
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 19 May 2022
Sec. Microbiotechnology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.874627
 Ramita Khanongnuch1*
Ramita Khanongnuch1* Rahul Mangayil1
Rahul Mangayil1 Ville Santala1
Ville Santala1 Anne Grethe Hestnes2
Anne Grethe Hestnes2 Mette Marianne Svenning2
Mette Marianne Svenning2 Antti J. Rissanen1
Antti J. Rissanen1
Methane (CH4) is a sustainable carbon feedstock for value-added chemical production in aerobic CH4-oxidizing bacteria (methanotrophs). Under substrate-limited (e.g., oxygen and nitrogen) conditions, CH4 oxidation results in the production of various short-chain organic acids and platform chemicals. These CH4-derived products could be broadened by utilizing them as feedstocks for heterotrophic bacteria. As a proof of concept, a two-stage system for CH4 abatement and 1-alkene production was developed in this study. Type I and Type II methanotrophs, Methylobacter tundripaludum SV96 and Methylocystis rosea SV97, respectively, were investigated in batch tests under different CH4 and air supplementation schemes. CH4 oxidation under either microaerobic or aerobic conditions induced the production of formate, acetate, succinate, and malate in M. tundripaludum SV96, accounting for 4.8–7.0% of consumed carbon from CH4 (C-CH4), while M. rosea SV97 produced the same compounds except for malate, and with lower efficiency than M. tundripaludum SV96, accounting for 0.7–1.8% of consumed C-CH4. For the first time, this study demonstrated the use of organic acid-rich spent media of methanotrophs cultivating engineered Acinetobacter baylyi ADP1 ‘tesA-undA cells for 1-alkene production. The highest yield of 1-undecene was obtained from the spent medium of M. tundripaludum SV96 at 68.9 ± 11.6 μmol mol Csubstrate–1. However, further large-scale studies on fermenters and their optimization are required to increase the production yields of organic acids in methanotrophs.
Methane (CH4) is the second most important greenhouse gas (GHG) after CO2, with a global warming potential (GWP) approximately 30 times higher than that of CO2 over a 100-year time horizon (IPCC, 2021). CH4 emissions from anthropogenic activities have been continuously increasing, accounting for approximately 60% of total CH4 emissions [based on top-down estimates reported by Saunois et al. (2020)]. Hence, stringent climate policy maintained over the next several decades, particularly regarding the use of CH4 as an energy source, is suggested to significantly reduce CH4 emissions (Harmsen et al., 2020). In environmental carbon flux, CH4-oxidizing bacteria are key regulators of CH4 abatement (Hanson and Hanson, 1996; Saarela et al., 2020). Due to its abundance and potential as a sustainable carbon feedstock, the development of biological processes for CH4 conversion to bio-based chemicals/liquid fuels is a promising and attractive research area (López et al., 2013; Sun et al., 2018; Liu et al., 2020).
Aerobic CH4-oxidizing bacteria (methanotrophs) containing methane monooxygenases use CH4 as the sole carbon and energy source and oxygen as an electron acceptor (Hanson and Hanson, 1996; Khider et al., 2021). Methanotrophs are classified into gammaproteobacterial (Type I and Type X) and alphaproteobacterial (Type II) methanotrophs based on their different pathways for formaldehyde assimilation into biomass, which is the ribulose monophosphate (RuMP) and serine cycles, respectively (Kalyuzhnaya et al., 2015; Guerrero-Cruz et al., 2021). Along with these pathways, CH4 can be converted into various value-added products, including methanol, single-cell protein, ectoine, and soluble metabolites (Ge et al., 2014; Sheets et al., 2016; Strong et al., 2016; Cantera et al., 2018). In various environmental processes and biological systems, methanotrophs have been reported to support other bacteria by producing organic carbon sources from CH4, such as wastewater treatment (Costa et al., 2000; Cao et al., 2019) and heavy metal bioremediation (Lai et al., 2016; Karthikeyan et al., 2021). The pathways for the excretion of organic acids (e.g., formate, acetate, lactate, and succinate) have been observed in experiments and deciphered from the genomes of Type I methanotrophs during O2-limiting conditions including the Embden–Meyerhof–Parnas pathway and tricarboxylic acid (TCA) cycle (Figure 1; Kalyuzhnaya et al., 2013; Gilman et al., 2017). In addition to O2-limiting conditions, acetate production was also recently detected in liquid cultures of methanotrophs incubated under aerobic conditions (Lee et al., 2021; Takeuchi and Yoshioka, 2021). These studies also showed the potential of CH4-derived organic acids in biotechnological applications by cultivating Type I methanotrophs and heterotrophs in co-culture systems, where the heterotrophs utilize organic acids produced by methanotrophs (Lee et al., 2021; Takeuchi and Yoshioka, 2021). For example, acetate produced by Methylocaldum marinum S8 has been successfully used as a growth medium for Cupriavidus necator cultivation (Takeuchi and Yoshioka, 2021). In addition, a co-culture of Methylococcus capsulatus Bath and engineered Escherichia coli SBA01 has been demonstrated for mevalonate production (Lee et al., 2021).
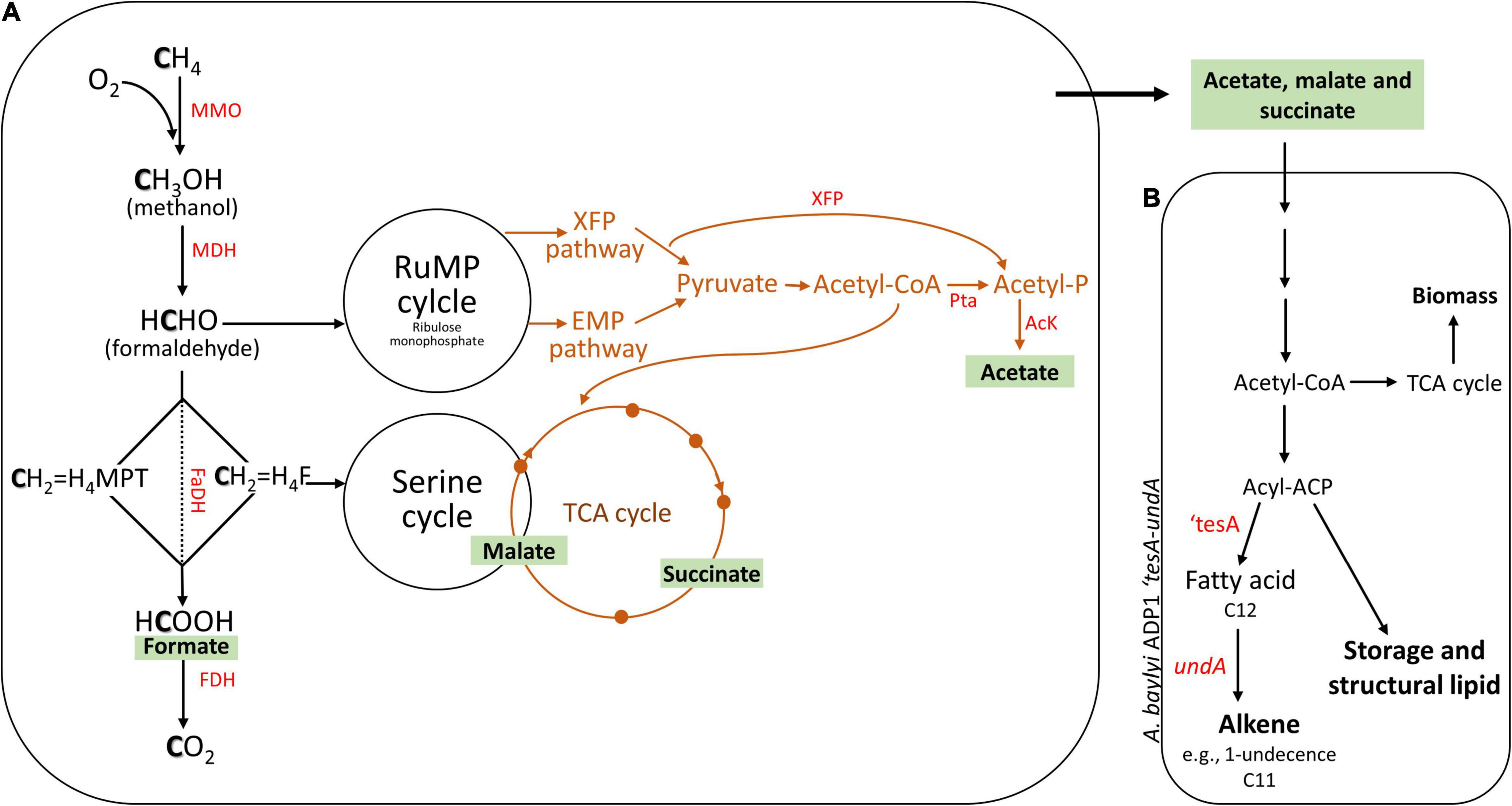
Figure 1. Principle of a two-stage bacterial process. The estimated pathway of the production of various organic acids could occur in methanotrophs (A). Organic acid-rich spent medium was fed to the engineered Acinetobacter baylyi ADP1 for alkene production (B). Aerobic CH4 oxidation pathway in Type I (RuMP cycle) and Type II (Serine cycle) methanotrophs (black arrows) and the possible pathway of CH4 oxidation under O2-limiting conditions via glycolysis-based CH4 fermentation mode (brown line). RuMP, ribulose monophosphate; EMP, Embden–Meyerhof–Parnas; TCA, tricarboxylic acid; MMO, methane monooxygenase; MDH, methanol dehydrogenase; FaDH, formaldehyde dehydrogenase; H4F, tetrahydrofolate pathway; H4MPT, tetrahydromethanopterin pathway; FDH, formate dehydrogenase; PTa, phosphate acetyltransferase; AcK, acetate kinase; XFP, phosphoketolase xylulose 5-phosphate/fructose 6-phosphate phosphoketolase; ‘tesA, thioesterase; undA, decarboxylase. Modified from Gilman et al. (2017) and Luo et al. (2019).
Acinetobacter baylyi ADP1, a naturally competent gram-negative gammaproteobacteria, has been recently attracted interest in bioengineering. Recently, A. baylyi ADP1 was used for lignin valorization into long-chain alkyl esters and 1-alkene compounds (e.g., 1-undecene) by developing an engineered A. baylyi ADP1 (‘tesA-undA) strain (Luo et al., 2019; Salmela et al., 2020). As a platform chemical, 1-undecene can be used in the chemical synthesis of medium-chain poly-α-olefins (C33), which are commonly used as lubricants (Salmela et al., 2020). Furthermore, the ability of A. baylyi ADP1 to utilize fermentation by-products generated by other bacteria has been exploited to broaden the metabolic landscape of biological production processes (Salmela et al., 2018; Mangayil et al., 2019). Hence, A. baylyi ADP1, as well as its engineered strain, is a promising candidate for the bioconversion of CH4-derived organic acids. However, it is critical to identify a suitable methanotroph partner for such applications. For instance, whether Type I or Type II methanotrophs are good candidates for converting CH4 to organic acids for A. baylyi ADP1 is not known; to our knowledge, a comparison of organic acid production yields between Type I and Type II methanotrophs has not been previously reported. Nevertheless, CH4-derived compounds are limited to short carbon chain carbon compounds (C2–C6), which could be further used as feedstock for various heterotrophic bacteria and can be easily engineered to extend the range of CH4-derived value-added (platform) chemicals.
This study aimed to demonstrate the production of organic acids in both Type I and Type II methanotrophs and its application as a growth medium for an engineered A. baylyi ADP1 strain with the aim of high-value product formation. Thus, the growth and metabolite production profiles of Type I and Type II methanotrophs, Methylobacter tundripaludum SV96 and Methylocystis rosea SV97, respectively, were investigated under different gas supplementation schemes. Next, the possibility of using the spent media of methanotrophs as a growth substrate for the cultivation of wild-type A. baylyi ADP1 was tested. Finally, the synthesis of 1-alkenes from CH4 was demonstrated using a two-stage process with a methanotroph, that is, M. tundripaludum SV96 and M. rosea SV97, and an engineered A. baylyi ADP1 (‘tesA-undA) strain (Luo et al., 2019).
M. tundripaludum SV96 and M. rosea SV97 isolated from Arctic wetland soils in Norway (Wartiainen et al., 2006a,b) were used in this study. Methanotrophs were cultivated on nitrate mineral salt (NMS) medium (DSMZ medium 921; initial pH ∼6.80) with the addition of 1 mM lanthanum chloride (LaCl3). The pre-inoculum was grown in 120-ml serum bottles containing 10-ml NMS medium, 20% CH4, and 80% air in headspace and incubated statically at 20°C. All experiments were conducted under sterile conditions, and the serum bottles used for methanotroph cultivation were sealed with butyl rubber stoppers and capped with aluminum crimps.
Wild-type and engineered A. baylyi ADP1 strains (Luo et al., 2019) carrying the plasmid pBAV1C-‘tesA-undA (A. baylyi ADP1 ‘tesA-undA) were used to evaluate the growth on the methanotroph spent media. For the pre-inoculum, A. baylyi ADP1 cells were inoculated in 10-ml culture tubes containing LB medium (5 g L–1 yeast extract, 10 g L–1 tryptone, and 5 g L–1 NaCl) supplemented with 0.5% glucose and 25 μg mL–1 chloramphenicol (for A. baylyi ADP1 ‘tesA-undA). The inoculated tubes were aerobically grown overnight at 30°C and 300 rpm. For 1-undecene synthesis, A. baylyi ADP1 ‘tesA-undA was induced with 0.5 mM cyclohexanone (Luo et al., 2019).
Batch tests were performed in 120-ml airtight serum bottles with a working volume of 15 ml of NMS medium. The tests were conducted in triplicates. The precultures of M. tundripaludum SV96 and M. rosea SV97 were inoculated at an initial optical density at 600 nm (OD600nm) of 0.02, and the growth, CH4 utilization, and organic acid profiles were monitored every 2 days for 14 days at 20°C under static conditions. Both methanotrophs were tested under the three different gas supplementation schemes (Figure 2). On day 0, the bottles were filled with 20% CH4 and 80% air into the headspace, accounting for the initial O2/CH4 molar ratio of ∼1.2, and incubated for 7 days. After the CH4 and O2 concentrations were depleted on day 7, the batch bottles were supplemented with three gas compositions into the headspace: test I: CH4 + air (20% CH4 and 80% air), test II: only CH4 (20% CH4 and 80% N2), and test III: only air (20% air and 80% N2). Bottles containing only NMS without bacterial cells were used as controls. The bottles were incubated for 7 days (days 8–14). Biomass growth (OD600mm), gas composition in the headspace, and organic acid accumulation in the liquid medium were monitored every 2 days.
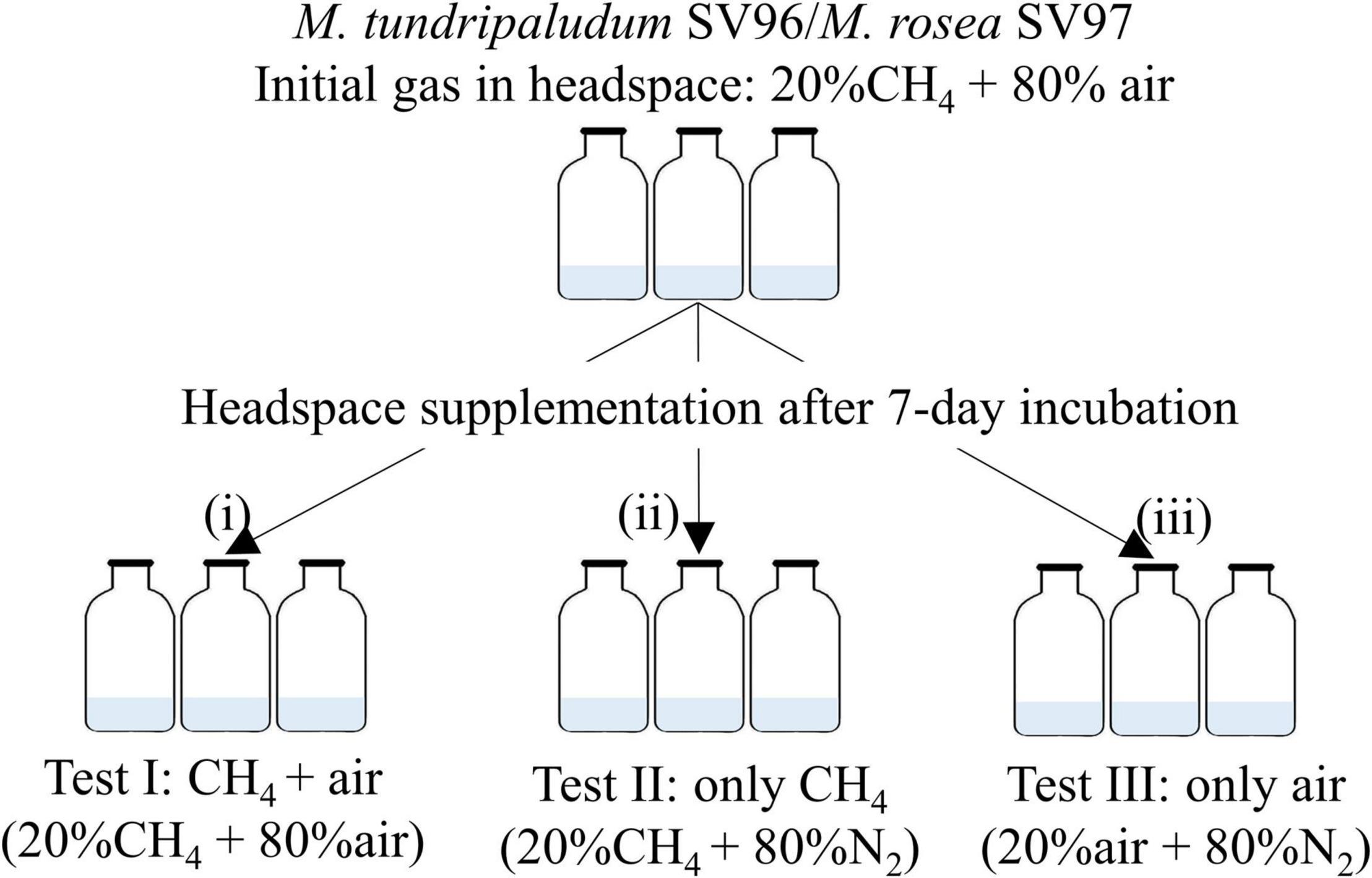
Figure 2. Experimental setup for methanotrophs under three different gas supplementation schemes applied on day 7 including test I: both CH4 and air added, test II only CH4 added, and test III only air added.
Prior to testing with an engineered A. baylyi ADP1, the capacity of A. baylyi ADP1 cells to utilize the spent media of methanotrophs was tested. To obtain methanotroph spent media, M. tundripaludum SV96 and M. rosea SV97 were cultivated in 500-ml airtight bottles with 60-ml NMS medium, as described previously (see section “Evaluation of Organic Acid Production by M. tundripaludum SV96 and M. rosea SV97”). To maximize the organic acid concentration, the bottle headspace was initially filled with 20% CH4 and 80% air and cultivated for 28 days under optimal conditions for M. tundripaludum SV96 and M. rosea SV97. The gaseous composition in the headspace and organic acid concentrations in the liquid medium were monitored twice a week.
After 28 days, the cultivation of methanotrophs was stopped, and spent media were used for A. baylyi ADP1 cultivation. The spent media was collected by centrifugation at 12,000 rpm for 10 min. The 50-ml supernatant of both methanotrophs was transferred to 250-ml sterile flasks and used for A. baylyi ADP1 cultivation. All tests were conducted in duplicate and incubated at 30°C and 300 rpm. After 4 h of cultivation, the liquid culture was collected to monitor the cell growth, organic acid concentration, and wax ester production. The spent media of methanotrophs without A. baylyi ADP1 were used as a contamination control (control 1), and A. baylyi ADP1 cells in NMS (fresh medium) were used to determine their background growth (control 2).
To obtain the methanotroph spent media, both M. tundripaludum SV96 and M. rosea SV97 were cultivated in 500-ml airtight bottles with 60-ml NMS medium, as described in section “Cultivation of Wild-Type A. baylyi ADP1 in Spent Media of Different Methanotrophs.” After 28 days, the spent media were collected and directly used for cultivation to identify any potential inhibitory effects on the growth of A. baylyi ADP1 cells imparted by the spent media supernatant (see section “Cultivation of Wild-Type A. baylyi ADP1 in Spent Media of Different Methanotrophs”). Thus, the original spent media, containing the methanotrophs and organic acids, devoid of any nutrient supplementation were employed as the growth medium for A. baylyi ADP1 cultivations in this experiment. The tests were conducted in quadruplicate in a sealed 20-ml glass tube containing 5 ml of the spent medium. Subsequently, A. baylyi ADP1 ‘tesA-undA cells (initial OD600nm, 0.02) were added to vials. After 1 h of incubation at 30°C and 300 rpm, 0.5 mM of cyclohexanone was added to the vials to induce 1-undecene synthesis and the vials were further incubated for 23 h at 30°C and 300 rpm. Liquid culture samples from M. tundripaludum SV96 and M. rosea SV97 cultivation without A. baylyi ADP1 ‘tesA-undA were included as experimental controls. The growth of A. baylyi ADP1 ‘tesA-undA was estimated based on the difference in OD600nm between the tests and controls after incubation for 24 h (ΔOD600nm).
Liquid samples were filtered through a 0.2 μm membrane (Chromafil® Xtra PET-20/25, Macherey-Nagel, Düren, Germany) prior to liquid metabolite analysis using a Shimadzu high-performance liquid chromatograph equipped with a Rezex RHM-Monosaccharide H+ column (Phenomenex, Torrance, CA, United States), as described by Okonkwo et al. (2018). The gas samples in the headspace (CH4, CO2, O2, and N2) were measured using a Shimadzu gas chromatograph GC-2014 equipped with a thermal conductivity detector and a Carboxen-1000 60/80 column (Agilent Technologies, Santa Clara, CA, United States). The oven temperature was held at 35°C for 3.75 min and then increased with the rate of 30°C min–1 until 150°C for 3 min. The injector and detector were 155 and 160°C, respectively. Helium was used as the carrier gas at 30 ml min–1.
1-undecene in the headspace was detected using solid phase microextraction gas chromatography–mass spectrometry, as previously described by Luo et al. (2019). Compounds were identified using the NIST/EPA/NIH Mass Spectral Library (NIST 05). Bacterial growth was measured as OD600nm using an Ultrospec 500 pro spectrophotometer (Amersham Biosciences, United Kingdom) and as cell dry weight (CDW) using the gravimetric method. The conversion factor between CDW and OD600nm obtained from the experimental measurements was 0.2914 g L–1 OD–1 (R2 = 0.9948) and 0.3015 g L–1 OD–1 (R2 = 0.9892) for M. tundripaludum SV96 and M. rosea SV97, respectively.
Statistical analysis was performed using Minitab 16.0 (United States). Significant differences in the obtained data sets (e.g., growth of the tested strains, gas concentrations and utilization, and concentrations and yields of the products produced by each strain) within the varied treatments were analyzed using one-way analysis of variance with Tukey’s multiple comparison tests at the 95% confidence interval, where P-value ≤ 0.05 was considered statistically significant.
Both M. tundripaludum SV96 and M. rosea SV97 used CH4 as the sole carbon and energy source and O2 as an electron acceptor. The biomass production of M. tundripaludum SV96 was greater than that of M. rosea SV97 in all tests (Figure 3). Highest biomass production of both methanotrophs was observed in the supplementation of both CH4 + air (test I) on day 14 at concentrations of 0.60 ± 0.03 and 0.48 ± 0.05 g CDW L–1 for M. tundripaludum SV96 and M. rosea SV97, respectively. In all tests, the growth yield of M. rosea SV97 was lower than that of M. tundripaludum SV96 (P < 0.05) because of the typical carbon assimilation pathway of Type I methanotrophs (RuMP), showing more efficient channeling of carbon from CH4 (C-CH4) to biomass than that of Type II methanotrophs (serine cycle) (Kalyuzhnaya et al., 2015).
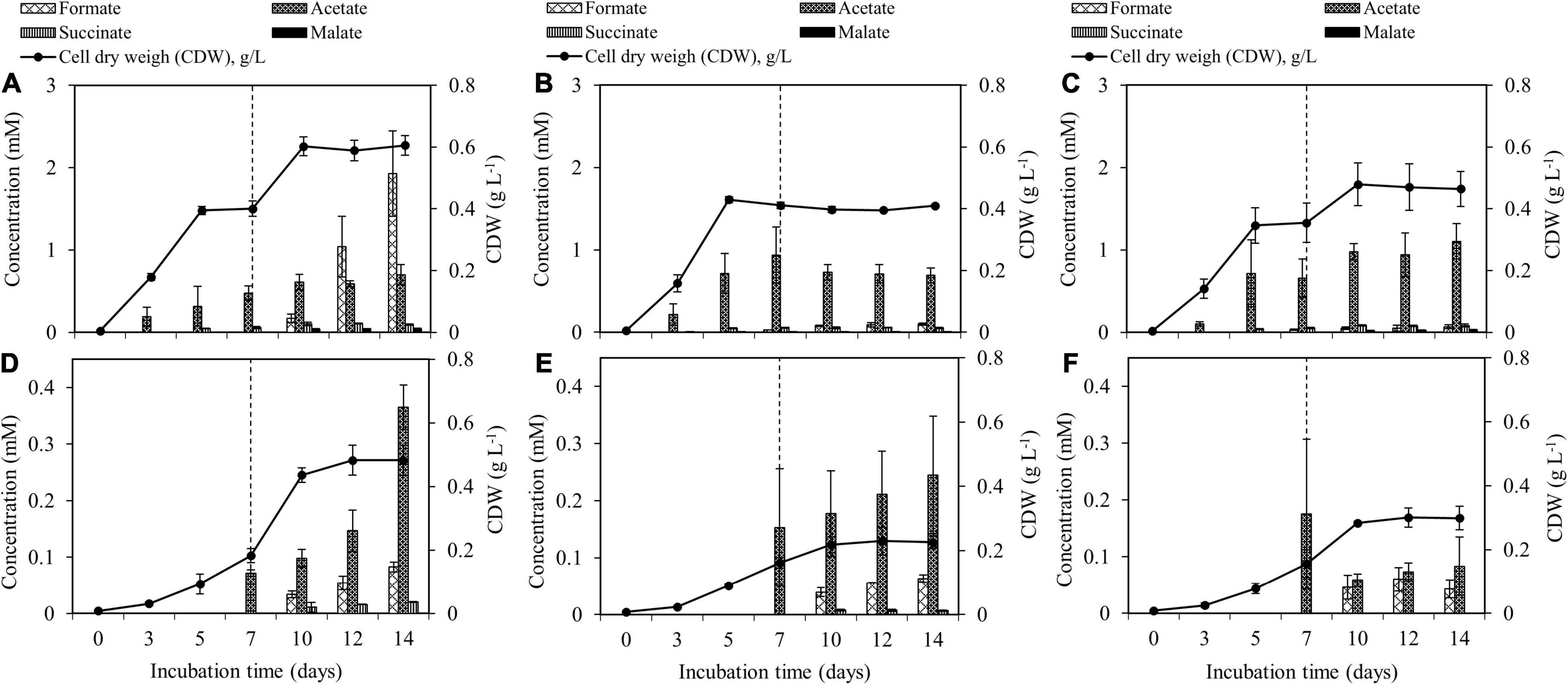
Figure 3. Accumulation of organic acid production and biomass production during the 14-day incubation of M. tundripaludum SV96 (A–C) and M. rosea SV97 (D–F) under three different gas supplementation schemes applied on day 7: (test I) both CH4 and air added (A,D), (test II) only CH4 added (B,E), and (test III) only air added (C,F). Error bars indicate the standard deviation of triplicate samples.
The spent media of both methanotrophs contained similar organic acid compounds, including formate, acetate, and succinate, whereas malate was only present in the spent medium of M. tundripaludum SV96. However, the results suggest that M. tundripaludum SV96 is more efficient and promising for CH4 conversion into organic acids than M. rosea SV97. Furthermore, the concentration and production yield of total organic acids present in the spent medium of M. tundripaludum SV96 were higher than those of M. rosea SV97 in all gas supplementation tests (P < 0.05) (Figure 3). The efficiency of C-CH4 conversion into organic acids was 4.8–7.0% and 0.7–1.8% (of consumed C-CH4) for M. tundripaludum SV96 and M. rosea SV97, respectively (Supplementary Table 1). This could be due to the different carbon assimilation pathways between the RuMP and serine pathways in Type I and Type II methanotrophs. The RuMP pathway efficiently links to the glycolytic pathway, where pyruvate is converted to organic acids, whereas the serine cycle of Type II methanotrophs has a high flux through acetyl-CoA to yield an intracellular storage compound such as polyhydroxybutyrate (PHB) under nutrient-deficient conditions (Kalyuzhnaya et al., 2015; Nguyen et al., 2021). PHB is also a native product of M. rosea SV97 (Wartiainen et al., 2006b). Regarding the production of organic acids in Type II methanotrophs, which has not been widely reported, Costa et al. (2000) observed methanotroph-driven CH4 conversion into acetate, which was subsequently consumed by heterotrophs in a denitrification bioreactor using CH4 as an electron donor. Vecherskaya et al. (2009) also observed succinate, acetate, and 2,3-butanediol excreted in the culture medium of the Type II methanotroph Methylocystis parvus during CH4 oxidation under microaerobic and anaerobic conditions. Based on the 13C analysis in their study, these organic acids were likely from PHB degradation under microaerobic conditions (5–10% O2) (Vecherskaya et al., 2009).
During the 14-day incubation period, organic acid concentrations gradually accumulated in the liquid culture of both methanotrophs in all gas supplementation tests, corresponding with the increase in their biomass production (Figure 3). On days 10–14 onward, three gas supplementation tests induced three different headspace conditions: microaerobic (O2-limited), anaerobic, and aerobic conditions. These conditions resulted in O2/CH4 molar ratios in the headspace of 0.2–0.3, <0.01, and 2–10 for the addition of CH4 + air (test I), CH4 (test II), and air (test III), respectively (Figure 4). Interestingly, the three gas supplementation tests in our study showed similar total organic acid production yields (Figures 5A,B). Lee et al. (2021) compared the production of organic acids in M. capsulatus Bath strain under various conditions, including aerobic, oxygen-limited, sulfur-limited, and nitrogen-limited conditions. The authors observed acetate production in all studied conditions, and nitrate-nitrogen limitation induced the highest acetate production (approximately 1.9 mmol-acetate g–1 CDW). Furthermore, previous studies on organic acid production by type I methanotrophs (Lee et al., 2021; Takeuchi and Yoshioka, 2021) have reported that acetate and formate are also produced under aerobic conditions but at lower concentrations than under O2-limited conditions. In our study, however, the three gas supplementation tests likely varied in the distribution of organic acids contained in the spent medium, particularly in M. tundripaludum SV96, a Type I methanotroph. Regarding organic acid production yields per consumed C-CH4, both formate (3.5%) and acetate (2.5%) were dominant in the test with CH4 + air supplementation (test I), whereas only acetate (5.1%) was dominant in the test with only air supplementation (test III) (Figures 5C,D). These results suggest that it is possible to target dominant organic acid compounds by the controlled feeding of CH4 and O2. The excretion of high formate concentration by M. tundripaludum SV96 during CH4 + air supplementation (test I) might be due to imbalanced growth during O2-limited conditions, which was previously reported in type I methanotrophs by Gilman et al. (2017). This also corresponded with the pH reduction observed in the test with CH4 + air supplementation (test I) (Supplementary Figure 1).
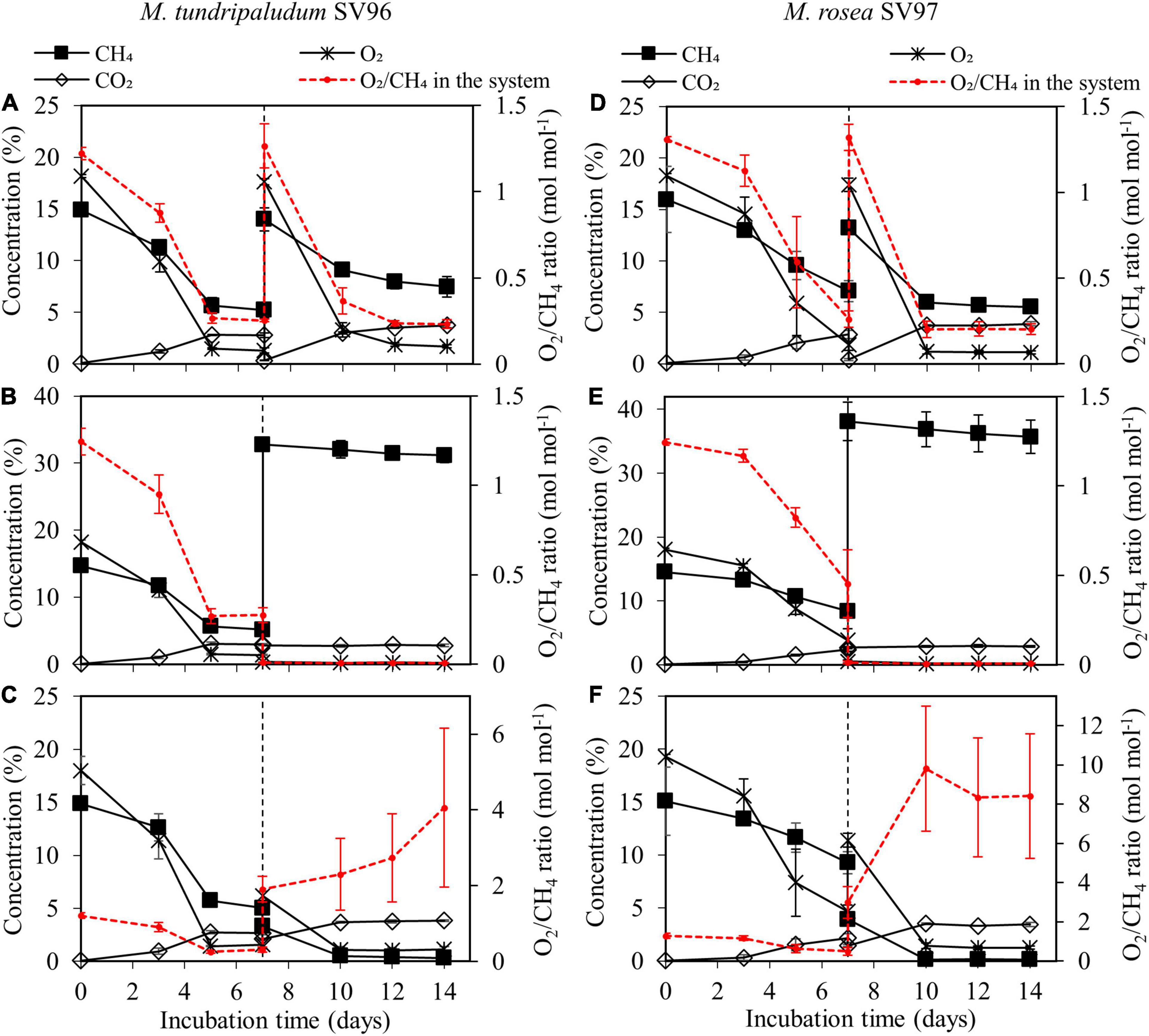
Figure 4. Gas compositions (CH4, O2, and CO2) in headspace during the 14-day incubation of M. tundripaludum SV96 (left column) and M. rosea SV97 (right column) under three different gas supplementation schemes applied on day 7: (test I) both CH4 and air added (A,D), (test II) only CH4 added (B,E), and (test III) only air added (C,F). Error bars indicate the standard deviation of triplicate samples.
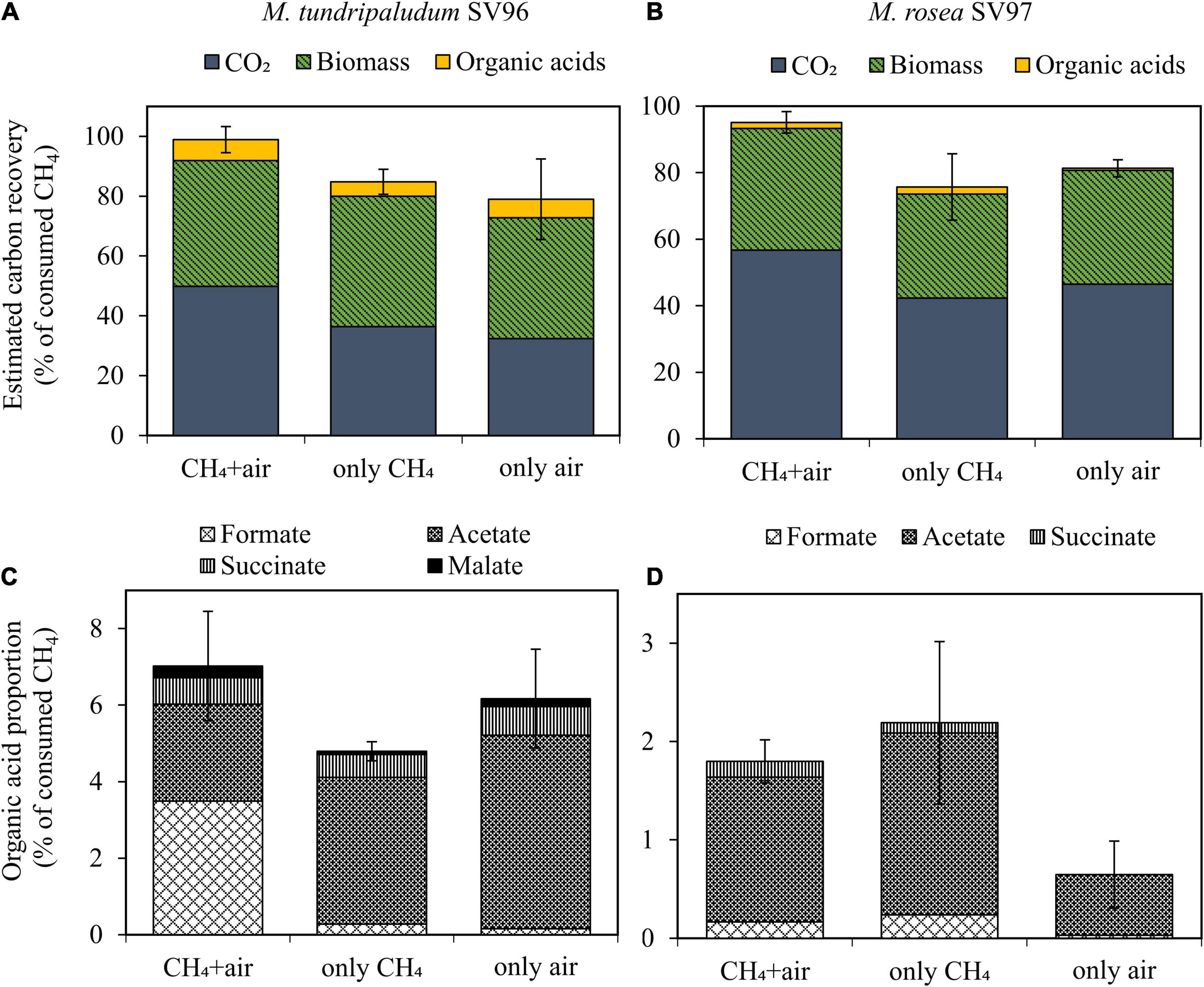
Figure 5. Carbon mass balance applied to CH4 oxidation to CO2, biomass, and organic acids (A,B) and distribution of each organic acid compound (C,D) of M. tundripaludum SV96 (left) and M. rosea SV97 (right) in three different gas supplementation tests for the 14-day incubation. Error bars indicate the standard deviation of sum of total yields in triplicate.
Compared to organic acid production in different Type I methanotroph species under O2-limited conditions, the M. tundripaludum SV96 used in our study showed comparable and higher organic acid yields per biomass production (CDW), that is, formate, acetate, and succinate (Table 1). These studies cultivated methanotrophs with initial headspace CH4 and O2 concentrations of 20 and 5%, respectively, to evaluate the organic acid production during microaerobic CH4 oxidation (Kalyuzhnaya et al., 2013; Gilman et al., 2015, 2017). However, in our study, M. tundripaludum SV96 was cultivated aerobically before allowing O2-limiting condition to occur. Further studies are required to determine whether prior cultivation under aerobic conditions enhances organic acid production under microaerobic conditions. In addition, the organic acid yields of M. tundripaludum SV96 obtained in our study (4.8–7.0% of consumed C-CH4) were higher than those of M. capsulatus Bath (<5% of consumed CH4) (Lee et al., 2021), whereas M. alcaliphilum 20Z enabled the convert 40–50% of the consumed CH4 into mostly acetate and formate under O2-limited conditions (20% CH4:5% O2) (Kalyuzhnaya et al., 2013).
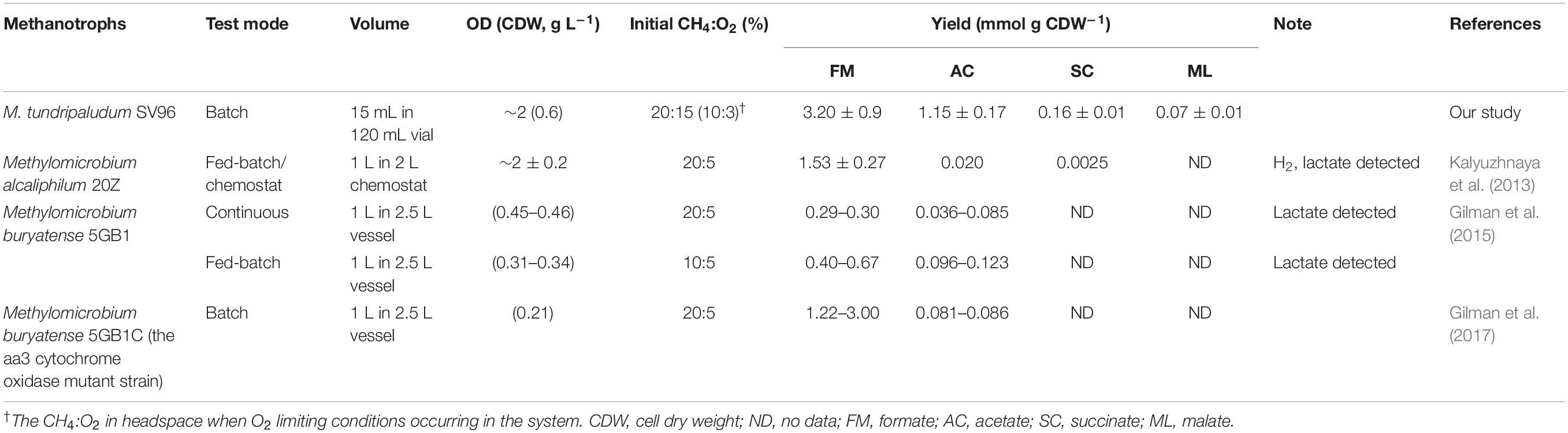
Table 1. Production of organic acids and other metabolites obtained from different Type I methanotrophs cultivated under O2-limiting conditions.
Wild-type A. baylyi ADP1 cells grew in methanotroph spent media containing formate, acetate, and succinate (and malate for M. tundripaludum SV96). During the 4-h incubation period, A. baylyi ADP1 cells incubated in the spent media from M. tundripaludum SV96 and M. rosea SV97 grew to an OD600nm of 0.14 ± 0.01 and 0.12 ± 0.03, respectively (Supplementary Figure 2). In this test, A. baylyi ADP1 cells likely utilized acetate, succinate, and malate as carbon sources for biomass assimilation (Supplementary Figure 2). However, formate is not utilized by cells as a carbon source, but it is used to maintain the cellular redox balance (Kannisto et al., 2015). The presence of fatty acid fractions derived from A. baylyi ADP1 biomass on thin layer chromatography plates also indicated the growth of A. baylyi ADP1 (Supplementary Figure 3). Typically, A. baylyi ADP1 produces wax esters as carbon storage compounds that are often associated with growth (Mangayil et al., 2019). However, they were not detected in this study (Supplementary Figure 3). This may have been due to the rapid degradation of accumulated wax ester under carbon-limiting conditions. Under such conditions, A. baylyi ADP1 cells utilize stored carbon to maintain cellular activities (Fixter et al., 1986; Salmela et al., 2018). The use of the spent media of methanotrophs for wax production is a possible direction for further study, but this approach likely requires a higher quantity of organic acids.
After confirming the growth of wild-type A. baylyi ADP1 on the methanotroph spent media, organic acid-rich spent media were used as carbon sources for 1-alkene production by engineered A. baylyi ADP1 ‘tesA-undA. The results showed that A. baylyi ADP1 ‘tesA-undA completely utilized acetate (0.3 mM), succinate (0.05 mM), and malate (0.2 mM) present in the spent mxsedia of methanotrophs, except formate, similar to the wild-type ADP1 cells (Figure 6A). The production of 1-undecene from M. tundripaludum SV96 spent medium (14.1 ± 2.7 μg L–1) was higher than that from M. rosea SV97 cultivations (1.0 ± 0.5 μg L–1) (Figure 6C), corroborating with the organic acid concentrations (Figures 6A,B). Likewise, the growth of A. baylyi ADP1 ‘tesA-undA was higher in the spent medium from M. tundripaludum SV96 (0.115 ± 0.06 OD600nm) than from M. rosea SV97 (0.070 ± 0.054 OD600nm) (Figure 6C). Production of 1-undecene was not detected in the control cultures (Supplementary Figure 4). In addition, the biomass growth detected in the methanotroph spent media was from solely A. baylyi ADP1 ‘tesA-undA as both methanotrophs could not grow without the presence of CH4.
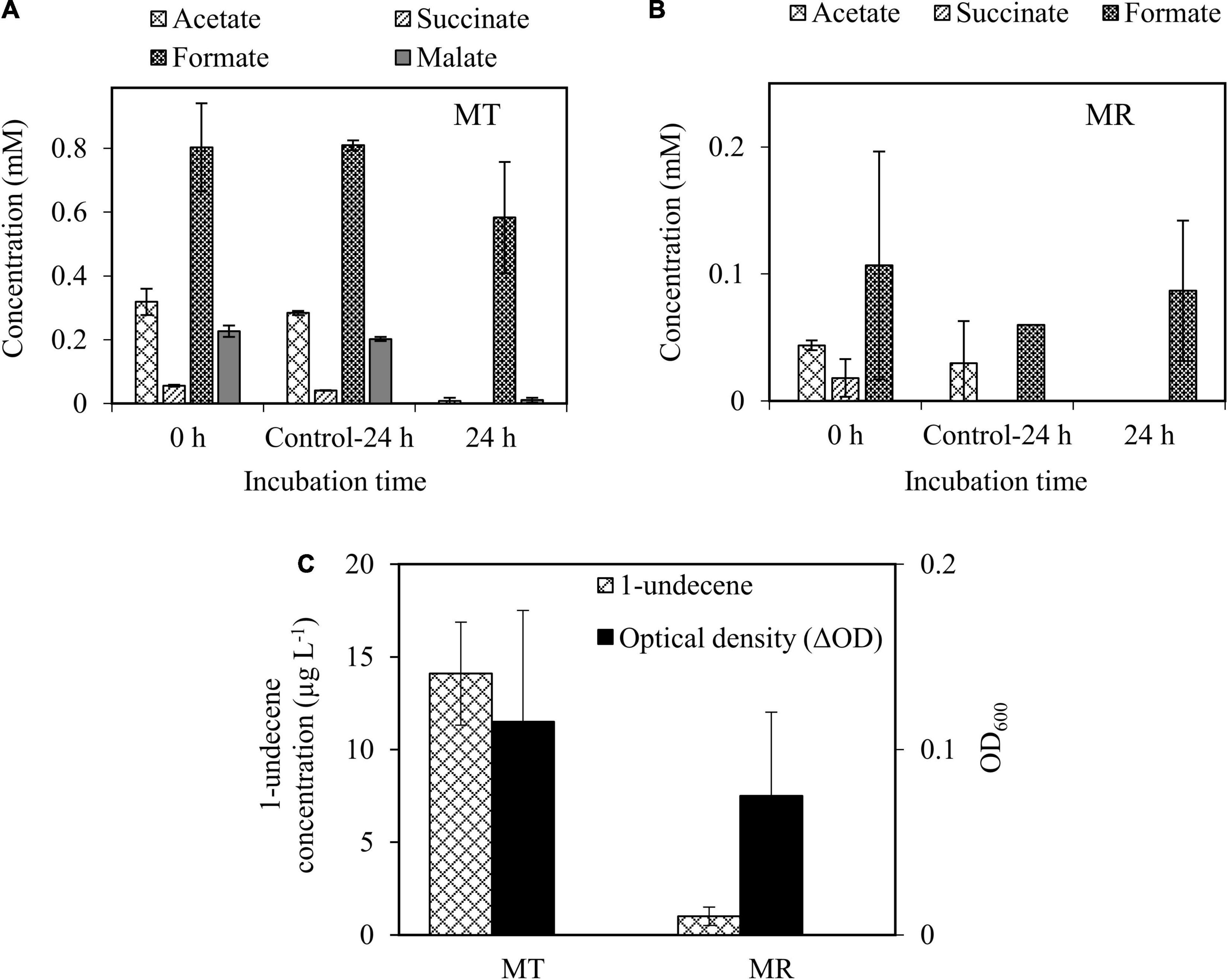
Figure 6. Organic acid utilization by A. baylyi ADP1 ‘tesA-undA in spent media obtained from the cultivation of M. tundripaludum SV96 (MT) (A) and M. rosea SV97 (MR) (B) under O2-limiting conditions. Growth and 1-undecene production from A. baylyi ADP1 ‘tesA-undA after 24-h incubation (C). ΔOD indicates the difference in OD600nm of A. baylyi ADP1 ‘tesA-undA between 0 and 24 h. Error bars indicate the standard deviation of four replicate samples. The spent media from methanotrophs fermentation without A. baylyi ADP1 were used as control (Control-24 h).
The obtained 1-undecene concentrations in this study (1.0 and 14.1 μg L–1) were lower than that previously reported for A. baylyi ADP1 ‘tesA-undA (Luo et al., 2019; Salmela et al., 2020). This phenomenon could be attributed to the higher substrate concentrations used in these studies. For example, Luo et al. (2019) developed a highly ferulate-tolerant A. baylyi ADP1 strain for 1-undecene production using adaptive laboratory evolution. The authors tested the use of a high concentration of ferulate (100 mM) as the sole carbon source and obtained a 1-undecene production titer of 72 ± 7.5 μg L–1, corresponding to a production yield of 1.0 μmol mol Csubstrate–1. In another study, Salmela et al. (2020) obtained 1-undecene concentration of up to ∼107 ± 8 μg L–1 from a two-stage system for 1-undecene production from cellulose which was converted into organic metabolites by Clostridium cellulolyticum (containing 5.2 mM glucose, 4.9 mM acetate, and 6.8 mM lactate). The authors reported the highest 1-undecene production yield of ∼35 μmol mol Csubstrate–1 using lactate as the substrate. Considering the 1-undecene production yield, the use of spent media of methanotrophs as a growth medium in our study was promising and comparable to those in previous studies, resulting in 68.9 ± 11.6 and 40.6 ± 19.8 μmol mol Csubstrate–1 for M. tundripaludum SV96 and M. rosea SV97, respectively. The carbon recovery obtained for 1-undecene production accounted for 0.065 and 0.045% of the total organic acids-carbon consumed by M. tundripaludum SV96 and M. rosea SV97, respectively (Supplementary Table 2). In our study, the spent media of methanotrophs likely did not contain intermediate compounds that were toxic to the growth and 1-undecene production of A. baylyi ADP1 ‘tesA-undA. Furthermore, the media can be directly used for cultivation without purification or additional downstream processing. However, in the future studies, the addition of some key macro/micronutrients should be evaluated, as it would benefit long-term process performance to increase 1-undecene concentration.
These results indicate that the spent media from microaerobic fermentation by methanotrophs are an excellent carbon source for heterotrophs. This two-stage bacterial process extends the range of CH4-derived products to the C11 compound (1-undecene). In further studies, the two-stage bacterial system will be scaled up and the process parameters will be optimized to be useful and competent for practical applications. In particular, the results obtained from our study show that different O2 and CH4 supplementation schemes in the headspace could lead to different concentrations and types of organic acids produced in the system. Furthermore, the contact time between methanotrophs and CH4 is important for maintaining active cells in fermenters and bioreactors (Guerrero-Cruz et al., 2021). In scale-up methanotroph cultivation, operational parameters such as O2 and CH4 inlet concentrations, dilution rates, and nitrogen sources should be optimized. The production of organic acids, particularly acetate and succinate, by methanotrophs has also been observed under aerobic conditions, suggesting potential strategies to develop a bioprocess system to co-cultivate methanotroph and A. baylyi ADP1 in a single system. In addition, the long-term effect of methanotroph cells in the spent media on the A. baylyi ADP1 growth and the 1-alkene production should be evaluated. For example, the spent media directly used as the A. baylyi ADP1 growth medium should be compared with the filtered and the centrifuged spent media prior to application.
A two-step bioprocess setup was designed for the successful integration of microaerobic CH4 fermentation with aerobic synthesis. This study provides a proof of concept for integrating GHG utilization and platform chemical production: the application of organic acids produced by methanotrophs for 1-undecene (C11) production. A Type I methanotroph, M. tundripaludum SV96, showed a higher potential for organic acid production than a Type II methanotroph, M. rosea SV97, under aerobic and microaerobic conditions. The organic acid-rich spent media of methanotrophs could be directly used as a medium for the cultivation of wild-type A. baylyi ADP1 and engineered A. baylyi ADP1 ‘tesA-undA without additional downstream processes or purification. Acetate, succinate, and malate contained in the spent media were completely utilized by A. baylyi ADP1 ‘tesA-undA for 1-undecene production. The highest yield of 1-undecene was obtained from the spent medium of M. tundripaludum SV96 at 68.9 ± 11.6 μmol mol Csubstrate–1. However, the long-term effect of the methanotroph spent media on the 1-undecene production and the system scale-up requires further studies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
RK involved in conceptualization, writing—original draft, formal analysis, investigation, and visualization. RM involved in supervision, conceptualization, writing—review and editing, formal analysis, and investigation. VS and AH involved in methodology and writing—review and editing. MS involved in supervision, methodology, and writing—review and editing. AR involved in supervision, conceptualization, formal analysis, investigation, writing—review and editing, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
This research work was supported by the Kone Foundation (grant number 201803224) and Academy of Finland (grant number 323214). Tampere University provided funding for publishing fees.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge Jin Luo for helping with using GC-MS for 1-undecene measurement. We thank the editor and reviewers for their constructive comments and suggestions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.874627/full#supplementary-material
Cantera, S., Muñoz, R., Lebrero, R., López, J. C., Rodríguez, Y., and García-Encina, P. A. (2018). Technologies for the bioconversion of methane into more valuable products. Curr. Opin. Biotechnol. 50, 128–135. doi: 10.1016/j.copbio.2017.12.021
Cao, Q., Liu, X., Ran, Y., Li, Z., and Li, D. (2019). Methane oxidation coupled to denitrification under microaerobic and hypoxic conditions in leach bed bioreactors. Sci. Total Environ. 649, 1–11. doi: 10.1016/j.scitotenv.2018.08.289
Costa, C., Dijkema, C., Friedrich, M., Garcõâ A-Encina, P., Fernaâ Ndez-Polanco, F., and Stams, A. J. M. (2000). Denitrification with methane as electron donor in oxygen-limited bioreactors. Appl. Microbiol. Biotechnol. 53, 754–762. doi: 10.1007/s002530000337
Fixter, L. M., Nagi, M. N., Mccormacks, J. G., and Fewson, C. A. (1986). Structure, distribution and function of wax esters in Acinetobacter calcoaceticus. J. Gen. Microbiol. 132, 3147–3157. doi: 10.1099/00221287-132-11-3147
Ge, X., Yang, L., Sheets, J. P., Yu, Z., and Li, Y. (2014). Biological conversion of methane to liquid fuels: status and opportunities. Biotechnol. Adv. 32, 1460–1475. doi: 10.1016/j.biotechadv.2014.09.004
Gilman, A., Fu, Y., Hendershott, M., Chu, F., Puri, A. W., Smith, A. L., et al. (2017). Oxygen-limited metabolism in the methanotroph Methylomicrobium buryatense 5GB1C. PeerJ 5:e3945. doi: 10.7717/peerj.3945
Gilman, A., Laurens, L. M., Puri, A. W., Chu, F., Pienkos, P. T., and Lidstrom, M. E. (2015). Bioreactor performance parameters for an industrially-promising methanotroph Methylomicrobium buryatense 5GB1. Microb. Cell Fact. 14:182. doi: 10.1186/s12934-015-0372-8
Guerrero-Cruz, S., Vaksmaa, A., Horn, M. A., Niemann, H., Pijuan, M., and Ho, A. (2021). Methanotrophs: discoveries, environmental relevance, and a perspective on current and future applications. Front. Microbiol. 12:678057. doi: 10.3389/fmicb.2021.678057
Harmsen, M., van Vuuren, D. P., Bodirsky, B. L., Chateau, J., Durand-Lasserve, O., Drouet, L., et al. (2020). The role of methane in future climate strategies: mitigation potentials and climate impacts. Clim. Change 163, 1409–1425. doi: 10.1007/s10584-019-02437-2
IPCC (2021). in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, eds V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, et al. (Cambridge: Cambridge University Press). Available online at: https://www.ipcc.ch/report/ar6/wg1/ (accessed November 16, 2021).
Kalyuzhnaya, M. G., Puri, A. W., and Lidstrom, M. E. (2015). Metabolic engineering in methanotrophic bacteria. Metab. Eng. 29, 142–152. doi: 10.1016/j.ymben.2015.03.010
Kalyuzhnaya, M. G., Yang, S., Rozova, O. N., Smalley, N. E., Clubb, J., Lamb, A., et al. (2013). Highly efficient methane biocatalysis revealed in a methanotrophic bacterium. Nat. Commun. 4:2785. doi: 10.1038/ncomms3785
Kannisto, M. S., Mangayil, R. K., Shrivastava-Bhattacharya, A., Pletschke, B. I., Karp, M. T., and Santala, V. P. (2015). Metabolic engineering of Acinetobacter baylyi ADP1 for removal of Clostridium butyricum growth inhibitors produced from lignocellulosic hydrolysates. Biotechnol. Biofuels 8:198. doi: 10.1186/s13068-015-0389-6
Karthikeyan, O. P., Smith, T. J., Dandare, S. U., Parwin, K. S., Singh, H., Loh, H. X., et al. (2021). Metal(loid) speciation and transformation by aerobic methanotrophs. Microbiome 9:156. doi: 10.1186/s40168-021-01112-y
Khider, M. L. K., Brautaset, T., and Irla, M. (2021). Methane monooxygenases: central enzymes in methanotrophy with promising biotechnological applications. World J. Microbiol. Biotechnol. 37:72. doi: 10.1007/s11274-021-03038-x
Lai, C. Y., Zhong, L., Zhang, Y., Chen, J. X., Wen, L. L., Shi, L. D., et al. (2016). Bioreduction of chromate in a methane-based membrane biofilm reactor. Environ. Sci. Technol. 50, 5832–5839. doi: 10.1021/acs.est.5b06177
Lee, H., Baek, J. I., Lee, J. Y., Jeong, J., Kim, H., Lee, D. H., et al. (2021). Syntrophic co-culture of a methanotroph and heterotroph for the efficient conversion of methane to mevalonate. Metab. Eng. 67, 285–292. doi: 10.1016/j.ymben.2021.07.008
Liu, T., Li, J., Khai Lim, Z., Chen, H., Hu, S., Yuan, Z., et al. (2020). Simultaneous removal of dissolved methane and nitrogen from synthetic mainstream anaerobic effluent. Environ. Sci. Technol. 54, 7629–7638. doi: 10.1021/acs.est.0c00912
López, J. C., Quijano, G., Souza, T. S. O., Estrada, J. M., Lebrero, R., and Muñoz, R. (2013). Biotechnologies for greenhouse gases (CH4. N2O, and CO2) abatement: state of the art and challenges. Appl. Microbiol. Biotechnol. 97, 2277–2303. doi: 10.1007/s00253-013-4734-z
Luo, J., Lehtinen, T., Efimova, E., Santala, V., and Santala, S. (2019). Synthetic metabolic pathway for the production of 1-alkenes from lignin-derived molecules. Microb. Cell Fact. 18:48. doi: 10.1186/s12934-019-1097-x
Mangayil, R., Efimova, E., Konttinen, J., and Santala, V. (2019). Co-production of 1,3 propanediol and long-chain alkyl esters from crude glycerol. New Biotechnol. 53, 81–89. doi: 10.1016/j.nbt.2019.07.003
Nguyen, D. T. N., Lee, O. K., Nguyen, T. T., and Lee, E. Y. (2021). Type II methanotrophs: a promising microbial cell-factory platform for bioconversion of methane to chemicals. Biotechnol. Adv. 47:107700. doi: 10.1016/j.biotechadv.2021.107700
Okonkwo, O., Lakaniemi, A. M., Santala, V., Karp, M., and Mangayil, R. (2018). Quantitative real-time PCR monitoring dynamics of Thermotoga neapolitana in synthetic co-culture for biohydrogen production. Int. J. Hydrog. Energy 43, 3133–3141. doi: 10.1016/j.ijhydene.2017.12.002
Saarela, T., Rissanen, A. J., Ojala, A., Pumpanen, J., Aalto, S. L., Tiirola, M., et al. (2020). CH4 oxidation in a boreal lake during the development of hypolimnetic hypoxia. Aquat. Sci. 82:19. doi: 10.1007/s00027-019-0690-8
Salmela, M., Lehtinen, T., Efimova, E., Santala, S., and Mangayil, R. (2018). Metabolic pairing of aerobic and anaerobic production in a one-pot batch cultivation. Biotechnol. Biofuels 11:187. doi: 10.1186/s13068-018-1186-9
Salmela, M., Lehtinen, T., Efimova, E., Santala, S., and Santala, V. (2020). Towards bioproduction of poly-α-olefins from lignocellulose. Green Chem. 22, 5067–5076. doi: 10.1039/d0gc01617a
Saunois, M., Stavert, A. R., Poulter, B., Bousquet, P., Canadell, J. G., Jackson, R. B., et al. (2020). The global methane budget 2000-2017. Earth Syst. Sci. Data 12, 1561–1623. doi: 10.5194/essd-12-1561-2020
Sheets, J. P., Ge, X., Li, Y. F., Yu, Z., and Li, Y. (2016). Biological conversion of biogas to methanol using methanotrophs isolated from solid-state anaerobic digestate. Bioresour. Technol. 201, 50–57. doi: 10.1016/j.biortech.2015.11.035
Strong, P. J., Kalyuzhnaya, M., Silverman, J., and Clarke, W. P. (2016). A methanotroph-based biorefinery: potential scenarios for generating multiple products from a single fermentation. Bioresour.Technol. 215, 314–323. doi: 10.1016/j.biortech.2016.04.099
Sun, M. T., Yang, Z. M., Fu, S. F., Fan, X. L., and Guo, R. B. (2018). Improved methane removal in exhaust gas from biogas upgrading process using immobilized methane-oxidizing bacteria. Bioresour. Technol. 256, 201–207. doi: 10.1016/j.biortech.2018.02.020
Takeuchi, M., and Yoshioka, H. (2021). Acetate excretion by a methanotroph, Methylocaldum marinum S8, under aerobic conditions. Biosci. Biotechnol. Biochem. 85, 2326–2333. doi: 10.1093/bbb/zbab150
Vecherskaya, M., Dijkema, C., Saad, H. R., and Stams, A. J. M. (2009). Microaerobic and anaerobic metabolism of a Methylocystis parvus strain isolated from a denitrifying bioreactor. Environ. Microbiol. Rep. 1, 442–449. doi: 10.1111/j.1758-2229.2009.00069.x
Wartiainen, I., Hestnes, A. G., McDonald, I. R., and Svenning, M. M. (2006a). Methylobacter tundripaludum sp. nov., a methane-oxidizing bacterium from Arctic wetland soil on the Svalbard islands, Norway (78° N). Int. J. Syst. Evol. Microbiol. 56, 109–113. doi: 10.1099/ijs.0.63728-0
Keywords: methane, methanotroph, organic acid production, 1-alkene, Acinetobacter baylyi ADP1
Citation: Khanongnuch R, Mangayil R, Santala V, Hestnes AG, Svenning MM and Rissanen AJ (2022) Batch Experiments Demonstrating a Two-Stage Bacterial Process Coupling Methanotrophic and Heterotrophic Bacteria for 1-Alkene Production From Methane. Front. Microbiol. 13:874627. doi: 10.3389/fmicb.2022.874627
Received: 12 February 2022; Accepted: 22 April 2022;
Published: 19 May 2022.
Edited by:
Obulisamy Parthiba Karthikeyan, South Dakota School of Mines and Technology, United StatesReviewed by:
Rajesh K. Sani, South Dakota School of Mines and Technology, United StatesCopyright © 2022 Khanongnuch, Mangayil, Santala, Hestnes, Svenning and Rissanen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramita Khanongnuch, cmFtaXRhLmtoYW5vbmdudWNoQHR1bmkuZmk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.