
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 31 May 2023
Sec. Microbe and Virus Interactions with Plants
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1132853
This article is part of the Research Topic Agricultural Sensors and Systems for Field Detection View all 21 articles
The amount of chemical fertilizer for vegetables is on the high level in China. The use of organic fertilizers to meet the nutrient requirement of crops will be an inevitable practice in sustainable agriculture. In this study, we compared the effects of pig manure fertilizer, rabbit manure fertilizer and chemical fertilizer on yield, quality of Brassica rapa var. Chinensis, soil physico-chemical properties and microbial community by using two consecutive seasons of three fertilizers in a pot experiment. The results were as follows: (1) In the first season, the fresh yield of Brassica rapa var. Chinensis applying chemical fertilizer was significantly (p ≤ 5%) higher than those of applying the pig manure and rabbit manure fertilizer, and the results were the opposite in the second season. The total soluble sugar concentration of fresh Brassica rapa var. Chinensis applying rabbit manure fertilizer was significantly (p ≤ 5%) higher than those of applying pig manure fertilizer and chemical fertilizer in the first season, and the NO3-N content of fresh Brassica rapa var. Chinensis on the contrary. (2) The organic fertilizer increased the concentration of total nitrogen, total phosphorus and organic carbon in soil in both two seasons. Rabbit manure fertilizer increased the soil pH and EC and significantly (p ≤ 5%) reduced the soil NO3-N content. (3) The pig manure and rabbit manure fertilizer significantly (p ≤ 5%) increased the diversity and abundance of soil bacterial of Brassica rapa var. Chinensis, but had no significant effect on soil fungi. Pearson correlation analysis showed that soil TN, TP, organic carbon content and EC were significantly correlated with soil bacterial α - diversity. There were significant differences (p ≤ 5%) in the bacterial community structures between three treatments in two seasons, and significant differences (p ≤ 5%) in the fungal community structures between fertilizer treatments while not between two seasons. Pig manure and rabbit manure fertilizer decreased the relative abundance of soil Acidobacteria and Crenarchaeota, rabbit manure fertilizer significantly increased the abundance of Actinobacteria in the second season. Distance-based redundancy analysis (dbRDA) showed that soil EC, TN, and organic carbon content were key physico-chemical factors in determining bacterial community structure in Brassica rapa var. Chinensis soil, and soil NO3-N, EC, SOC concentration and soil pH in the fungal community structure.
Vegetables are important agricultural products that are essential to the livelihoods of urban and rural residents. China is the world’s largest producer and consumer of vegetables, accounting for more than 40% of the world’s sown area and production(Huang et al., 2017). China’s total fertilizer dose is at a high level, much higher than the United States, the European Union and other developed countries. In particular, the amount of fertilizer applied to vegetables is high, with the average amount of fertilizer applied to vegetables being 445.5 kg/hm2 higher than the United States, and 471 kg/hm2 higher than the EU (China, T. M. O. A. A. R. A. O. T. P. S. R. O, 2017). According to statistics, the input of N, P2O5 and K2O in the greenhouse vegetables in China is 1.9, 5.4, 1.6 times of the recommended amount respectively, and the amount of chemical fertilizer nutrients was as high as 1,354.5 kg/hm2, which is 4.1 times the average amount of the national crop (Huang et al., 2017). In vegetable production, especially in protected vegetable production, the excessive and irrational use of chemical fertilizers not only leads to low fertilizer use and production efficiency, but also causes to a series of serious problems, such as the reduction of soil organic matter content, massive enrichment of available nutrients (nitrogen, phosphorus, etc.), secondary salinization, accumulation of heavy metals, edible parts of vegetables and excessive nitrate in groundwater, which seriously limits the sustainable development of China’s vegetable industry (He et al., 2016; Yang et al., 2016; Wang et al., 2018).
The application of organic fertilizer, which has long been proposed globally, helps to reduce agricultural dependence on chemical fertilizers, in order to prevent soil degradation (Jude and Vidah, 2008; Sun et al., 2019). Organic fertilizers can not only effectively promote the reduction and efficiency of chemical fertilizers (Wang et al., 2020b), and improve crop yield and quality (Zhang et al., 2019; Serri et al., 2021), but also enhance soil microbial diversity, biomass and activity (Gu et al., 2017; Huang et al., 2020), which can subsequently improve soil quality, and contribute to climate protection by increasing carbon sequestration in agricultural ecosystems (Burger and Jackson, 2003; Birkhofer et al., 2008; Zhang et al., 2016). Studies have shown that soil microorganisms are important components of soil ecosystems, and the community composition and diversity of soil microorganisms are important indicators of the ecological function of soil microbial communities (Dong et al., 2014). Organic fertilizers provide a variety of C compounds with different chemical compositions, ranging from readily degradable to stable, which can be utilized by soil microorganisms during mineralization to increase their growth rates and biomass (Lazcano et al., 2021). Thus, organic fertilizers have strong, short- and long-term effects on the soil microbiome and are the basis for supporting soil health by increasing microbial activity, microbial interactions and nutrient cycling (Lazcano et al., 2013; Ling et al., 2016).
With the rapid intensive and large-scale development of livestock production in China, the amount of manure is large and concentrated. According to statistics, the annual production of livestock and poultry manure in China is about 3.8 × 109 t in China (China, M. O. E. A. E. O. T. P. S. R. O., N. B. O. Statistics & M. O. A. A. R. A. O. T. P. S. R. O. China, 2010). By the end of 2019, the comprehensive utilization rate of national livestock and poultry manure reached 75% (China, T. M. O. A. A. R. A. O. T. P. S. R. O, 2017). Thus, the Chinese government attaches great importance to the resource utilization of livestock and poultry manure and encourages the use of organic fertilizers (Dong et al., 2019). Conventional organic fertilizer in China is mainly pig manure and poultry manure. Rabbit farming with regional characteristics is more common only in certain regions. Rabbits are herbivores animals and the crude fiber content of their feed is high. The crude fiber content of domestic rabbit feed is about 13%, and the crude cellulose content of fattening pig feed does not exceed 5%. Compared with pig manure, rabbit manure has a high crude fiber content and can be easily heated up and fermented during composting, and the pH value of both fresh rabbit manure and rabbit manure compost is relatively high. Previous research has shown that the long-term application of chemical fertilizers causes soil acidification and that organic fertilizers can increase soil pH and soil organic carbon and reduce soil acidification (Wang et al., 2019; Liu et al., 2021). Compared to chemical fertilizers, organic fertilizers can substantially increase the organic carbon content of the soil, thus promoting the diversity of soil microorganisms (Wang et al., 2019). In particular, farmers prefer to apply rabbit manure organic fertilizer on acidic soils to improve fruit yield and quality. However, the differences in the effects of chemical fertilizer, rabbit manure and pig manure organic fertilizer on the yield and quality of leafy green vegetables, the physico-chemical properties of the soil, and the microbial community within the soil are not clear.
We hypothesized that chemical fertilizer, rabbit manure organic fertilizer and pig manure organic fertilizer have different effects on soil physico-chemical properties and microbial community, and affect soil microbial community composition through higher pH and higher organic carbon content of organic fertilizer. In this study, we compared the effects of pig manure organic fertilizer, rabbit manure organic fertilizer and inorganic compound fertilizer on yield, quality of Brassica rapa var. Chinensis and soil physico-chemical properties by using two consecutive seasons of organic fertilizer application in a pot experiment. We also study the effect of different fertilizers on Brassica rapa var. Chinensis soil microbial community and ecosystem function, investigate soil micro-ecological mechanisms of different fertilizers to improve yield and quality of Brassica rapa var. Chinensis, to provide a theoretical basis for high-yield vegetable production and resource use of rabbit and pig manure, and to provide basic data support for the application of agricultural information technology between fertilizer, crop and soil interactions.
The experimental site for this study was located in the greenhouse of the Jiangsu Academy of Agricultural Sciences in Nanjing, China (32°02′N latitude, 118°52′E longitude). The temperature ranged from 6°C to 28°C from 17 March to 16 April, 2020, and from 11°C to 34°C from 18 April to 19 May 2020 in the greenhouse. The experimental soil was Magan soil which was collected from the experimental field around the greenhouse and the previous crop was wheat. The physical and chemical characteristics properties of the soil are shown in Table 1.
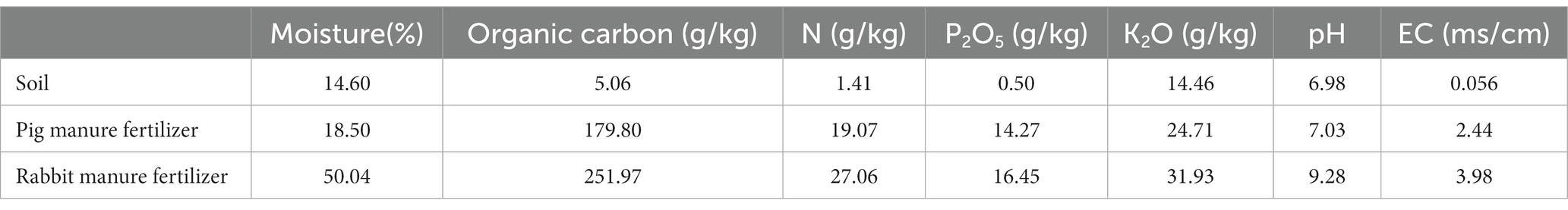
Table 1. The physical and chemical properties of experimental soil, the pig manure, and the rabbit manure used in the experiment (dry basis).
The variety of Brassica rapa var. Chinensis is heat resistant “605,” which was purchased from the seed store (Nangjing lvlingseeds, Nanjing, China). The two types of organic fertilizers were pig fermentation bed maturing bedding (pig manure fertilizer) and rabbit manure compost (rabbit manure fertilizer), the physical and chemical properties of which are shown in Table 1. The pig manure fertilizer, which was collected from the pig fermentation bed farm of the Luhe Animal Science Base, Jiangsu Academy of Agricultural Sciences, and which was the fermentation product of pig feces and urine, spent mushroom substrate and rice husk, on which pigs had been reared for 1 year. The rabbit dung fertilizer was produced at the organic fertilizer farm of the Luhe Animal Science Base, Jiangsu Academy of Agricultural Sciences, and which was the product of high temperature fermentation of mixed materials mainly consisting mainly of rabbit dung, Chinese medicine residues and cassava residues. The fresh rabbit dung was collected from the experimental rabbit farm of the Luhe Animal Science Base, Jiangsu Academy of Agricultural Sciences. The chemical fertilizer used in the experiment was the complex fertilizer with the ratio of nitrogen, phosphorus pentoxide and potassium oxide of 15–15-15 (Stanley, Linyi, China).
There were three treatments in the pot experiment (1) NPK chemical fertilizer, with an application rate of 300 kg N/hm2 (14.12 g/pot, including 2.12 g N/pot, 2.12 g P2O5/pot and 2.12 g K2O/pot), (2) pig manure organic fertilizer, (3) rabbit manure organic fertilizer, with an application rate of 600 kg N/hm2 for both pig manure (273 g/pot, including 4.24 g N/pot, 7.14 g P2O5/pot and 5.50 g K2O/pot) and rabbit manure (313 g/pot, including 4.24 g N/pot, 5.79 g P2O5/pot and 5.00 g K2O/pot) to account for differences in nutrient release and soil uptake during crop growth. Four pots were used for each treatment, for a total of 12 pots. The pot used was 40 cm high and 30 cm in diameter at the top. The experimental soil was mixed and sampled and then filled into the pots. All fertilizers were applied once before sowing 10 cm below the soil surface in two seasons. The first Brassica rapa var. Chinensis crop was sown on 17 March and harvested on 16 April 2020, the second Brassica rapa var. Chinensis crop was sown on 18 April and harvested on 19 May 2020. After emergence, 20 seedlings were planted per pot. The experimental samples from all 20 Brassica rapa var. Chinensis seedlings were collected at the same time as the Brassica rapa var. Chinensis crop was harvested. After harvesting of Brassica rapa var. Chinensis crop, 15 cm of soil was removed from the upper pot, mixed well and some of the soil was used for the post-harvest soil samples for each pot.
At harvest, the plants were cut at the soil surface and their fresh weight was determined using a digital balance. After washing the plants with distilled water, each Brassica rapa var. Chinensis sample was divided into three parts, snap frozen in liquid nitrogen and stored at −18°C for analysis of sugar content, Vc content and NO3-N content, respectively. After sieving (< 5 mm) and thorough mixing, each soil sample was divided into two parts: one part was stored at −18°C for the analysis of basic soil properties (pH, EC, NO3-N) and for DNA extraction, and the second part was air-dried at room temperature for the measurement of nutrients including nitrogen, phosphorus and organic carbon.
The total soluble sugar concentration of fresh Brassica rapa var. Chinensis samples was determined by the anthrone method(Sinay and Karuwal, 2014). Briefly, 0.5–1.0 g of fresh tissue from each Brassica rapa var. Chinensis sample and 15 mL of distilled water were placed in a stoppered test tube and incubated in a boiling water for 20 min, and then the test tube was cooled at room temperature. The extract solution was filtered into a 50 mL volumetric flask through double circle quantitative filter paper (Grade 2, Ge biotechnology Co., Ltd., Hangzhou, China) (extract twice). The residue of each sample was rinsed with distilled water into the volumetric flask with distilled water and then the volume was fixed on the scale. The diluted sample extract was pipetted 1.0 mL into a 20 mL graduated test tube. 5 mL of anthranilone concentrated sulfuric acid reagent was added one at a time to the test tube and shaken thoroughly, the test tube was immediately placed in a boiling water bath and each tube was kept warm for 10 min. Finally, the absorbance was read (l = 620 nm) in an ultra microplate spectrophotometer (BioTek epoch., Agilent Technologies, Inc., Vermont, USA). The amount of sugar in the extract was calculated using the standard linear equation for glucose and the total sugar content of the test samples was calculated.
The NO3–N concentration was measured using a rapid colourimetric salicylic acid nitration assay (Cataldo et al., 1975). 2.0 g fresh Brassica raps var. Chinensis samples, which were cut into pieces and mixed evenly, were added to 10 mL of deionized water in a 20 mL glass test tube. The tube was then placed in a boiling water bath for 30 min, removed and cooled with tap water.
The extract solution was filtered through quantitative filter paper (BioTek epoch, Agilent Technologies, Inc., Vermont, USA) into a 25 mL volumetric flask and the volume was fixed on the balance. Two portions of 0.1 mL of extract were transferred to two test tubes, 0.4 mL of salicylic acid was added to each test tube, mixed well and left at room temperature for 20 min. 9.5 mL of 8% NaOH was added to the test tube, the test tube was shaken well and then cooled to room temperature. Finally, the absorbance (l = 410 nm) was read in an ultra-micro microplate spectrophotometer (BioTek epoch., Agilent Technologies, Inc., Vermont, USA). The amount of NO3-N in the extract was calculated from the standard linear equation for KNO3 and the plant NO3-N content in the test samples was calculated.
Total nitrogen (TN) was determined by the Kjeldahl method using a Kjeldahl Nitrogen Detector (Kjeltec 8,400, FOSS Ltd., Denmark). Total phosphorus (TP) was determined using the method for the determination of total phosphorus in soil (GB9837-88). Total organic carbon (TOC) was determined using a TOC analyzer (Multi N/C 3100, Elementar Analysensysteme GmbH, Germany). Soil pH was determined using the potentiometric method (water: soil = 2.5: 1) with a pH meter (FiveEasy Plus pH/mV, Mettler-Toledo (Schweiz) GmbH, Switzerland). Soil EC (soil electrical conductivity, water: soil = 10: 1) was measured with a conductivity meter (EC215 Conductivity Meter, Hanna Instruments, Italy).
NO3–N in soil is determined by dual-wavelength ultraviolet spectrophotometry (Norman et al., 1985). Briefly, 10 g of the soil samples, which were frozen at – 18°C, 100 mL of 2 M KCL (1:5 soil to solution ratio) and 1 g of non-phosphate activated carbon powder were added to an Erlenmeyer flask, and shaken in a constant temperature (refrigerated) oscillator (Taicang Huamei instrument factory, Taicang, China) for 1 h. The extract solution was filtered through quantitative filter paper (BioTek epoch, Agilent Technologies, Inc., Vermont, USA) into a 50 mL plastic reagent bottle. And then the soil NO3–N concentration was measured by ultraviolet spectrophotometry using an ultra micro microplate spectrophotometer (BioTek epoch., Agilent Technologies, Inc., Vermont, USA) (GB/T32737-2016).
Differences in yield, Brassica rapa var. Chinensis quality and soil physicochemical characteristics between fertilizer treatments were determined using one-way analysis of variance (ANOVA). The Pearson’s coefficients were used to correlate yield, Brassica rapa var. Chinensis quality and soil physicochemical characteristics.
Total genomic DNA was extracted from 200 mg soil samples using the CTAB method. The concentration and purity of the extracted DNA was monitored on 1% agarose gels. The DNA was then stored at – 80°C until further processing. The v4 region of the bacterial 16S rRNA gene was amplified using the common primer pair F (5′-CCTAYGGGRBGCASCAG-3′) and R (5′-GGACTACNNGGGTATCTAAT - 3′) with the barcode. The ITS1 - 5f of the fungal gene was amplified with the common primer pair F (G5’ - GGAAGTAAAAGTCGTAACAAGG - 3’) and R (5′-GCTGCGTTCTTCATCGATGC-3′) with the barcode. PCR reactions were performed using 15 μl Phusion® High-Fidelity PCR Master Mix (New England Biolabs); 0.2 μM forward and reverse primers and approximately 10 ng template DNA. Thermal cycling conditions were as follows: an initial denaturation at 98°C for 1 min, followed by 30 cycles at 98°C for 10 s, 50°C for 30 s and 72°C for 30 s, with a final extension at 72°C for 5 min. Finally, an equal volume of 1X loading buffer (containing SYB green) was mixed with all PCR products and electrophoresed on a 2% agarose gel for detection. PCR products were mixed at equidensity. The mixed PCR products were then purified using Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were generated using a TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, USA) according to the manufacturer’s recommendations and index codes were added. Library quality was assessed using the Qubit@ 2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2,100 system. Finally, the library was sequenced on an Illumina NovaSeq platform and 250 bp paired-end reads were generated.
Mitochondria and chloroplast sequences were removed prior to analysis. Paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Paired-end reads were merged using FLASH (Magoč and Salzberg, 2011). Quality filtering of the raw tags was performed under specific filtering conditions (Q score for quality trimming = 19, minimum length = 3) to obtain the high-quality clean tags (Bokulich et al., 2013) according to the quality controlled process of Quantitative Insights Into Microbial Ecology (QIIME) (Caporaso et al., 2010). The tags were compared with the reference database (Silva database, https://www.arb-silva.de/) using the UCHIME algorithm (Edgar et al., 2011) to detect chimera sequences, and then the chimera sequences were removed (Haas et al., 2011). Finally, the effective Tags were obtained.
Sequence analysis was performed using Uparse software (Edgar, 2013) and sequences with ≥97% similarity were assigned to the same OTUs with the most abundant selected as the representative sequence for further annotation. For each representative sequence, the Silva database (Quast et al., 2012) was used based on the Mothur algorithm to annotate taxonomic information based on naive Bayesian classification for bacteria and Blast for fungi. Multiple sequence alignments were performed using the MUSCLE software (Edgar, 2004). OTU abundance information was normalized using a standard of sequence number corresponding to the sample with the fewest sequences, which was 55,582 for bacteria and 29,042 for fungi.
Alpha diversity is used to analyze the complexity of species diversity for a sample through 3 indices, including observed-species, Chao1, and Shannon. The Tukey test was used to analyze the differences between groups of the alpha diversity index. All these indices in our samples were calculated by QIIME (version 1.7.0) and displayed by R software (version 2.15.3). Beta diversity NMDS (Bray-Curits distance) was calculated by QIIME software (version 1.9.1) to evaluate the changes in microbial community structure. Based on the Unifrac distance, the amova function of the Mothur algorithm was used to analyze the differences between fertilizer treatment groups. In order to better reflect the non-linear structure of the ecological data, we performed the Non-Metric Multi-Dimensional Scaling (NMDS) of the nonlinear model based on Bray-Curtis distance. Pearson correlation approaches were used to correlate alpha-diversity with physicochemical characteristics of soils. Distance-based redundancy analysis (db-RDA) was then performed to further investigate the influence of soil physiochemical properties on bacteria and fungi according to relative phylum abundance R (version 3.2.2).
Table 2 shows the effect of different fertilizers on the yield and quality of Brassica rapa var. Chinensis. The fresh yield of Brassica rapa var. Chinensis in the first season were lower than that in the second season. In the first season, the fresh yield of Brassica rapa var. Chinensis (222.7 g/pot) with chemical fertilizer was significantly (p ≤ 5%) higher than that with pig manure and rabbit manure fertilizer, which were 202.3 g/pot and 136.7 g/pot, respectively. And the fresh yield of Brassica rapa var. Chinensis (638.7 g/pot) with pig manure fertilizer was significantly (p ≤ 5%) higher than that with rabbit manure and chemical fertilizer, which were 560.8 g/pot and 537.0 g/pot respectively, in the second season.
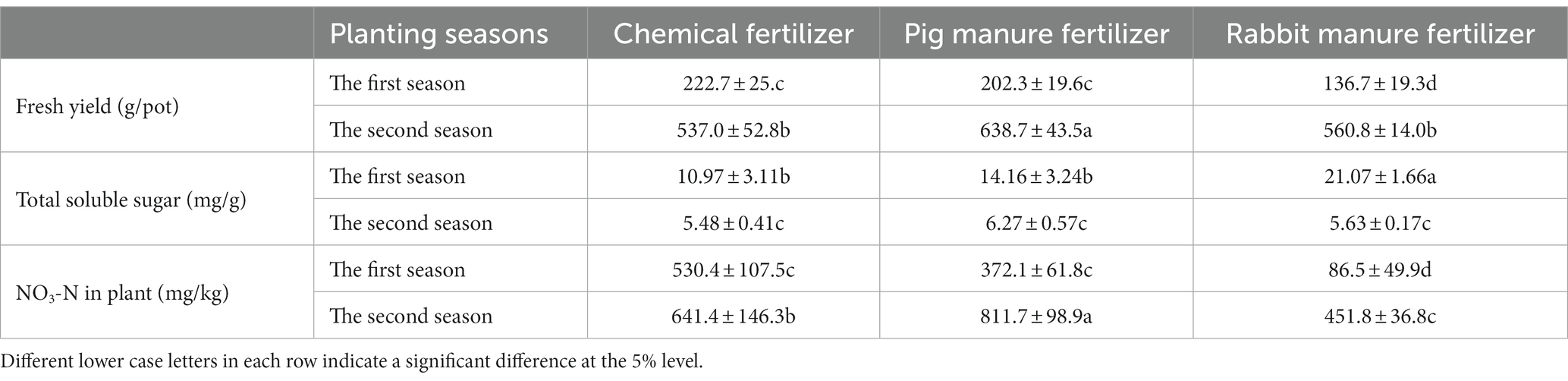
Table 2. Effect of different fertilizers on the yield and the quality of Brassica rapa var. Chinensis.
The total soluble sugar concentration of Brassica rapa var. Chinensis in the first season, which ranged from 10.97 mg/g to 21.07 mg/g, was higher significantly than that in the second season, which ranged from 5.48 mg/g to 6.27 mg/g. The total soluble sugar concentration of fresh Brassica rapa var. Chinensis using rabbit manure fertilizer (21.07 mg/g) was significantly higher (p ≤ 5%) than those using pig manure fertilizer and chemical fertilizer, which were 14.16 mg/g and 10.97 mg/g, respectively, in the first season, but there was no significant difference in the second season.
The NO3-N content of fresh Brassica rapa var. Chinensis in the second season was significantly higher than those in the first season (Table 2). The NO3-N content of fresh Brassica rapa var. Chinensis fertilized with chemical fertilizer (530.4 mg/kg) was significantly higher (p ≤ 5%) than those fertilized with pig manure and rabbit manure which were 372.1 mg/kg and 86.5 mg/kg, respectively, in the first season, that fertilized with pig manure fertilizer (811.7 mg/kg) was significantly higher (p ≤ 5%) than those fertilized with chemical fertilizer and rabbit manure fertilizer which were 645.4 mg/kg and 451.8 mg/kg, respectively, in the second season.
Table 3 shows the effect of the different fertilizers on the physical and chemical properties of the soil. Total nitrogen, total phosphorus and organic carbon in the soil were higher in the second season than in the first. In two seasons, soils treated with pig and rabbit manure fertilizers had significantly higher concentrations of total nitrogen, total phosphorus and organic carbon than those treated with chemical fertilizer. However, in the same season, there was no significant difference in total nitrogen, total phosphorus and organic carbon content in the soil after application of pig and rabbit manure fertilizer.
The rabbit and pig manure fertilizer increased the soil pH value from 6.60 and 6.41 at the end of the first season to 6.65 and 6.62 at the end of the second season, while the chemical fertilizer reduced the soil pH value from 6.63 at the end of the first season to 6.44 at the end of the second season. However, compared to the initial soil pH of 6.98 (Table 1), all fertilization treatments reduced the soil pH.
The soil ECs in the second season were significantly higher (p ≤ 5%) than those in the first season, and the order of the soil ECs order of the three fertilizers was: rabbit manure fertilizer (511 and 749 μs/cm) > chemical fertilizer (436 and 691 μs/cm) > pig manure fertilizer (423 and 625 μs/cm).
The soil NO3-N content in the second season, which ranged from 28.74 to 66.01 mg/kg, was significantly (p ≤ 5%) higher than that in the first season, which ranged from 65.18 to 146.06 mg/kg. Pig manure fertilizer and chemical fertilizer significantly increased soil NO3-N content compared to rabbit manure fertilizer. The C/N ratio in soil using pig manure and rabbit manure fertilizer, which increased over time, was significantly higher than that with chemical fertilizer, which decreased over time.
Table 4 shows that, there was a significant positive correlation between Brassica rapa var. Chinensis yield and soil EC, TN, TP, and nitrate, a significant negative correlation between Brassica rapa var. Chinensis total soluble sugar and soil EC and nitrate content, and a significant positive correlation between Brassica rapa var. Chinensis nitrate content and soil nitrate content.
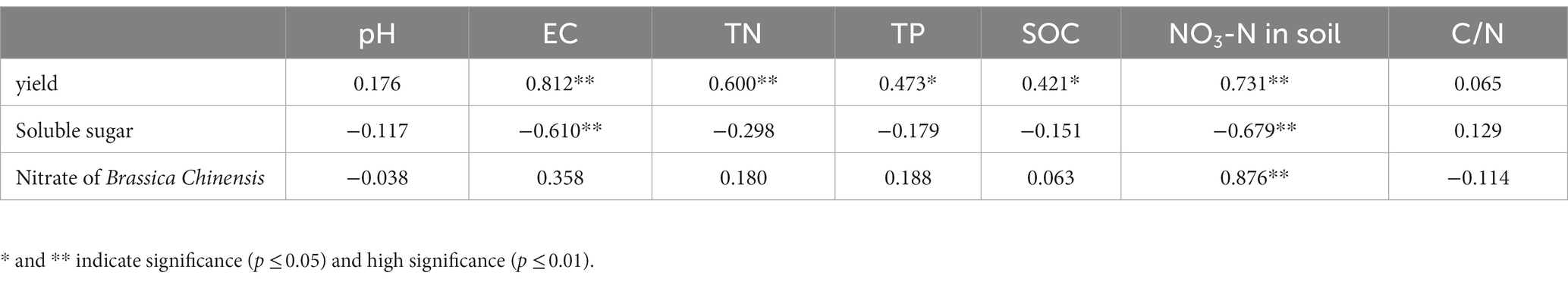
Table 4. Pearson’s correlation between yield and quality of Brassica rapa var. Chinensis and soil physico-chemical properties.
After the quality control, 1,477,222 and 1,556,272 valid tags were obtained from 16S rRNA and ITS rRNA gene sequencing. After 97% OTU clustering, 10,613 bacterial OTUs and 4,928 fungal OTUs were retained. Rarefaction curves showed that the sequencing effort was sufficient to describe the majority of the diversity in Brassica rapa var. Chinensis soil samples (Figure 1).
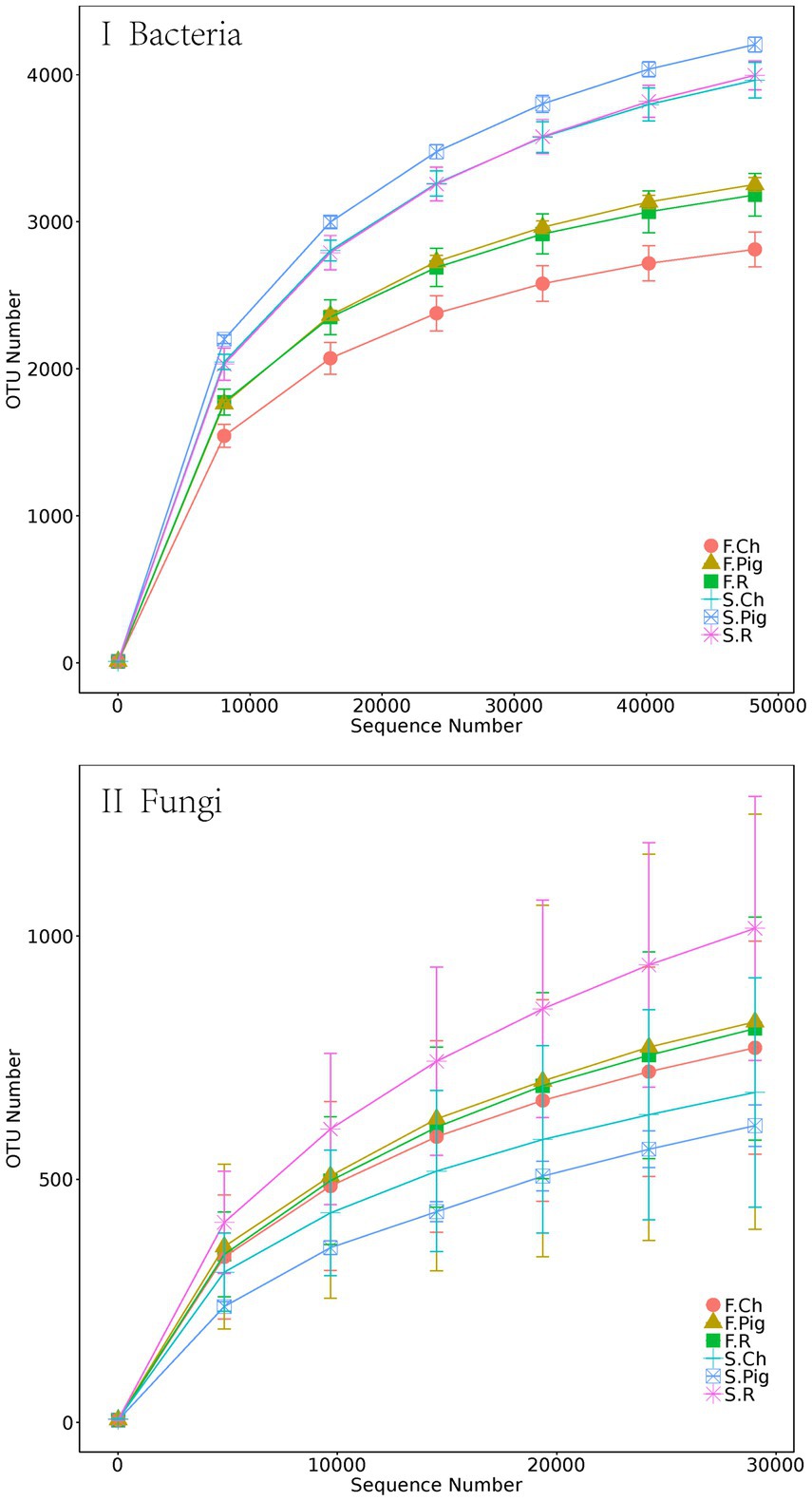
Figure 1. The rarefaction curves of bacterial and fungal rRNA sequencing depth and number of species number in Brassica rapa var. Chinensis soil.
The observed OTUs, Chao1 and Shannon indices (Table 5) were evaluated to estimate the alpha diversity of bacterial and fungal communities in Brassica rapa var. Chinensis soils under different fertilized treatments. According to Table 5, we found that the soil sample of each fertilizer treatment in the second season had the higher observed OTUs, Chao1 index and Shannon index of bacterial community than that in the first season, and there are significant difference between treatments. The observed OTUs, Chao1 index and Shannon index of pig and rabbit manure treatments were significantly higher than that of chemical fertilization treatment in the same season. These results indicated that the soil samples of pig manure and rabbit manure fertilizer had the higher level of bacterial diversity than that of chemical fertilizer in both two seasons. We found that the Observed OTUs, Chao1 index and Shannon index of fungal community of the soil sample of three fertilizer treatments in two seasons had no significant difference due to the large variance within treatments, but the Chao1 of organic fertilization treatment, especially rabbit manure fertilization treatment, was higher than that of chemical fertilizer treatment.
Analysis of molecular variance (AMOVA) showed that the differences in beta diversity were significant (bacteria: Fs = 6.76, p ≤ 0.001*; fungi: Fs = 2.40, p ≤ 0.001*) between the fertilizer treatment groups. The beta diversity (NMDS) of soil microbial communities of Brassica rapa var. Chinensis under fertilizer treatments was further investigated. The non-metric multi-dimensional scaling (NMDS) plot (Figure 2I) of the bacterial communities showed a clear separation among the soil bacterial of Brassica rapa var. Chinensis under two seasons and three fertilizer treatments, the bacterial communities were distinguished along MDS1 in two seasons and along MDS2 for different fertilizer treatments. These results indicated that there were significant differences (p ≤ 5%) in the bacterial community structure between three treatments in two seasons. And the distance between the three fertilizations in the second season was relatively close, indicating that the bacterial community of the three fertilizations in the second season was relatively similar. Thus, pig and rabbit manure fertilization had altered the structure of the soil bacterial community, and the difference in bacterial community structure between the three fertilizer treatments in the first season was significant compared to that in the second season. From the NMDS plot of fungal communities (Figure 2II), there was also a clear distinction between MDS1 and MDS2 of six groups of three fertilizer treatments in two seasons, the fungal communities were distinguished along MDS2 for three fertilizer treatments and not along MDS1 in two seasons. This result was consistent with the Amova analysis which showed that beta diversity was a significant difference in fungal community structure between fertilizer treatments.
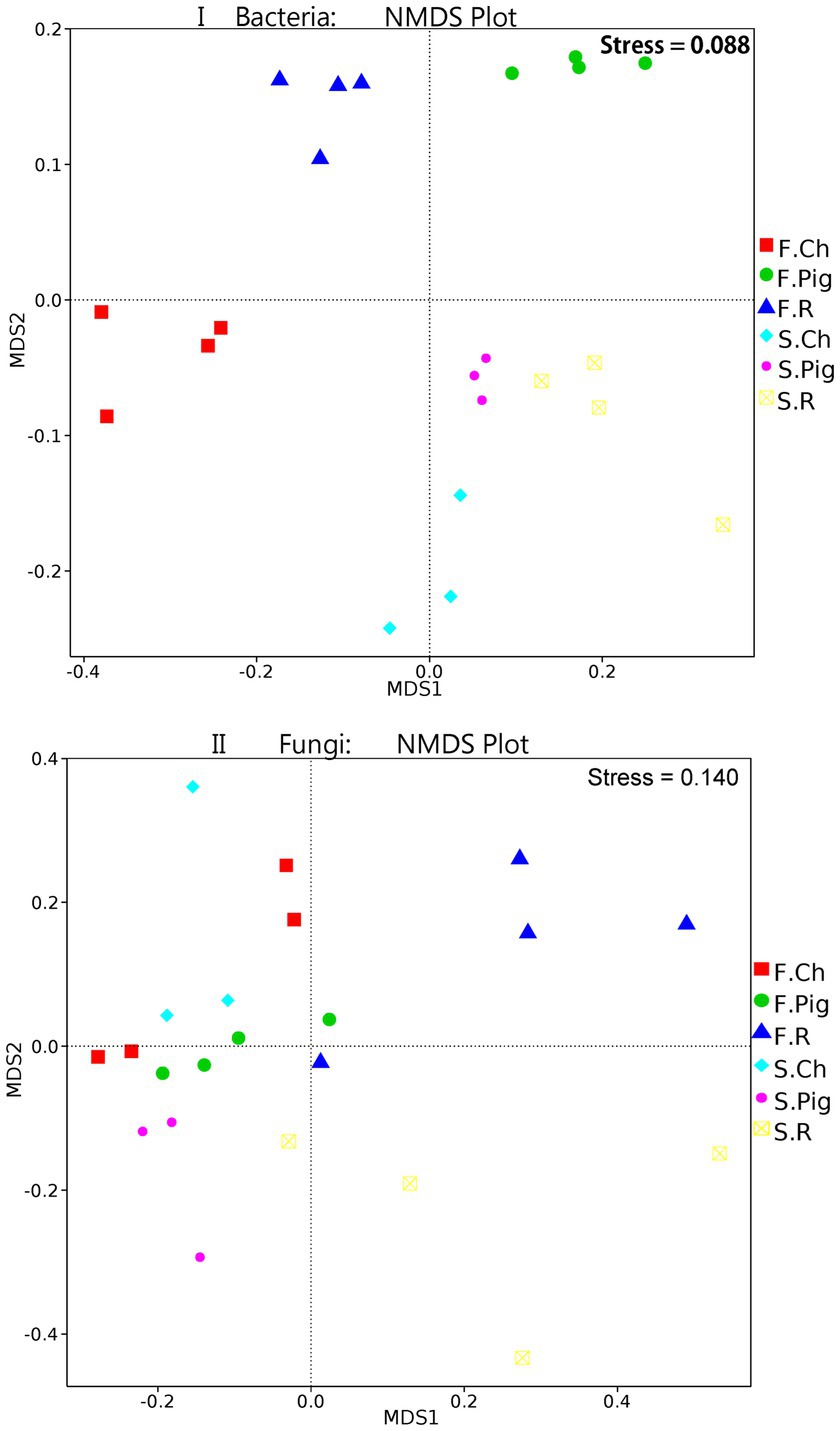
Figure 2. NMDS (Bray–Curits distance) of bacterial and fungal communities under three fertilization treatments in two seasons.
All bacterial OTUs were classified into 904 genera, 534 families and 78 phyla. The dominant phyla (relative sequence abundance ≥1%) across all samples were Proteobacteria (29.99%), Bacteroidota (11.25%), and Firmicutes (7.55%), Unidentified_Bacteria (14.86%), Acidobacteriota (7.55%), Actinobacteria (7.88%), Myxococcota (2.73%), Verrucomicrobiota (1.36%), Verrucomicrobia (3.00%) and Gemmatimonadetes (1.57%), representing 86.37% of the bacterial sequences (Figure 3I). The abundance of Acidobacteriota and Unidentified_Bacteria in the soil of the second season was significantly higher than that of the first season, the abundance of Bacteroidota and Crenarchaeota of the second season was significantly lower than that of the first season. Organic fertilization significantly decreased the abundance of soil Acidobacteriota and Crenarchaeota, increased the abundance of soil Unidentified Bacteria, Myxococcota and Gemmatimonadetes, and rabbit manure fertilization significantly increased the abundance of soil Actinobacteria (Figure 3III).
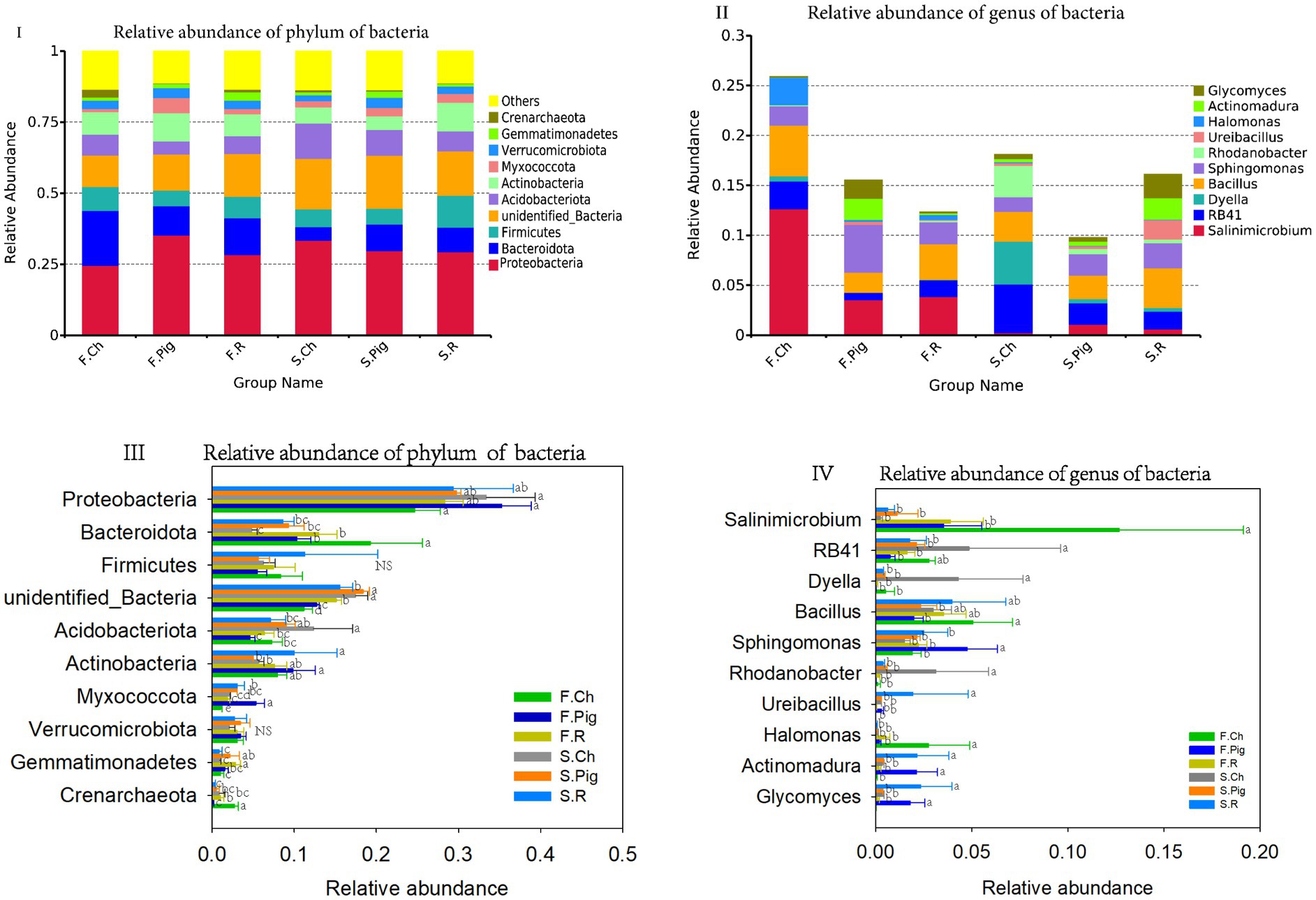
Figure 3. The relative abundance of phylum and genus of soil bacterial communities. Values are the mean of the three or four replicates of each treatment. Vertical bars represent the standard error, and bars with different letters within the same treatment indicate that there are significant differences between treatments at p ≤ 0.05. NS, not significant.
Four dominant genera (relative sequence abundance ≥1%) in all samples were Salinimicrobium (3.96%) and Sphingomonas (2.57%), which belong to Proteobacteria, and RB41 (2.22%) belongs to Acidobacteriota, Bacillus (3.38%) belong to Firmicutes, accounting for 12.12% of the bacterial sequences (Figure 3II). Among the top 10 dominant genura, the abundance of Dyella and Rhodanobacter in the soil of the second season was significantly higher than that of the first season, the abundance of Salinimicrobium and Sphingomonas in the second season was significantly lower than that of the first season. Pig and rabbit manure fertilizer significantly increased the abundance of Sphingomonas, Actinomadura and Glycomyces and decreased the abundance of Salinimicrobium, RB41, Dyella, Rhodanobacter and Halomonas (Figure 3IV).
All fungal OTUs were classified into 743 genera, 743 families and 18 phyla. The dominant phyla (relative sequence abundance ≥1%) in all samples were Ascomycota (64.95%), Olpidiomycota (7.90%), Basidiomycota (5.66%), Rozellomycota (5.27%), Chytridiomycota (2.47%), accounting for 85.96% of the fungal sequences (Figure 4I). The abundance of Ascomycota in the soil of the chemical and pig manure fertilization treatments was significantly higher than that of the rabbit manure fertilization treatment in both two seasons. The abundance of Olpidiomycota in the soil of rabbit manure fertilization treatments was significantly higher than that of the chemical and pig manure fertilization treatments in both two seasons. The abundance of Rozellomycota in the soil of the rabbit manure fertilization treatment was significantly higher than that of the other treatments in the second season.
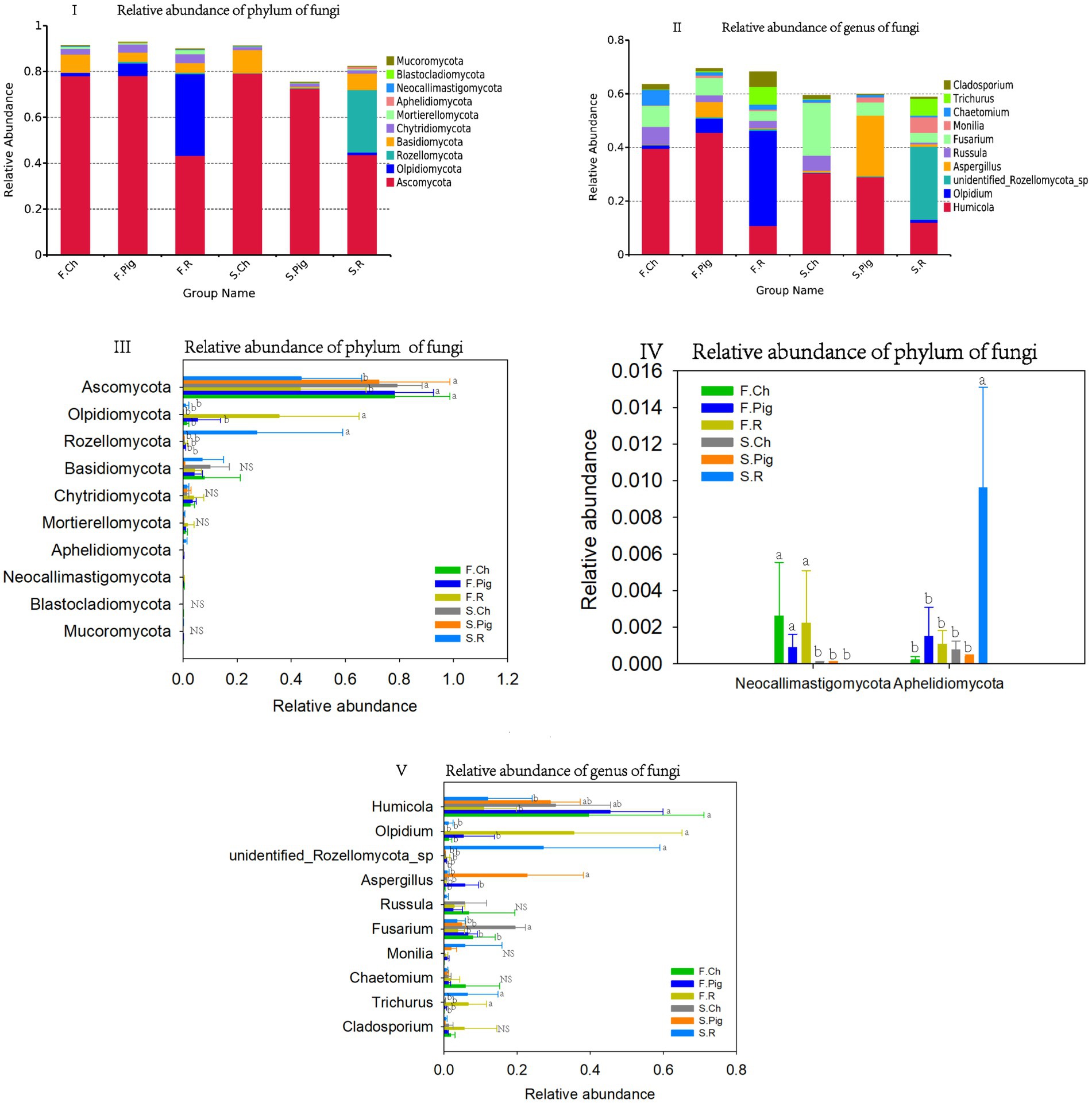
Figure 4. The relative abundance of the phylum and genus of the soil fungal communities. Values are the mean of the three or four replicates of each treatment. Vertical bars represent the standard error, and bars with different letters within the same treatment indicate that there are significant differences between treatments at p ≤ 0.05. NS, not significant.
Chemical fertilizer increased the abundance of soil Basidiomycota in the soil, and the abundance of Chytridiomycota was higher in the first season than in the second season (Figures 4III,IV).
The six dominant genera (relative sequence abundance ≥1%) in all samples were Humicola (27.74%), Olpidium (7.89%), Fusarium (7.27%), unidentified Rozellomycota sp. (5.20%), Aspergillus (4.49%), Russula (3.08%), Trichurus (2.47%), Chaetomium (2.04%), Cladosporium (1.82%) and Monilia (1.58%), accounting for 63.57% of the fungal sequences (Figure 4II). Among the top 10 dominant genera, rabbit manure fertilizer significantly reduced the abundance of Humicola, and increased the abundance of Olpidium and Trichurus. Pig manure fertilizer significantly increased the abundance of Aspergillus. Pig and rabbit manure fertilizer reduced the abundance of Fusarium (Figure 4V).
We estimated the Pearson correlation between soil physicochemical properties and microbial community alpha diversity measures (Table 6). We found that EC, TN, TP and SOC were significantly positively correlated (p ≤ 1%) with bacterial Chao 1, and negatively correlated with Goods coverage. TN, TP, and SOC concentrations were a significantly positively correlated (p ≤ 1%) with Shannon of the bacterial communities. Therefore, the EC, TN, TP and SOC concentrations were significantly correlated with alpha diversity in soil bacterial communities under three fertilizer treatments. However, the results showed that pH, EC, TN, TP, SOC, NO3-N and C/N had no significant correlations with the observed OTUs, Shannon, chao1, of the fungal communities.
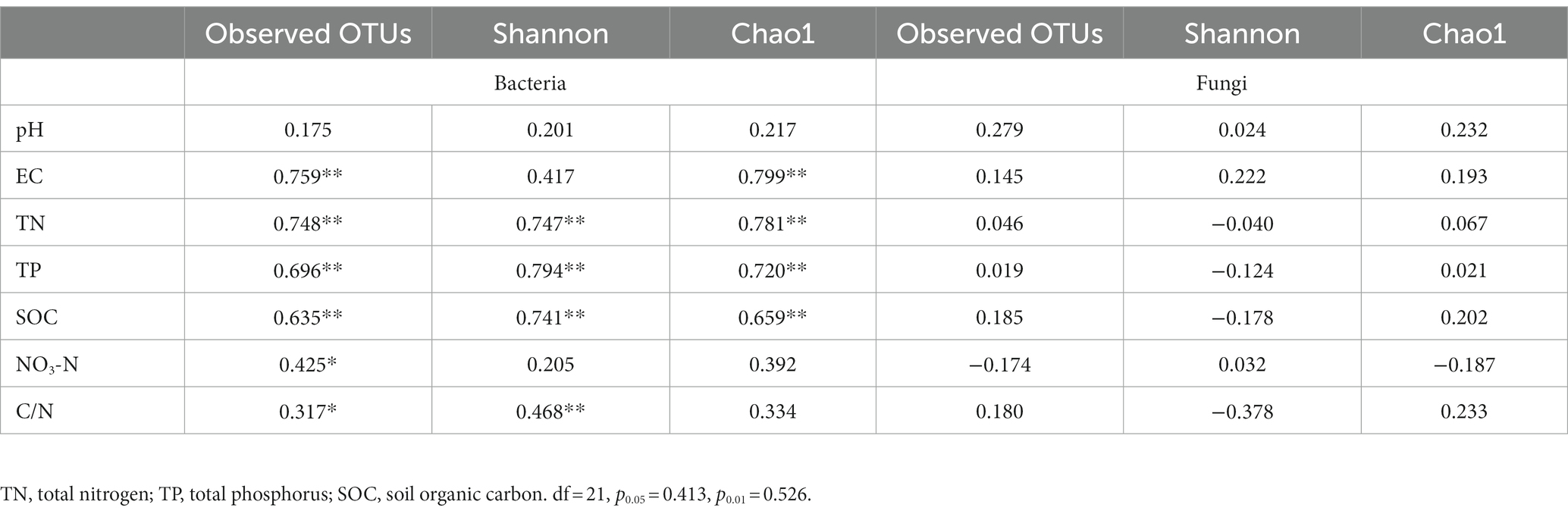
Table 6. Pearson’s correlation between soil physicochemical properties and alpha-diversity of soil microbial communities of Brassica rapa var. Chinensis.
To investigate the effect of the three fertilizer treatments on the composition of the bacterial and fungal communities and environmental factors, and to further reveal the relationship between soil physicochemical properties and microbial community structures, a distance-based redundancy analysis (db-RDA) of soil physicochemical properties and the relative abundance of OTUs of the microbial community was performed (Figure 5). The dbRDA1 and dbRDA2 explained 70.57% of the diversity of the bacterial community and 60.04% of the diversity of the fungal community. The bacterial community was clearly separated among the three fertilizer treatments in two seasons, and the fungal community was not clearly separated (Figure 5). The db-RDA plot (Figure 5I) clearly showed that soil EC, TN, and SOC concentrations were the three longest vectors, and they could be key physicochemical factors to assemble the bacterial community structure in the experimental soil. In the second season, the soil bacterial community of pig manure fertilization treatment was positively correlated with soil EC, that of rabbit manure fertilization treatment was positively correlated with soil TN content, and that of the chemical fertilization treatment was positively correlated with soil NO3−N content. The db-RDA plot (Figure 5II) clearly showed that soil NO3-N, EC, SOC concentration and pH were the four longest vectors. The soil fungal community of pig manure and chemical fertilization treatment was positively correlated with soil NO3-N in both two seasons, and the fungal community of rabbit manure fertilization treatment was positively correlated with soil EC, SOC, TP concentration and soil pH in the second season.
The aim of this study was to investigate the effect of organic manure on Brassica rapa var. Chinensis yield and quality, soil physicochemical properties, and soil microbial community composition. For sustainable agriculture, organic fertilization is inevitable in the future as it improves soil properties, increases crop productivity and maintains crop quality (Adekiya et al., 2020). In our study, chemical fertilization significantly increased the yield of Brassica rapa var. Chinensis in the first season compared to organic fertilization (Table 2), when the total nitrogen content of organic fertilization is twice that of chemical fertilization, which means that the mineralization rate of organic fertilization is slow, and the nutrients in the soil cannot meet the needs of Brassica rapa var. Chinensis. This is because the nitrate and ammonium roots of compound fertilizers can be directly absorbed by the plants after fertilization, whereas organic fertilizers must first be mineralized into inorganic nutrients by soil microbiota before being absorbed and assimilated by plants (Alizadeh et al., 2012). The supply of nitrogen from organic fertilizers depends on their rate of mineralization, but in the first season, soil temperatures are relatively low and the rate of mineralization of organic fertilizers is also low, resulting in inadequate nutrient supply (Geisseler et al., 2021; Cannavo et al., 2022). Although the yield of Brassica rapa var. Chinensis in the chemical fertilizer treatment was significantly lower than those of organic fertilizer treatments in the second season (Table 2), this is because the fertilizer is reapplied on the basis of the first season, which increases the total nutrient content in the soil, at the same time, the rate of soil mineralization increases with the increase in temperature and the nutrients in the soil applied with organic fertilizer can continuously meet the needs of the crop in the second season(Kelderer et al., 2008; Bebber and Richards, 2022; Cannavo et al., 2022). And that the application of organic fertilizers increased the input of organic carbon and nitrogen, replenished the carbon source, promoted the reproduction of soil microorganisms, and also improved the mineralization of organic fertilizers and increased the nutrients available in the soil to meet the needs of crops (Birkhofer et al., 2008; Anggraheni et al., 2019; Wang et al., 2020a).
Organic fertilizers (pig manure and rabbit manure fertilizer) significantly increased the soluble sugar content of Brassica rapa var. Chinensis, especially in the first season, and significantly decreased the nitrate nitrogen content of Brassica rapa var. Chinensis (Table 2), and these results were consistent with the results of previous studies (Xu et al., 2002; Li et al., 2017; Youssef and Eissa, 2017; Serri et al., 2021). Consistent with the results of our study (Table 3), previous research also found that organic fertilization significantly increased soil organic carbon, total nitrogen, total phosphorus and pH (Li X. et al., 2021), and chemical fertilization decreased soil pH (Li et al., 2019), but rabbit manure fertilizer increased soil EC compared to chemical fertilization (El-Mogy et al., 2020). Rabbit manure fertilizer decreased the nitrate content of Brassica rapa var. Chinensis (Table 3). The low nitrate content was caused by the lower nitrate content of rabbit manure fertilizer in soil (Li et al., 2017) and the correlation between soil nitrate content and Brassica rapa var. Chinensis nitrate content was significant and positive (p = 0.876). However, the nitrate content of vegetables and soil after application of pig manure was opposite to that of rabbit manure.
Using high-throughput sequencing, three fertilization treatments were compared in two seasons of Brassica rapa var. Chinensis soil. Fertilization with organic and chemical fertilizers profoundly affected the diversity, richness, structure and activity of soil microbial communities (Lazcano et al., 2013; Francioli et al., 2016; Gu et al., 2017). Previous studies suggested that organic fertilizer increased bacterial species’ richness and diversity (Lazcano et al., 2013) and significantly increased α-diversity, such as Chao1 and Shannon index of soil bacteria (Xue et al., 2018), and our results were consistent with these findings (Table 5). This is because organic fertilizer provides sufficient substrate for soil bacteria (Li et al., 2022) and a large number of soil microorganisms (Watts et al., 2010), leading to an increase in microbial diversity. On the other hand, organic fertilizer had no significant effect on fungal species’ richness and diversity (Francioli et al., 2016; Lin et al., 2019; Qi et al., 2022), and we have similar results (Table 5). This may be due to nutrient enrichment caused by fertilization (Jiang et al., 2021), for example, fertilizer increases fungal size, which reduces fungal biodiversity and changes community composition (Zhou et al., 2016). Based on the NMDS analysis, it was found that differences in bacterial community structure were highlighted among the three fertilization treatments in two seasons (Figure 2), this result is consistent with previous study (Gu et al., 2017; Wu et al., 2020). This may be because organic fertilizer provides a greater diversity of potential substrates for bacterial growth and reproduction, and at the same time bacteria in organic fertilizer could also increase soil enhance microbial biomass (Dong et al., 2014). Previous research on the sensitivity of fungi to fertilizer has been inconsistent. Studies suggested that fungi were not significantly affected by different fertilization treatments, and possibly because conventional tillage or invasive land management (e.g., fertilization) increased bacterial activity and reduced fungal activity, whereas fungi dominated under no-tillage or less invasive land management (de Vries et al., 2006). However, other studies have suggested that fungal communities are more resilient to environmental change than bacterial communities, and that bacterial communities have broader adaptive options (Lin et al., 2019). While our study results showed that differences in fungal community structure were highlighted between the three fertilizer treatments and not between the two seasons (Figure 2), this suggests that fungal community structure is strongly influenced by fertilizer type and is not affected by time (two seasons).
In this study, the abundance of unidentified bacteria and Acidobacteriota was significantly higher in the second season than in the first season, while the abundance of Bacteroidota and Crenarchaeota was lower than in the first season (Figure 3), indicating that bacteria are more sensitive to the environment (Lin et al., 2019). Proteobacteria are the most abundant bacteria in the soil in our experiment. Proteobacteria predominate in different soil environments and are mostly Gram-negative (Liang et al., 2018), which was expected to enhance the biological cycling of essential micro- or macro- nutrients and improve soil fertility and plant growth efficiency (Lesaulnier et al., 2008). In our study, Proteobacteria had an absolute advantages in different treatment groups, their abundance ranged from 23 to 37%, and 6 of the top 10 bacterial genera belonged to Proteobacteria. Previous research suggested that the relative abundance of Acidobacteria was negatively correlated with soil pH (Jones et al., 2009). In our study, pig manure and rabbit manure fertilizer increased soil pH and decreased the relative abundance of soil Acidobacteria, compared to chemical fertilizer in the second season (Figure 3III), which was consistent with previous study results (Ma et al., 2021). Compost application has been reported to decrease the relative abundance of Actinobacteria (Liang et al., 2018; Ma et al., 2021), but, our study showed that rabbit manure fertilizer significantly increased the abundance of Actinobacteria in the second season.
Our study results showed that organic fertilizer, especially rabbit manure, reduced the relative abundance of Ascomycota compared to chemical fertilizer (Figure 4), this trend may be due to the more stable form of organic substances after a fermentation process during the maturation of manure (Hannula et al., 2021). Similar results were found in previous studies which showed that mineral N fertilizer promoted fungal growth and organic fertilizer reduced fungal growth (Wang et al., 2017; Hannula et al., 2021). Basidiomycota are widely regarded as lignin decomposers and are therefore important for soil carbon cycling (Hanson et al., 2008). Our results showed that organic fertilizer reduced the abundance of soil Basidiomycota, as the beneficial function of Basidiomycota could be affected by high soil N level (Paungfoo-Lonhienne et al., 2015) and perhaps also by the inhibition of rabbit manure fertilizer.
According to Xue et al. (2018), soil properties, including nutrients (e.g., total C, total N, P,EC), are more correlated with the absolute abundance of microbes, and EC, clay content and pH accounted of the variation in soil microbial structure in southeast Australia. It has been reported that organic fertilizers can influence the structure of bacterial communities by altering soil properties in a soil type (Li P. et al., 2021; Iqbal et al., 2022). Wu et al. reported that the bacterial community was influenced by soil EC and soil carbon, while the fungal community was more influenced by alkaline nitrogen (Wu et al., 2020). Our study also found that pig manure fertilizer and rabbit manure fertilizer altered soil physicochemical properties (Table 3). Distance-based redundancy analysis (dbRDA) according to the relative abundance of OTUs further showed that, overall, soil EC, TN, and SOC concentration were the most important physicochemical factors to assemble the bacterial community structure in Brassica rapa var. Chinensis soil, and we also found that in the second season, soil EC and NO3-N content had the great effect on the soil bacterial community structure of pig manure fertilizer application and soil TN of rabbit manure fertilizer application (Figure 5I). dbRDA results showed that soil NO3-N, EC, SOC concentration and soil pH were fungal community structure in Brassica rapa var. Chinensis soil, and we also found that soil EC, SOC, pH had the great effect on the soil fungal community structure of rabbit manure fertilizer application and soil NO3-N of chemical fertilizer and pig manure fertilizer treatment soil (Figure 5II).
(1) With the increase of time, the advantage of using organic fertilizer to increase the yield of Brassica rapa var. Chinensis in the second season gradually emerged. The rabbit manure fertilizer significantly (p ≤ 5%) reduced the NO3-N content of fresh Brassica rapa var. Chinensis.
(2) The organic fertilizer increased the concentration of total nitrogen, total phosphorus and organic carbon in soil and the rabbit manure fertilizer increased soil pH and EC and significantly (p ≤ 5%) reduced soil NO3-N content.
(3) Organic fertilizer significantly (p ≤ 5%) improved the diversity and richness of soil bacteria in Brassica rapa var. Chinensis soil, but had no significant effect on soil fungi. There were significant differences (p ≤ 5%) in the bacterial community structures between different treatments in two seasons, and significant differences (p ≤ 5%) in the fungal community structures between fertilizer treatments, but not between two seasons.
(4) Soil EC, TN and organic carbon content were the most important physicochemical factors in determining the bacterial community structure in Brassica rapa var. Chinensis soils, and soil NO3-N, EC, SOC concentration and soil pH in the fungal community structure.
The raw sequence data reported in this study have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (https://ngdc.cncb.ac.cn/gsa), under the accession number CRA011178.
XZ, HG, and JY: conceptualization. XZ, JY, and XP: methodology. JL and PZ: software. LS and JY: validation. XZ, FQ, and XP: formal analysis. JY: investigation. GH: resources. XZ and JY: data curation. XZ: writing—original draft preparation, writing—review and editing, and project administration. FQ: visualization. JL: supervision. JY and XP: funding acquisition. All authors contributed to the article and approved the submitted version.
This research was funded by the the earmarked fund for China Agriculture Research System (grant number: CAR-43-G-2), the Jiangsu Characteristic Livestock and Poultry Industry Technology System (grant number: JATS[2022]244), Jiangsu Province Vice President of Science and Technology Project (grant number: FZ20220074), the Jiangsu Industry University Research Cooperation Project (590) and the One Zone, One Center Joint Special Project (grant number: 202212005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adekiya, A. O., Ejue, W. S., Olayanju, A., Dunsin, O., Aboyeji, C. M., Aremu, C., et al. (2020). Different organic manure sources and NPK fertilizer on soil chemical properties, growth, yield and quality of okra. Sci. Rep. 10:16083. doi: 10.1038/s41598-020-73291-x
Alizadeh, P., Fallah, S., and Raiesi, F. (2012). Potential N mineralization and availability to irrigated maize in a calcareous soil amended with organic manures and urea under field conditions. Int. J. Plant Prod. 6, 493–512. doi: 10.22069/IJPP.2012.762
Anggraheni, Y. G. D., Nuro, F., and Paradisa, Y. B.. (2019). Effect of organic fertilizer on growth and yield of chili pepper. in Proceedings The SATREPS Conference, 30–37. Available at: https://www.researchgate.net/publication/334315958_Effect_of_Organic_Fertilizer_on_Growth_and_Yield_of_Chili_Peppers
Bebber, D. P., and Richards, V. R. (2022). A meta-analysis of the effect of organic and mineral fertilizers on soil microbial diversity. Appl. Soil Ecol. 175:104450. doi: 10.1016/j.apsoil.2022.104450
Birkhofer, K., Bezemer, T. M., Bloem, J., Bonkowski, M., Christensen, S., Dubois, D., et al. (2008). Long-term organic farming fosters below and aboveground biota: implications for soil quality, biological control and productivity. Soil Biol. Biochem. 40, 2297–2308. doi: 10.1016/j.soilbio.2008.05.007
Bokulich, N. A., Subramanian, S., Faith, J. J., Gevers, D., Gordon, J. I., Knight, R., et al. (2013). Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59. doi: 10.1038/nmeth.2276
Burger, M., and Jackson, L. E. (2003). Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol. Biochem. 35, 29–36. doi: 10.1016/S0038-0717(02)00233-X
Cannavo, P., Recous, S., Valé, M., Bresch, S., Paillat, L., Benbrahim, M., et al. (2022). Organic fertilization of growing media: response of N mineralization to temperature and moisture. Horticulturae 8:152. doi: 10.3390/horticulturae8020152
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Cataldo, D., Maroon, M., Schrader, L. E., and Youngs, V. L. (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 6, 71–80. doi: 10.1080/00103627509366547
China, M. O. E. A. E. O. T. P. S. R. O., N. B. O. Statistics & M. O. A. A. R. A. O. T. P. S. R. O. China. (2010). Available at: First National Pollution Source Census Bulletin. http://www.stats.gov.cn/tjsj/tjgb/qttjgb/qgqttjgb/201002/t20100211_30641.htmL.
China, T. M. O. A. A. R. A. O. T. P. S. R. O. (2017). Carry out the action plan of substituting organic fertilizer for chemical fertilizer in fruit, vegetable and tea. Available at: http://www.moa.gov.cn/nybgb/2017/derq/201712/t20171227_6130977.htm.
de Vries, F. T., Hoffland, E., van Eekeren, N., Brussaard, L., and Bloem, J. (2006). Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 38, 2092–2103. doi: 10.1016/j.soilbio.2006.01.008
Dong, W.-Y., Zhang, X.-Y., Dai, X.-Q., Fu, X.-L., Yang, F.-T., Liu, X.-Y., et al. (2014). Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 84, 140–147. doi: 10.1016/j.apsoil.2014.06.007
Dong, H., Zuo, L., Wei, S., Zhu, Z., and Yin, F. (2019). Establish manure nutrient management plan to promote green development of integrated crop-livestock production system. Bull. Chin. Acad. Sci. 34, 180–189. doi: 10.16418/j.issn.1000-3045.2019.02.007
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
El-Mogy, M. M., Abdelaziz, S. M., Mahmoud, A. W. M., Elsayed, T. R., Abdel-Kader, N. H., and Mohamed, M. I. A. (2020). Comparative effects of different organic and inorganic Fertilisers on soil fertility, plant growth, soil microbial community, and storage ability of lettuce. Agriculture (Pol'nohospodárstvo) 66, 87–107. doi: 10.2478/agri-2020-0009
Francioli, D., Schulz, E., Lentendu, G., Wubet, T., Buscot, F., and Reitz, T. (2016). Mineral vs. organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 7:1446. doi: 10.3389/fmicb.2016.01446
Geisseler, D., Smith, R., Cahn, M., and Muramoto, J. (2021). Nitrogen mineralization from organic fertilizers and composts: literature survey and model fitting. J. Environ. Qual. 50, 1325–1338. doi: 10.1002/jeq2.20295
Gu, Y., Wang, Y., Lu, S.’., Xiang, Q., Yu, X., Zhao, K., et al. (2017). Long-term fertilization structures bacterial and archaeal communities along soil depth gradient in a paddy soil. Front. Microbiol. 8:1516. doi: 10.3389/fmicb.2017.01516
Haas, B. J., Gevers, D., Earl, A. M., Feldgarden, M., Ward, D. V., Giannoukos, G., et al. (2011). Chimeric 16S rRNA sequence formation and detection in sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504. doi: 10.1101/gr.112730.110
Hannula, S. E., Di Lonardo, D. P., Christensen, B. T., Crotty, F. V., Elsen, A., van Erp, P. J., et al. (2021). Inconsistent effects of agricultural practices on soil fungal communities across 12 European long-term experiments. Eur. J. Soil Sci. 72, 1902–1923. doi: 10.1111/ejss.13090
Hanson, C. A., Allison, S. D., Bradford, M. A., Wallenstein, M. D., and Treseder, K. K. (2008). Fungal taxa target different carbon sources in forest soil. Ecosystems 11, 1157–1167. doi: 10.1007/s10021-008-9186-4
He, X., Qiao, Y., Liu, Y., Dendler, L., Yin, C., and Martin, F. (2016). Environmental impact assessment of organic and conventional tomato production in urban greenhouses of Beijing city, China. J. Clean. Prod. 134, 251–258. doi: 10.1016/j.jclepro.2015.12.004
Huang, S., Tang, J., Li, C., Zhang, H., and Yuan, S. (2017). Reducing potential of chemical fertilizers and scientific fertilization countermeasure in vegetable production in China. J. Plant Nutr. Fertil. 23, 1480–1493. doi: 10.11674/zwyf.17366
Huang, R., Wang, Y., Liu, J., Gao, J., Zhang, Y., Ni, J., et al. (2020). Partial substitution of chemical fertilizer by organic materials changed the abundance, diversity, and activity of nirS-type denitrifying bacterial communities in a vegetable soil. Appl. Soil Ecol. 152:103589. doi: 10.1016/j.apsoil.2020.103589
Iqbal, A., He, L., Ali, I., Yuan, P., Khan, A., Hua, Z., et al. (2022). Partial substation of organic fertilizer with chemical fertilizer improves soil biochemical attributes, rice yields, and restores bacterial community diversity in a paddy field. Front. Plant Sci. 13:895230. doi: 10.3389/fpls.2022.895230
Jiang, S., An, X., Shao, Y., Kang, Y., Chen, T., Mei, X., et al. (2021). Responses of arbuscular mycorrhizal fungi occurrence to organic fertilizer: a meta-analysis of field studies. Plant Soil 469, 89–105. doi: 10.1007/s11104-021-05153-y
Jones, R. T., Robeson, M. S., Lauber, C. L., Hamady, M., Knight, R., and Fierer, N. (2009). A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 3, 442–453. doi: 10.1038/ismej.2008.127
Jude, J. O. O., and Vidah, N. M. (2008). An assessment of the use of mineral and organic fertilizers by smallholder farmers in Vhembe district, Limpopo province, South Africa. Afr. J. Agric. Res. 3, 357–362. doi: 10.46882/IJMF/1121
Kelderer, M., Thalheimer, M., Andreaus, O., Topp, A., Burger, R., and Schiatti, P.. (2008). “The mineralization of commercial organic fertilizers at 8 C temperature.” in Ecofruit-13th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing: Proceedings to the Conference from 18thFebruary to 20th February 2008 at Weinsberg/Germany, 160–166. Available at: https://orgprints.org/id/eprint/13665/1/160-166.pdf
Lazcano, C., Gómez-Brandón, M., Revilla, P., and Domínguez, J. (2013). Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils 49, 723–733. doi: 10.1007/s00374-012-0761-7
Lazcano, C., Zhu-Barker, X., and Decock, C. (2021). Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: a review. Microorganisms 9:983. doi: 10.3390/microorganisms9050983
Lesaulnier, C., Papamichail, D., McCorkle, S., Ollivier, B., Skiena, S., Taghavi, S., et al. (2008). Elevated atmospheric CO2 affects soil microbial diversity associated with trembling aspen. Environ. Microbiol. 10, 926–941. doi: 10.1111/j.1462-2920.2007.01512.x
Li, P., Kong, D., Zhang, H., Xu, L., Li, C., Wu, M., et al. (2021). Different regulation of soil structure and resource chemistry under animal-and plant-derived organic fertilizers changed soil bacterial communities. Appl. Soil Ecol. 165:104020. doi: 10.1016/j.apsoil.2021.104020
Li, G., Li, M., Wu, M., and Li, Z. (2022). Effects of chemical fertilizer combined with organic manure on Peanut rhizosphere bacterial community structure and co-occurrence network. Soil 54, 498–507. doi: 10.13758/j.cnki.tr.2022.03.009
Li, S., Li, J., Zhang, B., Li, D., Li, G., and Li, Y. (2017). Effect of different organic fertilizers application on growth and environmental risk of nitrate under a vegetable field. Sci. Rep. 7, 1–9. doi: 10.1038/s41598-017-17219-y
Li, X., Su, Y., Ahmed, T., Ren, H., Javed, M. R., Yao, Y., et al. (2021). Effects of different organic fertilizers on improving soil from newly reclaimed land to crop soil. Agriculture 11:560. doi: 10.3390/agriculture11060560
Li, J., Wan, X., Liu, X., Chen, Y., Slaughter, L. C., Weindorf, D. C., et al. (2019). Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Tillage Res. 195:104366. doi: 10.1016/j.still.2019.104366
Liang, B., Ma, C., Fan, L., Wang, Y., and Yuan, Y. (2018). Soil amendment alters soil physicochemical properties and bacterial community structure of a replanted apple orchard. Microbiol. Res. 216, 1–11. doi: 10.1016/j.micres.2018.07.010
Lin, Y., Ye, G., Kuzyakov, Y., Liu, D., Fan, J., and Ding, W. (2019). Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 134, 187–196. doi: 10.1016/j.soilbio.2019.03.030
Ling, N., Zhu, C., Xue, C., Chen, H., Duan, Y., Peng, C., et al. (2016). Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol. Biochem. 99, 137–149. doi: 10.1016/j.soilbio.2016.05.005
Liu, J., Shu, A., Song, W., Shi, W., Li, M., Zhang, W., et al. (2021). Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 404:115287. doi: 10.1016/j.geoderma.2021.115287
Ma, L., Gao, W., Luan, H., Tang, J., and MY, H. S. W. L. (2021). Soil microbial community characteristics in greenhouse vegetable production under different fertilization patterns based on metagenomic analysis. J. Plant Nutr. Fertil. 27, 403–416. doi: 10.11674/zwyf.20486
Magoč, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Norman, R. J., Edberg, J. C., and Stucki, J. W. (1985). Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 49, 1182–1185. doi: 10.2136/sssaj1985.03615995004900050022x
Paungfoo-Lonhienne, C., Yeoh, Y. K., Kasinadhuni, N. R., Lonhienne, T. G., Robinson, N., Hugenholtz, P., et al. (2015). Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 5:8678. doi: 10.1038/srep08678
Qi, Y., Wu, Z., Zhou, R., Hou, X., Yu, L., Cao, Y., et al. (2022). Nitrogen reduction with bio-organic fertilizer altered soil microorganisms, improved yield and quality of non-heading Chinese cabbage (Brassica campestris ssp. chinensis Makino). Agronomy 12:1437. doi: 10.3390/agronomy12061437
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Serri, F., Souri, M. K., and Rezapanah, M. (2021). Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 8:33. doi: 10.1186/s40538-021-00232-9
Sinay, H., and Karuwal, R. L. (2014). Proline and total soluble sugar content at the vegetative phase of six corn cultivars from Kisar Island Maluku, grown under drought stress conditions. Int. J. Adv. Agric. Res 2, 77–82. doi: 10.33500/ijaar.2014.02.008
Sun, Y., Qiu, T., Gao, M., Shi, M., Zhang, H., and Wang, X. (2019). Inorganic and organic fertilizers application enhanced antibiotic resistome in greenhouse soils growing vegetables. Ecotoxicol. Environ. Saf. 179, 24–30. doi: 10.1016/j.ecoenv.2019.04.039
Wang, N., Nan, H., and Feng, K. (2020a). Effects of reduced chemical fertilizer with organic fertilizer application on soil microbial biomass,enzyme activity and cotton yield. Chin. J. Appl. Ecol. 31, 173–181. doi: 10.13287/j.1001-9332.202001.022
Wang, J., Song, Y., Ma, T., Raza, W., Li, J., Howland, J. G., et al. (2017). Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 112, 42–50. doi: 10.1016/j.apsoil.2017.01.005
Wang, H., Xu, J., Liu, X., Zhang, D., Li, L., Li, W., et al. (2019). Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res. 195:104382. doi: 10.1016/j.still.2019.104382
Wang, X., Yang, Y., Zhao, J., Nie, J., Zang, H., Zeng, Z., et al. (2020b). Yield benefits from replacing chemical fertilizers with manure under water deficient conditions of the winter wheat–summer maize system in the North China plain. Eur. J. Agron. 119:126118. doi: 10.1016/j.eja.2020.126118
Wang, Y., Zhu, Y., Zhang, S., and Wang, Y. (2018). What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 199, 882–890. doi: 10.1016/j.jclepro.2018.07.222
Watts, D. B., Torbert, H. A., Feng, Y., and Prior, S. A. (2010). Soil microbial community dynamics as influenced by composted dairy manure, soil properties, and landscape position. Soil Sci. 175, 474–486. doi: 10.1097/SS.0b013e3181f7964f
Wu, Z., Li, H., Liu, Q., Ye, C., and Yu, F. (2020). Application of bio-organic fertilizer, not biochar, in degraded red soil improves soil nutrients and plant growth. Rhizosphere 16:100264. doi: 10.1016/j.rhisph.2020.100264
Xu, H., Wang, R., Xu, R., Mridha, M., and Goyal, S.. (2002). “Yield and quality of leafy vegetables grown with organic fertilizations.” in XXVI international horticultural congress: toward ecologically sound fertilization strategies for field vegetable production, vol. 627, 25–33. Available at: https://www.researchgate.net/publication/232041794_Yield_and_Quality_of_Leafy_Vegetables_Grown_with_Organic_Fertilizations
Xue, P.-P., Carrillo, Y., Pino, V., Minasny, B., and McBratney, A. (2018). Soil properties drive microbial community structure in a large scale transect in south eastern Australia. Sci. Rep. 8, 1–11. doi: 10.1038/s41598-018-30005-8
Yang, L., Huang, B., Mao, M., Yao, L., Niedermann, S., Hu, W., et al. (2016). Sustainability assessment of greenhouse vegetable farming practices from environmental, economic, and socio-institutional perspectives in China. Environ. Sci. Pollut. Res. 23, 17287–17297. doi: 10.1007/s11356-016-6937-1
Youssef, M., and Eissa, M. (2017). Comparison between organic and inorganic nutrition for tomato. J. Plant Nutr. 40, 1900–1907. doi: 10.1080/01904167.2016.1270309
Zhang, M., Li, B., and Xiong, Z. (2016). Effects of organic fertilizer on net global warming potential under an intensively managed vegetable field in southeastern China: a three-year field study. Atmos. Environ. 145, 92–103. doi: 10.1016/j.atmosenv.2016.09.024
Zhang, J., Zhuang, M., Shan, N., Zhao, Q., Li, H., and Wang, L. (2019). Substituting organic manure for compound fertilizer increases yield and decreases NH3 and N2O emissions in an intensive vegetable production systems. Sci. Total Environ. 670, 1184–1189. doi: 10.1016/j.scitotenv.2019.03.191
Keywords: Brassica rapa var. Chinensis, organic fertilizer, yield and quality, soil property, microbial community
Citation: Zhang X, Li J, Shao L, Qin F, Yang J, Gu H, Zhai P and Pan X (2023) Effects of organic fertilizers on yield, soil physico-chemical property, soil microbial community diversity and structure of Brassica rapa var. Chinensis. Front. Microbiol. 14:1132853. doi: 10.3389/fmicb.2023.1132853
Received: 10 January 2023; Accepted: 25 April 2023;
Published: 31 May 2023.
Edited by:
Claudia Goyer, Agriculture and Agri-Food Canada (AAFC), CanadaReviewed by:
Shuya Wang, Gansu Agricultural University, ChinaCopyright © 2023 Zhang, Li, Shao, Qin, Yang, Gu, Zhai and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pin Zhai, emhhaXBpbkBqYWFzLmFjLmNu;
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.