- 1The First College of Clinical Medical Science, China Three Gorges University, Yichang, China
- 2Hubei Key Laboratory of Tumor Microenvironment and Immunotherapy, Medical College, China Three Gorges University, Yichang, China
- 3The Institute of Infection and Inflammation, Medical College, China Three Gorges University, Yichang, China
- 4Affiliated Second People’s Hospital of China Three Gorges University, Yichang, China
- 5Department of Medical Biochemistry and Microbiology, Uppsala University, Uppsala, Sweden
- 6The People’s Hospital of China Three Gorges University, Yichang, China
Yersinia pseudotuberculosis is a foodborne zoonotic bacterium that is pathogenic to guinea pigs, rabbits, and mice. It also causes pseudotuberculosis in humans. However, it still lacked the scientific basis for control. Here, we found out that Ebselen (EbSe) exhibited synergistic antibacterial activity with silver nitrate (Ag+) against Y. pseudotuberculosis YpIII strain with high efficacy in vitro using UV-visible light absorption spectrum, 5,5’-dithiobis-(2-nitrobenzoic acid), laser scanning confocal microscope, flow cytometry, transmission electron microscopy and Western blotting assays. The depletion of total glutathione (GSH) amount and inhibition of thioredoxin reductase (TrxR) activity in thiol-dependent redox system revealed the destructiveness of EbSe-Ag+-caused intracellular oxidative stress. Furthermore, a YpIII-caused mice gastroenteritis model was constructed. EbSe-Ag+ significantly reduced bacterial loads with low toxicity. It also down-regulated the expression levels of interferon (IL)-1β and tumor necrosis factor-α, up-regulated the expression level of IL-10 on-site. All the in vivo results demonstrated the antibacterial activity and immune-modulatory property of EbSe-Ag+. Collectively, these results provided academic fundament for further analysis and development of EbSe-Ag+ as the antibacterial agents for pseudotuberculosis control.
Introduction
Yersiniosis, one of the “other infectious diarrheas” in humans, is an important foodborne zoonosis with wide range of clinical symptoms caused by the enteric pathogens Yersinia enterocolitica and Yersinia pseudotuberculosis, which is the third most common bacterial enteritis disease in European countries (Mecsas, 2019). Although human Y. pseudotuberculosis infections are less frequent than those caused by Y. enterocolitica worldwide, it has a wide animal reservoir, mostly in temperate and cold countries (Voskresenskaya et al., 2014). Pseudotuberculosis in animals can lead to tuberculosis-like symptoms, including localized tissue necrosis and granulomas in the liver, spleen, and lymph nodes (Aswal et al., 2020). Y. pseudotuberculosis infections in humans are acquired through the gastrointestinal tract by the ingestion of contaminated food products and the clinical manifestations of pseudotuberculosis are diarrhea, abdominal pain, and fever (Ohnishi et al., 2021; Rivas et al., 2021). The frequency of human infection caused by certain yersinia subgroups might be related to the frequency of exposure to specific animal sources (Le Guern et al., 2016). In some specific areas, Y. pseudotuberculosis may also cause a specific disease known as Far East scarlet-like fever for its clinical similarities to scarlet fever caused by group A streptococci (Dakić et al., 2005; Amphlett, 2016). Such atypical infections are severe, characterized by a strong inflammatory syndrome accompanying intestinal disorders (Eppinger et al., 2007).
As a “generalist,” Y. pseudotuberculosis deploys sophisticated virulence factors to effectively evade the host immune system (Bergsbaken et al., 2009; Lima-Junior et al., 2013; Reinhardt et al., 2018; Schneiders et al., 2021), and host factors are also essential for pathogen-induced cell death (Mares et al., 2021). Hypoxic environment of the intestine is critical for nutrient absorption, intestinal barrier function, and innate and adaptive immune responses in the intestine (Singhal and Shah, 2020). The oxygen deficiency during growth promotes increasing of pathogenic potential of Y. pseudotuberculosis (Bakholdina et al., 2009), the outcomes of artificial oxidative stress arouse our curiosity.
Known as thiol dependent redox system (TDRS), thioredoxin (Trx) and glutathione (GSH) systems are vital defenders against oxidative stress (Ren et al., 2020). Trx system consists of Trx and thioredoxin reductase (TrxR); The GSH system be formed from GSH, glutathione reductase (GR) and glutaredoxin (Grx). Both systems work as backup to maintain the intracellular redox homeostasis, participating in signal transduction and regulation, DNA repair and protein synthesis and repair, oxidation resistance and other biological processes. Ultimately, TDRS affects bacterial survival and death, activation, and proliferation (Ren et al., 2018, 2020).
Our previous work showed that a non-toxic seleno-organic drug, Ebselen (EbSe), has antibacterial effect against Gram-positive bacteria (Lu et al., 2013; Dong et al., 2019); meanwhile, it could work synergistically with silver nitrate (Ag+) to kill a couple of Gram-negative bacteria such as multidrug-resistant Escherichia coli (Zou et al., 2017) and Acinetobacter baumannii (Dong et al., 2020) by disrupting the TDRS and promoting the oxidative stress in bacteria (Zou et al., 2018; Dong et al., 2019). Thus, the in vitro and in vivo outcomes of EbSe-Ag+ against Y. pseudotuberculosis YpIII strain have been extensively investigated to develop its scientific basis for pseudotuberculosis control.
Materials and Methods
Mouse and Bacterial Strains
Kunming mice (male, 22–25 g) were purchased from China Three Gorges University, and the ethical permit approval of the Medical Animal Care and Welfare Committee of China Three Gorges University was obtained before using the mice for study. Every five mice were kept in an individual cage with a constant dark-light cycle in a conventional SPF animal house and were given free access to fundamental food as normal diet and water.
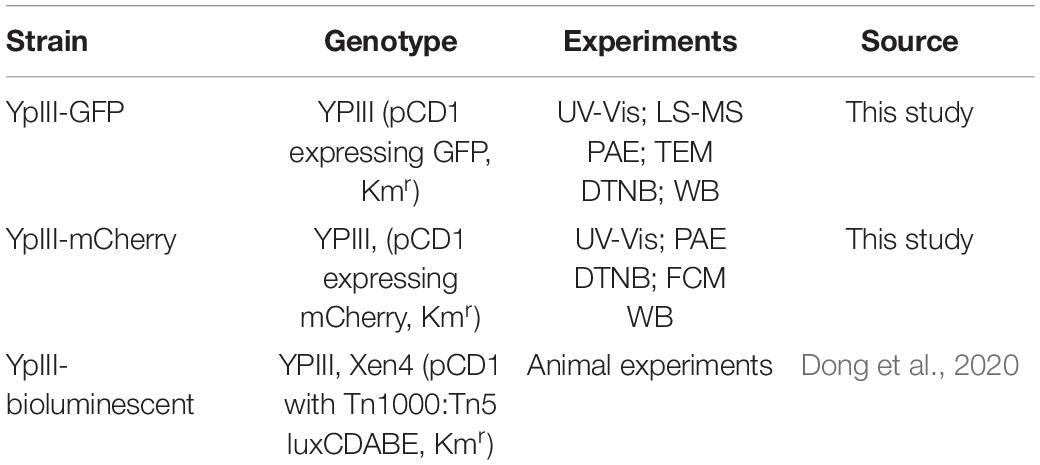
Yersinia pseudotuberculosis YpIII strains with different reporter systems on the virulence plasmid were constructed as described previously (Wang et al., 2016) and listed in Table 1. All experiments were carried out in the BSL2 laboratory.
Reagents
Luria Bertani (LB) medium (EMD Millipore), 2-phenyl-1, 2-benzisoselenazol-3(2H)-one (EbSe) (Daiichi), protease inhibitor cocktails (Roche), anti-Trx1 polyclonal antiserum (IMCO), rabbit anti-sheep IgG-HRP (Santa Cruz), goat anti-mouse H&L antibodies (Santa Cruz), IgG2a mouse monoclonal antibody (VIROGEN), CellROX™ Deep Red Reagent (Invitrogen), Anti-IL-1β antibody (Proteintech), anti-TNF-α antibody (Proteintech), anti-IL-10 antibody (Proteintech). Protein inhibitor cocktail (MedChemExpress). Silver nitrate and all the other reagents were from Sigma-Aldrich.
Inhibitory Effect of EbSe-Ag+ Against YpIII
The inhibitory effect of EbSe-Ag+ on the growth of YpIII was measured by UV-Vis at A600. YpIII cells were grown (26°C, 200 rpm) 8 h and diluted 1,000 times and treated with a serial concentration of EbSe-Ag+ for 24 h at 26°C in a 96-well plate, and the A600 were measured.
Bactericidal Effect of EbSe-Ag+ Against YpIII
The bactericidal activity of EbSe-Ag+ against YpIII was detected by laser scanning confocal microscopy (LSCM, A1R+, Nikon). YpIII cells were grown (26°C, 200 rpm) until an A600 of 0.4, and diluted 1,000 times, which were treated with 4 μM EbSe and 0.5 μM Ag+ for 24 h at 26°C. The bactericidal effect was observed by LSCM.
EbSe-Ag+ on YpIII Bacterial Morphology
YpIII was grown until an A600 of 0.4 and treated for 30 min with 80 μM EbSe and 5 μM Ag+. Cells were obtained by centrifugation (4°C, 12,000 rpm, 15 min) and fixed with 2.5% glutaraldehyde. The morphology of YpIII cells was observed by transmission electron microscopy (TEM, Hitachi H-7500).
Post-antibiotic Effect of EbSe-Ag+ Against YpIII
The post-antibiotic effect (PAE) of EbSe-Ag+ against YpIII was detected by UV-Vis. YpIII cells were grown (26°C, 200 rpm) until an A600 of 0.4, and diluted 1,000 times. Further, cells were treated with 4 μM EbSe and 0.5 μM Ag+ for 32 h at 26°C, 200 rpm. Then, drugs were removed by centrifugation (4°C, 10,000 rpm, 2 min) for three times using PBS (pH 7.4). The A600 of YpIII cells were further measured every 1 h for 6 h.
The Rescue Effect of DTT Against EbSe-Ag+-Treated YpIII
YpIII cells were grown (26°C, 200 rpm) until an A600 of 0.4, and diluted 1,000 times, which were treated with 4 μM EbSe and 0.5 μM Ag+ after pre-incubation with 4 μM dithiothreitol (DTT) for 30 min at 26°C, 200 rpm. The A600 of YpIII cells were measured.
The Disruption of Thioredoxin Reductase Activity and Glutathione Amount by EbSe-Ag+
YpIII cells were cultured until an A600 of 0.4 and incubated with 80 μM EbSe and 5 μM Ag+ for 30 min. YpIII cells were obtained by centrifugation (4°C, 5,000 rpm, 5 min), and a protein inhibitor cocktail (in 50 mmol/L Tris-EDTA buffer, pH 7.4) was added to decrease the protease activity. The cells were disrupted with sonication (240 W, 10 min), and the supernatants were obtained by centrifugation (4°C, 12,000 rpm, 10 min). The TrxR activity and total glutathione amount were detected in a 96-well plate by 5, 5’-dithiobis-(2-nitrobenzoic acid) assay. The activity of the untreated group was 100%. The protocols were performed as previously described (Lu et al., 2013; Zou et al., 2017; Wang P. et al., 2020).
Protein Expression Level of Thioredoxin 1 Upon Treatment With EbSe-Ag+
YpIII cells were cultured until an A600 of 0.4, which were incubated with 80 μM EbSe and 5 μM Ag+ for 30 min. After lysis by sonication, the cell lysates were obtained by centrifugation at 12,000 rpm for 20 min. Western blotting assay was performed with anti-Trx1 polyclonal antibody.
Protein S-glutathionylation Level Upon Treatment With EbSe-Ag+
YpIII cells were cultured until an A600 of 0.4, which were incubated with 80 μM EbSe and 5 μM Ag+ for 30 min. After lysis by sonication, the cell lysates were obtained by centrifugation at 12,000 rpm for 20 min. Total protein S-glutathionylation (S-PSSG) of 80 μM EbSe and 5 μM Ag+-treated YpIII cells were detected by Western blotting. Cells were cultured, washed, and resuspended in buffer containing 30 mmol/L Iodoacetamide (IAM). Western blotting assay was performed with IgG2a mouse monoclonal antibody for glutathione-protein complexes.
ROS Production by EbSe-Ag+ Treatment
YpIII were grown until an A600 of 0.4 in LB medium and incubated with 80 μM EbSe and 5 μM Ag+ for 30 min. The YpIII cells were stained with 5 μM CellROX™ Deep Red Reagent for 30 min at 37°C. After incubation, the reactive oxygen species (ROS) production was quantified by flow cytometry (BECKMAN COULTER, AW15093).
Acute Mice Gastroenteritis Model
All experiments were performed in accordance with the relevant guidelines and regulations approved by China Three Gorges University. 60 healthy male Kunming mice were randomly divided into 5 groups randomly (n = 12). The mice were infected via intragastric gavage with 100 μL 2 × 109CFU of YpIII as previously described (Wang et al., 2016) and were further administered i.p. with 20 mg/kg EbSe, 5 mg/kg Ag+, 20 mg/kg EbSe and 5 mg/kg Ag+ and DMSO on the 1-, 3-, and 5-days post-infection, separately.
Bioluminescent Imaging Analysis
Mice were deprived of food and water for 16 h prior to intragastric administration with YpIII-bioluminescent and were monitored for bioluminescent emission using IVIS lumina II (Caliper LifeSciences) at 1-, 4-, and 7-days post-infection. Mice were anesthetized using the XGI-8 gas anesthesia system (Caliper LifeSciences) prior to imaging with 2.5% IsoFluVet in oxygen (Orion Pharma Abbott Laboratories Ltd., Great Britain), and during imaging in 0.5% IsoFluVet. Images were acquired and analyzed using Living Image 4.5 (Caliper LifeSciences). To analyze bacterial localization within gastrointestinal tracts, mice were euthanized, the gastrointestinal tracts were removed, and imaged by bioluminescent imaging.
Immunohistochemical Analysis
Mice intestinal tracts were rolled, fixed in formalin (10%), and embedded in paraffin. Paraffin sections (4 μm thick) were used to detect cytokine levels by H&E stain and immunohistochemical (IHC) analysis. Anti-IL-1β, anti-TNF-α and anti-IL-10 antibodies were used for the analysis of cytokines in intestinal tracts.
Blood Routine Analysis
Mice blood was collected 7 days post-infection, and the white blood cells (WBCs) and neutrophils counts were detected. For investigation of liver and kidney function, blood was collected and centrifuged at 3,000 rpm for 10 min. Serum alanine transaminase (ALT), aspartate aminotransferase (AST), urea and creatinine were determined by fully automatic biochemical analyzer (Siemens, viva proE).
Statistical Analysis
Statistical analyses were performed by GraphPad Prism 6.0 (GraphPad Software). Means of data between two groups were contrasted using unpaired Student’s t test. Sample rates between two groups were tested with chi-square analysis. p-values of < 0.05 were significant.
Results
EbSe-Ag+ Has Strong Bactericidal Activity Against YpIII
The antibacterial effects of EbSe-Ag+ on the growth of YpIII-GFP and YpIII-mCherry were detected by UV-Vis. As shown in Figure 1, Ag+ alone inhibited YpIII growth with a 90% minimal inhibition concentration (MIC90) of 8 μM, while the addition of 2 μM EbSe effectively reduced the MIC90 of Ag+ to 0.5 μM (16 times). Meanwhile, 2 μM EbSe and 0.5 μM Ag+ showed no synergistic toxicity on human cells as described previously (Zou et al., 2017). In addition, the formula S = (FX0/F00) * (FY0/F00) – (FXY/F00) was used to calculate the synergistic effect of EbSe-Ag+. The results showed that the synergy indexes were 0.97 and 0.94, respectively, indicating EbSe-Ag+ has a strong synergistic antibacterial effect against YpIII (Supplementary Figure 1).
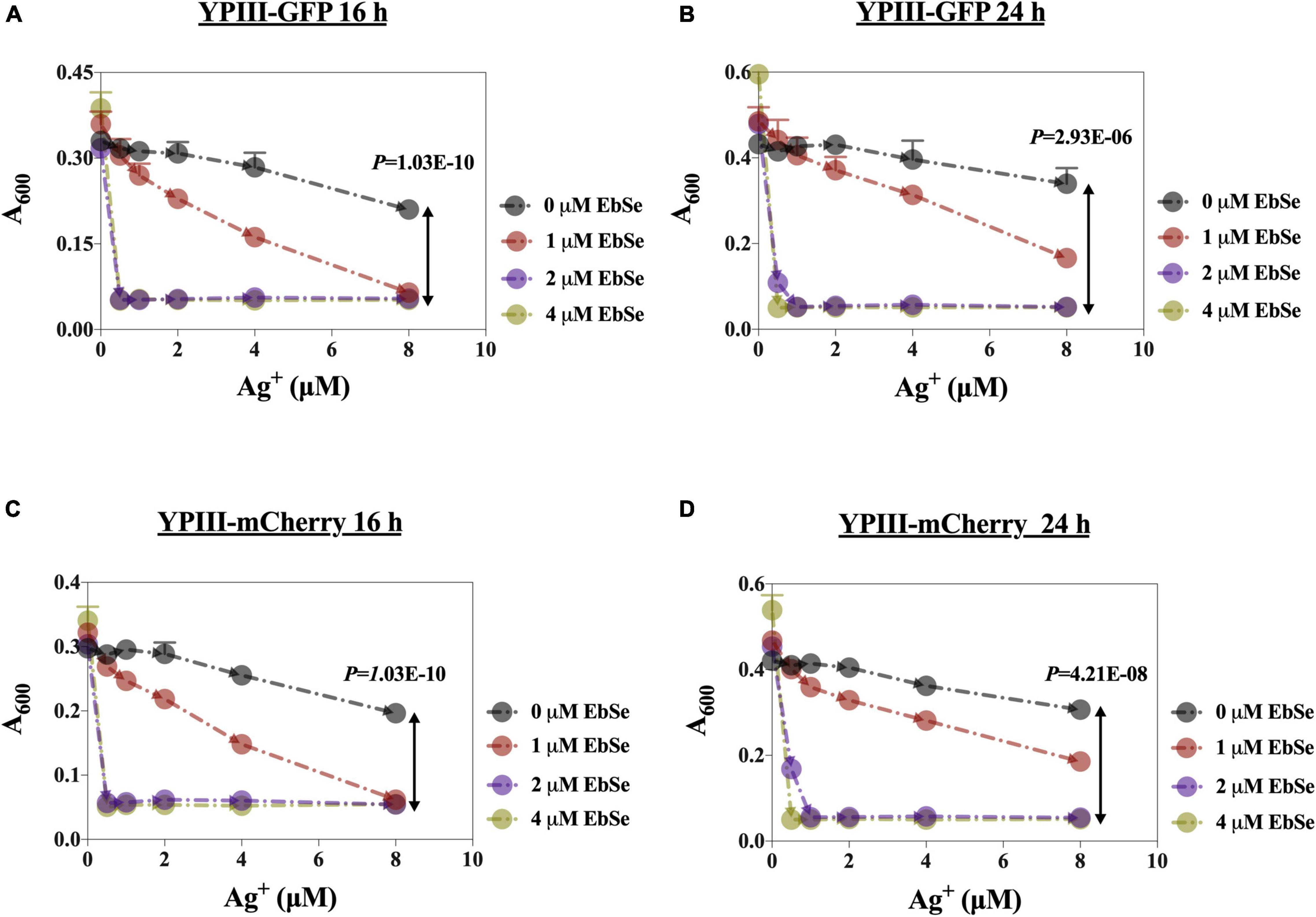
Figure 1. EbSe-Ag+ shows synergistic antibacterial activity against YpIII. YpIII-GFP and YpIII-mCherry were cultured to A600 of 0.4, diluted 1:1,000 times, and treated with EbSe-Ag+. (A,C) At 16 h; (B,D) at 24 h; (A,B) YpIII-GFP; (C,D) YpIII-mCherry.
Whether the antibacterial effect of EbSe-Ag+ is bacteriostatic or bactericidal was further investigated by LSCM and bacterial plating. 4 μM EbSe and 1 μM Ag+ were inoculated with YpIII-GFP and observed by LSCM (Figure 2). Meanwhile, the minimum bactericidal concentration (MBC) was calculated by plating (Supplementary Figure 2). Both results showed that the combination of 4 μM EbSe and 1 μM Ag+ has significant bactericidal effects against YpIII-GFP.
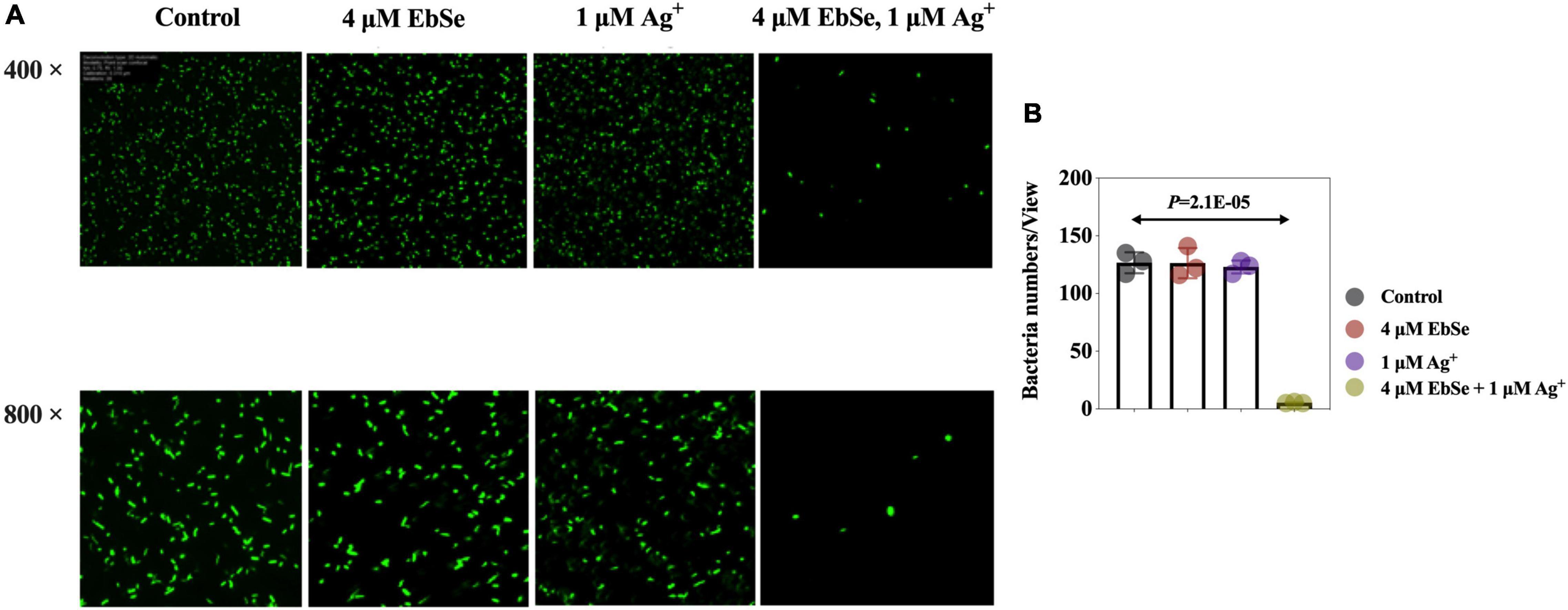
Figure 2. EbSe-Ag+ has a bactericidal effect against YpIII. YpIII-GFP were cultured to A600 of 0.4, diluted 1:1,000 times, and treated with either EbSe, Ag+ or EbSe-Ag+. (A) LSCM results showed EbSe-Ag+ has a bactericidal effect against YPIII-GFP; (B) quantitative values.
Further, the effect of EbSe-Ag+ on the morphology of YpIII was assessed by TEM. Normal YpIII-GFP has a smooth surface and a complete cell membrane and cell wall. After 30 min treatment with 80 μM EbSe and 5 μM Ag+, YpIII-GFP cells were deformed, cell membranes and cell walls were ruptured, and overflow of cell contents; meanwhile, 80 μM EbSe or 5 μM Ag+ treated cells showed no obvious morphological changes (Figure 3). The results showed that the morphology of YpIII-GFP changed significantly in EbSe-Ag+ treated group compared with the control group.
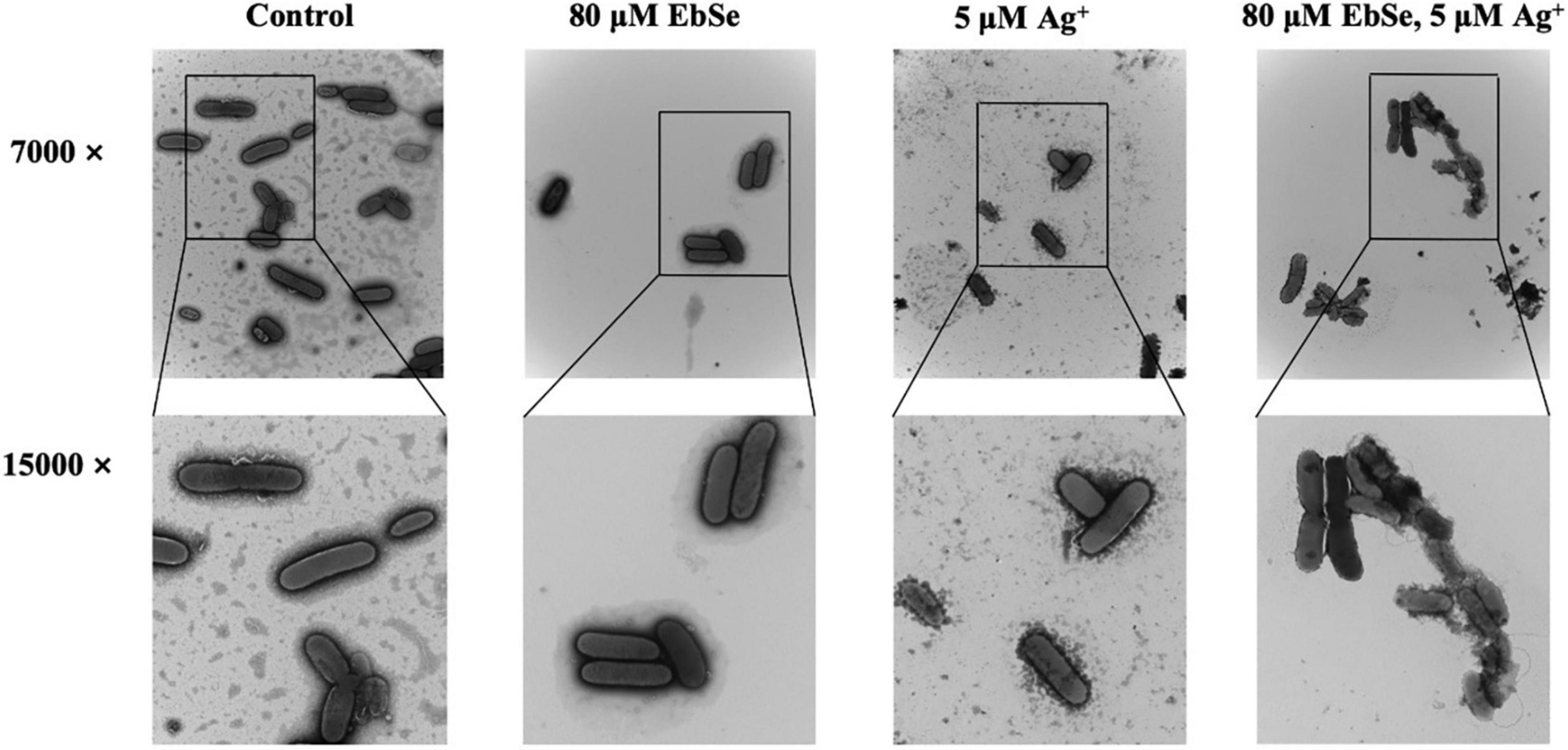
Figure 3. EbSe-Ag+ influences YpIII morphology. YpIII-GFP were cultured to A600 of 0.4 and treated with EbSe-Ag+. TEM results showed EbSe-Ag+ influences YPIII-GFP morphology.
EbSe-Ag+ Has Outstanding Post-antibiotic Effect Against YpIII
YpIII was cultured and co-cultivated with 8 μM EbSe and 0.5 μM Ag+ for 32 h. During this period, the A600 was measured every 4 h. 32 h post-treatment, the bacterial solution was centrifuged and washed in PBS by 3 times, and resuspended in LB. The A600 was further detected every 1 h for 6 h. The results show that the post-antibiotic effect (PAE) of EbSe-Ag+ was 4 h, which is longer than many clinical antibiotics, including quinolone (Figure 4).
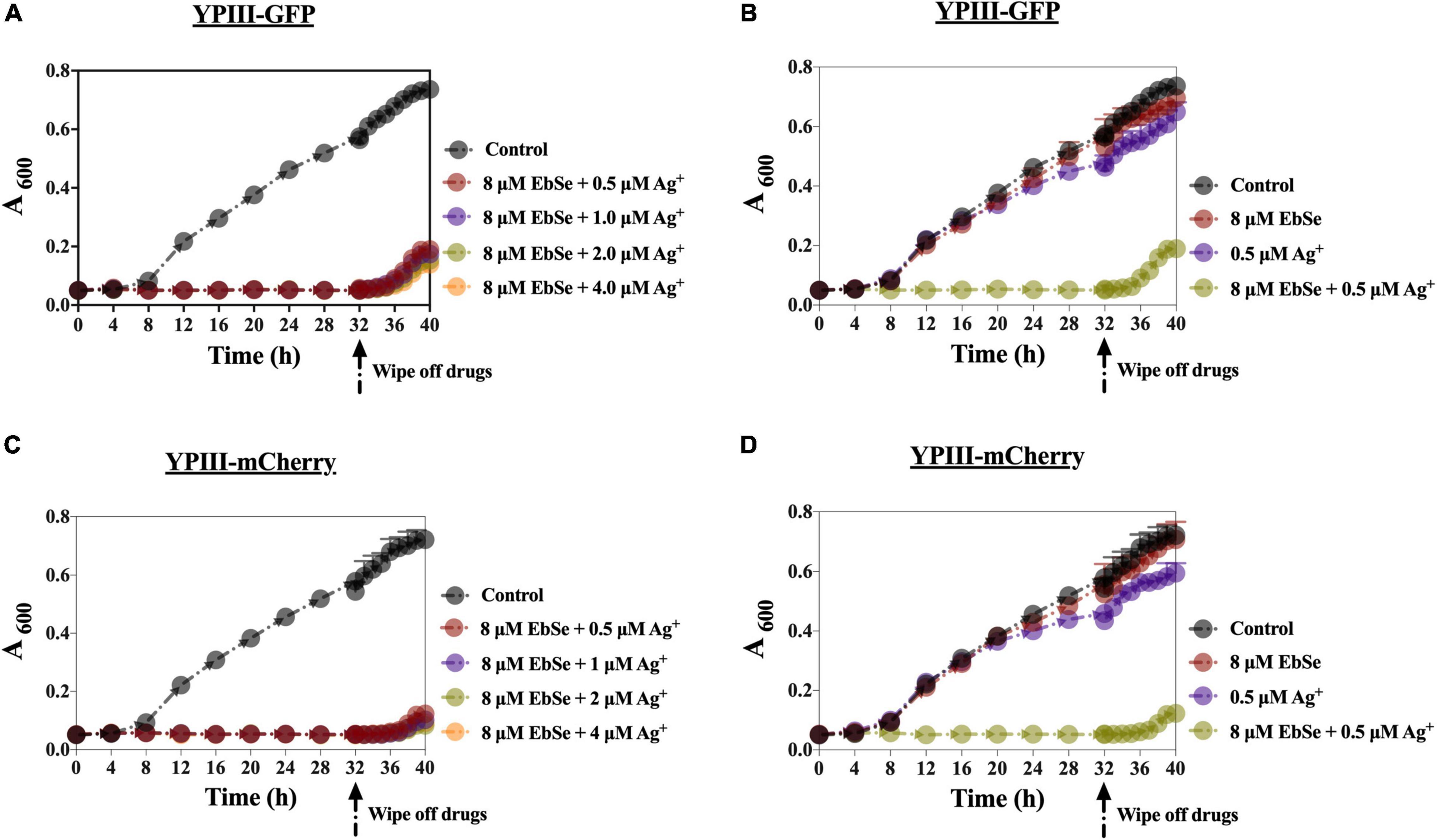
Figure 4. The PAE effect of EbSe-Ag+ against YpIII. YpIII-GFP and YpIII-mCherry were cultured to A600 of 0.4, diluted 1:1,000 times, and treated with EbSe-Ag+. The results showed EbSe-Ag+ has a PAE of 4 h. (A,C) 8 μM EbSe and a serial concentration of Ag+; (B,D) 8 μM EbSe and 0.5 μM Ag+; (A,B) YpIII-GFP; (C,D) YpIII-mCherry.
DTT Rescues YpIII From EbSe-Ag+ Treatment
The protective effect of DTT against EbSe-Ag+-treated YpIII was evaluated by UV-Vis and TEM assays. DTT was an organic reducing agent that can remove ROS, and 4 mmol/L DTT can effectively rescue YpIII from the bactericidal effect of 4 μM EbSe and 1 μM Ag+ (Figures 5A–F). Meanwhile, the TEM results showed that DTT could protect YpIII from EbSe-Ag+ caused morphological damage (Figure 5G). Both results indicated that the death of YpIII caused by EbSe-Ag+ was related to ROS production.
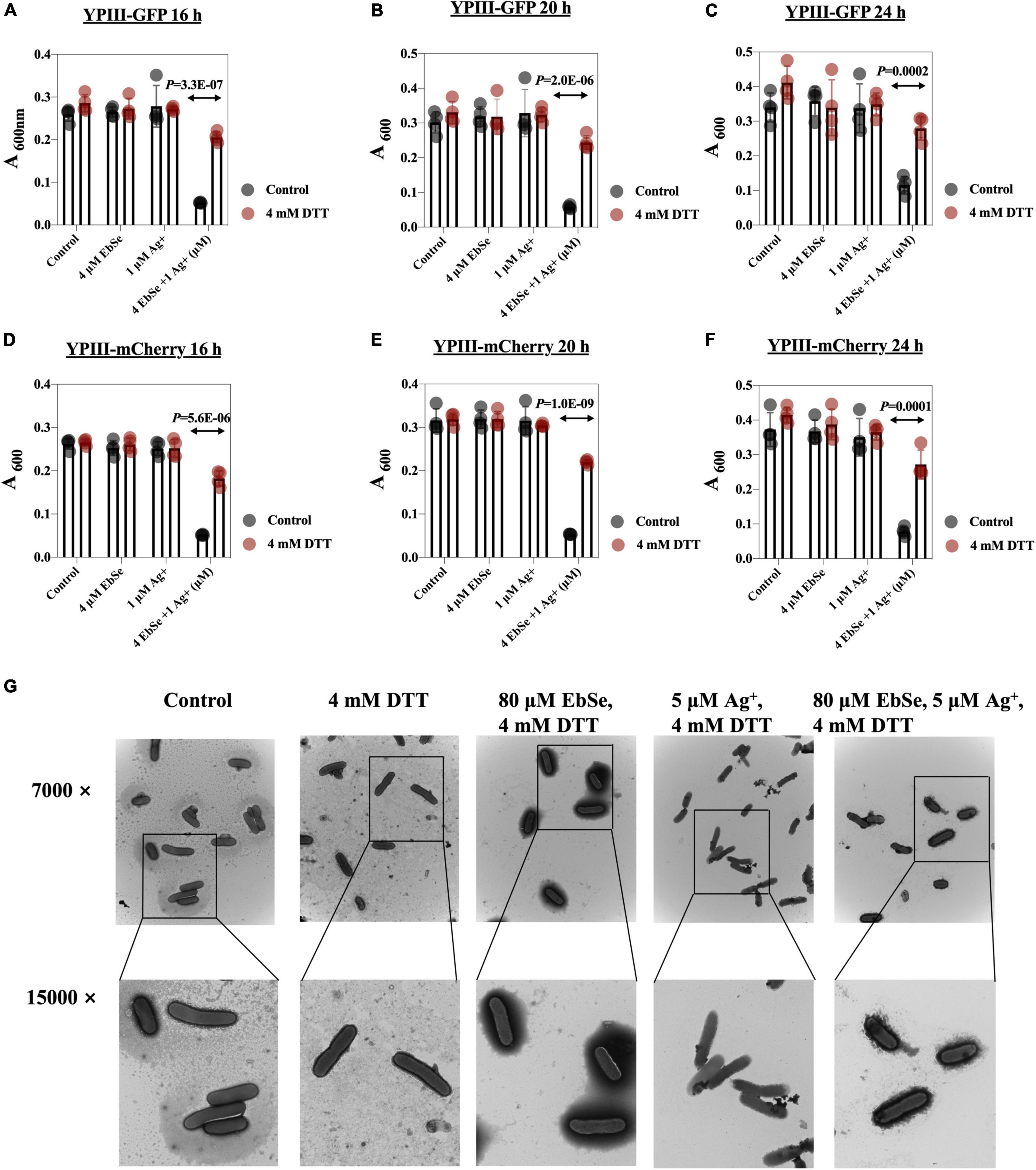
Figure 5. DTT protects YpIII from EbSe-Ag+. YpIII-GFP and YpIII-mCherry were cultured to A600 of 0.4, diluted 1:1,000 times, and treated with DTT followed with EbSe-Ag+. (A,D) At 16 h; (B,E) at 20 h; (C,F) at 24 h; (A–C) YpIII-GFP; (B–E) YpIII-mCherry. (G) YpIII-GFP was cultured to 0.4 and treated with EbSe-Ag+ with DTT, and the morphological changes were observed by TEM.
EbSe-Ag+ Up-Regulates the Intracellular ROS Level in YpIII
The mean fluorescent intensity (MFI) of ROS in YpIII-mCherry cells was detected by flow cytometry (Ezraty et al., 2017; Zou et al., 2017). The result showed that the ROS production level in YpIII cells treated with 80 μM EbSe and 5 μM Ag+ was significantly regulated when compared with the control (Figure 6).
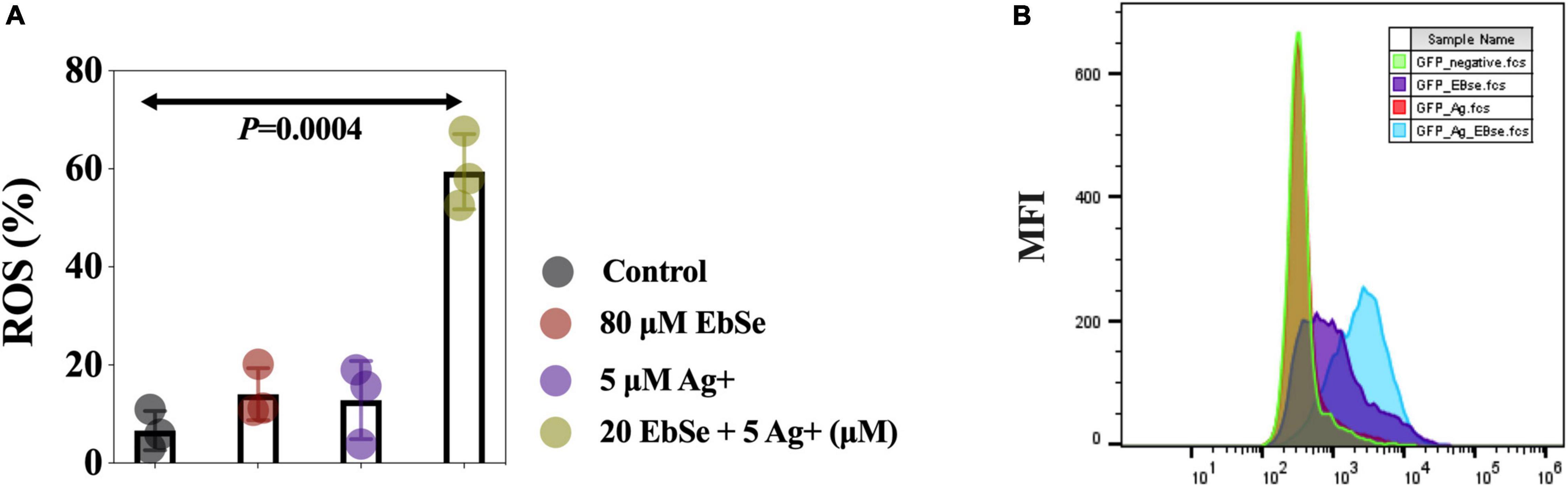
Figure 6. EbSe-Ag+ upregulates ROS production in YpIII. YpIII-mCherry was cultured to A600 of 0.4 and treated with EbSe-Ag+. (A) Cells were stained with 5 μM CellROX™ Deep Red Reagent for 30 min at 37°C and detected by FCM; (B) quantitative values.
EbSe-Ag+ Disrupts YpIII Thiol-Dependent Redox System
The effects of EbSe-Ag+ on bacterial TrxR activity and GSH amount in YpIII were measured by DTNB assay. The results showed that the combination of 80 μM EbSe and 5 μM Ag+ could efficiently inhibit the TrxR activity and deplete GSH amount when compared with the control (Figures 7A–D).

Figure 7. EbSe-Ag+ inhibits TrxR activity and depletes GSH in YpIII. YpIII-GFP and YpIII-mCherry were cultured to A600 of 0.4 and treated with EbSe-Ag+. DTNB assay was used to detect the TrxR activity and GSH amount. (A,B) At 30 min; (C,D) 0–5 min slope; (A,C) YpIII-GFP; (B,D) YpIII-mCherry.
Whether EbSe-Ag+ could affect protein Trx1 and S-PSSG expression levels were also analyzed by Western blot (Figures 8A–H). The result showed that Trx1 protein expression level had no difference, indicating the treatment of EbSe-Ag+ only influences enzyme activity. Meanwhile, protein S-PSSG was reduced by EbSe-Ag+ treatment compared with Ag+ or EbSe, which further reflected the loss of GSH.
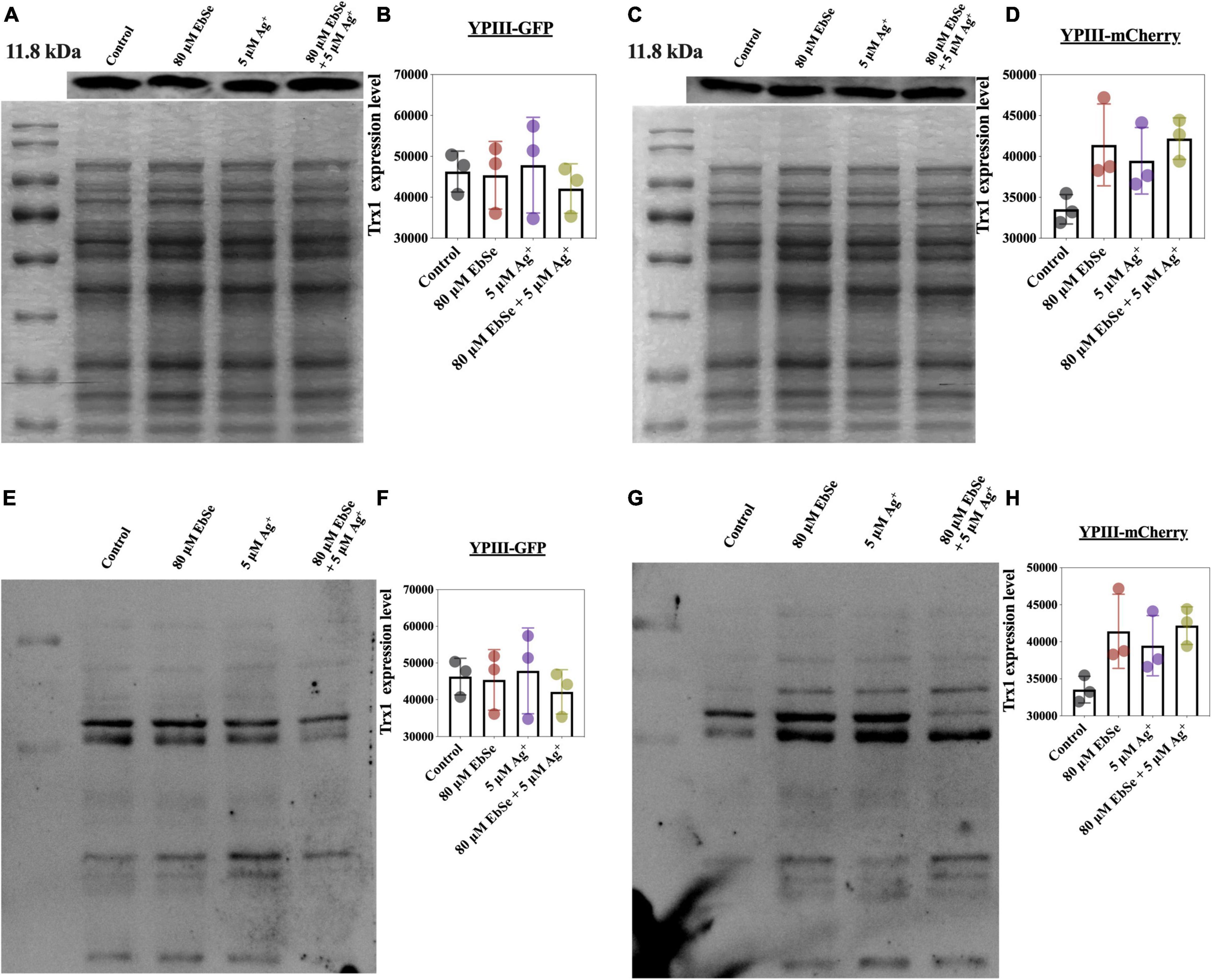
Figure 8. Effect of EbSe-Ag+ on Trx1 and S-PSSG expressions in YpIII. YpIII-GPF and YpIII-mCherry were cultured to A600 of 0.4 and treated with EbSe-Ag+. WB assay was used to detect (A,C) the Trx1 expression level and (E,G) S-PSSG expression level; (B,D) quantitative values of the Trx1 expression level; (F,H) quantitative values of the S-PSSG expression level; (A,B,E,F) YpIII-GFP; (C,D,G,H) YpIII-mCherry.
Taken together, the above results showed that EbSe-Ag+ inhibited TrxR activity and depleted GSH in YpIII. All the results suggested that EbSe-Ag+ could disrupt YpIII TDRS, and ROS production is one of the key mechanisms underlying its bactericidal activity.
EbSe-Ag+ Protects Mice From YpIII-Caused Gastroenteritis
Gastroenteritis is among the most common Y. pseudotuberculosis-caused infections (Amphlett, 2016). To evaluate whether EbSe-Ag+ could protect mice from YpIII-caused gastroenteritis, 60 mice were randomly divided into five groups, and 2 × 109 CFU/100 μL bioluminescent-YpIII strain Xen4 (Wang et al., 2016) were orally administrated to construct gastroenteritis models. Mice were further injected i.p. with 20 mg/kg EbSe, 5 mg/kg Ag+, 20 mg/kg EbSe and 5 mg/kg Ag+ and DMSO on days 1-, 3-, 5-, post-infection. Mice were monitored for bioluminescent emission at 1-, 4-, and 7-days post-infection using IVIS. To further analyze bacterial localization within gastrointestinal tracts, mice were euthanized, the gastrointestinal tracts were removed, and imaged by bioluminescent imaging. The results showed that EbSe-Ag+ led to a significant reduction in bacterial load on-site compared with the control group (Figure 9). These findings demonstrated that EbSe-Ag+ could effectively protect mice from YpIII-caused gastroenteritis.

Figure 9. EbSe-Ag+ protects mice from YpIII-caused pseudotuberculosis. YpIII-illuminous were orally administrated into mice, and further treated with EbSe-Ag+. (A,B) Mice were monitored for bioluminescent emission using IVIS lumina II (Caliper LifeSciences) at 1-, 4-, and 7-days post-infection. (C,D) Mice were euthanized, the gastrointestinal tracts were removed, and imaged by bioluminescent imaging to analyze bacterial localization within gastrointestinal tracts at 1-, 4-, and 7-days post-infection. IC: infection (illuminous) control.
EbSe-Ag+ Reduces Inflammatory Responses in Gastroenteritis Mice
Hematoxylin and eosin (H&E) staining was performed using the new formative tissues. Staining revealed that control mice had increased number of inflammatory cells. Meanwhile, EbSe-Ag+-treated mice had less scattered lymphocytes (Figure 10).
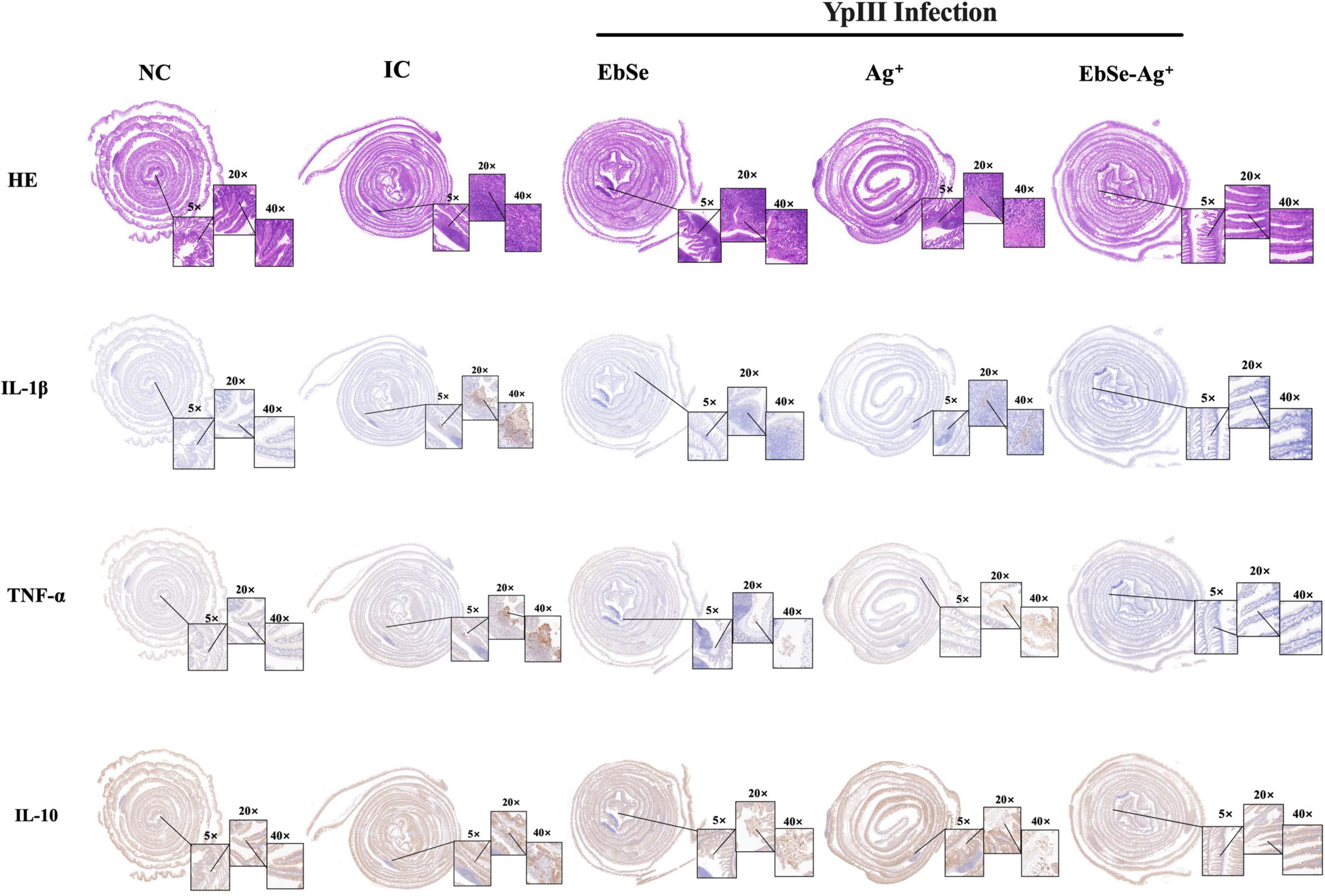
Figure 10. EbSe-Ag+ has immune-modulatory property. YpIII-illuminous were orally administrated into mice, and further treated with EbSe-Ag+. Mice intestinal tracts were rolled, fixed, and embedded. Paraffin sections were used to perform H&E stain and detect cytokine (IL-1β, TNF-α and IL-10) levels by IHC analysis.
An IHC assay was used to measure the presence of pro-inflammatory cytokines IL-1β and TNF-α and anti-inflammatory cytokine IL-10. As shown in Figure 10, EbSe-Ag+ could significantly down-regulate IL-1β and TNF-α and up-regulate IL-10 when compared to control mice.
The peripheral blood from different groups of mice was collected, and the results showed that EbSe-Ag+ statistically reduced the counts of WBCs (Figure 11A) and neutrophils (Figure 11B) compared with the control. Furthermore, ALT, AST, urea, and creatinine in mice blood serum were also detected, and the results showed that EbSe-Ag+ treatment reduced the ALT (Figure 11C), AST (Figure 11D) levels compared with Ag+ treatment.
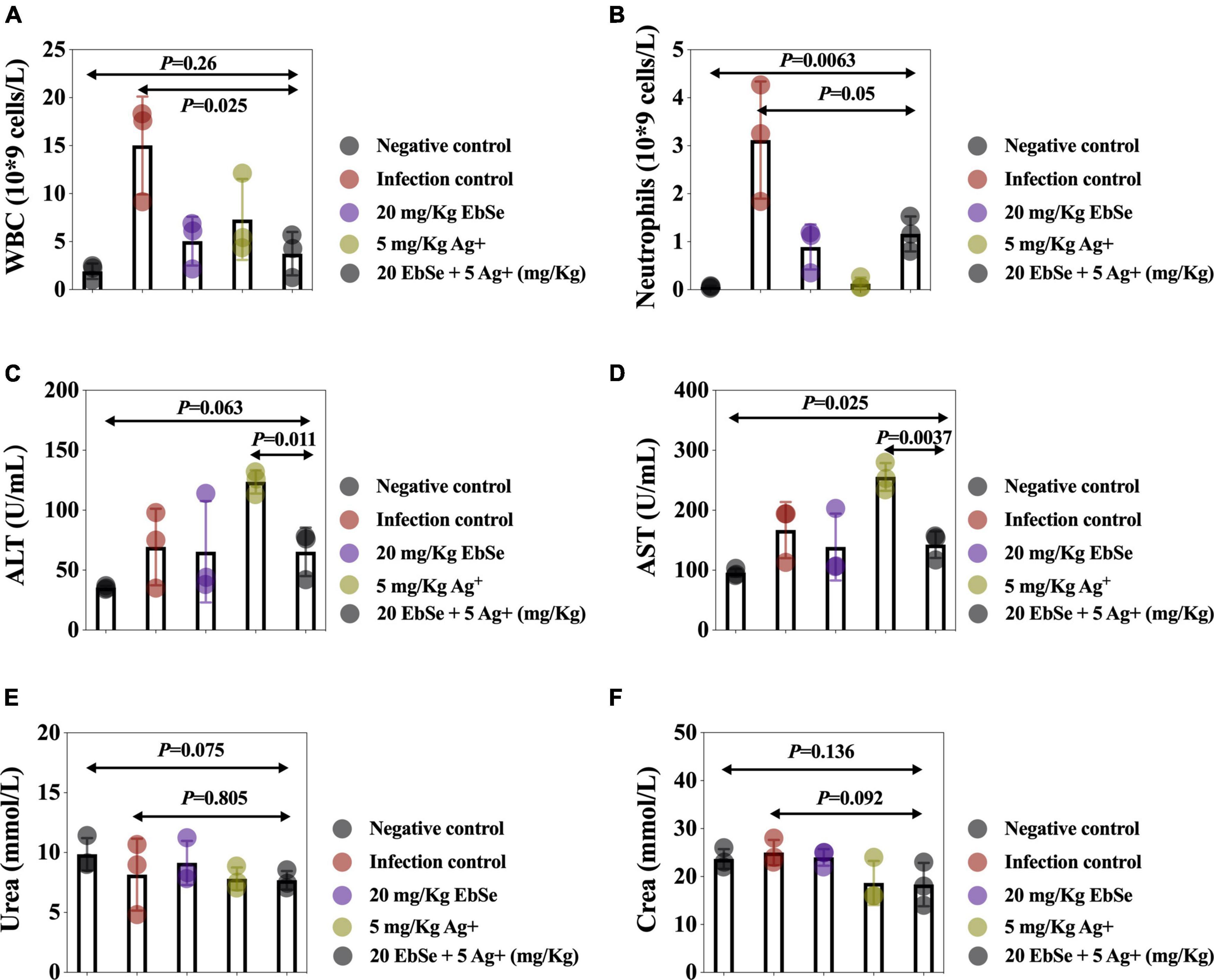
Figure 11. Routine blood analysis of EbSe-Ag+-treated mice. YpIII-illuminous were orally administrated into mice and further treated with EbSe-Ag+. Mice blood was collected 7 days post-infection, and the numbers of (A) WBC and (B) neutrophils, the amounts of (C) ALT, (D) AST, (E) Urea, and (F) creatine were detected.
Overall speaking, EbSe-Ag+ treatment exhibited significant effects in reducing the YpIII-caused inflammation with low toxicity.
Discussion
The study of the Gram-negative zoonotic bacteria Y. pseudotuberculosis has been at the forefront of cellular and molecular pathogenesis for over four decades (Davidson and Davis, 2020). Since Y. pseudotuberculosis has a very diverse host spectrum, it can infect virtually all warm-blooded animals, including humans, livestock, pets, and wild animals (Hashimoto et al., 2021). Therefore, given the growing fascination of human populations with wildlife (such as game meat), the pseudotuberculosis can be expected to increase. Here, we showed that EbSe work synergistically with Ag+ to eliminate Y. pseudotuberculosis YpIII strain in vitro. Briefly, UV-vis and LSCM assays verified that the pharmacological synergy was generated; TEM results revealed that EbSe-Ag+ caused the deformation, shrinkage, and content overflow of YpIII cells, suggesting the rupture and decomposition of the bacterial cell membrane. Further, DTT was used as organic reductant, and the UV-vis, TEM, and FCM results proved that DTT can protect YpIII from the bactericidal effect of EbSe-Ag+, indicating the antibacterial mechanism of EbSe-Ag+ is highly related to the overmuch accumulation of ROS.
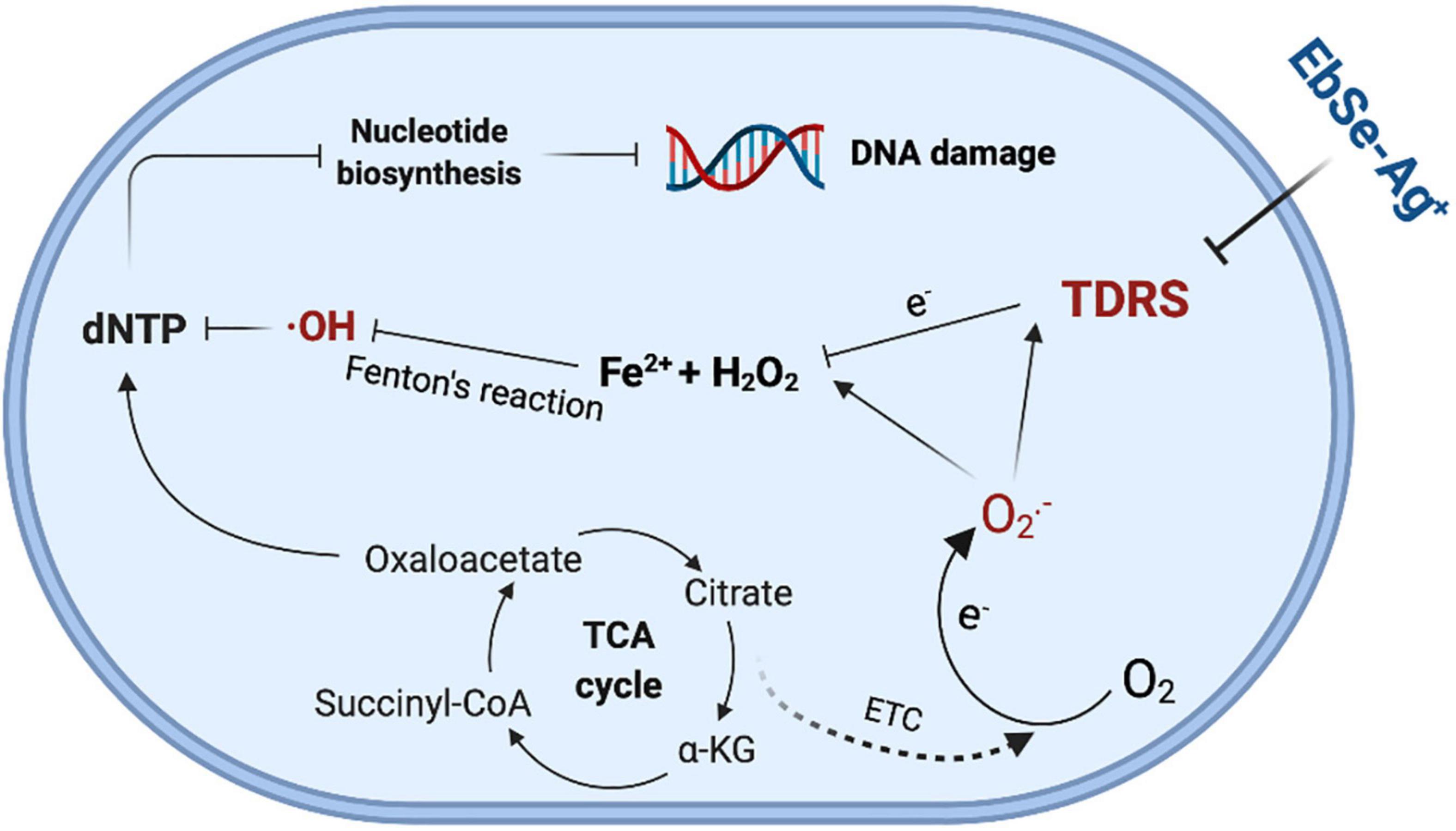
Known as a highly reactive oxygen-containing molecule, ROS is continuously produced by mitochondrial metabolism and eliminated by dedicated antioxidant systems (Rabinovitch et al., 2017). Since the excessive ROS oxidize proteins, lipids, and DNA (Lei et al., 2018), its intrinsic characteristics is the basis of the mechanism necessary for the survival and growth of organisms (Li et al., 2017), and the its homeostasis in bacteria plays a crucial role in regulating diverse physiological functions in bacteria (Zhang et al., 2017). Thus, the DTNB assays were performed, and the results showed that the activity of TrxR and the content of GSH were both significantly decreased by EbSe-Ag+, indicating the disrupt of redox homeostasis. Furthermore, the expression levels of Trx1 and S-PSSG were detected by Western blot as described previously (Zou et al., 2017). The results showed that the expression level of Trx1 maintained, while the level of S-PSSG decreased significantly after EbSe-Ag+ treatment. Collectively, our results indicated that EbSe-Ag+ affected the activity of the enzymes in Trx system rather than its expression; meanwhile, GSH system was significantly disrupted by consumption the GSH pool (Figure 12).
Using well-established experimental gastroenteritis in mice offers the opportunity to evaluate the preventive strategies for pseudotuberculosis (Wang et al., 2016). In the current study, the infected mice were treated by intraperitoneal administration of EbSe-Ag+. The mice were monitored for bioluminescent emission at regular intervals, and the therapeutic effect of EbSe-Ag+ was verified by a significant reduction in bacterial loads (Avican et al., 2017; Brodl et al., 2018; Fleiss and Sarkisyan, 2019). Pro-inflammatory cytokines, TNF-α, and IL-1β, which are produced during intestinal inflammation have important intestinal epithelial tight junction barrier-modulating actions (Kaminsky et al., 2021), highly related to inflammatory diseases of the gut (Xu et al., 2018). Our results showed that both TNF-α, and IL-1β were down-regulated by EbSe-Ag+, contributing to host defense during infection. Given that IL-10 is at the center of maintaining the delicate balance between effective immunity and tissue protection, it is not surprising that IL-10 is particularly important in maintaining the intestinal microbe-immune homeostasis, and functions to prevent excessive inflammation during the course of infection (Neumann et al., 2019). Our results showed that EbSe-Ag+ up-regulated IL-10 that provide a favorable outcome in host healing, which might be highly related to its recognized immune-modulatory, anti-inflammatory, and antioxidant activities (Ren et al., 2018). Neutrophils are critical components of immunity and can rapidly migrate to infected or injured tissue, performing central roles in both the innate and the acquired immune systems (Lemaitre et al., 2022). Upon their activation, release of signaling molecules by neutrophils help combat bacterial pathogens (Borregaard, 2010; Wang L. et al., 2020). The enteropathogenic bacterium YpIII successfully persists as an extracellular bacterium during infection by virtue of its translocation of virulence effectors that act in the cytosol of host immune cells to subvert phagocytosis and proinflammatory responses (Taheri et al., 2016). In our animal trial, the peripheral blood of mice was collected, and the neutrophil counts were detected. The result showed that EbSe-Ag+ can reduce the production of inflammatory cells. In addition, ALT and AST were monitored, and the results showed that the enzymes in the EbSe-Ag+ group were significantly lower than that of in the Ag+ group, proving that the EbSe-Ag+ could reduce the hepatotoxicity caused by Ag+ to a certain extent. In parallel, blood urea and serum creatinine were also monitored. The results illustrated that EbSe-Ag+ in this experiment has no obvious nephrotoxicity in mice.
Conclusion
Our in vitro study demonstrated that EbSe work synergistically with Ag+ to eliminate YpIII by disrupting TDRS; meanwhile, the in vivo experiment verified the antibacterial activity and immune-modulatory property of EbSe-Ag+. Taken together, all the results help to develop its scientific basis for pseudotuberculosis control.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by the ethical permit approval of the Medical Animal Care and Welfare Committee of China Three Gorges University (2019080Q) was obtained before using the mice for study.
Author Contributions
CD, WC, and PW performed in vitro experiments. JW and HW analyzed and interpreted the data. BL, KD, DG, and HC performed animal experiments. LZ was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
We are grateful for the support of the National Natural Science Foundation of China (32170191 and 81903105), the Natural Science Foundation of Hubei province (2021CFB497), the Health Commission of Hubei Province Foundation (WJ2019H528), and the Swedish Research Council (2018-02376).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.963901/full#supplementary-material
Supplementary Figure 1 | The S degree of EbSe-Ag+ against YpIII. UV-Vis assay was used to measure the survival of bacteria and the result showed that the S degree of 4 μM EbSe and 0.5 μM Ag+ was 0.97 and 0.94, respectively (in this figure). S = (FX0/F00) * (FY0/F00) - (FXY/F00).
Supplementary Figure 2 | EbSe-Ag+ showed bactericidal activity against YpIII. YpIII-GFP and YpIII-mCherry were cultured to A600 of 0.4, diluted 1:1,000 times, treated with EbSe-Ag+ and plated on LB medium.
References
Amphlett, A. (2016). Far east scarlet-like fever: a review of the epidemiology, symptomatology, and role of superantigenic toxin: Yersinia pseudotuberculosis-derived mitogen A. Open Forum Infect. Dis. 3:ofv202. doi: 10.1093/ofid/ofv202
Aswal, M., Garg, A., Singhal, N., and Kumar, M. (2020). Comparative in-silico proteomic analysis discerns potential granuloma proteins of Yersinia pseudotuberculosis. Sci. Rep. 10:3036. doi: 10.1038/s41598-020-59924-1
Avican, U., Doruk, T., Östberg, Y., Fahlgren, A., and Forsberg, Å (2017). The tat substrate SufI is critical for the ability of Yersinia pseudotuberculosis to cause systemic infection. Infect. Immun. 85, e00867–16. doi: 10.1128/IAI.00867-16
Bakholdina, S. I., Shubin, F. N., and Solov’eva, T. F. (2009). [Oxygen deficiency increases invasive activity and resistance of Yersinia pseudotuberculosis to heat stress]. Zh. Mikrobiol. Epidemiol. Immunobiol. 3, 18–23.
Bergsbaken, T., Fink, S. L., and Cookson, B. T. (2009). Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109. doi: 10.1038/nrmicro2070
Borregaard, N. (2010). Neutrophils, from marrow to microbes. Immunity 33, 657–670. doi: 10.1016/j.immuni.2010.11.011
Brodl, E., Winkler, A., and Macheroux, P. (2018). Molecular mechanisms of bacterial bioluminescence. Comput. Struct. Biotechnol. J. 16, 551–564. doi: 10.1016/j.csbj.2018.11.003
Dakić, I., Vukovic, D., Stepanović, S., Hauschild, T., Jezek, P., Petrás, P., et al. (2005). Survey of genes encoding staphylococcal enterotoxins, toxic shock syndrome toxin 1, and exfoliative toxins in members of the Staphylococcus sciuri group. J. Clin. Microbiol. 43, 4875–4876. doi: 10.1128/JCM.43.9.4875-4876.2005
Davidson, R. K., and Davis, K. M. (2020). Yersinia pseudotuberculosis: cultivation, storage, and methods for introducing DNA. Curr. Protoc. Microbiol. 59:e122. doi: 10.1002/cpmc.122
Dong, C., Wang, J., Chen, H., Wang, P., Zhou, J., Zhao, Y., et al. (2020). Synergistic therapeutic efficacy of ebselen and silver ions against multidrug-resistant Acinetobacter baumannii-induced urinary tract infections. Metallomics 12, 860–867. doi: 10.1039/d0mt00091d
Dong, C., Zhou, J., Wang, P., Li, T., Zhao, Y., Ren, X., et al. (2019). Topical therapeutic efficacy of ebselen against multidrug-resistant Staphylococcus aureus LT-1 targeting thioredoxin reductase. Front. Microbiol. 10:3016. doi: 10.3389/fmicb.2019.03016
Eppinger, M., Rosovitz, M. J., Fricke, W. F., Rasko, D. A., Kokorina, G., Fayolle, C., et al. (2007). The complete genome sequence of Yersinia pseudotuberculosis IP31758, the causative agent of far east scarlet-like fever. PLoS Genet. 3:e142. doi: 10.1371/journal.pgen.0030142
Ezraty, B., Gennaris, A., Barras, F., and Collet, J. F. (2017). Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 15, 385–396. doi: 10.1038/nrmicro.2017.26
Fleiss, A., and Sarkisyan, K. S. (2019). A brief review of bioluminescent systems (2019). Curr. Genet. 65, 877–882. doi: 10.1007/s00294-019-00951-5
Hashimoto, T., Takenaka, R., Fukuda, H., Hashinaga, K., Nureki, S. I., Hayashidani, H., et al. (2021). Septic shock due to Yersinia pseudotuberculosis infection in an adult immunocompetent patient: a case report and literature review. BMC Infect. Dis. 21:36. doi: 10.1186/s12879-020-05733-w
Kaminsky, L. W., Al-Sadi, R., and Ma, T. Y. (2021). IL-1beta and the intestinal epithelial tight junction barrier. Front. Immunol. 12:767456. doi: 10.3389/fimmu.2021.767456
Le Guern, A. S., Martin, L., Savin, C., and Carniel, E. (2016). Yersiniosis in France: overview and potential sources of infection. Int. J. Infect. Dis. 46, 1–7. doi: 10.1016/j.ijid.2016.03.008
Lei, H., Wang, G., Zhang, J., and Han, Q. (2018). Inhibiting TrxR suppresses liver cancer by inducing apoptosis and eliciting potent antitumor immunity. Oncol. Rep. 40, 3447–3457. doi: 10.3892/or.2018.6740
Lemaitre, J., Desjardins, D., Gallouët, A. S., Gomez-Pacheco, M., Bourgeois, C., Favier, B., et al. (2022). Expansion of immature neutrophils during siv infection is associated with their capacity to modulate T-cell function. Front. Immunol. 13:781356. doi: 10.3389/fimmu.2022.781356
Li, Y., Bai, H., Wang, H., Shen, Y., Tang, G., and Ping, Y. (2017). Reactive oxygen species (ROS)-responsive nanomedicine for RNAi-based cancer therapy. Nanoscale 10, 203–214. doi: 10.1039/C7NR06689A
Lima-Junior, D. S., Costa, D. L., Carregaro, V., Cunha, L. D., Silva, A. L., Mineo, T. W., et al. (2013). Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 19, 909–915. doi: 10.1038/nm.3221
Lu, J., Vlamis-Gardikas, A., Kandasamy, K., Zhao, R., Gustafsson, T. N., Engstrand, L., et al. (2013). Inhibition of bacterial thioredoxin reductase: an antibiotic mechanism targeting bacteria lacking glutathione. FASEB J. 27, 1394–1403. doi: 10.1096/fj.12-223305
Mares, C. A., Lugo, F. P., Albataineh, M., Goins, B. A., Newton, I. G., Isberg, R. R., et al. (2021). Heightened virulence of Yersinia is associated with decreased function of the YOPJ protein. Infect. Immun. 89:e0043021. doi: 10.1128/IAI.00430-21
Mecsas, J. (2019). Unraveling neutrophil– Yersinia interactions during tissue infection. F1000Res. 8, F1000FacultyRev–1046. doi: 10.12688/f1000research.18940.1
Neumann, C., Scheffold, A., and Rutz, S. (2019). Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 44:101344. doi: 10.1016/j.smim.2019.101344
Ohnishi, T., Nakazawa, M., Wada, N., Abe, J., and Kamimaki, I. (2021). Yersinia pseudotuberculosis infection accompanied by intussusception and incomplete Kawasaki disease in a 7-year-old Girl. Keio J. Med. 71, 50–52. doi: 10.2302/kjm.2021-0002-CR
Rabinovitch, R. C., Samborska, B., Faubert, B., Ma, E. H., Gravel, S. P., Andrzejewski, S., et al. (2017). AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 21, 1–9. doi: 10.1016/j.celrep.2017.09.026
Reinhardt, M., Hammerl, J. A., Kunz, K., Barac, A., Nöckler, K., and Hertwig, S. (2018). Yersinia pseudotuberculosis prevalence and diversity in wild boars in northeast Germany. Appl. Environ. Microbiol. 84, e00675–18. doi: 10.1128/AEM.00675-18
Ren, X., Zou, L., and Holmgren, A. (2020). Targeting bacterial antioxidant systems for antibiotics development. Curr. Med. Chem. 27, 1922–1939. doi: 10.2174/0929867326666191007163654
Ren, X., Zou, L., Lu, J., and Holmgren, A. (2018). Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free Radic. Biol. Med. 127, 238–247. doi: 10.1016/j.freeradbiomed.2018.05.081
Rivas, L., Strydom, H., Paine, S., Wang, J., and Wright, J. (2021). Yersiniosis in New Zealand. Pathogens 10:191. doi: 10.3390/pathogens10020191
Schneiders, S., Hechard, T., Edgren, T., Avican, K., Fällman, M., Fahlgren, A., et al. (2021). Spatiotemporal variations in growth rate and virulence plasmid copy number during Yersinia pseudotuberculosis infection. Infect. Immun. 89, e710–e720. doi: 10.1128/IAI.00710-20
Singhal, R., and Shah, Y. M. (2020). Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 295, 10493–10505. doi: 10.1074/jbc.REV120.011188
Taheri, N., Fahlgren, A., and Fallman, M. (2016). Yersinia pseudotuberculosis blocks neutrophil degranulation. Infect. Immun. 84, 3369–3378. doi: 10.1128/IAI.00760-16
Voskresenskaya, E., Savin, C., Leclercq, A., Tseneva, G., and Carniel, E. (2014). Typing and clustering of Yersinia pseudotuberculosis isolates by restriction fragment length polymorphism analysis using insertion sequences. J. Clin. Microbiol. 52, 1978–1989. doi: 10.1128/JCM.00397-14
Wang, H., Avican, K., Fahlgren, A., Erttmann, S. F., Nuss, A. M., Dersch, P., et al. (2016). Increased plasmid copy number is essential for Yersinia T3SS function and virulence. Science 353, 492–495. doi: 10.1126/science.aaf7501
Wang, L., Ai, Z., Khoyratty, T., Zec, K., Eames, H. L., van Grinsven, E., et al. (2020). ROS-producing immature neutrophils in giant cell arteritis are linked to vascular pathologies. JCI Insight 5:e139163. doi: 10.1172/jci.insight.139163
Wang, P., Wang, J., Xie, Z., Zhou, J., Lu, Q., Zhao, Y., et al. (2020). Depletion of multidrug-resistant uropathogenic Escherichia coli BC1 by ebselen and silver ion. J. Cell. Mol. Med. 24, 13139–13150. doi: 10.1111/jcmm.15920
Xu, T., Dong, Z., Wang, X., Qi, S., Li, X., Cheng, R., et al. (2018). IL-1beta induces increased tight junction permeability in bovine mammary epithelial cells via the IL-1beta-ERK1/2-MLCK axis upon blood-milk barrier damage. J. Cell. Biochem. 119, 9028–9041. doi: 10.1002/jcb.27160
Zhang, J., Li, X., Han, X., Liu, R., and Fang, J. (2017). Targeting the thioredoxin system for cancer therapy. Trends Pharmacol. Sci. 38, 794–808.
Zou, L., Lu, J., Wang, J., Ren, X., Zhang, L., Gao, Y., et al. (2017). Synergistic antibacterial effect of silver and ebselen against multidrug-resistant Gram-negative bacterial infections. EMBO Mol. Med. 9, 1165–1178. doi: 10.15252/emmm.201707661
Keywords: Yersinia pseudotuberculosis, Ebselen, silver nitrate, antibacterial activity, immune-modulatory property
Citation: Dong C, Chen W, Zou L, Liu B, Deng K, Guo D, Wang P, Chen H, Wang H and Wang J (2022) The Assessment on Synergistic Activity of Ebselen and Silver Ion Against Yersinia pseudotuberculosis. Front. Microbiol. 13:963901. doi: 10.3389/fmicb.2022.963901
Received: 08 June 2022; Accepted: 24 June 2022;
Published: 25 July 2022.
Edited by:
Hongliang Chai, Northeast Forestry University, ChinaReviewed by:
Dharmender K. Gahlot, Umeå University, SwedenEkaterina Psareva, I. M. Sechenov First Moscow State Medical University, Russia
Copyright © 2022 Dong, Chen, Zou, Liu, Deng, Guo, Wang, Chen, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Zou, em91bGlsaUBjdGd1LmVkdS5jbg==; Helen Wang, aGVsZW4ud2FuZ0BpbWJpbS51dS5zZQ==; Jun Wang, d2FuZ2pmb3hAY3RndS5lZHUuY29t
†These authors have contributed equally to this work
 Chuanjiang Dong1†
Chuanjiang Dong1† Wei Chen
Wei Chen Lili Zou
Lili Zou Helen Wang
Helen Wang