- 1Maestría en Química Aplicada, Facultad de Ciencias Exactas y Naturales, Universidad Técnica Particular de Loja, Loja, Ecuador
- 2Grupo de Investigación y Desarrollo de la Biotecnología BioSin-Biociencias, Quito, Ecuador
- 3Centro de Nanociencia y Nanotecnología, Universidad de las Fuerzas Armadas ESPE, Sangolquí, Ecuador
- 4Grupo de Investigación en Materiales y Ambiente, Departamento de Química, Universidad Técnica Particular de Loja (UTPL), Loja, Ecuador
Adaptation and microbial attachment mechanisms for the degradation of sulfide ores are mediated by the production of extracellular polymeric substances (EPS) and their role in biofilm formation. EPS production responds to induction mechanisms associated with environmental conditions. In this study, the double induction of EPS with galactose and high ferric iron concentrations in planktonic cells of Acidithiobacillus ferrooxidans, and their attachment on the surface of a polymetallic sulfide ore from Bella Rica-Azuay in Ecuador were evaluated. A. ferrooxidans cells were previously adapted to different concentrations of galactose [0, 0.15, and 0.25% (w/v)], using two ferrous iron concentrations as an energy source (9 and 18 g L–1) in a 9K culture medium. EPS production and its effect on mineral attachment were determined at the time point of maximal growth. The results obtained show a maximum cell attachment of 94.1% within 2 h at 0.15% of galactose and 18 g⋅L–1 of ferric iron concentration, compared to 71.4% without galactose and 9 g⋅L–1 of ferric iron. The maximum concentration of EPS was obtained with a 0.25% galactose concentration; however, it did not result in greater attachment compared to 0.15% galactose concentration. Through the combined induction of low galactose concentration and high ferric iron concentration, the percentage of bacterial attachment can be increased and, therefore, a possible increase in the rate of biooxidation and bioleaching could be obtained.
Introduction
Biooxidation and bioleaching processes allow to obtain the metals from low-grade sulfide ores (Srichandan et al., 2019) and have been widely accepted in the mining industry when conventional treatments are not economically feasible (Kaksonen et al., 2020). Biooxidation and bioleaching are carried out by acidophilic chemolithoautotrophic microorganisms capable of oxidizing reduced sulfur and/or iron compounds to obtain energy (Sajjad et al., 2019). Acidithiobacillus ferrooxidans (A. ferrooxidans) is the most characterized acidophilic bacterium due to its potential to oxidize iron and sulfur, with studies focused on specific genomic and metabolic features (Hödar et al., 2012; Campodonico et al., 2016; Latorre et al., 2016).
During biooxidation and bioleaching, microorganisms oxidize Fe2+ to Fe3+. The last one acts as an oxidizing agent, chemically oxidizing metal sulfides, reducing them to Fe2+ during mineral dissolution (Vera et al., 2013). The role of microorganisms is to regenerate the oxidizing agent Fe3+ (Rawlings and Johnson, 2007). This mechanism occurs at the interface between the microorganism and the mineral; in this process, the presence of extracellular polymeric substances (EPS) is required, which allows bacterial attachment to produce a biological attack on the mineral surface, forming a biofilm (Li et al., 2016).
In A. ferrooxidans, initial adhesion is mediated by EPS and type IV pili (Sand and Gehrke, 2006; Li et al., 2010). Type IV pili and EPS contain adhesins and outer membrane proteins that allow them to colonize the mineral surface (Yu et al., 2011; Tu et al., 2013). The functional groups of the EPS generate hydrophobic forces and electrostatic interactions that hold cells together with the mineral, contributing to the global stability of biofilm matrices (Li et al., 2016). The composition and quantity of EPS differ according to the type of substrate and energy source, in which the cells are cultured; thus, the attachment mechanisms also differ depending on the mineral surfaces (Li et al., 2016). In addition, it has been observed that the EPS composition of cells cultured on minerals, such as pyrite, is very similar to the EPS composition of cells cultured in the planktonic phase in a ferrous sulfate-containing medium, but with a lower amount of EPS (Gehrke et al., 1998; Rohwerder and Sand, 2007; Vu et al., 2009).
With the discovery of an alternative metabolic pathway that allows A. ferrooxidans to use galactose as a carbon source for EPS production (Barreto et al., 2005), strategies for inducing EPS in the presence of this sugar have been carried out, obtaining positive results (Bellenberg et al., 2012; Pavez et al., 2013; Saavedra et al., 2013b,2020). Furthermore, it has been observed that adapting A. ferrooxidans to high ferric ion concentrations produces greater amounts of EPS (Saavedra et al., 2013a), and, in the same way, the EPS produced by induction with galactose also allows A. ferrooxidans to tolerate high ferric ion concentrations (Saavedra et al., 2020). However, when biooxidizing bacteria, an excess of EPS has not always resulted in a greater attachment (Aguirre et al., 2017, 2018). With this background, being A. ferrooxidans the predominant microorganism in the microbial consortia of biooxidation cells, this study aimed to increase the bacteria attachment capacity to a refractory gold-bearing polymetallic sulfide ore by regulating the EPS production with both high-iron concentrations and galactose to increase its biooxidation potential.
Materials and methods
Polymetallic sulfide ore
The polymetallic sulfide ore used in the attachment assays was from Bella Rica, Azuay-Ecuador. X-ray diffraction analysis (Profile and structure analysis performed with TOPAS software of the equipment Bruker D8-Advance) shows the following minerals in percentages (%): 30.24 quartz, 34.26 pyrite, 0.41 chalcopyrite, 0.95 galena, 3.41 sphalerite, 15.19 orthoclase, 8.24 gypsum,0.89 apatite-(Sr-OH), 2.41 pyrrhotite, 2.61 arsenopyrite,0.92 cobalt pentlandite, and 0.46 magnetite. The ore was ground to a particle size of 75 μm, verified by the D80 of the ASTM#200 sieve.
Culture, adaptation, and growth kinetics of Acidithiobacillus ferrooxidans
The A. ferrooxidans strain used in this study was obtained from the biomining and bioprocess laboratory of Universidad Técnica Particular de Loja. Bacterial adaptation was carried out through successive cultures for 2 months in six treatments corresponding to the planned experiments (9 g⋅L–1 of Fe2+ supplemented with 0, 0.15, and 0.25% galactose, and 18 g⋅L–1 of Fe2+ with 0, 0.15, and 0.25% galactose). A. ferrooxidans was grown at 30°C and 180 rpm in 250 ml flasks with 50 ml of modified 9K culture medium (composition in g⋅L–1: KCl 0.004; MgSO4 7H2O 0.005; (NH4) H2PO4 0.15; CaCl2 0.0012) supplemented with FeSO4⋅7H2O for the treatments with 9 g⋅and 18 g⋅L–1 of ferrous iron, respectively. The pH was adjusted to 1.8 with H2SO4. The microorganisms were considered adapted when they achieved a cell number equivalent to the non-galactose treatments, and they completed the Fe2+ oxidation time that remained similar and constant in each iron concentration treatment. After adaptation, growth kinetics were determined for each condition from an initial inoculum of 1⋅107 cells⋅ml–1 in 9K medium at pH 1.8 by cell count and Fe2+ consumption. Each culture was followed until the Fe2+ concentration was close to zero, corresponding to iron oxidation greater than 90%. Cell numbers were determined with a Neubauer chamber of 0.02 μm of depth every 3 h. Fe2+ consumption, Fe3+ production, and total iron amount were determined at each point using the ferrozine method (Lovley and Phillips, 1987). The maximum specific growth rate (μmax), the yield of biomass (YX/S), and the volumetric productivity (Pv) were calculated with the Equations (1)–(3), respectively, in the exponential growth phase.
Where X is the cell concentration achieved at the end of the exponential growth phase (cell⋅ml–1); X0 is the initial cell concentration at the beginning of the exponential growth phase (cell⋅ml–1); Xm is the cell mass concentration (g⋅L–1); [Fe3+] is the ferric iron concentration (g⋅L–1); S is the Fe2+ concentration (g⋅L–1); t is the time (h); Δ represents the increment of the variable from the initial stage to the final stage, and Ln represents the natural logarithm. The Fe3+ generation rate (σP) was calculated as the slope of the Fe3+ concentration over time plot in the exponential growth phase.
Production and extraction of extracellular polymeric substances
Adapted microorganisms were cultured with 9 and 18 g⋅L–1 of Fe2+, supplemented with 0, 0.15, and 0.25% of galactose. The EPS extraction was performed at the time point of maximal growth when the Fe2+ concentration reached zero. EPS was extracted using DOWEX cation exchange resin (DOWEX™ Marathon C) as it is a technique that does not cause cell damage to the cultures, avoiding breaking microorganism membranes (Tapia et al., 2013). A proportion of 70 g of resin per gram of cell dry weight was used, taking as reference the 1.2⋅10–12 g/bacteria and the microbial count. The resin was previously hydrated with 20 ml of PBS buffer at pH 7 under stirring for 1 h. Approximately 40 ml of culture were centrifuged at 5,800 g for 10 min discarding the supernatant. The cells were resuspended on the hydrating DOWEX resin. Cell suspension was shaken at 100 rpm and 4°C for 4 h. Subsequently, the extract was centrifuged at 4,500 g for 5 min and the supernatant was filtered through a 0.2-μm membrane. The filtered extracts were dialyzed in 3.4 kDa membrane tubes for 24 h in ddH2O water. Protein and carbohydrate contents were quantified. The cell suspension precipitate was cultured in a 9K medium with 9 g⋅L–1 of Fe2+ and, in addition, a sample of microbial cultures was analyzed for the absorption spectrum carried out over the whole wavelength spectrum from 325 to 825 nm, before and after EPS extraction to verify that there was no cell lysis during extraction (Tapia et al., 2013).
Determination of carbohydrates and proteins in extracellular polymeric substances
Carbohydrates were determined by the sulfuric acid phenol method (Dubois et al., 1956), using glucose as the standard. Approximately 0.5 ml of 5% phenol was added to 1 ml of EPS extract, followed by 2.5 ml of concentrated sulfuric acid, and then mixed rapidly. The solution was cooled at room temperature for 10 min avoiding direct light exposure and then incubated at 30°C in a water bath for 30 min. The absorbance was read at 492 nm. The protein content in the EPS extract was determined by the bicinchoninic acid (BCA) method (Walker, 2009), using the Thermo Scientific™ Pierce™ BCA Protein Determination Kit.
Extracellular polymeric substances observation by transmission electron microscopy
The presence/absence of EPS was evaluated using a Transmission Electron Microscope (TEM, FEI, TECNAI, and G2 Spirit Twin). A drop (5 μl) of bacterial suspension obtained at the time point of maximal growth of each assay was deposited on a formvar/carbon-coated TEM-grid (300-mesh). Then, samples were negatively stained using phosphotungstic acid (PTA) 0.5% for 1 s. The TEM micrographs were obtained at 80 kV by using a TEM equipped with an Eagle 4k HR camera.
Microbial attachment assays
The bacterial attachment was determined by cell counting in the liquid phase and contrasted by qPCR using DNA extracted from the ore sample. Bacterial attachment assays were performed in 250 ml Erlenmeyer flasks with 100 ml of 9K culture medium, supplemented with 2% w/v of ore previously acidified to pH 1.8, with sulfuric acid under a nitrogen atmosphere to prevent chemical oxidation, and sterilized at 105°C for 24 h in dry heat. The mineral medium was inoculated with cells at peak growth time at concentrations of 1⋅108 cells⋅ml–1 and incubated for 7 h at 30°C and 180 rpm. Samples of 5 ml were taken at time intervals of 1, 10, 30, 60, 120, 180, 240, and 420 min. The samples were centrifuged at 6 g for 3 min and the number of bacteria in the liquid phase was determined by counting in a Neubauer chamber under a phase contrast microscope. The attachment percentage was determined by the difference between the initial number of inoculated cells and the number of cells remaining in the liquid phase. Attachment kinetics was contrasted by real-time PCR (qPCR) from the DNA extracted from the mineral phase of the samples at 1, 30, 60, and 180 min; each assay was performed in triplicate.
DNA extraction and qPCR
Attachment kinetics by qPCR was performed by quantifying the number of copies of DNA extracted from 0.1 g of ore, which is equivalent to 5 ml of mineral in suspension at 2% w/v. About 5 ml of ore suspension were taken at times of 1, 30, 60, and 180 min from the attachment assays and were centrifuged at 6 g for 3 min. The supernatant was immediately discarded. DNA of the cells attached to the precipitated ore was extracted using the FastDNA™ Spin Kit for Soil-MP Biomedicals, eluting in 50 μl of nuclease-free water. The qPCRs were performed with the primers designed by Aguirre et al. (2021): 5′-TCTTCGGATGCTACAG-3′ and 5′-CGSGTTACBTACACACT-3′, which amplify a fragment of 785 base pairs corresponding to the region 674-1450 of the 16S ribosomal RNA gene of A. ferrooxidans, using the EvaGreen qPCR Mastermix-ABM. The DNA copy number standards were obtained by PCR from DNA extracted from pure cultures of A. ferrooxidans, according to the specifications of the Wizard® Genomic DNA Purification Kit—Promega using the GoTaq® Green Master Mix—Promega, and subsequent PCR product purification using the Wizard® SV Gel and PCR Clean-Up System—Promega kit. The attachment percentage was determined by the difference between the initial cell number inoculated per gram of ore and the number of attached cells per gram of ore (number of DNA copies per gram of ore).
Statistical analysis
Differences between EPS production and attachment percentages were evaluated by ANOVA and subsequent Bonferroni’s post hoc test using the statistical software R version 4.1.1 (Supplementary material). Each experiment was performed in triplicate by analytical duplicate (n = 6).
Results
Microbial adaptation and growth parameters
As galactose is not used for the growth of A. ferrooxidans since it causes inhibition (Saavedra et al., 2020), a laboratory adaptation process was carried out through successive cultures for concentrations of 9 and 18 g⋅L–1 of iron in the presence of 0.15 and 0.25% of galactose. After 2 months of adaptation, the growth kinetics of A. ferrooxidans was evaluated at different concentrations of iron and galactose. Table 1 summarizes the kinetic parameters obtained.
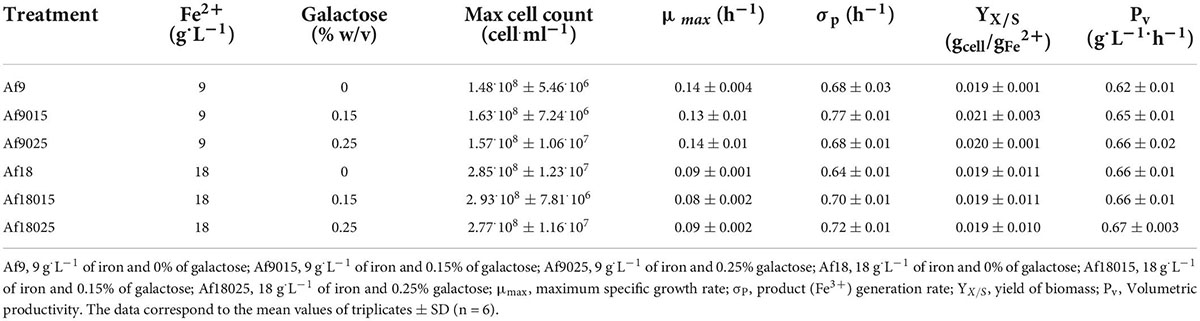
Table 1. Kinetic parameters of A. ferrooxidans cultured at different concentrations of iron and galactose.
A. ferrooxidans cultured with galactose exhibited a similar cell count compared with the treatments cultured without galactose for each iron concentration at the same period. Table 1 shows similar growth parameters for the different galactose concentration treatments denoting a complete adaptation. The volumetric productivity for Fe+3 remained relatively constant comparing 9 and 18 g⋅L–1 iron treatments.
Effect of galactose and ferric iron on the production of extracellular polymeric substances
EPS extractions were performed at peak growth time for each treatment, corresponding to 24 h for 9 g⋅L–1 iron culture, and 36 h for 18 g⋅L–1 iron culture. Total EPS production was quantified considering the amount of protein and carbohydrates (Figure 1).

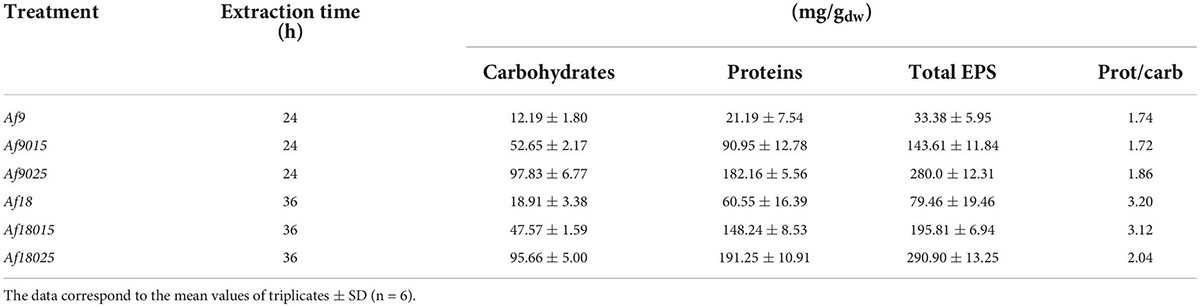
Figure 1. Carbohydrates and protein compositions of the EPS of A. ferrooxidans cultured at different concentrations of iron and galactose. Blue color: protein; Gray color: Carbohydrates. The data correspond to the mean values of triplicates ± SD (n = 6).
Figure 1 shows that EPS production increased, along with galactose concentration, for both 9 and 18 g⋅L–1 ferric iron treatments, reaching a higher concentration in cells cultured with 0.25% of galactose.
The media of EPS concentrations were 33.39, 143.61, and 280 mg/gdw for the induction with 9 g⋅L–1 of iron and 0, 0.15%, and 0.25% of galactose, respectively, and 83.81, 195.82, and 290.90 mg/gdw for the induction with 18 g⋅L–1 of iron and 0, 0.15%, and 0.25% of galactose, respectively. For 9 g⋅L–1 iron treatment, the EPS production increased 4.3 and 8.3 times in cells cultured with 0.15 and 0.25% of galactose; whereas, for 18 g⋅L–1 iron treatment, the EPS production increased 2.5, 5.8, and 8.7 times for cells cultured with 0,0.15, and 0.25% of galactose compared with the assays with 9g⋅L–1 without galactose.
Table 2 shows the composition of the EPS in terms of the carbohydrate and protein content for each induction treatment. Data shows a higher concentration of carbohydrates and proteins in the inductions with 0.25% of galactose, followed by the 0.15% treatments. The ratio of proteins per amount of carbohydrates in EPS remained relatively constant in the treatments with 9 g⋅L–1 of iron with increasing concentration of galactose, being 1.74 for the treatment without galactose, 1.72 and 1.86 for the induction with 0.15 and 0.25% of galactose. In 18 g⋅L–1 iron treatment, the protein/carbohydrate ratio was 3.1 and 3.2 for the induction with 0 and 0.15% of galactose, decreasing to 2.04 in the induction with 0.25% of galactose.
Comparing the EPS protein and carbohydrate content in the 18 g⋅L–1 iron treatment with the 9 g⋅L–1 iron treatment, it was observed that, for non-galactose assays, carbohydrates did not change significantly (p > 0.05), while protein content increased by 2.2 times. For 0.15 and 0.25% galactose assays, the carbohydrate content remained relatively constant, while the protein content, for the induction with 0.15% galactose, increased by 1.6 times and remained constant in the induction with 0.25% galactose. There was also no significant difference (p > 0.05) between the protein content comparing the treatments with 9 g⋅L–1 Fe and 0.15% galactose vs. the treatment with 18 g⋅L–1 iron and 0% galactose.
Extracellular polymeric substances visualization
EPS production in the planktonic phase was evidenced by TEM. Figure 2 shows that galactose-induced cells were surrounded by EPS (black arrows), whereas, in non-galactose treatments, there was no notable EPS visualization.
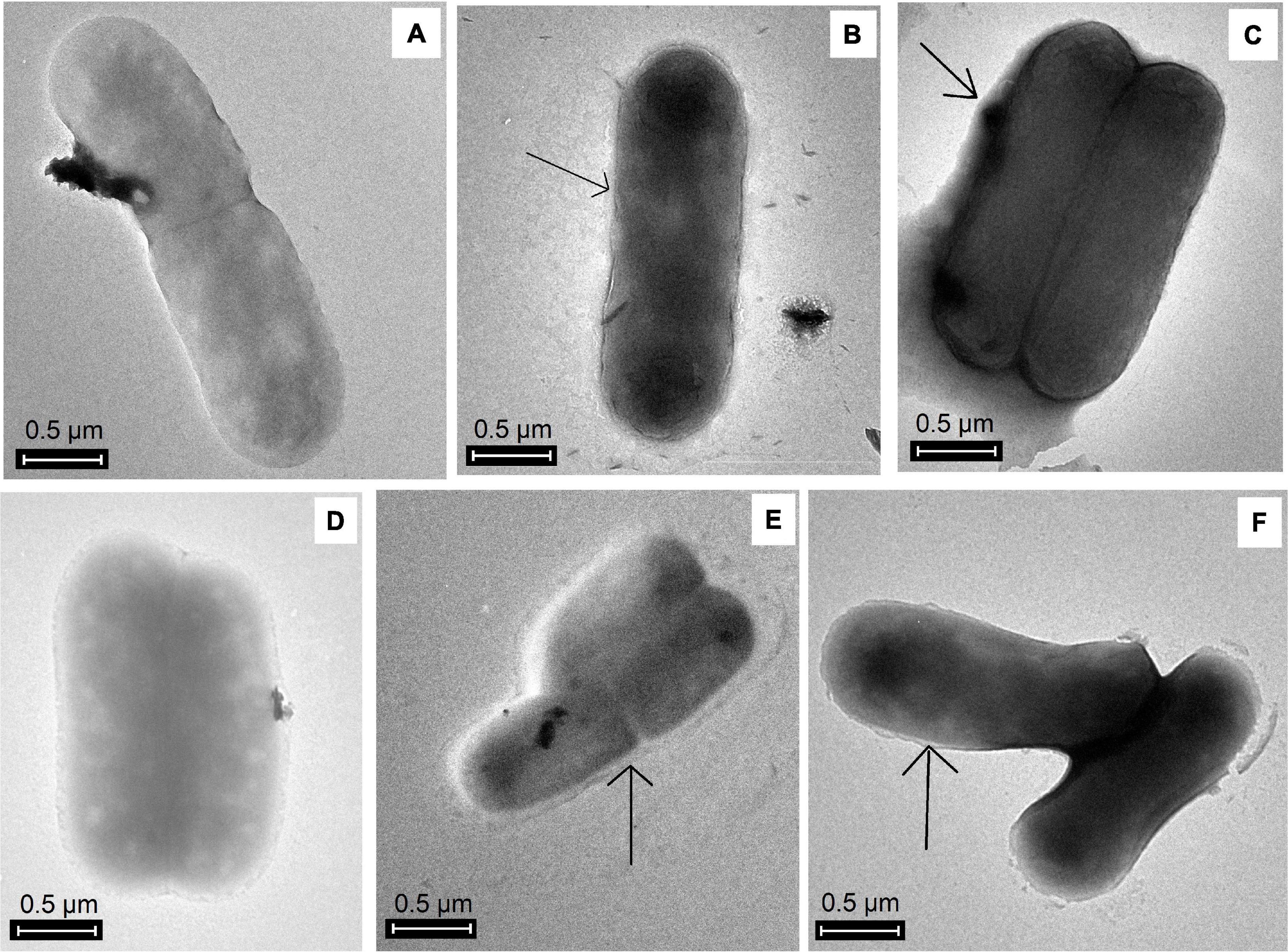
Figure 2. TEM images of EPS production by A. ferrooxidans in planktonic phase induced with different concentrations of iron and galactose. (A) Af9, (B) Af9015, (C) Af9025, (D) Af18, (E) Af18015, (F) Af18025. Black arrows show the presence of EPS.
Effect of extracellular polymeric substances induced with galactose and Fe3+ on the attachment of Acidithiobacillus ferrooxidans to the polymetallic sulfide ore
The attachment of A. ferrooxidans is more significant in the first hours of biooxidation (Rodríguez et al., 2003); thus, the assays were performed during the first 7 h. The results of bacterial attachment during the first 3 h (qPCR) and 7 h (cell count) of biooxidation are shown in Figure 3.
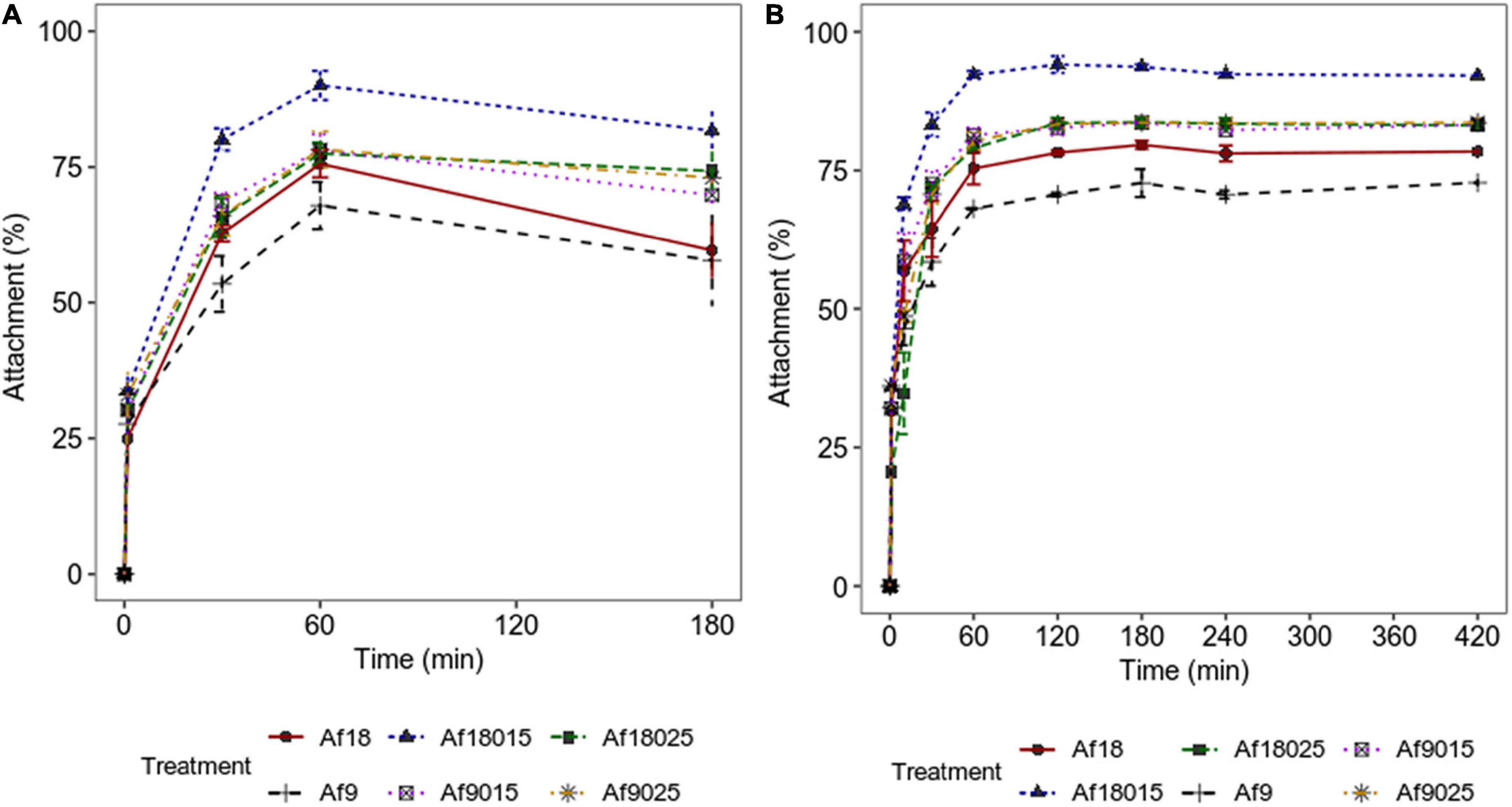
Figure 3. Attachment kinetics of A. ferrooxidans to a polymetallic sulfide ore. (A) Data obtained by qPCR. (B) Data obtained by counting cells in the planktonic phase using a Neubauer chamber (0.02 mm). Culture modified 9K medium supplemented with 2% polymetallic sulfide ore. The data correspond to the mean values of triplicates ± SD (n = 6).
Figure 3 shows how galactose and high-iron concentrations increase the attachment of A. ferrooxidans to the ore in the first hours of the process. The maximum attachment percentages achieved are shown in Figure 4.
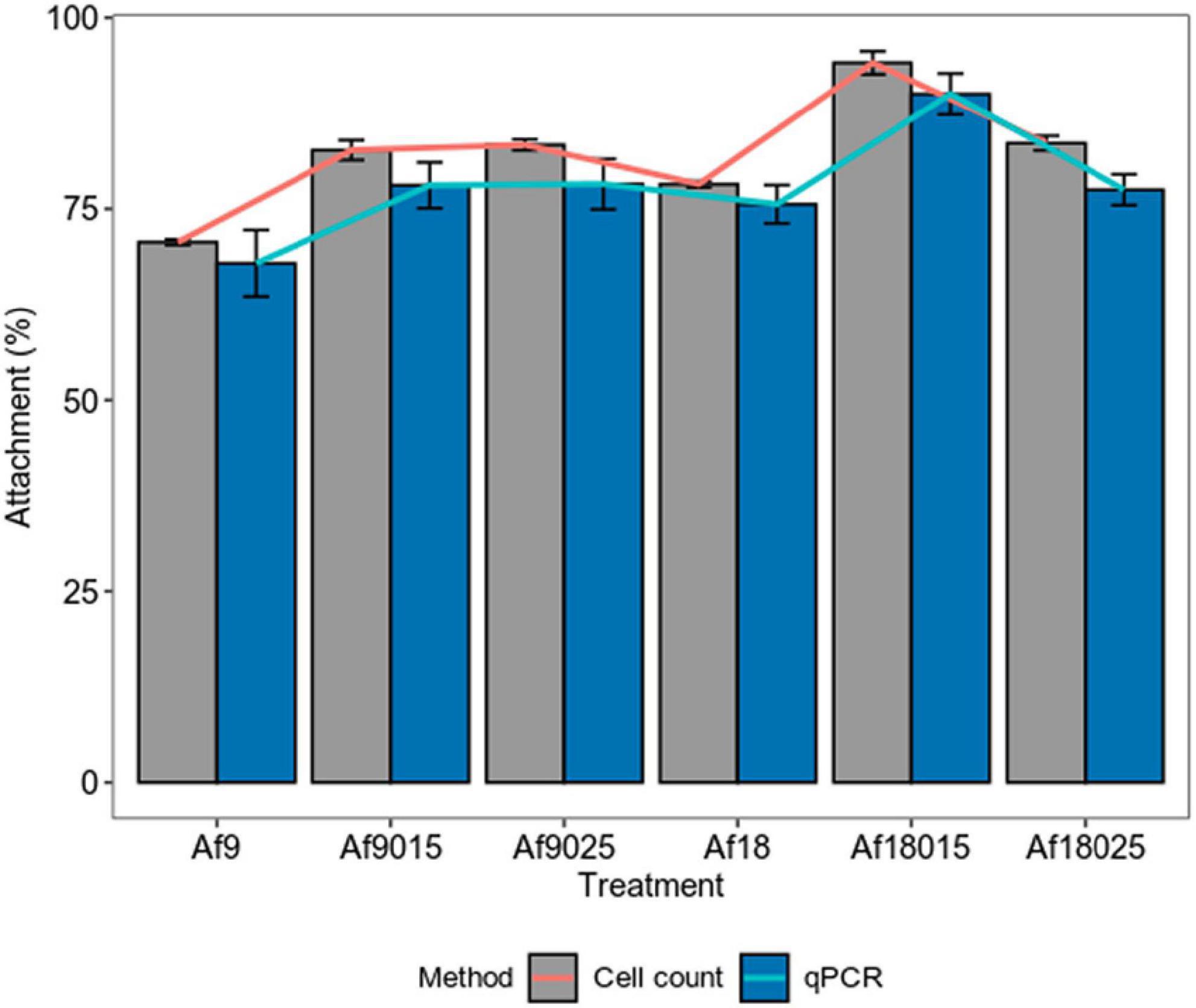
Figure 4. Maximum attachment percentage of A. ferrooxidans induced with different concentrations of iron and galactose to a polymetallic sulfide ore. Bars represent the maximum attachment percentage and lines represent the increase in attachment compared to the control assay (Af9). The data correspond to the mean values of triplicates ± SD (n = 6).
Figure 4 shows that for 9 g⋅L–1 iron treatment, the attachment percent increased by 12.3 and 12.9% measured by cell count, 10.3 and 10.4% as determined by qPCR for the induction, with 0.15 and 0.25% of galactose, respectively, compared to the control assays. On the other hand, in the induction with 18 g⋅L–1 of iron, the attachment percentage increased by 7.81, 23.7, and 13.1% measured by cell count, and 7.7, 22, and 9.6% determined by qPCR for the induction with 0, 0.15, and 0.25% of galactose, respectively. The maximum attachment percentage values reached were 70.4, 82.7, 83.3, 78.21, 94.1, and 83.5% measured by cell count, and 67.9, 78.2, 78.3,75.6, 89.9, and 77.5% determined by qPCR for the treatments Af9, Af9015, Af9025, Af18, Af18015, and Af18025, respectively, which suggest a higher attachment to the ore in case of a double induction in the production of EPS, with 0.15% galactose and 18 g⋅L–1 of iron.
Discussion
It has been reported that chemolithoautotrophic microorganisms, including A. ferrooxidans, show considerable sensitivity to organic compounds, such as galactose, which may result in a growth inhibitory effect (Shafia and Wilkinson, 1969; Tuovinen and Kelly, 1973; Saavedra et al., 2013b,2020; Aguirre et al., 2018). Glucose is not necessarily inhibitory, but it may allow for the growth of A. ferrooxidans in the absence of its inorganic energy source (Tabita and Lundgren, 1971). However, through successive cultures, a strain of A. ferrooxidans adapted to 0.15 and 0.25% galactose concentrations were obtained without observing a reduction of its maximum specific growth rate (μmax), with values within the ranges reported for kinetics of A. ferrooxidans in similar defined culture media, compared with the one used in this study that was between 0.07 and 0.14 h–1 (Braddock et al., 1984; Boon et al., 1999; Bryan et al., 2012; Saavedra et al., 2020); this implies a complete adaptation to galactose. Despite the increase in the energy source, the volumetric productivity, with respect to biomass and Fe3+ generation, remained relatively constant in treatments cultured with 9 and 18 g⋅L–1 of Fe2+, along with the rate of substrate consumption; thus, increasing only the total Fe2+ oxidation time, which results in doubling the microbial count in 18 g⋅L–1 of Fe2+ treatments compared to 9 g⋅L–1 of Fe2+ treatments.
Barreto et al. (2005) identified genes related to galactose metabolism (Leloir pathway) directed to the EPS production; EPS are part of the biofilm. A rapid biofilm formation on pyrite has been reported when A. ferrooxidans were exposed to D-galactose and glucose (Bellenberg et al., 2012), favoring the biooxidation of pyrite (Pavez et al., 2013). In addition, it has been observed that the cultivation of A. ferrooxidans at low concentrations of galactose increases the production of EPS, and it allows the bacteria to tolerate higher concentrations of Fe3+ (Saavedra et al., 2020). In the present study, a gradual increase in both carbohydrate and protein EPS contents was observed as galactose concentration increased. EPS presence visualized by TEM was only remarkable in galactose-induced cells. The maximum EPS yield was achieved in the inductions with 0.25% galactose with 9 and 18 g⋅L–1 of iron, without observing a significant difference (p > 0.05) between these two treatments. Saavedra et al. (2020) observed a higher production of EPS when A. ferrooxidans was grown with 0.35% galactose, but with a reduction in the cell density due to its sensitivity to carbohydrates; thus, it is counterproductive to cultivate A. ferrooxidans at concentrations higher than 0.25% galactose.
The double induction with iron and galactose in the EPS production was evidenced in cells cultured with 0.15% galactose mainly in the protein content, in which, protein composition increased by 1.6 times in 18 g⋅L–1 iron treatments compared with 9 g⋅L–1 iron cultured cells, with no significant increase in the carbohydrate content. While, in non-galactose treatments, the single effect of Fe3+ could be evidenced, where the protein content increased by 2.8 times, whereas the carbohydrate content remained relatively constant (p > 0.05). This variation in the EPS content suggests that the composition of EPS depends on the stress mechanisms applied to A. ferrooxidans when adapting to the environment. Culture conditions directly influence the EPS content of biooxidizing bacteria, and it has been observed that when cells are grown on soluble substrates, they produce fewer amounts of EPS compared to solid substrates (Gehrke et al., 1998; Harneit et al., 2006; Rohwerder and Sand, 2007; Vu et al., 2009). However, the EPS content in the planktonic phase can be increased in biooxidizing bacteria using soluble substances, such as galactose and ferric ion (Aguirre et al., 2018, 2021).
The attachment and biofilm formation are mediated by EPS and required during the ore biooxidation process (Li et al., 2016). EPS functional groups allow bacteria to interact with minerals through electrostatic interactions and hydrophobic forces, holding cells together and contributing to biofilm stability (Li et al., 2016). Attachment in biooxidizing bacteria occurs during the first hours of contact with the mineral (Rodríguez et al., 2003; Vardanyan et al., 2013), observing in such studies a maximum attachment percentage in the first 2 h of mineral contact in all treatments. However, the percentage of attachment to the mineral varied depending on the induction treatment in the planktonic phase. For the 9 g⋅L–1 iron without galactose treatment, a maximum attachment of 70.4 ± 0.3% was achieved with an EPS content of 27.44 ± 5.95 mg/gdw, while in induction with 0.15 and 0.25% galactose, the attachment percentage increased to 82.7 ± 3 and 83.3 ± 3.3% for EPS concentrations of 90.95 ± 12.78 and 182.16 ± 5.56 mg/gdw, respectively. Curiously, in 18-g⋅L–1 iron induction, the percentage of attachment was 78.2 ± 0.4% without galactose, while in induction with 0.15% galactose, it increased to a maximum of 94.1 ± 2.7 and only 83.5 ± 0.9% in the induction with 0.25%, for EPS contents of 60.55 ± 16.39, 148.24 ± 8.53, and 191.23 ± 10.25 mg/gdw, respectively. Although the amount of carbohydrates and lipids maintain biofilm stability, proteins play a fundamental role in the attachment (Tu et al., 2013). During cell attachment, outer membrane proteins from EPS and adhesins (Pili-associated proteins) molecularly interact irreversibly with the functional groups of the mineral, keeping cells attached (Li et al., 2010; Chandraprabha and Natarajan, 2013; Alfaro-Saldaña et al., 2019). When comparing the composition of the EPS in 0.15% galactose-induced treatments, it is observed that the proteins increased by 60% for the induction with 18 g⋅L–1 of iron compared to the induction with 9 g⋅L–1 (Table 2), suggesting a positive effect on attachment with protein increase. Such percentage of attachment is not verified in 0.25% galactose treatments, where the composition of proteins and carbohydrates do not vary significantly. On the other hand, when comparing the 0.15% galactose and 18 g⋅L–1 iron treatments, with 0.25% galactose, 9 and 18 g⋅L–1 iron treatments, the amount of carbohydrates was doubled for the inductions, with 0.25% galactose, and the proteins remain just a little above in the 0.25% galactose treatments. However, this high amount of carbohydrate results in less attachment compared to 0.15% galactose and 18 g⋅L–1 iron induction. Similar results were reported in previous work for A. thiooxidans, which reached a higher attachment with 0.15% galactose, compared with 0.25 and 0.35% galactose induction, with equally increasing concentrations of EPS according to galactose concentration (Aguirre et al., 2017). Those results indicate that excess EPS results are not always in higher attachment and that the combined induction of galactose and iron could modulate the EPS production to maximize the attachment.
Conclusion
Through the combined induction of galactose and ferric iron, the production of EPS in A. ferrooxidans was improved. The effect of the Fe3+ ion was mainly reflected in the protein content of EPS, while galactose increased the content of carbohydrates and proteins, suggesting that the biochemical composition and the number of EPS produced in A. ferrooxidans vary depending on the applied stress conditions. Despite an excess of EPS production, it did not maximize cell attachment. In this study, a higher attachment percentage (94%) was obtained for double induction with 18 g⋅L–1 of Fe3+ and 0.15% galactose, compared to treatments induced with 0.25% galactose (83.2% for 9 g⋅L–1 of Fe3+ and 83.5% for 18 g⋅L–1 of Fe3+) whose EPS carbohydrate content was almost double.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
EM and PA: conceptualization and formal analysis. EM, DJ-F, AD, and KV: methodology. PA and DJ-F: resources and funding acquisition, writing, and reviewing. EM: writing the original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
UTPL provided funding for this research through internal projects.
Acknowledgments
We thank Karlo Guerrero for revising and improving our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.951402/full#supplementary-material
References
Aguirre, P., Guerrero, K., Sanchez-Rodriguez, A., Gentina, J. C., and Schippers, A. (2018). Making sticky cells: Effect of galactose and ferrous iron on the attachment of Leptospirillum ferrooxidans to mineral surfaces. Res. Microbiol. 169, 569–575. doi: 10.1016/j.resmic.2018.08.005
Aguirre, P., Rodríguez, A. S., Gentina, J. C., and Schippers, A. (2017). Effect of galactose on EPS production and attachment of Acidithiobacillus thiooxidans to mineral surfaces. in Solid State Phenomena. Manuf. Sci. Technol. 262, 476–481. doi: 10.4028/www.scientific.net/SSP.262.476
Aguirre, P., Saavedra, A., Moncayo, E., Hedrich, S., Guerrero, K., and Gentina, J. C. (2021). Sticky Bacteria: Understanding the Behavior of a D-Galactose Adapted Consortium of Acidophilic Chemolithotroph Bacteria and Their Attachment on a Concentrate of Polymetallic Mineral. Front. Microbiol. 12:767639. doi: 10.3389/fmicb.2021.767639
Alfaro-Saldaña, E., Hernández-Sánchez, A., Araceli Patrón-Soberano, O., Astello-García, M., Alfredo Méndez-Cabañas, J., and Viridiana García-Meza, J. (2019). Sequence analysis and confirmation of the type IV pili-associated proteins PilY1, PilW and PilV in Acidithiobacillus thiooxidans. PLoS One 14:e0199854. doi: 10.1371/JOURNAL.PONE.0199854
Barreto, M., Jedlicki, E., and Holmes, D. S. (2005). Identification of a gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 71, 2902–2909. doi: 10.1128/AEM.71.6.2902-2909.2005
Bellenberg, S., Leon-Morales, C. F., Sand, W., and Vera, M. (2012). Visualization of capsular polysaccharide induction in Acidithiobacillus ferrooxidans. Hydrometallurgy 129–130, 82–89. doi: 10.1016/j.hydromet.2012.09.002
Boon, M., Ras, C., and Heijnen, J. J. (1999). The ferrous iron oxidation kinetics of Thiobacillus ferrooxidans in batch cultures. Appl. Microbiol. Biotechnol. 51, 813–819. doi: 10.1007/s002530051467
Braddock, J. F., Luong, H. V., and Brown, E. J. (1984). Growth kinetics of Thiobacillus ferrooxidans isolated from arsenic mine drainage. Appl. Environ. Microbiol. 48, 48–55. doi: 10.1128/aem.48.1.48-55.1984
Bryan, C. G., Davis-Belmar, C. S., van Wyk, N., Fraser, M. K., Dew, D., Rautenbach, G. F., et al. (2012). The effect of CO 2 availability on the growth, iron oxidation and CO 2-fixation rates of pure cultures of Leptospirillum ferriphilum and Acidithiobacillus ferrooxidans. Biotechnol. Bioeng. 109, 1693–1703. doi: 10.1002/bit.24453
Campodonico, M. A., Vaisman, D., Castro, J. F., Razmilic, V., Mercado, F., Andrews, B. A., et al. (2016). Acidithiobacillus ferrooxidans’s comprehensive model driven analysis of the electron transfer metabolism and synthetic strain design for biomining applications. Metab. Eng. Commun. 3, 84–96. doi: 10.1016/J.METENO.2016.03.003
Chandraprabha, M. N., and Natarajan, K. A. (2013). Role of outer membrane exopolymers of Acidithiobacillus ferrooxidans in adsorption of cells onto pyrite and chalcopyrite. Int. J. Mineral Proc. 123, 152–157. doi: 10.1016/j.minpro.2013.06.002
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Gehrke, T., Telegdi, J., Thierry, D., and Sand, W. (1998). Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 64, 2743–2747. doi: 10.1128/AEM.64.7.2743-2747.1998/ASSET/6B4E11C7-A881-4CC9-92DE-271D928F3293/ASSETS/GRAPHIC/AM0781249004.JPEG
Harneit, K., Göksel, A., Kock, D., Klock, J. H., Gehrke, T., and Sand, W. (2006). Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 83, 245–254. doi: 10.1016/j.hydromet.2006.03.044
Hödar, C., Moreno, P., Genova, A., di Latorre, M., Angélica, R. J., Maass, A., et al. (2012). Genome wide identification of Acidithiobacillus ferrooxidans (ATCC 23270) transcription factors and comparative analysis of ArsR and MerR metal regulators. BioMetals 25, 75–93. doi: 10.1007/S10534-011-9484-8
Kaksonen, A. H., Deng, X., Bohu, T., Zea, L., Khaleque, H. N., Gumulya, Y., et al. (2020). Prospective directions for biohydrometallurgy. Hydrometallurgy 195:105376. doi: 10.1016/j.hydromet.2020.105376
Latorre, M., Ehrenfeld, N., Cortés, M. P., Travisany, D., Budinich, M., Aravena, A., et al. (2016). Global transcriptional responses of Acidithiobacillus ferrooxidans Wenelen under different sulfide minerals. Bioresour. Technol. 200, 29–34. doi: 10.1016/J.BIORTECH.2015.09.110
Li, Q., Wang, Q., Zhu, J., Zhou, S., Gan, M., Jiang, H., et al. (2016). Effect of extracellular polymeric substances on surface properties and attachment behavior of Acidithiobacillus ferrooxidans. Minerals 6:100. doi: 10.3390/min6040100
Li, Y. Q., Wan, D. S., Huang, S. S., Leng, F. F., Yan, L., Ni, Y. Q., et al. (2010). Type IV Pili of Acidithiobacillus ferrooxidans are necessary for sliding, twitching motility, and adherence. Curr. Microbiol. 60, 17–24. doi: 10.1007/s00284-009-9494-8
Lovley, D. R., and Phillips, E. J. P. (1987). Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53:1536. doi: 10.1128/AEM.53.7.1536-1540.1987
Pavez, B., Saavedra, A., Díaz, M., and Gentina, J. C. (2013). Effect of Exogenous Galactose on EPS Production during Bioleaching of Pyrite by Acidithiobacillus ferrooxidans. Adv. Materials Res. 825, 125–128. doi: 10.4028/WWW.SCIENTIFIC.NET/AMR.825.125
Rawlings, D. E., and Johnson, D. B. (2007). The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 153, 315–324. doi: 10.1099/MIC.0.2006/001206-0
Rodríguez, Y., Ballester, A., Blázquez, M. L., González, F., and Munoz, J. A. (2003). Study of Bacterial Attachment During the Bioleaching of Pyrite, Chalcopyrite, and Sphalerite. Geomicrobiology 20, 131–141. doi: 10.1080/01490450303880
Rohwerder, T., and Sand, W. (2007). “Mechanisms and biochemical fundamentals of bacterial metal sulfide oxidation,” in Microbial Processing of Metal Sulfides, eds E. R. Donati and W. Sand (Dordrecht: Springer), 35–58. doi: 10.1007/1-4020-5589-7_2
Saavedra, A., Aguirre, P., and Gentina, J. C. (2020). Biooxidation of Iron by Acidithiobacillus ferrooxidans in the Presence of D-Galactose: Understanding Its Influence on the Production of EPS and Cell Tolerance to High Concentrations of Iron. Front. Microbiol. 11:759. doi: 10.3389/fmicb.2020.00759
Saavedra, A., Pavez, B., Diaz, M., and Gentina, J. C. (2013b). Galactose as inducer of the production of extracellular polymeric substances by Acidithiobacillus ferrooxidans. Adv. Materials Res. 285, 120–124. doi: 10.4028/www.scientific.net/AMR.825.120
Saavedra, A., Pavez, B., Diaz, M., and Gentina, J. C. (2013a). Eps production during adaptation of Acidithiobacillus ferrooxidans to high ferric ion concentration. Adv. Materials Res. 285, 115–119. doi: 10.4028/www.scientific.net/AMR.825.115
Sajjad, W., Zheng, G., Din, G., Ma, X., Rafiq, M., and Xu, W. (2019). Metals Extraction from Sulfide Ores with Microorganisms: The Bioleaching Technology and Recent Developments. Trans. Indian Institute Metals 72, 559–579. doi: 10.1007/S12666-018-1516-4
Sand, W., and Gehrke, T. (2006). Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron (III) ions and acidophilic bacteria. Res. Microbiol. 157, 49–56. doi: 10.1016/J.RESMIC.2005.07.012
Shafia, F., and Wilkinson, R. F. (1969). Growth of Ferrobacillus ferrooxidans on organic matter. J. Bacteriol. 97, 256–260. doi: 10.1128/JB.97.1.256-260.1969
Srichandan, H., Mohapatra, R. K., Parhi, P. K., and Mishra, S. (2019). Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 189:105122. doi: 10.1016/J.HYDROMET.2019.105122
Tabita, R., and Lundgren, D. G. (1971). Utilization of Glucose and the Effect of Organic Compounds on the Chemolithotroph Thiobacillus ferrooxidans. J. Bacteriol. 108:328. doi: 10.1128/JB.108.1.328-333.1971
Tapia, J. M., Muñoz, J., González, F., Blázquez, M. L., and Ballester, A. (2013). Sorption of ferrous and ferric iron by extracellular polymeric substances (EPS) from acidophilic bacteria. Prep. Biochem. Biotechnol. 43, 815–827. doi: 10.1080/10826068.2013.805624
Tu, B., Wang, F., Li, J., Sha, J., Lu, X., and Han, X. (2013). Analysis of Genes and Proteins in Acidithiobacillus ferrooxidans During Growth and Attachment on Pyrite Under Different Conditions. Geomicrobiology 30, 255–267. doi: 10.1080/01490451.2012.668608
Tuovinen, O. H., and Kelly, D. P. (1973). Studies on the growth of Thiobacillus ferrooxidans. Arch. Mikrobiol. 88, 285–298. doi: 10.1007/BF00409941
Vardanyan, A. K., Vardanyan, N. S., and Markosyan, L. M. (2013). Peculiarities of Adhesion and Bioleaching of Pyrite by New Isolated Leptospirillum Spp. Bacteria. Univer. J. Microbiol. Res. 1, 22–25. doi: 10.13189/UJMR.2013.010202
Vera, M., Schippers, A., and Sand, W. (2013). Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation–part A. Appl. Microbiol. Biotechnol. 97, 7529–7541. doi: 10.1007/S00253-013-4954-2
Vu, B., Chen, M., Crawford, R. J., and Ivanova, E. P. (2009). Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14, 2535–2554. doi: 10.3390/molecules14072535
Walker, J. M. (2009). “The Bicinchoninic Acid (BCA) Assay for Protein Quantitation,” in The Protein Protocols Handbook. Springer Protocols Handbooks, ed. J. M. Walker (Totowa, NJ: Humana Press), doi: 10.1007/978-1-59745-198-7_3
Keywords: attachment, Acidithiobacillus ferrooxidans, inducers, ferric iron, galactose
Citation: Moncayo EA, Debut A, Vizuete K, Jumbo-Flores D and Aguirre P (2022) Sticky bacteria: Combined effect of galactose and high ferric iron concentration on extracellular polymeric substances production and the attachment of Acidithiobacillus ferrooxidans on a polymetallic sulfide ore surface. Front. Microbiol. 13:951402. doi: 10.3389/fmicb.2022.951402
Received: 24 May 2022; Accepted: 16 August 2022;
Published: 12 September 2022.
Edited by:
Ales Lapanje, Institut Jožef Stefan (IJS), SloveniaReviewed by:
Axel Schippers, Federal Institute for Geosciences and Natural Resources, GermanyKaibin Fu, Southwest University of Science and Technology, China
Copyright © 2022 Moncayo, Debut, Vizuete, Jumbo-Flores and Aguirre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paulina Aguirre, cGlhZ3VpcnJlQHV0cGwuZWR1LmVj
 Eduardo A. Moncayo
Eduardo A. Moncayo Alexis Debut3
Alexis Debut3 Paulina Aguirre
Paulina Aguirre