- 1National Engineering Research Center of Biological Feed, Institute of Feed Research, Chinese Academy of Agricultural Sciences, Beijing, China
- 2China Oil Foodstuffs Corporation (COFCO) Nutrition and Health Research Institute, Beijing, China
- 3Technology Department, China Oil Foodstuffs Corporation (COFCO) (Beijing) Feed Technology Company Limited, Beijing, China
This study focused on evaluating the influence of Clostridium butyricum and Brevibacillus strains on egg production, egg quality, immune response and antioxidant function, apparent fecal amino acid digestibility, and jejunal morphology when supplemented as probiotics in the diets of laying hens in the peak phase. A total of 288 healthy 30-week-old Hy-Line Brown laying hens were arbitrarily assigned to four dietary groups, which included control diet and control diet supplemented with 0.02% C. butyricum zlc-17, C. butyricum lwc-13, or Brevibacillus zlb-z1, for 84 days. The results showed that dietary C. butyricum and Brevibacillus sp. exerted a positively significant influence (P ≤ 0.05) compared to the control group on the performance, egg quality, and physiological response of the birds. The diets could reduce mortality rate and enhance (P ≤ 0.05) egg weight and egg mass, egg production rate, and feed efficiency. Further analysis suggested that the probiotic strains can enhance (P ≤ 0.05) eggshell quality, Haugh unit, thick albumen content, and albumen height. Also, probiotics enhanced (P ≤ 0.05) the antioxidant status via increased antioxidant enzymes and jejunal morphology as evidenced by increased villi surface area (VSA), the ratio of villi height to crypt depth, villi width, and villi height, and a significant reduction in crypt depth. Besides, nutrient absorption and retention were enhanced, as apparent fecal amino acid digestibility of key essential amino acids was substantially improved in the diet-based group. The concentrations of immunoglobulin M and A (IgM and IgA) increased significantly (P ≤ 0.05) in the probiotics group and the same effect was notable for complement proteins (C3) and immune organ (Spleen). Conclusively, the supplementation of Clostridium butyricum zlc-17 in comparison to Clostridium butyricum lwc-13 and Brevibacillus zlb-z1 strains significantly (P ≤ 0.05) promoted the antioxidant status, modulated the intestinal structure, enhanced amino acid digestibility, and regulated the immunity index of the laying hens, which finally improves the laying performance and egg quality of the laying hens.
Introduction
Feed additives are often employed in poultry nutrition to enhance the health status of the birds, growth performance, and efficiency of production (Markowiak and Śliżewska, 2018). The utilization of synthetic antibiotics as feed additives in animal production is targeted mainly toward gut health; this appears beneficial, but such may not be the case in laying hen’s production due to issues related to egg safety. Also, the abrogation of antibiotic uses in animal diets according to European Parliament and Council Regulation EC No. 1831/2003 due to its adverse effects, such as drug resistance, residue effect, and environmental pollution (Wang et al., 2015), lends more evidence to its non-use in animal production despite its beneficial effect on animal health. To this end, other countries including China, the United States, and South Korea have also adopted antibiotic-free diets in animal production. In order to maintain an equilibrium between egg safety for consumers and animal health, feed additives, including probiotics, prebiotics, synbiotics, and organic acid, which tend to stimulate favorable growth and immune function in farm animals without adverse effects on animal product quality, have been advocated for Al-Khalaifah (2018).
Probiotics are often considered as “Live” micro-organisms, which when supplied in a substantial amount, provide the host with an improved health and welfare status (Food and Agriculture Organization and World Health Organization Expert Consultation, 2001). The underlying mechanism of probiotic actions, including the production of metabolites (short-chain organic fatty acids), immunostimulatory effects, alteration of gastrointestinal flora, and exclusive competitive binding to receptors (Sherman et al., 2009; Ahasan et al., 2015), accounts for the myriad of its positive influence on animal production and health in the poultry industry. Probiotics have been found to enhance laying performance (Mikulski et al., 2020; Macit et al., 2021; Xu et al., 2022), egg quality (Deng et al., 2021; Wang J. et al., 2021; Ray et al., 2022), immune response (Song et al., 2019; Deng et al., 2021; Pan et al., 2022), gut health (Abdel-Latif et al., 2018; Yang et al., 2020), and reduced oxidative stress response (Deng et al., 2021; Xu et al., 2022). Nevertheless, some studies reported that probiotics had no influence on egg production (Arpášová et al., 2016; Shi et al., 2020), egg quality: albumen quality (Souza et al., 2021) and eggshell quality (Wang W. W. et al., 2020), and antioxidant capacity (Forte et al., 2016). The probiotic strain used in the diet may be a contributory factor to the non-significant effect. Studies demonstrated that probiotics could be supplemented in the diet of laying hens as a single strain or a combination of different strains (Xiang et al., 2019; Yang et al., 2020). In the poultry industry, microorganisms often used as probiotics include colonizing species of Enterococcus. Streptococcus, Bacillus, Lactobacillus, and Clostridium.
Clostridium butyricum (CB) spores are highly stable anaerobic endospore-forming gram-positive bacteria, with a capacity to withstand higher temperatures and bile concentration (Kong et al., 2011); thus, they might be utilized in the diet of laying hens as safe feed additives. Previous pieces of literature showed that supplementation of CB in the diet of laying hens at different levels, 0.5 g/kg (Xiang et al., 2019), 0.9 g/kg (Wang et al., 2020), 1 × 109 CFU/kg (Wang et al., 2021), and 5 × 108 CFU/g (Zhan et al., 2019), exerted no negative effect, which lends more evidence of CB as safe feed additives. Our preliminary studies also revealed that CB could be supplemented at 0.02%. In previous research, the positive influence of CB on egg production and egg quality (Xiang et al., 2019; Zhan et al., 2019; Wang Y. et al., 2021) has been reported. Furthermore, C. butyricum can positively influence intestinal morphology and health (Zhang et al., 2011; Xiang et al., 2019; Wang Y. et al., 2021), probably because it can act as a source of nutrients for intestinal epithelium and modulate intestinal microflora and intestinal pH (Meimandipour et al., 2010; Zhang et al., 2016; Takahashi et al., 2018). Also, C. butyricum possesses the potential to improve antioxidant capacity and immune function (Zhan et al., 2019; Wang Y. et al., 2021) and nutrient absorption and utilization via the stimulation of enzymes and nutrient transporters (Wang W. W. et al., 2020). Therefore, the potential of CB to modulate gut health, antioxidant, and immune function may account for its beneficial influence on laying performance and egg quality. However, the C. butyricum species used in this study may differ from previous ones, where probably these species were reconstituted and designed to be more suitable for the peak phase of laying hens. In the same line, Bacillus strains are stable in an acidic gut environment, form biofilm in the small intestine, could be delivered in the form of spores (Jeong and Kim, 2014), and thus could be used as feed additives. The Bacillus spores were previously adopted as a feed additive to increase egg production (Mazanko et al., 2018; Zhou et al., 2020) and albumen quality (Wei et al., 2020; Zhou et al., 2020; Darsi and Zhaghari, 2021). The potential of Bacillus sp. to enhance nutrient utilization (Souza et al., 2021), intestine morphology (Yang et al., 2020; Wang J. et al., 2021), and serum antioxidant capacity (Zhou et al., 2020) has also been demonstrated. Thus, Bacillus strains can be supplemented in the diets of laying hens because of the potential to maintain the physiological status of the animals, which could translate to improved laying performance and egg quality. Furthermore, strains of Brevibacillus are producers of antibacterial and antifungal agents (Panda et al., 2014) and, hence, can be used for biological control. Previous reports showed that Brevibacillus brevis (FJAT-1501-BPA) can suppress the abundance of Staphylococcus aureus, E. Coli K88, and Salmonella typhimurium (Ge et al., 2009) while Brevibacillus laterosporus texasporus enhanced the intestine health of broiler birds (Purba et al., 2020), suggesting that the strain has the potential to be used as a probiotic in animal feeding. However, there exists a dearth of information on the utilization of Brevibacillus sp. in the diet of laying hens.
There are several studies on Clostridium sp. in laying hens, but the strains used in this study are newly created and designed specifically for peak-laying hens; moreover, studies on the effects of Brevibacillus sp. on egg production rate, egg quality, physiological status, and intestinal morphometric of laying hens rarely exist. Therefore, the current study investigated the dietary influence of Clostridium butyricum and Brevibacillus spores on egg quality, laying performance, amino acid digestibility, immune response, intestinal morphology, and antioxidant function in Hy-Line Brown laying hens.
Materials and methods
Ethics statement
The Animal Ethics and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences, Beijing, China consented to all protocols utilized in the current study with the animal ethics approval number CAAS. No.: 20200507S0600103.
Experimental design
Hy-Line Brown laying hens (n = 288, 30-week old) at the peak-laying phase (initial egg production rate = 89.0 ± 1.5%) with similar laying rates were arbitrarily assigned to one of four dietary groups, each of which consists of six replicates (n = 72 laying hens). The feeding trial included a 12-week test phase and a 2-week acclimation or a feed transfer period (lasted for 14 weeks; 30–44 weeks of age). On a daily basis, the laying hens were offered fresh water and feed ad lib, the birds were given routine vaccination, and management was based on the Hy-line International Online Management Guide. Throughout the feeding trial period, the laying hens were managed under a controlled house environment (on a daily basis): humidity (50–80%) and 16 h of light and temperature (24°C). During the feeding trial, the laying hens had stable good health as there was no disease outbreak, and invariably, no medications were offered.
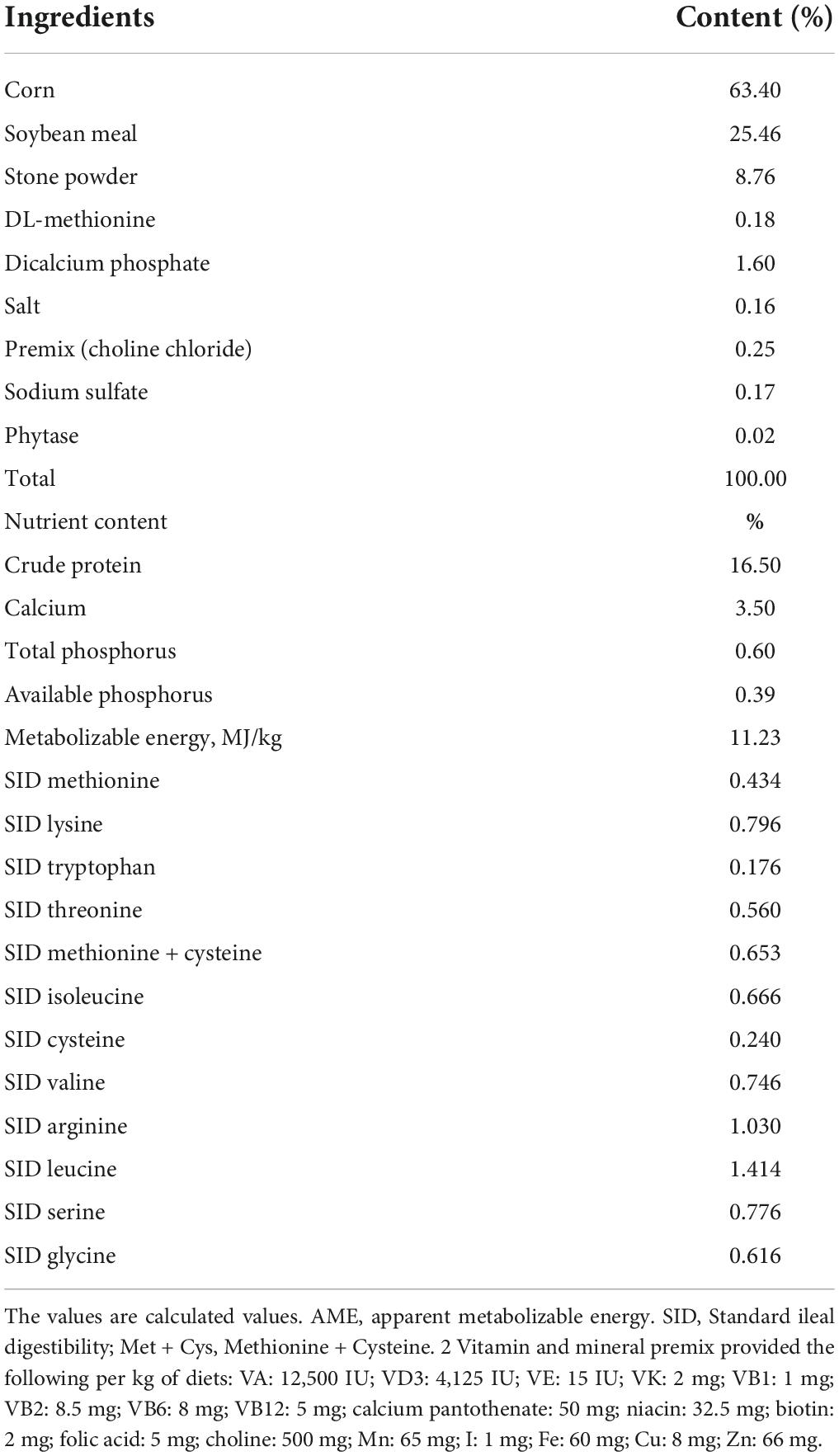
The diet groups consist of the control diet without supplementation of probiotics, basal diet + 0.02% of C. butyricum (zlc-17), basal diet + 0.02% of C. butyricum (lwc-17), and basal diet + 0.02% of Brevibacillus (zlb-z1). The viable count of three kinds of probiotic products is the same, i.e., 1 × 109 CFU/g. Prior to the feeding trial, the birds were fed laying hens’ diets; a basal diet of mashed corn and soybean. This diet was sufficient in all nutrients and met the necessary standards. The information regarding basal diet, nutrient level, and nutrient composition, is presented in Table 1, and the diet was in accordance with the nutrient formulation guide of the National Research Council (National Research and Council, 1994). The probiotics were purchased from COFCO Nutrition and Health Research Institute, Beijing, China.
Performance measurement
During the feeding experiment, which lasted for 12 weeks, the following records were taken per replicate on a daily basis, egg number, egg weight, damaged eggs, and mortality rate, and data collection on feed intake was done on a fortnight basis. Based on the collected data, the following calculations were deduced; hen day production (HDP), feed conversion ratio (FCR), average feed intake (ADFI), average egg weight (AEG), and egg mass for the whole trial period (π × VW × VH).
Sample collection and laboratory analysis of blood
After the feeding trial (12 weeks), 24 birds (six from each group, one per replicate) were separated and kept in other cages and subjected to a 12-h fast prior to slaughter. About 5 mL of blood was drawn from the wing vein for the measurement of whole blood and serum indices. The blood samples collected in a micro-anticoagulant tube were kept slant at a fixed point for a period of 30 min and then centrifuged (300 × g for 15 min) (Tang et al., 2018). The obtained serum was transferred to Eppendorf tubes (1.5 mL) and kept at a low temperature (−20°C). The blood samples were transported to the laboratory in an ice pack within 1 h of collection for hematology analysis.
An automated hematological analyzer (Model: BC-2800 Vet, Mindray, Shenzhen, China) was used for the hematological analysis. Prior to serum indices analysis, the serum was thawed and maintained at a low temperature (4°C) to prevent enzyme activation. The concentrations of malondialdehyde (MDA), glutathione transferase (GST), catalase (CAT), total antioxidant capacity (T-AOC), total superoxidase dismutase (T-SOD), and glutathione peroxidase (GSH-Px) in the serum were analyzed with the corresponding ELISA kits (A003-1, ml023160, A007-1-1, ml063644, A001-1-1, and ml061730) and spectrophotometrically measured (Shimadzu, model UV-1800, Tokyo, Japan). ML Bio and Jiancheng Bioengineering Institute (Nanjing, China) were sources of the ELISA kits. Concentrations of CAT, T-SOD, and T-AOC were expressed in micromoles per milliliter, GST and GSH-Px as nanograms per milliliter, and MDA as nanomoles per milliliter of serum. Serum concentrations of immunoglobulins, such as IgM, IgA, and IgG, and complement proteins C3 and C4 were determined with the appropriate ELISA kits (WLB-09120, WLB-091301, WLB-050501, E032-1-1, and E033-1-1), respectively, and measured with a microplate reader. The instructions of the manufacturers were stringently followed.
Intestine sample collection and jejunal morphology analysis
The birds were euthanized with pentobarbital sodium (100 mg/kg BW) intravenously and cut open while maintaining aseptic conditions. For each bird, the organs (magnum, heart, spleen, and liver) were separated and weighed immediately. The weights were expressed as a percentage of their body weight. For each bird, the small intestine samples were processed following an established procedure (Gungor and Erener, 2020). About 3 cm of jejunum were removed and flushed in saline solution to eliminate feed contents, then were immersed in 10% buffered formalin, and were kept under low temperature (4°C) for histology analysis. The jejunal tissue sections were embedded in paraffin blocks, and a 6-μm thickness of the tissue was subsequently cut, carefully placed on microscopic glass slides, and stained with a solution of hematoxylin and eosin. For slide examination, a microscope (Olympus BX43 microscope; Olympus Corp., Tokyo, Japan) was employed. To examine the jejunal morphology, 10 intact villi of each selected sample were measured, and the corresponding crypts were selected for measurement and an average value was obtained. The Villi height (VH) was obtained based on the measurement from the tip to the villus-crypt junction of each villus; the villus width (VW) was measured at the middle point of the villus; and crypt depth (CD) was obtained from the basement membrane up to the crypt–villus transition region with the aid of a software (Caseviewer Image). Also, the equation (π × VW × VH) was used to deduce the villi surface area (VSA), while (V/C) was used to obtain the ratio of villi height and crypt depth (Wang et al., 2016; Thiam et al., 2021).
Egg quality measurement
Following the end of 4, 8, and 12 weeks (a 4-week interval), three eggs per replicate (18 eggs) with a weight close to the range of that replicate were retrieved from each dietary group. The collected eggs were kept under room temperature, and egg quality was determined within 24 h of collection. Upon breakage of each egg, the albumen and the yolk were separated with the aid of an egg separator, and the weight of each was recorded. To measure the thick and thin albumen fractions, the weighed albumen was placed in a 60-mesh sieve at a time bound of 30 s, the thick portion of the albumen was glued to the sieve while the thin portion passed through the sieve as filtrate, and the corresponding weight of each fraction was recorded (Zhou et al., 2021). The eggshells were cleaned to remove any albumen fragments and then naturally dried for 48 h, and the weight was obtained. The proportion of the shell, the yolk, and the albumen relative to egg weight was expressed as shell or albumen or yolk weight/egg weight × 100 (Sarlak et al., 2021). The assessment of egg quality parameters, albumen height, Haugh Unit, and yolk color, was performed with an automatic egg analyzer (ORKA Food Technology Ltd., Ramat HaSharon, Israel). The eggshell breaking strength and eggshell thickness, which is expressed as an average measurement of three points (air cell, equator, and sharp end) (Mwaniki et al., 2018), were examined, respectively, with Egg Force Reader and Eggshell Thickness Gauge (ESTG-1, ORKA Technology Ltd., Ramat HaSharon, Israel).
Apparent fecal amino acid digestibility
At the end of the experiment (12th week), on a replicate basis, three birds were selected and kept in a cage fitted with a tray for the collection of fecal samples, and this lasted for 3 days. The fecal sample collection was done at an interval of 12 h, and the samples were kept at −20°C in tight-closed bags. At the point of collection, it was ensured that all external components, such as feed, feathers, and any other substances, were thoroughly removed from the samples to avoid contamination. The collected feces samples were thawed, weighed, and oven dried for 72 h at 65°C, after which it was broken and pulverized into a fine powder that can be sieved through a 0.05 mm mesh. For each metabolic cage, the feed intake and feces weight (dry matter basis) were recorded and used to determine the apparent fecal amino acid digestibility. The feed and fecal samples were further processed for amino acid analysis with HPLC while adopting the method proposed by Varzaru et al. (2013). The apparent fecal amino acid digestibility coefficient was computed; 1 − (amino acid concentration in feces × feces weight) ÷ (amino acid concentration in feed × feed intake) × 100%.
Statistical analysis
The experiment consists of four groups with six replications, each in a completely randomized design to ensure random allocation of birds to treatments. All the data generated in this study were subjected to a one-way analysis of variance (ANOVA), which ensures that there is no biasness with respect to data normality and equality of variance assumptions (Nwachukwu et al., 2021). Replicates were used as experimental units, and the data were presented as mean and pooled SEM, while the level of significance was considered at a p-value < 0.05. Duncan’s multiple range test was employed for post hoc comparison to ascertain the variations among the treatment groups. The statistical package used was SPSS software, version 17.0 (SPSS Inc., Chicago, II, United States) (Wang L. et al., 2020).
Results
Laying performance
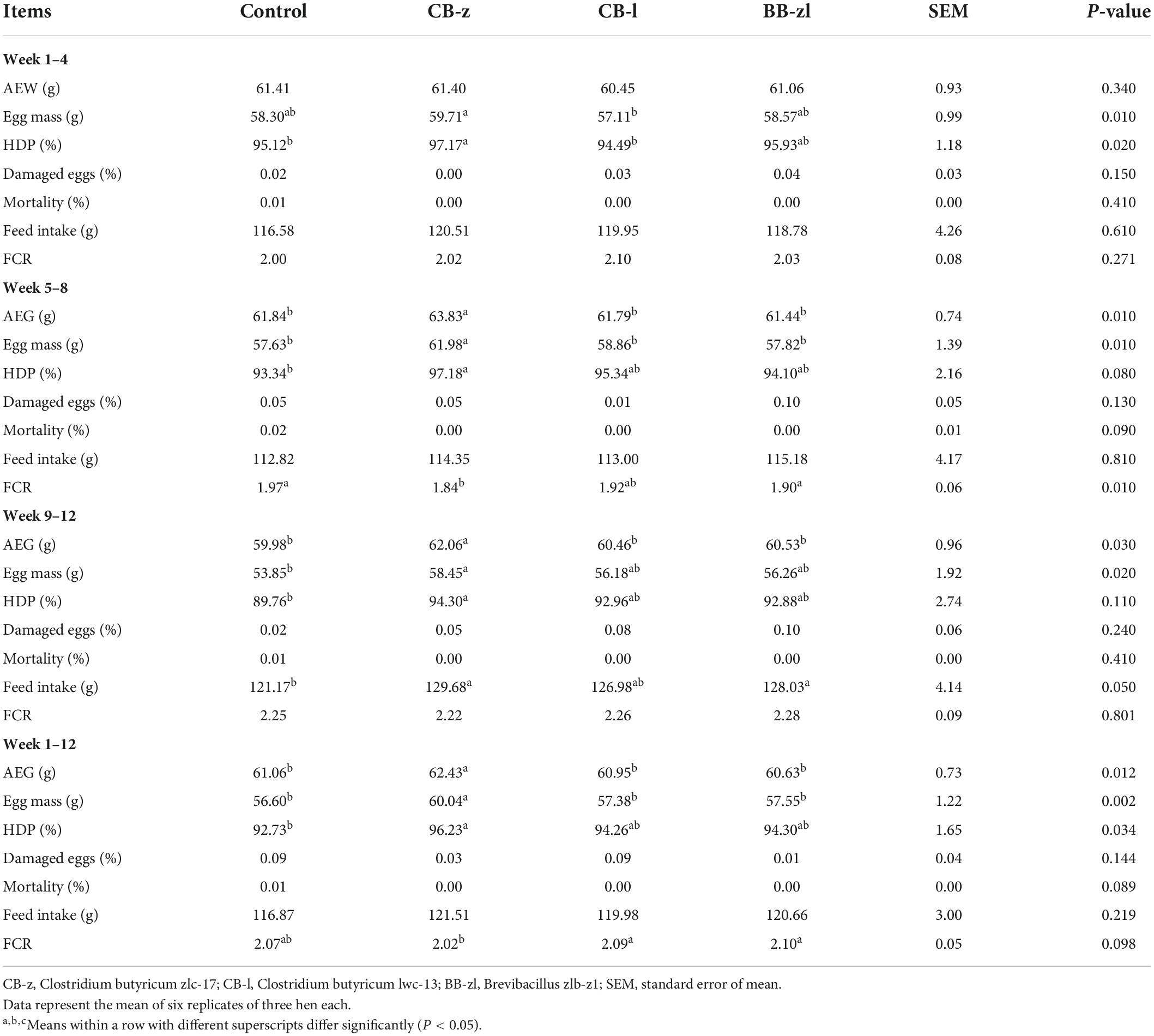
At 4-week intervals throughout the trial, the production performance indices (egg weight, egg mass, hen-day production, mortality and damaged egg rates, feed intake, and feed conversion) were analyzed for all groups. Table 2 presents the outcomes.
Egg weight was not influenced (P ≤ 0.05) by diets at the end of 4 weeks, but CB-z improved egg weight (P ≤ 0.05) at weeks 8 and 12, while egg weights for other groups did not vary (P ≥ 0.05) with control. Egg mass was increased (P ≤ 0.05) by dietary CB-z compared to control and other treatment groups at weeks 4, 8, and 12. Furthermore, at all sampling points, egg mass from the CB group had the highest value, while that of the Brevibacillus group was not significant (P ≥ 0.05) from the control. A significant increase (P ≤ 0.05) in egg production rate due to dietary treatments was observed throughout the feeding period. The egg production rate of the CB-z group was 4.7% higher (P ≤ 0.05) and varied from control and other treatment groups, while the Brevibacillus group had the lowest egg production rate among the treatment groups throughout the feeding trial. The egg production rate of the CB-l and Brevibacillus groups was not significant (P≥ 0.05) from control at all measuring points but numerically higher. Zero mortality rate was noticed in the probiotic-based groups but not in the control group, and damaged eggs were not influenced (P ≥ 0.05) by diets throughout the study period. Dietary influence on feed intake was not significant (P ≥ 0.05) at weeks 4 and 8 but was significant (P ≤ 0.05) at week 12. However, there was no variation (P ≥ 0.05) among the probiotic-based group. The FCR was enhanced (P ≤ 0.05) due to dietary influence at weeks 8 and 1–12 but not at other measuring points. Among the probiotic-based groups, the CB-z group recorded the lowest value, while no variation (P ≥ 0.05) between control and other treatments was found.
Egg quality assessment
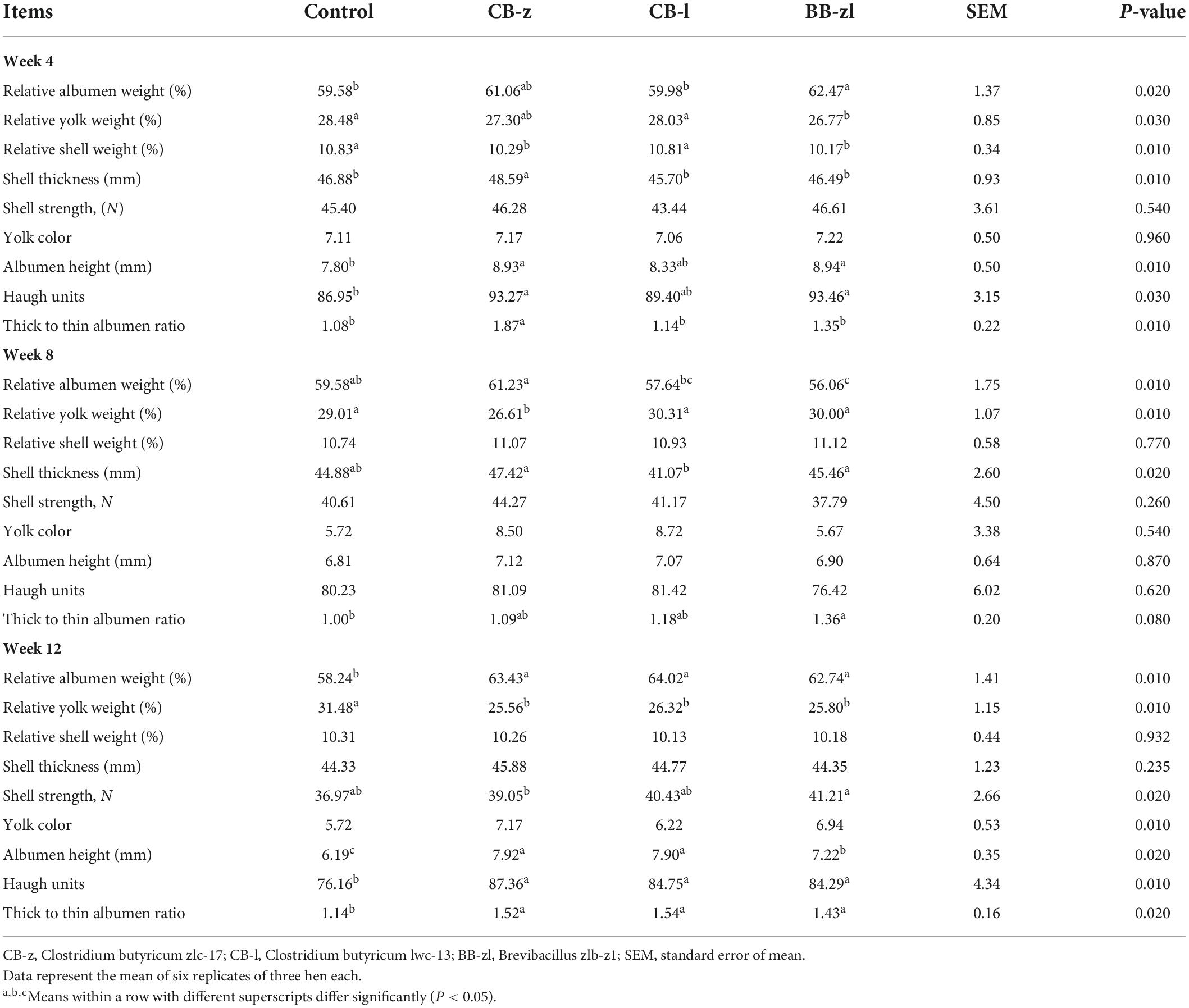
The results of egg quality determination are presented in Table 3. The relative weight of the albumen and the yolk differed statistically (P ≤ 0.05) between the control group and the dietary group throughout the feeding period, while the relative shell weight was only significant (P ≤ 0.05) at week 4 but not at other measuring points. The relative albumen weight consistently increased (P ≤ 0.05) due to the treatment effect at all measuring points, except week 4, while the relative yolk weight of the control group increased significantly compared to other treatments throughout the study. There was no variation (P ≥ 0.05) in relative albumen weight among the dietary groups during week 12, but the Brevibacillus group recorded the least and highest weight at weeks 4 and 12, respectively. Eggshell thickness was improved (P ≤ 0.05) by dietary probiotics at weeks 4 and 8, but no variation (P ≥ 0.05) was found at week 12. Eggshell strength and yolk color were not improved (P ≥ 0.05) by dietary treatments at weeks 4 and 8 but improved (≤ 0.05) by week 12. Eggshell strength did not vary (P ≥ 0.05) among the probiotic-based groups, while only the CB group differed from the control for yolk color. Haugh unit and albumen height were not influenced (P ≥ 0.05) by dietary probiotics at the end of week 4, but significant improvements (P ≤ 0.05) due to treatment effect were notable at the end of weeks 8 and 12. The Haugh unit and the albumen height of the probiotic-based groups were consistently higher (P ≤ 0.05) compared to the control. Among treatments, no significant variation (P ≥ 0.05) in the albumen height was found at the end of week 8, but the CB group was greater (P ≤ 0.05) than the Brevibacillus group at the end of week 12. Similarly, at the end of weeks 8 and 12, no statistical differences (P ≥ 0.05) were found for the Haugh unit among the treatment groups. Thick-to-thin albumen ratio was consistently significant (P ≤ 0.05) throughout the entire experiment. At the end of weeks 4 and 8, only the CB-z group and the Brevibacillus group were, respectively, significant (P ≤ 0.05) from the control, while all the treatment groups were significant from control at week 12, and no observable variations (P ≥ 0.05) among treatments were found.
Hematological and serum biochemical profiles
The hematological indices of laying hens fed dietary C. butyricum and Brevibacillus sp. are presented in Table 4. The blood indices such as WBC, MCV, MCH, PLT, heterophils, neutrophils, and H/L were influenced (P ≤ 0.05) by treatments, while other indices such as eosinophils, RBC, Hb, monocytes, PCV, and MCHC were not affected (P ≥ 0.05) by diets. Also, WBC count, heterophils, and lymphocytes were not significantly different (P ≥ 0.05) among the treatment groups. Only birds in the CB-z group recorded a lower H/L ratio among the supplemented groups with a level of significance (P ≤ 0.05) compared to the control group.
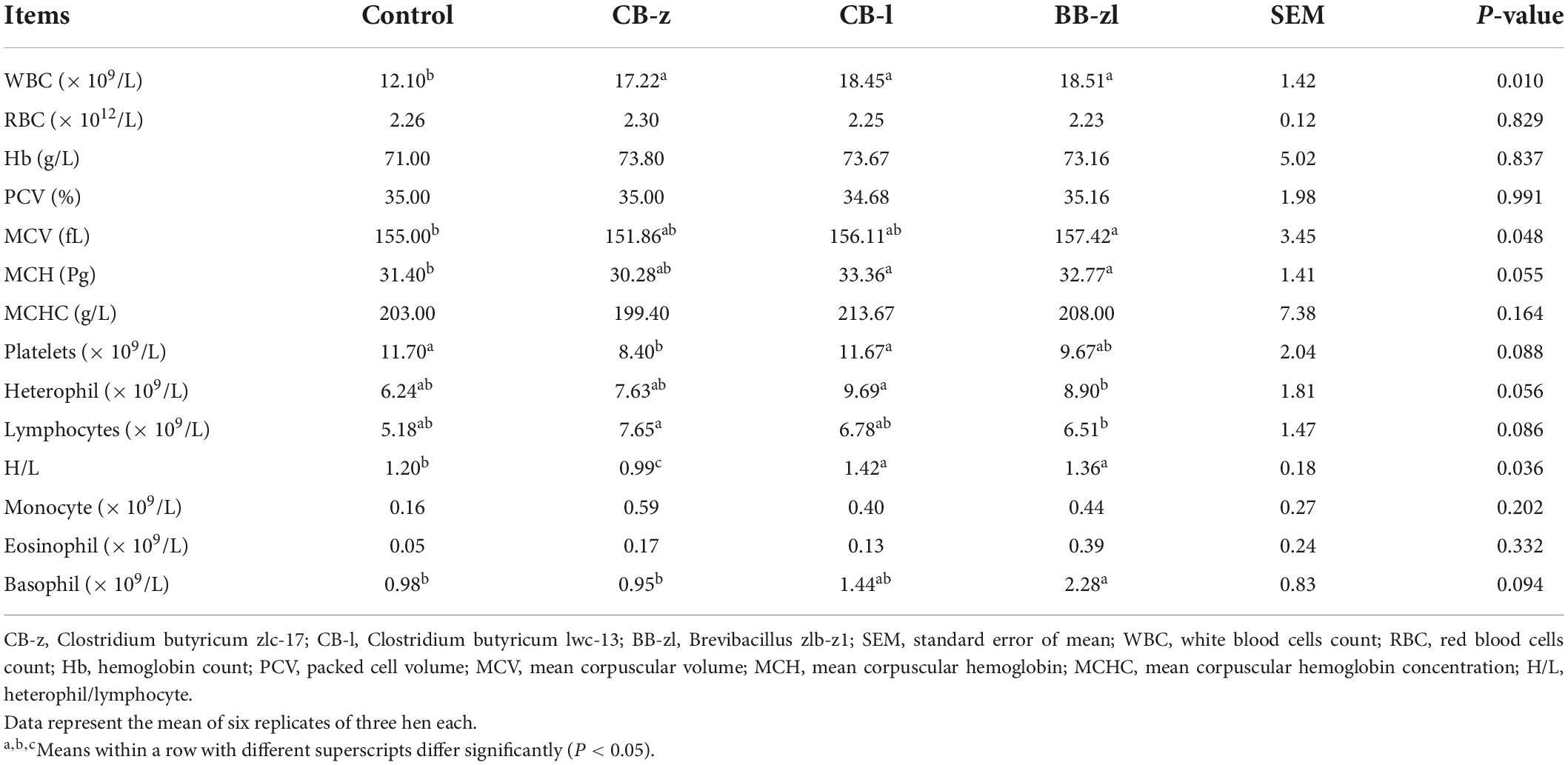
Table 4. Effects of Clostridium butyricum and Brevibacillus sp. on the hematological indices of laying hens.
The influence of dietary C. butyricum and Brevibacillus on the serum index, immunity and oxidant and antioxidant parameters, of laying hens is presented in Table 5. Serum MDA was significantly higher (P ≤ 0.05) in the control than in the treatment group, whereas the Brevibacillus group recorded the lowest concentration of the MDA content in the serum. The concentrations of the antioxidant enzymes T-SOD, GST, GSH-Px, and CAT were increased (P ≤ 0.05) due to dietary treatments, whereas T-AOC was not influenced (P ≥ 0.05) by the diets. The concentrations of serum IgM and IgA were influenced (P ≤ 0.05) by diets, but no variations (P ≥ 0.05) in IgG due to the dietary treatment were observed. The C. butyricum group had the highest immunoglobulin concentrations compared to the Brevibacillus group. Dietary treatments influenced (P ≤ 0.05) complement protein C3 (P ≤ 0.05) but not C4.
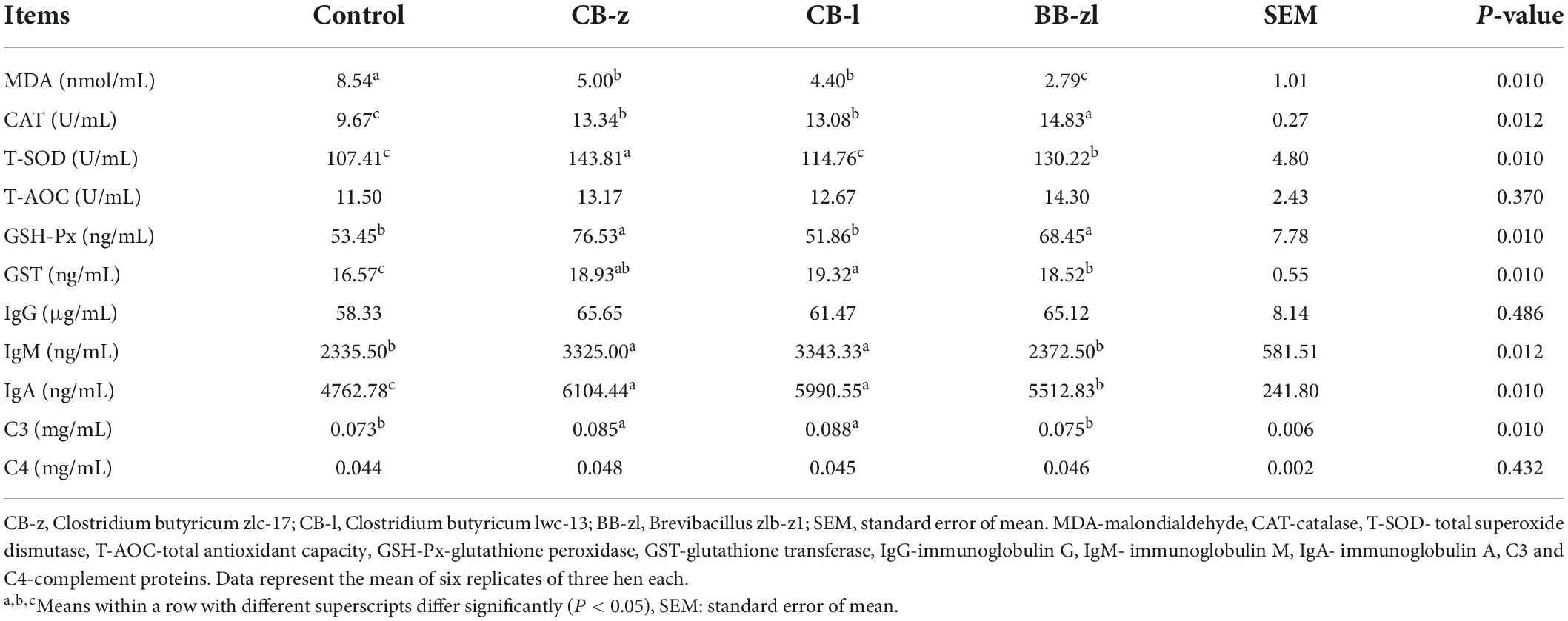
Table 5. Effects of Clostridium butyricum and Brevibacillus sp. on the serum antioxidant and immune capacity of laying hens.
Apparent fecal amino acid digestibility
Table 6 presents data on the apparent fecal amino acid digestibility of laying hens fed probiotics-based diets. There was a significant improvement (P ≤ 0.05) in the digestibility of essential amino acids (isoleucine, valine, leucine, methionine, histidine lysine, and phenylalanine) and non-essential amino acids (glycine, serine, methionine-cysteine, and tyrosine) and crude protein, due to influence of diets. Other amino acids including asparagine, threonine, glutamic acid, proline, alanine, cysteine, lysine, arginine, and tryptophan were not influenced (P ≥ 0.05) by treatments. Among the diet group, the CB-z group had the highest value for digestibility of all amino acids. No variations (P ≥ 0.05) exist between the Clostridium groups for all amino acid digestibility coefficients, while the Brevibacillus group differed in methionine-cysteine and serine from the C. butyricum group.
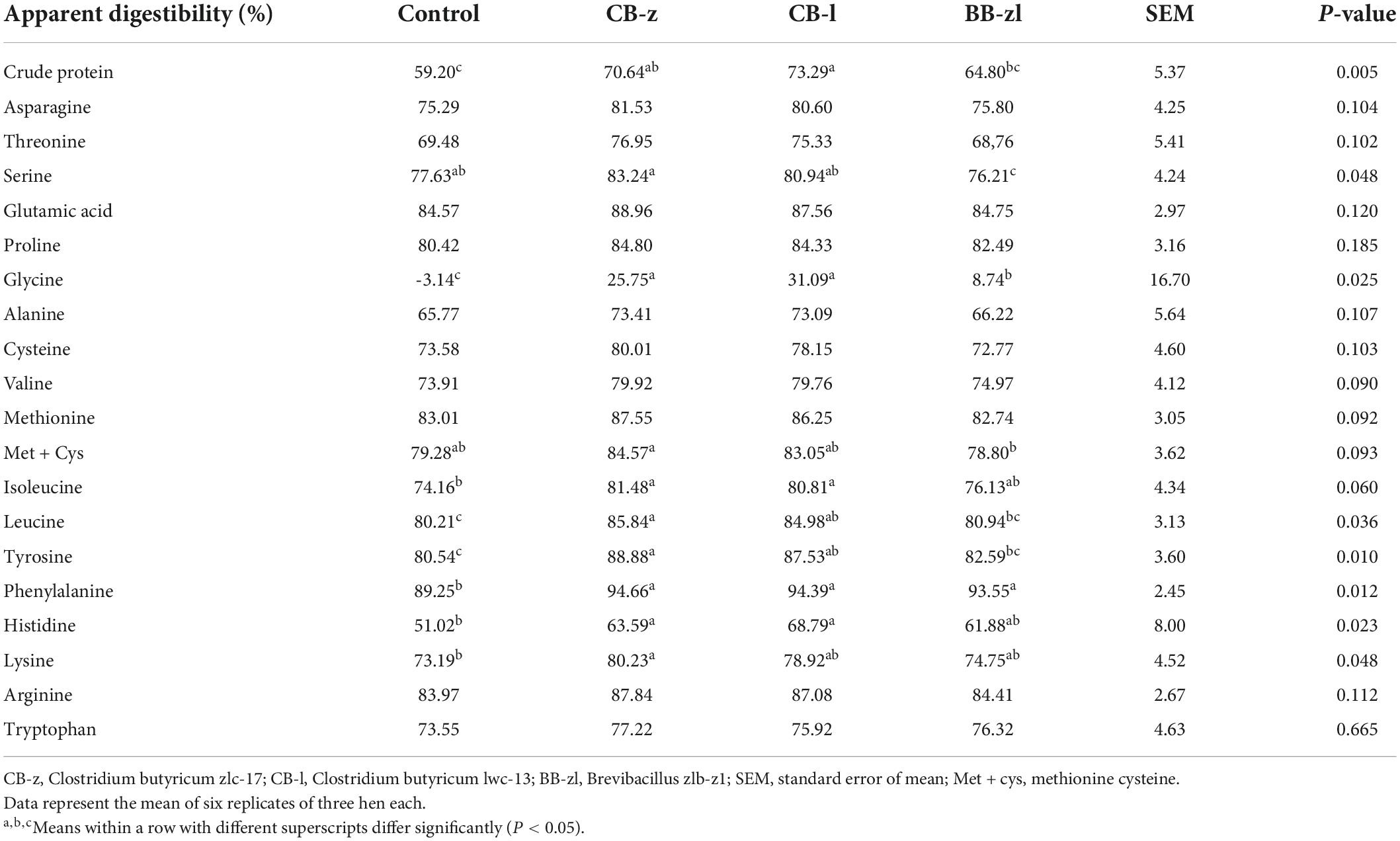
Table 6. Effects of Clostridium butyricum and Brevibacillus sp. on the apparent fecal amino acid digestibility coefficient of laying hens.
Relative organ weight and jejunal villi morphological structure
The organ weights of the magnum, the spleen, the heart, and the liver (expressed as relative weight) of laying hens were fed dietary C. butyricum and Brevibacillus, which are listed in Table 7. The relative weights of the magnum, the heart, and the liver were not statistically different (P ≥ 0.05) from the control, although the weight of the spleen was increased (P ≤ 0.05) due to dietary influence.
The morphological characteristics of the jejunal villi of laying hens fed C. butyricum and Brevibacillus are presented in Table 8. The villous indices (height, width, surface area), crypt depth, and villi height to crypt depth ratio were all influenced (P ≤ 0.05) by a probiotic-based diet. Villous height, width, surface area, and villi height to crypt depth were significantly increased (P ≤ 0.05), whereas crypt depth was reduced in the treatment group compared to the control. All villi morphometrics differed (P ≤ 0.05) among the treatments. The CB-z group recorded the highest value for villous surface area, height, and width, while the Brevibacillus group had the least value for crypt depth and the highest value for villi height to crypt depth ratio.
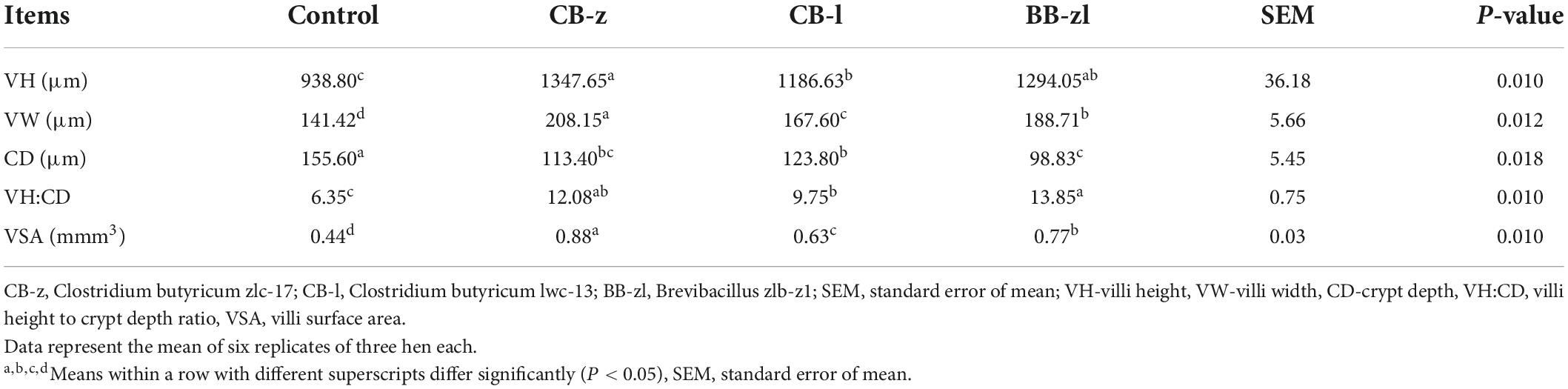
Table 8. Effects of Clostridium butyricum and Brevibacillus sp. on the jejunal villi morphometry of laying hens.
Discussion
Eggs are very important to consumers due to their high nutrient and biological quality; therefore, feeding laying hens with diets supplemented with natural feed additives, such as probiotics, can provide results that are vital for the commercial laying hens industry. The spores of Clostridium butyricum and Brevibacillus were used in the present study due to their stability in the gut and thus are regarded as safe use for poultry nutrition. Dietary supplementation with Clostridium butyricum and Brevibacillus sp. had no negative impact on the laying hens throughout the feeding period. This lends evidence that the newly designed strains of C. butyricum and Brevibacillus are suitable for peak-laying hens. They could also be used as a safe feed additive in the poultry industry as reported previously with other probiotic strains (Wei et al., 2020; Yang et al., 2020; Ahmat et al., 2021; César et al., 2022). There is a dearth of evidence on the influence of Brevibacillus sp. in the diet of laying hens, and Bacillus sp. would be used for comparison.
Laying performance
Improvement in egg production rate and egg weight is of critical economic value to the poultry industry. Probiotics, including Clostridium butyricum (Xiang et al., 2019; Zhan et al., 2019; Wang W. W. et al., 2020; Wang Y. et al., 2021) and Bacillus sp., B. velezensis (Wei et al., 2020), B. subtilis (Guo et al., 2017; Darsi and Zhaghari, 2021; Souza et al., 2021), and B. licheniformis (Pan et al., 2022), were reported to enhance egg production in laying hens. Also, combined Bacillus strains caused an 8% improvement in laying performance compared to the control (Yang et al., 2020). The increased egg production rate could be due to an improvement in nutrient utilization (Souza et al., 2021), which is accrued to a positive effect of probiotics on the beneficial gut microbial population (Xu et al., 2022) and gut morphology (Song et al., 2019). In the current study, our findings revealed that Clostridium butyricum and Brevibacillus significantly improved egg production rates. The improvement could be adducible to better nutrient utilization orchestrated by a decrease in stress response, enhanced gut health, and improved immune function, as observed in this study. This claim is supported by previous studies in laying hens, which demonstrated that probiotic-induced improvement in physiological indices, including immunity index, intestinal morphology, and antioxidant capacity culminated in significantly increased laying performance (Deng et al., 2021; Wang Y. et al., 2021; Pan et al., 2022). However, some studies found no probiotic-induced effect on egg production (Shi et al., 2020; Wang J. et al., 2021; Ray et al., 2022). The discrepancies may be due to the age of laying hens and probiotics composition and dosage. Enhanced laying performance often reflects egg mass output and egg weight. Our findings revealed a significant improvement in egg weight and egg mass in response to dietary probiotics, and this is in line with previous reports that probiotics exerted a beneficial effect on egg weight (Zhan et al., 2019; Wang Y. et al., 2021; Ray et al., 2022). The improvement suggests that probiotics are natural growth enhancers through efficient nutrient utilization. Nevertheless, some studies reported a non-significant influence of probiotics on egg weight (Xiang et al., 2019; Darsi and Zhaghari, 2021; Souza et al., 2021). Feed consumption (FI) and FCR are often used as indicators for feed utilization efficiency. In previous reports, probiotics including Clostridium butyricum (Xiang et al., 2019; Wang W. W. et al., 2020; Wang Y. et al., 2021), Bacillus sp. (Neijat et al., 2019; Yang et al., 2020; Pan et al., 2022), and combined probiotics strains (Ray et al., 2022; Xu et al., 2022) enhanced feed efficiency in laying hens. Similarly, in this study, Clostridium butyricum improved feed utilization, and consequently, the enhanced feed efficiency was a boost to the production potential of the animal. The improvement in feed efficiency may be the capacity of probiotics to enhance gut morphology and health via increased villi height for better nutrient absorption (Wang W. W. et al., 2020; Pan et al., 2022) and the production of bacteriocins and volatile bacteriostatic substances, which can suppress pathogen invasion (Chen et al., 2007). Conversely, there was no influence of probiotics on FCR (Upadhaya et al., 2019) and feed intake (Neijat et al., 2019). This may be due to the inclusion level of probiotics in the diets. Furthermore, in this study, the probiotic-based group recorded zero mortality rate relative to the control. In line with the current findings, Xiang et al. (2019) reported zero mortality rate in laying hens fed Clostridium butyricum. The zero-mortality observed in the diet group could be that continuous feeding of probiotics enhanced the health status of the birds as evidenced by gut integrity and better antioxidant and immune function. Conclusively, the improved egg weight, egg production rate, feed utilization efficiency, and egg mass are suggestive of the positive response of laying hens to dietary probiotics supplementation.
Egg quality
Maintenance of external (shell quality) and internal (albumen and yolk) components of the egg is of utmost priority in the laying hen’s industry, to gain consumers’ acceptance while meeting up with market demands. In the study, our findings showed improvement in eggshell thickness and eggshell strength in response to dietary probiotics. This corroborates the previous findings that supplementation of probiotics: B. subtilis PB6 in broiler breeder hens (Darsi and Zhaghari, 2021; Souza et al., 2021), B. subtilis (Guo et al., 2017; Fathi et al., 2018), and BLCC1-0238 (Upadhaya et al., 2019) in laying hens improved eggshell thickness. Also, Clostridium butyricum exerted a beneficial effect on eggshell thickness and eggshell strength (Zhan et al., 2019; Wang Y. et al., 2021). Probiotics have been found to enhance the growth of beneficial bacteria, and the proliferation of these microbes enhances the fermentation rate, which culminates in the accumulation of short-chain fatty acids (SCFAs) and a reduction in luminal pH (Forte et al., 2016). Also, the SCFAs enhance the growth and nourishment of intestinal villi structures for a better absorption rate (Zou et al., 2019). Thus, improvement in eggshell quality could be linked to the capacity of probiotics to increase the assimilation and retention levels of calcium in the serum of laying birds (Zhan et al., 2019; Attia et al., 2020), which would facilitate calcium deposition on the shell glands. Conversely, Wang W. W. et al. (2020) demonstrated that Clostridium butyricum had no influence on eggshell thickness and strength, while B. subtilis had no effect on eggshell thickness but enhanced eggshell strength (Upadhaya et al., 2019). These variations may be due to age and the laying phase of the hens. In addition, albumen quality is of great importance for the food processing and health industry, which utilizes the albumen as raw materials for further production of foods and drugs. Pieces of evidence demonstrated that Bacillus subtilis (Chen et al., 2019; Neijat et al., 2019; Yang et al., 2020) and B. velezensis (Wei et al., 2020) improved HU and albumen height in broiler breeders and laying hens. In another study, Bacillus strains improved HU and the protein index in laying hens (Mazanko et al., 2018). Also, Clostridium butyricum was also found to enhance albumen height (Wang W. W. et al., 2020; Wang Y. et al., 2021) and albumen crude protein (Xiang et al., 2019). The improvement in albumen quality due to dietary probiotics effect may be accrued to enhanced nutrient digestibility, which would improve protein synthesis. It has been reported that probiotics stimulate the activities of digestive enzymes that cause a resultant increase in nutrient utilization and protein digestibility (Ahiwe et al., 2020). In the present study, we observed a similar distinct increase in albumen indices (albumen height, Haugh unit, and thick-to-thin albumen ratio) in response to dietary probiotics. The current study, to the best of our knowledge, would be the first to report the influence of probiotics on the thick-to-thin albumen ratio. The improvement in albumen quality may reflect an increase in protein synthesis (Lei et al., 2013). The enhanced HU values could be accrued to better bioavailability of nutrients and better gross digestible energy due to probiotics. Also, the capacity of Clostridium butyricum and Brevibacillus to modulate the microflora composition in the body of an organism may have led to a beneficial effect on oviduct flora with consequent improvement in albumen synthesis. This claim is supported by the study of Camarda et al. (2000), which demonstrated that some pathogens may colonize the oviduct and impair its functions. The improvement in albumen quality indices suggests the production of high-quality eggs with better albumen viscoelasticity and shelf life (Zhang et al., 2020). On the contrary, Clostridium butyricum had no effect on albumen height, HU value (Zhan et al., 2019), and HU value (Wang W. W. et al., 2020). The discrepancies may be related to the type of probiotics used. Probiotics including Clostridium butyricum (Wang W. W. et al., 2020; Wang Y. et al., 2021) and Bacillus sp. (Liu et al., 2019; Zhou et al., 2020) improved yolk color in laying hens. The improvement in the yolk color may be accrued to the probiotic composition. In contrast, we observed no effect of diets on yolk color, similar to the results of Lei et al. (2013), which revealed that the dietary influence of B. licheniformis on egg yolk color was not significant. The non-significant effect on yolk color could be a reflection that dietary probiotics do not play a key role in the metabolism of xanthophyll, which is a non-nutritive component. According to the aforementioned research findings, Clostridium butyricum and Brevibacillus could be employed as secure feed additives in laying hen diets to enhance albumen and eggshell quality and two factors that are economically important and beneficial to the poultry sector.
Hematological indices, antioxidant capacity, and immune function
Farm animals are susceptible to oxidative stress, which often cause decreased immune function. Most often, blood parameters are used to investigate the level of stress in animals. In the present study, blood indices (WB, PLT, neutrophil, lymphocytes, basophils, H/L) were influenced by dietary probiotics. There are reports that Bacillus subtilis had no influence on blood WBC, RBC, or lymphocytes in laying hens (Shi et al., 2020) and broiler chickens (Park et al., 2018). In this study, the decreased H/L ratio is similar to the findings of César et al. (2022), and the decreased H/L ratio could be due to the immunomodulatory effect of the dietary bioactive components that can also suppress pathogenic conditions in poultry (Kogut and Klasing, 2009). The decrease in the H/L ratio due to dietary probiotics suggests that Clostridium butyricum and Brevibacillus could stabilize the health status of the laying hens. Therefore, the birds’ welfare can be improved under farm conditions, since probiotic supplementation in the diet of laying hens can be considered a dietary strategy for stress reduction in laying hens.
When there is a disequilibrium between the antioxidant system and reactive oxygen species (ROS) as output, oxidative stress becomes the norm, and the homeostatic balance of the animals is disrupted (Surai et al., 2019). Oxidative stress may impair reproductive performance because it induces the synthesis of ROS, which could disintegrate proteins and nucleic acid, and culminates in tissue damage (Pisoschi et al., 2021). There are pieces of evidence that probiotics could mask the negative effects of oxidative stress, enhance the activities of antioxidative enzymes and dietary CB; improved the activities of GSH-Px, CAT, and T-SOD with a concomitant decrease in the MDA content of the serum (Zhan et al., 2019), reduced the MDA content in the intestine (Xiang et al., 2019), improved serum antioxidant status but decreased T-AOC (Wang Y. et al., 2021) in laying hens. Also, Zhou et al. (2020) revealed that laying hens fed diets supplemented with B. amyloliquefaciens BLCC1-0238 had enhanced GSH-Px and GST activities but no effect was notable on the serum concentrations of the antioxidant system (CAT, T-AOC, T-SOD) and the MDA content. The increased antioxidant capacity could probably be due to the capacity of probiotics to increase the activities of antioxidant enzymes while the reduced T-AOC could be due to less occurrence of ROS in the body system. Our present findings revealed that C. butyricum and Brevibacillus enhanced the activities of GSH-Px, GST, CAT, and T-SOD and had no effect on T-AOC while reducing the MDA content. This lends evidence once again that probiotics including C. butyricum and Brevibacillus could modulate the antioxidant capacity of laying hens. An indication that the probiotics as feed additives could act as a boost to the antioxidant capacity of the host while lipid peroxidation activity is reduced. The enhanced antioxidant system may be due to the capacity of CB to synthesize butyrate and H2, which scavenge ROS and improve the activity of the antioxidative enzymes (Jahns et al., 2015). The lack of probiotic-diet effect on T-AOC could be that the body may be insensitive to such probiotic-mediated responses. All together, these findings indicate that C. butyricum and Brevibacillus could mitigate oxidative stress in laying hens through the stimulation of enzymatic components, which in turn supports better egg production rate and egg quality. An indication that these probiotics with antioxidant effects could be utilized in the laying industry as probiotic antioxidants.
The immunity index of animals is often measured based on the serum concentrations of immunoglobulins (IgM, IgA, and IgG) due to their key role in immune regulation and disease resistance (Mountzouris et al., 2010). Previous reports demonstrated that B. amyloliquefaciens significantly increased serum concentrations of IgG and IgA (Ahmat et al., 2021) and the probiotic complex (Deng et al., 2021), and B. subtilis (Qiu et al., 2021) enhanced the serum immunoglobulins of IgG, IgA, and IgM in broiler birds. There are pieces of evidence that B. amyloliquefaciens increased IgG and IgA levels (Zhou et al., 2020) and CB enhanced IgA, IgY, and IgM (Zhan et al., 2019) in laying hens. In a similar vein, our findings demonstrated that concentrations of the immunoglobulins (IgA and IgM) in the serum were significantly increased, while no effect was notable for IgG. Probiotics have been found to reduce the colonization of intestinal pathogens and stimulate the synthesis of natural antibodies (Haghighi et al., 2006). Thus, the improved immunoglobulin synthesis may be associated with the capacity of probiotics to exert immunomodulatory effects. Increased bioavailability of amino acids is critical to the synthesis of immunoglobulins (Azzam et al., 2015); therefore, the significant improvement may be adducible to improved digestibility of amino acids. Also, the small intestine acts as an immune protection barrier in animals (Patterson and Burkholder, 2003), and the improved gut integrity may favor the activities of intestinal mucosal cytokines, thereby enhancing the immune status of the birds. The immunity status of animals is often linked with the animal’s capacity to counteract oxidative stress via enhanced antioxidant capacity (Wan et al., 2018), and the enhanced activities of the antioxidant enzymes may be a contributory factor. In one study, B. subtilis C-3102 enhanced IgM concentration linearly but had no effect on serum concentrations of IgA and IgG (Liu et al., 2019). The discrepancies in the studies could be the environmental hygiene and the type of probiotics used. Complement proteins play key roles in immune function; thus, they are often used to assess the immune status of animals. Dietary supplementation of C. butyricum enhanced C3 concentration in broiler chickens (Zhang et al., 2016) and enhanced C3 and C4 concentration in laying hens (Zhan et al., 2019). Further analysis of the immunity index could be measured with the weight of the spleen, probably because the spleen is involved in cellular and humoral immunity of the body. Our findings showed that the relative weight of the spleen was improved with dietary Clostridium butyricum and similar to reports in laying hens (Awad et al., 2010; Zhan et al., 2019) and that of broilers (Chen et al., 2013; Ahmat et al., 2021). The aforementioned findings suggest that probiotics as nutritional components in the diets of laying hens can stimulate the local immune system in the gut, although the systemic effects are notable in the blood. The immunomodulatory effect of Clostridium butyricum and Brevibacillus on laying hens was evidenced in the enhanced level of immunoglobulins and the weight of the spleen. We could deduce that the improved immune status and the activities of antioxidant enzymes contributed immensely to the health status, which supported laying performance and production of eggs with better shell and albumen quality.
Apparent fecal amino acid digestibility
Data on the digestibility of amino acids using Clostridium butyricum and Brevibacillus sp. supplemented in laying hens’ feed are scarce. This study demonstrated that dietary probiotics can effectively improve the fecal digestibility of amino acids and crude protein. This study would be the first study to report the influence of probiotics on the apparent fecal digestibility of crude protein and amino acids. The digestibility of crude protein and most essential amino acids (Val, Met, Met-cys, Ile, Leu, Tyr, Phe, His) and non-essential amino acid (Glycine) was significantly high compared to the control. The enhanced digestibility may be due to the capacity of probiotics to positively influence the host by improving the intestinal structures and suppressing pathogens’ proliferation (Emami et al., 2020). It could also be that the probiotics used provided a favorable environment for the degradation of nutrients from feed. There are pieces of evidence that Clostridium butyricum could enhance secretion and activities of digestive enzymes (Zhang et al., 2016) and modulate gut microflora (Duan et al., 2018), which in turn could promote nutrient absorption. Also, in laying hens, probiotics enhanced nutrient retention, which acts as a catalyst for improved performance (Neijat et al., 2019). A study reported that in broiler diets supplemented with probiotics, increased ileal digestibility of nutrients leads to a corresponding increased performance (Mountzouris et al., 2010). The enhanced digestibility of crude protein may account for the improvement in albumen synthesis; it has been reported that diets with high crude protein values enhance albumen quality (Shim et al., 2013). Therefore, we deduced that the increased digestibility of amino acids was the basis of enhanced immunoglobulin secretions, laying performance, and egg quality.
Jejunal villi morphology
Digestion and absorption as key events in the digestive tract occur mainly in the jejunum, which is the part of the small intestine, and significant variations in this region could suggest changes in digestion and absorption capacity across the diets. The villi height, crypt depth, villi width, and surface area all reflect the gut integrity and strength of nutrient absorption capacity. The improved nutrient absorption capacity of the gut is evidenced by decreased crypt depth and increased villi height, villi width, and villi height to crypt depth ratio (Shamoto and Yamauchi, 2000). Whereas, shorter villi and deeper crypts cause less utilization of nutrients (Xu et al., 2020) because the energy needed for the metabolic process is diverted to gut cell renewal in response to normal sloughing or inflammatory response (Giannenas et al., 2014). There are shreds of evidence that C. butyricum increased jejunal villi height (Zhang et al., 2011) and ileal villi height (Wang W. W. et al., 2020) and villi height to crypt depth ratio but decreased crypt depth (Xiang et al., 2019) in laying hens. Also, C. butyricum increased villi height and villi height to crypt depth ratio but decreased crypt depth in broilers (Zhang et al., 2016; Abdel-Latif et al., 2018). In addition, Bacillus sp. increased jejunal villi height and villi height to crypt depth ratio and decreased crypt depth (Yang et al., 2020; Pan et al., 2022) in laying hens. The improvement in gut morphology may be accrued to the capacity of microorganisms present in the gut to extend the length of the intestine (Chen et al., 2016). Our findings are in tandem with the previous findings on enhanced villi height and villi height to crypt depth ratio, broader surface area, and decreased crypt depth due to dietary probiotics. The improved jejunal villi structures could be that Clostridium butyricum enhanced the digestion of carbohydrates and the synthesis of SCFAs (Shah et al., 2019), which supply nutrients to the intestinal goblet cells and protect intestinal epithelial cells (Liu et al., 2019; Guo et al., 2021). Conversely, C. butyricum had no distinct influence on the jejunum microscopic structures (Wang W. W. et al., 2020). This could be due to probiotic composition, age of laying hens, and duration of feeding. This suggests that the improvement in amino acid absorption probably was facilitated by improved intestinal structures. We could therefore deduce that improved villi structures, which provided larger absorption surface area, were culminated in enhanced utilization of nutrients in the feed and increased amino acid digestibility, which translated into increased laying performance and egg quality.
Conclusion
Clostridium butyricum and Brevibacillus spores improved protein synthesis and nutrient utilization while regulating gut function and health status in laying hens. The aforementioned findings revealed that the supplementation of Clostridium and Brevibacillus spores as probiotics in the diet of laying hens might be a promising safe feed additive and an enhancer for the intestinal health of laying hens. In comparison to other treatments, Clostridium butyricum (zlc-17) was more efficient in enhancing egg production rate, feed efficiency, albumen quality, immunoglobulin and antioxidant enzymes, amino acid digestibility, and jejunal villi microscopic structures. Hence, 0.02% of Clostridium butyricum (zlc-17) is suitable to be supplemented in the diet of laying hens at the peak phase.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was reviewed and approved by the Animal ethics and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences, Beijing, China. CAAS. No: 20200507S0600103.
Author contributions
KQ, T-HS, and S-GW: conceptualization. KQ, X-YC, Y-BS, and UO: resources data. UO: writing – original draft. KQ, JW, H-JZ, T-HS, and S-GW: supervision. KQ and UO: writing and editing. S-GW and G-HQ: funding. All authors reviewed and accepted this final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32072774), the National Key Research and Development Program of China (2021YFD1300204), and the Agricultural Science and Technology Innovation Program (ASTIP) of the Chinese Academy of Agricultural Sciences.
Conflict of interest
T-HS was employed by the COFCO Nutrition and Health Research Institute. Y-BS was employed by the company COFCO (Beijing) Feed Technology Company Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADFI, average feed intake; AEG, average egg weight; AH, albumen height; BB, Brevibacillus; CB, Clostridium butyricum; CAT, catalase; CD, crypt depth; FCR, feed conversion ratio; GST, glutathione transferase; GSH-Px, glutathione peroxidase; HDP, hen day production; HU, Haugh unit; H/L, heterophil to lymphocyte ratio; IgM, immunoglobulin M; IgG, immunoglobulin G; IgA, immunoglobulin A; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MDA, malondialdehyde; ROS, reactive oxygen species; RBC, red blood cells count; SCFA, short-chain fatty acid; TAO-C, Total antioxidant capacity; TSOD, Total peroxide dismutase; VH, Villi height; VW, villus width; VSA, villi surface area; V/C, villi height to crypt depth ratio; WBC, white blood cells count.
References
Abdel-Latif, M. A., Abd El-Hack, M. E., Swelum, A. A., Saadeldin, I. M., Elbestawy, A. R., Shewita, R. S., et al. (2018). Single and combined effects of Clostridium butyricum and Saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals 8:184. doi: 10.3390/ani8100184
Ahasan, A., Agazzi, A., Invernizzi, G., Bontempo, V., and Savoini, G. (2015). The beneficial role of probiotics in monogastric animal nutrition and health. J. Dairy Vet. Anim. Res. 2, 116–132. doi: 10.15406/jdvar.2015.02.00041
Ahiwe, E. U., Abdallh, M. E., Chang’a, E. P., Omede, A. A., Al-Qahtani, M., Gausi, H., et al. (2020). Influence of dietary supplementation of autolyzed whole yeast and yeast cell wall products on broiler chickens. Asian Australas. J. Anim. Sci. 33:579.
Ahmat, M., Cheng, J., Abbas, Z., Cheng, Q., Fan, Z., Ahmad, B., et al. (2021). Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens. Antibiotics 10:1427. doi: 10.3390/antibiotics10111427
Al-Khalaifah, H. (2018). Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 97, 3807–3815.
Arpášová, H. M., Kačániová, V., Pistová, B., Gálik, M., Fik, M., and Hleba, L. (2016). Effect of Probiotics and Humic Acid on Egg Production and Quality Parameters of Laying Hens Eggs. Sci. Papers 49, 1–9.
Attia, Y. A., Al-Harthi, M. A., and Abo El-Maaty, H. M. (2020). Calcium and cholecalciferol levels in late-phase laying hens: Effects on productive traits, egg quality, blood biochemistry, and immune responses. Front. Vet. Sci. 7:389. doi: 10.3389/fvets.2020.00389
Awad, W., Ghareeb, K., and Böhm, J. (2010). Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J. Anim. Physiol. Anim. Nutr. 94, 486–494. doi: 10.1111/j.1439-0396.2009.00933.x
Azzam, M. M., Dong, X. Y., Dai, L., and Zou, X. T. (2015). Effect of excess dietary L-valine on laying hen performance, egg quality, serum free amino acids, immune function and antioxidant enzyme activity. Br. Poult. Sci. 56, 72–78. doi: 10.1080/00071668.2014.989487
Camarda, A., Newell, D., Nasti, R., and Di Modugno, G. (2000). Genotyping Campylobacter jejuni strains isolated from the gut and oviduct of laying hens. Avian Dis. 44, 907–912.
Cé,sar, O., Maribel, J. F., César, A. S., and Robles, E. F. F. (2022). Effect of probiotic Bifidobacterium animalis as an alternative to growth-promoting antibiotics on performance, egg quality, and health parameters in young laying hens. Res. Square. doi: 10.21203/rs.3.rs-1204725/v1
Chen, J., Kuang, Y., Qu, K., Guo, S., Kang, K., and He, C. (2019). The effects and combinational effects of Bacillus subtilis and montmorillonite supplementation on performance, egg quality, oxidation status, and immune response in laying hens. Liv. Sci. 227, 114–119.
Chen, W., Wang, J., Yan, L., and Huang, Y. (2013). Evaluation of probiotics in diets with different nutrient densities on growth performance, blood characteristics, relative organ weight and breast meat characteristics in broilers. Br. Poult. Sci. 54, 635–641. doi: 10.1080/00071668.2013.825369
Chen, X. H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., et al. (2007). Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotech. 25, 1007–1014. doi: 10.1038/nbt1325
Chen, Y., Cheng, Y., Li, X., Zhang, H., Yang, W., Wen, C., et al. (2016). Dietary palygorskite supplementation improves immunity, oxidative status, intestinal integrity, and barrier function of broilers at early age. Anim. Feed Sci. Tech. 219, 200–209.
Darsi, E., and Zhaghari, M. (2021). Effects of Bacillus subtilis PB6 supplementation on productive performance, egg quality and hatchability in broiler breeder hens under commercial farm condition. J. Appl. Anim. Res. 49, 109–117.
Deng, Y., Xiong, X., Liu, X., He, C., Guo, S., Tang, S., et al. (2021). Palygorskite combined probiotics improve the laying performance, hatching performance, egg quality, plasma antioxidative status, and immune response of broiler breeders. Ital. J. Anim. Sci. 20, 1292–1301.
Duan, Y., Wang, Y., Dong, H., Ding, X., Liu, Q., Li, H., et al. (2018). Changes in the intestine microbial, digestive, and immune-related genes of Litopenaeus vannamei in response to dietary probiotic Clostridium butyricum supplementation. Front. Micro. 9:2191. doi: 10.3389/fmicb.2018.02191
Emami, N. K., Calik, A., White, M. B., Kimminau, E. A., and Dalloul, R. A. (2020). Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 7:572142. doi: 10.3389/fvets.2020.572142
Fathi, M., Al-Homidan, I., Al-Dokhail, A., Ebeid, T., Abou-Emera, O., and Alsagan, A. (2018). Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature. Ital. J. Anim. Sci. 17, 804–814.
Food and Agriculture Organization and World Health Organization Expert Consultation (2001). Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Córdoba: Food and Agriculture Organization of the United Nations and World Health Organization.
Forte, C., Moscati, L., Acuti, G., Mugnai, C., Franciosini, M., Costarelli, S., et al. (2016). Effects of dietary Lactobacillus acidophilus and Bacillus subtilis on laying performance, egg quality, blood biochemistry and immune response of organic laying hens. J. Anim. Phys. Anim. Nutr. 100, 977–987.
Ge, C., Liu, B., Lan, J., Huang, S., and Zhu, Y. (2009). The anti-bacterial activity research of biocontrol bacteria JK-2 on Fusarium oxysporum. Fujian J. Agric. Sci. 24, 29–34.
Giannenas, I., Tsalie, E., Triantafillou, E., Hessenberger, S., Teichmann, K., Mohnl, M., et al. (2014). Assessment of probiotics supplementation via feed or water on the growth performance, intestinal morphology and microflora of chickens after experimental infection with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Avian Pathol. 43, 209–216.
Gungor, E., and Erener, G. (2020). Effect of dietary raw and fermented sour cherry kernel (Prunus cerasus L.) on digestibility, intestinal morphology and caecal microflora in broiler chickens. Poult. Sci. 99, 471–478.
Guo, J. R., Dong, X. F., Liu, S., and Tong, J. M. (2017). Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult. Sci. 96, 1280–1289. doi: 10.3382/ps/pew389
Guo, L., Lv, J., Liu, Y., Ma, H., Chen, B., Hao, K., et al. (2021). Effects of Different Fermented Feeds on Production Performance, Cecal Microorganisms, and Intestinal Immunity of Laying Hens. Animals 11:2799. doi: 10.3390/ani11102799
Haghighi, H. R., Gong, J., Gyles, C. L., Hayes, M. A., Zhou, H., Sanei, B., et al. (2006). Probiotics stimulate production of natural antibodies in chickens. Clin. Vacc. Immunol. 13, 975–980.
Jahns, F., Wilhelm, A., Jablonowski, N., Mothes, H., Greulich, K. O., and Glei, M. (2015). Butyrate modulates antioxidant enzyme expression in malignant and non-malignant human colon tissues. Mol. Carcino. 54, 249–260. doi: 10.1002/mc.22102
Jeong, J., and Kim, I. (2014). Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 93, 3097–3103. doi: 10.3382/ps.2014-04086
Kogut, M. H., and Klasing, K. (2009). An immunologist’s perspective on nutrition, immunity, and infectious diseases: Introduction and overview. J. Appl. Poult. Res. 18, 103–110.
Kong, Q., He, G. Q., Jia, J. L., Zhu, Q. L., and Ruan, H. (2011). Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr. Microb. 2, 512–517. doi: 10.1007/s00284-010-9737-8
Lei, K., Li, Y., Yu, D., Rajput, I., and Li, W. (2013). Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 92, 2389–2395. doi: 10.3382/ps.2012-02686
Liu, X., Peng, C., Qu, X., Guo, S., Chen, J. F., He, C., et al. (2019). Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. J. Anim. Physiol. Anim. Nutr. 103, 182–190. doi: 10.1111/jpn.13022
Macit, M., Karaoglu, M., Celebi, S., Esenbuga, N., Yoruk, M. A., and Kaya, A. (2021). Effects of supplementation of dietary humate, probiotic, and their combination on performance, egg quality, and yolk fatty acid composition of laying hens. Trop. Anim. Health Prod. 53:63. doi: 10.1007/s11250-020-02546-6
Markowiak, P., and Śliżewska, K. (2018). The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut. Pathogens. 10:21.
Mazanko, M. S., Gorlov, I. F., Prazdnova, E. V., Makarenko, M. S., Usatov, A. V., Bren, A. B., et al. (2018). Bacillus probiotic supplementations improve laying performance, egg quality, hatching of laying hens, and sperm quality of roosters. Probi. Antim. Prot. 10, 367–373. doi: 10.1007/s12602-017-9369-4
Meimandipour, A., Shuhaimi, M., Soleimani, A., Azhar, K., Hair-Bejo, M., Kabeir, B., et al. (2010). Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 89, 470–476. doi: 10.3382/ps.2009-00495
Mikulski, D., Jankowski, J., Mikulska, M., and Demey, V. (2020). Effects of dietary probiotic (Pediococcus acidilactici) supplementation on productive performance, egg quality, and body composition in laying hens fed diets varying in energy density. Poult. Sci. 99, 2275–2285. doi: 10.1016/j.psj.2019.11.046
Mountzouris, K., Tsitrsikos, P., Palamidi, I., Arvaniti, A., Mohnl, M., Schatzmayr, G., et al. (2010). Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 89, 58–67. doi: 10.3382/ps.2009-00308
Mwaniki, Z., Neijat, M., and Kiarie, E. (2018). Egg production and quality responses of adding up to 7.5% defatted black soldier fly larvae meal in a corn-soybean meal diet fed to Shaver White Leghorns from wk 19 to 27 of age. Poult. Sci. 97, 2829–2835. doi: 10.3382/ps/pey118
National Research and Council (1994). Nutrient requirements of poultry: Ninth revised edition. Washington, DC: The National Academies Press.
Neijat, M., Shirley, R. B., Barton, J., Thiery, P., Welsher, A., and Kiarie, E. (2019). Effect of dietary supplementation of Bacillus subtilis DSM29784 on hen performance, egg quality indices, and apparent retention of dietary components in laying hens from 19 to 48 weeks of age. Poult. Sci. 98, 5622–5635. doi: 10.3382/ps/pez324
Nwachukwu, C. U., Aliyu, K. I., and Ewuola, E. O. (2021). Growth indices, intestinal histomorphology, and blood profile of rabbits fed probiotics-and prebiotics-supplemented diets. Trans. Anim. Sci. 5:txab096. doi: 10.1093/tas/txab096
Pan, X., Cai, Y., Kong, L., Xiao, C., Zhu, Q., and Song, Z. (2022). Probiotic Effects of Bacillus licheniformis DSM5749 on Growth Performance and Intestinal Microecological Balance of Laying Hens. Front. Nutr. 9:868093. doi: 10.3389/fnut.2022.868093
Panda, A. K., Bisht, S. S., DeMondal, S., Senthil Kumar, N., Gurusubramanian, G., and Panigrahi, A. K. (2014). Brevibacillus as a biological tool: A short review. Antonie Van Leeuwenhoek 105, 623–639. doi: 10.1007/s10482-013-0099-7
Park, J., Yun, H., and Kim, I. (2018). The effect of dietary Bacillus subtilis supplementation on the growth performance, blood profile, nutrient retention, and caecal microflora in broiler chickens. J. Appl. Anim. Res. 46, 868–872.
Patterson, J., and Burkholder, K. (2003). Application of prebiotics and probiotics in poultry production. Poult. Sci. 82, 627–631.
Pisoschi, A. M., Pop, A., Iordache, F., Stanca, L., and Predoi Serban, A. L. (2021). Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 209:112891. doi: 10.1016/j.ejmech.2020.112891
Purba, M. A., Pirzado, S. A., Cai, H., Haile, T. H., Zheng, A., Liu, J., et al. (2020). A study about Protective Effect of Brevibacillus laterosporus texasporus Culture on Broiler Chickens Infected with Salmonella Pullorum. Int. J. Sci. Tech. Mgt. 1, 68–78.
Qiu, K., Li, C., Wang, J., Qi, G. H., Gao, J., Zhang, H., et al. (2021). Effects of dietary supplementation with Bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front. Nutr. 8:786878. doi: 10.3389/fnut.2021.786878
Ray, B. C., Chowdhury, S. D., Das, S. C., Dey, B., Khatun, A., Roy, B. C., et al. (2022). Comparative effects of feeding single-and multi-strain probiotics to commercial layers on the productive performance and egg quality indices. J. Appl. Poult. Res. 31:100257.
Sarlak, S., Tabeidian, S. A., Toghyani, M., Shahraki, A. D. F., Goli, M., and Habibian, M. (2021). Effects of replacing inorganic with organic iron on performance, egg quality, serum and egg yolk lipids, antioxidant status, and iron accumulation in eggs of laying hens. Biol. Trace Elem. Res. 199, 1986–1999. doi: 10.1007/s12011-020-02284-8
Shah, M., Zaneb, H., Masood, S., Khan, R. S., Ashraf, S., Sikandar, A., et al. (2019). Effect of dietary supplementation of zinc and multi-microbe probiotic on growth traits and alteration of intestinal architecture in broiler. Prob. Antimicrob. Prot. 11, 931–937. doi: 10.1007/s12602-018-9424-9
Shamoto, K., and Yamauchi, K. (2000). Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult. Sci. 79, 718–723. doi: 10.1093/ps/79.5.718
Sherman, P. M., Ossa, J. C., and Johnson-Henry, K. (2009). Unraveling mechanisms of action of probiotics. Nutr. Clin. Pract. 24, 10–14.
Shi, H., Zhang, W. L., and Kim, I. H. (2020). Effects of dietary Bacillus subtilis RX7 and B2A supplementation on productive performance, egg quality, blood profiles, and excreta Salmonella counts in laying hens. Can. J. Anim. Sci. 100, 411–417.
Shim, M. Y., Song, E., Billard, L., Aggrey, S. E., Pesti, G. M., and Sodsee, P. (2013). Effects of balanced dietary protein levels on egg production and egg quality parameters of individual commercial layers. Poult. Sci. 92, 2687–2696. doi: 10.3382/ps.2012-02569
Song, D., Wang, Y., Lu, Z., Wang, W., Miao, H., Zhou, H., et al. (2019). Effects of dietary supplementation of microencapsulated Enterococcus fecalis and the extract of Camellia oleifera seed on laying performance, egg quality, serum biochemical parameters, and cecal microflora diversity in laying hens. Poult. Sci. 98, 2880–2887. doi: 10.3382/ps/pez033
Souza, O., Adams, C., Rodrigues, B., Krause, A., Bonamigo, R., Zavarize, K., et al. (2021). The Impact of Bacillus subtilis PB6 and Chromium Propionate on the Performance, Egg Quality and Nutrient Metabolizability of Layer Breeders. Animals 11:3084. doi: 10.3390/ani11113084
Surai, P. F., Kochish, I. I., Fisinin, V. I., and Kidd, M. T. (2019). Antioxidant defence systems and oxidative stress in poultry biology: An update.” Antioxidants 8:235. doi: 10.3390/antiox8070235
Takahashi, M., McCartney, E., Knox, A., Francesch, M., Oka, K., Wada, K., et al. (2018). Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim. Sci. J. 89, 895–905. doi: 10.1111/asj.13006
Tang, R. Y., Wu, Z. L., Wang, G. Z., and Liu, W. C. (2018). The effect of Bacillus amyloliquefaciens on productive performance of laying hens. Ital. J. Anim. Sci. 17, 436–441.
Thiam, M., Barreto Sánchez, A. L., Zhang, J., Zheng, M., Wen, J., Zhao, G., et al. (2021). Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken. Animals 11:3498. doi: 10.3390/ani11123498
Upadhaya, S. D., Rudeaux, F., and Kim, I. H. (2019). Efficacy of dietary Bacillus subtilis and Bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poult. Sci. 98, 4722–4728. doi: 10.3382/ps/pez184
Varzaru, I., Untea, A. E., Martura, T., Olteanu, M., Panaite, T. D., Schitea, M., et al. (2013). Development and validation of an RP-HPLC method for methionine, cystine and lysine separation and determination in corn samples. Ilie. Van. Rev. Chem. 64, 673–679.
Wan, J., Zhang, J., Chen, D. W., Yu, B., Huang, Z. Q., Mao, X. B., et al. (2018). Alginate oligosaccharide enhances intestinal integrity of weaned pigs through altering intestinal inflammatory responses and antioxidant status. RSC Adv. 8, 13482–13492. doi: 10.1039/c8ra01943f
Wang, J., Wang, W. W., Qi, G. H., Cui, C. F., Wu, S. G., Zhang, H. J., et al. (2021). Effects of dietary Bacillus subtilis supplementation and calcium levels on performance and eggshell quality of laying hens in the late phase of production. Poult. Sci. 100:100970. doi: 10.1016/j.psj.2020.12.067
Wang, L., Li, A., Shi, J., Liu, K., Cheng, J., Song, D., et al. (2020). Effects of different levels of cottonseed meal on laying performance, egg quality, intestinal immunity and hepatic histopathology in laying hens. Food Agric. Immunol. 31, 803–812.
Wang, W., Li, Z., Han, Q., Guo, Y., and Zhang, B.D’inca, R. (2016). Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 116, 1878–1888.
Wang, W. W., Wang, J., Zhang, H. J., Wu, S. G., and Qi, G. H. (2020). Effects of Clostridium butyricum on production performance and intestinal absorption function of laying hens in the late phase of production. Anim. Feed Sci. Techn. 264:114476.
Wang, X., Ryu, D., Houtkooper, R. H., and Auwerx, J. (2015). Antibiotic use and abuse: A threat. Poult. Sci. 93, 3097–3103.
Wang, Y., Wang, Y., Lin, X., Gou, Z., Fan, Q., and Jiang, S. (2021). Effects of Clostridium butyricum, sodium butyrate, and butyric acid glycerides on the reproductive performance, egg quality, intestinal health, and offspring performance of yellow-feathered breeder hens. Front. Microbio. 12:657542. doi: 10.3389/fmicb.2021.657542
Wei, C., Khalid, A., Hu, Q., Yang, R., Dai, B., Cheng, H., et al. (2020). Effect of Bacillus velezensis to substitute in-feed antibiotics on the production, blood biochemistry and egg quality indices of laying hens. BMC Vet. Res. 16:400. doi: 10.1186/s12917-020-02570-6
Xiang, Q., Wang, C., Zhang, H., Lai, W., Wei, H., and Peng, J. (2019). Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals 9:1110. doi: 10.3390/ani9121110
Xu, C., Wei, F., Yang, X., Feng, Y., Liu, D., and Hu, H. (2022). Lactobacillus salivarius CML352 Isolated from Chinese Local Breed Chicken Modulates the Gut Microbiota and Improves Intestinal Health and Egg Quality in Late-Phase Laying Hens. Microorganisms 10:726. doi: 10.3390/microorganisms10040726
Xu, Q., Azzam, M. M., Zou, X., and Dong, X. (2020). Effects of chitooligosaccharide supplementation on laying performance, egg quality, blood biochemistry, antioxidant capacity and immunity of laying hens during the late laying period. Ital. J. Anim. Sci. 19, 1180–1187.
Yang, J., Zhan, K., and Zhang, M. (2020). Effects of the use of a combination of two Bacillus species on performance, egg quality, small intestinal mucosal morphology, and cecal microbiota profile in aging laying hens. Probiot. Antimicrob. Prot. 12, 204–213. doi: 10.1007/s12602-019-09532-x
Ye, M., Wei, C., Khalid, A., Hu, Q., Yang, R., Dai, B., et al. (2020). Effect of Bacillus velezensis to substitute in-feed antibiotics on the production, blood biochemistry and egg quality indices of laying hens. BMC Vet. Res. 16, 1–8.
Zhan, H., Dong, X., Li, L., Zheng, Y., Gong, Y., and Zou, X. (2019). Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 98, 896–903. doi: 10.3382/ps/pey436
Zhang, B., Yang, X., Guo, Y., and Long, F. (2011). Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr. 65, 329–339. doi: 10.1080/1745039x.2011.568274
Zhang, J., Zhang, M., Liang, W., Geng, Z., and Chen, X. (2020). Green tea powder supplementation increased viscosity and decreased lysozyme activity of egg white during storage of eggs from Huainan partridge chicken. Ital. J. Anim. Sci. 19, 586–592.
Zhang, L., Zhang, L., Zhan, X. A., Zeng, X., Zhou, L., Cao, G., et al. (2016). Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotech. 7:3. doi: 10.1186/s40104-016-0061-4
Zhou, J. M., Qiu, K., Wang, J., Zhang, H. J., Qi, G. H., and Wu, S. G. (2021). Effect of dietary serine supplementation on performance, egg quality, serum indices, and ileal mucosal immunity in laying hens fed a low crude protein diet. Poult. Sci. 100:101465. doi: 10.1016/j.psj.2021.101465
Zhou, Y., Li, S., Pang, Q., and Miao, Z. (2020). Bacillus amyloliquefaciens BLCC1-0238 can effectively improve laying performance and egg quality via enhancing immunity and regulating reproductive hormones of laying hens. Prob. Antmb. Prot. 12, 246–252. doi: 10.1007/s12602-019-9524-1
Keywords: probiotics, laying hens, egg quality, gut health, immune response, antioxidant capacity
Citation: Obianwuna UE, Qiu K, Chang X, Zhang H, Wang J, Qi G, Sun T, Su Y and Wu S (2022) Enhancing egg production and quality by the supplementation of probiotic strains (Clostridium and Brevibacillus) via improved amino acid digestibility, intestinal health, immune response, and antioxidant activity. Front. Microbiol. 13:987241. doi: 10.3389/fmicb.2022.987241
Received: 06 July 2022; Accepted: 08 August 2022;
Published: 13 September 2022.
Edited by:
Wen-Chao Liu, Guangdong Ocean University, ChinaReviewed by:
Shimeng Huang, Jiangsu Academy of Agricultural Sciences, ChinaMohammad Goli, Islamic Azad University, Isfahan, Iran
Copyright © 2022 Obianwuna, Qiu, Chang, Zhang, Wang, Qi, Sun, Su and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tie-hu Sun, c3VudGllaHVAY29mY28uY29t; Shu-geng Wu, d3VzaHVnZW5nQGNhYXMuY24=
†These authors have contributed equally to this work
 Uchechukwu Edna Obianwuna
Uchechukwu Edna Obianwuna Kai Qiu
Kai Qiu Xin-yu Chang1
Xin-yu Chang1 Hai-jun Zhang
Hai-jun Zhang Jing Wang
Jing Wang Guang-hai Qi
Guang-hai Qi Shu-geng Wu
Shu-geng Wu