
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 09 June 2022
Sec. Infectious Agents and Disease
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.922042
This article is part of the Research Topic Infectious Diseases in Immunocompromised Hosts View all 4 articles
 Yutaro Ohki1†
Yutaro Ohki1† Mayuko Kawabe1†
Mayuko Kawabe1† Izumi Yamamoto1*
Izumi Yamamoto1* Haruki Katsumata1
Haruki Katsumata1 Yasuyuki Nakada1
Yasuyuki Nakada1 Akimitsu Kobayashi1
Akimitsu Kobayashi1 Fumihiko Urabe2
Fumihiko Urabe2 Jun Miki2
Jun Miki2 Hiroki Yamada2
Hiroki Yamada2 Takahiro Kimura2
Takahiro Kimura2 Nanae Matsuo1
Nanae Matsuo1 Yudo Tanno1
Yudo Tanno1 Tetsuya Horino3
Tetsuya Horino3 Ichiro Ohkido1
Ichiro Ohkido1 Hiroyasu Yamamoto1
Hiroyasu Yamamoto1 Takashi Yokoo1
Takashi Yokoo1Background: The mortality rate due to COVID-19 in kidney transplant recipients (KTRs) is 16.8 to 32%. Vaccination against COVID-19 is expected to contribute to the prevention of infection, severe disease, and mortality; however, it has been reported that the humoral response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine in KTRs is poor. Vaccination strategies against COVID-19 vary from country to country, and in Japan, the third dose is given 6 months after the second dose. Few studies have evaluated long-term humoral responses after the second dose of SARS-CoV-2 mRNA vaccine. In addition, the superiority of BNT162b2 vaccine and mRNA-1,273 vaccine in KTRs regarding humoral response is controversial.
Methods: Ninety-four KTRs were administered a second dose of the BNT162b2 or mRNA-1,273 vaccines, and anti-spike (anti-S) and anti-nucleocapsid (anti-N) SARS-CoV-2 antibody levels were measured 5 months (149.2 ± 45.5 days) later. The cutoff value of anti-S antibodies was defined ≥50 AU/ml and 1.4 Index for anti-N antibodies. The primary outcome was the rate of seropositivity, and factors associated with an appropriate humoral response were assessed by univariate and multivariate analysis.
Results: Of 94 KTRs, only 45 (47.9%) patients were positive for anti-S antibodies. The median anti-S SARS-CoV-2 IgG antibody titers was 35.3 (Interquartile range 3.8 to 159.7). Anti-N SARS-CoV-2 IgG antibodies in all patients were < 1.4 Index. Response to SARS-CoV-2 mRNA vaccines were 43.2 and 65% for BNT162b2 and mRNA-1,273, respectively (p = 0.152). In comparison with high-dose, low-dose of mycophenolic acid was a robust factor associated with an adequate humoral response.
Conclusion: The long-term humoral response after a second dose of SARS-CoV-2 mRNA vaccine in Japanese KTRs was poor. In comparison with high-dose, low-dose mycophenolic acid was related to an appropriate humoral response. Five months is too long to wait for a 3rd dose after 2nd dose of SARS-CoV-2 vaccine in KTRs. In this cohort, there was no statistical difference in humoral response to the BNT162b2 and mRNA-1,273 vaccines. Additional large observational studies and meta-analyses are needed to clarify the factors related to an appropriate humoral immune response to COVID-19 vaccination.
COVID-19, first reported in Wuhan, China, in December 2019, is an infectious disease caused by severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) (Wu and McGoogan, 2020). The alpha and gamma strains of COVID-19 were the first variants of concern (VoCs) and have been replaced by the omicron strain. In Japan, BNT162b2 and mRNA-1,273 vaccines were approved on 14 February 14 2021 and 21 May, 2021, respectively, and vaccination has been vigorously promoted. Unfortunately, although kidney transplant recipients (KTRs) are vulnerable to COVID-19, the rate of appropriate response to a first dose of these vaccines in KTRs is 17% (Boyarsky et al., 2021a), and after the second dose is 46% (Benotmane et al., 2021b; Boyarsky et al., 2021b; Cucchiari et al., 2021; Grupper et al., 2021; Rozen-Zvi et al., 2021; Russo et al., 2022). Therefore, some countries have started a third vaccination for KTRs, the effectiveness of which has been demonstrated (Benotmane et al., 2021a; Bertrand et al., 2021; Hall et al., 2021; Kamar et al., 2021). Vaccination strategies against COVID-19 vary from country to country, and in Japan, the third dose is given 6 months after the second dose.
Few studies have evaluated long-term (average 5 months) humoral responses after the second dose of SARS-CoV-2 mRNA vaccine. Therefore, we analyzed the long-term humoral response after a second dose of SARS-CoV-2 mRNA vaccine in Japanese KTRs and identified the associated factors.
Ninety-four KTRs attending the Division of Nephrology and Hypertension at Jikei University Hospital (Tokyo, Japan) were enrolled in this prospective observational study. The BNT162b2 or mRNA-1,273 vaccine was administered twice to all patients. The vaccine type was not assigned in our hospital. Blood samples were collected during the visit, and the anti-spike (anti-S) SARS-CoV-2 IgG (cutoff value ≥50.0 AU/ml) and anti-nucleocapsid (anti-N) SARS-CoV-2 IgG (cutoff value 1.40 Index) levels were measured using SARS-CoV-2 IgG II Quant Kits (Abbott©) 5 months (149.2 ± 45.5 days) after the second dose. Previous studies have established the usefulness of the assay used here: cutoff values of ≥50 AU/ml for anti-S SARS-CoV-2 IgG and 1.4 for anti-N SARS-CoV-2 IgG, respectively, have been shown to clearly stratify SARS-CoV-2 recent infected patients and vaccinated patient (Narasimhan et al., 2021). Age, sex, body mass index (BMI), primary kidney disease leading to end-stage kidney disease, and past medical history were obtained from the medical records. Medication information, including antihypertensive drugs, immunosuppressants, and iron or 1-α-OH-D3 supplementation, was obtained from prescription records. Routine biochemical measurements included serum albumin, creatinine, as well as complete blood counts. The serum samples were stored at −80°C prior to use. The Ethics Committee of the School of Medicine of Jikei University [approval number 33–314(10934)] approved the study protocol. Written informed consent was obtained from all patients prior to inclusion in the study. Study procedures were performed in accordance with the Declaration of Helsinki and its revisions.
Based on the anti-S SARS-CoV-2 IgG antibody cutoff values, the cohort was divided into antibody-positive (≥ 50 AU/ml) and -negative groups (< 50 AU/ml). Continuous variables with normal and non-normal distributions were compared by unpaired t-tests and Mann–Whitney tests, respectively. Dichotomous variables were compared between the groups by chi-squared tests. Data are presented as means and standard deviations (SDs) for continuous variables with normal distributions or medians and interquartile ranges (IQR) for continuous variables with non-normal distributions. The dosage of mycophenolate mofetil (MMF) varies from patient to patient because the dosage is adjusted according to the development of infection after transplantation. TAC, on the other hand, is treated as a dichotomous variable because its dosage is determined by measuring blood TAC target levels. For methylprednisolone, the dose is constant at 5 mg every other day for all patients 1 year after transplantation in our institution. Therefore, only MMF is treated as a continuous variable. Factors associated with an appropriate humoral response were evaluated using univariate and multivariate logistic regression models. To confirm the same trend with a different statistical model, univariate and multivariate liner regression models were performed to identify factors independently associated with anti-S SARS-CoV-2 IgG titers. Anti-S SARS-CoV-2 IgG antibody titers were pre-processed by converting to common logarithms (log10) to adjust for normality of distribution. The risks were evaluated by odd ratios (OR) and coefficients, such as β with 95% confidence intervals (95% CI). Differences with a two-sided value of p < 0.05 were considered significant. Data were analyzed using Stata 17.0 (Stata Corp LP, College Station, TX).
The mean age was 53.7 ± 11.7 years, males comprised 67% of the study population, and the mean time elapsed since kidney transplantation was 10.7 (IQR = 4.4 to 16.9) years. The frequencies of BNT162b2 and mRNA-1,273 vaccination were 79.6 and 20.4%, respectively. The anti-S SARS-CoV-2 IgG antibody titers are shown in Figure 1. The median titer was 27.5 (IQR = 3.0 to 153.0) in BNT162b2 and 97.1 (IQR = 17.0 to 369.2) in mRNA-1,273, respectively. It was no significant differences in anti-S SARS-CoV-2 IgG titers by vaccine types (p = 0.09). Overall, the median SARS-CoV-2 IgG S antibody titer was 35.3 (IQR = 3.8 to 159.7), and the appropriate humoral response rate was 47.8% using a cutoff value of ≥50.0 AU/ml. The subjects were divided into two groups according to immunogenicity, and the clinical characteristics of the two groups are shown in Table 1. There were significant differences between the two groups in time since last transplant, donor age, methylprednisolone, mycophenolic acid, mycophenolic acid dose, hemoglobin, lymphocyte count, serum creatinine, iron, and 1α-OH-D3 use. The percentages of subjects with an adequate humoral response to SARS-CoV-2 were 43.2 and 65% for BNT162b2 and mRNA-1,273 vaccines, respectively (p = 0.152). Anti-N SARS-CoV-2 IgG antibodies in all patients were < 1.4 Index.
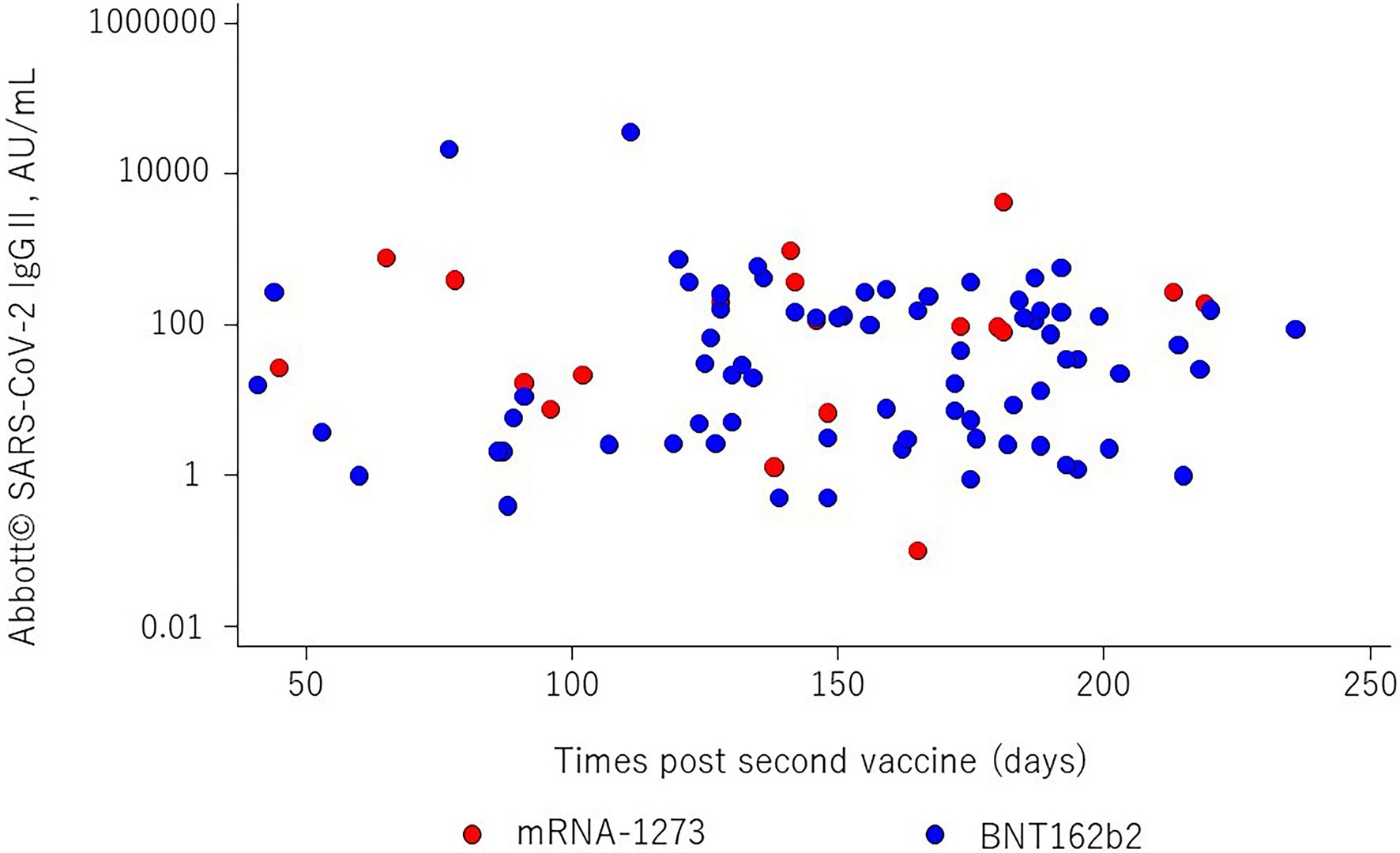
Figure 1. The relationship between SARS-CoV-2 IgG II antibody titers and times post second vaccine (days). The red and blue dots show mRNA-1,273 and BNT162b2, respectively. There is no statistical difference between two vaccines (p = 0.09).
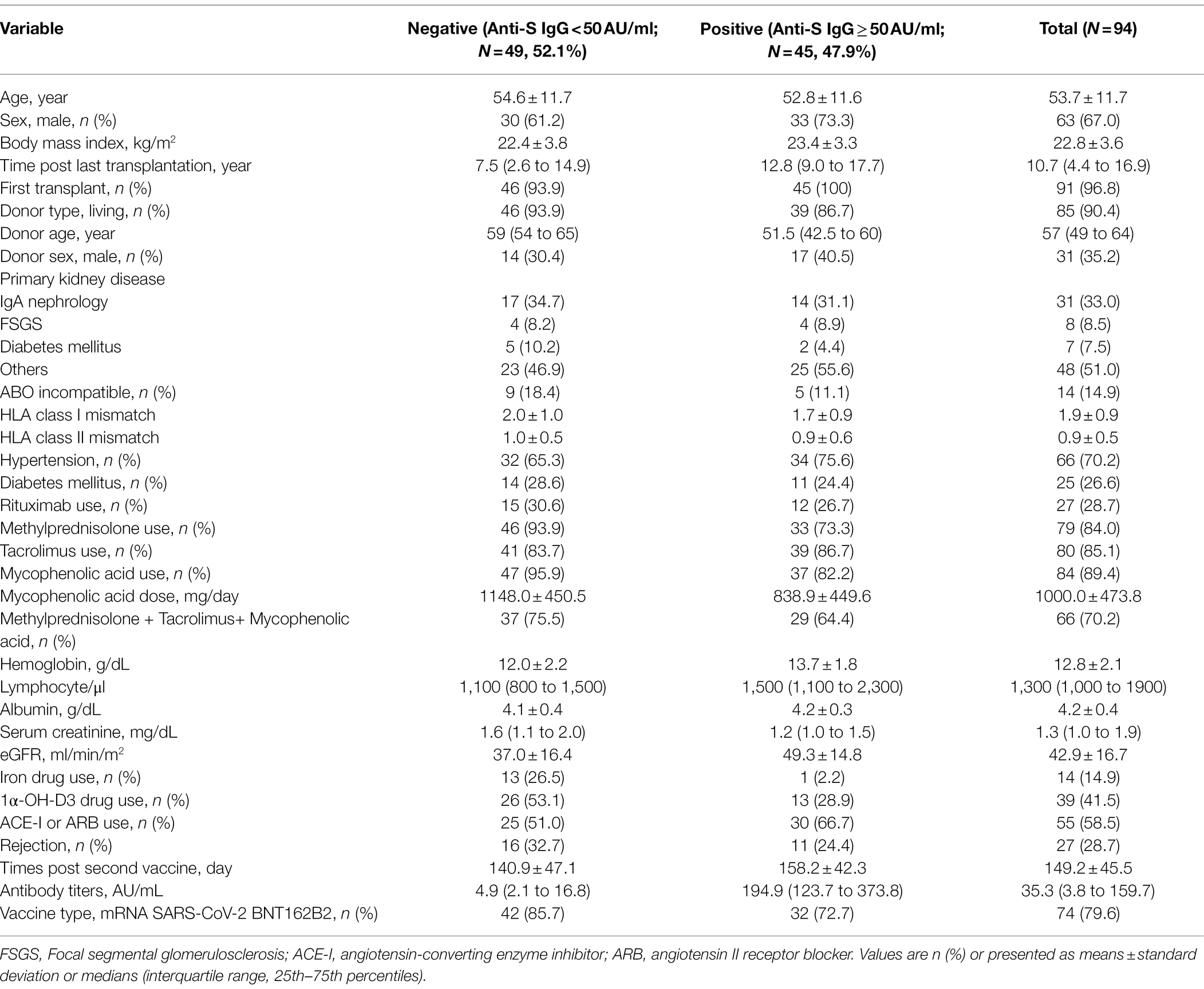
Table 1. Clinical characteristics of patients in kidney transplant recipients by anti-S SARS-CoV-2 antibody positivities.
In a multivariate logistic regression model, mycophenolic acid dose (OR = 0.998, 95% CI = 0.996 to 1.000), methylprednisolone (OR = 0.14, 95% CI = 0.02 to 0.85), high lymphocyte count (OR = 1.001, 95% CI = 1.000 to 1.002), and high hemoglobin level (OR = 1.67, 95% CI = 1.10 to 2.54) were associated with an appropriate humoral response (Table 2). Multivariate linear regression analysis showed that mycophenolic acid dose (β = −0.0004 95%, CI = −0.0009 to −0.00002), BMI (β = 0.06, 95% CI = 0.01 to 0.11), and time elapsed since the second dose (β = −0.005, 95% CI = −0.009 to 0.0002) were associated with an appropriate humoral response (Table 3).
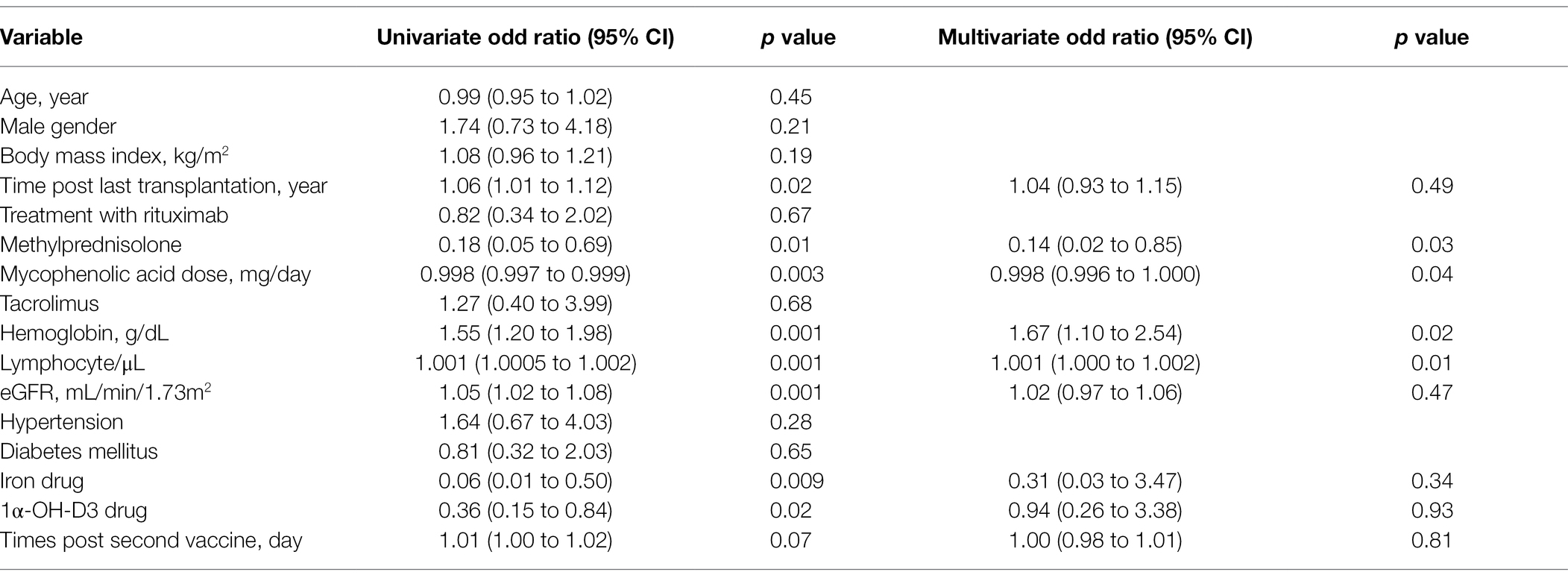
Table 2. Factors associated with antibody response by univariate and multivariate logistic regression models.
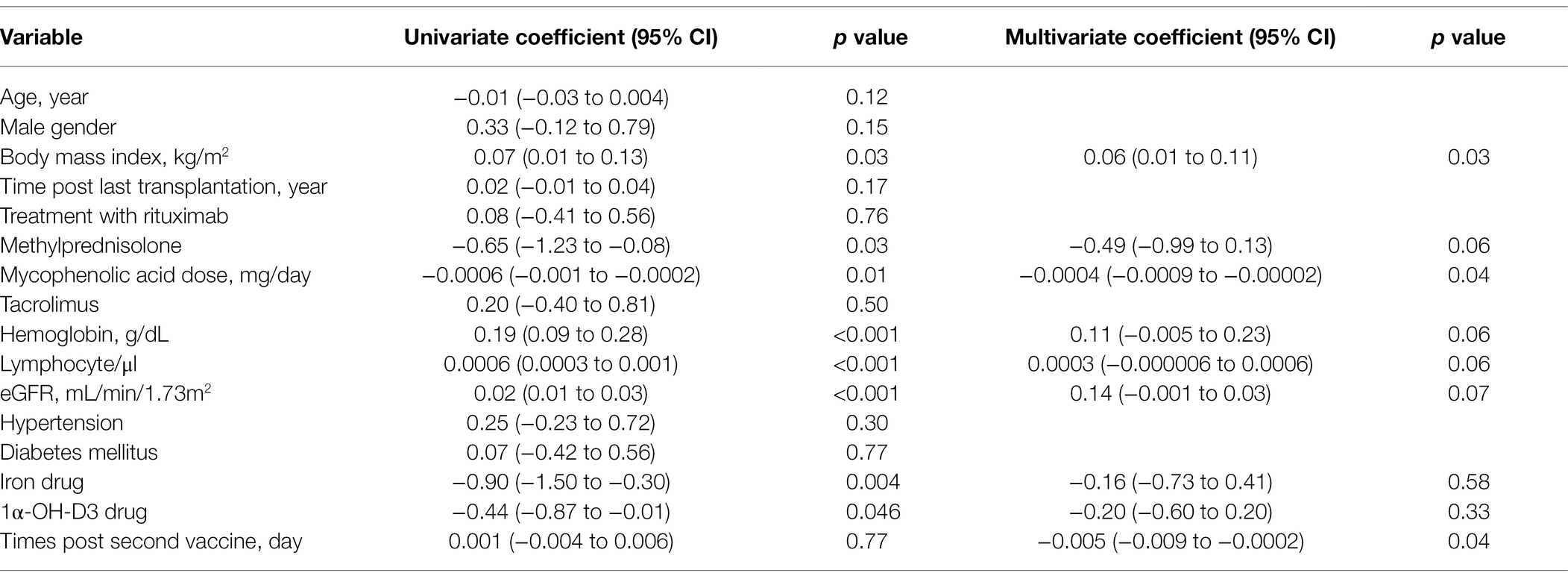
Table 3. Factors associated with anti-S SARS-CoV-2 antibody levels by univariate and multivariate liner regression models.
The total number of kidney transplants in Japan is 36,373, and the number of living patients is approximately 22,000 according to the 2020 Factbook of the Japanese Society of Transplantation (Fact Book, 2020). For KTRs, the COVID-19 mortality rate is reportedly 22 to 32%, higher than in the general population (Cravedi et al., 2020; Coll et al., 2021; De Meester et al., 2021). In the general population, BNT162b2 and mRNA-1,273 vaccines have an efficacy rate of 92 to 94.1% (Baden et al., 2021; Dagan et al., 2021). However, in KTRs, the rate of an appropriate humoral response is only 30.0 to 52.4% at 1 month after the second dose, and the antibody titer is low (Benotmane et al., 2021b; Boyarsky et al., 2021b; Cucchiari et al., 2021; Grupper et al., 2021; Rozen-Zvi et al., 2021; Russo et al., 2022). On this basis, the United States, Canada, Germany, and France gave priority to KTRs for a third dose. In Germany and Canada, an appropriate humoral response was observed in 61.2 and 60% of KTRs at 1 month after the third dose, respectively (Bertrand et al., 2021; Hall et al., 2021). In France, an appropriate humoral response was observed in 44 to 49% of KTRs (who did not respond to a second dose) at 1 month after the third dose (Benotmane et al., 2021a; Kamar et al., 2021). In this study, the long-term appropriate humoral response rate was 47.8% and the anti-S SARS-CoV-2 IgG antibody titers was very low at 35.3 (IQR = 3.8 to 159.7) AU/mL at 5 months (149.2 ± 45.5 days) after the second dose. Similarly, the appropriate response rate was 42% in Israel (Ben-Dov et al., 2022). Therefore, third doses should be administered to Japanese KTRs. In Japan, as of 1 March, 2022, the rate of third-dose vaccination is 20.4%, which does not differ according to pre-existing condition. In France, administration of a fourth dose elicited an appropriate humoral response in KTRs (Benotmane et al., 2022). The two vaccines may be less effective against omicron; however, additional doses elicit a response (Garcia-Beltran et al., 2022). Indeed, the CDC recommends a fourth dose for KTRs (Centre for Disease Control and Prevention, 2021).
The factors associated with an appropriate humoral response in a multivariate logistic model were a low mycophenolic acid dose, non-use of methylprednisolone, a high hemoglobin level, and a high lymphocyte count. The factors identified in a multivariate linear regression model were mycophenolic acid dose, BMI, and time elapsed since the second dose. Therefore, only a low mycophenolic acid dose was associated with an appropriate humoral response in KTRs. A high lymphocyte count (Ben-Dov et al., 2022) and fewer types of immunosuppressants (Grupper et al., 2021; Rozen-Zvi et al., 2021), are, as would be expected, reportedly associated with the lack of an appropriate humoral immune response. Data on the associations of hemoglobin and BMI with a humoral response in KTRs are sparse. Advanced age, decreased renal function, steroid pulse therapy, diabetes, history of cancer, serum ferritin level, and serum 25(OH)VitD level have also been reported to be associated with an inappropriate humoral immune response (Grupper et al., 2021; Rozen-Zvi et al., 2021; Ben-Dov et al., 2022). Although these factors were not associated with an appropriate humoral response in this study, analysis of a larger number of cases might have yielded associations.
The antibody positivity rate was not significantly different between the two vaccines. The rate of an appropriate humoral response rate for the BNT162b2 and mRNA-1,273 vaccines in KTRs was 46% (Boyarsky et al., 2021b) and 48% (Benotmane et al., 2021b) at 1 month after the second dose, 61.2% (Bertrand et al., 2021) and 60% (Hall et al., 2021) at 1 month after the third dose, and 44% (Kamar et al., 2021) and 49% (Benotmane et al., 2021a) in those who did not show an appropriate response to the second dose, respectively. These data were obtained from subjects of different races and nationalities. By contrast, the mRNA-1,273 vaccine reportedly has a higher antibody positivity rate than the BNT162b2 vaccine (Wijtvliet et al., 2021; Haller et al., 2022; Marinaki et al., 2022). Thus, additional studies are needed to evaluate the rates of an appropriate humoral response between the two vaccines.
The limitations of this study include its single-center design and small number of cases. In addition, we focused on antibody titers and did not evaluate cellular immunity or the link between antibody titer and prevention of infection or severe disease. Heterogeneity in antibody titer measurement techniques frankly limits quantitative comparison of results from different studies. Further study of the effects of third and fourth doses on antibody titers and the prevention of infection and severe disease is warranted.
In conclusion, less than half of KTRs had an appropriate humoral response and the antibody titer was low at 149.2 ± 45.5 days after the second dose of the mRNA SARS-Cov-2 vaccine. As in other countries, 5 months is too long to wait for a 3rd dose after 2nd dose of SARS-CoV-2 vaccine in KTRs. A third or fourth dose of the mRNA SARS-Cov-2 vaccine may be necessary in KTRs, who are susceptible to severe disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Committee of the School of Medicine of Jikei University [approval number 33–314(10934)] approved the study protocol. The patients/participants provided their written informed consent to participate in this study.
YO, MK, IY, and HY participated in the clinical practice, designed the study protocol, and drafted the manuscript. TY, HK, YN, AK, NM, YT, TH, and IO participated in the patient’s care and revised the manuscript. FU, JM, HY, and TK performed the kidney transplantation. TY is the divisional directors and supervised each author. All authors contributed to the preparation of the manuscript and approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Teruko Tamatsukuri from the Division of Nephrology and Hypertension, Jikei University School of Medicine for their expert assistance.
Baden, L. R., El Sahly, H. M., Essink, B., Kotloff, K., Frey, S., Novak, R., et al. (2021). Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl. J. Med. 384, 403–416. doi: 10.1056/NEJMoa2035389
Ben-Dov, I. Z., Oster, Y., Tzukert, K., Alster, T., Bader, R., Israeli, R., et al. (2022). Impact of tozinameran (BNT162b2) mRNA vaccine on kidney transplant and chronic dialysis patients: 5 months (149.2 ±45.5 days) months follow-up. J. Nephrol. 35, 153–164. doi: 10.1007/s40620-021-01210-y
Benotmane, I., Bruel, T., Planas, D., Fafi-Kremer, S., Schwartz, O., and Caillard, S. (2022). A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the delta variant in kidney transplant recipients. Kidney Int. 101, 1073–1076. doi: 10.1016/j.kint.2022.02.011
Benotmane, I., Gautier, G., Perrin, P., Olagne, J., Cognard, N., Fafi-Kremer, S., et al. (2021a). Antibodies response After a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients With minimal serologic response to 2 doses. JAMA 23:1065. doi: 10.1001/jama.2021.12339
Benotmane, I., Gautier-Vargas, G., Cognard, N., Olagne, J., Heibel, F., Braun-Parvez, L., et al. (2021b). Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 99, 1498–1500. doi: 10.1016/j.kint.2021.04.005
Bertrand, D., Hamzaoui, M., Lemée, V., Lamulle, J., Laurent, C., Etienne, I., et al. (2021). Antibodies and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 100, 1337–1340. doi: 10.1016/j.kint.2021.09.014
Boyarsky, B. J., Werbel, W. A., Avery, R. K., Tobian, A. A. R., Massie, A. B., Segev, D. L., et al. (2021a). Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 325, 1784–1786. doi: 10.1001/jama.2021.4385
Boyarsky, B. J., Werbel, W. A., Avery, R. K., Tobian, A. A. R., Massie, A. B., Segev, D. L., et al. (2021b). Antibodies response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325, 2204–2206. doi: 10.1001/jama.2021.7489
Centre for Disease Control and Prevention (2021). Available Online at: https://www.cdc.gov/coronavirus/2019cov/vaccines/recommendations/immuno.html (Accessed on March 5, 2022).
Coll, E., Fernández-Ruiz, M., Sánchez-Álvarez, J. E., Martínez-Fernández, J. R., Crespo, M., Gayoso, J., et al. (2021). COVID-19 in transplant recipients: The Spanish experience. Am. J. Transplant. 21, 1825–1837. doi: 10.1111/ajt.16369
Cravedi, P., Mothi, S. S., Azzi, Y., Haverly, M., Farouk, S. S., Pérez-Sáez, M. J., et al. (2020). COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am. J. Transplant. 20, 3140–3148. doi: 10.1111/ajt.16185
Cucchiari, D., Egri, N., Bodro, M., Herrera, S., Del Risco-Zevallos, J., Casals-Urquiza, J., et al. (2021). Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 21, 2727–2739. doi: 10.1111/ajt.16701
Dagan, N., Barda, N., Kepten, E., Miron, O., Perchik, S., Katz, M. A., et al. (2021). BNT162b2 mRNA Covid-19 vaccine in a Nationwide mass vaccination setting. N. Engl. J. Med. 384, 1412–1423. doi: 10.1056/NEJMoa2101765
De Meester, J., De Bacquer, D., Naesens, M., Meijers, B., Couttenye, M. M., and De Vriese, A. S. (2021). Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: A Regionwide registry study. J. Am. Soc. Nephrol. 32, 385–396. doi: 10.1681/ASN.2020060875
Fact Book (2020). On Organ Transplantation in Japan. Available online at: http://www.asas.or.jp/jst/pdf/factbook/factbook2020.pdf (Accessed on 5 March, 2022).
Garcia-Beltran, W. F., St Denis, K. J., Hoelzemer, A., Lam, E. C., Nitido, A. D., Sheehan, M. L., et al. (2022). mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell 185, 457–466. doi: 10.1016/j.cell.2021.12.033
Grupper, A., Rabinowich, L., Schwartz, D., Schwartz, I. F., Ben-Yehoyada, M., Shashar, M., et al. (2021). Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 21, 2719–2726. doi: 10.1111/ajt.16615
Hall, V. G., Ferreira, V. H., Ku, T., Ierullo, M., Majchrzak-Kita, B., Chaparro, C., et al. (2021). Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N. Engl. J. Med. 385, 1244–1246. doi: 10.1056/NEJMc2111462
Haller, M. C., Kaiser, R. A., Langthaler, S., Brandstetter, C., Apfalter, P., Kerschner, H., et al. (2022). Comparison of mRNA-1273 and BNT162b2 SARS-CoV-2 mRNA vaccine immunogenicity in kidney transplant recipients. Transpl. Int. 35:10026. doi: 10.3389/ti.2021.10026
Kamar, N., Abravanel, F., Marion, O., Couat, C., Izopet, J., and Del Bello, A. (2021). Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 385, 661–662. doi: 10.1056/NEJMc2108861
Marinaki, S., Degiannis, D., Roussos, S., Xagas, E., Tsoutsoura, P., Adamopoulos, S., et al. (2022). Head-to-head comparison of response rates to the two mRNA SARS-CοV-2 vaccines in a large cohort of solid organ transplant (SOT) recipients. Vaccines 10:190. doi: 10.3390/vaccines10020190
Narasimhan, M., Mahimainathan, L., Araj, E., Clark, A. E., Markantonis, J., Green, A., et al. (2021). Clinical evaluation of the Abbott Alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J. Clin. Microbiol. 59:e0038821. doi: 10.1128/JCM.00388-21
Rozen-Zvi, B., Yahav, D., Agur, T., Zingerman, B., Ben-Zvi, H., Atamna, A., et al. (2021). Antibodies response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin. Microbiol. Infect. 27, 1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028
Russo, G., Lai, Q., Poli, L., Perrone, M. P., Gaeta, A., Rossi, M., et al. (2022). SARS-COV-2 vaccination with BNT162B2 in renal transplant patients: risk factors for impaired response and immunological implications. Clin. Transpl. 36:e14495. doi: 10.1111/ctr.14495
Wijtvliet, V. P. W. M., Ariën, K. K., Abrams, S., Couttenye, M. M., Mestrez, F., Mariën, J., et al. (2021). mRNA-1273 vaccine (Moderna): a better option than BNT162b2 (Pfizer) in kidney transplant recipients and dialysis patients? Nephrol. Dial. Transplant. 37, 799–803. doi: 10.1093/ndt/gfab352
Keywords: SARS-CoV-2 vaccine, COVID-19, kidney transplant recipients, humoral response, anti-S SARS-CoV-2 IgG antibody titers
Citation: Ohki Y, Kawabe M, Yamamoto I, Katsumata H, Nakada Y, Kobayashi A, Urabe F, Miki J, Yamada H, Kimura T, Matsuo N, Tanno Y, Horino T, Ohkido I, Yamamoto H and Yokoo T (2022) Long-Term Humoral Response After a Second Dose of SARS-CoV-2 mRNA Vaccine in Japanese Kidney Transplant Recipients. Front. Microbiol. 13:922042. doi: 10.3389/fmicb.2022.922042
Received: 17 April 2022; Accepted: 19 May 2022;
Published: 09 June 2022.
Edited by:
Wenjuan Wu, Tongji University School of Medicine, ChinaReviewed by:
Andrew Karaba, Johns Hopkins University, United StatesCopyright © 2022 Ohki, Kawabe, Yamamoto, Katsumata, Nakada, Kobayashi, Urabe, Miki, Yamada, Kimura, Matsuo, Tanno, Horino, Ohkido, Yamamoto and Yokoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Izumi Yamamoto, aXp1bWkyNkBqaWtlaS5hYy5qcA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.