- 1College of Biological Engineering, Henan University of Technology, Henan, Zhengzhou, China
- 2College of Food and Biological Engineering (Liquor College), Henan University of Animal Husbandry and Economy, Zhengzhou, Henan, China
- 3International Education College, Henan Agricultural University, Zhengzhou, Henan, China
Daqu is a saccharifying and fermenting starter in the production of Chinese Baijiu; its quality directly affects the quality of Baijiu. The production of Daqu is highly environment-dependent, and after long-term natural domestication, it is rich in a wide variety of microorganisms with a stable composition, which provide complex and diverse enzymes and flavor (precursor) substances and microbiota for Jiupei (Fermented grains) fermentation. However, inoculation with a relatively stable microbial community can lead to a certain upper limit or deficiencies of the physicochemical properties (e.g., saccharification capacity, esterification capacity) of the Daqu and affect the functional expression and aroma formation of the Daqu. Targeted improvement of this problem can be proposed by selecting functional microorganisms to fortify the production of Daqu. This review introduced the isolation, screening, identification and functional characteristics of culture-dependent functional microorganisms in Baijiu-brewing, the core functional microbiota community of Daqu, and the related research progress of functional microorganisms fortified Daqu, and summarized the fortifying strategies of functional microorganisms, aiming to further deepen the application of functional microorganisms fortification in Daqu fermentation and provide ideas for the flavor regulation and quality control of Baijiu.
1. Introduction
As one of the six distilled liquors in the world, Chinese Baijiu is unique in that it uses a strategy of fermentation of raw materials while saccharifying and fermenting (Xu et al., 2017; He G. et al., 2019). Daqu is an essential starter for Chinese Baijiu, as it is rich in microbial communities, functional enzyme systems, and flavor precursors, and plays an important role in the initiation and smooth progress of Jiupei fermentation (Zheng et al., 2011; Wang B. W. et al., 2018). Microbiota from nature is naturally inoculated and assembled into Daqu’s open fermentation process, so it is abundant in bacteria, molds, yeasts, actinomycetes, and other microorganisms (Xu et al., 2017). As the most important microorganism in the brewing process of Baijiu, bacteria play a significant role in enhancing the flavor and quality of Baijiu and have always been the focus of research and hot spots (Du H. et al., 2021). As a class of brew microorganisms, yeast is not only the leading force in the alcoholic fermentation process of Baijiu, but also its aroma production performance is crucial to the quality and characteristics of different flavored Baijiu (Wang D. Q. et al., 2019). Mold in the process of fermentation of the Daqu produces protease, lipase, glycosylase, cellulase, and other rich and diverse enzyme system, with the ability of aldolization, esterification, etc., and the growth of mycelium is conducive to water volatilization and material utilization in the heart of the Daqu and has a vital role in the maturation of the Daqu fermentation (Li et al., 2019; Tang et al., 2022). Actinomyces have a variety of metabolic activities, including hydrolysis of starch, cellulose, protein, and pectin in raw grains, and the production of various secondary metabolites (Chen et al., 2022).
However, when Daqu is naturally enriched and inoculated with various microorganisms in the environment, in addition to assembling beneficial bacteria, bacteria harmful to Baijiu-brewing will inevitably intermingle, resulting in unstable quality, low yield, and high production cost of Baijiu production (Ke et al., 2019). Furthermore, due to environmental dependence, there are certain constraints on the inherent property of Daqu, such as the upper limit of enzyme activity (Zheng et al., 2015; Liu J. J. et al., 2018; Ma et al., 2022). In the early days, to solve such problems, scholars and breweries usually used functional microorganisms isolated from the brewing environment in the manufacture of Daqu or the development of compound bacterial agents to improve the structure and physicochemical properties of the Daqu microbial community and enhance the aromatic substances and flavor precursors of Daqu, thereby improving the yield and quality of the original Baijiu. The reason for choosing in-situ fortification is, firstly, to ensure the role of the Daqu in the brewing of Baijiu and to emphasize the innovation based on the traditional process, and secondly, the functional strain fortified Daqu can be used as a stable and effective carrier for the preservation of crude enzymes, microbiota and aroma substances. For example, adding Saccharomyces cerevisiae to Daqu can increase the content of nitrile enzyme and significantly degrade the content of cyanide in Daqu (Shen et al., 2021). Inoculation with Bacillus velezensis and Bacillus subtilis could improve the flavor profile of Daqu and significantly improve the contents of Tetramethyl pyrazine and phenyl ethanol (He G. et al., 2019). However, due to the limitation of research methods, the scientific mechanism of the consequence of functional microorganisms’ fortification on the Daqu is not clear, and often the fortification of functional microorganisms does not achieve the expected effect or lead to the degradation of the quality of Daqu.
In recent years, with the rapid development of ecological fermentation technology, researchers have reconstructed the fermentation microbiota, reorganized the core functional microbiota, and controlled the fermentation process to control the microbiota to produce target products by adjusting the microbiota inoculation ratio (Du H. et al., 2021). Studies have shown that the most effective way to control the microbial community of the mature Daqu is by directly adding microorganisms to change the initial microbiota (Wang et al., 2017). Yet, the key to the realization of the ecological fermentation of Daqu lies in the composition and succession of the microbial community and the analysis of metabolic characteristics of functional microorganisms. Only by clarifying the regulation of different microorganisms on Daqu flavor and physical and chemical indicators, can we further reconstruct fermentation microbiota or construct synthetic microbiota based on in situ system (Kong et al., 2014). Among them, the identification of core functional microorganisms is the primary condition for controlling fermentation. Presently, the core functional microorganisms in the fermentation process of Daqu have been identified based on metagenomics and other multi-omics approaches as well as statistical analysis methods such as partial least squares and Pearson correlation analysis. There has been some research, and under this premise, it is helpful to guide the screening of functional microorganisms.
Based on this, this paper systematically summarized the screening, identification, and functional characteristics of culture-dependent Baijiu-brewing functional microorganisms, the core microbial community in the fermentation process of Daqu, and the research progress of functional microorganisms fortified Daqu. Thinking and looking forward to the significance and effect of functional microbial community analysis, pure species isolation and screening, and microbial community recombination on flavor regulation and quality control of Daqu, which has far-reaching significance for the healthy and standardized development of Baijiu.
2. Definition and classification of Baijiu Daqu
Daqu is one of the Chinese Baijiu Jiuqus (starters). The main ingredient is raw wheat, supplemented by a percentage of barley and peas (Kang et al., 2022). The raw materials are moistened, grinder, and shaped into blocks and then sent to the incubation room to be naturally inoculated with environmental microorganisms for fermentation, and the finished product is named Daqu because of its shape (Zheng et al., 2011). The fermentation process of Daqu is open, so the enriched microbial community is complex and diverse. Microorganisms interact and evolve in Daqu to form a specific stable microbial community. Microbiota produces a variety of enzymes that play a vital role in the saccharification, alcoholic fermentation and flavor formation of starchy raw materials. Unlike Xiaoqu and Fuqu, Daqu is used as a starter to produce most of China’s famous Baijiu, such as Moutai, Wuliangye, Fenjiu, etc. In the Daqu type division, mainly in Daqu fermentation maximum temperature (High-temperature Daqu, Middle-temperature Daqu, Low-temperature Daqu), flavor characteristics (Maotai-flavor Baijiu Daqu, Luzhou-flavor Baijiu Daqu, Fen-flavor Baijiu Daqu, etc.), shape (Bag Daqu, Brick Daqu), production methods (Mechanical Daqu, Handmade Daqu), functional characteristics (Traditional Daqu, Fortified Daqu) to divide (Zheng et al., 2018; He G. et al., 2019; Zuo et al., 2020; Kang et al., 2022; Figure 1A).
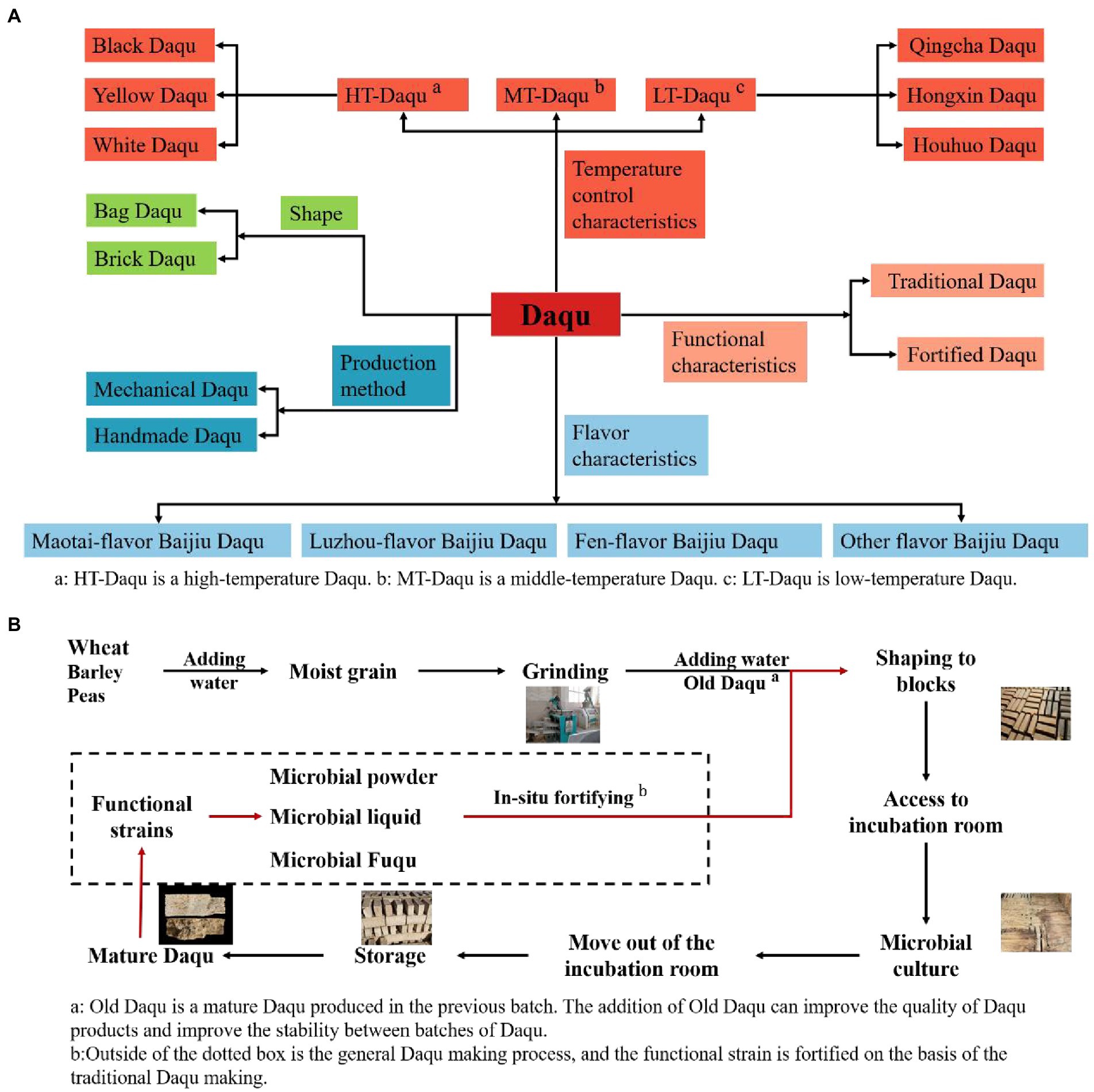
Figure 1. (A) Classification of Daqu according to different characteristics. (B) Sketch of the fortified Daqu production process.
The above classification has the potential for further. For example, High-temperature Daqu due to early fermentation temperature fluctuations will lead to the final Daqu color being different, divided into Black Daqu, Yellow Daqu, and White Daqu (Shi et al., 2022). Low-temperature Daqu was controlled at different temperatures during the fermentation stage to form three kinds of Daqu: Qingcha Daqu, Hongxin Daqu, and Houhuo Daqu (Cai et al., 2021). It seems that naming only focuses on the morphological differences, but it essentially reflects the changes in the metabolism of the microbial community caused by temperature changes. Each Daqu has its unique functional characteristics.
Yet, due to differences in the natural environment, there are often significant differences in the types, numbers, and proportions of microbiota in the Daqu from different geographical environments (Zheng et al., 2015; Ma et al., 2022). This difference is manifested in two aspects: firstly, the difference in the microbial community leads to a slight difference in flavor formation, and such influence is positive in a certain sense, creating a different sensory experience of Baijiu; secondly, the difference in the microbial community leads to defects in the physicochemical properties (such as esterification power and saccharification power) of Daqu, which to a certain extent directly affects the Baijiu wine quality and production cost.
The Daqu and Jiupei solid-state fermentation prohibits the addition of exogenous non-fermentative substances, such as the direct addition of various enzymes. In response to the adverse effects of differences in the microbial community, researchers improved the quality of Daqu by making fortified Daqu in the 1980s. Fortified Daqu improves quality and fermentation characteristics by adding artificially cultured pure functional microorganisms based on traditional Daqu production (Li W. W. et al., 2020). The production process of the Fortified Daqu is shown in Figure 1B.
3. Baijiu-brewing functional microorganisms
3.1. Definition and research methods of functional microorganisms (microbiota)
Baijiu fermentation is a complex natural fermentation system. The microorganisms involved in fermentation mainly come from Daqu microecology and environmental microecology, and the population and metabolism are complex, thus providing complex and diverse enzyme systems and flavor (precursor) substances for Baijiu fermentation, and giving Baijiu unique taste and flavor (Xiao et al., 2021). Among them, high-yielding enzymes (such as amylase, protease, cellulase, esterase, glucase, tannase, xylanase, pectinase, oxidoreductase, lyase, isomerase, etc) and aroma substances (such as ethyl caproate, ethyl acetate, ethyl butyrate, ethyl valerate, ethyl octanoate, 1-propanol, acetic acid, 2,3,5-Trimethylpyrazine and 2,3,5,6-tetramethylpyrazine, etc) were widely defined as Baijiu-brewing functional microorganisms (Zeng, 2016; Song et al., 2017; Ma et al., 2022; Zhu et al., 2022).
In the early stage, studies on functional microorganisms mainly focused on isolation and purification by traditional culture-dependent methods, identification by molecular biology techniques, enzyme activity determination, metabolic profiling and characterization of microorganisms with alcohol, aroma and enzyme production characteristics (Xing et al., 2018; Wu et al., 2021; Liu Y. B. et al., 2022). Compared with rapid and high-throughput microbial community analysis such as polymerase chain reaction-denatured gradient gel electrophoresis (PCR-DGGE) analysis of DNA extracts, pyrosequencing, and clonal library sequencing, the traditional culture-dependent method has the obvious advantage of obtaining pure strains that can be used for further experiments. However, due to the long cultivation time and complex operation method, the traditional cultivation-dependent method has the disadvantages of time-consuming and laborious.
In recent years, with the development of microbiome, targeted amplicon sequencing (16S rRNA gene of prokaryotes). The 18S rRNA and the Internal transcriptional spacer [ITS] gene of eukaryotes ushered in a new era of microbiome research, By clustering sequences, OTU division and comparison in databases (bacteria and archaea 16S rRNA database, fungi 18S rRNA database, fungi ITS database and functional gene database), the understanding of microbial composition and function of Daqu has been improved (Barooah et al., 2022). In addition, more advanced omics techniques such as metagenomics (which can not only provide insights into taxonomic classifications of the Daqu sequence at the species level, especially for the identification of culture-independent microorganisms, but also reveal details of microbiome assembly and underlying gene function) (Yang et al., 2021), Metatranscriptomics (can further explain the active microbial composition and enzyme profile in Daqu based on the overall expression of mRNA in the microbiome) (Song et al., 2017), Metaproteomics (which can detect changes in microbiome protein expression in Daqu and identify key enzyme-producing microbiome; Xia et al., 2022) further provides useful information on the activity and function of the microbiome in Daqu. But there is no doubt that the use of omics requires more financial support and the ability to analyze and mine large-scale data sets.
3.2. Traditional culture-dependent screening of functional microorganisms
It has been proved that there are abundant microorganism resources in Daqu and the brewing process (Xu et al., 2017; Wang D. Q. et al., 2019; Chen et al., 2022). The traditional dependent culture method mainly takes the characteristic flavor substances and process links of different flavor types as the guiding ideology to separate and screen the culturable microorganisms in the samples and study their functional characteristics (Zou et al., 2018). A large number of functional microorganisms were identified by screening from Daqu and brewing environment samples (Table 1). Among them, the functional yeasts are mainly Saccharomyces cerevisiae, Wickerhamomyces anomalus, Pichia kudriavzevii, and Saccharomycopsis fibuligera. Wickerhamomyces anomalus has good aroma and enzyme-producing characteristics and is enriched and screened in a variety of aromatic Baijiu Daqu and brewing environments. In addition, non-dominant yeast, such as Lodderomyces elongisporus and Hyphopichia burtonii, are unique in Maotai-flavor Baijiu Daqu, which contribute to alcohol production and aroma.
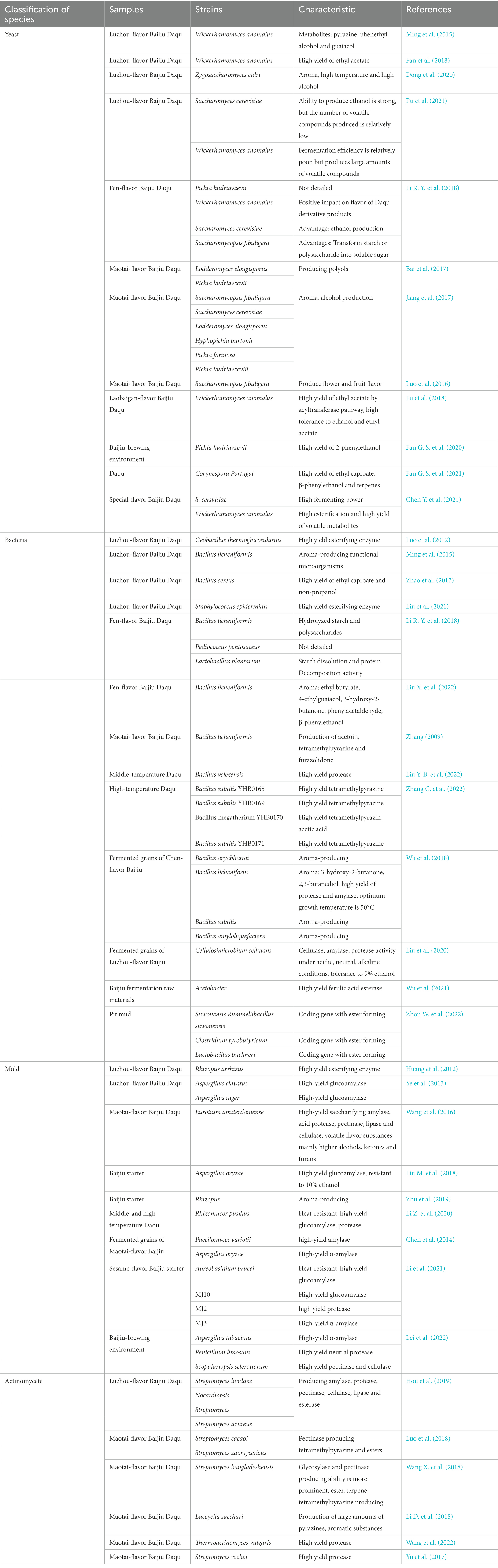
Table 1. Isolation, identification, and functional characteristics of Baijiu-brewing functional microorganisms.
The functional bacteria were mainly Bacillus. Bacillus is one of the important functional bacteria in the fermentation process of Daqu and fermented grains. It can secrete enzymes to hydrolyze starch, protein, pectin and other substrates, promote the smooth fermentation process, and produce health factors and aroma substances such as tetramethylpyrazine.
Aspergillus and Rhizopus showed higher glucoamylase and α-amylase activities. They are the key functional microorganisms to produce liquor, fragrance and enzyme during Daqu fermentation and Baijiu-brewing, and directly affect the fermentation quality of Daqu and the formation of Baijiu body flavor.
Actinomycetes also showed high enzyme production capacity, such as gelase, amylase, protease, which also play a certain role in the formation of aroma. The studies on actinomycetes mainly focus on Maotai-flavor Baijiu Daqu. Due to the high-temperature preparation process, high abundance of high temperature resistant actinomycetes are enriched. According to the fermentation test, actinomycetes showed a certain ability to produce tetramethylpyrazine, which contributed to the formation of the main aroma of Maotai-flavor Baijiu.
However, the microbiota of Daqu and its brewing environment is complex and diverse, and the traditional dependent culture technology can only obtain 0.1–1.0% of the microorganisms in the environment, which greatly limits the study of microbial community diversity, flavor characteristics and formation mechanism of Chinese Baijiu fermentation (Kang et al., 2022). Therefore, the identification of uncultured microorganisms has become an important subject for the in-depth understanding of Daqu and the Baijiu fermentation mechanism.
3.3. Study of functional microbiota based on multi-omics linkage
The dominant microflora with higher abundance in the brewing environment is generally considered as the core microbiota (Zhang J. et al., 2022). Traditionally, culture-dependent techniques have been used to isolate and identify species from Baijiu fermentation, and although these studies have shed some light on microbial populations, our understanding of the core functional microbiota in Baijiu fermentation is still limited. With the rapid development of histological technology in recent years, the study of microorganisms in Daqu is no longer limited to traditional culturable techniques, but researchers have used genomics, transcriptomics, proteomics, metabolomics and statistics to study the dominant microbial community, flavor-related microbiota and prevalent microbiota, thus further exploring the core functional microbiota in Daqu fermentation and Baijiu-brewing process (Wang S. L. et al., 2019). Song et al. (2017) combined high-throughput amplicon sequencing (16S rRNA gene amplicon sequencing and internal transcribed space amplicon sequencing), metatranscriptome sequencing technology, ultra-high performance liquid chromatography and headspace solid-phase microextraction-gas chromatography–mass spectrometry to determine the core microbial community of Daqu of Maotai-flavor Baijiu. The first stage of Daqu fermentation involves high-level alcohol (ethanol) production, with Schizosaccharomyces as the core functional microorganism. The second stage involves high levels of acid (lactic acid and acetic acid) production, and Lactobacillus is the core functional microorganism. Wang S. L. et al. (2019) used Chinese light-flavor Baijiu as a model system, based on its flavor production and symbiotic performance, revealed that the core microflora was Lactobacillus, Saccharomyces, Pichia, Geotrichum, and Candida. Synthetic microbial consortia capable of reproducing complex flavor metabolism were constructed using Lactobacillus acetotolerans, Pichia kudriavzevii, Geotrichum candidum, Candida vini, Saccharomyces cerevisiae.
In fact, more and more studies have used the above methods to obtain large-scale omics data sets and extended the research strategy of the Baijiu-brewing mechanism through gene function enrichment analysis and correlation analysis. Table 2 lists the research status of functional microbial composition, characteristics, and research strategies of some important samples of Daqu, pit mud, and fermented grains.
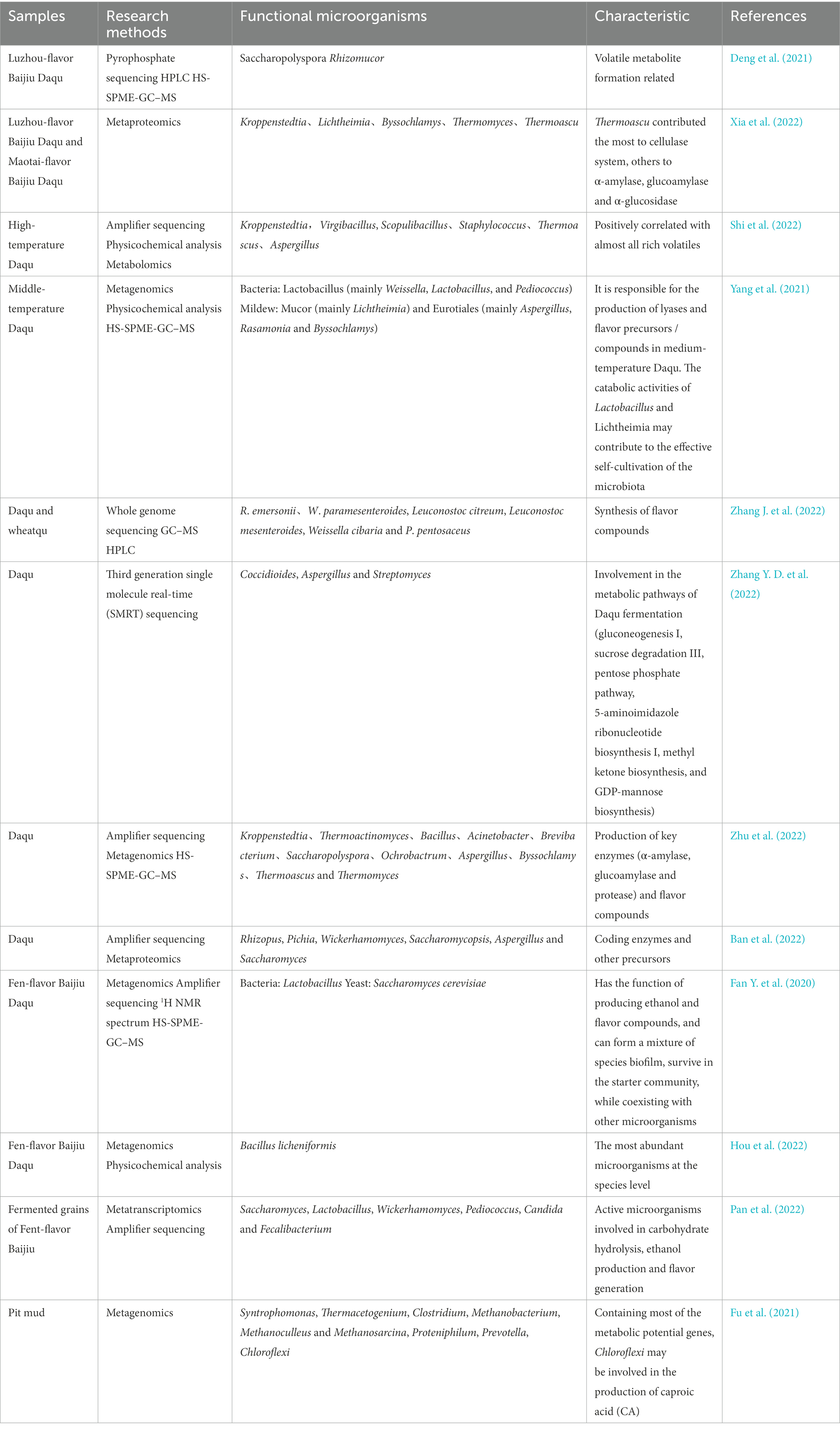
Table 2. Composition, functional characteristics, and research methods of core functional microbiota.
From the perspective of research methods, the characteristics of functional microbiomes determined by different combinations of techniques are not consistent. Most studies tend to establish an association network between the microbial community and flavor compounds, so as to define the core aromatic functional microflora; On this basis, further analysis of gene function from the DNA level, analysis of active microorganisms and enzyme profile from the RNA level, analysis of protein expression and enzyme producing microorganisms from the protein level can fully determine the core enzyme producing microorganisms in Daqu.
In addition, the functional microbiota composition of different types of Daqu samples was different. As mentioned earlier, Daqu can be classified according to fermentation temperature, flavor characteristics, etc. High temperature resistant bacteria such as Kroppenstedtia, Thermomyces, Thermoascus are mainly present in High-temperature Daqu or Maotai-flavor Baijiu Daqu; While Saccharomyces, Weissella, Lactobacillus and Pediococcus are the main functional bacteria in Middle-temperature Daqu and Fen-flavor Baijiu Daqu. We also noticed that the composition and species of core functional microorganisms in Fen-flavor Baijiu Daqu were similar to those in fermented grains of Fen-flavor Baijiu. The fermentation method is ground tank fermentation, and the microorganism mainly comes from Daqu, so the functional microbiome has certain similarities. We speculate that the functional microbial differences between Luzhou-flavor and Maotai-flavor Baijiu Daqu and fermented grains may be greater because the microbial sources of fermented grains of these two types are more extensive. For example, fermented grains of Luzhou-flavor Baijiu fermented in the cellar, pit mud microorganisms will participate in the fermentation, some methanogens (Methanobacterium, Methanoculleus and Methanosarcina) and Chloroflexi involved in the formation of caproic acid and ethyl caproate. Maotai-flavor Baijiu brewing process of high-temperature accumulation, environmental microorganisms will be further enriched.
4. Application strategy of functional microorganisms
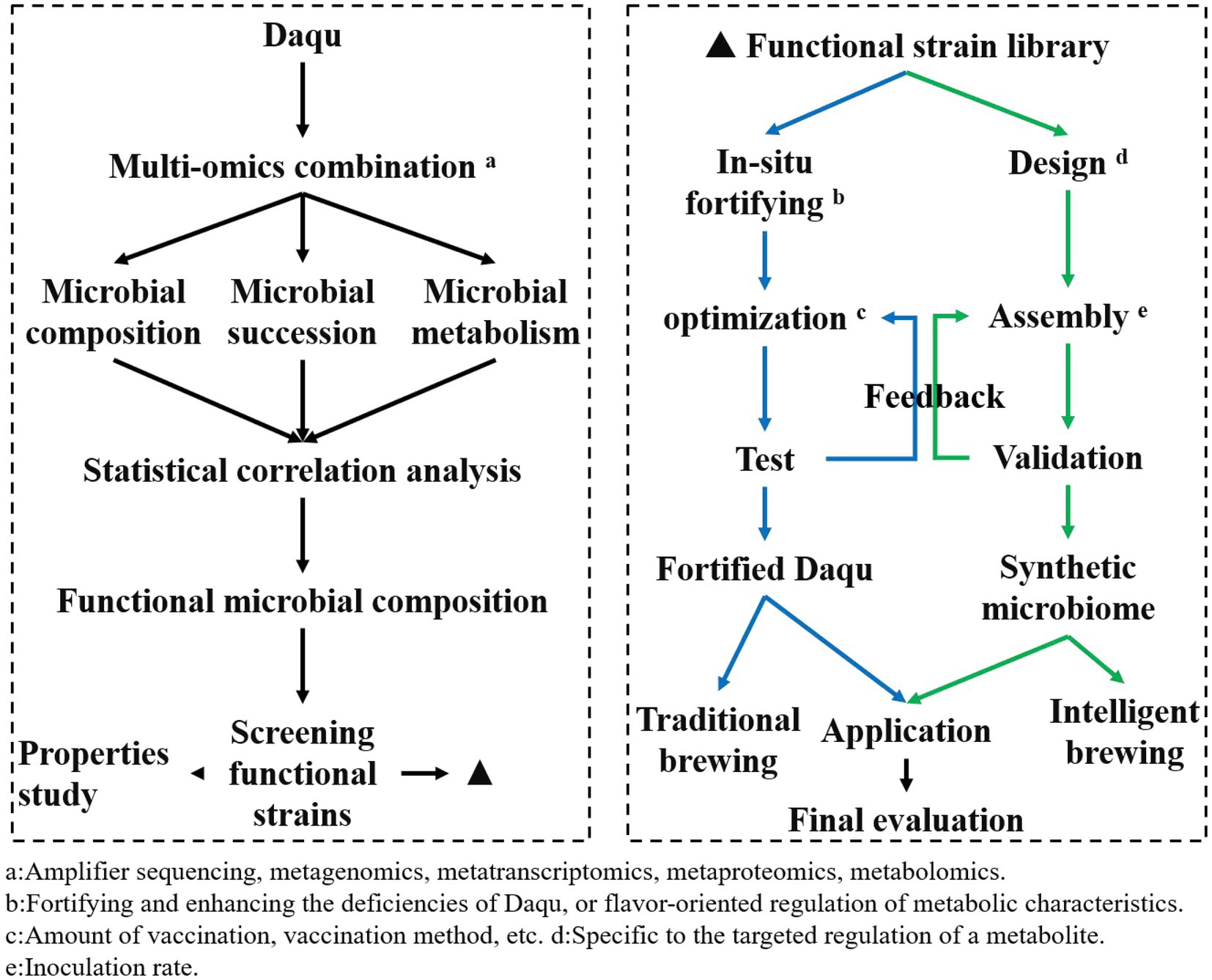
Determining the composition, succession law, and fermentation characteristics of core functional microorganisms in the fermentation process of Daqu is conducive to guiding the breeding of functional microorganisms, and further studying the interaction mechanism between strains based on breeding, laying a foundation for strengthening functional strains. Ecological fermentation indicates that adjusting the initial microbial community can reconstruct the fermented microbial community, recombine the core functional microbiota community, and control the fermentation process to control the community to efficiently produce the target product, achieving microbial community regulation through a top-down approach (Du R. et al., 2021). Besides, the identification of core microbiota is conducive to the construction of a synthetic microbiota. Simplified microbial flora is an effective way of directional regulation, which is of great significance for the directional, stable, and safe production of Daqu (Wang S. L. et al., 2019). The functional microorganism’s application strategy is shown in Figure 2.
In the first step, the microbial structure, succession law and metabolic characteristics of Daqu fermentation were analyzed, and the composition of functional microbial groups was determined by multi-omics combination combined with statistical correlation analysis (each omics technology has been introduced in section 3); on the one hand, to understand the mechanism of Daqu fermentation changes, provide a theoretical basis for the application of functional microorganisms; on the other hand, the composition and function of functional microorganisms are investigated to provide information support for screening functional microorganisms. The second step is to establish a functional strain library based on the screened functional microorganisms; the fermentation characteristics and safety of the screened strains were evaluated, and a strain characteristic classification information database was established for the subsequent selection of functional microbial strains. The third step is to construct in situ fortified microbial communities and synthetic microbial communities based on functional microorganisms; for functional microbial fortified Daqu, the inoculation method and inoculum number of functional microorganisms will be optimized and tested according to the need; for synthetic microbial communities, the composition and inoculation ratio will be determined and verified. This process is the most complex and challenging part of the application of functional microorganisms, the main premise and direction of the application of functional microorganisms are positive; then the existing problem is how to ensure the normal fermentation of Daqu under bioturbation and can achieve the purpose of fortification. If it is a synthetic microbiota, which microorganisms need to be combined, how to control the ratio of each strain, and whether the flavor characteristics and taste of Baijiu can be reproduced by using synthetic microbiota, these questions are still urgent to be solved. In the fourth step, evaluation of the application of fortified Daqu and synthetic microbiota; in actual production, Baijiu brewing is carried out using fortified Daqu and bacteriological agents constructed with synthetic microbiota, and the fermentation process (fermentation quality, raw material utilization rate, etc) and the quality of raw Baijiu (yield, flavor characteristics, taste, etc) are evaluated.
5. Functional microorganisms fortification
5.1. Fortification of functional yeast strains
Yeast is a kind of single-cell fungus, which is widely used in food, the chemical industry, medicine, and agriculture. Yeast is a very important microorganism in the Baijiu-brewing industry. Without yeast, there is no alcoholic beverage. Yeast mainly conducts alcoholic fermentation and produces aroma in Baijiu production, which is of great significance to Baijiu flavor and quality (Wang D. Q. et al., 2019).
Saccharomyces cerevisiae, Wickerhamomyces anomalus, Saccharomycopsis fibuligera, Pichia caribbica, and Pichia kudriavzeviia are the main yeasts in Daqu (Zheng et al., 2011; Wang D. Q. et al., 2019; Zhou Q. et al., 2022). In fact, Baijiu is particularly focused on the composition and content of its flavor, so the yeast fortification is mostly oriented towards producing high quality flavor. It was reported that the fortification application of Saccharomyces cerevisiae with high ethyl caproate yield increased the ethyl caproate content (Li W. W. et al., 2020). The use of high-yielding ester Saccharomyces cerevisiae fortification of the production of the Daqu not only increased the ester content, and can reduce the alcohol and aldehyde content, the content of beneficial aroma components of the Daqu partially enhanced (Jiang, 2017). However, subsequent studies found that Saccharomyces cerevisiae was the most capable of producing ethanol through fermentation but produced relatively less volatile compounds, while Wickerhamomyces anomalus was relatively less efficient in fermentation but produced the greatest amount of volatile compounds (Pu et al., 2021). Therefore, Wickerhamomyces anomalus is used more often to enhance the quality of Baijiu Daqu and Baijiu. Hong et al. (2021) replaced the traditional Daqu with functional microbial fortification with different ester-producing yeasts as variables to simulate the solid-state fermentation of Special-flavor Baijiu. Wickerhamomyces anomalus fortification increased the ethanol content in the fermented grains to 3.67% v/v and volatile matter content to 136.02 mg/kg, and fortification did not change the dominant bacterial genus relative abundance of Lactobacillus. Xu et al. (2021) isolated and purified the aroma-producing Wickerhamomyces anomalus Y87 from Luzhou-flavor Baijiu Daqu. It was used to strengthen the production of Daqu and Baijiu-brewing. The content of ethyl acetate in Baijiu increased by 36.6%, the content of fusel alcohol decreased by 7.1%, and the content of n-propanol decreased by 16.1%. The Baijiu had the typical characteristics of Light-flavor Xiaoqu Baijiu, with a mellow and sweet entrance and pure fragrance. In addition, according to the production requirements, different yeasts can be combined in different proportions to strengthen together. Pu et al. (2021) reported that two yeast strains, Saccharomyces cerevisiae, and Wickerhamomyces anomalus, were used in mixed culture to produce fortified Daqu, and when a 2% inoculum consisting of a 1:2 (v/v) ratio was used, greatest fermentation, saccharification, liquefaction, and esterification capacities were obtained as well as high levels of volatile compounds, and when the produced Baijiu was compared with the unfortified control Daqu, significantly higher levels of flavor compounds were found after fortification. Meanwhile, some low-abundance yeasts play an important role in flavor production, such as Clavispora lusitaniae fortified with increased ethyl caproate content (Li W. W. et al., 2020). The strengthening of Candida Y18 increased the relative abundance of total alcohols and total esters in fermented grains. The relative abundance of ethanol, isoamyl acetate, and ethyl acetate increased to a certain extent, and the aroma components of isoborneol ethanol, isobutanol, methyl benzoate, 2-phenylethyl acetate, and phenyl caproate were metabolized (Xing et al., 2018).
5.2. Fortification of functional bacterial strains
The whole enzyme production process of bacteria will directly affect the fermentation quality of Daqu and the brewing quality of Baijiu and is usually reflected in its protease, amylase, esterification enzyme, and other different types of enzyme characteristics, and produce rich flavor substances and its precursors. Bacillus is a kind of aerobic or facultative anaerobic bacillus, which can produce spores. Bacillus is widely distributed in the process of Baijiu-brewing and has high diversity, such as Bacillus licheniformis, Bacillus amyloliquefaciens, Bacillus subtilis, Bacillus tarda, Paenibacillus, Bacillus cereus, Bacillus pumilus, Bacillus methylotrophicus, etc.
Fortification of Daqu has a positive effect on the structure of microbial communities and the content of aroma components and the storage period of Daqu (Yan, 2012; Li et al., 2021). The fortification of Bacillus licheniformis significantly affected the physicochemical properties, microbial community diversity, and volatile fractions of the Luzhou-flavor Baijiu Daqu, with pyrazine and alcohol contents increasing by 5074.49 and 440.50%, respectively, and the fortification of Maotai-flavor Baijiu Daqu helped highlight the stylistic characteristics of its (Hu, 2016; Chen X. et al., 2021). The combination of Bacillus licheniformis and Bacillus velezensis fortification altered the relative abundance of 22 genera in the four layers (surface, fire ring, middle, and core) of the Daqu, with an increase in the abundance of Bacillus and Aspergillus and a decrease in the abundance of Thermoascomycetes, Lactobacillus, and some other genera, and a significant increase in the esterification capacity of the fire ring and surface layer and an increase in the content of alcohols, acids, and ketones (except esters) after inoculation (Xu et al., 2022). High 4-ethyl guaiacol (4-EG) producing strain Bacillus subtilis D-31, and tetramethylpyrazine (TTMP) producing strain Bacillus velezensis M-14 fortification, respectively, showed that D-31 fortification showed an increase in pyrazines, TTMP, esters and 4-EG compared to the control Daqu by 117.00, 130.54, 175.34, and 266.20%, respectively, while the results of M-14 fortification were 89.26, 149.44, 355.63, and 501.40%, and the pyrazines, TTMP, esters, and 4-EG of the co-cultivated Daqu fortification of the two bacteria combinations increased by 22.60, 16.74, 83.46, and 323.79%, while the co-cultivation of the fortified Daqu increased the saccharification and liquefaction power, and the content of volatile components increased (Wu et al., 2017). Functional microbial fortification has certain bioturbation effects and interferes with enzyme-encoded gene expression. The enrichment of Bacillus velezensis and Bacillus subtilis increased the abundance of Bacillus, Lactobacillus, and Candida albicans, and significantly improved the liquefaction ability, saccharification ability, and esterification ability. In addition, many enzymes involved in saccharification, ethanol fermentation, and aroma formation were predicted, and the abundance of their coding genes was significantly increased in fortified Daqu (He G. et al., 2019). B. velezensis and B. subtilis were jointly fortified with Daqu applied to the fermentation of fermented grains in different spatial locations, with varying degrees of influence on the microbial community structure and volatile aroma substances of the fermented grains, and with a high content of Baijiu skeletal flavor components, mainly including important esters and aromatic compounds (He G. Q. et al., 2019). Overall, bioaugmentation of inoculated B. velezensis and B. subtilis altered the microbial community to regulate their metabolic activities.
5.3. Fortification of functional mold strains
Molds in Daqu can not only form hyphae to penetrate the entire Daqu embryo to ensure the smooth discharge of moisture so as to avoid rancidity of Daqu, but also give Daqu saccharification, liquefaction, protein decomposition, and other hydrolysis ability and esterification ability, the formation of Baijiu style played an important role. It has been reported that the fortification of Eurotium cristatum has different effects on the number of microbial groups (molds, yeasts, bacteria and Eurotium cristatum), conventional physicochemical indicators (cultivation temperature, moisture, acidity and starch content) and enzymatic activity (fermentation power, liquefaction enzyme activity and glycolytic enzyme activity; Fan B. et al., 2021). Mixed fermentation of Monascus, Aspergillus oryzae and Rhizopus increased Baijiu yield and enriched flavor substances (Ma, 2022). The contents of ethyl propionate and ethyl 2-methylbutyrate in Paecilomyces variotii PV3 fortified Daqu increased significantly (Deng, 2022).
However, there are few reports on the use of mold strengthening. The potential influencing factors may be that the strengthening of mold destroys the steady state of Daqu microecology or fermented grains microecology. First, excessive proliferation of mold often leads to significant substrate hardening, affecting the Daqu and fermented grains fermentation process (Zhang et al., 2021); Second, the growth of filamentous fungi will produce secondary metabolites that affect microbial growth, such as resulting in the inhibition of ethanol metabolism of yeast (Wei et al., 2021); Third, the fortification of mold leads to greater saccharification power and rapid accumulation of reducing sugar content, which inhibits the growth and metabolism of yeast and disrupts the balance of bilateral fermentation of Daqu and fermented grains, resulting in some adverse effects (Zhang et al., 2015).
5.4. Multi-strain fortification and synthesis microbiota
In order to further analyze the interrelationship between functional microorganisms in the original ecological site, and to deepen the understanding of functional microorganism utilization, the researchers also conducted an investigation experiment on the effect of multiple functional strains of combination fortification of the Daqu. The study showed that the combination of multiple functional microorganisms’ fortification had a small perturbation effect on the composition and succession of the Daqu microbiota, and there was no significant difference between the microbiota in traditional Daqu and bio-enhanced Daqu (Li et al., 2017).
In addition, multi-strain fortification does not significantly affect the fermentation process, and can also improve the quality of the Daqu and Baijiu. Zhou et al. (2013) comprehensively utilized dozens of beneficial functional microorganisms in a variety of ways to fortify the Daqu in Baijiu production. Compared with the traditional Daqu, the mycelium of the fortified Daqu is fuller, the flavor of the Daqu is more prominent and richer, and the temperature change of the cellar using the fortified Daqu is fully in line with the best state of before slow, medium strong, and after slow down. Zeng (2016) obtained the fortified Daqu with the mixed ratio of 18: 6: 1 of functional microbial bacteria, molds and yeasts in the Daqu of Maotai-flavor Baijiu. The skin of the fortified Daqu was grayish white with brown color, the aroma was prominent, the section was covered with white spotted hyphae, and there was no foreign matter. The workshop application test showed that the Maotai-flavor of Baijiu was more prominent, the burnt aroma and paste aroma were more coordinated, the entrance was relatively smooth, the Baijiu body was full, the aftertaste was longer and slightly astringent, and the quality of the seventh round of Baijiu was improved. Liu et al. (2019) strengthened Maotai-flavor Daqu by optimizing the combination of 5 strains of functional microorganisms, and applied it to the production of Maotai-flavor Baijiu. The results showed that the flavor of Maotai-flavor Daqu was strong by adding an appropriate amount of functional microorganisms, and the flavor of Maotai-flavor Baijiu, flavor retention in empty cups, fineness and typicality were significantly improved. Wang (2017) constructed a composite functional microbial agent with Schizosaccharomyces pombe: Issatchenkia orientalis: Bacillus licheniformis + Rhizopus (106: 105: 105 + 1%), which was added to different months of Daqu or replaced different proportions of 4-month Daqu. The results showed that this group of functional microbial agents had the effect of increasing the yield of fermented ethanol and reducing the content of n-propanol, and had the function of improving the yield and quality of Baijiu.
As mentioned before (section 5.3) filamentous fungi have some instability in single fungal fortification applications, similarly in multi-strain combination fortification the growth characteristics of the fungus and the interactions with other microorganisms should be fully considered. Huo (2019) used filamentous fungus FBKL 3.0009, Pichia FBKL 2.0008 and Bacillus licheniformis FBKL 1.0199 to make fortified Daqu. Compared with the fortified Daqu without adding mold, the saccharifying power of the fortified Daqu was improved, and the saccharifying power of the fortified Daqu with more mold was significantly improved. This indicated that the addition of mold promoted the saccharifying power of the fortified Daqu, but the addition of mold inhibited the production of pyrazines.
Currently, the conversion of traditional fermented foods, including bread, wine, chocolate, pickles and wine from natural fermentation to controlled synthetic microbial fermentation is essential to achieve production stability of fermented foods. Compared with the above-mentioned traditional fermented foods, the research on synthetic microbiota in the brewing process of Baijiu is in its initial stage. On the one hand, the composition of the core functional microbiota in the Baijiu brewing process is not fully understood, the mechanism of microbial interactions is still not completely clear, and there are still some technical difficulties that need to be broken through as to how the synthetic microbiota can best fit the functions and roles of the Daqu microflora. On the other hand, synthetic microbiota replaces the role of Daqu (Daqu provides microorganisms, enzymes and substances for Baijiu fermentation), which is a newly emerged technology compared with traditional Baijiu fermentation, and innovates the Baijiu brewing process, but there is still a certain distance in a production application. Wang S. L. et al. (2019) used the fermentation of Chinese Light-flavor (Fen-flavor) Baijiu as a model system and used Lactobacillus, Saccharomyces, Pichia, Geotrichum, and Candida to construct a synthetic microbial community instead of Daqu as a starter. The synthetic core community produced 77.27% of the flavor compounds and showed a dynamic distribution similar to the natural Baijiu fermentation process.
In summary, the purposeful application of functional strains to Daqu fermentation can improve the performance of Daqu and improve Daqu flavor substances, and application to brewing production can increase the Baijiu yield and improve Baijiu quality. Based on the simplified synthetic microbiota applied to Baijiu brewing can achieve the reproduction of flavor metabolism to a certain extent.
6. Conclusion and prospect
The production process of Chinese Baijiu is extensive and multi-bacteria and multi-enzyme mixed fermentation. In addition to the application of the strains introduced above to the fortification and production of Daqu, there are still many strains that can be isolated and cultured and many strains that have not been able to be isolated in the fermentation process of Baijiu. It plays a metabolic regulation and other important roles, but the mechanism and conditions of action have not been fully explained due to the limitations of existing methods and research methods, which is not conducive to the stability of Baijiu quality and flavor regulation. Therefore, the core microflora formed by flavor compounds and the key factors affecting the core microflora needs to be further explored and explored. In addition, the culturability of uncultured functional microorganisms based on omics technology is an urgent problem to be solved in further research.
Daqu fermentation has strong environmental dependence, and there are many kinds of microorganisms. Different flavors of Baijiu have regional differences and brewing process differences. At the same time, a different flavor of Baijiu has different requirements for the flavor characteristics of the final Baijiu. Therefore, it is necessary to accurately determine the core functional microorganisms in the fermentation process of Daqu and fermented grains. Based on microbial analysis technology and metabolomics, the microbial community structure, succession law, and metabolic characteristics of the Daqu fermentation process were analyzed, and the research route of core microorganisms was determined by statistical analysis. It proved to be feasible. Although strains with high alcohol, enzyme, and specific flavor substances can be explored, Daqu fermentation and fermented grains fermentation are highly complex microecological interaction systems. Whether the fortified functional strains can have certain abundance and advantages in the fermentation system, whether they can maintain their high alcohol, enzyme, and aroma characteristics, and whether they can improve the quality of Daqu and Baijiu still need to be effectively verified.
Using functional strains to make fortified Daqu is to use its functional characteristics to change the quality of Daqu fermentation. The essence is that the proportion of initial microbial community inoculated with Daqu has changed, which in turn changes the interaction of microbial succession. Therefore, before fortifying functional microorganisms, it is necessary to further clarify the characteristics of interactions between microorganisms during Daqu fermentation.
On the basis of strengthened applied research the designed synthetic flora can be further utilized for flavor regulation and control in Daqu fermentation and fermented grains fermentation. However, in the future, there is a need to regulate the synthetic microbiota from various aspects such as community composition, community regulation, and metabolite regulation, which in turn will lead to the control and standardization of Baijiu quality.
Author contributions
HL: conceptualization, investigation, visualization, writing-original draft, and writing-review and editing. SL: writing-review and editing. YL: writing-review and editing, investigation, resources, and supervision. MH: writing-review and editing, investigation, resources, and supervision. CP: conceptualization, visualization, writing-review and editing, resources, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work supported by the Key Technologies Research and Development Program of Henan Province of China (No. 202102110130), Major Science and Technology Projects of Henan Province of China (No. 181100211400), and Food Science and Engineering Key Discipline Construction Project of Henan University of Animal Husbandry and Economy (No. XJXK202203).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, X., Qiu, S., Lei, A., Chen, M., Huang, W., and Wang, X. (2017). Screening of polyhydric alcohol-producing yeasts from Moutai-flavor baijiu during brewing. China Brewing 36, 58–62. https://kns.cnki.net/kcms/detail/11.1818.TS.20170523.1729.024.html
Ban, S. B., Chen, L. N., Fu, S. X., Wu, Q., and Xu, Y. (2022). Modelling and predicting population of core fungi through processing parameters in spontaneous starter (Daqu) fermentation. Int. J. Food Microbiol. 363:109493. doi: 10.1016/j.ijfoodmicro.2021.109493
Barooah, M., Joshi, S., and Bahar, B. (2022). Editorial: genomics and metabolomics of microbes in fermented food. Front. Microbiol. 13:2726. doi: 10.3389/fmicb.2022.892726
Cai, W. C., Wang, Y. R., Ni, H., Liu, Z. J., Liu, J. M., Zhong, J. A., et al. (2021). Diversity of microbiota, microbial functions, and flavor in different types of low-temperature Daqu. Food Res. Int. 150:110734. doi: 10.1016/j.foodres.2021.110734
Chen, Y., Cai, W., Chen, Y., Hong, L., Wu, X., Huang, B., et al. (2021). Study on screening of yeasts and perfuming performance in Daqu of special-flavor baijiu. J. Nanchang Univ. 45, 351–357. doi: 10.13764/j.cnki.ncdl.2021.04.005
Chen, X., Huang, J., Zhou, R., Zhang, S., Dong, Y., Wang, C., et al. (2021). Effects of fortifying patterns on the characteristics of strong flavor type Daqu. Food Ferm. Ind. 47, 50–55. doi: 10.13995/j.cnki.11-1802/ts.026190
Chen, B., Wu, Q., and Xu, Y. (2014). Filamentous fungal diversity and community structure associated with the solid state fermentation of Chinese Maotai-flavor liquor. Int. J. Food Microbiol. 179, 80–84. doi: 10.1016/j.ijfoodmicro.2014.03.011
Chen, C., Yang, H., Liu, J., Luo, H., and Zou, W. (2022). Systematic review of Actinomycetes in the baijiu fermentation microbiome. Foods 11:3551. doi: 10.3390/foods11223551
Deng, C. (2022). Characteristics of high-temperature Daqu with different grades for Nongxiang-Jiangxiang baijiu and the function of dominant thermophilic fungi. Wuhan (Hubei): Wuhan Polytechnic University.
Deng, Y. K., Huang, D., Han, B. L., Ning, X. Q., Yu, D., Guo, H. X., et al. (2021). Correlation: between autochthonous microbial diversity and volatile metabolites during the fermentation of Nongxiang Daqu. Front. Microbiol. 12:8981. doi: 10.3389/fmicb.2021.688981
Dong, Q., Pu, S., Liu, F., and Sun, Z. (2020). Screening, identification and fermentation characteristics of functional yeast from strong-flavor Daqu. China Brewing 39, 106–110. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNGZ202006021&DbName=CJFQ2020
Du, H., Ji, M., Xing, M. Y., Wang, X. S., and Xu, Y. (2021). The effects of dynamic bacterial succession on the flavor metabolites during baijiu fermentation. Food Res. Int. 140:109860. doi: 10.1016/j.foodres.2020.109860
Du, R., Yan, R., Wu, Q., and Xu, Y. (2021). The ecological fermentation technology: principle and its applications. Food Ferm. Ind. 47, 266–275. doi: 10.13995/j.cnki.11-1802/ts.025292
Fan, G. S., Cheng, L. J., Fu, Z. L., Sun, B. G., Teng, C., Jiang, X. Y., et al. (2020). Screening of yeasts isolated from baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech 10:275. doi: 10.1007/s13205-020-02267-5
Fan, Y., Huang, X. N., Chen, J. Y., and Han, B. Z. (2020). Formation of a mixed-species biofilm is a survival strategy for Unculturable lactic acid bacteria and Saccharomyces cerevisiae in Daqu, a Chinese traditional fermentation starter. Front. Microbiol. 11:138. doi: 10.3389/fmicb.2020.00138
Fan, G. S., Liu, P. X., Chang, X., Yin, H., Cheng, L. J., Teng, C., et al. (2021). Isolation and identification of a high-yield ethyl Caproate-producing yeast from Daqu and optimization of its fermentation. Front. Microbiol. 12:3744. doi: 10.3389/fmicb.2021.663744
Fan, G. S., Sun, B. G., Xu, D., Teng, C., Fu, Z. L., Du, Y. H., et al. (2018). Isolation and ldentifation of nigh-yield ethyl acetate-producing yeast from Gujinggony Daqu and its fermentation Charadecistics. J. Am. Soc. Brew. Chem. 76, 117–124. doi: 10.1080/03610470.2017.1396849
Fan, B., Wu, H., Yu, Y., Liu, A., and Xiong, X. (2021). The effect of dark tea fungus on the quality of wrapped starter culture process. Food Mach. 37, 27–37. doi: 10.13652/j.issn.1003-5788.2021.05.006
Fu, J. X., Chen, L., Yang, S. Z., Li, Y. Z., Jin, L., He, X. P., et al. (2021). Metagenome and analysis of metabolic potential of the microbial community in pit mud used for Chinese strong-flavor liquor production. Food Res. Int. 143:110294. doi: 10.1016/j.foodres.2021.110294
Fu, Z. L., Sun, B. G., Li, X. T., Fan, G. S., Teng, C., Alaa, A., et al. (2018). Isolation and characterization of a high ethyl acetate-producing yeast from Laobaigan Daqu and its fermentation conditions for producing high-quality baijiu. Biotechnol. Biotechnol. Equip. 32, 1218–1227. doi: 10.1080/13102818.2018.1492355
He, G. Q., Dong, Y., Huang, J., Wang, X. J., Zhang, S. Y., Wu, C. D., et al. (2019). Alteration of microbial community for improving flavor character of Daqu by inoculation with bacillus velezensis and Bacillus subtilis. Lebensm. Wiss. Technol. 111, 1–8. doi: 10.1016/j.lwt.2019.04.098
He, G., Huang, J., Zhou, R., Wu, C., and Jin, Y. (2019). Effect of fortified Daqu on the microbial community and flavor in Chinese strong-flavor liquor brewing process. Front. Microbiol. 10:56. doi: 10.3389/fmicb.2019.00056
Hong, L. X., Fu, G. M., Liu, T., Chen, Y. R., Wu, S. W., Cai, W. Q., et al. (2021). Functional microbial agents enhance ethanol contents and regulate the volatile compounds in Chinese baijiu. Food Biosci. 44:101411. doi: 10.1016/j.fbio.2021.101411
Hou, X., Sun, Z., Li, X., Chu, C., Pan, Q., Chen, H., et al. (2019). Lsolation, identification and enzyme-production of culturable Actinomycetes from Daqu applied in making strong-flavor liquor. Food Sci. Technol. 44, 28–35. doi: 10.13684/j.cnki.spkj.2019.12.006
Hou, Q. C., Wang, Y. R., Cai, W. C., Ni, H., Zhao, H. J., Zhang, Z. D., et al. (2022). Metagenomic and physicochemical analyses reveal microbial community and functional differences between three types of low-temperature Daqu. Food Res. Int. 156:111167. doi: 10.1016/j.foodres.2022.111167
Hu, B. (2016). Study on strengthening and characteristics of Maotai-flavor Daqu. MA, Guizhou University.
Huang, D., Shang, Z. C., Li, Y. L., Chu, Y. L., and Yuan, X. L. (2012). Isolation of Rhizopus producing esterifying enzyme and study on its enzyme production conditions. Adv. Mater. Res. 550, 1229–1232.
Huo, Y. (2019). Preliminary study on Maotai-flavor strengthened wheat Qu. Guiyang (Guizhou): Guizhou University.
Jiang, P. (2017). Production and application of a fragrant high Ester high temperature strengthened Daqu. Nanyang (Henan): Nanyang normal university.
Jiang, S., Qiu, S., Zou, J., Chen, L., Luo, X., and Wang, X. (2017). Preliminary studies on yeasts from sauce-flavor Daqu made by traditional and mechanical methods. China Brewing 36, 59–65. https://kns.cnki.net/kcms/detail/11.1818.TS.20170328.0909.026.html
Kang, J. M., Xue, Y. S., Chen, X. X., and Han, B. Z. (2022). Integrated multi-omics approaches to understand microbiome assembly in Jiuqu, a mixed-culture starter. Compr. Rev. Food Sci. Food Saf. 21, 4076–4107. doi: 10.1111/1541-4337.13025
Ke, T., Zhou, Y., Jiang, P., Li, J., and Ma, X. (2019). Production process and characteristics of medium and high temperature fortified Daqu. China Brewing 38, 47–52. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNGZ201902011&DbName=CJFQ2019
Kong, Y., Wu, Q., Zhang, Y., and Xu, Y. (2014). In situ analysis of metabolic characteristics reveals the key yeast in the spontaneous and solid-state fermentation process of Chinese light-style liquor. Appl. Environ. Microbiol. 80, 3667–3676. doi: 10.1128/AEM.04219-13
Lei, X., Zhao, D., Zheng, J., Qiao, Z., Liu, D., Zhang, X., et al. (2022). Lsolation and identification of the cultivable molds in the air of Baijiu-making environment of Wuliangye and the initial study of their enzyme producing ability. Food Ferm. Ind. 10:892. doi: 10.13995/j.cnki.11-1802/ts.030892
Li, W. W., Fan, G. S., Fu, Z. L., Wang, W. H., Xu, Y. Q., Teng, C., et al. (2020). Effects of fortification of Daqu with various yeasts on microbial community structure and flavor metabolism. Food Res. Int. 129:108837. doi: 10.1016/j.foodres.2019.108837
Li, D., Huang, W., Wang, X., Luo, X., and Qiu, S. (2018). Ldentification and flavor profile of a Thermoactinomycetaceae strain separated from Moutai-flavor Daqu. Food Sci. 39, 171–176. https://kns.cnki.net/kcms/detail/11.2206.TS.20170628.1637.174.html
Li, Z., Huang, D., Zhang, M., Guo, H., Hu, J., Luo, Z., et al. (2020). Isolation and ldentification of two heat-resistant molds in Daqu at medium-high temperature and comparison of their enzyme production characteristics. Food Ferm. Sci. Technol. 56, 1–7. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SKSF202003001&DbName=CJFQ2020
Li, P., Lin, W. F., Liu, X., Wang, X. W., Gan, X., Luo, L. X., et al. (2017). Effect of bioaugmented inoculation on microbiota dynamics during solid-state fermentation of Daqu starter using autochthonous of bacillus, Pediococcus, Wickerhamomyces and Saccharomycopsis. Food Microbiol. 61, 83–92. doi: 10.1016/j.fm.2016.09.004
Li, Y., Yang, Y., Zhang, L., Tan, H., Jiang, L., Guo, F., et al. (2019). Microbial growth/decline rules in the fermentation process of medium/high-temperature Daqu. Liquor-Making Sci. Technol. 5, 89–93. doi: 10.13746/j.njkj.2018322
Li, J., Zhang, Y., Wang, R., Li, D., Zhang, Y., Du, M., et al. (2021). Separation of Mold and analysis of enzyme production characteristics in sesame-flavor Liquor's yeast. Food Sci. Technol. 46, 33–37. doi: 10.13684/j.cnki.spkj.2021.05.006
Li, R. Y., Zheng, X. W., Zhang, X., Yan, Z., Wang, X. Y., and Han, B. Z. (2018). Characterization of bacteria and yeasts isolated from traditional fermentation starter (fen-Daqu) through a H-1 NMR-based metabolomics approach. Food Microbiol. 76, 11–20. doi: 10.1016/j.fm.2018.03.015
Liu, J. J., Chen, J. Y., Fan, Y., Huang, X. N., and Han, B. Z. (2018). Biochemical characterization and dominance of different hydrolases in different types of Daqu - a Chinese industrial fermentation starter. J. Sci. Food Agric. 98, 113–121. doi: 10.1002/jsfa.8445
Liu, Y. B., Fu, J. Y., Wang, L. L., Zhao, Z. J., Wang, H. H., Han, S. N., et al. (2022). Isolation, identification, and whole-genome sequencing of high-yield protease bacteria from Daqu of ZhangGong Laojiu. PLoS One 17:e0264677. doi: 10.1371/journal.pone.0264677
Liu, Y., Guan, G., Wan, Z., Xia, P., Du, R., and Wu, Q. (2019). Application of functional microorganisms in the production of Jiangxiang baijiu. Liquor-Making Sci. Technol. 12:98-104+118. doi: 10.13746/j.njkj.2019216
Liu, X., Li, H., Zhou, L., Yuan, Y., Li, B., Wang, X., et al. (2022). Isolation, screening and identification of aroma-producing bacteria in light-flavor baijiu Daqu. China Brewing 41, 96–100. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNGZ202205017&DbName=CJFQ2022
Liu, Y., Tang, Y., Zhao, Z., Hou, W., Sun, X., and Pan, C. (2021). Isolation and screening of high-yield esterase enzyme strains from Zhanggong Laojiu Daqu and optimization of the enzyme production conditions. J. Gansu Agric. Univ. 56, 150–159. doi: 10.13432/j.cnki.jgsau.2021.02.019
Liu, B., Zhou, K., Ye, L., Yu, Y., Li, X., Yan, P., et al. (2020). Identification and flavor analysis of an aroma-producing bacterium isolated from fermented grains of strong flavor baijiu in Songhe town. Food Sci. 41, 105–112. https://kns.cnki.net/kcms/detail/11.2206.TS.20191220.1413.056.html
Liu, M., Zhou, Y., Yuan, L., Liu, X., and Bian, M. (2018). The selection of high yield glucoamylase starter and the optimization of its solid fermentation production enzyme. Food Ferm. Ind. 44, 118–123. doi: 10.13995/j.cnki.11-1802/ts.017311
Luo, X., Qiu, S., Lu, A., Wang, X., and Chen, M. (2016). Isolation and identification of aroma-producing yeast in Moutai-flavor Daqu. Food Ferm. Indus. 42, 26–31. doi: 10.13995/j.cnki.11-1802/ts.201612005
Luo, X., Wang, X., and Qiu, S. (2018). Isolation, screening and aroma component analysis of 3 strains of actinomycetes from Moutai-flavor Daqu. China Brewing 37, 62–67. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNGZ201808016&DbName=CJFQ2018
Luo, H. B., Wei, C. H., and Yuan, X. L. (2012). Study on the metabolites of a bacteria strain producing esterifying Synthetase. Adv. Mater. Res. 550, 1080–1084.
Ma, P. (2022). Screening of molds with high esterification power, Saccharification power and liquefaction power and research on strengthening Daqu. Alaer (Xinjiang): Tarim University.
Ma, S. Y., Shang, Z. C., Chen, J., Shen, Y. J., Li, Z. J., Huang, D., et al. (2022). Differences in structure, volatile metabolites, and functions of microbial communities in Nongxiangxing daqu from different production areas. Lebensm. Wiss. Technol. 166:113784. doi: 10.1016/j.lwt.2022.113784
Ming, H., Guo, Z., Zhou, J., Chen, M., Xu, D., and Yao, X. (2015). Screening and ldentification of aroma-producing microorganisms in Luzhou-flavor Daqu. Mod. Food Sci. Technolo. 31, 186–191. doi: 10.13982/j.mfst.1673-9078.2015.4.030
Pan, Y. Y., Wang, Y., Hao, W. J., Duan, C. B., Wang, S. Y., Wei, J. W., et al. (2022). Metatranscriptomics unravel composition, drivers, and functions of the active microorganisms in light-flavor liquor fermentation. Microbiol. Spectr. 10:e0215121. doi: 10.1128/spectrum.02151-21
Pu, S. C., Zhang, Y., Lu, N., Shi, C. E., and Yan, S. B. (2021). Yeasts from Chinese strong flavor Daqu samples: isolation and evaluation of their potential for fortified Daqu production. AMB Express 11:176. doi: 10.1186/s13568-021-01337-y
Shen, T., Wu, Q., and Xu, Y. (2021). Biodegradation of cyanide with Saccharomyces cerevisiae in baijiu fermentation. Food Control 127:108107. doi: 10.1016/j.foodcont.2021.108107
Shi, W., Chai, L. J., Fang, G. Y., Mei, J. L., Lu, Z. M., Zhang, X. J., et al. (2022). Spatial heterogeneity of the microbiome and metabolome profiles of high-temperature Daqu in the same workshop. Food Res. Int. 156:111298. doi: 10.1016/j.foodres.2022.111298
Song, Z. W., Du, H., Zhang, Y., and Xu, Y. (2017). Unraveling Core functional microbiota in traditional solid-state fermentation by high-throughput amplicons and Metatranscriptomics sequencing. Front. Microbiol. 8:1294. doi: 10.3389/fmicb.2017.01294
Tang, J., Liu, L., Long, Y., Meng, Z., Wu, D., and Zhang, C. (2022). Physicochemical characteristics and fungal community structure of different colored Jiang-flavor Daqu. Food Sci. 43, 193–198. https://kns.cnki.net/kcms/detail/11.2206.TS.20211109.0839.008.html
Wang, S. (2017). Screening of functional bacillus producing Maotai-flavor liquor and study on compound functional intensified starter culture. Wuhan (Hubei): Wuhan Polytechnic University.
Wang, D. Q., Chen, L. Q., Yang, F., Wang, H. Y., and Wang, L. (2019). Yeasts and their importance to the flavor of traditional Chinese liquor: a review. J. Inst. Brew. 125, 214–221. doi: 10.1002/jib.552
Wang, F., Li, J., Zhao, Y., Tang, J., Liu, Z., and Peng, X. (2022). Screening of high-temperature actinomycetes in sauce-flavor Daqu and optimization of protease-producing conditions. China Brewing 41, 132–136. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNGZ202208022&DbName=CJFQ2022
Wang, X., Luo, X., and Qiu, S. (2018). Isolation and screening of a thermophilic actinomycete from Moutai-flavor Daqu and its characterization. China Brewing 37, 51–56. https://kns.cnki.net/kcms/detail/11.1818.TS.20180514.1616.020.html
Wang, P., Wu, Q., Jiang, X. J., Wang, Z. Q., Tang, J. L., and Xu, Y. (2017). Bacillus licheniformis affects the microbial community and metabolic profile in the spontaneous fermentation of Daqu starter for Chinese liquor making. Int. J. Food Microbiol. 250, 59–67. doi: 10.1016/j.ijfoodmicro.2017.03.010
Wang, S. L., Wu, Q., Nie, Y., Wu, J. F., and Xu, Y. (2019). Construction of synthetic microbiota for reproducible flavor compound metabolism in Chinese light-aroma-type liquor produced by solid-state fermentation. Appl. Environ. Microbiol. 85:18. doi: 10.1128/AEM.03090-18
Wang, B. W., Wu, Q., Xu, Y., and Sun, B. G. (2018). Specific volumetric weight-driven shift in microbiota compositions with Saccharifying activity change in starter for Chinese baijiu fermentation. Front. Microbiol. 9:2349. doi: 10.3389/fmicb.2018.02349
Wang, X., Xu, J., Zhou, H., Ban, S., and Qiu, S. (2016). Characterization of Eurotium cristatus lsolated from Maotai-flavored Daqu, a traditional Chinese Maotai-flavored liquor fermentation starter, for its abilities to produce enzymes and aromas. Food Sci. 37, 154–159. https://kns.cnki.net/kcms/detail/11.2206.ts.20160415.1533.012.html
Wei, J., Du, H., Zhang, H., Nie, Y., and Xu, Y. (2021). Mannitol and erythritol reduce the ethanol yield during Chinese baijiu production. Int. J. Food Microbiol. 337:108933. doi: 10.1016/j.ijfoodmicro.2020.108933
Wu, Q., Huang, J., Jiang, D., Zhou, R., Qiu, X., Wu, C., et al. (2017). Exploring the improvement of contents of pyrazines and 4-EG in Luzhou-flavor Daqu based on fortifying by co-culture. Food Sci. Technol. 42, 2–6. doi: 10.13684/j.cnki.spkj.2017.05.002
Wu, Z. Y., Yang, S. Q., Xu, L., Li, H. H., Sun, J. Y., Xu, Y. Q., et al. (2021). Screening and identifying microorganisms with feruloyl esterase activity in Chinese sesame-flavor Baijiu fermentation materials (Jiupei). J. Food Compost. Anal. 102:104069. doi: 10.1016/j.jfca.2021.104069
Wu, S., Yang, L., Yang, L., Yu, H., and Huang, Z. (2018). Isolation, identification and metabolite analysis of aroma-producing bacillus from fermented grains of Chen-xiang flavor baijiu. China Brewing 37, 35–40. https://kns.cnki.net/kcms/detail/11.1818.TS.20180209.1106.016.html
Xia, Y., Zhu, M., Du, Y. K., Wu, Z. Y., Gomi, K., and Zhang, W. X. (2022). Metaproteomics reveals protein composition of multiple saccharifying enzymes in nongxiangxing daqu and jiangxiangxing daqu under different thermophilic temperatures. Int. J. Food Sci. Technol. 57, 5102–5113. doi: 10.1111/ijfs.15818
Xiao, C., Yang, Y., Lu, Z. M., Chai, L. J., Zhang, X. J., Wang, S. T., et al. (2021). Daqu microbiota exhibits species-specific and periodic succession features in Chinese Baijiu fermentation process. Food Microbiol. 98:103766. doi: 10.1016/j.fm.2021.103766
Xing, X. Y., Wang, Y. H., Huo, N. R., and Wang, R. F. (2018). Candida ethanolica strain Y18 enhances aroma of Shanxi aged-vinegar. Food Sci. Technol. Res. 24, 1069–1081. doi: 10.3136/fstr.24.1069
Xu, Y. Q., Sun, B. G., Fan, G. S., Teng, C., Xiong, K., Zhu, Y. P., et al. (2017). The brewing process and microbial diversity of strong flavor Chinese spirits: a review. J. Inst. Brew. 123, 5–12. doi: 10.1002/jib.404
Xu, B. Y., Xu, S. S., Cai, J., Sun, W., Mu, D. D., Wu, X. F., et al. (2022). Analysis of the microbial community and the metabolic profile in medium-temperature Daqu after inoculation with bacillus licheniformis and bacillus velezensis. Lebensm. Wiss. Technol. 160:113214. doi: 10.1016/j.lwt.2022.113214
Xu, Y., Yang, Q., Zhang, L., Chen, J., Liu, Y., Chen, K., et al. (2021). Screening of high-yield ethyl acetate yeast and its application in the light-flavor Xiaoqu baijiu production. China Brewing 40, 76–80. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNGZ202108014&DbName=CJFQ2021
Yan, C. (2012). Analysis of bacterial community structure in high temperature Daqu and breeding of wine-making functional bacteria and study on strengthening Daqu. [master’s thesis]. Fuzhou (Fujian): Fujian Normal University.
Yang, Y., Wang, S. T., Lu, Z. M., Zhang, X. J., Chai, L. J., Shen, C. H., et al. (2021). Metagenomics unveils microbial roles involved in metabolic network of flavor development in medium-temperature daqu starter. Food Res. Int. 140:110037. doi: 10.1016/j.foodres.2020.110037
Ye, G. B., Hong, M. H., Huang, D., and Yang, X. D. (2013). Identification and primary application of two high-yield Glucoamylase Moulds from Luzhou-flavor Daqu. Adv. Mater. Res. 781, 1825–1829.
Yu, H., Huang, D., Chen, Z., Tang, J., Mao, X., Deng, L., et al. (2017). Lsolation of protease-producing actinomycetes from sauce-flavor Daqu and its protease-producing conditions. China Brewing 36, 64–68. https://kns.cnki.net/kcms/detail/11.1818.TS.20170313.1314.028.html
Zeng, D. (2016). Study on optimized combination and application of functional microorganisms in Maotai-flavor liquor production. [master’s thesis]. Guiyang (Guizhou): Guizhou university.
Zhang, R. (2009). Screening of bacteria producing soy sauce flavor and study of characteristic volatile compounds. [master’s thesis]. Wuxi (Jiangsu): Jiangnan University.
Zhang, H., Du, H., and Xu, Y. (2021). Volatile organic compound-mediated antifungal activity of Pichia spp. and its effect on the metabolic profiles of fermentation communities. Appl. Environ. Microbiol. 87, e02992–e02920. doi: 10.1128/AEM.02992-20
Zhang, J., Liu, S. P., Sun, H. L., Jiang, Z. F., Xu, Y. Z., Mao, J. Q., et al. (2022). Metagenomics-based insights into the microbial community profiling and flavor development potentiality of Baijiu Daqu and huangjiu wheat Qu. Food Res. Int. 152:110707. doi: 10.1016/j.foodres.2021.110707
Zhang, C., Pu, C., Bai, C., Tang, J., Long, Y., Li, Z., et al. (2022). Screening and ldentification of Moutai flavor producing bacteria from high-temperature Daqu and lts aroma producing characteristics. Food Sci. Technol. 47, 1–6. doi: 10.13684/j.cnki.spkj.2022.05.046
Zhang, Q., Wu, D. Y., Lin, Y., Wang, X. Z., Kong, H. N., and Tanaka, S. (2015). Substrate and product inhibition on yeast performance in ethanol fermentation. Energy Fuel 29, 1019–1027. doi: 10.1021/ef502349v
Zhang, Y. D., Xu, J. G., Ding, F., Deng, W. Y., Wang, X., Xue, Y. S., et al. (2022). Multidimensional profiling indicates the shifts and functionality of wheat-origin microbiota during high-temperature Daqu incubation. Food Res. Int. 156:111191. doi: 10.1016/j.foodres.2022.111191
Zhao, C. Q., Yan, X. L., Yang, S. T., and Chen, F. F. (2017). Screening of bacillus strains from Luzhou-flavor liquor making for high-yield ethyl hexanoate and low-yield propanol. Lebensm. Wiss. Technol. 77, 60–66. doi: 10.1016/j.lwt.2016.11.035
Zheng, X. W., Tabrizi, M. R., Nout, M. J. R., and Han, B. Z. (2011). Daqu - a traditional Chinese liquor fermentation starter. J. Inst. Brew. 117, 82–90. doi: 10.1002/j.2050-0416.2011.tb00447.x
Zheng, X. W., Yan, Z., Nout, M. J. R., Boekhout, T., Han, B. Z., Zwietering, M. H., et al. (2015). Characterization of the microbial community in different types of Daqu samples as revealed by 16S rRNA and 26S rRNA gene clone libraries. World J. Microbiol. Biotechnol. 31, 199–208. doi: 10.1007/s11274-014-1776-z
Zheng, J., Zhao, D., Peng, Z. F., Yang, K. Z., Zhang, Q., and Zhang, Y. K. (2018). Variation of aroma profile in fermentation process of Wuliangye baobaoqu starter. Food Res. Int. 114, 64–71. doi: 10.1016/j.foodres.2018.07.060
Zhou, X., Chen, X., Li, H., Gan, G., Zhang, L., and Jiang, L. (2013). The research and application of beneficial microorganisms in the production of liquor. Liquor Making 40, 33–40. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=NJZZ201305012&DbName=CJFQ2013
Zhou, Q., Ma, K., Song, Y., Wang, Z., Fu, Z., Wang, Y., et al. (2022). Exploring the diversity of the fungal community in Chinese traditional baijiu daqu starters made at low-, medium-and high-temperatures. Lebensm. Wiss. Technol. 162:113408. doi: 10.1016/j.lwt.2022.113408
Zhou, W., Xia, Y., Zhao, Y. J., Wang, Y., Wu, Z. Y., Suyama, T., et al. (2022). Study on the effect of key genes ME2 and adhE during Luzhou-flavor baijiu brewing. Foods 11:700. doi: 10.3390/foods11050700
Zhu, Q., Chen, L. Q., Peng, Z., Zhang, Q. L., Huang, W. Q., Yang, F., et al. (2022). Analysis of environmental driving factors on Core functional community during Daqu fermentation. Food Res. Int. 157:111286. doi: 10.1016/j.foodres.2022.111286
Zhu, L., Huang, Z., Wei, C., Deng, J., and Xie, J. (2019). Screening of aroma-producing Mold from Jiuqu and optimization of culture conditions. Food Res Dev 40, 193–197. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SPYK201910037&DbName=CJFQ2019
Zou, W., Zhao, C. Q., and Luo, H. B. (2018). Diversity and function of microbial Community in Chinese Strong-Flavor Baijiu Ecosystem: a review. Front. Microbiol. 9:671. doi: 10.3389/fmicb.2018.00671
Keywords: Daqu, Baijiu, functional microorganisms, microbiomics, fortification and regulation
Citation: Li H, Liu S, Liu Y, Hui M and Pan C (2023) Functional microorganisms in Baijiu Daqu: Research progress and fortification strategy for application. Front. Microbiol. 14:1119675. doi: 10.3389/fmicb.2023.1119675
Edited by:
Huaxi Yi, Ocean University of China, ChinaReviewed by:
Mingquan Huang, Beijing Technology and Business University, ChinaZheng-Hong Xu, Jiangnan University, China
Copyright © 2023 Li, Liu, Liu, Hui and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunmei Pan, c2lnZTUxODg4OEAxNjMuY29t
 Haideng Li1,2
Haideng Li1,2 Yanbo Liu
Yanbo Liu