- 1Key Laboratory of Biometallurgy Ministry of Education, School of Minerals Processing and Bioengineering, Central South University, Changsha, China
- 2Chenzhou Tobacco Company of Hunan Province, Chenzhou, China
- 3Hunan Renhe Environment Co., Ltd., Changsha, China
- 4Hubei Key Laboratory of Mineral Resources Processing and Environment, Wuhan University of Technology, Wuhan, Hubei, China
- 5Beijing Research Institute of Chemical Engineering and Metallurgy, Beijing, China
- 6Department of Civil and Environmental Engineering, University of Tennessee, Knoxville, Knoxville, TN, United States
- 7School of Computing, Clemson University, Clemson, SC, United States
Arsenic (As) is one of the most toxic metalloids that possess many forms. As is constantly migrating from abandoned mining area to the surrounding environment in both oxidation and reducing conditions, threatening human health and ecological safety. The biogeochemical reaction of As included oxidation, reduction, methylation, and demethylation, which is closely associated with microbial metabolisms. The study of the geochemical behavior of arsenic in mining areas and the microbial remediation of arsenic pollution have great potential and are hot spots for the prevention and remediation of arsenic pollution. In this study, we review the distribution and migration of arsenic in the mining area, focus on the geochemical cycle of arsenic under the action of microorganisms, and summarize the factors influencing the biogeochemical cycle of arsenic, and strategies for arsenic pollution in mining areas are also discussed. Finally, the problems of the risk control strategies and the future development direction are prospected.
1. Introduction
Arsenic (As) is a toxic metalloid element. Although arsenic As is not a metal, its toxicity is similar to that of heavy metal elements [such as antimony (Sb), cadmium (Cd), chromium (Cr), and lead (Pb)], therefore, arsenic is usually included when talking about heavy metal poisoning. These toxic heavy metals or metalloids are released from a large number of waste residues (mining waste rocks, tailings, and smelting slag) produced in the process of ore mining, beneficiation, and smelting. The released toxic elements further migrate to the surrounding environment under the action of surface runoff, rain, and snow infiltration, resulting in serious environmental pollution. Arsenic is one of the most toxic heavy metals and is classified as the Group 1 human carcinogen by the International Agency for Research on Cancer (IARC) (Zhou and Xi, 2018). Many areas are chronically contaminated with arsenic worldwide. For example, studies have shown that more than half of the Mississippi River Basin is at high risk of arsenic contamination (Yang et al., 2014).
Mining activity is one of the major factors leading to arsenic pollution. Arsenic is the 20th most abundant element in the earth's crust, and most of the As intercalate in gold, copper, lead, zinc, tin, nickel, or cobalt minerals in the form of sulfide (Mandal and Suzuki, 2002). Deposition of mining waste including mine rock, mine tailing, and smelting slags leads to serious heavy metal pollution in the surroundings, and arsenic is the main pollutant causing ecological risk to the environment around the mining area. The migration, transformation, and enrichment of arsenic in the soil–rice system are higher than those of other heavy metal elements such as lead and zinc. It threatens human health and ecological security.
In this article, the distribution and migration of arsenic, the geochemical cycle of arsenic under the action of microorganisms, the factors influencing the biogeochemical cycle of arsenic in the mining area, and the strategies for pollution control are summarized and prospected.
2. Distribution and migration of arsenic in the mining area
Heavy metal pollution around the mining area mainly originates from the oxidation and dissolution of sulfide ore. Weathering and leaching of abandoned rocks, mine tailings, and smelting slags that are produced during mining activity lead to As being released and dispersed into the surrounding environment (Okkenhaug et al., 2012). Therefore, mining waste containing As is the main source of arsenic contamination. Oxidation of sulfur will generate an acidic solution. Heavy metal ions in smelting slag, waste ore, and mine tailings diffuse into the surroundings through leaching effects (e.g., chemical acid leaching or microbial leaching) (Yan et al., 2017). Arseno-bearing sulfide (e.g., FeAsS) in the mine waste can be oxidized to form scorodite (i.e., FeAsO4). Except for sulfur oxidation leading to acid solubilization of As, arsenic can also be dissolved under reducing conditions. Studies have shown that a heap of tailings and slags in the mining area would cause reducing conditions, and iron oxide or iron hydroxide would be reductively dissolved, resulting in a large number of As in tailings and slag release into environments (Al-Abed et al., 2007). Both As and antimony (Sb) can be released under reduction conditions. As and Sb are homotopes, with similar chemical characteristics. In the mining areas, As is reported to have the highest environmental risk (Xue et al., 2023). As and Sb mainly occur in anion forms (i.e., arsenate and arsenite or antimonate and antimonite), which is different from other cation heavy metals, such as Cd, Pb, Zn, or Cu. Generally, in mining wastes with both Sb and As, Sb would be first released or whose release is stronger than As release, and thus, As would release after Sb. This may be due to the reason that the metabolism of Sb (both oxidation and reduction) may compete with As for electron acceptors or donors (Bagherifam et al., 2019) and lead to differences in As metabolism between As mining sites and other heavy metal contaminated environments. It is reported that the distribution of the arsenic content in different areas of mining varied from 70 to 5330 mg/kg (Otones et al., 2011), and the vertical distribution of As through soil profiles suggests a deposition mechanism of this element on the topsoils that involves both biotic and abiotic factors (Yang et al., 2020).
The chemical form and occurrence speciation of As would change through a series of biochemical reactions, where chemical forms and speciation determine the toxicity and bioavailability of arsenic. According to Tessier's sequential extraction method, heavy metals existed as five fractions, including exchangeable fraction, carbonate-bounded fraction, Fe/Mn oxide-bounded fraction, organic matter-bounded fraction, and residue fraction (Tessier et al., 1979). The speciation of heavy metals determines their solubility, mobility (Weng et al., 2002), and bioavailability (Zimmerman and Weindorf, 2010). It is reported that in soils, arsenic mainly existed in residual fraction, followed by organic matter-bounded fraction, Fe/Mn oxide-bounded fraction, carbonate-bounded fraction, and exchangeable fraction; among the fractions, residue state constituted 25–50%, and iron-manganese oxide bond state constituted 21 to 35% (Zhao et al., 2022). Even though the distribution pattern of fractions in mining sites differed between studies, the residual fraction was the main component (i.e., Yang et al., 2020; Zhao et al., 2022). In mining water, the main forms of arsenic are arsenate and arsenite. In the lower pH range (1 to 3), the main chemical forms of arsenate As (V) in acid mine drainage (AMD) were H3AsO4 and H2, while arsenite As (III) mainly exists in the form of H3AsO3. Yet at typical pH (4 to 9) for most surface and ground waters, As(V) is present as a negatively charged oxygen anion (e.g., H2 or ), while As(III) is present in the neutral (H3AsO3) form (Cheng et al., 2009). Published research on the distribution and migration of heavy metals in mining areas and their environmental risks was mainly based on the change in the occurrence forms and total amounts (Yang et al., 2020), while the distribution of As chemical forms (i.e., valence state) that highly determines toxicity was rarely reported. Different from some heavy metals (e.g., Pb and Cd) that mainly exist in inorganic forms, As can exist in both inorganic and organic forms. Inorganic arsenic has two valence states: As (III) (such as arsenite) and As (V) (such as arsenate). As (III) has strong water solubility and high mobility, while As (V) has relatively weak water solubility and low mobility, but this does not mean that As (V) is difficult to be bio-absorbed. For example, rice seedlings effectively absorb both As (V) and As (III) under liquid culture conditions. Under aerobic conditions, soil Fe (III) adsorbs As (V) and then decreases the bioavailability of As. Organic arsenic mainly includes methyl arsenide, sulfur-containing methyl arsenide, and chlorine-containing methyl arsenide. Methylated arsenic, on the one hand, can be microbial demethylated to produce inorganic arsenic, and on the other hand, it is further converted to volatile arsenyl hydrogen compounds [monomethylarsenic hydride (MMAsH2), dimethylarsenic hydride (DMAsH), and trimethylarsenic (TMAs)] (Yan et al., 2015). At present, it has also been found that in addition to TMAs, there are methyl arsenic chloride gas and methyl arsenic sulfur gas in the arsenic gas released by the geothermal environment (Planer-Friedrich et al., 2006). The biological effectiveness and toxicity of different forms of arsenic vary. Generally for living organisms, inorganic arsenic is significantly more toxic than organic arsenic. Dimethyl arsenates DMAs(V) and trimethylarsenic oxides (TMAsO) are significantly less toxic than As (III) (Cui et al., 2014), but toxicity increases substantially when DMAs (V) and TMAsO are reduced to dimethyl arsenites DMAs(III) and trimethylarsenic TMAs (III) (TMAs) (Styblo et al., 2000). Therefore, the environmental risk and health toxicity of arsenic can be reduced to some extent when inorganic arsenic in the environment or in living organisms is converted to less toxic organic arsenic or volatilized into the atmosphere as a gas. Since As in the same occurrence form can contain a variety of chemical forms with different toxicity (such as arsenite and arsenate can coexist in the exchange state), it is more in-depth and accurate to study the distribution, migration, and ecological risk of As pollution from the perspective of chemical valence states.
3. Microorganisms are the important driving force for the geochemical cycle of arsenic
Soil microbes and heavy metals can interact with each other. The diversity, abundance, and function of microorganisms can be significantly affected by heavy metals. With the increase in heavy metal concentration, the aggregation of the microbial community is more decisive. Heavy metal stress makes the prokaryotic community deterministic, but its effects on the assembly process of different microbes are different (Zhang Y. et al., 2020). The microbial community composition, as well as network interactions, was shifted to strengthen the adaptability of microorganisms to heavy metal contamination (Li et al., 2017). In addition, bacteria showed different reactions to heavy metals. For example, Anaerobic microbes, such as Anaerolineaceae, not only play important roles in shaping the microbial community in soils but also might be involved in regulating Cd solubility (Meng et al., 2019). Chlorella can biomineralize Pb under the promotion of montmorillonite to photosynthesis and urea hydrolysis (Tan et al., 2022). On the contrary, microorganisms are the core driving forces that lead to the transformation of different forms of arsenic.
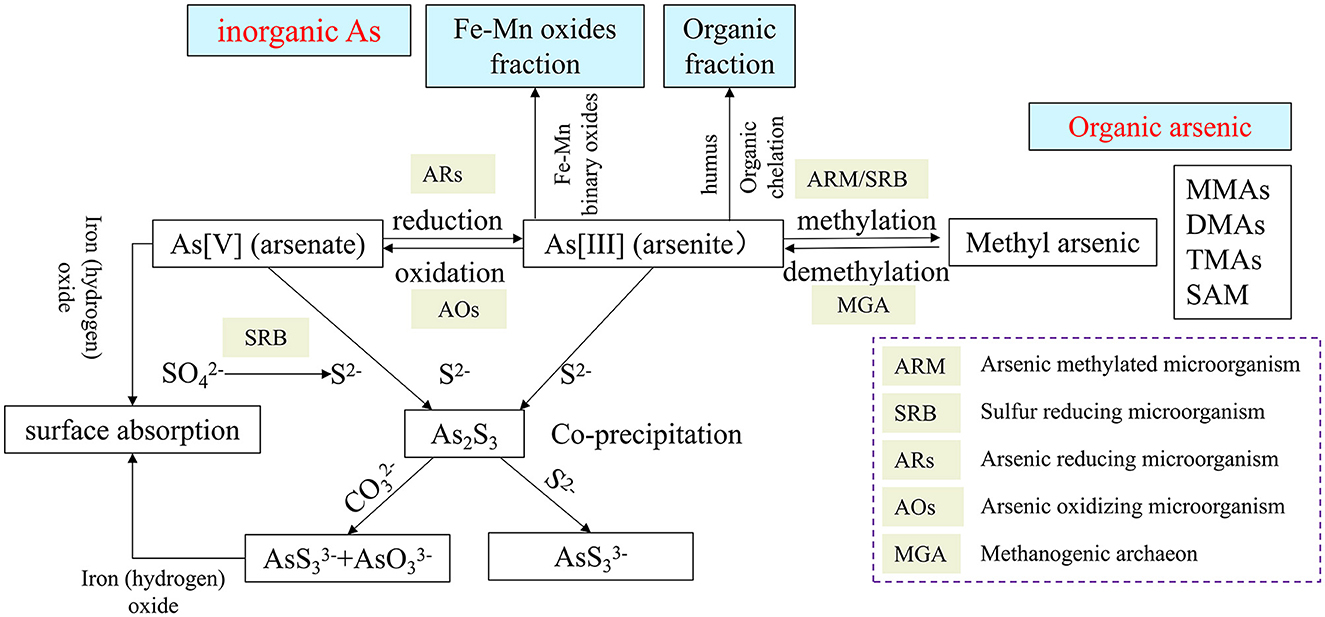
The transformation behavior of As (i.e., the geochemical behavior of arsenic) includes oxidation–reduction, methylation and demethylation, organic chelation, surface adsorption and dissociation, and ion co-precipitation. Of these, arsenic reduction, demethylation of organic arsenic, and dissociation of adsorbed arsenic form highly toxic As(III), leading to increased arsenic mobility and toxicity, while arsenic oxidation, methylation, organic chelation, and co-precipitation produce less toxic/mobile arsenates, volatile methyl arsenic (e.g., DMAs and TMAs), organically bound arsenic, and residual arsenic sulfide, reducing arsenic contamination (Bianco Prevot et al., 2018). Many geochemical processes of As involve the participation of microorganisms (Figure 1). Various direct or indirect As metabolic activities of microorganisms are the main driving forces for the geochemical behavior of As (Barrera-Diaz et al., 2012; Meng et al., 2019). Microorganisms can directly carry out arsenic reduction, arsenic oxidation, methylation, and demethylation and can also be indirectly involved in the oxidation of arsenic and ion co-precipitation. It should be mentioned that many microorganisms can conduct biological volatilization of arsenic to convert arsenic compounds into volatile derivatives, such as methylation of arsenic. Bio-volatilization of arsenic is a new subject in biogeochemistry and environmental health, which plays an important role in the global biogeochemical cycle of As and can also be used as a potential arsenic bioremediation method (Wang et al., 2014). Almost all microorganisms have been reported to possess an As resistance and metabolism gene (Zhu et al., 2017).
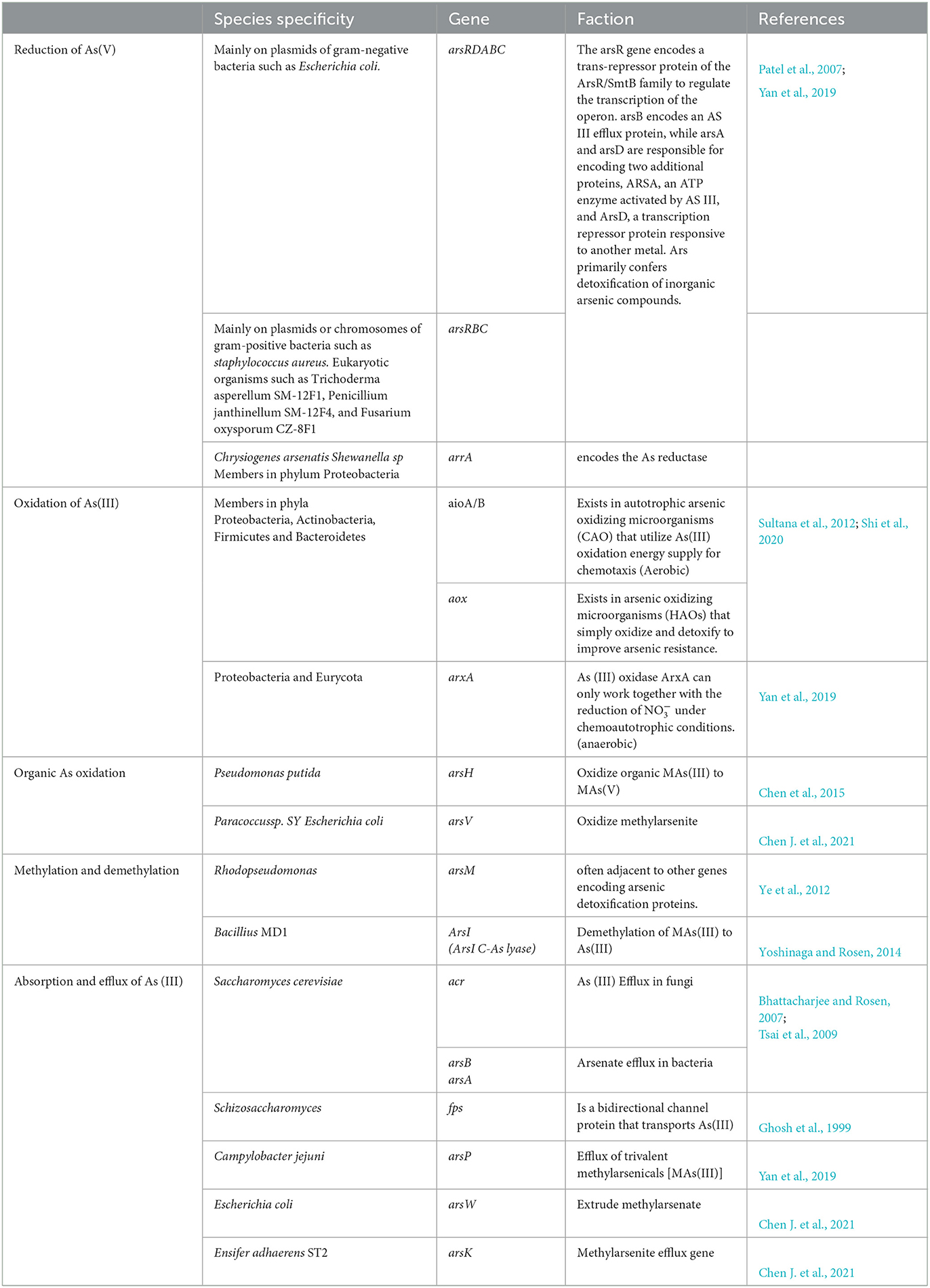
There are specific genes driving the As metabolism, including reduction, oxidation, methylation, or demethylation (Table 1). The genes and microorganisms containing As metabolism genes were widely detected in mine slag, fresh water, sediments, soil, hot spring, marine water, and other samples. Li et al. (2019) reported that microorganisms in the mining environments (i.e., acid mine drainage) can derive As metabolism genes such as arsA, arsR, and arsB through horizontal gene transfer (HGT), suggesting those genes may not have species specificity. Some others also reported HGT events of As metabolism genes, but the genes can also be vertically transferred (VGT) (Dunivin et al., 2019). As these processes dramatically alter the toxicity and bio-efficacy of arsenic, the study of microbial arsenic metabolism genes is important for understanding environmental arsenic metabolic processes and microbial remediation potential. In mining areas, the increase in arsenic concentration and the activation of arsenic in bedrock aquifers are caused by several geochemical processes, including bedrock weathering, oxidation of arsenopyrite and main sulfides in ore, the mixture of mine water and surface water, leaching arsenic alkali residue, and adsorption–desorption from iron/manganese oxide/hydroxide (Wen et al., 2018). Inorganic As is converted into organic As by methylation of soil microorganisms and volatilizes into the atmosphere, thus releasing and migrating As in gaseous forms (Bentley et al., 2002; Turpeinen et al., 2002). Volatile arsenic-containing compounds can be oxidized in the atmosphere and then enter into the soil or water with rain or atmospheric dry deposition, finally completing the circulation of As in the soil/water body and the atmosphere.
3.1. As reduction
The two mechanisms of microbial reduction of elemental arsenic include dissimilatory arsenic reduction and cytoplasmic arsenic reduction. Dissimilatory arsenic reduction refers to the reduction of As (V) to As (III) by microorganisms using compounds such as lactate and acetate as electron donors, and these microorganisms are known as dissimilatory arsenic-reducing prokaryotes (DARPs, dissimilatory arsenate-reducing prokaryotes) (Lukasz et al., 2014). More than 30 species of DARPs have been found, distributed in the genera Shewanella, Halomonas, and Bacillus. DARPs possess arrAB operon, which can be activated and expressed by As (V), and encode dissimilatory arsenic reductase to catalyze the reduction reaction of As (V) (Amend et al., 2014) [to form As (III)]. The reduction of cytoplasmic arsenic is catalyzed by a series of enzymes encoded by ars operons and carried out in the cytoplasm of microorganisms, where As (V) is reduced to As (III) with higher activity and then pump out of the cells by membrane carrier proteins encoded by arsB, thus achieving arsenic detoxification (Rosen, 2002). In addition, the As reduction has also been found in eukaryotic organisms such as Trichoderma asperellum SM-12F1, Penicillium janthinellum SM-12F4, and Fusarium oxysporum CZ-8F1 (Su et al., 2015).
3.2. As oxidation
As(III) can be oxidized to produce As(V) by arsenic-oxidizing microorganisms, under both aerobic and anaerobic conditions using either O2 or , respectively, as electron acceptor(s). The arsenic-oxidizing microbes include chemoautotrophic arsenic-oxidizing microorganisms (CAOs) and heterotrophic arsenic-oxidizing microorganisms (HAOs). CAOs can use oxygen as an electron acceptor to oxidize As (III) to produce As (V) catalyzed by arsenic oxidase enzyme that contains two subunits, a large subunit aioA (with molybdopterin and a [3Fe-4S] cluster) and smaller subunit aioB (with a Rieske-type [2Fe-2S] cluster). Another type of As(III) oxidase, arxA, was also identified in Alkalilimnicola ehrlichii strain MLHE-1 (Zargar et al., 2010). It was found that the arxA can only work together with the reduction of (Yan et al., 2019). HAOs can utilize arsenic oxidase in peripheral cytoplasm to catalyze As (III) to produce As (V) (Yan et al., 2019; Shi et al., 2020). The reported arsenic oxidation functional microorganisms include Rhizobium and Thermus aquaticus, and they were widely found in arsenic-contaminated soil, sediment, and water (Yamamura and Amachi, 2014). Furthermore, it reported the identification of the nemRA manipulator extracted from Enterobacteriaceae, which was shown to be involved in the oxidation of trivalent organic arsenic and to be regulated by the trivalent organic arsenic selective transcriptional repressor NemR (Shi et al., 2021). In addition, the organic methyl arsenite can be oxidized to methyl arsenate by the methyl arsenite-specific oxidase arsH or arsV and then be extruded by arsW, arsP, or arsK to increase microbial resistance to organic As (Chen et al., 2015; Chen J. et al., 2021).
3.3. As methylation
Microbial arsenic methylation generates volatile organic arsenic, which drives the biogeochemical cycle of arsenic from inorganic to organic. Biogenesis of arsenic methylation is widespread in nature, and arsenic methylation gene (arsM) is widely distributed in the genome of different species. Studies have shown that arsM orthologous protein is distributed in bacteria, archaea, and eukaryotes, including Cyanobacteria, Bacteroidetes, Firmicutes, and Proteobacteria (Zhao et al., 2013). Trimethyl arsenic is the main product of microbial methylated arsenic. It is shown that sulfate-reducing bacteria (SRB) could participate in the methylation process of inorganic arsenic, and their research results also indicated that methanogenic archaea were involved in the demethylation process of methylated arsenic (Chen et al., 2019).
3.4. As demethylation
Compared with microbial arsenic methylation, there are very limited studies associated with As demethylation in microbial cells. Yoshinaga and Rosen (2014) reported that Bacillus stain MD1 can use ArsI to demethylate MAs(III) to form As(III). Mycobacterium can demethylate monomethyl arsenite [MMAs (V)] or monomethyl arsenite [MMAs (III)] into a mixture of arsenite [As (V)] and arsenite [As (III)] (Lehr et al., 2003). In addition, the mixed cultures of Burkholderia and Streptomyces can be reduced and demethylated. It is proved that the demethylation of MMA (V) to As (III) is a two-step process (Yoshinaga et al., 2011). Moreover, some methylating microorganisms can demethylate methylsalicylic acid. Arsenic-methylating fungi, such as Fusarium oxysporum CZ-8F1, Penicillium janthinellum SM-12F4, and Trichoderma asperellum SM-12F1, can demethylate dimethyl arsonic acid [DMA (V)] to As (V) and As (III) (Su et al., 2015).
3.5. As bioleaching and co-precipitation
Microorganisms can also indirectly participate in the biogeochemical cycling of arsenic. Iron–sulfur-oxidizing microorganisms form acidic environments by oxidizing iron and sulfur elements in ores, resulting in the release of arsenic from ores, which are the main sources of arsenic pollution in mines (Wu et al., 2021). Microbial-mediated redox of iron has an important influence on the environmental behavior of arsenic, such as dissolution and release of arsenic, adsorption and precipitation, and morphological transformation. The extracellular electron transfer process of iron oxidation bacteria promotes the mineral phase transformation of iron and couples with arsenic passivation (Zhao et al., 2022). The product of manganese-oxidizing microorganisms, the biogenic manganese oxides, can oxidize As (III) to As (V), and microbial manganese oxidase (CumA) inhibits the expression activity of arsC gene of arsenic-reducing bacteria (Akhtar et al., 2013). Dissimilatory iron-reducing bacteria (DIRB), such as Geobacter and Shewanella, can reduce trivalent iron in iron (hydroxide) oxides to divalent iron, resulting in the deletion of arsenic adsorption sites and the release of adsorbed arsenic to activate metal ions in the solid phase (Liu et al., 2020). Geobacter can use acetic acid as a carbon source and electron donor to drive the reduction process of As (V) (Wang et al., 2016). Sulfate-reducing bacteria (SRBs) such as Desulfuromonas reduce sulfate to produce sulfide, which co-precipitates with trivalent arsenic in the soil to form insoluble arsenic sulfide, immobilizing free heavy metal ions (Sun et al., 2020). For instance, Klebsiella sp participated in the phosphate co-precipitation of Cr, and the highest removal rate of Cr reached 95% under soil conditions (Gupta et al., 2018). In the pH range of 3–12, the fixation rates of Cd, Pb, and Zn in soil by phosphate co-precipitation are 20–97.9%, 62.3–99.9%, and 28.6–98.7%, respectively (Chen et al., 1997). Phosphate-solubilizing microorganisms can solubilize phosphorus into phosphate that can co-precipitate with heavy metals to form arsenic phosphate minerals (Jiang et al., 2019).
4. Factors affecting arsenic biogeochemical cycle and risk control strategies in the mining area
Human activities, climatic conditions, geological background, and soil properties can directly or indirectly affect the biogeochemical cycling of arsenic through the action of microbial communities.
Soil physical (mineralogical characteristics, particle size, porosity, and climatic conditions) and chemical (temperature, pH, potential, and organic matter) (Abbasi et al., 2021) properties would affect the adsorption characteristics of heavy metals and, therefore, play important roles in regulating the geochemical behavior of arsenic (Burton et al., 2020). Soil oxidation–reduction potential (EH) has a strong effect on the migration and speciation of As in abandoned soil. Arsenic mobilization increases at moderately reducing conditions, while it reduces under oxic conditions (Mensah et al., 2022a). As can aggregate with the oxides and hydroxides of Fe resulting in bioavailability and mobility reduction (Bai et al., 2022). Therefore, As pollution and migration are affected by the existence of iron and manganese oxide and hydroxide adsorption (Wen et al., 2018). In addition, the rate (percentage) of soluble As in acidic soil is generally higher than that in alkaline soil. Carboxyl, phenol, and alcohol groups of soil organic matter can aggregate with metal ions to form complexes through inner cohesion and surface adsorption. As ions that are embedded in mineral lattice structures or stably complexed with complex organic matters are insoluble/insoluble in natural conditions, thereby affecting As mobility and toxicity (Egli et al., 2010).
Carbon, nitrogen, and sulfur compound can react with heavy metals or serve as electron donors or acceptors and, therefore, have a significant impact on As behavior. Sulfur element facilitates the conversion of iron oxide-bound arsenic to sulfide minerals, thereby enhancing its stability (Chen et al., 2022). In soil, phosphorus, particularly inorganic P, can release soil-retained As (mostly arsenate) by competing for adsorption sites (Wu et al., 2022). The addition of sodium sulfate and elemental sulfur reduced the mobility of As in the soil solution and the concentration of As (III), thus reducing the toxicity of As. Sulfate mainly affects the reduction process of sulfur and iron in soil and further affects the mobility and form of As in soil (Yan et al., 2022). Nitrogen is one of the most important basic elements in the biosphere, and different forms of nitrogen have different effects on the migration and transformation of As in soil. The addition of nitrate nitrogen can reduce the concentration of As in pore water in a short time, and the addition of high concentration ammonium nitrogen can reduce the concentration of As in the whole culture period (Liu et al., 2022). By promoting the reduction of Eh and inhibiting the reduction of dissimilated iron and the transformation of arsenic species, nitrate can be used as an effective modifier for the immobilization of As in soil (Chen Z. et al., 2021).
Human activities in the mining area such as smelting, waste/tailings management, and pollution control measures (such as pollution isolation, solidification stabilization, and phytoremediation) can directly or indirectly affect As distribution, migration, and toxicity (Wang et al., 2017; Ye et al., 2018). Studies showed that mining, dressing, and smelting activities in the mine area had the highest contribution rate of 46.6% to As pollution in the river sediments in the lower reaches of the mine area (Zhang et al., 2018). Therefore, it is necessary to clarify these questions:
(1) How do human activities affect the core driving force (microorganism) of the arsenic biogeochemical cycle? Human factors mainly include a large amount of arsenic released by human activities, which directly or indirectly enters the environment. The mining of mineral activities, the piling and leaching of coal gangue, and the discharge of bottom ash and fly ash from coal burning in mining areas will cause arsenic pollution to different degrees in the soil around the mining area. As we all know, the increase in arsenic concentration in the soil directly affects the activity of the soil microbial community. There is a negative correlation between the number of soil microbial communities and arsenic concentration. The chemical forms of arsenic in soil determine the transformation, migration, and toxicity of arsenic to a great extent, and the activities of soil microbial communities in turn affect the formation of arsenic and its compounds, so it may be possible to reduce arsenic pollution in mining areas by adjusting the structure of microbial communities (Simon, 2000). Studies have shown that the dominant phylum of soil biota is Proteus, Cyanobacteria, Actinomycetes, and Bacteroides, which play a key role in the formation of soil microbial communities of aioA and arsM. Soil organic carbon (OC), pH, and chlorophyll a (Chl a) are the most important environmental factors to change soil microbial communities, respectively, while it had a significant negative correlation with available As, As(III), and total As (Mao et al., 2023).
(2) How are the prevention and control approaches related to soil properties, microbial functions, As distribution and migration, and ecological toxicity? Biogeochemical cycling of As driven by microorganisms will affect the occurrence and chemical forms of As in ore areas, resulting in the migration and transformation of As and changes in ecological toxicity, such as pollution in the mining area and the surrounding environment. Previous studies on the migration, transformation, and ecological toxicity of As in the mining environment often neglected the key role of microorganisms in the biogeochemical cycle of As, which limited the research results to the immediate state of As pollution and lacked prediction on the changing trend of As pollution. As a result, the evaluation of As pollution remediation strategies lacked efficiency and predictability. Therefore, in-depth study on microbial diversity, composition, and function (such as ars operator, arrAB operator, cumA, sor, dirb, and other genes or functions) in the arsenic-contaminated environment of the mining area is needed. In combination with the distribution and migration law of arsenic element, scientific prediction is made on the changing trend of arsenic form and migration law in mining area environment, so as to provide a theoretical basis for the assessment and control of arsenic pollution risk in the mining area.
(3) What are the key factors that dominate As distribution and migration in mining areas, so as to provide theoretical basis and technical guidance for arsenic pollution prevention and control in areas with different climatic conditions, geological backgrounds, and soil properties? The forms of arsenic in the environment are changeable, so it is a research hot spot to study the changes and migration of arsenic in the environment. PH value is the primary factor to control the formation and transformation of secondary minerals. Studying the long-term stability of arsenic-containing secondary minerals in the mine environment and controlling arsenic migration behavior may be the key to improving the efficiency of arsenic pollution control and governance in future.
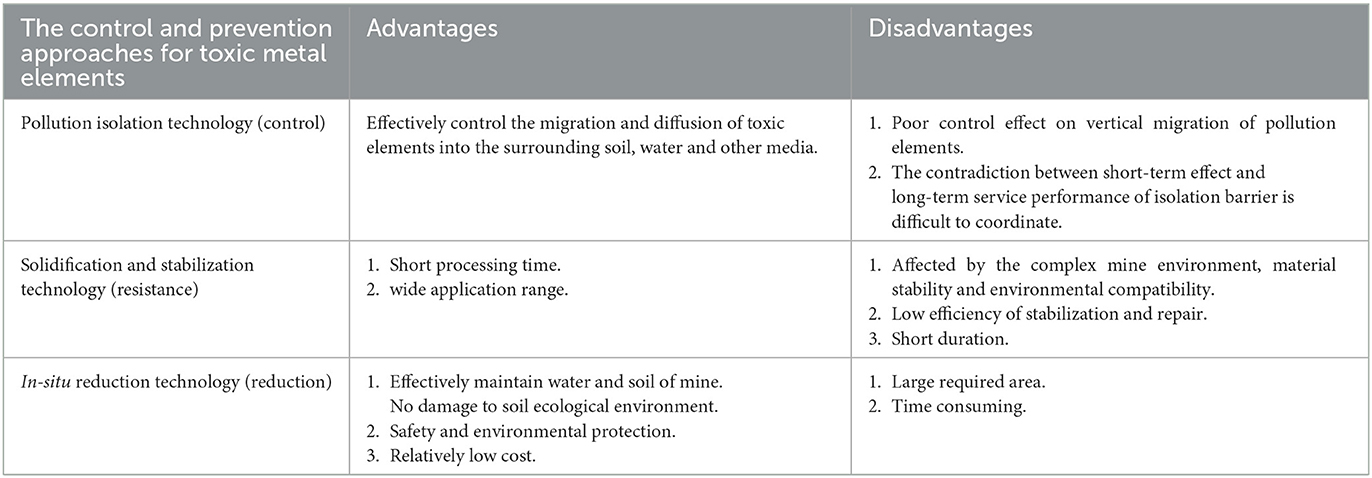
The control and prevention approaches for toxic metal elements in mining areas include pollution isolation technology (control), solidification and stabilization technology (resistance), and in situ reduction technology (reduction) (Table 2).
Pollution isolation technology can effectively control the migration and diffusion of toxic elements into the surrounding soil, water, and other media. Except for a series of construction technologies that have been developed, such as cutoff slurry walls using mainly thin walls, cement–bentonite–water slurries, sheet piles walls, jet grouting curtains, injection walls, and bored-pile cutoff walls, artificial ground freezing has been applied to pollution control in recent years (Anekwe and Isa, 2021; Rajendran et al., 2022). In this process, the pore water in the soil pores freezes and fills the gaps, which reduces the permeability of the soil and prevents the diffusion and transfer of pollutants (Bello et al., 2018). However, this technology mainly controls the horizontal lateral transfer of the pollution elements and has a poor control effect on the vertical migration of the pollution elements, and the contradiction between the short-term effect of the isolation barrier and the long-term service performance is difficult to coordinate.
Curing stabilization technology converts the soluble state of heavy metals in mine soils into insoluble states through the addition of passivating materials such as minerals, micro-nano, microorganisms, and chelator (Chen et al., 2009) that undergo characteristic adsorption, organic chelation and biomineralization, and chemical immobilization, physical adsorption, and physical encapsulation in situ to reduce the activity and mobility of contaminants and their diffusion in the mine. For instance, some microorganisms like cyanobacteria have a good remediation effect on arsenic in mining soil. Experiments show that the total As(T) and available As(a) in tailings soil decreased by 12.73 and 27.65%, respectively, after cyanobacteria inoculation (Qi et al., 2023). In addition to microorganisms, many nanomaterials can also be used to stabilize arsenic. Biochar-loaded nanoscale zero-valent iron (nZVI@BC) was prepared for remediation of arsenic-contaminated soil, which exhibited the best immobilization performance, significantly promoted the transformation from labile arsenic to iron–aluminum oxide-binding state (Song et al., 2022). It is noteworthy that there are quite a few examples of successfully using such methods. As an example, the use of citric acid and rhamnose as chelating agents has reportedly resulted in the removal of 83.65% of arsenic from contaminated soil around an abandoned smelter in China (Ke et al., 2020). Another example is that amorphous manganese oxide (AMO) was used as an amendment to chemically immobilize heavy metal-contaminated soil from a Czech lead smelter, resulting in a 52.64% reduction in reactive arsenic in the soil (Ettler et al., 2015). However, the strength of the reaction between the immobilized materials and heavy metal ions and the duration of action are affected by the mine's complex environment (climate, soil properties, and microorganisms), material stability, and environmental compatibility. The unclear geochemical behavior, biological driving mechanism, and environmental action principle of the toxic metal elements are the key bottlenecks that lead to the low efficiency and short duration of solidification and stabilization remediation. In addition, the lack of comprehensive and systematic data research in the mining area is also an important factor limiting the efficiency of solidification and stabilization restoration.
The in situ reduction technology is mainly based on the super heavy metal accumulation plants that can tolerate heavy metal toxicity and can extract heavy metals, which reduces the total amount of heavy metal elements in the mine environment. The plant roots can effectively maintain the water and soil in the mine to reduce the migration and diffusion of heavy metal elements into the surrounding environment. However, large polluted biomass needs to be treated properly. It still lacks green and efficient methods for treating polluted biomass. Plant root exudates can affect the speciation, migration, transformation, and ecological toxicity of metal elements in the environment. Moreover, it can change soil physical and chemical properties (organic matter content, water content, Eh, pH, and available nutrient content) and microbial community structure and function and indirectly affect microbial-mediated geochemical behavior of metal elements. The complex mechanisms remain to clarify further. At present, many studies and cases have proved the practicability of this method. For example, ryegrass can adsorb arsenic, and the use of manure alone and in combination with compost could improve the remediation efficiency of ryegrass on arsenic-contaminated soil (Mensah et al., 2022b). At the same time, many researchers have developed innovative methods to combine metal-tolerant plants with microbial inoculation and in situ immobilization as a means of phytoremediation (Xu et al., 2021).
The effectiveness of control and prevention measures for As or other toxic metal elements in mining areas can usually be assessed by measuring the bioavailability, fractional change and total change of As. In addition, risk assessment, including ecological risk assessment and health risk assessment, is also used to evaluate the effectiveness of control and prevention approaches. Other than accessing As in the remediated soil, the underground water is also used for assessment interventions (Xue et al., 2023). Chemical stabilization techniques reduce the risk of migration and diffusion of heavy metals in the environment by applying stabilizing materials to convert them from a highly reactive state to a less reactive state (Palansooriya et al., 2020) and are one of the main technical tools for risk management of heavy metal-contaminated soils, which are fast, economical, and efficient. In the practice of stabilization of heavy metal-contaminated soils, different leaching methods can be used, such as the AA method (using nitric sulfate), TCLP method (using acetic acid) (Cui et al., 2018), and DTPA method (using CaCl2-TEA-DTPA slow flush solution) (Zhang et al., 2022), which are used to determine the changes in the concentration of heavy metals in the active state of the soil before and after stabilization. The continuous grading method classifies heavy metals in soil into different active forms according to the extraction order, assesses the remediation effect of heavy metal stabilization in contaminated soil by the changes in the distribution of different heavy metal forms, and then investigates the stabilization mechanism (Zhang H. et al., 2020). The choice of specific solutions needs to be based on the remediation technology developed in different stages, scenarios, and soil heavy metal pollution control objectives required.
5. Conclusion and perspectives
The remediation of heavy metals such as As pollution will inevitably cause changes in biological and non-biological properties in the mining ecological system, in which the arsenic metabolism/transformation microorganisms will mediate the biogeochemical cycle process of arsenic, and finally affect the migration and transformation of arsenic and ecological toxicity. However, how do As metabolism/transformation microorganisms drive biogeochemical cycling of arsenic in mining areas? How can the key action factors of remediation approaches affect the migration and transformation of arsenic element and ecological toxicity? Such scientific problems are still unclear, which leads to the blindness in the formulation of control for heavy metal pollution in mining areas and the unsatisfactory effect of technical prevention and control.
At present, many studies developed a variety of new methods for simultaneous extraction and detection of arsenic chemical forms (Sun et al., 2015; Zhang et al., 2021), and the contribution of ore activities to arsenic pollution in the surrounding environment (farmland soil and sediment) was analyzed (Zhang et al., 2018), elucidating the biotoxicity and bioaccumulation process of arsenic with different chemical forms (Song et al., 2020; Cui et al., 2021), revealing the arsenic tolerance of arsenic-oxidizing microorganisms and arsenic oxidation mechanism (Li et al., 2019). On the basis of the aforementioned research, in future, one can use high-performance liquid chromatography–plasma mass spectrometry (HPLC-ICP-MS), in situ synchrotron radiation techniques (μ-XRF and μ-XANES), multi-omics analysis techniques, and system dynamics models to analyze the biogeochemical cycle process and microbial driving mechanism of arsenic by studying the occurrence/chemical morphological characteristics, distribution, and migration law of arsenic in mining areas, together with molecular bioinformatic tools, to make clear the key factors that affect the migration and transformation of arsenic and ecological toxicity and to reveal the internal mechanism of pollution control strategies to control arsenic pollution. The simulated arsenic pollution model mining area needs to be constructed, which should cover geological background, human activities, soil properties, arsenic metabolizing microorganisms, arsenic biogeochemical cycle process, arsenic mobility, and diffusion degree. A forward-looking arsenic pollution prevention and control strategies are expected to be put forward to provide a theoretical basis and technical guidance for the efficient prevention and control of arsenic pollution in the mining area.
Author contributions
FZ, JH, and DM wrote the manuscript. All authors helped in editing and completing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program by the Ministry of Science and Technology of China (2018YFE0110200) and the Key Research and Development Program of Hunan Province (Nos. 2020WK2022 and 2022SK2076).
Conflict of interest
XP was employed by Hunan Renhe Environment Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi, S., Lamb, D. T., Kader, M., Naidu, R., and Megharaj, M. (2021). The influence of long-term ageing on arsenic ecotoxicity in soil. J. Hazard. Mater. 407, 124819. doi: 10.1016/j.jhazmat.2020.124819
Akhtar, M. S., Chali, B., and Azam, T. (2013). Bioremediation of arsenic and lead by plants and microbes from contaminated soil. Res. Plant Sci. 1, 68–73. doi: 10.12691/plant-1-3-4
Al-Abed, S. R., Jegadeesan, G., Purandare, J., and Allen, D. (2007). Arsenic release from iron rich mineral processing waste: influence of pH and redox potential. Chemosphere 66, 775–782. doi: 10.1016/j.chemosphere.2006.07.045
Amend, J., Saltikov, C., Lu, G. S., and Hernandez-Maldonado, J. (2014). Microbial arsenic metabolism and reaction energetics. Rev. Mineral. Geochem. 79, 391–433. doi: 10.2138/rmg.2014.79.7
Anekwe, I. M., and Isa, Y. M. (2021). Wastewater and bioventing treatment systems for acid mine drainage-contaminated soil. Soil Sediment. Contam. 1–14. doi: 10.1080/15320383.2020.1863909
Bagherifam, S., Brown, T. C., Fellows, C. M., and Naidu, R. (2019). Bioavailability of arsenic and antimony in terrestrial ecosystems: a review. Pedosphere 29, 681–720. doi: 10.1016/S1002-0160(19)60843-X
Bai, Y., Tang, X., Sun, L., Yin, W., Hu, G., Liu, M., et al. (2022). Application of iron-based materials for removal of antimony and arsenic from water: sorption properties and mechanism insights. Chem. Eng. J. 431, 134143. doi: 10.1016/j.cej.2021.134143
Barrera-Diaz, C. E., Lugo-Lugo, V., and Bilyeu, B. (2012). A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 223-224, 1–12. doi: 10.1016/j.jhazmat.2012.04.054
Bello, A. O., Tawabini, B. S., Khalil, A. B., and Boland, C. R. (2018). Phytoremediation of cadmium-, lead- and nickel-contaminated water by phragmites australis in hydroponic systems. Ecol. Eng. 120, 126–133. doi: 10.1016/j.ecoleng.2018.05.035
Bentley, R., Chasteen, T. G., Bentley, R., and Chasteen, T. G. (2002). Microbial methylation of metalloids: arsenic, antimony and bismuth. Microbiol. Mol. Biol. Rev. 66, 250–71. doi: 10.1128/MMBR.66.2.250-271.2002
Bhattacharjee, H., and Rosen, B. P. (2007). “Arsenic metabolism in prokaryotic and eukaryotic microbes,” in Nies, D., Silver, S., eds Molecular Microbiology of Heavy Metals (Springer, Berlin). p. 371–406. doi: 10.1007/7171_2006_086
Bianco Prevot, A., Ginepro, M., Peracaciolo, E., Zelano, V., and De Luca, D. A. (2018). Chemical vs. bio-mediated reduction of hexavalent chromium. An in-vitro study for soil and deep waters remediation. Geoderma 312, 17–23. doi: 10.1016/j.geoderma.2017.09.032
Burton, E. D., Hockmann, K., and Karimian, N. (2020). Antimony sorption to goethite: effects of Fe(II)-catalyzed recrystallization. ACS Earth Space Chem 4, 476–487. doi: 10.1021/acsearthspacechem.0c00013
Chen, C., Huang, L., Li, K., Zhang, J., Xie, W-. Y., Lu, Y., et al. (2019). Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J. 13, 2523–2535. doi: 10.1038/s41396-019-0451-7
Chen J. Bhattacharjee H. Rosen B. P. (2015) ArsH is an organoarseical oxidase that confers resistance to trivalent forms of the herbicide monosodium methylarsenate the poultry growth promoter roxarsone. Mol. Microbiol. 96 1042–1052. doi: 10.1111/mmi.12988
Chen, J., Zhang, J., Wu, Y. F., Zhao, F. J., and Rosen, B. P. (2021). ArsV and ArsW provide synergistic resistance to the antibiotic methylarsenite. Environ. Microbiol. 23, 7550–7562. doi: 10.1111/1462-2920.15817
Chen, Q. Y., Tyrer, M., Hills, C. D., and Yang, X. M. (2009). Immobilisation of heavy metal in cement-based solidification/stabilisation: a review. Waste Manage. 29, 390–403. doi: 10.1016/j.wasman.2008.01.019
Chen, S., Zhang, C., Qiu, L., Li, Q., Zhang, K., Luo, H., et al. (2022). Biogeochemical transformation of sulfur and its effects on arsenic mobility in paddy fields polluted by acid mine drainage. Chemosphere 293, 133605. doi: 10.1016/j.chemosphere.2022.133605
Chen, X., Wright, J. V., Conca, J. L., and Peurrung, L. M. (1997). Evaluation of heavy metal remendiation using mineral apatite. Water Air Soil Pollut. 98, 57–78. doi: 10.1007/BF02128650
Chen, Z., An, L., Wei, H., Zhang, J., Zou, Q., Sun, M., et al. (2021). Nitrate alleviate dissimilatory iron reduction and arsenic mobilization by driving microbial community structure change. Surfaces Interfaces 26, 101421. doi: 10.1016/j.surfin.2021.101421
Cheng, H., Hu, Y., Jian, L., and Xu, B. (2009). Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J. Hazard. Mater. 165, 13–26. doi: 10.1016/j.jhazmat.2008.10.070
Cui, D., Zhang, P., Li, H., Zhang, Z., Song, Y., Yang, Z., et al. (2021). The dynamic changes of arsenic biotransformation and bioaccumulation in muscle of freshwater food fish crucian carp during chronic dietborne exposure. J. Environ. Sci. 100, 74–81. doi: 10.1016/j.jes.2020.07.005
Cui, M., Lee, Y., Choi, J., Kim, J., Han, Z., Son, Y., and Khim, J. (2018). Evaluation of stabilizing materials for immobilization of toxic heavy metals in contaminated agricultural soils in China. J. Clean. Prod. 193, 748–758 doi: 10.1016/j.jclepro.2018.05.105
Cui, M., Lee, Y., Choi, J., Kim, J., Han, Z., Son, Y., and Khim, J. (2014). The accumulation and toxicity of methylated arsenicals in endothelial cells: important roles of thiol compounds. Toxicol. Appl. Pharmacol. 198, 458–467. doi: 10.1016/j.taap.2003.10.023
Dunivin, T. K., Yeh, S. Y., and Shade, A. (2019). A global survey of arsenic-related genes in soil microbiomes. BMC Biol. 17, 45. doi: 10.1186/s12915-019-0661-5
Egli, M., Sartori, G., Mirabella, A., Giaccai, D., Favilli, F., Scherrer, D., et al. (2010). The influence of weathering and organic matter on heavy metals lability in silicatic, Alpine soils. Sci. Total Environ. 408, 931–946. doi: 10.1016/j.scitotenv.2009.10.005
Ettler, V., Tomasova, Z., Komarek, M., Mihaljevic, M., Sebek, O., Michalkova, Z., et al. (2015). The pH-dependent long-term stability of an amorphous manganese oxide in smelter-polluted soils: implication for chemical stabilization of metals and metalloids. Hazard Mater 286, 386e.394. doi: 10.1016/j.jhazmat.2015.01.018
Ghosh, M., Shen, J., and Rosen, B. P. (1999). Pathways of As (III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 96, 5001–5006. doi: 10.1073/pnas.96.9.5001
Gupta, P., Kumar, V., Usmani, Z., Rani, R., and Chandra, A. (2018). Phosphate solubilization and chromium (VI) remediation potential of Klebsiella sp. strain CPSB4 isolated from the chromium contaminated agricultural soil. Chemosphere 192, 318–27. doi: 10.1016/j.chemosphere.2017.10.164
Jiang, L., Liu, X., Yin, H., Liang, Y., Liu, H., Miao, B., et al. (2019). The utilization of biomineralization technique based on microbial induced phosphate precipitation in remediation of potentially toxic ions contaminated soil: a mini review. Ecotoxicol. Environ. Saf. 191, 110009. doi: 10.1016/j.ecoenv.2019.110009
Ke, X., Zhang, F. J., Zhou, Y., Zhang, H. J., Guo, G. L., Tian, Y., et al. (2020). Removal of Cd, Pb,Zn, Cu in smelter soil by citric acid leaching. Chemosphere 255, 126690. doi: 10.1016/j.chemosphere.2020.126690
Lehr, C. R., Polishchuk, E., Radoja, U., and Cullen, W. R. (2003). Demethylation of methylarsenic species by Mycobacterium neoaurum. Appl. Organomet. Chem. 17, 831e.834. doi: 10.1002/aoc.544
Li, L., Liu, Z., Meng, D., Liu, X., Li, X., Zhang, M., et al. (2019). Comparative genomic analysis reveals the distribution, organization, and evolution of metal resistance genes in the genus Acidithiobacillus. Appl. Environ. Microbiol. 85, e02153–02118. doi: 10.1128/AEM.02153-18
Li, X., Meng, D., Li, J., Yin, H., Liu, H., Liu, X., et al. (2017). Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 231, 908–917. doi: 10.1016/j.envpol.2017.08.057
Liu, H., Li, P., Wang, H., Qing, C., Tan, T., Shi, B., et al. (2020). Arsenic mobilization affected by extracellular polymeric substances (EPS) of the dissimilatory iron reducing bacteria isolated from high arsenic groundwater. Sci. Total Environ. 735, 139501. doi: 10.1016/j.scitotenv.2020.139501
Liu, L., Shen, R-. L., Zhao, Z-. Q., Ding, L-. J., Cui, H-. L., Li, G., et al. (2022). How different nitrogen fertilizers affect arsenic mobility in paddy soil after straw incorporation?. J. Hazard. Mater. 436, 129135. doi: 10.1016/j.jhazmat.2022.129135
Lukasz, D., Liwia, R., Aleksandra, M., and Aleksandra, S. (2014). Dissolution of arsenic minerals mediated by dissimilatory arsenate reducing bacteria: estimation of the physiological potential for arsenic mobilization. Biomed Res. Int. 841892. doi: 10.1155/2014/841892
Mandal, B. K., and Suzuki, K. T. (2002). Arsenic round the world: a review. Talanta 58, 201–235. doi: 10.1016/S0039-9140(02)00268-0
Mao, Q., Xie, Z., Pei, F., Irshad, S., Issaka, S., and Randrianarison, G. (2023). Indigenous cyanobacteria enhances remediation of arsenic-contaminated soils by regulating physicochemical properties, microbial community structure and function in soil microenvironment. Sci. Total Environ. 860, 160543. doi: 10.1016/j.scitotenv.2022.160543
Meng, D., Li, J., Liu, T., Liu, Y., Yan, M., Hu, J., et al. (2019). Effects of redox potential on soil cadmium solubility: insight into microbial community. J. Environ. Sci. China 75, 224–232. doi: 10.1016/j.jes.2018.03.032
Mensah, A. K., Marschner, B., Wang, J., Bundschuh, J., Wang, S-. L., Yang, P-. T., et al. (2022a). Reducing conditions increased the mobilisation and hazardous effects of arsenic in a highly contaminated gold mine spoil. J. Hazard. Mater. 436, 129238. doi: 10.1016/j.jhazmat.2022.129238
Mensah, A. K., Shaheen, S. M., Rinklebe, J., Heinze, S., and Marschner, B. (2022b). Phytoavailability and uptake of arsenic in ryegrass affected by various amendments in soil of an abandoned gold mining site. Environ. Res. 214, 113729. doi: 10.1016/j.envres.2022.113729
Okkenhaug, G., Zhu, Y-. G., He, J., Li, X., Luo, L., Mulder, J., et al. (2012). Antimony (Sb) and arsenic (As) in Sb mining impacted paddy soil from Xikuangshan, China: differences in mechanisms controlling soil sequestration and uptake in rice. Environ. Sci. Technol. 46, 3155–3162. doi: 10.1021/es2022472
Otones, V., Álvarez-Ayuso, E., García-Sánchez, A., Santa Regina, I., and Murciego, A. (2011). Arsenic distribution in soils and plants of an arsenic impacted former mining area. Environ. Pollut. 159, 2637–2647. doi: 10.1016/j.envpol.2011.05.027
Palansooriya, K. N., Shaheen, S. M., Chen, S. S., et al. (2020). Soil amendments for immobilization of potentially toxic elements in contaminated soils: acritical review. Environ. Int. 134, 105046. doi: 10.1016/j.envint.2019.105046
Patel, P. C., Goulhen, F., Boothman, C., Gault, A. G., Charnock, J. M., Kalia, K., et al. (2007). Arsenate detoxification in a Pseudomonad hypertolerant to arsenic. Arch. Microbiol. 187, 171–183. doi: 10.1007/s00203-006-0182-9
Planer-Friedrich, B., Lehr, C., Matschullat, J., et al. (2006). Speciation of volatile arsenic at geothermal features in yellowstone national park. Geochim. Cosmochim. Acta 700, 2480–2491. doi: 10.1016/j.gca.2006.02.019
Qi, Z., Han, Y., Afrane, S., Liu, X., Zhang, M., Crittenden, J., et al. (2023). Patent mining on soil pollution remediation technology from the perspective of technological trajectory. Environ. Pollut. 316, 120661. doi: 10.1016/j.envpol.2022.120661
Rajendran, S., Priya, T. A. K., Khoo, K. S., Hoang, T. K. A., Ng, H. S., Munawaroh, H. S. H., et al. (2022). A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 287, 132369. doi: 10.1016/j.chemosphere.2021.132369
Rosen, B. P. (2002). Biochemistry of arsenic detoxification. FEBS Lett. 529, 86–92. doi: 10.1016/S0014-5793(02)03186-1
Shi, K., Radhakrishnan, M., Dai, X., Rosen, B. P., and Wang, G. (2021). NemA catalyzes trivalent organoarsenical oxidation and is regulated by the trivalent organoarsenical-selective transcriptional repressor nemR. Environ. Sci. Technol. 55, 6485–94. doi: 10.1021/acs.est.1c00574
Shi, K., Wang, Q., and Wang, G. (2020). Microbial oxidation of arsenite: regulation, chemotaxis, phosphate metabolism and energy generation. Front. Microbiol. 11, 569282. doi: 10.3389/fmicb.2020.569282
Simon, T. (2000). The effect of nickel and arsenic on the occurrence and symbiotic abilities of native rhizobia. Rostlinna Vyroba 46, 63–68. doi: 10.1046/j.1439-0523.2000.00441.x
Song, P., Xu, H., Sun, S., Xiong, W., and Yang, Z. (2022). Remediation of arsenic-spiked soil by biochar-loaded nanoscale zero-valent iron: performance, mechanism, and microbial response. J. Clean. Prod. 380, 134985. doi: 10.1016/j.jclepro.2022.134985
Song, Y., Zhang, F., Li, H., Qiu, B., Gao, Y., Cui, D., et al. (2020). Antioxidant defense system in lettuces tissues upon various As species exposure. J. Hazard. Mater. 399. doi: 10.1016/j.jhazmat.2020.123003
Styblo, M., Del Razo, L. M., Vega, L., et al. (2000). Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol., 74, 289–299. doi: 10.1007/s002040000134
Su, S. M., Zeng, X. B., Feng, Q. F., Bai, L. Y., Zhang, L. L., Jiang, S., et al. (2015). Demethylation of arsenic limits its volatilization in fungi. Environ. Pollut. 204, 141e.144. doi: 10.1016/j.envpol.2015.04.025
Sultana, M., Vogler, S., Zargar, K., Schmidt, A. C., Saltikov, C., Seifert, J., et al. (2012). New clusters of arsenite oxidase and unusual bacterial groups in enrichments from arsenic-contaminated soil. Arch. Microbiol. 194, 623–635. doi: 10.1007/s00203-011-0777-7
Sun, J., Ma, L., Yang, Z., Lee, H., and Wang, L. (2015). Speciation and determination of bioavailable arsenic species in soil samples by one-step solvent extraction and high-performance liquid chromatography with inductively coupled plasma mass spectrometry. J. Sep. Sci. 38, 943–950. doi: 10.1002/jssc.201401221
Sun, R., Li, Y., Lin, N., Ou, C., Wang, X., Zhang, L., et al. (2020). Removal of heavy metals using a novel sulfidogenic AMD treatment system with sulfur reduction: Configuration, performance, critical parameters and economic analysis. Environ. Int. 136, 105457. doi: 10.1016/j.envint.2019.105457
Tan, J., Yi, H., Zhang, Z., Meng, D., Li, Y., Xia, L., et al. (2022). Montmorillonite facilitated Pb(II) biomineralization by Chlorella sorokiniana FK in soil. J. Hazard. Mater. 423, 127007. doi: 10.1016/j.jhazmat.2021.127007
Tessier, A., Campbell, P. G. C., and Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51, 844–851. doi: 10.1021/ac50043a017
Tsai, S. L., Singh, S., and Chen, W. (2009). Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr. Opin. Biotechnol. 20, 659–667. doi: 10.1016/j.copbio.2009.09.013
Turpeinen, R., Pantsar-Kallio, M., and Kairesalo, T. (2002). Role of microbes in controlling the speciation of arsenic and production of arsines in contaminated soils. Sci. Total Environ. 285, 133–145. doi: 10.1016/S0048-9697(01)00903-2
Wang, P., Sun, G., Jia, Y., Meharg, A. A., and Zhu, Y. A. (2014). Review on completing arsenic biogeochemical cycle: Microbial volatilization of arsines in environment. J. Environ. Sci. 26, 371–81. doi: 10.1016/S1001-0742(13)60432-5
Wang, R., Zhang, H., Sun, L., Qi, G., Chen, S., Zhao, X., et al. (2017). Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 7, 343. doi: 10.1038/s41598-017-00472-6
Wang, Y., Liu, T., and Li, F. (2016). Advances in the semiconductor-mediated electron transfer mechanism at microbe-mineral interface. Adv. Earth Sci. 31, 347–356. doi: 10.11867/j.issn.1001-8166.2016.04.0347
Wen, B., Zhou, A., Zhou, J., Liu, C., Huang, Y., Li, L., et al. (2018). Coupled S and Sr isotope evidences for elevated arsenic concentrations in groundwater from the world's largest antimony mine, Central China. J. Hydrol. 557, 211–221. doi: 10.1016/j.jhydrol.2017.12.013
Weng, L., Temminghoff, E. J. M., Lofts, S., Tipping, E., and Van Riemsdijk, W. H. (2002). Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ. Sci. Technol. 36, 4804–4810. doi: 10.1021/es0200084
Wu, B., Liu, F., Wenwen, F., Yang, T., Chen, G. H., He, Z., et al. (2021). Microbial sulfur metabolism and environmental implications. Sci. Total Environ. 146085. doi: 10.1016/j.scitotenv.2021.146085
Wu, J., Liang, J., Björn, L. O., Li, J., Shu, W., Wang, Y., et al. (2022). Phosphorus-arsenic interaction in the “soil-plant-microbe” system and its influence on arsenic pollution. Sci. Total Environ. 802, 149796. doi: 10.1016/j.scitotenv.2021.149796
Xu, D. M., Fu, R. B., Liu, H. Q., and Guo, X. P. (2021). Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: a critical review. J. Clean. Prod. 286, 124989. doi: 10.1016/j.jclepro.2020.124989
Xue, S. G., Korna, R., Fan, J. R., Ke, W. S., Lou, W., Wang, J. T., et al. (2023). Spatial distribution, environmental risks, and sources of potentially toxic elements in soils from a typical abandoned antimony smelting site. J. Environ. Sci. 127, 780–790. doi: 10.1016/j.jes.2022.07.009
Yamamura, S., and Amachi, S. (2014). Microbiology of inorganic arsenic: from metabolism to bioremediation. J. Biosci. Bioeng. 118, 1–9. doi: 10.1016/j.jbiosc.2013.12.011
Yan, G., Chen, X., Du, S., Deng, Z., Wang, L., and Chen, S. (2019). Genetic mechanisms of arsenic detoxification and metabolism in bacteria. Curr. Genet. 65, 329–338. doi: 10.1007/s00294-018-0894-9
Yan, S., Yang, J., Si, Y., Tang, X., Ma, Y., Ye, W., et al. (2022). Arsenic and cadmium bioavailability to rice (Oryza sativa L.) plant in paddy soil: influence of sulfate application. Chemosphere 307, 135641. doi: 10.1016/j.chemosphere.2022.135641
Yan, Y., Ye, J., Xue, X. M., and Zhu, Y. G. (2015). Arsenic demethylation by a C-As lyasein cyanobacterium Nostoc sp. PCC 7120. Environ. Sci. Technol. 49, 14350–14358. doi: 10.1021/acs.est.5b03357
Yan, L., Song, J., Chan, T., and Jing, C. (2017). Insights into antimony adsorption on TiO2: XAFS and DFT study. Environ. Sci. Technol. 51, 6335–6341. doi: 10.1021/acs.est.7b00807
Yang, J., Wang, S., Guo, Z., Deng, Y., Xu, M., Zhang, S., et al. (2020). Spatial distribution of toxic metal(loid)s and microbial community analysis in soil vertical profile at an abandoned nonferrous metal smelting site. Int. J. Environ. Res. Public Health 17, 7101. doi: 10.3390/ijerph17197101
Yang, N., Winkel, L., and Johannesson, K. H. (2014). Predicting geogenic arsenic contamination in shallow groundwater of South Louisiana, United States. Environ. Sci. Technol. 48–10. doi: 10.1021/es405670g
Ye, J., Rensing, C., Rosen, B. P., and Zhu, Y. G. (2012). Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 17, 155–162. doi: 10.1016/j.tplants.2011.12.003
Ye, L., Qiu, S., Li, X., Jiang, Y., and Jing, C. (2018). Antimony exposure and speciation in human biomarkers near an active mining area in Hunan, China. Sci. Total Environ. 640–641, 1–8. doi: 10.1016/j.scitotenv.2018.05.267
Yoshinaga, M., and Rosen, B. P. (2014). As lyase for degradation of environmental organoarsenical herbicides and animal husbandry growth promoters. Proc. Nat. Acad. Sci. 11121, 7701–6 doi: 10.1073/pnas.1403057111
Yoshinaga, M., Cai, Y., and Rosen, B. P. (2011). Demethylation of methylarsonic acid by a microbial community. Environ. Microbiome 13, 1205e.1215. doi: 10.1111/j.1462-2920.2010.02420.x
Zargar, K., Hoeft, S., Oremland, R., and Saltikov, C. W. (2010). Identification of a novel arsenite oxidase gene, arxA, in the haloalkaliphilic, arsenite-oxidizing bacterium Alkalilimnicola ehrlichii strain MLHE-1. J. Bacteriol. 192, 3755–3762. doi: 10.1128/JB.00244-10
Zhang, H., Shao, J., Zhang, S., and Zhang, X. (2020). Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J. Hazard. Mater. 390, 121349. doi: 10.1016/j.jhazmat.2019.121349
Zhang, M., Zhang, T., Zhou, L., Lou, W., Zeng, W., Liu, T., et al. (2022). Soil microbial community assembly model in response to heavy metal pollution. Environ. Res. 213, 113576. doi: 10.1016/j.envres.2022.113576
Zhang, Y., Wang, X., and Ji, H. (2020). Stabilization process and potential of agro-industrial waste on Pb-contaminated soil around Pb-Zn mining. Environ. Pollut. 260, 114069. doi: 10.1016/j.envpol.2020.114069
Zhang, Z., Lu, Y., Li, H., Tu, Y., Liu, B., Yang, Z., et al. (2018). Assessment of heavy metal contamination, distribution and source identification in the sediments from the Zijiang River, China. Sci. Total Environ. 645, 235–243. doi: 10.1016/j.scitotenv.2018.07.026
Zhang, Z., Lu, Y., Li, H., Zhang, N., Cao, J., Qiu, B., et al. (2021). Simultaneous separation of Sb(III) and Sb(V) by high performance liquid chromatography (HPLC)—inductively coupled plasma—mass spectrometry (ICP-MS) with application to plants, soils, and sediments. Anal. Lett. 54, 919–934. doi: 10.1080/00032719.2020.1788049
Zhao, F. J., Harris, E., Yan, J., Ma, J., Wu, L., Liu, W., et al. (2013). Arsenic methylation in soils and its relationship with microbial arsm abundance and diversity, and as speciation in rice. Environ. Sci. Technol. 47, 7147–7154. doi: 10.1021/es304977m
Zhao, J., Luo, Q., Ding, L., Fu, R., Zhang, F., Cui, C., et al. (2022). Valency distributions and geochemical fractions of arsenic and antimony in non-ferrous smelting soils with varying particle sizes. Ecotoxicol. Environ. Saf. 233, 113312. doi: 10.1016/j.ecoenv.2022.113312
Zhou, Q., and Xi, S. (2018). A review on arsenic carcinogenesis: epidemiology, metabolism, genotoxicity and epigenetic changes. Regulat. Toxicol. Pharmacol. 99, 78–88. doi: 10.1016/j.yrtph.2018.09.010
Zhu, Y. G., Xue, X-. M., Kappler, A., Rosen, B. P., and Meharg, A. A. (2017). Linking genes to microbial biogeochemical cycling: lessons from As. Environ. Sci. Technol. 51, 7326–7339. doi: 10.1021/acs.est.7b00689
Keywords: arsenic pollution, bio-geochemical processes, microbial communities, pollution control, mining area
Citation: Zhuang F, Huang J, Li H, Peng X, Xia L, Zhou L, Zhang T, Liu Z, He Q, Luo F, Yin H and Meng D (2023) Biogeochemical behavior and pollution control of arsenic in mining areas: A review. Front. Microbiol. 14:1043024. doi: 10.3389/fmicb.2023.1043024
Received: 13 September 2022; Accepted: 17 February 2023;
Published: 23 March 2023.
Edited by:
Xue Guo, Tsinghua University, ChinaReviewed by:
Yun Fang, Wuhan Institute of Technology, ChinaTianliang Zheng, Chengdu University of Technology, China
Copyright © 2023 Zhuang, Huang, Li, Peng, Xia, Zhou, Zhang, Liu, He, Luo, Yin and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delong Meng, RGVsb25nLm1lbmdAY3N1LmVkdS5jbg==
 Fan Zhuang1
Fan Zhuang1 Jingyi Huang
Jingyi Huang Xing Peng
Xing Peng Ling Xia
Ling Xia Zhenghua Liu
Zhenghua Liu Qiang He
Qiang He Feng Luo
Feng Luo Huaqun Yin
Huaqun Yin Delong Meng
Delong Meng