
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 07 April 2022
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.862882
This article is part of the Research TopicInteractions between Bioactive Food Ingredients and Intestinal MicrobiotaView all 22 articles
This study aimed to investigate the microbial structure and function in the rectum of weaned piglets with berberine supplementation. Twelve healthy 21-day-old Duorc × (Landrace × Large White) weaned piglets (similar body weight) were evenly divided into control and berberine groups and were fed a basal diet supplemented with 0 and 0.1% berberine, respectively. After 21 days, metagenomic sequencing analysis was performed to detect microbial composition and function in the rectum of weaned piglets. Results showed that there were 10,597,721,931–14,059,392,900 base pairs (bp) and 10,186,558,171–15,859,563,342 bp of clean data in the control and berberine groups, respectively. The Q20s of the control and berberine groups were 97.15 to 97.7% and 96.26 to 97.68%, respectively. The microorganisms in the berberine group had lower (p < 0.05) Chao1, alternating conditional expectation, Shannon, and Simpson indices at the species levels than those in the control group. Analysis of similarity showed that there were significant differences (p < 0.01) between the control and berberine groups at the genus and species levels of the gut microorganisms. Dietary berberine significantly increased (p < 0.05) the abundance of Subdoligranulum variabile, but decreased (p < 0.05) the abundance of Prevotella copri compared with the control group. Carbohydrate-active enzymes analysis revealed that the levels of polysaccharide lyases and carbohydrate esterases were lower (p < 0.05) in the berberine group than that in the control group. Linear discriminant analysis effect size analysis showed that berberine supplementation could induce various significant Kyoto Encyclopedia of Genes and Genomes pathways, including carbohydrate metabolism, environmental information processing, and microbial metabolism in diverse environments. In conclusion, our findings suggest that berberine could improve the composition, abundance, structure, and function of gut microbiome in the weaned piglets, potentially providing a suitable approach for the application of berberine in human and animal health.
Berberine is an isoquinoline alkaloid isolated from the traditional Chinese herb Coptis chinensis, which is widely used for its medicinal properties. Berberine has antidiarrheal, antibacterial, anti-inflammatory, antitumor, and hypoglycemic effects. It has good therapeutic effect on intestinal inflammation, diabetes, hypertension, and tumors (Fu et al., 2020; Huang et al., 2021; Patel, 2021). Specifically, the therapeutic effect of berberine on intestinal bacterial infection has been investigated for its potential use in clinical practice (Yu M. et al., 2020). Berberine helps in maintaining the intestinal health as it accumulates in the intestine easily and is beneficial in improving the imbalance in intestinal bacteria (Wu et al., 2020).
The intestinal microflora is a complex microbial system composed of a variety of microorganisms participating in numerous physiological processes of the body (Jin et al., 2019; Ma and Ma, 2019; Sun et al., 2020). The intestine harbors various microorganisms such as Lactobacillus, Bacillus, Enterobacter, Bifidobacterium, and Enterococcus. Initially, the gut microflora was thought to be closely related only to digestion and nutrient absorption; however, recent studies reported that it affects the body health by regulating metabolic diseases, such as obesity, diabetes, and cardiovascular diseases, as well as immune-related disorders (Schippa and Conte, 2014; Yadav and Jha, 2019). As the largest and most complex microecosystem of the body, intestinal microorganisms and their metabolites play an important role in animal health.
Limited studies have focused on the possible link between microbiome and function of gut microflora with berberine supplementation in weaned piglets. With the development of metagenomic high-throughput sequencing technology, it is possible to analyze a large number of microbial community species, abundance, and related biological information by performing total microbial DNA extraction from a specific environment and library construction (Fraher et al., 2012; Zhou et al., 2015; Quan et al., 2019). Thus, a large amount of information on non-culturable microbial flora can be obtained without the need of isolation and culture methods used in traditional microbial research (Guo et al., 2014; Walker et al., 2014). Currently, metagenomic sequencing technology has become an important tool to study intestinal environmental microorganisms. In the present study, this technique was used to characterize the microbial composition and function in the rectum of weaned pigs supplemented with berberine.
The animal experimental design was approved by the Animal Care and Use Committee of Anhui Science and Technology University. Twelve healthy 21-day-old Duorc × (Landrace × Large White) weaned piglets (similar body weight) were purchased from Qingxuan Agricultural Development Co., Ltd. (Bengbu, China), and equally divided into the control and berberine groups (six replicates and one pig/replicate). Pigs in the control and berberine groups were fed a basal diet supplemented with 0 and 0.1% berberine, respectively. Berberine chloride hydrate (purity ≥ 98%) was obtained from Aladdin Reagent Co., Ltd. (China). A basal diet (Table 1) was designed on the basis of National Research Council [NRC] (2012). Piglets could feed and drink water freely.
After 21 days, the stool samples were collected from the rectum of all piglets by rectal massage. These samples were immediately stored in liquid nitrogen (−196°C) for further analysis and metagenomic sequencing.
Genomic DNA was isolated from stool samples using Magen HiPure Bacterial DNA Kits (Guangzhou, China). The quality of genomic DNA was verified using Qubit Fluorometric Quantification and Nanodrop Spectrophotometers (Thermo Fisher Scientific, Waltham, MA, United States).
Metagenomic sequencing analysis was performed as described by Liu et al. (2020). Briefly, 12 metagenomic DNA libraries were constructed using NEBNext™ M Ltra® DNA Library Prep Kit (NEB, Ipswich, MA, United States) for Illumina. Polymerase chain reaction was used to amplify 300- to 400-bp-long DNA fragments. Metagenomic sequencing was carried out on an Illumina Novaseq 6000 platform at Gene Denovo Biotechnology Co., Ltd. (Guangzhou, China). Clean data were obtained from raw data using FASTP 18.0 software (Chen et al., 2018), which was used for further genome assembly.
Bioinformatics analysis of the metagenomic sequence was performed as described by Liu et al. (2020). Gene assembly and prediction were performed using MEGAHIT 11.2 and MetaGeneMark 3.38, respectively.
α Diversity refers to the richness of species/functions in an intestinal microbial environment, which indicates the balance state and living conditions of the gut microorganisms. Analysis of α diversity with Chao1, alternating conditional expectation (ACE), Shannon, and Simpson parameters were performed using the Python scikit-bio package.
Analysis of similarity (ANOSIM) is a test method for analyzing microbial community structure, which is used to test whether the difference between groups is significantly greater than that within groups. ANOSIM test was performed using the vegan R package.
The Venn graph was plotted using VennDiagram package in R project. Welch t and analysis of variance (ANOVA) tests were used to show the species with significant differences between the two groups. Prediction of carbohydrate-active enzymes (CAZy) was performed using the CAZy databases. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed using the DIAMOND software in the KEGG databases. Linear discriminant analysis effect size (LEfSe) analysis was performed by LEfSe software. All bioinformatics analyses were performed using the R software, and p < 0.05 indicates statistical significance.
Twelve metagenomic DNA libraries constructed from the control and berberine groups were sequenced on the Illumina Novaseq 6000 platform. As shown in Table 2, there were 10,622,678,100–14,120,565,000 base pairs (bp) and 10,226,653,500–15,941,277,000 bp of raw data in the control and berberine groups, respectively. After filtering these data, 10,597,721,931–14,059,392,900 bp and 10,186,558,171–15,859,563,34 bp of clean data were obtained in the control and berberine groups, respectively (Table 2). The Q20s (%) of the control and berberine groups were 97.15 to 97.7% and 96.26 to 97.68%, respectively (Table 2). Furthermore, the GC contents (%) of the control and berberine groups were 43.86 to 47.3% and 42.94 to 49.59%, respectively (Table 2). Negligible n (%) content was found in both groups (Table 2).
The microorganisms in the berberine group had lower (p < 0.05) Chao1, ACE, Shannon, and Simpson indices at the species levels than those in the control group (Figure 1).
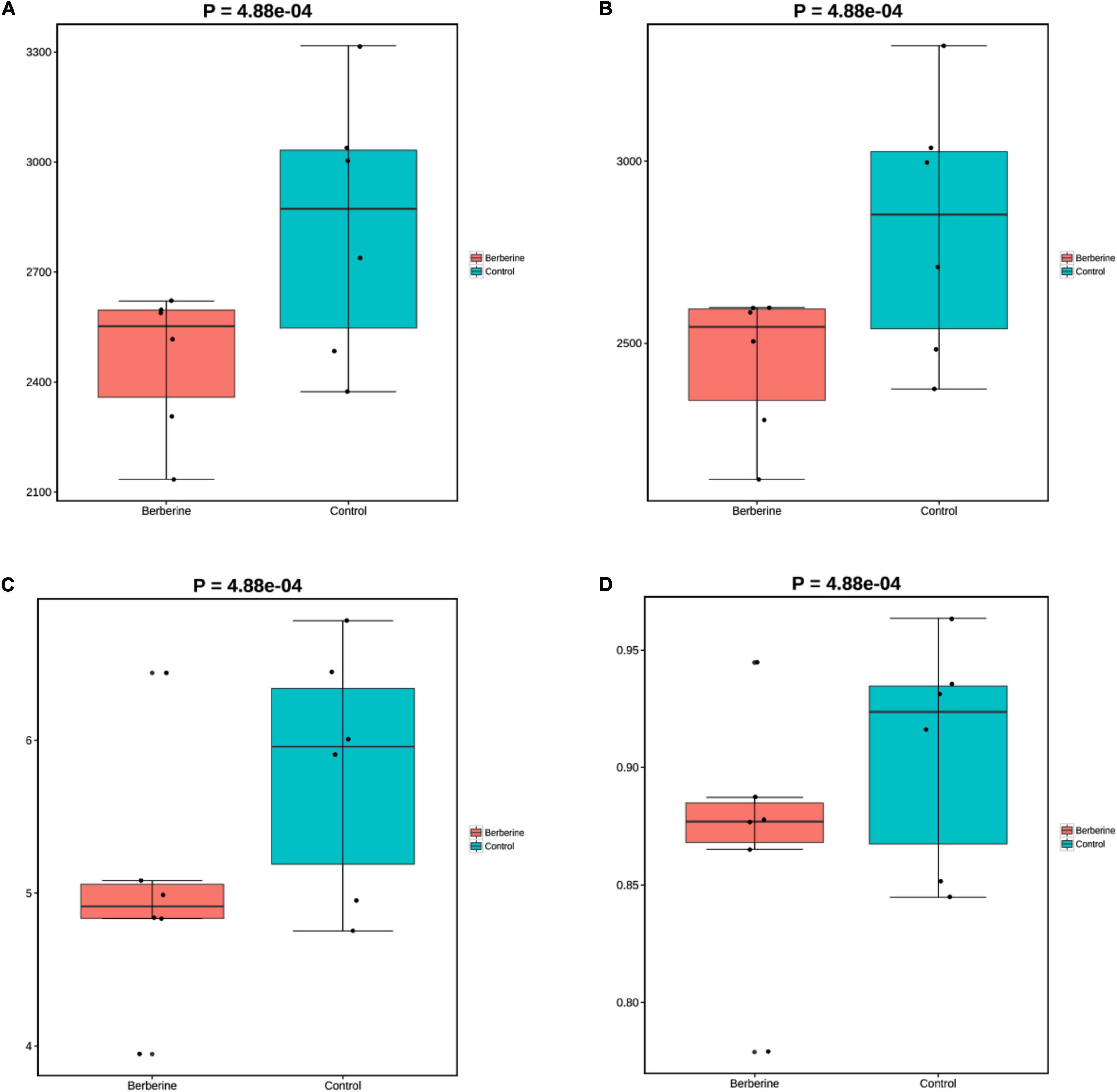
Figure 1. Effects of berberine on microbiome diversity (α-diversity analysis) of pig gut microbiome (A: Chao1; B: ACE; C: Shannon; D: Simpson).
As shown in Figure 2, there was significant difference (p < 0.01) between the control and berberine groups at the genus and species levels of the gut microorganisms.
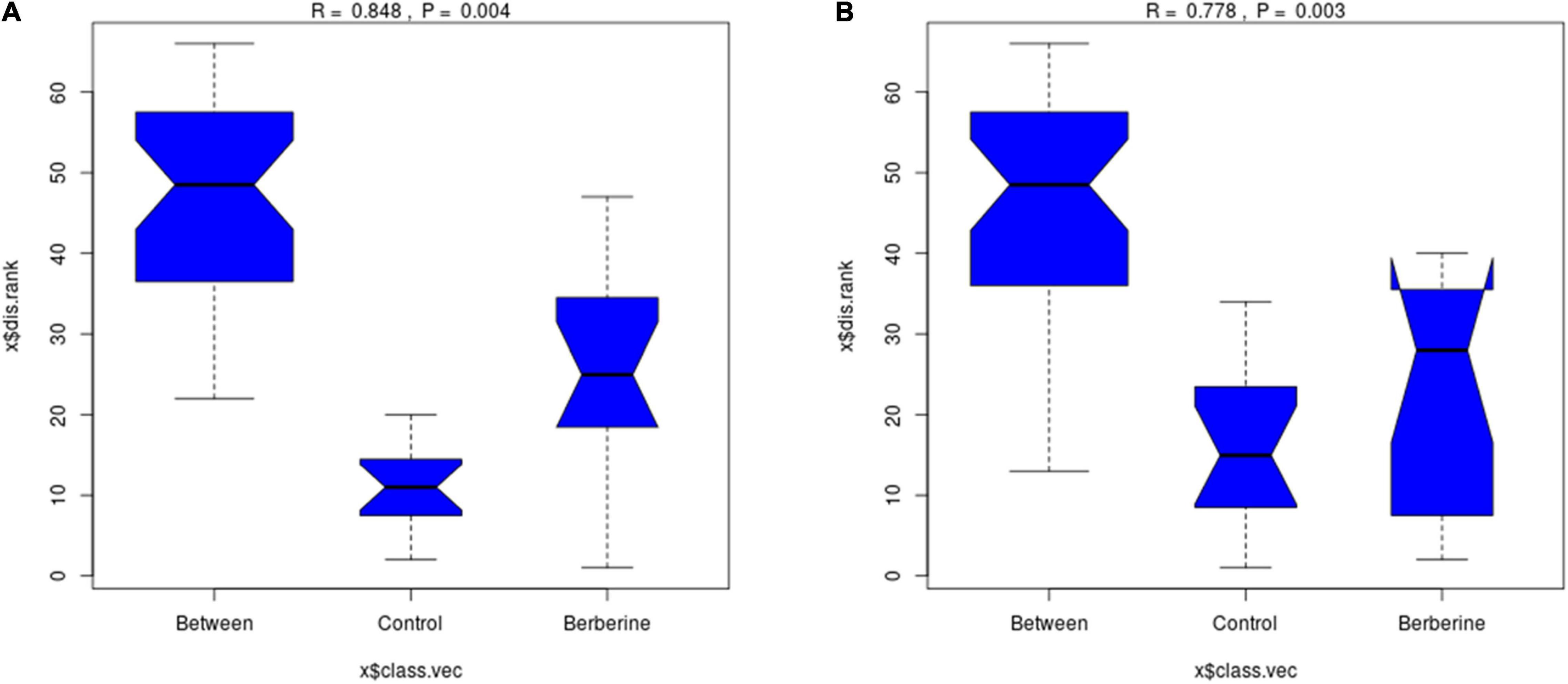
Figure 2. Analysis of similarity analysis between control and berberine groups (A: genus levels; B: species levels).
The species distribution in microbial communities of the different treatment groups has a certain degree of similarity and specificity. In order to understand the species differences, Venn diagram was used to show the common and unique information between the different groups based on the species abundance information of samples. As shown in Figure 3, a total of 3,218 microbial species were common in both groups; however, 1,314 and 525 microbial species were unique in the control and berberine groups, respectively.
Welch t-test showed that berberine supplementation significantly increased (p < 0.05) the abundance of Subdoligranulum variabile, Lactobacillus johnsonii, Parabacteroides distasonis, Fournierella massiliensis, Ruthenibacterium lactatiformans, Frisingicoccus caecimuris, and Gemmiger formicilis, but significantly decreased (p < 0.05) the abundance of Prevotella copri, Prevotella sp. P2-180, Prevotella sp. P4-76, Prevotella sp. AM42-24, Prevotella sp. 885, Prevotella sp. P5-50, Erysipelotrichaceae bacterium YH-PanP20, Prevotellaceae bacterium, and Phascolarctobacterium succinatutens compared with the control group (Figure 4). ANOVA test showed that berberine supplementation significantly increased (p < 0.05) the abundance of S. variabile, but significantly decreased (p < 0.05) the abundance of P. copri compared with the control group (Figure 5).
CAZy include glycoside hydrolases, glycosyl transferases, polysaccharide lyases (PLs), carbohydrate esterases (CEs), and auxiliary activities. As shown in Figure 6, PL and CE levels were lower in the berberine group than that in the control group.
The results of LEfSe analysis in the berberine group were significantly associated with various KEGG pathways, including carbohydrate metabolism, environmental information processing, microbial metabolism in diverse environments, drug metabolism cytochrome P450, cellular community prokaryotes, dioxin degradation, xylene degradation, Staphylococcus aureus infection, starch and sucrose metabolism, toluene degradation, and so on (Figure 7).
Early weaning of piglets can shorten the slaughter cycle of pigs and improve the reproductive performance of sows. However, incomplete development of intestinal microbiota in early weaned piglets may lead to intestinal irritability and reduced production performance (Moeser et al., 2017; Upadhaya and Kim, 2021). Antibiotics can improve the above conditions, but the problem of antibiotic residues needs to be addressed (Yang et al., 2019). Berberine, a traditional Chinese herbal extract, has strong antibacterial effect and is an effective antibiotic substitute (Xu et al., 2020; Zhu et al., 2021). However, few studies have focused on the effect of berberine on the gut microbiota in early weaned piglets. In this work, the macrogenomics and high-throughput sequencing techniques were used to investigate the effects of berberine on intestinal microbiome and function of early weaned piglets (weaning age: 21 days).
Metagenomic analysis involves the DNA extraction from all microorganisms in environmental samples directly followed by a metagenomic library construction and uses high-throughput sequencing technique to study the genetic composition and community functions of these microorganisms (Prayogo et al., 2020). Animal microbial population is distributed on the body surface, oral cavity, gastrointestinal tract, and reproductive tracts, but there are significant differences in the types and quantities of microorganisms based on dietary, species, sex, and age. The microbes in the gut are more numerous than the body’s other organs. The complexity of the environment and microorganisms results in less rigorous data analysis using traditional methods; however, the development of metagenomics and high-throughput sequencing technology has promoted the study of gut microbes considerably (Walker et al., 2014; Guo et al., 2021). In the present study, a major part of raw data (>99%) contributed to the clean data, and the Q20 (%) was more than 96% in the gut microbiomes of the control and berberine groups. A total of 68.7 and 68.3 billion bp clean reads were obtained by conducting metagenomic sequencing of the control and berberine groups, respectively. ANOSIM revealed that the control and berberine groups had significant differences at the genus and species levels of intestinal microorganisms in weaned piglets. These results suggest that the metagenomic sequencing data were reliable and sufficient to investigate the effects of berberine on the gut microbial diversity and function in weaned piglets.
Nutritional digestion and absorption, physiology, metabolism, intestinal barrier, immune function, and disease onset are affected by the composition, diversity, and functional changes in intestinal microflora (Chang and Martinez-Guryn, 2019; Liu et al., 2019). Accordingly, the stability of microecological environment plays a crucial role in animal health regulation. Berberine shows a broad-spectrum antibacterial effect against a variety of gram-positive, gram-negative, and drug-resistant bacteria (Yue et al., 2019; Jamshaid et al., 2020). The effects of berberine influence intestinal infectious disease development and body health through the inhibition of intestinal bacteria (Zhu et al., 2021). Studies have shown that berberine can directly regulate the structure of intestinal microbiota by reducing the number of intestinal microbes in a dose-dependent manner (Zhang et al., 2019). In metagenomics research, Chao1, ACE, Shannon, and Simpson indices are used to study the gut microbiome diversity. We found that dietary 0.1% berberine significantly decreased these indices at the species level compared with those in the control group, suggesting toward the efficacy of berberine in reducing the richness and diversity of intestinal microbiome. Similarly, research conducted by Zhang et al. (2019) revealed that berberine decreased the diversity and quantity of the intestinal microflora in db/db mice.
Berberine is not easily absorbed after oral administration; thus, it can maintain a high concentration in the gastrointestinal tract, providing the necessary conditions required for inhibiting the intestinal bacterial growth (Cheng et al., 2021). In addition, as an antibacterial drug, it can inhibit a variety of pathogenic bacteria and change the structure of intestinal microflora (Habtemariam, 2020; Yu C. et al., 2020; Cheng et al., 2021). The most common mechanism of berberine-mediated regulation of intestinal flora is to change the original dominant intestinal bacteria to maintain the microecological balance. Habtemariam (2020) suggested that the underlying mechanism for the multifunctional role of berberine was its regulation of gut microbiota. Zhang et al. (2012) showed that berberine with high-fat diet in rats could increase the abundance of Allobaculum and Blautia in the intestine. Dietary berberine also increased the abundance of beneficial bacteria including S. variabile, L. johnsonii, and P. distasonis, as shown in the present study. S. variabile improves gut mucosal immune response and inhibits food allergy in mice (Abdel-Gadir et al., 2019). L. johnsonii can promote growth, gut development, and intestinal microorganisms in pig, mice, and chicken, when used as a probiotic (Wang et al., 2017; He et al., 2019; Wang et al., 2020). The abundance of P. distasonis was negatively correlated with obesity, non-alcoholic fatty liver disease, diabetes, and other disease states, suggesting that it possibly plays a positive regulatory role in glucose and lipid metabolism (Wang et al., 2019). By contrast, dietary berberine decreased the abundance of P. copri, which leads to changes in microbiota metabolism and reduces interleukin-18 production. This aggravates the intestinal inflammation and may result in systemic autoimmunity (Ley, 2016).
Berberine can activate some signaling pathways and carbohydrate-related enzymes by improving intestinal microflora and health (Liao et al., 2020; Li et al., 2021). Findings from the present study revealed that berberine changed the structure, abundance, and function of gut microbiota in weaned piglets. Alterations in the gut microbiota lead to functional changes as well. Dietary berberine could markedly affect the CAZy activity of intestinal microflora. Similarly, Li et al. (2021) reported that berberine treatment affects the carbohydrate utilization by altering CAZy activity in the intestinal microflora. Alignment analysis based on KEGG database showed significantly enriched carbohydrate metabolism and environmental information processing pathways in the berberine group. Carbohydrate metabolism pathway mainly involves carbohydrate digestion to provide energy for microbial growth through fermentation in the large intestine, which leads to generation of volatile fatty acids and their derivatives to provide nutrition for the body (Tremaroli and Bäckhed, 2012). Environmental information processing pathway is related to the changes in intestinal microbiota (Arboleya et al., 2016).
In summary, there were microbial community and functional differences in the rectum of weaned piglets between the control and berberine groups. We demonstrated that berberine could improve the composition, abundance, structure, and function of gut microbiome in the weaned piglets. Our research might provide a novel scientific basis for the further development and application of berberine (such as replacing antibiotics) in the feed and food industries.
The raw sequencing data presented in the study are deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) repository, accession number PRJNA807368.
The animal study was reviewed and approved by the Animal Care and Use Committee of Anhui Science and Technology University.
HH and XB: data the collection and drafting the manuscript. HH, FZ, and XB: conceive and design the study. KX and KW: statistical analysis. FZ: critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the special Fund for Anhui Agriculture Research System (AGCYJSTX-05-15), the High-level Talents Introduction Foundation of Anhui Science and Technology University (DKYJ201802 and DKYJ202101), the Natural Science Key Foundation of Anhui Education Department (KJ2021A0868),the College Student Innovation and Entrepreneurship Project (202110879058; S202110879172), and Laboratory Open Project of Anhui Province Key Laboratory of Animal Nutrition Regulation and Health (APKLANRH202001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Gadir, A., Stephen-Victor, E., Gerber, G. K., Rivas, M. N., Wang, S., Harb, H., et al. (2019). Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat. Med. 25, 1164–1174. doi: 10.1038/s41591-019-0461-z
Arboleya, S., Sánchez, B., Solís, G., Fernández, N., Suárez, M., Hernández-Barranco, A. M., et al. (2016). Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota: a functional inference study. Int. J. Mol. Sci. 17:649. doi: 10.3390/ijms17050649
Chang, E. B., and Martinez-Guryn, K. (2019). Small intestinal microbiota: the neglected stepchild needed for fat digestion and absorption. Gut Microbes 10, 235–240. doi: 10.1080/19490976.2018.1502539
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, 884–890. doi: 10.1093/bioinformatics/bty560
Cheng, H., Liu, J., Tan, Y., Feng, W., and Peng, C. (2021). Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J. Pharm. Anal. 21, 117–119. doi: 10.1016/j.jpha.2021.10.003
Fraher, M. H., O’Toole, P. W., and Quigley, E. M. (2012). Techniques used to characterize the gut microbiota: a guide for the clinician. Nat. Rev. Gastroenterol. Hepatol. 9, 312–322. doi: 10.1038/nrgastro.2012.44
Fu, K., Xu, M., Zhou, Y., Li, X., Wang, Z., Liu, X., et al. (2020). The Satus quo and way forwards on the development of Tibetan medicine and the pharmacological research of Tibetan materia medica. Pharmacol. Res. 155:104688. doi: 10.1016/j.phrs.2020.104688
Guo, L., Zhang, D., Fu, S., Zhang, J., Zhang, X., He, J., et al. (2021). Metagenomic sequencing analysis of the effects of colistin sulfate on the pig gut microbiome. Front. Vet. Sci. 8:663820. doi: 10.3389/fvets.2021.663820
Guo, M., Huang, K., Chen, S., Qi, X., He, X., Cheng, W. H., et al. (2014). Combination of metagenomics and culture-based methods to study the interaction between ochratoxin A and gut microbiota. Toxicol. Sci. 141, 314–323. doi: 10.1093/toxsci/kfu128
Habtemariam, S. (2020). Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol. Res. 155:104722. doi: 10.1016/j.phrs.2020.104722
He, T., Zhu, Y. H., Yu, J., Xia, B., Liu, X., Yang, G. Y., et al. (2019). Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica infantis. Vet. Microbiol. 230, 187–194. doi: 10.1016/j.vetmic.2019.02.003
Huang, J., Feng, W., Li, S., Tang, H., Qin, S., Li, W., et al. (2021). Berberine exerts anti-cancer activity by modulating adenosine monophosphate-activated protein kinase (AMPK) and the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathways. Curr. Pharm. Des. 27, 565–574. doi: 10.2174/1381612826666200928155728
Jamshaid, F., Dai, J., and Yang, L. X. (2020). New development of novel berberine derivatives against bacteria. Mini Rev. Med. Chem. 20, 716–724. doi: 10.2174/1389557520666200103115124
Jin, M., Qian, Z., Yin, J., Xu, W., and Zhou, X. (2019). The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 23, 2343–2350. doi: 10.1111/jcmm.14195
Ley, R. E. (2016). Prevotella in the gut: choose carefully. Nat. Rev. Gastroenterol. Hepatol. 13, 69–70. doi: 10.1038/nrgastro.2016.4
Li, X., Su, C., Jiang, Z., Yang, Y., Zhang, Y., Yang, M., et al. (2021). Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbe 7:36. doi: 10.1038/s41522-021-00205-8
Liao, Z., Xie, Y., Zhou, B., Zou, B., Xiao, D., Liu, W., et al. (2020). Berberine ameliorates colonic damage accompanied with the modulation of dysfunctional bacteria and functions in ulcerative colitis rats. Appl. Microbiol. Biotechnol. 104, 1737–1749. doi: 10.1007/s00253-019-10307-1
Liu, H., Hu, L., Han, X., Zhao, N., Xu, T., Ma, L., et al. (2020). Tibetan sheep adapt to plant phenology in alpine meadows by changing rumen microbial community structure and function. Front. Microbiol. 11:587558. doi: 10.3389/fmicb.2020.587558
Liu, J., Wang, H. W., Lin, L., Miao, C. Y., Zhang, Y., and Zhou, B. H. (2019). Intestinal barrier damage involved in intestinal microflora changes in fluoride-induced mice. Chemosphere 234, 409–418. doi: 10.1016/j.chemosphere.2019.06.080
Ma, N., and Ma, X. (2019). Dietary amino acids and the gut-microbiome-immune axis: physiological metabolism and therapeutic prospects. Compr. Rev. Food Sci. Food Saf. 18, 221–242. doi: 10.1111/1541-4337.12401
Moeser, A. J., Pohl, C. S., and Rajput, M. (2017). Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim. Nutr. 3, 313–321. doi: 10.1016/j.aninu.2017.06.003
National Research Council [NRC] (2012). Nutrient Requirements of Swine. Washington, DC: National Academy Press.
Patel, P. (2021). A bird’s eye view on a therapeutically ‘wonder molecule’: berberine. Phytomed. Plus 1:100070. doi: 10.1016/j.phyplu.2021.100070
Prayogo, F. A., Budiharjo, A., Kusumaningrum, H. P., Wijanarka, W., Suprihadi, A., and Nurhayati, N. (2020). Metagenomic applications in exploration and development of novel enzymes from nature: a review. J. Genet. Eng. Biotechnol. 18:39. doi: 10.1186/s43141-020-00043-9
Quan, J., Cai, G., Yang, M., Zeng, Z., Ding, R., Wang, X., et al. (2019). Exploring the fecal microbial composition and metagenomic functional capacities associated with feed efficiency in commercial DLY pigs. Front. Microbiol. 10:52. doi: 10.3389/fmicb.2019.00052
Schippa, S., and Conte, M. P. (2014). Dysbiotic events in gut microbiota: impact on human health. Nutrients 6, 5786–5805. doi: 10.3390/nu6125786
Sun, M., Ma, N., He, T., Johnston, L. J., and Ma, X. (2020). Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. 60, 1760–1768. doi: 10.1080/10408398.2019.1598334
Tremaroli, V., and Bäckhed, F. (2012). Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249. doi: 10.1038/nature11552
Upadhaya, S. D., and Kim, I. H. (2021). The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets—a review. Animals 11:2418. doi: 10.3390/ani11082418
Walker, A. W., Duncan, S. H., Louis, P., and Flint, H. J. (2014). Phylogeny, culturing, and metagenomics of the human gut microbiota. Trends. Microbiol. 22, 267–274. doi: 10.1016/j.tim.2014.03.001
Wang, H., Ni, X., Qing, X., Zeng, D., Luo, M., Liu, L., et al. (2017). Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front. Microbiol. 8:1073. doi: 10.3389/fmicb.2017.01073
Wang, H., Sun, Y., Xin, J., Zhang, T., Sun, N., Ni, X., et al. (2020). Lactobacillus johnsonii BS15 prevents psychological stress–induced memory dysfunction in mice by modulating the gut–brain axis. Front. Microbiol. 11:1941. doi: 10.3389/fmicb.2020.01941
Wang, K., Liao, M., Zhou, N., Bao, L., Ma, K., Zheng, Z., et al. (2019). Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235. doi: 10.1016/j.celrep.2018.12.028
Wu, M., Yang, S., Wang, S., Cao, Y., Zhao, R., Li, X., et al. (2020). Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front. Pharmacol. 11:223. doi: 10.3389/fphar.2020.00223
Xu, X., Yang, C., Chang, J., Wang, P., Yin, Q., Liu, C., et al. (2020). Dietary supplementation with compound probiotics and berberine alters piglet production performance and fecal microbiota. Animals 10:511. doi: 10.3390/ani10030511
Yadav, S., and Jha, R. (2019). Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 10:2. doi: 10.1186/s40104-018-0310-9
Yang, H., Paruch, L., Chen, X., van Eerde, A., Skomedal, H., Wang, Y., et al. (2019). Antibiotic application and resistance in swine production in China: current situation and future perspectives. Front. Vet. Sci. 6:136. doi: 10.3389/fvets.2019.00136
Yu, C., Zhang, J., Qin, Q., Liu, J., Xu, J., and Xu, W. (2020). Berberine improved intestinal barrier function by modulating the intestinal microbiota in blunt snout bream (Megalobrama amblycephala) under dietary high-fat and high-carbohydrate stress. Fish Shellfish Immunol. 102, 336–349. doi: 10.1016/j.fsi.2020.04.052
Yu, M., Jin, X., Liang, C., Bu, F., Pan, D., He, Q., et al. (2020). Berberine for diarrhea in children and adults: a systematic review and meta-analysis. Therap. Adv. Gastroenterol. 13:1756284820961299. doi: 10.1177/1756284820961299
Yue, M., Tao, Y., Fang, Y., Lian, X., Zhang, Q., Xia, Y., et al. (2019). The gut microbiota modulator berberine ameliorates collagen-induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply. FASEB J. 33, 12311–12323. doi: 10.1096/fj.201900425RR
Zhang, W., Xu, J. H., Yu, T., and Chen, Q. K. (2019). Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 118:109131. doi: 10.1016/j.biopha.2019.109131
Zhang, X., Zhao, Y., Zhang, M., Pang, X., Xu, J., Kang, C., et al. (2012). Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One 7:e42529. doi: 10.1371/journal.pone.0042529
Zhou, J., He, Z., Yang, Y., Deng, Y., Tringe, S. G., and Alvarez-Cohen, L. (2015). High-throughput metagenomic technologies for complex microbial community analysis: open and closed formats. mBio 6:e02288-14. doi: 10.1128/mBio.02288-14
Keywords: weaned piglets, berberine, gut microbiota, metagenomic sequencing, microbial function
Citation: Hu H, Xu K, Wang K, Zhang F and Bai X (2022) Dissecting the Effect of Berberine on the Intestinal Microbiome in the Weaned Piglets by Metagenomic Sequencing. Front. Microbiol. 13:862882. doi: 10.3389/fmicb.2022.862882
Received: 26 January 2022; Accepted: 11 February 2022;
Published: 07 April 2022.
Edited by:
Zheng Ruan, Nanchang University, ChinaReviewed by:
Wei Zhang, Institute of Animal Husbandry and Veterinary Medicine, Anhui Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2022 Hu, Xu, Wang, Zhang and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhang, emhhbmdmZW5nQGFoc3R1LmVkdS5jbg==; Xi Bai, YmFpeGkxOTg3MzI2QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.