- College of Food Science, Sichuan Agricultural University, Ya’an, China
Bakery products are nutritious, but they are susceptible to fungal contamination, which leads to a decline in quality and safety. Chemical preservatives are often used to extend the shelf-life of bakery products, but long-term consumption of these preservatives may increase the risk of chronic diseases. Consumers increasingly demand food with fewer chemical preservatives. The application of lactic acid bacteria (LAB) as a novel biological preservative not only prolongs the shelf-life of bakery products but also improves the baking properties of bakery products. This review summarizes different types and action mechanisms of antifungal compounds produced by LAB, factors affecting the production of antifungal compounds, and the effects of antifungal LAB on bakery products, providing a reference for future applications of antifungal LAB in bakery products.
Introduction
Bakery products are among the most popular foods consumed daily by people throughout the world (ReportLinker, 2020). However, bakery products are susceptible to fungal contamination that greatly reduces their shelf-life, resulting in food waste and economic loss. Fungal contamination can also become a serious food safety issue due to the potential production and ingestion of toxic aflatoxins and other mycotoxins (Mahato et al., 2021). Inhibition of fungal growth on bakery products therefore offers significant economic and health benefits. The primary methods of inhibition that are being explored to extend the shelf-life of bakery products currently focus on physical methods (radio frequency sterilization, microwave sterilization, drying, pulsed-light, and low-pressure mercury lamp treatment) and chemical preservatives (calcium propionate, sorbate, benzoates, EDTA, nitrites, and sulfites; Salaheen et al., 2014). Although physical methods are better at maintaining taste, they simultaneously destroy the nutritional value of bakery products and are often costly. Calcium propionate and other chemical preservatives can be added directly to bakery products; however, long-term consumption of these preservatives may increase the risk of chronic disease (Qian et al., 2021). Food companies are interested in reducing the use of chemical preservatives because of the need of consumers for preservative-free foods (Sadeghi et al., 2019). Biological preservatives, on the other hand, are more consumer-friendly, ecologically sustainable, and have prospectively broad applications in controlling fungal contamination. Lactic acid bacteria (LAB) have gained attention as a potential biological preservative option since they are generally recognized as safe, and produce metabolites that can inhibit fungal growth. For example, Korcari et al. (2021) found that Pediococcus pentosaceus MB33 and Weissella cibaria CM32 exhibited high antifungal activity, enhancing the shelf-life of emmer bread. LAB also improve baking properties of bakery products including texture, specific volume, and flavors of bakery products (Ma et al., 2021). Several antifungal compounds produced by Leuconostoc citreum HO12 and Weissella koreensis HO20 improved the flavor and texture of bakery products (Choi et al., 2012). Therefore, investigating the application of antifungal LAB in bakery products has substantial value. While there are reviews on the application of antifungal LAB in foods, and mechanisms of antifungal substances, few of them have comprehensively focused on the application of LAB as biopreservatives in bakery products, and the antifungal mechanism of exopolysaccharides (EPS; Crowley et al., 2013a; Chen et al., 2021b; Nasrollahzadeh et al., 2022). The present review seeks to present an update on the antifungal compounds produced by LAB and their mechanism of action toward target fungi, factors affecting the production of antifungal compounds by LAB, and the application of LAB in bakery products.
Health Problems Caused by Fungal Contamination
The most common fungi related to spoilage of bakery products include Aspergillus, Penicillium, Fusarium, Mucor, Rhizopus, Candida, and Endopyrrhiza (Mohamad Asri et al., 2020). Fungal contamination not only reduces the quality of bakery products but also results in huge economic losses to both consumers and the bakery industry. In addition, some fungi (Aspergillus, Penicillium, Fusarium, etc.) may produce mycotoxins such as aflatoxin, ochratoxin, and zearalenone (Krnjaja et al., 2019; Drakopoulos et al., 2021). Aflatoxins are difuran ring toxoids mainly produced by certain strains of Aspergillus flavus and Aspergillus parasitica (Wang et al., 2022a). Aflatoxins are carcinogenic, teratogenic, and mutagenic and can damage the liver of animals (Ben Taheur et al., 2019). Aflatoxin B1 (AFB1) is the most toxic type of aflatoxin and is a significant risk factor for the development of hepatocellular carcinoma (HCC) in humans and animals (Fang et al., 2022). Ochratoxins are mycotoxins derived from Aspergillus and Penicillium, and their toxic effects include hepatotoxicity, carcinogenicity, nephrotoxicity, immunotoxicity, teratogenicity, mutagenicity, genotoxicity, embryotoxicity, as well as testicular toxicity (Amézqueta et al., 2012; Wang et al., 2022b). Zearalenone-producing fungi are mainly Fusarium. Zearalenone causes obvious estrogenic effects in both humans and animals, as well as serious malignant alterations and lesions in the female reproductive system (Sanad et al., 2022).
Overview of LAB
LAB are Gram-positive, non-spore-forming, facultatively anaerobic bacteria that produce a large amount of lactic acid during carbohydrate metabolism. LAB are widely distributed in nature, and include genus such as Aerococcus, Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Streptococcus, Tetragonococcus, Oenococcus, Weissella, and Vagococcus (Stiles and Holzapfel, 1997). LAB play a critical role in food fermentation to enhance food shelf-life (Pontonio and Rizzello, 2021). LAB induce rapid acidification of raw materials during fermentation, producing organic acids, CO2, H2O2, fatty acids, antifungal peptides, volatile compounds, and other antifungal compounds that inhibit fungal growth (Sadeghi et al., 2019). Moreover, mycotoxins can be detoxified by LAB with the help of one or more mechanisms. For instance, the utilization of metabolites and enzymes produced by LAB strains, adsorption of mycotoxins by LAB, or competitive relationship between LAB and other mycotoxin producing fungi (Ahlberg et al., 2015). LAB produce a variety of proteolytic enzymes, including cell-wall proteinases, peptide transporters, and ample intracellular peptidases, which are capable of biodegradation of mycotoxins to less toxic and less harmful compounds (Bangar et al., 2021). Citric acid and other organic acids produced by LAB also have a great effect on degrading aflatoxins. The results from Mendez-Albores et al. (2008) showed that the aflatoxin reduction was more effective when adding aqueous citric acid in the extrusion-cooking process. Ademola et al. (2021) found that lactic acid fermentation also reduced aflatoxin and fumonisin levels in maize. LAB cell wall including peptidoglycon and polysaccharides can adsorb mycotoxin, which also caused the toxin removal (Shetty and Jespersen, 2006). As a biological preservative, LAB significantly reduce the usage of chemical preservatives in bakery food. In addition, LAB also produce exopolysaccharides, ethanol, and volatile flavor substances during fermentation, which bring balanced bread sensory profiles. The α-glucan-producing Lactobacillus reuteri E81 was used in bread making, which improved the rheological properties, elasticity, and microstructure of bread dough, and the texture of bread (İspirli et al., 2020). Wu et al. (2022) found that sourdough fermented with Lactococcus lactis FCP1921 had improved volatile flavor substance abundance in bread when compared with ordinary wheat bread.
Antifungal Compounds Produced by LAB
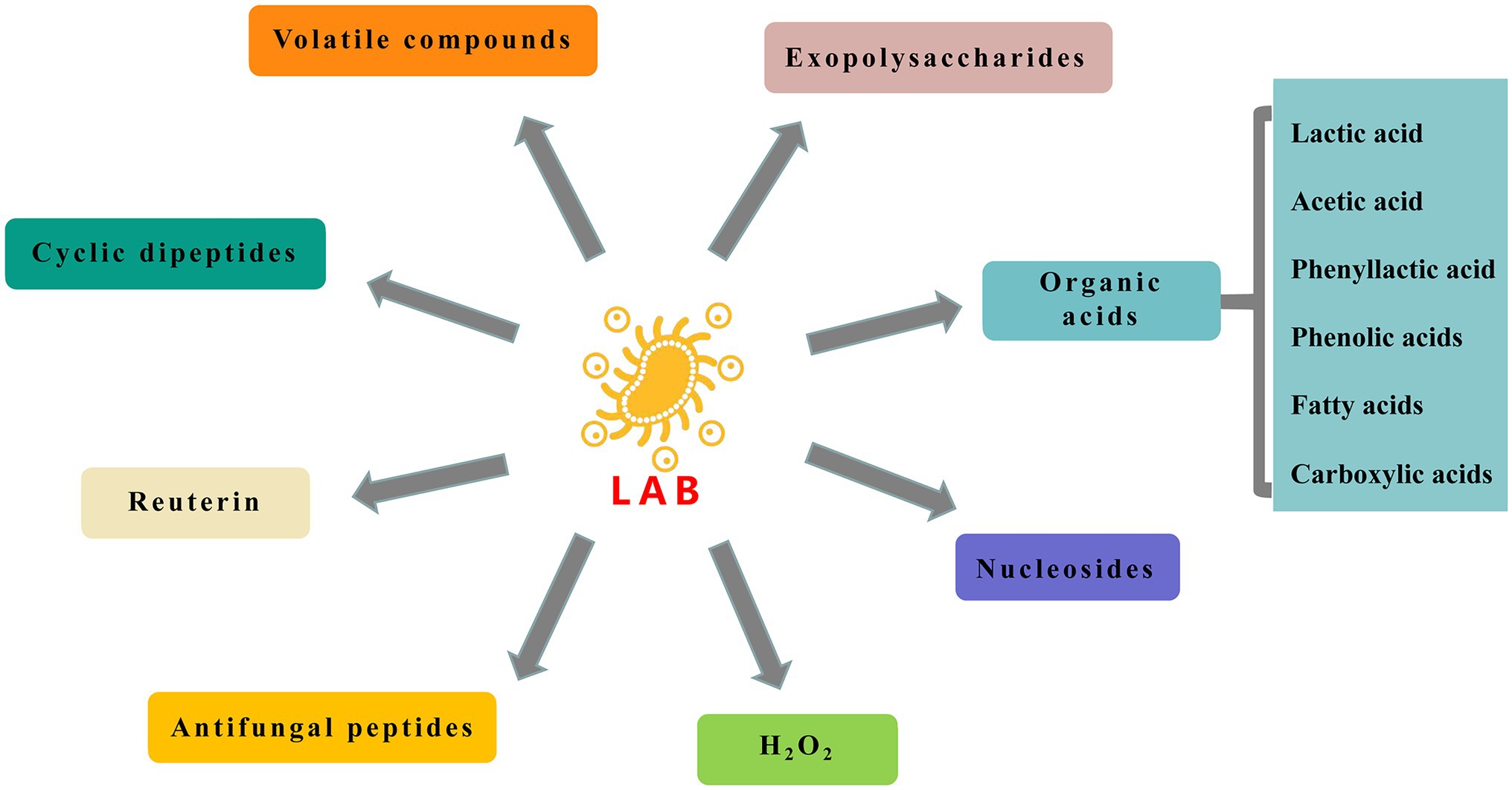
Metabolism of carbohydrates, proteins, lipids, and amino acids by LAB produces a variety of antifungal compounds discussed further here (Figure 1).
Organic Acids
LAB produce lactic acid, acetic acid, propionic acid, citric acid, phenyllactic acid, benzoic acid, and other organic acids. Most of these organic acids have antifungal properties; however, action mechanisms of some organic acids are still unclear. Studies have shown that acetic acid’s inhibition of the growth of pathogenic and spoilage bacteria and fungi may be due to its low pKa. The higher concentration of undissociated acids can traverse the cell membrane, then dissociate in the cell, resulting in acid stress (Jin et al., 2021). Relative to other organic acids, lactic acid demonstrated weaker inhibitory effects (Crowley et al., 2013a). With increased fermentation time, the lactic acid content of cell-free supernatants was positively correlated with antifungal activity, and lactic acid frequently played a synergistic role with other organic acids to ultimately enhance antifungal activity (Russo et al., 2017). Phenolic acids isolated from quinoa dough, including derivatives of cinnamic acid (p-coumaric acid, caffeic acid, and ferulic acid) and benzoic acid (vanillic acid, 4-hydroxybenzoic acid), also showed antifungal activity (Axel et al., 2016). Phenolic acids often exist in an insoluble form, or in a more complex cross-linked polymer, which can exert antioxidant and antifungal effects (John, 2008). Furthermore, some carboxylic acids produced by LAB, including 3-phenylpropionic acid, hydroxyphenyllactic acid, 3-(4-hydroxyphenyl) propionic acid, and 5-oxypyrroliden-2-carboxylic acid, showed inhibitory effects on Mucor ractosum and Penicillium (Leyva Salas et al., 2019). Honoré et al. (2016) isolated metabolites, such as 2-hydroxy-4-methylpentanoic acid, 2-hydroxy-3-phenylpropionic acid, and 2-hydroxy-3-(4-hydroxyphenylpropionic) propionic acid, from the cell-free supernatant of Lacticaseibacillus paracasei, which was positively correlated with inhibition of mold growth.
Phenyllactic Acid
Phenyllactic acid is derived from the catabolism of phenylalanine (Phe), which transfers its amino group to the ketoic acid receptor and then reduces the synthesized phenylpyruvate to phenyllactic acid (PLA) through dehydrogenase action (Vermeulen et al., 2006). PLA harbors broad spectrum antibacterial activity, which can destroy biofilm structures and inhibit the growth of pathogenic and spoilage bacteria and fungi. Dal Bello et al. (2007) isolated PLA, lactic acid, and two cyclic dipeptides from the cell-free supernatant of Lactobacillus plantarum FST1.7, all of which showed antifungal activity. Meanwhile, Gerez et al. (2009) obtained LAB with antifungal activity against Aspergillus, Fusarium, and Penicillium and also isolated PLA and acetic acid from these LAB. Le Lay et al. (2016a) detected PLA (22.04 mg/L) in the cell-free supernatant of Lactobacillus spicheri O15 with demonstrated antifungal activity. Like other organic acids, the antifungal activity of PLA is pH-dependent. At low pH, the undissociated form can easily pass through the cell membrane and accumulate within the cytoplasm, thereby causing loss of cell activity (Lambert and Stratford, 1999). At pH 3.5, the minimum concentration of PLA needed to inhibit 50% of the germination of different fungal spores was 0.03–0.07 mmol/L, while lactic acid and acetic acid were 80–180 and 0.9–18 mmol/L, respectively. Although PLA had higher antifungal activity than lactic acid and acetic acid, this was only true at high PLA concentrations and low pH (Gerez et al., 2009; Debonne et al., 2020). In addition, the minimum inhibitory concentration of PLA is different on different fungi. For example, PLA possesses MIC value of 2.5 mg/ml against Aspergillus fumigatus, in contrast to 20 mg/ml against Aspergillus niger (Lavermicocca et al., 2003; Ryan et al., 2011).
Fatty Acids
Fatty acids produced by LAB also have strong antifungal activity, and hexadecanoic acid, oleic acid, hexadecanoic acid, decanoic acid, and lauric acid isolated from antifungal LAB were confirmed to show inhibitory effects against Mucor ractosum and Penicillium common (Leyva Salas et al., 2019). In addition, 3-hydroxy-5-dodecenoic acid purified from Lactobacillus plantarum EM culture using solid-phase extraction and recycling preparative HPLC also exhibited a strong antifungal activity, with MIC of 0.21 g/L and 0.25 g/L against Aspergillus fumigatus and Bacillus cereus, respectively (Mun et al., 2019). Liang et al. (2017) extracted two kinds of fatty acids from cultures of Lactobacillus hammesii and Lactiplantibacillus plantarum and found that HUFA (unsaturated fatty acids with hydroxyl groups) effectively inhibited Aspergillus niger and Penicillum roqueforti but had poor inhibitory effects on Candida and other yeasts. Studies have shown that the antifungal activity of fatty acids is related to their structure; while unsaturated monohydroxy fatty acids have antifungal activity, saturated hydroxy fatty acids and unsaturated fatty acids do not (Black et al., 2013). Studies by Pohl et al. (2008) showed that the MICs of 13-HOE against Aspergillus niger and Penicillum roqueforti were 0.25 and 0.38 g/L, respectively. The MICs of 10-HOE against Aspergillus niger and Penicillum roqueforti were 0.42 and 0.38 g/l, respectively. The antifungal activity of hydroxy fatty acids might be due to their interaction with the cell membrane since distribution of hydroxy fatty acids into the fungal membrane increased membrane permeability (Sjogren et al., 2003).
H2O2
Some LAB produce H2O2, which has been proven to affect the growth and metabolism of foodborne pathogenic bacteria and fungi. As a strong oxidizer, H2O2 plunders electrons and molecules of nearby microorganisms and thus sterilizes by destroying protein molecular structure. Since LAB do not produce catalase, H2O2 cannot be decomposed, and therefore accumulates in the cell, preventing fungal growth (Nes et al., 1996). Bundgaard-Nielsen and Nielsen (1996) found that 3% H2O2 solution exhibited low antifungal activity against Penicillium, Cladosporium, Scopulariopsis, Aspergillus, and Eurotium but damaged conidia of seven fungal species. Martin and Maris (2012) tested the antifungal effect of H2O2 and 17 kinds of acids on fungi. The results showed that formic acid, acetic acid, propionic acid, oxalic acid, and lactic acid had synergistic effects with H2O2, resulting in stronger antifungal activity.
Antifungal Peptides
Antifungal peptides are the main antifungal compounds produced by LAB. Arulrajah et al. (2021) used Lactobacillus pentosus RK3 to ferment kenaf seeds to produce antifungal peptides, and eight cationic peptides were identified in the kenaf seed mixture, which showed inhibitory effects on Fusarium and Aspergillus niger. Among them, four peptides were shown to be similar to Gossypium mustelinum (cotton), two peptides corresponded to Gossypium barbadense (Sea-island cotton), and two were novel cationic de novo peptides. Similarly, Nionelli et al. (2020) identified nine peptides from bread hydrolysates fermented by Lactobacillus brevis, and these peptides prevented growth of Penicillium roqueforti. The inhibitory effect of antifungal peptides on fungi is mainly due to the interaction between negatively charged molecules of the fungal membrane and positively charged polypeptides, which destroys the membrane structure resulting in cell death (Rai et al., 2016). In addition, the activity of antifungal peptides produced by plant substrate fermentation was significantly affected by molecular weight, chemical structure, net charge, and hydrophobic ratio (Jakubczyk et al., 2020). Most of the antifungal peptides have low molecular weights, cationic charges, and low hydrophobicity (Muhialdin et al., 2016). Muhialdin et al. (2020) confirmed the above views, and they isolated a component targeting Aspergillus flavus from the cell-free supernatant of Lactiplantibacillus plantarum TE10 and 37 peptides identified from the isolated component—22 were cationic peptides.
Antifungal peptides also inhibited conidial germination, potentially through the inhibition of germ tube elongation after conidial wall breakdown (Puig et al., 2016). Leon et al. (2020) observed that the antifungal peptides AMPs LR14 produced by Lactiplantibacillus plantarum inhibit conidial germination. Fungal mycelia treated with antifungal peptides were wrinkled with no conidia detected, and the expression levels of brlA, a transcription factor involved in fungal meristem, were also significantly decreased.
Volatile Compounds
LAB produce several volatile compounds in the fermentation process, which not only improves the aroma profile but also inhibits fungal growth. Leyva Salas et al. (2019) isolated 35 volatile compounds from dairy products fermented by antifungal LAB, among which diacetyl and acetoin had antifungal activity and exhibited significant antifungal effects against Penicillium common and Mucor ractosum. Diacetyl, lactic acid, acetic acid, and acetoin were reported to be produced by catabolism of the two main carbon substrates present in dairy products, lactose and citrate (Urbach, 1995). Thus, acetoin and diacetyl with antifungal activity may be produced by lactic acid bacteria fermentation. Aunsbjerg et al. (2015) isolated diacetyl, the main volatile compound, from Lacticaseibacillus paracasei DGCC2132, which slowed down the growth of Penicillium for 5 days at concentrations above 75 μg/ml. The antifungal mechanisms of diacetyl may be due to the induction of ROS accumulation, which destroys the membrane structure resulting in the leakage of cellular materials and cell death (Shi and Knøchel, 2021). In addition, Coda et al. (2011) found that soluble extract of Lactiplantibacillus plantarum by Tris-HCI buffer contained ethyl acetate and ethanol, and confirmed that the conidia germination of Penicillium roqueforti was completely inhibited when ethyl acetate and ethanol concentrations were 6.81 and 1.69 mg/ml, respectively.
Cyclic Dipeptides
Cyclic dipeptides (CDPs) are metabolites widely synthesized by cyclodipeptide synthases or non-ribosomal peptide synthetases, by both prokaryotic and eukaryotic cells. CDPs are minimal cyclic peptides formed by inner cyclization of two amino acids amides and have various bioactive properties including anticancer, immunomodulatory, and antifungal activities (Rhee, 2004). Ström et al. (2002) reported for the first time that the production of antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) by LAB, and MIC value of cyclo(L-Phe-L-Pro) against Penicillium roqueforti and Aspergillus fumigatus was 20 mg/ml. Cyclo(L-His-L-Pro), Cyclo(L-Pro-L-Pro), Cyclo(L-Met-L-Pro), Cyclo(L-Leu-L-Pro), and Cyclo(L-Tyr-L-Pro) were isolated from the cell-free supernatant of Lactobacillus amylovorus LA 19280, and these cyclic dipeptides all showed antifungal activity, with MICs between 25 and 50 mg/ml against Aspergillus fumigatus (Ryan et al., 2011). In addition, cis-cyclo(L-Tyr-L-Pro), cis-cyclo(L-Val-L-Pro), cis-cyclo(L-Ser-L-Pro), cis-cyclo(L-Leu-L-Pro), and cis-cyclo(L-Phe-LPro) isolated from the cell-free supernatant of LAB with antifungal activity, had inhibitory effects on Ganoderma boninense, with MICs of 8.2, 8.1, 9.0, 8.4, 6.8 mmol/L, respectively (Kwak et al., 2014). Furthermore, Kwak et al. (2018) isolated 15 cyclic dipeptides containing proline and a single cyclic dipeptide without proline from Lactiplantibacillus plantarum LBP-K10, all of which exhibited antifungal activity. Combinations of multiple cyclic dipeptides showed higher antifungal activity than single cyclic dipeptides, with MICs between 18.6 and 22.7 mg/L against Candida albicans. Cyclic dipeptides can also inhibit fungal biofilm formation. Li et al. (2022b) isolated cyclo (leu-pro) and cyclo (phe-pro) from the cell-free supernatant of Lactiplantibacillus plantarum CCFM8724 and found that these two cyclic dipeptides decreased the expression of virulence genes in C. albicans (ALS3 and HWP1 genes), achieving a reduction of biofilm formation. Notably, cyclic dipeptides isolated by Ebrahimi et al. (2020) from the cell-free supernatant of P. pentosaceus also reduced aflatoxin content, and exhibited the most antifungal activity, reducing aflatoxin G1 and G2 by 82.06% and 87.32%, respectively.
Reuterin
Reuterin is a non-protein, broad-spectrum antimicrobial agent secreted by Limosilactobacillus reuteri, which controls growth of Gram-positive and Gram-negative bacteria, and fungi. It is water-soluble, heat-resistant, and highly stable (Cleusix et al., 2007). The mechanism of reuterin’s inhibition of fungi may be oxidative stress induced by modification of thiol groups in proteins and small molecules (Schaefer et al., 2010). The growth of Fusarium culmorum, Aspergillus niger, and Penicillium expansum spores were inhibited by reuterin produced by Limosilactobacillus reuteri R29; the cell-free supernatant with the highest concentration of reuterin completely prevented the growth of all three fungal spores. The MIC90 of reuterin against Fusarium culmorum was 4 mmol/L, while its MIC90 against both Aspergillus niger and Penicillium expansum was 8 mmol/L (Schmidt et al., 2018). Reuterin produced by Limosilactobacillus reuteri completely inhibited the growth of Penicillium expansum at a concentration of 10 mmol/L, and the concentration of reuterin was positively correlated with antifungal effects, within a specific range (Ortiz-Rivera et al., 2017).
Nucleoside (Cytidine)
Nucleosides are the basic elements of biological cells that maintain life, participate in the metabolic processes of DNA, and have a variety of anti-tumor, anti-viral, and antifungal functions (Dan et al., 2021). Ryan et al. (2011) isolated and identified two nucleosides with antifungal activity, cytidine and 2′ -deoxycytidine, from the cell-free supernatant of L. amylovorus LA 19280, and observed that these nucleosides showed antifungal activity against Aspergillus fumigatus, with MIC values >200 mg/ml. Chen et al. (2021a) also detected cytidine and 2′ -deoxycytidine in the cell-free supernatant of Lactobacillus kefiri M4 and Pediococcus acidilactici MRS-7, which retarded the growth of Penicillum expansum. In addition, nucleosides were also detected in the cell-free supernatant of other types of LAB, such as Lactiplantibacillus plantarun, Propionibacterium freudenreichii, Limosilactobacillus reuteri, and L. brevis (Le Lay et al., 2016a; Yépez et al., 2017). However, the studies of cytidine produced by LAB are limited, and cytidine’s antifungal mechanisms are still unclear.
Exopolysaccharides
Exopolysaccharides (EPSs) are biopolymers produced by LAB, mainly during their growth and metabolism period. EPS are secondary metabolites of microorganisms, can improve the texture and nutritional value of food, and inhibit growth of pathogens and fungi (Moradi et al., 2021). Rodrigues et al. (2005) reported that Kefiran, an edible, biodegradable, and water-soluble EPS produced by LAB, could prevent growth of bacteria and fungi, with MIC of 462 mg/L against C. albicans. Nehal et al. (2019) found that EPS produced by L. lactis F-MOU also exhibited significant antifungal effects against C. albicans, with MIC of 16 mg/ml. EPS inhibition of the growth of pathogenic and spoilage bacteria and fungi may be through the disordering of cell division, destruction of cell membrane and plasma membrane, and decomposition of DNA (Wu et al., 2010). EPS can interact with fungi, depending on their cell membrane permeability, then attack the respiratory chain and cell division machinery, leading to cell death (Abinaya et al., 2018). EPS can also act as a barrier, preventing the input of nutrients to pathogenic bacteria and fungi, thus slowing down their growth; this barrier effect might increase with increased polysaccharide concentration (Han et al., 2016). In addition, EPS could degrade the biofilm of C. albicans and reduce the adhesion of other fungi (Abinaya et al., 2018). Similarly, Wang et al. (2020) also found that EPS (composed of glucose, galactose, mannose, and arabinose) produced by Lactobacillus fermentum S1 showed activity against biofilms. EPS reduces hydrophobicity, zeta potential, and interactions between cells forming biofilms. The antibiofilm activity of EPS might be related to modifying the bacterial and fungal cell surfaces, inhibiting the attachment of pathogenic and fungal cells to the surface or downregulating the gene expression involved in biofilm formation (Wang et al., 2015).
Factors Affecting Production of Antifungal Compounds by Lactic Acid Bacteria
Different LAB produce different types of antifungal compounds. In addition to the species of LAB, parameters including temperature, time of incubation, nutritional factors, and growth medium also affect the production of antifungal compounds by LAB (Oliveira et al., 2014).
Crowley et al. (2013b) studied the effect of temperature on the production of antifungal compounds using six antifungal LAB, and they reported that the highest antifungal activity was recorded at 30°C. Valerio (2004) reported the effect of time of incubation on the production of antifungal compounds by Lactiplantibacillus plantarum ITM21B, and it was found that the maximum production of PLA and OH-PLA was reached after 72 h of incubation.
Mu et al. (2009) optimized the medium components for the production of PLA by Lactobacillus sp. SK007. And maximal PLA was obtained when the medium included 30 g/L glucose, 3 g/L K2HPO4, 5 g/L phenylpyruvic acid (PPA), 30 g/L yeast powder, 3 g/L CH3COONa, 47 g/L corn steep liquor, and 3 ml g/L Tween-80. Roy et al. (1996) found that compared to M17 and MRS growth media, the cell-free supernatant of L. lactis subsp. lactis CHD-28.3 in Elliker’s broth showed optimum antifungal activity against Aspergillus flavus. Magnusson and Schnürer (2001) reported that PLA yield were increased by the addition of peptides, α-ketoglutarate, citric acid in growth medium. Similar results were also obtained by Schmidt et al. (2018). The production of PLA was significantly increased by the addition of 1.5% phenylalanine (w/v) to MRS broth. And, a maximum yield of reuterin and the highest fungal inhibition were achieved by supplementation of 500 mmol/L glycerol and a reduced glucose content (1.5%) in MRS broth. The synthesis of reuterin was also correlated with temperature, pH, microbial concentration, oxygen concentration, and culture time (Ortiz-Rivera et al., 2017).
Application of Antifungal LAB in Bakery Products
Sourdough is a natural dough improver that can be used in making bakery products (Nionelli and Rizzello, 2016). Traditionally, the early sourdough (sourdough type I) is obtained after spontaneous fermentation of cereal flour using continuous backslopping. The microorganisms of spontaneous fermentation originate mainly from flours and processing equipment. However, sourdough type I has some disadvantages, including a long fermentation cycle and unstable properties. Therefore, the use of sourdough fermented with certain LAB species together with baker’s yeast for dough leavening is more attractive (Drakula et al., 2021). Sourdough fermentation improves the rheological properties of the dough and the nutritional properties of bakery products. This technique also has the potential to inhibit growth of fungi and extend the shelf-life of bakery products.
LAB Used to Extend the Shelf-Life of Bakery Products
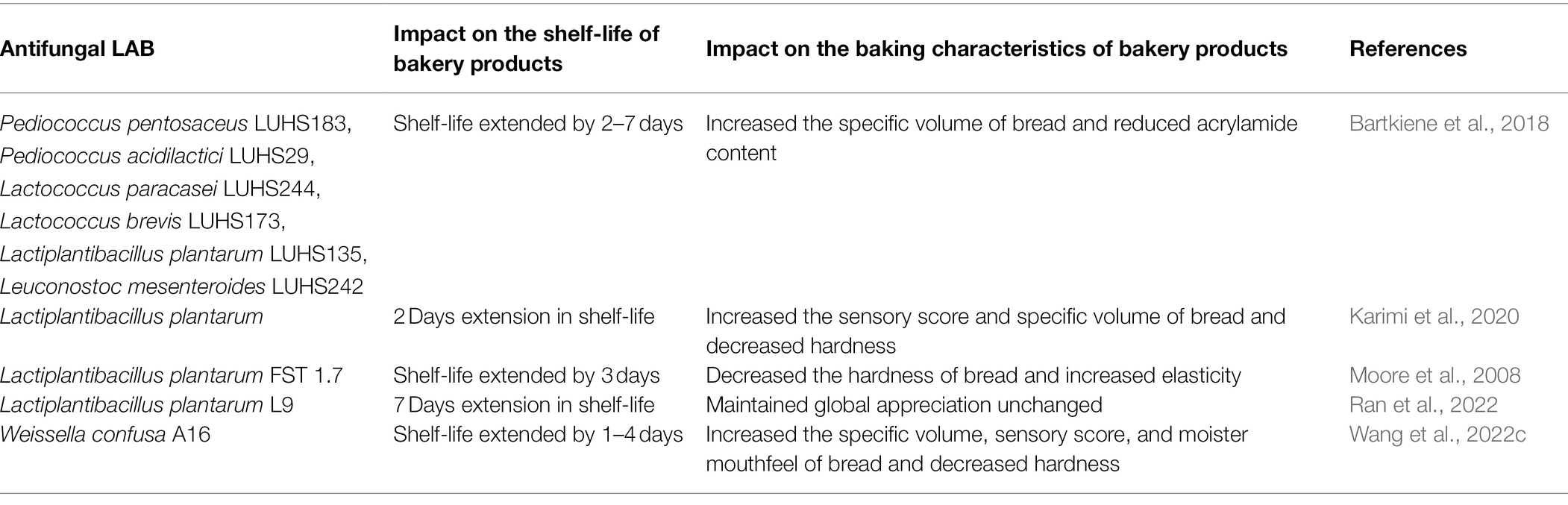
Sun et al. (2020) added Lactiplantibacillus plantarum LB-1 suspension, which had strong antifungal activity against Aspergillus ochraceus, Aspergillus niger, Fusarium graminearum, Aspergillus flavus, Aspergillus fumigatus, and Penicillium citrinum, into dough, and doubled the shelf-life of whole wheat bread, from 3 to 6 days. Sourdough inoculated with Lactiplantibacillus plantarum CH1 and Leuconostoc mesenteroides L1 showing antifungal activity was added to bread artificially contaminated with Aspergillus tubingensis or Aspergillus flavus, and the shelf-life of bread was extended by 1 day (Ouiddir et al., 2019). Bartkiene et al. (2018) confirmed that a combination of 15% antifungal Lactiplantibacillus plantarum LUHS135 sourdough, and cranberry coating increased the shelf-life of bread by 6 days, and the shelf-life increased with the amount of sourdough added. However, since excessive sourdough affected the quality of bread, the amount of sourdough added should be determined based on the specific strain selected.
Interestingly, LAB show different antifungal activity in sourdough made with different cereals. Axel et al. (2016) confirmed the above views, and they reported that Limosilactobacillus reuteri R29 and L. brevis R2 δ with antifungal activity were inoculated into quinoa and white rice flour, for bread making. However, the shelf-life of quinoa bread and white rice flour bread inoculated with the same LAB were different. The concentration of carboxylic acid in quinoa sourdough was much higher than that in rice sourdough, which indicated that the level of metabolites produced by LAB in different cereal sourdough varied. Quattrini et al. (2019) prepared wheat sourdough bread and flaxseed sourdough bread with antifungal LAB, and showed that wheat sourdough fermented with L. hammesii DSM16381, Lactiplantibacillus plantarum C264, and L. brevis C186 greatly extended the shelf-life of bread contaminated by Aspergillus niger; however, there was no significant effect on bread contaminated with Penicillium roqueforti. Furthermore, they also found that the combination of ricinoleic acid and wheat sourdough greatly extended the shelf-life of bread contaminated with Aspergillus niger or Penicillium roqueforti, compared with linseed sourdough fermented with the same LAB. More examples of using LAB to extend the shelf-life of bakery products can be found in Table 1.
LAB Used to Improve the Baking Characteristics of Bakery Products
Antifungal LAB not only prolong the shelf-life of bakery products but also improve the aroma profile and texture of bakery products (Moghaddam et al., 2020). Rizzello et al. (2011) baked using wheat germ sourdough, which not only produced bread with extended shelf-life but also with the lowest hardness and maximum specific volume. L. brevis AM7 with antifungal activity was inoculated into bread hydrolysate, and then the bread hydrolysate was employed for bread making. The specific volume of bread supplemented with 18% bread hydrolysate was improved compared to bread with lower percentages of bread hydrolysate (Nionelli et al., 2020). Bread containing hop extract (25%, vol/wt) sourdough also had a similar effect, prolonging the shelf-life of bread by 7 days, and enhancing its nutritional value, phytase activity, total phenolic content, and antioxidant activity. In addition, although bread with added hop extract had increased bitterness in taste and odor, and reduced sensory score, LAB fermentation overall improved the aroma profile and sensory acceptability of the bread (Nionelli et al., 2018).
The combination of antifungal LAB and fruit fermentation substrate also increased the shelf-life of bakery products and improved their texture, flavor, and nutritional value. The combination of LAB and pitaya inhibited growth of mold; LAB also promoted the release of phenolic acids from pitaya and the conversion of insoluble dietary fiber to soluble dietary fiber. These changes probably favored the formation of covalent and non-covalent linkages with S–S bonds in dough, helping to maintain the integrity of the gluten network structure (Omedi et al., 2021). After fermentation by LAB, the molecular properties of phenolic compounds in fruits can be changed to produce new derivative compounds with more bioactive potential, thereby increasing their antioxidative properties (Massot-Cladera et al., 2014; Septembre-Malaterre et al., 2018). In addition, LAB can use different kinds of fruit sugars to produce different types of EPS. EPS is a natural, high molecular weight polymer with high viscosity, which can be used as a stabilizer and thickener to improve the rheological properties, taste, porosity, and specific volume of bakery products (Li et al., 2022a). A comprehensive list of LAB with potential for improving the baking characteristics of bakery products is listed in Table 2.
LAB Used to Improve the Safety of Bakery Products
Grains, the raw material of bakery products, are prone to fungal contamination and may be contaminated by mycotoxins during the growing or storage period, potentially harming consumer health. LAB inhibit growth of fungi and reduce the contents of aflatoxins B1, B2, G1, and G2. LAB-induced degradation of mycotoxins is achieved with binding of the toxin to the bacterial cell wall (Sadeghi et al., 2019). Saladino et al. (2016) reported that Lactiplantibacillus plantarum strains used in fermentation of sourdough reduced bread Aflatoxin B1 levels by 99.9% as compared to the control.
In addition to mycotoxins, acrylamide, which is potentially carcinogenic and neurotoxic to human, is also a common contaminant of bakery products (Capuano and Fogliano, 2011). Fermentation with selected LAB strains can inhibit the growth of fungi and reduce acrylamide levels in bakery products, which improves the safety of bakery products. The addition of selected LAB, in combination with the antimicrobial cranberry-based coating, resulted in wheat bread with extended shelf-life and reduced acrylamide content. Moreover, the content of acrylamide gradually decreased with increasing amounts of sourdough (Bartkiene et al., 2018). The formation of acrylamide has primarily been related to reducing sugar content (Nguyen et al., 2016). LAB metabolize carbohydrates during fermentation, which reduces the reducing sugar content in dough, in turn leading to the reduction of acrylamide content in dough. In addition, the increase in organic acids also contributes to the reduction of acrylamide content in bakery products (Capuano and Fogliano, 2011).
Furthermore, the use of LAB as a biological preservative greatly reduces the addition of chemical preservatives. The results from Ryan et al. (2008) showed that the combination of sourdough and 0.1% calcium propionate effectively extended the shelf-life of bread, which was similar to bread supplemented with 0.3% calcium propionate alone. Luz et al. (2021) also found that relative to bread supplemented with 0.3% calcium propionate and 3 g/kg lactic acid, bread supplemented with 25 ml Lactiplantibacillus plantarum TR7 fermented whey had its shelf-life extended by 1 day, while the shelf-life of bread supplemented with 50 ml fermented whey was extended by 2 days. Ryan et al. (2011) also similarly found that 0.3% calcium propionate-supplemented bread had weaker antiseptic effects than bread fermented by L. amylovorus DSM 19280. The LAB-fermented bread also greatly reduced the addition of chemical preservatives and improved bakery products’ safety. More examples of the use of LAB to improve the safety of bakery products can be found in Table 3.
Summary and Prospect
In conclusion, LAB have extensive application prospects in bakery products. As a new kind of biological preservative, LAB can inhibit the growth of fungi through the production of organic acids, antifungal peptides, hydrogen peroxide, diacetyl, and other antifungal compounds, which greatly extends the shelf-life of bakery products and improves the baking characteristics and safety of products. The inhibitory effects of LAB on fungi are closely linked to the presence of antifungal compounds, with synergistic effects between various compounds. Therefore, LAB capable of producing a variety of antifungal compounds should be screened for use in bakery products. Additionally, we should also pay attention to LAB with antifungal activity and other properties that enhance the flavor profile and texture of bakery products. Lastly, LAB may be incorporated into packaging of bakery products for better protective function.
Author Contributions
RX: conceptualization and writing—original draft. SZ, YW, XA, QL, and JL: writing—original draft. BH: writing—original draft and writing—review and editing. KH, YY, and SL: writing—review and editing. AL: funding acquisition, writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the Sichuan Agricultural University “Shuang-Zhi Plan” foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abinaya, M., Vaseeharan, B., Divya, M., Vijayakumar, S., Govindarajan, M., Alharbi, N. S., et al. (2018). Structural characterization of Bacillus licheniformis Dahb1 exopolysaccharide-antimicrobial potential and larvicidal activity on malaria and Zika virus mosquito vectors. Environ. Sci. Pollut. R. 25, 18604–18619. doi: 10.1007/s11356-018-2002-6
Ademola, O., Saha Turna, N., Liverpool-Tasie, L. S. O., Obadina, A., and Wu, F. (2021). Mycotoxin reduction through lactic acid fermentation: evidence from commercial Ogi processors in Southwest Nigeria. Food Control 121:107620. doi: 10.1016/j.foodcont.2020.107620
Ahlberg, S. H., Joutsjoki, V., and Korhonen, H. J. (2015). Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 207, 87–102. doi: 10.1016/j.ijfoodmicro.2015.04.042
Amézqueta, S., Schorr-Galindo, S., Murillo-Arbizu, M., González-Peñas, E., López De Cerain, A., and Guiraud, J. P. (2012). OTA-producing fungi in foodstuffs: a review. Food Control 26, 259–268. doi: 10.1016/j.foodcont.2012.01.042
Arulrajah, B., Muhialdin, B. J., Qoms, M. S., Zarei, M., Hussin, A. S. M., Hasan, H., et al. (2021). Production of cationic antifungal peptides from kenaf seed protein as natural bio preservatives to prolong the shelf-life of tomato puree. Int. J. Food Microbiol. 359:109418. doi: 10.1016/j.ijfoodmicro.2021.109418
Aunsbjerg, S. D., Honoré, A. H., Marcussen, J., Ebrahimi, P., Vogensen, F. K., Benfeldt, C., et al. (2015). Contribution of volatiles to the antifungal effect of Lactobacillus paracasei in defined medium and yogurt. Int. J. Food Microbiol. 194, 46–53. doi: 10.1016/j.ijfoodmicro.2014.11.007
Axel, C., Brosnan, B., Zannini, E., Furey, A., Coffey, A., and Arendt, E. K. (2016). Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. Int. J. Food Microbiol. 239, 86–94. doi: 10.1016/j.ijfoodmicro.2016.05.006
Bangar, S. P., Sharma, N., Kumar, M., Ozogul, F., Purewal, S. S., and Trif, M. (2021). Recent developments in applications of lactic acid bacteria against mycotoxin production and fungal contamination. Food Biosci. 44:101444. doi: 10.1016/j.fbio.2021.101444
Bartkiene, E., Bartkevics, V., Lele, V., Pugajeva, I., Zavistanaviciute, P., Mickiene, R., et al. (2018). A concept of mould spoilage prevention and acrylamide reduction in wheat bread: application of lactobacilli in combination with a cranberry coating. Food Control 91, 284–293. doi: 10.1016/j.foodcont.2018.04.019
Bartkiene, E., Bartkevics, V., Lele, V., Pugajeva, I., Zavistanaviciute, P., Zadeike, D., et al. (2019). Application of antifungal lactobacilli in combination with coatings based on apple processing by-products as a bio-preservative in wheat bread production. J. Food Sci. Technol. 56, 2989–3000. doi: 10.1007/s13197-019-03775-w
Belz, M. C. E., Axel, C., Arendt, E. K., Lynch, K. M., Brosnan, B., Sheehan, E. M., et al. (2019). Improvement of taste and shelf life of yeasted low-salt bread containing functional sourdoughs using Lactobacillus amylovorus DSM 19280 and Weisella cibaria MG1. Int. J. Food Microbiol. 302, 69–79. doi: 10.1016/j.ijfoodmicro.2018.07.015
Ben Taheur, F., Mansour, C., Kouidhi, B., and Chaieb, K. (2019). Use of lactic acid bacteria for the inhibition of Aspergillus flavus and Aspergillus carbonarius growth and mycotoxin production. Toxicon 166, 15–23. doi: 10.1016/j.toxicon.2019.05.004
Black, B. A., Zannini, E., Curtis, J. M., and Ganzle, M. G. (2013). Antifungal hydroxy fatty acids produced during sourdough fermentation: microbial and enzymatic pathways, and antifungal activity in bread. Appl. Environ. Microbiol. 79, 1866–1873. doi: 10.1128/AEM.03784-12
Bundgaard-Nielsen, K., and Nielsen, P. V. (1996). Fungicidal effect of 15 disinfectants against 25 fungal contaminants commonly found in bread and cheese manufacturing. J. Food Prot. 59, 268–275. doi: 10.4315/0362-028x-59.3.268
Bustos, A. Y., Font de Valdez, G., and Gerez, C. L. (2018). Optimization of phenyllactic acid production by Pediococcus acidilactici CRL 1753. Application of the formulated bio-preserver culture in bread. Biol. Control 123, 137–143. doi: 10.1016/j.biocontrol.2018.05.017
Capuano, E., and Fogliano, V. (2011). Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT 44, 793–810. doi: 10.1016/j.lwt.2010.11.002
Chen, H., Ju, H., Wang, Y., Du, G., Yan, X., Cui, Y., et al. (2021a). Antifungal activity and mode of action of lactic acid bacteria isolated from kefir against Penicillium expansum. Food Control 130:108274. doi: 10.1016/j.foodcont.2021.108274
Chen, H., Yan, X., Du, G., Guo, Q., Shi, Y., Chang, J., et al. (2021b). Recent developments in antifungal lactic acid bacteria: application, screening methods, separation, purification of antifungal compounds and antifungal mechanisms. Crit. Rev. Food Sci. Nutr., 1–15. doi: 10.1080/10408398.2021.1977610 [Epub ahead of print]
Choi, H., Kim, Y. W., Hwang, I., Kim, J., and Yoon, S. (2012). Evaluation of Leuconostoc citreum HO12 and Weissella koreensis HO20 isolated from kimchi as a starter culture for whole wheat sourdough. Food Chem. 134, 2208–2216. doi: 10.1016/j.foodchem.2012.04.047
Cizeikiene, D., Juodeikiene, G., Paskevicius, A., and Bartkiene, E. (2013). Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control 31, 539–545. doi: 10.1016/j.foodcont.2012.12.004
Cleusix, V., Lacroix, C., Vollenweider, S., Duboux, M., and Le Blay, G. (2007). Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol. 7:101. doi: 10.1186/1471-2180-7-101
Coda, R., Cassone, A., Rizzello, C. G., Nionelli, L., Cardinali, G., and Gobbetti, M. (2011). Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: identification of novel compounds and long-term effect during storage of wheat bread. Appl. Environ. Microbiol. 77, 3484–3492. doi: 10.1128/AEM.02669-10
Crowley, S., Mahony, J., and van Sinderen, D. (2013a). Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 33, 93–109. doi: 10.1016/j.tifs.2013.07.004
Crowley, S., Mahony, J., and van Sinderen, D. (2013b). Broad-spectrum antifungal-producing lactic acid bacteria and their application in fruit models. Folia Microbiol. 58, 291–299. doi: 10.1007/s12223-012-0209-3
Dal Bello, F., Clarke, C. I., Ryan, L. A. M., Ulmer, H., Schober, T. J., Ström, K., et al. (2007). Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 45, 309–318. doi: 10.1016/j.jcs.2006.09.004
Dan, A., Hu, Y., Chen, R., Lin, X., Tian, Y., and Wang, S. (2021). Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali. Food Sci. Human Wellness 10, 401–407. doi: 10.1016/j.fshw.2021.04.002
Debonne, E., Baert, H., Eeckhout, M., Devlieghere, F., and Van Bockstaele, F. (2019). Optimization of composite dough for the enrichment of bread crust with antifungal active compounds. LWT 99, 417–422. doi: 10.1016/j.lwt.2018.10.020
Debonne, E., Vermeulen, A., Bouboutiefski, N., Ruyssen, T., Van Bockstaele, F., Eeckhout, M., et al. (2020). Modelling and validation of the antifungal activity of DL-3-phenyllactic acid and acetic acid on bread spoilage moulds. Food Microbiol. 88:103407. doi: 10.1016/j.fm.2019.103407
Drakopoulos, D., Sulyok, M., Krska, R., Logrieco, A. F., and Vogelgsang, S. (2021). Raised concerns about the safety of barley grains and straw: a Swiss survey reveals a high diversity of mycotoxins and other fungal metabolites. Food Control 125:107919. doi: 10.1016/j.foodcont.2021.107919
Drakula, S., Novotni, D., Čukelj Mustač, N., Voučko, B., Krpan, M., Hruškar, M., et al. (2021). Alteration of phenolics and antioxidant capacity of gluten-free bread by yellow pea flour addition and sourdough fermentation. Food Biosci. 44:101424. doi: 10.1016/j.fbio.2021.101424
Ebrahimi, M., Sadeghi, A., and Mortazavi, S. A. (2020). The use of cyclic dipeptide producing LAB with potent anti-aflatoxigenic capability to improve techno-functional properties of clean-label bread. Ann. Microbiol. 70:24. doi: 10.1186/s13213-020-01571-y
Facco Stefanello, R., Nabeshima, E. H., de Oliveira Garcia, A., Heck, R. T., Valle Garcia, M., Martins Fries, L. L., et al. (2019). Stability, sensory attributes and acceptance of panettones elaborated with Lactobacillus fermentum IAL 4541 and Wickerhamomyces anomallus IAL 4533. Food Res. Int. 116, 973–984. doi: 10.1016/j.foodres.2018.09.035
Fang, L., Zhao, B., Zhang, R., Wu, P., Zhao, D., Chen, J., et al. (2022). Occurrence and exposure assessment of aflatoxins in Zhejiang province, China. Environ. Toxicol. Pharmacol. 92:103847. doi: 10.1016/j.etap.2022.103847
Gerez, C. L., Fornaguera, M. J., Obregozo, M. D., Font De Valdez, G., and Torino, M. I. (2015). Antifungal starter culture for packed bread: influence of two storage conditions. Rev. Argent. Microbiol. 47, 118–124. doi: 10.1016/j.ram.2015.02.002
Gerez, C. L., Torino, M. I., Rollán, G., and Font de Valdez, G. (2009). Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control 20, 144–148. doi: 10.1016/j.foodcont.2008.03.005
Han, Q., Wu, Z., Huang, B., Sun, L., Ding, C., Yuan, S., et al. (2016). Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int. J. Biol. Macromol. 92, 116–124. doi: 10.1016/j.ijbiomac.2016.06.087
Honoré, A. H., Aunsbjerg, S. D., Ebrahimi, P., Thorsen, M., Benfeldt, C., Knochel, S., et al. (2016). Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal. Bioanal. Chem. 408, 83–96. doi: 10.1007/s00216-015-9103-6
İspirli, H., Özmen, D., Yılmaz, M. T., Sağdıç, O., and Dertli, E. (2020). Impact of glucan type exopolysaccharide (EPS) production on technological characteristics of sourdough bread. Food Control 107:106812. doi: 10.1016/j.foodcont.2019.106812
Izzo, L., Luz, C., Ritieni, A., Manes, J., and Meca, G. (2020). Whey fermented by using Lactobacillus plantarum strains: a promising approach to increase the shelf life of pita bread. J. Dairy Sci. 103, 5906–5915. doi: 10.3168/jds.2019-17547
Jakubczyk, A., Karas, M., Rybczynska-Tkaczyk, K., Zielinska, E., and Zielinski, D. (2020). Current trends of bioactive peptides-new sources and therapeutic effect. Foods 9:846. doi: 10.3390/foods9070846
Jin, J., Nguyen, T. T. H., Humayun, S., Park, S., Oh, H., Lim, S., et al. (2021). Characteristics of sourdough bread fermented with Pediococcus pentosaceus and Saccharomyces cerevisiae and its bio-preservative effect against Aspergillus flavus. Food Chem. 345:128787. doi: 10.1016/j.foodchem.2020.128787
John, V. (2008). Unique aspects of the grass cell wall John Vogel. Curr. Opin. Plant Biol. 11, 301–307. doi: 10.1016/j.pbi.2008.03.002
Karimi, N., Nikoo, M., Ahmadi Gavlighi, H., Piri Gheshlaghi, S., Regenstein, J. M., and Xu, X. (2020). Effect of pacific white shrimp (Litopenaeus vannamei) protein hydrolysates (SPH) and (−)-epigallocatechin gallate (EGCG) on sourdough and bread quality. LWT 131:109800. doi: 10.1016/j.lwt.2020.109800
Korcari, D., Secchiero, R., Laureati, M., Marti, A., Cardone, G., Rabitti, N. S., et al. (2021). Technological properties, shelf life and consumer preference of spelt-based sourdough bread using novel, selected starter cultures. LWT 151:112097. doi: 10.1016/j.lwt.2021.112097
Krnjaja, V., Mandić, V., Stanković, S., Obradović, A., Vasić, T., Lukić, M., et al. (2019). Influence of plant density on toxigenic fungal and mycotoxin contamination of maize grains. Crop Prot. 116, 126–131. doi: 10.1016/j.cropro.2018.10.021
Kwak, M. K., Liu, R., and Kang, S. O. (2018). Antimicrobial activity of cyclic dipeptides produced by Lactobacillus plantarum LBP-K10 against multidrug-resistant bacteria, pathogenic fungi, and influenza A virus. Food Control 85, 223–234. doi: 10.1016/j.foodcont.2017.10.001
Kwak, M. K., Liu, R., Kim, M. K., Moon, D., Kim, A. H., Song, S. H., et al. (2014). Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 52, 64–70. doi: 10.1007/s12275-014-3520-7
Lambert, R. J., and Stratford, M. (1999). Weak-acid preservatives: modelling microbial inhibition and response. J. Appl. Microbiol. 86, 157–164. doi: 10.1046/j.1365-2672.1999.00646.x
Lavermicocca, P., Valerio, F., and Visconti, A. (2003). Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 69, 634–640. doi: 10.1128/AEM.69.1.634-640.2003
Le Lay, C., Coton, E., Le Blay, G., Chobert, J. M., Haertle, T., Choiset, Y., et al. (2016a). Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 239, 79–85. doi: 10.1016/j.ijfoodmicro.2016.06.020
Le Lay, C., Mounier, J., Vasseur, V., Weill, A., Le Blay, G., Barbier, G., et al. (2016b). In vitro and in situ screening of lactic acid bacteria and propionibacteria antifungal activities against bakery product spoilage molds. Food Control 60, 247–255. doi: 10.1016/j.foodcont.2015.07.034
Leon, R., Ruiz, M., Valero, Y., Cardenas, C., Guzman, F., Vila, M., et al. (2020). Exploring small cationic peptides of different origin as potential antimicrobial agents in aquaculture. Fish Shellfish Immunol. 98, 720–727. doi: 10.1016/j.fsi.2019.11.019
Leyva Salas, M., Mounier, J., Maillard, M. B., Valence, F., Coton, E., and Thierry, A. (2019). Identification and quantification of natural compounds produced by antifungal bioprotective cultures in dairy products. Food Chem. 301:125260. doi: 10.1016/j.foodchem.2019.125260
Li, J., Ai, L., Xu, F., Hu, X., Yao, Y., and Wang, L. (2022a). Structural characterization of exopolysaccharides from Weissella cibaria NC516.11 in distiller grains and its improvement in gluten-free dough. Int. J. Biol. Macromol. 199, 17–23. doi: 10.1016/j.ijbiomac.2021.12.089
Li, J., Zhang, Q., Zhao, J., Zhang, H., and Chen, W. (2022b). Streptococcus mutans and Candida albicans biofilm inhibitors produced by Lactiplantibacillus plantarum CCFM8724. Curr. Microbiol. 79:143. doi: 10.1007/s00284-022-02833-5
Liang, N., Cai, P., Wu, D., Pan, Y., Curtis, J. M., and Gänzle, M. G. (2017). High-speed counter-current chromatography (HSCCC) purification of antifungal hydroxy unsaturated fatty acids from plant-seed oil and lactobacillus cultures. J. Agric. Food Chem. 65, 11229–11236. doi: 10.1021/acs.jafc.7b05658
Luz, C., Quiles, J. M., Romano, R., Blaiotta, G., Rodríguez, L., and Meca, G. (2021). Application of whey of mozzarella di Bufala Campana fermented by lactic acid bacteria as a bread biopreservative agent. Int. J. Food Sci. Technol. 56, 4585–4593. doi: 10.1111/ijfs.15092
Ma, S., Wang, Z., Guo, X., Wang, F., Huang, J., Sun, B., et al. (2021). Sourdough improves the quality of whole-wheat flour products: mechanisms and challenges—a review. Food Chem. 360:130038. doi: 10.1016/j.foodchem.2021.130038
Magnusson, J., and Schnürer, J. S. (2001). Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67, 1–5. doi: 10.1128/AEM.67.1.1
Mahato, D. K., Kamle, M., Sharma, B., Pandhi, S., Devi, S., Dhawan, K., et al. (2021). Patulin in food: a mycotoxin concern for human health and its management strategies. Toxicon 198, 12–23. doi: 10.1016/j.toxicon.2021.04.027
Martin, H., and Maris, P. (2012). Synergism between hydrogen peroxide and seventeen acids against five Agri-food-borne fungi and one yeast strain. J. Appl. Microbiol. 113, 1451–1460. doi: 10.1111/jam.12016
Massot-Cladera, M., Abril-Gil, M., Torres, S., Franch, À., Castell, M., and Pérez-Cano, F. J. (2014). Impact of cocoa polyphenol extracts on the immune system and microbiota in two strains of young rats. Br. J. Nutr. 112, 1944–1954. doi: 10.1017/S0007114514003080
Mendez-Albores, A., Martinez-Bustos, F., Gaytan-Martinez, M., and Moreno-Martinez, E. (2008). Effect of lactic and citric acid on the stability of B-aflatoxins in extrusion-cooked sorghum. Lett. Appl. Microbiol. 47, 1–7. doi: 10.1111/j.1472-765X.2008.02376.x
Moghaddam, M. F. T., Jalali, H., Nafchi, A. M., and Nouri, L. (2020). Evaluating the effects of lactic acid bacteria and olive leaf extract on the quality of gluten-free bread. Gene Rep. 21:100771. doi: 10.1016/j.genrep.2020.100771
Mohamad Asri, N., Muhialdin, B. J., Zarei, M., and Saari, N. (2020). Low molecular weight peptides generated from palm kernel cake via solid state lacto-fermentation extend the shelf life of bread. LWT 134:110206. doi: 10.1016/j.lwt.2020.110206
Moore, M. M., Bello, F. D., and Arendt, E. K. (2008). Sourdough fermented by Lactobacillus plantarum FST 1.7 improves the quality and shelf life of gluten-free bread. Eur. Food Res. Technol. 226, 1309–1316. doi: 10.1007/s00217-007-0659-z
Moradi, M., Guimarães, J. T., and Sahin, S. (2021). Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 40, 33–39. doi: 10.1016/j.cofs.2020.06.001
Mu, W., Chen, C., Li, X., Zhang, T., and Jiang, B. (2009). Optimization of culture medium for the production of phenyllactic acid by Lactobacillus sp. SK007. Bioresour. Technol. 100, 1366–1370. doi: 10.1016/j.biortech.2008.08.010
Muhialdin, B. J., Algboory, H. L., Kadum, H., Mohammed, N. K., Saari, N., Hassan, Z., et al. (2020). Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control 109:106898. doi: 10.1016/j.foodcont.2019.106898
Muhialdin, B. J., Hassan, Z., Bakar, F. A., and Saari, N. (2016). Identification of antifungal peptides produced by Lactobacillus plantarum IS10 grown in the MRS broth. Food Control 59, 27–30. doi: 10.1016/j.foodcont.2015.05.022
Mun, S. Y., Kim, S. K., Woo, E. R., and Chang, H. C. (2019). Purification and characterization of an antimicrobial compound produced by Lactobacillus plantarum EM showing both antifungal and antibacterial activities. LWT 114:108403. doi: 10.1016/j.lwt.2019.108403
Nasrollahzadeh, A., Mokhtari, S., Khomeiri, M., and Saris, P. E. J. (2022). Antifungal preservation of food by lactic acid bacteria. Foods 11:395. doi: 10.3390/foods11030395
Nehal, F., Sahnoun, M., Smaoui, S., Jaouadi, B., Bejar, S., and Mohammed, S. (2019). Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathog. 132, 10–19. doi: 10.1016/j.micpath.2019.04.018
Nes, I. E., Diep, D. B., Varstein, L. S. H., Brurberg, M. B., Eijsink, V., and Holo, H. (1996). Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 70, 113–128. doi: 10.1007/BF00395929
Nguyen, H. T., Van der Fels-Klerx, H. J., Peters, R. J., and Van Boekel, M. A. (2016). Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: part I: effects of sugar type. Food Chem. 192, 575–585. doi: 10.1016/j.foodchem.2015.07.016
Nionelli, L., Pontonio, E., Gobbetti, M., and Rizzello, C. G. (2018). Use of hop extract as antifungal ingredient for bread making and selection of autochthonous resistant starters for sourdough fermentation. Int. J. Food Microbiol. 266, 173–182. doi: 10.1016/j.ijfoodmicro.2017.12.002
Nionelli, L., and Rizzello, C. G. (2016). Sourdough-based biotechnologies for the production of gluten-free foods. Foods 5:65. doi: 10.3390/foods5030065
Nionelli, L., Wang, Y., Pontonio, E., Immonen, M., Rizzello, C. G., Maina, H. N., et al. (2020). Antifungal effect of bioprocessed surplus bread as ingredient for bread-making: identification of active compounds and impact on shelf-life. Food Control 118:107437. doi: 10.1016/j.foodcont.2020.107437
Oliveira, P. M., Zannini, E., and Arendt, E. K. (2014). Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: from crop farming to cereal products. Food Microbiol. 37, 78–95. doi: 10.1016/j.fm.2013.06.003
Omedi, J. O., Huang, J., Huang, W., Zheng, J., Zeng, Y., Zhang, B., et al. (2021). Suitability of pitaya fruit fermented by sourdough LAB strains for bread making: its impact on dough physicochemical, rheo-fermentation properties and antioxidant, antifungal and quality performance of bread. Heliyon 7:e08290. doi: 10.1016/j.heliyon.2021.e08290
Ortiz-Rivera, Y., Sanchez-Vega, R., Gutierrez-Mendez, N., León-Félix, J., Acosta-Muñiz, C., and Sepulveda, D. R. (2017). Production of reuterin in a fermented milk product by Lactobacillus reuteri: inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 100, 4258–4268. doi: 10.3168/jds.2016-11534
Ouiddir, M., Bettache, G., Leyva Salas, M., Pawtowski, A., Donot, C., Brahimi, S., et al. (2019). Selection of Algerian lactic acid bacteria for use as antifungal bioprotective cultures and application in dairy and bakery products. Food Microbiol. 82, 160–170. doi: 10.1016/j.fm.2019.01.020
Pohl, E. E., Voltchenko, A. M., and Rupprecht, A. (2008). Flip-flop of hydroxy fatty acids across the membrane as monitored by proton-sensitive microelectrodes. Biochim. Biophys. Acta Biomembr. 1778, 1292–1297. doi: 10.1016/j.bbamem.2008.01.025
Pontonio, E., and Rizzello, C. G. (2021). Editorial: ad-hoc selection of lactic acid bacteria for non-conventional food matrices fermentations: Agri-food perspectives. Front. Microbiol. 12:681830. doi: 10.3389/fmicb.2021.681830
Puig, M., Moragrega, C., Ruz, L., Calderon, C. E., Cazorla, F. M., Montesinos, E., et al. (2016). Interaction of antifungal peptide BP15 with Stemphylium vesicarium, the causal agent of brown spot of pear. Fungal Biol. 120, 61–71. doi: 10.1016/j.funbio.2015.10.007
Qian, M., Liu, D., Zhang, X., Yin, Z., Ismail, B. B., Ye, X., et al. (2021). A review of active packaging in bakery products: applications and future trends. Trends Food Sci. Technol. 114, 459–471. doi: 10.1016/j.tifs.2021.06.009
Quattrini, M., Liang, N., Fortina, M. G., Xiang, S., Curtis, J. M., and Ganzle, M. (2019). Exploiting synergies of sourdough and antifungal organic acids to delay fungal spoilage of bread. Int. J. Food Microbiol. 302, 8–14. doi: 10.1016/j.ijfoodmicro.2018.09.007
Rai, M., Pandit, R., Gaikwad, S., and Kovics, G. (2016). Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 53, 3381–3394. doi: 10.1007/s13197-016-2318-5
Ran, Q., Yang, F., Geng, M., Qin, L., Chang, Z., Gao, H., et al. (2022). A mixed culture of Propionibacterium freudenreichii and Lactiplantibacillus plantarum as antifungal biopreservatives in bakery product. Food Biosci. 47:101456. doi: 10.1016/j.fbio.2021.101456
ReportLinker. (2020). Global bakery products industry. https://www.reportlinker.com/p05069906/Global-Bakery-Products-Industry.html?utm_source=GNW (Accessed 10 May 2022).
Rhee, K. (2004). Cyclic dipeptides exhibit synergistic, broad spectrum antimicrobial effects and have anti-mutagenic properties. Int. J. Antimicrob. Agents 24, 423–427. doi: 10.1016/j.ijantimicag.2004.05.005
Rizzello, C. G., Cassone, A., Coda, R., and Gobbetti, M. (2011). Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem. 127, 952–959. doi: 10.1016/j.foodchem.2011.01.063
Rizzello, C. G., Verni, M., Bordignon, S., Gramaglia, V., and Gobbetti, M. (2017). Hydrolysate from a mixture of legume flours with antifungal activity as an ingredient for prolonging the shelf-life of wheat bread. Food Microbiol. 64, 72–82. doi: 10.1016/j.fm.2016.12.003
Rodrigues, K. L., Caputo, L. R., Carvalho, J. C., Evangelista, J., and Schneedorf, J. M. (2005). Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 25, 404–408. doi: 10.1016/j.ijantimicag.2004.09.020
Roy, U., Batish, V. K., Grover, S., and Neelakantan, S. (1996). Production of antifungal substance by Lactococcus lactis subsp. lactis CHD-28.3. Int. J. Food Microbiol. 32, 27–34. doi: 10.1016/0168-1605(96)01101-4
Russo, P., Arena, M. P., Fiocco, D., Capozzi, V., Drider, D., and Spano, G. (2017). Lactobacillus plantarum with broad antifungal activity: a promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 247, 48–54. doi: 10.1016/j.ijfoodmicro.2016.04.027
Ryan, L. A., Dal Bello, F., and Arendt, E. K. (2008). The use of sourdough fermented by antifungal LAB to reduce the amount of calcium propionate in bread. Int. J. Food Microbiol. 125, 274–278. doi: 10.1016/j.ijfoodmicro.2008.04.013
Ryan, L. A. M., Zannini, E., Dal Bello, F., Pawlowska, A., Koehler, P., and Arendt, E. K. (2011). Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 146, 276–283. doi: 10.1016/j.ijfoodmicro.2011.02.036
Sadeghi, A., Ebrahimi, M., Mortazavi, S. A., and Abedfar, A. (2019). Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 95, 298–307. doi: 10.1016/j.foodcont.2018.08.013
Saladino, F., Luz, C., Manyes, L., Fernández-Franzón, M., and Meca, G. (2016). In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 67, 273–277. doi: 10.1016/j.foodcont.2016.03.012
Salaheen, S., Peng, M., and Biswas, X. D. X. (2014). Microbial Food Safety and Preservation Techniques. Boca Raton: CRC Press.
Sanad, M. H., Farag, A. B., Bassem, S. A., and Marzook, F. A. (2022). Radioiodination of zearalenone and determination of Lactobacillus plantarum effect of on zearalenone organ distribution: in silico study and preclinical evaluation. Toxicol. Rep. 9, 470–479. doi: 10.1016/j.toxrep.2022.02.003
Schaefer, L., Auchtung, T. A., Hermans, K. E., Whitehead, D., Borhan, B., and Britton, R. A. (2010). The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 156, 1589–1599. doi: 10.1099/mic.0.035642-0
Schmidt, M., Lynch, K. M., Zannini, E., and Arendt, E. K. (2018). Fundamental study on the improvement of the antifungal activity of Lactobacillus reuteri R29 through increased production of phenyllactic acid and reuterin. Food Control 88, 139–148. doi: 10.1016/j.foodcont.2017.11.041
Septembre-Malaterre, A., Remize, F., and Poucheret, P. (2018). Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Food Res. Int. 104, 86–99. doi: 10.1016/j.foodres.2017.09.031
Shetty, P. H., and Jespersen, L. (2006). Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 17, 48–55. doi: 10.1016/j.tifs.2005.10.004
Shi, C., and Knøchel, S. (2021). Susceptibility of dairy associated molds towards microbial metabolites with focus on the response to diacetyl. Food Control 121:107573. doi: 10.1016/j.foodcont.2020.107573
Sjogren, J., Magnusson, J., Broberg, A., Schnurer, J., and Kenne, L. (2003). Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 69, 7554–7557. doi: 10.1128/AEM.69.12.7554-7557.2003
Stiles, M. E., and Holzapfel, W. H. (1997). Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 36, 1–29. doi: 10.1016/S0168-1605(96)01233-0
Ström, K., Sjögren, J., Broberg, A., and Schnürer, J. (2002). Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe–L-pro) and cyclo(L-Phe–trans-4-OH-L-pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 68, 4322–4327. doi: 10.1128/AEM.68.9.4322
Sun, L., Li, X., Zhang, Y., Yang, W., Ma, G., Ma, N., et al. (2020). A novel lactic acid bacterium for improving the quality and shelf life of whole wheat bread. Food Control 109:106914. doi: 10.1016/j.foodcont.2019.106914
Urbach, G. (1995). Contribution of lactic acid bacteria to flavour compound formation in dairy products. Int. Dairy J. 5, 877–903. doi: 10.1016/0958-6946(95)00037-2
Valerio, F. (2004). Production of phenyllactic acid by lactic acid bacteria: an approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 233, 289–295. doi: 10.1016/j.femsle.2004.02.020
Vermeulen, N., Gánzle, M. G., and Vogel, R. F. (2006). Influence of peptide supply and cosubstrates on phenylalanine metabolism of Lactobacillus sanfranciscensis DSM20451T and Lactobacillus plantarum TMW1.468. J. Agric. Food Chem. 54, 3832–3839. doi: 10.1021/jf052733e
Wang, L., Hua, X., Shi, J., Jing, N., Ji, T., Lv, B., et al. (2022b). Ochratoxin A: occurrence and recent advances in detoxification. Toxicon 210, 11–18. doi: 10.1016/j.toxicon.2022.02.010
Wang, F., Li, Z., Jia, H., Lu, R., Zhang, S., Pan, C., et al. (2022a). An ultralow concentration of Al-MOFs for turn-on fluorescence detection of aflatoxin B1 in tea samples. Food Chem. 383:132389. doi: 10.1016/j.foodchem.2022.132389
Wang, K., Niu, M., Song, D., Song, X., Zhao, J., Wu, Y., et al. (2020). Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 129, 206–214. doi: 10.1016/j.jbiosc.2019.07.009
Wang, Y., Xie, C., Pulkkinen, M., Edelmann, M., Chamlagain, B., Coda, R., et al. (2022c). In situ production of vitamin B12 and dextran in soya flour and rice bran: a tool to improve flavour and texture of B12-fortified bread. LWT 161:113407. doi: 10.1016/j.lwt.2022.113407
Wang, J., Zhao, X., Yang, Y., Zhao, A., and Yang, Z. (2015). Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 74, 119–126. doi: 10.1016/j.ijbiomac.2014.12.006
Wu, M., Pan, T., Wu, Y., Chang, S., Chang, M., and Hu, C. (2010). Exopolysaccharide activities from probiotic bifidobacterium: immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 144, 104–110. doi: 10.1016/j.ijfoodmicro.2010.09.003
Wu, S., Peng, Y., Xi, J., Zhao, Q., Xu, D., Jin, Z., et al. (2022). Effect of sourdough fermented with corn oil and lactic acid bacteria on bread flavor. LWT 155:112935. doi: 10.1016/j.lwt.2021.112935
Keywords: lactic acid bacteria, antifungal mechanisms, bakery products, biological preservative, fungal contamination
Citation: Liu A, Xu R, Zhang S, Wang Y, Hu B, Ao X, Li Q, Li J, Hu K, Yang Y and Liu S (2022) Antifungal Mechanisms and Application of Lactic Acid Bacteria in Bakery Products: A Review. Front. Microbiol. 13:924398. doi: 10.3389/fmicb.2022.924398
Edited by:
Rina Wu, Shenyang Agricultural University, ChinaReviewed by:
Francesca Valerio, Italian National Research Council, ItalyQiya Yang, Jiangsu University, China
Copyright © 2022 Liu, Xu, Zhang, Wang, Hu, Ao, Li, Li, Hu, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Hu, aHViaW4yNTU1QHNpbmEuY29t; Aiping Liu, bGFwZm9vZEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Aiping Liu
Aiping Liu Ruixia Xu
Ruixia Xu Shun Zhang
Shun Zhang Bin Hu*
Bin Hu* Shuliang Liu
Shuliang Liu