- 1Department of Microbiology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland
- 2Center for Translational Medicine, Warsaw University of Life Sciences (SGGW), Warszawa, Poland
Three distinct streptococcal species: Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus, belonging to the Streptococcus anginosus group (SAG), also known as Streptococcus milleri group, have been attracting clinicians and microbiologists, not only as oral commensals but also as opportunistic pathogens. For years they have been simply classified as so called viridans streptococci, and distinct species were not associated with particular clinical manifestations. Therefore, description of SAG members are clearly underrepresented in the literature, compared to other medically relevant streptococci. However, the increasing number of reports of life-threatening infections caused by SAG indicates their emerging pathogenicity. The improved clinical data generated with the application of modern molecular diagnostic techniques allow for precise identification of individual species belonging to SAG. This review summarizes clinical reports on SAG infections and systematizes data on the occurrence of individual species at the site of infection. We also discuss the issue of proper microbiological diagnostics, which is crucial for further clinical treatment.
Introduction
The Streptococcus group was described for milleri the first time by Guthof in 1956, after being isolated from dental abscesses. The species name was chosen to honor arguably one of the most important practicing dentists, W. D. Miller, who spent his time in Robert Koch’s Laboratory to identify the germs responsible for tooth decay. Miller’s chemo-parasitic theory, together with the description of “gelatinous microbic plaques,” now commonly known as “dental plaque,” provided the key elements of our modern concept of the etiology of dental caries (He and Shi, 2009). Due to the lack of a single international nomenclature and the lack of established phenotypic markers, the Anginosus group of the genus Streptococcus was a subject of taxonomic confusion. The name “Streptococcus milleri group” has been used by European and Japanese microbiologists, including all group members, while North American microbiologists have been using terms such as Streptococcus MG-intermedius and Streptococcus anginosus-constellatus. SMG is still in use, but the term “Streptococcus anginosus group” (SAG) proposed by Kawamura is preferred (Jensen et al., 2013). SAG consists of three species designated Streptococcus anginosus, Streptococcus constellatus, and Streptococcus intermedius. Recent data suggest that the Anginosus group exhibits higher taxonomic heterogeneity, including the following: S. anginosus subsp. anginosus (SAA) and subsp. whileyi, S. constellatus subsp. constellatus, subsp. pharyngis, and subsp. viborgensis, and S. intermedius. More recently, based on multilocus sequence analysis (MLSA), the new subspecies of S. anginosus (S. anginosus genomosubsp. vellorensis) was advocated and the type of SAA strain was clustered within the S. anginosus gssp. vellorensis (Whiley, et al., 1999; Jensen et al., 2013; Tabata et al., 2019).
The members of SAG are frequently found within different oral structures and tissues. S. intermedius and S. constellatus are often associated with dental plaque and periodontal diseases (Sitkiewicz, 2018), while S. anginosus colonizes the mucosal membranes of the oral cavity (Asam and Spellerberg, 2014). All species can also colonize the throat, nasopharynx, gastrointestinal tract, and genitourinary tract, caused by spreading from the oral cavity (Neumayr et al., 2010).
Until the 1990s (Vandamme et al., 1998) diagnostic procedures for the identification of streptococci and the classification of these bacteria into the S. anginosus group were based on phenotypic differences examined using biochemical tests. Among them was the Voges–Proskauer test (*Mac Faddin, 1980) that is positive for the S. anginosus group and not by other β-hemolytic streptococcal species (Coykendall et al., 1987). S. anginosus bacteria are positive for arginine dihydrolase and esculin hydrolysis and negative for mannitol and sorbitol fermentation (Gray, 2005). Differentiation in the production of sugar hydrolyzing enzymes, sialidase, and hyaluronidase, was also used to identify SAG (Whiley, et al., 1990). Grinwis and co-workers presented a very interesting analysis of the validity of using various SAG identification tests, including Lancefield grouping, hemolysis, production of hyaluronidase, chondroitin sulfatase, DNase, proteases, hydrogen peroxide, and PCR for the detection of the intermedilysin gene (ily) (Grinwis et al., 2010). Due to the multitude of tests required for precise diagnosis, SAG isolates were rarely identified at the species level before the 2000s when advanced molecular biology tests were introduced for the common practice. Therefore, SAGs have been considered for many years a part of the commensal human microbiota, and their presence in polymicrobial infection samples was neglected (Asam and Spellerberg, 2014). For these reasons, for many years, SAG species were not considered a source of serious clinical infections.
Streptococcus anginosus Group – Opportunistic Pathogens
The fast development of diagnostic methods based on molecular microbiology techniques revealed that SAG should be reclassified as opportunistic pathogens, as those species may cause invasive infections after entering sterile sites in the body (Clarridge et al., 2001).
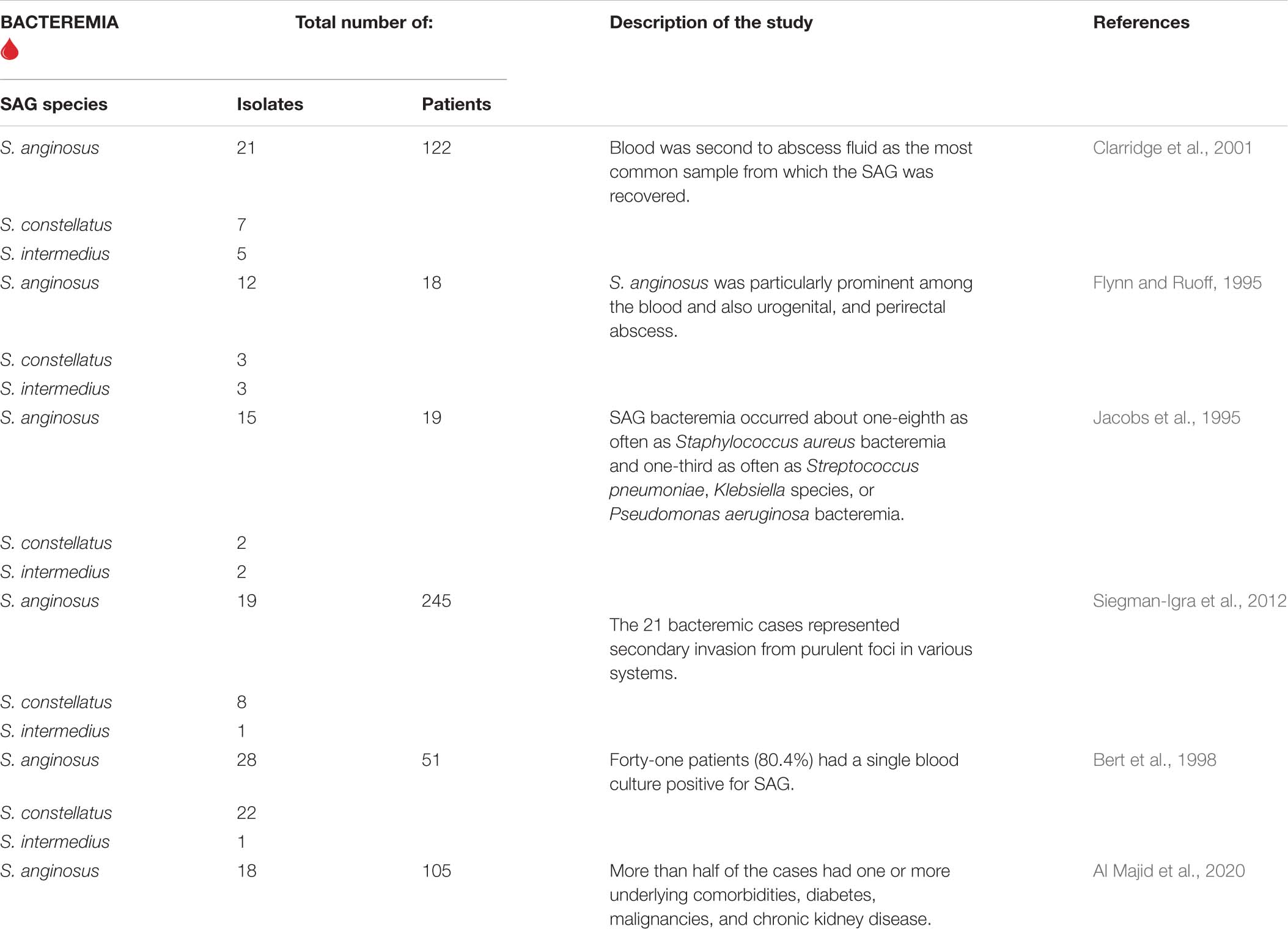
Bacteremia
The population study in 1989–2000 shows episodes of SAG bacteremia with an incidence of 0.93 per 100,000 population per year (Weightman et al., 2004). However, the epidemiological report from 2010 to 2017 of SAG-induced bloodstream infections presented an increase in annual incidence showing 3.7 cases per 100,000 inhabitants (Laupland et al., 2018). The increase in the prevalence index of SAG was clearly documented, although it remains elusive if it depends on the awareness of the clinician or the improvement of bacterial identification methods (OPEN QUESTION 1).
The systemic distribution of SAG leading to bacteremia is a consequence of the disruption of the mucosal barrier, which supports bacterial invasion into the underlying tissue (Bert et al., 1998).
The first evidence of bacteremia in association with gingivitis, periodontitis, and tooth brushing was reported by Forner et al. (2006). SAG can spread to the blood in people with oral infections, such as gingivitis and tooth abscesses (Terzi et al., 2016). Periodontitis is also a possible risk factor for the translocation of bacteria from the oral cavity into the bloodstream through ulcerated inflamed crevice, pocket epithelium, and adjacent gingival microcirculation (Dhotre et al., 2018). The authors observed a high similarity rate of the S. anginosus and S. constellatus isolates with distribution among subgingival plaque and blood samples. It is noteworthy that Lockhart et al. (2009) showed that even seemingly harmless gingival bleeding that occurs after brushing the tooth may be associated with an almost eightfold increase in the risk of bacteremia. As described above, the oral cavity can be a primary source of S. anginosus bacteriemia, but some authors indicate also the hepatobiliary system or the urinary tract (Suzuki et al., 2016). The others suggest esophagus and stomach as the source of SAG in cancer patients (Wu and Zheng, 2020), especially in patients with gastric tumors (Liu et al., 2019). Examples of SAG-related bacteremia are described in Table 1.
Among studies on SAG-induced mortality we can find the 3-year study (1990–1992), which showed that among examined 19 cases of SAG-associated bacteremia 5 patients died, pointing 26.3% of the mortality (Jacobs et al., 1994). On the other hand, another study conducted from 2015 to 2017 revealed only 6% overall mortality. The most recently published meta-analysis of 101 case reports published between 1996 and 2019 indicates that mortality rates among patients with SAG-associated bacteremia range between 10 and 16% (Issa et al., 2020).
Diseases Caused by Streptococcus anginosus Group
The latest clinical data indicate the presence of life-threatening infections induced by SAG, as reflected in the formation of various types of empyemas and abscesses. They are the most commonly isolated aerobes from peritonsillar abscesses next to the group A streptococci (GAS) (Galioto, 2017). A retrospective cohort study conducted between 2009 and 2015 showed that SAG-causing pyogenic infections were identified among 160/263 patients (60%) and included intraabdominal abscesses or peritonitis (69 patients, 43.1%), skin/soft tissue abscesses, arthritis or osteomyelitis (44 patients, 28.1%), empyema or lung abscesses (32 patients including one with empyema and intraabdominal infection, 20%) and intracranial abscesses (15 patients, 9.4%) (Kobo et al., 2017).
In particular, clinical data indicate the different rate of prevalence among SAG species, as well as tissue specificity. The retrospective analysis (2014–2019) of the 463 samples revealed 254 of S. anginosus (54.86%), 173 of S. constellatus (37.37%), and 36 of S. intermedius (7.77%) (Jiang et al., 2020). S. anginosus is more commonly isolated from gastrointestinal and genitourinary tract infections, S. constellatus has a propensity for the respiratory tract, while S. intermedius is responsible for most head and neck infections and infections of the central nervous system (CNS) (Clarridge et al., 2001). Similar data were recorded in studies carried out with 3–10 years old children. The authors identified S. anginosus in more than 70% of genitourinary tract infections, S. constellatus in skin and soft tissue diseases, and S. intermedius in head and neck diseases (Whiley, et al., 1992; Furuichi and Horikoshi, 2018).
Moreover, S. intermedius has the tendency to form abscesses and infection of deep tissues and is more likely to cause supportive, non-bacteremic infections, which require surgical intervention. On the contrary, S. anginosus and S. constellatus were associated with bacteremia, but have a lower incidence of pyogenic infection (Wu and Zheng, 2020).
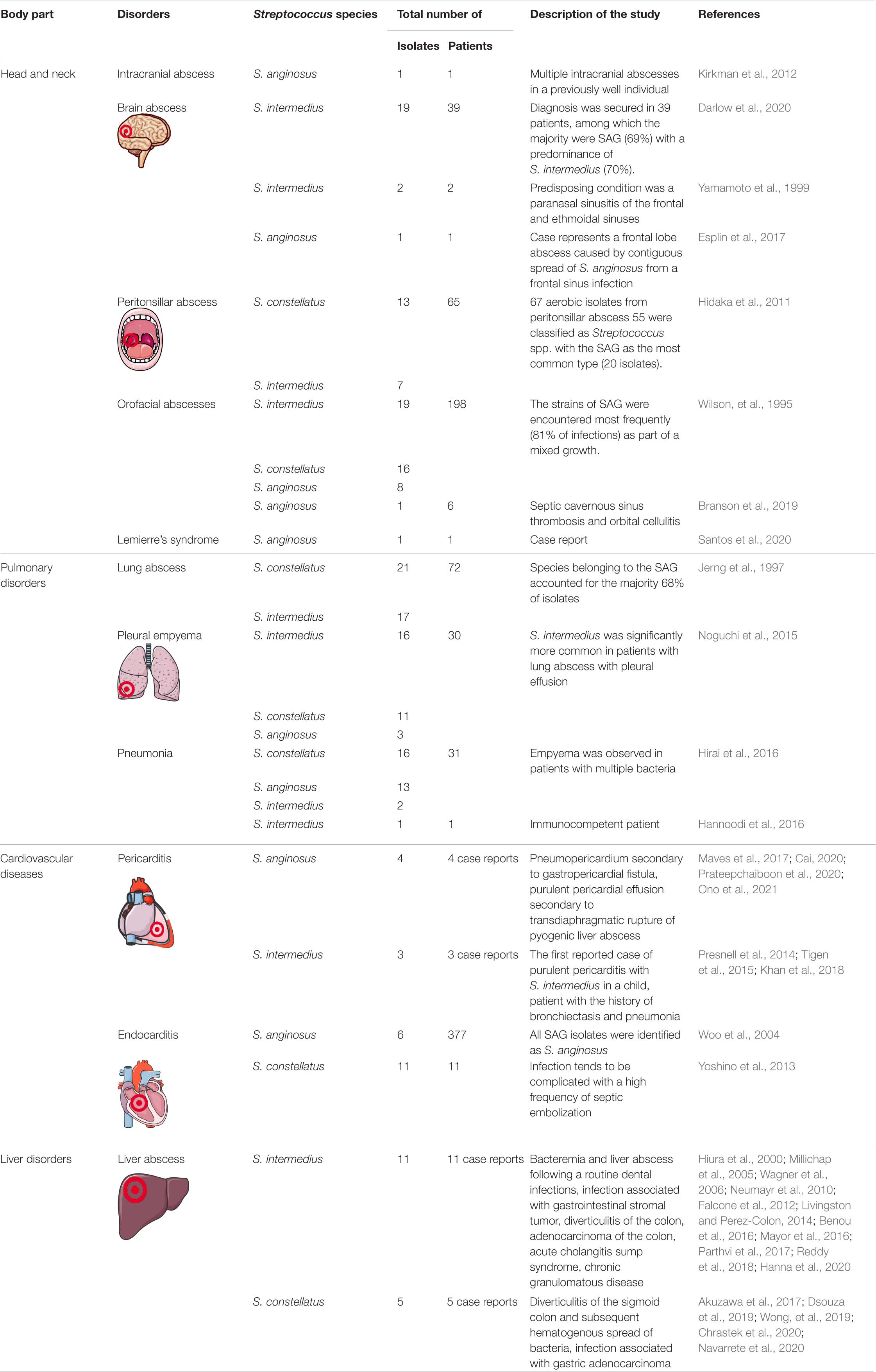
In the following, we describe the clinical data showing different tissue-specific SAG infection with their pathological consequences. Detailed information is presented in Table 2.
Head and Neck
Streptococcus anginosus group have a potential to extend in the head and neck, therefore their presence requires special attention considering the proximity of vital organs as CNS (Han and Kerschner, 2001).
Chronic maxillary sinusitis, which is often secondary to dental infection, is quite common among head and neck diseases. The main complication of sinusitis is the local spread of bacterial infection, causing periorbital or orbital cellulitis, cavernous sinus thrombosis, epidural, or brain abscess. Hutchin and co-workers emphasize the correlation between the occurrence of S. anginosus, sinusitis, and sinogenic subdural empyema. The authors proposed that the growth of microaerophilic SAG is favored as oxygen content decreases as a result of closure of the sinus ostia associated with mucosal edema and inflammation (Hutchin et al., 1999).
Peritonsillar abscess is an acute pharyngeal infection most common among adolescents and young adults and is considered a complication of acute tonsillitis or peritonsillar cellulitis (Passy, 1994). Interestingly, it was suggested that accelerated inflammation is an effect of coexistence of the SAG with other anaerobes, such as Fusobacterium necrophorum and Prevotella melaninogenica, but also Prevotella intermedia, Peptostreptococcus micros, Fusobacterium nucleatum, and Actinomyces odontolyticus (Jousimies-Somer et al., 1993; Hidaka et al., 2011). As the literature lists groups of bacteria associated with peritonsillar abscess, the open question is whether there is a profile of coexistence among the identified groups of bacteria (OPEN QUESTION 2).
An abscess of the brain can result from direct extension of cranial infections (e.g., osteomyelitis, sinusitis, and subdural empyema), penetrating head wounds (including neurosurgical procedures) or hematogenous spread (e.g., in bacterial endocarditis). It was shown that SAGs are significantly more likely than other bacteria to cause severe intracranial complications and neurological disorders in pediatric patients with rhinosinusitis. In the 50 cases identified, S. anginosus groups was the most commonly implicated bacterial pathogen in 14 (28%) (Deutschmann et al., 2013). In a retrospective analysis of cases of brain abscesses, SAGs were the most commonly isolated microorganisms in brain abscesses (Carpenter et al., 2007). The clinical association of S. intermedius with the tendency to form head and neck abscesses has long been recognized. Among the reported cases, there is also a case of a highly disseminated purulent infection caused by S. intermedius involving the brain, lungs, liver, and pleural cavity (Giuliano et al., 2012).
As described above, head and neck infections are caused by SAG, however, the routes of bacterial spread are poorly explored (OPEN QUESTION 3). Among the routes considered for brain infection are contiguous infection, hematogenous spread, direct implant, trauma or neurosurgery, and peripheral nerves (Kragha, 2016).
Pulmonary Disorders
Another possibility of SAG expansion is aspiration, which can lead to the development of pulmonary diseases, e.g., pneumonia, lung abscess, and pleural empyema. Several mechanisms have been suggested for SAG-causing thoracic infections, including aspiration of oral secretion, direct implantation by trauma or surgery, extension by contiguity, and hematogenous dissemination (Kobashi et al., 2008). The dissemination of SAG to the lungs from the oral cavity, leading to abscess, was demonstrated by the study by Mukae et al. (2016) The authors examined bronchoalveolar lavage fluid samples. Among the bacteria most frequently detected were SAG (15.3%), followed by Fusobacterium spp. (23.7%) (Mukae et al., 2016). The retrospective analysis carried out by Noguchi et al. (2015) revealed that among a total of 30 patients diagnosed with respiratory infection (pneumonia, lung abscess, and bacterial pleurisy only), S. intermedius, S. constellatus, and S. anginosus were identified in 16 (53.3%), 11 (36.7%), and 3 (10.0%) patients. Other retrospective studies describing the bacteriology and clinical features of empyema thoracis and lung abscess showed that among the 76 isolated strains of viridans streptococci, the most common were S. constellatus (21 strains), S. intermedius (17), and Streptococcus sanguis (10) (Jerng et al., 1997). Moreover, there is an increase in the number of case reports that indicate S. constellatus as a pulmonary pathogen (Morinaga et al., 2013; Elhussein and Hutchison, 2014; Price et al., 2015; Zhang et al., 2020; Vulisha et al., 2021). Taken together, the role of SAG in the pathogenesis of lung diseases is indisputable, with the leading role of S. constellatus and S. intermedius.
Cardiovascular Diseases
Bacterial pericarditis usually occurs as a secondary infection induced by contiguous spread from the surrounding intrathoracic area, including extension from the pulmonary, myocardial, and subdiaphragmatic site. It is also a consequence of hematogenous dissemination from distant organs (Masood et al., 2016). Purulent pericarditis can be caused by SAG due to dental problems (Li et al., 2013). Secondary pericarditis caused by S. anginosus has been reported four times since 2000, twice as a complication after S. intermedius infections. The primary purulent pericarditis is extremely rare, including one case in which S. anginosus culture grew confirmed and another caused by S. intermedius (Khan et al., 2018; Beom et al., 2021).
Among the etiological agents of infectious endocarditis, streptococci range from 60 to 80%. A detailed microbiological examination revealed that S. anginosus rarely induces infective endocarditis (0.1%) (Stryjewski et al., 2015). During a 5-year period (1997–2002), 6 cases of SAG endocarditis were documented (Woo et al., 2004). Infective endocarditis due to S. constellatus revealed non-specific initial symptoms, especially coughing, and complications of abscess formation and septic embolization. The choice of antibiotic agent for the treatment of infective endocarditis due to S. constellatus should receive special attention, because penicillin resistant strains have been documented in some cases (Yoshino et al., 2013).
Liver Abscesses
Streptococcus anginosus group have been found to cause infections within the abdominal cavity including liver abscesses, which are usually identified as polymicrobial. Microorganisms associated with liver abscesses include most commonly Gram-negative enteric bacteria (Escherichia coli, Klebsiella pneumoniae, Pseudomonas sp., and Proteus sp.), Gram-positive aerobes (SAG, Enterococcus sp., Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus sp.), anaerobic organisms (Bacteroides sp. and Fusobacterium), Actinomyces, Candida albicans, Salmonella typhi, Brucella melitensis, or other protozoa (Entamoeba histolytica and Echinococcus granulosus) (Ioannou et al., 2016). The study of Meddings et al. (2010) shows that among culture-positive patients with pyogenic liver abscess, the most common organisms isolated from pus were Streptococcus species (29.5%). A retrospective study of patients with pyogenic liver abscess presenting that the most common causative pathogens were SAG 25%, K. pneumoniae 21%, and E. coli 16%, respectively (Pang et al., 2011). From 2000, few clinical cases of liver abscess due to monospecies infection with S. intermedius were presented. Among the recent reports we can also find clinical studies describing multiple liver abscesses of mixed origin, with the presence of S. constellatus. The results of the investigation suggested that diverticulitis of the sigmoid colon and subsequent hematogenous spread of S. constellatus may have led to the formation of liver abscesses and bacteremia.
The data cited above indicate the dominance of S. constellatus and S. intermedius in the pathogenesis of liver abscess. Notably, it was revealed that these species replicate more rapidly in the presence of other species, including Eikenella corrodens (Young et al., 1996). A clinical report that studied liver abscess in patients showed that S. constellatus was the most common organism co-isolated with E. corrodens in liver abscess (n = 3), pleural effusion (n = 2), blood (n = 1), and brain abscess (n = 1). As streptococci and E. corrodens are members of endogenous flora in the mouth and upper respiratory tract, their coexistence in the liver abscess suggests the oral cavity as the common source of infection (Sheng et al., 2001). Moreover, it also suggests that the coexistence of SAG with other microorganisms could bidirectionally support the increase in their pathogenicity (OPEN QUESTION 4).
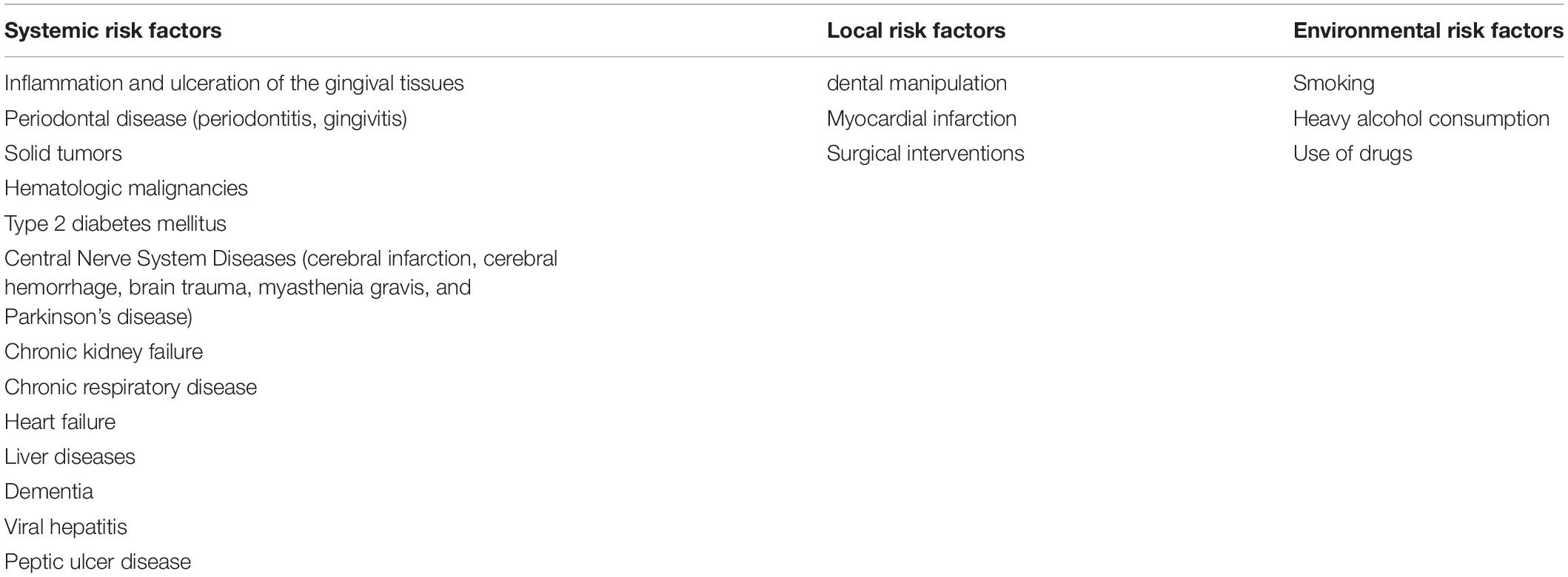
The Risk Factors for Streptococcus anginosus Group Infection
The incidence of SAG infection development increased with the occurrence of systemic diseases. The data indicate that clinically relevant predisposing factors were identified in 65% of the SAG infections studied. The most common were related to gastrointestinal tract pathology and included diverticular disease, cancer, biliary tract procedures and intestinal perforation, cirrhosis, Crohn’s disease, and variceal esophageal bleeding (Takeuchi et al., 2017). The significant common risk factors for blood infection were bariatric surgery, appendicular abscess, and appendectomy (Al Majid et al., 2020). The study by Jiang et al. (2020) describes risk factors for patients infected with SAG. A total of 210 of the 463 patients had major underlying diseases. Among them, 63 (30%) with solid tumors, 6 (2.86%) with hematologic malignancies, 70 (33.33%) with type 2 diabetes mellitus, 23 (10.95%) with diseases of the central nervous system (cerebral infarction, cerebral hemorrhage, brain trauma, myasthenia gravis, or Parkinson’s disease), 20 (9.52%) with chronic kidney failure, 12 (5.71%) with chronic respiratory disease, 6 (2.86%) with viral hepatitis, and 10 (4.77%) with connective tissue disease (Jiang et al., 2020).
Streptococcus anginosus group, as other microorganisms, have been shown to pass through the intestinal barrier and translocate to extra intestinal organs, thus increased intestinal permeability could be a plausible route of infection (Purohit et al., 2008). The presence of a cancerous lesion provides an insult to the normal colonic mucosa, allowing pathogens to invade the circulation. Therefore, colorectal carcinoma or rectal adenocarcinoma is a risk factor for SAG infection (Tzur et al., 2003; Lin et al., 2008; Masood et al., 2016). In addition, systemic S. anginosus infections have also been reported in patients with esophageal, gastric, and oral cancer (Sasaki et al., 2005; Masood et al., 2016). Another convincing report comes from the study of neutropenia patients. The authors revealed that the source of S. anginosus group infections is usually the gastrointestinal tract (GI). The study by Wenzler et al. (2015) found 18 cases (53% of 34 patients) with a gastrointestinal source of bacteremia; therefore, sepsis could have originated from an unidentified source of the GI tract. Siegman-Igra et al. (2012) reported 215 cases of SAG infections, the most common sources being the liver and other intraabdominal abscesses. In addition to cancer, there are many conditions that lead to a state of reduced immunity, which could predispose to SAG infection. Interesting data comes from the clinical case study of Reddy et al. (2018) who identified risk factors leading to the development of abscesses with the participation of S. intermedius. Among them, the authors revealed chronic diseases (56% of patients with type 2 diabetes, high blood pressure, AIDS, or cancer), unhealthy diet, alcohol, drug consumption, and smoking (33% patients) and surgical intervention, including dental work or laparoscopic cholecystectomy (11% cases) (Reddy et al., 2018). The age seems also to play a role in the course of disease as significantly higher mortality was reported in patients with SAG infections over 65 years of age, which was also associated with polymicrobial infections and immunosuppression (Al Majid et al., 2020). The list of risk factors is summarized in Table 3.
Identification Methods
The role of SAG in the pathogenesis of infectious diseases has been poorly described. This is a consequence of the difficulties in categorizing and identifying these microorganisms. Identification of S. anginosus group based on Lancefield groups is not applicable, as all species show high antigenic heterogeneity and include cross-reactive strains that react with Lancefield groups A, C, F, and G antisera (Grinwis et al., 2010). Therefore, commercial systems were developed, including: API 20 Strep and API Rapid 32 ID Strep (bioMérieux, France), the Fluo-Card Milleri kit (Flynn and Ruoff, 1995), or The VITEK® 2 system. Despite being simple and quick tests, these methods provide reliable identification at the group level, not species (Teles et al., 2011). There is no gold standard for the identification of SAG. Cultivation methods and the available biochemical test are not sufficient for precise identification of the species, therefore it is necessary to use molecular techniques (Wenzler et al., 2015; Kragha, 2016). Among them, two 16S rRNA real-time PCR assays were developed. The 16s_SA assay is specific for S. anginosus (100%), while the 16s_SCI assay is specific for S. constellatus and S. intermedius (100%). These assays can detect <10 genome equivalents in pure culture and >104 genome equivalents in sputum samples, making this a great tool for assessing the presence of SAG in complex polymicrobial samples. Note that it is not possible to distinguish between S. constellatus and S. intermedius by this method (Olson et al., 2010).
Another technique was designed on the basis of known streptococcal groESL sequences. The authors designed a pair of primers to differentiate the members of the SAG from other members of the viridans group streptococci (Liu et al., 2006). In order to further differentiate the species (S. anginosus and S. constellatus) among the anginosus group, amplification products were subsequently digested with the restriction enzymes. Therefore, the PCR restriction fragment length polymorphism (RFLP) could be used as a confirmatory test for the identification of the Anginosus group and differentiation between S. anginosus and S. constellatus. S. intermedius still remain unrecognized (Liu et al., 2006). Poyart et al. (1998) described the method to identify clinical streptococci isolates at the species level by determining the positions of their sodA gene that encodes a manganese-dependent enzyme.
To correctly identify S. intermedius, an accurate PCR identification system with the ily gene as a specific marker must be used. The amplification of fragment of the ily gene and its 3′-flanking region is specific for S. intermedius strains among all other streptococcal species (Teles et al., 2011). In recent studies, authors underline the promising potential of real-time PCR, rapid microarray assay, as well as mass spectrometry (MALDI-TOF-MS) in discriminating between the individual species of the S. anginosus group (Desar et al., 2008; Reißmann et al., 2010; Wenzler et al., 2015).
Accurate results were obtained for S. anginosus and S. constellatus which were identified at the species level by MALDI-TOF-MS (Woods et al., 2014). In addition, the developers of the MALDI Biotyper 3.1 software expand its database to cover, inter alia, S. intermedius strains to identify bacterial strains more accurately (Wei et al., 2021). The most promising diagnostic tool that can be used to identify and precisely distinguish between viridans streptococci is next generation sequencing (NGS). The low cost of sequencing, below cents per base, allows us to apply NGS for direct sequencing of bacteria from clinical samples. It is very useful to detect bacteria causing mixed infections, common in SAG infections. It speeds up the diagnostic process because the initial diagnosis does not need the growth of slowly growing SAG. Moreover, recent reports show that NGS combined with machine learning can be also used to predict MIC values (Nguyen et al., 2018). The disadvantage of NGS as widespread diagnostic tool is current lack of user friendly software that allow sequence based detection. However, due to rapid development of the technology it may change in the near future (Kozińska et al., 2019).
Jensen et al. (2013) presents re-examine the taxonomy SAG to seven distinct clusters based on MLSA combined with 16S rRNA gene sequence and phenotypic analyses. Isolates from newly described groups 3, 4, and 7 were of genetically homogeneous nature and contained β-hemolytic strains belonging to the Lancefield group C. The strains were mostly isolated from throat infections. The taxonomic presented at work will allow for a correct diagnosis which will facilitate a full understanding of the clinical significance (Jensen et al., 2013).
It is clear that the application of the molecular biology techniques has allowed for a more precise identification of SAG, thereby eliminating ambiguous or erroneous results that may have been unavoidable in the past (OPEN QUESTION 5).
Conclusion
The precise identification of viridans streptococci at the species level is of great clinical importance. Every year, there are more and more reports of life-threatening infections with SAG. The surprise of clinicians with the identification of SAG streptococci as the etiological factor of the cases studied shows how much more remains to be learned about clinical pictures and diagnostic best practices. The long list of risk factors predisposing to the development of serious infections induced by SAG pointing them as community acquired pathogens. Increased susceptibility of people and repeated documented indications of the clear participation of SAG in serious infections show that SAG appear as emerging opportunistic pathogens.
Open Questions
1. What is the real prevalence rate of SAG infections and the mortality rate?
2. Is there a profile of coexistence among the identified groups of bacteria?
3. Do SAGs have the ability to spread through the neural pathway?
4. Do SAGs act as independent pathogens, become harmful in relation to other microbes, or may support the pathogenicity of other microbes?
5. Is there a need to design a unified diagnostic scheme that leads to the precise identification of individual SAG species in clinical materials?
Author Contributions
MP-Z, IS, and JK created conception and design of the study and wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the National Science Center, Poland under 2018/29/B/NZ6/00624.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://smart.servier.com/).
References
Akuzawa, N., Hatori, T., Kitahara, Y., and Kurabayashi, M. (2017). Multiple liver abscesses and bacteremia caused by Streptococcus constellatus infection: a case report. Clin. Case Rep. 5:69. doi: 10.1002/ccr3.774
Al Majid, F., Aldrees, A., Barry, M., Binkhamis, K., Allam, A., and Almohaya, A. (2020). Streptococcus anginosus group infections: management and outcome at a tertiary care hospital. J. Infect. Public Health 13, 1749–1754. doi: 10.1016/j.jiph.2020.07.017
Asam, D., and Spellerberg, B. (2014). Molecular pathogenicity of Streptococcus anginosus. Mol. Oral Microbiol. 29, 145–155.
Benou, C., Walter, B., Schlitter, M., Wilhelm, D., Neu, B., and Schmid, R. (2016). Gastrointestinal stromal tumor as entry port for S. intermedius causing bacteremia and multiple liver abscesses. Case report and review of literature. Z. Gastroenterol. 54, 245–249. doi: 10.1055/s-0042-100628
Beom, J. W., Ko, Y., Boo, K. Y., Lee, J.-G., Choi, J. H., Joo, S. J., et al. (2021). A successfully treated case of primary purulent pericarditis complicated by cardiac tamponade and pneumopericardium. Acute Crit. Care 36, 70–74. doi: 10.4266/acc.2020.00234
Bert, F., Lancelin, M. B., and Zechovsky, N. L. (1998). Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin. Infect. Dis. 27, 385–387. doi: 10.1086/514658
Branson, S. V., McClintic, E., and Yeatts, R. P. (2019). Septic cavernous sinus thrombosis associated with orbital cellulitis: a report of 6 cases and review of literature. Ophthalmic Plast. Reconstr. Surg. 35, 272–280. doi: 10.1097/IOP.0000000000001231
Cai, Q. (2020). Streptococcus anginosus purulent pericarditis with cardiac tamponade after coronary artery bypass surgery. BMJ Case Rep. 13:e235862. doi: 10.1136/bcr-2020-235862
Carpenter, J., Stapleton, S., and Holliman, R. (2007). Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 26, 1–11.
Chrastek, D., Hickman, S., Sitaranjan, D., Vokshi, I., Kakisi, O., Kadlec, J., et al. (2020). Streptococcus constellatus causing empyema and sepsis, necessitating early surgical decortication. Case Rep. Infect. Dis. 2020:4630809. doi: 10.1155/2020/4630809
Clarridge, III. J. E., Attorri, S., Musher, D. M., Hebert, J., and Dunbar, S. (2001). Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin. Infect. Dis. 32, 1511–1515. doi: 10.1086/320163
Coykendall, A. L., Wesbecher, P. M., and Gustafson, K. B. (1987). Streptococcus milleri” Streptococcus constellatus, and Streptococcus intermedius are later synonyms of Streptococcus anginosus. Int. J. Syst. Evol. 37, 222–228.
Darlow, C., McGlashan, N., Kerr, R., Oakley, S., Pretorius, P., Jones, N., et al. (2020). Streptococcus intermedius as a leading agent of brain abscess: retrospective analysis of a UK cohort. MedRxiv [Preprint]. doi: 10.1101/2020.01.25.20018788
Desar, I. M., de Boer, M., Bens, C. C., Jacobs, J. A., Mouton, J. W., Dofferhoff, A. S., et al. (2008). Rapid and reliable identification of Streptococcus anginosus group isolates to the species level by real-time PCR and melting curve analysis. J. Microbiol. Methods 75, 372–374. doi: 10.1016/j.mimet.2008.06.022
Deutschmann, M. W., Livingstone, D., Cho, J. J., Vanderkooi, O. G., and Brookes, J. T. (2013). The significance of Streptococcus anginosus group in intracranial complications of pediatric rhinosinusitis. JAMA Otolaryngol. Head Neck Surg. 139, 157–160. doi: 10.1001/jamaoto.2013.1369
Dhotre, S., Jahagirdar, V., Suryawanshi, N., Davane, M., Patil, R., and Nagoba, B. (2018). Assessment of periodontitis and its role in viridans streptococcal bacteremia and infective endocarditis. Indian Heart J. 70, 225–232. doi: 10.1016/j.ihj.2017.06.019
Dsouza, R., Roopavathana, B., Chase, S., and Nayak, S. (2019). Streptococcus constellatus: a rare causative agent of pyogenic liver abscess. BMJ Case Rep. 12:e229738. doi: 10.1136/bcr-2019-229738
Elhussein, T. A., and Hutchison, S. J. (2014). Streptococcus constellatus community acquired pneumonia with subsequent isolated pulmonic valve endocarditis and abscess formation in a structurally normal heart. J. Cardiovasc. Ultrasound 22, 91–94. doi: 10.4250/jcu.2014.22.2.91
Esplin, N., Stelzer, J. W., All, S., Kumar, S., Ghaffar, E., and Ali, S. (2017). A case of Streptococcus anginosus brain abscess caused by contiguous spread from sinusitis in an immunocompetent patient. Cureus 9:e1745. doi: 10.7759/cureus.1745
Falcone, E. L., Hanses, S., Stock, F., Holland, S. M., Zelazny, A. M., and Uzel, G. (2012). Streptococcal infections in patients with chronic granulomatous disease: case report and review of the literature. J. Clin. Immunol. 32, 649–652.
Flynn, C. E., and Ruoff, K. L. (1995). Identification of” Streptococcus milleri” group isolates to the species level with a commercially available rapid test system. J. Clin. Microbiol. 33, 2704–2706. doi: 10.1128/jcm.33.10.2704-2706.1995
Forner, L., Larsen, T., Kilian, M., and Holmstrup, P. (2006). Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin Periodontol. 33, 401–407. doi: 10.1111/j.1600-051X.2006.00924.x
Furuichi, M., and Horikoshi, Y. (2018). Sites of infection associated with Streptococcus anginosus group among children. J. Infect. Chemother. 24, 99–102. doi: 10.1016/j.jiac.2017.09.011
Giuliano, S., Rubini, G., Conte, A., Goldoni, P., Falcone, M., Vena, A., et al. (2012). Streptococcus anginosus group disseminated infection: case report and review of literature. Infez. Med. 20, 145–154.
Gray, T. (2005). Streptococcus anginosus group: clinical significance of an important group of pathogens. Clin. Microbiol. Newsl. 27, 155–159.
Grinwis, M. E., Sibley, C. D., Parkins, M. D., Eshaghurshan, C. S., Rabin, H. R., and Surette, M. G. (2010). Characterization of Streptococcus milleri group isolates from expectorated sputum of adult patients with cystic fibrosis. J. Clin. Microbiol. 48, 395–401. doi: 10.1128/JCM.01807-09
Han, J. K., and Kerschner, J. E. (2001). Streptococcus milleri: an organism for head and neck infections and abscess. Arch. Otolaryngol. Head Neck Surg. 127, 650–654. doi: 10.1001/archotol.127.6.650
Hanna, A., Imam, Z., Odish, F., and Dalal, B. (2020). Multiple liver abscesses caused by S treptococcus intermedius bacteremia in the setting of a routine dental cleaning. BMJ Case Rep. 13:e233097. doi: 10.1136/bcr-2019-233097
Hannoodi, F., Ali, I., Sabbagh, H., and Kumar, S. (2016). Streptococcus intermedius causing necrotizing pneumonia in an immune competent female: a case report and literature review. Case Rep. Pulmonol. 2016:7452161 doi: 10.1155/2016/7452161
He, X. S., and Shi, W. Y. (2009). Oral microbiology: past, present and future. Int. J. Oral Sci. 1, 47–58.
Hidaka, H., Kuriyama, S., Yano, H., Tsuji, I., and Kobayashi, T. (2011). Precipitating factors in the pathogenesis of peritonsillar abscess and bacteriological significance of the Streptococcus milleri group. Eur. J. Clin. Microbiol. Infect. Dis. 30, 527–532. doi: 10.1007/s10096-010-1114-9
Hirai, J., Sakanashi, D., Haranaga, S., Kinjo, T., Hagihara, M., Kato, H., et al. (2016). Case-control study of pneumonia patients with Streptococcus anginosus group bacteria in their sputum. J. Infect. Chemother. 22, 794–799. doi: 10.1016/j.jiac.2016.08.014
Hiura, A., Kim, E.-C., Ikehara, T., Matsumura, Y., Mishima, K., and Ishida, I. (2000). Hepatic abscess as a complication of the sump syndrome. J. Hepatobiliary Pancreat. Surg. 7, 231–235.
Hutchin, M. E., Shores, C. G., Bauer, M. S., and Yarbrough, W. G. (1999). Sinogenic subdural empyema and Streptococcus anginosus. Arch. Otolaryngol. Head Neck Surg. 125, 1262–1266. doi: 10.1001/archotol.125.11.1262
Ioannou, A., Xenophontos, E., Karatsi, A., Petrides, C., Kleridou, M., and Zintilis, C. (2016). Insidious manifestation of pyogenic liver abscess caused by Streptococcus intermedius and Micrococcus luteus: a case report. Oxf. Med. Case Rep. 2016, 1–3. doi: 10.1093/omcr/omv071
Issa, E., Salloum, T., and Tokajian, S. (2020). From normal flora to brain abscesses: a review of Streptococcus intermedius. Front. Microbiol. 11:826. doi: 10.3389/fmicb.2020.00826
Jacobs, J. A., Pietersen, H. G., Stobberingh, E. E., and Soeters, P. B. (1994). Bacteremia involving the “Streptococcus milleri” group: analysis of 19 cases. Clin. Infect. Dis. 19, 704–713. doi: 10.1093/clinids/19.4.704
Jacobs, J. A., Pietersen, H. G., Stobberingh, E. E., and Soeters, P. B. (1995). Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. Clinical relevance, hemolytic and serologic characteristics. Am. J. Clin. Pathol. 104, 547–53. doi: 10.1093/ajcp/104.5.547
Jensen, A., Hoshino, T., and Kilian, M. (2013). Taxonomy of the Anginosus group of the genus Streptococcus and description of Streptococcus anginosus subsp. whileyi subsp. nov. and Streptococcus constellatus subsp. viborgensis subsp. nov. Int. J. Syst. Evol. Microbiol. 63, 2506–2519. doi: 10.1099/ijs.0.043232-0
Jerng, J.-S., Hsueh, P.-R., Teng, L.-J., Lee, L.-N., Yang, P.-C., and Luh, K.-T. (1997). Empyema thoracis and lung abscess caused by viridans streptococci. Am. J. Respir. Crit. Care Med. 156, 1508–1514.
Jiang, S., Li, M., Fu, T., Shan, F., Jiang, L., and Shao, Z. (2020). Clinical characteristics of infections caused by Streptococcus anginosus group. Sci. Rep. 10:9032.
Jousimies-Somer, H., Savolainen, S., Mäkitie, A., and Ylikoski, J. (1993). Bacteriologic findings in peritonsillar abscesses in young adults. Clin. Infect. Dis. 16, S292–S298.
Khan, M. S., Khan, Z., Banglore, B. S., Alkhoury, G., Murphy, L., and Georgescu, C. (2018). Primary purulent bacterial pericarditis due to Streptococcus intermedius in an immunocompetent adult: a case report. J. Med. Case Rep. 12, 1–5. doi: 10.1186/s13256-018-1570-x
Kirkman, M. A., Donaldson, H., and O’Neill, K. (2012). Multiple intracranial abscesses due to Streptococcus anginosus in a previously well individual. J. Neurol. Neurosurg. Psychiatry 83, 1231–1232. doi: 10.1136/jnnp-2012-303165
Kobashi, Y., Mouri, K., Yagi, S., Obase, Y., and Oka, M. (2008). Clinical analysis of cases of empyema due to Streptococcus milleri group. Jpn. J. Infect. Dis. 61, 484–486.
Kobo, O., Nikola, S., Geffen, Y., and Paul, M. (2017). The pyogenic potential of the different Streptococcus anginosus group bacterial species: retrospective cohort study. Epidemiol. Infect. 145, 3065–3069. doi: 10.1017/S0950268817001807
Kozińska, A., Seweryn, P., and Sitkiewicz, I. (2019). A crash course in sequencing for a microbiologist. J. Appl. Genet. 60, 103–111. doi: 10.1007/s13353-019-00482-2
Kragha, K. (2016). Multiple brain abscesses due to Streptococcus anginosus: prediction of mortality by an imaging severity index score. Case Rep. Radiol. 2016:7040352 doi: 10.1155/2016/7040352
Laupland, K. B., Pasquill, K., Parfitt, E. C., Dagasso, G., and Steele, L. (2018). Streptococcus anginosus group bloodstream infections in the western interior of British Columbia, Canada. Infect. Dis. 50, 423–428. doi: 10.1080/23744235.2017.1416163
Li, Q., Zi, J., Liu, F., and Li, D. (2013). Purulent pericarditis caused by a bad tooth. Eur. Heart J. 34, 862–862. doi: 10.1093/eurheartj/eht008
Lin, C. Y., Chao, P. C., Hong, G. J., Tsai, Y. T., Lee, C. Y., and Tsai, C. S. (2008). Infective endocarditis from Streptococcus viridans associated with colonic carcinoma: a case report. J. Card. Surg. 23, 263–265. doi: 10.1111/j.1540-8191.2007.00528.x
Liu, L.-C., Tsai, J.-C., Hsueh, P.-R., and Teng, L.-J. (2006). Rapid differentiation between members of the Anginosus group and Streptococcus dysgalactiae subsp. equisimilis within beta-hemolytic group C and G streptococci by PCR. J. Clin. Microbiol. 44, 1836–1838. doi: 10.1128/JCM.44.5.1836-1838.2006
Liu, X., Shao, L., Liu, X., Ji, F., Mei, Y., Cheng, Y., et al. (2019). Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine 40, 336–348. doi: 10.1016/j.ebiom.2018.12.034
Livingston, L. V., and Perez-Colon, E. (2014). Streptococcus intermedius bacteremia and liver abscess following a routine dental cleaning. Case Rep. Infect. Dis. 2014:954046. doi: 10.1155/2014/954046
Lockhart, P. B., Brennan, M. T., Thornhill, M., Michalowicz, B. S., Noll, J., Bahrani-Mougeot, F. K., et al. (2009). Poor oral hygiene as a risk factor for infective endocarditis–related bacteremia. J. Am. Dent. Assoc. 140, 1238–1244. doi: 10.14219/jada.archive.2009.0046
*Mac Faddin, J. F. (1980). Biochemical Tests for Identification of Medical Bacteria, 2nd Edn. (Baltimore, MD: Williams and Wilkins Co), 345–370.
Masood, U., Sharma, A., Lowe, D., Khan, R., and Manocha, D. (2016). Colorectal cancer associated with Streptococcus anginosus bacteremia and liver abscesses. Case Rep. Gastroenterol. 10, 769–774.
Maves, R. C., Tripp, M. S., Franzos, T., Wallace, S. C., Drinkwine, B. J., and Villines, T. C. (2017). Pyogenic pericarditis and cardiac tamponade due to Streptococcus anginosus in a combat theater. Open Forum Infect. Dis. 4:ofw267. doi: 10.1093/ofid/ofw267
Mayor, J. S., Robalo, M. M., Pacheco, A. P., and Esperança, S. (2016). Pyogenic liver abscess: uncommon presentation. BMJ Case Rep. 2016:bcr2016214841. doi: 10.1136/bcr-2016-214841
Meddings, L., Myers, R. P., Hubbard, J., Shaheen, A. A., Laupland, K. B., Dixon, E., et al. (2010). A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am. J. Gastroenterol. 105, 117–124. doi: 10.1038/ajg.2009.614
Millichap, J., McKendrick, A., and Drelichman, V. (2005). Streptococcus intermedius liver abscesses and colon cancer: a case report West Indian Med. J. 54:341. doi: 10.1590/s0043-31442005000500014
Morinaga, Y., Yanagihara, K., Gyotoku, H., Oshima, K., Izumikawa, K., Yamasaki, N., et al. (2013). Pulmonary artery pseudoaneurysm caused by Streptococcus constellatus. Int. J. Infect. Dis. 17, e1064-6. doi: 10.1016/j.ijid.2013.03.013
Mukae, H., Noguchi, S., Naito, K., Kawanami, T., Yamasaki, K., Fukuda, K., et al. (2016). The importance of obligate anaerobes and the Streptococcus anginosus group in pulmonary abscess: a clone library analysis using bronchoalveolar lavage fluid. Respiration 92, 80–89. doi: 10.1159/000447976
Navarrete, D., Patil, S., and Dandachi, D. (2020). Acute Streptococcus constellatus Pyogenic Liver Abscess Due to an Atypical Presentation of Sigmoid Diverticulitis Complicated by Pericolonic Abscess. Cureus 12:e10940. doi: 10.7759/cureus.10940
Neumayr, A., Kubitz, R., Bode, J., Bilk, P., and Häussinger, D. (2010). Multiple liver abscesses with isolation of Streptococcus intermedius related to a pyogenic dental infection in an immuno-competent patient. Eur. J. Med. Res. 15, 319–322. doi: 10.1186/2047-783x-15-7-319
Nguyen, M., Brettin, T., Long, S., Musser, J. M., Olsen, R. J., Olson, R., et al. (2018). Developing an in silico minimum inhibitory concentration panel test for Klebsiella pneumoniae. Sci. Rep. 8:421. doi: 10.1038/s41598-017-18972-w
Noguchi, S., Yatera, K., Kawanami, T., Yamasaki, K., Naito, K., Akata, K., et al. (2015). The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 15:133. doi: 10.1186/s12890-015-0128-6
Olson, A., Sibley, C., Schmidt, L., Wilcox, M., Surette, M., and Corbett, C. (2010). Development of real-time PCR assays for detection of the Streptococcus milleri group from cystic fibrosis clinical specimens by targeting the cpn60 and 16S rRNA genes. J. Clin. Microbiol. 48, 1150–1160. doi: 10.1128/JCM.02082-09
Ono, Y., Hashimoto, T., Sakamoto, K., Matsushima, S., Higo, T., Sonoda, H., et al. (2021). Effusive–constrictive pericarditis secondary to pneumopericardium associated with gastropericardial fistula. ESC Heart Fail. 8, 778–781. doi: 10.1002/ehf2.13135
Pang, T. C., Fung, T., Samra, J., Hugh, T. J., and Smith, R. C. (2011). Pyogenic liver abscess: an audit of 10 years’ experience. World J. Gastroenterol. 17:1622.
Parthvi, R., Amin, M., and Mehra, S. (2017). Antimicrobial therapy for pyogenic liver abscess secondary to Streptococcus intermedius bacteremia. Am. J. Ther. 24, e770–e771. doi: 10.1097/MJT.0000000000000537
Poyart, C., Quesne, G., Coulon, S., Berche, P., and Trieu-Cuot, P. (1998). Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 36, 41–47. doi: 10.1128/JCM.36.1.41-47.1998
Prateepchaiboon, T., Akarapatima, K., Pisudtinontakul, W., Rattanasupa, A., and Chang, A. (2020). A rare case of massive pericardial effusion due to spontaneous rupture of Streptococcus anginosus group liver abscess. J. Clin. Gastroenterol. 13, 1258–1264. doi: 10.1007/s12328-020-01196-3
Presnell, L., Maeda, K., Griffin, M., and Axelrod, D. (2014). A child with purulent pericarditis and Streptococcus intermedius in the presence of a pericardial teratoma: an unusual presentation. J. Thorac. Cardiovasc. Surg. 147, e23–e24. doi: 10.1016/j.jtcvs.2013.11.025
Price, K. E., Naimie, A. A., Griffin, E. F., Bay, C., and O’Toole, G. A. (2015). Tobramycin-treated Pseudomonas aeruginosa PA14 enhances Streptococcus constellatus 7155 biofilm formation in a cystic fibrosis model system. J. Bacteriol. 198, 237–247. doi: 10.1128/JB.00705-15
Purohit, V., Bode, J. C., Bode, C., Brenner, D. A., Choudhry, M. A., Hamilton, F., et al. (2008). Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 42, 349–361. doi: 10.1016/j.alcohol.2008.03.131
Reddy, S., Singh, K., and Hughes, S. (2018). Liver abscesses caused by Streptococcus intermedius in an immunocompromised patient. Cureus 10:e2107.
Reißmann, S., Friedrichs, C., Rajkumari, R., Itzek, A., Fulde, M., Rodloff, A. C., et al. (2010). Contribution of Streptococcus anginosus to infections caused by groups C and G streptococci, southern India. Emerg. Infect. Dis. 16:656. doi: 10.3201/eid1604.090448
Santos, F. V., Pires, S. X., Pereira, C., Gonçalves, L., Martins, S., and Aragão, I. (2020). Deep neck space infection and Lemierre’s syndrome caused by Streptococcus anginosus: a case report. IDCases 19:e00669. doi: 10.1016/j.idcr.2019.e00669
Sasaki, M., Yamaura, C., Ohara-Nemoto, Y., Tajika, S., Kodama, Y., Ohya, T., et al. (2005). Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 11, 151–156. doi: 10.1111/j.1601-0825.2005.01051.x
Sheng, W.-S., Hsueh, P.-R., Hung, C.-C., Teng, L.-J., Chen, Y.-C., and Luh, K.-T. (2001). Clinical features of patients with invasive Eikenella corrodens infections and microbiological characteristics of the causative isolates. Eur. J. Clin. Microbiol. Infect. Dis. 20, 231–236. doi: 10.1007/pl00011259
Siegman-Igra, Y., Azmon, Y., and Schwartz, D. (2012). Milleri group streptococcus—a stepchild in the viridans family. Eur. J. Clin. Microbiol. Infect. Dis. 31, 2453–2459. doi: 10.1007/s10096-012-1589-7
Sitkiewicz, I. (2018). How to become a killer, or is it all accidental? Virulence strategies in oral streptococci. Mol. Oral Microbiol. 33, 1–12.
Stryjewski, P. J., Kuczaj, A., Nowalany-Kozielska, E., and Nessler, J. (2015). Infective endocarditis caused by Streptococcus anginosus presenting with dehiscence of aortic valve and mitral vegetation in patient with bioprosthetic valves. Folia Cardiol. 10, 45–47.
Suzuki, H., Hase, R., Otsuka, Y., and Hosokawa, N. (2016). Bloodstream infections caused by Streptococcus anginosus group bacteria: a retrospective analysis of 78 cases at a Japanese tertiary hospital. J. Infect. Chemother. 22, 456–460. doi: 10.1016/j.jiac.2016.03.017
Tabata, A., Yamada, T., Ohtani, H., Ohkura, K., Tomoyasu, T., and Nagamune, H. (2019). β-Hemolytic Streptococcus anginosus subsp. anginosus causes streptolysin S-dependent cytotoxicity to human cell culture lines in vitro. J. Oral Microbiol. 11:1609839. doi: 10.1080/20002297.2019.1609839
Takeuchi, N., Naito, H., Yumoto, T., Tsukahara, K., Yamada, T., Osako, T., et al. (2017). Multiple Liver Abscesses Caused by Streptococcus constellatus: a Case Report. Surg. Infect. Case Rep. 2, 61–64.
Teles, C., Smith, A., Ramage, G., and Lang, S. (2011). Identification of clinically relevant viridans group streptococci by phenotypic and genotypic analysis. Eur. J. Clin. Microbiol. Infect. Dis. 30, 243–250. doi: 10.1007/s10096-010-1076-y
Terzi, H. A., Demiray, T., Koroglu, M., Cakmak, G., Ciftci, I. H., Ozbek, A., et al. (2016). Intra-abdominal abscess and primary peritonitis caused by Streptococcus anginosus. Jundishapur J. Microbiol. 9:e33863. doi: 10.5812/jjm.33863
Tigen, E. T., Sari, I., Ak, K., Sert, S., Tigen, K., and Korten, V. (2015). Giant Purulent Pericarditis with Cardiac Tamponade Due to Streptococcus intermedius Rapidly Progressing to Constriction. Echocardiography 32, 1318–1321. doi: 10.1111/echo.12919
Tzur, M., Liberman, S., Felzenstein, I., Cohen, R., Rivkind, A. I., and Almogy, G. (2003). Liver abscess caused by Streptococcus milleri: an uncommon presenting sign of silent colonic cancer. Isr. Med. Assoc. J. 5, 206–207.
Vandamme, P, Torck, U, Falsen, E, Pot, B, Goossens, H, and Kersters, K. (1998). Whole-cell protein electrophoretic analysis of viridans streptococci: evidence for heterogeneity among Streptococcus mitis biovars. Int. J. Syst. Bacteriol. 48, 117–125. doi: 10.1099/00207713-48-1-117
Vulisha, A. K., Sam, R., Nur, H., Bhardwaj, N., and Sirineni, S. (2021). Aggressive presentation of Streptococcus constellatus. Cureus 13:e14534.
Wagner, K. W., Schön, R., Schumacher, M., Schmelzeisen, R., and Schulze, D. (2006). Case report: brain and liver abscesses caused by oral infection with Streptococcus intermedius. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 102, e21–e23. doi: 10.1016/j.tripleo.2006.02.010
Wei, Y. S., Chang, Y. R., Tsai, Y. T., Yang, Y. T., Weng, S. H., Tseng, L. F., et al. (2021). The distribution of cultivable oral anaerobic microbiota identified by MALDI-TOF MS in healthy subjects and in patients with periodontal disease. J. Pharm. Biomed. 192:113647. doi: 10.1016/j.jpba.2020.113647
Weightman, N. C., Barnham, M. R., and Dove, M. (2004). Streptococcus milleri group bacteraemia in North Yorkshire. England (1989-2000). Indian J. Med. Res. 119, 164–167.
Wenzler, E., Chandrasekaran, V., Salvador, P., Anwar, M., Pancholi, P., and McGwire, B. S. (2015). Clinical and microbiological outcomes in patients with Streptococcus anginosus group bacteraemia identified through use of a rapid microarray assay. J. Med. Microbiol. 64, 1369–1374. doi: 10.1099/jmm.0.000176
Whiley, R., Beighton, D., Winstanley, T., Fraser, H., and Hardie, J. (1992). Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J. Clin. Microbiol. 30, 243–244. doi: 10.1128/jcm.30.1.243-244.1992
Whiley, R., Fraser, H., Hardie, J., and Beighton, D. (1990). Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the”. Streptococcus milleri group”. J. Clin. Microbiol. 28, 1497–1501.
Whiley, R., Hall, L., Hardie, J., and Beighton, D. (1999). A study of small-colony, β-haemolytic, Lancefield group C streptococci within the anginosus group: description of Streptococcus constellatus subsp. pharyngis subsp. nov., associated with the human throat and pharyngitis. Int. J. Syst. Evol. 49, 1443–1449. doi: 10.1099/00207713-49-4-1443
Wilson, M., Lewis, M., and Bishop, P. (1995). A preliminary study of the ‘Streptococcus milleri’ group and Prevotella in orofacial infection. Microb. Ecol. Health Dis. 8, 171–174.
Wong, Y. H., Huang, P. J., Chen, F. L., and Lee, W. S. (2019). Streptococcus constellatus septicemia complicating endocarditis and liver abscess associated with gastric adenocarcinoma. J. Microbiol. Immunol. Infect. 52, 1002–1003. doi: 10.1016/j.jmii.2019.10.007
Woo, P. C., Tse, H., Chan, K. M., Lau, S. K., Fung, A. M., Yip, K. T., et al. (2004). Streptococcus milleri” endocarditis caused by Streptococcus anginosus. Diagn. Microbiol. Infect. Dis. 48, 81–88.
Woods, K., Beighton, D., and Klein, J. L. (2014). Identification of the ‘Streptococcus anginosus group’ by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry. J. Med. Microbiol. 63, 1143–1147. doi: 10.1099/jmm.0.076653-0
Wu, H., and Zheng, R. (2020). Splenic abscess caused by Streptococcus anginosus bacteremia secondary to urinary tract infection: a case report and literature review. Open Med. 15, 997–1002. doi: 10.1515/med-2020-0117
Yamamoto, M., Fukushima, T., Ohshiro, S., Go, Y., Tsugu, H., Kono, K., et al. (1999). Brain abscess caused by Streptococcus intermedius: two case reports. Surg. Neurol. 51, 219–222.
Yoshino, Y., Kimura, Y., Sakai, T., Kanzaki, T., Seo, K., Koga, I., et al. (2013). Infective endocarditis due to a rare pathogen, Streptococcus constellatus, in a patient with gingivitis: a case report and review of the literature. Open Med. 8, 489–492.
Young, K. A., Allaker, R. P., Hardie, J. M., and Whiley, R. A. (1996). Interactions between Eikenella corrodens and ‘Streptococcus milleri-group’organisms: possible mechanisms of pathogenicity in mixed infections. Antonie Van Leeuwenhoek 69, 371–373. doi: 10.1007/BF00399626
Keywords: Streptococcus anginosus group, opportunistic pathogens, bacteremia, abscesses, empyema, clinical infection, Streptococcus milleri group
Citation: Pilarczyk-Zurek M, Sitkiewicz I and Koziel J (2022) The Clinical View on Streptococcus anginosus Group – Opportunistic Pathogens Coming Out of Hiding. Front. Microbiol. 13:956677. doi: 10.3389/fmicb.2022.956677
Received: 30 May 2022; Accepted: 17 June 2022;
Published: 08 July 2022.
Edited by:
Axel Cloeckaert, Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE), FranceReviewed by:
Hideaki Nagamune, Tokushima University, JapanMogens Kilian, Aarhus University, Denmark
Copyright © 2022 Pilarczyk-Zurek, Sitkiewicz and Koziel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Koziel, am9hbm5hLmtvemllbEB1ai5lZHUucGw=
 Magdalena Pilarczyk-Zurek
Magdalena Pilarczyk-Zurek Izabela Sitkiewicz
Izabela Sitkiewicz Joanna Koziel
Joanna Koziel