- 1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, China
- 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Lab Medicine, West China Hospital, Sichuan University, Chengdu, China
- 4West China Hospital, West China School Medicine, Sichuan University, Chengdu, China
Sporotrichosis has multiple clinical manifestations, and its cutaneous-disseminated form is uncommon and, in most cases, related to immunosuppressive conditions. We report the case of a 47-year-old male patient who presented with multiple cutaneous nodules and ulcers on the left upper limb and the right thigh, with no other comorbidities. Until the diagnosis was confirmed, the patient was initially given empiric antifungal treatment with itraconazole, which showed unsatisfactory results at a local hospital. Then, he was treated with voriconazole, which led to the slow improvement of his skin lesions. At one point during the voriconazole treatment course, the patient briefly self-discontinued voriconazole for economic reasons, and the lesions recurred and worsened. The patient was finally diagnosed with cutaneous-disseminated sporotrichosis based on the isolation and identification of Sporothrix globosa. Susceptibility testing revealed that the isolate was resistant to itraconazole, fluconazole, voriconazole, terbinafine, and amphotericin. Considering the patient's poor financial condition, potassium iodide was administered. After 1-month of therapy with potassium iodide, he reported rapid improvement of his skin lesions. The patient continued potassium iodide treatment for another 5 months until the full resolution of lesions was achieved.
Introduction
Sporotrichosis is a sub-acute to chronic subcutaneous mycosis caused by the ubiquitous, thermodimorphic fungus, Sporothrix complex (Valeriano et al., 2020). It occurs worldwide, predominantly in tropical and subtropical countries, such as Mexico, Central America, South America, and Africa (Barros et al., 2011). According to the immune status of the host, the load and location of the inoculation, and the thermal tolerance of the strain, sporotrichosis presents a series of clinical manifestations, which are clinically categorized into fixed cutaneous, lymphocutaneous, cutaneous-disseminated, and extracutaneous forms (Bonifaz and Tirado-Sánchez, 2017). Lymphocutaneous sporotrichosis is the most common form, while the cutaneous-disseminated form is uncommon and is mostly related to immunosuppressed individuals (Severo et al., 1999; Bonifaz and Vazquez-Gonzalez, 2010). Herein, a rare case of cutaneous-disseminated sporotrichosis in an immunocompetent man is presented.
Case presentation
A 47-year-old male patient presented to our hospital with multiple cutaneous nodules and ulcers on the left upper limb and the right thigh. He worked as a farmer in a rural region and had no pathological history. The lesions initially appeared 4 years ago on his middle finger of the left hand, where trauma occurred when he cut yak meat, and then, it gradually spread to the rest of his left upper limb and the right thigh. Over the past 4 years, he was hospitalized two times at a local hospital for presumed cutaneous invasive fungal infection and non-tuberculous Mycobacterium infection and had received empiric treatment with itraconazole, rifampicin, and levofloxacin for nearly 2 years with no obvious improvement. Then, the patient presented to the infection department of the West China Hospital and was hospitalized.
Blood investigations revealed an elevated erythrocyte sedimentation rate of 24 mm/h, an absolute CD3 lymphocyte count of 937 cell/μl, and an absolute CD8 lymphocyte count of 237 cell/μl. Absolute CD4 lymphocyte count, white blood cell count, and neutrophil percentage were normal. TB interferon-gamma release assay (TB-IGRA) was positive. Other blood investigations, including liver function, renal function, HIV, and viral hepatitis screening, were normal. Ultrasonography of the abdomen was also normal. Chest computed tomography (CT) revealed several sub-centimeter pulmonary nodules without lymphadenopathy. The culture of skin species for bacteria revealed Staphylococcus epidermidis, while that for fungi and mycobacteria was negative. Silver methenamine stain from biopsy tissue revealed several suspicious fungal spores. Detection of the skin tissue by the next-generation sequencing (NGS) was negative for bacteria, viruses, fungi, and mycobacteria.
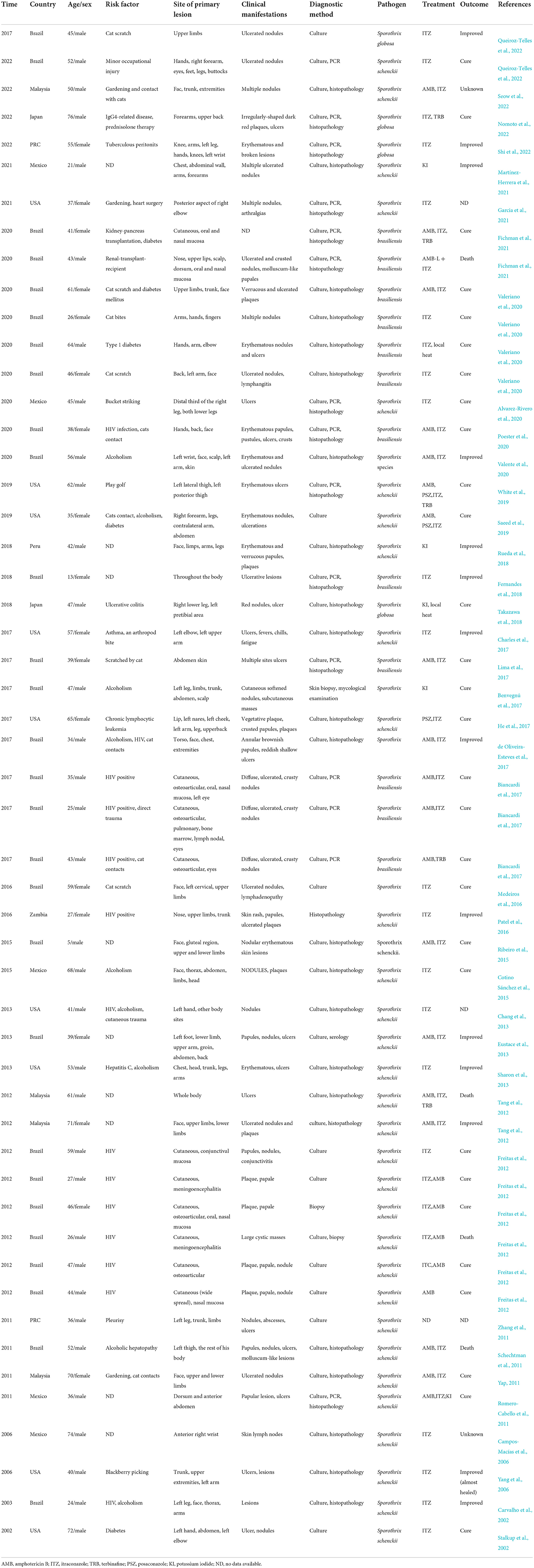
Before empiric antifungal treatment, the patient was referred to the dermatology clinic for screening for cutaneous fungal infection. Physical examination revealed large scattered verrucous and ulcerated nodules with overlying necrotic eschar on the left upper limb. Further examination of the trunk and extremities revealed scattered crusted papules and plaques on the right thigh (Figure 1). After skin tissue culture for fungi was performed in the dermatology clinic, the patient was discharged and started on empiric oral voriconazole 400 mg daily. The fungal culture on sabouraud dextrose agar (SDA) at 28°C for 10 days grew into grayish-white colonies (Figure 2A). Microscopic examination with slide culture and scanning electron microscope observations of the colony indicated the morphology of Sporothrix spp. (Figures 2B,C). The strain was identified as Sporothrix globosa by calmodulin gene (CAL) sequence analysis.
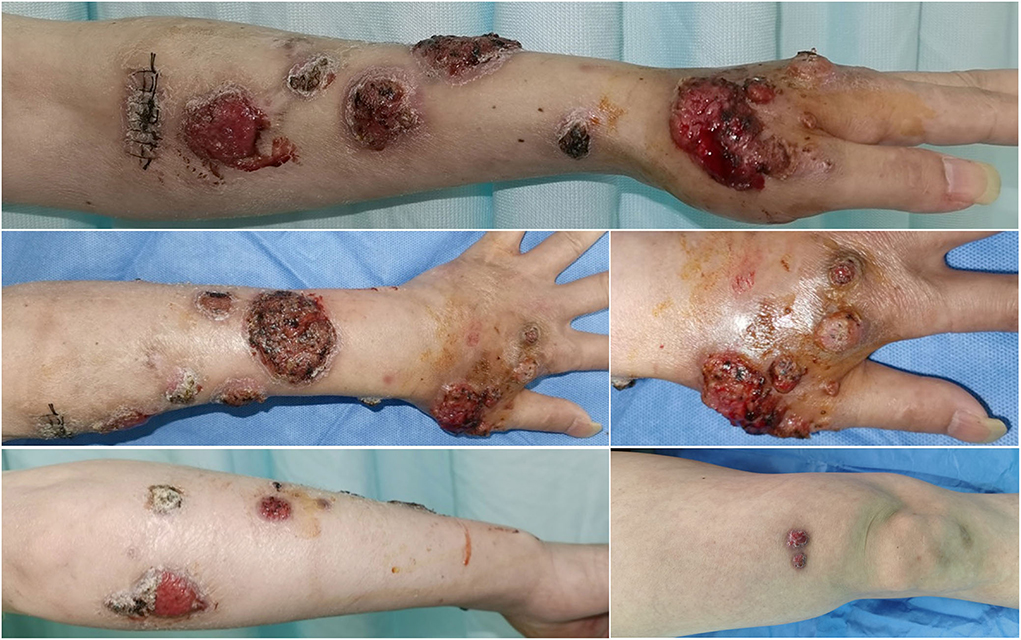
Figure 1. Multiple cutaneous lesions caused by Sporothrix globosa. A 47-year-old man with multiple verrucous and ulcerated nodules with overlying necrotic eschar on left upper limb, scattered crusted papules and plaques on right thigh.
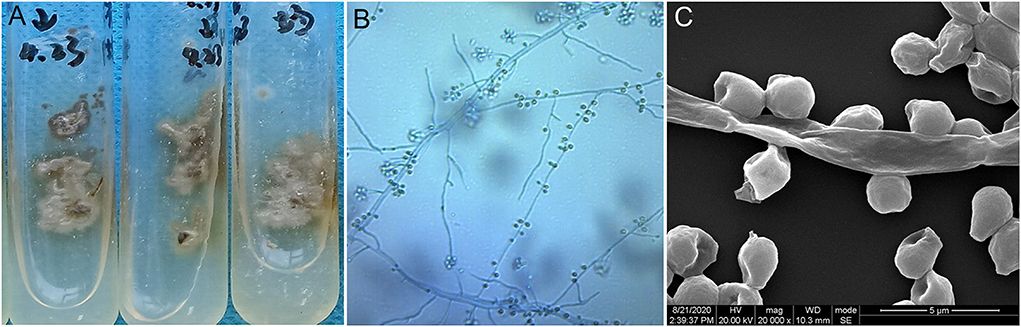
Figure 2. (A) Culture of Sporothrix globosa (Sabouraud dextrose agar, 28°C) grew grayish white colonies. (B,C) Slide culture and scanning electron microscope observations revealed thin hyphae and denticle microconidia like “daisy flowers”.
Based on the aforementioned evidence, a diagnosis of cutaneous-disseminated sporotrichosis was finally established, but the patient had not shown up for follow-up but continued antifungal therapy with voriconazole at a local hospital. During a telephone follow-up, the patient reported a slow improvement after voriconazole treatment. After 8 months of antifungal treatment, the patient self-discontinued voriconazole due to financial constraints, and the lesions recurred and worsened (Figure 3). He visited our dermatology clinic again for further treatment. An antifungal susceptibility test was performed by using the E-test (BIO KONT, China), which revealed that the isolate in this case was resistant to itraconazole, fluconazole, voriconazole, terbinafine, and amphotericin. Meanwhile, considering the patient's poor financial condition, a 10% solution of potassium iodide was administered. After 1-month of therapy with potassium iodide, there was a rapid improvement in his skin lesions. He continued potassium iodide treatment for another 5 months until there was a complete resolution of lesions (Figure 4). There was no recurrence at the 6-month follow-up.
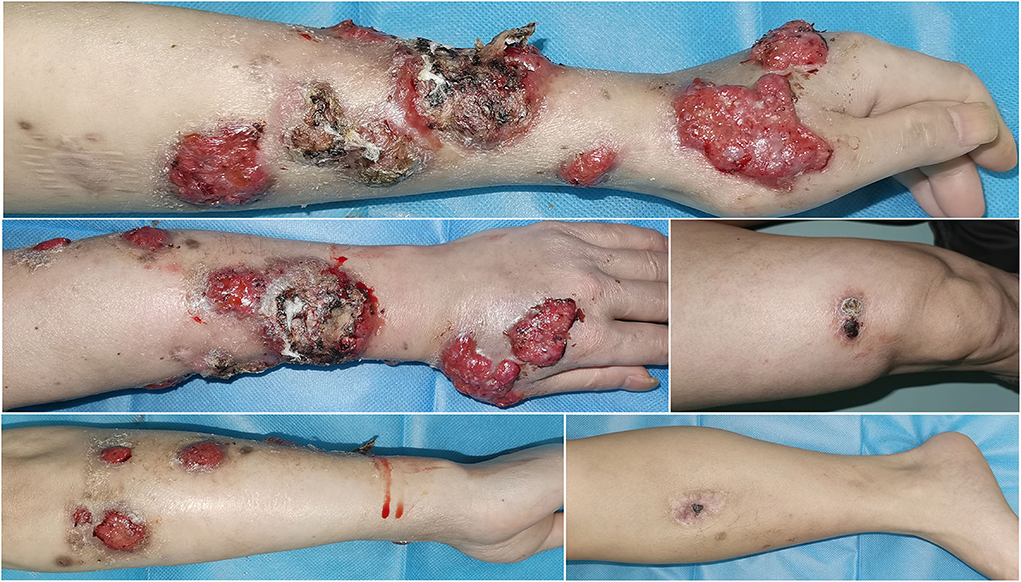
Figure 3. After self-discontinuation of voriconazole, the existing lesions (verrucous and ulcerated nodules on the left upper limb, and papules and plaques on the right thigh) recurred and worsened, extending to the right leg with crusted plaque.
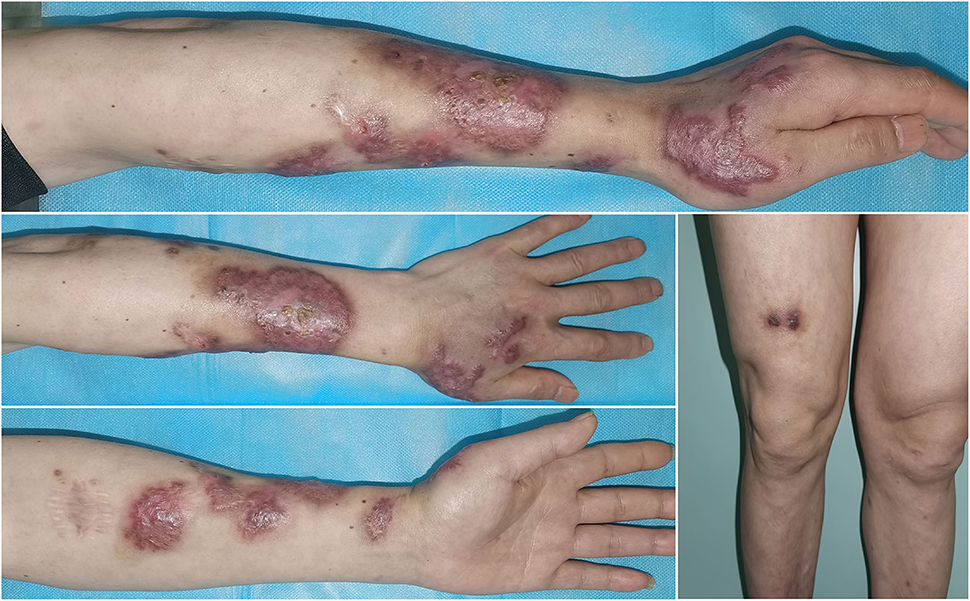
Figure 4. After 6-month therapy of potassium iodide, the patient achieved complete remission with only scarring remaining.
Discussion
Cutaneous-disseminated sporotrichosis (CDS) is characterized by multiple skin lesions at non-contiguous sites without extracutaneous involvement. Lesions of the fixed and lymphocutaneous forms may coexist in the same patient (Barros et al., 2011). The entity identified in this patient is a rare form of sporotrichosis, which only accounts for <1.75–8% of cases of Sporothrix infections (Song et al., 2013; Garcia et al., 2021). In China, the incidence of CDS is even lower. The cutaneous-disseminated form represented only 0.34% (14/4,969) of all sporotrichosis cases in a large-scale clinical epidemiological investigation of sporotrichosis reported from China (Lv et al., 2022).
CDS in most cases affffects immunodefificient individuals, frequently related to patients with HIV, hematologic cancer, diabetes mellitus, steroid treatment, chronic alcoholism, malnutrition, those who are pregnant and had undergone transplantation (Bonifaz and Tirado-Sánchez, 2017). There are few reports of immunocompetent individuals with disseminated lesions (Almeida-Paes et al., 2014), as in this patient. Dissemination in immunocompetent hosts has been linked to cat scratches, which cause multi-site repeat inoculations (Barros et al., 2011; Bonifaz and Tirado-Sánchez, 2017).
A literature search was performed in the PubMed database using the item “disseminated cutaneous sporotrichosis” for cases reported from January 2002 to June 2022 (Table 1). Of the 52 published cases of CDS found from the review, 35% were women and 65% were men, with an average age of 45.7 years (range 5–76 years). Among them, 28 patients were from Brazil, eight from the United States, five from Mexico, four from Malaysia, and two from China. HIV, diabetes, alcoholism, and a history of cat contact are the common predisposing factors. In the review, 22 published cases of CDS occur in hosts without obvious immunocompromised conditions.
Diagnosis of cutaneous-disseminated sporotrichosis is challenging due to its diverse clinical manifestations. The condition can affect any part of the body surface, presenting cutaneous features that include numerous ulcerated nodules and verrucous plaques (Saeed et al., 2019). This polymorphic presentation is distinct from the classic “sporotrichoid” appearance of the most common lymphocutaneous form of sporotrichosis. CDS can extend to mucous membranes, bones, joints, various organs, and systems and rapidly progress to fungemia (Bonifaz and Tirado-Sánchez, 2017).
Diagnosis of CDS is often delayed or misdiagnosed because its diverse clinical symptoms are easily confused with other conditions such as PG, Sweet's syndrome, tuberculosis, sarcoidosis, and other mycotic or parasitic infections, including cutaneous leishmaniasis (Saeed et al., 2019). Culture from tissue fragments, exudative lesions, scales, sputum, and blood remains a gold standard for diagnosis. Culture using sabouraud dextrose agar, incubated at 25–30°C, is a standard technique applied in most cases, but it is time-consuming (Barros et al., 2011). The histopathologic features of granulomatous inflammation with cigar-shaped organisms and asteroid bodies are supportive but have low sensitivity (Barros et al., 2011).
Itraconazole and amphotericin B are the most useful therapies for patients with CDS, as in the current review (Saeed et al., 2019; Valeriano et al., 2020). In refractory cases, different combination therapies can be considered. Potassium iodide, an inexpensive and fairly safe preparation, has been found to be consistently effective against Sporothrix. Potassium iodide and itraconazole in combination with thermotherapy are preferred therapeutic options in cutaneous-disseminated cases of sporotrichosis (Valeriano et al., 2020). The treatment with potassium iodide alone or combined with hyperthermia has also been reported in CDS, as in our case and the four published cases described in the review (Benvegnú et al., 2017; Rueda et al., 2018; Takazawa et al., 2018; Martínez-Herrera et al., 2021).
In summary, CDS is an uncommon clinical form of infection caused by Sporothrix, and it is even rarer in immunocompetent hosts. Due to the increased incidence of the condition, it is significant to maintain a high degree of suspicion in the presence of lesions similar to that reported here. A fungal culture is crucial to confirm the diagnosis of CDS. Although itraconazole and amphotericin B have been recommended for CDS, potassium iodide is a safe and effective alternative.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Biomedical Research Ethics Committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YR and KZ contributed to conception and design of the study. YZ and YK organized the database. XR and YD performed the statistical analysis. KZ wrote the first draft of the manuscript and sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was supported by the Natural Science Foundation of China (No. 81803150) and the HX-Academician project (HXYS19003) of West China Hospital, Sichuan University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almeida-Paes, R., de Oliveira, M. M. E., Freitas, D. F. S., do Valle, A. C. F., Zancope-Oliveira, R. M., and Gutierrez-Galhardo, M. C. (2014). Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl. Trop. Dis. 8, e3094. doi: 10.1371/journal.pntd.0003094
Alvarez-Rivero, V., Hernandez-Castro, R., Moreno-Coutiño, G., and Lozano-Platonoff, A. (2020). Disseminated sporotrichosis: an important differential diagnosis for venous ulcers. Adv. Skin Wound Care 33, 1–3. doi: 10.1097/01.ASW.0000666908.88965.35
Barros, M. B., de Almeida Paes, R., and Schubach, A. O. (2011). Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 24, 633–654. doi: 10.1128/CMR.00007-11
Benvegnú, A. M., Stramari, J., Dallazem, L. N., Chemello, R. M., and Beber, A. A. (2017). Disseminated cutaneous sporotrichosis in patient with alcoholism. Rev. Soc. Bras. Med. Trop. 50, 871–873. doi: 10.1590/0037-8682-0281-2017
Biancardi, A. L., Freitas, D. F., Vitor, R. D. A., Andrade, H. B., de Oliveira, M. M., do Valle, A. C., et al. (2017). Multifocal choroiditis in disseminated sporotrichosis in patients with HIV/AIDS. Retin. Cases Brief Rep. 11, 67–70. doi: 10.1097/ICB.0000000000000290
Bonifaz, A., and Tirado-Sánchez, A. (2017). Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J. Fungi 3, 6. doi: 10.3390/jof3010006
Bonifaz, A., and Vazquez-Gonzalez, D. (2010). Sporotrichosis: an update. G. Ital. Dermatol. Venereol. 145, 659–673.
Campos-Macías, P., Arenas, R., Vega-Memije, M., and Kawasaki, M. (2006). Sporothrix schenckii type 3D (mtDNA-RFLP): report of an osteoarticular case. J. Dermatol. 33, 295–299. doi: 10.1111/j.1346-8138.2006.00071.x
Carvalho, M. T. M., Castro, A. P. D., Baby, C., Werner, B., Filus Neto, J., and Queiroz-Telles, F. (2002). Cutaneous sporotrichosis in a patient with AIDS: report of a case. Rev. Soc. Bras. Med. Trop. 35, 655–659. doi: 10.1590/S0037-86822002000600018
Chang, S., Hersh, A. M., Naughton, G., Mullins, K., Fung, M. A., Sharon, V. R., et al. (2013). Disseminated cutaneous sporotrichosis. Dermatol. Online J. 19, 20401. doi: 10.5070/D31911020401
Charles, K., Lowe, L., Shuman, E., and Cha, K. B. (2017). Painful linear ulcers: a case of cutaneous sporotrichosis mimicking pyoderma gangrenosum. JAAD Case Rep. 3, 519–521. doi: 10.1016/j.jdcr.2017.07.014
Cotino Sánchez, A., Torres-Alvarez, B., Gurrola Morales, T., Méndez Martínez, S., Saucedo Gárate, M., and Castanedo-Cazares, J. P. (2015). Mycosis fungoides-like lesions in a patient with diffuse cutaneous sporotrichosis. Rev. Iberoam. Micol. (2015) 32, 200–203. doi: 10.1016/j.riam.2014.06.009
de Oliveira-Esteves, I. C. M. R., Almeida Rosa da Silva, G., Eyer-Silva, W. D. A., Basílio-de-Oliveira, R. P., de Araujo, L. F., Martins, C. J., et al. (2017). Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep. Infect. Dis. 2017, 4713140. doi: 10.1155/2017/4713140
Eustace, K. E., Sampaio, F. M., Lyra, M. R., Quintella, L., and do Valle, A. C. Cutaneous disseminated sporotrichosis complicated by osteomyelitis. Acta Derm. Venereol. (2013) 93, 192–193. doi: 10.2340/00015555-1403
Fernandes, B., Caligiorne, R. B., Coutinho, D. M., Gomes, R. R., Rocha-Silva, F., Machado, A. S., et al. (2018). A case of disseminated sporotrichosis caused by Sporothrix brasiliensis. Med. Mycol. Case Rep. 21, 34–36. doi: 10.1016/j.mmcr.2018.03.006
Fichman, V., Marques de Macedo, P., Francis Saraiva Freitas, D., Carlos Francesconi do Valle, A., Almeida-Silva, F., Reis Bernardes-Engemann, A., et al. (2021). Zoonotic sporotrichosis in renal transplant recipients from Rio de Janeiro, Brazil. Transpl. Infect. Dis. 23, e13485. doi: 10.1111/tid.13485
Freitas, D. F. S., de Siqueira Hoagland, B., do Valle, A. C. F., Fraga, B. B., de Barros, M. B., de Oliveira Schubach, A., et al. (2012). Sporotrichosis in HIV-infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med. Mycol. 50, 170–178. doi: 10.3109/13693786.2011.596288
Garcia, B. M., Bond, A. R., Barry, A. K., Steen, A. J., LeBoit, P. E., Ashbaugh, C., et al. (2021). Disseminated-cutaneous sporotrichosis in an immunocompetent adult. JAAD Case Rep. 26, 102−104. doi: 10.1016/j.jdcr.2021.03.003
He, Y., Ma, C., Fung, M., and Fitzmaurice, S. (2017). Disseminated cutaneous sporotrichosis presenting as a necrotic facial mass: case and review. Dermatol. Online J. 23, 13030./qt5zd47238. doi: 10.5070/D3237035747
Lima, R. B., Jeunon-Sousa, M. A., Jeunon, T., Oliveira, J. C., Oliveira, M. M. E., Zancopé-Oliveira, R. M., et al. (2017). Sporotrichosis masquerading as pyoderma gangrenosum. J. Eur. Acad. Dermatol. Venereol. 31, e539–e541. doi: 10.1111/jdv.14421
Lv, S., Hu, X., Liu, Z., Lin, Y., Wu, H., Li, F., et al. (2022). Clinical epidemiology of sporotrichosis in Jilin Province, China (1990-2019): a series of 4969 cases. Infect. Drug Resist. 11, 1753–1765. doi: 10.2147/IDR.S354380
Martínez-Herrera, E., Arenas, R., Hernández-Castro, R., Frías-De-León, M. G., and Rodríguez-Cerdeira, C. (2021). Uncommon clinical presentations of sporotrichosis: a two-case report. Pathogens 10, 1249. doi: 10.3390/pathogens10101249
Medeiros, K. B., Landeiro, L. G., Diniz, L. M., and Falqueto, A. (2016). Disseminated cutaneous sporotrichosis associated with ocular lesion in an immunocompetent patient. An. Bras. Dermatol. 91, 537–953. doi: 10.1590/abd1806-4841.20164859
Nomoto, Y., Higashi, Y., Uchida, Y., Fujii, K., Ooka, T., Kanekura, T., et al. (2022). Disseminated cutaneous sporotrichosis with intravascular granuloma. J. Dermatol. 49, e301–e302. doi: 10.1111/1346-8138.16429
Patel, A., Mudenda, V., Lakhi, S., and Ngalamika, O. A. (2016). 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep. Dermatol. Med. 2016, 9403690. doi: 10.1155/2016/9403690
Poester, V. R., Munhoz, L. S., Basso, R. P., Roca, B. M., Vieira, M. U., Melo, A. M., et al. (2020). Disseminated sporotrichosis with immune reconstitution inflammatory syndrome in an HIV patient: case report and review of the literature. Rev. Iberoam. Micol. 37, 97–99. doi: 10.1016/j.riam.2020.09.001
Queiroz-Telles, F., Cognialli, R. C., Salvador, G. L., Moreira, G. A., Herkert, P. F., Hagen, F., et al. (2022). Cutaneous disseminated sporotrichosis in immunocompetent patient: case report and literature review. Med. Mycol. Case Rep. 8, 31–34. doi: 10.1016/j.mmcr.2022.05.003
Ribeiro, B. N., Ribeiro, R. N., Penna, C. R., and Frota, A. C. (2015). Bone involvement by Sporothrix schenckii in an immunocompetent child. Pediatr. Radiol. 45, 1427–1430. doi: 10.1007/s00247-015-3299-7
Romero-Cabello, R., Bonifaz, A., Romero-Feregrino, R., Sánchez, C. J., Linares, Y., Zavala, J. T., et al. (2011). Disseminated sporotrichosis. BMJ Case Rep. 25, bcr1020103404. doi: 10.1136/bcr.10.2010.3404
Rueda, M., Torres, N., and Bravo, F. (2018). Disseminated cutaneous sporotrichosis: an unusual case. Dermatol. Online J. 24, 13030/qt2wk6r6w0. doi: 10.5070/D32411042003
Saeed, L., Weber, R. J., Puryear, S. B., Bahrani, E., Peluso, M. J., Babik, J. M., et al. (2019). Disseminated cutaneous and osteoarticular sporotrichosis mimicking pyoderma gangrenosum. Open Forum Infect Dis. 6, ofz395. doi: 10.1093/ofid/ofz395
Schechtman, R. C., Crignis, G. S., Pockstaller, M. P., Azulay-Abulafia, L., Quintella, L. P., Belo, M., et al. (2011). Molluscum-like lesions in a patient with sporotrichosis. An. Bras. Dermatol. 86, 1217–1219. doi: 10.1590/S0365-05962011000600028
Seow, W., Kammal, A., Jamil, N., Nor, W., and Kammal, C. (2022). Disseminated sporotrichosis in an immunocompetent patient: an unusual presentation. Clin. Case Rep. Int. 6, 1257–1260.
Severo, L. C., Festugato, M., Bernardi, C., and Londero, A. T. (1999). Widespread cutaneous lesions due to Sporothrix schenckii in a patient under a long-term steroids therapy. Rev. Inst. Med. Trop. 41, 59–62. doi: 10.1590/S0036-46651999000100010
Sharon, V. R., Kim, J., Sudhakar, S., Fung, M. A., and Maniar, A. (2013). Disseminated cutaneous sporotrichosis. Lancet Infect. Dis. 13, 95. doi: 10.1016/S1473-3099(12)70140-8
Shi, Y., Yu, Y., Li, S. S., and Cui, Y. (2022). Leukocytoclastic vasculitis caused by disseminated cutaneous sporotrichosis: a case report and review of the literature. Am. J. Dermatopathol. 44, 223–225. doi: 10.1097/DAD.0000000000002076
Song, Y., Li, S. S., Zhong, S. X., Liu, Y. Y., Yao, L., Huo, S. S., et al. (2013). Report of 457 sporotrichosis cases from Jilin province, northeast China, a serious endemic region. J. Eur. Acad. Dermatol. Venereol. 27, 313–318. doi: 10.1111/j.1468-3083.2011.04389.x
Stalkup, J. R., Bell, K., and Rosen, T. (2002). Disseminated cutaneous sporotrichosis treated with itraconazole. Cutis 69, 371–374.
Takazawa, M., Harada, K., Kakurai, M., Yamada, T., Umemoto, N., Sakai, T., et al. (2018). Case of pyoderma gangrenosum-like sporotrichosis caused by Sporothrix globosa in a patient with ulcerative colitis. J. Dermatol. 45, e226–e227. doi: 10.1111/1346-8138.14276
Tang, M. M., Tang, J. J., Gill, P., Chang, C. C., and Baba, R. (2012). Cutaneous sporotrichosis: a six-year review of 19 cases in a tertiary referral center in Malaysia. Int. J. Dermatol. 51, 702–708. doi: 10.1111/j.1365-4632.2011.05229.x
Valente, M. F., Diogo, A. B., Merlo, V. F. C., and Pegas, J. R. P. (2020). Disseminated cutaneous sporotrichosis: unusual presentation in an alcoholic patient. Rev. Inst. Med. Trop. São Paulo 62, e60. doi: 10.1590/s1678-9946202062060
Valeriano, C. A. T., Ferraz, C. E., Oliveira, M. M. E., Inácio, C. P., de Oliveira, E. P., Lacerda, A. M., et al. (2020). Cat-transmitted disseminated cutaneous sporotrichosis caused by Sporothrix brasiliensis in a new endemic area: case series in the northeast of Brazil. JAAD Case Re.p 6, 988–992. doi: 10.1016/j.jdcr.2020.07.047
White, M., Adams, L., Phan, C., Erdag, G., Totten, M., Lee, R., et al. (2019). Disseminated sporotrichosis following iatrogenic immunosuppression for suspected pyoderma gangrenosum. Lancet Infect. Dis. 19, e385–e391. doi: 10.1016/S1473-3099(19)30421-9
Yang, D. J., Krishnan, R. S., Guillen, D. R., Schmiege, L. M. III., Leis, P. F., and Hsu, S. (2006). Disseminated sporotrichosis mimicking sarcoidosis. Int. J. Dermatol. 45, 450–453. doi: 10.1111/j.1365-4632.2004.02363.x
Yap, F. B. (2011). Disseminated cutaneous sporotrichosis in an immunocompetent individual. Int. J. Infect. Dis. 15, e727–729. doi: 10.1016/j.ijid.2011.05.005
Keywords: cutaneous disseminated sporotrichosis, Sporothrix globosa, potassium iodide, itraconazole, voriconazole (VCZ)
Citation: Zhuang K, Dai Y, Zhou Y, Ke Y, Ran X and Ran Y (2022) Oral treatment with 10% potassium iodide solution for refractory cutaneous-disseminated sporotrichosis in an immunocompetent adult: Case report. Front. Microbiol. 13:994197. doi: 10.3389/fmicb.2022.994197
Received: 14 July 2022; Accepted: 23 August 2022;
Published: 28 October 2022.
Edited by:
Wanqing Liao, Shanghai Changzheng Hospital, ChinaReviewed by:
Gerson de Oliveira Paiva-Neto, Federal University of Amazonas, BrazilShuwen Deng, Suzhou High-tech Zone People's Hospital, China
Copyright © 2022 Zhuang, Dai, Zhou, Ke, Ran and Ran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuping Ran, cmFueXVwaW5nQHZpcC5zaW5hLmNvbQ==
 Kaiwen Zhuang
Kaiwen Zhuang Yaling Dai3
Yaling Dai3 Yuping Ran
Yuping Ran