
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 13 February 2023
Sec. Food Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1083917
This article is part of the Research Topic Gut Microbiota Modulation by Dietary Fiber on Human Health: Processes and Mechanisms View all 6 articles
Introduction: Lyophyllum decastes (Fr.) Singer polysaccharides (LDSPs) have been verified to possess strong biological properties. However, the effects of LDSPs on intestinal microbes and their metabolites have rarely been addressed.
Methods: The in vitro-simulated saliva-gastrointestinal digestion and human fecal fermentation were used to evaluate the effects of LDSPs on non-digestibility and intestinal microflora regulation in the present study.
Results: The results showed a slight increase in the content of the reducing end of the polysaccharide chain and no obvious change in the molecular weight during in vitro digestion. After 24 h in vitro fermentation, LDSPs were degraded and utilized by human gut microbiota, and LDSPs could be transformed into short-chain fatty acids leading to significant (p < 0.05) decrease in the pH of the fermentation solution. The digestion did not remarkably affect the overall structure of LDSPs and 16S rRNA analysis revealed distinct shifts in the gut microbial composition and community diversity of the LDSPs-treated cultures, compared with the control group. Notably, the LDSPs group directed a targeted promotion of the abundance of butyrogenic bacteria, including Blautia, Roseburia, and Bacteroides, and an increase in the n-butyrate level.
Discussion: These findings suggest that LDSPs might be a potential prebiotic to provide a health benefit.
Lyophyllum decastes (Fr.) Singer, commonly known as the fried chicken mushroom, has been recognized as a culinary delicacy in Asia with potential for the commercial culture and great economic importance (Arana-Gabriel et al., 2018). Apart from excellent flavor and texture, Lyophyllum decastes (Fr.) Singer is currently of great interest due to being a biologically rich source of various active substances (Pokhrel and Ohga, 2007). It is deserved to be mentioned that polysaccharides from Lyophyllum decastes (Fr.) Singer (LDSPs) exhibit a range of possible health benefits, including antioxidant, anticholinesterase, antibacterial, and antidiabetic (Miura et al., 2002; Pushpa and Purushothama, 2010; Tel et al., 2015; Deveci et al., 2021).
Increasing evidence demonstrates that the consumption of fungal-derived polysaccharides can result in specific changes in gastrointestinal microbiota. The composition and homeostasis of the gut microbial community have an intense and intimate linkage to human health and play a crucial role in a multitude of physiological functions involving metabolic, immunologic, and protective activities (Slavin, 2013). Polysaccharides derived from Ganoderma lucidum have been shown to alleviate rat Dextran sulfate (DSS)-induced colitis by stimulating the growth of beneficial bacteria, such as Ruminococcus_1, and reducing pathogens, such as Escherichia-Shigella (Xie et al., 2019). In addition, acting as a substrate for gut microorganisms, polysaccharides from fungi can facilitate gut microbiota to produce short-chain fatty acids (SCFAs) providing a health benefit in immune and metabolic disease (Sanna et al., 2019). For instance, Poria cocos polysaccharides significantly enhanced glucose and lipid metabolism and mitigated hepatic steatosis in ob/ob mice by elevating the levels of butyrate and butyrate-producing bacteria Lachnospiraceae and Clostridium (Sun et al., 2019). It was observed that the promising prophylactic and therapeutic properties of fungal polysaccharides might be attributed to their diversified abilities to restore gut microbial balance.
Meanwhile, different polysaccharides of fungal origin may possess distinct prebiotic properties, which could be critical to comprehend their modulation of gut microbiota composition (Jayachandran et al., 2018). An in vitro batch culture fermentation study examined the impact of edible mushrooms polysaccharides on aging gut microbiota characteristics, demonstrating that Pleurotus spp. and C. Cylindracea mushrooms induced a significant bifidogenic effect, while P. eryngii mushrooms simulated the growth of Lactobacillus spp. and butyrate-producing bacteria such as F. prausnitzii and E. rectale/Roseburia spp. group (Mitsou et al., 2020). Undeniably, fungal polysaccharides represent a vast and still untapped source assumed to be applied, but there is little known about the regulation of LDSPs on the intestinal microbiome and their metabolites.
We hypothesized that LDSPs should be neither digested nor absorbed; therefore, LDSPs could undergo fermentation in the colon and be utilized by the gut microbiota accompanied by changes in SCFAs. In this study, the simulated saliva-gastrointestinal digestion model was conducted to reveal the digestion behavior of LDSPs and the main changes in molecular weight (Mw) and the content of the reducing end of the polysaccharide chain (RC). In vitro anaerobic fermentation was performed, 16S rRNA sequencing was adopted to clarify the effect of LDSPs on gut microbial composition, and their fermentation characteristics were investigated by determining SCFAs production and pH value. This study was intended to provide evidence of the possible digestion and fermentation mechanism of LDSPs for application in new potential prebiotics.
Lyophyllum decastes (Fr.) Singer was collected from an agricultural product processing base in Taixing, Jiangsu Province, China. The enzymes, used in this study, including α-amylase (100 U/mg), pepsin (3,000 U/g), and pancreatic enzyme (4,000 U/g), were purchased from Sigma-Aldrich (St. Louis, United States). SCFA standards including acetic, propionic, butyric, valeric, isobutyric, and isovaleric acids were purchased from Aladdin (Shanghai, China). All other chemicals and solvents used were analytical grade.
The Lyophyllum decastes (Fr.) Singer was prepared using the method described by Wu et al. (2017) with slight modifications. Hot water extraction was used to obtain polysaccharides from Lyophyllum decastes (Fr.) Singer. In brief, the dried Lyophyllum decastes (Fr.) Singer powders (50.0 g) were extracted three times with ultrapure water (1:15, w/v) at 100°C for 1 h. Then, polysaccharides extracted from Lyophyllum decastes (Fr.) Singer was further concentrated and ultra-filtered (molar mass cutoff, 5.0 kDa) to remove impurities and finally freeze-dried.
The protein content, total phenol content, and total carbohydrate content of LDSPs were determined by the Bradford method, Folin–Ciocalteu method, and phenol–sulfuric acid method (Dubois et al., 1951; Bradford, 1976). The monosaccharide composition of LDSPs was according to Wang et al. (2019) with some modifications. Here, 2 M aqueous trifluoroacetic acid (TFA) was used to hydrolyze LDSPs at 110°C for 4 h. Then, High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD; Dionex ICS-5000+) equipped with the Dionex CarboPac PA20 analytical column (3 × 150 mm) was conducted to detect monosaccharides in hydrolyzed samples.
The Mw of LDSPs was determined using the modified method as previously reported (Chen G. et al., 2017) by high-performance size exclusion chromatography, equipped with a multi-angle laser light scattering and a refractive index detector (HPSEC-MALLS-RID Shimadzu, Kyoto, Japan). The separation of samples was applied on TSK-GEL G6000PWXL and G4000PWXL column (7.8 × 300 mm, TOSOH Crop., Tokyo, Japan). The sodium nitrate solution (0.15 mol/ml) was eluted and the flow rate was 0.5 ml/min, and the injection volume of the sample was 100 μl.
The simulated in vitro digestion was performed according to the previously published procedures (Brodkorb et al., 2019) with minor modifications. First, 25 mg of LDSPs were dissolved in 5 ml of ultrapure water. Subsequently, 4 ml of simulated salivary fluid (SSF) was preheated at 37°C in a water bath and then added into a 5 ml of LDSPs solution (10 mg/ml), CaCl2 solution (0.025 ml, 0.3 M), and α-amylase solution (75 U/ml, 0.5 ml). Ultrapure water was added to supplement the solution to 10 ml to mix LDSPs solution with SSF to achieve a final ratio of 1:1 (wt/wt). In addition, the ultrapure water and inulin solution (10 mg/ml) added to the simulated digestion medium were, respectively, used as the blank control (CON) group and positive control (INU); then, each group was kept in a 37°C shaking bath for 5 min.
Thereafter, the pH was adjusted to 3.0 with HCl (6 mol/L). Afterward, 8 ml of simulated gastric fluid (SGF) was preheated at 37°C in a water bath, followed by the addition of the previous stage of 10 ml simulated digestive fluid, CaCl2 solution (0.01 ml, 0.3 M), pepsin solution (2000 U/ml, 1 ml), and ultrapure water to supplement the solution to 20 ml to achieve a final ratio of 1:1 (wt/wt). The simulated digestion samples were incubated at 37°C. During the digestion process, an equal volume of simulated gastric fluid was taken out at the time points of 0, 1, 2, and 4 h for further analysis.
Next, the pH was adjusted to 7.0 with NaOH (6 mol/L). Next, 12 ml of simulated intestinal fluid (SIF) was preheated in a 37°C water bath, followed by the addition of the previous stage-simulated digestive fluid, bile salt solution (2.5 ml, 10 mM), CaCl2 solution (0.04 ml, 0.3 M), a pancreatic enzyme solution (0.5 g, 100 U/ml), and ultrapure water to supplement it to 40 ml. During the digestion process, an equal volume of simulated gastric fluid was taken out at the time points of 0, 1, 2, 4, and 6 h for further analysis.
The in vitro fermentation was performed based on the previous method with minor modifications (Lam et al., 2018). First, fresh fecal samples were collected from six healthy volunteers (three women and three men, 20–30 years of age) who maintained a regular diet and had not received antibiotics or prebiotic treatment for 3 months. The fecal samples were immediately mixed with sterilized 0.1 M phosphate-buffered saline (pH 7.0) to produce a 10% (w/v) fecal suspension. Then, 2.0 g of peptone, 2.0 g of yeast extract, 0.5 g of cysteine-HCl, 0.1 g of NaCl, 2.0 g of NaHCO3, 0.04 g of K2HPO4, 0.04 g of KH2PO4, 0.01 g of MgSO4 7H2O, 0.01 g of CaCl2 6H2O, 0.02 g of hemin, 0.5 g of bile salt, 2.0 ml of Tween 80, 10 μl of vitamin K1, and 1.0 ml of resazurin 1% (w/v) were dissolved in 1 L ultrapure water to obtain a basic medium. Finally, LDSPs were selected as 1% (w/v) carbon sources, and the medium without carbon sources and 1% (w/v) inulin were used as a blank control (CON) and positive control (INU). The medium was adjusted to pH 7.0 using 1 mol−1 HCl and placed in an anaerobic chamber at 37°C overnight to pre-reduce the media. Here, 1.0 ml of 10% (w/v) fecal slurry was added to a 9.0 ml medium and placed in the 37°C anaerobic chamber and incubated for 0, 6, 12, and 24 h. Then, the samples were collected immediately and stored at −80°C for further analysis.
The reducing end of the polysaccharide chain (RC) contents of the digestion and fermentation products were analyzed by the dinitrosalicylic acid (DNS) method using glucose as the standard (Li et al., 2020). The Mw and residual carbohydrate of the digestion and fermentation products were determined according to the previous method.
The pH of the fermentation system was measured with a standard pH meter (DELTA320, Mettler Toledo Co., Ltd. Shanghai, China). SCFAs were extracted by absolute ether following the method of Bai et al. (2021) and were analyzed by gas chromatography–mass spectrometry (GC–MS)-TQ8040 (Shimadzu, Kyoto, Japan) equipped with SH-Rtx®-WAX column (30 m × 0.25 mm i. d.; film thickness 0.25 μm). In brief, the fermented solution was centrifuged at 6000 g for 10 min. The 20 μl of 10% H2SO4 was added to acidify 500 μl of supernatant, and 500 μl of absolute ether was used to extract SCFAs. The mixtures were then centrifuged at 8000 g for 10 min at 4°C, and the phases were separated. The supernatant was taken and filtered using a 0.20 μm filter into a sample injection bottle. The temperature increased raised to 140°C at 7.5°C/min and held for 4 min, which then raised to 200°C at 60°C/min. Carrier gas helium was employed, the flow rate was 2.0 ml/min, the full scan mode in the m/z range was 20.0–300.0, and the injection volume was 1 μl. SCFA concentration was determined by the external standard method with corresponding standards.
After fermentation of 24 h, high-throughput sequencing technology of bacterial 16S rRNA was implemented to investigate the impact of polysaccharides on the gut microbiota. Each sample of 24 h blank control fermentation group (CON group), 24 h inulin fermentation group (INU group), and 24 h Lyophyllum decastes (Fr.) Singer fermentation group (LDSPs group) was extracted using Qiagen QIAamp Fast DNA Stool Mini Kit, according to the manufacturer’s instructions. The DNA extraction from all samples was visualized on 1% agarose gel electrophoresis. PCR amplification was performed using TransStart FastPfu DNA Polymerase, and the amplicons were purified using the AxyPrep DNA gel extraction kit (Axygen Bioscience, Union City, United States) and quantified using QuantiFluor™-ST fluorometer (Promega, Madison, United States). The major PCR products from the V3–V4 region of the 16S rRNA gene amplified with primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R(5′-GGACTACHVGGGTWTCTAAT-3′) were sequenced on the Illumina Miseq platform by Shanghai Majorbio Bio-pharm Technology Co. Ltd. (Shanghai, China).
The microbiological data were analyzed on the online platform Majorbio Cloud Platform.1 First, the double-ended reads were quality-controlled and filtered according to the sequencing quality, and the optimization data after quality control (QC) splicing are obtained according to the overlapping relationship between the double-ended reads. Then, the sequence noise reduction method (DADA2/Deblur) is used to process the optimization data to obtain Amplicon Sequence Variant (ASV) representing sequence and abundance information. All data, including alpha diversity, beta diversity, and the examination of the bacterial taxonomic compositions, were obtained from ASV. By using one-way ANOVA, the microbiota substantial difference analysis was obtained.
The other experiments were carried out 5-fold. Data were expressed as mean ± standard deviation (SD). Statistical analysis was carried out using SPSS (Version 17.0, Chicago, United States). One-way analysis of variance (ANOVA) followed by Tukey’s test at a 5% confidence level was used to calculate the significant difference.
The basic physical and chemical properties of purified LDSPs are shown in Table 1. As shown in Table 1, the total carbohydrate content, total phenol content, and protein content of LDSPs were 90.51, 3.82, and 1.95%, respectively. Moreover, the monosaccharide composition of LDSPs was glucose (82.14%), fructose (14.20%), galactose (2.49%), mannose (0.82%), fucose (0.20%), arabinose (0.10%), and rhamnose (0.04%). These results indicated that the main monosaccharide of LDSPs was glucose. HPGPC chromatogram showed the two peaks with an average molecular weight of 4.59 × 107 Da and 4.30 × 106 Da (1.14:1).
Salivary amylase and the severe pH in the simulated gastric fluid may play important roles in the digestion of non-starch polysaccharides (Zhang et al., 2021). Moreover, due to the presence of pancreatin and high concentration of bile acids, there may be changes in the chemical composition of polysaccharides after intestine digestion. After hydrolyzed by digestive fluid, the glycosidic of polysaccharides bonds is destroyed accompanied by the increase in reducing end. As shown in Table 2, the contents of RC in LDSPs did not change significantly after simulated saliva digestion, indicating that LDSPs were not affected by salivary amylase. During simulated gastric digestion and intestinal digestion, both LDSPs and INU were partially degraded, but RC content increased significantly (p < 0.05) in the INU group compared to the LDSPs group. Overall, the structures of LDSPs were not significantly influenced by simulated saliva-gastrointestinal fluid, so they could be neither digested nor absorbed and directed to the colon to be utilized by gut microbiota.
The digestion properties of LDSPs are also related to their changes in molecular weights (Wu et al., 2018). The molecular weight distributions of two peaks during digestion are shown in Table 3 and Supplementary Figure 1A. After in vitro-simulated digestion, the molecular weights of LDSPs did not change significantly (p < 0.05), in agreement with the changes in RC, which indicated that LDSPs were indigestible during in vitro digestion.
Lyophyllum decastes (Fr.) Singer was subjected to an in vitro fermentation model inoculated with human fecal microbiota. Recently, the accumulating evidence shows that large polysaccharides are indigestible in saliva-gastrointestinal fluid for the lack of corresponding enzymes but can be degraded by the intestinal microbiota to increase the reducing end of the polysaccharide chain (Guo et al., 2021). As shown in Figure 1C, the RC and residual carbohydrate content were detected at 6, 12, and 24 h of fermentation. The LDSPs group showed a significant (p < 0.05) increase in the contents of RC during 0–12 h fermentation and a decrease after 12 h of fermentation. Combined with the sharp (p < 0.05) reduction in residual carbohydrates in Figure 1B, it was clear that the LDSPs were degraded into polysaccharide chains with reducing ends by the gut microbiota before the first 12 h. In addition, the degradation rate of LDSPs was higher than the utilization rate, and with the rapid proliferation of microbiota, the polysaccharide chains with reducing ends were easily utilized by gut microbes; therefore, RC was gradually decreased during the 12–24 h of fermentation. Indeed, the total residual carbohydrate remarkably (p < 0.05) decreased to 39.31 and 81.59% in INU and LDSPs groups, respectively, after the in vitro fermentation for 24 h, suggesting that they can be partially or fully hydrolyzed by intestinal microbiota, which is determined by the physicochemical properties of polysaccharides (Hu et al., 2018).
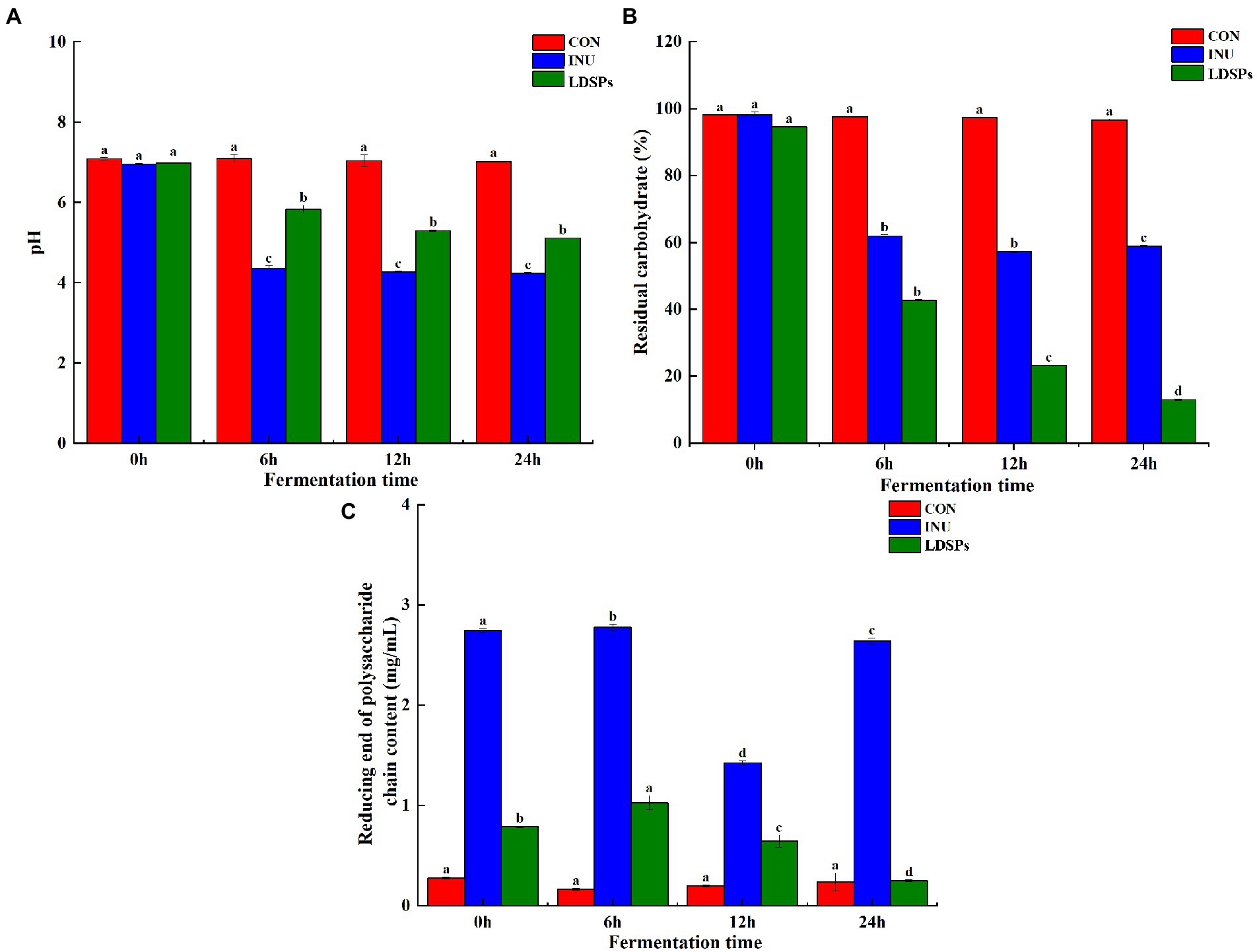
Figure 1. pH value (A), residual carbohydrate (B), and reducing end of polysaccharide chain content (C) during in vitro fermentation, respectively. Data are expressed as mean ± SD (n = 5), and a–d mean significantly different (p < 0.05) by a Tukey test in the same group with different time points.
The possible changes in molecular weight of LDSPs during in vitro fermentation were further investigated. Table 3 and Supplementary Figure 1B show the Mw changes in two peaks in LDSPs, and the Mw of LDSPs showed a slight decrease during 0–6 h and marginally decreased after 12 h of fermentation. These observations demonstrated that the microbiota went through a lag period in the initial 12 h and then degraded LDSPs quickly into polysaccharide chains with reducing ends for proliferation.
In the present work, the high-throughput sequencing analysis was conducted on samples after 24 h fecal fermentation to reveal the effect of the indigestible LDSPs on the microbial structure. The average Good’s coverage was 99.28%, indicating the 16S rRNA sequences identified in this study likely represent the majority of bacterial sequences present in the samples. Diversity data analysis of 60 samples was completed and obtained ranging from 3,530,423 to 1,472,821,519 reads, with an average sequence length of 418 bp. The Sobs index, Shannon index, Simpson index, hierarchical clustering analysis, and principal co-ordinate analysis (PCoA) are shown in Figure 2. The rarefaction curves for the Sobs index exhibits the numbers of observed species per sample, and each sample reached plateaus, indicating that the majority of the sequencing was already sufficient (Figure 2A). Both the community diversity and richness in the LDSPs group significantly decreased after 24 h of fermentation compared with the initial sample group (OR) but higher than that in the 24 h blank group (CON) and the 24 h inulin supplement group (INU). Furthermore, the PCoA is an important analytical method for β-diversity. The PCoA score plot was used to reveal the microbiota shifted in both LDSPs and INU groups (Figure 2F). The total alternation of principal component 1 (PC1; 55.87%) and principal component 2 (PC2; 36.91%) was 92.78%. PCoA results show that both the LDSPs group and the INU group were far away from the CON group, and the outcome of hierarchical clustering analysis (Figure 2E) was in line with the PCoA results, indicating that LDSPs and INU could significantly affect the structure of the microbial community.
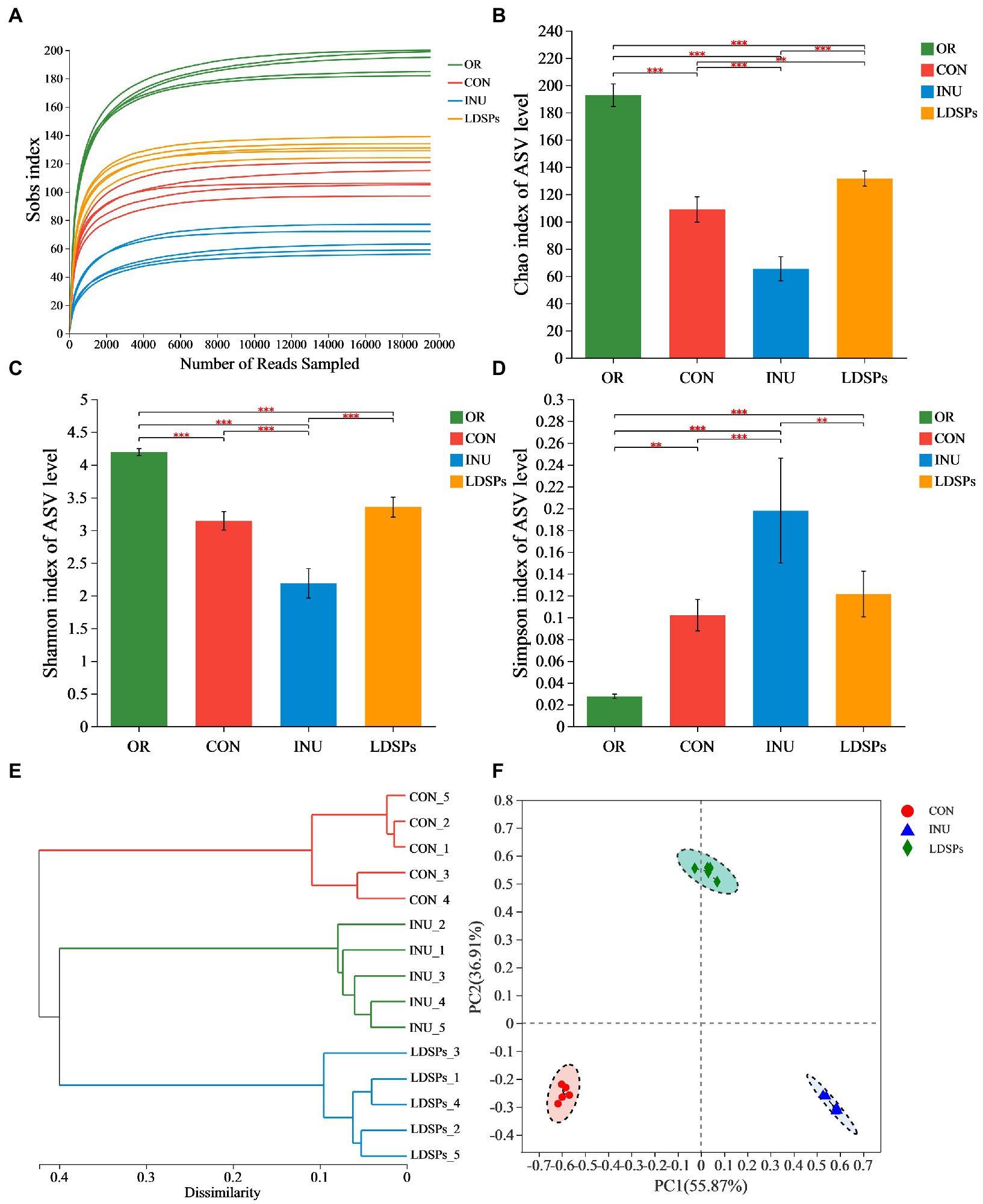
Figure 2. Alpha diversity analysis using the Student’s t-test for the Ace (A), Chao (B), Shannon (C), and Simpson indices (D) and β-diversity analysis using UPGMA for hierarchical clustering (E) and ANOSIM for PCoA (F) on ASV level of gut microbiota in groups (n = 5) after 24 h of fermentation. *p < 0.05, **p < 0.01, and ***p < 0.001, respectively; OR, the initial sample group, CON, the blank control (no additional carbon source supplement), INU, the positive control (INU supplement), and LDSPs, the experimental group (LDSPs supplement); ANOSIM, analysis of similarities; PCoA, principal co-ordinate analysis.
At the phylum level, the dominant bacterial communities comprised Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes (Figure 3A). Although the LDSPs group (85.75%) was noticeably richer in the relative abundance of Firmicutes compared with the CON group (27.66%), the Bacteroidetes to Firmicutes (B/F) ratio in the LDSPs group was significantly (p < 0.05) upregulated with 97% compared with the CON group (Figure 3B). Bacteroidetes is one of the major gut bacteria that could degrade polysaccharides, and the increased B/F ratio could alleviate obesity, which was considered one of the essential biological indicators (Ai et al., 2017). In the control group, an unsuitable ratio between protein and carbohydrate could result in increases in the number of potential pathogens due to disruption of the homeostasis of the gut micro-ecosystem with a higher abundance of Proteobacteria (Zhao et al., 2018). In addition, the LDSPs (4.10%) and INU (0.02%) also remarkably decrease the relative abundance of Proteobacteria compared with CON (65.28%), which might be attributed to the fact that LDSPs and INU could inhibit pathogens belonging to Proteobacteria such as Escherichia-Shigella. Actinobacteria, represented by the major probiotic bacteria Bifidobacterium, significantly increased in the INU group, compared with the CON group.
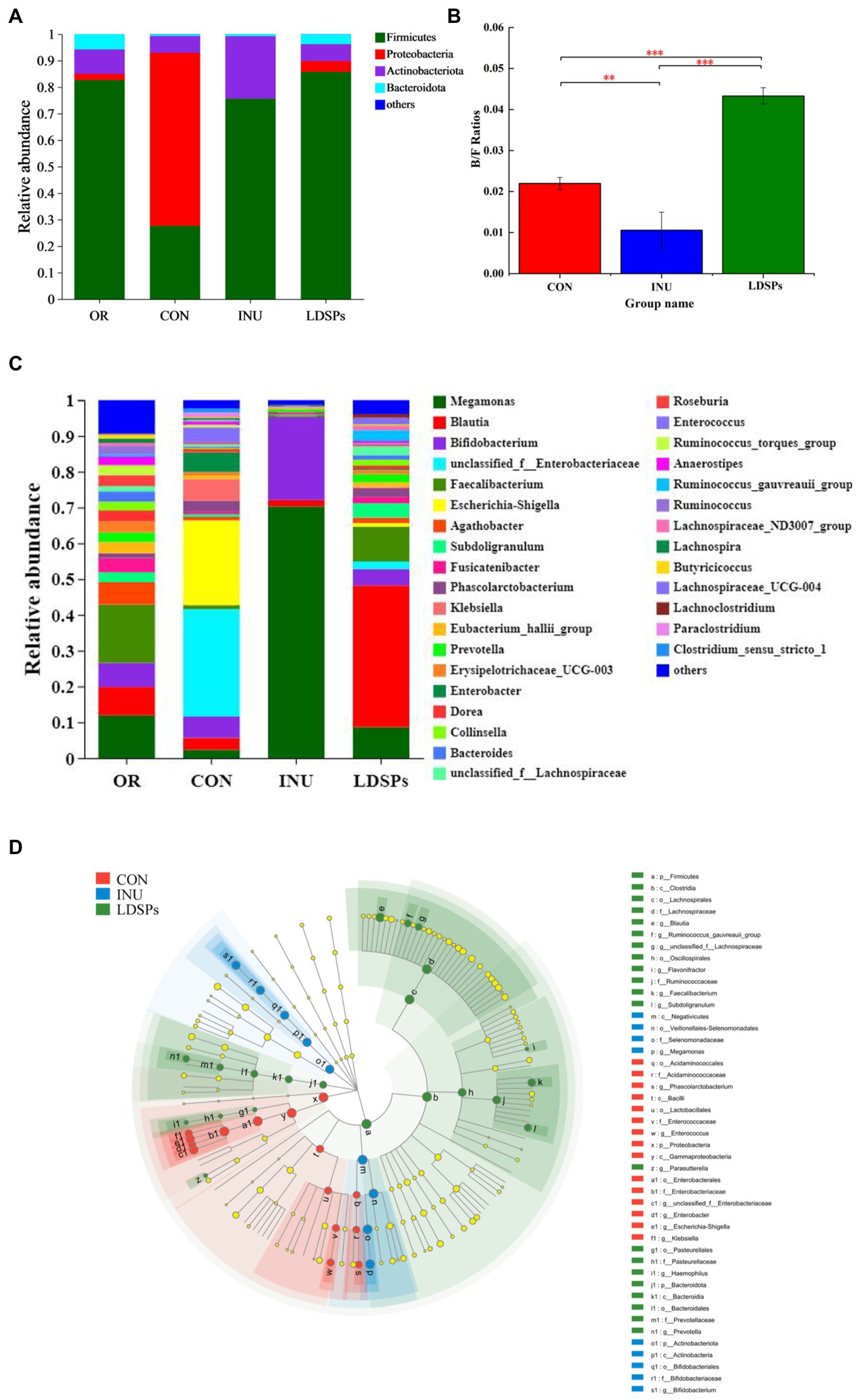
Figure 3. Relative abundance of gut microbiota community at the phylum (A) and genus (C) levels, Bacteroidetes/Firmicutes (B/F) ratios (B), and the comparison of microbiota from phylum level to genus level among the CON, INU, and LDSPs groups based on linear discriminant analysis effect size (LEfSe; D) after 24 h of fermentation. *p < 0.05, **p < 0.01, and ***p < 0.001, respectively; OR, the initial sample group, CON, the blank control (no additional carbon source supplement), INU, the positive control (INU supplement), and LDSPs, the experimental group (LDSPs supplement).
As shown in Figure 3C, three groups displayed different gut microbiota distributions at the genus level. The CON group was mainly composed of Escherichia-Shigella (23.72%), unclassified_f_Enterobacteriacece (30.02%), Enterobacter (5.51%), Klebsiella (6.02%), Bifidobacterium (5.94%), and Phascolarctobacterium (3.17%). However, Megamonas (70.30%) and Bifidobacterium (23.22%) became the dominant microbiota for the INU group after 24 h of fermentation, indicating that Megamonas might be the principal gut microbiota to degrade and utilize INU, similar to the result reported in a previous study (Chen B. D. et al., 2017). The LDSPs group also possessed higher levels of Blautia (39.59%), Faecalibacterium (9.69%), Dorea (3.62%), and Subdoligranulum (3.99%) than that of the CON group. The increasing works have suggested that Blautia is a kind of gut microbiota to promote the production of butyric acid (Cantu-Jungles et al., 2018).
The linear discriminant analysis effect size (LEfSe) in Figure 3D indicates the abundance of significantly different bacteria from the phylum level to the genus level in the CON, INU, and LDSPs groups. Prevotella has been reported to play an important role in glucose homeostasis and host metabolization (Si et al., 2017), and it was observed at a higher level in the LDSPs group than that in the CON and INU groups in this study. In addition, the increased Prevotella might be associated with the consumption of dietary fiber or carbohydrates (Kovatcheva-Datchary et al., 2015). Furthermore, clostridium and Lachnospiraceae, in the Firmicutes phylum, presented large increases in the LDSPs ferments and are known to be responsible for most of the butyrate produced in the human gut (Louis and Flint, 2009). The relative abundance of Bifidobacterium significantly increased in the INU group, which has been proved recently that could control serum cholesterol levels, prevent intestinal diseases, and modulate the immune system (Di Gioia et al., 2014). However, the lower abundance of Bifidobacterium in the LDSPs group, which might be a poor utilization of LDSPs by bifidobacterial, is consistent with the report that Bifidobacterium was not found in in vitro fermentation of polysaccharides from Fuzhuan brick tea (Chen G. et al., 2017). The probiotics can be broadly defined, and probiotics are live bacteria and yeasts, which are beneficial to human health when administrated in a viable form and in adequate amounts (Valdes et al., 2018). These results suggested that the commensal bacteria in the intestinal tract could break down indigestible LDSPs and that LDSPs could regulate gut microbiota dysbiosis by supporting the growth of beneficial bacteria and suppressing the proliferation of harmful bacteria, while the effects of LDSPs and INU on retarding dysbiosis were quite different.
Fermentation of polysaccharides by microbes in the colon results in the production of SCFAs, and SCFAs are involved in the reduction of gut pH levels (Thandapilly et al., 2018). As shown in Figure 1A, the pH values of the INU group and the LDSPs group were significantly (p < 0.05) lower than the 0 h after 24 h of fermentation. Furthermore, the pH values decreased with significant differences between the INU and LDSPs groups (p < 0.05), which might be related to their diverse chemical structure. A previous study showed that lower pH of the intestinal tract could promote the growth of probiotics and inhibit the reproduction of pathogens (Shoukat and Sorrentino, 2021). The concentrations of acetic acid, propionic acid, n-butyric acid, i-butyric acid, n-valeric acid, and i-valeric acid produced during in vitro fecal fermentation are shown in Table 4. Both INU and LDSPs groups noticeably promote the production of SCFAs, especially for the significant increase (p < 0.05) in propionic acid and n-butyric acid in the LDSPs group. The increased level of acetic acid was observed in the INU group.
Short-chain fatty acids have various positive effects on human health as prebiotic metabolites, and it has been confirmed that acetic acid is absorbed in the brain, heart, and peripheral tissues as an energy source (Kimura, 2014). Propionic acid produced in the intestinal tract improves tissue insulin sensitivity and suppresses cholesterol synthesis in the liver (Ding et al., 2019). In addition, butyric acid is the key to maintaining intestinal barrier integrity by providing energy for colonic epithelial cells and can also affect the host gene regulation, cell differentiation, and cell apoptosis (Ziegler et al., 2016). Moreover, the very high-level concentration of n-butyric acid exhibited in the LDSPs group might be primarily attributed to the relatively high abundance of Firmicutes according to previous reports (Fu et al., 2019).
Correlation analysis was conducted to further identify the relationship between the gut microbiota community and SCFAs. As shown in Figure 4, the level of butyrate has a significantly (p < 0.05) positive correlation to the abundance of Blautia, Faecalibacterium, and Bacteroides, which belong to members of butyrogenic Clostridium cluster XIVa (Cantu-Jungles et al., 2018). Faecalibacterium produces butyrate which is required for colonic epithelium repair and Treg cell production and plays a crucial role in human health (Faintuch and Faintuch, 2019). Moreover, the concentration of propionic acid was enhanced by the increase in the abundance of Collinsella. A previous study reported that the Collinsella genus correlated closely with the production of pro-inflammatory cytokine IL-17A and could ameliorate the permeability of the gut (Chen B. D. et al., 2017). The increases in the relative abundance of Lachnospiraceae and Prevotella in LDSPs was along with the promotion of total acid. Lachnospiraceae substantially involves the production of SCFAs that are positively correlated to the integrity of the epithelial barrier and immune activation, and its reduction in abundance is found in patients with Parkinson’s disease (Keshavarzian et al., 2020). The results provided strong evidence that LDSPs could exert health benefits by simulating the targeted abundance of beneficial bacteria to produce SCFAs.
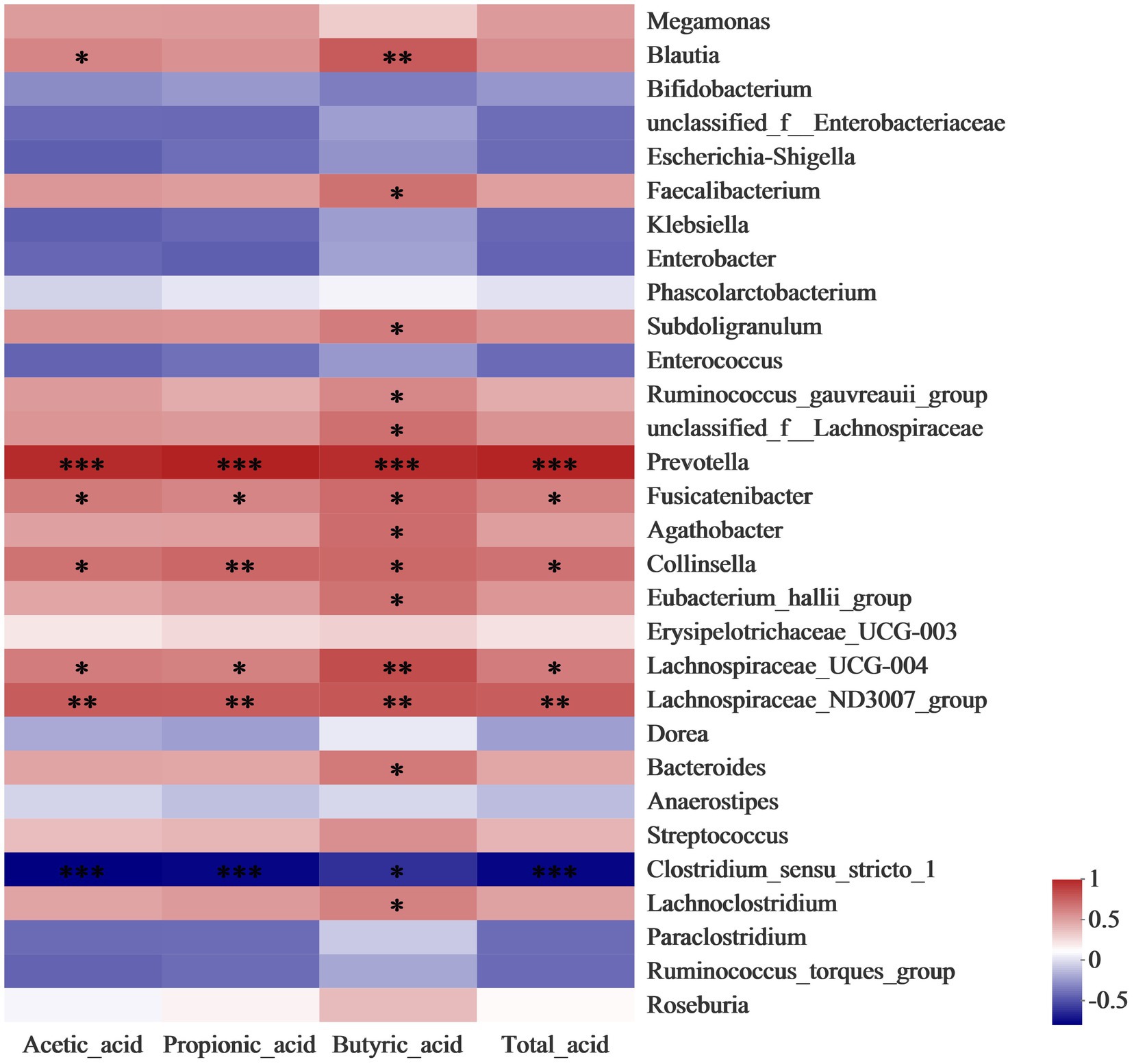
Figure 4. Heatmap analysis for correlation between gut microbiota community and SCFAs. *p < 0.05, **p < 0.01, and ***p < 0.001, respectively; CON, the blank control (no additional carbon source supplement), INU, the positive control (INU supplement), and LDSPs, the experimental group (LDSPs supplement); SCFAs, short-chain fatty acids.
In conclusion, we found that LDSPs were indigestible under simulated saliva-gastrointestinal digestion conditions and degraded and utilized by human gut microbiota after 24 h in vitro fermentation, resulting in a prominent increase in the concentration of SCFAs and a decrease in pH. Remarkably, the production of propionic acid and n-butyric acid after LDSPs fermentation causally correlated with the enrichments of Prevotella, Blautia, and Lachnospiraceae. Therefore, LDSPs may be a potential prebiotic for health benefits.
The data presented in the study are deposited in the NCBI repository (https://www.ncbi.nlm.nih.gov/bioproject/), accession number PRJNA930120.
FZ: experimental studies, data analysis, and writing. YX: experiment design, conceptualization, project administration, and revision. LP: data curation and statistical analysis. LY: experiment design and resources. YL: funding acquisition and conceptualization. DL: experimental studies. XL: statistical analysis. All authors contributed to the article and approved the submitted version.
This study was supported by the Shanghai Agriculture Applied Technology Development Program, China (grant no. X2021-02-08-00-12-F00797), and the Earmarked Fund for China Agriculture Research System, China (Grant No. CARS-20).
The authors would like to thank all the reviewers who participated in the review, as well as the online platform, Majorbio Cloud Platform (www.majorbio.com), for providing microbiological data analysis.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this study.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1083917/full#supplementary-material
Ai, C., Ma, N., Sun, X., Duan, M., Wu, S., Yang, J., et al. (2017). Absorption and degradation of sulfated polysaccharide from pacific abalone in in vitro and in vivo models. J. Funct. Foods 35, 127–133. doi: 10.1016/j.jff.2017.05.022
Arana-Gabriel, Y., Burrola-Aguilar, C., Garibay-Orijel, R., Matías-Ferrer, N., Franco-Maass, S., and Mata, G. (2018). Genetic characterization, evaluation of growth and production of biomass of strains from wild edible mushrooms of Lyophyllum of Central Mexico. Braz. J. Microbiol. 49, 632–640. doi: 10.1016/j.bjm.2017.12.002
Bai, J., Li, T., Zhang, W., Fan, M., Qian, H., Li, Y., et al. (2021). Systematic assessment of oat β-glucan catabolism during in vitro digestion and fermentation. Food Chem. 348:129116. doi: 10.1016/j.foodchem.2021.129116
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brodkorb, A., Egger, L., Alminger, M., Alvito, P., Assunção, R., Ballance, S., et al. (2019). INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 14, 991–1014. doi: 10.1038/s41596-018-0119-1
Cantu-Jungles, T. M., Ruthes, A. C., El-Hindawy, M., Moreno, R. B., Zhang, X., Cordeiro, L. M. C., et al. (2018). In vitro fermentation of Cookeina speciosa glucans stimulates the growth of the butyrogenic clostridium cluster XIVa in a targeted way. Carbohydr. Polym. 183, 219–229. doi: 10.1016/j.carbpol.2017.12.020
Chen, B. D., Sun, L. X., and Xuan, Z. X. (2017). Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J. Autoimmun. 83, 31–42. doi: 10.1016/j.jaut.2017.03.009
Chen, G., Xie, M., Wan, P., Chen, D., Ye, H., Chen, L., et al. (2017). Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 244, 331–339. doi: 10.1016/j.foodchem.2017.10.074
Deveci, E., Çayan, F., Tel-Çayan, G., and Duru, M. E. (2021). Inhibitory activities of medicinal mushrooms on α-amylase and α-glucosidase-enzymes related to type 2 diabetes. S. Afr. J. Bot. 137, 19–23. doi: 10.1016/j.sajb.2020.09.039
Di Gioia, D., Aloisio, I., Mazzola, G., and Biavati, B. (2014). Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl. Microbiol. Biotechnol. 98, 563–577. doi: 10.1007/s00253-013-5405-9
Ding, Y., Yan, Y., Peng, Y., Chen, D., Mi, J., Lu, L., et al. (2019). In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 125, 751–760. doi: 10.1016/j.ijbiomac.2018.12.081
Dubois, M., Gilles, K., Hamilton, J. K., Rebers, P. A., and Smith, F. (1951). A colorimetric method for the determination of sugars. Nature 168, 167–167. doi: 10.1038/168167a0
Faintuch, J., and Faintuch, S. (2019). Microbiome and metabolome in diagnosis, therapy, and other strategic applications. London, United Kingdom: Academic Press. 451–452.
Fu, X., Liu, Z., Zhu, C., Mou, H., and Kong, Q. (2019). Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 59, S130–S152. doi: 10.1080/10408398.2018.1542587
Guo, Y., Chen, X., Gong, P., Chen, F., Cui, D., and Wang, M. (2021). Advances in the in vitro digestion and fermentation of polysaccharides. Int. J. Food Sci. Technol. 56, 4970–4982. doi: 10.1111/ijfs.15308
Hu, J. L., Nie, S. P., Li, C., Wang, S., and Xie, M.-Y. (2018). Ultrasonic irradiation induces degradation and improves prebiotic properties of polysaccharide from seeds of Plantago asiatica L. during in vitro fermentation by human fecal microbiota. Food Hydrocoll. 76, 60–66. doi: 10.1016/j.foodhyd.2017.06.009
Jayachandran, M., Chen, J., Chung, S. S. M., and Xu, B. (2018). A critical review on the impacts of β -glucans on gut microbiota and human health. J. Nutr. Biochem. 61, 101–110. doi: 10.1016/j.jnutbio.2018.06.010
Keshavarzian, A., Engen, P., Bonvegna, S., and Cilia, R. (2020). The gut microbiome in Parkinson’s disease: a culprit or a bystander? Prog. Brain Res. 252, 357–450. doi: 10.1016/bs.pbr.2020.01.004
Kimura, I. (2014). Host energy regulation via SCFAs receptors, as dietary nutrition sensors, by gut microbiota. Yakugaku Zasshi 134, 1037–1042. doi: 10.1248/yakushi.14-00169
Kovatcheva-Datchary, P., Nilsson, A., Akrami, R., Lee, Y. S., De Vadder, F., Arora, T., et al. (2015). Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22, 971–982. doi: 10.1016/j.cmet.2015.10.001
Lam, K. L., Keung, H. Y., Ko, K. C., Kwan, H. S., and Cheung, P. C. K. (2018). In-vitro fermentation of beta-glucans and other selected carbohydrates by infant fecal inoculum: an evaluation of their potential as prebiotics in infant formula. Bioact. Carbohydr. Diet. Fibre 14, 20–24. doi: 10.1016/j.bcdf.2017.07.009
Li, X. J., Guo, R., Wu, X. J., Liu, X., Ai, L. Z., Sheng, Y., et al. (2020). Dynamic digestion of tamarind seed polysaccharide: indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr. Polym. 239:116194. doi: 10.1016/j.carbpol.2020.116194
Louis, P., and Flint, H. J. (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294, 1–8. doi: 10.1111/j.1574-6968.2009.01514.x
Mitsou, E. K., Saxami, G., Stamoulou, E., Kerezoudi, E., Terzi, E., Koutrotsios, G., et al. (2020). Effects of rich in Β-glucans edible mushrooms on aging gut microbiota characteristics: an in vitro study. Molecules 25:2806. doi: 10.3390/molecules25122806
Miura, T., Kubo, M., Itoh, Y., Iwamoto, N., Kato, M., Park, S. R., et al. (2002). Antidiabetic activity of Lyophyllum decastes in genetically type 2diabetic mice[J]. Biol. Pharm. Bull. 25, 1234–1237. doi: 10.1248/bpb.25.1234
Pokhrel, C., and Ohga, S. (2007). Submerged culture conditions for mycelial yield and polysaccharides production by Lyophyllum decastes. Food Chem. 105, 641–646. doi: 10.1016/j.foodchem.2007.04.033
Pushpa, H., and Purushothama, K. B. (2010). Antimicrobial activity of Lyophyllum decastes an edible wildmushroom. World J. Agric. Sci. 6, 506–509.
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vich Vila, A., Võsa, U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605. doi: 10.1038/s41588-019-0350-x
Shoukat, M., and Sorrentino, A. (2021). Cereal β-glucan: a promising prebiotic polysaccharide and its impact on the gut health. Int. J. Food Sci. Technol. 56, 2088–2097. doi: 10.1111/ijfs.14971
Si, J., You, H. J., Yu, J., Sung, J., and Ko, G. (2017). Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 21, 97–105. doi: 10.1016/j.chom.2016.11.010
Slavin, J. (2013). Fiber and prebiotics: mechanisms and health benefits. Nutrients 5, 1417–1435. doi: 10.3390/nu5041417
Sun, S. S., Wang, K., Ma, K., Bao, L., and Liu, H. W. (2019). An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 17, 3–14. doi: 10.1016/S1875-5364(19)30003-2
Tel, G., Ozturk, M., Duru, M. E., and Turkoglu, A. (2015). Antioxidant and anticholinesterase activities of five wild mushroom species with total bioactive contents. Pharm. Biol. 53, 824–830. doi: 10.3109/13880209.2014.943245
Thandapilly, S. J., Ndou, S. P., Wang, Y., Nyachoti, C. M., and Ames, N. P. (2018). Barley b-glucan increases fecal bile acid excretion and short chain fatty acid levels in mildly hypercholesterolemic individuals. Food Funct. 9, 3092–3096. doi: 10.1039/C8FO00157J
Valdes, A. M., Walter, J., Segal, E., and Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. BMJ 361:k2179. doi: 10.1136/bmj.k2179
Wang, L., Li, C., Huang, Q., Fu, X., and Liu, R. H. (2019). In vitro digestibility and prebiotic potential of a novel polysaccharide from Rosa roxburghii Tratt fruit. J. Funct. Foods 52, 408–417. doi: 10.1016/j.jff.2018.11.021
Wu, D. T., Deng, Y., Zhao, J., and Li, S. P. (2017). Molecular characterization of branched polysaccharides from Tremella fuciformis by asymmetrical flow field-flow fractionation and size exclusion chromatography. J. Sep. Sci. 40, 4272–4280. doi: 10.1002/jssc.201700615
Wu, D. T., Guo, H., Lin, S., Lam, S. C., Zhao, L., Lin, D. R., et al. (2018). Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Technol. 79, 171–183. doi: 10.1016/j.tifs.2018.07.016
Xie, J., Liu, Y., Chen, B., Zhang, G., Ou, S., Luo, J., et al. (2019). Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 63:1559. doi: 10.29219/fnr.v63.1559
Zhang, T., Wu, S., Ai, C., Wen, C., Liu, Z., Wang, L., et al. (2021). Galactofucan from Laminaria japonica is not degraded by the human digestive system but inhibits pancreatic lipase and modifies the intestinal microbiota. Int. J. Biol. Macromol. 166, 611–620. doi: 10.1016/j.ijbiomac.2020.10.219
Zhao, J., Liu, H., Michael, A. B., and Qiao, S. (2018). Dietary protein and gut microbiome composition and function. Curr. Protein Pept. Sci. 20, 145–154. doi: 10.2174/13892037196661805141454
Keywords: Lyophyllum decastes (Fr.) Singer, polysaccharides, simulated digestion, fermentation, short-chain fatty acids
Citation: Zhang F, Xiao Y, Pan L, Yu L, Liu Y, Li D and Liu X (2023) Effects of polysaccharides from Lyophyllum decastes (Fr.) Singer on gut microbiota via in vitro-simulated digestion and fermentation. Front. Microbiol. 14:1083917. doi: 10.3389/fmicb.2023.1083917
Received: 29 October 2022; Accepted: 09 January 2023;
Published: 13 February 2023.
Edited by:
Xiaowei Zhang, University of Shanghai for Science and Technology, ChinaReviewed by:
Tianming Yao, Purdue University, United StatesCopyright © 2023 Zhang, Xiao, Pan, Yu, Liu, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xiao, ✉ eS14aWFvbW5AMTYzLmNvbQ==; Ling Yu, ✉ eXVsaW5nQHNpdC5lZHUuY24=; Yanfang Liu, ✉ YWxpdS0xOTgwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.