
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 08 August 2022
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.919111
Gut microbiota plays a vital role in maintaining the health and immunity of wild birds. However, less is known about the comparison of fecal microbiota between different ecological groups of wild birds, particularly in the Yellow River National Wetland in Baotou, China, an important transit point for birds migrating all over the East Asia-Australian and Central Asian flyways. In this study, we characterized the fecal microbiota and potential microbial function in nine bird species of raptors, waders, and waterfowl using 16S rRNA gene amplicon sequencing to reveal the microbiota differences and interaction patterns. The results indicated that there was no significant difference in α-diversity, but a significant difference in β-diversity between the three groups of birds. The fecal bacterial microbiota was dominated by Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes in all groups of birds. Furthermore, we identified five bacterial genera that were significantly higher in raptors, five genera that were significantly higher in waders, and two genera that were more abundant in waterfowl. The bacterial co-occurrence network results revealed 15 and 26 key genera in raptors and waterfowls, respectively. The microbial network in waterfowl exhibited a stronger correlation pattern than that in raptors. PICRUSt2 predictions indicated that fecal bacterial function was significantly enriched in the antibiotic biosynthesis pathway in all three groups. Metabolic pathways related to cell motility (bacterial chemotaxis and flagellar assembly) were significantly more abundant in raptors than in waders, whereas waders were enriched in lipid metabolism (synthesis and degradation of ketone bodies and fatty acid biosynthesis). The fecal microbiota in waterfowl harbored more abundant vitamin B6 metabolism, RNA polymerase, and tyrosine and tryptophan biosynthesis. This comparative study revealed the microbial community structure, microbial co-occurrence patterns, and potential functions, providing a better understanding of the ecology and conservation of wild birds. Future studies may focus on unraveling metagenomic functions and dynamics along with the migration routine or different seasons by metagenomics or metatranscriptomics.
The coevolution of vertebrates and their gut microbiota can maintain homeostasis and improve the host dietary niches (Ley et al., 2008). An increasing amount of data from humans, cows, mice, chickens, insects, and fish has provided insight into the vital influence of gut microbes on host health, nutrition, immunity, and morphology (Muegge et al., 2011; Engel and Moran, 2013; Jing et al., 2021; Stressmann et al., 2021). Previous studies on the gut microbiota of bird species have focused on the effects of specific bacteria or bacterial pathogens, demonstrating the essential role of the gut microbiota (Benson et al., 2010). However, most of these studies have focused on only one or a few bird species. Few studies have compared the microbial community composition of bird species in different ecological groups, such as raptors, waders, and waterfowl, limiting our understanding of microbiota composition in multiple bird species.
Birds exhibit the most diverse range of ecological functions compared with other vertebrates and have a highly evolved lineage that is necessary for ecosystems (Zhan et al., 2017). Growing evidence has revealed that the dynamic gut microbial community of birds to adapt to their environment is affected by many factors such as host evolution, habitat environment, and human activity (Grond et al., 2019; Capunitan et al., 2020; San Juan et al., 2020). Additionally, the diet has been revealed as a major factor in the gut microbial composition in non-passerine birds (Xiao et al., 2021). A single host gene can have a significant effect on the diversity and structure of the gut microbiota (Spor et al., 2011). Furthermore, it has been shown that the gut microbiota of Darwin finches in the Galapagos Islands could be influenced by human activity (Knutie et al., 2019). Moreover, the habitat could also impact the gut microbiota of long-distance migratory swan geese (Wu et al., 2018). The gut microbiota of swan geese maintains a core group of species after long-distance migration, and it was found that host phylogeny is one of the main factors influencing the gut microbial community (Waite and Taylor, 2015). These studies provide a basic theoretical background for understanding gut microbiota and its function in different types of wild birds.
The significance of identifying the gut microbial community is that it provides a scientific basis for protecting endangered animals. Previous studies have confirmed that Proteobacteria is a diagnostic biomarker of reproductive dysfunction in crested ibises due to the abundance of Proteobacteria, and alpha-diversity indices were higher in sterile crested ibises than in healthy crested ibises (Ran et al., 2021). Similarly, the crested ibis chick growth rate was negatively associated with gut microbiota diversity and negatively correlated with the abundance of Dietziaceae, Halomonadaceae, Corynebacteriaceae, and Streptococci (Zhu et al., 2021). Furthermore, compared with the captive group of Alpine musk deer (Moschus chrysogaster), the Firmicutes-rich gut microbiota in the wild group enables individuals to maximize their energy intake from the cellulose, ensuring them to adapt to the wild environments (Sun et al., 2019). In addition, the fatal colibacillosis by MDR ESBL-producing Escherichia coli can threaten the critically endangered Brazilian merganser’s health, and in Orca whales, the potentially antibiotic-resistant pathogenic strains of Escherichia coli could contribute to the ongoing decline of this critically endangered population (Melendez et al., 2019; Fuentes-Castillo et al., 2021). Potential risks to human health will also be revealed by antibiotic-resistant bacteria in migratory birds because most wild birds are natural carriers of pathogenic bacteria (Cao et al., 2020). It is of significant importance to prevent the spread of infectious diseases in wild birds by studying the gut microbial composition (Zhao et al., 2018). The Yellow River National Wetland in Baotou is an important foraging location, which is a crossroad between the East Asia-Australian and Central Asian flyways, for migratory birds (Liu et al., 2019). A large number of different wild bird species inhabit this place. However, in recent years, the number of wild birds has declined owing to environmental changes and human activities. Therefore, it is urgently necessary to understand the gut microbiota structure and function in different wild birds in this region, providing an avenue for exploring the potential exchange of microbiota between different bird species and ecological groups.
In this study, we analyzed the fecal bacterial microbiota of three ecological bird groups (i.e., raptors, waders, and waterfowl), covering two endangered bird species, four national second-level protected species in China, and three common species at the Wildlife Conservation Center of Baotou (Inner Mongolia, China). Fecal microbial composition was identified by 16S rRNA gene amplicon sequencing, and microbiota function was predicted using PICRSUt2. The purpose of this study was to (i) reveal the fecal microbiota community structure, (ii) explore the microbial co-occurrence patterns, and (iii) compare the microbial potential function between raptors, waders, and waterfowl. This research enhances our understanding of the gut microbiota in different ecological groups of wild birds and provides novel insights into endangered bird conservation.
All 38 fresh fecal samples were collected from three ecological groups of raptors (MQ), waders (SQ), and waterfowl (YQ), covering nine bird species, including endangered species of cinereous vulture (Aegypius monachus) and steppe eagle (Aquila nipalensis); the national second-level protected species in China, such as upland buzzard (Buteo hemilasius), common kestrel (Falco tinnunculus), demoiselle crane (Anthropoides virgo), and Eurasian Eagle-owl (Bubo bubo); common species of greylag goose (Anser anser), ruddy shelduck (Tadorna ferruginea), and black swan (Cygnus atratus) at the Wildlife Conservation Center of Baotou (Inner Mongolia, China) in August 2021 (Supplementary Table 1). The samples in this study were collected from wild birds (either wounded wings or wounded legs), which were rescued and protected at the Wildlife Conservation Center of Baotou. Fresh fecal samples were collected as soon as the birds arrived at the wildlife conservation center. The birds that met the following conditions were sampled: (i) fresh wounds, (ii) minor injuries, and (iii) no diseases. Groups of waders and waterfowl bird species had deposited stools. Only the middle layer of the fecal ball was collected from these different individuals. Freshly dropped raptor samples were collected by scraping or syringe suction from the surface (Becker et al., 2020). Samples were immediately transferred into 5-ml sterile tubes, placed on dry ice, and stored at −80°C until further processing.
Genomic DNA was extracted from all the samples using the CTAB method (Honore-Bouakline et al., 2003). The extracted DNA was analyzed using a NANODROP LITE spectrophotometer (Thermo Scientific, United States) to evaluate DNA quantity and quality. We used the universal primers (341F [5′-CCTAYGGGRBGCASCAG-3′] and 806R [5′-GGACTACNNGGGTATCTAAT-3′]) to amplify the 16S rRNA gene V3–V4 region with 6 bp barcode unique to each sample. PCR was performed with 15 μl of Phusion High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM of forward and reverse primers, and 10 ng of template DNA. The PCR conditions were as follows: initial denaturation at 98°C for 1 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30 s, extension at 72°C for 30 s, and a final extension step at 72°C for 5 min. The PCR products were pooled and purified using the Qiagen Gel Extraction Kit (Qiagen, Germany). Illumina TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, United States) was used to produce sequencing libraries according to the manufacturer’s recommended protocol. After the detection of library quality, samples were sequenced using the Illumina NovaSeq platform with 250 bp paired-ended running mode. The sequencing service was provided by Novogene Co., Ltd., Tianjin, China. Raw reads were deposited and are available through the Sequence Read Archive under accession number SRR18553438.
All raw paired-end sequences were imported to the QIIME2 pipeline (version 2020.8.0) (Bolyen et al., 2019). Primers were removed using the Cutadapt plugin by “qiime cutadapt trim-paired” (–p-minimum-length 200). The DADA2 plugin (“qiime dada2 denoise-paired”) was used to generate denoised feature sequences (amplicon sequence variants, ASVs) and feature tables (-p-trim-left-f 0 –p-trim-left-r 0 –p-trunc-len-f 235 –p-trunc-len-r 220) (Callahan et al., 2016). Feature sequences with frequency ≤ 4 were discarded using “qiime feature-table filter-features –p-min-frequency 4.” Reference sequences were extracted from the SILVA database (release 132) using specific primers for the 16S V3-V4 region using “qiime feature-classifier extract-reads –p-min-length 200 –p-max-length 500” (Quast et al., 2013). The Naive Bayes classifier was trained for taxonomic annotation by the command line of “qiime feature-classifier fit-classifier-naive-bayes.” The ASVs assigned to mitochondria and chloroplast were excluded from the feature table (“qiime taxa filter-table –p-exclude mitochondria, chloroplast”) and feature sequences (“qiime taxa filter-seqs –p-exclude mitochondria, chloroplast”). We use the PICRUSt2 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) software1 to predict the functional abundance of microbiota (Douglas et al., 2020).
Alpha-diversity indices of microbiota (Chao1, Simpson, and Shannon) were calculated using the command line of “qiime diversity alpha.” These indices between groups were compared using the non-parametric Wilcoxon rank-sum test. Principle coordinate analysis (PCoA) plot was generated based on the Bray–Curtis distance. Permutational analysis of variance (PERMANOVA) was applied to test group differences based on Bray–Curtis distance matrix using vegan package (Oksanen et al., 2007). LEfSe (Linear Discriminant Analysis Effect Size) was used to test the difference in taxa abundance (LDA score ≥ 4.0 and p < 0.05) and functional abundance (LDA score ≥ 3.0 and p < 0.05) (Segata et al., 2011). The interaction network was constructed using a genus present in ≥60% of samples in MQ and YQ, respectively. Briefly, (i) we calculated the correlation coefficient using SparCC algorithm (Watts et al., 2019), (ii) the statistical significance of correlations was calculated from 1,000 bootstrap iterations, (iii) network (| SparCC| > 0.5 and adjusted p < 0.05) property calculation and module detection were employed using the igraph package, and (iv) Network visualization was performed using the Cystoscope 3.7.1 software (Shannon et al., 2003).
After quality control, a total of 2,139,722 effective sequences were obtained from all samples, with an average of 76,387 ± 4,908 (mean ± SD) in each sample (Supplementary Table 2). These sequences were denoised using DADA2 to obtain 6,807 amplicon sequence variants (ASVs). The rarefaction curve revealed an adequate sequence depth for covering the fecal bacterial community in birds based on the number of observed features and the Shannon index (Supplementary Figure 1). To determine the variations in fecal microbiota in MQ, SQ, and YQ, we characterized alpha diversity using the Chao1, Shannon, and Simpson indices. The results showed no significant differences among the three groups of birds (Figures 1A–C). The total number of ASVs in the MQ and YQ was significantly higher than that in the SQ (Supplementary Figure 1A). Although there was no significant difference in alpha diversity among the three groups of birds, Venn analysis indicated that MQ and YQ possessed 1,554 and 1,229 unique ASVs, respectively, more than that of SQ (387). Only 399 common ASVs were found in all three bird groups (Figure 1D). Principal coordinates analysis (PCoA) showed group-based separation of microbial communities based on Bray–Curtis dissimilarity (Figure 1E). PERMANOVA also verified that each ecological group had a significantly different microbial community structure (YQ vs. MQ: R2 = 0.288, p < 0.05; YQ vs. SQ: R2 = 0.200, p < 0.05; MQ vs. SQ: R2 = 0.319, p < 0.05). Moreover, there was a significant difference between the nine bird species (Supplementary Figure 2 and Supplementary Table 3).
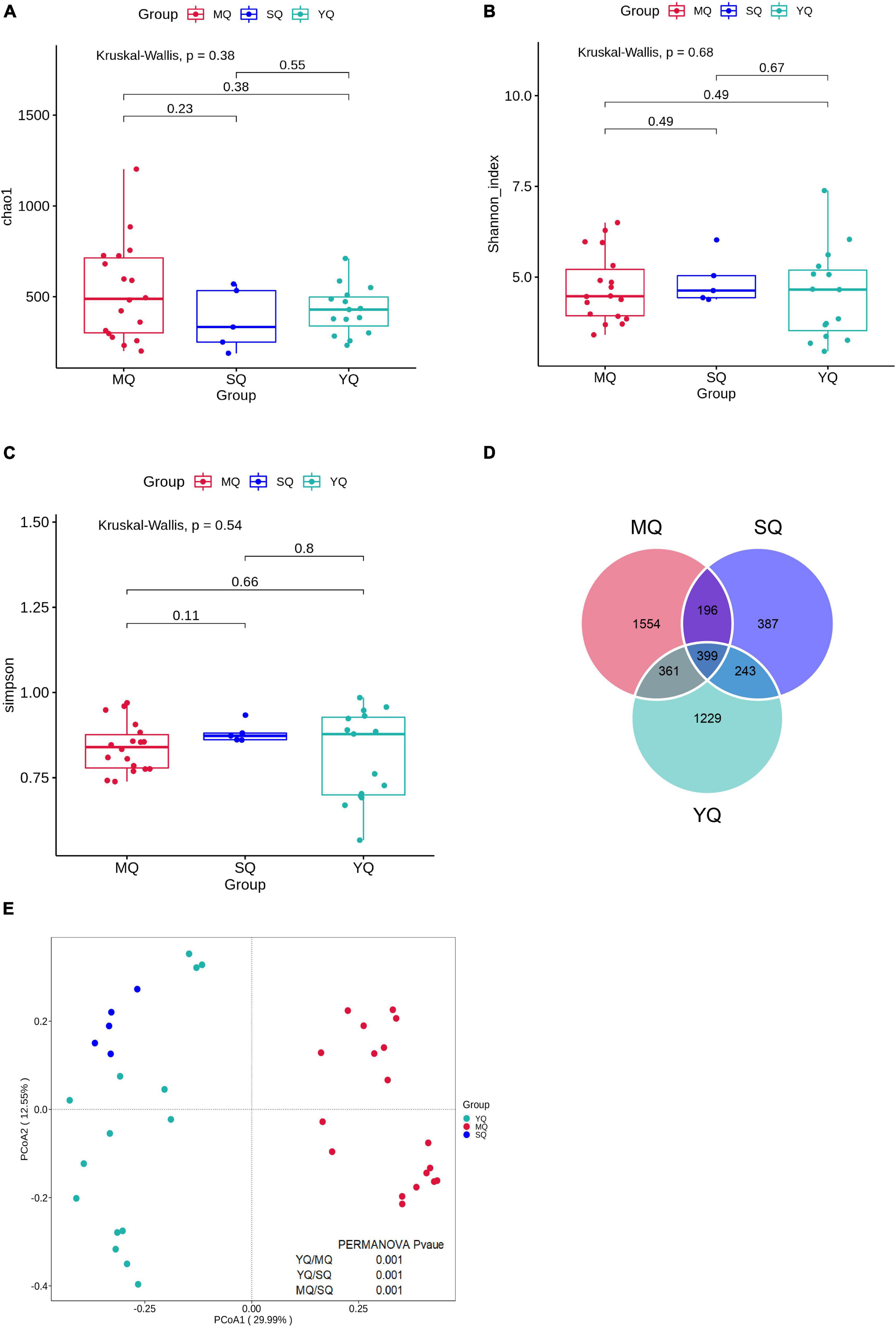
Figure 1. Alpha and beta diversity of fecal microbiota among three ecological groups of birds (MQ, SQ, and YQ). Alpha diversity was characterized by Chao1 index (A), Shannon index (B), and Simpson indices (C). Solid horizontal line within a box represents the median, the dots indicate the observed value, the box margins are the interquartile range (50% of the observations), and whisker lines extend for 1.5 times the interquartile range. There was no significant difference in Chao1 index, Shannon index, and Simpson indices (p > 0.05). Venn diagram of ASVs overlapping across MQ, SQ, and YQ based on ASV presence and absence (D). Principal coordinate analysis of fecal bacterial communities from the three groups of birds (E). MQ, raptors; SQ, waders; YQ, waterfowl.
All the ASVs were assigned to 40 phyla, 103 classes, 235 orders, 387 families, and 815 genera (including unclassified entries). We analyzed the bacterial composition of the fecal samples at the phylum and genus levels. The dominant phyla in the three ecological groups of birds were Firmicutes (70.49% in MQ, 77.71% in SQ, 80.55% in YQ), Proteobacteria (18.69%, 4.87%, 7.21%), Actinobacteria (6.02%, 15.09%, 9.71%), and Bacteroidetes (2.15%, 1.15%, 0.86%) (Figure 2A). As is shown in Figure 2B (Supplementary Table 4), the genus of Clostridium sensu stricto 1 occupied 28.16% in MQ, but only about 1.12% and 1.34% in the other two groups of SQ and YQ, respectively. We also observed increased genera in MQ but decreased genera in the other two groups, namely, Escherichia-Shigella and Paeniclostridium. The top four genera in the SQ were Weissella (45.01%), Corynebacterium 1 (8.91%), Lactobacillus (8.33%), and Staphylococcus (5.79%) (Supplementary Table 4). YQ was dominated by Romboutsia (17.68%), Turicibacter (16.05%), Streptococcus (15.50%), and Weissella (13.08%).
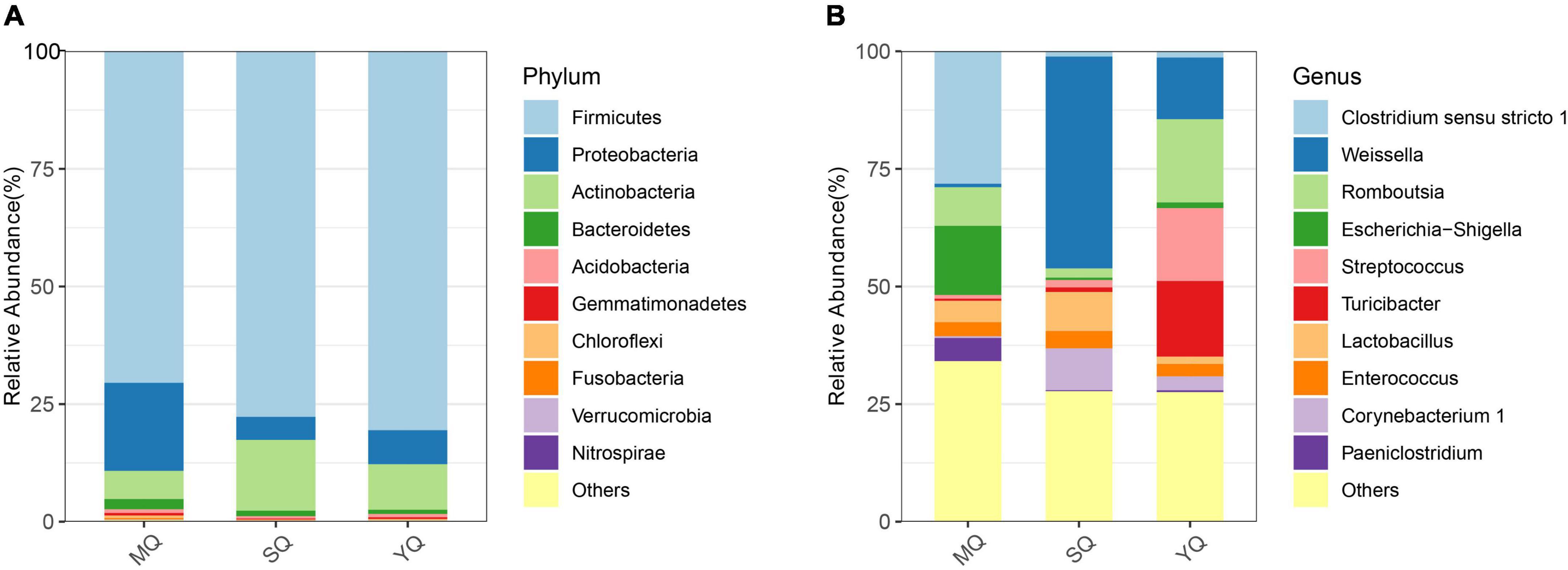
Figure 2. Bar chart of relative abundance. The relative abundance (%) of the top 10 abundant bacteria phyla (A) and genera (B) among the three ecological bird groups. The dominant phyla in three groups of birds consisted of Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes. Others, bacteria taxa with ≤1% abundance. MQ, raptors; SQ, waders; YQ, waterfowl.
The resulting network in MQ consisted of 78 nodes and 250 edges, with an average degree of 6.41. The clustering coefficient was 0.52, and network modularity was 0.66. We detected five modules in the MQ group, in which MQ-M5 was a highly connected sub-network (Figure 3A). The MQ-M5 module, mainly assigned to Firmicutes, had the highest degree (≥10) of nodes, defined as key nodes in this study, including the uncommonly seen genera Hathewaya (0.77%), Clostridium sensu stricto 4 (0.26%), Clostridium sensu stricto 15 (0.80%), Macrococcus (0.19%), uncultured Actinomycetales bacterium (0.15%), Pelagibius (0.05%), and Ilumatobacter (0.04%). However, the high relative abundances of genera, such as Clostridium sensu stricto 1 (28.2%), Escherichia-Shigella (14.7%), Romboutsia (8.22%), Paeniclostridium (4.90%), and Lactobacillus (4.53%), were low-degree nodes. Most of the key nodes had a substantial proportion of intra-module degree (>60%) and a small proportion of intra-module degree (Supplementary Table 5), indicating a tight intra-connection and sparse inter-connection in the MQ microbiota interaction network. The network in YQ contained 71 nodes and 286 edges with an average degree of 8.06, a clustering coefficient of 0.60, and a modularity of 0.61 (Figure 3B). Both MQ and YQ networks exhibited clustered topologies and modular structures. There were 26 key nodes in the YQ network (Supplementary Table 6). In YQ-M1 (mostly assigned to Proteobacteria and Firmicutes), the nodes of Klebsiella (0.80%) and Enterococcus (2.68%) had a large degree number of 15 and 14, respectively. Enterococcus was particularly associated with YQ-M3, in which most nodes belonged to Proteobacteria, Firmicutes, and Actinobacteria. YQ-M3 contained nine key nodes, among which Brevibacterium (relative abundance of 0.503%) had the largest degree number of 15, and Corynebacterium 1 was observed for the highest relative abundance of 2.99%. YQ-M4, which was mainly mapped to Firmicutes, was also a large module with nine key nodes. Interestingly, the modules in the bacterial network of YQ were stronger than those in MQ based on the proportion of inter-module degree (Supplementary Table 6), such as YQ-M1, YQ-M3, YQ-M5, YQ-M2, YQ-M4, and YQ-M5. Detailed statistical information and negative associations between the microbial co-occurrence networks in raptors and waterfowl are shown in Supplementary Figure 3 and Supplementary Tables 7, 8.
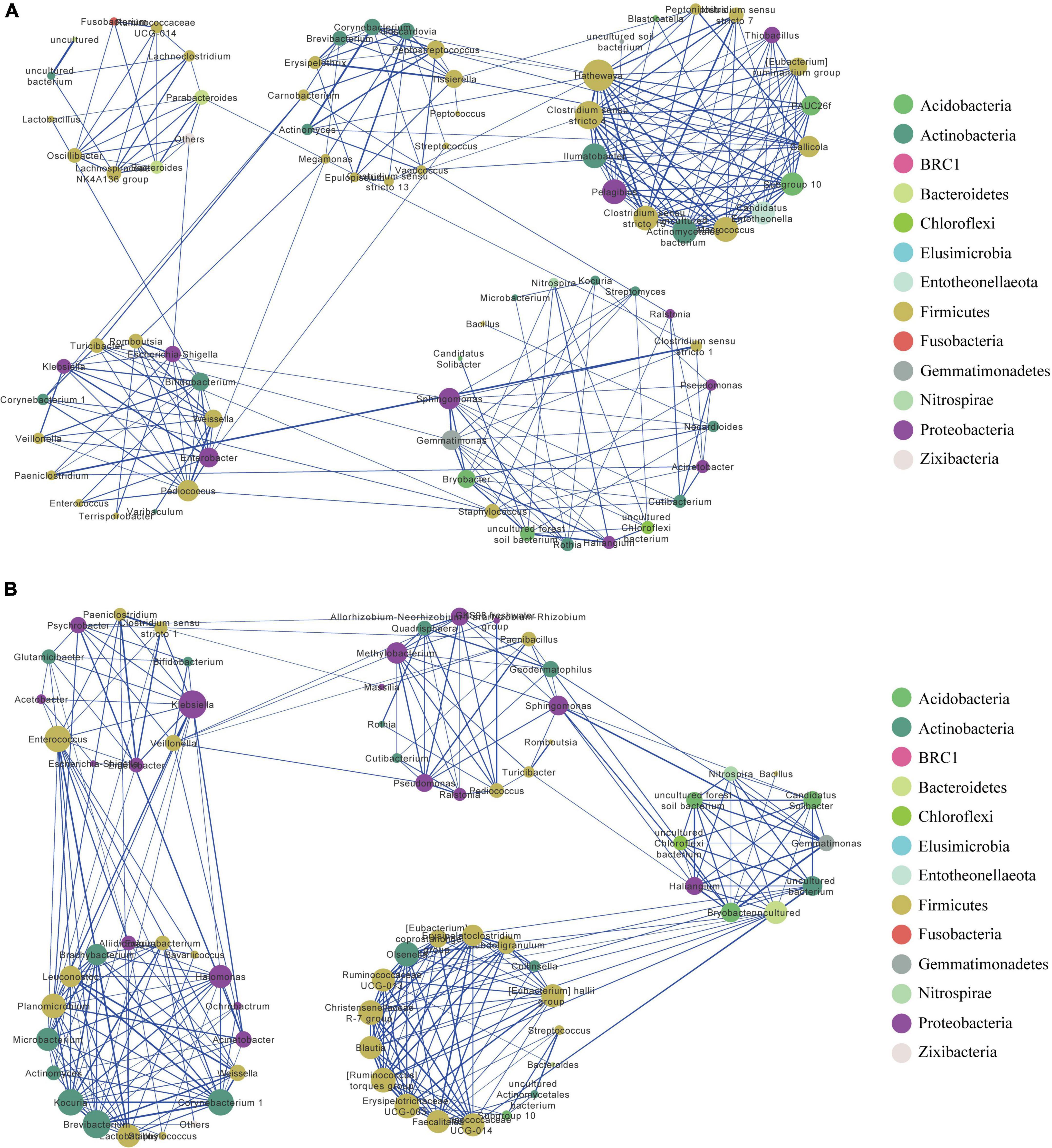
Figure 3. Bacterial co-occurrence network in MQ (A) and YQ (B) based on positive correlation analysis at the genus level. Nodes correspond to genus and edges to the correlation. Node size is proportional to the degree number. Node color represents the associated phylum for each genus. Edge width displays the strength of correlation. The blue edge indicates a positive correlation. Each large circle (e.g., MQ-M1 or YQ-M1) represents a module detected by Louvain method. MQ, raptors; SQ, waders; YQ, waterfowl.
Linear discriminant analysis effect size was performed to reveal differences in the bacterial microbiota between MQ, SQ, and YQ. The results showed that the biomarkers at the phylum level were Actinobacteria in SQ and Proteobacteria in MQ (LDA score ≥ 4, p < 0.05) (Figure 4A). At the genus level, we observed biomarkers for 12 taxa, namely, Turicibacter, Romboutsia, Weissella, Corynebacterium_1, Lactobacillus, Staphylococcus, Pediococcus, Clostridium_sensu_stricto_1, Escherichia_Shigella, Paeniclostridium, Micavibrionaceae.g_uncultured, and Epulopiscium (LDA ≥ 4.0, p < 0.05) (Supplementary Table 9). Among these 12 taxa, five genera were significantly higher in MQ, five were higher in SQ, and two were higher in YQ (Figure 4B). Moreover, there were five, six, nine, and nine significantly different taxa at the class, order, family, and species levels, respectively (Supplementary Figure 4).
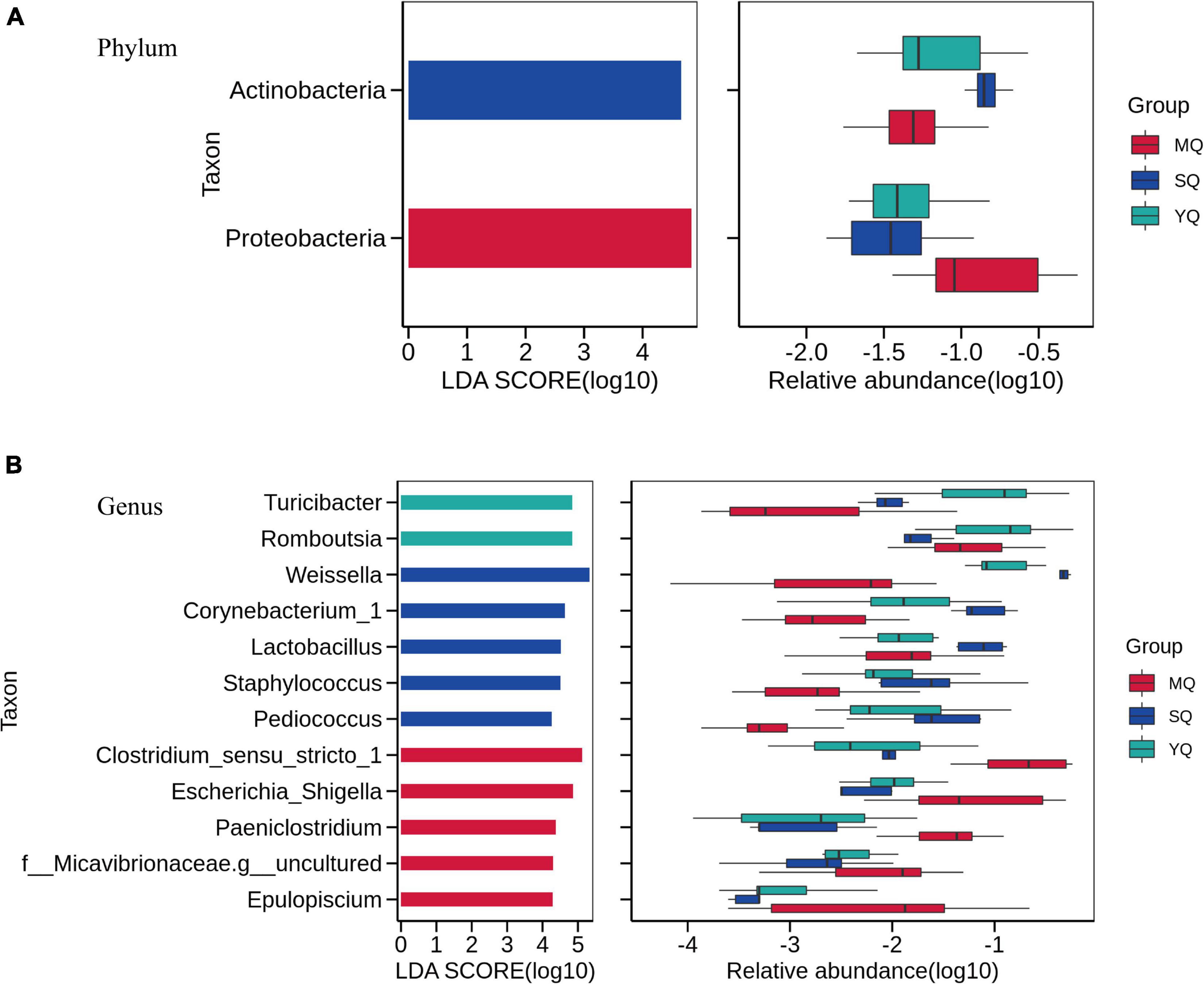
Figure 4. Linear discriminant analysis effect size (LEfSe) analysis. The linear discriminant analysis identified significantly different taxon between MQ, SQ, and YQ at the phylum (A) and genus (B) levels with a threshold of LDA score ≥ 4.0 and p < 0.05. Left, logarithm score of LDA analysis for each taxon. Right, relative abundance of different taxon; MQ, raptors; SQ, waders; YQ, waterfowl; LDA, linear discriminant analysis; LEfSe, LDA Effect Size.
To explore the functional profiling of the bacterial microbiota in different ecological bird groups, PIPCRUSt2 was used based on a 16S rRNA gene marker. PCoA results indicated significant clustering by diverse groups of birds based on the predicted KEGG ortholog (KOs) abundance (Figure 5A, YQ vs. MQ: R2 = 0.271, p < 0.01; YQ vs. SQ: R2 = 0.166, p < 0.01; MQ vs. SQ: R2 = 0.398, p < 0.01), which was consistent with the microbial community structure (Figure 1E). To determine the difference in metabolic potential in the fecal microbiota, the KEGG pathway was analyzed. LEfSe results (LDA score ≥ 3.0, p < 0.05) showed that the enriched functional classes in MQ mainly included biosynthesis of ansamycins (ko01051), bacterial chemotaxis (ko02030), and flagellar assembly (ko02040). The YQ group was dominated by vitamin B6 metabolism (ko00750); streptomycin biosynthesis (ko00521); RNA polymerase (ko03020); and phenylalanine, tyrosine, and tryptophan biosynthesis (ko00400). Functional pathways involved in the biosynthesis of vancomycin group antibiotics (ko01055), synthesis and degradation of ketone bodies (ko00072), and fatty acid biosynthesis (ko00061) were upregulated in the SQ group. In summary, there was a significant difference in fecal microbiota function among the three groups of birds (Figure 5B).
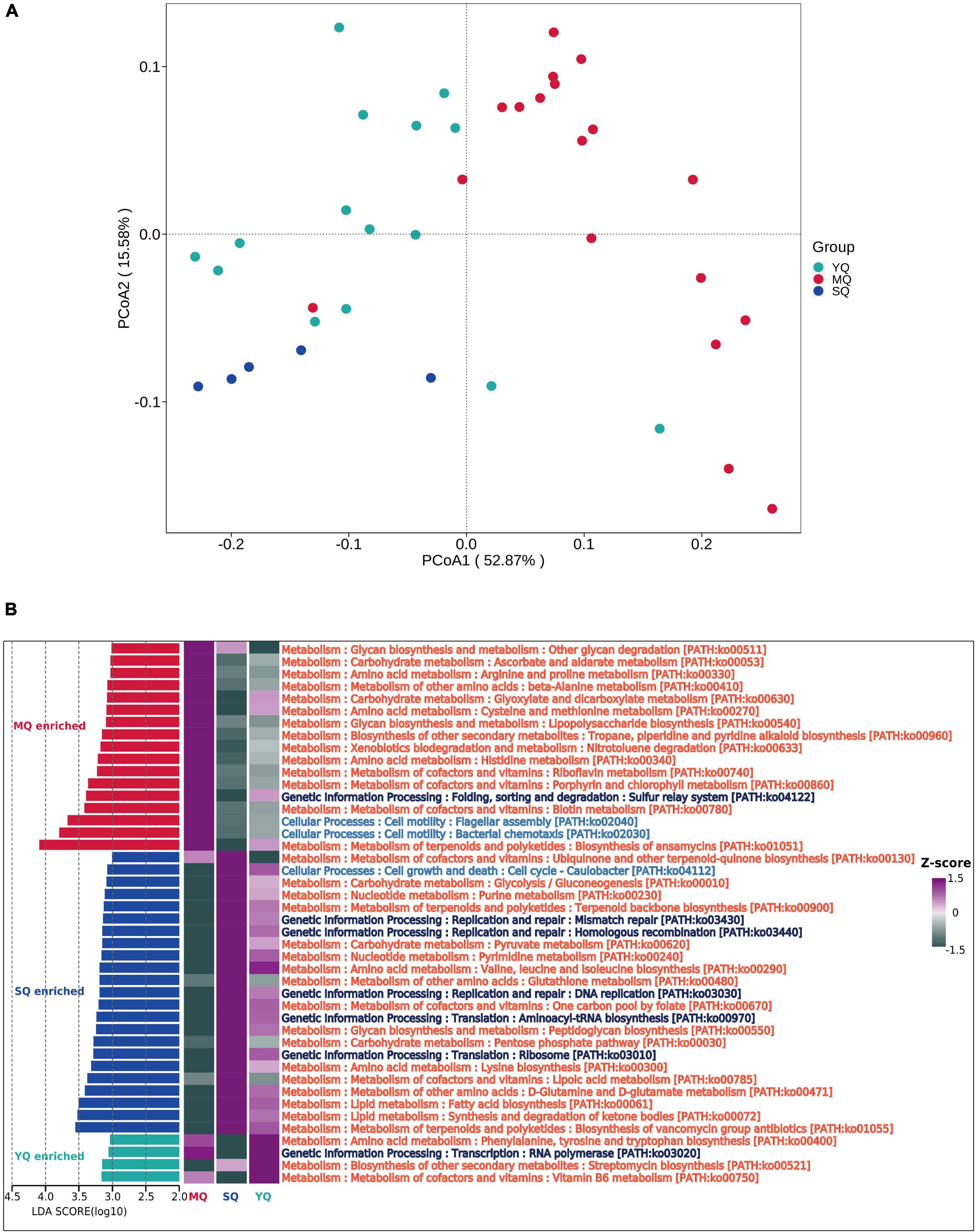
Figure 5. Microbial functional difference analysis between groups. (A) KOs principal coordinate analysis (PCoA) based on Bray–Curtis distance of predicted KO abundance. (B) Difference in the KEGG pathway identified by LEfSe (LDA score ≥ 3.0 and p < 0.05) between MQ, SQ, and YQ. Heat maps of differential pathways enrichment analysis based on KEGG. MQ, raptors; SQ, waders; YQ, waterfowl.
Gut microbiota exerts a major influence on food digestion, metabolism regulation, and immune protection in wild animals (Nicholson et al., 2012; Valdes et al., 2018). Both host species and diet have been suggested to be potential drivers modulating the gut microbial composition of wild animals (Xiao et al., 2021). The Yellow River National Wetland in Baotou, China, is an indispensable feeding station for migratory birds between the East Asian-Australian and Central Asian flyways (Liu et al., 2022). Thus, we selected wild birds to study the differences in the composition of the gut microbiota.
In this study, we compared the fecal microbiota composition of raptors, waders, and waterfowls. Although live in the Yellow River National Wetland in Baotou, these ecological birds have different habitat preferences. Waders have physical and behavioral adaptations for living near water. They live in waterflow areas, are strong swimmers, and spend much of their time on ponds, lakes, or rivers. Raptors can be found in tundra, desert, forests, and grasslands. Different living habit makes birds have different dietary preferences. It was observed that there was an insignificant difference in alpha diversity, but a significant difference in beta diversity, showing consistent species richness and evenness within fecal microbial communities in the three groups of birds, but a considerable divergence in microbial profiles between these groups. Our data showed that Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes were the dominant phyla, which is supported by several previous studies (Xenoulis et al., 2010; Waite et al., 2012; Dewar et al., 2013, 2014; Wang et al., 2016a,b; Zhao et al., 2017). Firmicutes can provide an energy source to hosts through the metabolism of polysaccharides, sugars, carbohydrates, and fatty acids (Flint et al., 2008; Tap et al., 2009). Most Actinobacteria are widely distributed in water and soil. These bacteria are easily transmitted to birds, suggesting that the bacterial community structure of birds is influenced by external bacterial transmission (Mikaelyan et al., 2015). Actinobacteria, which dominant in waders, are closely related to host fiber intake (Lee et al., 2015). This is consistent with the fact that the waders and waterfowl dietary preference of plants and grains. Carnivorous raptors are often regarded as pathogen vectors (Zhou et al., 2020). Consistent with previous studies, our research showed that the dominant flora of the gut microbiota of raptors were Proteobacteria, which have higher relative abundances than waders and waterfowl. It has been reported that Proteobacteria harbors the most microbial pathogens, such as Escherichia, Shigella, and Paeniclostridium, which were also detected in this study in raptors and could cause enteric disease in animals or diarrhea in humans (Kotloff et al., 2013; Nyaoke et al., 2020). Epulopiscium spp., which are dominant in the gut of raptors, are a group of large gram-positive bacteria that have a symbiotic relationship with their hosts and generally inhabit the gut of fish (Angert, 2021). Clostridium sensu stricto 1, also a significantly higher proportion of genera in raptors, is mostly a strictly anaerobic, fermenting bacteria. Clostridium perfringens, a member of Clostridium sensu stricto 1, was more abundant than any other member in this study (Supplementary Figure 4). C. perfringens is a rapidly growing opportunistic pathogen associated with intestinal diseases in humans and animals that secretes more than 20 virulent toxins (Kiu and Hall, 2018). In the fecal microbiota of waders and waterfowl, most genera, including Turicibacter, Romboutsia, Weissella, and Lactobacillus, were demonstrated as commensal bacteria, which were mainly driven by a diet with fiber-rich plant and grains, to maintain host health status and provide a host energy source by inhibiting inflammatory reactions, antimicrobial activity, or specific metabolic capability (Liu et al., 2014; Zhong et al., 2015; Milani et al., 2016; Ricaboni et al., 2016; Hernandez-Oaxaca et al., 2021). Besides, the usual habitat, migration paths, and host evolutionary also impact on the gut bacterial composition of birds (Loo et al., 2019; Lee et al., 2020; Skeen et al., 2021). Nevertheless, further exploration is still necessary to ensure taxonomic accuracy due to the low resolution of amplicon sequencing.
Correlation-based network analysis offers new insights into microbial community structure and its co-occurrence patterns, showing ecological interactions (Yeung et al., 2021). Modularity is an important indicator of ecosystem stability and resilience (Sharaf et al., 2019). The modularity of the microbial network in waders was greater than that in raptors, which may be the cause of the number of pathogens in the raptor gut. Node degree (or connectivity) is simply a measurement of the activity or importance it represents in a network (Wasserman and Faust, 1994). Hathewaya limosa (Clostridium limosum), which is usually found in animals and birds, is a pathogen that causes inflammatory diseases (Cato et al., 1970; Bistrom et al., 2016). In this study, there was a large degree of both positive and negative interaction networks in raptors (Supplementary Tables 5, 7). Clostridium sensu stricto 4 (also called Clostridium cluster IV or Clostridium leptum group) can produce short-chain fatty acids, and its decrease is associated with the expansion of gut pathogens (Livanos et al., 2018). We observed that Clostridium sensu stricto 4 had several negative connections with potential pathogens in raptor feces, such as Escherichia-Shigella, Paeniclostridium, Staphylococcus, Klebsiella, and Enterobacter (Supplementary Figure 3A). This may be due to the balance between beneficial microbes and pathogens, which maintains the stability of gut microecology. Additionally, there were also some pathogens, such as Klebsiella and Enterococcus, dominating the connectivity in the positive correlation network of waders (Figure 3B), which might be due to polluted water or soil. We speculated that these pathogens had an impact on the microbial co-occurrence patterns in the ecological bird groups. The clustering coefficient is typically used to describe the hierarchical properties of a network, representing how nodes are embedded in their neighborhood (Deng et al., 2012). This index in the microbial network of raptors was higher than that in waders, indicating a higher co-association among microbes in the gut of waders.
Although many microbial pathogens inhabit the guts of wild birds, the birds themselves are not affected by infection. It might be that the antimicrobial agents produced by the host or some commensal bacteria maintain a biological balance. This hypothesis was verified by the predicted microbial function in this study, in which we found that the pathways of antibiotic biosynthesis were enriched in the fecal microbiota of all ecological bird groups, such as ansamycins in raptors, streptomycin in waterfowl, and vancomycin in waders. Moreover, cell motility-related pathways, mainly flagellar assembly and bacterial chemotaxis, were overrepresented in raptor fecal microbiota, which may be driven by a high-fat diet (Hildebrandt et al., 2009). Ketone bodies are usually produced from the breakdown of fatty acids by ketogenesis in the liver during low food intake, carbohydrate-restrictive diets, or host starvation (Rui, 2014). This could offer an energy source for the host brain (Hasselbalch et al., 1994). The gut microbiota driven by ketone bodies also plays a key role in recovering the host immune imbalance (Cabrera-Mulero et al., 2019). Vitamin B6, an essential nutrient for animal hosts, is vital for regulating amino acid balance. However, the animals cannot produce it themselves, and it mainly comes from intestinal bacteria and diet. Inflammatory diseases occur due to vitamin B6 deficiency (Rall and Meydani, 1993). Additionally, RNA polymerase was upregulated in waterfowl, indicating the high activity of transcription in the fecal microbiota. The predicted functional differences in the microbiota may be driven by the different microbiota profiles induced by different diet compositions among raptors, waders, and waterfowl. Given the influence of pathogens on microbiota composition and microbial interaction patterns, it is urgent to accurately identify the pathogens inhabiting wild birds and to develop new strategies to reduce these pathogens.
In conclusion, we comprehensively examined the fecal microbiota, co-occurrence patterns, and microbiota function in different ecological groups of wild birds using amplicon sequencing and multi-statistical analysis. The differences in microbiota composition, microbiota function, and co-occurrence patterns were associated with diet composition, which may be due to habitat preferences. However, several limitations of this research should be noted: (i) sample collection of wild birds should be increased; (ii) advanced technologies such as metagenomics or metatranscriptomics should be adopted to explore the microbial functional genes, particularly for the pathogens; and (iii) the effect of dietary composition and environment on microbial community structure in wild birds should be quantified. In summary, this research on the fecal bacterial microbiota in raptors, waders, and waterfowl provides valuable insights into the microbial diversity and potential pathogens and provides theoretical knowledge for the protection of endangered animals.
The datasets generated for this study can be found in the NCBI/BioProject/PRJNA821628 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA821628).
The Animal Ethics and Welfare Committee (AEWC) of the Wildlife Conservation Center of Baotou and the College of Biological Science and Technology of Baotou Teachers’ College supported the progress of the project.
CZ and LL designed and performed the experiments. CZ and LG collected the fecal samples. CZ and LB analyzed the data and wrote the manuscript. All authors contributed to the article and approved the final manuscript.
Funds were provided by the High-Level Talents Introduced Scientific Research Startup Fund Project of Baotou Teacher’s College (Nos. BTTCRCQD2020-004 and BTTCRCQD2020-005) and Yellow River National Wetland Ecological Protection and High-Quality Development Research Institute of Baotou Teacher’s College (No. BSYHY202218).
We thank Shuxian Liu, Wildlife Conservation Center of Baotou, for helping with sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.919111/full#supplementary-material
Angert, E. R. (2021). Challenges Faced by Highly Polyploid Bacteria with Limits on DNA Inheritance. Genome Biol. Evol. 13:evab037. doi: 10.1093/gbe/evab037
Becker, A., Harrison, S. W. R., Whitehouse-Tedd, G., Budd, J. A., and Whitehouse-Tedd, K. M. (2020). Integrating Gut Bacterial Diversity and Captive Husbandry to Optimize Vulture Conservation. Front. Microbiol. 11:1025. doi: 10.3389/fmicb.2020.01025
Benson, A. K., Kelly, S. A., Legge, R., Ma, F., Low, S. J., Kim, J., et al. (2010). Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. U.S.A. 107, 18933–18938. doi: 10.1073/pnas.1007028107
Bistrom, M., Moisander-Jylha, A. M., Heinikainen, S., Pelkola, K., and Raunio-Saarnisto, M. (2016). Isolation of Clostridium limosum from an outbreak of metritis in farmed mink. Acta Vet. Scand. 58, 49. doi: 10.1186/s13028-016-0230-7
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Cabrera-Mulero, A., Tinahones, A., Bandera, B., Moreno-Indias, I., Macias-Gonzalez, M., and Tinahones, F. J. (2019). Keto microbiota: a powerful contributor to host disease recovery. Rev. Endocr. Metab. Disord. 20, 415–425. doi: 10.1007/s11154-019-09518-8
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Cao, J., Hu, Y., Liu, F., Wang, Y., Bi, Y., Lv, N., et al. (2020). Metagenomic analysis reveals the microbiome and resistome in migratory birds. Microbiome 8:26. doi: 10.1186/s40168-019-0781-8
Capunitan, D. C., Johnson, O., Terrill, R. S., and Hird, S. M. (2020). Evolutionary signal in the gut microbiomes of 74 bird species from Equatorial Guinea. Mol. Ecol. 29, 829–847. doi: 10.1111/mec.15354
Cato, E. P., Cummins, C., and Smith, L. D. (1970). Clostridium limosum andré in Prévot 1948, 165 amended description and pathogenic characteristics. Int. J. System. Evol. Microbiol. 20, 305–316.
Deng, Y., Jiang, Y. H., Yang, Y., He, Z., Luo, F., and Zhou, J. (2012). Molecular ecological network analyses. BMC Bioinform. 13:113. doi: 10.1186/1471-2105-13-113
Dewar, M. L., Arnould, J. P., Dann, P., Trathan, P., Groscolas, R., and Smith, S. (2013). Interspecific variations in the gastrointestinal microbiota in penguins. Microbiologyopen 2, 195–204. doi: 10.1002/mbo3.66
Dewar, M. L., Arnould, J. P., Krause, L., Dann, P., and Smith, S. C. (2014). Interspecific variations in the faecal microbiota of Procellariiform seabirds. FEMS Microbiol. Ecol. 89, 47–55. doi: 10.1111/1574-6941.12332
Douglas, G. M., Maffei, V. J., Zaneveld, J. R., Yurgel, S. N., Brown, J. R., Taylor, C. M., et al. (2020). PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38, 685–688. doi: 10.1038/s41587-020-0548-6
Engel, P., and Moran, N. A. (2013). The gut microbiota of insects - diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735. doi: 10.1111/1574-6976.12025
Flint, H. J., Bayer, E. A., Rincon, M. T., Lamed, R., and White, B. A. (2008). Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6, 121–131. doi: 10.1038/nrmicro1817
Fuentes-Castillo, D., Navas-Suarez, P. E., Gondim, M. F., Esposito, F., Sacristan, C., Fontana, H., et al. (2021). Genomic characterization of multidrug-resistant ESBL-producing Escherichia coli ST58 causing fatal colibacillosis in critically endangered Brazilian merganser (Mergus octosetaceus). Transbound. Emerg. Dis. 68, 258–266. doi: 10.1111/tbed.13686
Grond, K., Santo Domingo, J. W., Lanctot, R. B., Jumpponen, A., Bentzen, R. L., Boldenow, M. L., et al. (2019). Composition and Drivers of Gut Microbial Communities in Arctic-Breeding Shorebirds. Front. Microbiol. 10:2258. doi: 10.3389/fmicb.2019.02258
Hasselbalch, S. G., Knudsen, G. M., Jakobsen, J., Hageman, L. P., Holm, S., and Paulson, O. B. (1994). Brain metabolism during short-term starvation in humans. J. Cereb. Blood Flow Metab. 14, 125–131. doi: 10.1038/jcbfm.1994.17
Hernandez-Oaxaca, D., Lopez-Sanchez, R., Lozano, L., Wacher-Rodarte, C., Segovia, L., and Lopez Munguia, A. (2021). Diversity of Weissella confusa in Pozol and Its Carbohydrate Metabolism. Front. Microbiol. 12:629449. doi: 10.3389/fmicb.2021.629449
Hildebrandt, M. A., Hoffmann, C., Sherrill-Mix, S. A., Keilbaugh, S. A., Hamady, M., Chen, Y. Y., et al. (2009). High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137, 1716–24.e1–2. doi: 10.1053/j.gastro.2009.08.042
Honore-Bouakline, S., Vincensini, J. P., Giacuzzo, V., Lagrange, P. H., and Herrmann, J. L. (2003). Rapid diagnosis of extrapulmonary tuberculosis by PCR: impact of sample preparation and DNA extraction. J. Clin. Microbiol. 41, 2323–2329. doi: 10.1128/JCM.41.6.2323-2329.2003
Jing, Y., Yuan, Y., Monson, M., Wang, P., Mu, F., Zhang, Q., et al. (2021). Multi-Omics Association Reveals the Effects of Intestinal Microbiome-Host Interactions on Fat Deposition in Broilers. Front. Microbiol. 12:815538. doi: 10.3389/fmicb.2021.815538
Kiu, R., and Hall, L. J. (2018). An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 7:141. doi: 10.1038/s41426-018-0144-8
Knutie, S. A., Chaves, J. A., and Gotanda, K. M. (2019). Human activity can influence the gut microbiota of Darwin’s finches in the Galapagos Islands. Mol. Ecol. 28, 2441–2450. doi: 10.1111/mec.15088
Kotloff, K. L., Nataro, J. P., Blackwelder, W. C., Nasrin, D., Farag, T. H., Panchalingam, S., et al. (2013). Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382, 209–222. doi: 10.1016/S0140-6736(13)60844-2
Lee, C. Y., Peralta-Sanchez, J. M., Martinez-Bueno, M., Moller, A. P., Rabelo-Ruiz, M., Zamora-Munoz, C., et al. (2020). The gut microbiota of brood parasite and host nestlings reared within the same environment: disentangling genetic and environmental effects. ISME J. 14, 2691–2702. doi: 10.1038/s41396-020-0719-y
Lee, F. J., Rusch, D. B., Stewart, F. J., Mattila, H. R., and Newton, I. L. (2015). Saccharide breakdown and fermentation by the honey bee gut microbiome. Environ. Microbiol. 17, 796–815. doi: 10.1111/1462-2920.12526
Ley, R. E., Hamady, M., Lozupone, C., Turnbaugh, P. J., Ramey, R. R., Bircher, J. S., et al. (2008). Evolution of mammals and their gut microbes. Science 320, 1647–1651. doi: 10.1126/science.1155725
Liu, J., Xu, T., Zhu, W., and Mao, S. (2014). High-grain feeding alters caecal bacterial microbiota composition and fermentation and results in caecal mucosal injury in goats. Br. J. Nutr. 112, 416–427. doi: 10.1017/S0007114514000993
Liu, L., Liu, X., Du, C., Fang, H., Zhang, J., Li, W., et al. (2022). Spring diet and energy intake of whooper swans (Cygnus cygnus) at the Yellow River National Wetland in Baotou. China. PLoS One 17:e0264528. doi: 10.1371/journal.pone.0264528
Liu, L., Liu, X. G., Sun, Y., Pu, Z. H., Xu, H. Y., Li, W. X., et al. (2019). Trace Elements in the Feathers of Waterfowl from Nanhaizi Wetland, Baotou, China. Bull. Environ. Contam. Toxicol. 102, 778–783. doi: 10.1007/s00128-019-02596-z
Livanos, A. E., Snider, E. J., Whittier, S., Chong, D. H., Wang, T. C., Abrams, J. A., et al. (2018). Rapid gastrointestinal loss of Clostridial Clusters IV and XIVa in the ICU associates with an expansion of gut pathogens. PLoS One 13:e0200322. doi: 10.1371/journal.pone.0200322
Loo, W. T., Dudaniec, R. Y., Kleindorfer, S., and Cavanaugh, C. M. (2019). An inter-island comparison of Darwin’s finches reveals the impact of habitat, host phylogeny, and island on the gut microbiome. PLoS One 14:e0226432. doi: 10.1371/journal.pone.0226432
Melendez, D., Roberts, M. C., Greninger, A. L., Weissman, S., No, D., Rabinowitz, P., et al. (2019). Whole-genome analysis of extraintestinal pathogenic Escherichia coli (ExPEC) MDR ST73 and ST127 isolated from endangered southern resident killer whales (Orcinus orca). J. Antimicrob. Chemother. 74, 2176–2180. doi: 10.1093/jac/dkz159
Mikaelyan, A., Dietrich, C., Kohler, T., Poulsen, M., Sillam-Dusses, D., and Brune, A. (2015). Diet is the primary determinant of bacterial community structure in the guts of higher termites. Mol. Ecol. 24, 5284–5295. doi: 10.1111/mec.13376
Milani, C., Ticinesi, A., Gerritsen, J., Nouvenne, A., Lugli, G. A., Mancabelli, L., et al. (2016). Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci. Rep. 6:25945. doi: 10.1038/srep25945
Muegge, B. D., Kuczynski, J., Knights, D., Clemente, J. C., Gonzalez, A., Fontana, L., et al. (2011). Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332, 970–974. doi: 10.1126/science.1198719
Nicholson, J. K., Holmes, E., Kinross, J., Burcelin, R., Gibson, G., Jia, W., et al. (2012). Host-gut microbiota metabolic interactions. Science 336, 1262–1267. doi: 10.1126/science.1223813
Nyaoke, A. C., Navarro, M. A., Fresneda, K., Diab, S. S., Moore, J., Lyras, D., et al. (2020). Paeniclostridium (Clostridium) sordellii-associated enterocolitis in 7 horses. J. Vet. Diagn. Invest. 32, 239–245. doi: 10.1177/1040638720903738
Oksanen, J., Kindt, R., Legendre, P., O’Hara, B., Stevens, M. H. H., Oksanen, M. J., et al. (2007). The vegan: Community Ecology Package. Version:2.6-2
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Rall, L. C., and Meydani, S. N. (1993). Vitamin B6 and immune competence. Nutr. Rev. 51, 217–225. doi: 10.1111/j.1753-4887.1993.tb03109.x
Ran, J., Wan, Q. H., and Fang, S. G. (2021). Gut microbiota of endangered crested ibis: establishment, diversity, and association with reproductive output. PLoS One 16:e0250075. doi: 10.1371/journal.pone.0250075
Ricaboni, D., Mailhe, M., Khelaifia, S., Raoult, D., and Million, M. (2016). Romboutsia timonensis, a new species isolated from human gut. New Microbes New Infect. 12, 6–7. doi: 10.1016/j.nmni.2016.04.001
Rui, L. (2014). Energy metabolism in the liver. Compr. Physiol. 4, 177–197. doi: 10.1002/cphy.c130024
San Juan, P. A., Hendershot, J. N., Daily, G. C., and Fukami, T. (2020). Land-use change has host-specific influences on avian gut microbiomes. ISME J. 14, 318–321. doi: 10.1038/s41396-019-0535-4
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. doi: 10.1186/gb-2011-12-6-r60
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Sharaf, H., Rodrigues, R. R., Moon, J., Zhang, B., Mills, K., and Williams, M. A. (2019). Unprecedented bacterial community richness in soybean nodules vary with cultivar and water status. Microbiome 7:63. doi: 10.1186/s40168-019-0676-8
Skeen, H. R., Cooper, N. W., Hackett, S. J., Bates, J. M., and Marra, P. P. (2021). Repeated sampling of individuals reveals impact of tropical and temperate habitats on microbiota of a migratory bird. Mol. Ecol. 30, 5900–5916. doi: 10.1111/mec.16170
Spor, A., Koren, O., and Ley, R. (2011). Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 9, 279–290. doi: 10.1038/nrmicro2540
Stressmann, F. A., Bernal-Bayard, J., Perez-Pascual, D., Audrain, B., Rendueles, O., Briolat, V., et al. (2021). Mining zebrafish microbiota reveals key community-level resistance against fish pathogen infection. ISME J. 15, 702–719. doi: 10.1038/s41396-020-00807-8
Sun, Y., Sun, Y., Shi, Z., Liu, Z., Zhao, C., Lu, T., et al. (2019). Gut Microbiota of Wild and Captive Alpine Musk Deer (Moschus chrysogaster). Front. Microbiol. 10:3156. doi: 10.3389/fmicb.2019.03156
Tap, J., Mondot, S., Levenez, F., Pelletier, E., Caron, C., Furet, J. P., et al. (2009). Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 11, 2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x
Valdes, A. M., Walter, J., Segal, E., and Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. BMJ 361:k2179. doi: 10.1136/bmj.k2179
Waite, D. W., Deines, P., and Taylor, M. W. (2012). Gut microbiome of the critically endangered New Zealand parrot, the kakapo (Strigops habroptilus). PLoS One 7:e35803. doi: 10.1371/journal.pone.0035803
Waite, D. W., and Taylor, M. W. (2015). Exploring the avian gut microbiota: current trends and future directions. Front. Microbiol. 6:673. doi: 10.3389/fmicb.2015.00673
Wang, W., Cao, J., Yang, F., Wang, X., Zheng, S., Sharshov, K., et al. (2016a). High-throughput sequencing reveals the core gut microbiome of Bar-headed goose (Anser indicus) in different wintering areas in Tibet. Microbiologyopen 5, 287–295. doi: 10.1002/mbo3.327
Wang, W., Zheng, S., Sharshov, K., Cao, J., Sun, H., Yang, F., et al. (2016b). Distinctive gut microbial community structure in both the wild and farmed Swan goose (Anser cygnoides). J. Basic Microbiol. 56, 1299–1307. doi: 10.1002/jobm.201600155
Wasserman, S., and Faust, K. (1994). Social network analysis: Methods and applications.Cambridge, MA: Cambridge University Press.
Watts, S. C., Ritchie, S. C., Inouye, M., and Holt, K. E. (2019). FastSpar: rapid and scalable correlation estimation for compositional data. Bioinformatics 35, 1064–1066. doi: 10.1093/bioinformatics/bty734
Wu, Y., Yang, Y., Cao, L., Yin, H., Xu, M., Wang, Z., et al. (2018). Habitat environments impacted the gut microbiome of long-distance migratory swan geese but central species conserved. Sci. Rep. 8:13314. doi: 10.1038/s41598-018-31731-9
Xenoulis, P. G., Gray, P. L., Brightsmith, D., Palculict, B., Hoppes, S., Steiner, J. M., et al. (2010). Molecular characterization of the cloacal microbiota of wild and captive parrots. Vet. Microbiol. 146, 320–325. doi: 10.1016/j.vetmic.2010.05.024
Xiao, K., Fan, Y., Zhang, Z., Shen, X., Li, X., Liang, X., et al. (2021). Covariation of the Fecal Microbiome with Diet in Nonpasserine Birds. mSphere 6:e00308–21. doi: 10.1128/mSphere.00308-21
Yeung, M., Saingam, P., Xu, Y., and Xi, J. (2021). Low-dosage ozonation in gas-phase biofilter promotes community diversity and robustness. Microbiome 9:14. doi: 10.1186/s40168-020-00944-4
Zhan, J., Liu, M., Wu, C., Su, X., Zhan, K., and Zhao, G. Q. (2017). Effects of alfalfa flavonoids extract on the microbial flora of dairy cow rumen. Asian-Australas J. Anim. Sci. 30, 1261–1269. doi: 10.5713/ajas.16.0839
Zhao, G., Zhou, L., Dong, Y., Cheng, Y., and Song, Y. (2017). The gut microbiome of hooded cranes (Grus monacha) wintering at Shengjin Lake, China. Microbiologyopen 6:e00447. doi: 10.1002/mbo3.447
Zhao, N., Wang, S., Li, H., Liu, S., Li, M., Luo, J., et al. (2018). Influence of Novel Highly Pathogenic Avian Influenza A (H5N1) Virus Infection on Migrating Whooper Swans Fecal Microbiota. Front. Cell. Infect. Microbiol. 8:46. doi: 10.3389/fcimb.2018.00046
Zhong, Y., Nyman, M., and Fak, F. (2015). Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 59, 2066–2076. doi: 10.1002/mnfr.201500187
Zhou, L., Huo, X., Liu, B., Wu, H., and Feng, J. (2020). Comparative Analysis of the Gut Microbial Communities of the Eurasian Kestrel (Falco tinnunculus) at Different Developmental Stages. Front. Microbiol. 11:592539. doi: 10.3389/fmicb.2020.592539
Keywords: raptors, waders, waterfowl, fecal microbiota, co-occurrence network, metabolic pathways
Citation: Zhao C, Liu L, Gao L and Bai L (2022) A comprehensive comparison of fecal microbiota in three ecological bird groups of raptors, waders, and waterfowl. Front. Microbiol. 13:919111. doi: 10.3389/fmicb.2022.919111
Received: 13 April 2022; Accepted: 11 July 2022;
Published: 08 August 2022.
Edited by:
Lifeng Zhu, Nanjing Normal University, ChinaReviewed by:
Jonathan Badger, National Cancer Institute (NIH), United StatesCopyright © 2022 Zhao, Liu, Gao and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lige Bai, YmFpbGlnZUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.