- 1College of Animal Science and Technology, Hebei Agricultural University, Baoding, China
- 2College of Animal Science, Guizhou University, Guiyang, China
- 3Institute of Cereal and Oil Crops, Hebei Academy of Agriculture and Forestry Sciences, Shijiazhuang, China
- 4Key Laboratory for Feed Biotechnology of the Ministry of Agriculture and Rural Affairs, Institute of Feed Research, Chinese Academy of Agriculture Sciences, Beijing, China
- 5Precision Livestock and Nutrition Laboratory, Teaching and Research Centre (TERRA), Gembloux Agro-Bio Tech, University of Liège, Gembloux, Belgium
- 6College of Food Science and Technology, Hebei Agricultural University, Baoding, China
Carbohydrate is the most common macronutrient consumed across all phases of the diet and acts as a potential regulator in modulating the gut microbiota in animals. However, the influences of dietary non-fibrous carbohydrate (NFC) to neutral detergent fiber (NDF) in different ratios on gut microbiota, metabolites, intestinal immunity, and growth performance have not been fully explored. A total of 135 healthy weaned rabbits (45.1 ± 0.7 d of age) with an average body weight of 1.08 ± 0.07 kg were randomly divided into five groups. Under the same other nutrient levels, rabbits were fed diets with NFC/NDF ratios of 0.7 (T1), 1.0 (T2), 1.3 (T3), 1.6 (T4), and 1.9 (T5). During the 28-day experiment, T3 rabbits showed the highest final body weight and the lowest feed-to-weight ratio than T5 rabbits (P < 0.05) but no significant difference with T1 or T2 rabbits. The expression of cecal pro-inflammatory factors IL-1β and TNF-α was increased in the T4 and T5 than in those of other groups (P < 0.05). Conversely, the tight junction proteins (ZO-1, Claudin-1, and Occludin) were decreased to varying degrees in the T4 and T5 groups. The pH value in the cecal digesta of T5 rabbits was lower than that of T1, T2, and T3 (P < 0.05), while the concentration of volatile fatty acids and propionate was higher than those of T1, T2, and T3 rabbits (P < 0.05). In terms of gut microbiota, at the phylum level, the relative burden of Firmicutes and Actinobacteria in T2 rabbits was the highest (P < 0.05), and the relative burden of Proteobacteria in T5 rabbits was higher than that of other groups (P < 0.05). At the genus level, the relative burden of Ruminococcus was higher in T2 and T3 rabbits than that of other groups, and T5 rabbits have the lowest relative burden of Ruminococcus. Combination analysis showed that cecal metabolites were positively associated with fermentation-related phenotypes and the burden of Firmicutes (P < 0.05). In conclusion, different dietary NFC/NDF ratios can affect the intestinal immune response and growth performance of rabbits, and there was a positive effect when dietary NFC/NDF = 1.0–1.3.
1. Introduction
Intestinal microorganisms can reduce the pH of the intestinal content by degrading dietary fiber, which promotes the colonization of the acidophilic flora in the intestine, such as Lactobacillus, Bifidobacterium, Bacteroides, and Faecalibacterium (Holscher, 2017; Makki et al., 2018). Previous studies have reported that different types of fibers have a significant effect on the gut microbiome diversity in animals, and the number of fiber-degrading bacteria in the cecum increases when the fiber content of the diet is increased from 11 to 17% (Jin et al., 2018). Increasing dietary fiber can reduce the residence time of the digesta in the cecum and inhibits the growth of pathogenic microorganisms (Gidenne, 2015). Dietary fiber affects the growth and immune performance of rabbits by altering the structure of the gut microbiome and then influences intestinal immunity (Eberl, 2010; Zheng et al., 2020; Ye et al., 2021; Xue et al., 2022). It was found that dietary soluble fiber may protect the intestinal mucosa by limiting the penetration of acetylsalicylic acid into epithelial cells. Furthermore, volatile fatty acids (VFAs) produced by fermentative degradation of dietary fiber in the intestine can resist potentially infectious agents in vivo (Che et al., 2019; Shang et al., 2020; Guan et al., 2021). When the integrity of the intestinal epithelial barrier is compromised, potentially harmful macromolecules can easily pass through the intestinal epithelial barrier into the organism and result in autoimmune diseases (Iliev and Cadwell, 2021).
Neutral detergent fiber (NDF) can be degraded by intestinal microorganisms into VFA to supply energy for the organism. Studies have shown that dietary NDF levels could affect cecal-digesta pH and VFA concentration in rabbits, and the amount of anaerobic fungal increased with the increasing dietary NDF level, VFA produced by anaerobic fungal fermentation can promote the expression of intestinal tight junction proteins to avoid inflammatory reactions in the organism, which is beneficial to intestinal health (Zhu et al., 2017; Goulart et al., 2020). In addition, it has been reported that an increasing dietary NDF can improve the intestinal development and immune defense of rabbits (Zhu et al., 2017; Wu et al., 2019). However, excessive NDF can lead to a decrease in apparent nitrogen digestibility, which affects the growth performance of rabbits (Zhu et al., 2020).
Non-fibrous carbohydrates (NFCs) in diets are important components of carbohydrates that can provide a large amount of blood glucose to animals with negative energy balance (Hall, 2003). As a result, it was generally accepted that diets with high NFC concentration are more conducive to promoting the growth and development of the organism (Xue et al., 2019; Hernández-Castellano et al., 2020; Khattab et al., 2021). However, excessive NFC concentration can increase the odds of developing rumen acidosis and diarrhea, which also have a negative impact on growth performance (Xue et al., 2019; Li et al., 2022).
Recent studies have shown that an unbalanced NFC/NDF ratio in the diet can affect the disturbances in the rumen environment and a reduction in bacterial diversity of ruminants, which may eventually result in a reduction of growth performance (Pu et al., 2020; Chen et al., 2022). However, there are a very limited number of studies on monogastric animals. As a representative model system, rabbits have been extensively used as experimental models in biomedical research. This experiment was conducted to investigate the effects of different dietary NFC/NDF on gut microbiota, metabolites, and immune homeostasis in rabbits, and the result will also provide a scientific basis for determining the appropriate dietary NFC/NDF ratio of rabbits. We hypothesized that dietary NFC/NDF at a relatively low ratio (<1.4) had a positive effect on the growth and immune performance of rabbits, whereas a relatively high ratio (>1.4) had a negative effect.
2. Materials and methods
2.1. Animals, management, and experimental diets
A total of 135 healthy weaned New Zealand White rabbits (45.1 ± 0.7 d of age, average weight 1.08 ± 0.07 kg) were randomly divided into five groups, respectively. Each group was further allocated into 27 replicates, with a rabbit per replicate. Under the condition of the same other nutrient levels, rabbits were fed diets with NFC/NDF of 0.7 (T1), 1.0 (T2), 1.3 (T3), 1.6 (T4), and 1.9 (T5). The experiment period is 28 days. During the experiment, all rabbits were allowed to eat and drink freely. The rabbit dormitory was ensured with normal light and ventilation. The diet was formulated according to the nutritional requirements of the National Research Council (1977), using corn, bran, soybean meal, alfalfa meal, and oat meal as raw materials, formulated into pellet feed with a diameter × length of 4 × 10 mm. The feed composition is provided in Supplementary Table 1.
2.2. Sampling
In order to evaluate the growth performance parameters, body weight (BW) and feed consumption were measured; average daily gain (ADG), average daily feed intake (ADFI), and the feed conversion ratio (FCR) were calculated (g feed/g gain) for all phases. The number of dead rabbits with diarrhea was recorded during the experiment, and the mortality and diarrhea rates were calculated via the following formulas. Mortality (%) = (the number of dead rabbits/total number of experimental rabbits) × 100%. Diarrhea (%) = (the number of rabbits with diarrhea/total number of experimental rabbits) × 100%. On the 28th day of the trial, six rabbits from each replicate were randomly selected; blood samples were taken (2.5 mL) from the marginal vein of the ear using an anticoagulant-free vacuum test tube (5 mL) and immediately transported for analysis of routine hemogram with an auto hematology analyzer (Mindray BC-2800, Shenzhen, China). Afterward, the rabbits were euthanized according to AVMA (Association, 2001). The cecal tissue and content were immediately frozen in liquid nitrogen at −80°C till analyzed for mRNA, volatile fatty acid (VFA), and gut microbiota.
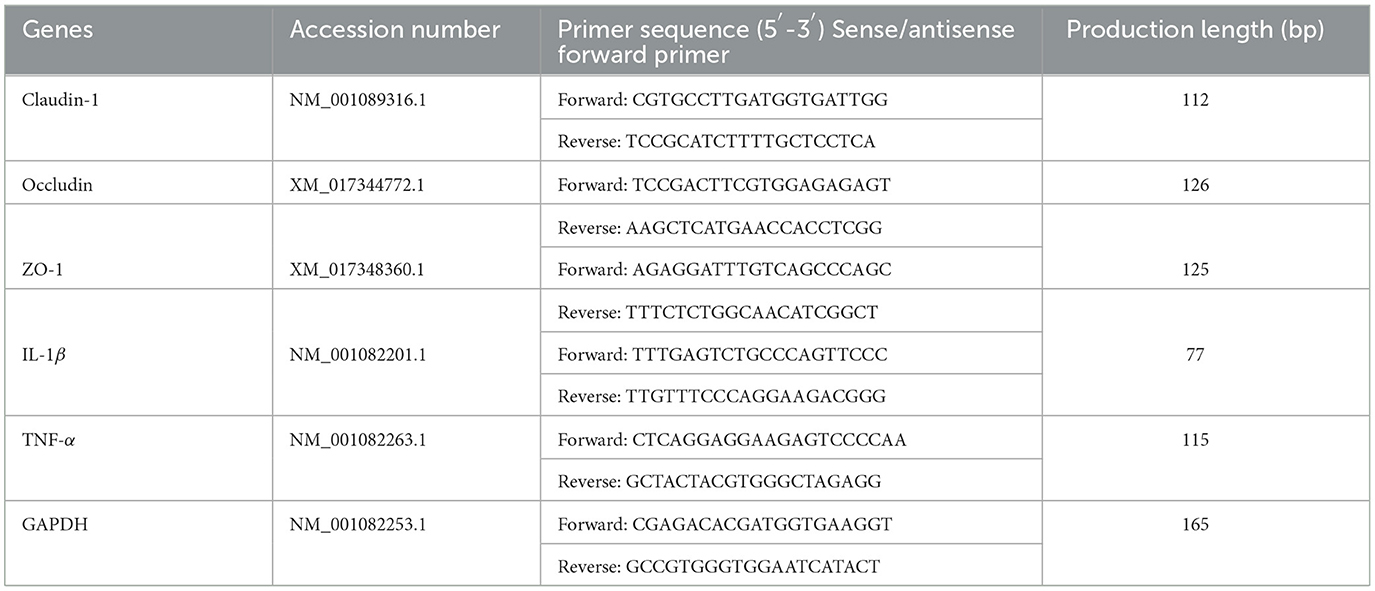
The mRNA expression of genes related to gut immunity interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), Claudin-1, Occludin, zonula occludens (ZO-1), and GAPDH in cecum tissues was measured by RT-qPCR. Primers were designed using Premier 5.0 software (Table 1), and GAPDH was used to normalize the expression of the targeted genes. Procedure: (1) The total RNA from the intestinal mucosa was isolated by TRI-zol reagent (TIANGEN, Beijing, China). (2) The total RNA was determined from OD 260/280. (3) Then, the total RNA was reversely transcribed into cDNA using the prime script of Fast Quant RT Kit (TIANGEN, Beijing, China), and qPCR was conducted. (4) The mRNA level of the relative gene was calculated using the 2−ΔΔCt method. Each sample was analyzed in triplicate and the results are reported as the geometric mean of the three results.
The VFA of the cecal content was measured by the Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, United States), according to the integration parameters and calibration curve. The pH value was detected potentiometrically using an automated pH analyzer (PHSJ-3F, INESA scientific instrument Co. Ltd, Shanghai, China). The NH3-N of the cecal content was measured by the phenol-sodium hypochlorite colorimetric method. A measure of 1 mL of sample solution or standard solution was added to 4 mL of HCl (0.2 mol/L) solution and then mixed well. After that, 0.4 mL of the mixture was added to 5 mL of phenol reagent, and 4 mL of sodium hypochlorite was added and mixed well. The sample was water bath-heated at 60°C for 10 min, and after cooling, the absorbance was measured at 545 nm wavelength (Weatherburn, 1967).
2.3. Cecal content DNA extraction, 16S rRNA amplification, and sequencing
A total of six cecal content samples from each group were randomly selected and used for the analysis of the gut microbiota. The workflow included DNA extraction, quality inspection, database sequencing, and 16S rRNA gene sequencing. Detailed methods are available in Supplementary material 2.
2.4. Statistical analysis
The results obtained were analyzed for power analysis in the G*Power package (version 3.1.4.), to ensure adequate power for the analysis. Afterward, experimental data were tested for normality by using the Shapiro–Wilk test of normality and for homogeneity of variances by using Levene's test. An overall test for treatment effect was first performed with a one-way analysis of variance (ANOVA). Linear and quadratic trends were conducted using orthogonal polynomial coefficients (Social Sciences 19.0 software, SPSS Inc., Chicago, IL, United States). The indexes were expressed as means with standard error of the mean (SEM) and significant main effect. P < 0.05 was considered a significant difference. GraphPad-Prism version 7.0 (San-Diego, CA, United States) and heatmap illustrator (HemI, version 1.0.3.7) software were used to draw the graphs and heatmaps, respectively.
3. Results
3.1. Effects of dietary NFC/NDF on growth performance
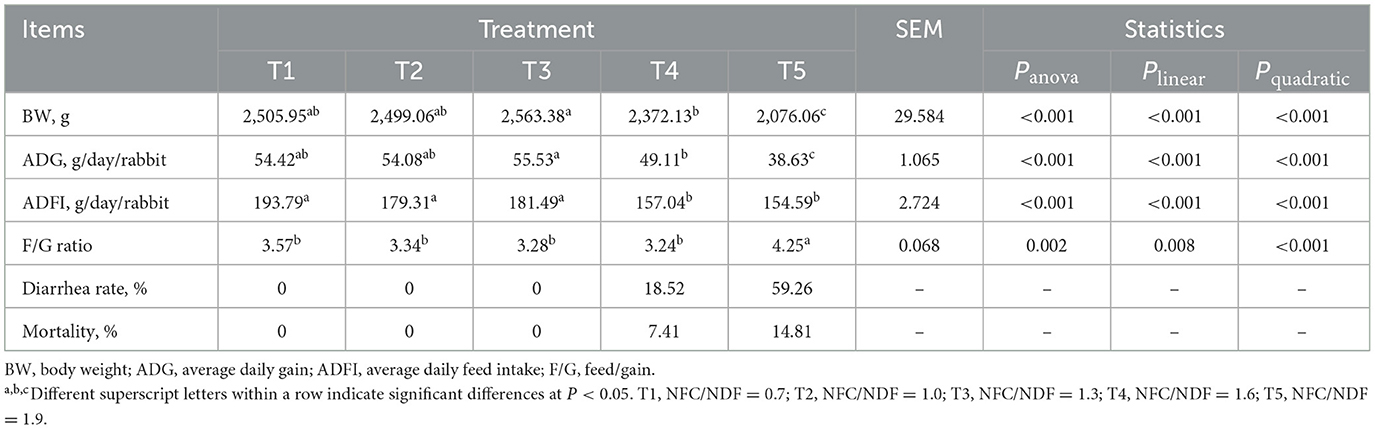
As presented in Table 2, the BW and ADG of rabbits in T5 were lower than the other groups (P < 0.05), and the ADFI of rabbits in T1, T2, and T3 was significantly higher than that in T4 and T5. Polynomial contrast analysis showed that the level of BW (Plin < 0.001, P quad < 0.001), ADG (Plin < 0.001, Pquad < 0.001), and ADFI (Plin < 0.001, Pquad < 0.001) displayed linear and quadratic patterns of decrease with increasing dietary NFC/NDF inclusion level, with T1, T2, T3, and T4 being higher than T5. However, the F/G ratio in T5 rabbits was higher than that in the other groups (P < 0.05, Plin < 0.001, and Pquad < 0.001), and the F/G ratio of rabbits in T3 was slightly lower than that in T1, T2, and T4 (P > 0.05). No individuals in the T1, T2, and T3 groups were found to have diarrhea or death. However, the diarrhea rates in the T4 and T5 groups were 18.52 and 59.26%, respectively, with the death rates being 7.41 and 14.81%, respectively.
3.2. Effects of dietary NFC/NDF on blood parameters
Figure 1 represents the blood parameters of rabbits. WBC and neutrophil in the blood of rabbits in the T5 group were higher than those in T1, T2, and T3 (P < 0.05), while there were no significant differences in lymphocyte and monocyte in all groups (P > 0.05).
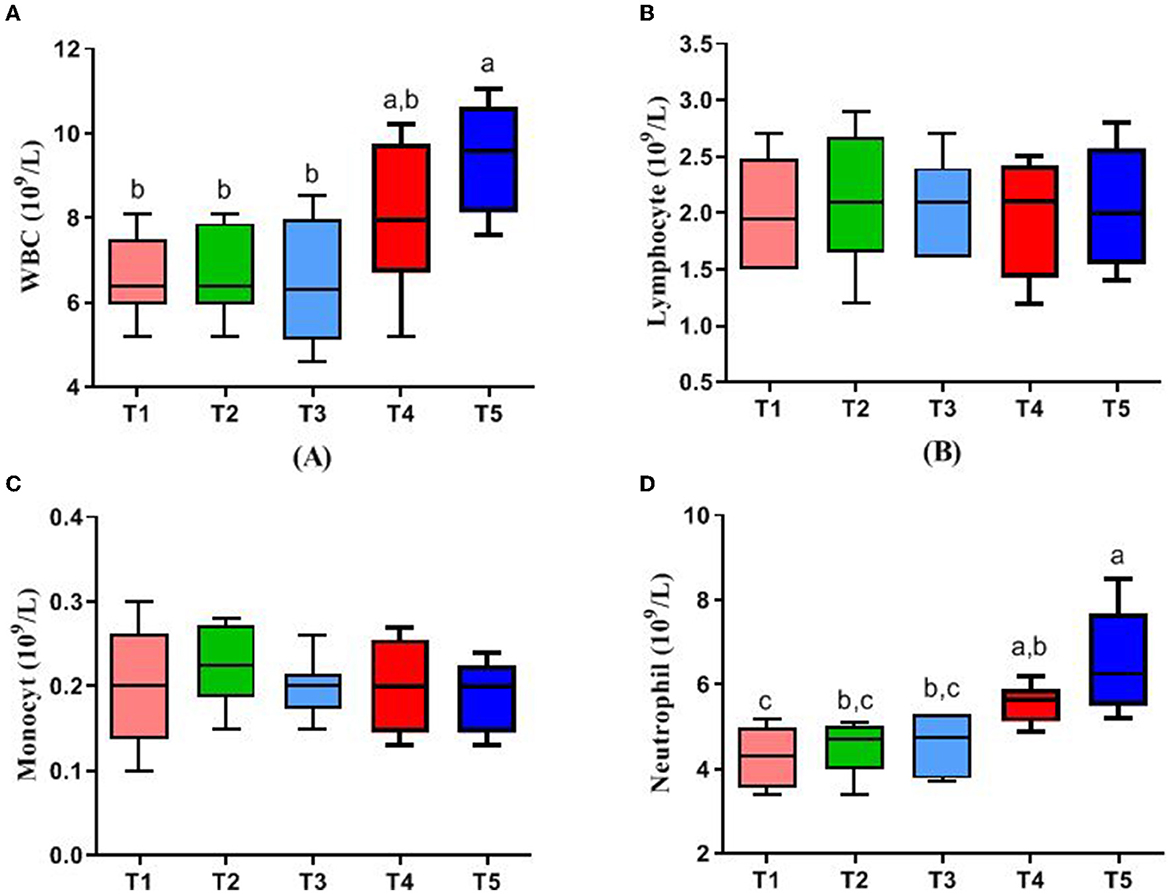
Figure 1. Effects of different ratios of dietary NFC/NDF on blood parameters. Data are indicated as means ± SEM (n = 6). (A) The impact on the number of white blood cells (WBC). (B) The impact on the number of lymphocytes. (C) The impact on the number of monocytes. (D) The impact on the number of neutrophils. T1, NFC/NDF = 0.7; T2, NFC/NDF = 1.0; T3, NFC/NDF = 1.3; T4, NFC/NDF = 1.6; T5, NFC/NDF = 1.9. a, b, and c Values, for the same parameter, with different superscripts are significantly different (P < 0.05).
3.3. Effects of dietary NFC/NDF on the expression of genes related to intestinal immunity
The results are shown in Figure 2. The genes associated with innate intestinal immunity, and the expression of IL-1β and TNF-α in T4 and T5 rabbits was significantly higher than that in T1, T2, and T3 (P < 0.05, Figures 2A, B). The genes associated with the intestinal barrier and the expression of Claudin-1 and Occludin in T2 and T3 rabbits were higher than that in other groups (P < 0.05, Figures 2C, D). ZO-1 in T2 rabbits was higher than that in T1, T4, and T5 (P < 0.05, Figure 2E).
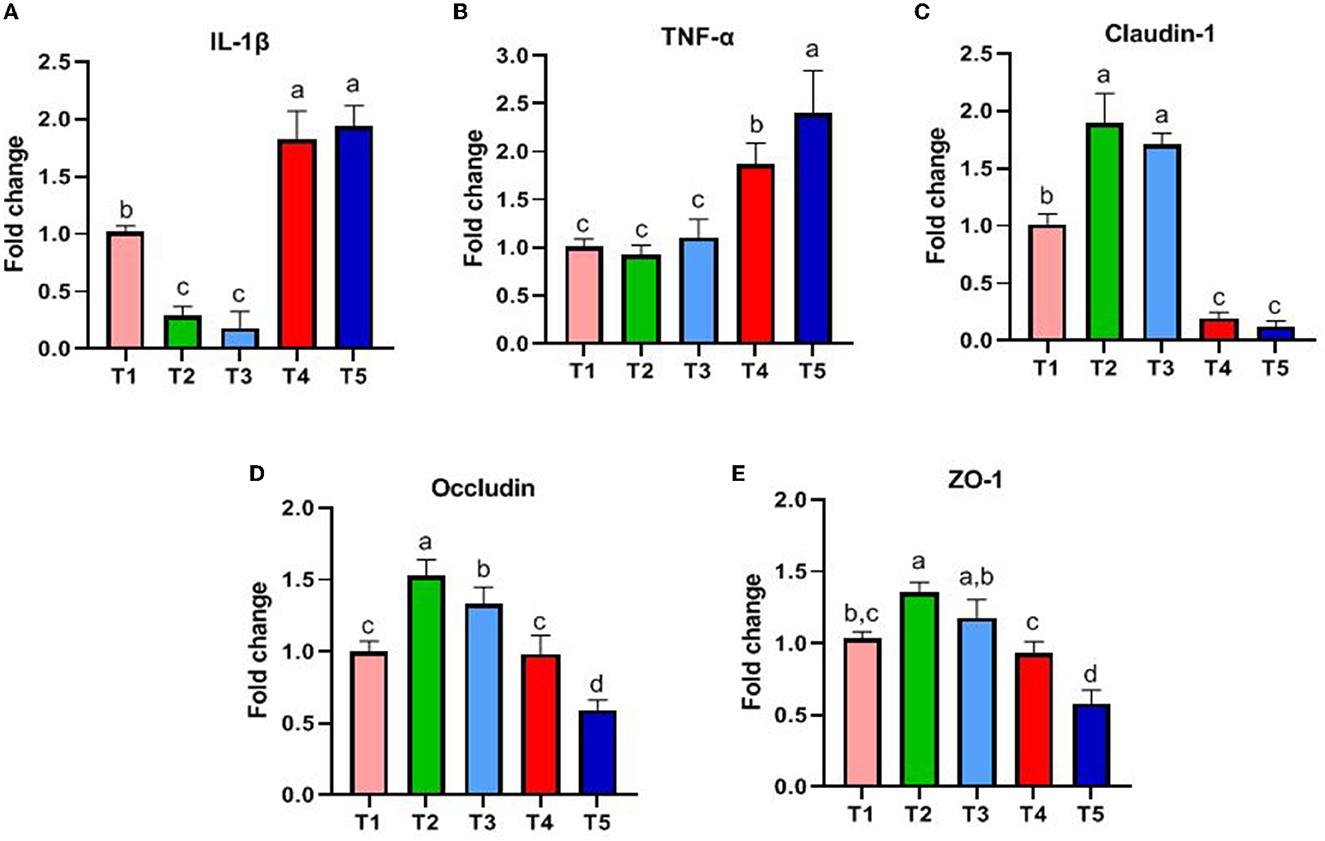
Figure 2. Effects of different ratios of dietary NFC/NDF on the expression of genes related to gut immunity. Data are indicated as means ± SEM (n = 6). (A) The impact on the expression of IL-1β. (B) The impact on the expression of TNF-α. (C) The impact on the expression of Claudin-1. (D) The impact on the expression of Occludin. (E) The impact on the expression of ZO-1. T1, NFC/NDF = 0.7; T2, NFC/NDF = 1.0; T3, NFC/NDF = 1.3; T4, NFC/NDF = 1.6; T5, NFC/NDF = 1.9. a, b, and c values for each intestinal segment with different superscripts are significantly different (P < 0.05).
3.4. Effects of dietary NFC/NDF on cecal pH and metabolites
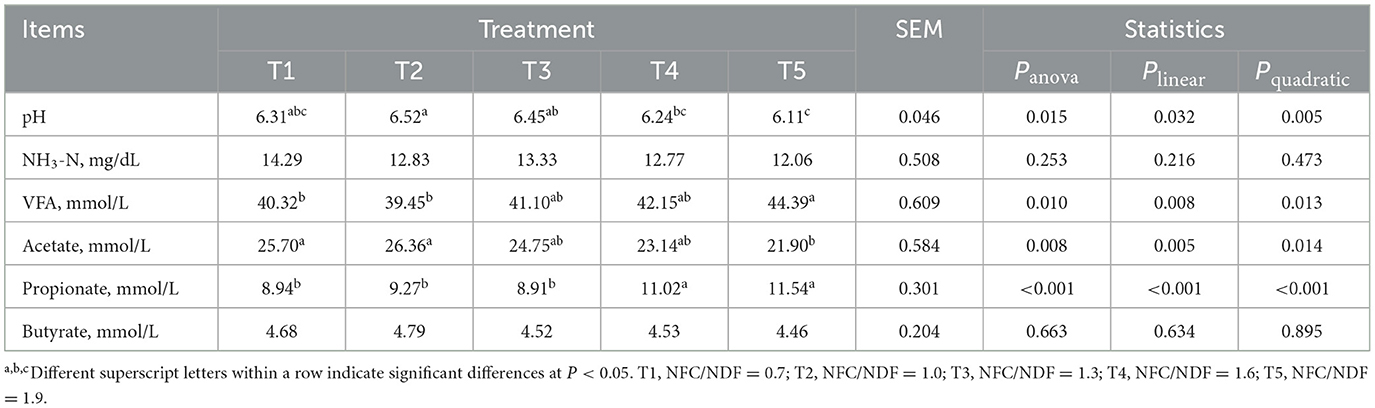
As shown in Table 3, we can conclude that the pH of the cecal content of T5 rabbits was lower than that of T1, T2, and T3 (P < 0.05). The pH decreased in a linear (Plin = 0.032) and a quadratic (Pquad = 0.005) fashion with increasing dietary NFC/NDF inclusion levels with T1, T2, and T3 being higher than T4 and T5. The level of VFA in T5 rabbits was higher than that in T1 and T2 (P < 0.05). With the increase in dietary NFC/NDF, the concentration of acetate decreased, which was highest in T1 and T2 rabbits than in other groups, especially compared with T5 (P < 0.05). However, the concentration of propionate in T5 rabbits was higher than that in T1, T2, and T3 (P < 0.05). Polynomial contrast analysis showed that the level of VFA (Pquad = 0.013) and propionate (Pquad < 0.001) displayed a quadratic pattern of increase with increasing dietary NFC/NDF inclusion level. But the expression of acetate displayed quadratic (Pquad = 0.014) and linear (Plin = 0.005) patterns of decrease. Among these five groups, no significant difference was observed in NH3-N and butyrate (P > 0.05).
3.5. Effects of dietary NFC/NDF on cecal microbial diversity
The Alpha diversity of the cecal microbiota of rabbits was influenced by dietary NFC/NDF (Figures 3A–C). Coverage value and Chao1 microbial diversity indices were not significantly altered by dietary NFC/NDF. In terms of species diversity, Shannon in T5 rabbits was lower than that in T2 and T3 (P < 0.05). The cecal microbiota samples from T1, T2, and T3 were clustered together and clearly separated from the cecal microbiota of T4 and T5 rabbits, as shown in Figure 3D.
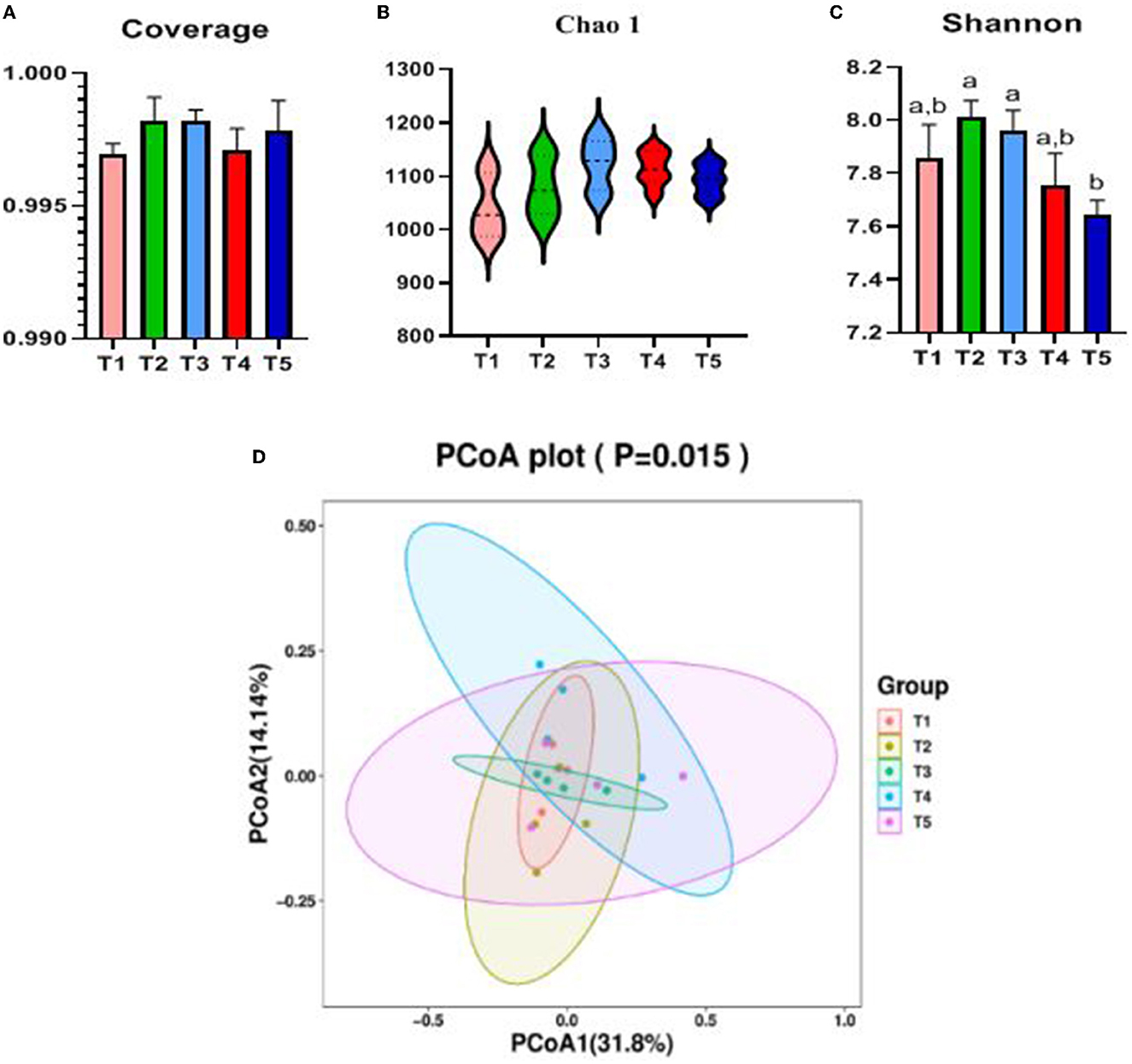
Figure 3. Effects of different ratios of dietary NFC/NDF on cecal microbial diversity. Data are indicated as means ± SEM (n = 6). (A) Coverage index of OUT level. (B) Chao1 index of OUT level. (C) Shannon index of OUT level. (D) β-diversity was estimated by the PCoA on the OUT level, respectively. T1, NFC/NDF = 0.7; T2, NFC/NDF = 1.0; T3, NFC/NDF = 1.3; T4, NFC/NDF = 1.6; T5, NFC/NDF = 1.9. a, b, and c Values for each intestinal segment with different superscripts are significantly different (P < 0.05).
3.6. Effects of dietary NFC/NDF on cecal microbial composition
3.6.1. Phylum level
We compared the microbial communities at the phylum level. The predominant phyla were Firmicutes, Bacteroidetes, Verrucomicrobia, and Actinobacteria accounted for 97.14, 96.01, 95.42, 96.77, and 94.84% for T1, T2, T3, T4, and T5 groups, respectively (Figure 4A, Table 4). Compared with the T2 rabbits, dietary NFC/NDF changed the relative burden of Firmicutes in the T1 (−3.36%; P > 0.05), T3 (-10.11%; P > 0.05), T4 (−12.90%; P > 0.05), and T5 (−26.32%; P < 0.01) groups. The relative burden of Bacteroidetes differed (P = 0.003) between the experimental treatments, displaying linear (Plin = 0.011) and quadratic (Pquad = 0.014) patterns of increase with increasing dietary NFC/NDF level, and the relative burden of Bacteroidetes in T2 rabbits was lower than that in T4 and T5 (P < 0.01) groups. The relative burden of Verrucomicrobia in T5 rabbits was higher than that in other groups (P < 0.01, Plin < 0.001, and Pquad < 0.001). Compared with the T2 rabbits, dietary NFC/NDF changed the relative burden of Actinobacteria in the T1 (−64.11%; P < 0.01), T3 (−28.05%; P < 0.01), T4 (−70.95%; P < 0.01), and T5 (−43.57%; P < 0.01) groups. However, the relative burden of Proteobacteria increased in a linear (Plin < 0.001) and a quadratic (Pquad < 0.001) fashion with increasing dietary NFC/NDF inclusion levels with T4 and T5 being higher than that in T1, T2, and T3 groups. The relative burdens of Patescibacteria, Tenericutes, Cyanobacteria, and Epsilonbacteraeota were all below 1%, and there were significant differences between all groups (P < 0.05).
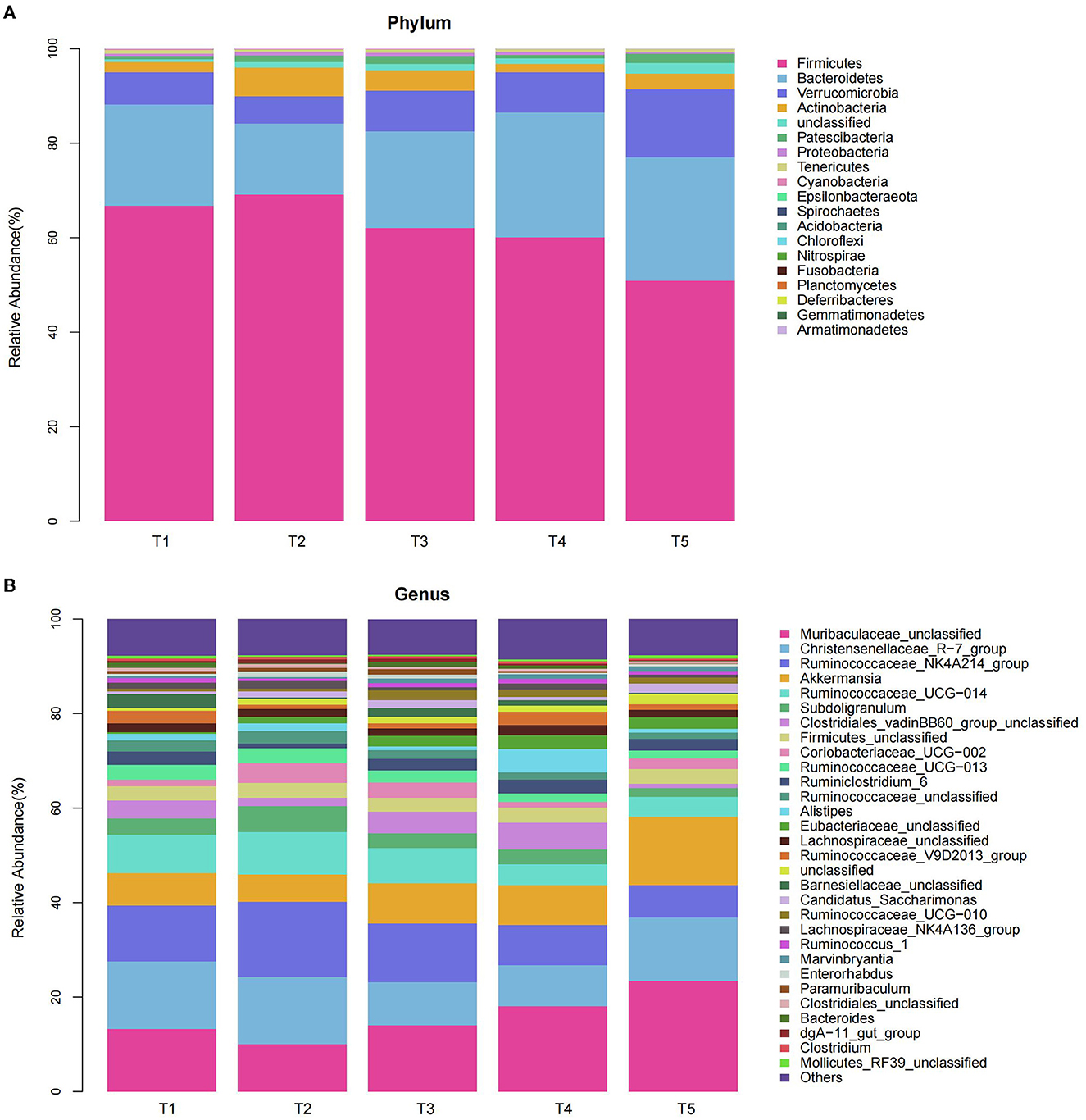
Figure 4. Effects of different ratios of dietary NFC/NDF on the relative abundances of bacterial Phylum (A) and Genus (B) in the cecal microbiota of rabbits. Data are indicated as means ± SEM (n = 6). T1, NFC/NDF = 0.7; T2, NFC/NDF = 1.0; T3, NFC/NDF = 1.3; T4, NFC/NDF = 1.6; T5, NFC/NDF = 1.9.
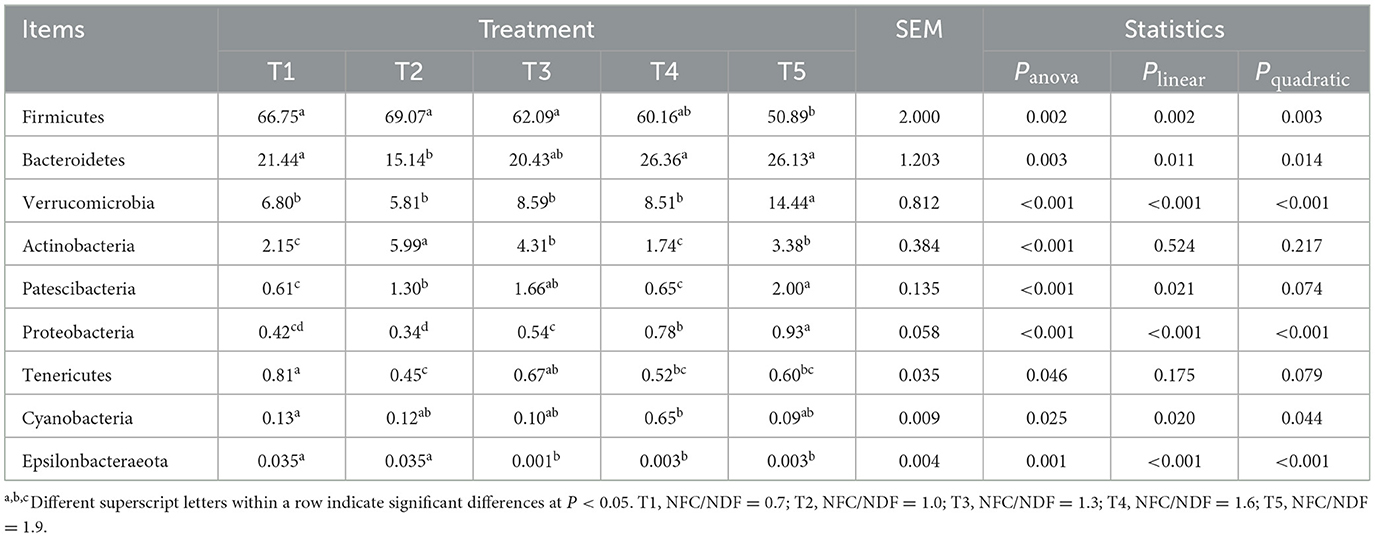
Table 4. Effects of different ratios of dietary NFC/NDF on the distribution of cecal microflora in rabbits at the phylum level %.
3.6.2. Genus level
We analyzed the microbial communities with a relative burden of >1% at the genus level (Figure 4B, Table 5). It was shown that the relative burdens of the Ruminococcus_NK4A214_group, Ruminococcus_UCG-014, and Ruminococcus_UCG-013 in Firmicutes of T2 and T3 rabbits were higher than those of T4 and T5 (P < 0.05). In addition, polynomial contrast analysis showed that the level of the relative burden of Ruminococcus_NK4A214_group (Pquad < 0.001), Ruminococcus_UCG-014 (Pquad < 0.001), Ruminococcus_UCG-013 (Pquad < 0.002), and Ruminococcaceae_unclassified (Pquad < 0.001) displayed a quadratic pattern of decrease with increasing dietary NFC/NDF inclusion level. Compared with the T2 rabbits, dietary NFC/NDF changed the relative burden of Coriobacteriaceae_UCG-002 in the T1 (−66.36%; P < 0.01), T3 (−25.00%; P < 0.01), T4 (−72.17%; P < 0.01), and T5 (−45.75%; P < 0.01) groups. Increasing dietary NFC/NDF inclusion level resulted in a quadratic pattern of increase for the relative burden of Muribaculaceae_unclassified (Pquad < 0.001) and Akkermansia (Pquad < 0.001) and resulted in a linear pattern of increase for the relative burden of Candidatus_Saccharimonas (Plin = 0.024). Meanwhile, these three microbial at T5 were higher than other groups (P < 0.05). In addition, the relative burden of the Subdoligranulum differed (P < 0.001) between the experimental treatments, displaying linear (Plin = 0.006) and quadratic (Pquad = 0.002) patterns of decrease with increasing dietary NFC/NDF levels. Meanwhile, the linear and quadratic patterns of decrease in the relative burden of Lachnospiraceae_NK4A136_group (Plin = 0.010) with increasing dietary NFC/NDF level were noted. Furthermore, both the relative burden of Subdoligranulum and Lachnospiraceae_NK4A136_group reached the highest at T2 and the lowest at T5.

Table 5. Effects of different ratios of dietary NFC/NDF on the distribution of cecal microflora in rabbits at the genus level %.
3.6.3. LDA effect size
The LDA effect size (LEfSe) was mainly used to obtain the final differential species by comparing between the groups. As shown in Figure 5, the most differential groups of cecum microorganisms were found in T2 rabbits and the least in T5 rabbits. A total of nine differential bacterial groups were screened in T2 rabbits for promoting intestinal health and cecum fermentation performance, namely, Clostridiales, Clostridia, Ruminococcaceae, Ruminococcaceae_NK4A214_group, and Ruminococcaceae_ UCG_014 in Firmicutes and Coriobacteriales, Coriobacteria, Coriobacteriaceae_UCG_002, and Eggerthellaceae in Actinobacteria. However, the harmful differential bacteria, Rickettsiales which is symbiotic with the host, are screened in T5 rabbits.

Figure 5. Cladogram of LEfSe multi-level species difference discriminant analysis (LDA > 2), different color nodes indicate microbial communities that are significantly enriched in the corresponding groups and significantly different between groups. Data are indicated as means ± SEM (n = 6). T1, NFC/NDF = 0.7; T2, NFC/NDF = 1.0; T3, NFC/NDF = 1.3; T4, NFC/NDF = 1.6; T5, NFC/NDF = 1.9.
3.7. Correlation analysis of altered cecal bacteria with pH, NH3-N, and VFA
Correlation analysis revealed that the burden of genus Ruminococcaceae_NK4A214_group, Ruminococcaceae_UCG−014, and Ruminococcaceae_UCG−013 was positively correlated with acetate and negatively correlated with propionate (P < 0.01) (Figure 6). Lachnospiraceae_NK4A136_group showed a strong positive correlation with butyrate and a negative correlation with VFA (P < 0.05). The genus Subdoligranulum showed a positive correlation with acetate and butyrate (P < 0.05). The abundance of genus Lachnospiraceae_unclassified and Alistipes was positively correlated with pH and negatively correlated with VFA (P < 0.05); moreover, the relative burden of Alistipes was negatively correlated with NH3-N (P < 0.01). Coriobacteriaceae_UCG−002 showed a positive correlation with VFA, acetate, and butyrate and negatively correlated with pH (P < 0.05). The abundance of genus Candidatus_Saccharimonas was positively correlated with VFA and negatively correlated with pH (P < 0.01). In addition, Muribaculaceae_unclassified and Akkermansia were negatively correlated with acetate and butyrate (P < 0.05).
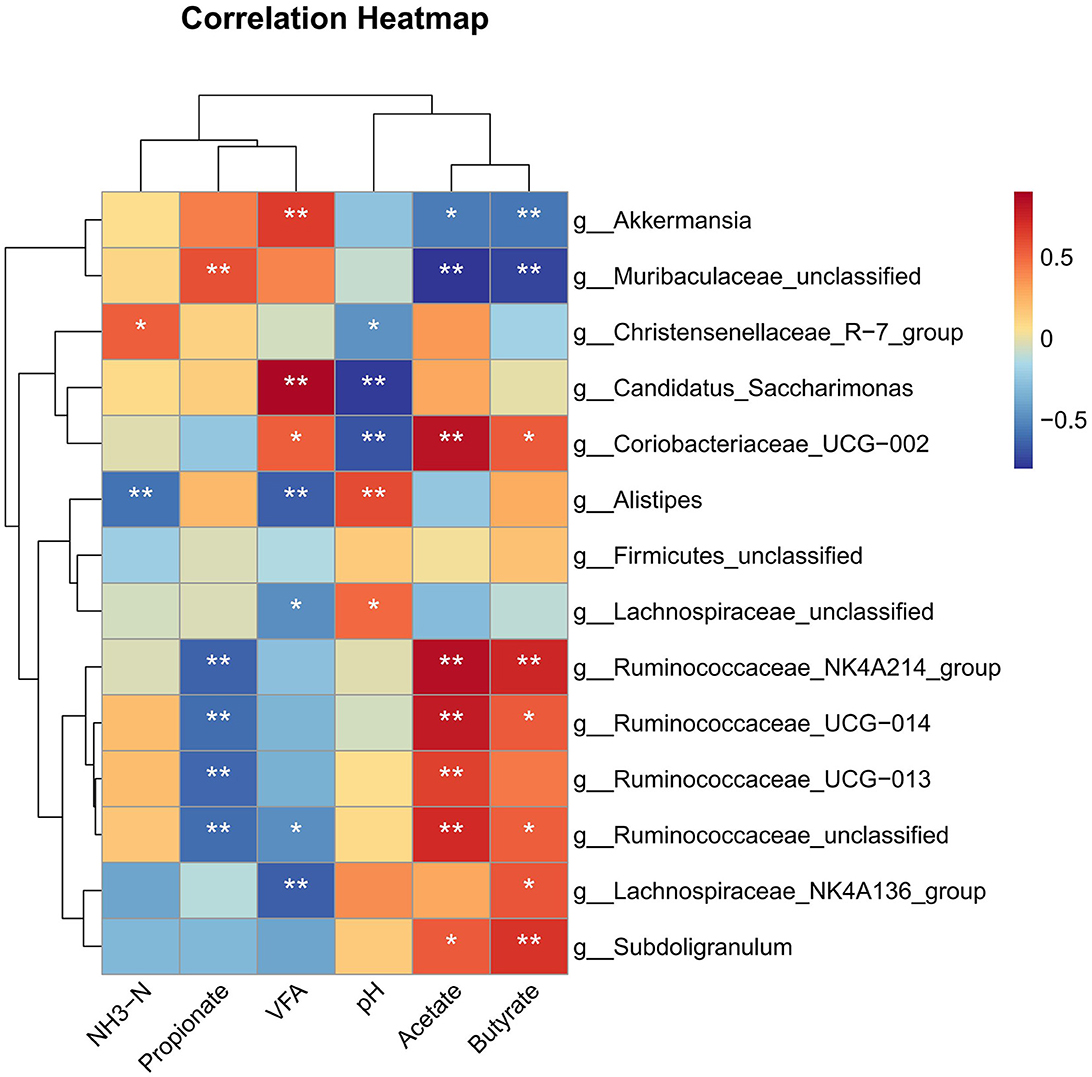
Figure 6. Spearman's rank correlation analysis (SRCA) between significantly modified microbiota, pH, NH3-N, and VFA concentrations of rabbits. *indicates P < 0.05 and **indicates P < 0.01. Red and blue colors represent positive and negative correlations, respectively. T1, NFC/NDF = 0.7; T2, NFC/NDF = 1.0; T3, NFC/NDF = 1.3; T4, NFC/NDF = 1.6; T5, NFC/NDF = 1.9.
4. Discussion
An appropriate level of dietary fiber is essential for animal growth and development, and excess or deficiency of fiber would limit the digestion and absorption of nutrients. We found that the ADG and ADFI of rabbits at NFC/NDF ratio = 0.7~1.3 were higher than those at NFC/NDF = 1.6–1.9, but the F/G, diarrhea rate, and mortality were lower than those at NFC/NDF = 1.6–1.9. A higher concentration of dietary fiber results in lower concentrations of other nutrients, and a compensatory increase in appetite as feedback which will lead to a higher F/G ratio. Meanwhile, the fiber can provide substrate for intestinal fermentation, produce VFA, maintain intestinal pH balance, and ensure normal activities of intestinal microorganisms; beneficial intestinal microorganisms and their fermentation products acting on antigen cells can improve the expression of immunoglobulins and macrophages in the body, thus regulating the immune function of the intestine, and nutritional diarrhea will be relieved. Moreover, NFC contains plant cell contents and the readily fermentable part, which will stimulate normal intestinal peristalsis, making the animals feel full. When dietary NFC is higher, the ADFI of rabbits is generally lower (Ranathunga et al., 2010; Chen et al., 2022).
Interleukins (IL) play an important role in immune cell activation and differentiation. However, high levels of IL may also induce an inflammatory response (Zhang et al., 2022). Il-1β and TNF-α are both pro-inflammatory cytokines, and TNF-α can promote the secretion of IL-1β (Torp et al., 2021). Immune diseases are often treated by blocking the expression of IL-1β clinically (Migliorini et al., 2020). Occludin, Claudins, and ZOs are the most important classes of genes related to intestinal immunity that play important roles in maintaining cell morphology and forming intestinal immunity (Wu et al., 2020; Tang et al., 2021). The expression of these genes will affect the function of the intestinal immunity and barrier. A summary of the main findings of this study is presented in Figure 7, and our results concluded that the expression of IL-1β and TNF-α genes induced by NFC/NDF = 0.7–1.3 was lower than that in NFC/NDF = 1.6–1.9. In parallel, the expression of Claudin-1, Occludin, and ZO-1 was higher at NFC/NDF = 1.0 and 1.3 than those at NFC/NDF = 1.6–1.9. It was found that appropriate levels of dietary fiber increased the numbers of lymphocytes in the rat (Fåk et al., 2015) and upregulated the gene expression of the anti-inflammatory cytokine IL-10 in the jejunum of a mouse, while downregulated the gene expression of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α (Xu et al., 2021). Moreover, appropriate levels of dietary fiber can increase the gene expression of Claudin-1, Occludin, and ZO-1 in the animal intestine (Chen et al., 2019; Beisner et al., 2021; Feng et al., 2022). Therefore, it was believed that intestinal immunity is better at NFC/NDF = 1.0 to 1.3 than that at NFC/NDF = 1.6 to 1.9. This may be due to the fact that dietary fiber can be fermented in the cecum to produce VFA, and meanwhile, it can increase the frequency of intestinal peristalsis and stimulate intestinal epithelial cells to secrete mucus (Mcrorie et al., 2020). Acetate and propionate can induce intestinal goblet cell differentiation and promote mucin synthesis (Yang et al., 2022). Acetate can also counteract TNF-α-induced epithelial barrier damage by regulating the expression of genes involved in the anti-inflammatory response in the intestine (Bach Knudsen et al., 2018; Al-Sadi et al., 2021) and reducing the transfer of virulence factors into the circulatory system (Aw and Fukuda, 2019). In addition, butyrate is the major energy source of intestinal epithelial cells, and it can effectively regulate the expression of intestinal immune factors, reduce intestinal permeability, and benefit intestinal health (Lenoir et al., 2020).
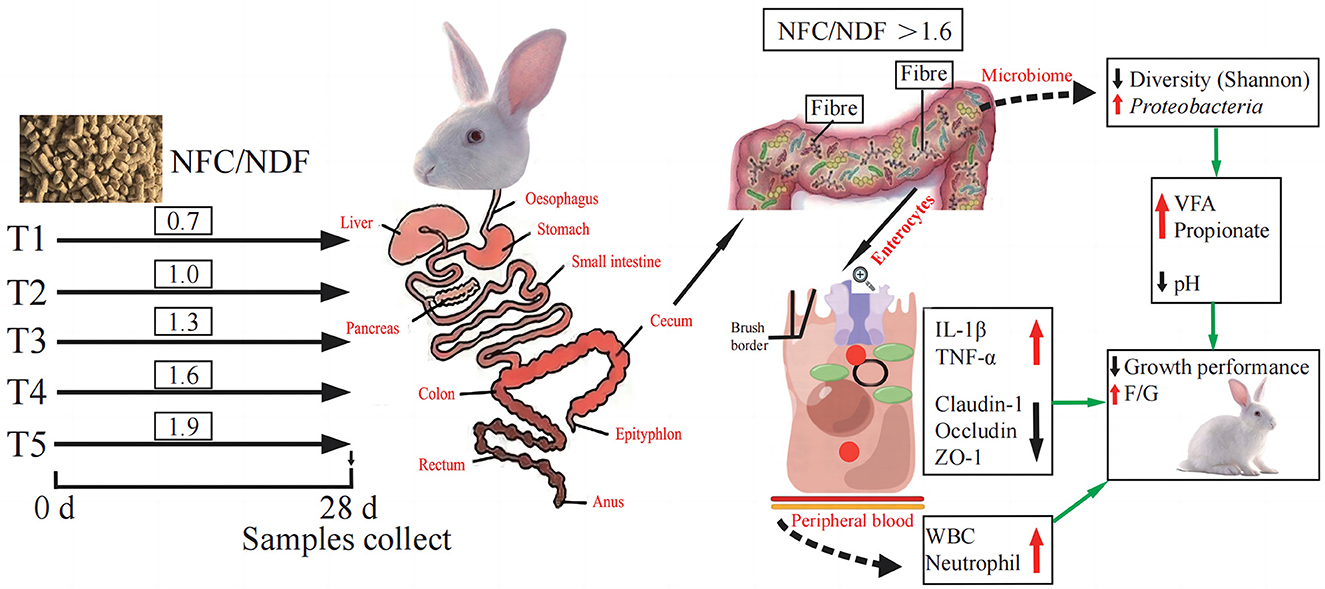
Figure 7. Schematic summary: effect of different ratios of dietary NFC/NDF on intestinal immunity, microbiota, and metabolites in rabbits.
The organic acids regulate the macrophage activities in the intestine and may contribute to intestinal immune and barrier functions (Nouri, 2022). The cecum microorganisms of rabbits can produce VFA by fermenting cellulose and other components (Makki et al., 2018), and then, VFA can be quickly absorbed by the hindgut, providing 40% of the maintenance energy for rabbits (Read et al., 2019). The concentration of VFA in the cecum is an important indicator of the fermentation activity of cecum microorganisms. As presented in Figure 7, in this study, the pH value and acetate in the cecum were lowest at NFC/NDF = 1.9, and the VFA and propionate were highest at NFC/NDF ratio = 1.9. It has been proven in ruminants that excessive organic acids can lead to the occurrence of rumen acidosis, and in this study, we also found that a high NFC ratio can lead to a decrease in the pH of the cecum. Decreasing the dietary NDF can increase the concentration of VFA in the cecum of rabbits, decrease the pH of the cecum, and improve the fermentation pattern of the cecum (Wu et al., 2019; Wang et al., 2022a). Therefore, it is considered that the lower the NFC/NDF in the diet, the higher the fiber contents in the diet, the less the fermentable substances, and the poorer the ability of intestinal fermentation to produce acids, resulting in an increase in the pH of the cecum. However, diets with high levels of NFC/NDF ratio can provide the body with more fermentable carbohydrates, which can be fully fermented in the cecum to produce organic acids. In this case, the content of fiber is reduced, the digestion time is shortened, and the secretion of digestive juices is reduced, thus lowering the pH of the intestine (Gidenne, 2015).
The cecal microbiota is formed in the long-term evolution process and maintains a relatively stable balance with the host (Wassie et al., 2022). The balanced symbiosis between the host and microbiota can maintain animal health and improve performance (Halliday et al., 2019). In this study, according to the Alpha and Beta diversity results, there were significant differences in the cecal microbial diversity of rabbits in each group, indicating that different dietary NFC/NDF ratios would change the cecal microbiota diversity and richness. Both the diversity and richness of microorganisms at the NFC/NDF ratio = 1.9 were lower than other groups. Most studies have shown that animals fed a high-fiber diet have richer gut microbiota than those fed a low-fiber diet (Trompette et al., 2014). The addition of soluble fiber to the diet causes more microbial fermentation, which helps control the colonization of foodborne pathogens and reduces the incidence of diarrhea (Li et al., 2018). Moreover, diets with low fiber can lead to a decrease in the proportion of beneficial bacteria in the intestine (Simpson and Campbell, 2015). The comprehensive analysis shows that different dietary NFC/NDF ratios could affect the cecal microbial diversity of rabbits and change the dynamic balance of cecal microorganisms. The higher the ratio is, the lower the bacterial diversity and richness are. A possible explanation was that with the rising NFC/NDF ratio, NFC will be over-fermented in the cecum and lower the pH value of the cecum, which eventually leads to inhibit the growth of beneficial bacteria that cannot tolerate acidic environments and promote the reproduction of harmful bacteria.
The composition of dietary can affect the composition of the intestinal microbiota in rabbits, and dietary fiber plays an important role in the structure of rabbit microbiota (Pi et al., 2021). Previous studies showed that dietary fiber can improve intestinal function and microbial composition (Sadeghi et al., 2015). This experiment showed that when NFC/NDF ratio = 1.0–1.3, Firmicutes had the higher relative burden and Bacteroidetes had a lower relative burden than that of the other three groups. Firmicutes and Bacteroidetes are the main components of intestinal symbionts (Monteils et al., 2008; Hills et al., 2019), both of which can ferment dietary fat and fiber to produce short-chain fatty acids (Turnbaugh et al., 2008; Wang et al., 2022b). Firmicutes are involved in the absorption of protein and carbohydrates and contain a large number of fiber-degrading bacteria (Holscher, 2017). Bacteroidetes can promote carbohydrate fermentation (Gidenne, 2015). When the dietary NFC/NDF ratio is low, the fiber can be fully fermented in the cecum, which is conducive to the growth and reproduction of cellulolytic bacteria, thereby improving the conversion efficiency of dietary fiber (Ma et al., 2015). Therefore, it is believed that when the dietary NFC/NDF ratio = 1.0–1.3, the environment in the cecum of the rabbits is more conducive to the reproduction of beneficial bacteria and can effectively degrade dietary fiber. The results of the genus level also proved that the NDF with a dietary NFC/NDF ratio = 1.0–1.3 can be fully fermented in the cecum to produce abundant short-chain fatty acids, and then provide a beneficial physiological environment for the cecal microbiota. It is conducive to the survival of beneficial microorganisms such as Ruminococcaceae, Lachnospiraceae, and Coriobacteriaceae in the cecum, which has a positive effect on the balance of intestinal flora. In addition, we found that when the NFC/NDF ratio = 1.9, the relative burden of Proteobacteria was higher than that of other groups, and the relative burden of Actinobacteria was lower than that of other groups. It has been reported that Proteobacteria, including Escherichia coli and Salmonella, can easily cause intestinal inflammation in animals, followed by diarrhea (Shin et al., 2015). Actinobacteria mainly exist in the digestive of herbivores and can promote digestion (Chen et al., 2016). This experiment shows that when the dietary NFC/NDF ratio = 1.9, the burden of harmful bacteria in the intestine will increase, which is a serious threat to intestinal health.
We found that the pH value and acetate concentration in the cecum of rabbits decreased, while the propionate concentration increased with the increase in the ratio, as the dietary NFC/NDF increased. Ruminococcaceae, one of the most abundant genera of Firmicutes, which is also a producer of VFA, can effectively degrade fibers (Holscher, 2017; Dou et al., 2022). The results showed that the relative burden of Ruminococcaceae_NK4A214_group, Ruminococcaceae_UCG-014, and Ruminococcaceae_UCG-013 was positively correlated with the concentration of acetate but negatively correlated with the concentration of propionate. It shows that the high dietary NFC/NDF ratio leads to the reduction of the relative abundance of Ruminococcaceae, the weakened ability to degrade fiber, and the reduction of the ability to produce acetate (Morrison and Miron, 2000). The Christensenellaceae_R-7_group is present in the gut microbiota of healthy hosts (Li et al., 2020). The results of this experiment showed that the relative burden of the Christensenellaceae_R-7_group was positively correlated with acetate and NH3-N. The Christensenellaceae_R-7_group belongs to Firmicutes and can improve the degradation of structural polysaccharides in the rumen. The genome of Firmicutes can encode glycoside hydrolase genes that degrade hemicellulose (Solden et al., 2018), and then maintain the health of the host by regulating the homeostasis of the intestinal environment. It is a short-chain fatty acid-producing bacteria that resist the invasion of pathogenic bacteria. Elevated acetate concentration causes an increased relative burden of the Christensenellaceae_R-7_group (Li et al., 2020). Previous studies have found that intestinal flora can provide nutrition and energy to the host by metabolizing carbohydrates and that Lachnospiraceae can break down straight-chain starch and the alpha-1,4 glycosidic bond in straight-chain starch to help animals utilize polysaccharides and fibrous material (Van Treuren and Dodd, 2020). This study showed that the relative burden of the Lachnospiraceae_NK4A136_group was positively correlated with butyrate (Cockburn et al., 2018). In addition, studies have confirmed that Lachnospiraceae can participate in the fermentation of fibrous substances or polysaccharides, and the main products are VFA such as butyric acid (Vacca et al., 2020), which is consistent with our results. From this, we conclude that cecal metabolites are determined by the degradation of fibrous and starch, and are positively associated with fermentation-related phenotypes and the burden of Firmicutes (Li et al., 2020).
5. Conclusion
Different dietary NFC/NDF can affect intestinal immune responses and the growth performance of rabbits. When dietary NFC/NDF = 1.0–1.3, the relative burden of Firmicutes was higher, which is more conducive to the decomposition of dietary fiber and the production of VFA. Dietary NFC/NDF = 1.0–1.3 can also improve the cecum microbial diversity of rabbits, which has a positive effect on the balance of intestinal flora. Importantly, it was also found that the cecal metabolites were determined by the degradation of fibrous and starch, which were positively associated with fermentation-related phenotypes and the burden of Firmicutes. Collectively, the mechanism may be associated with the appropriate NFC/NDF ratio, improving metabolites parameters by modulating gut microbiota.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI Sequence Read Archive (SRA) database, https://www.ncbi.nlm.nih.gov/sra, PRJNA913458.
Ethics statement
The animal study was reviewed and approved by all experimental procedures were approved by the Ethics Committee of Animal Experimentation of Hebei Agricultural University (Protocol 2021083).
Author contributions
SL, TL, and BC: conceptualization. SL, TL, and KW: methodology, investigation, software, data curation, and writing—original draft preparation. KW and CL: validation and resources. TL and CL: formal analysis. XC and BC: writing review, editing, and supervision. BC: project administration and funding acquisition. All authors have agreed to the final manuscript.
Funding
This research was funded by the Modern Agriculture Industry Technology System of Rabbit (CARS-43-B-2).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1146787/full#supplementary-material
References
Al-Sadi, R., Dharmaprakash, V., Nighot, P., Guo, S., Nighot, M., Do, T., et al. (2021). Bifidobacterium bifidum enhances the intestinal epithelial tight junction barrier and protects against intestinal inflammation by targeting the toll-like receptor-2 pathway in an NF-κB-independent manner. Int. J. Mol. Sci. 22, 8070. doi: 10.3390/ijms22158070
Association, A.P.O.E.a.V.M. (2001). 2000. Report of the AVMA panel on euthanasia. J. Am. Vet. Med. Assoc. 218, 669–696. doi: 10.2460/javma.2001.218.669
Aw, W., and Fukuda, S. (2019). Protective effects of bifidobacteria against enteropathogens. Microb. Biotechnol. 12, 1097–1100. doi: 10.1111/1751-7915.13460
Bach Knudsen, K. E., Lærke, H. N., Hedemann, M. S., Nielsen, T. S., Ingerslev, A. K., Gundelund Nielsen, D. S., et al. (2018). Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10, 1499. doi: 10.3390/nu10101499
Beisner, J., Filipe Rosa, L., Kaden-Volynets, V., Stolzer, I., Günther, C., Bischoff, S. C., et al. (2021). Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 12, 678360. doi: 10.3389/fimmu.2021.678360
Che, D., Adams, S., Wei, C., Gui-Xin, Q., Atiba, E. M., Hailong, J., et al. (2019). Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiologyopen 8, e00712. doi: 10.1002/mbo3.712
Chen, L., Zhang, H., Liu, G., and Sha, W. (2016). First report on the bacterial diversity in the distal gut of dholes (Cuon alpinus) by using 16S rRNA gene sequences analysis. J. Appl. Genet. 57, 275–283. doi: 10.1007/s13353-015-0319-0
Chen, T., Chen, D., Tian, G., Zheng, P., Mao, X., Yu, J., et al. (2019). Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model. Biomed. Res. Int. 2019, 7809171. doi: 10.1155/2019/7809171
Chen, Y., Gong, X., Huang, Y., Jiang, M., Zhan, K., Lin, M., et al. (2022). Growth performance, rumen fermentation and inflammatory response on holstein growing cattle treated with low and high non-fibrous carbohydrate to neutral detergent fiber ratio pelleted total mixed ration. Animals 12, 1306. doi: 10.3390/ani12081036
Cockburn, D. W., Suh, C., Medina, K. P., Duvall, R. M., Wawrzak, Z., Henrissat, B., et al. (2018). Novel carbohydrate binding modules in the surface anchored α-amylase of Eubacterium rectale provide a molecular rationale for the range of starches used by this organism in the human gut. Mol. Microbiol. 107, 249–264. doi: 10.1111/mmi.13881
National Research Council (1977). Nutrient Requirements of Rabbits: Second Revised Edition, 1977. Washington, DC: The National Academies Press.
Dou, Y., Yu, X., Luo, Y., Chen, B., Ma, D., Zhu, J., et al. (2022). Effect of fructooligosaccharides supplementation on the gut microbiota in human: a systematic review and meta-analysis. Nutrients 14, 3298. doi: 10.3390/nu14163298
Eberl, G. (2010). A new vision of immunity: homeostasis of the superorganism. Mucosal Immunol. 3, 450–460. doi: 10.1038/mi.2010.20
Fåk, F., Jakobsdottir, G., Kulcinskaja, E., Marungruang, N., Matziouridou, C., Nilsson, U., et al. (2015). The physico-chemical properties of dietary fibre determine metabolic responses, short-chain Fatty Acid profiles and gut microbiota composition in rats fed low- and high-fat diets. PLoS ONE 10, e0127252. doi: 10.1371/journal.pone.0127252
Feng, Z., Zhong, Y., He, G., Sun, H., Chen, Y., Zhou, W., et al. (2022). Yeast culture improved the growth performance, liver function, intestinal barrier and microbiota of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 120, 706–715. doi: 10.1016/j.fsi.2021.12.034
Gidenne, T. (2015). Dietary fibres in the nutrition of the growing rabbit and recommendations to preserve digestive health: a review. Animal 9, 227–242. doi: 10.1017/S1751731114002729
Goulart, R. S., Vieira, R. A., Daniel, J. L., Amaral, R. C., Santos, V. P., Toledo Filho, S. G., et al. (2020). Effects of source and concentration of neutral detergent fiber from roughage in beef cattle diets on feed intake, ingestive behavior, and ruminal kinetics. J. Anim. Sci. 98, skaa107. doi: 10.1093/jas/skaa107
Guan, Z. W., Yu, E. Z., and Feng, Q. (2021). Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 26, 6802. doi: 10.3390/molecules26226802
Hall, M. B. (2003). Challenges with non-fiber carbohydrate methods. J. Anim. Sci. 81, 3226–3232. doi: 10.2527/2003.81123226x
Halliday, M. J., Giles, H. E., Padmanabha, J., Mcsweeney, C. S., Dalzell, S. A., Shelton, H. M., et al. (2019). The efficacy of a cultured Synergistes jonesii inoculum to control hydroxypyridone toxicity in Bos indicus steers fed leucaena/grass diets. Animal Prod. Sci. 4, 696–708. doi: 10.1071/AN17853
Hernández-Castellano, L. E., Hernandez, L. L., and Bruckmaier, R. M. (2020). Review: endocrine pathways to regulate calcium homeostasis around parturition and the prevention of hypocalcemia in periparturient dairy cows. Animal 14, 330–338. doi: 10.1017/S1751731119001605
Hills, R. D. Jr., Pontefract, B.A., Mishcon, H.R., Black, C.A., Sutton, S.C., and Theberge, C.R. (2019). Gut microbiome: profound implications for diet and disease. Nutrients 11, 1613. doi: 10.3390/nu11071613
Holscher, H. D. (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8, 172–184. doi: 10.1080/19490976.2017.1290756
Iliev, I. D., and Cadwell, K. (2021). Effects of intestinal fungi and viruses on immune responses and inflammatory bowel diseases. Gastroenterology 160, 1050–1066. doi: 10.1053/j.gastro.2020.06.100
Jin, D. X., Zou, H. W., Liu, S. Q., Wang, L. Z., Xue, B., Wu, D., et al. (2018). The underlying microbial mechanism of epizootic rabbit enteropathy triggered by a low fiber diet. Sci. Rep. 8, 12489. doi: 10.1038/s41598-018-30178-2
Khattab, I. M., Abdel-Wahed, A. M., Anele, U. Y., Sallam, S. M., and El-Zaiat, H. M. (2021). Comparative digestibility and rumen fermentation of camels and sheep fed different forage sources. Anim. Biotechnol. 10, 1–10. doi: 10.1080/10495398.2021.1990939
Lenoir, M., Martín, R., Torres-Maravilla, E., Chadi, S., González-Dávila, P., Sokol, H., et al. (2020). Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 12, 1–16. doi: 10.1080/19490976.2020.1826748
Li, J., Xue, M., Zhang, L., Li, L., Lian, H., Li, M., et al. (2022). Integration of long non-coding RNA and mRNA profiling reveals the mechanisms of different dietary NFC/NDF ratios induced rumen development in calves. Animals 12, 650. doi: 10.3390/ani12050650
Li, Y., Lv, M., Wang, J., Tian, Z., Yu, B., Wang, B., et al. (2020). Dandelion (Taraxacum mongolicum Hand.-Mazz.) supplementation-enhanced rumen fermentation through the interaction between ruminal microbiome and metabolome. Microorganisms 9, 83. doi: 10.3390/microorganisms9010083
Li, Y., Yang, H., Xu, L., Wang, Z., Zhao, Y., Chen, X., et al. (2018). Effects of dietary fiber levels on cecal microbiota composition in geese. Asian Aust. J. Anim. Sci. 31, 1285–1290. doi: 10.5713/ajas.17.0915
Ma, T., Tu, Y., Zhang, N. F., Deng, K. D., and Diao, Q. Y. (2015). Effect of the ratio of non-fibrous carbohydrates to neutral detergent fiber and protein structure on intake, digestibility, rumen fermentation, and nitrogen metabolism in lambs. Asian Aust. J. Anim. Sci. 28, 1419–1426. doi: 10.5713/ajas.15.0025
Makki, K., Deehan, E. C., Walter, J., and Bäckhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715. doi: 10.1016/j.chom.2018.05.012
Mcrorie, J. W. Jr., Fahey, G.C. Jr, Gibb, R.D., and Chey, W.D. (2020). Laxative effects of wheat bran and psyllium: resolving enduring misconceptions about fiber in treatment guidelines for chronic idiopathic constipation. J. Am. Assoc. Nurse Pract. 32, 15–23. doi: 10.1097/JXX.0000000000000346
Migliorini, P., Italiani, P., Pratesi, F., Puxeddu, I., and Boraschi, D. (2020). The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 19, 102617. doi: 10.1016/j.autrev.2020.102617
Monteils, V., Cauquil, L., Combes, S., Godon, J. J., and Gidenne, T. (2008). Potential core species and satellite species in the bacterial community within the rabbit caecum. FEMS Microbiol. Ecol. 66, 620–629. doi: 10.1111/j.1574-6941.2008.00611.x
Morrison, M., and Miron, J. (2000). Adhesion to cellulose by ruminococcus albus: a combination of cellulosomes and Pil-proteins? FEMS Microbiol. Lett. 185, 109–115. doi: 10.1111/j.1574-6968.2000.tb09047.x
Nouri, A. (2022). Anticoccidial and immunogenic effectivity of encapsulated organic acids and anticoccidial drugs in broilers infected with Eimeria spp. Sci. Rep. 12, 17060. doi: 10.1038/s41598-022-20990-2
Pi, Y., Hu, J., Bai, Y., Wang, Z., Wu, Y., Ye, H., et al. (2021). Effects of dietary fibers with different physicochemical properties on fermentation kinetics and microbial composition by fecal inoculum from lactating sows in vitro. J. Sci. Food Agric. 101, 907–917. doi: 10.1002/jsfa.10698
Pu, X., Guo, X., Shahzad, K., Wang, M., Jiang, C., Liu, J., et al. (2020). Effects of dietary non-fibrous carbohydrate (NFC) to neutral detergent fiber (NDF) ratio change on rumen bacteria in sheep based on three generations of full-length amplifiers sequencing. Animals 10, 192. doi: 10.3390/ani10020192
Ranathunga, S. D., Kalscheur, K. F., Hippen, A. R., and Schingoethe, D. J. (2010). Replacement of starch from corn with non-forage fiber from distillers grains and soyhulls in diets of lactating dairy cows. J. Dairy Sci. 93, 1086–1097. doi: 10.3168/jds.2009-2332
Read, T., Fortun-Lamothe, L., Pascal, G., Le Boulch, M., Cauquil, L., Gabinaud, B., et al. (2019). Diversity and co-occurrence pattern analysis of cecal microbiota establishment at the onset of solid feeding in young rabbits. Front. Microbiol. 10, 973. doi: 10.3389/fmicb.2019.00973
Sadeghi, A., Toghyani, M., and Gheisari, A. (2015). Effect of various fiber types and choice feeding of fiber on performance, gut development, humoral immunity, and fiber preference in broiler chicks. Poult. Sci. 94, 2734–2743. doi: 10.3382/ps/pev292
Shang, Q., Ma, X., Liu, H., Liu, S., and Piao, X. (2020). Effect of fibre sources on performance, serum parameters, intestinal morphology, digestive enzyme activities and microbiota in weaned pigs. Arch. Anim. Nutr. 74, 121–137. doi: 10.1080/1745039X.2019.1684148
Shin, N. R., Whon, T. W., and Bae, J. W. (2015). Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503. doi: 10.1016/j.tibtech.2015.06.011
Simpson, H. L., and Campbell, B. J. (2015). Review article: dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 42, 158–179. doi: 10.1111/apt.13248
Solden, L. M., Naas, A. E., Roux, S., Daly, R. A., Collins, W. B., Nicora, C. D., et al. (2018). Interspecies cross-feeding orchestrates carbon degradation in the rumen ecosystem. Nat. Microbiol. 3, 1274–1284. doi: 10.1038/s41564-018-0225-4
Tang, M., Yuan, D., and Liao, P. (2021). Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 289, 117865. doi: 10.1016/j.envpol.2021.117865
Torp, C. K., Brüner, M., Keller, K. K., Brouwer, E., Hauge, E. M., Mcgonagle, D., et al. (2021). Vasculitis therapy refines vasculitis mechanistic classification. Autoimmun. Rev. 20, 102829. doi: 10.1016/j.autrev.2021.102829
Trompette, A., Gollwitzer, E. S., Yadava, K., Sichelstiel, A. K., Sprenger, N., Ngom-Bru, C., et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166. doi: 10.1038/nm.3444
Turnbaugh, P. J., Bäckhed, F., Fulton, L., and Gordon, J. I. (2008). Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223. doi: 10.1016/j.chom.2008.02.015
Vacca, M., Celano, G., Calabrese, F. M., Portincasa, P., Gobbetti, M., de Angelis, M., et al. (2020). The controversial role of human gut lachnospiraceae. Microorganisms 8, 573. doi: 10.3390/microorganisms8040573
Van Treuren, W., and Dodd, D. (2020). Microbial contribution to the human metabolome: implications for health and disease. Annu. Rev. Pathol. 15, 345–369. doi: 10.1146/annurev-pathol-020117-043559
Wang, J., Zhao, K., Kang, Z., Wang, M., Chen, Y., Fan, H., et al. (2022a). The multi-omics analysis revealed a metabolic regulatory system of cecum in rabbit with diarrhea. Animals 12, 1194. doi: 10.3390/ani12091194
Wang, X., Li, L., Bai, M., Zhao, J., Sun, X., Gao, Y., et al. (2022b). Dietary supplementation with Tolypocladium sinense mycelium prevents dyslipidemia inflammation in high fat diet mice by modulation of gut microbiota in mice. Front. Immunol. 13, 977528. doi: 10.3389/fimmu.2022.977528
Wassie, T., Cheng, B., Zhou, T., Gao, L., Lu, Z., Xie, C., et al. (2022). Microbiome-metabolome analysis reveals alterations in the composition and metabolism of caecal microbiota and metabolites with dietary Enteromorpha polysaccharide and Yeast glycoprotein in chickens. Front. Immunol. 13, 6125. doi: 10.3389/FIMMU.2022.996897/BIBTEX
Weatherburn, M. W. (1967). Phenol hipochlorite reaction for determination of ammonia. Anal. Chem. 39, 971–974. doi: 10.1021/ac60252a045
Wu, X., Gao, L. M., Liu, Y. L., Xie, C., Cai, L., Xu, K., et al. (2020). Maternal dietary uridine supplementation reduces diarrhea incidence in piglets by regulating the intestinal mucosal barrier and cytokine profiles. J. Sci. Food Agric. 100, 3709–3718. doi: 10.1002/JSFA.10410
Wu, Z., Zhou, H., Li, F., Zhang, N., and Zhu, Y. (2019). Effect of dietary fiber levels on bacterial composition with age in the cecum of meat rabbits. Microbiologyopen 8, e00708. doi: 10.1002/mbo3.708
Xu, Y., Xie, L., Tang, J., He, X., Zhang, Z., Chen, Y., et al. (2021). Morchella importuna flavones improve intestinal integrity in dextran sulfate sodium-challenged mice. Front. Microbiol. 12, 742033. doi: 10.3389/fmicb.2021.742033
Xue, B., Wu, M., Yue, S., Hu, A., Li, X., Hong, Q., et al. (2022). Changes in rumen bacterial community induced by the dietary physically effective neutral detergent fiber levels in goat diets. Front. Microbiol. 13, 820509. doi: 10.3389/fmicb.2022.820509
Xue, M., Wang, K., Wang, A., Li, R., Wang, Y., Sun, S., et al. (2019). MicroRNA sequencing reveals the effect of different levels of non-fibrous carbohydrate/neutral detergent fiber on rumen development in calves. Animals 9, 496. doi: 10.3390/ani9080496
Yang, Z., Liu, X., Wu, Y., Peng, J., and Wei, H. (2022). Effect of the microbiome on intestinal innate immune development in early life and the potential strategy of early intervention. Front. Immunol. 13, 936300. doi: 10.3389/fimmu.2022.936300
Ye, X., Zhou, L., Zhang, Y., Xue, S., Gan, Q. F., Fang, S., et al. (2021). Effect of host breeds on gut microbiome and serum metabolome in meat rabbits. BMC Vet. Res. 17, 24. doi: 10.1186/s12917-020-02732-6
Zhang, Y., Duan, X., Wassie, T., Wang, H. H., Li, T., Xie, C., et al. (2022). Enteromorpha prolifera polysaccharide-zinc complex modulates the immune response and alleviates LPS-induced intestinal inflammation via inhibiting the TLR4/NF-κB signaling pathway. Food Funct. 13, 52–63. doi: 10.1039/D1FO02171K
Zheng, D., Liwinski, T., and Elinav, E. (2020). Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506. doi: 10.1038/s41422-020-0332-7
Zhu, Y., Sun, Y., Wang, C., and Li, F. (2017). Impact of dietary fibre:starch ratio in shaping caecal archaea revealed in rabbits. J. Anim. Physiol. Anim. Nutr. 101, 635–640. doi: 10.1111/jpn.12585
Keywords: NFC/NDF, rabbit, intestinal immunity, metabolites, volatile fatty acids, propionate, gut microbiota
Citation: Li S, Liu T, Wang K, Li C, Wu F, Yang X, Zhao M, Chen B and Chen X (2023) The ratios of dietary non-fibrous carbohydrate (NFC) to neutral detergent fiber (NDF) influence intestinal immunity of rabbits by regulating gut microbiota composition and metabolites. Front. Microbiol. 14:1146787. doi: 10.3389/fmicb.2023.1146787
Received: 17 January 2023; Accepted: 20 March 2023;
Published: 20 April 2023.
Edited by:
Xin Wu, Chinese Academy of Sciences, ChinaReviewed by:
Fanlin Kong, China Agricultural University, ChinaYangchun Cao, Northwest A&F University, China
Copyright © 2023 Li, Liu, Wang, Li, Wu, Yang, Zhao, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Chen, eGNoZW4yQGd6dS5lZHUuY24=; Baojiang Chen, Y2hlbmJhb2ppYW5nQHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work
 Shuo Li1,2†
Shuo Li1,2† Tingting Liu
Tingting Liu Chong Li
Chong Li Baojiang Chen
Baojiang Chen Xiang Chen
Xiang Chen