
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 04 November 2022
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.945463
 Yi Wang1,2
Yi Wang1,2 Yuting Wen1,2
Yuting Wen1,2 Jiayin Wang1*
Jiayin Wang1* Xin Lai1
Xin Lai1 Ying Xu1
Ying Xu1 Xuanping Zhang1
Xuanping Zhang1 Xiaoyan Zhu1
Xiaoyan Zhu1 Chenglin Ruan1
Chenglin Ruan1 Yao Huang2
Yao Huang2Objective: To systematically evaluate the significance of Fusobacterium nucleatum (Fn) levels the clinicopathological impacts of cancer.
Methods: Literature from Pubmed, Embase, and Web of Science was retrieved to collect all English literatures on the correlation between Fn and cancer, and the quality of literatures collected was assessed based on the Newcastle-Ottawa Quality Assessment Scale. The heterogeneity and sensitivity were detected by Stata 14.0 software, and the correlation between Fn and cancer clinicopathological as the effect variables was assessed according to the odds ratio (OR) and 95% confidence interval (CI). The forest plot was drawn.
Results: A total of 19 articles meeting the inclusion criteria were selected. The incidence of Fn prevalence varied considerably (range: 6.1 to 83.3%) and was greater than 10% in 13 of 19 studies. Compared with those with no/low Fn levels, the high levels of Fn was positively associated with vascular invasion, nerve invasion, depth of invasion, and distant metastasis [vascular invasion: OR = 1.66, 95%CI(1.07, 2.57), I2 = 21.9%, fixed effect model; nerve invasion: OR = 1.36, 95%CI(1.00, 1.84), I2 = 43.1%, fixed effect model; infiltration depth: OR = 1.94, 95%CI(1.20, 3.15), I2 = 67.2%, random effect model; distant metastasis: OR = 1.80, 95%CI(1.23, 2.64), I2 = 3.4%, fixed effect model]. Patients with MLH1 methylation always present a higher Fn levels than those without methylation [OR = 2.53, 95%CI(1.42, 4.53), P = 0.01, I2 = 57.5%, random effect model]. Further, Fn was associatedwith the molecular characteristics of cancers [MSI-H Vs. MSS/MSI-low: OR = 2.92, 95%CI(1.61, 5.32), P = 0.01, I2 = 63.2%, random effect model; High Vs. Low/Negative CIMP: OR = 2.23, 95%CI(1.64, 3.03), P = 0.01, I2 = 64.2%, random effect model; KRAS mutation Vs. wild-type: OR = 1.24, 95%CI(1.04, 1.48), P = 0.02, I2 = 27.0%, fixed effect model; Present Vs. Abscent BRAF mutations: OR = 1.88, 95%CI(1.44, 2.45), P = 0.01, I2 = 24.2%, fixed effect model]. The cancer patients with high levels of Fn often have worse RFS than those with no/low Fn levels[OR = 1.14, 95%CI(0.61, 1.68), P = 0.01, I2 = 80.7%, random effect model].
Conclusion: This review and meta-analysis showed that Fn could be used to predict unfavorable prognosis and function as potential prognostic biomarkers in colorectal cancer (CRC). Our data may have implications for targeting Fn to develop strategies for cancer prevention and treatment.
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of global cancer deaths (Bray et al., 2018). Although great improvements in the diagnosis and treatment of CRC have been made, the prognosis is still not promising (Zhang et al., 2021). The prognosis of CRC depends on the clinicopathologic stage at the time of diagnosis. However, the disease stage alone cannot accurately predict the prognosis of individual patients (Chao et al., 2020). Therefore, identifying potential risk factors related to CRC prognosis is important in improving the survival of CRC patients.
Recent studies have shown a genera-specific shift in abundance between healthy microbiota present in normal tissues and potentially cancer-related bacteria in CRC (de Carvalho et al., 2019). Compared to normal tissues, Fusobacteria were enriched in human cancer tissues, especially Fusobacterium nucleatum (Fn; de Carvalho et al., 2019). Fn is a Gram-negative anaerobic microorganism that is indigenous to the human oral cavity (Kostic et al., 2012). The existing studies have reported that Fn may cause CRC by inducing inflammation and suppressing host immunity, possibly through modulating the E-cadherin/β-catenin pathway via FadA adhesion in Fn (Rubinstein et al., 2013). A previous work showed that the levels of Fn associates with CRC progression and it could be used as a prognosis biomarker for cancer (Gethings-Behncke et al., 2020). However, the correlation between the high levels of Fn and the clinicopathology and prognosis characteristics of CRC is still controversial.
Consider the case of clinicopathology, for example. A previous cohort study (Chen et al., 2020) showed that high Fn levels were positively associated with vascular and nerve invasion of cancer tissues, while another study (Lee et al., 2021) showed that Fn levels were not related to them. A study of Mima et al. (2016) showed the level of Fn in CRC tissue was associated with shorter survival, and may potentially serve as a prognostic biomarker. Contrarily, another study reported tumors with high levels of Fn had a better prognosis than those with low or negative levels of Fn in non-sigmoid colon cancers (Jeong et al., 2019). Yamamoto et al. (2021) confirmed that KRAS and BRAF mutation were related to high Fn levels, while Ito et al. (2015) believed that they were unrelated.
Based on the above controversial statements, we conducted a large sample integration study in this study to clarify the role of Fn infection in colorectal cancer patients.
A literature search on PubMed, Embase, and Web of Science was conducted to identify all primary studies relating to Fn and patient clinicopathological and prognosis published before 2022. The literature search used broad search terms that could ensure the inclusion of relevant literatures. We also reviewed the reference lists of the articles identified in the primary search for additional relevant studies. A specific search strategy was devised to include at least one keyword or Medical Subject Heading (MeSH) term from each of the following: (i) Neoplasia or Neoplasias or Neoplasm or Tumors or Cancer or Cancers or Malignancy or Malignancies or Malignant Neoplasms or Malignant Neoplasm or Neoplasm, Malignant or Neoplasms, Malignant or Benign Neoplasms or Neoplasms, Benign or Benign Neoplasm or Neoplasm, Benign; (ii) Bacteria or Fusobacteriaor or Fusobacterium or Fusobacterium nucleatum.
The inclusion criteria were as follows: (1) Tissue specimens from humans were obtained after surgical resection for the primary tumors. None of the patients received radiochemotherapy before surgery. (2) Original research articles were included in the final analysis. (3) Experiments were conducted using conventional quantitative polymerase chain reaction (qPCR) or 16S rRNA sequencing methodology.
The exclusion criteria are as follows: (1) Letters, abstracts, books, short conference abstracts/proceedings, and posters. (2) Cell and animal studies. (3) The publications on a small-sized corhort on the same topic from the same team (shared overlapped participants with large-sized studies). (4) Low quality studies (score < 5). (5) Neoadjuvant or adjuvant chemoradiotherapy.
The study design information and outcome data were independently extracted by two investigators (Yi Wang and Yuting Wen). We extracted data on the following: study characteristics (e.g., study design, year of publication, number of samples, types of cancer, population, clinicopathological features of patients, survival analysis), measurement methods, outcome measures, and statistical analysis. We assessed study quality using items from the Newcastle-Ottawa Quality Assessment Scale (NOS; Stang, 2010; Supplementary Table S1).
All statistical analyses were performed using Stata 14.0 (Stata Corp., College Station, TX, United States). Data were extracted from the original research articles and converted into 2 × 2 tables. The definitions of study characteristics, measurement methods, outcome measures were set by the authors of each paper, and the data were combined for analysis only if these definitions were sufficiently similar (determined by consensus). Pooled estimates and corresponding 95% CIs were represented by forest plots. Dichotomous variables [Mucus, Signet-ring cells, Lymphatic metastasis, Vascular invasion, Nerve invasion, Peridumoral lymphocytes (present VS. absent); differentiation (medium-low VS. high); infiltration depth (pT3/4 VS. pT1/2); regional lymph node metastasis (pN1/2 VS. pN0); distant metastasis (pM1 VS. pM0); Tumor Node Metastasis (III-IV VS. I-II); MSI (high VS. low); MLH1 methylation, CIMP status (high VS. low/negative); KRAS mutation, BRAF mutation (mutant VS. wild)] were analyzed with odds ratios (ORs) with mean differences at a 95% confidence interval (CI). Continuous variables were analyzed by the HR, and 95% CI was recorded. Recurrence free survival (RFS) was defined as the time from complete remission to recurrence or follow-up deadline.
Statistical heterogeneity across studies was evaluated with the Q statistics (p < 0.05 was considered significant) and the Inconsistency statistics (I2) (Higgins and Thompson, 2002). I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; and I2 = 75–100%, extreme heterogeneity. If no significant heterogeneity existed, the pooled estimates and the 95% CI were calculated using a fixed-effect model. If a significant heterogeneity was presented (I2 statistics no less than 50%), then a random-effect model was adopted. Sensitivity analysis was performed to explore the influence of an individual study on the pooled results, by deleting a single study each iteration from the pooled analysis. We used subgroup analysis to evaluate for potential sources of heterogeneity. All important results (p < 0.05) were analyzed using Egger’s and Begg’s tests for publication bias. A p value <0.10 was considered as suggesting the publication bias.
The literature screening process was presented in Figure 1 (The PRISMA 2020 statement. doi: 10.1136/BMJ.n71). Initial literature searches retrieved 1,601 articles, from which 984 were screened after excluding duplicates. The 325 citations were removed due to irrelevant publishing types or studies by a screening of the titles and abstracts. A total of 25 full-text articles were subsequently assessed for eligibility and 6 were removed due to low-quality scores (NOS quality scores<3). Finally, a total of 19 studies (Higgins and Thompson, 2002; Tahara et al., 2014; Mitsuhashi et al., 2015; Sun et al., 2016; Park et al., 2017; Yan et al., 2017; Yu et al., 2017; Yamaoka et al., 2018; Chen et al., 2019; Jeong et al., 2019; Kunzmann et al., 2019; Zhang et al., 2019; Boehm et al., 2020; Haruki et al., 2020; Serna et al., 2020; Egger et al., 2021; Lee et al., 2021; Nie et al., 2021; Yamamoto et al., 2021) were included in this meta-analysis which involved 4,920 participants (1,267 Fn-high and 3,653 Fn-no/low, respectively). Basic characteristics and Fn performance in cancer detection of these 19 studies (16 studies on CRC, 2 studies on GC, and 1 study on PAAD) were shown in Table 1. There was no disagreement among the authors as to whether the above studies should be included in this meta-analysis.
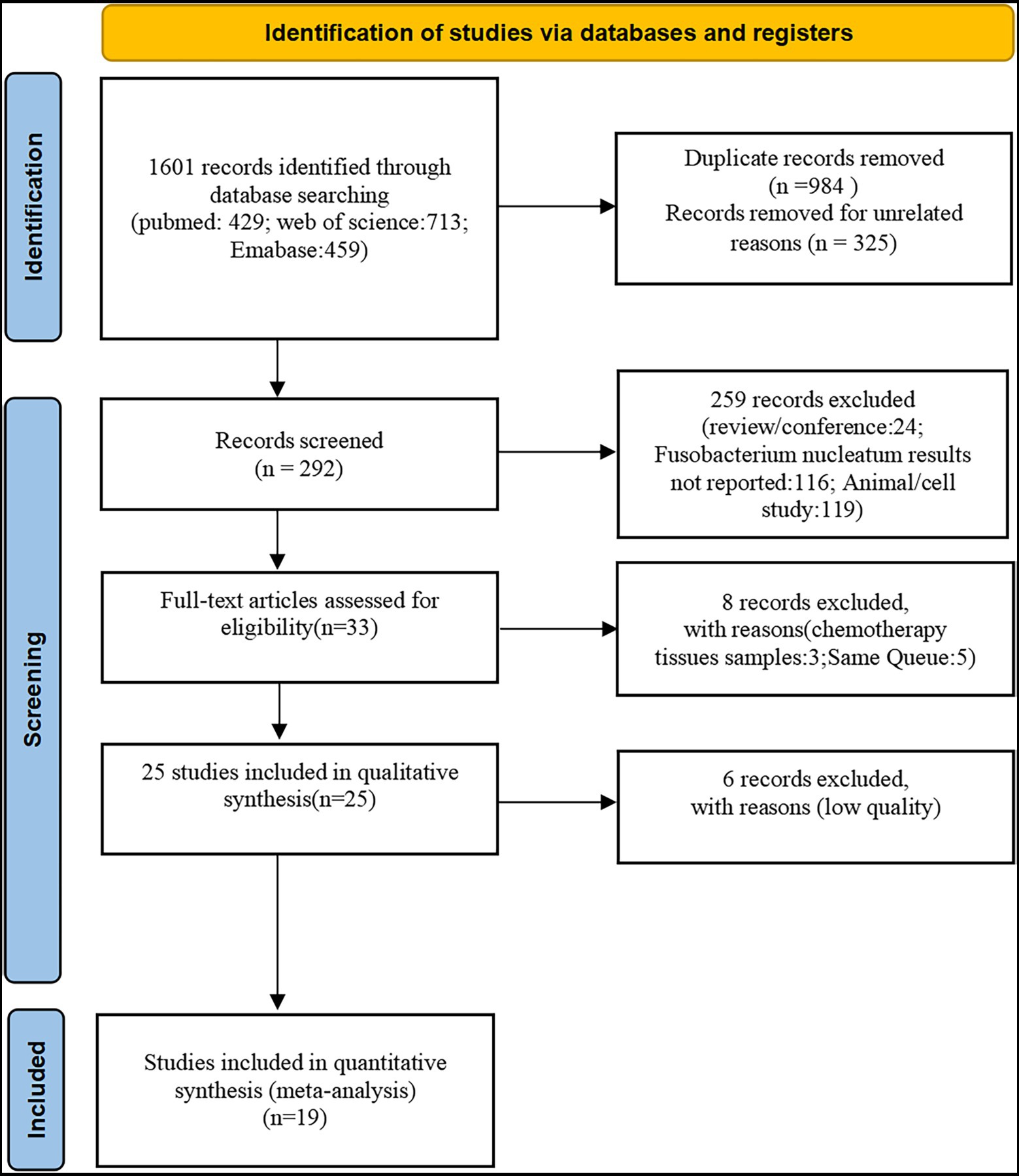
Figure 1. Study selection. A total of 19 eligible studies were included from 1,601 potentially relevant studies identified and reviewed.
19 studies (Tahara et al., 2014; Ito et al., 2015; Mitsuhashi et al., 2015; Mima et al., 2016; Sun et al., 2016; Park et al., 2017; Yan et al., 2017; Yu et al., 2017; Yamaoka et al., 2018; Chen et al., 2019; Jeong et al., 2019; Kunzmann et al., 2019; Zhang et al., 2019; Boehm et al., 2020; Haruki et al., 2020; Serna et al., 2020; Lee et al., 2021; Nie et al., 2021; Yamamoto et al., 2021) assessed the levels of Fn in cancer tissue samples. The heterogeneity test showed a result of I2 = 99.0%, indicating a heterogeneity among the studies. Therefore, the random-effect model was chosen. We further analyzed the subgroups according to race (Asian VS. No-Asian). It presented that the analysis results of each subgroup [Asian: I2 = 98.7%, P = 0.001, 0.31(0.29, 0.32); No-Asian: I2 = 98.8%, P = 0.001, 0.09(0.08, 0.10)] were basically consistent with the overall results. Thus, the subgroup analysis did not explain the heterogeneity. To further explore the potential source of the heterogeneity across the studies, we performed sensitivity analyses. First, when the 2 studies (Boehm et al., 2020; Nie et al., 2021) on GC and the studies (Mitsuhashi et al., 2015) on PAAD were excluded, there were still significant heterogeneity [I2 = 98.8%, P = 0.001, 0.21(0.2, 0.23)]. Then, the heterogeneity was still significant after removing each study (Supplementary Table S2). Thus, we refrained from meta-analysis of the Fn prevalence outcome. Across the 19 studies, the incidence of Fn prevalence varied considerably (range: 6.1 to 83.3%) and was greater than 10% in 13 of 19 studies.
Three studies (Park et al., 2017; Jeong et al., 2019; Lee et al., 2021) reported the correlation between mucus and the Fn levels in CRC tissues, while 9 studies (Sun et al., 2016; Park et al., 2017; Yan et al., 2017; Yamaoka et al., 2018; Chen et al., 2019; Jeong et al., 2019; Zhang et al., 2019; Boehm et al., 2020; Lee et al., 2021) reported the correlation between the differentiation and Fn levels in the CRC and GC tissues, 2 studies (Park et al., 2017; Lee et al., 2021) reported the correlation between the signet-ring cells and Fn levels in CRC tissues, 5 studies (Mitsuhashi et al., 2015; Park et al., 2017; Boehm et al., 2020; Lee et al., 2021; Nie et al., 2021) reported the correlation between the lymphatic metastasis and Fn levels in cancer tissues, two studies (Park et al., 2017; Nie et al., 2021) reported the correlation between peritumoral lymphocytes and Fn levels in CRC and GC tissues. The results revealed that there seems no statistically significance between the present and absent groups (mucus: I2 = 0, P = 0.54; differentiation: I2 = 0, P = 0.32; signet-ring: I2 = 0, P = 0.71; lymphatic metastasis: I2 = 0, P = 0.43; peritumoral lymphocytes: I2 = 0, P = 0.66). 14 studies (Ito et al., 2015; Mitsuhashi et al., 2015; Mima et al., 2016; Sun et al., 2016; Park et al., 2017; Yamaoka et al., 2018; Chen et al., 2019; Kunzmann et al., 2019; Zhang et al., 2019; Boehm et al., 2020; Haruki et al., 2020; Lee et al., 2021; Nie et al., 2021; Yamamoto et al., 2021) reported the correlation between TNM and Fn levels in CRC tissues. Nine studies (Mima et al., 2016; Sun et al., 2016; Yamaoka et al., 2018; Chen et al., 2019; Jeong et al., 2019; Boehm et al., 2020; Haruki et al., 2020; Lee et al., 2021; Yamamoto et al., 2021) reported the correlation between pN and Fn levels in CRC and GC tissues, the difference was not statistically significant [(III–IV VS. I–II): p = 0.12; (pN1/2 VS. pN0): p = 0.41] and with high heterogeneity [TNM: I2 = 53.4; pN: I2 = 74.1]. We performed a sensitivity analysis to further identify the possible origins of heterogeneity (see sensitivity analysis for details). The above results are provided in Supplementary Figures S1–S7.
Five studies (Sun et al., 2016; Chen et al., 2019; Zhang et al., 2019; Lee et al., 2021; Nie et al., 2021) reported the correlation between vascular invasion and the Fn levels in CRC tissues, while 5 studies (Park et al., 2017; Chen et al., 2019; Jeong et al., 2019; Zhang et al., 2019; Lee et al., 2021) reported the correlation between nerve invasion and Fn levels in CRC and GC tissues, 11 studies (Mima et al., 2016; Sun et al., 2016; Yan et al., 2017; Yamaoka et al., 2018; Chen et al., 2019; Jeong et al., 2019; Boehm et al., 2020; Haruki et al., 2020; Lee et al., 2021; Nie et al., 2021; Yamamoto et al., 2021) reported the correlation between infiltration depth (pT)and Fn levels in CRC and GC tissues, 7 studies (Sun et al., 2016; Yan et al., 2017; Yamaoka et al., 2018; Boehm et al., 2020; Haruki et al., 2020; Lee et al., 2021; Yamamoto et al., 2021) reported the correlation between distant metastasis (pM)and Fn levels in CRC and GC tissues. The difference between the two groups was statistically significant[vascular invasion (present VS. absent): P = 0.02; nerve invasion (present VS. absent): P = 0.04; pT(T3/4 VS. T1/2): P = 0.01; pM(M1 VS. M0): P = 0.01]. Furthermore, the present vascular invasion group showed high Fn levels compared to the absent group [OR = 1.66, 95%CI(1.07, 2.57), I2 = 21.9%, fixed effect model, Figure 2]. The present nerve invasion group showed a high Fn level compared to the absent group [OR = 1.36, 95%CI(1.00, 1.84), I2 = 43.1%, fixed effect model, Figure 3]. Higher Fn levels were observed in the pT3/4 group compared to the pT1/2 group [OR = 1.73, 95%CI(1.36, 2.21), I2 = 67.4%, random effect model, Figure 4]. Due to the existence of significant heterogeneity (I2 = 67.4%), we further performed the analyses to identify potential sources of heterogeneity (see sensitivity analysis for details). Higher Fn levels were observed in the pM1 group compared to pM0 group [pM: OR = 1.84, 95%CI(1.27, 2.65), I2 = 3.6%, fixed effect model, Figure 5]. The detailed results are shown in Table 2.
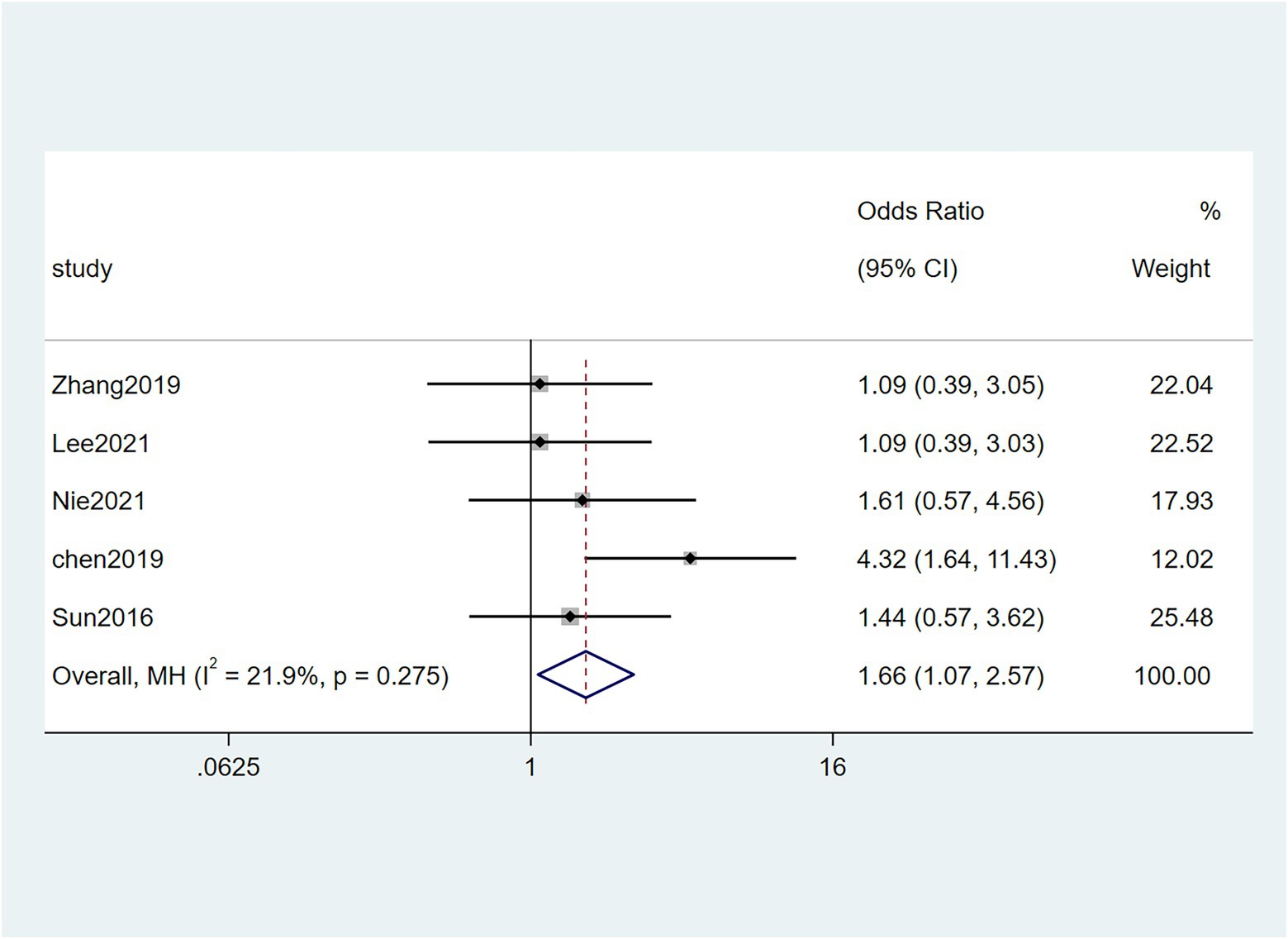
Figure 2. Forest plot of the relationship between Fusobacterium nucleatum and vascular invasion in cancer.

Figure 3. Forest plot of the relationship between Fusobacterium nucleatum and nerve invasion in cancer.
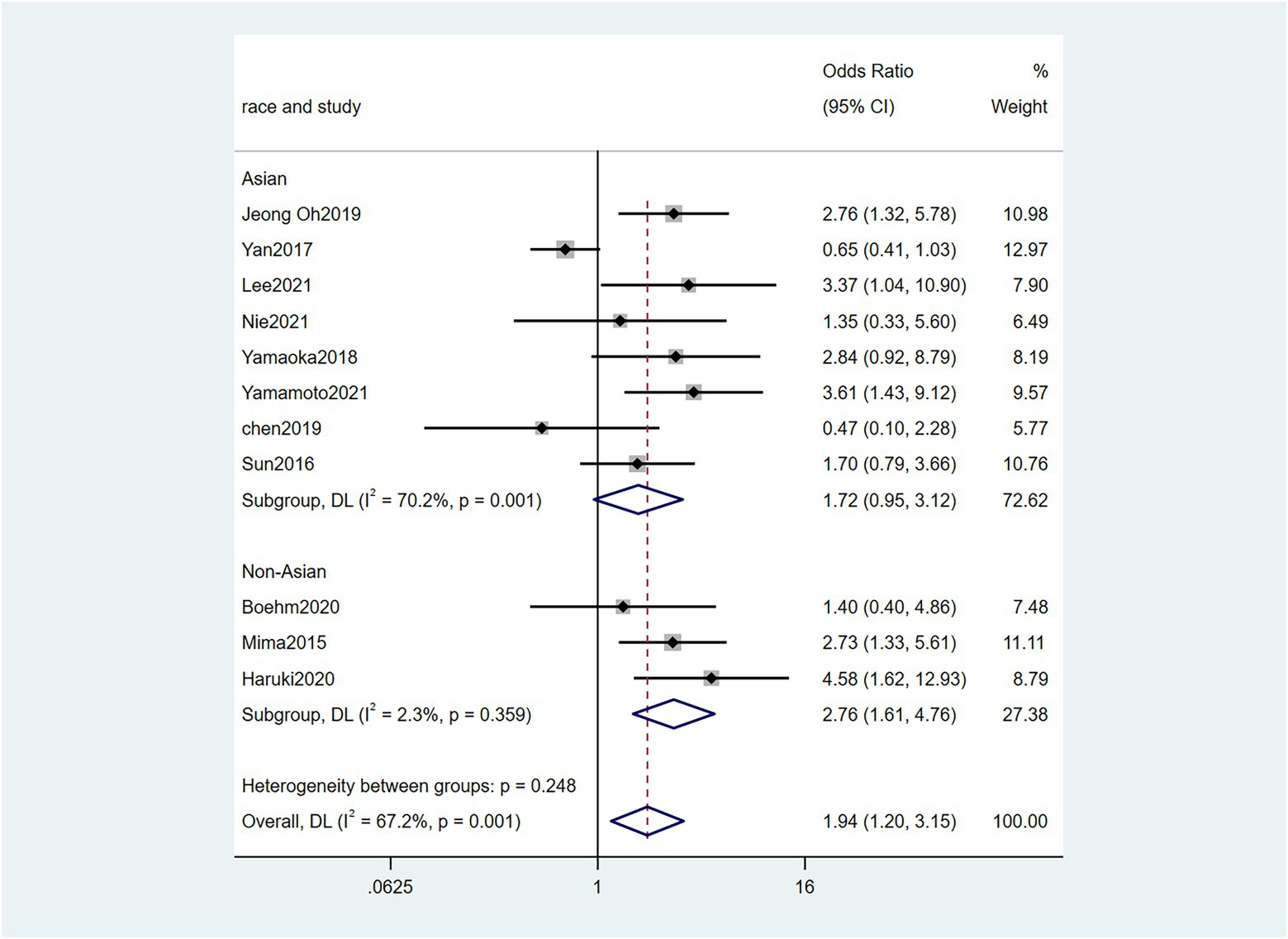
Figure 4. Forest plot of the relationship between Fusobacterium nucleatum and infiltration depth(pT) in cancer.
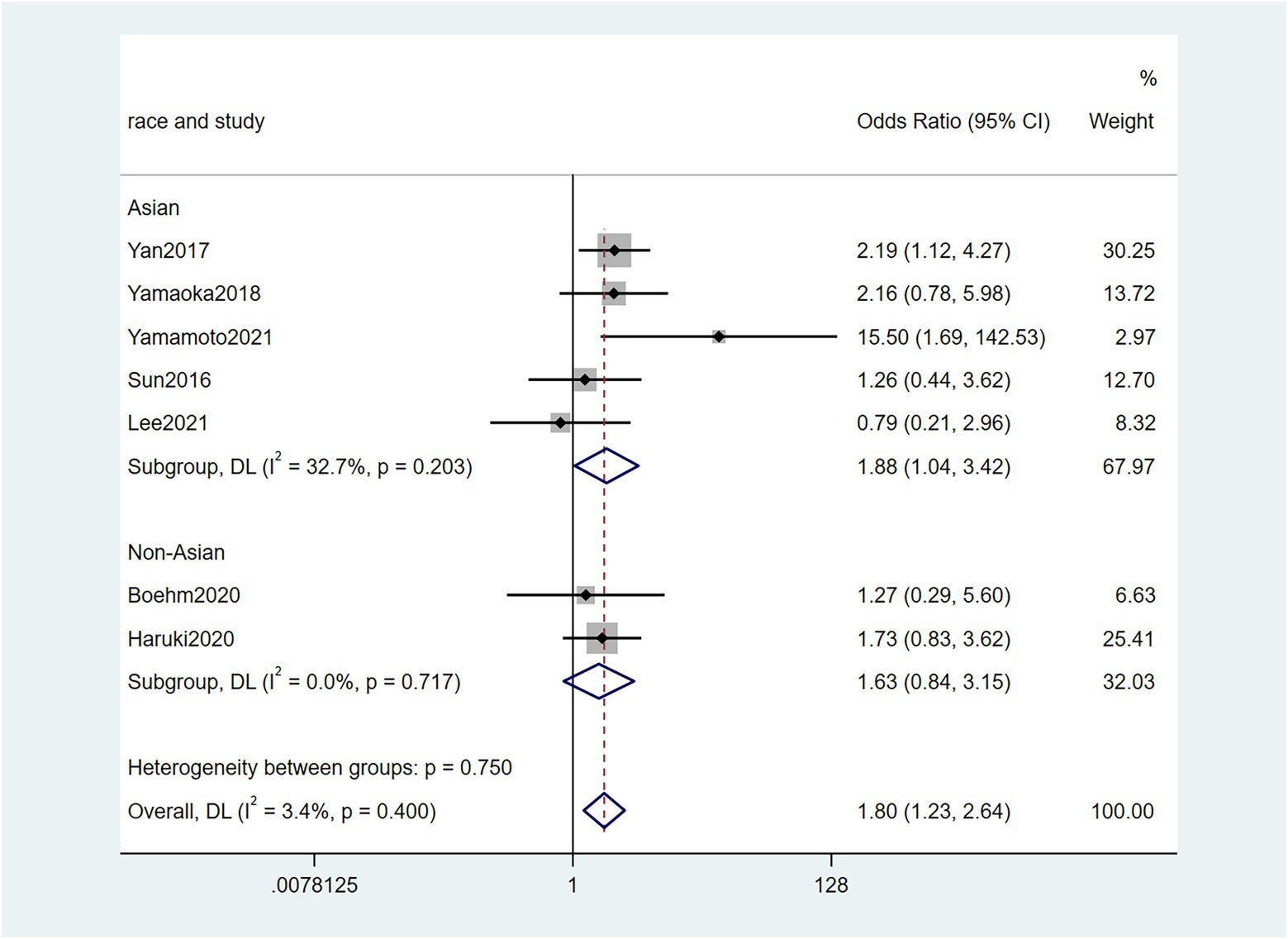
Figure 5. Forest plot of the relationship between Fusobacterium nucleatum and distant metastasis(pM) in cancer.
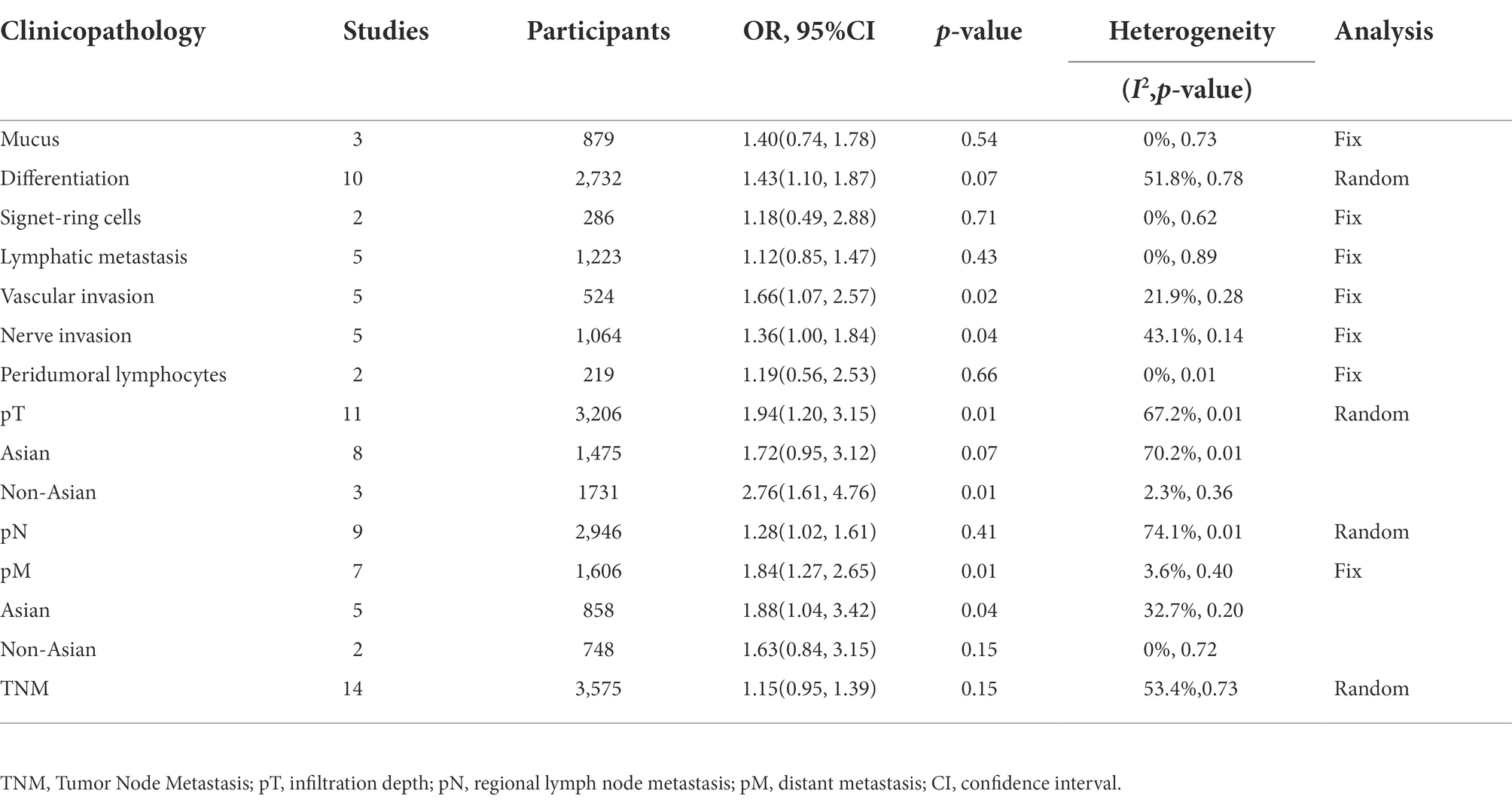
Table 2. The correlation between Fusobacterium nucleatum and the clinicopathological features of colorectal cancer.
To further investigate the effect of Fn levels in cancer, we extracted the molecular characteristic data from each study. 6 studies (Tahara et al., 2014; Ito et al., 2015; Mitsuhashi et al., 2015; Mima et al., 2016; Park et al., 2017; Yamaoka et al., 2018) were analyzed for the association between the Fn levels and MLH1 methylation status. The pooled results showed that patients with MLH1 methylation usually had a higher Fn level than those in the unmethylated group [OR = 2.53, 95%CI(1.42, 4.53), P = 0.01, I2 = 57.5%, random effect model, subgroups (Asian: P = 0.01, I2 = 0%; Non-Asian: P = 0.01, I2 = 0%), Figure 6]. Patients with MSI-high had the higher Fn levels compared to those with MSS/MSI-low from 5 studies(Ito et al., 2015; Chen et al., 2019; Jeong et al., 2019; Kunzmann et al., 2019; Haruki et al., 2020) [OR = 2.92, 95%CI(1.61, 5.32), P = 0.01, I2 = 63.2%, random effect model, subgroups (Asian: P = 0.01, I2 = 51.3%; Non-Asian: P = 0.01, I2 = 8.7%), Figure 7]. The pool of data from 6 studies (Tahara et al., 2014; Ito et al., 2015; Mitsuhashi et al., 2015; Mima et al., 2016; Park et al., 2017; Yamaoka et al., 2018) showed that a high level CIMP was positively correlated with the high levels of Fn [OR = 2.23, 95%CI(1.64, 3.03), P = 0.01, I2 = 64.2%, random effect model, subgroups (Asian: P = 0.19, I2 = 0%; Non-Asian: P = 0.01, I2 = 0%), Figure 8]. Higher Fn levels were observed in the KRAS mutation group compared to the wild group from 12 studies (Tahara et al., 2014; Ito et al., 2015; Mitsuhashi et al., 2015; Mima et al., 2016; Park et al., 2017; Yamaoka et al., 2018; Chen et al., 2019; Jeong et al., 2019; Kunzmann et al., 2019; Haruki et al., 2020; Lee et al., 2021; Yamamoto et al., 2021) [OR = 1.24, 95%CI(1.04, 1.48), P = 0.02, I2 = 27.0%, fixed effect model, Asian: P = 0.01, I2 = 2.5%; Non-Asian: P = 0.99, I2 = 44.4%), Figure 9]. Similarly, the pooled results of 11 studies (Tahara et al., 2014; Ito et al., 2015; Mima et al., 2016; Park et al., 2017; Chen et al., 2019; Jeong et al., 2019; Kunzmann et al., 2019; Haruki et al., 2020; Lee et al., 2021; Nie et al., 2021; Yamamoto et al., 2021) showed that BRAF mutation was positively associated with Fn levels [OR = 1.88, 95%CI(1.44, 2.45), P = 0.01, I2 = 24.2%, fixed effect model, Asian: P = 0.16, I2 = 35%; Non-Asian: P = 0.01, I2 = 0%), Figure 10]. The details of the above results are provided in Table 3.
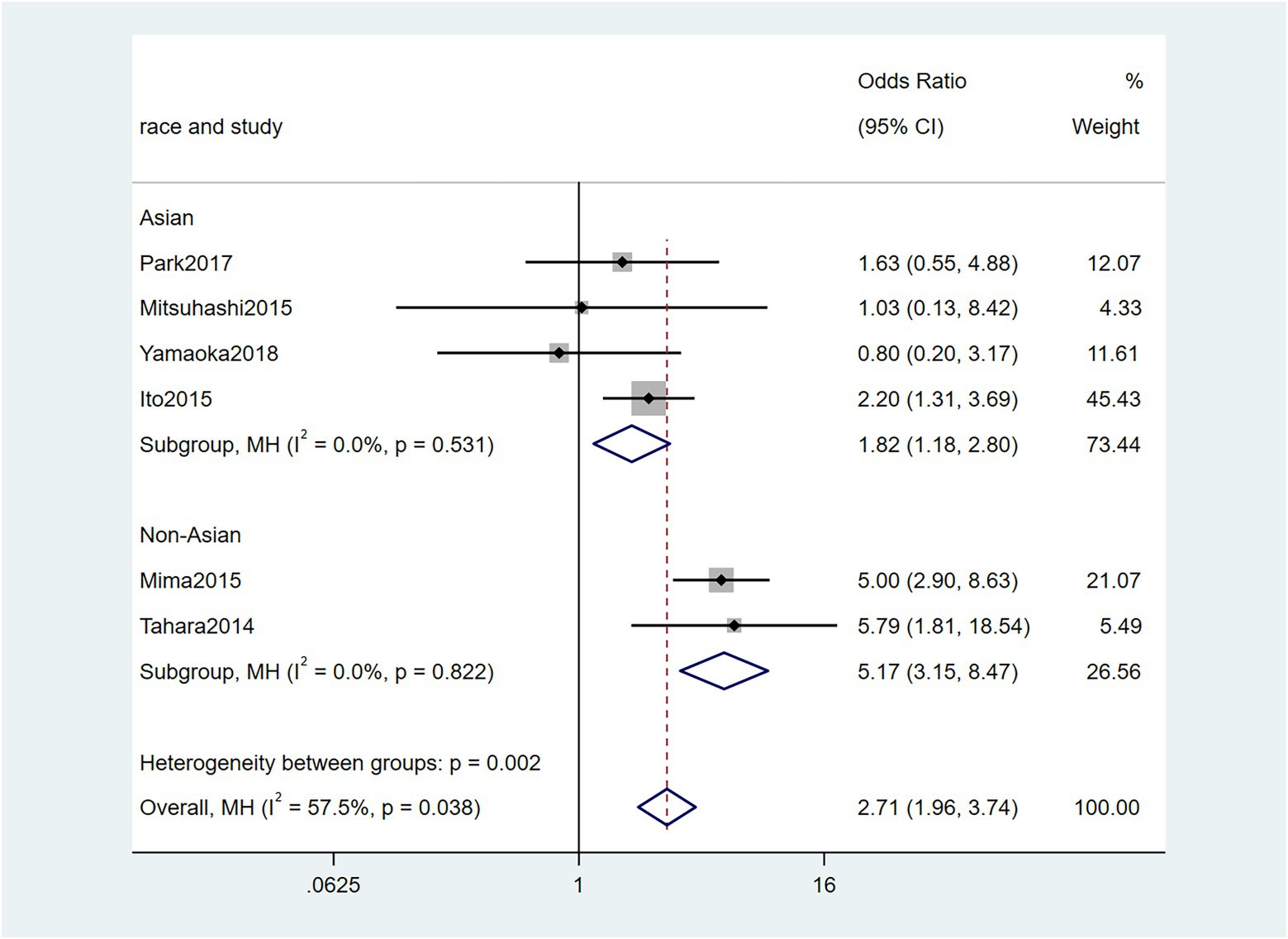
Figure 6. Forest plot of the relationship between Fusobacterium nucleatum and MLH1 methylation in cancer.
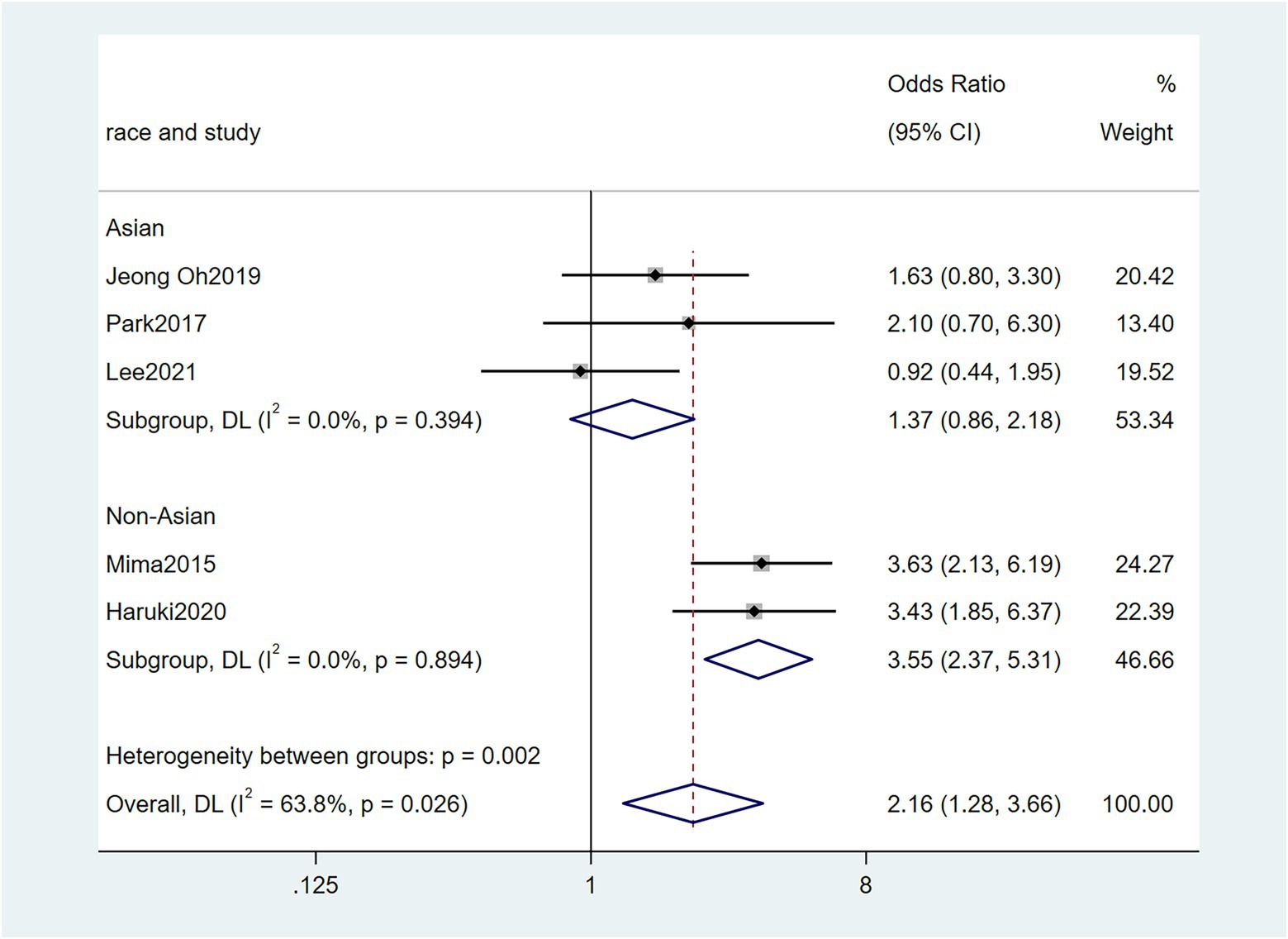
Figure 8. Forest plot of the relationship between Fusobacterium nucleatum and CIMP methylation in cancer.
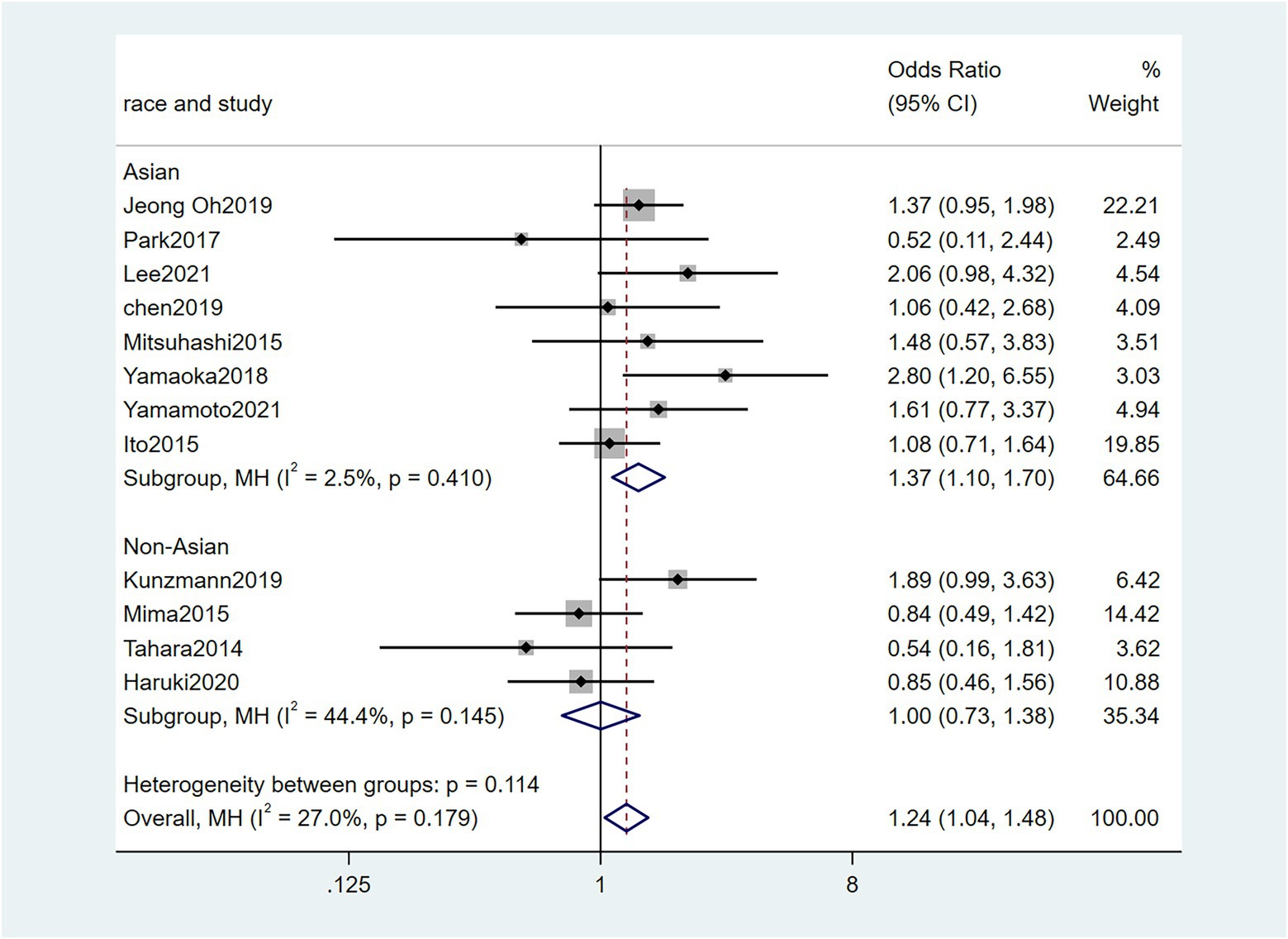
Figure 9. Forest plot of the relationship between Fusobacterium nucleatum and KRAS mutation in cancer.
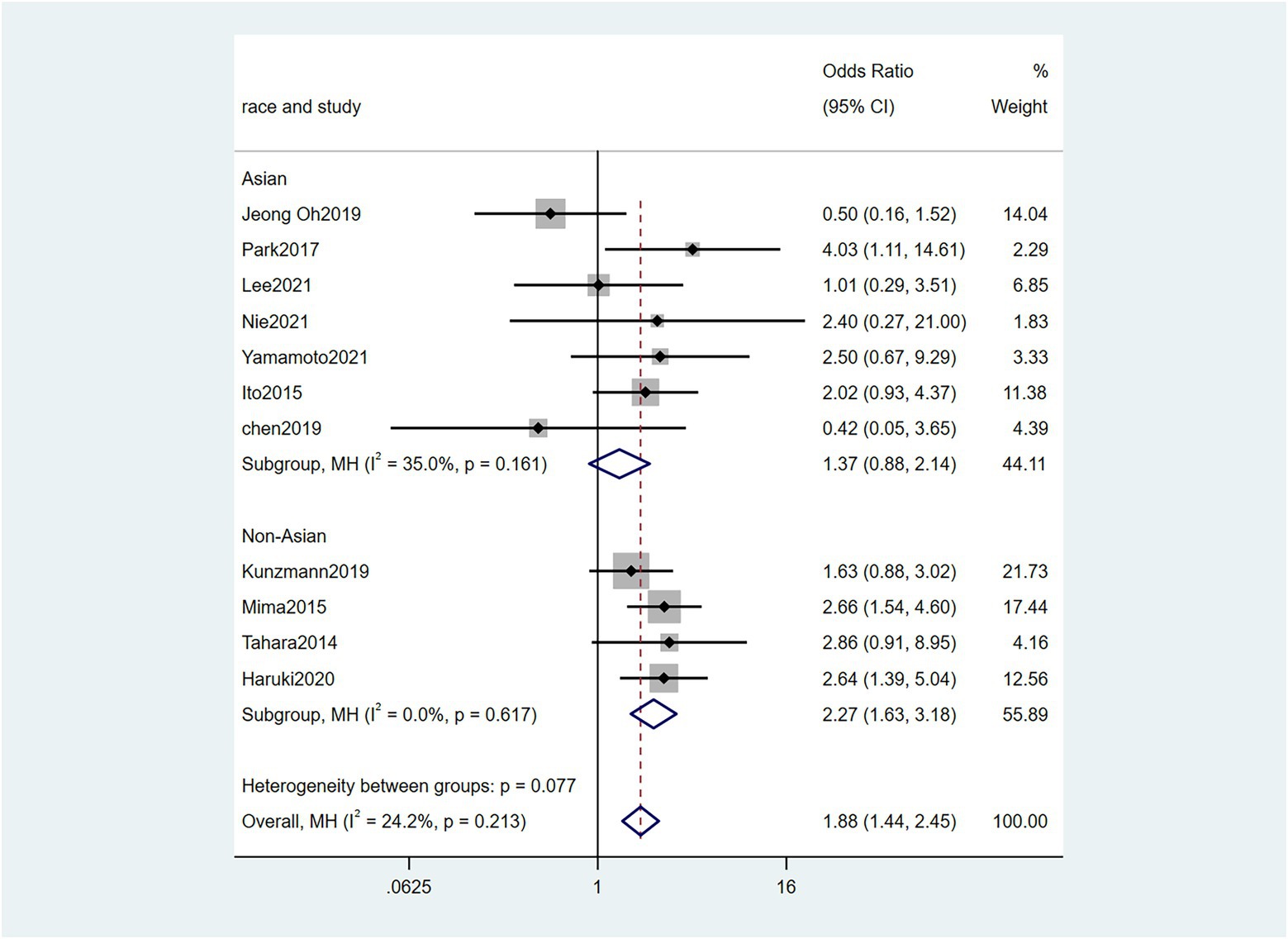
Figure 10. Forest plot of the relationship between Fusobacterium nucleatum and BRAF mutation in cancer.
Two studies (Zhang et al., 2019; Serna et al., 2020) reported the association between the RFS and Fn levels. Our results demonstrated that the CRC patients with high levels of Fn had a worse RFS than those with no/low levels of Fn [HR = 2.19, 95%CI(0.79, 3.58), P = 0.01, I2 = 42.8%, random effect model, shown in Figure 11].
For the comparisons with significant heterogeneity, we performed a sensitivity analysis in this meta-analysis. The sensitivity analysis was performed by omitting individual studies one by one to assess their effect on the aggregated results. When Yamamoto et al. (2021) was removed, the heterogeneity of pN directly decreased to 0%. The heterogeneity of pT was high (I2 = 67.4%), but there was no heterogeneity after removing the study of Yan et al. (2017) (I2 = 1.8%). The heterogeneity of MLH1 methylation was high (I2 = 57.5%), and the heterogeneity was I2 = 27% after the study of Mima et al. (2016) was removed. In addition, the heterogeneity of MSI status results was high (I2 = 63.2%). when the study of Jeong et al. (2019) was removed, the heterogeneity decreased (I2 = 0%). Heterogeneity in sensitivity analysis of CIMP status results remained high after each study was omitted sequentially. All results are provided in Supplementary Table S2.
To further clarify the heterogeneity source, subgroup analysis was performed according to race, and the results of MLH1 methylation, CIMP status indicated that race was the main source of heterogeneity. However, the results of pT, pN, and MSI status showed the heterogeneity did not decrease in the subgroup analyses of both Asian and No-Asian.
In this meta-analysis, three corresponding methods were used to evaluate publication bias: Begg’s adjusted rank correlation test, and Egger’s regression test. Statistical significance was also not observed according to Egger’s and Begg’s tests (Egger et al., 2021; P > 0.05; Table 4). There was no publication bias in our included studies. However, according to the limited number of literatures, the presence of a publication bias cannot be conclusively excluded.
Recently, increasing evidence suggests an important association between the Fn levels and CRC. Here, we performed a more comprehensive and detailed meta-analysis (Huangfu et al., 2021) on this association. To explore the correlation between the Fn infections and cancers more comprehensively, the scope of the literature search was not limited to CRC. Unfortunately, only 2 studies on GC and 1 study on PAAD passed the inclusion criteria. More research on Fn infections across pan-cancer should be considered in future.
Among the existing studies, Li et al. (2016) investigated 101 Chinese patients with CRC and reported that the Fn infection rate was as high as 87.1%. Another study (Mima et al., 2015) analyzed 598 CRC cases by qPCR, which found an Fn infection rate of 13.0% in tumor tissues. Nie et al. (2021) and Boehm et al. (2020) observed the Fn positivity in GC patients in 28.8 and 32.75%, respectively. The detection rate of Fusobacterium species in PAAD tissue specimens in the Mitsuhashi et al. (2015) was 8.8%. A report (Nosho et al., 2016) from Japan presented that the positive rate of Fn was 8.6%. Thus, no meta-analysis could be conducted due to the considerable heterogeneity in our study. Across these 19 studies, the incidence of Fn prevalence varied considerably (range: 6.1 to 83.3%) and was greater than 10% in 13 of 19 studies. The high heterogeneity observed could be due to variation in study design and/or population. However, as can be seen, Fn infections are already prevalent in cancer.
Our results indicated that the high Fn levels were significantly correlated with the presence of vascular and nerve invasion, suggesting that Fn may promote the aggressive potential of tumor cells. On the other hand, Lee et al. (2021) reported that vascular, and nerve invasion were not related to Fn levels (P > 0.05). This conclusion might be conducted due to a small corhort (Lee et al., 2021), compared to other research, among which the number of advanced cancer was only 36 cases. To our best knowledge, meta-analyse of the high Fn levels and vascular, nerve invasion has not been previously reported. As one of the main approaches to the pM1 of CRC, nerve invasion is closely related to the depth of tumor invasion and lymph node metastasis, which may affect postoperative tumor recurrence and metastasis to a certain extent and also reflect the prognosis of patients (Liebl et al., 2013; Ueno et al., 2014). Besides, we extracted the data on the correlation among pT, pN, pM, and Fn levels, and the results clearly showed that the high Fn levels were positively correlated with poor pathological status (pT3/4; pM1). Heterogeneity regarding the pooled data for pN was high (I2 = 74.1%), although the results seemed not statistically significant by a random-effect model. The main source of heterogeneity was introduced by the work of Yamamoto et al. (2021). In this study, the number of pN1/2 cases accounted for 10% of the total number of cases and that of other studies were in the range of 26–90%. These results are in excellent agreement with the published meta-analyses (Huangfu et al., 2021). A study (Casasanta et al., 2020) has shown that Fn directly induced metastasis by releasing cytokines, increasing NF-κB expression, and subsequently expressing KRT7 (Chen et al., 2020), increasing CARD3, and downregulating E-cadherin (Chen et al., 2020). Notably, another study has shown that Fn activates the β-catenin signaling pathway in CRC through LPS mediated Toll-like receptor 4 (TLR4)/ p21-activated kinase 1 (PAK1; Chen et al., 2017). TLR4 activates the β-catenin signaling pathway and forms intestinal tumors, while PAK1 is associated with CRC progression and metastasis (Wu et al., 2018). Mekenkamp et al. (2012) found that the presence of mucus in cancers indicates resistance to chemotherapy and also implies a worse prognosis for cancer patients. Multiple studies have shown that the signet ring cell carcinoma of CRC is often associated with lymph node metastasis, even if it is treated with radical resection, which is associated with poor prognosis (Mizushima et al., 2010; Hartman et al., 2013). Thus, to obtain a comprehensive study, we included signet ring cells and mucus factor, and the combined results showed no statistically significant difference.
In our study, the high Fn levels of cancer tissue were associated with key tumor molecular features of CRC, including MLH1 methylation, MSI-high, CIMP-high, KRAS mutation, and BRAF mutation, which were associated with clinical outcomes in CRC. The results of Jeong et al. (2019) considered no correlations between these molecular characteristics and Fn levels. A meta-analyse (Huangfu et al., 2021) also suggested no association between KRAS mutation and Fn levels. However, CRC arises from the transformation of normal mucosa to precancerous lesions, and colorectal adenoma progresses to cancer over several years. In the serrated polyp pathway, the genetic changes involved BRAF and KRAS mutation. Both KRAS and BRAF encode kinases belonging to the mitogen-activated protein kinase (MAPK) cascade that mediate cell signaling involved in cell proliferation, apoptosis, and differentiation (Yamane et al., 2014). Another molecular change is the MSI in serrated lesions, which is attributed to the loss of mismatch repair genes, often leading to increased susceptibility to the accumulation of gene mutations in regions containing microsatellites (Mizushima et al., 2010). MSI status has been evaluated as a prognostic and chemotherapy or immunotherapy response biomarker in CRC patients (Diao et al., 2021). Previous a report suggested that Fn was hypothesized to directly or indirectly interact with MSI-H status (Gao et al., 2021). A series of experimental studies have revealed that Fn may not be a mere passenger colorectal carcinogenesis and would play an active role in tumorigenesisi of MSI-H CRC (Kostic et al., 2013). Heterogeneity was substantial in the MSI status of the present meta-analysis, and the study of Jeong et al. (2019) was the primary source of heterogeneity. The samples in this study come from the patients diagnosed with stage II/III CRC, that may be a reason for its heterogeneity. There was a strong correlation between CIMP and serrated CRC. CIMP-high status as a potential biomarker to predict irinotecan-based chemotherapy regimens for CRC (Vilar and Gruber, 2010; Zhang et al., 2021). Results from subgroup analysis in the current study on MLH1 methylation and CIMP status suggested that race was a potential source of heterogeneity of the pooled results.
Previous meta-analyse (Huangfu et al., 2021) has shown that the high levels of Fn are strongly associated with poor outcomes in patients with CRC, including overall survival, disease-free survival, and cancer-specific survival. We also extracted the data on patients’ RFS and showed that CRC patients with high levels of Fn had worse RFS than patients with no/low Fn levels. Due to a lack of original data for some studies, HRs were extracted from the Kaplan–Meier survival curves, which may affect the accuracy of the results.
In conclusion, our study results show that the Fn could be quite useful to predict unfavorable prognosis and function as potential prognostic biomarkers in CRC. There is a discrepancy between our research and previous studies. Although Jeong et al. (2019) showed that the tumors with high levels of Fn had a better prognosis than those with low or negative levels of Fn in non-sigmoid colon cancers, the difference may be derived from the nature of study population. Their study samples were a well-selected and relatively-homogeneous cohort that contained only stage III or high-risk stage II CRCs treated with oxaliplatin-based adjuvant chemotherapy.
No doubt, certain limitations do exist in this study. We may miss some eligible studies published in other languages, while the unpublished data were not identified. In this case, the publication bias always cannot be absolutely excluded, although no significant publication bias was detected. In addition, there are still many unknowns in the study of bacteria and tumors. For example, whether a bacterial infection is a cause or effect has not been confirmed yet. The existing study (Nejman et al., 2020) has reported that bacteria exist in cancer tissues. We suggest the following questions for further research. How exactly do bacteria colonize tumor tissue? Whether it is because the bacteria invade the vascular epithelium and then the blood metastases to the cancer tissue, or whether the changes in the tumor microenvironment attract the colonization of the bacteria?
JW and YiW conceived and designed the study. YuW and XuZ evaluated study quality. Study tabulations were carried out by YiW and XL, with final review by XiZ, YX, CR, and YuW performed data analyses. YiW and YH drafted the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the Natural Science Basic Research Program of Shaanxi, grant number 2020JC-01. The article processing charge was funded by the Natural Science Basic Research Program of Shaanxi, grant number 2020JC-01.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.945463/full#supplementary-material
Boehm, E. T., Thon, C., Kupcinskas, J., Steponaitiene, R., Skieceviciene, J., Canbay, A., et al. (2020). Fusobacterium nucleatum is associated with worse prognosis in Lauren's diffuse type gastric cancer patients. Scientific reports 10:16240. doi: 10.1038/s41598-020-73448-8
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Casasanta, M. A., Yoo, C. C., Udayasuryan, B., Sanders, B. E., Umaña, A., Zhang, Y., et al. (2020). Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal. 13:eaba9157. doi: 10.1126/scisignal.aba9157
Chao, B., Ju, X., Zhang, L., Xu, X., and Zhao, Y. (2020). A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical cancer patients. Front. Oncol. 10:766. doi: 10.3389/fonc.2020.00766
Chen, Y., Chen, Y., Zhang, J., Cao, P., Su, W., Deng, Y., et al. (2020). Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics 10, 323–339. doi: 10.7150/thno.38870
Chen, Y., Lu, Y., Ke, Y., and Li, Y. (2019). Prognostic impact of the Fusobacterium nucleatum status in colorectal cancers. Medicine 98:e17221. doi: 10.1097/MD.0000000000017221
Chen, Y., Peng, Y., Yu, J., Chen, T., Wu, Y., Shi, L., et al. (2017). Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 8, 31802–31814. doi: 10.18632/oncotarget.15992
Chen, S., Su, T., Zhang, Y., Lee, A., He, J., Ge, Q., et al. (2020). Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microbes 11, 511–525. doi: 10.1080/19490976.2019.1695494
de Carvalho, A. C., de Mattos Pereira, L., Datorre, J. G., Dos Santos, W., Berardinelli, G. N., Matsushita, M. M., et al. (2019). Microbiota profile and impact of Fusobacterium nucleatum in colorectal cancer patients of barretos cancer hospital. Front. Oncol. 9:813. doi: 10.3389/fonc.2019.00813
Diao, Z., Han, Y., Chen, Y., Zhang, R., and Li, J. (2021). The clinical utility of microsatellite instability in colorectal cancer. Crit. Rev. Oncol. Hematol. 157:103171. doi: 10.1016/j.critrevonc.2020.103171
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (2021). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Gao, Y., Bi, D., Xie, R., Li, M., Guo, J., Liu, H., et al. (2021). Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal transduction and targeted therapy 6:398. doi: 10.1038/s41392-021-00795-x
Gethings-Behncke, C., Coleman, H. G., Jordao, H., Longley, D. B., Crawford, N., and Murray, L. J., et, al (2020). Fusobacterium nucleatum in the colorectum and its association with cancer risk and survival: a systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prevent., 29, 539–548. doi:doi: 10.1158/1055-9965.EPI-18-1295.
Hartman, D. J., Nikiforova, M. N., Chang, D. T., Chu, E., Bahary, N., Brand, R. E., et al. (2013). Signet ring cell colorectal carcinoma: a distinct subset of mucin-poor microsatellite-stable signet ring cell carcinoma associated with dismal prognosis. Am. J. Surg. Pathol. 37, 969–977. doi: 10.1097/PAS.0b013e3182851e2b
Haruki, K., Kosumi, K., Hamada, T., Twombly, T. S., Väyrynen, J. P., and Kim, S. A., et, al (2020). Association of autophagy status with amount of Fusobacterium nucleatum in colorectal cancer. J. Pathol., 250, 397–408. doi:doi: 10.1002/path.5381
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Huangfu, S. C., Zhang, W. B., Zhang, H. R., Li, Y., Zhang, Y. R., Nie, J. L., et al. (2021). Clinicopathological and prognostic significance of Fusobacterium nucleatum infection in colorectal cancer: a meta-analysis. J. Cancer 12, 1583–1591. doi: 10.7150/jca.50111
Ito, M., Kanno, S., Nosho, K., Sukawa, Y., Mitsuhashi, K., Kurihara, H., et al. (2015). Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int. J. Cancer 137, 1258–1268. doi: 10.1002/ijc.29488
Jeong, O., Kim, J. H., Bae, J. M., Kim, H. J., Cho, N. Y., and Kang, G. H. (2019). Prognostic Impact of Fusobacterium nucleatum depends on combined tumor location and microsatellite instability status in stage II/III colorectal cancers treated with adjuvant chemotherapy. J. Pathol. Translat. Med. 53, 40–49. doi: 10.4132/jptm.2018.11.29
Kostic, A. D., Chun, E., Robertson, L., Glickman, J. N., Gallini, C. A., Michaud, M., et al. (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215. doi: 10.1016/j.chom.2013.07.007
Kostic, A. D., Gevers, D., Pedamallu, C. S., Michaud, M., Duke, F., Earl, A. M., et al. (2012). Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298. doi: 10.1101/gr.126573.111
Kunzmann, A. T., Proença, M. A., Jordao, H. W., Jiraskova, K., Schneiderova, M., Levy, M., et al. (2019). Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 38, 1891–1899. doi: 10.1007/s10096-019-03649-1
Lee, J. A., Yoo, S. Y., Oh, H. J., Jeong, S., Cho, N. Y., and Kang, G. H., et, al (2021). Differential immune microenvironmental features of microsatellite-unstable colorectal cancers according to Fusobacterium nucleatum status. Cancer Immunol. Immunother. 70, 47–59. doi:doi: 10.1007/s00262-020-02657-x
Li, Y. Y., Ge, Q. X., Cao, J., Zhou, Y. J., Du, Y. L., Shen, B., et al. (2016). Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J. Gastroenterol. 22, 3227–3233. doi: 10.3748/wjg.v22.i11.3227
Liebl, F., Demir, I. E., Rosenberg, R., Boldis, A., Yildiz, E., Kujundzic, K., et al. (2013). The severity of neural invasion is associated with shortened survival in colon cancer. Clin. Cancer Res. 19, 50–61. doi: 10.1158/1078-0432.CCR-12-2392
Mekenkamp, L. J., Heesterbeek, K. J., Koopman, M., Tol, J., Teerenstra, S., Venderbosch, S., et al. (2012). Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur. J. Cancer 48, 501–509. doi: 10.1016/j.ejca.2011.12.004
Mima, K., Nishihara, R., Qian, Z. R., Cao, Y., Sukawa, Y., and Nowak, J. A., et, al (2016). Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut, 65, 1973–1980. doi:doi: 10.1136/gutjnl-2015-310101
Mima, K., Sukawa, Y., Nishihara, R., Qian, Z. R., Yamauchi, M., Inamura, K., et al. (2015). Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 1, 653–661. doi: 10.1001/jamaoncol.2015.1377
Mitsuhashi, K., Nosho, K., Sukawa, Y., Matsunaga, Y., Ito, M., Kurihara, H., et al. (2015). Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6, 7209–7220. doi: 10.18632/oncotarget.3109
Mizushima, T., Nomura, M., Fujii, M., Akamatsu, H., Mizuno, H., Tominaga, H., et al. (2010). Primary colorectal signet-ring cell carcinoma: clinicopathological features and postoperative survival. Surgery Today 40, 234–238. doi: 10.1007/s00595-009-4057-y
Nejman, D., Livyatan, I., Fuks, G., Gavert, N., Zwang, Y., Geller, L. T., et al. (2020). The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980. doi: 10.1126/science.aay9189
Nie, S., Wang, A., and Yuan, Y. (2021). Comparison of clinicopathological parameters, prognosis, micro-ecological environment and metabolic function of Gastric Cancer with or without Fusobacterium sp. infection. J. Cancer 12, 1023–1032. doi: 10.7150/jca.50918
Nosho, K., Sukawa, Y., Adachi, Y., Ito, M., Mitsuhashi, K., Kurihara, H., et al. (2016). Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 22, 557–566. doi: 10.3748/wjg.v22.i2.557
Park, H. E., Kim, J. H., Cho, N. Y., Lee, H. S., and Kang, G. H. (2017). Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virch. Archiv 471, 329–336. doi: 10.1007/s00428-017-2171-6
Rubinstein, M. R., Wang, X., Liu, W., Hao, Y., Cai, G., and Han, Y. W. (2013). Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206. doi: 10.1016/j.chom.2013.07.012
Serna, G., Ruiz-Pace, F., Hernando, J., Alonso, L., Fasani, R., Landolfi, S., et al. (2020). Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann. Oncol. 31, 1366–1375. doi: 10.1016/j.annonc.2020.06.003
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Sun, Y., An, Q. M., Tian, X. Y., Wang, Z. L., Guan, X. Y., Dong, B., et al. (2016). Fusobacterium nucleatum infection is correlated with tumor metastasis and postoperative survival of colorectal cancer patients in China. Translat. Cancer Res. 5, 579–588. doi: 10.21037/tcr.2016.10.45
Tahara, T., Yamamoto, E., Suzuki, H., Maruyama, R., Chung, W., Garriga, J., et al. (2014). Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 74, 1311–1318. doi: 10.1158/0008-5472.CAN-13-1865
Ueno, H., Shirouzu, K., Shimazaki, H., Kawachi, H., Eishi, Y., Ajioka, Y., et al. (2014). Histogenesis and prognostic value of myenteric spread in colorectal cancer: a Japanese multi-institutional study. J. Gastroenterol. 49, 400–407. doi: 10.1007/s00535-013-0822-1
Vilar, E., and Gruber, S. B. (2010). Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 7, 153–162. doi: 10.1038/nrclinonc.2009.237
Wu, Y., Wu, J., Chen, T., Li, Q., Peng, W., Li, H., et al. (2018). Fusobacterium nucleatum potentiates intestinal tumorigenesis in mice via a toll-like receptor 4/p21-activated kinase 1 cascade. Digest. Dis. Sci. 63, 1210–1218. doi: 10.1007/s10620-018-4999-2
Yamamoto, S., Kinugasa, H., Hirai, M., Terasawa, H., Yasutomi, E., Oka, S., et al. (2021). Heterogeneous distribution of Fusobacterium nucleatum in the progression of colorectal cancer. J. Gastroenterol. Hepatol. 36, 1869–1876. doi: 10.1111/jgh.15361
Yamane, L., Scapulatempo-Neto, C., Reis, R. M., and Guimarães, D. P. (2014). Serrated pathway in colorectal carcinogenesis. World J. Gastroenterol. 20, 2634–2640. doi: 10.3748/wjg.v20.i10.2634
Yamaoka, Y., Suehiro, Y., Hashimoto, S., Hoshida, T., Fujimoto, M., Watanabe, M., et al. (2018). Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J. Gastroenterol. 53, 517–524. doi: 10.1007/s00535-017-1382-6
Yan, X., Liu, L., Li, H., Qin, H., and Sun, Z. (2017). Clinical significance of Fusobacterium nucleatum, epithelial-mesenchymal transition, and cancer stem cell markers in stage III/IV colorectal cancer patients. Onco. Targets. Ther. 10, 5031–5046. doi: 10.2147/OTT.S145949
Yu, T., Guo, F., Yu, Y., Sun, T., Ma, D., Han, J., et al. (2017). Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563.e16. doi: 10.1016/j.cell.2017.07.008
Zhang, S., Yang, Y., Weng, W., Guo, B., Cai, G., Ma, Y., et al. (2019). Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Experiment. Clin. Cancer Res. 38:14. doi: 10.1186/s13046-018-0985-y
Zhang, X., Zhang, W., and Cao, P. (2021). Advances in CpG Island Methylator Phenotype Colorectal Cancer Therapies. Front. Oncol. 11:629390. doi: 10.3389/fonc.2021.629390
Keywords: cancer, colorectal cancer, meta-analysis, Fusobacterium nucleatum, review
Citation: Wang Y, Wen Y, Wang J, Lai X, Xu Y, Zhang X, Zhu X, Ruan C and Huang Y (2022) Clinicopathological differences of high Fusobacterium nucleatum levels in colorectal cancer: A review and meta-analysis. Front. Microbiol. 13:945463. doi: 10.3389/fmicb.2022.945463
Received: 17 May 2022; Accepted: 01 September 2022;
Published: 04 November 2022.
Edited by:
Jian Liu, Nankai University, ChinaReviewed by:
Yang Du, Annoroad Gene Technology, ChinaCopyright © 2022 Wang, Wen, Wang, Lai, Xu, Zhang, Zhu, Ruan and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayin Wang, d2FuZ2ppYXlpbkBtYWlsLnhqdHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.