
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 13 July 2022
Sec. Infectious Agents and Disease
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.923546
This article is part of the Research Topic Reviews in Vector-borne and Zoonotic Infectious Diseases View all 5 articles
 Sumaiya Adam1,2*
Sumaiya Adam1,2* Carmen Pheiffer1,3,4
Carmen Pheiffer1,3,4 Stephanie Dias3
Stephanie Dias3 Tsakane Hlongwane1,2
Tsakane Hlongwane1,2 Valerie Vannevel2
Valerie Vannevel2 Priya Soma-Pillay1,2
Priya Soma-Pillay1,2 Fareed Abdullah5,6
Fareed Abdullah5,6Despite many advances in medicine we are still faced with emerging pathogens. Pregnant women have been disproportionately affected by previous coronavirus outbreaks. The COVID-19 pandemic has not affected pregnant women as greatly as SARS-CoV and MERS, but has posed other challenges such as the need for quarantine and isolation, limited access to antenatal care, use of personal protective equipment (PPE), vaccine hesitancy and inequities in vaccine access and therapeutics between rich countries and the global south. This review will describe the impact of the significant coronaviruses on pregnancy, with special focus on the challenges being encountered by the SARS-CoV-2 global pandemic.
As we celebrate advances in obstetric care, improved maternal and perinatal outcomes, and a move toward personalized medicine, challenges such as human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) and emerging pathogens, still plague us (Abdelrahman et al., 2020; Di Mascio et al., 2020; Zhu et al., 2020; Matar et al., 2021). COVID-19, which is caused by the SARS-CoV-2 virus, has been associated with poor outcomes for mother (increased mortality and admission to critical care) and newborn [preterm labor and admission to the neonatal intensive care unit (NICU)] (Kim et al., 2015; Abdelrahman et al., 2020; Czeresnia et al., 2020; Di Mascio et al., 2020; Zhu et al., 2020; Matar et al., 2021). The SARS-CoV-2 pandemic and its manifestation in the global cohort of pregnant women reminds us of our vulnerabilities, gives us pause to reflect on the other novel infections that have challenged human health over the last 20 years; and to contemplate how we will react to future challenges to global health. This review will describe the impact of the significant coronaviruses on pregnancy, with special focus on the challenges being encountered by the SARS-CoV-2 global pandemic.
Coronaviruses are a group of pathogens with an animal reservoir that cause contagious, and sometimes fatal respiratory diseases in humans (Abdelrahman et al., 2020; Zhu et al., 2020). The coronaviruses were amongst the first major infectious pathogens to emerge in the 21st century. Coronaviruses can be spread via air in small airborne droplets of saliva, or indirectly spread via contaminated surfaces touched by an infected individual (Di Mascio et al., 2020; Zhu et al., 2020). The first coronavirus was identified in 2003. Since then, seven human coronaviruses have been identified, namely, Human coronavirus HKU1 (HCoV-HKU1), human coronavirus OC43 (HCoV-OC43), human coronavirus 229E (HCoV-229E), human coronavirus NL63 (HCoV-NL63), Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1 or SARS-CoV), and SARS-CoV-2. The last three viruses have resulted in severe illness in humans and warrant further discussion (Abdelrahman et al., 2020; Czeresnia et al., 2020; Di Mascio et al., 2020; Zhu et al., 2020).
Whilst there are many similarities between SARS-CoV-1, MERS, and SARS-CoV-2, there are also significant differences, which are summarized in Table 1.
SARS-CoV-1 causes a respiratory illness which was responsible for the 2002–2004 SARS outbreak (Abdelrahman et al., 2020; Di Mascio et al., 2020; Zhu et al., 2020; Matar et al., 2021). It is an enveloped, positive-sense, single-stranded RNA virus which infects the epithelial cells within the lungs. The virus enters the host cell by binding to angiotensin-converting enzyme 2 (ACE2) receptor. It infects humans, bats, and palm civets. SARS-CoV-1 caused a severe illness characterized by systemic symptoms of myalgia, headache, and fever, followed by the onset of respiratory symptoms such as mainly cough, dyspnea, and pneumonia, and lymphopenia within 2 weeks. In the SARS-CoV-1 outbreak of 2003, about 9% of patients with confirmed infection died (Abdelrahman et al., 2020; Czeresnia et al., 2020).
MERS-CoV is a viral respiratory disease that was first identified in Saudi Arabia in 2012 (Kim et al., 2015; Abdelrahman et al., 2020; Di Mascio et al., 2020; Zhu et al., 2020). Infection with MERS-CoV ranged from asymptomatic infection to typical symptoms such as fever, cough, shortness of breath, and occasional diarrhea. Pneumonia was common, but not always present. Dromedary camels, which are a major reservoir host for MERS-CoV, are implicated in the transmission of MERS-CoV to humans, but the exact role and route of transmission remains unknown. Approximately 35% of reported patients with MERS-CoV infection have died (Abdelrahman et al., 2020; Czeresnia et al., 2020; Di Mascio et al., 2020; Zhu et al., 2020).
SARS-CoV-2 is a novel severe acute respiratory syndrome coronavirus that causes variable symptoms including fever, cough, headache, fatigue, breathing difficulties, and loss of smell and taste (Czeresnia et al., 2020; Khalil et al., 2020; Matar et al., 2021). Symptoms usually begin within 14 days after exposure to the virus. The original source of viral transmission to humans remains unclear, but bats and pangolins have been implicated as animal reservoirs. At least a third of people who are infected remain asymptomatic. Most (81%) symptomatic patients develop mild to moderate symptoms, whilst 14% develop severe symptoms (dyspnea, hypoxia, or more than 50% lung involvement on imaging), and 5% suffer critical symptoms (respiratory failure, shock, or multiorgan dysfunction). Transmission is mainly via air contaminated by droplets and small airborne particles containing the virus (Abdelrahman et al., 2020; Czeresnia et al., 2020; Di Mascio et al., 2020; Zhu et al., 2020).
There are several variants of concern that have emerged. “Variants of Concern” have evidence of an increase in transmissibility, greater risk of severe disease, a significant reduction in neutralization by antibodies generated during previous infection or vaccination, or reduced effectiveness of treatments or vaccines (COVID-19 vaccines, 2022; Developing COVID-19 Vaccines, 2022). SARS-CoV-2 variants of interest are illustrated in Table 2.
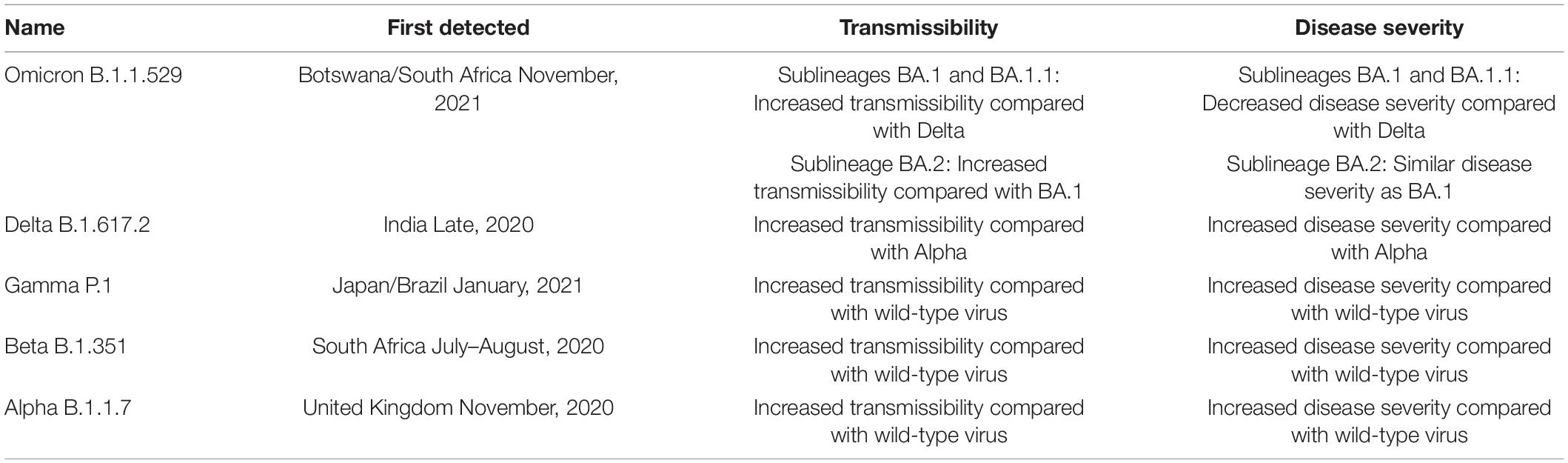
Table 2. SARS-CoV-2 variants of concern (World Health Organization, 2020).
Pregnancy is characterized by unique physiological changes to accommodate the growing fetus (Soma-Pillay et al., 2016). These physiological changes in the respiratory, cardiovascular, and immune systems predispose pregnant women to infections. The physiological adaptations of pregnancy are described in Table 3.
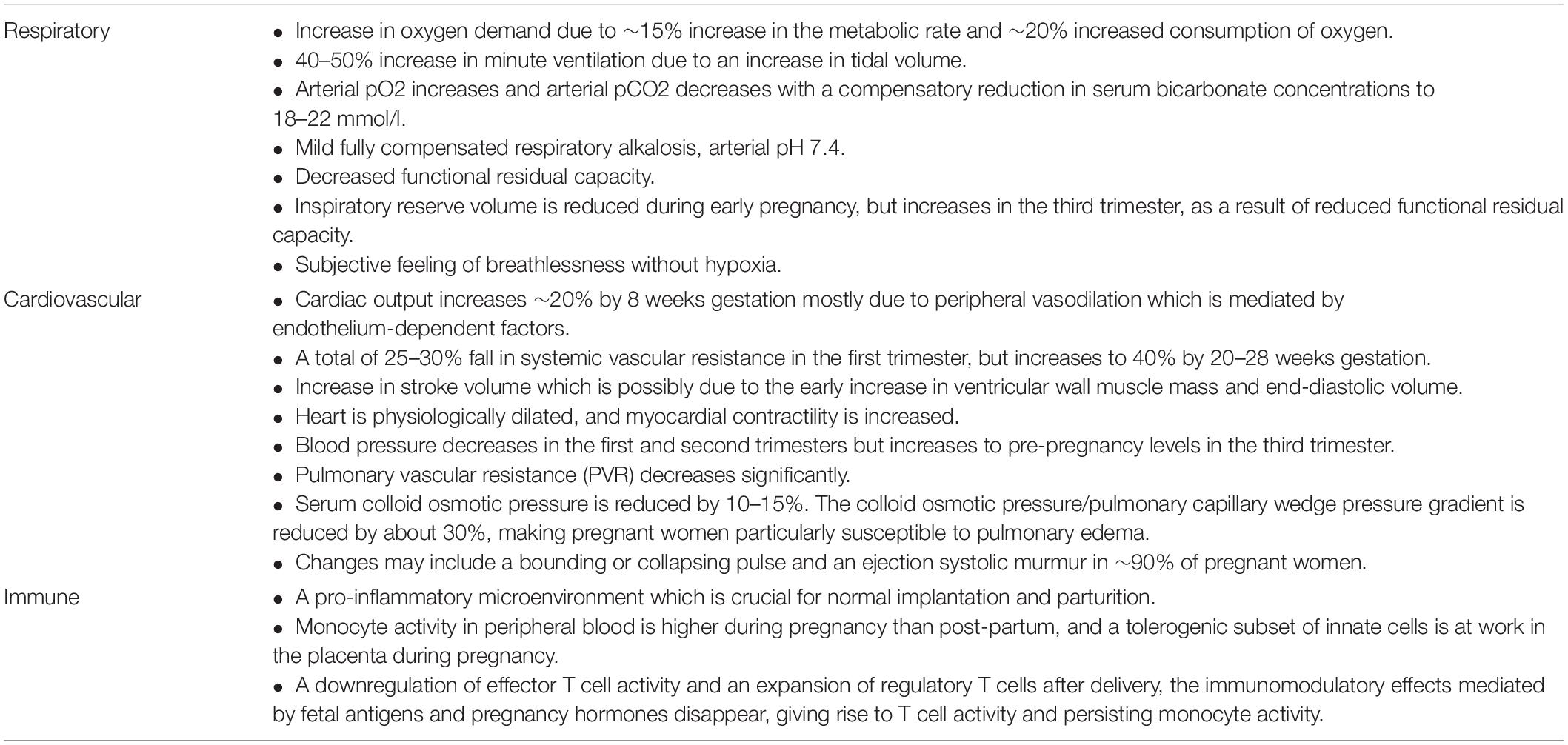
Table 3. Physiological changes of pregnancy that make pregnant women more susceptible to infection 2 (Soma-Pillay et al., 2016; Förger and Villiger, 2020).
The physiological adaptations of pregnancy are a fine balance that occurs throughout the three trimesters. Any disequilibrium, such as that created by a viral infection, may lead to maternal and fetal complications (Wong et al., 2004; Soma-Pillay et al., 2016; Förger and Villiger, 2020; Novoa et al., 2021).
The severity of viral pneumonias in pregnant women as evidenced especially by influenza outbreaks, may be attributed to the shift from humoral to cell-mediated immunity. In the pregnant woman’s immune system innate immune cells like natural killer (NK) cells and monocytes respond more strongly to invading pathogens. However, this is associated with some negatively regulated adaptive immune responses (Förger and Villiger, 2020). The swollen upper respiratory tract and restricted lung expansion in the last trimester make the pregnant woman more susceptible to respiratory pathogens such as SARS-CoV-2. Whilst these physiological changes are essential to the development and survival of the maternal-fetal interface, it does render the pregnant woman relatively immunocompromised. In the pro-inflammatory stages, especially in the first trimester, a pregnant woman will be more likely to develop a cytokine storm, which in the presence of respiratory changes may make a pregnant woman less tolerant to the effects of hypoxia. Hypoxia may lead to vasoconstriction and ultimately intrauterine growth restriction. In the third trimester, the growing pregnancy enhances the physiological changes. A viral infection in this time period may result in an increased secretion of T-helper-2 (Th2) cytokines that suppress inflammation, thus preserving the anti-inflammatory environment (Wong et al., 2004; Czeresnia et al., 2020).
The emergence of SARS-CoV-2 led to reflection on the experiences of SARS-CoV-1 and MERS to guide management. The SARS-CoV-1 outbreak infected more than 8,000 people globally and resulted in 919 deaths. Twelve pregnant women were reported to have SARS-CoV-1, of which four (57%) of the seven women infected in the first trimester had miscarriages, four (80%) of the five women infected in the second and early third trimester had preterm births and two (40%) of the five women had intrauterine fetal growth restriction (Wong et al., 2004, 2020; Turan et al., 2020). Placental findings in SARS-CoV-1 affected pregnancies showed maternal thrombosis and avascular villi suggesting fetal thrombosis, and reduced placental weight which was proportional to interval from SARS-CoV-1 infection to delivery (Wong et al., 2004, 2021). Pregnant women with SARS-CoV-1 were more likely to require mechanical ventilation, renal replacement therapy for acute injury, and develop coronavirus-related seizures. There was no evidence of trans-placental spread of SARS-CoV-1 (Czeresnia et al., 2020), and there were no pediatric fatalities (Ng et al., 2004).
Of the 2,496 confirmed cases of MERS, 852 (34.1%) people died. In one study of 660 patients with MERS, 11 (1.7%) cases were diagnosed in pregnant women. Of these seven (64%) women were admitted to intensive care, there were three (27%) maternal deaths, and three (27%) stillbirths. Again, there was no evidence of vertical transmission of MERS (Alserehi et al., 2016; Czeresnia et al., 2020).
Whilst only a small proportion of pregnant women were infected with SARS-CoV-1 and MERS, these women suffered severe complications. No effective antiviral therapy or vaccine have been found for either of these coronavirus infections. This led us to anticipate severe adverse outcomes in pregnant women infected with SARS-CoV-2.
The SARS-CoV-2 outbreak resulted in a pandemic with more than 3 million cases and approximately 230,000 deaths reported in the first 5 months, which is more deaths than cases in previous coronavirus outbreaks (see Table 1; Matar et al., 2021). Unlike SARS-CoV-1 and MERS-CoV, SARS-CoV-2 has reached epic global pandemic proportions with 520 million documented cases and 6,2 million deaths. Excess deaths have been estimated to be 14.9 million (range 13.3–16.6 million) and 18.2 million (Wang et al., 2021; World Health Organization, 2022b).
Pregnant women present with similar symptoms, though less frequent than the non-pregnant population. The majority of pregnant women with SARS-CoV-2 are asymptomatic and are usually screened due to more frequent contact with healthcare services and pregnancy-related admissions such as for delivery. Pregnant women with SARS-CoV-2 present with obstetric complications (Table 4) such as preterm labor, pre-eclampsia or HELLP (hemolytic anemia, elevated liver enzymes, low platelets)-syndrome like illnesses. Preterm births are increased in women infected with SARS-CoV-2, but are iatrogenic rather than a direct consequence of viral infection (Khalil et al., 2020; Novoa et al., 2021). Pregnant women with a body mass index (BMI) more than 35 kg/m2, pre-eclampsia, or underlying cardiopulmonary conditions should receive greater care when infected. In addition, women with confirmed SARS-CoV-2 infection should be monitored closely post-partum due to the risk of deterioration (Czeresnia et al., 2020; Khalil et al., 2020; Sakowicz et al., 2020; Novoa et al., 2021).
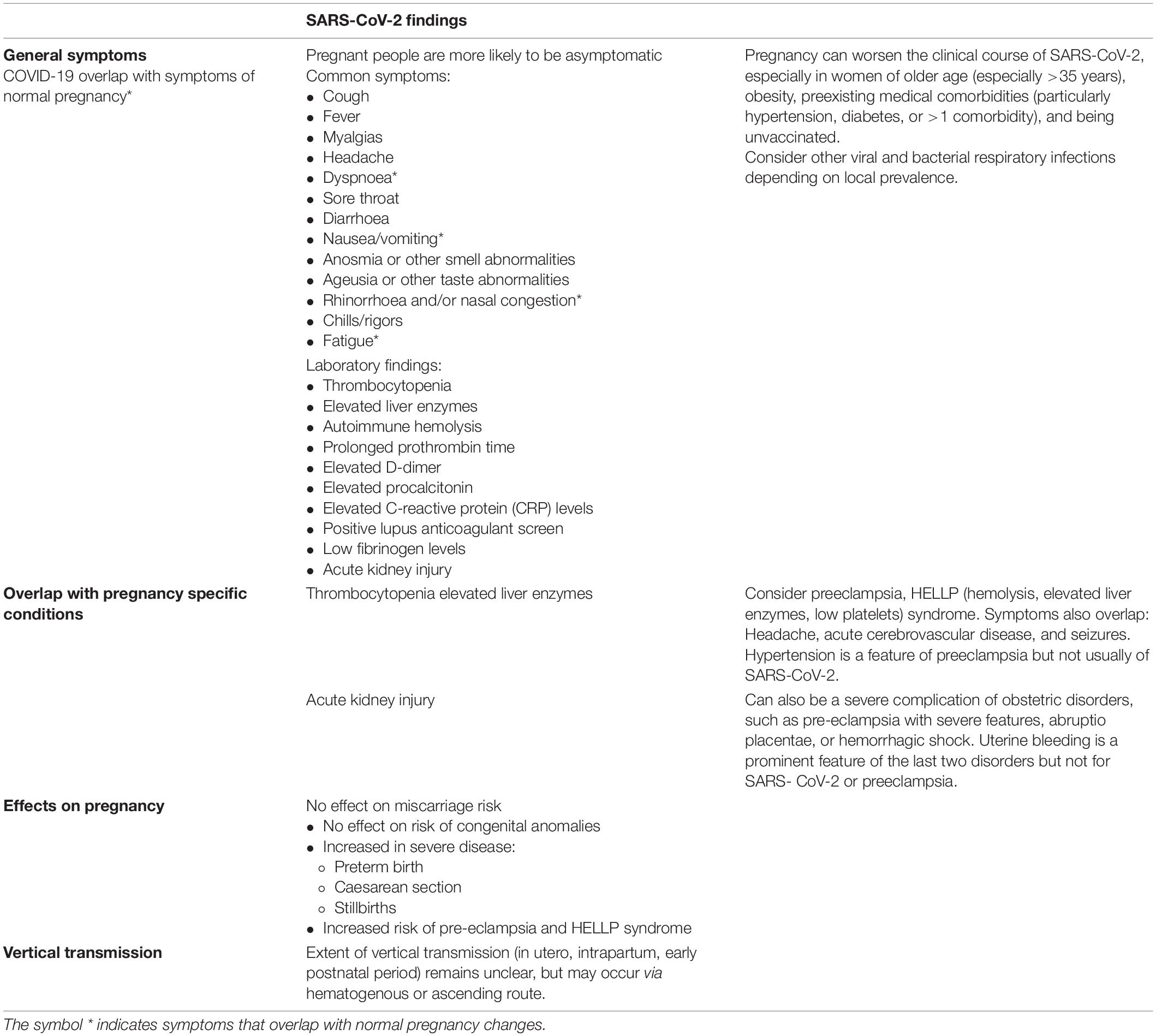
Table 4. Pregnancy related complications of SARS-CoV-2 (Goldshtein et al., 2021, 2022; Shimabukuro et al., 2021; Morgan et al., 2022).
In light of its increased transmissibility and spread especially with the Omicron variant, SARS-CoV-2 has resulted in a greater global burden of disease in pregnancy than SARS-CoV-1 and MERS (Zhu et al., 2020).
Pregnant women were amongst the highest risk cohorts in both the SARS-CoV-1 and MERS outbreaks. which is not the case with SARS-CoV-2 (Abdelrahman et al., 2020; Khalil et al., 2020; Zhu et al., 2020; Matar et al., 2021). Contrary to expectations, the pregnancy associated morbidity associated with SARS-CoV-2 is less severe than SARS-CoV-1 and MERS (Sakowicz et al., 2020). Compared with intensive care unit (ICU) admission and mortality rates in the previous coronavirus outbreaks, only 10% of pregnant women infected with SARS-CoV-2 required ICU admission for respiratory support (Novoa et al., 2021). The underlying reason for this still remains unclear but several theories have been postulated.
1) There may be a protective mechanism/barrier within the placenta which relies on the presence or absence of certain receptors/pathways (Sadeghi et al., 2021).
2) COVID-19 shows some peculiar pathogenetic, epidemiological, and clinical features which to date are not completely understood (Petrosillo et al., 2020). Some studies reveal that SARS-CoV-2 is very similar in structure and pathogenicity with SARS-CoV-1, but the most important structural protein, i.e., the spike protein (S), is slightly different in these viruses (Rabaan et al., 2020) and undergoing significant mutations over time leading to changing transmissibility and possibly severity of disease (Abdullah et al., 2022).
3) The familial hemophagocytic lymphohistiocytosis (HLH) gene mutations, which was found that patients with H1N1 influenza who experienced the “cytokine storm,” has not been found in COVID-19. Furthermore, the widespread infiltration of monocytes/macrophages in the lung tissue of COVID-19 patients and the peripheral monocyte trafficking and subsequent differentiation into macrophages in the lungs of COVID-19 patients contributes to pro- inflammatory responses and further activation of innate immune cells. The changes in the innate immune system during pregnancy, also, involve the pattern recognition receptors Toll-like receptors (TLRs). Infection with COVID-19 leads to pyroptosis of host cells and the release of danger associated molecular patterns (DAMPs) that can act as ligands for TLR molecules and trigger a greater inflammatory response (Sadeghi et al., 2021).
Further studies are needed to determine which factors result in higher susceptibility or are protective against COVID-19 during pregnancy.
Another question that has arisen during the SARS-CoV-2 pandemic is “Does SARS-CoV-2 cause pre-eclampsia?” Whilst there is an apparent link, probably due to the placental affectation by the viral infection, it is not clear whether this association is causal (Gajbhiye et al., 2020; Wong et al., 2021; Khalil et al., 2022). The risk factors for pre-eclampsia and SARS-CoV-2 infection, namely, Black or Asian ethnicity, overweight/obesity, and underlying chronic conditions overlap, which may be confounding factors. Whilst there are plausible theories that SARS-CoV-2 causes pre-eclampsia, clear evidence is lacking (Khalil et al., 2022).
Reports on vertical transmission of SARS-CoV-2 are conflicting. Three (9.1%) neonates born from 33 COVID-19 infected women in China were thought to have contracted SARS-CoV-2 in utero (Sakowicz et al., 2020). In a review of 441 cases Gajbhiye et al. (2020) report a vertical transmission rate of approximately 8%. Whilst the presence of SARS-CoV-2 in the bloodstream is evident, and that it uses the membrane bound ACE2 receptor, which is expressed in human placenta, to gain access to its target cell, current data do not support the premise of transmission in utero, even though the virus has been isolated in placenta in severe cases. This may be due to the placenta’s immunological barrier which prevents entry of pathogens (Wong et al., 2021). Current evidence does not support transmission via breastfeeding (Novoa et al., 2021).
SARS-CoV-2 unlike previous outbreaks, has been associated with an increase in gender-based violence and an exacerbation of mental health disorders. Pregnancy involves several changes at the social, biological, and psychological levels in mothers and their families. Pregnant and post-partum women have high prevalence rates of anxiety, depression, and insomnia, but during disasters, such as the current pandemic, the prevalence of mental disorders in prenatal and postnatal women are significantly higher than those in the general population. This may be attributed to the quarantine measures and disruptions in medical practices (Yan et al., 2020). Interestingly, a recent study reported that lockdown restrictions increase gestational diabetes mellitus (GDM), probably due to stress.
Over the last few months we have seen the emergence of the Omicron variant which spreads more easily than the original virus that causes COVID-19 and the Delta variant (Centers for Disease Control and Prevention, 2022). Infection with the Omicron variant causes less severe disease than infection with prior variants, although some people may still have severe disease, need hospitalization, and could die from the infection with this variant (Centers for Disease Control and Prevention, 2022). Unvaccinated individuals who contract the Omicron variant are at greatest risk of severe disease and adverse outcomes (Engjom et al., 2022).
SARS-CoV-2 in pregnancy presents with many challenges on the women’s physiological, immunological, and social level, it further challenges the health system [service provision, essential service continuation, healthcare provider safety, personal protective equipment (PPE), and labor/delivery practices, etc.] particularly in developing countries. PPE to protect against the spread of COVID-19 include wearing a medical mask, gown, glove, and eye protection. The use of PPE by attending healthcare workers during labor and delivery remains controversial. N95 (non-oil respirator) respirators, which are respirators that offer a higher level of protection instead of a facemask, are recommended for those healthcare workers performing or present during an aerosol-generating procedures (AGP). Despite the second stage of labor lasting up to 4 h and the healthcare worker being in close contact to women who are exerting extreme effort and may exhale vigorously, cough, shout, and vomit during this time, the second stage of labor is not considered an AGP. However, it is recommended that labor and delivery personnel exercise caution and protect themselves and other patients. The implications, indirect effects, and evidence is still evolving, and more research and data is needed (Adam et al., 2020; Palatnik and McIntosh, 2020).
The SARS-CoV-2 pandemic has showcased the rapid advances in science and technology with the speedy development of vaccines (COVID-19 vaccines, 2022; Developing COVID-19 Vaccines, 2022). The development of safe effective vaccines provides an ideal opportunity to alleviate some of burdens facing maternal and infant health. Vaccines are highly effective at preventing morbidity and mortality. It is recommended that all unvaccinated women planning pregnancy or pregnant and breastfeeding women receive the COVID-19 vaccine and booster doses as per local protocols, as soon as possible. The COVID-19 vaccines may be administered at the same time as other vaccines routinely administered in pregnancy or at the same time as Anti-D immunoglobin.
None of the recommended COVID-19 vaccines [Pfizer/BioNTech (Comirnaty) by Fosun Pharma, Pfizer; Moderna (Spikevax); Janssen/Johnson & Johnson by Janssen Pharmaceutical Companies] contain virus that replicates. Thus, they do not cause disease, but non-specific side-effects from activation of the immune system may occur (Brinkley et al., 2022). Women can be assured that data supports the safety of vaccine in pregnancy and lactation. Benefits of vaccination include reductions in maternal SARS-CoV-2 infection, severity of maternal COVID-19, perinatal death, COVID-19-related hospitalization among infants up to 6 months of age (American College of Obstetricians and Gynecologists, 2022; Brinkley et al., 2022; Fu et al., 2022). No harmful effects on fertility, embryo/fetal development, pregnancy outcome, parturition, or short-term postnatal development of offspring has been reported in association with vaccines. Side effects are similar in pregnant and non-pregnant females (Brinkley et al., 2022).
Whilst vaccine hesitancy initially appeared to be lower in lower- and middle-income countries (LMICs), that trend is changing as vaccines become more accessible. In South Africa, only 41,73% of women aged 18–34 have been vaccinated making the lack of vaccination the single biggest risk factor for severe disease and death in pregnant women (Latest Vaccine Statistics, 2022).
The inequitable access to vaccines by pregnant women in the global south compared to their peers in rich countries has significantly skewed the burden of severe disease and death across the geographic divide and requires ongoing urgent attention. The education of pregnant women and communities in these areas and access to safe SARS-CoV-2 vaccines will further the endeavors of equity in healthcare in the world’s most deserving areas (Kalafat et al., 2021; Magee et al., 2021; Vale et al., 2021).
The COVID-19 pandemic has highlighted the need for health system strengthening particularly in LMICs. Early in the pandemic, Robertson et al. (2020) warned of an 8–38% increase in maternal mortality in LMICs if routine and emergency services were not maintained by health systems. The global response to the COVID-19 pandemic has largely been centered on lockdowns to ensure physical distancing whilst trying to maintain essential health services. For many countries, this approach has resulted in a shift from essential healthcare services to providing emergency services together with COVID-19 care (Ahmed et al., 2021).
Lower- and middle-income countries, like South Africa, observed a 30% increase in maternal mortality during the first wave of COVID-19 infections (Pattinson et al., 2020). This increase in maternal deaths coincided with a significant decrease in usage of reproductive health services including contraceptive usage and attendance at termination of pregnancy clinics. Additionally, the lockdown resulted in a movement of women from metropolitan cities (areas of work) to their homes in rural areas. Rural provinces of Mpumalanga and Limpopo experienced excess pressure on already under-resourced health systems while metropolitan cities were overburdened with severe COVID-19 infection and an inability to manage other routine emergencies (Pattinson et al., 2020).
Important components of COVID-19 care include the provision of screening facilities, availability of PPE and vaccine coverage for healthcare workers. Ahmed et al. (2021) reported a shortage of screening facilities and PPE in three LMICs. There has been global disparities in vaccine distribution with high-income countries purchasing excess vaccines for their citizens with low vaccine coverage in most African countries. The World Health Organization has reported that only 1 in 4 (27%) African health workers are fully vaccinated against COVID-19 (World Health Organization, 2022a). This means that African healthcare workers remain dangerously exposed to severe COVID-19 infection. High vaccine coverage amongst healthcare workers is essential not only for their own protection but also for their patients and to ensure that health systems remain operation during a time of extreme need.
The SARS-CoV-2 pandemic has showcased the rapid advances in science and technology with the speedy development of vaccines (COVID-19 vaccines, 2022; Developing COVID-19 Vaccines, 2022). However, our achievements and unprecedented advances have posed their own challenges. We have seen that globalization and ease of travel have facilitated the spread of SARS-CoV-2. Advances in speed of communication and access to information (and misinformation) have allowed vaccine hesitancy and conspiracy theories to thrive. In addition, the rapid emergence of new strains of SARS-CoV-2 has resulted in political antagonism, especially directed at low-middle income countries.
Despite all our advances, the 21st century has also unmasked the prevailing social injustices, especially in healthcare. Pregnant women and children in under-resourced settings bear the brunt of morbidity and mortality, especially related to basic human needs such as access to food, safe drinking water and basic healthcare. This is especially true with the SARS-CoV-2 pandemic.
All authors have contributed equally to the conception and development of the manuscript, contributed to the article, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelrahman, Z., Li, M., and Wang, X. (2020). Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruses. Front. Immunol. 11:5529. doi: 10.3389/fimmu.2020.552909
Abdullah, F., Myers, J., Basu, D., Tintinger, G., Ueckermann, V., Mathebula, M., et al. (2022). Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 116, 38–42. doi: 10.1016/j.ijid.2021.12.357
Adam, S., Maswime, S., Soma-Pillay, P., Matjila, M., Chauke, L., Botha, M. H., et al. (2020). Judicious use of personal protective equipment to prevent the spread of covid-19 in maternity units. Afr. J. Obstet. Gynaecol. 26, 2–3. doi: 10.7196/SAJOG.2020.v26i1.1605
Ahmed, T., Rahman, A. E., Amole, T. G., Galadanci, H., Matjila, M., Soma-Pillay, P., et al. (2021). The effect of covid-19 on maternal newborn and child health (MNCH) services in bangladesh, nigeria and south africa: call for a contextualized pandemic in LMICs. Int. J. Equity Health 448:77. doi: 10.1186/s12939-021-01414-5
Alserehi, H., Wali, G., and Alshukairi, A. (2016). Impact of middle east respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis. 16:105.1.
American College of Obstetricians and Gynecologists (2022). COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. Practice advisory. Available online at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care#table-3 (accessed on May 30, 2022)
Brinkley, E., Mack, C. D., and Albert, L. (2022). COVID-19 vaccinations in pregnancy: comparative evaluation of acute side effects and self-reported impact on quality of life between pregnant and non-pregnant women in the united states. Am. J. Perinatol. doi: 10.1055/s-0042-1748158 [Epub ahead of print].
Centers for Disease Control and Prevention (2022). Omicron Variant: What You Need to Know. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html. (accessed February 8, 2020)
COVID-19 vaccines (2022). World Health Organisation. Available online at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines. (accessed May 24, 2022)
Czeresnia, R. M., Trad, A. T. A., Britto, I. S. W., Negrini, R., Nomura, M. L., Pires, P., et al. (2020). CoV-2 and pregnancy: a review of the facts. Rev. Bras Ginecol. Obstet. 42, 562–568. doi: 10.1055/s-0040-1715137
Developing COVID-19 Vaccines (2022). Centers for Disease Control and Prevention. Available online at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/distributing/steps-ensure-safety.html#:∼:text =FDA%20has%20now%20granted%20full,submitted%20to%20support%20the %20EUA. (accessed May 24, 2022)
Di Mascio, D., Khalil, A., Saccone, G., Rizzo, G., Buca, D., Liberati, M., et al. (2020). Outcome of coronavirus spectrum infections (SARS, MERS, covid-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2:100107. doi: 10.1016/j.ajogmf.2020.100107
Engjom, H., van den Akker, T., Aabakke, A., Ayras, O., Bloemenkamp, K., Donati, S., et al. (2022). Severe covid-19 in pregnancy is almost exclusively limited to unvaccinated women - time for policies to change. Lancet 2022:313. doi: 10.1016/j.lanepe.2022.100313
Förger, F., and Villiger, P. M. (2020). Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat. Rev. Rheumatol. 16, 113–122.
Fu, W., Sivajohan, B., and McClymont, E. (2022). Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int. J. Gynaecol. Obstet. 156:406. doi: 10.1002/ijgo.14008
Gajbhiye, R. K., Modi, D. N., and Mahale, S. D. (2020). Pregnancy outcomes, newborn complications and maternal-fetal transmission of SARS-CoV-2 in women with covid-19: a systematic review of 441 cases. medRxiv 2020:356. doi: 10.1101/2020.04.11.2006235
Goldshtein, I., Nevo, D., and Steinberg, D. M. (2021). Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA 326:728.
Goldshtein, I., Steinberg, D. M., and Kuint, J. (2022). Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 176:470. doi: 10.1001/jamapediatrics.2022.0001
Kalafat, E., Magee, L. A., von Dadelszen, P., O’Brien, P., and Khalil, A. (2021). SARS-CoV-2 vaccination in pregnancy: a unique opportunity for equity. Lancet 398:951. doi: 10.1016/S0140-6736(21)01756-6
Khalil, A., Kalafat, E., Benlioglu, C., O’Brien, P., Morris, E., Draycott, T., et al. (2020). SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. Clin. Med. 2020:100446. doi: 10.1016/j.eclinm.2020.100446
Khalil, A., Samara, A., Chowdhury, T., and O’Brien, P. (2022). Does covid-19 cause pre-eclampsia? Ultrasound Obstet. Gynecol. 59, 146–152. doi: 10.1002/uog.24809
Kim, Y. J., Cho, Y. J., and Kim, D. W. (2015). Complete genome sequence of middle east respiratory syndrome coronavirus KOR/KNIH/002_05_2015, isolated in south korea. Gen. Ann. 3:e787. doi: 10.1128/genomeA.00787-15
Latest Vaccine Statistics (2022). Available online at https://sacoronavirus.co.za/latest-vaccine-statistics/. (accessed May 24, 2022)
Magee, L. A., von Dadelszen, P., Kalafat, E., Duncan, E. L., O’Brien, P., Morris, E., et al. (2021). Covid-19 vaccination in pregnancy-number needed to vaccinate to avoid harm. Lancet Infect Dis. 21:1627. doi: 10.1016/S1473-3099(21)00691-5
Matar, R., Alrahmani, L., and Monzer, N. (2021). Clinical presentation and outcomes of pregnant women with coronavirus disease 2019: a systematic review and meta-analysis. Clin. Infect. Dis. 72, 521–533. doi: 10.1093/cid/ciaa828
Morgan, J. A., Biggio, J. R., and Martin, J. K. (2022). Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 139:107.
Naqvi, A. A. T., Fatima, K., and Mohammad, T. (2020). Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Bio. Biophys. Acta Mol. Basis Dis. 1866:165878. doi: 10.1016/j.bbadis.2020.165878
Ng, P. C., Leung, C. W., Chiu, W. K., Wong, S. F., and Hon, E. K. (2004). SARS in newborns and children. Biol. Neonate 85, 293–298. doi: 10.1159/000078174
Novoa, R. H., Quintana, W., Llancarí, P., Urbina-Quispe, K., Guevara-Ríos, E., and Ventura, W. (2021). Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 20A systematic review. Travel. Med. Infect. Dis. 39:1019. doi: 10.1016/j.tmaid.2020.101919
Palatnik, A., and McIntosh, J. J. (2020). Protecting labor and delivery personnel from covid-19 during the second stage of labor. Am. J. Perinatol. 37, 854–856. doi: 10.1055/s-0040-1709689
Pattinson, R., Fawcus, S., Gebhardt, S., Niit, R., Soma-Pillay, P., and Moodley, J. (2020). The effect of the first wave of covid-19 on use of maternal and reproductive health services and maternal deaths in south africa. O&G Forum 30, 36–44.
Petrosillo, N., Viceconte, G., Ergonul, O., Ippolito, G., and Petersen, E. (2020). Covid-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 26, 729–727. doi: 10.1016/j.cmi.2020.03.026
Rabaan, A. A., Al-Ahmed, S. H., Haque, S., Sah, R., Tiwari, R., Malik, Y. S., et al. (2020). SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 28, 174–184.
Robertson, T., Carter, E. D., Chou, V. B., Stegmuller, A. R., Jackson, B. D., Tam, Y., et al. (2020). Early estimates of the indirect effects of the covid-19 pandemic on maternal and child mortality on low-income and middle-income countries: a modelling study. Lancet Glob Health 8:e901. doi: 10.1016/S2214-109X(20)30229-1
Sadeghi, R. H., Joan, R., Nataly, S., Mark, A., Razavi, B. S., Majid, W., et al. (2021). The effects of COVID-19 on the placenta during pregnancy. Front. Immunol. 12:743022. doi: 10.3389/fimmu.2021.743022
Sakowicz, A., Ayala, A. E., Ukeje, C. C., Witting, C. S., Grobman, W. A., and Miller, E. S. (2020). Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am. J. Obstet. Gynecol. MFM 2:100198. doi: 10.1016/j.ajogmf.2020.100198
Shimabukuro, T. T., Kim, S. Y., and Myers, T. R. (2021). Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N. Eng. J. Med. 384:2273.
Soma-Pillay, P., Nelson-Piercy, C., Tolppanen, H., and Mebazaa, A. (2016). Physiological changes in pregnancy. Cardiovasc J. Afr. 27, 89–94. doi: 10.5830/CVJA-2016-021
Turan, O., Hakim, A., Dashraath, P., Jeslyn, W. J. L., Wright, A., and Abdul-Kadir, R. (2020). Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: a systematic review. Int. J. Gynecol. Obstet. 151:13329. doi: 10.1002/ijgo.13329
Vale, A. J. M., Fernandes, A. C. L., Guzen, F. P., Pinheiro, F. I., de Azevedo, E. P., and Cobucci, R. N. (2021). Susceptibility to covid-19 in pregnancy, labor, and postpartum period: immune system, vertical transmission, and breastfeeding. Front. Glob Woman’s Health. 2:602572. doi: 10.3389/fgwh.2021.602572
Wang, H., Paulson, K. R., Pease, S. A., and Watson, S. (2021). Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet 399, 1513–1536. doi: 10.1016/S0140-6736(21)02796-3
Wong, S. F., Chow, K. M., and Leung, T. N. (2004). Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 191, 292–297. doi: 10.1016/j.ajog.2003.11.019
Wong, T. C., Lee, Z. Y., Sia, T. L. L., Chang, A. K. W., and Chua, H. H. (2020). Miscarriage risk in COVID-19 infection [published online ahead of print, 2020 Aug 15]. Comput. Clin. Med. 1-4, 351. doi: 10.1007/s42399-020-00443-5
Wong, Y. P., Khong, T. Y., and Tan, G. C. (2021). The effects of covid-19 on placenta and pregnancy: what do we know so far? Diagnostics (Basel) 11:94. doi: 10.3390/diagnostics11010094
World Health Organization (2020). Tracking SARS-CoV-2 Variants. Available online at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed January 03, 2022).
World Health Organization (2022a). Only 1 in 4 African health workers fully vaccinated against Covid-November. Available online at: https://www.afro.who.int/news/only-1-4-african-health- workers-fully-vaccinated-against-Covid-19. (accessed January 3, 2022)
World Health Organization (2022b). WHO Coronavirus (COVID-19) Dashboard. Available online at https://covid19.who.int/. (accessed May 25, 2022).
Yan, H., Ding, Y., and Guo, W. (2020). Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front. Psychol. 11:3324. doi: 10.3389/fpsyg.2020.617001
Keywords: SARS-CoV-2, pregnancy, coronavirus, MERS, health systems
Citation: Adam S, Pheiffer C, Dias S, Hlongwane T, Vannevel V, Soma-Pillay P and Abdullah F (2022) Coronavirus and Pregnancy: The Challenges of the 21st Century: A Review. Front. Microbiol. 13:923546. doi: 10.3389/fmicb.2022.923546
Received: 19 April 2022; Accepted: 22 June 2022;
Published: 13 July 2022.
Edited by:
Arnon Gal, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Silvia Spoto, Policlinico Universitario Campus Bio-Medico, ItalyCopyright © 2022 Adam, Pheiffer, Dias, Hlongwane, Vannevel, Soma-Pillay and Abdullah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sumaiya Adam, c3VtYWl5YS5hZGFtQHVwLmFjLnph
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.