
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 24 May 2023
Sec. Virology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1194431
This article is part of the Research TopicThe Association Between Viral Infection and Human CancersView all 11 articles
Background: Uterine Cervical Carcinoma (UCC) is the most prevalent gynecological malignancy globally, with a rising incidence in recent years. Accumulating evidence indicates that specific viral infections, including human papillomavirus (HPV), Epstein-Barr virus (EBV), Hepatitis B and C viruses (HBV and HCV), and human herpesvirus (HHV), may contribute to UCC development and progression. Understanding the complex interplay between viral infections and UCC risk is crucial for developing novel preventative and therapeutic interventions.
Methods: This comprehensive review investigates the association between viral infections and UCC risk by examining the roles of various viral pathogens in UCC etiology and pathogenesis, and possible molecular mechanisms. Additionally, we evaluate current diagnostic methods and potential therapeutic strategies targeting viral infections for UCC prevention or treatment.
Results: The prevention of UCC has been significantly advanced by the emergence of self-sampling for HPV testing as a crucial tool, allowing for early detection and intervention. However, an essential challenge in UCC prevention lies in understanding how HPV and other viral coinfections, including EBV, HBV, HCV, HHV, HIV, or their concurrent presence, may potentially contribute to UCC development. The molecular mechanisms implicated in the association between viral infections and cervical cancer development include: (1) interference of viral oncogenes with cellular regulatory proteins, resulting in uncontrolled cell proliferation and malignant transformation; (2) inactivation of tumor suppressor genes by viral proteins; (3) evasion of host immune responses by viruses; (4) induction of a persistent inflammatory response, contributing to a tumor-promoting microenvironment; (5) epigenetic modifications that lead to aberrant gene expression; (6) stimulation of angiogenesis by viruses; and (7) activation of telomerase by viral proteins, leading to cellular immortalization. Additionally, viral coinfections can also enhance oncogenic potential through synergistic interactions between viral oncoproteins, employ immune evasion strategies, contribute to chronic inflammation, modulate host cellular signaling pathways, and induce epigenetic alterations, ultimately leading to cervical carcinogenesis.
Conclusion: Recognizing the implications of viral oncogenes in UCC etiology and pathogenesis is vital for addressing the escalating burden of UCC. Developing innovative preventative and therapeutic interventions requires a thorough understanding of the intricate relationship between viral infections and UCC risk.
Uterine cervical carcinoma (UCC) remains a major global health concern, accounting for significant morbidity and mortality in women worldwide. Despite considerable research efforts and advances in screening and prevention strategies, UCC continues to pose a substantial public health challenge, particularly in low- and middle-income countries (Srinath et al., 2022; Ton et al., 2022; Dau et al., 2023; Tin et al., 2023). A comprehensive understanding of the etiological factors underlying the development of UCC is crucial for devising improved prevention, early detection, and treatment strategies. Although extensive research has been conducted on the etiology and pathogenesis of UCC, the precise mechanisms underlying its development are not yet fully understood. Various factors, such as age (Basoya and Anjankar, 2022; Yuan et al., 2023), obesity (Frumovitz et al., 2014; Coffey et al., 2016; Sassenou et al., 2021; Bohn et al., 2022), hormonal imbalances (Zidi et al., 2020; Iversen et al., 2021; Lasche et al., 2022), genetic predisposition (Chandra et al., 2022; Liu et al., 2022b; Yadav et al., 2023a), and environmental exposures (Kycler et al., 2017; Korsakov et al., 2022), have been implicated in UCC development. Recently, a growing body of evidence has highlighted viral infections, particularly the Human Papillomavirus (HPV), as a key factor in UCC pathogenesis (Figueiredo et al., 2023; Gilham et al., 2023; Smith et al., 2023).
Viruses, as obligate intracellular parasites, are well-recognized for their ability to manipulate host cellular processes, potentially leading to malignant transformation. Several viruses, such as HPV, Epstein-Barr virus (EBV) (Abudoukadeer et al., 2015; Luo et al., 2019), and hepatitis B and C viruses (HBV and HCV) (Mahmood et al., 2002; Ferber et al., 2003), have been identified as oncogenic, playing crucial roles in the pathogenesis of various human cancers. Nevertheless, the association between viral infections and UCC remains an active area of investigation, with inconsistent findings reported in the literature.
This review aims to provide a comprehensive overview of the current state of knowledge regarding the relationship between viral infections and UCC risk. We will discuss the evidence supporting the involvement of various viruses in the development of UCC, focusing on their potential roles in oncogenesis, molecular mechanisms, and clinical implications. Furthermore, we will explore the challenges and future directions in the study of viral infections and UCC, emphasizing the need for well-designed epidemiological and molecular studies to better understand the intricate interplay between viruses and UCC malignancy. Ultimately, elucidating the role of viral infections in UCC may lead to novel preventive and therapeutic strategies for this prevalent and life-threatening disease.
There are over 100 different types of HPV, which are categorized into three risk groups: high-risk, intermediate-risk, and low-risk, based on their association with UCC (Table 1; Muñoz et al., 2003). High-risk HPV types are strongly associated with UCC, as they can cause persistent infections and lead to the development of precancerous lesions, which may eventually progress to cancer. The high-risk types include HPV strains 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 (Table 1). Notably, HPV 16 and 18 are the most common high-risk strains, responsible for approximately 70% of UCC cases worldwide. Two of these species, α9 (also known as HPV16-like) and α7 (also known as HPV18-like), are of particular importance in the context of UCC diagnosis (Table 1; Evans et al., 2022). HPV-16 is a high-risk HPV type belonging to the Alphapapillomavirus genus. It is responsible for nearly 50–60% of all cervical cancer cases, making it the most prevalent and oncogenic HPV type (Youn et al., 2020). The carcinogenic potential of HPV-16 is primarily attributed to the expression of two viral oncoproteins, E6 and E7. These oncoproteins play a crucial role in HPV-16-mediated cervical carcinogenesis by disrupting the normal cellular regulatory pathways. The E6 oncoprotein of HPV-16 targets the tumor suppressor protein p53, promoting its ubiquitin-mediated degradation and thus impairing its ability to regulate cell cycle progression, apoptosis, and DNA repair (Zhang et al., 2022). The E7 oncoprotein, on the other hand, binds to and inactivates the retinoblastoma protein (pRb), a key cell cycle regulator, leading to uncontrolled cell proliferation and genomic instability. Furthermore, E6 and E7 can cooperate to induce chromosomal aberrations, telomerase activation, and immortalization of infected cervical epithelial cells, eventually resulting in malignant transformation (Figure 1; Liu et al., 2019). The strong association between HPV-16 and UCC underscores the importance of HPV vaccination and screening programs to prevent infection and early detection of cervical precancerous lesions. Currently, available prophylactic HPV vaccines, such as the bivalent, quadrivalent, and nonavalent vaccines, provide protection against HPV-16 and other high-risk HPV types. These vaccination programs have been shown to significantly reduce the incidence of HPV-16-associated cervical intraepithelial neoplasia and invasive UCC (Pham et al., 2020). Moreover, persistent HPV-16 infection serves as a valuable biomarker for the early identification of women at high risk for cervical cancer development. Molecular testing for HPV-16 and other high-risk HPV types in cervical cancer screening programs can enhance the sensitivity and specificity of detecting cervical precancerous lesions, thereby improving the overall effectiveness of cervical cancer prevention strategies (Kim et al., 2020).
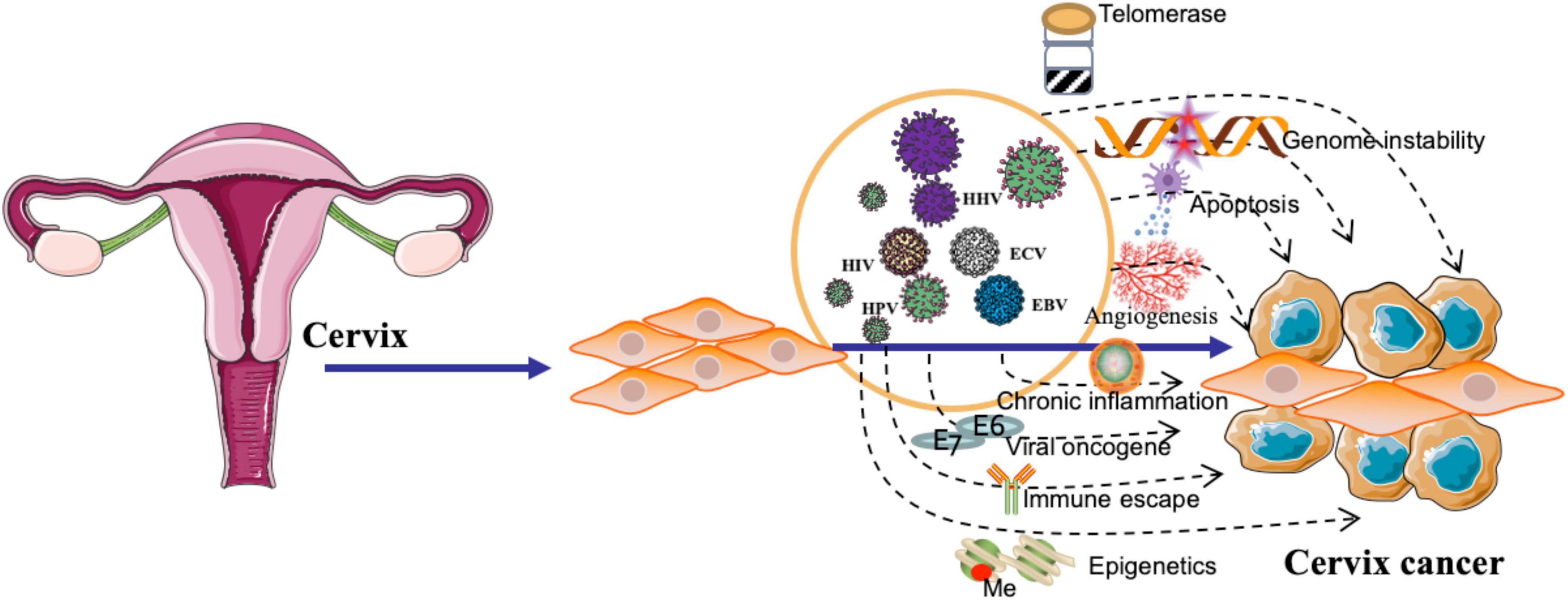
Figure 1. Viral pathogenesis of cervical cancer. The figure highlights the role of viral oncogenes in deregulating cellular processes, leading to uncontrolled cell proliferation and malignant transformation. Additionally, the figure emphasizes the involvement of viral infections in promoting immune escape, chronic inflammation, angiogenesis, and epigenetic modifications. Furthermore, the illustration demonstrates the influence of viruses on the activation of telomerase and the induction of genome instability, contributing to the development of cervical cancer.
Human papillomavirus α9 species includes HPV types 16, 31, 33, 35, 52, and 58. HPV α7 species includes HPV types 18, 39, 45, 59, and 68. These species comprise several high-risk HPV types, which are strongly associated with the development of UCC. UCC diagnosis typically involves a combination of screening and testing methods. Regular UCC screening, such as the Papanicolaou (Pap) test or liquid-based cytology, can identify abnormal cell changes in the cervix. If abnormal cells are detected, additional testing, such as HPV DNA testing or colposcopy, may be recommended. HPV DNA testing helps to identify high-risk HPV types, including those within the α9 and α7 species, that are associated with an increased risk of UCC. HPV α9 and α7 species, which encompass several high-risk HPV types, play a crucial role in the development of UCC. Regular screening, early detection, and appropriate follow-up care are vital for preventing the progression of precancerous lesions into invasive UCC. Additionally, HPV vaccination can help protect against the most common high-risk HPV types, including those in the α9 and α7 species, thereby reducing the risk of UCC. Intermediate-risk HPV types have a weaker association with UCC compared to high-risk types. They include HPV strains 68, 73, and 82 (Table 1). These types may contribute to the development of cancer in combination with other risk factors, but they are not as aggressive as high-risk types. Low-risk HPV types are not typically associated with UCC but can cause benign lesions, such as genital warts or mild dysplasia. These include HPV strains 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108 (Table 1). Although low-risk types do not generally lead to cancer, it is still essential to monitor and treat any HPV infection to maintain overall health. The relationship between UCC and HPV types is based on the risk categories. High-risk types are the primary cause of UCC, while intermediate-risk types have a weaker association, and low-risk types are generally not linked to the development of UCC. Over time, the accumulation of genetic mutations and chromosomal abnormalities in infected cells contributes to the progression from low-grade to high-grade precancerous lesions (cervical intraepithelial neoplasia, CIN) and, eventually, to invasive UCC.
There are several proposed mechanisms by which HPV might contribute to the development of UCC, such as by promoting genomic instability (Porter and Marra, 2022), deregulating cell cycle control (Singh et al., 2022), and inhibiting apoptosis (Figure 1; Yadav et al., 2023b). HPV may downregulate the expression of major histocompatibility complex (MHC) molecules, hindering antigen presentation and recognition by cytotoxic T cells (Ferrall et al., 2021). Viral infections can induce a persistent inflammatory response, characterized by the infiltration of immune cells and the release of pro-inflammatory cytokines, chemokines, and reactive oxygen species. This chronic inflammation may contribute to the formation of a tumor-promoting microenvironment (Hemmat and Bannazadeh Baghi, 2019); HPV infection may cause epigenetic modifications, such as DNA methylation (van den Helder et al., 2022), histone modifications (Lourenço de Freitas et al., 2021), and non-coding RNA regulation (Liu et al., 2022a), which can lead to the aberrant expression of genes involved in cell cycle regulation, apoptosis, and DNA repair, thereby facilitating carcinogenesis (Figure 1). HPV can disrupt the apoptotic machinery, thereby promoting the survival and proliferation of UCC (Liu et al., 2020). HPV can cause genome instability by integrating its viral DNA into the host genome. This integration disrupts the normal function of cellular genes and regulatory elements, leading to the dysregulation of cell cycle control and DNA repair mechanisms (Figure 1; Kamal et al., 2021).
Understanding the molecular biology of HPV infection and UCC development has been crucial in developing prevention and screening strategies, such as HPV vaccines and HPV-based UCC screening tests, to reduce the incidence and mortality of UCC worldwide (Table 1). HPV vaccines, such as Gardasil (Choi et al., 2023) and Cervarix (Roy et al., 2023), protect against the most common high-risk HPV types (particularly HPV 16 and 18). By preventing infection with these types, the vaccines can effectively reduce the likelihood of developing precancerous cervical lesions and, ultimately, UCC. Widespread vaccination has the potential to substantially decrease the overall incidence of UCC. Studies have already shown a decline in the prevalence of high-risk HPV infections and precancerous cervical lesions in vaccinated populations. When a significant portion of a population is vaccinated against HPV, it can create herd immunity, which means that even unvaccinated individuals will be indirectly protected due to reduced virus circulation in the population. Herd immunity, also known as community immunity, occurs when a substantial proportion of a population is immunized against a contagious disease, subsequently reducing the overall circulation of the pathogen and indirectly protecting unvaccinated individuals (Fine et al., 2011). The impact of herd immunity in UCC prevention is particularly relevant given the etiological role of HPV in the development of this malignancy. Persistent high-risk HPV infection is responsible for virtually all cases of UCC (Bosch et al., 2002). Therefore, achieving herd immunity through widespread HPV vaccination has the potential to considerably decrease the incidence of UCC. Several factors contribute to the development of herd immunity in the context of HPV vaccination. Firstly, the widespread vaccination of adolescents, both male and female, can significantly reduce the prevalence of high-risk HPV strains in the population. This reduced prevalence can lead to a decline in the transmission of HPV to unvaccinated individuals, thereby lowering their risk of developing UCC (Markowitz et al., 2012). Secondly, herd immunity can benefit specific population groups that may be at higher risk for HPV infection or cervical cancer but have lower vaccination rates. For example, certain minority or socioeconomically disadvantaged populations might face barriers to accessing HPV vaccination. Herd immunity can provide a measure of protection to these groups by reducing the overall circulation of high-risk HPV strains (Harper and DeMars, 2017). Recent studies have provided evidence of the positive impact of herd immunity on cervical cancer prevention. A study by Drolet et al. found that in countries with high HPV vaccination coverage, the prevalence of vaccine-targeted HPV types decreased by 83% among 13–19-year-old females and 66% among 20–24-year-old females, indicating a substantial reduction in the circulation of high-risk HPV strains (Drolet et al., 2019). Furthermore, the study observed a decrease in HPV prevalence among unvaccinated females, suggesting the presence of herd immunity.
However, it is important to note that HPV vaccines do not protect against all HPV types that can cause UCC, nor do they eliminate the risk entirely. Therefore, even vaccinated individuals should continue to undergo regular UCC screenings as recommended by healthcare professionals. With a reduction in the prevalence of high-risk HPV infections and UCC, there may be a reduced need for frequent UCC screenings (e.g., Pap smears or HPV tests) and associated treatments. This can lead to lower healthcare costs and improved quality of life for women. Self-sampling for HPV testing has emerged as a valuable tool in UCC prevention, offering several benefits that can help improve the overall effectiveness of screening programs and facilitate early detection of HPV infections, particularly among high-risk groups (Racey et al., 2013; Arbyn et al., 2018). Some key advantages of self-sampling for HPV testing during UCC prevention include: Self-sampling allows women to collect their samples in the privacy of their homes, without the need for a clinical appointment. This can lead to higher participation rates, especially among women who may be reluctant or unable to attend traditional clinic-based screenings due to cultural, logistical, or financial barriers (Sancho-Garnier et al., 2013; Gupta et al., 2018). Self-sampling provides a more comfortable and less invasive alternative to clinician-collected samples. Many women find self-sampling less intimidating and more acceptable, which can encourage them to undergo regular HPV testing as part of their UCC prevention routine (Waller et al., 2009; Arrossi et al., 2016). Self-sampling can help reduce costs associated with clinic visits, clinician time, and resources (Abuelo et al., 2014). By facilitating increased participation in HPV testing, self-sampling can contribute to more cost-effective UCC screening programs (Elfström et al., 2014). Self-sampling can improve access to UCC screening among hard-to-reach populations, such as women living in remote areas or those with limited access to healthcare services (Safaeian et al., 2007). By making HPV testing more accessible, self-sampling can help reduce health disparities and improve UCC prevention efforts in underserved communities (Bennett et al., 2018). With increased participation in HPV testing through self-sampling, more women can be screened for high-risk HPV infections. Early detection of these infections allows for timely intervention, such as close monitoring or treatment, to prevent the development of precancerous lesions and UCC (Ronco et al., 2014). It is important to note that the accuracy of self-sampling depends on the quality of the sample collected and the type of HPV test used (Arbyn et al., 2014). High-quality self-sampling kits and sensitive HPV tests are essential for reliable results (Petignat et al., 2007). In conclusion, self-sampling for HPV testing is an important tool in UCC prevention, as it can increase participation rates, improve access to screening, and facilitate early detection and intervention, ultimately contributing to a reduction in the incidence of UCC (Ogilvie et al., 2007; Nelson et al., 2017). It is important to consider other risk factors for UCC, such as obesity (Urbute et al., 2022), smoking (Table 1; Hildesheim et al., 2001; Malevolti et al., 2022), hormone replacement therapy, tamoxifen use, and a family history of Lynch syndrome (Kwolek et al., 2023), as these factors have more consistent associations with UCC risk.
Epstein-Barr virus, also known as human herpesvirus 4 (HHV-4), is a virus that has been associated with various types of cancers, including Burkitt’s lymphoma, Hodgkin’s lymphoma, nasopharyngeal carcinoma, and some types of stomach cancer (gastric carcinoma). However, the association between EBV and UCC is not well-established. Some studies have detected EBV DNA or viral proteins in UCC tissues (Blanco et al., 2020; Castro et al., 2020; Feng M. et al., 2021; Macleod and Reynolds, 2021), while others have not found any significant association between EBV infection and UCC (Table 2; De Oliveira et al., 1999; Noel et al., 2001).
Several mechanisms have been proposed to elucidate the role of EBV in the development of UCC. Two crucial viral proteins, Epstein-Barr nuclear antigen 1 (EBNA-1) and latent membrane protein 1 (LMP-1), have been implicated in the oncogenic process during EBV infection. EBNA-1 is a multifunctional protein involved in the replication, maintenance, and segregation of the EBV episome in latently infected cells. It is expressed in all EBV-associated malignancies and plays a pivotal role in the persistence of viral episomes within host cells. Additionally, EBNA-1 contributes to the immortalization of infected cells by altering cellular gene expression and promoting genomic instability. During clinical diagnosis, the detection of EBNA-1 expression may serve as a marker of latent EBV infection and its associated cervical malignancies (Hoseini Tabatabaie et al., 2023). LMP-1, on the other hand, is a transmembrane protein that functions as a constitutively active mimic of the tumor necrosis factor receptor (TNFR) family, stimulating multiple signaling pathways that promote cell survival, proliferation, and differentiation. LMP-1 exerts its oncogenic effects by activating the nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways. Detection of LMP-1 expression during clinical diagnosis can indicate the presence of an active EBV infection and suggest a more aggressive disease course, as LMP-1 is implicated in immune evasion, angiogenesis, and metastasis (Awasthi et al., 2023). A well-executed retrospective cohort study contributes to our understanding of the prevalence of EBV by targeting EBNA-1 and LMP-1 in cervical cancer specimens, with the authors reporting a 22.2% prevalence (n = 22) in their cohort of 99 patients. This is a valuable addition to the existing body of knowledge on EBV and cervical cancer. The authors provide evidence for the prognostic value of EBV status in cervical cancer patients, demonstrating that the 1-year and 5-year OS rates were higher in the EBV-positive group compared to the EBV-negative group. This finding highlights the potential clinical utility of EBV status as a prognostic biomarker (Table 2; Castro et al., 2020). EBV infection has been associated with epigenetic modifications in host cells, including DNA methylation (Fujii et al., 2022), histone modifications (Fujii et al., 2022), and non-coding RNA regulation (Kolesnik et al., 2021). EBV has developed various strategies to evade the host immune response, allowing the virus to persist in infected cells. This immune evasion can lead to chronic inflammation and contribute to the development of a favorable microenvironment for UCC initiation and progression (Figure 1; Pandey et al., 2021).
There have been some studies suggesting that coinfection with EBV and HPV may increase the risk of UCC development. The hypothesis is that the two viruses could act synergistically, with EBV potentially promoting HPV-induced cervical carcinogenesis (Table 2; da Carvalho and de Melo, 2019; Blanco et al., 2020). Co-infection with EBV, HPV, and human immunodeficiency virus (HIV) could potentially increase the risk of UCC development (Denny et al., 2008; Kelly et al., 2018). Infection with these high-risk types can lead to the formation of abnormal cervical cells, which may progress to UCC if not detected and treated early. HIV infection impairs the immune system, making it more difficult for the body to fight off infections, including HPV. Women with HIV are more likely to have persistent HPV infections, which can increase the risk of developing UCC (Clifford et al., 2017). Additionally, HIV-infected women are more likely to have faster progression from pre-cancerous lesions to invasive UCC compared to women without HIV. Although EBV is not directly linked to UCC, co-infection with HIV could further increase the risk due to the impaired immune response. Co-infections can complicate the clinical picture, and it is essential for healthcare providers (Table 2; Feng M. et al., 2021). HPV, EBV, and KSHV coinfections can stimulate angiogenesis (Figure 1), the formation of new blood vessels, by upregulating pro-angiogenic factors (e.g., VEGF and IL-8), which may promote tumor growth and metastasis (Dai et al., 2018; Hemmat and Bannazadeh Baghi, 2019). Upon coinfection, EBV and HPV oncoproteins have been shown to cooperatively activate several critical signaling pathways, including PI3K/AKT, MAPK/ERK, JAK/STAT, β-catenin, and p53 (Vranic et al., 2018). These pathways are known to regulate various cellular processes, such as cell survival, proliferation, differentiation, and migration, which are often dysregulated in cancer. Consequently, the simultaneous activation of these pathways by EBV and HPV oncoproteins can contribute to enhanced UCC development and progression.
Given the current state of research, the direct relationship between EBV and UCC risk remains unclear. Further studies are needed to investigate the potential association between EBV and uterine cancer, as well as the underlying mechanisms involved if such a link exists.
Hepatitis B viruses is a DNA virus that primarily infects the liver and can cause both acute and chronic hepatitis. HBV is a well-established risk factor for liver cancer, specifically hepatocellular carcinoma (HCC). However, the association between HBV infection and uterine cancer, particularly UCC, is not well-established. There is limited research on the potential relationship between HBV and uterine cancer, and the available studies do not provide sufficient evidence to establish a clear link between HBV infection and the risk of developing uterine cancer (Table 3). It is important to note that HBsAg (hepatitis B surface antigen) is associated with hepatitis B infection, which primarily affects the liver, while HPV is the main causative agent for UCC. Both HBV and HPV infection can potentially contribute to a weakened immune system, which may make it more difficult for the body to fight off other infections, including HPV. A weakened immune system could theoretically increase the risk of developing UCC in HPV positive individuals, but the primary risk factor remains the HPV infection itself (Table 3; Ferber et al., 2003; Luo et al., 2022). Although there is not a direct causal link between HBV infection and squamous cell UCC, some studies have suggested a potential association between the two. Serological markers of HBV infection, such as HBsAg, hepatitis B e antigen (HBeAg), and hepatitis B core antibody (anti-HBc), can provide information about the presence and stage of HBV infection in an individual. These markers may have prognostic value in certain cancer types (Table 3; Feng X. et al., 2021). The presence of HBsAg in serum indicates an ongoing infection, either acute or chronic (Lok and McMahon, 2009). HBeAg is a secreted viral protein that reflects active viral replication and is associated with high infectivity (Hadziyannis, 1995). Anti-HBc is an antibody produced in response to the HBcAg, which is a component of the viral nucleocapsid. The presence of anti-HBc indicates previous exposure to HBV, either resolved or ongoing (Chu and Liaw, 2010). In a study involving 277 cervical cancer patients, the seropositivity rates for HBsAg, HBeAg, and anti-HBc were found to be 4.33, 0.72, and 13.00%, respectively, indicating a potential relationship between HBV infection and cervical cancer (Wu et al., 2021). The detection of HBsAg and HBcAg in a subset of cervical cancer cases with seropositive HBsAg, as well as the increased risk of cervical cancer in individuals with both HBsAg and HPV positive, underscores the potential interplay between HBV infection and HPV in cervical cancer development. This observation highlights the importance of considering co-infections when examining UCC risk factors (Luo et al., 2022).
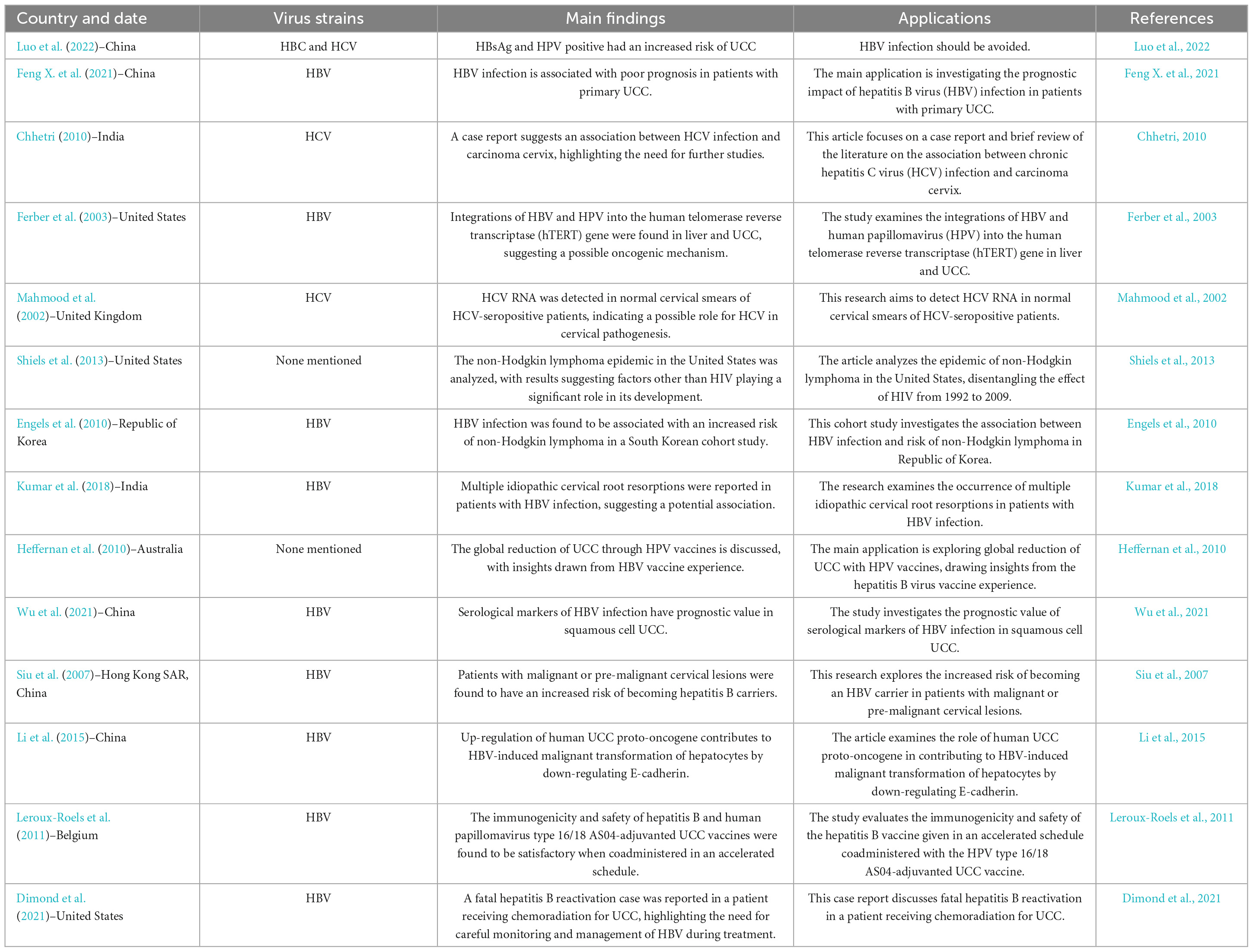
Table 3. The indirect association between hepatitis B virus (HBV) and hepatitis C virus (HCV) infections and the increased risk of developing uterine cervical Cancer (UCC).
Hepatitis C viruses is a well-known risk factor for liver cancer, particularly hepatocellular carcinoma. However, its association with uterine cancer, particularly UCC, is unclear and not well-established (Table 3; Mahmood et al., 2002; Chhetri, 2010). The prevalence of HBV and HCV infections was found among UCC patients compared to the general population. The association between chronic HBV infection and cervical cancer disappears among HPV-positive patients but remains significant for patients younger than 50 years after adjusting for HPV infection and parity. Co-infection with sexually transmitted infections (STIs), such as HIV, HBV, HCV, can also increase the risk of developing UCC. The seroprevalence of STIs among cervical cancer suspected women in Ethiopia, highlights a significant public health concern. The authors report an overall STI prevalence of 16.6% (67/403) in the study population, with the prevalence of HIV, HBV, HCV, and syphilis being 8.9, 2.5, 1, and 7.2%, respectively. This information helps to bridge the existing knowledge gap concerning the burden of coinfection in this UCC population (Abebe et al., 2021).
The clinical importance of EBV, HBV, and HCV in the etiology of cervical cancer has become an area of increasing interest among researchers and clinicians. The exact mechanisms by which EBV may contribute to cervical cancer development remain unclear, but possible explanations include the induction of genomic instability, inhibition of apoptosis, and promotion of cell proliferation (Figure 1). The possible mechanisms through which HBV may contribute to chronic inflammation, immune suppression, or molecular mimicry. Further investigation is warranted to determine the clinical relevance of HBV in the context of cervical cancer and to assess whether prevention and control of HBV infection may have implications for cervical cancer risk reduction. Similar to HBV, HCV is primarily implicated in liver diseases but has also been suggested as a possible co-factor in cervical cancer development. Some studies have reported an increased prevalence of HCV infection among cervical cancer patients, raising the possibility of an association between the two conditions. The potential mechanisms linking HCV to cervical cancer remain speculative and may involve chronic inflammation or immune dysregulation. In conclusion, the clinical importance of EBV, HBV, and HCV in cervical cancer etiology remains an emerging area of investigation. Although these viruses are not considered primary causative agents of cervical cancer, their potential role in the development of this malignancy warrants further study. Understanding the mechanisms through which these viruses may contribute to cervical carcinogenesis could have important implications for the prevention, diagnosis, and treatment of cervical cancer, ultimately improving patient outcomes and public health strategies.
Human cytomegalovirus (HCMV) has been associated with various malignancies, including glioblastoma and prostate cancer. It has been detected in some UCC tissues (Kienka et al., 2019; Khashman et al., 2020b; Ghadicolaee et al., 2021), but the relationship is not well-established. Human T-cell lymphotropic virus type 1 (HTLV-1) is a retrovirus known to be associated with adult T-cell leukemia/lymphoma (ATL) and a neurological disorder called HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Although HTLV-1 has not been directly implicated in UCC, some studies have suggested a potential link between HTLV-1 infection and an increased risk of developing UCC (Schierhout et al., 2020). The connection between HTLV-1 and UCC is not as well-established as that of HPV, which is the primary cause of UCC. However, there is some evidence to suggest that HTLV-1 infection might influence the persistence or progression of HPV infection, potentially increasing the risk of UCC in HTLV-1-infected women (Ibrahim Jaber and Qasim Dhumad, 2022; Rosadas and Taylor, 2022). Additionally, it has been suggested that HTLV-1 might indirectly contribute to UCC development by impairing the immune system and reducing the body’s ability to control HPV infection (Ibrahim Jaber and Qasim Dhumad, 2022).
Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is a virus primarily associated with Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. While KSHV has been extensively studied in relation to these conditions, its direct association with UCC is not well-established. Although KSHV and HPV are both viruses that can cause cancer, their roles in the development of UCC are distinct. HPV is considered the primary etiological agent for UCC, while KSHV has not been definitively linked to this malignancy. There have been some studies investigating the potential role of KSHV in UCC (Mukerebe, 2022), but the evidence is not yet strong enough to establish a direct association. Further research is needed to determine if KSHV plays a role in the development or progression of UCC or if it has any interaction with HPV in cervical carcinogenesis.
Human herpesvirus (HHV), particularly HHV-2, also known as herpes simplex virus type 2 (HSV-2), has been suggested to play a role in the development of UCC (Mukerebe, 2022). HSV-2 is primarily responsible for genital herpes infections, which can cause genital ulcers and increase the risk of acquiring other sexually transmitted infections (STIs), including HPV (Moharreri and Sohrabi, 2021). While HSV-2 itself is not considered a direct cause of UCC, its association with genital ulcers and the increased risk of acquiring HPV could potentially contribute to the development of UCC. Furthermore, HSV-2 infection might cause local inflammation and immunosuppression in the cervical area, which could facilitate the persistence and progression of HPV infection, ultimately leading to UCC (Vitali et al., 2020).
Human immunodeficiency virus is a virus that attacks the immune system, specifically the CD4 + T cells, which play a crucial role in immune response. HIV infection can progress to acquired immunodeficiency syndrome (AIDS) when the immune system is severely damaged, leaving the individual vulnerable to various opportunistic infections and cancers. There is a strong association between HIV and UCC. HIV infection weakens the immune system, making it difficult for the body to fight off HPV infections and prevent the progression of pre-cancerous lesions to UCC (Marima et al., 2021; Stelzle et al., 2021). HIV-infected women have a higher prevalence of HPV infection and are more likely to be infected with multiple high-risk HPV types. They are also more likely to have persistent HPV infections, which increases the risk of developing UCC (D’andrea et al., 2019). HIV-infected women tend to have a faster progression from HPV infection to the development of precancerous lesions and invasive UCC compared to women without HIV infection (Moscicki et al., 2019; Tawe et al., 2020). HIV-infected women may have a poorer response to UCC treatments, such as surgery, radiation, and chemotherapy, due to their compromised immune systems. To reduce the risk of UCC in HIV-infected women, regular UCC screening (e.g., Pap smears or HPV tests) is recommended. Additionally, the administration of the HPV vaccine can help protect against the high-risk HPV types responsible for UCC. Antiretroviral therapy (ART) can also help to improve the immune system in HIV-infected individuals, potentially reducing the risk of developing UCC (Shin et al., 2019). It is important to note that the link between many types of viruses and UCC is still not fully understood, and more research is needed to establish a clear relationship between the two. HPV remains the primary risk factor for UCC, and the prevention and control of HPV infection through vaccination and screening are the most effective strategies for reducing the incidence of UCC. More research is needed to determine the exact relationship between different virus types and UCC, as the current evidence is limited and inconclusive.
The future challenges for the viral etiology of UCC, particularly in relation to HPV, include (1) Improving vaccination coverage, although the HPV vaccine has proven effective in reducing the prevalence of high-risk HPV strains associated with UCC, improving vaccination coverage globally remains a challenge, especially in low- and middle-income countries where the burden of UCC is high; (2) Understanding HPV and other viral coinfections, such as EBV, may potentially play a role in UCC development. Further research is needed to elucidate the mechanisms and significance of these coinfections; (3) expanding screening programs: screening programs using HPV DNA testing have shown promise in detecting precancerous lesions, but implementing and expanding these programs worldwide, particularly in resource-limited settings, remains a challenge; (4) addressing disparities in access to care, disparities in access to preventive care, such as vaccination and screening, contribute to the high burden of UCC in certain populations. Efforts should be made to address socioeconomic, cultural, and logistical barriers to care; (5) developing novel therapeutic approaches, although current treatments for UCC, such as surgery, radiation, and chemotherapy, can be effective, there is a need for novel therapies that target the viral etiology of the disease, such as antiviral drugs and immunotherapies; (6) enhancing public awareness and education, public awareness and understanding of the role of HPV in UCC and the importance of vaccination and screening are essential to reduce the burden of the disease. Educational campaigns targeting various populations can help increase vaccine uptake and participation in screening programs; (7) investigating the role of viral genetic variation, the role of genetic variation within HPV types and its impact on UCC risk and vaccine efficacy needs further investigation; (8) Studying the impact of the HPV vaccine on non-cervical HPV-associated cancers, the HPV vaccine has the potential to prevent other HPV-associated cancers, such as oropharyngeal, anal, and penile cancers. Further research is needed to understand the long-term impact of vaccination on these cancers; (9) Addressing vaccine hesitancy: Vaccine hesitancy remains a challenge in certain populations, and efforts should be made to address misconceptions and promote confidence in the HPV vaccine, and (10) Long-term monitoring and surveillance, continued monitoring and surveillance of HPV prevalence, vaccine efficacy, and UCC incidence are crucial to assess the long-term impact of vaccination and screening programs and inform public health policy.
DC and TL: review conception, design, data collection, and quality analysis. TL and YY: the data extraction of the included studies, analysis, and interpretation of results. DC and YY: draft manuscript preparation and the critical revision of the manuscript. All authors reviewed the results and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abebe, M., Eshetie, S., and Tessema, B. (2021). Prevalence of sexually transmitted infections among cervical cancer suspected women at University of Gondar Comprehensive Specialized Hospital, North-west Ethiopia. BMC Infect. Dis. 21:378. doi: 10.1186/s12879-021-06074-y
Abudoukadeer, A., Niyazi, M., Aikula, A., Kamilijian, M., Sulaiman, X., Mutailipu, A., et al. (2015). Association of EBV and HPV co-infection with the development of cervical cancer in ethnic Uyghur women. Eur. J. Gynaecol. Oncol. 36, 546–550.
Abuelo, C., Levinson, K., Salmeron, J., Sologuren, C., Fernandez, M., and Belinson, J. (2014). The Peru cervical cancer screening study (PERCAPS): The design and implementation of a mother/daughter screen, treat, and vaccinate program in the Peruvian jungle. J. Commun. Health 39, 409–415. doi: 10.1007/s10900-013-9786-6
Ackermann, S., Renner, S., Fasching, P., Poehls, U., Bender, H., and Beckmann, M. (2005). Awareness of general and personal risk factors for uterine cancer among healthy women. Eur. J. Cancer Prev. 14, 519–524.
Arbyn, M., Castle, P., Schiffman, M., Wentzensen, N., Heckman-Stoddard, B., and Sahasrabuddhe, V. (2022a). Meta-analysis of agreement/concordance statistics in studies comparing self-vs clinician-collected samples for HPV testing in cervical cancer screening. Int. J. Cancer 151, 308–312.
Arbyn, M., Simon, M., de Sanjosé, S., Clarke, M., Poljak, P., Rezhake, R., et al. (2022b). Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: A systematic review and meta-analysis. Lancet Oncol. 23, 950–960.
Arbyn, M., Smith, S., Temin, S., Sultana, F., and Castle, P. (2018). Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 363:k4823. doi: 10.1136/bmj.k4823
Arbyn, M., Verdoodt, F., Snijders, P., Verhoef, V., Suonio, E., Dillner, L., et al. (2014). Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis. Lancet Oncol. 15, 172–183.
Arrossi, S., Ramos, S., Straw, C., Thouyaret, L., and Orellana, L. (2016). HPV testing: A mixed-method approach to understand why women prefer self-collection in a middle-income country. BMC Public Health 16:832. doi: 10.1186/s12889-016-3474-2
Awasthi, P., Dwivedi, M., Kumar, D., and Hasan, S. (2023). Insights into intricacies of the latent membrane protein-1 (LMP-1) in EBV-associated cancers. Life Sci. 313:121261. doi: 10.1016/j.lfs.2022.121261
Basoya, S., and Anjankar, A. (2022). Cervical cancer: Early detection and prevention in reproductive age group. Cureus 14:e31312. doi: 10.7759/cureus.31312
Bennett, K., Waller, J., Chorley, A., Ferrer, R., Haddrell, J., and Marlow, L. (2018). Barriers to cervical screening and interest in self-sampling among women who actively decline screening. J. Med. Screen. 25, 211–217. doi: 10.1177/0969141318767471
Bhatla, N., and Singhal, S. (2020). Primary HPV screening for cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 65, 98–108.
Blanco, R., Carrillo-Beltrán, D., Osorio, J., Calaf, G., and Aguayo, F. (2020). Role of Epstein-Barr virus and human papillomavirus coinfection in cervical cancer: Epidemiology, mechanisms and perspectives. Pathogens 9:685. doi: 10.3390/pathogens9090685
Bohn, J., Hernandez-Zepeda, M., Hersh, A., Munro, E., Kahn, J., Caughey, A., et al. (2022). Does obesity influence the preferred treatment approach for early-stage cervical cancer? A cost-effectiveness analysis. Int. J. Gynecol. Cancer 32, 133–140. doi: 10.1136/ijgc-2021-003004
Bosch, F., Lorincz, A., Muñoz, N., Meijer, C., and Shah, K. (2002). The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55, 244–265.
Brewster, W., Monk, B., Burger, R., Bergen, S., and Wilczynski, S. (1999). Does human papillomavirus have a role in cancers of the uterine corpus? Gynecol. Oncol. 75, 51–54.
Brown, D., Kjaer, S., Sigurdsson, K., Iversen, O., Hernandez-Avila, M., Wheeler, C., et al. (2009). The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J. Infect. Dis. 199, 926–935. doi: 10.1086/597307
Cameron, J., Dennis, D., Herrel, N., Chapple, A., and Hagensee, M. (2020). Risk of abnormal cervical cytology in HIV-infected women testing positive for both human papillomavirus and Epstein-Barr virus in genital tract specimens. Cancer Causes Control 31, 365–375. doi: 10.1007/s10552-020-01287-z
Castellsagué, X., Menéndez, C., Loscertales, M. P., Kornegay, J. R., dos Santos, F., Gómez-Olivé, F. X., et al., (2001). Human papillomavirus genotypes in rural Mozambique. Lancet 358, 1429–1430. doi: 10.1016/S0140-6736(01)06523-0
Castro, D., Vera, J., Soto-Becerra, P., López-Ilasaca, M., Yabar, A., Cámara, A., et al. (2020). Epstein-Barr virus and its prognostic value in a cohort of Peruvian women with cervical cancer. medRxiv [Preprint] doi: 10.1101/2020.08.04.20167841
Chandra, S., Sarkar, S., and Mandal, P. (2022). Identification of novel genetic and epigenetic regulators of different tissue types of cervical cancer. J. Obstet. Gynaecol. Res. 48, 3179–3190. doi: 10.1111/jog.15454
Chen, W., Sun, K., Zheng, R., Zeng, H., Zhang, S., Xia, C., et al. (2018). Cancer incidence and mortality in China, 2014. Chin. J. Cancer Res. 30, 1–12.
Chhetri, M. (2010). Chronic hepatitis C virus infection and carcinoma cervix–report of a case and brief review of literature. Apollo Med. 7, 61–63.
Choi, S., Ismail, A., Pappas-Gogos, G., and Boussios, S. (2023). HPV and cervical cancer: A review of epidemiology and screening uptake in the UK. Pathogens 12:298.
Chu, C., and Liaw, Y. (2010). Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir. Ther. 15, 133–143.
Clifford, G., Tully, S., and Franceschi, S. (2017). Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: A meta-analysis from HPV infection to cervical cancer. Clin. Infect. Dis. 64, 1228–1235. doi: 10.1093/cid/cix135
Coffey, K., Gaitskell, K., Beral, V., Canfell, K., Green, J., Reeves, G., et al. (2016). Past cervical intraepithelial neoplasia grade 3, obesity, and earlier menopause are associated with an increased risk of vulval cancer in postmenopausal women. Br. J. Cancer 115, 599–606. doi: 10.1038/bjc.2016.165
Costa, S., Verberckmoes, B., Castle, P., and Arbyn, M. (2023). Offering HPV self-sampling kits: An updated meta-analysis of the effectiveness of strategies to increase participation in cervical cancer screening. Br. J. Cancer 128, 805–813. doi: 10.1038/s41416-022-02094-w
Cuzick, J., Clavel, C., Petry, K., Meijer, C., Hoyer, H., Ratnam, S., et al. (2006). Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer 119, 1095–1101. doi: 10.1002/ijc.21955
da Carvalho, M., and de Melo, Y. (2019). Association between human papillomavirus and Epstein-Barr virus infections and cancer of the uterine cervix. Crit. Rev. Oncog. 24, 379–383.
Dai, L., Zhao, M., Jiang, W., Lin, Z., Del Valle, L., and Qin, Z. (2018). KSHV co-infection, a new co-factor for HPV-related cervical carcinogenesis? Am. J. Cancer Res. 8, 2176–2184.
D’andrea, F., Pellicanò, G., Venanzi Rullo, E., D’ALEO, F., Facciolà, A., Mical, C., et al. (2019). Cervical cancer in women living with HIV: A review of the literature. World Cancer Res. J. 6:e1224.
Dau, H., Trawin, J., Nakisige, C., Payne, B., Vidler, M., Singer, J., et al. (2023). The social and economic impacts of cervical cancer on women and children in low- and middle-income countries: A systematic review. Int. J. Gynaecol. Obstet. 160, 751–761. doi: 10.1002/ijgo.14395
de Lima, M., Neto, P., Lima, L., Gonçalves Júnior, J., Teixeira Junior, A., Teodoro, I., et al. (2018). Association between Epstein-Barr virus (EBV) and cervical carcinoma: A meta-analysis. Gynecol. Oncol. 148, 317–328.
De Oliveira, D., Monteiro, T., De Melo, W., Moreira, M., Alvarenga, M., and Bacchi, C. (1999). Lack of Epstein-Barr virus infection in cervical carcinomas. Arch. Pathol. Lab. Med. 123, 1098–1100.
de Sanjose, S., Quint, W., Alemany, L., Geraets, D., Klaustermeier, J., Lloveras, B., et al. (2010). Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 11, 1048–1056.
Denny, L., Boa, R., Williamson, A., Allan, B., Hardie, D., Stan, R., et al. (2008). Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet. Gynecol. 111, 1380–1387. doi: 10.1097/AOG.0b013e3181743327
Dimond, C., Negroiu, A., Hughes, D., and Patel, J. (2021). Fatal hepatitis B reactivation in a patient receiving chemoradiation for cervical cancer. J. Oncol. Pharm. Pract. 27, 1296–1301. doi: 10.1177/1078155220964256
Drolet, M., Bénard, É, Pérez, N., Brisson, M., and Hpv Vaccination Impact Study Group. (2019). Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 394, 497–509. doi: 10.1016/S0140-6736(19)30298-3
Elfström, K., Smelov, V., Johansson, A., Eklund, C., Nauclér, P., Arnheim-Dahlström, L., et al. (2014). Long term duration of protective effect for HPV negative women: Follow-up of primary HPV screening randomised controlled trial. BMJ 348:g130. doi: 10.1136/bmj.g130
Engels, E., Cho, E., and Jee, S. (2010). Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: A cohort study. Lancet Oncol. 11, 827–834.
Evans, A., Salnikov, M., Gameiro, S., Maleki Vareki, S., and Mymryk, J. (2022). HPV-positive and-negative cervical cancers are immunologically distinct. J. Clin. Med. 11:4825. doi: 10.3390/jcm11164825
Feng, M., Duan, R., Gao, Y., Zhang, H., Qiao, Y., Li, Q., et al. (2021). Role of Epstein-Barr virus and human papillomavirus coinfection in cervical intraepithelial neoplasia in Chinese women living with HIV. Front. Cell. Infect. Microbiol. 11:703259. doi: 10.3389/fcimb.2021.703259
Feng, X., Lu, H., Wei, Y., Guan, M., Wang, J., Liu, C., et al. (2021). Prognostic impact of hepatitis B virus infection in patients with primary cervical cancer. Cancer Med. 10, 8310–8319. doi: 10.1002/cam4.4358
Ferber, M. J., Montoya, D. P., Yu, C., Aderca, I., McGee, A., Thorland, E. C., et al. (2003). Integrations of the hepatitis B virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene 22, 3813–3820. doi: 10.1038/sj.onc.1206528
Ferrall, L., Lin, K., Roden, R., Hung, C., and Wu, T. (2021). Cervical cancer immunotherapy: Facts and hopes. Clin. Cancer Res. 27, 4953–4973. doi: 10.1158/1078-0432.CCR-20-2833
Figueiredo, D., Ribeiro, I., Penedones, A., Mendes, D., Alves, C., Batel-Marques, F., et al. (2023). Performance of Aptima-HPV in the cervical cancer screening program of Portugal: A cost-analysis. BMC Womens Health 23:96. doi: 10.1186/s12905-023-02219-0
Fine, P., Eames, K., and Heymann, D. (2011). “Herd immunity”: A rough guide. Clin. Infect. Dis. 52, 911–916. doi: 10.1093/cid/cir007
Frumovitz, M., Jhingran, A., Soliman, P., Klopp, A., Schmeler, K., and Eifel, P. (2014). Morbid obesity as an independent risk factor for disease-specific mortality in women with cervical cancer. Obstet. Gynecol. 124, 1098–1104. doi: 10.1097/AOG.0000000000000558
Fujii, T., Okabe, A., and Kaneda, A. (2022). Epigenetic contribution to tumorigenesis of host cells by Epstein-Barr virus infection. Chiba Med. J. 98, 1–7.
Ghadicolaee, S., Pazhoohan, M., Hasanzadeh, A., Nematolahi, M., Yahyapour, Y., Ranaee, M., et al. (2021). Low frequency of human cytomegalovirus in cancerous and precancerous cervical samples of Iranian women. Future Virol. 16, 399–405.
Gilham, C., Sargent, A., Crosbie, E., and Peto, J. (2023). Long-term risks of invasive cervical cancer following HPV infection: Follow-up of two screening cohorts in Manchester. Br. J. Cancer 128, 1933–1940. doi: 10.1038/s41416-023-02227-9
Giuliano, A., Sedjo, R., Roe, D., Harri, R., Baldwi, S., Papenfuss, M., et al. (2002). Clearance of oncogenic human papillomavirus (HPV) infection: Effect of smoking (United States). Cancer Causes Control 13, 839–846.
Gupta, S., Palmer, C., Bik, E., Cardenas, J., Nuñez, H., Kraal, L., et al. (2018). Self-sampling for human papillomavirus testing: Increased cervical cancer screening participation and incorporation in international screening programs. Front. Public Health 6:77. doi: 10.3389/fpubh.2018.00077
Hadziyannis, S. (1995). Hepatitis B e antigen negative chronic hepatitis B: From clinical recognition to pathogenesis and treatment. Viral Hepat Rev. 1, 7–36.
Harper, D., and DeMars, L. (2017). HPV vaccines–a review of the first decade. Gynecol. Oncol. 146, 196–204.
Heffernan, M., Garland, S., and Kane, M. (2010). Global reduction of cervical cancer with human papillomavirus vaccines: Insights from the hepatitis B virus vaccine experience. Sex. Health 7, 383–390. doi: 10.1071/SH09134
Hemmat, N., and Bannazadeh Baghi, H. (2019). Association of human papillomavirus infection and inflammation in cervical cancer. Pathog. Dis. 77:ftz048.
Hildesheim, A., Herrero, R., Castle, P. E., Wacholder, S., Bratti, M. C., Sherman, M. E., et al. (2001). HPV co-factors related to the development of cervical cancer: Results from a population-based study in Costa Rica. Br. J. Cancer 84, 1219–1226. doi: 10.1054/bjoc.2001.1779
Hoseini Tabatabaie, F., Hosseini, S., Hashemi, S., Safaie, A., and Sarvari, J. (2023). A preliminary sequence analysis of the Epstein-Barr virus nuclear antigen 1 (EBNA1) carboxy-terminal region in cervical and ovarian cancers. Iran. J. Pathol. 18, 24–32.
Huh, W., Joura, E., Giuliano, A., Iversen, O., de Andrade, R., Ault, K., et al. (2017). Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 390, 2143–2159. doi: 10.1016/S0140-6736(17)31821-4
Ibrahim Jaber, S., and Qasim Dhumad, B. (2022). Expression of HPV-16 L1 gene human papillomavirus (HTLV-1) identified by pap smear. Arch. Razi Inst. 77, 2125–2130.
Insinga, R., Dasbach, E., and Myers, E. (2003). The health and economic burden of genital warts in a set of private health plans in the United States. Clin. Infect. Dis. 36, 1397–1403. doi: 10.1086/375074
Iversen, L., Fielding, S., Lidegaard, O., and Hannaford, P. (2021). Contemporary hormonal contraception and cervical cancer in women of reproductive age. Int. J. Cancer doi: 10.1002/ijc.33585 [Epub ahead of print].
Joura, E., Giuliano, A., Iversen, O., Bouchard, C., Mao, C., Mehlsen, J., et al. (2015). A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 372, 711–723.
Kamal, M., Lameiras, S., Deloger, M., Morel, A., Vacher, S., Lecerf, C., et al. (2021). Human papilloma virus (HPV) integration signature in cervical cancer: Identification of MACROD2 gene as HPV hot spot integration site. Br. J. Cancer 124, 777–785.
Kelly, H., Weiss, H., Benavente, Y., de Sanjose, S., and Mayaud, P. (2018). Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. Lancet HIV 5, e45–e58. doi: 10.1016/s2352-3018(17)30149-2
Khashman, B., Al-Zahawi, F., Alwandawi, T., and Ali, M. J. (2020a). Expression of EBV latent membrane protein 1 (LMP1) in Iraqi women with cervical carcinoma. Biochem. Cell. Arch. 20:3263.
Khashman, B., Karim, S., Alhilli, H., and Ali, M. (2020b). Possible role of HCMV infection on the development of HPV positive cervical carcinoma in a group of Iraqi women. Biochem. Cell. Arch. 20, 1549–1552.
Kienka, T., Varga, M., Caves, J., Smith, J., and Sivaraman, V. (2019). Epstein-Barr virus, but not human cytomegalovirus, is associated with a high-grade human papillomavirus–associated cervical lesions among women in North Carolina. J. Med. Virol. 91, 450–456.
Kim, J., Kim, B., Jeon, D., Lee, C., Roh, J., Kim, J., et al. (2020). Type-specific viral load and physical state of HPV type 16, 18, and 58 as diagnostic biomarkers for high-grade squamous intraepithelial lesions or cervical Cancer. Cancer Res. Treat. 52, 396–405.
Kolesnik, M., Stepien, E., and Polz-Dacewicz, M. (2021). The role of microRNA (miRNA) as a biomarker in HPV and EBV-related cancers. J. Preclin. Clin. Res. 15, 104–110.
Korsakov, A., Kryukova, A., Troshin, V., Milushkina, O., and Lagerev, D. (2022). Cervical and endometrial cancer incidence in the female population from the bryansk region living in conditions of chemical, radioactive and combined environmental contamination (2000-2020). Life 12:1488. doi: 10.3390/life12101488
Kreimer, A., Struyf, F., Del Rosario-Raymundo, M., Hildesheim, A., Skinner, S., Wacholder, S., et al. (2015). Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica vaccine and PATRICIA trials. Lancet Oncol. 16, 775–786. doi: 10.1016/S1470-2045(15)00047-9
Kumar, V., Chawla, A., and Kaur, A. (2018). Multiple idiopathic cervical root resorptions in patients with hepatitis B virus infection. J. Endod. 44, 1575–1577. doi: 10.1016/j.joen.2018.06.017
Kwolek, D., Gerstberger, S., Tait, S., and Qiu, J. (2023). Ovarian, uterine, and vulvovaginal cancers: Screening, treatment overview, and prognosis. Med. Clin. 107, 329–355. doi: 10.1016/j.mcna.2022.10.016
Kycler, W., Kubiak, A., Rzymski, P., Wilczak, M., Trojanowski, M., Roszak, M., et al. (2017). Impact of selected environmental factors on attendance in the breast and cervical cancer early detection programme in the Wielkopolska province of Poland during 2007-2012. Ann. Agric. Environ. Med. 24, 467–471. doi: 10.26444/aaem/74481
Labani, S., Asthana, S., Sodhani, P., Gupta, S., Bhambhani, S., Pooja, B., et al. (2014). CareHPV cervical cancer screening demonstration in a rural population of north India. Eur. J. Obstet. Gynecol. Reprod. Biol. 176, 75–79. doi: 10.1016/j.ejogrb.2014.03.006
Lasche, M., Gallwas, J., and Grundker, C. (2022). Like brothers in arms: How hormonal stimuli and changes in the metabolism signaling cooperate, leading HPV infection to drive the onset of cervical cancer. Int. J. Mol. Sci. 23:5050. doi: 10.3390/ijms23095050
Lau, K., Hsu, Y., Lin, Y., Yeap, M., Lee, C., and Chen, K. (2021). Case history on Epstein-Barr Virus-associated smooth muscle tumor (EBV-SMT) of cranio-cervical junction in an immunocompetent patient. Br. J. Neurosurg. doi: 10.1080/02688697.2021.1932745 [Epub ahead of print].
Lee, S., Kang, D., Seo, S., Jeong, J., Yoo, K., Jeon, Y., et al. (2003). Multiple HPV infection in cervical cancer screened by HPVDNAChip™. Cancer Lett. 198, 187–192. doi: 10.1016/s0304-3835(03)00312-4
Leroux-Roels, G., Haelterman, E., Maes, C., Levy, J., De Boever, F., Licini, L., et al. (2011). Randomized trial of the immunogenicity and safety of the Hepatitis B vaccine given in an accelerated schedule coadministered with the human papillomavirus type 16/18 AS04-adjuvanted cervical cancer vaccine. Clin. Vaccine Immunol. 18, 1510–1518. doi: 10.1128/CVI.00539-10
Li, J., Dai, X., Zhang, H., Zhang, W., Sun, S., Gao, T., et al. (2015). Up-regulation of human cervical cancer proto-oncogene contributes to hepatitis B virus-induced malignant transformation of hepatocyte by down-regulating E-cadherin. Oncotarget 6, 29196–29209. doi: 10.18632/oncotarget.5039
Liu, X., Ma, H., Fei, L., Jiang, M., Xia, M., Bai, L., et al. (2020). HPV-mediated down-regulation of NOD1 inhibits apoptosis in cervical cancer. Infect. Agents Cancer 15:6. doi: 10.1186/s13027-020-0272-3
Liu, Y., Fan, P., Yang, Y., Xu, C., Huang, Y., Li, D., et al. (2019). Human papillomavirus and human telomerase RNA component gene in cervical cancer progression. Sci. Rep. 9:15926.
Liu, Y., Liu, H., Sheng, B., Pan, S., Wang, Z., and Zhu, X. (2022a). The functions of lncRNAs in the HPV-negative cervical cancer compared with HPV-positive cervical cancer. Apoptosis 27, 685–696.
Liu, Y., Zhang, Q., and Ni, R. (2022b). Association between genetic variants (rs920778, rs4759314, and rs217727) in LncRNAs and cervical cancer susceptibility in Chinese population: A systematic review and meta-analysis. Front. Genet. 13:988207. doi: 10.3389/fgene.2022.988207
Lourenço de Freitas, N., Deberaldini, M., Gomes, D., Pavan, A., Sousa, Â, Dos Santos, J., et al. (2021). Histone deacetylase inhibitors as therapeutic interventions on cervical cancer induced by human papillomavirus. Front. Cell. Dev. Biol. 8:592868. doi: 10.3389/fcell.2020.592868
Luo, C., Yu, S., Zhang, J., Wu, X., Dou, Z., Li, Z., et al. (2022). Hepatitis B or C viral infection and the risk of cervical cancer. Infect. Agents Cancer 17:54.
Luo, W., Feng, Y., Guo, R., Tang, L., Chen, L., Zhou, G., et al. (2019). Patterns of EBV-positive cervical lymph node involvement in head and neck cancer and implications for the management of nasopharyngeal carcinoma T0 classification. Oral Oncol. 91, 7–12. doi: 10.1016/j.oraloncology.2019.01.012
Macleod, C., and Reynolds, J. (2021). Human Papilloma Virus infection and cervical cancer among women who sell sex in Eastern and Southern Africa: A scoping review. Womens Health 17:17455065211058349. doi: 10.1177/17455065211058349
Mahmood, M., Baghestanian, M., Thomas, W. R., Battistutti, W., Pischinger, K., Schatten, C., et al. (2002). Detection of hepatitis C virus (HCV) RNA in normal cervical smears of HCV-seropositive patients. Clin. Infect. Dis. 35, 966–973. doi: 10.1086/342909
Malevolti, M., Lugo, A., Scala, M., Gallus, S., Gorini, G., Lachi, A., et al. (2022). Dose-risk relationships between cigarette smoking and cervical cancer: A systematic review and meta-analysis. Eur. J. Cancer Prev. 32, 171–183. doi: 10.1097/CEJ.0000000000000773
Marima, R., Hull, R., Lolas, G., Syrigos, K., Kgoebane-Maseko, M., Kaufmann, A., et al. (2021). The catastrophic HPV/HIV dual viral oncogenomics in concert with dysregulated alternative splicing in cervical cancer. Int. J. Mol. Sci. 22:10115. doi: 10.3390/ijms221810115
Markowitz, L., Tsu, V., Deeks, S., Cubie, H., Wang, S., Vicari, A., et al. (2012). Human papillomavirus vaccine introduction–the first five years. Vaccine 30, F139–F148.
Marlow, L., Waller, J., and Wardle, J. (2007). Public awareness that HPV is a risk factor for cervical cancer. Br. J. Cancer 97, 691–694.
Maskey, N., Thapa, N., Maharjan, M., Shrestha, G., Maharjan, N., Cai, H., et al. (2019). Infiltrating CD4 and CD8 lymphocytes in HPV infected uterine cervical milieu. Cancer Manag. Res. 11, 7647–7655.
Moharreri, M., and Sohrabi, A. (2021). Characteristics of hsv-2, M. genitalium and C. trachomatis in HPV genotypes associated with cervical intraepithelial neoplasia and genital infections. Infect. Disord. Drug Targets 21, 112–118. doi: 10.2174/1871526520666191231142317
Montoya-Fuentes, H., Suárez Rincón, A., Ramírez-Muñoz, M., Arévalo-Lagunas, I., Morán Moguel, M., Gallegos Arreola, M., et al. (2001). The detection of human papillomavirus 16, 18, 35 and 58 in cervical-uterine cancer and advanced degree of squamous intraepithelial lesions in Western Mexico: Clinical-molecular correlation. Ginecol. Obstet. Mexico 69, 137–142.
Moscicki, A., Flowers, L., Huchko, M., Long, M., MacLaughlin, K., Murphy, J., et al. (2019). Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J. Low. Genit. Tract Dis. 23, 87–101.
Mukerebe, C. (2022). Kaposi’s sarcoma-associated herpesvirus shedding in saliva and cervical secretions in Tanzanian women. Tanzania J. Health Res. 23, 157–158.
Muñoz, N., Bosch, F., de Sanjosé, S., Herrero, R., Castellsagué, X., Shah, K., et al. (2003). Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348, 518–527.
Muñoz-Bello, J., Carrillo-García, A., and Lizano, M. (2022). Epidemiology and molecular biology of HPV variants in cervical cancer: The state of the art in Mexico. Int. J. Mol. Sci. 23:8566. doi: 10.3390/ijms23158566
Nakagawa, J., Schirmer, J., and Barbieri, M. (2010). Human papillomavirus (HPV) and uterine cervical cancer. Rev. Bras. Enferm. 63, 307–311.
Nelson, E., Maynard, B., Loux, T., Fatla, J., Gordon, R., and Arnold, L. (2017). The acceptability of self-sampled screening for HPV DNA: A systematic review and meta-analysis. Sex. Transm. Infect. 93, 56–61. doi: 10.1136/sextrans-2016-052609
Noel, J., Lespagnard, L., Fayt, I., Verhest, A., and Dargent, J. (2001). Evidence of human papilloma virus infection but lack of Epstein-Barr virus in lymphoepithelioma-like carcinoma of uterine cervix: Report of two cases and review of the literature. Hum. Pathol. 32, 135–138. doi: 10.1053/hupa.2001.20901
Ogilvie, G., Krajden, M., Maginley, J., Isaac-Renton, J., Hislop, G., Elwood-Martin, R., et al. (2007). Feasibility of self-collection of specimens for human papillomavirus testing in hard-to-reach women. CMAJ 177, 480–483.
Okoye, J., Ngokere, A., Onyenekwe, C., Eze, U., and Obioma, O. (2023). Abstract C029: The pattern of mutant p53 protein expression in cervical cancer-associated single and co-infection with human papillomavirus and Epstein-Barr virus. Cancer Epidemiol. Biomarkers Prev. 32:C029.
Pandey, N., Chauhan, A., Raithatha, N., Patel, P., Khandelwal, R., Desai, A., et al. (2021). Influence of TLR4 and TLR9 polymorphisms and haplotypes on multiple hrHPV infections and HPV16 copy number in cervical cancer and cervicitis. Microb. Pathog. 159:105149. doi: 10.1016/j.micpath.2021.105149
Petignat, P., Faltin, D., Bruchim, I., Tramèr, M., Franco, E., and Coutlée, F. (2007). Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol. Oncol. 105, 530–535.
Pham, C., Juhasz, M., Sung, C., and Mesinkovska, N. (2020). The human papillomavirus vaccine as a treatment for human papillomavirus–related dysplastic and neoplastic conditions: A literature review. J. Am. Acad. Dermatol. 82, 202–212.
Plummer, M., de Martel, C., Vignat, J., Ferlay, J., Bray, F., and Franceschi, S. (2016). Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 4, e609–e616.
Porter, V., and Marra, M. (2022). The drivers, mechanisms, and consequences of genome instability in HPV-driven cancers. Cancers 14:4623. doi: 10.3390/cancers14194623
Racey, C., Withrow, D., and Gesink, D. (2013). Self-collected HPV testing improves participation in cervical cancer screening: A systematic review and meta-analysis. Can. J. Public Health 104, e159–e166. doi: 10.1007/bf03405681
Ronco, G., Dillner, J., Elfström, K., Tunesi, S., Snijders, P., Arbyn, M., et al. (2014). Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 383, 524–532. doi: 10.1016/S0140-6736(13)62218-7
Rosadas, C., and Taylor, G. (2022). HTLV-1 and co-infections. Front. Med. 9:812016. doi: 10.3389/fmed.2022.812016
Roy, V., Jung, W., Linde, C., Coates, E., Ledgerwood, J., Costner, P., et al. (2023). Differences in HPV-specific antibody Fc-effector functions following Gardasil® and Cervarix® vaccination. Npj Vaccines 8:39. doi: 10.1038/s41541-023-00628-8
Safaeian, M., Solomon, D., and Castle, P. (2007). Cervical cancer prevention—cervical screening: Science in evolution. Obstet. Gynecol. Clin. North Am. 34, 739–760. doi: 10.1016/j.ogc.2007.09.004
Sancho-Garnier, H., Tamalet, C., Halfon, P., Leandri, F., Le Retraite, L., Djoufelkit, K., et al. (2013). HPV self-sampling or the Pap-smear: A randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int. J. Cancer 133, 2681–2687. doi: 10.1002/ijc.28283
Sankaranarayanan, R., Nene, B., Shastri, S., Jayant, K., Muwonge, R., Budukh, A., et al. (2009). HPV screening for cervical cancer in rural India. N. Engl. J. Med. 360, 1385–1394.
Sasagawa, T., Shimakage, M., Nakamura, M., Sakaike, J., Ishikawa, H., and Inoue, M. (2000). Epstein-Barr virus (EBV) genes expression in cervical intraepithelial neoplasia and invasive cervical cancer: A comparative study with human papillomavirus (HPV) infection. Hum. Pathol. 31, 318–326. doi: 10.1016/s0046-8177(00)80245-2
Sassenou, J., Ringa, V., Zins, M., Ozguler, A., Paquet, S., Panjo, H., et al. (2021). Women with obesity in cervical cancer screening. The double penalty: Underscreening and income inequalities. Obes. Res. Clin. Pract. 15, 212–215. doi: 10.1016/j.orcp.2021.03.003
Schierhout, G., McGregor, S., Gessain, A., Einsiedel, L., Martinello, M., and Kaldor, J. (2020). Association between HTLV-1 infection and adverse health outcomes: A systematic review and meta-analysis of epidemiological studies. Lancet Infect. Dis. 20, 133–143. doi: 10.1016/S1473-3099(19)30402-5
Schiffman, M., Wentzensen, N., Wacholder, S., Kinney, W., Gage, J., and Castle, P. (2011). Human papillomavirus testing in the prevention of cervical cancer. J. Natl. Cancer Inst. 103, 368–383.
Serrano, B., Ibáñez, R., Robles, C., Peremiquel-Trillas, P., de Sanjosé, S., and Bruni, L. (2022). Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 154:106900.
Shiels, M., Engels, E., Linet, M., Clarke, C., Li, J., Hall, H., et al. (2013). The epidemic of non–hodgkin lymphoma in the United States: Disentangling the effect of HIV, 1992–2009U. S. NHL trends excluding HIV-infected cases, 1992–2009. Cancer Epidemiol. Biomarkers Prev. 22, 1069–1078.
Shin, S., Carpenter, C., Ekstrand, M., Wang, Q., Grover, S., Zetola, N., et al. (2019). Cervical cancer awareness and presence of abnormal cytology among HIV-infected women on antiretroviral therapy in rural Andhra Pradesh, India. Int. J. STD AIDS 30, 586–595. doi: 10.1177/0956462419825950
Singh, G., Sharma, S., and Singh, S. (2022). miR-34a negatively regulates cell cycle factor Cdt2/DTL in HPV infected cervical cancer cells. BMC Cancer 22:777. doi: 10.1186/s12885-022-09879-5
Siu, S., Cheung, T., Chan, P., Lin, C., and Lo, K. (2007). Patients with malignant or pre-malignant cervical lesion have increased risk of becoming hepatitis B carrier. J. Exp. Clin. Cancer Res. 26, 77–81.
Smith, J., Vaz, O., Gaber, C., Des Marais, A., Chirumamilla, B., Hendrickson, L., et al. (2023). Recruitment strategies and HPV self-collection return rates for under-screened women for cervical cancer prevention. PLoS One 18:e0280638. doi: 10.1371/journal.pone.0280638
Sosse, S., Tadlaoui, K., Benhassou, M., Elkarroumi, M., Elmzibri, M., and Ennaji, M. (2022). Viral co-infection of oncogenic human papillomavirus with Epstein–Barr Virus, human herpesvirus 8 and Herpes Simplex Virus type 2 in malignant cervical cancer. Int. Med. J. 30.
Srinath, A., van Merode, F., Rao, S., and Pavlova, M. (2022). Barriers to cervical cancer and breast cancer screening uptake in low-and-middle-income countries: A systematic review. Health Policy Plan 38, 509–527. doi: 10.1093/heapol/czac104
Stelzle, D., Tanaka, L., Lee, K., Ibrahim Khalil, A., Baussano, I., Shah, A., et al. (2021). Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 9, e161–e169.
Tawe, L., MacDuffie, E., Narasimhamurthy, M., Wang, Q., Gaseitsiwe, S., Moyo, S., et al. (2020). Human papillomavirus genotypes in women with invasive cervical cancer with and without human immunodeficiency virus infection in Botswana. Int. J. Cancer 146, 1667–1673.
Tin, K., Ngamjarus, C., Rattanakanokchai, S., Sothornwit, J., Aue-Aungkul, A., Paing, A., et al. (2023). Interventions to increase the uptake of cervical cancer screening in low- and middle-income countries: A systematic review and meta-analysis. BMC Womens Health 23:120. doi: 10.1186/s12905-023-02265-8
Ton, M., Swami, N., Germar, M., and Dee, E. (2022). HPV mRNA testing in cervical cancer screening: Implications for low- and middle-income countries. Int. J. Gynecol. Cancer 32, 1632–1633. doi: 10.1136/ijgc-2022-003959
Tota, J., Ramanakumar, A., Jiang, M., Dillner, J., Walter, S., Kaufman, J., et al. (2013). Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am. J. Epidemiol. 178, 625–634. doi: 10.1093/aje/kwt018
Urbute, A., Kjaer, S., Kesmodel, U., Frederiksen, K., and Thomsen, L. (2022). Women with obesity participate less in cervical cancer screening and are more likely to have unsatisfactory smears: Results from a nationwide Danish cohort study. Prev. Med. 159:107072. doi: 10.1016/j.ypmed.2022.107072
van den Helder, R., Steenbergen, R., van Splunter, A., Mom, C., Tjiong, M., Martin, I., et al. (2022). HPV and DNA methylation testing in urine for cervical intraepithelial neoplasia and cervical cancer detection. Clin. Cancer Res. 28, 2061–2068.
Vitali, D., Bagri, P., Wessels, J., Arora, M., Ganugula, R., Parikh, A., et al. (2020). Curcumin can decrease tissue inflammation and the severity of HSV-2 infection in the female reproductive mucosa. Int. J. Mol. Sci. 21:337. doi: 10.3390/ijms21010337
Vranic, S., Cyprian, F., Akhtar, S., and Al Moustafa, A. (2018). The role of Epstein-Barr Virus in cervical cancer: A brief update. Front. Oncol. 8:113. doi: 10.3389/fonc.2018.00113
Walboomers, J. M., Jacobs, M. V., Manos, M. M., Bosch, F. X., Kummer, J. A., Shah, K. V., et al. (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19.
Waller, J., Bartoszek, M., Marlow, L., and Wardle, J. (2009). Barriers to cervical cancer screening attendance in England: A population-based survey. J. Med. Screen. 16, 199–204. doi: 10.1258/jms.2009.009073
Wu, X., Li, L., Li, Y., Jiang, M., Li, K., Li, Z., et al. (2021). Prognostic value of serological markers of hepatitis B virus infection in squamous cell cervical cancer. J. Cancer 12, 6620–6628. doi: 10.7150/jca.61249
Yadav, C., Yadav, R., Chhabra, R., Nanda, S., Ranga, S., Kadian, L., et al. (2023a). Correction to: Overview of genetic and epigenetic regulation of human papillomavirus and apoptosis in cervical cancer. Apoptosis doi: 10.1007/s10495-023-01822-8 [Epub ahead of print].
Yadav, C., Yadav, R., Chabbra, R., Nanda, S., Ranga, S., Kadian, L., et al. (2023b). Overview of genetic and epigenetic regulation of human papillomavirus and apoptosis in cervical cancer. Apoptosis doi: 10.1007/s10495-023-01812-w [Epub ahead of print].
Yordanov, A., Ivanov, I., Dineva, T., Popovska, S., Karcheva, M., Strashilov, S., et al. (2020). Lymphoepithelioma-like carcinoma of the uterine cervix: Correlation with Epstein-Barr virus and human papillomavirus infection. A single-center experience. Eur. J. Gynaecol. Oncol. 41, 913–918. doi: 10.31083/j.ejgo.2020.06.2107
Yordanov, A., Karcheva, M., Betova, T., Ivanov, I., Dineva, T., and Strashilov, S. (2019). Immunohistochemical study of human papilloma virus and Epstein–Barr virus in patients with lymphoepithelioma-like carcinoma of the uterine cervix. Arch. Balk Med. Union 53, 680–684.
Youn, J., Hur, S., Woo, J., Kim, Y., Lim, M., Park, S., et al. (2020). Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: Interim results of a single-arm, phase 2 trial. Lancet Oncol. 21, 1653–1660. doi: 10.1016/S1470-2045(20)30486-1
Yuan, M., Zhao, X., Wang, H., Hu, S., and Zhao, F. (2023). Trend in cervical cancer incidence and mortality rates in China, 2006-2030: A Bayesian age-period-cohort modeling study. Cancer Epidemiol. Biomarkers Prev. doi: 10.1158/1055-9965.EPI-22-0674 [Epub ahead of print].
Zhang, J., Yu, G., Yang, Y., Wang, Y., Guo, M., Yin, Q., et al. (2022). A small-molecule inhibitor of MDMX suppresses cervical cancer cells via the inhibition of E6-E6AP-p53 axis. Pharmacol. Res. 177:106128. doi: 10.1016/j.phrs.2022.106128
Zidi, S., Sahli, M., Mezlini, A., and Yacoubli-Loueslati, B. (2020). Association of combined tobacco smoking, hormonal contraceptive use and status matrimonial with cervical cancer evolution in Tunisian women. Pathol. Oncol. Res. 26, 217–222. doi: 10.1007/s12253-018-0442-4
Keywords: human papillomavirus (HPV), Epstein-Barr virus (EBV), human herpesvirus (HHV), uterine cervical carcinoma (UCC), molecular mechanisms, hepatitis B and C viruses (HBV and HCV)
Citation: Chu D, Liu T and Yao Y (2023) Implications of viral infections and oncogenesis in uterine cervical carcinoma etiology and pathogenesis. Front. Microbiol. 14:1194431. doi: 10.3389/fmicb.2023.1194431
Received: 27 March 2023; Accepted: 05 May 2023;
Published: 24 May 2023.
Edited by:
Jinlin Li, Uppsala University, SwedenReviewed by:
Hussein O. M. Al-Dahmoshi, University of Babylon, IraqCopyright © 2023 Chu, Liu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daming Chu, Z2dsa2RkQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.