- 1College of Tropical Crops, Hainan University, Haikou, China
- 2State Key Laboratory of Black Soils Conservation and Utilization, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China
- 3Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China
Plant uptake of nitrogen is often associated with increased incidence of banana Fusarium wilt, a disease caused by the soil-borne fungus Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). However, the nitrogen metabolic preferences of Foc TR4 pathogens remain unknown. In this study, we investigated the ecophysiological patterns of Foc TR4 grown on different combinations of organic and inorganic nitrogen. Potato Dextrose Agar (PDA) and Rose Bengal Medium (RBM) were used as an organic nitrogen source, which was sequentially replaced with inorganic N (0, 50% or 90%) in the form 15NH4NO3 or NH415NO3 to reveal preferential assimilation of ammonium or nitrate. The results showed that mycelium biomass and nitrogen content decreased significantly, while the carbon content and C:N ratio increased in Foc TR4 grown on media containing inorganic nitrogen sources. Mycelium biomass was negatively correlated with C:N ratio. Mycelium 15N abundance increased significantly between the PDA50 + A50/RBM50 + A50 treatments (50% organic nitrogen+50%15NH4NO3) and the PDA10 + A90/RBM10 + A90 treatments (10% organic nitrogen+90%15NH4NO3). These results indicate that the higher C:N ratio reduced mycelium growth by reducing its biomass and diameter and showed that Foc TR4 preferred to use ammonium nitrogen to promote the growth. These findings suggest that treating banana crops with a combination of organic and inorganic (i.e., nitrate) nitrogen could be a better way to defend against Fusarium wilt of banana.
1. Introduction
Fusarium wilt of banana (FWB), induced by the soil-borne fungus Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4), is a serious disease affecting bananas that damages the vascular system and induces cell necrosis to reduce crop yield (Butler, 2013). Foc TR4 is highly pathogenic and can infect almost species of banana (Dita et al., 2018). The overuse of chemical nutrients, especially nitrogen (N), exacerbates disease resistance in Foc TR4. N is an important nutrient for plant growth and has long been known to play a key role in plant diseases (Fagard et al., 2014). Researchers have demonstrated that fungal pathogens need a high supply of N, but that N inputs also improve plant defense (Tavernier et al., 2007). However, excessive application of N fertilizer improves the availability of nitrogenous compounds for use by pathogens, exacerbating plant infections (Segura et al., 2021). Nitrate (NO3−) and ammonium (NH4+) are the two main forms of inorganic nitrogen absorbed and utilized by plants, and each has different effects on the occurrence and control of Fusarium wilt (Mur et al., 2017). Inorganic nutrients play an important role in the occurrence and development of many fungal and bacterial diseases (Walters and Bingham, 2007), and many of these nutrients can inhibit the occurrence of fungal diseases and mitigate the damage they cause (Deliopoulos et al., 2010). Previous work has shown that Foc number is positively correlated with NH4+ content, but negatively correlated to NO3− content (Zhou et al., 2017). Current evidence suggests that nitrate fertilizer reduces Fusarium wilt severity, whereas ammonium increases it (Wang et al., 2016). This relationship between N form and disease progression may be due to the acidifying effect of ammonium fertilizer, as soil acidification increases FWB severity (Teixeira et al., 2021; Segura et al., 2022). Fusaric acid (FA), the main fungal toxin produced by Foc TR4, is critical for FWB development (Wang et al., 2012). Nitrate increases organic acid metabolism and related gene expression, ammonium increases amino acid metabolism and related gene expression, resulting in amino acid accumulation in cucumber plants. This further stimulates FA production and Foc sporulation, increasing disease incidence (Wang et al., 2019). Moreover, previous studies have shown that mycelium growth can occur when amino acids are the only available N source (Solomon and Oliver, 2002). At present, there is not a universally applicable method for controlling FWB due to differences in how different soils affects Foc TR4’s physiological characteristics. The application of inorganic nitrogen fertilizer causes soil acidification, which may explain the frequent occurrence of banana soil-borne Fusarium wilt. Previous studies have focused mostly on the effect of different nitrogen forms on Foc TR4, but none have investigated which form of nitrogen is preferred by the pathogen. Therefore, it is important to investigate Foc TR4’s response mechanism to nitrogen fertilization in terms of physiological characteristics (Figure 1).
2. Materials and methods
2.1. Experimental design
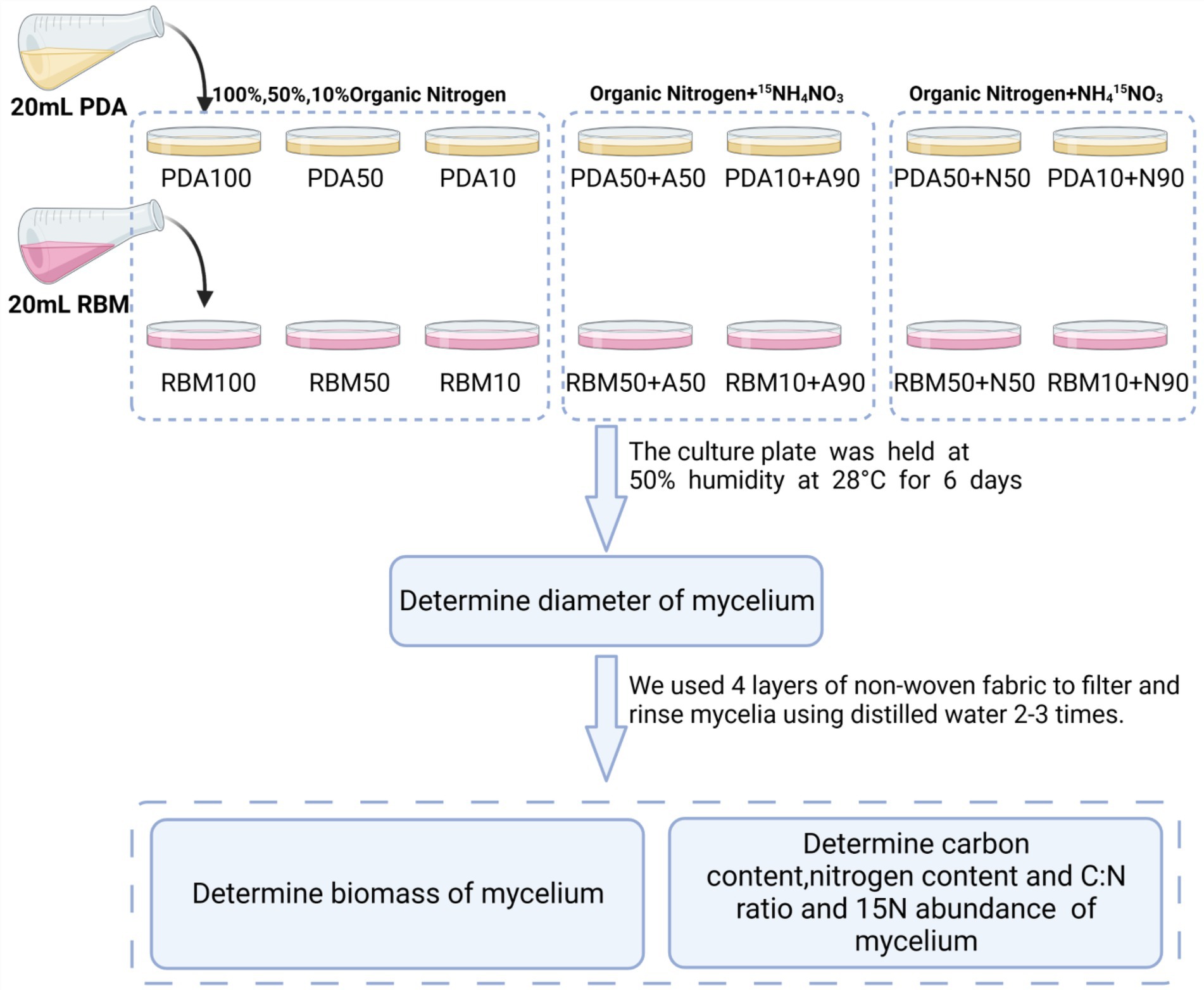
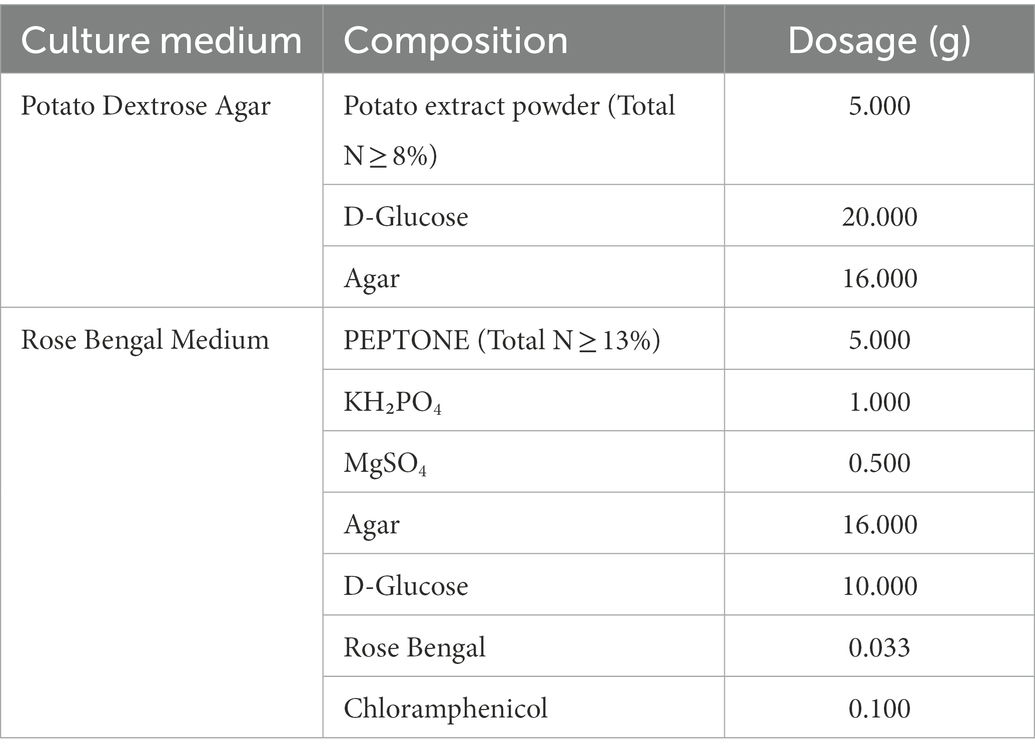
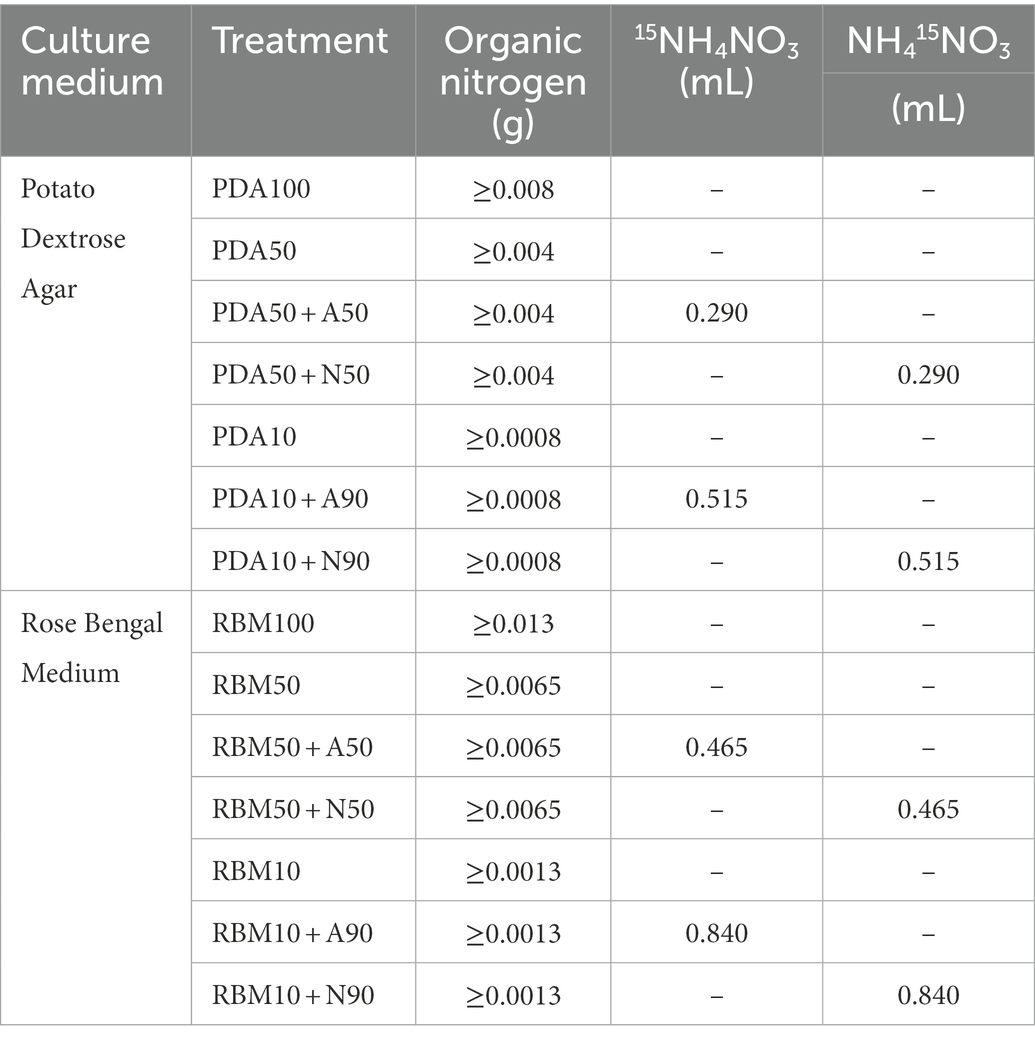
This experiment was conducted in 2021 at Hainan University (Haidian Campus). 15NH4NO3/NH415NO3 was added to Potato Dextrose Agar (PDA) and Rose Bengal Medium (RBM) to prepare the new culture medium with three replicates for each of the following composition: PDA100 (100% organic nitrogen), PDA50 (50% organic nitrogen), PDA50 + A50 (50% organic nitrogen+50%15NH4NO3), PDA50 + N50 (50% organic nitrogen+50% NH415NO3), PDA10 (10% organic nitrogen), PDA10 + A90 (10% organic nitrogen+90%15NH4NO3), PDA10 + N90 (10% organic nitrogen+90%NH415NO3), RBM100 (100% organic nitrogen), RBM50 (50% organic nitrogen), RBM50 + A50 (50% organic nitrogen+50%15NH4NO3), RBM50 + N50 (50% organic nitrogen+50% NH415NO3), RBM10 (10% organic nitrogen), RBM10 + A90 (10% organic nitrogen+90%15NH4NO3), RBM10 + N90 (10% organic nitrogen+90%NH415NO3). 20 mL culture solution and 15NH4NO3 / NH415NO3 solution were added to each plate, and each treatment had three replicates. Foc TR4 mycelium with the same growth and thickness as the culture medium was prepared by using a 1 cm diameter punch and was used to inoculate the new culture plate. The culture plate was held at 50% humidity at 28°C for 6 days. Media composition is presented in Table 1. A total of 1 L of culture medium was autoclaved at 121°C for 20–30 min. 0.40 g 15NH4NO3 / NH415NO3 (abundance of 15N:99%) was mixed with 10 ml of sterile water, and 0.04 g/ml 15NH4NO3 and NH415NO3 solution were prepared. Organic nitrogen (PDA and RBM) was added to each culture medium listed in Table 2.
We observed the growth of Foc TR4 mycelia, taking pictures and measuring growth after 2, 4, and 6 days. After the sixth day, we used four layers of non-woven fabric to filter and rinse mycelia using distilled water 2–3 times. Samples were dried in an oven at 105°C for 24 to a constant weight. Biomass was determined, after which mycelia were ground and crushed before being placed in a 5 ml test tube. Samples were sent to the Element and Stable Isotope Laboratory of the Analytical and Testing Center at Hainan University to determine the carbon content, nitrogen content, and 15N abundance using an isoprime precision mass spectrometer (Germany).
2.2. Statistical analyses
Microsoft Excel 2010 was used to organize the data and SPSS 24.0 software was used for statistical analysis of the data. One-way analysis of variance (ANOVA) and least significant difference (LSD) tests (p < 0.05) were used for multiple comparison analyses. Graphs were generated on Origin 2018.
3. Results
In addition to being significantly affected by nitrogen form, mycelium biomass and diameter were closely related to nitrogen uptake and utilization. Compared with the control (PDA100), biomass decreased significantly on PDA and RBM (p < 0.05). Biomass of the combined organic and inorganic nitrogen treatment was significantly lower than that of the pure organic nitrogen treatment (PDA100 or RBM100) on PDA and RBM. Foc TR4 preferentially utilized organic nitrogen rather than inorganic nitrogen (Figure 2A). In the combined organic and inorganic nitrogen treatment, mycelial diameter was higher on PDA compared to RBM (Figure 2B), indicating PDA is preferred for Foc TR4 growth. The carbon content, nitrogen content, and C:N ratio significantly influenced fungal growth. The mycelial carbon content in experimental treatments was 3.55, 3.28, 2.72, 5.13, 4.66, and 4.32% higher than the control. All experimental groups exhibited higher carbon content relative to the control, except the RBM50 + A50 group, which was 1.27% lower; however, differences between treatments within the RBM group were not significant (Figure 2C). In contrast, nitrogen content decreased on both PDA and RBM. On PDA, nitrogen content was 22.24, 13.53, 22.53, 58.09, 41.79, and 40.38% lower than in the control treatment (Figure 2D), suggesting that Foc TR4 increased carbon uptake and use in response to poor nitrogen utilization. The C:N ratio is an important indicator of nutrient utilization, and both high and low C:N ratios are detrimental to cell growth and exogenous protein expression and accumulation. The C:N ratios of mycelia in the experimental group increased significantly compared to the control group. The C:N ratios of mycelia in PDA10 + A90 and PDA10 + N90 treatments were 81.60 and 77.73% higher, respectively, than that of the control on PDA (p < 0.05). In addition, the C:N ratio in mycelia grown on RBM exhibited a similar pattern (Figure 2E). In the 15N abundance experiment, tracer uptake was higher on PDA50 + A50 than on PDA50 + N50 and higher in PDA10 + A90 than in PDA10 + N90, with rates of uptake 88.63 and 267.53% higher, respectively, than growth on PDA (p > 0.05). A similar pattern was observed in mycelia grown on RBM, suggesting that Foc TR4 prefers to use ammonium as a nitrogen source over nitrate when organic nitrogen is limited (Figure 2F).
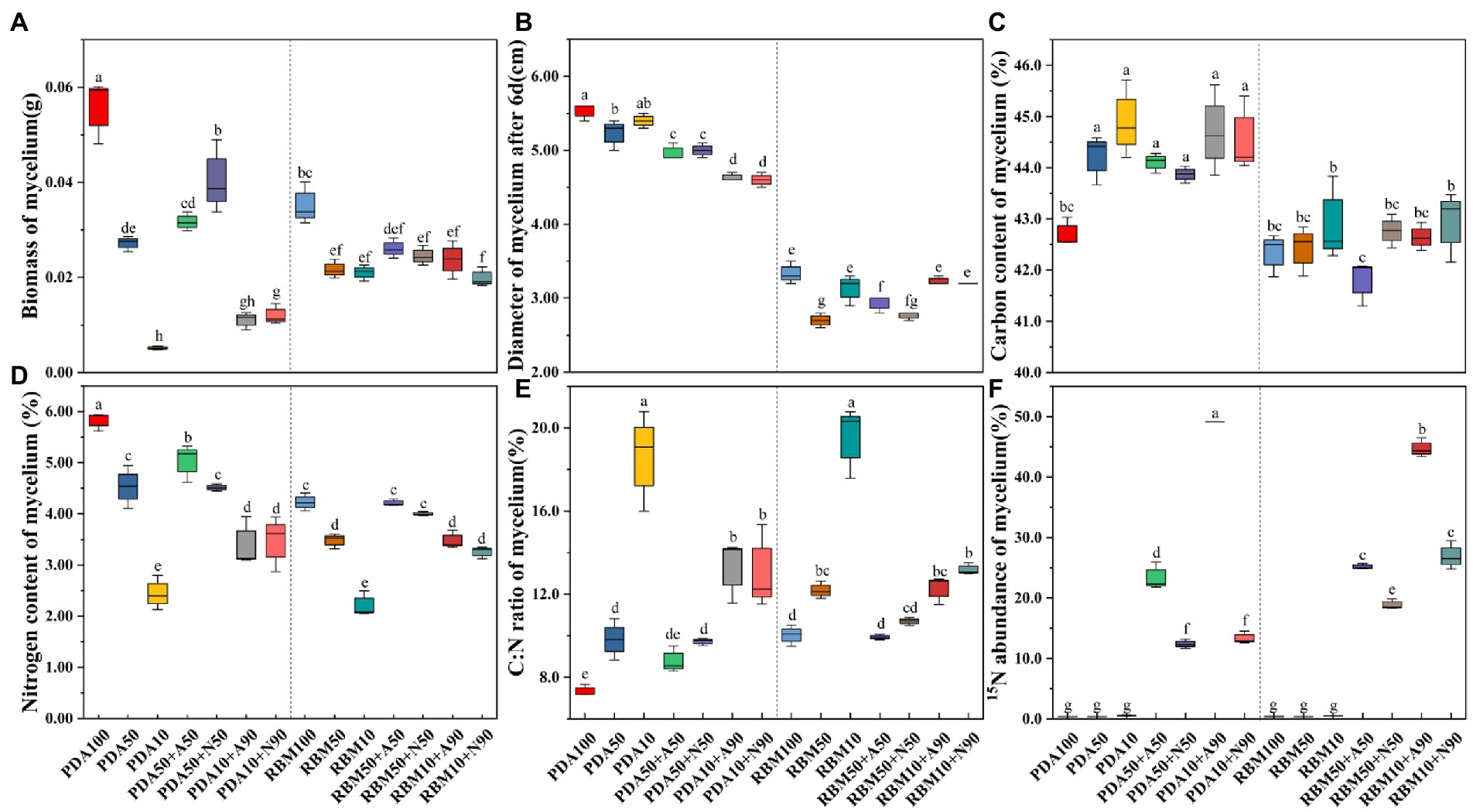
Figure 2. Foc TR4 prefers using ammonium to nitrate for growth when organic N is limited. Mycelium biomass on PDA and RBM (A). Mycelium diameter on PDA and RBM (B). Mycelium carbon content on PDA and RBM (C). Mycelium nitrogen content on PDA and RBM (D). Mycelium C:N ratio on PDA and RBM (E). Mycelium 15N abundance on PDA and RBM (F). Error bars represent standard errors (n = 3). Different letters indicate significant differences across different treatments (p<0.05).
We found that ammonium and nitrate assimilation was significantly higher in the 90% organic nitrogen reduction treatment compared to the 50% nitrogen reduction treatment; moreover, assimilation of inorganic nitrogen was higher in the PDA10 + 90 group than in all other treatments, indicating that reducing organic nitrogen availability increases the assimilation efficiency of inorganic nitrogen, especially ammonium (Figure 3A). Mycelium diameter was notably different between PDA and RBM treatments (Figure 3B). As the experiment proceeded, mycelium diameter gradually increased across all treatments, but the pattern on increase varied. For Foc TR4 grown on PDA, mycelium diameter increased rapidly after inoculation (Figure 3C); a similar rapid increase in mycelium diameter was observed for RBM, but not until the second day of growth (Figure 3D). Pearson correlation analysis showed that the biomass of mycelium was significantly correlated with nitrogen content and C:N ratio. Mycelium biomass was positively correlated with nitrogen content, but negatively correlated with C:N ratio. This suggests that the C:N ratio is one of the most important factors affecting the growth of Foc TR4. Carbon content was also significantly correlated with mycelial diameter (Figure 3E).
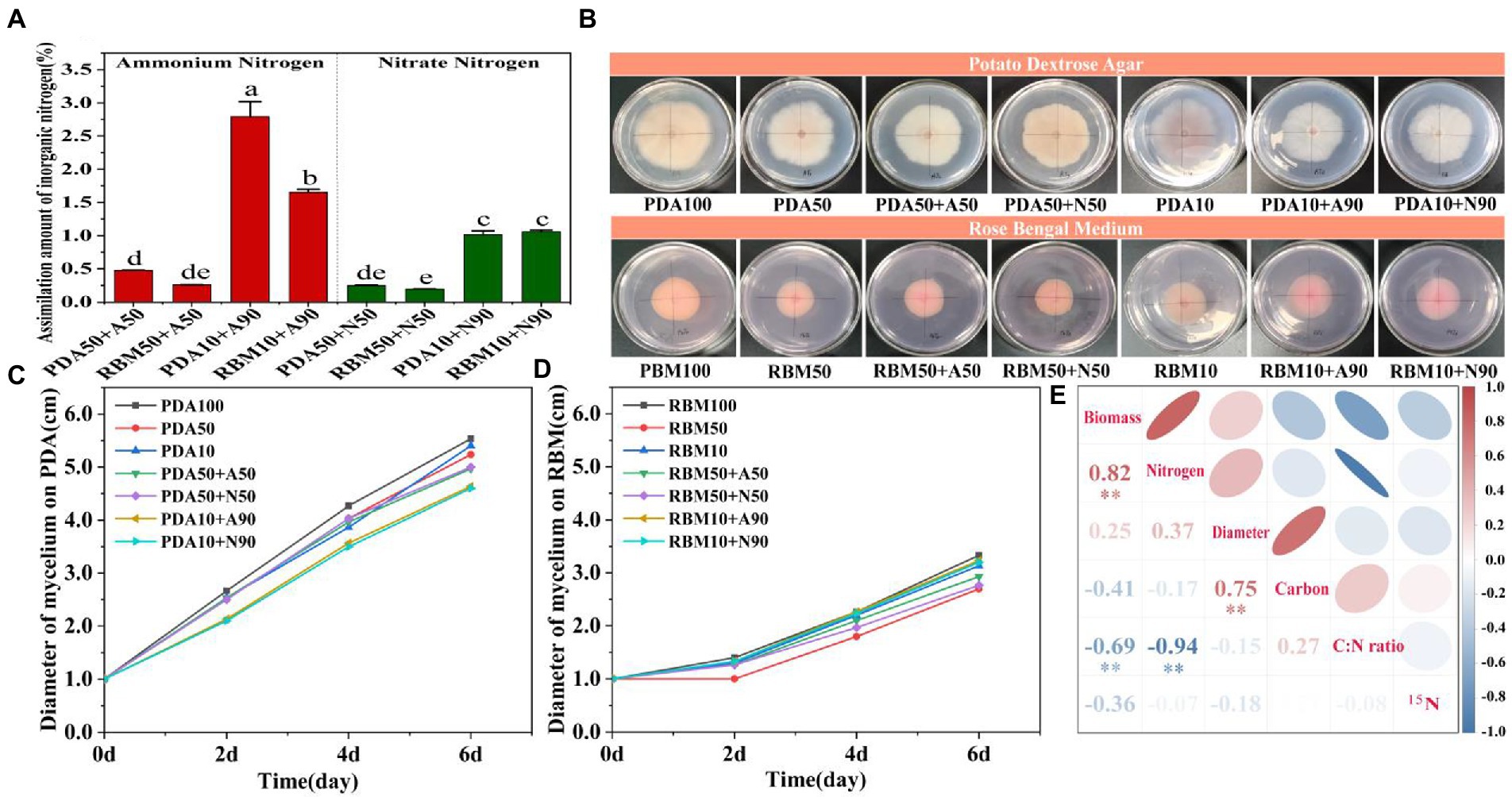
Figure 3. Inorganic nitrogen assimilation on PDA and RBM (A). Mycelium diameter 6 days post-inoculation on PDA and RBM (B). Change in mycelium diameter during incubation on PDA (C). Change in mycelium diameter during incubation on RBM (D). Pearson correlation analyses of biomass, diameter, nitrogen content, 15N abundance, carbon content, and C:N ratio (E). Blue indicates negative correlation, and red indicates positive correlation. Smaller shape and darker color indicates larger correlation coefficients, ** indicates p < 0.01.
4. Discussion
Fusarium wilt of banana is one of the most devastating soil-borne fungal diseases in banana production in the world. Nitrogen assimilation is necessary for the health growth of plants and strongly influences disease development and plant resistance. Previous work has found that nitrogen form influences the development and progression of Fusarium wilt disease (Gu et al., 2020). Pathogenic fungus can assimilate nitrogen occurring in different forms, such as ammonium nitrogen, nitrate nitrogen, and amino acids (Mur et al., 2017). Previous studies have shown that mycelium growth can occur when amino acids are the sole source of nitrogen (Solomon and Oliver, 2002). Zhou et al. (2019) showed that the content of total organic carbon, total organic nitrogen and available potassium in soil with blight was significantly higher than in healthy soil. Studies have shown that organic nitrogen sources such as beef extract and peptone promote the germination of cucumber wilt spores more effectively (Zhang et al., 2011). Bitter melon wilt can utilize multiple nitrogen sources, including ammonium and nitrate in organic nitrogen and inorganic nitrogen. However, the type of nitrogen required is differs across developmental and growth stages, and peptone is the form that most effectively promotes mycelial growth (Xiao et al., 2008). In this study, Fusarium wilt of banana preferentially used organic nitrogen over inorganic nitrogen, indicating that organic nitrogen is sufficient to support growth and that the addition of inorganic nitrogen will reduce the pathogen’s growth by reducing its biomass. It is generally understood that enhanced NH4+ availability increases plant susceptibility by enhancing the availability of apoplastic sugar, amino acids, and g-amino-n-butyric acid to the pathogen (Gupta et al., 2013). Interestingly, in this study, Foc TR4 prefered ammonium nitrogen in the presence of organic nitrogen, which is consistent with previous studies. At the same time, due to organic nitrogen limitation, fungal carbon content increased, but nitrogen content decreased, resulting in higher C:N ratios. This result has rarely been reported in previous studies.
5. Conclusion
In conclusion, the combination of organic and inorganic nitrogen treatments reduced mycelium biomass and nitrogen content significantly compared to an organic N source (PDA100 or RBM100); mycelium carbon content and C:N ratio increased significantly on PDA and RBM; and the mycelium biomass was negatively correlated with C:N ratio. A significant increase in 15N abundance was observed in mycelium grown on PDA50 + A50, PDA10 + A90, RBM50 + A50, and RBM10 + A90. These results indicate that mycelial biomass and diameter decreased as the C:N ratio increased, and that Foc TR4 preferentially utilized organic nitrogen over inorganic nitrogen. More importantly, Foc TR4 preferred to use ammonium nitrogen over nitrate to promote growth when organic N is limited. Therefore, we hypothesize that excessive application of ammonium nitrogen will aggravate the degree of banana wilt disease, while treating crops with a combination of organic and inorganic nitrogen fertilizers will reduce disease severity. This provides a new idea and reference for using nitrogen fertilization in banana cultivation management in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZJ conceived and designed the experiment. CH, PF, YL, and JL conducted the experiment. ZJ and CH analyzed data. YR contributed to materials and reagents. CH and JM wrote the article with assistance from all coauthors. All authors have read and approved the final manuscript.
Funding
This study was supported by the Project of Sanya Yazhou Bay Science and Technology City (SCKJ-JYRC-2022-94), the Strategic Priority Research Program of Chinese Academy of Sciences (XDA28020203), and the National Natural Science Foundation of China (91751204, 32160750, and 31672239).
Acknowledgments
We would like to thank Joseph Elliot at the University of Kansas for his assistance with English language and grammatical editing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Deliopoulos, T., Kettlewell, P. S., and Hare, M. C. (2010). Fungal disease suppression by inorganic salts: a review. Crop Prot. 29, 1059–1075. doi: 10.1016/j.cropro.2010.05.011
Dita, M., Barquero, M., Heck, D., Mizubuti, E. S. G., and Staver, C. P. (2018). Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 9:1468. doi: 10.3389/fpls.2018.01468
Fagard, M., Launay, A., Clement, G., Courtial, J., Dellagi, A., Farjad, M., et al. (2014). Nitrogen metabolism meets phytopathology. J. Exp. Bot. 65, 5643–5656. doi: 10.1093/jxb/eru323
Gu, Z., Wang, M., Wang, Y., Zhu, L., Mur, L. A. J., Hu, J., et al. (2020). Nitrate stabilizes the rhizospheric fungal community to suppress fusarium wilt disease in cucumber. Mol. Plant-Microbe Interact. 33, 590–599. doi: 10.1094/MPMI-07-19-0198-R
Gupta, K. J., Brotman, Y., Segu, S., Zeier, T., Zeier, J., Persijn, S. T., et al. (2013). The form of nitrogen nutrition affects resistance against pseudomonas syringae pv. Phaseolicola in tobacco. J. Exp. Bot. 64, 553–568. doi: 10.1093/jxb/ers348
Mur, L. A. J., Simpson, C., Kumari, A., Gupta, A. K., and Gupta, K. J. (2017). Moving nitrogen to the Centre of plant defence against pathogens. Ann. Bot. 119, mcw179–mcw709. doi: 10.1093/aob/mcw179
Segura, R. A., Stoorvogel, J. J., García, F., Salacinas, M., Kema, G. H. J., and Sandoval, J. A. (2021). Evaluating the potential of soil management to reduce the effect of Fusarium oxysporum f. sp. cubense in banana (Musa AAA). Euro. J. Plant Pathol. 160, 441–455. doi: 10.1007/s10658-021-02255-2
Segura, R. A., Stoorvogel, J. J., and Sandoval, J. A. (2022). The effect of soil properties on the relation between soil management and fusarium wilt expression in Gros Michel bananas. Plant Soil 471, 89–100. doi: 10.1007/s11104-021-05192-5
Solomon, P. S., and Oliver, R. P. (2002). Evidence that gamma-aminobutyric acid is a major nitrogen source during cladosporium Fulvum infection of tomato. Planta 214, 414–420. doi: 10.1007/s004250100632
Tavernier, V., Cadiou, S., Pageau, K., Lauge, R., Reisdorf, M., Langin, T., et al. (2007). The plant nitrogen mobilization promoted by colletotrichum lindemuthianum in phaseolus leaves depends on fungus pathogenicity. J. Exp. Bot. 58, 3351–3360. doi: 10.1093/jxb/erm182
Teixeira, L., Heck, D., Nomura, E., Vieira, H., and Dita, M. (2021). Soil attributes, plant nutrition, and fusarium wilt of banana in São Paulo Brazil. Tropic Plant Pathol. 46, 443–454. doi: 10.1007/s40858-021-00428-2
Walters, D. R., and Bingham, I. J. (2007). Influence of nutrition on disease development caused by fungal pathogens: implications for plant disease control. Ann. Appl. Biol. 151, 307–324. doi: 10.1111/j.1744-7348.2007.00176.x
Wang, M., Gu, Z., Wang, R., Guo, J., Ling, N., Firbank, L. G., et al. (2019). Plant primary metabolism regulated by nitrogen contributes to plant-pathogen interactions. Plant Cell Physiol. 60, 329–342. doi: 10.1093/pcp/pcy211
Wang, M., Ling, N., Dong, X., Zhu, Y. Y., Shen, Q. R., and Guo, S. W. (2012). Thermographic visualization of leaf response in cucumber plants infected with the soil-borne pathogen Fusarium oxysporum f. sp. cucumerinum. Plant Physiol. Biochem. 61, 153–161. doi: 10.1016/j.plaphy.2012.09.015
Wang, M., Sun, Y., Gu, Z., Wang, R., Sun, G., Zhu, C., et al. (2016). Nitrate protects cucumber plants against Fusarium oxysporum by regulating citrate exudation. Plant Cell Physiol. 57, 2001–2012. doi: 10.1093/pcp/pcw124
Xiao, C. H., Yu, X. M., He, Y. Y., Deng, X. C., and Tang, E. (2008). Studies on the biological characteristics of the pathogen of cucurbit wilt. Plant Prot. 02, 83–86.
Zhang, W., Liu, B. Y., Gai, Z. S., and Wang, Y. Z. (2011). Biological characteristics of cucumber wilt disease pathogen. Shandong Agric. Sci. 10, 70–73. doi: 10.14083/j.issn.1001-4942.2011.10.014
Zhou, D. B., Jing, T., Chen, Y. F., Wang, F., Qi, D. F., Feng, R. J., et al. (2019). Deciphering microbial diversity associated with fusarium wilt-diseased and disease-free banana rhizosphere soil. BMC Microbiol. 19:161. doi: 10.1186/s12866-019-1531-6
Keywords: 15N tracing technology, preference, Foc TR4, nitrogen forms, banana
Citation: He C, Jia Z, Fan P, Ruan Y, Liang Y, Ma J and Li J (2023) 15N tracing reveals preference for different nitrogen forms of Fusarium oxysporum f. sp. cubense tropical race 4. Front. Microbiol. 14:1102720. doi: 10.3389/fmicb.2023.1102720
Edited by:
Yong Yuan, Guangdong University of Technology, ChinaReviewed by:
Zhenke Zhu, Institute of Subtropical Agriculture (CAS), ChinaLaith Khalil Tawfeeq Al-Ani, Universiti Sains Malaysia, Malaysia
Copyright © 2023 He, Jia, Fan, Ruan, Liang, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongjun Jia, ✉ amlhQGlnYS5hYy5jbg==; ✉ amlhQGlzc2FzLmFjLmNu
 Chen He
Chen He Zhongjun Jia2,3*
Zhongjun Jia2,3* Jingjing Ma
Jingjing Ma