
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 18 May 2023
Sec. Terrestrial Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1187572
The land damaged by coal mining can be recovered to healthy condition through various reclamation methods. Fertilization is one of the effective methods to improve soil fertility and microbial activity. However, the effects of coal-derived compound fertilizers (SH) on bacterial communities in coal mining subsidence areas still remain unclear. Here, we studied the effects on the nutrient characteristics and bacterial communities in fertilizer-reclaimed soil (CK, without fertilizer; CF, common compound fertilizers; SH, coal-derived compound fertilizers) in coal mining subsidence areas and we applied SH with four different nitrogen application rates (90, 135, 180, and 225 kg/hm2). The results showed that the application of SH significantly increased the contents of available nitrogen (AN), available phosphorus (AP), available potassium (AK), total phosphorus (TP) and soil organic matter (SOM) compared with CK, as well as the bacterial richness (Chao1) and diversity (Shannon) in reclaimed soil that increased first and then decreased with the increase of nitrogen application. Under the same nitrogen application rate (135 kg/hm2), the nutrient content, Chao1 and Shannon of SH2 treatments were higher than those of CF treatment. Meanwhile, SH increased the relative abundance of Proteobacteria, Actinobacteria and Gemmatimonadetes. LEfSe analysis indicated that the taxa of Acidobacteria and Actinobacteria were significantly improved under SH treatments. Canonical correspondence analysis (CCA) and Variance partitioning analysis (VPA) showed that SOM was the most important factor affecting the change of bacterial community structure in reclaimed soil. In conclusion, application of SH can not only increase nutrient content and bacterial diversity of reclaimed soil, but also improve bacterial community structure by increasing bacterial abundance.
Coal is an important source of energy in the world, and China is the largest producer and consumer of coal in the world (Feng et al., 2019). Large-scale land subsidence due to coal mining occurs in many areas (Guo et al., 2018) and causes many environmental problems such as soil erosion, soil quality degradation, soil ecosystem imbalance, etc. (Kumar et al., 2015; Ahirwal and Maiti, 2016; de Quadros et al., 2016; Ma et al., 2019). At the same time, a large number of solid wastes produced in mining area, which cannot be degraded in a short time, brings potential danger to the surrounding environment. Moreover, there are also some problems such as poor soil structure and low fertility in mining area (Heneghan et al., 2008). And land reclamation is an effective strategy to solve the conflict between coal mining and land resource protection (Hu Z. et al., 2014), soil reclamation aims to restore the nutrient properties and try to recover the soil from mining area to original condition through a series of reclamation methods (Upadhyay et al., 2016). Based on engineering, chemical and other measures, the reclaimed soil can quickly evolve to mellow soil, at the same time contribute to the increase the content of soil nutrient and promote the growth of crops. Therefore, relatively high ecological and economic benefits can be achieved in a short period of time with proper management of conservation methods (Feng et al., 2019). However, traditional fertilization method is presently a common method of soil reclamation in mining area. And due to excessive use of fertilizer and nitrogen addition, fertilizer nutrient use efficiency decreases so that it is not conducive to the sustainable development of agriculture (Carvalho, 2017; Chen et al., 2018). Therefore, it is very important to restore the quality of reclaimed soil in mining area, and it has become the current direction of development to use compound fertilizer developed from industrial and mining wastes to restore the quality of reclaimed soil.
Actually, industrial and mining wastes are not friendly enough, which mainly cause the area of land resources decreased and environment pollution if the wastes without properly solution. However, it is of great significance to explore the utilization in agricultural production because of the special structure and good physical and chemical properties, as well as make more land resources available. More importantly, coal slime, weathered coal and other industrial and mining wastes can improve soil properties, which has been increasingly widely used as a fertilizer synergist in agriculture (Leita et al., 2009; DiDonato and Hatcher, 2017; Amoah-Antwi et al., 2021). However, directly to apply the slime and weathered coal will not significantly stimulate microbial activity even with the supplement of nitrogen fertilizer (Tran et al., 2015), and slime is prone to cause secondary pollution due to water loss. A previous study showed that the combination of coal-derived compound fertilizer and microbial fertilizer produced from solid wastes (weathered coal, coal slime, fly ash and coal gangue) had significant effects on soil fertility, crop growth and utilization efficiency of water and fertilizers (Guo et al., 2016). Liu et al. (2017) revealed that there were different effects on soil enzyme activity and crop yield between different amounts of coal-derived compound fertilizers. The best effect was obtained when the amount of coal-derived compound fertilizer was 4,115 kg/hm2, and the yield of maize was increased by 6.96% ~ 218.90% and the urease activity was increased by 49.20% ~ 98.61% compared with the other two amounts. In addition, Fan et al. (2020) found that adding humic acid to the coal-derived compound fertilizers was more conducive to the improvement of soil carbon pool management index compared with ordinary coal-derived compound fertilizers. Besides, it was found that 135 kg/hm2 of nitrogen application had the best effect on the improvement of soil organic carbon and carbon pool management index in the treatment of different nitrogen application rates. Soil microorganisms are the most active part of soil, which are involved in the formation of soil structure (Sun et al., 2016) and crucial to vegetation establishment, soil formation and nutrient transformation. Especially in the early stage of reclamation (Machulla et al., 2005; Li et al., 2013), the composition and distribution of soil microbial community are highly sensitive to the changes in soil environment (Liu et al., 2020), which may have a huge impact on ecosystem function (Wu et al., 2017). Microorganisms are positively related to the degradation and transformation of various pollutants (Gianfreda and Rao, 2004), playing an essential role in soil restoration of mining area. Soil microorganisms can be used as a standard to evaluate the success of reclamation (Feng et al., 2019). However, researches on coal-derived compound fertilizers mainly focus on the effects of soil fertility and physicochemical properties, and few researches have explored the effects of coal-derived compound fertilizers on reclaimed soil microorganisms. How coal-derived compound fertilizers affect reclaimed soil bacterial communities in mining areas is still poorly understood.
Humic acid has large inner surface, good adsorption, adhesion and colloid dispersion, which can effectively reduce water loss from slime. At the same time, the combination of humic acid and various inorganic fertilizers contributes to the improvement of soil physicochemical properties and bioactivity (Muhammad et al., 2019; De Corato, 2020), and can also improve soil quality and increase fertilizer utilization efficiency (Chen et al., 2017), promote crop yield and quality (Ahmad et al., 2018; Khan et al., 2018). Therefore, this research adopts the coal-derived compound fertilizer, which is made up of solid waste and humic acid from industrial and mining areas and common chemical fertilizer, and sets up four kinds of different nitrogen application rates at the same time, aiming to test the hypothesis that different nitrogen application rates of coal-derived compound fertilizers would affect soil nutrients, bacterial diversity and community composition. The purpose of this study was to determine (i) whether and how the coal-derived compound fertilizer affects soil properties and changes soil bacterial community structure; (ii) determine which bacterial groups will be significantly affected by coal-derived compound fertilizer; and (iii) which soil properties contribute to changes of soil bacterial diversity and community composition. It is expected to provide basis for reutilization of solid waste, rational fertilization and reclamation of soil, and effective guidance for ecological restoration of degraded soil in coal-mining subsidence areas.
The experimental area is located in Luojianggou Village, Wangqiao Town, Xiangyuan County, Shanxi Province (112°42′-113°14′E and 36°23′-36°44′N). It has a warm temperate continental monsoon with an average temperature of 8°C – 9°C, the temperature from July to September is the highest, with an average of 23.4°C, and the annual average precipitation is 532.8 mm. The frost-free period is about 166 days. Before the subsidence, the land was flat and the soil was fertile. It subsidenced and turned into poor dry land, and the productivity of the land decreased seriously. The soil type is calcareous cinnamon soil, the basic characters of which were as follows (Table 1).
The tested fertilizers include two kinds, common compound fertilizer (CF, N: urea (46%), P2O5: single superphosphate (16%), K2O: potassium sulfate (52%)): the mass ratio of N, P2O5 and K2O is 25:10:10, produced by Shanxi Yefeng Chemical Fertilizer Co., Ltd. Coal-derived compound fertilizer (SH) is made of coal slime, humic acid, urea, calcium superphosphate and potassium ore powder in a certain proportion. The mass ratio of N, P2O5 and K2O is 25:10:10, and the organic mass is 26.65%.
The field experiment included six treatments: CK (no fertilizer), conventional compound fertilizer (CF, N 135 kg/hm2) and four coal-derived compound fertilizer treatments with nitrogen application rate of 90 kg/hm2 (SH1), 135 kg/hm2 (SH2), 180 kg/hm2 (SH3), 225 kg/hm2 (SH4), the contents of the base fertilizer in treatments of CF was 540 kg/hm2, SH1–SH4, respectively, were 360, 540, 720, 900 kg/hm2. All treatments had 3 replicates, 100 m2 (10 m × 10 m) per plot, randomly arranged, 18 plots in total. The maize variety planted is Dafeng 30, produced by Shanxi Dafeng Seed Industry Co., Ltd. Its growing period is 150 days, the planting density is 60,000 plants per hectare, and there is no irrigation during the whole growing period. Fertilizer is applied as a base fertilizer to the soil once a year before sowing.
Soil samples were collected after the maize harvest on September 28th, 2021. A total of 18 soil samples (6 treatments × 3 replicates) were analyzed. Soil samples from each plot were collected at 5 sampling points at a depth of 0 ~ 20 cm and mixed to form a single sample. After the removal of visible plant residues and stones, each sample was divided into two parts: one was stored at −80°C for DNA extraction, the other part is air-dried, ground and sifted to remove debris for chemical property analysis. All the chemical properties were determined by routine methods (Bao, 2000). Soil organic matter (SOM) content was measured by the method of potassium dichromate oxidation (K2Cr2O7), and available nitrogen (AN) content was measured using the alkaline hydrolysis-diffusion method. The available phosphorus (AP) was extracted with 0.5 mol/L NaHCO3 solution before being assayed using the colorimetric molybdenum blue method. The available potassium (AK) was extracted using 1 mol/L NH4OAc solution before being assayed via flame photometry. Total phosphorus (TP) was digested with sulfuric acid-perchloric acid and then measured via molybdenum blue.
Total DNA was extracted from 0.25 g of each soil sample using the HiPure Soil DNA Kits (Magen, Guangzhou, China). DNA concentration and purity were determined using NanoDrop ND-2000 photometer (Thermo Scientific, Wilmington, DE, United States). The V3-V4 region of the 16S rRNA gene was amplified using paired primers 341F (5′-CCTACGGGNBGCASCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′). The PCR reaction conditions were as follows: 95°C predenaturation for 5 min; 95°C denaturation for 1 min, 60°C annealing for 1 min, 72°C extension for 1 min, 30 cycles; and final 7 min extension at 72°C. The gel-purified products were used to construct a library, and they were sequenced on an Illumina MiSeq platform (Gene Denovo Biotechnology Co., Ltd., Guangzhou, China).
After the 16S rRNA gene fragment was sequenced, the obtained reads were filtered to remove adaptor sequences, low-quality sequences, and sequences with more than 10% of unknown nucleotides (N). The chimeras were checked and removed using the UCHIME (Edgar et al., 2011). The clean reads were clustered into operational taxonomic units (OTUs) of ≥ 97% similarity using UPARSE (Edgar, 2013; version 9.2.64). The sequence with highest abundance was selected as representative sequence within each OTU. The representative OTU sequences were classified into organisms by a naive Bayesian model using RDP classifier (version 2.2) based on SILVA database (version 132),1 with the confidence threshold value of 1. BioProject number: PRJNA944927.
One-way analysis of variance (ANOVA), and Tukey’s HSD test were used to compare the means for each environmental variable, with a significance level of p < 0.05. Non-metric multidimensional scaling plots (NMDS) were used to evaluate the overall differences in bacterial community composition, based on the Bray-Curtis distances. Alpha Diversity Indices, including Shannon and Chao1, were calculated using Mothur (Schloss et al., 2009). Adonis test was used to analyze the explanatory degree of the sample difference, and the permutation test was used to analyze the statistical significance of the groups. The correlation between soil bacterial community structure and environmental factors was tested using Mantel’s test. Canonical correspondence analysis (CCA) was performed using the R project “Vegan” package (version 2.5.3) and Variance partitioning analysis (VPA) was based on the species and environment factor abundances table, with the envfit test used to assess the significance of the impact of each factor in the CCA model, in order to clarify the influence of environmental factors on community composition. Pearson correlation coefficients between environmental factors and alpha diversity were calculated using the R project “psych” package. To identify taxa which were significantly affected by fertilization, we carried out the linear discriminant analysis (LDA) effect size (LEfSe; Segata et al., 2011), biomarkers examined in this study are consistent with the following standards: (i) minimum LDA score (log10 value) for discriminative features are ≥3 and (ii) alpha value for the factorial Kruskal-Wallis test between groups ≤0.05.
The results showed that the contents of AN, AP and TP were significantly increased after fertilization compared with CK (p < 0.05), and the overall order was SH > CF > CK (Table 2). While under SH2 treatment with the same nitrogen application rate, the results indicated that the effect of coal-derived compound fertilizer on soil nutrient content was better than that of common compound fertilizer. In particular, the contents of AN, AP, AK, SOM and TP in SH2 treatment were significantly increased by 17.46, 43.62, 24.43, 26.51, and 19.58% compared with CF (p < 0.05), respectively. In addition, the contents of AN, AP, AK, SOM and TP in SH treatments increased first and then decreased with the increase of nitrogen application, and the contents in SH3 treatment were the highest, respectively, increased by 58.46, 112.65, 35.00, 26.51, and 19.58%, which was significantly different from that in CF treatment (p < 0.05).
Bacterial diversity and richness (Figure 1). Compared with CK, fertilization could effectively improve bacterial richness (Chao1) and diversity (Shannon) in reclaimed soil. At the same nitrogen application rate, Chao1 in SH2 was significantly higher than that in CF (p < 0.05), and Shannon in SH2 was higher than that in CF though not significantly. In addition, Chao1 and Shannon in SH treatments increased initially and decreased afterwards with the increase of nitrogen application. Specifically, Chao1 was highest in SH2 treatment while Shannon in SH3 treatment.
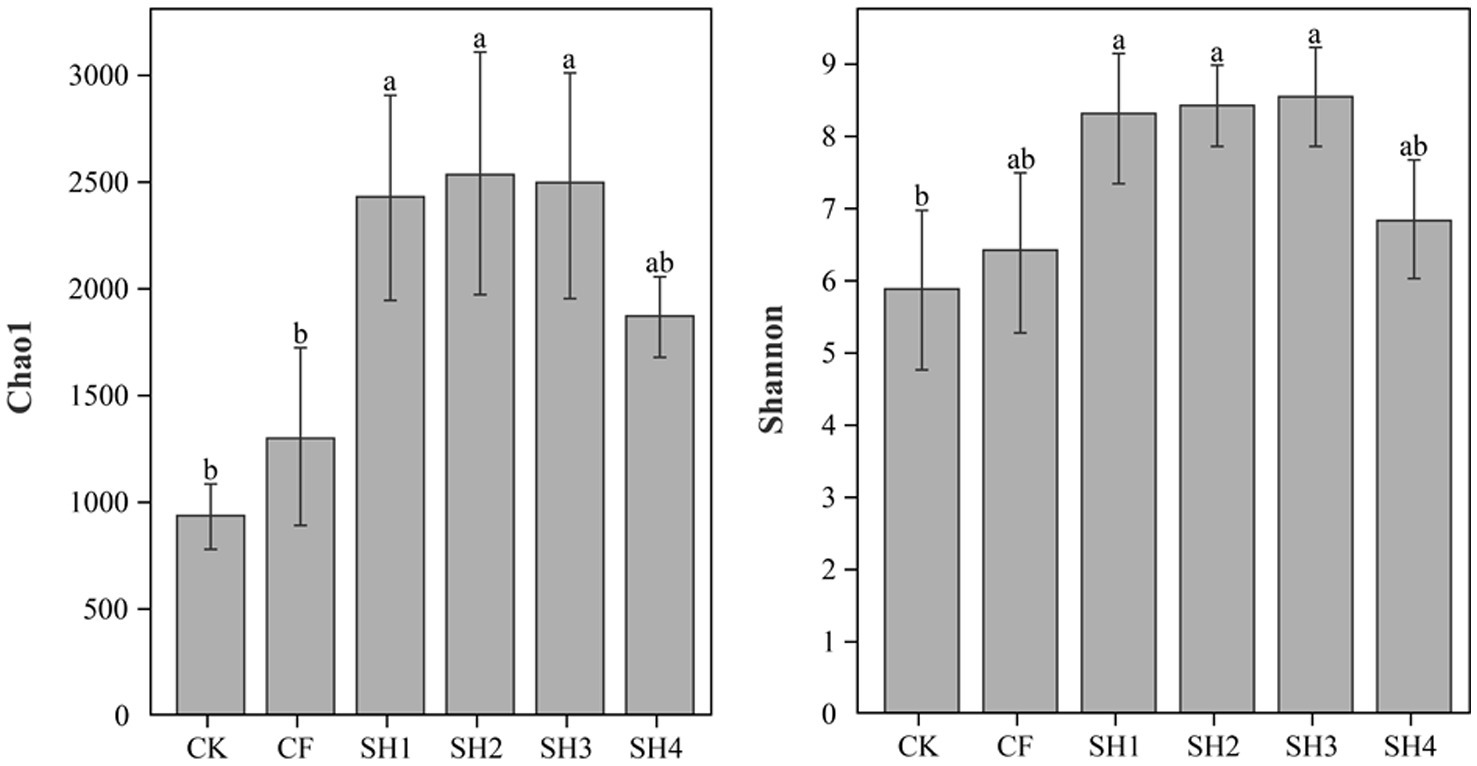
Figure 1. Comparison of estimated alpha diversity indices of 16 s rRNA gene libraries for clustering at 97% similarity, obtained from high throughput sequencing analysis. Data are the means, n = 4, error bars represent standard error. Bars with the same letter are not significantly different at p ≤ 0.05.
We evaluated the taxonomic composition of bacteria, with Proteobacteria, Actinobacteria, Gemmatimonadetes, Planctomycetes, and Acidobacteria being the dominant phyla in reclaimed soil (Figure 2). Compared with CK, the relative abundance of Proteobacteria, Actinobacteria and Gemmatimonadetes were increased in SH treatments, while the relative abundance of Planctomycetes was decreased in SH treatments. And the highest relative abundance of Proteobacteria was detected in SH4 treatment (Supplementary Table S1), which was significantly different from CK (p < 0.05). Besides, the relative abundance of Gemmatimonadetes and Acidobacteria were highest in SH2 treatment (p < 0.05) and the highest relative abundance of Actinobacteria was found in SH3 all significantly different from CK (p < 0.05). CF treatment increased the relative abundance of Proteobacteria, Actinobacteria and Planctomycetes, while decreased the abundance of Gemmatimonadetes and Acidobacteria.
To determine whether fertilization led to shifts in soil bacterial communities, we profiled the structural changes in bacterial communities (Genus level) using NMDS based on Bray-Curtis dissimilarities (Figure 3). There were significant differences between bacterial communities and CK in different fertilization treatments, and the stress of 0.048 also indicated that NMDS fit well. There were also significant differences among the four SH nitrogen application rates. In addition, Adonis (Permanova) test confirmed that there was significant difference in soil microbial community among different treatments (R2 = 0.3925, p < 0.01).
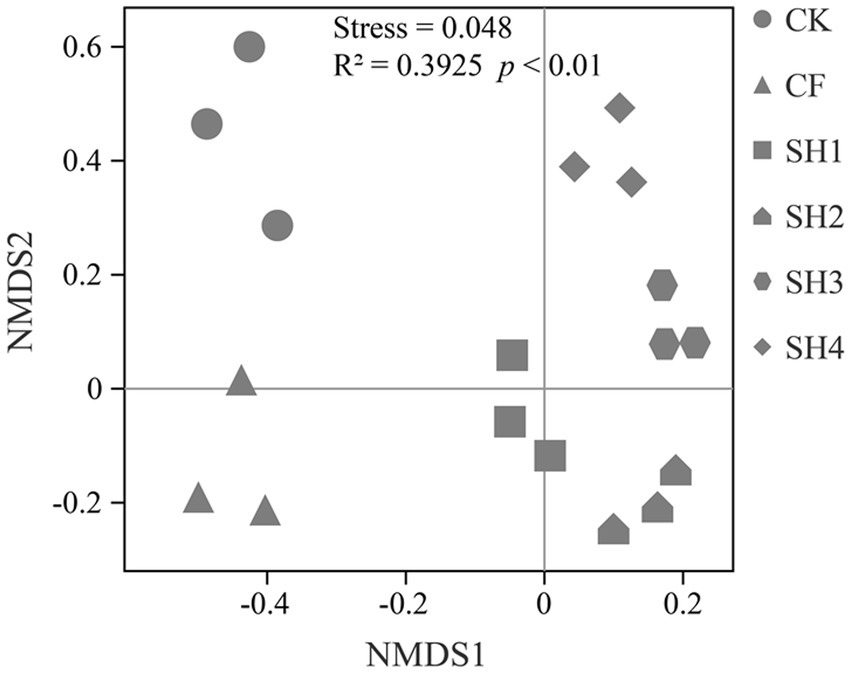
Figure 3. Effects of fertilization on bacterial communities composition (Genus level) in reclaimed soil. Non-metric multidimensional scaling (NMDS) of bacterial communities based on Bray-Curtis dissimilarities.
We further identified high-dimensional biomarkers at each taxonomic level using LEfSe and explored the significantly changed taxa (Figure 4). The results showed that the taxa of RF32 (LDA score = 4.48) changed significantly in CK. However, Bradyrhizobiaceae (LDA score = 3.71) and Brevundimonas (LDA score = 3.06) were significantly altered in SH1, while Polyangiaceae (LDA score = 3.27) was significantly altered in SH2. In addition, we found that the abundance of Firmicutes and Fusobacteria was the highest in CK and CF treatments, and only three taxa from Firmicutes were significantly decreased in SH1 and SH4 treatments including Streptococcaceae (LDA score = 3.12), Streptococcus (LDA score = 3.10), and Guilliermondii (LDA score = 3.09). Meanwhile, the Acidobacteria taxa and Actinobacteria taxa were only found in SH treatments.
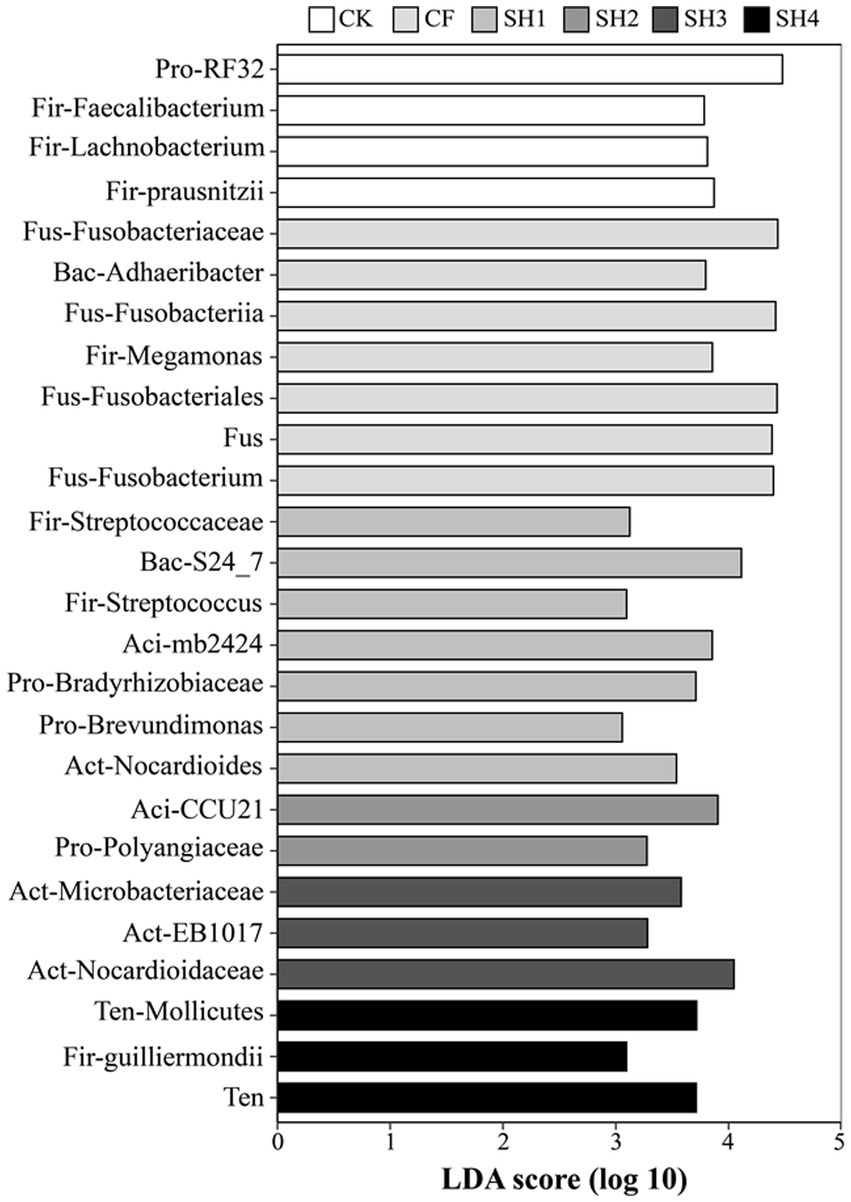
Figure 4. In reclaimed soils, key phylogenetic phenotypes of bacteria were significantly altered between treatments using linear discriminant analysis (LDA) effect sizes (LEfSe). Pro, Proteobacteria; Fir, Firmicutes; Fus, Fusobacteria; Bac, Bacteroidetes; Aci, Acidobacteria; Act, Actinobacteria; Ten, Tenericutes; Aci, Acidobacteria; Act, Actinobacteria; Ten, Tenericutes.
The Mantel test for the association between bacterial community structure (Bray-Curtis distance) and soil properties indicated that AN, AP, TP, and SOM were significantly correlated with bacterial community structure (p < 0.05, Supplementary Table S2). The relationship among soil nutrients, bacterial diversity and bacterial community structure was determined using the PLSPM (Figure 5). The results of the PLSPM revealed that soil nutrients had significant positive correlation with bacterial diversity (0.606) and bacterial community structure (0.494, p < 0.001). Bacterial diversity had significant positive relationships with bacterial community structure (0.532, p < 0.001). Meanwhile, soil nutrients can also affect the bacterial community structure indirectly through soil microbial diversity.
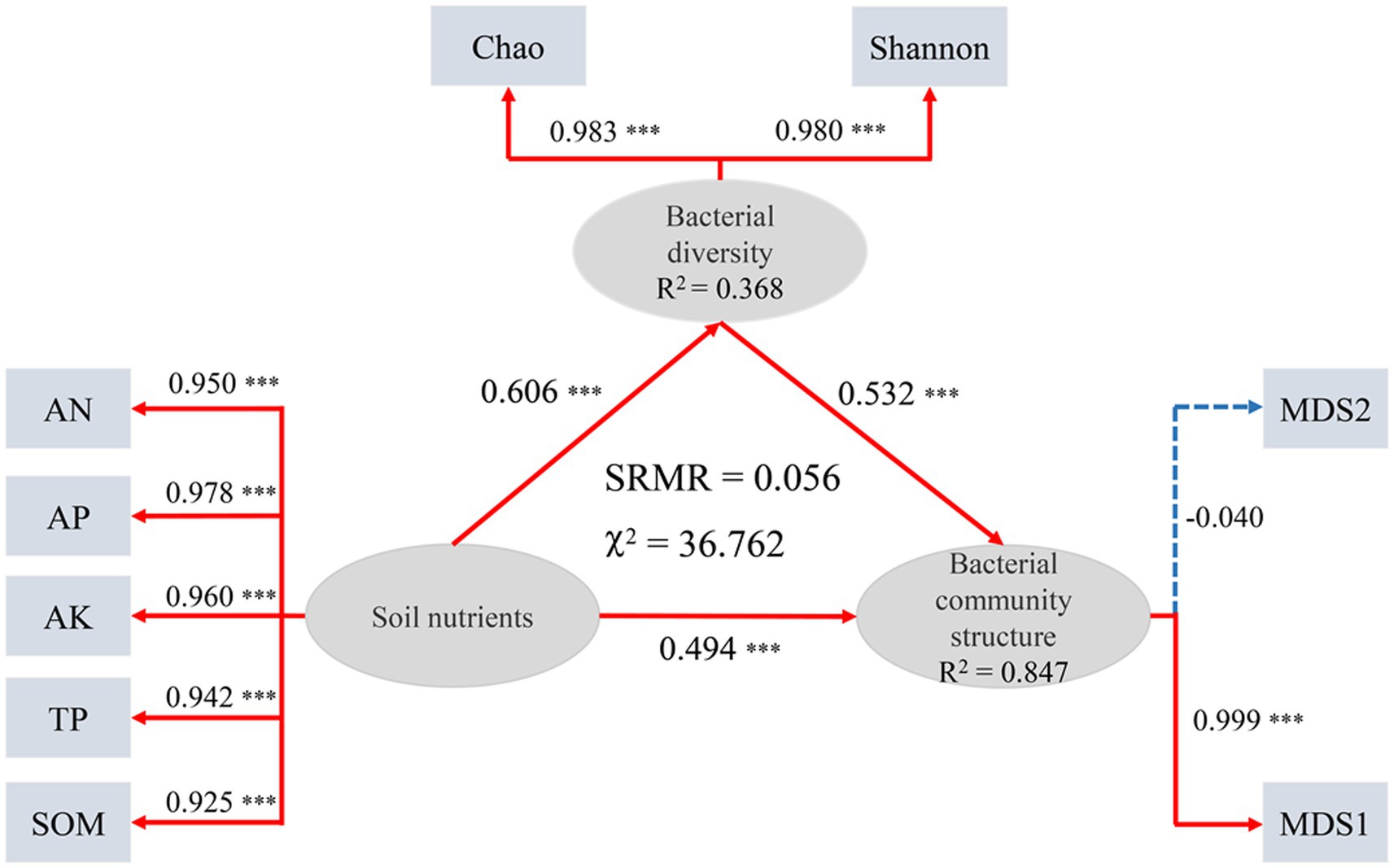
Figure 5. Partial Least Squares Path Modeling (PLSPM). Each oval shape represents an observed variable (i.e., measured) and box represents latent variable (i.e., constructs). Red lines represent positive effects and blue lines represent negative effect. Dashed arrows show that coefficients did not differ significantly from 0 (p > 0.05). Numbers on the lines in the PLSPM model are the ‘total effects’ values, ***p < 0.001.
CCA results directly show the effect of environmental factors on soil bacterial communities (Figure 6). At the Genus level, the five soil parameters accounted for 79.82% of the total variation, with the first and second axes, respectively, accounting for 61.84 and 17.44%, respectively. The bacterial communities in soil from SH treatments were positively correlated with the concentrations of AN, AP, AK, TP and SOM. Among these, SOM (R2 = 0.6985, p = 0.001) was the most important environmental factor affecting the variation of bacterial community. Besides, variance partitioning analysis (VPA) showed that SOM accounted for 10.66% of the total Variance and followed by AP 9.92% (Figure 7), which was further verified that SOM was the most important factor affecting the variation of bacterial community structure in reclaimed soil. In addition, AN, AP, AK and TP were also significantly correlated with the variance of bacterial community (Supplementary Table S3).
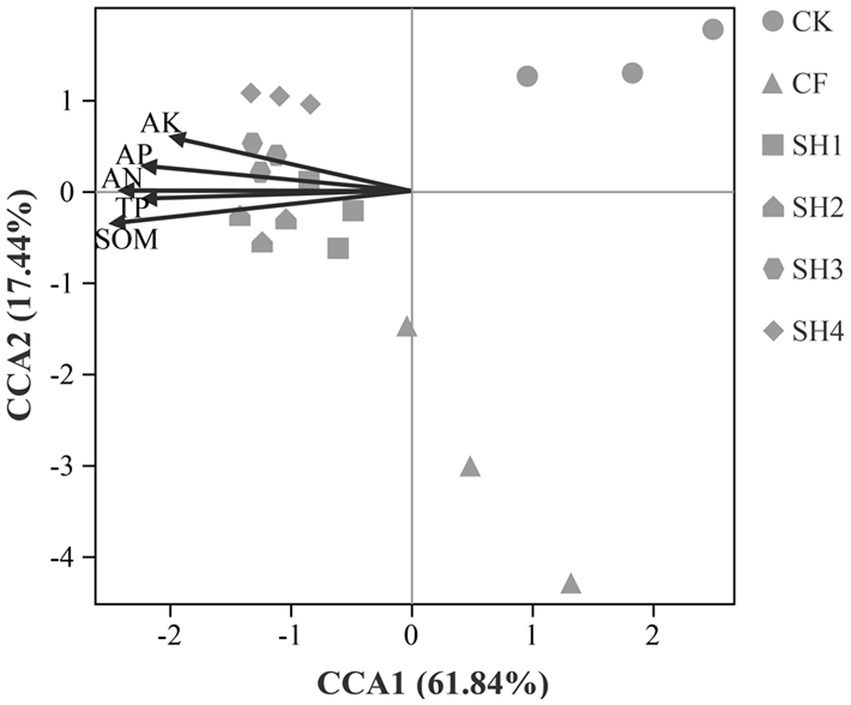
Figure 6. Canonical correspondence analysis indicated the potential relationship between soil environmental variables and soil bacterial community (Genus level).
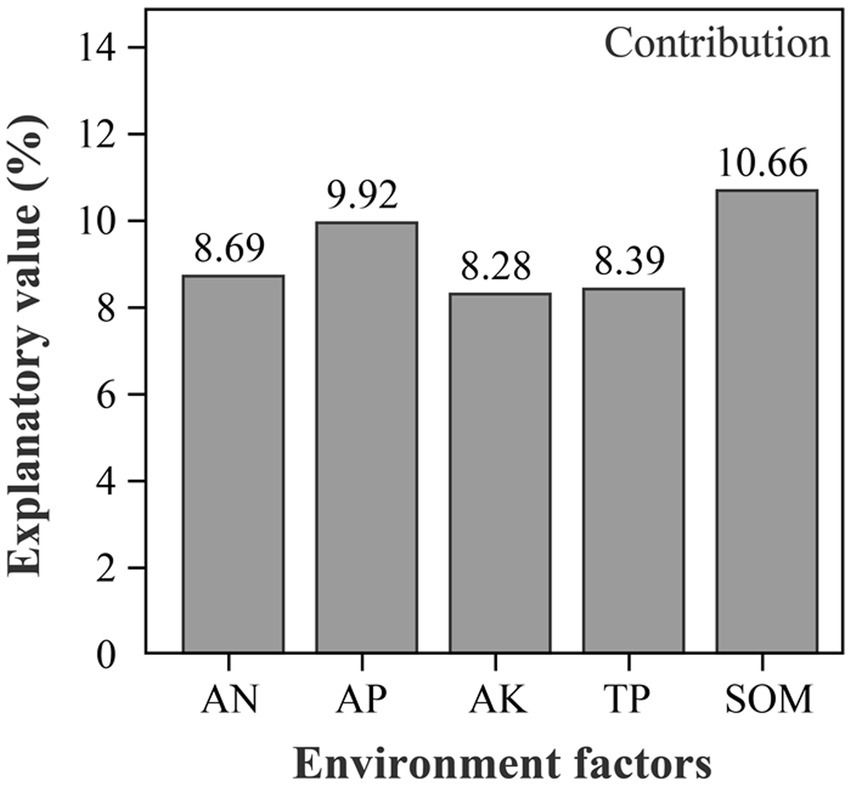
Figure 7. VPA analysis of the contribution of environmental factors to the total variation of species.
Fertilization can effectively improve the soil’s total and available nutrients, and accelerate soil maturity (Sun et al., 2016). Excessive use of chemical fertilizers hardens the soil, reduces soil fertility and pollutes the natural environment (Pahalvi et al., 2021). Low-rank coals and their derivatives are rich in micronutrients and are also valuable sources of organic matter containing large amounts of humus (Akimbekov et al., 2021), which can be used as an alternative source for the management of soil fertility. During our reclamation, coal-derived compound fertilizers significantly increased soil nutrients (AN, AP, AK, SOM, and TP; Table 2), especially in SH3 treatment with 180 kg/hm2 nitrogen application, and the results were similar to those in this study (Li et al., 2019; Dehsheikh et al., 2020). In addition, SH2 had a better effect on soil nutrient content than CF under the same nitrogen application rate (135 kg/hm2), which may be due to the addition of humic acid to coal-derived compound fertilizer. The results indicated that the combination of humic acid and inorganic fertilizer can effectively improve the utilization rate of fertilizer (Chen et al., 2017). It is worth noting that when the nitrogen application rate increased to 225 kg/hm2 (SH4), soil nutrient content decreased in different degrees, which indicated that the effect of combined application of coal-derived compound fertilizer and suitable nitrogen amount on soil nutrient was better, and excessive nitrogen application had a negative effect on SOM synthesis and decomposition (Galloway et al., 2008), while soil acidification caused by excess nitrogen will prevent inorganic phosphorus from adsorbing to mineral surfaces (Cusack et al., 2016). Reducing the amount of chemical fertilizer combined application is more beneficial to the growth of soil nutrient level (Zhong et al., 2010; Zhao et al., 2016).
Soil microbial diversity is critical to the integrity, function and long-term sustainability of soil ecosystems (Kandeler et al., 2006; Cheng et al., 2018), and restoration of microbial diversity is a key issue in regenerating soil systems. In this study, we analyzed microbial diversity and richness using Shannon and Chao1, respectively. The results showed that SH could effectively increase the bacteria alpha diversity bacteria compared with CK treatment. At the same nitrogen rate (135 kg/hm2), the bacteria alpha diversity in SH2 treatment was higher than that in CF treatment, which indicated that the application of coal-derived compound fertilizer promoted the recovery of soil microorganisms (Figure 1). Interestingly, Chao1 and Shannon also showed a trend of first increasing and then decreasing with the increase of nitrogen application in SH, which is consistent with the trend of nutrient content change in reclaimed soil, there is ample evidence that an increase in nitrogen input leads to a decrease in bacterial diversity (Zhou et al., 2015), and that high mineral nitrogen content may create stress conditions that inhibit bacterial growth, resulting in a significant decrease in bacterial diversity (Shen et al., 2016). Moreover, the subsidy-stress hypothesis states that higher levels of nitrogen addition results in reduced diversity, while moderate levels of amendment can promote microbial diversity (Odum et al., 1979; Odum, 1985). The application of exogenous organic matter is often crucial to improve soil fertility and nutrient management. The addition of humic acids to inorganic fertilizers can change soil microbial community structure, improve soil microbial diversity and soil nutrient content (Li et al., 2019; Wang et al., 2022), which were similar to the results of this experiment. To sum up, coal-derived compound fertilizer can effectively improve soil nutrients and promote the recovery of soil microbial diversity, and SH3 (180 kg/hm2 nitrogen application) is the most suitable.
NMDS and Adonis (Permanova) test showed that fertilization resulted in significant differences in soil bacterial community composition, and there were also significant differences in SH among four nitrogen application rates (Figure 2). According to the analysis of the changes in taxa composition (Figure 3), the relative abundance of Proteobacteria in the reclaimed soil showed an overall increasing trend with the increase of SH nitrogen application, while Acidobacteria decreased with the increase of SH nitrogen application (Supplementary Table S1), which are consistent with previous studies (Ling et al., 2017; Wang et al., 2018). The changes in bacterial composition after the increase of nitrogen application can be explained by the eutrophication hypothesis (Fierer et al., 2007). And Proteobacteria can adapt to resource-rich (carbon-rich) environments well. Moreover, as a co-trophic group, Proteobacteria has a rapid growth rate and is more likely to grow under nutrient-rich conditions (Fierer et al., 2007, 2012), while nutrient-poor populations are conducive to growth under low-carbon conditions.
In the study, Proteobacteria, Actinobacteria and Acidobacteria were the main phylums from reclaimed soil in mining area, which was consistent with other researches about soil microbial community. Among these, Proteobacteria was the key phylums with the highest relative abundance in reclaimed soil, due to it could survive in extreme environment via carbon cycling and nitrogen fixing processes (Kasemodel et al., 2019). We found that soil nutrient contents (AN, AP, AK, TP, and SOM) were positively correlated with the abundance of Proteobacteria, Actinobacteria and Gemmatimonadetes, while negatively correlated with the abundance of Firmicutes and Fusobacteria (Supplementary Figure S1). The LEfSe analysis also showed that Actinobacteria taxa were only found in the treatments with high soil nutrient content (SH1 – SH3; Figure 4), indicating that Nocardioides, Microbacteriaceae, EB1017, Nocardioidaceae were positively correlated with soil nutrient contents. Studies have shown that Actinobacteria plays an important role in improving agricultural soil quality (Francioli et al., 2016), participates in organic matter turnover and carbon cycling, supplements the nutrient supply in soils and is an important component of humus formation (Anandan et al., 2016). In this study, Nocardioides, Microbacteriaceae, and Nocardioidaceae are all belong to Actinomycetales. Previous reports have shown that members of Actinomycetales have many ecological roles, such as the potential to inhibit the growth of a variety of plant pathogens (Sprusansky et al., 2005; Jeffrey et al., 2007), to promote plant growth, and it also can help to solubilize phosphate, produce siderophore and fix nitrogen (Bhatti et al., 2017). In addition, Actinomycetes do not pollute the environment; on the contrary, they help maintain a biological balance between soil and nutrient cycling. However, the taxa of Firmicutes and Fusobacteria were only found in CK, CF and SH1 with low nitrogen application, indicating that the taxa of Firmicutes and Fusobacteria were negatively correlated with soil nutrient content. Our results suggest that Proteobacteria and Actinobacteria are the dominant phyla in reclaimed soils (Figure 3), which is consistent with previous studies (Lauber et al., 2009; Rastogi et al., 2010; Zhan and Sun, 2014). The consistency of dominant phyla in different mining areas shows that these bacteria play an important role in soil improvement and have extensive adaptability to soil environment in mining areas.
The interactions between soil chemical properties, biological properties, and microbial communities have triggered debates about the mechanisms of nutrient cycling and ecosystem processes, and understanding these complex interactions is essential for the proper functioning of biomes. Numerous soil properties play critical roles in shaping bacterial community structure and diversity when the soil is subjected to fertilization (Dangi et al., 2012; Sun et al., 2016; Yu et al., 2019), and improving soil fertility leads to higher bacterial abundance and diversity (Cui et al., 2012; Li et al., 2014). In this study, we found that Chao1 and Shannon were positively correlated with SOM, AP and TP (p < 0.001, p < 0.01, and p < 0.05), and the results showed that the accumulation of SOM and nutrients could well explain the higher bacterial richness and diversity index in reclaimed soil. We also found that most abundant phyla were significantly associated with one or more selected soil properties, underscoring the critical role of SOM and nutrients in shaping the abundance and diversity of soil bacterial communities (Supplementary Figure S1).
Previous studies have shown that SOM and AP are major factors influencing the composition of bacterial communities in soil (Fierer et al., 2012; Hu Y. et al., 2014; Liu et al., 2014; Yu et al., 2019), which is consistent with our study. For one thing, the reclaimed soil is exposed to poor soil nutrients and low SOM content. While the content of SOM influence ecosystem function more, it would induce the change of bacterial community structure if increased SOM in a short time (Zhang et al., 2023). For another thing, SOM can effectively promote the growth and recovery of bacteria in reclaimed soil, while fertilization can apparently improve the content of SOM and apply energy for the activities of microorganisms, which contribute to the increase of soil microbial diversity and influence the composition of bacterial community. In this study, the results of CCA and Mantel test indicated that SOM was the main factor influencing bacterial community structure in reclaimed soil (Figure 6; Supplementary Table S2). Many biological factors directly affect carbon mineralization in soils, and carbon sources are one of the most important factors affecting microbial communities (Blagodatskaya and Kuzyakov, 2008). VPA and correlation analysis further showed that SOM had a positive effect on soil microbial diversity (Figure 7). Studies have shown that the increase of SOM can promote soil agglomeration, improve soil physical properties and nutrients, thereby promoting the growth and recovery of bacteria in reclaimed soil (Li L. et al., 2021). Meanwhile, SOM plays a key role in plant growth and directly affects soil bacterial diversity (Zeng et al., 2016; Li W. et al., 2021; Xu et al., 2021). Moreover, as a reservoir of carbon and nitrogen sources, continuous decomposition of SOM can contribute to the improvement of bacterial community diversity by producing multiple substrates for the microbiota (Li et al., 2014).
The purpose of soil reclamation in coal mining subsidence area is to reconstruct productive, healthy and sustainable ecosystem of land use after mining. Increasing soil nutrients and enriching microbial population through fertilization is an effective method for ecological restoration in mining areas (Mummey et al., 2002; Cao et al., 2020; Li et al., 2020; Yan et al., 2021). In our study, soil nutrient and bacterial community diversity increased significantly after 4 years of reclamation. However, the reconstruction of soil microbial community in coal mining subsidence area depends not only on the mode of reclamation, but also on the time of reclamation. The study on the reclamation of mining area shows that the most important restoration stage of microbial community is 5 ~ 20 years after reclamation, and the difference is mainly related to fertilization, soil properties and vegetation (Dangi et al., 2012; Li et al., 2015; Cheng et al., 2018). Therefore, there is still a need for continuous monitoring of soil nutrients and microbial communities in selected sites, taking into account the effects of fertilization and the timing of reclamation.
In conclusion, the application of coal-deriver compound fertilizer can promote soil bacterial diversity and community composition by improving soil nutrients, and improve beneficial bacterial groups that play a significant role in ecosystem function. After 4 years of reclamation, the abundance and diversity of soil bacteria increased with the increase of soil nutrients. In addition, fertilization could effectively increase the abundance of soil bacteria, and it is more obvious that coal-derived compound fertilizer could positively affect bacterial diversity, especially SOM, which is critical for the formation of major bacterial populations.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
HM provided the resources for performing the experiment, and helped to came up with the idea for this article. SW was responsible for the experiment process, data curation, original draft writing, and article revised. JZ, XW, CQ, and JH contributed to review and editing of manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by the State Key Program of National Natural Science of China (Grant number U1710255-3), National Natural Science Foundation of China (41907215), Natural Science Research Project of Shanxi Province (202103021224171) and Technology Innovation Fund of Shanxi Agricultural University (2017ZZ08).
We are thankful for “Gene Denovo Biotechnology Co., Ltd. (Guangzhou, China)” for their help in analyzing the pyrosequencing data. The data were analyzed on the Omicsmart (www.omicsmart.com) and Majorbio (www.majorbio.com) free online cloud platforms.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1187572/full#supplementary-material
Ahirwal, J., and Maiti, S. K. (2016). Assessment of soil properties of different land uses generated due to surface coal mining activities in tropical Sal (Shorea robusta) forest, India. Catena 140, 155–163. doi: 10.1016/j.catena.2016.01.028
Ahmad, T., Khan, R., and Nawaz Khattak, T. (2018). Effect of humic acid and fulvic acid based liquid and foliar fertilizers on the yield of wheat crop. J. Plant Nutr. 41, 2438–2445. doi: 10.1080/01904167.2018.1527932
Akimbekov, N., Digel, I., Abdieva, G., Ualieva, P., and Tastambek, K. (2021). Lignite biosolubilization and bioconversion by Bacillus sp.: the collation of analytical data. Biofuels 12, 247–258. doi: 10.1080/17597269.2020.1753936
Amoah-Antwi, C., Kwiatkowska-Malina, J., Fenton, O., Szara, E., Thornton, S. F., and Malina, G. (2021). Holistic assessment of biochar and brown coal waste as organic amendments in sustainable environmental and agricultural applications. Water Air Soil Pollut. 232, 1–25. doi: 10.1007/s11270-021-05044-z
Anandan, R., Dharumadurai, D., and Manogaran, G. P. (2016). “An introduction to actinobacteria” in Actinobacteria-basics and biotechnological applications. eds. D. Dhanasekaran and Y. Jiang (Rijeka: IntechOpen)
Bao, S. D. (2000). Agricultural chemical analysis of soil. China Agriculture Press:Beijing. In Chinese.
Bhatti, A. A., Haq, S., and Bhat, R. A. (2017). Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 111, 458–467. doi: 10.1016/j.micpath.2017.09.036
Blagodatskaya, Е., and Kuzyakov, Y. (2008). Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertil. Soils 45, 115–131. doi: 10.1007/s00374-008-0334-y
Cao, Y., Zhou, B., Wang, X., Meng, H., Zhang, J., Li, L., et al. (2020). Different fertilization treatments in coal mining-affected soils change bacterial populations and enable soil reclamation. Ann. Microbiol. 70, 1–11. doi: 10.1186/s13213-020-01589-2
Carvalho, F. P. (2017). Pesticides, environment, and food safety. Food Energy Sec 6, 48–60. doi: 10.1002/fes3.108
Chen, X., Kou, M., Tang, Z., Zhang, A., Li, H., and Wei, M. (2017). Responses of root physiological characteristics and yield of sweet potato to humic acid urea fertilizer. PLoS One 12:e0189715. doi: 10.1371/journal.pone.0189715
Chen, J., Luo, Y., Van Groenigen, K. J., Hungate, B. A., Cao, J., Zhou, X., et al. (2018). A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 4:eaaq1689. doi: 10.1126/sciadv.aaq1689
Cheng, Z., Zhang, F., Gale, W. J., Wang, W., Sang, W., and Yang, H. (2018). Effects of reclamation years on composition and diversity of soil bacterial communities in Northwest China. Can. J. Microbiol. 64, 28–40. doi: 10.1139/cjm-2017-0362
Cui, J., Liu, C., Li, Z., Wang, L., Chen, X., Ye, Z., et al. (2012). Long-term changes in topsoil chemical properties under centuries of cultivation after reclamation of coastal wetlands in the Yangtze Estuary, China. Soil Tillage Res. 123, 50–60. doi: 10.1016/j.still.2012.03.009
Cusack, D. F., Karpman, J., Ashdown, D., Cao, Q., Ciochina, M., Halterman, S., et al. (2016). Global change effects on humid tropical forests: evidence for biogeochemical and biodiversity shifts at an ecosystem scale. Rev. Geophys. 54, 523–610. doi: 10.1002/2015RG000510
Dangi, S. R., Stahl, P. D., Wick, A. F., Ingram, L. J., and Buyer, J. S. (2012). Soil microbial community recovery in reclaimed soils on a surface coal mine site. Soil Sci. Soc. Am. J. 76, 915–924. doi: 10.2136/sssaj2011.0288
De Corato, U. (2020). Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: a review under the perspective of a circular economy. Sci. Total Environ. 738:139840. doi: 10.1016/j.scitotenv.2020.139840
de Quadros, P. D., Zhalnina, K., Davis-Richardson, A. G., Drew, J. C., Menezes, F. B., Flávio, A., et al. (2016). Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 98, 195–203. doi: 10.1016/j.apsoil.2015.10.016
Dehsheikh, A. B., Sourestani, M. M., Zolfaghari, M., and Enayatizamir, N. (2020). Changes in soil microbial activity, essential oil quantity, and quality of Thai basil as response to biofertilizers and humic acid. J. Clean. Prod. 256:120439. doi: 10.1016/j.jclepro.2020.120439
DiDonato, N., and Hatcher, P. G. (2017). Alicyclic carboxylic acids in soil humic acid as detected with ultrahigh resolution mass spectrometry and multi-dimensional NMR. Org. Geochem. 112, 33–46. doi: 10.1016/j.orggeochem.2017.06.010
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Fan, X., Liu, B., Meng, H., Hong, J., and Zhang, J. (2020). The effects of coal-derived compound fertilizers on soil carbon and its formations in reclaimed soil in coal mining areas. J. Irrig. Drain. 39, 75–81. doi: 10.13522/j.cnki.ggps.2019115
Feng, Y., Wang, J., Bai, Z., and Reading, L. (2019). Effects of surface coal mining and land reclamation on soil properties: a review. Earth Sci. Rev. 191, 12–25. doi: 10.1016/j.earscirev.2019.02.015
Fierer, N., Bradford, M. A., and Jackson, R. B. (2007). Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364. doi: 10.1890/05-1839
Fierer, N., Lauber, C. L., Ramirez, K. S., Zaneveld, J., Bradford, M. A., and Knight, R. (2012). Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 6, 1007–1017. doi: 10.1038/ismej.2011.159
Francioli, D., Schulz, E., Lentendu, G., Wubet, T., Buscot, F., and Reitz, T. (2016). Mineral vs. organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 7:1446. doi: 10.3389/fmicb.2016.01446
Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z., Freney, J. R., et al. (2008). Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892. doi: 10.1126/science.1136674
Gianfreda, L., and Rao, M. A. (2004). Potential of extra cellular enzymes in remediation of polluted soils: a review. Enzym. Microb. Technol. 35, 339–354. doi: 10.1016/j.enzmictec.2004.05.006
Guo, H., YingHe, X., JianPing, H., Ying, W., Sheng, B., and Libin, G. (2016). Effects of coal-derived compound fertilizer on soil nutrient, corn yield and water and fertilizer use efficiency in reclaimed area. J. Soil Water Conserv. 30, 213–218. doi: 10.13870/j.cnki.stbcxb.2016.02.037
Guo, X.-M., Zhao, T.-Q., Chang, W.-K., Xiao, C.-Y., and He, Y.-X. (2018). Evaluating the effect of coal mining subsidence on the agricultural soil quality using principal component analysis. Chilean J. Agric. Res. 78, 173–182. doi: 10.4067/S0718-58392018000200173
Heneghan, L., Miller, S. P., Baer, S., Callaham, M. A. Jr., Montgomery, J., Pavao-Zuckerman, M., et al. (2008). Integrating soil ecological knowledge into restoration management. Restor. Ecol. 16, 608–617. doi: 10.1111/j.1526-100X.2008.00477.x
Hu, Y., Xiang, D., Veresoglou, S. D., Chen, F., Chen, Y., Hao, Z., et al. (2014). Soil organic carbon and soil structure are driving microbial abundance and community composition across the arid and semi-arid grasslands in northern China. Soil Biol. Biochem. 77, 51–57. doi: 10.1016/j.soilbio.2014.06.014
Hu, Z., Yang, G., Xiao, W., Li, J., Yang, Y., and Yu, Y. (2014). Farmland damage and its impact on the overlapped areas of cropland and coal resources in the eastern plains of China. Resour. Conserv. Recycl. 86, 1–8. doi: 10.1016/j.resconrec.2014.01.002
Jeffrey, L., Sahilah, A., Son, R., and Tosiah, S. (2007). Isolation and screening of actinomycetes from Malaysian soil for their enzymatic and antimicrobial activities. J. Trop. Agric. Food Sci. 6:159. doi: 10.4172/2155-952x.c1.064
Kandeler, E., Mosier, A. R., Morgan, J. A., Milchunas, D. G., King, J. Y., Rudolph, S., et al. (2006). Response of soil microbial biomass and enzyme activities to the transient elevation of carbon dioxide in a semi-arid grassland. Soil Biol. Biochem. 38, 2448–2460. doi: 10.1016/j.soilbio.2006.02.021
Kasemodel, M. C., Sakamoto, I. K., Varesche, M. B. A., and Rodrigues, V. G. S. (2019). Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci. Total Environ. 675, 367–379. doi: 10.1016/j.scitotenv.2019.04.223
Khan, R., Khan, M., Khan, A., Saba, S., Hussain, F., and Jan, I. (2018). Effect of humic acid on growth and crop nutrient status of wheat on two different soils. J. Plant Nutr. 41, 453–460. doi: 10.1080/01904167.2017.1385807
Kumar, S., Maiti, S. K., and Chaudhuri, S. (2015). Soil development in 2–21 years old coalmine reclaimed spoil with trees: a case study from Sonepur-Bazari opencast project, Raniganj Coalfield, India. Ecol. Eng. 84, 311–324. doi: 10.1016/j.ecoleng.2015.09.043
Lauber, C. L., Hamady, M., Knight, R., and Fierer, N. (2009). Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 75, 5111–5120. doi: 10.1128/AEM.00335-09
Leita, L., Margon, A., Pastrello, A., Arčon, I., Contin, M., and Mosetti, D. (2009). Soil humic acids may favour the persistence of hexavalent chromium in soil. Environ. Pollut. 157, 1862–1866. doi: 10.1016/j.envpol.2009.01.020
Li, Y., Chen, L., and Wen, H. (2015). Changes in the composition and diversity of bacterial communities 13 years after soil reclamation of abandoned mine land in eastern China. Ecol. Res. 30, 357–366. doi: 10.1007/s11284-014-1230-6
Li, Y., Chen, L., Wen, H., Zhou, T., Zhang, T., and Gao, X. (2014). 454 pyrosequencing analysis of bacterial diversity revealed by a comparative study of soils from mining subsidence and reclamation areas. J. Microbiol. Biotechnol. 24, 313–323. doi: 10.4014/jmb.1309.09001
Li, Y., Fang, F., Wei, J., Wu, X., Cui, R., Li, G., et al. (2019). Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: a three-year experiment. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-019-48620-4
Li, L., Li, T., Meng, H., Xie, Y., Zhang, J., and Hong, J. (2021). Effects of seven-year fertilization reclamation on bacterial community in a coal mining subsidence area in Shanxi, China. Int. J. Environ. Res. Public Health 18:12504. doi: 10.3390/ijerph182312504
Li, F., Men, S., Zhang, S., Huang, J., Puyang, X., Wu, Z., et al. (2020). Responses of low-quality soil microbial community structure and activities to application of a mixed material of humic acid, biochar, and super absorbent polymer. J. Microbiol. Biotechnol. 30, 1310–1320. doi: 10.4014/jmb.2003.03047
Li, W., Zhang, F., Cui, G., Wang, Y., Yang, J., Cheng, H., et al. (2021). Effects of bio-organic fertilizer on soil fertility, microbial community composition, and potato growth. Sci. Asia 47:347. doi: 10.1371/10.2306/scienceasia1513-1874.2021.039
Li, J., Zheng, Y., Yan, J., Li, H., Wang, X., He, J., et al. (2013). Effects of different regeneration scenarios and fertilizer treatments on soil microbial ecology in reclaimed opencast mining areas on the Loess Plateau, China. PLoS One 8:e63275. doi: 10.1371/journal.pone.0063275
Ling, N., Chen, D., Guo, H., Wei, J., Bai, Y., Shen, Q., et al. (2017). Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 292, 25–33. doi: 10.1016/j.geoderma.2017.01.013
Liu, Y., Hong, J., Guo, H., Wei, Y., and Liu, L. (2017). Effects of applying coal-derived compound fertilize on soil nutrients, enzyme activities and corn yield in reclaimed soil. J. Shanxi Agric. Sci. 45, 1786–1790.
Liu, J., Sui, Y., Yu, Z., Shi, Y., Chu, H., Jin, J., et al. (2014). High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of Northeast China. Soil Biol. Biochem. 70, 113–122. doi: 10.1016/j.soilbio.2013.12.014
Liu, H., Wang, C., Xie, Y., Luo, Y., Sheng, M., Xu, F., et al. (2020). Ecological responses of soil microbial abundance and diversity to cadmium and soil properties in farmland around an enterprise-intensive region. J. Hazard. Mater. 392:122478. doi: 10.1016/j.jhazmat.2020.122478
Ma, K., Zhang, Y., Ruan, M., Guo, J., and Chai, T. (2019). Land subsidence in a coal mining area reduced soil fertility and led to soil degradation in arid and semi-arid regions. Int. J. Environ. Res. Public Health 16:3929. doi: 10.3390/ijerph16203929
Machulla, G., Bruns, M. A., and Scow, K. M. (2005). Microbial properties of mine spoil materials in the initial stages of soil development. Soil Sci. Soc. Am. J. 69, 1069–1077. doi: 10.2136/sssaj2004.0271
Muhammad, Q., Huang, J., Waqas, A., Li, D., Liu, S., Zhang, L., et al. (2019). Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res. 198:104569. doi: 10.1016/j.still.2019.104569
Mummey, D. L., Stahl, P. D., and Buyer, J. S. (2002). Soil microbiological properties 20 years after surface mine reclamation: spatial analysis of reclaimed and undisturbed sites. Soil Biol. Biochem. 34, 1717–1725. doi: 10.1016/S0038-0717(02)00158-X
Odum, E. P. (1985). Trends expected in stressed ecosystems. Bioscience 35, 419–422. doi: 10.2307/1310021
Odum, E. P., Finn, J. T., and Franz, E. H. (1979). Perturbation theory and the subsidy-stress gradient. Bioscience 29, 349–352. doi: 10.2307/1307690
Pahalvi, H. N., Rafiya, L., Rashid, S., Nisar, B., and Kamili, A. N. (2021). “Chemical fertilizers and their impact on soil health” in Microbiota and biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs. eds. G. H. Dar, R. A. Bhat, M. A. Mehmood, and K. R. Hakeem (Cham: Springer), 1–20.
Rastogi, G., Osman, S., Vaishampayan, P. A., Andersen, G. L., Stetler, L. D., and Sani, R. K. (2010). Microbial diversity in uranium mining-impacted soils as revealed by high-density 16S microarray and clone library. Microb. Ecol. 59, 94–108. doi: 10.1007/s00248-009-9598-5
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 16, 608–617. doi: 10.1111/j.1526-100X.2008.00477.x
Shen, W., Ni, Y., Gao, N., Bian, B., Zheng, S., Lin, X., et al. (2016). Bacterial community composition is shaped by soil secondary salinization and acidification brought on by high nitrogen fertilization rates. Appl. Soil Ecol. 108, 76–83. doi: 10.1016/j.apsoil.2016.08.005
Sprusansky, O., Stirrett, K., Skinner, D., Denoya, C., and Westpheling, J. (2005). The bkdR gene of Streptomyces coelicolor is required for morphogenesis and antibiotic production and encodes a transcriptional regulator of a branched-chain amino acid dehydrogenase complex. J. Bacteriol. 187, 664–671. doi: 10.1128/JB.187.2.664-671.2005
Sun, L., Xun, W., Huang, T., Zhang, G., Gao, J., Ran, W., et al. (2016). Alteration of the soil bacterial community during parent material maturation driven by different fertilization treatments. Soil Biol. Biochem. 96, 207–215. doi: 10.1016/j.soilbio.2016.02.011
Tran, C. K. T., Rose, M. T., Cavagnaro, T. R., and Patti, A. F. (2015). Lignite amendment has limited impacts on soil microbial communities and mineral nitrogen availability. Appl. Soil Ecol. 95, 140–150. doi: 10.1016/j.apsoil.2015.06.020
Upadhyay, N., Verma, S., Pratap Singh, A., Devi, S., Vishwakarma, K., Kumar, N., et al. (2016). Soil ecophysiological and microbiological indices of soil health: a study of coal mining site in Sonbhadra, Uttar Pradesh. J. Soil Sci. Plant Nutr. 16, 778–800. doi: 10.4067/S0718-95162016005000056
Wang, D., Chen, X., Tang, Z., Liu, M., Jin, R., Zhang, A., et al. (2022). Application of humic acid compound fertilizer for increasing sweet potato yield and improving the soil fertility. J. Plant Nutr. 45, 1933–1941. doi: 10.1080/01904167.2022.2046064
Wang, C., Liu, D., and Bai, E. (2018). Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 120, 126–133. doi: 10.1016/j.soilbio.2018.02.003
Wu, W., Dong, C., Wu, J., Liu, X., Wu, Y., Chen, X., et al. (2017). Ecological effects of soil properties and metal concentrations on the composition and diversity of microbial communities associated with land use patterns in an electronic waste recycling region. Sci. Total Environ. 601-602, 57–65. doi: 10.1016/j.scitotenv.2017.05.165
Xu, Q., Fu, H., Zhu, B., Hussain, H. A., Zhang, K., Tian, X., et al. (2021). Potassium improves drought stress tolerance in plants by affecting root morphology, root exudates and microbial diversity. Meta 11:131. doi: 10.3390/metabo11030131
Yan, X., Wang, J., Hu, X., Yu, B., Gao, B., Li, Z., et al. (2021). Contrasting effects of microbial fertiliser and organic fertiliser on soil bacterial community in coal mine dump of Inner Mongolia. Chem. Ecol. 37, 384–398. doi: 10.1080/02757540.2021.1886283
Yu, H., Ling, N., Wang, T., Zhu, C., Wang, Y., Wang, S., et al. (2019). Responses of soil biological traits and bacterial communities to nitrogen fertilization mediate maize yields across three soil types. Soil Tillage Res. 185, 61–69. doi: 10.1016/j.still.2018.08.017
Zeng, J., Liu, X., Song, L., Lin, X., Zhang, H., Shen, C., et al. (2016). Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 92, 41–49. doi: 10.1016/j.soilbio.2015.09.018
Zhan, J., and Sun, Q.-Y. (2014). Development of microbial properties and enzyme activities in copper mine wasteland during natural restoration. Catena 116, 86–94. doi: 10.1016/j.catena.2013.12.012
Zhang, X., Li, D., Liu, Y., Li, J., and Hu, H. (2023). Soil organic matter contents modulate the effects of bacterial diversity on the carbon cycling processes. J. Soils Sediments 23, 911–922. doi: 10.1007/s11368-022-03336-3
Zhao, J., Ni, T., Li, J., Lu, Q., Fang, Z., Huang, Q., et al. (2016). Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl. Soil Ecol. 99, 1–12. doi: 10.1016/j.apsoil.2015.11.006
Zhong, W., Gu, T., Wang, W., Zhang, B., Lin, X., Huang, Q., et al. (2010). The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 326, 523–522. doi: 10.1007/s11104-009-0099-6
Keywords: coal-derived compound fertilizer, soil reclamation, bacterial community, soil nutrients, fertilization treatment
Citation: Meng H, Wang S, Zhang J, Wang X, Qiu C and Hong J (2023) Effects of coal-derived compound fertilizers on soil bacterial community structure in coal mining subsidence areas. Front. Microbiol. 14:1187572. doi: 10.3389/fmicb.2023.1187572
Received: 16 March 2023; Accepted: 03 May 2023;
Published: 18 May 2023.
Edited by:
Reeta Goel, GLA University, IndiaReviewed by:
Yongjie Yu, Nanjing University of Information Science and Technology, ChinaCopyright © 2023 Meng, Wang, Zhang, Wang, Qiu and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huisheng Meng, bWVuZ2h1aXNoZW5nQHN4YXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.