
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 05 August 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.981181
This article is part of the Research Topic Worldwide Emergence of Drug-Resistant fungi: from Basic to Clinic, Volume II View all 13 articles
Cutaneous candidiasis is one of the most prevalent mycotic infections caused by Candida species. The severity of infection mounts faster when the species shows antifungal resistance. In the current retrospective study, we aimed to analyze the occurrence, causes of cutaneous candidiasis, and antifungal susceptibility pattern of Candida isolates from Skin and Venereal Diseases Prevention and Control Hospital of Shantou, located in eastern Guangdong, China. The laboratory data of all patients (n = 3,113) suffering from various skin and venereal infections during January 2012 to December 2021 was analyzed through Excel and GraphPad prism. Our analysis indicate that cutaneous candidiasis was 22.29% (n = 694), of which 78.53% (n = 554) of patients were males and 21.47% (n = 149) of patients were females. The median age of patients with cutaneous candidiasis was 38-year [interquartile range (30–48)]. Most cases occurred in the adult age group (19–50 years). Regarding the species type, the Candida albicans were prominently detected (n = 664, 95.68%), while non-C. albicans were found only in 30 (4.32%) patients, which were C. glabrata (n = 18), C. krusei (n = 8), C. tropicalis (n = 3), and C. parapsilosis (n = 1). The C. albicans susceptibility rate for terbinafine, miconazole, voriconazole, itraconazole, fluconazole, ketoconazole, nystatin, 5-flucytosine and amphotericin B were 10.83, 29.32, 59.39, 78.53, 85.28, 87.75, 99.59, 99.41, and 100%, respectively. Finally, all C. glabrata isolates were found susceptible to all tested azole drugs with exception to miconazole against which 8.33% of isolates showed resistance. The findings of this study will help healthcare officials to establish better antifungal stewardship in the region.
Cutaneous candidiasis is a superficial infection of skin and mucus membranes caused by yeast from genus Candida (Nurdin et al., 2021). Candida albicans is the most common specie responsible for candidiasis in humans; however other species like C. glabrata, C. tropicalis, C. krusei, C. parapsilosis, and many others are also causing skin infections (Bhattacharya et al., 2020). The C. albicans is an opportunistic yeast that mainly causes infections in immunocompromised patients or those with nutritional deficiencies and endocrine disorders. Besides these, local factors like xerostomia, ulcerations, radiation-induced mucositis, trauma-induced skin damage, and skin maceration increase the morbidity rate (Sadeghi et al., 2019). Types of cutaneous candidiasis are candidal vulvovaginitis, candidal balanitis, congenital candidiasis, candidal diaper dermatitis, oral candidiasis, intertrigo, decubital candidiasis, paronychia, perianal dermatitis, and erosio interdigitalis blastomycetica. Pustules, papules, ulcerations, and vesicles are typical signs of cutaneous candidiasis (Edwards, 2015). A study reported that 7% of all inpatients and 1% of all outpatients’ visits to dermatological hospitals had cutaneous candidiasis (Taudorf et al., 2019). The mortality rate of cutaneous infections is relatively low; nevertheless, if the infections remain enigmatic or untreated for a long time, they might cause systematic and invasive candidiasis, with an approximately 25–50% mortality rate (Tortorano et al., 2021).
The global therapeutic guidelines for rare yeast infections are available, but cutaneous candidiasis remains unaddressed (Chen et al., 2021). Physicians prescribe various topical and oral antifungal agents combined with antibacterial, anti-inflammatory, and corticosteroid drugs for its treatment. Common topical antifungal drugs for cutaneous candidiasis are clotrimazole, nystatin, and miconazole. The terbinafine, ketoconazole, and fluconazole are also studied as systematic therapeutic agents for cutaneous candidiasis (Taudorf et al., 2019). Due to the lack of national guidelines for treating cutaneous candidiasis, the misuse of available antifungal drugs occurs, which endorses antifungal drug resistance (Markogiannakis et al., 2021). Antimicrobial resistance is a worldwide health concern; prolonged hospital stays, increased patient cost burden, and mortality rates (Ur Rahman et al., 2018).
Locally and country-wise antifungal drug resistance surveillance studies need to be performed to depict the current scenario. These surveillance studies will help physicians and healthcare officials to properly manage and treat infections (Badali and Wiederhold, 2019). Therefore, the purpose of the current study was to retrospectively analyze the prevalence of cutaneous candidiasis reported over the past 10 years in the Skin and Venereal Diseases Prevention and Control Hospital of Shantou in eastern Guangdong, China. Furthermore, the current study sorted out candidiasis in different age groups and gender, data about various Candida species, and their antifungal drug susceptibility profiles.
The current retrospective study was conducted at Skin and Venereal Diseases Prevention and Control Hospital of Shantou city, Guangdong, China. Data about cutaneous candidiasis were obtained from laboratory records of the hospital for the past 10 years (January 2012 to December 2021).
The data of all patients with cutaneous infections were obtained for which direct microscopy, candida growth culture, and antifungal susceptibility tests had performed. The patient’s age, gender, date of sample collection, sample type, Candida species type, and the antifungal susceptibility profile for each species were obtained from laboratory records and saved in an Excel sheet for further analysis.
In routine, every patient with cutaneous fungal infections was first recommended for direct microscopy with potassium hydroxide to visualize fungal pathogens. The positive samples with the Candida-like growth were cultured on CHROMagar-Candida medium to examine and identify Candida following the standard protocol (Saud et al., 2020). Furthermore, the antifungal susceptibility tests for positive Candida cultures were performed using CLSI-recommended broth microdilution methods or ATB fungus-2 kit. From 2012 to 2018, antifungal susceptibility tests were performed for fluconazole, miconazole, terbinafine, ketoconazole, itraconazole, and nystatin according to CLSI broth microdilution method (Espinel-Ingroff, 2022). Onward 2019, the tests were performed by ATB fungus-2 kit, and the tested antifungal agents were 5-flucytosine, voriconazole, fluconazole, amphotericin B, and itraconazole. The susceptibility, intermediated, and resistant results were interpreted according to the CLSI M60 or epidemiological cutoff values guidelines (CLSI, 2017; Procop, 2020).
The patients’ data were classified into four groups depending on age: infants; less than 1 year of age, pediatrics; aged from 1 to 18 years, adults; aged from 18 to 65 years, and older adults; ages greater than 65. The adult age group was further divided into four groups: group I; 18–30 years of age, group II; 31–40 years of age, group III; 41–50 years of age, group IV; 50–65 years of age. The number and percentage of Candida species in each age group, patient gender type, and year of the report were noted. The antifungal susceptibility patterns for each Candida species were amalgamated over the past decade. The percentage of susceptible, intermediate, and resistant Candida species against the examined antifungal drugs was determined.
Moreover, the year-wise antifungal susceptibility pattern of C. albicans was determined. The trend of year-wise susceptibility patterns of fluconazole and itraconazole were resolved. The data numeration and percentages were calculated by Microsoft Excel 2016, while the statistical analysis and graphs constructions were performed by GraphPad prism v.8.0 software. The total number of C. albicans cases occurred each year, and the number of cases in different age groups of patients were compared using student’s t-tests. Furthermore, gender base significance was calculated by ratio paired t-test. Statistical significance was calculated by two-tailed tests, and p < 0.05 was considered statistically significant.
In the past 10 years, 3,113 patients with cutaneous mycosis were examined by direct microscopy and fungal routine culture, in which 694 (22.29%) were diagnosed with cutaneous candidiasis. Among the candidiasis patients, 545 (78.53%) were male, and 149 (21.47%) were female. Regarding the Candida species type, the C. albicans were prominently detected in 664/694 (95.68%) patients, while non-C. albicans were found only in 30/694 (4.32%) patients. The high number of C. albicans were reported in year 2013 (n = 121, 18.22%), followed by 2012 (n = 111, 16.71%) and 2018 (n = 103, 15.51%). Among the 30 non-C. albicans species C. glabrata were reported in 18/694 (2.59%) patients, C. krusei in 8/694 (1.15%), C. tropicalis in 3/694 (0.43%), and C. parapsilosis was detected only in one patient (n = 1/694, 0.14%). The year-wise incidence of C. albicans and non-C. albicans species are presented in Figure 1.
The median age of patients was 38-year, range (from 8 months to 82 years), interquartile range (30–48 years). Most cases occurred in the adult age group (19–50 years). For C. albicans only three (0.45%) cases were reported in infants, and seven (1.05%) were from the pediatric group; three were male, and four were female pediatric patients. From the older adult group, 18 (2.71%) C. albicans were isolated, of which 17 were from male patients, and only one was from a female patient. From the adult age group (19–65 years), a total of 636 (95.78%) cases were reported, of which 500/636 (78.61%) were from males, and 136/636 (21.38%) were from female patients. In the current study, we found that the C. albicans causing cutaneous candidiasis occurred in a high proportion in males (n = 522 out of 664, 78.61%) compared to females (n = 142 out of 664, 21.39%; p-value = 0.0001). Among the age groups, the adult age group II for males and adult age group I for females were more vulnerable to C. albicans. The year-wise occurrence of C. albicans in different age groups and gender and their statistical significance (p-values) are summarized in Table 1.
The number of non-C. albicans (n = 30 out of 694, 4.32%) were relatively much smaller than C. albicans detected in the present study. Four different types of non-C. albicans species were detected, which were C. glabrata (n = 18 out 30, 60%), C. krusei (n = 8 out 30, 26.67%), C. tropicalis (n = 3 out of 30, 10%) and C. parapsilosis (n = 1 out of 30, 3.33%). The number of non-C. albicans isolates from male patients (n = 22 out of 30, 73.33%) were high compared to females (n = 8 out of 30, 26.67%). The number of different non-C. albicans species concerning different age and gender groups are presented in Table 2.
For C. albicans the lowest antifungal susceptibility was reported for terbinafine, with only 10% of the isolates susceptible out of 157 tested strains. Among the azole class, miconazole showed the lowest susceptibility with 29.32% out of 440 tested isolates, while 40.23% of isolates were intermediate and 30.45% resistant. Besides, itraconazole and fluconazole, two of the most extensively used antifungal drugs, have resistance rates of 16.10 and 9.34%, respectively. Among the other tested antifungal drugs, only two isolates in 2012 were intermediate-resistant to nystatin, while only one isolate in 2021 was found resistant to 5-flucytosine, and none of the isolates exhibited resistance to amphotericin B. The antifungal susceptibility profile of all tested antifungal agents against the C. albicans is summarized in Table 3.
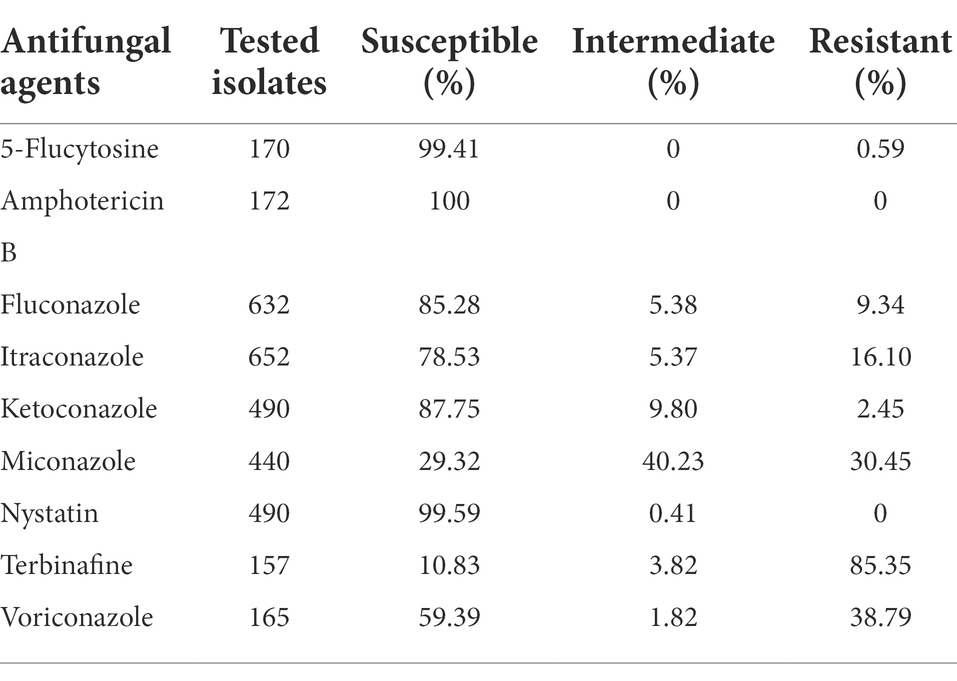
Table 3. Antifungal susceptibility profile of C. albicans for all tested drugs in the past 10 years.
The terbinafine was tested in 2012 and 2013 and showed the highest resistance, 84.47, and 90.47%, respectively. In 2014, the highest resistance was reported against miconazole, which was 57.14% out of 21 tested isolates. From 2015 to 2018, the resistance rate was comparatively lower except for the miconazole, which was 18.18% in 2015, 9.23% in 2016, 10.52% in 2017, and 12.74% in 2018. Onward 2019, antifungal susceptibility tests were performed by ATB fungus-2 kit, in which the highest resistance was observed for itraconazole, fluconazole, and voriconazole. In 2019 resistance to itraconazole, voriconazole, and fluconazole were 53.16, 53.42, and 36.98%, in 2020 it was 40.81, 38.77, and 32.65%, while in 2021 it was 27.90, 13.95, and 13.95%, respectively. Year-wise antifungal susceptibility profiles of C. albicans against all tested drugs are presented in Figure 2.
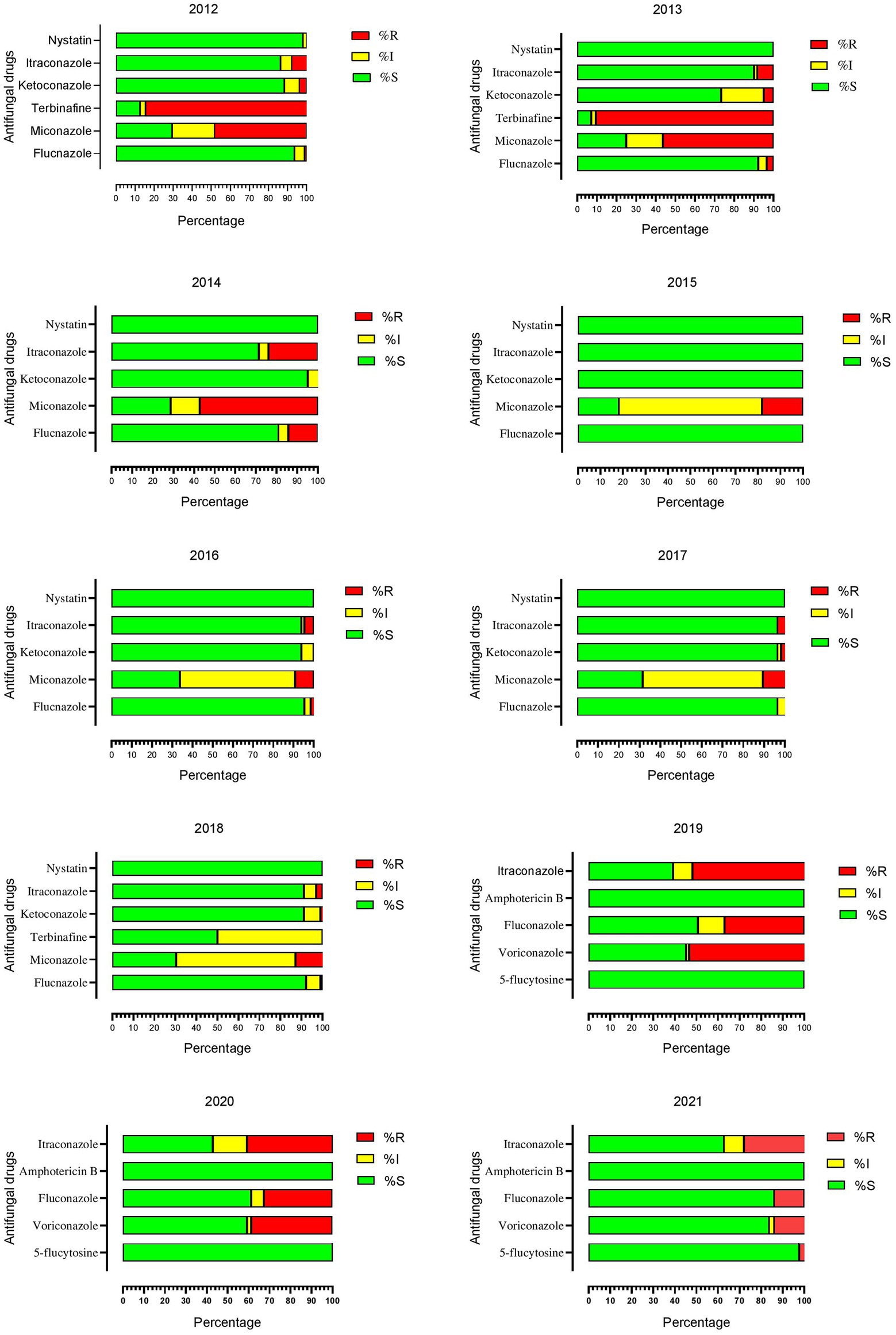
Figure 2. Year-wise antifungal susceptibility patterns of Candida albicans against all tested drugs.
From a broader perspective, the last 10 years’ fluconazole and itraconazole resistance patterns showed little similarity. However, the combined susceptibility rate of fluconazole was 85.28%, while itraconazole was 78.53%. The lowest resistance rates were noted in 2018 and reached the highest for both drugs in the next 2 years (2019 and 2020). The similarity in pattern between these two drugs indicates that the exact molecular mechanism might be involved in developing resistance against azole drugs. The trend of fluconazole and itraconazole susceptibility patterns over time is shown in Figure 3.
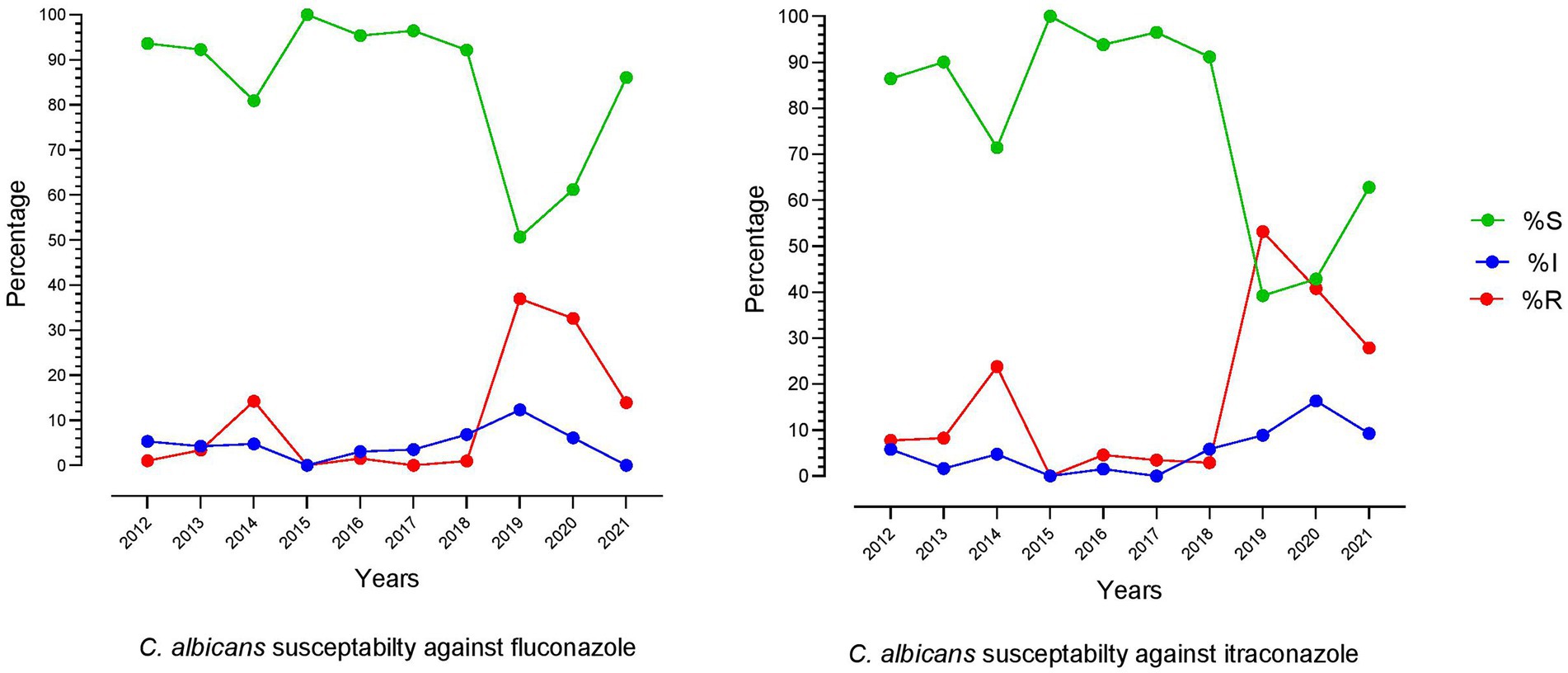
Figure 3. Trend of fluconazole and itraconazole susceptibility patterns of C. albicans over the last 10 years (2012–2021).
The C. parapsilosis showed resistance to fluconazole, itraconazole, and voriconazole and was susceptible to amphotericin B and 5-flucytosine. Among the three C. tropicalis isolates, resistance to terbinafine and miconazole were observed in only one isolate. For C. krusei, 80% of isolates were resistant to terbinafine out of five tested strains, while all other isolates were susceptible to amphotericin B and 5-flucytosine. For C. glabrata, one isolate detected in 2021 was resistant to 5-flucytosine; however, it was susceptible to amphotericin B and all tested azole drugs. Similarly, another isolate of C. glabrata reported in 2013 showed resistance to nystatin, the only nystatin-resistant isolate in the current study. The antifungal susceptibility profile of C. glabrata is summarized in Table 4.
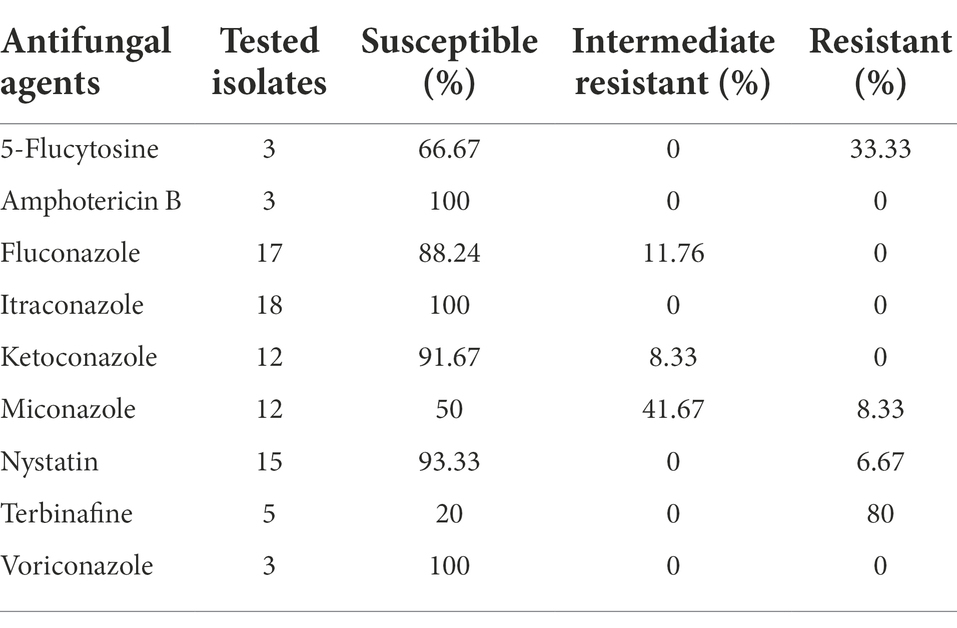
Table 4. Antifungal susceptibility profile of C. glabrata for all tested drugs in the past 10 years.
The prevalence of cutaneous candidiasis varies regarding the geography, demography of patients, type of the fungal pathogen, and many other environmental factors (Kühbacher et al., 2017). In the current study, the prevalence rate of cutaneous candidiasis over the past decade was 22.29% which makes it different from other regions of the world, where it is reported to be 40.5% in Iran, 57% in Serbia, and 82.9% in Brazil (de Albuquerque Maranhão et al., 2019; Otašević et al., 2019; Khodadadi et al., 2021). The difference in the prevalence might be due to different environmental conditions and the social status of the populations (Zareshahrabadi et al., 2021). In the present study, the infection rate was highly reported in the male population (78.53%) compared to females (21.47%). In different countries, gender-based prevalence varies; a previous study from China, Italy, and France reported an equal proportion of males and females infected with cutaneous mycosis (Vena et al., 2012; Cai et al., 2016; Faure-Cognet et al., 2016). Studies from South Korea and Chile reported a high proportion of Candida-infected females (Cruz Ch et al., 2011; Yoon et al., 2014). However, a study from Iran showed resemblance to our finding, with high cases of cutaneous candidiasis among males (Zamani et al., 2016). These contradictions depend on differences in occupational activities, personal hygiene, and exposure to contamination of male and female populations (de Albuquerque Maranhão et al., 2019). In our study, cutaneous candidiasis mainly occurred in the adult age group (from 19 to 50 years). Some other studies reported that patients below 20 are more vulnerable to candidiasis; however, the studies from Iran, South Korea, and India agreed with our findings (Nawal et al., 2012; Yoon et al., 2014; Cai et al., 2016; Khodadadi et al., 2021). The main reason for the adult age group’s link with cutaneous candidiasis might be that these populations have more involvement in job markets and social activities with a high chance of exposure to Candida infections (Khodadadi et al., 2021).
More than 200 Candida species have been identified, of which over 15 are known for human pathogenicity, among which the C. albicans are highly reported (Palese et al., 2018). Similarly, in our study, 95.68% of cutaneous candidiasis was caused by C. albicans. The high infection rate of C. albicans is due to its ability to grow in different morphological forms like true hyphae, pseudo-hyphae, and unicellular budding yeast, which enhance its virulence and invading host cell activity (Nam et al., 2022). Moreover, underlying diseases, immunosuppressive states, antibiotic therapy, and skin environment variation are the factors due to which the commensal C. albicans switched into a true pathogen (Palese et al., 2018). In the present study, the C. glabrata was detected in 18 cases, the highest among non-C. albicans species. This differs from the studies reported in Cameroon, Nigeria, and India, where C. tropicalis are more prevalent than C. glabrata (Verma et al., 2021). However, a similar drift was observed in North America and many European countries (Song et al., 2020). After C. glabrata, the C. krusei was reported as the second high in number among the non-C. albicans species. It is a matter of concern because C. krusei is one of the multidrug-resistant species and is intrinsically resistant to fluconazole (Jamiu et al., 2021).
In the current study, the C. albicans show high resistance to terbinafine, i.e. 85.35% of 157 tested isolates. Similarly, for C. glabrata and C. krusei, 80% of the isolates were resistant to terbinafine. This high resistance might be due to the weak inhibitory activity of terbinafine against all Candida species except C. parapsilosis (Ameen et al., 2014; Noguchi et al., 2019). In the azole class, the high resistance (30.45%) was reported for miconazole, while 40.23% of 440 tested C. albicans isolates were intermediated resistant. The high resistance to miconazole is due to its improper usage as a topical therapeutic agent for cutaneous candidiasis (Taudorf et al., 2019).
Similarly, high resistance was reported for voriconazole; 38.79% out of 175 tested isolates. For fluconazole and itraconazole, the resistant rate was 9.34 and 16.10%, while 5.38 and 5.37% of the isolate were intermediate resistant, respectively. In this study, the azoles are comparatively less susceptible than polyenes and flucytosine. The high resistance to azole might be due to its inappropriate usage in agriculture and clinical settings in China (Zhou et al., 2022). Moreover, the fungistatic nature of azole drugs imposes a robust direct selection of antifungal-resistant species (Das et al., 2019). Only one isolate of C. albicans and C. glabrata show resistance to 5-flucytosine. For nystatin, only one C. glabrata isolate was resistant, and two C. albicans were intermediate resistant, while none of the isolates was resistant to amphotericin B. According to a report, a single antifungal agent and corticosteroid drugs are good options for curing cutaneous candidiasis (Taudorf et al., 2019). Hence, based on our findings, we suggest nystatin, a topical antifungal agent, and a corticosteroid drug for curing cutaneous candidiasis in our regions.
Moreover, it is suggested that the general population not to take antifungal drugs without a proper diagnosis of mycotic infections and prescriptions from a dermatologist. The laboratory screening of candidiasis needs to be performed molecularly or by MALDI TOF MS to correctly identify Candida species (Zhao et al., 2018). Furthermore, national guidelines for treating cutaneous mycotic infections need to be developed for proper medication and to halt the incidence of antifungal-resistant pathogens.
The current study has some limitations; our study was based on a single center in the eastern Guangdong province. Hence our findings might not be generalized to other regions because the prevalence of cutaneous candidiasis varies due to environmental and socio-economic factors (Dhillon and Chopra, 2022). Moreover, our study was based on the available laboratory records; therefore, clinical and detailed demographic features were not analyzed. To provide new insights, from now on, we intend to collect the clinical and patient demographic data for future research work and scientific base treatment of cutaneous candidiasis. On a vaster glimpse, the premise of this study entails significant epidemiological findings that are valuable for scheming approaches to improve the management of cutaneous candidiasis.
In the current study, we summarized the significant updated data about the prevalence of cutaneous candidiasis, species distribution, and antifungal susceptibility patterns of Candida species. Over the past decade of surveillance, C. albicans was a primary cause of cutaneous candidiasis, and resistances to terbinafine and azole were prominent. The C. glabrata were reported in high number among the few non-C. albicans isolates. Amphotericin B and 5-flucytosine were more susceptible drugs in the last 3 years. Over the years, nystatin has shown excellent activity against all Candida species. National trends of antifungal susceptibility and continuous monitoring are needed. The epidemiological outcomes of the present study will provide baselines for more in-depth research and help healthcare officials to tackle the challenges of antifungal resistance.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Skin and Venereal Diseases Prevention and Control Hospital of Shantou city, Guangdong, China. The ethics committee waived the requirement of written informed consent for participation.
HB and YZ: study idea and plan. HB and BH: attainment of data. HB, MS, and XC: analysis and interpretation of data. HB, MAS, and YZ: drafting of the manuscript. YZ and MAS: critical revision of the manuscript for important intellectual content. YZ: administrative, technical, material support, and institutional study supervision. All authors contributed to the article and approved the submitted version.
This study is funded by the Second Affiliated Hospital of Shantou University Medical College to HBil and his mentor YZ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ameen, M., Lear, J., Madan, V., Mohd Mustapa, M., Richardson, M., Hughes, J., et al. (2014). British association of dermatologists' guidelines for the management of onychomycosis 2014. Br. J. Dermatol. 171, 937–958. doi: 10.1111/bjd.13358
Badali, H., and Wiederhold, N. P. (2019). Antifungal resistance testing and implications for management. Curr. Fungal Inf. Rep. 13, 274–283. doi: 10.1007/s12281-019-00354-6
Bhattacharya, S., Sae-Tia, S., and Fries, B. C. (2020). Candidiasis and mechanisms of antifungal resistance. Antibiotics (Basel) 9:312. doi: 10.3390/antibiotics9060312
Cai, W., Lu, C., Li, X., Zhang, J., Zhan, P., Xi, L., et al. (2016). Epidemiology of superficial fungal infections in Guangdong, southern China: a retrospective study from 2004 to 2014. Mycopathologia 181, 387–395. doi: 10.1007/s11046-016-9986-6
Chen, S. C., Perfect, J., Colombo, A. L., Cornely, O. A., Groll, A. H., Seidel, D., et al. (2021). Global guideline for the diagnosis and management of rare yeast infections: an initiative of the ECMM in cooperation with Isham and ASM. Lancet Infect. Dis. 21, E375–E386. doi: 10.1016/S1473-3099(21)00203-6
CLSI (2017). M60. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
Cruz Ch, R., Ponce, E. E., Calderón, R. L., Delgado, V. N., Vieille, O. P., and Piontelli, L. E. (2011). Superficial mycoses in the city of Valparaiso, Chile: period 2007-2009. Rev. Chil. Infectol. 28, 404–409. doi: 10.4067/S0716-10182011000600002
Das, K. H., Mangayarkarasi, V., and Sen, M. (2019). Antifungal resistant in non-Albicans Candida species are emerging as a threat to antenatal women with vulvovaginal candidiasis. Biomed. Pharmacol. J. 12, 1369–1378. doi: 10.13005/bpj/1765
de Albuquerque Maranhão, F. C., Oliveira-Júnior, J. B., Dos Santos Araújo, M. A., and Silva, D. M. W. (2019). Mycoses in northeastern Brazil: epidemiology and prevalence of fungal species in 8 years of retrospective analysis in Alagoas. Braz. J. Microbiol. 50, 969–978. doi: 10.1007/s42770-019-00096-0
Dhillon, A., and Chopra, A. (2022). An observational study of infant dermatoses at a tertiary care health center in Delhi region. Egypt. J. Dermatol. Venerol. 42:115. doi: 10.4103/ejdv.ejdv_21_21
Edwards, J. E. (2015). “258- Candida Species,” in Mandell, Douglas, and Bennett's principles and practice of infectious diseases (Eighth Edition). eds. J. E. Bennett, R. Dolin, and M. J. Blaser (Philadelphia, PA: W.B. Saunders).
Espinel-Ingroff, A. (2022). Commercial methods for antifungal susceptibility testing of yeasts: strengths and limitations as predictors of resistance. J. Fungi 8:309. doi: 10.3390/jof8030309
Faure-Cognet, O., Fricker-Hidalgo, H., Pelloux, H., and Leccia, M. T. (2016). Superficial fungal infections in a French teaching hospital in Grenoble area: retrospective study on 5470 samples from 2001 to 2011. Mycopathologia 181, 59–66. doi: 10.1007/s11046-015-9953-7
Jamiu, A. T., Albertyn, J., Sebolai, O. M., and Pohl, C. H. (2021). Update on Candida Krusei, a potential multidrug-resistant pathogen. Med. Mycol. 59, 14–30. doi: 10.1093/mmy/myaa031
Khodadadi, H., Zomorodian, K., Nouraei, H., Zareshahrabadi, Z., Barzegar, S., Zare, M. R., et al. (2021). Prevalence of superficial-cutaneous fungal infections in Shiraz, Iran: a five-year retrospective study (2015-2019). J. Clin. Lab. Anal. 35:E23850. doi: 10.1002/jcla.23850
Kühbacher, A., Burger-Kentischer, A., and Rupp, S. (2017). Interaction of Candida species with the skin. Microorganisms 5:32. doi: 10.3390/microorganisms5020032
Markogiannakis, A., Korantanis, K., Gamaletsou, M. N., Samarkos, M., Psichogiou, M., Daikos, G., et al. (2021). Impact of a non-compulsory antifungal stewardship program on overuse and misuse of antifungal agents in a tertiary care hospital. Int. J. Antimicrob. Agents 57:106255. doi: 10.1016/j.ijantimicag.2020.106255
Nam, M., Kim, S. H., Jeong, J. H., Kim, S., and Kim, J. (2022). Roles of the pro-apoptotic factors Canma111 and Caybh3 in apoptosis and virulence Of Candida Albicans. Sci. Rep. 12:7574. doi: 10.1038/s41598-022-11682-y
Nawal, P., Patel, S., Patel, M., Soni, S., and Khandelwal, N. (2012). A study of superficial mycoses in tertiary care hospital. J Age 6:11. Available at: https://typeset.io/papers/a-study-of-superficial-mycoses-in-tertiary-care-hospital-5eg4oww8k3
Noguchi, H., Matsumoto, T., Kimura, U., Hiruma, M., Kano, R., Yaguchi, T., et al. (2019). Fungal Melanonychia caused by Candida Parapsilosis successfully treated with oral Fosravuconazole. J. Dermatol. 46, 911–913. doi: 10.1111/1346-8138.15024
Nurdin, R. S. C., Vitayani, S., Amin, S., Kadir, D., Djamaluddin, W., and Adriani, A. (2021). Cutaneous candidiasis caused by Candida Kefyr. Pan Afr. Med. J. 38:178. doi: 10.11604/pamj.2021.38.178.28054
Otašević, S., Momčilović, S., Golubović, M., Ignjatović, A., Rančić, N., Đorđević, M., et al. (2019). Species distribution and epidemiological characteristics of superficial fungal infections in southeastern Serbia. Mycoses 62, 458–465. doi: 10.1111/myc.12900
Palese, E., Nudo, M., Zino, G., Devirgiliis, V., Carbotti, M., Cinelli, E., et al. (2018). Cutaneous candidiasis caused by Candida Albicans in a young non-immunosuppressed patient: an unusual presentation. Int. J. Immunopathol. Pharmacol. 32:2058738418781368. doi: 10.1177/2058738418781368
Procop, G. W. (2020). Epidemiological Cutoff Values for Antifungal Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute.
Sadeghi, G., Ebrahimi-Rad, M., Shams-Ghahfarokhi, M., Jahanshiri, Z., Ardakani, E. M., Eslamifar, A., et al. (2019). Cutaneous candidiasis in Tehran-Iran: from epidemiology to multilocus sequence types, virulence factors and antifungal susceptibility of etiologic Candida species. Iran J. Microbiol. 11, 267–279. doi: 10.18502/ijm.v11i4.1463
Saud, B., Bajgain, P., Paudel, G., Shrestha, V., Bajracharya, D., Adhikari, S., et al. (2020). Fungal infection among diabetic and nondiabetic individuals in Nepal. Interdiscip. Perspect. Infect. Dis. 2020:7949868. doi: 10.1155/2020/7949868
Song, Y., Chen, X., Yan, Y., Wan, Z., Liu, W., and Li, R. (2020). Prevalence and antifungal susceptibility of pathogenic yeasts in China: a 10-year retrospective study in a teaching hospital. Front. Microbiol. 11:1401. doi: 10.3389/fmicb.2020.01401
Taudorf, E. H., Jemec, G. B. E., Hay, R. J., and Saunte, D. M. L. (2019). Cutaneous candidiasis-an evidence-based review of topical and systemic treatments to inform clinical practice. J. Eur. Acad. Dermatol. Venereol. 33, 1863–1873. doi: 10.1111/jdv.15782
Tortorano, A. M., Prigitano, A., Morroni, G., Brescini, L., and Barchiesi, F. (2021). Candidemia: evolution of drug resistance and novel therapeutic approaches. Infect. Drug Resist. 14, 5543–5553. doi: 10.2147/IDR.S274872
Ur Rahman, S., Ali, T., Ali, I., Khan, N. A., Han, B., and Gao, J. (2018). The growing genetic and functional diversity of extended spectrum beta-lactamases. Biomed. Res. Int. 2018:9519718. doi: 10.1155/2018/9519718
Vena, G. A., Chieco, P., Posa, F., Garofalo, A., Bosco, A., and Cassano, N. (2012). Epidemiology of dermatophytoses: retrospective analysis from 2005 to 2010 and comparison with previous data From 1975. New Microbiol. 35, 207–213. Available at: https://pubmed.ncbi.nlm.nih.gov/22707134/
Verma, R., Pradhan, D., Hasan, Z., Singh, H., Jain, A. K., and Khan, L. A. (2021). A systematic review on distribution and antifungal resistance pattern of Candida species in the Indian population. Med. Mycol. 59, 1145–1165. doi: 10.1093/mmy/myab058
Yoon, H. J., Choi, H. Y., Kim, Y. K., Song, Y. J., and Ki, M. (2014). Prevalence of fungal infections using National Health Insurance Data From 2009-2013. South Korea. Epidemiol. Health 36:E2014017. doi: 10.4178/epih/e2014017
Zamani, S., Sadeghi, G., Yazdinia, F., Moosa, H., Pazooki, A., Ghafarinia, Z., et al. (2016). Epidemiological trends of dermatophytosis in Tehran, Iran: a five-year retrospective study. J. Mycol. Med. 26, 351–358. doi: 10.1016/j.mycmed.2016.06.007
Zareshahrabadi, Z., Totonchi, A., Rezaei-Matehkolaei, A., Ilkit, M., Ghahartars, M., Arastehfar, A., et al. (2021). Molecular identification and antifungal susceptibility among clinical isolates of dermatophytes in shiraz, Iran (2017-2019). Mycoses 64, 385–393. doi: 10.1111/myc.13226
Zhao, Y., Tsang, C. C., Xiao, M., Chan, J. F. W., Lau, S. K. P., Kong, F., et al. (2018). Yeast identification by sequencing, biochemical kits, Maldi-Tof Ms and rep-PCR DNA fingerprinting. Med. Mycol. 56, 816–827. doi: 10.1093/mmy/myx118
Keywords: cutaneous candidiasis, antifungal resistance, C. albicans, retrospective study, China
Citation: Bilal H, Hou B, Shafiq M, Chen X, Shahid MA and Zeng Y (2022) Antifungal susceptibility pattern of Candida isolated from cutaneous candidiasis patients in eastern Guangdong region: A retrospective study of the past 10 years. Front. Microbiol. 13:981181. doi: 10.3389/fmicb.2022.981181
Received: 29 June 2022; Accepted: 25 July 2022;
Published: 05 August 2022.
Edited by:
Wenjie Fang, Shanghai Changzheng Hospital, ChinaReviewed by:
Mohsan Ullah Goraya, University of Agriculture, Faisalabad, PakistanCopyright © 2022 Bilal, Hou, Shafiq, Chen, Shahid and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuebin Zeng, emVuZ195YkAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.