
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 01 June 2022
Sec. Microbe and Virus Interactions with Plants
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.893393
The antagonistic potential of bacteria obtained from the nest of Odontotermes formosanus was assessed against Fusarium oxysporum f. sp. cucumerinum (FOC). Of 30, seven termite nest-associated bacteria strains had biocontrol potential. Among them, the strain YC-9 showed the strongest antifungal activity toward FOC. Phylogenetic analysis of the 16S rRNA amplified product of YC-9 revealed its identification as Bacillus siamensis. The in vivo antifungal activity experiment showed that the application of YC-9 at 108 cfu/ml significantly reduced the cucumber wilt incidence with a control efficacy of 73.2%. Furthermore, plant growth parameters such as fresh weight, dry weight, plant height, and root height were significantly improved by 42.6, 53.0, 20.8, and 19.3%, respectively. We found that inoculation with B. siamensis YC-9 significantly increased the activity of defensive enzymes such as peroxidase (POD), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) in diseased cucumber roots, thereby raising the resistance. PCR using gene-specific primers revealed that B. siamensis YC-9 contains biosynthetic genes for known antibiotics, including bacillomycin, iturin, and surfactin. Chemical analysis of the cultivation of B. siamensis YC-9 resulted in the isolation of five metabolites, including hexadecanoic acid (1), cyclo-(L-phenylalanylglycine) (2), cyclo-(L-trans-Hyp-L-Leu) (3), C15-surfactin (4), and macrolactin A (5), the structures of which were identified by the analysis of NMR spectroscopic data and MS. Among them, the compound 4 showed significant antifungal activity against conidial germination of FOC with an IC50 value of 5.1 μg/ml, which was comparable to that of the positive control, cycloheximide (IC50 value of 2.6 μg/ml). Based on these findings, this study suggests that termite-nest associated B. siamensis YC-9 could be a potential biological control agent for integrated control of soil-borne diseases like cucumber Fusarium wilt.
Cucumber (Cucumis sativus L.) is one of the most important economic crops worldwide, and it is popular and favored by consumers for its distinct aromas and flavors (Shan et al., 2021). It is well known for its softness and succulence and contains a variety of nutrients, such as potassium, copper, manganese, phosphorus, pantothenic acid, dietary fibers, and vitamins (Cao et al., 2020). However, cucumber is susceptible to many pathogens (Lebeda et al., 2007). Cucumber Fusarium wilt, induced by the pathogen Fusarium oxysporum f. sp. cucumerinum (FOC), is a typical soil-borne fungal disease and also one of the most important cucumber diseases worldwide (Cao et al., 2020). The disease could reduce 10–30% of cucumber production and cause quality degradation, which results in serious economic losses (Li et al., 2009; Shi et al., 2016).
Chemical controls can effectively protect plants from infectious pathogens (Zhang et al., 2018). However, the drawbacks of chemical fungicides are obvious when pathogen resistance to pesticides, food safety, and environmental quality are considered. As an efficient, environmentally friendly, and sustainable method, biocontrol is used to protect plants against soil-borne diseases (Ben Abdallah et al., 2016; Koch et al., 2018; Yadav et al., 2021).
Many microbial strains are currently used as biological control agents (BCAs) (Chen et al., 2020), including yeast, bacteria, fungi, and actinomycetes (Sharma et al., 2009; Wisniewski et al., 2016; Sujarit et al., 2020). In the past, BCAs were mainly isolated from habitats such as soil, and it is currently difficult to isolate new strain resources due to long-term repetitive studies (Saxena et al., 2020). Therefore, to discover new BCA resources, it is necessary to find new microbial resources in special habitats. As a biological material, termite nest is an important microbial resource. The Streptomyces of termites’ feces in an underground nest could produce a series of bioactive metabolites that provided termites with a certain degree of protection against some insect pathogenic fungi (Chouvenc et al., 2018). For example, Oberpaul et al. (2020) isolated, screened, and identified new species with the potential of anti-pathogenic fungi from termite nests by high-throughput culture. However, there are few reports on termite nest-related microorganisms.
In this study, we evaluated the ability of Bacillus siamensis YC-9, identified from among 30 isolates obtained from the nest of O. formosanus, to function as a plant growth promoting bacterium and control cucumber Fusarium wilt. To investigate the mechanism of its activity, an induced resistance experiment was designed to detect changes in the enzyme activities of resistance-related enzymes in cucumber roots. We also performed an examination of known antibiotic related genes, and further attempts were made to isolate and identify the active metabolites.
Nests of O. formosanus were collected in July 2017 in Jiangyin City, Jiangsu Province, China. The termite was authenticated by Professor Jianguo Wang (Department of Plant Protection, College of Agriculture, Jiangxi Agricultural University). More than three termite nests were grinded in mortar, followed by the treatment with an equal volume of 70% (v/v) ethanol for 4 h at room temperature to kill vegetative cells. Subsequently, the material was washed three times with sterile water. The diluted sample was coated onto the Luria-Bertani (LB) culture medium (NaCl 10 g, peptone 10 g, yeast extract 5 g, and agar 15 g, in 1 L of distilled water), which was supplemented with 0.1% sodium taurocholate in order to promote spore germination (Browne et al., 2016). All mediums were cultured at 37°C for 24 h. After 2 days, a total of 30 germinal microorganisms were conserved on slant LB medium and stored at 4°C.
All the bacteria isolates were initially screened to assess their antifungal activity against F. oxysporum f. sp. cucumerinum (FOC) using a dual culture method on potato dextrose agar (PDA) plates (Ribeiro et al., 2021). A single 6 mm plug of the pathogen from 4 days of growth was inoculated in the center of the PDA medium. The isolates with 1 μl bacterial suspension (108 cfu/ml) were spot inoculated at three sites equidistant from the center of Petri plates. PDA media inoculated with the pathogen alone were used as control. All the plates were cultured continuously at 28°C. After 4 days, the diameter of the pathogen colony (cm) was recorded. The inhibition percentage (I) was calculated using the following formula (Nasr et al., 2019):
I (%) = [(R – r)/R] × 100
where r and R are the radial growth of fungal pathogens in dual plate culture and control plates, respectively.
The antifungal spectrum of the selected strain YC-9 against the following plant pathogens was detected by the dual culture method described above: F. oxysporum f. sp. vasinfectum, Alternaria solani, Colletotrichum graminicola, Curvularia lunata, Corynespora cassiicola, F. oxysporum f. sp. mornordicae, Botrytis cinerea, and Fusarium graminearum. All plant fungal pathogens were obtained from the Department of Microbiology, School of Life Sciences, Anhui Agricultural University, Hefei, China.
The antagonistic isolates were identified using 16S rRNA gene sequences and phylogenetic analysis (Gorai et al., 2021). Total DNA was extracted using an Aidlab DNA extraction kit (Aidlab Biotechnologies Co., Ltd., Beijing, China). Primers 27F (5′-AGAGTTTGATCATGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) were used as amplification primers. Polymerase chain reaction amplification (PCR) was utilized on the basis of the following steps: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 90 s, and a final extension at 72°C for 10 min, with 30 cycles in total. DNA sample was sequenced by General Biol (Anhui) Co., Ltd. Additionally, 16S rRNA gene sequence-based phylogenetic analysis was performed by the Maximum Likelihood method with the MEGA 6.0 software. A bootstrap analysis with 1,000 replicates was performed to calculate node support.
Plump and uniform cucumber seeds (Jinyan No. 4) were selected and soaked with 2% NaClO for 10 min, then soaked with 75% alcohol for 30 s, and washed three times with sterilized water. The seeds were then sowed in plastic cups (50 × 50 × 70 mm) filled with sterilized soil. After 15 days, 20 ml of YC-9 suspension (108 cfu/ml) was slowly injected into the cucumber rhizosphere. The same volume of sterile water was added as a control. There were 25 repeats of the treatment and control groups. After 5 days, 20 ml of FOC conidial suspension (105 spores/ml) was inoculated in the rhizosphere of all cucumber seedlings. The planted cups were placed in an illumination incubator at 28°C with light (12 h)/dark (12 h) cycles. At 15 days after fungal inoculation, wilt development in each cucumber plant was rated using the Yang et al. (2011) scale: 0 (whole plant was healthy); 1 (<10% of leaves wilted); 2 (11–20% of leaves wilted); 3 (21–50% of leaves wilted); 4 (50–100% of leaves wilted); and 5 (the plant was dead). The disease index was transformed to percent disease index (PDI) before analysis of variance. The disease indices and biocontrol efficiency were calculated according to the following formulas:
Percent disease index (%) = Σ (rating × number of plants rated)/(total number of plants × highest rating) × 100
Biocontrol efficiency (%) = (disease index of control – disease index of treated)/disease index of control × 100
The preliminary preparation of the experiment is the same as that in the “In vivo Antifungal Activity in a Pot Experiment” section. After 30 days of inoculation with YC-9 or sterile water, the plant growth characteristics were measured by the modified method developed by Wang et al. (2020). The heights of the aerial and underground parts were measured as the plant and root height, respectively. After washing the root soil and removing the surface moisture with absorbent paper, the cucumber seedlings were weighed to get the fresh weight. The dry weight was weighed after the seedlings were dried at 65°C for 24 h.
After 15 days of cultivation, the cucumber seedlings were inoculated with different treatments, including sterile water (A), YC-9 suspension (B), FOC conidia suspension (C), and challenge inoculation with FOC after 48 h of YC-9 suspension inoculation (D). The experiment was set up for three replicates. An amount of 0.2 g of root samples was collected on the 2nd, 4th, 6th, 8th, and 10th days after inoculation and homogenized with liquid nitrogen. The homogenized tissues were rinsed with 1 ml of ddH2O (25 mM borate buffer for the PAL assay) at 4°C. The tissue extracts were centrifuged at 5,000 × g for 5 min at 4°C. The supernatant was stored at –80°C for the enzymatic activity assays.
The PAL activity was measured according to the modified method of Mori et al. (2001). A volume of 200 μl of enzyme extract was mixed with 2 ml of 0.15 mM borate buffer (pH 8.8) and 0.8 ml of 56.25 μM L-phenylalanine and incubated at 37°C for 30 min. A volume of 0.1 ml of HCl at a concentration of 5 M was added to terminate the reaction. The enzyme activity was measured spectrophotometrically at 290 nm. The unit of enzyme activity was determined as the amount of plant fresh weight that was required to change the optical density by 1.0 for 1 h.
The activity of PPO (EC 1.10.3.2) was determined by the modified method developed by Cavalcanti et al. (2007). A volume of 50 μl of enzyme extract was mixed with 1.5 ml of 0.2 M pyrocatechol and 1.5 ml of 0.05 M phosphate buffer (pH 6.8) and further incubated at 30°C for 2 min. The enzyme activity was measured spectrophotometrically at 398 nm. The unit of enzyme activity was determined as the amount of plant fresh weight required to change the optical density by 1.0 for 1 h.
The activity of POD was determined by the method described by Choudhary (2011) with little modification. A volume of 20 μl of enzyme extract was mixed with 1.95 ml of 0.2 M acetate buffer (pH 5.0), 1 ml of 0.1% O-methoxyphenol, and 1 ml of 0.08% hydrogen peroxide, and incubated at 25°C for 1 h. The peroxidase (POD) activity was measured spectrophotometrically at 470 nm. The unit of enzyme activity was determined as the amount of plant fresh weight required to change the optical density by 1.0 for 1 h.
The detection of encoding genes of known antibiotics was determined by the method described by Gorai et al. (2021) with less modification. A PCR reaction was carried out in the mixture, which contained 1 μl (50 ng) of DNA template of YC-9, 1 μl of each primer (which was listed in Table 1), 12.5 μl of Premix Taq (LA Taq Version 2.0), and 9.5 μl of ddH2O. PCR amplification was utilized on the basis of the following steps: pre-denaturation at 94°C for 10 min, denaturation at 94°C for 45 s, annealing at 55°C for 45 s, extension at 72°C for 2 min, and a final extension at 72°C for 10 min, with 35 cycles in total. The amplified product was subjected to electrophoresis with 1% agarose gel. The results were observed and recorded by a gel imager.
The fermentation of strain YC-9 was conducted according to the methods described in detail previously (Liu and Sun, 2021). The single colony of B. siamensis YC-9 strain was inoculated into a 250-ml flask containing 100 ml of LB medium and cultured at 180 r/min for 24 h at 28°C. Then, 5 ml of this seed culture was inoculated into a 1-L flask containing 500 ml of LB medium (× 39) and cultured at 180 r/min for 3 days at 28°C. The culture broth (19.5 L) was filtered by gauze to afford the supernatant. The supernatant was extracted with EtOAc (3 × 20 L) at room temperature. The EtOAc phase was evaporated in vacuo to afford a crude extract and then subjected to silica gel column elution with a stepwise gradient of CH2Cl2/MeOH (100:0–100:8, v/v) to give five fractions (Fr1-Fr5). Fr1 (CH2Cl2/MeOH, 100:0, v/v) was further fractionated on a silica gel column, eluting with a stepwise gradient of petroleum ether (PE)/ethyl acetate (EA) (100:0–100:10, v/v) to give compound 1 (9.5 mg). Fr3 (CH2Cl2/MeOH, 100:2, v/v) was repeatedly chromatographed over a silica gel column to give compound 2 (22.7 mg). Fr5 (CH2Cl2/MeOH, 100:8, v/v) was repeatedly chromatographed over a silica gel column to give compound 3 (46.8 mg). The remaining part of Fr5 was loaded onto a Sephadex LH-20 column (100% MeOH) to yield compounds 4 (38.4 mg) and 5 (6.1 mg). Due to the limitation of separation means, no monomer compound was isolated and purified from Fr2 and Fr4.
Structural identifications of the metabolites were made on the basis of the spectroscopic data and mass spectrometry. Nuclear magnetic resonance (NMR) spectra were recorded on an Agilent II DD2 instrument operating at 600 MHz for 1H and 150 MHz for 13C, while the DEPT and 2D spectra (COSY, HMQC, and HMBC) were obtained using the standard Agilent software. Chemical shifts were given in parts per million (δ) downfield from the TMS internal standard. The electrospray ionization mass spectrometry (ESI–MS) spectra were determined on a micrOTOF II mass spectrometer (Mariner Mass 5304, United States).
The inhibitory activities of the secondary metabolites of strain YC-9 against spore germination of FOC were assessed as reported previously with slight changes (Zhang et al., 2013). FOC spores were obtained from PDA plates of 5-day-old cultures. All secondary metabolites were made to obtain solutions with serial concentrations in aqueous solution (1% acetone). A tested compound solution (60 μl) was added to a spore suspension (60 μl, 105 spores/ml). Then, aliquots of 40 μl of spore suspension from each were placed on separate glasses in triplicate. Cycloheximide (Hefei Bomei Biotechnology Co., Ltd.) was used as the positive control. The negative control was treated with the above aqueous solution alone. Slides containing spores were incubated in a moisture chamber at 28°C for 6 h, after which approximately 100 spores were examined under a light microscope to determine the percentage of germinated spores. The percentage of spore germination inhibition was calculated from mean values as follows:
Inhibition (%) = (A – B)/A × 100
where A and B are the percentages of germinated spores in the control and the sample, respectively.
The contribution and significance of the treatments were determined using a one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test or Student’s t-test to determine significant differences between treatment means (p < 0.05) with the SPSS 20.0 software. All experiments were performed with at least three independent replicates, unless otherwise stated.
In total, 30 bacterial strains were isolated from the nest of the termite, O. formosanus, seven of which exhibited strong antifungal activity against FOC with the inhibition rates ranging from 45.7 to 72.5% in the dual culture (Figure 1). Among them, the strain YC-9 showed the strongest antifungal activity against FOC, with an inhibition rate of 72.5% (Supplementary Figure 1). Therefore, the strain YC-9 was selected as the objective strain in this study to further investigate its biocontrol effect.
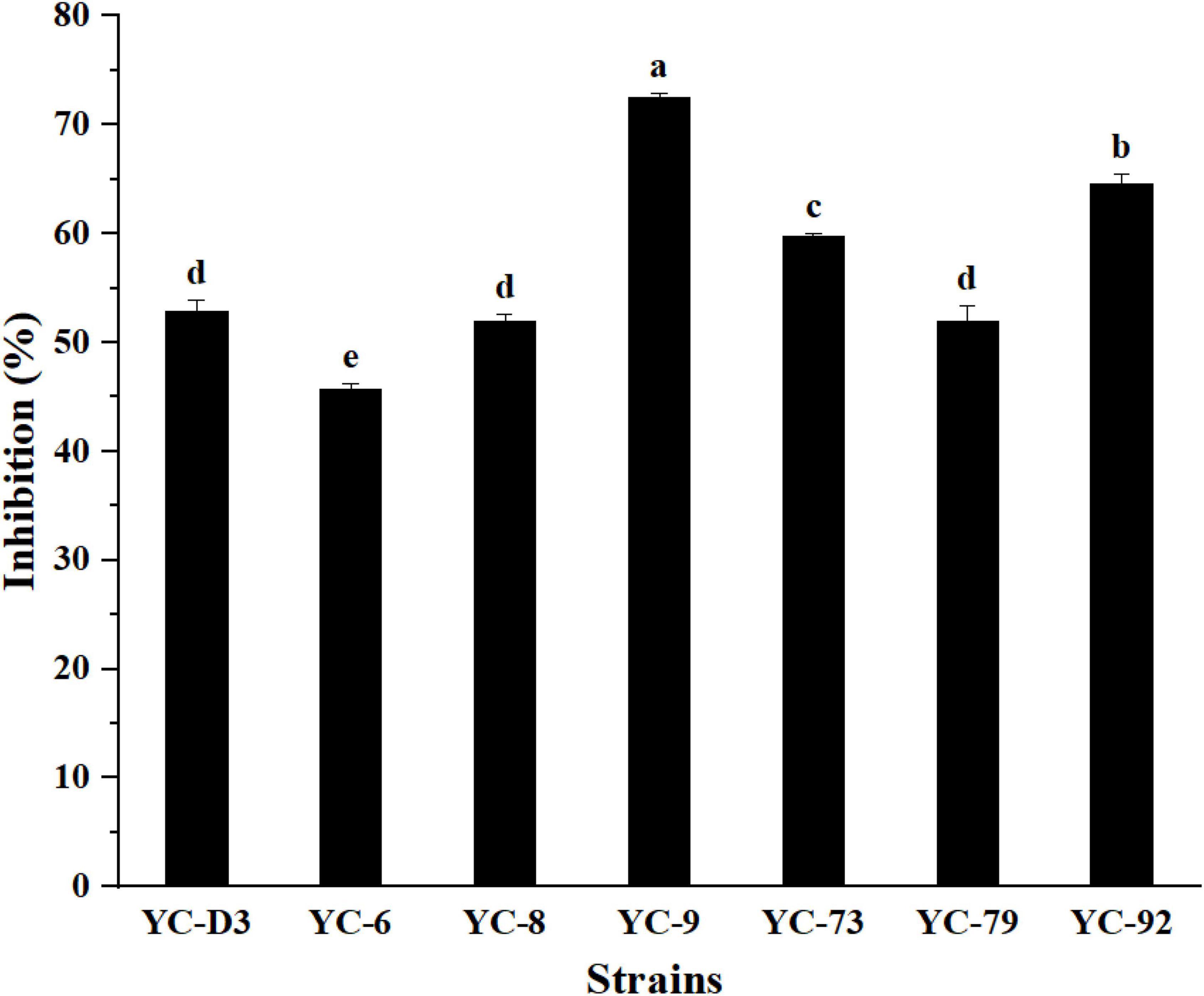
Figure 1. The inhibitory efficiency of seven bacterial strains against FOC. Bars with the same letters are not significantly different according to Duncan’s multiple range test at P < 0.05. Error bars represent the standard deviation from three replicates.
The inhibitory activities of the strain YC-9 against eight selected pathogenic fungi are presented in Figure 2 and Supplementary Figure 2. The results showed that the strain YC-9 had the most potent inhibitory effect on all tested fungi except for F. graminearum. The strain YC-9 was found to have broad spectrum antifungal activity and could be applied to biological control of other crop diseases.
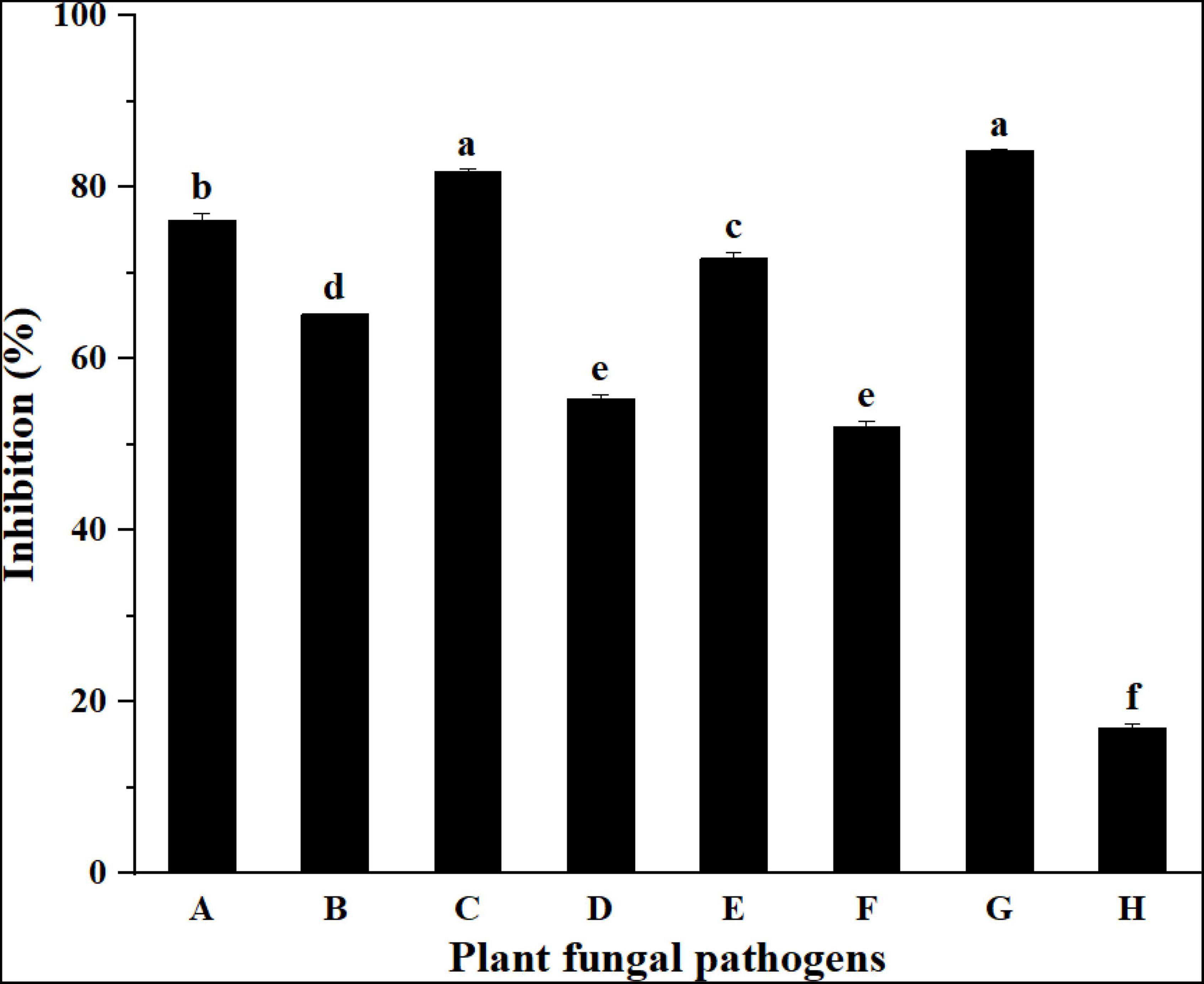
Figure 2. Inhibition activities of strain YC-9 against plant fungal pathogens. A: F. oxysporum f. sp. vasinfectum; B: A. solani; C: C. graminicola; D: C. lunata; E: C. cassiicola; F: F. oxysporum f. sp. mornordicae; G: B. cinerea; H: F. graminearum. Bars with the same letters are not significantly different according to Duncan’s multiple range test at P < 0.05. Error bars represent the standard deviation from three replicates.
A 1,431 base pair (bp) strain of the 16S rRNA gene was amplified and sequenced. The sequence was then queried against the EzBioCloud database. Relevant type strains were selected to construct a phylogenetic tree using the maximum-likelihood method (Kumar et al., 2021). The phylogenetic analysis revealed that the strain YC-9 and B. siamensis clustered on the same branch, with 99.86% sequence similarity for the 16S rRNA gene sequence (Figure 3), indicating the strain YC-9 as B. siamensis.
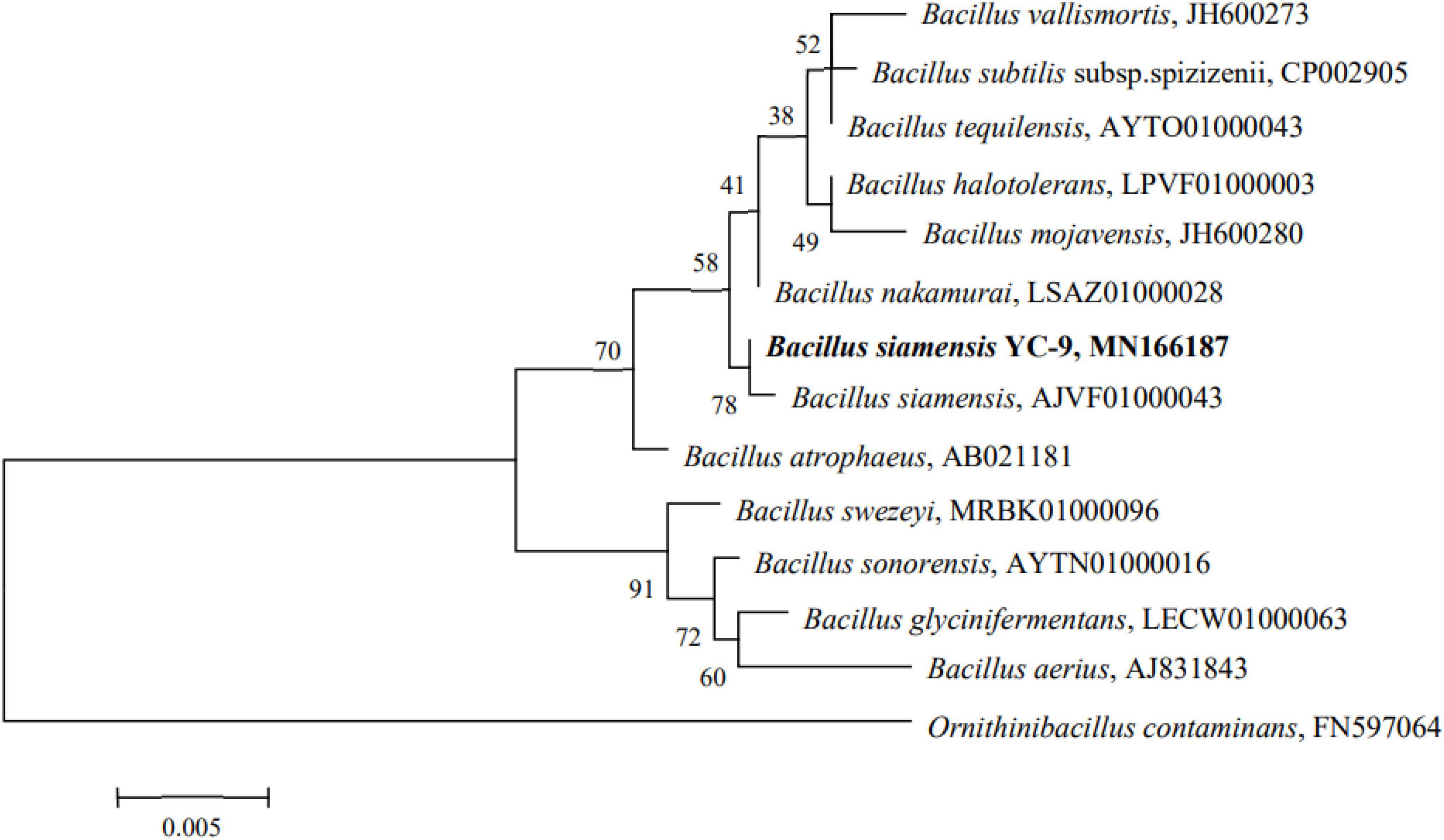
Figure 3. Phylogenetic tree of strain YC-9 based on maximum-likelihood method analysis of 16S rRNA sequences data.
In vivo challenge experiment was performed to check the ability of B. siamensis YC-9 to reduce the disease severity. After 20 days of treatment with conidial suspension of FOC (108 cfu/ml), 32.8% disease severity was observed for control. However, only 8.8% of disease severity was recorded due to the application of B. siamensis YC-9 even after pathogenic treatment (Supplementary Figures 3, s4). The calculated control efficacy was 73.2%. This observation strongly suggested that the B. siamensis YC-9 had significant suppression activity against FOC even after treatment with the pathogenic conidia in high concentration.
The effects of inoculation with B. siamensis YC-9 on cucumber growth characteristics are presented in Table 2. The results showed that B. siamensis YC-9 increased the fresh weight by 42.6%, dry weight by 53.0%, plant height by 20.8%, and root length by 19.3%. Thus, the application of B. siamensis YC-9 could promote cucumber growth in comparison with those grown without the experimental strain.
The resistance-related enzymes (POD, PPO, and PAL) activities in the roots of cucumber seedlings were measured at different times after pathogen inoculation (Figure 4). The results showed that the POD activity of cucumber treated with B. siamensis YC-9 was significantly higher than other treatments after pathogen inoculation and increased to the top level of 2,401 U/g⋅Fw⋅h on the 4th day, while the group A (CK), group B (only B. siamensis YC-9 was inoculated), and group C (only FOC was inoculated) were only 818, 1,244, and 1,024 U/g⋅Fw⋅h, respectively. The activities of PPO and PAL after pathogen inoculation were the overall upward trend, and their activities were maximum on the 10th day with 380 and 18.6 U/g⋅Fw⋅h, respectively. The results showed that B. siamensis YC-9 treatment could significantly increase the activities of PAL, PPO, and POD for some period of time after treatment in contrast to other treatments.
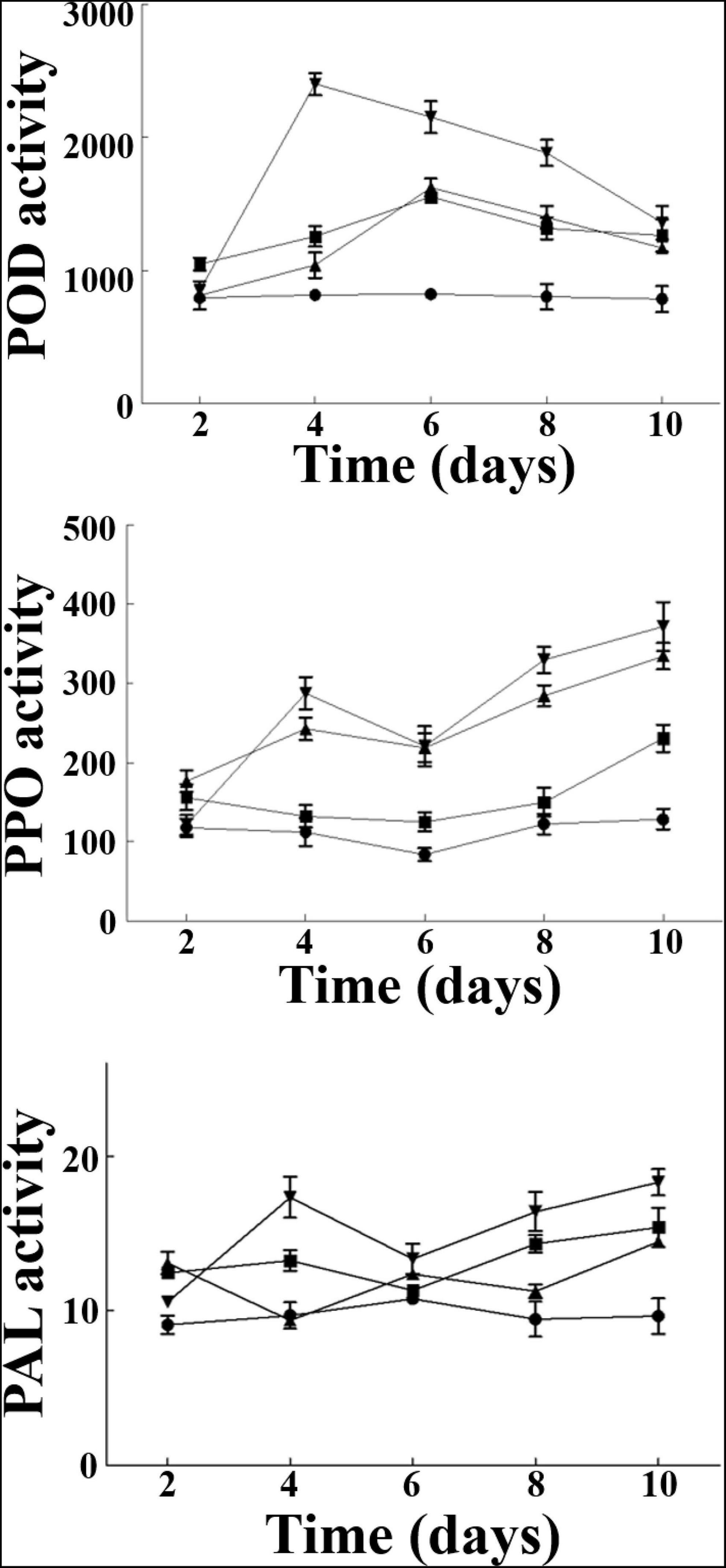
Figure 4. Effect of B. siamensis YC-9 on POD, PPO, and PAL activities (U/g⋅Fw⋅h) of cucumber root. A(●): CK; B(■): only YC-9 suspension were inoculated; C(▲): only FOC suspension were inoculated; D(▼): challenge inoculation with FOC suspension after 48 h of YC-9 suspension inoculation. Each data point represents the mean ± standard deviation of three replicates in each treatment group. Error bars represent the standard deviation from three replicates.
The presence of genes for biosynthesis of antibiotics such as 2,4-diacetyl phloroglucinol (DAPG), pyrrolnitrin (PRN), bacillomycin, fengycin, iturin, and surfactin in B. siamensis YC-9 was confirmed by PCR analysis with gene-specific primers. The primer groups and PCR amplification conditions were listed in Table 1. The primer set SRFAF/R amplified a 1,300-bp fragment from the srfAA gene, involved in the biosynthesis of surfactin. The primer set FEND1F/R amplified a 964-bp fragment from the fenD gene, involved in the biosynthesis of fengycin. The primer set BACC1F/R amplified an 875-bp fragment from the bmyB gene, involved in the biosynthesis of bacillomycin. The primer set ItuC-F/R amplified a 465-bp fragment from ituC gene, involved in the biosynthesis of iturin. The primer sets for Phl2a and Phl2b amplified a 745-bp fragment from phlD gene, involved in the biosynthesis of DAPG. The primer sets for PrnCf/r amplified a 719-bp fragment from the prnC gene, involved in the biosynthesis of PRN. The results of electrophoresis showed that B. siamensis YC-9 contained genes for biosynthesis of bacillomycin, iturin, and surfactin (Supplementary Figure 5).
Chromatographic separation of the ethyl acetate extracts from the filtrate of B. siamensis YC-9 afforded five secondary metabolites (Figure 5), which were identified as hexadecanoic acid (1) (Aiyelaagbe et al., 2019), cyclo-(L-phenylalanylglycine) (2) (Zhuravleva et al., 2011), cyclo-(L-trans-Hyp-L-Leu) (3) (Xiang et al., 2020), C15-surfactin (4) (Tang et al., 2007), and macrolactin A (5) (Schneider et al., 2007) by spectroscopic data (Supplementary Figures 6–10) analyses, and the comparison of their derivative data in the literature.
The isolated secondary metabolites from the culture of strain YC-9 were further evaluated for their antifungal activities by using the spore germination inhibition assay. Figure 6 showed C15-surfactin (4) had significantly antifungal activity against spore germination of FOC when compared to cycloheximide co-assayed as a positive reference and had higher antifungal activity with the increasing concentration. The calculated IC50 value of antifungal activity for C15-surfactin (4) was 5.1 μg/ml, which was comparable to that of the positive control, cycloheximide (IC50 value of 2.6 μg/ml). However, the other secondary metabolites were not active against spore germination (IC50 > 50 μg/ml).
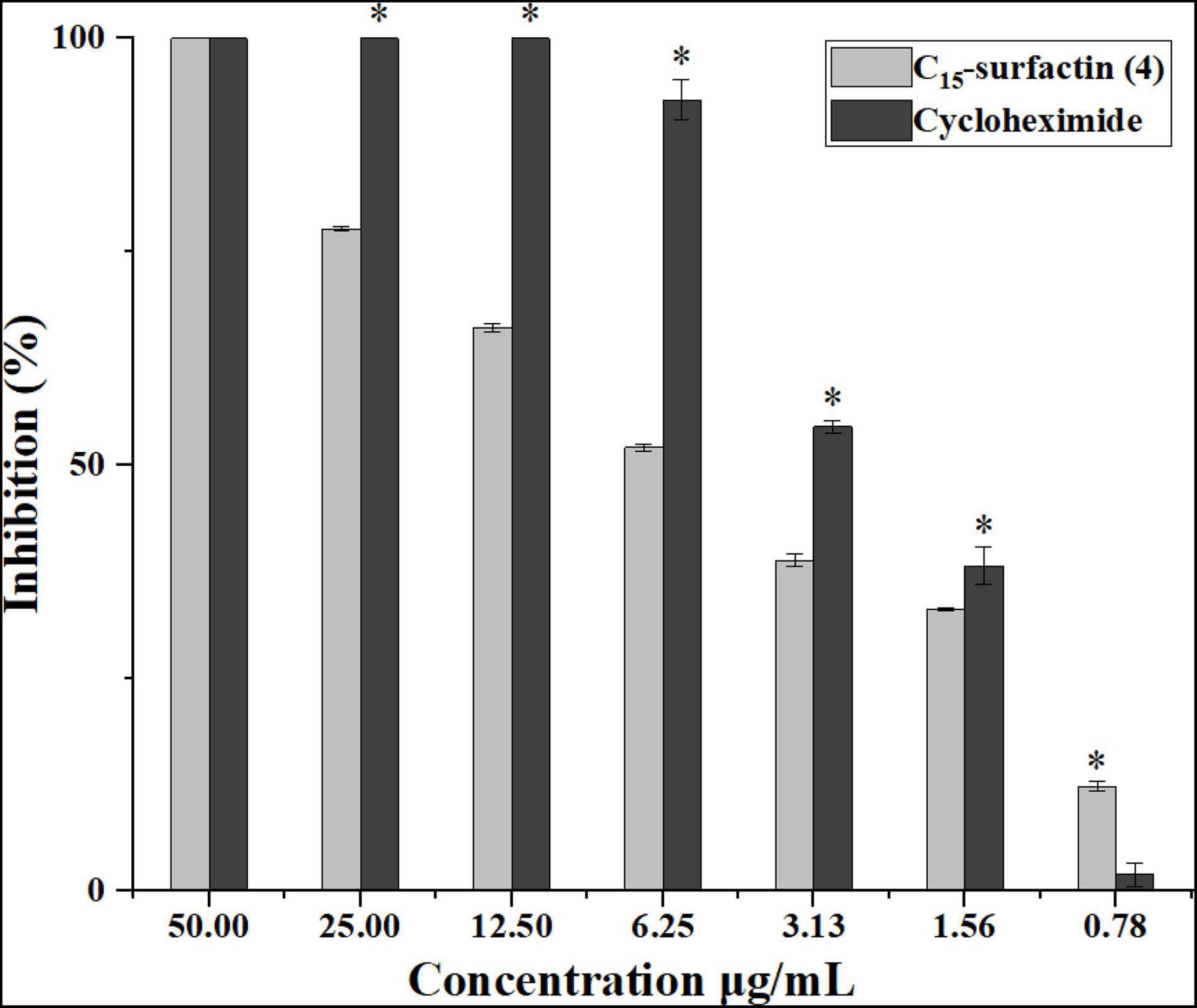
Figure 6. Effect of compound (4) (C15-surfactin) and referenced cycloheximide on conidia germination of FOC. Asterisks indicate a significant difference between the control (cycloheximide) and compound (4) treated conidia (t-test; *P < 0.05). Error bars represent the standard deviation from three replicates.
Insect-associated microbiota had enormous potential to discover novel therapeutic agents against pathogens (Jang and Kikuchi, 2020). In fact, a recent comprehensive survey among diverse insects of 15 orders revealed that insect-associated Streptomyces inhibited more antimicrobial-resistant bacteria than soil-derived Streptomyces (Chevrette et al., 2019). As a biological material, termite nest is an important microbial resource. Due to its unique environment, the bacteria in a termite nest are more likely to have good antimicrobial activity than those in a normal environment (Long et al., 2010). For example, Neonectria discophora SNB-CN63 isolated from a termite nest has strong antimicrobial activity (Nirma et al., 2015). We isolated and screened a termite nest associated with B. siamensis YC-9 with significant antagonistic activity against FOC, which indicated the possibility that neglected insect symbiotic microorganisms could be a good source for microbial biocontrol agents.
Many genera of bacteria have latent capacity as biological control agents (BCAs), including genera of Bacillus, Azospirillum, Pseudomonas, Burkholderia, and Enterobacter (Xie et al., 2021). Species of Bacillus are the most commonly commercialized biocontrol agents due to their high tolerance of environmental stress, plant-growth promoting properties, and production of antimicrobial compounds (Khan et al., 2020; Rojas-Solis et al., 2020). Among the 30 bacterial isolates in this study, B. siamensis YC-9 displayed potent antifungal activity against FOC in in vitro experiments, exhibiting an inhibition rate of 72.5%. The results of the further pot test demonstrated that an application of B. siamensis YC-9 prior to inoculation of roots with FOC could significantly reduce both the incidence and severity of cucumber Fusarium wilt. Although the results of the pot experiment cannot predict the outcome of field trials, the demonstrated ability to reduce disease severity indicates that B. siamensis YC-9 has a promising potential for use under field conditions.
Plant growth-promoting bacteria (PGPB) can enhance plant yield and control phytopathogens, constituting the most widely studied and increasingly used tool in modern agriculture (Emmanuel and Babalola, 2020). Bacillus is one of the more widely studied PGPB, which is partly due to its production of ACC deaminase, indole-3-acetic acid (IAA), organic acids, siderophores, and phosphate solubilization (Khan et al., 2020; Shin et al., 2021). Our study showed that the application of B. siamensis YC-9 significantly increased the dry weight, fresh weight, plant height, and root length of cucumbers in pot experiments. Therefore, insect-associated microbes may be an important source of PGPB. However, the mechanism of promoting growth needs to be further examined.
Microbial-induced systemic resistance is an important aspect of the biological control of plant diseases. Enzymes such as PPO, PAL, and POD are associated with systemic-induced resistance in plant tissues (Qiu et al., 2022). In this study, B. siamensis YC-9 was demonstrated to have the ability to enhance the activity of POD, PAL, and PPO in cucumber roots when the seedlings were challenged with FOC, which contributed to a reduction in disease severity. Although many studies have shown that B. siamensis could promote plant growth, induce host resistance, and improve plant tolerance to abiotic stress (Lee et al., 2018; Xie et al., 2021), few studies have been conducted on the use of B. siamensis to prevent and treat cucumber diseases, especially cucumber Fusarium wilt. To the best of our knowledge, this is the first report on the use of B. siamensis to induce systemic resistance against FOC in the cucumber seedling roots.
The production of antifungal metabolites may be the most famous and important mechanism used by biocontrol bacteria to limit the pathogens’ invasion into host plant tissues. For example, lipopeptide production played a major role in the successful control of cucumber Fusarium wilt by B. subtilis SQR 9 (Cao et al., 2012). Bacillomycin is another member of the iturin family produced by bacilli with a strong antifungal spectrum (Caulier et al., 2019). Purified surfactins can also inhibit the growth of some fungi (Ma et al., 2020; Li et al., 2021). A PCR was used to determine the antibiosis mechanisms of B. siamensis YC-9 by screening six genes involved in the biosynthesis of antibiotics. Amplicons of the expected sizes were detected as ituC for iturin, srfAA for surfactin, and bmyB for bacillomycin synthesis. These findings suggest that B. siamensis YC-9 could produce multiple antibiotics as a biocontrol agent. Due to the limitations of separation methods, we only isolated C15-surfactin and related metabolites in the stage of separating secondary metabolites.
The use of B. siamensis YC-9 isolated from the termite nest was shown to have potential for the prevention and control of cucumber Fusarium wilt, which indicated the possibility that neglected insect symbiotic microorganisms could be a good source for microbial biocontrol agents. The biological control ability of B. siamensis YC-9 against FOC appeared to be due to several mechanisms, including the induction of host resistance and the secretion of antifungal metabolites. The bioactive compound C15-surfactin was successfully identified from the ethyl acetate extract of fermentation filtrate. It could significantly inhibit the germination of FOC spores, and the effect was comparable to that of the positive control, cycloheximide. A pot experiment also proved that B. siamensis YC-9 had an obvious growth-promoting effect on cucumber seedlings. Therefore, the termite-nest-associated B. siamensis YC-9 could be a potential biological control agent for integrated control of soil-borne diseases such as cucumber Fusarium wilt.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
YZ and KK designed the research. YZ supervised the study. LZ performed the experiments, analyzed the data, and wrote the manuscript. JW performed the experiments and analyzed the results. All authors revised the manuscript and approved the final version for submission.
This study was co-financed by the National Natural Science Foundation of China (32011540382 and 31770007) and Anhui Outstanding Youth Science Fund Project (2108085J18). This study was supported under the framework of international cooperation program managed by the National Research Foundation of Korea (2020K2A9A2A06037042).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.893393/full#supplementary-material
Aiyelaagbe, O. O., Negi, A. S., Kaneez, F., Luqman, S., Oguntoye, S. O., Kumar, S. B., et al. (2019). Isolation and antiproliferative activity of triterpenoids and fatty acids from the leaves and stem of Turraea vogelii Hook. f. ex benth. Nat. Prod. Res. 33, 296–301. doi: 10.1080/14786419.2018.1446133
Alvarez, F., Castro, M., Principe, A., Borioli, G., Fischer, S., Mori, G., et al. (2012). The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J. Appl. Microbiol. 112, 159–174. doi: 10.1111/j.1365-2672.2011.05182.x
Bakthavatchalu, S., Shivakumar, S., and Sulla, S. B. (2013). Molecular detection of antibiotic related genes from Pseudomonas aeruginosa FP6, an antagonist towards Rhizoctonia solani and Colletotrichum gloeosporioides. Turk. J. Biol. 37, 289–295. doi: 10.3906/biy-1207-56
Ben Abdallah, R. A., Mokni-Tlili, S., Nefzi, A., Jabnoun-Khiareddine, H., and Daami-Remadi, M. (2016). Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Nicotiana glauca organs. Biol. Control 97, 80–88. doi: 10.1016/j.biocontrol.2016.03.005
Browne, H. P., Forster, S. C., Anonye, B. O., Kumar, N., Neville, B. A., Stares, M. D., et al. (2016). Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533, 543–546. doi: 10.1038/nature17645
Cao, P., Li, C. X., Wang, H., Yu, Z. Y., Xu, X., Wang, X. J., et al. (2020). Community structures and antifungal activity of root-associated endophytic actinobacteria in healthy and diseased cucumber plants and Streptomyces sp. HAAG3-15 as a promising biocontrol agent. Microorganisms. 8:236. doi: 10.3390/microorganisms8020236
Cao, Y., Xu, Z. H., Ling, N., Yuan, Y. J., Yang, X. M., Chen, L. H., et al. (2012). Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Sci. Hortic-Amsterdam. 135, 32–39. doi: 10.1016/j.scienta.2011.12.002
Caulier, S., Nannan, C., Gillis, A., Licciardi, F., Bragard, C., and Mahillon, J. (2019). Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 10:302. doi: 10.3389/fmicb.2019.00302
Cavalcanti, F. R., Resende, M. L. V., Carvalho, C. P. S., Silveira, J. A. G., and Oliveira, J. T. A. (2007). An aqueous suspension of Crinipellis perniciosa mycelium activates tomato defence responses against Xanthomonas vesicatoria. Crop Prot. 26, 729–738. doi: 10.1016/j.cropro.2006.06.012
Chen, C., Cao, Z., Li, J., Tao, C., Feng, Y. N., and Han, Y. L. (2020). A novel endophytic strain of Lactobacillus plantarum CM-3 with antagonistic activity against Botrytis cinerea on strawberry fruit. Biol. Control. 148:104306. doi: 10.1016/j.biocontrol.2020.104306
Chevrette, M. G., Carlson, C. M., Ortega, H. E., Thomas, C., Ananiev, G. E., Barns, K. J., et al. (2019). The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 10:516. doi: 10.1038/s41467-019-08438-0
Choudhary, D. K. (2011). Plant growth-promotion (PGP) activities and molecular characterization of rhizobacterial strains isolated from soybean (Glycine max L. Merril) plants against charcoal rot pathogen, Macrophomina phaseolina. Biotechnol. Lett. 33, 2287–2295. doi: 10.1007/s10529-011-0699-0
Chouvenc, T., Elliott, M. L., Sobotnik, J., Efstathion, C. A., and Su, N. Y. (2018). The termite fecal nest: a framework for the opportunistic acquisition of beneficial soil Streptomyces (Actinomycetales: Streptomycetaceae). Environ. Entomol. 47, 1431–1439. doi: 10.1093/ee/nvy152
Emmanuel, O. C., and Babalola, O. O. (2020). Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 239:126569. doi: 10.1016/j.micres.2020.126569
Gorai, P. S., Ghosh, R., Mandal, S., Ghosh, S., Chatterjee, S., Gond, S. K., et al. (2021). Bacillus siamensis CNE6-a multifaceted plant growth promoting endophyte of Cicer arietinum L. having broad spectrum antifungal activities and host colonizing potential. Microbiol. Res. 252:126859. doi: 10.1016/j.micres.2021.126859
Jang, S., and Kikuchi, Y. (2020). Impact of the insect gut microbiota on ecology, evolution, and industry. Curr. Opin. Insect Sci. 41, 33–39. doi: 10.1016/j.cois.2020.06.004
Khan, M. S., Gao, J. L., Chen, X. Q., Zhang, M. F., Yang, F. P., Du, Y. F., et al. (2020). The endophytic bacteria Bacillus velezensis Lle-9, isolated from Lilium leucanthum, harbors antifungal activity and plant growth-promoting effects. J. Microbiol. Biotechn. 30, 668–680. doi: 10.4014/jmb.1910.10021
Koch, E., Becker, J. O., Berg, G., Hauschild, R., Jehle, J., Köhl, J., et al. (2018). Biocontrol of plant diseases is not an unsafe technology. J. Plant Dis. Prot. 125, 121–125. doi: 10.1007/s41348-018-0158-4
Kumar, M., Charishma, K., Sahu, K. P., Sheoran, N., Patel, A., Kundu, A., et al. (2021). Rice leaf associated Chryseobacterium species: an untapped antagonistic flavobacterium displays volatile mediated suppression of rice blast disease. Biol. Control 161:104703. doi: 10.1016/j.biocontrol.2021.104703
Lebeda, A., Ryder, E. J., Grube, R., Dolezalová, I., and Kristková, E. (2007). “Lettuce (Asteraceae Lactuca spp.),” in Genetic Resources, Chromosome Engineering, and Crop Improvement, Vol. 3, Vegetable Crops, ed. R. J. Singh (Boca Raton: CRC Press), 377–472.
Lee, Y. H., Jang, S. J., Han, J. H., Bae, J. S., Shin, H., Park, H. J., et al. (2018). Enhanced tolerance of chinese cabbage seedlings mediated by Bacillus aryabhattai H26-2 and B. siamensis H30-3 against high temperature stress and fungal infections. Plant Pathology J. 34, 555–566. doi: 10.5423/PPJ.OA.07.2018.0130
Li, J., Yang, Q., Zhang, S. M., Wang, Y. X., and Zhao, X. Y. (2009). Evaluation of biocontrol efficiency and security of a Bacillus subtilis strain B29 against cucumber Fusarium wilt in field. China Veg. 2, 30–33.
Li, M. S. M., Piccoli, D. A., McDowell, T., MacDonald, J., Renaud, J., and Yuan, Z. C. (2021). Evaluating the biocontrol potential of Canadian strain Bacillus velezensis 1B-23 via its surfactin production at various pHs and temperatures. BMC Biotechnol. 21:31. doi: 10.1186/s12896-021-00690-x
Liu, W. X., and Sun, C. M. (2021). C-17-fengycin B, produced by deep-sea-derived Bacillus subtilis, possessing a strong antifungal activity against Fusarium solani. J. Oceanol. Limnol. 39, 1938–1947. doi: 10.1007/s00343-020-0215-2
Long, Y. H., Xie, L., Liu, N., Yan, X., Li, M. H., Fan, M. Z., et al. (2010). Comparison of gut-associated and nest-associated microbial communities of a fungus-growing termite (Odontotermes yunnanensis). Insect Sci. 17, 265–276. doi: 10.1111/j.1744-7917.2010.01327.x
Ma, Z. W., Zhang, S. Y., Zhang, S. H., Wu, G. Y., Shao, Y., Mi, Q. F., et al. (2020). Isolation and characterization of a new cyclic lipopeptide surfactin from a marine-derived Bacillus velezensis SH-B74. J. Antibiot. 73, 863–867. doi: 10.1038/s41429-020-0347-9
Mori, T., Sakurai, M., and Sakuta, M. (2001). Effects of conditioned medium on activities of PAL. CHS, DAHP synthase (DS-Co and DS-Mn) and anthocyanin production in suspension cultures of Fragaria ananassa. Plant Sci. 160, 355–360. doi: 10.1016/S0168-9452(00)00399-X
Nasr, A., Zhou, X. X., Huang, S. P., Wang, Y., Li, X. N., and Zhu, G. P. (2019). Comparative effects of some extraction solvents on the antimicrobial activity of Eucalyptus camaldulensis leaf, bud, capsule and seed crude extracts. Nat. Prod. Res. 33, 2560–2565. doi: 10.1080/14786419.2018.1455049
Nirma, C., Eparvier, V., and Stien, D. (2015). Antibacterial llicicolinic acids C and D and llicicolinal from Neonectria discophora SNB-CN63 isolated from a termite nest. J. Nat. Prod. 78, 159–162. doi: 10.1021/np500080m
Oberpaul, M., Zumkeller, C. M., Culver, T., Spohn, M., Mihajlovic, S., Leis, B., et al. (2020). High-throughput cultivation for the selective isolation of acidobacteria from termite nests. Front. Microbiol 11:597628. doi: 10.3389/fmicb.2020.597628
Qiu, Y., Yan, H. H., Sun, S. M., Wang, Y. Q., Zhao, X. R., and Wang, H. Y. (2022). Use of Bacillus velezensis SDTB022 against tobacco black shank (TBS) and the biochemical mechanism involved. Biol. Control 165:104785. doi: 10.1016/j.biocontrol.2021.104785
Ribeiro, I. D. A., Bach, E., Moreira, F. D., Muller, A. R., Rangel, C. P., Wilhelm, C. M., et al. (2021). Antifungal potential against Sclerotinia sclerotiorum (Lib.) de Bary and plant growth promoting abilities of Bacillus isolates from canola (Brassica napus L.) roots. Microbiol. Res. 248:126754. doi: 10.1016/j.micres.2021.126754
Rojas-Solis, D., Vences-Guzman, M. A., Sohlenkamp, C., and Santoyo, G. (2020). Bacillus toyonensis COPE52 modifies lipid and fatty acid composition, exhibits antifungal activity, and stimulates growth of tomato plants under saline conditions. Curr. Microbiol. 77, 2735–2744. doi: 10.1007/s00284-020-02069-1
Saxena, A. K., Kumar, M., Chakdar, H., Anuroopa, N., and Bagyaraj, D. J. (2020). Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 128, 1583–1594. doi: 10.1111/jam.14506
Schneider, K., Chen, X. H., Vater, J., Franke, P., Nicholson, G., Borriss, R., et al. (2007). Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J. Nat. Prod. 70, 1417–1423. doi: 10.1021/np070070k
Shan, N., Xiang, Z. J., Sun, J. Y., Zhu, Q. L., Xiao, Y., Wang, P. T., et al. (2021). Genome-wide analysis of valine-glutamine motif-containing proteins related to abiotic stress response in cucumber (Cucumis sativus L.). BMC Plant Biol. 21:492. doi: 10.1186/s12870-021-03242-9
Sharma, R. R., Singh, D., and Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol. Control 50, 205–221. doi: 10.1016/j.biocontrol.2009.05.001
Shi, L., Du, N., Yuan, Y., Shu, S., Sun, J., and Guo, S. (2016). Vinegar residue compost as a growth substrate enhances cucumber resistance against the Fusarium wilt pathogen Fusarium oxysporum by regulating physiological and biochemical responses. Environ. Sci. Pollut. Res. Int. 23, 1–11. doi: 10.1007/s11356-016-6798-7
Shin, J. H., Park, B. S., Kim, H. Y., Lee, K. H., and Kim, K. S. (2021). Antagonistic and plant growth-promoting effects of Bacillus velezensis BS1 isolated from rhizosphere soil in a pepper field. Plant Pathology J. 37, 307–314. doi: 10.5423/PPJ.NT.03.2021.0053
Stankovic, S., Mihajlovic, S., Draganic, V., Dimkic, I., Vukotic, G., Beric, T., et al. (2012). Screening for the presence of biosynthetic genes for antimicrobial lipopeptides in natural isolates of Bacillus sp. Arch. Biol. Sci. 64, 1425–1432. doi: 10.2298/ABS1204425S
Sujarit, K., Pathom-aree, W., Mori, M., Dobashi, K., Shiomi, K., and Lumyong, S. (2020). Streptomyces palmae CMU-AB204T, an antifungal producing-actinomycete, as a potential biocontrol agent to protect palm oil producing trees from basalstem rot disease fungus. Ganoderma boninense. Biol. Control. 148:104307. doi: 10.1016/j.biocontrol.2020.104307
Tang, J. S., Gao, H., Hong, K., Yu, Y., Jiang, M. M., Lin, H. P., et al. (2007). Complete assignments of 1H and 13C NMR spectral data of nine surfactin isomers. Magn. Reson. Chem. 45, 792–796. doi: 10.1002/mrc.2048
Velusamy, P., Immanuel, J. E., Gnanamanickam, S. S., and Thomashow, L. (2006). Biological control of rice bacterial blight by plant-associated bacteria producing 2,4-diacetylphloroglucinol. Can. J. Microbiol. 52, 56–65. doi: 10.1139/W05-106
Wang, S. S., Ji, B. Y., Su, X. H., Li, H. W., Dong, C. M., Chen, S. Q., et al. (2020). Isolation of endophytic bacteria from Rehmannia glutinosa Libosch and their potential to promote plant growth. J. Gen. Appl. Microbiol. 66, 279–288. doi: 10.2323/jgam.2019.12.001
Wisniewski, M., Droby, S., Norelli, J., Liu, J., and Schena, L. (2016). Alternative management technologies for postharvest disease control: the journey from simplicity to complexity. Postharvest Biol. Technol. 122, 3–10. doi: 10.1016/j.postharvbio.2016.05.012
Xiang, W. X., Liu, Q., Li, X. M., Lu, C. H., and Shen, Y. M. (2020). Four pairs of proline-containing cyclic dipeptides from Nocardiopsis sp. HT88, an endophytic bacterium of Mallotus nudiflorus L. Nat. Prod. Res. 34, 2219–2224. doi: 10.1080/14786419.2019.1577834
Xie, Z. Y., Li, M. M., Wang, D. K., Wang, F. L., Shen, H., Sun, G. J., et al. (2021). Biocontrol efficacy of Bacillus siamensis LZ88 against brown spot disease of tobacco caused by Alternaria alternata. Biol. Control 154:104508. doi: 10.1016/j.biocontrol.2020.104508
Yadav, K., Damodaran, T., Dutt, K., Singh, A., Muthukumar, M., Rajan, S., et al. (2021). Effective biocontrol of banana Fusarium wilt tropical race 4 by a Bacillus rhizobacteria strain with antagonistic secondary metabolites. Rhizosphere-neth. 18:100341. doi: 10.1016/j.rhisph.2021.100341
Yang, X. M., Chen, L. H., Yong, X. Y., and Shen, Q. R. (2011). Formulations can affect rhizosphere colonization and biocontrol efficiency of Trichoderma harzianum SQR-T037 against Fusarium wilt of cucumbers. Biol. Fert. Soils 47, 239–248. doi: 10.1007/s00374-010-0527-z
Zhang, J. J., Luan, F., Li, Q., Gu, G. D., Dong, F., and Guo, Z. Y. (2018). Synthesis of novel chitin derivatives bearing amino groups and evaluation of their antifungal activity. Mar. Drugs 16:380. doi: 10.3390/md16100380
Zhang, Y. L., Li, S., Jiang, D. H., Kong, L. C., Zhang, P. H., and Xu, J. D. (2013). Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. J. Agr. Food Chem. 61, 1521–1524. doi: 10.1021/jf305210u
Keywords: Bacillus siamensis, Fusarium oxysporum f. sp. cucumerinum, antifungal activity, C15-surfactin, biocontrol
Citation: Zhou L, Wang J, Wu F, Yin C, Kim KH and Zhang Y (2022) Termite Nest Associated Bacillus siamensis YC-9 Mediated Biocontrol of Fusarium oxysporum f. sp. cucumerinum. Front. Microbiol. 13:893393. doi: 10.3389/fmicb.2022.893393
Received: 15 March 2022; Accepted: 06 May 2022;
Published: 01 June 2022.
Edited by:
Motaher Hossain, Bangabandhu Sheikh Mujibur Rahman Agricultural University, BangladeshReviewed by:
Shekhar Jain, Mandsaur University, IndiaCopyright © 2022 Zhou, Wang, Wu, Yin, Kim and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinglao Zhang, emhhbmd5bEBhaGF1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.