- 1Key Laboratory for Humid Subtropical Eco-Geographical Processes of the Ministry of Education, School of Geographical Sciences, Fujian Normal University, Fuzhou, China
- 2Institute of Oceanography, College of Geography and Oceanography, Minjiang University, Fuzhou, China
- 3School of Agriculture and Food, Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Parkville, VIC, Australia
- 4State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China
The excessive usage of nitrogen (N) fertilizers can accelerate the tendency of global climate change. Biological N fixation by diazotrophs contributes substantially to N input and is a viable solution to sustainable agriculture via reducing inorganic N fertilization. However, how manure application influences the abundance, community structure and assembly process of diazotrophs in soil aggregates is not fully understood. Here, we investigated the effect of manure amendment on diazotrophic communities in soil aggregates of an arable soil. Manure application increased soil aggregation, crop yield and the abundance of nifH genes. The abundance of nifH genes increased with aggregate sizes, indicating that diazotrophs prefer to live in larger aggregates. The abundance of nifH genes in large macroaggregates, rather than in microaggregates and silt and clay, was positively associated with plant biomass and crop yield. Both manure application and aggregate size did not alter the Shannon diversity of diazotrophs but significantly changed the diazotrophic community structure. The variation of diazotrophic community structure explained by manure application was greater than that by aggregate size. Manure application promoted the relative abundance of Firmicutes but reduced that of α-Proteobacteria. Stochastic processes played a dominant role in the assembly of diazotrophs in the control treatment. Low-rate manure (9 Mg ha−1) application, rather than medium-rate (18 Mg ha−1) and high-rate (27 Mg ha−1) manure, significantly increased the relative importance of deterministic processes in diazotrophic community assembly. Taken together, our findings demonstrated that long-term manure application increased nifH gene abundance and altered the community structure and assembly process of diazotrophs in soil aggregates, which advanced our understanding of the ecophysiology and functionality of diazotrophs in acidic Ultisols.
Introduction
The overuse of mineral N fertilizers in arablex soils has resulted in a series of environmental issues, such as water eutrophication, soil acidification, and nitrous oxide (N2O) emissions (Gallejones et al., 2012; Shcherbak et al., 2014), and accerelated the global climate change. Biological nitrogen (N) fixation, a process converting atmospheric dinitrogen (N2) into plant- or microorganism-available ammonium, is an important source of N input in ecosystems (Galloway et al., 2004). More importantly, biological N fixation is commonly recognized as an environmental-friendly approach alternative to fertilization to meet the N requirements of plants (Cleveland et al., 1999; Wang J. et al., 2020). Diazotrophs are the major biological engines for fixing atmospheric N into nitrogenous reactive compounds via the nitrogenase enzyme (Kuypers et al., 2018). The nifH gene, encoding nitrogenase, serves as an ideal biomarker to detect the abundance and community structure of diazotrophs without the need for cultivation (Reardon et al., 2014; Pajares and Bohannan, 2016; Tang et al., 2021).
Many biotic and abiotic variables can regulate the abundance and community structure of diazotrophs, including temperature (Fu et al., 2014), soil moisture (Feng et al., 2019), soil pH (Wang Y.et al., 2017), nutrient contents (Zheng et al., 2019), and agronomic practices (Xun et al., 2018). Fertilizer is an important abiotic factor affecting the diazotrophic communities, which has become a growing concern in the past decade (Berthrong et al., 2014; Liao et al., 2018; Chen H. et al., 2021). In general, the application of mineral fertilizer, especially N fertilizer, suppresses N fixation and diazotrophs, and alters diazotrophic community composition (Fan et al., 2019; Chen H. et al., 2021; Chen L. et al., 2021). However, the influence of organic fertilizers on diazotrophic abundance was controversial in previous findings. Chen H. et al. (2021) found that organic fertilizers application increased the abundance and activity of diazotrophs in a vertisol. Hu et al. (2018) demonstrated that manure application enhanced the abundance of nifH genes in a Mollisol. In contrast, manure amendment reduced the abundance of diazotrophs in an Ultisol through increasing soil pH (Lin et al., 2018a). To unveil the underlying mechanisms, it is imperative to investigate the effect of manure application on the abundance and community structure of diazotrophs in agroecosystems.
Soil has a complex hierarchical structure containing soil aggregates and pore space (Wilpiszeski et al., 2019). Different sized soil aggregates vary in their physicochemical properties, resulting in the variation of microbial community at the aggregate level (Trivedi et al., 2017; Han et al., 2018). Nutrients and labile C content are generally higher in macroaggregates and therefore select copiotrophic microbes, while higher recalcitrant C and lower nutrients content in microaggregates favor oligotrophic microorganisms (Trivedi et al., 2017). Bacterial community structures in aggregates have been well explored (Smith et al., 2014; Zheng et al., 2021), while less efforts have been devoted to study the impact of soil aggregation on the abundance and community structure of diazotrophs, especially under manure application conditions.
The community assembly mechanism of microorganisms is a central topic in microbial ecology (Kinnunen et al., 2018; Jiao et al., 2020). Although it is accepted that both deterministic and stochastic processes are important in microbial assembly, which process is more important remains largely unclear (Tripathi et al., 2018; Ning et al., 2020; Zhou et al., 2021). It has been shown that soil organic carbon and aggregate sizes could regulate the relative importance of different processes in the assembly of microorganisms (Dini-Andreote et al., 2015; Zheng et al., 2021). However, how manure application influences microbial community assembly remains largely unknown. Despite of some recent progress (Feng et al., 2018; Wang J. et al., 2020), we have limited knowledge of the relative contributions of deterministic and stochastic processes in diazotrophic community assembly, especially considering different sized soil aggregates in Ultisols.
The objectives of this study were (i) to explore the effect of pig manure application on the abundance, diversity, community structure and assembly process of diazotrophs in soil aggregates of Ultisols and (ii) to link the abundance of diazotrophs in soil aggregates to plant biomass and crop yield. We hypothesized that manure application would decrease abundance and alter community structure and assembly process of diazotrophs, due to the increased availability of N.
Materials and methods
Field experimental description and sampling
The experimental site was located at the Yingtan, Jiangxi Province, China (28°15′20″N, 116°55′30″E). This site has a typical subtropical monsoon climate, with an annual average precipitation of 1795 mm and temperature of 17.6°C. The tested soil is originated from the quaternary red clay and classified as an Ultisol. The long-term fertilization experiment began in 2002 and included four treatments with three replicates (12 plots in total): CK (without manure application and did not receive any type of fertilizer), M9 (receiving 9 Mg ha−1 of manure), M18 (receiving 18 Mg ha−1 of manure) and M27 (receiving 27 Mg ha−1 of manure). The manure application rate in M9 is approximately to the rate frequently used in local area. The experimental plots have received the designated manure applications yearly since 2002. The manure used in this study was pig manure collected from local farms and was stockpiled for 3 months. The characteristics of pig manure are shown in Supplementary Table 1. The cropping system is summer peanut followed by winter fallow. At harvest, plant biomass (belowground plus aboveground) and kernel samples were collected for analysis and oven dried at 65°C to a constant moisture level.
Soils were collected in October 2019 from a depth of 0–20 cm. Ten soil cores were collected at random from each plot and combined to form one soil sample. Soil samples were placed on ice and transported to the lab. Visible plant debris and stones were picked out and the remaining soils were passed through an 8 mm sieve and divided into two subsamples. One was used for aggregate fractionation, and the other was for soil variables determination. The methods for soil variables determination, such as pH, soil organic matter (SOM), total nitrogen (TN), ammonium, nitrate, and available phosphorus (AP) have been described previously by Lin et al. (2018b). Briefly, pH was determined by a glass electrode with a 1:5 soil-to-water ratio. SOM and TN were measured using the wet oxidation redox titration and micro-Kjeldahl methods, respectively. Ammonium and nitrate were analyzed by a continuous-flow analyzer. AP was measured using the molybdenum blue method.
Soil aggregate fractionation
A wet-sieving technique was utilized for the fractionation of soil aggregate according to Elliott (1986) and Ye et al. (2021). Four fractions of soil aggregates for each sample were obtained: large macroaggregates (>2 mm), small macroaggregates (0.25–2 mm), microaggregates (0.053–0.25 mm), and silt and clay (<0.053 mm). An aliquot of each fraction was freeze-dried for DNA extraction.
DNA extraction and quantitative PCR (qPCR)
DNA was extracted from 0.25 g freeze-dried aggregate soils using the MoBio PowerSoil™ DNA Isolation Kits (Qiagen, Carlsbad, CA) following the manufacturer's recommendations. The quality of the extracted DNA was evaluated using 1.2% agarose gel electrophoresis. The gene copy numbers of nifH were determined in a CFX96 Optical Real-Time Detection System as described by Lin et al. (2018a), using the primers nifHF/nifHR (Töwe et al., 2010). The amplification always resulted in a single peak with efficiencies of 92–95%, and R2 of 0.994–0.999.
Sequencing and bioinformatics analysis
The primer pair used for high-throughput sequencing was the same as that for qPCR. The paired-end sequencing (2 × 300 bp) was conducted on an Illumina MiSeq platform by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). After sequencing, the raw nifH reads were quality-filtered and merged using FLASH (version 1.2.7). The resulting reads were processed using QIIME v1.8.0 (Caporaso et al., 2010) to remove low quality sequences. After sorting sequences, barcode and primer sequences were eliminated. Chimeras and sequences with low similarity to the nifH sequence were subsequently discarded (Lin et al., 2018a), and the remaining high-quality sequences at a 95% similarity cutoff were assigned to the same OTUs using UPARSE (Edgar, 2013). Representative sequences from each OTU were taxonomically classified by constructing neighbor-joining phylogenetic trees in MEGA-X. The sequences were rarified before Shannon diversity calculation in QIIME. All sequences have been deposited in the DNA Data Bank of Japan under the accession number DRA014094.
Data analysis
Statistical analyses were conducted using SPSS Statistics for Windows v 25.0 (IBM, Armonk, NY). The significance of difference in soil variables, plant biomass, peanut yield, the abundance of nifH genes among different treatments were evaluated by one-way analysis of variance (ANOVA) followed by least significant difference test. Pearson's correlation coefficients were used to determine the relationships between the abundance of nifH genes in aggregates and plant biomass or peanut yield. Non-metric multidimensional scaling (NMDS) analysis was performed using metaMDS function in the vegan package on the R platform (Version 4.1.0). Permutational multivariate analysis of variance (PERMANOVA) was conducted to determine the effects of manure applications and aggregate sizes on diazotrophic community structure using the adonis function in the vegan package. The relative importance of diazotrophic community assembly processes was evaluated by a phylogenetic bin-based null model framework, iCAMP (Ning et al., 2020). The analysis was conducted using the iCAMP (version 1.3.2) with appropriate default settings on a Galaxy platform (http://ieg3.rccc.ou.edu:8080). The significance of difference in the relative importance of specific ecological process was calculated based on bootstrapping with 1,000 replicates.
Results
Soil physicochemical variables, plant biomass and peanut yield
Compared to CK treatment, low-rate and medium-rate manure applications significantly decreased soil pH (Supplementary Table 2). However, the contents of SOM, TN, , and AP increased with the application rate of manure, with the highest values recorded in the M27 treatment. The mass proportion of large macroaggregates was 1.76% in CK, and the value increased to 5.62 with high-rate manure applications (Supplementary Figure 1). Conversely, high-rate manure applications significantly decreased the mass proportion of microaggregates from 39.93% in CK to 34.27%. The plant biomass and peanut yield in CK was 1503 and 1054 kg ha−1, respectively. The application of manure generally increased the plant biomass and yield of peanut, with the highest values found in M18 (Figure 1).
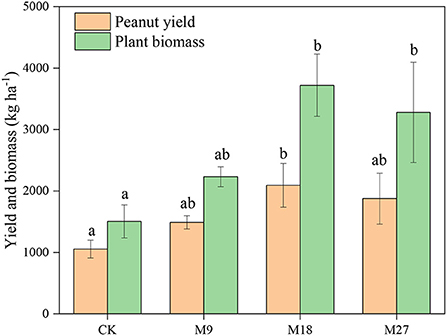
Figure 1. Peanut yield and plant biomass under long-term manure application. Vertical bars represent standard error (n = 3). Different letters denote significant differences (p < 0.05) between different treatments.
The abundance of nifH genes in soil aggregates and their correlation with plant biomass and yield
Manure application and aggregate size, and their interactions significantly influenced the abundance of nifH genes (Figure 2). In general, nifH gene abundance increased with the application rate of manure, regardless of aggregate sizes. The abundance of nifH genes was always higher in macroaggregates than in microaggregates and silt and clay. Pearson correlation analysis revealed that the abundance of nifH genes was significantly associated with plant biomass and peanut yield in large macroaggregates, and with plant biomass in small macroaggregates, but not in microaggregates and silt and clay (Figure 3; Supplementary Table 3).
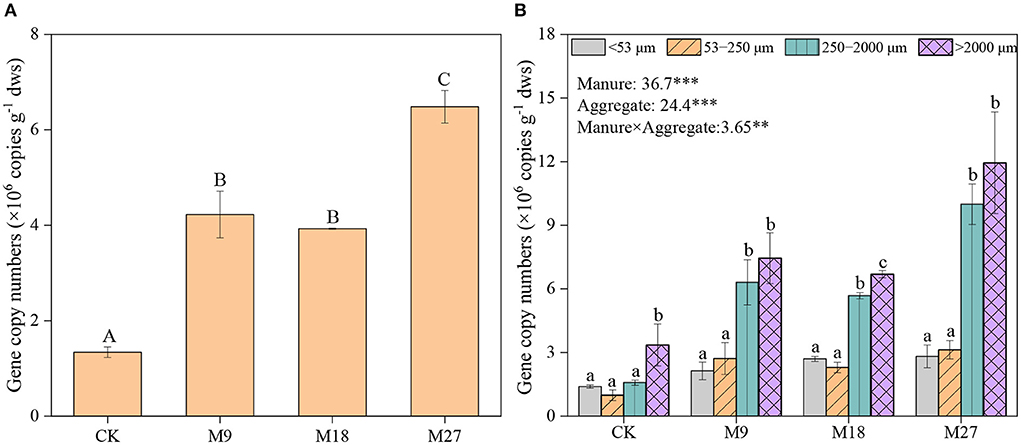
Figure 2. Abundance of nifH gene in soils (A) and aggregates (B) following long-term manure application. Vertical bars represent standard error (n = 3). Different lowercase letters denote significant differences (p < 0.05) between aggregate sizes in the same treatment, and different capital letters denote significant differences between different treatments. *** and ** indicate statistically significant at the 0.001 and 0.01 probability levels, respectively, while the numbers are F values by two-way ANOVA.
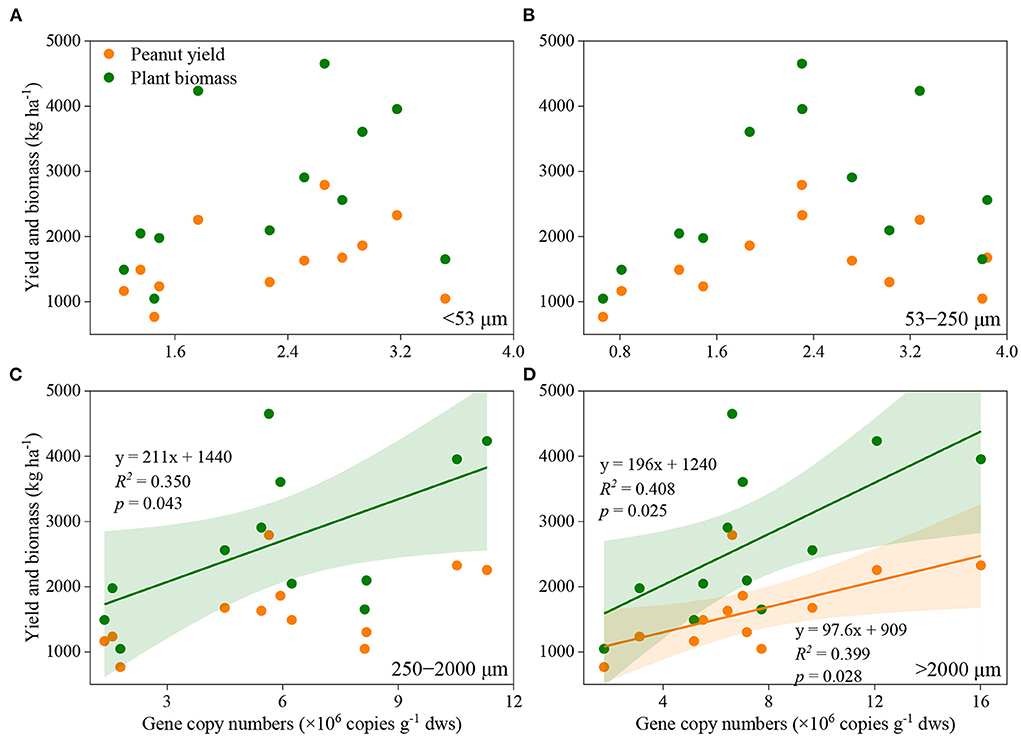
Figure 3. Association between peanut yield or plant biomass and the abundance of nifH gene in silt and clay (A), microaggregates (B), small macroaggregates (C), and large mcroaggregates (D). The shaded area indicates the 95% confidence interval of the regression models.
Diversity and community structure of diazotrophs
A total of 1,008,453 high-quality nifH sequences were obtained from 48 soil aggregate samples, with an average of 21,009 sequences per sample. Both manure application and aggregate sizes did not influence the Shannon diversity of diazotrophs (Supplementary Figure 2). However, the PERMANOVA analysis showed that manure application and soil aggregation significantly influenced the community structure of diazotrophs (Figure 4). The variation explained by manure application was 19.9%, which was higher than that explained by soil aggregate sizes (10.9%).
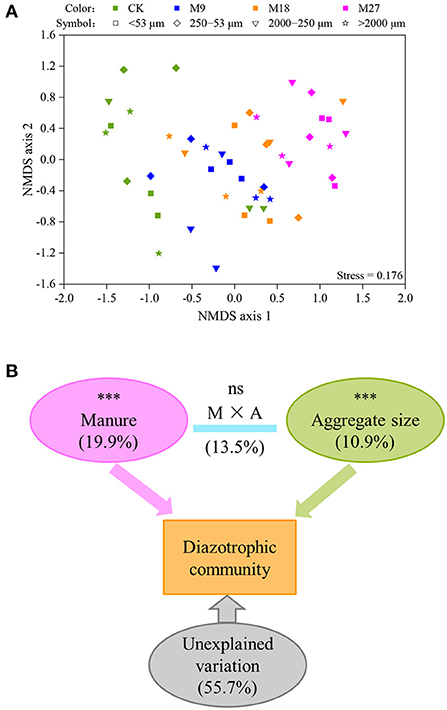
Figure 4. NMDS (A) and PERMANOVA (B) analysis of the communities of diazotrophs. Different colors represent treatments and different symbols indicate aggregate sizes in panel (A). In panel (B), the numbers are the percent of variation explained, and the letters M and A denote manure and aggregate sizes, respectively. Asterisks (***) denote significant differences at p < 0.001.
Phylogenetic analysis showed that the sequences of nifH genes could be affiliated with four phylotypes, i.e., Cyanobacteria, α-Proteobacteria, γ-Proteobacteria and Firmicutes (Figure 5). Cyanobacteria was the dominant diazotrophs in the tested soils, accounting for 46.48% of the detected diazotrophic sequences (Figure 6). The application of low-rate manure, rather than medium-rate and high-rate manure, significantly increased the relative abundance of Cyanobacteria. The relative abundance of Firmicutes was 0.09% in the CK and increased to 42.62% after application of high-rate manure. The relative abundance of α-Proteobacteria and γ-Proteobacteria was the highest in the CK treatment and was reduced by the application of manure. Soil aggregate sizes did not significantly affect the relative abundances of Cyanobacteria, α-Proteobacteria, γ-Proteobacteria and Firmicutes (Supplementary Figure 3).
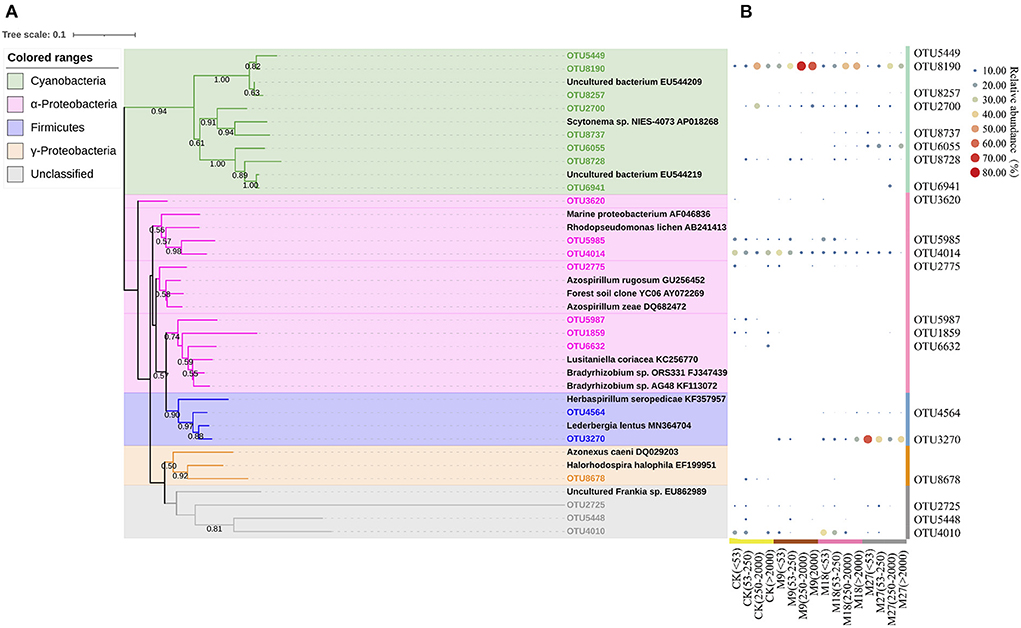
Figure 5. Phylogenetic tree shows the relationship between the representative sequences from this study and reference sequences from GenBank (A). The colored ranges indicate different phylotypes of diazotrophs. Bootstrap values of > 50% based on 1,000 replicates are shown next to the branches. The scale bar represents 0.1 nucleic acid sequence divergence. Panel (B) shows the heatmap of the relative abundance of each OTU to the diazotrophic sequences in each treatment. The relative abundances of OTUs are indicated by bubble size.
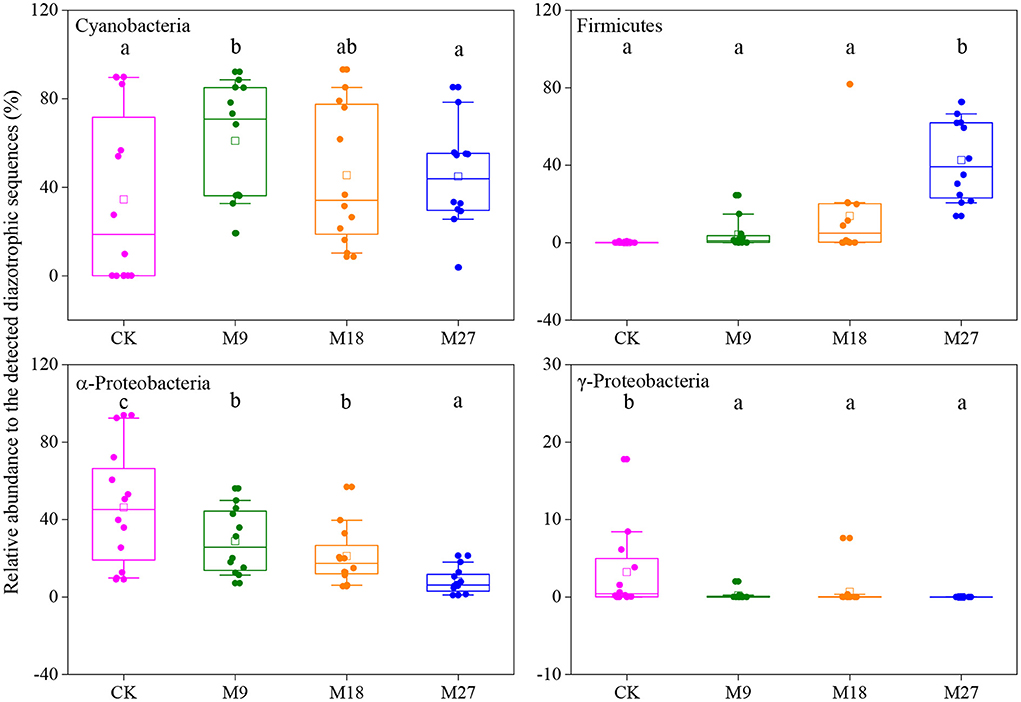
Figure 6. Box plots of main diazotrophic phyla affected by long-term manure applications. Boundaries of boxes indicate the first and third quartiles, and lines and squares within boxes represent the median and average, respectively. Whiskers indicate the 10th and 90th percentiles, and all the data are shown as dots. Different letters represent significant differences between treatments (p < 0.05).
Stochastic vs. deterministic diazotrophic community assembly
We investigated the community assembly mechanisms of diazotrophs using iCAMP. We found that homogenous selection, dispersal limitation, and drift and others were the main processes driving the community assembly of diazotrophs (Figure 7). Compared with CK, the application of low-rate manure significantly increased the relative importance of homogenous selection, while decreased the relative importance of dispersal limitation, and drift and others. The application of low-rate manure significantly decreased the relative contribution of stochastic process in diazotrophic community assembly. In addition, the relative importance of stochastic process in diazotrophic community assembly was also lower in the small macroaggregates than in other sizes of aggregates.
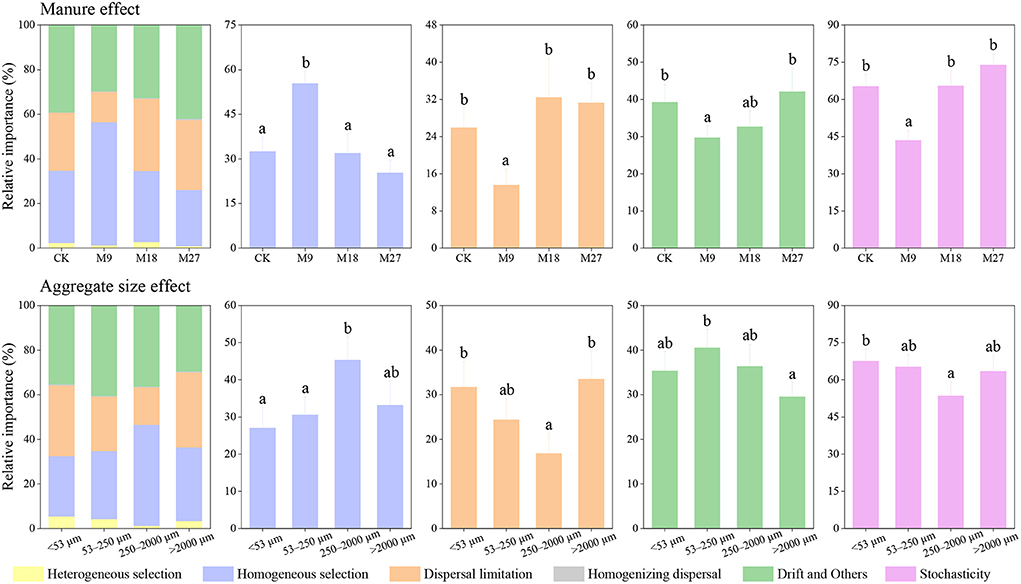
Figure 7. Relative importance of different ecological processes in diazotrophic community assembly based on iCAMP. Different letters denote significant differences (p < 0.05) based on bootstrapping with 1,000 replications. Stochasticity was estimated by the sum of dispersal limitation, homogenizing dispersal, and drift and others.
Discussion
Effect of manure and aggregate size on diazotrophic abundance in acidic Ultisols
Compared with NPK, the combined application of NPK and pig manure has been shown to reduce the abundance of nifH genes in an acidic Ultisol, possibly because of the promotion of soil pH (Lin et al., 2018a). Indeed, the diazotrophic communities in Ultisols have been acclimatized to acidic environments, and the increase of soil pH might be detrimental for them. However, this is not the case in this study, since we found that compared with control, the application of pig manure did not increase soil pH but increased the abundance of nifH genes. This result also rejected our hypothesis that the input of large amount of nutrients by manure applications would increase N availability and suppress the growth of diazotrophs. Several reasons may account for the increase of nifH genes by pig manure applications. Firstly, the majority of diazotrophs are heterotrophic or mixotrophic, and pig manure amendments provide C and energy sources for their growth (Rahav et al., 2016). The increase of SOC has been frequently found to stimulate the growth of diazotrophs (Perez et al., 2014; Liu et al., 2019), and the improvement of C substrate stimulates N fixation (Orr et al., 2012; Wang C. et al., 2017). Secondly, numerous studies have demonstrated the importance of phosphorus (P) availability in the growth of diazotrophs (Yang et al., 2019; Wang C. et al., 2020), since biological N fixation is energetically expensive and requires P for ATP synthesis (Olivares et al., 2013). However, Ultisols are well-known for low P availability, due to their strong P-fixation capacity by soil minerals. Thus, manure application could increase nifH gene abundance through substantially promoting the available P status. Thirdly, manure application increased the proportion of large macroaggregates, and the abundance of nifH genes increased with the increase of aggregate sizes. In general, SOC and nutrients in the macroaggregates are more abundant than those in the microaggregates (Trivedi et al., 2015; Ye et al., 2021), which could provide more C and energy sources for diazotrophs. Moreover, the cultivation of crops favored the growth of diazotrophs in larger aggregates, since microbes are more likely to be affected by plant roots in the macroaggregates than in the microaggregates (Zheng et al., 2018).
Besides biological N fixation, diazotrophs stimulate plant growth through diverse mechanisms, such as improving soil nutrient uptake efficiency, providing phytohormones to the host, and enhancing the host tolerance against stress (Dobbelaere et al., 2003; Shin et al., 2016; Pankievicz et al., 2021). As a result, the abundances of diazotrophs such as Azospirillum and Burkholderia, have been frequently found to be positively correlated with crop biomass and yield (Skonieski et al., 2019; Chen L. et al., 2021). Thus, it is reasonable to observe the close association between the abundance of nifH genes and plant performance. However, in this study, we found a significant correlation between the abundance of nifH genes in macroaggregates and plant biomass, rather than in microaggregates, indicating that the diazotrophs in macroaggregates more likely contribute to plant performance. As a result, the abundance of nifH genes in large macroaggregates might be a good indicator for plant performance in acidic Ultisols. It should be noted that only DNA was retrieved and analyzed in this study, and the higher abundance of nifH genes does not necessarily mean the higher activity of nitrogen fixation. Further investigations of the nifH transcription and nitrogen fixation activity are required in the future studies.
Effect of manure and aggregate size on diazotrophic community structure in acidic Ultisols
Pig manure application significantly altered the diazotrophic community structure in this study. Previous studies have shown that the application of mineral or organic fertilizers introduced a large amount of nutrients and C sources into soils, which significantly influenced the community structure of diazotrophs (Hu et al., 2018; Liao et al., 2018; Chen H. et al., 2021). Shi et al. (2021) further showed that organic manure over mineral fertilization primarily determined the stability of diazotrophic community structure in an upland chromic cambisol. Moreover, aggregate size also significantly influenced the community structure of diazotrophs, which was in line with most of previous studies that aggregate size classes substantially affected soil microbial community structure (Lin et al., 2019; Wang et al., 2021). Indeed, various sizes of aggregates could provide heterogeneous niches for diazotrophs and selected different diazotrophic phylotypes. However, pig manure was more important than aggregate sizes in regulating the community structure of diazotrophs, indicating the diazotrophic communities in acidic Ultisols are more prone to be influenced by resource availability than by the sizes of niches.
Cyanobacteria have been frequently reported to be dominant diazotrophs in aquatic environments (Liao et al., 2018; Wang et al., 2019). Cyanobacteria were also found to be dominant in the tested soils, indicating that the photoautotrophic diazotrophic species could also thrive in upland soils with high temperature and precipitation, due to their ability to endure repeated desiccation and hydration (Teng et al., 2009; Tang et al., 2021). Li et al. (2018) showed that Cyanobacteria may require more N than other diazotrophs, and thus the application of low-rate manure increased the relative abundance of Cyanobacteria through providing more nutrients. However, this effect might be counteracted by the addition of large amount of organic C in the medium-rate and high-rate manure treatments, since the autotrophic Cyanobacteria were less competitive than heterotrophic diazotrophs when large amount of organic C was applied. Moreover, the application of pig manure, especially in the high-rate, increased the relative abundance of Firmicutes. Firmicutes are generally the most abundant bacterial phylum in manure, and are able to degrade the complex organic compounds and thrive in soils after fertilization (Rieke et al., 2018; Ye et al., 2022). Numerous studies have shown that the application of manure increased the relative abundance of Firmicutes in soils (Francioli et al., 2016; Xun et al., 2016; Ye et al., 2019). In contrast, manure application reduced the relative abundance of α-Proteobacteria. This is out of our expectation since α-Proteobacteria are generally regarded as copiotrophic microorganisms (Eilers et al., 2010; Hu et al., 2018). It is likely that the α-Proteobacteria diazotrophs were less competitive than diazotrophic Firmicutes after pig manure applications and were out-competed in the manure-amended treatments, due to the overwhelming dominant role of Firmicutes in these treatments. Moreover, different OTUs within a diazotrophic groups may respond differently to manure applications (Figure 5B), indicating the highly diverse physio-ecological characteristics of diazotrophs in acidic Ultisols.
Effect of manure and aggregate size on diazotrophic community assembly in acidic Ultisols
We found that stochastic processes played a dominant role in the assembly of diazotrophs in CK. However, the application of low-rate manure, but not medium-rate and high-rate manure, significantly increased the relative importance of deterministic processes in diazotrophic community assembly. The possible explanation is that low-rate manure reduced soil pH in the tested soils. Soil pH was generally regarded as an important factor driving the assembly of diazotrophs (Wang Y.et al., 2017), and the lower pH in acidic soils would aggravate the stress and strengthen environmental filtering (Feng et al., 2018). As a result, the application of low-rate manure reduced soil pH, which further caused an increasing dominance of deterministic processes in diazotrophic community assembly. Although medium-rate manure also decreased soil pH, it increased the nutrient content to a greater extent. A previous study has shown that the relative importance of stochastic processes in microbial community assembly increased with the addition of nutrients (Zhou et al., 2014), since the elevation of nutrient content was beneficial for microbial growth, which would stimulate the stochastic processes (Wang J. et al., 2020). Thus, the increasing deterministic processes by reducing soil pH was counteracted by the increasing stochastic processes by promoting nutrient content in the medium-rate manure treatment. The variation of soil pH and nutrient content after fertilization might play a crucial role in determining the assembly processes of diazotrophs (Wang J. et al., 2020). Moreover, we found that the relative importance of stochastic processes in diazotrophic community assembly was the lowest in the 250–2000 μm aggregates, indicating that aggregate sizes could also play an important role in regulating the assembly of diazotrophic communities. However, the underlying mechanisms require further investigations.
Conclusions
Our study provided novel evidence that both manure application and soil aggregation increased the abundance of nifH genes. The abundance of nifH genes in large macroaggregates was positively associated with plant biomass and peanut yield, which was not the case in microaggregates and silt and clay. Manure applications had a greater role than aggregate sizes in regulating the community structure of diazotrophs. Manure applications increased the relative abundance of Firmicutes while reduced that of α-Proteobacteria. Moreover, the assembly of diazotrophs in CK was mainly dominated by stochastic processes, and low-rate manure application increased the relative importance of deterministic processes in diazotrophic community assembly. Together, our study suggested that manure application could substantially influence the abundance, community structure and assembly process of diazotrophs in aggregates of acidic Ultisols.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
YL designed the research and draft the manuscript. GY and JF conducted the experiment and performed the data analysis. H-WH revised the manuscript. J-ZH supervised all aspects of experimentation and manuscript preparation. All authors contributed to the final version.
Funding
This work was financially supported by the National Natural Science Foundation of China (42077041 and 41930756).
Acknowledgments
The authors are grateful to Yingtan National Agro-ecosystem Field Experiment Station for providing soil samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.965293/full#supplementary-material
References
Berthrong, S. T., Yeager, C. M., Gallegos-Graves, L., Steven, B., Eichorst, S. A., Jackson, R. B., et al. (2014). Nitrogen fertilization has a stronger effect on soil nitrogen-fixing bacterial communities than elevated atmospheric CO2. Appl. Environ. Microbiol. 80, 3103–3112. doi: 10.1128/AEM.04034-13
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chen, H., Zheng, C., Qiao, Y., Du, S., Li, W., Zhang, X., et al. (2021). Long-term organic and inorganic fertilization alters the diazotrophic abundance, community structure, and co-occurrence patterns in a vertisol. Sci. Total Environ. 766, 142441. doi: 10.1016/j.scitotenv.2020.142441
Chen, L., Li, K. K., Shi, W. J., Wang, X. L., Wang, E. T., Liu, J. F., et al. (2021). Negative impacts of excessive nitrogen fertilization on the abundance and diversity of diazotrophs in black soil under maize monocropping. Geoderma 393, 114999. doi: 10.1016/j.geoderma.2021.114999
Cleveland, C. C., Townsend, A. R., Schimel, D. S., Fisher, H., Howarth, R. W., Hedin, L. O., et al. (1999). Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochem. Cycle 13, 623–645. doi: 10.1029/1999GB900014
Dini-Andreote, F., Stegen, J. C., Van Elsas, J. D., and Salles, J. F. (2015). Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA. 112, E1326–E1332. doi: 10.1073/pnas.1414261112
Dobbelaere, S., Vanderleyden, J., and Okon, Y. (2003). Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 22, 107–149. doi: 10.1080/713610853
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Eilers, K. G., Lauber, C. L., Knight, R., and Fierer, N. (2010). Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 42, 896–903. doi: 10.1016/j.soilbio.2010.02.003
Elliott, E. (1986). Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 50, 627–633. doi: 10.2136/sssaj1986.03615995005000030017x
Fan, K., Delgado-Baquerizo, M., Guo, X., Wang, D., Wu, Y., Zhu, M., et al. (2019). Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 7, 143. doi: 10.1186/s40168-019-0757-8
Feng, J., Penton, C. R., He, Z., Van Nostrand, J. D., Yuan, M. M., Wu, L., et al. (2019). Long-term warming in Alaska enlarges the diazotrophic community in deep soils. MBio 10, e02521–e02518. doi: 10.1128/mBio.02521-18
Feng, M., Adams, J. M., Fan, K., Shi, Y., Sun, R., Wang, D., et al. (2018). Long-term fertilization influences community assembly processes of soil diazotrophs. Soil Biol. Biochem. 126, 151–158. doi: 10.1016/j.soilbio.2018.08.021
Francioli, D., Schulz, E., Lentendu, G., Wubet, T., Buscot, F., and Reitz, T. (2016). Mineral vs. organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 7, 1446. doi: 10.3389/fmicb.2016.01446
Fu, F. X., Yu, E., Garcia, N. S., Gale, J., Luo, Y., Webb, E. A., et al. (2014). Differing responses of marine N2 fixers to warming and consequences for future diazotroph community structure. Aquat. Microb. Ecol. 72, 33–46. doi: 10.3354/ame01683
Gallejones, P., Castellon, A., Del Prado, A., Unamunzaga, O., and Aizpurua, A. (2012). Nitrogen and sulphur fertilization effect on leaching losses, nutrient balance and plant quality in a wheat–rapeseed rotation under a humid Mediterranean climate. Nutr. Cycl. Agroecosys. 93, 337–355. doi: 10.1007/s10705-012-9520-2
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., et al. (2004). Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226. doi: 10.1007/s10533-004-0370-0
Han, S., Li, X., Luo, X. S., Wen, S. L., Chen, W. L., and Huang, Q. Y. (2018). Nitrite-oxidizing bacteria community composition and diversity are influenced by fertilizer regimes, but are independent of the soil aggregate in acidic subtropical red soil. Front. Microbiol. 9, 885. doi: 10.3389/fmicb.2018.00885
Hu, X., Liu, J., Zhu, P., Wei, D., Jin, J., Liu, X., et al. (2018). Long-term manure addition reduces diversity and changes community structure of diazotrophs in a neutral black soil of northeast China. J. Soil Sedim. 18, 2053–2062. doi: 10.1007/s11368-018-1975-6
Jiao, S., Yang, Y., Xu, Y., Zhang, J., and Lu, Y. (2020). Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 14, 202–216. doi: 10.1038/s41396-019-0522-9
Kinnunen, M., Dechesne, A., Albrechtsen, H. J., and Smets, B. F. (2018). Stochastic processes govern invasion success in microbial communities when the invader is phylogenetically close to resident bacteria. ISME J. 12, 2748–2756. doi: 10.1038/s41396-018-0202-1
Kuypers, M. M. M., Marchant, H. K., and Kartal, B. (2018). The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 16, 263–276. doi: 10.1038/nrmicro.2018.9
Li, Y., Pan, F., and Yao, H. (2018). Response of symbiotic and asymbiotic nitrogen-fixing microorganisms to nitrogen fertilizer application. J. Soil Sedim. 19, 1948–1958. doi: 10.1007/s11368-018-2192-z
Liao, H., Li, Y., and Yao, H. (2018). Fertilization with inorganic and organic nutrients changes diazotroph community composition and N-fixation rates. J. Soil Sedim. 18, 1076–1086. doi: 10.1007/s11368-017-1836-8
Lin, Y., Ye, G., Kuzyakov, Y., Liu, D., Fan, J., and Ding, W. (2019). Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 134, 187–196. doi: 10.1016/j.soilbio.2019.03.030
Lin, Y., Ye, G., Liu, D., Ledgard, S., Luo, J., Fan, J., et al. (2018a). Long-term application of lime or pig manure rather than plant residues suppressed diazotroph abundance and diversity and altered community structure in an acidic Ultisol. Soil Biol. Biochem. 123, 218–228. doi: 10.1016/j.soilbio.2018.05.018
Lin, Y., Ye, G., Luo, J., Di, H. J., Liu, D., Fan, J., et al. (2018b). Nitrosospira cluster 8a plays a predominant role in the nitrification process of a subtropical Ultisol under long-term inorganic and organic fertilization. Appl. Environ. Microbiol. 84, e01031–e01018. doi: 10.1128/AEM.01031-18
Liu, X., Liu, C., Gao, W., Xue, C., Guo, Z., Jiang, L., et al. (2019). Impact of biochar amendment on the abundance and structure of diazotrophic community in an alkaline soil. Sci. Total Environ. 688, 944–951. doi: 10.1016/j.scitotenv.2019.06.293
Ning, D., Yuan, M., Wu, L., Zhang, Y., Guo, X., Zhou, X., et al. (2020). A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 11, 4717. doi: 10.1038/s41467-020-18560-z
Olivares, J., Bedmar, E. J., and Sanjuán, J. (2013). Biological nitrogen fixation in the context of global change. Mol. Plant Microbe Interact. 26, 486–494. doi: 10.1094/MPMI-12-12-0293-CR
Orr, C. H., Leifert, C., Cummings, S. P., and Cooper, J. M. (2012). Impacts of organic and conventional crop management on diversity and activity of free-living nitrogen fixing bacteria and total bacteria are subsidiary to temporal effects. PLoS ONE 7, e52891. doi: 10.1371/journal.pone.0052891
Pajares, S., and Bohannan, B. J. (2016). Ecology of nitrogen fxing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. 7, 1045. doi: 10.3389/fmicb.2016.01045
Pankievicz, V. C. S., do Amaral, F. P., Ane, J. M., and Stacey, G. (2021). Diazotrophic bacteria and their mechanisms to interact and benefit cereals. Mol. Plant Microbe Interact. 34, 491–498. doi: 10.1094/MPMI-11-20-0316-FI
Perez, P. G., Ye, J., Wang, S., Wang, X., and Huang, D. (2014). Analysis of the occurrence and activity of diazotrophic communities in organic and conventional horticultural soils. Appl. Soil Ecol. 79, 37–48. doi: 10.1016/j.apsoil.2014.03.006
Rahav, E., Giannetto, M., and Bar-Zeev, E. (2016). Contribution of mono and polysaccharides to heterotrophic N2 fixation at the eastern Mediterranean coastline. Sci. Rep. 6, 1–11. doi: 10.1038/srep27858
Reardon, C. L., Gollany, H. T., and Wuest, S. B. (2014). Diazotroph community structure and abundance in wheat–fallow and wheat–pea crop rotations. Soil Biol. Biochem. 69, 406–412. doi: 10.1016/j.soilbio.2013.10.038
Rieke, E. L., Soupir, M. L., Moorman, T. B., Yang, F., and Howe, A. C. (2018). Temporal dynamics of bacterial communities in soil and leachate water after swine manure application. Front. Microbiol. 9, 3197. doi: 10.3389/fmicb.2018.03197
Shcherbak, I., Millar, N., and Robertson, G. P. (2014). Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA. 111, 9199–9204. doi: 10.1073/pnas.1322434111
Shi, W., Zhao, H. Y., Chen, Y., Wang, J. S., Han, B., Li, C. P., et al. (2021). Organic manure rather than phosphorus fertilization primarily determined asymbiotic nitrogen fixation rate and the stability of diazotrophic community in an upland red soil. Agric. Ecosyst. Environ. 319, 107535. doi: 10.1016/j.agee.2021.107535
Shin, W., Islam, R., Benson, A., Joe, M. M., Kim, K., Gopal, S., et al. (2016). Role of diazotrophic bacteria in biological nitrogen fixation and plant growth improvement. Korean J. Soil Sci. Fert. 49, 17–29. doi: 10.7745/KJSSF.2016.49.1.017
Skonieski, F. R., Viégas, J., Martin, T. N., Mingotti, C. C. A., Naetzold, S., Tonin, T. J., et al. (2019). Effect of nitrogen topdressing fertilization and inoculation of seeds with Azospirillum brasilense on corn yield and agronomic characteristics. Agronomy 9, 812. doi: 10.3390/agronomy9120812
Smith, A. P., Marín-Spiotta, E., de Graaff, M. A., and Balser, T. C. (2014). Microbial community structure varies across soil organic matter aggregate pools during tropical land cover change. Soil Biol. Biochem. 77, 292–303. doi: 10.1016/j.soilbio.2014.05.030
Tang, H., Zhang, N., Ni, H., Xu, X., Wang, X., Sui, Y., et al. (2021). Increasing environmental filtering of diazotrophic communities with a decade of latitudinal soil transplantation. Soil Biol. Biochem. 154, 108119. doi: 10.1016/j.soilbio.2020.108119
Teng, Q., Sun, B., Fu, X., Li, S., Cui, Z., and Cao, H. (2009). Analysis of nifH gene diversity in red soil amended with manure in Jiangxi, South China. J. Microbiol. 47, 135–141. doi: 10.1007/s12275-008-0184-1
Töwe, S., Albert, A., Kleineidam, K., Brankatschk, R., Dümig, A., Welzl, G., et al. (2010). Abundance of microbes involved in nitrogen transformation in the rhizosphere of Leucanthemopsis alpina (L.) Heywood grown in soils from different sites of the Damma glacier forefield. Microb. Ecol. 60, 762–770. doi: 10.1007/s00248-010-9695-5
Tripathi, B. M., Stegen, J. C., Kim, M., Dong, K., Adams, J. M., and Lee, Y. K. (2018). Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 12, 1072–1083. doi: 10.1038/s41396-018-0082-4
Trivedi, P., Delgado-Baquerizo, M., Jeffries Thomas, C., Trivedi, C., Anderson Ian, C., Lai, K., et al. (2017). Soil aggregation and associated microbial communities modify the impact of agricultural management on carbon content. Environ. Microbiol. 19, 3070–3086. doi: 10.1111/1462-2920.13779
Trivedi, P., Rochester, I. J., Trivedi, C., Van Nostrand, J. D., Zhou, J., Karunaratne, S., et al. (2015). Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 91, 169–181. doi: 10.1016/j.soilbio.2015.08.034
Wang, C., Zheng, M., Song, W., Wen, S., Wang, B., Zhu, C., et al. (2017). Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 113, 240–249. doi: 10.1016/j.soilbio.2017.06.019
Wang, C., Zheng, M. M., and Shen, R. F. (2020). Diazotrophic communities are more responsive to maize cultivation than phosphorus fertilization in an acidic soil. Plant Soil 452, 499–512. doi: 10.1007/s11104-020-04596-z
Wang, J., Li, Q., Shen, C., Yang, F., Wang, J., and Ge, Y. (2020). Significant dose effects of fertilizers on soil diazotrophic diversity, community composition, and assembly processes in a long-term paddy field fertilization experiment. Land Degrad. Dev. 32, 420–429. doi: 10.1002/ldr.3736
Wang, X., Bian, Q., Jiang, Y., Zhu, L., Chen, Y., Liang, Y., et al. (2021). Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil Biol. Biochem. 153, 108062. doi: 10.1016/j.soilbio.2020.108062
Wang, X., Liu, B., Ma, J., Zhang, Y., Hu, T., Zhang, H., et al. (2019). Soil aluminum oxides determine biological nitrogen fixation and diazotrophic communities across major types of paddy soils in China. Soil Biol. Biochem. 131, 81–89. doi: 10.1016/j.soilbio.2018.12.028
Wang, Y., Li, C., Kou, Y., Wang, J., Tu, B., Li, H., et al. (2017). Soil pH is a major driver of soil diazotrophic community assembly in Qinghai-Tibet alpine meadows. Soil Biol. Biochem. 115, 547–555. doi: 10.1016/j.soilbio.2017.09.024
Wilpiszeski, R. L., Aufrecht, J. A., Retterer, S. T., Sullivan, M. B., Graham, D. E., Pierce, E. M., et al. (2019). Soil aggregate microbial communities: towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 85, e00324–e00319. doi: 10.1128/AEM.00324-19
Xun, W., Li, W., Huang, T., Ren, Y., Xiong, W., Miao, Y., et al. (2018). Long-term agronomic practices alter the composition of asymbiotic diazotrophic bacterial community and their nitrogen fixation genes in an acidic red soil. Biol. Fert. Soils 54, 329–339. doi: 10.1007/s00374-018-1264-y
Xun, W., Zhao, J., Xue, C., Zhang, G., Ran, W., Wang, B., et al. (2016). Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol. 18, 1907–1917. doi: 10.1111/1462-2920.13098
Yang, L., Bai, J., Zeng, N., Zhou, X., Liao, Y., Lu, Y., et al. (2019). Diazotroph abundance and community structure are reshaped by straw return and mineral fertilizer in rice-rice-green manure rotation. Appl. Soil Ecol. 136, 11–20. doi: 10.1016/j.apsoil.2018.12.015
Ye, G., Banerjee, S., He, J. Z., Fan, J., Wang, Z., Wei, X., et al. (2021). Manure application increases microbiome complexity in soil aggregate fractions: results of an 18-year field experiment. Agric. Ecosyst. Environ. 307, 107249. doi: 10.1016/j.agee.2020.107249
Ye, G., Fan, J., Hu, H. W., Chen, J., Zhong, X., Chen, J., et al. (2022). Short-term cellulose addition decreases microbial diversity and network complexity in an Ultisol following 32-year fertilization. Agric. Ecosyst. Environ. 325, 107744. doi: 10.1016/j.agee.2021.107744
Ye, G., Lin, Y., Liu, D., Chen, Z., Luo, J., Bolan, N., et al. (2019). Long-term application of manure over plant residues mitigates acidification, builds soil organic carbon and shifts prokaryotic diversity in acidic Ultisols. Appl. Soil Ecol. 133, 24–33. doi: 10.1016/j.apsoil.2018.09.008
Zheng, M., Zhou, Z., Luo, Y., Zhao, P., and Mo, J. (2019). Global pattern and controls of biological nitrogen fixation under nutrient enrichment: a meta-analysis. Global Change Biol. 25, 3018–3030. doi: 10.1111/gcb.14705
Zheng, W., Zhao, Z., Gong, Q., Zhai, B., and Li, Z. (2018). Responses of fungal–bacterial community and network to organic inputs vary among different spatial habitats in soil. Soil Biol. Biochem. 125, 54–63. doi: 10.1016/j.soilbio.2018.06.029
Zheng, W., Zhao, Z., Lv, F., Wang, R., Wang, Z., Zhao, Z., et al. (2021). Assembly of abundant and rare bacterial and fungal sub-communities in different soil aggregate sizes in an apple orchard treated with cover crop and fertilizer. Soil Biol. Biochem. 156, 108222. doi: 10.1016/j.soilbio.2021.108222
Zhou, J., Deng, Y., Zhang, P., Xue, K., Liang, Y., Van Nostrand, J. D., et al. (2014). Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci. USA. 111, E836–E845. doi: 10.1073/pnas.1324044111
Keywords: diazotroph, soil aggregates, manure application, assembly process, agroecosystem
Citation: Lin Y, Ye G, Hu H-W, Fan J and He J-Z (2022) Manure applications alter the abundance, community structure and assembly process of diazotrophs in an acidic Ultisol. Front. Microbiol. 13:965293. doi: 10.3389/fmicb.2022.965293
Received: 09 June 2022; Accepted: 27 July 2022;
Published: 12 August 2022.
Edited by:
Olli H. Tuovinen, The Ohio State University, United StatesReviewed by:
Chris Sedlacek, University of Vienna, AustriaSarah Shawver, Columbia College, United States
Copyright © 2022 Lin, Ye, Hu, Fan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianbo Fan, amJmYW5AaXNzYXMuYWMuY24=; Ji-Zheng He, anpoZUBmam51LmVkdS5jbg==
 Yongxin Lin
Yongxin Lin Guiping Ye2
Guiping Ye2 Hang-Wei Hu
Hang-Wei Hu