- 1Department of Gastroenterology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China
- 2Department of Gastroenterology, Shanghai Changzheng Hospital, Second Military Medical University, Shanghai, China
There are few studies on the changes of gut microbiota in patients with gallstones, especially in patients with asymptomatic gallstones, and there are some deficiencies in these studies, for instance, the effects of metabolic factors on gut microbiota are not considered. Here, we selected 30 asymptomatic gallstone patients from the survey population, and 30 controls according to the age and BMI index matching principle. The 16SrDNA technology was used to detect and compare the structural differences in the gut microbiota between the two groups. Compared with healthy controls, the abundance of gut microbiota in patients with gallstones increased significantly, while the microbiota diversity decreased. At the level of phylum, both groups were dominated by Firmicutes, Bacteroides, Proteobacteria, and Actinobacteria. At the genus level, there were 15 species with significant differences in abundance between the two groups. Further subgroup analysis found that only unclassified Lactobacillales showed differences in the intestines of gallstones patients with hypertension, non-alcoholic fatty liver disease, or patients with elevated BMI (≧24). The structure of gut microbiota in patients with gallstones changed significantly, and this might be related to the occurrence of gallstones, rather than metabolic factors such as hypertension, non-alcoholic fatty liver disease, and obesity.
Introduction
Gallstone disease (GSD) represents a major public health problem worldwide with a prevalence ranging from 3.2 to 35.2% (Wang et al., 2018) and a frequent cause of hospital admissions, associated with high costs because of treatment and morbidity [Everhart et al., 1999; European Association for the Study of the Liver [EASL], 2016; Shabanzadeh, 2018]. Although most patients are asymptomatic during their lifetime, 10–20% will develop symptomatic gallstone disease, the consequences of which can range from simple biliary colic to potentially life-threatening complications such as cholecystitis, Gallstone ileus, choledocholithiasis, Cholestatic jaundice, cholangitis, acute biliary pancreatitis, and rarely, gallbladder cancer (Portincasa et al., 2016; Soreide, 2017). Gallstone formation is multifactorial, such as advancing age, being female, genes/ethnicity, obesity, rapid weight loss, diet, drugs, and activity. Some risk factors are unalterable, and some factors can be modified (Shaffer, 2006; Lammert et al., 2016).
The microorganisms living in the gastrointestinal cavity collectively referred to as gut microbiota or intestinal microbiota are a hotspot in recent years. The rapid advances made during the past decade suggest that the gut microbiota constitutes an important environmental factor that contributes to metabolic diseases, such as obesity, diabetes, dyslipidemia, and hypertension (Karlsson et al., 2013; Fandriks, 2017). However, those diseases are closely related to the formation of gallstones (Shaffer, 2006; Song et al., 2020), suggesting gut microbiota is likely to be associated with the gallstones. Kawai et al. (2002) found that gram-positive cocci are associated with the formation of pure cholesterol stones. It is considered that abnormal metabolism and secretion of cholesterol and bile acids as the primary pathophysiological defect in the formation of gallstones (Wang et al., 2009), and gut microbiota regulate bile acid metabolism by reducing bile acid pool size and composition (Sayin et al., 2013). Many biliary diseases are also affected by gut microbiota, such as primary biliary cholangitis/cirrhosis (Bogdanos et al., 2005; Lemoinne and Marteau, 2017) and primary sclerosing cholangitis (Tabibian et al., 2013). Meanwhile, the biliary microbiome has been proved to facilitate the formation of brown pigment stones (Wang et al., 2018). However, little is known about the correlation between gut microbiota dysbiosis and the formation of gallstones, especially which one and how the gut microbiota acts on the formation of asymptomatic gallstones. Consequently, for developing a greater understanding of the connection between GSD and the microflora communities in the body and then developing strategies a better understanding of the gut bacterial tract in asymptomatic gallstone patients is crucial.
Here, we selected 30 patients with asymptomatic gallstones from the survey population, and 30 control populations according to their age and BMI index. The 16SrDNA technology was used to detect and compare the structural differences of intestinal microbiota between two groups, to better understand the role bacteria play in the pathogenesis of asymptomatic gallstones.
Materials and Methods
Studied Subjects and Sample Collection
This work was based on the Three-Year Action Program of Shanghai Municipality for Strengthening the Construction of the Public Health System (no. GWIV-27.7), a cross-sectional health survey of Shanghai residents (Song et al., 2020). Among the 4,038 participants, 30 patients with asymptomatic gallstones were selected, and 30 cases without gallstones were selected according to the sex, age, and BMI index matching principle. All the subjects had no history of biliary surgery and had no history of taking antibiotics, probiotics, immunosuppressants, or traditional Chinese medicines for at least 3 months before sample collection. Criteria for diagnosis of gallstones: All the participants were examined by B-ultrasound after fasting for at least 8 h. Gallstones were identified based on the presence of strong echoes with or without acoustic shadows and rolling stone signs in the gallbladder lumen or in the gallbladder sludge. Metabolic disorders, such as hypertension, diabetes, and non-alcoholic fatty liver disease were diagnosed according to established standards (Jian-Gao and Chinese Liver Disease Association, 2010; Bakris et al., 2019; Jia et al., 2019). Body mass index (BMI) (kg/m2) was calculated by dividing weight (kg) by the square of height (m).
Sample Preparation, Molecular Methods, and Bioinformatics
Stool samples were obtained and placed in a fecal collection box, which was refrigerated and transported to the laboratory by KingMed Cold Chain Logistics (Shanghai, China) within 2 h, and stored at −80°C until analysis. 16SrDNA sequencing was completed by Shanghai Majorbio Bio-pharm Technology Co., Ltd. DNA extraction was carried out according to the E.Z.N.A.® DNA Kit (Omega BioTek, United States) protocol to enable amplification of the 16S V3-V4 region of the DNA gene, primers were designed based on the universal primer set, 338F: ACTCCTACGGGAGGCAGCAG; 806R: GGACTACHVGGGTWTCTAAT. The PCR products were detected and quantified with a mini fluorometer (Quantus™ Fluorometer, Promega, United States). The NEXTFLEX Rapid DNA-Seq kit (Bioo Scientific, United States) was used for library construction, and the Miseq PE300 platform of Illumina was used for sequencing. The quality control of the original sequencing sequence was performed by Trimmomatic software. Spliced by FLASH software, after filtering and removing the low-quality and short-length sequences, the UCHIME software was used to eliminate the chimera and obtain valid sequence data. The UPARSE software (version 7.11) was used to perform OTU clustering and analysis of sequences based on 97% similarity. The alpha diversity analysis was conducted to reveal the microbial diversity indices, such as the Chao, ACE, and Simpson diversity indices. The Wilcoxon signed-rank test was used to test the significance among groups on these indices. The beta diversity analysis was carried out using UniFrac to compare the results of PCoA at the OTU level with the community ecology package, R-forge (vegan 2.0 package was used to generate a PCA figure; the differences among the groups were tested). The hierarchical clustering analysis was performed using the Primer 5 software (Primer-E Ltd., United Kingdom). The original data are accessible at NCBI-BioProject under the accession number PRJNA822035.
Statistical Methods
Statistical analyses were performed by using SPSS version 18 (IBM, Armonk, NY, United States). Measurement data were presented as mean ± standard deviation (SD). Comparison between the two groups was performed by the Wilcoxon rank-sum test. Statistical significance was set at *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001. p ≤ 0.05 was considered statistically significant.
Results
Demographic Characteristics
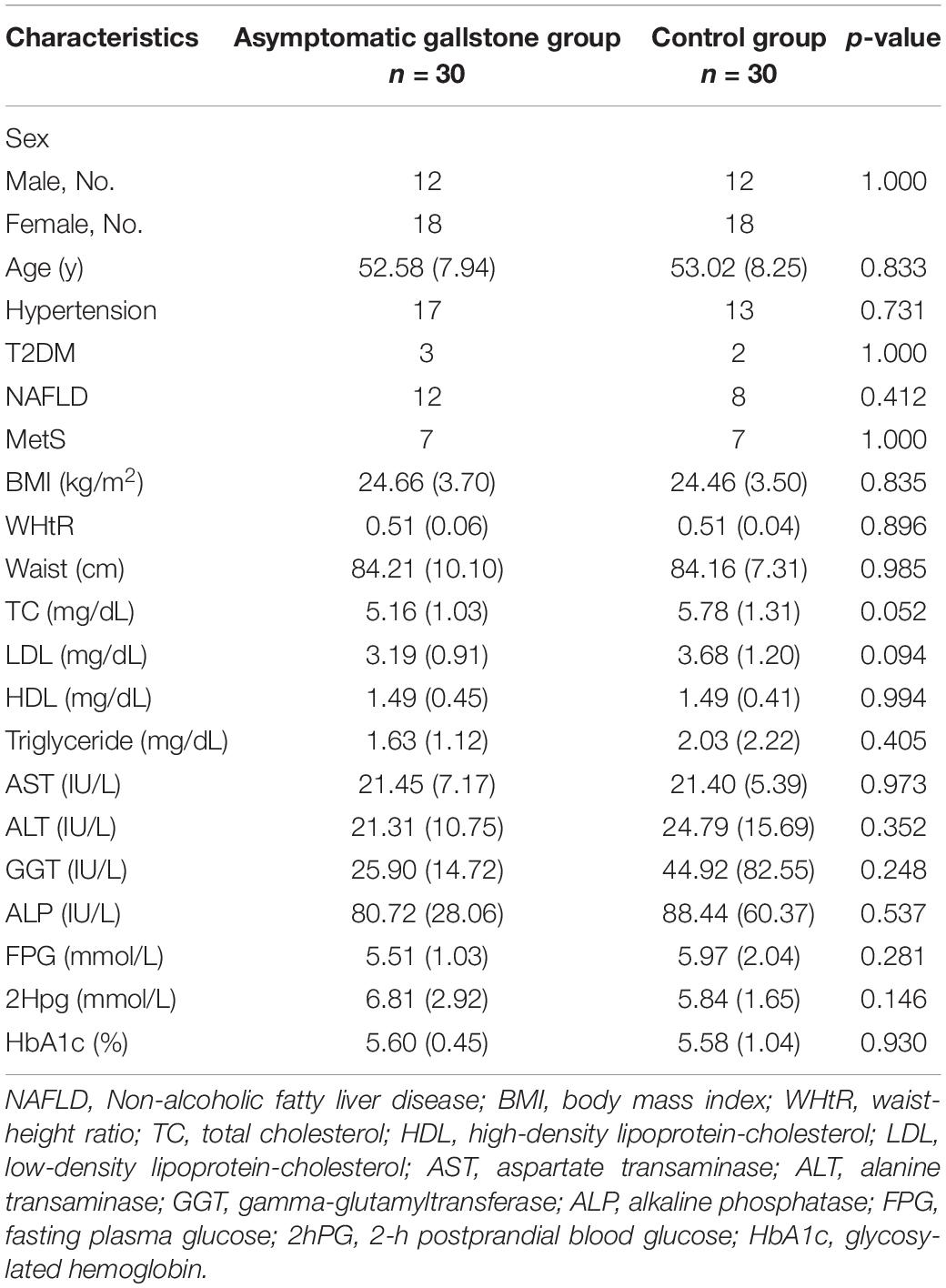
A total of 60 subjects (30 in the asymptomatic gallstone group and 30 in the control group) were analyzed. As shown in Table 1, the two groups were comparable with respect to the clinical characteristics of age, sex, BMI, hypertension, diabetes, NAFLD, and Mets. There were also no significant differences in the laboratory results, such as lipid metabolism index, glucose metabolism index, and liver function.
Gut Microbial Dysbiosis in Asymptomatic Gallstone Patients
Since the development of microbiota-targeted therapies is an intriguing new avenue for many diseases, changes in microbiome composition or function must first be carefully shown to contribute to GSD. To investigate the specific changes of microbiota in samples from asymptomatic gallstone patients, we performed 16SrDNA sequencing on samples collected from all the participants. We obtained a total of 3,579,322 effective sequences and 1,479,043,036 base pairs from DNA extracted from fecal samples of asymptomatic gallstone patients (n = 30) and controls (n = 30). The average length of each sequence was 413.2 base pairs. To investigate the diversity of gut microbiota in the two groups, we used the alpha diversity analysis. The results showed that the abundance of gut microbiota in the asymptomatic gallstones group was significantly increased compared with the control group (median chao1 index of 390.57 vs. 346.07 in the controls, p = 0.0364; median ace index of 389.65 vs. 348.32 in the controls, p = 0.0292), while the diversity of gut microbiota was decreased (median Simpson index of 0.13 vs. 0.11 in the controls, p = 0.0183) (Figure 1A). The principal coordinates analysis showed that there was no significant difference in beta diversity between the two groups (Figure 1B).
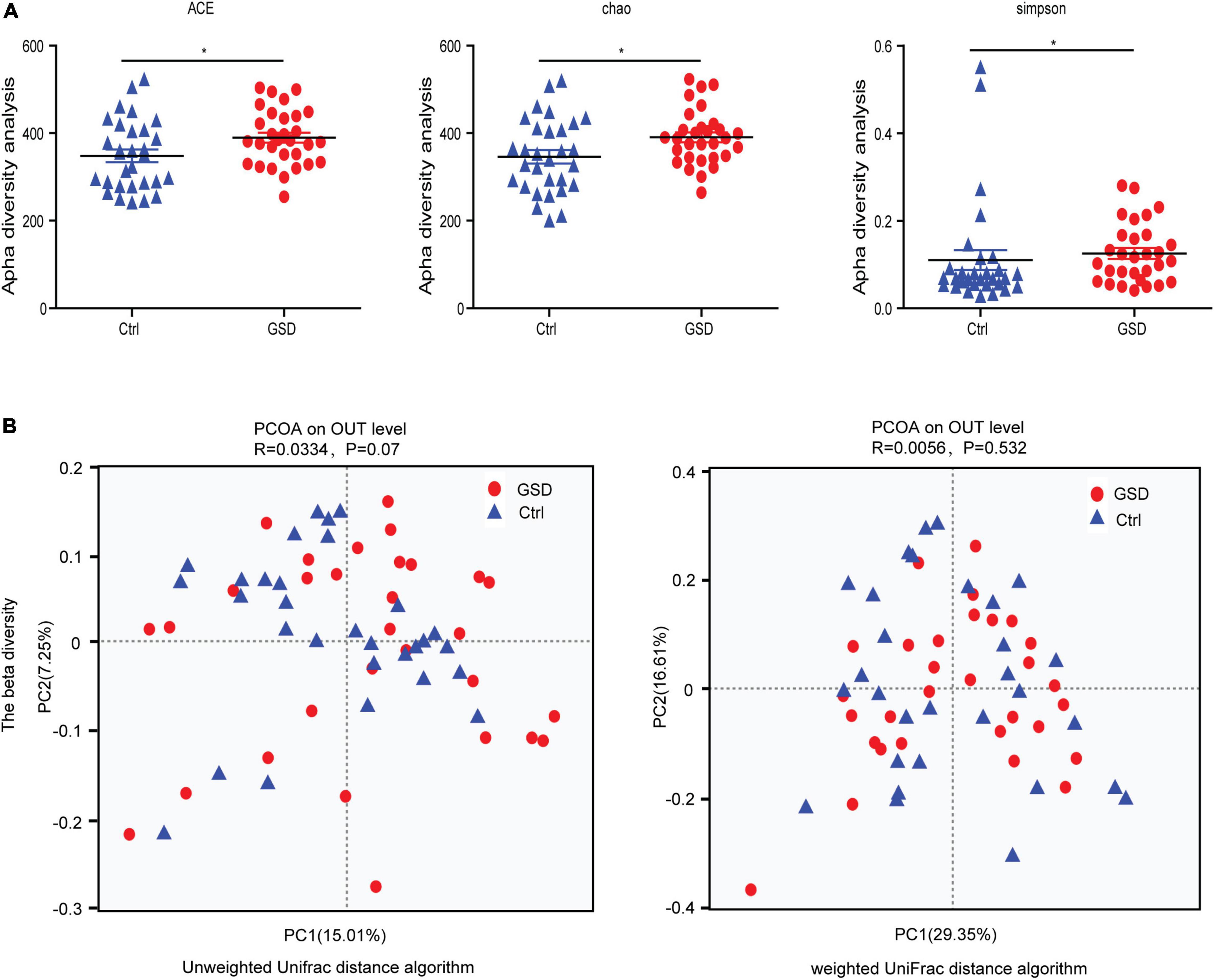
Figure 1. Microbial community characteristics generated by 16SrDNA sequencing. (A) Boxplots of the alpha-diversity indices in the asymptomatic gallstone patients and controls. From left to right, observed ACE, Chao, and Simpson index. (B) Principal coordinates analysis showed the composition of the asymptomatic gallstone patients and controls. *P < 0.05.
We next investigated the richness and evenness of the gut microbiota in all samples at the genus, species, and gene levels. At the phylum level, the top 4 abundance in descending order were Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. The average abundance of each was 62.63, 17.60, 12.51, and 5.37% in the gallstone group and 59.62, 18.64, 13.78, and 7.52% in the control group (Supplementary Table 1). However, there were also some samples with large variations in the abundance of the microbiota. For example, the abundance of Actinobacteria increased significantly in control-05, and Proteobacteria was the main bacterial phylum in the control-20 (Figure 2A).
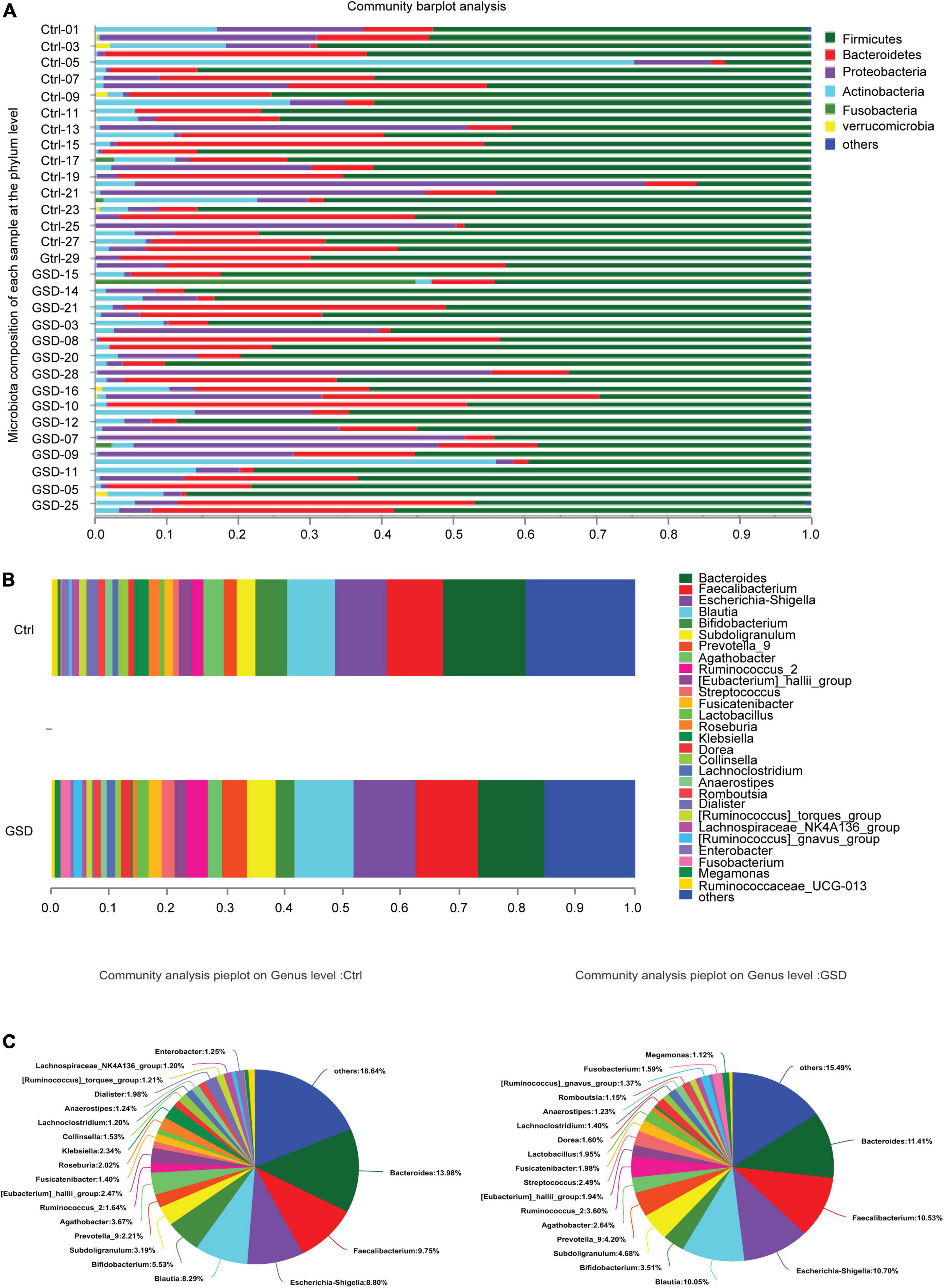
Figure 2. The microbiota composition of each sample at the genus and phylum level. (A) Microbiota composition of each sample at the phylum level. (B,C) The proportion of gut microbiota abundance in two groups (genus level). GSD, asymptomatic gallstone group; Ctrl, control group. *P < 0.05; **P < 0.01.
According to the proportion of microbiota abundance, the main groups ranked forward were Bacteroides, Faecalibacterium, Escherichia-Shigella, Blautia, Bifidobacterium, Prevotella_9, Subdoligranulum, Agathobacte, Ruminococcus_2, and [Eubacterium]_hallii_group at the genus level, while there was little difference between in 2 groups (Figure 2B). However, there was an increment abundance of Streptococcus, Lactobacillus, Dorea, Romboutsia, Fusobacterium, and Megamonas, while decrement of Klebsiella, Roseburia, Collinsella, Dialister, and Enterobacerin asymptomatic gallstone patients (Figure 2C).
Further abundance analysis showed that there were 15 genera with significant differences between the two groups at the genus level. Megamonas, Comamonas, Ruminococcaceae_UCG-014, Coprobacillus, Adlercreutzia, unclassified_p_Firmicutes, Morganella, CHKCI002, and Tyzzerella_4 were in relatively higher abundance in asymptomatic gallstone groups. Ruminococcaceae_UCG-008, Sutterella, GCA-900066755, Butyricicoccus, unclassified_o_Lactobacillales, and Lachnospiraceae_ND3007_group were significantly lower abundance in asymptomatic gallstone groups. Moreover, there were no Morganella and CHKCI002 detected in control patients and no Ruminococcaceae_UCG-008 in asymptomatic gallstone patients. It suggested that the declined abundance of Ruminococcaceae_UCG-008 and increased abundance of Morganella and CHKCI002 may act on the formation of GSD (Figures 3A,B).
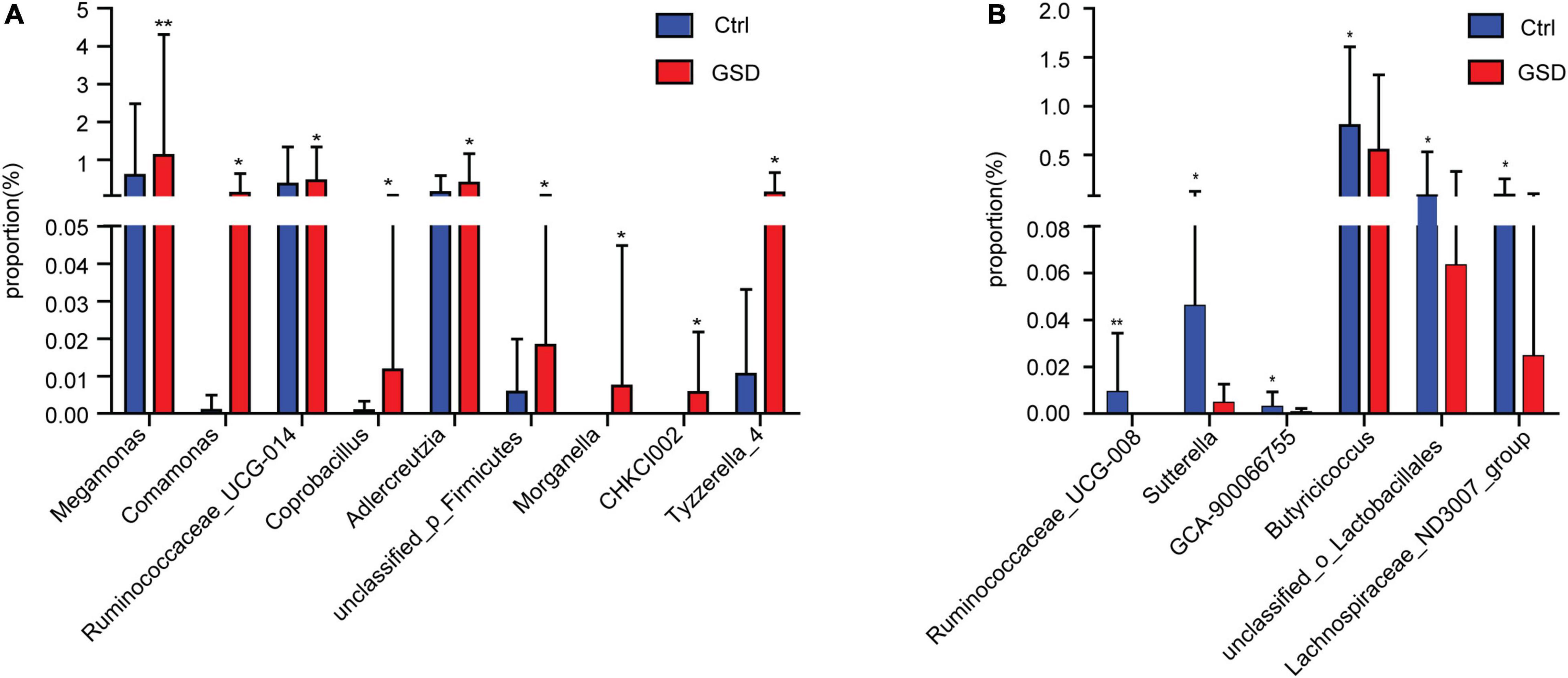
Figure 3. Comparison of gut microbiota abundance between asymptomatic gallstone group and control group at the genus level. (A) Genera with increasing abundance in asymptomatic gallstone group. (B) Genera with decreasing abundance in asymptomatic gallstone group. *P < 0.05; **P < 0.01.
Gut Microbial Dysbiosis in Asymptomatic Gallstone Patients Accompanied by Other Diseases
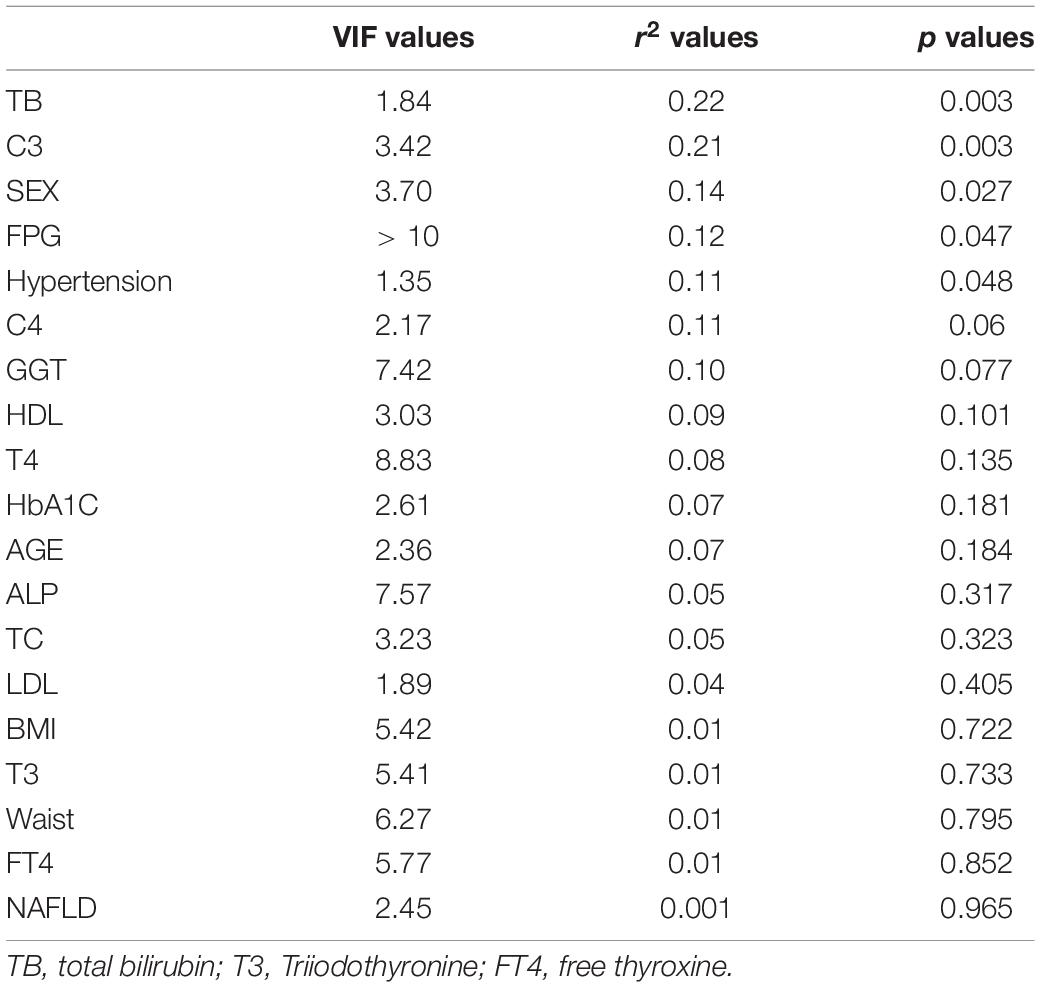
As we all know, hypertension and obesity are important risk factors for gallstones. Recently, the immune system was considered to be involved in cholesterol cholelithogenesis (Maurer et al., 2009). Here, we found that gender, hypertension, TBil, C3, C4, FPG, and GGT had a great influence on the results of flora with distance-based redundancy analysis (Figure 4A, Table 2 and Supplementary Table 2). The correlation analysis showed that Streptococcus and Lactobacillus were positively correlated with complement C3 and C4, while Roseburia and Eubacterium _ hallii_ Group were negatively correlated with C4 level. In addition, Eubecterium_hallii_Group and Lachnoclostridium, Collinella were positively correlated with hypertension (Figure 4B). To determine the effect of other diseases on microbial composition, we further analyzed the subgroups of patients with elevated BMI (≧24), non-alcoholic fatty liver disease, and hypertension in the gallstone group. At the genus level, there were eight bacterial taxa that displayed different abundance between asymptomatic gallstone patients with BMI ≧ 24 and BMI < 24, and all of them were increased in asymptomatic gallstone patients accompanied by BMI ≧ 24 (Figure 4C). Moreover, 11 bacterial taxa were found to display different abundance between asymptomatic gallstone patients accompanied by hypertension and without hypertension, while 7 were increased (Figure 4D) and 4 were decreased in asymptomatic gallstone patients accompanied by hypertension (Figure 4E). There were 13 bacterial taxa that displayed different abundance between asymptomatic gallstone patients accompanied by NAFLD and without NAFLD. Among them, 11 genera were enriched and 2 genera were decreased in asymptomatic gallstone patients accompanied by NAFLD. The abundance of Streptococcus in asymptomatic gallstone patients accompanied by NAFLD was mostly significantly ascended (Figure 4F). Furthermore, we found that among the 15 species with significant differences in genus level between the two groups, none of them showed differences in the three subgroups except unclassified_ Lactobacillales.
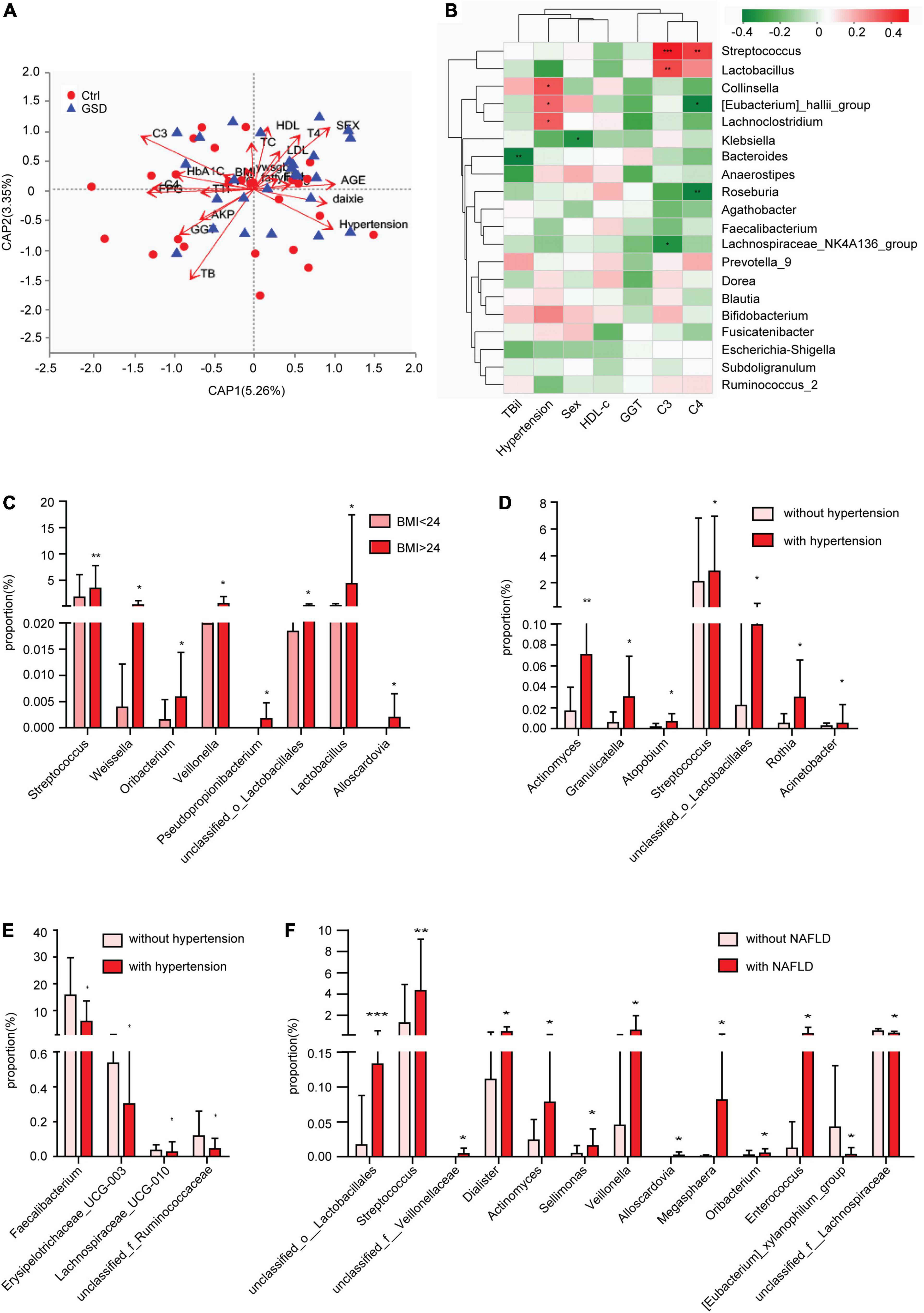
Figure 4. Gut microbial dysbiosis in asymptomatic gallstone patients accompanied with other diseases. (A) Influence index with distance-based redundancy analysis. (B) The relationship between different species (the top 20 abundance, p < 0.1 and VIF < 10) and clinical factors. (C) Differences of gut microbiota composition in asymptomatic gallstone patients with BMI < 24 or BMI ≧ 24 (genus level). (D,E) Differences of gut microbiota composition in asymptomatic gallstone patients with or without hypertension (genus level). (F) Differences of gut microbiota composition in asymptomatic gallstone patients with or without NAFLD (genus level). *P < 0.05; **P < 0.01.
Discussion
It is now well accepted that the pathogenesis of GSD is determined by genetic background, hepatic hypersecretion of biliary cholesterol, rapid precipitation of solid cholesterol crystals in bile, gallbladder dysmotility, and intestinal factors (with increased absorption of cholesterol, slow intestinal motility, and dysbiosis) (Di Ciaula et al., 2018). Intestinal dysbiosis makes a significant contribution to the development of GSD. Many reports are underlining the association of the gut microbiota with the pathogenesis of GSD in mice and humans. It was found that in the feces of mice with cholesterol gallstones (induced by a lithogenic diet), the Firmicutes/Bacteroidetes ratio and the Firmicutes content decreased, and the richness and alpha diversity of the microbiota also significantly reduced (Wang et al., 2017). Studies with experimental animal models indicated that enterohepatic helicobacters promote the formation of cholesterol gallstones in vivo (Maurer et al., 2005). The cholecystectomy could affect the gut microbiota, and the abundance of B. obeum and V. parvula, which have azoreductase activity, was increased in the cholecystectomy group (Yoon et al., 2019). Wang et al. (2020) found that the diversity of intestinal bacteria and the abundances of certain phylogroups significantly decreased, especially Firmicutes in the gallstone group. Keren et al. (2015) showed that intestinal microbial diversity, the abundances of the genus Roseburia and the species Bacteroides uniformis were decreased, and those of the family Ruminococcaceae and the genus Oscillospira were increased in patients with gallstones compared with the controls. Gallstone patients had higher overall concentrations of fecal bile acids, and Oscillospira may predispose individuals to cholesterol gallstones for it correlated negatively with primary bile acids and fecal cholesterol concentration and positively with the secondary bile lithocholic acid in the feces. In our study, we focused on the asymptomatic gallstone patients and found that the abundance of gut microbiota in the asymptomatic gallstones group was significantly increased compared with the control group, while the diversity of gut microbiota was also decreased. Although the main bacteria composition of the two groups is similar, there was an increment abundance of Streptococcus, Lactobacillus, Dorea, Romboutsia, Fusobacterium, and Megamonas, while decrement of Klebsiella, Roseburia, Collinsella, Dialister, and Enterobacerin asymptomatic gallstone patients. Some of them were consistent with many studies (Grigor’eva, 2020). Previous studies have shown that obesity is associated with an elevated Firmicutes/Bacteroidetes ratio in the gut microbiota (Grigor’eva, 2020), and obesity is a major risk factor for developing GSD. However, there was no significant difference in Firmicutes/Bacteroidetes ratio between the asymptomatic gallstone patients (3.56) and controls (3.20) in our study.
The human gut microbiota was composed of nine kinds of bacterial phyla, and dominated by four: Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteriat (Tap et al., 2009). Although there was no significant difference of the four bacterial phyla between the asymptomatic gallstone patients and controls in our study, we found that the abundance of Ruminococcaceae_UCG-008, Sutterella, GCA-900066755, Butyricicoccus, unclassified_o_Lactobacillales, and Lachnospiraceae_ND3007_group were significantly decreased in asymptomatic gallstone patients at the genus level. Among them, Ruminococcaceae_UCG-008, Butyricicoccus, Butyricicoccus, unclassified_o_Lactobacillales, and Lachnospiraceae_ND3007_group belong to the phylum of Firmicutes. Butyricicoccus species are considered autochthonous microbes predominantly colonizing the mucosa-associated mucus layer of the colon of mice and humans. It was decreased in patients with inflammatory bowel disease (Eeckhaut et al., 2013). The evidence from different studies shows that Lachnospiraceae might influencemetabolic syndrome, obesity, diabetes, liver diseases, IBD, and CKD (Vacca et al., 2020). Megamonas has not previously been reported as a dominant genus in gut microbiome studies with European and American subjects but was found in studies with Chinese individuals. The genus Megamonas was detected as highly abundant in colorectal cancer (Yachida et al., 2019). Morganella is an opportunistic secondary invader, acting on summer diarrhea, was founded in many cases (such as brain abscess, septicemia, tubo-ovarian abscess, and postoperative foot infection in a diabetic). It has been isolated from human urine, gallbladder, stool, sputum, and other respiratory samples, and assorted wound sites (O’Hara et al., 2000). The Comamonas have been shown to be capable of catabolizing a wide range of organic substrates, such as amino acids, carboxylic acids, steroids, and aromatic compounds. Some Comamonas species have been suggested to be involved in some invasive infections, like appendicitis, bacteremia, and meningitis (Wu et al., 2018). All Megamonas, Morganella, and Comamonas were significantly increased in asymptomatic gallstone patients. This indicated that the occurrence of gallstones may be related to the increase of pathogenic bacteria and the decrease of beneficial bacteria.
Our previous study revealed that metabolic diseases (such as hypertension and obesity) were closely related to the formation of gallstones (Song et al., 2020). Studies have shown that obesity and NAFLD were associated with an elevated Firmicutes/Bacteroidetes ratio in the gut microbiota (Leung et al., 2016; Grigor’eva, 2020). Evidence from the fecal microbiota transplantation (FMT) and interventions that target the gut microbiota, such as a high-fiber diet, probiotics, and antibiotics, reflected that gut dysbiosis plays a role in the genesis of hypertension (Li et al., 2017; Wilck et al., 2017; Yang et al., 2018). On the other hand, animal studies in which the gut microbiota are manipulated, and observational studies in patients with NAFLD, have provided considerable evidence that dysbiosis contributes to the pathogenesis of NAFLD. All of this indicated that the change in gut dysbiosis in our study may be related to NAFLD, obesity, or hypertension. Distance-based redundancy analysis also showed that hypertension, C3, and C4 had a great influence on the results of flora. Here, streptococcus and lactobacillus were positively correlated with complement C3 and C4, while Roseburia and Eubacterium_hallii_Group were negatively correlated with the C4 level. In addition, Eubecterium_hallii_Group and Lachnoclostridium, Collinella were positively correlated with hypertension. The abundance of Klebsiella in men is higher than that in women. However, among the 15 species with significant differences in genus level in the asymptomatic gallstone patients, there were only unclassified-o-Lactobacillales significantly different between the patients with BMI≧24 or BMI < 24, with or without hypertension, with or without NAFLD. These indicate that most of the 15 species are specially related to gallstones. Streptococcus significantly increased in the asymptomatic gallstone patients with BMI≧24, hypertension, or NAFLD. It revealed that Streptococcus plays an important role in the pathogenesis of obesity, hypertension, and NAFLD.
In conclusion, our data confirmed that the diversity of the gut microbiota in asymptomatic gallstone patients was decreased, while the abundance was significantly increased compared with the control group. The gut microbiota in patients with asymptomatic gallstone have shown a significant increase in the phyla Firmicutes (Megamonas, Coprobacillus, Ruminococcaceae_UCG-014, unclassified_p_Firmicutes, Tyzzerella_4), Actinobacteria (Adlercreutzia, CHKCI002), Proteobacteria (Comamonas, Morganella), and a significant decrease in the phyla Firmicutes (Ruminococcaceae_UCG-008, unclassified_o_Lactobacillales, GCA-900066755, Butyricicoccus, Lachnospiraceae_ND3007_group), and Proteobacteria (Sutterella). Most of them are especially associated with the asymptomatic gallstone, not with obesity, NAFLD, and hypertension.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/, PRJNA822035.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Changzheng Hospital (no. 2016SL039). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XZ and L-YC obtained funding. S-TS and L-YC designed the study and wrote the manuscript. S-TS performed the experiments and contributed to the analysis and interpretation of data. L-YC performed the statistical analysis. XZ provided material support. W-FX proposed initial proposal and revised the manuscript.
Funding
This work was funded by the Medical Discipline Construction Project of the Pudong Health Committee of Shanghai (Grant No. PWYgf2021-02), and the Health and Technology Project of the Pudong Health Committee of Shanghai (Grant No. PW2021A-38).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Mei-Dong Xu (Shanghai, China) for aiding the efforts of the authors.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.882265/full#supplementary-material
Footnotes
References
Bakris, G., Ali, W., and Parati, G. (2019). ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J. Am. Coll. Cardiol. 73, 3018–3026. doi: 10.1016/j.jacc.2019.03.507
Bogdanos, D. P., Baum, H., Okamoto, M., Montalto, P., Sharma, U. C., Rigopoulou, E. I., et al. (2005). Primary biliary cirrhosis is characterized by IgG3 antibodies cross-reactive with the major mitochondrial autoepitope and its Lactobacillus mimic. Hepatology 42, 458–465. doi: 10.1002/hep.20788
Di Ciaula, A., Wang, D. Q., and Portincasa, P. (2018). An update on the pathogenesis of cholesterol gallstone disease. Curr. Opin. Gastroenterol. 34, 71–80. doi: 10.1097/MOG.0000000000000423
Eeckhaut, V., Machiels, K., Perrier, C., Romero, C., Maes, S., Flahou, B., et al. (2013). Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 62, 1745–1752.
European Association for the Study of the Liver [EASL] (2016). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J. Hepatol. 65, 146–181. doi: 10.1016/j.jhep.2016.03.005
Everhart, J. E., Khare, M., Hill, M., and Maurer, K. R. (1999). Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 117, 632–639. doi: 10.1016/s0016-5085(99)70456-7
Fandriks, L. (2017). Roles of the gut in the metabolic syndrome: an overview. J. Intern. Med. 281, 319–336. doi: 10.1111/joim.12584
Grigor’eva, I. N. (2020). Gallstone disease, obesity and the firmicutes/bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 11:13. doi: 10.3390/jpm11010013
Jia, W., Weng, J., Zhu, D., Ji, L., Lu, J., Zhou, Z., et al. (2019). Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 35:e3158. doi: 10.1002/dmrr.3158
Jian-Gao, F., and Chinese Liver Disease Association (2010). Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Gan Zang Bing Za Zhi 18, 163–166.
Karlsson, F., Tremaroli, V., Nielsen, J., and Backhed, F. (2013). Assessing the human gut microbiota in metabolic diseases. Diabetes 62, 3341–3349. doi: 10.2337/db13-0844
Kawai, M., Iwahashi, M., Uchiyama, K., Ochiai, M., Tanimura, H., and Yamaue, H. (2002). Gram-positive cocci are associated with the formation of completely pure cholesterol stones. Am. J. Gastroenterol. 97, 83–88. doi: 10.1111/j.1572-0241.2002.05425.x
Keren, N., Konikoff, F. M., Paitan, Y., Gabay, G., Reshef, L., Naftali, T., et al. (2015). Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ. Microbiol. Rep. 7, 874–880. doi: 10.1111/1758-2229.12319
Lammert, F., Gurusamy, K., Ko, C. W., Miquel, J. F., Mendez-Sanchez, N., Portincasa, P., et al. (2016). Gallstones. Nat. Rev. Dis. Primers 2:16024.
Lemoinne, S., and Marteau, P. (2017). Gut microbial profile in primary biliary cholangitis: towards bioindicators. Clin. Res. Hepatol. Gastroenterol. 41, 507–508. doi: 10.1016/j.clinre.2017.06.002
Leung, C., Rivera, L., Furness, J. B., and Angus, P. W. (2016). The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425.
Li, J., Zhao, F., Wang, Y., Chen, J., Tao, J., Tian, G., et al. (2017). Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5:14. doi: 10.1186/s40168-016-0222-x
Maurer, K. J., Carey, M. C., and Fox, J. G. (2009). Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology 136, 425–440. doi: 10.1053/j.gastro.2008.12.031
Maurer, K. J., Ihrig, M. M., Rogers, A. B., Ng, V., Bouchard, G., Leonard, M. R., et al. (2005). Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology 128, 1023–1033. doi: 10.1053/j.gastro.2005.01.008
O’Hara, C. M., Brenner, F. W., and Miller, J. M. (2000). Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 13, 534–546. doi: 10.1128/CMR.13.4.534
Portincasa, P., Di Ciaula, A., de Bari, O., Garruti, G., Palmieri, V. O., and Wang, D. Q. (2016). Management of gallstones and its related complications. Expert Rev. Gastroenterol. Hepatol. 10, 93–112. doi: 10.1586/17474124.2016.1109445
Sayin, S. I., Wahlstrom, A., Felin, J., Jantti, S., Marschall, H. U., Bamberg, K., et al. (2013). Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235. doi: 10.1016/j.cmet.2013.01.003
Shabanzadeh, D. M. (2018). Incidence of gallstone disease and complications. Curr. Opin. Gastroenterol. 34, 81–89.
Shaffer, E. A. (2006). Gallstone disease: epidemiology of gallbladder stone disease. Best Pract. Res. Clin. Gastroenterol. 20, 981–996. doi: 10.1016/j.bpg.2006.05.004
Song, S. T., Shi, J., Wang, X. H., Guo, Y. B., Hu, P. F., Zhu, F., et al. (2020). Prevalence and risk factors for gallstone disease: a population-based cross-sectional study. J. Dig. Dis. 21, 237–245. doi: 10.1111/1751-2980.12857
Soreide, K. (2017). Gallstone disease and cancer risk: finding the bug in the system. Gastroenterology 152, 1825–1828. doi: 10.1053/j.gastro.2017.04.028
Tabibian, J. H., Talwalkar, J. A., and Lindor, K. D. (2013). Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed. Res. Int. 2013:389537. doi: 10.1155/2013/389537
Tap, J., Mondot, S., Levenez, F., Pelletier, E., Caron, C., Furet, J. P., et al. (2009). Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 11, 2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x
Vacca, M., Celano, G., Calabrese, F. M., Portincasa, P., Gobbetti, M., and De Angelis, M. (2020). The controversial role of human gut lachnospiraceae. Microorganisms 8:573. doi: 10.3390/microorganisms8040573
Wang, D. Q., Cohen, D. E., and Carey, M. C. (2009). Biliary lipids and cholesterol gallstone disease. J. Lipid Res. 50 (Suppl), S406–S411.
Wang, Q., Hao, C., Yao, W., Zhu, D., Lu, H., Li, L., et al. (2020). Intestinal flora imbalance affects bile acid metabolism and is associated with gallstone formation. BMC Gastroenterol. 20:59. doi: 10.1186/s12876-020-01195-1
Wang, Q., Jiao, L., He, C., Sun, H., Cai, Q., Han, T., et al. (2017). Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 17:74. doi: 10.1186/s12876-017-0629-2
Wang, Y., Qi, M., Qin, C., and Hong, J. (2018). Role of the biliary microbiome in gallstone disease. Expert Rev. Gastroenterol. Hepatol. 12, 1193–1205. doi: 10.1080/17474124.2018.1533812
Wilck, N., Matus, M. G., Kearney, S. M., Olesen, S. W., Forslund, K., Bartolomaeus, H., et al. (2017). Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589. doi: 10.1038/nature24628
Wu, Y., Zaiden, N., and Cao, B. (2018). the core- and pan-genomic analyses of the genus comamonas: from environmental adaptation to potential virulence. Front. Microbiol. 9:3096. doi: 10.3389/fmicb.2018.03096
Yachida, S., Mizutani, S., Shiroma, H., Shiba, S., Nakajima, T., Sakamoto, T., et al. (2019). Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976. doi: 10.1038/s41591-019-0458-7
Yang, T., Richards, E. M., Pepine, C. J., and Raizada, M. K. (2018). The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456. doi: 10.1038/s41581-018-0018-2
Keywords: gut microbial, asymptomatic gallstone, metabolic diseases, 16SrDNA, hypertension, non-alcoholic fatty liver disease, obesity
Citation: Song S-T, Cai L-Y, Zeng X and Xie W-F (2022) Gut Microbial Profile in Asymptomatic Gallstones. Front. Microbiol. 13:882265. doi: 10.3389/fmicb.2022.882265
Received: 28 February 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Hein M. Tun, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Jingwan Zhang, The Chinese University of Hong Kong, ChinaCong He, The First Affiliated Hospital of Nanchang University, China
Copyright © 2022 Song, Cai, Zeng and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Zeng, emVuZ3hpbm1kMTk3OEAxNjMuY29t; Wei-Fen Xie, d2VpZmVueGllQG1lZG1haWwuY29tLmNu
†These authors have contributed equally to this work and share first authorship
 Sen-Tao Song
Sen-Tao Song Ling-Yan Cai
Ling-Yan Cai Xin Zeng
Xin Zeng Wei-Fen Xie2*
Wei-Fen Xie2*