- 1Key Laboratory of Feed Biotechnology of Ministry of Agriculture and Rural Affairs, Laboratory of Quality and Safety Risk Assessment for Animal Products on Feed Hazards (Beijing) of Ministry of Agriculture and Rural Affairs, Institute of Feed Research, Chinese Academy of Agricultural Sciences, Beijing, China
- 2Wilmar (Shanghai) Biotechnology Research & Development Center Co., Ltd, Shanghai, China
The present study was conducted to evaluate the effects of Clostridium butyricum (CB) and fructooligosaccharide (FOS) singly or combined, on performance, egg quality, amino acid digestibility, jejunal morphology, immune function and antioxidant capacity in peak-phase laying hens. A total of 288 Hy-Line Brown laying hens (30 weeks of age) were randomly assigned to 4 dietary groups that included basal diet, basal diet +0.02% of CB (zlc-17: 1 × 109 CFU/g) (PRO), basal diet +0.6% FOS (PRE), and basal diet +0.02% CB + 0.6% FOS (SYN) for 12 weeks. Each treatment had 6 replicates with 12 birds each. The results demonstrated that probiotics (PRO), prebiotics (PRE) and synbiotics (SYN) (p ≤ 0.05), respectively, exerted a positive effect on the performance and physiological response of the birds. There were significant increases in egg production rate, egg weight, egg mass, daily feed intake and reduced number of damaged eggs. and zero mortality rate due to dietary PRO, PRE and SYN (p ≤ 0.05) respectively. Also, feed conversion was improved by PRO (p ≤ 0.05). In addition, egg quality assessment showed that; eggshell quality was increased by PRO (p ≤ 0.05) and albumen indices (Haugh unit, thick albumen content, and albumen height) were enhanced by PRO, PRE and SYN (p ≤ 0.05). Further analysis showed that PRO, PRE and SYN (p ≤ 0.05), reduced heterophil to lymphocyte ratio, increased antioxidant enzymes and immunoglobulin concentration. Although spleen index was higher for PRO (p ≤ 0.05) group. The significant increase in villi height, villi width, villi height to crypt depth ratio and reduced crypt depth were obvious for PRO, PRE, and SYN (p ≤ 0.05). Furthermore, improved nutrient absorption and retention evidenced by increased digestibility of crude protein and amino acids, were notable for PRO, PRE, and SYN (p ≤ 0.05) group. Collectively, our findings revealed that dietary CB and FOS alone, or combined, enhanced productive performance, egg quality, amino acid digestibility, jejunal morphology, and physiological response in peak-phase laying hens. Our results would provide direction on nutritional strategies for gut enhancers and better physiological response of peak laying hens.
Introduction
Poultry products (meat and eggs) constitutes one of the major sources of animal protein for humans. The demand for these products is on the rise due to ever increasing population and farmers must meet up with this demand, hence poultry production has increased significantly over the years. In a bid to meet up with this enormous task, feed additives such as synthetic antibiotics are commonly used as animal growth-promoters and an antimicrobial agent to enhance production and gut health. Nevertheless, synthetic antibiotics cannot be used in laying hen industry due to related safety risks with eggs (Xiang et al., 2019), and other negative effects such as drug-resistant bacteria, drug residue in animal tissue and alteration of microbiota balance, which poses a threat to both animal and consumers health. In this dimension, beneficial microorganisms such as prebiotics, probiotics and synbiotics may hold considerable promise as safe feed additives and could be harnessed by poultry industry (Adhikari et al., 2018; Salah et al., 2018; Ding et al., 2019; Macit et al., 2021).
Probiotics (PRO) are considered as live organisms or microbial feed supplements targeted at modulating the gastrointestinal tract by enhancing intestinal microbial balance (Xu et al., 2022). Probiotics have been shown to enhance laying performance (Xiang et al., 2019; Wang et al., 2021; Pan et al., 2022) and egg quality (Zhan et al., 2019; Wang et al., 2021). The additive influence of probiotics on poultry birds including the capacity of probiotics; to enhance gut development and integrity, improve and suppress immune and inflammatory response respectively, enhance feed intake and nutrient utilization by increasing activity of digestive enzymes and intestine health (Mikulski et al., 2020; Souza et al., 2021; Pan et al., 2022) have been documented. Probiotics commonly used as in-feed additives in poultry nutrition include; colonizing species of Streptococcus, Lactobacillus, Clostridium, Bacillus, and Enterococcus. Clostridium butyricum (CB) is a gram-positive anaerobe, mainly present in in the intestinal tract of humans and animals (Yang et al., 2010). The suitability of CB as feed additive in poultry diets are attributable to its spore-forming capacity and production of short chain fatty acids (SCFAs) such as butyric acid (Liu et al., 2018), survivability in low pH environment (Kong et al., 2011), stimulation of digestive enzymes (Duan et al., 2018) and enhancing activity of nutrient transporters (Wang et al., 2020). Myriad of beneficial effects of CB on chickens have been reported; favor growth of beneficial microbes and suppress pathogen colonization (Yang et al., 2012; Zhang et al., 2014), enhance activity of antioxidant enzymes (Liao et al., 2015; Zhan et al., 2019), immune function (Zhan et al., 2019), intestinal morphology, and oviduct health (Wang et al., 2021). These in turn promote nutrient absorption and physiological response for improved laying performance (Xiang et al., 2019; Wang et al., 2020, 2021) and albumen quality (Xiang et al., 2019; Obianwuna et al., 2022b). Nevertheless, colonization of probiotics in the gut is reliant on many factors, including nutritional status of the host, health, age, genetics, intestinal pH and availability of fermentation substrate (prebiotics). Hence, prebiotics are crucial to the survival potentials of probiotics in the gut.
Prebiotics (PRE) are referred to as indigestible foods or feed ingredients that beneficially affect the host via selective stimulation of growth of one or few numbers of bacteria in the colon (Mookiah et al., 2014). There are evidences in literatures that utilization of prebiotics as feed additives in poultry diets could enhance laying performance (Guo et al., 2020; Xu et al., 2020; Obianwuna et al., 2022a), egg quality (Li et al., 2017; Guo et al., 2020), and physiological response (Adhikari et al., 2018; Xu et al., 2020). Prebiotics are known to modulate the intestinal microflora via increase in probiotic bacteria such as Lactobacillus and Bifidobacterium (Alavi et al., 2012), inhibit colonization of gut by pathogenic bacteria via competitive adhesion to their binding sites on the intestinal mucosa (Adhikari et al., 2018). Thus, the prebiotic effect enhance proliferation of healthy microecological balance in the gut. Common prebiotics utilized in poultry diets include; lactulose, inulin, transgalactooligosaccharides, galactooligosaccharides, and fructooligosaccharide (FOS). FOS are non-digestible oligosaccharides due to presence of β-linkages: β-2,1 fructosyl-fructose and β-2,6 fructosyl-fructose (Zhao et al., 2013). This linkage accounts for its resistance to digestive enzymes of monogastric and selectively serves as substrate for proliferation of Lactobacillus spores in the intestinal tract (Ricke, 2015). Fermentation of FOS in the gut is associated with production of acetic acid and lactate acid (Rossi et al., 2005; Yang et al., 2008), which accounts for array of effects; reduction of pathogen colonization in the gut (Xu et al., 2003; Li et al., 2008) and ovary (Donalson et al., 2008; Adhikari et al., 2018), improved immune response in the host (Xu et al., 2003), enhanced villi morphometrics in the jejunum and increased enzyme activity for better nutrient absorption (Xu et al., 2003; Obianwuna et al., 2022a). This health promoting effects enhance performance and physiological status of the birds. In addition, the interaction between prebiotics and probiotics may enhance; the probiotics adaptation to the substrate (prebiotics), probiotics multiplication and invariably its synergetic effect (Hamasalim, 2016).
Combined form of probiotics and prebiotics as an in-feed additive is often considered as a synbiotics (SYN; Hassanpour et al., 2013). Synbiotic products have the capacity to enhance proliferation of existing beneficial strains in the gut and as well sustain the survival and growth of new probiotic strains (Likotrafiti et al., 2016). Previous reports showed that dietary synbiotics (FOS (prebiotics) + Lactobacillus.plantarum 15–1 (probiotic)) enhanced the intestinal health and immune response of broilers challenged with E. coli O78 (Ding et al., 2019). Several probiotic strains and FOS combined as a synbiotic product enhanced growth performance in broiler under E. coli challenge (Luoma et al., 2017). In another study, Zarei et al. (2018) reported that a synbiotic product (whey powder and probiotics) supplemented in broilers diets improved intestinal microflora and intestinal morphology. In addition, combination of bacillus and inulin (Abdelqader et al., 2013), probiotic and isomaltooligosaccharides (Tang et al., 2017) enhanced egg production and beneficial microbial composition in laying hens. In ovo feeding of synbiotics (whey powder and Pediococcus acidilactici) enhanced caecal microbial diversity and upregulated pathways crucial to increased egg production and egg quality (Pineda-Quiroga et al., 2019). However, Mohebbifar et al. (2013) reported no significant effect of probiotics or prebiotics on performance, egg quality and blood parameters of laying hens. In one study, Youssef et al. (2013) revealed that commercial synbiotics product improved egg production, egg mass but had no effect on albumen quality.
Evidently, in-feed application of feed additives such as probiotics, prebiotics alone or in combination (synbiotics) has the potential to modulate the gut environment and physiological response, which beneficially influence growth performance, health of the animals and product quality. Our preliminary research suggested that inclusion of FOSFOS at 0.6% and CB at 0.02% level had no adverse effect on the laying hens. Previous literatures have also shown that CB can be supplemented in the diet of laying hens at various levels; 0.5 g/kg (Xiang et al., 2019), 0.9 g/kg (Wang et al., 2020), without negative effects. Also, FOS have been included at 0.4% (Xu et al., 2003) levels in broilers diets, 0.5–1.0% (Adhikari et al., 2018), 200 mg/kg (Li et al., 2017), and 0.2–0.6% (Feng and Xia, 2019) in diet of breeder hens. To date, there are few studies which have examined effects of combined probiotic and prebiotics as a single product (Synbiotic) on laying hens and to the best of our knowledge our present study is the first to examine a combined effect of FOS and CB as a synbiotic. Therefore, the present study investigated the effects of probiotics (CB), or prebiotics (FOS) and a combination of the two (CB + FOS) on laying performance, egg quality, amino acid digestibility, intestinal morphology, antioxidant and immune function in peak-phase laying hens.
Materials and methods
Ethics statement
All the experimental procedures adopted in this study were in accordance to that approved by the Institutional Animal Care and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences, Beijing, China (CAAS.No20200507).
Experimental design
Hy-Line Brown laying hens at the peak-laying phase (30-week old, laying rate = 89.0 ± 1.5%, and n = 288) with laying rate of similar range were randomly allocated to one of four treatment groups with 6 replicates each (n-72). The experiment lasted for 13 weeks; a 12-week test period and a 1-week feed transition period. The controlled environment of the pen house was maintained daily on: Temperature (24°C), humidity (50–80%), and 16 h of light. The laying hens were offered fresh feed (mash form) and water ad lib on a daily basis while being managed based on Hy-line International Online Management Guide (Hy-Line W-98 Commercial Management Guide, 2004–2006). Throughout the feeding trial, the health of the birds was stable and no medications were administered except for routine vaccination.
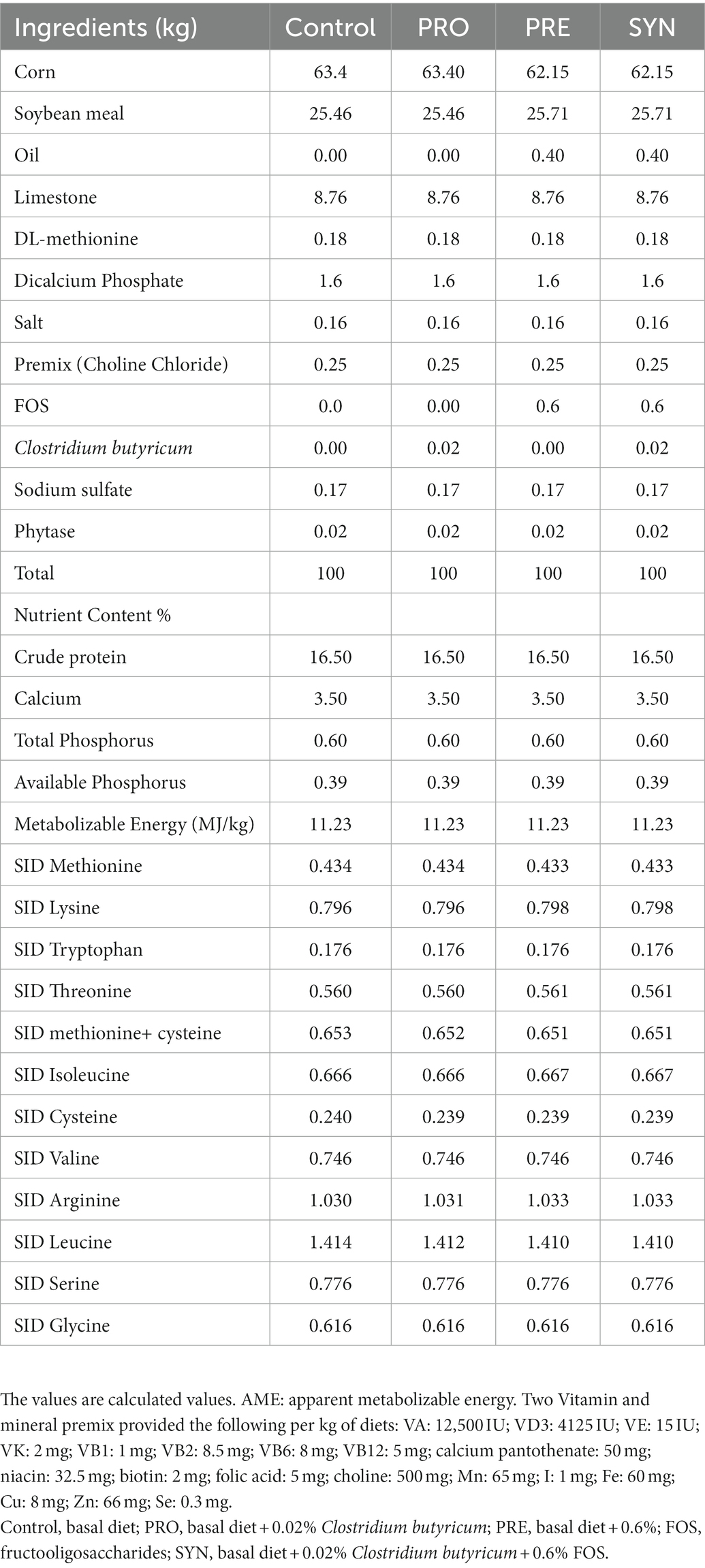
A 2 × 2 factorial arrangement with two levels of C. butyricum (0 and 0.02%) and two levels of FOS (0 and 0.6%) was designed in the present study. The dietary treatments consist of basal diet without the feed additives, basal diet+0.02% of C. butyricum (zlc-17: 1 × 109 CFU/g) (PRO), basal diet +0.6% FOS (FOS, 99% purity) (PRE), and basal diet +0.02% C. butyricum + 0.6% FOS (SYN). Before the commencement of the feeding experiment, the birds were offered laying hens’ diet which is corn-soybean meal-based mash feed. The nutrient composition and nutrient level of the diet are presented in Table 1. The basal diet nutrient composition and levels are consistent with the standard proposed by National Research and Council (1994). The feed additives (C. butyricum and FOS) were provided by COFCO Nutrition and Health Research Institute (Beijing, China).
Measurement of laying performance parameters
During the experiment, egg production rate, egg weight, damaged eggs (shells, misshapen and cracked) and mortality rate were recorded daily on replicate basis. Feed consumption was recorded every fortnight on replicate basis. The data collected was used to calculate the following variables; egg mass, average egg weight (AEW), hen-day production (HDP), average daily feed intake (ADFI), and feed conversion ratio (FCR) for the whole feeding period. The HDP and the ADFI were adjusted for mortality.
Egg quality assessment
On the last day of each of the 4-week period (week 4, 8, and 12), respectively, three eggs were collected from each replicate with the weight close to replicate range (18 eggs per treatment group). The collected eggs were placed under room temperature and assessment of egg quality was done within 24 h. Each egg was weighed, recorded, then broken and the albumen and yolk are separated with the egg separator. The individual components were then weighed, respectively. The eggshells were cleaned of albumen remains and air-dried for 48 h, then the eggshell weight was obtained. The relative proportion of albumen, yolk and shell was obtained as their weight/egg weight × 100% (Sarlak et al., 2021). Eggshell thickness (average of 3 points (air cell, equator, and sharp end) around the eggshell) (Mwaniki et al., 2018) and egg shell breaking strength were, respectively, determined with Eggshell Thickness Gauge and Egg Force Reader (ESTG-1, ORKA Technology Ltd., Ramat HaSharon, Israel). Albumen height, Haugh Unit and yolk color measurements were detected with an automatic Egg Analyzer (ORKA Food Technology Ltd., Ramat HaSharon, Israel). Further analysis of egg quality; the weighed albumen was separated further by moving it through using a 60-mesh sieve and allow it to stand for 30 s. the thick portion remained glued on the sieve and was weighed as thick albumen while the filtrate was weighed as thin albumen (Zhou et al., 2021).
Determination of serum and blood biochemical indices
At the end of the study period (12th week), 24 birds [six birds from each group (1 per replicate)] were selected for sample collection after 12 h fasting. About 5 ml of blood were collected from the jugular veins, then kept in a micro-anticoagulant tube in slanting position for 30 min, centrifuged at 1,500 x g for 15 min (Tang et al., 2017). The harvested plasma was transferred to Eppendorf tubes (1.5 ml) and stored at –20°C until analysis. The whole blood samples were put together in an ice pack and transported to the laboratory for hematology analysis within 1 h of collection. For hematological indices analysis, an automated hematology analyzer (Model: BC-2800 Vet, Mindray, Shenzhen, China) was used. Red blood cell indices, MCH, MCV and mean cell hemoglobin concentration (MCHC), were calculated (Jain, 1993).
Before analysis of biochemical indices, the serum was thawed at 4°C and kept at low temperature during the whole process in order to avoid activation of enzymes. Determination of glutathione peroxidase (GST), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), catalase (CAT), and malondialdehyde (MDA) were achieved using an assay kits from ML Bio and Jiancheng Bioengineering Institute (Nanjing, China), and measured spectrophotometrically (Shimadzu, model UV-1800, Tokyo, Japan). Serum concentrations of immunoglobulins A (IgA), immunoglobulins G (IgG), immunoglobulins M (IgM) and complement proteins; C3 and C4 were determined with the corresponding chicken ELISA kits. All standards were tested in duplicate and concentrations of IgA, IgG, IgM, C3, and C4 were determined using standard curves constructed from the standards run on the plate. All the ELISA kits adopted in the study are of high specificity and sensitivity for chickens, and all procedures were done according to the manufacturer’s instructions.
Intestine sample collection and jejunal morphology analysis
At the end of the study (12th week), 24 birds [six birds from each group (1 per replicate)] were selected for sample collection after 12 h fasting. The selected birds were euthanized with pentobarbital sodium (100 mg/kg BW) intravenously and cut open under aseptic conditions. The heart, liver, magnum and spleen of each bird were weighed and the organ index was calculated weight of organ (g)/body weight (g) × 100%. The jejunal samples of each bird were processed following that established in Gungor and Erener (2020). About three-centimeter length of tissue from jejunum were flushed in physiological saline solution and placed in 10% buffered formalin, kept at 4°C for analysis.
The fixed jejunal samples were processed and stained with hematoxylin–eosin, while adopting the standard techniques for histology examination (Yamauchi et al., 2010). Images were observed with the aid of a microscope (An Olympus BX43 microscope; Olympus Corp., Tokyo, Japan). The villi morphometrics were obtained; ten intact villi of each sample and corresponding crypts were selected, measured, and the average value was obtained. The measurements were made with a software (Caseviewer Image). The measurements include; villi height (VH: measured from the top of the villus to the villus-crypt junction), the villus width (VW: was measured at the middle point of the villus), crypt depth (CD: from the base up to the crypt–villus transition region), the VH-CD ratio (measured as VH/CD), villi surface area (VSA: π × VW × VH) (Wang et al., 2016; Thiam et al., 2021).
Apparent fecal amino acid digestibility
At the end of 12 weeks, 3 birds were chosen from each replicate and put in a metabolic cage with collection tray for fecal samples. The fecal samples were collected on replicate basis at 12 h intervals daily for 3 days. Contamination of fecal samples were avoided by ensuring that feed, feathers and other foreign materials were completely excluded from fecal samples during collection. The fecal samples were then weighed, pooled together for each treatment group, and kept in sealed bags at –20°C (Zhou et al., 2021). Prior to amino acid analysis, the fecal samples were thawed and oven-dried at 65°C for 72 h, then weighed again and pulverized into finely ground powder that can pass through a 0.05 mm mesh. Amino acids determinations from samples of feed and feces was performed by HPLC, following an established method by Varzaru et al. (2013). The HPLC system was Finnigan Surveyor Plus and HyperSil BDS C18 column, size 250 × 4.6 mm, 5 μm (Thermo-Electron Corporation, Waltham, MA). For apparent fecal amino acid digestibility analysis, the feed intake and the fecal weight (dry matter basis) from each metabolic cage were calculated. The apparent fecal amino acid digestibility was calculated as: Apparent Amino acid Digestibility = 1 ˗ (amino acid concentration in the feces ×feces weight)/(amino acid concentration in the feed × feed intake) × 100%.
Statistical analysis
The experiment consists of four groups with 6 replications each, in a completely randomized design to ensure random allocation of birds to treatments. All the data generated in this study were subjected to using one-way analysis of variance (ANOVA), this ensures that there is no biasness in respect to data normality and equality of variance assumptions (Nwachukwu et al., 2021). Replicates were used as experimental units, the data were presented as mean and pooled standard error of mean (SEM), while the level of significance was considered at p ≤ 0.05. Overall differences between the dietary treatments were analyzed using one-way analysis of variance (ANOVA), and the general linear model procedure. The statistical package used was SPSS software, version 17.0 (SPSS Inc., Chicago, II, United States).
Results
We conducted two trials at the same time. This design can save trial resources and did not affect the research objectives of the two trials. Although the data from two groups are shared, the data analysis is completely different, and one part of the trial have been published in Obianwuna et al. (2022b).
Laying performance
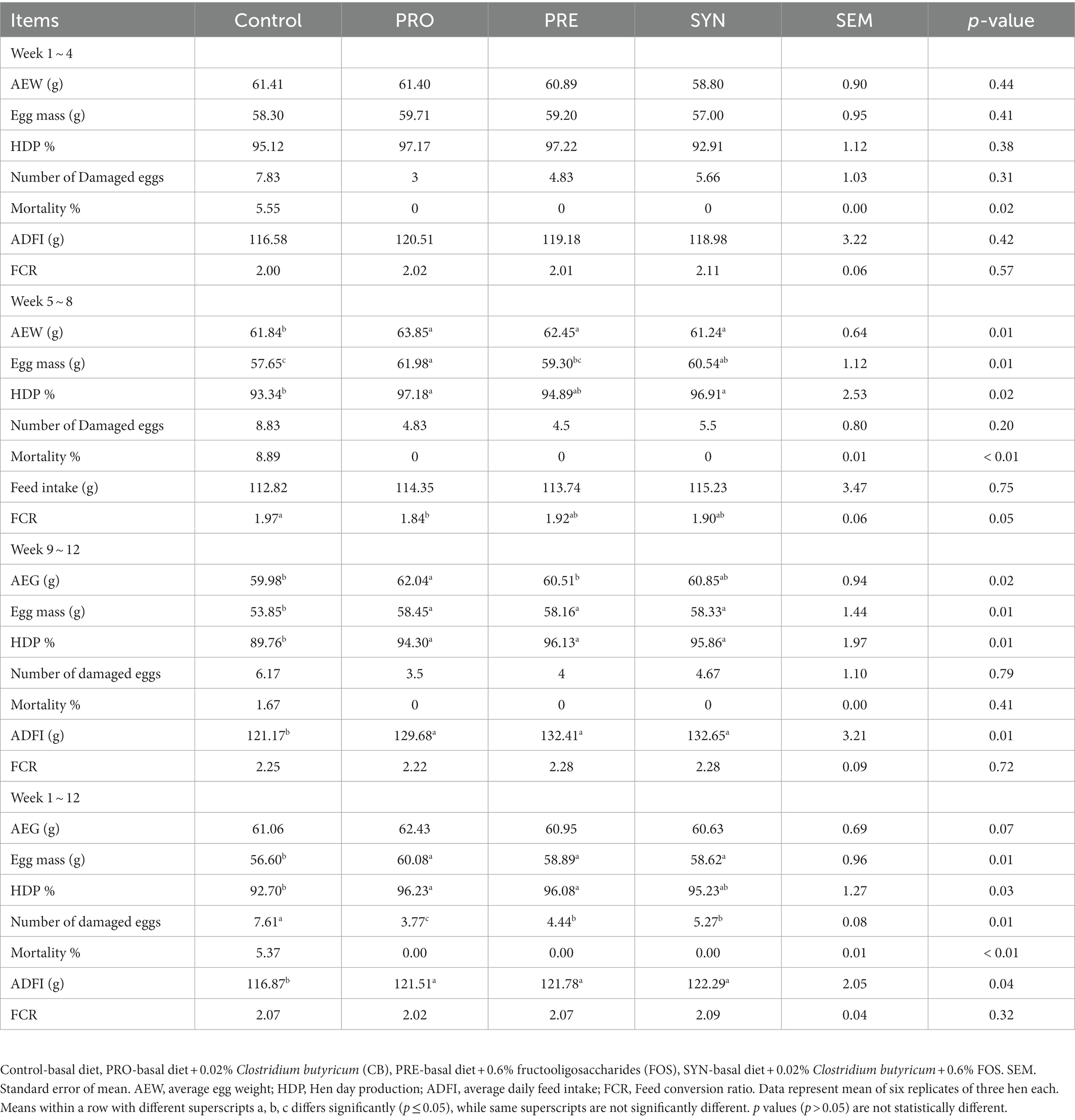
The effects of PRO, PRE and SYN on egg production and performance of laying hens are presented in Table 2. The results of this study demonstrated that at the end of week 4, average egg weight, egg mass, hen-day production, feed intake, feed conversion ratio and number of damaged eggs were not influenced by each of the PRO, PRE, and SYN (p > 0.05) group, while mortality rate was influenced by PRO, PRE and SYN (p ≤ 0.05) respectively. At the end of week 8; average egg weight and mortality rate were influenced by the PRO, PRE and SYN (p ≤ 0.05), egg mass and hen-day production were increased by PRO and SYN (p ≤ 0.05) while the effect of PRE (p > 0.05) was comparable to control, feed conversion ratio was enhanced by the PRO (p ≤ 0.05) but no diet effect was observed for PRE and SYN (p > 0.05) group, and no treatment effect (p > 0.05) was observed for feed intake and number of damaged eggs. At the end of week 12; average egg weight was higher for PRO (p ≤ 0.05) group but the effects of PRE and SYN (p > 0.05) were not significant, egg mass, hen-day production, mortality rate and feed intake were improved by PRO, PRE and SYN (p ≤ 0.05), number of damaged eggs and feed intake were not influenced by the dietary treatments (p > 0.05). The results for the overall period (weeks 1–12) showed that; average egg weight, and feed conversion ratio were not influenced by neither PRO, PRE nor SYN (p > 0.05), egg mass, feed intake, mortality rate and number of damaged eggs were influenced by PRO, PRE and SYN (p ≤ 0.05), and hen-day production was increased by PRO and PRE (p ≤ 0.05) but the effect of SYN (p > 0.05) was comparable to the control.
Egg quality assessment
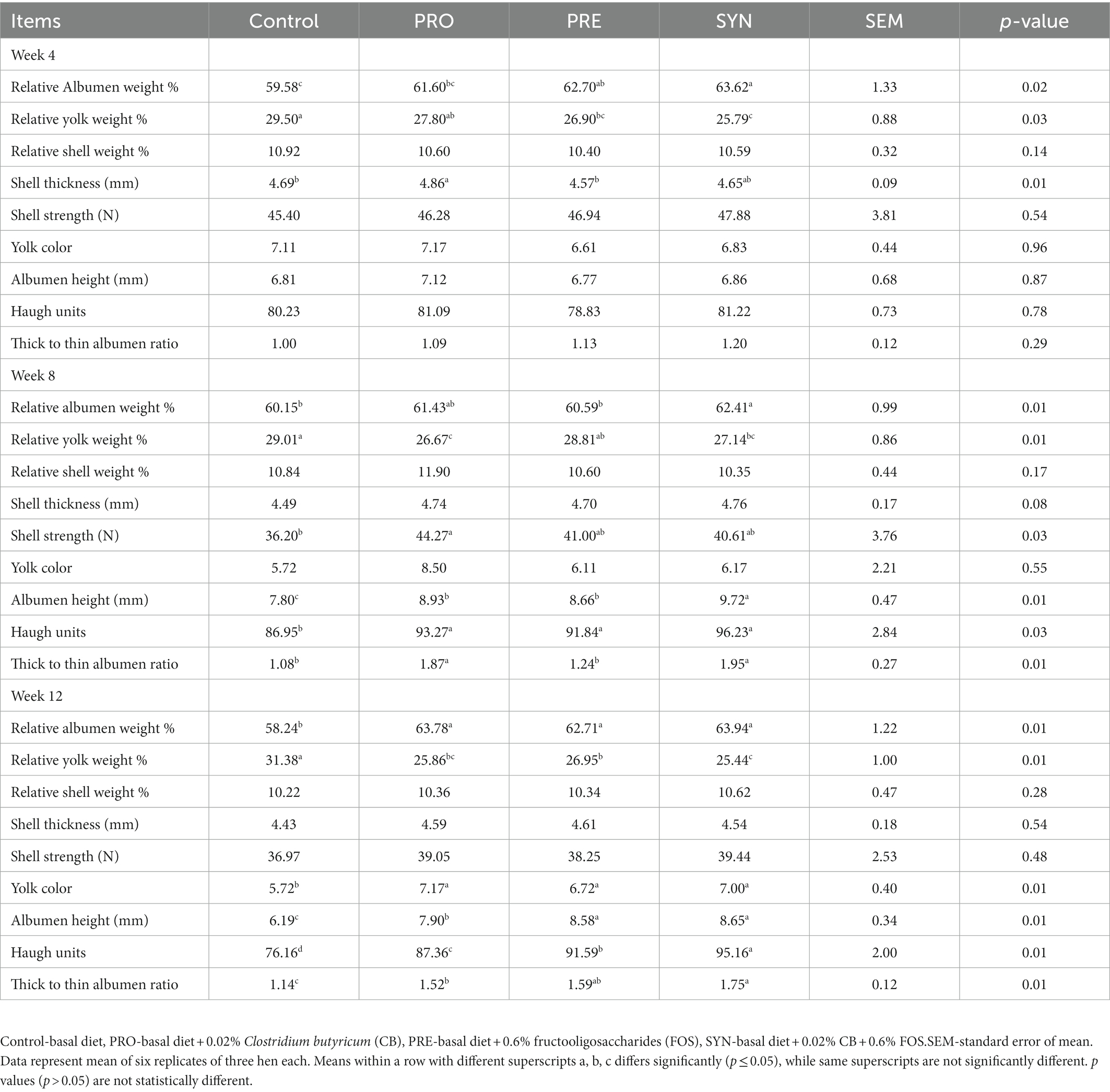
The effects of PRO, PRE and SYN on egg quality of laying hens are presented in Table 3. At the end of week 4; the relative weight of albumen and yolk were influenced by PRE and SYN (p ≤ 0.05) while no significant effect was observed for PRO (p > 0.05), eggshell thickness was enhanced by PRO (p ≤ 0.05) but the effects of PRE and SYN (p > 0.05) were not significant. The eggshell strength, yolk color and albumen indices (albumen height, Haugh unit, and thick to thin albumen ratio) were not influenced by PRE, PRO nor SYN (p > 0.05). At the end of week 8; eggshell thickness, yolk color and eggshell weight were not influenced by any of PRO, PRE, and SYN (p > 0.05), relative weight of albumen was enhanced by SYN (p ≤ 0.05) while the effects of PRO and PRE (p > 0.05) were not significant, relative yolk weight was influenced by PRO and SYN (p ≤ 0.05) but not PRE (p > 0.05), eggshell strength was enhanced by PRO (p ≤ 0.05) but the effects of PRE and SYN (p > 0.05) were comparable to control, albumen height and Haugh unit were significantly increased by PRO, PRE and SYN (p ≤ 0.05) respectively, and thick to thin albumen ratio was enhanced by PRO and SYN (p ≤ 0.05) but not PRE (p > 0.05). At the end of week 12; the relative weight of albumen and yolk, yolk color, and albumen indices (albumen height, Haugh unit and thick to thin albumen ratio), were significantly improved by PRO, PRE and SYN, respectively. Also, eggshell thickness, eggshell strength, and eggshell weight were not influenced by any of PRO, PRE, and SYN (p > 0.05). The effect of SYN on albumen indices were numerically higher compared to PRO and PRE, at the end of week 8 and 12.
Hematological and serum biochemical indices
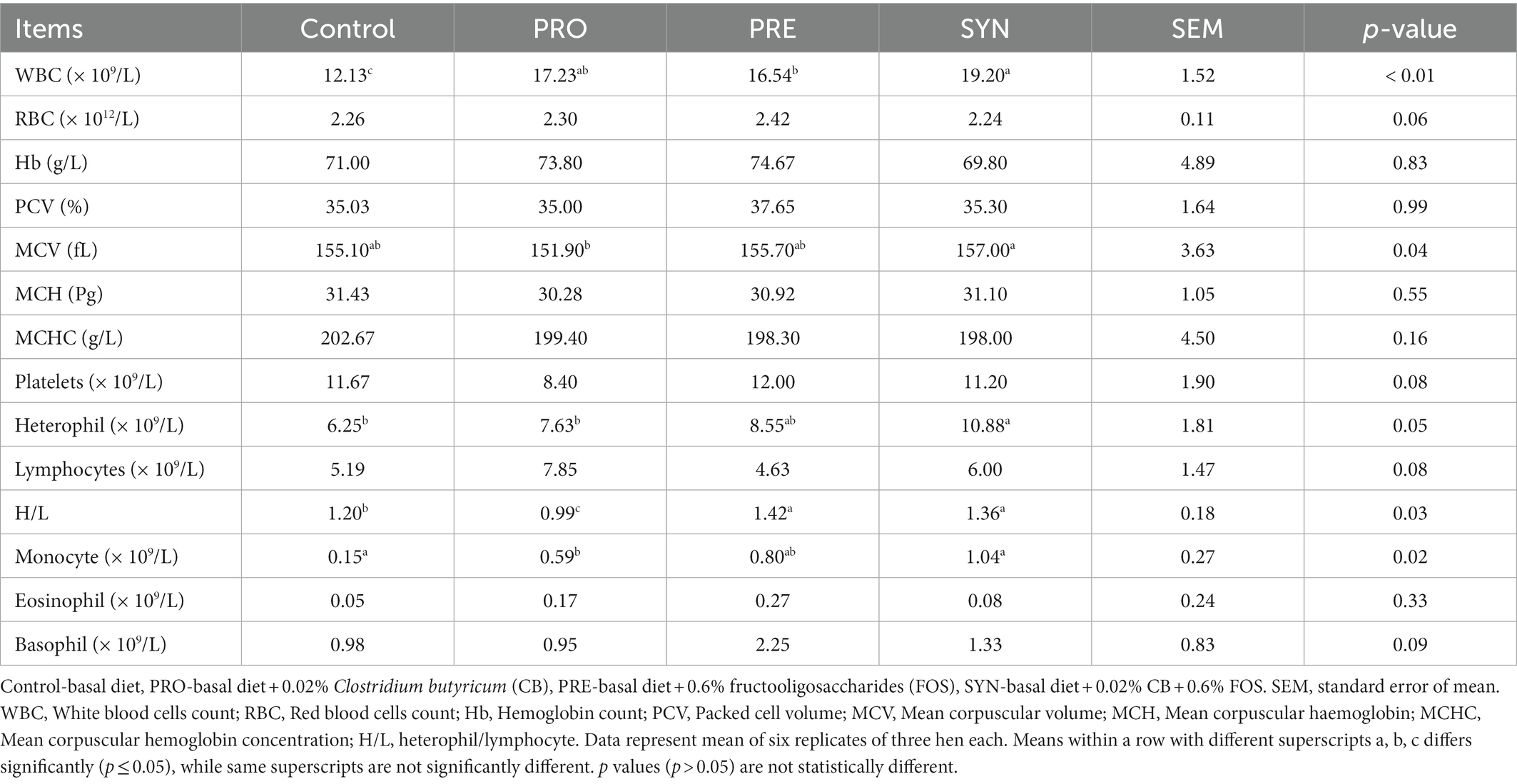
The effects of PRO, PRE and SYN on the hematological indices of laying hens are presented in Table 4. Hematological indices including WBC and H/L ratio were significantly influenced by PRO, PRE and SYN (p ≤ 0.05) respectively. The H/L values for PRO was significantly lower (p ≤ 0.05), compared to that of PRE and SYN. The effect of PRO (p ≤ 0.05) on MCV and monocytes, and SYN (p ≤ 0.05) on heterophil were significant but not PRE (p > 0.05) effect. Other indices including; RBC, Hb, PCV, MCH, MCHC, platelets, lymphocytes, basophil and eosinophil were not influenced by any of the PRO, PRE and SYN (p > 0.05).
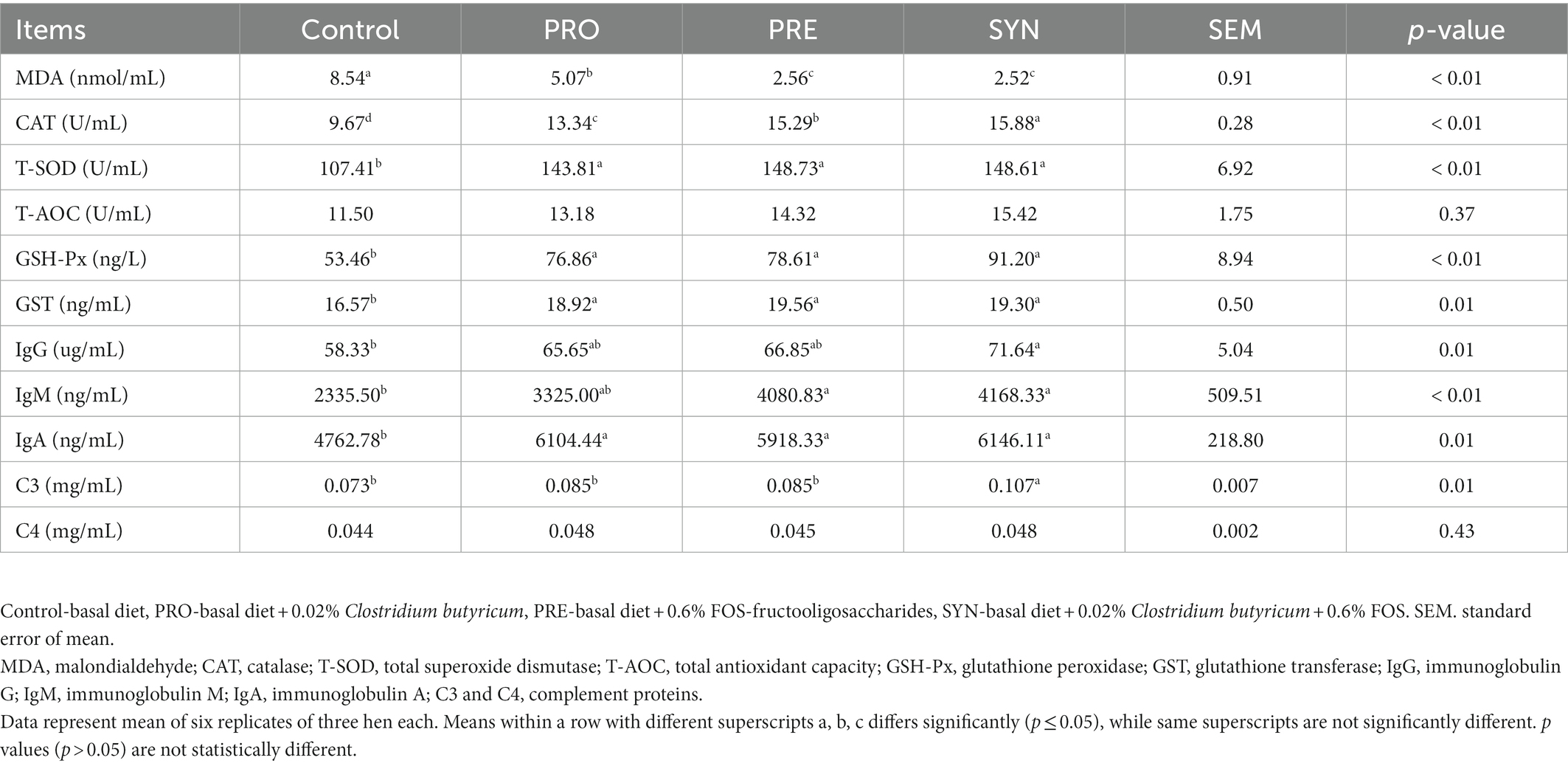
The effects of PRO, PRE and SYN on serum biochemical indices of laying hens are shown in Table 5. The serum biochemical indices were significantly influenced by dietary PRO, PRE and SYN (p ≤ 0.05) respectively, as evidenced by increased antioxidant enzymes (T-SOD, CAT, GSH-Px and GST) and reduced level of MDA, compared to the control group. Antioxidant enzymes; T-SOD, CAT, GSH-Px and GST, were numerically higher in the SYN group compared to other dietary treatments. Among dietary treatments, there were no significant differences in T-SOD, GSH-Px and GST (p > 0.05), while no influence of neither PRE, PRO nor SYN was notable for T-AOC (p > 0.05). The concentration of IgA was enhanced by each of the PRO, PRE and SYN (p ≤ 0.05) treatments, IgM was increased by PRE and SYN (p ≤ 0.05) but not by PRO (p > 0.05), IgG and complement protein C3 was increased by SYN (p ≤ 0.05) but no treatment effect was notable for PRE and PRO (p > 0.05). The complement protein C4 was not influenced by any of the PRO, PRE and SYN (p > 0.05).
Apparent fecal amino acid digestibility
The effects of PRO, PRE and SYN on the apparent fecal amino acid digestibility of laying hens are listed in Table 6. The digestibility of crude protein was enhanced by the PRO, PRE and SYN (p ≤ 0.05), respectively. Significant differences due to dietary treatments (p ≤ 0.05), were notable for digestibility of essential amino acids (threonine, isoleucine, leucine, phenylalanine, lysine, arginine and tryptophan) and non-essential amino acids (serine, glycine, cysteine, and tyrosine). Threonine, serine, phenylalanine, and lysine were influenced by PRO and PRE (p ≤ 0.05) while the effect of SYN (p > 0.05) was comparable to control, cysteine, isoleucine, leucine, arginine, and tyrosine were improved by PRO (p ≤ 0.05) but the effects of PRE and SYN (p > 0.05) was not notable, glycine was enhanced by PRO, PRE and SYN (p ≤ 0.05), respectively and tryptophan was increased by PRE and SYN (p ≤ 0.05) but not PRE (p > 0.05). There was no significant effect of PRO, PRE and SYN (p > 0.05) on digestibility of other amino acids.
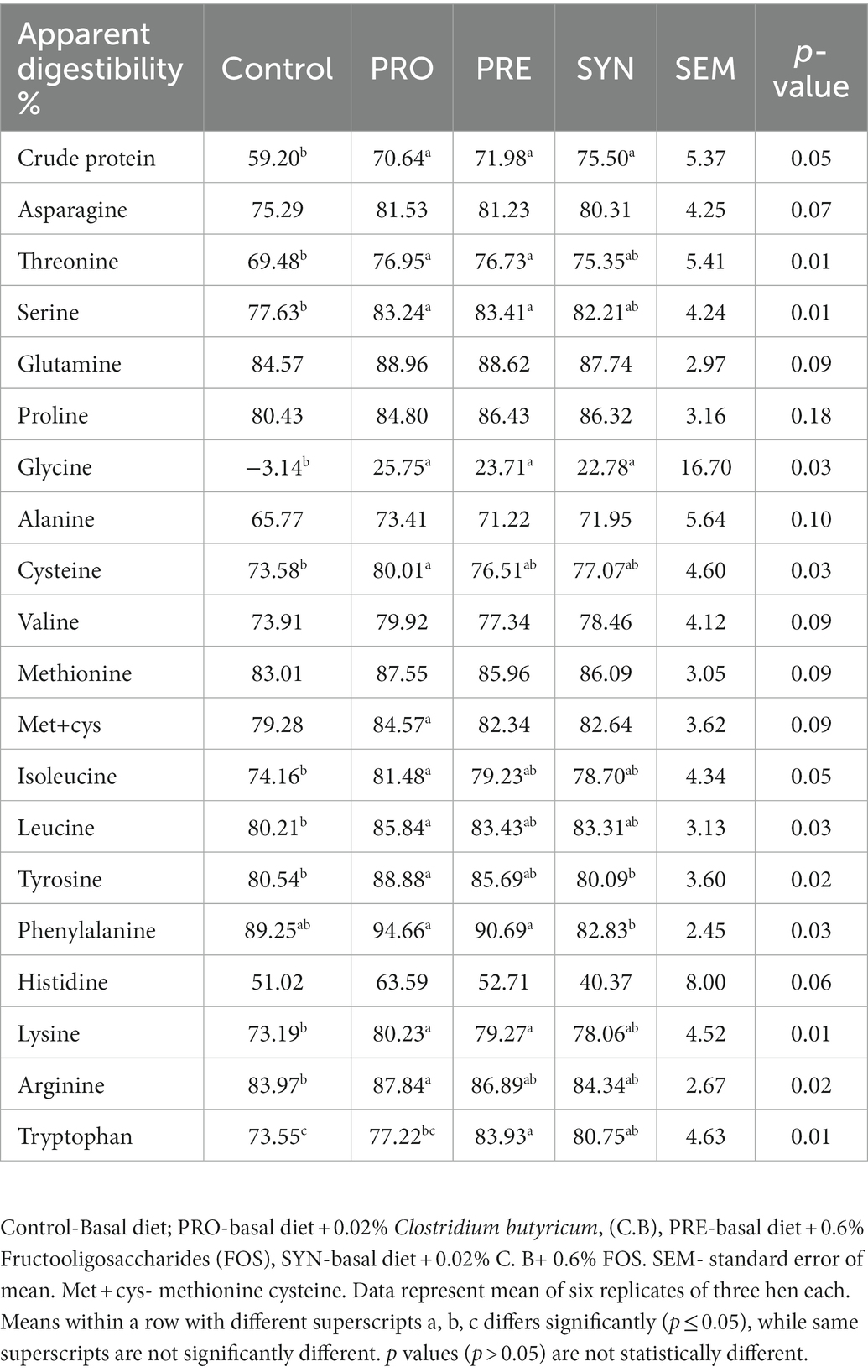
Table 6. Effects of dietary treatments on apparent fecal amino acid digestibility coefficient of laying hens.
Organ index and jejunal villi morphological structure
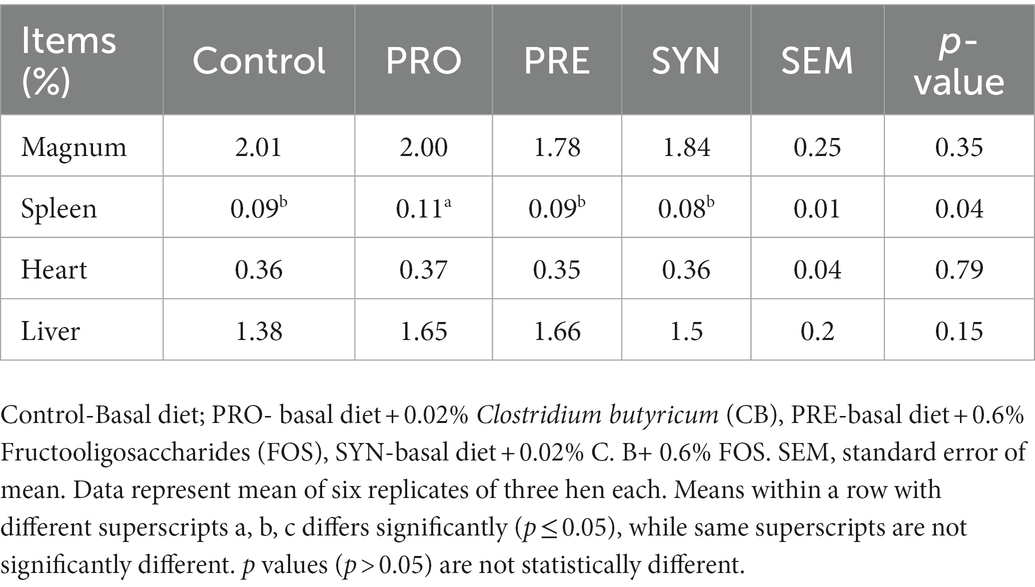
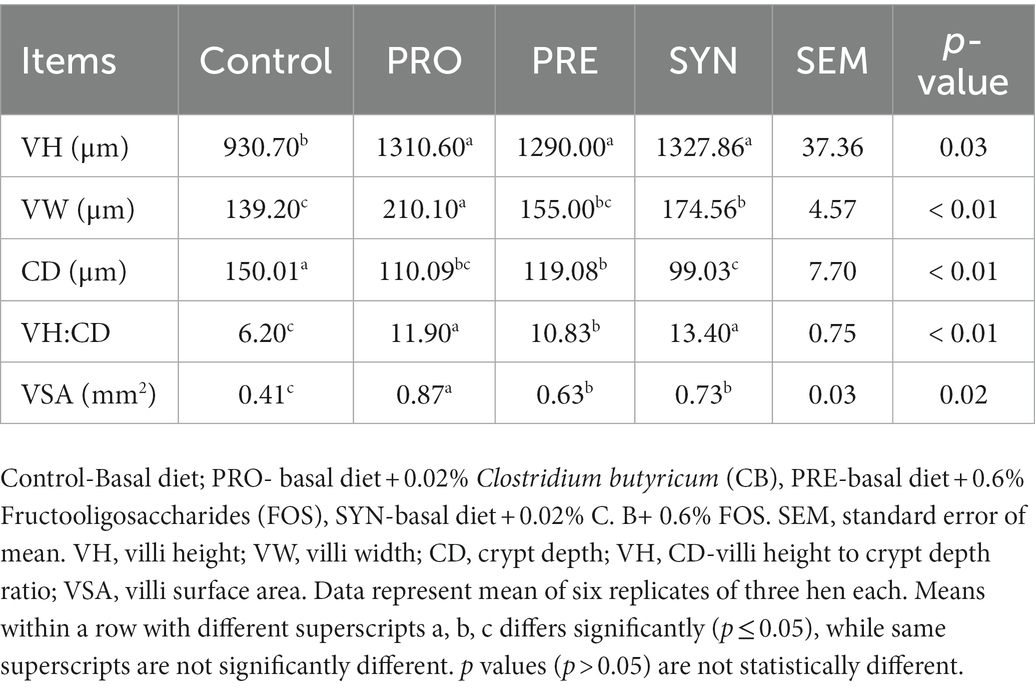
The organ weights of the magnum, spleen, heart, and liver (expressed as organ index) of laying hens offered dietary PRO, PRE and SYN are listed in Table 7. The organ index of magnum, heart, and liver were not influenced by any of the PRO, PRE and SYN (P 0.05) group. The organ index of the spleen was increased by PRO (p ≤ 0.05) while no significant effect was reported for PRE and SYN (P 0.05). The effects of PRO, PRE and SYN on the jejunal villi morphometry of laying hens are presented in Table 8. There were significant increases in VH, VSA, VH:CD and decrease in CD due to dietary effect of PRO, PRE and SYN (p ≤ 0.05) respectively. The villi width was increased by PRO and SYN (p ≤ 0.05) but not PRE (P 0.05). All villi morphometrics were numerically higher in the PRO and SYN group compared to that of PRE.
Discussion
To promote intestinal gut health and animal product quality in poultry industry, attempts have been made to enhance utilization of natural feed additives which are safe. Earlier reports have shown that novel feed additives such as prebiotics, probiotics or synbiotics could be used in poultry production as growth and gut enhancers (Yang et al., 2020; Song et al., 2022). Thus, the present research lends supporting evidence that supplementation of CB, FOS singly or their combination in diet of laying hens improved laying performance, egg quality, apparent fecal amino acid digestibility, blood indices and serum biochemical parameters.
Laying performance and egg quality
In the present study, our findings revealed that probiotics, prebiotics and synbiotics enhanced laying performance, egg mass, feed intake and feed efficiency. Our findings are in line with previous reports on the beneficial effects of dietary probiotics (Darsi and Zhaghari, 2021; Souza et al., 2021; Pan et al., 2022), prebiotics (Xu et al., 2020; Zhou et al., 2021) and synbiotics (Luoma et al., 2017; Song et al., 2022) on egg production rate. There are evidences of improved laying performance due to supplementation of CB (Xiang et al., 2019;Zhan et al., 2019; Wang et al., 2021) and FOS (Li et al., 2017; Obianwuna et al., 2022a) in diets of laying hens. Further, CB (Zhan et al., 2019; Wang et al., 2021), FOS (Li et al., 2017), and synbiotics (Tang et al., 2017), have been reported to enhance egg mass. In addition, we observed an increased feed intake in the probiotics and synbiotics group while feed efficiency was significantly improved with probiotics. This is in line with other reports in laying hens, that CB enhanced feed efficiency (Xiang et al., 2019; Wang et al., 2020), while synbiotics enhanced feed intake (Tang et al., 2017). The positive effects of diets on laying performance, egg mass and feed efficiency could be attributable to availability of nutrients necessary for metabolism, maintenance and egg production (Markowiak and Śliżewska, 2018), probably because probiotics and prebiotics modulate gut structure for efficient nutrient absorption (Swiatkiewicz et al., 2015; Tang et al., 2017; Xiang et al., 2019). Also, gut fermentation effects are linked with production of short-chain fatty acids which nourish the enterocytes, protect the villi from pathogen invasion and invariably increase microvilli structures for nutrient absorption (Hajiaghapour and Rezaeipour, 2018; Liu et al., 2018). Thus, the probiotic-prebiotic fermentation effect on the gut (Yousaf et al., 2016; Xiang et al., 2019) explains the synergistic effect on laying performance. In addition, the improved crude protein digestibility of key amino acids essential for egg production may be a key factor to improved performance (Phuoc et al., 2019). Conversely, there are also reports that probiotics (Shi et al., 2020; Ray et al., 2022), CB (Wang et al., 2020), prebiotics (Li et al., 2017), FOS (Li et al., 2017), and synbiotics (Tang et al., 2017), had no influence on laying performance. Similarly, no effect on feed efficiency (Upadhaya et al., 2019) and feed intake (Abdelqader et al., 2013) due to dietary probiotics and synbiotics, respectively. The variations may be explained by the differences in viable counts of microorganisms, strains, and supplementation dosage. Further analysis showed that, zero mortality was recorded in feed additive groups, which agrees with previous reports on zero mortality due to dietary CB (Xiang et al., 2019) and prebiotics (Li et al., 2017). The enhancement effect of the feed additives on the animal health may explain the zero-mortality status. These aforementioned positive findings on laying performance and feed efficiency can be accrued to better utilization of dietary feed additives by the birds.
Egg quality is critical to consumer acceptance of table eggs and utilization by the food industry. Our findings revealed that egg shell strength was enhanced with feed additives compared to the control. This is line with previous reports that dietary CB (Xiang et al., 2019; Zhan et al., 2019) and prebiotics (Li et al., 2017; Xu et al., 2020) improved egg shell strength in laying hens. The increased eggshell strength and eggshell thickness may be due to fermentation effect in the gut which favors mineral absorption and retention with resultant effect on shell gland (Świątkiewicz et al., 2010). Contrarily, there are evidences that CB (Wang et al., 2020), FOS (Li et al., 2017), and synbiotics (Song et al., 2022) had no effect on egg shell strength. The non-significant influence on egg shell strength may be due to age of the hens and laying phase (Guo et al., 2020). Yolk color is an intrinsic trait that is of priority to consumers. In this study, we observed a significant increase in yolk color at the end of the feeding trial. In line with our findings, CB (Wang et al., 2020, 2021) and prebiotics (Guo et al., 2020) enhanced yolk color in laying hens. The enhanced yolk color may be due to potentials of the feed additives to degrade nutrients in the feed and long-term absorption may influence yolk color though the underlying principle may not be clear. Although some studies found no significant improvement in yolk color with probiotics (Lei et al., 2013) and prebiotics (Li et al., 2017). The variations in studies may be due to the composition of the feed supplement used. In addition, improved albumen quality would mean extended egg shelf life during storage and more value for its utilization by the health and food industry (Sun et al., 2016; Zhang et al., 2020). Our findings demonstrated a significant improvement in albumen quality (albumen height, Haugh unit and thick to thin albumen ratio) due to probiotics, prebiotics and the two-fold synergistic effect of synbiotics. Dietary CB have been reported to enhance albumen height (Zhan et al., 2019; Wang et al., 2020, 2021; Obianwuna et al., 2022b) and albumen crude protein (Xiang et al., 2019). Prebiotics including marine derived polysaccharides and FOS enhance albumen height and Haugh unit (Guo et al., 2020; Obianwuna et al., 2022a). Increased albumen height and Haugh unit reflects abundance of ovomucin in thick albumen fraction (Aobai et al., 2020). In the study of He et al. (2017), low protein diets could alter albumen secretion in the magnum due to reduction in albumen proteins, ovomucin inclusive. The study of Zhou et al. (2021) highlighted the critical role of high dietary crude protein and amino acid in albumen synthesis. The improvement in albumen quality may be explained by enhanced crude protein and amino acid digestibility that is crucial to albumen synthesis. This claim is supported in the previous reports that prebiotics and probiotics enhance protein digestibility and utilization (Zhang et al., 2016; Ahiwe et al., 2020). Further, the previous studies of Adhikari et al. (2018) and Wang et al. (2021) have demonstrated that FOS and CB respectively, can enhance oviduct health in laying hens. Thus, stable oviduct health may be a contributory factor to improved albumen synthesis. Conversely, CB (Xiang et al., 2019), prebiotics (Xu et al., 2020), and synbiotics (Song et al., 2022), were reported to have no effect on albumen height and Haugh unit. Variations may be due to different compounds that make up the feed additives and the supplementation dosage. Taken together, the improved egg quality may be adducible to nutrients bioavailability and efficient utilization.
Hematological indices, antioxidant capacity, and immune function.
Hematological indices are used for health status indicators in organisms (Etim et al., 2014). Our results showed that feed additives increased RBC, WBC, Platelet and decreased H/L ratio compared to the control. The increased RBC implies that the animal is not prone to anemia and an indication of efficient nutrient utilization which in turn promotes erythropoiesis. The potentials of probiotics to cause a significant reduction in H/L was notable in the study. In a likely manner, probiotics, prebiotics or synbiotics decreased H/L ratio in laying hens (Tang et al., 2017) and broiler chickens (Salah et al., 2018). Thus, the lower H/L ratio suggests immunostimulatory nature of probiotics and the stability of animal health. On the other hand, Akinleye et al. (2008), found no effect of dietary prebiotics on hematological indices of broilers while Ghasemi et al. (2014) reported that synbiotics did not influence H/L ratio. The divergent views may be related to the hygiene condition of the farm where birds are kept. Our findings suggest that these feed additives could improve the health and welfare of the animals under farm conditions.
Serum biochemical indices are often used to measure the physiological response of the organisms to the supplemented diets. Serum immunoglobulins are used as critical indicators to assess the non-specific immunity status of the organism (Wu et al., 2019), and serum concentrations of IgA, IgM, and IgG can be modulated by dietary supplementations (Sun et al., 2020). In the current study, enhanced immunoglobulin (IgM, IgA, and IgG) concentrations due to dietary feed additives were notable. This is in line with previous evidences that dietary CB increased concentrations of IgA, IgG, and IgM (Zhan et al., 2019), IgA and IgM (Obianwuna et al., 2022b) in laying hens, and IgA, IgG, and IgM in broilers (Zhang et al., 2014). Also, FOS have been found to enhance concentrations of IgA and IgM (Ding et al., 2019), IgA (Al-Khalaifa et al., 2019) in broilers, and IgA, IgM, and IgG (Obianwuna et al., 2022a) in laying hens. In same lieu, synbiotics enhanced the concentrations of the immunoglobulins IgG in laying hens (Song et al., 2022) and IgA concentrations in laying hens challenged with salmonella (Luoma et al., 2017). The enhanced immunity status depicts the capacity of prebiotics to act as growth enhancers for probiotic gut microbes, stimulating an indirect effect on the host immune system (Xu et al., 2003). Therefore, the two-fold synergistic effect could be the immunomodulatory effect of probiotics and prebiotics. Also, we observed that the organ index of the spleen significantly improved in the probiotics group compared to the control. The result was in line with previous reports that dietary probiotics increased relative weight of the spleen in broilers (Chen et al., 2013) and spleen index in laying hens (Zhan et al., 2019). Further analysis, showed that complement proteins C3 was enhanced in the synbiotics group but no effect of dietary treatments was observable for C4. Although previous reports demonstrated that C3 and C4 concentrations were increased due to CB supplementation in laying hens (Zhan et al., 2019) and broilers (Zhang et al., 2014). The improved immune status is an indication on the immunomodulatory effects of probiotics, prebiotics and synbiotics and the potentials to support the immune system component of chickens.
Normal cellular metabolisms often lead to generation of free oxygen radicals and lipid peroxidation (Shi et al., 2020), often indicated by MDA which is an oxidative biomarker (Puvača et al., 2015). The protective effect of antioxidant enzymes (CAT, GSH-Px, GST, and T-SOD) on biological membranes from oxidative damage have been reported (Zhang et al., 2014). In the present study, supplementation of dietary probiotics, prebiotics and synbiotics significantly increased CAT, T-SOD, GSH-Px and GST, decreased MDA but no dietary treatment effect was found for T-AOC. In line with our results, literature have shown that dietary CB enhanced activities of CAT, T-SOD, GSH-Px but had no effect on T-AOC and MDA in laying hens (Zhan et al., 2019). In one study, CB reduced T-AOC but no adverse effect on the antioxidant defense system was found (Wang et al., 2021). Also, prebiotics enhanced activities of T-SOD, CAT, and GSH-Px, while decreasing MDA content (Li et al., 2017; Guo et al., 2020; Obianwuna et al., 2022a). In similar lieu, synbiotics enhanced GSH-Px, TSOD, T-AOC, reduce MDA in the serum (Song et al., 2022), decreased MDA and increased GSH but had no effect on CAT (Salah et al., 2018). The enhanced antioxidant function could probably be due to capacity of the feed additives to activate the upregulation of the enzymes in the antioxidant defense system that can scavenge ROS, and reduce lipid peroxidation (Mohammed et al., 2019). The non-significant effect of the treatments on T-AOC suggests that the treatments enhanced the antioxidant system of the host, thus lowering the activity of T-AOC. The reduced lipid peroxidation and increased enzyme activity may have reduced the susceptibility of the biological membranes to oxidative damage (Yuan et al., 2014). Therefore, this could promote the physiological response and systems crucial to egg production, egg quality and animal health. The beneficial effect of the feed additives on health of the laying hens reflects in enhanced hematological indices, immunoglobulin synthesis and antioxidant capacity, which could explain the improved laying performance and egg quality.
Apparent fecal amino acid digestibility
There is limited information on the influence of feed additives; CB, FOS alone or in combination, on apparent fecal digestibility of crude protein and amino acids in laying hens. This study to the best of our knowledge would be the first to report the effect of dietary CB and FOS in a combined form on fecal digestibility of crude protein and amino acids in laying hens. In the present study, the fecal digestibility of crude protein and all key amino acids except proline and alanine were significantly improved in response to dietary feed additives. In the study of Emami et al. (2012), supplementation of FOS in broiler diets had no influence on crude protein digestibility while FOS enhanced methionine digestibility in cecectomized roosters (Biggs and Parsons, 2007). The variations may be due to inclusion level. Improved digestibility of crude protein and amino acids and consequent reduced loss in fecal samples, suggest that gut integrity enhanced amino acid bioavailability and absorption, rather than degradation in the gut. Prebiotics and probiotics have been found to regulate growth of beneficial microbes and suppress pathogens invasion in the gut (Kumar et al., 2019; Wang et al., 2021). It could then be that the improved microecological environment of the gut, provided support for gut integrity and nutrient absorption capacity. Amino acids including isoleucine and total sulfur amino acids (TSAA) are crucial to albumen synthesis (Phuoc et al., 2019; Parenteau et al., 2020), thus, enhanced digestibility of these amino acids may have contributed to the better albumen quality. It could be deduced that the significant improvement in crude protein digestibility, amino acid absorption and utilization, culminated in availability of circulating amino acids which the body utilized for metabolism and other physiological processes including egg production, immunoglobulins and albumen synthesis.
Jejunal villi morphology
The small intestine is the main site for digestion and absorption of nutrients. Enhanced nutrient absorption capacity of the gut is a function of microvilli structures; shallow CD, and increased VH, VH/CD, which allows for maturity of intestinal mucosa and efficient utilization ratio of energy for gut development (Adibmoradi et al., 2006). Our findings showed that dietary probiotics, prebiotics and synbiotics, enhanced jejunal villi morphology. This corroborates the submissions of earlier reports on influence of prebiotics on jejunal villi morphology of laying hens (Ding et al., 2018; Guo et al., 2020). Also, our findings agree with previous reports in literatures that demonstrated enhancement effect of CB on ileal VH (Wang et al., 2020), VH, VH/CD and decreased CD (Xiang et al., 2019), and jejunal VH, VH/CD and decreased CD (Wang et al., 2021) in laying hens. In addition, Zarei et al. (2018) showed that the probiotic, prebiotic and synbiotics significantly increased villus height, villus height: CD ratios and decreased CD. The increased villus height, width, surface area and VH/CD, suggests an increased surface area with greater capacity for nutrient absorption (Adhikari et al., 2018). Prebiotics including FOS, acts as key substrates for probiotics in the intestinal tract, this in turn stimulates the growth of beneficial bacteria, which further promotes intestinal integrity (Sugiharto, 2016; Holscher, 2017). Probiotics (Hajiaghapour and Rezaeipour, 2018; Vieco-Saiz et al., 2019), CB (Liu et al., 2018; Wang et al., 2021), and FOS (Ding et al., 2019) are known to produce large amount of SCFAs, which provide energy for the intestinal epithelial cells (Zou et al., 2019; Salvi and Cowles, 2021), and prevention of pathogen adhesion to the GIT, thus increasing synbiotic intestinal microflora that supports villi development. Therefore, the enhanced gut morphology and two-fold synergistic effect of the synbiotics is attributable to adequate nutrient supply and beneficial microecological environment. However, Samli et al. (2007) found no significant effect of synbiotics (whey powder plus probiotics), on gut morphology of broilers. Also, FOS had no significant effect on the ileal morphology of laying hens (Adhikari et al., 2018). The variations could be due to microbial composition of the feed supplements. It could be deduced that the improved gut function increased bioavailability of nutrients, which reflect in enhanced crude protein and amino acid digestibility, egg production rate, albumen synthesis, and health status of the birds.
Conclusion
Dietary supplementation of probiotics; CB, prebiotics; FOS on a singly basis and a combination of FOS and CB as synbiotics enhanced laying performance, albumen quality, amino acid digestibility, jejunal villi morphology, immune response and antioxidant capacity in laying hens at peak phase. The significant effect of synbiotics on albumen quality and physiological response of the birds suggests the superiority of the synbiotics over singly basis of probiotics or prebiotics. The study shows that CB and FOS either singly or combined could be used as one of dietary strategies to enhance physiological health, egg production, and albumen quality of laying hens, thus boosting poultry production.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Animal Care and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences, Beijing, China (CAAS No. 20200507).
Author contributions
KQ, LH, and SW: Conceptualization. KQ, LH, and UO: Resources data. UO: Writing original draft. KQ, JW, HZ, LH, and SW: Supervision. KQ and UO: Writing and Editing. SW and GQ: Funding. This final version of the manuscript has been reviewed and accepted by all the authors.
Funding
This study was supported by Beijing Innovation Consortium of Agriculture Research System, the National Key Research and Development Program of China (2022YFC2105005), the National Natural Science Foundation of China (32072774), and the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202111).
Conflict of interest
L-lH was employed by Wilmar (Shanghai) Biotechnology Research & Development Centre Co., Ltd., Shanghai, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADFI, average feed intake, AEG, average egg weight, AH, albumen height, CB, Clostridium butyricum, CAT, catalase, CD, crypt depth, FCR, feed conversion ratio, FOS, fructooligosaccharide, GST, glutathione transferase, GSH-Px, glutathione peroxidase, HDP, hen day production, HU, Haugh unit, H/L, heterophil to lymphocyte ratio, IgM, immunoglobulin M, IgG, immunoglobulin G, IgA, immunoglobulin A, MCH, mean corpuscular hemoglobin, MCV, mean corpuscular volume, MDA, malondialdehyde, ROS, reactive oxygen species, RBC, red blood cells count, SCFA, short-chain fatty acid, TAO-C, total antioxidant capacity, TSOD, total superoxide dismutase, VH, Villi height, VW, villus width, VSA, villi surface area, V/C villi height to crypt depth ratio, WBC, white blood cells count,
References
Abdelqader, A., Al-Fataftah, A.-R., and Daş, G. (2013). Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim. Feed Sci. Technol. 179, 103–111. doi: 10.1016/j.anifeedsci.2012.11.003
Adhikari, P., Cosby, D. E., Cox, N. A., Franca, M. S., Williams, S. M., Gogal, R. M., et al. (2018). Effect of dietary fructooligosaccharide supplementation on internal organs, salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with salmonella enteritidis. Poult. Sci. 97, 2525–2533. doi: 10.3382/ps/pey101
Adibmoradi, M., Navidshad, B., Seifdavati, J., and Royan, M. (2006). Effect of dietary garlic meal on histological structure of small intestine in broiler chickens. J. Poult. Sci. 43, 378–383. doi: 10.2141/jpsa.43.378
Ahiwe, E. U., Abdallh, M. E., Chang’a, E. P., Omede, A. A., Al-Qahtani, M., Gausi, H., et al. (2020). Influence of dietary supplementation of autolyzed whole yeast and yeast cell wall products on broiler chickens. Asian Aust. J. Anim. Sci. 33, 579–587. doi: 10.5713/ajas.19.0220
Akinleye, S., Iyayi, E., and Afolabi, K. (2008). The performance, haematology and carcass traits of broilers as affected by diets supplemented with or without biomin a natural growth promoter. World J. Agric. Sci. 4, 467–470.
Alavi, S., Zakeri, A., Kamrani, B., and Pourakbari, Y. (2012). Effect of prebiotics, probiotics, acidfire, growth promoter antibiotics and synbiotic on humural immunity of broiler chickens. Glob. Vet. 8, 612–617.
Al-Khalaifa, H., Al-Nasser, A., Al-Surayee, T., Al-Kandari, S., Al-Enzi, N., Al-Sharrah, T., et al. (2019). Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 98, 4465–4479. doi: 10.3382/ps/pez282
Aobai, T., Xue, Z., Yuanyuan, S., and Xin, L. (2020). Potential role of ovomucin and its peptides in modulation of intestinal health: a review. Int. J. Biol. Macromol. 160, 385–392. doi: 10.1016/j.ijbiomac.2020.06.148
Biggs, P., and Parsons, C. (2007). The effects of several oligosaccharides on true amino acid digestibility and true metabolizable energy in cecectomized and conventional roosters. Poult. Sci. 86, 1161–1165. doi: 10.1093/ps/86.6.1161
Chen, W., Wang, J., Yan, L., and Huang, Y. (2013). Evaluation of probiotics in diets with different nutrient densities on growth performance, blood characteristics, relative organ weight and breast meat characteristics in broilers. Br. Poult. Sci. 54, 635–641. doi: 10.1080/00071668.2013.825369
Darsi, E., and Zhaghari, M. (2021). Effects of Bacillus subtilis PB6 supplementation on productive performance, egg quality and hatchability in broiler breeder hens under commercial farm condition. J. Appl. Animl. Res. 49, 109–117. doi: 10.1080/09712119.2021.1893738
Ding, X., Li, D., Bai, S., Wang, J., Zeng, Q., Su, Z., et al. (2018). Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 97, 874–881. doi: 10.3382/ps/pex372
Ding, S., Wang, Y., Yan, W., Li, A., Jiang, H., and Fang, J. (2019). Effects of lactobacillus plantarum 15-1 and fructooligosaccharides on the response of broilers to pathogenic Escherichia coli O78 challenge. PLoS One 14:e0212079. doi: 10.1371/journal.pone.0212079
Donalson, L., McReynolds, J., Kim, W., Chalova, V., Woodward, C., Kubena, L., et al. (2008). The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, salmonella Enteritidis infection, and intestinal shedding in laying hens. Poult. Sci. 87, 1253–1262. doi: 10.3382/ps.2007-00166
Duan, Y., Wang, Y., Dong, H., Ding, X., Liu, Q., Li, H., et al. (2018). Changes in the intestine microbial, digestive, and immune-related genes of Litopenaeus vannamei in response to dietary probiotic clostridium butyricum supplementation. Front. Microb. 9:2191. doi: 10.3389/fmicb.2018.02191
Emami, N. K., Samie, A., Rahmani, H., and Ruiz-Feria, C. (2012). The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim. Feed Sci. Technol. 175, 57–64. doi: 10.1016/j.anifeedsci.2012.04.001
Etim, N. N., Williams, M. E., Akpabio, U., and Offiong, E. E. (2014). Haematological parameters and factors affecting their values. Agric. Sci. 2, 37–47. doi: 10.12735/as.v2i1p37
Feng, Z., and Xia, Z. (2019). Effects of dietary fructo-oligosaccharides on laying performance and serum biochemical parameters of yellow broiler breeder hens, In: E3S Web of Conferences: EDP Sciences, 131.
Ghasemi, H. A., Kasani, N., and Taherpour, K. (2014). Effects of black cumin seed (Nigella sativa L.), a probiotic, a prebiotic and a synbiotic on growth performance, immune response and blood characteristics of male broilers. Lives Sci. 164, 128–134. doi: 10.1016/j.livsci.2014.03.014
Gungor, E., and Erener, G. (2020). Effect of dietary raw and fermented sour cherry kernel (Prunus cerasus L.) on digestibility, intestinal morphology and caecal microflora in broiler chickens. Poult. Sci. 99, 471–478. doi: 10.3382/ps/pez538
Guo, Y., Zhao, Z.-H., Pan, Z.-Y., An, L.-L., Balasubramanian, B., and Liu, W.-C. (2020). New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 99, 2100–2107. doi: 10.1016/j.psj.2019.12.032
Hajiaghapour, M., and Rezaeipour, V. (2018). Comparison of two herbal essential oils, probiotic, and mannan-oligosaccharides on egg production, hatchability, serum metabolites, intestinal morphology, and microbiota activity of quail breeders. Live Sci. 210, 93–98. doi: 10.1016/j.livsci.2018.02.007
Hamasalim, H. J. (2016). Synbiotic as feed additives relating to animal health and performance. Adv. Microbiol. 6, 288–302. doi: 10.4236/aim.2016.64028
Hassanpour, H., Moghaddam, A. Z., Khosravi, M., and Mayahi, M. (2013). Effects of synbiotic on the intestinal morphology and humoral immune response in broiler chickens. Live Sci 153, 116–122. doi: 10.1016/j.livsci.2013.02.004
He, T., Zhang, H., Wang, J., Wu, S., Yue, H., and Qi, G. (2017). Proteomic comparison by iTRAQ combined with mass spectrometry of egg white proteins in laying hens (Gallus gallus) fed with soybean meal and cottonseed meal. PLoS One 12:e0182886. doi: 10.1371/journal.pone.0182886
Holscher, H. D. (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microb. 8, 172–184. doi: 10.1080/19490976.2017.1290756
Hy-Line W-98 Commercial Management Guide , (2004–2006). Hy-line Int, West Des Moines, IA: Commercial Management Guide.
Kong, Q., He, G.-Q., Jia, J.-L., Zhu, Q.-L., and Ruan, H. (2011). Oral administration of clostridium butyricum for modulating gastrointestinal microflora in mice. Curr. Microbiol. 62, 512–517. doi: 10.1007/s00284-010-9737-8
Kumar, S., Shang, Y., and Kim, W. K. (2019). Insight into dynamics of gut microbial community of broilers fed with fructooligosaccharides supplemented low calcium and phosphorus diets. Front Vet. Sci. 6:95. doi: 10.3389/fvets.2019.00095
Lei, K., Li, Y., Yu, D., Rajput, I., and Li, W. (2013). Influence of dietary inclusion of bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 92, 2389–2395. doi: 10.3382/ps.2012-02686
Li, D., Ding, X., Zhang, K., Bai, S., Wang, J., Zeng, Q., et al. (2017). Effects of dietary xylooligosaccharides on the performance, egg quality, nutrient digestibility and plasma parameters of laying hens. Anim. Feed Sci. Technol. 225, 20–26. doi: 10.1016/j.anifeedsci.2016.12.010
Li, X., Qiang, L., and Xu, C. (2008). Effects of supplementation of fructooligosaccharide and/or Bacillus subtilis to diets on performance and on intestinal microflora in broilers. Arch. Anim. Breed. 51, 64–70. doi: 10.5194/aab-51-64-2008
Liao, X., Ma, G., Cai, J., Fu, Y., Yan, X., Wei, X., et al. (2015). Effects of clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 94, 662–667. doi: 10.3382/ps/pev038
Likotrafiti, E., Tuohy, K. M., Gibson, G. R., and Rastall, R. (2016). Antimicrobial activity of selected synbiotics targeted for the elderly against pathogenic Escherichia coli strains. Int. J. Food Sci. Nutr. 67, 83–91. doi: 10.3109/09637486.2015.1134444
Liu, H., Wang, J., He, T., Becker, S., Zhang, G., Li, D., et al. (2018). Butyrate: a double-edged sword for health? Adv. Nutr. 9, 21–29. doi: 10.1093/advances/nmx0009
Luoma, A., Markazi, A., Shanmugasundaram, R., Murugesan, G., Mohnl, M., and Selvaraj, R. (2017). Effect of synbiotic supplementation on layer production and cecal salmonella load during a salmonella challenge. Poult. Sci. 96, 4208–4216. doi: 10.3382/ps/pex251
Macit, M., Karaoglu, M., Celebi, S., Esenbuga, N., Yoruk, M. A., et al. (2021). Effects of supplementation of dietary humate, probiotic, and their combination on performance, egg quality, and yolk fatty acid composition of laying hens. Trop. Anim. Health Prod. 53, 1–8. doi: 10.1007/s11250-020-02546-6
Markowiak, P., and Śliżewska, K. (2018). The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 10, 1–20. doi: 10.1186/s13099-018-0250-0
Mikulski, D., Jankowski, J., Mikulska, M., and Demey, V. (2020). Effects of dietary probiotic (Pediococcus acidilactici) supplementation on productive performance, egg quality, and body composition in laying hens fed diets varying in energy density. Poult. Sci. 99, 2275–2285. doi: 10.1016/j.psj.2019.11.046
Mohammed, A., Jiang, S., Jacobs, J., and Cheng, H. W. (2019). Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poult. Sci. 98, 4408–4415. doi: 10.3382/ps/pez246
Mohebbifar, A., Kashani, S., Afsari, M., and Torki, M. (2013). Effects of commercial prebiotic and probiotics of diet on performance of laying hens, egg traits and some blood parameters. Ann. Res. Rev. Biol. 3, 921–934.
Mookiah, S., Sieo, C. C., Ramasamy, K., Abdullah, N., and Ho, Y. W. (2014). Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 94, 341–348. doi: 10.1002/jsfa.6365
Mwaniki, Z., Neijat, M., and Kiarie, E. (2018). Egg production and quality responses of adding up to 7.5% defatted black soldier fly larvae meal in a corn-soybean meal diet fed to shaver white leghorns from wk 19 to 27 of age. Poult. Sci. 97, 2829–2835. doi: 10.3382/ps/pey118
National Research and Council (1994). Nutrient Requirements of Poultry Ninth Revised Edition. Washington, DC: The National Academies Press.
Nwachukwu, C. U., Aliyu, K. I., and Ewuola, E. O. (2021). Growth indices, intestinal histomorphology, and blood profile of rabbits fed probiotics-and prebiotics-supplemented diets. Trans. Anim. Sci. 5:txab096. doi: 10.1093/tas/txab096
Obianwuna, U. E., Chang, X.-Y., Wang, J., Zhang, H.-J., Qi, G.-H., Qiu, K., et al. (2022a). Dietary fructooligosaccharides effectively facilitate the production of high-quality rggs via improving the physiological status of laying hens. Foods 11:1828. doi: 10.3390/foods11131828
Obianwuna, U. E., Qiu, K., Chang, X.-Y., Zhang, H.-J., Wang, J., Qi, G.-H., et al. (2022b). Enhancing egg production and quality by the supplementation of probiotic strains (clostridium and Brevibacillus) via improved amino acid digestibility, intestinal health, immune response, and antioxidant activity. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.987241
Pan, X., Cai, Y., Kong, L., Xiao, C., Zhu, Q., and Song, Z. (2022). Probiotic effects of bacillus licheniformis DSM5749 on growth performance and intestinal microecological balance of laying hens. Front. Nut.r 9. doi: 10.3389/fnut.2022.868093
Parenteau, I. A., Stevenson, M., and Kiarie, E. G. (2020). Egg production and quality responses to increasing isoleucine supplementation in shaver white hens fed a low crude protein corn-soybean meal diet fortified with synthetic amino acids between 20 and 46 weeks of age. J. Poult. Sci. 99, 1444–1453. doi: 10.1016/j.psj.2019.10.064
Phuoc, T., Dung, N., and Manh, L. (2019). Effects of dietary total sulphur amino acids to lysine ratio on performance, nitrogen utilization of ac layers (black-boned chicken). S. Afr. J. Anim. Sci. 49, 156–165. doi: 10.4314/sajas.v49i1.18
Pineda-Quiroga, C., Borda-Molina, D., Chaves-Moreno, D., Ruiz, R., Atxaerandio, R., Camarinha-Silva, A., et al. (2019). Microbial and functional profile of the ceca from laying hens affected by feeding prebiotics, probiotics, and synbiotics. Microorganisms 7:123. doi: 10.3390/microorganisms7050123
Puvača, N., Kostadinović, L., Popović, S., Lević, J., Ljubojević, D., Tufarelli, V., et al. (2015). Proximate composition, cholesterol concentration and lipid oxidation of meat from chickens fed dietary spice addition (Allium sativum, Piper nigrum, Capsicum annuum). Anim. Prod. Sci. 56, 1920–1927. doi: 10.1071/AN15115
Ray, B., Chowdhury, S., Das, S., Dey, B., Khatun, A., Roy, B., et al. (2022). Comparative effects of feeding single-and multi-strain probiotics to commercial layers on the productive performance and egg quality indices. J. Appl. Poult. Res. 31:100257. doi: 10.1016/j.japr.2022.100257
Ricke, S. (2015). Potential of fructooligosaccharide prebiotics in alternative and nonconventional poultry production systems. Poult. Sci. 94, 1411–1418. doi: 10.3382/ps/pev049
Rossi, M., Corradini, C., Amaretti, A., Nicolini, M., Pompei, A., Zanoni, S., et al. (2005). Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 71, 6150–6158. doi: 10.1128/AEM.71.10.6150-6158.2005
Salah, A. S., El-Tarabany, M. S., and Ali, M. A. (2018). Impact of dietary supplementation with a synbiotic, organic acids or their combination on growth performance, carcass traits, economic efficiency, jejunum histomorphometry and some blood indices of broiler chickens. Anim. Prod. Sci. 59, 1318–1326. doi: 10.1071/AN18156
Salvi, P. S., and Cowles, R. A. (2021). Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells 10:1775. doi: 10.3390/cells10071775
Samli, H. E., Senkoylu, N., Koc, F., Kanter, M., and Agma, A. (2007). Effects of enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 61, 42–49. doi: 10.1080/17450390601106655
Sarlak, S., Tabeidian, S. A., Toghyani, M., Shahraki, A. D. F., Goli, M., and Habibian, M. (2021). Effects of replacing inorganic with organic iron on performance, egg quality, serum and egg yolk lipids, antioxidant status, and iron accumulation in eggs of laying hens. Biol. Trace Elem. Res. 199, 1986–1999. doi: 10.1007/s12011-020-02284-8
Shi, H. T., Wang, B. Y., Bian, C. Z., Han, Y. Q., and Qiao, H. X. (2020). Fermented Astragalus in diet improved laying performance, egg quality, antioxidant and immunological status and intestinal microbiota in laying hens. AMB Exp. 10:159. doi: 10.1186/s13568-020-01092-6
Song, D., Wang, W., Chen, B., Li, A., Song, G., Cheng, J., et al. (2022). Dietary supplemental synbiotic–yucca extract compound preparation modulates production performance, immune status and faecal microflora diversity in laying hens. Food Agric. Immunol. 33, 360–376. doi: 10.1080/09540105.2022.2080187
Souza, O., Adams, C., Rodrigues, B., Krause, A., Bonamigo, R., Zavarize, K., et al. (2021). The impact of Bacillus subtilis PB6 and chromium propionate on the performance, egg quality and nutrient metabolizability of layer breeders. Animals 11:3084. doi: 10.3390/ani11113084
Sugiharto, S. (2016). Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 15, 99–111. doi: 10.1016/j.jssas.2014.06.001
Sun, X., Chakrabarti, S., Fang, J., Yin, Y., and Wu, J. (2016). Low-molecular-weight fractions of Alcalase hydrolyzed egg ovomucin extract exert anti-inflammatory activity in human dermal fibroblasts through the inhibition of tumor necrosis factor–mediated nuclear factor κB pathway. Nutr. Res. 36, 648–657. doi: 10.1016/j.nutres.2016.03.006
Sun, X., Yue, S.-Z., Qiao, Y.-H., Sun, Z.-J., Wang, C., and Li, H.-F. (2020). Dietary supplementation with selenium-enriched earthworm powder improves antioxidative ability and immunity of laying hens. Poult. Sci. 99, 5344–5349. doi: 10.1016/j.psj.2020.07.030
Świątkiewicz, S., Koreleski, J., and Arczewska, A. (2010). Laying performance and eggshell quality in laying hens fed diets supplemented with prebiotics and organic acids. Czech J. Anim. Sci. 55, 294–306. doi: 10.17221/207/2009-CJAS
Swiatkiewicz, S., Swiatkiewicz, M., Arczewska-Wlosek, A., and Jozefiak, D. (2015). Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J. Anim. Physiol. Anim. Nutr. 99, 1–12. doi: 10.1111/jpn.12222
Tang, S. G. H., Sieo, C. C., Ramasamy, K., Saad, W. Z., Wong, H. K., and Ho, Y. W. (2017). Performance, biochemical and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic and synbiotic. BMC Vet. Res. 13, 1–12. doi: 10.1186/s12917-017-1160-y
Thiam, M., Barreto Sánchez, A. L., Zhang, J., Zheng, M., Wen, J., Zhao, G., et al. (2021). Association of heterophil/lymphocyte ratio with intestinal barrier function and immune response to salmonella enteritidis infection in chicken. Animals 11:3498. doi: 10.3390/ani11123498
Upadhaya, S. D., Rudeaux, F., and Kim, I. H. (2019). Efficacy of dietary Bacillus subtilis and bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poult. Sci. 98, 4722–4728. doi: 10.3382/ps/pez184
Varzaru, I., Untea, A. E., Martura, T., Olteanu, M., Panaite, T. D., Schitea, M., et al. (2013). Development and validation of an RP-HPLC method for methionine, cystine and lysine separation and determination in corn samples. Ilie. Van. Rev. Chem. 64, 673–679.
Vieco-Saiz, N., Belguesmia, Y., Raspoet, R., Auclair, E., Gancel, F., Kempf, I., et al. (2019). Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 10:57. doi: 10.3389/fmicb.2019.00057
Wang, W., Li, Z., Han, Q., Guo, Y., Zhang, B., and D’inca, R. (2016). Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 116, 1878–1888. doi: 10.1017/S0007114516004116
Wang, Y., Wang, Y., Lin, X., Gou, Z., Fan, Q., and Jiang, S. (2021). Effects of clostridium butyricum, sodium butyrate, and butyric acid glycerides on the reproductive performance, egg quality, intestinal health, and offspring performance of yellow-feathered breeder hens. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.657542
Wang, W.-W., Wang, J., Zhang, H.-J., Wu, S.-G., and Qi, G.-H. (2020). Effects of clostridium butyricum on production performance and intestinal absorption function of laying hens in the late phase of production. Anim. Feed Sci. Technol. 264:114476. doi: 10.1016/j.anifeedsci.2020.114476
Wu, Y., Zhen, W., Geng, Y., Wang, Z., and Guo, Y. (2019). Effects of dietary enterococcus faecium NCIMB 11181 supplementation on growth performance and cellular and humoral immune responses in broiler chickens. Poult. Sci. 98, 150–163. doi: 10.3382/ps/pey368
Xiang, Q., Wang, C., Zhang, H., Lai, W., Wei, H., and Peng, J. (2019). Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals (Basel) 9. doi: 10.3390/ani9121110
Xu, Q., Azzam, M. M. M., Zou, X., and Dong, X. (2020). Effects of chitooligosaccharide supplementation on laying performance, egg quality, blood biochemistry, antioxidant capacity and immunity of laying hens during the late laying period. Ital. J. Anim. Sci. 19, 1180–1187. doi: 10.1080/1828051x.2020.1827991
Xu, Z., Hu, C., Xia, M., Zhan, X., and Wang, M. (2003). Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 82, 1030–1036. doi: 10.1093/ps/82.6.1030
Xu, C., Wei, F., Yang, X., Feng, Y., Liu, D., and Hu, Y. (2022). Lactobacillus salivarius CML352 isolated from Chinese local breed chicken modulates the gut microbiota and improves intestinal health and egg quality in late-phase laying hens. Microorganisms 10:726. doi: 10.3390/microorganisms10040726
Yamauchi, K. E., Incharoen, T., and Yamauchi, K. (2010). The relationship between intestinal histology and function as shown by compensatory enlargement of remnant villi after midgut resection in chickens. Anatomical. Rec. Adv. Integ. Anat. Evol. Biol. 293, 2071–2079. doi: 10.1002/ar.21268
Yang, C., Cao, G., Ferket, P., Liu, T., Zhou, L., Zhang, L., et al. (2012). Effects of probiotic, clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 91, 2121–2129. doi: 10.3382/ps.2011-02131
Yang, Y., Iji, P., Kocher, A., Mikkelsen, L., and Choct, M. (2008). Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic Escherichia coli challenge. Br. Poult. Sci. 49, 550–559. doi: 10.1080/00071660802290408
Yang, J., Zhan, K., and Zhang, M. (2020). Effects of the use of a combination of two bacillus species on performance, egg quality, small intestinal mucosal morphology, and cecal microbiota profile in aging laying hens. Prob. Antim. Prot. 12, 204–213. doi: 10.1007/s12602-019-09532-x
Yang, X., Zhang, B., Guo, Y., Jiao, P., and Long, F. (2010). Effects of dietary lipids and clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult. Sci. 89, 254–260. doi: 10.3382/ps.2009-00234
Yousaf, M., Ijaz, A., Ashraf, K., Rashid, M., Hafeez, A., Zaneb, H., et al. (2016). Comparative effects of different dietary concentrations of β-galacto-oligosaccharides on growth performance, feed conversion efficiency and organs development in broilers. J. Anim. Plant. Sci 26, 1603–1608.
Youssef, A. W., Hassan, H., Ali, H., and Mohamed, M. (2013). Effect of probiotics, prebiotics and organic acids on layer performance and egg quality. Asian J. Poult. Sci. 7, 65–74. doi: 10.3923/ajpsaj.2013.65.74
Yuan, C., Song, H. H., Zhang, X. Y., Jiang, Y. J., Zhang, A. T., Azzam, M. M., et al. (2014). Effect of expanded cottonseed meal on laying performance, egg quality, concentrations of free gossypol in tissue, serum and egg of laying hens. Anim. Sci. J. 85, 549–554. doi: 10.1111/asj.12169
Zarei, A., Lavvaf, A., and Motamedi Motlagh, M. (2018). Effects of probiotic and whey powder supplementation on growth performance, microflora population, and ileum morphology in broilers. J. Appl. Anim. Res. 46, 840–844. doi: 10.1080/09712119.2017.1410482
Zhan, H., Dong, X., Li, L., Zheng, Y., Gong, Y., and Zou, X. (2019). Effects of dietary supplementation with clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 98, 896–903. doi: 10.3382/ps/pey436
Zhang, L., Cao, G., Zeng, X., Zhou, L., Ferket, P., Xiao, Y., et al. (2014). Effects of clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 93, 46–53. doi: 10.3382/ps.2013-03412
Zhang, Z., Liu, D., Yi, B., Liao, Z., Tang, L., Yin, D., et al. (2014). Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 10, 2255–2262. doi: 10.3892/mmr.2014.2544
Zhang, J., Zhang, M., Liang, W., Geng, Z., and Chen, X. (2020). Green tea powder supplementation increased viscosity and decreased lysozyme activity of egg white during storage of eggs from Huainan partridge chicken. Ital. J. Anim. Sci. 19, 586–592. doi: 10.1080/1828051x.2020.1769512
Zhang, L., Zhang, L., Zhan, X. A., Zeng, X., Zhou, L., Cao, G., et al. (2016). Effects of dietary supplementation of probiotic, clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 7, 1–9. doi: 10.1186/s40104-016-0061-4
Zhao, P., Wang, J., and Kim, I. (2013). Effect of dietary Levan fructan supplementation on growth performance, meat quality, relative organ weight, cecal microflora, and excreta noxious gas emission in broilers. J. Anim. Sci. 91, 5287–5293. doi: 10.2527/jas.2012-5464
Zhou, J.-M., Qiu, K., Wang, J., Zhang, H.-J., Qi, G.-H., and Wu, S. G. (2021). Effect of dietary serine supplementation on performance, egg quality, serum indices, and ideal mucosal immunity in laying hens fed a low crude protein diet. Poult. Sci. 100:101465. doi: 10.1016/j.psj.2021.101465
Zhou, J.-M., Zhang, H.-J., Wu, S.-G., Qiu, K., Fu, Y., Qi, G.-H., et al. (2021). Supplemental xylooligosaccharide modulates intestinal mucosal barrier and cecal microbiota in laying hens fed oxidized fish oil. Front. Microbiol. 12:179. doi: 10.3389/fmicb.2021.635333
Keywords: Clostridium butyricum, laying performance, fructooligosaccharides, synbiotics, egg quality
Citation: Obianwuna UE, Qiu K, Wang J, Zhang H-j, Qi G-h, Huang L-l and Wu S-g (2023) Effects of dietary Clostridium butyricum and fructooligosaccharides, alone or in combination, on performance, egg quality, amino acid digestibility, jejunal morphology, immune function, and antioxidant capacity of laying hens. Front. Microbiol. 14:1125897. doi: 10.3389/fmicb.2023.1125897
Edited by:
Weiwei Wang, South China Agricultural University, ChinaReviewed by:
Yibing Wang, Guangdong Academy of Agricultural Sciences, ChinaChao Wen, Nanjing Agricultural University, China
Copyright © 2023 Obianwuna, Qiu, Wang, Zhang, Qi, Huang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-ling Huang, ✉ aGxpbmdsaW5nMjAwNkAxNjMuY29t; Shu-geng Wu, ✉ d3VzaHVnZW5nQGNhYXMuY24=
†These authors have contributed equally to this work
 Uchechukwu Edna Obianwuna
Uchechukwu Edna Obianwuna Kai Qiu
Kai Qiu Jing Wang
Jing Wang Hai-jun Zhang
Hai-jun Zhang Guang-hai Qi
Guang-hai Qi Ling-ling Huang2*
Ling-ling Huang2* Shu-geng Wu
Shu-geng Wu