Abstract
Biological seed dressing is a cost-effective means to protect plant roots from pathogens. Trichoderma is generally considered as one of the most common biological seed dressings. However, there is still a dearth of information on the effects of Trichoderma on microbial community of rhizosphere soil. High-throughput sequencing was used to analyze the effects of Trichoderma viride and a chemical fungicide on microbial community of soybean rhizosphere soil. The results showed that both T. viride and chemical fungicide could significantly reduce the disease index of soybean (15.11% for Trichoderma and 17.33% for Chemical), while no significant difference was observed between them. Both T. viride and chemical fungicide could affect the structure of rhizosphere microbial community, they increased the β-diversity of microbial community and significantly reduce the relative abundance of Saprotroph-Symbiotroph. Chemical fungicide could reduce the complexity and stability of co-occurrence network. However, T. viride is beneficial for maintaining network stability and increasing network complexity. There were 31 bacterial genera and 21 fungal genera significantly correlated with the disease index. Furthermore, several potential plant pathogenic microorganisms were also positively correlated with disease index, such as Fusarium, Aspergillus, Conocybe, Naganishia, and Monocillium. From this work, T. viride may be used as a substitute for chemical fungicide to control soybean root rot and be more friendly to soil microecology.
1. Introduction
Soybean is an important legume crop, but is susceptible to many diseases, such as root rot, charcoal rot, sclerotinia stem rot and soybean cyst. The majority of losses are attributed to microbial diseases. Root rot has become one of the most destructive soil-borne diseases of soybean, which causes yield reduction and serious economic losses. The soybean yield loss caused by root rot is 10–60%, or even no yield in serious cases (Hartman et al., 2015). Root rot has been listed as one of the destructive diseases impairing soybean yield because of its wide distribution, strong destruction and difficult control (Chang et al., 2018).
Seed dressing can control pathogens early and raise healthy plant stand, which is considered as a simple, economical and effective method to control root-rot disease. Many chemical fungicides are now formulated as seed dressings, the chemicals are delivered into the soil along with the seed, which can reduce the number of pathogens or inhibit their reproduction, and protect germinating seedlings (Labanya et al., 2018). Although the use of chemical fungicides in plant disease management is acknowledged to be effective, many studies also have shown that chemical control of soil-borne pathogens is challenging because chemistries can adversely affect beneficial soil microbes to disrupt the ecological balance of rhizosphere microbial communities (Roth et al., 2020), and also pollutes the environment to threaten human health (Ali et al., 2021). Biological control of plant pathogens is a more promising method to control soil-borne diseases and is recognized by many people. Trichoderma is genetically very diverse, and it is also tolerant of a range of recalcitrant environmental pollutants (Tripathi et al., 2013). Trichoderma is well documented as potential growth promoting and effective biological control agents for many crop plants (Verma et al., 2007; Savazzini et al., 2009; Ferreira and Musumeci, 2021). Trichoderma populations can be established relatively easily in several types of soil and can continue to existence for months, because they grow rapidly and also because of their abundant conidiation (John et al., 2010; Tripathi et al., 2013). Biological control agents control soil-borne diseases mainly through competition with pathogens, parasitism and producing antifungal compounds (Verma et al., 2007; Savazzini et al., 2009). Owing to its antagonistic effect on inimical organism, Trichoderma viride ranks as one of the most potential biocontrol agents against several soil and seed borne pathogens (Bayoumi et al., 2019; Khan et al., 2021).
Rhizosphere soil microbiomes can form complex interactions with plants and play an important role in the health of plants in natural environments, including nutrient uptake, growth promotion, stress tolerance and resistance to pathogens (Trivedi et al., 2020). A balanced and stable relationship between plants and rhizosphere microbial communities may be the key to maintaining plant health and inhibiting plant diseases. Understanding the community composition of rhizosphere microbial community and its response to chemical or biological control treatment is critical for root rot control. This work aims to explore the control effect of T. viride on soybean disease and the potential impact of T. viride on rhizosphere microbial communities, and provide theoretical basis for the application of T. viride in the sustainable management of plant diseases.
2. Materials and methods
2.1. Seed dressing treatment
The chemical fungicide Cruiser® seed dressing consisting of 22.6% thiamethoxam, 2.2% fludioxonil and 2.2% difenoconazole (Syngenta Group China, Beijing, China) applied at the ratio of 3.3 mL/kg seeds, according to manufacturer’s instructions. T. viride spores were applied at the rate of 15 g/kg seeds and appropriate amount of sterile water was added to the group. The T. viride spores (1 × 108 CFU/g) were provided by the Ecology Institute of Shandong Academy of Sciences. The blank group was treated with sterile water at the ratio of 3.3 mL/kg seeds. Before sowing, the seeds and seed dressing were uniformly mixed by stirring repeatedly.
2.2. Experimental design and soil sample collection
The experiment was carried out at the research station in Shentang village (Jining City, Shandong Province). The region belongs to a warm temperate monsoon climate with sufficient rainfall and sunshine in summer, which is suitable for the growth of soybean crops. In June 2020, three plots were selected for experiment at the research station. And the area of each plot was 5 hectares. The experiment was designed with three seed-dressing treatments. The Control group was a control, which used the same amount of sterile water instead of seed coating agent. Chemical group was the chemical fungicide Cruiser® seed dressing. Trichoderma group was a T. viride seed dressing.
In this study, all treatments were carried out under the same weather and field management conditions. After 30 days of sowing, 5 quadrats (5 × 5 m) were randomly selected in each plot, and 5 sampling points were selected by the diagonal method for each plot. Soybean plants and root-attached soil were collected at each sampling point for collection of rhizosphere soil samples and observation of soybean root rot disease. And 5 samples in each quadrat were taken as one parallel sample. The rhizosphere soil samples were collected according to the description of Bulgarelli et al. (2012). Briefly, shaken off the loosely attached soil, and then collected ca. 1-mm of soil covering the roots with sterile brush. After removing plant debris, the collected soil samples were stored at −80°C.
2.3. Evaluation of disease index and growth characteristic of soybean
The disease index, plant weight and plant height of soybean were evaluated by 50 plants randomly collected from each treatment. At harvest stage, soybeans were harvested from 3 plots (50 m2) from each experimental field to determine the yield. And the seed weight and pod number per plant of soybean were determined with 50 plants randomly collected from each treatment. The disease severity scale of soybean was evaluated according to the section “Fungicides control soybean root rot” in GB/T 17980.88–2004 pesticide field efficacy test guidelines (Grade 0 = no diseased spots on the stem and taproot of the plant; Grade 1 = a few disease spots on the stem and taproot; Grade 3 = many diseased spots on the stem or taproot, with the diseased spot area accounting for 1/4 to 1/2 of the total area of the stem and root; Grade 5 = multiple and large diseased spots on the stem and taproot, with the diseased spot area accounting for 1/2 to 3/4 of the total area of the stem and root; Grade 7 = disease spots on the stem or taproot are connected into patches, forming a phenomenon of wrapping around the stem, but the root is not dead; Grade 9 = root necrosis, plant aboveground parts withering or death), and then the disease index was calculated based on the above evaluation results. The disease index was calculated as follows:
2.4. DNA extraction and sequencing
Total genomic DNA of rhizosphere soil was extracted using the FastDNA spin for soil kit (MP Biomedicals, USA) following the manufacturer’s instructions. All the DNA samples were stored at −20°C before sequencing. High-throughput sequencing was performed using an Illumina platform at the Novogene Biinformatics Technology Co., Ltd. For bacteria, the 16S rDNA was amplified using universal primers 515F/806R (5′-GTGCCAGCMGCCGCGGTAA-3′/5′-GGACTACHVGGGTWTCTAAT-3′). And for fungi, the second nuclear ribosomal internal transcribed spacer (ITS1) region was amplified using primers ITS1F/ITS2 (5′-CTTGGTCATTTAGAGGAAGTAA-3′/5′-GCTGCGTTCTTCATCGATGC-3′). Raw sequences have been stored in the NCBI Sequence Read Archive under the BioProject accession number PRJNA951875. Sequences were analyzed with the QIIME (Version 1.7.0) data analysis package (Caporaso et al., 2010). Generated operational taxonomy units (OTUs) were defined by clustering at a similarity threshold of 97%.
2.5. Statistical analysis
All microbial community 16S rDNA and ITS amplicon sequencing data analyses were carried out in R 4.1.3 based on normalized OTUs table. Bacterial and fungal alpha diversity indices (including the OTU richness, Shannon and phylogenetic diversity indices) were calculated using the “vegan” and “picante” packages in the R statistical computing environment. The statistical significance was analyzed using analysis of variance (ANOVA) followed by Tukey’s honest significant difference (HSD) post hoc tests, and p < 0.05 was regarded as significant. Nonmetric multidimensional scaling (NMDS) analysis was conducted using the metaMDS functions from the R package “vegan” with the Bray-Curtis metric. The Bray–Curtis distance was calculated using the vegdist function in the “vegan” package. Three nonparametric statistical tests were applied to determine the differences in microbial community structure of three treatments, including permutational multivariate analysis of variance (Adonis), analysis of similarity (ANOSIM), and multiple response permutation procedure (MRPP). And values were considered statistically different when p < 0.05. FUNGuild1 database was applied to predict fungal functions (Nguyen et al., 2016). According to the annotation results obtained by FUNGuild software, the confidence scores of ‘Highly Probable’ and ‘Probable’ were accepted in this study (Chen et al., 2020). The relative abundances of each trophic mode group for the three treatments were compared using a heat map. Similarity and clustering relationships of distinctive OTUs for each treatment were presented in a Venn diagram. Spearman’s correlation for abundant genera and disease indices were calculated using the corr. Test function in the R package “psych.” To quantify the assembly processes of microbial community, the nearest taxon index (NTI) was calculated by the null-model theory using the R package “picante” (Kembel et al., 2010), followed by the beta nearest taxon index (βNTI) for pairwise phylogenetic turnover between communities (Guan et al., 2021). βNTI values >2 or <−2 indicate the dominance of stochastic processes in shaping the community composition, whereas βNTI values between −2 and 2 indicate the dominance of deterministic processes (Dini-Andreote et al., 2015). To further identify the OTUs preferentially associated with seed-dressing treatments, the “DESeq2” package in R based on the negative binomial distribution was used to compute log 2-fold changes (log2FC) of OTUs. The OTUs were selected only when the adjusted p value was <0.05 and the absolute log2FC was >1 from the DESeq2 analysis. For all comparisons and statistical tests, the Benjamini–Hochberg approach was applied to adjust the p-values, and alpha = 0.05 was set as the significance threshold (Kunz et al., 2019).
2.6. Microbial network analysis
Co-occurrence network analysis was performed following Molecular Ecological Network Analyses Pipeline to elucidate the effects of different treatments on the overall microbial ecological network (Deng et al., 2012). The top 304 genera (>0.01% per samples) were retained and the genera with low frequencies (present in <3 samples) were removed for analysis to improve the reliability of network construction. After threshold scanning through RMT-based approach, the networks were constructed with an identical similarity threshold (St = 0.97) (Deng et al., 2012). Gephi was used to visualize the co-occurrence networks. The connectivity of each node was determined based on within-module connectivity (Zi) and among-module connectivity (Pi) (Guimera and Nunes Amaral, 2005). The potential keystone genera were defined based on the values of Zi and Pi, with thresholds of 2.5 and 0.62, respectively (Wu et al., 2022). The natural connectivity was used to evaluate network robustness to assess the difference in network stability for the three treatments (Pan et al., 2021). The network stability is derived by removing nodes in the static network to assess how quickly robustness degraded (Peng and Wu, 2016; Fan et al., 2018).
3. Results
3.1. Disease index and growth characteristic of soybean
The three treatments (Control, Chemical and Trichoderma) had different effects on the disease index and growth characteristic of soybean (Figure 1). The disease index of Control was 28.89–44.44%, while that of Chemical and Trichoderma were 13.33–17.78% and 15.56–20.00%, respectively. The disease index of both Chemical and Trichoderma were significantly lower than that of the Control (p < 0.05). The disease index of Trichoderma was slightly higher than that of the Chemical, but no significant difference was observed between the two treatments. No significant differences in the height and weight of soybean plants were observed among the three treatments. At the harvest stage, the pod number and seed weight per plant increased significantly in Chemical and Trichoderma, and the pod number of Trichoderma was significantly higher than that of Chemical. The soybean yield increased by 26.77 and 23.79% in Chemical and Trichoderma, respectively.
Figure 1
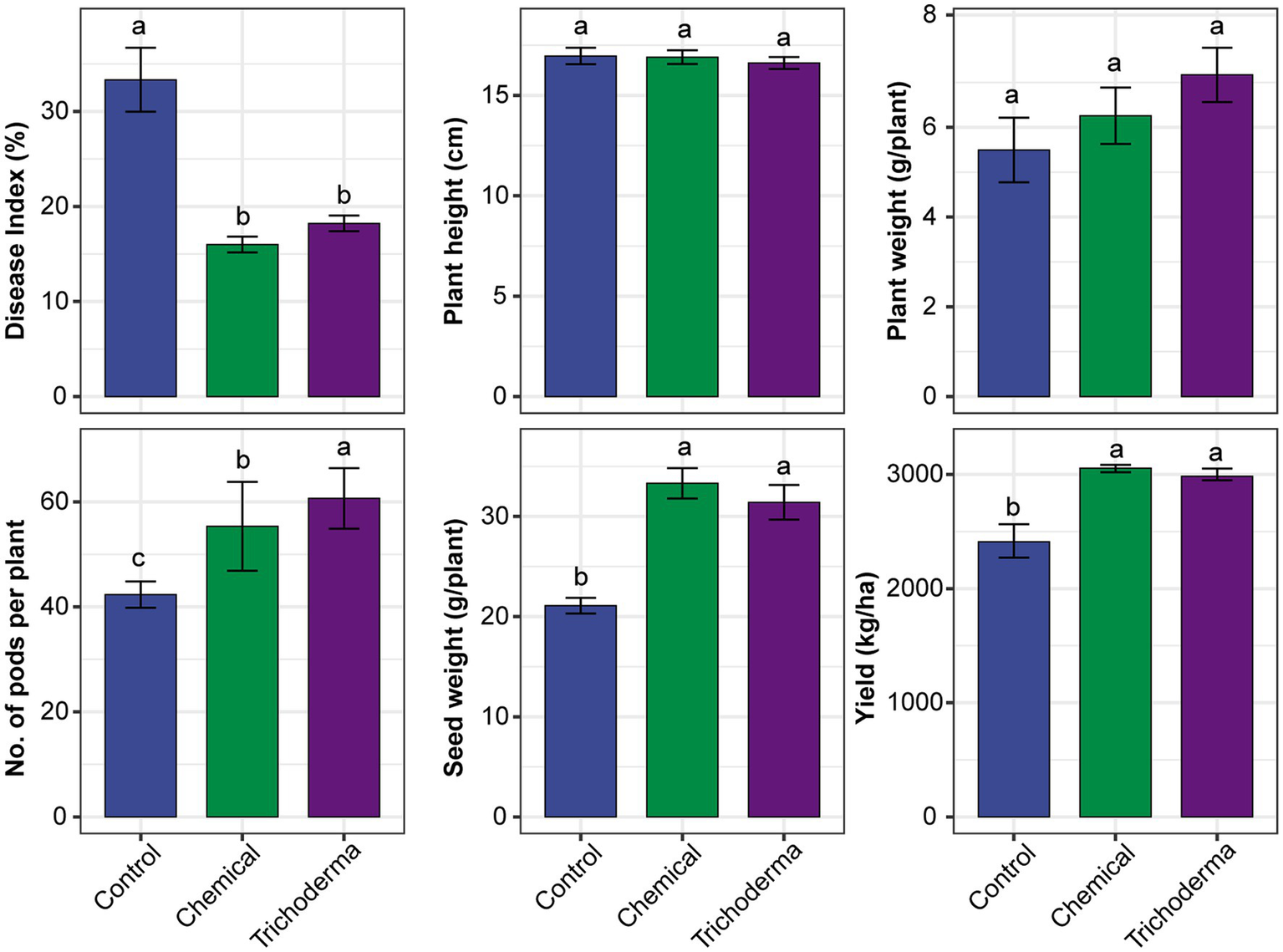
Effects of different treatments on the disease index and growth characteristic of soybean. Different letters indicate significant difference between treatments (p < 0.05).
3.2. Alpha diversity analysis of microbial communities
Statistical significance analysis of alpha diversity indicated that the bacterial diversity among the three treatments was not significantly different (Table 1). Analysis of fungal alpha diversity showed that all alpha diversity indices in Trichoderma had no significant difference with the control, while the phylogenetic diversity and OTUs richness indices in Chemical were significantly higher than those in Control and Trichoderma (p < 0.05). In summary, the effect of T. viride on the alpha diversity of rhizosphere microbial community was limited, while chemical fungicide changed the richness and phylogenetic diversity of rhizosphere fungal community.
Table 1
| Comparisons | Treatment | OTUs richness | Shannon | Phylogenetic diversity |
|---|---|---|---|---|
| Bacteria | Control | 1333.60 ± 32.58a | 6.41 ± 0.02a | 73.80 ± 1.24a |
| Chemical | 1348.80 ± 57.62a | 6.36 ± 0.08a | 72.93 ± 2.45a | |
| Trichoderma | 1263.20 ± 60.24a | 6.27 ± 0.08a | 70.25 ± 2.47a | |
| Fungi | Control | 192.60 ± 8.82b | 3.82 ± 0.08a | 36.26 ± 0.88b |
| Chemical | 224.00 ± 7.33a | 4.01 ± 0.10a | 42.06 ± 1.19a | |
| Trichoderma | 176.00 ± 5.55b | 3.91 ± 0.06a | 34.52 ± 0.90b |
Comparison of alpha diversity indices of rhizosphere bacterial and fungal communities under different treatments (Control, Chemical, and Trichoderma).
Results show Mean ± SE. Different letters indicate significant difference between treatments (p < 0.05).
3.3. Beta diversity analysis of microbial communities
The bacterial and fungal community structure of the different treatments was visualized using the NMDS analysis. The soil samples of three treatments (Control, Chemical and Trichoderma) did not form significantly different clusters in the ordination space (Figures 2A,B). The analysis of the Bray–Curtis dissimilarity showed that there was no significant difference in the β-diversity of the microbial communities between the Chemical and Trichoderma treatments (Figures 2C,D). And the β-diversity of bacterial and fungal communities was significantly higher in the Chemical and Trichoderma treatments than that in the Control (p < 0.05). The non-parametric multivariate statistical tests, Adonis, ANOSIM and MRPP at the OTUs level indicated significant differences in microbial community among the three treatments (p < 0.05 in all cases) (Supplementary Table S1), and the differences among fungal communities were more significant (p < 0.01).
Figure 2
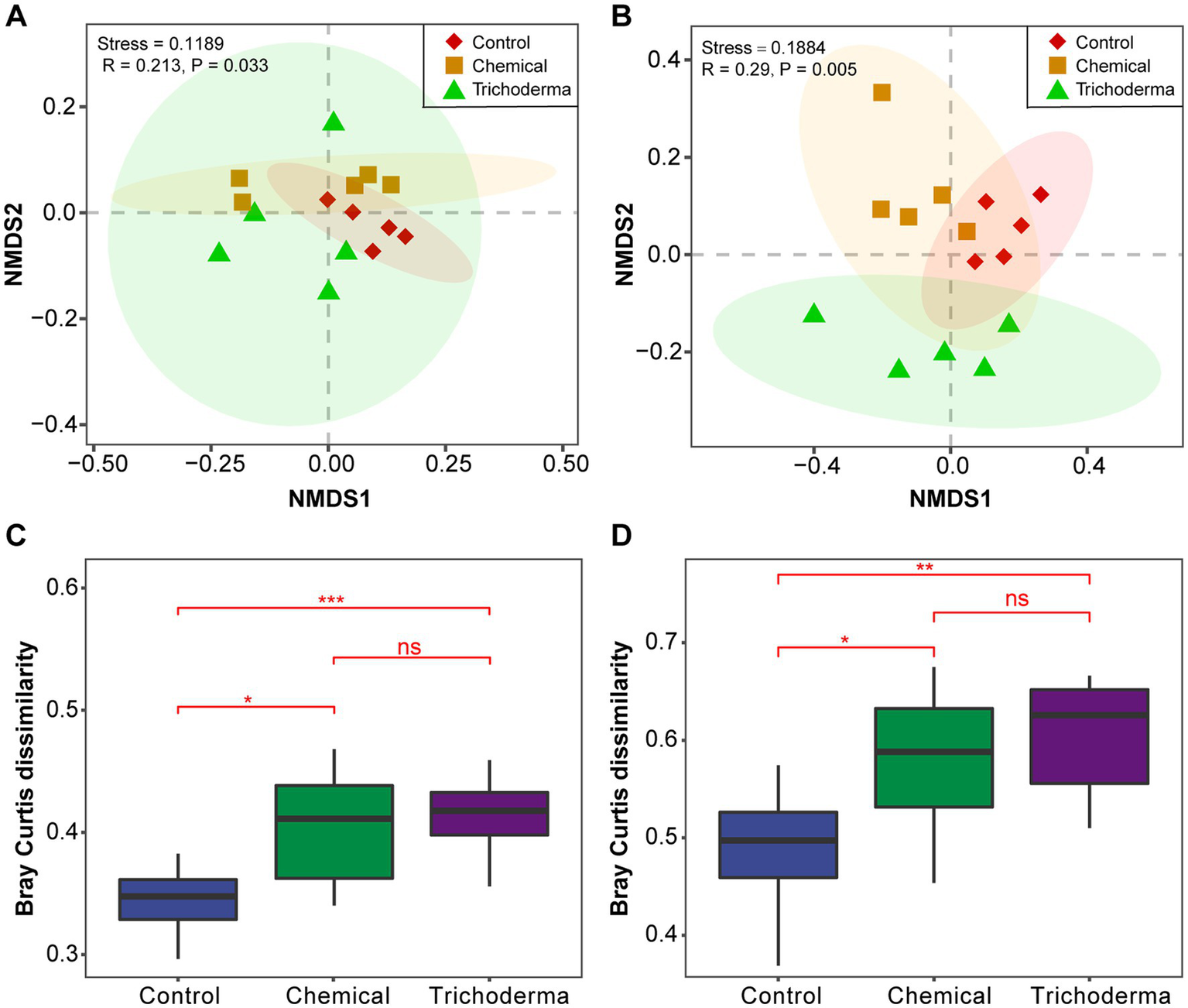
Beta diversity analysis of microbial communities. NMDS ordination of the Bray–Curtis dissimilarity of bacterial (A) and fungal (B) communities, Confidence ellipses level at 0.95. Effect of different treatments on bacterial (C) and fungal (D) communities dissimilarity, “ns” no significance, *p < 0.05, **p < 0.01, ***p < 0.001.
Stochastic and deterministic were two types of ecological processes that influence the microbial community assembly. To compare the relative importance of the two processes of the rhizosphere microbial community of each group, we calculated the βNTI values of bacterial and fungal communities, respectively (Supplementary Figure S1). Both processes drove the assembly of microbial community composition in soybean rhizosphere. Deterministic was the main force that influenced the bacterial community assembly (80% for Control, 70% for Chemical and 70% for Trichoderma) (Supplementary Figure S1A). However, the majority of the βNTI values for the rhizosphere fungal community compositions fell between −2 and 2 in the three treatments (Supplementary Figure S1B), suggesting that stochastic processes dominated the assembly of the rhizosphere fungal community compositions. In general, neither Chemical nor Trichoderma alter the assembly process of the microbial community.
3.4. Microbial community composition
A total of 2080 bacterial OTUs and 477 fungal OTUs were identified from all samples. For bacteria, a total of 1950, 1977, and 1970 OTUs were found from the samples derived from Control, Chemical and Trichoderma, respectively. For fungi, 355, 407, and 350 OTUs were found from the three treatments, respectively (Figure 3). The majority of OTUs were shared by three treatments. And more unique OTUs were identified in Chemical treatment, indicating that the chemical fungicide has a stronger stimulating effect on bacterial and fungal communities.
Figure 3
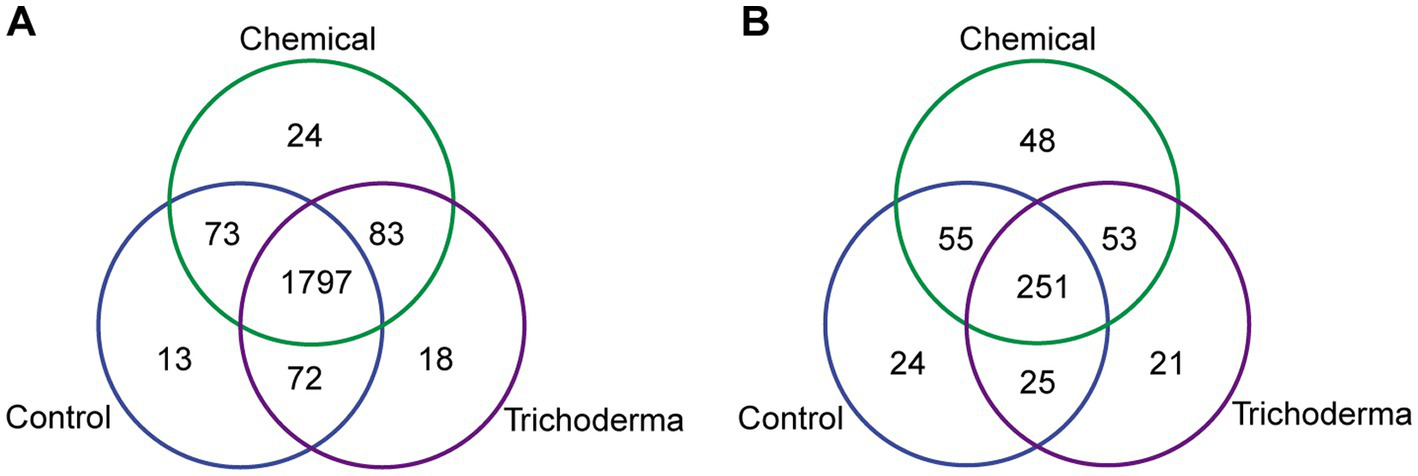
Venn diagram for the number of shared and unique bacterial (A) and fungal (B) OTUs under different treatments.
The analysis of the relative abundance of dominant bacteria and fungi (relative abundance >0.1%) at the phylum level indicated that both T. viride and chemical fungicide had influence on the microbial community composition (Figure 4). The dominant OTUs of bacterial in the three treatments belonged to 19 phyla, while those of fungi belonged to 7 phyla. Proteobacteria and Ascomycota were the most dominant phylum of bacteria and fungi, with relative abundances of 33.93–38.38% and 49.44–65.85%, respectively. For the bacterial community, statistical analysis showed that Planctomycetes, Patescibacteria, and Myxococcota were significant different among the three treatments (ANOVA, p < 0.05). For the fungal community, Ascomycota and Mortierellomycota were significant different among the different treatments (ANOVA, p < 0.05). The relative abundance of Ascomycota and Myxococcus in the Control were lower than those of the other two treatments, while Mortierellomycota was higher. And the Planctomycetes and Patescibacteria were found maximum in Chemical and minimum in Trichoderma.
Figure 4
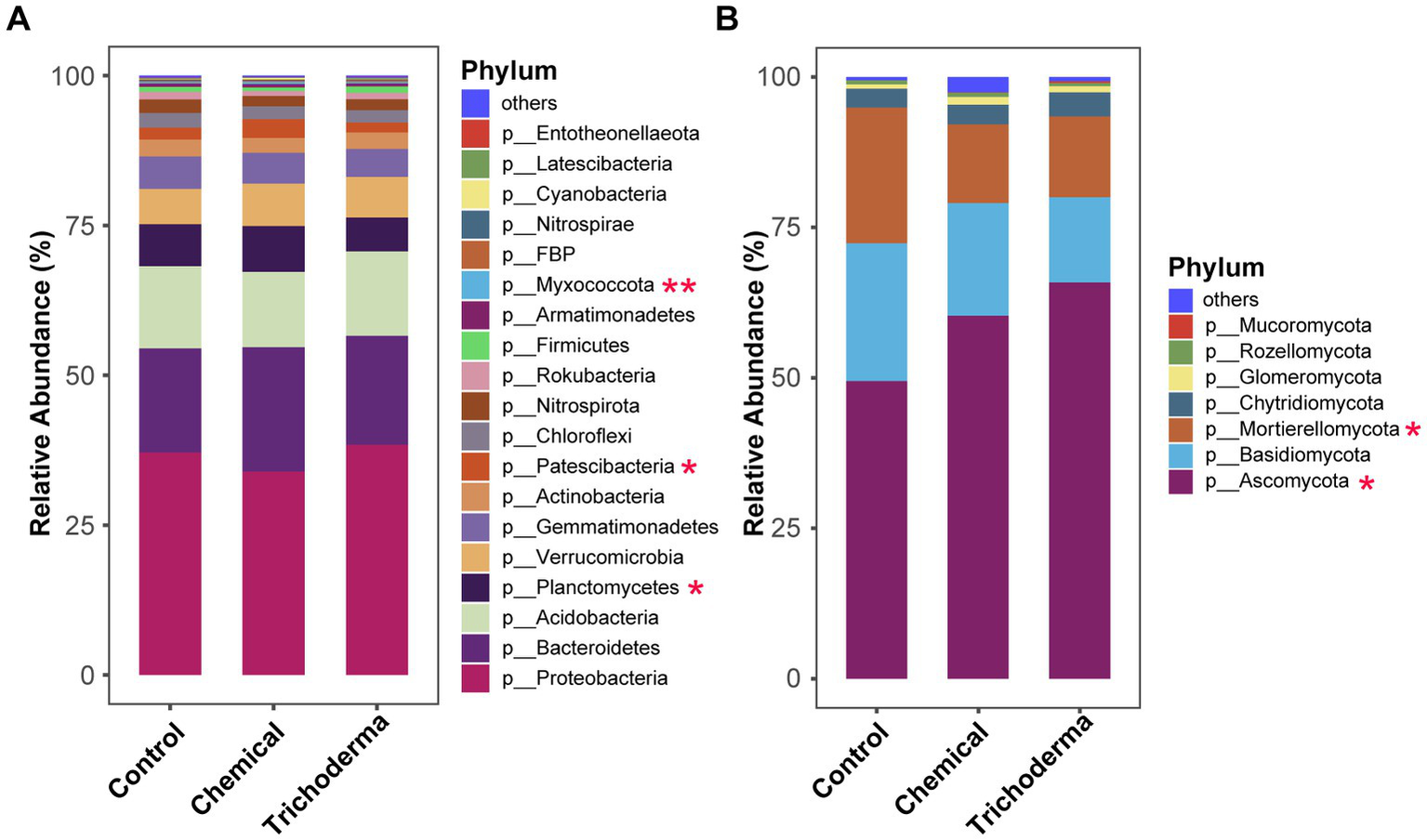
Relative abundance of bacterial (A) and fungal (B) communities at the phylum level of rhizosphere soil under different treatments (Control, Chemical and Trichoderma). “Others” represent the phyla with a relative abundance of less than 0.1%. *p < 0.05, **p < 0.01.
From the result of DESeq2 algorithm, the fungal community was more sensitive to chemical fungicide and T. viride than bacterial community (Figure 5). There were 12 OTUs and 18 OTUs were influenced by chemical fungicide and T. viride treatments, respectively. And among them, 9 and 6 OTUs were significantly correlated with the disease index of soybean, respectively. In the Chemical, the relative abundance of 2 bacterial OTUs and 7 fungal OTUs increased, while that of 4 fungal OTUs decreased. In the Trichoderma, 1 bacterial OTUs and 7 fungal OTUs were enriched, while 3 bacterial OTUs and 7 fungal OTUs were reduced. These OTUs with significant differences in relative abundance belonged to Proteobacteria, Bacteroidetes, Ascomycota, Basidiomycota, or Chytridiomycota (Figure 5). Especially, 1 bacterial OTUs and 2 fungal OTUs were enriched in both Chemical and Trichoderma, and 4 fungal OTUs were significantly reduced (absolute log2FC > 1, padj < 0.05), which belonged to Proteobacteria, Ascomycota and Chytridiomycota. Most of the identified OTUs belonged to Ascomycota, indicating that Ascomycota may be more associated with soybean root rot and is easily affected by control measures. In addition, only 3 fungal OTUs were significantly enriched in Chemical compared to Trichoderma, and there was no significant difference in the relative abundance of bacterial OTUs between Chemical and Trichoderma.
Figure 5
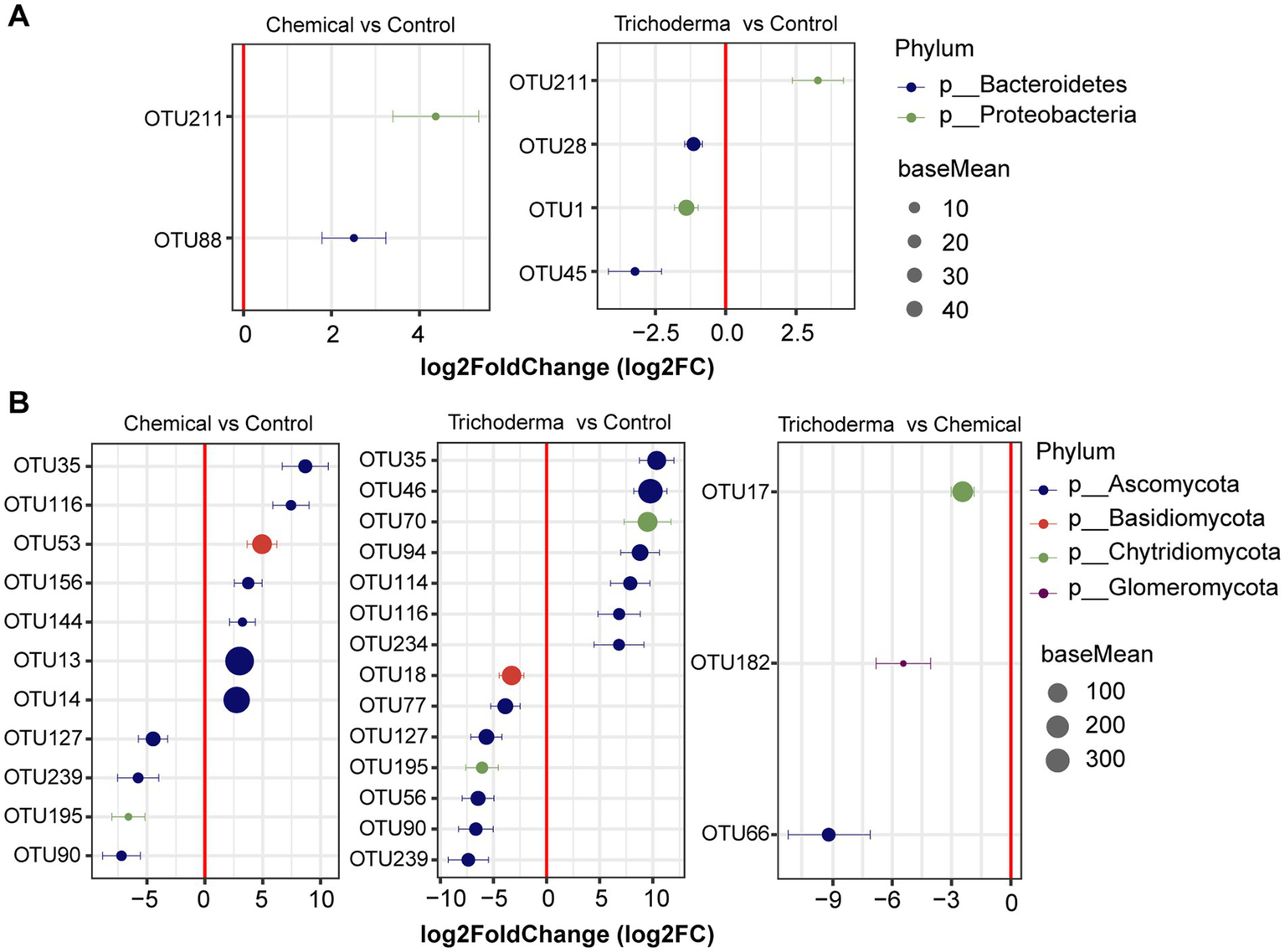
Differentially abundant bacterial (A) and fungal (B) OTUs identified in the rhizosphere soil from different treatments. The absolute log2FC was >1 and FDR adjusted p-values of <0.05 were considered to be differentially abundant.
3.5. FUNGuild analysis of fungal communities
FUNGuild was used to predict the nutritional and functional groups of the fungal communities. The results of FUNGuild showed that seven trophic modes could be classified, including Pathotroph, Pathotroph-Saprotroph, Pathotroph-Saprotroph-Symbiotroph, Pathotroph-Symbiotroph, Saprotroph, Saprotroph-Symbiotroph and Symbiotroph (Figure 6). Of the total 286 (59.96%) fungal OTUs assigned to trophic modes, only 224 (46.96%) OTUs had an assignment with highly probable and probable confidence ranks. The Saprotroph and Saprotroph-Symbiotroph were the major fungal guilds, with the OTUs percentages being 19.09–27.82% and 12.92–22.55%, respectively (Figure 6). The relative abundance of Saprotroph-Symbiotroph in Chemical and Trichoderma treatments (12.92 and 13.26%, respectively) was reduced compared to Control (22.55%), while Saprotroph showed the reverse trend. Statistical analysis showed that only the difference of Saprotroph-Symbiotroph among the three treatments was significant (p < 0.05).
Figure 6
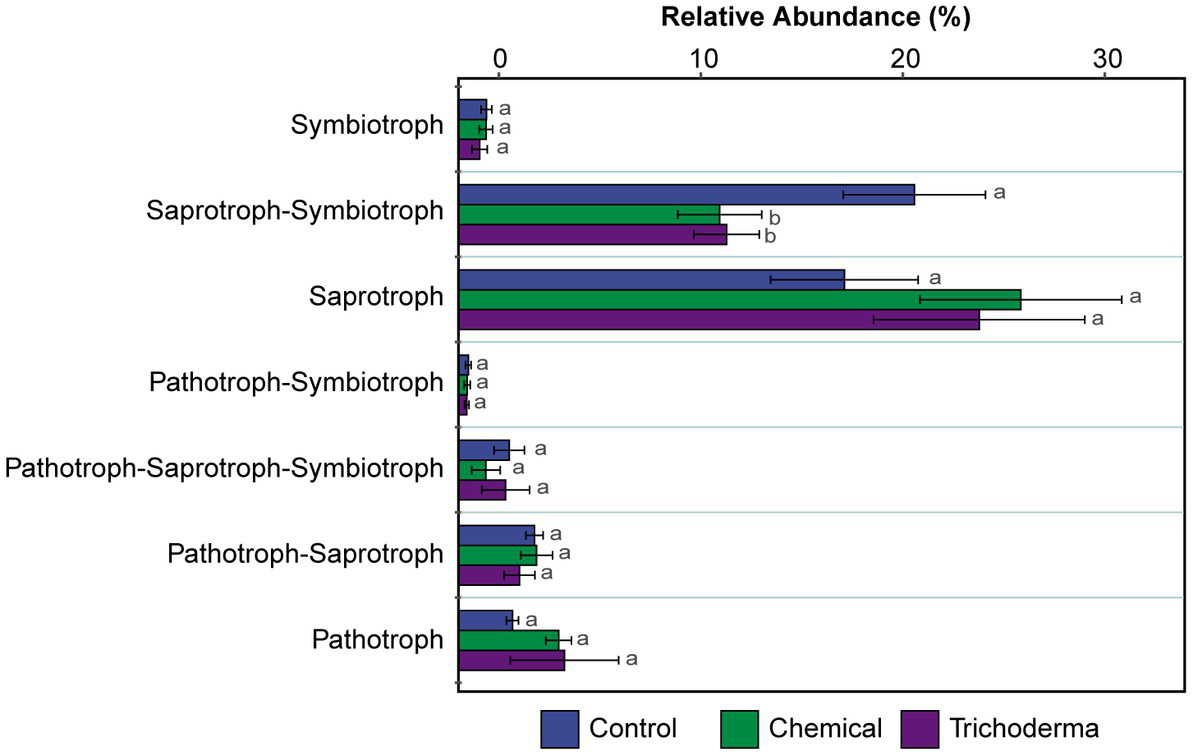
The relative abundance of trophic modes assigned by FUNGuild for fungal communities. Different letters indicate significant difference between treatments (p < 0.05).
3.6. Co-occurrence network analysis of microbial communities
The microbial co-occurrence networks of Control, Chemical and Trichoderma consisted of 269, 248, and 268 nodes (genera), respectively (Figure 7). Most nodes of the three networks were primarily assigned to Proteobacteria (29.37% for Control, 29.44% for Chemical and 30.97% for Trichoderma). Ascomycota (23.42% for Control, 26.61% for Chemical and 21.64% for Trichoderma) and Basidiomycota (5.20% for Control, 6.05% for Chemical and 5.97% for Trichoderma) were the most abundant fungal nodes. Specifically, the proportion of fungal nodes in the network of the Chemical (35.89%) was higher than that of the Control (30.86%) and Trichoderma (30.22%). A total of 766, 499 and 818 co-occurrence relationships were inferred for the consensus microbial network of Control, Chemical and Trichoderma, respectively. Compared with the Control (68.80%), both Chemical (59.32%) and Trichoderma (60.02%) had less positive interaction in the microbial community, suggesting that they exhibited stronger competition among genera. Network degree for microbial nodes obeyed a power-law distribution (p < 0.001 in all cases) (Supplementary Figure S2), indicating non-random community assembly may be the characteristic of the three microbial communities (Barberan et al., 2012).
Figure 7
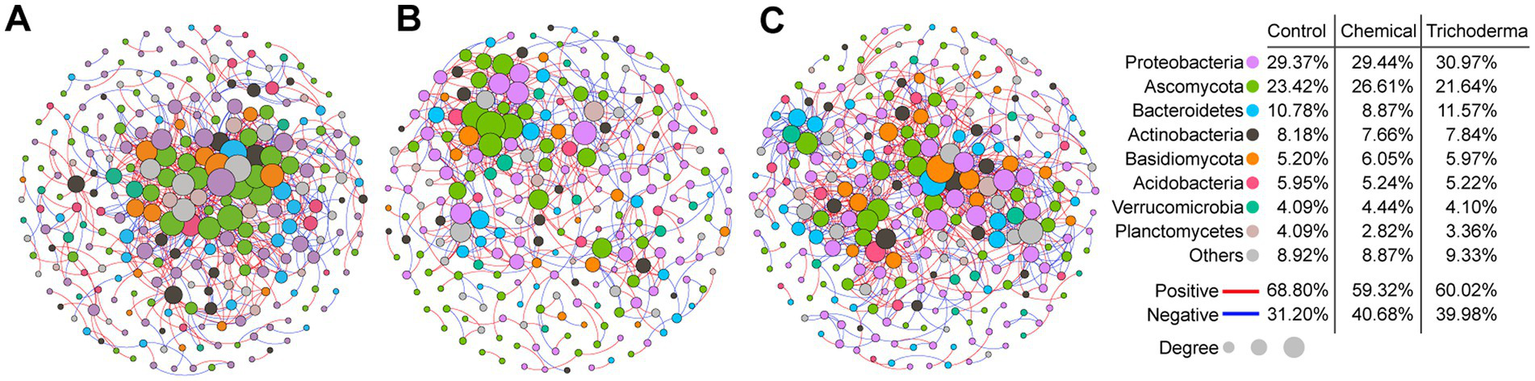
Co-occurrence networks of microbial genera in Control (A), Chemical (B), and Trichoderma (C). The size of each node is proportional to the number of connections (degree). The color of connections between two nodes represents a positive (red) or a negative correlation (blue). The node colors show various phyla.
Multiple network topological metrics revealed that the microbial networks of the three treatments have different complexity and stability (Table 2). Compared with the Control network, Trichoderma had higher parameters of edge numbers, modularity, graph density, average degree and average clustering coefficient, and a lower parameter of average path distance. However, the average path distance of Chemical was higher than that of the control, while the node numbers, edge numbers, graph density and average degree were lower than that of the control. The high modularity (0.616 for Control, 0.736 for Chemical and 0.648 for Trichoderma) of the three networks illustrated that they all have modular structures (modularity values >0.4) (Newman, 2006; Ju and Zhang, 2015).
Table 2
| Network metrics | Control | Chemical | Trichoderma |
|---|---|---|---|
| Nodes | 269 | 248 | 268 |
| Edges | 766 | 499 | 818 |
| Modularity | 0.616 | 0.736 | 0.648 |
| Graph density | 0.021 | 0.016 | 0.023 |
| Average degree | 5.695 | 4.024 | 6.104 |
| Average clustering coefficient | 0.234 | 0.327 | 0.314 |
| Average path distance | 4.733 | 5.068 | 4.67 |
Topological properties of networks associated with soybean rhizosphere microbiome under different treatments.
Most genera identified in the three treatments were peripherals (97.40% for Control, 94.35% for Chemical and 95.90% for Trichoderma), which have most of their links inside their modules (Supplementary Figure S3A). Only 2.6, 5.65 and 4.1% of the genera in Control, Chemical and Trichoderma were designated as keystone genera, respectively (Pi >0.62 or Zi > 2.5). For Control, there were 3 module hubs, which are most closely related to Actinobacteria (2 genera) and Proteobacteria (1 genus), and 4 connectors belonging to Actinobacteria (2 genera), Ascomycota (1 genus) and Proteobacteria (1 genus). For Chemical, there were 9 module hubs belonging to Ascomycota (3 genera), Proteobacteria (3 genera), Basidiomycota (1 genus), Bacteroidetes (1 genus) and Actinobacteria (1 genus), and 5 connectors belonging to Ascomycota (4 genera) and Proteobacteria (1 genus). For Trichoderma, there were 8 module hubs belonging to Ascomycota (2 genera), Basidiomycota (3 genera), Bacteroidetes (1 genus), Actinobacteria (1 genus) and Proteobacteria (1 genus), and 3 connectors belonging to Ascomycota (1 genus), Basidiomycota (1 genus) and Myxococcota (1 genus). The relative abundance of keystone genera in Chemical and Trichoderma increased, most of which belonged to fungi. Moreover, the relative abundance of the keystone genera in Trichoderma was the highest (Supplementary Figure S3B).
The natural connectivity in all treatments gradually decreased with the increasing number of removed nodes (Figure 8). Compared with the Control network, the natural connectivity in the Chemical network was reduced, while that in the Trichoderma network was reduced little. The evidence implied that chemical fungicide may reduce the stability of microbial network, while T. viride does not. The higher network stability in Control may be due to the fact that these keystone genera (module hubs and connectors) influence the community structure through strong biotic interactions with the host or with other microbial taxa, rather than through their own high abundance (Supplementary Figure S3B and Figure 8).
Figure 8
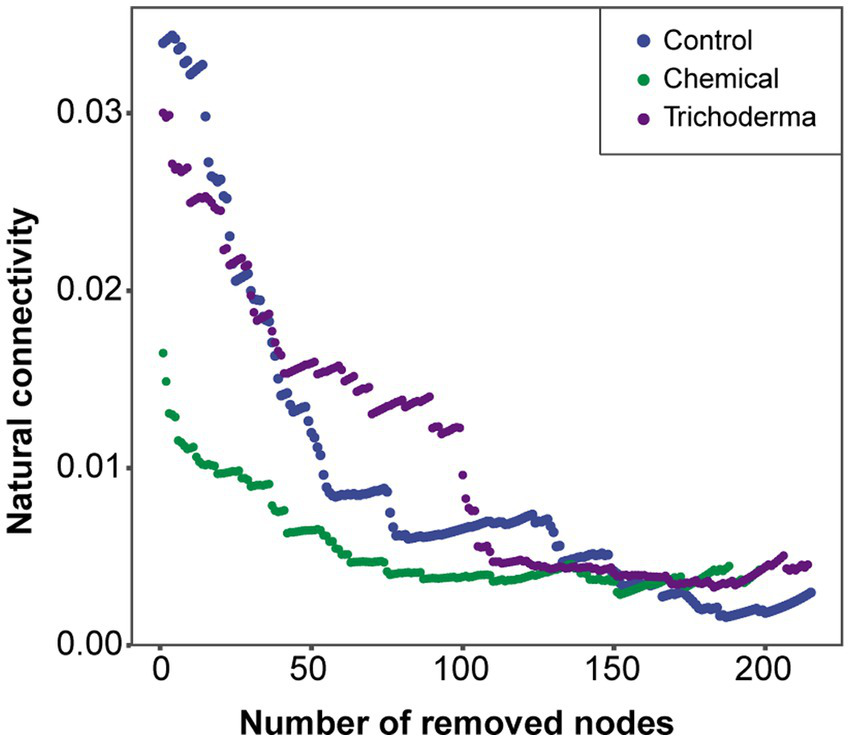
The natural connectivity of co-occurrence networks in three treatments.
3.7. Correlation between disease index and microbial communities
Associations between the abundant genera (abundance >0.1%) and disease index were analyzed by Spearman’s correlation coefficient (Supplementary Table S2). The relative abundance of 31 bacterial genera and 21 fungal genera were significantly correlated with the disease index of soybean, and there were 12 (38.71%) bacterial genera and 10 (47.62%) fungal genera positively correlated (r > 0, p < 0.05) with the disease index. For bacteria, the genera Chiayiivirga, Salmonella, Rhodobacter, Mucilaginibacter, Pseudoxanthomonas, Pedobacter, Opitutus, Stenotrophobacter, Rhizobacter, Acidovorax, Kaistia, Neorhizobium, Acinetobacter, Dyadobacter, Oligoflexus, Microterricola, Lacunisphaera, Pantoea, and Asticcacaulis were negatively correlated with the disease index, while the genera MND1, Bradyrhizobium, Tumebacillus, Pir4_lineage, UTBCD1, Marmoricola, WX53, Blastopirellula, Duganella, Paraburkholderia, Caballeronia and OLB17 showed a significant positive correlation. For fungi, the genera Coprinellus, Schizothecium, Waitea, Sarocladium, Filobasidium, Limonomyces, Zopfiella, Acremonium, Symmetrospora, and Torula were significantly negatively correlated with the disease index, while the genera Lecythophora, Exophiala, Monocillium, Chrysosporium, Trichoderma, Conocybe, Tausonia, Fusarium, Coniochaeta, Aspergillus, and Naganishia showed a significant positive correlation. Among the different genera that significantly correlated with the disease index, Coprinellus (r = −0.786, p < 0.001) exhibited an extremely significant negative correlation, while Lecythophora (r = 0.772, p < 0.001) showed an extremely significant positive correlation.
4. Discussion
4.1. Trichoderma viride reduced soybean root rot
Trichoderma species are used widely as biocontrol agents because they have many benefits on plant growth and have been proven to play a key role in controlling the plant diseases for several times (Sharma et al., 2011; Shahid and Khan, 2018). Among the various species of the Trichoderma, T. viride is considered to be one of the effective biocontrol agents for controlling root diseases of plants such as groundnut, mung bean, onion and so on (Manjula et al., 2004; Shahid and Khan, 2018; Bayoumi et al., 2019). The differences in control effects between studies may be related to the differences in plant types, regions and microbial communities (Li et al., 2020). In this study, the experimental results showed that T. viride and chemical fungicides reduced the disease incidence by 15.11 and 17.33% against soybean root rot, respectively (Figure 1). The control efficiency of T. viride was almost as high as that of chemical fungicide, and similar result had also been reported by Sui et al. (2022). Moreover, like other biocontrol agents, T. viride may also have the added benefit of protecting soil environment, improving plant growth and yield of soybean (Butt et al., 2001; John et al., 2010; Tan et al., 2017; Shahid and Khan, 2018).
4.2. The effects of Trichoderma viride on rhizosphere microbial community structure
The structure of soil microbial community has a crucial impact on soil quality and function (Janvier et al., 2007). The rhizosphere microbial community is also considered to be critical to improve crop productivity, resist soil-borne diseases, maintain soil ecosystem function and long-term sustainability (Trivedi et al., 2020). In the prevention and control of soybean root rot, special attention should be paid to the impact of the use of biological control agents and chemical fungicides on the rhizosphere microbial community (Wille et al., 2021). The rich root exudates have been shown to affect microbial communities in the rhizosphere soil (Butler et al., 2003). Pathogen attack can result in higher production of plant exudates, which contain proteins and extracellular DNA with antimicrobial functions (Basu et al., 2006; Minh Tran et al., 2016), thus influencing rhizobiome composition (Benizri et al., 2007). On the one hand, Trichoderma and chemical fungicides can directly affect rhizosphere microbial community. On the other hand, they can also indirectly affect the microbial community by affecting the production of plant root exudates (Howell, 2006).
In this study, T. viride did not significantly affect the alpha diversity of the rhizosphere bacterial and fungal communities compared to chemical fungicide that caused weakly significant differences in fungal richness (Table 1). This was similar to the effect of Trichoderma reported by Labanya et al. (2018) on the microbial community. On the contrary, Fu et al. (2019) and Sui et al. (2022) reported that treating soil with Trichoderma would elicit changes in soil microbial diversity. These effects may be related to the dosage of Trichoderma seed dressings (Liu and Hsiang, 1994). The microbial beta-diversity increased significantly in both Chemical and Trichoderma (Figure 2), implying that the decrease of soybean disease may be related to the increase of microbial beta-diversity. The differences between fungal communities were more significant than those of bacteria (Supplementary Table S1), which may be because the fungal community was more sensitive to T. viride and chemical fungicide than the bacterial community.
Exploring the formation and maintenance mechanisms of species diversity is an important scientific issue in soil microbial research (Nemergut et al., 2013). It is generally accepted that the rhizosphere microbial community is mainly profoundly shaped by metabolites produced by plant root (Bulgarelli et al., 2013). Consistent with previous studies on rhizosphere microbial community (Mendes et al., 2014; Fan et al., 2017), deterministic was the main force that influenced the bacterial community assembly (Supplementary Figure S1). However, the stochastic process in this study drove fungal community assembly. From this study, the fungal community may have the greater influence on the disease index of soybean root rot than bacterial community (Figure 5). The bacteria and fungi that changed significantly in the experiment may have a close relationship with the disease of soybean root rot. In this study, several potential plant pathogenic microorganisms were also positively correlated with disease index, such as Fusarium, Aspergillus, Conocybe, Naganishia, and Monocillium (Girlanda et al., 2001; Tekaia and Latgé, 2005; Liu et al., 2018; Horváth et al., 2020; Li et al., 2020). FUNGuild predicted the nutrient and functional groups of fungal community, which showed that the relative abundance of Saprotroph-Symbiotroph reduced in Chemical and Trichoderma treatments (Figure 6). Therefore, the Saprotroph-Symbiotroph in this work may play the most important role in soybean root rot.
4.3. The effects of Trichoderma viride on the co-occurrence network of microbial community
Hassani et al. have demonstrated that plant growth and fitness may be altered in beneficial ways by enhanced interactions within rhizosphere bacterial communities (Hassani et al., 2018). In this work, network analysis revealed the microbial community network of Trichoderma consisted of the most nodes and edges, whereas the microbial community network of Chemical comprised of the least nodes and edges (Table 2). We observed that T. viride and chemical fungicide increased the proportion of negative interactions in rhizosphere microbial network. The increase of negative interaction is helpful to improve the tolerance of rhizosphere microbial communities to external pressures, and thus promote plant performance and fosters plant growth (Coyte et al., 2015; de Vries et al., 2018). The high stability and complexity of the rhizosphere microbial network may be beneficial to enhance the resistance to disturbance and the soil ecosystem’s multifunctionality (Ding et al., 2023). The differential complexity reflects the difference of protection or destruction of seed dressings on rhizosphere microbial community structure. Previous studies also have suggested that fungicides can significantly reduce the microbial network complexity (Han et al., 2021, 2022; Zhang et al., 2021). While T. viride can enhance the complexity of microbial network to resist the attack of pathogens, which plays a key role in protecting soil ecology and sustainable agricultural development. The keystone genera have important ecological functions and play important roles in network structure, which can be identified as targets for microbial modulation to maintain crop health and improve productivity (Olesen et al., 2007; Fan et al., 2018). We found that more fungal genera were identified as keystone genera in the network of Trichoderma and Chemical, which may effectively enhance the resistance of soybean to pathogens (Supplementary Figure S3). In particular, chemical fungicide significantly reduced the stability of the microbial network, which may be a potential risk for the sustainable development of agriculture, while T. viride did not (Figure 8). In conclusion, compared with chemical fungicide, T. viride can enhance the complexity of rhizosphere soil microbial network, maintain the stability of the network, and be more beneficial to protect soil microecology.
5. Conclusion
This study compared the effects of T. viride and chemical fungicide on the control of soybean root rot, the diversity and composition of rhizosphere microbial community. The results indicated that T. viride and chemical fungicide could significantly reduce the disease index of soybean root rot and increase soybean yield. Both T. viride and chemical fungicide could increase the β-diversity of microbial community and significantly reduce the relative abundance of Saprotroph-Symbiotroph. Specially, our study showed that T. viride is beneficial to increase the complexity of co-occurrence network and maintain the stability of network, which is different from chemical fungicide. In addition, there were 31 bacterial genera and 21 fungal genera significantly correlated with the disease index (p < 0.05). Furthermore, the Coprinellus genus showed an extremely significant negative correlation, while Lecythophora showed an extremely significant positive correlation. For the prevention and control of soybean root rot, we may pay more attention to these microorgnisms that are significantly related to the disease index in the future. In summary, T. viride may be used as a substitute for chemical fungicide to control soybean root rot and create a healthier soil microecology.
Funding
This study was supported by the National Key R&D Program of China (2018YFD0201005).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA951875.
Author contributions
JQ conceived the idea and designed the experiment. PG, KQ, LM, BZ, and YZ performed the experiment. XG collected the data and samples. PG and YH performed the data analyses and wrote the manuscript. PG, KQ, YH, LM, BZ, YZ, XG, and JQ reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1204688/full#supplementary-material
Footnotes
References
1
AliS.UllahM. I.SajjadA.ShakeelQ.HussainA. (2021). “Environmental and health effects of pesticide residues” in Sustainable agriculture reviews, Vol. 48 eds, InamuddinM. I.LichtfouseA. E. (Gewerbestrasse, Cham, Switzerland: Springer), 311–336. doi: 10.1007/978-3-030-54719-6_8
2
BarberanA.BatesS. T.CasamayorE. O.FiererN. (2012). Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J.6, 343–351. doi: 10.1038/ismej.2011.119
3
BasuU.FrancisJ. L.WhittalR. M.StephensJ. L.WangY.ZaianeO. R.et al. (2006). Extracellular proteomes of Arabidopsis thaliana and Brassica napus roots: analysis and comparison by mud PIT and LC-MS/MS. Plant Soil286, 357–376. doi: 10.1007/s11104-006-9048-9
4
BayoumiY.TahaN.ShalabyT.AlshaalT.El-RamadyH. (2019). Sulfur promotes biocontrol of purple blotch disease via Trichoderma spp. and enhances the growth, yield and quality of onion. Appl. Soil Ecol.134, 15–24. doi: 10.1016/j.apsoil.2018.10.011
5
BenizriE.NguyenC.PiuttiS.Slezack-DeschaumesS.PhilippotL. (2007). Additions of maize root mucilage to soil changed the structure of the bacterial community. Soil Biol. Biochem.39, 1230–1233. doi: 10.1016/j.soilbio.2006.12.026
6
BulgarelliD.RottM.SchlaeppiK.Van Themaat, EV. L.AhmadinejadN.AssenzaF.et al. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature488, 91–95. doi: 10.1038/nature11336
7
BulgarelliD.SchlaeppiK.SpaepenS.Van ThemaatE. V. L.Schulze-LefertP. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol.64, 807–838. doi: 10.1146/annurev-arplant-050312-120106
8
ButlerJ. L.WilliamsM. A.BottomleyP. J.MyroldD. D. (2003). Microbial community dynamics associated with rhizosphere carbon flow. Appl. Environ. Microbiol.69, 6793–6800. doi: 10.1128/AEM.69.11.6793-6800.2003
9
ButtT. M.JacksonC.MaganN. (2001). Fungi as biocontrol agents: progress problems and potential. CABI. doi: 10.1079/9780851993560.0000
10
CaporasoJ. G.KuczynskiJ.StombaughJ.BittingerK.BushmanF. D.CostelloE. K.et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods7, 335–336. doi: 10.1038/nmeth.f.303
11
ChangX.DaiH.WangD.ZhouH.HeW.FuY.et al. (2018). Identification of fusarium species associated with soybean root rot in Sichuan Province, China. Eur. J. Plant Pathol.151, 563–577. doi: 10.1007/s10658-017-1410-7
12
ChenY.TianW.ShaoY.LiY. J.LinL. A.ZhangY. J.et al. (2020). Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Arch. Microbiol.202, 1157–1171. doi: 10.1007/s00203-020-01830-1
13
CoyteK. Z.SchluterJ.FosterK. R. (2015). The ecology of the microbiome: networks, competition, and stability. Science350, 663–666. doi: 10.1126/science.aad2602
14
de VriesF. T.GriffithsR. I.BaileyM.CraigH.GirlandaM.GweonH. S.et al. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun.9, 3033–3012. doi: 10.1038/s41467-018-05516-7
15
DengY.JiangY.-H.YangY.HeZ.LuoF.ZhouJ. (2012). Molecular ecological network analyses. BMC Bioinform13, 1–20. doi: 10.1186/1471-2105-13-113
16
DingL.TianL.LiJ.ZhangY.WangM.WangP. (2023). Grazing lowers soil multifunctionality but boosts soil microbial network complexity and stability in a subtropical grassland of China. Front. Microbiol.13:1027097. doi: 10.3389/fmicb.2022.1027097
17
Dini-AndreoteF.StegenJ. C.Van ElsasJ. D.SallesJ. F. (2015). Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci.112, E1326–E1332. doi: 10.1073/pnas.1414261112
18
FanK.CardonaC.LiY.ShiY.XiangX.ShenC.et al. (2017). Rhizosphere-associated bacterial network structure and spatial distribution differ significantly from bulk soil in wheat crop fields. Soil Biol. Biochem.113, 275–284. doi: 10.1016/j.soilbio.2017.06.020
19
FanK.WeisenhornP.GilbertJ. A.ChuH. (2018). Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem.125, 251–260. doi: 10.1016/j.soilbio.2018.07.022
20
FerreiraF. V.MusumeciM. A. (2021). Trichoderma as biological control agent: scope and prospects to improve efficacy. World J. Microbiol. Biotechnol.37:90. doi: 10.1007/s11274-021-03058-7
21
FuJ.XiaoY.WangY. F.LiuZ. H.YangK. J. (2019). Trichoderma affects the physiochemical characteristics and bacterial community composition of saline–alkaline maize rhizosphere soils in the cold-region of Heilongjiang Province. Plant Soil436, 211–227. doi: 10.1007/s11104-018-03916-8
22
GirlandaM.PerottoS.Moenne-LoccozY.BergeroR.LazzariA.DefagoG.et al. (2001). Impact of biocontrol Pseudomonas fluorescens CHA0 and a genetically modified derivative on the diversity of culturable fungi in the cucumber rhizosphere. Appl. Environ. Microbiol.67, 1851–1864. doi: 10.1128/AEM.67.4.1851-1864.2001
23
GuanY.JiangN.WuY.YangZ.BelloA.YangW. (2021). Disentangling the role of salinity-sodicity in shaping soil microbiome along a natural saline-sodic gradient. Sci. Total Environ.765:142738. doi: 10.1016/j.scitotenv.2020.142738
24
GuimeraR.Nunes AmaralL. A. (2005). Functional cartography of complex metabolic networks. Nature433, 895–900. doi: 10.1038/nature03288
25
HanL.KongX.XuM.NieJ. (2021). Repeated exposure to fungicide tebuconazole alters the degradation characteristics, soil microbial community and functional profiles. Environ. Pollut.287:117660. doi: 10.1016/j.envpol.2021.117660
26
HanL.XuM.KongX.LiuX.WangQ.ChenG.et al. (2022). Deciphering the diversity, composition, function, and network complexity of the soil microbial community after repeated exposure to a fungicide boscalid. Environ. Pollut.312:120060. doi: 10.1016/j.envpol.2022.120060
27
HartmanG. L.ChangH. X.LeandroL. F. (2015). Research advances and management of soybean sudden death syndrome. Crop Prot.73, 60–66. doi: 10.1016/j.cropro.2015.01.017
28
HassaniM.DuránP.HacquardS. (2018). Microbial interactions within the plant holobiont. Microbiome6, 58–17. doi: 10.1186/s40168-018-0445-0
29
HorváthE.SipiczkiM.CsomaH.MiklósI. (2020). Assaying the effect of yeasts on growth of fungi associated with disease. BMC Microbiol.20, 320–310. doi: 10.1186/s12866-020-01942-0
30
HowellC. R. (2006). Understanding the mechanisms employed by Trichoderma virens to effect biological control of cotton diseases. Phytopathology96, 178–180. doi: 10.1094/PHYTO-96-0178
31
JanvierC.VilleneuveF.AlabouvetteC.Edel-HermannV.MateilleT.SteinbergC. (2007). Soil health through soil disease suppression: which strategy from descriptors to indicators?Soil Biol. Biochem.39, 1–23. doi: 10.1016/j.soilbio.2006.07.001
32
JohnR. P.TyagiR.PrévostD.BrarS. K.PouleurS.SurampalliR. (2010). Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot.29, 1452–1459. doi: 10.1016/j.cropro.2010.08.004
33
JuF.ZhangT. (2015). Bacterial assembly and temporal dynamics in activated sludge of a full-scale municipal wastewater treatment plant. ISME J.9, 683–695. doi: 10.1038/ismej.2014.162
34
KembelS. W.CowanP. D.HelmusM. R.CornwellW. K.MorlonH.AckerlyD. D.et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics26, 1463–1464. doi: 10.1093/bioinformatics/btq166
35
KhanI. H.JavaidA.AhmedD. (2021). Trichoderma viride controls Macrophomina phaseolina through its DNA disintegration and production of antifungal compounds. Int. J. Agric. Biol.25, 888–894. doi: 10.17957/IJAB/15.1743
36
KunzI. G.ReedK. J.MetcalfJ. L.HasselD. M.ColemanR. J.HessT. M.et al. (2019). Equine fecal microbiota changes associated with anthelmintic administration. J. Equine Vet.77, 98–106. doi: 10.1016/j.jevs.2019.01.018
37
LabanyaR.KumarN.BhattM. (2018). Effect of fungicides and bioagents on rhizosphere microbial population, microbial biomass carbon and dehydrogenase activity in soybean (Glycine max L. Merrill) soil. Int. J. Fauna and Biological Stud.6, 102–107. doi: 10.13057/nusbiosci/n060108
38
LiJ.PhilpJ.LiJ.WeiY.LiH.YangK.et al. (2020). Trichoderma harzianum inoculation reduces the incidence of Clubroot disease in Chinese cabbage by regulating the rhizosphere microbial community. Microorganisms8:1325. doi: 10.3390/microorganisms8091325
39
LiuL.ChenS.ZhaoJ.ZhouX.WangB.LiY.et al. (2018). Watermelon planting is capable to restructure the soil microbiome that regulated by reductive soil disinfestation. Appl. Soil Ecol.129, 52–60. doi: 10.1016/j.apsoil.2018.05.004
40
LiuL. X.HsiangT. (1994). Bioassays for benomyl adsorption and persistence in soil. Soil Biol. Biochem.26, 317–324. doi: 10.1016/0038-0717(94)90280-1
41
ManjulaK.KishoreG. K.GirishA.SinghS. (2004). Combined application of Pseudomonas fluorescens and Trichoderma viride has an improved biocontrol activity against stem rot in groundnut. Plant Pathol. J.20, 75–80. doi: 10.5423/PPJ.2004.20.1.075
42
MendesL. W.KuramaeE. E.NavarreteA. A.Van VeenJ. A.TsaiS. M. (2014). Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J.8, 1577–1587. doi: 10.1038/ismej.2014.17
43
Minh TranT.MacintyreA.KhokhaniD.HawesM.AllenC. (2016). Extracellular DNases of Ralstonia solanacearum modulate biofilms and facilitate bacterial wilt virulence. Environ. Microbiol.18, 4103–4117. doi: 10.1111/1462-2920.13446
44
NemergutD. R.SchmidtS. K.FukamiT.OneillS. P.BilinskiT. M.StanishL. F.et al. (2013). Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev.77, 342–356. doi: 10.1128/MMBR.00051-12
45
NewmanM. E. J. (2006). Modularity and community structure in networks. Proc. Natl. Acad. Sci.103, 8577–8582. doi: 10.3389/fgene.2021.701331
46
NguyenN. H.SongZ.BatesS. T.BrancoS.TedersooL.MenkeJ.et al. (2016). FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol.20, 241–248. doi: 10.1016/j.funeco.2015.06.006
47
OlesenJ. M.BascompteJ.DupontY. L.JordanoP. (2007). The modularity of pollination networks. Proc. Natl. Acad. Sci.104, 19891–19896. doi: 10.1073/pnas.0601602103
48
PanZ.ChenY.ZhouM.McallisterT. A. (2021). Microbial interaction-driven community differences as revealed by network analysis. Comput. Struct. Biotechnol. J.19, 6000–6008. doi: 10.1016/j.csbj.2021.10.035
49
PengG.-S.WuJ. (2016). Optimal network topology for structural robustness based on natural connectivity. Phys. Stat Mech. Appl.443, 212–220. doi: 10.1016/j.physa.2015.09.023
50
RothM. G.WebsterR. W.MuellerD. S.ChilversM. I.FaskeT. R.MathewF. M.et al. (2020). Integrated management of important soybean pathogens of the United States in changing climate. J. Integrat. Pest Manag.11, 1–28. doi: 10.1093/jipm/pmaa013
51
SavazziniF.LongaC. M. O.PertotI. (2009). Impact of the biocontrol agent Trichoderma atroviride SC1 on soil microbial communities of a vineyard in northern Italy. Soil Biol. Biochem.41, 1457–1465. doi: 10.1016/j.soilbio.2009.03.027
52
ShahidS.KhanM. R. (2018). Evaluation of biocontrol agents for the management of root-rot of mung bean caused by Macrophomina phaseolina. Indian Phytopathol.72, 89–98. doi: 10.1007/s42360-018-0098-8
53
SharmaP.KumarV. P.RameshR.SaravananK.DeepS.SharmaM.et al. (2011). Biocontrol genes from Trichoderma species: a review. Afr. J. Biotechnol.10, 19898–19907. doi: 10.5897/ajbx11.041
54
SuiL.LiJ.PhilpJ.YangK.WeiY.LiH.et al. (2022). Trichoderma atroviride seed dressing influenced the fungal community and pathogenic fungi in the wheat rhizosphere. Sci. Rep.12:9677. doi: 10.1038/s41598-022-13669-1
55
TanY.CuiY.LiH.KuangA.LiX.WeiY.et al. (2017). Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol. Res.194, 10–19. doi: 10.1016/j.micres.2016.09.009
56
TekaiaF.LatgéJ.-P. (2005). Aspergillus fumigatus: saprophyte or pathogen?Curr. Opin. Microbiol.8, 385–392. doi: 10.1016/j.mib.2005.06.017
57
TripathiP.SinghP. C.MishraA.ChauhanP. S.DwivediS.BaisR. T.et al. (2013). Trichoderma: a potential bioremediator for environmental clean up. Clean Techn. Environ. Policy15, 541–550. doi: 10.1007/s10098-012-0553-7
58
TrivediP.LeachJ. E.TringeS. G.SaT.SinghB. K. (2020). Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol.18, 607–621. doi: 10.1038/s41579-020-0412-1
59
VermaM.BrarS. K.TyagiR.SahaiV.PrévostD.ValéroJ.et al. (2007). Bench-scale fermentation of Trichoderma viride on wastewater sludge: rheology, lytic enzymes and biocontrol activity. Enzym. Microb. Technol.41, 764–771. doi: 10.1016/j.enzmictec.2007.06.013
60
WilleL.KurmannM.MessmerM. M.StuderB.HohmannP. (2021). Untangling the pea root rot complex reveals microbial markers for plant health. Front. Plant Sci.12:737820. doi: 10.3389/fpls.2021.737820
61
WuB.WangP.DevlinA. T.SheY.ZhaoJ.XiaY.et al. (2022). Anthropogenic intensity-determined assembly and network stability of Bacterioplankton communities in the Le’an river. Front. Microbiol.13:806036. doi: 10.3389/fmicb.2022.806036
62
ZhangH.SongJ.ZhangZ.ZhangQ.ChenS.MeiJ.et al. (2021). Exposure to fungicide difenoconazole reduces the soil bacterial community diversity and the co-occurrence network complexity. J. Hazard. Mater.405:124208. doi: 10.1016/j.jhazmat.2020.124208
Summary
Keywords
soybean root rot, seed dressing, Trichoderma viride, chemical fungicide, rhizosphere microbial community, co-occurrence network
Citation
Gao P, Qi K, Han Y, Ma L, Zhang B, Zhang Y, Guan X and Qi J (2023) Effect of Trichoderma viride on rhizosphere microbial communities and biocontrol of soybean root rot. Front. Microbiol. 14:1204688. doi: 10.3389/fmicb.2023.1204688
Received
12 April 2023
Accepted
15 May 2023
Published
02 June 2023
Volume
14 - 2023
Edited by
Md. Motaher Hossain, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Bangladesh
Reviewed by
Md. Tanbir Rubayet, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Bangladesh; Bo Zhou, Shandong Agricultural University, China
Updates
Copyright
© 2023 Gao, Qi, Han, Ma, Zhang, Zhang, Guan and Qi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junshan Qi, qi999@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.