. Still, the employment of analytical techniques enabling subpopulations differentiation is highly desirable (Steć et al., 2022). In addition, the quantity of bacterial EVs that could represent their CNS-effective but safe dose requires elucidation.
Numerous works have proved intensive secretion of EVs by bacteria under unfavorable stress conditions, e.g., misfolded proteins accumulation (McBroom and Kuehn, 2007; Roier et al., 2016), DNA damage or perforation of peptidoglycan, and reflecting cell condition at the death (Toyofuku et al., 2019). It is intriguing how such stress-induced EVs produced by psychobiotics may exert any beneficial effect to the human. The phenomenon may be partially explained by the nature of parental cells (Güttsches et al., 2012; Ashrafian et al., 2019). However, the bacterial EVs may be produced also under physiological conditions during their logarithmic growth (Baeza et al., 2021; Laurin et al., 2022). Moreover, the reason for bacteria to produce EVs remains a major paradox (McMillan and Kuehn, 2021), and these issues require further studies.
Also, the EVs from psychobiotic bacteria tested in the CNS-relevant models have been obtained in precisely defined conditions (Choi et al., 2019; Yaghoubfar et al., 2020; Choi et al., 2022). Nevertheless, EVs composition and size can change drastically, depending on environment and growth conditions (Ñahui Palomino et al., 2021). The potential effect of variable host physiological status on probiotic bacteria EVs generation and action requires further attention. Moreover, psychobiotic bacteria EVs may change the properties of other bacterial vesicles (McMillan and Kuehn, 2021) or host-derived EVs (Imamiya et al., 2023), further entangling their mechanism of action.
Although convincing evidence supports the role of bacterial EVs in the psychobiotics’ mechanism of action, many questions need to be addressed, with some being raised above. Once solved, psychobiotic bacteria-derived EVs have potential to become a new generation postbiotics.
Author contributions
MK: conceptualization, supervision, and project administration. LB and MK: methodology and visualization. LB, SD, KW, and MK: literature search and writing—original draft. EK: funding acquisition. LB, SD, KW, EK, and MK: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by Medical University of Lodz (Łódź, Poland; grant number 503/5-108-03/503-51-001-19-00). The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish it.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ait-Belgnaoui, A., Colom, A., Braniste, V., Ramalho, L., Marrot, A., Cartier, C., et al. (2014). Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 26, 510–520. doi: 10.1111/nmo.12295
Aizawa, E., Tsuji, H., Asahara, T., Takahashi, T., Teraishi, T., Yoshida, S., et al. (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 202, 254–257. doi: 10.1016/j.jad.2016.05.038
Al-Nedawi, K., Mian, M. F., Hossain, N., Karimi, K., Mao, Y. K., Forsythe, P., et al. (2015). Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 29, 684–695. doi: 10.1096/fj.14-259721
Artigas, F. (2013). Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 137, 119–131. doi: 10.1016/j.pharmthera.2012.09.006
Ashrafian, F., Shahriary, A., Behrouzi, A., Moradi, H. R., Keshavarz Azizi Raftar, S., Lari, A., et al. (2019). Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front. Microbiol. 10:2155. doi: 10.3389/fmicb.2019.02155
Baeza, N., Delgado, L., Comas, J., and Mercade, E. (2021). Phage-mediated explosive cell lysis induces the formation of a different type of O-IMV in Shewanella vesiculosa M7T. Front. Microbiol. 12:713669. doi: 10.3389/fmicb.2021.713669
Bajic, S. S., Cañas, M. A., Tolinacki, M., Badia, J., Sánchez, B., Golic, N., et al. (2020). Proteomic profile of extracellular vesicles released by Lactiplantibacillus plantarum BGAN8 and their internalization by non-polarized HT29 cell line. Sci. Rep. 10:21829. doi: 10.1038/s41598-020-78920-z
Banks, W. A., Sharma, P., Bullock, K. M., Hansen, K. M., Ludwig, N., and Whiteside, T. L. (2020). Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 21:4407. doi: 10.3390/ijms21124407
Behzadi, E., Mahmoodzadeh Hosseini, H., and Imani Fooladi, A. A. (2017). The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb. Pathog. 110, 1–6. doi: 10.1016/j.micpath.2017.06.016
Bowman, M. A., and Daws, L. C. (2019). Targeting serotonin transporters in the treatment of juvenile and adolescent depression. Front. Neurosci. 13:156. doi: 10.3389/fnins.2019.00156
Branton, W. G., Ellestad, K. K., Maingat, F., Wheatley, B. M., Rud, E., Warren, R. L., et al. (2013). Brain microbial populations in HIV/AIDS: α-proteobacteria predominate independent of host immune status. PLoS One 8:e54673. doi: 10.1371/journal.pone.0054673
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain-gut Axis in psychiatric and inflammatory disorders. Front. Psychol. 9:44. doi: 10.3389/fpsyt.2018.00044
Champagne-Jorgensen, K., Mian, M. F., McVey Neufeld, K. A., Stanisz, A. M., and Bienenstock, J. (2021). Membrane vesicles of Lacticaseibacillus rhamnosus JB-1 contain immunomodulatory lipoteichoic acid and are endocytosed by intestinal epithelial cells. Sci. Rep. 11:13756. doi: 10.1038/s41598-021-93311-8
Chao, L., Liu, C., Sutthawongwadee, S., Li, Y., Lv, W., Chen, W., et al. (2020). Effects of probiotics on depressive or anxiety variables in healthy participants under stress conditions or with a depressive or anxiety diagnosis: a Meta-analysis of randomized controlled trials. Front. Neurol. 11:421. doi: 10.3389/fneur.2020.00421
Chen, Y., Xu, J., and Chen, Y. (2021). Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 13:2099. doi: 10.3390/nu13062099
Choi, J., Kim, Y. K., and Han, P. L. (2019). Extracellular vesicles derived from Lactobacillus plantarum increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp. Neurobiol. 28, 158–171. doi: 10.5607/en.2019.28.2.158
Choi, J., Kwon, H., Kim, Y. K., and Han, P. L. (2022). Extracellular vesicles from gram-positive and gram-negative probiotics remediate stress-induced depressive behavior in mice. Mol. Neurobiol. 59, 2715–2728. doi: 10.1007/s12035-021-02655-9
Choi, J. H., Moon, C. M., Shin, T. S., Kim, E. K., McDowell, A., Jo, M. K., et al. (2020). Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 52, 423–437. doi: 10.1038/s12276-019-0359-3
Cohen Kadosh, K., Basso, M., Knytl, P., Johnstone, N., Lau, J., and Gibson, G. (2021). Psychobiotic interventions for anxiety in young people: a systematic review and meta-analysis, with youth consultation. Transl. Psychiatry 11:11. doi: 10.1038/s41398-021-01422-7
Cuesta, C. M., Guerri, C., Ureña, J., and Pascual, M. (2021). Role of microbiota-derived extracellular vesicles in gut-brain communication. Int. J. Mol. Sci. 22:4235. doi: 10.3390/ijms22084235
de Rodovalho, V. R., da Luz, B. S. R., Rabah, H., do Carmo, F. L. R., Folador, E. L., Nicolas, A., et al. (2020). Extracellular vesicles produced by the probiotic Propionibacterium freudenreichii CIRM-BIA 129 mitigate inflammation by modulating the NF-κB pathway. Front. Microbiol. 11:1544. doi: 10.3389/fmicb.2020.01544
Del Toro-Barbosa, M., Hurtado-Romero, A., Garcia-Amezquita, L. E., and García-Cayuela, T. (2020). Psychobiotics: mechanisms of action, evaluation methods and effectiveness in applications with food products. Nutrients 12, 389. doi: 10.3390/nu12123896
Den, H., Dong, X., Chen, M., and Zou, Z. (2020). Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment — a meta-analysis of randomized controlled trials. Aging (Albany NY) 12, 4010–4039. doi: 10.18632/aging.102810
Dey, G., and Mookherjee, S. (2021). Probiotics-targeting new milestones from gut health to mental health. FEMS Microbiol. Lett. 368:fnab 096. doi: 10.1093/femsle/fnab096
Dinan, T. G., Stanton, C., and Cryan, J. F. (2013). Psychobiotics: a novel class of psychotropic. Biol. Psychiatry 74, 720–726. doi: 10.1016/j.biopsych.2013.05.001
Ding, Y., Bu, F., Chen, T., Shi, G., Yuan, X., Feng, Z., et al. (2021). A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 105, 8411–8426. doi: 10.1007/s00253-021-11622-2
El Dib, R., Periyasamy, A. G., de Barros, J. L., França, C. G., Senefonte, F. L., Vesentini, G., et al. (2021). Probiotics for the treatment of depression and anxiety: a systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN. 45, 75–90. doi: 10.1016/j.clnesp.2021.07.027
Emery, D. C., Shoemark, D. K., Batstone, T. E., Waterfall, C. M., Coghill, J. A., Cerajewska, T. L., et al. (2017). 16S rRNA next generation sequencing analysis shows Bacteria in Alzheimer’s post-mortem brain. Front. Aging Neurosci. 9:195. doi: 10.3389/fnagi.2017.00195
Florez, C., Raab, J. E., Cooke, A. C., and Schertzer, J. W. (2017). Membrane distribution of the Pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa. MBio 8, e01034–e01047. doi: 10.1128/mBio.01034-17
Frankiensztajn, L. M., Elliott, E., and Koren, O. (2020). The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 62, 76–82. doi: 10.1016/j.conb.2019.12.003
Goh, K. K., Liu, Y. W., Kuo, P. H., Chung, Y. C. E., Lu, M. L., and Chen, C. H. (2019). Effect of probiotics on depressive symptoms: a meta-analysis of human studies. Psychiatry Res. 282:112568. doi: 10.1016/j.psychres.2019.112568
Gradowski, M., Baranowski, B., and Pawłowski, K. (2020). The expanding world of protein kinase-like families in bacteria: forty families and counting. Biochem. Soc. Trans. 48, 1337–1352. doi: 10.1042/BST20190712
Gray, N. A., Milak, M. S., DeLorenzo, C., Ogden, R. T., Huang, Y. Y., Mann, J. J., et al. (2013). Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol. Psychiatry 74, 26–31. doi: 10.1016/j.biopsych.2012.11.012
Güttsches, A. K., Löseke, S., Zähringer, U., Sonnenborn, U., Enders, C., Gatermann, S., et al. (2012). Anti-inflammatory modulation of immune response by probiotic Escherichia coli Nissle 1917 in human blood mononuclear cells. Innate Immun. 18, 204–216. doi: 10.1177/1753425910396251
Haas-Neill, S., and Forsythe, P. (2020). A budding relationship: bacterial extracellular vesicles in the microbiota-gut-brain Axis. Int. J. Mol. Sci. 21:8899. doi: 10.3390/ijms21238899
Han, E. C., Choi, S. Y., Lee, Y., Park, J. W., Hong, S. H., and Lee, H. J. (2019). Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood-brain barrier in mice. FASEB J. 33, 13412–13422. doi: 10.1096/fj.201901575R
Heidarzadeh-Rad, N., Gökmen-Özel, H., Kazemi, A., Almasi, N., and Djafarian, K. (2020). Effects of a Psychobiotic supplement on serum brain-derived neurotrophic factor levels in depressive patients: a post hoc analysis of a randomized clinical trial. J. Neurogastroenterol. Motil. 26, 486–495. doi: 10.5056/jnm20079
Hemarajata, P., and Versalovic, J. (2013). Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 6, 39–51. doi: 10.1177/1756283X12459294
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document: the international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Huang, R., Ning, H., Yang, L., Jia, C., Yang, F., Xu, G., et al. (2017). Efficacy of probiotics on anxiety: a Meta-analysis of randomized controlled trials. Neuropsychiatry 7, 862–71. doi: 10.4172/Neuropsychiatry.1000291
Imamiya, R., Shinohara, A., Yakura, D., Yamaguchi, T., Ueda, K., Oguro, A., et al. (2023). Escherichia coli-derived outer membrane vesicles relay inflammatory responses to macrophage-derived exosomes. MBio 14:e0305122. doi: 10.1128/mbio.03051-22
Jakubec, M., Maple-Grødem, J., Akbari, S., Nesse, S., Halskau, Ø., and Mork-Jansson, A. E. (2020). Plasma-derived exosome-like vesicles are enriched in lyso-phospholipids and pass the blood-brain barrier. PLoS One 15:e0232442. doi: 10.1371/journal.pone.0232442
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Johnson, D., Thurairajasingam, S., Letchumanan, V., Chan, K. G., and Lee, L. H. (2021). Exploring the role and potential of probiotics in the field of mental health: major depressive disorder. Nutrients 13:1728. doi: 10.3390/nu13051728
Karbownik, M. S., Sokołowska, P., and Kowalczyk, E. (2023). Gut microbiota metabolites differentially release Gliotransmitters from the cultured human astrocytes: a preliminary report. Int. J. Mol. Sci. 24:6617. doi: 10.3390/ijms24076617
Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H., and Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 38, 522–528. doi: 10.1016/j.clnu.2018.04.010
Kelly, J. R., Borre, Y., O’Brien, C., Patterson, E., El Aidy, S., Deane, J., et al. (2016). Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019
Kim, J. H., Jeun, E. J., Hong, C. P., Kim, S. H., Jang, M. S., Lee, E. J., et al. (2016). Extracellular vesicle–derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J. Allergy Clin. Immunol. 137, 507–516.e8. doi: 10.1016/j.jaci.2015.08.016
Kim, W., Lee, E. J., Bae, I. H., Myoung, K., Kim, S. T., Park, P. J., et al. (2020). Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 9:1793514 doi: 10.1080/20013078.2020.1793514
Kobayashi, H., Uematsu, K., Hirayama, H., and Horikoshi, K. (2000). Novel toluene elimination system in a toluene-tolerant microorganism. J. Bacteriol. 182, 6451–6455. doi: 10.1128/JB.182.22.6451-6455.2000
Laurin, D., Mercier, C., Quansah, N., Robert, J. S., Usson, Y., Schneider, D., et al. (2022). Extracellular vesicles from 50,000 generation clones of the Escherichia coli long-term evolution experiment. Int. J. Mol. Sci. 23:14580. doi: 10.3390/ijms232314580
Lee, J., Kim, O. Y., and Gho, Y. S. (2016). Proteomic profiling of gram-negative bacterial outer membrane vesicles: current perspectives. Proteom. Clin. Appl. 10, 897–909. doi: 10.1002/prca.201600032
Lee, K. E., Kim, J. K., Han, S. K., Lee, D. Y., Lee, H. J., Yim, S. V., et al. (2020). The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome 8:107. doi: 10.1186/s40168-020-00881-2
Li, M., Lee, K., Hsu, M., Nau, G., Mylonakis, E., and Ramratnam, B. (2017). Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 17:66. doi: 10.1186/s12866-017-0977-7
Liu, Y., Defourny, K. A. Y., Smid, E. J., and Abee, T. (2018). Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 9:1502. doi: 10.3389/fmicb.2018.01502
Liu, R. T., Walsh, R. F. L., and Sheehan, A. E. (2019). Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 102, 13–23. doi: 10.1016/j.neubiorev.2019.03.023
Liu, N., Yang, D., Sun, J., and Li, Y. (2023). Probiotic supplements are effective in people with cognitive impairment: a meta-analysis of randomized controlled trials. Nutr. Rev. nuac113. doi: 10.1093/nutrit/nuac113
Magalhães-Guedes, K. T. (2022). Psychobiotic therapy: method to reinforce the immune system. Clin. Psychopharmacol. Neurosci. 20, 17–25. doi: 10.9758/cpn.2022.20.1.17
Mashburn-Warren, L., Howe, J., Garidel, P., Richter, W., Steiniger, F., Roessle, M., et al. (2008). Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69, 491–502. doi: 10.1111/j.1365-2958.2008.06302.x
Matsumoto, J., Stewart, T., Banks, W. A., and Zhang, J. (2017). The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr. Pharm. Des. 23, 6206–6214. doi: 10.2174/1381612823666170913164738
McBroom, A. J., and Kuehn, M. J. (2007). Release of outer membrane vesicles by gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63, 545–558. doi: 10.1111/j.1365-2958.2006.05522.x
McMillan, H. M., and Kuehn, M. J. (2021). The extracellular vesicle generation paradox: a bacterial point of view. EMBO J. 40:e108174. doi: 10.15252/embj.2021108174
Messaoudi, M., Violle, N., Bisson, J. F., Desor, D., Javelot, H., and Rougeot, C. (2011). Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R 0175) in healthy human volunteers. Gut Microbes 2, 256–261. doi: 10.4161/gmic.2.4.16108
Mohammadi, G., Dargahi, L., Peymani, A., Mirzanejad, Y., Alizadeh, S. A., Naserpour, T., et al. (2019). The effects of probiotic formulation pretreatment (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) on a lipopolysaccharide rat model. J. Am. Coll. Nutr. 38, 209–217. doi: 10.1080/07315724.2018.1487346
Morad, G., Carman, C. V., Hagedorn, E. J., Perlin, J. R., Zon, L. I., Mustafaoglu, N., et al. (2019). Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano 13, 13853–13865. doi: 10.1021/acsnano.9b04397
Morales-Prieto, D. M., Murrieta-Coxca, J. M., Stojiljkovic, M., Diezel, C., Streicher, P. E., Henao-Restrepo, J. A., et al. (2022). Small extracellular vesicles from peripheral blood of aged mice pass the blood-brain barrier and induce glial cell activation. Cells 11:625. doi: 10.3390/cells11040625
Morelli, L, and Patrone, V. (2015). “Chapter 3—probiotic microorganisms for shaping the human gut microbiota–mechanisms and efficacy into the future,” in Diet-Microbe Interactions in the Gut. (eds.) K. Tuohy and D. Del Rio (San Diego: Academic Press), 27–40.
Ñahui Palomino, R. A., Vanpouille, C., Costantini, P. E., and Margolis, L. (2021). Microbiota–host communications: bacterial extracellular vesicles as a common language. PLoS Pathog. 17:e1009508. doi: 10.1371/journal.ppat.1009508
Ng, Q. X., Peters, C., Ho, C. Y. X., Lim, D. Y., and Yeo, W. S. (2018). A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 228, 13–19. doi: 10.1016/j.jad.2017.11.063
O’Riordan, K. J., Collins, M. K., Moloney, G. M., Knox, E. G., Aburto, M. R., Fülling, C., et al. (2022). Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 546:111572. doi: 10.1016/j.mce.2022.111572
Oliphant, K., and Claud, E. C. (2022). Early probiotics shape microbiota. Nat. Microbiol. 7, 1506–1507. doi: 10.1038/s41564-022-01230-9
Park, T. E., Mustafaoglu, N., Herland, A., Hasselkus, R., Mannix, R., FitzGerald, E. A., et al. (2019). Hypoxia-enhanced blood-brain barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 10:2621. doi: 10.1038/s41467-019-10588-0
Partrick, K. A., Rosenhauer, A. M., Auger, J., Arnold, A. R., Ronczkowski, N. M., Jackson, L. M., et al. (2021). Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci. Rep. 11:3763. doi: 10.1038/s41598-021-83284-z
Pérez-Cruz, C., Carrión, O., Delgado, L., Martinez, G., López-Iglesias, C., and Mercade, E. (2013). New type of outer membrane vesicle produced by the gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Appl. Environ. Microbiol. 79, 1874–1881. doi: 10.1128/AEM.03657-12
Peter, J., Fournier, C., Durdevic, M., Knoblich, L., Keip, B., Dejaco, C., et al. (2018). A microbial signature of psychological distress in irritable bowel syndrome. Psychosom. Med. 80, 698–709. doi: 10.1097/PSY.0000000000000630
Pirolli, N. H., Bentley, W. E., and Jay, S. M. (2021). Bacterial extracellular vesicles and the gut-microbiota brain Axis: emerging roles in communication and potential as therapeutics. Adv. Biol. 5:e2000540. doi: 10.1002/adbi.202000540
Reis, D. J., Ilardi, S. S., and Punt, S. E. W. (2018). The anxiolytic effect of probiotics: a systematic review and meta-analysis of the clinical and preclinical literature. PLoS One 13:e199041. doi: 10.1371/journal.pone.0199041
Roier, S., Zingl, F. G., Cakar, F., Durakovic, S., Kohl, P., Eichmann, T. O., et al. (2016). A novel mechanism for the biogenesis of outer membrane vesicles in gram-negative bacteria. Nat. Commun. 7:10515. doi: 10.1038/ncomms10515
Rutsch, A., Kantsjö, J. B., and Ronchi, F. (2020). The gut-brain Axis: how microbiota and host Inflammasome influence brain physiology and pathology. Front. Immunol. 11:604179. doi: 10.3389/fimmu.2020.604179
Sarkar, A., Lehto, S. M., Harty, S., Dinan, T. G., Cryan, J. F., and Burnet, P. W. J. (2016). Psychobiotics and the manipulation of Bacteria–gut–brain signals. Trends Neurosci. 39, 763–781. doi: 10.1016/j.tins.2016.09.002
Schrijver, I. A., van Meurs, M., Melief, M. J., Wim Ang, C., Buljevac, D., Ravid, R., et al. (2001). Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain 124, 1544–1554. doi: 10.1093/brain/124.8.1544
Schroeder, B. O., and Bäckhed, F. (2016). Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089. doi: 10.1038/nm.4185
Schwechheimer, C., and Kuehn, M. J. (2015). Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619. doi: 10.1038/nrmicro3525
Seo, M. K., Park, E. J., Ko, S. Y., Choi, E. W., and Kim, S. (2018). Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2, 4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 101, 8662–8671. doi: 10.3168/jds.2018-15014
Steć, A., Jońca, J., Waleron, K., Waleron, M., Płoska, A., Kalinowski, L., et al. (2022). Quality control of bacterial extracellular vesicles with Total protein content assay, nanoparticles tracking analysis, and capillary electrophoresis. Int. J. Mol. Sci. 23:4347. doi: 10.3390/ijms23084347
Stentz, R., Carvalho, A. L., Jones, E. J., and Carding, S. R. (2018). Fantastic voyage: the journey of intestinal microbiota-derived microvesicles through the body. Biochem. Soc. Trans. 46, 1021–1027. doi: 10.1042/BST20180114
Suda, K., and Matsuda, K. (2022). How microbes affect depression: underlying mechanisms via the gut–brain Axis and the modulating role of probiotics. Int. J. Mol. Sci. 23:1172. doi: 10.3390/ijms23031172
Suzuki, A., Stern, S. A., Bozdagi, O., Huntley, G. W., Walker, R. H., Magistretti, P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cells 144, 810–823. doi: 10.1016/j.cell.2011.02.018
Takahashi, S. (2021). Neuroprotective function of high glycolytic activity in astrocytes: common roles in stroke and neurodegenerative diseases. Int. J. Mol. Sci. 22:6568. doi: 10.3390/ijms22126568
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7:1535750. doi: 10.1080/20013078.2018.1535750
Tillmann, S., Awwad, H. M., Eskelund, A. R., Treccani, G., Geisel, J., Wegener, G., et al. (2018). Probiotics affect one-carbon metabolites and Catecholamines in a genetic rat model of depression. Mol. Nutr. Food Res. 62:e1701070. doi: 10.1002/mnfr.201701070
Toyofuku, M., Nomura, N., and Eberl, L. (2019). Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24. doi: 10.1038/s41579-018-0112-2
Tremblay, A., Lingrand, L., Maillard, M., Feuz, B., and Tompkins, T. A. (2021). The effects of psychobiotics on the microbiota-gut-brain axis in early-life stress and neuropsychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 105:110142. doi: 10.1016/j.pnpbp.2020.110142
Turnbull, L., Toyofuku, M., Hynen, A. L., Kurosawa, M., Pessi, G., Petty, N. K., et al. (2016). Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 7:11220. doi: 10.1038/ncomms11220
Vandendriessche, C., Balusu, S., Van Cauwenberghe, C., Brkic, M., Pauwels, M., Plehiers, N., et al. (2021). Importance of extracellular vesicle secretion at the blood-cerebrospinal fluid interface in the pathogenesis of Alzheimer’s disease. Acta Neuropathol. Commun. 9:143. doi: 10.1186/s40478-021-01245-z
Wijeratne, T., and Sales, C. (2021). Understanding why post-stroke depression may be the norm rather than the exception: the anatomical and Neuroinflammatory correlates of post-stroke depression. J. Clin. Med. 10:1674. doi: 10.3390/jcm10081674
Wurdinger, T., Gatson, N. N., Balaj, L., Kaur, B., Breakefield, X. O., and Pegtel, D. M. (2012). Extracellular vesicles and their convergence with viral pathways. Adv. Virol. 2012:767694. doi: 10.1155/2012/767694
Yaghoubfar, R., Behrouzi, A., Ashrafian, F., Shahryari, A., Moradi, H. R., Choopani, S., et al. (2020). Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 10:22119. doi: 10.1038/s41598-020-79171-8
Yang, Z., Gao, Z., Yang, Z., Zhang, Y., Chen, H., Yang, X., et al. (2022). Lactobacillus plantarum-derived extracellular vesicles protect against ischemic brain injury via the micro RNA-101a-3p/c-Fos/TGF-β axis. Pharmacol. Res. 182:106332. doi: 10.1016/j.phrs.2022.106332
Yang, H., Liu, Y., Cai, R., Li, Y., and Gu, B. (2021). A narrative review of relationship between gut microbiota and neuropsychiatric disorders: mechanisms and clinical application of probiotics and prebiotics. Ann. Palliat. Med. 10, 2304–2302313. doi: 10.21037/apm-20-1365
Yao, S., Xu, M. D., Wang, Y., Zhao, S. T., Wang, J., Chen, G. F., et al. (2023). Astrocytic lactate dehydrogenase a regulates neuronal excitability and depressive-like behaviors through lactate homeostasis in mice. Nat. Commun. 14:729. doi: 10.1038/s41467-023-36209-5
Zhou, F., Ebea, P., Mutai, E., Wang, H., Sukreet, S., Navazesh, S., et al. (2022). Small extracellular vesicles in Milk cross the blood-brain barrier in murine cerebral cortex endothelial cells and promote dendritic complexity in the Hippocampus and brain function in C57BL/6J mice. Front. Nutr. 9:838543. doi: 10.3389/fnut.2022.838543
Keywords: probiotics, psychobiotics microorganisms, postbiotic, neuropsychiatric disorder, mental health, extracellular vesicles, gut brain axis, mechanism of action
Citation: Bleibel L, Dziomba S, Waleron KF, Kowalczyk E and Karbownik MS (2023) Deciphering psychobiotics’ mechanism of action: bacterial extracellular vesicles in the spotlight. Front. Microbiol. 14:1211447. doi: 10.3389/fmicb.2023.1211447
Edited by:
Zhaojie Li, Qingdao Agricultural University, ChinaReviewed by:
Huaxi Yi, Ocean University of China, ChinaCopyright © 2023 Bleibel, Dziomba, Waleron, Kowalczyk and Karbownik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michał Seweryn Karbownik, bWljaGFsLmthcmJvd25pa0B1bWVkLmxvZHoucGw=
 Layla Bleibel
Layla Bleibel Szymon Dziomba2
Szymon Dziomba2 Krzysztof Franciszek Waleron
Krzysztof Franciszek Waleron Michał Seweryn Karbownik
Michał Seweryn Karbownik