
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 26 January 2023
Sec. Systems Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1070917
Periodontal disease has been suggested to be linked to adverse pregnancy outcomes such as preterm birth, low birth weight, and preeclampsia. Adverse pregnancy outcomes are a significant public health issue with important clinical and societal repercussions. This article systematically reviews the available epidemiological studies involving the relationship between periodontal disease and adverse pregnancy outcomes over the past 15 years, and finds a weak but independent association between adverse pregnancy outcomes and periodontal disease. The bidirectional association and the potential mechanisms are then explored, focusing on three possible mechanisms: inflammatory reaction, oral microorganisms and immune response. Specifically, elevated systemic inflammation and increased periodontal pathogens with their toxic products, along with a relatively suppressed immune system may lead to the disruption of homeostasis within fetal-placental unit and thus induce adverse pregnancy outcomes. This review also explains the possible mechanisms around why women are more susceptible to periodontal disease. In conclusion, pregnant women are more likely to develop periodontal disease due to hormonal changes, and periodontal disease has also been suspected to increase the incidence of adverse pregnancy outcomes. Therefore, in order to lessen the risk of adverse pregnancy outcomes, both obstetricians and dentists should pay attention to the development of periodontal diseases among women during pregnancy.
In 2013, the Joint EFP/AAP (European Federation of Periodontology/American Academy of Periodontology) Workshop published an updated consensus report focusing on periodontal diseases and adverse pregnancy outcomes (Sanz et al., 2013).
Adverse pregnancy outcomes are serious public health issues with wide-ranging social and economic effects (Bobetsis et al., 2020), and many studies have shown their association with periodontal diseases. Preterm birth (PT), which is defined as delivery prior to 37 full weeks (<259 days), is the primary cause of neonatal death in the first 4 weeks of life (Zi et al., 2014; Iheozor-Ejiofor et al., 2017). Low birth weight (LBW), defined as a weight less than 2,500 g at birth, is typical for infants born preterm and/or with intrauterine restricted growth conditions (IUGR). Additionally, LBW infants are more likely to experience adverse outcomes, such as an increased mortality rate. Preeclampsia (PE) is a multisystem pregnancy condition that affects around 2–10% of pregnant mothers and is a major risk factor for preterm birth and slow infant growth. Hypertension and proteinuria in pregnant women were characterized after the 20th week of gestation (Zi et al., 2014; Gare et al., 2021). It should be noted that, “adverse pregnancy outcome” is a broad term that extends beyond these noted conditions.
Periodontal disease was identified as a possible risk factor for PT as early as 1996 (Offenbacher et al., 1996). Particularly since Han et al. (2006) reported direct evidence of the first oral- utero translocation in 2006, suggesting that the oral cavity was the source of the Bergeyella strain found in the patient’s intrauterine illness, a great deal of effort has been placed in the field of association between oral health and pregnancy over the last 16 years. While some facets of the association between periodontal disease and adverse pregnancy outcomes have been clarified, the potential relationship between them remains controversial and the underlying mechanisms must be better revealed and elucidated.
Given the relatively high incidence of worsened dental health among pregnant women and the devastating consequences of adverse pregnancy outcomes, combined with the reality that oral diseases are largely both curable and avoidable, this review will focus on recent literature reporting the relationship between oral health and pregnancy complications. It will also discuss possible mechanisms of this association. This review assesses the issue from a bidirectional and reciprocal relationship; that is, not only how oral health affects the outcomes of pregnancy, but also how some of the physiological changes that occur during pregnancy can alter oral cavity conditions.
According to epidemiological evidence, preterm birth, low birth weight, pre-eclampsia, and other adverse pregnancy outcomes may be associated with periodontal disease. Forty studies published in the last 15 years and indicating a relationship between periodontal disease and adverse pregnancy outcomes were identified after a search on PubMed following the process shown in the flowchart (Figure 1) below. These studies have been summarized in Table 1. Inclusion criteria were as follows: (1) original publications reporting data from randomized and non-randomized controlled trials, case–control, cross-sectional or cohort studies on the association between periodontal condition and adverse pregnancy outcomes; (2) women during reproductive age; (3) sufficient data such as relative risk (RR), the odds ratio (OR), hazard ratio (HR), p values or 95% confidence interval (CI) were available (4) choose the most recent and comprehensive study when there are overlapping ones. Exclusion criteria were as follows: (1) inadequate or confusing case definitions and unavailable data; (2) papers with abstract only; (3) animal research; (4) literature reviews, comments, letters or replies; (5) languages other than English.
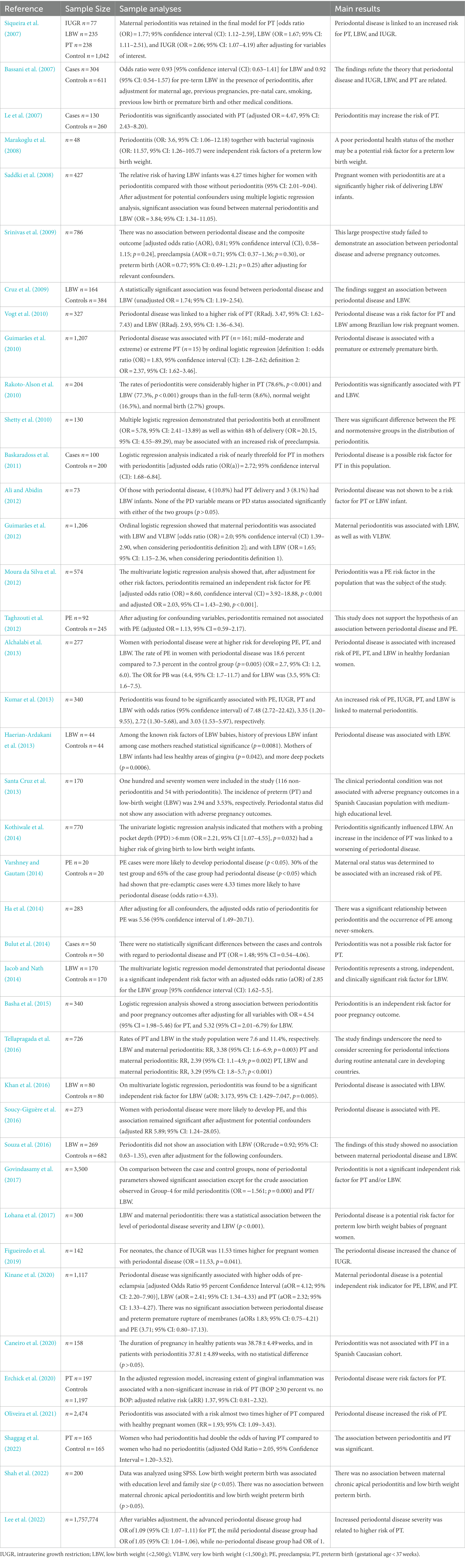
Table 1. Epidemiological studies that reported the association between adverse pregnancy outcomes and periodontal disease published in the last 15 years.
A total of 1,071 articles were identified in the first search. After headlines, abstract, and data screening, 863 articles were excluded for being irrelevant. The remaining 208 papers were read and assessed in their entirety, and 40 articles (n = 1,781,311 participants) were selected for analysis. These identified studies focused on low birth weight, preterm birth, preeclampsia and intrauterine growth restriction. Of the selected studies, 31 suggested a correlation between adverse pregnancy outcomes and periodontal disease (ORs ranging from 0.92 to 20.15) and nine found no evidence of an association (ORs ranging from 0.71 to 1.56). A summary of evidence has been listed in Table 2.
Adverse pregnancy outcomes include PT, LBW/VLBW, PE, IUGR, spontaneous miscarriage, gestational diabetes, fetal injury and stillbirth. For PT, one cohort study (including 1,757,774 pregnant women) by Lee et al. (2022) showed that the more severe the periodontal disease, the higher the risk of PT after variables adjustment. Another cohort study conducted in Africa indicated that women with periodontal disease were twice as likely to PT as women with healthy oral conditions. Low hemoglobin was also demonstrated to be a risk factor for PT (Shaggag et al., 2022). For LBW, Kinane et al. (2020) recruited 1,117 women with singleton delivery and found that periodontal disease was an independent risk indicator for LBW (aOR = 2.41; 95% CI: 1.34–4.33 after adjustment for age, parity, and previous history). However, a prospective cross-sectional study including 3,500 pregnant women suggested no significant association between periodontal parameters and LBW (Govindasamy et al., 2017). For PE, Varshney and Gautam (2014) found that women with PE are 4.33 times more likely to have periodontal disease than normal pregnancies. Coincidentally, the prospective study by Ha et al. (2014) to evaluate the link between periodontal health and PE in a never-smoking population showed that there was a significant relationship between periodontal disease and PE among pregnant women at 21–24 weeks of gestation. Maternal periodontal disease and IUGR have rarely been investigated in recent studies, but a retrospective cohort study has shown an 11.53 times increase in the probability of IUGR in pregnant women with severe periodontal disease (Figueiredo et al., 2019). In addition, adverse pregnancy outcomes such as spontaneous miscarriage, gestational diabetes, and stillbirth have been studied, but their association with periodontal disease requires further investigation (Bobetsis et al., 2020). The evidence suggests there is a correlation between periodontal disease and adverse pregnancy outcomes. Although 31 articles indicated significant association between adverse pregnancy outcomes and periodontal disease, other conflicting studies did not report results of statistical significance between them, possibly due to variations in clinical parameters of periodontal disease assessment, variability in study populations, inclusion of pregnant women in different gestation periods, variation in disease severity and extension, inadequate data analyses, discrepancies of types of the diseases (aggressive and chronic periodontal disease) and so on (Zi et al., 2014; Figuero et al., 2020). Overall, much of the research points to a link between worsening periodontal health and a higher rate of adverse pregnancy outcomes (Bobetsis et al., 2020).
Periodontal conditions can affect pregnancy outcomes. What exactly are the biological mechanisms behind this? Two major pathways have been hypothesized in the consensus report from the Joint EFP/AAP Workshop on periodontitis and systematic diseases. One is indirect mechanisms, largely mediated by periodontitis-associated elevation of inflammatory mediators which can break the homeostasis of placental barrier. The other is direct mechanisms, mainly associated with oral microorganism translocation and the toxic component secretion on site. Both can trigger a metastatic infection within the fetal-placental unit (Figuero et al., 2020). In addition, based on the literature review, an aberrant shift in maternal immune response during pregnancy also seems to play a role in adverse pregnancy outcomes (Zi et al., 2014). These three postulated pathways will be described below.
A series of studies have been conducted to explore the association between elevated serum levels of inflammatory cytokines and adverse pregnancy outcomes. According to some studies, women with subclinical intra-amniotic infection had considerably higher levels of maternal blood inflammation-associated cytokines than healthy women. For example, a study by Perunovic et al. (2016) showed that PT women had worse periodontal parameters and significantly increased levels of prostaglandin E2 (PGE2) and interleukin-6 (IL-6), both of which are labor triggers and therefore contribute to the preterm birth. However, few contradicting findings without discernible differences have also been published. An observational case/control study by Mesa et al. (2016) showed that no relationship was found between PT/LBW and the markers of systemic inflammatory response assessed such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β).
In the subgingival region, gram-negative microaerophilic and anaerobic bacteria generate large quantities of proinflammatory mediators. Increased production of inflammatory mediators of periodontal origin may initiate metastatic inflammation including the placenta-fetal unit by blood circulation (Zi et al., 2014; Figuero et al., 2020). Studies in animals showed that periodontal pathogen infections in mothers raise levels of circulating IL-1β, IL-6, interleukin-17 (IL-17), and TNF-α and cause PT (Ao et al., 2015). Periodontal infections and their byproducts lead to the release of Cyclooxygenase-2 (COX-2), IL-8, interferon-γ (IFN-γ), and TNF-α secretion and/or apoptosis in placental tissues/cells in vitro models (Ren and Du, 2017). Focusing on a few specific inflammation-related factors, TNF-α has been identified as a potential mediator of overexpression of endothelial activation and injury, a key pathogenetic mechanism of PE (Fitzmaurice et al., 2004). IL-6 may be associated with polycystic ovary syndrome, which increases the risk of PE and PT (Piltonen, 2016). Elevated levels of proinflammatory cytokines, in particular interleukins IL-6, IL-1β, and TNF-α are associated with PT as compared to levels found at term birth (Lyon et al., 2010). In addition, elevated levels of prostaglandins in the chorion can lead to cervical ripening and uterine contractions, ultimately leading to an increased risk of PT (Bobetsis et al., 2020).
As mentioned above, increased periodontal origin inflammatory mediators may initiate metastatic inflammation in the placenta. Additionally, the periodontal microorganisms together with their byproducts can trigger an inflammatory cascade via hematogenous dissemination. Microbial pattern recognition receptors, such as Toll-like Receptors (TLR), recognize these circulating microbes and signal pro-inflammatory pathways in the placenta (chorion, metamorphosis and trophoblast cells; Guleria and Pollard, 2000). Furthermore, signaling the periodontal pathogens in gingival tissues helps release additional inflammatory agents that can spread through the system. The final result is that circulating microorganisms together with their by-products initiate an inflammatory response at the fetal-placental unit, indirectly (Bobetsis et al., 2020; Figuero et al., 2020; Genco and Sanz, 2020).
Regardless of how inflammatory response is induced, the exacerbation of inflammatory processes causes the shift of the uterus from a quiescent to a contractile state, which may result in PT, fetal injury, LBW and so on (Romero et al., 2014; Zi et al., 2014).
Notably, bacteria and proinflammatory cytokines in the infected periodontal tissues are released into the systemic circulation and can increase C-reactive protein levels through an acute response in the liver of pregnant women, which can lead to adverse pregnancy outcomes such as PE, PT and IUGR (Paraskevas et al., 2008; Bobetsis et al., 2020). Acute-phase reactants can trigger secondary reactions, known as metastatic inflammation, in the fetal-placental unit. In other words, intrauterine inflammatory response can be amplified by increased plasma C-reactive protein, which can lead to adverse pregnancy outcomes through tissue damage, complement activation and induction of proinflammatory cytokines.
Specifically, the enhanced inflammatory cytokine levels in the feto-placental unit stimulate uterine contractility, exacerbate cervical ripening, induce endothelial dysfunction, cause the rupture of fetal membrane and eventually leads to an increased risk for IUGR, LBW, VLBW, PE, PT and so on (Bobetsis et al., 2020; Gómez et al., 2020). Figure 2 presents the inflammatory pathway and its role in adverse pregnancy outcomes.
The fetal placental unit may not be as sterile as previously thought, with nearly a third of placental specimens confirmed to contain intracellular bacteria in the substrate, the tissue layer and the underside of the maternal-fetus interface (Pelzer et al., 2017; Park et al., 2022). Moreover, a placenta microbiome study reported that the placental microbiome is in fact more closely related to the oral microbiome than to the urogenital tract microbiome, which is what is generally expected. This finding suggested a link between oral microbiome and potential adverse pregnancy outcomes (Aagaard et al., 2014; Zi et al., 2014).
The infected periodontal tissues acts as a reservoir for bacteria that can transfer from periodontal tissues to the fetal placenta unit and trigger a metastatic infection (Zi et al., 2014; Bobetsis et al., 2020; Genco and Sanz, 2020). Periodontal pathogens, such as Fusobacterium nucleatum, Porphyromonas gingivalis, Filifactor alocis, Campylobacter rectus, Tannerella forsythia, Prevotella nigrescens, and Parvimonas micra, among others, were detected in the amniotic fluid from mothers with periodontal disease (Han et al., 2006; Andonova and Iliev, 2021; Narita and Kodama, 2022).
How do the oral microorganisms with their by-products actually contribute to adverse pregnancy outcomes? The pathogenic subgingival microorganisms make the translocation to the bloodstream possible, that is, they cause bacteremia (Figuero et al., 2020). The dissemination of symbiotic and pathogenic microbes in the blood can lead to the establishment of metastatic infections in various parts of the fetal-placental unit, such as amniotic fluid, choriodecidual space, placenta, chorioamniotic membrane, umbilical cord, and the fetus (Bobetsis et al., 2020).
A unique adhesin, namely Fad A, plays an important role in F. nucleatum (a Gram-negative anaerobe frequently associated with adverse pregnancy outcomes) colonization in vivo (Liu et al., 2007; Han et al., 2010). It is not only an adhesin but also an invasin (Han, 2015). Vascular endothelial-cadherin, a member of the cadherin family and a cell–cell connection protein, has been recognized as an endothelium receptor for Fad A, a necessary component for F. nucleatum to bind to endothelial cells. Due to the enhanced endothelial permeability, the bacteria are able to pass through loosening junctions in the endothelium and penetrate the placental barrier (Fardini et al., 2011).
Lipopolysaccharide (LPS), synthesized by pathogenic microorganisms, is one of the most important virulence factors. Porphyromonas gingivalis is the main pathogen of periodontal disease (Gómez et al., 2020; Mei et al., 2020). Porphyromonas gingivalis LPS induces IL-8 and IL-6 production via TLR-2 in chorion-derived cells and can increase expression of COX-2, IL-8 and TNF-α in human trophoblast-8 in an NF-κB-dependent fashion (Hasegawa-Nakamura et al., 2011; Ao et al., 2015). Aggregatibacter actinomycetemcomitans LPS (Aa-LPS) induces apoptosis in human trophoblasts via the mitochondria-dependent pathway by increasing levels of caspase 9, caspase 3, caspase 2, cytochrome c and so on (Li et al., 2011).
Moreover, there are many different molecular mechanisms specific to different microorganisms. For example, P. gingivalis can induce a decrease of CD56+ dNK cells and a rise in CD16+ dNK cells in the first trimester. It may also interrupt the function of stromal cells that are frequently linked to uNK cells and CD68+ macrophages in a paracrine manner (Reyes et al., 2017). Inadequate remodeling of the myometrial segments of the uterine spiral arteries, known as defective deep placentation (DDP), may also be a common mechanism of P. gingivalis inducing adverse pregnancy outcomes (Brosens et al., 2011). And in a dose-dependent manner, Campylobacter rectus challenge dramatically increased both mRNA and protein levels of TNF-α and IL-6 in human trophoblasts (Arce et al., 2010).
Lastly, circulating microorganisms together with their by-products may also trigger a direct inflammatory reaction in the uterus, which have been covered in detail in the indirect infection section (Zi et al., 2014; Bobetsis et al., 2020; Genco and Sanz, 2020).
The infection induced by the microbial community in subgingival sites of periodontal disease patients leads to a maternal immune response to pathogenic bacteria and their products, and the elevated serum inflammatory cytokines produced by the immune system play an adverse role to the pregnancy. Nonetheless, this side-effect is listed as the third mechanism in this review, in addition to the indirect and direct effects.
Maternal immune responses play a dual role throughout pregnancy. On the one hand, the mother and her fetus must be shielded from external pathogens. On the other hand, the embryo/fetus expresses paternal antigens that serve as an allograft, which have to be tolerated by the mother during the whole pregnancy period (Zi et al., 2014; Bobetsis et al., 2020). Altogether, pregnancy characteristically presents with physiological immune tolerance.
Even in the early stages of pregnancy, the maternal immune system experiences significant changes. Specifically, some substances contained in seminal fluid can promote the shift of dendritic cells (DCs) to be more tolerogenic. This promotes a conversion from Th17 and T helper-1 (Th1) toward a T regulatory cells (Treg) and Th2. Treg may be involved in inhibiting maternal effector T cells, such as Th17 cells, in peripheral blood. In addition, antibodies secreted by B cells protect the presence of paternal antigens in trophoblasts once they enter the fetal-maternal interface. Last but not least, a wide range of molecules play a role in immune tolerance at the interface. For example, molecules secreted or produced by the trophoblast itself modulate the phenotype of function of immune cells, which can make DCs turn or remain immature and thus tolerogenic. When it comes to molecules secreted by innate immune system cells, they can positively influence trophoblast physiology while helping maternal T cells become or remain resistant to paternal antigens expressed by the fetus (Zenclussen, 2013; Bobetsis et al., 2020).
These physiological processes are so delicate that if any triggering mechanisms disturb them may break the balance and result in adverse pregnancy outcomes. Unfortunately, the infection of periodontal microbes triggers a switch in the maternal immune response to a pathogenic pro-inflammatory response, disrupting the homeostasis at the maternal-fetal interface and diminishing these immunological privileges throughout pregnancy (Zi et al., 2014).
Certain infectious diseases, even subclinical infections, may lead to an overall bias toward type 1, resulting in an increase in the number and activity of Th1/Th17 cells. The Th1 response activates decidual macrophages, which release toxic amounts of TNF-α and nitric oxide, leading to deleterious effects to the fetus. Overall, it appears that an imbalance of Th17/Treg proportion is associated with adverse pregnancy outcomes (Zenclussen, 2013; Zi et al., 2014).
The B cell response induced by infection cannot be neglected. Periodontal infection by P. gingivalis can cause atopobiosis to the placenta and induce inflammation (Gómez et al., 2020). Generally, the infection levels of P. gingivalis are correlated with the antibody response to the pathogens (Saraiva et al., 2014). A study has shown that LBW was linked to a higher maternal serum antibody level against P. gingivalis at mid-trimester (Dasanayake et al., 2001). Interestingly, in women with severe periodontitis, the risk of adverse pregnancy outcomes was higher when the antibody response to periodontal pathogens is lower (Zi et al., 2014).
Although a great deal of prospective studies have shown a positive association between oral condition and adverse pregnancy, the findings are far from conclusive when it comes to clinical interventions. There are still some controversial studies reporting that treating periodontal disease does not reduce the incidence of adverse pregnancy outcomes (Macones et al., 2010; Polyzos et al., 2010). Michalowicz et al. (2009) stated that non-surgical mechanical periodontal treatment did not significantly alter the level of inflammatory mediators in serum and these markers were not associated with adverse pregnancy outcomes such as PT and LBW. A study performed by Penova-Veselinovic et al. (2015) showed periodontal treatment can lower the levels of specific inflammatory mediators in gingival crevicular fluid, but no discernible difference in pregnancy outcomes was observed between the treatment and control groups. Reddy et al. conducted a randomized clinical study to establish the effect of non-surgical periodontal therapy on pregnancy outcomes in women with periodontal diseases. Phase-I periodontal therapy was given to the treatment group, while only oral hygiene guidance was imparted to the control group. The results showed no statistically significant difference in pregnancy outcomes between the groups. However, this study concluded that periodontal diseases enhanced the serum IgM antibody concentration, which may lead to a higher prevalence of PT and LBW in the control group (Reddy et al., 2014). Additionally, a multivariate logistic analysis performed by López et al. (2005) showed that women with periodontal diseases were at a higher incidence of PT/LBW than women who received periodontal therapy before 28 weeks of gestation.
As in previous literature, recent studies have demonstrated that periodontal diseases are risk factors for adverse pregnancy outcomes. However, the impact of periodontal treatment on the prevention of adverse pregnancy outcomes remains a contentious topic, despite the fact that the majority of non-surgical mechanical treatments for pregnant women with periodontal disease have shown improvements in clinical parameters regarding oral health. Given the complexity of the clinical problem, the inconsistent data could be attributed to variations in periodontal disease diagnosis criteria, the effectiveness of periodontal treatment strategies, individual differences in maternal responses, differences in disease severity, and so on. Notably, significant associations have been reported with antenatal factors and periodontal status leading to adverse pregnancy outcomes. For example, maternal stress is a risk factor for PT, which may be related to the production of adrenocorticotropic hormone-releasing hormone (CRH; Mannem and Chava, 2011; Romero et al., 2014). Other relative heterogeneities in the study population, such as smoking, age, ethnicity, and education level may also influence pregnancy outcomes (Huck et al., 2011).
How can the occurrence of adverse pregnancy outcomes be minimized? First, periodontal treatment before pregnancy is recommended. The first 12 weeks of pregnancy are crucial for fetal organogenesis and therefore aggressive periodontal treatment is not recommended during this period. From a biological point of view, treatment given in the second trimester may be too late because the pathogenicity potential of the microbial community and the severity of the periodontal disease increase throughout the pregnancy and interventions at this stage cannot influence pathogens already present in the placenta (Zi et al., 2014; Cobb et al., 2017). Next, surgical treatment and the use of antibiotics can be taken into account. More aggressive treatments such as surgical periodontal treatment can better improve periodontal conditions, especially for patients with severe periodontal disease (Heitz-Mayfield et al., 2002).The additional application of antibiotics including amoxicillin and metronidazole may serve as an effective intervention to get rid of periodontal diseases (Leitich et al., 2003; Keestra et al., 2015). However, given the specificity of the pregnant population, the fear of teratogenicity may mean that there is still a long way to go before these can be implemented. While undergoing more thorough treatment, dentists prescribing medication to pregnant women should specifically follow the Food and Drug Administration (FDA) regulations regarding the use of medication in pregnancy. Lastly, there should be interdisciplinary cooperation between obstetricians and dentists in order to efficiently identify risk factors for adverse pregnancies and to provide timely and effective interventions. Preventive oral health care can also be promoted as part of prenatal care. Obstetricians, as the health care professionals most commonly contacted by women during pregnancy, should educate on the importance of maintaining good oral hygiene and promptly remind them to receive necessary dental care.
Periodontal health impacts the pregnancy process, and vice versa. In other words, not only may periodontal disease interfere with pregnancy but also periodontal conditions tend to worsen during pregnancy due to specific physiological alterations (Kapila, 2021).
During pregnancy, a woman’s body goes through significant hormonal changes and organ system adaptations, as well as changes in the oral cavity. Hormones, as specific regulatory molecules, play important roles in modulating the periodontal tissue responses and may change periodontal tissue responses to microbial plaque, which could exacerbate the severity of periodontal disease (Güncü et al., 2005; Yokoyama et al., 2005). During pregnancy, a woman’s sex hormones levels fluctuate wildly. Progesterone and estrogen, which work through various biochemical mechanisms to quiet or activate the myometrial smooth muscle cervical composition and reach peak plasma levels by the end of the third trimester, mediate the majority of the hormonal regulation of labor and birth (Bobetsis et al., 2020; Figuero et al., 2020). These hormone changes make the host more susceptible to periodontal disease.
Increased sensitivity to stimuli occurs in the gingiva during pregnancy (Terzic et al., 2021). For example, pregnant women are more susceptible to inflammation and symptoms often take place in the second or third month of pregnancy. When probed, the gingiva seem red, swollen, sensitive to stimulation, larger, and prone to bleeding (Huck et al., 2011; Gare et al., 2021).
Sex hormones can modulate the production of cytokines. The temporary elevation of certain sex hormones such as progesterone and estrogen can induce proinflammatory cytokines including IL-6, IL-8, and IL-1β to be released in the tissue, which has been associated with an increase in the extent, prevalence and intensity of gingival inflammation (Bobetsis et al., 2020; Figuero et al., 2020). Moreover, progesterone increases the synthesis of prostaglandins, particularly PGE2, which can amplify the clinical manifestations of gingival inflammation by increasing vascular capillarity and permeability (Markou et al., 2009).
In addition to the vascular system, connective tissue is also a major target of hormones during pregnancy. The migratory cells, fibroblasts, and extracellular matrix can also be affected (Laine, 2002). Progesterone plays an important role in increasing the production of vascular endothelial growth factor (VEGF) in human gingival fibroblasts (HGF; Yokoyama et al., 2005). And it can dilate the gingival capillaries and increase capillary permeability by stimulating the endothelial cells through inhibiting cellular antioxidant effect and increasing oxidative stress (Prakash et al., 2012; Yuan et al., 2016). The changes in vascular responses and connective tissue turnover in the periodontium indirectly contribute to the increased gingival inflammation (Silva de Araujo Figueiredo et al., 2017).
During pregnancy, the surge of hormonal levels triggers oral tissue responses (Cornejo Ulloa et al., 2021), which means changes in the composition or abundance of oral microorganisms occur relative to postpartum or non-pregnant status. This shift may lead to a potentially more hazardous microbial community (Zi et al., 2014).
Pregnancy, especially in the early stages, accelerates the growth of bacteria in the oral cavity and makes it easier for periodontal pathogens to colonize there (Fujiwara et al., 2017). A major change in the oral microbiome during pregnancy is increased microbial load (Neuman and Koren, 2017). Research examining the prevalence of seven common bacterial species in the oral cavity found that early pregnancy had considerably higher overall cultivable microbial counts compared to non-pregnant women. It is worth noting that the bacteria count of P. gingivalis and A. actinomycetemcomitans, two of the main periodontal pathogens, is elevated in pregnant women as well (Fujiwara et al., 2017). Progesterone levels in the first trimester are positively correlated with P. gingivalis, and this relevance suggests that progesterone levels during this period promote the growth of P. gingivalis (Massoni et al., 2019). This phenomenon can be explained by the fact that both estradiol and progesterone could substitute vitamin K, which serves as an essential growth factor for P. gingivalis, and therefore stimulates P. gingivalis growth and elevates gingival inflammation (Kornman and Loesche, 1982). It is also consistent with the fact that both progesterone and estradiol are significantly elevated during pregnancy. Moreover, increased levels of anaerobic species such as A. actinomycetemcomitans and Parvimonas micra may also induce a shift in the microbial communities on mucosal surfaces, which can lead to pro-inflammatory immune responses (Zi et al., 2014).
The maturation and selection of thymocytes, cell proliferation, MHC-II expression, cell migration and cytokine generation are all immunological processes that are modulated by sex hormones (Ortiz-Sánchez et al., 2021). The severity of periodontal diseases can be exacerbated by immune suppression during pregnancy, including the altered lymphocyte response, suppression of T-cell activity, decreased antibody production and depressed phagocytosis and neutrophil chemotaxis (Boyapati et al., 2021).
Specifically, during pregnancy, the immune system is adapted to be able to tolerate the fetus, a potential antigen source. Thus, both in the fetal-maternal interface and the peripheral blood, an immune response shift from Th1 and Th17 to Th2 and Treg cells takes place (Zi et al., 2014; Bobetsis et al., 2020). Also, functional changes in polymorphonuclear leukocytes include alterations and decreases of chemotaxis, as well as adherence and inhibition of the neutrophil respiratory burst, which can worsen the periodontal condition (Morelli et al., 2018). Additionally, proinflammatory cytokines such as IFN-γ and TNF-α may also decrease with the increase of estrogen, which has been observed in experimental models (Soldan et al., 2003). These modifications affect the defensive system of periodontal tissues, making gingival tissue less efficient at resisting the inflammatory challenges produced by bacteria (Gare et al., 2021; Raju and Berens, 2021).
The mother’s immune system is more vulnerable during this special period, making her body more susceptible to illnesses. It has been shown that human gingiva is a target tissue for increases in estrogen and progesterone. Moreover, periodontal microvascularization can be caused by estradiol. These changes in oral tissues lead to a transition toward a more anaerobic flora (Pucci et al., 2021), which favors the growth of periodontal pathogens.
Emotional and psychosocial stress are factors of periodontal disease. The emotional fluctuation during pregnancy could increase the mother’s risk for periodontal disease, but the precise role of stress in the pathogenesis of periodontal diseases is unknown (Pihlstrom et al., 2005). Moreover, some women may modify their dietary habits during the first trimester of pregnancy, such as consuming more carbohydrates. And vomiting during this period increases the acidity of saliva (Morelli et al., 2018). As mentioned above, pregnancy aggravates gingiva bleeds due to the elevated concentration of estrogens, and the bleeding may make women feel unwilling to brush their teeth because of hemophobia (Terzic et al., 2021).
This review discusses the bidirectional relationship between periodontal disease and adverse pregnancy outcomes and elucidates the potential mechanisms. We further explored the underlying logic behind this bidirectional relationship from three possible pathways building on existing research.
Although current mechanistic and clinical intervention studies need to be further developed, clarification of the relationship between specific periodontal pathogens, inflammatory factors and adverse pregnancy outcomes can help to develop effective preventive intervention strategies for specific populations. Ultimately, periodontal disease is relatively both preventable and treatable, whereas adverse pregnancy outcomes can be a huge burden to the family and society. Therefore, prenatal periodontal treatment is a decent option because it improves oral health, advances general health, and reduces the risk of deleterious effects to the pregnant women and their fetuses.
XW and XF contributed to the conception and design of the work, drafting the manuscript, made final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LY and CZ contributed to the interpretation of data for the work, made the figures, drafting the manuscript, made final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RH contributed to the conception and design of the work, revised the manuscript, made final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
This study is partially supported by National Natural Science Foundation of China (NSFC31800114) to RH.
We thank Sara Alhaffar for the language proof reading.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aagaard, K., Ma, J., Antony, K. M., Ganu, R., Petrosino, J., and Versalovic, J. (2014). The placenta harbors a unique microbiome. Sci. Transl. Med. 6:237ra65. doi: 10.1126/scitranslmed.3008599
Alchalabi, H. A., Al Habashneh, R., Jabali, O. A., and Khader, Y. S. (2013). Association between periodontal disease and adverse pregnancy outcomes in a cohort of pregnant women in Jordan. Clin. Exp. Obstet. Gynecol. 40, 399–402.
Ali, T. B. T., and Abidin, K. Z. (2012). Relationship of periodontal disease to pre-term low birth weight infants in a selected population--a prospective study. Community Dent. Health 29, 100–105. doi: 10.1922/CDH_2639TaiyebAli06
Andonova, I., and Iliev, V. (2021). Oral anaerobic microflora and pregnancy complication. Open Access Maced. J. Med. Sci. 9, 1681–1685. doi: 10.3889/oamjms.2021.7601
Ao, M., Miyauchi, M., Furusho, H., Inubushi, T., Kitagawa, M., Nagasaki, A., et al. (2015). Dental infection of Porphyromonas gingivalis induces preterm birth in mice. PLoS One 10:e0137249. doi: 10.1371/journal.pone.0137249
Arce, R. M., Diaz, P. I., Barros, S. P., Galloway, P., Bobetsis, Y., Threadgill, D., et al. (2010). Characterization of the invasive and inflammatory traits of oral campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast cultures. J. Reprod. Immunol. 84, 145–153. doi: 10.1016/j.jri.2009.11.003
Basha, S., Shivalinga Swamy, H., and Noor Mohamed, R. (2015). Maternal periodontitis as a possible risk factor for preterm birth and low birth weight – a prospective study. Oral Health Prev. Dent. 13, 537–544. doi: 10.3290/j.ohpd.a34053
Baskaradoss, J. K., Geevarghese, A., and Kutty, V. R. (2011). Maternal periodontal status and preterm delivery: a hospital based case-control study. J. Periodontal Res. 46, 542–549. doi: 10.1111/j.1600-0765.2011.01371.x
Bassani, D. G., Olinto, M. T. A., and Kreiger, N. (2007). Periodontal disease and perinatal outcomes: a case-control study. J. Clin. Periodontol. 34, 31–39. doi: 10.1111/j.1600-051X.2006.01012.x
Bobetsis, Y. A., Graziani, F., Gürsoy, M., and Madianos, P. N. (2020). Periodontal disease and adverse pregnancy outcomes. Periodontol. 83, 154–174. doi: 10.1111/prd.12294
Boyapati, R., Cherukuri, S. A., Bodduru, R., and Kiranmaye, A. (2021). Influence of female sex hormones in different stages of women on periodontium. J Midlife Health 12, 263–266. doi: 10.4103/jmh.jmh_142_21
Brosens, I., Pijnenborg, R., Vercruysse, L., and Romero, R. (2011). The “great obstetrical syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 204, 193–201. doi: 10.1016/j.ajog.2010.08.009
Bulut, G., Olukman, O., and Calkavur, S. (2014). Is there a relationship between maternal periodontitis and pre-term birth? A prospective hospital-based case-control study. Acta Odontol. Scand. 72, 866–873. doi: 10.3109/00016357.2014.919663
Caneiro, L., Lopez-Carral, J. M., Martin-Lancharro, P., Linares, A., Batalla, P., and Blanco-Carrion, J. (2020). Periodontitis as a preterm birth risk factor in Caucasian women: a cohort study. Oral Health Prev. Dent. 18, 77–84. doi: 10.3290/j.ohpd.a44116
Cobb, C., Kelly, P., Williams, K., Babbar, S., Angolkar, M., and Derman, R. (2017). The oral microbiome and adverse pregnancy outcomes. Int. J. Women's Health 9, 551–559. doi: 10.2147/IJWH.S142730
Cornejo Ulloa, P., Krom, B. P., and van der Veen, M. H. (2021). Sex steroid hormones as a balancing factor in Oral host microbiome interactions. Front. Cell. Infect. Microbiol. 11:714229. doi: 10.3389/fcimb.2021.714229
Costa, E. M., de Araujo Figueiredo, C. S., Martins, R. F. M., Ribeiro, C. C. C., Alves, C. M. C., Sesso, M. L. T., et al. (2019). Periodontopathogenic microbiota, infectious mechanisms and preterm birth: analysis with structural equations (cohort—BRISA). Arch. Gynecol. Obstet. 300, 1521–1530. doi: 10.1007/s00404-019-05355-x
Cruz, S. S., Costa, M. D. C. N., Gomes-Filho, I. S., Rezende, E. J. C., Barreto, M. L., Dos Santos, C. A. S. T., et al. (2009). Contribution of periodontal disease in pregnant women as a risk factor for low birth weight. Community Dent. Oral Epidemiol. 37, 527–533. doi: 10.1111/j.1600-0528.2009.00492.x
Dasanayake, A. P., Boyd, D., Madianos, P. N., Offenbacher, S., and Hills, E. (2001). The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J. Periodontol. 72, 1491–1497. doi: 10.1902/jop.2001.72.11.1491
Erchick, D. J., Khatry, S. K., Agrawal, N. K., Katz, J., LeClerq, S. C., Rai, B., et al. (2020). Risk of preterm birth associated with maternal gingival inflammation and oral hygiene behaviours in rural Nepal: a community-based, prospective cohort study. BMJ Open 10:e036515. doi: 10.1136/bmjopen-2019-036515
Fardini, Y., Wang, X., Témoin, S., Nithianantham, S., Lee, D., Shoham, M., et al. (2011). Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 82, 1468–1480. doi: 10.1111/j.1365-2958.2011.07905.x
Figueiredo, M. G. O. P., Takita, S. Y., Dourado, B. M. R., Mendes, H. D. S., Terakado, E. O., Nunes, H. R. D. C., et al. (2019). Periodontal disease: repercussions in pregnant woman and newborn health—a cohort study. PLoS One 14:e0225036. doi: 10.1371/journal.pone.0225036
Figuero, E., Han, Y. W., and Furuichi, Y. (2020). Periodontal diseases and adverse pregnancy outcomes: mechanisms. Periodontol. 83, 175–188. doi: 10.1111/prd.12295
Fitzmaurice, C., Efraim, S. B., Vervoort, M., Visser, W., and Wallenburg, H. C. S. (2004). Tumor necrosis factor-α in whole blood cultures of preeclamptic patients and healthy pregnant and nonpregnant women. Hypertens. Pregnancy 23, 319–329. doi: 10.1081/PRG-200030334
Fujiwara, N., Tsuruda, K., Iwamoto, Y., Kato, F., Odaki, T., Yamane, N., et al. (2017). Significant increase of oral bacteria in the early pregnancy period in Japanese women. J. Investig. Clin. Dent. 8:e12189. doi: 10.1111/jicd.12189
Gare, J., Kanoute, A., Meda, N., Viennot, S., Bourgeois, D., and Carrouel, F. (2021). Periodontal conditions and pathogens associated with pre-eclampsia: a scoping review. Int. J. Environ. Res. Public Health 18:7194. doi: 10.3390/ijerph18137194
Genco, R. J., and Sanz, M. (2020). Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol. 83, 7–13. doi: 10.1111/prd.12344
Gómez, L. A., De Avila, J., Castillo, D. M., Montenegro, D. A., Trujillo, T. G., Suárez, L. J., et al. (2020). Porphyromonas gingivalis placental Atopobiosis and inflammatory responses in women with adverse pregnancy outcomes. Front. Microbiol. 11:591626. doi: 10.3389/fmicb.2020.591626
Govindasamy, R., Dhanasekaran, M., Varghese, S., Balaji, V., Karthikeyan, B., and Christopher, A. (2017). Maternal risk factors and periodontal disease: a cross-sectional study among postpartum mothers in Tamil Nadu. J. Pharm. Bioallied Sci. 9, S50–S54. doi: 10.4103/jpbs.JPBS_88_17
Guimarães, A. N., Silva-Mato, A., Miranda Cota, L. O., Siqueira, F. M., and Costa, F. O. (2010). Maternal periodontal disease and preterm or extreme preterm birth: an ordinal logistic regression analysis. J. Periodontol. 81, 350–358. doi: 10.1902/jop.2009.090527
Guimarães, A. N., Silva-Mato, A., Siqueira, F. M., Cyrino, R. M., Cota, L. O. M., and Costa, F. O. (2012). Very low and low birth weight associated with maternal periodontitis. J. Clin. Periodontol. 39, 1024–1031. doi: 10.1111/jcpe.12000
Guleria, I., and Pollard, J. W. (2000). The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 6, 589–593. doi: 10.1038/75074
Güncü, G. N., Tözüm, T. F., and Cağlayan, F. (2005). Effects of endogenous sex hormones on the periodontium--review of literature. Aust. Dent. J. 50, 138–145. doi: 10.1111/j.1834-7819.2005.tb00352.x
Ha, J.-E., Jun, J.-K., Ko, H.-J., Paik, D.-I., and Bae, K.-H. (2014). Association between periodontitis and preeclampsia in never-smokers: a prospective study. J. Clin. Periodontol. 41, 869–874. doi: 10.1111/jcpe.12281
Haerian-Ardakani, A., Eslami, Z., Rashidi-Meibodi, F., Haerian, A., Dallalnejad, P., Shekari, M., et al. (2013). Relationship between maternal periodontal disease and low birth weight babies. Iran J. Reprod. Med. 11, 625–630.
Han, Y. W. (2015). Fusobacterium nucleatum: a commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147. doi: 10.1016/j.mib.2014.11.013
Han, Y. W., Fardini, Y., Chen, C., Iacampo, K. G., Peraino, V. A., Shamonki, J. M., et al. (2010). Term stillbirth caused by oral Fusobacterium nucleatum. Obstet. Gynecol. 115, 442–445. doi: 10.1097/AOG.0b013e3181cb9955
Han, Y. W., Ikegami, A., Bissada, N. F., Herbst, M., Redline, R. W., and Ashmead, G. G. (2006). Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J. Clin. Microbiol. 44, 1475–1483. doi: 10.1128/JCM.44.4.1475-1483.2006
Hasegawa-Nakamura, K., Tateishi, F., Nakamura, T., Nakajima, Y., Kawamata, K., Douchi, T., et al. (2011). The possible mechanism of preterm birth associated with periodontopathic Porphyromonas gingivalis. J. Periodontal Res. 46, 497–504. doi: 10.1111/j.1600-0765.2011.01366.x
Heitz-Mayfield, L. J. A., Trombelli, L., Heitz, F., Needleman, I., and Moles, D. (2002). A systematic review of the effect of surgical debridement vs. non-surgical debridement for the treatment of chronic periodontitis. J. Clin. Periodontol. 29, 92–102. doi: 10.1034/j.1600-051X.29.s3.5.x
Huck, O., Tenenbaum, H., and Davideau, J.-L. (2011). Relationship between periodontal diseases and preterm birth: recent epidemiological and biological data. J. Pregnancy 2011:164654. doi: 10.1155/2011/164654
Iheozor-Ejiofor, Z., Middleton, P., Esposito, M., and Glenny, A.-M. (2017). Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst. Rev. 2017:CD005297. doi: 10.1002/14651858.CD005297.pub3
Jacob, P. S., and Nath, S. (2014). Periodontitis among poor rural Indian mothers increases the risk of low birth weight babies: a hospital-based case control study. J. Periodont. Implant Sci. 44, 85–93. doi: 10.5051/jpis.2014.44.2.85
Kapila, Y. L. (2021). Oral health’s inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol. 87, 11–16. doi: 10.1111/prd.12398
Keestra, J. A. J., Grosjean, I., Coucke, W., Quirynen, M., and Teughels, W. (2015). Non-surgical periodontal therapy with systemic antibiotics in patients with untreated aggressive periodontitis: a systematic review and meta-analysis. J. Periodontal Res. 50, 689–706. doi: 10.1111/jre.12252
Khan, N. S., Ashraf, R. N., Noor, S., Mahmood-Ur-Rahman, N., Mashhadi, S. F., Rashid, Z., et al. (2016). Association of maternal periodontitis with low birth weight in newborns in a tertiary care hospital. J. Ayub Med. Coll. Abbottabad 28, 120–125.
Kinane, D., Bouchard, P., Brunet-Llobet, L., Lahor-Soler, E., Mahande, M. J., and Masenga, G. (2020). The association between periodontal disease and adverse pregnancy outcomes in northern Tanzania: a cross-sectional study. Afr. Health Sci. 18, 601–611. doi: 10.4314/ahs.v18i3.18
Kornman, K. S., and Loesche, W. J. (1982). Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect. Immun. 35, 256–263. doi: 10.1128/iai.35.1.256-263.1982
Kothiwale, S. V., Desai, B. R., Kothiwale, V. A., Gandhid, M., and Konin, S. (2014). Periodontal disease as a potential risk factor for low birth weight and reduced maternal haemoglobin levels. Oral Health Prev. Dent. 12, 83–90. doi: 10.3290/j.ohpd.a31224
Kumar, A., Basra, M., Begum, N., Rani, V., Prasad, S., Lamba, A. K., et al. (2013). Association of maternal periodontal health with adverse pregnancy outcome. J. Obstet. Gynaecol. Res. 39, 40–45. doi: 10.1111/j.1447-0756.2012.01957.x
Laine, M. A. (2002). Effect of pregnancy on periodontal and dental health. Acta Odontol. Scand. 60, 257–264. doi: 10.1080/00016350260248210
Le, H. T. T., Jareinpituk, S., Kaewkungwal, J., and Pitiphat, W. (2007). Increased risk of preterm birth among non- smoking, non- alcohol drinking women with maternal periodontitis. Southeast Asian J. Trop. Med. Public Health 38, 586–593.
Lee, Y.-L., Hu, H.-Y., Chou, S.-Y., Lin, C.-L., Cheng, F.-S., Yu, C.-Y., et al. (2022). Periodontal disease and preterm delivery: a nationwide population-based cohort study of Taiwan. Sci. Rep. 12:3297. doi: 10.1038/s41598-022-07425-8
Leitich, H., Brunbauer, M., Bodner-Adler, B., Kaider, A., Egarter, C., and Husslein, P. (2003). Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am. J. Obstet. Gynecol. 188, 752–758. doi: 10.1067/mob.2003.167
Li, Y., Shibata, Y., Zhang, L., Kuboyama, N., and Abiko, Y. (2011). Periodontal pathogen Aggregatibacter actinomycetemcomitans LPS induces mitochondria-dependent-apoptosis in human placental trophoblasts. Placenta 32, 11–19. doi: 10.1016/j.placenta.2010.10.007
Liu, H., Redline, R. W., and Han, Y. W. (2007). Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J. Immunol. 179, 2501–2508. doi: 10.4049/jimmunol.179.4.2501
Lohana, M. H., Suragimath, G., Patange, R. P., Varma, S., and Zope, S. A. (2017). A prospective cohort study to assess and correlate the maternal periodontal status with their pregnancy outcome. J. Obstet. Gynecol. India 67, 27–32. doi: 10.1007/s13224-016-0920-0
López, N. J., Da Silva, I., Ipinza, J., and Gutiérrez, J. (2005). Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J. Periodontol. 76, 2144–2153. doi: 10.1902/jop.2005.76.11-S.2144
Lyon, D., Cheng, C.-Y., Howland, L., Rattican, D., Jallo, N., Pickler, R., et al. (2010). Integrated review of cytokines in maternal, cord, and Newborn blood: part I—associations with preterm birth. Biol. Res. Nurs. 11, 371–376. doi: 10.1177/1099800409344620
Macones, G., Parry, S., Nelson, D., Strauss, J., Ludmir, J., Cohen, A., et al. (2010). Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the periodontal infections and prematurity study (PIPS). Am. J. Obstet. Gynecol. 202, 147.e1–147.e8. doi: 10.1016/j.ajog.2009.10.892
Mannem, S., and Chava, V. (2011). The relationship between maternal periodontitis and preterm low birth weight: a case-control study. Contemp. Clin. Dent. 2, 88–93. doi: 10.4103/0976-237X.83067
Marakoglu, I., Gursoy, U. K., Marakoglu, K., Cakmak, H., and Ataoglu, T. (2008). Periodontitis as a risk factor for preterm low birth weight. Yonsei Med. J. 49, 200–203. doi: 10.3349/ymj.2008.49.2.200
Markou, E., Eleana, B., Lazaros, T., and Antonios, K. (2009). The influence of sex steroid hormones on gingiva of women. Open Dent. J. 3, 114–119. doi: 10.2174/1874210600903010114
Massoni, R. S. D. S., Aranha, A. M. F., Matos, F. Z., Guedes, O. A., Borges, Á. H., Miotto, M., et al. (2019). Correlation of periodontal and microbiological evaluations, with serum levels of estradiol and progesterone, during different trimesters of gestation. Sci. Rep. 9:11762. doi: 10.1038/s41598-019-48288-w
Mei, F., Xie, M., Huang, X., Long, Y., Lu, X., Wang, X., et al. (2020). Porphyromonas gingivalis and its systemic impact: current status. Pathogens 9:944. doi: 10.3390/pathogens9110944
Mesa, F., Pozo, E., O’Valle, F., Puertas, A., Magan-Fernandez, A., Rosel, E., et al. (2016). Relationship between periodontal parameters and plasma cytokine profiles in pregnant woman with preterm birth or low birth weight. Clin. Oral Investig. 20, 669–674. doi: 10.1007/s00784-015-1553-x
Michalowicz, B. S., Novak, M. J., Hodges, J. S., DiAngelis, A., Buchanan, W., Papapanou, P. N., et al. (2009). Serum inflammatory mediators in pregnancy: changes after periodontal treatment and association with pregnancy outcomes. J. Periodontol. 80, 1731–1741. doi: 10.1902/jop.2009.090236
Morelli, E., Broadbent, J., Leichter, J., and Thomson, W. (2018). Pregnancy, parity and periodontal disease. Aust. Dent. J. 63, 270–278. doi: 10.1111/adj.12623
Moura da Silva, G., Coutinho, S. B., Piscoya, M. D. B. V., Ximenes, R. A. A., and Jamelli, S. R. (2012). Periodontitis as a risk factor for preeclampsia. J. Periodontol. 83, 1388–1396. doi: 10.1902/jop.2012.110256
Narita, Y., and Kodama, H. (2022). Identification of the specific microbial community compositions in saliva associated with periodontitis during pregnancy. Clin. Oral Investig. 26, 4995–5005. doi: 10.1007/s00784-022-04468-z
Neuman, H., and Koren, O. (2017). The pregnancy microbiome. Nestle Nutr. Inst. Workshop Ser. 88, 1–10. doi: 10.1159/000455207
Offenbacher, S., Katz, V., Fertik, G., Collins, J., Boyd, D., Maynor, G., et al. (1996). Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 67, 1103–1113. doi: 10.1902/jop.1996.67.10s.1103
Oliveira, L. J. C., Cademartori, M. G., Schuch, H. S., Barros, F. C., Silveira, M. F., Correa, M. B., et al. (2021). Periodontal disease and preterm birth: findings from the 2015 Pelotas birth cohort study. Color. Dis. 27, 1519–1527. doi: 10.1111/odi.13670
Ortiz-Sánchez, B. J., Legorreta-Herrera, M., and Rodriguez-Sosa, M. (2021). Influence of gestational hormones on the bacteria-induced cytokine response in periodontitis. Mediat. Inflamm. 2021, 1–12. doi: 10.1155/2021/5834608
Paraskevas, S., Huizinga, J. D., and Loos, B. G. (2008). A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 35, 277–290. doi: 10.1111/j.1600-051X.2007.01173.x
Park, D.-Y., Park, J. Y., Lee, D.-G., Hwang, I., and Kim, H.-S. (2022). Leaky gum: the revisited origin of systemic diseases. Cells 11:1079. doi: 10.3390/cells11071079
Pelzer, E., Gomez-Arango, L. F., Barrett, H. L., and Nitert, M. D. (2017). Review: maternal health and the placental microbiome. Placenta 54, 30–37. doi: 10.1016/j.placenta.2016.12.003
Penova-Veselinovic, B., Keelan, J. A., Wang, C. A., Newnham, J. P., and Pennell, C. E. (2015). Changes in inflammatory mediators in gingival crevicular fluid following periodontal disease treatment in pregnancy: relationship to adverse pregnancy outcome. J. Reprod. Immunol. 112, 1–10. doi: 10.1016/j.jri.2015.05.002
Perunovic, N. D., Rakic, M. M., Nikolic, L. I., Jankovic, S. M., Aleksic, Z. M., Plecas, D. V., et al. (2016). The association between periodontal inflammation and labor triggers (elevated cytokine levels) in preterm birth: a cross-sectional study. J. Periodontol. 87, 248–256. doi: 10.1902/jop.2015.150364
Pihlstrom, B. L., Michalowicz, B. S., and Johnson, N. W. (2005). Periodontal diseases. Lancet 366, 1809–1820. doi: 10.1016/S0140-6736(05)67728-8
Piltonen, T. T. (2016). Polycystic ovary syndrome: endometrial markers. Best Pract. Res. Clin. Obst. Gynaecol. 37, 66–79. doi: 10.1016/j.bpobgyn.2016.03.008
Polyzos, N., Polyzos, I. P., Zavos, A., Valachis, A., Mauri, D., Papanikolaou, E., et al. (2010). Obstetric outcomes after treatment of periodontal disease during pregnancy: systematic review and meta-analysis. BMJ 341:c7017. doi: 10.1136/bmj.c7017
Prakash, S., Nayak, R., Choudhury, G. K., Deshpande, S., Ashok, K., and Spoorthi, B. (2012). The role of plasma female sex hormones on gingivitis in pregnancy: a clinicobiochemical study. J. Contemp. Dent. Pract. 13, 760–763. doi: 10.5005/jp-journals-10024-1225
Pucci, R., Cassoni, A., Di Carlo, D., Della Monaca, M., Romeo, U., and Valentini, V. (2021). Severe odontogenic infections during pregnancy and related adverse outcomes. Case report and systematic literature review. Trop. Med. Infect. Dis. 6:106. doi: 10.3390/tropicalmed6020106
Raju, K., and Berens, L. (2021). Periodontology and pregnancy: an overview of biomedical and epidemiological evidence. Periodontol. 87, 132–142. doi: 10.1111/prd.12394
Rakoto-Alson, S., Tenenbaum, H., and Davideau, J.-L. (2010). Periodontal diseases, preterm births, and low birth weight: findings from a homogeneous cohort of women in Madagascar. J. Periodontol. 81, 205–213. doi: 10.1902/jop.2009.090351
Reddy, B. V. R., Tanneeru, S., and Chava, V. K. (2014). The effect of phase-I periodontal therapy on pregnancy outcome in chronic periodontitis patients. J. Obstet. Gynaecol. 34, 29–32. doi: 10.3109/01443615.2013.829029
Ren, H., and Du, M. (2017). Role of maternal periodontitis in preterm birth. Front. Immunol. 8:139. doi: 10.3389/fimmu.2017.00139
Reyes, L., Phillips, P., Wolfe, B., Golos, T. G., Walkenhorst, M., Progulske-Fox, A., et al. (2017). Porphyromonas gingivalis and adverse pregnancy outcome. J. Oral Microbiol. 9:1374153. doi: 10.1080/20002297.2017.1374153
Romero, R., Dey, S. K., and Fisher, S. J. (2014). Preterm labor: one syndrome, many causes. Science 345, 760–765. doi: 10.1126/science.1251816
Saddki, N., Bachok, N., Hussain, N. H. N., Zainudin, S. L. A., and Sosroseno, W. (2008). The association between maternal periodontitis and low birth weight infants among Malay women. Community Dent. Oral Epidemiol. 36, 296–304. doi: 10.1111/j.1600-0528.2007.00383.x
Santa Cruz, I., Herrera, D., Martin, C., Herrero, A., and Sanz, M. (2013). Association between periodontal status and pre-term and/or low-birth weight in Spain: clinical and microbiological parameters. J. Periodontal Res. 48, 443–451. doi: 10.1111/jre.12024
Sanz, M., and Kornman, K., working group 3 of the joint EFP/AAP workshop (2013). Periodontitis and adverse pregnancy outcomes: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J. Periodontol. 84, S164–S169. doi: 10.1902/jop.2013.1340016
Saraiva, L., Rebeis, E. S., Martins, E. D. S., Sekiguchi, R. T., Ando-Suguimoto, E. S., Mafra, C. E. S., et al. (2014). IgG sera levels against a subset of periodontopathogens and severity of disease in aggressive periodontitis patients: a cross-sectional study of selected pocket sites. J. Clin. Periodontol. 41, 943–951. doi: 10.1111/jcpe.12296
Shaggag, L. M., Alhabardi, N., and Adam, I. (2022). The association between maternal periodontitis and preterm birth: a case-control study in a low-resource setting in Sudan, Africa. Medicina 58:632. doi: 10.3390/medicina58050632
Shah, H., Nisar, N., Hassan, A., and Butt, S. (2022). Association between maternal chronic apical periodontitis (CAP) and low birth weight preterm birth (LBWPT). J. Pak. Med. Assoc. 72, 436–439. doi: 10.47391/JPMA.0921
Shetty, M., Shetty, P. K., Ramesh, A., Thomas, B., Prabhu, S., and Rao, A. (2010). Periodontal disease in pregnancy is a risk factor for preeclampsia. Acta Obstet. Gynecol. Scand. 89, 718–721. doi: 10.3109/00016341003623738
Silva de Araujo Figueiredo, C., Gonçalves Carvalho Rosalem, C., Costa Cantanhede, A. L., Abreu Fonseca Thomaz, É. B., and Fontoura Nogueira Da Cruz, M. C. (2017). Systemic alterations and their oral manifestations in pregnant women. J. Obstet. Gynaecol. Res. 43, 16–22. doi: 10.1111/jog.13150
Siqueira, F. M., Cota, L. O. M., Costa, J. E., Haddad, J. P. A., Lana, Â. M. Q., and Costa, F. O. (2007). Intrauterine growth restriction, low birth weight, and preterm birth: adverse pregnancy outcomes and their association with maternal periodontitis. J. Periodontol. 78, 2266–2276. doi: 10.1902/jop.2007.070196
Soldan, S. S., Retuerto, A. I. A., Sicotte, N. L., and Voskuhl, R. R. (2003). Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J. Immunol. 171, 6267–6274. doi: 10.4049/jimmunol.171.11.6267
Soucy-Giguère, L., Tétu, A., Gauthier, S., Morand, M., Chandad, F., Giguère, Y., et al. (2016). Periodontal disease and adverse pregnancy outcomes: a prospective study in a low-risk population. J. Obstet. Gynaecol. Can. 38, 346–350. doi: 10.1016/j.jogc.2016.02.012
Souza, L. M., Cruz, S. S. Da, Gomes-Filho, I. S., Barreto, M. L., Passos-Soares, J. S., Trindade, S. C., et al. (2016). Effect of maternal periodontitis and low birth weight—a case control study. Acta Odontol. Scand. 74, 73–80. doi: 10.3109/00016357.2015.1049374
Srinivas, S. K., Sammel, M. D., Stamilio, D. M., Clothier, B., Jeffcoat, M. K., Parry, S., et al. (2009). Periodontal disease and adverse pregnancy outcomes: is there an association? Am. J. Obstet. Gynecol. 200, 497.e1–497.e8. doi: 10.1016/j.ajog.2009.03.003
Taghzouti, N., Xiong, X., Gornitsky, M., Chandad, F., Voyer, R., Gagnon, G., et al. (2012). Periodontal disease is not associated with preeclampsia in Canadian pregnant women. J. Periodontol. 83, 871–877. doi: 10.1902/jop.2011.110342
Tellapragada, C., Eshwara, V. K., Bhat, P., Acharya, S., Kamath, A., Bhat, S., et al. (2016). Risk factors for preterm birth and low birth weight among pregnant Indian women: a hospital-based prospective study. J. Prev. Med. Public Health 49, 165–175. doi: 10.3961/jpmph.16.022
Terzic, M., Aimagambetova, G., Terzic, S., Radunovic, M., Bapayeva, G., and Laganà, A. S. (2021). Periodontal pathogens and preterm birth: current knowledge and further interventions. Pathogens 10:730. doi: 10.3390/pathogens10060730
Varshney, S., and Gautam, A. (2014). Poor periodontal health as a risk factor for development of pre-eclampsia in pregnant women. J. Indian Soc. Periodontol. 18, 321–325. doi: 10.4103/0972-124X.134569
Vogt, M., Sallum, A. W., Cecatti, J. G., and Morais, S. S. (2010). Periodontal disease and some adverse perinatal outcomes in a cohort of low risk pregnant women. Reprod. Health 7:29. doi: 10.1186/1742-4755-7-29
Yokoyama, M., Hinode, D., Masuda, K., Yoshioka, M., and Grenier, D. (2005). Effect of female sex hormones on campylobacter rectus and human gingival fibroblasts. Oral Microbiol. Immunol. 20, 239–243. doi: 10.1111/j.1399-302X.2005.00222.x
Yuan, X.-H., Fan, Y.-Y., Yang, C.-R., Gao, X.-R., Zhang, L.-L., Hu, Y., et al. (2016). Progesterone amplifies oxidative stress signal and promotes NO production via H2O2 in mouse kidney arterial endothelial cells. J. Steroid Biochem. Mol. Biol. 155, 104–111. doi: 10.1016/j.jsbmb.2015.09.029
Zenclussen, A. C. (2013). Adaptive immune responses during pregnancy. Am. J. Reprod. Immunol. 69, 291–303. doi: 10.1111/aji.12097
Keywords: periodontal diseases, periodontal pathogens, adverse pregnancy, inflammation, immune response
Citation: Wen X, Fu X, Zhao C, Yang L and Huang R (2023) The bidirectional relationship between periodontal disease and pregnancy via the interaction of oral microorganisms, hormone and immune response. Front. Microbiol. 14:1070917. doi: 10.3389/fmicb.2023.1070917
Received: 15 October 2022; Accepted: 11 January 2023;
Published: 26 January 2023.
Edited by:
George Tsiamis, University of Patras, GreeceReviewed by:
Girish Suragimath, Krishna Institute of Medical Sciences Deemed University, IndiaCopyright © 2023 Wen, Fu, Zhao, Yang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruijie Huang, ✉ cnVpam1odWFuZ0BnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Ruijie Huang http://orcid.org/0000-0003-3211-518X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.