- 1College of Life Sciences, Northeast Forestry University, Harbin, China
- 2Key Laboratory for Enzyme and Enzyme-like Material Engineering of Heilongjiang, Harbin, China
Plant-associated microbes play important roles in plant health and disease. Mortierella is often found in the plant rhizosphere, and its possible functions are not well known, especially in medical plants. Mortierella alpina isolated from ginseng soil was used to investigate its effects on plant disease. The promoting properties and interactions with rhizospheric microorganisms were investigated in a medium. Further, a pot experiment was conducted to explore its effects on ginseng root rot disease. Physicochemical properties, high-throughput sequencing, network co-occurrence, distance-based redundancy analysis (db-RDA), and correlation analysis were used to evaluate their effects on the root rot pathogen. The results showed that Mortierella alpina YW25 had a high indoleacetic acid production capacity, and the maximum yield was 141.37 mg/L at 4 days. The growth of M. alpina YW25 was inhibited by some probiotics (Bacillus, Streptomyces, Brevibacterium, Trichoderma, etc.) and potential pathogens (Cladosporium, Aspergillus, etc.), but it did not show sensitivity to the soil-borne pathogen Fusarium oxysporum. Pot experiments showed that M. alpina could significantly alleviate the diseases caused by F. oxysporum, and increased the available nitrogen and phosphorus content in rhizosphere soil. In addition, it enhanced the activities of soil sucrase and acid phosphatase. High-throughput results showed that the inoculation of M. alpina with F. oxysporum changed the microbial community structure of ginseng, stimulated the plant to recruit more plant growth-promoting bacteria, and constructed a more stable microbial network of ginseng root. In this study, we found and proved the potential of M. alpina as a biocontrol agent against F. oxysporum, providing a new idea for controlling soil-borne diseases of ginseng by regulating rhizosphere microorganisms.
Introduction
Ginseng (Panax ginseng C. A. Meyer), a member of the Araliaceae family, is a valuable medicinal plant with multiple functions, such as enhancing organ functioning, inhibiting inflammation, preventing tumors and diseases, and potentially inhibiting COVID-19 (Han et al., 2018; Li et al., 2020b; Lee and Rhee, 2021). It is estimated that the global ginseng market, including ginseng root and the processed products, is worth $2,084 million (Baeg and So, 2013). Ginseng is cultivated in many countries, such as China, United States, and South Korea. As a perennial plant, ginseng grows in cold and humid environments and is prone to various diseases during growth. F. oxysporum can cause many plant diseases, such as tomato wilt, potato dry rot, and soybean root rot (Palmieri et al., 2020; Han et al., 2021; Ren et al., 2021). Root rot is a serious soil-borne disease of ginseng that damages ginseng of all ages and can lead to crop failure in severe cases (Farh et al., 2018). F. oxysporum is one of the main pathogens causing root rot in ginseng (Punja et al., 2008).
Currently, ginseng soil-borne diseases are mainly controlled by chemical agents. The frequent use of chemical fungicides has led to many problems, including increased pathogen resistance to fungicides, destruction of the soil microenvironment, high levels of toxic substances in ginseng, and environmental pollution (Wang et al., 2021a). Biological control is an environmentally friendly method of controlling plant diseases using beneficial microorganisms to regulate the microbiological composition of the soil. This effectively protects plants from pathogenic microorganisms while gradually leading to positive microbial community succession. A large number of commercial microbial agents have been developed from endophytic and rhizospheric microorganisms, such as Bacillus, Pseudomonas, and Trichoderma (John et al., 2010; You et al., 2016; De Silva et al., 2019). Different plants have different physiological characteristics and rhizospheric soil microdomains. Consequently, screening microorganisms from native plants and rhizospheric soil can easily enhance the effectiveness of biocides (Bagy et al., 2019; Azabou et al., 2020). Therefore, it can become an effective means to control soil-borne diseases.
Mortierella, due to its ability to degrade organic pollutants, can be used for soil remediation. Mortierella has also been detected in the rhizosphere and bulk soils of many plants (Liu et al., 2021; Tong et al., 2021; Xiang et al., 2021; Zhou et al., 2021). Some studies have indicated that Mortierella is related to soil disease inhibition, and may inhibit the diseases caused by Fusarium and participate in the transformation of phosphorus in soil. This is beneficial for soil health and nutrient absorption in plants from soil (Li et al., 2020a; Liu et al., 2020a). However, another study reported that Mortierella was a dominant plant pathogen (Guo et al., 2021). This controversy suggests that the effects of Mortierella on plants may be species-specific. In addition, the current analysis of Mortierella disease inhibition has not reached a consensus on whether Mortierella achieves inhibition and growth promotion in pathogenic microorganisms by affecting the bacterial or fungal community in soil or plants (Li et al., 2020a; Guo et al., 2021). Therefore, it is vital to discuss possible plant-specific probiotic effects of microbial species. This will contribute to better defining the scope of action and functions of biocontrol microorganisms.
Mortierella has also been detected in the rhizosphere of Panax ginseng (Li et al., 2018; Liu et al., 2020b). However, its possible function during Panax ginseng cultivation remains unclear. Our previous research showed that Mortierella accounted for different proportions of fungal communities under different soil planting conditions, with the highest proportion in forest soil and the lowest in 4-year ginseng-cultivated soil. This indicates that there is a positive correlation between Mortierella and the health of ginseng cultivation soil (Wang et al., 2021b). However, it is unclear whether it can be used as a possible biocontrol fungus to improve the resistance of ginseng to soil-borne diseases.
Here, an M. alpina strain YW25 isolated from ginseng rhizosphere soil was inoculated into the ginseng rhizosphere to test its possible pathogen resistance and biocontrol potential during Panax ginseng cultivation. F. oxysporum strain YFW32, which causes ginseng root rot, was used as the pathogen. In this study, we aimed to determine (1) the effects of inoculation of native M. alpina on ginseng and rhizosphere soil; (2) whether M. alpina has the ability to help plants resist the invasion of pathogens; and (3) if it does, how is the underlying mechanism?
Materials and Methods
Microbial Strains
Mortierella alpina YW25 was isolated from ginseng rhizosphere soil, and F. oxysporum YFW32 was isolated from diseased ginseng roots. The above strain sequences were been stored in DDBJ/EMBL/GenBank using DDBJ quick annotation and submission tool (DFAST),1 and their login numbers were LC663965 and LC656545, respectively. The strains were stored at −80°C and then streaked on PDA plates, cultured at 28°C for 7 days, and transferred twice for subsequent tests.
Analysis of Growth-Promoting Potential of Mortierella alpina
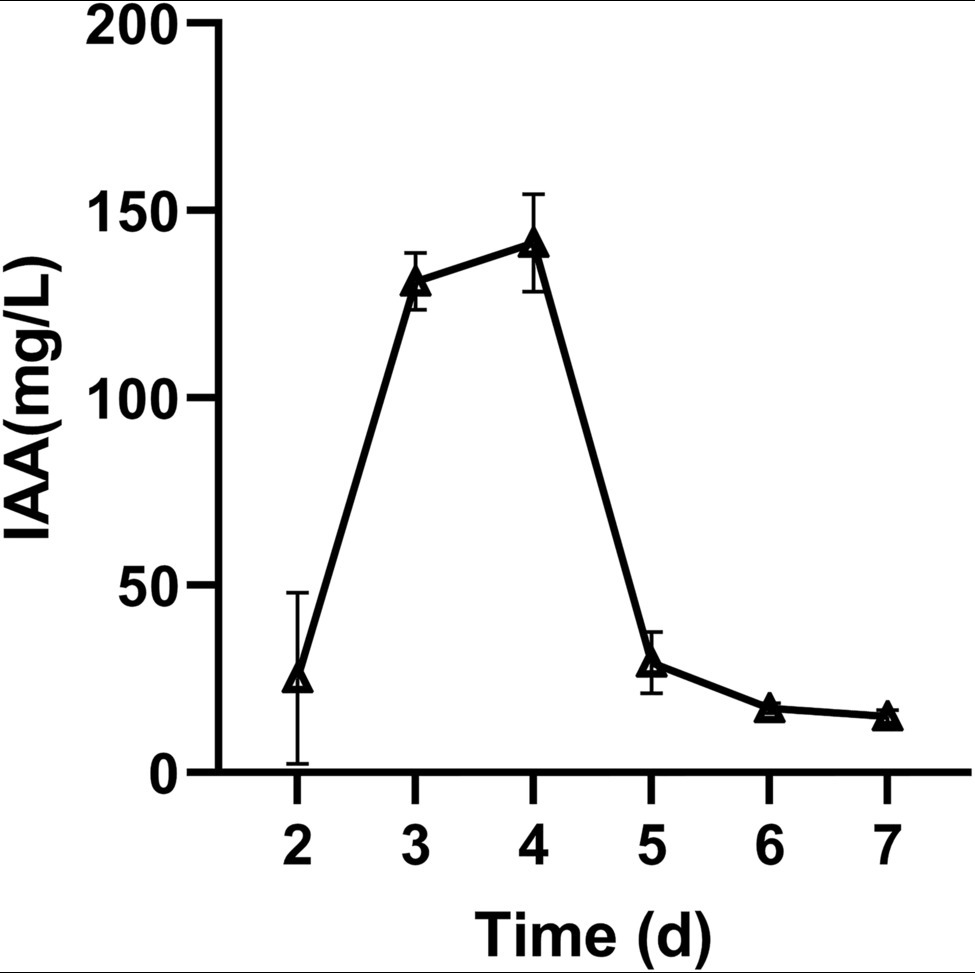
The Salkowski colorimetric method (Gordon and Weber, 1951) was used to evaluate the IAA production capacity of M. alpina YW25. Briefly, six PDA plugs with M. alpina YW25 mycelia (5 mm diameter) were inoculated in flasks containing 100 ml PDB liquid medium and 3 mM tryptophan. The flasks were maintained at 28°C for 2–7 days at 180 rpm. Uninoculated medium was used as the control. Then, 2 ml of culture was centrifuged at 10,000 rpm and 4°C for 10 min. The supernatant was mixed with Salkowski reagent in equal volumes, and the reaction was developed at 25°C in the dark for 30 min. The absorbance was measured at 535 nm. A calibration curve was established for calculating IAA concentration (5–100 mg/L) at 535 nm using pure IAA. The values were averaged over triplicates.
The solubility of M. alpina YW25 inorganic phosphorus was evaluated using Pikovskaya’s (PVK) medium (Pikovskaya, 1948). The 1 L medium consisted of 10 g glucose, 0.3 g NaCl, 0.3 g KCl, 0.5 g (NH4)2SO4, 0.3 g MgSO4·7H2O, 0.03 g MnSO4·4H2O, 0.03 g FeSO4·7H2O, 5 g Ca3(PO4)2, 18 g agar, and 1 L distilled water, and was adjusted to pH 7.0–7.2. M. alpina YW25 was inoculated into plates containing PVK agar medium. The inoculated plates were incubated in the dark at 28°C for 7 days. Clear halos were observed around the colonies, which indicated that the isolate solubilized inorganic phosphate. Lecithin (P7443, Sigma-Aldrich, United States) was used instead of Ca3(PO4)2 to evaluate its ability to dissolve organophosphorus (Wei et al., 2018). This was carried out by the same process as inorganic phosphorus. The phosphate solubility index (SI), which is the whole diameter zone (diameter of halo + diameter of colony) ÷ colony diameter, was used to evaluate the phosphorus solubility of the strain. The values were averaged over triplicates.
Chrome Azurol S (CAS; Schywn and Nielands, 1987) was used to evaluate the siderophore production capacity of M. alpina YW25. PDA plugs with M. alpina YW25 mycelia (5 mm diameter) were inoculated on CAS plates and incubated at 28°C for 7 days. The formation of an orange halo around the colony was observed. Larger halos had darker colors, which indicated a higher yield of siderophores. Six PDA plugs with M. alpina YW25 mycelia were inoculated in 100 ml of PDA liquid medium. The flasks were maintained at 28°C for 7 days at 180 rpm. Subsequently, 2 ml of culture at 4°C was centrifuged at 10,000 rpm for 10 min. The supernatant was mixed with CAS solution in equal volumes, and the reaction was carried out at 25°C in the dark for 1 h. The absorbance was detected at 630 nm (A), and the uninoculated medium was used as the control (Ar). Siderophores produced by the isolate were measured as percent siderophore units (% SU), and were calculated according to the following formula: % SU = (Ar–A) ÷ Ar × 100. The values were averaged over triplicates (Machuca and Milagres, 2003).
PDA plugs containing M. alpina YW25 mycelia were inoculated on PDA plates containing 0.2% soluble starch, 0.5% carboxymethyl cellulose, 0.5% xylan, 1% pectin, and 1% skim milk powder and cultured at 28°C for 7 days to evaluate the activity of amylase, cellulase, xylanase, pectinase, and protease, respectively. The plate containing 0.2% soluble starch was treated with a 1% iodine solution. A transparent halo around the colony indicated amylase activity. Congo red solution (0.2%) was added to the plates containing 0.5% carboxymethyl cellulose and 0.5% xylan. Following this, the plates were washed with 1 M NaCl. Yellow halos were observed around the colonies, which indicated cellulase and xylanase activities, respectively. When 1% cetyl trimethyl ammonium bromide (CTAB) was added to the plate containing 1% pectin, a transparent halo appeared around the colony, which indicated pectinase activity. After the fungi were cultured on PDA plates containing 1% skim milk powder, a transparent hydrolytic halo appeared around the colony, which indicated protease activity (Sopalun and Iamtham, 2020; Liu et al., 2020c; Sopalun et al., 2021).
Six PDA plugs with M. alpina YW25 mycelia (5 mm diameter) were inoculated in flasks containing 100 ml YM liquid medium (Papagianni and Moo-Young, 2002). The flasks were maintained at 28°C for 5 days at 180 rpm. Mix 1 ml culture supernatant in equal volume with a phosphate buffer (pH 7.0) containing 1% (w/v) casein, and incubated for 10 min at 30°C. Two milliliter of 0.4 M trichloro acetic (TCA) acid was added to terminate the reaction. The mixture containing the culture supernatant was incubated for 30 min at 25°C followed by centrifugation at 10,000 rpm for 5 min. Five microliter of 0.4 M Na2CO3 was then mixed with the supernatant (1 ml) and after 10 min, 1 ml of Folin reagent was added to each tube. The tubes were allowed to stand for 30 min at 30°C and then the absorbance was measured at 660 nm. Similar approach was used to prepare the control except casein was added only after the reaction was stopped. 1 U = the amount of enzyme required to liberate one microgram (1 μg/ml) of tyrosine under the assay conditions described (Chimbekujwo et al., 2020). The values were averaged over triplicates.
In vitro Analysis of Interactions Between Mortierella alpina and Rhizosphere Microorganisms
The interaction between M. alpina YW25 and ginseng rhizosphere microorganisms was evaluated in vitro using the plate confrontation method (Cong et al., 2019). The 17 fungi, 15 bacteria, and two actinomycetes used for confrontation were isolated from ginseng rhizosphere soil. M. alpina YW25 and rhizosphere fungi were symmetrically and equidistantly inoculated on a PDA plate 2.5 cm away from the center, and cultured at 28°C in the dark for 7 days. M. alpina YW25 was placed in the center of LB and Gao’s No.1 plates, and bacterial and actinomycete colonies, respectively, were picked out with sterilized toothpicks. The bacterial and actinomycete colonies were inoculated symmetrically and equidistantly at a distance of 2.5 cm from the M. alpina YW25 block on the plate, and cultured at 28°C in the dark for 5 days. The plate inoculated with M. alpina YW25 was used as the control. All processing settings were triplicated. Inhibition rate (%) = (colony radius of control group − colony radius of treatment group)/colony radius of control group × 100 (Cong et al., 2019). The inhibition of M. alpina YW25 by rhizosphere microorganisms was divided into four grades: − (no inhibition), + (inhibition rate < 30%), ++ (inhibition rate 30–60%), and +++ (inhibition rate > 60%).
Experimental Design and Sample Collection
The PDA plugs containing mycelia of M. alpina YW25 and F. oxysporum YFW32 with a diameter of 5 mm were cultured for 7 days at 28°C in PDA liquid medium separately at 180 rpm. The mycelium was filtered using gauze and diluted with sterile water to prepare a 1.2 × 107/ml spore suspension, which was used for pot inoculation of ginseng. Three treatment groups were established: single inoculation of M. alpina YW25 (MA), single inoculation with F. oxysporum YFW32 (FO), and inoculation with M. alpina YW25 and F. oxysporum YFW32 (MA_FO).
Potted soil (not autoclaved) contains 25.05 mg/kg nitrate nitrogen, 0.69 mg/kg ammonium nitrogen, 1.18 mg/kg available phosphorus, 292.25 mg/kg available potassium, total nitrogen 10.2 mg/g, total phosphorus 8.59 mg/g, total potassium 20 mg/g, and organic matter 0.35 g/g and the pH was 6.96. Three-year-old ginseng seedings were planted in each pot and inoculated by root irrigation. In MA and FO treatments, each pot (1.5 kg flower soil) was inoculated with 10 ml spore suspension. In MA_FO treatment, M. alpina YW25 and F. oxysporum YFW32 spore suspensions were inoculated with 5 ml each, and 10 ml sterile water was used as the control (CK). The setup for each treatment was repeated five times.
Ginseng was harvested after 70 days of pot planting. It was carefully uprooted and gently shaken to remove loosely adhered soil from the roots. Subsequently, all ginseng rhizosphere soil samples from the same treatment were mixed, and the rhizosphere soil sample of the treatment was formed. The rhizosphere soil samples were divided into two parts, and one of these was immediately stored in a −80°C refrigerator for the detection of soil microbial diversity. The other was air-dried indoors and stored at room temperature after filtering through a 2 mm sieve for determination of various soil physical and chemical properties. After washing and drying five ginseng plants in each treatment, the length and fresh weight of ginseng plants were measured by scale and balance. The ginseng plants were then divided into root and aboveground parts. After surface disinfection, the samples were quickly frozen in liquid nitrogen and then stored at −80°C for the detection of ginseng microbial diversity and plant defense enzymes.
Measurement of Soil Physicochemical Properties and Plant Defense Enzymes
Soil pH was measured using a pH meter (S010, Horiba, Japan). Nitrate and ammonium nitrogen were determined by 2 mol/L KCl extraction spectrophotometry (Li et al., 2021b). Available phosphorus was determined by NaHCO3 extraction and molybdenum–antimony resistance spectrophotometry (Yuan et al., 2020). Kjeldahl was used to determine total nitrogen (Arunrat et al., 2022). Total phosphorus was determined by sodium hydroxide alkali fusion–molybdenum–antimony anti spectrophotometry (Liu et al., 2022). Total potassium and available potassium were determined by flame atomic absorption spectrophotometry (Li et al., 2021a), and organic matter was determined by the loss-of-burning method (Salehi et al., 2011). Soil urease activity was determined by indophenol colorimetry (Adetunji et al., 2021), and the activities of soil catalase, acid phosphatase, and sucrase were determined using kits (Suzhou Grace Biotechnology Co. Ltd.; Zhou et al., 2020).
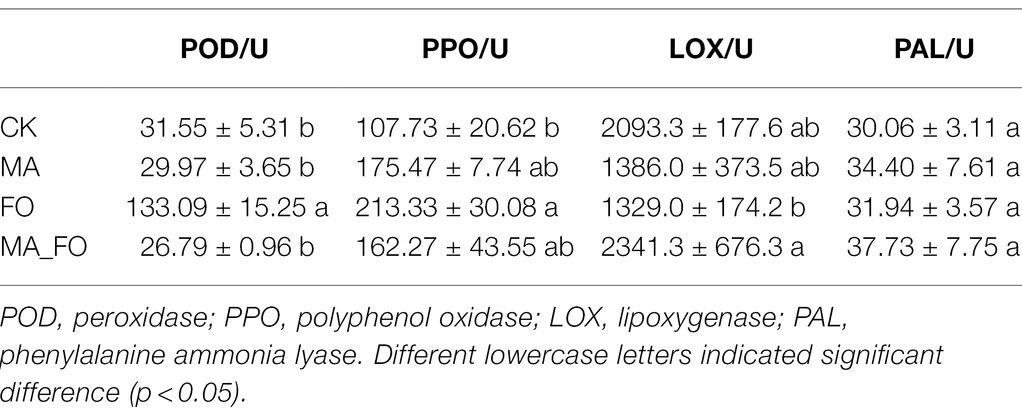
Harvested fresh ginseng root tissue was used to detect plant defense enzymes. The activities of peroxidase (POD), polyphenol oxidase (PPO), lipoxygenase (LOX), and phenylalanine ammonia lyase (PAL) were determined using microplate kits (NO. G0107W, NO. G0113W, NO. G0906W, and NO. G0114W, respectively, Suzhou Grace Biotechnology Co. Ltd.; Cheng et al., 2020; Yang et al., 2020).
High-Throughput Sequencing and Analysis of 16S rDNA and Internal Transcribed Spacer Regions
High-throughput Illumina sequencing was used to characterize the microbial community structure in the soil and plant samples (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). The V3-V4 regions of the soil bacterial 16S rRNA genes were amplified using the primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′; Xu et al., 2019). To assess the ginseng bacterial community, two sets of primers targeting the V3-V4 region of 16S rRNA gene were designed. The first-round reaction was amplified with primers 799F (5′-AACMGGATTAGATACCCKG-3′) and 1392R (5′-ACGGGCGGTGTGTRC-3′; Cui et al., 2020). The second-round reaction was amplified with primers 799F (5′-AACMGGATTAGATACCCKG-3′) and 1193R (5′-ACGTCATCCCCACCTTCC-3′; Bulgarelli et al., 2015). The ITS1F-ITS2R region of the ginseng fungal gene was amplified using the primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′; Sun et al., 2018). Specific primers with barcodes were synthesized according to the designated sequencing region, and then the samples were amplified using a thermocycler (GeneAmp® 9700, ABI, United States). The raw reads were deposited into the NCBI sequence read archive (SRA) under the submission ID SUB10895992.2
Bacterial PCR reactions were performed in triplicate, with 4 μl 5× FastPfu Buffer, 2 μl 2.5 mM dNTPs, 0.8 μl 5 μM forward primer, 0.8 μl 5 μM reverse primer, 0.4 μl FastPfu Polymerase, 0.2 μl bovine serum albumin (BSA), and 10 ng template DNA in a 20 μl reaction volume. The thermal cycling conditions for prokaryotic 16S rRNA gene from soil bacteria fragment amplification were as follows: 3 min at 95°C, 30 cycles of 30 s at 95°C, 30 s at 55°C, 45 s at 72°C, and 10 min at 72°C. The 16S rRNA gene from ginseng bacterial fragments was amplified in two rounds, and the thermal cycling conditions of amplification were as follows: first round: 3 min at 95°C, 27 cycles of 30 s at 95°C, 30 s at 55°C, 45 s at 72°C; and 10 min at 72°C; second round: 3 min at 95°C, 13 cycles of 30 s at 95°C, 30 s at 55°C, 45 s at 72°C, and 10 min at 72°C. Fungal PCR reactions were performed in triplicate with 2 μl 10× rTaq Buffer, 2 μl 2.5 mM dNTPs, 0.8 μl 5 μM forward primer, 0.8 μl 5 μM reverse primer, 0.2 μl rTaq polymerase, 0.2 μl BSA, and 10 ng template DNA in a 20 μl reaction volume. The thermal cycling conditions for prokaryotic ITS gene fragment amplification were as follows: 3 min at 95°C, 30 cycles of 30 s at 95°C, 30 s at 55°C, 45 s at 72°C, and 10 min at 72°C. The PCR products were identified by 2% agarose gel electrophoresis, purified using an AxyPrep DNA gel extraction kit (Axygen, Corning, NY, United States), and quantified using a QuantiFluor™-ST Blue Fluorescence Quantification System (Promega).
The amplified sub-library was sequenced on an Illumina PE250 platform (Biozeron, Shanghai, China). The effective sequences of all samples were obtained according to the barcode, and Trimmomatic (version 0.36; Lohse et al., 2012) filtration was used to remove reads with an average mass of less than 20 in 50 bp. Sequences were assembled using FLASH with a minimum overlap of 10 bp and a maximum mismatch ratio of 0.2 (Magoc and Salzberg, 2011). The RDP classifier Bayesian algorithm (Wang et al., 2007; version 2.2) was used to classify the representative sequences of each operational taxonomic unit (OTU) with 97% similarity.3 The bacterial 16S rRNA comparison database was Silva (Release138; Quast et al., 2013)4 and the fungal ITS comparison database was Unite (Release 8.0; Koljalg et al., 2013).5
Co-occurrence Network Analysis
A co-occurrence network based on the Spearman correlation coefficient matrix was constructed by NetworkX to study the relationship and interaction between bacteria and fungi in the aboveground and root of ginseng under different inoculation treatments. OTUs with relative abundance greater than 0.01% in each treatment were screened for OTU with subsequent correlation network construction. The most important interaction was highlighted, and the Spearman correlation threshold was set to 0.7, p < 0.05. Each node represents an OTU, and each edge represents a strong and significant correlation between the different nodes. Networks were visualized using the Gephi platform.6 Topological features (average degree and modularity) of the networks were calculated using NetworkX on the free online platform of Majorbio Cloud Platform.7
Statistical Analysis
GraphPad Prism 8.3.0 was used to draw line and bar charts. SPSS 19.0 was used for one-way analysis of variance (ANOVA), and the significance level was p < 0.05. For the high-throughput Illumina sequencing data, Adonis test, Student’s t-test along with alpha diversity, db-RDA, and linear regression analyses were performed using the online platform of Majorbio Cloud.8
Result
Growth-Promoting Potential of Mortierella alpina YW25
To detect the plant growth-promoting potential of M. alpina YW25, the capacities of IAA and siderophore production, phosphorus solubilization, and hydrolase activity of M. alpina YW25 were determined. The results showed that it had a high ability to produce IAA, and the highest IAA concentration in PDB liquid medium containing 3 mM tryptophan reached 141.37 mg/L at 4 days (Figure 1). The ability to produce siderophores and dissolved phosphorus was not detected in M. alpina YW25. No hydrolase activities of M. alpina YW25, except for protease activity was 5.5 U/ml after 5 days.
In vitro Analysis of Mortierella alpina YW25 and Rhizosphere Microbes Interactions
The interaction between M. alpina YW25 and a variety of microorganisms in the ginseng rhizosphere were studied by plate confrontation experiments (Table S1 and Figure 2). Bacillus species (Bacillus siamensis, B. velezensis, B. toyonensis, B. cereus, and B. zhangzhouensis) significantly inhibited the growth of M. alpina YW25 among the 15 bacterial strains isolated from the ginseng rhizosphere. Streptomyces tricolor and Brevibacterium frigoritolerans (actinomycetes) also showed significant inhibition of M. alpina YW25. Fungi isolated from ginseng rhizosphere, such as Trichoderma koningiopsis, T. viridescens, T. harzianum, T. velutinum, Rhizopus oryzae, Penicillium citrinum, P. chrysogenum, Aspergillus ochraceus, A. flavus, Cladosporium anthropophilum, and C. cladosporioides, also inhibited the growth of M. alpina YW25. However, there was no obvious interaction between F. oxysporum YFW32 and M. alpina YW25.
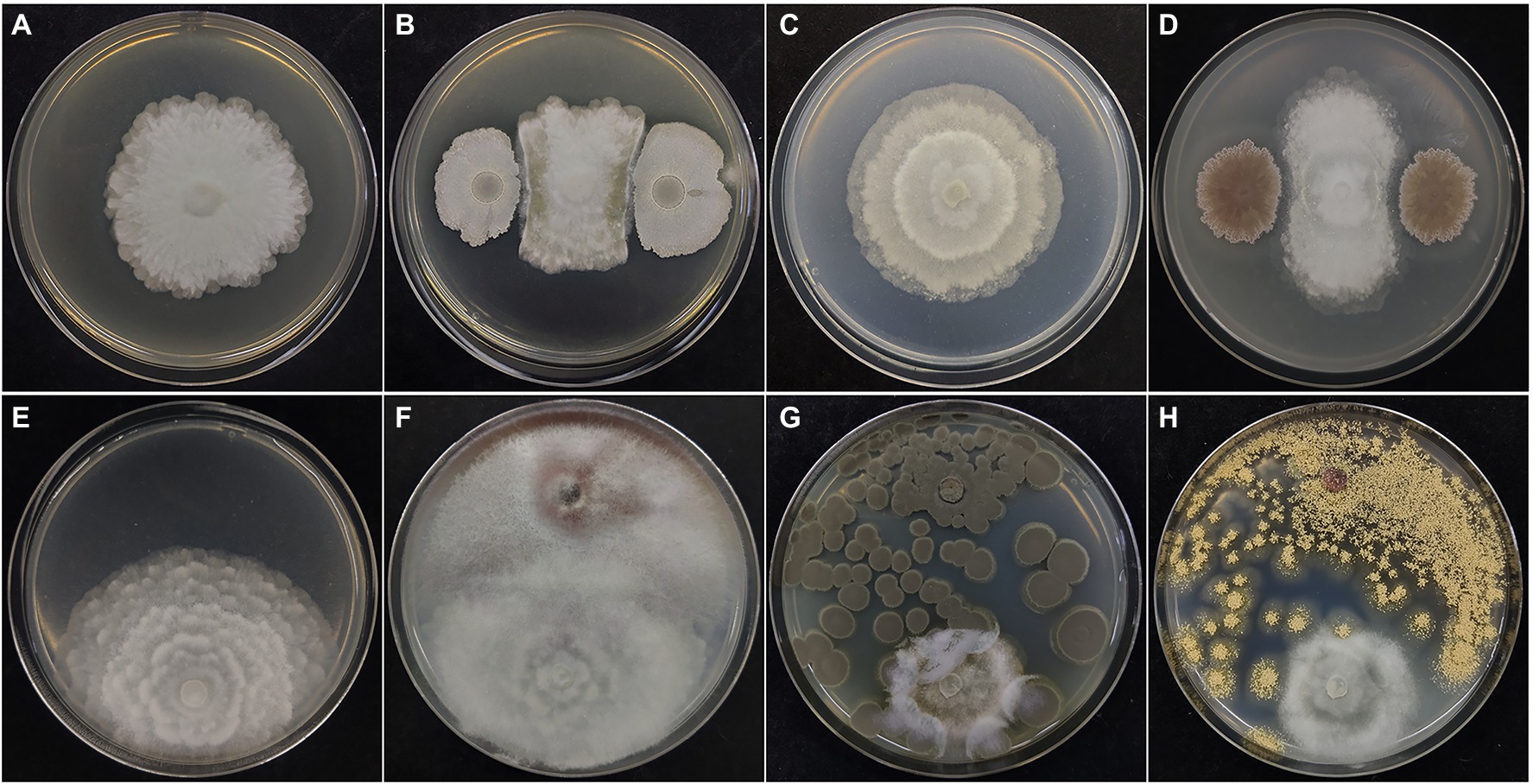
Figure 2. Results of confrontation between Mortierella alpina YW25 and rhizosphere microorganisms. As the control, Mortierella alpina YW25 was inoculated separately on LB (A), Gao’s No.1 (C) medium for 5 days, and on PDA medium 7 days (E). Mortierella alpina YW25 was co-cultured with Bacillus velezensis (B) on LB medium, Streptomyces tricolor (D) on Gao’s No.1 medium at 28°C for 5 days, and with Fusarium oxysporum YFW32 (F), Penicillium citrinum (G), Aspergillus ochraceus (H) on PDA medium at 28°C for 7 days.
Effects of Inoculation With Mortierella alpina YW25 on Panax ginseng
To determine the effects of M. alpina YW25 inoculation on ginseng, plant height, root length, and fresh weight of both aboveground and root regions were measured (Figure 3). The defense enzymes of ginseng roots were also determined (Table 1). In the FO treatment, leaves withered and roots were infected and decomposed (Figure 3A). Ginseng in the other treatments showed no disease symptoms. The root length of FO was significantly lower than that of the other treatments (p < 0.05), and there was no significant difference between MA and CK. There was no significant difference in fresh weight of ginseng aboveground. The fresh weight of ginseng roots in FO was significantly lower than that in the other treatments. The fresh weight of ginseng roots in CK was significantly higher than that in MA, but there was no significant difference between CK and MA (Figure 3B). In the FO treatment, POD and PPO activities were 133.09 and 213.33, respectively, which were significantly higher than those in CK (p < 0.05). LOX activity was significantly lower, whereas PAL activity showed no significant change. Compared with CK, the activities of PPO, LOX, and PAL in the MA_FO were higher, but the activities of the four plant defense enzymes in the MA and MA_FO treatments were not significantly different (Table 1).
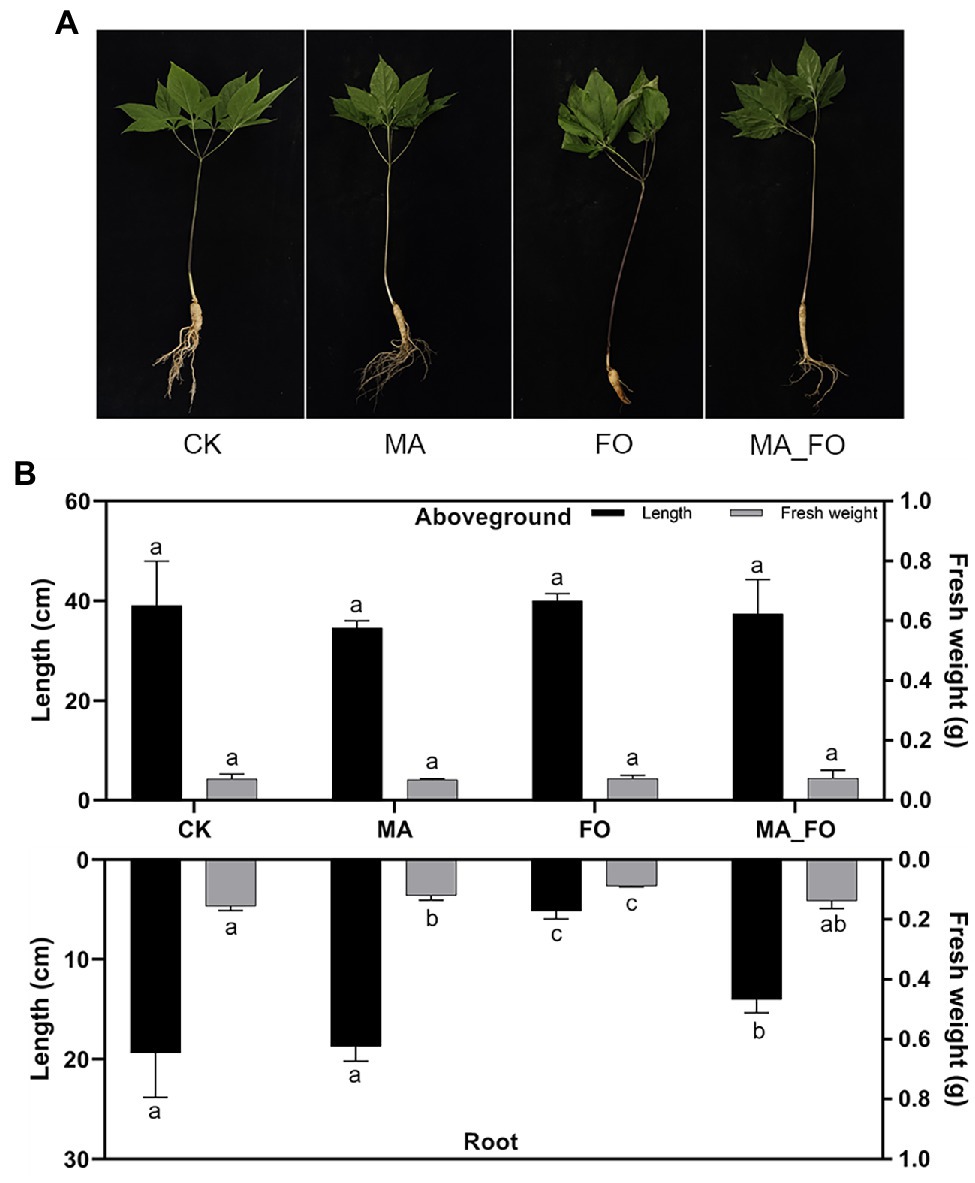
Figure 3. Growth status of ginseng under different inoculation treatments. (A) Photos of ginseng under different inoculation treatments. (B) The length and fresh weight of different parts of ginseng under different inoculation treatments. CK: control, FO: Fusarium oxysporum YFW32, MA: Mortierella alpina YW25, MA_FO: Mortierella alpina YW25 and Fusarium oxysporum YFW32, the same as below. The length and fresh weight of aboveground and root of ginseng under different treatments were analyzed for difference significance separately, and different lowercase letters indicated significant difference (p < 0.05).
Effects of Mortierella alpina YW25 Inoculation on Soil Physicochemical and Enzymatic Properties
Soil pH, ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3−-N), total nitrogen (TN), available phosphorus (AP), total phosphorus (TP), available potassium (AK), total potassium (TK), urease (Urease), catalase (CAT), sucrase (SC), and acid phosphatase (ACP) were measured in ginseng roots (Table 2).
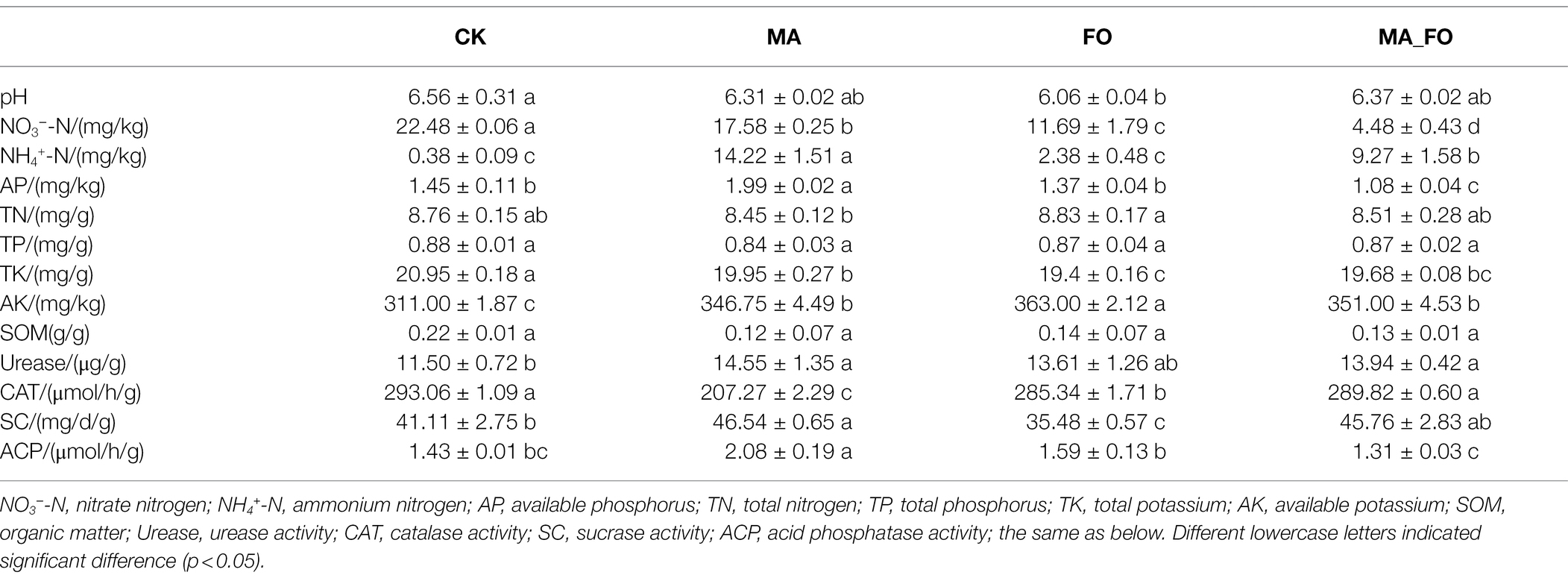
Table 2. Physicochemical and enzyme activity characteristics of ginseng rhizosphere soil under different inoculation treatments.
As shown in the table, there were no significant differences in pH, TN, and TP among the MA, MA_FO, and CK treatments. The NO3−-N and TK content in MA rhizosphere soil was significantly lower than those in CK, and the NH4+-N, AP, and AK content was significantly higher than those in CK (p < 0.05). In the FO treatment, soil pH was significantly lower than that in CK, but the soil AK content was significantly higher than that of the other treatments (p < 0.05). The content of NO3−-N, AP, and AK in the MA_FO rhizosphere soil was significantly lower, but the content of NH4+-N was significantly higher than that in FO (p < 0.05). The available nitrogen (NH4+-N and NO3−-N) and AP content in the MA treatment was significantly higher than those in the MA_FO treatment. In addition, the activity of urease, SC, and ACP in MA soil was significantly higher, while the activity of CAT was significantly lower than that in CK (p < 0.05). The activities of CAT and SC in the MA_FO treatment were significantly higher, while the activity of ACP was significantly lower than that in FO.
Effects of Mortierella alpina YW25 Inoculation on Plant and Soil Microbiome
The Shannon index was used to evaluate the soil microbial diversity of ginseng aboveground, roots, and rhizosphere under different treatments (Figure 4). The bacterial diversity in the ginseng rhizosphere soil was higher than that in the plant (p < 0.05) in all treatments. In the FO and MA treatments, soil fungal diversity was significantly lower than that in the CK and MA_FO treatments (p < 0.05). There was no significant difference between MA and CK in the diversity of bacteria and fungi in the aboveground parts of ginseng. In the MA treatment, the fungal diversity was significantly lower in the roots of ginseng than in CK and FO. Further, FO and MA_FO showed no significant differences in the diversity of bacteria in the aboveground parts of ginseng. Compared with FO, the bacterial diversity significantly increased, and the fungal diversity significantly decreased in the roots of ginseng in MA_FO (p < 0.05).

Figure 4. Shannon diversity index of bacterial (A) and fungi (B) community structure at different ecological niches (Aboveground: aboveground of ginseng; Root: root of ginseng; Soil: rhizosphere soil). Different lowercase letters indicate significant difference (p < 0.05).
There were also significant differences in the bacterial (Adonis, R2 = 0.2943, p = 0.001) and fungal (Adonis, R2 = 0.4483, p = 0.001) community structures in different parts of ginseng. Visual circos of microorganisms in aboveground and root of ginseng were constructed using bacteria and fungi genera, respectively, with relative abundance greater than 1% to evaluate the relationship between microorganisms and samples in different parts of ginseng (Figure 5).
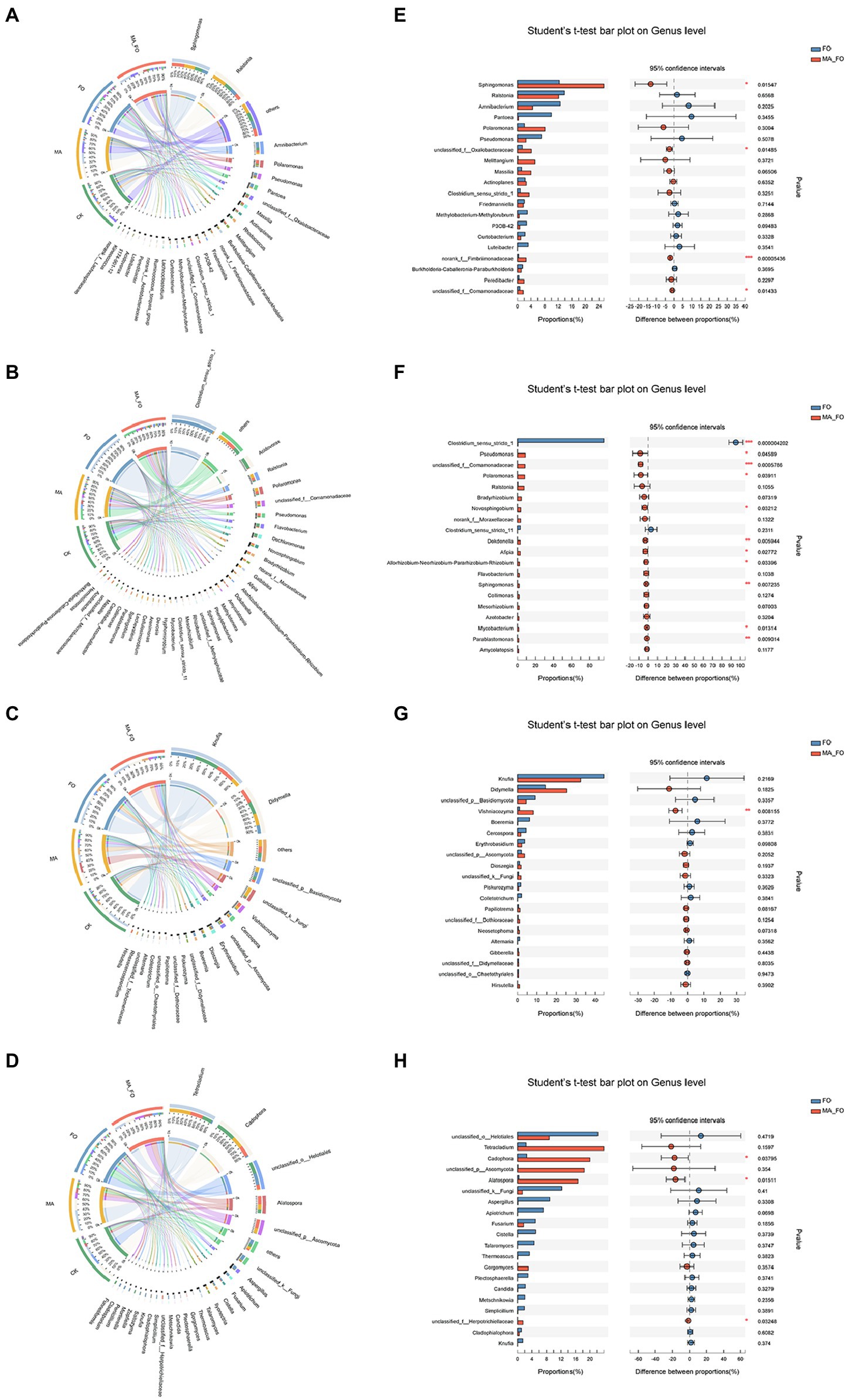
Figure 5. Analysis of microbial composition and differences in ginseng. Effects of different inoculation treatments on internal bacteria ((A) aboveground of ginseng; (B) root of ginseng) and fungi ((C) aboveground of ginseng; (D) root of ginseng) of ginseng. The left semicircle represents the different inoculation treatments. The right semicircle represents the dominant genera and proportions of each genus in different samples. Student’s t-test was used to test the significance of differences between FO and MA_FO at the genus level ((E) aboveground bacteria of ginseng; (F) root bacteria of ginseng; (G) aboveground fungi of ginseng; (H) root fungi of ginseng). The y-axis represents the species names at the genus level, the x-axis represents the average relative abundance in different groups of species, and the columns with different colors represents different groups. The far right is the value of p, *p < 0.05; **p < 0.01; ***p < 0.001.
The relationship between bacteria of aboveground ginseng parts and different treatments is shown in Figure 5A. Sphingomonas, Ralstonia, Amnibacterium, and Polaromonas were the main bacterial genera in the aboveground ginseng. The proportions of Sphingomonas and Polaromonas in MA_FO treatment were 34% and 51%, respectively, and Ralstonia had the highest distribution in MA treatment (41%). Compared with the FO treatment, the relative abundance of Sphingomona, Oxalobacteraceae, Fimbriimonadaceae, and Comamonadaceae in the MA_FO treatment was significantly increased (Figure 5B). Clostridium is the main bacterial genus of FO-treated ginseng roots and had a relative abundance of 96%. The main bacterial genera in CK roots were Acidovorax, Flavobacterium, and Dechioromonas with relative abundances of 28%, 9.9%, and 11%, respectively (Figure 5A). The bacterial diversity in ginseng roots between the MA and MA_FO treatments was significantly higher than that between the CK and FO treatments (p < 0.05), and there was no significant difference between the MA and MA_FO treatments. Compared to FO, the relative abundances of Pseudomonas, Comamonadaceae, Polaromonas, Novosphingobium, Dokdonella, Afipia, Rhizobium, Sphingomonas, Mycobacterium, and Parablastomonas increased significantly in ginseng roots after MA_FO treatment (Figure 5B).
Knufia and Didymella are the main fungal genera in the aboveground parts of ginseng. The relative abundance of Knufia in the MA treatment was 15%, which was significantly lower than that in the other treatments (p < 0.05; Figure 5A). In MA_FO, the species of potential plant pathogens in the aboveground parts of ginseng, such as Didymella, Cercospora, Boeremia, and Alternaria, were less than that of FO, and the relative abundance of Vishniacozyma was significantly higher than that of FO (p < 0.05; Figure 5B). Among the ginseng roots treated with CK, MA, and MA_FO, Tetracladium, Helotiales, Cadophora, and Alatospora were the main fungal genera. The relative abundances of Helotiales (1.3% and 8.8%) and Cadophora (22% and 20%) in MA and MA_FO were lower than those in CK, and Tetracladium (41% and 24%) and Alatospora (9.9% and 17%, respectively) were significantly higher than those in CK (Figure 5A). The relative abundance of Cadophora and Alatospora in the FO treatment was significantly lower than that in the MA_FO treatment (p < 0.05; Figure 5B). However, in terms of the distribution of fungi in different treatments, Aspergillus, Plectosphaerella, Candida, Cladosporium, and Cladophialophora had the highest distribution proportion in the FO treatment (89%, 100%, 83%, 91%, and 56%; Figure 5A).
The co-occurrence networks of bacterial and fungal communities significantly varied in different parts of ginseng and among the different treatments (Figure 6, Tables 3 and 4). Except for FO treatment, the bacterial network structure of ginseng root was generally more complex (based on the number of edges and nodes, and average degree) than that of the aboveground parts of ginseng. Among all bacterial networks, the FO-treated bacterial network of ginseng root was the simplest (nodes: 13; edges: 32; average degree: 4.932). Compared with FO treatment, MA_FO had a higher proportion of negative correlation between aboveground and root bacterial networks (aboveground: 42.88%; root: 39.6%) and modularity (aboveground: 0.713; root: 0.646). The number of edges and nodes, average degree, and modularity of the bacterial networks in the aboveground and root of ginseng in MA were higher than those of the control, but the proportion of negative correlation was lower than that of the control. MA treatment had a more complex bacterial network (based on the number of edges and nodes, and average degree) than MA_FO, but MA_FO might have a more stable bacterial network (based on the negative correlation ratio).

Figure 6. Analysis of microbial co-occurrence network in ginseng under different inoculation treatments. The nodes are colored according to bacterial and fungal phylum. Node size indicates the relative abundance of OTU. Edge color represents positive (blue) and negative (orange) correlations.
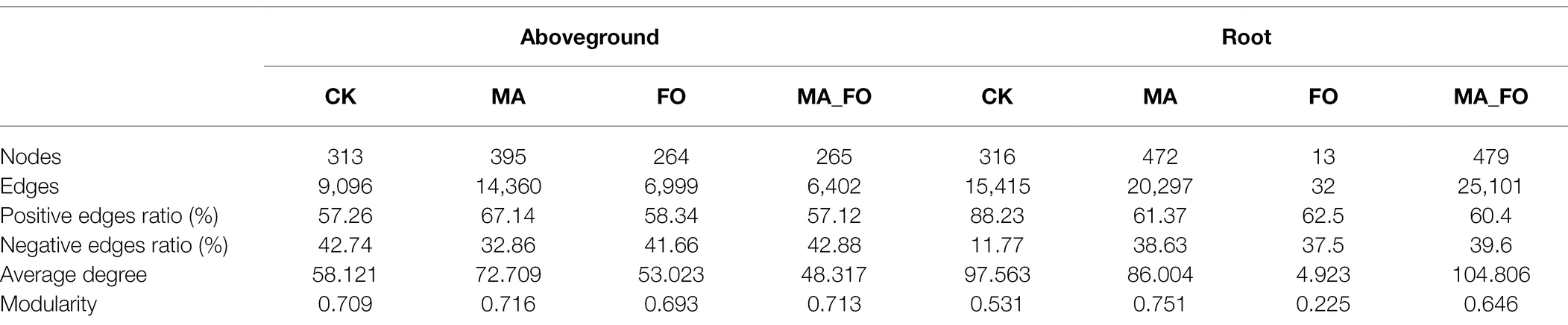
Table 3. Key topological features of bacterial networks in aboveground and root of ginseng under different inoculation treatments.
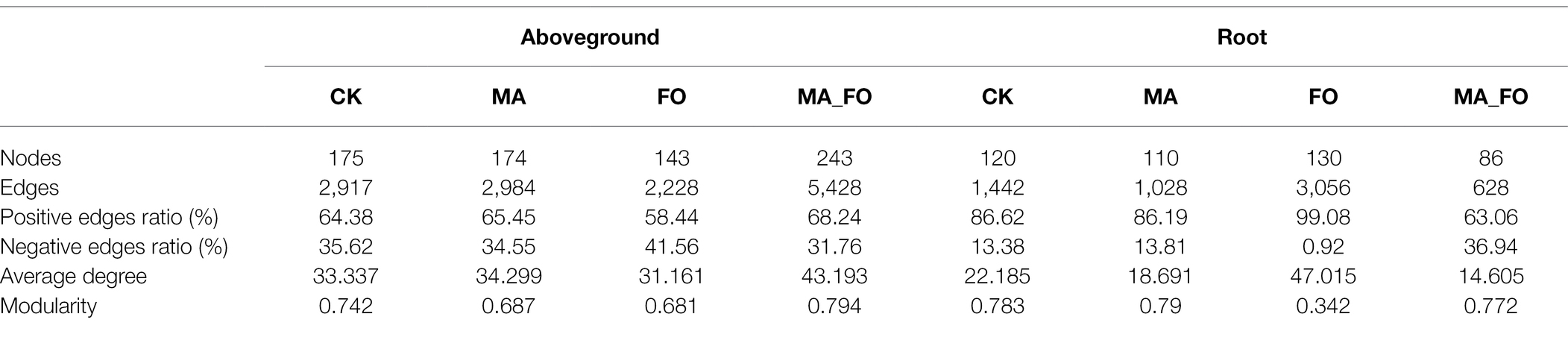
Table 4. Key topological features of fungal networks in aboveground and root of ginseng under different inoculation treatments.
The fungal network of ginseng is simpler since it has fewer nodes and edges compared to the bacterial network (Figure 6; Table 4). In contrast to the bacterial networks, the fungal network structure of the aboveground parts of ginseng is generally more complex than that of the root (based on the number of edges and nodes, and average degree); FO being the exception. The number of edges and nodes, average degree, and modularity of the fungal network in the aboveground parts of ginseng treated with MA_FO were higher than those in FO, while the opposite trend was observed in the root network of ginseng. However, the proportion of negative correlation was lower than that of FO. The fungal network of ginseng roots had a very high positive correlation ratio under the treatment of FO (99.08%). The number of edges and nodes, and average degree of the fungal networks in the aboveground ginseng treated with MA were higher than those of the control, but the proportion of negative correlation and modularity were lower than those of the control. The fungal networks of ginseng roots showed the opposite trend.
The bacterial community structure of ginseng rhizosphere soil under FO treatment was significantly different from that under the other treatments (Figure 7A). Pseudarthrobacter was the bacterium with the highest relative abundance in the rhizosphere soil of ginseng (5.5%–9.6%). The relative abundances of Flavobacterium, Marmoricola, Gaiella, and Ellin6070 in FO were significantly higher than those in the other treatments (p < 0.05; Figure 8). There were no significant differences in soil fungal diversity and community structure between CK and MA_FO, but the diversity of FO and MA soils decreased significantly, and the fungal communities of FO and MA soil were significantly separated in CAP1 (36.02%). This suggests that FO and MA soils had different community structures (Figure 7B). Fusarium and Mortierella could colonize soil and become the dominant genera in FO and MA, with relative abundances of 41.3% and 57%, respectively. The relative abundance of Fusarium in MA_FO was significantly lower than that in FO, but the abundance of Pseudeurotium and Schizothecium was significantly higher (Figure 8).
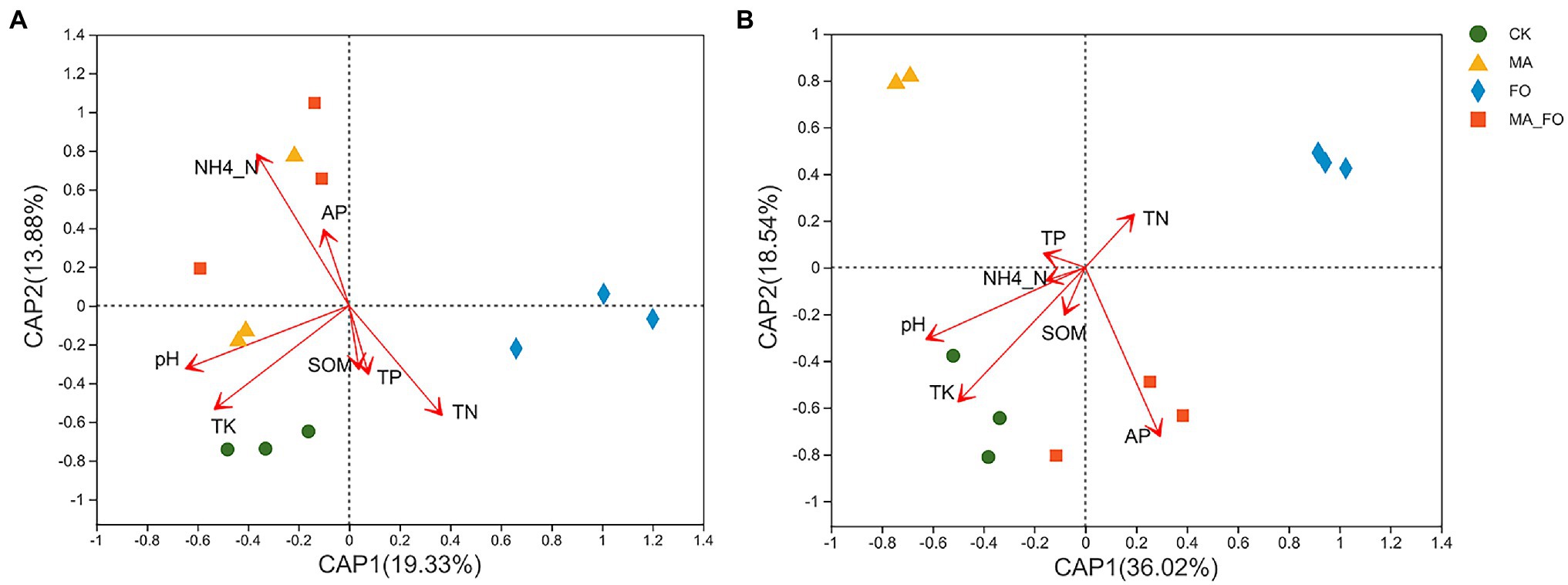
Figure 7. db-RDA analysis of soil microbial community and environmental factors. (A) bacterial community; (B) fungal community. Arrows represent environmental factors.
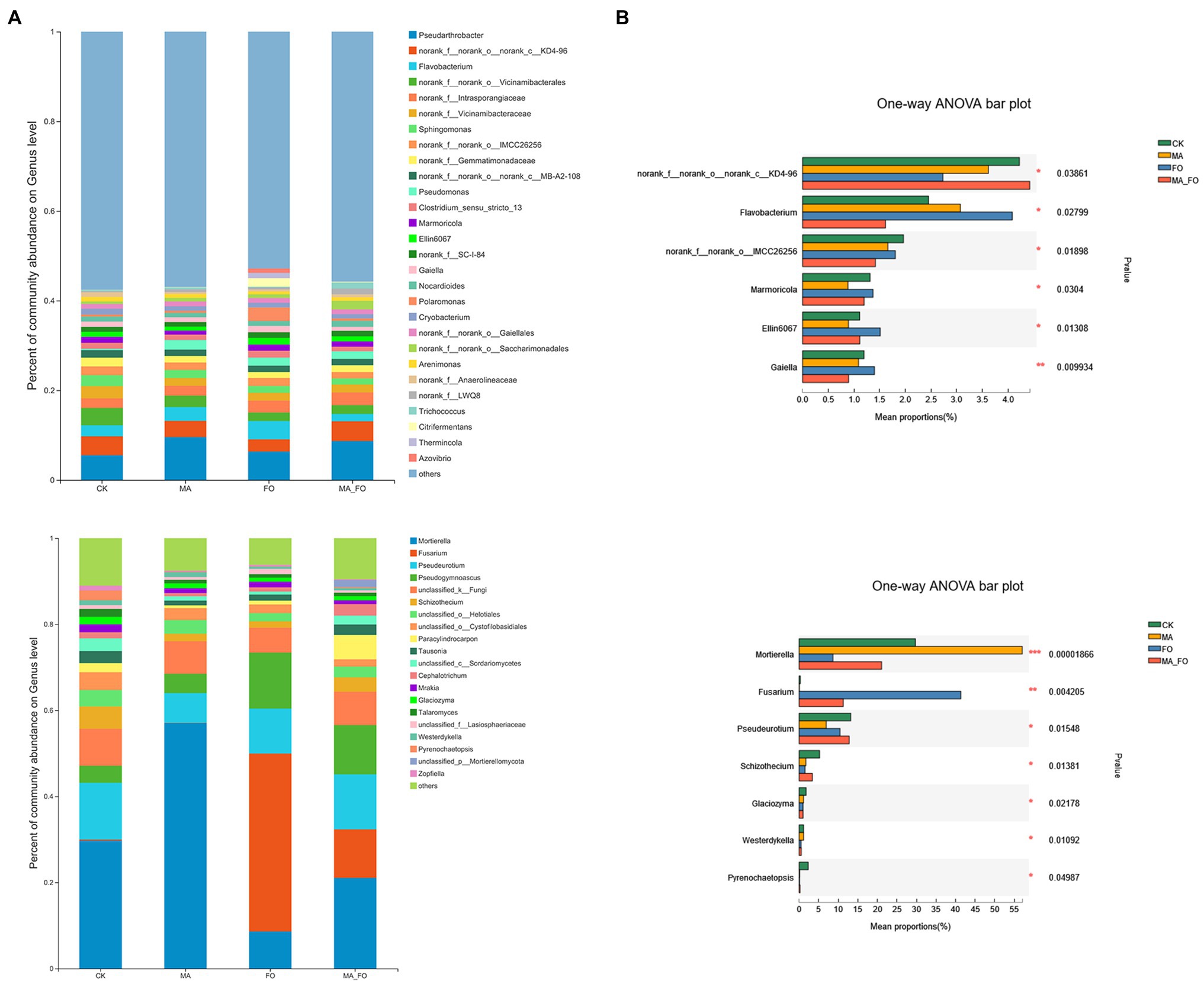
Figure 8. Analysis of microbial composition and differences in ginseng rhizosphere soil. (A) Relative abundance at the genus level of bacteria and fungi, where “others” represents species with relative abundance less than 1% in all samples; (B) One-way univariate analysis of variance (ANOVA) was used to test the significance of differences between groups at the genus level of bacteria and fungi. The y-axis represents the species names at the genus level, the x-axis represents the average relative abundance in different groups of species, and the columns with different colors represents different groups. Far right is the value of p, *p < 0.05; **p < 0.01; ***p < 0.001.
Correlation Between Soil Microorganisms and Physicochemical and Enzymatic Factors in Ginseng Rhizosphere
db-RDA and linear regression analyses were used to analyze the effects of soil physicochemical factors and enzyme activities on the soil microbial community structure. Using the variance inflation factor (VIF) to judge the collinearity between different soil physicochemical factors, the physicochemical factors with VIF > 10 in soil physical and chemical indicators were screened and removed. NO3−-N (VIF = 40.01) and AK (VIF = 72.16) were removed because they strongly correlated with other physicochemical factors.
The selected physicochemical factors were compared with soil bacteria (Figure 7A) and fungi (Figure 7B) for db-RDA analysis based on the Bray–Curtis distance. The results showed that pH (r2 = 0.5745, p = 0.011), NH4+-N (r2 = 0.9168, p = 0.001), TN (r2 = 0.5517, p = 0.032), and TK (r2 = 0.631, p = 0.023) were significantly correlated with bacterial community structure. Further, pH (r2 = 0.5775, p = 0.002), AP (r2 = 0.738, p = 0.007), and TK (r2 = 0.6945, p = 0.011) were significantly correlated with fungal community structure.
Linear regression analysis was used to evaluate the degree of explanation of the activity of sucrase, urease, ACP, and catalase to the variation in soil bacterial and fungal community structure (Supplementary Figure S1). Sucrase activity was significantly correlated with the community structure of soil bacteria (R2 = 0.5216, p = 0.008) and fungi (R2 = 0.4544, p = 0.0162). There was no significant correlation between urease, ACP, and catalase, and soil bacterial and fungal community structure.
Discussion
Growth-Promoting Characteristics of Mortierella alpina YW25 and Its in vitro Interaction With Rhizospheric Microorganisms
Plant-associated microbes are known to play important roles in plant health and disease (Kwak et al., 2018). Roots absorb water and inorganic nutrients from the soil and secrete organic exudates to shape the microbial diversity and structure of the soil (Bulgarelli et al., 2013). Exploring the interaction between plant and soil microbes and rhizospheric microorganisms is vital to prevent and suppress diseases, promote plant growth, or improve plant stress resistance (Mendes et al., 2013).
In this study, ginseng rhizosphere microorganisms were selected for the plate confrontation test to preliminarily study the interaction between M. alpina YW25 and the rhizosphere microorganisms. The results showed that the mycelial growth of M. alpina YW25 was inhibited by some probiotics in the rhizosphere, such as Bacillus, Streptomyces, Brevibacterium, Trichoderma, and Penicillium (Lee et al., 2021; Zhao et al., 2021a,b), as well as by some potential pathogens, such as Cladosporium and Aspergillus (Carolina Virginia et al., 2021; Tan et al., 2021). Antimicrobial substances (lipopeptides, antibiotics, and volatile organic compounds) secreted by Bacillus and metabolites of Streptomyces (e.g. quercetin) may inhibit the growth of M. alpina YW25 mycelia on plates (Awla and Rashid, 2020; Chen et al., 2020). However, it showed no sensitivity to other microorganisms, such as Fusarium, Bjerkandera adusta, Trametes, Trichaptum abietinum (Supplementary Table S1), and the soil-borne pathogen F. oxysporum during co-cultivation. The results of the pot experiment showed that Mortierella was negatively correlated with Fusarium and Trichoderma in ginseng rhizosphere soil (Spearman, −0.95 and −0.72). These negative correlations between Mortierella and Fusarium have also been observed in other systems (Hong et al., 2020; Xiang et al., 2021), which indicated that Mortierella did not directly inhibit the growth of Fusarium.
Effects of Inoculation With Native Mortierella alpina YW25 on Physicochemical Properties of Ginseng Plants and Rhizosphere Soil
In this study, when M. alpina YW25 was singularly inoculated, the leaves of the ginseng plants expanded, fibrous roots developed, and it did not show any disease symptoms. There was no significant difference between the aboveground and root lengths of ginseng compared with CK (Figure 3B). This indicates that inoculation of M. alpina YW25 in ginseng rhizosphere did not result in ginseng root disease. We evaluated the growth-promoting characteristics of M. alpina YW25 and found that it had a high IAA production capacity, with a maximum value of 141.37 mg/L, which was much higher than the IAA yield of reported strains (Bader et al., 2020; Galeano et al., 2021). However, no obvious growth-promoting effect was observed in the ginseng plants. A study involving M. capitata inoculation showed that it could increase maize biomass and promote plant growth (Li et al., 2020a). This difference may be attributed to the different microbial or plant species in this study.
Interactions with microbial species and network modularity affect the community stability (Coyte et al., 2015). Compared with CK, MA significantly increased the diversity of root bacteria and significantly decreased the diversity of root fungi, but there was no significant difference in the microbial diversity of the aboveground parts of ginseng (Figure 4). In the co-occurrence network (Figure 6), MA was more complex than CK. Compared with CK, ginseng roots in MA had more edges and nodes in the bacterial network and fewer edges and nodes in the fungal network. Moreover, in the MA treatment, the ginseng root microbial network had a higher negative correlation ratio and modularity. The results showed that inoculation of M. alpina YW25 could increase the complexity of the bacterial community structure in ginseng root, while reducing the complexity and improving the stability of the ginseng root fungal community.
The results of this study showed that a single inoculation of M. alpina YW25 had significant effects on some nutrient content in ginseng rhizosphere soil. Phosphorus can enhance drought and disease resistance in plants and promote their growth and development. A lack of phosphorus leads to a significant decrease in crop yield (Elhaissoufi et al., 2021). The results of this study showed that the AP content and ACP activity of ginseng rhizosphere soil treated with MA were significantly higher than those in other treatments. Hence, inoculation with Mortierella increased the AP content in soil (Spearman, 0.70). This was the same as observed in previous studies (Li et al., 2020a; Guo et al., 2021), and indicated that Mortierella could dissolve inorganic phosphorus in soil. In addition, oxalates are also synthesized and released to help plants or mycorrhizal fungi obtain phosphorus (Qiang et al., 2021). However, M. alpina YW25 did not show phosphorus solubility in the PVK plate. The difference between plate cultivation and pot experimentation might be because dissolving phosphorus in the pot experiment was realized by regulating rhizosphere microorganisms. Compared with CK, Actinobacteria (Pseudarthrobacter, Microbacterium, and Microlunatus) and Rhizobium were significantly enriched in MA (Supplementary Figure S2). These microorganisms have their own biophosphorus conversion activity (Pindi, 2012). In addition, the content of soil available nitrogen (NH4+-N and NO3−-N) after M. alpina YW25 inoculation was significantly higher than that in FO. The AP content in the rhizosphere soil was positively correlated with the available nitrogen content (NH4+-N and NO3−-N; Spearman, 0.26 and 0.62), but negatively correlated with the available potassium content (Spearman, −0.36). The results showed that nitrogen and phosphorus availabilities were driven by each other between plants and soil (Xu et al., 2020). Phosphorus in soil also increases the retention of nitrogen in soil–plant systems, thereby reducing nitrogen loss due to soil leaching (Mehnaz et al., 2019).
Mortierella alpina YW25 Could Aid in Plant Resistance Against Pathogenic Invasion After MA_FO Treatment
In FO treatment, ginseng plant showed the typical characteristics of root rot disease with wilted leaves, brown and rotten root (Punja et al., 2008). However, in the MA_FO treatment, the leaves of ginseng expanded and the roots did not show browning symptoms. This indicates that the treatment with MA_FO in ginseng rhizosphere could effectively resist root rot caused by F. oxysporum YFW32. Plant defense enzymes (POD, PPO, and PAL) in ginseng roots were detected while exploring the reason for disease resistance (Table 1), but these results are different from those of previous studies (Nandhini et al., 2018). Pattern recognition receptors located on the surface of plants can recognize microbe- or pathogen-associated molecular patterns, and this recognition can then stimulate cascade defense signals resulting in induced systemic resistance (ISR) in plants (Bukhat et al., 2020). ISR is associated with defense enzymes, such as POD, PPO, and PAL. When plants are under biotic stress, these enzymes are induced to help resist pathogens (Appu et al., 2021). Invasion of F. oxysporum YFW32 significantly increased POD and PPO activities in ginseng roots. However, the activities of these enzymes were not significantly increased in MA_FO, which suggests that M. alpina YW25 may not induce plant resistance of ginseng to resist pathogen invasion.
Furthermore, we investigated the plant-associated microbiomes. The structure of the plant microbiome is influenced by complex interactions between the host, microorganisms, and related environmental factors, such as climate, soil, and cultivation practices (Kmgd et al., 2020). Treatment with MA_FO had a significant effect on the ginseng microbiome (Figure 5). Compared with FO, treatment with MA_FO significantly increased bacterial diversity and decreased fungal diversity in ginseng roots. The relative abundances of Pseudomonas, Comamonadaceae (e.g., Polaromonas), Sphingomonadaceae (Sphingomonas and Novosphingobium), and Rhizobium in MA_FO were significantly higher than those in FO (Figure 5B). This result is similar to that of previous research on American ginseng with Trichoderma atroviride inoculation (Li et al., 2022). These root microbiotas showed high antagonistic ability against root-associated fungi (Duran et al., 2018). In addition, the relative abundance of some potential plant growth-promoting microorganisms, such as Vishniacozyma, Cadophora, and Alatospora, was higher in the MA_FO treatment than in FO (Bizabani and Dames, 2015; Artigas et al., 2017; Lutz et al., 2020). Therefore, we speculated that M. alpina YW25 may enrich plant growth-promoting microorganisms by stimulating ginseng plants, and absorbing more nutrients for plant growth while inhibiting the invasion and proliferation of potential pathogens.
The plant microbial community structure of ginseng treated with MA_FO was different from that treated with FO, and the effect of MA_FO treatment on root microbial community was greater than that of aboveground ginseng (Figure 6). In the ginseng root, the number of nodes and edges of the bacterial network in FO was much lower than that in the control, and the number of nodes and edges in the fungal network was higher than that in CK. This indicated that FO reduced the complexity of the bacterial network, but increased the complexity of the fungal network in the ginseng root. The same results were observed in co-occurrence networks of peppers infected with Fusarium wilt disease (Gao et al., 2021). The complexity of microbial networks may be related to alpha diversity (Fan et al., 2018). The modularity and negative correlation of the bacterial and fungal networks of ginseng roots treated with FO were also much lower than those of the control. Low modularity and negative correlation may increase the unstable effect of the community (Grilli et al., 2016; Hernandez et al., 2021). Compared with FO, MA_FO increased the complexity of the root bacterial network, reduced the complexity of the root fungal network (based on the number of edges, nodes, and average degree), and improved the stability of the root microbial community (based on modularity and negative correlation ratio).
In this study, we found that ginseng rhizosphere-inoculated fungi had an effect on soil properties and the rhizosphere soil microbial community (Table 2 and Figure 8). First, pH was significantly correlated with the changes in rhizosphere soil bacterial and fungal communities (R2 = 0.5745 and 0.5775, respectively; Figure 7), which was the main factor affecting soil microbial diversity and community structure (Kang et al., 2021). Second, soil NH4+-N content had the strongest correlation with soil bacterial community structure (R2 = 0.9168; Figure 7A), and the soil physical and chemical factors had the greatest influence on the composition of the rhizosphere bacterial community (Liu et al., 2020a). Compared with the FO treatment, the activities of NH4+-N, sucrase, and catalase in soil increased significantly in the MA_FO treatment. This suggested that when treated in MA_FO, MA may help improve soil fertility, provide more nutrients for plants and soil microorganisms, and bioremediate the soil (Stepniewska et al., 2009; Sellami et al., 2022).
The fungal diversity of the rhizosphere soil in MA_FO was significantly higher than that in the FO treatment, but there was no significant change in bacterial diversity (Figure 4). Mortierella inoculation with Fusarium significantly reduced the relative abundance of Fusarium in soil (Figure 8B), and there was no significant difference in the soil fungal community structure between the two treatments (Figure 7B). Previous studies have also shown that the abundance of Mortierella in soil was significantly negatively correlated with diseases in plants, such as apple (Wang et al., 2018), vanilla (Xiong et al., 2017), eggplant (Ogundeji et al., 2021), celery, and watermelon (Liu et al., 2020a). In conclusion, inoculation with M. alpina YW25 significantly inhibited the proliferation of F. oxysporum in ginseng rhizosphere soil but did not affect the health of the rhizosphere soil.
To conclude, M. alpina YW25 had the maximum yield of IAA at 4 days (141.37 mg/L). Inoculation of M. alpina in ginseng rhizosphere significantly alleviated the pathogenicity of F. oxysporum in ginseng plants, increased the content of available nitrogen and phosphorus in rhizosphere soil, and improved the activities of soil sucrase and ACP. M. alpina inoculation with F. oxysporum had the greatest effect on the microbial community in the ginseng roots and had a greater effect on the fungal community than on the bacterial community. M. alpina inoculation helped ginseng recruit more plant growth-promoting microorganisms, change the microbial structure of ginseng roots, and build a more stable microbial network of ginseng roots. Thus, it inhibited potential pathogens, effectively prevented the invasion of pathogens, and ensured healthy plant growth. Therefore, M. alpina helped Panax ginseng resist F. oxysporum infection by mainly regulating the fungal community in the root.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, Biosample No. SAMN24474502.
Author Contributions
YW and HY conceived and designed the experiment. YW and LW performed the experiment. YW, LW, and MS analyzed the data. YW wrote the paper. HW, MZ, and HY guided the research work and thoroughly reviewed and corrected English language of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Fundamental Research Funds for the Central Universities (No. 2572020DR08 and No. 2572020DP07).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Qiang Ye (Yanbian Korean Autonomous Prefecture Academy of Agricultural Sciences) for providing the ginseng seedings. The authors would also like to thank Hongyan Zhao (College of Agronomy, Yanbian University, Yanji, China) for the detection of physicochemical indexes.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.850917/full#supplementary-material
Footnotes
2. ^https://submit.ncbi.nlm.nih.gov/subs/sra/SUB10895992/overview
3. ^http://sourceforge.net/projects/rdpclassifier/
References
Adetunji, A. T., Ncube, B., Meyer, A. H., Olatunji, O. S., Mulidzi, R., and Lewu, F. B. (2021). Soil pH, nitrogen, phosphatase and urease activities in response to cover crop species, termination stage and termination method. Heliyon 7:e05980. doi: 10.1016/j.heliyon.2021.e05980
Appu, M., Ramalingam, P., Sathiyanarayanan, A., and Huang, J. (2021). An overview of plant defense-related enzymes responses to biotic stresses. Plant Gene. 27:100302. doi: 10.1016/j.plgene.2021.100302
Artigas, J., Rossi, F., Gerphagnon, M., and Mallet, C. (2017). Sensitivity of laccase activity to the fungicide tebuconazole in decomposing litter. Sci. Total Environ. 584–585, 1084–1092. doi: 10.1016/j.scitotenv.2017.01.167
Arunrat, N., Sereenonchai, S., and Hatano, R. (2022). Effects of fire on soil organic carbon, soil total nitrogen, and soil properties under rotational shifting cultivation in northern Thailand. J. Environ. Manag. 302:113978. doi: 10.1016/j.jenvman.2021.113978
Awla, H. K., and Rashid, T. S. (2020). HPLC fractionation: A comparative analysis of anti-fungal compounds from different Streptomyces isolates inhibiting Colletotrichum acutatum. Biocatal. Agric. Biotechnol. 27:101688. doi: 10.1016/j.bcab.2020.101688
Azabou, M. C., Gharbi, Y., Medhioub, I., Ennouri, K., Barham, H., Tounsi, S., et al. (2020). The endophytic strain bacillus velezensis OEE1: an efficient biocontrol agent against Verticillium wilt of olive and a potential plant growth promoting bacteria. Biol. Control 142:104168. doi: 10.1016/j.biocontrol.2019.104168
Bader, A. N., Salerno, G. L., Covacevich, F., and Consolo, V. F. (2020). Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud. Univ. Sci. 32, 867–873. doi: 10.1016/j.jksus.2019.04.002
Baeg, I. H., and So, S. H. (2013). The world ginseng market and the ginseng (Korea). J. Ginseng Res. 37, 1–7. doi: 10.5142/jgr.2013.37.1
Bagy, H. M. M. K., Hassan, E. A., Nafady, N. A., and Dawood, M. F. A. (2019). Efficacy of arbuscular mycorrhizal fungi and endophytic strain Epicoccum nigrum ASU11 as biocontrol agents against blackleg disease of potato caused by bacterial strain Pectobacterium carotovora subsp. atrosepticum PHY7. Biol. Control 134, 103–113. doi: 10.1016/j.biocontrol.2019.03.005
Bizabani, C., and Dames, J. (2015). Effects of inoculating Lachnum and Cadophora isolates on the growth of Vaccinium corymbosum. Microbiol. Res. 181, 68–74. doi: 10.1016/j.micres.2015.08.005
Bukhat, S., Imran, A., Javaid, S., Shahid, M., Majeed, A., and Naqqash, T. (2020). Communication of plants with microbial world: exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 238:126486. doi: 10.1016/j.micres.2020.126486
Bulgarelli, D., Garrido-Oter, R., Munch, P. C., Weiman, A., Droge, J., Pan, Y., et al. (2015). Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17, 392–403. doi: 10.1016/j.chom.2015.01.011
Bulgarelli, D., Schlaeppi, K., Spaepen, S., van Themaat, E. V. L., and Schulze-Lefert, P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64, 807–838. doi: 10.1146/annurev-arplant-050312-120106
Carolina Virginia, T., Javier Néstor, A., Adrián Dario, C., and Graciela Noemí, P. (2021). Cladosporium species causing “Cladosporium rot” on “Bosc” pear fruit in Argentina. Rev. Argent. Microbiol. 53, 75–77. doi: 10.1016/j.ram.2019.11.006
Chen, K., Tian, Z. H., He, H., Long, C. A., and Jiang, F. T. (2020). Bacillus species as potential biocontrol agents against citrus diseases. Biol. Control 151:104419. doi: 10.1016/j.biocontrol.2020.104419
Cheng, B., Raza, A., Wang, L., Xu, M., Lu, J. J., Gao, Y., et al. (2020). Effects of multiple planting densities on lignin metabolism and lodging resistance of the strip intercropped soybean stem. Agronomy 10:1177. doi: 10.3390/agronomy10081177
Chimbekujwo, K. I., Inuwa, J. M., and Moses, A. O. (2020). Purification, characterization and optimization conditions of protease produced by Aspergillus brasiliensis strain BCW2. Scientific African. 8:e00398. doi: 10.1016/j.sciaf.2020.e00398
Cong, Y. Z., Fan, H. D., Ma, Q. F., Lu, Y., Xu, L., Zhang, P. Y., et al. (2019). Mixed culture fermentation between Rhizopus nigricans and Trichoderma pseudokoningii to control cucumber Fusarium wilt. Crop Prot. 124:104857. doi: 10.1016/j.cropro.2019.104857
Coyte, K. Z., Schluter, J., and Foster, K. R. (2015). The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666. doi: 10.1126/science.aad2602
Cui, B. J., Hu, C., Fan, X. Y., Cui, E. P., Li, Z. Y., Ma, H. H., et al. (2020). Changes of endophytic bacterial community and pathogens in pepper (Capsicum annuum L.) as affected by reclaimed water irrigation. Appl. Soil Ecol. 156:103627. doi: 10.1016/j.apsoil.2020.103627
De Silva, N. I., Brooks, S., Lumyong, S., and Hyde, K. D. (2019). Use of endophytes as biocontrol agents. Fungal Biol. Rev. 33, 133–148. doi: 10.1016/j.fbr.2018.10.001
Duran, P., Thiergart, T., Garrido-Oter, R., Agler, M., Kemen, E., Schulze-Lefert, P., et al. (2018). Microbial Interkingdom interactions in roots promote Arabidopsis survival. Cell 175, 973–983.e14. doi: 10.1016/j.cell.2018.10.020
Elhaissoufi, W., Ghoulam, C., Barakat, A., Zeroual, Y., and Bargaz, A. (2021). Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 1–16. doi: 10.1016/j.jare.2021.08.014
Fan, K. K., Weisenhorn, P., Gilbert, J. A., and Chu, H. Y. (2018). Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 125, 251–260. doi: 10.1016/j.soilbio.2018.07.022
Farh, M. E., Kim, Y. J., Kim, Y. J., and Yang, D. C. (2018). Cylindrocarpon destructans/Ilyonectria radicicola-species complex: causative agent of ginseng root-rot disease and rusty symptoms. J. Ginseng Res. 42, 9–15. doi: 10.1016/j.jgr.2017.01.004
Galeano, R. M. S., Franco, D. G., Chaves, P. O., Giannesi, G. C., Masui, D. C., Ruller, R., et al. (2021). Plant growth promoting potential of endophytic Aspergillus Niger 9-p isolated from native forage grass in Pantanal of Nhecolândia region. Brazil. Rhizosphere 18:100332. doi: 10.1016/j.rhisph.2021.100332
Gao, M., Xiong, C., Gao, C., Tsui, C. K. M., Wang, M. M., Zhou, X., et al. (2021). Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 9:187. doi: 10.21203/rs.3.rs-113338/v1
Gordon, S. A., and Weber, R. P. (1951). Colorimetric estimation of Indoleacetic acid. Plant Physiol. 26, 192–195. doi: 10.1104/pp.26.1.192
Grilli, J., Rogers, T., and Allesina, S. (2016). Modularity and stability in ecological communities. Nat. Commun. 7. doi: 10.1038/ncomms12031
Guo, N., Li, L., Cui, J. Q., and Cai, B. Y. (2021). Effects of Funneliformis mosseae on the fungal community in and soil properties of a continuously cropped soybean system. Appl. Soil Ecol. 164:103930. doi: 10.1016/j.apsoil.2021.103930
Han, S. Y., Chen, J. X., Zhao, Y. J., Cai, H. S., and Guo, C. H. (2021). Bacillus subtilis HSY21 can reduce soybean root rot and inhibit the expression of genes related to the pathogenicity of Fusarium oxysporum. Pestic Biochem. Phys. 178:104916. doi: 10.1016/j.pestbp.2021.104916
Han, S. Y., Kim, J., Kim, E., Kim, S. H., Seo, D. B., Kim, J. H., et al. (2018). AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J. Ginseng Res. 42, 496–503. doi: 10.1016/j.jgr.2017.06.003
Hernandez, D. J., David, A. S., Menges, E. S., Searcy, C. A., and Afkhami, M. E. (2021). Environmental stress destabilizes microbial networks. ISME J. 15, 1722–1734. doi: 10.1038/s41396-020-00882-x
Hong, S., Jv, H. L., Lu, M., Wang, B. B., Zhao, Y., and Ruan, Y. Z. (2020). Significant decline in banana Fusarium wilt disease is associated with soil microbiome reconstruction under chilli pepper-banana rotation. Eur. J. Soil Biol. 97:103154. doi: 10.1016/j.ejsobi.2020.103154
John, R. P., Tyagi, R. D., Prevost, D., Brar, S. K., Pouleur, S., and Surampalli, R. Y. (2010). Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot. 29, 1452–1459. doi: 10.1016/j.cropro.2010.08.004
Kang, E., Li, Y., Zhang, X., Yan, Z., Wu, H., Li, M., et al. (2021). Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 774:145780. doi: 10.1016/j.scitotenv.2021.145780
Kmgd, A., Fht, A., As, A., Maa, A., and Ac, B. (2020). Plant microbiome – an account of the factors that shape community composition and diversity. Curr. Plant Biol. 23:100161. doi: 10.1016/j.cpb.2020.100161
Koljalg, U., Nilsson, R. H., Abarenkov, K., Tedersoo, L., Taylor, A. F. S., Bahram, M., et al. (2013). Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 22, 5271–5277. doi: 10.1111/mec.12481
Kwak, M. J., Kong, H. G., Choi, K., Kwon, S. K., and Kim, J. F. (2018). Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 36, 1100–1109. doi: 10.1038/nbt.4232
Lee, S. M., Kong, H. G., Song, G. C., and Ryu, C. M. (2021). Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 15, 330–347. doi: 10.1038/s41396-020-00785-x
Lee, W. S., and Rhee, D. K. (2021). Corona-Cov-2 (COVID-19) and ginseng: comparison of possible use in COVID-19 and influenza. J. Ginseng Res. 45, 535–537. doi: 10.1016/j.jgr.2020.12.005
Li, J., Awasthi, M. K., Zhu, Q., Chen, X. Y., Wu, F. Q., Wu, F. Y., et al. (2021a). Modified soil physicochemical properties promoted sequestration of organic and inorganic carbon synergistically during revegetation in desertified land. J. Environ. Chem. Eng. 9:106331. doi: 10.1016/j.jece.2021.106331
Li, X., Chu, S. F., Lin, M. Y., Gao, Y., Liu, Y. J., Yang, S. W., et al. (2020b). Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 203:112627. doi: 10.1016/j.ejmech.2020.112627
Li, Z. B., Fu, J. F., Zhou, R. J., and Wang, D. (2018). Effects of phenolic acids from ginseng rhizosphere on soil fungi structure, richness and diversity in consecutive monoculturing of ginseng. Saudi J Biol Sci. 25, 1788–1794. doi: 10.1016/j.sjbs.2018.07.007
Li, H. M., Toh, R., Wei, Y. L., Wang, Y. L., Hu, J. D., An, S. H., et al. (2022). Microbiomes across root compartments are shaped by inoculation with a fungal biological control agent. Appl. Soil Ecol. 170:104230. doi: 10.1016/j.apsoil.2021.104230
Li, Q. J., Zhang, D. Q., Cheng, H. Y., Song, Z. X., Ren, L. R., Hao, B. Q., et al. (2021b). Chloropicrin alternated with dazomet improved the soil's physicochemical properties, changed microbial communities and increased strawberry yield. Ecotox Environ Safe. 220:112362. doi: 10.1016/j.ecoenv.2021.112362
Li, F., Zhang, S. Q., Wang, Y., Li, Y., Li, P. P., Chen, L., et al. (2020a). Rare fungus, Mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol. Biochem. 151:108017. doi: 10.1016/j.soilbio.2020.108017
Liu, S., Ahmed, S., Zhang, C. G., Liu, T. X., Shao, C. L., and Fang, Y. W. (2020c). Diversity and antimicrobial activity of culturable fungi associated with sea anemone Anthopleura xanthogrammica. Electron. J. Biotechnol. 44, 41–46. doi: 10.1016/j.ejbt.2020.01.003
Liu, L. L., Huang, X. Q., Zhang, J. B., Cai, Z. C., Jiang, K., and Chang, Y. Y. (2020a). Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 148:107909. doi: 10.1016/j.soilbio.2020.107909
Liu, X., Lu, X., Zhao, W. Q., Yang, S., Wang, J. W., Xia, H. T., et al. (2022). The rhizosphere effect of native legume Albizzia julibrissin on coastal saline soil nutrient availability, microbial modulation, and aggregate formation. Sci. Total Environ. 806:150705. doi: 10.1016/j.scitotenv.2021.150705
Liu, N., Shao, C., Sun, H., Liu, Z. B., Guan, Y. M., Wu, L. J., et al. (2020b). Arbuscular mycorrhizal fungi biofertilizer improves American ginseng (Panax quinquefolius L.) growth under the continuous cropping regime. Geoderma 363:114155. doi: 10.1016/j.geoderma.2019.114155
Liu, C. X., Zhou, Y., Qin, H., Liang, C. F., Shao, S., Fuhrmann, J. J., et al. (2021). Moso bamboo invasion has contrasting effects on soil bacterial and fungal abundances, co-occurrence networks and their associations with enzyme activities in three broadleaved forests across subtropical China. Forest Ecol Manag. 498:119549. doi: 10.1016/j.foreco.2021.119549
Lohse, M., Bolger, A. M., Nagel, A., Fernie, A. R., Lunn, J. E., Stitt, M., et al. (2012). RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 40, W622–W627. doi: 10.1093/nar/gks540
Lutz, M. C., Lopes, C. A., Sosa, M. C., and Sangorrin, M. P. (2020). Semi-commercial testing of regional yeasts selected from North Patagonia Argentina for the biocontrol of pear postharvest decays. Biol. Control 150:104246. doi: 10.1016/j.biocontrol.2020.104246
Machuca, A., and Milagres, A. M. F. (2003). Use of CAS-agar plate modified to study the effect of different variables on the siderophore production by Aspergillus. Lett. Appl. Microbiol. 36, 177–181. doi: 10.1046/j.1472-765X.2003.01290.x
Magoc, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Mehnaz, K. R., Keitel, C., and Dijkstra, F. A. (2019). Phosphorus availability and plants alter soil nitrogen retention and loss. Sci. Total Environ. 671, 786–794. doi: 10.1016/j.scitotenv.2019.03.422
Mendes, R., Garbeva, P., and Raaijmakers, J. M. (2013). The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37, 634–663. doi: 10.1111/1574-6976.12028
Nandhini, M., Rajini, S. B., Udayashankar, A. C., Niranjana, S. R., Lund, O. S., Shetty, H. S., et al. (2018). Diversity, plant growth promoting and downy mildew disease suppression potential of cultivable endophytic fungal communities associated with pearl millet. Biol. Control 127, 127–138. doi: 10.1016/j.biocontrol.2018.08.019
Ogundeji, A. O., Li, Y., Liu, X. J., Meng, L. B., Sang, P., Mu, Y., et al. (2021). Eggplant by grafting enhanced with biochar recruits specific microbes for disease suppression of Verticillium wilt. Appl. Soil Ecol. 163:103912. doi: 10.1016/j.apsoil.2021.103912
Palmieri, D., Vitale, S., Lima, G., Di Pietro, A., and Turra, D. (2020). A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 11:5264. doi: 10.1038/s41467-020-18994-5
Papagianni, M., and Moo-Young, M. (2002). Protease secretion in glucoamylase producer Aspergillus niger cultures: fungal morphology and inoculum effects. Process Biochem. 37, 1271–1278. doi: 10.1016/S0032-9592(02)00002-X
Pikovskaya, R. I. (1948). Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya
Pindi, P. K. (2012). Liquid microbial consortium – a potential tool for sustainable soil health. J. Fertil. Pest. 3. doi: 10.4172/2155-6202.1000124
Punja, Z. K., Wan, A., and Goswami, R. S. (2008). Root rot and distortion of ginseng seedling roots caused by Fusarium oxysporum. Can. J. Plant Pathol. 30, 565–574. doi: 10.1080/07060660809507556
Qiang, W., He, L. L., Zhang, Y., Liu, B., Liu, Y., Liu, Q. H., et al. (2021). Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in Southwest China. Catena 202:105251. doi: 10.1016/j.catena.2021.105251
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Ren, J., Tong, J., Li, P. H., Huang, X. Q., Dong, P., and Ren, M. Z. (2021). Chitosan is an effective inhibitor against potato dry rot caused by Fusarium oxysporum. Physiol Mol Plant P., 113:101601. doi: 10.1016/j.pmpp.2021.101601
Salehi, M. H., Beni, O. H., Harchegani, H. B., Borujeni, I. E., and Motaghian, H. R. (2011). Refining soil organic matter determination by loss-on-ignition. Pedosphere 21, 473–482. doi: 10.1016/S1002-0160(11)60149-5
Schywn, B., and Nielands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. doi: 10.1016/0003-2697(87)90612-9
Sellami, K., Couvert, A., Nasrallah, N., Maachi, R., Abouseoud, M., and Amrane, A. (2022). Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: a review. Sci. Total Environ. 806:150500. doi: 10.1016/j.scitotenv.2021.150500
Sopalun, K., and Iamtham, S. (2020). Isolation and screening of extracellular enzymatic activity of endophytic fungi isolated from Thai orchids. S. Afr. J. Bot. 134, 273–279. doi: 10.1016/j.sajb.2020.02.005
Sopalun, K., Laosripaiboon, W., Wachirachaikarn, A., and Iamtham, S. (2021). Biological potential and chemical composition of bioactive compounds from endophytic fungi associated with Thai mangrove plants. S. Afr. J. Bot. 141, 66–76. doi: 10.1016/j.sajb.2021.04.031
Stepniewska, Z., Wolinska, A., and Ziomek, J. (2009). Response of soil catalase activity to chromium contamination. J. Environ. Sci. 21, 1142–1147. doi: 10.1016/S1001-0742(08)62394-3
Sun, X. D., Lyu, G. Z., Luan, Y. S., Zhao, Z. H., Yang, H., and Su, D. (2018). Analyses of microbial community of naturally homemade soybean pastes in Liaoning Province of China by Illumina Miseq sequencing. Food Res. Int. 111, 50–57. doi: 10.1016/j.foodres.2018.05.006
Tan, C., Deng, J. L., Zhang, F., Zhu, Z., Yan, L. J., Zhang, M. J., et al. (2021). CWI pathway participated in vegetative growth and pathogenicity through a downstream effector AflRlm1 in Aspergillus flavus. Iscience. 24:103159. doi: 10.1016/j.isci.2021.103159
Tong, A. Z., Liu, W., Liu, Q., Xia, G. Q., and Zhu, J. Y. (2021). Diversity and composition of the Panax ginseng rhizosphere microbiome in various cultivation modesand ages. BMC Microbiol. 21:18. doi: 10.1186/s12866-020-02081-2
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 73, 5261–5267. doi: 10.1128/Aem.00062-07
Wang, R., Liang, X., Long, Z., Wang, X., Yang, L., Lu, B., et al. (2021a). An LCI-like protein APC2 protects ginseng root from Fusarium solani infection. J. Appl. Microbiol. 130, 165–178. doi: 10.1111/jam.14771
Wang, Y., Wang, L. W., Zhao, H. Y., Zhao, M., and Yang, H. Y. (2021b). Characteristics of nutrients and microbial community composition of different Panax ginseng cultivation soil (in Chinese). Chin. Agric. Sci. Bull. 38, 60–68. doi: 10.11924/j.issn.1000-6850.casb2021-0564
Wang, G. S., Yin, C. M., Pan, F. B., Wang, X. B., Xiang, L., Wang, Y. F., et al. (2018). Analysis of the fungal Community in apple replanted soil around Bohai Gulf. Hortic. Plant J. 4, 175–181. doi: 10.1016/j.hpj.2018.05.003
Wei, Y. Q., Zhao, Y., Lu, Q., Cao, Z. Y., and Wei, Z. M. (2018). Organophosphorus-degrading bacterial community during composting from different sources and their roles in phosphorus transformation. Bioresour. Technol. 264, 277–284. doi: 10.1016/j.biortech.2018.05.088
Xiang, L., Wang, M., Jiang, W. T., Wang, Y. F., Chen, X. S., Yin, C. M., et al. (2021). Key indicators for renewal and reconstruction of perennial trees soil: microorganisms and phloridzin. Ecotox Environ Safe. 225:112723. doi: 10.1016/j.ecoenv.2021.112723
Xiong, W., Li, R., Ren, Y., Liu, C., Zhao, Q. Y., Wu, H. S., et al. (2017). Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 107, 198–207. doi: 10.1016/j.soilbio.2017.01.010
Xu, L. X., Han, Y. S., Yi, M., Yi, H. L., Guo, E. H., and Zhang, A. Y. (2019). Shift of millet rhizosphere bacterial community during the maturation of parent soil revealed by 16S rDNA high-throughput sequencing. Appl. Soil Ecol. 135, 157–165. doi: 10.1016/j.apsoil.2018.12.004
Xu, M. P., Zhong, Z. K., Sun, Z. Y., Han, X. H., Ren, C. J., and Yang, G. H. (2020). Soil available phosphorus and moisture drive nutrient resorption patterns in plantations on the loess plateau. Forest Ecol Manag. 461:117910. doi: 10.1016/j.foreco.2020.117910
Yang, L., Zhou, J. H., Lai, L. M., Sun, Q. L., Yi, S. G., Jiang, L. H., et al. (2020). Evaluating physiological changes of grass and semishrub species with seasonality for understanding the process of shrub encroachment in semiarid grasslands. Funct. Plant Biol. 47, 628–638. doi: 10.1071/Fp19194
You, C., Zhang, C. S., Kong, F. Y., Feng, C., and Wang, J. (2016). Comparison of the effects of biocontrol agent Bacillus subtilis and fungicide metalaxyl-mancozeb on bacterial communities in tobacco rhizospheric soil. Ecol. Eng. 91, 119–125. doi: 10.1016/j.ecoleng.2016.02.011
Yuan, X. B., Niu, D. C., Weber-Grullon, L., and Fu, H. (2020). Nitrogen deposition enhances plant-microbe interactions in a semiarid grassland: The role of soil physicochemical properties. Geoderma 373:114446. doi: 10.1016/j.geoderma.2020.114446
Zhao, J., Liu, T., Liu, W. C., Zhang, D. P., Dong, D., Wu, H. L., et al. (2021a). Transcriptomic insights into growth promotion effect of Trichoderma afroharzianum TM2-4 microbial agent on tomato plants. J Integr Agr. 20, 1266–1276. doi: 10.1016/S2095-3119(20)63415-3
Zhao, X. B., Liu, X. T., Zhao, H., Ni, Y. X., Lian, Q. G., Qian, H. M., et al. (2021b). Biological control of Fusarium wilt of sesame by Penicillium bilaiae 47M-1. Biol. Control 158:104601. doi: 10.1016/j.biocontrol.2021.104601
Zhou, Y. J., Jia, X., Han, L., Liu, Z., Kang, S. Z., and Zhao, Y. H. (2021). Fungal community diversity in soils along an elevation gradient in a Quercus aliena var. acuteserrata forest in Qinling Mountains. China. Appl Soil Ecol. 167:104104. doi: 10.1016/j.apsoil.2021.104104
Zhou, B., Zhao, L. X., Wang, Y. B., Sun, Y., Li, X. J., Xu, H. J., et al. (2020). Spatial distribution of phthalate esters and the associated response of enzyme activities and microbial community composition in typical plastic-shed vegetable soils in China. Ecotox. Environ. Safe. 195:110495. doi: 10.1016/j.ecoenv.2020.110495
Keywords: Mortierella alpina, Panax ginseng, microbial community, Fusarium oxysporum, resistance
Citation: Wang Y, Wang L, Suo M, Qiu Z, Wu H, Zhao M and Yang H (2022) Regulating Root Fungal Community Using Mortierella alpina for Fusarium oxysporum Resistance in Panax ginseng. Front. Microbiol. 13:850917. doi: 10.3389/fmicb.2022.850917
Edited by:
Prem Lal Kashyap, Indian Institute of Wheat and Barley Research (ICAR), IndiaReviewed by:
Omar Abdelraouf Hewedy, University of Menoufia, EgyptAkansha Jain, Bose Institute, India
Copyright © 2022 Wang, Wang, Suo, Qiu, Wu, Zhao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhao, ODIxOTE1MTNAMTYzLmNvbQ==; Hongyan Yang, eWFuZ2h5QG5lZnUuZWR1LmNu
 Yan Wang1,2
Yan Wang1,2 Min Zhao
Min Zhao Hongyan Yang
Hongyan Yang