
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 24 August 2023
Sec. Microbe and Virus Interactions with Plants
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1168203
 Aboagye Kwarteng Dofuor1*
Aboagye Kwarteng Dofuor1* Naa Kwarley-Aba Quartey2
Naa Kwarley-Aba Quartey2 Angelina Fathia Osabutey3
Angelina Fathia Osabutey3 Akua Konadu Antwi-Agyakwa4
Akua Konadu Antwi-Agyakwa4 Kwasi Asante5
Kwasi Asante5 Belinda Obenewa Boateng5
Belinda Obenewa Boateng5 Fred Kormla Ablormeti5
Fred Kormla Ablormeti5 Hanif Lutuf6
Hanif Lutuf6 Jonathan Osei-Owusu7
Jonathan Osei-Owusu7 Joseph Harold Nyarko Osei8
Joseph Harold Nyarko Osei8 William Ekloh9
William Ekloh9 Seyram Kofi Loh10
Seyram Kofi Loh10 Joseph Okani Honger11
Joseph Okani Honger11 Owusu Fordjour Aidoo1
Owusu Fordjour Aidoo1 Kodwo Dadzie Ninsin1
Kodwo Dadzie Ninsin1Mango anthracnose disease (MAD) is a destructive disease of mangoes, with estimated yield losses of up to 100% in unmanaged plantations. Several strains that constitute Colletotrichum complexes are implicated in MAD worldwide. All mangoes grown for commercial purposes are susceptible, and a resistant cultivar for all strains is not presently available on the market. The infection can widely spread before being detected since the disease is invincible until after a protracted latent period. The detection of multiple strains of the pathogen in Mexico, Brazil, and China has prompted a significant increase in research on the disease. Synthetic pesticide application is the primary management technique used to manage the disease. However, newly observed declines in anthracnose susceptibility to many fungicides highlight the need for more environmentally friendly approaches. Recent progress in understanding the host range, molecular and phenotypic characterization, and susceptibility of the disease in several mango cultivars is discussed in this review. It provides updates on the mode of transmission, infection biology and contemporary management strategies. We suggest an integrated and ecologically sound approach to managing MAD.
Mango anthracnose disease (MAD) is a worldwide disease that is extremely destructive to mangoes before and after harvest. The disease damages the infected mango trees, leading to low yield and quality of fruits. MAD can cause a 100% loss of yield in orchards that are not well taken care of and where the environment is good for the disease to spread. The disease occurs in almost all regions that produce the crop, and its high economic losses have prompted a wealth of research focusing on postharvest losses (Cheng et al., 2022; Silué et al., 2022), damage (Wu et al., 2022), characterization (Ismail and El-Ganainy, 2022), diagnosis and classification (Alberto et al., 2022; Patil et al., 2022; Prabu and Chelliah, 2022), genomics (Ciofini et al., 2022; Kumari et al., 2022), control (Evangelista-Martínez et al., 2022; Liang et al., 2022) and resistance management (Janamatti et al., 2022).
The Colletotrichum species complex is responsible for the fungal disease known as MAD. Weir et al. (2012) used morphological and molecular techniques to determine that roughly twenty-two species and one subspecies make up the C. gloeosporioides complex. However, several C. gloeosporioides isolates in various locations worldwide are characterized by characteristic black, expanding lesions on mango plant parts, including fruits, leaves, flowers, petioles, twigs, and stems. Many of these isolates, including C. alienum, C. fructicola, C. siamense, C. tropicale, and C. asianum, have significantly damaged millions of mango trees in Mexico, China, and India (Weir et al., 2012; Li et al., 2019; Tovar-Pedraza et al., 2020). In the United States, C. aeschynomenes, C. musae, and C. nupharicola are associated with anthracnose in mangoes (Su et al., 2011; Weir et al., 2012). Several articles such as Ciofini et al. (2022), Paudel et al. (2022), Jenny et al. (2019), Honger et al. (2015), Nelson (2008), and Arauz (2000) have reviewed MAD. However, all the reviews have a narrow focus on particular geographical areas, including Ghana (Kankam et al., 2022) and India (Maske et al., 2022). Though the economic impacts and management of the disease in many countries have been investigated by different authors (e.g., Arauz, 2000), these studies were confined to a small geographical region.
In contrast to previous articles on mango anthracnose, this review provides a concise summary of the current state of knowledge about MAD from a global viewpoint, including the most up-to-date information on its history, economic importance, epidemiology, early detection methods and management strategies. It highlights the rapid diagnosis of MAD using a computer vision system and a species-specific PCR assay to slow down the spread of the disease into disease-free areas. The biological relationships between the pathogen, environmental conditions, and host plant susceptibility have been better understood due to early detection technologies, genome sequencing, and machine learning.
This review is well-timed because MAD is spreading rapidly to disease-free countries like Indonesia (Benatar et al., 2021), Vietnam (Li et al., 2020) and Cuba (Manzano León et al., 2018). MAD continues to be a severe problem in many regions; for instance, the detection of new strains in Peru (Vilcarromero-Ramos et al., 2022) and China (Ahmad et al., 2021) threatens the livelihood of millions of people that depend on the crop. The potential of different strains of the fungus coexisting within a country; for example, in the northeast of Brazil, where C. asianum, C. fructicola, C. tropicale, C. karstii, and C. dianesei are present (Lima et al., 2013a,b) highlights the importance of this review. We also provide an update on the taxonomic status of the Colletotrichum taxa linked with various MAD, which has changed since the introduction of molecular techniques.
Globally, Colletotrichum species was first reported by Corda (1831). Since then, the disease has been infecting many crops and trees, including mangoes worldwide (Figure 1; Ciofini et al., 2022). MAD occurs in several countries, including Côte d’Ivoire, Ethiopia, Ghana, Nigeria, South Africa in Africa; Australia in Oceania; Bangladesh, China, India, Indonesia, Taiwan in Asia; and Colombia, Mexico, and Peru in the Americas. The disease mainly affects the leaves, tissues, peduncle, pedicle, twig, stem, fruit, and pulp. Different species of MAD may be part of a complex or just a singleton, and these fungal infections have been reported in different countries. Members of the Colletoctrichum genus are the predominant pathogens that cause MAD. They are made up of about 200 species that are tentatively placed into 15 species complexes and singletons (Talhinhas et al., 2018; Guevara-Suarez et al., 2022). Several mango species tolerant to anthracnose diseases have been determined through screening after inoculating these mango species with Colletotrichum asianum (Grice et al., 2022). Moreover, many working groups from different geographical regions or countries, such as Ethiopia and Nigeria, while studying the MAD distribution, symptoms, pathogenicity, etiology, incidence, and severity, have also reported MAD infections of different parts of mango (Awa et al., 2012; Chala et al., 2014; Tucho et al., 2014; Benatar et al., 2021). Ismail et al. (2015) reported the recovery of C. gloeosporioides, C. kahawae subsp. ciggaro, and C. karstii for the first time in Italy.
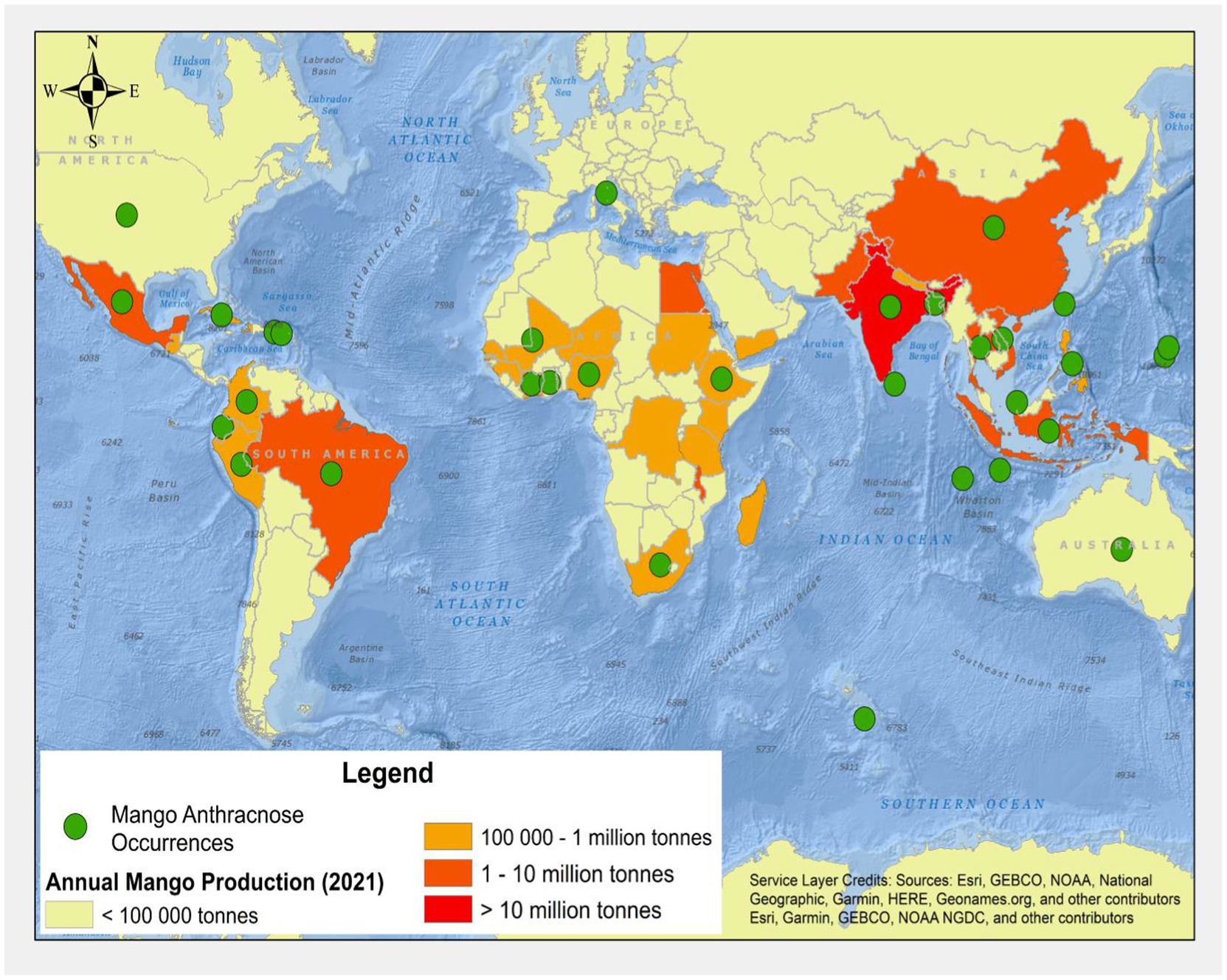
Figure 1. Global map of mangoes production and known distribution records for mango anthracnose [refer to Table 1 for further details (FAOSTAT, 2021)].
Reports of the C. gloeosporioides species complex, including C. alienum, C. asianum, C. fructicola, C. siamense, and C. tropicale, were identified in Mexico in 2019 (Tovar-Pedraza et al., 2020). Among these, C. alienum was reported for the first time in Mexico. The C. alienum infections have spread into many anthracnose disease endemic areas; for instance, it was reported in China in 2020 (Ahmad et al., 2021). Another type of MAD discovered in 2019 was the Colletotrichum scovillei (Qin et al., 2019). Table 1 further explains the history and distribution of MAD.
MAD represents the most severe fungal disease restricting the cultivation and commercialization of mango fruits internationally. C. gloeosporioides, the disease’s causative organism, represents one of MAD’s most economically important agents that can impact the sorting, packaging, shipping, storage, and sale of mango fruits (Bhagwat et al., 2015). In many regions, pre-harvest infections from organisms like fungi, viruses, bacteria, and nematodes have led to low mango harvests (Chowdhury and Rahim, 2009). MAD is one example of a postharvest disease that drastically reduces fruit quality, leading to substantial economic losses (Akem, 2006). The economic and scientific importance of postharvest damage from MAD is that it lowers fruit quality and shelf life, thereby influencing export quality standards (Kankam et al., 2022).
MAD typically causes yield losses through a decline in either the quantity or quality of harvested mango fruits. According to local reports, MAD poses a severe threat to Ghana’s mango crop, causing a 30% output loss in one of its districts (Honger et al., 2014). Similar to how anthracnose disease is responsible for roughly 39% of yield loss in mango cultivation in India (Prakash and Srivastava, 1987). MAD causes a 30–60% annual loss of mango, with potential damage of 100% under ideal conditions (Kamle and Kumar, 2016). In Gondunglegi, Indonesia, yield loss due to anthracnose was reported to be 50.28% by Kumari et al. (2017) and Arauz (2000), while in Himachathe l Pradesh, India, the postharvest loss was reported to be 29.6% from 1990 to 1992 (Sharma and Verma, 2007). In Hyderabad, 20 to 30% of mango fruits rotted due to C. gloeosporioides. According to Hossain and Ahmed (1994), MAD and stem end rot diseases account for a 25–30% loss in mango yield in Bangladesh. Mangoes in Thailand were lost at a rate of 62.8% during harvest and 63.2% in the markets due to MAD, according to research by Sardsud et al. (2003). Anthracnose can cause a 30–60% annual loss in China’s mango harvest (Li et al., 2019).
Severe problems in nurseries and orchards may appear under crowded and moist conditions (Lai and Simon, 2013). The incidence may increase significantly under favorable environmental conditions. Damage to foliage, a reduction in flower production, and yield losses have all been attributed to C. gloeosporioides.
Humid climates are also ideal for the spread of anthracnose fungus (Arauz, 2000; Akem, 2006). The disease has a prevalence of about 100% in fruits grown in damp or very humid environments (Arauz, 2000; Akem, 2006; Chowdhury and Rahim, 2009). MAD can also induce postharvest deterioration, leading to the fruit being rejected by consumers.
MAD is widely recognized as a significant challenge to southern Ethiopia’s mango farming business’s sustainability (Chala et al., 2014). Mango exporting countries like Ghana suffer the most as a result of the disparity in revenue between export and local markets, despite this, producers and sellers continue to sell low-quality fruits on the local market (Kankam et al., 2022).
The expense of controlling MAD could have repercussions for the economy. Farmers have used a variety of fungicides to prevent the fungus from spreading. Smallholder farmers, who may lack the capital to invest in such suggested management tactics, may profoundly feel the economic effects of these controls. In addition, fungi can become resistant to fungicides if too many are used, necessitating the use of more potent or hazardous compounds.
MAD is among the most widespread diseases that can attack mango at any time, whether when it is still in the field, in transportation, or storage. Mango orchards may show symptoms of the disease on the mango trees’ leaves, twigs, and fruits (Figure 2). It can also be found on the blossoms (Uddin et al., 2018). The disease’s symptoms show up as oval or irregular brown to deep brown sunken point-sized spots of varying sizes spread all over the leaf surface, most prominently on young leaves but also on older leaves. As the leaves age, these spots develop into larger lesions that are either circular or irregular in shape and surrounded by a red halo (Qin et al., 2019).
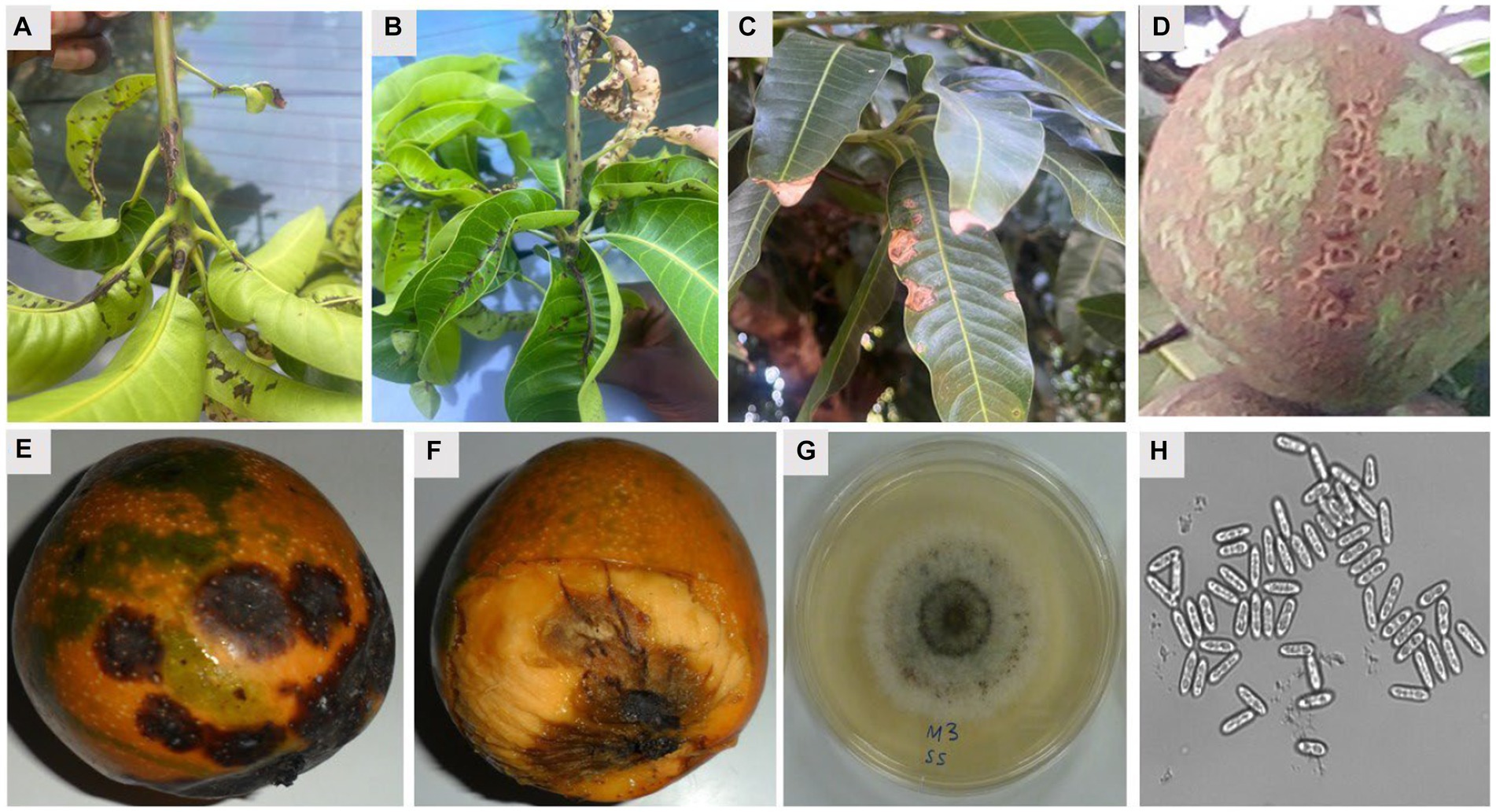
Figure 2. Symptoms of mango anthracnose disease and cultural and morphological features of C. gloeosporioides causing anthracnose disease of mango. (A,B) Symptomatic leaves and twigs, (C) symptomatic mature leaves, (D) the alligator skin effect symptoms on unripe fruit, (E) typical sunken spot symptoms of mango anthracnose, (F) a ripe fruit with the pericarp removed to show the penetration of the dark lesions of the anthracnose disease on the fruit pulp, (G) white mycelial growth on PDA showing presence of acervuli ring in the middle of the culture, and (H) short conical spores with rounded edges (H). Mg × 200.
The fungus can produce blossom blight on the inflorescence. Lengthy lesions ranging from dark grey to black can appear in the stalk. The panicles and open flowers also develop tiny black spots that eventually grow and kill the plant. Flowers that have been blighted become brittle and dark brown to black (Alemu et al., 2014). The fungus sometimes invades the twigs, stems and branches of the mango tree. Symptoms on twigs manifest as small, expanded oval and necrotic lesions that eventually consolidate and disperse. The fungus can invade twigs during severe infections and cause dieback (Tovar-Pedraza et al., 2020).
MAD is most common on immature fruits and during transport and storage but can occur at any stage during the fruit’s life cycle. The young fruits are either aborted or mummified, and infection on larger fruits may remain latent or dormant until the ripening of the fruit, where black, sunken necrotic lesions appear on the fruit peel and increase rapidly in size (De Souza et al., 2013). Tear staining, caused by spore-laden water droplets from diseased twigs and leaves spreading across the fruit and infecting the surface, is another symptom of anthracnose damage to fruit (Mabbett, 2014). Several other diseases attack the mango plant aside from anthracnose. Some of these diseases have symptoms similar to anthracnose, and others have distinct symptoms. A couple of such equally important diseases of mango are compared with anthracnose.
Alternaria leaf spot disease is similar to anthracnose disease because it mostly attacks the young, tender leaves and is characterized by brown spots. Anthracnose, however, shows up as irregular, oval-shaped spots all over the leaf’s surface while in Alternaria leaf spot, typical round spots are uniformly distributed across the leaf’s lamina and more noticeable on the leaf’s underside. Symptoms of bacterial canker disease on leaves include yellowing and eventual leaf drop, as well as wet, irregular to angular, elevated lesions that are sometimes clustered near the leaf apex. While anthracnose leaf spots are large, scab disease spots are tiny and cause the leaf to become distorted and wrinkled before falling off prematurely. A powdery fungus growth coating on the leaves also characterizes powdery mildew disease.
Black-banded or black velvety mycelial growth on leaf veins and midribs is a telltale sign of disease (Gautam et al., 2017), while red rust is characterized by small, circular lesions that appear on the top leaf surfaces and coalesce toward the midribs (Patrice et al., 2020). Stem end rot is a disease that affects fruits and causes these fruits to turn black at the stem end, eventually spreading to cover about half of the fruit. Though the damaged skin retains its firmness, the rot sets into the pulp, which gives off a foul odor. Additionally, water-soaked sores on fruits progressively develop into cankers, which break open and release a gummy slime due to bacterial canker disease. Conversely, MAD causes black spots, which are initially spherical, then transform into big irregular blotches across the entire fruit while in storage. Large, gaping crevices form at these sites, allowing the fungus to eat its way deep into the fruit (Sefu et al., 2015).
The necrotic regions on the twigs become increasingly longer and black due to the twig blight disease. The leaves begin to drop and eventually dry out and fall off. Very immature branches begin to dry out from the tips down. Dieback disease causes twigs to wilt and die from the top down, especially in older trees, and then leaves to wilt and die, giving the impression of a fire scorch (Saeed et al., 2017). Young, green twigs get discolored and black, eventually dying from the top down (Malik et al., 2014). Mango twigs eventually succumb to MAD during extremely severe outbreaks and vice versa.
For powdery mildew, the distinguishing symptoms of the disease are whitish powdery fungus growth coating on flowers. Affected flowers and young fruits that have reached marble size may drop prematurely (Ajitomi et al., 2020). The panicles and open flowers also develop tiny black spots that eventually grow and kill the plant. Flowers affected by anthracnose are dry and dark brown to black in hue (Rosman et al., 2019). It is estimated that the disease causes 30–60% damage economically, which can climb to 100% in fruits produced during humid seasons or at the commencement of rains (Kamle and Kumar, 2016).
MAD causes the most harm in wet environments during the flowering and fruit-setting periods. Flowering and early fruit development are susceptible to mango infections (Sarkar, 2016). Some cutting-edge strategies for early diagnosis of anthracnose disease in mangoes include the application of Modified Rotational Kernel Transform Features (Ullagaddi and Raju, 2017), computational biology, and image processing analytic methods (Khan et al., 2019).
Conserved signal transduction pathways control fungi growth, development, and reproduction (Cannon et al., 2012; Gan et al., 2013). The mitogen-activated protein (MAP) kinase cascades have been shown to be responsible for the infection-related morphogenesis in pathogenic fungi like C. gloeosporioides (Yong et al., 2013; Zhou Z. et al., 2017). Yong et al. (2013), in their study isolated and characterized the MAK kinase gene Cgl-SLT2 from C. gloeosporioides, and indicated the full involvement of MAPK in conidiation, appressorium formation, polarized growth, and the pathogenicity of the filamentous fungus. The MAPK cascade, which consists of three conserved kinases, the MAP kinase (MAPK), MAP kinase kinase (MEK), and MAP kinase kinase kinase (MEKK), is a pivotal signaling pathway sensing and relaying extracellular signals to control gene expression (Yong et al., 2013). The fungal MAPK cascade together with the calcium-calcineurin pathway, which functions via calmodulin, the Ca2+ binding protein, calcineurin, and the calmodulin-dependent serotonin-threonine phosphatase (Zhou Z. et al., 2017), targets a wide range of downstream effectors including enzymes, other proteins, and transcription effectors. Thus, triggered by a host of stimuli, the two pathways regulate basic cell function and stress response. Like other pathogenic fungi that attack plants, C. gloeosporioides secret proteolytic enzymes such as pectate lyase (PEL), endopolygalacturonase (PG) and pectin lyase (PNL) (Zhou Z. et al., 2017). These essential pathogenic functionaries can depolymerize polysaccharides present in the primary cell wall of the host during infection and colonization (Zhou Z. et al., 2017). Zhou Z. et al. (2017) reported that C. gloeosporioides required the ABC (ATP-binding cassette) protein CgABCF2 for appressorial formation and plant infection as well as for sexual and asexual reproduction.
Many of the studies conducted on C. gloeosporioides depend on evidence from previous works which stated that the sequence data in the domain 2 (D2) region of the rDNA could efficiently reveal information about the type of leucyl-tRNA synthetase (LARS) and relationships within C. gloeosporioides (Cannon et al., 2012; Sharma et al., 2013). A recent study caused mutations within the WT strain W16 of C. gloeosporioides via ATMT (Agrobacterium tumefaciens-Mediated Transformation) to study genes associated with conidiation (Wu et al., 2016). The insertional mutagenesis populations were then isolated, and conidiation assays were conducted on 59 conidial production-variation transformants (Wu et al., 2016). The study conclusively found the oligopeptide transporter (Opt) to be involved in C. gloeosporioides conidiation. The team also identified 19 putative genes including CgOPT2 (oligopeptide transporter protein), CgMCT1 (monocarboxylate transporter protein), CgCOP9 (Cop9 signalosome subunit 6 protein), CgMRP1 (multidrug resistance- associated protein 5), CgRXT2 (Rxt2-like protein) and CgRRN3 (specific transcription initiation factor RRN3). Interestingly, while the WT strain W16 contained 19 genes, 11 of these genes were not detected in T-DNA insertional mutants that were also sequenced (Wu et al., 2016). The team inferred from the observational differences in phenotypes that variations in conidiation and production of albino hyphae could be attributed to the absence of those 11 genes (Wu et al., 2016).
Early in the 20th century, research teams successfully isolated and characterized phenotypes of C. gloeosporioides using morphological features. Relying on morphology of appressoria and conidiomata conidia dimensions, three biological groups within the C. gloeosporioides complex were determined (Sharma and Kulshrestha, 2015). These were C. gloeosporioides var. gloeosporioides which had unlobed or slightly lobed appressoria and conidial widths of 4.5–5.5 μm; C. gloeosporioides var. minor of similar appearance but with mean conidial widths of 3.0–4.2 μm, and a third phenotype that closely matches the definitions classifying C. crassipes with lobed appressoria and conidial widths of 4.5–5.5 μm (Wu et al., 2016).
On the molecular level, phylogenetic relationships in the Colletotrichum spp. have been successfully identified through sequencing in the internal transcribed spacers 1 and 2 (ITS1 and ITS2) regions and glyceraldehyde-3-phosphate dehydrogrenase (GAPDH) gene (Sharma and Kulshrestha, 2015; Pardo-De la Hoz et al., 2016). Using an additional genomic region, ApMat, the three strains identified in the C. gloeosporioides complex were refined and confirmed (Doyle et al., 2013; Pardo-De la Hoz et al., 2016). From work in the intergenic regions of apn2 and MAT1-2-1 (ApMat) genes, 22 phenotypes (Supplementary Table S1) have been identified (Cannon et al., 2012), and four distinct phylogenies (C. fructicola, C. frgariae sensu stricto, C. melanocaulon and C. jasmine-sambac) within the complex described (Doyle et al., 2013). Subsequently, another team using the same approach confirmed that these four and five potentially novel Colletotrichum lineages, yet to be assigned specie names, were associated with anthracnose in mango tissues (Sharma et al., 2013).
Intervention strategies against C. gloeosporioides have been explored as molecular knowledge of the pathogenic fungus delves deeper. For instance, Yong et al. (2013) showed conclusively that the MAPK gene Cgl-SLT2 is required for appressorium formation, conidiation and pathogenicity in C. gloeosporioides. Subsequently, the mutant Cgl-slt2 showed defective hyphae formation and sporulation compared to the wild type. Other mutagenic studies are exploring using T-DNA (transferred-DNA) to yield fewer pathogenic phenotypes.
Pathogenic fungi of plants need to align with the seasonal growth stages of the host plant for a successful transmission and infection cycle. For instance, since most plants shed leaves and remain dormant during the autumn and winter seasons, plants’ pathogenic fungi typically adopt corresponding dormancy strategies until spring (Marcianò et al., 2021). These strategies include the production of spores, the development of sclerotia, hiding within the host plant, finding shelter in the ground, or moving to another active host plant during adverse conditions (Marcianò et al., 2021). When conditions become favorable, spores may be transmitted by water, wind, or animals to a susceptible host for infection.
At least three major infection strategies are implicated in the molecular pathogenesis of plant fungal pathogens. Fungal strategies may involve preventing host recognition, inhibiting host defense mechanisms, and hijacking host cellular machinery (Rodriguez-Moreno et al., 2018). Specifically, to penetrate the plant host, pathogenic fungi may secrete cell wall-degrading carbohydrate-active enzymes such as glycoside hydrolases, glycosyltransferases, polysaccharide lyases, carbohydrate esterases, and redox enzymes (Lombard et al., 2014). Plant fungi may also modify the components of their cell walls through the accumulation of specialized carbohydrates to prevent degradation by plant chitinases (Fujikawa et al., 2012) or the secretion of carbohydrate-binding effectors to inhibit chitin-mediated host response (de Jonge et al., 2010; Sanchez-Vallet et al., 2013).
Moreover, pathogenic fungi can hijack the host defense system by subversion of reactive oxygen species, modification of host pH, regulation of hormone signaling, and inhibition of host proteases (Rodriguez-Moreno et al., 2018). In most of these infection cycles, fungal effector proteins, secondary metabolites, host-specific toxins, and small RNAs (sRNA) are increasingly gaining prominence as critical regulators and mediators in the mechanism of molecular pathogenesis (Rodriguez-Moreno et al., 2018).
Colletotrichum gloeosporioides is typically dormant in the dry season, especially during extremes of temperature, low humidity, and sunlight (Sharma and Kulshrestha, 2015). However, optimal growth of the pathogen is favored at high humidity, pH range of 5.8 to 6.5, and temperature of 25–28°C (Sharma and Kulshrestha, 2015). These favorable conditions stimulate the release of spores from acervuli of seeds, leaves, fruits, or trash of hosts. Dispersal of spores may occur through air currents, insects, water splash or other forms of direct transmission (Sharma and Kulshrestha, 2015). After successful dispersal, C. gloeosporioides utilizes a hemibiotrophic mode of infection to penetrate, develop and spread within a susceptible host plant (Münch et al., 2008). Through this mode, the infection of host plants usually consists of two stages: penetration and colonization (Marcianò et al., 2021). Melanized appressoria are initially formed to aid in host penetration and harmless formation of primary hyphae in a biotrophic phase of infection, followed by the secondary formation of hyphae in a necrotrophic phase that is potentially deleterious to the host (Münch et al., 2008). This leads to the spread of spores on the surface of infected tissues that are subsequently dispersed to repeat the transmission and infection cycle of the pathogen (Figure 2). Thus, the disease moves through a cycle of dissemination of asexual spores (conidia), inoculation of spores into susceptible part of the host, development of symptoms in fruiting bodies (acervuli), infection of a host, further development of disease, reproduction of pathogen, and survival of pathogen (Paudel et al., 2022). Glomerella cingulata, the name typically given to the sexual stage (teleomorph) of the same pathogen, may induce dark, long-necked perithecia with clavate asci that are relatively rarely observed (Figure 3).
Several C. gloeosporioides genes play critical roles in host defense mechanisms during infection. Most of these genes interact with a pH-responsive transcription factor (pacC) that regulates the expression of approximately 5% fungal genome involved in transport, oxidative damage, and cell wall degradation (Alkan et al., 2013). The expression of pectate lyase by pelB induces the degradation of the plant cell wall in a pH-dependent manner under the regulation of pacC (Drori et al., 2003; Kramer-Haimovich et al., 2006). A PepCYP product homologous to cytochrome P450 with a heme-containing domain involved in host defense mechanisms during pathogen invasion and colonization is also expressed in a ripening–dependent manner by C. gloeosporioides (Oh et al., 1999).
Several genes also mediate pathogenesis in the fungus. CgRac1 protein regulates morphogenesis, nuclear division, and germination via potential concentration in conidia and hyphal tips (Nesher et al., 2011). The expression of cgOPT1 varies in resting and germinating spores during mycelia development (Chagué et al., 2009). Several nitrogen-metabolism-related genes (GDH2, GS1, GLT, and MEP) are differentially expressed to aid in the induction of ammonia accumulation and fungal pathogenicity (Miyara et al., 2012). The expression of Bcl-2 protein plays a role in cell death and survival of the fungus through processes such as pathogenicity, conidial germination, and mycelium growth (Barhoom and Sharon, 2007). CgCTR2, a putative vacuolar copper transporter, is critical in cellular copper balance during the initial stages of pathogenesis (Barhoom et al., 2008). The expression of pel1 and pel2 proteins also plays vital roles in pathogenesis during the neurotropic phase of fungal infection (Shih et al., 2000). The expression of the cgDN3 gene of C. gloeosporioides is stimulated during the early stages of infection to aid in the modulation of a hypersensitive-like response by a compatible host (Stephenson et al., 2000). The chip6 gene plays an essential role in conidial germination and appressorium formation (Kim et al., 2002). Moreover, two pectin lyase genes are differentially expressed during fungal pathogenesis: pnl-2 is highly expressed in the necrotrophic phase of infection, and pnl-1 may be observed in both necrotrophic and biotrophic phases (Wei et al., 2002).
The pathogen responsible for severe anthracnose infections in Brazil’s southeast was identified, and disease susceptibility among a global collection of mango germplasm was assessed, based on a survey. Colletotrichum was the most common pathogen of commercial mangoes in the region, and the cultivars ‘Ubà,’ ‘Quinzenga,’ ‘Amarelinha da Sementeira,’ ‘Aroeira,’ and ‘Correjo’ were particularly vulnerable to C. asianum infections (Vitale et al., 2020). The disease is notably less severe on ‘Ourinho’ and ‘Lita’ cultivars but less on ‘Mallika’. None of the accessions tested was resistant, and commercial cultivars generally cannot deliver appropriate qualitative and quantitative yields under humid environmental conditions without regularly applying fungicide sprays (Arauz, 2000). Mango (‘Palmer,’ ‘Keith,’ and ‘Tommy Arkins’) from the orchards of Northeastern Brazil were used to test the virulence of five species of Colletotrichum (C. asianum, C. dianesei, C. fructicola, C. karstii, and C. tropicale). Neither the ‘Keith’ nor the ‘Palmer’ cultivars were affected by C. karstii, suggesting that it is not pathogenic to these species (Lima et al., 2015). The Tommy Atkins cultivar was more tolerant to infection from all Colletotrichum species tested, and the alternate host plants were all susceptible to the Colletotrichum spp. In Australia, ‘Carrie’, ‘Tommy Atkins’, ‘Saigon’ cultivars are resistant to C. gloeosporioides, while ‘Kensington Pride’ shows moderate resistance to the disease (Nelson, 2008; Kankam et al., 2022). In the Philippines, ‘Palmer’, ‘Siam’, ‘Velei-Colomban’, and ‘Joe Welch’ are resistant cultivars, whereas ‘Fernandin’, ‘Arumanis’, ‘Edward’, ‘Gedong’, and Tjenkir’ are fairly resistant to the disease. Moreover, ‘Paris’, ‘Fairchild’, and ‘Rapoza’ show resistance to C. gloeosporioides in Hawai‘I, with only ‘Haden’ showing moderate resistance to the pathogen. In Florida, the ‘Zill’ shows resistance to C. gloeosporioides.
In addition to mango, other hosts of C. gloeosporioides include Musa species, avocado (Persea americana Mill.) and guava (Psidium guajava L.) (Nelson, 2008; Moraes et al., 2013). The pathogen has also been found in apples (Malus domestica Borkh.) (Munir et al., 2016), papaya (Carica papaya L.) (Maharaj and Rampersad, 2012) and leaves, tubers and seeds of yam (Dioscorea alata L.) (Abang et al., 2002). Besides, capsicum, coffee, eggplant, and tomato are susceptible to MAD. Lima et al. (2015) suggest alternative host plants (papaya, banana, guava and bell pepper) are susceptible to C. asianum, C. dianesei, C. fructicola, C. karstii and C. tropicale. There are many strains of the pathogen that can infect non-mango host plants, which include ornamental lupine (Lupinus hartwegii L.), marsh lupine (Lupinus polyphyllus Lindl.), various herbs such as angelica (Archangelica officinalis Hoffm.), thyme (Thymus vulgaris L.) caraway (Carum carvi L.) and elder (Sambucus nigra L.) (Paulitz, 1995). Anthracnose symptoms have been observed in mango cv. R2E2 when C. alienum B.S. Weir and P.R. Johnst., C. kahawae subsp. cigarro B.S. Weir and P.R. Johnst., and C. theobromicola Delacr., were isolated from avocados (Grice et al., 2022). In Venezuela, Coffee Berry Borer -infested and -uninfested branches and twigs, as well as ripe and green berries with indications of anthracnose, were all found to be positive for C. siamense and C. alienum (Castillo et al., 2022). The dragon fruit, or Hylocereus undatus, is a species of the Hylocereus that often has a white pulp and scarlet or pink skin. One of the most widespread worldwide phytopathogens responsible for postharvest anthracnose in dragon fruits is Colletotrichum spp. (Bordoh et al., 2020). The disease infects fruits in the field, during transportation, and during cold storage, thereby reducing the shelf life of the fruits. Colletotrichum scovillei causes anthracnose symptoms in bananas, mangoes, wampi (Clausena lansium, Rutaceae), and onions (Zhou Y. et al., 2017; Qin et al., 2019; Lin et al., 2020; Lopes et al., 2021).
Though C. tamarilloi is associated with tamarillo in Colombia and Ecuador, the fungus also attacks mango and onion in Columbia and Brazil, respectively (Damm et al., 2012; Pardo-De la Hoz et al., 2016; Caicedo et al., 2017; Lopes et al., 2021). In China, C. gigasporum was isolated from mango (Li et al., 2019), and the same species attacks coffee in Mexico and China (Cristóbal-Martínez et al., 2017; Cao et al., 2019). In Brazil, C. gigasporum, was recorded as a secondary fungal disease on Annona spp. (Costa et al., 2019). Colletotrichum alienum attacks many crops, including Aquilaria sinensis and Camellia sinensis in China (Liu et al., 2017, 2020; Ahmad et al., 2021). In Australia, the pathogen has been isolated from Fragaria × ananassa, Grevillea sp., and Nerium oleander (Liu et al., 2013; Schena et al., 2014; Shivas et al., 2016). Moreover, in New Zealand, the pathogen has been reported from Malus domestica (Weir et al., 2012), Persea americana in Australia, New Zealand and Israel (Weir et al., 2012; Sharma et al., 2017). Colletotrichum cigarro occurs in mango in Colombia and Italy (Ismail et al., 2015; Pardo-De la Hoz et al., 2016). Colletotrichum asianum is associated with mangoes but has been reported on avocados (Persea americana) in Indonesia (Zhafarina et al., 2021). Colletotrichum cigarro has been isolated from mango in Colombia and Italy (Ismail et al., 2015; Pardo-De la Hoz et al., 2016). The species also occurs in plants, such as Kunzea ericoides in New Zealand (Weir et al., 2012), Areca catechu in China (Zhang et al., 2020) and apple (Malus domestica) in Belgium and the USA (Grammen et al., 2019; McCulloch et al., 2020). C. endophyticum is associated with mango fruits and leaves but also occurs in Camellia sinensis (Wang et al., 2016), and coffee (Coffea arabica and C. robusta) leaves and fruits (Cao et al., 2019). The pathogen is of grave concern in Southeast Asia to tea, coffee and mango plantations (Talhinhas and Baroncelli, 2021). C. perseae is associated with MAD (Hofer et al., 2021) and avocado (Talhinhas and Baroncelli, 2021). C. queenslandicum affects papaya and avocado in Australia, cashew in Brazil (Veloso et al., 2018), and coffee in Fiji (Weir et al., 2012). The pathogen has been found in a variety of other host plants, including Citrus latifolia in the United States (Kunta et al., 2018), Licania tomentosa in Brazil (Lisboa et al., 2018), Litchi chinensis in Australia (Anderson et al., 2013; Shivas et al., 2016), Nephelium lappaceum in Puerto Rico (Serrato-Diaz et al., 2014; Shivas et al., 2016). C. liaoningense attacks mango in China (Li et al., 2019). However, the pathogen can also occur in Solanum pseudocapsicum (Liu et al., 2021). In mangoes, larger lesions are observed on hosts at temperatures ranging between 25 and 30°C, though many species exhibit varying thermal requirements for maximal pathogenicity in the fruits (Lima et al., 2015). Table 2 lists a range of mango cultivars with varying levels of resistance and susceptibility.
Traditionally, MAD is identified using certain morphological features such as mycelial growth, conidia size, colony color, texture, and presence and absence of setae (Abera et al., 2016; Ashraful et al., 2017). Although these features are still used with other techniques to identify and characterize the causative organisms of the disease, the differences in these physical features are not adequate to separate the different species of Colletotrichum. This was confirmed in a study where it was shown that differences exist between identical specimens grown under different laboratory conditions (Mo et al., 2018). According to Peres et al. (2002), there is an overlap between the conidial morphology and cultural characteristics, thus making the use of these features unreliable.
Biochemical reactions and immunoassays have been used to distinguish between bacterial, fungal, protozoan, and plant species based on differences in certain structural features associated with these organisms. Some of these reactions have been employed to separate the different species of MAD causative agents. Honger et al. (2014) used the casein hydrolysis method to distinguish between C. gloeosporioides and C. acutatum in mango isolates from Ghana. Their study found that C. gloeosporioides and C. acutatum tested negative and positive, respectively for the casein hydrolysis method. This result further confirmed that the two species are different; hence, they concluded that the main causative species in Ghana was C. gloeosporioides and not C. acutatum.
Using indirect ELISA and western blotting techniques, Theerthagiri et al. (2016) could distinguish between the various causative agents of the disease. For the western blotting results, they found a 40 kDa molecular weight protein in C. gloeosporioides which was different from other Colletotrichum species. The indirect ELISA data gave higher titer values of polyclonal antibodies to protein extracts of C. gloeosporioides compared to other fungal species, further confirming that it differs from the other Colletotrichum species.
With technological advancement, molecular techniques have been employed to augment morphological and biochemical detection methods. Molecular techniques are accurate, rapid, specific, and sensitive in detecting the causative organisms of the disease and thus help in understanding the mechanism involved in disease pathology and management (Kamle et al., 2013). Molecular techniques have been developed based on species’ ribosomal DNA (rDNA) differences. The internal transcribed spacer (ITS) regions (ITS 1 and ITS 4) within the nuclear ribosomal gene cluster have mainly been found to be a good site for the design of specific primers for the detection of the causative agents of the disease (Freeman et al., 2000; Kamle et al., 2013; Zakaria et al., 2015). In a study to distinguish between Colletotrichum species, species-specific primers MKCgF and MKCgR of amplicon size 380 bp were designed. The study showed that the primers could amplify all isolates of C. gloeosporioides but not the other species, such as C. acutatum, C. falcatum and C. capsici. It was therefore suggested that the MKCgF and MKCgR would be a good marker for distinguishing between species-specific Colletotrichum and hence valuable for developing a rapid and sensitive a diagnostic PCR assay for early detection and management of the disease (Kamle et al., 2013). In another study from Ghana using 480 bp species-specific primers CgInt and ITS 4, it was observed that the C. gloeosporioides complex showed amplification products at 480 bp while C. acutatum did not. Phylogenetic analysis also showed that the C. acutatum clade clustered far away from the C. gloeosporioides complex, thus indicating that the two species are different (Honger et al., 2014).
Although the ITS gene sequence is instrumental in identifying Colletotrichum species, it cannot distinguish between closely related species (Cannon et al., 2012). This, therefore, has led to the use of multiple gene sequences to differentiate between and within species (Weir et al., 2012; Dela Cueva et al., 2021). Due to the drawback of using ITS, other genes such as actin, β – tubulin, chitin synthase, glyceraldehyde-3-phosphate dehydrogenase, calmodulin, and glutamine synthetase have also been studied to explore the differences among Colletotrichum species (Weir et al., 2012; Lima et al., 2013a,b; Dela Cueva et al., 2021). Lima et al. (2013a,b), in their study to identify the Colletotrichum species associated with MAD in northeastern Brazil, used sets of primers for the glyceraldehyde-3-phosphate, actin, β – tubulin, calmodulin, and glutamine synthetase genes. It was found that the primers were able to amplify five different species from the C. gloeosporioides complex namely C. asianum, C. fructicola, C. tropicale, C. karstii, and C. dianesei. A similar result was obtained in mango leaves in which C. asianum, C. fructicola and C. siamense were identified using glyceraldehyde-3-phosphate dehydrogenase, partial actin, β – tubulin, and chitin synthase (Mo et al., 2018). Therefore, the findings suggest that these genes could be used to detect different strains within a species complex.
Current methods focus on designing and developing imaging processing and algorithms that have aided in automated disease detection. Accurate techniques such as camera-assisted image analysis (Corkidi et al., 2006) and a computer vision system based on ultraviolet light (Alberto et al., 2022) have been employed in the visual detection of the disease. However, this is only effective if the symptoms appear on the skin of the fruits hence unable to detect the disease at an early stage. A hyperspectral imaging spectrum based on spectroscopy and computer vision has been developed for early detection of the disease. The technique has been shown to provide information about the spatial distribution of components in the mango plant for easy detection and classification under different infection levels (Siriptrawan, 2021). Another computer study was done using a Matrix Laboratory (MATLAB) based disease detection system. The system employs a Gray Level Co-occurrence Matrix (GLCM) algorithm, which can extract the features of the disease, and an SVM classifier that classifies the disease type based on the extracted features from the GLCM. The study observed that the system yields a 90% accuracy of automated detection and provides appropriate preventive and curative solutions (Veling et al., 2019). Thus, a combination of different detection methods can ensure efficient and accurate disease detection.
In Taiwan, systemic fungicides known as Benzimidazoles are used to control C. gloeosporioides (Chung et al., 2010). Certain strains of this fungus show different levels of resistance to Benzimidazole. There are known C. gloeosporioides isolates from resistant crops, but we still need a complete picture of their molecular features (Chung et al., 2010). Benzimidazole-resistant strains have been found in Japan (Tashiro et al., 2012). There appears to be a spectrum of resistance among the species in the C. gloeosporioides Species Complex (CGSC), as determined by genetic analysis (Yokosawa et al., 2017). It has been reported that benomyl-resistant C. gloeosporioides f.sp. malvae was isolated from uv-irradiated, actively developing mycelium (Holmström-Ruddick and Mortensen, 1995). Mycoherbicide agents for round-leaved mallow can be registered for this plant. Benomyl foliar spray has been shown to be quite effective in preventing the spread of C. gloeosporioides; nevertheless, some strains have become resistant to the spray after repeated administration. The amino acid sequence at the benzimidazole binding site can change because of a point mutation in a β-tubulin gene resulting in fungal resistance to benomyl (Maymon et al., 2006). Two β-tubulin genes have been characterized in some Colletotrichum spp., namely TUB1 and TUB2 (Maymon et al., 2006).
Colletotrichum gloeosporioides has become a global problem causing significant economic damage (Chung et al., 2010) with some strains being resistant and difficult to control using specific chemicals. The microorganism has been classified taxonomically using microscopic and morphological characteristics. Resistance strains are microorganisms that do not yield to the expected effect of chemicals. Some phenolic lipids in living organisms include alkyl phenols, alkyl resorcinols, anarcadic acids, and alkyl catechols (Supriya et al., 2020). Alkylresorcinols impart resistance to plants and other living organisms against abiotic and biotic stresses (Supriya et al., 2020). They do not only elicit defensive action in plants grown in biotic stress conditions, but the alkylresorcinols extracted from rye can inhibit the mycelial growth of Fusarium culmorum and Rhizoctonia solani. There are also reports of resistance by C. gloeosporioides to systemic fungicides named Benzimidazoles (Chung et al., 2010). Different strains have different levels of pathogenicity to different plants (Sanchez-Arizpe et al., 2021).
Natural disease resistance in Mango varieties is being exploited as a control against anthracnose disease in mango fruits (Gong et al., 2013). Different cultivars show different levels of disease resistance. A study was carried out on the resistance of two mango varieties (‘Keitt’ and ‘Zill’) to anthracnose disease. C. gloeosporioides infection of young or commercially ripe fruit resulted in reduced lesion diameters on ‘Keitt’ fruit compared to ‘Zill’ fruit (Gong et al., 2013). When non-inoculated fruits were harvested at commercial maturity, “Keitt” showed a lower disease index than ‘Zill’, indicating that ‘Keitt’ was more disease resistant than ‘Zill’ (Gong et al., 2013). More significant amounts of hydrogen peroxide, total phenolic compounds, and lignin were found in ‘Keitt’ fruit during development and storage compared to ‘Zill’ fruit. Additionally, ‘Keitt’ fruit had higher contents of hydrogen peroxide and lignin in harvested fruit early during storage (Gong et al., 2013). These findings highlight the importance of defense enzymes and chemicals in mango fruit’s resistance to MAD. It has been proposed that these could be utilized as markers to screen for mango cultivars with increased resistance to post-harvest illnesses (Gong et al., 2013).
Resistant varieties vary in their ability to suppress disease development in the crop. The varieties may have specific biochemical differences which enable them to suppress disease development, and humanity can exploit the suppressive ability to overcome the problems of resistant strains. In order to understand these biochemical differences, a study was conducted in India to find the constitutive antifungal phenolic lipids, phenolics contents, and antioxidant activities in a resistant variety of mango known as ‘Kensingtonpride’ and two susceptible varieties known as ‘Badami’ and ‘Raspuri’; their resistance and susceptibility were to the MAD (Supriya et al., 2020). Both the 5-n-pentadecyl resorcinol and total phenolics levels were measured using phytochemical analysis. Furthermore, the antioxidant potential of the mango peel methanolic extracts was evaluated using in vitro DPPH assay. The results demonstrate that early in fruit growth (at 30DFS), ‘Kensington pride,’ as opposed to ‘Badami’ and ‘Raspuri,’ has the highest concentration of 5-n-pentadecyl resorcinol in the mango fruit peel extract. High levels (p ≤ 0.05) of constitutive antifungal 5-n-pentadecyl resorcinol in anthracnose-resistant mango cultivar (‘Kensington pride’) was endogenously produced and retained during the early stage of fruit development. During plants’ growing, maturing, and ripening stages, this molecule confers resistance to disease, and it may serve as a foundation of the plant’s defense mechanism against anthracnose (Supriya et al., 2020).
The resistant varieties of mango contain fungi toxic levels of antifungal resorcinols, which enable the mangoes to resist infection (Karunanayake et al., 2014). In China, it has been shown that by manipulating temperatures and carbendazim concentrations, the strains of C. gloesporioides resistant to Benzamidazole can be effectively controlled (Lin et al., 2016). Screening of mango hybrids against the disease is also a step taken by several research institutes (Bally et al., 2010). It is advisable to use fungicides to which the strains are not resistant. Mancozeb and Copper Oxychloride are two fungicides currently being used, and the latter is known to increase fruit set (Uddin et al., 2018).
Management plans must be cost-effective, efficient, and safe for the environment, consumers, and agricultural workers. Pre-harvest, postharvest, or, ideally, a mix of both treatments are effective ways to control anthracnose. A wide range of options are available for dealing with MAD in the field. Fungicide spraying, proper cultural techniques, and the choice of cultivars are the primary components.
The varied responses of cultivars to the disease in various regions have prevented resistance from being consistently employed as a method of controlling MAD. With an appropriate mango variety, growers can grow a crop with fewer incidences of anthracnose, resulting in an increased yield and fruit of superior quality. Resistance also reduces the need for fungicides to protect the crop, making it more cost-effective and sustainable (Leadbeater, 2014).
Wet conditions or high relative humidity are necessary for the disease to flourish (Božič and Kanduč, 2021). Therefore, locating farms in areas with a distinct dry season is ideal, as this promotes disease-free fruit growth. Additionally, it has been suggested that field sanitation procedures incorporate collecting and burning trash from fallen trees and fruit (Sosnowski et al., 2009).
Fungicide use has received the majority of the focus and attention in the fight against anthracnose. Inflorescence and fruit damage are minimized with the administration of fungicides. Different fungicides may be utilized depending on where the exported fruit will go. Non-systemic fungicides like zineb, maneb, or captan, applied weekly during blossoming and then monthly during fruit development, were shown in early experiments to be effective against the disease (Sardrood and Goltapeh, 2018). Applying copper oxychloride or copper oxychloride in conjunction with zineb in pre-harvest management is recommended for anthracnose control in South Africa every 14 days in wet conditions and every 28 days in dry conditions (Akem, 2006). In addition, after the fruit had begun to set, mancozeb and copper oxychloride were alternated every month until harvest. Although copper fungicides are recommended, they are not as effective as dithiocarbamates when dealing with high disease pressure and hence pose a worry for phytotoxicity on mango blossoms (Thind and Hollomon, 2018). Fungicides with an after-infection activity, such as benzimidazoles and imidazole prochloraz, are effective against MAD. Scheduled applications of benomyl, sometimes in conjunction with protectant fungicides, have been used to halt the development of resistance in pathogen populations. Both preventative and curative applications of prochloraz have been made (Chiangsin et al., 2016).
Prioritizing the reduction of dormant infections on mangoes could be a mainstay of postharvest management strategies for MAD. Anthracnose in ripe fruit has been a target of postharvest treatments for a long time. It has been shown that hot water dips are useful for controlling anthracnose (Mirshekari et al., 2012). Commercially, latent infection is removed using heat, chemicals, or a mix of both approaches. It has been suggested to expose subjects to temperatures between 50 and 55°C for exposure durations ranging from 3 to 15 min (Mirshekari et al., 2012). To control anthracnose, a variety of fungicides have been used after harvest. Prochloraz and Thiabendazole can be utilized. However, their effectiveness varies with the severity of the condition (Shi et al., 2020). One benefit of benzimidazole fungicides like benomyl or thiabendazole is that they work well to suppress stem-end rot on mangoes brought on by Lasiodiplodia theobromae (Pat.), which is regarded as the second most significant postharvest disease of mango in tropical regions (Shi et al., 2020). Postharvest techniques, including a controlled environment and cold storage, maintain resistance to deterioration by postponing the ripening phase. The potential benefit of this strategy has several restrictions. Mangoes suffer damage at temperatures below 10 to 13°C because they are susceptible to chilling (Patel et al., 2016). Disease naturally develops if the fruit is allowed to ripe in natural settings. There have been limited and inconsistent attempts at postharvest biological control of MAD. Numerous microorganisms have been implicated in the cases of MAD as having antagonistic relationships with the disease’s causal agent (C. gloeosporioides) (Kankam et al., 2022). For example, Trichoderma spp. Bacillus spp. and Pseudomonas spp. are known for their antagonistic properties against plant pathogens (Zin and Badaluddin, 2020). They have been reported to be effective in reducing the incidence of MAD by competing with C. gloeosporioides for nutrients and space. However, there is currently no evidence of this finding’s commercial implementation, although it appears to be quite promising.
Recent studies have also revealed the success of using botanicals to cure MAD (Alemu et al., 2014). The in vitro mycelial growth of C. gloeosporioides has been demonstrated to be suppressed by aqueous extracts of the leaves of Eucalyptus camaldulensis and Azadirachta indica. Anthracnose on mango trees was reduced in both occurrence and severity after foliar application of the extracts, even in field settings (Haider et al., 2020). When applied to mangoes before storage, aqueous extracts of Ruta chalepensis at a concentration of 50 grams of the powdered plant material in 100 mL of distilled water effectively decreased the incidence of anthracnose disease, maintaining quality, and making the fruits more marketable (Alemu et al., 2014).
The inadequacies and inconsistent efficacy of postharvest treatments demonstrate the need for an integrated strategy in managing MAD. Figure 4 provides a schematic illustration of the various management strategies employed to deal with MAD before and after harvest.
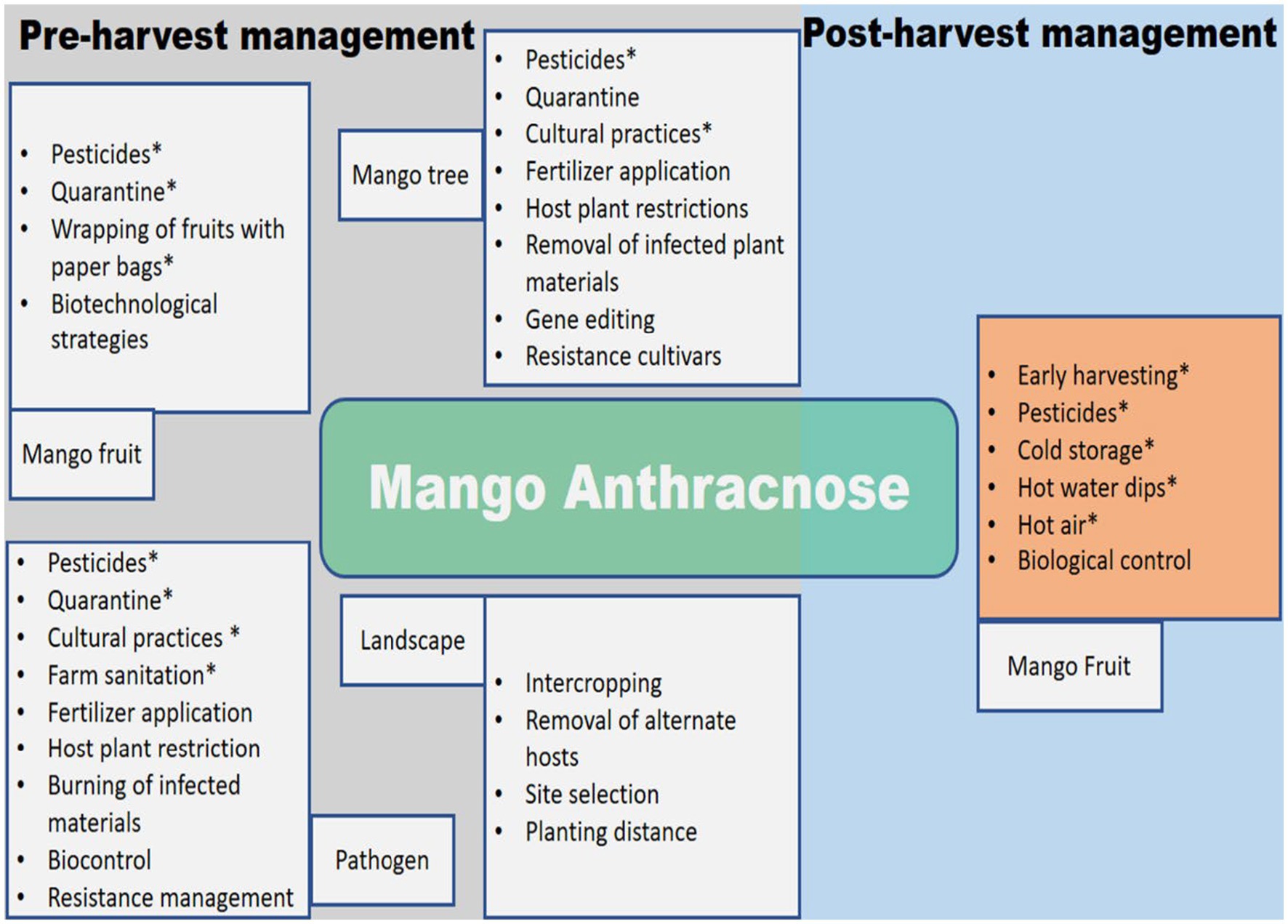
Figure 4. Schematic diagram for the management of mango anthracnose. Options that have had an influence in at least some field settings are indicated by an asterisk (*).
MAD is still a widespread fungal disease that lowers production and quality worldwide. Colletotrichum is endemic to nearly all places where mangoes are grown, posing a significant threat to the industry worldwide. Decay patches from dark brown to black appear on leaves, twigs, flowers, and fruits during the pre-and postharvest stages of the disease. The pathogenic fungus that causes MAD tends to infest unripe mango fruits and become dormant until the fruit ripens. The extensive use of synthetic fungicides to prevent the occurrences of MAD can lead to environmental pollution, water pollution, soil compaction, and ecological problems, which then affect the sustainability of mango production. Research on the biological characteristics and behavior of the pathogen, epidemiology, and management of MAD has recently increased. The availability of genomic data on C. gloeosporioides has provided a better understanding and knowledge of the host-fungal interaction. This has also assisted in developing new methods for rapidly detecting C. gloeosporioides.
Significant progress in MAD management has been made, especially concerning fungicides and timing treatments, postharvest treatments, and resistant cultivars. However, improper identification of closely related species of the pathogen that causes MAD remains a significant challenge in managing the disease. This has been attributed to the similarities in the morphological traits of pathogens and their symptoms on the host plant. Plant breeding projects for developing new disease-resistant mango cultivars could benefit from a more in-depth understanding of the disease’s epidemiology. There is little known about C. gloeosporioides’ population structure, genetic diversity, or sensitivity to varying fungicide concentrations. Further research on the population, diversity, and fungicides sensitivity of the pathogen is vital for developing effective management strategies for the disease.
MAD remains the most crucial economic disease worldwide in almost all mango production areas. It requires pre-harvest and postharvest strategies for effective control because it affects nearly all of the plant’s above-ground portions. Timely application of appropriate fungicides in the field, good cultural practices, resistant cultivars, and appropriate postharvest treatments, such as dip treatments and refrigeration, are only some of the management methods that have been called for to lessen the impact of this threat. However, picking the best control approach requires more information about this condition and public awareness.
Alternatives to fungicides have been developed in light of rising awareness of the fungicides’ deleterious effects on human health, the presence of fungicide residues in mango fruits, and the contamination of the natural environment that results from their widespread use. Essential oils, botanicals, and oxalic acid treatments have all been shown to be effective alternatives to chemical control, especially in locations where the use of synthetic fungicides is prohibited. Microbial agents and biological control of the MAD have also broadened developmental prospects for establishing environmentally friendly pest management.
Furthermore, resistance breeding is vital to the control of MAD. Research on host-pathogen interaction and identification of fungal genes underlying virulence, phytotoxins of C. gloeosporioides, and its pathway genes will improve knowledge of resistance mechanisms and management of the disease. Invading fungi usually secrete plant growth regulators such as auxin, cytokines, ethylene, gibberellic acid, jasmonic acid, and salicylic acid. These growth regulators distract plants’ levels of endogenous hormones, weakening their defense mechanism against the pathogenic fungus. More targets for the development of innovations or fungicides against C. gloeosporioides can be found through a systematic investigation of the manufacturing and signal transduction pathways of pathogenic fungal hormones.
Pathogenic fungi also release effector proteins, which serve crucial functions in plant cells and modify the interaction between pathogens and their hosts. Little is known about the fungal effectors that cause mango disease. The host-plant relationship, the pathogenic fungus’s pathogenic processes, and host plants’ disease-resistance mechanisms may all benefit from functional investigations and comparative analyses of the C. gloeosporioides effector protein.
The key motivating factors for creating alternative techniques to reduce MAD include the recent understanding of health dangers, the rising customer preference for healthy agricultural products, and the environmental pollution connected with fungicide usage. Further research should be encouraged on host-pathogenic fungal interaction, transmission, and effective control techniques. Shortly, research on MAD should be geared toward developing techniques that address many environmental factors and pathogenic fungal resistance. Future research on developing and deploying electronic and disease sensors for rapid detection of C. gloeosporioides on-site and entry points of disease-free regions are recommended to minimize the spread of MAD.
For instance, smartphone-based fingerprinting of leaf volatiles has may be to detect the late blight of tomatoes. In addition, a microneedle patch coupled with a loop-meditated isothermal amplification-based sensor may be developed to detect coinfections of late blight and spotted wilt virus in tomatoes. These strategies could be adopted for MAD and a more comprehensive and coordinated surveillance and monitoring strategies for MAD that includes all stakeholders, government, and non-government organizations would help to reduce the migration of the disease pathogens.
Furthermore, research programs that focus on disease surveillance through risk modeling, bioinformatics tools, and geospatial analytic tools for mapping and analyzing data about the pathogen and its host must be deployed to respond to potential anthracnose threats in mango production. Global sharing of data or information by researchers and policymakers, and identification of a global hot spot for new outbreaks of MAD will be needed to manage the disease. There is still more to learn about MAD, its detection, and causal organism and management strategies. Future research areas should include; how climate change affects the spread and management of MAD, sensors for on-site detection, and the deployment of gene editing, nanoparticles, and nanotechnology for managing the diseases.
AD and OA: study-conceived and designed, writing–original draft, review, and editing. NQ, AO, AA-A, BB, FA, KA, HL, SL, JO-O, JO, WE, and JH: writing–original draft. KN: review and editing. All authors approved the submitted version.
The authors thank Frederick Leo Sossah for his insightful comments and contributions to earlier versions of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1168203/full#supplementary-material
Abang, M. M., Winter, S., Green, K. R., Hoffmann, P., Mignouna, H. D., and Wolf, G. A. (2002). Molecular identification of Colletotrichum gloeosporioides causing yam anthracnose in Nigeria. Plant Pathol. 51, 63–71. doi: 10.1046/j.0032-0862.2001.00655.x
Abera, A., Lemessa, F., and Adunga, G. (2016). Morphological characteristics of Colletotrichum species associated with mango (Mangifera indica L.) in Southwest Ethiopia. Food Sci. Qual. Manag. 48, 106–115.
Ahmad, T., Wang, J., Zheng, Y., Mugizi, A. E., Moosa, A., Nie, C., et al. (2021). First record of Colletotrichum alienum causing postharvest anthracnose disease of mango fruit in China. Plant Dis. 105:1852. doi: 10.1094/PDIS-09-20-2074-PDN
Ajitomi, A., Takushi, T., Sato, Y., Arasaki, C., and Ooshiro, A. (2020). First report of powdery mildew of mango caused by Erysiphe quercicola in Japan. J. Gen. Plant Pathol. 86, 316–321. doi: 10.1007/s10327-020-00918-2
Akem, C. N. (2006). Mango anthracnose disease: present status and future research priorities. Plant Pathol. J. 5, 266–273. doi: 10.3923/ppj.2006.266.273
Alberto, L. R., Ardila, C. E. C., and Ortiz, F. A. P. (2022). A computer vision system for early detection of anthracnose in sugar mango (Mangifera indica) based on UV-A illumination. Inf. Process. Agric. 10, 204–215. doi: 10.1016/j.inpa.2022.02.001
Alemu, K., Ayalew, A., and Woldetsadik, K. (2014). Antifungal activity of plant extracts and their applicability in extending the shelf-life of mango fruits. Food Sci. Qual. Manag. 33, 47–53.
Alkan, N., Meng, X., Friedlander, G., Reuveni, E., Sukno, S., Sherman, A., et al. (2013). Global aspects of pacC regulation of pathogenicity genes in Colletotrichum gloeosporioides as revealed by transcriptome analysis. Mol. Plant-Microbe Interact. 26, 1345–1358. doi: 10.1094/MPMI-03-13-0080-R
Anderson, J. M., Aitken, E. A. B., Dann, E. K., and Coates, L. M. (2013). Morphological and molecular diversity of Colletotrichum spp. causing pepper spot and anthracnose of lychee (Litchi chinensis) in Australia. Plant Pathol. 62, 279–288. doi: 10.1111/j.1365-3059.2012.02632.x
Arauz, L. F. (2000). Mango anthracnose: economic impact and current options for integrated managaement. Plant Dis. 84, 600–611. doi: 10.1094/PDIS.2000.84.6.600
Ashraful, A., Sanjoy, K. A., and Mahtalat, A. (2017). Morphological characterization of Colletotrichum gloeosporioiedes identified from anthracnose of Mangifera indica L. Asian J. Plant. Pathol. 11, 102–117. doi: 10.3923/ajppaj.2017.102.117
Awa, O. C., Samuel, O., Oworu, O. O., and Sosanya, O. (2012). First report of fruit anthracnose in mango caused by Colletotrichum gloeosporioides in southwestern Nigeria. Int. J. Sci. Technol. Res. 1, 30–34.
Bally, I. S., Akem, C. N., Dillon, N. L., Grice, C., Lakhesar, D., and Stockdale, K. (2010). Screening and breeding for genetic resistance to anthracnose in mango. In IX International Mango Symposium 992 (pp. 239–244)
Barhoom, S., Kupiec, M., Zhao, X., Xu, J. R., and Sharon, A. (2008). Functional characterization of CgCTR2, a putative vacuole copper transporter that is involved in germination and pathogenicity in Colletotrichum gloeosporioides. Eukaryot. Cell 7, 1098–1108. doi: 10.1128/EC.00109-07
Barhoom, S., and Sharon, A. (2007). Bcl-2 proteins link programmed cell death with growth and morphogenetic adaptations in the fungal plant pathogen Colletotrichum gloeosporioides. Fungal Genet. Biol. 44, 32–43. doi: 10.1016/j.fgb.2006.06.007
Benatar, G. V., Wibowo, A., and Suryanti, (2021). First report of Colletotrichum asianum associated with mango fruit anthracnose in Indonesia. Crop Prot. 141:105432. doi: 10.1016/j.cropro.2020.105432
Bhagwat, R. G., Mehta, B. P., Patil, V. A., and Sharma, H. (2015). Screening of cultivars/varieties against mango anthracnose caused by Colletotrichum gloeosporioides. Int. J. Environ. Agric. Res. 1, 21–23.
Bordoh, P. K., Ali, A., Dickinson, M., Siddiqui, Y., and Romanazzi, G. (2020). A review on the management of postharvest anthracnose in dragon fruits caused by Colletotrichum spp. Crop Prot. 130:105067. doi: 10.1016/j.cropro.2019.105067
Božič, A., and Kanduč, M. (2021). Relative humidity in droplet and airborne transmission of disease. J. Biol. Phys. 47, 1–29. doi: 10.1007/s10867-020-09562-5
Caicedo, J. D., Lalangui, K. P., Pozo, A. N., Cevallos, P. A., Arahana, V. S., and Méndez, K. S. (2017). Multilocus molecular identification and phylogenetic analysis of Colletotrichum tamarilloi as the causal agent of Tamarillo (Solanum betaceum) anthracnose in the Ecuadorian highlands. Europ. J. Plant Pathol. 148, 983–996. doi: 10.1007/s10658-017-1155-3
Cannon, P. F., Damm, U., Johnston, P. R., and Weir, B. S. (2012). Colletotrichum: current status and future directions. Stud. Mycol. 73, 181–213. doi: 10.3114/sim0014
Cao, X. R., Xu, X. M., Che, H. Y., West, J. S., and Luo, D. Q. (2019). Characteristics and distribution of Colletotrichum species in coffee plantations in Hainan, China. Plant Pathol. 68, 1146–1156. doi: 10.1111/ppa.13028
Castillo, S. R. M., Miller, S., and Stewart, J. (2022). Colletotrichum spp. and other fungi associated with anthracnose on Coffea arabica L. in Mérida state, Venezuela. Summa Phytopathol. 48, 99–111. doi: 10.1590/0100-5405/245876
Chagué, V., Maor, R., and Sharon, A. (2009). CgOpt1, a putative oligopeptide transporter from Colletotrichum gloeosporioides that is involved in responses to auxin and pathogenicity. BMC Microbiol. 9, 1–12. doi: 10.1186/1471-2180-9-173
Chala, A., Getahun, M., Alemayehu, S., and Tadesse, M. (2014). Survey of mango anthracnose in southern Ethiopia and in-vitro screening of some essential oils against Colletotrichum gloeosporioides. Int. J. Fruit Sci. 14, 157–173. doi: 10.1080/15538362.2013.817899
Cheng, Y. J., Wu, Y. J., Lee, F. W., Ou, L. Y., Chen, C. N., Chu, Y. Y., et al. (2022). Impact of storage condition on chemical composition and antifungal activity of pomelo extract against Colletotrichum gloeosporioides and anthracnose in post-harvest mango. Plan. Theory 11:2064. doi: 10.3390/plants11152064
Chiangsin, R., Wanichkul, K., Guest, D. I., and Sangchote, S. (2016). Reduction of anthracnose on ripened mango fruits by chemicals, fruit bagging, and postharvest treatments. Australas. Plant Pathol. 45, 629–635. doi: 10.1007/s13313-016-0456-x
Chowdhury, M. N., and Rahim, M. A. (2009). Integrated crop management to control anthracnose (Colletotrichum gloeosporioides) of mango. J. Agric. Ext. Rural Dev., 115–120. doi: 10.3329/jard.v7i1.4430
Chung, W. H., Chung, W. C., Peng, M. T., Yang, H. R., and Huang, J. W. (2010). Specific detection of benzimidazole resistance in Colletotrichum gloeosporioides from fruit crops by PCR-RFLP. New Biotechnol. 27, 17–24. doi: 10.1016/j.nbt.2009.10.004
Ciofini, A., Negrini, F., Baroncelli, R., and Baraldi, E. (2022). Management of Post-Harvest Anthracnose: current approaches and future perspectives. Plan. Theory 11:1856. doi: 10.3390/plants11141856
Corda, A. C. I. (1831). “Die Pilze Deutschlands” in Deutschlands Flora in Abbildungen nach der Natur mit Beschreibungen. ed. J. Sturm, vol. 3 (Nürnberg: Sturm), 33–64.
Corkidi, G., Balderas-Ruíz, K. A., Taboada, B., Serrano-Carreón, L., and Galindo, E. (2006). Assessing mango anthracnose using a new three-dimensional image-analysis technique to quantify lesions on fruit. Plant Pathol. 55, 250–257. doi: 10.111/j.1365-3059.2005.01321.x
Costa, J. F., Kamei, S. H., Silva, J. R. A., Miranda, A. R. G. D. S., Netto, M. B., da Silva, S. J. C., et al. (2019). Species diversity of Colletotrichum infecting Annona spp. in Brazil. Europ. J. Plant Pathol. 153, 1119–1130. doi: 10.1007/s10658-018-01630-w
Cristóbal-Martínez, A. L., de Jesús Yáñez-Morales, M., Solano-Vidal, R., Segura-León, O., and Hernández-Anguiano, A. M. (2017). Diversity of Colletotrichum species in coffee (Coffea arabica) plantations in Mexico. Eur. J. Plant Pathol. 147, 605–614. doi: 10.1007/s10658-016-1029-0
Damm, U., Cannon, P. F., Woudenberg, J. H. C., and Crous, P. W. (2012). The Colletotrichum acutatum species complex. Stud. Mycol. 73, 37–113. doi: 10.3114/sim0010
De Jonge, R., Peter van Esse, H., Kombrink, A., Shinya, T., Desaki, Y., Bours, R., et al. (2010). Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science 329, 953–955. doi: 10.1126/science.1190859
De Souza, A., Delphino Carboni, R. C., Wickert, E., de Macedo Lemos, E. G., and de Goes, A. (2013). Lack of host specificity of Colletotrichum spp. isolates associated with anthracnose symptoms on mango in Brazil. Plant Pathol. 62, 1038–1047. doi: 10.1111/ppa.12021
Dela Cueva, F. M., Laurel, N. R., Dalisay, T. U., and Sison, M. L. J. (2021). Identification and characterisation of Colletotrichum fructicola, C. tropicale and C. theobromicola causing mango anthracnose in the Philippines. Arch. Phytopathol. 54, 1989–2006. doi: 10.1080/03235408.2021.1968234
Dembele, D. D., Camara, B., Grechi, I., Rey, J. Y., and Kone, D. (2019). Pre and postharvest assessment of mango anthracnose incidence and severity in the north of Côte d’Ivoire. Int. J. Biol. Chem. Sci. 10, 33–43. doi: 10.3923/IJAR.2015.33.43
Doyle, V. P., Oudemans, P. V., Rehner, S. A., and Litt, A. (2013). Habitat and host indicate lineage identity in Colletotrichum gloeosporioides sl from wild and agricultural landscapes in North America. PLoS One 8:e62394. doi: 10.1371/journal.pone.0062394
Drori, N., Kramer-Haimovich, H., Rollins, J., Dinoor, A., Okon, Y., Pines, O., et al. (2003). External pH and nitrogen source affect secretion of pectate lyase by Colletotrichum gloeosporioides. Appl. Environ. Microbiol. 69, 3258–3262. doi: 10.1128/AEM.69.6.3258-3262.2003
Evangelista-Martínez, Z., Ek-Cen, A., Torres-Calzada, C., and Uc-Várguez, A. (2022). Potential of Streptomyces sp. strain AGS-58 in controlling anthracnose-causing Colletotrichum siamense from post-harvest mango fruits. J. Plant Pathol. 104, 553–563. doi: 10.1007/s42161-022-01104-3
Feng, G., Zhang, X. S., Zhang, Z. K., Ye, H. C., Liu, Y. Q., Yang, G. Z., et al. (2019). Fungicidal activities of camptothecin semisynthetic derivatives against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Technol. 147, 139–147. doi: 10.1016/j.postharvbio.2018.09.019
Freeman, S., Minz, D., Jurkevitch, E., Maymon, M., and Shabi, E. (2000). Molecular analyses of Colletotrichum species from almond and other fruits. Phytopathology 90, 608–614. doi: 10.1094/PHYTO.2000.90.6.608
Fujikawa, T., Sakaguchi, A., Nishizawa, Y., Kouzai, Y., Minami, E., Yano, S., et al. (2012). Surface α-1, 3-glucan facilitates fungal stealth infection by interfering with innate immunity in plants. PLoS Pathog. 8:e1002882. doi: 10.1371/journal.ppat.1002882
Gan, P., Ikeda, K., Irieda, H., Narusaka, M., O'Connell, R. J., Narusaka, Y., et al. (2013). Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 197, 1236–1249. doi: 10.1111/nph.12085
Gautam, C., Prabhu, H. V., and Nargund, V. B. (2017). In vitro evaluation of fungicides, botanicals and bioagents against Peziotrichum corticolum causing black banded disease of mango. Int. J. Curr. Microbiol. Appl. Sci. 6, 652–661. doi: 10.20546/ijcmas.2017.603.076
Gong, D. Q., Zhu, S. J., Gu, H., Zhang, L. B., Hong, K. Q., and Xie, J. H. (2013). Disease resistance of ‘Zill’and ‘Keitt’mango fruit to anthracnose in relation to defence enzyme activities and the content of anti-fungal substances. J. Hortic. Sci. Biotechnol. 88, 243–250. doi: 10.1080/14620316.2013.11512962
Grammen, A., Wenneker, M., Van Campenhout, J., Pham, K. T. K., Van Hemelrijck, W., Bylemans, D., et al. (2019). Identification and pathogenicity assessment of Colletotrichum isolates causing bitter rot of apple fruit in Belgium. Eur. J. Plant Pathol. 153, 47–63. doi: 10.1007/s10658-018-1539-z
Grice, K. R. E., Bally, I. S. E., Wright, C. L., Maddox, C., Ali, A., and Dillon, N. L. (2022). Mango germplasm screening for the identification of sources of tolerance to anthracnose. Australas. Plant Pathol. 52, 27–41. doi: 10.1007/s13313-022-00899-0
Guevara-Suarez, M., Cárdenas, M., Jiménez, P., Afanador-Kafuri, L., and Restrepo, S. (2022). Colletotrichum species complexes associated with crops in northern South America: a review. Agronomy 12:548. doi: 10.3390/agronomy12030548
Haider, E., Khan, M. A., Atiq, M., Shahbaz, M., and Yaseen, S. (2020). Phytoextracts as management tool against fungal diseases of vegetables. Int. J. Biosci. 16, 303–314. doi: 10.12692/ijb/16.3.303-314
Hofer, K. M., Braithwaite, M., Braithwaite, L. J., Sorensen, S., Siebert, B., Pather, V., et al. (2021). First report of Colletotrichum fructicola, C. perseae, and C. siamense causing anthracnose disease of avocado (Persea americana) in New Zealand. Plant Dis. 105:1564. doi: 10.1094/PDIS-06-20-1313-PDN
Holmström-Ruddick, B., and Mortensen, K. (1995). Factors affecting pathogenicity of a benomyl-resistant strain of Colletotrichum gloeosporioides f. sp. malvae. Mycol. Res. 99, 1108–1112. doi: 10.1016/S0953-7562(09)80780-6
Honger, J. O., Offei, S. K., Oduro, K. A., and Odamtten, G. T. (2015). Nature of mango anthracnose in Ghana: implications for the control of the disease. Ghana J. Agric. Sci. 49, 53–67.
Honger, J. O., Offei, S. K., Oduro, K. A., Odamtten, G. T., and Nyaku, S. T. (2014). Identification and species status of the mango biotype of Colletotrichum gloeosporioides in Ghana. Eur. J. Plant Pathol. 140, 455–467. doi: 10.1007/s10658-014-0480-z
Hossain, A. A., and Ahmed, A. (1994). A monograph on mango varieties of Bangladesh. Dhaka: Bangladesh Agricultural Research Institute.
Ismail, A. M., Cirvilleri, G., Yaseen, T., Epifani, F., Perrone, G., and Polizzi, G. (2015). Characterisation of Colletotrichum species causing anthracnose disease of mango in Italy. J. Plant Pathol., 167–171. doi: 10.4454/JPP.V97I1.011
Ismail, A. M., and El-Ganainy, S. M. (2022). Characterization of Colletotrichum species associating with anthracnose disease of mango in Egypt. J. Plant Dis. Prot. 129, 449–454. doi: 10.1007/s41348-021-00538-8
Janamatti, A. T., Kumar, A., Kaur, C., Gogoi, R., Varghese, E., and Kumar, S. (2022). Fumigation by bacterial volatile 2, 5-dimethylpyrazine enhances anthracnose resistance and shelf life of mango. Eur. J. Plant Pathol. 164, 209–227. doi: 10.1007/s10658-022-02551-5
Jenny, F., Sultana, N., Islam, M., Khandaker, M. M., and Bhuiyan, M. A. B. (2019). A review on anthracnose of mango caused by Colletotrichum gloeosporioides. Bangladesh J. Plant Phytopathol. 35, 65–74.
Kadam, J. A., Shimpi, S. B., Biradar, R. P., and Chate, S. V. (2002). Review on post harvest diseases and management of mango fruits. J. Plant Pathol. Microbiol. 13:1000635.
Kamle, M., and Kumar, P. (2016). Colletotrichum gloeosporioides: pathogen of anthracnose disease in mango (Mangifera indica L.). Curr. Trends Plant Dis. Diagn. Manag. Pract., 207–219. doi: 10.1007/978-3-319-27312-9_9
Kamle, M., Pandey, B. K., Kumar, P., and Kumar, M. (2013). A species-specific PCR based assay for rapid detection of mango anthracnose pathogen Colletotrichum gloeosporioides Penz. And Sacc. J. Plant Pathol. Microbiol. 4:184. doi: 10.4172/2157-7471.1000184
Kankam, F., Larbi-Koranteng, S., Adomako, J., Kwodaga, J. K., Akpatsu, I. B., Danso, Y., et al. (2022). “Anthracnose disease of mango: epidemiology, impact and management options” in Current and emerging challenges in the diseases of trees (London: IntechOpen)
Karunanayake, K. O. L. C., Sinniah, G. D., Adikaram, N. K. B., and Abayasekara, C. L. (2014). Cultivar differences in antifungal activity and the resistance to postharvest anthracnose and stem-end rot in mango (Mangifera indica L.). Australas. Plant Pathol. 43, 151–159. doi: 10.1007/s13313-013-0257-4
Khan, M. S., Uandai, S. B., and Srinivasan, H. (2019). Anthracnose disease diagnosis by image processing, support vector machine and correlation with pigments. J. Plant Pathol. 101, 749–751. doi: 10.1007/s42161-019-00268-9
Kim, Y. K., Wang, Y., Liu, Z. M., and Kolattukudy, P. E. (2002). Identification of a hard surface contact-induced gene in Colletotrichum gloeosporioides conidia as a sterol glycosyl transferase, a novel fungal virulence factor. Plant J. 30, 177–187. doi: 10.1046/j.1365-313x.2002.01284.x
Kramer-Haimovich, H., Servi, E., Katan, T., Rollins, J., Okon, Y., and Prusky, D. (2006). Effect of ammonia production by Colletotrichum gloeosporioides on pelB activation, pectate lyase secretion, and fruit pathogenicity. Appl. Environ. Microbiol. 72, 1034–1039. doi: 10.1128/AEM.72.2.1034-1039.2006
Kumari, C., Sharma, M., Kumar, V., Sharma, R., Kumar, V., Sharma, P., et al. (2022). Genome editing technology for genetic amelioration of fruits and vegetables for alleviating post-harvest loss. Bioengineering 9:176. doi: 10.3390/bioengineering9040176
Kumari, P., Singh, R., and Punia, R. (2017). Survival of Colletotrichum gloeosporioides causing anthracnose of mango at different depths and durations in soil. J. Pharmacogn. Phytochem. 6, 2194–2198.
Kunta, M., Park, J. W., Vedasharan, P., da Graça, J. V., and Terry, M. D. (2018). First report of Colletotrichum queenslandicum on persian lime causing leaf anthracnose in the United States. Plant Dis. 102:677. doi: 10.1094/PDIS-09-17-1382-PDN
Lai, A. A., and Simon, S. (2013). Post harvest management of anthracnose rot of mango (Mangifera indica l.). Ann. Plant Prot. Sci. 21, 121–124.
Leadbeater, A. J. (2014). Plant health management: fungicides and antibiotics, encyclopedia of agriculture and food systems. Biology. doi: 10.1016/B978-0-444-52512-3.00179-0
Li, Q., Bu, J., Shu, J., Yu, Z., Tang, L., Huang, S., et al. (2019). Colletotrichum species associated with mango in southern China. Sci. Rep. 9:18891. doi: 10.1038/s41598-019-54809-4
Li, Q., Shu, J., Tang, L., Huang, S., Guo, T., Mo, J., et al. (2020). First report of mango leaf anthracnose caused by Colletotrichum asianum in Vietnam. Plant Dis. 104:1558. doi: 10.1094/PDIS-09-19-1830-PDN
Liang, Y. S., Fu, J. Y., Chao, S. H., Tzean, Y., Hsiao, C. Y., Yang, Y. Y., et al. (2022). Postharvest application of Bacillus amyloliquefaciens PMB04 fermentation broth reduces anthracnose occurrence in mango fruit. Agriculture 12:1646. doi: 10.3390/agriculture12101646
Lima, N. B., De Batista, M. V. A., De Morais, M. A., Barbosa, M. A., Michereff, S. J., Hyde, K. D., et al. (2013a). Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 61, 75–88. doi: 10.1007/s13225-013-0237-6
Lima, N. B., Lima, W. G., Tovar-Pedraza, J. M., Michereff, S. J., and Câmara, M. P. (2015). Comparative epidemiology of Colletotrichum species from mango in northeastern Brazil. Eur. J. Plant Pathol. 141, 679–688. doi: 10.1007/s10658-014-0570-y
Lima, N. B., Marques, M. W., Michereff, S. J., Morais, M. A. Jr., Barbosa, M. A. G., and Camara, M. P. S. (2013b). First report of mango anthracnose caused by Colletotrichum karstii in Brazil. Plant Dis. 97:1248. doi: 10.1094/PDIS-01-13-0002-PDN
Lin, C. H., Long, X. P., Li, Z. P., Zhang, Y., He, J. J., Liu, W. B., et al. (2020). First report of anthracnose of Clausena lansium caused by Colletotrichum scovillei in China. Plant Dis. 104:1557. doi: 10.1094/PDIS-08-19-1765-PDN
Lin, T., Xu, X. F., Dai, D. J., Shi, H. J., Wang, H. D., and Zhang, C. Q. (2016). Differentiation in development of benzimidazole resistance in Colletotrichum gloeosporioides complex populations from strawberry and grape hosts. Australas. Plant Pathol. 45, 241–249. doi: 10.1007/s13313-016-0413-8
Lisboa, D. O., Silva, M. A., Pinho, D. B., Pereira, O. L., and Furtado, G. Q. (2018). Diversity of pathogenic and endophytic Colletotrichum isolates from Licania tomentosa in Brazil. For. Pathol. 48:e12448. doi: 10.1111/efp.12448
Liu, Y., An, F., Zhang, Y., Fu, C., and Su, Y. (2021). First report of anthracnose on Jerusalem cherry caused by Colletotrichum liaoningense in Shandong, China. Plant Dis. 105:2248. doi: 10.1094/PDIS-01-21-0124-PDN
Liu, F., Damm, U., Cai, L., and Crous, P. W. (2013). Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of Proteaceae. Fungal Divers. 61, 89–105. doi: 10.1007/s13225-013-0249-2
Liu, H. N., Liu, J. A., and Zhou, G. Y. (2020). First report of Colletotrichum alienum causing anthracnose on Aquilaria sinensis in China. Plant Dis. 104:283. doi: 10.1094/PDIS-01-19-0155-PDN
Liu, L. P., Shu, J., Zhang, L., Hu, R., Chen, C. Q., Yang, L. N., et al. (2017). First report of post-harvest anthracnose on mango (Mangifera indica) caused by Colletotrichum siamense in China. Plant Dis. 101:833. doi: 10.1094/PDIS-08-16-1130-PDN
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M., and Henrissat, B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495. doi: 10.1093/nar/gkt1178
Lopes, L. H. R., Boiteux, L. S., Rossato, M., Aguiar, F. M., Fonseca, M. E., Oliveira, V. R., et al. (2021). Diversity of Colletotrichum species causing onion anthracnose in Brazil. Eur. J. Plant Pathol. 159, 339–357. doi: 10.1007/s10658-020-02166-8
Maharaj, A., and Rampersad, S. N. (2012). Genetic differentiation of Colletotrichum gloeosporioides and C. truncatum associated with anthracnose disease of papaya (Carica papaya L.) and bell pepper (Capsium annuum L.) based on ITS PCR-RFLP fingerprinting. Mol. Biotechnol. 50, 237–249. doi: 10.1007/s12033-011-9434-2
Malik, M. T., Ammar, M., Ranan, M., Rehman, A., and Bally, I. S. (2014). Chemical and cultural management of die back disease of mango in Pakistan. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): IV 1111 (pp. 363–368).
Manzano León, A. M., Serra Hernández, W., García Pérez, L., Crespo, K., and Guarnaccia, V. (2018). First report of leaf anthracnose caused by Colletotrichum grossum on mango (Mangifera indica) in Cuba. J. Plant Pathol. 100:329. doi: 10.1007/s42161-018-0040-z
Marcianò, D., Mizzotti, C., Maddalena, G., and Toffolatti, S. L. (2021). The dark side of Fungi: how they cause diseases in plants. Front. Young Minds 9:315. doi: 10.3389/frym.2021.560315
Marikar, F. M. M. T., Sivakumar, D., and Wijerathnam, R. W. (2008). Biological control of rambutan post-harvest anthracnose (Colleotrichum gloeosporioides) by combined treatment of Trichoderma harzianum-TrH40 culture filtrates and calcium salts. Food Biotechnol. 22, 326–337. doi: 10.1080/08905430802458412
Maske, J. M., Masih, S., and Verma, O. P. (2022). A review on morphological and molecular characterization of Colletotrichum species associated with mango anthracnose in Konkan region of Maharashtra state. J. Pharm. Innov. 11, 1577–1581.
Maymon, M., Zveibil, A., Pivonia, S., Minz, D., and Freeman, S. (2006). Identification and characterization of benomyl-resistant and-sensitive populations of Colletotrichum gloeosporioides from statice (Limonium spp.). Phytopathology 96, 542–548. doi: 10.1094/PHYTO-96-0542
McCulloch, M. J., Gauthier, N. W., and Vaillancourt, L. J. (2020). First report of bitter rot of apple caused by a Colletotrichum sp. in the C. kahawae clade in Kentucky. Plant Dis. 104:289. doi: 10.1094/PDIS-06-19-1247-PDN
Mirshekari, A., Ding, P., Kadir, J., and Ghazali, H. M. (2012). Effect of hot water dip treatment on postharvest anthracnose of banana var Berangan. Afr. J. Agric. Res. 7, 6–10. doi: 10.5897/AJAR11.056
Miyara, I., Shnaiderman, C., Meng, X., Vargas, W. A., Diaz-Minguez, J. M., Sherman, A., et al. (2012). Role of nitrogen-metabolism genes expressed during pathogenicity of the alkalinizing Colletotrichum gloeosporioides and their differential expression in acidifying pathogens. Mol. Plant-Microbe Interact. 25, 1251–1263. doi: 10.1094/MPMI-01-12-0017-R
Mo, J., Zhao, G., Li, Q., Solangi, G. S., Tang, L., Guo, T., et al. (2018). Identification and characterization of Colletotrichum species associated with mango anthracnose in Guangxi, China. Plant Dis. 102, 1283–1289. doi: 10.1094/PDIS-09-17-1516-RE
Moraes, S. R. G., Tanaka, F. A. O., and Massola Júnior, N. S. (2013). Histopathology of Colletotrichum gloeosporioides on guava fruits (Psidium guajava L.). Rev. Bras. Frutic. 35, 657–664. doi: 10.1590/S0100-29452013000200039
Münch, S., Lingner, U., Floss, D. S., Ludwig, N., Sauer, N., and Deising, H. B. (2008). The hemibiotrophic lifestyle of Colletotrichum species. J. Plant Physiol. 165, 41–51. doi: 10.1016/j.jplph.2007.06.008
Munir, M., Amsden, B., Dixon, E., Vaillancourt, L., and Gauthier, N. W. (2016). Characterization of Colletotrichum species causing bitter rot of apple in Kentucky orchards. Plant Dis. 100, 2194–2203. doi: 10.1094/PDIS-10-15-1144-RE
Nesher, I., Minz, A., Kokkelink, L., Tudzynski, P., and Sharon, A. (2011). Regulation of pathogenic spore germination by CgRac1 in the fungal plant pathogen Colletotrichum gloeosporioides. Eukaryot. Cell 10, 1122–1130. doi: 10.1128/EC.00321-10
Ntsoane, M. L., Zude-Sasse, M., Mahajan, P., and Sivakumar, D. (2019). Quality assesment and postharvest technology of mango: A review of its current status and future perspectives. Sci. Hortic. 249, 77–85. doi: 10.1016/j.scienta.2019.01.033
Oh, B. J., Ko, M. K., Kim, Y. S., Kim, K. S., Kostenyuk, I., and Kee, H. K. (1999). A cytochrome P450 gene is differentially expressed in compatible and incompatible interactions between pepper (Capsicum annuum) and the anthracnose fungus, Colletotrichum gloeosporioides. Mol. Plant-Microbe Interact. 12, 1044–1052. doi: 10.1094/MPMI.1999.12.12.1044
Onyeani, C. A., and Amusa, N. A. (2015). Incidence and severity of anthracnose in mango fruits and its control with plant extracts in south West Nigeria. Int. J. Agric. Res. 10, 33–43. doi: 10.3923/IJAR.2015.33.43
Pardo-De la Hoz, C. J., Calderón, C., Rincón, A. M., Cárdenas, M., Danies, G., López-Kleine, L., et al. (2016). Species from the Colletotrichum acutatum, Colletotrichum boninense and Colletotrichum gloeosporioides species complexes associated with tree tomato and mango crops in Colombia. Plant Pathol. 65, 227–237. doi: 10.1111/ppa.12410
Patel, B., Tandel, Y. N., Patel, A. H., and Patel, B. L. (2016). Chilling injury in tropical and subtropical fruits: A cold storage problem and its remedies: A review. Int. J. Environ. Sci. Technol. 5, 1882–1887.
Patil, R. Y., Gulvani, S., Waghmare, V. B., and Mujawar, I. K. (2022). Image based anthracnose and red-rust leaf disease detection using deep learning. Telkomnika 20, 1256–1263. doi: 10.12928/telkomnika.v20i6.24262
Patrice, N. D., Alain, H., Bertrand, M. S., Norbert, K. T., Nourou, K. N., Brice, T. T., et al. (2020). First report of red rust disease caused by Cephaleuros virescens on mango (Mangifera indica) tree in Cameroon. Int. J. Phytopathol. 9, 187–193. doi: 10.33687/phytopath.009.03.3432
Paudel, A., Poudel, P., and Yogi, M. (2022). Insights on the mango anthracnose and its management. J Plant Pathol. Res. 4, 81–90. doi: 10.36959/394/629
Paulitz, T. C. (1995). First report of Colletotrichum gloeosporioides on lupine in Canada. Plant Dis. 79:319. doi: 10.1094/PD-79-0319D
Peres, N. A., Kuramae, E. E., Dias, M. S., and De Souza, N. L. (2002). Identification and characterization of Colletotrichum spp. affecting fruit after harvest in Brazil. J. Phytopathol. 150, 128–134. doi: 10.1046/j.1439-0434.2002.00732.x
Pérez-Mora, J. L., Mora-Romero, G. A., Beltrán-Peña, H., García-León, E., Lima, N. B., Camacho-Tapia, M., et al. (2021). First report of Colletotrichum siamense and C. gloeosporioides causing anthracnose of Citrus spp. in Mexico. Plant Dis. 105:496. doi: 10.1094/PDIS-08-20-1743-PDN
Prabu, M., and Chelliah, B. J. (2022). Mango leaf disease identification and classification using a CNN architecture optimized by crossover-based levy flight distribution algorithm. Neural Comput. Appl. 34, 7311–7324. doi: 10.1007/s00521-021-06726-9
Prakash, O., and Srivastava, K. C. (1987). Mango diseases and their management. A world review. Today and Tomorrow's Printers and Publishers: New Delhi.
Qin, L. P., Zhang, Y., Su, Q., Chen, Y. L., Nong, Q., Xie, L., et al. (2019). First report of anthracnose of Mangifera indica caused by Colletotrichum scovillei in China. Plant Dis. 103:1043. doi: 10.1094/PDIS-11-18-1980-PDN
Reyes-Perez, J. J., Hernandez-Montiel, L. G., Vero, S., Noa-Carrazana, J. C., Quiñones-Aguilar, E. E., and Rincón-Enríquez, G. (2019). Postharvest biocontrol of Colletotrichum gloeosporioides on mango using the marine bacterium Stenotrophomonas rhizophila and its possible mechanisms of action. J. Food Sci. Technol. 56, 4992–4999. doi: 10.1007/s13197-019-03971-8
Rodriguez-Moreno, L., Ebert, M. K., Bolton, M. D., and Thomma, B. P. (2018). Tools of the crook-infection strategies of fungal plant pathogens. Plant J. 93, 664–674. doi: 10.1111/tpj.13810
Rosman, N. F., Asli, N. A., Abdullah, S., and Rusop, M. (2019). “Some common disease in mango” in AIP conference proceedings, vol. 2151 (New York: AIP Publishing LLC), 020019.
Saeed, E. E., Sham, A., AbuZarqa, A., Al Shurafa, K. A., Al Naqbi, T. S., Iratni, R., et al. (2017). Detection and management of mango dieback disease in the United Arab Emirates. Int. J. Mol. Sci. 18:2086. doi: 10.3390/ijms18102086
Sanchez-Arizpe, A., Galindo-Cepeda, M. E., Arispe-Vazquez, J. L., Genis-Velazquez, R., Vazquez-Badillo, M. E., and Antonio-Bautista, A. (2021). Natural resistance of two Mango'mangifera indica'l. commercial cultivars to anthracnose caused by'Colletotrichum gloeosporioides' Penz. Penz. and Sacc. Aust. J. Crop Sci. 15, 1198–1203. doi: 10.21475/ajcs.21.15.08.p3347
Sardrood, B. P., and Goltapeh, E. M. (2018). “Effect of agricultural chemicals and organic amendments on biological control Fungi” in Sustainable Agriculture Reviews. ed. E. Lichtfouse, vol. 12 (Berlin: Springer), 217–359.
Sardsud, U., Sardsud, V., and Singkaew, S. (2003). Postharvest loss assessment of mango cv. Nam Dok Mai. Warasan Witthayasat Kaset. Available at: https://agris.fao.org/agris-search/search.do?recordID=TH2005000687 (Accessed February 1, 2023).
Sarkar, A. K. (2016). Anthracnose diseases of some common medicinally important fruit plants. J. Med. Plant Res. 4, 233–236.
Schena, L., Mosca, S., Cacciola, S. O., Faedda, R., Sanzani, S. M., Agosteo, G. E., et al. (2014). Species of the Colletotrichum gloeosporioides and C. boninense complexes associated with olive anthracnose. Plant Pathol. 63, 437–446. doi: 10.1111/ppa.12110
Sefu, G., Satheesh, N., and Berecha, G. (2015). Effect of essential oils treatment on anthracnose (Colletotrichum gloeosporioides) disease development, quality and shelf life of mango fruits (Mangifera indica L). Am. Eurasian J. Agric. Environ. Sci. 15, 2160–2169. doi: 10.5829/idosi.aejaes.2015.15.11.96140
Serrato-Diaz, L. M., Rivera-Vargas, L. I., and French-Monar, R. D. (2014). First report of Neofusicoccum mangiferae causing rachis necrosis and inflorescence blight of mango (Mangifera indica) in Puerto Rico. Plant Dis. 98:570. doi: 10.1094/PDIS-08-13-0878-PDN
Sharma, M., and Kulshrestha, S. (2015). Colletotrichum gloeosporioides: an anthracnose causing pathogen of fruits and vegetables. Biosci. Biotechnol. Res. Asia 12, 1233–1246. doi: 10.13005/bbra/1776
Sharma, G., Kumar, N., Weir, B. S., Hyde, K. D., and Shenoy, B. D. (2013). The ApMat marker can resolve Colletotrichum species: a case study with Mangifera indica. Fungal Divers. 61, 117–138. doi: 10.1007/s13225-013-0247-4
Sharma, G., Maymon, M., and Freeman, S. (2017). Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Sci. Rep. 7:15839. doi: 10.1038/s41598-017-15946-w
Sharma, A., and Verma, K. S. (2007). In vitro cross pathogenicity and management of Colletotrichum gloeosporioides causing anthracnose of mango. Ann. Plant Prot. Sci 15, 186–188.
Shi, N., Ruan, H., Jie, Y., Chen, F., and Du, Y. (2020). Sensitivity and efficacy of fungicides against wet bubble disease of Agaricus bisporus caused by Mycogone perniciosa. Eur. J. Plant Pathol. 157, 873–885. doi: 10.1007/s10658-020-02047-0
Shih, J., Wei, Y., and Goodwin, P. H. (2000). A comparison of the pectate lyase genes, pel-1 and pel-2, of Colletotrichum gloeosporioides f. sp. malvae and the relationship between their expression in culture and during necrotrophic infection. Gene 243, 139–150. doi: 10.1016/s0378-1119(99)00546-6
Shivas, R. G., Tan, Y. P., Edwards, J., Dinh, Q., Maxwell, A., Andjic, V., et al. (2016). Colletotrichum species in Australia. Australas. Plant Pathol. 45, 447–464. doi: 10.1007/s13313-016-0443-2
Silué, Y., Nindjin, C., Cissé, M., Kouamé, K. A., Mbéguié-A-Mbéguié, D., Lopez-Lauri, F., et al. (2022). Hexanal application reduces postharvest losses of mango (Mangifera indica L. variety" Kent") over cold storage whilst maintaining fruit quality. Postharvest Biol. Technol. 189:111930. doi: 10.1016/j.postharvbio.2022.111930
Siriptrawan, U. (2021). Early detection of anthracnose on mango fruit using hyperspectral imaging. Rep. Grant. Res. Asahi Glass Foundation 90:81. doi: 10.50867/afreport.2021_081
Sosnowski, M. R., Fletcher, J. D., Daly, A. M., Rodoni, B. C., and Viljanen-Rollinson, S. L. H. (2009). Techniques for the treatment, removal and disposal of host material during programmes for plant pathogen eradication. Plant Pathol. 58, 621–635. doi: 10.1111/j.1365-3059.2009.02042.x
Stephenson, S. A., Hatfield, J., Rusu, A. G., Maclean, D. J., and Manners, J. M. (2000). CgDN3: an essential pathogenicity gene of Colletotrichum gloeosporioides necessary to avert a hypersensitive-like response in the host Stylosanthes guianensis. Mol. Plant-Microbe Interact. 13, 929–941. doi: 10.1094/MPMI.2000.13.9.929
Su, Y. Y., Noireung, P., Liu, F., Hyde, K. D., Moslem, M. A., Bahkali, A. H., et al. (2011). Epitypification of Colletotrichum musae, the causative agent of banana anthracnose. Mycoscience 52, 376–382. doi: 10.1007/s10267-011-0120-9
Supriya, A., Kumar, A., and Kudachikar, V. B. (2020). A comparison investigation on antioxidant activities, constitutive antifungal phenolic lipids and phenolics contents of anthracnose resistant and susceptible mango fruit cultivars. Int. J. Fruit Sci. 20, 692–704. doi: 10.1080/15538362.2019.1668332
Talhinhas, P., and Baroncelli, R. (2021). Colletotrichum species and complexes: geographic distribution, host range and conservation status. Fungal Divers. 110, 109–198. doi: 10.1007/s13225-021-00491-9
Talhinhas, P., Loureiro, A., and Oliveira, H. (2018). Olive anthracnose: a yield-and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 19, 1797–1807. doi: 10.1111/mpp.12676
Tashiro, N., Manabe, K., and Ide, Y. (2012). Emergence and frequency of highly benzimidazole-resistant Colletotrichum gloeosporioides, pathogen of Japanese pear anthracnose, after discontinued use of benzimidazole. J. Gen. Plant Pathol. 78, 221–226. doi: 10.1007/s10327-012-0375-9
Theerthagiri, A., Govindasamy, S., Thiruvengadam, R., and Ramasamy, S. (2016). Immunoassay-based techniques for early and specific detection of latent postharvest anthracnose in mango. J. Phytopathol. 164, 318–329. doi: 10.1111/jph.12459
Thind, T. S., and Hollomon, D. W. (2018). Thiocarbamate fungicides: reliable tools in resistance management and future outlook. Pest Manag. Sci. 74, 1547–1551. doi: 10.1002/ps.4844
Torres-Calzada, C., Tapia-Tussell, R., Higuera-Ciapara, I., and Perez-Brito, D. (2013). Morphological, pathological and genetic diversity of Colletotrichum species responsible for anthracnose in papaya (Carica papaya L). Eur. J. Plant Pathol. 135, 67–79. doi: 10.1007/s10658-012-0065-7
Tovar-Pedraza, J. M., Mora-Aguilera, J. A., Nava-Diaz, C., Lima, N. B., Michereff, S. J., Sandoval-Islas, J. S., et al. (2020). Distribution and pathogenicity of Colletotrichum species associated with mango anthracnose in Mexico. Plant Dis. 104, 137–146. doi: 10.1094/PDIS-01-19-0178-RE
Tucho, A., Lemessa, F., and Berecha, G. (2014). Distribution and occurrence of mango anthracnose (Colletotrichum gloesporioides penz and sacc) in humid agro-ecology of Southwest Ethiopia. Plant Pathol. J. 13, 268–277. doi: 10.3923/ppj.2014.268.277
Uddin, M., Shefat, S., Afroz, M., and Moon, N. (2018). Management of anthracnose disease of mango caused by Colletotrichum gloeosporioides: A review. Acta Sci. Agric. 2, 169–177. doi: 10.35248/2157-7471.22.13.635
Ullagaddi, S. B., and Raju, S. V. (2017). “Disease recognition in mango crop using modified rotational kernel transform features” in 2017 4th international conference on advanced computing and communication systems (ICACCS) (New York: IEEE), 1–8.
Veling, P. S., Kalelkar, R. S., Ajgaonkar, L. V., Mestry, N. V., and Gawade, N. N. (2019). Mango disease detection by using image processing. IJRASET 7, 3717–3726. doi: 10.22214/ijraset.2019.4624
Veloso, J. S., Câmara, M. P., Lima, W. G., Michereff, S. J., and Doyle, V. P. (2018). Why species delimitation matters for fungal ecology: Colletotrichum diversity on wild and cultivated cashew in Brazil. Fungal boil. 122, 677–691. doi: 10.1016/j.funbio.2018.03.005
Vilcarromero-Ramos, R. L., Díaz-Valderrama, J. R., Caetano, A. C., Huamán-Pilco, J., and Mansilla-Córdova, P. J. (2022). First report of anthracnose caused by Colletotrichum asianum on mango (Mangifera indica) in Peru. Plant Dis. 107:2245. doi: 10.1094/PDIS-10-22-2357-PDN
Vitale, A., Alfenas, A. C., de Siqueira, D. L., Magistà, D., Perrone, G., and Polizzi, G. (2020). Cultivar resistance against Colletotrichum asianum in the world collection of mango germplasm in southeastern Brazil. Plan. Theory 9:182. doi: 10.1094/PDIS.2000.84.6.600
Wang, Y. C., Hao, X. Y., Wang, L., Xiao, B., Wang, X. C., and Yang, Y. J. (2016). Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 6:35287. doi: 10.1038/srep35287
Wei, Y., Shih, J., Li, J., and Goodwin, P. H. (2002). Two pectin lyase genes, pnl-1 and pnl-2, from Colletotrichum gloeosporioides f. sp. malvae differ in a cellulose-binding domain and in their expression during infection of Malva pusilla. Microbiol. 148, 2149–2157. doi: 10.1099/00221287-148-7-2149
Weir, B. S., Johnston, P. R., and Damm, U. (2012). The Colletotrichum gloeosporioides species complex. Stud. Mycol. 73, 115–180. doi: 10.3114/sim0011
Wu, Y., Cheng, J. H., and Sun, D. W. (2022). Subcellular damages of Colletotrichum asianum and inhibition of mango anthracnose by dielectric barrier discharge plasma. Food Chem. 381:132197. doi: 10.1016/j.foodchem.2022.132197
Wu, J., Ji, Z., Wang, N., Chi, F., Xu, C., Zhou, Z., et al. (2016). Identification of conidiogenesis-associated genes in Colletotrichum gloeosporioides by Agrobacterium tumefaciens-mediated transformation. Curr. Microbiol. 73, 802–810. doi: 10.1007/s00284-016-1131-8
Yokosawa, S., Eguchi, N., Kondo, K. I., and Sato, T. (2017). Phylogenetic relationship and fungicide sensitivity of members of the Colletotrichum gloeosporioides species complex from apple. J. Gen. Plant Pathol. 83, 291–298. doi: 10.1007/s10327-017-0732-9
Yong, H. Y., Bakar, F. D. A., Illias, R. M., Mahadi, N. M., and Murad, A. A. (2013). Cgl-SLT2 is required for appressorium formation, sporulation and pathogenicity in Colletotrichum gloeosporioide. Braz. J. Microbiol. 44, 1241–1250. doi: 10.1590/S1517-83822013000400031
Zakaria, L., Juhari, N. Z., Vijaya, S. I., and Anuar, I. S. M. (2015). Molecular characterization of Colletotrichum isolates associated with anthracnose of mango fruit. Sains Malays. 44, 651–656. doi: 10.17576/jsm-2015-4405-02
Zhafarina, S., Wibowo, A., and Widiastuti, A. (2021). Multi-genetic analysis of Colletotrichum spp. associated with postharvest disease of fruits anthracnose in special region of Yogyakarta, Indonesia. Pak. J. Biol. Sci. PJBS 24, 53–65. doi: 10.3923/pjbs.2021.53.65
Zhang, H., Wei, Y., and Shi, H. (2020). First report of anthracnose caused by Colletotrichum kahawae subsp. ciggaro on Areca in China. Plant Dis. 104:1871. doi: 10.1094/PDIS-12-19-2628-PDN
Zhou, Y., Huang, J. S., Yang, L. Y., Wang, G. F., and Li, J. Q. (2017). First report of banana anthracnose caused by Colletotrichum scovillei in China. Plant Dis. 101:381. doi: 10.1094/PDIS-08-16-1135-PDN
Zhou, Z., Wu, J., Wang, M., and Zhang, J. (2017). ABC protein CgABCF2 is required for asexual and sexual development, appressorial formation and plant infection in Colletotrichum gloeosporioides. Microb. Pathog. 110, 85–92. doi: 10.1016/j.micpath.2017.06.028
Keywords: mango disease, anthracnose, epidemiology, detection, host range, management strategies, Colletotrichum gloeosporioides
Citation: Dofuor AK, Quartey NK-A, Osabutey AF, Antwi-Agyakwa AK, Asante K, Boateng BO, Ablormeti FK, Lutuf H, Osei-Owusu J, Osei JHN, Ekloh W, Loh SK, Honger JO, Aidoo OF and Ninsin KD (2023) Mango anthracnose disease: the current situation and direction for future research. Front. Microbiol. 14:1168203. doi: 10.3389/fmicb.2023.1168203
Received: 17 February 2023; Accepted: 03 August 2023;
Published: 24 August 2023.
Edited by:
Lei Huang, Purdue University, United StatesReviewed by:
Porfirio Gutierrez-Martinez, Instituto Tecnológico de Tepic, MexicoCopyright © 2023 Dofuor, Quartey, Osabutey, Antwi-Agyakwa, Asante, Boateng, Ablormeti, Lutuf, Osei-Owusu, Osei, Ekloh, Loh, Honger, Aidoo and Ninsin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aboagye Kwarteng Dofuor, YWtkb2Z1b3JAdWVzZC5lZHUuZ2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.