- 1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, China
- 2Jiangxi Institute of Respiratory Disease, The First Affiliated Hospital of Nanchang University, Nanchang, China
- 3Yichun People's Hospital, Yichun, China
- 4Department of Infectious Diseases, The First Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, China
- 5Department of Clinical Laboratory, Medical Center of Burn Plastic and Wound Repair, The First Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, China
- 6National Regional Center for Respiratory Medicine, China-Japan Friendship Jiang Xi Hospital, Nanchang, Jiangxi, China
Background: The worldwide dissemination of K. pneumoniae isolates is a significant public health concern, as these organisms possess a unique capacity to acquire genetic elements encoding both resistance and hypervirulence. This study aims to investigate the epidemiological, resistance, and virulence characteristics of K. pneumoniae isolates that carry both virulence plasmids and blaOXA-48-like genes in a tertiary hospital in China.
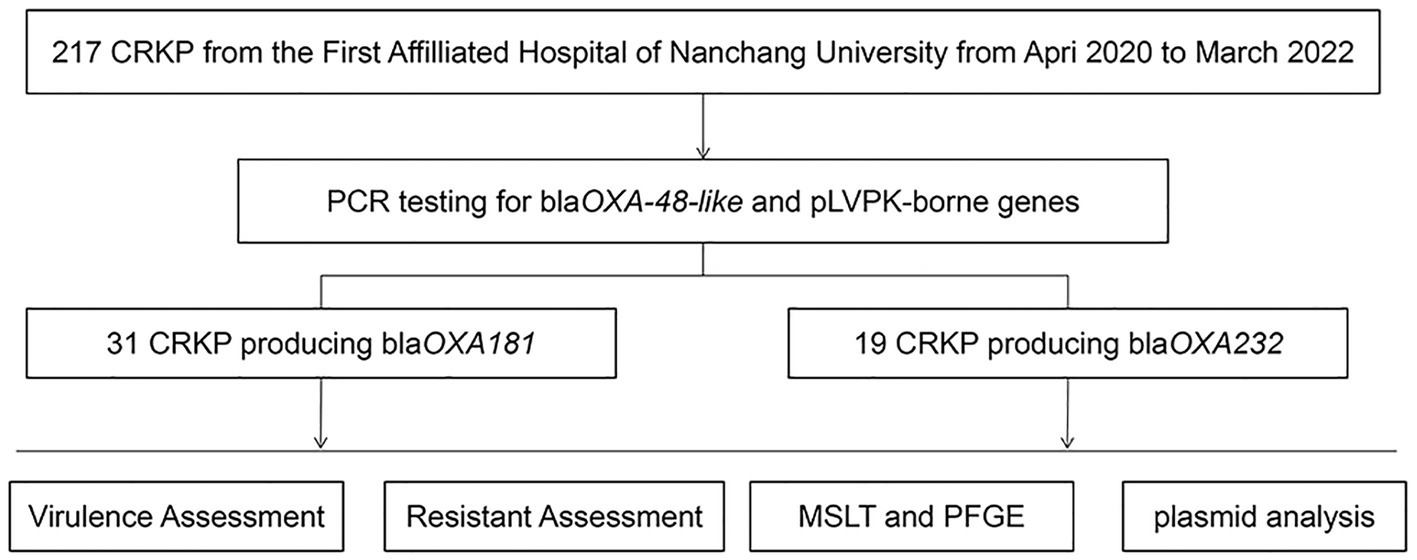
Methods: A total of 217 clinical isolates of carbapenem-resistant K. pneumoniae (CRKP) were collected between April 2020 and March 2022. The antimicrobial susceptibility test was conducted to evaluate the drug resistance profile. All isolates were screened for the presence of genes encoding carbapenemases (blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48-like), ESBLs genes (blaCTX-M, blaSHV, blaTEM), and virulence plasmid pLVPK-borne genes (rmpA, rmpA2, iucA, iroB, and peg344) using polymerase chain reaction (PCR) amplification. Clonal lineages were assigned using multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE). The plasmid incompatibility groups were identified using PCR-based replicon typing (PBRT). The transferability of carbapenemase-encoding plasmids and pLVPK-like virulence plasmids was assessed via conjugation. The plasmid location of rmpA2 was determined using S1-Pulsed Field Gel Electrophoresis (S1-PFGE) and southern blotting hybridization. The virulence potential of the isolates was assessed using the string test, capsular serotyping, serum killing assay and a Galleria mellonella larval infection model.
Results: Of the 217 CRKP clinical isolates collected, 23% were identified as carrying blaOXA-48-like genes. All blaOXA-48-like isolates exhibited resistance to commonly used clinical antimicrobial agents, except for ceftazidime/avibactam, colistin, tigecycline, trimethoprim-sulfamethOXAzole, polymyxin B, and nitrofurantoin. The main common OXA-48-like carbapenemase enzymes were found to be blaOXA-181 and blaOXA-232. MLST and PFGE fingerprinting analysis revealed clonal transmission and plasmid transmission. OXA-48-like producing CRKP isolates mainly clustered in K64 ST11 and K47 ST15. Results of the string Test, serum killing assay (in vitro) and Galleria mellonella infection model (in vivo) indicated hypervirulence. PBRT showed that the blaOXA-181 and blaOXA-232 producing hypervirulent carbapenem-resistant Klebsiella pneumoniae (Hv-CRKP) were mainly carried on ColE-type, IncF, and IncX3. Eight clinical isolates of hv-CRKP were identified as carrying three carbapenem-resistant genes (blaKPC, blaOXA-181 or OXA-232, and blaNDM-1). Moreover, Southern blotting hybridization revealed that all eight isolates had a pLVPK-like virulent plasmid (138.9–216.9 kb) with an uneven number and size of plasmid.
Conclusion: In our investigation, we have observed the emergence of hv-CRKP carrying blaOXA-48-like genes, which identified two genetic relationships: clonal transmission and plasmid transmission. PBRT analysis showed that these genes were mainly carried on ColE-type, IncF, and IncX3 plasmids. These isolates have been shown to be hypervirulent in vitro and in vivo. Additionally, eight clinical isolates of hv-CRKP were identified as carrying three carbapenem-resistant genes (blaKPC, blaOXA-181 or OXA-232, and blaNDM-1) and carrying a pLVPK-like virulent plasmid. Hence, our findings highlight the need for further investigation and active surveillance of hypervirulent OXA-48-like producing Hv-CRKP isolates to control their transmission.
1. Introduction
The Antimicrobial resistance (AMR) is a serious threat to global health, according to the World Health Organization. Among clinical pathogens, K. pneumoniae is particularly concerning due to its propensity to acquire multidrug resistance and hypervirulence-encoding mobile genetic elements (Yang et al., 2021). Carbapenem resistance in K. pneumoniae is often mediated by plasmid-encoded carbapenemase enzymes, such as blaKPC, blaNDM, and blaOXA-48-like enzyme (Han et al., 2020).
Hypervirulent K. pneumoniae, first identified from cases of liver abscess, has been increasingly reported worldwide (Russo and Marr, 2019). A recent study demonstrated that iroB, iucA, peg-344, rmpA, and rmpA2 were the most accurate molecular markers for defining hvKP, all of which have been shown to be located in the virulence plasmid (Russo et al., 2018). In recent years, more and more K. pneumoniae isolates integrating both hypervirulence and carbapenem resistance phenotypes have been identified, creating hypervirulent and carbapenem-resistant K. pneumoniae that result in devastating clinical outcomes (Yang et al., 2022).
Surveillance studies have revealed that OXA-48-like β-lactamases are among the 2nd or 3rd most common carbapenemases found in Enterobacterales globally (Pitout et al., 2019). OXA-48-like carbapenemases are mainly found in K. pneumoniae isolates submitted from hospital sites and have been increasing toward the end of surveillance periods (de Jonge et al., 2016; Karlowsky et al., 2017). Data from global surveillance programs such as SMART (Karlowsky et al., 2017) and INFORM (de Jonge et al., 2016) show that 27% of carbapenemase-producing Enterobacterales (CPE; n = 1,615) carry blaOXA-48-like carbapenemases (compared to 55% blaKPCs and 26% blaNDMs). In some regions, such as the Middle East, North Africa, and certain European countries like Belgium and Spain, OXA-48-like enzymes were the most prevalent carbapenemases among Enterobacterales (Pitout et al., 2019).
In recent years, cases of OXA-48-like K. pneumoniae isolates have been on the rise in China. For instance, OXA-232-producing CRKP was first isolated from five neonatal patients in China in 2017 (Yin et al., 2017), while the first report of OXA-181-producing K. pneumoniae from the fecal specimen of a patient in China was in 2020 (Liu et al., 2020). Subsequent reports have documented an increasing number of blaOXA-48-like K. pneumoniae isolates in China (Liu et al., 2020; Shi et al., 2020; Jia et al., 2021). In December 2016, the draft genome sequences of three hypervirulent CRKP isolates from India were reported to harbor blaOXA genes (blaOXA-232, blaOXA-181, and blaOXA-1) along with the rmpA2 gene (Shankar et al., 2016). While China reported the emergence of OXA-232 carbapenemase-producing K. pneumoniae carrying a pLVPK-like virulence plasmid among elderly patients in February 2019, these isolates were not hypervirulent despite carrying a virulence plasmid (Shu et al., 2019). This study aims to investigate the resistance mechanisms and molecular epidemiology of hypervirulent Klebsiella pneumoniae isolates producing OXA-48-like carbapenemases in a Chinese tertiary hospital.
2. Materials and methods
2.1. Bacterial isolates and definitions
Between April 2020 and March 2022, the First Affiliated Hospital of Nanchang University in China collected 217 unique clinical carbapenem-resistant K. pneumoniae isolates, characterized by minimum inhibitory concentrations (MICs) of ertapenem >0.5 μg/mL, imipenem >4 μg/mL or meropenem >8 μg/mL. All isolates were identified using the VITEK 2 automated system (bioMerieux, Marcy l’Etoile, France) and the MALDI-TOF MS system (Bruker Daltonics, Billerica, MA, United States) and stored at −80°C until use. The MIC of tigecycline was determined through the E-test (AB Biodisk, Solna, Sweden) on Mueller-Hinton media. Susceptibility to colistin and tigecycline was determined according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines,1 while susceptibilities to other agents were interpreted using the Clinical and Laboratory Standards Institute (CLSI) breakpoints (document M100-S32).
All isolates were screened for the presence of genes encoding carbapenemases (blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48-like), ESBLs genes (blaCTX-M, blaSHV, blaTEM), and virulence plasmid pLVPK-borne genes (rmpA, rmpA2, iucA, iroB, and peg344) using polymerase chain reaction (PCR) amplification, as previously described (Liu et al., 2019). PCR products were visualized by agarose gel electrophoresis and sequencing, and the sequence analysis of PCR products was conducted by Sangon Biotech (Shanghai, China) and aligned in blaST searches in the NCBI Genbank. Isolates positive for blaOXA-48-like genes and virulence genes were further studied.
2.2. Clinical data collection
The clinical data used in this study were obtained from the Electronic Medical Records of inpatients at the First Affiliated Hospital of Nanchang University. The data included patient demographics, date of isolation, clinical diagnosis, specimens, ward admission, antimicrobial treatment, and hospitalization outcomes. The study and consent procedures were approved by the Ethical Committee of the First Affiliated Hospital of Nanchang University.
2.3. Molecular epidemiology analysis: multilocus sequence typing and pulsed-field gel electrophoresis
MLST and PFGE was used to evaluate the genetic relatedness of isolates positive for blaOXA-48-like genes and virulence genes.
MLST was conducted in accordance with the protocol outlined on the Pasteur Institute MLST website, using seven conserved housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB). The resulting MLST amplicons were purified and sequenced by Sangon Biotech in Shanghai, China, and compared to those in the MLST database to determine the sequence type (ST).
PFGE using XbaI from TaKaRa was performed. DNA fragments were then separated via a CHEF DR III apparatus (Bio-Rad, Richmond, CA, United States), with Salmonella serotype Braenderup isolate H9812 serving as a molecular marker. Subsequently, BioNumerics software version 7.6 was utilized to construct a tree diagram using the unweighted Pair-Group Method with Arithmetic means (UPGMA) and the Dice similarity coefficient (SD) with a 1.5% position tolerance. Isolates were considered genetically similar if their Dice coefficient correlation exceeded 80%, in line with the “possibly related (4–6 bands difference)” criteria developed by Tenover et al. (1995).
2.4. Plasmid analyses
2.4.1. Plasmid conjugation
Conjugation was employed to evaluate the transferability of plasmids carrying carbapenemases (blaKPC, blaOXA-181 or OXA-232, and blaNDM-1) and pLVPK-like virulence plasmid. Eight clinical isolates of CR-hvKP carrying carbapenem-resistant genes (blaKPC, blaOXA-181 or OXA-232, and blaNDM-1) were used as donors, while rifampicin-resistant E. coli EC600 was used as the recipient. Both donor and recipient isolates were cultured in Luria-Bertani broth (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl) at 37°C with shaking (180 rpm) until they reached their exponential growth phase (OD600 = 0.4–0.6). The overnight cultures were then mixed in a 1:1 ratio and incubated at 37°C for 16–20 h. After incubation, 100 μL of the sample was spread onto MH agar plates containing imipenem (5 μg/mL), potassium tellurite (5 μg/mL), and rifampicin (600 μg/mL).
2.4.2. PCR-based replicon typing (PBRT)
Plasmid incompatibility groups were determined using PBRTas previously described in literature (Carattoli et al., 2005; Carattoli, 2009; Carattoli, 2011). PBRT was used to tract the plasmids conferring drug resistance in epidemiological of transconjugants and isolates positive for blaOXA-48-like genes and virulence genes. The identified plasmid incompatibility groups included HI1, HI1b, HI2, I1-γ, L/M, N, FIA, FIB, FIC, FIIA, F, K, B/O, W, Y, P, A/C, T, X, X1, X2, X3, and X4.
2.4.3. S1-pulsed field gel electrophoresis and southern blotting hybridization
Plasmid characteristics were assessed by S1-PFGE. Southern blotting hybridization was performed to determine the plasmid location of the virulence plasmid with a rmpA2 gene. In brief, the isolates were embedded in 1% Seakem Gold agarose and digested with S1-nuclease (Takara, Otsu, Japan) at 37°C for 30 min, and plasmids were separated on a CHEF DR III apparatus (Bio-Rad, Richmond, CA, United States) for 18 h at 14°C, using a 0.8% agarose gel and run conditions of 6 V/cm and pulse times ranging from 2.16 s to 63.8 s. Plasmid molecular mass standards covering a range from 20.5 kb to 1,135 kb, isolated from Salmonella serotype Braenderup isolate H9812, were used. The transferred plasmids on the S1-PFGE gel were transferred to Hybond-N+ membranes (Amersham), following a previously described protocol (Liu et al., 2017). The probe labeling for rmpA2 and hybridization were conducted using the DIG-High Prime DNA Labeling and Detection Starter Kit I, following the manufacturer’s instructions (CAT.NO.11745832910, Roche, Mannheim, Germany).
2.5. Virulence assessment of transformant
2.5.1. Hyperviscous phenotype detection (string test)
For isolates that were positive for all the aforementioned virulence genes, hypermucoviscosity was defined as present when the viscous string was longer than 5 mm when colonies were stretched on an agar plate.
2.5.2. Serum killing assay
In addition, we performed a serum killing assay to determine in vitro virulence, as described in previous literature (Liu et al., 2017). Briefly, serum was collected from healthy individuals and stored at −80°C. An inoculum of 106 CFU mid-log phase bacteria was incubated with 75% pooled human serum, and viable counts were recorded at 0, 1, 2, and 3 h of incubation at 37°C and 200 rpm. Each isolate was tested at least three times. The reaction to serum killing was classified into six grades and categorized as highly sensitive (grade 1 or 2), intermediately sensitive (grade 3 or 4), or resistant (grade 5 or 6). Grade 1 indicated viable counts <10% of the inoculum after 1 and 2 h, and < 0.1% after 3 h. Grade 2 referred to viable counts between 10 and 100% of the inoculum after 1 h and < 10% after 3 h. Grade 3 indicated viable counts exceeding those of the inoculum after 1 h but <100% after 2 and 3 h. Grade 4 referred to viable counts >100% of the inoculum after both 1 and 2 h but <100% after 3 h. Grade 5 referred to viable counts >100% of the inoculum at 1, 2, and 3 h, which decreased during the third hour. Grade 6 referred to viable counts that exceeded those of the inoculum at 1, 2, and 3 h and increased throughout this period. Isolates K. pneumonia ATCC 700603 and the hvKP isolates NTUH-K2044 were used as negative and positive controls, respectively, with serum killing sensitivity of grade 2 and resistance of grade 5.
2.5.3. Galleria mellonella infection model
The larvae of Galleria mellonella (Gm) was an infection model for the virulent to evaluate study virulence of gram-negative bacteria isolates (Ennis and Sells, 1968; Asai et al., 2022), so we evaluated in vivo virulence using the Galleria mellonella infection model to assess hypervirulence. Microbial virulence in the G. mellonella infection model is typically assessed within 5 d and the most commonly used end point is the survival rate at different time points (Asai et al., 2022). Specific experimental steps was as previously described (Mclaughlin et al., 2014). In brief, 10 pathogen-free G. mellonella larvae weighing between 250 and 350 mg (purchased from Tianjin Huiyude Biotech Company, Tianjin, China) were used for each isolate. A mid-log-phase culture was washed and diluted with PBS, and each larva was inoculated by injecting 1 × 106 CFU in a 10 ul aliquot into the hemocoel via the rear left pro leg. Survival rate was recorded every 24 h for 4 days, and larvae were kept in petri dishes at 37°C in the dark. All experiments were conducted in triplicate. The calculation of the LD50 value has been proposed to define hypervirulence in the Galleria mellonella infection model for K. pneumoniae isolates (Li et al., 2020). The HvKP isolate NTUH-K2044 and PBS were used as controls for high and low virulence, respectively. Statistical analyses were performed and visualized using GraphPad Prism 8.0.
2.6. Statistical analyses
The statistical analysis was performed using SPSS version 17.0 (SPSS, Chicago, IL, United States). Categorical variables were compared using either the chi-square test or Fisher’s exact test, and a p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Prevalence of CRKP co-carrying pLVPK-like virulence plasmid and blaOXA-48-like carbapenemases genes in a Chinese tertiary hospital
A total of 217 clinical isolates of CRKP were collected from our hospital between April 2020 and March 2022. Among these isolates, 50 (23%) carried both the pLVPK-like virulence plasmid and blaOXA-48-like carbapenemase genes (Figure 1). These clinical isolates were obtained from various clinical specimens, including blood (Pitout et al., 2019), pus (Li et al., 2020), sputum (Khan et al., 2019), and urine (Asai et al., 2022) (Table 1). The ICU occupancy rate for these patients was 62% (31/50), and the overall mortality rate among inpatients involved in the outbreak was 52% (26/50). The median age of patients was 54.7 ± 12.6 years, and the male-to-female ratio was 2.3 (Table 1).
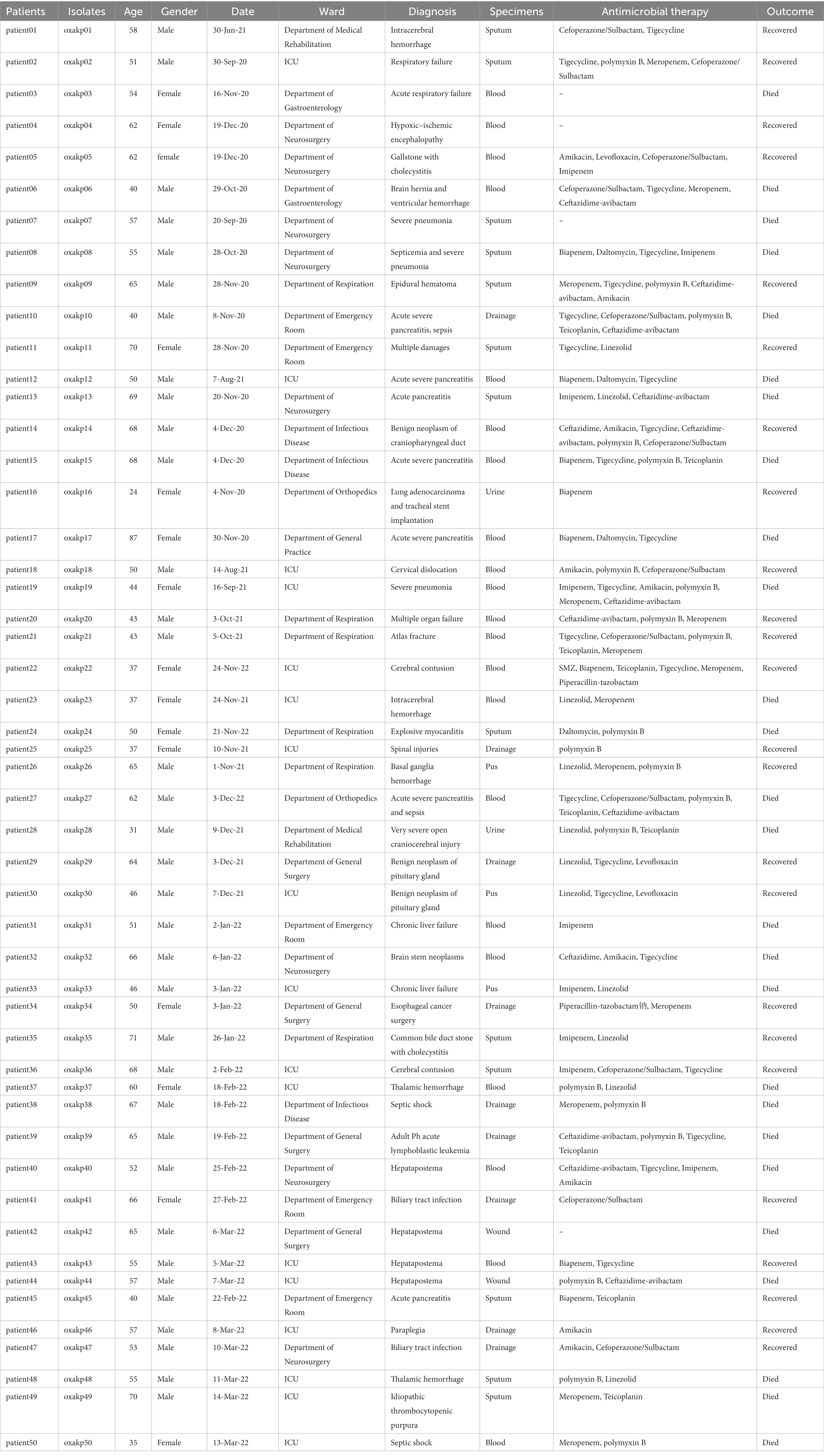
Table 1. The clinical data of patients infected with K. pneumoniae isolates co-carrying pLVPK virulence plasmid and blaOXA-48-like carbapenemases genes.
Upon sequence comparison with GenBank, we found that OXA-181 (62%, 31/50) and OXA-232 (38%, 19/50) were the most common carbapenemases identified among the OXA-48-like carbapenemases. All the isolates were found to be positive for the presence of blaCTX-M and blaTEM genes. Additionally, the blaSHV gene was detected in over 80% of the isolates. As shown in Figure 2, eight clinical isolates of CRKP carried three carbapenem-resistant genes, including five isolates producing blaKPC + NDM + OXA-181 and three isolates producing blaKPC + NDM + OXA-232.
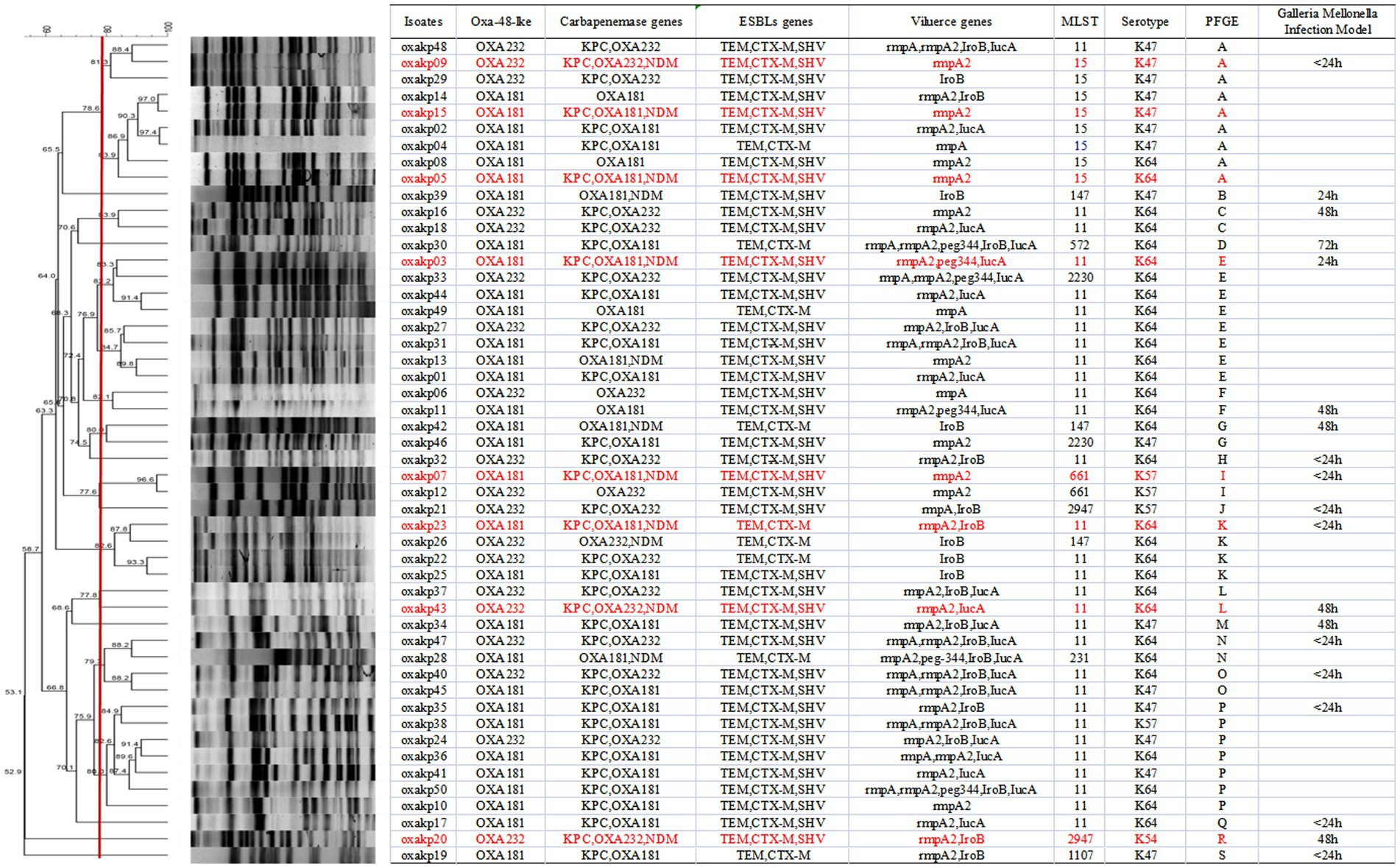
Figure 2. PFGE typing of 50 clinical OXA-48-like positive CRKP isolates. Genomic DNA from each research strains was digested using Xba I and the digests were subjected to PFGE to generate diagnostic genomic DNA fragmentation fingerprints. The dendrogram of the PFGE profiles was clustered by the UPGAMA on the basis of the Dice similarity by the bionumber software. The red line delineates 80% of the boundary. The strains producing carbapenemases (kpc, OXA, and NDM) are indicated in red font.
3.2. Molecular characteristics
MLST analysis of 50 isolates of OXA-48-like producing CRKP identified nine distinct sequence types (STs), as demonstrated in Figure 2. The most frequently encountered ST was ST11, which accounted for 30 out of 50 isolates, followed by ST15, which was found in eight isolates. No notable differences were observed in the STs of isolates carrying blaOXA-181 versus those carrying blaOXA-232. PFGE analysis demonstrated that CRKP isolates producing both blaOXA-181 and blaOXA-232 displayed 19 distinct PFGE patterns, respectively, as depicted in Figure 2. Notably, Cluster A, E, and P exhibited clonal relatedness. Furthermore, both clonal and plasmid transmission was observed based on PFGE analysis. The combined results of PFGE and MLST analysis showed that CRKP isolates co-carrying pLVPK-like virulence plasmid and blaOXA-181 and OXA-232 resistant plasmid mainly clustered in ST11 and ST15 isolates.
3.3. Resistant assessment of Klebsiella pneumoniae clinical isolates co-carrying pLVPK-like virulence plasmid and blaOXA-181 and OXA-232 resistant genes
Figure 3 present the antibacterial susceptibility of 50 OXA-48-like producing K. pneumoniae isolates. All isolates exhibited resistance to commonly used clinical antimicrobial agents, except for ceftazidime/avibactam, colistin, tigecycline, trimethoprim-sulfamethOXAzole, polymyxin B, and nitrofurantoin. Specifically, the clinical isolates in this study demonstrated complete resistance to Piperacillin-tazobactam, Ticarcillin-clavulanic acid, Cefazolin, Cefepime, Cefoperazone/Sulbactam, Ceftazidime, Ceftriaxone, Aztreonam, and Imipenem (100%). The rates of antibacterial resistance to LevoflOXAcin, CiproflOXAcin, Meropenem, Ertapenem, and Doxycycline were 96, 96, 94, 90, and 90%, respectively, with 48/50, 48/50, 47/50, 45/50, and 45/50 isolates exhibiting resistance to each drug, respectively. Furthermore, these isolates were fully sensitive to Polymixin B and Nitrofurantoin. The tigecycline and colistin MICs were each <1 μg/mL, except for six isolates that had a TGC zone diameter of 4, 4, 8, 8, 8, and 8 mm.
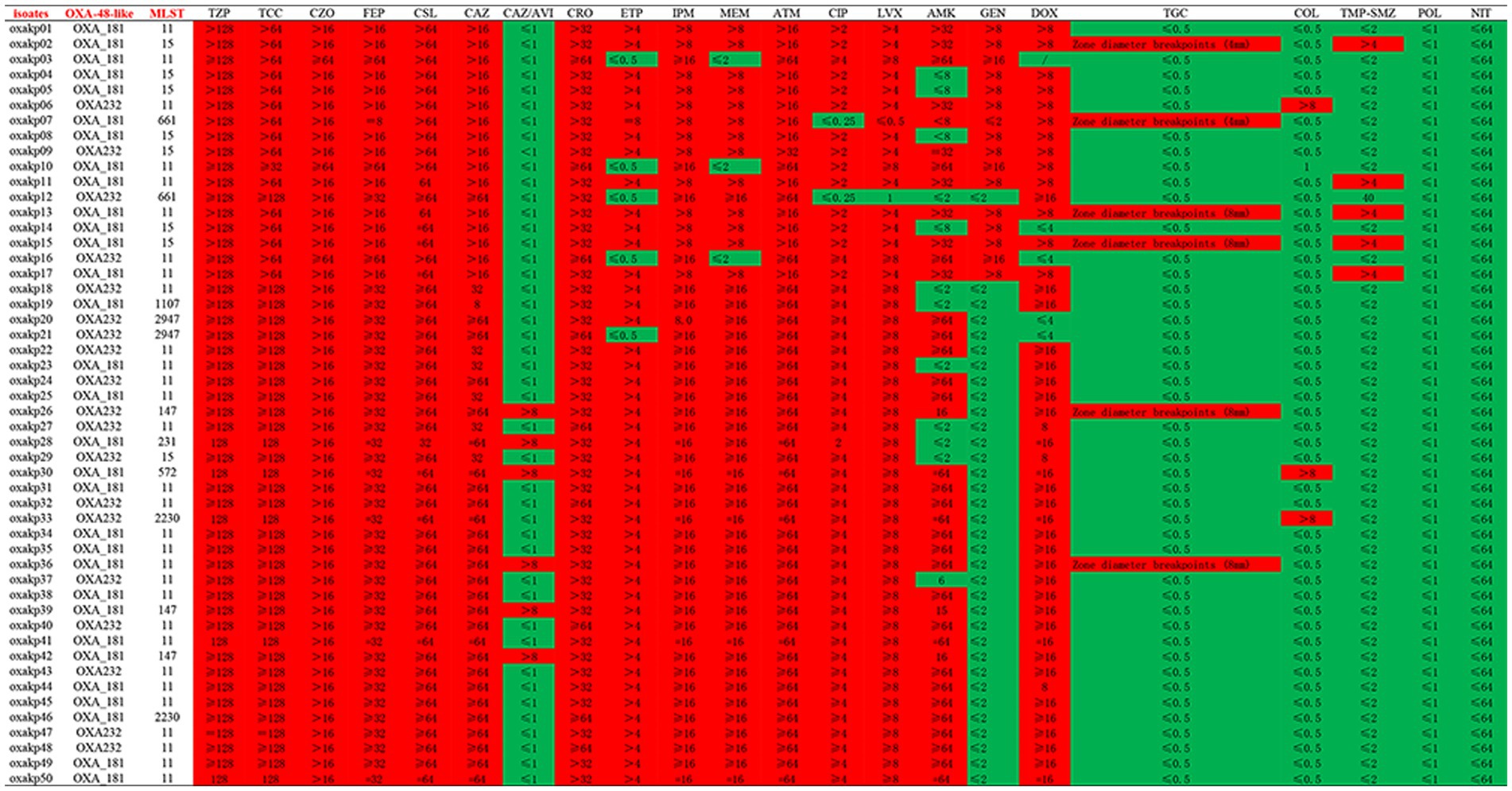
Figure 3. In vitro activities of antimicrobial agents and disinfectants against CRKP isolates co-carrying pLVPK virulence plasmid and blaOXA-48-like carbapenemases genes.
Figure 2 illustrates that all these 50 CRKP carried at least one carbapenemase genes (blaKPC, blaNDM, blaOXA) or ESBL genes (blaCTX-M, blaSHV, blaTEM). As depicted in Figure 2, our study identified eight clinical isolates of Hv-CRKP that carried three carbapenem-resistant genes, namely five of blaKPC + OXA-181 + NDM-1 and three of blaKPC + OXA-233 + NDM-1. To our knowledge, this is the first report of the co-production of three carbapenemase genes (blaKPC + NDM + OXA181 or OXA232) and the pLVPK-like virulence plasmid in CRKP isolates.
The plasmid-borne resistance to blaOXA-181 and OXA-232 producing CRKP was mainly attributed to ColE-type plasmids (100%, 50/50), IncF plasmids (72%, 36/50), and IncX3 plasmids (26%, 13/50), with IncX3 plasmids always associated with blaNDM (Figure 4). To evaluate the transferability of these resistant plasmids, we selected the aforementioned eight isolates that carry three carbapenemase resistance genes and performed the plasmid conjugation experiment. Fortunately, all eight bacterial isolates were successfully conjugated.
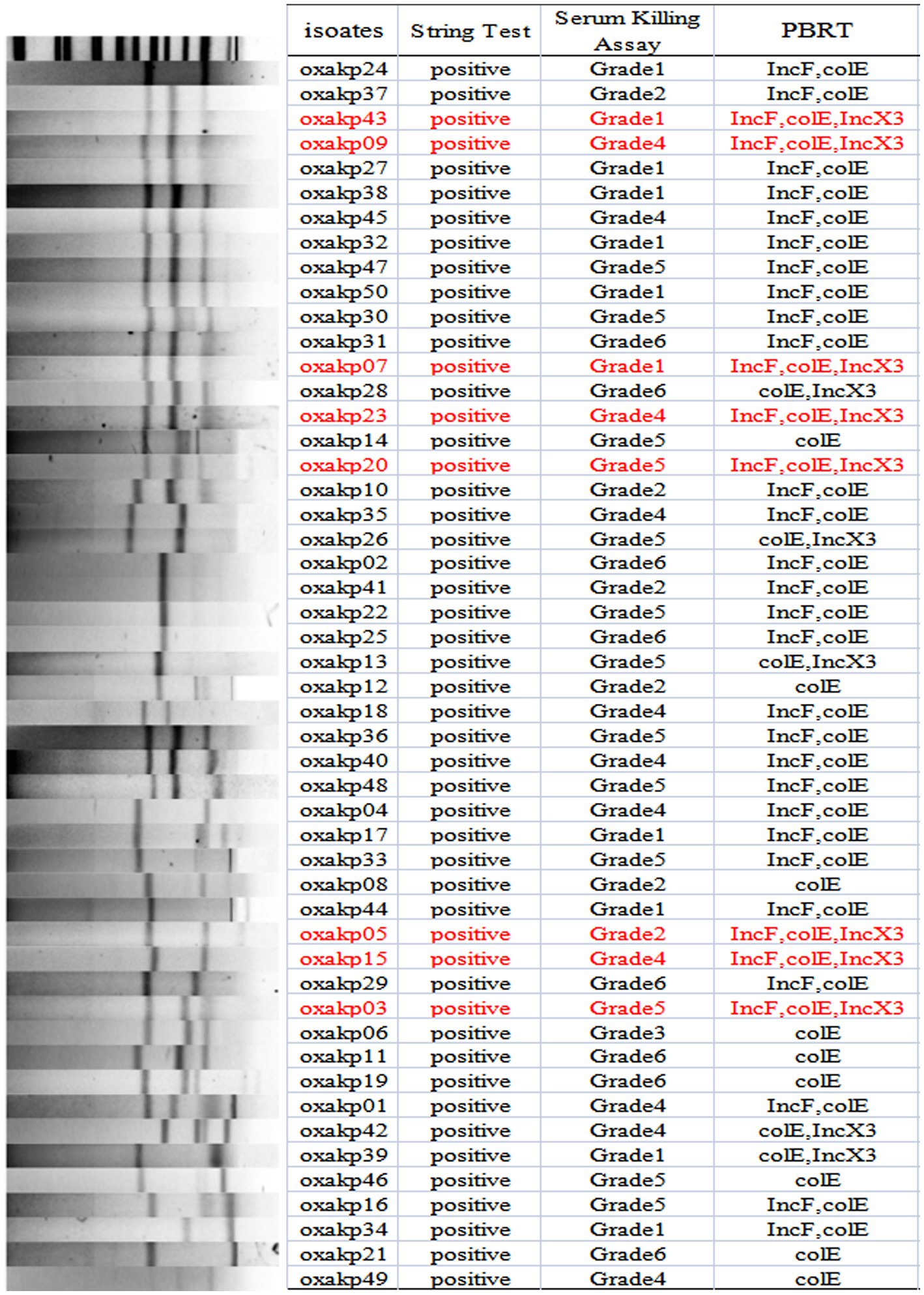
Figure 4. S1-PFGE typing of 50 clinical OXA-48-like Positive K. pneumoniae isolates. Genomic DNA from each research strains was digested using S1 and the digests were subjected to DNA fragments were separated with a CHEF DR III apparatus.
3.4. Virulence assessment of Klebsiella pneumoniae clinical isolates co-carrying p-LVPK-like virulence plasmid and blaOXA-181 and OXA-232 resistant genes
As showed in Figure 2, it was observed that all blaOXA-48-like positive CR-hvKP isolates harbored at least one virulence gene located on a pLVPK-like virulence plasmid (including iroB, iucA, peg-344, rmpA, and rmpA2 genes). The string test was positive for all these isolates. Capsular serotyping showed that 30 isolates were K64, 15 were K47, 4 were K57, and 1 was K54, while K1 and K2 types were not detected. No significant difference was observed between the blaOXA-181 and blaOXA-232 groups. Our findings suggest that K. pneumoniae isolates co-carrying p-LVPK-like virulence plasmids and blaOXA-181 and OXA-232 resistant plasmids are mainly clustered in K64 and K47 isolates.
The presence of hypermucoviscosity and plasmid-borne genes resembling pLVPK in the isolated strains suggests hypervirulence. In vitro experiments confirmed that the strains exhibited serum resistance, with a survival rate of approximately 78% after 60 min of incubation with serum (Figure 4). To assess virulence in vivo, one isolate was randomly selected from each typing cluster based on PFGE. The results, shown in Figure 2, indicate that 19 CRKP isolates were chosen. When a 106 CFU suspension of the isolates was used to infect Galleria mellonella larvae, 18 isolates had a survival rate of less than 48 h, which was similar to that of a virulent strain of NUTH-K2044 (Figure 2). Eight CRKP isolates, which co-produced three carbapenemases (blaKPC, blaOXA-181 or OXA-232, and blaNDM-1) (Figure 5), were found to harbor a pLVPK-like virulent plasmid (ranging from 138.9 to 216.9 kb) as determined by Southern blotting hybridization of the virulence gene rmpA2 (Figure 6).
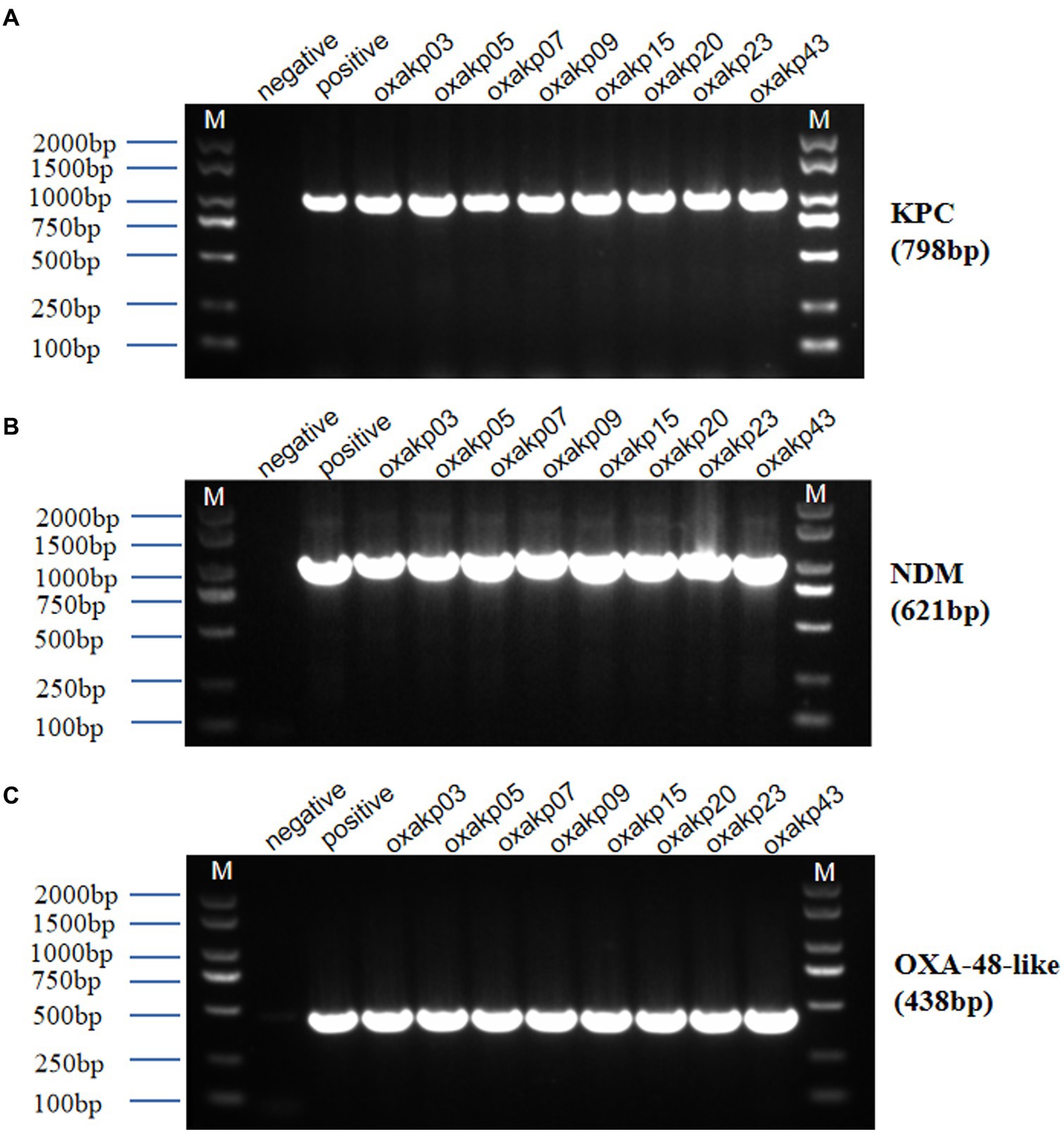
Figure 5. Agarose gel electrophoresis was performed for eight clinical isolates of Hv-CRKP carrying three carbapenem-resistant genes (A–C). The gel showed PCR products of expected lengths for the blaKPC gene (approximately 798 bp), blaNDM gene (approximately 621 bp), and blaOXA-48-like gene (approximately 438 bp). M:2000 bp size marker, Negative Control did not show amplification.
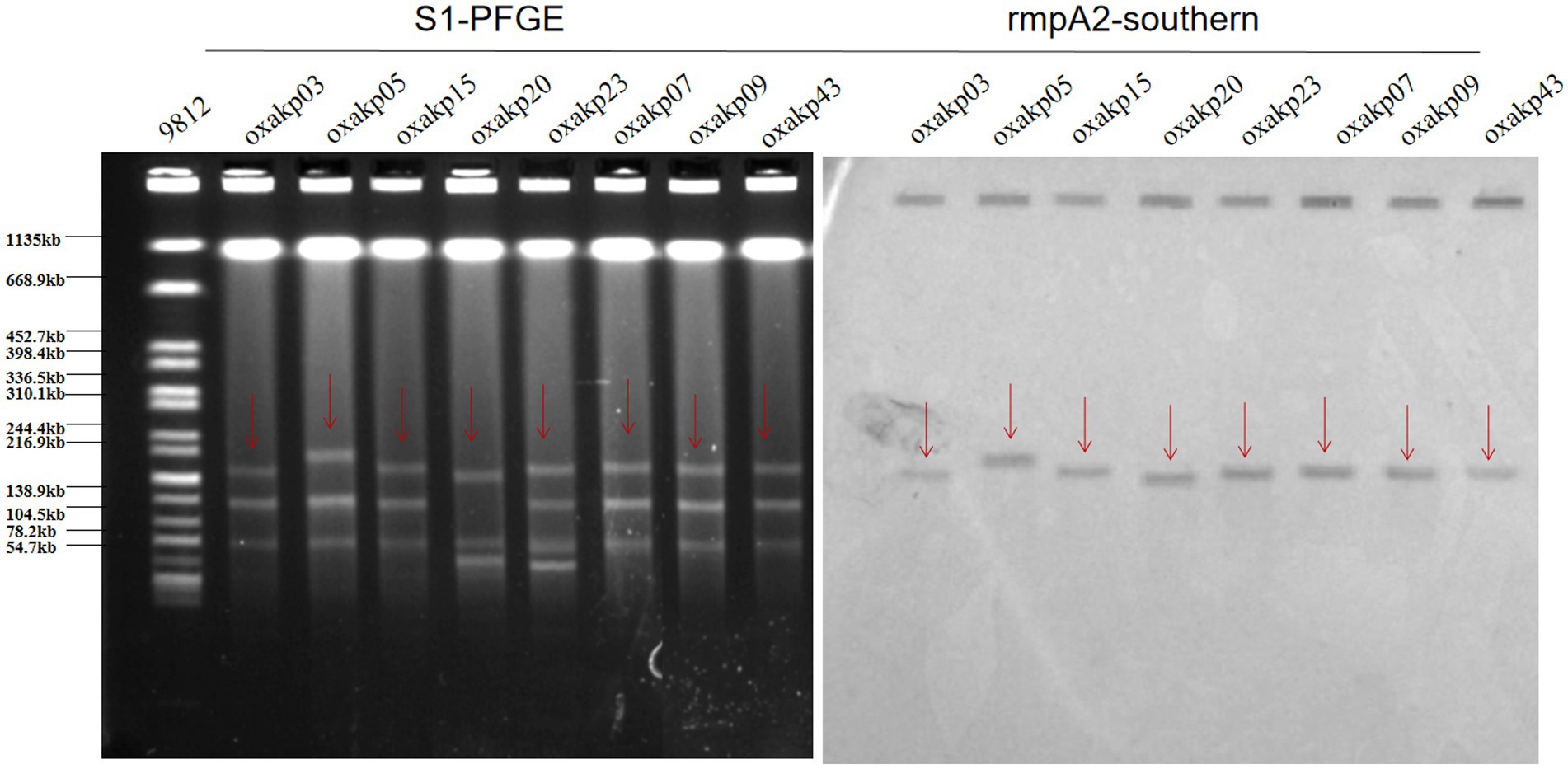
Figure 6. S1-PFGE patterns and rmpA2-southern of the eight hv-CRKP strains co-producing three carbapenemases harbored a pLVPK-like virulent plasmid (ranging from 138.9 to 216.9 kb).
4. Discussion
Bacteria carrying blaOXA-48, blaOXA-181, and blaOXA-232 are emerging globally, particularly in K. pneumoniae and E. coli, but their incidence is likely underreported due to laboratory detection challenges (Pitout et al., 2019). The rapid emergence and spread of multidrug-resistant (MDR) and hypervirulent K. pneumoniae isolates is a growing concern in clinical settings (Zhang et al., 2020). In recent years, two evolutionary pathways in K. pneumoniae have converged, leading to the emergence of carbapenem-resistant and hypervirulent isolates through plasmid recombination and fusion events. Consequently, carbapenem-resistant K. pneumoniae (CRKP) and hypervirulent K. pneumoniae (hvKP) have merged (Yang et al., 2021). The carriage of a virulence plasmid containing two capsular polysaccharide (CPS) regulator genes (rmpA and rmpA2) and several siderophore gene clusters (iucABCD/iutA/iroBCDN clusters) is thought to contribute to the hypermucoviscous phenotype of hvKP (Russo et al., 2018; Russo and Marr, 2019). Globally, there has been a growing incidence of MDR and hvKP isolates carrying the blaOXA-48, blaOXA-181, and blaOXA-232 genes (Pitout et al., 2019), likely resulting from two converging evolutionary pathways of K. pneumoniae involving plasmid recombination and fusion events (Zhang et al., 2020; Yang et al., 2021). We report the emergence of hvKP isolates carrying both blaOXA-232 and blaOXA-181 recovered from patients at a teaching hospital in southern China. The high ICU occupancy (62%) and mortality rates (52%) of patients infected with these bacteria highlight the importance of monitoring this isolate. In recent years, an increasing number of K. pneumoniae isolates with integrated hypervirulence and carbapenem resistance phenotypes have been identified, resulting in devastating clinical outcomes (Yang et al., 2022). Our research team found that OXA-resistant isolates with hypervirulence had begun to prevail in China during our active surveillance of CRKP isolates.
In our study, all blaOXA-48-like positive CR-hvKP isolates exhibited resistance to commonly used clinical antimicrobial agents and harbored at least one virulence gene located on a pLVPK-like virulence plasmid, including iroB, iucA, peg-344, rmpA, and rmpA2 genes. Although OXA-48-like represents weak activity of carbapenemase (Oueslati et al., 2015), all these isolates demonstrated high resistance to carbapenem antimicrobial agents such as imipenem (100%), meropenem (94%), and ertapenem (90%), consistent with the results of another study (Jia et al., 2021). In addition, we detected at least one extended-spectrum β-lactamase (ESBL) gene, such as blaCTX-M, blaSHV, or blaTEM, which might contribute to the observed drug-resistant phenotype.
Horizontal transmission of resistance genes via mobile plasmids is a common dissemination mechanism for carbapenemase-producing Enterobacteriaceae (CPE), resulting in rapid spread of resistance genes across diverse isolates and hosts (Pulss et al., 2018). The high proportion of ST11 (30/50) among the blaOXA-48-like positive CR-hvKP isolates in our study suggests common clonal origins (Figure 2). Our study further revealed that three clusters of isolates (A, E, and P) were closely related (Figure 2), with PFGE patterns and MLST demonstrating both clonal and plasmid transmission. These findings suggest that horizontal gene transfer/plasmid transfer plays a crucial role in the dissemination of CR-hvKP strains. Notably, we found a high prevalence of triple positivity for multiple carbapenemases in eight isolates (five producing blaKPC + NDM + OXA-181 and three producing blaKPC + NDM + OXA-232). Our results are consistent with the continuous global emergence of multidrug-resistant strains (Hu et al., 2014; Khan et al., 2019), which can be sustained by diverse mechanisms, such as R plasmids or transposons (Miller et al., 2014).
In our study, we identified three plasmid replicons (ColE, IncF, and IncX3) with high frequency in our isolates. Previous research has demonstrated that the blaNDM gene can be found in various types of plasmids, including those in the IncF, IncFII, IncN, and IncX3 incompatibility groups (Zhu et al., 2016). The initial acquisition of the OXA-181 gene occurred through the mediation of ISEcp1, which subsequently integrated into Tn2013 and was found on several plasmids such as ColE2, IncX3, IncN1, and IncT. On the other hand, the genetic environment surrounding blaOXA-232 is very similar to that of blaOXA-181, with the former differing from the latter by only one amino acid substitution (Pitout et al., 2019). Plasmids harboring blaKPC genes, ranging in size from 10 to 300 kb, are commonly found in various incompatibility groups, such as IncF, IncI, IncA/C, IncN, IncX, IncR, IncP, IncU, IncW, IncL/M, and ColE (Chen et al., 2014). In our plasmid conjugation experiment, eight isolates carrying three carbapenemase resistance genes were successfully conjugated. However, Potron A. and colleagues discovered that the plasmid-mediated carbapenem-resistance gene blaOXA-232 was located on a small, non-conjugative plasmid called pOXA-232, which carried a ColE-type backbone (Potron et al., 2013; Abdul Momin et al., 2017; Weng et al., 2020). Our research team discovered for the first time the emergence of super resistant bacteria due to the lack of reports on blaKPC + NMD + OXA resistant strains of super carbapenem. Our next challenge is to investigate how these three resistant plasmids can facilitate transfer in vivo, as well as the mechanism underlying their coexistence.
pLVPK-like virulent plasmids often have a strong correlation with high hypervirulent phenotypes in K. pneumoniae. Several experiments to confirm the virulent phenotype of these blaOXA-48-like positive CR-hvKP isolates: hypermucoviscosity (String Test), serum killing assay (in vitro) and Galleria mellonella infection model (in vivo), these isolates have been shown to be hypervirulent. We performed the localization of virulence plasmids for eight strains that carried three resistance plasmids at the same time. Southern blotting hybridization determined that these CRKP carried a pLVPK-like virulent plasmid (138.9–216.9 kb) with uneven numbers and sizes of plasmids. These findings suggest the emergence of hv-CRKP isolates that simultaneously carry three carbapenemases and a virulence plasmid. We speculate that our hv-CRKP isolate may have acquired a virulence plasmid during the evolution of the drug-resistant isolate.
Given these findings, it is essential to carefully monitor and conduct follow-up studies to gain further insights into the epidemiology of multidrug-resistant strains, as well as the possible evolution of successful plasmids and transposition modules that contain three antimicrobial resistance genes (blaOXA-48-like + NDM + KPC) of clinical relevance. Our active surveillance of CRKP isolates led to the discovery of OXA-resistant isolates with hypervirulence that have become prevalent in China. These hypervirulent OXA-resistant isolates carry a pLVPK-like virulence plasmid containing the iroB, iucA, peg-344, rmpA, and rmpA2 genes. Furthermore, we found eight clinical isolates of hv-CRKP carrying three carbapenem-resistant genes: blaKPC, blaOXA-181 or OXA-232, and blaNDM-1. This is the first report of the co-production of three carbapenemase genes (blaKPC + NDM + OXA181 and blaKPC + NDM + OXA232) in CRKP isolates, highlighting the need for active surveillance to control further transmission.
5. Conclusion
This study reports the emergence of hypervirulent OXA-48-like-producing hv-CRKP in our hospital. Based on PFGE and MLST results, two genetic relationships were identified: clonal and plasmid transmission. The most common OXA-like carbapenemases were blaOXA-181 and blaOXA-232, which predominantly clustered in K64 ST11 and K47 ST15 isolates. Notably, we identified the co-production of three carbapenemases genes (blaKPC + NDM + OXA181 or OXA232) and a pLVPK-like virulence plasmid in hv-CRKP isolates, which to our knowledge, has not been previously reported. These hv-CRKP isolates carried a pLVPK-like virulence plasmid ranging from 138.9 to 216.9 kb. Therefore, implementation of effective infection control measures is urgently needed to prevent further spread in the region.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
The study was designed by WZ and YL, while T-xP, W-yL, and PL conducted the experiments. SL, Z-yH, and D-DW carried out the analysis. T-XX, PL, and W-JL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Youth Science Foundation of China (grant number 82102411 and 82200194); The Natural Science Key Project of Jiangxi Province (grant numbers 20202ACBL206025 and 20202ACBL206023); the Project of Science and Technology Innovation Talents in Jiangxi (grant number JSXQ2019201102); Jiangxi Provincial Department of Science and Technology (grant number 20202ZDB01016); the Science and technology plan of Jiangxi Health Committee (grant number SKJP-220212554); the Clinical Research Nurture Project of the First Affiliated Hospital of Nanchang University (grant number YFYLCYJPY202001); and Youth Scientific Research Foundation of the First Affiliated Hospital of Nanchang University (grant number YFYPY202115); and the Science and Technology Plan of Jiangxi Provincial Health Commission (202130137), the Natural Science Foundation of Jiangxi province (grant number 20224BAB216084), The first affiliated hospital of Nanchang University Young Talents Scientific Research Breeding Fund (grant number YFYPY202114) and Science and Technology Research Project of Jiangxi Provincial Department of Education (grant number GJJ2001448).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Abdul Momin, M. H. F., Liakopoulos, A., Phee, L. M., and Wareham, D. W. (2017). Emergence and nosocomial spread of carbapenem-resistant oxa-232-producing klebsiella pneumoniae in brunei darussalam. J. Glob. Antimicrob. Resist. 9, 96–99. doi: 10.1016/j.jgar.2017.02.008
Asai, M., Li, Y., Spiropoulos, J., Cooley, W., Everest, D. J., Kendall, S. L., et al. (2022). Galleria mellonella as an infection model for the virulent mycobacterium tuberculosis h37rv. Virulence 13, 1543–1557. doi: 10.1080/21505594.2022.2119657
Carattoli, A. (2009). Resistance plasmid families in enterobacteriaceae. Antimicrob. Agents Chemother. 53, 2227–2238. doi: 10.1128/aac.01707-08
Carattoli, A. (2011). Plasmids in gram negatives: molecular typing of resistance plasmids. Int. J. Med. Microbiol. 30, 1654–1658. doi: 10.1016/j.ijmm.2011.09.003
Carattoli, A., Bertini, A., Villa, L., Falbo, V., Hopkins, K. L., and Threlfall, E. J. (2005). Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63, 219–228. doi: 10.1016/j.mimet.2005.03.018
Chen, L., Mathema, B., Chavda, K. D., Deleo, F. R., Bonomo, R. A., and Kreiswirth, B. N. (2014). Carbapenemase-producing klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 22, 686–696. doi: 10.1016/j.tim.2014.09.003
de Jonge, B. L., Karlowsky, J. A., Kazmierczak, K. M., Biedenbach, D. J., Sahm, D. F., and Nichols, W. W. (2016). In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible enterobacteriaceae isolates collected during the inform global surveillance study (2012 to 2014). Antimicrob. Agents Chemother. 60, 3163–3169. doi: 10.1128/aac.03042-15
Ennis, H. L., and Sells, B. H. (1968). Breakdown and re-formation of polysomes in escherichia coli during inhibition of protein synthesis. Biochim. Biophys. Acta 16, 1503–1508. doi: 10.1016/0005-2787(68)90126-3
Han, R., Shi, Q., Wu, S., Yin, D., Peng, M., Dong, D., et al. (2020). Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant enterobacteriaceae isolated from adult and children patients in China. Front. Cell. Infect. Microbiol. 10:314. doi: 10.3389/fcimb.2020.00314
Hu, L., Zhong, Q., Shang, Y., Wang, H., Ning, C., Li, Y., et al. (2014). The prevalence of carbapenemase genes and plasmid-mediated quinolone resistance determinants in carbapenem-resistant enterobacteriaceae from five teaching hospitals in Central China. Epidemiol. Infect. 14, 21972–21977. doi: 10.1017/s0950268813002975
Jia, H., Zhang, Y., Ye, J., Xu, W., Xu, Y., Zeng, W., et al. (2021). Outbreak of multidrug-resistant oxa-232-producing st15 klebsiella pneumoniae in a teaching hospital in Wenzhou, China. Infect. Drug Resist. 14, 4395–4407. doi: 10.2147/idr.S329563
Karlowsky, J. A., Lob, S. H., Kazmierczak, K. M., Badal, R. E., Young, K., Motyl, M. R., et al. (2017). In vitro activity of imipenem against carbapenemase-positive enterobacteriaceae isolates collected by the smart global surveillance program from 2008 to 2014. J. Clin. Microbiol. 55, 1638–1649. doi: 10.1128/jcm.02316-16
Khan, F. A., Söderquist, B., and Jass, J. (2019). Prevalence and diversity of antibiotic resistance genes in swedish aquatic environments impacted by household and hospital wastewater. Front. Microbiol. 10:688. doi: 10.3389/fmicb.2019.00688
Li, G., Shi, J., Zhao, Y., Xie, Y., Tang, Y., Jiang, X., et al. (2020). Identification of hypervirulent klebsiella pneumoniae isolates using the string test in combination with galleria mellonella infectivity. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1673–1679. doi: 10.1007/s10096-020-03890-z
Liu, Y., Du, F. L., Xiang, T. X., Wan, L. G., Wei, D. D., Cao, X. W., et al. (2019). High prevalence of plasmid-mediated quinolone resistance determinants among serotype k1 hypervirulent klebsiella pneumoniae isolates in China. Microb. Drug Resist. 25, 681–689. doi: 10.1089/mdr.2018.0173
Liu, C., Fang, Y., Zeng, Y., Lu, J., Sun, Q., Zhou, H., et al. (2020). First report of oxa-181-producing klebsiella pneumoniae in China. Infect. Drug Resist. 13, 995–998. doi: 10.2147/idr.S237793
Liu, Y., Liu, P. P., Wang, L. H., Wei, D. D., Wan, L. G., and Zhang, W. (2017). Capsular polysaccharide types and virulence-related traits of epidemic kpc-producing klebsiella pneumoniae isolates in a chinese university hospital. Microb. Drug Resist. 23, 901–907. doi: 10.1089/mdr.2016.0222
Mclaughlin, M. M., Advincula, M. R., Malczynski, M., Barajas, G., Qi, C., and Scheetz, M. H. (2014). Quantifying the clinical virulence of klebsiella pneumoniae producing carbapenemase klebsiella pneumoniae with a galleria mellonella model and a pilot study to translate to patient outcomes. BMC Infect. Dis. 14:31. doi: 10.1186/1471-2334-14-31
Miller, W. R., Munita, J. M., and Arias, C. A. (2014). Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti-Infect. Ther. 12, 1221–1236. doi: 10.1586/14787210.2014.956092
Oueslati, S., Nordmann, P., and Poirel, L. (2015). Heterogeneous hydrolytic features for oxa-48-like β-lactamases. J. Antimicrob. Chemother. 70, 1059–1063. doi: 10.1093/jac/dku524
Pitout, J. D. D., Peirano, G., Kock, M. M., Strydom, K. A., and Matsumura, Y. (2019). The global ascendency of oxa-48-type carbapenemases. Clin. Microbiol. Rev. 33:e00102-19. doi: 10.1128/CMR.00102-19
Potron, A., Rondinaud, E., Poirel, L., Belmonte, O., Boyer, S., Camiade, S., et al. (2013). Genetic and biochemical characterisation of oxa-232, a carbapenem-hydrolysing class d β-lactamase from enterobacteriaceae. Int. J. Antimicrob. Agents 41, 325–329. doi: 10.1016/j.ijantimicag.2012.11.007
Pulss, S., Stolle, I., Stamm, I., Leidner, U., Heydel, C., Semmler, T., et al. (2018). Multispecies and clonal dissemination of oxa-48 carbapenemase in enterobacteriaceae from companion animals in Germany, 2009-2016. Front. Microbiol. 9:1265. doi: 10.3389/fmicb.2018.01265
Russo, T. A., and Marr, C. M. (2019). Hypervirulent klebsiella pneumoniae. Clin. Microbiol. Rev. 32:e00001-19. doi: 10.1128/CMR.00001-19
Russo, T. A., Olson, R., Fang, C. T., Stoesser, N., Miller, M., Macdonald, U., et al. (2018). Identification of biomarkers for differentiation of hypervirulent klebsiella pneumoniae from classical K. pneumoniae. J. Clin. Microbiol. 56:e00776-18. doi: 10.1128/jcm.00776-18
Shankar, C., Nabarro, L. E., Devanga Ragupathi, N. K., Muthuirulandi Sethuvel, D. P., Daniel, J. L., Doss, C. G., et al. (2016). Draft genome sequences of three hypervirulent carbapenem-resistant klebsiella pneumoniae isolates from bacteremia. Genome Announc. 4:e01081-16. doi: 10.1128/genomeA.01081-16
Shi, Q., Han, R., Guo, Y., Zheng, Y., Yang, Y., Yin, D., et al. (2020). Emergence of st15 klebsiella pneumoniae clinical isolates producing plasmids-mediated rmtf and oxa-232 in China. Infect. Drug Resist. 13, 3125–3129. doi: 10.2147/idr.S257298
Shu, L., Dong, N., Lu, J., Zheng, Z., Hu, J., Zeng, W., et al. (2019). Emergence of oxa-232 carbapenemase-producing klebsiella pneumoniae that carries a plvpk-like virulence plasmid among elderly patients in China. Antimicrob. Agents Chemother. 63:e02246-18. doi: 10.1128/aac.02246-18
Tenover, F. C., Arbeit, R. D., Goering, R. V., Mickelsen, P. A., Murray, B. E., Persing, D. H., et al. (1995). Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33, 2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995
Weng, X., Shi, Q., Wang, S., Shi, Y., Sun, D., and Yu, Y. (2020). The characterization of oxa-232 carbapenemase-producing st437 klebsiella pneumoniae in China. Can. J. Infect. Dis. Med. Microbiol. 2020:5626503. doi: 10.1155/2020/5626503
Yang, X., Dong, N., Chan, E. W., Zhang, R., and Chen, S. (2021). Carbapenem resistance-encoding and virulence-encoding conjugative plasmids in klebsiella pneumoniae. Trends Microbiol. 29, 65–83. doi: 10.1016/j.tim.2020.04.012
Yang, X., Sun, Q., Li, J., Jiang, Y., Li, Y., Lin, J., et al. (2022). Molecular epidemiology of carbapenem-resistant hypervirulent klebsiella pneumoniae in China. Emerg. Microbes Infect. 11, 841–849. doi: 10.1080/22221751.2022.2049458
Yin, D., Dong, D., Li, K., Zhang, L., Liang, J., Yang, Y., et al. (2017). Clonal dissemination of oxa-232 carbapenemase-producing klebsiella pneumoniae in neonates. Antimicrob. Agents Chemother. 61:e00385-17. doi: 10.1128/aac.00385-17
Zhang, Y., Jin, L., Ouyang, P., Wang, Q., Wang, R., Wang, J., et al. (2020). Evolution of hypervirulence in carbapenem-resistant klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J. Antimicrob. Chemother. 75, 327–336. doi: 10.1093/jac/dkz446
Keywords: Klebsiella pneumoniae, carbapenem-resistance, mobilized virulence factors, blaOXA gene, antimicrobial-resistant genes
Citation: Li P, Luo W-y, Xiang T-X, Peng T-x, Luo S, He Z-y, Liao W, Wei D-D, Liu P, Wan L-g, Zhang W and Liu Y (2023) Isolation of Hv-CRKP with co-production of three carbapenemases (blaKPC, blaOXA-181 or OXA-232, and blaNDM-1) and a virulence plasmid: a study from a Chinese tertiary hospital. Front. Microbiol. 14:1182870. doi: 10.3389/fmicb.2023.1182870
Edited by:
Ximin Zeng, The University of Tennessee, Knoxville, United StatesReviewed by:
Hussein O. M. Al-Dahmoshi, University of Babylon, IraqMahmoud M. Tawfick, Al-Azhar University, Egypt
Copyright © 2023 Li, Luo, Xiang, Peng, Luo, He, Liao, Wei, Liu, Wan, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, emhhbmd3ZWlsaXV4aW5AMTYzLmNvbQ==; Yang Liu, bHkxMzc2NzE2MDQ3NEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ping Li
Ping Li Wan-ying Luo
Wan-ying Luo Tian-Xin Xiang
Tian-Xin Xiang Ting-xiu Peng1,2
Ting-xiu Peng1,2 La-gen Wan
La-gen Wan Wei Zhang
Wei Zhang Yang Liu
Yang Liu