
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 24 March 2022
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.851271
 Tianqi Xia1,2†
Tianqi Xia1,2† Tianwei Wang1†
Tianwei Wang1† Jiahao Sun1,2
Jiahao Sun1,2 Weixiong Shi1,2
Weixiong Shi1,2 Yayong Liu1,2
Yayong Liu1,2 Fuqing Huang1,2
Fuqing Huang1,2 Jiaqi Zhang1,2
Jiaqi Zhang1,2 Jin Zhong1,2*
Jin Zhong1,2*
Sesbania cannabina (SC) is a protein-rich roughage that thrives under moderate-severe saline-alkali (MSSA) soils with the potential to relieve the shortage of high nutritive forage. Sweet sorghum (SS) also tolerates MSSA soils and contains rich fermentable carbohydrates which could improve the fermentation quality in mixed silage. The present study investigated the silage quality, bacterial community, and metabolome in the mixed silage of SC and SS (SC-SS) with or without lactic acid bacterial (LAB) inoculants. Four ratios (10:0, 7:3, 5:5, and 3:7) of SC and SS were treated with sterile water or LAB inoculants (homofermentative Companilactobacillus farciminis and Lactiplantibacillus plantarum, and heterofermentative Lentilactobacillus buchneri and Lentilactobacillus hilgardii), which were analyzed after 60 days of ensiling. Results revealed that LAB inoculation improved the fermentation quality by increasing the lactic acid content and decreasing the ammonia nitrogen and butyric acid contents compared with the untreated group. LAB inoculation also raised the relative feed value by reducing indigestible fibers [e.g., neutral detergent fiber (NDF), acid detergent fiber, and hemicellulose]. Microbial and metabolomic analysis indicated that LAB inoculants could modify the bacterial community and metabolome of SC-SS silage. In co-ensiling samples except for SC alone silage, L. buchneri and L. hilgardii were the dominant species. Metabolites with bioactivities like anti-inflammatory, antioxidant, antimicrobial, and anti-tumor were upregulated with LAB inoculation. Furthermore, correlation analysis demonstrated that active metabolites (e.g., glycitin, glabrene, alnustone, etc.) were positively correlated with L. buchneri, while tripeptides (e.g., SPK, LLK, LPH, etc.) were positively correlated with L. hilgardii. Adequately describing the SC-SS silage by multi-omics approach might deepen our understanding of complicated biological processes underlying feature silages fermentation. Moreover, it may also contribute to screening of targeted functional strains for MSSA-tolerating forage to improve silage quality and promote livestock production.
Ensiling plays a vital role to help livestock survive winters and dry seasons in many countries around the world by preservation of fresh forage. Additionally, reserving protein-rich forage is also important because plant-derived proteins are a prerequisite for livestock products. However, the productivity of protein-rich forages, like alfalfa, is inadequate in the moderate-severe saline-alkali (MSSA) region which affects the nutrient uptake of the plants (Wang W. et al., 2021). Therefore, addressing the relationship between the supplement of protein-rich roughage and the requirement of ruminants in the MSSA region remains a significant challenge.
Sesbania cannabina (SC) is a protein-rich annual herbaceous legume that thrives under adverse environments (e.g., saline-alkali and waterlogged soils) and grows rapidly in the MSSA region (Shahjalal and Topps, 2000). It can fix atmospheric nitrogen and contains 25% protein content (Kitonga et al., 2019), comparable to the “queen of forages” alfalfa. Besides that, the dry matter (DM) yields of SC (45 t ha–1 year–1) were much higher than that of alfalfa (30 t ha–1 year–1) when the water quality was similar (Nanduri, 2013). Therefore, SC can compensate the shortage of protein-rich forages in the MSSA region. However, the ensiling of SC alone is difficult because of its high protein content, high buffering capacity, and low water-soluble carbohydrate (WSC) level.
Co-ensiling of gramineous-legume forages has proved advantageous in balancing nutrients and fermentation quality (Ni et al., 2018). Sweet sorghum (SS) is a conventional crop with abundant fermentable carbohydrate content over 20% DM (dos Passos Bernardes et al., 2014). SC and SS are both suitable for cultivation in the MSSA region due to their tolerance of soil types, fertilizers, and rainfall patterns. Moreover, co-ensiling of legumes and SS could more easily achieve high-quality silage due to their chemical composition: protein-rich in the former and WSC-rich in the latter (Ni et al., 2018; Wang J. et al., 2021). Therefore, co-ensiling of SC and SS is a feasible way to produce quality silage in the MSSA region.
Lactic acid bacteria (LAB) could reduce pH rapidly and regulate microbial community during the fermentation process (Weinberg and Muck, 1996). Our previous studies indicated that LAB inoculants, including both homofermentative and heterofermentative strains, improved the fermentation quality by modifying the microbial community and metabolome (Wang et al., 2020; Sun et al., 2021). Homofermentative LAB is the most common additive converting WSC into lactic acid (LA) and rapidly decreasing pH to inhibit the growth of pathogens, while that of heterofermentative LAB is the representative additive to improve aerobic stability. We inoculated four strains including homofermentative Lactiplantibacillus plantarum (Lac. plantarum, formerly Lactobacillus plantarum) B90 and Companilactobacillus farciminis (Com. farciminis, formerly Lactobacillus farciminis) GMX4, and heterofermentative Lentilactobacillus buchneri (Len. buchneri, formerly Lactobacillus buchneri) NX205 and Lentilactobacillus hilgardii (Len. hilgardii, formerly Lactobacillus hilgardii) 60TS-2. Lac. plantarum B90 and Len. hilgardii 60TS-2 could improve the fermentation quality and aerobic stability and reduce the DM loss of sugarcane top silage (Wang et al., 2020). Com. farciminis GMX4 and Len. buchneri NX205 were isolated from high-quality silage of legume and SS, respectively. LAB inoculants could also regulate the concentration of metabolites (Guo et al., 2018; Xu et al., 2019). For example, Lac. plantarum accumulated some functional phenolic compounds of sainfoin silage (Xu et al., 2020). Moreover, a previous study found that metabolites from SC have a biological function (Zhou et al., 2018). Therefore, we hypothesized that LAB inoculants can improve the fermentation quality and increase the active metabolites of SC-SS silage.
To the best of our knowledge, few studies have investigated the fermentation quality, microbial community, and metabolome of SC-SS silage with or without LAB inoculants. Thus, the current study was aimed to determine whether LAB inoculants would improve the fermentation quality, change the bacterial community, or upregulate the functional metabolites concentration in co-ensiled SC-SS silage, providing the theoretical basis for taking full advantage of forage growth in the MSSA region.
SC (LuJing 5, at the pod-bearing stage) and SS (KeTian 14, at the milk-ripe stage) were harvested from an experimental field (salinity = 0.5%, pH = 9.13) of the Yellow River Delta Modern Agricultural Technology Innovation Center (37°67′N, 118°90′E) at Dongying Shandong Province, China, on September 9, 2020. The harvested fresh materials were cut into a particle size of 2.0 cm by a crop chopper. The chopped SC and SS were mixed at ratios of 10:0, 7:3, 5:5, and 3:7. The strains Lac. plantarum B90 (CGMCC No. 13318), Com. farciminis GMX4 (CGMCC No. 19434), Len. hilgardii 60TS-2 (CGMCC No. 19435), and Len. buchneri NX205 (CGMCC No. 16534) were used as compound microbial inoculants. These strains were preserved in 25% glycerin at −80°C. Before utilization, these strains were recovered in de Man, Rogosa, Sharpe (MRS) agar (Oxoid) at 37°C for 48 h under anaerobic conditions. The monoclonal was picked and cultivated in 5 ml MRS broth (Oxoid) at 37°C under anaerobic conditions to OD600 = 1.0. Then, the bacterial cultures were transferred to a 500-ml culture flask for proliferation. Finally, 10-fold gradient dilution coating was used to determine the number of viable bacteria. The inoculants were sprayed onto the SC-SS silage at a concentration of 106 cfu/g fresh weight as the LAB group. An equal volume of sterile water was sprayed onto the SC-SS silage for the control (CK) group. The mixed materials (500 g) were packed into polyethylene bags (45 cm × 50 cm) and vacuum sealed. All bags were ensiled at room temperature (21–30°C) for 60 days.
The DM content of the silage samples was determined by drying to a constant weight (Ni et al., 2017). Samples of 10 g mixed silage were blended with 90 ml sterilized water and shaken for 30 min. The resulting silage extract of 30 ml was used to measure pH (HANNA; Italy). The silage extract of 1 ml was filtered through a 0.22 μm filter for organic acids analyses (Wang et al., 2020). The silage extract of 2 ml was used to measure ammonia nitrogen (NH3-N) (Broderick and Kang, 1980). The contents of crude protein (CP), acid detergent fiber (ADF), acid detergent lignin (ADL), and neutral detergent fiber (NDF) were measured with reference to the Association of Official Analytical Chemists (Van Soest et al., 1991). The WSC content was determined according to prior work (Arthur Thomas, 1977). NDF and ADF were used to calculate hemicellulose (HC) and relative feed value (RFV) based on formulas as follows:
where DDM = digestible dry matter and DMI = dry matter intake.
The DNA extraction of fresh samples and silages was performed with a DNA isolation kit (18815ES50, Yeasen, Shanghai, China). PCR amplification of the full-length 16S rRNA gene for single-molecule real-time (SMRT) sequencing was performed with the forward primer V1–V9 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer V1–V9 R (5′-GNTACCTTGTTACGACTT-3′). The PCR products were mixed and purified. Then sequencing libraries were prepared using SMRTbell™ Template Prep Kit (PacBio) and sequenced on the PacBio Sequel platform. Raw sequences were extracted using Circular Consensus Sequencing software to obtain raw reads. The reads were compared using the UCHIME algorithm, and chimeric reads were removed to obtain the optimized sequences. Sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs). Then, the taxonomic information was obtained with SSUrRNA Database. To study the phylogenetic relationship of different OTUs, sequences were analyzed with MUSCLE software (Version 3.8.31). Alpha diversity and beta diversity were obtained with QIIME (Version 1.9.1) based on the output-normalized data.
Samples of 10 g SC-SS silage were ground with liquid nitrogen using grinding miller, and then 100 mg of the above crushed samples were resuspended with 53% methanol and centrifuged. Finally, the supernatant was injected into the liquid chromatography tandem mass spectrometry (LC-MS/MS) system to analyze by a Vanquish UHPLC system (Thermo Fisher Scientific, Germany) coupled with an Orbitrap Q Exactive™ HF mass spectrometer (Thermo Fisher Scientific, Germany) in Novogene Co., Ltd. Samples were injected onto a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) using a 17-min linear gradient at a flow rate of 0.2 ml/min. The eluents for the positive polarity mode were eluent A (0.1% formic acid (FA) in water) and eluent B (methanol). The eluents for the negative polarity mode were eluent A (5 mM ammonium acetate, pH 9.0) and eluent B (methanol). The raw data files generated by ultra-high performance liquid chromatography (UHPLC)-MS/MS were processed by the Compound Discoverer 3.1 (CD3.1, Thermo Fisher Scientific) to perform peak alignment, peak picking, and quantitation for each metabolite. After that, the peak intensities were normalized to the total spectral intensity for predicting the molecular formula based on additive ions, molecular ion peaks, and fragment ions. Then, the peaks were matched with the mzCloud, mzVault, and MassList database to obtain the accurate qualitative and relative quantitative results. These metabolites were annotated using the Human Metabolome Database (HMDB).1 The metabolites with variable importance in the projection (VIP) > 1, p-value < 0.05, and fold changes ≥ 2 or ≤ 0.5 were considered to be differential metabolites. Volcano plots were used to filter metabolites of interest based on log2 (fold change) and −log10 (p-value) of metabolites.
Metabolites were screened based on HMDB. Firstly, metabolites detected simultaneously in three mixed ratios (7:3, 5:5, and 3:7) group with definite classification to HMDB database were chosen. Then, metabolites of phenylpropanoids and polyketides superclass with fold changes more than 6 in four ratios (10:0, 7:3, 5:5, and 3:7) group were selected.
Data were shown with means ± standard deviation (SD). The fermentation quality was analyzed with two-way ANOVA using GraphPad 8.0.2, and the nutritional quality and alpha diversity data were analyzed with SPSS 25. The correlations between bacterial taxonomic profile and silage quality variables were analyzed and plotted with Canoco 5. The correlations between bacterial taxonomic profile and metabolites were analyzed and plotted with R 4.0.2 software packages. Significance was declared at p < 0.05.
The pH value and organic contents of SC-SS silage were affected (p < 0.05) by the LAB inoculants (Figure 1). The pH value of the LAB group was significantly lower in every ratio compared with the CK group, especially in the 7:3 ratio reducing from 5.03 to 3.98. The LA content of the LAB group was significantly higher in every ratio, especially in the 7:3 ratio increasing by 61% compared with the CK group. LAB inoculation only considerably decreased the acetic acid (AA) content in the SC alone (10:0) silage but did not significantly affect the AA contents in the other ratios silage. The ratios of LA/AA were gradually increased as the proportion of SS increased in the mixed silage, and the highest LA/AA ratio was observed in the LAB group of the 3:7 ratio. Although the butyric acid (BA) content was higher in the SC alone silage in both LAB and CK groups, no BA was detected in the LAB group of other ratios. Lower NH3-N content was observed in the LAB group compared with the CK group, especially in the 7:3 ratio (reduced by 39%). Our results showed that LAB inoculants improved the fermentation quality by reducing the pH, increasing the LA content, and decreasing the BA and NH3-N contents.
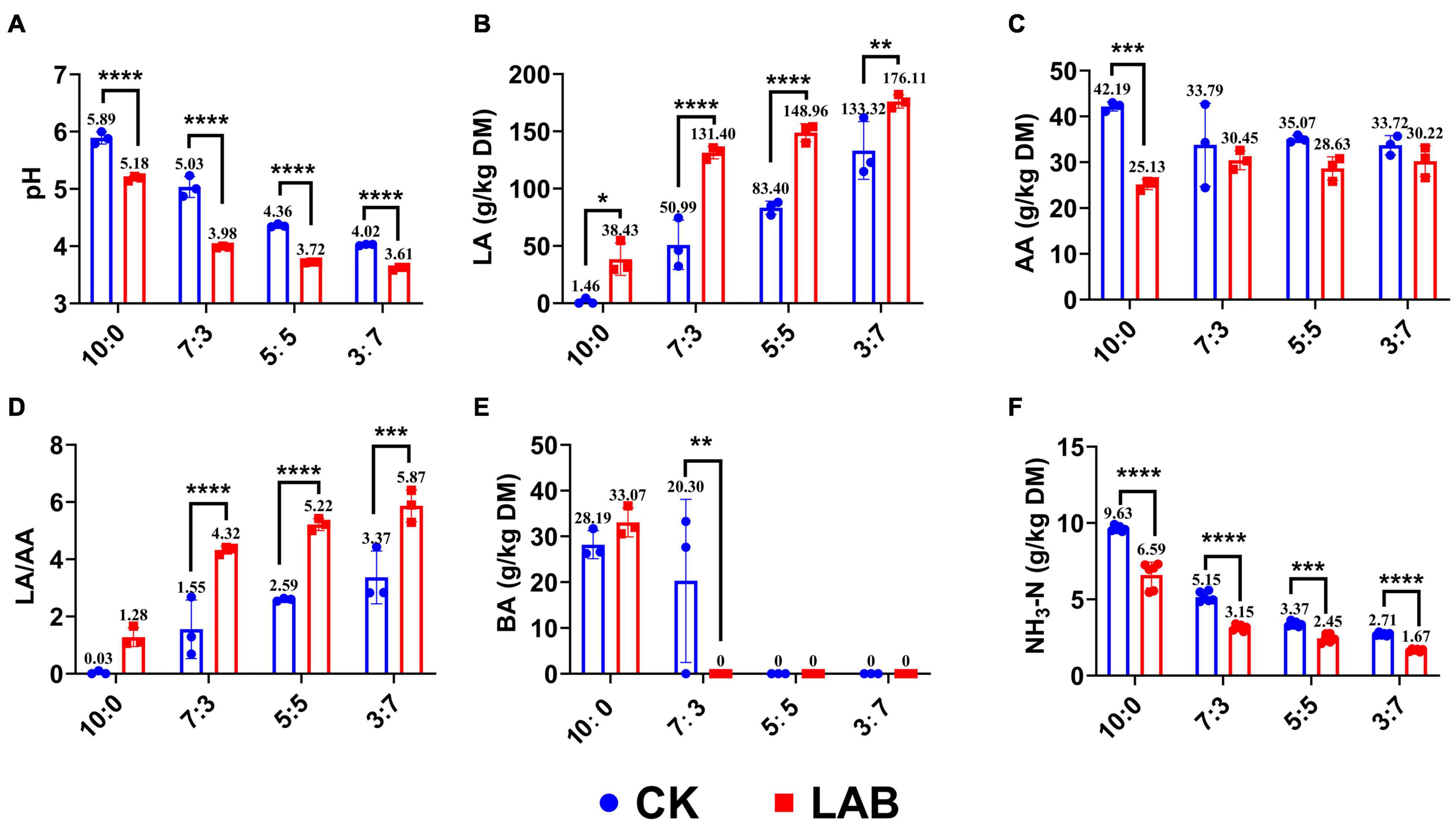
Figure 1. Fermentation characteristics of SC-SS silage. (A) pH value. (B) The content of lactic acid. (C) The content of acetic acid. (D) The ratio of lactic acid to acetic acid. (E) The content of butyric acid. (F) The content of ammonium-N. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
The nutrition characteristics of SC-SS silage are shown in Table 1. The DM content of SC-SS silage ranged from 23.13 to 25.07% DM, and LAB group showed higher (p < 0.001) DM contents compared with the CK group. For example, the LAB group (25.07%) resulted in 8% higher DM content compared with the CK group (23.8%) at the 5:5 ratio. LAB inoculants significantly (p < 0.001) increased the CP content compared to the CK group, especially in the 7:3 ratio increased by 10.23%. The WSC content increased (p < 0.001) with the increase in the proportion of SS in mixed silages, and it was significantly higher in the LAB group than that of the CK group. The structural carbohydrate contents were lower in the LAB group compared to the CK group in all ratios. The NDF and ADF contents decreased with LAB inoculation, and the lowest contents were found in the 7:3 ratio (54.07 and 42.90%DM, respectively). The HC content was reduced with LAB treatment, and maximum reduction (18.35%) was found in the 7:3 proportion. In contrast, all ratios found increased RFV in LAB-treated silages, especially in the 7:3 proportion (increased by 21.38%). Our results showed that LAB inoculation improved the nutritional quality by reducing the indigestible fiber contents (e.g., NDF and ADF).
The bacterial community was identified via SMRT sequencing. The alpha diversity of the bacterial community in ensiled samples was analyzed (Table 2). The indexes of Shannon, Simpson, and chao1 were lower in the LAB group compared with the CK group at every ratio.
The bacterial community of fresh and ensiled samples is demonstrated in Figure 2. At the genus level, the dominant genus was Bacillus in the pre-ensiled forage accounting for 78.09 and 91.35% in the fresh SC and SS, respectively. However, the relative abundance of Bacillus sharply decreased in SC-SS silage (lower than 3%) after fermentation. In contrast, Lactobacillus was the dominant genus in all the treatments except for the SC alone silage of the CK group after 60 days fermentation (Figure 2A and Supplementary Table 1). Other genera with relative abundance over 1% also include Dialister (33.26% vs. 24.31%) and Enterococcus (1.84% vs. 28.07%) in LAB and CK groups of SC alone silage.
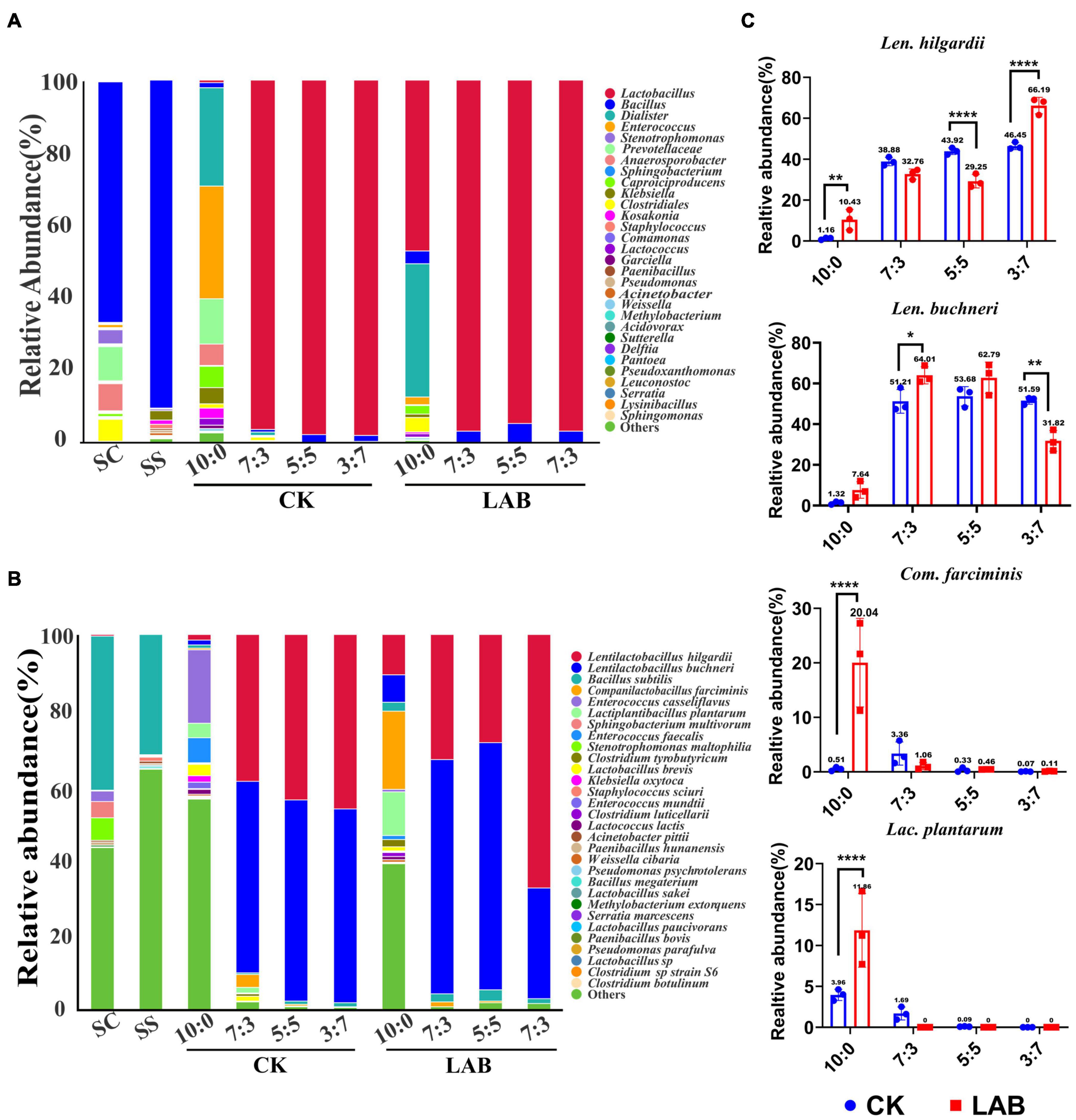
Figure 2. The bacterial community of SC-SS silage. (A) Bacterial composition at the genus level. (B) Bacterial composition at the species level. CK, untreated group; LAB, lactic acid bacteria inoculation group. (C) Relative abundance of Com. farciminis, Lac. plantarum, Len. buchneri, Len. hilgardii in the SC-SS silage, respectively. *p < 0.05; **p < 0.01; ****p < 0.0001.
At the species level, Bacillus subtilis was the dominant species in the fresh SC (41.28%) and SS (31.83%). Fresh SC had low proportions of Len. hilgardii (0.14%), Len. buchneri (0.05%), Lac. plantarum (0.03%), and Com. farciminis (0.07%), while that of fresh SS had low proportions of Len. buchneri (0.01%), and Com. farciminis (0.03%). In the SC-SS silage, the heterofermentative LABs (Len. hilgardii and Len. buchneri) were dominant species (accounting for approximately 95.51% of total species) after 60 days fermentation, followed by the homofermentative LABs (Lac. plantarum and Com. farciminis). The effects of LAB inoculants on relative abundance were inconsistent for these four species. For example, the relative abundance of Len. buchneri in the LAB group was significantly higher than that of the CK group in the 7:3 proportion, while no similar results were observed for Len. hilgardii (Figure 2C). However, in the SC silage, the relative abundances of Len. hilgardii (p < 0.01), Len. buchneri (p > 0.05), Lac. plantarum (p < 0.0001), and Com. farciminis (p < 0.0001) were higher in the LAB group compared to the CK group (Figure 2C and Supplementary Table 2). Furthermore, the average relative abundance exceeds 1% also containing B. subtilis and Enterococcus casseiflavus. Our results indicated the microbial inoculants have limited influence on the bacterial community after 60 days of fermentation.
A total of 862 metabolites annotated to the HMDB database were detected in SC-SS silage (Figure 3A). The main components of metabolites are lipids and lipid-like molecules, organic acids and derivatives, phenylpropanoids and polyketides, and organ heterocyclic compounds, accounting for 22.27, 18.45, 17.17, and 15.66%, respectively. The Venn diagram demonstrated common and specific metabolites in comparison combinations (Figure 3B). The four comparison combinations had 13 common metabolites. The numbers of specific metabolites of the comparison combinations in the ratios of 10:0, 7:3, 5:5, and 3:7 were 418, 209, 55, and 27, respectively (Figure 3B). The change level was compared between the LAB and CK groups in four mixed ratios. The identified differentially expressed metabolites were shown by a volcano plot (Figure 3C). The number of upregulated metabolites was gradually increased with the increasing proportion of SC in SC-SS silage (90, 178, 229, and 248 in the 3:7, 5:5, 7:3 and10:0 ratio groups, respectively).
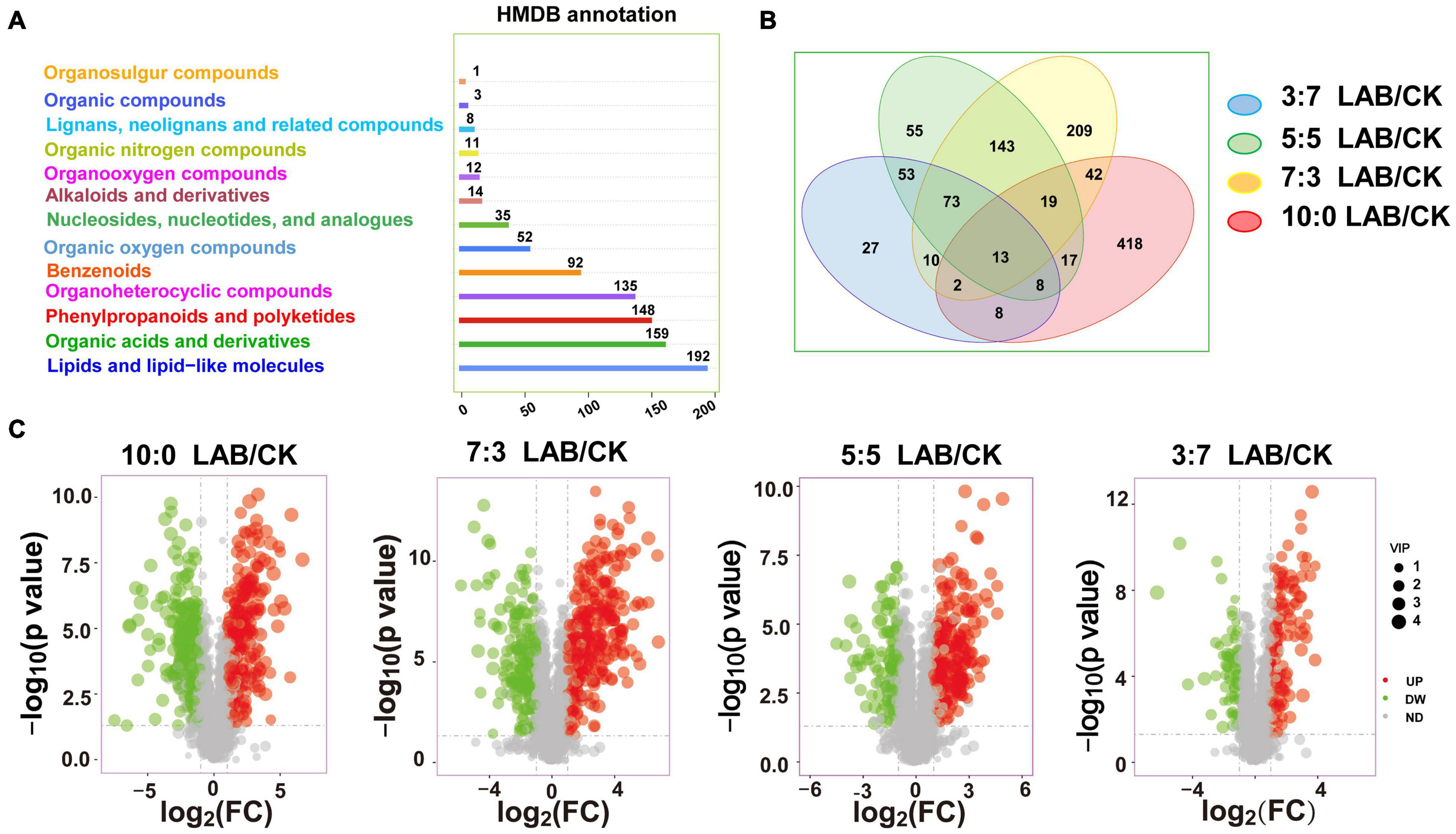
Figure 3. The metabolome of SC-SS silage. (A) Classification annotation of metabolites in HMDB database. (B) Venn diagram depicting specific or common metabolites of SC-SS silage. (C) Volcano plot of the differentially expressed metabolites (LAB/CK). UP, upregulated metabolites; DW, downregulated metabolites; ND, no difference.
A total of 54 metabolites were screened with definite superclass to the HMDB database (Table 3). In comparison with the CK silages, LAB-treated silages contained higher levels of metabolites with bioactivity, such as anti-inflammatory activity metabolites (e.g., glycitin, lithospermic acid, and psoralidin), antioxidant activity metabolites (e.g., isoferulic acid, sinapinic acid, and moslosooflavone), anti-tumor activity metabolites (e.g., alnustone), and anti-fungal activity metabolites (e.g., 3-phenyllactic acid). LAB inoculation increased the relative content of free amino acids (e.g., L-arginine) and tripeptides (e.g., VLK, LPH, TLK, etc.). Moreover, LAB inoculation caused sugar accumulations, such as D-(+)-maltose, α-lactose, raffinose, and maltotriitol. Furthermore, the level of histamine (harmful metabolites) was decreased in LAB-treated silages.
Redundancy analysis (RDA) revealed the correlation between fermentation characteristics and major bacteria (the relative abundance > 1%) at the genus level (Figure 4). The position over the two dimensions on the figure demonstrated how variables clustered. The angle between the fermentation factors and bacteria indicates the correlation. An angle (significantly) less than 90° shows (intense) positive correlations, otherwise negative. The length of the line represents the degree of correlation between the two variables. The longer the line, the greater the correlation, and vice versa. Lactobacillus was positively correlated with LA content while negatively correlated with BA and NH3-N contents. However, Dialister and Enterococcus were positively associated with AA, BA, and NH3-N contents but negatively correlated with LA (Figure 4A).
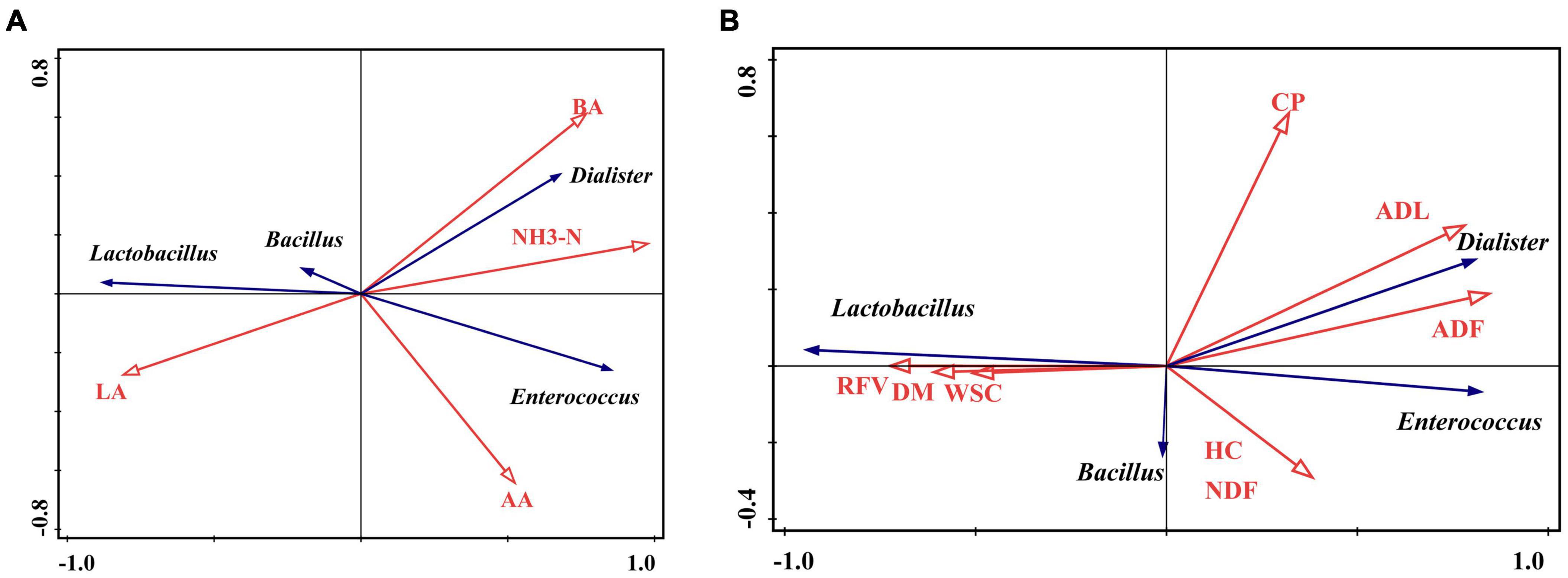
Figure 4. Redundancy analysis (RDA) plot showing the correlations between fermentation (A) and nutrition (B) characteristics and the bacterial community. The orange arrow line represents fermentation and nutrition characteristics. The blue arrow line represents bacteria at the genus level. The angle between the orange arrow line and blue represents the correlation. The angle ≤ 90° represents a positive correlation, otherwise negative. The length of the arrow line represents the contribution of a factor to the bacterial community. The longer the line is, the greater the contribution is.
The correlation between nutrition characteristics and four genera are shown in Figure 4B. Lactobacillus was positively correlated with DM, WSC, and RFV while negatively correlated with ADF, ADL, HC, and NDF. However, Enterococcus and Dialister were positively correlated with ADF, ADL, HC, and NDF but negatively correlated with WSC, DM, and RFV.
The heatmap shows the correlations between the keystone species and metabolites (Figure 5). Active metabolites, including glycitin, glabrene, and alnustone, were positively correlated (p < 0.01) with Len. buchneri, while that of psoralidin and isoferulic acid were positively correlated (p < 0.01) with Len. hilgardii. The metabolites of organic oxygen compounds superclass, like raffinose, galactinol, and maltotriitol, were positively correlated (p < 0.01) with Len. hilgardii, while that of D-(+)-maltose were positively correlated (p < 0.01) with Len. buchneri. Tripeptides, like SPK, LLK, SLK, RMK, etc., were positively correlated (p < 0.01) with Len. hilgardii. However, these metabolites were negatively correlated (p < 0.01) with Lac. plantarum and Com. farciminis. Raffinose and galactinol were also negatively correlated (p < 0.01) with Lac. plantarum and Com. farciminis, while that of oxytetracycline, esculin, and caffeic aldehyde were positively correlated.
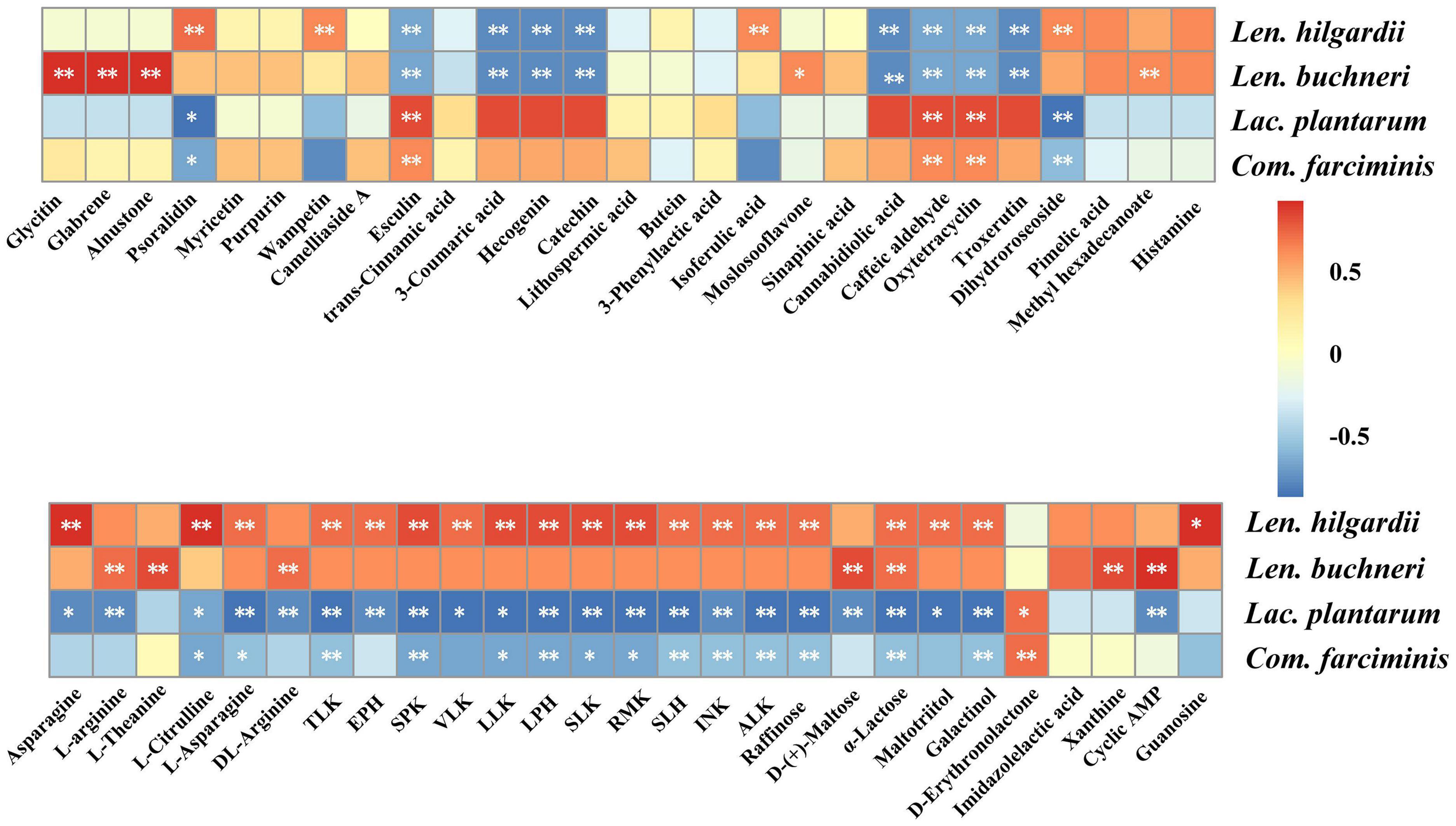
Figure 5. Spearman correlations between metabolites and inoculants. The correlations > 0.5 and < −0.5 were annotated significantly. p-values are shown as **p ≤ 0.01, *0.01 < p ≤ 0.05.
In the present study, the fermentation quality of co-ensiled protein-rich SC with WSC-rich SS was better than that of SC alone, such as the lower pH value, the higher LA content, and the lower BA and NH3-H contents. These results agreed with the reports of Chen et al. (2017) and Ni et al. (2018), who mixed gramineous crops with legume forage at different ratios. LAB inoculants further improved the fermentation quality based on SC-SS silage, which could be explained by the fact that Lactobacillus significantly reduced the pH value by increasing the LA content and decreasing the BA and NH3-H contents (Figure 3A). This may be due to LAB converting WSC into organic acids leading to rapid acidification of silage (Mcdonald et al., 1991). LA is a vital organic acid during ensiling because of its firm acidity (pKa = 3.86) for pH reduction. Rapid pH reduction can inhibit the fermentation of BA-producing and NH3-N-producing bacteria (e.g., Clostridium, Enterobacter) (Kung and Shaver, 2001). Therefore, the composition of the organic acids is an indicator to evaluate the quality of silage. In quality silage, LA usually accounts for 60–70% of the total organic content, and BA should approach 0% (Mcdonald et al., 1991). In this study, the LA makes up the majority of total acid, and BA is absent in LAB-treated SC-SS silage (7:3, 5:5, and 3:7 ratios), which indicated that LAB inoculants play a crucial role in the fermentation quality.
The DM content is an essential indicator of the nutritional preservation of forages (Hu et al., 2009). We found that LAB inoculation significantly increased DM content comparable to a previous study: Lac. plantarum or a combination of LAB inoculation increased DM concentration in the alfalfa and other legumes, temperate and tropical grasses, and the mixture of grass and legume (Oliveira et al., 2017). We also found that LAB inoculation significantly (p < 0.01) increased the contents of CP and WSC and decreased the structural carbohydrate contents (e.g., NDF, ADF, and HC). Previous researchers found similar results in which Lac. plantarum treatment increased the contents of WSC and CP in mixed silages of amaranth and rice straw (Mu et al., 2020), and Lac. plantarum application decreased the contents NDF and ADF in Pennisetum sinese silage (Li et al., 2020). It may be caused by the regulation of the bacterial community via LAB inoculants in the SC-SS silage, especially the proportion of Lactobacillus that relates to the nutritional quality. Fibers like NDF and ADF are negatively correlated with voluntary feed intake and digestibility of livestock (Mcdonald et al., 1991). As expected, the LAB group had a higher RFV than that of the CK group, indicating that inoculated LAB improved the nutritional quality of SC-SS silage. Moreover, we found that the LAB-treated group at the 7:3 ratio has abundant CP content (16.92% DM) and favorable fermentation quality (pH = 3.98, without BA). Therefore, co-ensiling SS and SC at 7:3 ratio is applicable. However, the SC-SS silage contained quantities of indigestible fiber. So it is necessary to develop more powerful LAB inoculants to further reduce the fiber contents.
As reported previously, LAB inoculation decreased the bacterial diversity (Liu et al., 2019). In our research, Lactobacillus became the dominant genus in the all silages after 60 days of being ensiled, which is analogous to prior work: Lactobacilli were the dominant genus after ensiling 96 samples in Southwest China (Guan et al., 2018). Furthermore, the species of Len. buchneri and Len. hilgardii were dominant, while that of Com. farciminis and Lac. plantarum were not dominant. Our results may explain that Len. buchneri and Len. hilgardii are more adaptive to the stable fermentation stage niche than that of Lac. plantarum and Com. farciminis. Prior work reported that Lac. plantarum dominated at the early fermentation stage (before 14 days) of ensiled alfalfa, while Len. buchneri played a role at the stable fermentation phase (after 30 days) (Guo et al., 2018). Ferrero et al. (2019) also found that the relative abundance of Len. hilgardii and Len. buchneri were higher in the stable fermentation phase than that in the early stage. Surprisingly, the bacterial community of SC-SS silage without LAB inoculants was also dominated by Len. buchneri and Len. hilgardii, and their relative abundance was nearly unaffected by the additives. This may reflect that Len. buchneri and Len. hilgardii were adapted to the nutritional characteristics of SC-SS silage.
In this study, the differential metabolites were analyzed by metabolomics. The comparison between metabolites concentration of the LAB and CK groups suggested that LAB inoculation caused dramatic changes in the metabolome. The correlations between inoculants and metabolites showed that most of the metabolites were positively correlated with heterofermentative LAB but were negatively correlated with homofermentative LAB. We found that LAB inoculation increased the level of some compounds in the superclass of phenylpropanoids and polyketides in agreement with the previous work which showed that ensiling caused the accumulation of some phenolic compounds in sainfoin silage (Xu et al., 2020). The anti-inflammatory metabolite glycitin and the anti-osteoporosis metabolite glabrene were positively correlated (p < 0.01) with Len. buchneri. This may be because Len. buchneri upregulated the isoflavone biosynthesis pathway which was similar to that observed in crop corn silage (Chen et al., 2019; Liu et al., 2021; Xu et al., 2021). The concentrations of most organic acids and their derivatives, including SPK, LPH, SLK, L-arginine, DL-arginine, etc., were upregulated in the LAB-treated silages. Tripeptides are attractive therapeutic agents and have potential applications in neurology, hematology, endocrinology, etc. (Santos et al., 2012). L-Arginine serves as a precursor of protein synthesis with pharmacological effects on reduction of vascular disease and improvement of immunity (Gad, 2010). In our experiments, tripeptides (e.g., LLK, TLK, SPK, etc.) and free amino acids were positively correlated (p < 0.01) with Len. hilgardii while negatively correlated with Lac. plantarum, broadening previous results that Len. buchneri was positively correlated with amino acids while negatively correlated with Lac. plantarum (Xu et al., 2021). Organic oxygen compounds, including D-(+)-maltose, raffinose, and α-lactose, accumulated in the LAB group during ensiling, which is similar to a previous study (Xu et al., 2020). Raffinose, α-lactose, and galactinol were positively correlated with Len. hilgardii while negatively correlated with Lac. plantarum and Com. farciminis. D-(+)-Maltose was positively correlated with Len. buchneri while negatively correlated with Lac. plantarum. The results observed for Lac. plantarum and Com. farciminis were opposite to those for Len. buchneri and Len. hilgardii with free amino acids, tripeptides, and sugars, which may be due to the different metabolic pathways of protein biosynthesis and sugar fermentation in homofermentative and heterofermentative LAB (Guo et al., 2018). Lactose can be utilized by LAB to produce acids and flavor, but it cannot be utilized by pathogenic or spoilage organisms (Vos and Vaughan, 2010). We found that LAB inoculation resulted in accumulated α-lactose, which is beneficial for reducing pH and inhibiting the growth of pathogens during ensiling, according to previous report (Hu et al., 2020). In contrast, organic nitrogen compounds, like histamine with toxicological characteristics, were downregulated with LAB treatment. Because of the difference between raw materials and inoculated LAB, we also detected other anti-inflammatory compounds such as psoralidin and alnustone and antioxidant compounds like lithospermic acid, which were not reported in the previous research. Therefore, the correlations between inoculants and the metabolites provided crucial information for screening of targeted LAB for quality silages, with extending bioactivity being beneficial to livestock performance and production.
In conclusion, LAB inoculation decreased the contents of NH3-N and BA but increased the LA content in SC-SS silage. LAB inoculation decreased the level of indigestible fibers (NDF, ADF, and HC), affecting livestock feed intake, but increased the essential nutrients (DM, CP, and WSC) for growth and production. Furthermore, LAB inoculation upregulated the sugars, free amino acids, peptides, and active metabolites but downregulated the harmful metabolites. Our results suggest that LAB inoculants, especially the heterofermentative Len. hilgardii and Len. buchneri, are potential additives for co-ensiling of SC with SS. Moreover, we expect the screening of targeted LAB for distinctive forage by metabolomics to broaden silages function for enhancing livestock performance in feed efficiency, milk yield, meat production, etc.
The data presented in the study are deposited in the NCBI repository, accession number PRJNA796383.
JZho: funding acquisition, supervision, and conceptualization. TX: writing original draft and visualization. TW: investigation, methodology, and visualization. WS and JZha: data curation and supervision. YL: supervision and project administration. FH: resources and methodology. JS: reviewing. All authors contributed to the article and approved the submitted version.
This research was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA26040201) and the Key Deployment Project of Chinese Academy of Sciences (KFZD-SW-113).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.851271/full#supplementary-material
Arthur Thomas, T. (1977). An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 28, 639–642. doi: 10.1002/jsfa.2740280711
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Chen, L., Guo, G., Yuan, X. J., Zhang, J., Wen, A. Y., Sun, X. H., et al. (2017). Effect of ensiling whole crop oat with lucerne in different ratios on fermentation quality, aerobic stability and in vitro digestibility on the Tibetan plateau. J. Anim. Physiol. Anim. Nutr. 101, e144–e153. doi: 10.1111/jpn.12577
Chen, Y., Guo, S., Jiang, K., Wang, Y., Yang, M., and Guo, M. (2019). Glycitin alleviates lipopolysaccharide-induced acute lung injury via inhibiting NF-κB and MAPKs pathway activation in mice. Int. Immunopharmacol. 75:105749. doi: 10.1016/j.intimp.2019.105749
dos Passos Bernardes, A., Tremblay, G. F., Bélanger, G., Brégard, A., Seguin, P., and Vanasse, A. (2014). Sugar Yield of Sweet Pearl Millet and Sweet Sorghum as Influenced by Harvest Dates and Delays Between Biomass Chopping and Pressing. Bioenergy Res. 8, 100–108. doi: 10.1007/s12155-014-9504-y
Ferrero, F., Piano, S., Tabacco, E., and Borreani, G. (2019). Effects of conservation period and Lactobacillus hilgardii inoculum on the fermentation profile and aerobic stability of whole corn and sorghum silages. J. Sci. Food Agric. 99, 2530–2540. doi: 10.1002/jsfa.9463
Gad, M. Z. (2010). Anti-aging effects of l-arginine. J. Adv. Res. 1, 169–177. doi: 10.1016/j.jare.2010.05.001
Guan, H., Yan, Y., Li, X., Li, X., Shuai, Y., Feng, G., et al. (2018). Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour Technol. 265, 282–290. doi: 10.1016/j.biortech.2018.06.018
Guo, X., Ke, W., Ding, W., Ding, L., Xu, D., and Wang, W. (2018). Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 8:357. doi: 10.1038/s41598-017-18348-0
Hu, W., Schmidt, R. J., McDonell, E. E., Klingerman, C. M., and Kung, L. Jr. (2009). The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 92, 3907–3914. doi: 10.3168/jds.2008-1788
Hu, Z., Niu, H., Tong, Q., Chang, J., Yu, J., Li, S., et al. (2020). The Microbiota Dynamics of Alfalfa Silage During Ensiling and After Air Exposure, and the Metabolomics After Air Exposure Are Affected by Lactobacillus casei and Cellulase Addition. Front. Microbiol. 11:519121. doi: 10.3389/fmicb.2020.519121
Kitonga, K., Sartie, A. M., Hanson, J., Nils, Teufel, Jones, C. S., Jamora, N., et al. (2019). The Benefits of ILRI Forages to Livestock Producers in East Africa. Genebank Impacts Poster. Kenya: ILRI, 4.
Kung, L., and Shaver, R. (2001). Interpretation and use of silage fermentation analysis reports. Focus Forage 3, 1–5.
Li, F., Ke, W., Ding, Z., Bai, J., Zhang, Y., Xu, D., et al. (2020). Pretreatment of Pennisetum sinese silages with ferulic acid esterase-producing lactic acid bacteria and cellulase at two dry matter contents: fermentation characteristics, carbohydrates composition and enzymatic saccharification. Bioresour Technol. 295:122261. doi: 10.1016/j.biortech.2019.122261
Liu, B., Huan, H., Gu, H., Xu, N., Shen, Q., and Ding, C. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Liu, J., Deng, X., Liang, X., and Li, L. (2021). The phytoestrogen glabrene prevents osteoporosis in ovariectomized rats through upregulation of the canonical Wnt/β−catenin signaling pathway. J. Biochem. Mol. Toxicol. 35:e22653. doi: 10.1002/jbt.22653
Mcdonald, P., Henderson, A. R., and Heron, S. (1991). The Biochemistry of Silage. Southampton: Chalcombe publications.
Mu, L., Xie, Z., Hu, L., Chen, G., and Zhang, Z. (2020). Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 315:123772. doi: 10.1016/j.biortech.2020.123772
Nanduri, K. R. (2013). Sesbania: a promising forage legume for the arabian peninsula. Biosalinity News 14, 6–8.
Ni, K., Wang, F., Zhu, B., Yang, J., Zhou, G., Pan, Y., et al. (2017). Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 238, 706–715. doi: 10.1016/j.biortech.2017.04.055
Ni, K., Zhao, J., Zhu, B., Su, R., Pan, Y., Ma, J., et al. (2018). Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 265, 563–567. doi: 10.1016/j.biortech.2018.05.097
Oliveira, A. S., Weinberg, Z. G., Ogunade, I. M., Cervantes, A. A. P., Arriola, K. G., Jiang, Y., et al. (2017). Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 100, 4587–4603. doi: 10.3168/jds.2016-11815
Santos, S., Torcato, I., and Castanho, M. A. (2012). Biomedical applications of dipeptides and tripeptides. J. Pept. Sci. 98, 288–293. doi: 10.1002/bip.22067
Shahjalal, M., and Topps, J. H. (2000). Feeding sesbania leaves as a sole feed on growth and nutrient utilization in goats. Asian Aust. J. Anim. 13, 487–489. doi: 10.5713/ajas.2000.487
Sun, J., Wang, T., Huang, F., Liu, Y., Shi, W., Ma, C., et al. (2021). Silage fermentation: a potential microbial approach for the forage utilization of cyperus esculentus L. By-product. Fermentation 7:273. doi: 10.3390/fermentation7040273
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Vos, W. M., and Vaughan, E. E. (2010). Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol. Rev. 15, 217–237. doi: 10.1111/j.1574-6976.1994.tb00136.x
Wang, J., Yang, B. Y., Zhang, S. J., Amar, A., Chaudhry, A. S., Cheng, L., et al. (2021). Using mixed silages of sweet sorghum and alfalfa in total mixed rations to improve growth performance, nutrient digestibility, carcass traits and meat quality of sheep. Animal 15:100246. doi: 10.1016/j.animal.2021.100246
Wang, T., Teng, K., Cao, Y., Shi, W., Xuan, Z., Zhou, J., et al. (2020). Effects of Lactobacillus hilgardii 60TS-2, with or without homofermentative Lactobacillus plantarum B90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bioresour. Technol. 312:123600. doi: 10.1016/j.biortech.2020.123600
Wang, W., Ge, J., Yang, H., Yin, F., Huang, T., and Kuai, J. (2021). Adaptation of feed crops to saline-alkali soil stress and effect of improving saline-alkali soil. Acta Agron. Sin. 1–14.
Weinberg, Z. G., and Muck, R. E. (1996). New trends in development and use of inoculants for silage. FEMS Microbiol. Rev. 19, 53–68. doi: 10.1111/j.1574-6976.1996.tb00253.x
Xu, D., Ding, W., Ke, W., Li, F., Zhang, P., and Guo, X. (2019). Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 9:3299. doi: 10.3389/fmicb.2018.03299
Xu, D., Ding, Z., Wang, M., Bai, J., Ke, W., Zhang, Y., et al. (2020). Characterization of the microbial community, metabolome and biotransformation of phenolic compounds of sainfoin (Onobrychis viciifolia) silage ensiled with or without inoculation of Lactobacillus plantarum. Bioresour. Technol. 316, 123910. doi: 10.1016/j.biortech.2020.123910
Xu, D., Wang, N., Rinne, M., Ke, W., Weinberg, Z. G., Da, M., et al. (2021). The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 14, 561–576. doi: 10.1111/1751-7915.13623
Keywords: Sesbania cannabina, co-ensiling, lactic acid bacteria, bacterial community, metabolome
Citation: Xia T, Wang T, Sun J, Shi W, Liu Y, Huang F, Zhang J and Zhong J (2022) Modulation of Fermentation Quality and Metabolome in Co-ensiling of Sesbania cannabina and Sweet Sorghum by Lactic Acid Bacterial Inoculants. Front. Microbiol. 13:851271. doi: 10.3389/fmicb.2022.851271
Received: 09 January 2022; Accepted: 01 February 2022;
Published: 24 March 2022.
Edited by:
Jose Antonio Curiel, Instituto Nacional de Investigación y Tecnología Agroalimentaria (INIA), SpainReviewed by:
Francesca Anna Ramires, National Research Council (CNR), ItalyCopyright © 2022 Xia, Wang, Sun, Shi, Liu, Huang, Zhang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Zhong, emhvbmdqQGltLmFjLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.