- 1Division of Plant Pathology, Indian Agricultural Research Institute, New Delhi, India
- 2CPGSAS, CAU (Imphal) Umiam, Imphal, India
- 3Department of Plant Pathology, College of Horticulture Bagalkot, Bagalkot, Karnataka, India
- 4Krishi Vigyan Kendra, Khowai, Tripura, India
- 5Department of Plant Pathology, Assam Agricultural University, Jorhat, Assam, India
- 6Krishi Vigyan Kendra-Kamrup, Azara, Assam Agricultural University, Guwahati, Assam, India
- 7College of Agriculture, Navsari Agricultural University, Bharuch, Gujarat, India
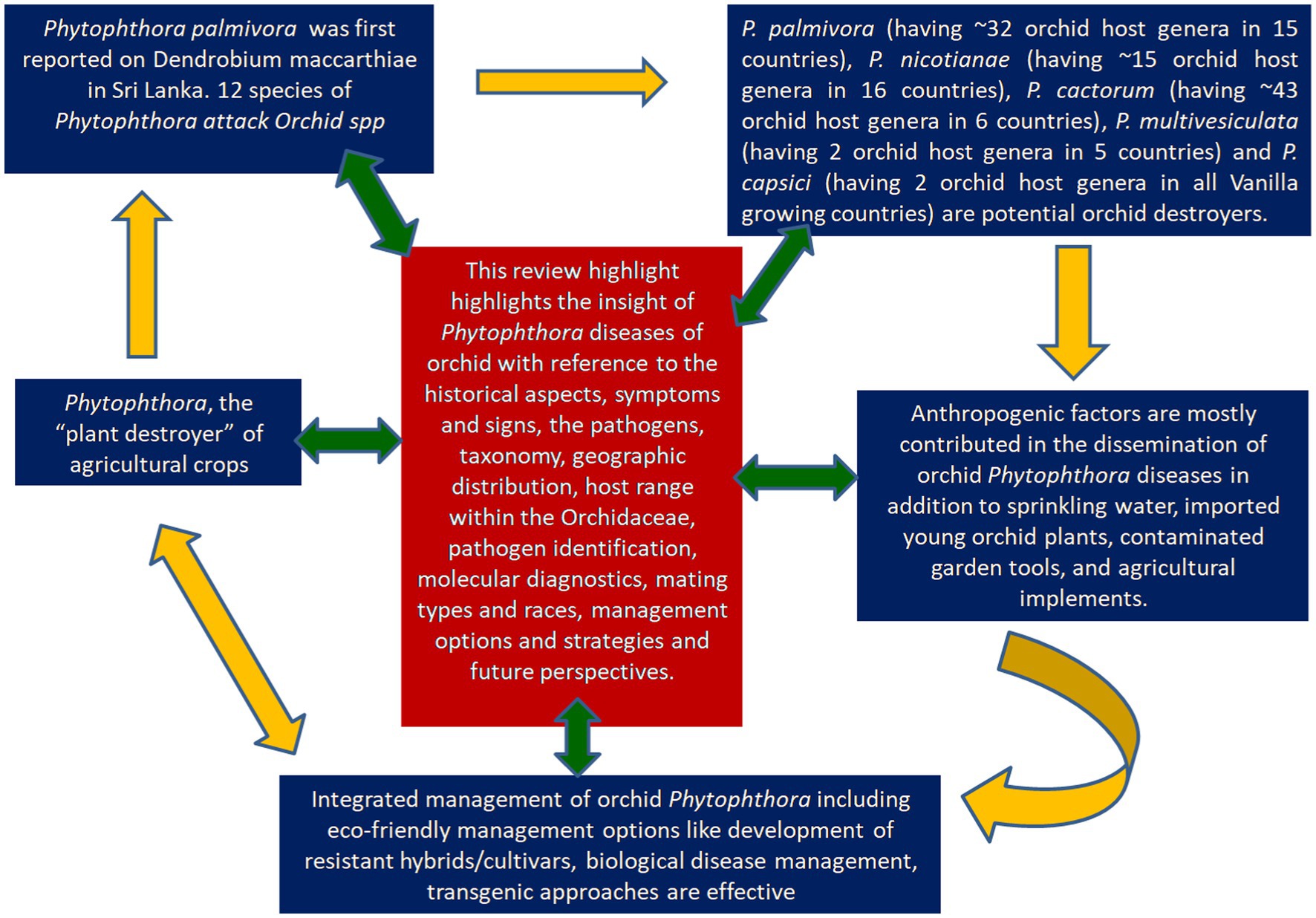
Anton de Bary first coined the genus, Phytophthora, which means “plant destroyer”, viewing its devastating nature on potatoes. Globally plants have faced enormous threat from Phytophthora since its occurrence. In fact, a century ago, Phytophthorapalmivora was first reported on Dendrobium maccarthiae in Sri Lanka. Since then, members of beautiful flowering crops of the family Orchidaceae facing the destructive threat of Phytophthora. Several Phytophthora species have been recorded to infect orchids with economic loss worldwide. To date, orchids are attacked by 12 species of Phytophthora. Five Phytophthora species (P. palmivora, P. nicotianae, P. cactorum, P. multivesiculata, P. meadii) are the major pathogenic Oomycetous Chromista” rather than true fungi frequently occurred on Orchidaceae. Phytophthora palmivora (having ~32 orchid host genera in 15 countries), Phytophthora nicotianae (having ~15 orchid host genera in 16 countries), Phytophthora cactorum (having ~43 orchid host genera in 6 countries), Phytophthora multivesiculata (having 2 orchid host genera in 5 countries) and Phytophthora capsici (having 2 orchid host genera in all Vanilla growing countries) are potential destroyers of Orchidaceae. Most of them are water loving Oomycetes cause disease in moist environments (> 80% RH) at 16–28°C. In artificially constructed orchidaria, anthropogenic factors are mostly contributed to the dissemination Phytophthora diseases in addition to many other factors. Water management, clean cultivation, and agro-chemicals are the major options for effective management of orchid Phytophthora, as the eco-friendly management options like development of resistant hybrids/cultivars, biological disease management, transgenic approaches, RNAi technology remained in the infant stage. In this review, we intended to highlight the insight of Phytophthora diseases associated with the orchid disease with reference to the historical aspect of the diseases, symptoms and signs, the pathogens, taxonomy, geographic distribution, host range within the Orchidaceae, pathogen identification, molecular diagnostics, mating types and races, management options and strategies and future perspectives.
Introduction
Orchids are economically highly valued ornamental flowering plants belonging to one of the largest plant families, Orchidaceae. These are traded commercially across the globe in the form of ornamental plants, cut flowers, and potted plants (Hinsley et al., 2018). As per the recent report, the total number of species is approximately 29,199 (Govaerts et al., 2017), and approximately 1, 25, 000 hybrid orchids are registered in the world (Jangyukala and Hemanta, 2021). They exhibit huge diversity with respect to the size, shape, and color of the flower. Furthermore, these are also popular for their durability and exquisite appearance. The value of the global orchid industry is approximately 400 billion US dollars (DITP News, 2015).
The advancement in commercial orchid production technology has increased the trade of orchid cut-flowers and potted plants several times around the world. However, the commercial production of orchids is challenged by an array of biotic as well as abiotic stresses across the globe. The biotic stresses comprise thrips, scale insects, mites, cockroaches, snails, slugs, nematodes, bacteria, fungi, and viruses, which infect the crop at different stages of production (Jensen, 1959; Farr et al., 1989; Hu et al., 1993; Uchida and Sipes, 1998). The two most important bacterial species infecting orchids are Acidovorax avenae subsp. cattleyae (Syn: Pseudomonas cattleyae), inciting bacterial brown spot disease (Miller, 1990), and Pectobacterium chrysanthemi (Syn: Erwinia chrysanthemi), inciting bacterial soft rot (Chan et al., 2005; Cating et al., 2008). Among the fungal diseases, black rot caused by species of Phytophthora and Pythium, anthracnose caused by species of Colletotrichum, Fusarium rots (Foster, 1955; Srivastava et al., 2018), flower spot (Botrytis sp.), and rusts are considered to be important. Major black rot caused by Phytophthora is known to be one of the most deadly diseases in orchids. The very name Phytophthora was described as a plant destructor or plant destroyer by de Bary (1876). There have been 116 species in total in the genus Phytophthora, infecting a range of crops across the globe. Several Phytophthora species have been recorded to inflict economic losses worldwide by the infection of orchids (Uchida, 1994; Erwin and Ribeiro, 1996; Orlikowski and Szkuta, 2006; Cating et al., 2009). Although the nature of the damage and economic losses depend on the growth stages and organs of infected orchid crops, global data on the intensity of the diseases in different countries and the actual losses incurred by different Phytophthora diseases on orchids have remained obscure. Furthermore, consolidated information on various aspects of the destructive Phytophthora diseases on orchids is lacking in the literature. This review aimed to highlight recent developments in Phytophthora diseases associated with orchids regarding symptoms and signs, the pathogens, taxonomy, geographic distribution, host range within the Orchidaceae, and management strategies, along with future perspectives.
Historical perspective of Phytophthora species infecting Orchidaceae
There are several species of Phytophthora recorded among the various members of the family Orchidaceae. However, a brief historical aspect of important species of Phytophthora infecting orchids is enumerated below:
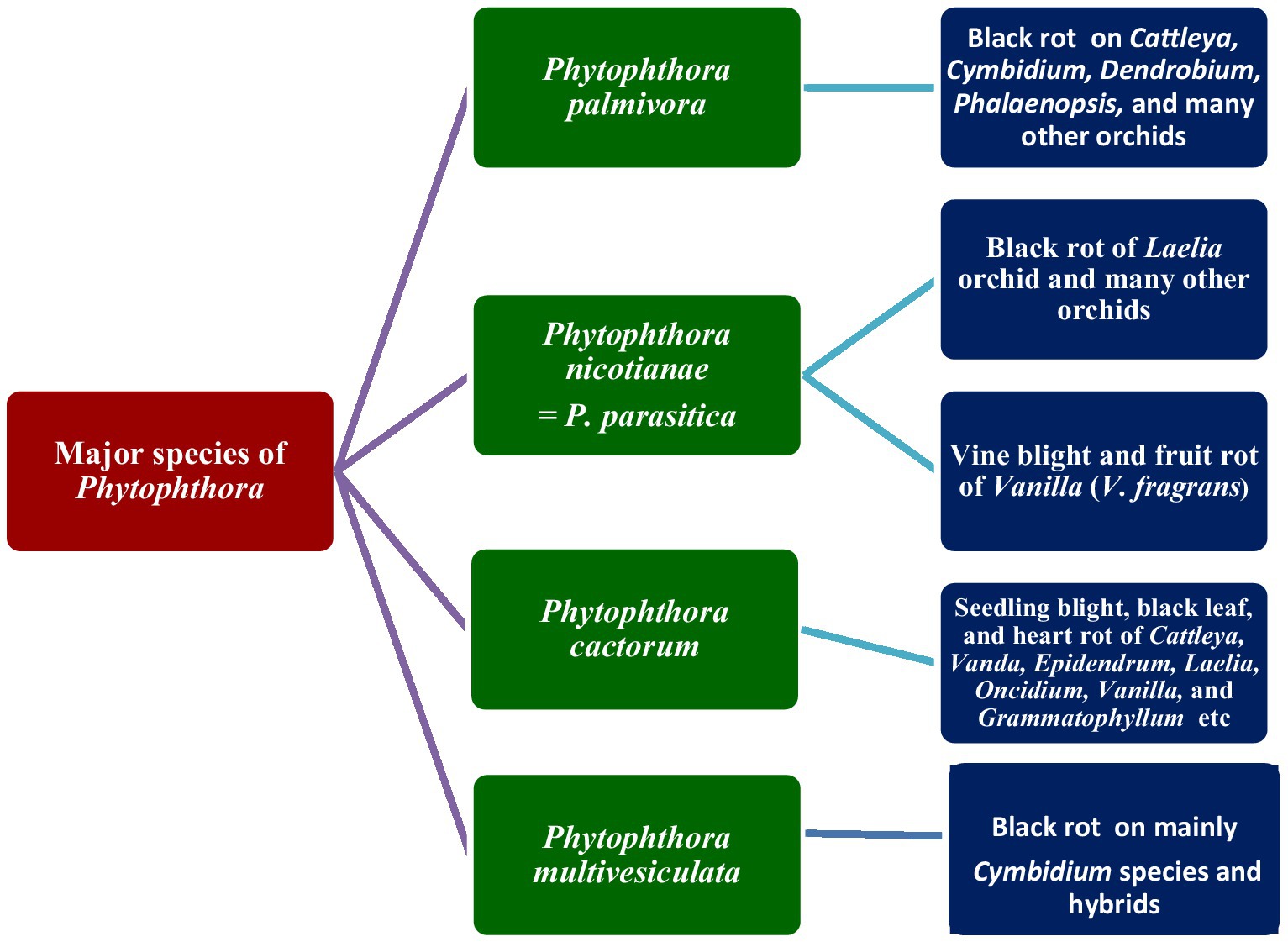
Phytophthora palmivora Butler
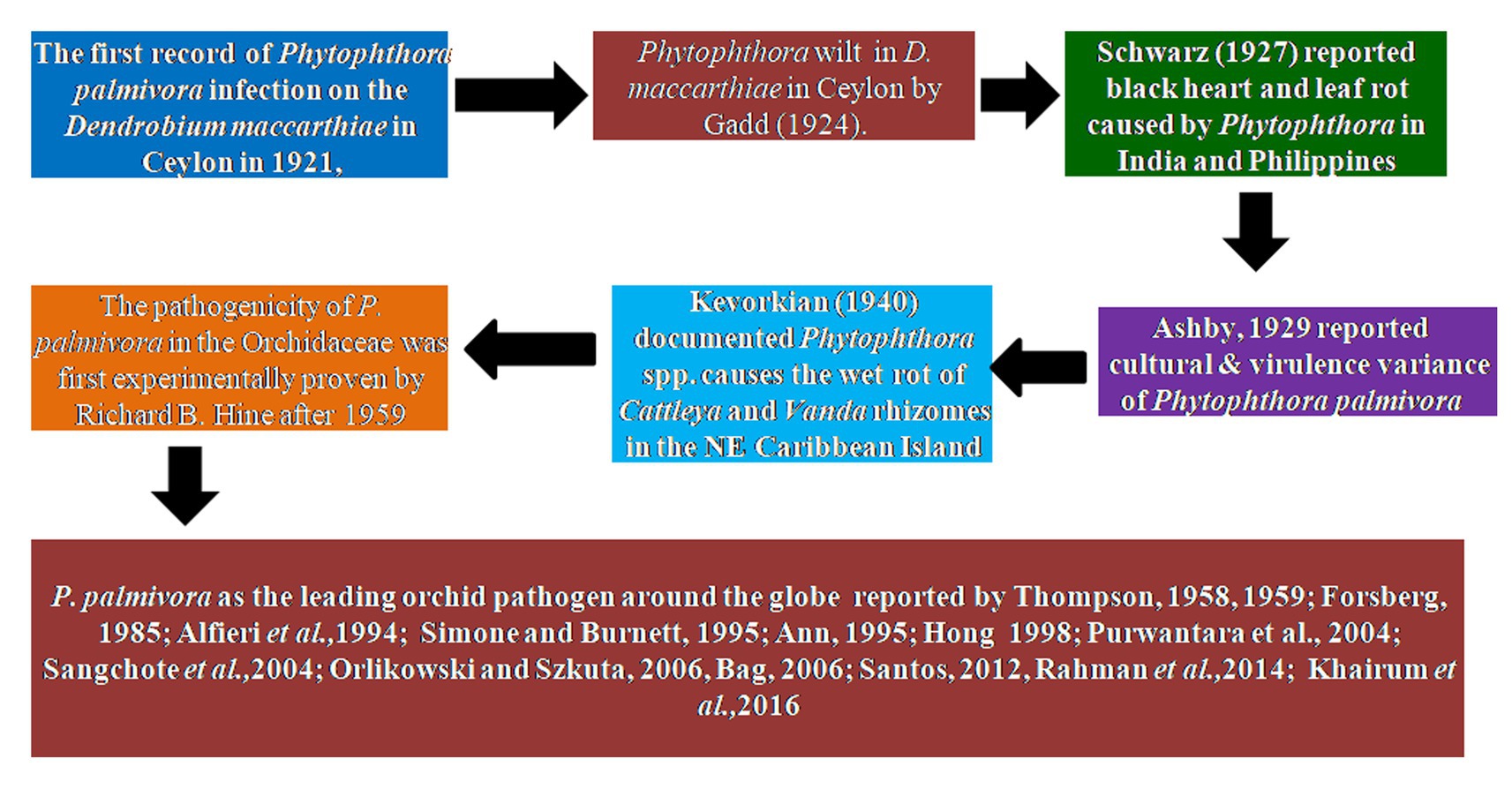
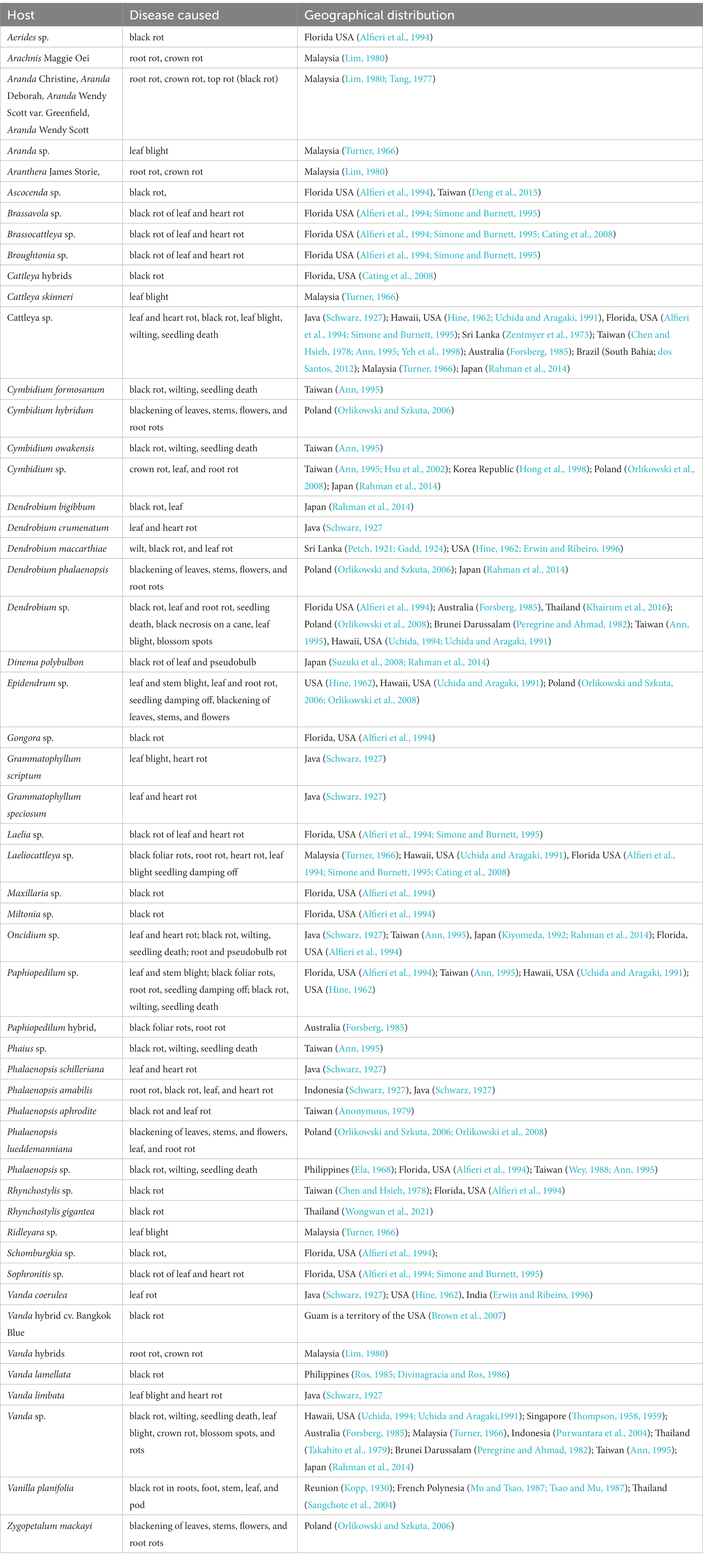
The first record of Phytophthora infection on Dendrobium maccarthiae was reported from Ceylon (presently Sri Lanka) in 1921, in ibid which constitutes the oldest literature. The fungus was identified as Phytophthora palmivora (Petch, 1921) and reported to be responsible for the wilt disease of D. maccarthiae in Ceylon by Gadd (1924). Later, it was reported by Schwarz (1927) that several indigenous and imported orchid varieties from India and the Philippines were attacked by Phytophthora in Java (presently Indonesia). The disease was named black heart and leaf rot of orchids as characterized by the rotting of the heart and leaves with the final discoloration of affected areas and drooping of foliage (Figure 1; Supplementary File 1). Several species in Orchidaceae, viz., Cattleya sp., Dendrobium crumenatum, Grammatophyllum speciosum, Oncidium sp., Phalaenopsis amabilis, Phalaenopsis schilleriana, Vanda coerulea, and Vanda limbata, were invaded by this fungus. The strains of the fungus varied significantly in cultural characteristics and virulence with reference to their hosts. Initially, this rot disease was believed to be caused by the tropical fungus Phytophthora omnivora de Bary, which was considered synonymous with Phytophthora faberi, but later on, the fungus was identified as P. palmivora by Ashby (1929a). Interestingly, a contemporary agronomist, Kopp (1930), described a disease of Vanilla (family: Orchidaceae) in Reunion that resulted in black lesions and rot on the stems, leaves, and pods. These spots contained abundant Phytophthora sporangia similar to those of P. palmivora Butler (=P. faberi Maubl. at that time). It might be the same lineage that caused the pod rot in Cocoa. Subsequently, Kevorkian (1940) documented that Phytophthora spp. caused the wet rot of Cattleya and Vanda rhizomes in the northeastern Caribbean island of Puerto Rico. However, the name “Black rot of orchids” was first coined by Rossetti (1943) while giving an account of a disease of Cattleya and Vanda in Puerto Rico and Stanhopea saccata in an unnamed locality. The name black rot is used to designate a disease caused by pythiaceous fungi of more than one species, mainly including the genera Pythium or Phytophthora (Limber, 1946). The symptoms of P. palmivora (Bult.) were adequately described on mature plants of Vanda orchids, and it was reported that P. palmivora was a parasite of orchids in Singapore (Thompson, 1959). The pathogenicity of P. palmivora in the Orchidaceae was first experimentally proven by Richard B. Hine, who was working as a plant pathologist at the University of Hawaii, USA. He demonstrated that plants in the orchid genera Dendrobium, Cattleya, Epidendrum, Paphiopedilum, and Vanda were susceptible to P. palmivora (Bult.) when inoculated with suspensions of zoospores or mycelium on wounded leaves. However, a natural infection of Cattleya and Vanda by P. palmivora in the field was reported in Hawaii (Hine, 1962). Thereafter, several scientific reports confirmed that P. palmivora (Bult.) is the leading orchid pathogen in various countries around the globe (Thompson, 1958, 1959; Forsberg, 1985; Alfieri et al., 1994; Ann, 1995; Simone and Burnett, 1995; Hong et al., 1998; Yeh et al., 1998; Portales, 2004; Purwantara et al., 2004; Sangchote et al., 2004; Bag, 2006; Orlikowski and Szkuta, 2006; dos Santos, 2012; Rahman et al., 2014; Khairum et al., 2016).
Phytophthora nicotianae Breda de Haan
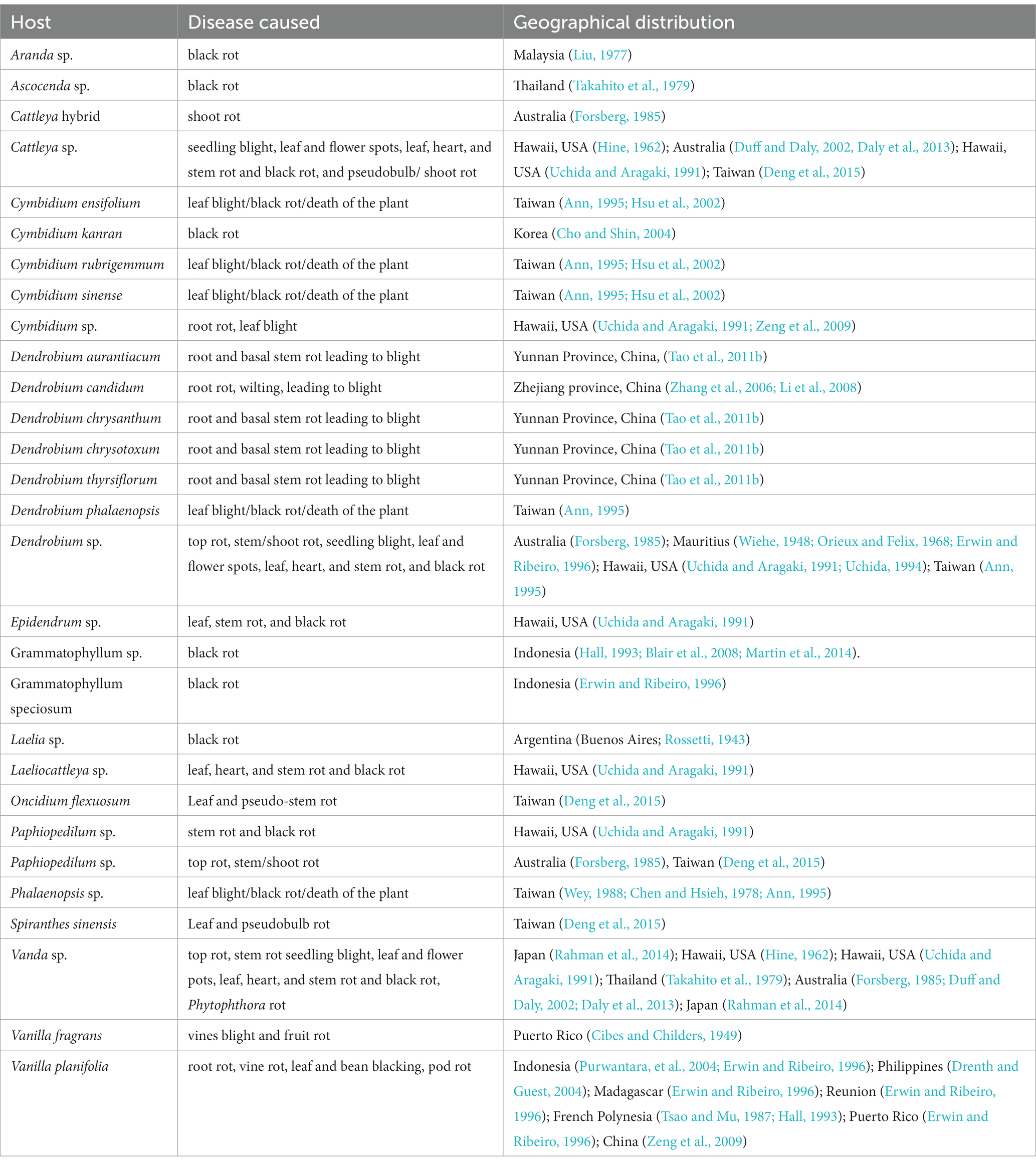
The black rot disease on the Laelia orchid was reported to be caused by Phytophthora parasitica Dastur (=P. nicotianae Breda de Haan) in Buenos Aires (Argentina) by Rossetti (1943) who first brought it to the attention of the scientific community. Vine blight and fruit rot of Vanilla (V. fragrans) caused by Phytophthora parasitica were further reported in Puerto Rico (Cibes and Childers, 1949). Subsequently, Phytophthora parasitica was repeatedly confirmed as a phytopathogen of orchids in predominant orchid-growing countries (Chen and Hsieh, 1978; Takahito et al., 1979; Forsberg, 1985; Wey, 1988; Uchida and Aragaki, 1991; Uchida, 1994; Ann, 1995; Zhang et al., 2006; Tao et al., 2011a; Daly et al., 2013).
Phytophthora cactorum (Leb. and Cohn) Schröeter
The occurrence of Phytophthora cactorum on orchids was first reported by Burnett in Florida, USA, in a series of scientific reports (Burnett, 1957, 1958, 1965, 1974). The fungus P. cactorum was reported to cause seedling blight, black leaf, and heart rot in Cattleya, Vanda, Epidendrum, Laelia, Oncidium, Vanilla, and Grammatophyllum in Florida. Later, occurrences of P. cactorum on orchids were also reported in several other countries (Arnold, 1986; Uchida and Aragaki, 1991; Pereira et al., 1993; Uchida, 1994; Claudio, 2003).
Phytophthora multivesiculata Ilieva, Man in ‘t Veld, Veenbaas-Rijks and R. Pieters
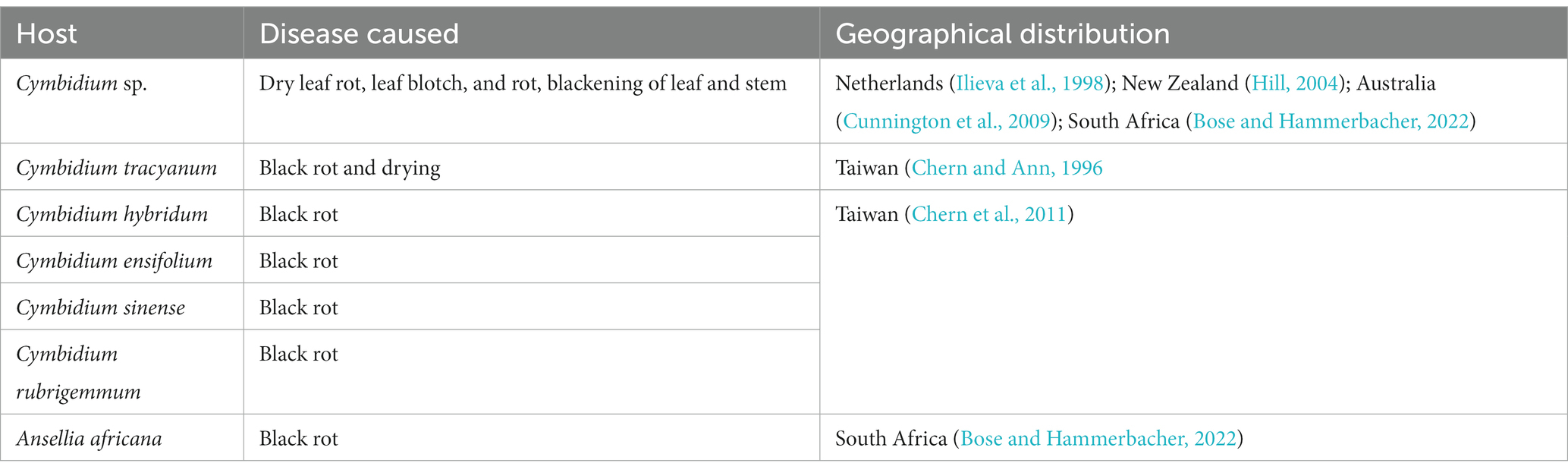
At the end of the 20th century, a new species of Phytophthora was isolated and characterized from blackened leaves and stems of naturally infected Cymbidium orchids in the Netherlands. The species was described as Phytophthora multivesiculata (Ilieva et al., 1998). Subsequently, the disease was reported exclusively on Cymbidium in New Zealand (Hill, 2004), Australia (Cunnington et al., 2009), Taiwan (Chern et al., 2011), and South Africa (Bose and Hammerbacher, 2022).
Other species of Phytophthora reported on orchids
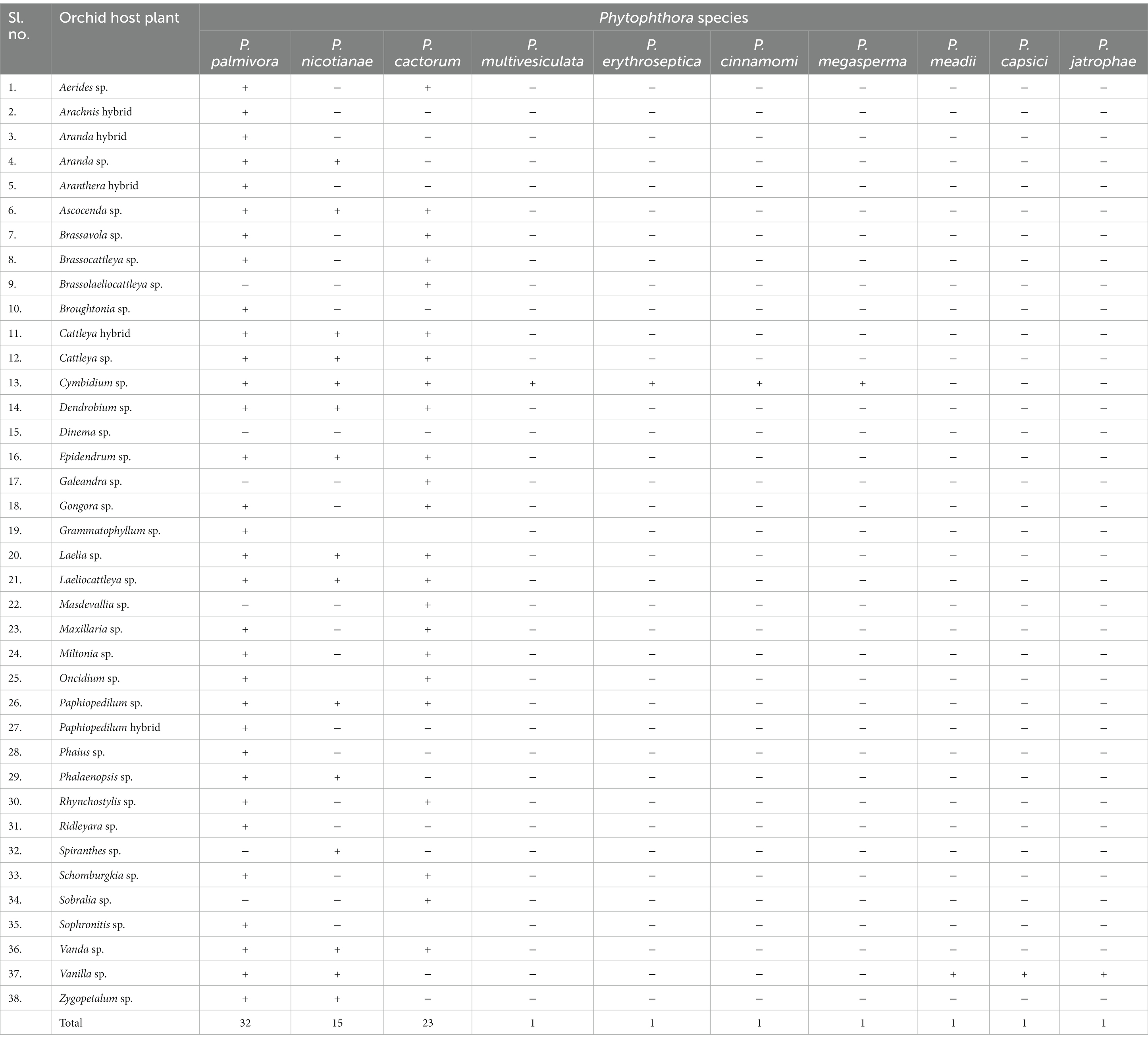
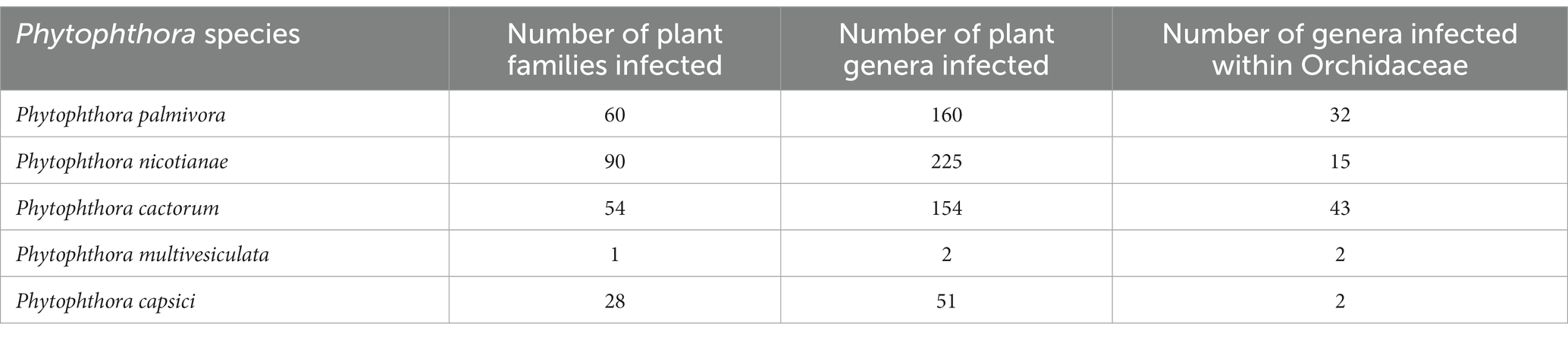
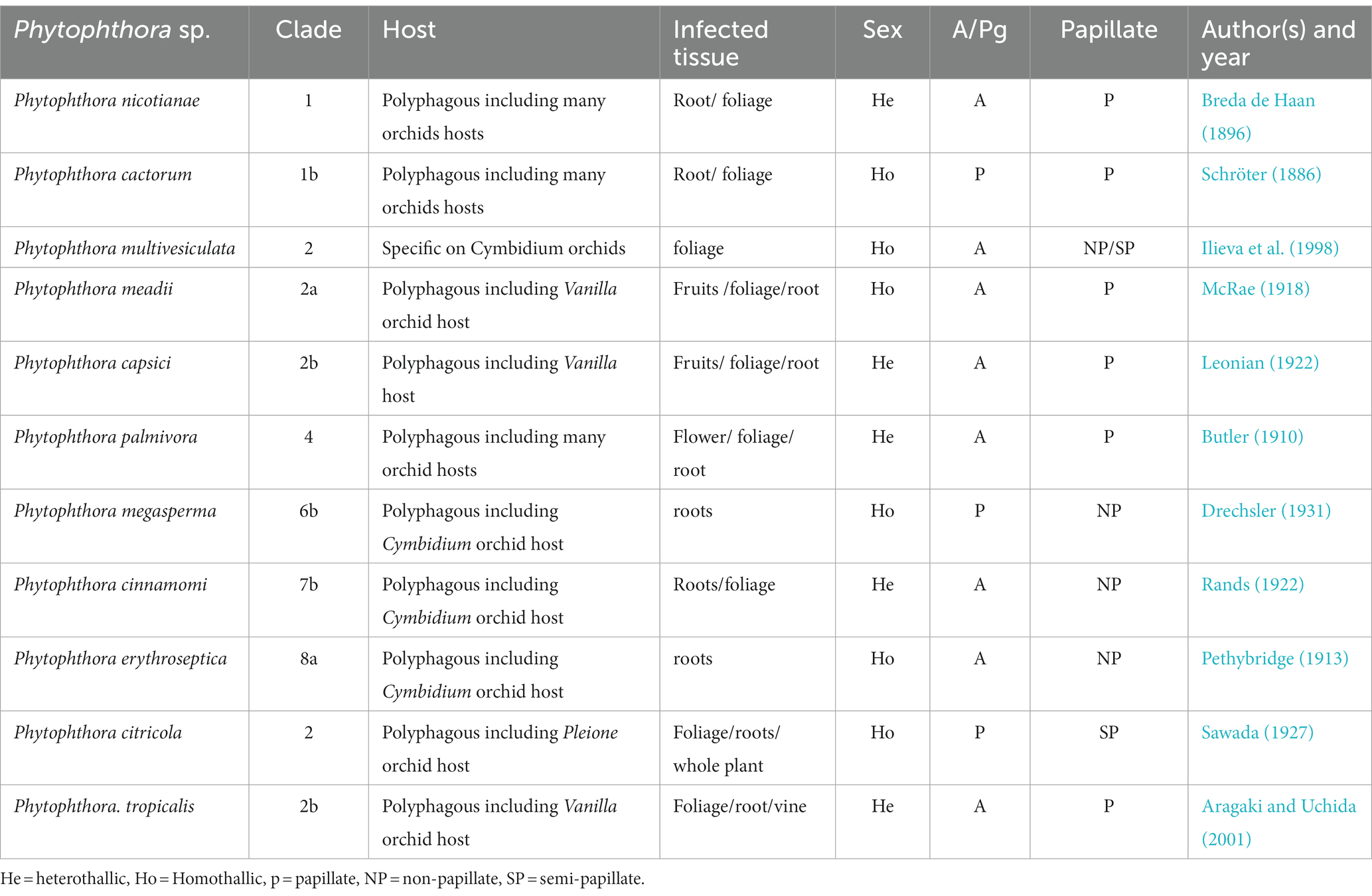
Phytophthora erythroseptica var. erythroseptica in Australia (Hall, 1989; Shivas, 1989), Phytophthora cinnamomi in Hawaii, USA (Uchida and Aragaki, 1991; Uchida, 1994), Phytophthora meadii in India (Bhai and Thomas, 2000; Bhai and Dhanesh, 2008), Phytophthora capsici in Indonesia (Andriyani et al., 2008), Phytophthora citricola in Taiwan (Ann, 2000), Phytophthora syringae in New South Wales, Australia (Cunnington et al., 2009), Phytophthora tropicalis in (Tahiti) French Polynesia (Aragaki and Uchida, 2001), Phytophthora jatrophae in French Polynesia and Puerto Rico (Bouriquet, 1954) as well as unidentified species of Phytophthora in Taiwan (Chern and Ann, 1996), Costa Rica (Rivera and Corrales, 2007), and Japan (Rahman et al., 2014) were found in the scientific literature. However, their frequency was found to be lower. Currently, it is globally accepted that five major Phytophthora species, viz., P. palmivora Butler, P. nicotianae Breda de Haan, P. cactorum (Leb. and Cohn) Schröeter, P. multivesiculata Ilieva et al., and P. meadii are responsible for orchid diseases that are globally known by different vernacular names as black rot, crown rot, brown rot, stem and leaf rot, heart and leaf rot, top and shoot rot, and leaf spot, including seedling rot and damping off (Tao et al., 2011a). An elaborate list of species of Phytophthora infecting various members of Orchidaceae is presented in Table 1.
Global distribution of Phytophthora species infecting orchids
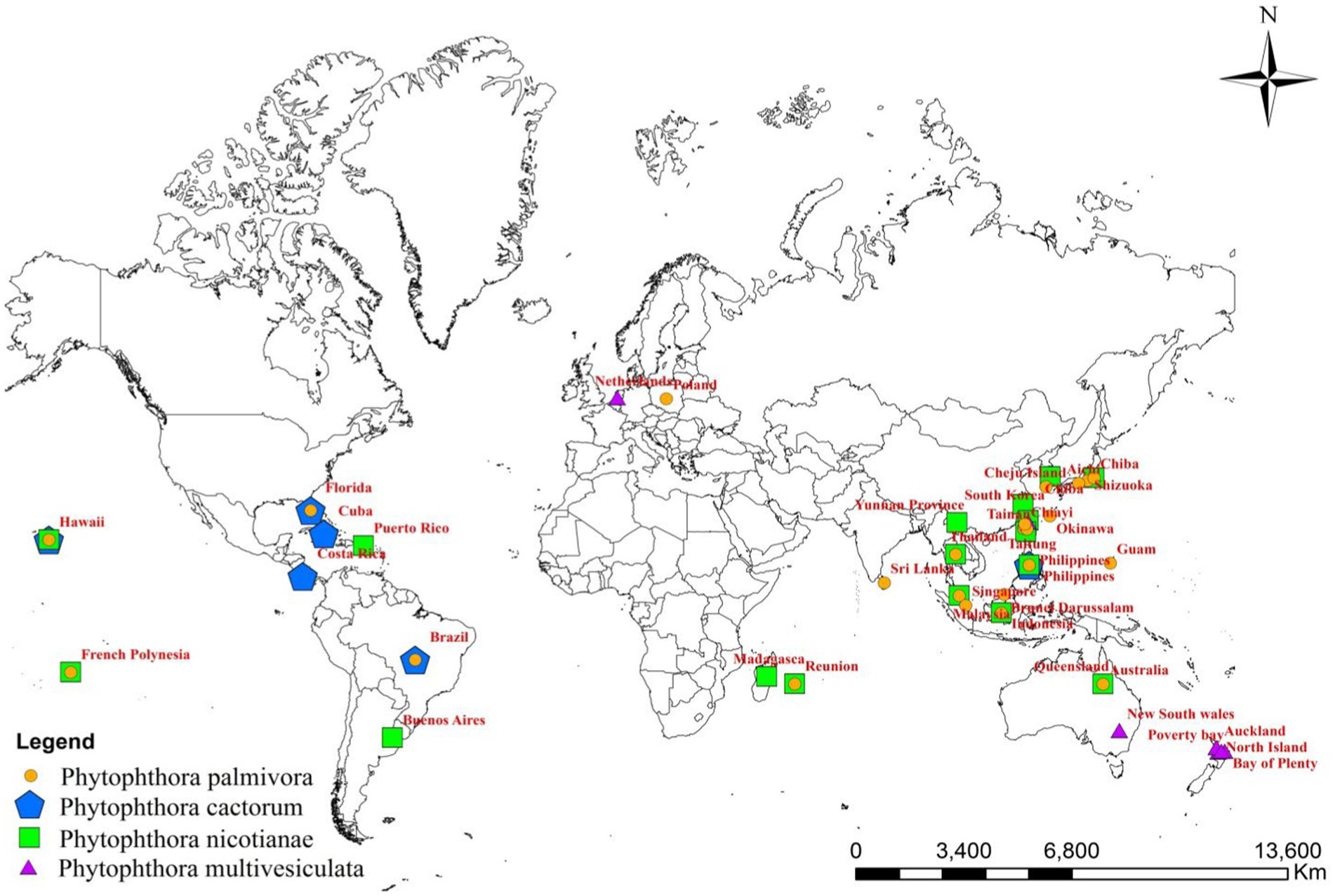
Although the genera and species of cultivated orchids are variable from continent to continent, orchids are globally distributed (Hinsley et al., 2018). When the orchid–fungi pathogen system is considered, Phytophthora cannot be separated from orchids. With the variation in the distribution of orchid hosts in various geographical locations, the distribution of Phytophthora spp. also varied widely on various continents (Figure 2).
Among the Phytophthora species documented as pathogens on orchids, P. palmivora appeared to be the most widely distributed one throughout the globe. P. palmivora has been recorded on one or more orchid hosts in multiple countries, such as Australia (Forsberg, 1985); Brazil (Santos dos Santos, 2012); Brunei Darussalam (Peregrine and Ahmad, 1982); Florida, USA (Alfieri et al., 1994; Simone and Burnett, 1995; Erwin and Ribeiro, 1996; Cating et al., 2008); French Polynesia (Mu and Tsao, 1987; Tsao and Mu, 1987); Guam, a territory of USA (Brown et al., 2007); Hawaii, USA (Hine, 1962; Uchida and Aragaki, 1991; Uchida, 1994); India (Erwin and Ribeiro, 1996); Java, Indonesia (Schwarz, 1927; Purwantara et al., 2004); Japan (Kiyomeda, 1992; Suzuki et al., 2008; Rahman et al., 2014); Korea Republic (Hong et al., 1998); Malaysia (Turner, 1966; Lim, 1980); Philippines (Ros, 1985; Divinagracia and Ros, 1986); Poland (Orlikowski and Szkuta, 2006; Orlikowski et al., 2008); Reunion (Kopp, 1930); Sri Lanka (Petch, 1921; Gadd, 1924; Zentmyer et al., 1973); Taiwan (Chen and Hsieh, 1978; Wey, 1988; Ann, 1995; Yeh et al., 1998; Hsu et al., 2002); and Thailand (Takahito et al., 1979; Sangchote et al., 2004; Khairum et al., 2016; Wongwan et al., 2021).
The distribution of P. nicotianae Breda de Haan is cosmopolitan (Cline et al., 2008). It has been reported on wide varieties of orchid species and hybrids in Argentina (Buenos Aires’ Rossetti, 1943); Australia (Forsberg, 1985; Daly et al., 2013); French Polynesia (Tsao and Mu, 1987; Hall, 1993); Hawaii, USA (Hine, 1962; Uchida and Aragaki, 1991; Uchida, 1994); Indonesia (Hall, 1993; Erwin and Ribeiro, 1996; Purwantara et al., 2004; Blair et al., 2008; Martin et al., 2014); Japan (Rahman et al., 2014); Korea (Cho and Shin, 2004); Madagascar (Erwin and Ribeiro, 1996); Malaysia (Liu, 1977); Mauritius (Wiehe, 1948; Orieux and Felix, 1968; Erwin and Ribeiro, 1996); the Philippines (Portales, 2004); Puerto Rico (Cibes and Childers, 1949; Erwin and Ribeiro, 1996); Reunion (Erwin and Ribeiro, 1996); Taiwan (Chen and Hsieh, 1978; Wey, 1988; Ann, 1995; Hsu et al., 2002); Thailand (Takahito et al., 1979); Yunnan Province, China (Tao et al., 2011a); Zhejiang province, China (Zhang et al., 2006; Li et al., 2008); and Hainan Island, China (Zeng et al., 2009).
Based on the pathogenic nature of Orchidaceae, the distribution of P. cactorum is restricted (Figure 1; Table 2) in Florida and New and Old-World countries (McMillan et al., 2009). Brazil (Pereira et al., 1993; Mendes et al., 1998); Costa Rica (Claudio, 2003); Cuba (Arnold, 1986); Florida, USA (Burnett, 1957; Burnett, 1965; Alfieri et al., 1994; Simone and Burnett, 1995; Cating et al., 2008; McMillan et al., 2010); and Hawaii, USA (Raabe et al., 1981; Uchida and Aragaki, 1991; Uchida, 1994) are major countries on the American continent where P. cactorum is frequently observed on orchids. Among Asian countries, P. cactorum has been occasionally recorded on orchids in India (Erwin and Ribeiro, 1996) and the Philippines (Schwarz, 1927; Erwin and Ribeiro, 1996).
As far as the orchid hosts are concerned, P. multivesiculata is mainly distributed in European countries, especially in the Netherlands (Ilieva et al., 1998; Figure 1; Table 2). Later, it was found in the North Island of New Zealand, prevalent in Northland, Auckland, the Bay of Plenty, and Poverty Bay, where Cymbidiums are grown commercially (Hill, 2004). Arguably, P. multivesiculata was also reported in Australia (Cunnington et al., 2009). In the recent past, an aberrant strain of P. multivesiculata was recorded in Taiwan (Chern et al., 2011). Very recently, the presence of P. multivesiculata was also documented in Pretoria, South Africa (Bose and Hammerbacher, 2022).
The presence of P. erythroseptica var. erythroseptica was recorded in Australia (Hall, 1989; Shivas, 1989), P. cinnamomi in Hawaii (Uchida and Aragaki, 1991; Uchida, 1994) and Phytophthora megasperma var. megasperma in New Zealand (Laundon, 1978; Boesewinkel, 1982).
P. meadii and P. capsici were established as pathogens of vanilla in India (Bhai and Thomas, 2000; Bhai and Dhanesh, 2008) and Indonesia (Andriyani et al., 2008), respectively; whereas P. palmivora was recorded in French Polynesia (Tsao and Mu, 1987) and Thailand (Sangchote et al., 2004) as Vanilla pathogen (Table 2). Unconfirmed reports of Phytophthora tropicalis in French Polynesia (Aragaki and Uchida, 2001) and P. jatrophae in French Polynesia and Puerto Rico (Bouriquet, 1954) as Vanilla pathogens were also available in the literature.
Host range of Phytophthora species within the Orchidaceae
Phytophthora palmivora Butler
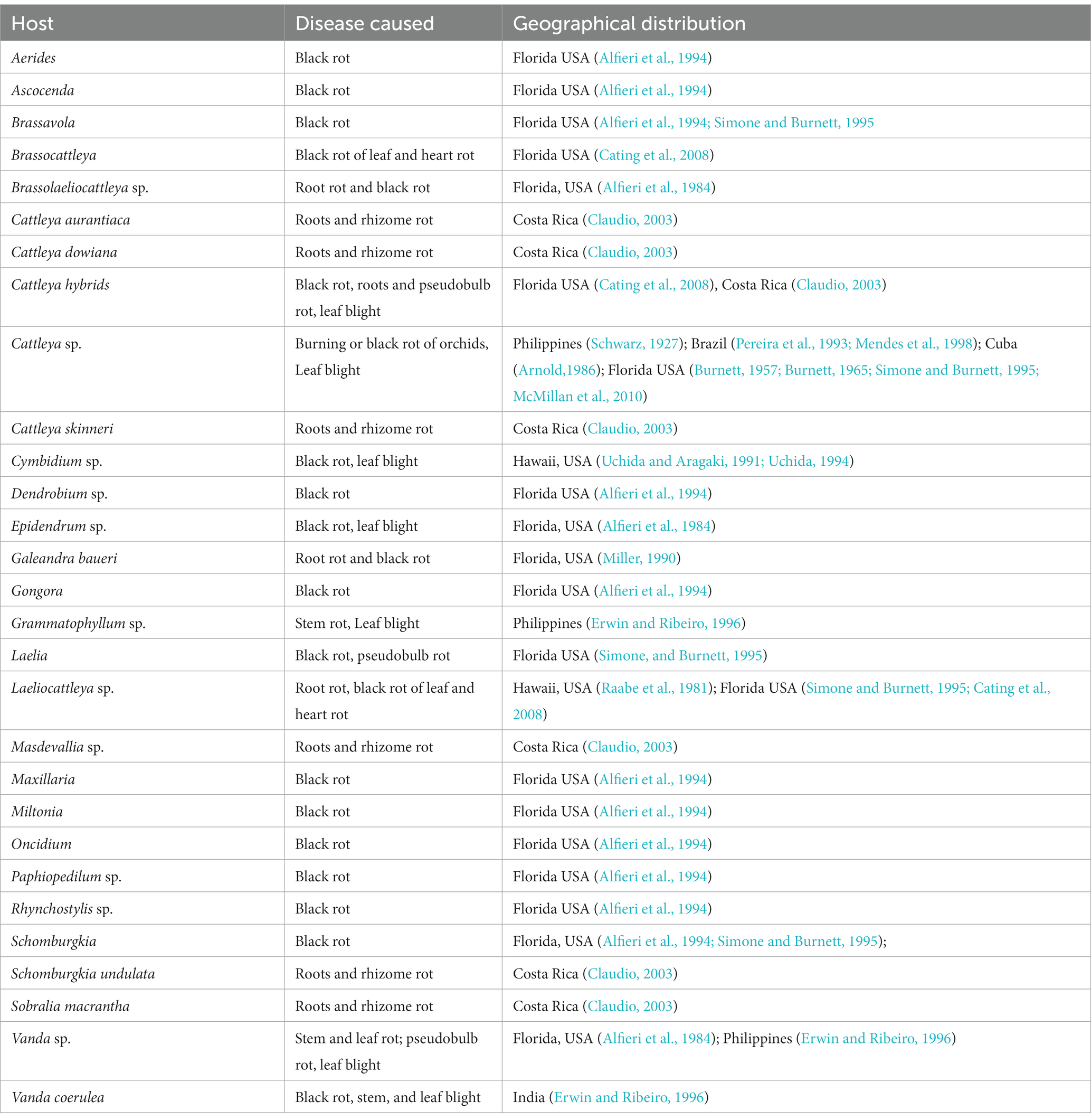
Many genera and species of orchids, along with their hybrids, are acknowledged as the hosts of Phytophthora. According to recent literature, P. palmivora, a prominent species infecting orchids, was reported to have a large number of hosts belonging to 160 genera of 60 plant families (Cline et al., 2008; Table 2; Figure 3; Supplementary File 2). Within the Orchidaceae, several species, hybrids, and intergeneric hybrids were reported to be infected by P. palmivora (Table 3). The major genera and species include Aerides sp. (Alfieri et al., 1994); Arachnis Maggie Oei (Lim, 1980); Aranda sp. (Turner, 1966); Aranda Christine, Aranda Deborah, Aranda Wendy Scott var. Greenfield (Lim, 1980), Aranda Wendy Scott (Tang, 1977); Aranthera James Storie (Lim, 1980); Ascocenda sp. (Alfieri et al., 1994); Brassavola sp. (Alfieri et al., 1994; Simone and Burnett, 1995); Brassocattleya sp. (Alfieri et al., 1994; Simone and Burnett, 1995; Cating et al., 2008); Broughtonia sp. (Alfieri et al., 1994; Simone and Burnett, 1995); Cattleya hybrids (Cating et al., 2008); Cattleya skinneri (Turner, 1966); Cattleya sp. (Schwarz, 1927; Hine, 1962; Turner, 1966; Zentmyer et al., 1973; Chen and Hsieh, 1978; Forsberg, 1985; Uchida and Aragaki, 1991; Alfieri et al., 1994; Ann, 1995; Simone and Burnett, 1995; Yeh et al., 1998; dos Santos, 2012; Rahman et al., 2014); Cymbidium formosanum (Ann, 1995); Cymbidium hybridum (Orlikowski and Szkuta, 2006); Cymbidium owakensis (Ann, 1995); Cymbidium sp. (Ann, 1995; Hong et al., 1998; Hsu et al., 2002; Orlikowski et al., 2008; Rahman et al., 2014); Dendrobium bigibbum (Rahman et al., 2014); Dendrobium crumenatum (Schwarz, 1927); D. maccarthiae (Petch, 1921; Gadd, 1924; Hine, 1962; Erwin and Ribeiro, 1996); Dendrobium phalaenopsis (Orlikowski and Szkuta, 2006); Dendrobium sp. (Peregrine and Ahmad, 1982; Forsberg, 1985; Uchida and Aragaki, 1991; Alfieri et al., 1994; Uchida, 1994; Ann, 1995; Orlikowski et al., 2008; Khairum et al., 2016); Dinema polybulbon (Suzuki et al., 2008; Rahman et al., 2014); Epidendrum sp. (Hine, 1962; Uchida and Aragaki, 1991; Orlikowski and Szkuta, 2006; Orlikowski et al., 2008); Gongora sp. (Alfieri et al., 1994); Grammatophyllum scriptum (Schwarz, 1927); Grammatophyllum speciosum (Schwarz, 1927); Laelia sp. (Alfieri et al., 1994; Simone and Burnett, 1995); Laeliocattleya sp. (Turner, 1966; Uchida and Aragaki, 1991; Alfieri et al., 1994; Simone and Burnett, 1995; Cating et al., 2008); Maxillaria sp. (Alfieri et al., 1994); Miltonia sp. (Alfieri et al., 1994); Oncidium sp. (Schwarz, 1927; Kiyomeda, 1992; Alfieri et al., 1994; Ann, 1995; Rahman et al., 2014); Paphiopedilum sp. (Hine, 1962; Uchida and Aragaki, 1991; Alfieri et al., 1994; Ann, 1995); Paphiopedilum hybrid (Forsberg, 1985); Phaius sp. (Ann, 1995); Phalaenopsis achilleriana, Phalaenopsis amabilis (Schwarz, 1927); Phalaenopsis aphrodite (Anonymous, 1979); Phalaenopsis lueddemanniana (Orlikowski and Szkuta, 2006; Orlikowski et al., 2008); Phalaenopsis sp. (Ela, 1968; Wey, 1988; Alfieri et al., 1994; Ann, 1995); Rhynchostylis gigantea (Wongwan et al., 2021); Rhynchostylis sp. (Chen and Hsieh, 1978; Alfieri et al., 1994); Ridleyara sp. (Turner, 1966); Schomburgkia (Alfieri et al., 1994); Sophronitis sp. (Alfieri et al., 1994; Simone and Burnett, 1995); Vanda coerulea (Schwarz, 1927; Hine, 1962; Erwin and Ribeiro, 1996); Vanda hybrid cv. Bangkok Blue (Brown et al., 2007); Vanda hybrids (Lim, 1980); Vanda lamellata (Ros, 1985; Divinagracia and Ros, 1986); Vanda limbata (Schwarz, 1927); Vanda sp. (Thompson, 1958, 1959; Turner, 1966; Takahito et al., 1979; Peregrine and Ahmad, 1982; Forsberg, 1985; Uchida and Aragaki, 1991; Uchida, 1994; Ann, 1995; Purwantara et al., 2004; Rahman et al., 2014); Vanilla planifolia (Kopp, 1930; Mu and Tsao, 1987; Tsao and Mu, 1987; Sangchote et al., 2004); and Zygopetalum mackayi (Orlikowski and Szkuta, 2006).
Phytophthora nicotianae Breda de Haan
With its cosmopolitan distribution and polyphagous nature, P. nicotianae is known to have hosts in 255 genera in 90 plant families (Cline et al., 2008), including orchid hosts of at least 15 genera within the family Orchidaceae (Tables 1, 4). The orchid hosts reported to be infected by this pathogen are Aranda sp. (Liu, 1977); Ascocenda sp. (Takahito et al., 1979); Cattleya hybrid (Forsberg, 1985); Cattleya sp. (Hine, 1962; Uchida and Aragaki, 1991; Duff and Daly, 2002; Daly et al., 2013); Cymbidium ensifolium (Ann, 1995; Hsu et al., 2002); Cymbidium kanran (Cho and Shin, 2004); Cymbidium rubrigemmum (Ann, 1995; Hsu et al., 2002); Cymbidium sinense (Ann, 1995; Hsu et al., 2002); Cymbidium sp. (Uchida and Aragaki, 1991; Zeng et al., 2009); Dendrobium aurantiaca (Tao et al., 2011a); Dendrobium candidum (Zhang et al., 2006; Li et al., 2008); Dendrobium chrysanthum (Tao et al., 2011a); Dendrobium chrysotoxum (Tao et al., 2011a); Dendrobium phalaenopsis (Ann, 1995); Dendrobium sp. (Wiehe, 1948; Orieux and Felix, 1968; Forsberg, 1985; Uchida and Aragaki, 1991; Uchida, 1994; Ann, 1995; Erwin and Ribeiro, 1996); Dendrobium thyrsiflorum (Tao et al., 2011b); Epidendrum sp. (Uchida and Aragaki, 1991); Grammatophyllum sp. (Hall, 1993; Blair et al., 2008; Martin et al., 2014); Grammatophyllum speciosum (Erwin and Ribeiro, 1996); Laelia sp. (Rossetti, 1943); Laeliocattleya sp. (Uchida and Aragaki, 1991); Paphiopedilum sp. (Forsberg, 1985; Uchida and Aragaki, 1991); Phalaenopsis sp. (Chen and Hsieh, 1978; Wey, 1988; Ann, 1995); Vanda sp. (Hine, 1962; Takahito et al., 1979; Forsberg, 1985; Uchida and Aragaki, 1991; Duff and Daly, 2002; Daly et al., 2013; Rahman et al., 2014); Vanilla fragrans (Cibes and Childers, 1949); and Vanilla planifolia (Tsao and Mu, 1987; Hall, 1993; Erwin and Ribeiro, 1996; Portales, 2004; Purwantara et al., 2004; Zeng et al., 2009).
Phytophthora cactorum (Leb. and Cohn) Schröeter
Phytophthora cactorum (Leb. and Cohn) Schröeter is another phytopathogen of crop plants, consisting of 154 genera in 54 plant families (Cline et al., 2008), including 22 genera of orchids within the family Orchidaceae (Tables 1, 5). Reported orchid hosts are Aerides sp. (Alfieri et al., 1994); Ascocenda sp. (Alfieri et al., 1994); Brassavola sp. (Alfieri et al., 1994; Simone and Burnett, 1995); Brassocattleya sp. (Cating et al., 2008); Brassolaeliocattleya sp. (Alfieri et al., 1984); Cattleya aurantiaca (Claudio, 2003); Cattleya dowiana (Claudio, 2003); Cattleya hybrids (Claudio, 2003; Cating et al., 2008); Cattleya sp. (Schwarz, 1927; Burnett, 1957, 1965; Arnold, 1986; Pereira et al., 1993; Simone and Burnett, 1995; Mendes et al., 1998; McMillan et al., 2010);Cattleya skinneri (Claudio, 2003); Cymbidium sp. (Uchida and Aragaki, 1991; Uchida, 1994); Dendrobium sp. (Alfieri et al., 1994); Epidendrum sp. (Alfieri et al., 1984); Galeandra baueri (Miller, 1990); Gongora sp. (Alfieri et al., 1994); Grammatophyllum sp.(Erwin and Ribeiro, 1996); Laelia sp. (Simone and Burnett, 1995); Laeliocattleya sp. (Raabe et al., 1981; Simone and Burnett, 1995; Cating et al., 2008);Masdevallia sp. (Claudio, 2003); Maxillaria sp. (Alfieri et al., 1994); Miltonia sp. (Alfieri et al., 1994); Oncidium sp. (Alfieri et al., 1994); Paphiopedilum sp. (Alfieri et al., 1994); Rhynchostylis sp. (Alfieri et al., 1994); Schomburgkia sp. (Alfieri et al., 1994; Simone and Burnett, 1995); Schomburgkia undulata (Claudio, 2003); Sobralia macrantha (Claudio, 2003); Vanda sp. (Alfieri et al., 1984; Erwin and Ribeiro, 1996); and Vanda coerulea (Erwin and Ribeiro, 1996).
Phytophthora multivesiculata Ilieva et al.
Phytophthora multivesiculata was first reported on the boat orchid Cymbidium in the Netherlands (Ilieva et al., 1998). This fungal species is known to have a restricted host only in Orchidaceae to date (Table 6). Therefore, it exhibits pathogenicity only against species of Cymbidium. The reported hosts are Cymbidium sp. (Ilieva et al., 1998; Hill, 2004; Cunnington et al., 2009; Bose and Hammerbacher, 2022), namely Cymbidium tracyanum (Chern and Ann, 1996); Cymbidium hybridum, Cymbidium ensifolium, Cymbidium sinense, and Cymbidium rubrigemmum (Chern et al., 2011).Very recently, P. multivesiculata was documented to cause black rot on the Leopard orchid Ansellia africana in Pretoria, South Africa (Bose and Hammerbacher, 2022).
Diversity of symptoms and signs on orchids by different species of Phytophthora
Since the first report of Phytophthora palmivora occurrence on D. maccarthiae in Ceylon (Petch, 1921) causing wilt (Gadd, 1924) and an adequate description of symptoms of the disease caused by this pathogen on seedlings or matured hybrids of Vanda orchids in Singapore, numerous reports of Phytophthora diseases on orchids have been accumulated (Thompson, 1959). Summing up all the up-to-date literature on Phytophthora diseases of orchids, it is evident that Phytophthora generates a variety of symptoms in infected plants. According to the observation of Orlikowski and Szkuta (2006), symptoms may vary with the age of host plants, species, and organs attacked. In reality, major Phytophthora diseases of orchids are widely known as black rot (Ann, 1995; Erwin and Ribeiro, 1996; Yeh et al., 1998; Brown et al., 2007; Cating et al., 2010; Chern et al., 2011), wilt (Gadd, 1924; Hine, 1962), crown rot (Hong et al., 1998), heart and leaf rot (Schwarz, 1927; Hine, 1962; Chen and Hsieh, 1978), stem and leaf rot (Orlikowski and Szkuta, 2006), top and shoot rot (Daly et al., 2013), roots and rhizome rot (Claudio, 2003), blight (Li et al., 2008), bud and flower blight (Uchida, 1994), and seedling blight (Tao et al., 2011a) or damping off (Uchida and Aragaki, 1991; Uchida, 1994) in different countries. All these orchid diseases caused by Phytophthora spp. are known by the common name Phytophthora rots. Variation in the name of the disease is based on the severity of the disease symptoms on specific organs of the orchid hosts. The symptoms of major species are presented here.
Phytophthora palmivora
Uchida has a long history of involvement with the research of orchid diseases at the University of Hawaii in Honolulu, USA, and has successfully enumerated various symptoms of Phytophthora diseases on a galaxy of tropical orchids, covering several phenological stages of crop growth from seedling to budding and flowering (Uchida, 1994). Symptoms of leaf and pseudobulb rot caused by P. palmivora on Cattleya, Laeliocattleya, and related hybrids are often dark brown to black. After the initiation of leaf infection, the fungus moves quickly through the leaves and pseudobulb; the leaves turn black within a few weeks and fall. Before the fall of the leaf, infection is initiated in the pseudobulbs, which causes gradual rot of the pseudobulbs where the fungus harbors for longer periods. Young plants are killed rapidly, but adult plants decline gradually. In community pots, seedling blight or damping is also observed due to overcrowding (Uchida and Aragaki, 1991).
Blossom, bud, and spike rots were also caused by P. palmivora in Dendrobium and Vanda orchids. It results in the production of light brown watery lesions with slightly darker centers on flowers (Thompson, 1958, 1959; Uchida and Aragaki, 1991) which may be similar to that of Botrytis flower spots (gray mold). However, gray mold with a mass of powdery gray spores is not present in the case of Phytophthora blight or flower spots (Uchida and Aragaki, 1991). The sign of Phytophthora crown rot in the sympodial orchid, Cymbidium, starts at the basal region of an infected plant and progresses upward to the lower leaves. Subsequently, typical water-soaked lesions appeared on the lower leaves, and the plant became wilted, blighted, and died. A study by Orlikowski and Szkuta (2006) recorded symptoms of P. palmivora rot in Polish greenhouse orchids based on their observation of Cymbidium, Dendrobium phalaenopsis, Epidendrum, Phalaenopsis lueddemanniana, and Zygopetalum mackayi. P. palmivora mostly attacks the leaves that gradually spread onto the stems and roots, and its symptoms are evident as irregular yellow-brown or black areas in different parts of the plants. On Phalaenopsis, yellow to brown tongue-shaped spots appeared mainly on the leaf base, while they spread upward, invading the subsequent upper leaves that rot and die. Furthermore, the infection spreads to the next higher leaf, resulting in the detachment of leaves from the main plants after turning brown to black. When the disease moves downward, it ultimately reaches the roots, causing root rots. As per our knowledge, no report of rots on the flower was reported from Poland. Rotting was observed in tissue culture seedlings of Dendrobium nobile in community pots during hardening. Furthermore, rotting in mature potted plants started from the base with gradual progression to the top, causing defoliation (Figures 4A–D). Severely infected Cattleya plants turn black; leaves and stems get detached from each other (Figure 5). On Cymbidium, severe black rot symptoms on new side shoots, leaves, and pseudobulbs and mass destruction of orchid plants were reported (Figures 6A–D) in Darjeeling and Sikkim, India (Bag, 2006).

Figure 4. Phytophthora rot on Dendrobium nobile in different growth stages. (A) Damping off symptoms in community pot during hardening of tissue cultured plantlets. (B) Rotting of side shoots in the potted plan. (C) Matured plant showing progressing rotting of stems and defoliation. (D) Matured plant showing rotting on top of the growing plants.

Figure 6. (A) New shoot rot caused by Phytophthora in potted Cymbidium orchid. (B) Phytophthora infected shoots of different ages turning black. (C) Black leaf blight incited by Phytophthora on Cymbidium leaves. (D) Mass destruction of Phytophthora-infected Cymbidium orchids in Nursery.
Phytophthora nicotianae
The earliest report of black rot disease on Laelia orchids was published by Rossetti (1943). The study described distinct symptoms of the disease caused by Phytophthora parasitica (= nicotianae) in Buenos Aires (the capital city of Argentina). The disease was characterized by a dark brown, flaccid rot of the pseudobulb tissues and sharply defined discoloration of the leaves, accompanied by sharply defined discoloration of the leaves. The infected leaves get detached from the main plant and the fungus further progresses until they are completely blackened. The pseudobulbs initially remain attached to the rhizome; however, later on, they wither as the infection progresses. As observed by Takahito et al. (1979), the infected parts such as buds, leaves, and stems of Vanda and Ascocenda with P. nicotianae show light brown to brown water-soaked lesions that turn into dark brown to black, followed by the death of the plants eventually. Subsequently, similar symptoms were also reported in various orchid hosts from many other countries (Uchida and Aragaki, 1991; Uchida, 1994; Ann, 1995; Duff and Daly, 2002; Daly et al., 2013). The seedling blight of Dendrobium candidum caused by P. nicotianae was reported from Zhejiang province, China (Zhang et al., 2006; Li et al., 2008). Typical root and basal stem rot symptoms that progress in an acropetal direction, causing the death of plants in a few days, were recorded in hardened tissue cultured seedlings of Dendrobium in the field. The rotten tap roots show brownish-black to black sunken lesions that frequently extend up to the stems. Typical brownish-black lesions are visible in older plants on the tender top parts, causing top wilt and defoliation. Later, Tao et al. (2011a) reported similar root and basal stem rot leading to blight and defoliation caused by P. nicotianae on D. aurantiacum, D. chrysanthum, D. thyrsiflorum, and D. chrysotoxum in Yunnan Province, China.
Phytophthora cactorum
In Florida, USA, P. cactorum has been reported to cause seedling blight, black leaf, and heart rot in Cattleya, Vanda, Epidendrum, Laelia, Oncidium, Vanilla, and Grammatophyllum (Burnett, 1957, 1958, 1965, 1974). Unlike Hawaii, P. cactorum is a major pathogen of Cattleya species and its hybrids in Florida and New and Old-World countries. This fungus infects leaves, pseudobulbs, rhizomes, and flower buds (McMillan et al., 2010). The symptomatic expression of black rot disease caused by P. cactorum on the Cattleya species (C. skinneri and C. aurantiaca), including its hybrids, was enumerated by Claudio (2003) in the Central Valley of Costa Rica. The disease was also found to attack Schomburgkia undulata, Masdevallia, and Sobralia macrantha.
The infection initiates in the roots and rhizomes; later, the fungus spreads to the aerial parts of all the infected plants, causing decay and dark discoloration. The characteristic symptoms of the disease include immediate detachment of the leaves from the main stem or the leaves becoming brittle at the point of attachment to pseudobulbs and ultimately falling on the ground or the growing medium. The leaves become necrotic and develop a thin, floury layer on the plant surface, which has sporangia of the fungus under highly moist conditions.
Phytophthora multivesiculata
As per current knowledge, P. multivesiculata is reported to be the pathogen of only Cymbidium orchids, and no other host. During the formation of the new species “multivesiculata” under the genus “Phytophthora,” the original symptom of rot disease caused by P. multivesiculata on naturally infected Cymbidium in the Netherlands was described by Ilieva et al. (1998). Naturally infected plants were recorded to have dry rot of leaves that showed a change in color to brown with typical horizontal zebra CV-like stripes (approximately 0.5 cm wide). A lighter discoloration in the middle and a dark brown to black color in the margin was observed. The base of the pseudobulbs showed water-soaked brownish-black discolored tissue. Under artificial inoculation, a typical brown rot was visible in 6 days, but the typical zebra-like stripes were not as prominent as a natural infection on Cymbidium leaves. The symptoms were found to be more or less similar on mature Cymbidium leaves and pseudobulbs in New Zealand. A characteristic internal, blue-black, or purplish-brown discoloration along with a sour odor was observed in infected young pseudobulbs. The roots remained grayish-white, unaffected while the rest of the plant had turned brown (Hill, 2004). In Taiwan, Chern et al. (2011) briefed the symptoms of black rot caused by the aberrant strain of P. multivesiculata. Prominent initial symptoms include the formation of bleached water-soaked spots on leaves and pseudo-stems, which later turn black within 3–5 days. The premature drooping of infected leaves may also occur. In cases of severe rots in pseudo-stems and leaves, most of the infected plants eventually die. In general, this pathogen causes leaf blotches and rots of pseudobulbs/stems in seedlings or mature plants. However, no report of Cymbidium root and flower infection by P. multivesiculata was reported.
Needless to say, Phytophthora induces a variety of types of disease symptoms in infected orchid hosts, which are reported in various terms: seedling blight, root rot, top and shoot rot, heart and leaf rot, black rot, and crown rot, the end result of which is “the blackening of invaded parts and defoliation,” justifying the universally accepted term “Black rot of orchids” given by Rossetti (1943).
Taxonomy of Phytophthora infecting orchid
Traditionally, all species of Phytophthora are known as Oomycetous fungi. They belong to the order Peronosporales, family Pythiaceae, class Oomycetes, and phylum Oomycota under the Kingdom “Fungi.” Currently, with the advancement in molecular, chemical, and ultra-structural studies, species of Phytophthora are considered to be “fungal-like organisms” or “pseudo fungi” with characteristics that are closer to the “heterokont,” biflagellate algae. They are chlorophyll-deficient “colourless algae.” According to the present concept, five important unique characteristics differentiate the Oomycota as a distinct group of an organism that is closer to the heterokont algae “Chromista” (Rossman and Palm, 2006). These characteristics include (a) the diploid state of vegetative mycelium, (b) the presence of β-glucans and cellulose in the cell wall, (c) sexual reproduction by heterogametangia (i.e., by oogonium and antheridium), (d) asexual reproduction through the production of motile heterokonts and (e) mitochondria with tubular cristae. Therefore, these are shifted into the separate Kingdom “Chromista” (Agrios, 1997) or “Straminopila” (Alexopoulos et al., 1996) rather than the true fungi kingdom “Fungi.” The taxonomic information is available in the first published monograph, “Phytophthora Diseases Worldwide,” by Erwin and Ribeiro (1996). Based on multiple loci, a detailed phylogeny of the genus Phytophthora was given by Blair et al. (2008). In addition, online taxonomic databases can be consulted using www.phytophthora.org for an instant requirement for Phytophthora identification (Park et al., 2008). Recently, Kroon et al. (2012) reported key details for the identification and delimitation of Phytophthora species, describing 10 clades in the genus Phytophthora, which accommodates 116 species, 15 of which still await valid publication.
Characteristics and features of major Phytophthora species infecting orchid
Phytophthora palmivora
This destructive pathogen was first identified as Phytophthora omnivora (Massee, 1899) and Pythium palmivorum (Butler, 1907) as the causal organisms of cocoa black pod disease and the destructive palm disease, respectively. Later on, both pathogens were merged into a single pathogen and renamed Phytophthora palmivora by Butler (1910). At present, it is globally accepted that P. palmivora causes black rot disease in both monopodial as well as sympodial orchids. According to the study of Hong et al. (1998), P. palmivora infecting Cymbidium produces uniform hyaline hyphae, no hyphal swellings, readily form chlamydospores (32–48 μm) in the medium, sporangiophores sympodially branched or sometimes terminal, zoosporangia form readily on agar and in water, zoosporangia conspicuously papillate, ellipsoid to ovoid (Erwin and Ribeiro, 1996; Hong et al., 1998), size 36–80 × 26–40 μm (average 57 × 34 μm), highly deciduous with short pedicel of 3–4 μm. The fungus can be characterized as heterothallic, oogonia globose, smooth 25–28 μm (average 26.5 μm) diameter with spherical oospores of 20–24 μm (average 22.4 μm) diameter, antheridia amphigynous, and oogonia aplerotic (Table 7). The optimum temperature for growth was 27–30° C, including a minimum temperature of 8°C and a maximum temperature of 33°C.
In Taiwan, the Cattleya black rot pathogen P. palmivora was studied by Yeh et al. (1998). According to their observation, the basic characteristics of this isolate were similar to those of P. palmivora infecting Cymbidium in the Republic of Korea. However, it was noted that the size of asexual (sporangia size 44.3–51.0 × 26.1–29.7 μm, with L/W ratios 1.61–1.75) and sexual structures (oogonia 23.9–32.2 μm diameter; oospores 20.3–27.6 μm diameter) were slightly different, but within the standard size of typical P. palmivora. The optimum, minimum, and maximum temperature requirements for mycelial growth were 24–32, 10, and 35°C, respectively. All host species of P. palmivora share similarities in basic distinguishing characteristics, but the size of various asexual and sexual structures may vary with isolates of different host species (Brasier and Griffin, 1979; Figure 7). A range of various morphological parameters for isolates of different hosts can be observed in “Phytophthora Diseases Worldwide” (Erwin and Ribeiro, 1996). Phylogenetically, P. palmivora belongs to clade 4 (Kroon et al., 2012; Martin et al., 2012).
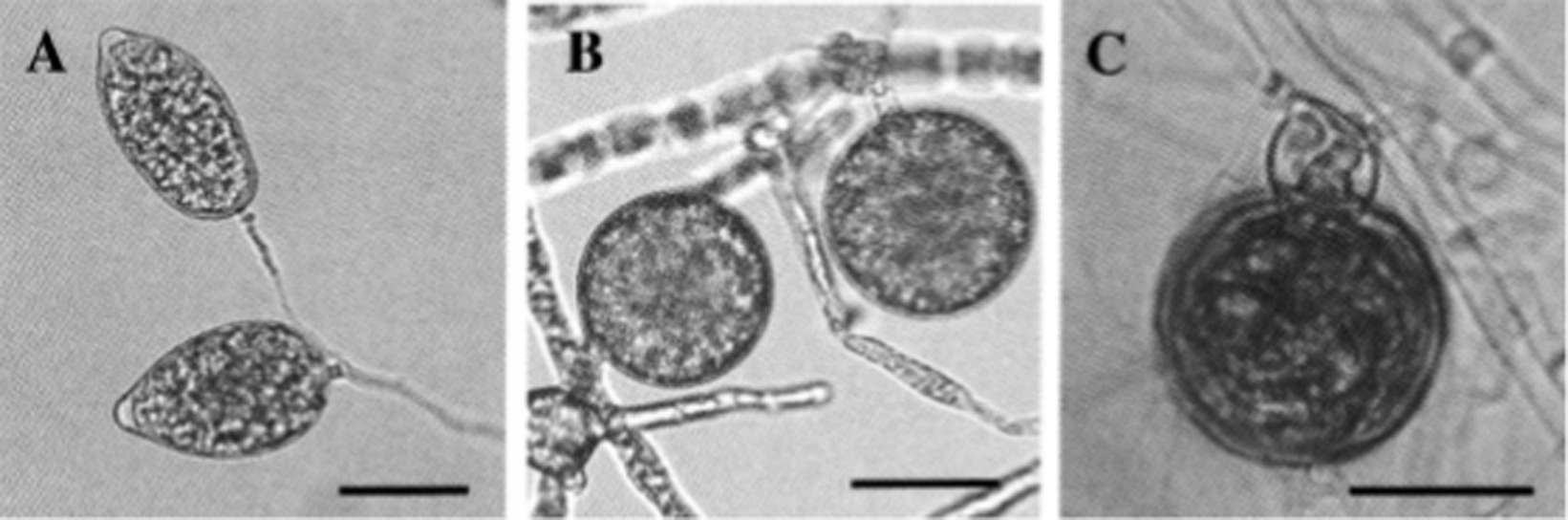
Figure 7. Morphological characteristics of Phytophthora palmivora on Dinema polybulbon orchid. (A) Sporangia, (B) chlamydospores, and (C) oospore (bar = 30 μm; Courtesy: Suzuki et al., 2008).
Phytophthora nicotianae
The nomenclature of P. nicotianae has been under debate for a long time. Based on the detailed comparative morphological studies, Ho and Jong (1989) opined that P. nicotianae and P. parasitica should be considered single species. Following the International Code of Botanical Nomenclature, P. nicotianae, which has priority, should be used as the correct name throughout the world. Over the past few decades, the binomial name P. nicotianae has gained official recognition. The fungal colonies show cottony growth on V8A, CMA, and OMA with non-septate hyphae, hyaline (av. 5.05 μm wide), and no hyphal swellings. It consists of rounded, thick-walled rounded chlamydospores, either intercalary or terminal, measuring 19.71–49.58 μm (average diameter 30.15 μm). Sporangia are non-deciduous, terminal or occasionally intercalary, and conspicuously papillate; shapes may be different, such as pyriform, obpyriform, ellipsoid, ovoid, or spherical, measuring 23.07–70.31 × 17.09–57.91 μm (av. 46.20 × 34.89 μm) with mean L/W ratios of 1.34. Antheridia are amphigynous (Table 7) and round to short cylindrical; oogonia are smooth and globose, measuring 17.38–31.03 μm (av. 24.04 μm) in diameter (Figure 8). No antheridia or oogonia production is observed in single cultures until provided with appropriate mating strains (Tao et al., 2011b). P. nicotianae is a clade 1 Phytophthora species (Kroon et al., 2012).
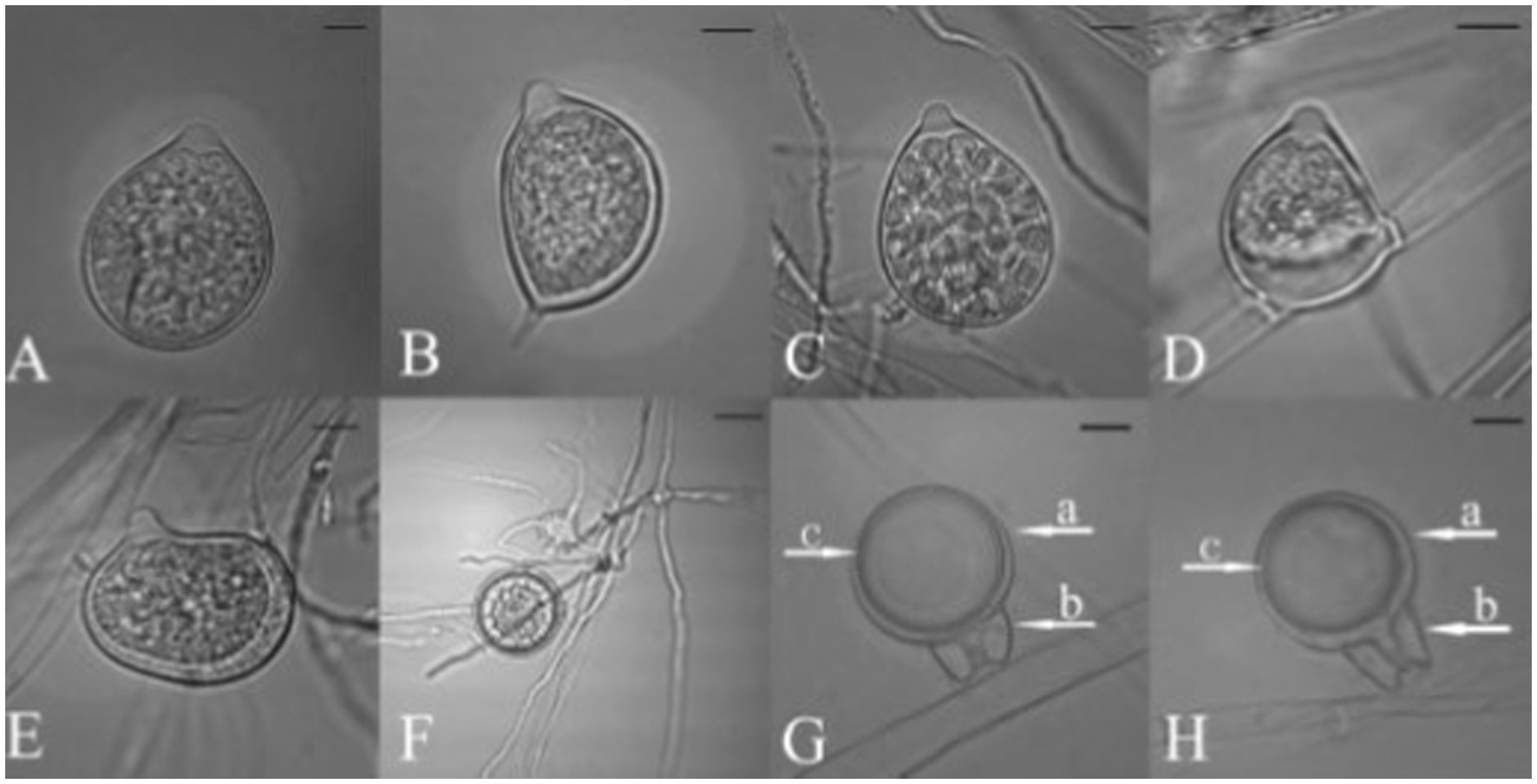
Figure 8. Morphological characteristics of Phytophthora nicotianae, (A–E) Papillate Sporangia, (F) Chlamydospore, (G,H) spherical oospore, a: Oogonia, b: antheridia (bar = 10 μm; Courtesy: Tao et al., 2011b).
Phytophthora cactorum
This fungus is also one of the causal pathogens of black rot in orchids. It mainly occurs in temperate regions. P. cactorum was first described as a pathogen of cactus in 1870. Since then, it has been reported on several plant species worldwide, infecting approximately 150 plant genera belonging to at least 250 known plant species (Erwin and Ribeiro, 1996). For decades, it has been well-established as an orchid pathogen in many temperate countries. P. cactorum can proliferate on a range of standard laboratory mediums, including PDA, CMA, LBA, and V8A. PDA and V8A show white fungal colonies. Mycelium is hyaline, coenocytic, and readily develops zoosporangia on culture media or host tissue maintained in a humidity chamber under constant fluorescent light at 25–28°C (Cating et al., 2010). Zoosporangia is typically ellipsoidal to pear-shaped to spherical (size 30 × 26 μm), usually terminal, papillate, easily detachable, and pedicel less than 4 μm long. Owing to the homothallic nature of the fungus, it is easy for it to develop sexual structures on standard laboratory media (Erwin and Ribeiro, 1996; Gallegly and Hong, 2008). Antheridia are paragynous (Table 7), spherical to irregularly clavate, and measure 13 × 15 (−21) μm. Oogonia are spherical or taper at the base and measure 25–32 μm in diameter. Oospores have an aplerotic shape with a diameter of 20–26 μm. Chlamydospores are usually not formed; however, they may be terminal or intercalary, measuring 20–40 μm in diameter if produced. P. cactorum is a clade 1b Phytophthora species (Kroon et al., 2012).
Phytophthora multivesiculata
Phytophthora multivesiculata has been reported as a causal agent of leaf blotch and rots of pseudobulbs in Cymbidium. The disease was first reported by Ilieva et al. (1998) with the speciation of this fungus under the genus Phytophthora. Colonies of P. multivesiculata on V8, PDA, OA, and CA show moderately fluffy aerial mycelium, a bit denser in the center of the colony on PDA; on CMA, mycelium is submerged (Ilieva et al., 1998). In CMA, the major hyphae are coenocytic, branched, and 6 mm wide; the hyphal swellings are distinctive characteristics of the fungus (Figure 9). Numerous hyphal swellings were common on agar and in water culture. Hyphal swelling is usually described as spherical, elliptical, catenulate, and grouped. Hyphal swellings typically vary from 14 to 36 mm in diameter, new branches develop at sharp angles, and no chlamydospores were reported. The fungus is reported to be homothallic, as both asexual and sexual productions are observed. Sexual structures are produced on both agar and liquid media as well as in plant tissues. Sporangiophores are usually long, slim (2.0–3.0 μm), mostly twisted, and borne singly on the sporangiophores. Sympodial arrangements of sporangia are also recorded occasionally (Ilieva et al., 1998). Sporangia are produced in both solid and liquid media, measuring 45.0 μm (30.0–60.0 μm) × 33.0 μm (20.0–41.0 μm). They may be ovoid, obpyriform, non-papillate, or semi-papillate, and exit pores range from 8.0 to 14.0 μm wide. Antheridia are amphigynous (95%), and some are diclinous (Table 7). It can be irregularly spherical or ellipsoidal. Oogonia are spherical and smooth-walled and vary from 28.0 to 50.0 μm on V8 medium. Oospores are mainly aplerotic and vary from 24.0 to 42.0 μm (Ilieva et al., 1998).
The characteristics of P. multivesiculata resemble the biological features of P. porri and P. megasperma. However, with critical examination, P. multivesiculata can be distinguished from P. porri and P. megasperma based on morphology, pathogenicity, and temperature requirements (Figure 9). Although differences are very small, a clear-cut distinction prevails among these species. To date, P. porri is known to be pathogenic to the genus Allium and Brassica, but P. multivesiculata is pathogenic to the genus Cymbidium. P. porri has slow growth and low optimum and maximum growth temperatures. P. multivesiculata exhibits a considerably higher growth rate at 20°C than P. porri, but lower than those observed for P. megasperma. The maximum growth temperature for P. multivesiculata was 35°C. Typical catenulate hyphal swellings for P. multivesiculata may be observed occasionally in P. Porri, but their appearance is mainly in a more radiating manner. Hyphal swellings are more frequent and abundant in P. multivesiculata than in P. megasperma. Furthermore, antheridia in P. multivesiculata are mainly amphigynous (95%); they are mostly paragynous in P. megasperma. An aberrant strain of P. multivesiculata from Taiwan on Cymbidium was reported by Chern et al. (2011). Chain-like catenulate hyphal swellings of Taiwanese isolates were similar to those of Netherlands isolates of P. multivesiculata. However, sporangia, oogonia, and oospores are larger in the Taiwanese isolates than in the Netherlands isolates. The oogonial wall of Taiwanese isolates is echinulate, but Netherlands isolates have smooth wall oogonia. In addition, the maximum growth temperature for the Taiwanese isolate of P. multivesiculata is 29°C, whereas the maximum growth temperature for the Netherlands isolates is 35°C. P. multivesiculata exhibits isozyme profiles that differ from the profiles of P. megasperma and P. porri types (Ilieva et al., 1998). P. multivesiculata belongs to a clade 2 Phytophthora species (Kroon et al., 2012).
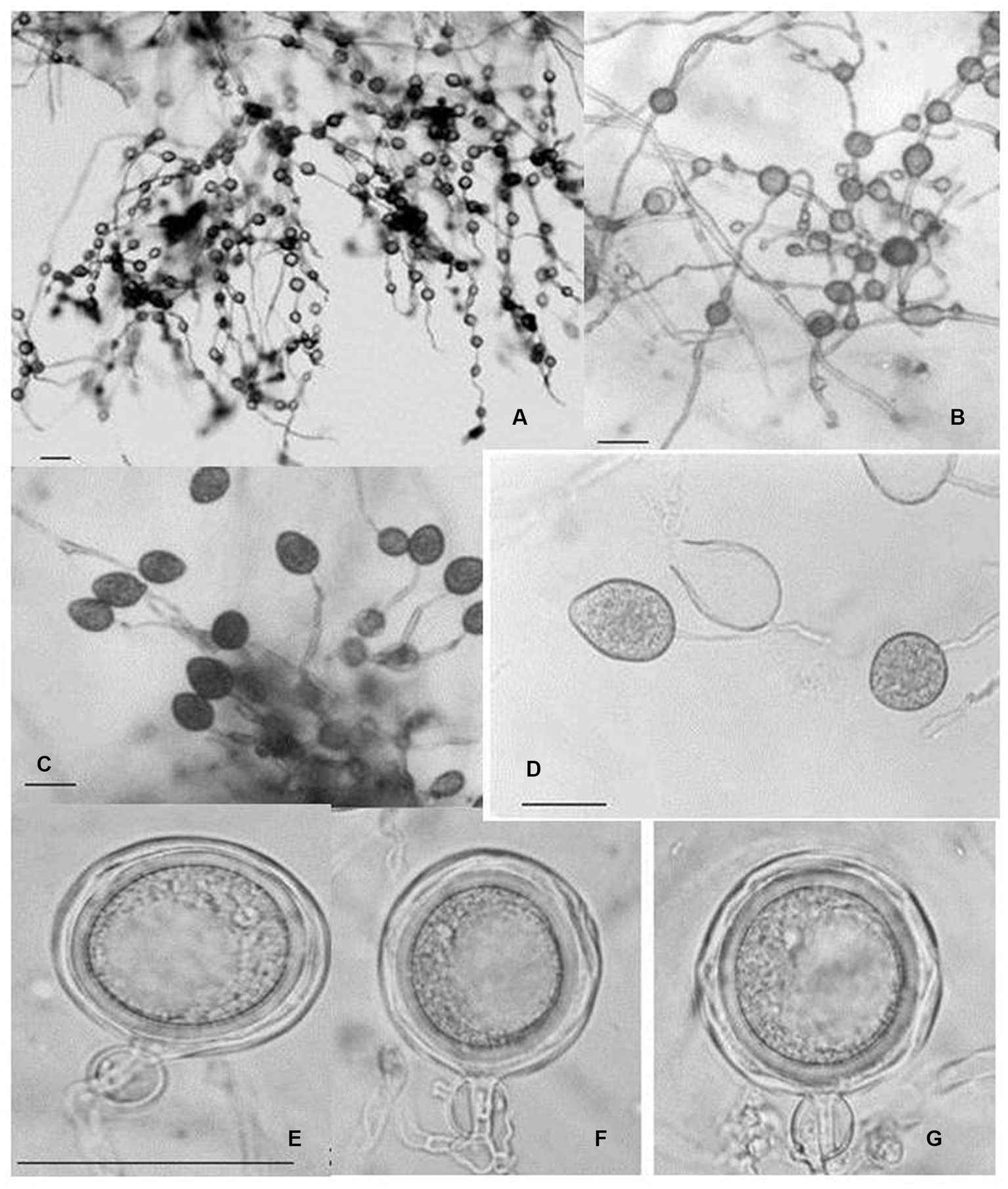
Figure 9. Morphological characteristics of P. multivesiculata infecting Cymbidium hybridum: (A, B) hyphal swellings in chain; (C) primary sporangia, (D) proliferation of Sporangia; (E–G) oospores (bar = 50 mm; Courtesy: Chern et al., 2011).
Phylogenetic relationship among orchid infecting Phytophthora species
A comprehensive phylogenetic analysis of Phytophthora species comprising 39 sequences of 6 notable orchid-infecting species such as P. palmivora, P. cactorum, P. nicotianae, P. capsici, P. tropicalis, and P. multivesiculata was carried out, which exhibited diverse host affinities toward orchids such as Vanda, Dendrobium, Oncidium, Cymbidium, Phalaenopsis, Cattleya, Pleione, and Vanilla. The investigation led to the emergence of four distinct clades, as visually depicted in Figures 10A,B. Remarkably, P. multivesiculata was observed to cluster within clade I, while P. palmivora species formed a cohesive group in clade IV. Additionally, the study unveiled a close relationship between P. tropicalis and P. capsici within clade II and a clustering of P. cactorum with P. nicotianae in clade III. These findings contribute valuable insights into the evolutionary dynamics and relatedness among the Phytophthora species infecting orchids studied.
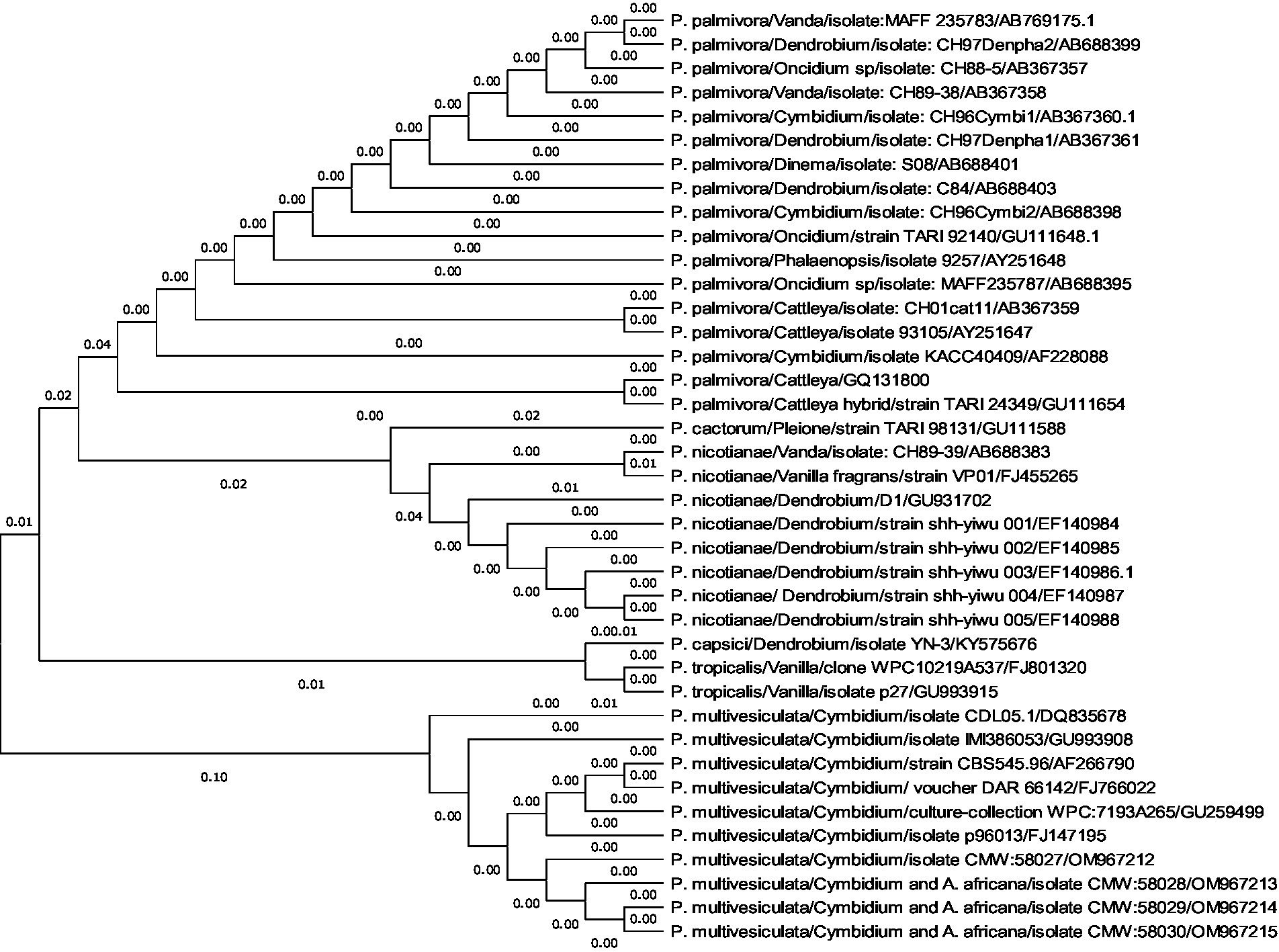
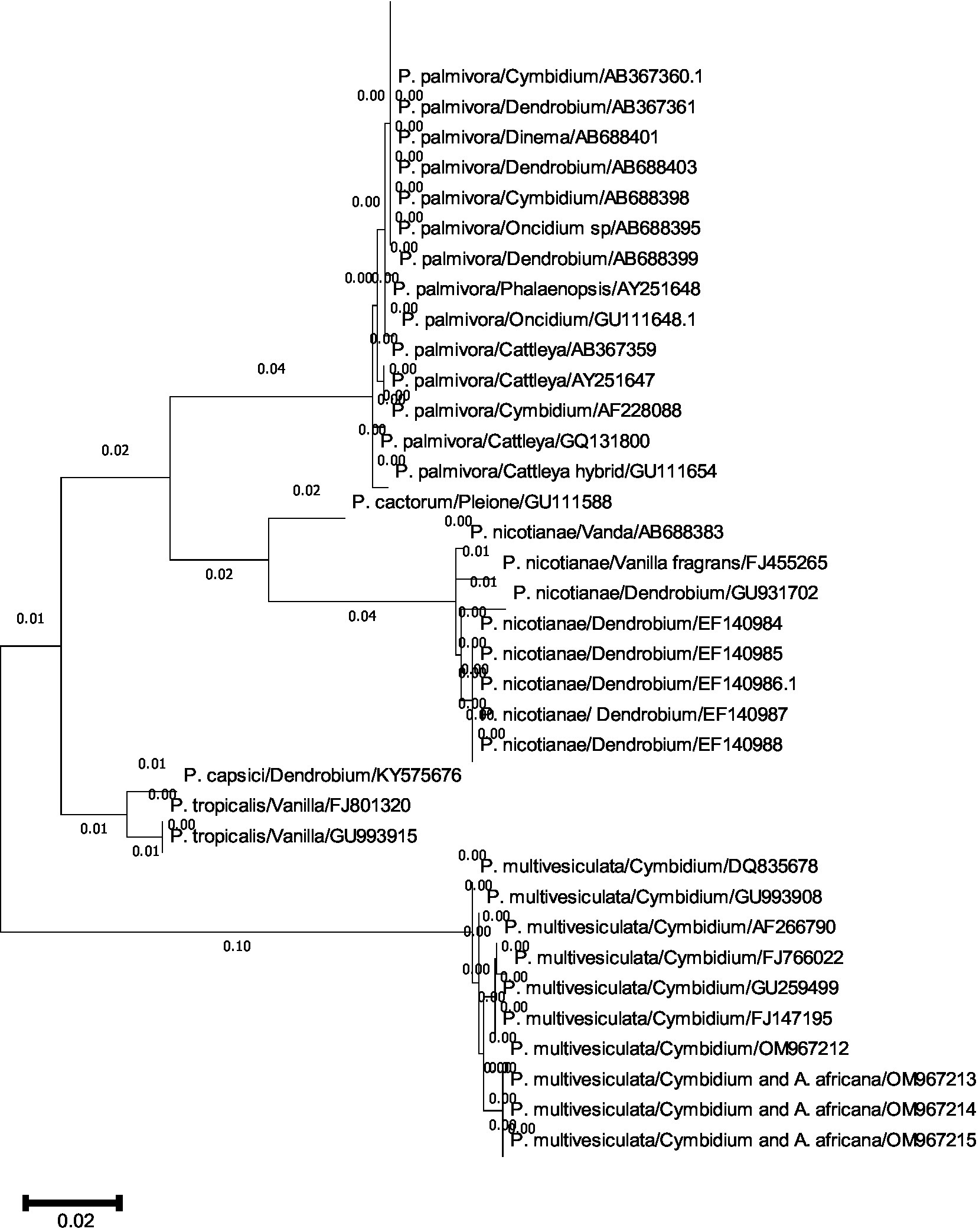
Figures 10. (A,B) Evolutionary analysis by maximum likelihood method. (A) Phylogenetic relationship of different species of Phytophthora infecting orchids. (B) Phylogenetic relationship of different species of Phytophthora infecting orchids.
The evolutionary history was inferred by using the maximum likelihood method and the Tamura–Nei model (Tamura and Nei, 1993). The tree with the highest log-likelihood (−2779.26) is shown. The initial tree(s) for the heuristic search were obtained automatically by applying neighbor-join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura–Nei model and then selecting the topology with a superior log-likelihood value. This analysis involved 39 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Non-coding. There were a total of 964 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 (Tamura et al., 2021).
Mating types and races of Phytophthora infecting orchids
The production of oospores in Phytophthora is very important from an evolutionary point of view for pathogenic variability, fitness of the conventional hosts, and their constantly changing patterns with the continuous evolution of new man-made hybrids. In some of the species of Phytophthora (called heterothallic), two opposite sexual types are prerequisite for the formation of oospores through sexual reproduction. Other groups of Phytophthora (called homothallic) do not require this specificity, but they can produce oospores themselves. Based on the production of oospores in paired cultures, “mating type” or “compatibility type” A1 and A2 in Phytophthora were first conceptualized by Gallegly and Galindo (1958) using the potato-Phytophthora infestans pathosystem. It is globally used for all Phytophthora species, which consist of only two mating types. After 30 years, another mating type, “A1A2,” was introduced by Ko (1988); therefore, there are three mating types, A1, A2, and A1A2, in the genus Phytophthora at present.
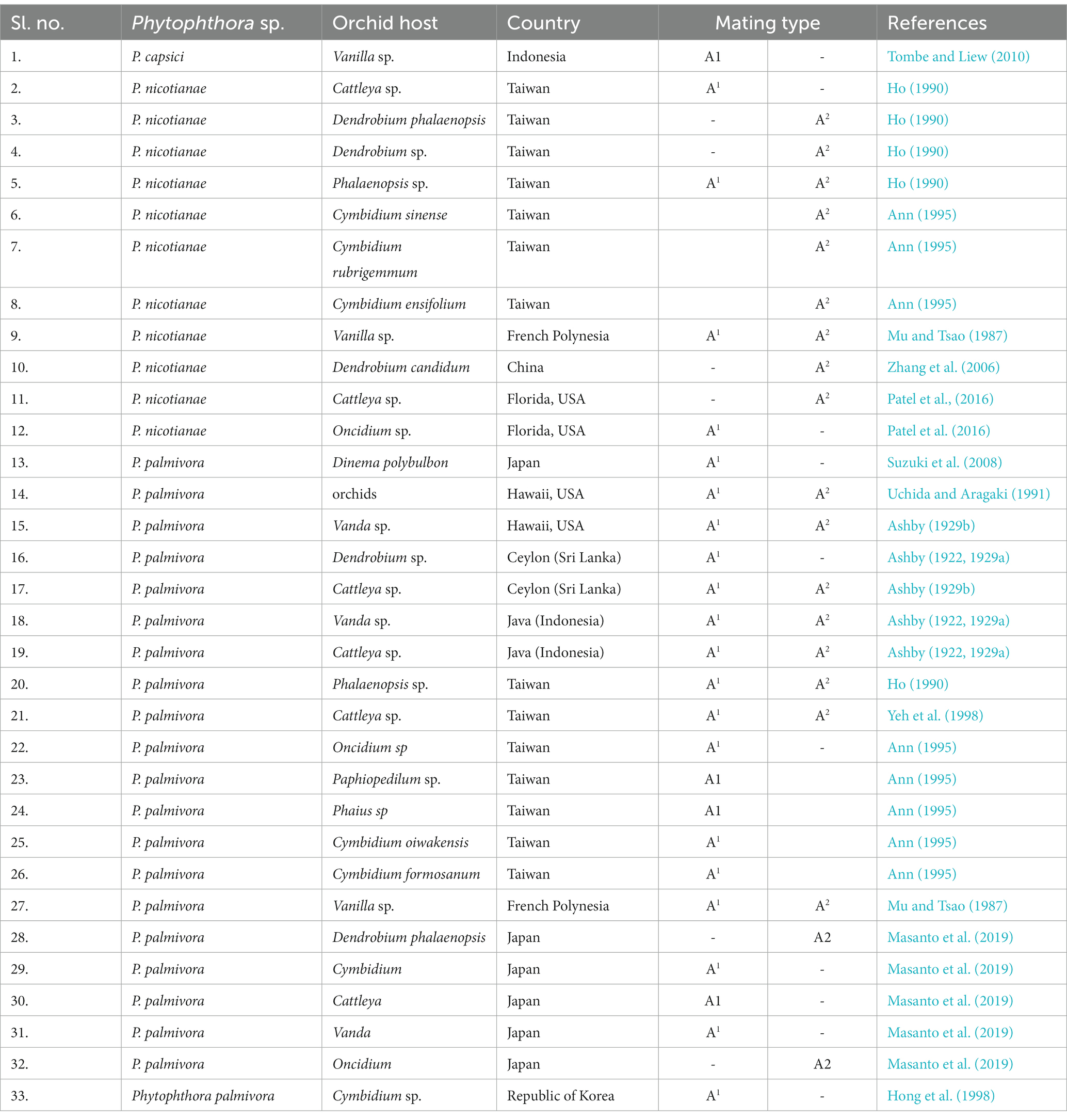
Among the Phytophthora species infecting orchids, P. palmivora, P. nicotianae, P. cinnamomi, and P. capsici are heterothallic species, and so they can multiply asexually as well as sexually (Erwin and Ribeiro, 1996). In the presence of both mating types, sexual reproduction leads to the production of oospores, which ensures a longer period of natural overwintering in addition to genetic variability in the germination of oospores, making races or pathotypes of the pathogen more complex. Both mating types A1 and A2 (Table 8) have been reported for P. palmivora infecting various orchid genera in French Polynesia (Mu and Tsao, 1987); Hawaii, USA (Ashby, 1929b; Uchida and Aragaki, 1991); Ceylon (Sri Lanka; Ashby, 1929b); Java (Ashby, 1922, 1929a); and Taiwan (Ho, 1990; Yeh et al., 1998). However, only the A1 mating type was reported from the Republic of Korea (Hong et al., 1998), Sri Lanka (Ashby, 1922, 1929a), and Japan (Suzuki et al., 2008).
Both mating types A1 and A2 were recorded for P. nicotianae in Taiwan (Ho, 1990) and French Polynesia (Mu and Tsao, 1987), infecting Phalaenopsis and Vanilla orchids, respectively, and other P. nicotianae isolates were either A1 or A2 in Taiwan and Florida (Patel et al., 2016). Reports of races that are common in heterothallic species of Phytophthora, viz., 24 races in P. capsici causing pepper root rot (Barchenger et al., 2018), are in the public domain. Interestingly, no races of P. palmivora, P. nicotianae, or P. capsici infecting orchids have been reported globally to date, as per our knowledge. One reason could be that sufficient scientific attention was not paid to this aspect with special reference to orchido-phytopathogenic P. palmivora, P. nicotianae, and P. capsici on various species or hybrids of the orchid world over. Another reason could be that the genomic organization of present-day interspecific, intergeneric, and/or multi-generic hybrids is so complex that the successful establishment of differential lines/ hosts of orchids (based on R-gene) for race identification may not be possible or is a researchable issue for future attention.
Epidemiology of Phytophthora species infecting orchids
The epidemiological aspects of the species of Phytophthora infecting orchids have not been studied well under field conditions, unlike the other Phytophthora species infecting commercially cultivated fields and horticultural crops. Most of the available literature on epidemiology is based only on in vitro observations. Perhaps Hine (1962) was the first scientist to study the influence of temperature and length of wetness period on the mycelial growth, zoosporangia, and zoospore formation in P. palmivora infecting Vanda, Cattleya, Epidendrum, and Dendrobium in Hawaii. The minimum and optimum temperatures for mycelial growth on V8A medium were observed to be 10°C and 28–31°C, respectively. The fungus can grow up to 25°C but not at 37°C. The zoosporangia and chlamydospore formation start after 3 days on V8A medium and stems or leaves of Vanda orchids at a temperature between 20 and 31°C. Zoospore formation requires optimum water temperatures between 15 and 25°C and remains active for the longest time (5 h) in water at 25°C. No zoospores are visible at a water temperature of 28°C. In Vanda, wounded leaves can be infected with zoospores at temperatures between 15 and 31°C. Generally, the disease does not occur during the hotter months of the year, even if there is sufficient moisture on the plant surface because of the inhibition of zoospore formation above 25°C. According to Yeh et al. (1998), the minimum, optimum, and maximum temperatures for mycelial growth of P. palmivora causing black rot of Vanda are 10, 24–32, and 35°C, respectively. The optimum temperature requirement for zoosporangia production on V8A and the leaf surface of Cattleya is 24°C. The maximum number of zoosporangia is produced at 100% RH, whereas no zoosporangia are produced below 80% RH. Zoosporangia germinate directly at an optimum temperature of 24°C whereas indirect germination of zoosporangia can occur from 8 to 32°C with an optimum temperature of 16°C. No zoospores are formed at 35°C.
A comparative study was conducted by Hsieh (1984) on the temperature requirements for zoosporangia formation and germination and chlamydospore formation in P. palmivora and P. nicotianae. Both produce zoosporangia between 12 and 32°C (optimum at 24–28°C for P. palmivora and 24°C for P. nicotianae), and a large number of zoosporangia are formed at 95% RH but no zoosporangia are formed at 85% RH. Both pathogens produce abundant zoosporangia at 90–100% RH on the disease leaves of orchids. They prefer to produce more zoosporangia on diseased leaves (such as Vanda sp.) than chlamydospores at 90–95% RH; however, at a lower range of RH of 80–85% and a higher temperature of 36°C, only chlamydospores are produced. Young zoosporangia germinate indirectly between 8 and 28°C, and mature zoosporangia germinate directly between 20 and 28°C. Chlamydospore germination takes place at a temperature range of 8–37°C, with an optimum temperature of 28 and 24°C for P. palmivora and P. nicotianae, respectively. They also observed that a favorable temperature for disease development is 16–36°C, with an optimum of 28°C. At the saturated condition of relative humidity, lesion development was faster but decreased in drier conditions on the diseased leaves of orchids.
Phytophthora multivesiculata is host-specific to the Cymbidium orchid. However, epidemiological data on this pathogen in orchids are limited. P. multivesiculata on Cymbidium in the Netherlands was reported by Ilieva et al. (1998). They observed that the disease occurs on the leaves after prolonged periods of rain or on the indoor plant after irrigation. P. multivesiculata grows at a faster rate on the V8A medium at 20°C, and the maximum temperature for growth is 25°C (Ilieva et al., 1998). The temperature range for growth of an atypical (aberrant strain) P. multivesiculata that causes black rot on Cymbidium species is reported to be 10–29°C in Taiwan, and there is practically no growth at 30°C, which is 6°C less than that of typical P. multivesiculata (with 35°C as the highest growth temperature) described from the Netherland. The optimum growth temperature of Taiwanese isolate is reported to be 24°C on a V8A medium (Chern et al., 2011).
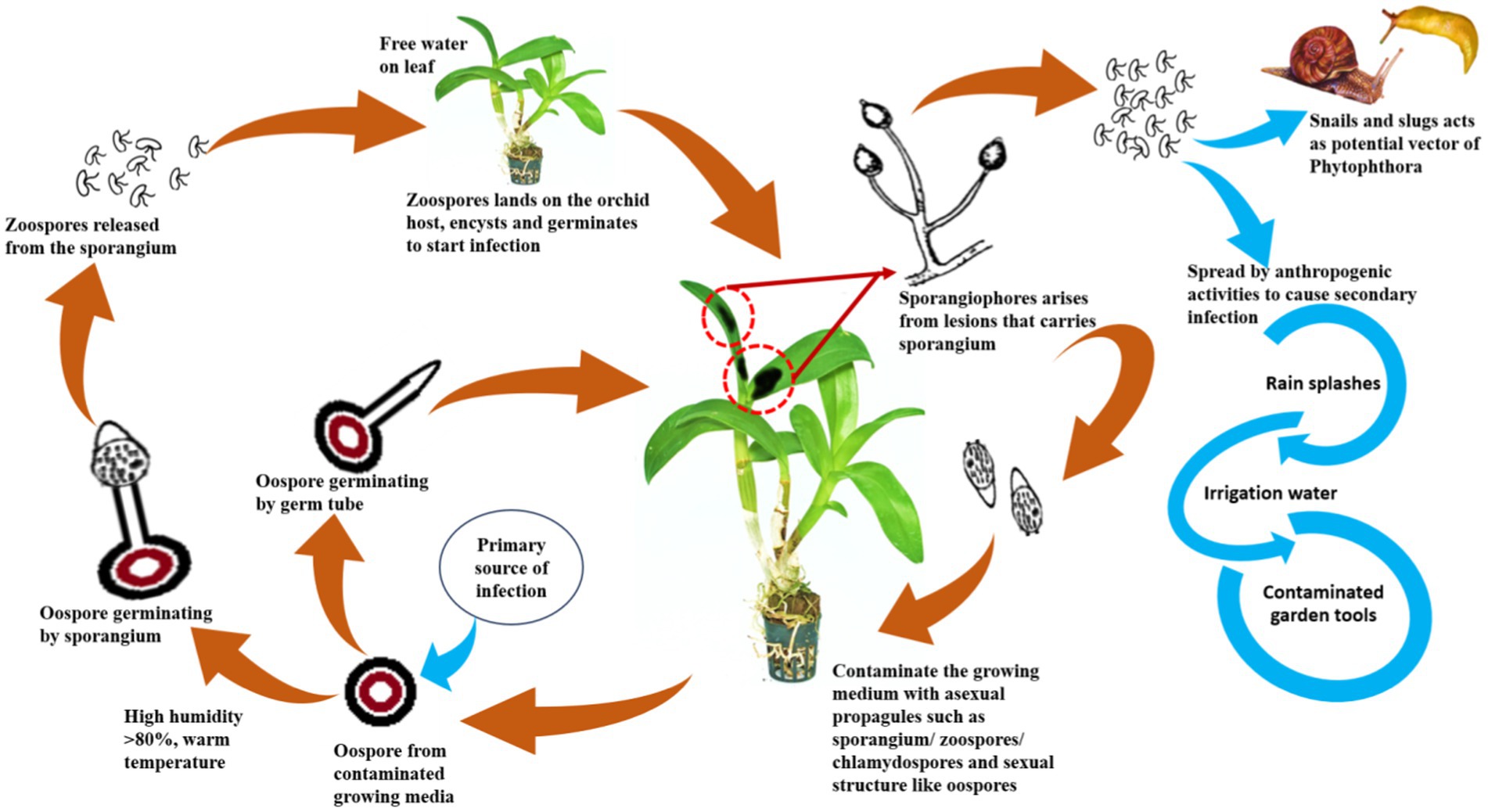
Dissemination of orchids infecting Phytophthora
To develop management strategies, it is crucial to understand the dispersal of plant pathogens, particularly water molds. Since most of the orchids are grown under controlled conditions in artificially constructed polyhouses/net houses, anthropogenic factors are mostly contributed to the spread or dissemination of orchid Phytophthora diseases. The spores may spread from one plant to another neighboring plant by watering or water splashing; thus, irrigation water can act as a medium for the spread of zoospores (Uchida, 1994). P. palmivora is reported to be spread by sprinkling water and by the import of young plant materials into greenhouse crops (Orlikowski et al., 2008). Contaminated garden tools, agricultural implements, plant transport trailers, or carts can also passively spread Phytophthora. Snails and slugs are also considered potential agents for the spread of Phytophthora zoospores by either carrying the zoospores on their bodies or by ingesting the zoospores and later discharging the viable zoospores through excreta on the plant surface (Uchida and Aragaki, 1991; Figure 11). The intercontinental movement of planting material can passively disseminate Phytophthora. It was observed that imported prefinished Cattleya liners from Thailand are often infected with P. cactorum during monsoon seasons in Florida, USA (McMillan et al., 2010).
Molecular diagnostic for major orchids infecting Phytophthora
Orchids are legally or illegally traded across the world (Fay, 2015), due to which live orchid plant materials may carry Phytophthora either on plant surfaces or in potting media from one country to another, where it may or may not be present as a serious pathogen. Hence, the diagnosis of orchid diseases caused by Phytophthora species is of paramount importance for advocating an appropriate management strategy and regulation of the movement of this destructive phytopathogen through traded orchid materials. Proper diagnosis of any Phytophthora species infecting orchids depends on the pathogenicity and morphology of the pathogenic fungi or through molecular techniques. Symptoms, signs, and pathogenicity tests of isolated fungi give vital clues about the hosts, but these cannot always provide the true identity of the Phytophthora species. The reality is that the diagnosis of orchid Phytophthora based on symptomatic observation is hard and difficult to distinguish from those caused by the phytobacterial orchid pathogen Erwinia carotovora subsp. carotovora (Su and Leu, 1992), and possibly it becomes worse because these orchid pathogens may simultaneously infect orchids (Tsai et al., 2006).
Traditionally, the identification of the orchid Phytophthora is based on the characteristics of mycelium, hyphal swellings, branching of sporangiophores, shape and size of zoosporangia, presence of chlamydospores with their size and position, formation of antheridia, and oogonia along with their position (either paragynous or amphigynous) and oospores characters that confirm the fungi up to the level of the genus “Phytophthora.” Diagnostic tools used certainly depend on the ease of available facilities at the diagnostic laboratory or research units. Prior to the development of advanced diagnostic molecular techniques, most mycologists, or specifically plant pathologists, used conventional techniques of culturing the fungi on specific media for sporulation or fructification, followed by microscopic observation to define the fungi up to species level using identification keys given by stalwart mycologists’ time to time. Often, identification based on taxonomic keys leads to erroneous identification when compared with molecular data.
Therefore, nowadays molecular diagnostic data with multiple parameters are thought to be essential for the correct identification of the Phytophthora species infecting orchids. Sequencing the internal transcribed spacer (ITS) regions of rDNA is conventionally used as a molecular diagnostic tool to distinguish between P. cactorum, P. palmivora, and P. nicotianae, as well as many other Phytophthora species (Brasier and Griffin, 1979; Cooke and Duncan, 1997; Cooke et al., 2000). Based on the sequence analysis of rDNA-ITS regions, P. palmivora causes black rot of Dinema polybulbon in Japan (Suzuki et al., 2008), P. nicotianae causing Dendrobium candidum blight in China (Zhang et al., 2006), and P. multivesiculata causing leaf blotch and rot on Cymbidium orchids in New Zealand (Hill, 2004) have been identified (Figure 7).
However, the variation in DNA sequence in the ITS regions may not be enough to discriminate among closely related Phytophthora species (Martin et al., 2014), so it essentially necessitates the use of multiple criteria for species identification. Sequencings of the ITS1, 5.8S rRNA gene, and ITS2 regions of a Phytophthora isolate were carried out to identify P. palmivora causing black rot on Cattleya orchids in Florida (Cating et al., 2010). P. nicotianae causing blight on Dendrobium aurantiacum, Dendrobium chrysanthum, Dendrobium chrysotoxum, and Dendrobium thyrsiflorum was confirmed by using multiple molecular data along with morphology and pathogenicity tests in Yunnan Province, China. The gene sequences targeting multiple genes such as ITS1, 5.8S rRNA, ITS2, and β-tubulin of the pathogen were analyzed and compared to those of other known P. nicotianae available on GenBank. The results were used to confirm the identification of the orchid pathogen, P. nicotianae infecting different Dendrobium species (Tao et al., 2011b).
Isozyme analysis has proven to be a powerful tool in Phytophthora taxonomy (Oudemans and Coffey, 1991a,b). Isozyme analysis, along with the ITS sequence of a suspected Phytophthora isolate, can provide further insights into the pathogen for species differentiation among closely related Phytophthora species. Profiles of isozymes such as MDH, i.e., malate dehydrogenase; MDHP, i.e., a malic enzyme; and isocitrate dehydrogenase (IDH) of Phytophthora sp. infecting Cymbidium orchids and closely related P. porri and P. megasperma were analyzed, and it was found that the IDH pattern of Phytophthora sp. infecting Cymbidium is different from those of P. porri and P. megasperma. Furthermore, the ITS sequence of Phytophthora sp. causing blackening of leaves and stems on Cymbidium orchids in the Netherlands has been determined as P. multivesiculata, which was found to be unique but closely related to that of P. citricola. However, P. multivesiculata has amphigynous antheridia and hyphal swellings, whereas P. citricola has paragynous antheridia and no hyphal swellings. Thus, P. multivesiculata, infecting Cymbidium orchids, was identified as a new species of Phytophthora (Ilieva et al., 1998). An aberrant strain of Phytophthora sp. causing black rot of Cymbidium orchids in Taiwan was precisely identified as P. multivesiculata based on analysis of soluble protein patterns closely related to Phytophthora species and the sequence of ITS regions, including ITS1-5.8S rDNA-ITS2 plus, partial 18S rRNA and 28S rRNA of Phytophthora sp. infecting Cymbidium orchids (Chern et al., 2011).
In the era of global trade of orchids, quick and faster detection techniques are in great demand, particularly in quarantine stations in airports or land frontiers or in plant disease diagnostic laboratories, where the diagnosis of Phytophthora diseases in orchids is carried out. Based on the traditional method of isolation in pure culture of Phytophthora from disease plants, followed by identification based on morphology, this is indeed laborious and time-consuming. A rapid and simple process of diagnosis, i.e., nested PCR assay, has been developed for fast and accurate detection of orchid Phytophthora pathogens at the National Taiwan University, Taiwan (Tsai et al., 2006). In this unique diagnostic technique, fungal DNA is directly extracted from infected orchid (Oncidium sp.) host tissue, which is then subjected to PCR amplification with a Phytophthora-specific primer set. Amplification of DNA fragments of approximately 1 kb confirms the presence of Phytophthora pathogens in the infected orchid sample. Subsequently, nested PCR is run to identify the species of Phytophthora using the amplified product of the first PCR as the template DNA and species-specific oligonucleotides as the species primers (Pal1s/Pal2a for P. palmivora and Paris/Par2a for P. parasitica = nicotianae). Amplification of specific DNA fragments indicates the presence of either P. palmivora or P. nicotianae or both in the infected orchid host (Tsai et al., 2006). Nested PCR assay, indeed, offers sensitive and rapid detection of orchid Phytophthora pathogens. If properly designed, this assay can also be used to detect Phytophthora pathogens from contaminated potting media of orchids and thereby greatly contribute to the early diagnosis of destructive Phytophthora diseases of orchids. Various sequence-based PCR techniques along with specific primers are available for the detection of Phytophthora spp. infecting orchids (Table 9) and they can be used suitably for the diagnosis of Phytophthora diseases of orchids.
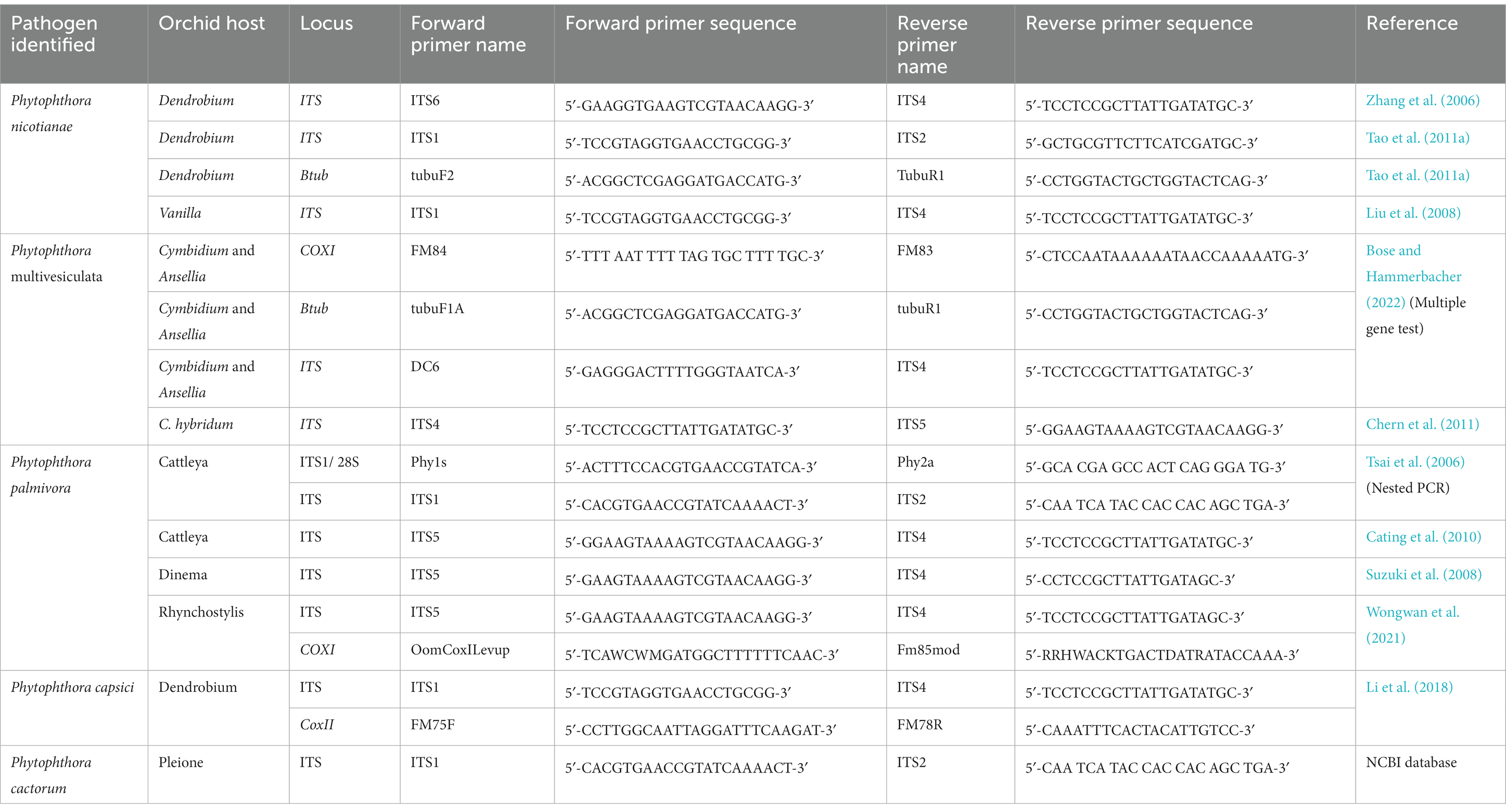
Table 9. Sequence-based PCR detection of different Phytophthora species infecting orchids with primer pairs.
Management strategies and options
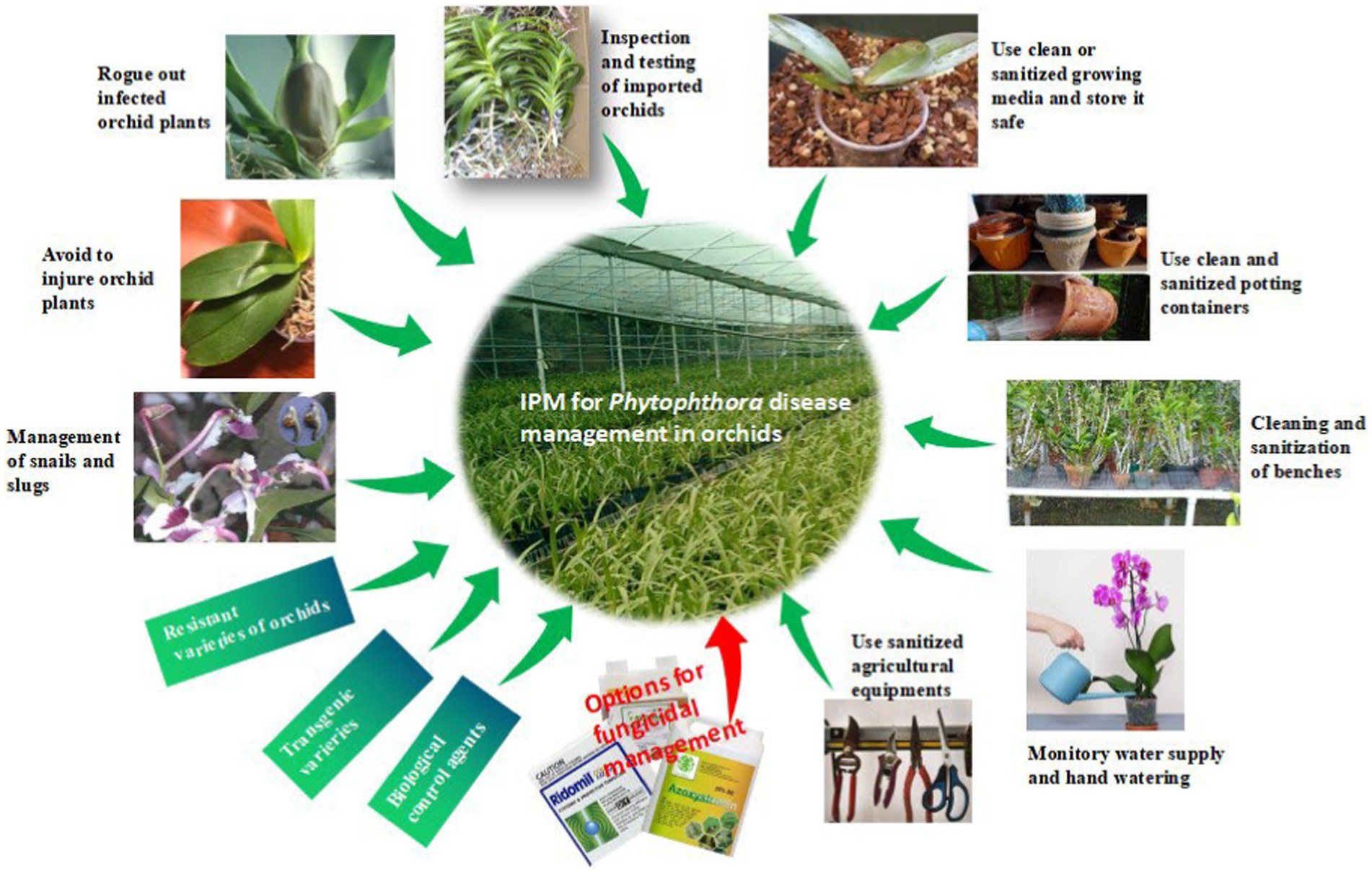
Often, we come across the popular statement “maintenance of beauty is a costly affair.” This statement is aptly applicable to orchids. Unlike openly cultivated fields and other horticultural crops, the orchid crop requires special attention in every stage of the production process, starting from the vegetative phase to flowering and the ultimate sale of the flower to consumers, which certainly involves some extra cost. It may be noted that an exquisitely beautiful orchid hybrid requires special attention for hardening and early establishment in a community pot with proper watering and nutrition after the micropropagation; otherwise, seedlings will be aggravated with so-called water mold, Phytophthora or Pythium, causing seedling blight, and there may be a chance of losing the costly orchid hybrid developed by the breeder for the first time. Sterilized potting media with optimum watering is required to protect seedlings from damping off during the initial establishment of the seedlings. The integrated management strategies of Phytophthora in Orchid are presented in Figure 12, and each of the components is described thereafter.
Maintenance of optimum water level
Phytophthora spp. are water molds; thus, they require water for the development of zoosporangia and zoospores and the further germination of zoospores on the orchid hosts. As a result, water used for irrigation acts as a dispersal medium for the spread of zoospores infecting other plants due to water splashing or watering (Uchida, 1994). Furthermore, zoospores, which are motile in free moisture, are the ultimate infective propagules on orchid hosts and can move fast when free water is readily available. Naturally, high moisture accelerates disease as well as fungal life cycles. The virtual effect of growth and development of pathogens largely aids the development of Phytophthora diseases rather than the direct effect of high levels of water on the plant. It is extremely crucial to regulate excess watering and reduce high relative humidity (RH) to manage Phytophthora diseases of orchids in small amateur households as well as commercial orchid establishments. All these can be successfully achieved by constructing solid-covered greenhouses or glass houses with good ventilation, reducing prolonged periods of wetness, and using well-drained potting media (Uchida and Aragaki, 1991; Cating et al., 2009). However, growers always need to remain alert with long-term holistic strategies and actions, including preventive, mechanical, and chemical treatment throughout the crop life, for the successful management of Phytophthora diseases of orchids. In addition, to keep Phytophthora diseases under control, growers should also keep in mind the following points during the production process:
Growing media and its storage
The growing medium for orchid production plays an important role in the management of disease, as this pathogen is soil-borne. The media used should be fresh and free from the inoculum of Phytophthora. Even at the stage of repotting, one should take care of media, as the reuse of old media may potentially contaminate and cause the mortality of seedlings. Freshly purchased growing media should be stored in unopened bags in a closed room. The sealed bags of growing media can also be stored on a concrete floor covered with a polythene sheet. Concrete floors should be periodically washed with bleach (1:3 ratio of sodium hypochlorite to water). Growing media in opened bags, left in an unattended condition for a long time, may get contaminated with species of Phytophthora, which may cause the initiation of black rot in the next season if potted with negligence.
Potting containers
The containers used for the growing of orchids also play an equally important role as media, as they can also carry the potential inoculum of Phytophthora. It is advisable to go for new containers wherever it is possible, or the old containers should be treated with a bleaching solution (1:3 ratio of sodium chlorite and water) with agitation for at least 10 min.
Cleaning and sanitization of benches
Unlike openly cultivated fields and horticultural crops, orchids are not grown on ground soil. They are grown in earthen pots or plastic containers that are placed on raised benches for sufficient aeration. Benches may be made of iron, wooden planks, or a combination of both items. The bench surface should be at least 1 m above the ground soil to avoid splashing from the ground below. It will be better if the benches are kept on the concrete floor to avoid the soil-inhabiting Phytophthora from reaching the orchid plants kept on the benches. Single-layer benches are ideal; however, multi-layer horizontal benches are not recommendable because, while watering, excess water might drip on the orchid plants kept on the lower benches from the plants kept on the upper benches. Dripping water will carry the inoculum of Phytophthora to lower plants. Sanitization of benches by using available bleaches or disinfectants is also crucial. Several preparations, such as 25% chlorine bleach, 25% pine oil cleaner, 50% rubbing alcohol, 50% denatured ethanol, and 5% quaternary ammonium salts (Cating et al., 2009), are available for sanitization. The wooden portion of benches or wooden benches is difficult to sanitize because they are porous. Regular removal of algae, scum, mildew, and dirt from benches by scrubbing is also required to keep the orchid house clean.
Quality of water used
Any type of surface water, such as ponds, reservoirs, or even fountain water in hilly areas, can be a potential source of Phytophthora zoospores and should not be used unless it is disinfected. In case of hand watering, the hose and wands should be sanitized with a solution of bleach and kept hanging.
Sanitization of agricultural equipment
Tools such as separating knives, root trimmers (secateurs), and pruning scissors should be sanitized regularly with a bleached solution. Glass, plastic, cloth, or other non-metallic tools, pots, and equipment can be disinfested with freshly prepared 10% Clorox, Physan, or Consan (Uchida and Aragaki, 1991). During repotting, disinfected tools and latex hand gloves may be used. Other potential carriers of Phytophthora spores, such as plant transport trailers or carts, should also be sanitized with a bleached solution.
Season of import of planting materials
As Phytophthora spp. are water-loving molds and reproduce zoosporangia and zoospores in high temperatures and saturated moisture conditions, the time of import of orchid materials may also be taken into consideration while importing important breeding lines or extraordinary hybrids for further growth. A study by McMillan et al. (2010) noted that imports of prefinished Cattleya orchid liners from Thailand to Florida (USA) during the monsoon season are often infected with P. cactorum, whereas Cattleya liners imported during the dry season are found to be free from P. cactorum.
Avoidance of wounding orchid plants
Wounds are a prerequisite for the infection of orchid plants by P. palmivora (Hine, 1962; Ann, 1995). One should take a lot of care so that the least amount of injuries are inflicted on any part of the orchid plant while they are in transit for sale or exhibition. Furthermore, care should be taken to minimize injuries during various intercultural operations in the greenhouse.
Isolation of newly introduced orchid plants
New orchid plants introduced in nurseries, greenhouses, or commercial growing hubs may be the potential carriers of Phytophthora spp., either on the surface of the plant as a latent infection or carried with the potting media. Therefore, newly introduced orchid plants should be kept in isolation for at least 6 weeks (Cating et al., 2009) to observe the presence of the disease through the manifestation of disease symptoms. The infected orchid plants are removed, and healthy plants are allowed to grow after fungicide application in the growing hub. This reduces the possibility of Phytophthora’s introduction into the growing area. Sometimes, introduced or imported orchids with visual symptoms of Phytophthora are drenched with fungicides such as Banrot, Truban, Physan 27, Heritage, Stature, Aliette, Subdue Maxx, and Insignia to salvage some of the plants, and severely infected plants are discarded (McMillan et al., 2010).
Management of snails and slugs in the orchidarium
Snails and slugs invade the orchid hubs and feed on the green parts of the orchid plants. They not only damage the orchid crops by feeding on leaves and flowers but also work as passive carriers of Phytophthora when they enter the commercial growing hub of orchids. Helix spp. (snails) and Philomycus spp. (slugs) are considered potential agents for the spread of Phytophthora pathogens in Taiwan (Hsieh, 1984). These soft-bodied animals may carry pathogen zoospores either on their bodies or by ingesting zoospores during the feeding of diseased plant tissue and later excreting viable zoospores on the plant surface (Uchida and Aragaki, 1991).
Chemical disease management
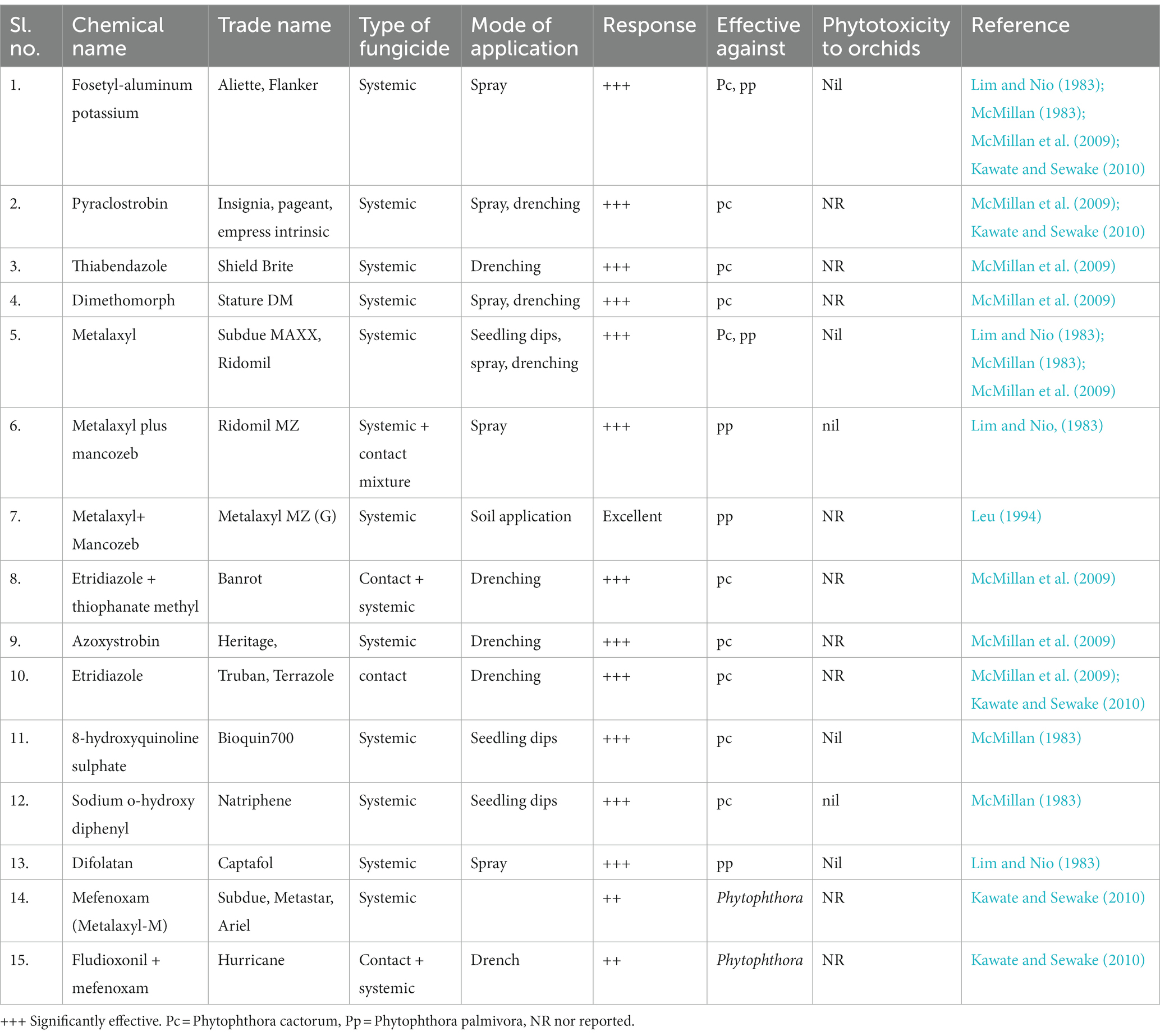
Regular practice of the above-mentioned preventive and mechanical control measures can minimize the intensity of Phytophthora disease in orchids in commercial growing hubs but may not always control a cent percent of the disease. In this condition, options for the application of chemical fungicides can be considered. Early-diagnosed disease can be treated by drenching the plant with a protective fungicide such as Turban or Terrazole. For advanced stages of the disease, systemic fungicides such as Aliette or Subdue will be more effective. Captan, Dithane M 45, and Physan 20 are also recommended for the control of black rot (Jones, 2002). Cattleya leaf and flower bud infection caused by P. cactorum was effectively managed with the preventive sprays of Aliette, Insignia, Stature, and Subdue Maxx. However, preventive drenching with fungicides Aliette, Banrot, Heritage, Insignia, Shield Brite, Stature, Subdue Maxx, and Turban is found to be significantly effective for controlling P. cactorum in community pots for Cattleya seedlings (McMillan et al., 2009). Various fungicides used for the management of Phytophthora diseases of orchids in various countries are listed below (Table 10).
Future perspectives
Since the aegis of wild orchid hunting, much has been achieved, from the domestication of wild orchids to their commercialization in global markets. Once upon a time, beautiful orchids were within the grasp of only the elite classes of people, but now it is brought to the open market for all classes due to the concerted effort of orchid biologists and orchid fraternities. At the same time, orchid health management, with special reference to Phytophthora diseases, has also progressed substantially. However, in the present context of soil and water pollution and global health hazards, eco-friendly options for disease management, viz., the development of resistant hybrids/cultivars, biological disease management, transgenic approaches, and RNAi technology, need encouragement.
Resistance sources of orchids against Phytophthora diseases
In the 21st century, scientists from various sections, such as agriculture or horticulture, are in search of resistant sources either in the existing landraces or exotic world germplasm for prospective use in the development of durable resistant hybrids (maybe they are orchids or other crops). An extensive survey of global literature indicates that at present there are no resistant varieties of any category of orchids against Phytophthora. Even so, there is no report of natural resistance in wild species or landraces of orchids against Phytophthora diseases. Attempts should be made for a systemic search for any novel resistant gene among wild and landraces of orchids that can be incorporated into popular susceptible hybrids/cultivars using suitable molecular techniques. Alternatively, tolerance of orchids could be enhanced against Phytophthora spp. in question by the insertion of chitinase and glucanase genes. Since orchids are mostly propagated using tissue culture techniques, an attempt was made for the in vitro selection of Dendrobium “Earsukul” PLBs (protocorm-like bodies) resistant to P. palmivora using P. palmivora CF (culture filtrate) containing α-elicitin. Several putative mutants resistant to P. palmivora in detached leaf assay have been selected in Thailand. One such Dendrobium mutant resistant to all isolates of P. palmivora is “SUT13E18-A,” which can be used as a resistant source in a future breeding program (Khairum et al., 2016, 2018).
Biological control of Phytophthora diseases of orchids
Although a large number of country-specific commercial formulations of biocontrol agents are available for the management of plant diseases (Sevugapperumal et al., 2016; Hyder et al., 2017). However, very limited bioagents are available for the management of Phytophthora diseases in orchids worldwide. In a recent study, Pseudomonas aeruginosa RS1 was reported to have a good inhibitory effect on P. palmivora, causing orchid black rot. The antifungal proteins from Pseudomonas aeruginosa RS1 were identified as an active compound for inhibiting the growth of P. palmivora. The effective protein molecules are identified through LC/MS analysis. The proteins are found to be closely identified with three broad groups, such as catalase, chitin-binding protein, and protease. Partially purified proteins from P. aeruginosa RS1 caused abnormal growth and hyphal elongation in P. palmivora (Sowanpreecha and Rerngsamran, 2018). Similarly, in a parallel study, Streptomyces similanensis strain 9X166 has shown high antagonistic activity against P. palmivora which causes black rot in orchids in both in vitro and in vivo assays. Endo β-1,3-glucanase produced by the actinomycetes Streptomyces similanensis is the active principle for antagonistic activity. Consequently, Streptomyces sp. 9X166 culture filtrate, containing β-1,3-glucanase, can degrade freeze-dried as well as the living mycelium of P. palmivora. Therefore, β-1,3-glucanase-producing Streptomyces sp. can be an effective biocontrol agent for black rot of orchids (Sakdapetsiri et al., 2016). For mass production of Streptomyces similanensis 9X166, solid-state fermentation using agro-industrial substrates is being standardized in Thailand. Up to 60 days, the product retained 106CFU/g of Streptomyces similanensis 9X166 in a dried solid which can effectively inhibit cent percent of P. palmivora in living orchids (Sakdapetsiri et al., 2019).
Biotechnological and transgenic approaches for the Phytophthora management in orchids
Needless to say, biotechnological interventions have immensely contributed to the improvement of orchids in various aspects: phylogenetic studies, embryology, tissue culture, micropropagation, somaclonal variation, germplasm conservation, and mycorrhizal technology in orchid seed germination and natural establishment and diagnosis of orchid viruses (Hossain et al., 2013). Genetic engineering has further contributed to the improvement of complex orchid traits such as flower color, vase life, and genetic control of flower morphogenesis through the transformation of orchids (Hossain et al., 2013), where conventional breeding could not satisfactorily enlighten the stable path. However, biotechnological interventions, including transgenic approaches, did not contribute much to the development of Phytophthora disease-resistant varieties in orchids, except in a few cases of viral and bacterial diseases. Recently, Sjahril et al. (2006) carried out an Agrobacterium-mediated transformation in Phalaenopsis orchid (Phalaenopsis Wataboushi ‘#6.13’) with the incorporation of the wasabi defensin gene (WjAMP-1) in Japan. The transformed Phalaenopsis orchid overexpressed resistance to soft rot pathogen Erwinia carotovora subsp. carotovora. In Indonesia, a different method, the particle bombardment approach, was successfully used to incorporate the wasabi defensin gene into Phalaenopsis orchids for the development of transgenic Phalaenopsis amabilis resistant to soft rot bacterial Erwinia carotovora subsp. carotovora (Mariani, 2016). A transgenic Oncidium orchid resistant to soft rot bacteria (Erwinia carotovora subsp. carotovora) has been developed by incorporation of sweet pepper ferredoxin-like protein (pflp) gene into Oncidium (cv. Sherry Baby OM 8) using Agrobacterium tumefaciens as a vector in China (Liau et al., 2003). Transgenic Dendrobium orchids cloned with the CymMV coat protein gene expressed Cymbidium virus-resistant capacity (Petchthai et al., 2015). All of these can, therefore, strongly support the idea that the genetic transformation of orchids was very successful in the development of durable resistance against individual phytopathogens. At the same time, there is also a graceful breakthrough by a different group of researchers to develop transgenic orchids with multiple disease resistance against more than one phytopathogen by gene staking through double transformation. In Taiwan, transgenic Phalaenopsis orchids, which were developed by the incorporation of CymMV coat protein (CP) and sweet pepper ferrodoxin-like protein (pflp) by double transformation, expressed dual resistance to the Cymbidium mosaic virus and the soft rot bacterium Erwinia carotovora subsp. carotovora (Chan et al., 2005). However, a breakthrough is certainly zero when one narrows down its search to the contribution of biotechnology, including transgenic approaches for the development of disease-resistant orchids against any species of Phytophthora. To date, there is not a single hybrid or transgenic orchid in the global market to showcase that it is resistant to Phytophthora diseases of orchids. Here, the focus needs to be on the future of supporting the orchid business vis a vis world orchid trade.
Concluding remarks
Since the potato late blight outbreak caused by Phytophthora infestans in 1845 and the worst Irish Potato Famine (1845–1849) in Europe in the 19th century, intensive studies have been done on host–pathogen interaction, resistant breeding, identification of races/pathotypes, identification of R-genes, and the development of genetically modified crop varieties involving Phytophthora with many of its field and horticultural crops worldwide. However, there is a lack of information regarding host–pathogen interaction between any Phytophthora sp. and orchid host in the entire Plant Pathology literature. About a century has passed since the first report of Phytophthora palmivora infection on Dendrobium maccarthiae in Ceylon in 1921 (Petch, 1921), and hardly any scientific literature is there to support the status of race or pathotype of any Phytophthora species infecting orchids. For developing resistant varieties of orchids against Phytophthora spp. in any country, defining the prevalent races or pathotype structures is a prerequisite. A lot of literature is available on conventional breeding for the improvement of flower characteristics, such as big, durable, and fascinating flowers, in addition to the enhancement of quality features such as flower color, vase durability attribute, shape, and architecture and genetic engineering also showed a successful breakthrough for changing complex characters in orchids, such as novel flower color (Chia et al., 2001) and increased vase life (Chia et al., 2001), with the identification of specific genes involved for those characters. Although more than one hundred thousand interspecific, intergeneric, or multi-generic hybrids of orchids were developed through conventional breeding worldwide, breeding for the development of resistant varieties against Phytophthora diseases of orchids is still absent in the orchid industry. Breakthroughs on the patterns of inheritance of resistance against Phytophthora spp. in orchids are yet to come. Data on the availability of the R-gene in orchids also remained obscure. Identification of resistance sources in orchid species and landraces is needed to be strengthened; otherwise, the development of durable resistant orchid hybrids against any Phytophthora sp. will remain a dream.
Author contributions
TB conceived and designed the study and wrote the first draft of the manuscript with a graphical presentation and tables. PD supervised the final draft of the manuscript, designed the graphical abstract, and arranged the table. RK prepared the phylogenetic trees. MH, MM, AC, GD, MK, and RW checked the drafted manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors acknowledge the ICAR-NRC for Orchid, Sikkim; the Division of Plant Pathology, ICAR-IARI, New Delhi; and the Central Agricultural University (Imphal) for support rendered during the preparation of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1139811/full#supplementary-material
References
Alexopoulos, C. J., Mims, C. W., and Blackwell, M., (1996). Introductory mycology. 4th Ed. John Wiley & Sons, Inc., New York, USA.
Alfieri, S. A. Jr., Langdon, K. R., Wehlburg, C., and Kimbrough, J. W. (1984). Index of plant diseases in Florida (revised). Florida Dept. Agric. and consumer Serv. Div. Plant Ind. Bull. 11, 1–389.
Alfieri, A. S. Jr., Longdon, K. R., Kimbrough, J. W., El-Gholl, N. E., and Wehlburg, C. (1994). Disorders of Plants In Florida. Florida Department of Agriculture & consumer Services, Division of Plant Industry, Bulletin 14, 1–1114.
Andriyani, N. D., Wahyuno, D., Manohar,, and Gunawan, A. W. (2008). Phytophthora capsici is the causal agent of shoot rot disease of Vanilla in Indonesia. J. Biol. Indonesia 5, 227–234. (in Indonesian)
Ann, P. J. (2000). New diseases and records of flowering potted plants caused by Phytophthora species in Taiwan. Plant Pathol. Bull. 9, 1–10.
Aragaki, M., and Uchida, J. Y. (2001). Morphological distinctions between Phytophthora capsici and P. tropicalis sp. nov. Mycologia 93, 137–145. doi: 10.1080/00275514.2001.12061285
Arnold, G. R. W. (1986). Lista de Hongos Fitopatogenos de Cuba. Cuba, La Habana: Ministerio de Cultura Editorial Cientifico-Tecnica, 207.
Ashby, S. F. (1922). Oospores in cultures of Phytophthora faberi. Kew Bull. Misc. Inform. 1922, 257–262. doi: 10.2307/4129946
Ashby, S. F. (1929a). Strains and taxonomy of Phytophthora palmivora Bulter (P. faberi Maubl.). Brit. Mycol. 14, 18–38. doi: 10.1016/S0007-1536(29)80025-3
Ashby, S. F. (1929b). Further note on the production of sexual organs in paired cultures of species of Phytophthora. Brit. Mycol. Soc. Trans. 14, 254–260. doi: 10.1016/S0007-1536(29)80012-5
Bag, T. K. (2006). Pseudo bulb rot: A threatening disease of cymbidium orchidin the hills of Sikkim Himalaya (Abst.). The paper has been presented in the “international symposium on agriculturally important microorganisms: Conservation, utilization, bioremediation and ecological significance” held at University of Calcutta, Bally Gunj Circular Road, Kolkata from 23-25th February 2006. P 114–115.
Barchenger, D. W., Sheu, Z. M., Kumar, S., Lin, S. W., Burlakoti, R. R., and Bosland, P. W. (2018). Race characterization of Phytophthora root rot on capsicum in Taiwan as a basis for anticipatory resistance breeding. Phytopathology PHYTO-08-17-0289-R 108, 964–971. doi: 10.1094/PHYTO-08-17-0289-R
Bhai, S. R., and Dhanesh, J. (2008). Occurrence of fungal diseases in vanilla (Vanilla planifolia) in Kerala. J. Spices Arom. Crops 17, 140–148.
Bhai, S. R., and Thomas, J. (2000). Phytophthora rot a new disease of vanilla (Vanilla planifolia) in India. J. Spices Arom. Crops 9, 73–75.
Blair, J. E., Coffey, M. D., Park, S. K., Geiser, D. M., and Kang, S. (2008). A multilocus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 45, 266–277. doi: 10.1016/j.fgb.2007.10.010
Boesewinkel, H. J. (1982). A list of 142 new plant disease recordings from New Zealand and short notes on three diseases. Australas. Plant Pathol. 11, 40–43. doi: 10.1071/APP9820040
Bose, T., and Hammerbacher, A. (2022). The first report of Phytophthora multivesiculata causing black rot of Cymbidium and Ansellia africana from South Africa. Plant Dis. 2022:623. doi: 10.1094/PDIS-03-22-0623-PDN
Bouriquet, G. (1954). Le vanillier et la vanille dans le monde. Encyclopédie Biologique XLVI. Paris VI: Lechevalier.
Brasier, C. M., and Griffin, M. J. (1979). Taxonomy of Phytophthora palmivora on cocoa. Trans. Br. Mycol. Soc. 72, 111–143. doi: 10.1016/S0007-1536(79)80015-7
Breda de Haan, J. van . (1896). De bibitziekte in de Deli-tabak veroorzaakt door Phytophthora nicotianae (The root disease in Deli-tobacco caused by Phytophthora nicotianae). Meded. S. Lands Plantentiun 15. (In Dutch). Available at: https://biotanz.landcareresearch.co.nz/references/8c8d434c-264f-4873-9224-5512b0809687
Brown, R., Wall, G., and Schlub, R. (2007). Phytophthora black rot of orchid. Guam plant Disease report. University of Guam, College of Natural and Applied Science.
Burnett, H.C. (1974). Black rot of orchids. In Orchid diseases, bulletin, Florida Department of Agricultural and Consumer Services, No. 10, Florida, USA, pp. 8–9.
Butler, E. J. (1907). An account of the genus Pythium and some Chytridiaceae. Mem. Dep. Agric. India. Bot. Ser. 1, 82–84.
Butler, E. J. (1910). Report of the Imperial mycologist, 1918-1919. Sci. Rep. Agric. Res. Inst. Pusa, 68–85.
Cating, R. A., Hong, J. C., Palmateer, A. J., Stiles, C. M., and Dickstein, E. R. (2008). First report of Dickeya chrysanthemi (Erwinia chrysanthemi) on Vanda orchids in Florida. Plant Dis. 92:977. doi: 10.1094/PDIS-92-6-0977A
Cating, R. A., Palmateer, A. J., Stiles, C. M., and Rayside, P. A. (2010). Black rot of orchids caused by Phytophthora cactorum and Phytophthora palmivora in Florida. Plant Health Progress 11, 39–43. doi: 10.1094/PHP-2010-0614-01-DG
Cating, R. A., Palmateer, A. J., Stiles, C. M., Rayside, P. A., and Davison, D. A., (2009). Black rot of orchids caused by Phytophthora palmivora and Phytophthora cactorum. University Florida IFAS Extension, Publication No. pp: 260. Available at: http://edis.ifas.ufl.edu/pp260
Chan, Y. L., Lin, K. H., Sanjaya, L. L. J., Chen, W. H., and Chan, M. T. (2005). Gene stacking in Phalaenopsis orchid enhances dual tolerance to pathogen attack. Transgenic Res. 14, 279–288. doi: 10.1007/s11248-005-0106-5
Chen, J. S., and Hsieh, S. P. Y. (1978). Phytophthora black rot of Phalaenopsis in Taiwan. Plant Prot. Bull. 20, 161–170.
Chern, L. L., and Ann, P. J. (1996). Black rot of Cymbidium orchid caused by an unidentified Phytophthora species. Plant Pathol. Bull. 5, 200–201.
Chern, L. L., Ann, P. J., and Wang, I. T. (2011). Cymbidium black rot caused by an aberrant strain of Phytophthora multivesiculata in Taiwan. Plant Pathol. Bull. 20, 1–10.
Chia, T. F., Lim, A. Y. H., Luan, Y., and Ng, I. (2001). “Transgenic Dendrobium (Orchid)” in Biotechnology in agriculture and forestry (Vol. 48) transgenic crops III. ed. Y. P. S. Bajaj (Germany: Springer Verlag Berlin Heidel berg), 95–106.
Cho, W. D., and Shin, H. D. (2004). List of plant diseases in Korea. Fourth Edn, Gangnam-gu, Seoul, Republic of Korea: Korean Society of Plant Pathology, 779.
Cibes, H. R., and Childers, N. F. (1949). Republic of Federal Experimental Station. Puerto Rico. 1948. p. 27.
Claudio, C. B. (2003). Study of the black decay of the orchids caused by Phytophthora sp. in collections of the central valley of Costa Rica. Lankesteriana 7, 179–180.
Cline, E. T., Farr, D. F., and Rossman, A. Y. (2008). A synopsis of Phytophthora with accurate scientific names, host range, and geographic distribution. Plant Health Progress 9, 32–43. doi: 10.1094/PHP-2008-0318-01-RS
Cooke, D. E. L., Drenth, A. J. M., Duncan, J. M., Wagels, G., and Brasier, C. M. (2000). A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 30, 17–32. doi: 10.1006/fgbi.2000.1202
Cooke, D. E. L., and Duncan, J. M. (1997). Phylogenetic analysis of Phytophthora species based on the ITS1 and ITS2 sequences of ribosomal DNA. Mycol. Res. 101, 667–677. doi: 10.1017/S0953756296003218
Cunnington, J. H., de Alwis, S. K., and Priest, M. (2009). Some notes on Phytophthora syringae and P. Multivesiculata in Australia. Aust Plant Dis Notes 4, 42–43. doi: 10.1071/DN09017
Daly, A., and Conde, B. and Duff, J . (2013). Orchid diseases in the Northern Territory. Agnote, 568: no.13.
de Bary, A. (1876). Researches into the nature of the potato fungus Phytophthora infestans. J. Roy. Agric. Soc. Eng. Series 212, 239–269.
Deng, T. C., Chen, C. Y., Hseu, S. H., and Chen, D. Y. (2015). New records of plant diseases occurred in Taiwan in the 21st Century – Supplementary List of Plant Diseases in Taiwan (2015). In: Proceeding of the Symposium on Important Crop Diseases in Taiwan and their controls edited by Deng, TC, Chen CC, Tsai, CH, Tsai, JN and Hsieh, TF. 2015. TARI Publication No. 184. P147–199.
DITP News (2015). Tropical Thai orchids are in high demand around the world. Thai Trade Center Los Angeles. Available at: www.thailandhorizon.com.
Divinagracia, G. G, and Ros, L. B. (1986). Pathogenicity of Phytophthora palmivora (Butl.) Butler causing black rot of orchids [Philippines]. Anniversary and annual convention of the Pest control Council of the Philippines, Iloilo City (Philippines), May 8–10, 1986.
dos Santos, C. D. (2012). (Fungi and oomycetes associated with native and cultivated species of orchids in southern Bahia) Fungos e Oomicetoassociadosaespéciesnativas e cultivadas de orquídeas no Sul Da Bahia. Dissertation submitted for title Master's Degree in Plant Production, State of Santa Cruz. Submitted to the State University of Santa Cruz. pp. 1–74
Drechsler, C. (1931). A crown rot of hollyhocks caused by Phytophthora megasperma n. sp. J. Wash. Acad. Sci. 21, 513–526.
Drenth, A., and Guest, D. I., (ed.) (2004). Diversity and management of Phytophthora in Southeast Asia. ACIAR Monograph No. 114, pp 1–238.
Duff, J., and Daly, A. (2002). Orchid diseases in the Northern Territory. Agnote,568. I3. Available at: www.primaryindustry.nt.gov.au.
Erwin, D. C., and Ribeiro, O. K. (1996). Phytophthora diseases worldwide. American Phytopathological Society, St. Paul, MN.
Farr, D. F., Bills, G. F., and Chamuris, G. P., And Rossman, A. Y. (1989). Fungi on plants and plant products in the United States. The American Phytopathological Society, St. Paul, MN. 1, 252 pp.
Fay, MF . (2015). Undocumented trade in species of Orchidaceae: examples from Asia, the eastern Mediterranean region and Africa. PC22 Inf 6. Available at: https://cites.org/sites/default/files/eng/com/pc/22/Inf/E-PC22-Inf-06.pdf
Forsberg, L. I. (1985). Phytophthora species on ornamental plants in Queensland. Australas. Plant Pathol. 14, 18–20. doi: 10.1071/APP9850018
Gallegly, M. E., and Galindo, J. (1958). Mating Types and oospores of Phytophthora infestans in nature in Mexico. Phytopathology 48, 274–277.
Gallegly, M. C., and Hong, C. (2008). Phytophthora: identifying species by morphology and DNA fingerprints. APS Press –the American Phytopathological society, St. Paul, Minnesota, 158 pp
Govaerts, R., Bernet, P., Kratochvil, K., Gerlach, G., Carr, G., Alrich, P., et al. (2017). World checklist of Orchidaceae. Kew: Facilitated by the Royal Botanic Gardens. Available at: http://apps.kew.org/wcsp (Accessed March 23, 2017).
Hall, G. (1989). Unusual or interesting records of plant pathogenic oomycetes. Plant Pathol. 38, 604–611. doi: 10.1111/j.1365-3059.1989.tb01458.x
Hall, G. (1993). An integrated approach to the analysis of variation in Phytophthora nicotianae and a redescription of the species. Mycol. Res. 97, 559–574. doi: 10.1016/S0953-7562(09)81179-9
Hill, C. F. (2004). First report of Phytophthora multivesiculata on cymbidium orchids in New Zealand. Australas. Plant Pathol. 33, 603–604. doi: 10.1071/AP04070
Hine, R. B. (1962). Pathogenicity of Phytophthora palmivora in the Orchidaceae. Plant Dis. Rep. 46, 643–645.
Hinsley, A., De Boer, H. J., Fay, M. F., LM, G. S. W. G., Gunasekara, R. S., Kumar, P., et al. (2018). A review of the trade in orchids, and its implications for conservation. Bot. J. Linn. Soc. 186, 435–455. doi: 10.1093/botlinnean/box083
Hong, S. Y., Jee, H. J., and Hyun, S. W. (1998). First report of Phytophthora palmivora in Cheju Island as the causal pathogen of Phytophthora crown rot of Cymbidium. Korean J. Plant Pathol. 14, 725–728.
Hossain, M. M., Kant, R., Van, P. T., Budi Winarto, B., Zeng, S., and da Silva, J. A. T. (2013). The application of biotechnology to orchids. Crit. Rev. Plant Sci. 32, 69–139. doi: 10.1080/07352689.2012.715984
Hsieh, S. P. Y. (1984). Ecology of black rot fungi of orchids and its control. Taiwan Agric. Res. Inst. J. Spec. Publ. Number 14, 121–130.
Hsu, S. T., Chang, T. T., Chang, C. A., Tsai, J. L., and Sai, T. T. (2002). List of plant diseases in Taiwan. Phytopathological Society Publication, Taichung. Taiwan (in Chinese).
Hu, J. S., Ferreira, S., Wang, M., and Xu, M. Q. (1993). Detection of Cymbidium mosaic virus, Odontoglossum ringspot virus, tomato spotted wilt virus, and Potyviruses infecting orchids in Hawaii. Plant Dis. 77, 464–468. doi: 10.1094/PD-77-0464
Hyder, S., Inam-ul-Haq, M., Bibi, S., Humayun, A., Malik, A. H., Ghuffar, S., et al. (2017). Novel potential of Trichoderma spp. as a biocontrol agent. J. Entomol. Zool. Stud. 5, 214–222.
Ilieva, E., Man In't Veld, W. A., Veenbaas-Rijks, W., and Pieters, R. (1998). Phytophthora multivesiculata, a new species causing rot in Cymbidium. Eur. J. Plant Pathol. 104, 677–684. doi: 10.1023/A:1008628402399
Jangyukala, M., and Hemanta, L. (2021). Review on advances in production of hybrids in orchids. J. Crop Sci. Technol. 10, 15–20. doi: 10.37591/RRJoCST
Jensen, D. D. (1959). “Virus diseases in orchids” in The orchids: A scientific survey. ed. C. L. Withner (New York, NY: The Ronald Press Company), 431–458.
Jones, Susan . (2002). Orchid ailments. Orchids. (October issue), pp. 932–933. Available at: www.aos.org.
Kawate, M., and Sewake, K. T. (2010). Pest management strategic plan for potted orchid production in Hawaii. Summary of a workshop held on September 30, 2010 Hilo, Hawaii. Available at: https://ipmdata.ipmcenters.org/documents/ pmsps/ HI_orchid_PMSP.pdf [Accessed July 21, 2014].
Khairum, A., Poolsawat, O., Pornbungkerd, P., Tharapreuksapong, A., and Wongkaew, S., And Tantasawat, P. A. (2018). Effects of culture media on Phytophthora palmivora growth, α-elicitin production and toxicity to Dendrobium. Notulae Bot. Horti. Agrobotanici Cluj-Napoca, 46, 630–638. doi: 10.15835/nbha46211076
Khairum, A., Tharapreuksapong, A., Wongkaew, S., and Tantasawat, P. A. (2016). Cultural characteristics and pathogenicity analysis of Phytophthora palmivora, causal pathogen of black rot in orchids. International conference on agricultural, food, biological and health sciences (AFBHS-16) August 22–24, Kuala Lumpur (Malaysia).
Ko, W. H. (1988). Hormonal heterothallism and homothallism in Phytophthora. Annu. Rev. Phytopathol. 26, 57–73. doi: 10.1146/annurev.py.26.090188.000421
Kopp, A. (1930). Plant diseases in Reunion (Foreign Title: Les maladies des Plantes a la Reunion). Rev. Botanique Appl. 10, 281–287. doi: 10.3406/jatba.1930.4856
Kroon, L. P. N. M., Brouwer, H., de Cock, A. W. A. M., and Govers, F. (2012). The genus Phytophthora anno 2012. Phytopathology 102, 348–364. doi: 10.1094/PHYTO-01-11-0025
Laundon, G. F. (1978). New host records of plant disease fungi in New Zealand. N. Z. J. Agric. Res. 21, 705–707. doi: 10.1080/00288233.1978.10427471
Leonian, L. H. (1922). Stem and fruit blight of peppers caused by Phytophthora capsii sp. nov. Phytopathology 12, 401–408.
Leu, L. S. (1994). Control of orchid black rot induced by Phytophthora palmivora by metalaxyl. Plant Prot. Bull. 36, 107–115.
Li, D. L., Cao, J. F., Huo, C., Rajput, N. A., and Zhao, Z. J. (2018). First report of Phytophthora capsici causing blight and root rot of Dendrobium candidum in China. Plant Dis. 102, 685–686. doi: 10.1094/PDIS-06-17-0799-PDN
Li, J., Zhang, J. Z., Wu, X. P., Shan, W. X., and Tong, X. U. (2008). The causal agent of Dendrobium candidum blight disease. Mycosystema 27, 171–176.
Liau, C. H., Lu, J. C., Prasad, V., Hsiao, H., You, S. J., Lee, J., et al. (2003). The sweet pepper ferredoxin-like protein (pflp) conferred resistance against soft rot disease in Oncidium orchid. Transgenic Res. 12, 329–336. doi: 10.1023/A:1023343620729
Lim, T. K. (1980). Orchid disease and their control in Malaysia. Paper presented at the second southeast Asian symposium on plant diseases in the tropics, Bangkok, Thailand, October 20–26, 1980.
Lim, T. K., and Nio, H. L. (1983). Control of Phytophthora palmivora on orchids with some new systemic and standard fungicides. Pertanika 6, 34–39.
Liu, P. S. W. (1977). A supplement to a host list of plant diseases in Sabah, Malaysia. Phytopathol. Pap. 21, 1–49.
Liu, A., Zeng, T., Zeng, H., Cheng, X., and Sang, L. (2008). A survey of Vanilla Phytophthora disease in Hainan, China and identification of its pathogen species. Chin. J. Trop. Crops 29, 803–807.
Mariani, T. S. (2016). Genetic transformation of Phalaenopsis amabilis with resistance to soft rot disease, herbicide and glowing in the dark by particle bombardment method. Int. Res. J. Nat. Sci. 4, 1–11.
Martin, F. N., Abad, Z. G., Balci, Y., and Ivors, K. (2012). Identification and detection of Phytophthora: reviewing our progress, identifying our needs. Plant Dis. 96, 1080–1103. doi: 10.1094/PDIS-12-11-1036-FE
Martin, F. N., Blair, J. E., and Coffey, M. D. (2014). A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet. Biol. 66, 19–32. doi: 10.1016/j.fgb.2014.02.006
Masanto,, Hieno, A., Wibowo, A., Subandiyah, S., Shimizu, M., Suga, H., et al. (2019). Genetic diversity of Phytophthora palmivora isolates from Indonesia and Japan using rep-PCR and microsatellite markers. J. Gen. Plant Pathol. 85, 367–381. doi: 10.1007/s10327-019-00853-x
McMillan, R. T. Jr. (1983). Black rot control of door yard orchids. Proc. Fla. State Hort. Soc. 96, 136–137.
McMillan, R. T. Jr., Palmateer, A., and Cating, R. A. (2009). Problems in controlling Phytophthora cactorum on Cattleya orchids. Proc. Fla. State Hort. Soc. 122, 426–428.
McMillan, R. T., Palmateer, A. J., and Cating, R. A. (2010). Phytophthora cactorum a serious problem on prefinished Cattleya orchid liners from Thailand. Phytopathology 100:S174.
McRae, W. (1918). Phytophthora meadii n. sp. on Hevea brasiliensis. Mem. Dep. Agric. India Bot. Ser. 9, 219–273.
Mendes, M.A.S., da Silva, V.L., and Dianese, J.C. (1998). Fungosem Plants no Brasil. Embrapa-SPI/Embrapa-Cenargen, Brasilia, 555.
Miller, J. W. (1990). Bacterial brown spot of orchid caused by Pseudomonas cattleyae. Plant Pathology Circular No. 330. Florida Department of Agriculture and Consumer Service.
Mu, L., and Tsao, P. H. (1987). “Identities of Phytophthora isolates causing Vanilla blight and root rot in French Polynesia.” in Abstracts of the annual meeting of the APS. Phytopathology. 77(12): 104.
Orieux, L., and Felix, S. (1968). List of plant diseases in Mauritius. Phytopathol. Pap. 7, 1–48. (23735)
Orlikowski, L. B., Ptaszek, M., Trzewik, A., and Orlikowska, T. (2008). Increase of plant threat by Phytophthora species in Poland. Phytopathol. Pol. 48, 39–43.
Orlikowski, L. B., and Szkuta, G. (2006). Phytophthora rot of some orchids-new disease in Poland. Phytopathol. Pol. 40, 57–61.
Oudemans, P., and Coffey, M. D. (1991a). Isozyme comparison within and among worldwide sources of three morphologically distinct species of Phytophthora. Mycol. Res. 95, 19–30. doi: 10.1016/S0953-7562(09)81358-0
Oudemans, P., and Coffey, M. D. (1991b). Revised systematics of twelve papillate Phytophthora species based on isozyme analysis. Mycol. Res. 95, 1025–1046. doi: 10.1016/S0953-7562(09)80543-1
Park, J., Park, B., Veeraraghavan, N., Blair, J. E., Geiser, D. M., Isard, S., et al. (2008). Phytophthora database: a cyberinfrastructure supporting the identification and monitoring of Phytophthora. Plant Dis. 92, 966–972. doi: 10.1094/PDIS-92-6-0966
Patel, J. S., Vitoreli, A., Palmateer, A. J., El-Sayed, A., Noman, D. J., Goss, E. M., et al. (2016). Characterization of Phytophthora Spp. isolated from ornamental plants in Florida. Plant Dis. 100, 500–509. doi: 10.1094/PDIS-05-15-0598-RE
Peregrine, W. T. H., and Ahmad, K. B. (1982). Brunei: A first annotated list of plant diseases and associated organisms. Phytopathol. Pap. 27, 1–87.
Pereira, G. F., Laranjeira, D., Menezes, M., and Oliveira, S. M. (1993). Occurrence of Phytophthora cactorum associated to burning or black rot of orchids in the state of Pernambuco. Fitopatol. Brazil 18:347.
Petchthai, U., Chuphrom, A., and Huehne, P. S. (2015). Recovery of virus-infected Dendrobium orchids by constitutive expression of the Cymbidium mosaic virus coat protein gene. Plant Cell Tissue Organ Cult. 120, 597–606. doi: 10.1007/s11240-014-0626-x
Pethybridge, G. H. (1913). On the rotting of potato tubers by a new species of Phytophthora having a method of sexual reproduction hitherto undescribed. Sci. Proc. R. Dublin Soc. 13, 529–565.
Portales, L. A. (2004). “Phytophthora diseases in the Philippines” in Diversity and management of Phytophthora in South East Asia. eds. A. Drenth and D. I. Guest (Canberra, Australia: ACIAR), 90–93.
Purwantara, A, Manohara, D, and Warokka, JS. (2004). Phytophthora diseases in Indonesia. Pp. 70–76 In: Diversity and Management of Phytophthora in Southeast Asia. (Eds. André Drenth and David I. Guest), Australian Centre for International Agricultural Research Canberra. ACIAR Monograph No. 114, p. 238.
Raabe, R.D., Conners, I.L., and Martinez, A.P. (1981). Checklist of plant diseases in Hawaii. College of Tropical Agriculture and Human Resources, University of Hawaii. Information Text Series No. 22. Hawaii Inst. Trop. Agric. Human Resources, p. 313.
Rahman, M. Z., Uematsu, S., Coffey, M. D., Uzuhashi, S., Suga, H., and Kageyama, K. (2014). Re-evaluation of Japanese Phytophthora isolates based on molecular phylogenetic analyses. Mycoscience 55, 314–327. doi: 10.1016/j.myc.2013.11.005
Rands, R. D. (1922). Streepkanker van Kaneel, veroorzaakt door Phytophthora cinnamomi n. sp. (Stripe canker of cinnamon caused by Phytophthora cinnamomi n. sp.), Meded. Inst. Plantenziekten 54. (In Dutch).
Rivera, G., and Corrales, G. (2007). Phytosanitary problems that threaten the conservation of orchids in Costa Rica. Lankesteriana 7, 347–352.
Ros, L.B. (1985). Survival, dissemination, ingress and infection of the orchid strain of Phytophthora palmivora (Butl.) Butler on Vanda lamellata Lind. Ph.D. thesis. University of the Philippines at Los Banos, College, Laguna. pp. 84.
Rossman, A. Y., and Palm, M. E. (2006). Why are Phytophthora and other Oomycota not true Fungi? Outlook Pest Manag. 17, 217–219. doi: 10.1564/17oct08
Sakdapetsiri, C., Fukuta, Y., Aramsirirujiwet, Y., Shirasaka, N., and Kitpreechavanich, V. (2016). Antagonistic activity of endo-1,3-glucanase from a novel isolate, Streptomyces sp. 9X166, against black rot in orchids. J. Basic Microbiol. 56, 469–479. doi: 10.1002/jobm.201500709
Sakdapetsiri, C., Fukuta, Y., Aramsirirujiwet, Y., Shirasaka, N., Tokuyama, S., and Kitpreechavanich, V. (2019). Solid state fermentation, storage and viability of Streptomyces similanensis 9X166 using agro-industrial substrates against Phytophthora palmivora-induced black rot disease in orchids. Biocontrol Sci. Tech. 29, 276–292. doi: 10.1080/09583157.2018.1553027
Sangchote, S., Poonpolgul, S., Sdoodee, R., Kanjanamaneesathian, M., and Baothong, T. and Lumyong, P . (2004). Phytophthora diseases in Thailand. pp. 77–82. In: Diversity and Management of Phytophthora in Southeast Asia.(Eds. André Drenth and David I. Guest), Australian Centre for International Agricultural Research Canberra. ACIAR Monograph No. 114, p. 238.
Sawada, K. (1927). Descriptive catalogue of the Formosan fungi III. Dep. Agric. Gov. Res. Inst. Formosa Rep. 27, 1–62.
Schröter, J. (1886). Pilze. F. Cohn (ed). Kryptogamen-Flora von Schlesien. 1. J.U. Kern’s Verlag, Breslau.
Schwarz, M. B. (1927). The heart and leaf rot of orchids caused by Phytophthora omnivora De by. (Foreign Title: Het hartenbladrot van Orchideeen, veroovzaakt door Phytophthora omnivore Be By.) H. van Ingen, Soerabaja pp. 7 pp.
Sevugapperumal, N., Renukadevi, P., and Aiyanathan, E. (2016). Exploring the potential of Trichoderma for the Management of Seed and Soil-Borne Diseases of crops.
Shivas, R. G. (1989). Fungal and bacterial diseases of plants in Western Australia. J. R. Soc. West. Aust. 72, 1–62.
Simone, G. W., and Burnett, H. C. (1995). “Diseases caused by bacteria and fungi” in Orchid pests and diseases (Delray Beach, FL: Amer. Orchid Soc).
Sjahril, R., Chin, D. P., Khan, R. S., Yamamura, S., Nakamura, I., Amemiya, Y., et al. (2006). Transgenic Phalaenopsis plants with resistance to Erwinia carotovora produced by introducing wasabi defensin gene using the Agrobacterium method. Plant Biotechnol. 23, 191–194. doi: 10.5511/plantbiotechnology.23.191
Sowanpreecha, R., and Rerngsamran, P. (2018). Biocontrol of orchid-pathogenic mold, Phytophthora palmivora, by antifungal proteins from Pseudomonas aeruginosars1. Mycobiology 46, 129–137. doi: 10.1080/12298093.2018.1468055
Srivastava, S., Kadooka, C., and Uchida, J. Y. (2018). Fusarium species as pathogen on orchids. Microbiol. Res. 207, 188–195. doi: 10.1016/j.micres.2017.12.002
Su, C. C., and Leu, L. S. (1992). Soft rot of Oncidium "Gower Ramsey" and Cymbidium sp. caused by Erwinia carotovora subsp. carotovora. Plant Pathol. Bull. 1, 190–195.
Suzuki, M., Kageyama, K., Togawa, M., and Uchwam, T. (2008). First report of Phytophthora rot of Dinema in Japan. Ann. Rep. Kanto-Tosan Plant Prot. Soc. 55, 89–92.
Takahito, S., Ubol, K., and Thanawatt, K. (1979). Cross-inoculation of Phytophthora spp. isolated from some economic plants in Thailand. Tech. Bull., Trop. Agr. Res. Center, Japan No. 12. pp. 32.
Tamura, K., Glen Stecher, G., and Kumar, S. (2021). MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Molecular Biology and Evolution, 38, 3022–3027. doi: 10.1093/molbev/msab120
Tamura, K.and Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 10, 512–526. doi: 10.1093/oxfordjournals.molbev.a040023
Tang, S. Y. (1977). The Phytophthora top rot (black rot) disease of orchid (Aranda Wendy Scotts). Singapore: Chung ChingJitPao Ltd.
Tao, Y. H., Ho, H. H., and Wu, Y. X. And He, Y. Q. (2011a). Phytophthora nicotianae causing Dendrobium blight in Yunnan Province, China. Int. J. Plant Pathol., 2:177–186, doi: 10.3923/ijpp.2011.177.186
Tao, Y., Zeng, F., Ho, H. H., Wei, J. G., Wu, Y. X., Yang, L. L., et al. (2011b). Pythium vexans causing stem rot of Dendrobium in Yunnan Province, China. J. Phytopathol. 159, 255–259. doi: 10.1111/j.1439-0434.2010.01756.x
Thompson, A. (1958). A disease of orchid caused by the fungus Phytophthora palmivora. MAHA Magazine 15, 61–66.
Thompson, A. (1959). Phytophthora palmivora-A parasite of orchids in Singapore. Malay Agric. J. 42, 83–92.
Tombe, M., and Liew, E. C. Y. (2010) in Fungal diseases of Vanilla. Chapter 8. eds. E. Odoux and M. Grisoni (NY: CRC Press, Taylor and Francic group), 125, 369–140.
Tsai, H. L., Huang, L. C., Ann, P. J., and Liou, R. F. (2006). Detection of orchid Phytophthora disease by nested PCR. Bot. Stud. 47, 379–387.
Tsao, P. H., and Mu, L. (1987). “Phytophthora blight and root rot of Vanilla in French Polynesia: occurrence and causal species.” in 11th International Congress of Plant Protection. Manila (Philippines). October 5–9 1987. p. 2.
Turner, G. J. (1966). New records of plant diseases in Sarawak for the years 1963 and 1964. Gardeners Bull. Dermatol. Sin. 21, 393–402.
Uchida, J. Y. (1994). Diseases of orchids in Hawaii. Plant Dis. 78, 220–224. doi: 10.1094/PD-78-0220
Uchida, J. Y., and Aragaki, M. (1991). Phytophthora diseases of orchids in Hawaii. University of Hawaii, HITAHR. Res. Ext. Ser. 129, 1–7.
Uchida, J.Y., and Sipes, B.S.. (1998). Foliar nematodes on orchids in Hawaii. Publication PD-13, CTAHR, University of Hawaii. pp. 1–7.
Wey, G. C. (1988). Occurrence and investigation of important diseases on Phalaenopsis in Taiwan. Rep. Taiwan Sugar Res. Inst. 122, 31–41.
Wiehe, P. O. (1948). The plant diseases and fungi recorded from Mauritius. Mycol. Pap. 24, 1–39. (23736)
Wongwan, T., Haituk, S., Senwanna, C., Toanun, C., Intaparn, P., and Cheewangkoon, R. (2021). New host record of Phytophthora palmivoracausing black rot on Rhynchostylis gigantea in Thailand. Chiang Mai J. Sci. 48, 942–951.
Yeh, J. T., Hsieh, S. P. Y., and Ann, P. J. (1998). Physiological and morphological characteristics of Phytophthora palmivora causing black rot of Cattleya in Taiwan. Plant Prot. Bull. 7, 85–93.
Zeng, H. C., Ho, H. H., and Zheng, F. C. (2009). A survey of Phytophthora species on Hainan Island of South China. J. Phytopathol. 157, 33–39. doi: 10.1111/j.1439-0434.2008.01441.x
Zentmyer, G. A., Mitchell, D. J., Jefferson, L., Roheim, J., and Carnes, D. (1973). Distribution of mating types of Phytophthoras palmivora. Phytopathology 63, 663–667. doi: 10.1094/Phyto-63-663
Keywords: Phytophthora, Orchidaceae, destructive pathogen, epidemiology, integrated management
Citation: Bag TK, Dutta P, Hubballi M, Kaur R, Mahanta M, Chakraborty A, Das G, Kataky M and Waghunde R (2024) Destructive Phytophthora on orchids: current knowledge and future perspectives. Front. Microbiol. 14:1139811. doi: 10.3389/fmicb.2023.1139811
Edited by:
Miha Humar, University of Ljubljana, SloveniaReviewed by:
Ram Pal, National Research Centre For Orchids (ICAR), IndiaNakkeeran S., Tamil Nadu Agricultural University, India
Copyright © 2024 Bag, Dutta, Hubballi, Kaur, Mahanta, Chakraborty, Das, Kataky and Waghunde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tusar Kanti Bag, dHVzYXIuYmFnQGdtYWlsLmNvbQ==; Pranab Dutta, cHJhbmFiZHV0dGE3NEBnbWFpbC5jb20=
 Tusar Kanti Bag
Tusar Kanti Bag Pranab Dutta
Pranab Dutta Manjunath Hubballi
Manjunath Hubballi Ravpreet Kaur1
Ravpreet Kaur1