- 1Clinical Microbiology Laboratory, 2nd Affiliated Hospital of Zhejiang University, School of Medicine, Zhejiang University, Hangzhou, China
- 2State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai, China
- 3Infection Control Department, 2nd Affiliated Hospital of Zhejiang University, School of Medicine, Zhejiang University, Hangzhou, China
Pseudomonas aeruginosa is one of the most common opportunistic pathogens causing severe nosocomial infections for its patterns of multidrug resistance, particularly for carbapenems. Timely epidemiological surveillance could greatly facilitate infection control of P. aeruginosa and many deadly pathogens alike. IR Biotyper (IRBT), is a novel real-time typing tool, based on a Fourier-transform infrared (FTIR) spectroscopy system. It is critical to comprehensively establish and evaluate the feasibility of IRBT in P. aeruginosa strain typing. In the current study, we first established standards and schemes for its routine laboratory application, and we found that Mueller–Hinton agar plates give better discriminatory power than blood agar plates. Data showed that the cut-off value of 0.15 with an additional 0.025 range was optimal. Secondly, 27 clinically isolated carbapenem-resistant P. aeruginosa (CRPA) strains collected from October 2010 to September 2011 were evaluated for typing effectiveness by comparing IRBT to the other commonly used typing methods, such as multi-locus sequence typing (MLST), pulsed-field gel electrophoresis (PFGE) and whole-genome sequencing (WGS)-based typing. When using WGS-based typing as the reference method, the typing method of FTIR spectroscopy (AR = 0.757, SID = 0.749) could better cluster P. aeruginosa strains than MLST and in silico serotyping (AR = 0.544, SID = 0.470). Though PFGE showed the highest discriminatory power, low concordance was observed between PFGE and the other methods. Above all, this study demonstrates the utility of the IRBT as a quick, low-cost, real-time typing tool for the detection of CRPA strains.
Introduction
Pseudomonas aeruginosa plays a leading role in infections caused by gram-negative bacilli, especially in critically ill and immunocompromised patients, including ventilator-associated pneumonia, wound infections, and respiratory infections from cystic fibrosis (Lyczak et al., 2000; De Bentzmann and Plesiat, 2011). Due to its properties of biofilms-forming, P. aeruginosa can easily survive and spread on the surfaces of hospital equipment and facilities, making it an important source of nosocomial infections and outbreaks (Alipour et al., 2017; Bicking Kinsey et al., 2017). Recently, the increasing incidence of multidrug resistance (MDR), particularly for carbapenems, has further exacerbated P. aeruginosa infections and increased mortality (Livermore, 2002; Rossolini and Mantengoli, 2005; Horcajada et al., 2019).
Early detection of cross-transmissions is extremely important in limiting the spread of multidrug-resistant bacterial strains and quickly implementing hygiene control measures to block the spread and minimize the damages. Rapid detection of pathogen cross-transmission in healthcare settings remains challenging, multi-locus sequence typing (MLST), in silico serotyping, pulsed-field gel electrophoresis (PFGE), and whole-genome sequencing-based typing (WGS) are several methods that are commonly used at present (Hammer et al., 2003; Waters et al., 2012; Martak et al., 2020),. However, many limitations remain in adapting these methods for regular surveillance, including high costs, labor, time-consuming, and especially the need for professional and technical personnel. Therefore, a convenient, rapid, and reliable bacterial typing technique is urgently required for routine clinical microbiology laboratories.
Fourier transform Infrared Spectroscopy (FT-IR) is an emerging phenotyping technique that is established on a comparison of infrared light absorption spectra patterns and varies according to the whole microorganism cell composition (i.e., lipids, proteins, or polysaccharides). Compared to molecular biology methods, the quick, easy, and low-cost features of FT-IR are highly attractive for routine surveillance (Naumann et al., 1991; Novais et al., 2019). Previous studies of FT-IR typing were commonly based on hardware and manual third-party statistics analysis software. In 2017, the integrated IR Biotyper (IRBT) system that can automatically perform spectra treatment and further comparison by multivariate data analysis tool was launched by Bruker Daltonics. Since then, more research on the potential of FT-IR to differentiate and identify particular groups of strains has been explored in the contexts of clinical or food microbiology (Cordovana et al., 2021; Lombardo et al., 2021; Hu et al., 2021b; Li et al., 2022), particularly for Gram-negative bacteria associated with hospital-acquired infections (Martak et al., 2019).
To our knowledge, no comprehensive estimates of FT-IR typing schemes for clinical P. aeruginosa have ever been published. The objectives of the current study are to evaluate the typing effectiveness of clinically isolated carbapenem-resistant P. aeruginosa (CRPA) as well as to establish standards and schemes for its routine laboratory application, providing the reference basis for the integration of FT-IR into laboratory epidemiological surveillance and outbreak investigations.
Materials and methods
Bacterial isolates
Thirty-eight CRPA isolates were randomly chosen from our previously sequenced genomes from the routine clinical microbiological laboratory of the Second Affiliated Hospital of Zhejiang University from 2010 to 2012. Detailed clinical information is shown in Supplementary Table 1. Before the experiments, all the isolates were re-identified using Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) at the species level (Bruker Daltonik GmbH, Bremen, Germany). The selected strains were divided into two groups for the method optimization (n = 11) and comparison (n = 27) respectively. All the isolates were revived on Columbia blood agar plates (BA; Autobio Diagnostics, Zhengzhou, China) and Mueller–Hinton agar plates (MH) (Oxoid, Wesel, Germany) for 24 ± 2 h at 35°C to optimize the process. MH medium was then chosen for further comparison experiments.
Pulsed-field gel electrophoresis
PFGE was performed as previously described with slight modification (Hu et al., 2012). Genomic DNAs of the 38 CRPA isolates were digested using the restriction enzyme SpeI. Salmonella enterica serotype Braenderup H9812 was used as a size marker. A dendrogram based on Dice similarity coefficients was generated from the homology matrix with a coefficient of 0.5% using the unweighted pair group method using arithmetic averages (UPGMA) to describe the relationships among PFGE profiles through the UVIBand software (Bio-Rad). Isolates were considered the same PFGE group if their dice similarity index was ≥80%.
Genome sequencing
Genomic DNAs of all 38 CRPA isolates were extracted from overnight cultures by using a Wizard genomic DNA purification kit (Promega, Beijing, China) and were subjected to whole-genome sequencing using 150 bp paired-end strategy with the Illumina HiSeq X10 platform (Illumina, San Diego, CA, United States). Raw reads were trimmed and assembled to contigs using SPAdes version 3.11. Acquired antimicrobial resistance genes (ARGs), MLST, and in silico serotyping were determined via the Center for Genomic Epidemiology website of.1 Single-nucleotide polymorphisms (SNPs) were called by the gingr online software of Harvestsuite.2 The interpretation of WGS-based typing is more complicated, for which the number of differences depends on the strains collected. Here, we set the allele differences ≤10 as homologous. Previous studies chose a threshold of 14 (Mellmann et al., 2016) or 20 (Martak et al., 2020) alleles of difference for P. aeruginos. However, this threshold was not suitable for our study as it would have created too few clusters (data not shown).
IRBT spectrum acquisition and analysis
An optimal sample preparation method (H2O-EtOH) reported in our previous study was applied in the current research (Hu et al., 2021b). Specifically, one inoculation loopful of bacteria, which was grown at 35°C for 24 ± 2 h on BA and MH agar plates, was suspended in 100 μl sterile H2O in 1.5 ml vials containing sterile metal rods (Bruker Daltonik, Bremen, Germany), and then 100 μl 70% (vol/vol) ethanol was added after vortex. Subsequently, 15 μl of the homogeneous suspension was taken and spotted onto the 96-well IRBT silicon sample plate (Bruker Daltonik, Bremen, Germany). The sample plate was dried at 37°C for approximately 20 min and four parallel tests were prepared for each sample. The measurements were carried out using an IRBT System (Bruker Daltonik, Bremen, Germany) running the IRBT software (Version 2.0) with the default analysis settings as recommended by the manufacturer. Generally, spectra (4000–500 cm−1) of isolates and backgrounds were acquired. For each spectrum, 64 scans were collected in single-beam mode with 4 cm−1 resolution at a rate of 3 scans/s. And the spectra range of 1,200–900 cm−1 was automatically processed, normalized, and second derivatives by OPUS 7.5 software (Bruker Optics GmbH). Data that did not meet the default quality criteria were excluded in further analysis. All qualified data were analyzed using offline IRBT Client by building dendrograms using the Euclidian distance and average linkage clustering method following the manufacturer’s suggestion.
Calculation of clustering concordance
The concordance of IRBT clustering in comparison to MLST, in silico serotyping, PFGE, and WGS typing methods was determined by calculation of the 95% confidence interval adjusted rand index (Adjusted Rand index, AR) using the online tool.3 The Simpson’s index of diversity (SID) was used to evaluate the discriminative ability of each typing method, and the probability of two unrelated isolates separated from the experimental strain set clustering into different typing groups was calculated.
Results
Determination of FT-IR clustering cut-off value of Pseudomonas aeruginosa typing
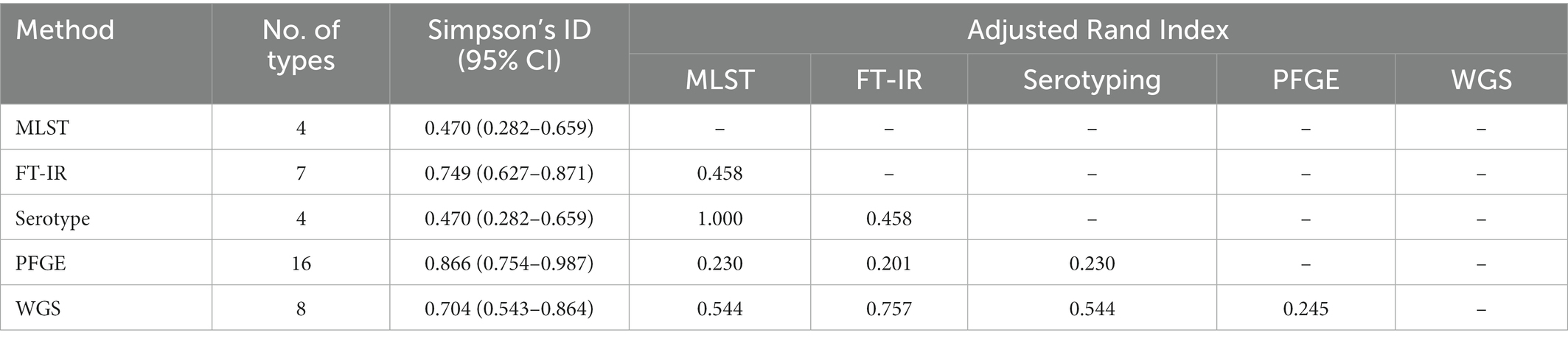
The culture medium has been determined before the clustering cut-off value, 11 CRPA isolates of 5 ST types were inoculated on BA and MH agar medium separately and incubated for 24 h ± 2 h. After incubation and spectra acquisition, projects were used to build the dendrograms for each medium using MLST and WGS-based typing data as a reference. As a result of four replicate experiments, albeit the superior growth on the blood agar was to that of MH agar, which was facilitating sample preparation, the failure to correctly discriminate among three distinct genotypes ST463/G1 (1201), ST782/G4 (1204), and ST242/G5 (1205) on BA agar plate (Figure 1; Supplementary Table 2), was considered an “unacceptable error.” Based on this, the MH medium was selected for the subsequent experiments.
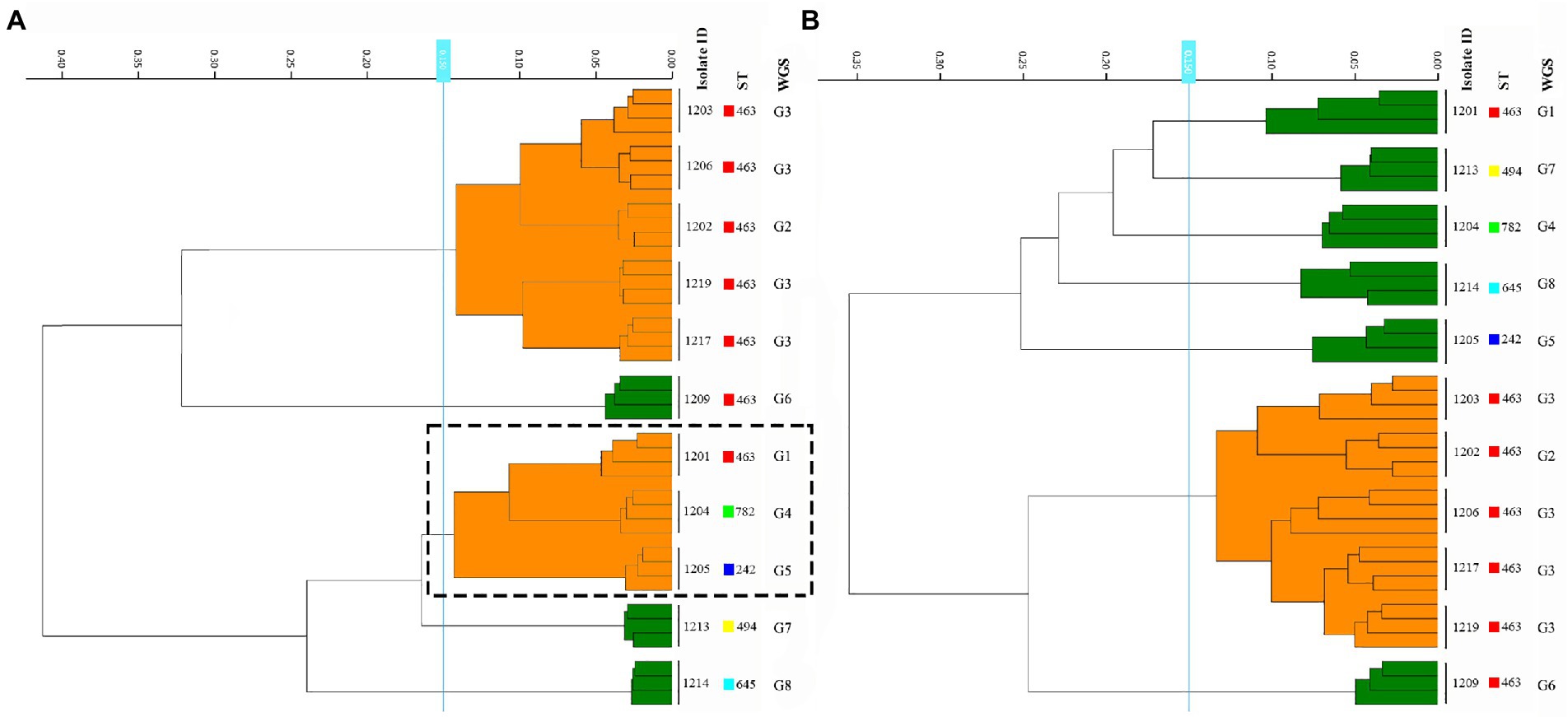
Figure 1. Comparison of the results from the BA and MH agar plate among the 11 P. aeruginosa strains. (A,B) represent samples prepared in BA and MH medium, respectively. The dashed box showed three distinct ST types that were misclustered together by FT-IR in the BA medium.
The determination of the FT-IR clustering cut-off value was assessed by the concordance of FT-IR types using WGS-based typing as the reference, Adjusted Rand index (AR) was calculated at a different cut-off of clustering (Figure 2). The optimal cut-off varied from 0.132 to 0.173 (AR, 0.711) with one discordance with the WGS-based typing results (Figure 2). Accordingly, 0.15 was used as the COV with an additional 0.025 range in the following experiments.
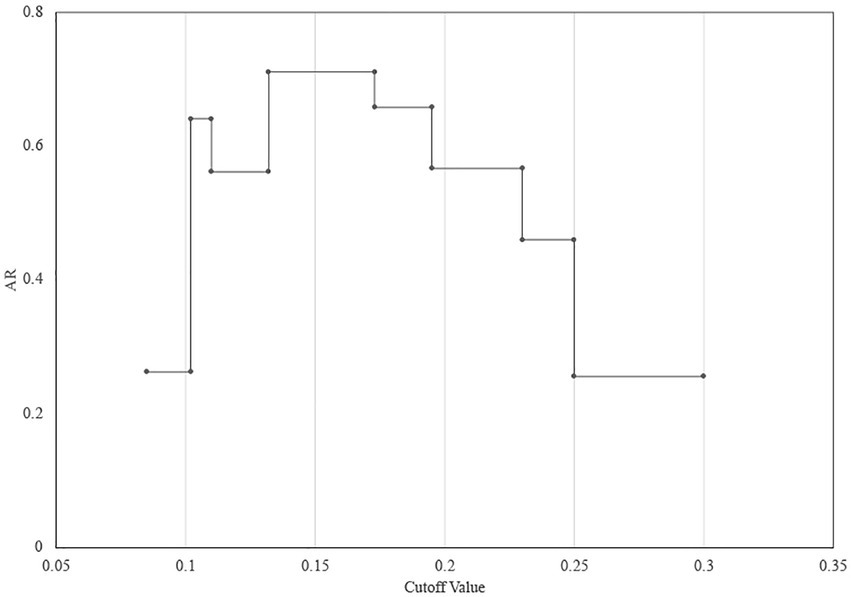
Figure 2. Determination of the FT-IR clustering cut-off value using WGS as the reference. The maximum Adjusted Rand index (AR = 0.711) was reached with a cut-off value range of 0.132–0.173.
Comparison of different typing methods for Pseudomonas aeruginosa
Twenty-seven CRPA isolates collected from different wards over 1 year from October 2010 to September 2011 were analyzed. According to the WGS-based typing results (Figure 3; Supplementary Table 3), 27 isolates could be classified into 8 different types (A-H), with three clusters containing more than one isolate (A, B, G) and an additional 5 singletons. The most predominant type A contained 14 isolates from 5 different locations (SICU, NICU, Neurosurgery, Burns, and ICU). Four MLST types were identified from the 27 isolates, of which ST463 was the dominant ST, which occupied 70.4% (19/27), demonstrating that ST463 was the most prevalent type transmitted within our hospital in the year 2010 and 2011, in combination with its high potential virulence risk had been reported before (Hu et al., 2021a), more awareness should be expected. In silico serotyping of these strains was completely congruent with MLST, where serotypes O11, O5, O7, and O4 correspond to ST1076, ST554, ST337, and ST463, respectively. Likewise, O4 was the main serotype. The conventional method PFGE showed a greater discriminatory power (SID = 0.866) by classifying 27 isolates into 16 types (a-p), of which all were singletons except for types a, f, and c (more than 2 isolates). Using the COV range of FT-IR as defined above, the 27 isolates were classified into seven IR types (1–7), four of which contained more than one isolate and the other three were singletons. The concordance analysis of the four methods was calculated through the Adjusted Rand’s index. The highest AR value of 0.757 was obtained between IRBT and WGS, followed by MLST and Serotype typing (AR = 0.544) (Table 1).
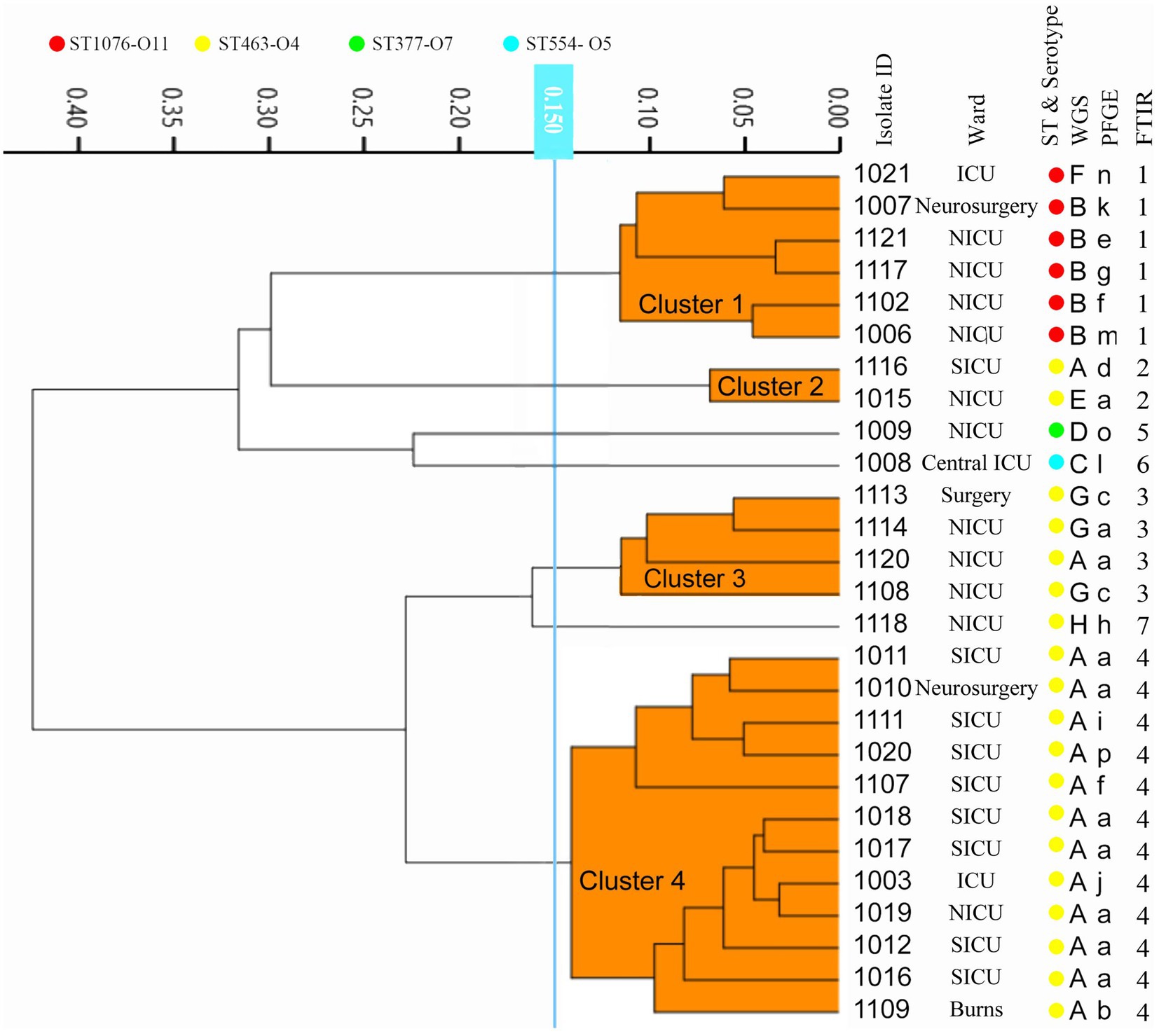
Figure 3. The dendrogram was obtained automatically by FT-IR. The lineages of IRBT, WGS-based typing, PFGE, STs, and serotypes are given for each isolate.
Evaluation of the capacity for detection of the CRPA outbreak transmission routes
According to the internal 2–1-1 criteria setting for CRPA outbreak identification: two or more CRPAs isolated from the same ward within a month will be identified as a potential nosocomial outbreak and investigation and interventions need to be commenced. By this criteria, five potential outbreaks were included in this study, each from Neurosurgery (1), NICU (2), and SICU (2).
WGS-based typing confirmed four of these nosocomial outbreaks (Figure 4), differing from the conventional results by ruling out one potential outbreak from the NICU, as the four CRPAs isolated in a short period were identified into different subtypes by WGS-based typing. Furthermore, for CRPAs isolated in the NICU from July to September 2011, even though they were isolated from the same location and shared the same drug-resistant phenotype and even resistance genotype (KPC+), the WGS-based typing classified them as different types, which indicates different transmission routes might be involved.

Figure 4. Overview of the capacity of different methods for detection of the CRPA outbreak transmission routes. Different Colors represent outbreaks occurring at different locations: Orange: Neurosurgery; Green: NICU; Yellow: SICU. Each rectangular box represents one outbreak and the number in the box is the relevant isolates ID. Different transmission routes from the same location during the same period are indicated with a gray background. The values in brackets indicate an estimate of the reporting time.
Discussion
Previous studies have reported a good correlation between commonly used typing methods and FT-IR for different species, showing the ability of FT-IR to decipher the links between epidemiologically-related isolates. However, the COV values automatically allocated by FT-IR systems do not always precisely reflect the sub-species level discrepancies (Rakovitsky et al., 2020), which makes it important to standardize the sample preparation process and the internal reference COV range before formally incorporating IRBT into their laboratory routines.
Accordingly, in this study, with the confirmed cultivation conditions, we first identified internal COV criteria by using the best AR-matching method, using WGS results as references. The 0.15 ± 0.025 range we obtained was comparatively smaller to the unofficial reference from the manufacturer but much smaller than Martak’s results of 0.184–0.374 (Martak et al., 2019), albeit the same optimal AR-matching method was used. The explanation behind this discrepancy may lie in the reference method used in the two studies, the reference method used in Martak’s research was MLST, whereas in the current study more discriminating WGS-based typing had been selected. This further illustrates the significance of the reference method selected when establishing a laboratory’s routine using FT-IR typing parameters and procedure, i.e., the depth of the epidemiological investigation should first be identified. The same discordance is also reflected in the results on Klebsiella pneumoniae by the two laboratories, where the variation in reference methods led to differences in the COV (Martak et al., 2019).
According to the comparison of different typing methods, the discriminatory power of FT-IR was higher than that of MLST and phenotypic serotype typing, which is consistent with our previous study (Hu et al., 2021b). PFGE demonstrated the highest discriminatory power in the present study, but its lack of consistency with other methods decreased its reference significance for in-hospital epidemiological investigations. A previous study about Salmonella enterica typing methods also showed that strains differed by 0 SNPs presented distinct PFGE subtypes (Kozyreva et al., 2016), suggesting that the distinct PFGE types did not necessarily correlate with increased genetic distance between isolates, PFGE results would be misleading for these isolates, and the relative discriminatory power of different subtyping methods might depend on the strains tested. FT-IR showed the highest concordance with WGS-based typing compared to the other three methods, and the relatively higher resolution allows FT-IR to infer more elaborate genomic linkages or discrepancies than MLST and serotyping, this is indicated by FT-IR can further subdivide the predominantly isolated ST463 into three IR types (2, 3, and 4).
Correctly recognizing outbreaks is the first and most critical step toward effective prevention of in-hospital transmission. This correctness includes finding out the true outbreak and ruling out false positives. In the present study using the WGS as the reference method, 4 out of 5 in-hospital criteria-based outbreaks were confirmed, while the 4 CRPAs from a suspected outbreak in the NICU isolated in 2010 were excluded from relevance. The same results were with PFGE and FT-IR. Due to relatively lower discriminatory power, the same conclusions could not be reached by MLST and in silico serotyping. PFGE’s excessively high resolution classified a portion of potentially relevant strains into different types, resulted in a false alert dismissal, and failed to instruct timely and efficient tracking and preventive measures. Besides, we also observed different transmissions occurring in the same location over a short period, such as outbreaks in the NICU in 2011. The presence of two independent transmission routes was detected by multiple methods, giving more detailed evidence for better implementation of corrective interventions.
Conclusion
As a phenotyping method, our study found that despite standardized schemes for sample preparation, some instability was observed in results when performing a comprehensive analysis of data from different laboratories or batches. Differences in the spectrum caused by operational/system variation will lead to higher COV, further research into the causes and resolutions is required. However, based on the current results, the potential of FT-IR as an epidemiological tool for clinical CRPA is well established. In practice, we recommend the use of FT-IR as a rapid real-time screening option to acquire efficient information for timely preventive and corrective action, the results of which can be further examined and studied using the more sophisticated method like WGS-based typing. This multi-methods program will establish a complete surveillance and investigation system for nosocomial infections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical approval was approved by the Ethics Committee of The Second Affiliated Hospital of Zhejiang University, School of Medicine (Number: 2020–319). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RZ and YH: conceptualization and validation. DJ and KZ: methodology. WS, CL, and HZ: formal analysis and data curation. KZ, YH, and RZ: writing, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1068872/full#supplementary-material
Footnotes
References
Alipour, N., Karagoz, A., Taner, A., Gaeini, N., Alipour, N., Zeytin, H., et al. (2017). Outbreak of hospital infection from biofilm-embedded pan drug-resistant Pseudomonas aeroginosa, due to a contaminated bronchoscope. J. Prev. Med. 2, 1–9. doi: 10.21767/2572-5483.100014
Bicking Kinsey, C., Koirala, S., Solomon, B., Rosenberg, J., Robinson, B. F., Neri, A., et al. (2017). Pseudomonas aeruginosa outbreak in a neonatal intensive care unit attributed to hospital tap water. Infect. Control Hosp. Epidemiol. 38, 801–808. doi: 10.1017/ice.2017.87
Cordovana, M., Mauder, N., Kostrzewa, M., Wille, A., Rojak, S., Hagen, R. M., et al. (2021). Classification of salmonella enterica of the (Para-)typhoid fever group by Fourier-transform infrared (FTIR) spectroscopy. Microorganisms 9:853. doi: 10.3390/microorganisms9040853
De Bentzmann, S., and Plesiat, P. (2011). The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol. 13, 1655–1665. doi: 10.1111/j.1462-2920.2011.02469.x
Hammer, A. S., Pedersen, K., Andersen, T. H., Jørgensen, J. C., and Dietz, H. H. (2003). Comparison of Pseudomonas aeruginosa isolates from mink by serotyping and pulsed-field gel electrophoresis. Vet. Microbiol. 94, 237–243. doi: 10.1016/S0378-1135(03)00103-2
Horcajada, J. P., Montero, M., Oliver, A., Sorlí, L., Luque, S., Gómez-Zorrilla, S., et al. (2019). Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 32:e00031-19. doi: 10.1128/CMR.00031-19
Hu, Y. Y., Cai, J. C., Zhang, R., Zhou, H. W., Sun, Q., and Chen, G. X. (2012). Emergence of Proteus mirabilis harboring blaKPC-2 and qnrD in a Chinese hospital. Antimicrob. Agents Chemother. 56, 2278–2282. doi: 10.1128/AAC.05519-11
Hu, Y., Peng, W., Wu, Y., Li, H., Wang, Q., Yi, H., et al. (2021a). A potential high-risk clone of Pseudomonas aeruginosa ST463. Front. Microbiol. 12:670202. doi: 10.3389/fmicb.2021.670202
Hu, Y., Zhou, H., Lu, J., Sun, Q., Liu, C., Zeng, Y., et al. (2021b). Evaluation of the IR Biotyper for Klebsiella pneumoniae typing and its potentials in hospital hygiene management. Microb. Biotechnol. 14, 1343–1352. doi: 10.1111/1751-7915.13709
Kozyreva, V. K., Crandall, J., Sabol, A., Poe, A., Zhang, P., et al. (2016). Laboratory investigation of salmonella enterica serovar Poona outbreak in California: comparison of pulsed-field gel electrophoresis (PFGE) and whole genome sequencing (WGS) results. PLoS Curr 8. doi: 10.1371/currents.outbreaks.1bb3e36e74bd5779bc43ac3a8dae52e6
Li, X., Zhu, L., Wang, X., Li, J., and Tang, B. (2022). Evaluation of IR Biotyper for Lactiplantibacillus plantarum typing and its application potential in probiotic preliminary screening. Front. Microbiol. 13:823120. doi: 10.3389/fmicb.2022.823120
Livermore, D. M. (2002). Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34, 634–640. doi: 10.1086/338782
Lombardo, D., Cordovana, M., Deidda, F., Pane, M., and Ambretti, S. (2021). Application of Fourier transform infrared spectroscopy for real-time typing of Acinetobacter baumannii outbreak in intensive care unit. Future Microbiol. 16, 1239–1250. doi: 10.2217/fmb-2020-0276
Lyczak, J. B., Cannon, C. L., and Pier, G. B. (2000). Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist1*address for correspondence: Channing Laboratory, 181 Longwood avenue, Boston, MA 02115, USA. Microbes Infect. 2, 1051–1060. doi: 10.1016/S1286-4579(00)01259-4
Martak, D., Meunier, A., Sauget, M., Cholley, P., Thouverez, M., Bertrand, X., et al. (2020). Comparison of pulsed-field gel electrophoresis and whole-genome-sequencing-based typing confirms the accuracy of pulsed-field gel electrophoresis for the investigation of local Pseudomonas aeruginosa outbreaks. J. Hosp. Infect. 105, 643–647. doi: 10.1016/j.jhin.2020.06.013
Martak, D., Valot, B., Sauget, M., Cholley, P., Thouverez, M., Bertrand, X., et al. (2019). Fourier-transform InfraRed spectroscopy can quickly type gram-negative bacilli responsible for hospital outbreaks. Front. Microbiol. 10:1440. doi: 10.3389/fmicb.2019.01440
Mellmann, A., Bletz, S., Boking, T., Kipp, F., Becker, K., Schultes, A., et al. (2016). Real-time genome sequencing of resistant bacteria provides precision infection control in an institutional setting. J. Clin. Microbiol. 54, 2874–2881. doi: 10.1128/JCM.00790-16
Naumann, D., Helm, D., and Labischinski, H. (1991). Microbiological characterizations by FT-IR spectroscopy. Nature 351, 81–82. doi: 10.1038/351081a0
Novais, Â., Freitas, A. R., Rodrigues, C., and Peixe, L. (2019). Fourier transform infrared spectroscopy: unlocking fundamentals and prospects for bacterial strain typing. Eur. J. Clin. Microbiol. Infect. Dis. 38, 427–448. doi: 10.1007/s10096-018-3431-3
Rakovitsky, N., Frenk, S., Kon, H., Schwartz, D., Temkin, E., Solter, E., et al. (2020). Fourier transform infrared spectroscopy is a new option for outbreak investigation: a retrospective analysis of an extended-Spectrum-Beta-lactamase-producing Klebsiella pneumoniae outbreak in a neonatal intensive care unit. J. Clin. Microbiol. 58. doi: 10.1128/JCM.00098-20
Rossolini, G. M., and Mantengoli, E. (2005). Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 11, 17–32. doi: 10.1111/j.1469-0691.2005.01161.x
Keywords: carbapenem-resistant P. aeruginosa, Fourier-transform infrared spectroscopy, Nosocomial infection investigation, strain typing, WGS-based typing
Citation: Hu Y, Zhu K, Jin D, Shen W, Liu C, Zhou H and Zhang R (2023) Evaluation of IR Biotyper for carbapenem-resistant Pseudomonas aeruginosa typing and its application potential for the investigation of nosocomial infection. Front. Microbiol. 14:1068872. doi: 10.3389/fmicb.2023.1068872
Edited by:
Irene Burckhardt, Medical Microbiology and Hygiene, University Hospital Heidelberg, GermanyReviewed by:
Miriam Cordovana, Bruker Daltonik GmbH, GermanyCarla Rodrigues, Institut Pasteur, France
Copyright © 2023 Hu, Zhu, Jin, Shen, Liu, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Zhang, ✉ emhhbmctcm9uZ0B6anUuZWR1LmNu
 Yanyan Hu
Yanyan Hu Kun Zhu
Kun Zhu Dingping Jin3
Dingping Jin3 Congcong Liu
Congcong Liu Hongwei Zhou
Hongwei Zhou Rong Zhang
Rong Zhang