- 1Institute of Technology and Life Sciences—National Research Institute, Raszyn, Poland
- 2Department of Animal Nutrition, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Jabłonna, Poland
- 3Department of Physical Chemistry, Medical University of Białystok, Białystok, Poland
Plant growth-promoting bacteria are one of the most interesting methods of controlling fungal phytopathogens. These bacteria can participate in biocontrol via a variety of mechanisms including lipopeptide production, hydrolytic enzymes (e.g., chitinase, cellulases, glucanase) production, microbial volatile organic compounds (mVOCs) production, and induced systemic resistance (ISR) triggering. Among the bacterial genera most frequently studied in this aspect are Bacillus spp. including Bacillus pumilus. Due to the range of biocontrol traits, B. pumilus is one of the most interesting members of Bacillus spp. that can be used in the biocontrol of fungal phytopathogens. So far, a number of B. pumilus strains that exhibit biocontrol properties against fungal phytopathogens have been described, e.g., B. pumilus HR10, PTB180, B. pumilus SS-10.7, B. pumilus MCB-7, B. pumilus INR7, B. pumilus SE52, SE34, SE49, B. pumilus RST25, B. pumilus JK-SX001, and B. pumilus KUDC1732. B. pumilus strains are capable of suppressing phytopathogens such as Arthrobotrys conoides, Fusarium solani, Fusarium oxysporum, Sclerotinia sclerotiorum, Rhizoctonia solani, and Fagopyrum esculentum. Importantly, B. pumilus can promote plant growth regardless of whether it alters the native microbiota or not. However, in order to increase its efficacy, research is still needed to clarify the relationship between the native microbiota and B. pumilus. Despite that, it can already be concluded that B. pumilus strains are good candidates to be environmentally friendly and commercially effective biocontrol agents.
1. Introduction
Plant diseases cause enormous losses in agricultural production through decreasing yields and the quality of crops, resulting in great economic losses (Rashad and Moussa, 2020). Globally, losses due to plant diseases are estimated to more than 15% for unprotected crops (Chatterjee et al., 2016; Asad, 2022). Importantly, more than 70% of these diseases are caused by fungal phytopathogens (Liu et al., 2017). The most widespread fungal phytopathogens belong to genera such as Alternaria, Aspergillus, Botrytis, Cladosporium, Verticillium, Pythium, Fusarium, and Rhizoctonia (Djonovic et al., 2007; Zhang et al., 2016; Doehlemann et al., 2017; Krylov et al., 2018; Li and Chen, 2019; Tyśkiewicz et al., 2022). Over the last few decades, chemical fungicides have been the most popular solution for controlling phytopathogens, with recent annual use exceeding one million tonnes per year. However, ongoing climate change and agricultural pollution are boosting the development of eco-friendly agricultural products (Wierzchowski et al., 2021; Zielewicz et al., 2021; Heyi et al., 2022; Wróbel et al., 2022), including safe plant protection products (Mhatre et al., 2018; Köhl et al., 2019; Khan et al., 2022; Lahlali et al., 2022). Environmentally friendly methods of reducing plant phytopathogens include biological control with beneficial bacterial strains (Durán et al., 2018; Pascale et al., 2020). Bacteria are the most ubiquitous known organisms found in the environment and are not evenly distributed. For example, the density of bacteria around the roots of plants is usually considerably higher than the density found in the soil as a whole, which is influenced by root exudates containing, among other things, sugars, amino acids, or organic acids that provide a source of energy for bacteria (Carvalhais et al., 2015; Ojuederie et al., 2019; Basu et al., 2021; Khan et al., 2021; Bhat et al., 2023). Importantly, many bacterial strains are found in or around rhizosphere soil and have the ability to enhance plant growth, hence they are called plant growth-promoting bacteria (PGPB). Besides, PGPB also include endophytic bacteria (Morales-Cedeño et al., 2021). PGPB are capable of promoting plant growth either directly or indirectly. Direct plant growth promotion traits include, e.g., atmospheric nitrogen fixation, indolyl-3-acetic acid (IAA) production, cytokinins, gibberellins production, 1-aminocyclopropane-1-carboxylic acid deaminase (ACC), and phosphorus solubilization (Prakash and Arora, 2019; Dobrzyński et al., 2022a; Ferrusquía-Jiménez et al., 2022; Kaur et al., 2022; Miljaković et al., 2022; Sarmiento-López et al., 2022). Meanwhile, biocontrol traits are described as an indirect mechanism of plant growth enhancement, e.g., production of antibiotics including cyclic lipopeptides, chitinase and glucanase, and triggering of induced systemic resistance (ISR) (Shahid et al., 2021; Dobrzyński et al., 2022a; Mirskaya et al., 2022). The solutions above are in agreement with the contemporary trends introduced by the strategic programs of the EU and are in line with the principles of the European Green Deal (EGD) and the EU Biodiversity Strategy for 2030, which highlight the importance and necessity of agricultural biologicalization and agroecology development, and an increase in the area of ecological crops (Montanarella and Panagos, 2021).
In terms of agricultural application, one of the most interesting bacteria are members of the genus Bacillus. It includes mainly Gram-positive, spore-forming bacteria which have a wide distribution in many environments including soil (Nicholson, 2002; Pudova et al., 2022; Yakovleva et al., 2022). Importantly, Bacillus spp. strains show significant resistance to environmental stresses, for instance drought, irradiation, UV light, and low nutrient availability–which also increases their potential to enhance plant growth (Nicholson et al., 2000). So far, many species of the genus Bacillus have been described as PGPB, e.g., B. subtilis (Siahmoshteh et al., 2018), B. licheniformis (Gomaa, 2012), B. amyloliquefaciens (Siahmoshteh et al., 2018), B. megaterium (Mannaa and Kim, 2018), B. cereus (Chauhan et al., 2016), B. thuringiensis (Raddadi et al., 2007; Gomaa, 2012), B. laterosporus (Sun et al., 2021), B. vallismortis (Castaldi et al., 2021), B. badius (Zhu et al., 2021), B. velezensis (Azabou et al., 2020), B. endophyticus (Chauhan et al., 2016; Zhang et al., 2019). In terms of biocontrol agents, the best studied species of Bacillus spp. is B. subtilis. Due to its properties, including ISR induction, and the lipopeptides or hydrolytic enzymes production, it has already been commercialized as a fungal phytopathogen control agent and currently, there are many preparations on the market containing it in the formulation (Hashem et al., 2019; Samaras et al., 2021). Nevertheless, according to the literature, B. pumilus is not inferior in terms of biocontrol; for instance, B. pumilus strains can also trigger ISR and produce numerous lipopeptides and enzymes involved in the suppression of fungal phytopathogens. It suggests that the species has a great potential for more frequent commercialization (Jeong et al., 2014). However, before this can happen, it is necessary to look a little deeper into the effectiveness of potential PGPB. Hence, this review aims to gather and summarize the information on the potential of B. pumilus in biocontrol and to indicate research gaps that should be filled to increase the effectiveness of B. pumilus as a biocontrol agent and contribute to increased interest in commercialization.
2. Bacillus pumilus biofilm formation
Biofilm is a form of bacterial community that is considered to be the most widespread form of bacterial life in the natural environment (it also occurs in artificial environments, e.g., thrives on plastic). Bacterial cells in biofilms have a higher degree of organization and therefore more advantages than single cells. In a biofilm, bacterial cells form multicellular aggregations encapsulated in a matrix that generally consists of exopolysaccharides (EPS) and fiber proteins as well as extracellular DNA (eDNA) (Sutherland, 2001; Flemming and Wingender, 2010; Diehl et al., 2018; Flemming and Wuertz, 2019). According to the occupied surface, biofilms can be divided into submerged (solid-liquid interface), colonic (solid-air interface), or pellicles (liquid-air interface). Submerged and pellicle biofilms differ in terms of access to oxygen and nutrients, and the preferred niche for biofilm formation appears to depend on the bacterial species (Diehl et al., 2018). For example, B. subtilis is mainly known for its architecturally complex colonies and formation of wrinkled pellicle, while, e.g., Pseudomonas aeruginosa can show either a pellicle or submerged lifestyle (Tjalsma et al., 2000; Gao et al., 2015; Mielich-Süss and Lopez, 2015).
Bacterial biofilm formation is regulated by different genes and activated by different environmental factors. In terms of the mechanism of biofilm formation, one of the best known bacterial species is Bacillus subtilis; its mechanism depends mainly on the de-repression of the epsA-O and tapA-sipW-tasA operons which are responsible for coding enzymes involved in exopolysaccharides (EPS) synthesis and the amyloid fibril protein TasA (Branda et al., 2004, 2006). De-repression occurs when the Spo0A regulator reaches a threshold level of phosphorylation and leads to repression of the abrB which acts as a repressor of the previously mentioned operons (epsA-O and tapA-sipW-tasA). In addition, Spo0A upregulates the expression of sinI, which is responsible for the repression of the matrix gene, sinR (Fujita et al., 2005; Kearns et al., 2005).
Interestingly, it has been documented that 23 genes (identified by a random transposon insertion mutagenesis) are responsible for the regulation of biofilm formation in B. cereus strain AR156, including the comER gene which plays a significant role in both biofilm and spore formation, and is thought to be a part of the regulatory pathway responsible for activation of Spo0A (Xu et al., 2014; Huang et al., 2021; Kulkova et al., 2023). It is conceivable that, due to the affinity to B. subtilis and B. cereus, most of the molecular mechanisms of biofilm formation in B. pumilus may be similar to those in the aforementioned species. Nevertheless, there are still no studies helping to understand the process of biofilm formation in B. pumilus and therefore there is an urgent need for future studies.
Effective promotion of plant growth is associated with colonization of the rhizosphere (in the case of rhizobacteria) or plant tissue (in the case of endophytes). In turn, the intensity of soil and plant colonization is linked to biofilm formation, which allows better accessibility to nutrients and thus better biocontrol efficiency (Bhattacharyya and Jha, 2012). In contrast, environmental factors that affect biofilm development include pH, temperature, humidity, oxygen, and metal ions in the rhizosphere or plant (Dutta and Podile, 2010; Zhou et al., 2016). In the case of B. pumilus, there is a little research on biofilm formation, which could enhance plant growth promotion. Nevertheless, Zhu et al. (2020a) conducted a biofilm study on B. pumilus HR10 which has previously been described as a rhizosphere bacterium involved in biocontrol, including the control of plant phytopathogens and supporting in mycorrhiza formation. Namely, the authors observed a noticeable ease in the formation of a stable biofilm structure on the medium surface. The optimum temperature for the B. pumilus HR10 biofilm was 37°C, while the optimum pH was 7.0. Additionally, the study found that the biofilm was not very sensitive to an acidic and alkaline environment. Furthermore, it was proven that the majority of polysaccharide components of plant root exudates enhanced biofilm formation by B. pumilus HR10, with glucose having the largest stimulating impact. Besides, low concentrations of such elemental ions as sodium, potassium, calcium, iron, and magnesium promoted biofilm formation (Zhu et al., 2020a). Another study on the biofilm in B. pumilus was also conducted on strain HR10 (Zhu et al., 2000b). The study compared the properties of the wild-type strain B. pumilus with mutants. Among other things, the authors found that the EPS and protein content produced by the mutants was significantly reduced compared to those in the wild-type bacterial strain, and the swarming ability of both types of HR10 strains was positively correlated with biofilm production. Furthermore, an experiment to determine the degree of colonization of the root system of Pinus massoniana seedlings proved that the wild strain could colonize and persist, while the biofilm-free mutants showed a poor ability to colonize (Zhu et al., 2020b). Moreover, another strain of B. pumilus FAB10 was able to form an abundant biofilm, produce ACC deaminase, and solubilize phosphorus (in vitro) (Ansari and Ahmad, 2019). Interestingly, the authors demonstrated the development of the biofilm of the FAB10 strain at various concentrations of NaCl in vitro, and then, using electron microscopy, proved that this strain has the ability to colonize wheat roots at various concentrations of NaCl (0 to 250 mM) in a pot experiment (Ansari and Ahmad, 2019).
Moreover, recent studies indicate that the synthesis of lipopeptide antibiotics, especially surfactin and fengycin, is involved in biofilm formation not only by B. subtilis but also likely by B. pumilus, which may be a key factor for successful colonization of inoculated plant roots (Penha et al., 2020; Zhu et al., 2020b).
3. Bacillus pumilus triggered induced systemic resistance (ISR)
Plant growth-promoting bacteria is able to modulate the plant immune system for reaction to a wide range of phytopathogens without direct contact with them. This type of action is called induced systemic resistance (ISR). Importantly, ISR is long-term and permanently protects plants. Bacteria exhibiting these traits are considered biocontrol agents (Pieterse et al., 2014; Stringlis et al., 2018). The mechanism of ISR activation by PGPB is still not fully understood. Despite that, a few ISR triggering traits have been proposed, including lipopeptides, volatile organic compounds (VOCs), flagellin, lipopolysaccharides, and siderophores (Chowdhury et al., 2015; Romera et al., 2019; Ayaz et al., 2021; Yu et al., 2022). In plants, ISR is modulated by the jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) pathways (Shoresh et al., 2005; Yu et al., 2022).
Protection due to ISR triggered by the genus Bacillus has been recorded in various diseases, e.g., bacterial pathogens, systemic viruses, root-knot nematodes, late blight diseases, leaf-spotting fungal, crown-rotting fungal pathogen, stem-blight fungal pathogen, and blue mold (Choudhary and Johri, 2009; Li and Chen, 2019; Nie et al., 2019). One of the bacteria of the genus Bacillus that elicit ISR against fungal phytopathogens are B. pumilus strains. For instance, Enebak and Carey (2000) noted a potential elicitation of ISR in Pinus taeda by B. pumilus strains including INR7, SE52, SE34, and SE49 against a fungal phytopathogen Cronartium quercuum which contributes to a disease called fusiform rust. Moreover, ISR triggering by B. pumilus can decrease the severity of anthracnose which is caused by another fungus–Colletotrichum orbiculare; the experiment was conducted on cucumbers in field trials (Wei et al., 1996). Subsequently, Jeong et al. (2014) studied the commercial B. pumilus strain INR7 (Bayer Crop Science) which has the ability to ISR elicitation and is used in plant biological control against Colletotrichum orbiculare, Rhizoctonia solani, and Fusarium spp. Using the Illumina Genome Analyzer IIx, researchers detected 3 gene clusters present in B. pumilus INR7 that encode lipopeptide synthetases including surfactin, bacillibactin, and bacilysin, which may be involved in triggering ISR. Besides, B. pumilus strain INR7, B. subtilis GB03, and Curtobacterium flaccumfaciens were tested individually and in a consortium for biological control of various cucumber phytopathogens including Colletotrichum orbiculare. In greenhouse trials, B. pumilus in the consortium was shown to have a more effective effect in suppressing pathogens compared to the use of B. pumilus alone. In field trials, the efficacy of the consortium in eliciting ISR was confirmed–the consortium inhibited anthracnose symptoms (Raupach and Kloepper, 1998). Interestingly, B. pumilus KUDC1732 also suppressed gray leaf spot caused by Stemphylium lycopersici in peppers (Son et al., 2014).
Furthermore, B. pumilus strains are also capable of elicit ISR against bacterial phytopathogens including Pseudomonas syringae pv. lachrymans, Xanthomonas axonopodis (Jeong et al., 2014; Li et al., 2020), or Erwinia tracheiphila (Jeong et al., 2014) and a fungus-like organisms–Oomycetes including Peronospora tabacina (Zhang et al., 2004).
In conclusion, it is worth adding that in the case of ISR elicitation by B. amyloliquefaciens, FZB42 is probably the main mechanism in controlling phytopathogens (Chowdhury et al., 2015). Therefore, in B. pumilus it may be similar but there is still a lack of studies that could confirm this fact.
4. Lipopeptides synthesized by Bacillus pumilus
Lipopeptide antibiotics are one of the most commonly produced antibiotics by bacteria of the genus Bacillus (Saggese et al., 2022). Lipopeptides are low molecular weight biosurfactants, biodegradable, non-toxic, stable, and environmentally friendly (Meena and Kanwar, 2015; Toral et al., 2018). These compounds are synthesized by non-ribosomal peptide synthases (NRPSs) which are encoded by a cluster of genes, for instance: bmy (bacillomycin), bac (bacilysin), srf (surfactin), and fen (fengycins) (Luo et al., 2015; Kim et al., 2017; Fazle Rabbee and Baek, 2020). In addition, gene clusters encoding polyketide synthases (PKSs) that are involved in the synthesis of polyketides (PKs), e.g., macrolactin (mln), bacillaene (bae), and difficidin (dfn), have also been detected in Bacillus spp.; PKSs exhibit similar properties to lipopeptides (Chen et al., 2007; Fazle Rabbee and Baek, 2020). The cyclic lipopeptides of Bacillus spp. are represented by three major families: surfactins, iturins, and fengycins (Penha et al., 2020). For instance, lipopeptides belonging to the surfactin family (e.g., surfactin, bamilocyn, lichenysin, halobacilin, pumilacidin) detected in B. pumilus strains are heptapeptides and have antifungal and antimicrobial activities. In addition, they play an important role in biofilm formation and induce systemic immunity in plants (Abbas et al., 2019; Miljaković et al., 2020). Recent studies have shown that surfactin produced by B. pumilus strains HR10 and PTB180 may be involved in the prevention growth of the Rhizoctonia solani in Pinus massoniana seedlings and may inhibit Botrytis cinerea mycelial growth and conidia germination on tomato (Zhu et al., 2020b; Bouchard-Rochette et al., 2022). Furthermore, using Matrix-Assisted Laser Desorption–Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) analysis of lipopeptides, Labiadh et al. (2021) demonstrated a diverse variety of molecules in B. pumilus such as surfactins, pumilacidin, and kurstakin, which exhibited antifungal ability against Arthrobotrys conoides and Fusarium solani.
Some heptapeptides of the iturin family (e.g., iturin, bacillomycin, mycosubtilin, mixirins, bacillopeptins, mojavensin, subtulene) and decapeptides of the fengycin family (e.g., fengycin, plipastatin, maltacin) have been found in B. pumilus strains; they can inhibit a wide range of fungi and some bacteria (Miljaković et al., 2020). For instance, surfactin and fengycin B extracted from the strain B. pumilus W-7 can inhibit the growth of Phytophthora infestans (oomycete, fungus-like organisms) in potatoes. Importantly, the mechanism of antifungal action of fengycin B is based on direct inhibition of the fungus, while surfactin induces defense responses in potato by enhancing the expression of genes encoding antioxidant enzymes including pod, pal, and cat; it indicates that the two metabolites act in a synergistic way (Wang et al., 2020). Furthermore, antifungal activity against Sclerotinia sclerotiorum was also attributed to B. pumilus strain YSPMK11 (isolated from the cauliflower endorhizosphere) which produced iturin A and surfactin. In a 2-year experiment under field conditions, B. pumilus strain YSPMK11 reduced disease severity caused by Sclerotinia sclerotiorum in cauliflower by 93% (Kaushal et al., 2017). Interestingly, isolated from date palm, strain B. pumilus showed antagonistic activity against Rhizoctonia solani, Botrytis cinerea R16, Galactomyces geotrichum MUCL 28959, and Verticillium longisporum O1, which is associated with the presence of genes encoding lipopeptide synthases of the mycosubtilin and bacillomycin (iturin family) and the pumilacidin (surfactin family), which inhibit fungal growth (El Arbi et al., 2016).
Bacillus pumilus is also able to suppress bacterial phytopathogens. For instance, an in vitro study by Nikolić et al. (2019) showed that a mixture of Bacillus pumilus SS-10.7 and B. amyloliquefaciens strains SS-12.6 and SS-38.4 was able to synthesize lipopeptides such as surfactin, fengycin A, and iturin A which inhibited the growth of Pseudomonas syringae pv. aptata on sugar beet.
In addition, there are reports that B. pumilus strains produce other antibiotic substances including bacilysin (dipeptide antibiotic), tetaine (dipeptide antibiotic), bacircine, amino sugar NTD, bacteriocin (antimicrobial peptide), phenazine (heterocyclic antibiotic), amicoumacin A, paenilamicin, and subtilin, which also exhibit antibacterial and antifungal properties (Sansinenea and Ortiz, 2011; Özcengiz and Ögulur, 2015; Padaria et al., 2016; Toymentseva et al., 2019; Maksimova et al., 2021; Islam et al., 2022; Khatoon et al., 2022).
Finally, it is worth mentioning that most antibiotic substances can be found in relatively low concentrations near the roots colonized by PGBP of the genus Bacillus. The exception is surfactin, which has been detected in the root environment in much higher concentrations, accounting for more than 90% of the total lipopeptides. For instance, such lipopeptides as iturin and fengycin have been found in much smaller amounts, and moreover, most of the antibacterial polyketides and other bioactive compounds have so far not been reported at all in the environment of roots colonized by the members of the genus Bacillus (Nihorimbere et al., 2012; Debois et al., 2014). Therefore, it is suggested that perhaps surfactin is one of the main substances involved in the suppression of plant pathogens by Bacillus spp. Moreover, it is speculated that antibiotic compounds are not so heavily involved in the direct suppression of phytopathogens, but are more related to ISR elicitation (Chowdhury et al., 2015).
5. Hydrolytic enzymes produced by Bacillus pumilus
Other biocontrol agents are hydrolytic enzymes including chitinase, cellulase, glucanase, and protease, which break down components of the fungal cell wall (Branda et al., 2004, 2006; Mielich-Süss and Lopez, 2015). Chitinases, cellulases, glucanases, and proteases are enzymes produced by various bacteria such as Aeromonas, Azospirillum, Bradyrhizobium, Serratia, Vibrio, Streptomyces, Bacillus, and fungi such as Aspergillus, Fusarium Trichoderma, and Penicillium, plants, Actinobacteria, arthropods, etc. (Juturu and Wu, 2014; Bělonožníková et al., 2022; Díaz-Díaz et al., 2022; Dobrzyński et al., 2022b; Fatima et al., 2022; Gómez-de la Cruz et al., 2022; Yin et al., 2023).
Both B. pumilus and related species are able to synthesize the above-mentioned hydrolytic enzymes under various environmental conditions, which makes them potential biocontrol agents (Martínez-Zavala et al., 2020; Dobrzyński et al., 2021; Dimkić et al., 2022). Among hydrolytic enzymes, chitinolytic enzymes are the most important for the biocontrol of fungal phytopathogens. Chitinases are encoded by a number of genes that include, e.g., chiA, chiB, chiC and chiD, chiF, chiG, chiR, chiW, and chiX (Alam et al., 1996; Hamilton et al., 2014; Danişmazoğlu et al., 2015; Martínez-Zavala et al., 2020; Azizoglu et al., 2021; Kumar et al., 2022). The efficacy of chitinases produced by B. pumilus against fungi has been documented in a few studies. For instance, Rishad et al. (2017) reported that B. pumilus MCB-7 showed chitinase activity (3.36 U mL–1) even at temperatures up to 60°C and high saline concentration. Both crude and purified chitinase exhibited fungistatic activity against important agricultural phytopathogens such as Fusarium oxysporum, Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, and Ceratorhiza hydrophila (Rishad et al., 2017). Moreover, based on clear zones on chitin agar, Gurav et al. (2017) isolated the strain B. pumilus RST25. The strain exhibited the highest chitinase activity at 96 h (51.7 U mL–1) of culture under submerged conditions. Importantly, in vitro assay of B. pumilus RST25 chitinolytic potential (crude and purified enzyme) showed significant antifungal activity against pathogens such as F. solani and A. niger. Moreover, after wheat (Triticum aestivum) seeds inoculation, the strain RST25 was able to reduce fungal infections (Gurav et al., 2017). Another study also showed that B. pumilus (strain JUBCH08) is capable of antagonistic activity against F. oxysporum (45% antagonism after dual plate analysis); the highest chitinase activity of B. pumilus JUBCH08 was obtained at 35°C after 72 h of submerged fermentation. Interestingly, the chitinase was thermostable and active in alkaline conditions, which indicates the possibility of the use of this strain in biotechnological application (Bhattacharya et al., 2016). Furthermore, Agarwal et al. (2017) conducted a study on biocontrol Rhizoctonia solani and Fusarium oxysporum using B. pumilus MSUA3; the authors observed a significant decrease in the disease index of Fagopyrum esculentum after B. pumilus MSUA3 inoculation in gnotobiotic conditions.
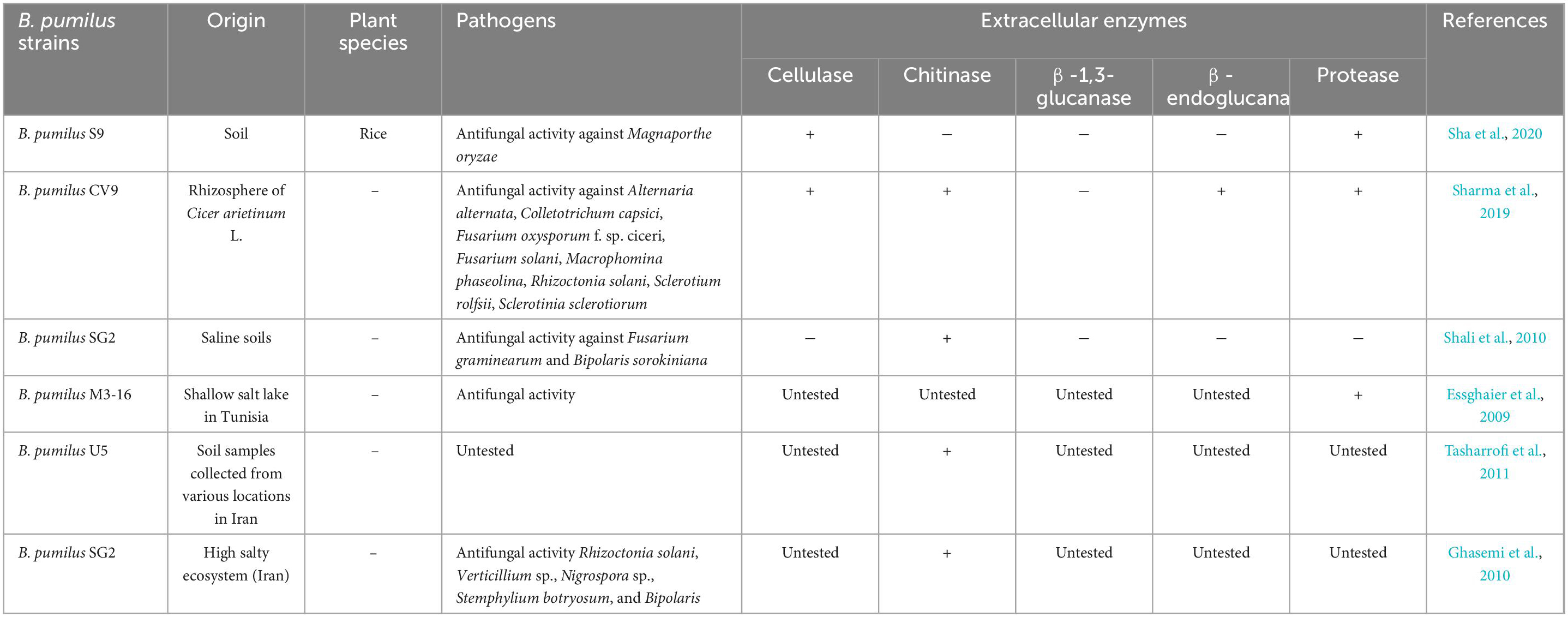
In terms of other lytic enzymes, B. pumilus JK-SX001 is able to produce protease and cellulase (Ren et al., 2013). As evidenced, metabolites of strain JK-SX001 could significantly suppress the growth of three phytopathogens including Phomopsis macrospora, Cytospora chrysosperma, and Fusicoccum aesculi. Importantly, this strain exhibited antagonistic activity against the above-mentioned pathogens on seedlings of polar under greenhouse conditions. Besides, B. pumilus JK-SX001 enhanced plant growth by direct stimulation, e.g., improved biomass production and shoot length (Ren et al., 2013). Antifungal activity has also been documented by other authors, as shown in Table 1.
6. Microbial volatile organic compounds as fungal growth-limiting agents
Microbial volatile organic compounds (mVOCs) that can be produced by bacteria are also considered a biocontrol agent (Hassan et al., 2017). VOCs are low molecular weight compounds that readily evaporate and diffuse at ordinary temperatures and pressures (Schulz-Bohm et al., 2017; Poveda, 2021). VOCs mainly belong to following groups of chemical compounds: alcohols (e.g., 2-methylbutan-1-ol, ethanol, 2-phenylethanol), esters, aldehydes, alkanes, alkenes, acids, ketones, benzenoids, pyrazines, terpenes, sulfur compounds, and nitrogen compounds (Buzzini et al., 2003; Fialho et al., 2011; Schmidt et al., 2015). Whereas mVOCs that exhibit antifungal activity include for example caryophyllene, dimethyl disulfide, dimethyl trisulfide, hydrogen cyanide, 2-methylpyrazine, S-methyl thioacetate, 2,3,6-trimethylphenol, undecan-2-one, dodecan-2-one, dodecan-2-one, nonan-2-one, decan-2-one, benzonitrile, and acetoin (Arrebola et al., 2010; Schmidt et al., 2015; Tyc et al., 2015; He et al., 2020). Importantly, in most cases bacterial VOCs are found in low concentrations and have a complex composition. Moreover, their concentration and abundance is influenced by many factors, such as the type of medium, culture conditions, and physiological states of the microorganisms (Elkahoui et al., 2015; Tyc et al., 2015). It is also worth adding that the antifungal efficacy of mVOCs-producing bacteria depends on the composition of these compounds and, as a rule, it is a composition of several or more compounds that can effectively inhibit fungal growth (Poveda, 2021).
Microorganisms capable of suppressing fungal growth through mVOCs include representatives of genera such as Bacillus spp. (Liu et al., 2008; Arrebola et al., 2010), Streptomyces spp. (Wang et al., 2013), Pseudomonas spp. (Elkahoui et al., 2015; Rojas-Solís et al., 2018), Tsukamurella spp., Dyella sp., Janthinobacterium spp., Chryseobacterium sp. (Tyc et al., 2015), and Collimonas spp. (Garbeva et al., 2014). While members of the genus Bacillus capable of producing mVOCs include bacteria such as Bacillus amyloliquefaciens (Gotor-Vila et al., 2017), Bacillus subtilis (Arrebola et al., 2010), and members of related genus Paenibacillus polymyxa (Liu et al., 2008).
Bacteria of the genus Bacillus are capable of producing compounds that inhibit the growth of fungi such as Penicillium sp. (Andersen et al., 1994), Trichoderma sp., Botrytis cinerea (Gotor-Vila et al., 2017) Fusarium oxysporum (Minerdi et al., 2009; Yuan et al., 2012), Botryosphaeria berengeriana (Zhang et al., 2010), and Colletotrichum gloeosporioides (Lee et al., 2012). However, there are several broad-spectrum antifungal strains against plant fungal diseases. So far, in the case of B. pumilus, there have been few studies describing the possibility of mVOCs production (against fungal phytopathogens) by this bacterial species. In this regard, Liu et al. (2008) conducted a study on B. pumilus isolated from the rhizosphere of cucumber–B. pumilus BSH-4 and B. pumilus ZB13. The aforementioned mVOCs-mediated strains inhibited the growth of fungi such as Sclerotinia sclerotiorum, Botrytis cinerea, Alternaria brassicae, Alternaria solani, Ascochyta citrullina, Fusarium oxysporum, Fusarium graminearum, Cercospora kikuchii, Rhizoctonia solani, Phoma arachidicola, and Verticillium dahliae. In addition, the authors, using GC-MS, showed that the mVOCs produced by either strains of B. pumilus include compounds, e.g., 2,4-decadienal, diethyl phthalate, n-hexadecanoic acid, and oleic acid. Interestingly, it was also shown that, compared to B. subtilis BL02 and Paenibacillus polymyxa BMP-11, either strains of B. pumilus produced less volatile substances, which may have been influenced by factors such as the natural dissimilarity of these species of bacteria and the conditions of cultivation including the type of medium and temperature (Liu et al., 2008). Furthermore, Morita et al. (2019) studied the antifungal effect of volatile organic compounds produced by B. pumilus TM-R, which was cultured on four different media; its antifungal activity was evaluated against several fungal species including Fusarium oxysporum, Cladosporium cladosporioides, Alternaria alternata, Curvularia lunata, Aspergillus niger, Cladosporium cladosporioides, and Penicillium italicum. In dual plate experiment, the bacteria clearly inhibited the growth of five fungi: Fusarium oxysporum, Cladosporium cladosporioides, Alternaria alternata, Curvularia lunata, Cladosporium cladosporioides, and Penicillium italicum, but enhanced the growth of Aspergillus niger. In contrast, in tests conducted on a larger volume (12-L medium), the level of antifungal activity was lower, but despite this, the B pumilus TM-R cultured on TMEA medium still seeded a high level of growth inhibition of the pathogens tested, especially against P. italicum (growth suppression levels reached 93%). Interestingly, the authors detected 32 mVOCs in the tested strain using the GC-MS technique. The content of the compounds and their concentrations were dependent on the culture media. The authors identified dominant mVOCs (that limit fungal growth) including ethanol, S-(−)-2-methylbutylamine, methyl isobutyl ketone, and 5-methyl-2-heptanone (Morita et al., 2019).
Besides, B. pumilus in combination with B. amyloliquefaciens and Exiguobacterium acetylicum has been shown to inhibit the growth of Peronophythora litchii (in vitro) and disease on litchi fruit (in vivo) through the secretion of mVOCs such as α-farnezen, 1- (2-aminophenyl) etanon, benzotiazol (Zheng et al., 2019).
Importantly, as mentioned in an earlier section, studies conducted on other Bacillus spp. suggest that mVOCs are involved in ISR induction and may be a major mechanism of phytopathogens control (Chowdhury et al., 2015).
7. Bacillus pumilus effect on the indigenous microbiota and its post-inoculation monitoring of abundance
As evidenced by research, it is assumed that the structure of the microbial community colonizing plant roots is important for the plant health and resistance to pathogens. Thus, the impact of plant growth-promoting bacteria on the native microbiota and their survival in soil and plant tissues (in the case of endophytes) may be crucial for the efficiency of their application (Manfredini et al., 2021; Kulkova et al., 2023; Wróbel et al., 2023). For instance, after the application against poplar canker, the abundance of endophytic strain, B. pumilus JK-SX001 (GFP-labeled strain and evaluated by confocal laser scanning microscopy) has persisted for a long time and its content was higher in roots and stems than in leaves (Huang et al., 2012). Furthermore, B. pumilus SQR-N43 was also analyzed using GFP tagging and after 2 weeks from the application of concentration of 108 and 109 cells g–1 as well as 105 and 106 cells g–1 (study on biocontrol of Rhizoctonia solani) the biofilm on the roots was observed. Importantly, colonization of the root by this strain has been recorded in the root apex and in certain regions of the elongation and differentiation zone of plant roots (Huang et al., 2012). Persistence of B. pumilus after application was also studied by qPCR technique. Win et al. (2018) showed that after 2 and 5 weeks from inoculation, the population of B. pumilus TUAT-1 in the rhizosphere of rice was stable. Interestingly, using denaturing gradient gel electrophoresis (PCR-DGGE), Kang et al. (2013) conducted a study on the persistence of B. pumilus WP8 in soil. The authors showed that the abundance of B. pumilus WP8 was stable up to 40 days in bulk soil. Besides, the strain changed the native soil bacterial community, particularly the dominant structure. However, these changes and persistence of this PGPB strain did not interfere with the efficiency of PGPB strain (Kang et al., 2013). Another study on the effects of B. pumilus on the indigenous microbiota was carried out using phospholipid fatty acid (PLFA) analysis (Probanza et al., 2001). Application of B. pumilus with B. licheniformis contributed to a change of the rhizosphere microbiota of stone pine (Pinus pinea), despite the low number of these strains at the end of the study. However, also in this case, these changes did not interfere with the efficiency of PGPB strain (Probanza et al., 2001). Moreover, Ramos et al. (2003) also documented the changes in the native microbiota of the rhizosphere [Alnus glutinosa (L.) Gaertn] after B. pumilus application using PLFA analysis (Ramos et al., 2003). Importantly, Win et al. (2018) assessed the response of the native microbiota to B. pumilus (strain TUAT-1) introduction using Next-Generation Sequencing (NGS); the study included microbiota of bulk soil, as well as rhizosphere and root endosphere of rice. For instance, B. pumilus application caused an increase in the population of Acidobacteriales and Desulfuromonadales and decreased the number of Xanthomonadales. Interestingly, the introduction of PGPB does not always result in alterations to the native bacterial community of soil or plant tissue. Dos Santos et al. (2022) did not observe the impact of B. subtilis application on the soybean microbial community.
Finally, it is worth adding that the mechanism of the impact of PGPB on the microbiota is still not fully understood and depends on a number of variables, e.g., chemical properties of soil, plant root exudates, plant development stage, and structure of indigenous microbiota of treated plants and soil type (Manfredini et al., 2021). In conclusion, it should also be noted that shifting the native microbiota in the right direction by PGPB may be one of the key factors in suppressing the phytopathogens (Chowdhury et al., 2015).
8. Conclusion
Bacillus pumilus is capable of producing lipopeptides, hydrolytic enzymes (e.g., chitinase and cellulase), and VOCs, and importantly is involved in triggering ISR. Thus, this species is a very good PGPB exhibiting a number of beneficial traits that are used to biocontrol a large number of fungal phytopathogens. However, there is still not a lot of studies using the strains on plants, particularly with the emphasis on studies in field conditions. Moreover, the mechanism of ISR elicitation by B. pumilus and other members of Bacillus spp. is still not fully elucidated. Based on the current knowledge, future research addressing this issue should focus specifically on the ISR induction by antibiotic lipopeptides, especially surfactin, which was detected in large quantities around the roots after the inoculation of B. amyloliquefaciens. Besides, due to the multitude of factors determining the composition of the native plant and soil microbiota, there is also a need for further research to determine the impact of B. pumilus on the native microbiota and its survival in bulk soil, rhizosphere, and plant tissues. Furthermore, studies profiling the transcriptome should be carried out in parallel, which would assess the RNA occurrence of key biocontrol agents in response to B. pumilus application. These studies should be conducted with recommended NGS sequencing techniques for the evaluation of microbiota after inoculation and qPCR for post-inoculation monitoring. This approach will allow a deeper insight into the relationship between the native microbiota and the inoculant and find some patterns which may have a direct impact on the efficiency of B. pumilus in biocontrol. Importantly, already commercialized strains should also be studied in order to make future formulations more effective and safe for the native microbiota.
Author contributions
JD contributed to the conception of the review. JD, ZJ, IK, PK, and KK wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This review was supported by a grants of Medical University of Bialystok no: B.SUB.23.359, SUB1/NN/22/001/2201 and the National Science Center, Poland project OPUS2021/43/B/NZ7/0 1903.
Acknowledgments
Many thanks to Katarzyna Rafalska for help in revising the English language.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, A., Khan, S. U., Khan, W. U., Saleh, T. A., Khan, M. H. U., Ullah, S., et al. (2019). Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: alternatives to chemical pesticides. C. R. Biol. 342, 124–135. doi: 10.1016/j.crvi.2019.05.002
Agarwal, M., Dheeman, S., Dubey, R. C., Kumar, P., Maheshwari, D. K., and Bajpai, V. K. (2017). Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol. Res. 205, 40–47. doi: 10.1016/j.micres.2017.08.012
Alam, M., Mizutani, T., Isono, M., Nikaidou, N., and Watanabe, T. (1996). Three chitinase genes (chiA, chiC and chiD) comprise the chitinase system of Bacillus circulans WL-12. J. Ferment. Bioeng. 82, 28–36. doi: 10.1016/0922-338X(96)89450-5
Andersen, R. A., Hamiltonkemp, T. R., Hildebrand, D. F., Mccracken, C. T., Collins, R. W., and Fleming, P. D. (1994). Structure-antifungal activity relationships among volatile C-6 and C-9 aliphatic-aldehydes, ketones, and alcohols. J. Agric. Food Chem. 42, 1563–1568. doi: 10.1021/jf00043a033
Ansari, F. A., and Ahmad, I. (2019). Isolation, functional characterization and efficacy of biofim forming rhizobacteria under abiotic stress conditions. Anton. Leeuw. Int. J. G. 112, 1827–1839. doi: 10.1007/s10482-019-01306-3
Arrebola, E., Sivakumar, D., and Korsten, L. (2010). Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control 53, 122–128. doi: 10.1016/j.biocontrol.2009.11.010
Asad, S. A. (2022). Mechanisms of action and biocontrol potential of trichoderma against fungal plant diseases-a review. Ecol. Complexity 49:100978. doi: 10.1016/j.ecocom.2021.100978
Ayaz, M., Ali, Q., Farzand, A., Khan, A. R., Ling, H., and Gao, X. (2021). Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against meloidogyne incognita. Int. J. Mol. Sci. 22:5049. doi: 10.3390/ijms22095049
Azabou, M. C., Gharbi, Y., Medhioub, I., Ennouri, K., Barham, H., Tounsi, S., et al. (2020). The endophytic strain Bacillus velezensis OEE1: an efficient biocontrol agent against Verticillium wilt of olive and a potential plant growth promoting bacteria. Biol. Control 142:104168. doi: 10.1016/j.biocontrol.2019.104168
Azizoglu, Z., Yilmaz, S., Azizoğlu, U., Karaborklu, S., Temizgül, R., and Ayvaz, A. (2021). Molecular characterization of the chitinase genes of native Bacillus thuringiensis isolates and their antagonistic activity against three important phytopathogenic fungi. Biologia 76, 2745–2755. doi: 10.1007/s11756-021-00802-0
Basu, A., Prasad, P., Das, S. N., Kalam, S., Sayyed, R., Reddy, M., et al. (2021). Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13:1140. doi: 10.3390/su13031140
Bělonožníková, K., Hýsková, V., Vašková, M., Křížek, T., Čokrtová, K., Vaněk, T., et al. (2022). Seed Protection of Solanum lycopersicum with Pythium oligandrum against Alternaria brassicicola and Verticillium albo-atrum. Microorganisms 10:1348. doi: 10.3390/microorganisms10071348
Bhat, M. A., Mishra, A. K., Jan, S., Bhat, M. A., Kamal, M. A., Rahman, S., et al. (2023). Plant growth promoting Rhizobacteria in plant health: a perspective study of the underground interaction. Plants 12:629. doi: 10.3390/plants12030629
Bhattacharya, S., Das, A., Samadder, S., and Rajan, S. S. (2016). Biosynthesis and characterization of a thermostable, alkali-tolerant chitinase from Bacillus pumilus JUBCH08 displaying antagonism against phytopathogenic Fusarium oxysporum. 3 Biotech 6:87. doi: 10.1007/s13205-016-0406-x
Bhattacharyya, P. N., and Jha, D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microb. Biot. 28, 1327–1350. doi: 10.1007/s11274-011-0979-9
Bouchard-Rochette, M., Machrafi, Y., Cossus, L., Thuy An Nguyen, T., Antoun, H., Droit, A., et al. (2022). Bacillus pumilus PTB180 and Bacillus subtilis PTB185: production of lipopeptides, antifungal activity, and biocontrol ability against Botrytis cinerea. Biol. Control. 170:104925. doi: 10.1016/j.biocontrol.2022.104925
Branda, S. S., Chu, F., Kearns, D. B., Losick, R., and Kolter, R. (2006). A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59, 1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x
Branda, S. S., Gonzalez-Pastor, J. E., Dervyn, E., Ehrlich, S. D., Losick, R., and Kolter, R. (2004). Genes involved in formation of structured multicellular communities by Bacillus subtilis. J. Bacteriol. 186, 3970–3979. doi: 10.1128/JB.186.12.3970-3979.2004
Buzzini, P., Martini, A., Cappelli, F., Pagnoni, U. M., and Davoli, P. A. (2003). Study on volatile organic compounds (VOCS) produced by tropical Ascomycetous yeasts. Antonie Leeuwenhoek 84, 301–311. doi: 10.1023/A:1026064527932
Carvalhais, L. C., Dennis, P. G., Badri, D. V., Kidd, B. N., Vivanco, J. M., and Schenk, P. M. (2015). Linking jasmonic acid signaling, root exudates, and rhizosphere microbiomes. Mol. Plant Microbe Interact. 28, 1049–1058. doi: 10.1094/MPMI-01-15-0016-R
Castaldi, S., Petrillo, C., Donadio, G., Piaz, F. D., Cimmino, A., Masi, M., et al. (2021). Plant growth promotion function of Bacillus sp. strains isolated from salt-pan rhizosphere and their biocontrol potential against macrophomina phaseolina. Int. J. Mol. Sci. 22:3324. doi: 10.3390/ijms22073324
Chatterjee, S., Kuang, Y., Splivallo, R., Chatterjee, P., and Karlovsky, P. (2016). Interactions among filamentous fungi Aspergillus niger, Fusarium verticillioides and Clonostachys rosea: fungal biomass, diversity of secreted metabolites and fumonisin production. BMC Microbiol. 16:83. doi: 10.1186/s12866-016-0698-3
Chauhan, A. K., Maheshwari, D. K., Kim, K., and Bajpai, V. K. (2016). Termitarium-inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 colonizing Curcuma longa L.: Isolation, characterization, and evaluation of their biocontrol and plant-growth-promoting activities. Can. J. Microbiol. 62, 880–892. doi: 10.1139/cjm-2016-0249
Chen, X. H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., et al. (2007). Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 25, 1007–1014. doi: 10.1038/nbt1325
Chowdhury, S., Hartmann, A., Gao, X., and Borriss, R. (2015). Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 – a review. Front. Microbiol. 6:780. doi: 10.3389/fmicb.2015.00780
Choudhary, D. K., and Johri, B. N. (2009). Interactions of Bacillus spp. and plants–with special reference to induced systemic resistance (ISR). Microbiol. Res. 164, 493–513. doi: 10.1016/j.micres.2008.08.007
Danişmazoğlu, M., Demir, I., Sezen, K., Muratoğlu, H., and Nalcacioglu, R. (2015). Cloning and Expression of chitinase A, B, and C (ChiA, ChiB, ChiC) genes from Serratia marcescens originating from Helicoverpa armigera and determining their activities. Turk. J. Biol. 39, 78–87. doi: 10.3906/biy-1404-31
Debois, D., Jourdan, E., Smargiasso, N., Thonart, P., de Pauw, E., and Ongena, M. (2014). Spatiotemporal monitoring of the antibiome secreted by Bacillus biofilms on plant roots using MALDI Mass Spectrometry imaging. Anal. Chem. 86, 4431–4438. doi: 10.1021/ac500290s
Díaz-Díaz, M., Bernal-Cabrera, A., Trapero, A., Medina-Marrero, R., Sifontes-Rodríguez, S., Cupull-Santana, R. D., et al. (2022). Characterization of Actinobacterial Strains as potential biocontrol agents against Macrophomina phaseolina and Rhizoctonia solani, the main soil-borne pathogens of phaseolus vulgaris in Cuba. Plants 11:645. doi: 10.3390/plants11050645
Diehl, A., Roske, Y., Ball, L., Chowdhury, A., Hiller, M., Molière, N., et al. (2018). Structural changes of TasA in biofilm formation of Bacillus subtilis. Proc. Natl. Acad. Sci. U.S. A. 115, 3237–3242. doi: 10.1073/pnas.1718102115
Dimkić, I., Janakiev, T., Petrović, M., Degrassi, G., and Fira, D. (2022). Plant-Associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms - A review. Physiol. Mol. Plant. Pathol. 117:101754. doi: 10.1016/j.pmpp.2021.101754
Djonovic, S., Vittone, G., Mendoza-Herrera, A., and Kenerley, C. M. (2007). Enhanced biocontrol activity of Trichoderma virens transformants constitutively coexpressing beta-1,3- and beta-1,6-glucanase genes. Mol. Plant Pathol. 8, 469–480. doi: 10.1111/j.1364-3703.2007.00407.x
Dobrzyński, J., Jakubowska, Z., and Dybek, B. (2022a). Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 13:1069053. doi: 10.3389/fmicb.2022.1069053
Dobrzyński, J., Wróbel, B., and Górska, E. B. (2022b). Cellulolytic properties of a potentially lignocellulose-degrading Bacillus sp. 8E1A strain isolated from bulk soil. Agronomy 12:665. doi: 10.3390/agronomy12030665
Dobrzyński, J., Wierzchowski, P., Stȩpien, W., and Górska, E. B. (2021). The reaction of cellulolytic and potentially cellulolytic spore-forming bacteria to various types of crop management and farmyard manure fertilization in bulk soil. Agronomy 11:772. doi: 10.3390/agronomy11040772
Doehlemann, G., Ökmen, B., Zhu, W., and Sharon, A. (2017). Plant pathogenic fungi. Microbiol. Spectr. 5, FUNK–0023–2016. doi: 10.1128/microbiolspec.FUNK-0023-2016
Dos Santos, R. M., Cueva-Yesquén, L. G., Garboggini, F. F., Desoignies, N., and Rigobelo, E. C. (2022). Inoculum concentration and mineral fertilization: effects on the endophytic microbiome of soybean. Front. Microbiol. 13:900980. doi: 10.3389/fmicb.2022.900980
Durán, P., Thiergart, T., Garrido-Oter, R., Agler, M., Kemen, E., Schulze-Lefert, P., et al. (2018). Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 175, 973–983. doi: 10.1073/pnas.1414592112
Dutta, S., and Podile, A. R. (2010). Plant growth-promoting rhizobacteria (PGPR): the bugs to debug the root zone. Crit. Rev. Microbiol. 36, 232–244. doi: 10.3109/10408411003766806
El Arbi, A., Rochex, A., Chataigné, G., Béchet, M., Lecouturier, D., Arnauld, S., et al. (2016). The Tunisian oasis ecosystem is a source of antagonistic Bacillus spp. producing diverse antifungal lipopeptides. Res. Microbiol. 167, 46–57.
Elkahoui, S., Djebali, N., Yaich, N., Azaiez, S., Hammami, M., Essid, R., et al. (2015). Antifungal activity of volatile compounds-producing Pseudomonas P2 strain against Rhizoctonia solani. World J. Microbiol. Biotechnol. 31, 175–185. doi: 10.1007/s11274-014-1772-3
Enebak, S. A., and Carey, W. A. (2000). Evidence for induced systemic protection to fusiform rust in loblolly pine by plant growth-promoting rhizobacteria. Plant Dis. 84, 306–308. doi: 10.1094/PDIS.2000.84.3.306
Essghaier, B., Bejji, M., Jijakli, H., Boudabous, A., and Sadfi-Zouaoui, N. (2009). High salt-tolerant protease from a potential biocontrol agent Bacillus pumilus M3-16. Ann. Microbiol. 59, 553–558. doi: 10.1007/BF03175145
Fatima, I., Hakim, S., Imran, A., Ahmad, N., Imtiaz, M., Ali, H., et al. (2022). Exploring biocontrol and growth-promoting potential of multifaceted PGPR isolated from natural suppressive soil against the causal agent of chickpea Wilt. Microbiol. Res. 260:127015. doi: 10.1016/j.micres.2022.127015
Fazle Rabbee, M., and Baek, K.-H. (2020). Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules 25:4973. doi: 10.3390/molecules25214973
Ferrusquía-Jiménez, N. I., González-Arias, B., Rosales, A., Esquivel, K., Escamilla-Silva, E. M., Ortega-Torres, A. E., et al. (2022). Elicitation of Bacillus cereus-Amazcala (B.c-A) with SiO2 nanoparticles improves its role as a plant growth-promoting bacteria (PGPB) in chili pepper plants. Plants 11:3445. doi: 10.3390/plants11243445
Fialho, M. B., De Moraes, M. H. D., Tremocoldi, A. R., and Pascholati, S. F. (2011). Potential of antimicrobial volatile organic compounds to control Sclerotinia sclerotiorum in bean seeds. Pesqui. Agropecu. Bras. 46, 137–142. doi: 10.1590/S0100-204X2011000200004
Flemming, H. C., and Wingender, J. (2010). The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. doi: 10.1038/nrmicro2415
Flemming, H. C., and Wuertz, S. (2019). Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 17, 247–260. doi: 10.1038/s41579-019-0158-9
Fujita, M., González-Pastor, J. E., and Losick, R. (2005). High and low threshold genes in the Spo0A regulon of Bacillus subtilis. J. Bacteriol. 187, 1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005
Gao, T., Foulston, L., Chai, Y., Wang, Q., and Losick, R. (2015). Alternative modes of biofilm formation by plant-associated Bacillus cereus. Microbiologyopen 4, 452–464. doi: 10.1002/mbo3.251
Garbeva, P., Hordijk, C., Gerards, S., and de Boer, W. (2014). Volatiles produced bythe mycophagous soil bacterium collimonas. FEMS Microbiol. Ecol. 87, 639–649. doi: 10.1111/1574-6941.12252
Ghasemi, S., Ahmadian, G., Jelodar, N. B., Rahimian, H., Ghandili, S., Dehestani, A., et al. (2010). Antifungal Chitinases from Bacillus Pumilus SG2: preliminary report. World J. Microbiol. Biotechnol. 26, 1437–1443. doi: 10.1007/s11274-010-0318-6
Gomaa, E. Z. (2012). Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: their potential in antifungal biocontrol. J. Microbiol. 50, 103–111. doi: 10.1007/s12275-012-1343-y
Gómez-de la Cruz, I., Guillén-Navarro, K., Huerta-Palacios, G., García-Fajardo, L. V., and Martínez-Bolaños, M. (2022). Enzyme Activity of three mycoparasite isolates and their effect on coffee leaf rust (Hemileia vastatrix Berk. & Br.). Symbiosis 88, 47–59. doi: 10.1007/s13199-022-00885-6
Gotor-Vila, A., Teixidó, N., Di Francesco, A., Usall, J., Ugolini, L., Torres, R., et al. (2017). Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 64, 219–225. doi: 10.1016/j.fm.2017.01.006
Gurav, R., Tang, J., and Jadhav, J. (2017). Novel chitinase producer Bacillus pumilus RST25 isolated from the shellfish processing industry revealed antifungal potential against phyto-pathogens. Int. Biodeterior. Biodegradation. 125, 228–234. doi: 10.1016/j.ibiod.2017.09.015
Hamilton, J. J., Marlow, V. L., Owen, R. A., Costa, M., de, A. A., Guo, M., et al. (2014). Holin and an endopeptidase are essential for chitinolytic protein secretion in Serratia Marcescens. J. Cell. Biol. 207, 615–626. doi: 10.1083/jcb.201404127
Hashem, A., Tabassum, B., and Allah, E. F. A. (2019). Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 26, 1291–1297. doi: 10.1016/j.sjbs.2019.05.004
Hassan, N., Nakasuji, S., Elsharkawy, M. M., Naznin, H. A., Kubota, M., Ketta, H., et al. (2017). Biocontrol potential of an endophytic Streptomyces sp. strain MBCN152-1 against Alternaria brassicicola on cabbage plug seedlings. Microb. Environ. 32, 133–141. doi: 10.1264/jsme2.ME17014
He, C.-N., Ye, W.-Q., Zhu, Y.-Y., and Zhou, W.-W. (2020). Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules 25:3360. doi: 10.3390/molecules25153360
Heyi, E. A., Dinka, M. O., and Mamo, G. (2022). Assessing the impact of climate change on water resources of upper awash river sub-basin, Ethiopia. J. Water Land Dev. 52, 232–244. doi: 10.24425/jwld.2022.140394
Huang, Q., Zhang, Z., Liu, Q., Liu, F., Liu, Y., Zhang, J., et al. (2021). SpoVG is an important regulator of sporulation and affects biofilm formation by regulating Spo0A transcription in Bacillus cereus 0–9. BMC Microbiol. 21:172. doi: 10.1186/s12866-021-02239-6
Huang, X., Zhang, N., Yong, X., Yang, X., and Shen, Q. (2012). Biocontrol of Rhizoctonia Solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol. Res. 167, 135–143. doi: 10.1016/j.micres.2011.06.002
Islam, T., Rabbee, M. F., Choi, J., and Baek, K. H. (2022). Biosynthesis, molecular regulation, and application of bacilysin produced by Bacillus species. Metabolites 12:397. doi: 10.3390/metabo12050397
Jeong, H., Choi, S. K., Kloepper, J. W., and Ryu, C. M. (2014). Genome sequence of the plant endophyte Bacillus pumilus INR7, triggering induced systemic resistance in field crops. Genome. Announc. 2:e01093-14. doi: 10.1128/genomeA.01093-14
Juturu, V., and Wu, J. C. (2014). Microbial cellulases: engineering production and applications. Renew. Sustain. Energy Rev. 33, 188–203. doi: 10.1016/j.rser.2014.01.077
Kang, Y., Shen, M., Wang, H., and Zhao, Q. A. (2013). Possible mechanism of action of plant growth-promoting rhizobacteria (PGPR) strain Bacillus pumilus WP8 via regulation of soil bacterial community structure. J. Gen. Appl. Microbiol. 59, 267–277. doi: 10.2323/jgam.59.267
Kaur, M., Vyas, P., Rahi, P., and Sharma, S. (2022). Chlorpyrifos and carbofuran-tolerant phosphate-solubilising Arthrobacter oxydans and Bacillus flexus improved growth and phosphorus content in potato in pesticide-amended soils. Potato. Res. 65, 213–231. doi: 10.1007/s11540-021-09520-1
Kaushal, M., Kumar, A., and Kaushal, R. (2017). Bacillus pumilus strain YSPMK11 as plant growth promoter and bicontrol agent against Sclerotinia sclerotiorum. 3. Biotech. 7:90. doi: 10.1007/s13205-017-0732-7
Kearns, D. B., Chu, F., Branda, S. S., Kolter, R., and Losick, R. (2005). A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 55, 739–749. doi: 10.1111/j.1365-2958.2004.04440.x
Khan, A. R., Mustafa, A., Hyder, S., Valipour, M., Rizvi, Z. F., Gondal, A. S., et al. (2022). Bacillus spp. as Bioagents: uses and application for sustainable agriculture. Biology 11:1763. doi: 10.3390/biology11121763
Khan, N., Ali, S., Shahid, M. A., Mustafa, A., Sayyed, R. Z., and Curá, J. A. (2021). Insights into the Interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells 10:1551. doi: 10.3390/cells10061551
Khatoon, Z., Orozco-Mosqueda, C., Huang, S., Nascimento, F. X., and Santoyo, G. (2022). “Peptide antibiotics produced by Bacillus species: first line of attack in the biocontrol of plant diseases,” in Bacilli in agrobiotechnology, eds M. Tofazzal Islam, M. Rahman, and P. Pandey (Cham: Springer), 31–46. doi: 10.1007/978-3-030-85465-2_2
Kim, S. Y., Lee, S. Y., Weon, H. Y., Sang, M. K., and Song, J. (2017). Complete genome sequence of Bacillus velezensis M75, a biocontrol agent against fungal plant pathogens, isolated from cotton waste. J. Biotechnol. 241, 112–115. doi: 10.1016/j.jbiotec.2016.11.023
Köhl, J., Kolnaar, R., and Ravensberg, W. J. (2019). Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front. Plant Sci. 10:845. doi: 10.3389/fpls.2019.00845
Krylov, V. B., Petruk, M. I., Glushko, N. I., Khaldeeva, E. V., Mokeeva, V. L., Bilanenko, E. N., et al. (2018). Carbohydrate specificity of antibodies against phytopathogenic fungi of the Aspergillus genus. Appl. Biochem. Microbiol. 54, 522–527. doi: 10.1134/S0003683818050095
Kulkova, I., Dobrzyński, J., Kowalczyk, P., Bełżecki, G., and Kramkowski, K. (2023). Plant growth promotion using Bacillus cereus. Int. J. Mol. Sci. 24:9759. doi: 10.3390/ijms24119759
Kumar, M., Chakdar, H., Pandiyan, K., Thapa, S., Shahid, M., Singh, A., et al. (2022). Bacterial chitinases: genetics, engineering and applications. World. J. Microbiol. Biotechnol. 38:252. doi: 10.1007/s11274-022-03444-9
Labiadh, M., Dhaouadi, S., Chollet, M., Chataigne, G., Tricot, C., Jacques, P., et al. (2021). Antifungal lipopeptides from Bacillus strains isolated from rhizosphere of citrus trees. Rhizosphere 19:100399. doi: 10.1016/j.rhisph.2021.100399
Lahlali, R., Ezrari, S., Radouane, N., Kenfaoui, J., Esmaeel, Q., el Hamss, H., et al. (2022). Biological control of plant pathogens: a global perspective. Microorganisms 10:596. doi: 10.3390/microorganisms10030596
Lee, H. J., Park, K. C., Lee, S. H., Bang, K. H., Park, H. W., Hyun, D. Y., et al. (2012). Screening of antifungal Bacillus Spp. against Alternaria blight pathogen (Alternaria Panax) and Anthracnose pathogen (Colletotrichum gloeosporioides) of ginseng. Korean J. Med. Crop. Sci. 20, 339–344. doi: 10.7783/KJMCS.2012.20.5.339
Li, W., Lee, S. Y., Cho, Y. J., Ghim, S. Y., and Jung, H. Y. (2020). Mediation of induced systemic resistance by the plant growth-promoting rhizobacteria Bacillus Pumilus S2-3-2. Mol. Biol. Rep. 47, 8429–8438. doi: 10.1007/s11033-020-05883-9
Li, Y., and Chen, S. (2019). Fusaricidin produced by Paenibacillus Polymyxa WLY78 induces systemic resistance against Fusarium Wilt of cucumber. Int. J. Mol. Sci. 20:5240. doi: 10.3390/ijms20205240
Liu, K., Newman, M., McInroy, J. A., Hu, C. H., and Kloepper, J. W. (2017). Selection and assessment of plant growth promoting rhizobacteria for biological control of multiple plant diseases. Phytopathology 107, 928–936. doi: 10.1094/phyto-02-17-0051-r
Liu, W. W., Wei, M., Zhu, B. Y., Du, Y. C., and Feng, L. (2008). Antagonistic activities of volatiles from four strains of Bacillus spp. and Paenibacillus spp. against soil-borne plant pathogens. Agric. Sci. China 7, 1104–1114. doi: 10.1016/S1671-2927(08)60153-4
Luo, C., Liu, X., Zhou, H., Wang, X., and Chen, Z. (2015). Nonribosomal peptide synthase gene clusters for lipopeptide biosynthesis in Bacillus subtilis 916 and their phenotypic functions. Appl. Environ. Microbiol. 81, 422–431. doi: 10.1128/AEM.02921-14
Maksimova, E. M., Vinogradova, D. S., Osterman, I. A., Kasatsky, P. S., Nikonov, O. S., Milón, P., et al. (2021). Multifaceted mechanism of amicoumacin a inhibition of bacterial translation. Front. Microbiol. 12:618857. doi: 10.3389/fmicb.2021.618857
Manfredini, A., Malusà, E., Costa, C., Pallottino, F., Mocali, S., Pinzari, F., et al. (2021). Current methods, common practices, and perspectives in tracking and monitoring bioinoculants in soil. Front. Microbiol. 12:698491. doi: 10.3389/fmicb.2021.698491
Mannaa, M., and Kim, K. D. (2018). Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: study II. Mycobiology 46, 52–63. doi: 10.1080/12298093.2018.1454015
Martínez-Zavala, S. A., Barboza-Pérez, U. E., Hernández-Guzmán, G., Bideshi, D. K., and Barboza-Corona, J. E. (2020). Chitinases of Bacillus thuringiensis: phylogeny, modular structure, and applied potentials. Front. Microbiol. 10:3032. doi: 10.3389/fmicb.2019.03032
Meena, K. R., and Kanwar, S. S. (2015). Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. Biomed. Res. Int. 2015:473050. doi: 10.1155/2015/473050
Mhatre, P. H., Karthik, C., Kadirvelu, K., Divya, K. L., Venkatasalam, E. P., Srinivasan, S., et al. (2018). Plant growth promoting rhizobacteria (PGPR): a potential alternative tool for nematodes bio-control. Biocatal. Agricult. Biotechnol. 17, 119–128. doi: 10.1016/j.bcab.2018.11.009
Mielich-Süss, B., and Lopez, D. (2015). Molecular mechanisms involved in Bacillus subtilis biofilm formation. Environ. Microbiol. 17, 555–565. doi: 10.1111/1462-2920.12527
Miljaković, D., Marinković, J., and Balešević-Tubić, S. (2020). The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8:1037. doi: 10.3390/microorganisms8071037
Miljaković, D., Marinković, J., Tamindžić, G., Ðorđević, V., Tintor, B., Milošević, D., et al. (2022). Bio-priming of soybean with Bradyrhizobium japonicum and Bacillus Megaterium: strategy to improve seed germination and the initial seedling growth. Plants 11:1927. doi: 10.3390/plants11151927
Minerdi, D., Bossi, S., Gullino, M. L., and Garibaldi, A. (2009). Volatile organic compounds: a potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain Msa 35. Environ. Microbiol. 11, 844–854. doi: 10.1111/j.1462-2920.2008.01805.x
Mirskaya, G. V., Khomyakov, Y. V., Rushina, N. A., Vertebny, V. E., Chizhevskaya, E. P., Chebotar, V. K., et al. (2022). Plant development of early-maturing spring wheat (Triticum aestivum L.) under inoculation with Bacillus sp. V2026. Plants 11:1817. doi: 10.3390/plants11141817
Montanarella, L., and Panagos, P. (2021). The relevance of sustainable soil management within the European Green Deal. Land Use Policy 100:104950. doi: 10.1016/j.landusepol.2020.104950
Morales-Cedeño, L. R., Orozco-Mosqueda, M., del, C., Loeza-Lara, P. D., Parra-Cota, F. I., de los Santos-Villalobos, S., et al. (2021). Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: fundamentals, methods of application and future perspectives. Microbiol. Res. 242:126612. doi: 10.1016/j.micres.2020.126612
Morita, T., Tanaka, I., Ryuda, N., Ikari, M., Ueno, D., and Someya, T. (2019). Antifungal spectrum characterization and identification of strong volatile organic compounds produced by Bacillus pumilus TM-R. Heliyon 5:e01817. doi: 10.1016/j.heliyon.2019.e01817
Nicholson, W. L. (2002). Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 59, 410–416. doi: 10.1007/s00018-002-8433-7
Nicholson, W. L., Munakata, N., Horneck, G., Melosh, H. J., and Setlow, P. (2000). Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64, 548–572. doi: 10.1128/mmbr.64.3.548-572.2000
Nie, P., Chen, C., Yin, Q., Jiang, C., Guo, J., Zhao, H., et al. (2019). Function of MiR825 and MiR825* as negative regulators in Bacillus cereus AR156-Elicited systemic resistance to Botrytis Cinerea in Arabidopsis thaliana. Int. J. Mol. Sci. 20:5032. doi: 10.3390/ijms20205032
Nihorimbere, V., Cawoy, H., Seyer, A., Brunelle, A., Thonart, P., and Ongena, M. (2012). Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol. Ecol. 79, 176–191. doi: 10.1111/j.1574-6941.2011.01208.x
Nikolić, I., Berić, T., Dimkić, I., Popović, T., Lozo, J., Fira, D., et al. (2019). Biological control of Pseudomonas syringae pv. aptata on sugar beet with Bacillus pumilus SS-10.7 and Bacillus amyloliquefaciens (SS-12.6 and SS-38.4) strains. J. Appl. Microbiol. 126, 165–176. doi: 10.1111/jam.14070
Ojuederie, O. B., Olanrewaju, O. S., and Babalola, O. O. (2019). Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: implications for sustainable agriculture. Agronomy 9:712. doi: 10.3390/agronomy9110712
Özcengiz, G., and Ögulur, I. (2015). Biochemistry, genetics and regulation of bacilysin biosynthesis and its significance more than an antibiotic. N. Biotechnol. 32, 612–619. doi: 10.1016/j.nbt.2015.01.006
Padaria, J. C., Tarafdar, A., Raipuria, R., Lone, S. A., Gahlot, P., Shakil, N. A., et al. (2016). Identification of phenazine-1-carboxylic acid gene (phc CD) from Bacillus pumilus MTCC7615 and its role in antagonism against Rhizoctonia solani. J. Basic Microbiol. 56, 999–1008. doi: 10.1002/jobm.201500574
Pascale, A., Proietti, S., Pantelides, I. S., and Stringlis, I. A. (2020). Modulation of the root microbiome by plant molecules: the basis for targeted disease suppression and plant growth promotion. Front. Plant Sci. 10:1741. doi: 10.3389/fpls.2019.01741
Penha, R. O., Vandenberghe, L. P. S., Faulds, C., Soccol, V. T., and Soccol, C. R. (2020). Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: recent studies and innovations. Planta 251:70. doi: 10.1007/s00425-020-03357-7
Pieterse, C. M. J., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C. M., and Bakker, P. A. H. M. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. doi: 10.1146/annurev-phyto-082712-102340
Poveda, J. (2021). Beneficial effects of microbial volatile organic compounds (MVOCs) inplants. Appl. Soil Ecol. 168:104118. doi: 10.1016/j.apsoil.2021.104118
Prakash, J., and Arora, N. K. (2019). Phosphate-solubilizing Bacillus sp. enhances growth, phosphorus uptake and oil yield of Mentha arvensis. L. 3 Biotech. 9:126. doi: 10.1007/s13205-019-1660-5
Probanza, A., Mateos, J. L., Lucas Garcia, J. A., Ramos, B., De Felipe, M. R., and Gutierrez Manero, F. J. (2001). Effects of inoculation with PGPR Bacillus and Pisolithus tinctorius on Pinus pinea L. growth, bacterial rhizosphere colonization, and mycorrhizal infection. Microb. Ecol. 41, 140–148. doi: 10.1007/s00248000008
Pudova, D. S., Toymentseva, A. A., Gogoleva, N. E., Shagimardanova, E. I., Mardanova, A. M., and Sharipova, M. R. (2022). Comparative genome analysis of two Bacillus pumilus strains producing high level of extracellular hydrolases. Genes 13:409. doi: 10.3390/genes13030409
Raddadi, N., Cherif, A., Ouzari, H., Marzorati, M., Brusetti, L., Boudabous, A., et al. (2007). Bacillus thuringiensis beyond insect biocontrol: plant growth promotion and biosafety of polyvalent strains. Ann. Microbiol. 57, 481–494. doi: 10.1007/BF03175344
Ramos, B., Lucas García, J. A., Probanza, A., Domenech, J., and Javier Gutierrez Mañero, F. (2003). Influence of an indigenous European alder (Alnus glutinosa (L.) Gaertn) Rhizobacterium (Bacillus pumilus) on the growth of ader and its rhizosphere microbial community structure in two soils. New For. 25, 149–159. doi: 10.1023/A:1022688020897
Rashad, Y. M., and Moussa, T. A. (2020). “Biocontrol agents for fungal plant diseases management,” in Cottage industry of biocontrol agents and their applications, eds M. Saleh, M. Abu-hashim, and N. El-Wakeil (Cham: Springer), 337–363. doi: 10.1007/978-3-030-33161-0_11
Raupach, G. S., and Kloepper, J. W. (1998). Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 88, 1158–1164. doi: 10.1094/PHYTO.1998.88.11.1158
Ren, J. H., Li, H., Wang, Y. F., Ye, J. R., Yan, A. Q., and Wu, X. Q. (2013). Biocontrol potential of an endophytic Bacillus pumilus JK-SX001 against poplar canker. Biol. Control 67, 421–430. doi: 10.1016/j.biocontrol.2013.09.012
Rishad, K. S., Rebello, S., Shabanamol, P. S., and Jisha, M. S. (2017). Biocontrol potential of halotolerant bacterial chitinase from high yielding novel Bacillus pumilus MCB-7 autochthonous to mangrove ecosystem. Pestic. Biochem. Physiol. 137, 36–41. doi: 10.1016/j.pestbp.2016.09.005
Rojas-Solís, D., Zetter-Salmón, E., Contreras-Pérez, M., Rocha-Granados, M. D. C., Macías-Rodríguez, L., and Santoyo, G. (2018). Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 13, 46–52. doi: 10.1016/j.bcab.2017.11.007
Romera, F. J., García, M. J., Lucena, C., Martínez-Medina, A., Aparicio, M. A., Ramos, J., et al. (2019). Induced systemic resistance (ISR) and fe deficiency responses in dicot plants. Front. Plant. Sci. 10:287. doi: 10.3389/fpls.2019.00287
Saggese, A., De Luca, Y., Baccigalupi, L., and Ricca, E. (2022). An antimicrobial peptide specifically active against Listeria monocytogenes is secreted by Bacillus pumilus SF214. BMC Microbiol. 22:3. doi: 10.1186/s12866-021-02422-9
Samaras, A., Nikolaidis, M., Antequera-Gómez, M., Cámara-Almirón, J., Romero, D., Moschakis, T., et al. (2021). Whole genome sequencing and root colonization studies reveal novel insights in the biocontrol potential and growth promotion by Bacillus subtilis MBI 600 on cucumber. Front. Microbiol. 11:600393. doi: 10.3389/fmicb.2020.600393
Sansinenea, E., and Ortiz, A. (2011). Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 33, 1523–1538. doi: 10.1007/s10529-011-0617-5
Sarmiento-López, L. G., López-Meyer, M., Maldonado-Mendoza, I. E., Quiroz-Figueroa, F. R., Sepúlveda-Jiménez, G., and Rodríguez-Monroy, M. (2022). Production of indole-3-acetic acid by Bacillus circulans E9 in a low-cost medium in a bioreactor. J. Biosci. Bioeng. 134, 21–28. doi: 10.1016/j.jbiosc.2022.03.007
Schmidt, R., Cordovez, V., De Boer, W., Raaijmakers, J., and Garbeva, P. (2015). Volatile affairs in microbial interactions. ISME J. 9, 2329–2335. doi: 10.1038/ismej.2015.42
Schulz-Bohm, K., Martín-Sánchez, L., and Garbeva, P. (2017). Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 8:2484. doi: 10.3389/fmicb.2017.02484
Sha, Y., Zeng, Q., and Sui, S. (2020). Screening and application of Bacillus strains isolated from nonrhizospheric rice soil for the biocontrol of rice blast. Plant. Pathol. J. 36, 231–243. doi: 10.5423/PPJ.OA.02.2020.0028
Shahid, I., Han, J., Hanooq, S., Malik, K. A., Borchers, C. H., and Mehnaz, S. (2021). Profiling of metabolites of Bacillus spp. and their application in sustainable plant growth promotion and biocontrol. Front. Sustain. Food Syst. 5:605195. doi: 10.3389/fsufs.2021.605195
Shali, A., Ghasemi, S., Ahmadian, G., Ranjbar, G., Dehestani, A., Khalesi, N., et al. (2010). Bacillus pumilus SG2 chitinases induced and regulated by chitin, show inhibitory activity against Fusarium graminearum and bipolaris sorokiniana. Phytoparasitica 38, 141–147. doi: 10.1007/s12600-009-0078-8
Sharma, A., Kashyap, P. L., Srivastava, A. K., Bansal, Y. K., and Kaushik, R. (2019). Isolation and characterization of halotolerant bacilli from chickpea (Cicer Arietinum L.) rhizosphere for plant growth promotion and biocontrol traits. Eur J Plant Pathol 153, 787–800. doi: 10.1007/s10658-018-1592-7
Shoresh, M., Yedidia, I., and Chet, I. (2005). Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 95, 76–84. doi: 10.1094/PHYTO-95-0076
Siahmoshteh, F., Hamidi-Esfahani, Z., Spadaro, D., Shams-Ghahfarokhi, M., and Razzaghi-Abyaneh, M. (2018). Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 89, 300–307. doi: 10.1016/j.foodcont.2017.11.010
Son, J. S., Sumayo, M., Hwang, Y. J., Kim, B. S., and Ghim, S. Y. (2014). Screening of plant growth-promoting rhizobacteria as elicitor of systemic resistance against gray leaf spot disease in pepper. Appl. Soil. Ecol. 73, 1–8. doi: 10.1016/j.apsoil.2013.07.016
Stringlis, I. A., Yu, K., Feussner, K., de Jonge, R., Van Bentum, S., Van Verk, M. C., et al. (2018). MYB72-Dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. U.S.A. 115, E5213–E5222. doi: 10.1073/pnas.1722335115
Sun, Y. F., Liu, Z., Li, H. Y., Zheng, Z. H., Ji, C. L., Guo, Q., et al. (2021). Biocontrol effect and mechanism of Bacillus laterosporus Bl13 against early blight disease of tomato. Chinese J. Appl. Ecol. 32, 299–308. doi: 10.13287/j.1001-9332.202101.036
Sutherland, I. W. (2001). The biofilm matrix–an immobilized but dynamic microbial environment. Trends Microbiol. 9, 222–227. doi: 10.1016/s0966-842x(01)02012-2011
Tasharrofi, N., Adrangi, S., Fazeli, M., Rastegar, H., Khoshayand, M. R., and Faramarzi, M. A. (2011). Optimization of chitinase production by Bacillus pumilus using plackett-burman design and response surface methodology. Iran J. Pharm. Res. 10, 759–768.
Tjalsma, H., Stöver, A. G., Driks, A., Venema, G., Bron, S., and van Dijl, J. M. (2000). Conserved serine and histidine residues are critical for activity of the ER-type signal peptidase SipW of Bacillus subtilis. J. Org. Chem. 275, 25102–25108. doi: 10.1074/jbc.M002676200
Toral, L., Rodríguez, M., Béjar, V., and Sampedro, I. (2018). Antifungal activity of lipopeptides from Bacillus methylotrophicus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 9:1315. doi: 10.3389/fmicb.2018.01315
Toymentseva, A. A., Pudova, D. S., and Sharipova, M. R. (2019). Identification of secondary metabolite gene clusters in the genome of Bacillus pumilus strains 7P and 3-19. BioNanoScience 9, 313–316.
Tyc, O., Zweers, H., de Boer, W., and Garbeva, P. (2015). Volatiles in inter-specific bacterial interactions. Front. Microbiol. 6:1412. doi: 10.3389/fmicb.2015.01412
Tyśkiewicz, R., Nowak, A., Ozimek, E., and Jaroszuk-Ściseł, J. (2022). Trichoderma: the current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 23:2329. doi: 10.3390/ijms23042329
Wang, C., Wang, Z., Qiao, X., Li, Z., Li, F., Chen, M., et al. (2013). Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 341, 45–51. doi: 10.1111/1574-6968.12088
Wang, Y., Zhang, C., Liang, J., Wang, L., Gao, W., Jiang, J., et al. (2020). Surfactin and fengycin b extracted from Bacillus pumilus W-7 provide protection against potato late blight via distinct and synergistic mechanisms. Appl. Microbiol. Biotechnol. 104, 7467–7481. doi: 10.1007/s00253-020-10773-y
Wei, G., Kloepper, J. W., and Tuzun, S. (1996). Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Biol. Control. 86, 221–224.
Wierzchowski, P. S., Dobrzyński, J., Mazur, K., Kierończyk, M., Wardal, W. J., Sakowski, T., et al. (2021). Chemical properties and bacterial community reaction to acidified cattle slurry fertilization in soil from Maize cultivation. Agronomy 11:601. doi: 10.3390/agronomy11030601
Win, K. T., Oo, A. Z., Ohkama-Ohtsu, N., and Yokoyama, T. (2018). Bacillus pumilus strain TUAT-1 and nitrogen application in nursery phase promote growth of Rice plants under field conditions. Agron. J. 8, 216. doi: 10.3390/agronomy8100216
Wróbel, B., Zielewicz, W., Paszkiewicz-Jasińska, A., Spychalski, B., and Jakubowska, Z. (2022). Effect of harvest date on structural carbohydrates and lignin content in meadow sward in different pluvio-thermal conditions. J. Water Land Dev. 50, 60–66. doi: 10.24425/jwld.2022.142305
Wróbel, M., Śliwakowski, W., Kowalczyk, P., Kramkowski, K., and Dobrzyński, J. (2023). Bioremediation of heavy metals by the genus Bacillus. Int. J. Environ. Res. Public Health 20:4964. doi: 10.3390/ijerph20064964
Xu, Y. B., Chen, M., Zhang, Y., Wang, M., Wang, Y., Huang, Q. B., et al. (2014). The phosphotransferase system gene ptsI in the endophytic bacterium Bacillus cereus is required for biofilm formation, colonization, and biocontrol against wheat sharp eyespot. FEMS Microbiol. Lett. 354, 142–152. doi: 10.1111/1574-6968.12438
Yakovleva, G., Kurdy, W., Gorbunova, A., Khilyas, I., Lochnit, G., and Ilinskaya, O. (2022). Bacillus pumilus proteome changes in response to 2, 4, 6-trinitrotoluene-induced stress. Biodegradation 33, 593–607. doi: 10.1007/s10532-022-09997-8
Yin, J., Bai, R., Yuan, L., and Huang, J. (2023). Application of ceriporia Lacerata HG2011 as biocontrol agent against multiple phytopathogenic fungi and Oomycetes. Pesticide Biochem. Physiol. 190:105316. doi: 10.1016/j.pestbp.2022.105316
Yu, Y., Gui, Y., Li, Z., Jiang, C., Guo, J., and Niu, D. (2022). Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 11:386. doi: 10.3390/plants11030386
Yuan, J., Raza, W., Shen, Q., and Huang, Q. (2012). Antifungal activity of Bacillus amyloliquefaciens Njn-6 volatile compounds against Fusarium oxysporum f. sp. Cubense. Appl. Environ. Microbiol. 78, 5942–5944. doi: 10.1128/AEM.01357-12
Zhang, L. L., Cheng, Y. H., Ding, F. B., Liu, Y. Z., Liu, C. H., and Chen, Z. Y. (2010). Inhibition Effect of 2 Bacillus subtilis on Botryosphaeria berengeriana f. sp. piricola in vitro. Fruit Sci. 27, 823–827.
Zhang, S., Reddy, M. S., and Kloepper, J. W. (2004). Tobacco growth enhancement and blue mold disease protection by Rhizobacteria: relationship between plant growth promotion and systemic disease protection by PGPR strain 90-166. Plant. Soil. 262, 277–288. doi: 10.1023/B:PLSO.0000037048.26437.fa
Zhang, D. D., Wang, X. Y., Chen, J. Y., Kong, Z. Q., Gui, Y. J., Li, N. Y., et al. (2016). Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci. Rep. 6:27979. doi: 10.1038/srep27979
Zhang, S., Zheng, Q., Xu, B., and Liu, J. (2019). Identification of the fungal pathogens of postharvest disease on peach fruits and the control mechanisms of B. subtilis JK-14 strain. Toxins 11:322. doi: 10.3390/toxins11060322
Zheng, L., Situ, J. J., Zhu, Q. F., Xi, P. G., Zheng, Y., Liu, H. X., et al. (2019). Identification of volatile organic compounds for the biocontrol of postharvest litchi fruit pathogen Peronophythora litchii. Postharvest Biol. Technol. 155, 37–46. doi: 10.1016/j.postharvbio.2019.05.009
Zhou, D., Huang, X. F., Chaparro, J. M., Badri, D. V., Manter, D. K., Vivanco, J. M., et al. (2016). Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant. Soil. 401, 259–272. doi: 10.1007/s11104-015-2743-7
Zhu, L., Wang, Y., Lu, J., Liu, S., Min, Y., Liu, X., et al. (2021). Complete genome sequence of Bacillus badius NBPM-293, a plant growth-promoting strain isolated from rhizosphere soil. Am. Soc. Microbiol. 10:e0097721. doi: 10.1128/MRA.00977-21
Zhu, M. L., Wang, Y. H., Dai, Y., Wu, X. Q., and Ye, J. R. (2020a). Effects of different culture conditions on the biofilm formation of Bacillus pumilus HR10. Curr. Microbiol. 77, 1405–1411. doi: 10.1007/s00284-020-01944-1
Zhu, M. L., Wu, X. Q., Wang, Y. H., and Dai, Y. (2020b). Role of biofilm formation by Bacillus pumilus HR10 in biocontrol against pine seedling damping-off disease caused by Rhizoctonia solani. Forests 11:652. doi: 10.3390/f11060652
Zielewicz, W., Swȩdrzyński, A., Dobrzyński, J., Swȩdrzyńska, D., Kulkova, I., Wierzchowski, P. S., et al. (2021). Effect of forage plant mixture and biostimulants application on the yield, changes of botanical composition, and microbiological soil activity. Agronomy 11:1786. doi: 10.3390/agronomy11091786
Keywords: biological control, eco-friendly agent, spore-forming bacteria, Bacillus, pathogenic organisms
Citation: Dobrzyński J, Jakubowska Z, Kulkova I, Kowalczyk P and Kramkowski K (2023) Biocontrol of fungal phytopathogens by Bacillus pumilus. Front. Microbiol. 14:1194606. doi: 10.3389/fmicb.2023.1194606
Received: 27 March 2023; Accepted: 03 July 2023;
Published: 25 July 2023.
Edited by:
Jingping Ge, Heilongjiang University, ChinaReviewed by:
Oluwaseyi Samuel Olanrewaju, North-West University, South AfricaOrlando Borras-Hidalgo, Qilu University of Technology, China
Copyright © 2023 Dobrzyński, Jakubowska, Kulkova, Kowalczyk and Kramkowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jakub Dobrzyński, ai5kb2Jyenluc2tpQGl0cC5lZHUucGw=; Zuzanna Jakubowska, ei5qYWt1Ym93c2thQGl0cC5lZHUucGw=
 Jakub Dobrzyński1*
Jakub Dobrzyński1* Zuzanna Jakubowska
Zuzanna Jakubowska Paweł Kowalczyk
Paweł Kowalczyk Karol Kramkowski
Karol Kramkowski