- 1State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Science, Nanjing, China
- 2University of Chinese Academy of Sciences, Beijing, China
Endorheic lakes in arid regions of Northwest China are generally vulnerable and sensitive to accelerated climate change and extensive human activities. Therefore, a better understanding of the self-purification capacity of ecosystems, such as denitrification, is necessary to effectively protect these water resources. In the present study, we measured unamended and amended denitrification rates of Lake Bosten by adding the ambient and extra nitrate isotopes in slurry incubations. Meanwhile, we investigated the abundances and community structure of nitrous oxide-reducing microorganisms using qPCR and high-throughput sequencing, respectively, in the surface sediments of Lake Bosten to study denitrification potential in endorheic lakes of arid regions as well as the response of those denitrifiers to climatically induced changes in lake environments. Amended denitrification rates increased by one order of magnitude compared to unamended rates in Lake Bosten. The great discrepancy between unamended and amended rates was attributed to low nitrate availability, indicating that Lake Bosten is not operating at maximum capacity of denitrification. Salinity shaped the spatial heterogeneity of denitrification potential through changes in the abundances and species diversity of denitrifiers. Climate change had a positive effect on the water quality of Lake Bosten so far, through increased runoff, decreased salinity, and enhanced denitrification. But the long-term trajectories of water quality are difficult to predict alongside future glacier shrinkage and decreased snow cover.
Introduction
Endorheic lakes, as one of the major components of endorheic water systems, are the primary available water sources in arid regions and play significant roles in the social and economic development of those regions (Tao et al., 2015; Xu et al., 2020). High degrees of continentality and isolation make endorheic lakes particularly susceptible and vulnerable to climate and environmental change (Yapiyev et al., 2017; Huang et al., 2020). Due to the discharge of agricultural, industrial, and urban wastewaters, endorheic waters have been increasingly threatened by eutrophication in recent decades (Arce et al., 2013; Menberu et al., 2021; Sun et al., 2021; Diaz-Torres et al., 2022). More importantly, endorheic lakes are land-locked drainage networks where water does not drain into other water bodies (Yapiyev et al., 2017). Due to the lack of outlets, the removal of inflowing pollutants depend primarily on the self-purification capacity of ecosystems (Arce et al., 2013; Valiente et al., 2018). Therefore, a better understanding of the self-purification capacity and ecological resilience of endorheic lakes is necessary to effectively protect the water resources in arid regions.
Denitrification, the process of reducing nitrate to dinitrogen (N2), is considered the primary pathway for permanent nitrogen (N) removal in aquatic ecosystems (Seitzinger, 1988). It plays an important role in alleviating N pollution and maintaining the self-purification capacity of ecosystems (Roland et al., 2018). The availability of both nitrate and organic carbon are considered the most important factors limiting denitrification rates (Seitzinger, 1988; Seitzinger et al., 2006; Xia et al., 2017; Jiang et al., 2020). Salinity also has an important influence on denitrification (Koop-Jakobsen and Giblin, 2010; Arce et al., 2013; Zhou et al., 2016). Many endorheic lakes are naturally saline, as the evaporative concentration process leads to salt accumulation (Yapiyev et al., 2017), which comprises a diverse array of salts such as calcium, sodium, potassium, sulfate, carbonate, and chloride (Heinrichs and Walker, 2006). The change in salinity can cause decrease or increase in cytoplasmic volume by imposing considerable osmotic stress on the relevant microbes such as nitrifiers and denitrifiers, resulting in the loss of metabolic activity (Ardon et al., 2013; Zhao et al., 2013b; Neubauer et al., 2019). In addition, salt water containing sulfate may have inhibitory or promoting effects on nitrate reduction due to sulfide toxicity or by providing electron donors for chemoautotrophic processes from the reduction of sulfate (Aelion and Warttinger, 2010; Zhu et al., 2018; Murphy et al., 2020). A consensus on these mechanisms remains elusive, probably due to the differences in microbial community structure involved in N cycling, which is sensitive to salinity variation (Herbert et al., 2015; Zhou et al., 2016). Lakes are hotspots of denitrification with high N removal efficiency due to the long residence times of water (Wollheim et al., 2008; Finlay et al., 2013). Previous research about denitrification has mainly focused on out-flowing lakes. We lack a direct understanding of the denitrification potential of endorheic lakes in arid regions.
Climate change introduces a new challenge for endorheic lakes (Tao et al., 2015; Zhang et al., 2017). In Northwest China, temperatures have risen markedly in recent decades and faster than in the surrounding regions (Shi et al., 2007; Yang et al., 2020). Climate change can influence lakes either through higher temperatures or by changes in salinity (Mosley, 2015; Greaver et al., 2016), the latter is particularly influential in arid and semiarid lakes (Brucet et al., 2012; Lin et al., 2017). Climate change is affecting the hydrological cycle with more frequent and intense precipitation, altered snow accumulation and melt, and changes in evaporation (Sorg et al., 2012; Zhou et al., 2015), leading to large-scale changes in lake salinity (Jeppesen et al., 2015; Rusuli et al., 2015). Thus, how denitrification in endorheic lakes will respond to climatically induced changes in lake salinity is a key question.
Lake Bosten used to be the largest freshwater lake in the endorheic basins of China and is of great importance as a water supply for local domestic use and industrial and agricultural production (Zhou et al., 2015; Wang et al., 2018b). Since the 1960s, Lake Bosten suffers from salinization and eutrophication mainly caused by accelerated climate change and extensive human activities (Guo et al., 2015b; Fontana et al., 2019). It has changed from an oligotrophic freshwater lake to a mesotrophic oligosaline lake (Tang et al., 2012; Dai et al., 2013). Lake Bosten represents an interesting feature, which has a natural salinity gradient (Tang et al., 2012; Dai et al., 2013). It provides an ideal ecosystem in which to study the denitrification capacity of endorheic lakes in arid regions as well as its response to climatically induced changes in lake salinity.
Slurry incubations incorporating the 15N isotope-tracing technique have been used as a powerful tool for estimating potential denitrification rates in the field (Song et al., 2013; Yin et al., 2014; Jiang et al., 2020) and simulation experiments (Salk et al., 2017; Wang et al., 2018a; Murphy et al., 2020). Although denitrification rates in slurries are usually significantly higher than under in situ conditions due to the disruption of natural profiles of sediments (Laverman et al., 2006), slurry incubation is highly repeatable and flexible (Yin et al., 2014; Salk et al., 2017). It is suitable for exploring the relative differences in denitrification potential and estimating nitrate limitations by adding extra nitrate (amended) and not adding extra nitrate (unamended) to the slurries. During denitrification, nitrite and nitrous oxide reductase enzymes can catalyze the reduction of nitrite and nitrous oxide, which are encoded by the nirS/nirK and nosZI/nosZII genes, respectively (Franklin et al., 2017; Neubauer et al., 2019; Murphy et al., 2020). These genes commonly used as an amplicon for quantifying or sequencing denitrifiers (Throback et al., 2004). Quantifying the potential denitrification rates, combined with the abundance of key genes, can provide a comprehensive understanding of lake denitrification potential (Murphy et al., 2020; Broman et al., 2021; Yang et al., 2022).
Here, we examine unamended and amended denitrification rates at 17 sampling sites in Lake Bosten through the addition of extra nitrate (100 μmol L−1) or otherwise to slurries, in order to estimate potential denitrification rates. We determine the abundance of nirS and nosZI genes in surface sediments through qPCR to estimate the genetic potential of denitrification. In addition, high throughput sequencing using nosZI gene as an amplicon was conducted to estimate the microbial community structure mediating denitrification. We test the following hypotheses: (1) low nitrate concentration may be a proximate control of denitrification rates in Lake Bosten; (2) the sediment of different areas in Lake Bosten varies in denitrification potential due to the difference in nitrate and salinity levels; and (3) based on earlier studies about the influence of salinity on denitrification, we hypothesize that climate change can influence lake denitrification via changes in salinity. Our study provides insights into the influence of changes in salinity on N cycling in endorheic lakes and can be used to predict future response of water quality to climate change in arid regions.
Materials and Methods
Study Area
Lake Bosten is located in the lowest area of the intermontane Yanqi Basin between the Taklimakan Desert and Tien Shan, Northwestern China. Winds come mainly from the southwest, indicating dominant influences of westerlies throughout the summer season (Ma et al., 2020; Yao et al., 2022). The mean annual temperature was ~9.1°C, and the annual precipitation was 76.3 mm. Lake Bosten has a surface area of ~1,005 km2, a maximum depth of 16 m, and an average depth of 8 m. Water temperature and pH ranged from 23.5 to 24.9°C and from 8.8 to 9.5, respectively (Supplementary Table S1).
The Kaidu River is the most important tributary in the area and accounts for about 83% of the lake's annual water inflow runoff, which is supplied by melting ice, precipitation, and groundwater (Chen et al., 2008; Rusuli et al., 2016). Other rivers are small seasonal rivers, such as the Huangshui River, which contributes to almost all the remaining 17% of inflow runoff (Chen et al., 2008; Rusuli et al., 2016). A pumping station was constructed in the southwestern part of Lake Bosten, which pumps lake water into the Peacock River to adjust the outflow of the lake (Yu et al., 2015; Zhou et al., 2017).
The freshwater inflow from the Kaidu River is removed by the pumping station at the southwestern margin of Lake Bosten, shaping a freshwater region in the southwest of Lake Bosten (Kaidu River Estuary: KRE). The drainage channels of agricultural production are mainly located in the northwestern coastal area (NCA), making this area the most heavily polluted in Lake Bosten. The rest of the area is the main lake area (MLA) (Figure 1).
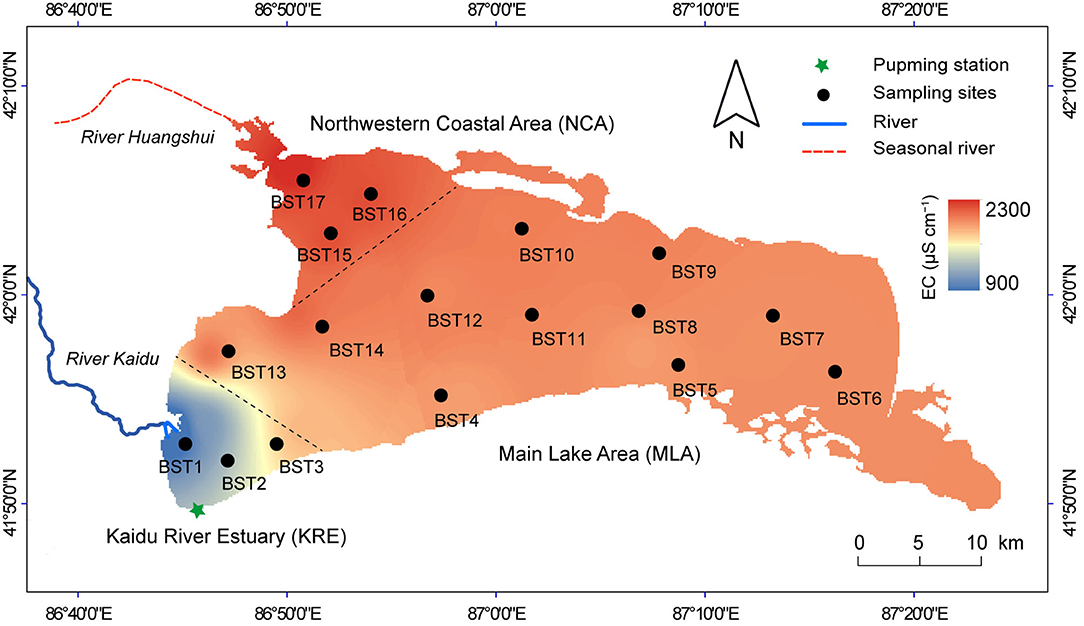
Figure 1. Map of Lake Bosten, showing study area, sampling sites and electrical conductivity (EC) in water column. Electrical conductivity was the average value from 2010 to 2019, which derived from the long-term monitoring of the institute of Lake Bosten. The lake area was divided into three subregions according to the EC gradient: Kaidu River Estuary (KRE), Main Lake Area (MLA) and Northwestern Coastal Area (NCA).
Collection and Pre-Treatment of Samples
Water and sediment samples, from 17 sites at Lake Bosten, were collected in September 2019. Water samples from the top (50 cm below the surface), middle, and bottom (50 cm above the sediment) were collected using a polymethyl methacrylate water sampler (UWITEC, Austria) and then mixed. Water samples for dissolved nutrient analyses were filtered using 0.2 μm nylon syringe filters immediately following the mixture in the field. The surface sediments (about 3 cm depth) were collected with a 60 cm long gravity corer (UWITEC, Austria), and then placed in clean airtight plastic bags. Water temperature (WT), pH, dissolved oxygen (DO), and salinity were measured at each sampling site in situ, with a multiparameter water quality sonde (YSI 6600V2, USA). All collected sediment and water samples were kept in a cool and shaded place, and subsequently delivered to the Institute of Lake Bosten.
Measurements of Unamended and Amended Denitrification Rates
Unamended and amended denitrification rates were measured using slurry incubations incorporating the 15N isotope-tracing technique (Yin et al., 2014; Jiang et al., 2020). Sediment samples were mixed with lake water at a ratio of 1:10 to make homogenized slurries. The slurry was stirred continuously and purged with helium for 40 min. The purged slurries were distributed into 12 ml vials (Labco Exetainer, UK), and then immediately sealed. Subsequently, preincubation was conducted at the in situ temperature on a shaker table (200 rpm) for 24 h, to eliminate residual nitrate and DO. After preincubation, a certain amount of 15N-nitrate (99 atom%) solution was injected into a group of vials to determine the unamended denitrification rates according to the nitrate concentrations of the water column in Lake Bosten. Simultaneously, a high 15N-nitrate level (a final concentration of 100 μmol L−1) was injected into another group of vials to estimate the amended potential rates. Subsequently, three of the vials were randomly selected from each group and immediately preserved with the addition of 200 μL ZnCl2 as the initial samples for the measurement of denitrification rates. The remaining vials were incubated under the same conditions for 2, 4, and 8 h, and then used to measure the production of 29N2 and 30N2.
Dissolved 29N2 and 30N2 were determined using membrane inlet mass spectrometry (MIMS) analysis (Kana et al., 1998). The unamended and amended denitrification rates were calculated according to the following equation:
where R (μmol N kg−1 h−1) indicates the measured 15N-based unamended or amended denitrification rates, K is the slope calculated from the concentration of 15N-N2 vs. incubation time (Supplementary Figure S1), V (L) is the volume of the incubation vial, and W (kg) denotes the dry weight of the sediment.
DNA Extraction and Real-Time Quantitative PCR (qPCR)
Genomic DNA was extracted from the sediment samples using the FastDNA™ Spin kit for soil (MP Biomedicals) according to the manufacturer's instructions. The quality and quantity of DNA were checked using agarose gel electrophoresis and using a NanoDrop ND-1000 UV/Vis spectral photometer. The quality of DNA extracted in each sampling site was exhibited in Supplementary Table S2. Extracts were stored at −80°C prior to gene quantification. The sampling site of BST06 and BST13 were removed from the following molecular biological analysis due to low DNA quality.
qPCR was used to quantify functional genes for enzymes of nitrification (AOB and AOA) (Rotthauwe et al., 1997; Francis et al., 2005) and denitrification (nirS and nosZI) (Throback et al., 2004). Triplicate qPCR reactions were set up for each sample. The primers, thermocycling conditions, and relevant references are included in the supplemental information (Supplementary Table S3). The total qPCR reaction volume of 20 μl contained 10 μl of SYBR green qPCR Master Mix, 1 μl of forward primer, 1 μl of reverse primer, 7 μl of ddH2O, and 1 μl of the template (DNA). Reactions were performed using an Eco™ Real-Time PCR System. Melting curves were checked for each reaction to confirm the purity of the amplified products. Standard curves were obtained using 10-fold serial gradient dilutions of standard plasmids containing targeted genes with known copy numbers. The qPCR amplification efficiencies and other calibration curve parameters are listed in Supplementary Table S4. Gene abundance was calculated based on the constructed standard curve and then converted into copies per gram of dry sediment.
High-throughput Sequencing
The microbial reduction of nitrous oxide to N2 is catalyzed by nitrous oxide reductase. The nosZI gene was sequenced to explore the microbial community structure performing complete denitrification across the lake using the primer pairs nosZ-F/nosZ1622R (Throback et al., 2004). The purified DNA products were sent to Shanghai Personal Biotechnology Co., Ltd for high-throughput sequencing using the Illumina MiSeq platform. Paired-end sequencing reads were merged using FLASH (Fast Length Adjustment of Short reads, v1.2.11) (Magoc and Salzberg, 2011). Adapters and primers were trimmed off all the reads using Cutadapt (v1.9.1). Sequences shorter than 400 bp, lower than 25 quality scores, and suspected to be chimeras were discarded using USEARCH (v10.0.240) (Edgar and Flyvbjerg, 2015). The sequences were initially grouped at 97% of the sequence similarities, and representative sequences were translated and compared to the nosZ reference sequence in GenBank using BLASTx. Frameshift errors were removed. Subsequently, the high-quality sequences were aligned based on the amino acid residues and grouped based on 85% of the nucleotide sequence similarities to form a new OTU table for the subsequent analysis.
Chemical Analysis of Water and Sediment Physicochemical Parameters
Dissolved organic carbon (DOC) was determined using a TOC analyzer (Teledyne Tekmar, Torch, USA). Acid volatile sulfides (AVS) in sediments were determined with the following methods. Sulfide in sediment samples was converted into H2S by HCl extraction for 1 h. Subsequently, released H2S was captured in a NaOH solution with a continuous N2 flow. The dissolved sulfide concentration in the NaOH solution was measured spectrophotometrically by the methylene blue method. The other physicochemical parameters, including total nitrogen (TN), nitrate, ammonium, sulfate, sediment total organic carbon (STOC), and sediment total nitrogen (STN) were determined following standard methods detailed in Jiang et al. (2020).
Statistical Analyses
The statistical analyses were performed using R 3.5.3 and the RStudio 1.1.462 interface. Kruskal–Wallis rank tests and one-way ANOVA were used to evaluate the differences in environmental factors, gene abundances, species diversity, and denitrification rates among areas, determining statistically significant differences when p < 0.05. Alpha diversity indices, unconstrained PCoA analysis, and redundancy analysis (RDA) were constructed with the “vegan” package in R. Pearson correlations were used to examine the relationships between parameters of denitrification and environmental properties. Data visualization was performed using the package “ggplot2” in R.
Results
The Physicochemical Parameters of the Water and Sediment in Lake Bosten
In Lake Bosten, dissolved oxygen and salinity ranged from 6.9 to 10.8 mg L−1 and from 0.51‰ to 0.81‰, respectively. Dissolved oxygen in NCA was significantly higher than that in KRE and MLA, while salinity in KRE was significantly lower than that in MLA and NCA. The concentrations of nitrate and TN exhibited a decreasing trend from KRE to NCA. Especially for nitrate, the values in KRE were 3–4 times higher than the values in MLA and NCA, which ranged from 0.1 to 0.4 mg L−1. The concentrations of TN ranged from 0.7 to 1.3 mg L−1, and the values in KRE were significantly higher than that in NCA. In contrast, the opposite trend was found for sulfate, DOC, STOC, STN, and AVS, which ranged from 140 to 413 mg L−1, from 5.3 to 12.0 mg L−1, from 10.0 to 58.3 mg g−1, from 2.2 to 6.1 mg g−1 and from 3.9 to 26.1 μmol g−1, respectively. Ammonium concentrations ranged from 0.1 to 0.4 mg L−1, and there was no significant variation among the three areas in Lake Bosten (Figure 2).
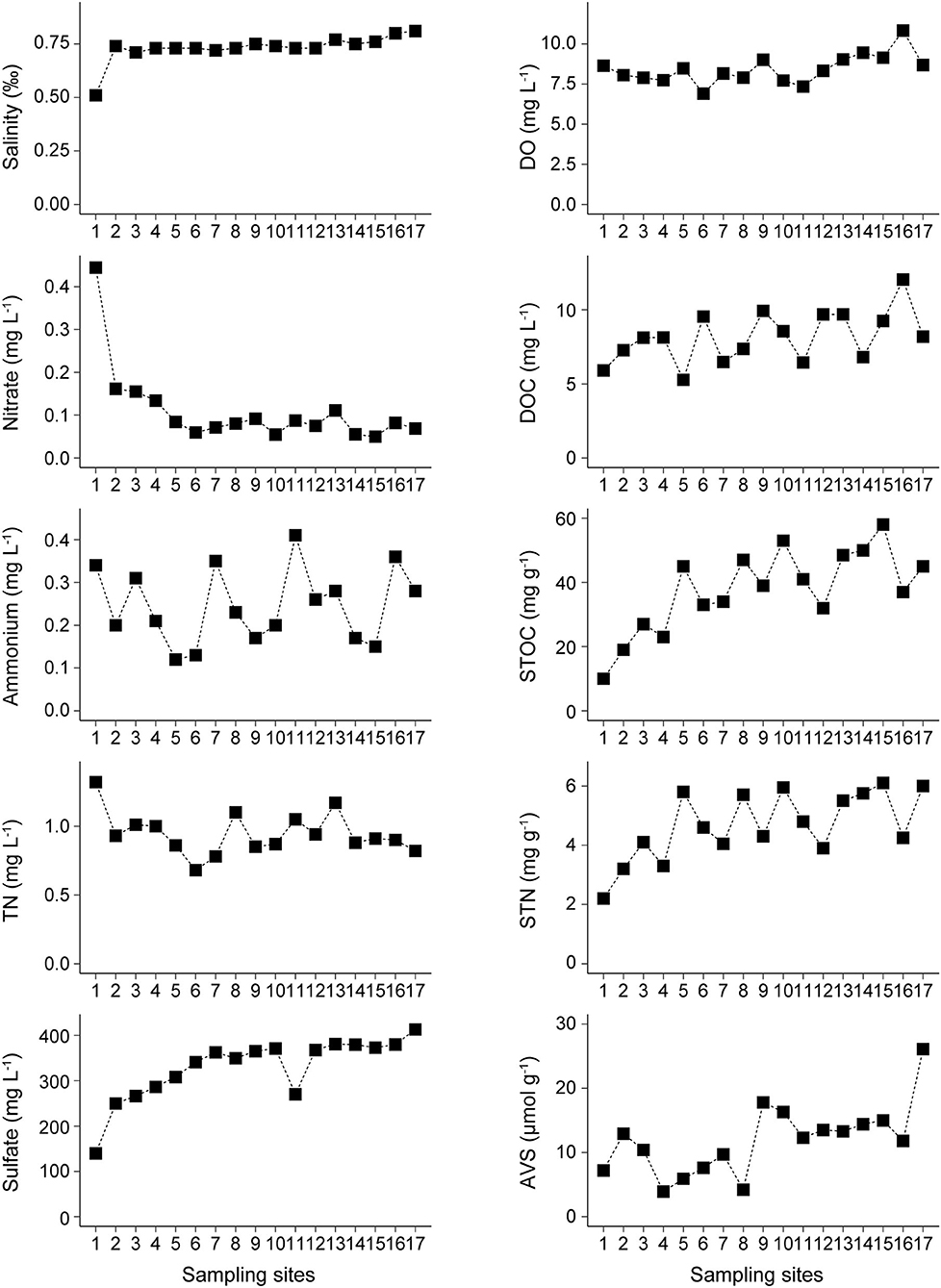
Figure 2. Spatial distribution of the physicochemical parameters in the overlying water [Salinity, Dissolved Oxygen (DO), Nitrate, Ammonium, Total Nitrogen (TN), Sulfate and Dissolved Organic Carbon (DOC)] and sediments [Sediment Total Organic Carbon (STOC), Sediment Total Nitrogen (STN) and Acid volatile sulfides (AVS)] in Lake Bosten.
Unamended and Amended Denitrification Rates
Unamended and amended denitrification rates ranged from 2.24 to 6.08 μmol N kg−1 h−1 and from 12.87 to 77.63 μmol N kg−1 h−1, respectively, in Lake Bosten, and the amended rates were about 8.9 times those of the unamended rates (Figures 3A,B). These sampling sites showed considerable variation in amended denitrification rates. Generally, the amended rates were highest in KRE, intermediate in MLA, and lowest in NCA. In contrast, in unamended denitrification rates, the differences were small among sampling sites in Lake Bosten.
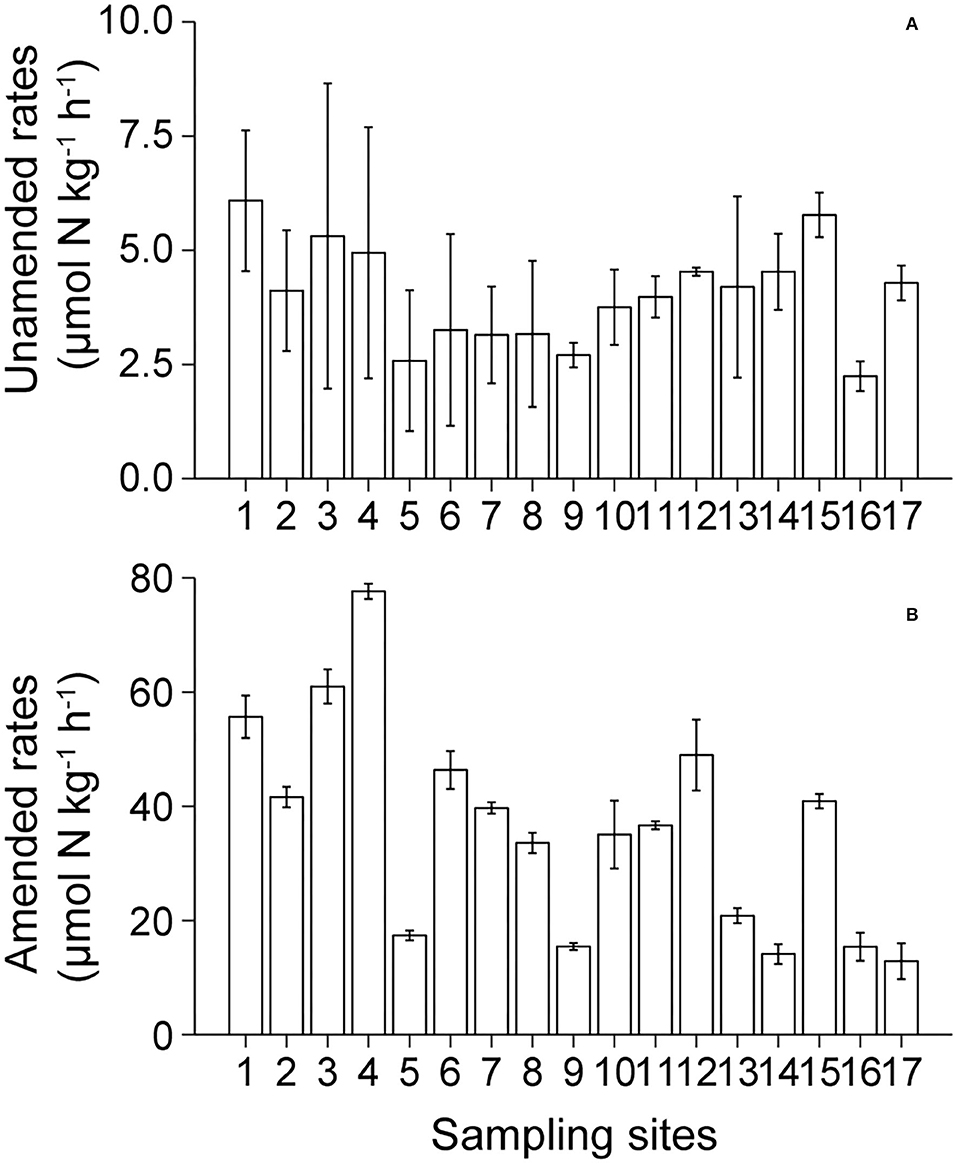
Figure 3. Spatial distribution of the unamended (A) and amended (B) denitrification rates in Lake Bosten.
The Abundance of Functional Genes for Nitrification and Denitrification
The bacterial amoA gene copy numbers ranged from 9.45 × 102 copies g−1 of sediment at site BST13 to 8.30 × 104 copies g−1 of sediment at site BST4 (Figure 4A). The archaeal amoA gene copy numbers ranged from undetected values to 1.15 × 104 copies g−1 of sediment, and there was a decreasing trend from KRE to NCA (Figure 4B). The abundance of the AOB gene was higher than the abundance of the AOA gene in most of the sampling sites.
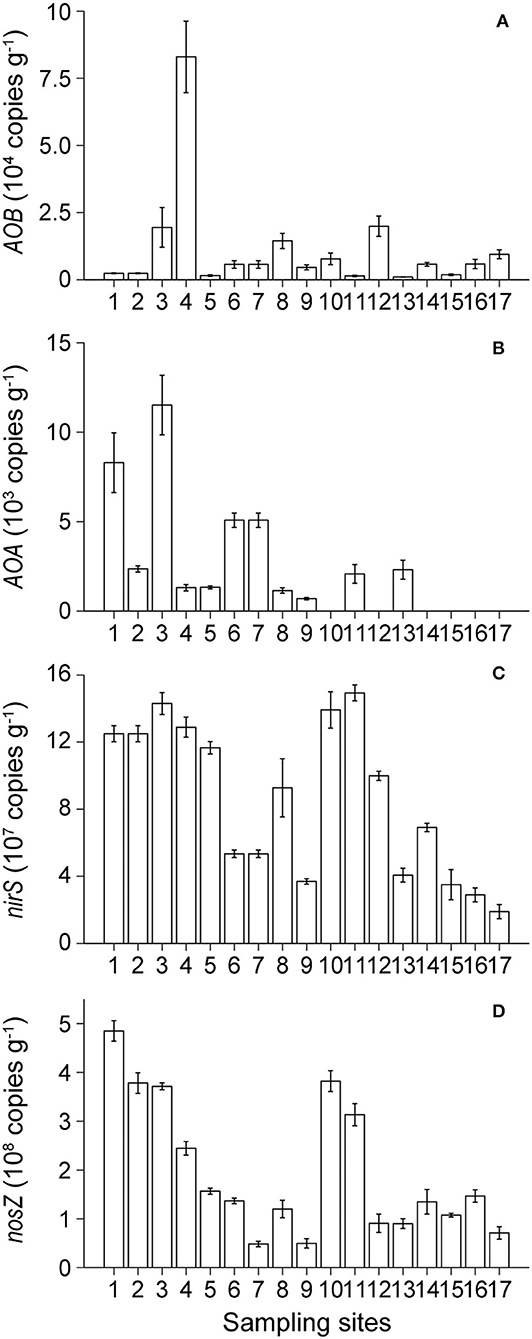
Figure 4. Spatial distribution of the abundances of AOB (A), AOA (B), nirS (C), and nosZI (D) genes in Lake Bosten.
Copy numbers of the nirS gene and nosZI genes ranged from 1.89 × 107 to 1.49 × 108 copies g−1 of sediment and from 4.84 × 107 to 4.85 × 108 copies g−1 of sediment, respectively (Figures 4C,D). The abundance of the nosZI gene exceeded the abundance of the nirS gene at most sites. There was a decreasing trend in the abundance of the nirS gene and nosZI gene from KRE to NCA.
The Community Structure of Denitrifying Microorganisms
Alpha-diversity analysis revealed a decreasing trend in the diversity indexes of Richness, Shannon, and Simpson from KRE to NCA (Supplementary Figure S2). Unconstrained principal coordinates analysis (PCoA) with Bray–Curtis distance showed the community variation of nitrous oxide reducers among different sampling sites (Supplementary Figure S3). The results showed that the first two axes explained 87.3% of the microbial community variation. The microbial community of nitrous oxide reducers was significant differences among the three areas in Lake Bosten (p < 0.05). The forward selection procedure in RDA revealed that the variation in the microbial community of nitrous oxide reducer was significantly explained by salinity and ammonium, which described 21.3 and 21.5% of the total variation, respectively (Figure 5).
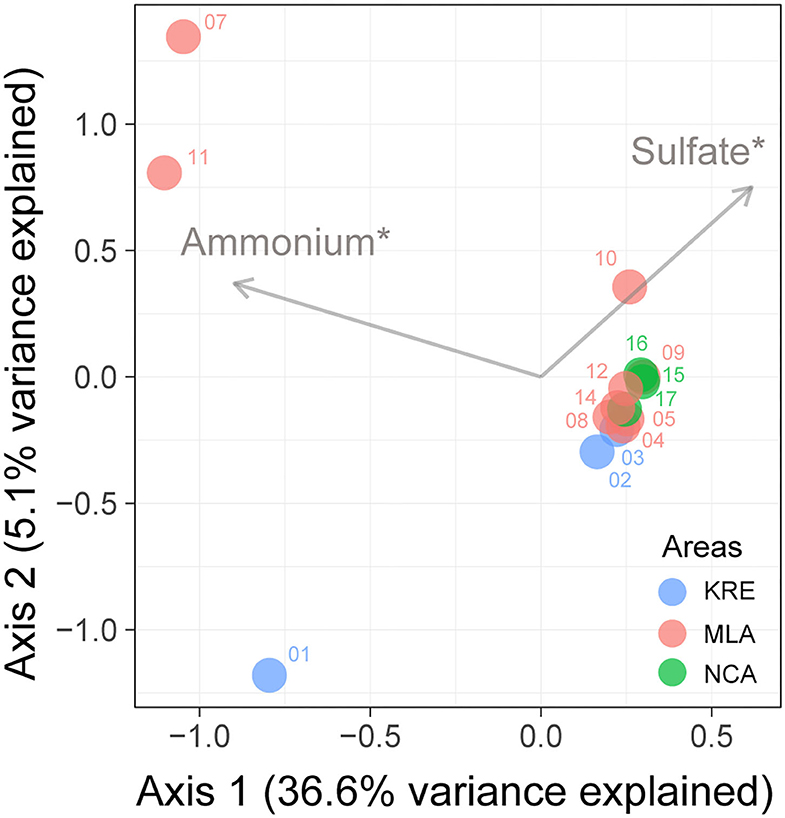
Figure 5. Redundancy analyses (RDA) ordination plots showing the significant environmental factors in structuring variations in the community composition of nitrous oxide reducer in Lake Bosten. Significance levels: *corrected p-value < 0.05. KRE, Kaidu River Estuary; MLA, Main Lake Area; NCA, Northwestern Coastal Area.
Discussion
Nitrate Availability Limited Unamended Denitrification Rates
The main objective of this study was to explore the denitrification potential of Lake Bosten and its response to increasing inputs of nutrients and changes in salinity. Amended denitrification rates increased by one order of magnitude compared to unamended rates in response to additional nitrate at most of the sites, indicating the high potential of denitrification in Lake Bosten. The availability of nitrate is considered one of the most important limiting factors for denitrification in lakes and other aquatic ecosystems (Pina-Ochoa and Alvarez-Cobelas, 2006; Seitzinger et al., 2006). In Lake Bosten, nitrate concentrations in the water positively correlated with unamended denitrification rates (Table 1), indicating that low nitrate concentrations may limit unamended denitrification rates. Nitrate can be supplied as an external input or by in situ nitrification. The coupled nitrification-denitrification process usually dominates N removal in ecosystems of nitrate limitation (Koop-Jakobsen and Giblin, 2010; Xia et al., 2017). In Lake Bosten, the abundance of either the AOB or AOA gene was low compared to the equivalent values in most freshwater lakes by at least one order of magnitude (Wu et al., 2010; Hou et al., 2013; Zhao et al., 2013a; Bollmann et al., 2014; Liu et al., 2014). Meanwhile, the concentrations of ammonium were significantly higher than those of nitrate, perhaps indicating that nitrification was inhibited. Therefore, both the low nitrate concentrations and abundances of nitrifiers may limit denitrification rates in Lake Bosten.
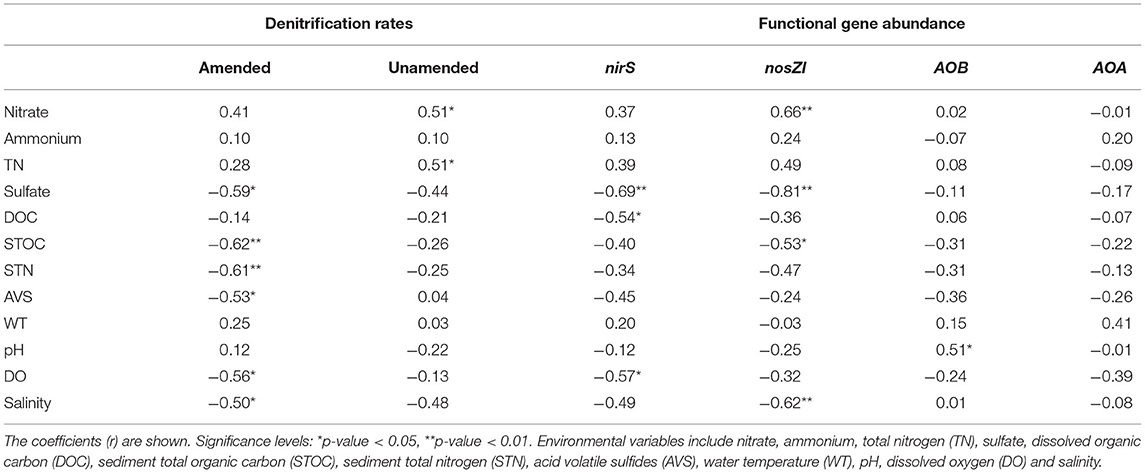
Table 1. Pearson's correlation analysis between denitrification rates (amended and unamended) and environmental variables (n = 17) and between functional gene abundance (nirS, nosZI, AOB, and AOA) and environmental variables (n = 15).
The Influence of Changes in Salinity on Denitrification
In Lake Bosten, there is heterogeneity in denitrification potential. The sediments in KRE had the highest amended denitrification rates. Nitrate loads mainly derive from the transportation of the Kaidu River in Lake Bosten (Yu et al., 2015; Zhou et al., 2017), which exhibited an obvious decline in nitrate from the estuary to the east section (Figure 2). This phenomenon indirectly reflects a high potential of N removal in KRE. In contrast, the denitrification potential in NCA was low, suggesting that other environmental factors constrained denitrification except for nitrate availability.
Amended denitrification rates were negatively correlated with salinity and sulfate concentrations in Lake Bosten (Table 1), perhaps indicating that salinity is a key regulating factor of denitrification. Climate change is affecting the hydrological cycle of Lake Bosten and consequent effects on concentration or dilution will alter lake salinity (Rusuli et al., 2015; Liu and Bao, 2020). In addition, there is a continuous input of salinity from salt leaching associated with the agricultural irrigation of the northwestern basin (Tang et al., 2012; Guo et al., 2015b). In the past 60 years, Lake Bosten has evolved from freshwater to an oligosaline lake. Salinity has become a dominant factor shaping the bacterial community in the water and sediment of Lake Bosten (Tang et al., 2012; Dai et al., 2013).
Salinity can affect denitrification in many respects. Considerable studies have reported that salinity directly inhibits the metabolic activity of denitrification, through osmotic stress and sulfide toxicity (Ruiz-Romero et al., 2009; Ardon et al., 2013; Zhao et al., 2013b; Neubauer et al., 2019). Sulfate is the main component of salt anions in Lake Bosten, which is consistent with the changes in salinity (r = 0.83, p < 0.01). High sulfate can enhance the accumulation of toxic sulfide in sediments (Aelion and Warttinger, 2010; Zhu et al., 2018; Murphy et al., 2020) and then inhibit denitrification rates. Furthermore, the influence of salinity on denitrification depends on the long-term salinity adaptation of denitrifying microorganisms (Franklin et al., 2017; Wang et al., 2018a; Murphy et al., 2020). Many studies have shown that salinity is the most important factor in structuring communities of denitrifying microorganisms, such as nosZ-denitrifiers (Cai et al., 2019; Wang et al., 2019; Han et al., 2021), and that salinity can affect denitrification rates by altering the abundance and diversity of denitrifying microorganism. In Lake Bosten, there was a significant correlation between the abundance of the nirS and nosZI genes (r = 0.79, p < 0.01). Salinity or sulfate was negatively correlated with the abundance of nirS and nosZI, respectively, and the latter was the stronger. The nitrous oxide reducers are more sensitive to osmotic stress and ionic toxicity than the nitrite reducers (Laverman et al., 2007; Zhou et al., 2016). In addition, the species diversity of nitrous oxide reducers significantly decreased with the increase of salinity or sulfate in Lake Bosten (p < 0.01). Elevated salinity appears to decrease the abundance and diversity of nitrous oxide reducers, resulting in incomplete denitrification.
Salinity also indirectly affects denitrification by inhibiting nitrification rates or altering the physicochemical environment (Herbert et al., 2015; Zhou et al., 2016; Franklin et al., 2017). Previous research also suggests that toxic sulfide can inhibit microbial activity related to nitrification (Herbert et al., 2015; Wang et al., 2020), while some investigations conducted in other habitats showed moderate salinity in favor of nitrification (Zhou et al., 2016; Wang et al., 2018a). In this study, the abundances of AOB and AOA genes were not correlated with salinity, sulfate, and AVS, indicating that the differences in gene abundances were not attributed to the changes in salinity. Further research is needed to reveal the underlying mechanisms of low gene abundance mediated nitrification in Lake Bosten. In addition, amended denitrification rates were negatively correlated with STOC and DO in Lake Bosten. Elevated salinity might constrain the activities of microbes and reduce the deposition of organic carbon and the consumption of DO, thus inhibiting the anaerobic denitrifying process. Neubauer et al. (2019) also reported that denitrification rates were reduced due to the decrease in oxygen demand induced by salinization.
The Response of Endorheic Lakes to Climate Change in Northwest China
The arid region of Northwest China has been getting warmer and wetter in recent decades because of the enhancement of the westerly circulation (Yang et al., 2021; Yao et al., 2022). Glacial melt leads to increased runoff, which carries a large amount of silt as well as plant and animal residues (Sorg et al., 2012; Guo et al., 2015b). Meanwhile, the decomposition of organic matter and the release of nutrients may be enhanced by the increasing temperature (Wik et al., 2016; Jane et al., 2021). Thus, climate warming may lead to an increase in nutrient input into lakes from the watershed. For example, climate warming and consequent glacial melt led to the increasing inflow of the Kaidu River into Lake Bosten in 2013 (Guo et al., 2015a). Meanwhile, nitrate concentration in the Kaidu River estuary has been fluctuating but increasing in recent years (Supplementary Figure S4). However, a decreasing trend in nitrate concentration was observed in Lake Bosten. This may be because the increase in water level led to the decrease in salinity and nitrate concentrations through dilution. In addition, the estuary area of the Kaidu River has excess capacity to remove additional nitrate, which plays an important role in regulating and alleviating external nitrate loads, especially with the decrease of salinity.
In Northwest China, the influence of climate change on the water quality of endorheic lakes is complicated. On the one hand, the external nutrient inputs may increase, accompanied by an increase in the quantity of runoff and climate warming, but on the other hand, the pollution will be alleviated due to dilution and the decrease of salinity. Although climate change had a positive effect on the water quality of Lake Bosten so far, long-term trajectories of water quality are difficult to predict. Accelerated climate warming will cause glacier shrinkage and decreased snow cover in the future (Sorg et al., 2012; Zhou et al., 2015). This brings large uncertainties into predictions of the changes in runoff, water level, and salinity. Effective management decisions are essential for maintaining the water quantity, salinity, and denitrification capacity of lakes in the face of accelerated climate change and extensive human activities.
Data Availability Statement
The data presented in the study are deposited in the China National Center for Bioinformation, accession number CRA006560 (https://www.cncb.ac.cn/).
Author Contributions
XJ: conceptualization, methodology, investigation, writing—original draft, visualization, and funding acquisition. CL and YH: investigation and data curation. KS: formal analysis and project administration. XT: writing—review and editing. GG and BQ: conceptualization, writing—review andediting, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Natural Science Foundation of China (grant numbers: 41790423 and U2003205) and National Key R&D Program of China (grant numbers: 2019YFA0607100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Institute of Lake Bosten, Environmental Protection Bureau of Bayingolin Mongolia Autonomous Prefecture, Korle, China for providing the data, instruments and labs.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.922546/full#supplementary-material
References
Aelion, C. M., and Warttinger, U. (2010). Sulfide inhibition of nitrate removal in coastal sediments. Estuaries Coasts 33, 798–803. doi: 10.1007/s12237-010-9275-4
Arce, M. I., Gomez, R., Suarez, M. L., and Vidal-Abarca, M. R. (2013). Denitrification rates and controlling factors in two agriculturally influenced temporary Mediterranean saline streams. Hydrobiologia 700, 169–185. doi: 10.1007/s10750-012-1228-4
Ardon, M., Morse, J. L., Colman, B. P., and Bernhardt, E. S. (2013). Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob. Change Biol. 19, 2976–2985. doi: 10.1111/gcb.12287
Bollmann, A., Bullerjahn, G. S., and McKay, R. M. (2014). Abundance and diversity of ammonia-oxidizing archaea and bacteria in sediments of trophic end members of the Laurentian Great Lakes, Erie and Superior. PLoS ONE 9, e97068. doi: 10.1371/journal.pone.0097068
Broman, E., Zilius, M., Samuiloviene, A., Vybernaite-Lubiene, I., Politi, T., Klawonn, I., et al. (2021). Active DNRA and denitrification in oxic hypereutrophic waters. Water Res. 194, 13. doi: 10.1016/j.watres.2021.116954
Brucet, S., Boix, D., Nathansen, L. W., Quintana, X. D., Jensen, E., Balayla, D., et al. (2012). Effects of temperature, salinity and fish in structuring the macroinvertebrate community in shallow lakes: implications for effects of climate change. PLoS ONE 7, e30877. doi: 10.1371/journal.pone.0030877
Cai, Y., Zhang, X., Li, G., Dong, J., Yang, A., Wang, G., et al. (2019). Spatiotemporal distributions and environmental drivers of diversity and community structure of nosZ-type denitrifiers and anammox bacteria in sediments of the Bohai Sea and North Yellow Sea, China. J. Oceanol. Limnol. 37, 1211–1228. doi: 10.1007/s00343-019-8200-3
Chen, X., Wu, J., and Hu, Q. (2008). Simulation of climate change impacts on streamflow in the Bosten Lake Basin using an artificial neural network model. J. Hydrol. Eng. 13, 180–183. doi: 10.1061/(ASCE)1084-0699(2008)13:3(180)
Dai, J., Tang, X., Gao, G., Chen, D., Shao, K., Cai, X., et al. (2013). Effects of salinity and nutrients on sedimentary bacterial communities in oligosaline Lake Bosten, northwestern China. Aquat. Microb. Ecol. 69, 123–134. doi: 10.3354/ame01627
Diaz-Torres, O., Lugo-Melchor, O. Y., de Anda, J., Pacheco, A., Yebra-Montes, C., Gradilla-Hernandez, M. S., et al. (2022). Bacterial dynamics and their influence on the biogeochemical cycles in a subtropical hypereutrophic lake during the rainy season. Front. Microbiol. 13, 832477. doi: 10.3389/fmicb.2022.832477
Edgar, R. C., and Flyvbjerg, H. (2015). Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 31, 3476–3482. doi: 10.1093/bioinformatics/btv401
Finlay, J. C., Small, G. E., and Sterner, R. W. (2013). Human influences on nitrogen removal in lakes. Science 342, 247–250. doi: 10.1126/science.1242575
Fontana, L., Sun, M., Huang, X., and Xiang, L. (2019). The impact of climate change and human activity on the ecological status of Bosten Lake, NW China, revealed by a diatom record for the last 2000 years. Holocene 29, 1871–1884. doi: 10.1177/0959683619865586
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E., and Oakley, B. B. (2005). Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. U. S. A. 102, 14683–14688. doi: 10.1073/pnas.0506625102
Franklin, R. B., Morrissey, E. M., and Morina, J. C. (2017). Changes in abundance and community structure of nitrate-reducing bacteria along a salinity gradient in tidal wetlands. Pedobiologia 60, 21–26. doi: 10.1016/j.pedobi.2016.12.002
Greaver, T. L., Clark, C. M., Compton, J. E., Vallano, D., Talhelm, A. F., Weaver, C. P., et al. (2016). Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 6, 836–843. doi: 10.1038/nclimate3088
Guo, M., Wu, W., Zhou, X., Chen, Y., and Li, J. (2015a). Investigation of the dramatic changes in lake level of the Bosten Lake in northwestern China. Theor. Appl. Climatol. 119, 341–351. doi: 10.1007/s00704-014-1126-y
Guo, M., Zhou, X., Li, J., Wu, W., and Chen, Y. (2015b). Assessment of the salinization processes in the largest inland freshwater lake of China. Stoch. Environ. Res. Risk Assess. 29, 1823–1833. doi: 10.1007/s00477-014-0995-z
Han, B., Mo, L. Y., Fang, Y. T., Di, H. J., Wang, J. T., Shen, J. P., et al. (2021). Rates and microbial communities of denitrification and anammox across coastal tidal flat lands and inland paddy soils in East China. Appl. Soil Ecol. 157, 103768. doi: 10.1016/j.apsoil.2020.103768
Heinrichs, M. L., and Walker, I. R. (2006). Fossil midges and palaeosalinity: potential as indicators of hydrological balance and sea-level change. Quat. Sci. Rev. 25, 1948–1965. doi: 10.1016/j.quascirev.2006.01.022
Herbert, E. R., Boon, P., Burgin, A. J., Neubauer, S. C., Franklin, R. B., Ardon, M., et al. (2015). A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, 43. doi: 10.1890/es14-00534.1
Hou, J., Song, C., Cao, X., and Zhou, Y. (2013). Shifts between ammonia-oxidizing bacteria and archaea in relation to nitrification potential across trophic gradients in two large Chinese lakes (Lake Taihu and Lake Chaohu). Water Res. 47, 2285–2296. doi: 10.1016/j.watres.2013.01.042
Huang, J., Yang, J., Jiang, H., Wu, G., Liu, W., Wang, B., et al. (2020). Microbial responses to simulated salinization and desalinization in the sediments of the Qinghai-Tibetan lakes. Front. Microbiol. 11, 1772. doi: 10.3389/fmicb.2020.01772
Jane, S. F., Hansen, G. J. A., Kraemer, B. M., Leavitt, P. R., Mincer, J. L., North, R. L., et al. (2021). Widespread deoxygenation of temperate lakes. Nature 594, 66–70. doi: 10.1038/s41586-021-03550-y
Jeppesen, E., Brucet, S., Naselli-Flores, L., Papastergiadou, E., Stefanidis, K., Noges, T., et al. (2015). Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 750, 201–227. doi: 10.1007/s10750-014-2169-x
Jiang, X., Gao, G., Zhang, L., Tang, X., Shao, K., and Hu, Y. (2020). Denitrification and dissimilatory nitrate reduction to ammonium in freshwater lakes of the Eastern Plain, China: influences of organic carbon and algal bloom. Sci. Total Environ. 710, 136303. doi: 10.1016/j.scitotenv.2019.136303
Kana, T. M., Sullivan, M. B., Cornwell, J. C., and Groszkowski, K. M. (1998). Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol. Oceanogr. 43, 334–339. doi: 10.4319/lo.1998.43.2.0334
Koop-Jakobsen, K., and Giblin, A. E. (2010). The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnol. Oceanogr. 55, 789–802. doi: 10.4319/lo.2009.55.2.0789
Laverman, A. M., Canavan, R. W., Slomp, C. P., and Van Cappellen, P. (2007). Potential nitrate removal in a coastal freshwater sediment (Haringvliet Lake, The Netherlands) and response to salinization. Water Res. 41, 3061–3068. doi: 10.1016/j.watres.2007.04.002
Laverman, A. M., Van Cappellen, P., van Rotterdam-Los, D., Pallud, C., and Abell, J. (2006). Potential rates and pathways of microbial nitrate reduction in coastal sediments. FEMS Microbiol. Ecol. 58, 179–192. doi: 10.1111/j.1574-6941.2006.00155.x
Lin, Q., Xu, L., Hou, J., Liu, Z., Jeppesen, E., and Han, B. P. (2017). Responses of trophic structure and zooplankton community to salinity and temperature in Tibetan lakes: implication for the effect of climate warming. Water Res. 124, 618–629. doi: 10.1016/j.watres.2017.07.078
Liu, B., Li, Y., Zhang, J., Zhou, X., and Wu, C. (2014). Abundance and diversity of ammonia-oxidizing microorganisms in the sediments of Jinshan Lake. Curr. Microbiol. 69, 751–757. doi: 10.1007/s00284-014-0646-0
Liu, Y., and Bao, A. (2020). Exploring the effects of hydraulic connectivity scenarios on the spatial-temporal salinity changes in Bosten Lake through a model. Water 12, 40. doi: 10.3390/w12010040
Ma, L., Abuduwaili, J., and Liu, W. (2020). Environmentally sensitive grain-size component records and its response to climatic and anthropogenic influences in Bosten Lake region, China. Sci. Rep. 10, 1–10. doi: 10.1038/s41598-020-57921-y
Magoc, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Menberu, Z., Mogesse, B., and Reddythota, D. (2021). Evaluation of water quality and eutrophication status of Hawassa Lake based on different water quality indices. Appl. Water Sci. 11, 61. doi: 10.1007/s13201-021-01385-6
Mosley, L. M. (2015). Drought impacts on the water quality of freshwater systems; review and integration. Earth-Sci. Rev. 140, 203–214. doi: 10.1016/j.earscirev.2014.11.010
Murphy, A. E., Bulseco, A. N., Ackerman, R., Vineis, J. H., and Bowen, J. L. (2020). Sulphide addition favours respiratory ammonification (DNRA) over complete denitrification and alters the active microbial community in salt marsh sediments. Environ. Microbiol. 22, 2124–2139. doi: 10.1111/1462-2920.14969
Neubauer, S. C., Piehler, M. F., Smyth, A. R., and Franklin, R. B. (2019). Saltwater intrusion modifies microbial community structure and decreases denitrification in tidal freshwater marshes. Ecosystems 22, 912–928. doi: 10.1007/s10021-018-0312-7
Pina-Ochoa, E., and Alvarez-Cobelas, M. (2006). Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81, 111–130. doi: 10.1007/s10533-006-9033-7
Roland, F. A., Darchambeau, F., Borges, A. V., Morana, C., De Brabandere, L., Thamdrup, B., et al. (2018). Denitrification, anaerobic ammonium oxidation, and dissimilatory nitrate reduction to ammonium in an East African Great Lake (Lake Kivu). Limnol. Oceanogr. 63, 687–701. doi: 10.1002/lno.10660
Rotthauwe, J. H., Witzel, K. P., and Liesack, W. (1997). The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63, 4704–4712. doi: 10.1128/AEM.63.12.4704-4712.1997
Ruiz-Romero, E., Alcantara-Hernandez, R., Cruz-Mondragon, C., Marsch, R., Luna-Guido, M. L., and Dendooven, L. (2009). Denitrification in extreme alkaline saline soils of the former lake Texcoco. Plant Soil 319, 247–257. doi: 10.1007/s11104-008-9867-y
Rusuli, Y., Li, L., Ahmad, S., and Zhao, X. (2015). Dynamics model to simulate water and salt balance of Bosten Lake in Xinjiang, China. Environ. Earth Sci. 74, 2499–2510. doi: 10.1007/s12665-015-4257-2
Rusuli, Y., Li, L., Li, F., and Eziz, M. (2016). Water-level regulation for freshwater management of Bosten Lake in Xinjiang, China. Water Supply 16, 828–836. doi: 10.2166/ws.2016.002
Salk, K. R., Erler, D. V., Eyre, B. D., Carlson-Perret, N., and Ostrom, N. E. (2017). Unexpectedly high degree of anammox and DNRA in seagrass sediments: description and application of a revised isotope pairing technique. Geochim. Cosmochim. Acta 211, 64–78. doi: 10.1016/j.gca.2017.05.012
Seitzinger, S., Harrison, J. A., Bohlke, J. K., Bouwman, A. F., Lowrance, R., Peterson, B., et al. (2006). Denitrification across landscapes and waterscapes: a synthesis. Ecol. Appl. 16, 2064–2090. doi: 10.1890/1051-0761(2006)016(2064:dalawa)2.0.co;2
Seitzinger, S. P. (1988). Denitrification in freshwater and coastal marine ecosystems-ecological and geochemical significance. Limnol. Oceanogr. 33, 702–724. doi: 10.4319/lo.1988.33.4_part_2.0702
Shi, Y., Shen, Y., Kang, E., Li, D., Ding, Y., Zhang, G., et al. (2007). Recent and future climate change in northwest China. Clim. Change 80, 379–393. doi: 10.1007/s10584-006-9121-7
Song, G. D., Liu, S. M., Marchant, H., Kuypers, M. M. M., and Lavik, G. (2013). Anammox, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment. Biogeosciences 10, 6851–6864. doi: 10.5194/bg-10-6851-2013
Sorg, A., Bolch, T., Stoffel, M., Solomina, O., and Beniston, M. (2012). Climate change impacts on glaciers and runoff in Tien Shan (Central Asia). Nat. Clim. Chang. 2, 725–731. doi: 10.1038/nclimate1592
Sun, H., Lu, X., Yu, R., Yang, J., Liu, X., Cao, Z., et al. (2021). Eutrophication decreased CO2 but increased CH4 emissions from lake: a case study of a shallow Lake Ulansuhai. Water Res. 201, 117363. doi: 10.1016/j.watres.2021.117363
Tang, X., Xie, G., Shao, K., Sai, B., Chen, Y., and Gao, G. (2012). Influence of salinity on the bacterial community composition in Lake Bosten, a large oligosaline lake in arid northwestern China. Appl. Environ. Microbiol. 78, 4748–4751. doi: 10.1128/AEM.07806-11
Tao, S., Fang, J., Zhao, X., Zhao, S., Shen, H., Hu, H., et al. (2015). Rapid loss of lakes on the Mongolian Plateau. Proc. Natl. Acad. Sci. U. S. A. 112, 2281–2286. doi: 10.1073/pnas.1411748112
Throback, I. N., Enwall, K., Jarvis, A., and Hallin, S. (2004). Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 49, 401–417. doi: 10.1016/j.femsec.2004.04.011
Valiente, N., Carrey, R., Otero, N., Soler, A., Sanz, D., Munoz-Martin, A., et al. (2018). A multi-isotopic approach to investigate the influence of land use on nitrate removal in a highly saline lake-aquifer system. Sci. Total Environ. 631–632, 649–659. doi: 10.1016/j.scitotenv.2018.03.059
Wang, F., Yan, Z., Liu, Y., Sun, C., and Ji, M. (2020). Nitrogen removal and abundances of associated functional genes in rhizosphere and non-rhizosphere of a vertical flow constructed wetland in response to salinity. Ecol. Eng. 158, 106015. doi: 10.1016/j.ecoleng.2020.106015
Wang, H., Gilbert, J. A., Zhu, Y., and Yang, X. (2018a). Salinity is a key factor driving the nitrogen cycling in the mangrove sediment. Sci. Total Environ. 631–632, 1342–1349. doi: 10.1016/j.scitotenv.2018.03.102
Wang, J., Kan, J., Qian, G., Chen, J., Xia, Z., Zhang, X., et al. (2019). Denitrification and anammox: understanding nitrogen loss from Yangtze Estuary to the east China sea (ECS). Environ. Pollut. 252, 1659–1670. doi: 10.1016/j.envpol.2019.06.025
Wang, Y., Zhou, X., and Engel, B. (2018b). Water environment carrying capacity in Bosten Lake basin. J. Clean Prod. 199, 574–583. doi: 10.1016/j.jclepro.2018.07.202
Wik, M., Varner, R. K., Anthony, K. W., MacIntyre, S., and Bastviken, D. (2016). Climate-sensitive northern lakes and ponds are critical components of methane release. Nat. Geosci. 9, 99–105. doi: 10.1038/ngeo2578
Wollheim, W. M., Vörösmarty, C. J., Bouwman, A., Green, P., Harrison, J., Linder, E., et al. (2008). Global N removal by freshwater aquatic systems using a spatially distributed, within-basin approach. Glob. Biogeochem. Cycle 22, GB2026. doi: 10.1029/2007GB002963
Wu, Y., Xiang, Y., Wang, J., Zhong, J., He, J., and Wu, Q. L. (2010). Heterogeneity of archaeal and bacterial ammonia-oxidizing communities in Lake Taihu, China. Environ. Microbiol. Rep. 2, 569–576. doi: 10.1111/j.1758-2229.2010.00146.x
Xia, X., Liu, T., Yang, Z., Michalski, G., Liu, S., Jia, Z., et al. (2017). Enhanced nitrogen loss from rivers through coupled nitrification-denitrification caused by suspended sediment. Sci. Total Environ. 579, 47–59. doi: 10.1016/j.scitotenv.2016.10.181
Xu, W., Gao, Q., He, C., Shi, Q., Hou, Z., and Zhao, H. (2020). Using ESI FT-ICR MS to characterize dissolved organic matter in salt lakes with different salinity. Environ. Sci. Technol. 54, 12929–12937. doi: 10.1021/acs.est.0c01681
Yang, H. Q., Xu, J. H., Chen, Y. N., Li, D. H., Zuo, J. P., Zhu, N. N., et al. (2020). Has the Bosten Lake Basin been dry or wet during the climate transition in Northwest China in the past 30 years? Theor. Appl. Climatol. 141, 627–644. doi: 10.1007/s00704-020-03209-0
Yang, J. H., Zhang, Q., Lu, G. Y., Liu, X. Y., Wang, Y. H., Wang, D. W., et al. (2021). Climate transition from warm-dry to warm-wet in eastern Northwest China. Atmosphere 12, 15. doi: 10.3390/atmos12050548
Yang, Z., Lu, L., Cheng, Z., Xian, J., Yang, Y., Liu, L., et al. (2022). Dissimilatory nitrate reduction in urban lake ecosystems: a comparison study between closed and open lakes in Chengdu, China. Water Res. 214, 118218. doi: 10.1016/j.watres.2022.118218
Yao, J., Chen, Y., Guan, X., Zhao, Y., Chen, J., and Mao, W. (2022). Recent climate and hydrological changes in a mountain–basin system in Xinjiang, China. Earth-Sci. Rev. 226, 103957. doi: 10.1016/j.earscirev.2022.103957
Yapiyev, V., Sagintayev, Z., Inglezakis, V. J., Samarkhanov, K., and Verhoef, A. (2017). Essentials of endorheic basins and lakes: a review in the context of current and future water resource management and mitigation activities in Central Asia. Water 9, 798. doi: 10.3390/w9100798
Yin, G., Hou, L., Liu, M., Liu, Z., and Gardner, W. S. (2014). A novel membrane inlet mass spectrometer method to measure for isotope-enrichment experiments in aquatic ecosystems. Environ. Sci. Technol. 48, 9555–9562. doi: 10.1021/es501261s
Yu, Z., Wang, X., Zhang, E., Zhao, C., and Liu, X. (2015). Spatial distribution and sources of organic carbon in the surface sediment of Bosten Lake, China. Biogeosciences 12, 6605–6615. doi: 10.5194/bg-12-6605-2015
Zhang, G., Yao, T., Shum, C. K., Yi, S., Yang, K., Xie, H., et al. (2017). Lake volume and groundwater storage variations in Tibetan Plateau's endorheic basin. Geophys. Res. Lett. 44, 5550–5560. doi: 10.1002/2017gl073773
Zhao, D., Zeng, J., Wan, W., Liang, H., Huang, R., and Wu, Q. (2013a). Vertical distribution of ammonia-oxidizing archaea and bacteria in sediments of a eutrophic lake. Curr. Microbiol. 67, 327–332. doi: 10.1007/s00284-013-0369-7
Zhao, W., Wang, Y., Liu, S., Pan, M., Yang, J., and Chen, S. (2013b). Denitrification activities and N2O production under salt stress with varying COD/N ratios and terminal electron acceptors. Chem. Eng. J. 215, 252–260. doi: 10.1016/j.cej.2012.10.084
Zhou, H., Chen, Y., Perry, L., and Li, W. (2015). Implications of climate change for water management of an arid inland lake in Northwest China. Lake Reserv. Manag. 31, 202–213. doi: 10.1080/10402381.2015.1062834
Zhou, L., Zhou, Y., Hu, Y., Cai, J., Bai, C., Shao, K., et al. (2017). Hydraulic connectivity and evaporation control the water quality and sources of chromophoric dissolved organic matter in Lake Bosten in arid northwest China. Chemosphere 188, 608–617. doi: 10.1016/j.chemosphere.2017.09.006
Zhou, M., Butterbach-Bahl, K., Vereecken, H., and Brüggemann, N. (2016). A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Change Biol. 23:1338. doi: 10.1111/gcb.13430
Keywords: nitrate availability, salinity, arid region, Northwest China, species diversity, endorheic lake
Citation: Jiang X, Liu C, Hu Y, Shao K, Tang X, Gao G and Qin B (2022) Salinity-Linked Denitrification Potential in Endorheic Lake Bosten (China) and Its Sensitivity to Climate Change. Front. Microbiol. 13:922546. doi: 10.3389/fmicb.2022.922546
Received: 18 April 2022; Accepted: 13 June 2022;
Published: 14 July 2022.
Edited by:
Antonio Castellano-Hinojosa, University of Florida, United StatesReviewed by:
Jordi Catalan, Spanish National Research Council (CSIC), SpainMaria Cavaco, University of Alberta, Canada
Copyright © 2022 Jiang, Liu, Hu, Shao, Tang, Gao and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Gao, Z3VhbmdnYW9AbmlnbGFzLmFjLmNu
 Xingyu Jiang
Xingyu Jiang Changqing Liu
Changqing Liu Yang Hu
Yang Hu Keqiang Shao
Keqiang Shao Xiangming Tang
Xiangming Tang Guang Gao
Guang Gao Boqiang Qin
Boqiang Qin