
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 03 August 2022
Sec. Microbiotechnology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.965934
This article is part of the Research Topic Basidiomycete Fungi: From Biosystematics and Biodiversity to Biotechnology View all 21 articles
For thousands of years, sanghuang is distinctive as a general designation for a group of precious and rare Chinese medicinal mushrooms. Numerous investigations have revealed that polysaccharide is one of the important biological active ingredients of sanghuang with various excellent biological activities, including antioxidant, anti-aging, anti-tumor, immunomodulatory, anti-inflammatory, anti-diabetic, hepatoprotective, and anti-microbial functionalities. For the past two decades, preparation, structural characterization, and reliable bioactivities of the polysaccharides from fruiting bodies, cultured mycelia, and fermentation broth of sanghuang have been arousing extensive interest, and particularly, different strains, sources, and isolation protocols might result in obvious discrepancies in structural features and bioactivities. Therefore, this review summarizes the recent reports on preparation strategies, structural features, bioactivities, and structure-activity relationships of sanghuang polysaccharides, which will enrich the knowledge on the values of natural sanghuang polysaccharides and support their further development and utilization as therapeutic agents, vaccines, and functional foods in tonic and clinical treatment.
Sanghuang, also called as “sang'er,” “sangchen,” and “sanghuanggu,” was first recorded in the medicinal utilization dating back to 2000 years in China's Shennong's Herbal Classic of Materia Medica. Compendium of Materia Medica written by Shi-Zhen Li in the Ming Dynasty also clearly described its medicinal effects (Wu, 2012; Zhu and Cui, 2016). Since then, sanghuang has been around and consumed as a food and versatile medicine in Chinese traditional medicine due to its excellent activities to treat dysentery, metrorrhagia, amenorrhea, aging, poisoning, and digestion (Wu, 2012; Hsieh et al., 2013; Zhang et al., 2016). Until 1968, modern pharmacological investigation on sanghuang was unlocked based on the finding of its remarkable anti-tumor activity by Ikekawa et al. (1968). As a group of ancient, precious, natural, and medicinal fungal resources, sanghuang has been validated by extensive scholars to have fascinating therapeutic functionalities, such as anti-tumor (Huang et al., 2011; He et al., 2021a), antioxidant (Liu et al., 1997; Shon et al., 2003; Chen et al., 2016), anti-bacterial (Lu et al., 2020; Ma et al., 2021), anti-virus (Mirzadeh et al., 2020), anti-angiogenic (Lee Y. S. et al., 2010), anti-platelet (Kamruzzaman et al., 2011), anti-inflammatory (Kim B. C. et al., 2006; Lin et al., 2017), immunoregulatory (Meng et al., 2016; Azeem et al., 2018; Jung and Kang, 2020), and hepatoprotective activities (Shan et al., 2019; Yang et al., 2020), treating diabetes (Hwang et al., 2005; Ajith and Janardhanan, 2021), hyperlipidemia (Rony et al., 2014), and cardiovascular (Lee et al., 2007; Su et al., 2017) diseases, etc.
Polysaccharides, which act as “biological response modifiers,” are macromolecules with long complex chains made of aldose or ketose joined by glycosidic bonds and are involved in many biological processes (Leung et al., 2006; Barbosa and de Carvalho Junior, 2020). Currently, the polysaccharides extracted from natural resources have drawn widespread concern around the world for their safety, non-toxic, and, thus, potential applications in functional food and biomedical products (Singdevsachan et al., 2016; Liu L. Q. et al., 2019; Yang M. Y. et al., 2019; Wang H. et al., 2021). Sanghuang has long been recognized as a class of famous medicinal mushrooms and contains a huge variety of biologically active ingredients, including polysaccharides (Yang et al., 2020), flavonoids (Wu et al., 2019) triterpenoids (Rajachan et al., 2020), polyphenols (Hwang et al., 2015; Liu et al., 2017), alkaloids (Wu et al., 2019), enzymes (Wang et al., 2020a,b), lipids (He et al., 2021b), and other compounds (Chen et al., 2016; Martinez-Medina et al., 2021), which have been manifested to possess various biological activities (Aida et al., 2009; Reis et al., 2017; Wen et al., 2019; Shi et al., 2020; Yang et al., 2020; Ajith and Janardhanan, 2021). Especially, the most dominant active component of sanghuang is a polysaccharide, which exerts various health-promoting effects, including free-radical scavenging ability (Gong et al., 2020), anti-tumor (Chakraborty et al., 2019), anti-microbial (He et al., 2021b), anti-inflammatory (Hou et al., 2020), neuroprotective (Chen et al., 2019), hepatoprotective (Yuan et al., 2019), anti-diabetic (Gong et al., 2020), and immunomodulatory (Meng et al., 2016) activities. An ever-growing number of structurally multifarious polysaccharides have been prepared from the fruiting bodies, cultured mycelia, and fermentation broth of sanghuang, by large quantities of extraction and isolation methods, such as hot water extraction, and ultrasonic- and enzymatic-assisted extraction, as well as substantial purification protocols, such as ethanol precipitation and column chromatography filled with different packings (Zhang Y. et al., 2015; Ren et al., 2019; Liu Y. H. et al., 2020; Mirzadeh et al., 2020; Leong et al., 2021). It is worth noting that the varied sources of original strains and preparation strategies can result in differences in the structures and properties of polysaccharides. Moreover, the bioactivities of polysaccharides are speculated to be significantly affected by their chemical structures, including molecular weights (Mws), monosaccharide compositions, uronic acid contents, primary structures, water solubilities, spatial configurations, and positions and types of glycosidic linkages and branches, sulfates contents, polymer charges, etc. (Villares et al., 2012; Meng et al., 2016; Yan et al., 2017; Wang W. J. et al., 2020; He et al., 2021b), but the accurate structure-bioactivity relationships are still not quite clear.
In this review, the evolution of sanghuang species and their names, preparation strategies, and emerging techniques of structural characterization, as well as biological activities detected of a series of overcovered bioactive polysaccharides from the fruiting bodies, cultured mycelia, and fermentation broth of sanghuang in recent years, were systematically summarized. In addition, the relationships between structural features and biological activities were discussed, and the future development trends of sanghuang polysaccharides were also prospected, aiming at benefiting the comprehensive understanding of the polysaccharides from natural medicinal fungal resources and providing valuable guidance for future research and applications on sanghuang polysaccharides-based therapeutic drugs, vaccines, and health-promoting functional foods in tonic and clinical treatment. The schematic illustration of preparation, structural elucidation, and bioactivity determination of sanghuang polysaccharides was demonstrated in Figure 1. The overall highlights of this review were visualized in Figure 2.
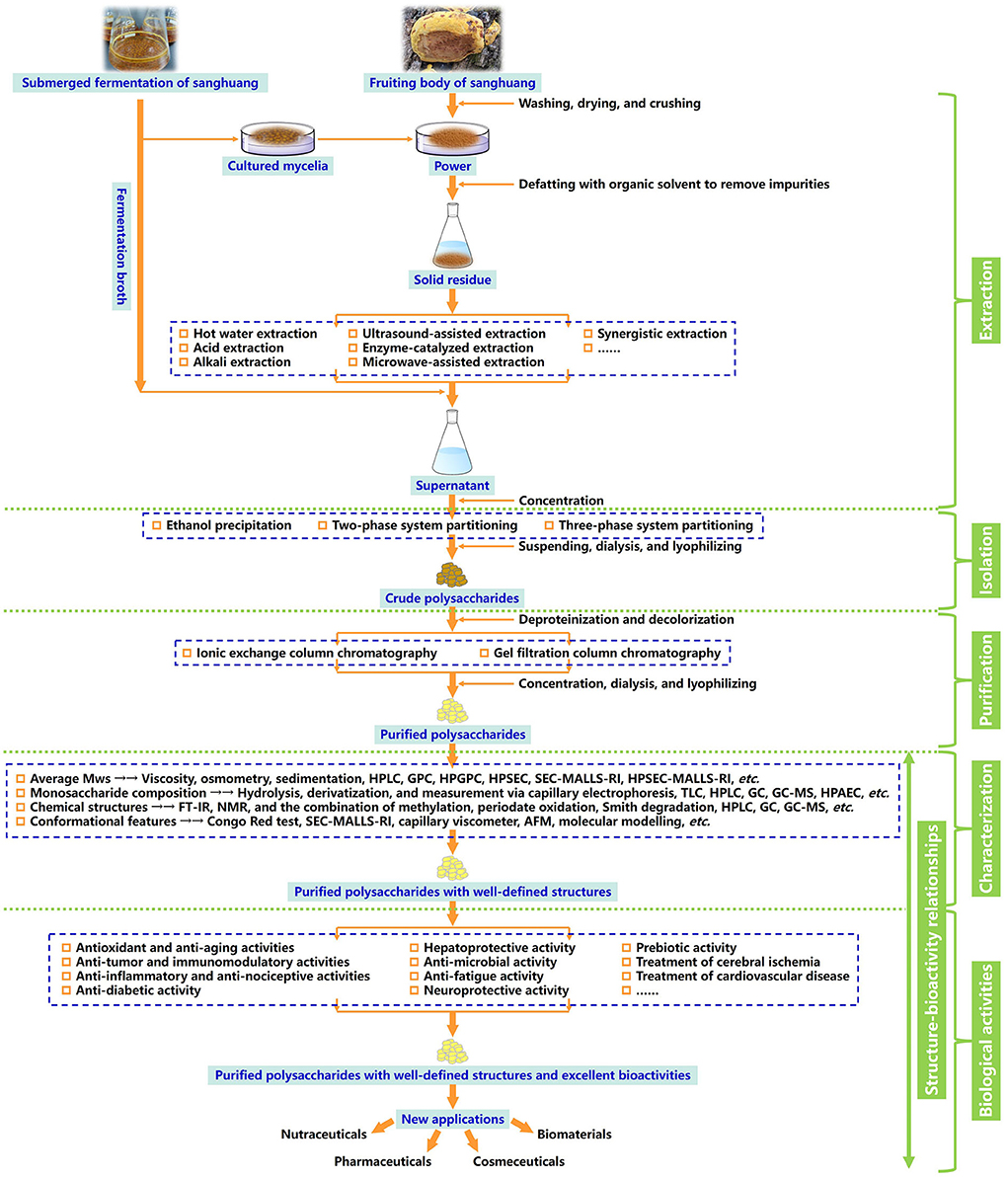
Figure 1. Schematic illustration of preparation, structural elucidation, and bioactivity determination of the polysaccharides derived from the naturally medicinal fungal resources sanghuang.
Herein, the sanghuang polysaccharides have been reported in recent years and the detailed information regarding their strains, sources, extraction, isolation and purification methods, Mws, monosaccharide compositions, structural features, bioactivities, involved mechanisms, and structure-bioactivity relationships, as well as their references, were integrated into Supplementary Tables 1, 2.
There was much controversy and confusion concerning the sanghuang species and their accurate scientific names in the past. The term Phellinus linteus (Berk. & M.A. Curtis) Teng had been universally believed as the scientific name for sanghuang (Teng, 1939). Dai and Xu (1998) found that P. linteus was a species of Central America, not Asia. The species distributed in China, Japan, and Korea was considered to be P. baumii Pilát. During this period, some scholars supported that the scientific name of sanghuang was P. igniarius (L.) Quél. (Liu, 1978). Wu et al. (2012) published the authentic sanghuang as a new species, Inonotus sanghuang Sheng H. Wu, T. Hatt. & Y.C. Dai that only grows on Morus. Then, Zhou et al. (2016) proposed the new genus Sanghuangporus Sheng H. Wu, L.W. Zhou & Y.C. Dai to amend I. sanghuang and its closely related species, and S. sanghuang (Sheng H. Wu, T. Hatt. & Y.C. Dai) Sheng H. Wu, L.W. Zhou & Y.C. Dai had become the scientific name for the sanghuang species growing on Morus. Wu et al. (2020) found a new species, S. vitexicola, from tropical Taiwan, China. In 2021, a new species, S. subbaumii Shan Shen, Y.C. Dai & L.W. Zhou, belonging to this genus had been discovered by Shen et al. (2021). To date, the genus Sanghuangporus consists of 15 species, which are widely distributed across cold temperate to subtropical and tropical zones. However, some species only appeared in specific regions and form a strict parasitic relationship with their host tree species; for example, S. sanghuang only grows on Morus (Zhu et al., 2019). Some researchers also endorsed that the sanghuang recorded in ancient Materia Medica might be I. hispidus (Bull.) P. Karst. (Bao et al., 2013). Consequently, the generalized sanghuang comprises the genera Sanghuangporus, Phellinus, Inonotus, and other species in the family Hymenochaetaceae, characterized by their yellow-brown and hard fruiting bodies (Wu and Dai, 2020).
The extraction, isolation, and purification of polysaccharides are fundamental to further research on their monosaccharide compositions, Mws, primary structures, types and degrees of branching, configurations, bioactivities, and structure-activity relationships (Zhang et al., 2007; Ferreira et al., 2015; He et al., 2021b; Maity et al., 2021). To effectively improve the purity, the fruiting bodies, cultured mycelia, or fermentation broth are usually amenable to an array of extraction, isolation, and purification steps, such as defatting with an organic solvent to remove lipids and other impurities, extraction using hot water, acid, alkali, or other assisted treatment, subsequent fractional precipitation by ethanol with various concentrations, deproteination with a Sevag reagent or protease enzymolysis, decolorization by resin or activated carbon, dialysis, concentration, lyophilization, and final refinement through ionic exchange column chromatography, gel filtration chromatography, affinity chromatography, etc. (Shi, 2016; Ren et al., 2019; Shi et al., 2020; Huang et al., 2021). Nevertheless, the obstacles to the acquirement of the purified functional polysaccharides from sanghuang is the structural changes and loss in activity caused by unsuitable approaches and operating conditions. It is essential to seek the appropriate schemes for extraction, isolation, and purification of these biological macromolecules according to their characteristics, to ensure that sanghuang polysaccharides can be commercialized to a large extent.
Fungal polysaccharides can be classified by their locations in cells, namely, intracellular polysaccharides from the fruiting bodies and cultured mycelia and exopolysaccharides from the fermentation broth. Generally, most exopolysaccharides are water soluble, and can be directly extracted by the water, acidic, or alkaline solutions (Cheng et al., 2018; Tang et al., 2020; Leong et al., 2021; Wu et al., 2022a). Hot water extraction as the most classical, convenient, and common extraction method should be operated using water at ≥50°C for a long duration of 1.5–10 h. Yan et al. (2016b) extracted an antioxidant activity-containing polysaccharide from the P. linteus mycelia through immersing the defatted mycelia into distilled water at 95°C for 8 h, then extracted twice. Similar extraction protocols were deployed by Ge et al. (2009) for gaining a water-soluble polysaccharide from the fruiting bodies of sanghuang mushroom P. baumii using hot water extraction (extracted thrice for 2 h each at 100°C). However, this extraction approach has several drawbacks containing the high reaction temperature, large material consumption, prolonged extraction time, etc. (Kang et al., 2019). Extraction conditions, including temperature, time, and the ratio of raw material to solvent are the important factors influencing the extraction yield of sanghuang polysaccharides (Huang et al., 2012; Tang et al., 2020). Wang et al. (2014a) extracted polysaccharides from the P. nigricans mycelia and verified the effects of time, temperature, frequency, and the ratio of water to raw material via central composite design in response surface methodology. The optimal conditions were extraction time of 2.8 h, the ratio of water to the raw material of 28, frequency of 5, and the temperature of 95°C. Under these conditions, the maximal yield of crude polysaccharides was 15.33 ± 0.21%. An increase in extraction temperature can accelerate the extraction yield of polysaccharides from sanghuang into the water to a certain level, followed by possible loss, due to the thermal degradation and structural modification at an excessive temperature, even resulting in decreases in Mws, as evidenced by Guo et al. (2010) and Zheng et al. (2019). A longer extraction time also usually shows a promotion in extraction yield (Guo et al., 2010). Li et al. (2019) found that an excessively lower ratio of water to raw material may lead to enhanced viscosity and incomplete extraction, while the comparatively higher ratio may incur an increased difficulty of further separation and purification, impacting the overall processing time, costs, and resources. Therefore, water-material ratio acts a key role in the hot water extraction of polysaccharides.
In acid or alkali extraction, acids such as HCl or (NH4)2C2O4, and alkalis such as NaOH or NaBH4, are devoted to drive the release of polysaccharides from sanghuang (Zhang et al., 2007; Yan et al., 2017; Tang et al., 2020). Wang Z. B. et al. (2014) adopted the successive extraction, including hot water, 1% (NH4)2C2O4, and 1.25 M NaOH/.05% NaBH4 to get P. linteus polysaccharides. The alkali extract with a high Mw and the highest carbohydrate and uronic acid contents exhibited stronger bioactivities when compared with other extracts. Wang K. et al. (2020) also employed 1.25 M NaOH/.05% NaBH4 to extract polysaccharides from the P. linteus mycelia, and the resulting fraction composed of Ara, Xyl, Glc, and Gal with the molar ratio of 5.5:7.8:1.8:1 exerted radical scavenging capacities and antioxidant activities in vitro and in vivo. The reason for the acid or alkali extraction often brings out the elevated extraction yields of polysaccharides is that destruction of cell walls of fruiting bodies and cultured mycelia of sanghuang and the degradation of fiber structures by acid or alkali solution, hence, allowing the release of more intracellular polysaccharides and transformation of water-insoluble into water-soluble fractions (Zhang Y. et al., 2015).
As fruiting bodies and cultured mycelia are incompletely water-soluble, other alternatives, such as ultrasound, enzyme, and microwave, which can provoke the breakdown of the cell walls of mushrooms and improve the solubility of polysaccharides, are introduced to assist in harvesting the higher yield of polysaccharides (Ebringerová and Hromádková, 2010; Cheng et al., 2012; Marić et al., 2018; Shi et al., 2019; Chang et al., 2021; Wu et al., 2022b). Zhang et al. (2014) established a flat-plate ultrasound technique to stimulate the production of the polysaccharide from the P. igniarius mycelial fermentation. Optimal conditions obtained by a three-factor-three-level Box-Behnken design were ultrasound treatment time of 65 min, duty cycle time of 25 s, and culture time of 3.8 d that gave a maximal polysaccharide yield of 1.8002 g/L, which increased by ~22.64%, compared with the treatment without ultrasonication. LSCM observations revealed that ultrasound can change the morphology and structure and of fungal mycelia, and then, accelerate the mass transfer of metabolites. Also, Liu et al. (2019a) inspected that the yield of I. hispidus polysaccharide with ultrasonic-assisted extraction was 39% higher than that with the non-ultrasound control group, suggesting that ultrasound-assisted extraction is a more effective method for polysaccharide extraction using its cavitation generated by rapid formation, implosion of the tremendous bubbles in mobile phase, and release of a vast of hydrodynamic forces, thus, driving the cell wall disruption, mass transfer, osmotic force, etc. (You et al., 2014; Chen X. H. et al., 2021; Wu et al., 2022b). Efficient degradation of the cell walls of mushrooms can be achieved by enzymes, producing more polysaccharides from the inside cells, whose extraction rates depend on the type and amounts of enzymes, temperature, pH, the ratio of liquid to solid material, reaction time, etc. (Marić et al., 2018; Cui and Zhu, 2021). A new procedure for the enzyme-catalyzed extraction of polysaccharides from P. igniarius was developed by Xu et al. (2016). Based on the single-factor experiment and Box-Behnken design, the optimum technological conditions were pH 5.79, reaction time of 1.5 h, extraction temperature of 55.02°C, and enzyme dosage of 3.04%, respectively. Under these conditions, the extraction rate of polysaccharides was increased by 49.5% compared with conventional hot water extraction. However, the higher costs of enzymes and sensitivity to harsh surroundings are the main shortcomings of enzyme-assisted extraction (Marić et al., 2018). Microwave is a radio wave with the frequency range at 300–3 × 105 MHz, which appears via interactions between polar molecules and is influenced by the external pressure, temperature, and time, whereupon being advocated to be a feasible tool for extraction of polysaccharide macromolecules (Singh et al., 2012; Mirzadeh et al., 2020). Gao et al. (2017) extracted the P. igniarius polysaccharides from six different origins using the microwave extraction method, and the resulting Gansu polysaccharide was identified to have a great potential as a natural anti-tumor agent with immunoregulatory activities. Furthermore, synergistic treatments for incorporating the superiority of these extraction methods can serve as the other beneficial choices in extracting active substances from sanghuang (Shi, 2016; Leong et al., 2021). For example, Ying et al. (2019) optimized the extraction procedure of polysaccharides from the fruiting bodies of P. igniarius by using the ultrasonic-microwave synergistic approach. Rank order from the highest to the lowest effects of the three extraction factors on the polysaccharides yields was ultrasonic power > extraction time > microwave power. The consequences displayed that the polysaccharides extracted by the synergistic method performed higher antioxidant and anti-tumor activities, which implied that the combination of ultrasound and microwave comes as a time-saving and high-yield method for polysaccharides assisted extraction.
Subsequently, the aqueous extracts or fermentation broth of sanghuang can be subjected to ethanol precipitation with ~95% (V/V) ethanol, in addition to fractional precipitation with various gradients of ethanol or acid precipitation with acetate acid, to isolate the crude polysaccharides mixed with proteins and pigments. Aqueous two-phase system and three-phase partitioning as alternative tools referring to the sequential mixing of organic solvent and salt with crude extracts or suspensions to reach the different dispense phases have been exploited for the efficient extraction and separation of polysaccharides as well (Chew et al., 2019; Wang et al., 2019b; Wu Y. et al., 2022). Wang et al. (2019a) engaged in three-phase partitioning to directly isolate a bioactive exopolysaccharide from the cultured broth of P. baumii and gave the maximal extraction yield of 52.09% under the following conditions: 20% (w/v) (NH4)2SO4 concentration, 1.0:1.5 (V/V) ratio of broth to t-butanol, 30 min, and 35°C. This polysaccharide with high carbohydrate and uronic acid contents demonstrated dramatically radical-scavenging, antioxidant, α-amylase and α-glycosidase inhibitory, as well as macrophage-stimulating activities.
To further eliminate the biological wastes, and then acquire the homogenized samples, the crude polysaccharides should go through a set of adsorbents and eluents, including chemical/enzymatic deproteinization, decolorization by resin or activated carbon, dialysis to remove small molecules, concentration, and freeze-drying, followed by ionic exchange column chromatography (DEAE-Cellulose, DEAE-Sepharose, DEAE-Sephadex, etc.) to seize the neutral and acidic polysaccharides, and gel filtration column chromatography (Sepharose, Sephadex, Sephacryl, etc.) to capture the polysaccharides with different Mws, eluting with suitable eluates and speeds, collecting, dialyzing, condensing, and lyophilizing (Fang and Ding, 2007; Tang et al., 2020; Leong et al., 2021). During the entire steps, the associated carbohydrate and protein concentrations of each polysaccharide fraction were measured via the phenol-sulfuric acid method with Glc as a standard (DuBois et al., 1956) and the Bradford method with bovine serum albumin as a standard (Bradford, 1976). Yang et al. (2007) utilizes the hot water extraction, ethanol precipitation, DEAE-Sepharose Fast-Flow, and High-Resolution Sephacryl S-400 column chromatography to isolate a new P. igniarius fruiting bodies-derived heteropolysaccharide, whose structural investigation was carried out with HPLC, HPAEC, GC-MS, methylation analysis, and NMR spectroscopy techniques. Xue et al. (2011) separated a 17-kDa water-soluble polysaccharide from the P. baumii mycelia taking advantage of the hot water extraction, ethanol precipitation, and DEAE-Sephadex A-50 and LPLC-Sephadex G-75 chromatography methods, as well as confirming that this polysaccharide owned immunomodulatory and anti-tumor activities in vitro. Two different polysaccharides from the submerged mycelia culture of P. mori were the ethanol precipitated and gel filtration chromatographed by Cao et al. (2013). Besides, they substantiated that the polysaccharides had (1 z → 4)-linked Manp backbones, with branches of (1 → 4)-linked glucosyl residues and (1 → 3,4)-linked Galp residues. Cheng et al. (2020a) fractionated a novel exopolysaccharide from the liquid culture broth of S. sanghuang by using ethanol precipitation, DEAE-Sepharose Fast Flow, and Sephacryl S-100 HR gel column chromatography. This exopolysaccharide, exclusively composed of Man, consisted of 1,3-linked and 1,2-linked α-D-Manp with substitution at O-6 of 1,2-linked α-D-Manp by 1,6-linked α-D-Manp residues and terminal α-D-Manp residues, and exerted potential anti-tumor activity against the growth of HepG2 and MCF7 in vitro.
Up to present, the versatile natural polysaccharides derived from sanghuang have been prepared and structurally characterized, as listed in Supplementary Table 1, because of their plentiful promising bioactivities (Zong et al., 2012; Meng et al., 2016; Ren et al., 2019; Gong et al., 2020). It is widely accepted that the functions and behaviors of these complex biological macromolecules are dramatically affected by their physicochemical and structural properties, which are involved in composition and structural uniqueness, mainly covering Mws, sequences of monosaccharide residues, configurations and conformation of isomers, types and numbers of glycosidic linkages, skeleton lengths, chain compositions and aggregations, presences and positions of branches and functional groups, branching degrees, lengths of side chains, 3D conformation, etc. (Ferreira et al., 2015; Ruthes et al., 2016; Wang J. Q. et al., 2016; Xie et al., 2020). Although there exist some restrictions for accessing the fine structures in all hierarchies due to the structural diversity and variability of macromolecules, the basic chemical structures of purified sanghuang polysaccharides have been probed and identified by combined means of the chemical, instrumental, and biological techniques, including hydrolysis (Cao et al., 2019), methylation analysis (Sun et al., 2021), periodate oxidation (Nypelö et al., 2021), Smith degradation (Villares et al., 2012), Congo Red test (Guo X. Y. et al., 2021), capillary viscometer (Yuan et al., 2018), Zeta-sizer Nano instrument (Miao et al., 2020), UV-visible spectrophotometry (Guo X. Y. et al., 2021), HPLC (Yang et al., 2007), HPSEC (Wang et al., 2014b), HPSEC-MALLS-RI (Cheong et al., 2015), HPGPC (Ren et al., 2019), TLC (Yang P. et al., 2019), GC (Lo et al., 2011), MS (Liu et al., 2021), GC-MS (Ma et al., 2016), HPAEC (Zhang Z. F. et al., 2015), FT-IR (Qian et al., 2009), NMR spectroscopy (1H, 13C, COSY, DEPT, HMBC, HMQC, HSQC, NOESY, and TOCSY) (Doost et al., 2019; Yao et al., 2021), XRD (Qian et al., 2009; Guo X. Y. et al., 2021), DLS (Wang Z. B. et al., 2014), DSC (Guo X. Y. et al., 2021), TGA (Guo X. Y. et al., 2021), AFM (Guo X. Y. et al., 2021), enzymatic analysis (Karaki et al., 2016; Mitchell et al., 2021), immune analysis (Sharma et al., 2019), etc. Recently, some emerging methods have also been applied for the structural characterization of polysaccharides (Fabijanić et al., 2019; Li Y. T. et al., 2020; Nandita et al., 2021).
Polysaccharides, as a structurally diverse class of biological macromolecules, commonly possess the relatively higher Mws, which have been determined by viscosity measurement (Yan et al., 2016a), osmometry (Gong et al., 2020), sedimentation (Ren et al., 2019), HPLC (Jiang et al., 2015), GPC (Wu et al., 2013), HPGPC (Zhang et al., 2018; Wu Y. et al., 2022), HPSEC (Lee S. M. et al., 2010), SEC-MALLS-RI (Jia et al., 2017; Cheng et al., 2020a), HPSEC-MALLS-RI (Li et al., 2015; Wang et al., 2019b), etc. Pei et al. (2015) used HPGPC to estimate the average Mw of a polysaccharide prepared from the alkaline extract of the P. linteus mycelia as 3.43 × 105 kDa. Li et al. (2015), by virtue of HPSEC-MALLS-RI, unearthed that the Mws of nine polysaccharides from the fruiting bodies, submerged mycelia, and solid-state-fermented products of P. baumii, decreased with the increasing precipitated ethanol concentrations. Mws of the polysaccharides from the fruiting bodies and submerged mycelia ranged from 19.8 to 1.89 × 103 kDa and 2.11 × 103 to 2.01 × 104 kDa, respectively. Some lower-Mw fractions were detected in the solid fermented products. As summarized in Supplementary Table 1, the polysaccharides derived from various sanghuang species and experimental conditions exhibit wide Mws ranging from 1 to 1 × 106 kDa.
A correlation between the antioxidative potency and Mw of polysaccharides had been well-established according to the substantial research (Wang J. Q. et al., 2016; Yan et al., 2016a; Gong et al., 2020; Wu Y. et al., 2022). It is pronounced that the antioxidation and free radicals scavenging of low-Mw polysaccharides are superior to that of the polysaccharides with high Mws, on account of more active intramolecular hydrogen bonding effect of O–H and electron-donating substituents (Xing et al., 2005). Wu Y. et al. (2022) separated a low-Mw polysaccharide from the P. linteus mycelia by using an aqueous two-phase system based on choline chloride ([Chol]Cl)/K2PO4, which owned more prominent scavenging effect for radicals when compared to the higher-Mw polysaccharide obtained using the ethanol precipitation and isolation protocols. Previous studies have also raised that several physical, chemical, or enzymatic-assisted treatments, such as ultrasound, microwave, acid, alkali, and cellulase, could be applied for degrading polysaccharides into low-Mw fragments, thereby, influencing their antioxidant abilities (Karaki et al., 2016; Wang J. Q. et al., 2016; Mirzadeh et al., 2020; Chen X. H. et al., 2021). Yan et al. (2016a) physically degraded a P. linteus mycelia-derived polysaccharide using ultrasound to garner a low-Mw and low-intrinsic-viscosity fraction, performing the stronger hydroxyl radical scavenging capacity and higher values of Trolox equivalent antioxidant capacity (TEAC) and ferric-reducing ability of plasma (FRAP). Wang Z. B. et al. (2014) extracted and partially purified three polysaccharides from the P. linteus mycelia using hot water, 1% (NH4)2C2O4, and 1.25 M NaOH/.05% NaBH4 and found that the polysaccharide with higher amounts of lower Mw fractions and uronic acid contents showed the stronger DPPH radical-scavenging capacity. However, contradictory findings also exist. Yuan et al. (2018) covered that a polysaccharide from the P. igniarius mycelia with high Mw and intrinsic viscosity could effectively scavenge hydroxyl radicals, partly eliminating DPPH radicals and chelate ferrous ions. Besides, El Enshasy and Hatti-Kaul (2013) pointed out that the low-Mw polysaccharides could penetrate the immune systems and demonstrate stimulatory activity, whereas the advantage of high-Mw polysaccharides might be ascribed to the better binding affinity of the receptors of immune cells.
Polysaccharides can be divided into homo- and hetero-polysaccharides duo to the various monosaccharides in compositions. For heteropolysaccharides consisting of a series of monosaccharides mainly embracing Glc, GlcA, Gal, GalA, Ara, Fuc, Fru, Man, Rha, and Xyl, their structures and chemical properties could be affected by the different types and sequences of monosaccharides and glycosidic bonds (Lo et al., 2011; Ren et al., 2019). Confirmation of monosaccharide composition normally involves the liberation of monosaccharides through hydrolysis and derivatization (Zhang et al., 2020; Liu et al., 2021), then subsequent measurement of the liberated monosaccharides via various detection techniques, including capillary electrophoresis (Baker et al., 2008), TLC (Liu and Wang, 2007), HPLC (Kim G. Y. et al., 2006; Wan et al., 2020), GC (Luo et al., 2010; Pei et al., 2015), GC-MS (Yang et al., 2007; Suabjakyong et al., 2015), HPAEC (Yang et al., 2009; Wu et al., 2013; Jin et al., 2016), etc. A neutral polysaccharide was isolated and purified from the cultured mycelia of S. sanghuang by DEAE Sepharose Fast Flow and Sephacryl S-100 columns (Cheng et al., 2020b). This polysaccharide was primarily composed of Glc, indicated via acid hydrolysis and GC-MS detection, as well as having the potential inhibitory activities against α-amylase and α-glucosidase and hypoglycemic effects on in vitro insulin resistance of HepG2. Jin et al. (2016) employed hot water extraction, delignification, ethanol precipitation, DEAE-52 cellulose anion-exchange, and Sephadex G-100 columns to fractionate a polysaccharide from the fruiting bodies of P. baumii. HPAEC analysis delineated that this polysaccharide comprised Fuc, Ara, Gal, Glc, Xyl, and Man at molar ratios of 2.19:1.27:5.85:43.22:2.73:4.18, and that Glc was the predominant monosaccharide.
Correlational studies notarized that the biological proficiency of polysaccharides is dependent on the ratios of different monosaccharides in compositions (Lo et al., 2011; Yang et al., 2020). Two proteoglycans isolated from the mycelia and fermentation broth of P. nigricans through submerged fermentation were elucidated to possess the similar average Mws and consist of Glc, Gal, Man, Ara, and Fuc in the molar ratios of 3.26:8.77:6.44:1:1.35 and 20.06:8.72:6.94:1:0.76, respectively. The anti-tumor and immunomodulating activities of mycelia polysaccharides were higher than those of the broth fraction, which may be related to, in part, the variations in ratios of monosaccharides in compositions (Li et al., 2008). Jia et al. (2017) separated two water-soluble polysaccharides with the similar Mws but different monosaccharide compositions from the fruiting bodies of P. vaninii, and by means of MTT assay identified that the alkali-extracted polysaccharide exhibited the higher inhibition effect on HepG2 and HeLa than the hot water-extracted portion in vitro. On reason for the results happened maybe the significant shift on monosaccharide relative contents in polysaccharides caused by the alkaline solution treatment. Similar evidence was also figured out by Jiang et al. (2015), who isolated and purified two polysaccharides from the mycelia and culture medium of P. pini, both neutral heteropolysaccharides comprising Man, Gal, and Glc with molecular ratios of 2.99:1.00:0.34 and 38.40:1.00:1.76, respectively. The antioxidant activities of scavenging DPPH and hydroxyl radicals, chelating ferrous ions, and reducing ferric ions of mycelia polysaccharide were stronger than those of the culture medium portion, suggesting that the antioxidant activities could be impacted by the multiple ratios of monosaccharides in compositions. Apart from bioactivities, the multiplicity in monosaccharide compositions and molar ratios may correspond to the original materials, sources, extraction, isolation, and purification methods, etc., which is authenticated by the up-mentioned examples as well.
At present, different tools, such as FT-IR, NMR spectroscopy, and the combination of methylation, periodate oxidation, Smith degradation, HPLC, GC, and GC-MS, have been devoted to clarifying the chemical structures of sanghuang polysaccharides, including α/β configurations, branching degrees, types and positions of branches and functional groups, patterns and chirality of glycosidic bonds, positions and linkage sequences of the residues in glycosidic bonds, etc. (Liu and Wang, 2007; Mei et al., 2015; Ma et al., 2019; Cheng et al., 2020a; Sun et al., 2021). FT-IR could preliminarily analyze the vibrations of atoms or functional groups existed in polysaccharides, owing to the unique wavenumber range to the adsorption bond, hereby, providing the information of anomeric configurations and glycosidic bonds (Qian et al., 2009; Ren et al., 2019). For instance, the characteristic peak at 855–833 cm−1 is indicative of the α configuration of Glc, whereas the higher wavenumber ranging from 905 to 876 cm−1 signifies the β configuration (Zhang, 1994). NMR spectroscopy has been claimed as a vital technology for almost full-structural identification of the underivatized polysaccharides, such as monosaccharide compositions, positions and patterns of branching and glycosidic linkages, and types of cyclic structures, replying on the resonance spectroscopy and Zeeman, splitting generated by the atomic nucleus in an external magnetic field after adsorbing electromagnetic waves with the certain frequencies transiting from one spin to another (Yao et al., 2021). NMR spectroscopy is also a nondestructive testing tool in which the samples are not destroyed, and the resulting data are accurate and reliable, but the “pure” polysaccharide is highly required for preparation (Gong et al., 2020). Interpretation of functional groups is based on the comparison of their chemical shifts and coupling constants with the spectral data documented in previous literature containing the relevant polysaccharides (Villares et al., 2012; Yao et al., 2021). Methylation of polysaccharides plays an important role in sample preparation in the determination of the monosaccharide compositions, anomeric configurations, occurrence of branches, and positions of glycosidic linkages (Villares et al., 2012). This overall course involves several steps: the complex carbohydrates are methylated, then hydrolyzed by acids, and the newly constructed hydroxyl groups are acetylated, then deciphered via HPLC, GC, or GC-MS. It is particularly noted that FT-IR is often used to examine whether the characteristic adsorption band at 3,000–3,500 cm−1 is completely disappeared to ensure that all the hydroxyl groups in polysaccharides are absolutely methylated (Sims et al., 2018). Periodate oxidation as a routine choice also has been used for elucidating the structures of polysaccharides referring to the cleavage of C–C bonds via oxidation reaction caused by periodate ion attack, accordingly, forming dialdehyde, aldehyde, or formic acid. Since each C–C bond cleavage consumes one molecule of periodate ion, the position and type of glycosidic linkages, branching degree, and chain composition, as well as aggregation, can be defined by the consumption of periodate ion and the amounts of corresponding products released (Jiang et al., 2016; Nypelö et al., 2021). Smith degradation is accomplished by the reduction of periodate-oxidized products, acid hydrolyzed, and subsequent HPLC, GC, or GC-MS analysis, whereby, inferring the linkage sequences of the monosaccharide residues in glycosidic bonds (Wasser, 2002).
Liu and Wang (2007) prepared a water-soluble polysaccharide from the fruiting bodies of P. ribis by hot water extraction, ethanol precipitation, DEAE-cellulose, and Superdex 30-column chromatography, and with the help of FT-IR, NMR spectroscopy, GLC-MS, methylation analysis, periodate oxidation, and Smith degradation investigated it to be a β-D-glucan containing a (1 → 4), (1 → 6)-joined backbone, with a single β-D-Glc at the C-3 position of (1 → 6)-joined glucosyl residue every eight residues, along the main chain. Preliminary activity tests in vitro revealed that this polysaccharide could stimulate the proliferation of spleen lymphocytes. A heteropolysaccharide, fractionated by Yang et al. (2009) from the fruiting bodies of P. igniarius via hot aqueous extraction, ethanol precipitation, DEAE-Sepharose anion-exchange, and gel filtration chromatography, was elaborated to have a skeleton consisting of 1,6-disubstituted-3-O-methyl-α-D-Glcp residue, 1,3,6-trisubstituted-α-D-Manp residue, 1,4-disubstituted-α-D-Galp residue, and 1,2-disubstituted-α-D-Galp residue, and have a 1-substituted-α-L-Fucp terminal attached to O-3 of a Manp residue, on the basis of methylation and NMR studies (1H, 13C, COSY, TOCSY, ROESY, HSQC, and HMBC). Bioactivity assays conducted in vitro displayed that this heteropolysaccharide stimulated the proliferation of mice spleen lymphocytes. Jiang et al. (2016) gained a P. pini exopolysaccharide that contained a backbone of (1 → 2)-linked Man, being heavily substituted via (1 → 6)-glycosidic bonds with (1 → 3)-linked Man, and terminated mainly with Man, as well as a small amount of Gal and Glc, as affirmed by partial hydrolysis with acid, periodate oxidation and Smith degradation, methylation, and NMR spectroscopy. Immunological experiments showed that the P. pini exopolysaccharide demonstrated the high macrophage-activating ability, consequently augmenting phagocytosis and enhancing the production of NO, TNF-α, and reactive oxygen species (ROS). Yan et al. (2016c) using hot water extraction, ethanol precipitation, DEAE-Sepharose FF, and Sephacryl S-400 HR column chromatography purified a polysaccharide from an ammonium oxalate extract of the P. linteus mycelia. Results of methylation analysis, FT-IR, and NMR spectroscopy indicated that the backbone of this polysaccharide was composed of (1 → 4)-α-D-Glcp, (1 → 2)-α-D-Xylp, and (1 → 3)-α-D-Araf residues, whereas the (1 → 6)-α-D-Manp residues formed branches at the O-2 position with 1-linked-α-D-Glcp terminal residues. From the antioxidative activity measurements in vivo, this polysaccharide enhanced the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), and reduced the malondialdehyde (MDA) levels in serum and liver of the D-Gal-treated aging mice in a concentration-dependent manner, as well as effectively stimulated the immune systems of aging mice.
These findings also provide the considerably valuable clues and new insights for elaborating the structure-bioactivity relationships of sanghuang polysaccharides, that is, the complexity of structures of polysaccharides is significantly associated with their biological functionalities (Ferreira et al., 2015; Qu et al., 2020; Song et al., 2021). For instance, Ara 1 → 4 and Man 1 → 2 linkages of the branched chains in polysaccharides are positively correlated with their FRAP, whereas Glc 1 → 6 and Ara 1 → 4 linkages are related with their abilities on scavenging DPPH radicals (Lo et al., 2011). Polysaccharides abundant in the → 3)-β-D-Glcp-(1 → linkage strongly abate their inflammatory responses, and so do the polysaccharide plentiful in rich sequences of the side-chain units (Nie et al., 2017; Qu et al., 2020). An exopolysaccharide from the fermentation broth of S. sanghuang was purified by ethanol precipitation, DEAE-52 cellulose, and Sepharose CL-6B columns, as well as was disclosed to comprise a skeleton of → 4)-β-Manp-(1 → 4)-α-Araf-(1 → 3,4)-α-Glcp-(1 → 3,4)-α-Glcp-(1 → 3,4)-α-Glcp-(1 → 3,4)-α-Glcp-(1 → 3,4)-α-Glcp-(1 → 6)-α-Galp-(1 → 4)-β-Manp-(1 → and five branches, including four α-D-Glcp-(1 → and one α-D-Manp-(1 → with the aid of FT-IR, methylation, and 1H, 13C, COSY, DEPT, TOCSY, ROESY, HSQC, and HMBC NMR spectroscopy, and, hence, increase antioxidant enzymes activities and diminish lipofuscin levels, and ameliorate histopathological hepatic lesions and apoptosis in hepatocytes of the D-Gal-aged mice (Ma et al., 2019). Sun et al. (2021) isolated a 46-kDa heteropolysaccharide from the fruiting bodies of P. baumii via hot water extraction, ethanol precipitation, DEAE-Sepharose Fast Flow, and Sephadex G-200 gel-permeation column chromatography, and found it had a backbone containing 1,3-linked β-D-Glcp and 1,6-linked α-D-Galp residues, being attached to Araf, Manp, and Galp units as oligosaccharidic side chains to the skeleton at C-6 position of some Glcp. Moreover, this polysaccharide displayed markedly anti-inflammatory activity in the LPS-stimulated macrophage RAW264.7 cells by downregulating phosphorylation of the signal transducer and activator of transcription 1 and increasing the tissue repairing genes, as well as alleviated the dextran sodium sulfate-inducible ulcerative colitis in mice, presumably attributed to the copious branched chains and → 3)-β-D-Glcp-(1 → linkages.
Conformation of polysaccharides is delimited as their 3D structures in liquid or solid-state through bonding or spacing physical forces resulting from their chemical structures, including spherical, random coil, single-helix, double-helix, triplex-helix, rod-like, etc. (Zhang et al., 2020; Guo X. Y. et al., 2021). Likewise, biological activities of polysaccharides are drastically affected by their conformational features. More flexible and extended conformation, as well as good solubilities, confer polysaccharides to have more opportunities to bind to cell membranes and have stronger interactions with the correlated receptors, which lead to the higher antioxidant, anti-tumor, and immunomodulatory activities (Falch et al., 2000; Zheng et al., 2017; Guo X. Y. et al., 2021). Based on the data of Mw and monosaccharide composition, Wang Z. B. et al. (2014), making use of DLS and Congo Red test, proved the three partially purified polysaccharides extracted from the P. linteus mycelia using hot water, acid, and alkali to exist as compact random coils in aqueous solutions. In vitro antioxidant assays verified that the acid- and alkali-extracted polysaccharides exerted the stronger scavenging capacities and antioxidant activities in a dose-dependent manner, imputed to the chemical structures and conformational features. Jia et al. (2017) illustrated that one fraction isolated from the fruiting bodies of P. vaninii by hot water was a high-branched heteropolysaccharide with a globular shape, whereas another fraction extracted by NaOH solution was a β-1,3-D-glucan branched with β-1,6-D-Glc and adopted more extended and flexible random coils conformation, as checked by FT-IR, acid hydrolysis and GC-MS, 13C NMR spectroscopy, and SEC-MALLS-RI and capillary viscometer. MTT assay indicated that the NaOH-treated polysaccharide had the stronger inhibition activity against HeLa and HepG2 in vitro, which revealed that the presence of β-glucan, high chain rigidity, good water solubility, and moderate Mw of the derivatives in an aqueous solution were beneficial to the increase in cytotoxicity on the growth of tumors. A polysaccharide was obtained by Yuan et al. (2018) from the P. igniarius mycelia, and was elucidated to exhibit a triple helical structure and have a high Mw, a high intrinsic viscosity, and a linear repeating backbone composing of Glcp, Galp, and Manp joined by α-(1 → 4), α-(1 → 3), and α-(1 → 6) linkages, and single α-terminal-D-Glcp as side chains of 6-O-connected to the main chain through HPGPC, capillary viscometer, acid hydrolysis and HPLC, FT-IR, methylation analysis and GC-MS, NMR spectroscopy, and Congo Red test. Consequences of the antioxidant and anti-tumor tests delineated that this polysaccharide with triple helix conformation, and only α-type glycosidic bond promoted the positive scavenging effects against DPPH and hydroxyl radicals, and ferrous ions chelating activity in vitro, as well as possessed the potent anti-tumor activity against the growth of HT29 and MCF7 in vitro. Cheng et al. (2020a,b) isolated two polysaccharides from the liquid culture broth and mycelia of S. sanghuang. In addition to the tests of acid hydrolysis and GC-MS, FT-IR, methylation analysis and GC-MS, and NMR spectroscopy (1H, 13C, COSY, TOCSY, HSQC, HMBC, and NOESY), the two polysaccharides were ascertained to exist in flexible chain conformation in 0.1 M NaNO3 by using AFM, SEC-MALLS-RI, and 3D molecular modeling. Further, the two polysaccharides showed the potential for either anti-tumor or hypoglycemic effects.
From what was described above, it can be seen that deeper and accurate identifying the conformational features of sanghuang polysaccharides contributes helpfully to probe their biological and functional activities, even in designing biomaterials for clinic therapy and vaccines (Zafar et al., 2016; Makvandi et al., 2020; Alturki, 2021; Prateeksha et al., 2021). There have been many powerful techniques applied in explaining the architecture of an individual polysaccharide, including the Congo Red test, SEC-MALLS-RI, capillary viscometer, AFM, XRD, molecular modeling, circular dichroism, DSC, DLS, etc. The Congo Red test is among the commonly used method because of the low demand of operation and the needlessness of advanced instrument. Congo Red, an acidic dye, can bind with the triple helical polysaccharides to form complexes, which induce a characteristic birefringence shift in the adsorption wavelength when compared with the original Congo Red, therefore, reflecting the presence of triple-helix structures of polysaccharides (Ogawa et al., 1972; Wang Z. B. et al., 2014; Guo X. Y. et al., 2021). Conformational features of the polysaccharides can also be incarnated by some latent parameters, such as intrinsic viscosity (η), hydrodynamic radius (Rh), radius of gyration (Rg), molar mass per contour length (ML), persistence length (q), chain diameter (d), Huggins constant (k′), and Mark-Houwink equation exponent (α), which can be monitored through SEC-MALLS-RI and capillary viscometer and calculated on the basis of related equations (Jia et al., 2017; Cheng et al., 2020a,b; Guo X. Y. et al., 2021). AFM has emerged as a visual tool for investigating the shape, size, and aggregated morphology of macromolecular chains to further characterize the conformation of polysaccharides (Cheng et al., 2020a,b; Guo X. Y. et al., 2021). Lately, molecular modeling plays a novel role in predicting the 3D structures and exploring the mechanisms of polysaccharides by mimicking the spatial conformation of biological macromolecules and their intramolecular interactions (Cheng et al., 2020a; Guo X. Y. et al., 2021).
Nowadays, the worldwide population is rapidly aging so considerable attention had been paid to a balanced diet and health needs. People are urgent to find more alternative active ingredients in our daily food and health care products, and sanghuang polysaccharides are in line with this (Aida et al., 2009; Yang M. Y. et al., 2019; Li N. Y. et al., 2021; Martinez-Medina et al., 2021; Yadav and Negi, 2021). Innumerable works, in vitro and in vivo, have been implemented to substantiate that the sanghuang polysaccharides have multiple biological activities and health benefits, such as antioxidant and anti-aging (Wang et al., 2014a; Yuan et al., 2018; Zuo et al., 2021), anti-tumor and immunomodulatory (Chen et al., 2011; Liu et al., 2018; Wan et al., 2020), anti-inflammatory and anti-nociceptive (Li et al., 2015; Wang et al., 2019; Sun et al., 2021), anti-diabetic (Kim et al., 2010; Wu et al., 2016; Khursheed et al., 2020), anti-microbial (Lee S. M. et al., 2010; Reis et al., 2014), anti-fatigue (Zhong, 2020), hepatoprotective (Liu et al., 2019b; Zhang et al., 2019; Chen C. et al., 2020), neuroprotective (Liu Y. H. et al., 2015; Yang P. et al., 2019) activities, etc. Even though the bioactivities of sanghuang polysaccharides are intensively mined, scientific research focusing on deciphering the structure-bioactivity relationships and underlying mechanisms is still relatively insufficient. The available information regarding the biological activities of sanghuang polysaccharides are provided in Supplementary Table 2 and discussed in detail below.
It is widely accepted that ROS, including hydroxyl radical (·OH), superoxide anion (·O), and hydrogen peroxide, produced during the metabolism by normal cells play an essential part in the homeostasis in organisms (Barja, 2004; Wang J. Q. et al., 2016). Nevertheless, an excess of ROS, either credited to cellular stress and/or disordered metabolism and enzymatic systems, can trigger disfunction, cause DNA damage, and ultimately be involved in the pathogenesis of a variety of human diseases, such as aging, diabetes, rheumatoid arthritis, inflammation, cardiovascular disease, atherosclerosis, cerebral ischemia, and cancer (Benzie, 2000; Lu and Finkel, 2008; Espinosa-Diez et al., 2015; Li C. P. et al., 2021; Yuan et al., 2021). Many examples demonstrated that the sanghuang polysaccharides, which are capable of balancing the overproduction of ROS and protecting the living organisms against these diseases through capturing free radicals and/or promoting antioxidant enzymes activities, have been pursued as the potential ROS scavengers and antioxidants (Azeem et al., 2018; Forman and Zhang, 2021; He et al., 2021b). As concluded in Supplementary Table 2, sanghuang polysaccharides exhibited significant antioxidant activities in comparison with the untreated groups using both in vivo and in vitro assays, reflecting in scavenging superoxide anions and hydroxyl, DPPH, and ABTS radicals, reducing power, chelating metal ions, enhancing cell viability, the activities of SOD, CAT, and GSH-Px, and levels of ascorbic acid and TEAC, decreasing cell apoptosis, MDA content, and the activities of myeloperoxidase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactic dehydrogenase, upregulating the mRNA expression of IL-10, NAD(P)H quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase, catalytic subunit, and glutamate-cysteine ligase, and modifier subunit, as well as downregulating the levels of TNF-α and IL-1β mRNA. On the one hand, sanghuang polysaccharides act as the hydrogen atom donators to radicals by the weak dissociation energies of O–H bonds, then, emerging with stronger scavenging capacities against free radicals in terminating radical chain reactions. In addition, the existence of two or more of the following functional groups, such as O–H, S–H, –COOH, –PO3H2, C=O, –NR2, –S–, and –O–, in the structures of sanghuang polysaccharides is in favor of metal chelating activities. Antioxidant enzymes also contribute to prevent cells, cell membranes, cellular DNA, lipids, and proteins from oxidative stress. They were produced by stimulation of sanghuang polysaccharides and can catalyze the breakdown of lipid hydroperoxides and hydrogen peroxides to stable forms (Wang J. Q. et al., 2016; Cheng et al., 2018).
Xu et al. (2017) achieved the maximum yield of P. vaninii polysaccharide when Suc was employed as a carbon source. Likewise, Suc was also the best carbon source from the perspective of antioxidant activity, owing to the high Gal content, moderate Mw, and nearly globular shape form of the polysaccharide, as confirmed by Mw estimation, monosaccharide composition analysis, and FT-IR spectroscopy. A degraded polysaccharide from the P. linteus mycelia was caught through hot water extraction, (NH4)2C2O4 and NaOH/NaBH4 treatment, ethanol precipitation, and ultrasonication. The degraded fraction with a low Mw and viscosity, by contrast, demonstrated the stronger hydroxyl radical scavenging capacity and higher TEAC and FRAP (Yan et al., 2016a). Zhang et al. (2014) took advantage of a flat-plate ultrasound technology to harvest a polysaccharide from the P. igniarius mycelia, and using in vitro assays indicated that the polysaccharide with a lower Mw and higher amounts of carbohydrate, uronic acid, hydroxyl groups, and monosaccharide Rha had stronger antioxidant activities. The findings were in accordance with Yuan et al. (2018) and Wu Y. et al. (2022) who found that the polysaccharides from the P. igniarius mycelia with lower Mw and higher contents of electrophilic groups facilitated the liberation of hydrogen from O–H bonds and motivated the higher antioxidant capacities. It was suggested that strain species, sources, and culture media and extraction, isolation, and purification methods could impose great effects on the antioxidant and anti-aging properties of sanghuang polysaccharides. Besides, the activities depend chiefly on the structural characteristics, including the amounts of carbohydrate, uronic acid, and sulfate and reducing end, Mws, viscosities, monosaccharide constituents, conjugates, positions and types of glycosidic linkages and branches, etc. For example, polysaccharides with more complex structures and lower Mws, involving more side-branches and residues, could alleviate the aging process to varying extents (Ma et al., 2019). The carboxyl groups substituted at C-5 position of the main chain could activate the chelating effects, hence, reinforcing the antioxidant capabilities of sugars (He et al., 2012). Thus, the performance of sanghuang polysaccharide is not a behavior of a single factor but a multifaceted function of two or several factors (Benzie, 2000; Wang et al., 2013; Wang J. Q. et al., 2016; Huang et al., 2017). Furthermore, chemical modification, including sulfation, carboxymethylation, acetylation, phosphorylation, benzoylation, quaternization, and periodate oxidation, can influence the antioxidant activities of polysaccharides as well, attributed to the weaker dissociation energy of hydrogen bonds by introducing the substituted groups into their structures, and the decrease in anomeric carbon (Bedini et al., 2017; Shah et al., 2018; de Almeida and da Silva, 2021).
Until now, rapid increases in morbidity of malignant tumors with high mortality have become the great challenges for global public health, characterized by the uncontrollable division and proliferation of abnormal cells (Bode and Dong, 2009; Klein et al., 2021). Even though conspicuous progress has been achieved on the treatment of carcinomas by traditional methods, including surgical interventions, radiotherapy, chemotherapy, bone marrow transplant, immunotherapy, and hormone therapy, chemotherapeutical agents endure severe side effects, such as targeting non-specificity and heavily opportunistic resistance to drugs (Schirrmacher, 2019). Hence, the quest of studying and developing new potential anti-tumor candidates, particularly derived from natural resources, is an eager demand, which not only diagnose and cure the human malignancies and directly induce the tumor cells apoptosis, but can also be functionalized as the immunomodulators to suffer from adverse stress and strengthen body's immune defense against the development of cancer cells (Moradali et al., 2007; de Silva et al., 2012; Kothari et al., 2018; Sindhu et al., 2021). Mushroom polysaccharides were first reported for anti-tumor properties by Byerrum et al. (1957). Ikekawa et al. (1968) declared that P. linteus showed an inhibitory rate of 96.7% against S180. Ever since, numerous sanghuang polysaccharides have been prepared, and in vivo animal and in vitro research have shown them to display striking anti-tumor and immunostimulant activities across a wide range of cancer cell lines, such as S180 (Chen et al., 2011; Mei et al., 2015), B16 (Kim G. Y. et al., 2006), H22 (Gao et al., 2017), HepG2 (Cheng et al., 2020a), Bel7402 (Pei et al., 2015), A549 (Ying et al., 2017), SGC7901 (Liu et al., 2016), HT29 (Yuan et al., 2018), SW480 (Li S. C. et al., 2015), HCT8 (Pei et al., 2015), MCF7 (Wan et al., 2020), Hela (Ying et al., 2019), etc.
A 250-kDa P. linteus polysaccharide, consisted primarily of repeating α-D-Glc(1 → 4)-α-D-Glc(1 → 6) units, was validated to harbor stronger anti-proliferative effect against S180 through lowering the expression level of anti-apoptotic Bcl-2 protein and increasing the level of pro-apoptotic protein Bax, which, in turn, facilitated the release of cytochrome c from mitochondrial intermembrane compartment into cytosol, thus, causing apoptotic cell death (Mei et al., 2015). Similar results were obtained by Li et al. (2004) and Wang G. B. et al. (2012), which also implied that the sanghuang polysaccharide-induced apoptosis was interrelated with a decrease in Bcl-2 level and an increase in the release of cytochrome c. Zhong et al. (2013) courted an insight into the action mechanism of P. linteus polysaccharide triggered-HT29 apoptosis, namely, remarkably downregulating the expression of cyclin D1, cyclin E, and CDK2, as well as increasing the expression of p27Kip, thus leading to the S-phase cell cycle arrest in HT29. Wan et al. (2020) extracted and characterized a water-soluble S. vaninii polysaccharide, which was qualified for effectively inhibiting the proliferation of MCF7, stopping the cell cycle at G2 phase, accelerating apoptosis, and reducing the migratory and invasive powers, through driving the activation of p53-related genes and downregulating the expression of matrix metalloproteinase (MMP). A sulfated polysaccharide constructed by sulfation of a glucan from the fruiting bodies of P. ribi, using chlorosulfonic acid protocol, exerted anti-cancer activity, which is the anti-angiogenic activity by intervention of proliferation, migration, and formation of vascular endothelial growth factor (VEGF) in EA.hy926, with the mechanism as downregulating the protein expression of VEGF and VEGF receptor-1 and restricting the phosphorylation of VEGF receptor-2, protein kinase B, and extracellular signal-regulated kinase (Liu et al., 2018). Xue et al. (2011) obtained a P. baumii polysaccharide and exposed it to the RAW264.7 macrophages, accompanied by noticeable increases in the cellular proliferative rate, NO production, and expression levels of IL-1β, IL-18, IL-6, IL-12p35, and IL-12p40 genes. MTT assay and cell cycle analysis suggested that this polysaccharide could markedly suppress the proliferation of HepG2 in a dosage-dependent manner by inducing the cell cycle arrest at S phase, incurring apoptosis. As displayed by the study of Liu et al. (2016), a homogeneous polysaccharide purified from the fruiting bodies of P. baumii had an antiproliferative effect on Hela and SGC7901 by mediating the block of cell division in G0/G1 and S phases, improvement of immune cell phagocytic activity, and secretion of TNF-α and IL-6. Two polysaccharides, isolated by Li et al. (2008) from the P. nigricans mycelia through submerged fermentation and culture medium, both owned the anti-tumor activties against S180 in vivo. Further experiments certified that no direct cytotoxic activity against S180 was monitored, but apparent increases in lymphocytes proliferation and production of NO and TNF-α in microphages were observed, indicating that anti-cancer effects of the two carbohydrates are not directly tumoricidal but rather immunostimulating. Tremendous evidence have authenticated that the possible anti-tumor mechanisms of sanghuang polysaccharides are involved in the direct cancer inhibition activities, including cell-cycle arrest, induction of apoptosis, anti-angiogenesis, and inhibition of metastasis, including proliferation, invasion, migration, and adhesion (Zhang et al., 2007; Khan et al., 2019; Hyder and Dutta, 2021). More importantly, sanghuang polysaccharides can assist a host to boost the immunomodulatory activities to fight against cancer cells, so-called as biological response modifiers. They rely on either the direct activation of various immune cells like macrophages, T-lymphocytes, B-lymphocytes, natural killer cells, and dendritic cells, or potentiation of the release of various cell signal messengers, inflammatory mediators, and cytokines, such as IL-2, IL-4, IL-10, IL-1β, IFN-γ, IFN-β, TNF-α, cyclooxygenase-2, and NO (Wasser, 2002; Zhu et al., 2007; Lin et al., 2016; Singdevsachan et al., 2016; Chakraborty et al., 2019; Yin et al., 2021).
It is well-documented that the anti-cancer activities of sanghuang polysaccharides are notably decided by their refined structures in all hierarchies, such as Mws, chemical compositions, configurations of glycosidic linkages, backbones and branched chains, types of substituent groups, 3D conformation, etc. (Zhang et al., 2007; Meng et al., 2016; Yan et al., 2017). Only by scrutinizing their structures may explain their properties and offer the valuable direction for purpose screening, modification, and synthesis. In general, the larger the Mws and the better water solubility of sanghuang polysaccharides, the stronger the anti-tumor activities (Li S. C. et al., 2015; Pei et al., 2015). Kim et al. (2003) got an acidic proteo-heteroglycan mixed both with α- and β-linkages, substituted with linear D-(1,3), branched D-(1,6) and terminal D-residues at C-6 position, and illuminated that the structural features like 3D conformation, branching ratio, and molecular complexity are quite crucial for the anti-tumor activity by the rapid proliferation of spleen cells and stimulation of humoral host defense and macrophage function. Moreover, P. vaninii polysaccharide, a β-glucan with good water solubility and relatively high chain stiffness, pose beneficial to promotion of the anti-tumor capacities by having more chances to bind to cell membrane and higher cytotoxicity to cancer cells (Jia et al., 2017).
Inflammation is a comprehensive, natural self-protective response of organism mediated by the actions of cells in innate immune system, to address the injury or damage caused by mechanical, chemical, or microbial stimuli, such as infections, irritants, ultraviolet light irradiation, pathogens, and allergens. Among these cells, macrophages play a pivotal role during inflammation, including the surplus of inflammatory mediators, like NO by inducible nitric oxide synthase, prostaglandin E2 by cyclooxygenase-2, and ROS, as well as the increase in expression of some pro-inflammatory cytokines, such as TNF-α, IFN-γ, IL-6, and IL-1β, which are extremely linked to a wide variety of diseases (Shi, 2016; Nie et al., 2017; Du et al., 2018; Yang P. et al., 2019; Hou et al., 2020). A large body of studies have discovered that sanghuang polysaccharides own the desired anti-inflammatory and anti-nociceptive activities. A selenium-enriched polysaccharide from the P. igniarius mycelia could act a positive influence on skin repairing in mice through lowering the expression of IL-6, TNF-α, and VEGF over cascade (Luo L. J. et al., 2021). Ma et al. (2019) disclosed that a polysaccharide from the S. sanghuang broth reinforced the immune organ efficacy and mitigated the histopathological hepatic lesions and apoptosis in hepatocytes of the D-Gal-aged mice in a dose-dependent behavior. Sanghuang-derived polysaccharides are capable of attenuating inflammation and pushing tissue healing by downregulating the phosphorylation level of STAT-1 and the expression level of STAT-1 targeted genes, such as inducible nitric oxide synthase, TNF-α, IL-1β, IL-2, IL-6, and IL-12 in an LPS-induced macrophage RAW264.7 model (Xie et al., 2019; Sun et al., 2021; Zuo et al., 2021). Their anti-inflammatory and anti-nociceptive effects originate from blocking and inhibiting the activation of MAPK and peroxisome proliferator-activated receptor (PPAR) signaling pathways (Hu et al., 2018; Yin et al., 2021).
Unlike other types of sugars, polysaccharides extracted from the mushrooms sanghuang do not raise the concentration of glucose in blood but possess the robust anti-diabetic activities, primarily by inhibiting the β-cells apoptosis in pancreatic islets, affecting the activities of glucose-metabolizing enzymes, such as α-amylase and α-glucosidase, and enhancing the glucose disposal, synthesis of hepatic glycogen, and insulin sensitivity, as well as activating the expression of adenosine monophosphate-activated protein kinase and PPAR-γ modulators (Wu et al., 2016; Azeem et al., 2018; Ajith and Janardhanan, 2021). One study confirmed that the treatment with a polysaccharide from the P. linteus mycelia could suppress the development of autoimmune diabetes in NOD mice not only through delaying the transfer of diabetes by spleen cells from NOD mice into NOD/SCID mice but also through adjusting the expression of pro-inflammatory cytokines, including IFN-γ, IL-2, IL-4, and TNF-α by Th1 and Th2 cells (Kim et al., 2010). Oral administration with a water-soluble crude polysaccharide from the P. linteus mycelia at 100 mg/kg body weight/d could distinctively cut down the blood glucose level by 35.60% in the alloxan-revulsive diabetic mice (Zhao et al., 2014). Another report warranted that a neutral polysaccharide from the S. sanghuang mycelia owned the potential inhibitory activities against α-amylase and α-glucosidase and had the hypoglycemic impacts on in vitro insulin resistance of HepG2, implying that this polysaccharide may be suitable as the functional anti-diabetic drugs for therapeutic cure (Cheng et al., 2020b). In-depth study suggested that many various molecules, such as protein kinase B, insulin receptor substrate-2, and fork head transcription factor-1, have engaged in regulating the intracellular procedure of insulin signals inside cells (Wang P. C. et al., 2016; Wu et al., 2016; Feng et al., 2018).
Abundant investigations have proven that sanghuang polysaccharides can be the hepatoprotective agents by virtue of enhancing liver metabolism, reducing the levels of lipid peroxidation and expression of inflammatory mediators, restraining oxidative stress and histopathological fibrogenesis, inducing apoptosis and cycle arrest in hepatic stellate cells, as well as improving the activities of antioxidative enzymes (Shan et al., 2019; Yuan et al., 2019; Qu et al., 2020). Liu et al. (2019b) verified that the extracellular I. hispidus exopolysaccharide from fermentation broth performed the liver protective ability on acute alcoholic liver injury in mice with the help of extending the duration of righting reflex, shortening the duration of recovery, and decreasing the liver index and levels of ALT, AST, and MDA, as well as increasing the levels of alcohol dehydrogenase, CAT, and SOD. Deeper research reported that this polysaccharide was able to activate the nuclear factor E2-related factor 2 signaling pathway, and increase the mRNA expression levels of downstream-related antioxidant enzymes, such as CAT, Cu-Zn SOD, and NQO1. Compared with the untreated groups, the P. linteus polysaccharide treatment harbored a notable reduction in liver fibrosis in a thioacetamide-induced liver damage mice model via regulation of the oxidative stress pathways, heat shock pathways, and metabolic pathways, referring to a total of 13 differentially expressed proteins, including actin, tubulin α-1C chain, preprohaptoglobin, hemopexin, galectin-5, glutathione S-transferase α4, branched chain keto acid dehydrogenase heterotetrameric E1 subunit-α, glutathione S-transferase κ, glyceraldehyde-3-phosphate dehydrogenase, thiosulfate sulfurtransferase, betaine-homocysteine S-methyltransferase 1, quinoid dihydropteridine reductase, and ribonuclease UK114 (Wang H. L. et al., 2012). Chen C. et al. (2020) evaluated the hepatoprotective effect of an isolated P. linteus polysaccharide against the acetaminophen-stimulated liver injury in mice, which was attributed to its antioxidant functions, by decreasing cytochrome P450 2E1 expression and hepatic release of cytokines, improving the levels of phase II enzymes, exhibiting hepatic-repairing performance, and accelerating metabolism of acetaminophen.
Sanghuang polysaccharides are known to show the potential of anti-bacterial, anti-fungal, and anti-viral activities, whose assessment is a valuable concept for the rational design to targeted drugs for infectious diseases (Bach et al., 2019; Liu Z. H. et al., 2020; Guo Y. X. et al., 2021; Wang Z. C. et al., 2021). Reis et al. (2014) tested the anti-microbial effects of a polysaccharide extracted from P. linteus against an array of Gram-positive and Gram-negative bacteria and filamentous fungi. It was observed that the P. linteus polysaccharide powerfully inhibited the proliferation of Staphylococcus aureus, Bacillus cereus, Micrococcus flavus, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli, Enterobacter cloacae, Aspergillus fumigatus, A. versicolor, A. ochraceus, A. niger, Trichoderma viride, Penicillium funiculosum, Pe. ochrochloron, and Pe. verrucosum. Two polysaccharides purified from P. pini exerted the anti-viral activities against coxsackie virus B3, a non-enveloped virus, and herpes simplex virus-1, an enveloped virus, as well as possibly against influenza viruses, together with the decreases in neuraminidase activities in a concentration-dependent manner, revealing that these polysaccharides can be applied for antivirals after herpesvirus infection (Lee S. M. et al., 2010). More noteworthy, the abilities of polysaccharides derived from natural resources, especially mushrooms sanghuang, to modulate the immune response and cyto-protect pulmonary, make them the latent candidates for combination therapy in severe cases of COVID-19 (Chen R. R. et al., 2020; Chen X. Y. et al., 2020; Barbosa and de Carvalho Junior, 2021).
Except for the biological activities mentioned above, sanghuang polysaccharides have also possessed other efficacies, principally including anti-fatigue activity, neuroprotective activity, prebiotic activity, etc., which are as followed. Zhong (2020) affirmed that a polysaccharide from the fermentation broth of P. igniarius had the conspicuous anti-fatigue impacts on sports fatigue. Liu Y. H. et al. (2015) and Yang P. et al. (2019) both found the neurotrophic activities of the polysaccharides isolated from the fruiting bodies of P. ribis through facilitating the neurite outgrowth of nerve growth of the factor-stimulated PC12 and inhibiting cell apoptosis, meaning they are the promising choices for treatment of neurodegenerative diseases, such as Alzheimer's. The mechanism was associated with the attenuation of PC12 death by increasing the MMP level and decreases in the protein expression of cytochrome c. Many investigations have validated that intake of sanghuang polysaccharides with lower digestibility in gastric acidity could be helpful for the use of probiotics, increases in the number of probiotics, amelioration of gut microbiota, and treatment of gastrointestinal diseases (Liu L. Q. et al., 2019; Niu et al., 2021; Song et al., 2021).
Additionally, sanghuang polysaccharides have received more and more attention from the therapeutic effects against cerebral ischemia, where the mechanism includes resisting oxidative stress, relieving inflammation and deactivation of the related signaling pathways and pro-inflammatory cytokines, inhibiting neurotoxicity, provoking the expression of anti-apoptotic proteins, holding mitochondrial homeostasis, and motivating cerebral angiogenesis (Meng et al., 2021; Yuan et al., 2021).
Notably, the versatile functions of sanghuang polysaccharides primarily reply on their antioxidant activities. The reason is that by upregulating the antioxidant capacities of quenching free radicals and/or accelerating antioxidant enzyme activities, sanghuang polysaccharides can withstand the oxidative stress, aging, carcinoma, inflammation, diabetes, bacteria, virus, fatigue, liver damage and neurodegenerative, gastrointestinal, cardiovascular, and cerebral diseases, as well as enhance the immunostimulating abilities (Benzie, 2000; Wang J. Q. et al., 2016; Huang et al., 2017).
Sanghuang, distinguished as one of the most popular medicinal mushrooms in traditional Chinese medicine, has fascinated humans for centuries since it can resist and remedy dysentery, metrorrhagia, amenorrhea, aging, poisoning, digestion, allergy, and cancer. Polysaccharides are among the major ingredients of sanghuang responsible for multiple bioactivities, including antioxidant and anti-aging, anti-tumor and immunomodulatory, anti-inflammatory and anti-nociceptive, anti-diabetic, anti-microbial, anti-fatigue, hepatoprotective, and neuroprotective activities, which have earned huge interest and attention as a potential source of various therapeutic drugs, vaccines, and functional foods.
In this historical and scientific background, this review provides a systematic and thorough description of the evolution of species and their names, preparation, structural characterization, and related bioactivities of sanghuang polysaccharides, and builds upon a valuable and profound guidance for the development and utilization of natural resources-based nutraceuticals, pharmaceuticals, and cosmeceuticals. An ever-increasing number of bioactive polysaccharides have been isolated from the fruiting bodies, cultured mycelia, and fermentation broth of sanghuang species. The structural features of these carbohydrates, including Mws, sugar compositions, configurations and patterns of isomers, backbone structures, types and numbers of glycosidic linkages, positions and degrees of branching, as well as 3D conformation, have been deciphered. Although the significant progress has been garnered focusing on the sanghuang polysaccharides, there remain some issues required to be desperately addressed.
First, the taxonomy on synonym and homonym of the species names in sanghuang often confuses their utilization, thus, anxiously desiderating unification and normalization of these mushrooms. As well-known complex macromolecules, polysaccharides from different sources of raw strains, and preparation strategies can result in the differentiation of chemical properties, structural characteristics, and bioactivities due to the physical, chemical, and/or biochemical mechanisms, so hunting for new sanghuang resources and artificial cultivation contributes to their healthy utilization. Current extraction, isolation, and purification protocols largely confine to the analytical or preparative purposes in laboratory scales, and they may not be reasonable for each kind of polysaccharide. New, feasible, economic, and efficient strategies should be developed, and a related database for the optimized cultivation and preparation of polysaccharides derived from each sanghuang species should be established for standardization, industrialization, and commercialization. More attempts regarding the genetic and metabolic engineering should be conducted to harvest the higher yield of sanghuang polysaccharides, lower cost, shorten cycle, and reuse wastes.
The bioactivities of sanghuang polysaccharides are the results of a combination of various factors. Although many chemical structures and bioactivities of sanghuang polysaccharides and their derivatives have been elucidated, understanding of the relationships between structures and bioactivities, as well as the accurate impactful factors and cellular and molecular mechanisms of actions underlying their bioactivities still lack depth and precision. It is of imperative importance to construct the analytically high-resolution techniques and deploy the massive in vitro and in vivo experiments, as well as clinical trials, to further guarantee the consistency and reliability, which is vital to control the usage quality and structural characteristics of sanghuang polysaccharides. Another meaningful route is by means of modification, including physical (ultrasonication, microwave treatment, radiation exposure, steam explosion, hyperthermy, high pressure homogenization, and plasma copolymerization), chemical (sulfation, phosphorylation, carboxymethylation, selenization, methylation, acetylation, benzoylation, quaternization, and periodate oxidation), or biological (enzymatic degradation) treatment to clarify the structure-activity relationships; a steerable polysaccharide will be created and its properties can be predicted (Karaki et al., 2016; Xu et al., 2019).
Previous studies have validated that the administration of sanghuang extracts seems to be non-toxic or hypotoxic (Huo et al., 2020; Li I. C. et al., 2020). For efficient uses in nutraceuticals, pharmaceuticals, and cosmeceuticals, the assessment of safety, adverse effects, and efficacies of sanghuang polysaccharides is imminently necessitated in deciding the normative dosage and administration duration in the next steps, such as acute oral toxicity test, chromosome aberration test, bone marrow micronucleus test, and clinical test.
Discovery of other novel activities of sanghuang polysaccharides is a fresh frontier, which can be combined with other materials to improve their characteristics and be reflected in other physical properties, entitling them as the safe and effective additives. The combination of artificial materials, such as nanocomposites, can perfectly enhance the non-toxicity, surface area, tensile strength, biocompatibility, biodegradability, and environmental adaptation, imparting them to exhibit the ideal features, and be subjected to the packing biomaterials, emulsions, and immunostimulants for carrying and maintaining the functionalities of foods and drugs (Mohan et al., 2019; Zhang R. Y. et al., 2020; Chen C. W. et al., 2021; Luo M. C. et al., 2021; Mahendiran et al., 2021; Zhang et al., 2021). Also, a database with respect to the sources, structures, active sites, bioactivities, involved mechanisms, and structure-activity relationships of sanghuang polysaccharides should be set up to better design delivery systems, as well as accelerate scientific and rational protection, development, and application of the medicinal mushrooms sanghuang.
B-KC and JS conceived and designed manuscript. HW, J-XM, and MZ collated references. JS and HW wrote manuscript. All authors had reviewed this manuscript before submission.
This work was supported by the National Natural Science Foundation of China (Nos. U2003211 and 32070016), the Scientific and Technological Tackling Plan for the Key Fields of Xinjiang Production and Construction Corps (No. 2021AB004), and the Beijing Forestry University Outstanding Young Talent Cultivation Project (No. 2019JQ03016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.965934/full#supplementary-material
Aida, F. N. N. A., Shuhaimi, M., Yazid, M., and Maaruf, A. G. (2009). Mushroom as a potential source of prebiotics: a review. Trends Food Sci. Technol. 20, 567–575. doi: 10.1016/j.tifs.2009.07.007
Ajith, T. A., and Janardhanan, K. K. (2021). Antidiabetic properties of medicinal mushrooms with special reference to Phellinus species: a review. Nat. Prod. J. 11, 120–126. doi: 10.2174/2210315510666200124124540
Alturki, A. M. (2021). Rationally design of electrospun polysaccharides polymeric nanofiber webs by various tools for biomedical applications: a review. Int. J. Biol. Macromol. 184, 648–665. doi: 10.1016/j.ijbiomac.2021.06.021
Azeem, U., Dhingra, G. S., and Shri, R. (2018). Pharmacological potential of wood inhabiting fungi of genus Phellinus Quél.: an overview. J. Pharmacogn. Phytochem. 7, 1161–1171.
Bach, F., Zielinski, A. A. F., Helm, C. V., Maciel, G. M., Pedro, A. C., Stafussa, A. P., et al. (2019). Bio compounds of edible mushrooms: in vitro antioxidant and antimicrobial activities. LWT-Food Sci. Technol. 107, 214–220. doi: 10.1016/j.lwt.2019.03.017
Baker, J. R., Kim, J. S., and Park, S. Y. (2008). Composition and proposed structure of a water-soluble glycan from the Keumsa Sangwhang Mushroom (Phellinus linteus). Fitoterapia 79, 345–350. doi: 10.1016/j.fitote.2008.03.002
Bao, H. Y., Wang, C. Y., and Bau, T. (2013). Herbalogical textual research on “sanghuang”. Mycosystema 32, 70–78.
Barbosa, J. R., and de Carvalho Junior, R. N. (2020). Occurrence and possible roles of polysaccharides in fungi and their influence on the development of new technologies. Carbohyd. Polym. 246, 116613. doi: 10.1016/j.carbpol.2020.116613
Barbosa, J. R., and de Carvalho Junior, R. N. (2021). Polysaccharides obtained from natural edible sources and their role in modulating the immune system: biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 108, 223–235. doi: 10.1016/j.tifs.2020.12.026
Barja, G. (2004). Free radicals and aging. Trends Neurosci. 27, 595–600. doi: 10.1016/j.tins.2004.07.005
Bedini, E., Laezza, A., Parrilli, M., and Iadonisi, A. (2017). A review of chemical methods for the selective sulfation and desulfation of polysaccharides. Carbohyd. Polym. 174, 1224–1239. doi: 10.1016/j.carbpol.2017.07.017
Benzie, I. F. F. (2000). Evolution of antioxidant defence mechanisms. Eur. J. Nutr. 39, 53–61. doi: 10.1007/s003940070030
Bode, A. M., and Dong, Z. G. (2009). Cancer prevention research-then and now. Nat. Rev. Cancer 9, 508–516. doi: 10.1038/nrc2646
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Byerrum, R., Clarke, D., Lucas, E., Ringler, R., Stevens, J., and Stock, C. C. (1957). Tumor inhibitors in Boletus edulis and other Holobasidiomycetes. Antibiot. Chemother. 7, 1.
Cao, C. L., Peng, F., and Cui, B. K. (2013). Chemical characterization and structure of exopolysaccharides from submerged culture of new medicinal mushroom from China, Phellinus mori (higher basidiomycetes). Int. J. Med. Mushrooms 15, 57–69. doi: 10.1615/IntJMedMushr.v15.i1.70
Cao, C. Y., Wang, L. L., Wang, L. L., Gong, Y., Ai, C. Q., Wen, C. R., et al. (2019). A strategy to identify mixed polysaccharides through analyzing the monosaccharide composition of disaccharides released by graded acid hydrolysis. Carbohyd. Polym. 223, 115046. doi: 10.1016/j.carbpol.2019.115046
Chakraborty, I., Sen, I. K., Mondal, S., Rout, D., Bhanja, S. K., Maity, G. N., et al. (2019). Bioactive polysaccharides from natural sources: a review on the antitumor and immunomodulating activities. Biocatal. Agr. Biotechnol. 22, 101425. doi: 10.1016/j.bcab.2019.101425
Chang, C., Zhao, J. H., Yu, W. J., Chen, Q. H., Qin, L. W., Wu, X. L., et al. (2021). Extraction technology and determination of polysaccharide from Sanghuangporus vaninii. Chem. Reag. 43, 973–978. doi: 10.13822/j.cnki.hxsj.2021007987
Chen, C., Liu, X., Qi, S. S., Dias, A. C. P., Yan, J. K., and Zhang, X. Y. (2020). Hepatoprotective effect of Phellinus linteus mycelia polysaccharide (PL-N1) against acetaminophen-induced liver injury in mouse. Int. J. Biol. Macromol. 154, 1276–1284. doi: 10.1016/j.ijbiomac.2019.11.002
Chen, C. W., Wang, Y. T., Zhang, D. G., Wu, X. W., Zhao, Y., Shang, L. R., et al. (2021). Natural polysaccharide based complex drug delivery system from microfluidic electrospray for wound healing. Appl. Materialstoday 23, 101000. doi: 10.1016/j.apmt.2021.101000
Chen, H., Tian, T., Miao, H., and Zhao, Y. Y. (2016). Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: a review. Fitoterapia 113, 6–26. doi: 10.1016/j.fitote.2016.06.009
Chen, L., Pan, J. Z., Li, X., Zhou, Y., Meng, Q. L., and Wang, Q. (2011). Endo-polysaccharide of Phellinus igniarius exhibited anti-tumor effect through enhancement of cell mediated immunity. Int. Immunopharmacol. 11, 255–259. doi: 10.1016/j.intimp.2010.11.033
Chen, R. R., Li, Y. J., Chen, J. J., and Lu, C. L. (2020). A review for natural polysaccharides with anti-pulmonary fibrosis properties, which may benefit to patients infected by 2019-nCoV. Carbohyd. Polym. 247, 116740. doi: 10.1016/j.carbpol.2020.116740
Chen, W. H., Tan, H. Y., Liu, Q., Zheng, X. H., Zhang, H., Liu, Y. H., et al. (2019). A review: the bioactivities and pharmacological applications of Phellinus linteus. Molecules 24, 1888. doi: 10.3390/molecules24101888
Chen, X. H., Wang, Z. R., and Kan, J. Q. (2021). Polysaccharides from ginger stems and leaves: effects of dual and triple frequency ultrasound assisted extraction on structural characteristics and biological activities. Food Biosci. 42, 101166. doi: 10.1016/j.fbio.2021.101166
Chen, X. Y., Han, W. W., Wang, G. X., and Zhao, X. (2020). Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 164, 331–343. doi: 10.1016/j.ijbiomac.2020.07.106
Cheng, J. W., Song, J. L., Liu, Y., Lu, N., Wang, Y. B., Hu, C. J., et al. (2020a). Conformational properties and biological activities of α-D-mannan from Sanghuangporus sanghuang in liquid culture. Int. J. Biol. Macromol. 164, 3568–3579. doi: 10.1016/j.ijbiomac.2020.08.112
Cheng, J. W., Song, J. L., Wei, H. L., Wang, Y. B., Huang, X. B., Liu, Y., et al. (2020b). Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 164, 3305–3314. doi: 10.1016/j.ijbiomac.2020.08.202
Cheng, L. Z., Wang, Y. F., He, X. X., and Wei, X. L. (2018). Preparation, structural characterization and bioactivities of Se-containing polysaccharide: a review. Int. J. Biol. Macromol. 120, 82–92. doi: 10.1016/j.ijbiomac.2018.07.106
Cheng, W., Qin, J. Z., Du, J. G., and Zhang, C. H. (2012). Optimization of ultrasonic-assisted enzymatic extraction of polysaccharide from Phellinus igniarius. Mod. Food Sci. Technol. 28, 662–666. doi: 10.13982/j.mfst.1673-9078.2012.06.025
Cheong, K. L., Wu, D. T., Zhao, J., and Li, S. P. (2015). A rapid and accurate method for the quantitative estimation of natural polysaccharides and their fractions using high performance size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detector. J. Chromatogr. A 1400, 98–106. doi: 10.1016/j.chroma.2015.04.054
Chew, K. W., Ling, T. C., and Show, P. L. (2019). Recent developments and applications of three-phase partitioning for the recovery of proteins. Sep. Purif. Rev. 48, 52–64. doi: 10.1080/15422119.2018.1427596
Cui, R. B., and Zhu, F. (2021). Ultrasound modified polysaccharides: a review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 107, 491–508. doi: 10.1016/j.tifs.2020.11.018
Dai, Y. C., and Xu, M. Q. (1998). Studies on the medicinal polypore, Phellinus baumii, and its kin, P. linteus. Mycotaxon 67, 191–200.
de Almeida, W. S., and da Silva, D. A. (2021). Does polysaccharide quaternization improve biological activity? Int. J. Biol. Macromol. 182, 1419–1436. doi: 10.1016/j.ijbiomac.2021.05.012
de Silva, D. D., Rapior, S., Fons, F., Bahkali, A. H., and Hyde, K. D. (2012). Medicinal mushrooms in supportive cancer therapies: an approach to anti-cancer effects and putative mechanisms of action. Fungal Divers. 55, 1–35. doi: 10.1007/s13225-012-0151-3
Doost, A. S., Akbari, M., Stevens, C. V., Setiowati, A. D., and der Meeren, P. V. (2019). A review on nuclear overhauser enhancement (NOE) and rotating-frame overhauser effect (ROE) NMR techniques in food science: basic principles and applications. Trends Food Sci. Technol. 86, 16–24. doi: 10.1016/j.tifs.2019.02.001
Du, B., Zhu, F. M., and Xu, B. J. (2018). An insight into the anti-inflammatory properties of edible and medicinal mushrooms. J. Funct. Foods 47, 334–342. doi: 10.1016/j.jff.2018.06.003
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356.
Ebringerová, A., and Hromádková, Z. (2010). An overview on the application of ultrasound in extraction, separation and purification of plant polysaccharides. Cent. Eur. J. Chem. 8, 243–257. doi: 10.2478/s11532-010-0006-2
El Enshasy, H. A., and Hatti-Kaul, R. (2013). Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol. 31, 668–677. doi: 10.1016/j.tibtech.2013.09.003
Espinosa-Diez, C., Miguel, V., Mennerich, D., Kietzmann, T., Sánchez-Pérez, P., Cadenas, S., et al. (2015). Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 6, 183–197. doi: 10.1016/j.redox.2015.07.008
Fabijanić, I., CavuŽić, D., and Zubak, A. M. (2019). Meningococcal polysaccharides identification by NIR spectroscopy and chemometrics. Carbohyd. Polym. 216, 36–44. doi: 10.1016/j.carbpol.2019.03.102
Falch, B. H., Espevik, T., Ryan, L., and Stokke, B. T. (2000). The cytokine stimulating activity of (1 → 3)-β-D-glucans is dependent on the triple helix conformation. Carbohyd. Res. 329, 587–596. doi: 10.1016/S0008-6215(00)00222-6
Fang, J. N., and Ding, K. (2007). Bioactivities, isolation and purification methods of polysaccharide. Chin. J. Nat. Med. 5, 338–347.
Feng, H., Zhang, S. J., Wan, J. M. F., Gui, L. F., Ruan, M. C., Li, N., et al. (2018). Polysaccharides extracted from Phellinus linteus ameliorate high-fat high-fructose diet induced insulin resistance in mice. Carbohyd. Polym. 200, 144–153. doi: 10.1016/j.carbpol.2018.07.086
Ferreira, S. S., Passos, C. P., Madureira, P., Vilanova, M., and Coimbra, M. A. (2015). Structure-function relationships of immunostimulatory polysaccharides: a review. Carbohyd. Polym. 132, 378–396. doi: 10.1016/j.carbpol.2015.05.079
Forman, H. J., and Zhang, H. Q. (2021). Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 20, 689–709. doi: 10.1038/s41573-021-00233-1
Gao, W. W., Wang, W. D., Sun, W. J., Wang, M. F., Zhang, N., and Yu, S. W. (2017). Antitumor and immunomodulating activities of six Phellinus igniarius polysaccharides of different origins. Exp. Ther. Med. 14, 4627–4632. doi: 10.3892/etm.2017.5191
Ge, Q., Zhang, A. Q., and Sun, P. L. (2009). Structural investigation of a novel water-soluble heteropolysaccharide from the fruiting bodies of Phellinus baumii Pilát. Food Chem. 114, 391–395. doi: 10.1016/j.foodchem.2008.09.010
Gong, P., Wang, S. Y., Liu, M., Chen, F. X., Yang, W. J., Chang, X. N., et al. (2020). Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: a mini-review. Carbohyd. Res. 494, 108037. doi: 10.1016/j.carres.2020.108037
Guo, X., Zou, X., and Sun, M. (2010). Optimization of extraction process by response surface methodology and preliminary characterization of polysaccharides from Phellinus igniarius. Carbohyd. Polym. 80, 344–349. doi: 10.1016/j.carbpol.2009.11.028
Guo, X. Y., Kang, J., Xu, Z. Y., Guo, Q. B., Zhang, L. F., Ning, H. F., et al. (2021). Triple-helix polysaccharides: formation mechanisms and analytical methods. Carbohyd. Polym. 262, 117962. doi: 10.1016/j.carbpol.2021.117962
Guo, Y. X., Chen, X. F., and Gong, P. (2021). Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 183, 1753–1773. doi: 10.1016/j.ijbiomac.2021.05.139
He, P. X., Geng, L. J., Wang, J. Z., Wang, Z., Mao, D. B., and Xu, C. P. (2012). Purification, characterization and bioactivity of an extracellular polysaccharide produced from Phellinus igniarius. Ann. Microbiol. 62, 1697–1707. doi: 10.1007/s13213-012-0427-6
He, P. Y., Hou, Y. H., Yang, Y., and Li, N. (2021a). The anticancer effect of extract of medicinal mushroom Sanghuangprous vaninii against human cervical cancer cell via endoplasmic reticulum stress-mitochondrial apoptotic pathway. J. Ethnopharmacol. 279, 114345. doi: 10.1016/j.jep.2021.114345
He, P. Y., Zhang, Y., and Li, N. (2021b). The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: a review. Food Funct. 12, 1856–1881. doi: 10.1039/D0FO02342F
Hou, C. Y., Chen, L. L., Yang, L. Z., and Ji, X. L. (2020). An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 153, 248–255. doi: 10.1016/j.ijbiomac.2020.02.315
Hsieh, P. W., Wu, J. B., and Wu, Y. C. (2013). Chemistry and biology of Phellinus linteus. BioMedicine 3, 106–113. doi: 10.1016/j.biomed.2013.01.002
Hu, T., Lin, Q. L., Guo, T., Yang, T., Zhou, W. H., Deng, X. F., et al. (2018). Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohyd. Polym. 200, 487–497. doi: 10.1016/j.carbpol.2018.08.021
Huang, G. L., Chen, F., Yang, W. J., and Huang, H. L. (2021). Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 109, 564–568. doi: 10.1016/j.tifs.2021.01.038
Huang, G. L., Mei, X. Y., and Hu, J. C. (2017). The antioxidant activities of natural polysaccharides. Curr. Drug Targets 18, 1296–1300. doi: 10.2174/1389450118666170123145357
Huang, H. Y., Chieh, S. Y., Tso, T. K., Chien, T. Y., Lin, H. T., and Tsai, Y. C. (2011). Orally administered mycelial culture of Phellinus linteus exhibits antitumor effects in hepatoma cell-bearing mice. J. Ethnopharmacol. 133, 460–466. doi: 10.1016/j.jep.2010.10.015
Huang, S. Q., Ding, S. D., and Fan, L. P. (2012). Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 50, 1183–1187. doi: 10.1016/j.ijbiomac.2012.03.019
Huo, J. X., Sun, Y. Q., Zhong, S., Li, Y. G., Yang, R. C., Xia, L. J., et al. (2020). Safety evaluation of aqueous extracts of Sanghuangporus vaninii fruiting body in Sprague-Dawley rats. Food Sci. Nutr. 8, 5107–5113. doi: 10.1002/fsn3.1811
Hwang, B. S., Lee, I. K., Choi, H. J., and Yun, B. S. (2015). Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 25, 3256–3260. doi: 10.1016/j.bmcl.2015.05.081
Hwang, H. J., Kim, S. W., Lim, J. M., Joo, J. H., Kim, H. O., Kim, H. M., et al. (2005). Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sci. 76, 3069–3080. doi: 10.1016/j.lfs.2004.12.019
Hyder, M. S., and Dutta, S. D. (2021). Mushroom-derived polysaccharides as antitumor and anticancer agent: a concise review. Biocatal. Agr. Biotechnol. 35, 102085. doi: 10.1016/j.bcab.2021.102085
Ikekawa, T., Nakanishi, M., Uehara, N., Chihara, G., and Fukuoka, F. (1968). Antitumor action of some basidiomycetes, especially Phellinus linteus. Jpn. J. Cancer Res. 59, 155–157.
Jia, X. W., Gao, M. Q., Li, M. Z., Wu, Y., Zeng, Y., and Xu, C. P. (2017). Molecular characterization of two polysaccharides from Phellinus vaninii Ljup and their cytotoxicity to cancer cell lines. Anti-Cancer Age Med. Chem. 17, 1–8. doi: 10.2174/1871520617666170912144956
Jiang, P., Yuan, L., Cai, D. L., Jiao, L. L., and Zhang, L. P. (2015). Characterization and antioxidant activities of the polysaccharides from mycelium of Phellinus pini and culture medium. Carbohyd. Polym. 117, 600–604. doi: 10.1016/j.carbpol.2014.10.013
Jiang, P., Yuan, L., Huang, G. H., Wang, X. L., Li, X., Jiao, L. L., et al. (2016). Structural properties and immunoenhancement of an exopolysaccharide produced by Phellinus pini. Int. J. Biol. Macromol. 93, 566–571. doi: 10.1016/j.ijbiomac.2016.09.020
Jin, Q. L., Zhang, Z. F., Lv, G. Y., Cai, W. M., Cheng, J. W., Wang, J. G., et al. (2016). Antioxidant and DNA damage protecting potentials of polysaccharide extracted from Phellinus baumii using a delignification method. Carbohyd. Polym. 152, 575–582. doi: 10.1016/j.carbpol.2016.07.027
Jung, G. H., and Kang, J. H. (2020). Efficacy of Phellinus linteus (sanghuang) extract for improving immune functions. Medicine 99, 3. doi: 10.1097/MD.0000000000018829
Kamruzzaman, S. M., Endale, M., Oh, W. J., Park, S. C., Kim, T. H., Lee, I. K., et al. (2011). Antiplatelet activity of Phellinus baumii methanol extract is mediated by cyclic AMP elevation and inhibition of collagen-activated integrin-αIIbβ3 and MAP kinase. Phytother. Res. 25, 1596–1603. doi: 10.1002/ptr.3450
Kang, Q. Z., Chen, S. S., Li, S. F., Wang, B., Liu, X., Hao, L. M., et al. (2019). Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 124, 1137–1144. doi: 10.1016/j.ijbiomac.2018.11.215
Karaki, N., Aljawish, A., Humeau, C., Muniglia, L., and Jasniewski, J. (2016). Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: a review. Enzyme Microb. Technol. 90, 1–18. doi: 10.1016/j.enzmictec.2016.04.004
Khan, T., Date, A., Chawda, H., and Patel, K. (2019). Polysaccharides as potential anticancer agents: a review of their progress. Carbohyd. Polym. 210, 412–428. doi: 10.1016/j.carbpol.2019.01.064
Khursheed, R., Singh, S. K., Wadhwa, S., Gulati, M., and Awasthi, A. (2020). Therapeutic potential of mushrooms in diabetes mellitus: role of polysaccharides. Int. J. Biol. Macromol. 164, 1194–1205. doi: 10.1016/j.ijbiomac.2020.07.145
Kim, B. C., Choi, J. W., Hong, H. Y., Lee, S. A., Hong, S., Park, E. H., et al. (2006). Heme oxygenase-1 mediates the anti-inflammatory effect of mushroom Phellinus linteus in LPS-stimulated RAW264.7 macrophages. J. Ethnopharmacol. 106, 364–371. doi: 10.1016/j.jep.2006.01.009
Kim, G. Y., Lee, J. Y., Lee, J. O., Ryu, C. H., Choi, B. T., Jeong, Y. K., et al. (2006). Partial characterization and immunostimulatory effect of a novel polysaccharide-protein complex extracted from Phellinus linteus. Biosci. Biotechnol. Biochem. 70, 1218–1226. doi: 10.1271/bbb.70.1218
Kim, G. Y., Park, H. S., Nam, B. H., Lee, S. J., and Lee, J. D. (2003). Purification and characterization of acidic proteo-heteroglycan from the fruiting body of Phellinus linteus (Berk. and M.A. Curtis) Teng. Bioresour. Technol. 89, 81–87. doi: 10.1016/S0960-8524(02)00273-0
Kim, H. M., Kang, J. S., Kim, J. Y., Park, S. K., Kim, H. S., Lee, Y. J., et al. (2010). Evaluation of antidiabetic activity of polysaccharide isolated from Phellinus linteus in non-obese diabetic mouse. Int. Immunopharmacol. 10, 72–78. doi: 10.1016/j.intimp.2009.09.024
Klein, W. M. P., O'Connell, M. E., Bloch, M. H., Czajkowski, S. M., Green, P. A., Han, P. K. J., et al. (2021). Behavioral research in cancer prevention and control: emerging challenges and opportunities. JNCI J. Natl. Cancer Inst. 114, djab139. doi: 10.1093/jnci/djab139
Kothari, D., Patel, S., and Kim, S. K. (2018). Anticancer and other therapeutic relevance of mushroom polysaccharides: a holistic appraisal. Biomed. Pharmacother. 105, 377–394. doi: 10.1016/j.biopha.2018.05.138
Lee, S. H., Oh, I. S., Kim, Y. I., Jun, S. C., So, S. S., and Kim, H. G. (2007). Phellinus extracts inhibit migration and matrix metalloproteinase secretion in porcine coronary artery endothelial cells. Biotechnol. Bioproc. Eng. 12, 100–105. doi: 10.1007/BF03028633
Lee, S. M., Kim, S. M., Lee, Y. H., Kim, W. J., Park, J. K., Park, Y. I., et al. (2010). Macromolecules isolated from Phellinus pini fruiting body: chemical characterization and antiviral activity. Macromol. Res. 18, 602–609. doi: 10.1007/s13233-010-0615-9
Lee, Y. S., Kim, Y. H., Shin, E. K., Kim, D. H., Lim, S. S., Lee, J. Y., et al. (2010). Anti-angiogenic activity of methanol extract of Phellinus linteus and its fractions. J. Ethnopharmacol. 131, 56–62. doi: 10.1016/j.jep.2010.05.064
Leong, Y. K., Yang, F. C., and Chang, J. S. (2021). Extraction of polysaccharides from edible mushrooms: emerging technologies and recent advances. Carbohyd. Polym. 251, 117006. doi: 10.1016/j.carbpol.2020.117006
Leung, M. Y. K., Liu, C., Koon, J. C. M., and Fung, K. P. (2006). Polysaccharide biological response modifiers. Immunol. Lett. 105, 101–114. doi: 10.1016/j.imlet.2006.01.009
Li, C. P., Duan, S. S., Li, Y. P., Pan, X., and Han, L. R. (2021). Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: a review. Carbohyd. Polym. 261, 117876. doi: 10.1016/j.carbpol.2021.117876
Li, G., Kim, D. H., Kim, T. D., Park, B. J., Park, H. D., Park, J. I., et al. (2004). Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 216, 175–181. doi: 10.1016/j.canlet.2004.07.014
Li, I. C., Chen, C. C., Sheu, S. J., Huang, I. H., and Chen, C. C. (2020). Optimized production and safety evaluation of hispidin-enriched Sanghuangporus sanghuang mycelia. Food Sci. Nutr. 8, 1864–1873. doi: 10.1002/fsn3.1469
Li, N. Y., Wang, C. F., Georgiev, M. I., Bajpai, V. K., Tundis, R., Simal-Gandara, J., et al. (2021). Advances in dietary polysaccharides as anticancer agents: structure-activity relationship. Trends Food Sci. Technol. 111, 360–377. doi: 10.1016/j.tifs.2021.03.008
Li, R. X., Wang, Y. T., Xia, J. F., Luo, D. Q., and Wang, T. C. (2019). Optimization of extraction process of polysaccharide of Phellinus igniarius mycelium and analysis of its antioxidant activity in vitro. Chin. Agr. Sci. Bull. 35, 143–150.
Li, S. C., Yang, X. M., Ma, H. L., Yan, J. K., and Guo, D. Z. (2015). Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohyd. Polym. 133, 24–30. doi: 10.1016/j.carbpol.2015.07.013
Li, T. T., Yang, Y., Liu, Y. F., Zhou, S., Yan, M. Q., Wu, D., et al. (2015). Physicochemical characteristics and biological activities of polysaccharide fractions from Phellinus baumii cultured with different methods. Int. J. Biol. Macromol. 81, 1082–1088. doi: 10.1016/j.ijbiomac.2015.09.001
Li, X., Jiao, L. L., Zhang, X., Tian, W. M., Chen, S., and Zhang, L. P. (2008). Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int. Immunopharmacol. 8, 909–915. doi: 10.1016/j.intimp.2008.02.008
Li, Y. T., Guo, X., Tian, Y. H., Zhang, T. L., Luo, Z. W., Liu, X. J., et al. (2020). Methylation in combination with temperature programming enables rapid identification of polysaccharides by ambient micro-fabrication glow discharge plasma (MFGDP) desorption ionization mass spectrometry. Talanta 218, 121156. doi: 10.1016/j.talanta.2020.121156
Lin, C. J., Lien, H. M., Lin, H. J., Huang, C. L., Kao, M. C., Chen, Y. A., et al. (2016). Modulation of T cell response by Phellinus linteus. J. Biosci. Bioeng. 121, 84–88. doi: 10.1016/j.jbiosc.2015.05.008
Lin, W. C., Deng, J. S., Huang, S. S., Wu, S. H., Lin, H. Y., and Huang, G. J. (2017). Evaluation of antioxidant, anti-inflammatory and anti-proliferative activities of ethanol extracts from different varieties of Sanghuang species. RSC Adv. 7, 7780–7788. doi: 10.1039/C6RA27198G
Liu, D., Tang, W., Yin, J. Y., Nie, S. P., and Xie, M. Y. (2021). Monosaccharide composition analysis of polysaccharides from natural sources: hydrolysis condition and detection method development. Food Hydrocolloid. 116, 106641. doi: 10.1016/j.foodhyd.2021.106641
Liu, F., Ooi, V. E. C., and Chang, S. T. (1997). Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 60, 763–771.
Liu, K., Xiao, X., Wang, J. L., Chen, C. Y. O., and Hu, H. G. (2017). Polyphenolic composition and antioxidant, antiproliferative, and antimicrobial activities of mushroom Inonotus sanghuang. LWT-Food Sci. Technol. 82, 154–161. doi: 10.1016/j.lwt.2017.04.041
Liu, L. Q., Li, M. Z., Yu, M. L., Shen, M. Y., Wang, Q., Yu, Y., et al. (2019). Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 121, 743–751. doi: 10.1016/j.ijbiomac.2018.10.083
Liu, M. M., Zeng, P., Li, X. T., and Shi, L. G. (2016). Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 91, 1199–1205. doi: 10.1016/j.ijbiomac.2016.06.086
Liu, X., Hou, R. L., Xu, K. Q., Chen, L., Wu, X. P., Lin, W. X., et al. (2019a). Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus. Int. J. Biol. Macromol. 123, 468–476. doi: 10.1016/j.ijbiomac.2018.11.069
Liu, X., Hou, R. L., Yan, J. J., Xu, K. Q., Wu, X. P., Lin, W. X., et al. (2019b). Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 129, 41–49. doi: 10.1016/j.ijbiomac.2019.02.011
Liu, Y. H., Liu, C. H., Jiang, H. Q., Zhou, H. L., Li, P. L., and Wang, F. S. (2015). Isolation, structural characterization and neurotrophic activity of a polysaccharide from Phellinus ribis. Carbohyd. Polym. 127, 145–151. doi: 10.1016/j.carbpol.2015.03.057
Liu, Y. H., Tang, X., Li, Y. L., Zou, Q., Wu, X. P., and Chen, T. (2020). A review about the research of extraction, purification and structure analysis of active polysaccharide. Chin. J. Ethnomed. Ethnopharm. 29, 67–73.
Liu, Y. H., and Wang, F. S. (2007). Structural characterization of an active polysaccharide from Phellinus ribis. Carbohyd. Polym. 70, 386–392. doi: 10.1016/j.carbpol.2007.04.019
Liu, Y. H., Xu, J. Z., Zong, A. Z., Wang, J. H., Liu, Y. G., Jia, W., et al. (2018). Anti-angiogenic activity and mechanism of a chemically sulfated natural glucan from Phellinus ribis. Int. J. Biol. Macromol. 107, 2475–2483. doi: 10.1016/j.ijbiomac.2017.10.134
Liu, Z. H., Niu, F. J., Xie, Y. X., Xie, S. M., Liu, Y. N., Yang, Y. Y., et al. (2020). A review: natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed. Pharmacother. 129, 110469. doi: 10.1016/j.biopha.2020.110469
Lo, T. C. T., Chang, C. A., Chiu, K. H., Tsay, P. K., and Jen, J. F. (2011). Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohyd. Polym. 86, 320–327. doi: 10.1016/j.carbpol.2011.04.056
Lu, L. X., Li, Y., Lu, Y., Zhu, Y. F., Xu, J., and Lin, J. M. (2020). Study on the antibacterial properties of Phellinus igniarius extract. Chin. J. Disinfect. 37, 406–409. doi: 10.11726/j.issn.1001-7658.2020.06.002
Lu, T., and Finkel, T. (2008). Free radicals and senescence. Exp. Cell Res. 314, 1918–1922. doi: 10.1016/j.yexcr.2008.01.011
Luo, J. G., Liu, J., Sun, Y., Ye, H., Zhou, C. H., and Zeng, X. X. (2010). Medium optimization, preliminary characterization and antioxidant activity in vivo of mycelial polysaccharide from Phellinus baumii Pilát. Carbohyd. Polym. 81, 533–540. doi: 10.1016/j.carbpol.2010.03.010
Luo, L. J., Wang, Y. X., Zhang, S., Guo, L., Jia, G. T., Lin, W. P., et al. (2021). Preparation and characterization of selenium-rich polysaccharide from Phellinus igniarius and its effects on wound healing. Carbohyd. Polym. 264, 117982. doi: 10.1016/j.carbpol.2021.117982
Luo, M. C., Zhang, X. Y., Wu, J., and Zhao, J. M. (2021). Modifications of polysaccharide-based biomaterials under structure-property relationship for biomedical applications. Carbohyd. Polym. 266, 118097. doi: 10.1016/j.carbpol.2021.118097
Ma, J. X., Cai, C. S., Liu, J. J., Gao, S., Zhao, G. Z., and He, X. W. (2021). In vitro antibacterial and antitumor activity of total triterpenoids from a medicinal mushroom Sanghuangporus sanghuang (Agaricomycetes) in liquid fermentation culture. Int. J. Med. Mushrooms 23, 27–39. doi: 10.1615/IntJMedMushrooms.2021038916
Ma, X. K., Guo, D. D., Peterson, E. C., Dun, Y., and Li, D. Y. (2016). Structural characterization and anti-aging activity of a novel extracellular polysaccharide from fungus Phellinus sp. in mammalian system. Food Funct. 7, 3468–3479. doi: 10.1039/C6FO00422A
Ma, X. K., She, X., Peterson, E. C., Wang, Y. Z., Zheng, P., Ma, H. Y., et al. (2019). A newly characterized exopolysaccharide from Sanghuangporus sanghuang. Int. J. Biol. Macromol. 57, 812–820. doi: 10.1007/s12275-019-9036-4
Mahendiran, B., Muthusamy, S., Sampath, S., Jaisankar, S. N., Popat, K. C., Selvakumar, R., et al. (2021). Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: a review. Int. J. Biol. Macromol. 183, 564–588. doi: 10.1016/j.ijbiomac.2021.04.179
Maity, P., Sen, I. K., Chakraborty, I., Mondal, S., Bar, H., Bhanja, S. K., et al. (2021). Biologically active polysaccharide from edible mushrooms: a review. Int. J. Biol. Macromol. 172, 408–417. doi: 10.1016/j.ijbiomac.2021.01.081
Makvandi, P., Ghomi, M., Ashrafizadeh, M., Tafazoli, A., Agarwal, T., Delfi, M., et al. (2020). A review on advances in graphene-derivative/polysaccharide bionanocomposites: therapeutics, pharmacogenomics and toxicity. Carbohyd. Polym. 250, 116952. doi: 10.1016/j.carbpol.2020.116952
Marić, M., Grassino, A. N., Zhu, Z. Z., Barba, F. J., Brnčić, M., and Brnčić, S. R. (2018). An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 76, 28–37. doi: 10.1016/j.tifs.2018.03.022
Martinez-Medina, G. A., Chávez-González, M. L., Verma, D. K., Prado-Barragán, L. A., Martínez-Hernández, J. L., Flores-Gallegos, A. C., et al. (2021). Bio-functional components in mushrooms, a health opportunity: ergothionine and huitlacohe as recent trends. J. Funct. Foods 77, 104326. doi: 10.1016/j.jff.2020.104326
Mei, Y. X., Zhu, H., Hu, Q. M., Liu, Y. Y., Zhao, S. M., Peng, N., et al. (2015). A novel polysaccharide from mycelia of cultured Phellinus linteus displays antitumor activity through apoptosis. Carbohyd. Polym. 124, 90–97. doi: 10.1016/j.carbpol.2015.02.009
Meng, H. H., Jin, W. F., Yu, L., Xu, S. C., Wan, H. T., and He, Y. (2021). Protective effects of polysaccharides on cerebral ischemia: a mini-review of the mechanisms. Int. J. Biol. Macromol. 169, 463–472. doi: 10.1016/j.ijbiomac.2020.12.124
Meng, X., Liang, H. B., and Luo, L. X. (2016). Antitumor polysaccharides from mushrooms: a review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohyd. Res. 424, 30–41. doi: 10.1016/j.carres.2016.02.008
Miao, J. N., Regenstein, J. M., Qiu, J. Q., Zhang, J. Q., Zhang, X. P., Li, H. X., et al. (2020). Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia: a review. Int. J. Biol. Macromol. 150, 102–113. doi: 10.1016/j.ijbiomac.2020.02.054
Mirzadeh, M., Arianejad, M. R., and Khedmat, L. (2020). Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: a review. Carbohyd. Polym. 229, 115421. doi: 10.1016/j.carbpol.2019.115421
Mitchell, D. A., Moreira, I., and Krieger, N. (2021). Potential of time-stepping stochastic models as tools for guiding the design and operation of processes for the enzymatic hydrolysis of polysaccharides: a review. Bioresour. Technol. 323, 124559. doi: 10.1016/j.biortech.2020.124559
Mohan, K., Ravichandran, S., Muralisankar, T., Uthayakumar, V., Chandirasekar, R., Seedevi, P., et al. (2019). Potential uses of fungal polysaccharides as immunostimulants in fish and shrimp aquaculture: a review. Aquaculture 500, 250–263. doi: 10.1016/j.aquaculture.2018.10.023
Moradali, M. F., Mostafavi, H., Ghods, S., and Hedjaroude, G. A. (2007). Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 7, 701–724. doi: 10.1016/j.intimp.2007.01.008
Nandita, E., Bacalzo, N. P., Ranque, C. L., Amicucci, M. J., Galermo, A., and Lebrilla, C. B. (2021). Polysaccharide identification through oligosaccharide fingerprinting. Carbohyd. Polym. 257, 117570. doi: 10.1016/j.carbpol.2020.117570
Nie, Y., Lin, Q. L., and Luo, F. J. (2017). Effects of non-starch polysaccharides on inflammatory bowel disease. Int. J. Mol. Sci. 18, 1372. doi: 10.3390/ijms18071372
Niu, W., Chen, X. Q., Xu, R. L., Dong, H. M., Yang, F. Y., Wang, Y., et al. (2021). Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: a review. Carbohyd. Polym. 254, 117189. doi: 10.1016/j.carbpol.2020.117189
Nypelö, T., Berke, B., Spirk, S., and Sirviö, J. A. (2021). Review: periodate oxidation of wood polysaccharides—modulation of hierarchies. Carbohyd. Polym. 252, 117105. doi: 10.1016/j.carbpol.2020.117105
Ogawa, K., Tsurugi, J., and Watanabe, T. (1972). Complex of gel-forming β-1,3-D-glucan with congored in alkaline solution. Chem. Lett. 1, 689–692.
Pei, J. J., Wang, Z. B., Ma, H. L., and Yan, J. K. (2015). Structural features and antitumor activity of a novel polysaccharide from alkaline extract of Phellinus linteus mycelia. Carbohyd. Polym. 115, 472–477. doi: 10.1016/j.carbpol.2014.09.017
Prateeksha, Sharma, V. K., Liu, X., Oyarzún, D. A., Abdel-Azeem, A. M., Atanasov, A. G., Hesham, A. E. L., et al. (2021). Microbial polysaccharides: an emerging family of natural biomaterials for cancer therapy and diagnostics. Semin. Cancer Biol. doi: 10.1016/j.semcancer.2021.05.021
Qian, J. Y., Chen, W., Zhang, W. M., and Zhang, H. (2009). Adulteration identification of some fungal polysaccharides with SEM, XRD, IR and optical rotation: a primary approach. Carbohyd. Polym. 78, 620–625. doi: 10.1016/j.carbpol.2009.05.025
Qu, J. L., Huang, P., Zhang, L., Qiu, Y., Qi, H., Leng, A. J., et al. (2020). Hepatoprotective effect of plant polysaccharides from natural resources: a review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 161, 24–34. doi: 10.1016/j.ijbiomac.2020.05.196
Rajachan, O., Sangdee, A., Kanokmedhakul, K., Tontapha, S., Amornkitbamrung, V., and Kanokmedhakul, S. (2020). Cyclofarnesane sesquiterpenoids from the fungus Sanghuangporus sp. Phytochem. Lett. 37, 17–20. doi: 10.1016/j.phytol.2020.03.007
Reis, F. S., Barreira, J. C. M., Calhelha, R. C., van Griensven, L. J. I. D., Cirić, A., Glamočlija, J., et al. (2014). Chemical characterization of the medicinal mushroom Phellinus linteus (Berkeley and Curtis) Teng and contribution of different fractions to its bioactivity. LWT Food Sci. Technol. 58, 478–485. doi: 10.1016/j.lwt.2014.04.013
Reis, F. S., Martins, A., Vasconcelos, M. H., Morales, P., and Ferreira, I. C. F. R. (2017). Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 66, 48–62. doi: 10.1016/j.tifs.2017.05.010
Ren, Y., Bai, Y. P., Zhang, Z. D., Cai, W. L., and Flores, A. R. (2019). The preparation and structure analysis methods of natural polysaccharides of plants and fungi: a review of recent development. Molecules 24, 3122. doi: 10.3390/molecules24173122
Rony, K. A., Ajith, T. A., Nima, N., and Janardhanan, K. K. (2014). Hypolipidemic activity of Phellinus rimosus against triton WR-1339 and high cholesterol diet induced hyperlipidemic rats. Environ. Toxicol. Pharmcol. 37, 482–492. doi: 10.1016/j.etap.2014.01.004
Ruthes, A. C., Smiderle, F. R., and Iacomini, M. (2016). Mushroom heteropolysaccharides: a review on their sources, structure and biological effects. Carbohyd. Polym. 136, 358–375. doi: 10.1016/j.carbpol.2015.08.061
Schirrmacher, V. (2019). From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 54, 407–419. doi: 10.3892/ijo.2018.4661
Shah, N. N., Soni, N., and Singhal, R. S. (2018). Modification of proteins and polysaccharides using dodecenyl succinic anhydride: synthesis, properties and applications—a review. Int. J. Biol. Macromol. 107, 2224–2233. doi: 10.1016/j.ijbiomac.2017.10.099
Shan, L., Liu, Z. N., Ci, L. L., Shuai, C., Lv, X. W., and Li, J. (2019). Research progress on the anti-hepatic fibrosis action and mechanism of natural products. Int. Immunopharmacol. 75, 105765. doi: 10.1016/j.intimp.2019.105765
Sharma, N., Hanif, S., Upadhyay, D., and Chhikara, M. K. (2019). Inhibition ELISA as a putative tool for the identification and quantification of meningococcal A and X polysaccharides at various stages of vaccine development. J. Immunol. Methods 473, 112634. doi: 10.1016/j.jim.2019.112634
Shen, S., Liu, S. L., Jiang, J. H., and Zhou, L. W. (2021). Addressing widespread misidentifications of traditional medicinal mushrooms in Sanghuangporus (Basidiomycota) through ITS barcoding and designation of reference sequences. IMA Fungus 12, 1–21. doi: 10.1186/s43008-021-00059-x
Shi, H., Zhang, M., and Devahastin, S. (2020). New development of efficient processing techniques on typical medicinal fungi: a review. Food Rev. Int. 36, 39–57. doi: 10.1080/87559129.2019.1613663
Shi, L. (2016). Bioactivities, isolation and purification methods of polysaccharides from natural products: a review. Int. J. Biol. Macromol. 92, 37–48. doi: 10.1016/j.ijbiomac.2016.06.100
Shi, Y. B., Bai, W. D., Zhao, W. H., Qian, M., and Bai, Y. L. (2019). Optimization of enzymatic hydrolysis-assisted extraction of polysaccharides from Phellinus igniarius. Farm Prod. Process. 9, 29–32+35. doi: 10.16693/j.cnki.1671-9646(X).2019.09.042
Shon, M. Y., Kim, T. H., and Sung, N. J. (2003). Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 82, 593–597. doi: 10.1016/S0308-8146(03)00015-3
Sims, I. M., Carnachan, S. M., Bell, T. J., and Hinkley, S. F. R. (2018). Methylation analysis of polysaccharides: technical advice. Carbohyd. Polym. 188, 1–7. doi: 10.1016/j.carbpol.2017.12.075
Sindhu, R. K., Goyal, A., Das, J., Neha, Choden, S., and Kumar, P. (2021). Immunomodulatory potential of polysaccharides derived from plants and microbes: a narrative review. Carbohyd. Polym. Technol. Appl. 2, 100044. doi: 10.1016/j.carpta.2021.100044
Singdevsachan, S. K., Auroshree, P., Mishra, J., Baliyarsingh, B., Tayung, K., and Thatoi, H. (2016). Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: a review. Bioact. Carbohyd. Diet. Fibre 7, 1–14. doi: 10.1016/j.bcdf.2015.11.001
Singh, V., Kumar, P., and Sanghi, R. (2012). Use of microwave irradiation in the grafting modification of the polysaccharides: a review. Prog. Polym. Sci. 37, 340–364. doi: 10.1016/j.progpolymsci.2011.07.005
Song, Q. Q., Wang, Y. K., Huang, L. X., Shen, M. Y., Yu, Y., Yu, Q., et al. (2021). Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 140, 109858. doi: 10.1016/j.foodres.2020.109858
Su, H. H., Chu, Y. C., Liao, J. M., Wang, Y. H., Jan, M. S., Lin, C. W., et al. (2017). Phellinus linteus mycelium alleviates myocardial ischemia-reperfusion injury through autophagic regulation. Front. Pharmcol. 8, 175. doi: 10.3389/fphar.2017.00175
Suabjakyong, P., Nishimura, K., Toida, T., and van Griensven, L. J. L. D. (2015). Structural characterization and immunomodulatory effects of polysaccharides from Phellinus linteus and Phellinus igniarius on the IL-6/IL-10 cytokine balance of the mouse macrophage cell lines (RAW264.7). Food Funct. 6, 2834–2844. doi: 10.1039/C5FO00491H
Sun, Y. Q., Huo, J. X., Zhong, S., Zhu, J. X., Li, Y. G., and Li, X. J. (2021). Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohyd. Polym. 268, 118214. doi: 10.1016/j.carbpol.2021.118214
Tang, W., Liu, D., Yin, J. Y., and Nie, S. P. (2020). Consecutive and progressive purification of food-derived natural polysaccharide: based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 99, 76–87. doi: 10.1016/j.tifs.2020.02.015
Teng, S. C. (1939). A Contribution to Our Knowledge of the Higher Fungi of China. Beijing: Academia Sinica Press, 1–614.
Villares, A., Mateo-Vivaracho, L., and Guillamón, E. (2012). Structural features and healthy properties of polysaccharides occurring in mushrooms. Agriculture 2, 452–471. doi: 10.3390/agriculture2040452
Wan, X. L., Jin, X., Xie, M. L., Liu, J., Gontcharov, A. A., Wang, H., et al. (2020). Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 163, 865–877. doi: 10.1016/j.ijbiomac.2020.06.279
Wang, G. B., Dong, L. L., Zhang, Y. Y., Ji, Y. Y., Xiang, W. H., and Zhao, M. (2012). Polysaccharides from Phellinus linteus inhibit cell growth and invasion and induce apoptosis in HepG2 human hepatocellular carcinoma cells. Biologia 67, 247–254. doi: 10.2478/s11756-011-0160-9
Wang, H., Liu, Y. M., Qi, Z. M., Wang, S. Y., Liu, S. X., Li, X., et al. (2013). An overview on natural polysaccharides with antioxidant properties. Curr. Med. Chem. 20, 2899–2913. doi: 10.2174/0929867311320230006
Wang, H., Qian, K., Si, J., and Cui, B. K. (2021). Research advances on polysaccharides from sanghuang. Mycosystema 40, 895–911. doi: 10.13346/j.mycosystema.200364
Wang, H. L., Wu, G., Park, H. J., Jiang, P. P., Sit, W. H., van Griensven, L. J. L. D., et al. (2012). Protective effect of Phellinus linteus polysaccharide extracts against thioacetamide-induced liver fibrosis in rats: a proteomics analysis. Chin. Med. 7, 23. doi: 10.1186/1749-8546-7-23
Wang, J. Q., Hu, S. Z., Nie, S. P., Yu, Q., and Xie, M. Y. (2016). Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 5692852. doi: 10.1155/2016/5692852
Wang, K., Ding, Z. C., Pei, J. J., and Yan, J. K. (2020). Antioxidant activities of polysaccharides from Phellinus linteus mycelia by alkaline extraction. Sci. Technol. Food Ind. 41, 289–294. doi: 10.13386/j.issn1002-0306.2020.01.047
Wang, L., Yao, L., Jin, Y. W., Jin, C. Y., Dong, Y., Shou, D., et al. (2019). Activation effect of human TLR4 signaling pathway by polysaccharide from Phellinus igniarius. Chin. J. Mod. Appl. Pharm. 36, 1178–1182. doi: 10.13748/j.cnki.issn1007-7693.2019.10.002
Wang, P. C., Zhao, S., Yang, B. Y., Wang, Q. H., and Kuang, H. X. (2016). Anti-diabetic polysaccharides from natural sources: a review. Carbohyd. Polym. 148, 86–97. doi: 10.1016/j.carbpol.2016.02.060
Wang, W. J., Xue, C. H., and Mao, X. Z. (2020). Radioprotective effects and mechanisms of animal, plant and microbial polysaccharides. Int. J. Biol. Macromol. 153, 373–384. doi: 10.1016/j.ijbiomac.2020.02.203
Wang, X. T., Sun, T. T., Sun, J., Wang, S. X., Ma, Y. S., Liu, Z. C., et al. (2020a). Molecular cloning, characterisation, and heterologous expression of farnesyl diphosphate synthase from Sanghuangporus baumii. Mol. Biotechnol. 62, 132–141. doi: 10.1007/s12033-019-00231-0
Wang, X. T., Wang, S. X., Xu, X. R., Sun, J., Ma, Y. S., Liu, Z. C., et al. (2020b). Molecular cloning, characterization, and heterologous expression of an acetyl-CoA acetyl transferase gene from Sanghuangporus baumii. Protein Expres. Purif. 170, 105592. doi: 10.1016/j.pep.2020.105592
Wang, Y. Y., Ma, H. L., Ding, Z. C., Yang, Y., Wang, W. H., Zhang, H. N., et al. (2019a). Three-phase partitioning for the direct extraction and separation of bioactive exopolysaccharides from the cultured broth of Phellinus baumii. Int. J. Biol. Macromol. 123, 201–209. doi: 10.1016/j.ijbiomac.2018.11.065
Wang, Y. Y., Ma, H. L., Yan, J. K., Wang, K. D., Yang, Y., Wang, W. H., et al. (2019b). Three-phase partitioning system with dimethyl carbonate as organic phase for partitioning of exopolysaccharides from Phellinus baumii. Int. J. Biol. Macromol. 131, 941–948. doi: 10.1016/j.ijbiomac.2019.03.149
Wang, Z. B., Pei, J. J., Ma, H. L., Cai, P. F., and Yan, J. K. (2014). Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohyd. Polym. 109, 49–55. doi: 10.1016/j.carbpol.2014.03.057
Wang, Z. C., Sun, Q., Zhang, H. R., Wang, J. Q., Fu, Q. Z., Qiao, H. Z., et al. (2021). Insight into antibacterial mechanism of polysaccharides: a review. LWT Food Sci. Technol. 150, 111929. doi: 10.1016/j.lwt.2021.111929
Wang, Z. Y., Wang, C. Y., and Quan, Y. (2014a). Extraction of polysaccharides from Phellinus nigricans mycelia and their antioxidant activities in vitro. Carbohyd. Polym. 99, 110–115. doi: 10.1016/j.carbpol.2013.08.073
Wang, Z. Y., Zhou, F., and Quan, Y. (2014b). Antioxidant and immunological activity in vitro of polysaccharides from Phellinus nigricans mycelia. Int. J. Biol. Macromol. 64, 139–143. doi: 10.1016/j.ijbiomac.2013.11.038
Wasser, S. P. (2002). Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 60, 258–274. doi: 10.1007/s00253-002-1076-7
Wen, Y., Wan, Y. Z., Qiao, C. X., Xu, X. F., Wang, J., and Shen, Y. (2019). Immunoregenerative effects of the bionically cultured Sanghuang mushrooms (Inonotus sanghuang) on the immunodeficient mice. J. Ethnopharmacol. 245, 112047. doi: 10.1016/j.jep.2019.112047
Wu, D. T., An, L. Y., Liu, W., Hu, Y. C., Wang, S. P., and Zou, L. (2022a). In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 156, 111185. doi: 10.1016/j.foodres.2022.111185
Wu, D. T., He, Y., Fu, M. X., Gan, R. Y., Hu, Y. C., Peng, L. X., et al. (2022b). Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocolloid. 122, 107085. doi: 10.1016/j.foodhyd.2021.107085
Wu, J. J., Shi, S. S., Wang, H. J., and Wang, S. C. (2016). Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: a review. Carbohyd. Polym. 144, 474–494. doi: 10.1016/j.carbpol.2016.02.040
Wu, M. D., Cheng, M. J., Chen, Y. L., Chan, H. Y., Hsieh, S. Y., Chen, I. C., et al. (2019). Secondary metabolites from the fermented whole broth of fungal strain Sanghuangporus sanghuang. Chem. Nat. Compd. 55, 36–40. doi: 10.1007/s10600-019-02610-0
Wu, S. H., and Dai, Y. C. (2020). Species clarification of the medicinal fungus Sanghuang. Mycosystema 39, 781–794. doi: 10.13346/j.mycosystema.190354
Wu, S. H., Dai, Y. C., Hattori, T., Yu, T. W., Wang, D. M., Parmasto, E., et al. (2012). Species clarification for the medicinally valuable 'sanghuang' mushroom. Bot. Stud. 53, 135–149.
Wu, S. H., Wei, C. L., and Chang, C. C. (2020). Sanghuangporus vitexicola sp. nov. (Hymenochaetales, Basidiomycota) from tropical Taiwan. Phytotaxa 475, 43–51. doi: 10.11646/phytotaxa.475.1.4
Wu, S. J., Liaw, C. C., Pan, S. Z., Yang, H. C., and Ng, L. T. (2013). Phellinus linteus polysaccharides and their immunomodulatory properties in human monocytic cells. J. Funct. Foods 5, 679–688. doi: 10.1016/j.jff.2013.01.011
Wu, Y., Liu, H., Li, Z. H., Huang, D. Y., Nong, L. Z., Ning, Z. X., et al. (2022). Purification of polysaccharides from Phellinus linteus by using an aqueous two-phase system and evaluation of the physicochemical and antioxidant properties of polysaccharides in vitro. Prep. Biochem. Biotechnol. 52, 89–98. doi: 10.1080/10826068.2021.1911815
Xie, L. M., Shen, M. Y., Hong, Y. Z., Ye, H. D., Huang, L. X., and Xie, J. H. (2020). Chemical modifications of polysaccharides and their anti-tumor activities. Carbohyd. Polym. 229, 115436. doi: 10.1016/j.carbpol.2019.115436
Xie, Z. L., Wang, Y., Huang, J. Q., Qian, N., Shen, G. Z., and Chen, L. H. (2019). Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264.7 macrophages. Int. J. Biol. Macromol. 129, 61–67. doi: 10.1016/j.ijbiomac.2019.02.023
Xing, R. E., Liu, S., Guo, Z. Y., Yu, H. H., Wang, P. B., Li, C. P., et al. (2005). Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorg. Med. Chem. 13, 1573–1577. doi: 10.1016/j.bmc.2004.12.022
Xu, C. P., Yu, J. W., Zhao, S. S., Wu, S. S., He, P. X., Jia, X. W., et al. (2017). Effect of carbon source on production, characterization and bioactivity of exopolysaccharide produced by Phellinus vaninii Ljup. An. Acad. Bras. Ciênc. 89, 2033–2041. doi: 10.1590/0001-3765201720150786
Xu, Y., Wu, Y. J., Sun, P. L., Zhang, F. M., Linhardt, R. J., and Zhang, A. Q. (2019). Chemically modified polysaccharides: synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 132, 970–977. doi: 10.1016/j.ijbiomac.2019.03.213
Xu, Y., Zhao, X. Y., Cao, H., Sheng, S., Wang, J., and Wu, F. A. (2016). Enzyme-catalyzed extraction and antioxidant activity of polysaccharides from Phellinus igniarius. Curr. Top. Nutraceut. Res. 14, 171–180.
Xue, Q., Sun, J., Zhao, M. W., Zhang, K. Y., and Lai, R. (2011). Immunostimulatory and anti-tumor activity of a water-soluble polysaccharide from Phellinus baumii mycelia. World J. Microbiol. Biotechnol. 27, 1017–1023. doi: 10.1007/s11274-010-0545-x
Yadav, D., and Negi, P. S. (2021). Bioactive components of mushrooms: processing effects and health benefits. Food Res. Int. 148, 110599. doi: 10.1016/j.foodres.2021.110599
Yan, J. K., Pei, J. J., Ma, H. L., Wang, Z. B., and Liu, Y. S. (2017). Advances in antitumor polysaccharides from Phellinus sensu lato: production, isolation, structure, antitumor activity and mechanisms. Crit. Rev. Food Sci. Nutr. 57, 1256–1269. doi: 10.1080/10408398.2014.984802
Yan, J. K., Wang, Y. Y., Ma, H. L., and Wang, Z. B. (2016a). Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 29, 251–257. doi: 10.1016/j.ultsonch.2015.10.005
Yan, J. K., Wang, Y. Y., Ma, H. L., Wang, Z. B., and Pei, J. J. (2016b). Structural characteristics and antioxidant activity in vivo of a polysaccharide isolated from Phellinus linteus mycelia. J. Taiwan Inst. Chem. Eng. 65, 110–117. doi: 10.1016/j.jtice.2016.05.052
Yan, J. K., Wang, Y. Y., Wang, Z. B., Ma, H. L., Pei, J. J., and Wu, J. Y. (2016c). Structure and antioxidative property of a polysaccharide from an ammonium oxalate extract of Phellinus linteus. Int. J. Biol. Macromol. 91, 92–99. doi: 10.1016/j.ijbiomac.2016.05.063
Yang, M. Y., Belwal, T., Devkota, H. P., Li, L., and Luo, Z. S. (2019). Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: a comprehensive review. Trends Food Sci. Technol. 92, 94–110. doi: 10.1016/j.tifs.2019.08.009
Yang, P., Jin, J., Liu, Q., Ma, D. M., Li, J., Zhang, Y. Q., et al. (2019). Optimization of degradation conditions with PRG, a polysaccharide from Phellinus ribis, by RSM and the neuroprotective activity in PC12 cells damaged by Aβ25−35. Molecules 24, 3010. doi: 10.3390/molecules24163010
Yang, Y., Ji, J., Di, L. Q., Li, J. S., Hu, L. H., Qiao, H. Z., et al. (2020). Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages. Carbohyd. Polym. 241, 116355. doi: 10.1016/j.carbpol.2020.116355
Yang, Y., Ye, L. B., Zhang, J. S., Liu, Y. F., and Tang, Q. J. (2009). Structural analysis of a bioactive polysaccharide, PISP1, from the medicinal mushroom Phellinus igniarius. Biosci. Biotechnol. Biochem. 73, 134–139. doi: 10.1271/bbb.80546
Yang, Y., Zhang, J. S., Liu, Y. F., Tang, Q. J., Zhao, Z. G., and Xia, W. S. (2007). Structural elucidation of a 3-O-methyl-D-galactose-containing neutral polysaccharide from the fruiting bodies of Phellinus igniarius. Carbohyd. Res. 342, 1063–1070. doi: 10.1016/j.carres.2007.02.019
Yao, H. Y. Y., Wang, J. Q., Yin, J. Y., Nie, S. P., and Xie, M. Y. (2021). A review of NMR analysis in polysaccharide structure and conformation: progress, challenge and perspective. Food Res. Int. 143, 110290. doi: 10.1016/j.foodres.2021.110290
Yin, Z. H., Liang, Z. H., Li, C. Q., Wang, J. M., Ma, C. Y., and Kang, W. Y. (2021). Immunomodulatory effects of polysaccharides from edible fungus: a review. Food Sci. Hum. Well. 10, 393–400. doi: 10.1016/j.fshw.2021.04.001
Ying, R. F., Huang, M. G., Wang, Y. S., Wu, C. E., Li, T. T., and Fan, G. J. (2019). Ultrasonic-microwave synergistic assisted extraction and activity of polysaccharides from Phellinus igniarius. Food Res. Dev. 40, 82–88. doi: 10.12161/j.issn.1005-6521.2019.21.015
Ying, R. F., Wu, C. E., Huang, M. G., and Wang, Y. S. (2017). Anti-tumor activity of polysaccharides from Phellinus igniarius fruiting body and mycelium. China Food Addit. 12, 57–61.
You, Q. H., Yin, X. L., and Ji, C. W. (2014). Pulsed counter-current ultrasound-assisted extraction and characterization of polysaccharides from Boletus edulis. Carbohyd. Polym. 101, 379–385. doi: 10.1016/j.carbpol.2013.09.031
Yuan, Q. H., Yuan, Y., Zheng, Y., Sheng, R., Liu, L., Xie, F., et al. (2021). Anti-cerebral ischemia reperfusion injury of polysaccharides: a review of the mechanisms. Biomed. Pharmacother. 137, 111303. doi: 10.1016/j.biopha.2021.111303
Yuan, Q. X., Zhao, L. Y., Li, Z. H., Harqin, C., Peng, Y. F., and Liu, J. K. (2018). Physicochemical analysis, structural elucidation and bioactivities of a high-molecular-weight polysaccharide from Phellinus igniarius mycelia. Int. J. Biol. Macromol. 120, 1855–1864. doi: 10.1016/j.ijbiomac.2018.09.192
Yuan, Y., Che, L. H., Qi, C., and Meng, Z. L. (2019). Protective effects of polysaccharides on hepatic injury: a review. Int. J. Biol. Macromol. 141, 822–830. doi: 10.1016/j.ijbiomac.2019.09.002
Zafar, R., Zia, K. M., Tabasum, S., Jabeen, F., Noreen, A., and Zuber, M. (2016). Polysaccharide based bionanocomposites, properties and applications: a review. Int. J. Biol. Macromol. 92, 1012–1024. doi: 10.1016/j.ijbiomac.2016.07.102
Zhang, H. N., Ma, H. L., Liu, W., Pei, J. J., Wang, Z. B., Zhou, H. J., et al. (2014). Ultrasound enhanced production and antioxidant activity of polysaccharides from mycelial fermentation of Phellinus igniarius. Carbohyd. Polym. 113, 380–387. doi: 10.1016/j.carbpol.2014.07.027
Zhang, H. N., Ma, H. L., Zhou, C. S., Yan, Y., Yin, X. L., and Yan, J. K. (2018). Enhanced production and antioxidant activity of endo-polysaccharides from Phellinus igniarius mutants screened by low power He–Ne laser and ultraviolet induction. Bioact. Carbohyd. Diet. Fibre 15, 30–36. doi: 10.1016/j.bcdf.2016.11.006
Zhang, J. J., Li, Y., Zhou, T., Xu, D. P., Zhang, P., Li, S., et al. (2016). Bioactivities and health benefits of mushrooms mainly from China. Molecules 21, 938. doi: 10.3390/molecules21070938
Zhang, M., Cui, S. W., Cheung, P. C. K., and Wang, Q. (2007). Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 18, 4–19. doi: 10.1016/j.tifs.2006.07.013
Zhang, R. Y., Belwal, T., Li, L., Lin, X. Y., Xu, Y. Q., and Luo, Z. S. (2020). Recent advances in polysaccharides stabilized emulsions for encapsulation and delivery of bioactive food ingredients: a review. Carbohyd. Polym. 242, 116388. doi: 10.1016/j.carbpol.2020.116388
Zhang, W. (1994). Biochemical Technology of Carbohydrate Complexes. Hangzhou: Zhejiang University Press, 193–200.
Zhang, Y., Wang, C. F., Liu, C. X., Wang, X., Chen, B. J., Yao, L. M., et al. (2020). Recent developments in stigma maydis polysaccharides: isolation, structural characteristics, biological activities and industrial application. Int. J. Biol. Macromol. 150, 246–252. doi: 10.1016/j.ijbiomac.2020.01.294
Zhang, Y., Zhang, S. M., Wang, F., Wang, L., Liu, M. J., and Liang, Y. (2015). Review on extraction methods of plant polysaccharide of these years. Farm Prod. Process. 6, 65–68+72. doi: 10.3969/jissn.1671-9646(X).2015.06.021
Zhang, Z., Hao, G. Y., Liu, C., Fu, J. Q., Hu, D., Rong, J. H., et al. (2021). Recent progress in the preparation, chemical interactions and applications of biocompatible polysaccharide-protein nanogel carriers. Food Res. Int. 147, 110564. doi: 10.1016/j.foodres.2021.110564
Zhang, Z. F., Lv, G. Y., Cheng, J. W., Cai, W. M., Fan, L. F., and Miao, L. X. (2019). Characterization and biological activities of polysaccharides from artificially cultivated Phellinus baumii. Int. J. Biol. Macromol. 129, 861–868. doi: 10.1016/j.ijbiomac.2019.02.082
Zhang, Z. F., Lv, G. Y., Song, T. T., Jin, Q. L., Huang, J. B., Fan, L. F., et al. (2015). Comparison of the preliminary characterizations and antioxidant properties of polysaccharides obtained from Phellinus baumii growth on different culture substrates. Carbohyd. Polym. 132, 397–399. doi: 10.1016/j.carbpol.2015.06.006
Zhao, C., Liao, Z. S., Wu, X. Q., Liu, Y. L., Liu, X. Y., Lin, Z. X., et al. (2014). Isolation, purification, and structural features of a polysaccharide from Phellinus linteus and its hypoglycemic effect in alloxan-induced diabetic mice. J. Food Sci. 79, H1002–H1010. doi: 10.1111/1750-3841.12464
Zheng, X., Lu, F. Z., Xu, X. J., and Zhang, L. N. (2017). Extended chain conformation of β-glucan and its effect on antitumor activity. J. Mater. Chem. B 5, 5623–5631. doi: 10.1039/C7TB01324H
Zheng, Y., Cui, J., Chen, A. H., Zong, Z. M., and Wei, X. Y. (2019). Optimization of ultrasonic-microwave assisted extraction and hepatoprotective activities of polysaccharides from Trametes orientalis. Molecules 24, 147. doi: 10.3390/molecules24010147
Zhong, C. (2020). Anti-fatigue effect of fermentation broth polysaccharose of Phellinus igniarius. Edible Fungi China 39, 46–48. doi: 10.13629/j.cnki.53-1054.2020.06.012
Zhong, S., Ji, D. F., Li, Y. G., Lin, T. B., Lv, Z. Q., and Chen, H. P. (2013). Activation of P27kip1-cyclin D1/E-CDK2 pathway by polysaccharide from Phellinus linteus leads to S-phase arrest in HT-29 cells. Chem. Biol. Interact. 206, 222–229. doi: 10.1016/j.cbi.2013.09.008
Zhou, L. W., Vlasák, J., Decock, C., Assefa, A., Stenlid, J., Abate, D., et al. (2016). Global diversity and taxonomy of the Inonotus linteus complex (Hymenochaetales, Basidiomycota): Sanghuangporus gen. nov., Tropicoporus excentrodendri and T. guanacastensis gen. et spp. nov., and 17 new combinations. Fungal Divers. 77, 335–347. doi: 10.1007/s13225-015-0335-8
Zhu, L., and Cui, B. K. (2016). Progress on the studies of medicinal mushrooms “sanghuang” group. J. Fungal Res. 14, 201–209. doi: 10.13341/j.jfr.2014.6402
Zhu, L., Song, J., Zhou, J. L., Si, J., and Cui, B. K. (2019). Species diversity, phylogeny, divergence time, and biogeography of the genus Sanghuangporus (Basidiomycota). Front. Microbiol. 10, 812. doi: 10.3389/fmicb.2019.00812
Zhu, T., Guo, J., Collins, L., Kelly, J., Xiao, Z. J., Kim, S. H., et al. (2007). Phellinus linteus activates different pathways to induce apoptosis in prostate cancer cells. Brit. J. Cancer 96, 583–590. doi: 10.1038/sj.bjc.6603595
Zong, A. Z., Cao, H. Z., and Wang, F. S. (2012). Anticancer polysaccharides from natural resources: a review of recent research. Carbohyd. Polym. 90, 1395–1410. doi: 10.1016/j.carbpol.2012.07.026
Keywords: sanghuang, polysaccharide, preparation strategy, structural characterization, biological activity
Citation: Wang H, Ma J-H, Zhou M, Si J and Cui B-K (2022) Current advances and potential trends of the polysaccharides derived from medicinal mushrooms sanghuang. Front. Microbiol. 13:965934. doi: 10.3389/fmicb.2022.965934
Received: 10 June 2022; Accepted: 04 July 2022;
Published: 03 August 2022.
Edited by:
Jing-Kun Yan, Dongguan University of Technology, ChinaReviewed by:
Ding-Tao Wu, Chengdu University, ChinaCopyright © 2022 Wang, Ma, Zhou, Si and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Si, amluZ3NpMTc4OEAxMjYuY29t; Bao-Kai Cui, Y3VpYmFva2FpQGJqZnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.