
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 14 September 2022
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.942208
 Ming Deng1,2,3†
Ming Deng1,2,3† Zupeng Xiao1,2,3†
Zupeng Xiao1,2,3† Guangbin Liu1,2,3
Guangbin Liu1,2,3 Baoli Sun1,2,3
Baoli Sun1,2,3 Yongqing Guo1,2,3
Yongqing Guo1,2,3 Xian Zou4
Xian Zou4 Dewu Liu1,2,3
Dewu Liu1,2,3 Zhenwei Yang1*
Zhenwei Yang1* Yaokun Li1,2,3*
Yaokun Li1,2,3*In this study, silage Pennisetum sinese Roxb-based diet was replaced with fermented pineapple residue (FPR) at the replacement ratio of 0% (CON), 25% (T25), and 50% (T50) in fattening Simmental bulls for 30 days to evaluate the effects of FPR on growth performance, serum indexes, and ruminal characteristics. A total of 30 Simmental bulls (546 ± 44 kg initial BW) were allocated to three groups according to a completely randomized design. On day 30, the slaughter performance and meat quality were determined. Rumen fluids were collected for analyzing the rumen fermentation parameters and microbiota composition on day 30. The results showed that the average daily weight gain increased (P < 0.05) as the proportion of FPR rose. Within treatments, the T25 group reached more profit (5.34 RMB per day per bull) than CON while T50 was 3.69. The content of crude fat, cysteine, and proline in the muscle of T50 increased significantly (P < 0.05). The amounts of tyrosine, proline, and phenylalanine were significantly increased in the T25 (P < 0.05). The beta diversity analysis showed significant differences among the rumen bacterial flora of each group (P < 0.05). In the T25 group, the relative abundance of Spirochaetes decreased significantly (P < 0.05). The relative abundance of Lachnospiraceae_bacterium_RM44 was significantly lower (P < 0.05). Thus, FPR could improve the growth performance, economic benefits, and meat quality without adverse effects on ruminal characteristics.
After the United Nations Conference on Environment and Development in 1992 (Thomas, 1992), sustainable development became the consensus of countries worldwide. However, many countries are facing issues with the development of sustainable agriculture. Pineapple, the third most produced tropical fruit in China, plays an important role in the agricultural economy. In 2019, pineapple production in Guangdong Province exceeded one million tons and accounted for more than 60% of the production in China (Statistics Bureau of Guangdong Province, 2019). More than 30% of the pineapple residue is inedible pomace (Ketnawa et al., 2012), which may cause environmental pollution and ecological problems if not used properly. A previous study has shown that inedible pineapple pomace has ~19.8% cellulose, 11.7% hemicellulose, and abundant nutrients such as minerals and vitamins (Bardiya et al., 1996). Another study has shown that pineapple waste is physically and chemically suitable for making nursery pots (Jirapornvaree et al., 2017). Pineapple waste material has been used as a substrate for bromelain, organic acids, and ethanol; it can also be used in industrial processes such as fermentation and bioactive component extraction (Atul et al., 2013). Choi et al. (2021) found that feed-finishing Hanwoo steers with pineapple by-products had no adverse effects on growth and carcass performances. Fermentation can be used to process and convert pineapple residue into animal feed (Gowda et al., 2015). A previous study has shown that the addition of 20% fermented pineapple residue (FPR) replacing yellow corn in the basic diet can decrease the abdominal fat percentage of broiler chickens (Mandey et al., 2018). In sheep, pineapple by-product silage in diets could completely replace elephant grass and might reduce production costs without changing the consumption and performance (Cutrim et al., 2013). Wittayakun et al. (2019) found that pineapple waste silage-based diets had no significant impact on rumen fermentation, blood metabolites, and thyroid hormone responses. Hattakum et al. (2019) also found that ruminal pH, ammonia-nitrogen, and volatile fatty acid concentrations were not significantly different when pineapple stem by-products were used to feed Holstein steer. When 40% silage pineapple stem starch was used as roughage to feed Holstein steers, it can improve the feed conversion ratio by promoting short-chain fatty acids production in the rumen (Khongpradit et al., 2020). The addition of 25% silage pineapple residue as roughage can also positively promote weight gain of growing local Myanmar cattle (Kyawt et al., 2020). Considering that the availability of FPR could gain economic and environmental benefits, it might contribute to the sustainable development of agriculture when using FPR as a feedstuff to feed bulls. Therefore, this study aims to analyze the appropriate proportion of FPR replacing silage Pennisetum sinese Roxb (SPR), which is widely used in China as roughage in the basic diet of Simmental bull, and to evaluate its impact on growth performance, meat quality, and ruminal characteristics.
All experimental procedures and sample collection methods complied with the Regulation on the Administration of Laboratory Animals (CLI.2.293192, 2017 Revision, State Council, China) and were performed in strict accordance with the Institutional Animal Care and Use Committees of South China Agricultural University (approval no. 2018-P002).
The FPR was obtained from BOYA Biotechnology Co., Ltd (Leizhou, Guangdong, China). The raw pineapple peel was squeezed to maintain 78%−80% initial moisture, then evenly sprayed with a mixed lactic acid bacterium (Lactobacillus plantarum GIM1.191) and yeast (Saccharomyces cerevisiae GIM2.133) liquid. Finally, the FPR mixture was pressed into polyethylene bags (50 kg each) and fermented anaerobically for 20 days.
The experiment was conducted in a beef cattle company in Yunfu, Guangdong, China. A total of 30 healthy Simmental bulls (20 months old, 546 ± 44 kg weight) were used in a completely randomized design for a 3-day adaptation period and a 30-day experimental period. They were randomly divided into three groups in an open sawdust-bedded cowshed: the CON group (no FPR or control, fed basic diet), T25 (25% FPR replaced SPR), and T50 (50% FPR replaced SPR). All bulls were fed a total mixed ration (TMR) at 10:00 and 16:00, and water was provided ad libitum. To meet nutritional requirements, the TMR was based on SPR and rice straw as the main forage components and corn flour as the major concentrate component, according to NRC standards (NRC, 2016). The ingredients and nutrient composition of the three diets are shown in Table 1. The remaining feed was collected and recorded daily at 8:30.
The samples of FPR and FPR (days 0 and 20) were analyzed for dry matter (DM), crude protein (CP), ether extract (EE), and ash according to the AOAC International guidelines (AOAC, 1990). The neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were determined using the method reported by Van Soest et al. (1991). These contents were determined from water extract, while wet FPR (20 g) was transferred to a glass bottle filled with 180 ml of deionized water, sealed, mixed, and stored at 4°C overnight (Fang et al., 2016). Then, the water extract was passed through filter paper, and the filtrate pH was measured using a glass-electrode pH meter (Horiba D-21, Horiba, Tokyo, Japan). The FPR had low DM, CP, NDP, ADF, and ash of 21.15, 6.66, 63.46, 33.03, and 4.33% of DM basis, respectively (Table 2). Additionally, it had high starch of 3.2%.
On days 1, 12, and 24, the feed offered to the bulls was sampled and used for nutrient analysis and chemical analysis. The methods of nutrient determination, including CP, NDF, and ADF, were consistent with the method described in the “Nutritional compositions” section. Chemical analysis of the calcium (Ca) and phosphorus (P) contents was performed using inductively coupled plasma spectroscopy (Chemists and Horwitz, 1990).
The cattle were weighed before the morning feeding on days 1 and 30, and the average daily gain and feed weight ratio were calculated. Eight bulls were randomly selected from each group for the slaughter test. They were fasted for 12 h, and water was withheld for 3 h before slaughter. After being stunned, the cattle were slaughtered according to a general process, including hanging upside down, slaughtering, bloodletting, skinning, removing head and tail, and eviscerating. The live weight was recorded before slaughter and carcass weight after slaughter. Then the samples of the longissimus thoracis (LT) were excised between the 12th and 13th rib. After measuring the eye muscle area, pH, and flesh color of LT, cut into two uniform pieces vertically. One piece was put on ice for 24 h to measure drip loss, centrifugal water loss rate, pH and shear force, and the other was frozen in dry ice for the later determination of nutritional indicators.
The outline of the LT cross-section was delineated with sulfuric acid paper, and the eye muscle area was calculated with ADOBE PDF (version 1.2, San Jose Co., Ltd., CA, United States) after scanning. At 45 min, 24 h, and 48 h after slaughter, the pH was determined using a pH meter (FE28-Standard, METTLER-TOLEDO Co., Ltd., Shanghai, China) in the cut surface of the LT. A colorimeter (NR10QC, 3nh Co., Ltd., Shenzhen, China) was used to measure meat color on the surface of the LT about 45 min after slaughter. The meat samples were cut into 1 cm thick pieces, wrapped in plastic bags, heated to 70°C, and taken out to cool. The meat was cut into strips with a cross-section of 1 cm × 1 cm along the fiber direction, and then the shear force was measured using a tenderness meter (TA. XTPlus, SANHAO Co., Ltd., Suzhou, China). The meat was cut into long strips (5 cm × 2 cm × 2 cm) and weighted as N1. After being packed into plastic bags and hanging suspended for 24 h in a 4°C freezer, the weight was scored as N2. Drip loss% = (N2/N1) × 100%. Then, after centrifugation (1,500 × g, 30 min at 4°C), the centrifugal water loss rate was calculated. Analyzing DM, CP, EE, and ash of meat according to the AOAC method and amino acids using a full-automatic amino acid analyzer (LA8080, Hitachi Co., Ltd., Tokyo, Japan).
Rumen fluid samples were collected from all the bulls on the last day by a rumen tube before the morning feeding. To avoid the contamination of oral saliva, the first 20 ml of rumen fluid was discarded. Approximately 150 ml of rumen fluid sample from each bull was collected and then strained through four layers of cheesecloth. The filtrate was dispensed into 50-ml centrifuge tubes (REF430829, Corning Life Science Co., Ltd.) and 2-ml storage tubes. The samples in the storage tubes were put into liquid nitrogen and then transferred to a −80°C laboratory refrigerator for future use.
The pH of rumen fluid in centrifuge tubes was immediately measured by a pH meter (FE28-Standard, METTLER-TOLEDO Co., Ltd., Shanghai, China). Then, the samples were centrifuged at 5,000 × g for 15 min (BR4I, Thermo Co., Ltd., NY, United States) to collect the supernatant. The supernatant was divided into three 15-ml centrifuge tubes. Two tubes were used to measure volatile fatty acid (VFA) (acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, and valeric acid) content and ammonia nitrogen (NH3-N) concentration using a gas chromatograph (SP-3420, BEIFENGRUILI Co., Ltd., Beijing, China) and ELIASA (ST-360, KEHUA Co., Ltd., Shenzhen, China), respectively, according to Erwin et al. (1961) and Broderick and Kang (1980), and were stored at −20°C, and the remaining tube was stored at −80°C as a spare.
The total genomic DNA was extracted from rumen fluid samples using the modified cetyltrimethylammonium bromide/sodium dodecyl sulfate method (Zhang et al., 2021). The DNA samples were tested for integrity using 1% agarose gel electrophoresis, and their concentration was determined using a Qubit fluorometer (Nanodrop2000/2000C, Thermo Co., Ltd., NY, United States). Then, DNA was diluted to 1 ng/μl using sterile water according to the concentration. The V1–V9 regions of the 16S ribosomal DNA (rDNA) genes were amplified by polymerase chain reaction using the TransStart® FastPfu DNA Polymerase Kit (TransGen Biotech Co., Ltd., Beijing, China). In detail, the amplification was performed with the universal primers (forward primer, 27F: AGAGTTTGATCCTGGCTCAG; reverse primer, 149R: GNTACCTTGTTACGACTT). Sequencing libraries were generated using the SMRTbellTM Template Preparation Kit (Pacific Bioscience, CA, United States) on the PacBio Sequel sequencer.
Single-end reads were assigned to samples based on their unique barcode in the adaptor sequence. Quality filtering of the raw reads was performed to obtain high-quality clean reads according to the PacBio SMRT Portal Provisioning Agreement. The reads were compared with the reference database using the UCHIME algorithm (http//www.drive5.com/usearch/manual/uchime_algo.html) to detect chimeric sequences (Haas et al., 2011; Quast et al., 2012), and clean reads were finally obtained using the Uparse software (Uparse v7.0.1001) Edgar RCUPARSE, 2013. Sequences with ≥97% similarity were assigned to the same operational taxonomic units (OTUs). For each representative OTU, the Silva Database (https://www.arb-silva.de/) was used to annotate taxonomic information based on the Mothur algorithm (Quast et al., 2012). Alpha diversity was applied to analyze the complexity of species diversity within groups, including the observed species, Chao1, Shannon, and ACE indices. Beta diversity analysis was used to evaluate differences between groups using nonmetric multidimensional scaling (NMDS). All these indices were calculated using the quantitative insights into microbial ecology (QIIME) pipeline (Version 1.7.0).
The data were analyzed using the INFLUENCE Statement and GLM Model of SAS (version 9.4; SAS Institute Inc., Cary, NC, United States). A CONTRAST Statement was used to analyze the effects of each index between treatment and control. The test results were presented as the mean and standard error of the mean (SEM), with P < 0.05, indicating a significant difference, and P < 0.01, indicating a highly significant difference. Growth performance, meat quality indicators, rumen fermentation parameters, and relative abundance of rumen flora were analyzed using the analytical model I: Yi = μ + Pi + εi, where Yi is the dependent variable value of the bull in different treatments, μ is the overall mean, Pi is the dietary treatment effect, and εi is the random error. The KENWARDROGERS method is used to perform DOF correction.
The FPR had low DM, CP, NDP, ADF, and ash of 21.15, 6.66, 63.46, 33.03, and 4.33% of DM basis, respectively (Table 2). Additionally, it had high starch of 3.2.%.
The daily dry matter intake (DMI) of the control and treatments were approximately similar (Figure 1). The average daily weight gain of the T25 and T50 groups, respectively, increased by 0.17 and 0.29 kg, and DMI/weight gain significantly (P < 0.05) decreased (Table 3). According to the purchase and sale prices, the benefit of fattening each bull per day improved from ¥3.52 (CON) to ¥ 8.86 (T25) and ¥7.21 (T50; Table 4).
Fermented pineapple residue did not adversely affect the slaughter performance and beef sensory quality (Table 5). The crude fat indicators were significantly higher (P < 0.05) in T50 than in CON (Table 6). The content of cysteine, glycine, histidine, phenylalanine, proline, and tyrosine in treatments was raised (P < 0.05; Table 7), indicating that FPR can improve the amino acid composition of meat.
The FPR increases (P < 0.05) the rumen fluid pH while CON was 7.05, T25 was 7.18, and T50 was 7.26 (Table 8). The concentrations of isobutyric acid and isovaleric acid significantly (P < 0.05) decreased in T25 (0.83 mmol/L) and T50 (0.70 mmol/L) while CON was 0.94, whereas isovaleric acid descent (P < 0.05) in T25 (0.88 mmol/L) and T50 (0.62 mmol/L) while CON was 1.92. Butyrate raised (P < 0.05) while CON was 7.60%, T25 was 8.87%, and T50 was 9.65%. Thus, FPR had a regulating effect on the fluctuation range of the rumen fermentation parameters.
The V1–V9 regions of the 16S rDNA were enriched, and 356,162 raw reads were collected using high-throughput analysis. After quality control, each sample produced 11,339 valid sequences with a read length of 1,447 nucleotides. Venn diagram analysis yielded 4,119 unique OTU candidates with 97% sequence similarity, and 1,331 candidates shared across all samples were defined as core OTUs (Figure 2). The core OTUs were ~32.31% of the total candidates, whereas 456, 447, and 480 OTUs were identified as unique in the CON, T25, and T50 groups, respectively. A total of 21 phyla, 26 kingdoms, 46 orders, 66 families, 102 genera, and 97 species were found using the OTU annotations. The main bacterial phyla were Firmicutes, Bacteroidetes, and Tenericutes (48.62, 38.19, and 5.65%, respectively; Figure 3). At the species level (Figure 4), Rumen_bacterium_YS3 (1.32%) was the most common species. Unclassified bacteria accounted for 93.45% of the OTUs, while the identified secondary strains accounted for 97.03%.
Observed species, Chao1, Shannon, Simpson, ACE, and PD_whole_tree were used to evaluate the microbial diversity after FPR treatment (Figure 5). The addition of FPR had no significant effect on the above-mentioned indexes, but the diversity and richness tended to decrease as the proportion of FPR increased. The diversity and richness of the T50 group were the lowest. The rumen flora of the groups was roughly distributed in the same area (Figure 6A). The sample distances were more concentrated within each group, presenting three different colonies as a whole; this indicated that FPR affected the main bacterial groups in the rumen. The locations of the sample points in each group were not completely separated (Figure 6B), and the area of intersection of sample colonies in each group was the smallest for CON and T50, which indicated that 25% FPR affected more than 50% but not vigorously. The differences between and within bacterial groups showed obvious discrimination under the nonlinear structure (Figure 6C); the samples were clustered more centrally within each treatment, and the groups were well distinguished.
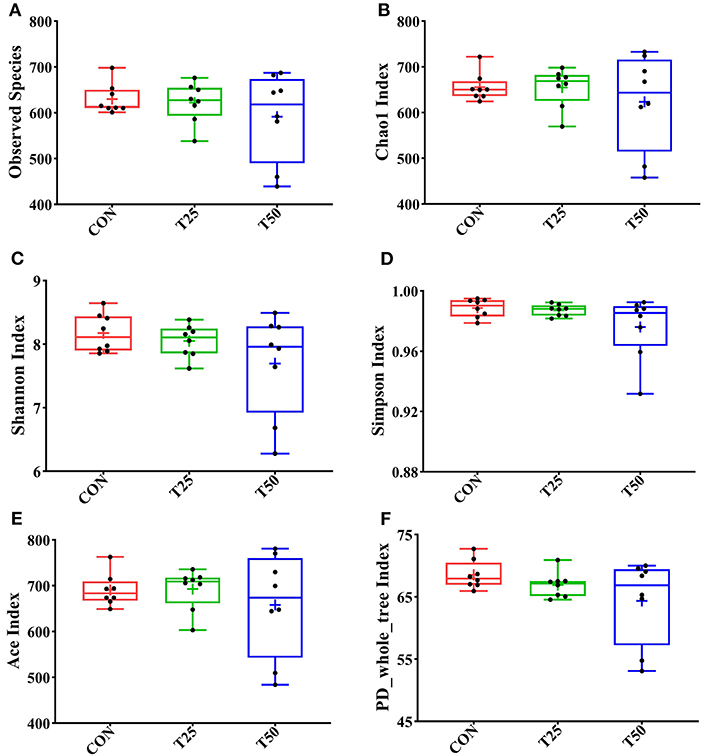
Figure 5. Alpha diversity analysis of rumen flora. (A) The number of observed species; (B) Chao1 index of species richness; (C) Shannon index of species diversity; (D) Simpson index of diversity; (E) Ace index of species richness; and (F) phylogenetic tree index. The “+” symbol in the box plot represents the mean of the within-group exponent.
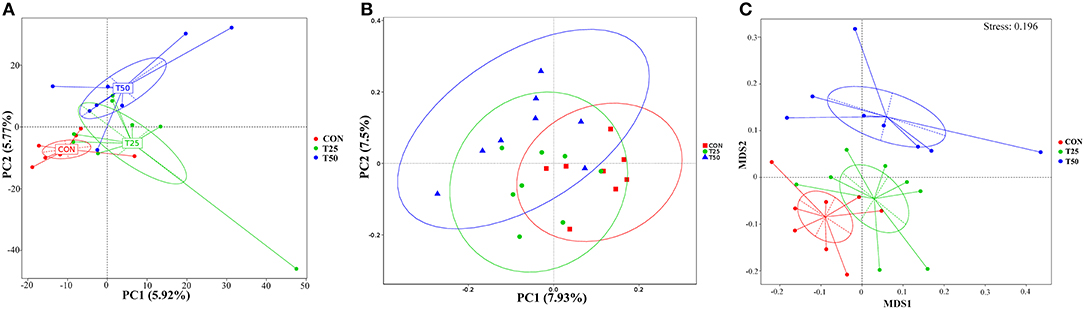
Figure 6. Differences of bacteria between groups. (A) PCA main coordinate axis analysis; (B) PCoA main coordinate axis analysis; (C) NMDS nonmetric multidimensional scale analysis.
A total of 25 different bacterial strains were statistically detected between the groups, with nine species in CON, two in T25, and 14 in T50 (Figure 7A). The phylogenetic tree showed multiple clades (Figure 7B). The evolutionary routes of the three treatment groups were mixed with each other, indicating they had similar evolutionary directions. This showed that the environment created in the rumen was not the same when the proportion of FPR was different, so the rumen microbes followed different evolutionary directions.
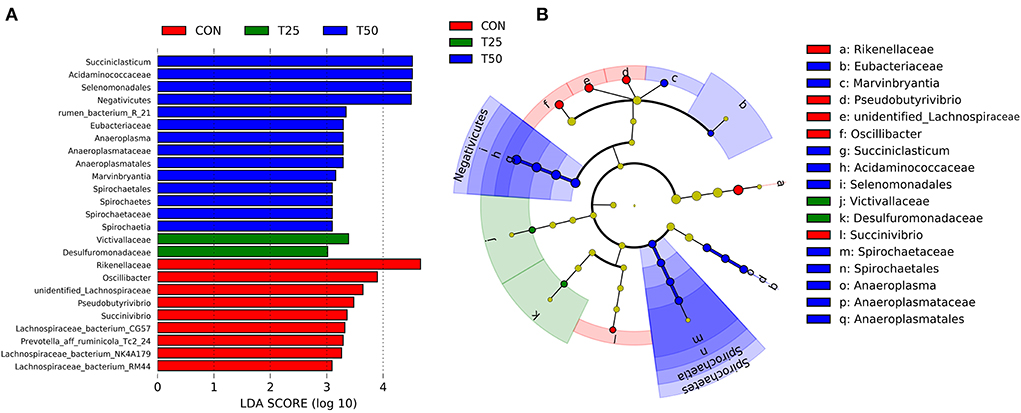
Figure 7. LEfSe analysis of rumen microflora. (A) LDA bar chart; (B) LEfSe evolution branch diagram. The graph reflects the affiliation of flora populations between groups at the species to phyla level, with node size corresponding to the average relative abundance of the corresponding taxon.
To analyze the correlations of FPR and rumen microbiota, Pearson correlation analysis was performed and then found that four phyla, seven genera, and three species were related to the rumen fermentation parameters (Figure 8). At the genus level, unidentified_Rikenellaceae and Psedobutyrivibrio were positively (P < 0.05) correlated to pH, whereas Succiniclasticum was significantly (P < 0.05) negatively correlated. Fibrobacter was negatively (P < 0.05) correlated to NH3-N while Pirellula was positive. Alcaligenes was positively (P < 0.05) correlated to lactic acid and butyric acid. Marvinbryantia was correlated to butyric acid positively (P < 0.05). At the species level, Lachnospiraceae_bacterium_RM and Butyrivibrio_fibrisolvens were negatively (P < 0.05) correlated to pH. Butyrivibrio_fibrisolvens was positively (P < 0.05) correlated to NH3-N. Alcaligenes_faecalis was significantly correlated to lactic acid and butyric acid (P < 0.05).
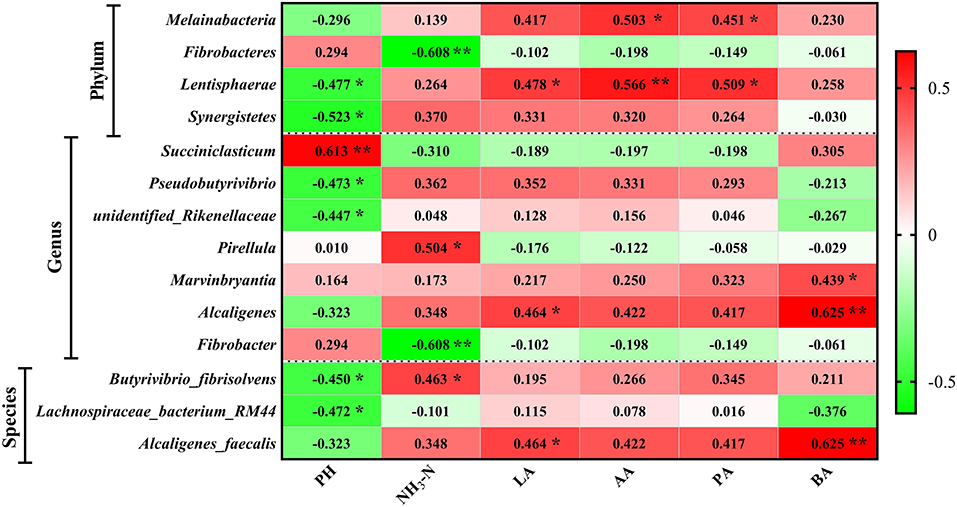
Figure 8. Correlation of fermentation parameters and flora. *indicates P < 0.05, and **indicates P < 0.01.
Agricultural by-products may be used to solve the feed shortage problem. In this study, the nutrient levels of FPR (~21% DM, 6% CP, 63% NDF, and 33% ADF) were lower than SPR (~32% DM, 7% CP, 72% NDF, and 43% ADF). However, the addition of FPR resulted in higher average daily weight gain and a lower DMI-weight-gain ratio. Thus, the treatments generated more net profit. This could be attributed to the addition of lactic acid bacteria and yeast to the pineapple residue during fermentation, which may have improved the digestibility of feed and energy efficiency in rumen (Niba et al., 2009; Kaewpila et al., 2021; So et al., 2021), cause the organic acids produced during fermentation contributed on structural carbohydrate hydrolysis (Wang et al., 2018). The addition of FPR had no adverse effects on the slaughter performance and beef sensory quality, which is similar to the findings of Hattakum et al. (2019), Liu et al. (2020), and Mello et al. (2021). Intramuscular fat deposition is influenced by numerous factors, such as breed, genotype, age, and nutrition (Jeong et al., 2013). Luccia et al. (2013) and Zhang and Guan (2019) found that improving dietary energy levels would increase the intramuscular fat content and decrease shear force, thus improving meat quality. A significant increase in crude fat content was observed in T50, whereas no differential shear force was detected. The possible mechanism of this strange phenomenon needs further study.
Isovalerate and isobutyrate are branched-chain VFAs (BCVFAs) produced by rumen microbial deamination and decarboxylation of leucine and valine. BCVFAs can improve NDF degradability, bacterial protein synthesis, and bacterial growth rate (Kajikawa et al., 2002; Zhang et al., 2013). Liu et al. (2020) found the addition of BCVFAs accelerated the growth of Holstein dairy calves. However, this study showed that isobutyric acid and isovaleric acid decreased significantly in the treatments. Considering the higher feed conversion rate in treatments, the relevant BCVFAs dynamically change in a complex process when the NDF reaches a lower level. The OTUs of the groups were relatively similar, but the components of the microflora were significantly different, with T50 showing the lowest diversity and richness. This was also reflected in the changes in the rumen microecological environment, such as pH (CON, 7.05; T25, 7.18; T50, 7.26) and NH3-N (CON, 8.81; T25, 7.56; T50, 6.51; mg/100 ml). Therefore, the phylogenetic composition of the rumen microbes was quite different.
Members of Succiniclasticum are involved in converting succinate to propionate and contributing to fiber metabolism in ruminants (van Gylswyk et al., 1997). Furthermore, Succiniclasticum abundance has been positively correlated to feed efficiency (Auffret et al., 2020; Clemmons et al., 2020). Daghio et al. (2021) and Du et al. (2021) found that Succiniclasticum was positively correlated to body weight, which is consistent with our results. Ma et al. (2021) observed that Succiniclasticum was positively related to NH3-N, isobutyrate, and isovalerate levels, which is inconsistent with our results. Lachnospiraceae has been reported to be correlated to feed efficiency in beef cattle (Li and Guan, 2017; Hernandez et al., 2021). The decreased relative abundance of unidentified_Lachnospiraceae shows that FPR contributes to intestinal health. Ma et al. (2019) found that unidentified_Lachnospiraceae was positively correlated to SOD and GSH when mice were fed with a high-fat diet, but it was negative in this study. It concluded that the FPR contributes to reducing oxidative stress damage in the gut. The increased relative abundance of Oscillibacter may lead to metabolic diseases (Naseribafrouei et al., 2014; Cheung et al., 2019). Pseudobutyrivibrio is related to sugar phosphorylation metabolism (Kasperowiczb et al., 2010), and Anaeroplasma has been found to be associated with lipid metabolic diseases and short-chain fatty acid metabolism (Granado-Serrano et al., 2019; Velazquez et al., 2019). This may partly explain the decline of isobutyric acid and isovaleric acid varied in T50 cause.
The results indicate that FPR increased growth performance and did not affect the major VFA content of the rumen or the diversity and richness of the rumen flora. The net profit of each bull in treatments had improved. Synthetically considering the economic benefits and growth performance, 25% FPR in diet has a positive impact on feeding bull. However, the specific mechanisms need to be studied further.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by Administration of Laboratory Animals (CLI.2.293192, 2017 Revision, State Council, China) Institutional Animal Care and Use Committees of South China Agricultural University (Approval No. 2018-P002).
This study was conceived and designed by YL and DL. The experiments was performed by ZY and MD. The data were analyzed by ZY and ZX. The manuscript was mainly written by ZY and ZX with the assistance of GL, BS, YG, and YL. All authors read and approved the final manuscript.
This study was supported by the Guangdong Provincial Promotion Project of Modern Seed Industry, GuangDong Basic and Applied Basic Research Foundation-Enterprise (Wens) Joint Fund (2019B1515210017), Special Fund of Agricultural Development and Rural Work in Guangdong Province Beef Cattle Concentrate Feed and Roughage Research and Development, and Popularization and Application in South China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
HSPD1, heat shock protein family D member 1; SIFs, sperm intrinsic factors; FPR, fermented pineapple residue; SPR, silage Pennisetum sinese Roxb; DM, dry matter; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; VFA, volatile fatty acid; NH3-N, ammonia nitrogen; OTU, operational taxonomic unit; DMI, dry matter intake.
AOAC. (1990). Official Methods of Analysis. 15th edn. Washington DC: Association of Official Analytical Chemists.
Atul, U., Jeewan, P. L., and Shinkichi, T. (2013). Utilization of pineapple waste: a review. J. Food Sci. Tech. 6, 10–18. doi: 10.3126/jfstn.v6i0.8255
Auffret, M. D., Stewart, R. D., Dewhurst, R. J., Duthie, C. A., Watson, M., Roehe, R., et al. (2020). Identification of microbial genetic capacities and potential mechanisms within the rumen microbiome explaining differences in beef cattle feed efficiency. Front. Microbiol. 11, 1229. doi: 10.3389/fmicb.2020.01229
Bardiya, N., Somayaji, D., and Khanna, S. (1996). Biomethanation of banana peel and pineapple waste. Bioresource Technol. 58, 73–76. doi: 10.1016/S0960-8524(96)00107-1
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. Anim. Sci. J. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Chemists, A. A., and Horwitz, W. (1990). Official Methods of Analysis, 15th ed. Arlington, VA: AOAC.
Cheung, S. G., Goldenthal, A. R., Uhlemann, A. C., Mann, J. J., Miller, J. M., et al. (2019). Systematic review of gut microbiota and major depression. Front. Psychiatry 10, 34. doi: 10.3389/fpsyt.2019.00034
Choi, Y., Lee, S., and Na, Y. (2021). Effects of a pineapple (Ananas comosus L.) cannery by-product on growth performance and carcass characteristics in finishing Hanwoo steers. Anim. Biosci. 34, 2. 233–242. doi: 10.5713/ajas.20.0234
Clemmons, B. A., Powers, J. B., Campagna, S. R., Seay, T. B., Embree, M. M., Myer, P. R., et al. (2020). Rumen fluid metabolomics of beef steers differing in feed efficiency. Metabolomics 16, 23. doi: 10.1007/s11306-020-1643-x
Cutrim, D. O., Alves, K. S., Neiva, J. N. M., Oliveira, d. E., Mezzomo, L. R., Elias, R., et al. (2013). Replacement levels of elephant grass by moist pineapple by-product silage in diets of Santa Inês crossbred sheep: performance and digestibility. Trop. Anim. Health Prod. 45, 585–592. doi: 10.1007/s11250-012-0263-5
Daghio, M., Ciucci, F., Buccioni, A., Cappucci, A., Casarosa, L., Serra, A., et al. (2021). Correlation of breed, growth performance, and rumen microbiota in two tustic cattle breeds reared under different conditions. Front. Microbiol. 12, 652031. doi: 10.3389/fmicb.2021.652031
Du, E., Guo, W., Zhao, N., Chen, F., Fan, Q., et al. (2021). Effects of diets with various levels of forage rape (Brassica napus) on growth performance, carcass traits, meat quality and rumen microbiota of Hu lambs. J Sci Food Agr. 102, 1281–1291. doi: 10.1002/jsfa.11466
Edgar RCUPARSE. (2013). Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 10, 996–998. doi: 10.1038/nmeth.2604
Erwin, E. S., Marco, G. J., and Emery, E. M. (1961). Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. Anim. Sci. J. 44, 1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6
Fang, J., Cao, Y., Matsuzaki, M., and Suzuki, H. (2016). Effects of apple pomace proportion levels on the fermentation quality of total mixed ration silage and its digestibility, preference and ruminal fermentation in beef cows. Anim. Sci. J. 87, 217–223. doi: 10.1111/asj.12410
Gowda, N. K. S., Vallesha, N. C., Awachat, V. B., Anandan, S., Pal, D. T., Prasad, C. S., et al. (2015). Study on evaluation of silage from pineapple (Ananas comosus) fruit residue as livestock feed. Trop. Anim. Health Prod. 47, 557–561. doi: 10.1007/s11250-015-0762-2
Granado-Serrano, A. B., Martín-Garí, M., Sánchez, V., Solans, M. R., Berdún, R., Ludwig, I. A., et al. (2019). Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 9, 102–107. doi: 10.1038/s41598-019-38874-3
Haas, B. J., Gevers, D., Earl, A. M., Feldgarden, M., Ward, D. V., Giannoukos, G., et al. (2011). Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504. doi: 10.1101/gr.112730.110
Hattakum, C., Kanjanapruthipong, J., Nakthong, S., Wongchawalit, J., Piamya, P., Sawanon, S., et al. (2019). Pineapple stem by-product as a feed source for growth performance, ruminal fermentation, carcass, and meat quality of Holstein steers. S. Afr. J. Anim. Sci. 49, 147–155. doi: 10.4314/sajas.v49i1.17
Hernandez, E., Guan, L. L., Goonewardene, L. A., Li, M., Mujibi, D. F., Stothard, P., et al. (2021). Correlation of particular bacterial PCR-denaturing gradient gel electrophoresis patterns with bovine ruminal fermentation parameters and feed efficiency traits. Appl. Environ. Microbiol. 76, 6338–6350. doi: 10.1128/AEM.01052-10
Jeong, J., Bong, J., Kim, G. D., Joo, S. T., Lee, H. J., Baik, M., et al. (2013). Transcriptome changes favoring intramuscular fat deposition in the longissimus muscle following castration of bulls. J. Anim. Sci. 91, 4692–4704. doi: 10.2527/jas.2012-6089
Jirapornvaree, I., Suppadit, T., and Popan, A. (2017). Use of pineapple waste for production of decomposable pots. Int. J. Recycling Org. 6, 345–350. doi: 10.1007/s40093-017-0183-5
Kaewpila, C., Gunun, P., Kesorn, P., Subepang, S., Thiputen, S., Cai, Y., et al. (2021). Improving ensiling characteristics by adding lactic acid bacteria modifies in vitro digestibility and methane production of forage-sorghum mixture silage. Sci Rep. 11, 1968. doi: 10.1038/s41598-021-81505-z
Kajikawa, H., Mitsumori, M., and Ohmomo, S. (2002). Stimulatory and inhibitory effects of protein amino acids on growth rate and efficiency of mixed ruminal bacteria. J. Dairy Sci. 85, 2015–2022. doi: 10.3168/jds.S0022-0302(02)74278-1
Kasperowiczb, A., Pristaša, P., Piknováa, M., Javorskýa, P., Guczyńskab, W., Michałovskib, T., et al. (2010). Fructanolytic and saccharolytic enzymes of Treponema zioleckii strain kT. Anaerobe 16, 387–392. doi: 10.1016/j.anaerobe.2010.03.003
Ketnawa, S., Chaiwut, P., and Rawdkuen, S. (2012). Pineapple wastes: a potential source for bromelain extraction. Food Bioprod. Process. 90, 385–391. doi: 10.1016/j.fbp.2011.12.006
Khongpradit, A., Boonsaen, P., Homwong, N., Suzuki, Y., Koike, S., Sawanon, S., et al. (2020). Effect of pineapple stem starch feeding on rumen microbial fermentation, blood lipid profile, and growth performance of fattening cattle. Anim. Sci. J. 91, e13459. doi: 10.1111/asj.13459
Kyawt, Y. Y., Win, K. S., Mu, K. S., Aung, A., and Aung, M. (2020). Feeding pineapple waste silage as roughage source improved the nutrient intakes, energy status and growth performances of growing Myanmar local cattle. J. Adv. Vet. Anim. Res. 7, 436–441. doi: 10.5455/javar.2020.g439
Li, F., and Guan, L. L. (2017). Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microbiol. 83, e00061–e00017. doi: 10.1128/AEM.00061-17
Liu, Y. R., Wang, C., Liu, Q., Guo, G., Huo, W. J., Zhang, Y. L., et al. (2020). Effects of branched-chain volatile fatty acids and fibrolytic enzyme on rumen development in pre- and post-weaned Holstein dairy calves. Anim. Biotechnol. 31, 512–519. doi: 10.1080/10495398.2019.1633340
Luccia, A. D., Satriani, A., Barone, C. M. A., Colatruglio, P., Gigli, S., Occidente, M., et al. (2013). Effect of dietary energy content on the intramuscular fat depots and triglyceride composition of river buffalo meat. Meat Sci. 65, 1379–1389. doi: 10.1016/S0309-1740(03)00060-3
Ma, H., Zhang, B., Hu, Y., Wang, J., Liu, J., Qin, R., et al. (2019). Correlation analysis of intestinal redox state with the gut microbiota reveals the positive intervention of tea polyphenols on hyperlipidemia in high fat diet fed mice. J. Agr. Food Chem. 67, 7325–7335. doi: 10.1021/acs.jafc.9b02211
Ma, Y., Wang, H., and Li, C. (2021). Response of sheep rumen fermentation and microbial communities to feed infected with the endophyte Epichloë gansuensis as evaluated with rumen-simulating technology. J. Microbiol. 59, 718–728. doi: 10.1007/s12275-021-1113-9
Mandey, J. S., Tulung, B., Leke, J. R., and Sondakh, B. F. J. (2018). “Performance and carcass quality of broiler chickens fed diet containing pineapple waste meal fermented by “ragi tape”,” in: IOP Conference Series: Earth and Environmental Science 102, 1. doi: 10.1088/1755-1315/102/1/012042
Mello, B. L. B., Fernandes, A. M., de Oliveira, T. S., Leonel, F. P., Glória, L. S., Silva, R. S., et al. (2021). Feed intake, digestibility, and energy contents in growing bull fed pineapple crop waste silage in different planes of nutrition. Trop. Anim. Health Pro. 53, 188. doi: 10.1007/s11250-021-02640-3
Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linløkken, A., Wilson, R., et al. (2014). Correlation between the human fecal microbiota and depression. Neurogastroent Motil. 26, 1155–1162. doi: 10.1111/nmo.12378
Niba, A. T., Beal, J. D., Kudi, A. C., and Brooks, P. H. (2009). Potential of bacterial fermentation as a biosafe method of improving feeds for pigs and poultry. Afr. J. Biotechnol. 8, 1758–1767. doi: 10.1186/1471-2105-10-130
NRC (National Research Council). (2016). Nutrient Requirements of Beef Cattle. 8th ed. Washington, D.C.: National Academy Press.
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
So, S., Cherdthong, A., and Wanapat, M. (2021). Growth performances, nutrient digestibility, ruminal fermentation and energy partition of Thai native steers fed exclusive rice straw and fermented sugarcane bagasse with Lactobacillus, cellulase and molasses. J Anim Physiol An N. 106, 1. doi: 10.1111/JPN.13563
Statistics Bureau of Guangdong Province. (2019). Available online at: http://stats.gd.gov.cn/tjkx185/content/post_2878171.html (accessed June 8, 2022).
Thomas, C. (1992). The United Nations Conference on Environment and Development (UNCED) of 1992 in Context. Environ Politi. 1, 250–261. doi: 10.1080/09644019208414053
van Gylswyk, N. O., Hippe, H., and Rainey, F. A. (1997). Schwartzia succinivorans gen. nov., sp. nov., another ruminal bacterium utilizing succinate as the sole energy source. Int. J. Syst. Bacteriol. 4791, 155–159. doi: 10.1099/00207713-47-1-155
Van Soest, P. V., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Velazquez, K. T., Enos, R. T., Bader, J. E., Sougiannis, A. T., Carson, M. S., Chatzistamou, I., et al. (2019). Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 11, 619–637. doi: 10.4254/wjh.v11.i8.619
Wang, S., Guo, G., Li, J., Chen, L., Dong, Z., and Shao, T. (2018). Improvement of fermentation profile and structural carbohydrate compositions in mixed silages ensiled with fibrolytic enzymes, molasses and Lactobacillus plantarum MTD-1. Ital J Anim Sci. 18, 328–335. doi: 10.1080/1828051X.2018.152
Wittayakun, S., Innsree, W., Inaree, S., Chainetr, W., and Kongngoen, N. (2019). Effect of protein to metabolizable energy ratio in pineapple waste silage-based diets on performance of holstein heifers. J. Anim. Health Prod. 7, 4. 158–165. doi: 10.17582/journal.jahp/2019/7.4.158.165
Zhang, H., and Guan, W. (2019). The response of gene expression associated with intramuscular fat deposition in the longissimus dorsi muscle of Simmental × Yellow breed cattle to different energy levels of diets. Anim. Sci. J. 90, 493–503. doi: 10.1111/asj.13170
Zhang, H. L., Chen, Y., Xu, X. L., and Yang, Y. X. (2013). Effects of branched-chain amino acids on in vitro ruminal fermentation of wheat straw. Asian-Australas. J. Anim. Sci. 26, 523–528. doi: 10.5713/ajas.2012.12539
Keywords: fermentation, pineapple, serum indexes, meat quality, rumen microbiota, Simmental bull
Citation: Deng M, Xiao Z, Liu G, Sun B, Guo Y, Zou X, Liu D, Yang Z and Li Y (2022) The effects of fermented pineapple residue on growth performance, meat quality, and rumen microbiota of fattening Simmental bull. Front. Microbiol. 13:942208. doi: 10.3389/fmicb.2022.942208
Received: 12 May 2022; Accepted: 21 July 2022;
Published: 14 September 2022.
Edited by:
Giovanni Tarantino, University of Naples Federico II, ItalyReviewed by:
Fangfang Zhao, Yangzhou University, ChinaCopyright © 2022 Deng, Xiao, Liu, Sun, Guo, Zou, Liu, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaokun Li, eWtsaUBzY2F1LmVkdS5jbg==; Zhenwei Yang, NDkzMjk0MjE5QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.