
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 26 August 2022
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.978940
This article is part of the Research Topic Gastrointestinal Nutrients-Microbiome-Host Interactions in Livestock View all 43 articles
 Chuang Li1,2
Chuang Li1,2 Ning Chen2,3
Ning Chen2,3 Xingxing Zhang2,3
Xingxing Zhang2,3 Khuram Shahzad4
Khuram Shahzad4 Ruxin Qi1
Ruxin Qi1 Zhenbin Zhang1
Zhenbin Zhang1 Zhiqi Lu1
Zhiqi Lu1 Yue Lu1
Yue Lu1 Xiang Yu1
Xiang Yu1 Muhammad Hammad Zafar1
Muhammad Hammad Zafar1 Mengzhi Wang1,2,3*
Mengzhi Wang1,2,3* Wujun Liu5*
Wujun Liu5*Silage is rich in nutrients, which can make up for the lack of seasonal roughage, and has a certain promotion effect on the intensive feeding of ruminants. In addition, silage can maintain the rumen function of ruminants to a certain extent and reduce the risk of rumen acidosis and abomasum translocation. The purpose of this study was to investigate the effects of the mixed silage of Chinese cabbage waste and rice straw (mixed silage) on antioxidant performance, rumen microbial population, and fermentation metabolism of Hu sheep. The 16 healthy Hu sheep (eight rams and eight ewes, 39.11 ± 1.16 kg, 5.5 months) were randomly divided into two groups (the control group and the mixed silage group) with eight animals (four rams and four ewes) in each group. The control group was fed with farm roughage (peanut seedlings, corn husk, and high grain shell) as forage, and the mixed silage group was fed with the mixed silage as forage. The results showed that the mixed silage had no effect on the growth performance of Hu sheep (p > 0.05). Ruminal butyric acid, total volatile fatty acids (TVFA), and ammonia nitrogen (NH3-N) concentration in the mixed silage group were increased, whereas the pH was decreased (p < 0.05). The blood and rumen total antioxidants capacity (T-AOC) concentration in the mixed silage group was higher, and the malondialdehyde (MDA) content in rumen, serum, liver, and kidney was lower than that in the control group (p < 0.05). PCoA and ANOSIM results of Illumina sequencing indicated that the mixed silage affected the bacterial composition of the rumen microbes. The mixed silage increased the proportion of Prevotellaceae UCG-004 which was in a positive correlation with Vitamin C (Vc). In addition, PICRUSt functional prediction analysis showed that ascorbate and aldarate metabolism were up-regulated in the mixed silage group (p < 0.05). In conclusion, higher contents of VC and acid detergent fiber (ADF) in the mixed silage were beneficial to the growth and reproduction of Prevotellaceae UCG-004, resulting in increased production of the butyric acid significantly upregulated the metabolism of ascorbate and aldarate metabolism, thereby improving the antioxidant properties of Hu sheep.
As the global population increases, so does the demand for animal products. The growing demand for animal products has also created a huge requirement for animal feed, and the efficient use of existing feed is essential for efficient livestock farming and food safety (Makkar and Ankers, 2014). Chinese vegetable production accounts for 50% of the world’s total production (FAOSTAT, 2016), but it is also accompanied by a large amount of vegetable waste. Vegetable waste, such as cabbage tails, accounts for the majority of food waste, especially in supermarkets, fresh food markets and households (Ma et al., 2017). The rational use of vegetable waste can not only reduce environmental pollution (water and soil pollution, bacteria reproduction, etc.) but also reduce the harm to consumers’ health (Qiao et al., 2020). In addition, vegetable tails contains minerals, vitamins and other nutrients, which can promote the antioxidant properties of ruminants (Seong et al., 2016; Sahoo et al., 2021). The annual production of rice straw in China is about 21 million t/year, accounting for about 47% of the straw production (Wang et al., 2010; Chen, 2016). Fresh crop stalks dry and wilt rapidly, resulting in reduced nutrients such as moisture and soluble substances (Liu et al., 2019). Furthermore, The lower nutritional value and higher silica content of straw results in lower feed utilization and digestibility of rice straw (Cherdthong et al., 2020; Suntara et al., 2020).
There is a certain complementary effect between Chinese cabbage waste and rice straw in terms of physical structure, nutrients, and water content. Air-dried straw stalks and Chinese cabbage waste are available to complete common silage (Ren et al., 2018). Previous studies showed that vegetable waste can be mixed with straw for silage, leading to the higher rate of utilization, for example, broccoli by-products were mixed with wheat straw (Partovi et al., 2020); corn stover were mixed with cabbage (Ren et al., 2018), co-ensiling of sugar beet waste, and wheat straw (Hillion et al., 2018).
Silage treatment can be used for feed utilization of unconventional feed, and has certain positive impacts on the health status, physiology and rumen microorganisms of ruminants (Bampidis and Robinson, 2006; Abd El Tawab et al., 2020). Broccoli by-product and wheat straw (ratio 69:31) mixed feed did not affect rumen fermentation parameters in Fasander lambs (Partovi et al., 2020). In addition, adding 10% silage mulberry leaves to the total mixed diet of Hanwoo cattle can improve the activities of antioxidant enzymes such as total superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px; Cheong et al., 2012). Hassanat et al. (2013) found that by increasing the proportion of maize silage in the diet, the richness and diversity of bacterial communities was decreased, but the number of total bacteria and Prevotella spp., was increased which favored the propionate production.
The rumen environment and microflora structure are crucial to the digestion and absorption of nutrients, production performance, and health. The rumen is home to a large number of microorganisms that grow in an anaerobic environment and can adhere to the feed surface and ferment (Palma-Hidalgo et al., 2021). Diet level, geographic distribution, feeding management, and health status are several factors that affect the rumen environment and microbial community structure while the diet level is the biggest factor affecting rumen fermentation and microbial population changes (Henderson et al., 2015; van Lingen et al., 2016).
We hypothesized that the mixed silage could improve the rumen environment and microbial flora structure, thereby enhancing the antioxidant properties of Hu sheep. Therefore, in the present study, we fed Hu sheep mixed silage to explore its effects on the antioxidant properties, rumen fermentation and microorganisms, so as to provide some reference for the application of mixed silage in Hu sheep.
The rice straw was collected from the experimental farms of Yangzhou University in Jiangsu Province. The Chinese cabbage waste was collected from the East Garden Farmers Market in Yangzhou City, Jiangsu Province, China. Lactobacillus plantarum and cellulase used in the experiment were purchased from Guangzhou Lvhui Biotechnology Co., Ltd.
The rice straw and Chinese cabbage tails were cut to 2–3 cm, and then were mixed thoroughly with Lactobacillus plantarum and cellulase in a ratio of 4:6. Lactobacillus plantarum 0.035 g/kg and cellulase 0.25 g/kg were added to each kilogram of mixed silage. After mixing, it was put into silage bags, vacuum-treated with a Meggis vacuum machine, sealed in a cool place for 45 days, and then silaged and sampled after anaerobic fermentation.
After the mixed silage was completed, the contents of vitamin A (VA), vitamin B2 (VB2), VC, and vitamin E (VE) were determined by commercial kits (Shanghai Enzyme Link Biotechnology Co., Ltd.).
We selected 16 healthy Hu sheep with an initial weight of 39.97 ± 1.22 kg and an age of about 5.5 months, and randomly divided them into two groups (n = 8, four rams and four ewes). We took the sheep farm roughage (peanut seedlings, corn husks and high grain husks) as the control group, and the mixed silage group took the mixed silage as the roughage. Both the control group and the mixed silage group were fed 50% roughage and 50% concentrate (based on dry matter (DM), Table 1). Feed formulations were designed according to the nutritional requirements of mutton sheep (NRC, 2007). The feeding experiment was carried out for a total of 5 weeks. The first week was the pre-feeding period, and the remaining period from the second to the fifth week was the experimental period. The experimental Hu sheep were raised in single pens, and the sheep house was cleaned and disinfected before the experiment. All sheep were uniformly dewormed and immunized before entering into the sheep house. In the pre-feeding period and the formal experimental period, each Hu sheep was fed twice a day (8:00 and 18:00). During the experiment, each Hu sheep was free to get clean drinking water.
We recorded the fasting weight of each sheep before the formal start and end of morning feeding, that is, the initial weight (IW) and final weight (FW), to calculate the average daily gain (ADG). The amount of feed and leftovers fed to each sheep was recorded during the experiment to calculate the mean daily feed intake (DMI). Finally, the feed-to-weight ratio (F/W) was calculated according to ADG and DMI.
Before morning feed, 5 Hu sheep were randomly selected from the experimental group and the control group for slaughter after fasting for 24 h and having no access to water for 2 h. The sheep were euthanized by injecting thiopental sodium (0.125 mg/kg BW) and potassium chloride (5–10 ml), and then immediately bloodletting. After slaughtering, the rumen fluid was collected. About 50 ml of rumen fluid from each sheep was collected and filtered with four layers of gauze, and the pH value was measured by a pH meter (Mettler Toledo, Switzerland) immediately. Then, it was sub-packed into four 15 ml centrifuge tubes and stored at −80°C for analysis. Rumen fluid for VFA determination requires the addition of deproteinized solution (100 g metaphosphoric acid and 0.6 g crotonic acid per L) in a 1:1 ratio. During slaughter, we immediately collected blood, muscle (longissimus dorsi), rumen, kidney, spleen, liver, and small intestine (duodenum, jejunum, ileum) samples of Hu sheep.
The content of NH3-N in rumen fluid was determined by phenol sodium hypochlorite colorimetry (Broderick and Kang, 1980). Microprotein (MCP) content was determined by the trichloroacetic acid method (Cotta and Russell, 1982). The content of VFA in rumen fluid was determined by gas chromatography (GC-14B, Kyoto, Japan) meteorological chromatography internal standard method (Pan et al., 2016), and the TVFA and the ratio of acetate to propionate were calculated. The instruments and conditions of gas chromatography were the same as Zhang’s method (Zhang et al., 2021).
The levels of superoxide dismutase (SOD), total antioxidant capacity (T-AOC), GSH-Px, malondialdehyde (MDA) and Catalase (CAT) in blood, muscle (longissimus dorsi), rumen, kidney, spleen, liver, and small intestine (duodenum, jejunum and ileum) samples were measured. The kit for index determination was purchased from Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China.
After the rumen fluid samples were thawed, the total microbial DNA of the rumen fluid was extracted using the HiPure Soil DNA Kit (purchased from D3142B, Magen Biotechnology Co., Ltd., GuangZhou, China). The extracted DNA was tested for purity and concentration (OD260/280 and OD260/230) with an ultra-micro spectrophotometer (NzanoDrop-1000, Thermo Fisher Scientific Co., Ltd., United States), and then 1% agarose gel electrophoresis was used.
Amplification was performed using bacterial V3-V4 variable region universal primers 341F (5′-CCTACGGGNGGCWGCAG-3′), 806R (5′-GGACTACHVGGGTWTCTAAT-3′). PCR used 2× Phanta Master Mix, 30 μl reaction system: 10 ng Genomic DNA, 15 μl 2 × Phanta Master Mix, 1 μl Bar-PCR primer F (10 μM), 1 μl Primer R (10 μM), ddH2O supplemented to 30 μl. Reaction program: initial denaturation at 95°C for 5 min; 27 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and elongation at 72°C for 45 s; stretch for 10 min at 72°C. After PCR amplification, the obtained products were detected by 2% gel electrophoresis. The PCR products with correct band size and appropriate concentration were sequenced on the computer. The sequencing company was Genepioneer Biotechnologies Co., Ltd (Nanjing, China) and the platform was Illumina miseq.
We used SPSS Statistics V20.0 Software to test the normal distribution and homogeneity of the data. Vitamin content (VA, VB2, VC and VE) and rumen fermentation parameters (pH, VFA, MCP and NH3-N) were tested in SPSS using independent samples T-test.
Species richness (Chao1 index) and diversity (Simpson and Shannon index) were calculated with the R language picante package and the difference test was performed with Mann–Whitney U nonparametric rank-sum test using IBM SPSS Statistics 20.0 software. Venn diagrams and Principal coordinate analysis (PCoA) diagrams were drawn through the ggplot2 package of the R language. At the same time, the similarity analysis (ANOSIM) calculated by the vegan package of R language shows the similarity between groups, where 0 = indistinguishable, 1 = identical. Determination of ANOSIM’s p-value based on 999 permutation tests. Canonical correlation analysis (CCA) was used to analyze the influence of environmental factors on microorganisms, which was performed through the vegan and ggrepel packages and visualized with ggplot2. Only those bacterial taxa with an abundance >0.1% in at least one sample were analyzed. KEGG microbial gene function prediction analysis was performed using PICRUSt according to previous protocol (Zhang et al., 2022). The Pearson correlations between differential flora and rumen fermentation parameters, as well as differential flora and differential metabolic pathways, were completed by the complot package in R language. When the correlation coefficient (R) was >|0.65| and the p-value was <0.05, the bacterial community and rumen fermentation parameters were considered to be significantly correlated (Wang et al., 2016).
In Table 2, the contents of VB2 and VC in the mixed silage group were higher than those in the control group (p < 0.05), and there was no difference in the contents of VA and VE (p > 0.05).
It can be seen from Figure 1 that the mixed silage did not affect the DMI (Figure 1A), ADG (Figure 1B) and F/W (Figure 1C) of Hu sheep (p > 0.05).
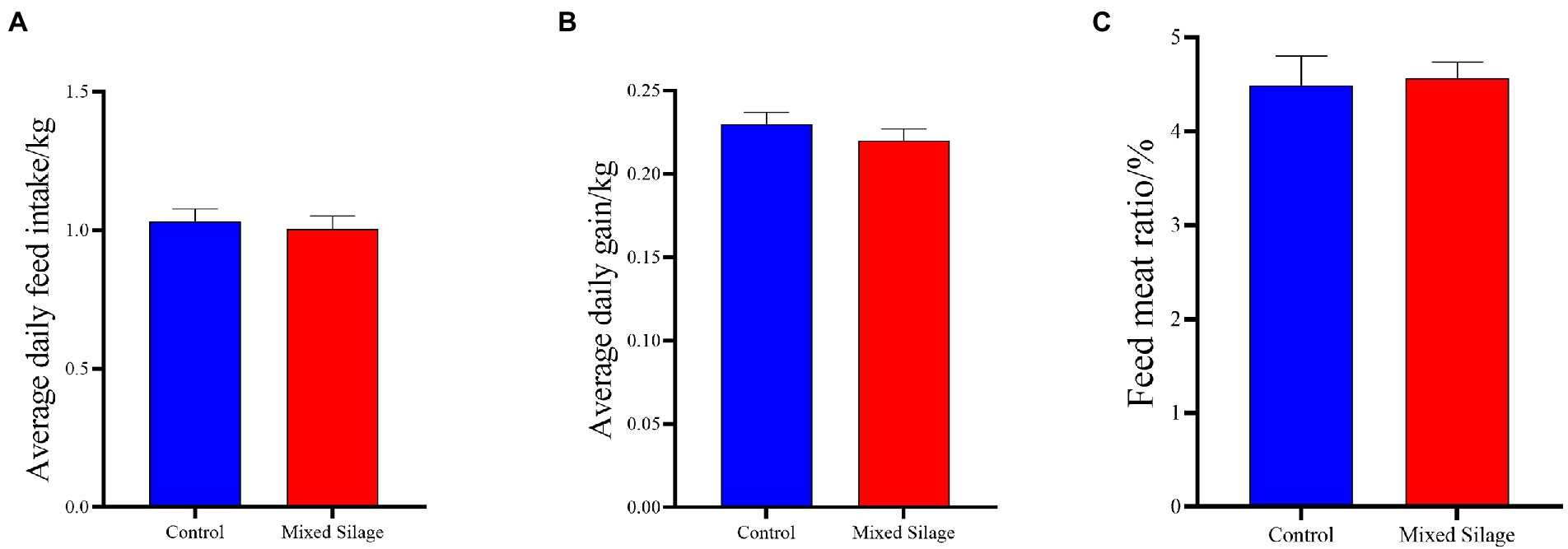
Figure 1. Effects of the mixed silage on growth performance of Hu sheep. (A) Average daily feed intake; (B) Average daily gain; (C) Feed meat ratio. Control: Based on peanut seedling, corn husk and sorghum shell for roughage in the diet; Mixed Silage: Based on the mixed silage for roughage in the diet.
Figure 2 shows the comparison of antioxidant properties of blood and organs between mixed silage group and control group. Compared with the control group, the mixed silage increased the concentration of T-AOC in serum and SOD in kidney (p < 0.05). In addition, the content of MDA in serum, liver and kidney decreased (p < 0.05). The effects of the mixed silage on intestinal antioxidant performance of Hu sheep is shown in Figure 3. The mixed silage could increase the content of T-AOC and CAT in rumen and duodenum. In addition, it could reduce the content of MDA in rumen (p < 0.05). The results showed (Table 3) that the content of MDA in the mixed silage group was lower (p < 0.05), while the antioxidant indexes such as T-AOC, SOD, GSH-Px, and CAT were higher than those in the control groups with no differences (p > 0.05).
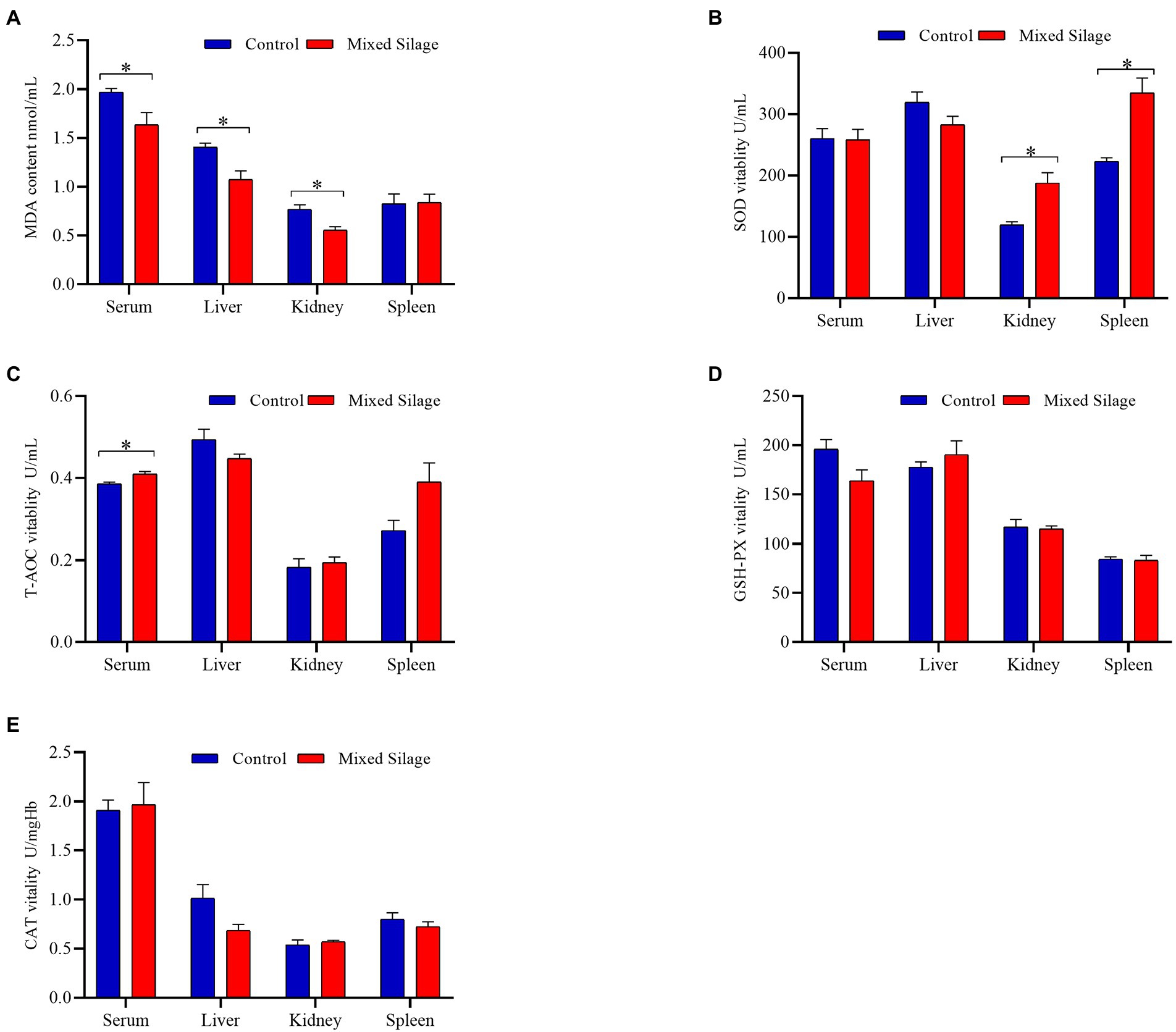
Figure 2. Effects of the mixed silage on antioxidant capacity of Hu sheep. (A) MDA content. (B) SOD vitality. (C) T-AOC vitality. (D) GSH-PX vitality. (E) CAT vitality. Control: Based on peanut seedling, corn husk and sorghum shell for roughage in the diet; Mixed Silage: Based on the mixed silage for roughage in the diet. “*” means indicates a significant difference (p < 0.05). No “*” indicates that the difference is not significant (p > 0.05). The same as below.
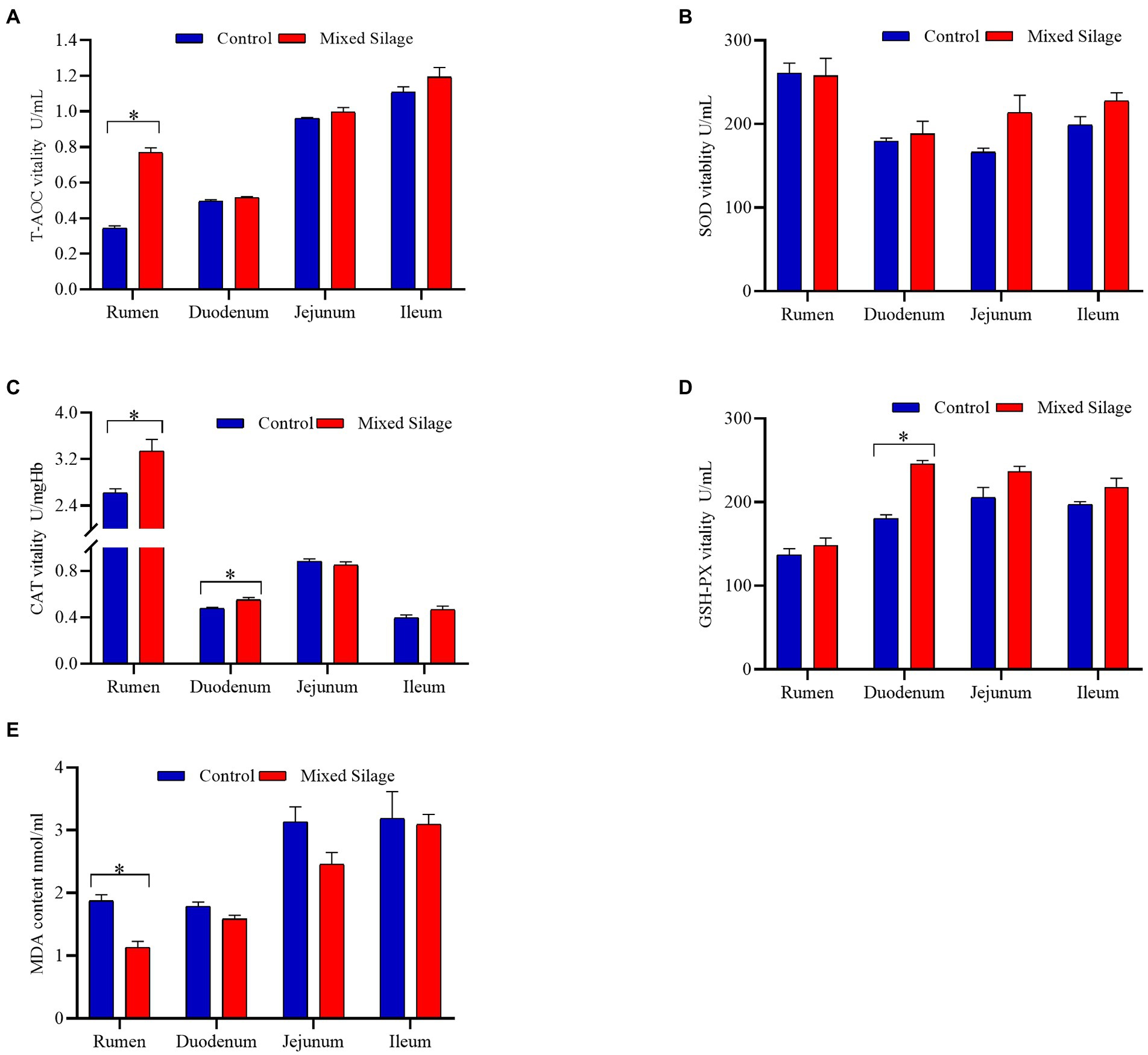
Figure 3. Effects of the mixed silage on antioxidant capacity in the digestive tract of fattening Hu sheep. (A) T-AOC vitality. (B) SOD vitality. (C) CAT vitality. (D) GSH-PX vitality. (E) MDA content. Control: Based on peanut seedling, corn husk and sorghum shell for roughage in the diet; Mixed Silage: Based on the mixed silage for roughage in the diet.
The results in the Table 4 showed that the mixed silage could reduce the pH and the concentrations of isovalerate and isobutyrate (p < 0.05). In addition, it increased the concentrations of TVFA, NH3-N, butyrate, and valerate in rumen fluid (p < 0.05).
The results in the Supplementary Table 1 showed that the OTU, Chao1 index, Shannon index and Simpson index of the silage group were higher than those of the control group (p < 0.05). It can be seen from the species accumulation curve (Supplementary Figure 1A) that when the number of samples taken reaches 8, the detection rate of new species is further slowed down, and the species accumulation curve becomes flat (close to the horizontal state), indicating that adding new sequencing samples was important for the discovery of rumen. The contribution of new microbial species was getting smaller and smaller, so the sequencing samples in this experiment can completely cover most of the microorganisms in the rumen environment of Hu sheep. Clustering of sequences with 97% similarity revealed that the two groups shared 1,187 OTUs, 348 were shared by the silage group, and 172 were exclusively shared by the control group (Supplementary Figure 1B). PCoA based on Bray–Curtis distance was drawn for comparison of the two treatments. As it can be seen from Figure 4A that the samples of the mixed silage group were completely separated from the samples of the control group. The results of ANOSIM analysis showed that mixed silage could affect the rumen microflora structure of the fattening Hu sheep (R = 0.536, p = 0.011).
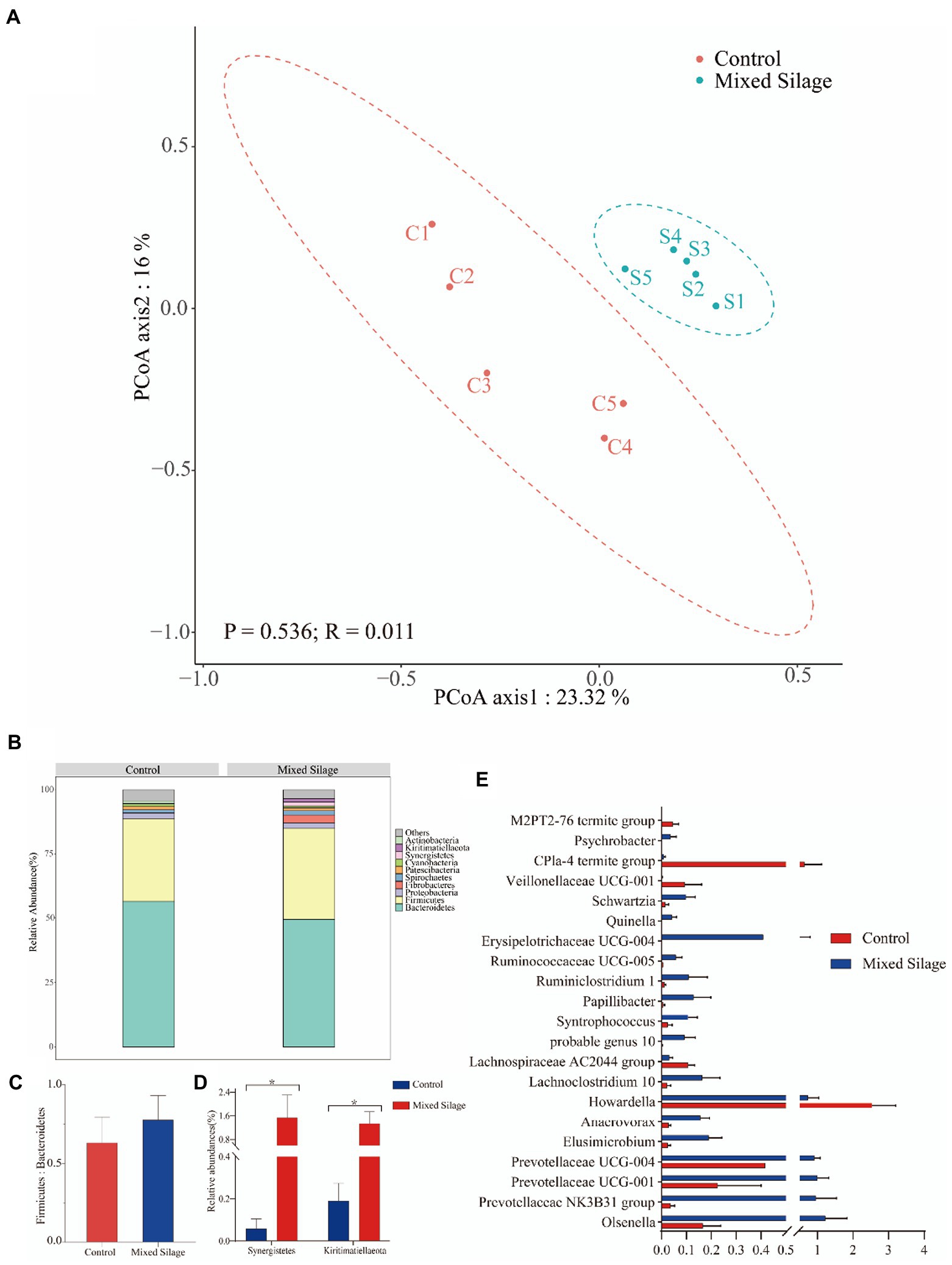
Figure 4. Structure of rumen bacterial flora in Hu Sheep. (A) Principal coordinate analysis (PCoA) of the bacterial community structure of ruminal microbiota in the control and mixed silage groups. (B) The composition of the top 10 phyla in rumen fluid. (C) Ratio of the proportion of phyla Firmicutes to the proportion of Bacteroidetes. (D) The phyla with the difference in the top 10 phyla. (E) The proportion of the bacterial genus with significant differences among the two groups. Generas with a proportion >0.1% in any one sample will be analyzed. Control: Based on peanut seedling, corn husk and sorghum shell for roughage in the diet; Mixed Silage: Based on the mixed silage for roughage in the diet. PCoA plots were constructed using Bray–Curits distance.
At the phyla level, Bacteroidetes and Firmicumtes were the dominant phyla in both the mixed silage group and the control group (Figure 4B). Furthermore, the ratio of Firmicutes to Bacteroidetes was not different between the two groups (p > 0.05; Figure 4C). Compared with the control group, the mixed silage group increased the proportion of Synergistetes and Kiritimatiellaeota in rumen fluid (p < 0.05; Figure 4D). At the genera level, 21 genera (relative abundance of more than 0.1%) were different between the two groups (p < 0.05; Figure 4E). Compared with the control group, the mixed silage group increased the proportion of Olsenella, Prevotellaceae NK3B31 group, Prevotellaceae UCG-001, Prevotellaceae UCG-004, Elusimicrobium, Anaerovorax, Lachnoclostridium 10, probable genus 10, Papillibacter, Syntrophococcus, Erysipelotrichaceae, Ruminiclostridium 1, Ruminococcaceae UCG-005, Quinella, Schwartzia, Veillonellaceae UCG 001 and Psychrobacter (p < 0.05), but decreased the proportion of Howardella, Lachnospiraceae AC2044 group, Veillonellaceae UCG 001, CPla 4 termite group and M2PT2 76 termite group relative abundance (p < 0.05).
The relationship between the rumen bacterial community and rumen fermentation parameters were analyzed by Canonical correspondence analysis (CCA). As shown in Figure 5A, the relationship between the rumen microflora and isovalerate (p = 0.002), NH3-N (p = 0.003), butyrate (p = 0.021), pH (p = 0.022), valerate (p = 0.048) and TVFA (p = 0.064) contributed to the flora.
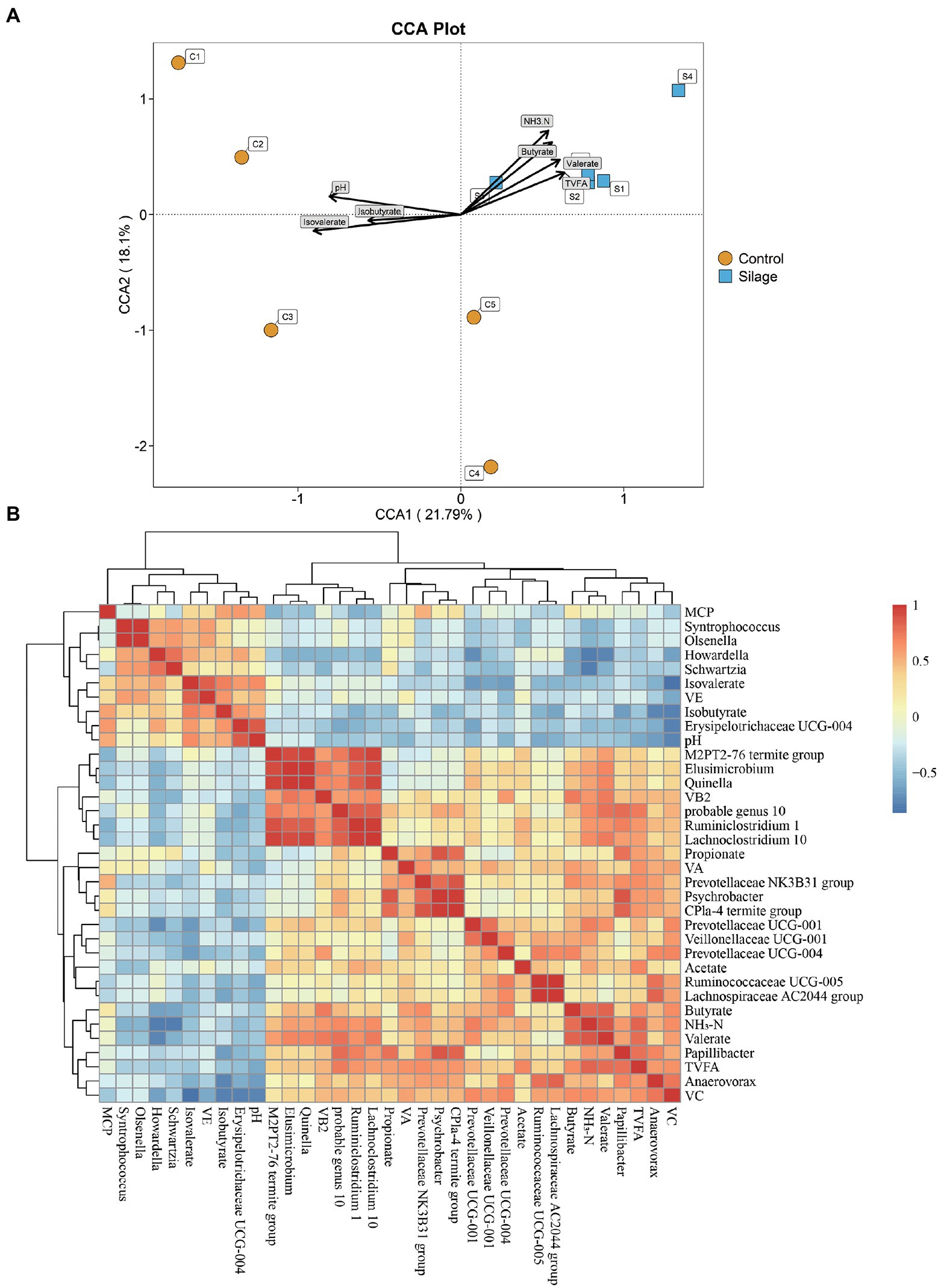
Figure 5. Correlation results between rumen microbiota structure and fermentation parameters or vitamins. (A) CCA to prove the associations between the rumen fermentation parameters and the bacterial community structure. The length of an arrow expresses the relative influence of its corresponding rumen fermentation parameters on the distribution of the bacterial community analyzed. Centroid is displayed for each treatment. The samples are represented by circles and square symbols, respectively. (B) Correlation analyses between proportion of differential bacteria genera and ruminal fermentation parameters and vitamins.
As shown in Figure 5B, there was a negative correlation between pH and Anaerovorax, and a positive correlation with Erysipelotrichaceae UCG-004 (p < 0.05). NH3-N showed a positive correlation with Prevotellaceae UCG-001, Lachnoclostridium 10 and probable genus 10, but a negative correlation with Schwartzia and Howardella (p < 0.05). There was a positive correlation between TVFA and Prevotellaceae NK3B31 group, Papillibacter and probable genus 10 (p < 0.05). Propionate showed a positive correlation with Psychrobacter, Papillibacte and CPla-4 termite group (p < 0.05). Isobutyrate showed a negative correlation with Anaerovorax and Papillibacter. Butyrate showed a positive correlation with Prevotellaceae NK3B31 and Prevotellaceae UCG 004 (p < 0.05). Valerate showed a positive correlation with Quinella, Elusimicrobium, Lachnoclostridium 10 and probable genus 10 (p < 0.05). VB2 was positively correlated with Elusimicrobium, Quinella, and Lachnoclostridium 10 (p < 0.05). VC was positively correlated with Anaerovorax, Prevotellaceae UCG-004 and Prevotellaceae UCG-001, but negatively correlated with Erysipelotrichaceae UCG-004 (p < 0.05).
There were 10 significantly different metabolic pathways in the microbiota prediction function between the mixed silage group and the control group (Figure 6A). The mixed silage up-regulated epithelial cell signaling in helicobacter pylori infection, phosphotransferase system (PTS), primary immunodeficiency and ascorbate and aldarate metabolism (p < 0.05), while downregulated aminobenzoate degradation, ethylbenzene degradation, glycolysis/gluconeogenesis, lysine degradation, propanoate metabolism and spliceosome metabolic pathways (p < 0.05).
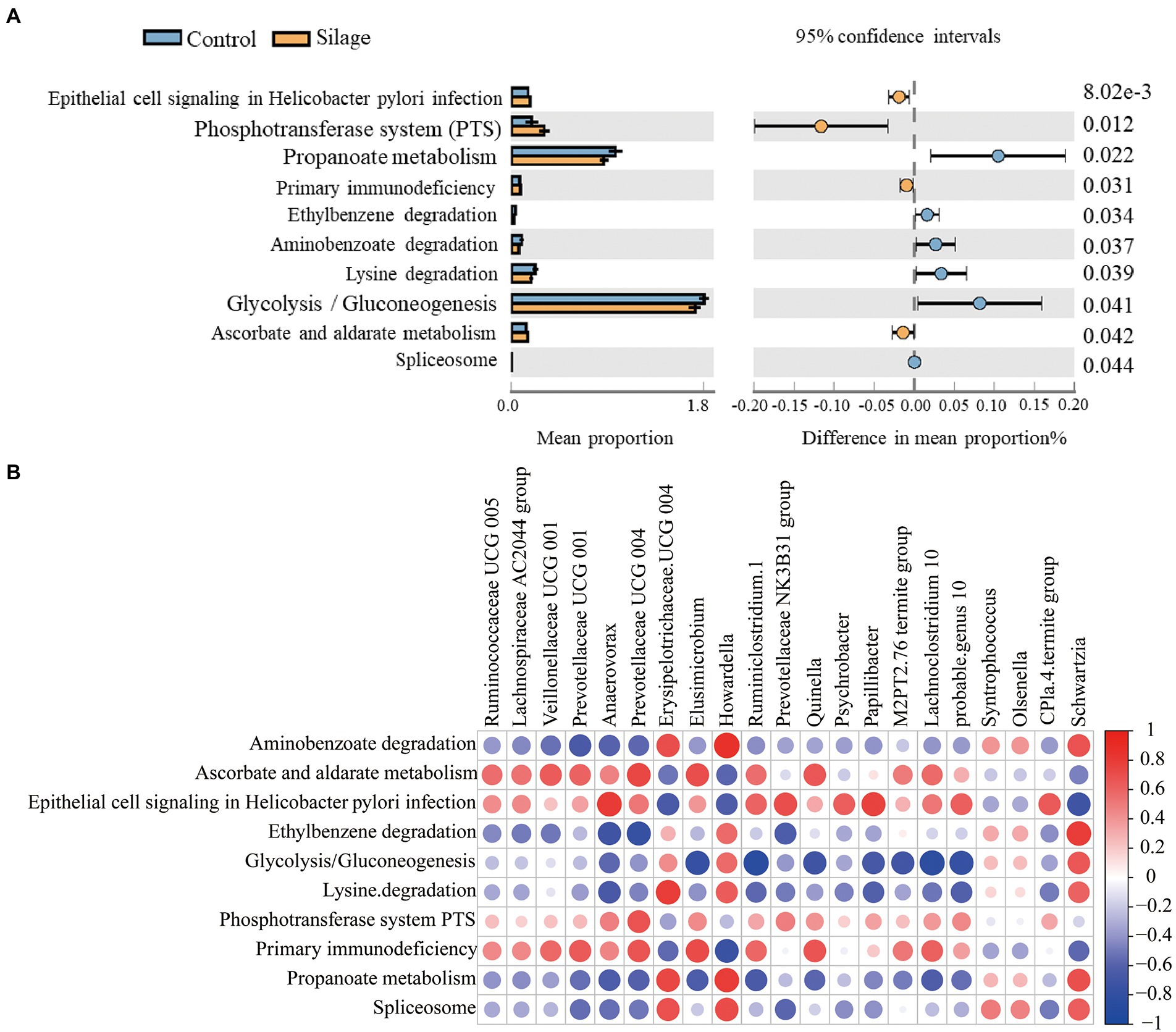
Figure 6. Prediction of metabolic pathways and their correlation with rumen microbes. (A) Analysis of differences in functional prediction pathways of rumen flora between the control group and the mixed silage group. (B) Correlation analysis between differential microorganisms and differential pathways. In this graph, each row represents differential metabolic pathways and each column represents differential microorganisms at the genera level. Red circles indicate positive correlations, and blue circles indicate negative correlations.
As shown in Figure 6B, aminobenzoate degradation had a positive correlation with Erysipelotrichaceae UCG-004, Howardella and Schwartzia, and a negative correlation with Prevotellaceae UCG-001 (p < 0.05). Ascorbate and aldarate metabolism were positively correlated with Veillonellaceae UCG-001, Prevotellaceae UCG-004, Elusimicrobium and Quinella (p < 0.05). Epithelial cell signaling in helicobacter pylori infection was positively correlated with Anaerovorax, Prevotellaceae NK3B31 group, Papillibacter and CPla-4 termite group, while negatively correlated with Erysipelotrichaceae UCG-004 and Schwartzia (p < 0.05). Ethylbenzene degradation showed a positive correlation with Schwartzia and a negative correlation with Anaerovorax and Prevotellaceae UCG-004 (p < 0.05). Glycolysis/gluconeogenesis showed a positive correlation with Schwartzia, and a negative correlation with Elusimicrobium, Ruminiclostridium 1, Quinella, Papillibacter, M2PT2–76 termite group, Lachnoclostridium 10, and probable genus 10 (p < 0.05). Lysine degradation had a positive correlation with Erysipelotrichaceae UCG-004 and Howardella, while a negative correlation with Anaerovorax (p < 0.05). Phosphotransferase system (PTS) had a positive correlation with Prevotellaceae UCG-004 (p < 0.05). Primary immunodeficiency was positively correlated with Prevotellaceae UCG-001, Prevotellaceae UCG-004 and Elusimicrobium, but negatively correlated with Quinella (p < 0.05). Spliceosome had a positive correlation with Erysipelotrichaceae UCG-004 and Howardella (p < 0.05). In addition, propanoate metabolism was found negatively correlated with Ruminiclostridium 1 (p < 0.05).
The production, utilization and elimination of free radicals in the body are in a dynamic balance. When this balance is broken, the body produces oxidative stress, the reactive oxygen species cause lipid peroxidation, giving rise MDA as a final product (Honaramooz et al., 2011). The complex antioxidant system in the body is influenced by dietary intake of antioxidants (taurine, etc.), vitamins (VC and VE), and minerals (Zn etc.; Agarwal and Gupta, 2005). SOD is an intracellular antioxidant enzyme that protects against oxidative stress (Bhatia et al., 2003). CAT exists in red blood cells and peroxides in certain tissues, and can make H2O2 react to generate water and oxygen molecules (Zhao et al., 2017). Serum T-AOC is primarily considered to be a proxy for the balance between oxidative and antioxidant compounds in the body, and it provides more biologically relevant information than any other individual antioxidants (Ghiselli et al., 2000). The results showed that T-AOC in serum and rumen of the mixed silage group were significantly higher than those in the control group. SOD activity in the kidney was observed significantly higher, while CAT activity in the rumen and duodenum was seen higher significantly. In addition, MDA content in the muscle, serum, liver, kidney and rumen of the mixed silage group was significantly lower than that of the control group. This may be caused by the higher content of VC in the mixed silage. Sahoo et al. (2021) results showed that the addition of fresh fruit and vegetable tails to the diet increased the ability of T-AOC in the blood of ewes. VC can be used to maintain the oxidation state of cells and remove other oxidizing substances, so as to improve the T-AOC of cells (Sordillo and Aitken, 2009). A study by Seifzadeh et al. (2022) also showed that by adding 20 mg of VC to prenatal dairy cow diets significantly reduced blood MDA concentration. By adding 50 mg/ml VC to drinking water can increase the level of T-AOC in the myocardium and serum of chickens, and improves its antioxidant capacity (Yin et al., 2020). In addition, by adding VC to the diet can also increase the activity of MnSOD (a kind of SOD) in chicken liver and kidney (Ozturk-Urek et al., 2001).
Partovi et al. (2020) fed Fasander lambs with a mixed diet of broccoli by-products and wheat straw (ratio 69:31) and found that the mixed diet did not affect the rumen fermentation of lambs. In our experiment, the mixed silage increased the concentrations of TVFA, NH3-N and butyric acid in the rumen fluid, while the pH of the rumen fluid decreased. The normal range of rumen pH is 5.5–7.0, but it may also reach to 7.5 (Kim et al., 2012; Anantasook et al., 2013). The pH of the rumen in the two groups of diets was within the normal range, whereas the pH of the mixed silage group was lower than that of the control group, which may be caused by the increase of TVFA. The NH3-N in the rumen originates from the degradation of endogenous nitrogenous substances or exogenous nitrogenous substances, and is also the main substance for the synthesis of MCP, which can reflect the utilization of nitrogenous substances by rumen microorganisms to a certain extent. All animals in both groups had NH3-N concentrations in the rumen above 5 mg/dl, which is the minimum concentration required to ensure optimal growth of rumen microorganisms (Satter and Slyter, 1974). The differences in NH3-N in the two groups of diets indicated that the mixed silage could affect the release of N in the rumen and the efficiency of MCP synthesis after being absorbed by microorganisms. The increase of VFA content in the mixed silage group may be caused by the increase of rumen microbial reproduction and activity. VFAs which account for 75% of the total energy requirements provided by ruminants, are the main product of rumen microbial fermentation, and are critical to the overall metabolism of ruminants (Bergman, 1990; Dijkstra, 1994). Butyrate can effectively stimulate the proliferation and growth of the rumen epithelium (Gorka et al., 2018). The increase of butyric acid in the mixed silage group in this experiment may be due to the increase of butyric acid-producing bacteria in the rumen.
The rumen microbes are complex and diverse, and play an important role in the digestion of nutrients and the functioning of the rumen. Diet type, structure and feeding method can significantly affect the species and quantity of ruminant microorganisms in ruminants (van Lingen et al., 2016). The mixed silage increased the species diversity and richness of rumen microorganisms, which may be due to the higher metabolizable and digestible energies of the silage group, promoted the growth and reproduction of microorganisms, and improved the richness and diversity of microorganisms. The results of PCoA and ANOSIM clearly showed that the mixed silage significantly improved the rumen microbial composition. Additionally, CCA analysis showed that among the different rumen fermentation parameters, isovalerate, NH3-N, butyrate, pH, valerate and TVFA had the greatest influence on rumen microorganisms.
The ecosystem of rumen microorganisms is dominated by its core flora which is not affected by the nutrients from feed sources (Petri et al., 2012; Henderson et al., 2015). Bacteroidea and Firmicutes are two dominant microflora in the structure of rumen microbial flora (Singh et al., 2012; Pitta et al., 2014). Previous studies have shown that the main function of Bacteroidea members is to hydrolyze proteins and degrade carbohydrates, while Firmicutes members are mainly involved in energy utilization (Wu et al., 2011; Chen et al., 2015). The ratio of Firmicutes to Bacteroidetes is generally considered to be related to the metabolic potential of the intestinal microbiota and can regulate the metabolism, physiology and health of the host (Tilg and Kaser, 2011; Clemente et al., 2012). Our results showed that the mixed silage did not have a significant effect on Firmicutes, Bacteroidetes or their ratios, which is consistent with previous studies (Zhang et al., 2014). Notably, at the level of the top 10 phyla in relative abundance, the mixed silage increased the proportion of Synergistetes and Kiritimatiellaeota. Synergistetes is part of the gut microbiota of healthy animals and can degrade amino acids (Leser et al., 2002; Menes and Muxi, 2002; Godon et al., 2005). Studies have pointed out that Synergistetes isolated from sheep rumen can not only utilize arginine and histidine to improve nitrogen utilization, but also degrade toxins present in feed (McSweeny et al., 1993; Jumas-Bilak et al., 2009). Kiritimatiellaeota is a supergroup of the planktonic Chlamydia verrucobacterium (PVC) whose primary function is to break down complex polysaccharides and glycoproteins (Spring et al., 2016; Mu et al., 2020).
The experimental results showed that the proportions of Prevotellaceae NK3B31 group, Prevotellaceae UCG-001 and Prevotellaceae UCG-004 were increased in the mixed silage group. Prevotellaceae UCG-001, Prevotellaceae UCG-004 and Prevotellaceae NK3B31 group, all belong to the Prevotellaceae family. The results of association analysis showed that Prevotellaceae UCG-004 was positively correlated with Vc, ascorbate and aldarate metabolism. In mammals, VC is abundant and an effective water-soluble antioxidant for reducing oxidative stress, but its impact on the rumen ecology is unknown (Ghanem et al., 2008; Matsui, 2012). The use of antioxidants to improve animal health and product quality has received increasing attention (Schelling et al., 1995). The basic physiological needs of the organism are thought to be met by L-ascorbic acid (or VC) generated in the liver of ruminants (Ranjan et al., 2012). Compared to other animals, ruminants may require vitamin supplementation during feeding because microbes in the rumen completely destroy VC in the feed (Kim et al., 2012; Ranjan et al., 2012). Another in vitro study has shown that high doses of VC have a certain positive effect on rumen microorganisms (Tagliapietra et al., 2013). Therefore, it is speculated that the higher VC content in the mixed silage group could increase the proportion of Prevotellaceae UCG-004, but it remains to be studied. Prevotellaceae is positively correlated with the content of ADF and NDF in the diet (An et al., 2020). In this experiment, the ADF content in the mixed silage group was higher, which may be another reason for the increase in the relative abundance of Prevotellaceae UCG-004. Prevotellaceae UCG-004 can ferment carbohydrates and produce SCFAs including acetate and butyrate. The process of butyrate metabolism is linked to the interconversion of “pentose and glucuronic acid,” and the “ascorbate and aldarate metabolism” through dehydrogenases together (Heinritz et al., 2016; Shi et al., 2020).
In summary, the mixed silage could improve rumen fermentation and enhance the antioxidant capacity of Hu sheep. In this experiment, the mixed silage also increased the concentrations of TVFA, NH3-N and butyric acid in the rumen, whereas the pH of the rumen fluid was reduced. In addition, the mixed silage increased the activity of T-AOC in blood, SOD in liver and kidney, and decreased the content of MDA in blood. The improvement of the antioxidant capacity of Hu sheep may be directly caused by the higher VC content in the mixed silage, or it may be caused by the up-regulation of ascorbate and aldarate metabolism in Hu sheep. The upregulation of ascorbate and aldarate metabolism may be related to the increased proportion of Prevotellaceae UCG-004 caused by higher VC or ADF content in the mixed silage.
The datasets provided in this study can be found in the online repository. The name and login number of the repository are as follows: NCBI SRA Bioproject, login number: PRJNA795847.
The animal study was reviewed and approved by Yangzhou Veterinary Animal Welfare Committee of the Ministry of agriculture of China [Yangzhou, China, license No. syxk (Su) 2019-0029]. Written informed consent was obtained from the owners for the participation of their animals in this study.
MW and CL designed this study and wrote the manuscript of this study. CL, XZ, RQ, ZZ, ZL, YL, MZ, and XY conducted experiments and collected samples. Animal data were analyzed by CL, MZ, XZ, ZZ, YL, NC, and ZL. CL, KS, NC, WL, and MW performed bioinformatics analysis and visualization of data and draft manuscripts. All authors contributed to the article and approved the submitted version.
This research was funded by the National 14th and 13th Five-Year Plan Key Research and Development Program (2021YFD1600702, 2018YFD0502100, 2017YFD0800200), the key program of State Key Laboratory of Sheep Genetic Improvement and Healthy Production (2021ZD07, NCG202232), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China.
The author hereby expresses his gratitude to the Siyang County Huifeng Goat Breeding Professional Cooperative (Suqian, Jiangsu) for the Hu sheep and breeding sites provided.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.978940/full#supplementary-material
Abd El Tawab, A. M., Kholif, A. E., Hassan, A. M., Matloup, O. H., El-Nor, S. A. A., Olafadehan, O. A., et al. (2020). Feed utilization and lactational performance of Friesian cows fed beet tops silage treated with lactic acid bacteria as a replacement for corn silage. Anim. Biotechnol. 31, 473–482. doi: 10.1080/10495398.2019.1622556
Agarwal, A., and Gupta, S. (2005). Role of reactive oxygen species in female reproduction. Part I. oxidative stress: a general overview. Agro Food Ind. Hi Tech 16, 21–25.
An, X. J., Zhang, L. Y., Luo, J., Zhao, S. G., and Jiao, T. (2020). Effects of oat Hay content in diets on nutrient metabolism and the rumen microflora in sheep. Animals 10:2341. doi: 10.3390/ani10122341
Anantasook, N., Wanapat, M., Cherdthong, A., and Gunun, P. (2013). Effect of plants containing secondary compounds with palm oil on feed intake, digestibility, microbial protein synthesis and microbial population in dairy cows. Asian-Australas J. Anim. Sci. 26, 820–826. doi: 10.5713/ajas.2012.12689
Bampidis, V. A., and Robinson, P. H. (2006). Citrus by-products as ruminant feeds: a review. Anim. Feed Sci. Tech. 128, 175–217. doi: 10.1016/j.anifeedsci.2005.12.002
Bergman, E. (1990). Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70, 567–590. doi: 10.1152/physrev.1990.70.2.567
Bhatia, S., Shukla, R., Madhu, S. V., Gambhir, J. K., and Prabhu, K. M. (2003). Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 36, 557–562. doi: 10.1016/S0009-9120(03)00094-8
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Chen, X. G. (2016). Economic potential of biomass supply from crop residues in China. Appl. Energy 166, 141–149. doi: 10.1016/j.apenergy.2016.01.034
Chen, Y.-B., Lan, D.-L., Tang, C., Yang, X.-N., and Li, J. (2015). Effect of DNA extraction methods on the apparent structure of yak rumen microbial communities as revealed by 16S rDNA sequencing. Pol. J. Microbiol. 64, 29–36. doi: 10.33073/pjm-2015-004
Cheong, S. H., Kim, K. H., Jeon, B. T., Park, P. J., Hwang, I. H., Choi, N. J., et al. (2012). Effect of mulberry silage supplementation during late fattening stage of Hanwoo (Bos taurus coreanae) steer on antioxidative enzyme activity within the longissimus muscle. Anim. Prod. Sci. 52, 240–247. doi: 10.1071/AN11087
Cherdthong, A., Suntara, C., and Khota, W. (2020). Lactobacillus casei TH14 and additives could modulate the quality, gas kinetics and the in vitro digestibility of ensilaged rice straw. J. Anim. Physiol. Anim. Nutr. (Berl) 104, 1690–1703. doi: 10.1111/jpn.13426
Clemente, J. C., Ursell, L. K., Parfrey, L. W., and Knight, R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270. doi: 10.1016/j.cell.2012.01.035
Cotta, M. A., and Russell, J. B. (1982). Effect of peptides and amino acids on efficiency of rumen bacterial protein synthesis in continuous culture. J. Dairy Sci. 65, 226–234. doi: 10.3168/jds.S0022-0302(82)82181-4
Dijkstra, J. (1994). Production and absorption of volatile fatty acids in the rumen. Livest. Sci. 39, 61–69. doi: 10.1016/0301-6226(94)90154-6
FAOSTAT (2016). Food and Agriculture Data (FAO, 2016). Available at: http://www.fao.org/faostat/en/#home
Ghanem, A. M., Jaber, L. S., Said, M. A., Barbour, E. K., and Hamadeh, S. K. (2008). Physiological and chemical responses in water-deprived Awassi ewes treated with vitamin C. J. Arid Environ. 72, 141–149. doi: 10.1016/j.jaridenv.2007.06.005
Ghiselli, A., Serafini, M., Natella, F., and Scaccini, C. (2000). Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic. Biol. Med. 29, 1106–1114. doi: 10.1016/S0891-5849(00)00394-4
Godon, J. J., Moriniere, J., Moletta, M., Gaillac, M., Bru, V., and Delgenes, J. P. (2005). Rarity associated with specific ecological niches in the bacterial world: the 'Synergistes' example. Environ. Microbiol. 7, 213–224. doi: 10.1111/j.1462-2920.2004.00693.x
Gorka, P., Kowalski, Z. M., Zabielski, R., and Guilloteau, P. (2018). Invited review: use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 101, 4785–4800. doi: 10.3168/jds.2017-14086
Hassanat, F., Gervais, R., Julien, C., Masse, D. I., Lettat, A., Chouinard, P. Y., et al. (2013). Replacing alfalfa silage with corn silage in dairy cow diets: effects on enteric methane production, ruminal fermentation, digestion, N balance, and milk production. J. Dairy Sci. 96, 4553–4567. doi: 10.3168/jds.2012-6480
Heinritz, S. N., Weiss, E., Eklund, M., Aumiller, T., Louis, S., Rings, A., et al. (2016). Intestinal microbiota and microbial metabolites are changed in a pig model fed a high-fat/low-fiber or a low-fat/high-fiber diet. PLoS One 11:e0154329. doi: 10.1371/journal.pone.0154329
Henderson, G., Cox, F., Ganesh, S., Jonker, A., Young, W., Janssen, P. H., et al. (2015). Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep.-UK 5, 1–15. doi: 10.1038/srep14567
Hillion, M. L., Moscoviz, R., Trably, E., Leblanc, Y., Bernet, N., Torrijos, M., et al. (2018). Co-ensiling as a new technique for long-term storage of agro-industrial waste with low sugar content prior to anaerobic digestion. Waste Manag. 71, 147–155. doi: 10.1016/j.wasman.2017.10.024
Honaramooz, A., Schlatt, S., Orwig, K., and Kim, N. H. (2011). Recent advances in reproductive technologies. Vet. Med. Int. 2011:915031. doi: 10.4061/2011/915031
Jumas-Bilak, E., Roudiere, L., and Marchandin, H. (2009). Description of 'Synergistetes' phyl. Nov and emended description of the phylum 'Deferribacteres' and of the family Syntrophomonadaceae, phylum 'Firmicutes'. Int. J. Syst. Evol. Microbiol. 59, 1028–1035. doi: 10.1099/ijs.0.006718-0
Kim, J. H., Mamuad, L. L., Yang, C. J., Kim, S. H., Ha, J. K., Lee, W. S., et al. (2012). Hemato-biochemical and cortisol profile of Holstein growing-calves supplemented with vitamin C during summer season. Asian-Australas J. Anim. Sci. 25, 361–368. doi: 10.5713/ajas.2011.11438
Leser, T. D., Amenuvor, J. Z., Jensen, T. K., Lindecrona, R. H., Boye, M., and Moller, K. (2002). Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68, 673–690. doi: 10.1128/AEM.68.2.673-690.2002
Liu, B. Y., Huan, H. L., Gu, H. R., Xu, N. X., Shen, Q., and Ding, C. L. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Ma, Y., Yin, Y., and Liu, Y. (2017). A holistic approach for food waste management towards zero-solid disposal and energy/resource recovery. Bioresour. Technol. 228, 56–61. doi: 10.1016/j.biortech.2016.12.090
Makkar, H. P., and Ankers, P. (2014). A need for generating sound quantitative data at national levels for feed-efficient animal production1. Anim. Prod. Sci. 54, 1569–1574. doi: 10.1071/AN14377
Matsui, T. (2012). Vitamin C nutrition in cattle. Asian-Australas J. Anim. Sci. 25, 597–605. doi: 10.5713/ajas.2012.r.01
McSweeny, C. S., Allison, M. J., and Mackie, R. I. (1993). Amino acid utilization by the ruminal bacterium Synergistes jonesii strain 78-1. Arch. Microbiol. 159, 131–135. doi: 10.1007/BF00250272
Menes, R. J., and Muxi, L. (2002). Anaerobaculum mobile sp nov., a novel anaerobic, moderately thermophilic, peptide-fermenting bacterium that uses crotonate as an electron acceptor, and emended description of the genus Anaerobaculum. Int. J. Syst. Evol. Microbiol. 52, 157–164. doi: 10.1099/00207713-52-1-157
Mu, D. S., Zhou, L. Y., Liang, Q. Y., Chen, G. J., and Du, Z. J. (2020). Tichowtungia aerotolerans gen. Nov., sp. nov., a novel representative of the phylum Kiritimatiellaeota and proposal of Tichowtungiaceae fam. Nov., Tichowtungiales Ord. Nov. and Tichowtungiia class. Nov. Int. J. Syst. Evol. Microbiol. 70, 5001–5011. doi: 10.1099/ijsem.0.004370
National Research Council (NRC). (2007). Nutrient Requirements of Small Ruminants, Sheep, Goats, Cervids, and New World Camelids. Washington, DC: National Academy Press.
Ozturk-Urek, R., Bozkaya, L. A., and Tarhan, L. (2001). The effects of some antioxidant vitamin- and trace element-supplemented diets on activities of SOD, CAT, GSH-Px and LPO levels in chicken tissues. Cell Biochem. Funct. 19, 125–132. doi: 10.1002/cbf.905
Palma-Hidalgo, J. M., Jiménez, E., Popova, M., Morgavi, D. P., Martín-García, A. I., Yáñez-Ruiz, D. R., et al. (2021). Inoculation with rumen fluid in early life accelerates the rumen microbial development and favours the weaning process in goats. Anim. Microbiome. 3, 1–21. doi: 10.1186/s42523-021-00073-9
Pan, X., Yang, L., Xue, F., Xin, H., Jiang, L., Xiong, B., et al. (2016). Relationship between thiamine and subacute ruminal acidosis induced by a high-grain diet in dairy cows. J. Dairy Sci. 99, 8790–8801. doi: 10.3168/jds.2016-10865
Partovi, E., Rouzbehan, Y., Fazaeli, H., and Rezaei, J. (2020). Broccoli byproduct-wheat straw silage as a feed resource for fattening lambs. Transl. Anim. Sci. 4:txaa078. doi: 10.1093/tas/txaa078
Petri, R. M., Forster, R. J., Yang, W., McKinnon, J. J., and McAllister, T. A. (2012). Characterization of rumen bacterial diversity and fermentation parameters in concentrate fed cattle with and without forage. J. Appl. Microbiol. 112, 1152–1162. doi: 10.1111/j.1365-2672.2012.05295.x
Pitta, D. W., Kumar, S., Vecchiarelli, B., Shirley, D. J., Bittinger, K., Baker, L. D., et al. (2014). Temporal dynamics in the ruminal microbiome of dairy cows during the transition period. J. Anim. Sci. 92, 4014–4022. doi: 10.2527/jas.2014-7621
Qiao, Y. Y., Xu, F. F., Ming, X., Feng, S. W., Ji, Y. Y., Jiang, Y., et al. (2020). Valorization of vegetable waste via pyrolysis: thermal behavior, volatiles release, and products analysis from its extractives. Energy Fuel 34, 1896–1907. doi: 10.1021/acs.energyfuels.9b03970
Ranjan, R., Ranjan, A., Dhaliwal, G. S., and Patra, R. C. (2012). L-ascorbic acid (vitamin C) supplementation to optimize health and reproduction in cattle. Vet. Q. 32, 145–150. doi: 10.1080/01652176.2012.734640
Ren, H., Wang, C., Fan, W., Zhang, B., Li, Z., and Li, D. (2018). Effects of formic acid or acetic acid on the mixed-storage quality of air-dried maize Stover and cabbage wastes and microbial community analysis. Food Technol. Biotechnol. 56, 71–82. doi: 10.17113/ftb.56.01.18.5455
Sahoo, A., Sarkar, S., Lal, B., Kumawat, P., Sharma, S., and De, K. (2021). Utilization of fruit and vegetable waste as an alternative feed resource for sustainable and eco-friendly sheep farming. Waste Manag. 128, 232–242. doi: 10.1016/j.wasman.2021.04.050
Satter, L. D., and Slyter, L. L. (1974). Effect of ammonia concentration of rumen microbial protein production in vitro. Br. J. Nutr. 32, 199–208. doi: 10.1079/bjn19740073
Schelling, G. T., Roeder, R. A., Garber, M. J., and Pumfrey, W. M. (1995). Bioavailability and interaction of vitamin A and vitamin E in ruminants. J. Nutr. 125, 1799S–1803S. doi: 10.1093/jn/125.suppl_6.1799S
Seifzadeh, S., Seifdavati, J., Abdi-Benemar, H., Salem, A. Z. M., Sharifi, R. S., and Elghandour, M. M. M. Y. (2022). Dietary vitamin C in pre-parturient dairy cows and their calves: blood metabolites, copper, zinc, iron, and vitamin C concentrations, and calves growth performance. Trop. Anim. Health Pro. 54:54. doi: 10.1007/s11250-022-03061-6
Seong, G. U., Hwang, I. W., and Chung, S. K. (2016). Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp Pekinensis) leaves. Food Chem. 199, 612–618. doi: 10.1016/j.foodchem.2015.12.066
Shi, B. C., Lux, R., Klokkevold, P., Chang, M., Barnard, E., Haake, S., et al. (2020). The subgingival microbiome associated with periodontitis in type 2 diabetes mellitus. ISME J. 14, 519–530. doi: 10.1038/s41396-019-0544-3
Singh, K. M., Jakhesara, S. J., Koringa, P. G., Rank, D. N., and Joshi, C. G. (2012). Metagenomic analysis of virulence-associated and antibiotic resistance genes of microbes in rumen of Indian buffalo (Bubalus bubalis). Gene 507, 146–151. doi: 10.1016/j.gene.2012.07.037
Sordillo, L. M., and Aitken, S. L. (2009). Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunop. 128, 104–109. doi: 10.1016/j.vetimm.2008.10.305
Spring, S., Bunk, B., Sproer, C., Schumann, P., Rohde, M., Tindall, B. J., et al. (2016). Characterization of the first cultured representative of Verrucomicrobia subdivision 5 indicates the proposal of a novel phylum. ISME J. 10, 2801–2816. doi: 10.1038/ismej.2016.84
Suntara, C., Cherdthong, A., Uriyapongson, S., Wanapat, M., and Chanjula, P. (2020). Comparison effects of Ruminal Crabtree-negative yeasts and Crabtree-positive yeasts for improving ensiled Rice straw quality and Ruminal digestion using In vitro gas production. J. Fungi. 6:109. doi: 10.3390/jof6030109
Tagliapietra, F., Cattani, M., Hansen, H. H., Bittante, G., and Schiavon, S. (2013). High doses of vitamin E and vitamin C influence in vitro rumen microbial activity. Anim. Feed Sci. Tech. 183, 210–214. doi: 10.1016/j.anifeedsci.2013.05.010
Tilg, H., and Kaser, A. (2011). Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investigation 121, 2126–2132. doi: 10.1172/JCI58109
van Lingen, H. J., Plugge, C. M., Fadel, J. G., Kebreab, E., Bannink, A., and Dijkstra, J. (2016). Thermodynamic driving force of hydrogen on rumen microbial metabolism: a theoretical Investig. PLoS One 11:e0161362. doi: 10.1371/journal.pone.0161362
Wang, Y. J., Bi, Y. Y., and Gao, C. Y. (2010). The assessment and utilization of straw resources in China. Agric. Sci. China 9, 1807–1815. doi: 10.1016/S1671-2927(09)60279-0
Wang, W. M., Li, C., Li, F., Wang, X. J., Zhang, X. X., Liu, T., et al. (2016). Effects of early feeding on the host rumen transcriptome and bacterial diversity in lambs. Sci. Rep.-UK 6, 1–14. doi: 10.1038/srep32479
Wu, G. D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y.-Y., Keilbaugh, S. A., et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. doi: 10.1126/science.1208344
Yin, B., Di, L., Tang, S., and Bao, E. (2020). Vitamin CNa enhances the antioxidant ability of chicken myocardium cells and induces heat shock proteins to relieve heat stress injury. Res. Vet. Sci. 133, 124–130. doi: 10.1016/j.rvsc.2020.09.008
Zhang, Z., Shahzad, K., Shen, S., Dai, R., Lu, Y., Lu, Z., et al. (2021). Altering dietary soluble protein levels With decreasing crude protein may be a potential strategy to improve nitrogen efficiency in Hu sheep based on rumen microbiome and metabolomics. Front. Nutr., 8:815358. doi: 10.3389/fnut.2021.815358
Zhang, R. Y., Zhu, W. Y., Zhu, W., Liu, J. X., and Mao, S. Y. (2014). Effect of dietary forage sources on rumen microbiota, rumen fermentation and biogenic amines in dairy cows. J. Sci. Food Agric. 94, 1886–1895. doi: 10.1002/jsfa.6508
Zhang, Z., Wei, W., Yang, S., Huang, Z., Li, C., Yu, X., et al. (2022). Regulation of dietary protein solubility improves ruminal nitrogen metabolism in vitro: role of bacteria–protozoa interactions. Nutrients 14:2972. doi: 10.3390/nu14142972
Keywords: mixed silage, Hu sheep, antioxidant properties, high-throughput sequencing, bacterial community
Citation: Li C, Chen N, Zhang X, Shahzad K, Qi R, Zhang Z, Lu Z, Lu Y, Yu X, Zafar MH, Wang M and Liu W (2022) Mixed silage with Chinese cabbage waste enhances antioxidant ability by increasing ascorbate and aldarate metabolism through rumen Prevotellaceae UCG-004 in Hu sheep. Front. Microbiol. 13:978940. doi: 10.3389/fmicb.2022.978940
Received: 27 June 2022; Accepted: 01 August 2022;
Published: 26 August 2022.
Edited by:
Junhua Liu, Nanjing Agricultural University, ChinaReviewed by:
Yang Cao, Heilongjiang Bayi Agricultural University, ChinaCopyright © 2022 Li, Chen, Zhang, Shahzad, Qi, Zhang, Lu, Lu, Yu, Zafar, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengzhi Wang, bWVuZ3poaXdhbmd5ekAxMjYuY29t; Wujun Liu, d3VqdW5saXUxMDI2QHhqYXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.