- Research Center, King Fahad Medical City, Riyadh, Saudi Arabia
Since its emergence in China in 2019, the SARS-CoV-2 virus has affected all countries worldwide. The virus is easily transmitted from one person to another via infected aerosols or contaminated surfaces. Unlike its counterparts, the prognosis of COVID-19 ranges from asymptomatic to critical disease or death. Several factors play a role in determining the severity of the disease in infected patients. Among others, is the pre-existence of an underlying medical condition such as diabetes, cancer, and others. Furthermore, although children are less prone to the severe form of the COVID-19 disease, they require attention due to the report of many atypical presentations of the infection, post-asymptomatic exposure. In the Middle East, little is known about the prognosis of the SARS-CoV-2 infection in high-risk categories, notably patients with diabetes, cancer, and pregnant women. The aim of this review is to summarize the current knowledge about this group of population in the middle eastern region as well as to highlight the gap in the literature. We have found that the majority of the papers were from the Gulf countries. Although, few studies were conducted; high-risk patients appear to have an increased risk of morbidity and mortality from COVID-19 compared to their counterparts. Higher levels of inflammatory markers, C-reactive protein, erythrocyte sedimentation rate, D-dimer, and ferritin levels were also observed. Children are often asymptomatic or present with atypical presentations. More studies should be conducted to determine the clinical biomarkers of COVID-19 in high-risk categories to help in patient risk stratification and management in the middle eastern population.
Introduction
Coronaviruses (CoVs) are positive single-stranded RNA viruses that have large spike protein molecules on their surface giving them a “crown-like” shape (Pellett et al., 2014). The subfamily of the Coronaviridae family has been classified into four genera: alpha, beta, delta, and gamma (Cascella et al., 2022). Within these, seven are known to infect humans: NL63 and 229E from the alpha-CoV and HKU1, OC43, SARS-COV, MERS-COV, and SARS-CoV-2 from the betaCoV. SARS-CoV-2 is a novel coronavirus that shares around 80% nucleotide sequence similarity with SARS-CoV (Abdelrahman et al., 2020). Following its first detection in China, in 2019, SARS-CoV-2 became a worldwide pandemic that up to 13 April 2022, affected 499,119,316 cases and resulted in 6,185,242 deaths (World Health Organization, 2022b). Compared to SARS-COV and MERS-COV, SARS-CoV-2 appears to be more infectious and contagious (Abdelrahman et al., 2020). During the pandemic, multiple variants have been described. Among these, few were characterized by the WHO as “variants of concerns” (VOCs) due to their either enhanced transmissibility, increased virulence, or decreased effectiveness of the current diagnostics, therapeutics, and vaccines (World Health Organization, 2022a). To date, five VOCs have been identified: Alpha (B.1.1.7), first described in the United Kingdom in late December, Beta (B.1.351) first reported in South Africa in December 2020, Delta (B.1.617.2) identified in India in December in 2020, Gamma (P.1), identified in Brazil in January in 2021, and Omicron (B.1.1.529) reported in South Africa in November 2021 (Cascella et al., 2022). Healthcare workers (HCW) were at the frontlines to confront and combat this pandemic. Between January 2020 and May 2021, the WHO estimated a 115,500 HCWs’ death from COVID-19 (WHO departmental news, 2021). As of April 2020, in Italy, around 10,000 HCWs got infected including 74 that have died (Chersich et al., 2020).
The COVID-19 disease ranges from asymptomatic to pauci-symptomatic to severe illness (Cascella et al., 2022). The center of disease and prevention center mentioned that people with certain medical conditions such as diabetes, cancer, and pregnant women have increased risk of being “very sick” from SARS-CoV-2 infection. This means that an infected person will be more likely hospitalized, admitted to an intensive care unit (ICU), require ventilation to assist in breathing, or in the worst-case scenario die (Center for Disease Control and Prevention, 2022). SARS-CoV-2 can infect all ages including those under 18 years of age and neonates. Compared to adults, the clinical course is often milder and asymptomatic in children; however, it has been reported that this group is more susceptible to co-infection, and can present with atypical symptoms making thus diagnosis of COVID-19 more challenging (Li et al., 2020; Ben-Shimol et al., 2021; Shahin et al., 2021).
In the Middle East, in adults, the first confirmed cases of SARS-CoV-2 were in the United Arab Emirates (UAE) on the 29th of January 2020, followed by Lebanon in 21 February and Bahrain, Kuwait, Oman, and Iraq in the 24th of February of the same year (Alwahaibi et al., 2021). In infants, the first case was reported in Lebanon early in the year 2020 (Mansour et al., 2020). The Middle East is a sensitive area culturally, politically, and economically. Over the past few years, the region was subject to continuous population mobilization in view of the multiple war crisis as well as socio-economic conflicts. This region includes 14 countries: Egypt, Iran, United Arab Emirates, Kingdom of Saudi Arabia (KSA), Qatar, Kuwait, Bahrain, Sultanate of Oman, Yemen, Lebanon, Jordan, Palestine, and Syria. The aim of this review is to summarize the current knowledge about the clinical impact of COVID-19 on healthcare workers, adults with underlying medical conditions as well as younger ages in this distinct area of the world.
Healthcare workers
Prognosis and source of infection
Healthcare workers are the building blocks of the healthcare system. According to the WHO definition, healthcare workers are all people engaged in the process of enhancing health. These include nurses, midwives, physicians, paramedical staff but also support staff, hospital managers, and community workers (El-Raey et al., 2021). During the SARS-CoV-2 pandemic, HCW had to work for many hours, over many shifts, at maximum capacity, and in some settings with limited protection (The Lancet, 2020). This made them at increased risk of contracting the infection. During their work, HCWs face excessive exhaustion, psychological distress due to separation from family, own risk of exposure and infection, and risk of introducing the infection to colleagues, family members, and hospitalized patients (Chersich et al., 2020).
In Egypt, studies have shown that most of the infected HCWs are nurses and physicians (Abou-ElWafa et al., 2021; El-Sokkary et al., 2021; Mostafa et al., 2021). This could be due to work overload, extended use/re-use, or suboptimal use of personal protective equipment (PPE), and the transmission of the virus from a HCW to another. Environmental factors can also play a role, such as hospital air, devices, and surfaces contaminated by infectious aerosols (Abou-ElWafa et al., 2021). One study conducted by Musa et al. compared the prevalence of SARS-CoV-2 infection in HCW in different hospital departments and found that those working in emergency medicine or gastroenterology have higher rates of infection compared to those in other departments including oncology and pediatrics. Indeed, HCWs in these settings are in frequent encounters with aerosol-generating procedures in patients with unknown SARS-CoV-2 status (Rana, 2020; Musa et al., 2021). Other studies in Egypt, on the other hand, have shown that patients’ cleaners/transporters and administrative employees are more affected by SARS-CoV-2 in terms of infection compared to other frontline workers (Kassem et al., 2020; Abdelmoniem et al., 2021; Musa et al., 2021). This finding could be attributed to the fact that in many cases, HCWs with more patient-near contact have greater awareness about infection control measures and the proper use of PPE (Musa et al., 2021). Most of the affected HCW in this country, presented with asymptomatic infection detected by serological testing or nasopharyngeal screening (Abdelmoniem et al., 2021; El-Sokkary et al., 2021; Mostafa et al., 2021). Among symptomatic HCW, the most common symptoms reported were fever, sore throat, headache, and myalgia (Kassem et al., 2020; Musa et al., 2021). In one study, 48.5% of those infected had moderate disease, 30.4% had mild disease and 21.1% had severe/critical disease. The rate of hospitalization was 35.8% with 3.9% of them being admitted to the ICU (El-Raey et al., 2021). Disease severity of COVID-19 was independently related to the associated chronic diseases in the affected HCW. No death was reported; this is except for the aforementioned study where the death rate was found to be 0.5%. The younger age of the participating HCW with less likely accompanied comorbidities could be the reason behind this observation (El-Raey et al., 2021). Healthcare facility and contact with a confirmed COVID-19 case during work were the most common sources of infection for HCWs (Kassem et al., 2020; Abou-ElWafa et al., 2021; El-Raey et al., 2021; Mostafa et al., 2021). Interestingly, many healthcare workers admitted that they do not know exactly where they were infected (Abou-ElWafa et al., 2021; El-Raey et al., 2021). This emphasizes on the role played by “asymptomatic silent spreaders of COVID-19″ in the transmission of SARS-CoV-2 from one person to another. HCW with unknown infectious source could have acquired the virus from patients at the hospital, work colleagues, relatives, friends, or household contacts (Abou-ElWafa et al., 2021).
In Iran, nurses, personnel of the emergency wards, and physicians constituted also the majority of the infected cases among HCW (Balou et al., 2021; Sabetian et al., 2021). In one study, it was found that HCW with rotational shifts were more likely to get infected compared to those with fixed shifts. This higher susceptibility could reflect different COVID-19 exposures among evening, afternoon, and morning shifts, in addition to the weakened immune system influenced by night shifts as reported by other studies (Loef et al., 2019; Lankarani et al., 2021). Other risk factors identified for the acquisition of SARS-CoV-2 among HCW in Iran include age, non-proper disposal of used PPE/infectious wastes, and anxiety about getting COVID-19 (Lankarani et al., 2021). The source of SARS-CoV-2 infection among Iranian HCW was mainly via occupational exposure (Goshayeshi et al., 2021; Sabetian et al., 2021). In fact, it is worth mentioning that occupational exposure is not restricted to contact with SARS-CoV-2 infected patients solely but also includes contaminated work environment and disregard of safety precautions in contaminated workspace (Sabetian et al., 2021). The COVID-19 disease among HCW in Iran is manifested mainly by an asymptomatic infection followed by a symptomatic one with atypical symptoms such as myalgia and cough and symptomatic with typical symptoms such as cough and fever (Armin et al., 2020; Goshayeshi et al., 2021; Mortezagholi et al., 2021; Sabetian et al., 2021). Twenty hospital admissions, six ICU admissions, seven mechanical ventilation requirement, and two deaths were reported among HCW of this country (Armin et al., 2020; Goshayeshi et al., 2021; Sabetian et al., 2021).
In the Kingdom of Saudi Arabia, two studies found that there is no statistical difference between different job categories and hospital departments in the acquisition of SARS-CoV-2 among HCW (Alshahrani et al., 2020; Alroqi et al., 2021). In another two studies, it was found that nurses were the most commonly affected (Al Bujayr et al., 2021; Barry et al., 2021). One plausible explanation for these findings is the standardization of the Saudi national guidelines for PPE that are adopted from the guidelines of the WHO (Alroqi et al., 2021). In addition, the healthcare workforce in KSA relies mainly on the nursing-related occupation that have more frequent contact and longer exposure time with patients (Barry et al., 2021). In this country, hospital and community settings played both the role of an infection source for HCW (Barry et al., 2020; Alshamrani et al., 2021). Healthcare-associated infections include exposure to a positive COVID-19 patient as well as exposure to other infected HCW (Al Bujayr et al., 2021). Community-acquired infections, on the other hand, include social gatherings, shared transportation, returning travelers, and family members (Barry et al., 2021). Indeed, in one study, it was found that the odds of testing seropositive for COVID-19 IgG are higher among those that have an infected family member and were not associated with ICU employment or involvement in the intubation and close contact with infected patients (Farsi et al., 2021). This emphasizes that infection control measures should be implemented, followed, and strictly monitored in and outside the clinical setting. Infected HCW in Saudi Arabia are mostly asymptomatic (Alshahrani et al., 2020; Barry et al., 2020). The most common symptoms reported in symptomatic patients include gastrointestinal ones (Alshahrani et al., 2020), cough, fever, body aches (Alroqi et al., 2021; Barry et al., 2021), headache, and dry throat (Alhabbab et al., 2021). Notably, in one study, the majority of infected healthcare workers were symptomatic with 198 reported deaths. Death was mostly related to the age groups of 46 years and above (Al Bujayr et al., 2021). Interestingly, Alshamrani et al. conducted a study where they compared the clinical outcomes of infected HCW and non-HCW. They found that the higher risk of ICU admission and hospitalization increases three and two times, respectively in non-HCW compared to HCW (Alshamrani et al., 2021). This could be attributed to the lower comorbidities, younger age, and better awareness of the healthcare personnel (Alshamrani et al., 2021; Gholami et al., 2021).
In Qatar, the least affected groups of healthcare workers were the clinical employees compared to outsourced and non-clinical staff (Al-Kuwari et al., 2021; Alishaq et al., 2021a). Al-Kuwari et al. found no significant difference of SARS-CoV-2 rates among employees who worked in a COVID-19 healthcare facility versus other ones (Al-Kuwari et al., 2021). Al-Ajmi et al., on the other hand, found that midwives and nurses have the highest rate of SARS-CoV-2 infection followed by non-clinical support service staff, administrative employees, allied healthcare professionals, physicians, and others (Alajmi et al., 2020). In that same report, the majority of the infected HCW reported working in a non-COVID-19 facility (Alajmi et al., 2020). Regarding risk factors, one study found that HCW aged <45 years have a higher infection rate (Al-Kuwari et al., 2021); whereas in another, it was found that, compared to age groups <30, older ages were significantly associated with a lower risk of infection (Alishaq et al., 2021a). HCW are a heterogeneous group of population and the risk of infection may differ based on several variables, including exposure to an unrecognized infection among patients (Alajmi et al., 2020), level of community exposure, and adherence to infection control measures. As for the disease presentation, only one study conducted found that two-thirds of the included HCW are symptomatic with fever, cough and sore throat being the most common symptoms reported. The hospitalization rate was 15.4%, and of the survey respondents in this study, nine, four, and two required supplemental oxygen were ICU admitted and required mechanical ventilation, respectively. Furthermore, zero death was reported (Alajmi et al., 2020).
In the Sultanate of Oman, most of the infected HCWs were nurses followed by doctors (Al Abri et al., 2021; Al Maskari et al., 2021; Al-Siyabi et al., 2021). Indeed, Maskari et al. found that being a doctor or a nurse is significantly associated with the acquisition of COVID-19 inside the hospital (Al Maskari et al., 2021). One study reported that nurses followed by administrative and supporting services staff are the most affected groups compared to medical doctors (Al-Maani et al., 2021). Nurses are frontline workers, and specific procedures such as aerosol-generating ones, electrocardiography, and others involved in direct patient contact (incubation assistance, suctioning, and manipulation of oxygen face masks) increase their risk of SARS-CoV-2 infection (Loeb et al., 2004; Chou et al., 2020; Al Abri et al., 2021). Moreover, on the contrary, Al-Naamani et al. found that supportive staff and not nurses or physicians are the mostly affected by the virus (Al-Naamani et al., 2021). Throughout the studies, we found that older age (above 50 years; Al-Naamani et al., 2021), availability of N95 masks, reuse of personal protective equipment, inadequate sanitization and disinfection of hospital surfaces and medical equipment, lack of education and training of cleaners, small size of the facility restricting social distancing, poor ventilation, and patient overcrowding are all risk factors that increases the chance of contracting the COVID-19 disease inside the healthcare facility (Al Abri et al., 2021). The sources of SARS-CoV-2 among healthcare workers in Oman appear to be both hospital and community acquired (Al Abri et al., 2021). In one study, community acquisition was the most common (Al Maskari et al., 2021) while in another one, hospital acquisition was the most common (Al-Siyabi et al., 2021). Interestingly, in both studies, in roughly equal percentages of HCW, the source of infection could not be determined. Inside the hospital, noncompliance with social distancing and wearing masks in eating times, in addition to patients’ exposure, are all routes of transmission of COVID-19 among HCW (Al Maskari et al., 2021; Al-Siyabi et al., 2021). Regarding the clinical presentation of COVID-19 among HCW in Oman, it was found that the majority presented with a mild illness with five hospital admissions, one ICU admission, and zero death reported. The most common symptoms were fever, headache, cough, and sore throat (Al Lawati et al., 2021; Al Maskari et al., 2021; Al-Siyabi et al., 2021).
In the Levant (Lebanon, Syria, Jordan, and Palestine) and the rest of the Gulf countries, scarce studies exist on the clinical impact of COVID-19 on HCW. In the UAE, it was found that support staff including housekeeping, facility and catering staff, porters, and security guards are more likely to get infected with SARS-CoV-2 compared to the non-support ones. Non-support HCW include physicians, nurses, allied health professionals, and administrative staff (Park et al., 2021).
In Kuwait, Al-Youha et al. reported that the likelihood of contracting the SARS-CoV-2 infection is significantly associated with working as a nurse and wearing gloves (Al Youha et al., 2021). A possible explanation for this latter finding is that, due to poor hand hygiene practices, extended gloves use could result in greater contamination. Furthermore, due to possible PPE shortages, HCWs are less likely to change their gloves when dealing with different patients and procedures (Neuwirth et al., 2020). Contact with COVID-19 patients was reported in more than two-thirds of the included HCW in the aforementioned study in Kuwait (Al Youha et al., 2021).
In Yemen, one study reported 17-infected HCWs after contact with two confirmed COVID-19 patients inside the hospital; only two of the infected HCW needed hospitalization (Al-Sakkaf et al., 2021). In Syria, a report from the North West region reported that due to the protracted conflict in the area, HCWs face several challenges related to inadequate PPE, insufficient resources, poor infection and prevention control measures/practices, and severe under-staffing. The first confirmed case in this area was a doctor working at a border-located hospital. Thereafter, a cluster was noted among hospital staff, in addition to several community ones. Five physicians and one nurse have died (Almhawish et al., 2021). In Israel, it was reported that one COVID-19-positive ophthalmologist transmitted the infection to a visiting patient that was not wearing a mask (Saban et al., 2020). On the other hand, Temkin et al. reported the asymptomatic infection of a nurse working in a COVID-19 unit (Temkin and Healthcare Worker COVID-19 Surveillance Working Group, 2021).
Taking it overall, in the middle eastern countries, notably in Egypt, Iran, and KSA where many studies were conducted, a substantial proportion of healthcare workers infected with SARS-CoV-2 were asymptomatic. Worldwide speaking, the percentage of asymptomatic infections among HCW varies from 0% to 8.2% in the United States (Campbell et al., 2020; Jameson et al., 2020; Stock et al., 2020), 2.2% in France (Guery et al., 2020), 2.7% in Italy (Cavicchiolo et al., 2020), 5% in Spain (Moncunill et al., 2021), 7.5% in the United Kingdom (Hellewell et al., 2021), and 11.1% in Indonesia (Hidayat et al., 2020). The varying numbers could be explained by regional differences in the incidence of COVID-19, together with the difference in the baseline features of the pandemic in each country. Indeed, it has been suggested that among asymptomatic HCW, higher rates of positivity can be expected when the incidence of the virus in the general population increases; this is possibly due to a higher probability of exposure to a confirmed infected case outside the hospital (Jabs et al., 2022). The main concern about asymptomatic carriers, as mentioned earlier, is the unrecognizable transmission of COVID-19 from the asymptomatic patient to other high-risk subjects in the clinical setting as well as in the community. Throughout the literature, it remains under debate the extent to which asymptomatic infections contribute to disease transmission and continuous spread. In fact, what is pretty sure is that asymptomatic carriers are infectious. The level of infectivity is however complex and linked to several factors including viral load, duration of viral shedding, age, comorbidities, and immune responses (Wang et al., 2022). In a systematic review conducted by Byambasuren et al., it was found that asymptomatic infection with SARS-CoV-2 is unlikely to be the main driver of community transmission (Byambasuren et al., 2020). On the other hand, Yang et al. found that from October 2020 to February 2021, out of five COVID-19 outbreaks in China, four were caused by asymptomatic infections (Yang et al., 2021). In the Middle East, the impact of asymptomatic carriage on the spread of SARS-CoV-2 cannot be deduced since no studies explored the viral load, duration of viral shedding nor the immune responses in asymptomatic healthcare workers or other patients’ categories.
Breakthrough infections
It is worth mentioning that all the studies addressing COVID-19 in HCW (as well as other in other patients’ categories), in the middle eastern region, were conducted before the vaccination campaigns have started, and thus the effect of vaccines could have not impacted the findings. Frontline workers were the first to take the COVID-19 vaccine in all middle eastern countries. Vaccination campaigns started in December 2020 in KSA (Assiri et al., 2021), UAE (Sahib, 2020), Bahrain (Gazette, 2020), Kuwait (Al-Ayyadhi et al., 2021), Qatar (Alishaq et al., 2021b), Sultanate of Oman (Al Rawahi et al., 2022), Jordan (Al-Shaikh et al., 2021), and Israel (Rosen et al., 2021); January 2021 in Egypt (ElSharkawy, 2021); February 2021 in Iran (Heidari and Jafari, 2021), Palestine (Mukhtar, 2021), and Lebanon (Mumtaz et al., 2021); March 2021 in Yemen (Reuters, 2021); and May 2021 in Syria (WHO in Syria, 2021) and Iraq (Abdulah, 2021).
Breakthrough infections defined as the detection of SARS-CoV-2 RNA or antigen in a respiratory specimen post ≥14 days after the receipt of all recommended doses of the COVID-19 vaccine (Center for Disease Control and Prevention, 2021) were described in HCW from KSA, Qatar, and Israel. For instance, in the Kingdom of Saudi Arabia, in one study, 20 HCWs who all received the first dose and four only received the second dose of the COVID-19 vaccine were infected. The majority were physicians and nurses. The infection was asymptomatic in seven, and the rest exhibited mild-to-moderate disease. The most common symptoms were fever, cough, headache, malaise, and sore throat. In the majority of the included HCW in this study, the source of infection could not be recognized (Alshamrani et al., 2022). In Qatar, Alishaq et al. reported 164 breakthrough infections among HCWs. History of contact with a confirmed case was independently associated with a higher risk of infection. Interestingly, the presence of comorbidities was not associated with a higher risk of breakthrough infection. Moreover, almost all job families, i.e., clinical and non-clinical, support staff had a higher risk of breakthrough infection compared to nurses (Alishaq et al., 2021b). In Israel, one study found that symptomatic breakthrough infections occurred in eight HCW fully vaccinated compared to 38 unvaccinated ones. Asymptomatic infection, on the other hand, occurred in 19 and 17 fully vaccinated and unvaccinated HCW, respectively (Angel et al., 2021). Oster et al., reported one hospitalization in HCW who got infected with SARS-CoV-2 after at least 2 weeks of receiving the 2nd dose of the COVID-19 vaccine; this is versus two hospitalizations in the unvaccinated group, with no death recorded in both of them. As for the source of infection, exposure to a positive household member was more common in vaccinated subjects vs. unvaccinated ones, with a significant statistical difference (Oster et al., 2021). Similarly, in another study, the main source of breakthrough infection in HCW was also community-related. In this latter, the most common symptoms were influenza-like illness including fever, chills, cough, headache, and sore throat (Amit et al., 2021). Moreover, in a study conducted by Bergwerk et al., on breakthrough infections with follow-up, up to 6 weeks, “long COVID-19 symptoms” were reported; these included persistent cough, fatigue, weakness, dyspnea, or myalgia, in addition to prolonged loss of smell. Similar to the study in KSA, HCW experienced mainly mild disease followed by an asymptomatic one (Bergwerk et al., 2021). Interestingly, in this study among 33 isolates of breakthrough infections tested for a variant of concern, 28 were found to be the B.1.1.7 (alpha) variant. According to the authors, at the time of the investigation, this variant was the most commonly spread in Israel and accounted for up to 94.5% of the SARS-CoV-2 isolates (Bergwerk et al., 2021; Haas et al., 2021; Kustin et al., 2021).
High-risk population
As mentioned in the introduction, COVID-19 patients with underlying medical conditions, especially those with an immunocompromised system, either due to an underlying disease or being on immunosuppressive medications, are at a higher risk of developing severe complications and poor prognosis of the SARS-CoV-2 infection (Kim et al., 2021). In diabetic patients, for example, several studies revealed their higher vulnerability to some infectious diseases. This is could be in particular due to their high levels of blood glucose, on which the virus may thrive (Robert et al., 2021), and can damage the immune system defense mechanisms, triggering diabetes-related problems such as nerve damage. Furthermore, the impaired blood flow increases the patient’ susceptibility to infection (Jeong et al., 2020; Al Hayek et al., 2020b). As for cancer patients, poor functional status could increase their risk of poor outcomes for COVID-19 (Blimark et al., 2015; Mousavi et al., 2021). Other categories such as hemodialysis (HD) patients and solid organ transplant patients can also experience poor prognosis of COVID-19. This is owing to their weak immune system and their comorbidity/multimorbidity (Cheng et al., 2020; Kenarkoohi et al., 2022). In this section, we will review the current knowledge about the prognosis and clinical outcomes of SARS-CoV-2 infection in high-risk categories in the Middle East.
Diabetes
In the Kingdom of Saudi Arabia, the most common symptoms of SARS-CoV-2 infection in type 1 diabetic patients (T1DM) observed in one study were nausea, vomiting, followed by fever, cough, sore throat, abdominal pain, and dyspnea (Al Hayek et al., 2020a). Type 1 Diabetic patients with COVID-19 were found to have a greater comorbidity percentage and lower mean of Vitamin D levels, in addition to higher ferritin and average D dimer levels. This is compared to non-infected diabetic patients and control groups (Ahmed et al., 2021). Diabetes biomarker levels including fasting blood glucose (FBG) and HbA1c were also higher in infected patients with T1DM. This result is in accordance with the findings of a study conducted in China that reported higher levels of FBG in diabetic patients infected with the SARS-CoV-2 virus (Cai et al., 2020). Indeed, studies have shown that FBG and Hb1Ac are associated with the progression of the COVID-19 illness in diabetic subjects (Wang et al., 2020; Ling et al., 2021). The rate of hospitalization of T1DM infected with SARS-CoV-2 ranges from 21.9% to 48% (Al Hayek et al., 2020a). The most common reason for hospitalization was diabetic ketoacidosis, followed by hyperglycemia, bacterial pneumonia, COVID-19 pneumonia, fever, and sore throat (Al Hayek et al., 2020a). Similarly, high incidence of ketoacidosis was also observed in hospitalized patients because of COVID-19, in Germany and in India (Kamrath et al., 2020; Reddy et al., 2020). In type 2 diabetic COVID-19 patients, ages between 70 and 79 and above 80 years are more likely to be hospitalized compared to those <40 years old. Furthermore, those with higher HbA1c levels, presence of comorbidities such as chronic kidney disease (CKD), chronic pulmonary disease, cerebrovascular disease, cardiovascular disease (CVD), hypertension, and insulin-treated ones are more likely to get hospitalized (Al Hayek et al., 2020b). Alguwaihes et al. found that SARS-CoV-2 infected DM patients have significantly lower survival time and higher death rates compared to non-infected patients with diabetes. However, after adjustment for confounders like age, sex, body mass index (BMI), and pre-existing medical conditions; diabetes was not associated with mortality (Alguwaihes et al., 2020). The result is in line with a study conducted in the United States that found no association between diabetes, and risk of ICU admission, mechanical ventilation, and mortality (Suleyman et al., 2020). In contrast, another study conducted in the United Kingdom found that one-third of COVID-19 patients who died in hospitals had diabetes (Hillson, 2020). The lack of association between diabetes and poor clinical outcomes does not eliminate the fact that diabetic patients have a major risk of poor prognosis of COVID-19; however, this suggests that the increased risk of poor outcome is due to the cumulative effect of the presence of diabetes mellitus together with other chronic conditions (Alguwaihes et al., 2020; Apicella et al., 2020; Maddaloni et al., 2020).
In Iran, one study has shown that the most common symptoms of COVID-19 in hospitalized patients with diabetes, defined as fasting plasma glucose ≥126 mg/dl, were fever, dry cough, and dyspnea (Akbariqomi et al., 2020). At admission, laboratory findings indicated increased white blood cells (WBCs) counts and neutrophils and decreased lymphocyte count compared to infected patients without diabetes. This shows that infected patients with DM experienced a severer viral infection. Furthermore, blood urea nitrogen levels were also increased in COVID-19 DM patients, suggesting the occurrence of kidney damage (Akbariqomi et al., 2020). The mortality rate among DM patients infected with SARS-CoV-2 was found to be 22% in one study. The clinical outcome did not however differ significantly between patients with well-controlled and poorly-controlled DM (Raoufi et al., 2020). In infected patients, the mortality rate was higher in those with DM compared to those without (Akbariqomi et al., 2020; Pazoki et al., 2021). Interestingly, unlike what has been reported in KSA, a study conducted by Akbariqomi et al., found that compared to COVID-19 patients lacking DM and any other comorbidity, those that are infected and have DM only, still experience more complications and deaths (Akbariqomi et al., 2020). In Iran, it was found that advanced age, addiction, high levels of blood urea nitrogen, and alkaline phosphatase are associated significantly with increased odds of death in DM COVID-19 patients (Borzouei et al., 2021).
In the UAE, one study has shown that among hospitalized COVID-19 patients with diabetes (including known and newly diagnosed diabetes), 80.8% had moderate-to-severe disease, and 19.4% had mild disease without pneumonia. In terms of the severity of the COVID-19 illness, no statistical difference was found between those with pre-existing diabetes and those with a newly diagnosed one. Only, the requirement for mechanical ventilation, and mortality rate were significantly higher in patients with newly diagnosed diabetes compared to those with a pre-existing one (Hafidh et al., 2020). Bhatti et al. conducted a study and found that at admission, laboratory findings of infected diabetic patients (87.4% being type 2 diabetes) indicated higher levels of fibrinogen, D-dimer, ferritin, and C-reactive protein (CRP) in those admitted to the ICU. These biomarkers can help in assessing in advance the more likely clinical course of the disease, and the patient subsequent need for intensive care (Bhatti et al., 2020). The length of hospital stay, in this same study, was longer in ICU admitted patients. The mortality rate was 4.9% among those who were admitted to the ICU. Similar to the study conducted in the UAE, the most common comorbidities detected in these patients were hypertension, ischemic heart disease, and dyslipidemia (Bhatti et al., 2020).
In Qatar, one study compared the clinical outcomes of hospitalized COVID-19 patients among type 2 diabetics and non-diabetics. It was found that diabetic patients had a higher prevalence of chronic kidney disease, hypertension, congestive heart failure, and cardiac dysfunction. They had also significantly higher percentages of pneumonia, severe pneumonia, and acute respiratory distress syndrome (ARDS). Hematological speaking, diabetic patients had significantly higher CRP levels, absolute neutrophil counts, but lower counts of lymphocytes and eosinophils compared to the non-diabetics. CRP was correlated significantly with the duration of stay in the intensive care unit, as well as to the duration of oxygen supplementation (Soliman et al., 2020b; Table 1). In Kuwait, diabetes was found to be associated with ICU admission in hospitalized COVID-19 patients after adjustment of confounding factors (Al-Sabah et al., 2020). In this study, diabetes was defined as a fasting blood glucose level of ≥126 mg/dl (Al-Sabah et al., 2020). In Egypt, Emara et al. reported three cases of type 2 diabetic patients who presented at the outpatient department with diabetic ketoacidosis. A few days later, these patients developed fever and hypoxemia and turned out to be SARS-CoV-2 positive. All three cases were hypertensive and dyslipidemics, and one case passed away due to severe hypoxemia (Emara et al., 2020). In Palestine, it was found that COVID-19 patients with diabetes were more likely to be hospitalized in comparison to those without diabetes (Hamdan et al., 2021). In Israel, one study found that in SARS-CoV-2 diabetic patients, HemoglobinA1c ≥9% significantly increased the risk of hospitalization (Merzon et al., 2021).
The poorer outcome observed in diabetic patients infected with SARS-CoV-2 in the majority of the middle eastern countries is supported by findings from China (Chen N et al., 2020), where it was found that the rates of mortality are higher in diabetic patients compared to the general population with COVID-19. Another study from the United States reported a longer length of hospital stay as well as a higher mortality in COVID-19 patients with diabetes versus those without (Bode et al., 2020). Furthermore, a nationwide study conducted in Sweden indicated that increased risk of hospitalization and ICU admission, in addition to death, was associated independently with type 2 diabetes (Rawshani et al., 2021).
Cancer
In Iran, several studies assessed the clinical outcome of COVID-19 in cancer patients (Mousavi et al., 2021; Rakhsha et al., 2021; Shahidsales et al., 2021). In one, it was found that compared to hospitalized patients without malignancy, malignant patients have an increased risk of death and mechanical ventilation; although these latter have less rates of comorbidities (Shahidsales et al., 2021). No factor such as the type of malignancy neither stage of the disease or recent oncological treatment could predict death in infected cancer patients. Similarly, in another study, the type of cancer could not predict mortality in hospitalized COVID-19 patients. The mortality rate was however higher in those with a history of lung cancer with lung metastasis due to non-pulmonary cancer and in those who received cytotoxic chemotherapy within the last 14 days (compared to those who were on cancer treatment more than 2 weeks prior to admission with COVID-19; Mousavi et al., 2021). This finding is in agreement with a study conducted by Dai et al., who found that the mortality rate among COVID-19 patients with lung cancer is higher compared to other cancer types and that the cancer stage directly correlates with mortality (Dai et al., 2020). In contrast, another study in the United Kingdom argued that lung cancer patients are not specifically subject to a higher mortality when infected (Lee et al., 2020). More studies with a larger sample size are needed to confirm whether lung cancer patients have a higher risk of death from COVID-19. As for COVID-19 prognosis, in patients with malignancies in Iran, dyspnea, fever, dry cough, and gastrointestinal symptoms were the most commonly reported. During hospitalization, most of the patients received oxygen therapy, around half received invasive ventilation and only 10% received a non-invasive one. Admission to an ICU was reported in around half of the hospitalized patients with cancer. The most common complications were acute respiratory distress syndrome, sepsis, septic shock, and pulmonary thromboembolism. In this same aforementioned study, death was reported in almost half of the patients (Mousavi et al., 2021). This rate was similar to another report from Iran where 4 out of 7 patients with cancer died due to COVID-19 (Aznab, 2020). Asymptomatic infection detected by serological testing was reported in one study in Iran, conducted by Arab et al. (2021).
In the United Arab Emirates, the majority of studies were conducted in asymptomatic cancer patients. In one, none of the patients developed any COVID-19-related symptoms (Iskanderian et al., 2020), whereas in the other two studies, symptoms subsequently appeared (Al-Shamsi et al., 2020, 2021). The SARS-CoV-2 infection was mild in the majority of the reported cases with few requiring hospital and ICU admission. Only five deaths were reported (Al-Shamsi et al., 2020, 2021; Nawazani et al., 2020). These findings emphasize the need of a scheduled screening for cancer patients regardless of their symptom presentation as these can act as hidden reservoirs for nosocomial transmission of SARS-CoV-2 among high-risk population and critically ill patients.
In Qatar, only two cases of SARS-CoV-2 infection among cancer patients were reported. In one, the patient presented with COVID-19 symptoms in addition to absolute lymphocytosis that with further workup found out to be chronic lymphocytic leukemia (CLL). The patient had moderate disease that did not progress to a severe form as expected. This is according to the authors might be due to the defective immune response in CLL patients that prevented cytokine storm and the subsequent multi-organ involvement (Ali et al., 2020). The second case reported in Qatar was in a patient with hair cell leukemia, who presented with severe respiratory symptoms but recovered later (Kohla et al., 2020). In Oman, it was found that the fatal outcome was higher in cancer patients with COVID-19 compared to those without. Cardiac diseases, hypertension and diabetes were likely risk factors for SARS-CoV-2 infection in patients with malignancy (Al Bahrani et al., 2021). In Israel, one study conducted on patients with hematological malignancies and infected with SARS-CoV-2 found that age > 70 years, hospitalization, severe/critical disease, hypertension with severe/critical disease, and active hemato-oncological treatment with hospitalization were associated with mortality. Around one-third of the patients developed severe/critical respiratory infection. Death was reported in one-fifth of the infected patients with cancer (Levy et al., 2021). In a recent meta-analysis conducted by Di Felice et al., it was found that infected patients with cancer have a twofold higher risk of experiencing a severe form of the COVID-19 illness as well as ICU admission compared to non-cancer patients. The association of SARS-CoV-2 infection with mortality in patients with malignancies was stronger in studies from Asia than from those of North America and Europe (Di Felice et al., 2022). In our region, studies on COVID-19 patients with cancer are scarce and include only a small number of subjects. More studies with a larger sample size are needed to draw a definitive conclusion about the prognosis of COVID-19 in this group of patients. Regarding antiviral treatment, Levy et al. found that remdesivir treatment was associated with decreased mortality in hemato-oncological patients infected with SARS-CoV-2 (Levy et al., 2021). No other studies in the Middle East explored the relationship between the administered therapeutic regimen and the clinical outcome of COVID-19.
Other high-risk categories
In Iran, one study has shown that in COVID-19 patients, chronic kidney disease was associated with an increased risk of mortality (Mirjalili et al., 2021). On the other hand, in patients undergoing hemodialysis, hereditary kidney failure and blood group A were associated with higher COVID-19 morbidity. Compared to SARS-CoV-2-infected patients without hemodialysis requirement, it was shown that those “who require hemodialysis”, have only higher absolute counts of WBC, and polymorphonuclears. Clinical biomarkers for COVID-19 in hemodialysis patients should be therefore more explored to help better management of the disease in this group of people. Moreover, a study conducted also in hemodialysis patients infected with SARS-CoV-2 reported that the most common symptoms were dyspnea and cough. The majority of the patients were hospitalized, 12.9% needed intensive care and 16.1% died (Kenarkoohi et al., 2022).
In kidney transplant recipients, in Iran, the most common symptoms observed in COVID-19 patients were myalgia, cough, fever followed by headache, shortness of breath, and sore throat. Acute kidney injury (AKI) was the most common complication observed, and death occurred in half of the patients due to the SARS-CoV-2 infection (Rahimzadeh et al., 2021). The mortality rate was higher in ICU patients versus those who did not require intensive care. Pre-transplantation diabetes was the only factor associated with an increased risk death in COVID-19 patients (Rahimzadeh et al., 2021). In another study, fever and cough were also the most common symptoms observed in COVID-19 kidney transplant patients. Diabetes mellitus and hypertension were considered underlying diseases in this group (Molaei et al., 2021). As for the source of infection, around one-fourth acquired the virus from a family member (Rahimzadeh et al., 2021). This highlights that kidney transplant recipients should keep their own distance even within their families.
In the Kingdom of Saudi Arabia, one study conducted on COVID-19 patients with end-stage kidney disease showed that the mortality risk increases significantly with age and concurrent cardiovascular diseases. High D-dimer levels, neutrophilia, lymphocytopenia, and high blood urea nitrogen levels were also significant risk factors for death in this population. The most common complications resulting in death were septic shock, respiratory failure, and respiratory distress syndrome (Hakami et al., 2021). As for the symptoms, the most commonly reported were fever, shortness of breath, and cough. The majority of the patients were admitted to the hospital general ward with 17% only requiring ICU and intubation (Hakami et al., 2021). Al Maghrabi et al. reported four cases of fully vaccinated solid organ transplants (three renal and one liver), infected with SARS-CoV-2. All patients were hospitalized and admitted to the ICU. Two were infected with the alpha variant and died (one renal and one liver transplants) due to sepsis/septic shock and sepsis/septic shock with cytomegalovirus reactivation, respectively. The other two patients recovered with one being infected with the Beta variant and the other with the Delta variant (Almaghrabi et al., 2022). In Israel, one study reported six hemodialysis patients who got infected with SARS-CoV-2 after >7 days of taking the second dose of the mRNA BNT162b2 vaccine. Three patients experienced moderate disease, two mild, and one had severe illness. In two patients, the virus variant was determined and found to be B.1.1.7 – UK (L5F[S]; Yanay et al., 2021). No other studies reporting breakthrough infections in high-risk patients were conducted in the middle eastern region.
Pregnant women and neonates
In the literature, studies have shown that the risk of SARS-CoV-2 acquisition in pregnant women is the same compared to the general population (Docherty et al., 2020). However due to the immunological and physiological changes that occur normally during pregnancy; this category might be at increased risk of severe illness. In fact, the maternal immune system faces challenges in tolerating the fetus, and at the same time defending the body from microbial infections (Hazari et al., 2021). Physiological changes, on the other hand, include cardiopulmonary ones such as respiratory tract edema, diaphragm elevation, and increased consumption of oxygen (Schwartz and Graham, 2020). All of these changes make pregnant women a vulnerable population. This is supported by the fact that previous coronaviruses like the severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) were associated with increased maternal mortality and adverse outcomes in pregnancy and delivery (Samadi et al., 2021).
In Iran, the majority of pregnant women were infected during their third trimester (Abedzadeh-Kalahroudi et al., 2021b; Vaezi et al., 2021; Vizheh et al., 2021). This is in accordance with other studies in the Middle East as well as in other worldwide countries such as the United States (Hirshberg et al., 2020) and China (Chen H et al., 2020). In Iranian pregnant women, the most common reported symptoms of COVID-19 were fever, cough, and dyspnea (Kazemi Aski et al., 2020, 2021; Motlagh et al., 2020; Pirjani et al., 2020; Sattari et al., 2020; Akbarian-Rad et al., 2021; Alipour et al., 2021; Samadi et al., 2021; Vaezi et al., 2021; Vizheh et al., 2021; Abedzadeh-Kalahroudi et al., 2021b). Lymphopenia and elevated C-reactive protein levels (Pirjani et al., 2020; Akbarian-Rad et al., 2021; Vaezi et al., 2021; Abedzadeh-Kalahroudi et al., 2021a,b), in addition to Elevated lactate dehydrogenase (LDH; Kazemi Aski et al., 2020; Abedzadeh-Kalahroudi et al., 2021a), were the most common laboratory findings observed. The SARS-CoV-2 infection in this population presented mainly as a mild-to-moderate disease (Kazemi Aski et al., 2020; Vizheh et al., 2021). Samadi et al. found that the presence of underlying medical conditions is significantly associated with disease severity of COVID-19. In addition, infected pregnant women are more likely to develop a critical stage of the disease with increasing gestational and maternal age when having underlying diseases (Samadi et al., 2021). This in accordance with other studies conducted in the United States and the United Kingdom where it was found that hospitalized women with COVID-19 and have comorbidities, are more prone to severe illness (Knight et al., 2020; Zambrano et al., 2020). Although the majority of the pregnant women with COVID-19 were hospitalized, a minority of ICU admissions and maternal death were observed (Pirjani et al., 2020; Sattari et al., 2020; Alipour et al., 2021; Abedzadeh-Kalahroudi et al., 2021a). The most common mode of delivery of infected pregnant women was a cesarean section, performed mostly under obstetric indication (Pirjani et al., 2020; Alipour et al., 2021; Vaezi et al., 2021; Vizheh et al., 2021; Abedzadeh-Kalahroudi et al., 2021a), rather than due to complications of COVID-19 (Abedzadeh-Kalahroudi et al., 2021a). In one study only, it was found that the severity of the coronavirus disease was the only factor effective in increasing the rate of cesarean delivery (Samadi et al., 2021). In another study, COVID-19 in pregnancy was associated significantly with a higher risk of cesarean section (Alipour et al., 2021). Compared to pregnant women without COVID-19, those infected have lower gestational age (Pirjani et al., 2020; Alipour et al., 2021), and higher rate of ICU admission (Pirjani et al., 2020). Furthermore, it was reported that these latter have poorer maternal outcomes and higher rates of pre-eclampsia, preterm labor, and fetal distress (Alipour et al., 2021; Abedzadeh-Kalahroudi et al., 2021a). On the other hand, compared to non-pregnant infected women, pregnant women with COVID-19 have a lower frequency of severe disease, acute respiratory distress syndrome, and shorter mean duration of hospitalization (Vizheh et al., 2021; Table 1). In their study, Ghelichkhani et al. found that compared to infected pregnant women without underlying diseases, preterm labor, preeclampsia, and eclampsia are significantly higher in those having pre-medical conditions (Ghelichkhani et al., 2021). Interestingly, in one study, infected pregnant women, in their second and third trimesters without pre-existing medical conditions, were compared to their infected familial and household members, and found that the maternal outcomes were more severe (Hantoushzadeh et al., 2020). As for neonates born to infected mothers, in most of the studies, no vertical transmission was observed (Akbarian-Rad et al., 2021; Vaezi et al., 2021; Abedzadeh-Kalahroudi et al., 2021b). Scattered reports however reported the transmission of the SARS-CoV-2 virus from the infected mother to her newborn (Kamali Aghdam et al., 2020; Rashidian et al., 2020; Sattari et al., 2020; Zamaniyan et al., 2020; Abolhasan et al., 2021; Rabiei et al., 2021; Abedzadeh-Kalahroudi et al., 2021a). According to several studies (Alzamora et al., 2020; Penfield et al., 2020; Wu et al., 2020), it seems that vertical transmission increases when the mother experience a severe or critical course of the disease (Alipour et al., 2021; Farhadi et al., 2021; Vizheh et al., 2021). Viral load in the infected mother may be another contributing factor for the vertical transmission (Qiu et al., 2020; Fan et al., 2021). Neonatal death due to COVID-19 was only reported in four cases throughout the studies in Iran (Rashidian et al., 2020; Moeindarbary et al., 2021; Vizheh et al., 2021).
In the Kingdom of Saudi Arabia, very few studies explored the clinical impact of COVID-19 in pregnant women. One study found that the most common symptoms were cough, fever, and dyspnea (Al-Matary et al., 2021). The majority of infected pregnant women did not have comorbidities and experienced a mild or moderate disease (Alhamoud et al., 2020; Al-Matary et al., 2021; AlQurashi et al., 2021). A small proportion only, required intensive care and respiratory support as they developed symptoms of pneumonia; this finding was reported in pregnant women between 26 and 36 weeks of gestational age (Al-Matary et al., 2021). The low mortality rate and ICU admission reported from Iran and KSA are similar to the one observed in several studies worldwide conducted on pregnant women infected with SARS-CoV-2 (Allotey et al., 2020; Elshafeey et al., 2020; Juan et al., 2020; Smith et al., 2020). In pregnant women with COVID-19 in this country, vaginal delivery was more prevalent than the cesarian one (Algadeeb et al., 2020; Alhamoud et al., 2020; Al-Matary et al., 2021; AlQurashi et al., 2021). The most frequent adverse pregnancy outcomes were premature followed by fetal distress, and preeclampsia which occurred in pregnant women <37 weeks of gestational age (Al-Matary et al., 2021). No vertical transmission was reported (Al-Matary et al., 2021; AlQurashi et al., 2021). This is except for one case where the female infant experienced severe respiratory symptoms together with persistent pulmonary hypertension that led consequently to her death (Algadeeb et al., 2020).
In the United Arab Emirates, one study has shown that fever followed by myalgia, sore throat, cough, and shortness of breath are the most common symptoms observed in pregnant women infected with SARS-CoV-2. Similar to what is observed in other middle eastern countries, pregnant patients are mostly asymptomatic or have mild-to-moderate disease (Hazari et al., 2021). Severe COVID-19 illness, ICU admission, and intubation were observed in 10 infected pregnant women with prior comorbidities, of whom eight were in their third trimester, one in her second, and one in her first trimester. The mode of delivery was mostly performed based on obstetric indication, except for few cases where the lower segment cesarean section was done because of COVID-19 pneumonia (Hazari et al., 2021). Compared to non-pregnant women with COVID-19, infected pregnant women had more severe symptoms, ICU admissions, and complications. Furthermore, C-reactive protein and D-dimer levels were significantly higher in pregnant patients (Hazari et al., 2021). Fortunately, two studies have shown a minimal risk of vertical transmission as well as symptomatic infection of COVID-19 in neonates born to infected mothers (Hazari et al., 2021; Kaushal et al., 2021). In Qatar, two cases of infected pregnant women, one in her 26th week and one in her 32th week of gestation, were reported. Both had typical symptoms of COVID-19, i.e., fever, shortness of breath, cough, and sore throat. Cesarian section was performed for fetal safety with both newborns being negative for SARS-CoV-2 (Yaqoub et al., 2020; Khatib et al., 2021). Another interesting study conducted in Qatar, on 16 vaccinated and 370 unvaccinated pregnant women infected with SARS-CoV-2, found that 47% of the isolates were the Beta variant (B.1.351), 19% the alpha variant (B.1.1.7), and 34% were variants of unknown status. Eight severe cases and one critical case were recorded with all being in the unvaccinated group. Moreover, six were in their 1st trimester of pregnancy and two were in their second (Butt et al., 2021).
In Kuwait, one study reported that the most common symptoms of COVID-19 in pregnant women were fever and cough. The median gestational age of infected pregnant women in this study was 29 weeks with half of them being in their third trimester and the majority (up to 91%) having no pre-existing medical conditions. Vertical transmission was minimal with only two neonates out of 167, testing positive for SARS-CoV-2. One newborn was asymptomatic, while the other one required intensive care and was discharged later in a good condition (Ayed et al., 2020). In Bahrain, high incidence of COVID-19 was reported in pregnant women in their third trimester. Pregnant women were mostly asymptomatic with the symptomatic ones experiencing mild disease without the need for ICU admission. The complications by trimester of pregnancy were statistically significant for miscarriage where 25%–26% reported in the 1st trimester, 40% in the second, and 0% in the third one. No vertical transmission was observed (Sunder et al., 2022). In the Sultanate of Oman, it was shown that the risk of ICU admission, preterm labor, pre-eclampsia, and emergency lower segment C-section, in addition to thromboembolic complications, are increased in pregnant women with COVID-19. Similarly in this study, all neonates tested negative for the SARS-CoV-2 virus (Santhosh et al., 2021). In Egypt, only one study was conducted on pregnant women with COVID-19. In this latter, it was found that compared to non-pregnant women with COVID-19, pregnant patients tend to have more severe symptoms in the form of clinical pneumoniae, are more likely to be hospital and ICU admitted, need invasive mechanical ventilator, and experience severe outcomes of infection (BahaaEldin et al., 2021; Table 1).
In Jordan, one case of an infected pregnant woman in her third trimester, and who gave birth through C-section due to obstetric indication was reported. The newborn was SARS-CoV-2 negative and the mother was discharged later in a good general condition (AlZaghal et al., 2020). In Israel, scarce studies exist on the prognosis of COVID-19 in pregnant women. Two studies have shown that the most common symptom observed in this population is cough (Harel et al., 2021) and that the disease presents mostly as a mild or an asymptomatic one (Rottenstreich et al., 2021). The majority of pregnant women were in their third trimester at the time of COVID-19 diagnosis and delivery (Mohr-Sasson et al., 2020; Barber et al., 2021; Harel et al., 2021; Rottenstreich et al., 2021). Vaginal delivery was the most common, followed by C-section that was mainly performed based on obstetric indication and not due to the SARS-CoV-2 infection (Mohr-Sasson et al., 2020; Barber et al., 2021; Rottenstreich et al., 2021). Compared to non-pregnant patients, it was found in one study that the relative lymphocyte counts to white blood cells and the levels of WBC and absolute neutrophil counts are significantly reduced and increased in pregnant patients, respectively (Mohr-Sasson et al., 2020; Barber et al., 2021; Table 1). Furthermore, non-pregnant infected women are more likely to have chronic diseases and be hospitalized (Barber et al., 2021). With regard to neonates born to infected mothers, no vertical transmission was reported (Barber et al., 2021; Harel et al., 2021; Rottenstreich et al., 2021). In pregnant women, only one study was conducted to compare SARS-CoV-2 infected pregnant women vaccinated with a 1st dose of COVID-19 vaccine versus unvaccinated ones. This study was conducted in Israel and found that there is no notable difference in the rate of symptomatic infection, SARS-CoV-2-related hospitalization, or maternal complications such as pre-eclampsia, abortions, still birth, and maternal death between both groups (Goldshtein et al., 2021).
Children
At the international context, many descriptive studies were conducted in children infected with SARS-CoV-2. However, little is known about this category in the middle eastern countries.
In Iran, the clinical outcome of SARS-CoV-2 infection in children is well described compared to other countries in the Middle East. It was found that the most common symptoms reported in children, i.e., <18 years old, are cough and dyspnea (Rabizadeh et al., 2020; Fahimzad et al., 2021) and fever and cough (Hosseninasab et al., 2020; Mahmoudi et al., 2020; Armin et al., 2021; Hoseinyazdi et al., 2021; Mamishi et al., 2021; Soleimani et al., 2021; Keshavarz Valian et al., 2022). Gastrointestinal symptoms including vomiting, nausea, and diarrhea were reported but to a lesser extent (Hosseninasab et al., 2020; Mamishi et al., 2020; Armin et al., 2021; Fahimzad et al., 2021; Pourakbari et al., 2021). Interestingly, neurological symptoms such as seizure, fatigue, and headache were also described (Cheraghali et al., 2021; Fahimzad et al., 2021). In one study, it was found that among hospitalized children with acute respiratory infection, those with COVID-19 had a higher frequency of respiratory distress and fever compared to those without (Hosseninasab et al., 2020). Furthermore, laboratory findings showed that hospitalized children with COVID-19 are more likely to have elevated inflammatory markers such as C-reactive protein, erythrocyte sedimentation rate (ESR), liver enzymes like alanine aminotransferase and aspartate aminotransferase (Armin et al., 2021; Hoseinyazdi et al., 2021; Mamishi et al., 2021); leukocytosis and leukopenia were also observed in one study (Armin et al., 2021). Prognosis of COVID-19 in Iranian children ranges from mild (Armin et al., 2021; Fahimzad et al., 2021) to severe disease (Mahmoudi et al., 2020; Ghazizadeh Esslami et al., 2021; Mamishi et al., 2021). In two studies, the SARS-CoV-2 infection manifested as a hyperinflammatory syndrome with multi-organ involvement similar to Kawasaki disease shock syndrome (Mamishi et al., 2021) and as MIS-C (Mamishi et al., 2020). In one study that reported severe infection in children, 51% of the patients had underlying medical conditions such as chronic kidney disease, neurological disorders, and others (Mahmoudi et al., 2020). In COVID-19 children, compared to those admitted to the general ward, ICU patients had more acute respiratory distress syndrome, shock, and acute cardiac injury as complications (Fahimzad et al., 2021). The mortality rate appears to be low in infected children of Iran (Hosseninasab et al., 2020; Armin et al., 2021; Hoseinyazdi et al., 2021; Mamishi et al., 2021; Pourakbari et al., 2021), ranging from 0 death (Esmaeili Dooki et al., 2020; Soleimani et al., 2021) to 11% (Mahmoudi et al., 2020; Mamishi et al., 2020; Armin et al., 2021). Interestingly, in one study the mortality rate was 20%, however, this was in children with underlying malignancies, namely acute lymphocytic and acute myeloid leukemia (Navaeian et al., 2021). The source of SARS-CoV-2 infection in the majority of the studies described in Iran was essentially contact history with suspected or confirmed family member with COVID-19 (Mahmoudi et al., 2020; Mamishi et al., 2020, 2021; Ghazizadeh Esslami et al., 2021; Navaeian et al., 2021). In one study, the source of infection was mainly unknown (Soleimani et al., 2021) and in another one, one case was infected during hospitalization (Mamishi et al., 2021).
In the Kingdom of Saudi Arabia, studies have shown a dominant benign prognosis of COVID-19 disease in children. A substantial portion were asymptomatic (Ahmad et al., 2021; Almuzaini et al., 2021; Hijazi et al., 2021) with this rate ranging from 27.9% (Mosalli et al., 2021) to 54.6% (Alharbi et al., 2021). Among symptomatic ones, the most common reported symptoms were fever, cough, runny nose, and shortness of breath (Harbi et al., 2020; Ahmad et al., 2021; Alharbi et al., 2021; Alshengeti et al., 2021; Mosalli et al., 2021; Shahin et al., 2021; AlGhamdi et al., 2022). Gastrointestinal symptoms were also described but to a lesser extent (Almoosa et al., 2020; Alharbi et al., 2021; Almuzaini et al., 2021; Alnajjar et al., 2021; Shahin et al., 2021). In some patients, the infection was first asymptomatic and presented later as a complicated course in the form of MIC-S (Almoosa et al., 2020; Al Qahtani et al., 2021 Shahin et al., 2021; Table 2). Among symptomatic ones, the rate of hospitalization ranged from 4.4% (Hijazi et al., 2021) to 9.6% (Alharbi et al., 2021). Similar to what was observed in Iran, it was found that pre-existing comorbidities, notably cardiac diseases, malignancies, and neurological and metabolic disorders, in addition to higher frequency of lower respiratory and gastrointestinal symptoms, are significantly associated with hospitalization in infected children (Alharbi et al., 2021); an observation that was also described in other studies (DeBiasi et al., 2020; Hendler et al., 2021; Woodruff et al., 2022). Moeller et al., conducted a multicenter study across 174 centers in Europe, found that children with chronic respiratory conditions might be at a higher risk of experiencing a severe form of COVID-19 (Moeller et al., 2020). In hospitalized COVID-19 children, the majority were admitted to the general ward and did not require intensive care (Alshengeti et al., 2021; Asseri et al., 2021; Almuzaini et al., 2021; Alnajjar et al., 2021; Hijazi et al., 2021; Kari et al., 2021b; AlGhamdi et al., 2022). On the other hand, those that needed intensive care had more frequent shortness of breath, higher absolute neutrophil counts and ferritin levels, and lower oxygen saturation, and albumin levels compared to those admitted to the general ward in the hospital (Asseri et al., 2021). Another study in KSA found that higher levels of creatinine values, leukocytes, and transaminases, in addition to worse renal functions, are significantly associated with ICU admission (Alharbi et al., 2021). Furthermore, the length of hospital stay was significantly correlated with the presence of comorbidities, leucopenia (Kari et al., 2021b), high levels of absolute neutrophil count (Shahin et al., 2021) as well as inflammatory markers such as ferritin, D-dimer, ESR and CRP (Kari et al., 2021b; Shahin et al., 2021). Indeed, the laboratory findings showed that among hospitalized patients, lymphocytopenia and leukopenia, in addition to elevated levels of ferritin, D-dimer, and C-reactive proteins, were encountered (Almoosa et al., 2020; Alnajjar et al., 2021; Mosalli et al., 2021; Shahin et al., 2021). The mortality rate was very low in the infected children of this country, with this latter not exceeding four deaths among all reports (Almoosa et al., 2020; Alnajjar et al., 2021; Alshengeti et al., 2021; Asseri et al., 2021; Hijazi et al., 2021; Kari et al., 2021b; Mosalli et al., 2021; AlGhamdi et al., 2022). Interestingly, in one study, it was found that 21% of hospitalized children infected with SARS-CoV-2 developed acute kidney injury. Children with AKI had a higher number of comorbidities compared to those with normal kidney function. Furthermore, AKI was significantly associated with more frequent ICU admissions as well as mortality. Residual renal impairment upon discharge occurred in 9% of those with AKI. This latter was influenced significantly by the presence of comorbidities, hypoxia, hypotension, heart failure, acute respiratory distress syndrome, hypernatremia, and high CRP level (Kari et al., 2021a). Like other countries, the main source of infection in Saudi children was family members, notably the father or the mother (Alnajjar et al., 2021; Shahin et al., 2021).
Al-Fraij et al. conducted a study both in KSA and Kuwait, where symptomatic children infected with SARS-CoV-2 and admitted to the ICU were explored. It was found that fever and cough were the most common symptoms at the time of ICU admission. The common cause of ICU admission was respiratory failure. On the other hand, low platelet counts, high procalcitonin, circulatory compromise, and the presence of comorbidities, such as hematological malignancies and neurological disorders, were associated with death in this group of population (Alfraij et al., 2021).
In Qatar, only two studies were conducted on Children with COVID-19. One included seven children who fulfilled the criteria of MIS-C according to WHO. The most common symptoms were fever, rash and gastrointestinal symptoms, and to a lesser extent upper respiratory tract ones. All cases included in the aforementioned study had high levels of procalcitonin, CRP, and ferritin levels, in addition to deranged coagulation profile. Five children required intensive care for inotropic support who later on were discharged in good condition (Hasan et al., 2021; Table 2). Notably, all children tested positive for COVID-19 antibodies, and only one tested positive by PCR test from nasopharyngeal swab; supporting thus the post-infectious nature of the MIS-C disease in this group (Hasan et al., 2021). The other study in Qatar was a case report of an 8-month-old infant with newly diagnosed diabetes (Soliman et al., 2020a). In Oman, the most common symptoms among symptomatic children infected with SARS-CoV-2 were fever (Alqayoudhi et al., 2021) followed by other respiratory and gastrointestinal symptoms (Al Yazidi et al., 2021). Hospitalized as well as critically ill patients were more likely to have pre-existing medical conditions. Moreover, intensive care was independently associated with leukocytosis and elevated C-reactive protein levels (Al Yazidi et al., 2021). No mortality was recorded (Al Yazidi et al., 2021; Al Sabahi et al., 2021; Alqayoudhi et al., 2021). As seen in other countries, family members were the main source of infection (Al Yazidi et al., 2021). Interestingly, in one study, it was found that 50 out of 1,026 children with COVID-19 were able to transmit the virus to 107 patients including 86 adults and 21 children (Alqayoudhi et al., 2021). In the UAE, one study found that the most common symptoms in infected children were fever, cough, and rhinorrhea. Patients presented with mild-to-moderate disease with no severe symptoms or mortality being reported. Furthermore, high levels of CRP and lactate dehydrogenase were significantly associated with symptomatic children. History of contact with family or household member was reported in the majority of infected patients (Ennab et al., 2021). In Iraq, Salih et al., found that fever was the common symptom detected in children with COVID-19. In this study, it was found that 62% of the cases showed signs of severe disease in the form of MIS-C. Around one-third required ICU admission with the majority of these being among MIS-C patients. Mortality was related to primary COVID-19 infection and to MIS-C cases (Salih et al., 2022). Another study reported the first case of delta variant B.1.617.2 in Iraq which was detected in a 6 years old female who presented at the hospital with fever, headache, severe abdominal pain, vomiting, and diarrhea. She was successfully treated and was discharged from the hospital in good condition (Essa et al., 2021). In Bahrain, AlGhoozi et al. reported a case where SARS-CoV-2 infection was possibly the trigger of Henoch-Schonlein purpura in a 4 years old boy (AlGhoozi and AlKhayyat, 2021).
In Egypt, fever, dry cough, polypnea, fatigue, headache, and shock were among the most common symptoms observed in children with COVID-19 (Shafiek et al., 2021; Saleh et al., 2021). Interestingly, in one study, in a subset of patients, the disease manifested by atypical presentations including deep venous thrombosis, acute pancreatitis, and MIS-C (Saleh et al., 2021; Table 2). Higher levels of D-dimer were significantly associated with disease severity (Shafiek et al., 2021; Saleh et al., 2021). The mortality rate was 5.3%, with these constituting 20.4% of the severe cases (Saleh et al., 2021). Definite contact with a SARS-CoV-2 infected family member was the main source of infection (Shafiek et al., 2021; Saleh et al., 2021). Sherif et al. conducted a study on infected children with type 1 diabetes and found that all four cases required ICU admission with the common symptoms being fever with respiratory or gastrointestinal ones. Three of the four cases presented with an MIS-C-like picture (Sherif et al., 2021). On the other hand, studies conducted on cancer children with COVID-19 showed that these can be either asymptomatic (Ebeid et al., 2021) or symptomatic with mainly mild-to-moderate form of the illness (Hammad et al., 2021; Hamdy et al., 2021). The mortality rate was as low as 6.5% after the exclusion of septicemia and cancer progression in one study (Hammad et al., 2021) and almost the half in another one (Hamdy et al., 2021). Interestingly, in both of the aforementioned studies, the poor outcome was mainly observed in those with hematological malignancies. In a study conducted in the United Kingdom, it was found that patients with hematological malignancies are at an increased risk of critical SARS-CoV-2 infection (Lee et al., 2020). On the contrary, another study in the US did not find an increased mortality in this type of cancer patients (Kuderer et al., 2020). Whether the effect observed is a true impact of COVID-19 or is due to the higher chemotherapy intensity given in these patients, should be more investigated in future studies.
In Israel, fever and cough were the most common symptoms observed in symptomatic children infected with SARS-CoV-2 (Ben-Shimol et al., 2021; Shapiro Ben David et al., 2021). Seizures in patients with prior neurological disorder were also reported (Kurd et al., 2021). Meyer et al., reported a case of an 8-month-old infant who contracted severe gastrointestinal symptoms, mild carditis, and significant hypoalbuminemia, following an asymptomatic infection with SARS-CoV-2 (Orlanski-Meyer et al., 2020). COVID-19 prognosis in Israeli children appears to be mainly mild (Ben-Shimol et al., 2021; Shapiro Ben David et al., 2021). In one study, pediatric inflammatory multisystem syndrome (PMIS) was detected in a subset of patients. Mechanical ventilation, intensive care in addition to lymphopenia, higher rates of neutrophils/lymphocytes, and elevated CRP and troponin levels were most common among PMIS patients compared to those with mild and moderate/severe disease (Ben-Shimol et al., 2021). No mortality was recorded. In one study that included 1,032 children with COVID-19, it was found that contact with a confirmed case was the most common source of infection followed by school exposure and unknown source (Shapiro Ben David et al., 2021). Among those who acquired the infection via a confirmed case, the majority were throughout infected parents. On the other hand, those who got the infection from school were mainly asymptomatic and were detected via contact tracing (Shapiro Ben David et al., 2021). The role of children in the transmission of SARS-CoV-2 appears to be less prominent than the one of adults (Dattner et al., 2021). Children cases are mainly secondary rather than primary (Shapiro Ben David et al., 2021). Only one study conducted by Somekh et al., explored the prognosis of COVID-19 in children in two different periods where the former SARS-CoV-2 variants (mainly GH and GR clades) were circulating in Israel versus when the B.1.1.7 variant was introduced. Results revealed a lower rate of hospitalization when the B.1.1.7 was introduced; however, the percentage of hospitalized children with poor outcomes, i.e., severe illness and/or death was not different between the two periods (Somekh et al., 2021). This implies that although B.1.1.7 appear to be more contagious but is not necessarily more severe, similar with what has been observed in other reports (Frampton et al., 2021; Galloway et al., 2021). Last but not least, in Jordan, the rate of asymptomatic vs. symptomatic children with COVID-19 was almost half, half. The most prominent symptoms were nasal congestion, generalized malaise, and headache. All children had infected parents (Kilani et al., 2021). In Lebanon, only one case of COVID-19 in children was reported (Mansour et al., 2020).
The favorable outcome of COVID-19 seen in children in the middle eastern population is similar to the one reported in other worldwide regions including Europe and China (Götzinger et al., 2020; Badal et al., 2021). Lower expression of angiotensin-converting enzyme 2 in children, differences in the immune system status as well as lower frequency of underlying medical conditions in children compared to adults has been suggested to play a role in the mild form of the COVID-19 illness in this population (Zimmerman et al., 2013; Falahi et al., 2021).
Conclusion
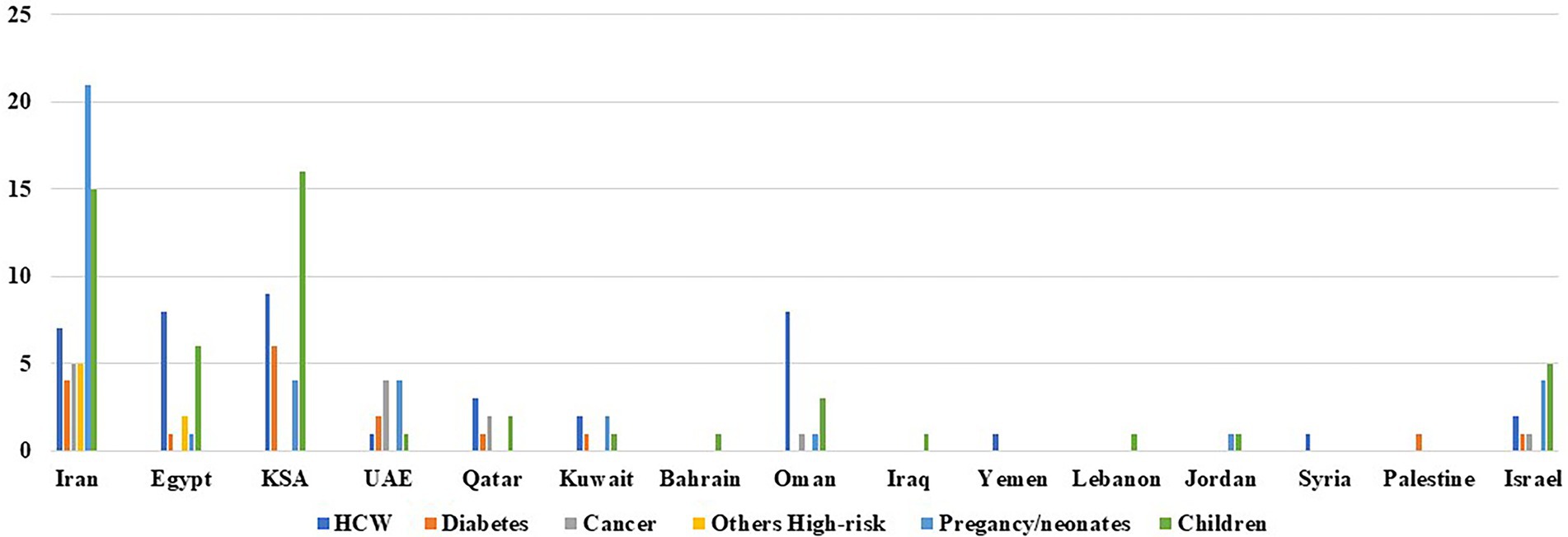
In this review, we have highlighted several important issues regarding SARS-CoV-2 infection in the middle eastern region. First, there is a substantial proportion of asymptomatic carriage of among healthcare workers. This necessitates the implementation of a routine-based screening, not based on the presence of symptoms solely. This is in order to limit the transmission of the virus from the asymptomatic healthcare personnel to other patients, colleagues, or community members. This measure should be however tailored based on each country’s resource capacity. This is especially relevant in the Levant region, where financial and political crises are still ongoing. In fact, this is manifested in this review by the scarcity of studies exploring the prognosis of COVID-19 in different population categories in this region of the Middle East (Figure 1). High-risk patients appear to be at a higher risk of increased morbidity and mortality from SARS-CoV-2 infection compared to the general population (Table 1). Studies aimed to perform risk stratification of these high-risk patients are of paramount importance in the region. In fact, highlighting the clinical biomarkers during infection in these categories can be useful in the early suspicion of the disease, predicting clinical outcome, framing hospital and ICU admission/discharge, risk stratification; in addition to rationalizing therapies and assessing patients’ response to it. Unfortunately, enough data on which biomarkers can be used is still lacking in the Middle East. This also applies to infected pregnant women with their neonates. The vertical transmission of SARS-CoV-2 from the mother to the neonate is still controversial. Culturing of amniotic fluid, umbilical cord blood, in addition to neonatal nasopharyngeal swab is essential to explore this hypothesis. This should be done aseptically and at several time points after delivery. Last but not least, for children, although few studies were conducted; the current knowledge shows that SARS-CoV-2 infection is manifested as a benign form. This is not to omit, that physicians should be at the same time aware of the atypical presentations of COVID-19 in this group of population (Table 2). It is important to note also that the findings of this review are mainly based on studies conducted on unvaccinated people. The role of vaccine, viral load, variant of concerns, antiviral treatment in the prognosis and spread of COVID-19 in the categories described in this review should be addressed in future studies. This is in order to know the current true impact of this disease on different patients’ categories in the middle eastern region.
Author contributions
All authors conceived and designed the review. ID wrote the manuscript. WA corrected the manuscript. All authors contributed to manuscript revision, and approved the final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelmoniem, R., Fouad, R., Shawky, S., Amer, K., Elnagdy, T., Hassan, W. A., et al. (2021). SARS-CoV-2 infection among asymptomatic healthcare workers of the emergency department in a tertiary care facility. J. Clin. Virol. 134:104710. doi: 10.1016/j.jcv.2020.104710
Abdelrahman, Z., Li, M., and Wang, X. (2020). Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza A respiratory viruses. Front. Immunol. 11:552909. doi: 10.3389/fimmu.2020.552909
Abdulah, D. M. (2021). Prevalence and correlates of COVID-19 vaccine hesitancy in the general public in Iraqi Kurdistan: A cross-sectional study. J. Med. Virol. 93, 6722–6731. doi: 10.1002/jmv.27255
Abedzadeh-Kalahroudi, M., Sehat, M., Vahedpour, Z., and Talebian, P. (2021a). Maternal and neonatal outcomes of pregnant patients with COVID-19: A prospective cohort study. Int. J. Gynaecol. Obstet. 153, 449–456. doi: 10.1002/ijgo.13661
Abedzadeh-Kalahroudi, M., Sehat, M., Vahedpour, Z., Talebian, P., and Haghighi, A. (2021b). Clinical and obstetric characteristics of pregnant women with covid-19: A case series study on 26 patients. Taiwan. J. Obstet. Gynecol. 60, 458–462. doi: 10.1016/j.tjog.2021.03.012
Abolhasan, C. F., Ghassemzadeh, M., Attarian, M., Abbariki, E., Nateghian, A., Ghanbari, B., et al. (2021). Transplacental transmission of SARS-CoV-2 infection: A case report from Iran. Arch. Pediatr. Infect. Dis. 9, 1–6. doi: 10.5812/pedinfect.108582
Abou-ElWafa, H. S., El-Gilany, A. H., and Albadry, A. A. (2021). Self-reported COVID-19 among physicians: An Egyptian online study during the pandemic. F1000Research 10, 785. doi: 10.12688/f1000research.53931.1
Ahmad, N., Eltawel, M., Khan, W. M., Essa, M. F., Alharbi, T., and Al-Sudairy, R. (2021). COVID-19 in pediatric cancer patients: How concerned we should be? Lessons learned from a single center in middle east. J. Pediatr. Hematol. Oncol. 43, 279–280. doi: 10.1097/MPH.0000000000002013
Ahmed, A. S., Alotaibi, W. S., Aldubayan, M. A., Alhowail, A. H., Al-Najjar, A. H., Chigurupati, S., et al. (2021). Factors affecting the incidence, progression, and severity of COVID-19 in type 1 diabetes mellitus. Biomed. Res. Int. 2021, 1–9. doi: 10.1155/2021/1676914
Akbarian-Rad, Z., Mojaveri, M. H., Bouzari, Z., Sadeghi, F., Yahyapour, Y., Rad, M. N., et al. (2021). Neonatal outcomes in pregnant women infected with COVID-19 in Babol, north of Iran: A retrospective study with short-term follow-up. Infect. Dis. Obstet. Gynecol. 2021, 1–5. doi: 10.1155/2021/9952701
Akbariqomi, M., Hosseini, M. S., Rashidiani, J., Sedighian, H., Biganeh, H., Heidari, R., et al. (2020). Clinical characteristics and outcome of hospitalized COVID-19 patients with diabetes: A single-center, retrospective study in Iran. Diabetes Res. Clin. Pract. 169:108467. doi: 10.1016/j.diabres.2020.108467
Al Abri, Z. G. H., Al Zeedi, M. A. S. A., and Al Lawati, A. A. (2021). Risk factors associated with COVID-19 infected healthcare workers in Muscat governorate, Oman. J. Prim. Care Community Health 12, 215013272199545. doi: 10.1177/2150132721995454
Al Bahrani, B. J., Mehdi, I., Khamis, F. A., Al Farsi, A. M., Fahdi, F. A., and Al Lawati, N. A. (2021). COVID-19 amongst cancer patients: An experience from Oman. J. Pak. Med. Assoc. 71, 2563–2569. doi: 10.47391/JPMA.11915
Al Bujayr, A. A., Aljohar, B. A., Bin Saleh, G. M., Alanazi, K. H., and Assiri, A. M. (2021). Incidence and epidemiological characteristics of COVID-19 among health care workers in Saudi Arabia: A retrospective cohort study. J. Infect. Public Health 14, 1174–1178. doi: 10.1016/j.jiph.2021.08.005
Al Hayek, A. A., Robert, A. A., Alotaibi, Z. K., and Al Dawish, M. (2020a). Clinical characteristics of hospitalized and home isolated COVID-19 patients with type 1 diabetes. Diabetes Metab. Syndr. 14, 1841–1845. doi: 10.1016/j.dsx.2020.09.013
Al Hayek, A. A., Robert, A. A., Matar, A. B., Algarni, A., Alkubedan, H., Alharbi, T., et al. (2020b). Risk factors for hospital admission among COVID-19 patients with diabetes. A study from Saudi Arabia. Saudi Med. J. 41, 1090–1097. doi: 10.15537/smj.2020.10.25419
Al Lawati, A., Khamis, F., Al Habsi, S., and Al Dalhami, K. (2021). Risk of COVID-19 infection in healthcare workers exposed during use of non-invasive ventilation in a tertiary care hospital in Oman. Oman Med. J. 36:e236. doi: 10.5001/omj.2021.110
Al Maskari, Z., Al Blushi, A., Khamis, F., Al Tai, A., Al Salmi, I., Al Harthi, H., et al. (2021). Characteristics of healthcare workers infected with COVID-19: A cross-sectional observational study. Int. J. Infect. Dis. 102, 32–36. doi: 10.1016/j.ijid.2020.10.009
Al Qahtani, M., Uddin, M. S., Al Fulayyih, S., Al Baridi, S., and Hamid, Z. (2021). An 11-year-old saudi arabian girl who presented with multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection with coronary artery aneurysm and cardiac involvement: A case report. Am. J. Case Rep. 22:e933053. doi: 10.12659/AJCR.933053
Al Rawahi, B., Al Wahaibi, A., Al Khalili, S., Al Balushi, A. Y. M., Al-Shehi, N., Al Harthi, K., et al. (2022). The impact of the acceleration of COVID-19 vaccine deployment in two border regions in Oman. IJID Regions 3, 265–267. doi: 10.1016/j.ijregi.2022.03.020
Al Sabahi, A., Al Maskari, N., Al Busaidi, I., Albiroty, K. A., Alkhamisy, A., Al Hinai, K., et al. (2021). Coronavirus disease 2019 infection in children with sickle cell disease: Case series from Oman. J. Pediatr. Hematol. Oncol. 43, e975–e978. doi: 10.1097/MPH.0000000000002061
Al Yazidi, L. S., Al Hinai, Z., Al Waili, B., Al Hashami, H., Al Reesi, M., Al Othmani, F., et al. (2021). Epidemiology, characteristics and outcome of children hospitalized with COVID-19 in Oman: A multicenter cohort study. Int. J. Infect. Dis. 104, 655–660. doi: 10.1016/j.ijid.2021.01.036
Al Youha, S., Alowaish, O., Ibrahim, I. K., Alghounaim, M., Abu-Sheasha, G. A., Fakhra, Z., et al. (2021). Factors associated with SARS-CoV-2 infection amongst healthcare workers in a COVID-19 designated hospital. J. Infect. Public Health 14, 1226–1232. doi: 10.1016/j.jiph.2021.08.012
Alajmi, J., Jeremijenko, A. M., Abraham, J. C., Alishaq, M., Concepcion, E. G., Butt, A. A., et al. (2020). COVID-19 infection among healthcare workers in a national healthcare system: The Qatar experience. Int. J. Infect. Dis. 100, 386–389. doi: 10.1016/j.ijid.2020.09.027
Al-Ayyadhi, N., Ramadan, M. M., Al-Tayar, E., Al-Mathkouri, R., and Al-Awadhi, S. (2021). Determinants of hesitancy towards COVID-19 vaccines in State of Kuwait: An exploratory internet-based survey. Risk Manage. Healthcare Policy 14, 4967–4981. doi: 10.2147/RMHP.S338520
Alfraij, A., Bin Alamir, A. A., Al-Otaibi, A. M., Alsharrah, D., Aldaithan, A., Kamel, A. M., et al. (2021). Characteristics and outcomes of coronavirus disease 2019 (COVID-19) in critically ill pediatric patients admitted to the intensive care unit: A multicenter retrospective cohort study. J. Infect. Public Health 14, 193–200. doi: 10.1016/j.jiph.2020.12.010
Algadeeb, K. B., AlMousa, H. H., AlKadhem, S. M., Alduhilan, M. O. 2nd, and Almatawah, Y. (2020). A novel case of severe respiratory symptoms and persistent pulmonary hypertension in a Saudi neonate with SARS-CoV-2 infection. Cureus 12:e10472. doi: 10.7759/cureus.10472
AlGhamdi, A., Al Talhi, Y., Al Najjar, A., Sobhi, A., Al Juaid, A., Ibrahim, A., et al. (2022). Epidemiology, clinical characteristics and risk factors of COVID-19 among children in Saudi Arabia: A multicenter chart review study. BMC Pediatr. 22, 86. doi: 10.1186/s12887-021-02959-8
AlGhoozi, D. A., and AlKhayyat, H. M. (2021). A child with henoch-schonlein purpura secondary to a COVID-19 infection. BMJ Case Rep. 14:e239910. doi: 10.1136/bcr-2020-239910
Alguwaihes, A. M., Al-Sofiani, M. E., Megdad, M., Albader, S. S., Alsari, M. H., Alelayan, A., et al. (2020). Diabetes and covid-19 among hospitalized patients in Saudi Arabia: A single-Centre retrospective study. Cardiovasc. Diabetol. 19:205. doi: 10.1186/s12933-020-01184-4
Alhabbab, R. Y., Alsaieedi, A., Algaissi, A., Almahboub, S., Al-Raddadi, R. M., Shabouni, O. I., et al. (2021). Seroprevalence of SARS-CoV-2 binding and neutralizing antibodies in healthcare workers during the epidemic peak in referral hospitals and quarantine sites: Saudi Arabia. Viruses 13, 1413. doi: 10.3390/v13071413
Alhamoud, A. H., Matary, F., Bukhari, S., Kelantan, M., and Bajahzer, M. (2020). Outcomes of an in vitro fertilization pregnancy with COVID-19 and the perinatal outcome in Riyadh, Saudi Arabia. Cureus 12:e12296. doi: 10.7759/cureus.12296
Alharbi, M., Kazzaz, Y. M., Hameed, T., Alqanatish, J., Alkhalaf, H., Alsadoon, A., et al. (2021). SARS-CoV-2 infection in children, clinical characteristics, diagnostic findings and therapeutic interventions at a tertiary care center in Riyadh, Saudi Arabia. J. Infect. Public Health 14, 446–453. doi: 10.1016/j.jiph.2020.12.034
Ali, E., Badawi, M., Abdelmahmuod, E., Kohla, S., and Yassin, M. A. (2020). Chronic lymphocytic leukemia concomitant with COVID 19: A case report. Am. J. Case Rep. 21:e926062. doi: 10.12659/AJCR.926062
Alipour, Z., Samadi, P., Eskandari, N., Ghaedrahmati, M., Vahedian, M., Khalajinia, Z., et al. (2021). Relationship between coronavirus disease 2019 in pregnancy and maternal and fetal outcomes: Retrospective analytical cohort study. Midwifery 102:103128. doi: 10.1016/j.midw.2021.103128
Alishaq, M., Jeremijenko, A., Al-Kanaani, Z., Nafady-Hego, H., Jboor, D. H., Saba, R., et al. (2021a). Prevalence and risk factors for SARS-CoV-2 infection and seroprevalence among clinical and non-clinical staff in a national healthcare system. PLoS One 16:e0257845. doi: 10.1371/journal.pone.0257845
Alishaq, M., Nafady-Hego, H., Jeremijenko, A., Al Ajmi, J. A., Elgendy, M., Vinoy, S., et al. (2021b). Risk factors for breakthrough SARS-CoV-2 infection in vaccinated healthcare workers. PLoS One 16:e0258820. doi: 10.1371/journal.pone.0258820
Al-Kuwari, M. G., Abdul Malik, M. A., Al-Nuaimi, A. A., Abdulmajeed, J., Al-Romaihi, H. E., Semaan, S., et al. (2021). Epidemiology characteristics of COVID-19 infection amongst primary health care workers in Qatar: March-october 2020. Front. Public Health 9:679254. doi: 10.3389/fpubh.2021.679254
Allotey, J., Stallings, E., Bonet, M., Yap, M., Chatterjee, S., Kew, T., et al. (2020). Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ (Clinical Research Ed.) 370:m3320. doi: 10.1136/bmj.m3320
Al-Maani, A., Al Wahaibi, A., Al-Sooti, J., Al Abri, B., Al Shukri, I., AlRisi, E., et al. (2021). The role of supporting services in driving SARS-CoV-2 transmission within healthcare settings: A multicenter seroprevalence study. Int. J. Infect. Dis. 107, 257–263. doi: 10.1016/j.ijid.2021.04.071
Almaghrabi, R. S., Alhamlan, F. S., Dada, A., Al-Tawfiq, J. A., Al Hroub, M. K., Saeedi, M. F., et al. (2022). Outcome of SARS-CoV-2 variant breakthrough infection in fully immunized solid organ transplant recipients. J. Infect. Public Health 15, 51–55. doi: 10.1016/j.jiph.2021.11.021
Al-Matary, A., Almatari, F., Al-Matary, M., AlDhaefi, A., Alqahtani, M. H. S., Alhulaimi, E. A., et al. (2021). Clinical outcomes of maternal and neonate with COVID-19 infection – multicenter study in Saudi Arabia. J. Infect. Public Health 14, 702–708. doi: 10.1016/j.jiph.2021.03.013
Almhawish, N., Karah, N., Elferruh, Y., Aksh, A., and Abbara, A. (2021). Protecting healthcare workers in conflict zones during the COVID-19 pandemic: Northwest Syria. J. Infect. 82, 186–230. doi: 10.1016/j.jinf.2021.01.027
Almoosa, Z. A., Al Ameer, H. H., AlKadhem, S. M., Busaleh, F., AlMuhanna, F. A., and Kattih, O. (2020). Multisystem inflammatory syndrome in children, the real disease of COVID-19 in pediatrics: a multicenter case series from al-Ahsa, Saudi Arabia. Cureus 12:e11064. doi: 10.7759/cureus.11064
Almuzaini, Y., Alsohime, F., Subaie, S. A., Temsah, M. H., Alsofayan, Y., Alamri, F., et al. (2021). Clinical profiles associated with SARS-CoV-2 infection and complications from coronavirus disease-2019 in children from a national registry in Saudi Arabia. Ann. Thoracic Med. 16, 280–286. doi: 10.4103/atm.atm_709_20
Al-Naamani, K., Al-Jahdhami, I., Al-Tamtami, W., Al-Amri, K., Al-Khabori, M., Sinani, S. A., et al. (2021). Prevalence and persistence of SARS-CoV2 antibodies among healthcare workers in Oman. J. Infect. Public Health 14, 1578–1584. doi: 10.1016/j.jiph.2021.09.006
Alnajjar, A. A., Dohain, A. M., Abdelmohsen, G. A., Alahmadi, T. S., Zaher, Z. F., and Abdelgalil, A. A. (2021). Clinical characteristics and outcomes of children with COVID-19 in Saudi Arabia. Saudi Med. J. 42, 391–398. doi: 10.15537/smj.2021.42.4.20210011
Alqayoudhi, A., Al Manji, A., Al Khalili, S., Al Maani, A., Alkindi, H., Alyaquobi, F., et al. (2021). The role of children and adolescents in the transmission of SARS-CoV-2 virus within family clusters: A large population study from Oman. J. Infect. Public Health 14, 1590–1594. doi: 10.1016/j.jiph.2021.09.008
AlQurashi, M. A., Alattas, A., Shirah, B., Mustafa, A., Al-Hindi, M. Y., Alrefai, A., et al. (2021). Clinical characteristics of newborn infants delivered to pregnant women with laboratory-confirmed COVID-19: A single-center experience from Saudi Arabia. Cureus 13:e18573. doi: 10.7759/cureus.18573
Alroqi, F., Masuadi, E., Alabdan, L., Nogoud, M., Aljedaie, M., Abu-Jaffal, A. S., et al. (2021). Seroprevalence of SARS-CoV-2 among high-risk healthcare workers in a MERS-CoV endemic area. J. Infect. Public Health 14, 1268–1273. doi: 10.1016/j.jiph.2021.08.029
Al-Sabah, S., Al-Haddad, M., Al-Youha, S., Jamal, M., and Almazeedi, S. (2020). COVID-19: Impact of obesity and diabetes on disease severity. Clinical Obesity 10:e12414. doi: 10.1111/cob.12414
Al-Sakkaf, E., Ghaleb, Y., Al-Dabis, E., Qairan, M., Al Amad, M., Al Serouri, A., et al. (2021). First COVID-19 cases with high secondary infection among health workers, sana'a capital, April 2020: Lessons learned and future opportunities. Int. J. Infect. Dis. 110, S6–S10. doi: 10.1016/j.ijid.2021.04.022
Alshahrani, M. S., Alnimr, A., Alnassri, S., Alfarag, S., Aljehani, Y., and Alabdali, M. (2020). Prevalence of the SARS-CoV-2 infection among post-quarantine healthcare workers. J. Multidiscip. Healthc. 13, 1927–1936. doi: 10.2147/JMDH.S279469
Al-Shaikh, A., Muthu, N., Aidyralieva, C., Profili, M. C., and Bellizzi, S. (2021). COVID-19 vaccine roll-out in middle-income countries: Lessons learned from the Jordan experience. Vaccine 39, 4769–4771. doi: 10.1016/j.vaccine.2021.06.078
Alshamrani, M. M., El-Saed, A., Al Zunitan, M., Almulhem, R., and Almohrij, S. (2021). Risk of COVID-19 morbidity and mortality among healthcare workers working in a large tertiary care hospital. Int. J. Infect. Dis. 109, 238–243. doi: 10.1016/j.ijid.2021.07.009
Alshamrani, M. M., Farahat, F. M., El-Saed, A., Alzunitan, M., Alsaedi, A., El Gammal, A., et al. (2022). Post-vaccination SARS-CoV-2 infection among healthcare workers in tertiary care hospitals in Saudi Arabia: A case series. J. Infect. Public Health 15, 10–12. doi: 10.1016/j.jiph.2021.11.015
Al-Shamsi, H. O., Coomes, E. A., Aldhaheri, K., and Alrawi, S. (2021). Serial screening for COVID-19 in asymptomatic patients receiving anticancer therapy in the United Arab Emirates. JAMA Oncol. 7, 129–131. doi: 10.1001/jamaoncol.2020.5745
Al-Shamsi, H. O., Coomes, E. A., and Alrawi, S. (2020). Screening for COVID-19 in asymptomatic patients with cancer in a hospital in the United Arab Emirates. JAMA Oncol. 6, 1627–1628. doi: 10.1001/jamaoncol.2020.2548
Alshengeti, A., Alahmadi, H., Barnawi, A., Alfuraydi, N., Alawfi, A., Al-Ahmadi, A., et al. (2021). Epidemiology, clinical features, and outcomes of coronavirus disease among children in al-Madinah, Saudi Arabia: A retrospective study. Int. J. Pediatr. Adolesc. Med. 9, 136–142. doi: 10.1016/j.ijpam.2021.11.001
Al-Siyabi, N., Al-Lawati, A., Al-Lawati, M., Al-Wahibi, I., Al-Nasseri, M., Al-Maashari, A., et al. (2021). Characteristics and immunoglobulin G seropositivity among COVID-19 positive healthcare workers in a tertiary care hospital in Oman. Oman Med. J. 36:e302. doi: 10.5001/omj.2021.116
Alwahaibi, N., Al Maskari, M., Al Dhahli, B., Al Issaei, H., and Al-Jaaidi Shadia Al Bahlani, S. (2021). One-year review of COVID-19 in the Arab world. Qatar Med. J. 2021, 66. doi: 10.5339/qmj.2021.66
AlZaghal, L. A., AlZaghal, N., Alomari, S. O., Obeidat, N., Obeidat, B., and Hayajneh, W. A. (2020). Multidisciplinary team management and cesarean delivery for a Jordanian woman infected with SARS-CoV-2: A case report. Case Rep. Women's Health 27:e00212. doi: 10.1016/j.crwh.2020.e00212
Alzamora, M. C., Paredes, T., Caceres, D., Webb, C. M., Valdez, L. M., and La Rosa, M. (2020). Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol. 37, 861–865. doi: 10.1055/s-0040-1710050
Amit, S., Beni, S. A., Biber, A., Grinberg, A., Leshem, E., and Regev-Yochay, G. (2021). Postvaccination COVID-19 among healthcare workers, Israel. Emerg. Infect. Dis. 27, 1220–1222. doi: 10.3201/eid2704.210016
Angel, Y., Spitzer, A., Henig, O., Saiag, E., Sprecher, E., Padova, H., et al. (2021). Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA 325, 2457–2465. doi: 10.1001/jama.2021.7152
Apicella, M., Campopiano, M. C., Mantuano, M., Mazoni, L., Coppelli, A., and Del Prato, S. (2020). COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8, 782–792. doi: 10.1016/S2213-8587(20)30238-2
Arab, M., Noei Teymoordash, S., Talayeh, M., Ghavami, B., Javadi, A., and Nouri, B. (2021). Evaluation of serologic changes of IgG and IgM antibodies associated with SARS-CoV-2 in cancer patients: A cohort seroprevalence study. Asian Pac. J. Cancer Prev. 22, 1667–1670. doi: 10.31557/APJCP.2021.22.6.1667
Armin, S., Karbasian, F., Hoseinialfatemi, S. M., Ghanaie, R. M., Tabatabaei, S. R., Fahimzad, S. A., et al. (2020). Prevalence of SARS-CoV-2 specific antibodies in the staff of a children's hospital, in Tehran, Iran. Jundishapur J. Microbiol. 13, 1–6. doi: 10.5812/jjm.108592
Armin, S., Mirkarimi, M., Pourmoghaddas, Z., Tariverdi, M., Jafrasteh, A., Marhamati, N., et al. (2021). Iranian pediatric COVID-19 epidemiology and clinical characteristics. Can. J. Infect. Dis. Med. Microbiol. 2021, 4914371, 1–4914375. doi: 10.1155/2021/4914371
Asseri, A. A., Alzaydani, I., Al-Jarie, A., Albishri, A., Alsabaani, A., Almaghrabi, M. K., et al. (2021). Clinical characteristics and laboratory abnormalities of hospitalized and critically ill children with coronavirus disease 2019: A retrospective study from Saudi Arabia. Int. J. General Med. 14, 1949–1958. doi: 10.2147/IJGM.S311831
Assiri, A., Al-Tawfiq, J. A., Alkhalifa, M., Al Duhailan, H., Al Qahtani, S., Dawas, R. A., et al. (2021). Launching COVID-19 vaccination in Saudi Arabia: Lessons learned, and the way forward. Travel Med. Infect. Dis. 43:102119. doi: 10.1016/j.tmaid.2021.102119
Ayed, A., Embaireeg, A., Benawadh, A., Al-Fouzan, W., Hammoud, M., Al-Hathal, M., et al. (2020). Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy Childbirth 20, 754. doi: 10.1186/s12884-020-03461-2
Aznab, M. (2020). Evaluation of COVID 19 infection in 279 cancer patients treated during a 90-day period in 2020 pandemic. Int. J. Clin. Oncol. 25, 1581–1586. doi: 10.1007/s10147-020-01734-6
Badal, S., Thapa Bajgain, K., Badal, S., Thapa, R., Bajgain, B. B., and Santana, M. J. (2021). Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: A systematic review and meta-analysis. J. Clin. Virol. 135:104715. doi: 10.1016/j.jcv.2020.104715
BahaaEldin, H., El Sood, H. A., Samy, S., Khader, Y., AbdelFatah, M., Hassany, M., et al. (2021). COVID-19 outcomes among pregnant and nonpregnant women at reproductive age in Egypt. J. Public Health (Oxf.) 43, iii12–iii18. doi: 10.1093/pubmed/fdab376
Balou, H. A., Yaghubi Kalurazi, T., Joukar, F., Hassanipour, S., Shenagari, M., Khoshsorour, M., et al. (2021). High seroprevalence of SARS-CoV-2 (COVID-19)-specific antibodies among healthcare workers: A cross-sectional study in guilan, Iran. J. Environ. Public Health 2021, 1–8. doi: 10.1155/2021/9081491
Barber, E., Kovo, M., Leytes, S., Sagiv, R., Weiner, E., Schwartz, O., et al. (2021). Evaluation of SARS-CoV-2 in the vaginal secretions of women with COVID-19: Aprospective study. J. Clin. Med. 10, 2735. doi: 10.3390/jcm10122735
Barry, M., AlMohaya, A., AlHijji, A., Akkielah, L., AlRajhi, A., Almajid, F., et al. (2020). Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J. Epidemiol. Global Health 10, 214–221. doi: 10.2991/jegh.k.200806.002
Barry, M., Robert, A. A., Temsah, M. H., Abdul Bari, S., Akhtar, M. Y., Al Nahdi, F., et al. (2021). COVID-19 community transmission among healthcare workers at a tertiary care cardiac center. Med. Sci. (Basel, Switzerland) 9, 49. doi: 10.3390/medsci9030049
Ben-Shimol, S., Livni, G., Megged, O., Greenberg, D., Danino, D., Youngster, I., et al. (2021). COVID-19 in a subset of hospitalized children in Israel. J. Pediatric Infect. Dis. Soc. 10, 757–765. doi: 10.1093/jpids/piab035
Bergwerk, M., Gonen, T., Lustig, Y., Amit, S., Lipsitch, M., Cohen, C., et al. (2021). Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 385, 1474–1484. doi: 10.1056/NEJMoa2109072
Bhatti, R., Khamis, A. H., Khatib, S., Shiraz, S., and Matfin, G. (2020). Clinical characteristics and outcomes of patients with diabetes admitted for COVID-19 treatment in Dubai: Single-Centre cross-sectional study. JMIR Public Health Surveill. 6:e22471. doi: 10.2196/22471
Blimark, C., Holmberg, E., Mellqvist, U. H., Landgren, O., Björkholm, M., Hultcrantz, M., et al. (2015). Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica 100, 107–113. doi: 10.3324/haematol.2014.107714
Bode, B., Garrett, V., Messler, J., McFarland, R., Crowe, J., Booth, R., et al. (2020). Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 14, 813–821. doi: 10.1177/1932296820924469
Borzouei, S., Mohammadian-Khoshnoud, M., Omidi, T., Bashirian, S., Bahreini, F., Heidarimoghadam, R., et al. (2021). Predictors of COVID-19 related death in diabetes patients: A case-control study in Iran. Diabetes Metab. Syndr. 15:102149. doi: 10.1016/j.dsx.2021.05.022
Butt, A. A., Chemaitelly, H., Al Khal, A., Coyle, P. V., Saleh, H., Kaleeckal, A. H., et al. (2021). SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J. Clin. Invest. 131:e153662. doi: 10.1172/JCI153662
Byambasuren, O., Cardona, M. B. K., and Glasziou, P. (2020). Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: Systematic review and meta-analysis. JAMMI 5, 223–234. doi: 10.3138/jammi-2020-0030
Cai, Y., Shi, S., Yang, F., Yi, B., Chen, X., Li, J., et al. (2020). Fasting blood glucose level is a predictor of mortality in patients with COVID-19 independent of diabetes history. Diabetes Res. Clin. Pract. 169:108437. doi: 10.1016/j.diabres.2020.108437
Campbell, M., Datta, R., Wyllie, A., Casanovas-Massana, A., Handoko, R., Sewanan, L., et al. (2020). Clinical and epidemiological features of healthcare workers detected with coronavirus disease. Open Forum Infect. Dis. 7. doi: 10.1093/ofid/ofaa439.686
Cascella, M., Rajnik, M., Aleem, A., Dulebohn, S. C., and Di Napoli, R. (2022). Features, Evaluation, and Treatment of Coronavirus (COVID-19). Stat Pearls (). Treasure Island, FL Stat Pearls Publishing LLC.
Cavicchiolo, M. E., Trevisanuto, D., Lolli, E., Mardegan, V., Saieva, A. M., Franchin, E., et al. (2020). Universal screening of high-risk neonates, parents, and staff at a neonatal intensive care unit during the SARS-CoV-2 pandemic. Eur. J. Pediatr. 179, 1949–1955. doi: 10.1007/s00431-020-03765-7
Center for Disease Control and Prevention. (2021). COVID-19 vaccine breakthrough infections reported to CDC — United States, January 1–April 30, 2021. Available at: https://www.cdc.gov/mmwr/volumes/70/wr/mm7021e3.htm
Center for Disease Control and Prevention. (2022). People with certain medical conditions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
Chen, H., Guo, J., Wang, C., Luo, F., Yu, X., Zhang, W., et al. (2020). Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 395, 809–815. doi: 10.1016/S0140-6736(20)30360-3
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395, 507–513. doi: 10.1016/S0140-6736(20)30211-7
Cheng, Y., Luo, R., Wang, K., Zhang, M., Wang, Z., Dong, L., et al. (2020). Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 97, 829–838. doi: 10.1016/j.kint.2020.03.005
Cheraghali, F., Barati, L., Amanian, D., Shahkar, L., Najafinejad, M., Naziri, H., et al. (2021). A case series of pediatric COVID-19 with complicated symptoms in Iran. Futur. Virol. 16, 649–656. doi: 10.2217/fvl-2021-0091
Chersich, M. F., Gray, G., Fairlie, L., Eichbaum, Q., Mayhew, S., Allwood, B., et al. (2020). COVID-19 in Africa: Care and protection for frontline healthcare workers. Glob. Health 16, 46. doi: 10.1186/s12992-020-00574-3
Chou, R., Dana, T., Buckley, D. I., Selph, S., Fu, R., and Totten, A. M. (2020). Epidemiology of and risk factors for coronavirus infection in health care workers: A living rapid review. Ann. Intern. Med. 173, 120–136. doi: 10.7326/M20-1632
Dai, M., Liu, D., Liu, M., Zhou, F., Li, G., Chen, Z., et al. (2020). Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 10, 783–791. doi: 10.1158/2159-8290.CD-20-0422
Dattner, I., Goldberg, Y., Katriel, G., Yaari, R., Gal, N., Miron, Y., et al. (2021). The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput. Biol. 17:e1008559. doi: 10.1371/journal.pcbi.1008559
DeBiasi, R. L., Song, X., Delaney, M., Bell, M., Smith, K., Pershad, J., et al. (2020). Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J. Pediatr. 223, 199–203.e1. doi: 10.1016/j.jpeds.2020.05.007
Di Felice, G., Visci, G., Teglia, F., Angelini, M., and Boffetta, P. (2022). Effect of cancer on outcome of COVID-19 patients: A systematic review and meta-analysis of studies of unvaccinated patients. eLife 11, 1–17. doi: 10.7554/eLife.74634
Docherty, A. B., Harrison, E. M., Green, C. A., Hardwick, H. E., Pius, R., Norman, L., et al. (2020). Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ (Clinical Research Ed.) 369:m1985. doi: 10.1136/bmj.m1985
Ebeid, F. S. E., Ragab, I. A., Elsherif, N. H. K., Makkeyah, S., Mostafa, S., Eltonbary, K., et al. (2021). COVID-19 in children with cancer: A single low-middle income center experience. J. Pediatr. Hematol. Oncol. 43, e1077–e1081. doi: 10.1097/MPH.0000000000002025
El-Raey, F., Alboraie, M., Youssef, N., Yousef, A., Abdelmoaty, A. A., Hassan, E., et al. (2021). Predictors for severity of SARS-CoV-2 infection among healthcare workers. J. Multidiscip. Healthc. 14, 2973–2981. doi: 10.2147/JMDH.S335226
Elshafeey, F., Magdi, R., Hindi, N., Elshebiny, M., Farrag, N., Mahdy, S., et al. (2020). A systematic scoping review of COVID-19 during pregnancy and childbirth. Int. J. Gynaecol. Obstet. 150, 47–52. doi: 10.1002/ijgo.13182
ElSharkawy, L. (2021). Egypt's coronavirus vaccination campaign: A timeline. Available at: https://english.ahram.org.eg/NewsContent/1/64/404860/Egypt/Politics-/Egypts-coronavirus-vaccination-campaign-A-timeline.aspx
El-Sokkary, R. H., Daef, E., El-Korashi, L. A., Khedr, E. M., Gad, D., Mohamed-Hussein, A., et al. (2021). Sero-prevalence of anti-SARS-CoV-2 antibodies among healthcare workers: a multicenter study from Egypt. J. Infect. Public Health 14, 1474–1480. doi: 10.1016/j.jiph.2021.09.011
Emara, M. H., Mazid, U., Atta, M. A., Elshahat, S., and Mahros, A. M. (2020). Ketonuria with or without ketoacidosis as the presenting manifestation of SARS-CoV-2 (COVID-19) among uncontrolled type 2 diabetic patients. Med. Hypotheses 144:110226. doi: 10.1016/j.mehy.2020.110226
Ennab, F., ElSaban, M., Khalaf, E., Tabatabaei, H., Khamis, A. H., Devi, B. R., et al. (2021). Clinical characteristics of children with COVID-19 in the United Arab Emirates: cross-sectional multicenter study. JMIR Pediatrics Parenting 4:e29049. doi: 10.2196/29049
Esmaeili Dooki, M., Mehrabani, S., Sorkhi, H., Nikpour, M., Tabatabaie, M., Mohammadi, M., et al. (2020). COVID-19 and digestive system in children: a retrospective study. Arch. Iran. Med. 23, 782–786. doi: 10.34172/aim.2020.104
Essa, R. A., Ahmed, S. K., Bapir, D. H., Rasul, S. A., Khdir, A. A., and Abubakr, C. P. (2021). Clinical features and laboratory findings first case of B. 1.617.2 (delta) variant concern (VOC) in Iraq. Ann. Med. Surg. 69, 102814. doi: 10.1016/j.amsu.2021.102814
Fahimzad, A., Sedighi, I., Pak, N., Khalili, M., Farahmand, M., Shokrollahi, M., et al. (2021). A comparative analysis of clinical characteristics and laboratory findings of COVID-19 between intensive care unit and non-intensive care unit pediatric patients: a multicenter, retrospective, observational study from Iranian network for research in viral. Front. Emerg. Med. 5, 1–11. doi: 10.18502/fem.v5i4.6692
Falahi, S., Abdoli, A., and Kenarkoohi, A. (2021). Claims and reasons about mild COVID-19 in children. New Microbes New Infect. 41:100864. doi: 10.1016/j.nmni.2021.100864
Fan, C., Lei, D., Fang, C., Li, C., Wang, M., Liu, Y., et al. (2021). Perinatal transmission of 2019 coronavirus disease-associated severe acute respiratory syndrome coronavirus 2: should we worry? Clin. Infect. Dis. 72, 862–864. doi: 10.1093/cid/ciaa226
Farhadi, R., Mehrpisheh, S., Ghaffari, V., Haghshenas, M., and Ebadi, A. (2021). Clinical course, radiological findings and late outcome in preterm infant with suspected vertical transmission born to a mother with severe COVID-19 pneumonia: a case report. J. Med. Case Rep. 15, 213. doi: 10.1186/s13256-021-02835-0
Farsi, S. H., Alandijany, T. A., Radwi, M., Farsi, A., Bahaaziq, W., Abushoshah, I., et al. (2021). Prevalence of COVID-19 antibodies among operating room and critical care staff at a tertiary teaching hospital: a cross-sectional study. Saudi Med. J. 42, 742–749. doi: 10.15537/smj.2021.42.7.20210348
Frampton, D., Rampling, T., Cross, A., Bailey, H., Heaney, J., Byott, M., et al. (2021). Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. The lancet. Infect. Dis. 21, 1246–1256. doi: 10.1016/S1473-3099(21)00170-5
Galloway, S. E., Paul, P., Mac Cannell, D. R., Johansson, M. A., Brooks, J. T., Mac Neil, A., et al. (2021). Emergence of SARS-CoV-2 B.1.1.7 lineage – United States, December 29, 2020–January 12, 2021. MMWR Morb. Mortal. Wkly Rep. 70, 95–99. doi: 10.15585/mmwr.mm7003e2
Gazette, S. (2020). Bahrain approves emergency use of COVID-19 vaccine among frontline healthcare workers. Available at https://saudigazette.com.sa/article/599906
Ghazizadeh Esslami, G., Mamishi, S., Mahmoudi, S., Navaeian, A., Behfar, M., Hamidieh, A. A., et al. (2021). COVID-19 in transplant recipient children: an Iranian referral hospital-based study. Acta Bio-Med. 92:e2021095. doi: 10.23750/abm.v92i2.11189
Ghelichkhani, S., Jenabi, E., Jalili, E., Alishirzad, A., and Shahbazi, F. (2021). Pregnancy outcomes among SARS-CoV-2-infected pregnant women with and without underlying diseases: a case-control study. J. Med. Life 14, 518–522. doi: 10.25122/jml-2021-0157
Gholami, M., Fawad, I., Shadan, S., Rowaiee, R., Ghanem, H., Hassan Khamis, A., et al. (2021). COVID-19 and healthcare workers: a systematic review and meta-analysis. Int. J. Infect. Dis. 104, 335–346. doi: 10.1016/j.ijid.2021.01.013
Goldshtein, I., Nevo, D., Steinberg, D. M., Rotem, R. S., Gorfine, M., Chodick, G., et al. (2021). Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. Jama. 326, 728–735. doi: 10.1001/jama.2021.11035
Goshayeshi, L., Akbari Rad, M., Bergquist, R., Allahyari, A., Hashemzadeh, K., and MUMS Covid-19 Research Team, et al. (2021). Demographic and clinical characteristics of severe covid-19 infections: a cross-sectional study from Mashhad University of Medical Sciences, Iran. BMC Infect. Dis. 21:656. doi: 10.1186/s12879-021-06363-6
Götzinger, F., Santiago-García, B., Noguera-Julián, A., Lanaspa, M., Lancella, L., Calò Carducci, F. I., et al. (2020). COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc. Health 4, 653–661. doi: 10.1016/S2352-4642(20)30177-2
Guery, R., Delaye, C., Brule, N., Nael, V., Castain, L., Raffi, F., et al. (2020). Limited effectiveness of systematic screening by nasopharyngeal RT-PCR of medicalized nursing home staff after a first case of COVID-19 in a resident. Medecine Et Maladies Infectieuses 50, 748–750. doi: 10.1016/j.medmal.2020.04.020
Haas, E. J., Angulo, F. J., McLaughlin, J. M., Anis, E., Singer, S. R., Khan, F., et al. (2021). Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 397, 1819–1829. doi: 10.1016/S0140-6736(21)00947-8
Hafidh, K., Abbas, S., Khan, A., Kazmi, T., Nazir, Z., and Aldaham, T. (2020). The clinical characteristics and outcomes of COVID-19 infections in patients with diabetes at a tertiary care center in the UAE. Dubai Diabetes Endocrinol. J. 26, 158–163. doi: 10.1159/000512232
Hakami, A., Badedi, M., Elsiddig, M., Nadeem, M., Altherwi, N., Rayani, R., et al. (2021). Clinical characteristics and early outcomes of hospitalized COVID-19 patients with end-stage kidney disease in Saudi Arabia. Int. J. General Med. 14, 4837–4845. doi: 10.2147/IJGM.S327186
Hamdan, M., Badrasawi, M., Zidan, S., Sayarah, A., Zahra, L. A., Dana, S., et al. (2021). Risk factors associated with hospitalization owing to COVID-19: a cross-sectional study in Palestine. J. Int. Med. Res. 49, 3000605211064405. doi: 10.1177/03000605211064405
Hamdy, R., El-Mahallawy, H., and Ebeid, E. (2021). COVID-19 infection in febrile neutropenic pediatric hematology oncology patients. Pediatr. Blood Cancer 68:e28765. doi: 10.1002/pbc.28765
Hammad, M., Shalaby, L., Sidhom, I., Sherief, N., Abdo, I., Soliman, S., et al. (2021). Management and outcome of coronavirus disease 2019 (COVID-19) in pediatric cancer patients: a single Centre experience from a developing country. Clin. Lymphoma Myeloma Leuk. 21, e853–e864. doi: 10.1016/j.clml.2021.07.025
Hantoushzadeh, S., Shamshirsaz, A. A., Aleyasin, A., Seferovic, M. D., Aski, S. K., Arian, S. E., et al. (2020). Maternal death due to COVID-19. Am. J. Obstet. Gynecol. 223, 109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030
Harbi, S., Kobeisy, S. A. N., Mehdawi, R. S., Bashammakh, D. S., and Mosalli, R. (2020). Pediatric COVID-19 patients in Jeddah, Saudi Arabia: clinical, laboratory and radiological aspects. J. Biomed. Sci. 9, 1–4. doi: 10.36648/2254-609X.9.3.7
Harel, L., Eliasi, E., Jaffe Lifshitz, S., Schindler, Y., Rosen, D., Olteanu, I., et al. (2021). Does the presence of symptoms affect pregnancy outcomes in third trimester in women with SARS-CoV-2. J. Matern. Fetal. Neonatal. Med. 1-8, 1–8. doi: 10.1080/14767058.2021.1956895
Hasan, M. R., Al Zubaidi, K., Diab, K., Hejazi, Y., Bout-Tabaku, S., Al-Adba, B., et al. (2021). COVID-19 related multisystem inflammatory syndrome in children (MIS-C): A case series from a tertiary care pediatric hospital in Qatar. BMC Pediatr. 21, 267. doi: 10.1186/s12887-021-02743-8
Hazari, K. S., Abdeldayem, R., Paulose, L., Kurien, N., Almahloul, Z., Mohammad, H., et al. (2021). Covid-19 infection in pregnant women in Dubai: a case-control study. BMC Pregnancy Childbirth 21, 658. doi: 10.1186/s12884-021-04130-8
Heidari, M., and Jafari, H. (2021). Challenges of COVID-19 vaccination in Iran: in the fourth wave of pandemic spread. Prehosp. Disaster Med. 36, 659–660. doi: 10.1017/S1049023X21000777
Hellewell, J., Russell, T. W., SAFER Investigators and Field Study Team, Crick COVID-19 Consortium, CMMID COVID-19 working group, Beale, R., and Kucharski, A. J. (2021). Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 19, 106. doi: 10.1186/s12916-021-01982-x
Hendler, J. V., Miranda Do Lago, P., Müller, G. C., Santana, J. C., Piva, J. P., and Daudt, L. E. (2021). Risk factors for severe COVID-19 infection in Brazilian children. The Braz. J. Infect. Dis. 25:101650. doi: 10.1016/j.bjid.2021.101650
Hidayat, R., Aini, N., Ilmi, A. F. N., Azzahroh, F., and Giantini, A. (2020). Test, trace, and treatment strategy to control COVID-19 infection among hospital staff in a COVID-19 referral hospital in Indonesia. Acta Med. Indones. 52, 206–213.
Hijazi, L. O., Alaraifi, A. K., and Alsaab, F. (2021). Otolaryngology manifestations of COVID-19 in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 144:110701. doi: 10.1016/j.ijporl.2021.110701
Hillson, R. (2020). COVID-19: diabetes and death. A call to action. Practical Diabetes 37, 76–78. doi: 10.1002/pdi.2271
Hirshberg, J. S., Stout, M. J., and Raghuraman, N. (2020). Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York city hospitals. Ame. J. Obstetrics Gynecol. MFM 2:100162. doi: 10.1016/j.ajogmf.2020.100162
Hoseinyazdi, M., Esmaeilian, S., Jahankhah, R., Teimouri, A., Sherbaf, F. G., Rafiee, F., et al. (2021). Clinical, laboratory, and chest CT features of severe versus non-severe pediatric patients with COVID-19 infection among different age groups. BMC Infect. Dis. 21:560. doi: 10.1186/s12879-021-06283-5
Hosseninasab, A., Bafti, M. S., Ebrahimi, S., Anjomshoaa, A., Zerandi, F. S., Jafari, M., et al. (2020). Coronavirus disease 2019 in children with acute respiratory infection: a study from southeastern Iran. Shiraz E Med. J. 21, 1–7. doi: 10.5812/semj.108377
Iskanderian, R. R., Karmstaji, A., Mohamed, B. K., Alahmed, S., Masri, M. H., Choufani, E., et al. (2020). Outcomes and impact of a universal COVID-19 screening protocol for asymptomatic oncology patients. Gulf J. Oncolog. 1, 7–12.
Jabs, J. M., Schwabe, A., Wollkopf, A. D., Gebel, B., Stadelmaier, J., Erdmann, S., et al. (2022). The role of routine SARS-CoV-2 screening of healthcare-workers in acute care hospitals in 2020: a systematic review and meta-analysis. BMC Infect. Dis. 22, 587. doi: 10.1186/s12879-022-07554-5
Jameson, A. P., Biersack, M. P., Sebastian, T. M., and Jacques, L. R. (2020). SARS-CoV-2 screening of asymptomatic healthcare workers. Infect. Control Hosp. Epidemiol. 41, 1229–1231. doi: 10.1017/ice.2020.361
Jeong, I. K., Yoon, K. H., and Lee, M. K. (2020). Diabetes and COVID-19: global and regional perspectives. Diabetes Res. Clin. Pract. 166:108303. doi: 10.1016/j.diabres.2020.108303
Juan, J., Gil, M. M., Rong, Z., Zhang, Y., Yang, H., and Poon, L. C. (2020). Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet. Gynecol. 56, 15–27. doi: 10.1002/uog.22088
Kamali Aghdam, M., Jafari, N., and Eftekhari, K. (2020). Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect. Dis. 52, 427–429. doi: 10.1080/23744235.2020.1747634
Kamrath, C., Mönkemöller, K., Biester, T., Rohrer, T. R., Warncke, K., Hammersen, J., et al. (2020). Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA 324, 801–804. doi: 10.1001/jama.2020.13445
Kari, J. A., Shalaby, M. A., Albanna, A. S., Alahmadi, T. S., Alherbish, A., and Alhasan, K. A. (2021a). Acute kidney injury in children with COVID-19: a retrospective study. BMC Nephrol. 22, 202. doi: 10.1186/s12882-021-02389-9
Kari, J. A., Shalaby, M. A., Albanna, A. S., Alahmadi, T. S., Sukkar, S. A., Mohamed Nur, H. A. H., et al. (2021b). Coronavirus disease in children: a multicentre study from the Kingdom of Saudi Arabia. J. Infect. Public Health 14, 543–549. doi: 10.1016/j.jiph.2021.01.011
Kassem, A. M., Talaat, H., Shawky, S., Fouad, R., Amer, K., Elnagdy, T., et al. (2020). SARS-CoV-2 infection among healthcare workers of a gastroenterological service in a tertiary care facility. Arab J. Gastroenterol. 21, 151–155. doi: 10.1016/j.ajg.2020.07.005
Kaushal, M., Tulasi, S. Y. V., Kaushal, A., Rakhecha, A., Memon, R., Ramasamy, K., et al. (2021). Rooming in and breast feeding practices and rate of horizontal transmission of SARS-CoV-2 virus in 5 UAE hospitals—an observational study. J. Neonatol. 35, 183–188. doi: 10.1177/09732179211060613
Kazemi Aski, S., Norooznezhad, A. H., Shamshirsaz, A. A., Mostafaei, S., Aleyasin, A., Nabavian, S. M., et al. (2021). Clinical features and risk factors associated with acute respiratory distress syndrome in pregnant women diagnosed with COVID-19: a multi-center case-control study. J. Matern. Fetal. Neonatal. Med. 1-5, 1–5. doi: 10.1080/14767058.2021.1872062
Kazemi Aski, S., Sharami, S. H., Hosseinzadeh, F., Hesni, E., Dalil Heirati, S. F., Ghalandari, M., et al. (2020). Risk factors, clinical symptoms, laboratory findings and imaging of pregnant women infected with COVID-19 in north of Iran. Arch. Iran. Med. 23, 856–863. doi: 10.34172/aim.2020.114
Kenarkoohi, A., Maleki, M., Ghiasi, B., Bastani, E., Pakzad, I., Bonyadi, M., et al. (2022). Prevalence and clinical presentation of COVID-19 infection in hemodialysis patients. J. Nephropathol. 11, e7–e6. doi: 10.34172/jnp.2022.07
Keshavarz Valian, N., Pourakbari, B., Asna Ashari, K., Hosseinpour Sadeghi, R., and Mahmoudi, S. (2022). Evaluation of human coronavirus OC43 and SARS-CoV-2 in children with respiratory tract infection during the COVID-19 pandemic. J. Med. Virol. 94, 1450–1456. doi: 10.1002/jmv.27460
Khatib, M. Y., Olagundoye, V. O., Elshafei, M. S., El Khatib, F. M., Mohamed, A. S., and Nashwan, A. J. (2021). Management considerations for a critically ill 26-gestational week patient with COVID-19: a case report. Clin. Case Rep. 9, 1721–1724. doi: 10.1002/ccr3.3886
Kilani, M. M., Odeh, M. M., Shalabi, M., Al Qassieh, R., and Al-Tamimi, M. (2021). Clinical and laboratory characteristics of SARS-CoV2-infected paediatric patients in Jordan: serial RT-PCR testing until discharge. Paediatrics Int. Child Health 41, 83–92. doi: 10.1080/20469047.2020.1804733
Kim, L., Garg, S., O'Halloran, A., Whitaker, M., Pham, H., Anderson, E. J., et al. (2021). Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin. Infect. Dis. 72, e206–e214. doi: 10.1093/cid/ciaa1012
Knight, M., Bunch, K., Vousden, N., Morris, E., Simpson, N., Gale, C., et al. (2020). Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ (Clinical Research Ed.) 369:m2107. doi: 10.1136/bmj.m2107
Kohla, S., Ibrahim, F. A., Aldapt, M. B., ELSabah, H., Mohamed, S., and Youssef, R. (2020). A rare case of hairy cell leukemia with unusual loss of CD123 associated with COVID-19 at the time of presentation. Case Rep. Oncol. 13, 1430–1440. doi: 10.1159/000512830
Kuderer, N. M., Choueiri, T. K., Shah, D. P., Shyr, Y., Rubinstein, S. M., Rivera, D. R., et al. (2020). Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 395, 1907–1918. doi: 10.1016/S0140-6736(20)31187-9
Kurd, M., Hashavya, S., Benenson, S., and Gilboa, T. (2021). Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children. Seizure 92, 89–93. doi: 10.1016/j.seizure.2021.08.017
Kustin, T., Harel, N., Finkel, U., Perchik, S., Harari, S., Tahor, M., et al. (2021). Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat. Med. 27, 1379–1384. doi: 10.1038/s41591-021-01413-7
Lankarani, K. B., Honarvar, B., Omidifar, N., Pakdin, M., Moghadami, M., Shokripour, M., et al. (2021). Prevalence of anti-SARS-CoV-2 antibody in hospital staff in double-center setting: a preliminary report of a cohort study from Iran. Shiraz E Med. J. 22, 1–14. doi: 10.5812/semj.112681
Lee, L. Y. W., Cazier, J. B., Starkey, T., Briggs, S. E. W., Arnold, R., Bisht, V., et al. (2020). COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 21, 1309–1316. doi: 10.1016/S1470-2045(20)30442-3
Levy, I., Lavi, A., Zimran, E., Grisariu, S., Aumann, S., Itchaki, G., et al. (2021). COVID-19 among patients with hematological malignancies: a national Israeli retrospective analysis with special emphasis on treatment and outcome. Leuk. Lymphoma 62, 3384–3393. doi: 10.1080/10428194.2021.1966782
Li, B., Zhang, S., Zhang, R., Chen, X., Wang, Y., and Zhu, C. (2020). Epidemiological and clinical characteristics of COVID-19 in children: a systematic review and meta-analysis. Front. Pediatr. 8:591132. doi: 10.3389/fped.2020.591132
Ling, P., Luo, S., Zheng, X., Cai, G., and Weng, J. (2021). Elevated fasting blood glucose within the first week of hospitalization was associated with progression to severe illness of COVID-19 in patients with preexisting diabetes: a multicenter observational study. J. Diabetes 13, 89–93. doi: 10.1111/1753-0407.13121
Loeb, M., McGeer, A., Henry, B., Ofner, M., Rose, D., Hlywka, T., et al. (2004). SARS among critical care nurses, Toronto. Emerg. Infect. Dis. 10, 251–255. doi: 10.3201/eid1002.030838
Loef, B., Nanlohy, N. M., Jacobi, R. H. J., van de Ven, C., Mariman, R., van der Beek, A. J., et al. (2019). Immunological effects of shift work in healthcare workers. Sci. Rep. 9, 18220. doi: 10.1038/s41598-019-54816-5
Maddaloni, E., D'Onofrio, L., Alessandri, F., Mignogna, C., Leto, G., Pascarella, G., et al. (2020). Cardiometabolic multimorbidity is associated with a worse covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II). Cardiovasc. Diabetol. 19:164. doi: 10.1186/s12933-020-01140-2
Mahmoudi, S., Mehdizadeh, M., Shervin Badv, R., Navaeian, A., Pourakbari, B., Rostamyan, M., et al. (2020). The coronavirus disease 2019 (COVID-19) in children: a study in an Iranian children's referral hospital. Inf. Drug Resist. 13, 2649–2655. doi: 10.2147/IDR.S259064
Mamishi, S., Heydari, H., Aziz-Ahari, A., Shokrollahi, M. R., Pourakbari, B., Mahmoudi, S., et al. (2021). Novel coronavirus disease 2019 (COVID-19) outbreak in children in Iran: atypical CT manifestations and mortality risk of severe COVID-19 infection. J. Microbiol. Immunol. Infect. 54, 839–844. doi: 10.1016/j.jmii.2020.07.019
Mamishi, S., Movahedi, Z., Mohammadi, M., Ziaee, V., Khodabandeh, M., Abdolsalehi, M. R., et al. (2020). Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol. Infect. 148:e196. doi: 10.1017/S095026882000196X
Mansour, A., Atoui, R., Kanso, K., Mohsen, R., Fares, Y., and Fares, J. (2020). First case of an infant with COVID-19 in the middle east. Cureus 12, e 7520. doi: 10.7759/cureus.7520
Merzon, E., Green, I., Shpigelman, M., Vinker, S., Raz, I., Golan-Cohen, A., et al. (2021). Haemoglobin A1c is a predictor of COVID-19 severity in patients with diabetes. Diabetes Metab. Res. Rev. 37:e3398. doi: 10.1002/dmrr.3398
Mirjalili, H., Dastgheib, S. A., Shaker, S. H., Bahrami, R., Mazaheri, M., Sadr-Bafghi, S. M. H., et al. (2021). Proportion and mortality of iranian diabetes mellitus, chronic kidney disease, hypertension and cardiovascular disease patients with COVID-19: a meta-analysis. J. Diabetes Metab. Disord. 20, 905–917. doi: 10.1007/s40200-021-00768-5
Moeindarbary, S., Dadgar, S., Layegh, P., Shahriari, Z., Fayyaz, F., Danesteh, S., et al. (2021). Extensive bilateral diffuse infiltrates and deterioration of lung following infection with severe acute respiratory syndrome coronavirus 2 in a pregnant woman: a case report. J. Med. Case Rep. 15, 588. doi: 10.1186/s13256-021-03156-y
Moeller, A., Thanikkel, L., Duijts, L., Gaillard, E. A., Garcia-Marcos, L., Kantar, A., et al. (2020). COVID-19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 6, 00409-02020. doi: 10.1183/23120541.00409-2020
Mohr-Sasson, A., Chayo, J., Bart, Y., Meyer, R., Sivan, E., Mazaki-Tovi, S., et al. (2020). Laboratory characteristics of pregnant compared to non-pregnant women infected with SARS-CoV-2. Arch. Gynecol. Obstet. 302, 629–634. doi: 10.1007/s00404-020-05655-7
Molaei, H., Khedmat, L., Nemati, E., Rostami, Z., and Saadat, S. H. (2021). Iranian kidney transplant recipients with COVID-19 infection: clinical outcomes and cytomegalovirus coinfection. Transpl. Infect. Dis. 23:e13455. doi: 10.1111/tid.13455
Moncunill, G., Mayor, A., Santano, R., Jiménez, A., Vidal, M., Tortajada, M., et al. (2021). SARS-CoV-2 seroprevalence and antibody kinetics among health care workers in a Spanish hospital after 3 months of follow-up. J. Infect. Dis. 223, 62–71. doi: 10.1093/infdis/jiaa696
Mortezagholi, S., Rostamzadeh, D., Alinejad, M., Younesi, V., Tabarsi, P., and Shabani, M. (2021). Prevalence of anti-SARS-CoV-2 specific antibodies in health-care workers compared to general population during an early phase of the pandemic, Tehran-Iran. Iranian J. Immunol. 18, 82–92. doi: 10.22034/iji.2021.88168.1851
Mosalli, R. M., Kobeisy, S. A. N., Al-Dajani, N. M., Ateeg, M. A., Ahmed, M. A., Meer, W. M., et al. (2021). Coronavirus disease in children: a single-center study from western Saudi Arabia. Int. J. Pediatr. 2021, 1–5. doi: 10.1155/2021/9918056
Mostafa, A., Kandil, S., El-Sayed, M. H., Girgis, S., Hafez, H., Yosef, M., et al. (2021). Universal COVID-19 screening of 4040 health care workers in a resource-limited setting: an Egyptian pilot model in a university with 12 public hospitals and medical centers. Int. J. Epidemiol. 50, 50–61. doi: 10.1093/ije/dyaa173
Motlagh, A. J., Esmaelzadeh Saeieh, S., Parhigar, O., and Salehi, L. (2020). An asthmatic pregnant woman with COVID-19: a case report study. Respir. Med. Case. Rep. 31:101296. doi: 10.1016/j.rmcr.2020.101296
Mousavi, S. A., Rostami, T., Kiumarsi, A., Rad, S., Rostami, M., Motamedi, F., et al. (2021). COVID-19 and cancer: a comparative case series. Cancer Treat. Res. Commun. 27:100339. doi: 10.1016/j.ctarc.2021.100339
Mukhtar, I. (2021). Palestine to receive 125,000 coronavirus jabs. Available at: https://www.aa.com.tr/en/latest-on-coronavirus-outbreak/palestine-to-receive-125-000-coronavirus-jabs/2190000
Mumtaz, G. R., El-Jardali, F., Jabbour, M., Harb, A., Abu-Raddad, L. J., and Makhoul, M. (2021). Modeling the impact of COVID-19 vaccination in Lebanon: a call to speed-up vaccine roll out. Vaccine 9, 697. doi: 10.3390/vaccines9070697
Musa, S., Abdel Alem, S., Amer, K., Elnagdy, T., Hassan, W. A., Ali, M. A., et al. (2021). Prevalence of SARS-CoV-2 infection and dynamics of antibodies response among previously undiagnosed healthcare workers in a university hospital: a prospective cohort study. J. Infect. Public Health 14, 1466–1473. doi: 10.1016/j.jiph.2021.06.001
Navaeian, A., Mahmoudi, S., Pourakbari, B., Bakhtiari, M., Khodabandeh, M., Abdolsalehi, M. R., et al. (2021). COVID-19 infection in children with underlying malignancies in Iran. J. Basic Clin. Physiol. Pharmacol. 33, 79–84. doi: 10.1515/jbcpp-2021-0057
Nawazani, A., Ghanaim, M., and Tariq, S. (2020). Case of COVID-19 infection and polycythaemia presenting with massive acute pulmonary embolism. BMJ Case Rep. 13:e237390. doi: 10.1136/bcr-2020-237390
Neuwirth, M. M., Mattner, F., and Otchwemah, R. (2020). Adherence to personal protective equipment use among healthcare workers caring for confirmed COVID-19 and alleged non-COVID-19 patients. Antimicrob. Resist. Infect. Control 9, 199. doi: 10.1186/s13756-020-00864-w
Orlanski-Meyer, E., Yogev, D., Auerbach, A., Megged, O., Glikman, D., Hashkes, P. J., et al. (2020). Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus-2 in an 8-week-old infant. J. Pediatric Infect. Dis. Soc. 9, 781–784. doi: 10.1093/jpids/piaa137
Oster, Y., Benenson, S., Yochi Harpaz, L., Buda, I., Nir-Paz, R., Strahilevitz, J., et al. (2021). Association between exposure characteristics and the risk for COVID-19 infection among health care workers with and without BNT162b2 vaccination. JAMA Netw. Open 4:e2125394. doi: 10.1001/jamanetworkopen.2021.25394
Park, S. S., Oh, H. Y., and Hong, D. J. (2021). Mass screening of healthcare personnel for SARS-CoV-2 in the northern emirates. J. Hosp. Infect. 108, 52–54. doi: 10.1016/j.jhin.2020.10.008
Pazoki, M., Chichagi, F., Hadadi, A., Kafan, S., Montazeri, M., Kazemian, S., et al. (2021). Association of clinical characteristics, antidiabetic and cardiovascular agents with diabetes mellitus and COVID-19: a 7-month follow-up cohort study. J. Diabetes Metab. Disord. 20, 1545–1555. doi: 10.1007/s40200-021-00901-4
Pellett, P. E., Mitra, S., and Holland, T. C. (2014). Basics of virology. Handb. Clin. Neurol. 123, 45–66. doi: 10.1016/B978-0-444-53488-0.00002-X
Penfield, C. A., Brubaker, S. G., Limaye, M. A., Lighter, J., Ratner, A. J., Thomas, K. M., et al. (2020). Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstetrics Gynecol. MFM 2:100133. doi: 10.1016/j.ajogmf.2020.100133
Pirjani, R., Hosseini, R., Soori, T., Rabiei, M., Hosseini, L., Abiri, A., et al. (2020). Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J. Travel Med. 27, taaa158. doi: 10.1093/jtm/taaa158
Pourakbari, B., Mahmoudi, S., Mahmoudieh, Y., Eshaghi, H., Navaeian, A., Rostamyan, M., et al. (2021). SARS-CoV-2 RNAaemia in children: an Iranian referral hospital-based study. J. Med. Virol. 93, 5452–5457. doi: 10.1002/jmv.27065
Qiu, L., Liu, X., Xiao, M., Xie, J., Cao, W., Liu, Z., et al. (2020). SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin. Infect. Dis. 71, 813–817. doi: 10.1093/cid/ciaa375
Rabiei, M., Soori, T., Abiri, A., Farsi, Z., Shizarpour, A., and Pirjani, R. (2021). Maternal and fetal effects of COVID-19 virus on a complicated triplet pregnancy: a case report. J. Med. Case Rep. 15:87. doi: 10.1186/s13256-020-02643-y
Rabizadeh, S., Hajmiri, M., Rajab, A., Emadi Kouchak, H., and Nakhjavani, M. (2020). Severe diabetic ketoacidosis and coronavirus disease 2019 (COVID-19) infection in a teenage patient with newly diagnosed diabetes. J. Pediatric Endocrinol. Metab. 33, 1241–1243. doi: 10.1515/jpem-2020-0296
Rahimzadeh, H., Tamehri Zadeh, S. S., Khajavi, A., Saatchi, M., Reis, L. O., Guitynavard, F., et al. (2021). The tsunami of COVID-19 infection among kidney transplant recipients: a single-center study from Iran. J. Epidemiol. Global Health 11, 389–396. doi: 10.1007/s44197-021-00015-3
Rakhsha, A., Mahboubi-Fooladi, Z., and Jafari, A. (2021). Simultaneous development of COVID-19 pneumonia and pulmonary metastasis in a known case of chondrosarcoma: a case report. J. Med. Case Rep. 15, 218. doi: 10.1186/s13256-021-02753-1
Rana, S. S. (2020). Risk of COVID-19 transmission during gastrointestinal endoscopy. J. Digestive Endoscopy. 11, 27–30. doi: 10.1055/s-0040-1712076
Raoufi, M., Khalili, S., Mansouri, M., Mahdavi, A., and Khalili, N. (2020). Well-controlled vs poorly-controlled diabetes in patients with COVID-19: are there any differences in outcomes and imaging findings? Diabetes Res. Clin. Pract. 166:108286. doi: 10.1016/j.diabres.2020.108286
Rashidian, T., Sharifi, N., Fathnezhad-Kazemi, A., Mirzamrajani, F., Nourollahi, S., and Ghaysouri, A. (2020). Death of a neonate with suspected coronavirus disease 2019 born to a mother with coronavirus disease 2019 in Iran: a case report. J. Med. Case Rep. 14, 186. doi: 10.1186/s13256-020-02519-1
Rawshani, A., Kjölhede, E. A., Rawshani, A., Sattar, N., Eeg-Olofsson, K., Adiels, M., et al. (2021). Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: a nationwide retrospective cohort study. Lancet Reg. Health Eur. 4:100105. doi: 10.1016/j.lanepe.2021.100105
Reddy, P. K., Kuchay, M. S., Mehta, Y., and Mishra, S. K. (2020). Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab. Syndr. 14, 1459–1462. doi: 10.1016/j.dsx.2020.07.050
Reuters,. (2021). Yemen starts COVID-19 vaccination campaign. Available at: https://www.reuters.com/business/healthcare-pharmaceuticals/yemen-starts-covid-19-vaccination-campaign-2021-04-20/
Robert, A. A., Al Saeed, A., and Al Dawish, M. A. (2021). COVID-19 among people with diabetes mellitus in Saudi Arabia: current situation and new perspectives. Diabetes Metab. Syndr. 15:102231. doi: 10.1016/j.dsx.2021.102231
Rosen, B., Waitzberg, R., and Israeli, A. (2021). Israel's rapid rollout of vaccinations for COVID-19. Isr. J. Health Policy Res. 10, 6. doi: 10.1186/s13584-021-00440-6
Rottenstreich, A., Tsur, A., Braverman, N., Kabiri, D., Porat, S., Benenson, S., et al. (2021). Vaginal delivery in SARS-CoV-2-infected pregnant women in Israel: a multicenter prospective analysis. Arch. Gynecol. Obstet. 303, 1401–1405. doi: 10.1007/s00404-020-05854-2
Saban, O., Levy, J., and Chowers, I. (2020). Risk of SARS-CoV-2 transmission to medical staff and patients from an exposure to a COVID-19-positive ophthalmologist. Graefe's Arch. Clin. Exp. Ophthal. 258, 2271–2274. doi: 10.1007/s00417-020-04790-w
Sabetian, G., Moghadami, M., Hashemizadeh Fard Haghighi, L., Shahriarirad, R., Fallahi, M. J., Asmarian, N., et al. (2021). COVID-19 infection among healthcare workers: a cross-sectional study in Southwest Iran. Virol. J. 18, 58. doi: 10.1186/s12985-021-01532-0
Sahib, K. (2020). United Arab Emirates starts its vaccination campaign against COVID-19. Available at: https://atalayar.com/en/content/united-arab-emirates-starts-its-vaccination-campaign-against-covid-19
Saleh, N. Y., Aboelghar, H. M., Salem, S. S., Ibrahem, R. A., Khalil, F. O., Abdelgawad, A. S., et al. (2021). The severity and atypical presentations of COVID-19 infection in pediatrics. BMC Pediatr. 21, 144. doi: 10.1186/s12887-021-02614-2
Salih, A. F., Hamasalih, K., Rahman, H. S., and Mohammed, G. A. (2022). Pediatric COVID-19 infection in sulaimaniyah governorate, Iraq. Am. J. Otolaryngol. 43:103199. doi: 10.1016/j.amjoto.2021.103199
Samadi, P., Alipour, Z., Ghaedrahmati, M., and Ahangari, R. (2021). The severity of COVID-19 among pregnant women and the risk of adverse maternal outcomes. Int. J. Gynaecol. Obstet. 154, 92–99. doi: 10.1002/ijgo.13700
Santhosh, J., Al Salmani, M., Khamis, F., Ali Al Ubaidani, S., and Al-Zakwani, I. (2021). Clinical characteristics of COVID-19 in pregnant women: a retrospective descriptive single-center study from a tertiary hospital in Muscat, Oman. Int. J. Gynaecol. Obstet. 152, 270–274. doi: 10.1002/ijgo.13427
Sattari, M., Bashirian, S., Masoumi, S. Z., Shayan, A., Jenabi, E., Ghelichkhani, S., et al. (2020). Evaluating clinical course and risk factors of infection and demographic characteristics of pregnant women with COVID-19 in Hamadan province, west of Iran. J. Res. Health Sci. 20:e00488. doi: 10.34172/jrhs.2020.22
Schwartz, D. A., and Graham, A. L. (2020). Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 12, 194. doi: 10.3390/v12020194
Shafiek, H. K., El Lateef, H. M. A., Boraey, N. F., Nashat, M., Abd-Elrehim, G. A. B., Abouzeid, H., et al. (2021). Cytokine profile in Egyptian children and adolescents with COVID-19 pneumonia: a multicenter study. Pediatr. Pulmonol. 56, 3924–3933. doi: 10.1002/ppul.25679
Shahidsales, S., Aledavood, S. A., Joudi, M., Molaie, F., Esmaily, H., and Javadinia, S. A. (2021). COVID-19 in cancer patients may be presented by atypical symptoms and higher mortality rate, a case-controlled study from Iran. Cancer Rep. 4, e1378. doi: 10.1002/cnr2.1378
Shahin, W., Rabie, W., Alyossof, O., Alasiri, M., Alfaki, M., Mahmoud, E., et al. (2021). COVID-19 in children ranging from asymptomatic to a multi-system inflammatory disease: a single-center study. Saudi Med. J. 42, 299–305. doi: 10.15537/smj.2021.42.3.20200625
Shapiro Ben David, S., Rahamim-Cohen, D., Tasher, D., Geva, A., Azuri, J., and Ash, N. (2021). COVID-19 in children and the effect of schools reopening on potential transmission to household members. Acta Paediatrica (Oslo, Norway: 1992) 110, 2567–2573. doi: 10.1111/apa.15962
Sherif, E. M., Elhenawy, Y. I., Matter, R. M., Aly, H. H., Thabet, R. A., and Fereig, Y. A. (2021). Clinical characteristics and outcome of hospitalized children and adolescent patients with type 1 diabetes during the COVID-19 pandemic: data from a single center surveillance study in Egypt. J. Pediatr. Endocrinol. Metab. 34, 925–936. doi: 10.1515/jpem-2021-0099
Smith, V., Seo, D., Warty, R., Payne, O., Salih, M., Chin, K. L., et al. (2020). Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One 15:e0234187. doi: 10.1371/journal.pone.0234187
Soleimani, G., Akbarirad, F., Shafighi Shahri, E., and Sajjadi, S. M. (2021). Demographic, clinical, and paraclinical characteristics of COVID-19 pediatric cases in Southeast Iran. Antimicrob. Resist. Infect. Control 10:165. doi: 10.1186/s13756-021-01020-8
Soliman, A. T., Al-Amri, M., Alleethy, K., Alaaraj, N., Hamed, N., and De Sanctis, V. (2020a). Newly-onset type 1 diabetes mellitus precipitated by COVID-19 in an 8-month-old infant. Acta Biomed. 91 [ahead of print]. doi: 10.23750/abm.v91i3.10074
Soliman, A. T., Prabhakaran Nair, A., Al Masalamani, M. S., De Sanctis, V., Abu Khattab, M. A., Alsaud, A. E., et al. (2020b). Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus versus nondiabetic symptomatic patients with COVID-19: a comparative study. Acta Biomed. 91:e2020010. doi: 10.23750/abm.v91i3.10214
Somekh, I., Stein, M., Karakis, I., Simões, E. A. F., and Somekh, E. (2021). Characteristics of SARS-CoV-2 infections in Israeli children during the circulation of different SARS-CoV-2 variants. JAMA Netw. Open 4:e2124343. doi: 10.1001/jamanetworkopen.2021.24343
Stock, A. D., Bader, E. R., Cezayirli, P., Inocencio, J., Chalmers, S. A., Yassari, R., et al. (2020). COVID-19 infection among healthcare workers: serological findings supporting routine testing. Front. Med. 7:471. doi: 10.3389/fmed.2020.00471
Suleyman, G., Fadel, R. A., Malette, K. M., Hammond, C., Abdulla, H., Entz, A., et al. (2020). Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw. Open 3:e2012270. doi: 10.1001/jamanetworkopen.2020.12270
Sunder, A., Varghese, B., Darwish, B., Shaikho, N., and Rashid, M. (2022). Impacts and effects of COVID-19 infection in pregnancy. Saudi Med. J. 43, 67–74. doi: 10.15537/smj.2022.43.1.20210694
Tayebi Khosroshahi, H., Mardomi, A., Niknafs, B., Farnood, F., Shekarchi, M., Salehi, S., et al. (2021). Current status of COVID-19 among hemodialysis patients in the East Azerbaijan province of Iran. Hemodial. Int. 25, 214–219. doi: 10.1111/hdi.12907
Temkin, Healthcare Worker COVID-19 Surveillance Working Group, (2021). Extremely low prevalence of asymptomatic COVID-19 among healthcare workers caring for COVID-19 patients in israeli hospitals: a cross-sectional study. Clin. Microbiol. Infect. 27, 130.e1–130.e4.
The Lancet (2020). COVID-19: protecting health-care workers. Lancet 395:922. doi: 10.1016/j.cmi.2020.09.040
Vaezi, M., Mirghafourvand, M., and Hemmatzadeh, S. (2021). Characteristics, clinical and laboratory data and outcomes of pregnant women with confirmed SARS-CoV-2 infection admitted to al-Zahra tertiary referral maternity center in Iran: a case series of 24 patients. BMC Pregnancy Childbirth 21:378. doi: 10.1186/s12884-021-03764-y
Vizheh, M., Muhidin, S., Aghajani, F., Maleki, Z., Bagheri, F., Hosamirudsari, H., et al. (2021). Characteristics and outcomes of COVID-19 pneumonia in pregnancy compared with infected nonpregnant women. Int. J. Gynaecol. Obstet. 153, 462–468. doi: 10.1002/ijgo.13697
Wang, Z., Du, Z., and Zhu, F. (2020). Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res. Clin. Pract. 164:108214. doi: 10.1016/j.diabres.2020.108214
Wang, Y., Zheng, K., Gao, W., Lv, J., Yu, C., Wang, L., et al. (2022). Asymptomatic and pre-symptomatic infection in coronavirus disease 2019 pandemic. Med. Rev. (Berlin, Germany) 2, 66–88. doi: 10.1515/mr-2021-0034
WHO departmental news. (2021). Health and care worker deaths during COVID-19. Available at: https://www.who.int/news/item/20-10-2021-health-and-care-worker-deaths-during-covid-19#:~:text=WHO%20estimates%20that%20between%2080,in%20the%20world's%20pandemic%20response
WHO in Syria (2021). Syria completes the first phase of COVID-19 vaccination campaign. Available at: http://www.emro.who.int/syria/news/syria-completes-the-first-phase-of-covid-19-vaccination-campaign.html
Woodruff, R. C., Campbell, A. P., Taylor, C. A., Chai, S. J., Kawasaki, B., Meek, J., et al. (2022). Risk factors for severe COVID-19 in children. Pediatrics 149:e2021053418. doi: 10.1542/peds.2021-053418
World Health Organization (2022a). Tracking SARS-CoV-2 variants. Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
World Health Organization (2022b). WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/
Wu, Y. T., Liu, J., Xu, J. J., Chen, Y. F., Yang, W., Chen, Y., et al. (2020). Neonatal outcome in 29 pregnant women with COVID-19: a retrospective study in Wuhan, China. PLoS Med. 17:e1003195. doi: 10.1371/journal.pmed.1003195
Yanay, N. B., Freiman, S., Shapira, M., Wishahi, S., Hamze, M., Elhaj, M., et al. (2021). Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 99, 1496–1498. doi: 10.1016/j.kint.2021.04.006
Yang, C., Zhang, S., Lu, S., Yang, J., Cheng, Y., Liu, Y., et al. (2021). All five COVID-19 outbreaks during epidemic period of 2020/2021 in China were instigated by asymptomatic or pre-symptomatic individuals. J. Biosaf. Biosecur. 3, 35–40. doi: 10.1016/j.jobb.2021.04.001
Yaqoub, S., Ahmad, S., Mansouri, Z., Pallivalapila, A., El Kassem, W., Maslamani, M., et al. (2020). Management of life-threatening acute respiratory syndrome and severe pneumonia secondary to COVID-19 in pregnancy: a case report and literature review. Clin. Case Rep. 9, 137–143. doi: 10.1002/ccr3.3485
Zamaniyan, M., Ebadi, A., Aghajanpoor, S., Rahmani, Z., Haghshenas, M., and Azizi, S. (2020). Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat. Diagn. 40, 1759–1761. doi: 10.1002/pd.5713
Zambrano, L. D., Ellington, S., Strid, P., Galang, R. R., Oduyebo, T., Tong, V. T., et al. (2020). Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status – United States, january 22-october 3, 2020. MMWR Morb. Mortal. Wkly Rep. 69, 1641–1647. doi: 10.15585/mmwr.mm6944e3
Keywords: SARS-CoV-2, diabetes, cancer, children, pregnancy
Citation: Dandachi I and Aljabr W (2022) Prognosis of COVID-19 in the middle eastern population, knowns and unknowns. Front. Microbiol. 13:974205. doi: 10.3389/fmicb.2022.974205
Edited by:
Eduardo Rodriguez-Noriega, Civil Hospital of Guadalajara, MexicoReviewed by:
Yongfen Xu, Institut Pasteur of Shanghai (CAS), ChinaOmbretta Turriziani, Sapienza University of Rome, Italy
Copyright © 2022 Dandachi and Aljabr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waleed Aljabr, d2FsamFickBrZm1jLm1lZC5zYQ==
 Iman Dandachi
Iman Dandachi Waleed Aljabr
Waleed Aljabr