
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 15 February 2022
Sec. Virology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.846747
This article is part of the Research TopicThe Viral Evasion of Antiviral Innate ImmunityView all 71 articles
 Zhihua Ren1†
Zhihua Ren1† Guilin Jia1†
Guilin Jia1† Hongyi He1†
Hongyi He1† Ting Ding1
Ting Ding1 Yueru Yu1
Yueru Yu1 ZhiCai Zuo1
ZhiCai Zuo1 Yanchun Hu1
Yanchun Hu1 Zhijun Zhong1
Zhijun Zhong1 Shumin Yu1
Shumin Yu1 Huidan Deng1
Huidan Deng1 Liuhong Shen1
Liuhong Shen1 Suizhong Cao1
Suizhong Cao1 Guangneng Peng1
Guangneng Peng1 Ya Wang1
Ya Wang1 Dongjie Cai1
Dongjie Cai1 Liping Gou1
Liping Gou1 Xiaoping Ma1
Xiaoping Ma1 Haifeng Liu1
Haifeng Liu1 Ziyao Zhou1
Ziyao Zhou1 Youtian Deng1
Youtian Deng1 Dingyong Yang2*
Dingyong Yang2* Junliang Deng1*
Junliang Deng1*
Porcine deltacoronavirus (PDCoV) is an emerging porcine intestinal coronavirus in recent years, which mainly causes different degrees of vomiting and diarrhea in piglets and has caused great harm to the swine husbandry worldwide since its report. Selenium is an essential trace element for organisms and has been demonstrated to have antiviral effects. In this study, pig kidney epithelial (LLC-PK) cells were used to study the antiviral activity of selenomethionine (Se-Met) (2, 4, 8, and 16 μM) against PDCoV by detecting the replication of the virus, the expression of the mitochondrial antiviral signal protein (MAVS) protein, and the phosphorylation of interferon regulatory factor-3 (IRF-3), IFN-α, and IFN-β, and the changes in glutathione content, glutathione peroxidase, superoxide dismutase activity, and hydrogen peroxide content in the cells. The results showed that Se-Met at higher than physiological concentrations (16 μM) could significantly inhibit the replication of PDCoV in LLC-PK cells and enhance the expression of MAVS protein and the phosphorylation of IRF-3. In addition, Se-Met also improved the intracellular production of IFNα/β and antioxidant capacity with increasing doses. These data suggest that the availability of selenium through selenomethionine supports the antiviral response in porcine kidney cells, and the specific mechanism is attributed to the improved cellular antioxidant capacity and activation of the MAVS pathway by Se-Met.
Coronaviruses are single-stranded positive capsular RNA viruses that mainly infect mammals and birds (Niederwerder and Hesse, 2018). According to the differences of genetic characteristics and serology, it can be divided into four genera: Alphacoronavirus, Betacoronavirus, Gamacoronavirus, and Deltacoronavirus (Ma et al., 2015). Among them, PDCoV belongs to the genus Deltacoronavirus in the Coronaviridae family and is a newly discovered porcine enteropathogenic coronavirus, which is mainly characterized by digestive system symptoms, such as diarrhea, dehydration, and varying degrees of vomiting in piglets (Xu et al., 2018; Yin et al., 2020). PDCoV was first reported in Hong Kong in 2012 and is now a pandemic worldwide (Zhang, 2016). The outbreak of PDCoV has caused serious economic losses to swine farming, but there is no widely used drug and vaccine in production. Therefore, we need to find a drug or nutrient with an antiviral effect commonly used in production to fight PDCoV infection.
As an essential nutrient element, selenium mainly exists in organic and inorganic selenium. Selenoprotein and selenoamino acids are the most common organic selenium, and selenomethionine (Se-Met) is the most common selenium form ingested by the organisms from food (Weekley and Harris, 2013). The antioxidant and immunomodulatory functions of selenium are most important, and these functions are mainly exerted by selenoproteins, such as glutathione peroxidase (GSH-PX) and thioredoxin reductase (TrxR). GSH-PX 1-4 is involved in hydrogen peroxide (H2O2) signal transduction and maintaining the cellular redox state (Brigelius-Flohe and Maiorino, 2013). In addition, GPX1, GPX4, and TrxR1 are also the most abundant selenoproteins in a variety of immune cells, and they play an important role in T cell proliferation and NK cell activation (Chu et al., 1992; Wingler and Brigelius-Flohe, 1999; Lei et al., 2007; Huang et al., 2012; Ingold et al., 2018).
Innate immunity acts as the first line of defense against pathogenic microorganisms. RIG-I-like receptor (RLR) is a member of innate immunity, which plays a significant role against RNA viruses (Ren et al., 2020). RLR recognizes the virus and can activate the mitochondrial antiviral signal protein (MAVS) and interferon regulatory factor (IRF), which in turn secrete interferon (IFN) to achieve antiviral effects (Hou et al., 2011). Currently, selenium has been found to have antiviral effects, including against coxsackie virus, influenza virus, human immunodeficiency virus (HIV), and porcine circovirus (PCV) in humans and animals (Beck et al., 1994; Schrauzer and Sacher, 1994; Nelson et al., 2001; Jaspers et al., 2007; Qian et al., 2018; Guillin et al., 2019; Bermano et al., 2021). The antiviral effect of selenium is mainly attributed to its antioxidant and immunomodulatory effects. For example, Se-Met (2, 4 mM) can inhibit PCV2 replication by inhibiting H2O2-mediated oxidative stress (Chen et al., 2012). In 450 HIV-1 seropositive patients, daily supplementation with 200 μg of yeast selenium not only decreased the viral load of HIV-1 but also increased the number of CD4+ T cells (Hurwitz et al., 2007).
Given the current situation of PDCoV, which is seriously harmful, there is no specific drug treatment. In this assay, we first examined the replication effect of Se-Met on PDCoV in vitro. Then, we further explored the potential mechanism of PDCoV inhibition by Se-Met from the perspective of innate immunity and anti-oxidation. It provides some theoretical basis and guiding significance for reducing PDCoV infection from antiviral nutrition.
Pig Kidney Epithelial (LLC-PK) cells were provided by Professor Zhanyong Wei of Henan Agricultural University. Cells were cultured in Minimum Essential Medium (MEM, Solarbio). MEM was supplemented with 8% fetal bovine serum (FBS, Gibco), 1% HEPES (Gibco), 100 IU/mL penicillin, and 100 IU/mL streptomycin solution. Se-Met was purchased from Sigma, United States. Referring to the results of Pan’s study (Pan et al., 2008), we confirmed that 16 μM Se-Met was not toxic to cells. Se-Met was diluted to 2, 4, 8, and 16 μM with MEM.
PDCoV HNZK-04 strain (provided by Professor Zhanyong Wei, Henan Agricultural University) was used in this study. PDCoV was propagated in a maintenance medium (MEM supplemented with 1% antibiotics, 1% HEPES, and 5 μg/mL trypsin) containing LLC-PK cells. The number of infectious PDCoV particles was determined based on the 50% tissue culture infectious dose (TCID50) in LLC-PK cells, according to the method described by Zhai’s study (Zhai et al., 2019).
To understand the inhibitory activity of Se-Met (2, 4, 8, and 16 μM) on PDCoV, cells were added to six-well plates and allowed to grow to about 90%. The virus stock was diluted to 100 TCID50 and inoculated into cells for 1 h. The virus liquid was discarded, washed twice with D-Hanks, and Se-Met solution was added and cultured at 37°C for 24 h. The virus infection control group (group V) and the blank control group (group C) were randomly within the plates. At last, the viral load of each group was measured.
RNA from LLC-PK cells was extracted using TRIzol reagent (Invitrogen) and cDNA synthesis using cDNA Synthesis Super (TransGen Biotech) according to the manufacturer’s instructions. Then, RT-qPCR was performed using Perfect Start™ Green qPCR Super (TransGen Biotech). The virus was determined by absolute RT qPCR, and the primers for the PDCoV M gene are shown in Table 1. The 2–ΔΔCT method was used to differentiate between control and treated cells.
According to the kit instructions (Jiangsu MEIMIAN Co., Ltd. China), expression of IFN-α and IFN-β was determined using ELISA and RT qPCR. Primers for RT qPCR are shown in Table 1, and β-actin was used as a reference gene (Chen et al., 2012). There are three independent replicates done for treatment.
The cell pellet was collected at the end of cell culture after washing and centrifugation. One milliliter of the extract was added to the cell pellet, and the cells were disrupted by sonication for subsequent testing. The contents of glutathione (GSH) and H2O2, as well as the activities of GSH-PX and superoxide dismutase (SOD), were measured using kits (Nanjing Jiancheng Bioengineering Institute, China). There are three independent replicates were done for treatment.
The expression of MAVS protein and the phosphorylation of IRF-3 in innate immunity was examined using Western blot to determine whether Se-Met affects innate immunity. The following primary antibodies were used: Anti-Phospho-IRF-3 (Ser396), Monoclonal Antibody (MA5-14947) (Invitrogen Corporation, NY, United States), Anti-IRF-3 Polyclonal antibody (11312-1-AP), and Anti-MAVS antibody (14341-1-AP) (Proteintech Group, Wuhan, China).
Results were expressed as the means ± standard deviation (SD). The test data were analyzed for the significance of difference by one-way ANOVA using SPSS 26 (P < 0.05).
LLC-PK cells were treated with different concentrations of Se-Met (2, 4, 8, and 16 μM) after inoculation with PDCoV. The copy number of the PDCoV M gene in each group was detected using RT qPCR 24 h after virus infection. The results are shown in Figure 1. The 4 and 8 μM Se-Met could significantly inhibit the copy number of virus M gene (0.01 < P < 0.05). Moreover, after the virus was treated with 16 μM Se-Met, the replication was extremely decreased (P < 0.01). In conclusion, Se-Met can inhibit the replication of PDCoV in a dose-dependent manner, with 16 μM Se-Met having the best effect.
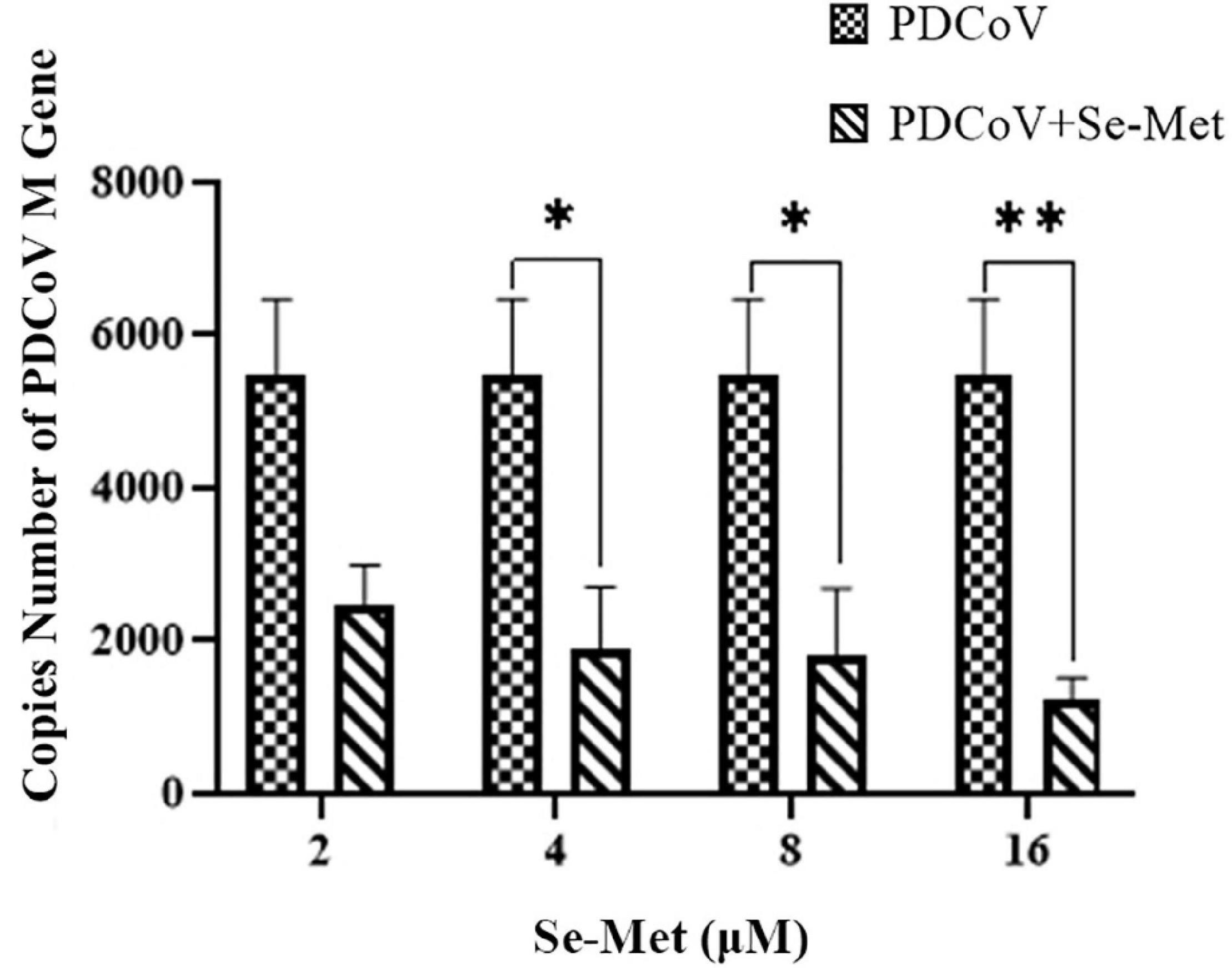
Figure 1. Antiviral effect of Se-Met against PDCoV. LLC-PK cells were incubated with PDCoV for 1 h, and 2, 4, 8, and 16 μM of Se-Met was added. *0.01 < P < 0.05, **P < 0.01.
Evasion of innate immunity has emerged as a way for the virus to maintain replication. We treated LLC-PK cells with Se-Met in four concentrations to further investigate whether Se-Met inhibits viral replication by improving cellular immunity. First, we used Western blot to detect the expression of MAVS protein and phosphorylation of IRF-3 intracellularly and then used ELISA and RT-qPCR to detect the changes of IFN-α and IFN-β. Western blot results showed that PDCoV was able to significantly reduce the protein expression of MAVS and the phosphorylation of IRF-3 (0.01 < P < 0.05). After Se-Met treatment, the protein expression of MAVS and the phosphorylation of IRF-3 were significantly increased in all concentration groups (P < 0.01, Figure 2). We can see from Figure 3 that all concentrations of Se-Met significantly increased the production of IFNα/β in cells compared with group V (P < 0.01).
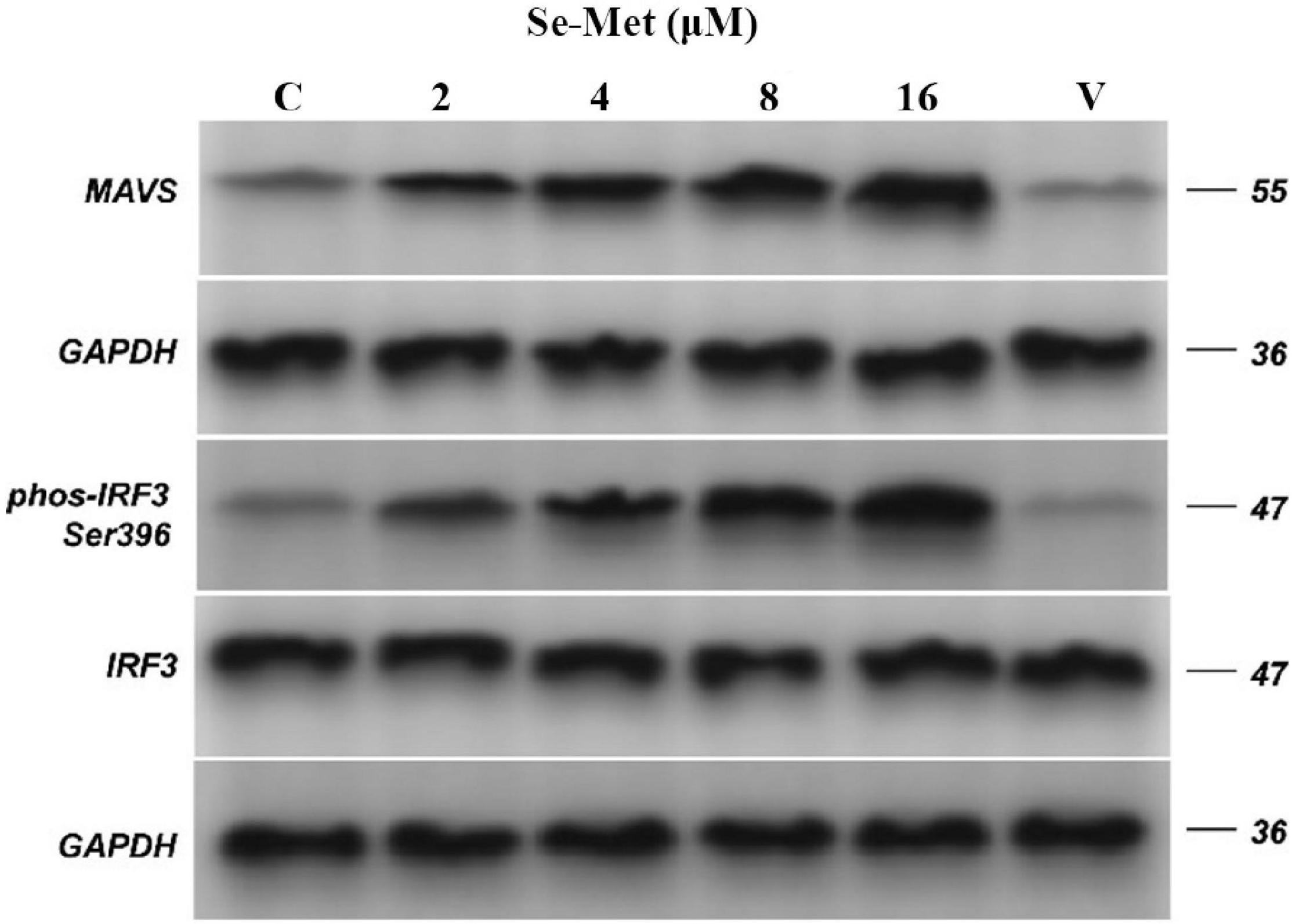
Figure 2. Effects of Set-Met on the expression of MAVS protein and the phosphorylation of IRF-3 induced by PDCoV. LLC-PK cells were incubated with PDCoV for 1 h, and 2, 4, 8, and 16 μM of Se-Met was added. The expression of MAVS protein and the phosphorylation of IRF-3 were detected by western blot. Results were mean ± SD for three individual experiments.
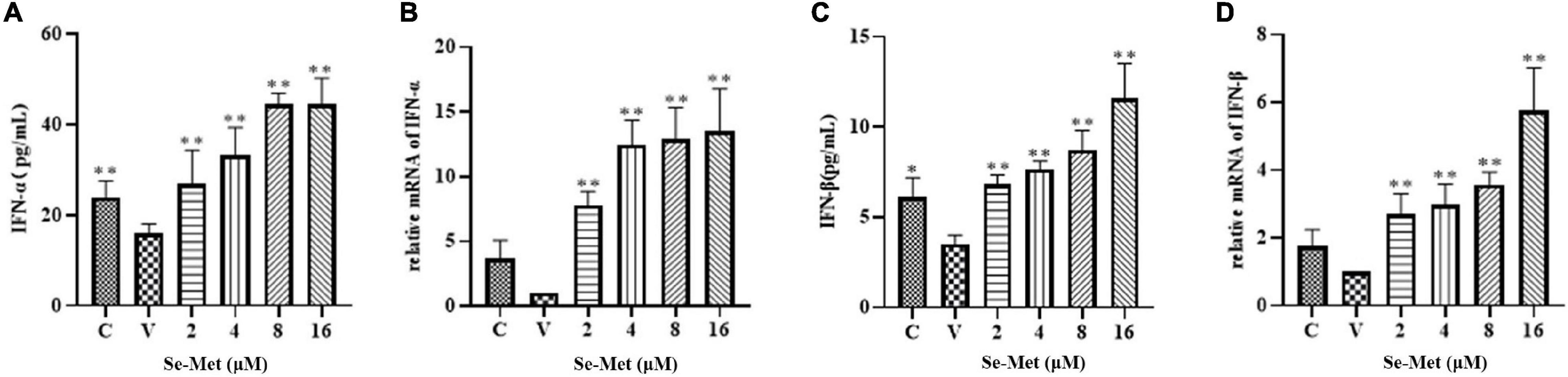
Figure 3. Effects of Set-Met on the changes of IFN-α/β in cells induced by PDCoV. LLC-PK cells were incubated with PDCoV for 1 h, and 2, 4, 8, and 16 μM of Se-Met was added. Changes in IFN-α/β were detected by ELISA (A,C) and RT qPCR (B,D). *0.01 < P < 0.05, **P < 0.01.
The effect of Set-Met on oxidative/antioxidant factors of LLC-PK cells induced by PDCoV. Se-Met and virus were applied to LLC-PK cells using the modalities described above. After cytocentrifugation, cells were disrupted with ultrasound and used to detect GSH-Px, H2O2, SOD, and GSH. As shown in Figure 4, PDCoV was able to reduce the activity of GSH-PX in the cells significantly (0.01 < P < 0.05), and although the contents of SOD and GSH were also decreased, they did not change significantly (P > 0.5). We found that 16 μM Se-Met was able to significantly increase the activity of GSH-PX and the content of SOD (P < 0.01). After PDCoV was treated with 8 and 16 μM Se-Met, the H2O2 content was significantly reduced (0.01 < P < 0.05). In summary, PDCoV induces oxidative stress in cells; however, Se-Met can alleviate this damage.
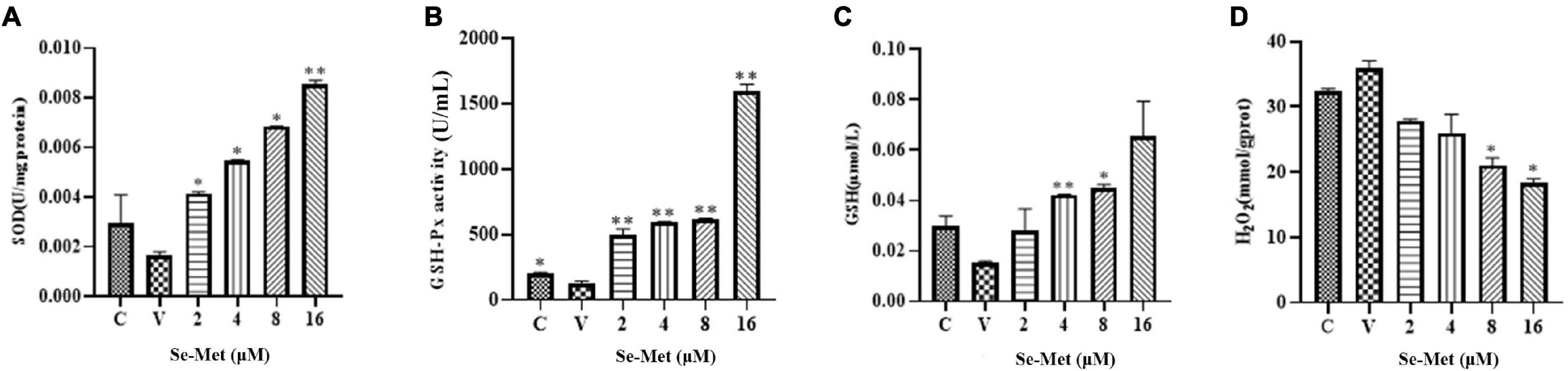
Figure 4. Effects of Set-Met on the changes of oxidative/antioxidant indexes in cells induced by PDCoV. LLC-PK cells were incubated with PDCoV for 1 h, and 2, 4, 8, and 16 μM of Se-Met was added. (A) Changes in SOD. (B) Changes in GSH-PX. (C) Changes in GSH. (D) Changes in H2O2. *0.01 < P < 0.05, **P < 0.01.
Since PDCoV was first reported in 2012, PDCoV infections have been reported in regions every year, and new lineages have been slowly discovered. PDCoV is currently seriously damaging the development of the pig industry, but there are no commercial vaccines and antiviral drugs commonly used in the breeding industry. To develop a good drug against PDCoV infection, we found that the nutrient selenium has biological functions such as antiviral, antioxidant, and immunomodulatory (Guillin et al., 2019; Bermano et al., 2021). Whether Se-Met, as an organoselenium, has an inhibitory effect on the infection of PDCoV is unknown. In this experiment, we first determined that the TCID50 of PDCoV for LLC-PK cells was 10–4⋅15/0.1 mL. Subsequently, referring to the results of Pan’s study (Pan et al., 2008), we confirmed that 16 μM Se-Met did not affect the growth of cells, which was used as the maximum effective concentration of Se-Met in this experiment for the determination of subsequent antiviral and antioxidant assays.
Given the current situation that there is no specific drug treatment for PDCoV, some people have successively explored the potential of broad-spectrum antiviral drugs in treating PDCoV. For example, lithium chloride and diammonium glycyrrhizinate could inhibit PDCoV replication in LLC-PK cells in a dose-dependent manner (Zhai et al., 2019). In addition, Zhang explored the inhibitory effect of LJ001 on PDCoV in three ways (Zhang et al., 2020). He found that the antiviral effect of pretreatment was not significant, which may be because the receptor sites available for virus attachment on the cell membrane are altered after the cells are pretreated with the drug, which affects the fusion of virus and cells (Zhang et al., 2020). Studies on the inhibition of viral replication by selenium have been reported, for example, HIV, Coxsackie virus, influenza virus, and PCV (Beck et al., 1994; Schrauzer and Sacher, 1994; Nelson et al., 2001; Jaspers et al., 2007; Qian et al., 2018). In this experiment, PDCoV was treated with Se-Met in a post-treatment manner. The results showed that Se-Met significantly inhibits PDCoV replication, and 16 μM Se-Met has the best effect; 16 μM Se-Met showed better antiviral activity, but 16 μM was far higher than the normal physiological concentration, and too high concentration of selenium would be counterproductive (Sivertsen et al., 2007; Zhao et al., 2016). We found that Se-Met at 8 and 16 μM had similar antiviral activity, and combined with the dangers of high concentrations of selenium, we believe that Se-Met at 8 μM is in line with clinical application. However, if we want to treat PDCoV, 8 μM Se-Met will not necessarily be clinically effective, so we also need to combine in vivo experiments.
After virus infection, the oxidative stress state of the organism can destroy the immune system in the body, which in turn facilitates virus replication (Zhang Z. et al., 2019). GSH-PX is an important enzyme in the biological function of selenium, and it achieves antioxidant effects by scavenging peroxides in the body (Tian et al., 2021). It was previously reported that selenium could alleviate virus-induced oxidative stress. For example, in the PK-15 cell model of PCV infection, 6 μM Se-Met can inhibit the increase of PCV2 replication by enhancing the activity of GSH-PX1 and inhibiting the production of H2O2 (Chen et al., 2012). SOD is an important antioxidant enzyme in organisms that scavenges superoxide radicals. Styblo et al. (2007) found that after mice were infected with the influenza virus, selenium supplementation significantly increased the SOD activity of the mouse liver. In addition, oseltamivir is an effective antiviral drug. Nano-selenium is surface-modified by oseltamivir, which significantly inhibits ROS generation induced by H1N1 (Li et al., 2017). This experiment verified that Se-Met could inhibit the replication of PDCoV in LLC-PK cells, and the GSH-Px, H2O2, GSH, and SOD changes in the virus groups and the treatment groups were further compared. The experimental results are consistent with previous results on other viruses; that is, PDCoV inhibits the viability of cellular GSH-Px, increasing the content of H2O2 in the cell, which in turn causes oxidative stress. The addition of Se-Met can significantly increase the ability of GSH-PX and GSH in cells. From this, we can infer that Se-Met can alleviate the oxidative stress caused by PDCoV by improving the antioxidant capacity of cells, thereby achieving the effect of inhibiting virus replication.
During virus infection and replication, innate immunity acts as the first line of defense of the immune response, clearing the virus from the host. As a key receptor for the recognition of RNA viruses, RLR can activate the downstream MAVS after binding to the virus, which in turn stimulates the expression of IRF and nuclear factor kappa-B (Chen et al., 2018; Ren et al., 2020). Then, the release of IFN, a key cytokine for the host to see viral immunity, is stimulated by IRF. However, viruses have been able to evade or fight the host’s immune system in various ways in continuous evolution (Wang et al., 2016; Kouwaki et al., 2021). It has been found that Hepatitis B can competitively bind MAVS with the help of lactate and further interfere with the binding of RLR to MAVS to achieve the effect of evading innate immunity (Zhang W. et al., 2019). Then, At least eight proteins encoded by severe acute respiratory syndrome coronavirus have been identified as interferon antagonists (Devaraj et al., 2007; Kopecky-Bromberg et al., 2007; Wathelet et al., 2007; Siu et al., 2009). In addition, PDCoV was also found to evade host immune responses, and it not only avoided IFN-β activation but also inhibited IFN-β production induced by SeV or Poly (I: C) (Luo et al., 2016). This reason may be attributed to the degradation of interferon by the ubiquitin proteasome encoded by PDCoV (Ji et al., 2020). Based on previous studies, we hope to improve the antiviral effect of host innate immunity by adding Se-Met. As expected, Se-Met could significantly increase the expression of MAVS protein, the phosphorylation of IRF-3, and the mRNA level of IFN-α/β. Therefore, we conclude that Se-Met can activate MAVS and IRF-3 in innate immunity and secrete a series of IFN to inhibit PDCoV replication.
In summary, this study suggests that Se-Met could inhibit PDCoV replication in a dose-dependent manner. The underlying mechanism may be attributed to the activation of MAVS and IRF-3 in innate immunity by Se-Met, which in turn secretes a series of cytokines. In addition, it may also be because Se-Met can improve the antioxidant capacity of cells.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
ZR, ZCZ, JD, YH, ZJZ, and SY contributed to the conception and design of the study. TD, HH, and YY performed the statistical analysis. GJ wrote the first draft of the manuscript. The rest of the authors reviewed and revised the manuscript. All authors reviewed the manuscript, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GSH, glutathione; GSH-PX, glutathione peroxidase; HIV, human immunodeficiency virus; H2O2, hydrogen peroxide; IRF-3, interferon regulatory factor-3; IFN, interferon; LLC-PK, Pig Kidney Epithelial; MAVS, mitochondrial antiviral signal protein; PDCoV, Porcine deltacoronavirus; PCV, Porcine circovirus; RLR, RIG-I-like receptor; Se-Met, selenomethionine; SOD, superoxide dismutase; TrxR, thioredoxin reductase; TCID50, tissue culture infectious dose.
Beck, M. A., Kolbeck, P. C., Rohr, L. H., Shi, Q., Morris, V. C., and Levander, O. A. (1994). Benign human enterovirus becomes virulent in selenium-deficient mice. J. Med. Virol. 43, 166–170. doi: 10.1002/jmv.1890430213
Bermano, G., Meplan, C., Mercer, D. K., and Hesketh, J. E. (2021). Selenium and viral infection: are there lessons for COVID-19? Br. J. Nutr. 125, 618–627. doi: 10.1017/S0007114520003128
Brigelius-Flohe, R., and Maiorino, M. (2013). Glutathione peroxidases. Biochim. Biophys. Acta 1830, 3289–3303. doi: 10.1016/j.bbagen.2012.11.020
Chen, X., Liu, S., Goraya, M. U., Maarouf, M., Huang, S., and Chen, J. L. (2018). Host Immune Response to Influenza A Virus Infection. Front. Immunol. 9:320. doi: 10.3389/fimmu.2018.00320
Chen, X., Ren, F., Hesketh, J., Shi, X., Li, J., Gan, F., et al. (2012). Selenium blocks porcine circovirus type 2 replication promotion induced by oxidative stress by improving GPx1 expression. Free Radic. Biol. Med. 53, 395–405. doi: 10.1016/j.freeradbiomed.2012.04.035
Chu, F. F., Esworthy, R. S., Doroshow, J. H., Doan, K., and Liu, X. F. (1992). Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood 79, 3233–3238.
Devaraj, S. G., Wang, N., Chen, Z., Chen, Z., Tseng, M., Barretto, N., et al. (2007). Regulation of IRF-3-dependent innate immunity by the papain-like protease domain of the severe acute respiratory syndrome coronavirus. J. Biol. Chem. 282, 32208–32221. doi: 10.1074/jbc.M704870200
Guillin, O. M., Vindry, C., Ohlmann, T., and Chavatte, L. (2019). Selenium. Selenoproteins and Viral Infection. Nutrients 11:2101. doi: 10.3390/nu11092101
Hou, F., Sun, L., Zheng, H., Skaug, B., Jiang, Q. X., and Chen, Z. J. (2011). MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461. doi: 10.1016/j.cell.2011.06.041
Huang, Z., Rose, A. H., and Hoffmann, P. R. (2012). The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 16, 705–743. doi: 10.1089/ars.2011.4145
Hurwitz, B. E., Klaus, J. R., Llabre, M. M., Gonzalez, A., and Lawrence, P. J. (2007). Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch. Intern. Med. 167, 148–154. doi: 10.1001/archinte.167.2.148
Ingold, I., Berndt, C., Schmitt, S., Doll, S., and Poschmann, G. (2018). Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 172, 409-422. doi: 10.1016/j.cell.2017.11.048
Jaspers, I., Zhang, W., Brighton, L. E., Carson, J. L., Styblo, M., and Beck, M. A. (2007). Selenium deficiency alters epithelial cell morphology and responses to influenza. Free Radic. Biol. Med. 42, 1826–1837. doi: 10.1016/j.freeradbiomed.2007.03.017
Ji, L., Wang, N., Ma, J., Cheng, Y., Wang, H., Sun, J., et al. (2020). Porcine deltacoronavirus nucleocapsid protein species-specifically suppressed IRF7-induced type I interferon production via ubiquitin-proteasomal degradation pathway. Vet. Microbiol. 250:108853. doi: 10.1016/j.vetmic.2020.108853
Kopecky-Bromberg, S. A., Martinez-Sobrido, L., Frieman, M., Baric, R. A., and Palese, P. (2007). Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 81, 548–557. doi: 10.1128/JVI.01782-06
Kouwaki, T., Nishimura, T., Wang, G., and Oshiumi, H. (2021). RIG-I-Like Receptor-Mediated Recognition of Viral Genomic RNA of Severe Acute Respiratory Syndrome Coronavirus-2 and Viral Escape From the Host Innate Immune Responses. Front. Immunol. 12:700926. doi: 10.3389/fimmu.2021.700926
Lei, X. G., Cheng, W. H., and McClung, J. P. (2007). Metabolic regulation and function of glutathione peroxidase-1. Annu. Rev. Nutr. 27, 41–61. doi: 10.1146/annurev.nutr.27.061406.093716
Li, Y., Lin, Z., Guo, M., Xia, Y., Zhao, M., Wang, C., et al. (2017). Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 12, 5733–5743. doi: 10.2147/IJN.S140939
Luo, J., Fang, L., Dong, N., Fang, P., Ding, Z., Wang, D., et al. (2016). Porcine deltacoronavirus (PDCoV) infection suppresses RIG-I-mediated interferon-beta production. Virology 495, 10–17. doi: 10.1016/j.virol.2016.04.025
Ma, Y., Zhang, Y., Liang, X., Lou, F., Oglesbee, M., Krakowka, S., et al. (2015). Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio 6:e00064. doi: 10.1128/mBio.00064-15
Nelson, H. K., Shi, Q., Van Dael, P., Schiffrin, E. J., Blum, S., Barclay, D., et al. (2001). Host nutritional selenium status as a driving force for influenza virus mutations. FASEB. J. 15, 1727–1738.
Niederwerder, M. C., and Hesse, R. A. (2018). Swine enteric coronavirus disease: a review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transbound. Emerg. Dis. 65, 660–675. doi: 10.1111/tbed.12823
Pan, Q., Huang, K., He, K., and Lu, F. (2008). Effect of different selenium sources and levels on porcine circovirus type 2 replication in vitro. J. Trace Elem. Med. Biol. 22, 143–148. doi: 10.1016/j.jtemb.2008.02.002
Qian, G., Liu, D., Hu, J., Gan, F., Hou, L., Zhai, N., et al. (2018). SeMet attenuates OTA-induced PCV2 replication promotion by inhibiting autophagy by activating the AKT/mTOR signaling pathway. Vet. Res. 49:15. doi: 10.1186/s13567-018-0508-z
Ren, Z., Ding, T., Zuo, Z., Xu, Z., Deng, J., and Wei, Z. (2020). Regulation of MAVS Expression and Signaling Function in the Antiviral Innate Immune Response. Front. Immunol. 11:1030. doi: 10.3389/fimmu.2020.01030
Schrauzer, G. N., and Sacher, J. (1994). Selenium in the maintenance and therapy of HIV-infected patients. Chem. Biol. Interact. 91, 199–205. doi: 10.1016/0009-2797(94)90040-x
Siu, K. L., Kok, K. H., Ng, M. J., Poon, V. K. M., Yuen, K. Y., Zheng, B. J., et al. (2009). Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J. Biol. Chem. 284, 16202–16209. doi: 10.1074/jbc.M109.008227
Sivertsen, T., Vie, E., Bernhoft, A., and Baustad, B. (2007). Vitamin E and selenium plasma concentrations in weanling pigs under field conditions in Norwegian pig herds. Acta. Vet. Scand. 49:1. doi: 10.1186/1751-0147-49-1
Styblo, M., Walton, F. S., Harmon, A. W., Sheridan, P. A., and Beck, M. A. (2007). Activation of superoxide dismutase in selenium-deficient mice infected with influenza virus. J. Trace Elem. Med. Biol. 21, 52–62. doi: 10.1016/j.jtemb.2006.11.001
Tian, R., Geng, Y., Yang, Y., Seim, I., and Yang, G. (2021). Oxidative stress drives divergent evolution of the glutathione peroxidase (GPX) gene family in mammals. Integr. Zool. 16, 696–711. doi: 10.1111/1749-4877.12521
Wang, D., Fang, L., Shi, Y., Zhang, H., Gao, L., Peng, G., et al. (2016). Porcine Epidemic Diarrhea Virus 3C-Like Protease Regulates Its Interferon Antagonism by Cleaving NEMO. J. Virol. 90, 2090–2101. doi: 10.1128/JVI.02514-15
Wathelet, M. G., Orr, M., Frieman, M. B., and Baric, R. S. (2007). Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 81, 11620–11633. doi: 10.1128/JVI.00702-07
Weekley, C. M., and Harris, H. H. (2013). Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 42, 8870–8894. doi: 10.1039/c3cs60272a
Wingler, K., and Brigelius-Flohe, R. (1999). Gastrointestinal glutathione peroxidase. Biofactors 10, 245–249. doi: 10.1002/biof.5520100223
Xu, Z., Zhong, H., Zhou, Q., Du, Y., Chen, L., Zhang, Y., et al. (2018). A Highly Pathogenic Strain of Porcine Deltacoronavirus Caused Watery Diarrhea in Newborn Piglets. Virol. Sin. 33, 131–141. doi: 10.1007/s12250-018-0003-8
Yin, L., Chen, J., Li, L., Guo, S., Xue, M., Zhang, J., et al. (2020). Aminopeptidase N Expression, Not Interferon Responses, Determines the Intestinal Segmental Tropism of Porcine Deltacoronavirus. J. Virol. 94, e00480–20. doi: 10.1128/JVI.00480-20
Zhai, X., Wang, S., Zhu, M., He, W., Pan, Z., and Su, S. (2019). Antiviral Effect of Lithium Chloride and Diammonium Glycyrrhizinate on Porcine Deltacoronavirus In Vitro. Pathogens 8:144. doi: 10.3390/pathogens8030144
Zhang, J. (2016). Porcine deltacoronavirus: overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 226, 71–84. doi: 10.1016/j.virusres.2016.05.028
Zhang, W., Wang, G., Xu, Z. G., Tu, H., Hu, F., Dai, J., et al. (2019). Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 178, 176–189. doi: 10.1016/j.cell.2019.05.003
Zhang, Y., Xia, L., Yuan, Y., Li, Q., Han, L., Yang, G., et al. (2020). Rhodanine derivative LJ001 inhibits TGEV and PDCoV replication in vitro. Virus Res. 289:198167. doi: 10.1016/j.virusres.2020.198167
Zhang, Z., Rong, L., and Li, Y. P. (2019). Flaviviridae Viruses and Oxidative Stress: implications for Viral Pathogenesis. Oxid. Med. Cell Longev. 2019:1409582. doi: 10.1155/2019/1409582
Keywords: porcine deltacoronavirus, antiviral activities, antioxidant, selenomethionine, innate immunity
Citation: Ren Z, Jia G, He H, Ding T, Yu Y, Zuo Z, Hu Y, Zhong Z, Yu S, Deng H, Shen L, Cao S, Peng G, Wang Y, Cai D, Gou L, Ma X, Liu H, Zhou Z, Deng Y, Yang D and Deng J (2022) Antiviral Effect of Selenomethionine on Porcine Deltacoronavirus in Pig Kidney Epithelial Cells. Front. Microbiol. 13:846747. doi: 10.3389/fmicb.2022.846747
Received: 31 December 2021; Accepted: 24 January 2022;
Published: 15 February 2022.
Edited by:
Chunfu Zheng, University of Calgary, CanadaReviewed by:
Geert Janssens, Ghent University, BelgiumCopyright © 2022 Ren, Jia, He, Ding, Yu, Zuo, Hu, Zhong, Yu, Deng, Shen, Cao, Peng, Wang, Cai, Gou, Ma, Liu, Zhou, Deng, Yang and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingyong Yang, NDk4MTI3NjE3QHFxLmNvbQ==; Junliang Deng, ZGVuZ2psMjEzQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.